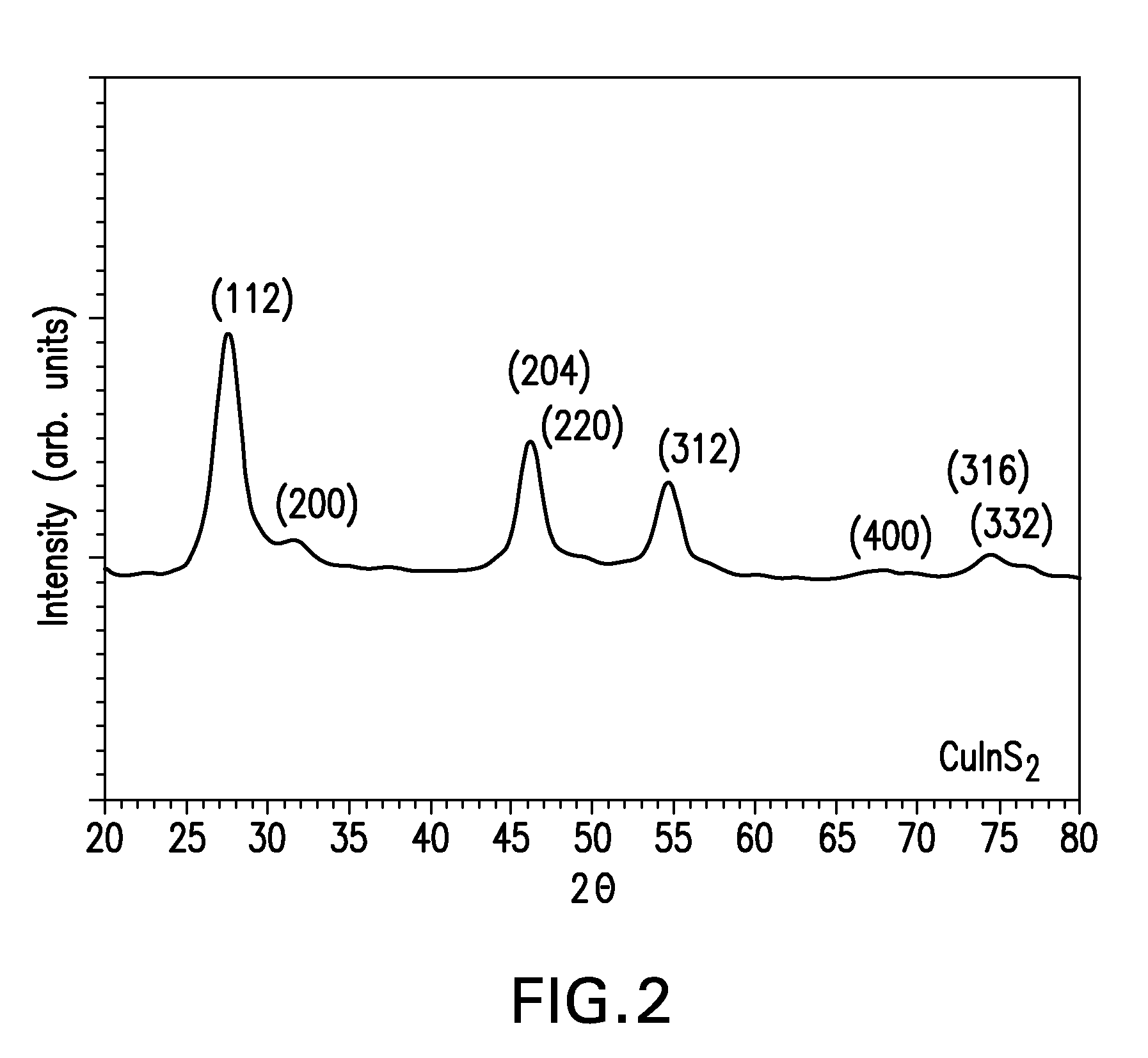
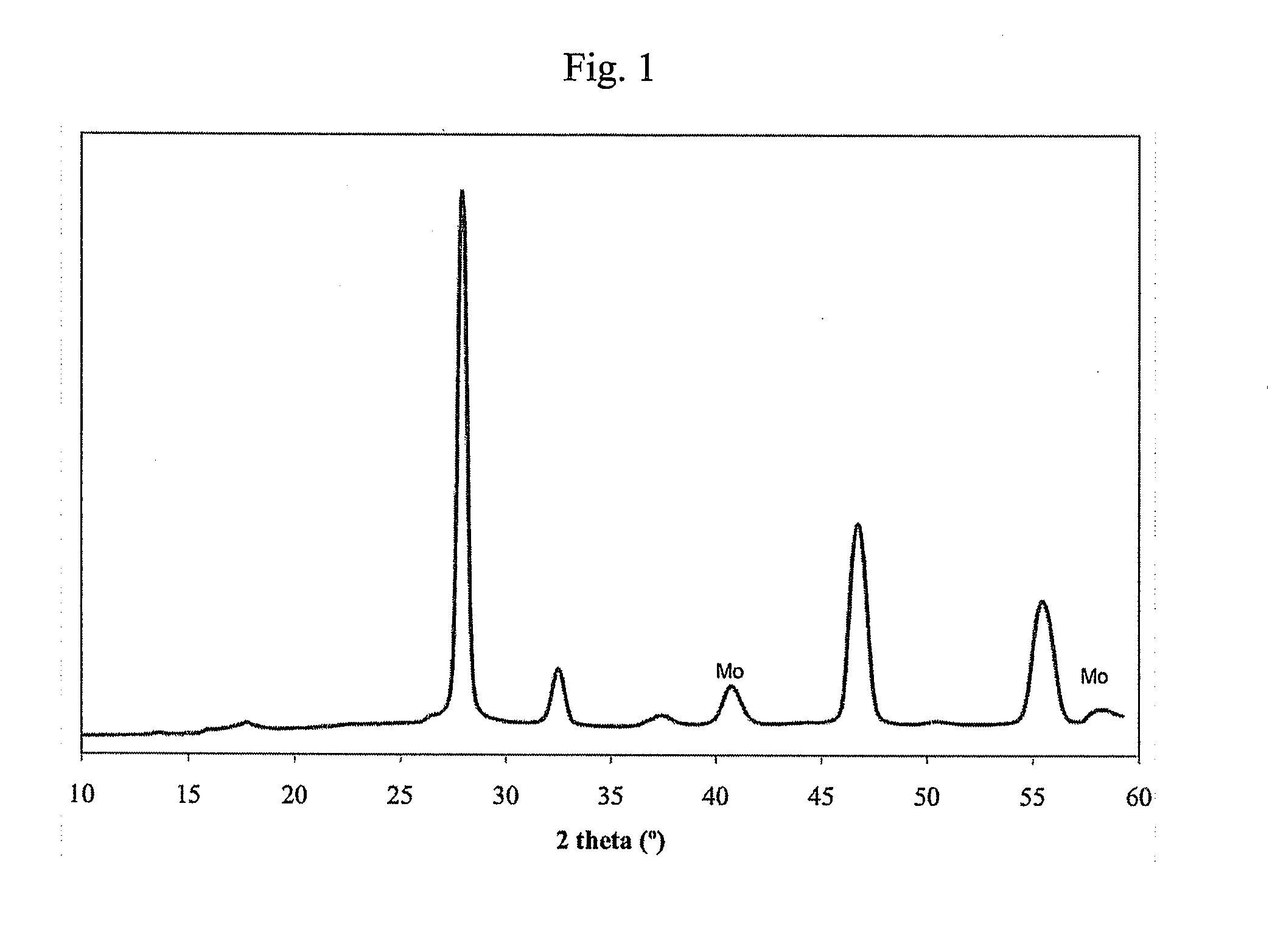
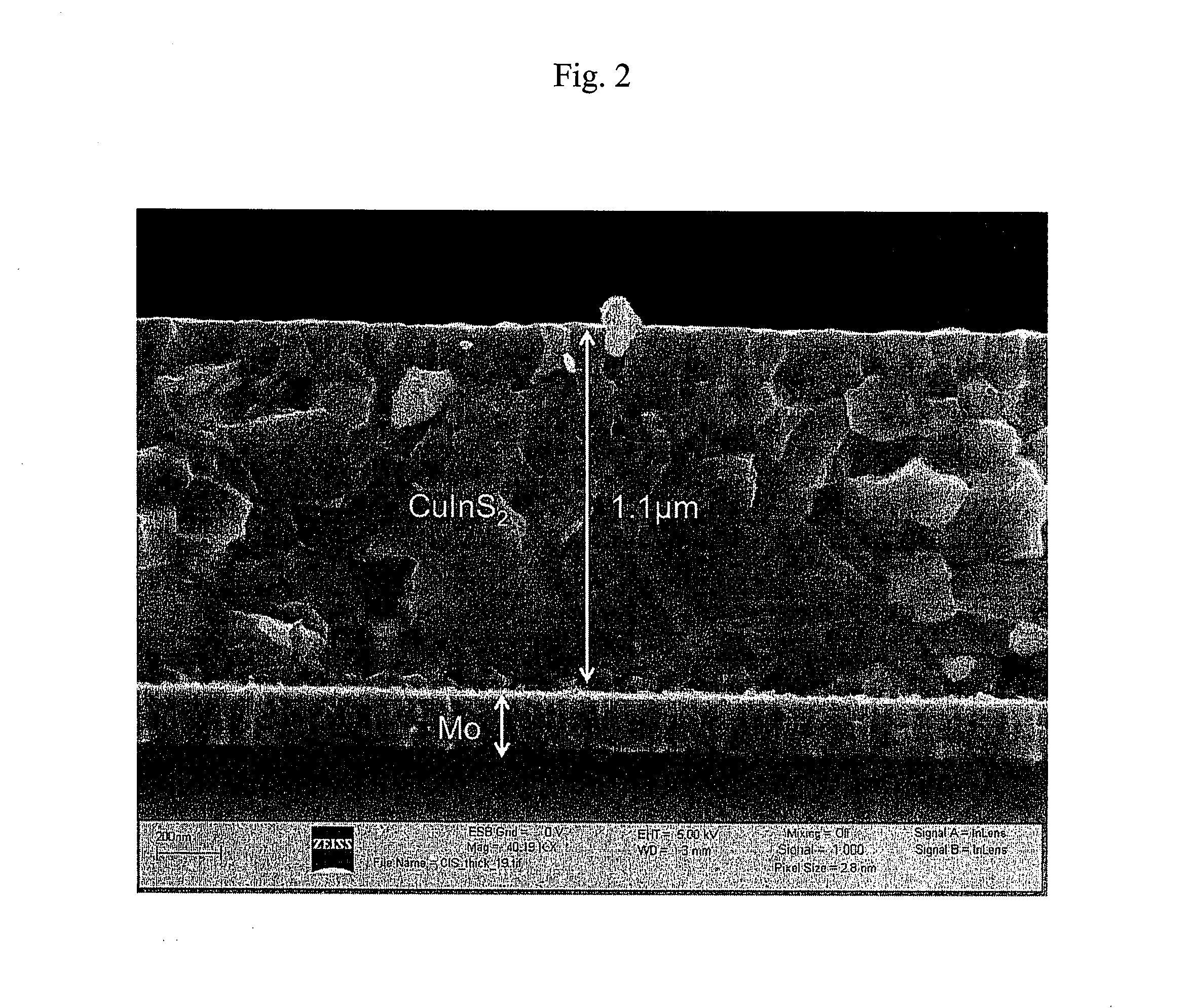
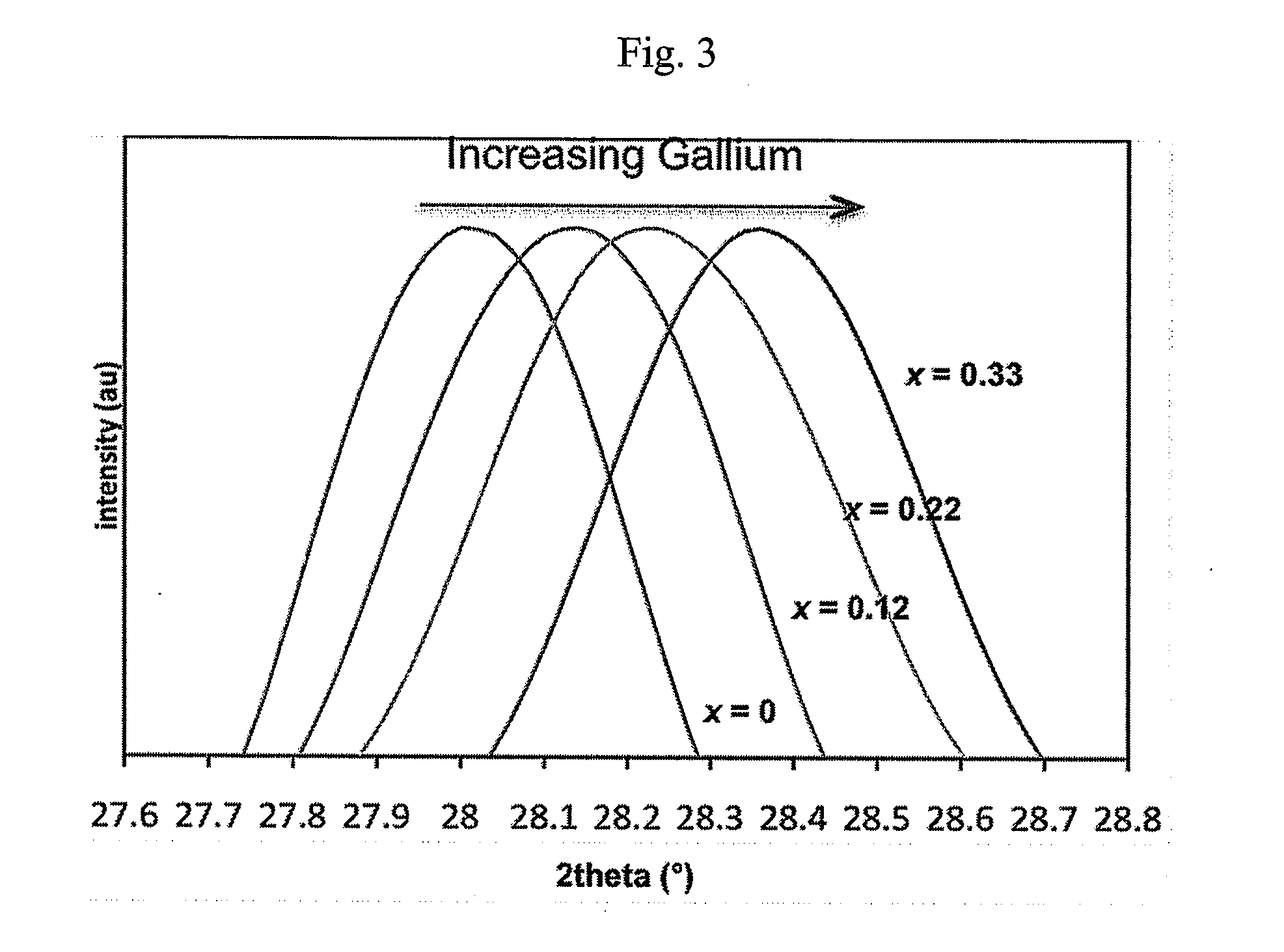
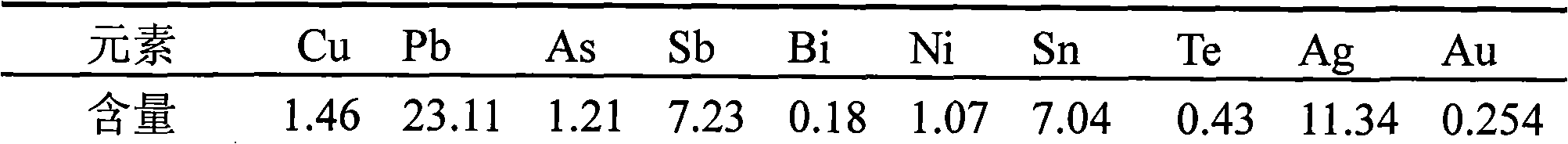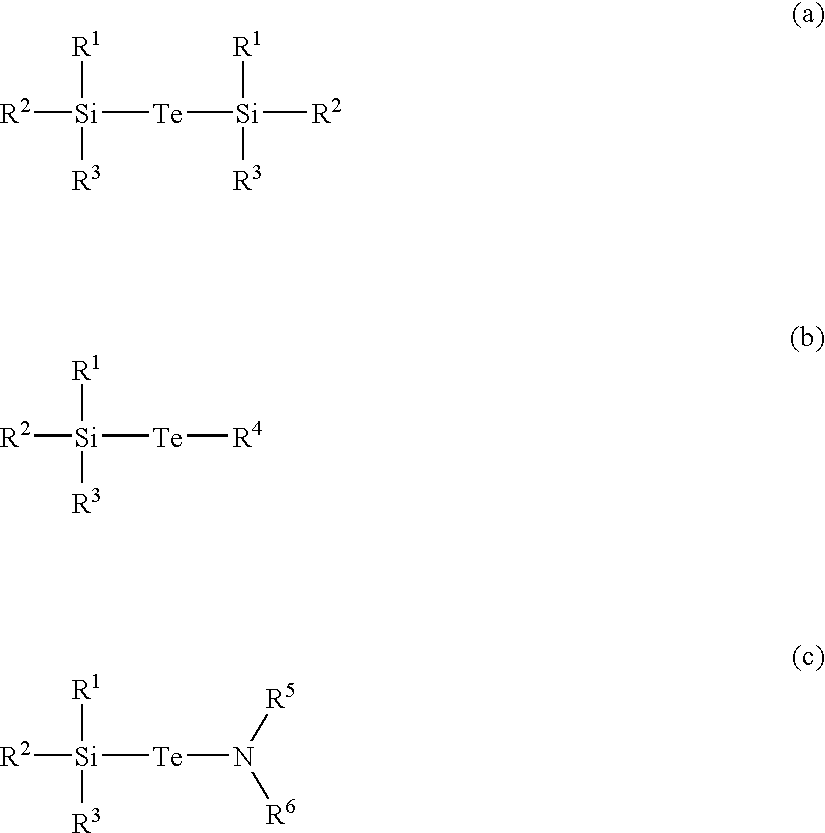Patents
Literature
407results about "Selenium/tellurium compunds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
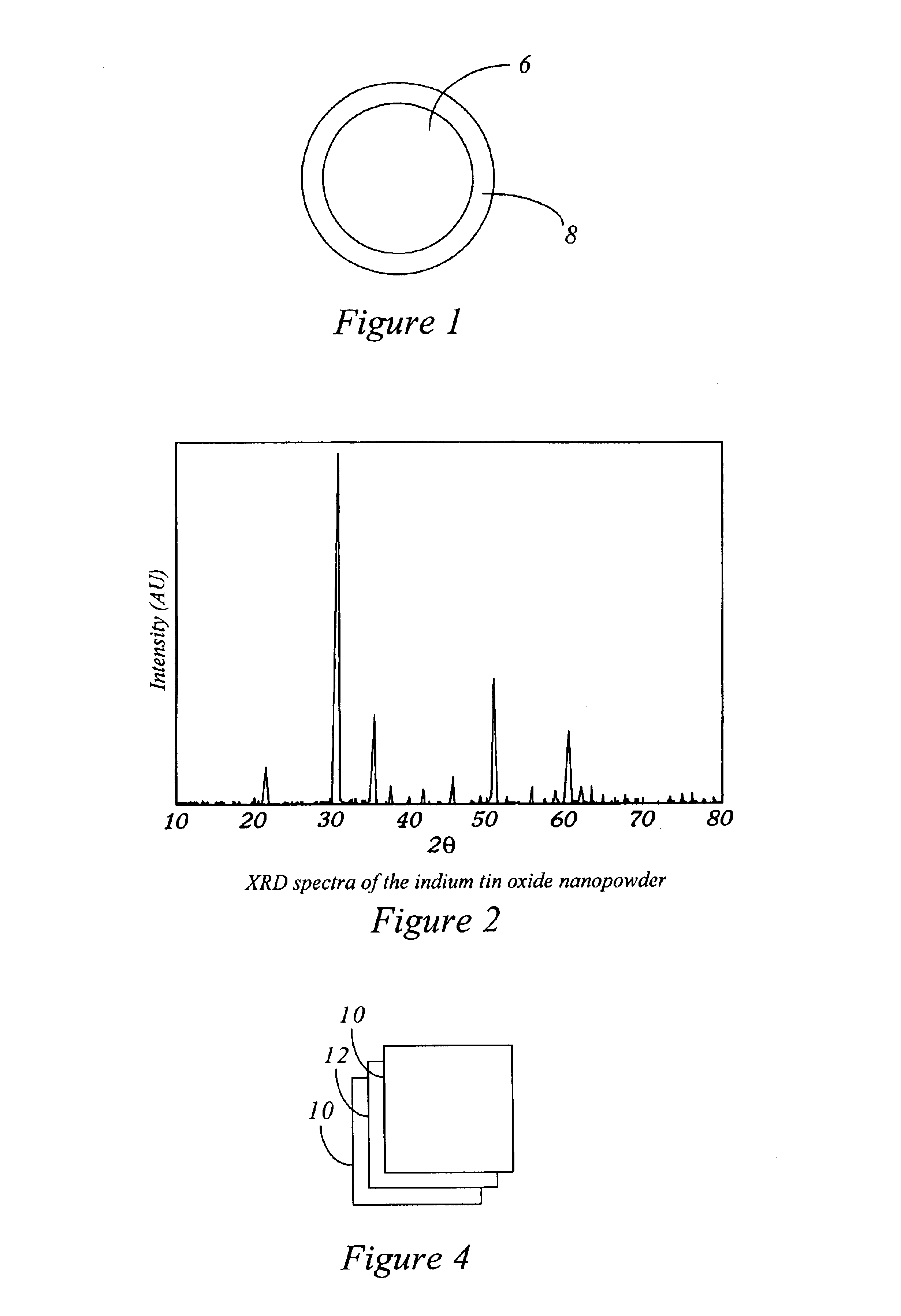
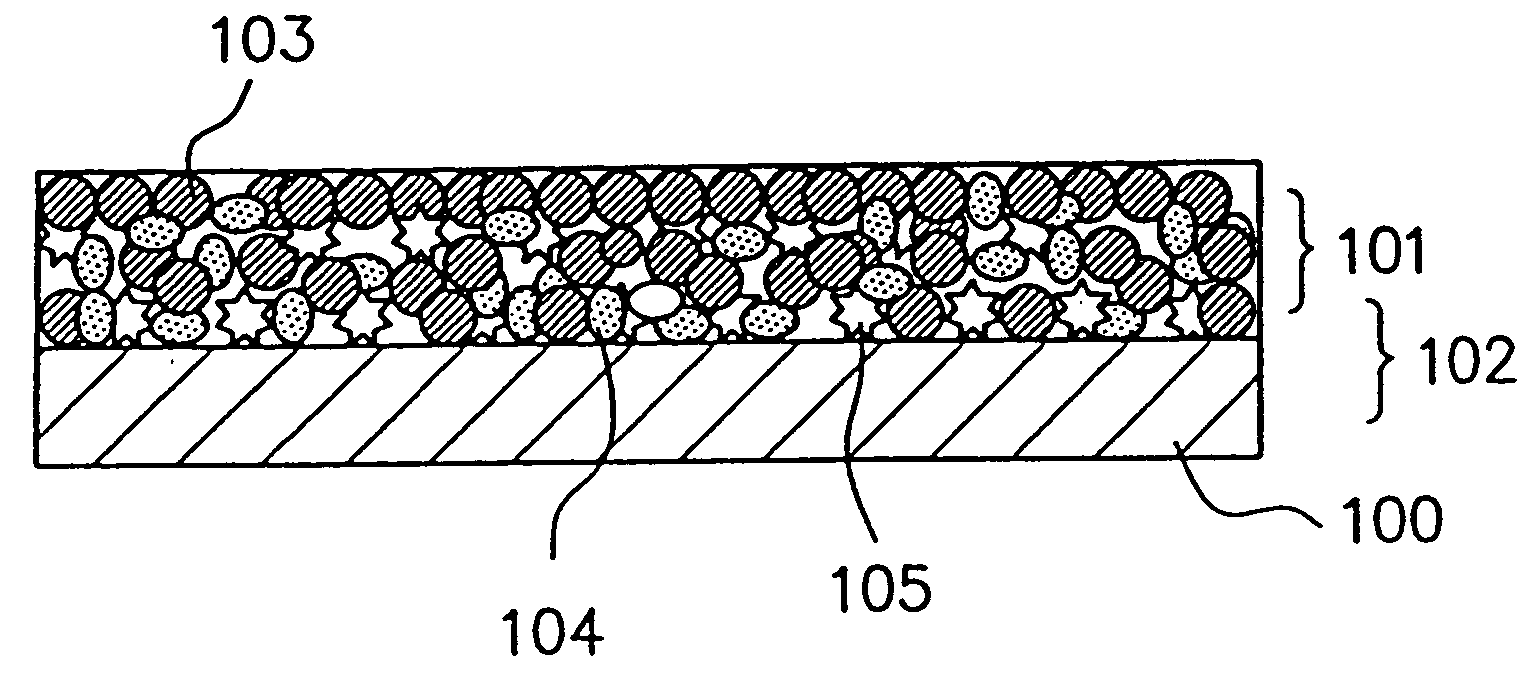
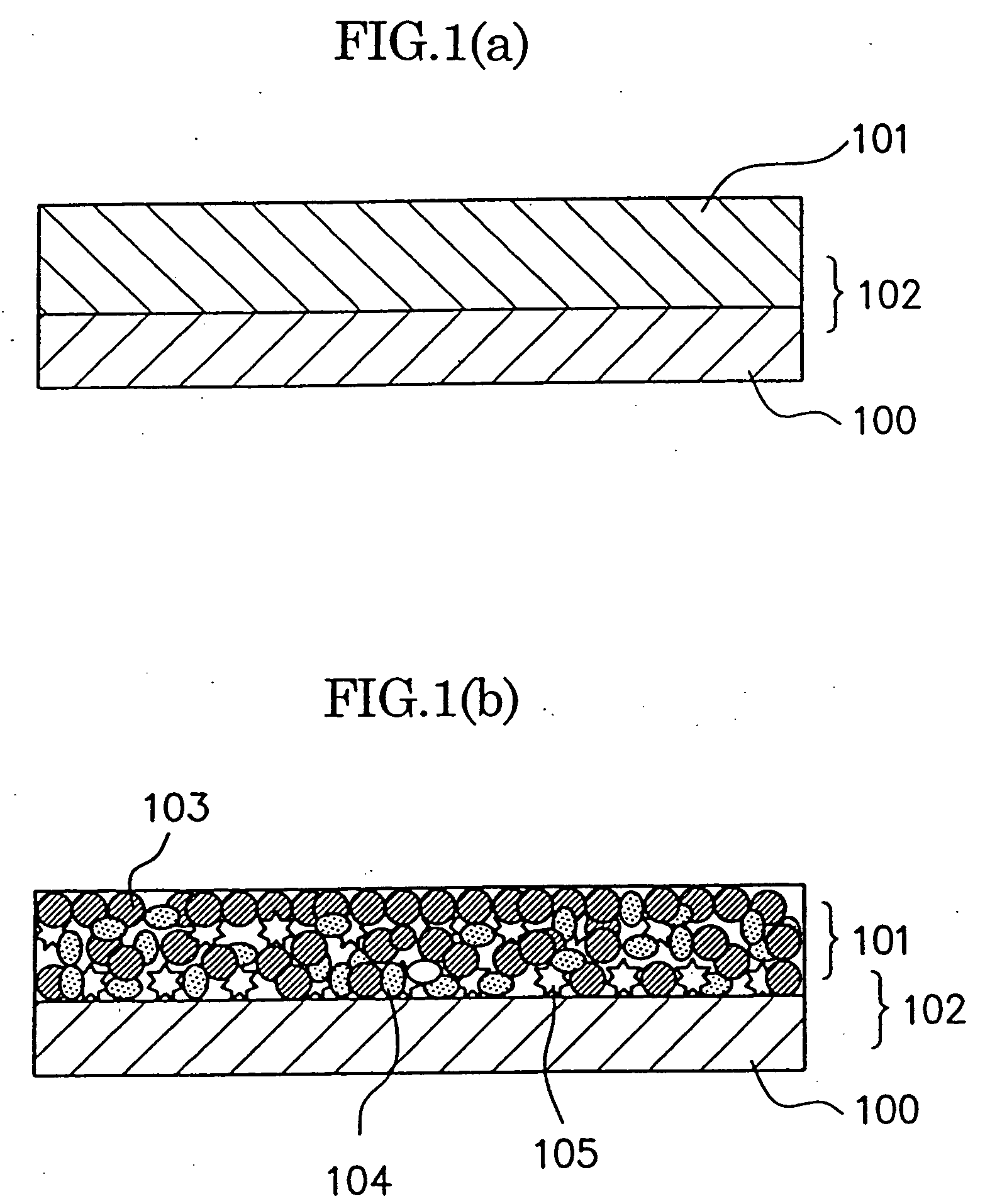
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS20080206124A1Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumPhysical chemistry
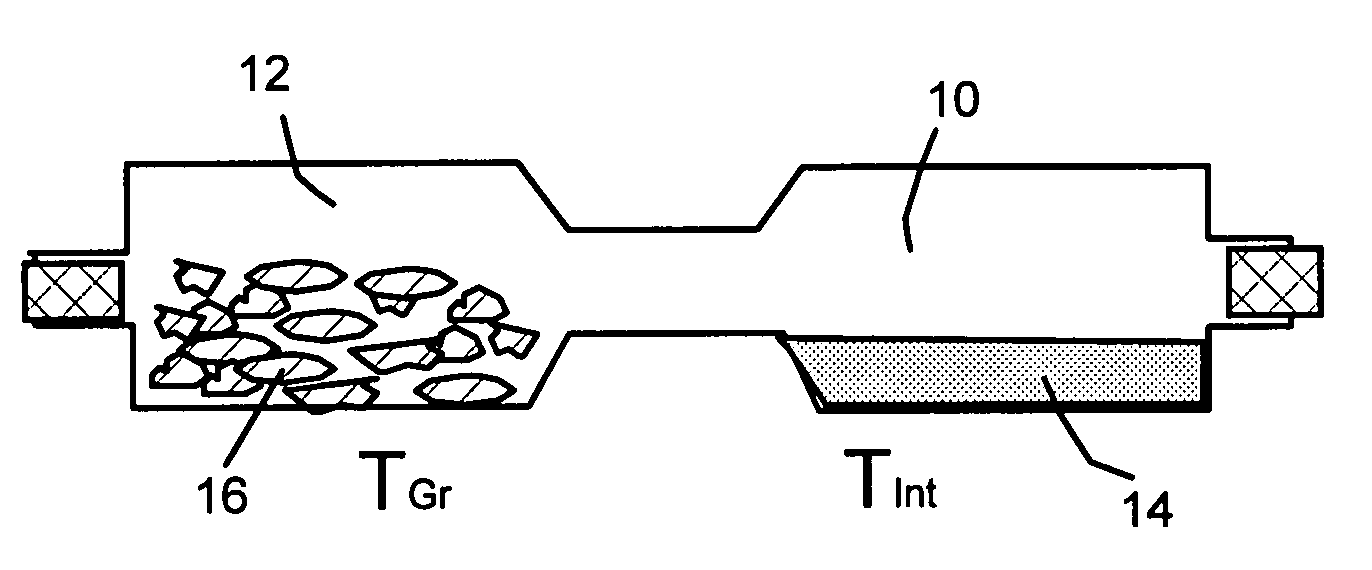
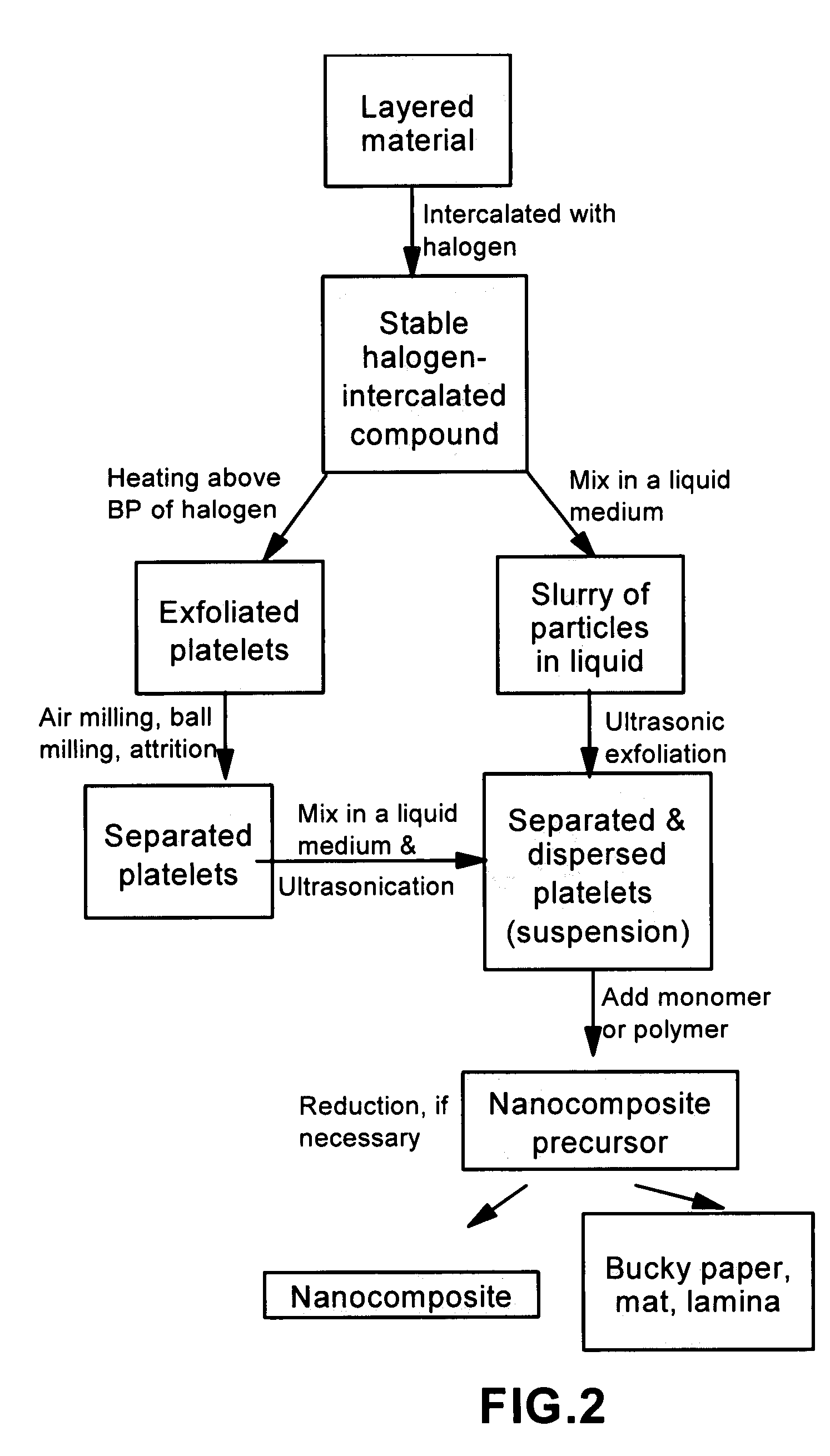
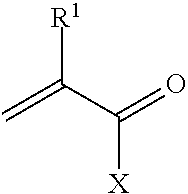
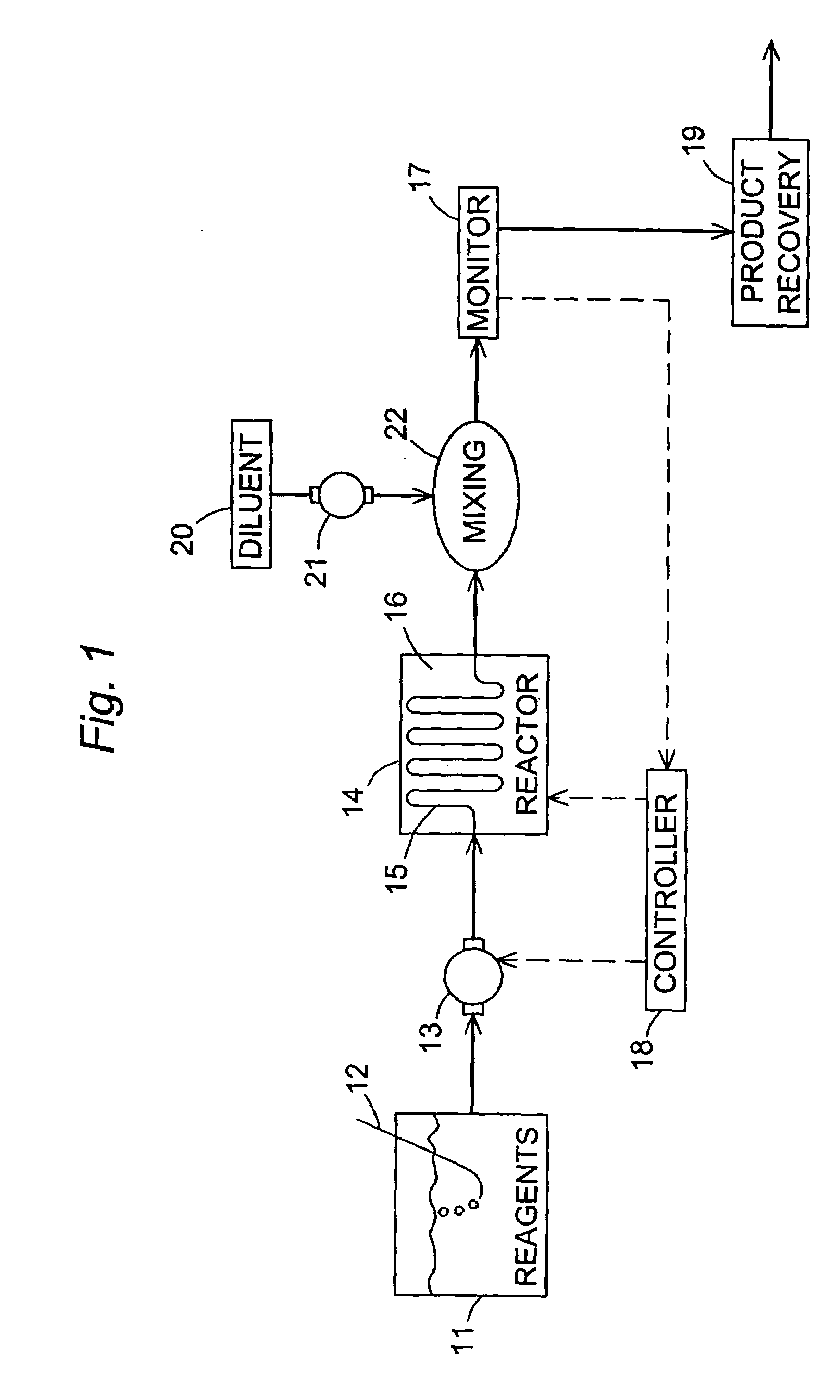
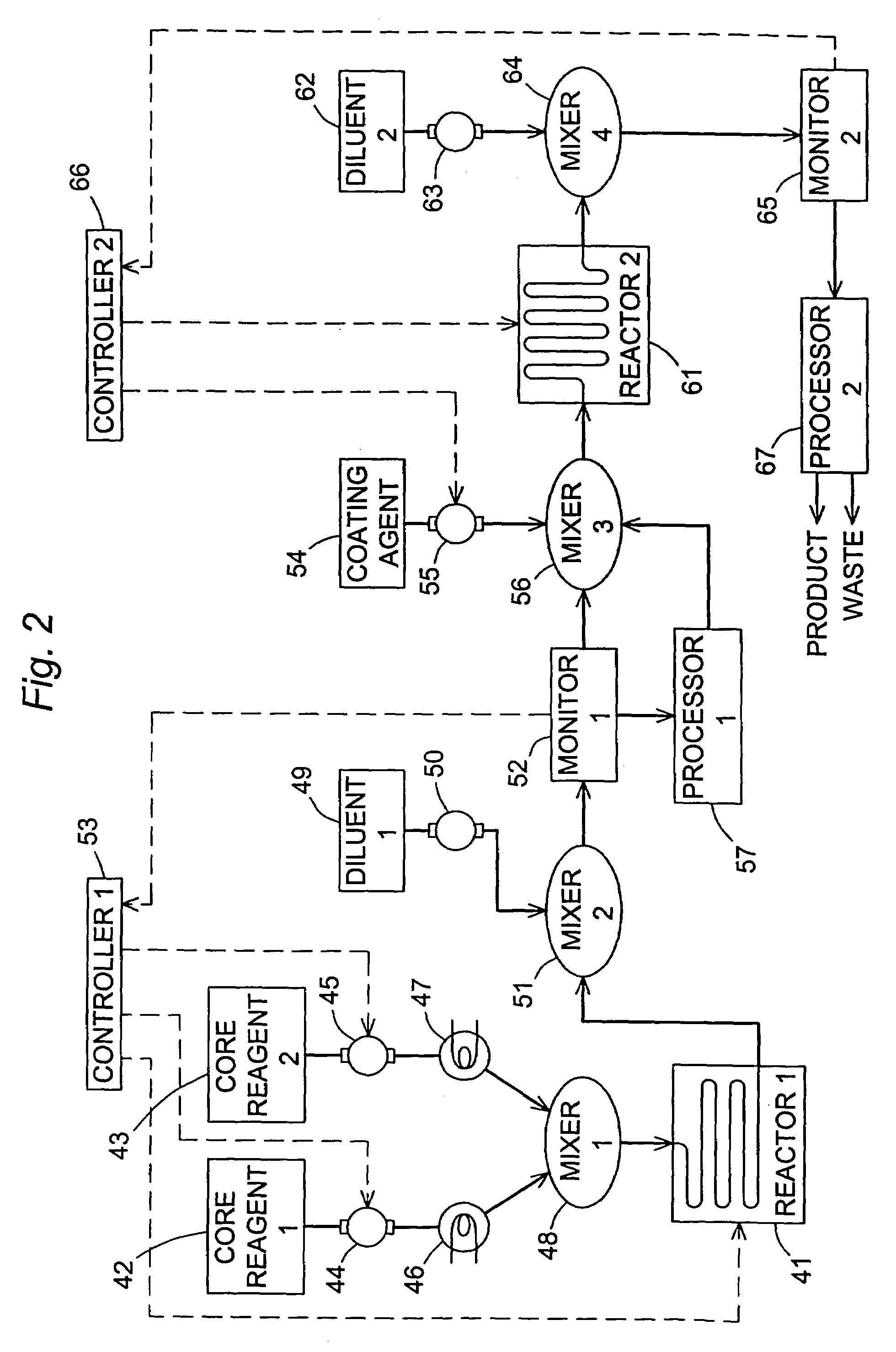
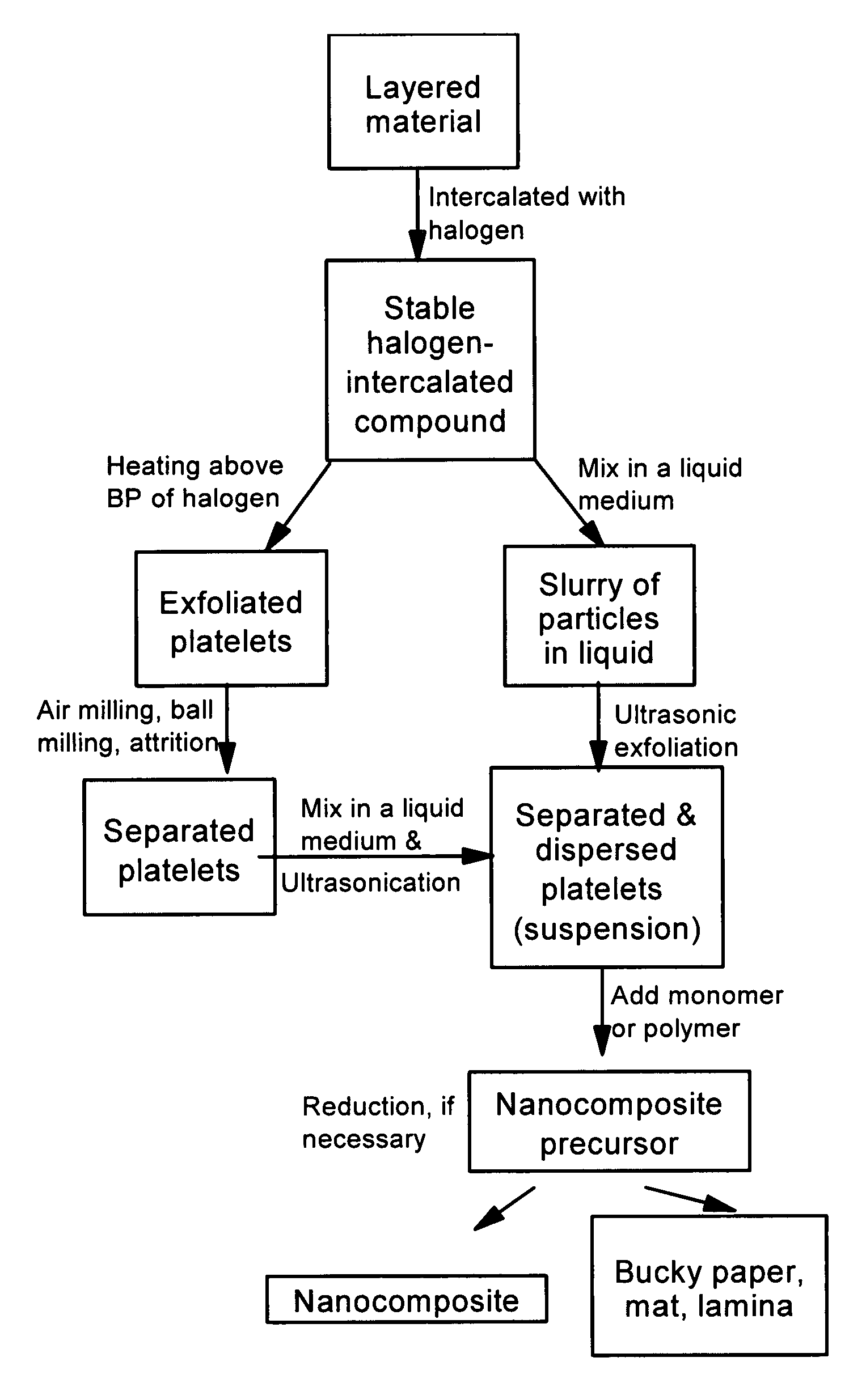
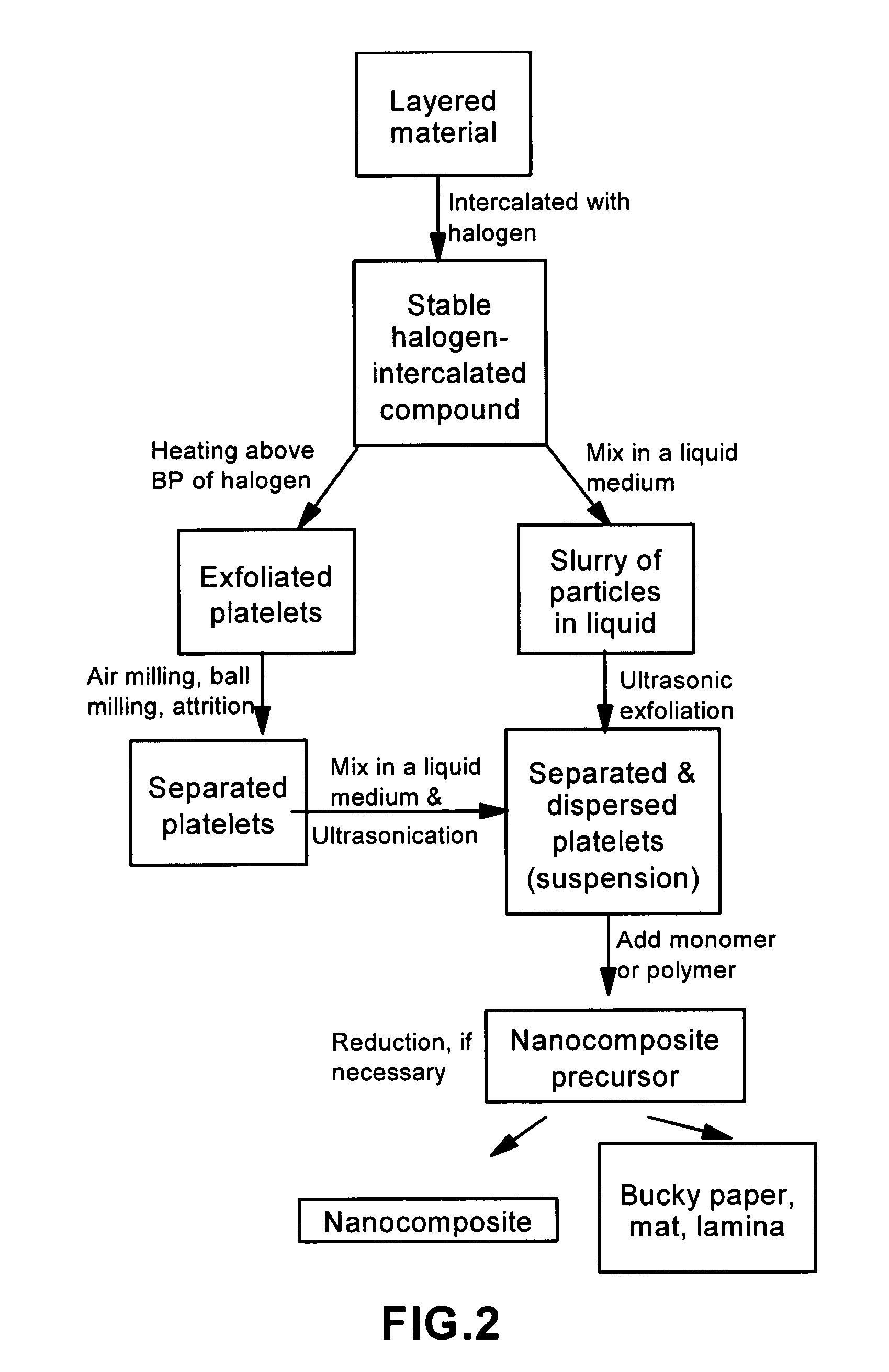
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
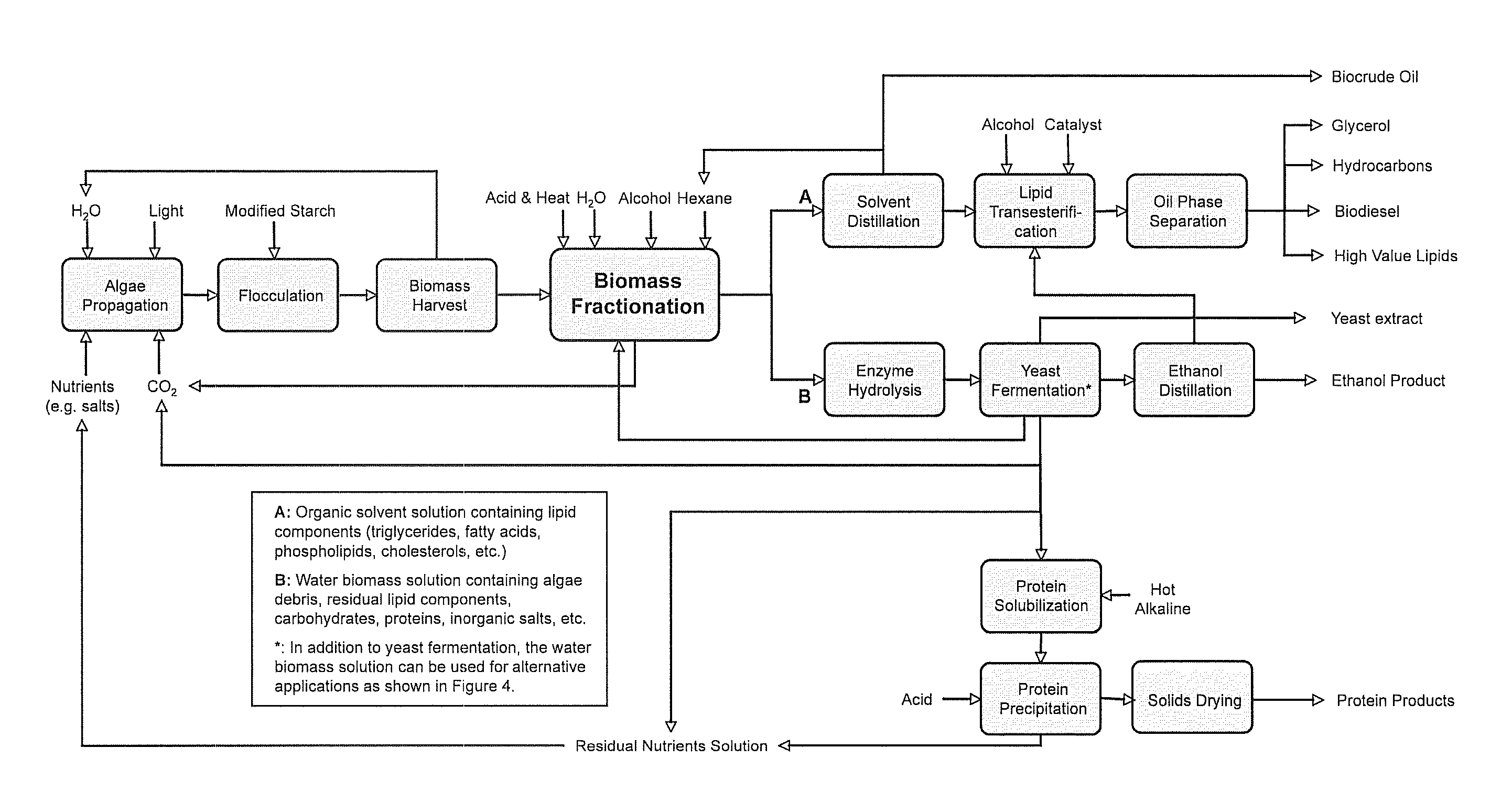
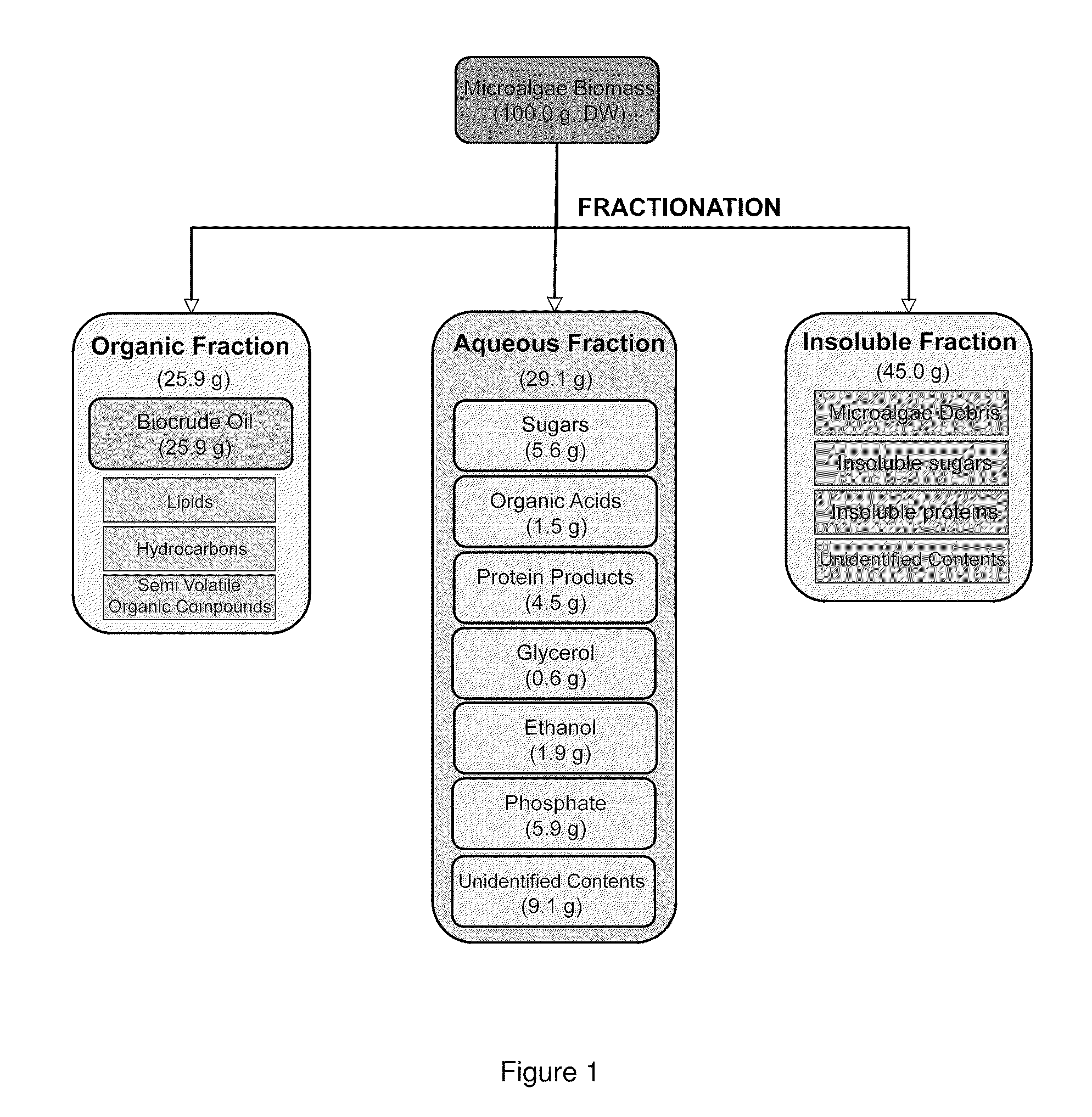
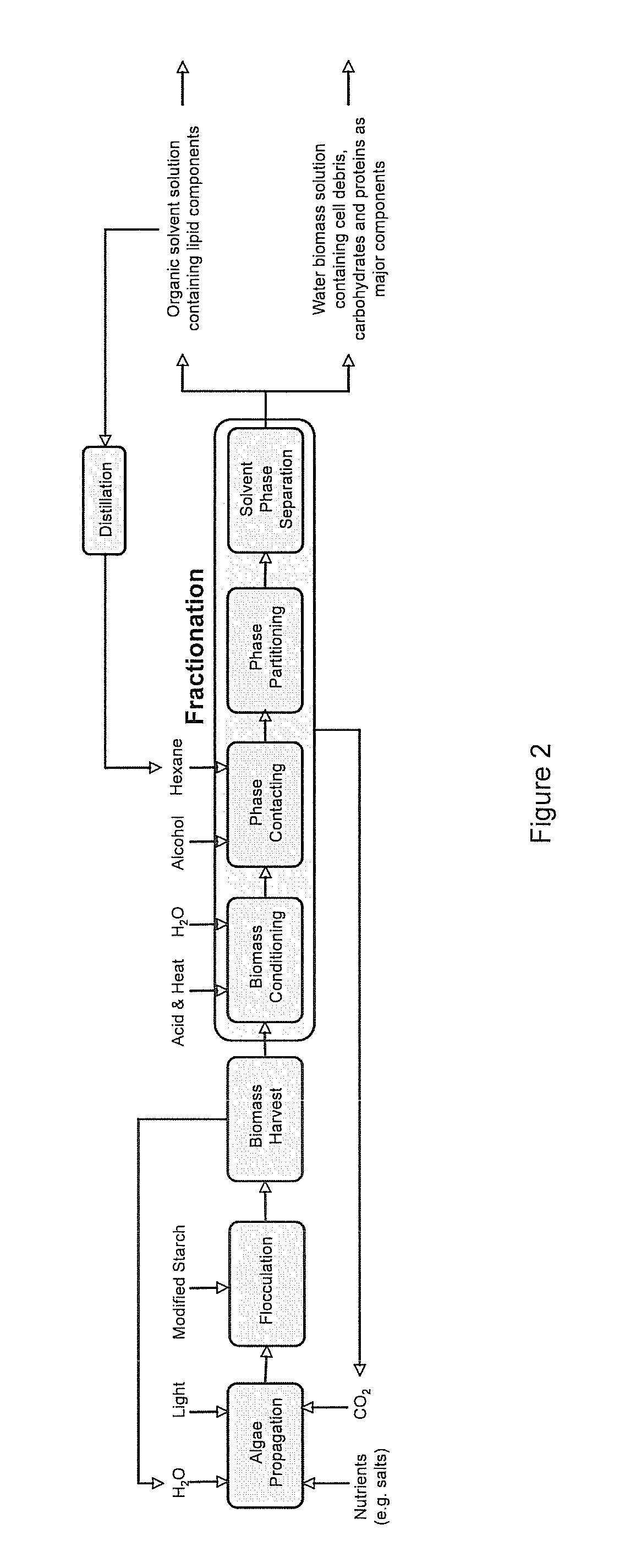
Algae biomass fractionation
A method of fractionating biomass, by permeability conditioning biomass suspended in a pH adjusted solution of at least one water-based polar solvent to form a conditioned biomass, intimately contacting the pH adjusted solution with at least one non-polar solvent, partitioning to obtain an non-polar solvent solution and a polar biomass solution, and recovering cell and cell derived products from the non-polar solvent solution and polar biomass solution. Products recovered from the above method. A method of operating a renewable and sustainable plant for growing and processing algae.
Owner:VALICOR
Polymer nanocomposite implants with enhanced transparency and mechanical properties for administration within humans or animals
Polymer nanocomposite implants with nanofillers and additives are described. The nanofillers described can be any composition with the preferred composition being those composing barium, bismuth, cerium, dysprosium, europium, gadolinium, hafnium, indium, lanthanum, neodymium, niobium, praseodymium, strontium, tantalum, tin, tungsten, ytterbium, yttrium, zinc, and zirconium. The additives can be of any composition with the preferred form being inorganic nanopowders comprising aluminum, calcium, gallium, iron, lithium, magnesium, silicon, sodium, strontium, titanium. Such nanocomposites are particularly useful as materials for biological use in applications such as drug delivery, biomed devices, bone or dental implants.
Owner:PPG IND OHIO INC
Nanotechnology for drug delivery, contrast agents and biomedical implants
A nanocomposite structure comprising a nanostructured filler or carrier intimately mixed with a matrix, and methods of making such a structure. The nanostructured filler has a domain size sufficiently small to alter an electrical, magnetic, optical, electrochemical, chemical, thermal, biomedical, or tribological property of either filler or composite by at least 20%.
Owner:PPG IND OHIO INC
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
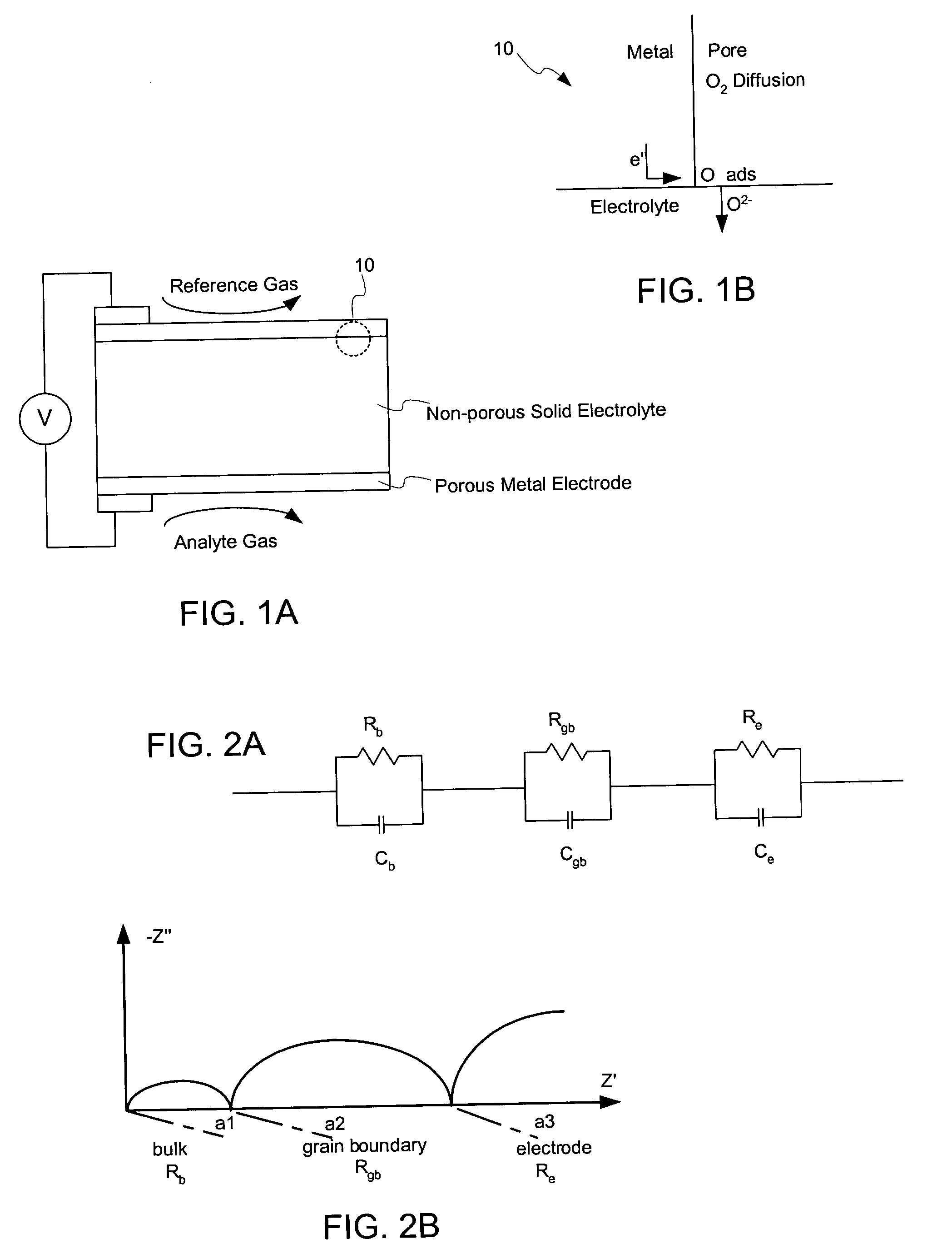
Nanostructured powders and related nanotechnology
InactiveUS7081267B2Low costReduce operating costsNitrogen compoundsSelenium/tellurium compundsThermal energyOxygen
Methods to manufacture nanoscale particles comprising metals, alloys, intermetallics, ceramics are disclosed. The thermal energy is provided by plasma, internal energy, heat of reaction, microwave, electromagnetic, direct electric arc, pulsed electric arc and / or nuclear. The process is operated at some stage above 3000K and at high velocities. The invention can be utilized to prepare nanopowders for nanostructured products and devices such as ion conducting solid electrolytes for a wide range of applications, including sensors, oxygen pumps, fuel cells, batteries, electrosynthesis reactors and catalytic membranes.
Owner:PPG IND OHIO INC
Alloyed semiconductor quantum dots and concentration-gradient alloyed quantum dots, series comprising the same and methods related thereto
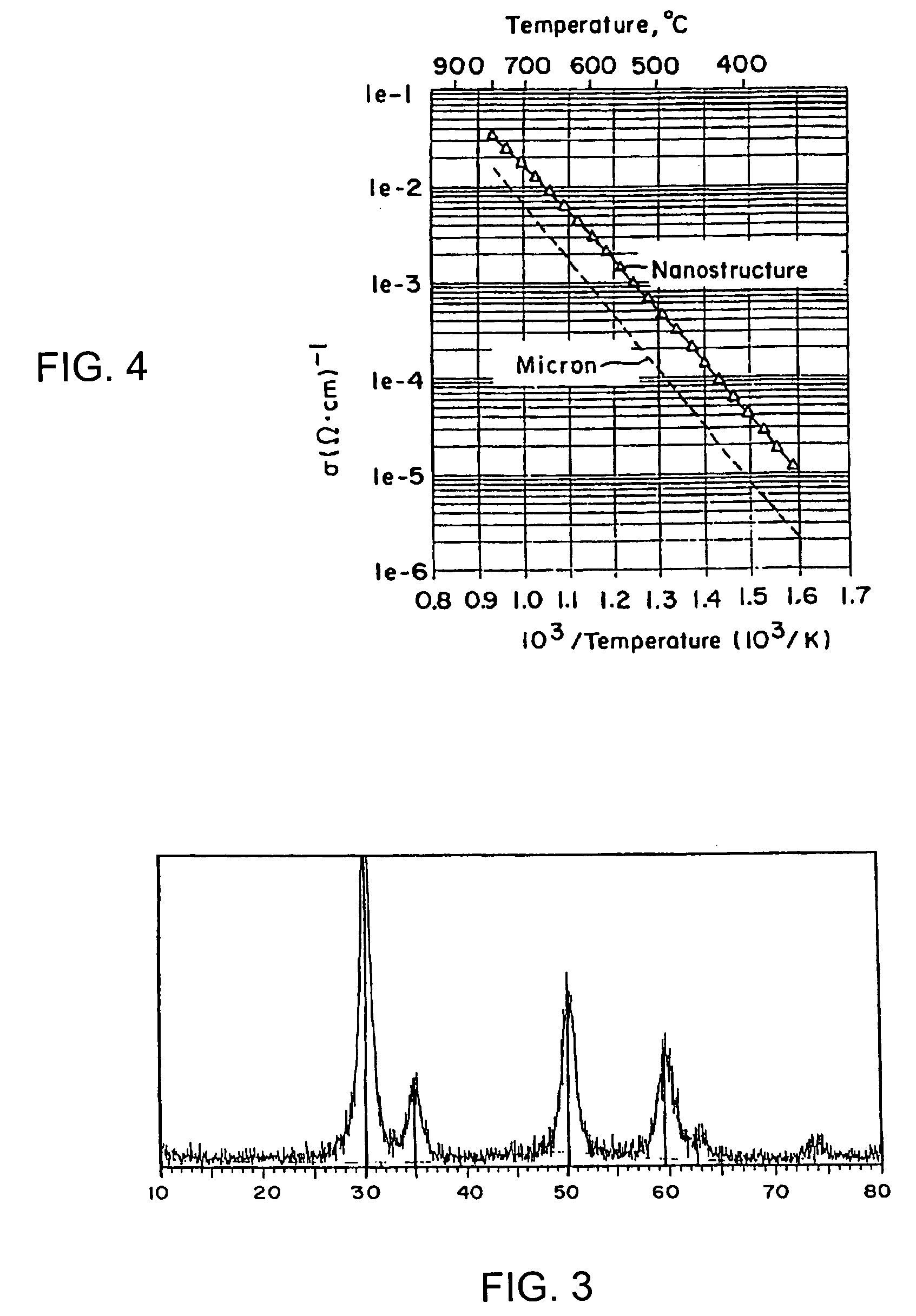
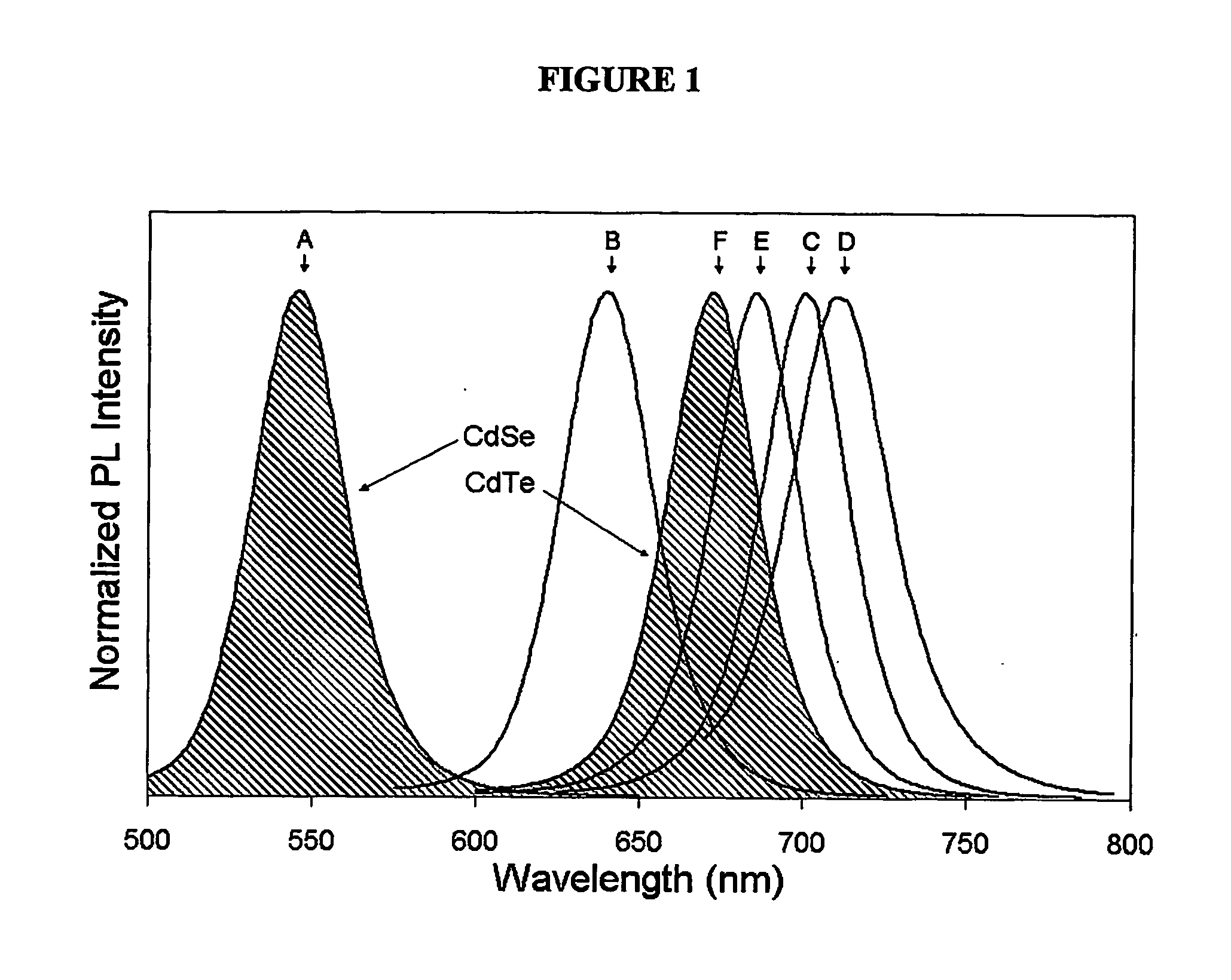
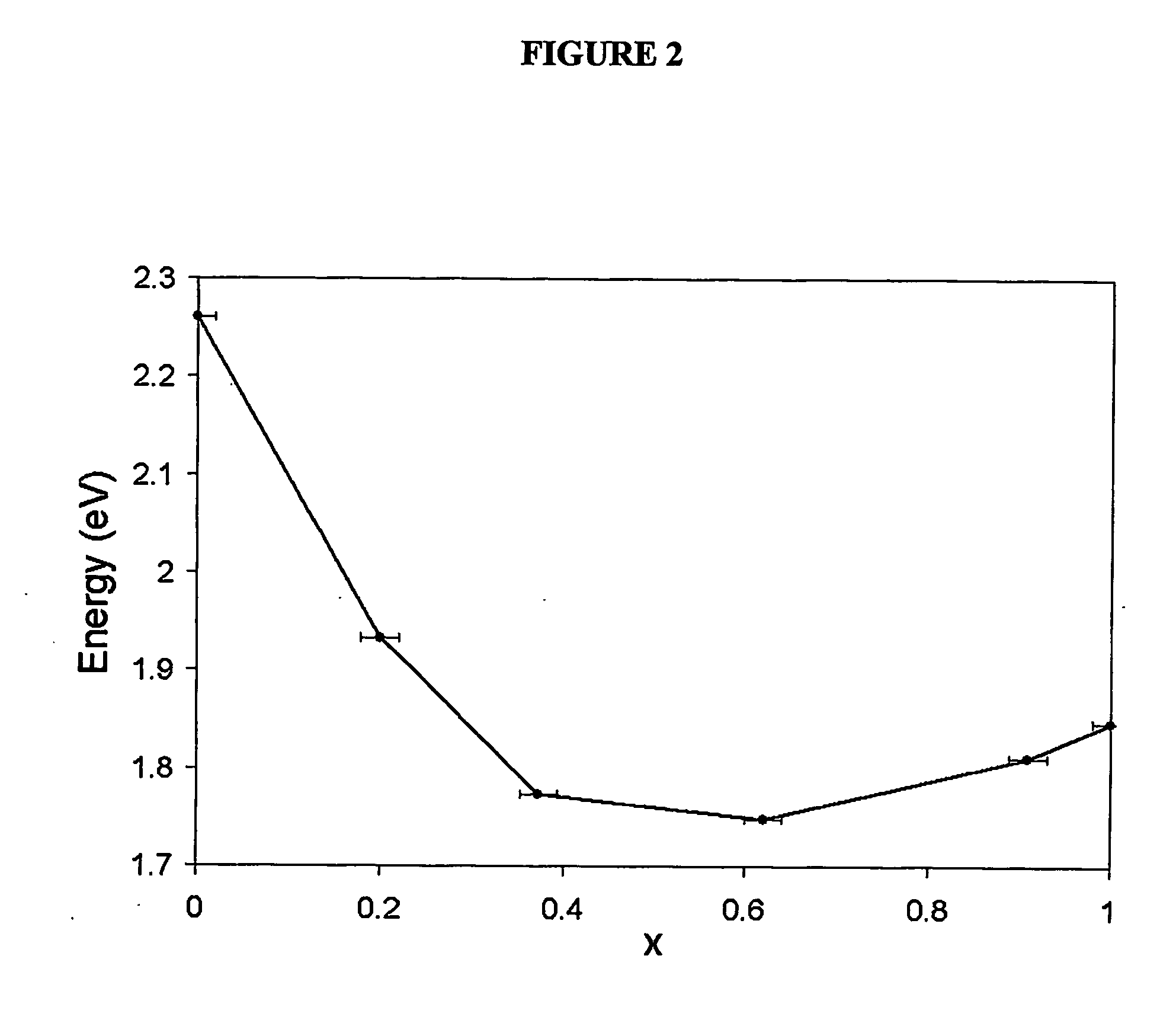
An alloyed semiconductor quantum dot comprising an alloy of at least two semiconductors, wherein the quantum dot has a homogeneous composition and is characterized by a band gap energy that is non-linearly related to the molar ratio of the at least two semiconductors; a series of alloyed semiconductor quantum dots related thereto; a concentration-gradient quantum dot comprising an alloy of a first semiconductor and a second semiconductor, wherein the concentration of the first semiconductor gradually increases from the core of the quantum dot to the surface of the quantum dot and the concentration of the second semiconductor gradually decreases from the core of the quantum dot to the surface of the quantum dot; a series of concentration-gradient quantum dots related thereto; in vitro and in vivo methods of use; and methods of producing the alloyed semiconductor and concentration-gradient quantum dots and the series of quantum dots related thereto.
Electrode material for anode of rechargeable lithium battery, electrode structural body using said electrode material, rechargeable lithium battery using said electrode structural body, process for producing said electrode structural body, and process for producing said rechargeable lithium battery
InactiveUS20050175901A1Prolonged discharging cycle lifeLarge capacityNitrogen compoundsSelenium/tellurium compundsElectrochemical responseParticulates
An electrode material for an anode of a rechargeable lithium battery, containing a particulate comprising an amorphous Sn.A.X alloy with a substantially non-stoichiometric ratio composition. For said formula Sn.A.X, A indicates at least one kind of an element selected from a group consisting of transition metal elements, X indicates at least one kind of an element selected from a group consisting of O, F, N, Mg, Ba, Sr, Ca, La, Ce, Si, Ge, C, P, B, Pb, Bi, Sb, Al, Ga, In, Tl, Zn, Be, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, As, Se, Te, Li and S, where the element X is not always necessary to be contained. The content of the constituent element Sn of the amorphous Sn.A.X alloy is Sn / (Sn+A+X)=20 to 80 atomic %. An electrode structural body for a rechargeable lithium battery, comprising said electrode material for an anode and a collector comprising a material incapable of being alloyed with lithium in electrochemical reaction, and a rechargeable lithium battery having an anode comprising said electrode structural body.
Owner:CANON KK
III-V semiconductor nanocrystal complexes and methods of making same
ActiveUS20060001119A1High Luminescence Quantum YieldMaterial nanotechnologyFrom normal temperature solutionsLuminescence quantum yieldSemiconductor nanocrystals
A semiconductor nanocrystal complex that is stable and has high luminescent quantum yield. The semiconductor nanocrystal complex has a semiconductor nanocrystal core of a III-V semiconductor nanocrystal material. A method of making a semiconductor nanocrystal complex is also provided. The method includes synthesizing a semiconductor nanocrystal core of a III-V semiconductor nanocrystal material, and forming a metal layer on the semiconductor nanocrystal core after synthesis of the semiconductor nanocrystal core.
Owner:SAMSUNG ELECTRONICS CO LTD
Semiconductor nanocrystal complexes and methods of making same
ActiveUS20060014040A1Material nanotechnologyLiquid surface applicatorsLattice mismatchSemiconductor nanocrystals
A semiconductor nanocrystal complex including a metal layer formed on the outer surface of a semiconductor nanocrystal core after synthesis of the semiconductor nanocrystal core and a method for preparing a nanocrystal complex comprising forming a metal layer on a semiconductor nanocrystal core after synthesis of the semiconductor nanocrystal core. The metal layer may passivate the surface of the semiconductor nanocrystal core and protect the semiconductor nanocrystal core from the effects of oxidation. Also provided is a semiconductor nanocrystal complex with a shell grown onto the metal layer formed on the semiconductor nanocrystal core. In this embodiment, the metal layer may prevent lattice mismatch between the semiconductor shell and the semiconductor nanocrystal core.
Owner:SAMSUNG ELECTRONICS CO LTD
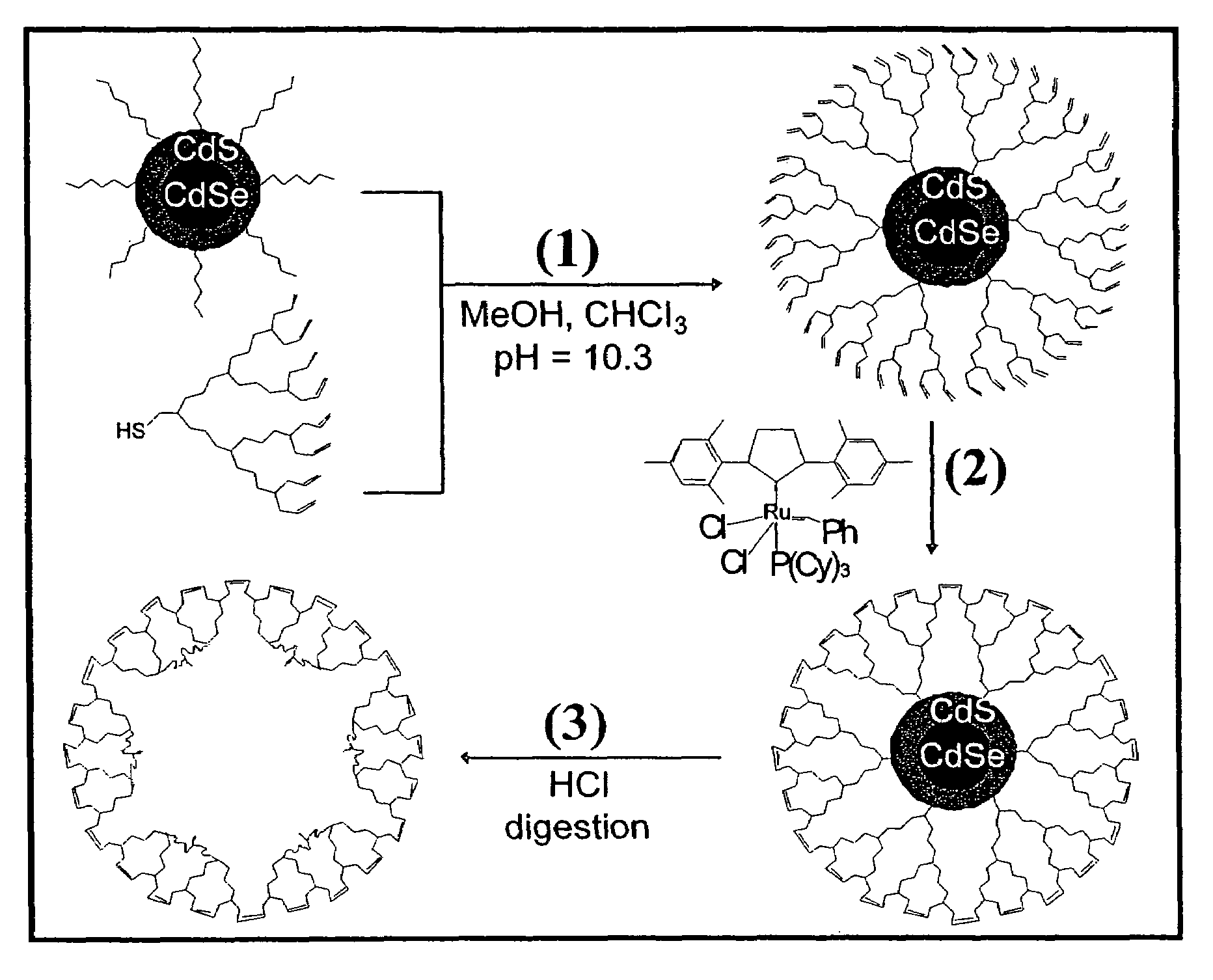
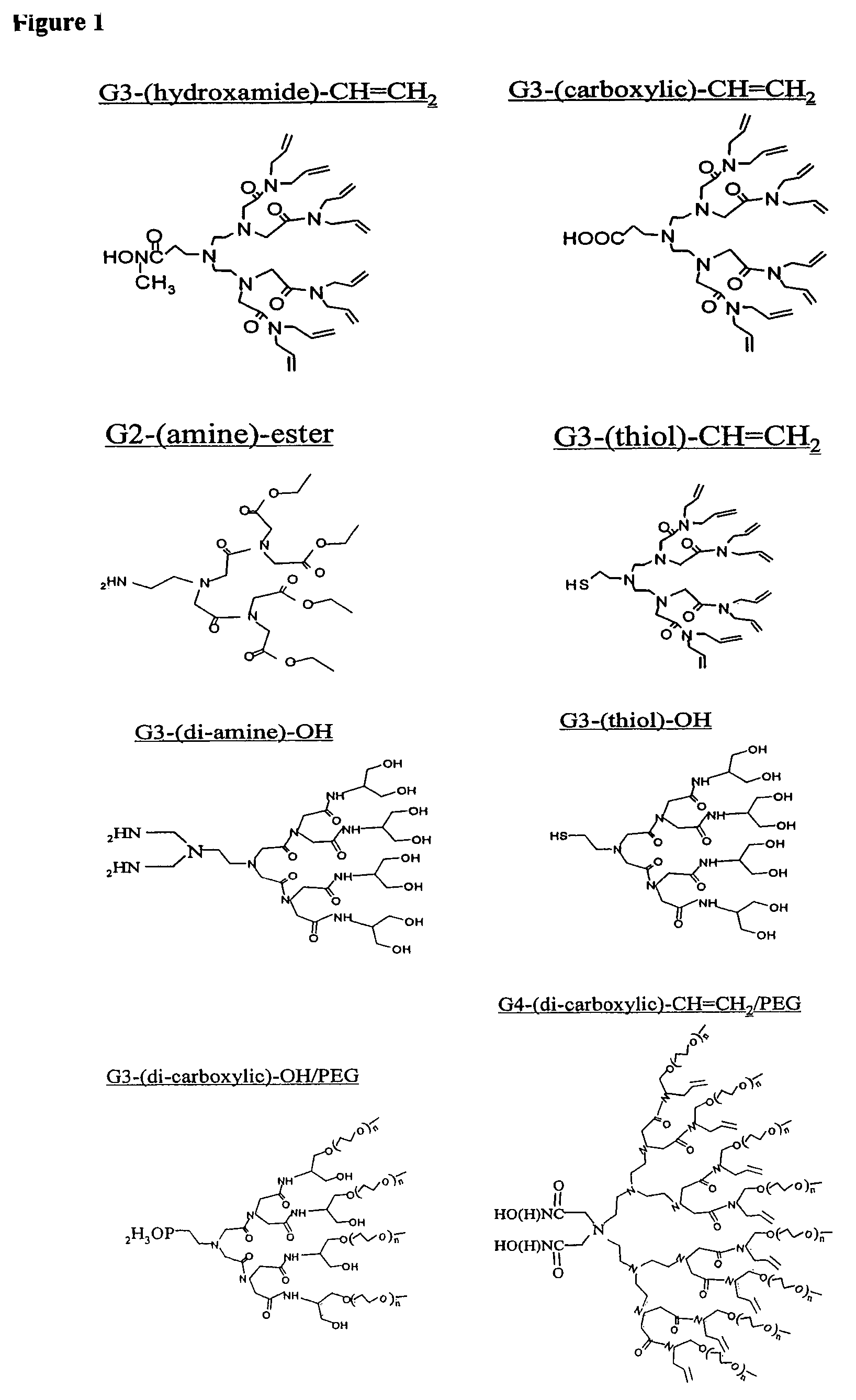
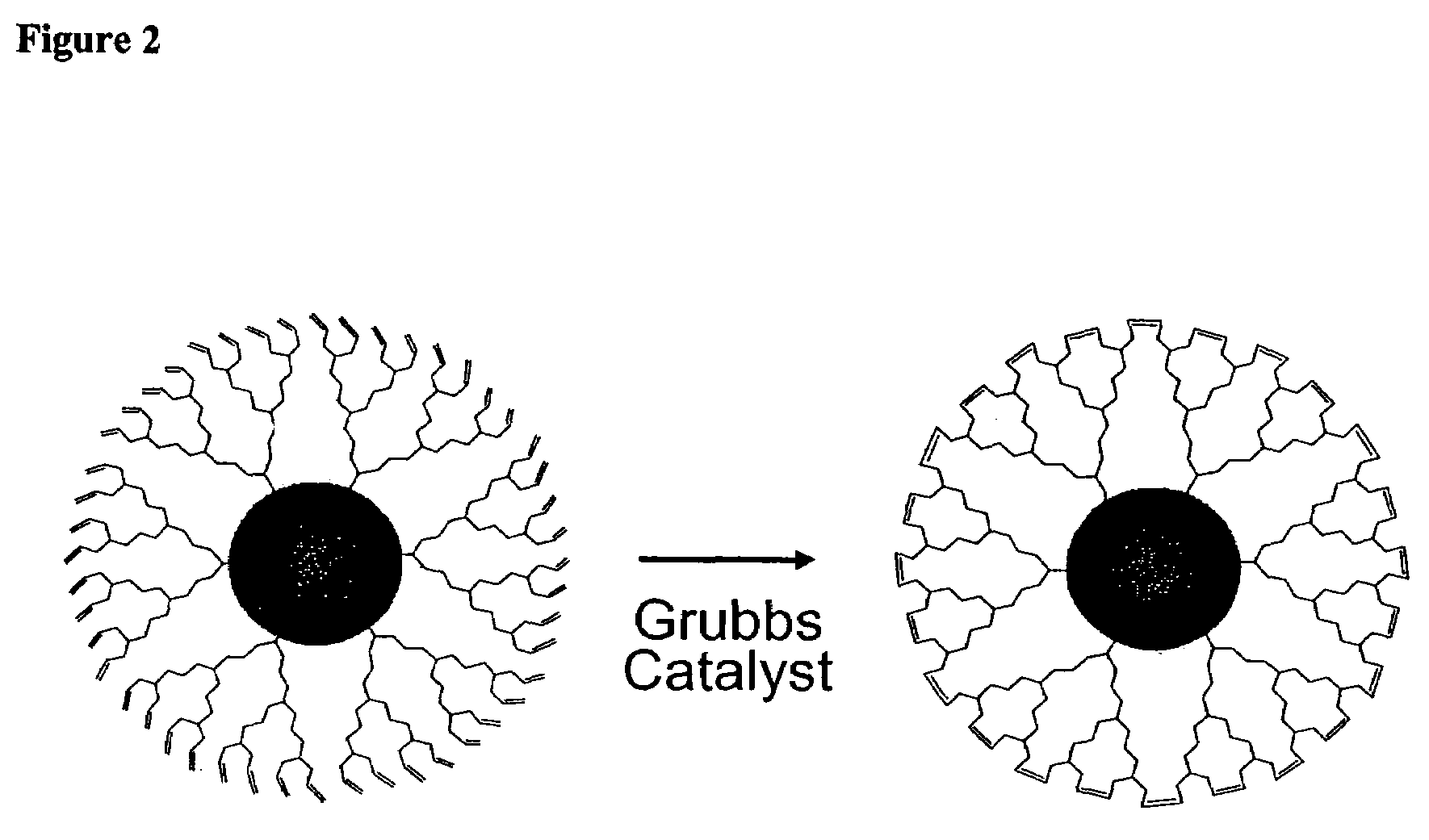
Nanocrystals in ligand boxes exhibiting enhanced chemical, photochemical, and thermal stability, and methods of making the same
ActiveUS7273904B2Improve stabilityEasy to detectMaterial nanotechnologyFrom normal temperature solutionsDendrimerCross-link
Dendron ligands or other branched ligands with cross-linkable groups were coordinated to colloidal inorganic nanoparticles, including nanocrystals, and substantially globally cross-linked through different strategies, such as ring-closing metathesis (RCM), dendrimer-bridging methods, and the like. This global cross-linking reaction sealed each nanocrystal within a dendron box to yield box-nanocrystals which showed dramatically enhanced stability against chemical, photochemical and thermal treatments in comparison to the non-cross-linked dendron-nanocrystals. Empty dendron boxes possessing a very narrow size distribution were formed by the dissolution of the inorganic nanocrystals contained therein upon acid or other etching treatments.
Owner:BEIJING JINGTAI MEIKANG BIOTECH CO LTC +1
Method for preprocessing anode sludge and recovering dissipated metal
InactiveCN101338368AHigh recovery rateSimple processSelenium/tellurium compundsProcess efficiency improvementAcetic acidSludge
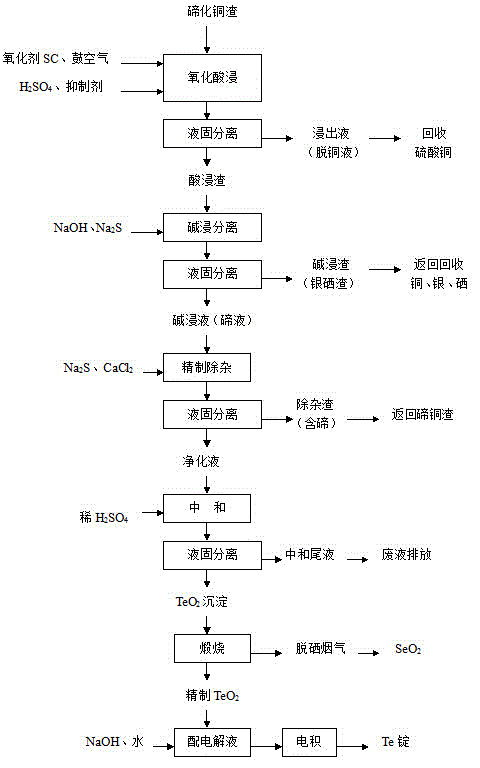
The invention relates to a method for pre-processing anode mud and recycling rare metals. The invention carries out pre-processing on the anode mud of copper and lead ions and recycling the rare metals; firstly the anode mud is extracted by an acid liquid; a primary acid extraction liquid and the primary decopper anode mud are obtained by filtering; the primary decopper anode mud after being stirred and grinded with sodium carbonate is extracted by metric acid or acetic acid to obtain the deleading anode mud; after sulfated roasting for steaming selenium is carried out on the primary decopper anode mud or the deleading anode mud, the steaming selenium anode mud is extracted by the acid liquid; a secondary acid extraction liquid and the secondary decopper anode mud are obtained by filtering. The acid extraction liquid recycles the selenium and Te by reducing or recycles the slag of selenium and Te by directly adding alkali to react in the acid extraction liquid. The secondary acid extraction liquid after recycling the Te by reducing uses alkali or adds water to dilute and adjust the pH value of a filter liquid and obtain the slag of bismuth by filtering. The method of the invention has a simple flow, a high recycling rate of selenium, Te and bismuth; the noble metals can be enriched and the recycling rate of gold and silver can be remarkably improved.
Owner:CENT SOUTH UNIV
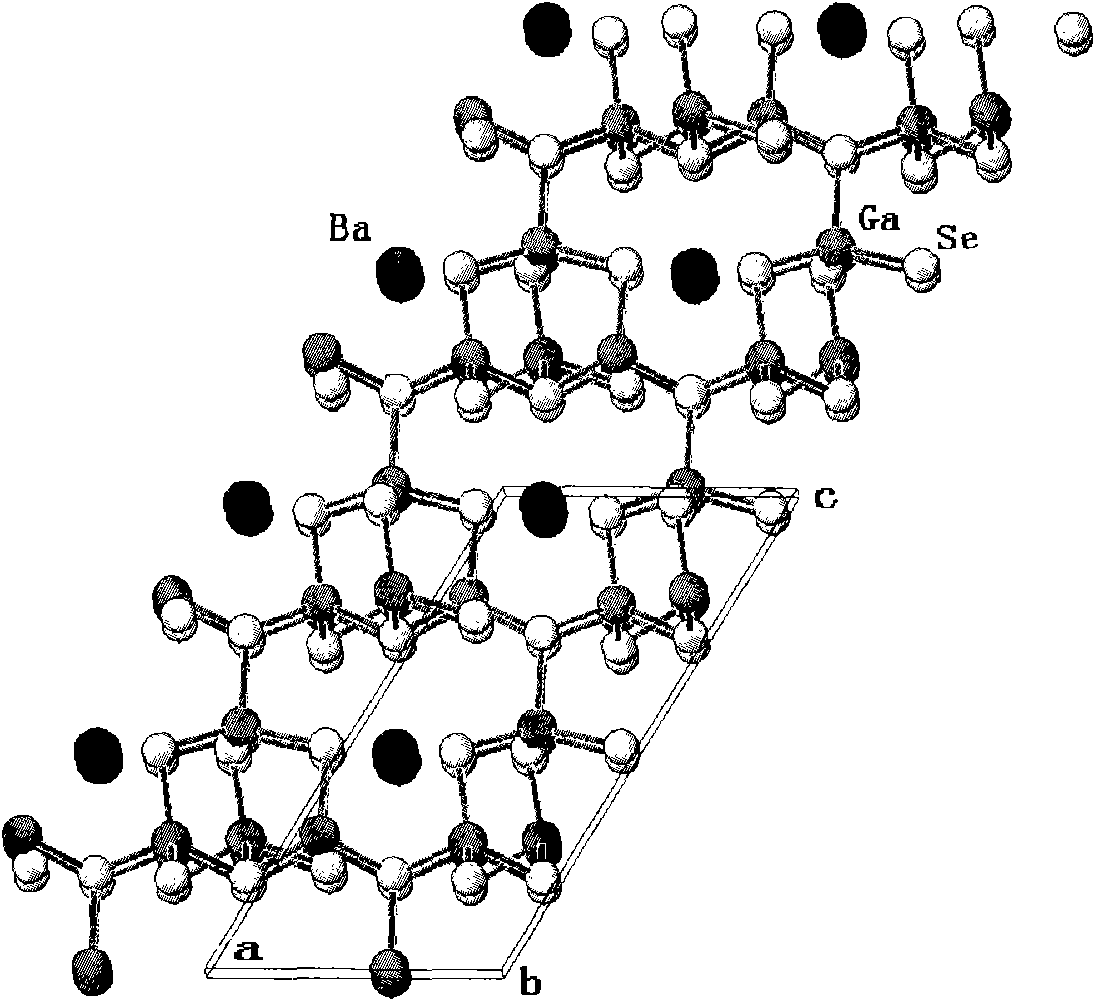
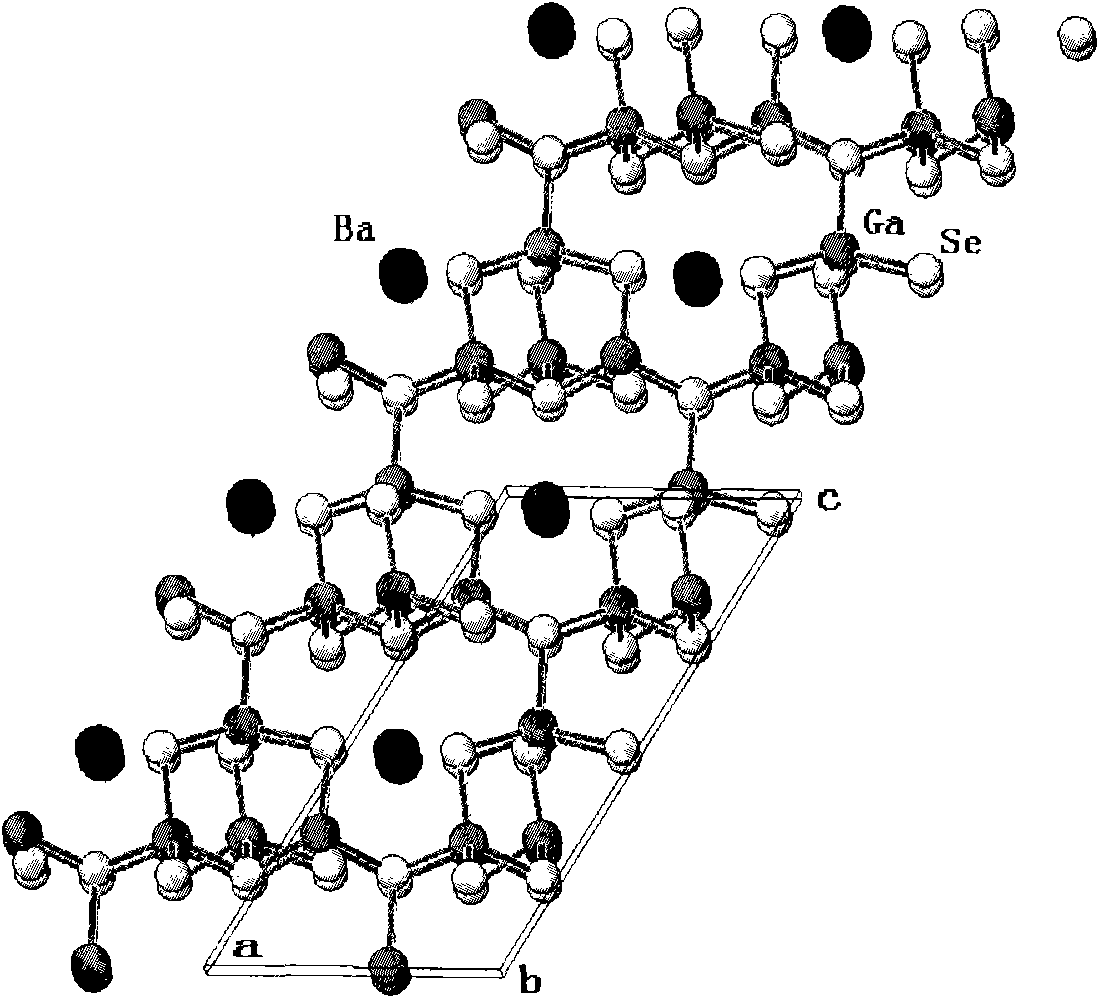
BaCa4Se7 compound, BaCa4Se7 nonlinear optical crystal, preparation method and application
ActiveCN101767778AWide band of light transmissionHigh hardnessPolycrystalline material growthAfter-treatment detailsNonlinear optical crystalOptical transparency
The invention relates to a BaCa4Se7 compound, a BaCa4Se7 nonlinear optical crystal, a preparation method and application. The BaCa4Se7 compound is prepared by adopting a solid-phase reaction, and the BaCa4Se7 nonlinear optical crystal is grown by adopting a high-temperature melt spontaneous crystallization method, a flux growth method or a Bridgman method. In the growth of the BaCa4Se7 nonlinear optical crystal, the crystal is easy to grow and transparent and has no package and the advantages of higher growing speed, low cost, easy obtaining of a crystal in a larger size, and the like. The obtained BaCa4Se7 nonlinear optical crystal has the advantages of wider optical transparency range, larger hardness, good mechanical property, difficult cracking and deliquescence, easy processing and storage, and the like. The BaCa4Se7 nonlinear optical crystal can be used for preparing a nonlinear optical device.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
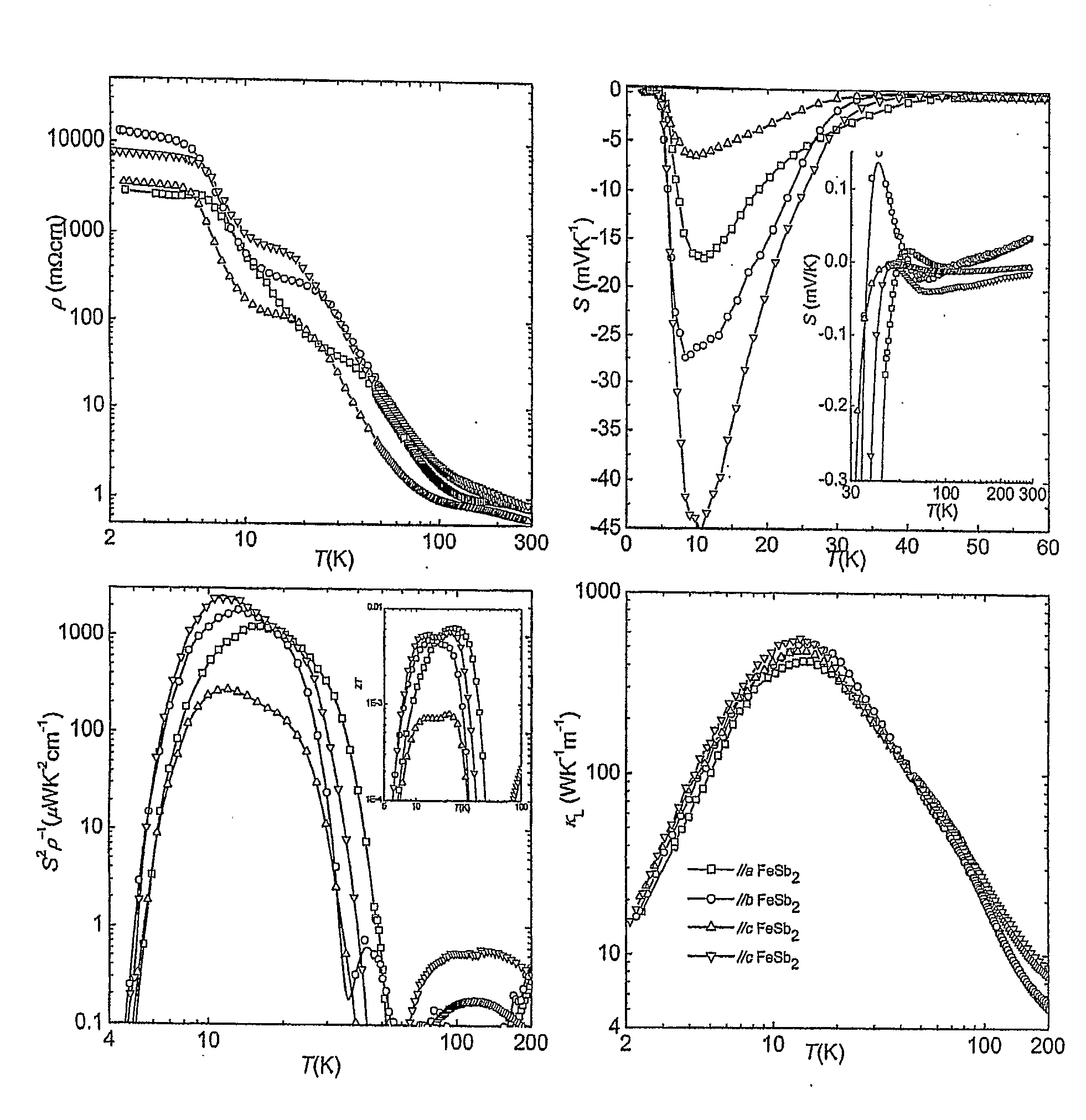
Use of thermoelectric materials for low temperature thermoelectric purposes
InactiveUS20100139730A1Polycrystalline material growthSelenium/tellurium compundsThermoelectric materialsPower factor
The invention relates to the use of a thermoelectric material for thermoelectric purposes at a temperature of 150 K or less, said thermoelectric material is a material corresponding to the stoichiometric formula FeSb2, wherein all or part of the Fe atoms optionally being substituted by one or more elements selected from the group comprising: Sc, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, La, Hf, Ta, W, Re, Os, Tr, Pt, Au, Hg, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu and a vacancy; and wherein all or part of the Sb atoms optionally being substituted by one or more elements selected from the group comprising: P, As, Bi, S, Se, Te, B, Al, Ga, In, Tl, C, Si, Ge, Sn, Pb and a vacancy; with the proviso that neither one of the elements Fe and Sb in the formula FeSb2 is fully substituted with a vacancy, characterised in that said thermoelectric material exhibits a power factor (S2σ) of 25 μW / cmK2 or more at a temperature of 150 K or less. The invention also relates to thermoelectric materials per se falling within the above definition.
Owner:AARHUS UNIV +1
Preparation of metal chalcogenides from reactions of metal compounds and chalcogen
A method of preparing metal chalcogenides from elemental metal or metal compounds has the following steps: providing at least one elemental metal or metal compound; providing at least one element from periodic table groups 13-15; providing at least one chalcogen; and combining and heating the chalcogen, the group 13-15 element and the metal at sufficient time and temperature to form a metal chalcogenide. A method of functionalizing the surface of semiconducting nanoparticles has the following steps: providing at least one metad compound; providing one chalcogenide having a cation selected from the group 13-15 (B, Al, Ga, In, Si, Ge, Sn, Pb, P, As, Sb and Bi); dissolving the chalcogenide in a first solution; dissolving the metal compound in a second solution; providing and dissolving a functional capping agent in at least one of the solutions of the metal compounds and chalcogenide; combining all solutions; and maintaining the combined solution at a proper temperature for an appropriate time.
Owner:ARIZONA STATE UNIVERSITY
Preparation of metal chalcogenides from reactions of metal compounds and chalcogen
InactiveUS20060239882A1Rare earth metal sulfidesSelenium/tellurium compundsSufficient timeNanoparticle
A method of preparing metal chalcogenides from elemental metal or metal compounds has the following steps: providing at least one elemental metal or metal compound; providing at least one element from periodic table groups 13-15; providing at least one chalcogen; and combining and heating the chalcogen, the group 13-15 element and the metal at sufficient time and temperature to form a metal chalcogenide. A method of functionalizing the surface of semiconducting nanoparticles has the following steps: providing at least one metad compound; providing one chalcogenide having a cation selected from the group 13-15 (B, Al, Ga, In, Si, Ge, Sn, Pb, P, As, Sb and Bi); dissolving the chalcogenide in a first solution; dissolving the metal compound in a second solution; providing and dissolving a functional capping agent in at least one of the solutions of the metal compounds and chalcogenide; combining all solutions; and maintaining the combined solution at a proper temperature for an appropriate time.
Owner:ARIZONA STATE UNIVERSITY
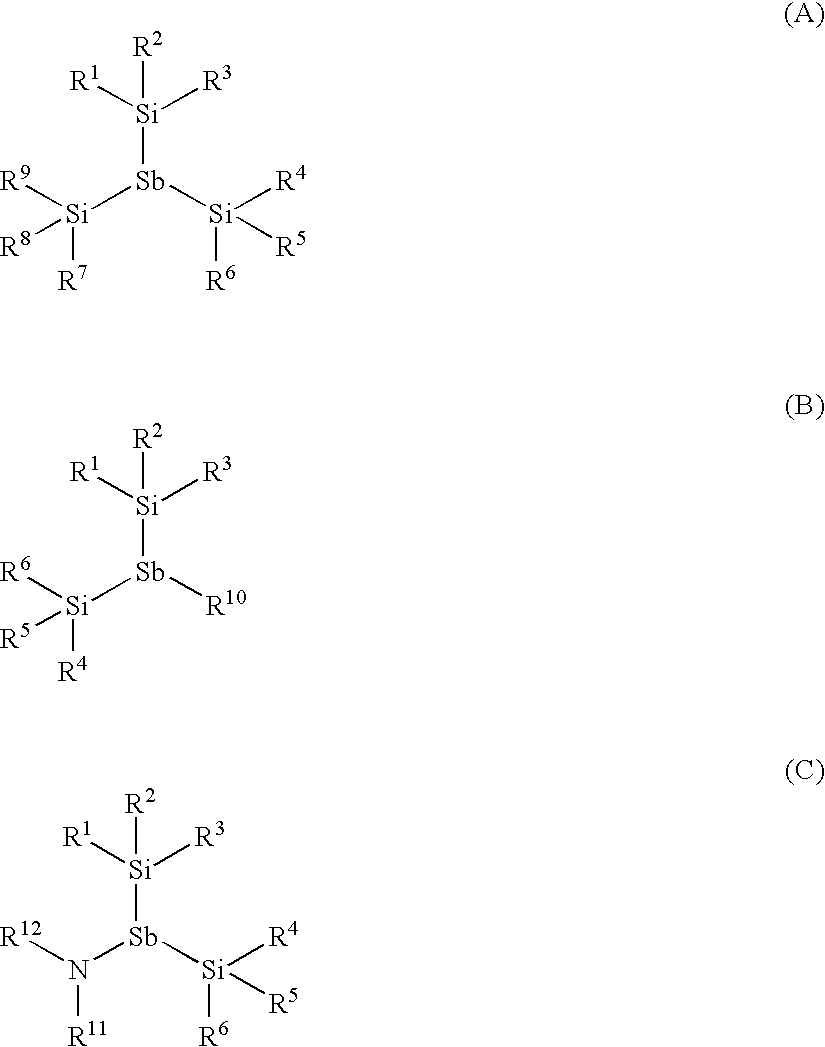
Antimony Precursors for GST Films in ALD/CVD Processes
The present invention is a process of making a germanium-antimony-tellurium alloy film using a process selected from the group consisting of atomic layer deposition and chemical vapor deposition, wherein a silylantimony precursor is used as a source of antimony for the alloy film. Novel silylantimony compounds are also disclosed.
Owner:VERSUM MATERIALS US LLC
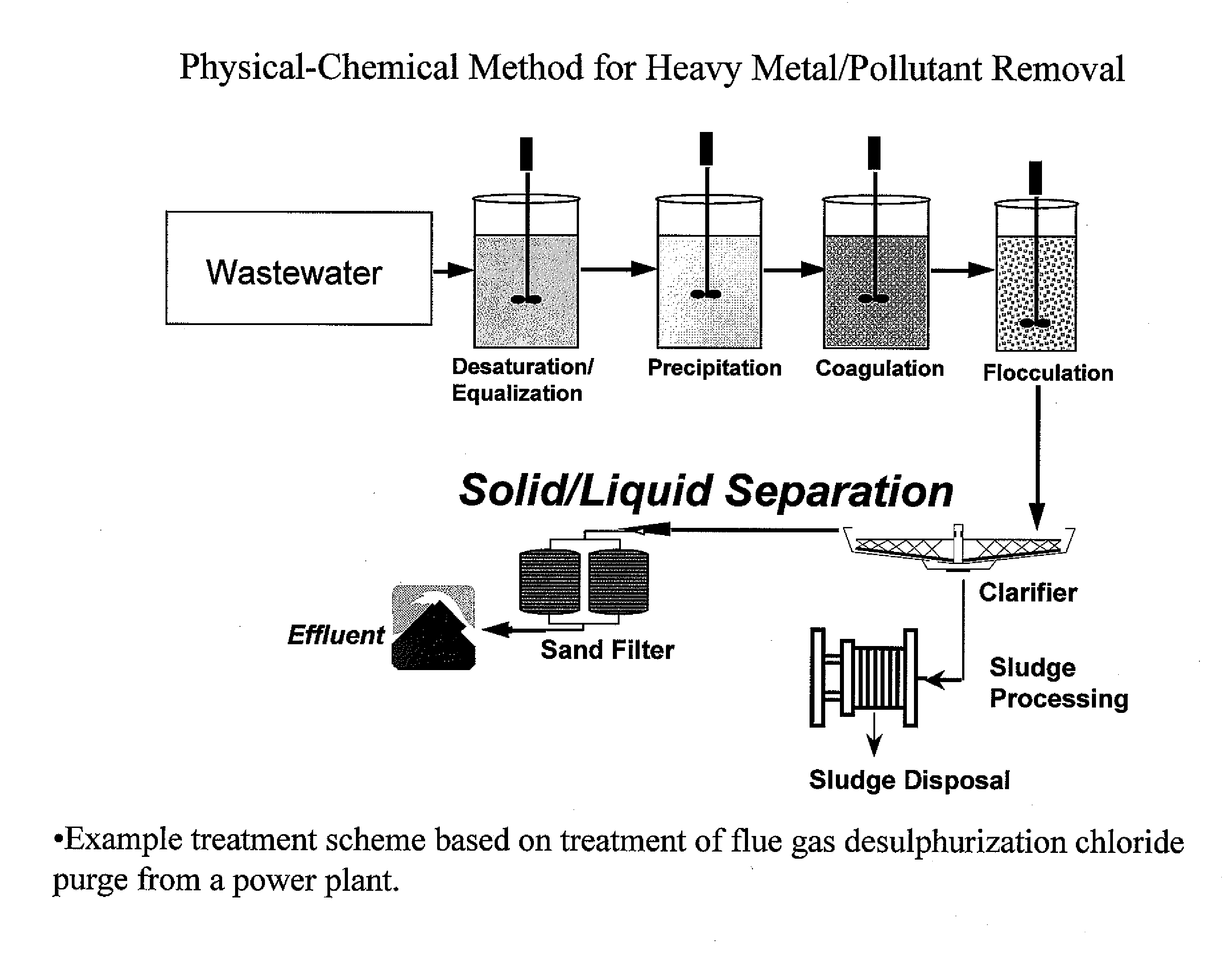
Metal scavenging polymers and uses thereof
ActiveUS20110243819A1Dispersed particle filtrationSelenium/tellurium compundsWastewater systemsMonomer
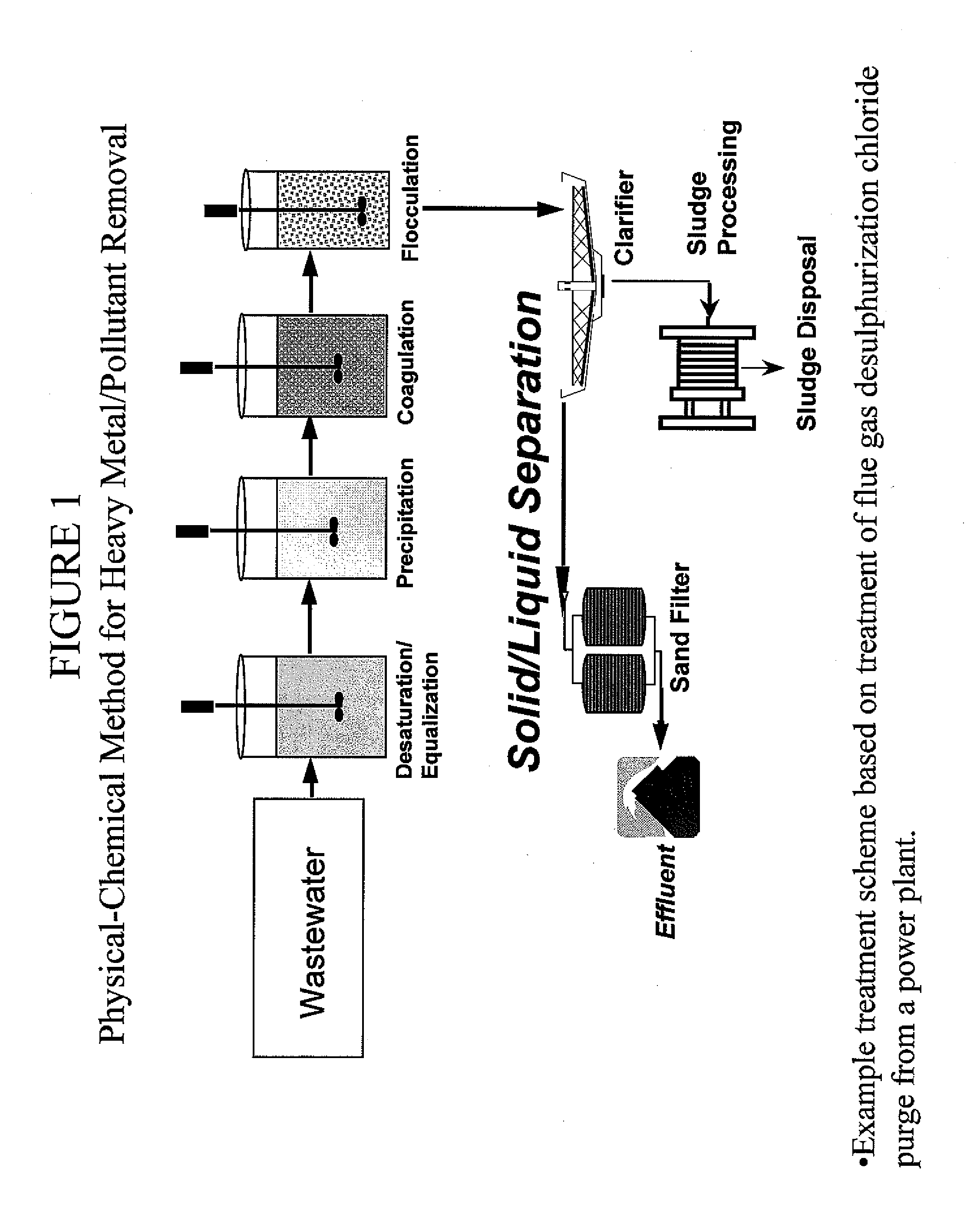
Uses for a composition comprising a polymer derived from at least two monomers: acrylic-x and an alkylamine, wherein said polymer is modified to contain a functional group capable of scavenging one or more compositions containing one or more metals are disclosed. These polymers have many uses in various mediums, including wastewater systems.
Owner:ECOLAB USA INC
Nanoparticles and methods of making and using
A nanoparticle composition is disclosed comprising a copper indium gallium selenide, a copper indium sulfide, or a combination thereof. Also disclosed is a layer comprising the nanoparticle composition. A photovoltaic device comprising the nanoparticle composition and / or the absorbing layer is disclosed. Also disclosed are methods for producing the nanoparticle compositions, absorbing layers, and photovoltaic devices described herein.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Metal chalcogenide aqueous precursors and processes to form metal chalcogenide films
InactiveUS20110206599A1Selenium/tellurium compundsPhosphorus sulfur/selenium/tellurium compoundsSimple Organic CompoundsFormate
Metal chalocogenide precursor solutions are described that comprise an aqueous solvent, dissolved metal formate salts and a chalcogenide source composition. The chalcogenide source compositions can be organic compounds lacking a carbon-carbon bond. The precursors are designed to form a desired metal chalcogenide upon thermal processing into films with very low levels of contamination. Potentially contaminating elements in the precursors form gaseous or vapor by-products that escape from the vicinity of the product metal chalcogenide films.
Owner:INPRIA CORP
CIGS powder, CIGS target, CIGS film and preparation method thereof
InactiveCN101613091ALow costSmall grain sizeSelenium/tellurium compundsVacuum evaporation coatingCompression moldingManufactured material
The invention provides CIGS powder, a CIGS target, a CIGS film and a preparation method thereof. The CIGS powder has a pure CuInxGa1-xSe2 phase, wherein x is more than 0 and is less than 1; and the CIGS target has a homogeneous CuInxGa1-xSe2 phase, and is obtained from the CIGS powder through cold isostatic pressing or compression molding and then sintering. The method for preparing the CIGS film comprises the steps of: depositing a layer of film by the CIGS target through a magnetron sputtering method, and then performing heat treatment on the film. The relative density of the CIGS target reaches over 95 percent, and the CIGS target has uniform components, has the homogeneous CuInxGa1-xSe2 phase, and has low production cost and steady performance. The preparation technology for the CIGS film greatly simplifies the prior process, and improves the utilization rate of raw materials and production efficiency.
Owner:CENT SOUTH UNIV
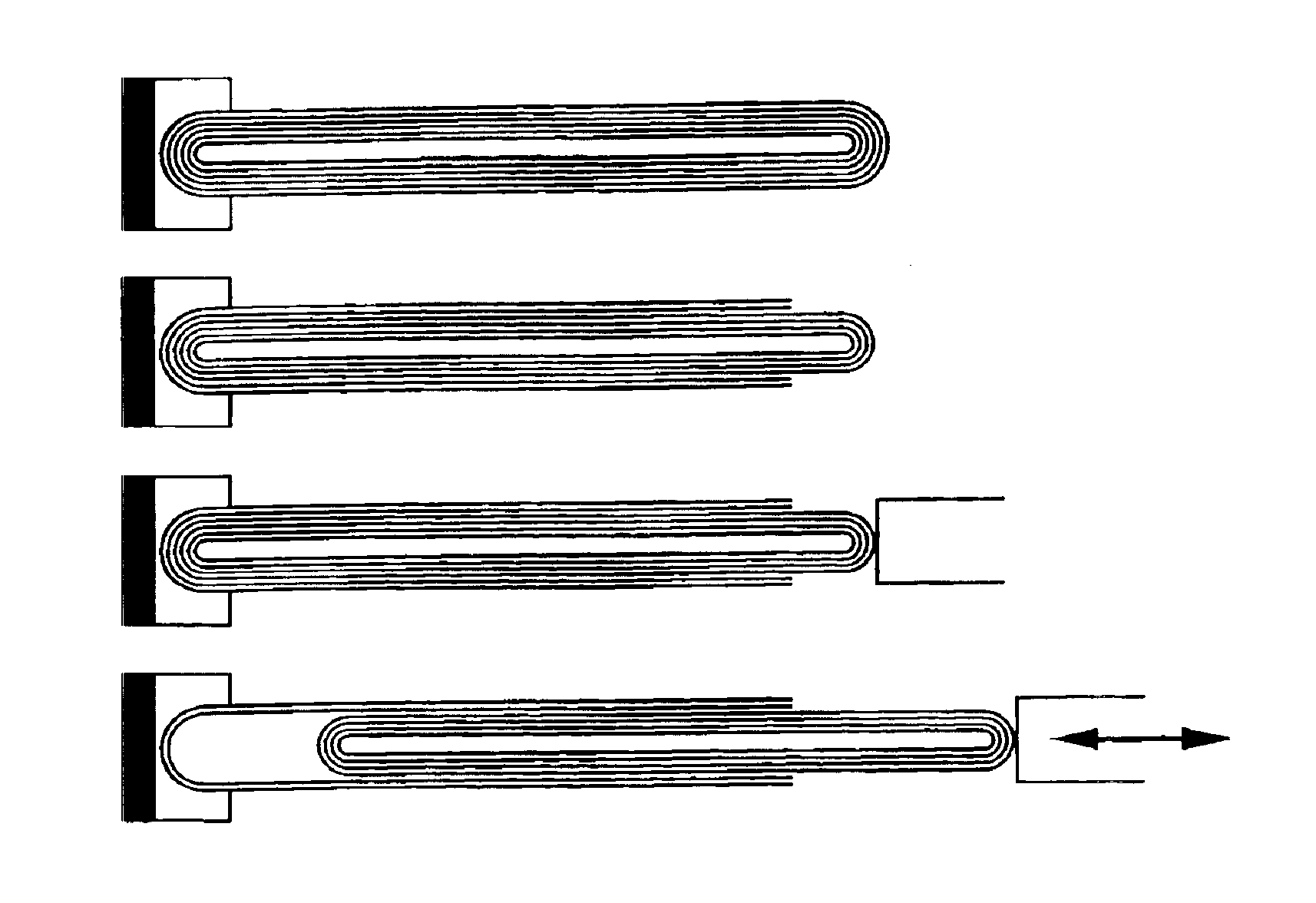
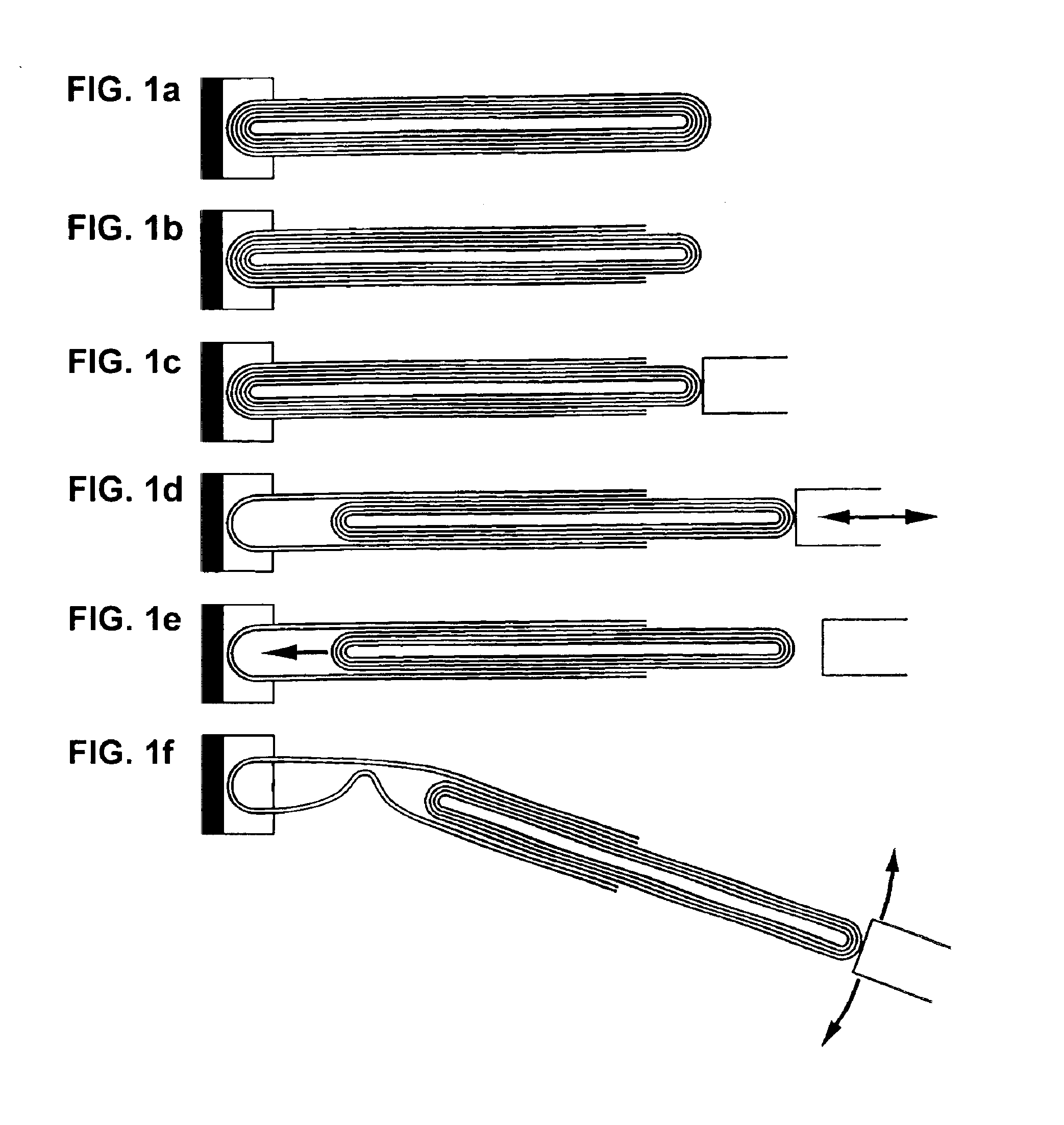
Telescoped multiwall nanotube and manufacture thereof
InactiveUS6874668B2Overcome disadvantagesMaterial nanotechnologyNitrogen compoundsNanomanipulatorConstant force
The invention relates to a method for forming a telescoped multiwall nanotube. Such a telescoped multiwall nanotube may find use as a linear or rotational bearing in microelectromechanical systems or may find use as a constant force nanospring. In the method of the invention, a multiwall nanotube is affixed to a solid, conducting substrate at one end. The tip of the free end of the multiwall nanotube is then removed, revealing the intact end of the inner wall. A nanomanipulator is then attached to the intact end, and the intact, core segments of the multiwall nanotube are partially extracted, thereby telescoping out a segment of nanotube.
Owner:RGT UNIV OF CALIFORNIA
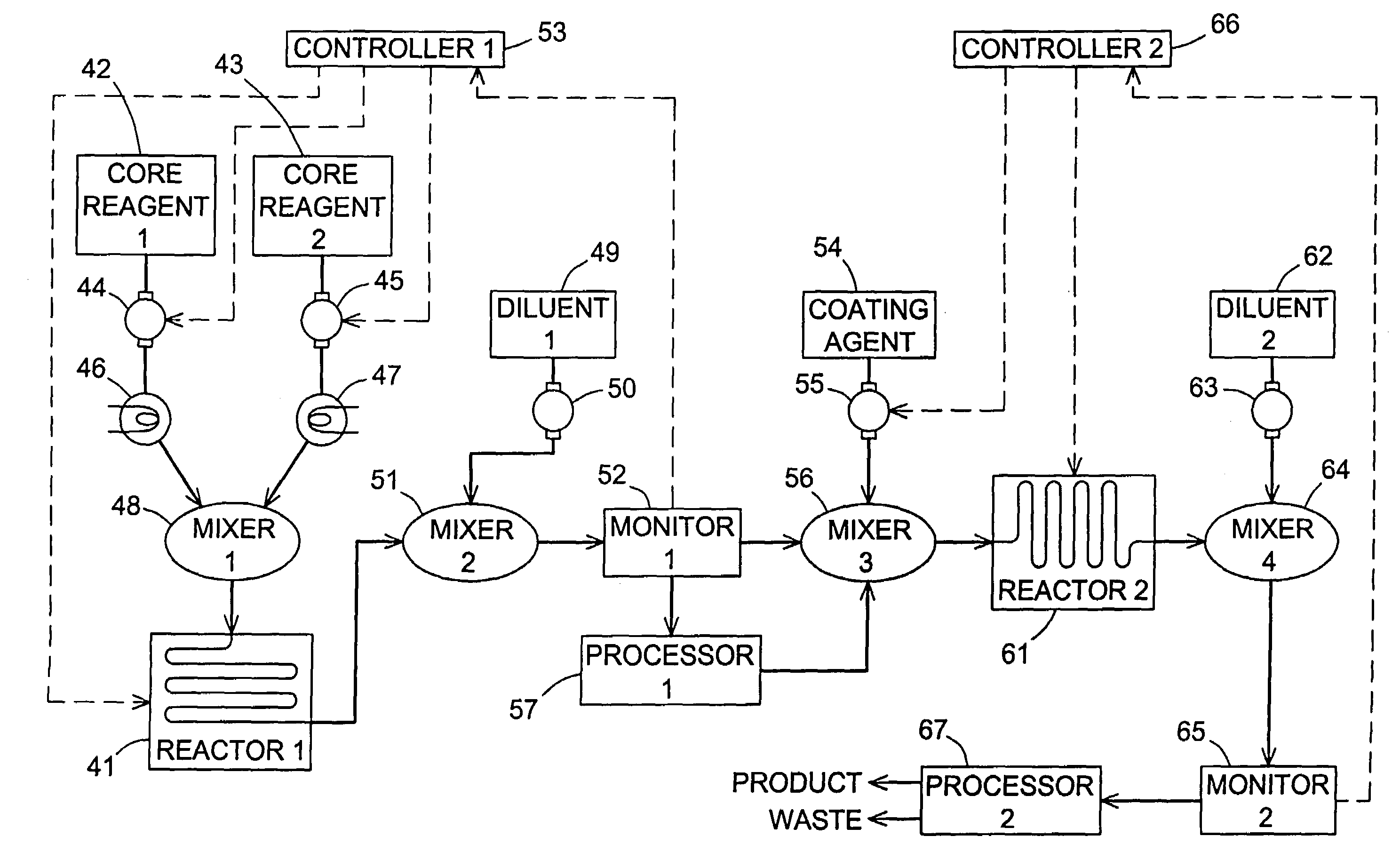
Flow synthesis of quantum dot nanocrystals
Owner:INVITROGEN
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS7892514B2Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumHalogen
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
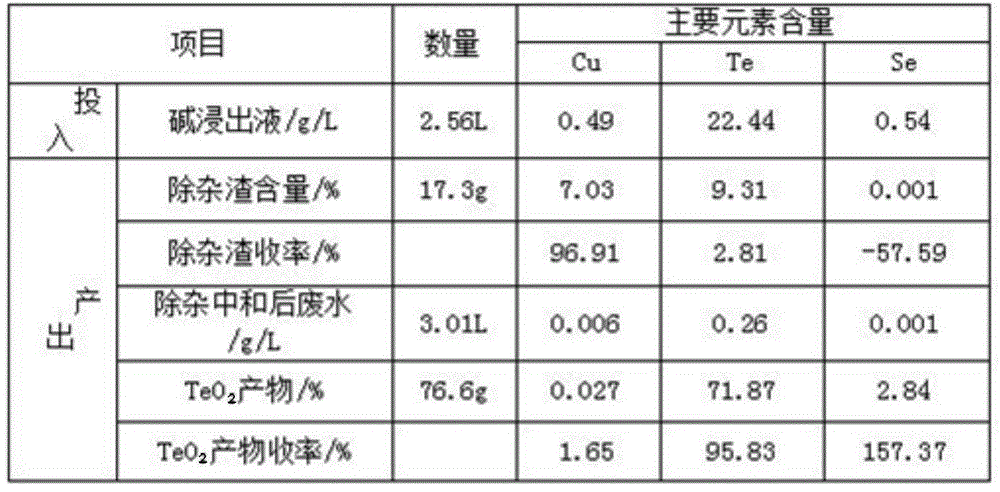
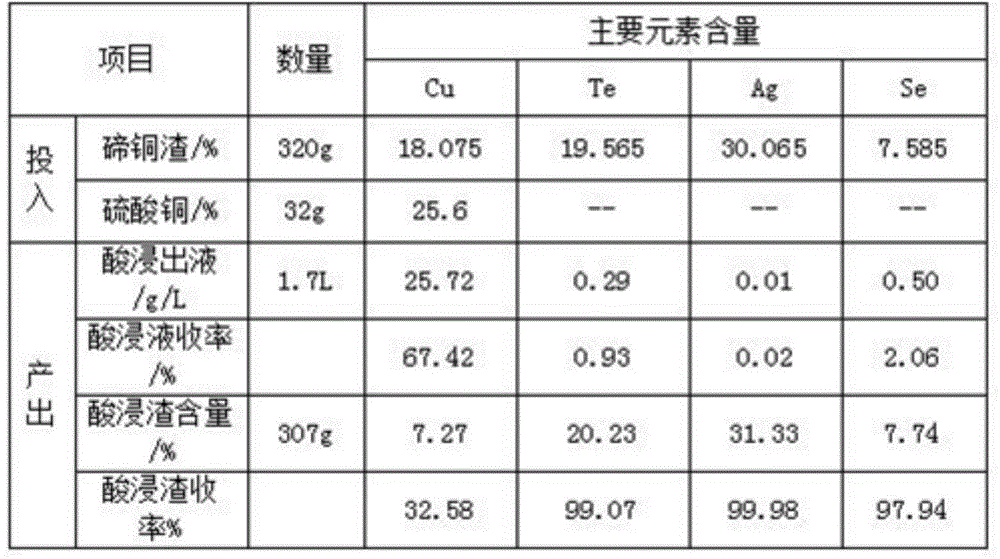
Method for comprehensively recycling silver, selenium, tellurium and copper from telluride copper slag
The invention discloses a method for comprehensively recycling silver, selenium, tellurium and copper from telluride copper slag. The method comprises the following steps of (1), oxidizing acid leaching, wherein the telluride copper slag is added into a sulfuric acid solution containing an oxidizing agent to be heated and stirred so as to be leached, after filtering, copper sulfate leaching liquid and acidic leaching residues are obtained, and the leaching liquid is conveyed to a furnace for copper recycling; and (2) alkali leaching separation, wherein the acidic leaching residues are added into sodium hydroxide solutions to be leached, sodium tellurite solutions and basic leached residues are obtained, the basic leached residues are sent to the KALDO furnace for smelting, so that the silver and the selenium are recycled, and after purification, tellurium deposition, forging and electrolysis of the alkali leaching liquid, refined tellurium is obtained. According to the method, the recycling rate of the silver, the selenium, the tellurium and the copper is high, the silver, the selenium, the tellurium and the copper are not lost, the concentration ratio is high, the silver, the selenium, the tellurium and the copper can be separated from other impurities well, the pollution to the environment is small, the technology is simple, and needed equipment cost is low.
Owner:NORTHWEST RES INST OF MINING & METALLURGY INST
Low-cost method for synthesizing ZnxCd1-xSe (x is more than or equal to zero and less than or equal to 1) and related core/shell structured semiconductor nanocrystals thereof
InactiveCN101891162AReduce usageAvoid inconvenienceSelenium/tellurium compundsLuminescent compositionsQuantum yieldTrioctylphosphine
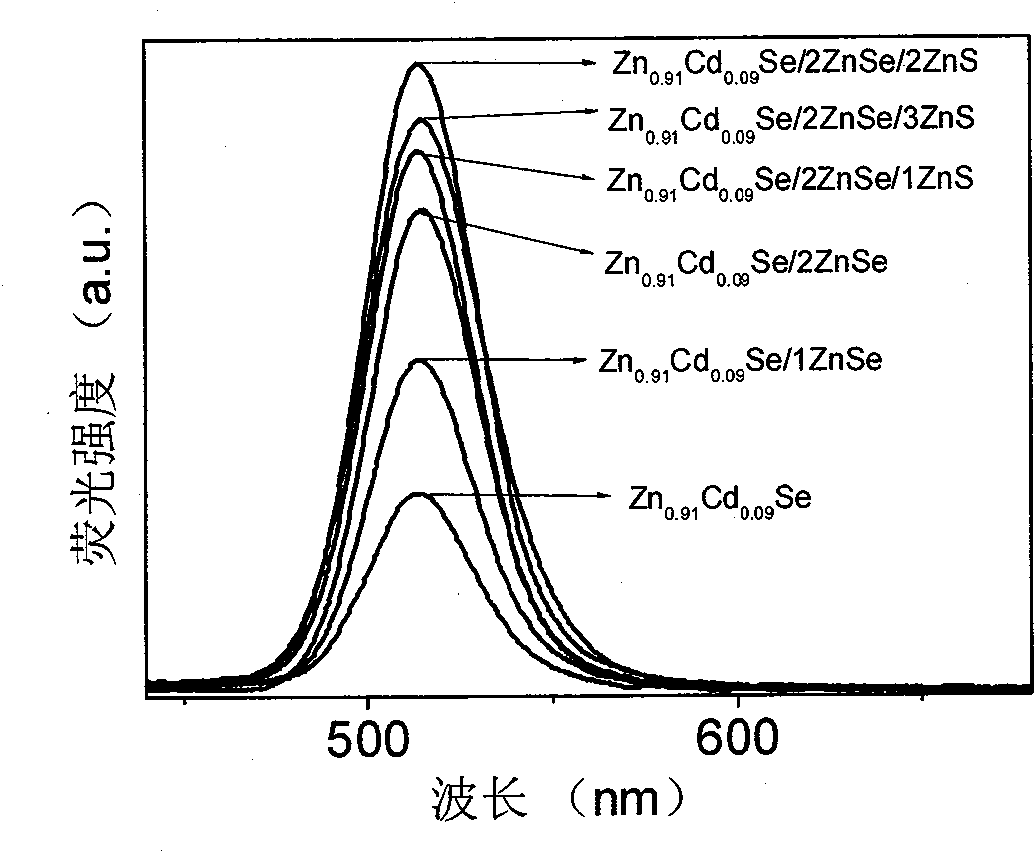
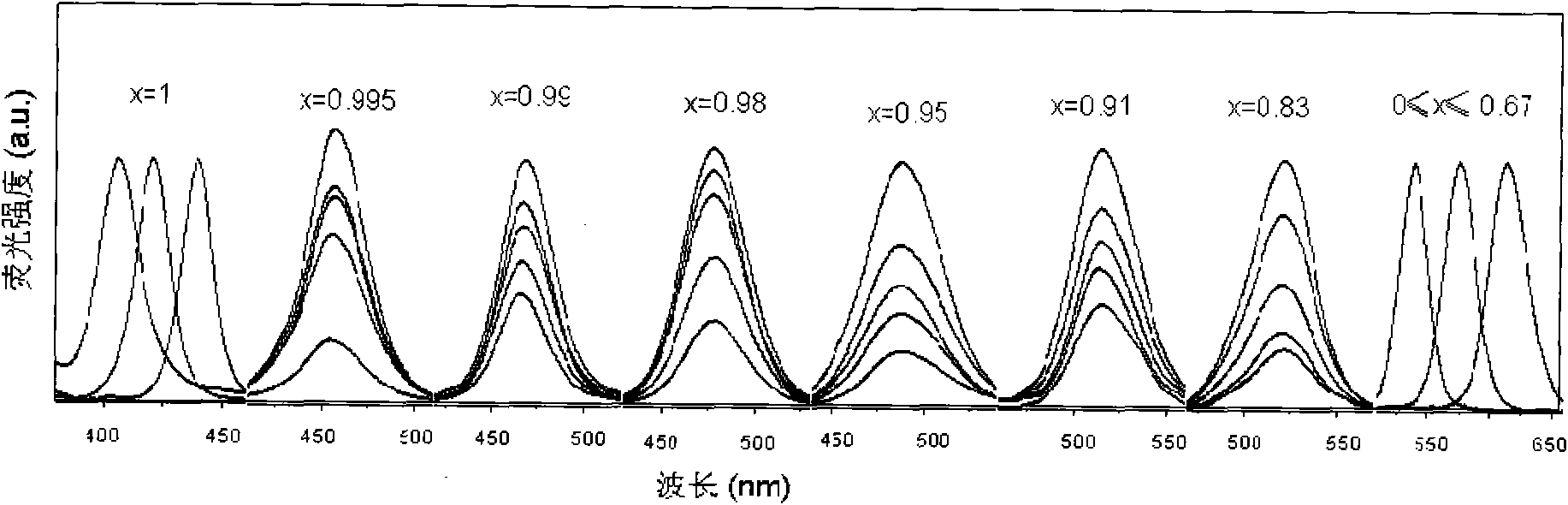
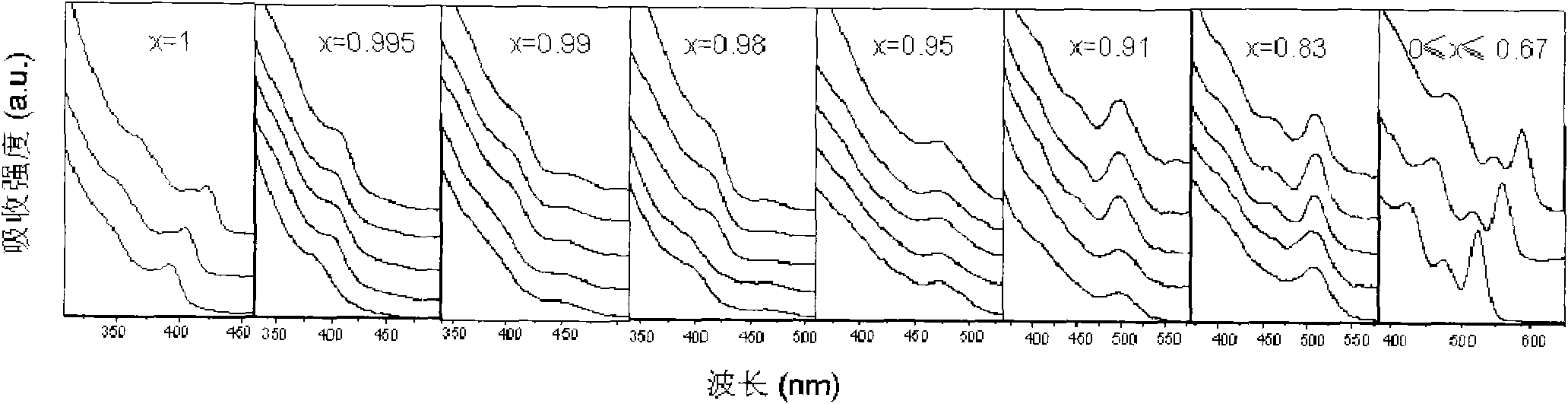
The invention provides a method for synthesizing ZnxCd1-xSe (x is more than or equal to 0 and less than or equal to 1) and ZnxCd1-xSe / ZnSe, ZnxCd1-xSe / ZnS and ZnxCd1-xSe core / shell structured semiconductor nanocrystals thereof by using long-chain fatty acid salts of cadmium and zinc as precursors of cadmium and zinc, which is the most economical and environment-friendly method for synthesizing high-quality ZnxCd1-xSe and related core / shell structured semiconductor nanocrystals thereof currently. The method avoids using tributylphosphine (TBP) or trioctylphosphine (TOP) dissolved elementary selenium as the precursor of selenium currently in the world, but adopts octadecylene (ODE) dissolved elementary selenium as the precursor of selenium; and the obtained nanocrystals have the quality equal to that of nanocrystals which are synthesized when the TBP or TOP dissolved selenium powder is used as the precursor of selenium. The method is called a phosphine-free method, has the advantages of simple synthesizing process, good repeatability, safety, environmental protection, no need of glove box, and cost conservation of over 60 percent. The synthesized ZnxCd1-xSe and related core / shell structured nanocrystals have a fluorescence range of between 400 and 650nm, and have the advantages of uniform particle size distribution, high efficiency of fluorescent quantum yield (40 to 70 percent) and narrow full width at half maximum. The method is also suitable for synthesizing the ZnxCd1-xS, HgxCd1-xSe, ZnxCd1-xTe nanocrystals and related core / shell structures thereof. More importantly, the method can synthesize high-quality nanocrsytals on a large scale, and has enormous application value both in laboratory synthesis and industrial synthesis.
Owner:HENAN UNIVERSITY
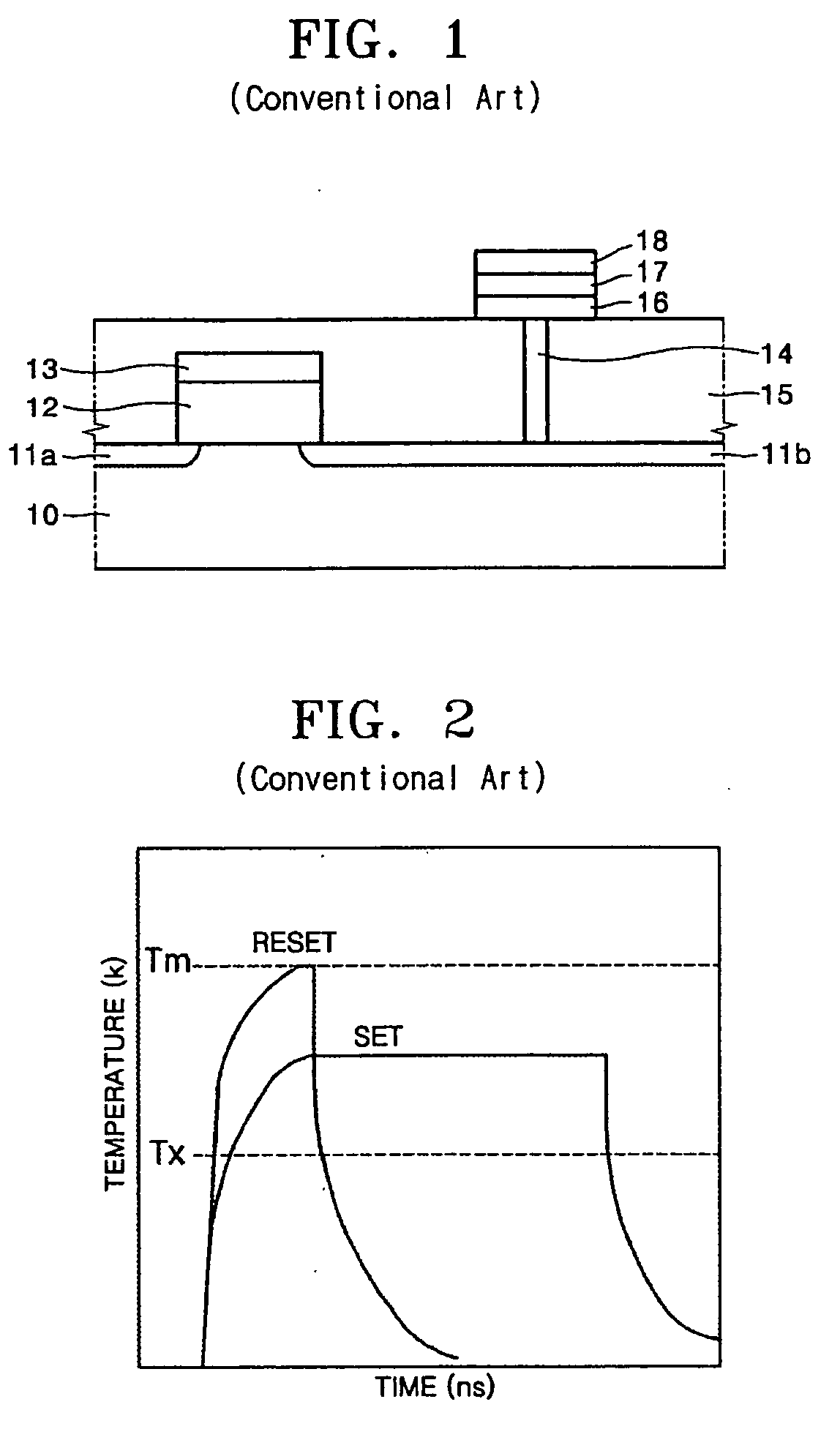
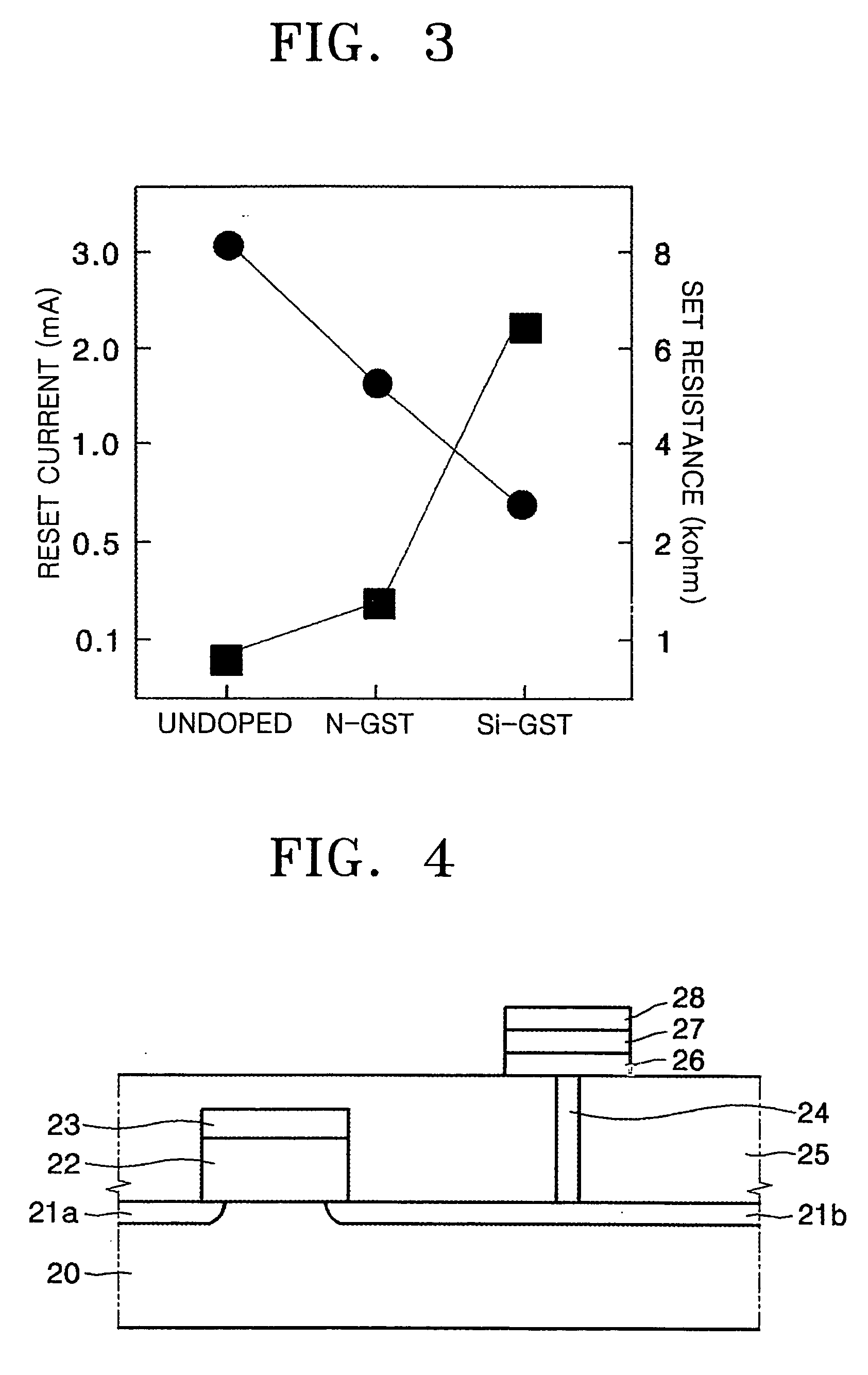
Precursor, thin layer prepared including the precursor, method of preparing the thin layer and phase-change memory device
InactiveUS20080258127A1Uniform thicknessImprove performanceGroup 4/14 element organic compoundsSelenium/tellurium compundsDeposition temperaturePhase-change memory
A Te precursor containing Te, a 15-group compound (for example, N) and / or a 14-group compound (for example, Si), a method of preparing the Te precursor, a Te-containing chalcogenide thin layer including the Te precursor, a method of preparing the thin layer; and a phase-change memory device. The Te precursor may be deposited at lower temperatures for forming a Te-containing chalcogenide thin layer doped with a 15-group compound (for example, N) and / or a 14-group compound (for example, Si). For example, the Te precursor may employ plasma enhanced chemical vapor deposition (PECVD) or plasma enhanced atomic layer deposition (PEALD) at lower deposition temperatures. The GST phase-change layer doped with a 15-group compound (for example, N) and / or a 14-group compound (for example, Si) formed by employing the Te precursor may have a decreased reset current, and thus when a memory device including the same is employed, its integration may be possible, and operation with higher capacity and / or higher speed may be possible.
Owner:SAMSUNG ELECTRONICS CO LTD
Method for synthesizing copper-indium-selenium nanocrystalline
InactiveCN101698472ANo pollution in the processImprove reaction efficiencySelenium/tellurium compundsNitrogen gasSolvent
The invention discloses a method for synthesizing copper-indium-selenium nanocrystalline and relates to a solar cell material. The invention provides a method for synthesizing copper-indium-selenium nanocrystalline with simple technological steps. The method comprises the following steps: mixing dodecyl mercaptan and oleyl amine to obtain a mixed solvent; adding an elementary substance selenium, and dispersing to obtain an evenly dispersed elementary substance solution; adding cuprous chloride and indium chloride into an oleic acid-octadecylene mixed solvent, heating while stirring, vacuumizing, so that the vacuum degree of the system is lower than -0.1 MPa, and introducing nitrogen to obtain an oleic acid-octadecylene composition of cupric salts and indium salts in the nitrogen atmosphere, wherein the oleic acid-octadecylene composition of cupric salts and indium salts is a light-brown transparent solution; and injecting the prepared selenium solution into the oleic acid-octadecylene composition of cupric salts and indium salts, heating to carry out the reaction, centrifugating to obtain a precipitate, and respectively washing the precipitate with methenyl chloride and ethanol at least once to obtain the copper-indium-selenium nanocrystalline.
Owner:XIAMEN UNIV
Copper-zinc-tin-sulfur or copper-zinc-tin-selenium target for absorbed layer of thin-film solar battery, preparation method for target and application of target
InactiveCN102372302AImprove efficiencyReduce porosityTin compoundsSelenium/tellurium compundsHigh densitySulfur
The invention provides a copper-zinc-tin-sulfur or copper-zinc-tin-selenium target for an absorbed layer of a thin-film solar battery. The target is characterized in that: the molar ratio of Cu:Zn:Sn:S is (1.8-2.2):(0.8-1.2):(0.8-1.2):(3.6-4.4) in the target or the molar ratio of Cu:Zn:Sn:Se is (1.8-2.2):(0.8-1.2):(0.8-1.2):(3.6-4.4), wherein Cu2ZnSnX4 (X is S or Se) is taken as a main phase in the target. The invention also provides a method for preparing the target and application of the target to the preparation of films of absorbed layers of solar batteries. A Cu2ZnSnX4 (X is S or Se) film can be directly prepared through magnetron sputtering or pulsed laser deposition. The process is simple; and the prepared target has uniform components and high density, and is particularly suitable for preparing Cu2ZnSnX4 (X is S or Se) absorbed layers of thin-film solar batteries through magnetron sputtering and pulsed laser deposition systems.
Owner:EAST CHINA NORMAL UNIV
Method for recovering rare precious metals from solution
ActiveCN101928834AAdaptable to changeEasy to separate and recycleSelenium/tellurium compundsProcess efficiency improvementSlagZinc
The invention relates to a method for recovering rare precious metals from a solution, which recovers the rare precious metals from the solution by adopting an SO2 direct-reduction process. The method comprises the following steps of: regulating the acidity of the solution containing the rare precious metals, adding a compound containing chloridions, introducing SO2 to react for a certain time under a heating condition, and then filtering to obtain rare precious metal slag; lixiviating the rare precious metal slag by using a Na2SO3 solution, and filtering to obtain separated selenium slag and a solution containing selenium; adding sulfuric acid to the solution containing the selenium, heating, and then filtering to obtain selenium slag; lixiviating the separated selenium slag by using a mixed solution of the sulfuric acid and hydrogen peroxide, and filtering to obtain precious metal concentrates and a solution containing tellurium; adding the compound containing the chloridions to the solution containing the tellurium, introducing SO2 for reduction, and filtering to obtain tellurium powder. The invention recovers the rare precious metals contained in the solution through the reduction by using the SO2; and in addition, compared with a zinc powder reduction method, the method has the advantages of simplicity, easy operation, low cost and high comprehensive utility ratio.
Owner:CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com