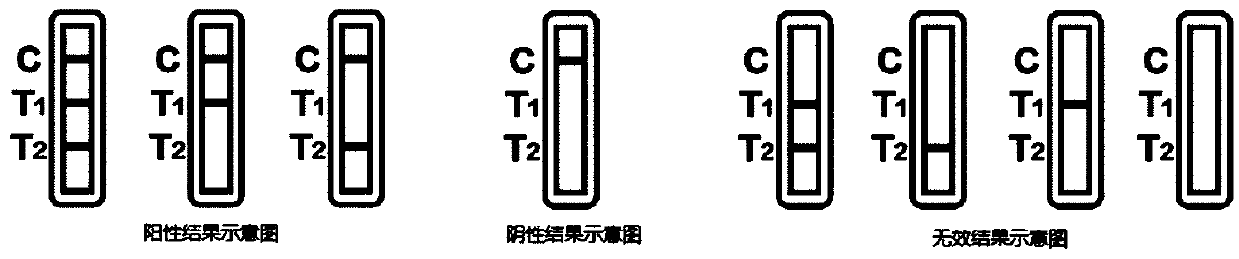
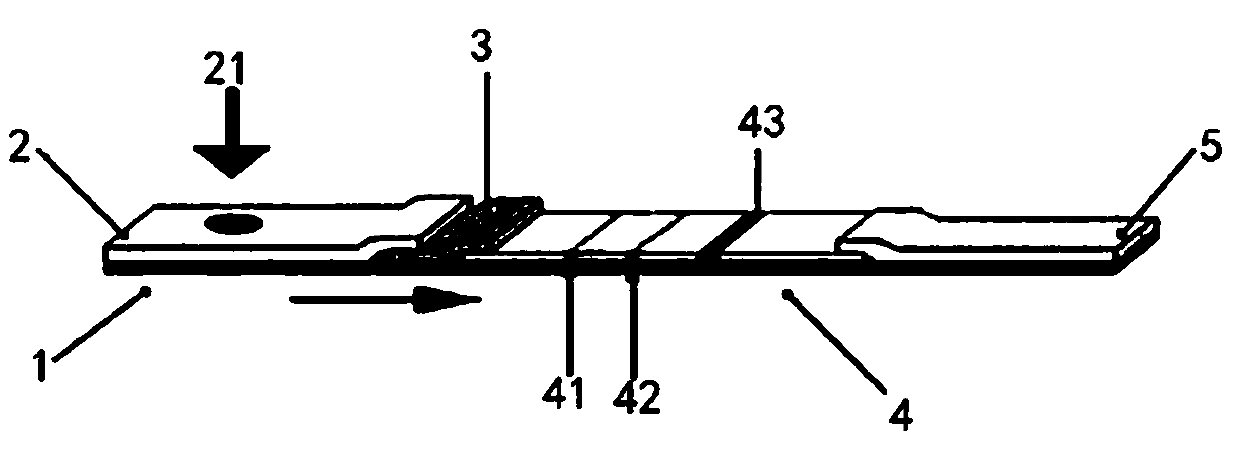
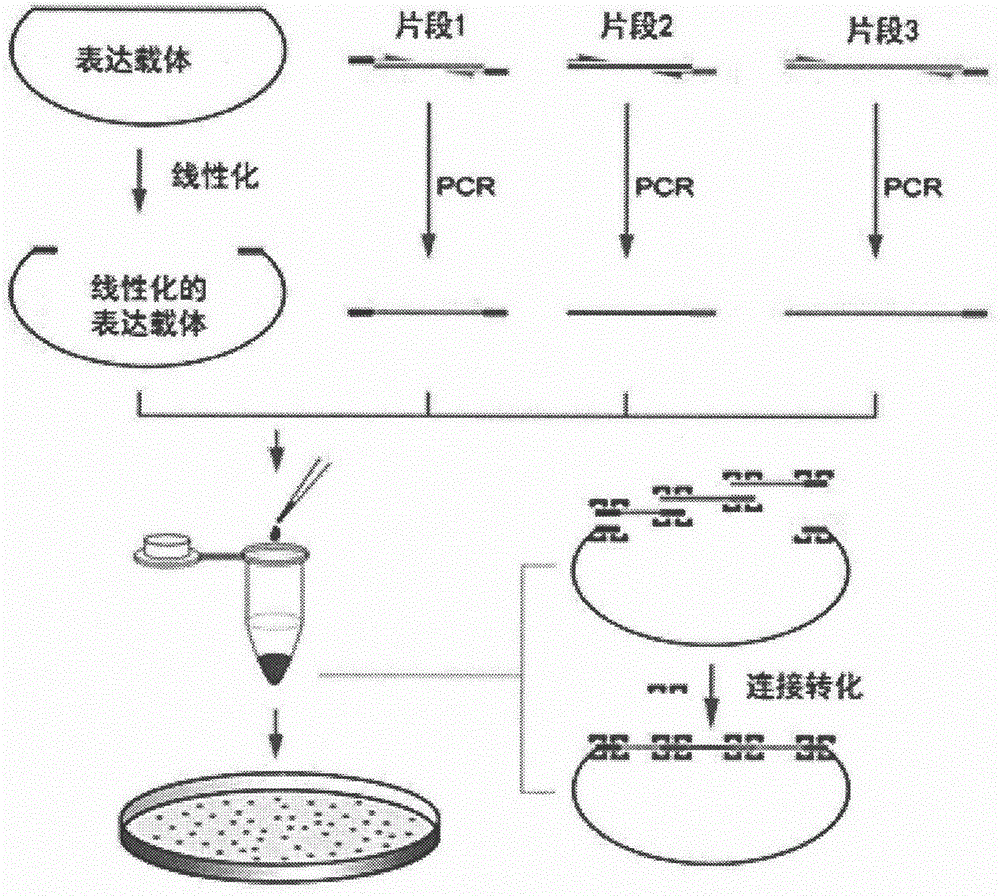
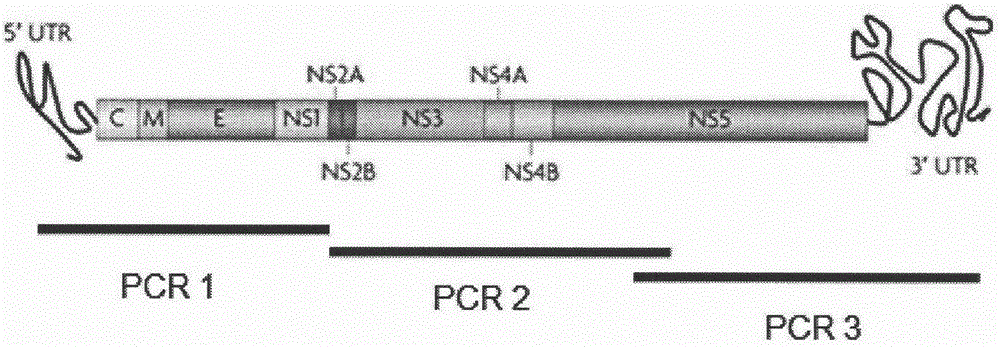
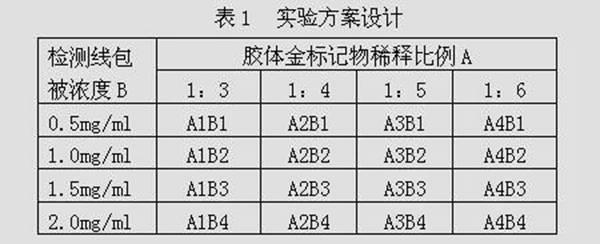
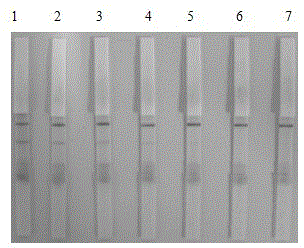
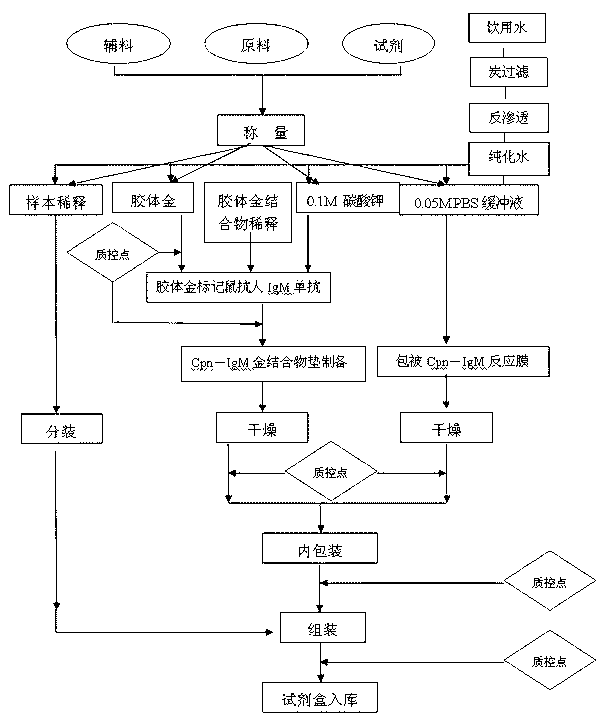
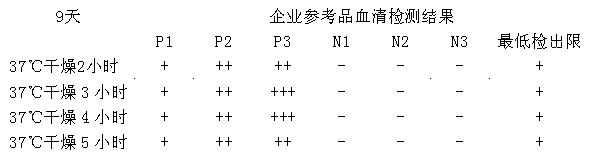
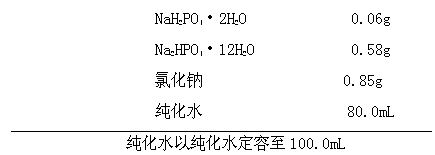
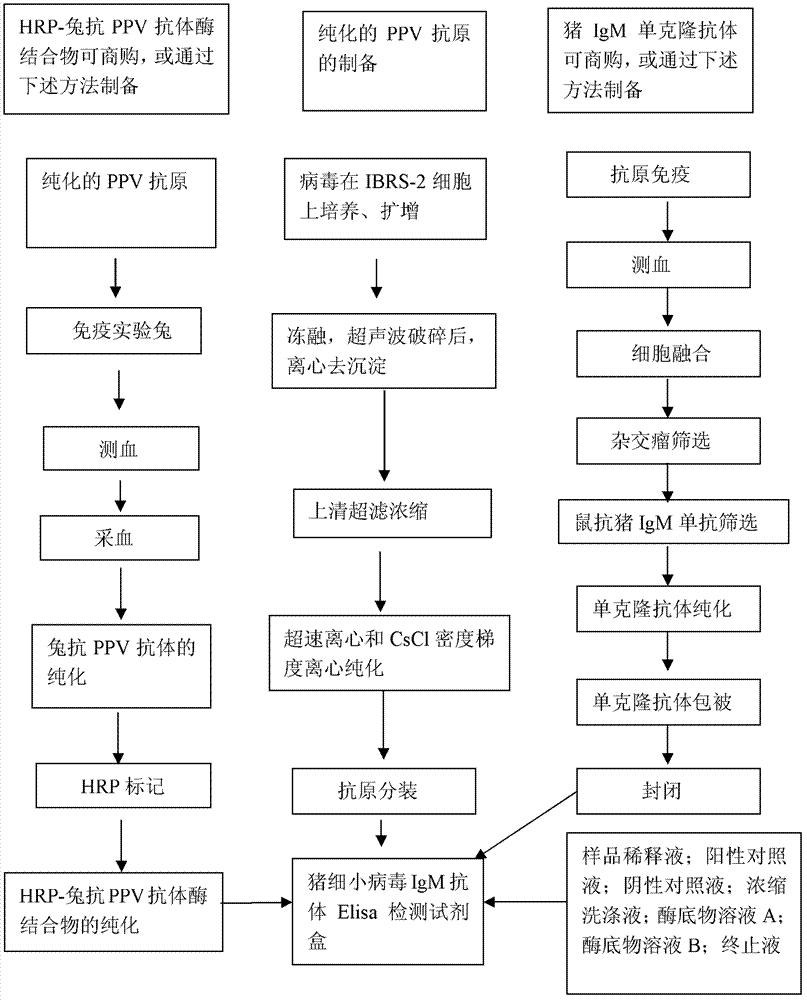
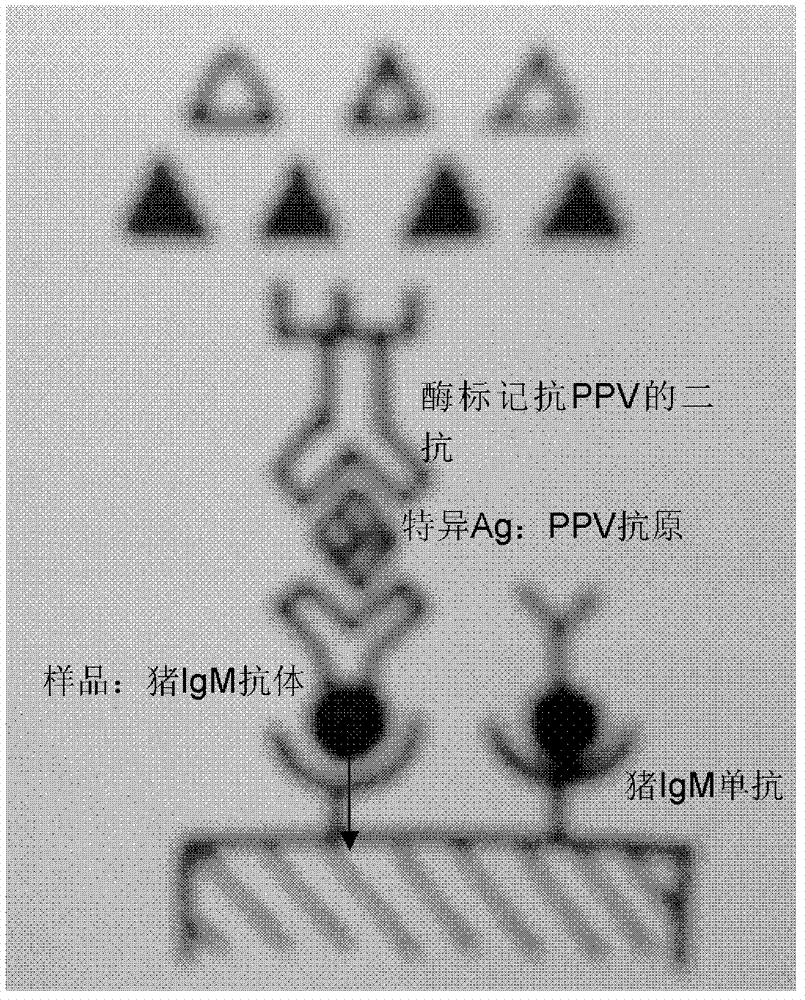
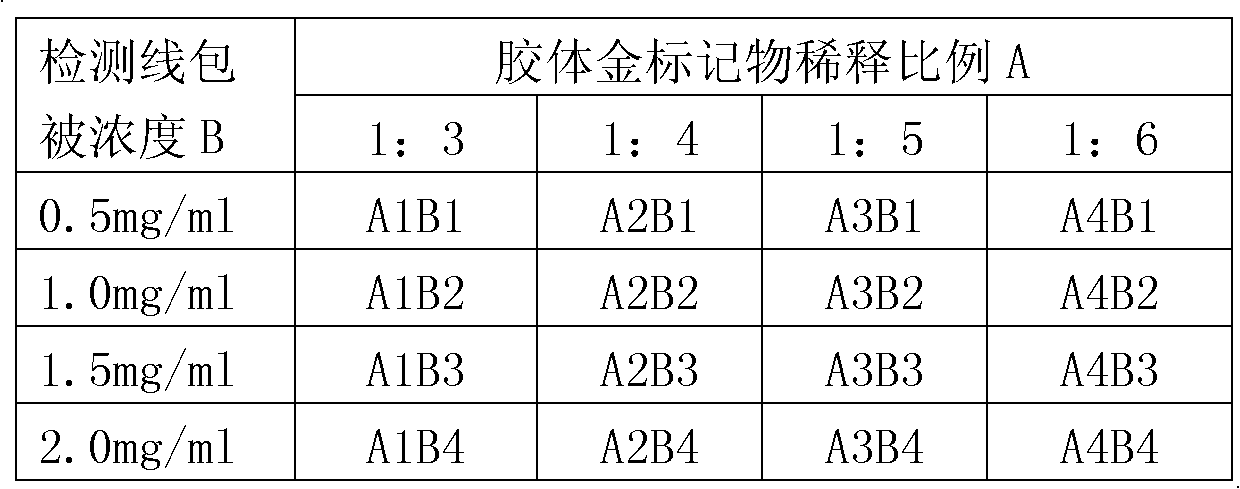
Patents
Literature
74 results about "IgM.monoclonal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
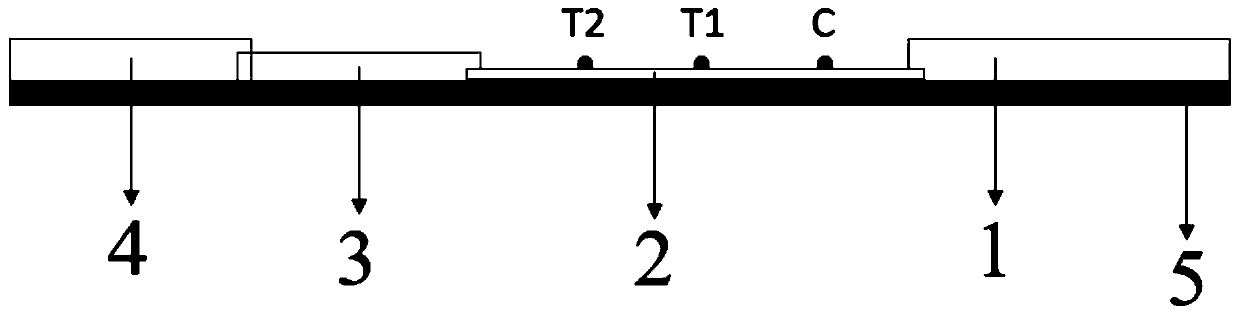
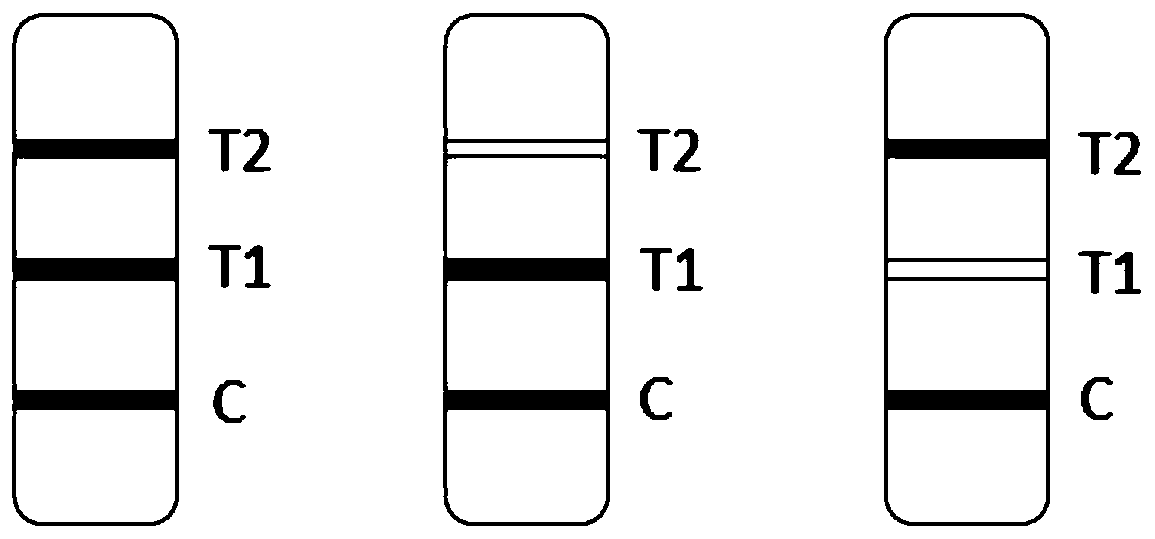
Novel fluorescence immunochromatography test strip for joint inspection of SARS-CoV-2 IgG-IgM antibodies of coronaviruses
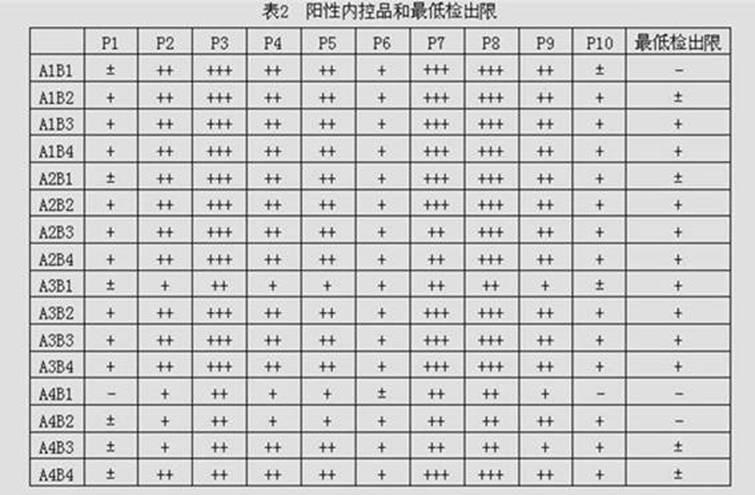
PendingCN111426844ARapid Quantitative DetectionHigh sensitivityBiological testingImmunoassaysIgm antibodyStructural protein
The invention discloses a novel fluorescence immunochromatography test strip for joint inspection of SARS-CoV-2 IgG-IgM antibodies of coronaviruses. The test strip comprises a bottom plate, a sample pad, a combination pad, a nitrocellulose membrane and a water absorption pad, wherein the sample pad, the combination pad, the nitrocellulose membrane and the water absorption pad are sequentially connected end to end and adhered to the bottom plate; the combination pad is coated with an SARS-CoV-2 structural protein-marker and a goat anti-rabbit IgG-marker, and the nitrocellulose membrane is provided with a detection line T1 coated with a mouse anti-human IgG monoclonal antibody, a detection line T2 coated with a mouse anti-human IgM monoclonal antibody and a quality control line C coated withrabbit IgG. When the test strip is used for quantitatively detecting SARS-CoV-2 IgG and IgM antibodies, the detection sensitivity is high, and the specificity is good and can reach 96%; the batch-to-batch difference is small, and good repeatability is achieved; the test strip can be stored for half a year at normal temperature without reducing the sensitivity and has good stability; the test strip is simple to operate and low in cost, can quickly and quantitatively detect the levels of SARS-CoV-2 IgG and IgM antibodies in a human body, assists a nucleic acid detection means, and provides powerful support for epidemic situations.
Owner:NANJING AGRICULTURAL UNIVERSITY
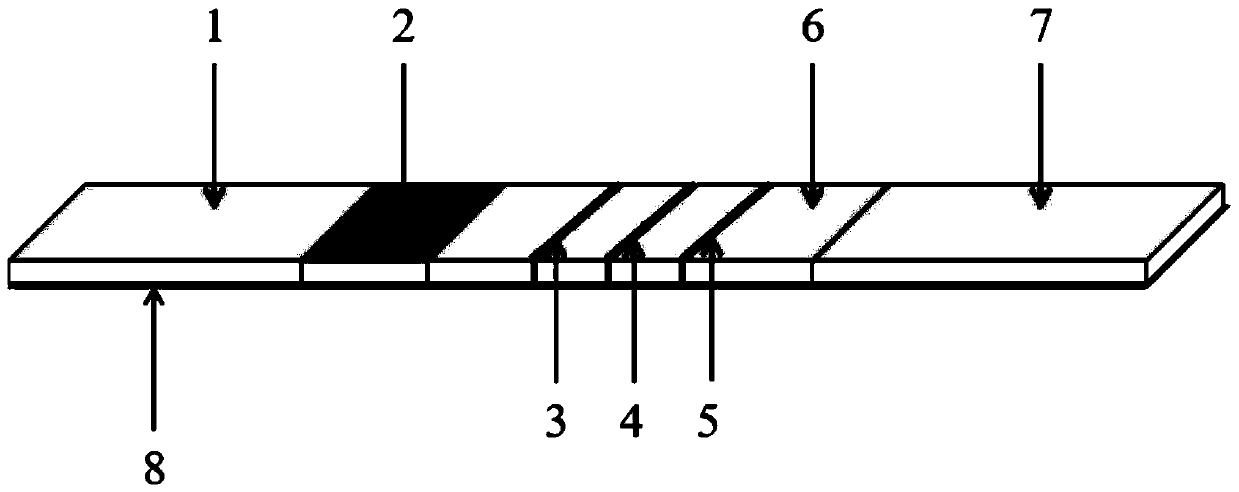
ELISA (enzyme-linked immuno sorbent assay) detection kit of porcine pseudorabies virus IgM antibody
InactiveCN103308684AStrong specificityHigh sensitivityVirus peptidesFermentationIgm antibodyHorseradish peroxidase
The invention relates to an ELISA (enzyme-linked immuno sorbent assay) detection kit of a porcine pseudorabies virus IgM antibody, and a preparation method and application thereof. The ELISA detection kit of the porcine pseudorabies virus IgM antibody disclosed by the invention comprises an elisa plate of enveloping an IgM monoclonal antibody, sealing liquid, sample diluting liquid, detecting antigen, enzyme conjugate, concentrated scrubbing solution, enzyme substrate A solution, enzyme substrate B solution and stop buffer; the detecting antigen is prepared from purified porcine pseudorabies virus gE protein, structure protein gB and structure protein gD; and the enzyme conjugate is horse radish peroxidase-anti-gE, gB or gD protein enzyme compound. The specificity of the kit disclosed by the invention can be up to 100%; the sensitivity is 1:640; and the kit can be used for early diagnosis of porcine pseudorabies virus infection.
Owner:WUHAN CHOPPER BIOLOGY
Novel coronavirus detection test strip as well as preparation method and application thereof
InactiveCN111426840AImprove accuracySimple and fast operationBiological testingImmunoassaysIgm antibodyMonoclonal antibody agent
The invention provides a novel coronavirus detection test strip as well as a preparation method and application thereof. The test strip comprises a binding pad and an analysis membrane, and the binding pad is coated with a luminescent substance labeled 2019-nCoV recombinant antigen and a mouse anti-human HCG monoclonal antibody; a T2 detection line, a T1 detection line and a quality control line are sequentially arranged on the analysis membrane along the chromatography direction; the T2 detection line is coated with a mouse anti-human IgG monoclonal antibody, the T1 detection line is coated with a mouse anti-human IgA monoclonal antibody and a mouse anti-human IgM monoclonal antibody, and the quality control line is coated with a goat anti-mouse IgG polyclonal antibody. The test paper card can simultaneously determine the positive conditions of IgA antibody, IgM antibody and IgG antibody in serum of a patient, can more accurately detect the early antibody level condition in the body of the patient, assists in judging different periods of novel coronavirus infection of the patient, and improves the sensitivity and specificity of novel coronavirus detection.
Owner:北京中检安泰诊断科技有限公司
Antibodies And Epitopes Specific To Misfolded Prion Protein
The present invention relates to antibodies and immunogenic peptides specific to misfolded prion protein (PrP, e g, PrPSc), and uses thereof. The immunogenic peptides comprise the amino acid sequence tyrosine-methionine-leucine (YML). The antibodies or peptides can be used for treating or preventing a disease or disorder associated with misfolded PrP, including cancer. In particular, a IgM monoclonal antibody designated “1A1” was generated using a peptide consisting of the sequence GGYMLGS (i e, SEQ ID NO 8), which corresponds to residues 126-132 of human PrP 1A1 recognizes misfolded PrP, but not normal PrP.
Owner:THE UNIV OF BRITISH COLUMBIA
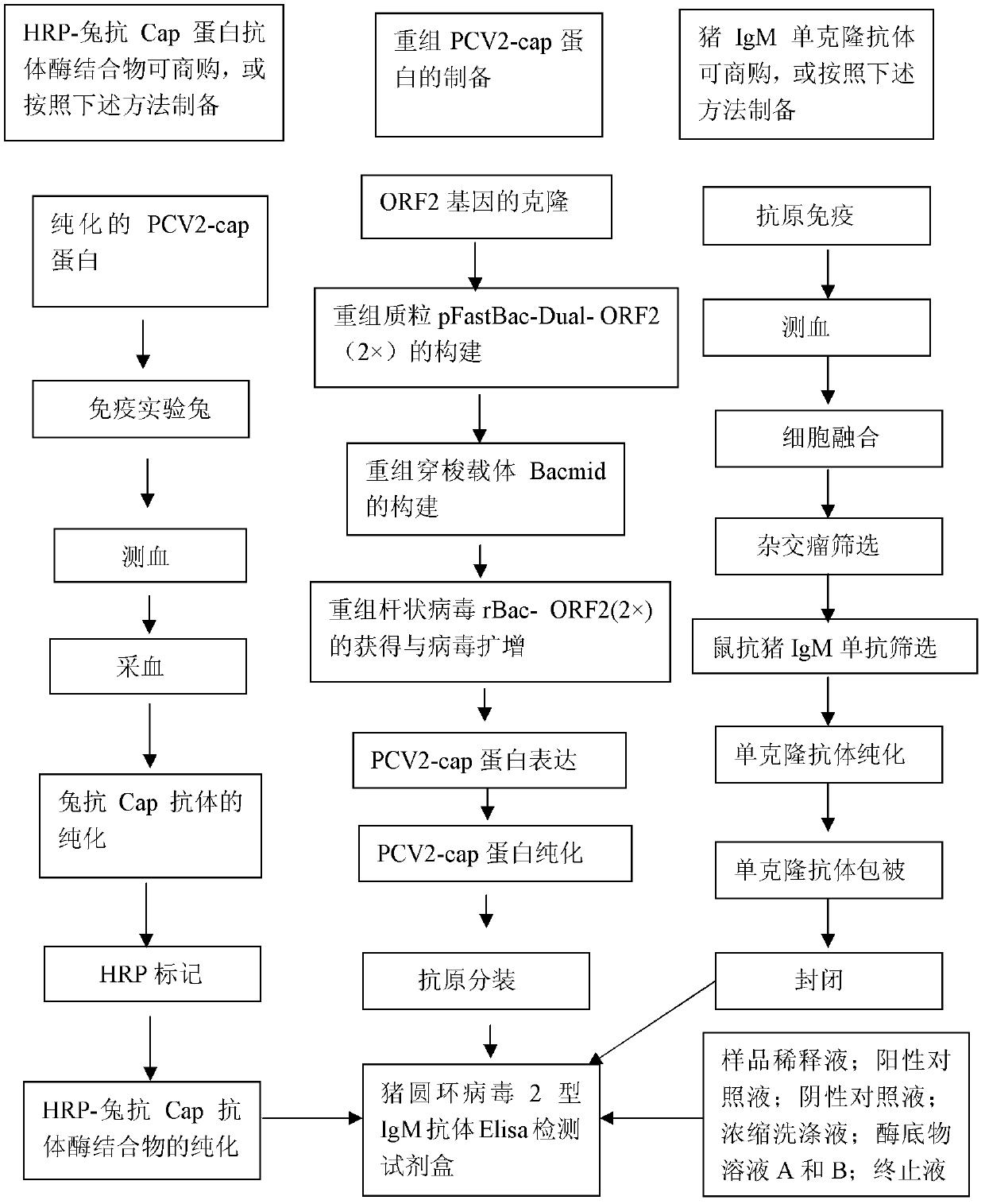
ELISA (Enzyme-Linked Immunosorbent Assay) detection kit for porcine circovirus type 2 IgM antibody and preparation method of ELISA detection kit
InactiveCN103308688AOvercoming defects such as low sensitivityHigh sensitivityBiological testingIgm antibodyHorseradish peroxidase
The invention relates to an ELISA (Enzyme-Linked Immunosorbent Assay) detection kit for a porcine circovirus type 2 IgM antibody as well as a preparation method and application of the ELISA detection kit. The ELISA detection kit for the porcine circovirus type 2 IgM antibody comprises an ELISA plate for coating a pig IgM monoclonal antibody, a confining liquid, a sample diluent, an antigen for detection, an enzyme conjugate, a concentrated washing liquid, an enzyme substrate solution A, an enzyme substrate solution B and a stop solution, wherein the antigen for detection is a purified porcine circovirus type 2 nucleocapsid protein (PCV2-Cap protein) antigen, and the enzyme conjugate is a horse radish peroxidase-antiCap protein antibody enzyme conjugate. The kit provided by the invention can realize 100% of specificity expression, has the high sensitivity of 1:800, and can be used for the early diagnosis of PCV2 infection of a swinery.
Owner:WUHAN CHOPPER BIOLOGY
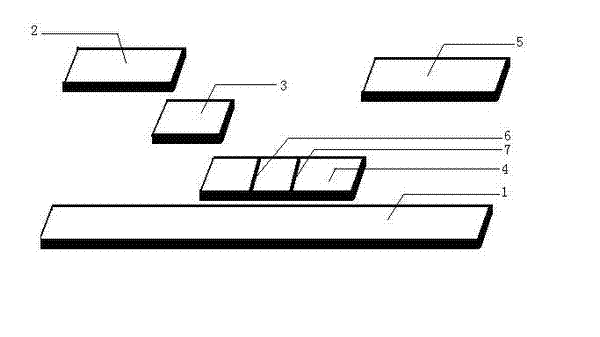
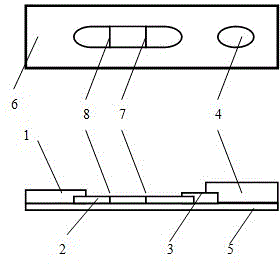
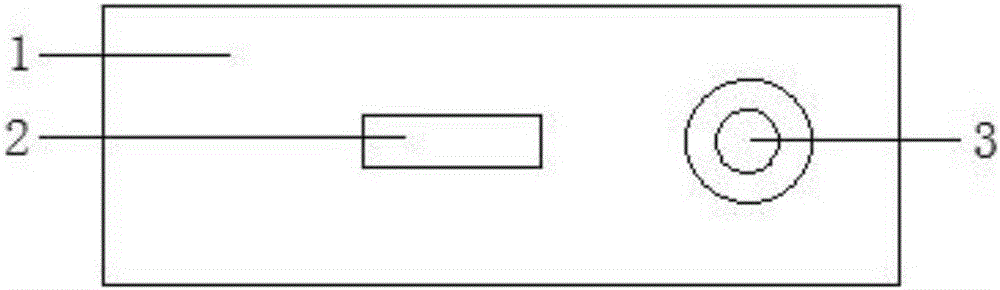
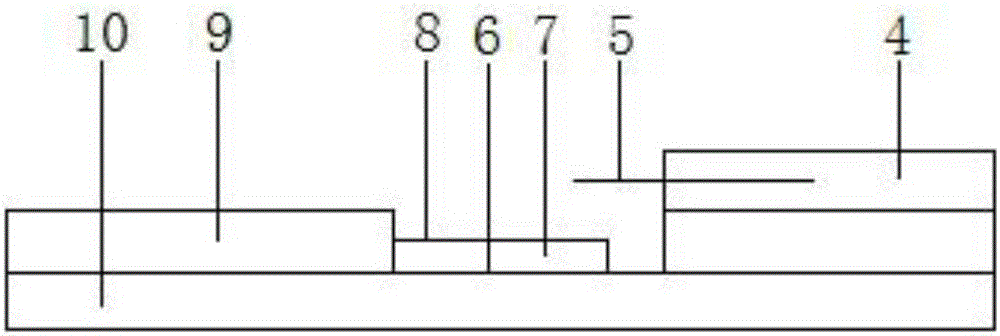
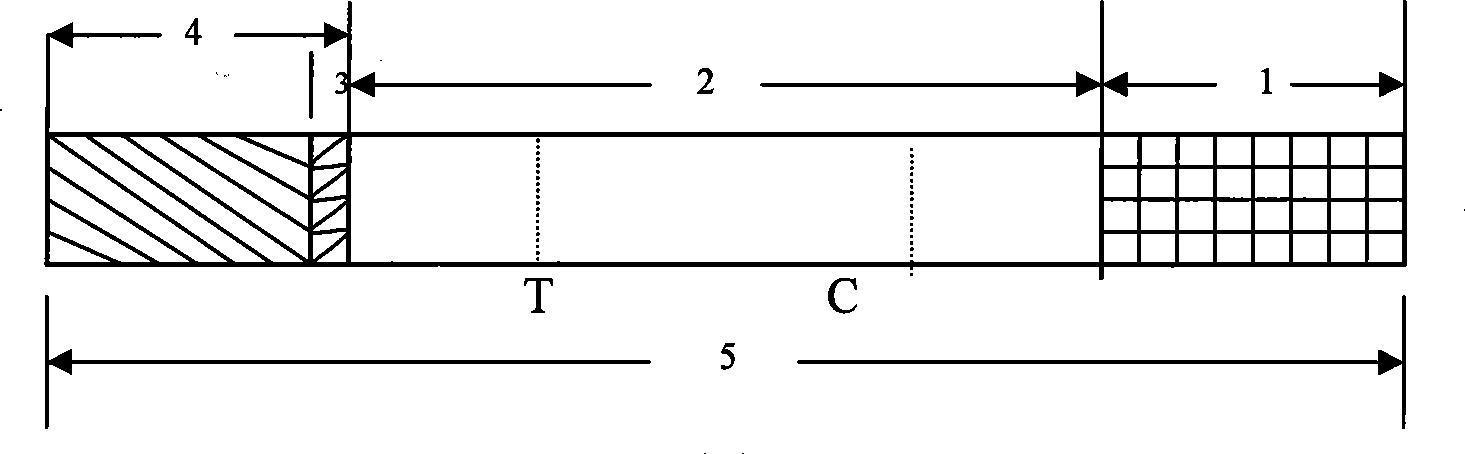
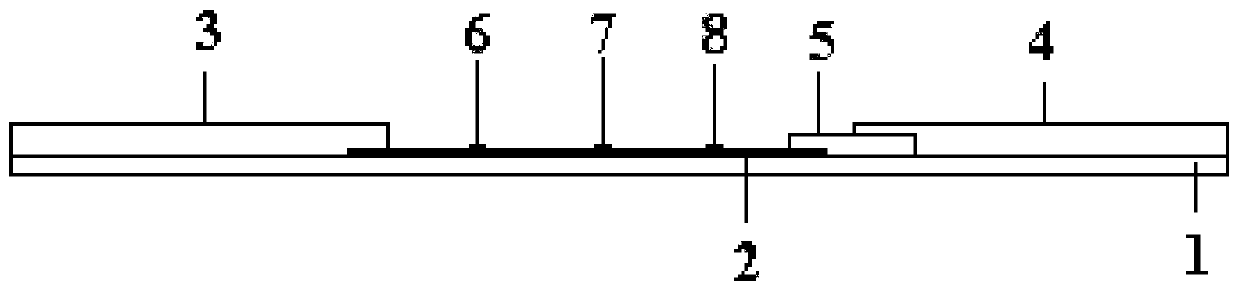
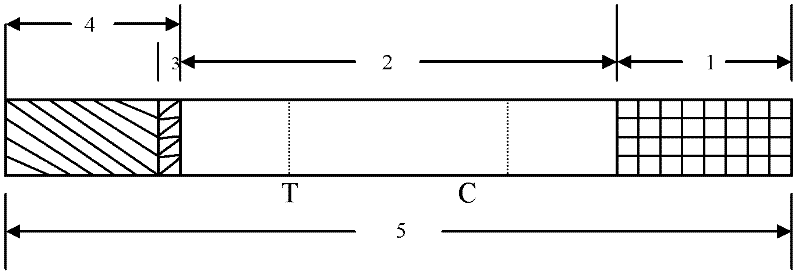
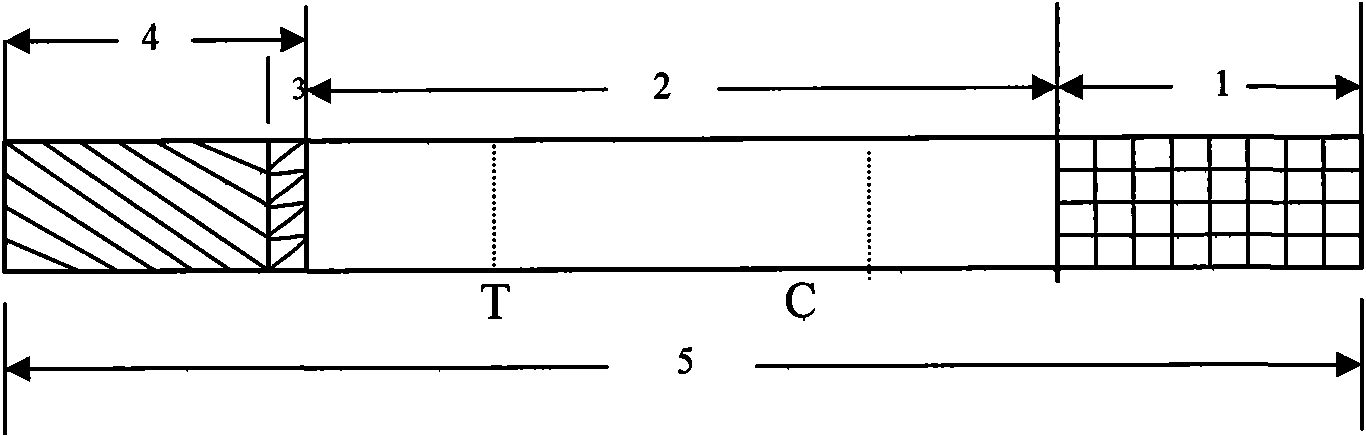
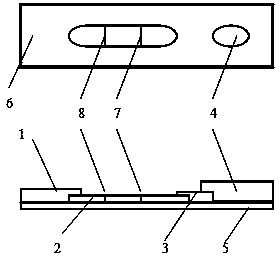
Detection reagent of treponema pallidum IgM (Immunoglobulin M) antigen colloidal gold method and preparation method thereof
The invention discloses a detection reagent of a treponema pallidum IgM (Immunoglobulin M) antigen colloidal gold method and a preparation method thereof. The reagent comprises a gold conjugate pad (3), a cellulose nitrate reaction membrane (4), a sample pad (2), water absorbing paper (5) and a PVC (Polyvinyl Chloride) back lining (1), wherein the sample pad, the gold conjugate pad, the cellulose nitrate reaction membrane and the water absorbing paper are sequentially and mutually laminated and adhered on the PVC back lining; the sample pad is laminated on the gold conjugate pad for 2-3 mm, the gold conjugate pad is laminated on the cellulose nitrate reaction membrane for 2-3 mm, and the water absorbing paper is laminated on the cellulose nitrate reaction membrane for 2-3 mm; the gold conjugate pad is coated with an anti-human IgM monoclonal antibody-colloidal gold conjugate; and positions of a quality control region (7) and a detection region (6) of the cellulose nitrate reaction membrane are coated with goat anti-mouse IgG antibody or rabbit anti-mouse IgG antibody and specific gene recombinant treponema pallidum antigen respectively. A production process of a product provided by the invention is simple and easy to control; the detection reagent is easy, convenient and quick in detection; and a result can be read in 25 minutes and is not affected by the IgG antibody and is accurate and reliable.
Owner:BEIJING BIONEOVAN
Preparation of Zika virus multi-segment fusion protein and IgG/IgM antibody detection kit
InactiveCN106841601AQuick checkDisease diagnosisAgainst vector-borne diseasesBiotin-streptavidin complexIgm antibody
The invention aims at providing a simple and quick Zika virus detection kit. The kit optimally selects fusion expression protein as diagnostic antigen; an anti-human IgG monoclonal antibody A374, an anti-human IgM monoclonal antibody A371 and a biotin-BSA conjugate are respectively coated on a nitrocellulose membrane as a detection line and a quality control line; colloidal gold labeled fusion expression protein and colloidal gold labeled streptavidin and other reagents are matched; and an immunochromatography capture method principle is used for qualitative detection of Zika virus specific IgM antibody and IgG antibody in human serum, thereby realizing quick and specific diagnosis of Zika virus infection.
Owner:GUANGZHOU DARUI BIOTECH
Mycoplasma pneumoniae IgM antibody colloid gold method detecting kit and preparation method thereof
InactiveCN102305856AObvious detectabilityReduce the effectMaterial analysisPaper tapeMurine antibody
The invention relates to a mycoplasma pneumoniae IgM antibody colloid gold method detecting kit which comprises a nitrocellulose membrane detecting line coated recombinant antigen MP-Ag, a quality control line coated goat anti-mouse IgG antibody and a gold labeled pad coated mouse anti-human IgM monoclonal antibody which is marked by colloidal gold, wherein the recombinant mycoplasma pneumoniae antigen is colorless transparent liquid, has the concentration of larger than 2mg / ml and is detected by SDS-PAG; the goat anti-mouse IgG antibody is colorless transparent liquid and has the concentration of larger than 4mg / ml; the mouse anti-human IgM monoclonal antibody is colorless transparent liquid, has the concentration of larger than 2mg / ml and is detected by SDS-PAGE; and the sample loading amount uses two strips under the condition of 10 microliters. The mycoplasma pneumoniae IgM antibody colloid gold rapid detecting test paper tape uses multiepitope recombinant antigen as a raw materials, has the characteristics of simpler operation, low cost, high specificity and high sensitivity, can carry out single detection, is easy for popularization and has obvious detecting and control effect on the mycoplasma pneumoniae IgM antibody.
Owner:北京中检安泰诊断科技有限公司
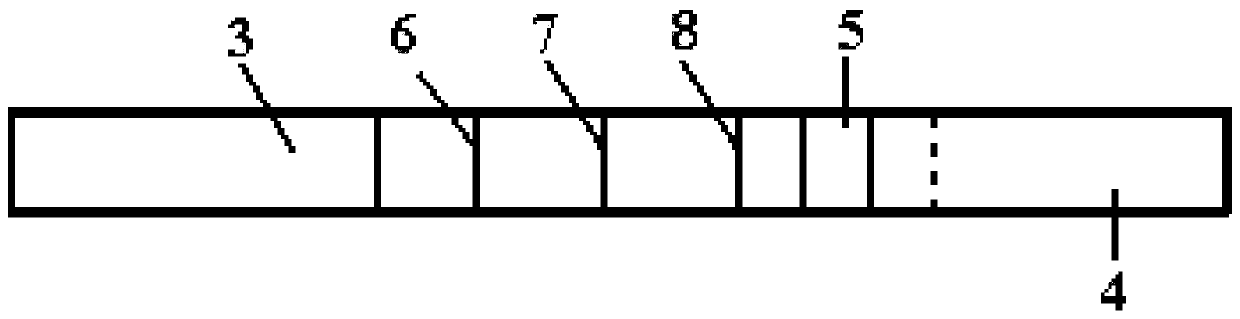
Porcine pseudorabies virus gE IgM antibody colloidal gold immunochromatograohic assay test strip as well as preparation method and application thereof
InactiveCN103983781AImprove detection accuracyImprove featuresBiological material analysisProtein.monoclonalAssay
The invention discloses a porcine pseudorabies virus gE IgM antibody colloidal gold immunochromatograohic assay test strip as well as a preparation method and application thereof. The test strip comprises a sample absorption region, a colloidal gold labeled probe region, a solidifying antibody region, a water absorption region, a bottom plate and a clamping shell, wherein the sample absorption region, the colloidal gold labeled probe region, the solidifying antibody region and the water absorption region are sequentially bonded on the bottom plate and are interlapped in the clamping shell; the sample absorption region is coated with purified porcine pseudorabies virus gE protein; the colloidal gold labeled probe region is coated with colloidal gold labeled porcine pseudorabies virus gE protein monoclonal antibody; the solidifying antibody region is sequentially provided with a detection line T coated with mouse anti-pig IgM monoclonal antibody and a control line C coated with goat anti-mouse IgG. The test strip is simple to operate, good in repeatability, high in sensitivity, rapid and intuitive in result, simple in process and low in cost, can be mass-prepared, and is suitable for mass field test and early diagnosis for porcine pseudorabies virus infection in swinery.
Owner:WUHAN CHOPPER BIOLOGY
Chlamydia pneumoniae IgM (immunoglobulin M) colloidal golden method kit and preparation method thereof
InactiveCN102749446AObvious detectabilityReduce the effectMaterial analysisMurine antibodyChlamydiae
The invention discloses a chlamydia pneumoniae IgM (immunoglobulin M) colloidal golden method kit which comprises recombinant chlamydia pneumoniae antigens enveloped on a nitrocellulose membrane detection line, goat anti-rat IgG (immunoglobulin G) antibodies enveloped on a quality control line and rat anti-human IgM monoclonal antibodies which have colloidal gold labels and are enveloped on a gold label pad, the concentration of the chlamydia pneumoniae antigens ranges from 1mg / ml to 2mg / ml and is measured by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), the concentration of the goat anti-rat IgG (immunoglobulin G) antibodies ranges from 1mg / ml to 3mg / ml, and the concentration of the rat anti-human IgM monoclonal antibodies ranges from 5g / mL to 30g / mL and is measured by the SDS-PAGE. The chlamydia pneumoniae IgM colloidal golden method kit has the advantages that the kid is speedy, simple, convenient and accurate and is high in sensitivity, and a judgment result can be read after integral operation time of dozens of minutes; and a colloidal gold quick detecting paper strip is made of the multi-epitope recombinant antigens and is simple and convenient to operate, low in cost, good in specificity and high in sensitivity, can be used for single-component detection and is popularized easily, and detection and control effects for chlamydia pneumoniae IgM are obvious.
Owner:北京中检安泰诊断科技有限公司
Detection method of hemorrhagic fever with renal syndrome IgM antibodies and reagent kit
ActiveCN103033616AReduce the impact of stabilityExtended shelf lifeMaterial analysisSerum igeIgm antibody
The invention relates to the technical field of biological detection, in particular to a detection method of hemorrhagic fever with renal syndrome IgM (immunoglobulin M) antibodies and a reagent kit. The detection method comprises the steps that hemorrhagic fever with renal syndrome antigens are fixed on a membrane; to-be-detected serum is added; the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum are combined with the antigens fixed on the membrane; gold labeled operating fluid containing mouse anti-human IgM monoclonal antibodies is added; the mouse anti-human IgM monoclonal antibodies are combinec with the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum; a red color is shown; scrubbing liquid is added finally; and the redundant gold labeled operating fluid and other impurities are washed out. The reagent kit has the characteristics of longer quality guarantee period, less cross reaction, better color rendering performance and the like.
Owner:山东康华生物医疗科技股份有限公司
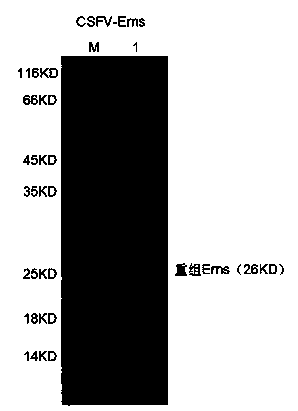
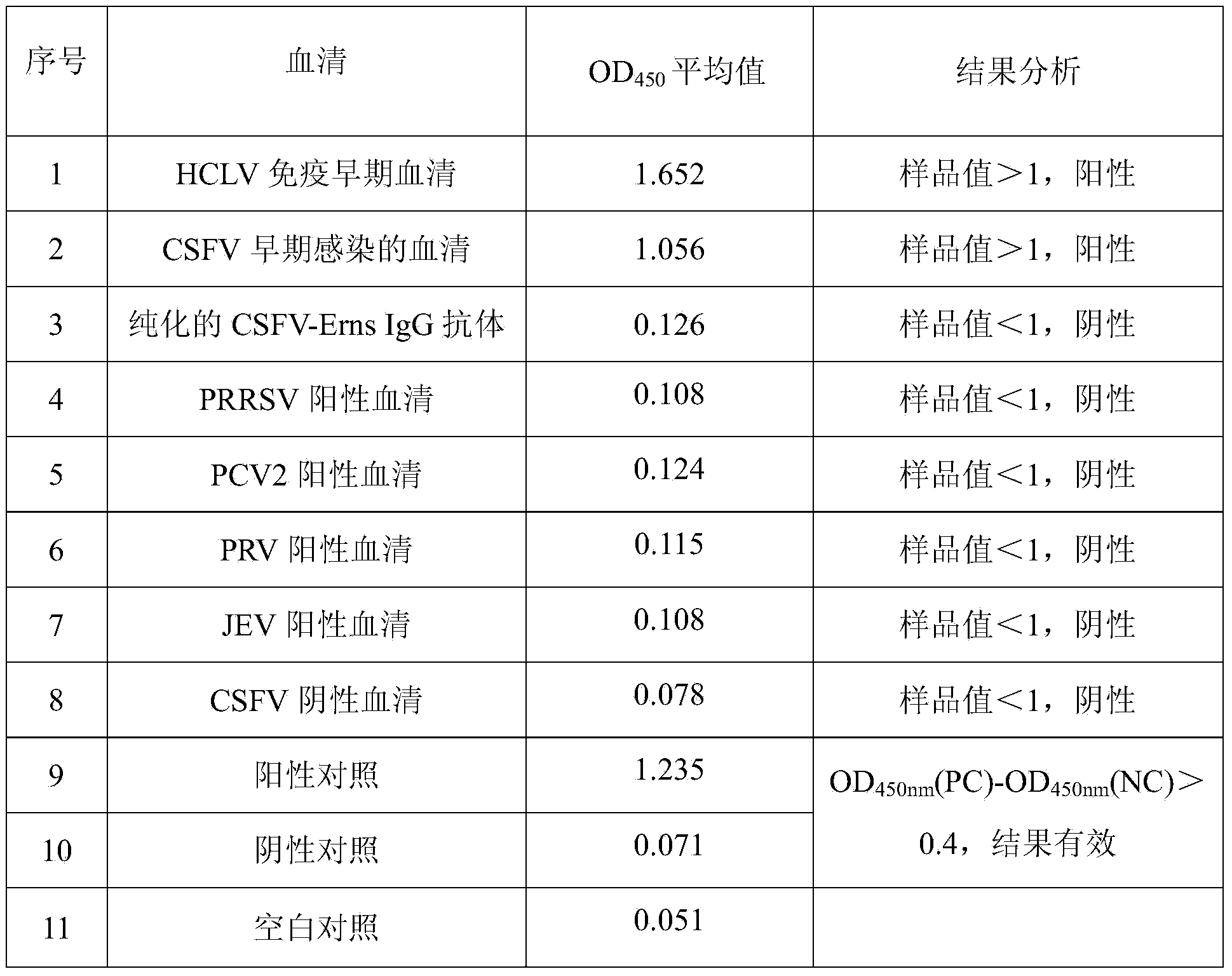
ELISA kit for detecting hog cholera virus Erns IgM antibody
InactiveCN103983782AHigh sensitivityImprove featuresMaterial analysisChromogenic SubstratesElisa kit
The invention discloses an ELISA kit for detecting a hog cholera virus Erns IgM antibody, and belongs to the fields of a biotechnology and diagnosis research of an animal-borne disease. The ELISA kit comprises an elisa plate of enveloping an anti-swine IgM monoclonal antibody, a sample diluent, a positive control and a negative control, a detection antigen (hog cholera virus Erns protein), washing concentrate, an enzyme conjugate, an enzyme chromogenic substrate and a stopping solution. The IgM antibody is detected by adopting a capture method, the specific hog cholera virus Erns IgM antibody is detected by adopting a sandwich method, the specificity of the prepared kit can be up to 100%, the sensitivity is 1:800, and the ELISA kit can be applied to diagnosis of swine fever virus infection and assessment of immune efficacy of a hog cholera vaccine.
Owner:WUHAN CHOPPER BIOLOGY
Preparation method of respiratory syncytial virus antigen and rapid respiratory syncytial virus antibody detection kit prepared from antigen
InactiveCN105859843AImprove featuresIncreased sensitivitySsRNA viruses negative-senseVirus peptidesEscherichia coliInclusion bodies
The invention relates to a respiratory syncytial virus antigen for diagnosing respiratory syncytial virus infection, a method for preparing the antigen, a method and a reagent for detecting respiratory syncytial virus infection by using the antigen, and belongs to the field of clinical medical detection. The artificially synthesized optimized respiratory syncytial virus F1 gene was constructed as a prokaryotic expression vector, and Escherichia coli was used to express the respiratory syncytial virus F1 antigen, and inclusion bodies with three-dimensional structure and immunity were obtained by renaturation by dialysis, gradient dilution and gel chromatography. Active recombinant respiratory syncytial virus F1 protein antigen. The present invention applies colloidal gold immunochromatography technology, uses engineered expressed respiratory syncytial virus F1 antigen as detection antigen, uses high-efficiency anti-human IgG or IgM monoclonal antibody as capture antibody, and filters red blood cells with blood filter membrane, according to "indirect method" The principle is to prepare a rapid detection kit for respiratory syncytial virus antibody, which is used to detect respiratory syncytial virus antibody in human serum, plasma or whole blood. It has the advantages of simple detection method, accurate and rapid result display, and no need for special equipment.
Owner:LANZHOU YAHUA BIOTECH
Test paper strip for rapidly detecting brucellosis IgM antibody colloidal gold
InactiveCN101363862ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisBrucella IgMSpecific igm
The invention provides a test strip for rapid detection of a Brucella IgM antibody, which comprises a reaction film and a conjugate release pad. The reaction film has a detection band simultaneously coated with Brucella specific antigen bp26, and a quality control band coated with a double-antibody. The conjugate release pad is coated with colloidal golden labeled anti-human IgM monoclonal antibody. A membrane chromatography indirect sandwich method is adopted to detect the Brucella specific IgM antibody in a specimen. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and early diagnosis, and has auxiliary effect on the diagnosis of Brucella infection.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
ELISA (enzyme-linked immuno sorbent assay) detection kit for porcine parvovirus IgM (immunoglobulin m) antibodies as well as preparation method and application of ELISA detection kit
InactiveCN103364552AOvercoming defects such as low sensitivityHigh sensitivityMaterial analysisAntigenHorseradish peroxidase
The invention relates to an ELISA (enzyme-linked immuno sorbent assay) detection kit for porcine parvovirus IgM (immunoglobulin m) antibodies as well as a preparation method and application of the ELISA detection kit. The ELISA detection kit for the porcine parvovirus IgM antibodies comprises an ELISA plate coating a porcine IgM monoclonal antibody, sealing fluid, a sample diluent, a detection antigen, an enzyme conjugate, a concentrated cleaning solution, a zymolyte solution A, a zymolyte solution B and a stop solution, wherein the detection antigen is a purified porcine parvovirus, and the enzyme conjugate is a horse-radish peroxide enzyme-anti-PPV (porcine parvovirus) antibody enzyme conjugate. The specificity of the ELISA detection kit provided by the invention reaches 100% and the sensitivity of the ELISA detection kit reaches up to 1:800, so that the ELISA detection kit can be used for performing early diagnosis on PPV infection of a swinery.
Owner:WUHAN CHOPPER BIOLOGY
A colloidal gold method detection kit for hepatitis A virus igm antibody and preparation method thereof
InactiveCN102288758AReduce the effectHigh sensitivityMaterial analysisAgainst vector-borne diseasesHepatitis APolyacrylamide gel electrophoresis
The invention discloses a colloidal gold detection kit for hepatitis A virus IgM antibody, comprising a recombinant antigen HAV-Ag coated on a nitrocellulose membrane detection line, a goat anti-mouse IgG antibody and a gold label coated on a quality control line The mouse anti-human IgM monoclonal antibody labeled with colloidal gold coated on the pad, the recombinant hepatitis A virus antigen concentration was greater than 2mg / ml, and was determined by SDS-PAGE; the goat anti-mouse IgG antibody concentration was greater than 4mg / ml; The concentration of the mouse anti-human IgM monoclonal antibody was greater than 2 mg / ml, which was determined by SDS-PAGE, and the loading amount was two bands under the condition of 10 μl. The beneficial effects of the invention are as follows: the hepatitis A virus IgM antibody detection kit has the characteristics of rapidity, simplicity, accuracy and high sensitivity, and the whole operation time only takes 20 minutes to interpret the results. The colloidal gold rapid detection test strip is based on the multi-epitope recombinant antigen as the raw material. It has the characteristics of easier operation, low cost, good specificity and high sensitivity. It can be detected in a single batch and is easy to popularize. The detection and control effect is obvious.
Owner:北京中检安泰诊断科技有限公司
Detection paper for simultaneously and quantitatively detecting IgG and IgM contents of novel coronavirus and method
PendingCN111458505ASolving Unquantifiable ProblemsHigh sensitivityFluorescence/phosphorescenceMicrosphereIgG.monoclonal
The invention discloses detection paper for simultaneously detecting IgG and IgM of the novel coronavirus and a method. A nitrocellulose membrane in the detection paper is provided with a first detection strip sprayed with an anti-human IgM monoclonal antibody, a second detection strip sprayed with an anti-human IgG monoclonal antibody and a quality control strip sprayed with a monoclonal antibodyof the specific protein of anti-novel coronavirus 2019-nCoV, wherein the first detection strip, the second detection strip and the quality control strip are distributed at intervals; a conjugate padis sprayed with rare earth ion nanosphere labeled specific protein of novel coronavirus 2019-nCoV. The test strip can detect the IgG and IgM contents of novel coronavirus 2019-nCoV at the same time, which provides convenience for the clinical diagnosis of novel coronavirus 2019-nCoV.
Owner:浙江理工大学绍兴生物医药研究院有限公司 +1
ABO-CDE blood type grouping reagent card and preparation method thereof
InactiveCN102331504AEnsure stabilityImprove buffering effectBiological testingE AntibodyStandardization
The invention discloses an AB / CDE blood type grouping reagent card. The reagent card is provided with eight microcolumn gel tubes, namely a gel tube containing an immune globulin (IgM) monoclonal anti-A antibody, a gel tube containing an IgM monoclonal anti-B antibody, a gel tube containing an IgM monoclonal anti-D antibody, a gel tube containing an IgM monoclonal anti-C antibody, a gel tube containing an IgM monoclonal anti-E antibody, a gel tube which is used for negative control and contains a gel buffer solution and two gel tubes which are used for reverse typing and contain gel buffer solutions. The invention provides standard for the ABO-CDE blood type grouping; and the ABO-CDE blood type grouping reagent card which conforms to standard can be used in all hospitals and blood transfusion services to accurately group ABO-CDE blood types, so conditions are created for guaranteeing transfusion safety.
Owner:JIANGSU ZHONGSHENG MEDICAL DIAGNOSTIC REAGENT
Test strip for detecting enterovirus 71 (EV71) IgM antibody
ActiveCN102253210ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisNitrocelluloseVASCULAR ADHESION PROTEIN 1
The invention provides a rapid test strip for detecting an enterovirus 71 (EV71) IgM antibody. In the invention, EV71 gene antigenic vascular adhesion protein-1 (VAP1) and anti-mouse IgG are coated on a nitrocellulose (NC) membrane in combination with a colloidal gold labeled anti-human IgM monoclonal antibody, and the specific EV71 IgM antibody in an infected human specimen is detected by utilizing a membrane chromatography capture method. The rapid test strip provided by the invention has the advantages of being simple, convenient and rapid in operation, having no need of special instrumentand equipment and professional training, ensuring clear and easily-distinguished results and being easy for popularization; and the rapid test strip is applicable to a basic level as well as field test and early diagnosis, and plays a role in assisting the EV71 infection diagnosis.
Owner:辽宁迪浩生物科技有限公司
Coxsackie virus type a16 (ca16) igm antibody detection test strip
ActiveCN102262156ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCoxsackievirus a16Nitrocellulose
The invention provides a detection test strip for a coxsackie virus A16 type (CA16) IgM (Immune Globulin M) antibody. According to the detection test strip, a nitrocellulose membrane (NC membrane) is coated by a CA16 gene antigen (VP1) and an anti-mouse IgG; and by combining a tihuman IgM monoclonal antibody labeled by colloidal gold, a CA16 specificity IgM antibody in an infected human speciment is detected by applying a membrane chromatography capture method. The test strip for detection, which is disclosed by the invention, has the advantages of simpleness, convenience, quickness and fastness in operation, no need of special instrument or special training, distinct and easily-distinguished result and easiness for popularization; and the detection test strip is suitable for field detection and early-stage diagnosis and assisting function for CA16 infected diagnosis.
Owner:辽宁迪浩生物科技有限公司
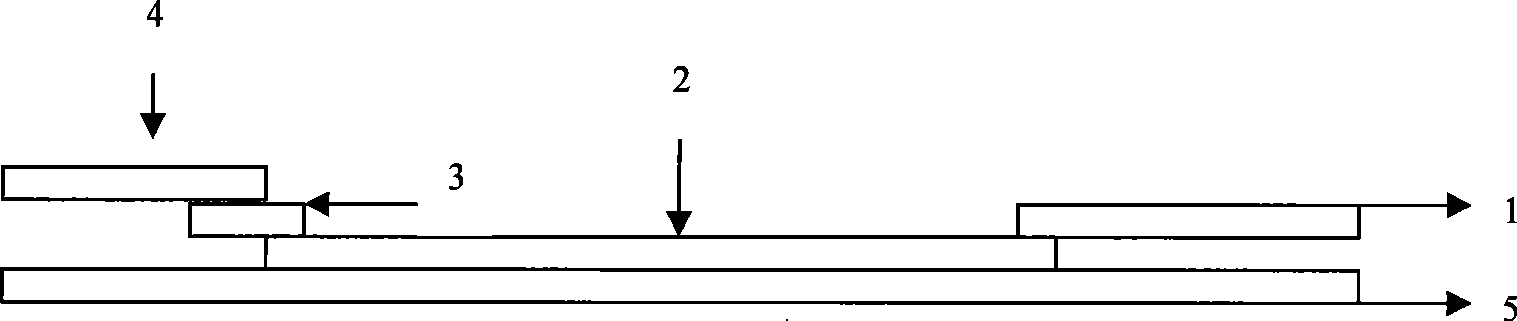
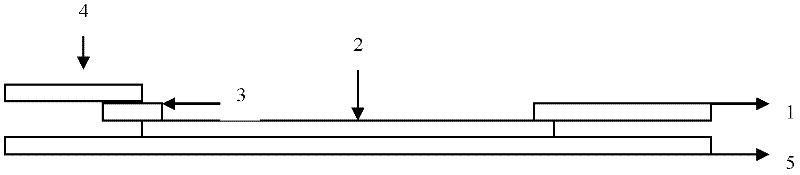
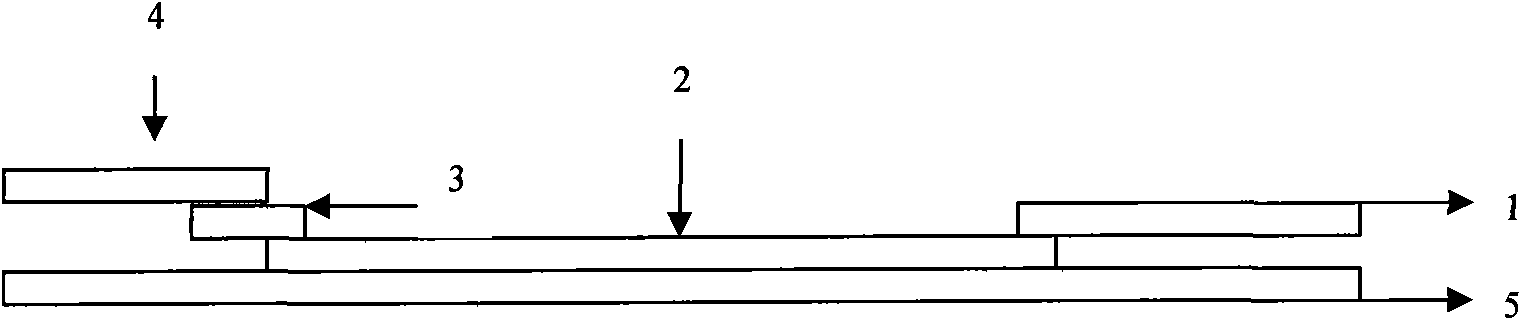
EB virus capsid antigen IgM antibody colloidal gold method detection reagent and preparation method thereof
The present invention discloses an EB virus capsid antigen IgM antibody colloidal gold method detection reagent and a preparation method thereof. The reagent comprises a gold conjugate pad (3), a nitrocellulose reaction membrane (4), a sample pad (2), a rheumatoid factor treatment pad (1), water absorption paper (5) and a PVC backing (8), wherein a length of the rheumatoid factor treatment pad is approximately 1 / 2 of a length of the sample pad, the sample pad, the gold conjugate pad, the nitrocellulose reaction membrane and the water absorption paper are sequentially and mutually laminated and adhered on the PVC backing, the gold conjugate pad is coated with an anti-human IgM monoclonal antibody-colloidal gold conjugate, and positions of a quality control area (7) and a detection area (6) of the nitrocellulose reaction membrane are respectively coated with goat anti-mouse IgG antibody or rabbit anti-mouse IgG antibody and specific gene recombinant EB virus capsid antigen. According to the present invention, a production process is simple and easy to control, test results can be obtained within 25 minutes, self-detection can be achieved according to operations in a manufacturer's instruction, test results are not affected by the rheumatoid factor, sensitivity is high, specificity is good, and results are accurate and reliable.
Owner:BEIJING BIONEOVAN
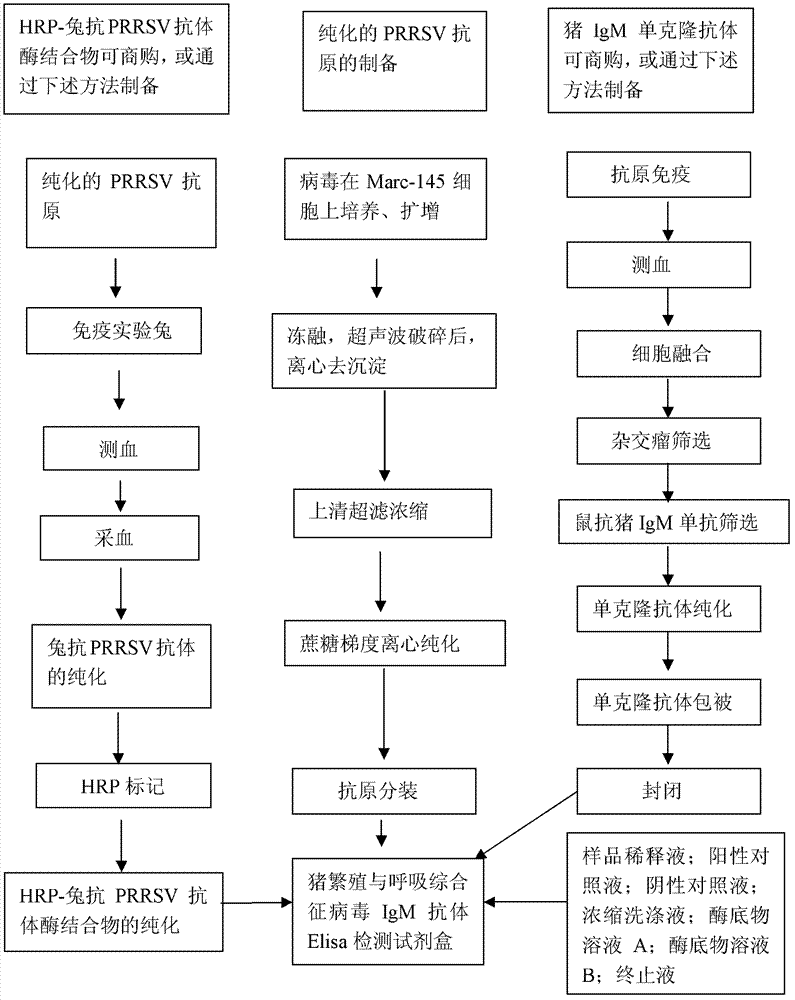
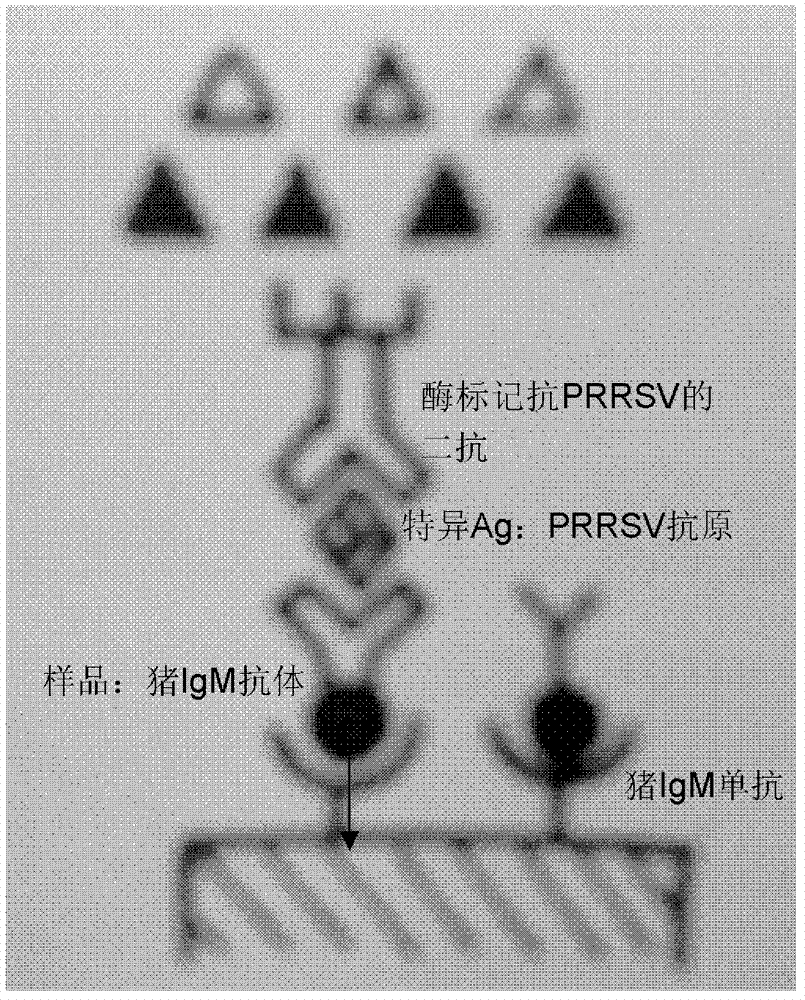
ELISA (enzyme-linked immuno sorbent assay) detection kit for porcine reproductive and respiratory syndrome virus IgM (immunoglobulin m) antibodies as well as preparation method and application of ELISA detection kit
InactiveCN103364551AOvercoming defects such as low sensitivityHigh sensitivityRecovery/purificationMaterial analysisAntigenSorbent
The invention relates to an ELISA (enzyme-linked immuno sorbent assay) detection kit for porcine reproductive and respiratory syndrome virus IgM (immunoglobulin m) antibodies as well as a preparation method and application of the ELISA detection kit. The ELISA detection kit for the porcine reproductive and respiratory syndrome virus IgM antibodies comprises an ELISA plate coating a porcine IgM monoclonal antibody, sealing fluid, a sample diluent, a detection antigen, an enzyme conjugate, a concentrated cleaning solution, a zymolyte solution A, a zymolyte solution B and a stop solution, wherein the detection antigen is a purified porcine reproductive and respiratory syndrome virus, and the enzyme conjugate is a horse-radish peroxide enzyme-anti-PRRSV (porcine reproductive and respiratory syndrome virus) antibody enzyme conjugate. The specificity of the ELISA detection kit provided by the invention reaches 100% and the sensitivity of the ELISA detection kit reaches up to 1:800, so that the ELISA detection kit can be used for performing early diagnosis on PRRSV infection of a swinery.
Owner:WUHAN CHOPPER BIOLOGY
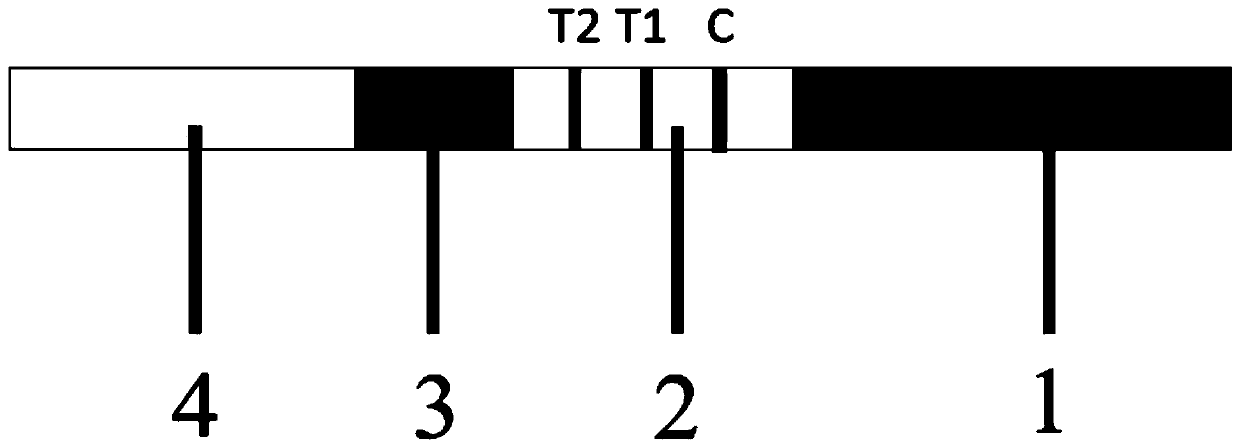
Novel coronavirus IgG/IgM antibody detection kit and detection method thereof
PendingCN111505276AEasy to detectReduce the risk of missed detectionMaterial analysisNitrocelluloseCoronavirus antibody
The invention provides a novel coronavirus IgG / IgM antibody detection kit and a detection method thereof, which relate to the technical field of novel coronavirus detection. The novel coronavirus IgG / IgM antibody detection kit comprises a sample pad, a colloidal gold pad coated with a colloidal gold labeled novel coronavirus S antigen, a nitrocellulose membrane coated with a detection strip T1 containing an anti-human IgG monoclonal antibody, a detection strip T2 containing an IgM monoclonal antibody, a quality control strip C containing a goat anti-mouse IgG antibody, and water absorption filter paper which are sequentially adhered to a reaction support. The kit can simplify the detection process of the novel coronavirus, covers the front, middle and later periods of the disease course, and reduces the risk of missing detection; dependence on any experimental instrument, environment or operator is avoided, the operation is simple and convenient, the detection period is short, and theinterpretation is easy; in addition, special instruments and equipment are not needed, professional training is not needed, adaptability is high, and monitoring and inspection at any time and any place are facilitated.
Owner:SHANGHAI JIAO TONG UNIV +1
AB/Rh blood type grouping reagent card and preparation method thereof
InactiveCN102331505AImprove buffering effectSuitable buffer rangeBiological testingGroup A - bloodBuffer solution
The invention discloses an AB / Rh blood type grouping reagent card and a preparation method thereof. The reagent card is provided with at least one group of microcolumn gel tubes; each group of microcolumn gel tubes comprise four microcolumn gel tubes, namely a gel tube containing an immune globulin (IgM) monoclonal A-resistant antibody, a gel tube containing an IgM monoclonal B-resistant antibody, a gel tube containing an IgM monoclonal D-resistant antibody and a gel tube which is used for negative control and contains a gel buffer solution. The invention provides standard for the ABO / Rh(D) blood type grouping; and the ABO / Rh(D) blood type grouping reagent card which conforms to standard can be used in all hospitals and blood transfusion services to accurately group ABO / Rh(D) blood types, so conditions are created for guaranteeing transfusion safety.
Owner:JIANGSU ZHONGSHENG MEDICAL DIAGNOSTIC REAGENT
Immunochromatography detection kit for simple herpes virus II type IgM antibody
The invention belongs to the field of biological medicines, and particularly relates to an immunochromatography detection kit for a simple herpes virus II type IgM antibody. The kit is formed by assembling a reagent strip and a plastic card, wherein the reagent strip comprises a base plate; a reaction membrane with a detection line and a quality control line is adhered to the base plate; a colloidal gold pad and a sample pad are stacked and adhered to one end, which is close to the detection line, of the reaction membrane in sequence; an absorption pad is stacked and adhered to one end, which is close to the quality control line, of the reaction membrane; the detection line is coated with a simple herpes virus II type antigen; the quality control line is coated with a goat anti-mouse IgG antibody; and the colloidal gold pad is coated with a colloidal gold labeled mouse anti-human IgM monoclonal antibody. The kit disclosed by the invention can obviously reduce the influence of environmental factors (such as low temperature and high temperature) on a detection process and a detection result, and guarantee the sensitivity, the accuracy and the stability of the detection result.
Owner:武汉融颐生物科技有限公司
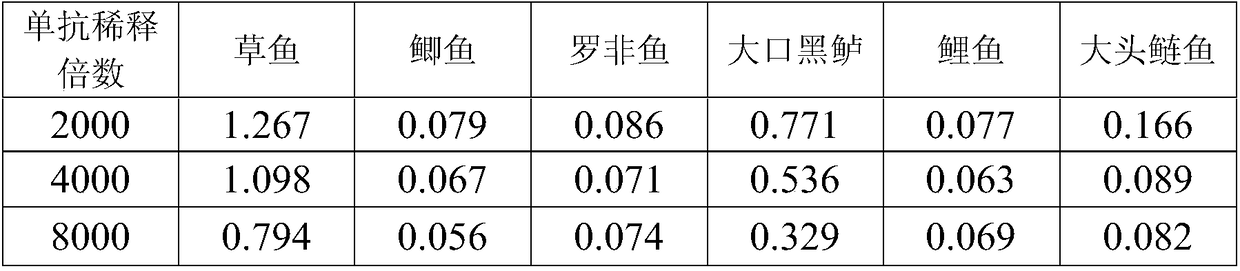
Grass carp IgM monoclonal antibody, and preparation method and applications thereof
The invention discloses a grass carp IgM monoclonal antibody, and a preparation method thereof and applications thereof. The grass carp IgM monoclonal antibody is produced by a hybridoma cell strain having a preservation number of C2017130; and the applications of the grass carp IgM monoclonal antibody comprise an application of the grass carp IgM monoclonal antibody in the preparation of a reagent or a kit for detecting the Ig level of fish, an application in the preparation of a drug for treating viral infections in the fish, an application in the preparation of a reagent for evaluating immune effects of fish vaccines, and an application in the evaluation of the immune function of the fish. The invention also provides the hybridoma cell strain. The grass carp IgM monoclonal antibody canimprove the sensitivity of the serum antibody detection process, and has a good application prospect.
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST
Leptospira IgM antibody quick detection test strip
ActiveCN101592665ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisIgm antibodyColloidal gold
The invention provides a quick detection test strip for detecting leptospira IgM antibody, comprising a reaction film and a combination releasing pad; wherein the reaction film is provided with a detection zone capable of wrapping leptospira specific antigen ompL1 and LipL32 and a quality control zone encasing secondary antibody IgG, and the combination releasing pad is encased by anti-human IgM monoclonal antibody marked by colloidal gold. Leptospira specific IgM antibody in a specimen is detected by applying membrane chromatography capturing method. The test strip of the invention is applied to detection and is easy, convenient, fast and short-cut to operate, special instrument or equipment or professional training are not needed, the result is clear and easy to recognize, and the operation is easy, thus being easy to popularize, being applicable to basic level, field detection and early diagnosis, and being beneficial to leptospira infection diagnosis.
Owner:辽宁迪浩生物科技有限公司
Test paper strip for detecting encephalitis virus IgM antibody colloidal gold, method for making same and applications
ActiveCN101363866ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCelluloseIgm antibody
The invention provides a test strip for rapid detection of Japanese encephalitis virus IgM antibody. A Japanese encephalitis virus E gene antigen domain III and a double-antibody IgM III coat a nitrate cellulose film (NC film), and a membrane chromatography indirect sandwich method is adopted to detect the Japanese encephalitis virus specific IgM antibody in an infected human body specimen in combination with a colloidal gold labeled antihuman IgM monoclonal antibody. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and epidemiological investigation, and has auxiliary effect on the diagnosis of Japanese encephalitis virus infection.
Owner:辽宁迪浩生物科技有限公司
Dengue fever NS1 antigen and IgG/IgM antibody two-joint inspection test strip, detection card and kit
ActiveCN112362870AShow immune response statusEasy to operateBiological testingImmunoassaysNitrocelluloseIgm antibody
The invention relates to a dengue fever NS1 antigen and IgG / IgM antibody two-joint inspection test strip, which comprises a dengue fever NS1 antigen test strip and a dengue fever IgG / IgM antibody teststrip, and is characterized in that the dengue fever NS1 antigen test strip comprises a first gold labeled pad layer and a first nitrocellulose membrane, and the first gold labeled pad layer is immobilized with a dengue fever NS1 monoclonal antibody colloidal gold compound; and the first nitrocellulose membrane is coated with detection lines of four serotypes of dengue fever NS1 monoclonal antibodies, the dengue fever IgG / IgM antibody test strip comprises a second gold labeled pad layer and a second nitrocellulose membrane, and the second gold labeled pad layer is immobilized with four serotypes of dengue fever recombinant antigen colloidal gold compounds. The second nitrocellulose membrane is coated with a detection line T1 of a mouse anti-human IgG monoclonal antibody and a detection line T2 of a mouse anti-human IgM monoclonal antibody; and according to the test strip, IgM / IgG antibodies and NS1 antigens can be jointly detected through one-time operation, the operation process is simplified, the immune response reaction state of an organism is effectively diagnosed, and the purpose of real POCT detection is achieved.
Owner:山东康华生物医疗科技股份有限公司
Porcine circovirus type 2 (PCV2) IgM antibody colloidal gold immunochromatographic assay test paper, and preparation method and application thereof
InactiveCN103969451AOvercome the disadvantage of low sensitivityImprove featuresBiological testingCircovirusProtein.monoclonal
The invention discloses a porcine circovirus type 2 (PCV2) IgM antibody colloidal gold immunochromatographic assay test paper, and a preparation method and application thereof. The test paper comprises a sample absorption region, a colloidal gold labeled probe region, a curing antibody region, a water absorption region, a backboard and a shell, wherein the sample absorption region, colloidal gold labeled probe region, curing antibody region and water absorption region are sequentially attached to the backboard and arranged in the shell in a superposition mode. A purified PCV2 Cap protein is coated on the sample absorption region; a colloidal gold labeled PCV2 Cap protein monoclonal antibody is coated on the colloidal gold labeled probe region; and the curing antibody region is sequentially provided with a detection line T coated mouse anti-pig IgM monoclonal antibody and a control line C coated goat anti-mouse IgG. The test paper is simple to operate, has the advantages of favorable repetitiveness, high sensitivity, quick and visual results, simple technique and low cost, can implement mass preparation, is suitable for mass field detection for basic level, and can be used for early quick diagnosis on swinery PCV infections.
Owner:WUHAN CHOPPER BIOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com