Solid acid catalyst as well as preparation method and application of solid acid catalyst
A solid acid catalyst and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic chemistry, etc., can solve the problems of low utilization rate of active sites, difficulty in bare leakage, etc., and achieve cycle stability and utilization The effect of high yield and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 0.3 g of zirconium hydrogen phosphate and disperse it in 20 mL of deionized water, ultrasonically stir for 3 hours, then add 10 mL of 0.1 mol / L 1-propylamine, and ultrasonically treat for 3 hours in an ice bath to obtain exfoliated zirconium hydrogen phosphate nanosheets. Then drop 10mL of 0.1mol / L HCl into it, and wash by centrifugation until there is no Cl in the filtrate. - In the presence of protonated zirconium hydrogen phosphate nanosheets.
[0032] (2) Preparation of solid acid catalyst
[0033] Using ion exchange method, 0.3655g Zn(AC) 2 Dissolve in 50mL deionized water, add the above-mentioned protonated zirconium hydrogen phosphate nanosheets, wherein Zn(AC) 2 The molar ratio of zinc ions to zirconium hydrogen phosphate is 2:1. Stir and sonicate for 2 hours, then reflux at 80°C for 12 hours. After the reaction is finished, let it stand for 12 hours, set the centrifugal speed to 20000r / min, centrifuge for 2 minutes, wash with deionized water until th...
Embodiment 2
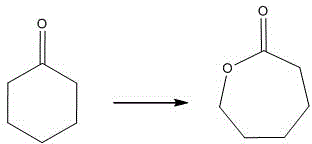
[0036] When the solid acid catalyst prepared in Examples 1-2 is applied to the Baeyer-Villiger oxidation reaction, the specific method can be: weigh 0.05g of solid acid, add 0.3951g of cyclohexanone, 2.2391g of 30wt.% hydrogen peroxide, at 70°C React for 12 hours, the reaction chemical formula is as figure 1 shown. After the reaction, the catalyst was centrifugally separated, and the catalyst was washed with hydrogen peroxide to carry out the next round of reaction. A total of 5 catalytic reactions were performed. After the reaction solution was filtered, the gas-mass spectrometer was used to analyze the yield of the product caprolactone. The results are shown in Table 1.
[0037] Example 3
Embodiment 3
[0039] Weigh 1.2 g of zirconium hydrogen phosphate and disperse it in 50 mL of deionized water, ultrasonically stir for 5 hours, then add 4 mL of 1 mol / L 1-propylamine, and then ultrasonically treat for 5 hours in an ice bath to obtain exfoliated zirconium hydrogen phosphate nanosheets . Then drop 4 mL of 1 mol / L HCl into it, and wash by centrifugation until there is no Cl in the filtrate. - In the presence of protonated zirconium hydrogen phosphate nanosheets.
[0040] (2) Preparation of solid acid catalyst
[0041] In this example, the ion exchange method is adopted, and the molar ratio of yttrium ion to zirconium hydrogen phosphate is 3:1, and 1.7684g C 2 h 3 o2 Y was dissolved in 60 mL of deionized water, and then 1.2 g of the above-mentioned protonated zirconium hydrogen phosphate nanosheets were added, and stirred at 90° C. for 12 hours. After the reaction was completed, let it stand for 5 hours, set the centrifugal speed to 10000 r / min, centrifuged for 15 minutes to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com