Use of thermoelectric materials for low temperature thermoelectric purposes
a thermoelectric and thermoelectric technology, applied in the field of thermoelectric materials, can solve the problems of no commercial device based on the nernst effect, no excellent thermoelectric properties of these compounds, and low zt/sub>n/sub>values for commercial applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-12
Preparation of the Thermoelectric Materials
example 1
Preparation of the Prior Art Composition FeSb2 (in Sb Flux)
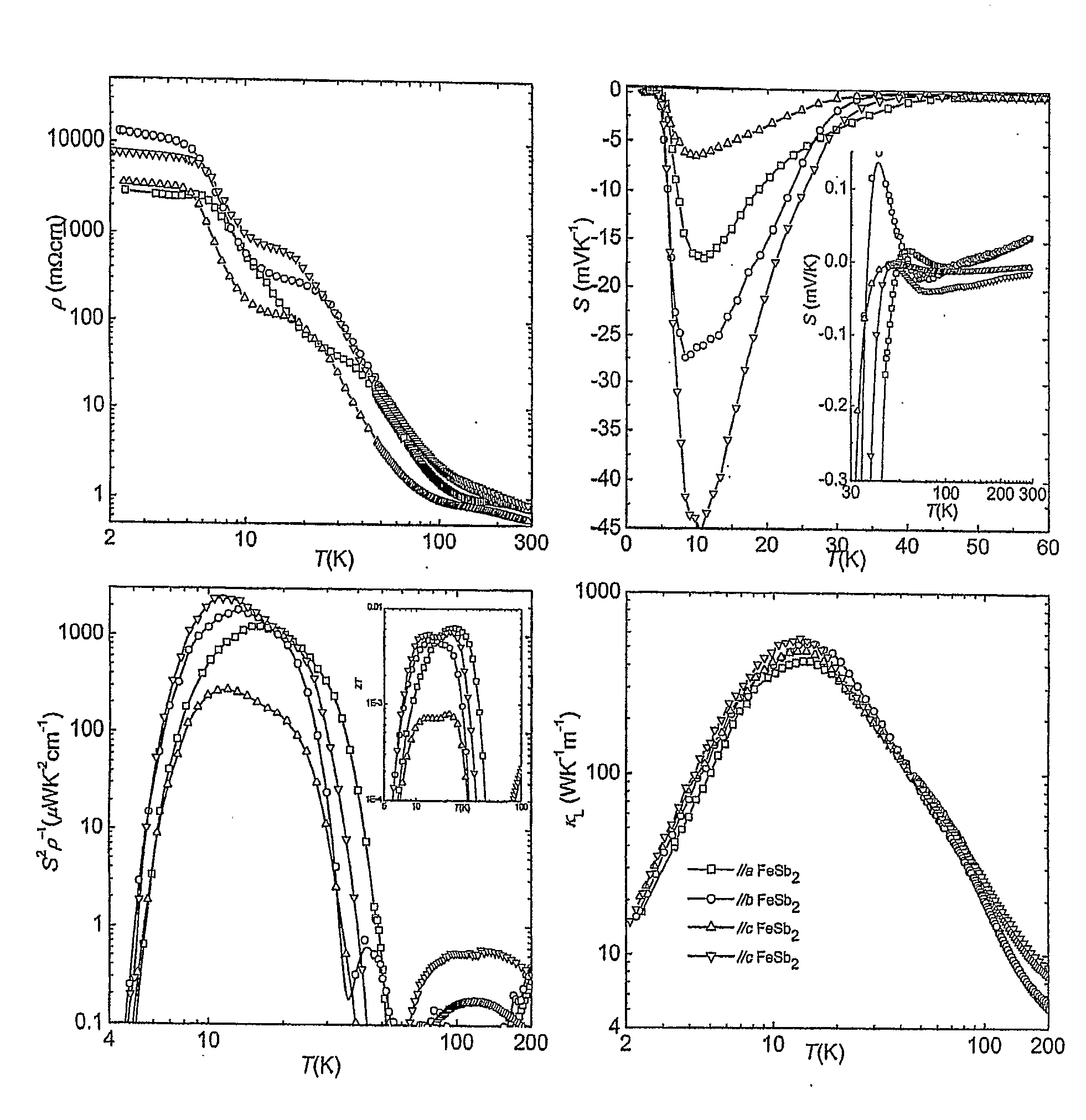
[0173]Pure FeSb2 samples are prepared by a flux method. 0.95882 g bulk Fe (Alfa Aesar Puratronic® 99.995% metals basis) and 24.04118 g bulk Sb (Alfa Aesar Puratronic® 99.9999% metals basis) are mixed in an alumina crucible which is sealed inside an evacuated quartz ampoule. The ampoule is isolated with mineral wool and heated fast (over approximately 6 hours) to 1050° C. and left there for 2 hours, followed by cooling to 775° C. over 14 hours and finally cooling to 640° C. over 15 days. The Sb-flux is removed by centrifuging at 690° C. on top of small broken quartz pieces inside an evacuated quartz ampoule. To remove any remaining Sb-flux on the FeSb2 samples they are cleaned in an ultra sonic bath of Aqua Regia for 3-8 minutes. Relatively large single crystals are obtained. The resulting samples can be seen in FIG. 1-3.
example 2
Preparation of the Prior Art Composition FeSb2 (in Bi Flux)
[0174]The samples are prepared by a flux method using a melt with nominal stoichiometry Fe8Sb16.1Bi75.9. 0.61137 g bulk Fe (Alfa Aesar Puratronic® 99.995% metals basis) and 2.68264 g bulk Sb (Alfa Aesar Puratronic® 99.9999% metals basis) and 21.70598 g bulk Bi (Strem chemicals 99.999+% metals basis) are mixed in an alumina crucible which is sealed inside an evacuated quartz ampoule. The ampoule is isolated with mineral wool and heated fast (over approximately 6 hours) to 1050° C. and left there for 2 hours, followed by cooling to 775° C. over 14 hours and finally cooling to 640° C. over 15 days. The flux is removed by centrifuging at 690° C. on top of small broken quartz pieces inside an evacuated quartz ampoule.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com