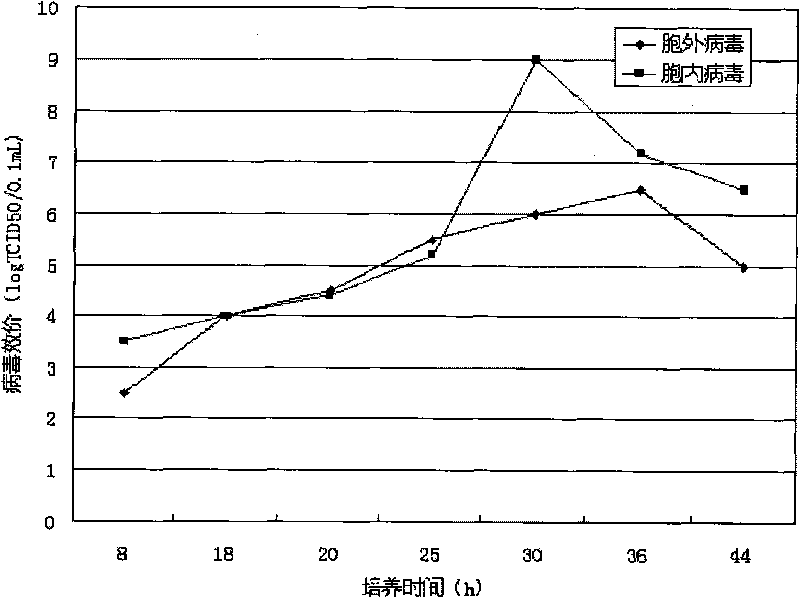
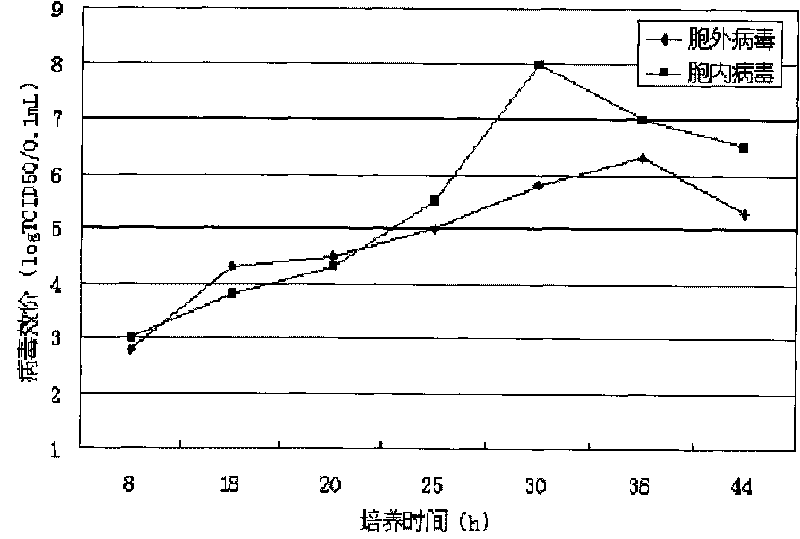
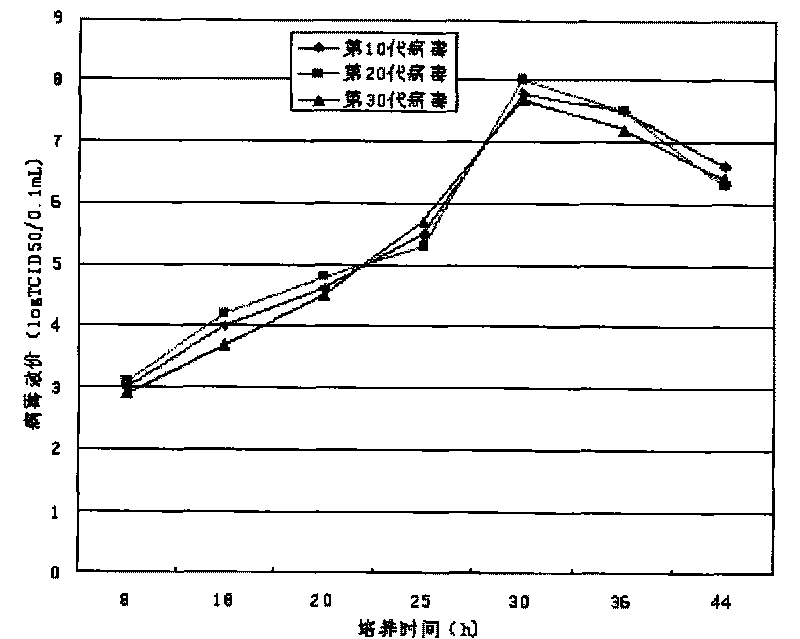
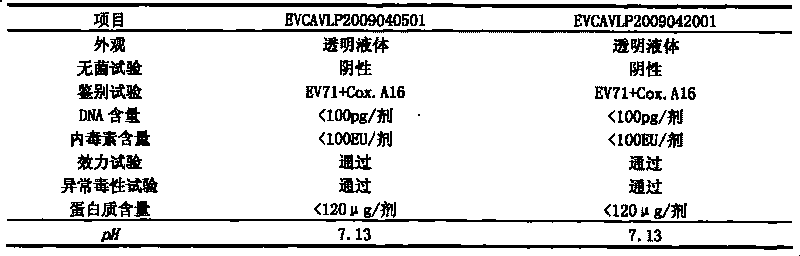
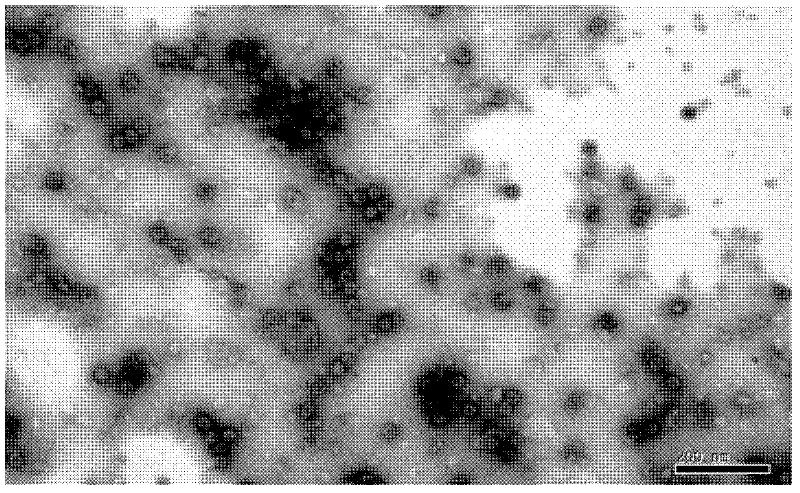
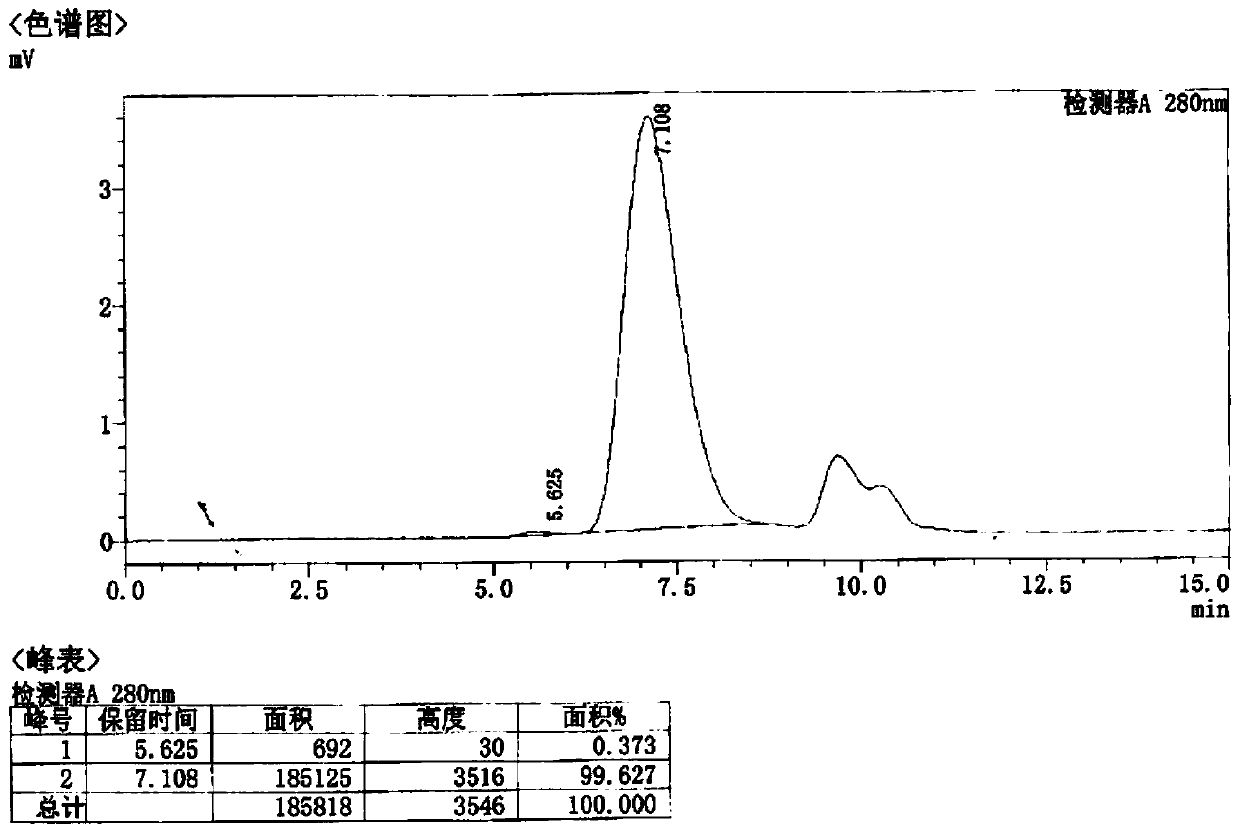
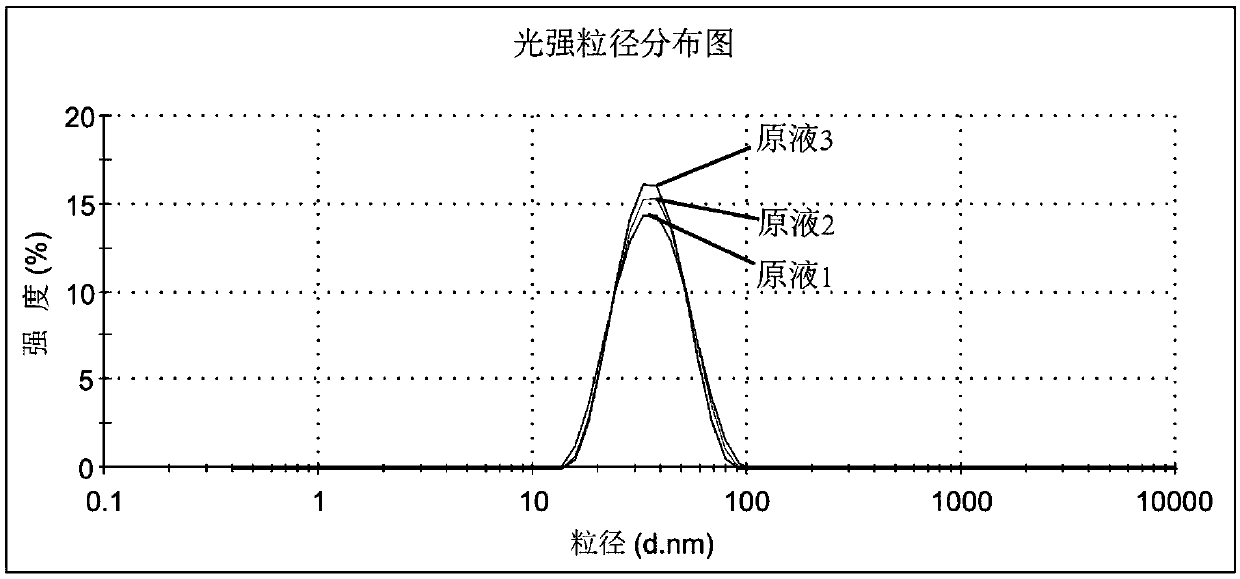
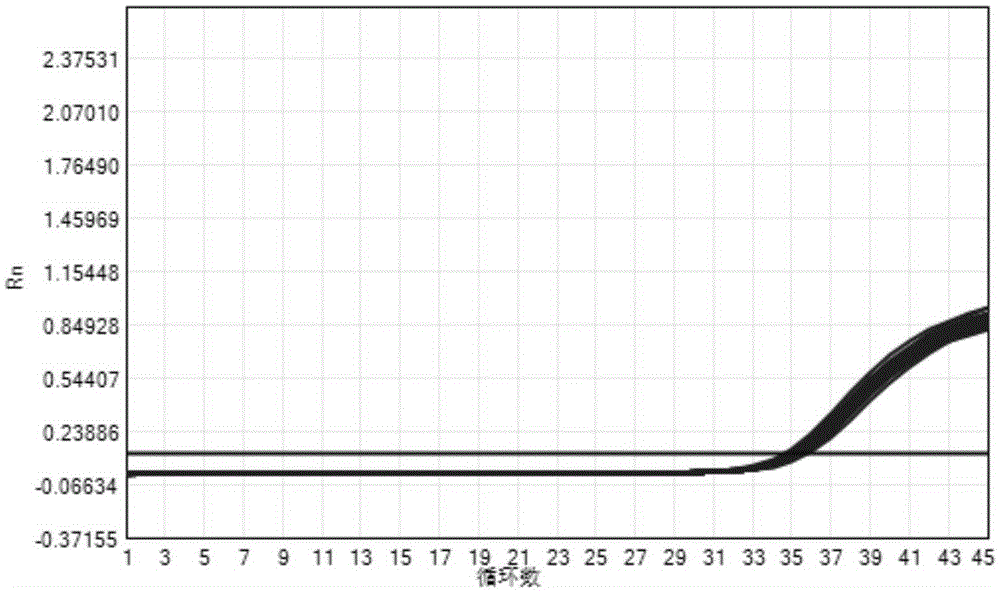
Patents
Literature
74 results about "Coxsackievirus a16" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Univalent and bivalent inactivated vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695570AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
The invention discloses a univalent and bivalent inactivated vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: screening vaccine strains for the hand-foot-and-mouth disease; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; collecting virus suspension; inactivating the virus; ultra-filtering, concentrating and purifying the virus suspension to obtain vaccine stock solution; and finally preparing the univalent and bivalent inactivated vaccine. The univalent and bivalent inactivated vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695569AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
The invention discloses a univalent and bivalent gene engineered subunit vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: respectively obtaining recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD by gene engineering means, respectively efficiently coexpressing similar SeQ ID No.1 EV71 P1 and Se Q ID No.2 Cox.A16 P1 and 3CD proteins in insect cells, and respectively self-assembling into EV71 VLP and Cox.A16 VLP; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; lysing the cells, ultra-filtering and purifying virus suspension; and further preparing the univalent and bivalent vaccine. The vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as kit thereof
InactiveCN101886138AEasy to detectHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Reverse transcription polymerase chain reaction
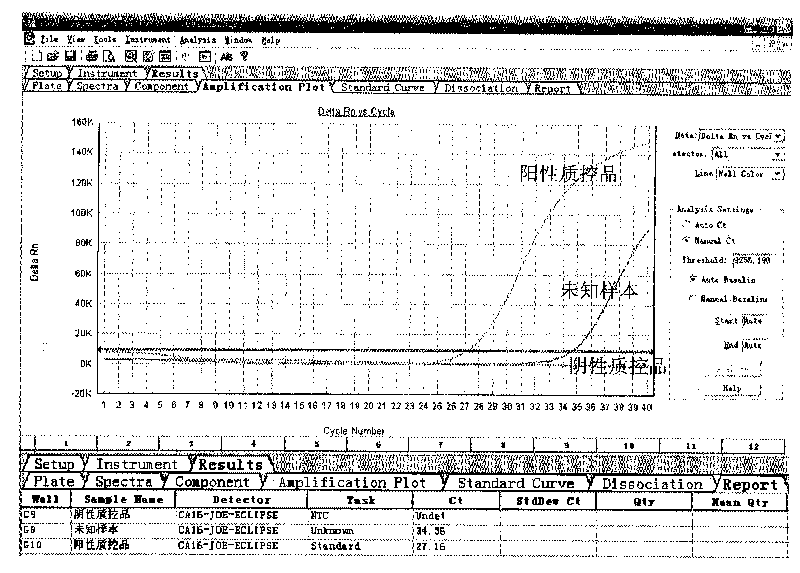
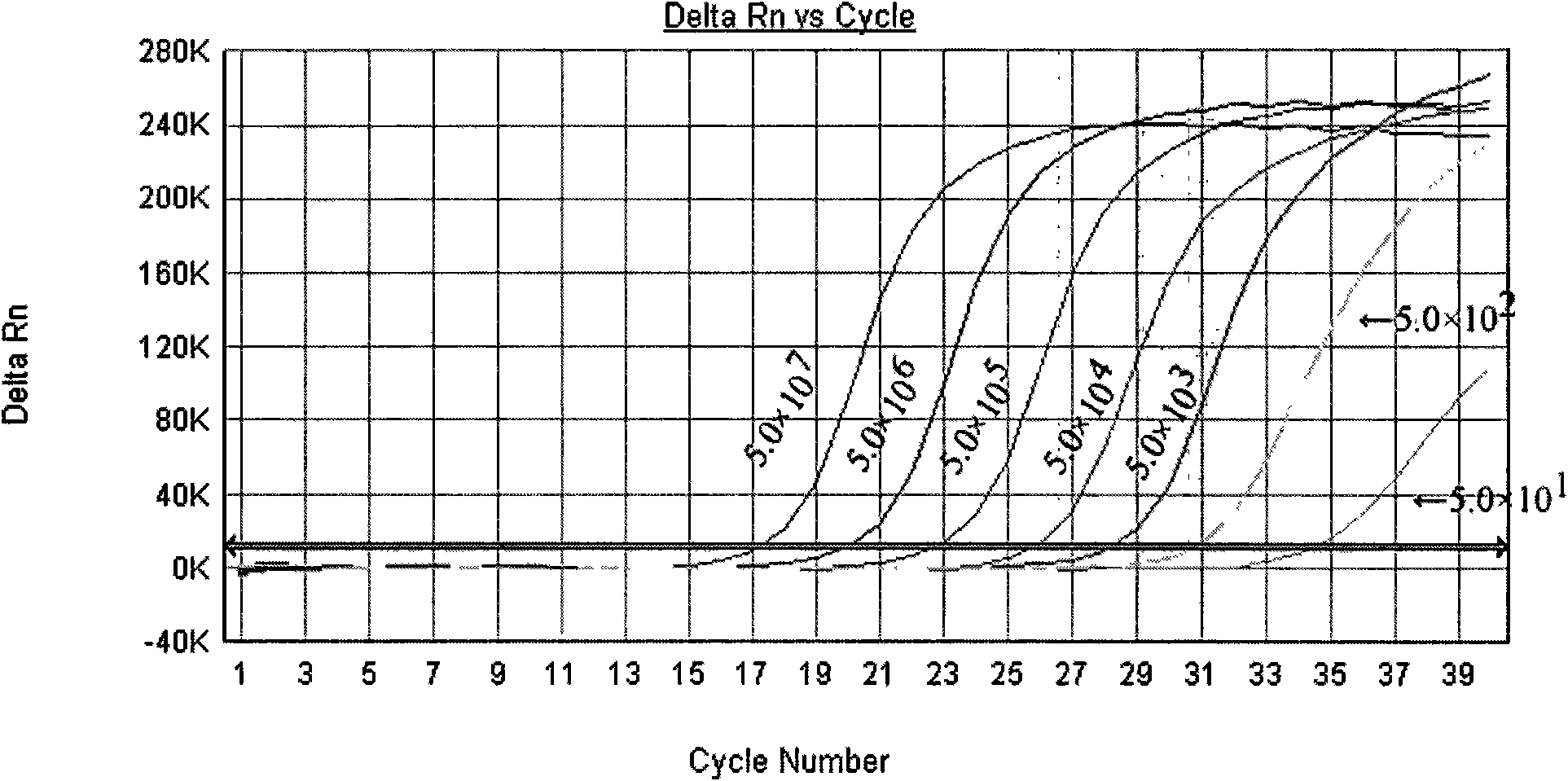
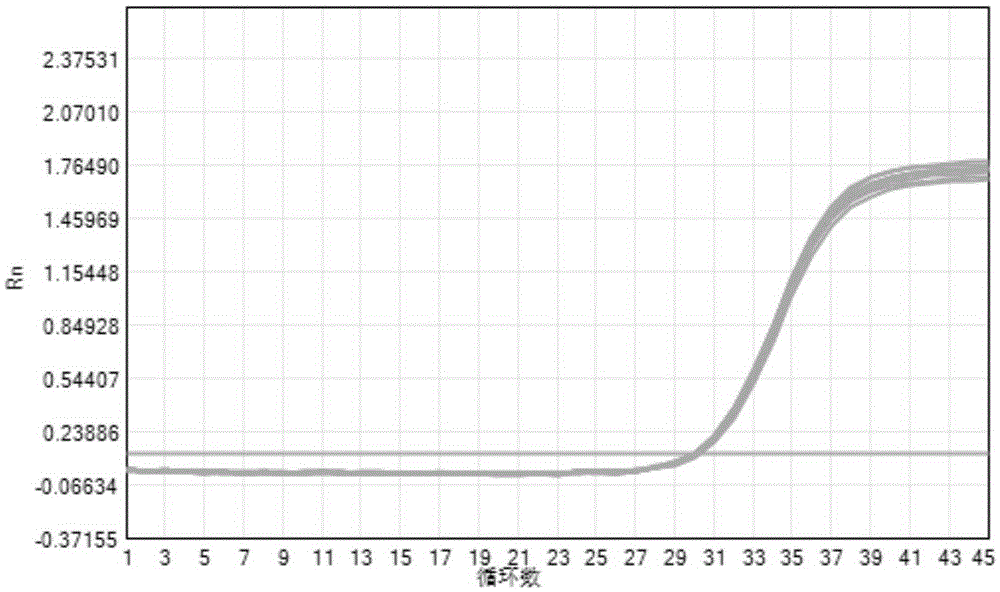
The invention provides a three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as a kit thereof. The method can rapidly and accurately detect the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of enterovirus in a sample. The method comprises the following steps of: (1) acquiring and conveying a sample of an infected patient or a suspected patient; (2) preprocessing the sample and extracting RNA; (3) detecting the sample by adopting a one-step PCR-three-color fluorescent probe in-vitro amplification method; and (4) analyzing the corresponding sample according to the fluorescence intensity of each amplification reaction after the amplification reaction is finished, thereby judging the existence of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus in the acquired sample and being capable of carrying out accurate quantitation (a figure 3) on the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus. The invention realizes the aim of carrying out rapid and accurate combined detection of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus.
Owner:BEIJING SUOAO BIOTECH
Quick test paper for detecting enterovirus and method for preparing same
ActiveCN101650366AFacilitate on-site screening workStrong specificityMaterial analysisCelluloseEnterovirus
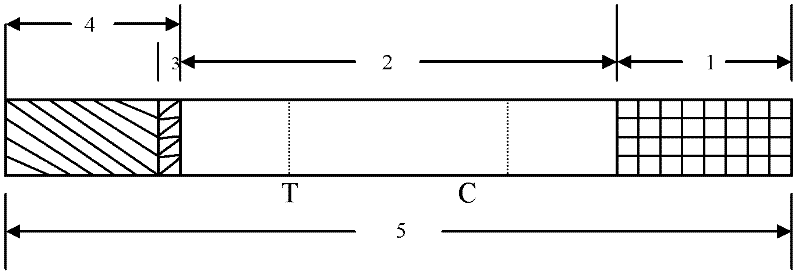
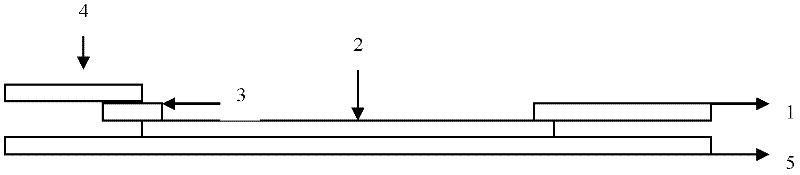
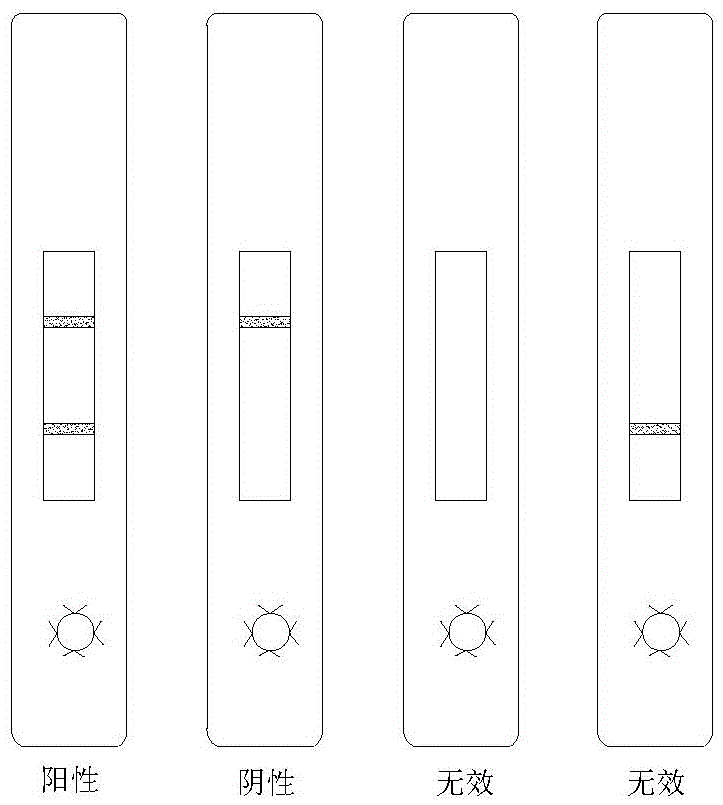
The invention discloses a quick test paper for detecting enterovirus and a method for preparing the same. The test paper detects enterovirus EV71 and Coxsackievirus A16 viruses in a sample by the immunochromatography adopting marking by colorful particles. The quick test strip is formed by sequentially overlapping and bonding a sample adsorption liquid layer, a colorful particle storing pad, a cellulose nitrate membrane and a water adsorption board on a bottom board, wherein the colorful particle storing pad is coated with a monoclonal antibody with a colorful particle mark; and the cellulose nitrate membrane is provided with a detection line sprayed by the monoclonal antibody of the EV71 and / or Coxsackievirus A16 and a control line sprayed by the polyclonal antibody of anti-mouse IgG. The test paper can quickly detects the EV71 and Coxsackievirus A16 viruses in the detected sample at the same time or respectively, finds the epidemic situation caused by the virus infection as early as possible and has the advantages of being easily operated, quickly getting results, avoiding special operators and the like.
Owner:万志静 +1
Coxsackie virus A16 type virus-like particle vaccine
InactiveCN102465144AHigh neutralizing activityAntiviralsViruses/bacteriophagesCoxsackievirus a16Spatial configuration
The invention relates to a coxsackie virus A16 type virus-like particle vaccine. The inventor fortuitously finds that proteins which have better spatial configurations and are suitably cut can be obtained by expressing P1 protein of CVA16 and 3CD protein of CVA16 by infecting insect cells with rhabdovirus. The proteins can be automatically assembled into a virus-like particle which has high immunogenicity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Preparation method for recombinant coxsackie virus A16 like particle and applications thereof
InactiveCN102533797AIncrease productionFungiInactivation/attenuationYeastHand-foot-and-mouth disease
The invention discloses a preparation method for a recombinant coxsackie virus A16 like particle, which comprises the following steps: (1) cloning a P1 gene and a 3CD gene of a coxsackie virus A16 to a target plasmid to obtain a recombinant expression vector; (2) transforming a target yeast cell by using the recombinant expression vector obtained in the step (1) to obtain a recombinant yeast cellfor expressing the P1 gene and the 3CD gene; and (3) cracking the recombinant yeast cell obtained in the step (2), and separating to obtain the recombinant coxsackie virus A16 like particle. The recombinant coxsackie virus A16 like particle can be prepared in a yeast expression system by the method provided by the invention. Compared with a wild-type P1 gene and a wild-type 3CD gene, the yield ofthe coxsackie virus A16 like particle in the yeast expression system is greatly increased through the optimization of codons of the P1 gene and the 3CD gene, and the recombinant coxsackie virus A16 like particle can be further used for producing candidate preventive vaccines and pharmaceutical compositions for infant hand-foot-and-mouth diseases.
Owner:BEIJING UNIV OF TECH
Coxsackie virus A16 virus strain, uses of strain, vaccine and preparation method of vaccine
ActiveCN104099301AEffective immune activityObvious paralysisSerum immunoglobulinsImmunoglobulins against virusesCoxsackievirus a16Antiviral drug
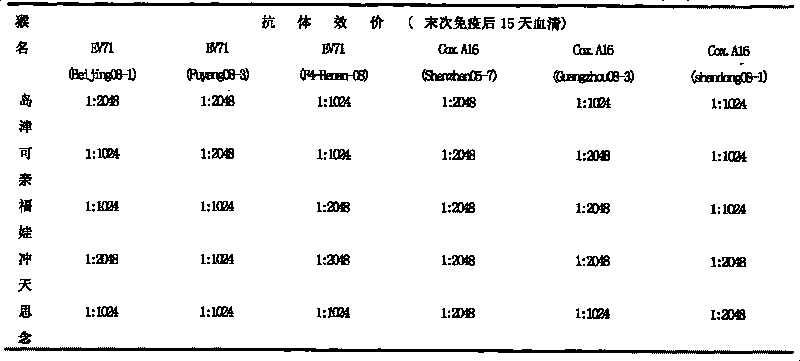
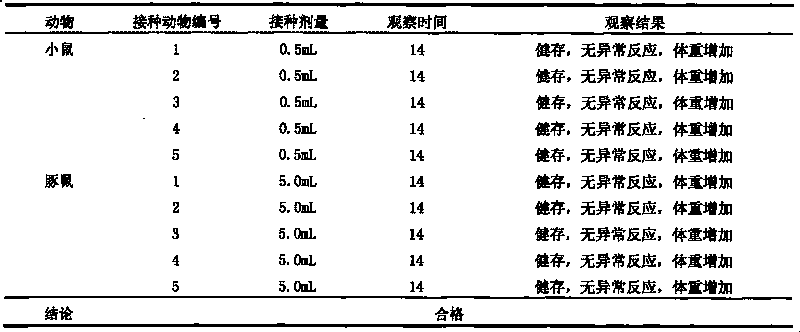
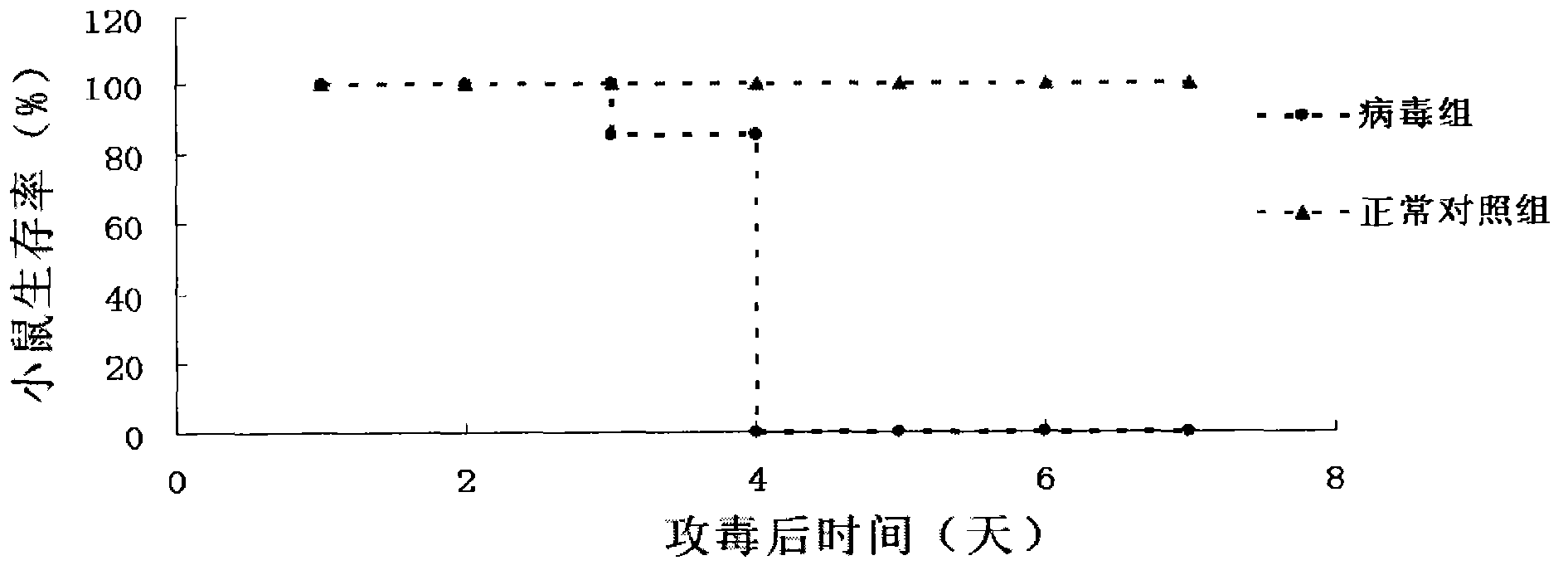
The invention provides a Coxsackie virus A16 virus strain, uses of the strain, a vaccine and a preparation method of the vaccine. The preservation number of the Coxsackie virus A16 virus strain is CGMCC No.6954. The CA16 virus strain has strong virulence, can be used for evaluating the CA16 vaccine, and can also be used for researching the CA16 virus infection mechanism. A method for establishing a Coxsackie virus A16 infected animal model provided by the invention can provide a stable animal model, and provides a foundation for the development of the Coxsackie virus A16 vaccine, the screening of antiviral drugs and the researches of the CA16 virus infection mechanism. The vaccine prepared by using the CA16 virus has effective immune activity.
Coxsackie virus A16-type virus strain and applications thereof
ActiveCN102533671AStable titerImproving immunogenicitySerum immunoglobulinsMicroorganism based processesSequence analysisImmunogenicity
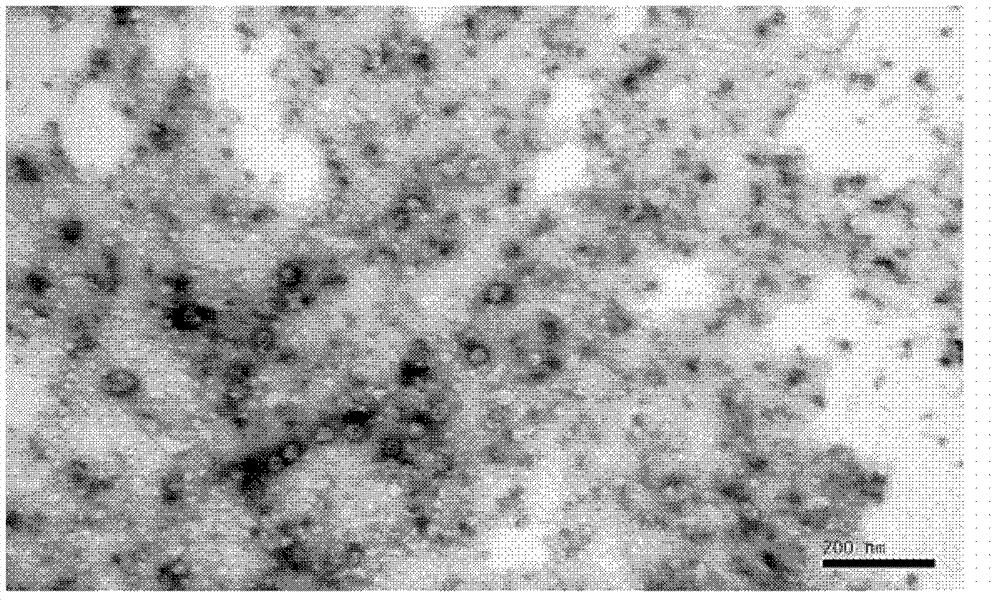
The invention provides a coxsackie virus A16-type virus strain. The collection number of the coxsackie virus A16-type virus strain is CGMCC No.5372, wherein CGMCC refers to China General Microbiological Culture Collection Center. The virus is a 20-face stereoscopic symmetrical sphere under observation through an electron microscope, and the diameter of the virus is 23-30nm. VP1 conserved region sequence analysis and mass spectrum analysis are respectively performed on the virus strain, and a result shows a CA16 virus. The CA16 virus can be efficiently proliferated in Vero cells (African green monkey kidney cells), and the virus titer can reach 6.61g CCID50 / ml. Moreover, the virus strain has no external pollution, better immunogenicity and a good effect.
Owner:SINOVAC BIOTECH
Viral particles as immunogens against enterovirus infection and production thereof
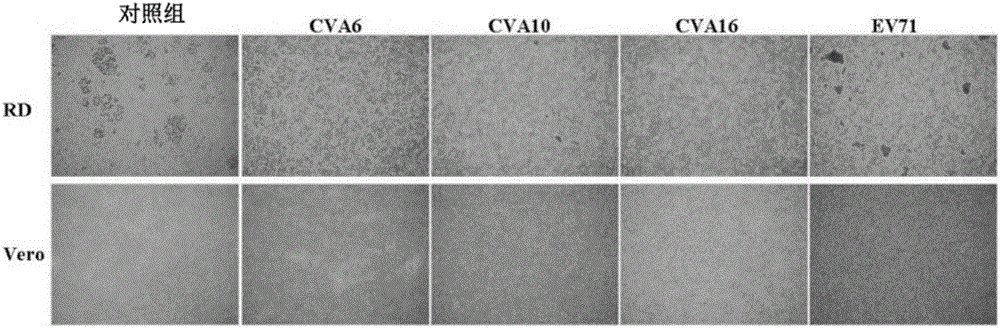
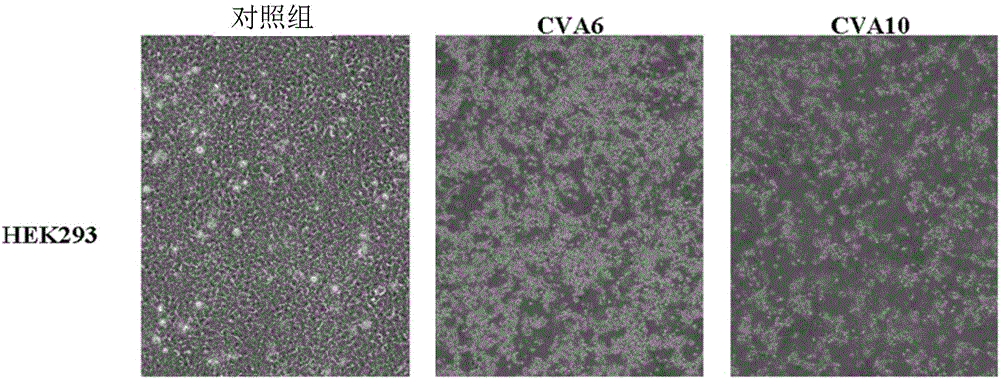
The present invention relates to viral particles as immunogens against enterovirus infection and a method of producing the same. Specifically, the present invention features that human embryo kidney 293 (HEK 293) cells are used to produce viral particles of Enterovirus A, particularly Coxsackievirus A6 (CVA6) particles or Coxsackievirus A10 (CVA10) particles or both and optionally additional viral particles of other Enterovirus A e.g. Coxsackievirus A16 (CVA16) and / or Enterovirus A71 (EV71). The yield of the viral particles in HEK 293 cells is unexpectedly high and effective to induce an immune response against enterovirus infection, especially CVA6 and CVA10. The present invention also relates to an immunogenic composition against enterovirus infection for human use comprising the viral particles as described herein and a method of preventing enterovirus infection or a disease as caused, particularly Hand-Foot-Mouth diseases (HFMD), by administering the immunogenic composition to a subject in need thereof.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH +1
Coxsackievirus A16-type virus strain and use thereof
ActiveCN103087994AGenetic stabilityStable titerSerum immunoglobulinsTransferasesSequence analysisDisease
The invention provides a coxsackievirus A16-type virus strain and a use thereof. The coxsackievirus A16-type virus strain has the preservation number of CGMCC No. 5371. The full-length sequence analysis and the mass spectrometry analysis on a VP1 protein produced by the coxsackievirus A16-type virus strain prove that the oxsackievirus A16-type virus strain is a good CA16 virus strain which is not polluted by allothigenes and has good immunogenicity. The CA16 virus strain can efficiently proliferate in Vero cells and has virus titer of 7.41g CCID 50 / ml. The CA16 virus strain or a vaccine prepared from the CA16 virus strain can be used for preventing diseases caused by CA16 viruses, and has the characteristics of stable titer, good immunogenicity and less immunizing dose.
Owner:SINOVAC BIOTECH
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
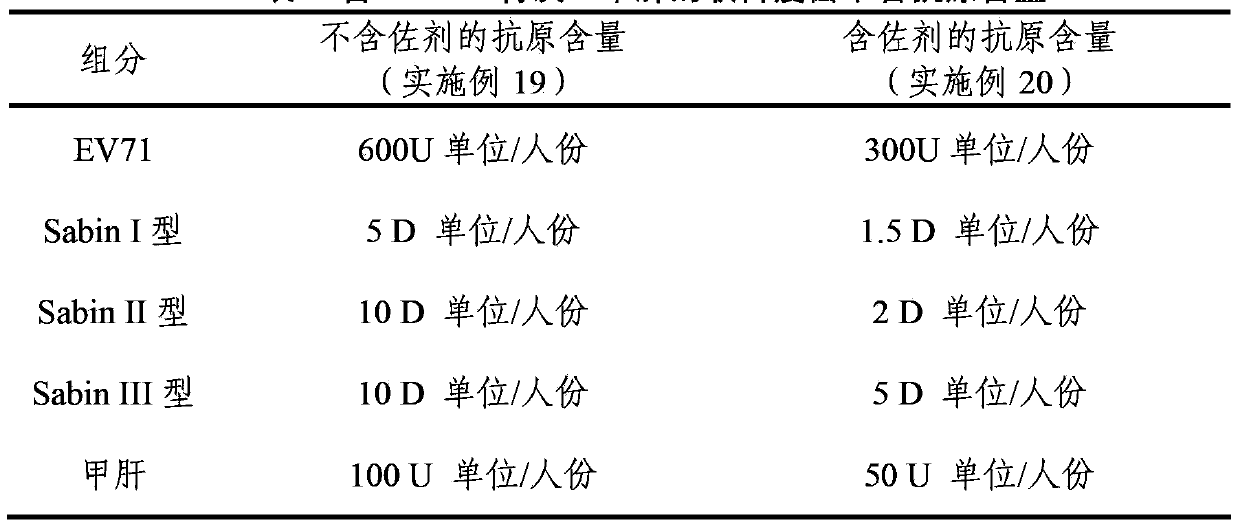
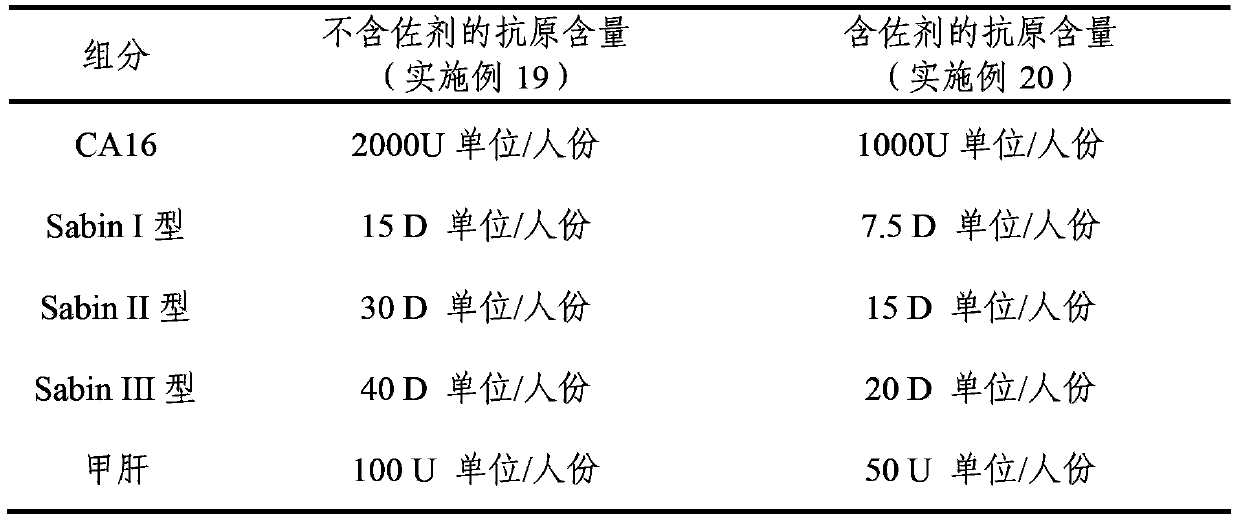
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
IgM antibody joint detection device and method for coxsackievirus A16 and enterovirus 71
PendingCN105044362ASimple structureNovel ideaMaterial analysis by observing effect on chemical indicatorDisease diagnosisCelluloseCoxsackievirus a16
The invention relates to a joint detection device and a preparation method of helicobacter pylori urease antibodies IgM and IgG. The helicobacter pylori urease antibodies are prepared from a nitrocellulose membrane, a glass fiber adsorbing a colloidal gold labeled helicobacter pylori urease antigen and a mouse IgG, a sample pad, absorbent paper and other auxiliary materials, wherein the materials are adhered together, and the nitrocellulose membrane contains a purified high-specificity mouse-anti-human IgM and IgG antibodies, and a goat-anti-mouse antibody in a solid phase manner. The joint detection device has the advantages that the structure is simple, the conception is novel, the nitrocellulose membrane are coated with the anti-human IgM and IgG antibodies, so that the specificity is strong, and the helicobacter pylori urease antibodies IgM and IgG in a specimen are simultaneously detected without increasing the production operation complexity. Proper gold spray buffer and sample pad treating fluid are matched to effectively improve the reaction sensitivity on the basis of guaranteeing complete release of immunocolloidal gold, and under the same threshold, the use amount of the immunocolloidal gold can be reduced to save the cost. The detection device is high in sensitivity, strong in specificity, simple, convenient and strong in practicability and can realize the time-saving aim during operation.
Owner:吉林双正医疗科技有限公司
A16 type strain of Coxsackie virus and application of the strain
ActiveCN102559606AStable titerImproving immunogenicitySerum immunoglobulinsImmunoglobulins against virusesSequence analysisCoxsackievirus a16
The invention provides an A16 type strain of Coxsackie virus, which has the preservation number of CGMCC No. 5373. When observed with an electronic microscope, the virus is in the shape of an icosahedral three-dimensional symmetrical sphere with the diameter of 23-30nm. VP1 conserved region sequence analysis and mass spectrum analysis are respectively conducted for the strain, and the results indicate that the strain is CA 16 virus which can be efficiently proliferated in Vero cells, and the virus titer can reach 7.01g CCID50 / ml, and furthermore, the strain is free of extraneous contamination, and has good immunogenicity and excellent effects.
Owner:SINOVAC BIOTECH
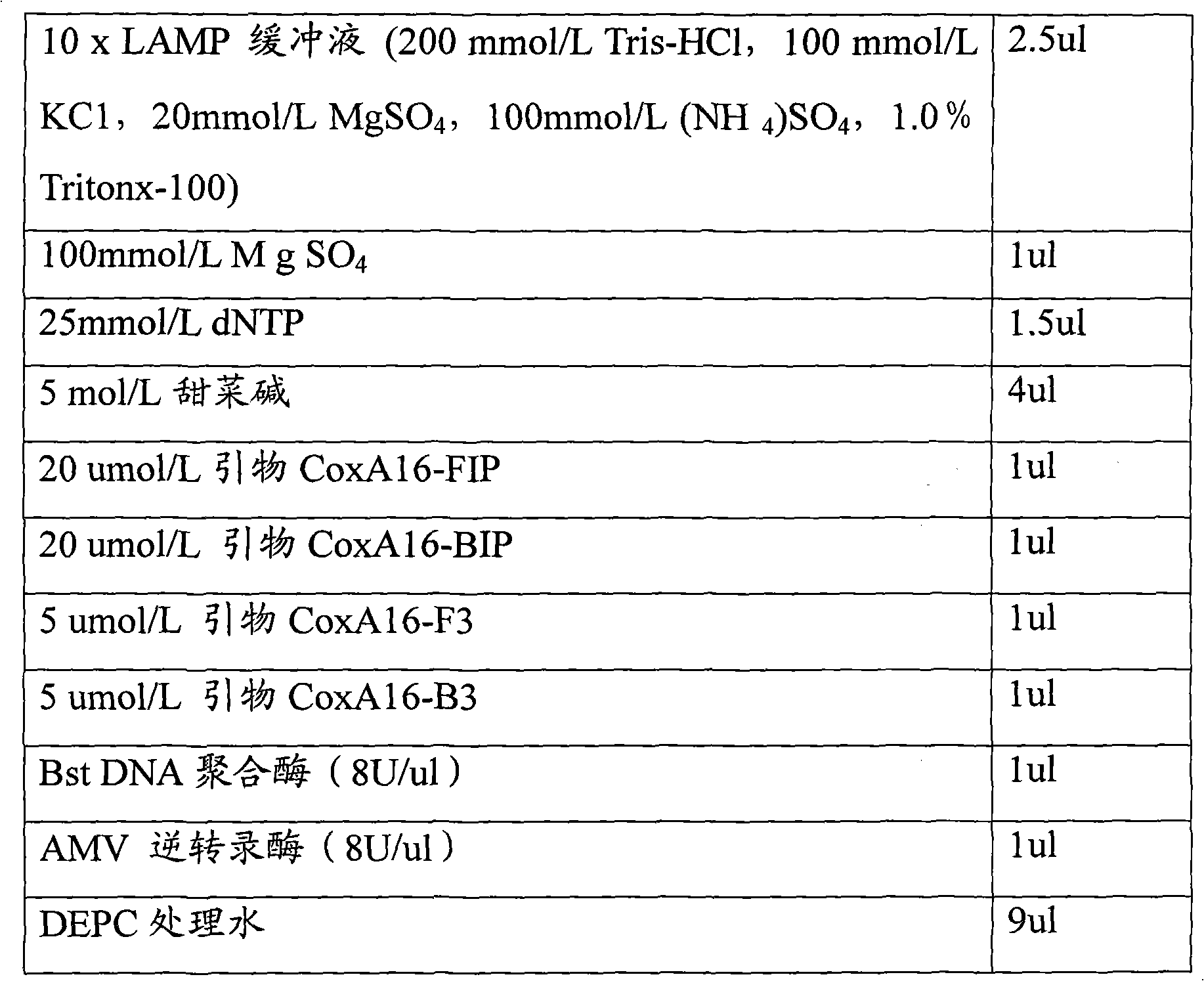
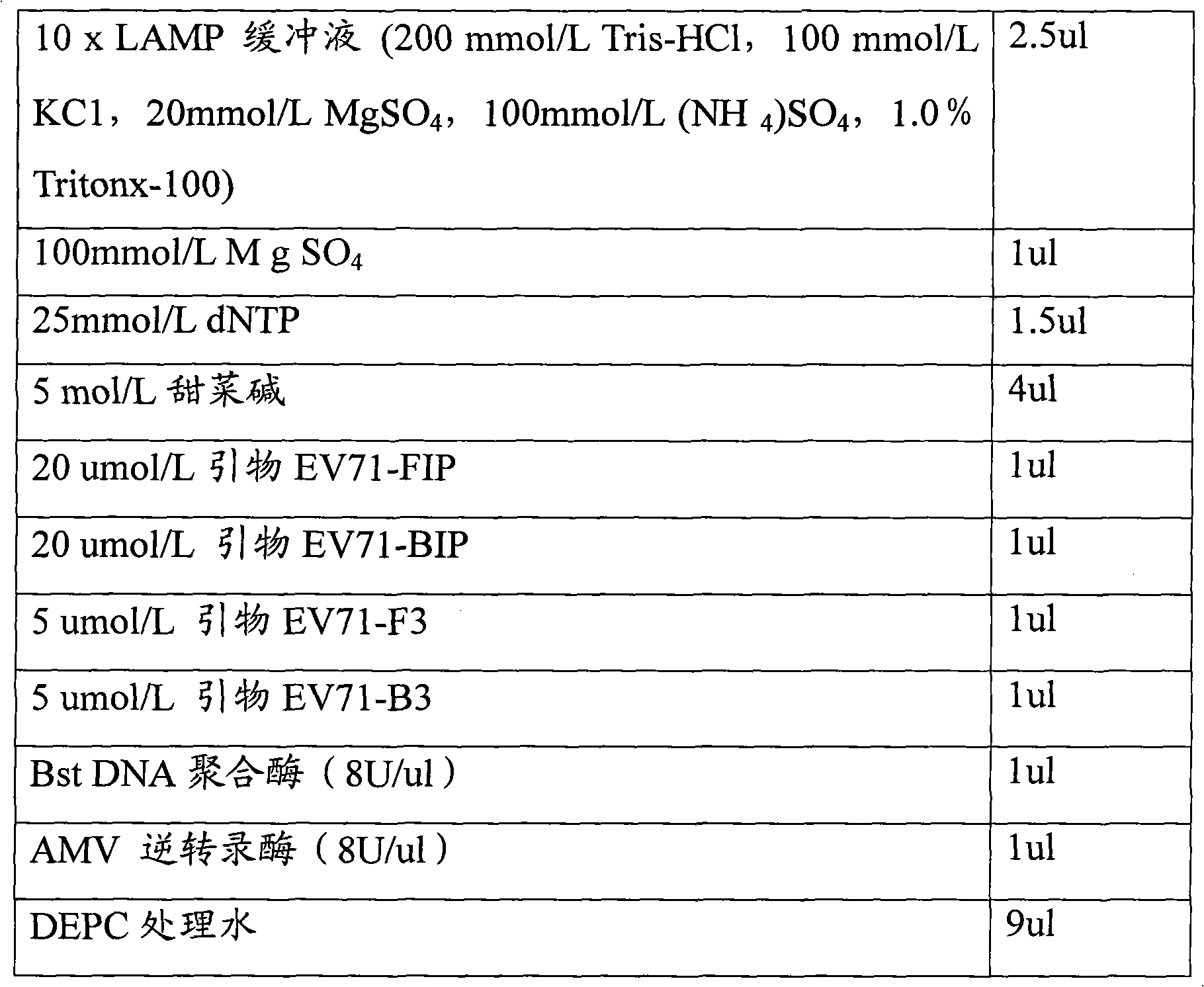
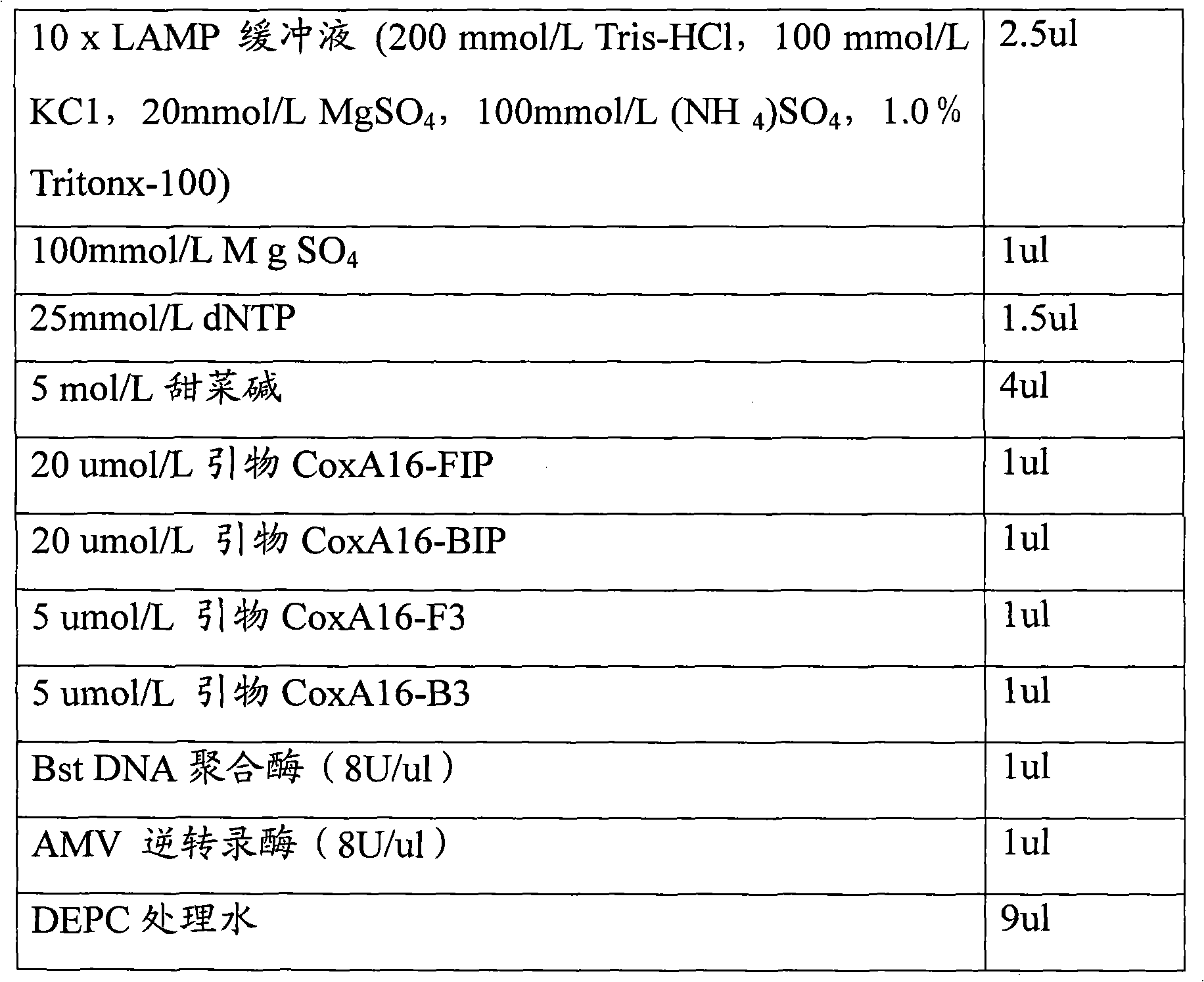
Loop-mediated isothermal amplification assay kit and detection method of hand, foot and mouth disease
InactiveCN102242223ASuitable for field applicationStrong specificityMicrobiological testing/measurementHand-foot-and-mouth diseaseCoxsackievirus a16
The invention belongs to the field of biotechnology, and relates to a loop-mediated isothermal amplification (LAMP) assay kit of Coxsackie A16 (Cox A16) and Enterovirus 71 (EV 71) which are main pathogens of hand, foot and mouth disease, and an establishment method and an application of the assay kit. The assay kit comprises four LAMP primers and LAMP reaction liquid for detecting Coxsackie A16, and four LAMP primers and LAMP reaction liquid for detecting Enterovirus 71. Tests prove that the assay kit has the advantages of good specificity and sensitivity, rapid amplification rapid, high efficiency and simple identification. A detection system provided by the invention can rapidly, conveniently, efficiently, high specifically and high sensitively detect Coxsackie A16 and Enterovirus 71 without complex apparatuses thus can satisfy well clinical detection requirements of hand, foot and mouth disease, and is suitable for a large-scale promotion and an application.
Owner:上海吉美生物工程有限公司
Purification of recombinant coxsackievirus A16 (CA16) virus-like particles, application thereof in vaccine and vaccine
ActiveCN109680026AImproving immunogenicityImprove securitySsRNA viruses positive-senseViral antigen ingredientsPurification methodsUltrafiltration
The present invention provides a method for purifying recombinant coxsackievirus A16 (CA16) virus-like particles, an application thereof in an vaccine, and the vaccine. The method comprises carrying out high density fermentation culture of recombinantly expressed engineering bacteria and methanol-induced expression of CA16 viroid granulin, carrying out centrifugation for collecting thalli, performing high-pressure homogenization and breaking, and purifying the supernatant by ultrafiltration, ion exchange chromatography, hydroxyapatite chromatography and molecular sieve chromatography and the like. The CA16 viroid particle vaccine provided by the invention has good immunogenicity, safety, immunological characteristics and biological activity. The process is simple, and the purification is performed by chromatographic methods. The purification method is more conducive to linear amplification compared with density gradient centrifugation, and can be used in large-scale preparation and purification, and a high-purity (more than 99%) virus-like particle (VLP) stock solution can be obtained and can be used to prepare a vaccine for preventing CA16 infection, and has good economic value and application prospects.
Owner:深圳鑫泰康生物科技有限公司 +1
Nucleic acid fluorescence PCR detection kit for universal enterovirus, coxsackievirus A16 and enterovirus 71
InactiveCN105420411AHigh sensitivityHigh amplification efficiencyMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16RNA extraction
The embodiment of the invention discloses a nucleic acid fluorescence PCR detection kit for a universal enterovirus, a coxsackievirus A16 and an enterovirus 71. The nucleic acid fluorescence PCR detection kit comprises an RNA extracting solution, an RNA eluant, internal standards, a PCR reaction solution, an EV / CA16 / EV71 enzyme mixed solution, EV / CA16 / EV71 positive reference substances and EV / CA16 / EV71 negative reference substances. According to the kit, the universal enterovirus, the coxsackievirus A16 and the enterovirus 71 can be simultaneously detected in a same sample, but other pathogen RNA cannot be detected, the detection sensitivity can reach 400 copie / ml, the detection range is 4.0 E+02-4.0E+08 copies / ml, and a reliable experimental evidence is supplied to early diagnosis on infection of the universal enterovirus, the coxsackievirus A16 and the enterovirus 71.
Owner:SANSURE BIOTECH INC
Kit for detecting coxsackie virus A16-type nucleic acid and detection method thereof
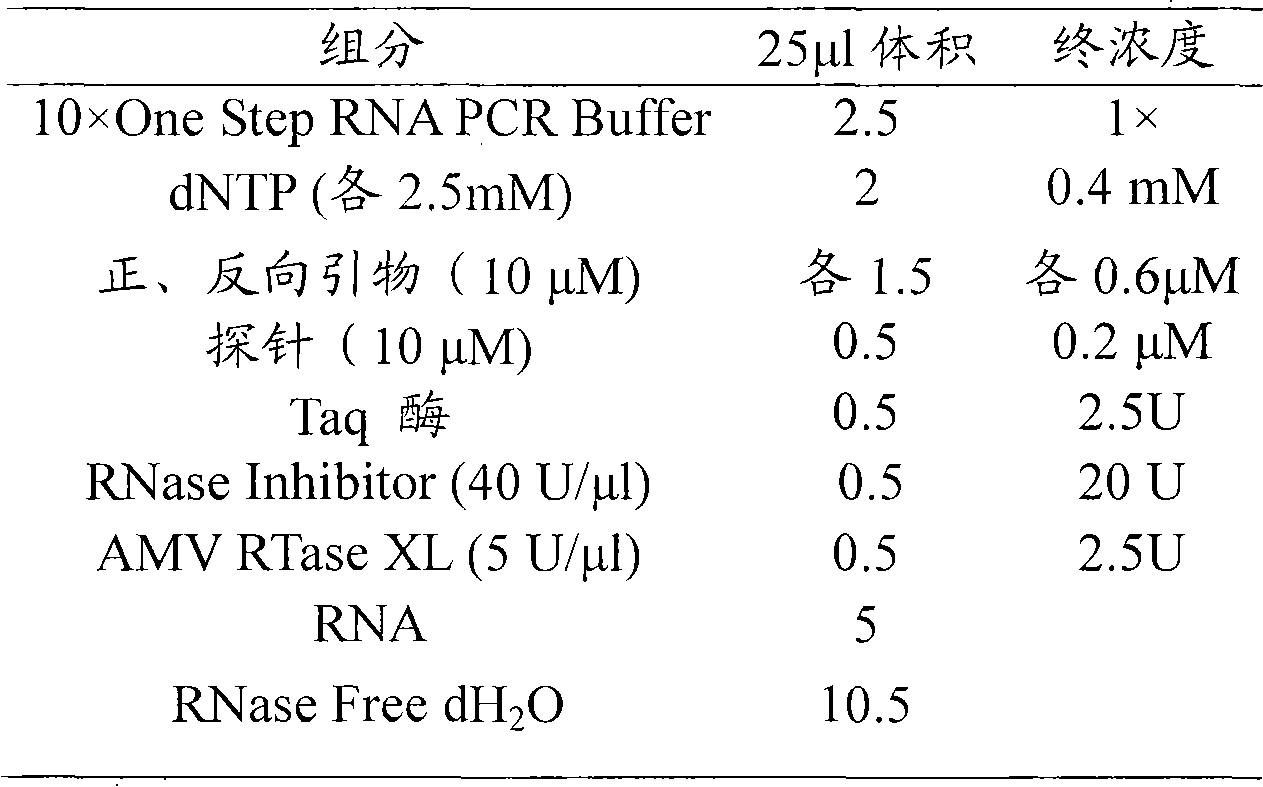
ActiveCN101713003ASimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceForward primerFluorescence
The invention discloses a kit for detecting coxsackie virus A16-type nucleic acid and a detection method thereof. The kit comprises PCR reaction enzyme, PCR reaction liquid, a negative quality control product and a positive quality control product, wherein the PCR reaction enzyme contains a hot start Taq enzyme, a MLV reverse transcriptase and a RNA enzyme inhibitor; the PCR reaction liquid contains DEPC treating water, dNTPs, 10*PCR Buffer, a coxsackie virus A16-type forward primer, a coxsackie virus A16-type reverse primer and a coxsackie virus A16-type probe, the sequence of the coxsackie virus A16-type forward primer is 5'-CGCTGCCGATACTGAAGCACCG-3', the sequence of the coxsackie virus A16-type reverse primer is 5'-CTGTCTCCGCGGCTTGTAG-3', the sequence of the coxsackie virus A16-type probe is 5'-ACAGATTAGGCACTGGTGTTGTACCGTA-3'; the negative quality control product is the DEPC treating water; and the positive quality control product is the prepared transcription in vitro RNA standard product. The method for fast detecting the sequence of the coxsackie virus A16-type nucleic acid by using a real-time fluorescence quantitative PCR technology has the advantages of specificity, sensitiveness, high speed as well as simple and convenient operation.
Owner:IPE BIOTECHNOLOGY CO LTD
Hand-foot-mouth disease detection reagent kit and its detection method
InactiveCN102643927ASuitable for field applicationStrong specificityMicrobiological testing/measurementHand-foot-and-mouth diseaseCoxsackievirus a16
The invention belongs to biological field, and relates to a reagent kit for detecting main pathogen Coxsackievirus A16 (Cox A16) and enterovirus 71 of hand-foot-mouth disease, construction method and uses thereof. The reagent kit comprises reaction solutions for detecting four primers of Coxsackievirus A16 and four primers of enterovirus 71.As detected, the reagent kit has a good performance of specificity, sensitivity, rapid amplification, high efficiency and simple identification. The detection system of the invention can detect Coxsackievirus A16 and enterovirus 71 quickly, conveniently, high-effectively, high-specifically and highly-sensitive under temperature of 64 DEG C without need of complicated instruments, which can preferably satisfy clinical detection for hand-foot-mouth disease and be easily popularized in large scope.
Owner:刘志学 +1
Coxsackie virus A16 type RT-LAMP (reverse transcription-loop-mediated isothermal amplification) nucleic acid assay kit
InactiveCN102373293ALow costStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationHand-foot-and-mouth diseaseCoxsackievirus a16
The invention relates to the field of biological assay technical application, and in particular relates to a coxsackie virus A16 type RT-LAMP (reverse transcription-loop-mediated isothermal amplification) nucleic acid assay kit. The kit comprises RT-LAMP reaction solutions containing four RT-LAMP primers, as well as 10000X SYBR Green I dye. The kit has the characteristics of good specificity, high sensitivity, simple operation method, quick assay, easily judged result, low cost and the like, can well fulfill the current urging requirement for field assay of hand-foot-and-mouth disease, can beused for field quick assay of disease prevention and control institutions, hospitals and kindergartens, is easy for large-scale popularization and application in grass-roots places, and has wide market prospect and great economical and social benefit.
Owner:何雅青 +2
Primer for coxsackie virus A16 nucleic acid detection, probe and kit
InactiveCN101676406AHigh sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationForward primerCoxsackievirus a16
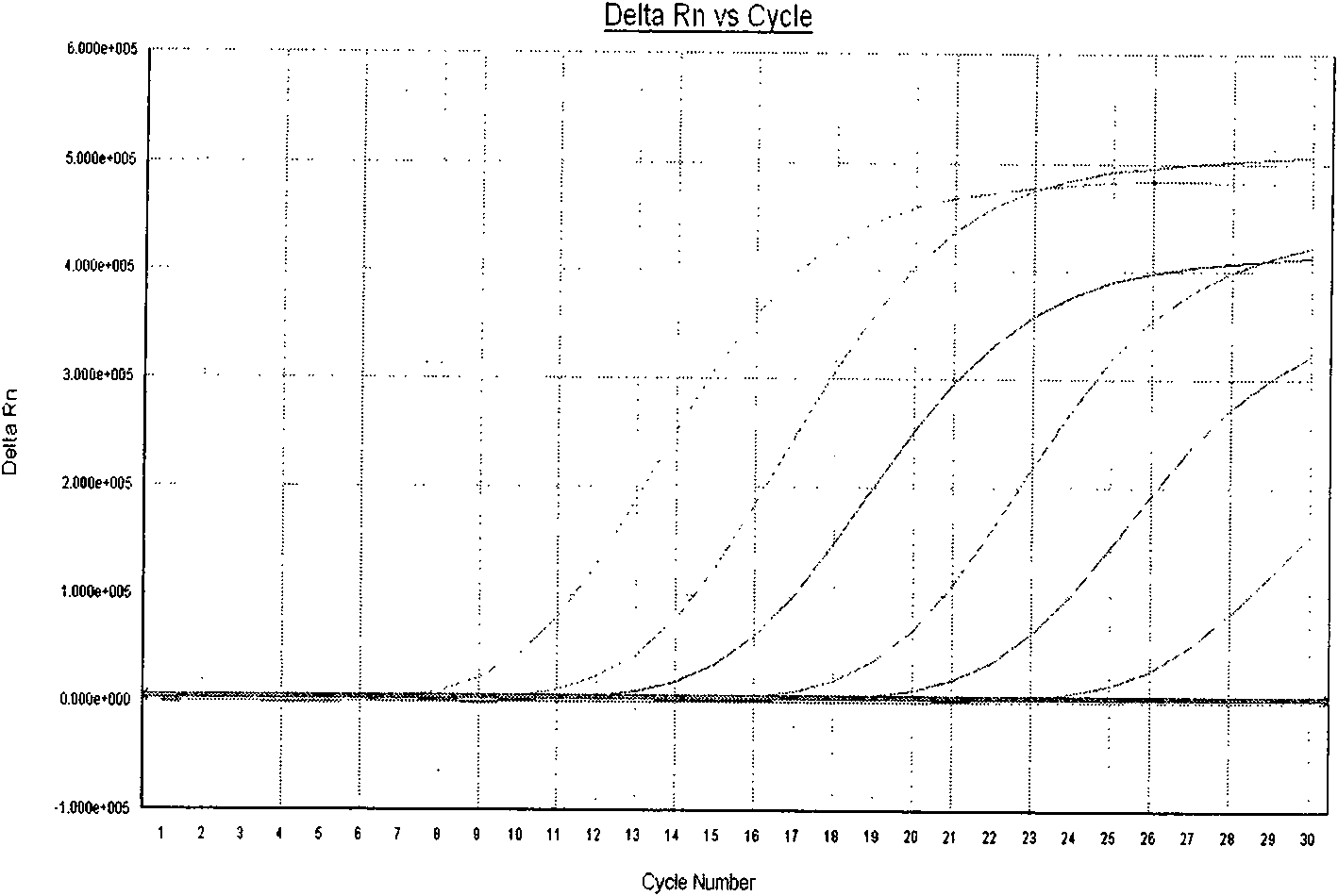
The invention relates to a primer for coxsackie virus A16 nucleic acid detection, probe and kit, wherein the nucleotide sequence of the primer is that: forward primer 5'-GAACCATCACTCCACACAGGAG-3'; backward primer 5'-GTACCCGTGGTGGGCATTG-3' and the nucleotide sequence of the probe is 5'-CAGCCATTGGGAATTTCTTTAGCCGTG-3'. The invention also provides a method for detecting coxsackie virus A16 nucleic acid. Sample RNA is used as template and the above primer and probe are subjected to real-time fluorescence RT-PCR amplification and the result is predicated based on the amplification curve after each circulation. The primer specificity is good, the detection method is quick and simple, the accuracy and the sensitivity is high and the invention provides scientific reference for etiologic diagnosis and differential diagnosis of hand-foot-mouth disease and relative diseases.
Owner:何雅青 +1
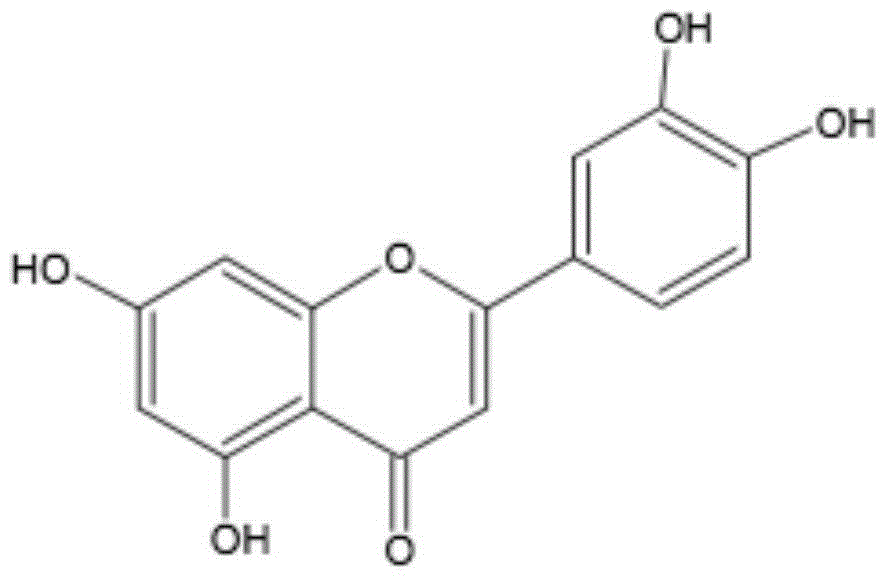
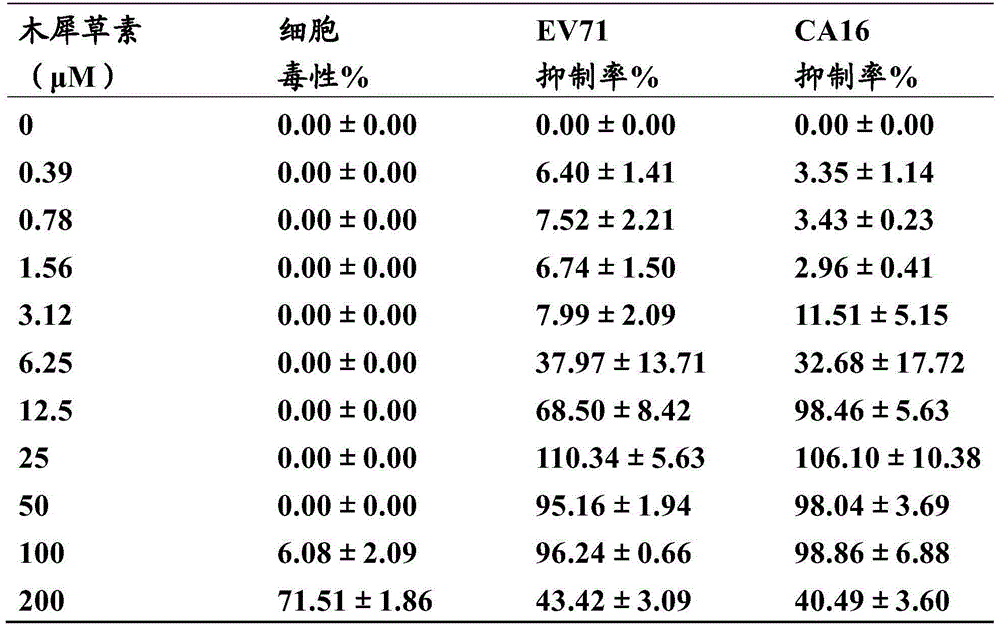
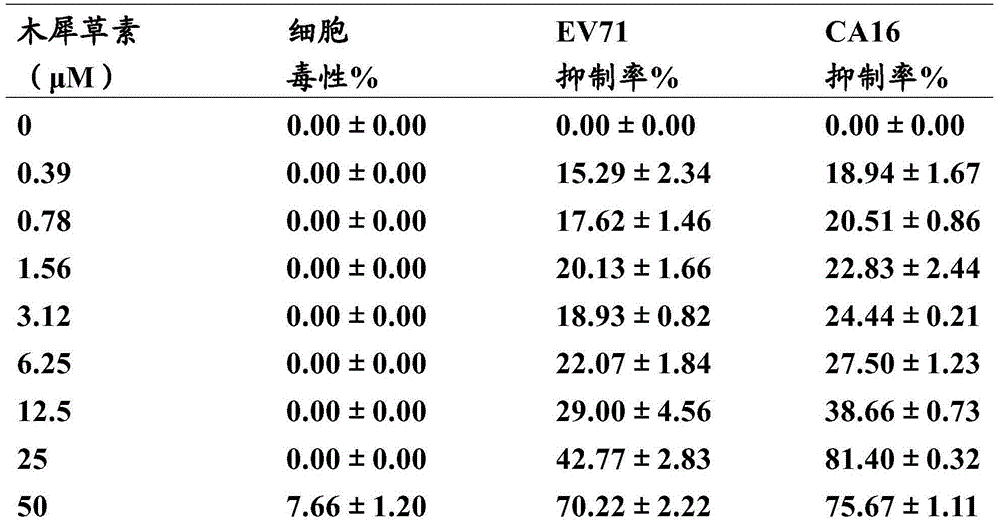
New application of luteolin
The invention relates to new application of luteolin, in particular to application of luteolin in preparing medicine for preventing and treating hand-foot-mouth diseases, particularly, medicine used for resisting enterovirus 71 and / or coxsackievirus A16.
Owner:JILIN UNIV +1
Coxsackie virus type A16 (CA16) real-time fluorescent nucleic acid isothermal amplification detection kit
ActiveCN103388032AReduce pollutionEfficient captureMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Throat swab
The invention discloses a Coxsackie virus type A16 (CA16) real-time fluorescent nucleic acid isothermal amplification detection kit comprising reagents such as a capturing probe, CA16 amplification primers T7 primer and nT7 primer, a CA16 detection probe, M-MLV reverse transcriptase, T7 RNA polymerase, and the like. The kit can be used for detecting CA16 RNA in throat swab or stool, and has the characteristics of high specificity, high sensitivity (reaching 10copies / reaction), low pollution (amplification product RNA can be easily degraded under natural environment), and fast detection (conventionally detection can be finished within 60min). The kit can perform important effect in clinical diagnosis of CA16 early-stage infection, and can be widely applied.
Owner:SHANGHAI RENDU BIOTECH
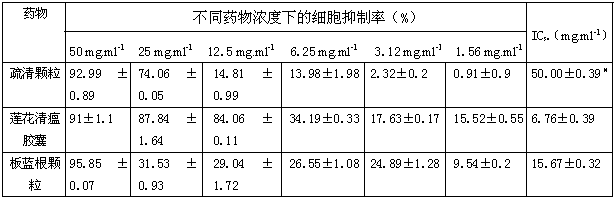
Application of Shuqing granules in preparing medicine for resisting hand-foot-and-mouth disease pathogens
InactiveCN102920944ALow priceWide range of medicinesAntiviralsAluminium/calcium/magnesium active ingredientsBiotechnologyCoxsackievirus a16
Owner:吉林华康药业股份有限公司
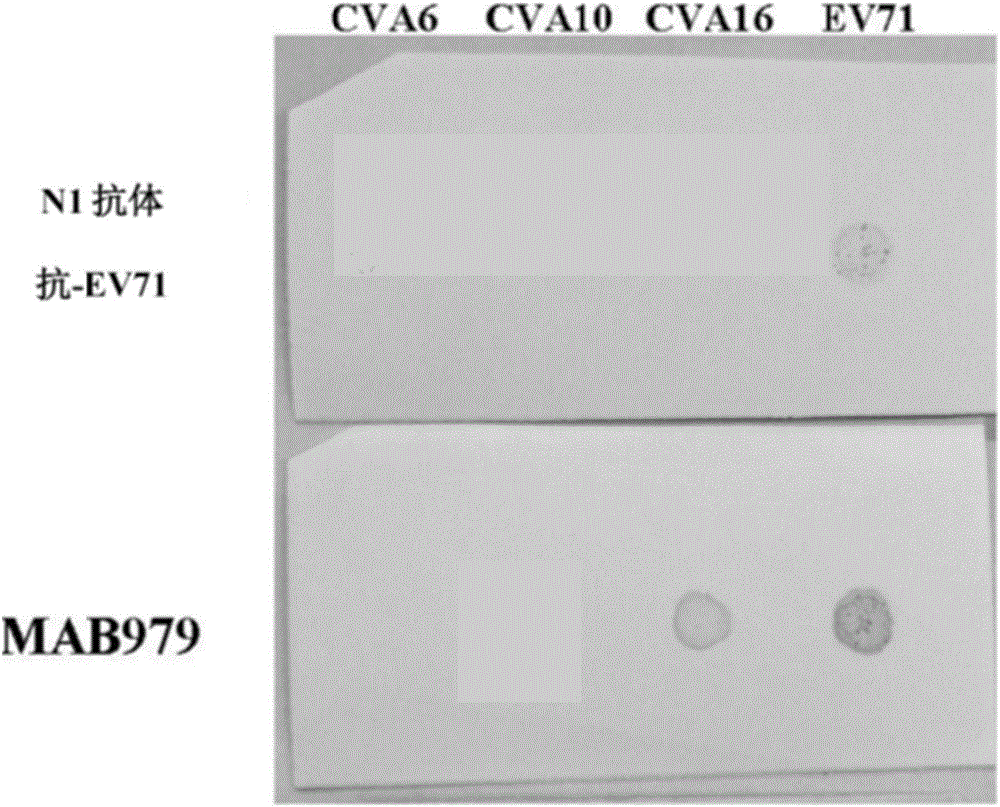
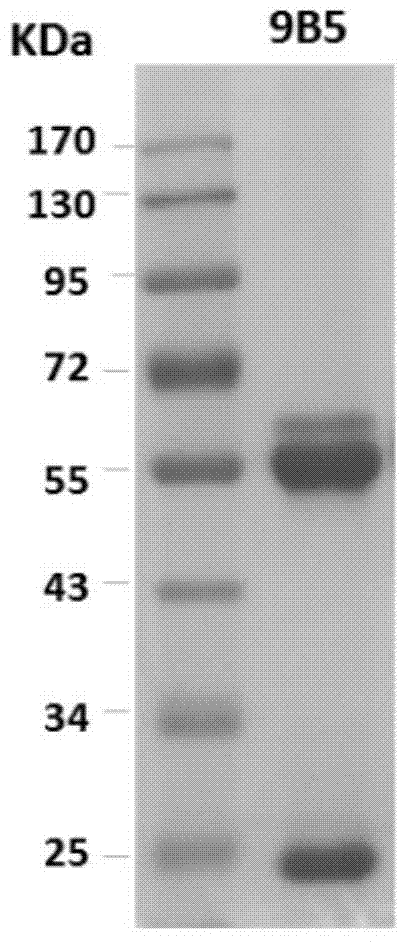
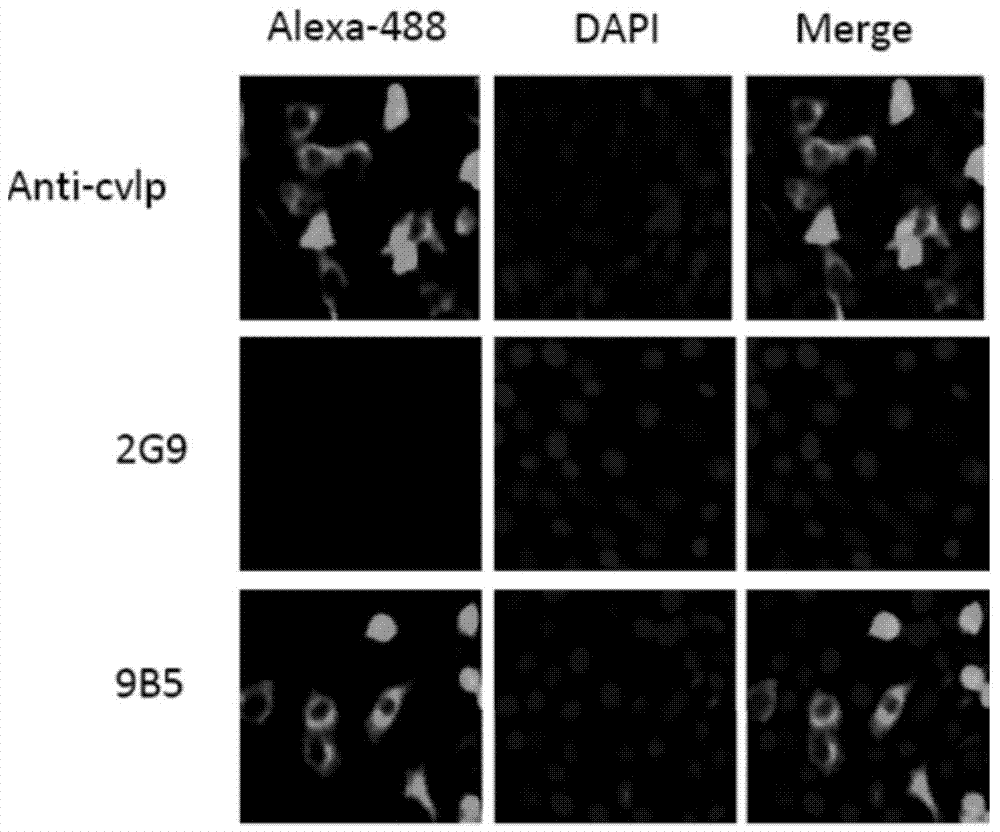
Preparation and application of anti-Coxsackie virus A16 monoclonal antibody
The invention relates to preparation and application of an anti-Coxsackie virus A16 (CA16) monoclonal antibody. The invention reveals a mouse source anti-CA16 monoclonal antibody, which has good binding specificity, can be used for sensitive detection of Coxsackie A16 virus, and has strong anti-virus infection capacity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Coxsackie virus type a16 (ca16) igm antibody detection test strip
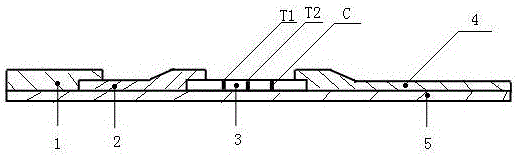
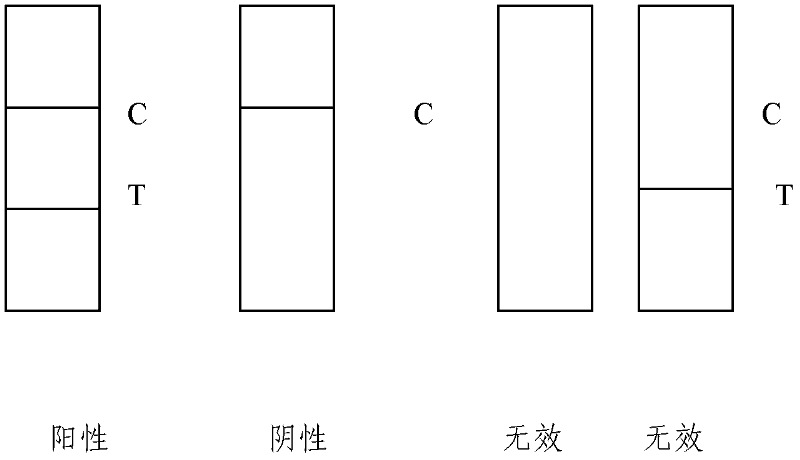
ActiveCN102262156ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCoxsackievirus a16Nitrocellulose
The invention provides a detection test strip for a coxsackie virus A16 type (CA16) IgM (Immune Globulin M) antibody. According to the detection test strip, a nitrocellulose membrane (NC membrane) is coated by a CA16 gene antigen (VP1) and an anti-mouse IgG; and by combining a tihuman IgM monoclonal antibody labeled by colloidal gold, a CA16 specificity IgM antibody in an infected human speciment is detected by applying a membrane chromatography capture method. The test strip for detection, which is disclosed by the invention, has the advantages of simpleness, convenience, quickness and fastness in operation, no need of special instrument or special training, distinct and easily-distinguished result and easiness for popularization; and the detection test strip is suitable for field detection and early-stage diagnosis and assisting function for CA16 infected diagnosis.
Owner:辽宁迪浩生物科技有限公司
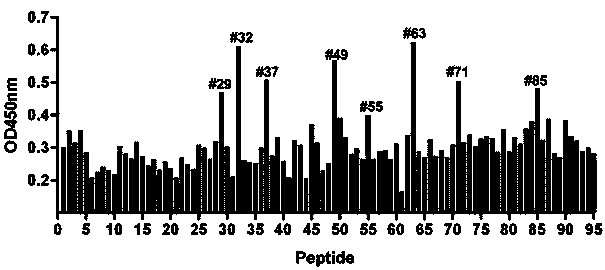
Conservative neutralizing epitope polypeptide of Coxsackievirus A16 and application thereof
The invention relates to a conservative neutralizing epitope polypeptide of Coxsackievirus A16 and application thereof. Specifically, the invention provides the neutralizing epitope polypeptide of Coxsackievirus A16, and also provides a binding molecule able to specifically bind with the neutralizing epitope polypeptide, antiserum containing the binding molecule, a kit for detecting the Coxsackievirus A16, a detection method and a composition inhibiting the Coxsackievirus A16. The invention also provides application of the neutralizing epitope polypeptide, the binding molecule and the antiserum in preparation of drugs for preventing and treating hand, foot and mouth disease and diagnostic agents for diagnosing hand, foot and mouth disease. According to the invention, 6 linear neutralizing epitopes on CA16VP1 are identified for the first time, the comparison result of different genotype CA16 sequences shows that the epitopes are very conservative, and the serum brought about by immunizing mice with the neutralizing epitope polypeptide can neutralize homologous and heterogenous CA16 strains. The results have important guiding significance on development of Coxsackievirus A16 vaccines and diagnostic kits.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Nucleic acid fluorescent PCR detection kit for coxsacki evirus A16 and human enter ovirus 71
InactiveCN105368986AHigh purityHigh yieldMicrobiological testing/measurementMicroorganism based processesEnterovirusRNA extraction
The embodiment of the invention discloses a nucleic acid fluorescent PCR detection kit for coxsacki evirus A16 and human enter ovirus 71. The nucleic acid fluorescent PCR detection kit comprises the following components: RNA extract solution, RNA eluant, internal standard substance, PCR reaction liquid, CA16 / EV 71 enzyme mixed liquid, CA16 / EV 71 positive control substance and CA16 / EV 71 negative control substance. According to the nucleic acid fluorescent PCR detection kit, two kinds of enteroviruses including the coxsacki evirus A16 and human enter ovirus 71 are simultaneously detected in one sample, other pathogen RNAs can not be detected, and RNAs are extracted by a paramagnetic particle method, so that the detection sensitivity, accuracy and stability are improved, the detection sensitivity achieves 400 copie / ml, the detection range is (4.0E+02)-(4.0E+08) copies / ml, and reliabile experimental evidences are provided for early diagnosis of the infection of the coxsacki evirus A16 and the human enter ovirus 71.
Owner:SANSURE BIOTECH INC
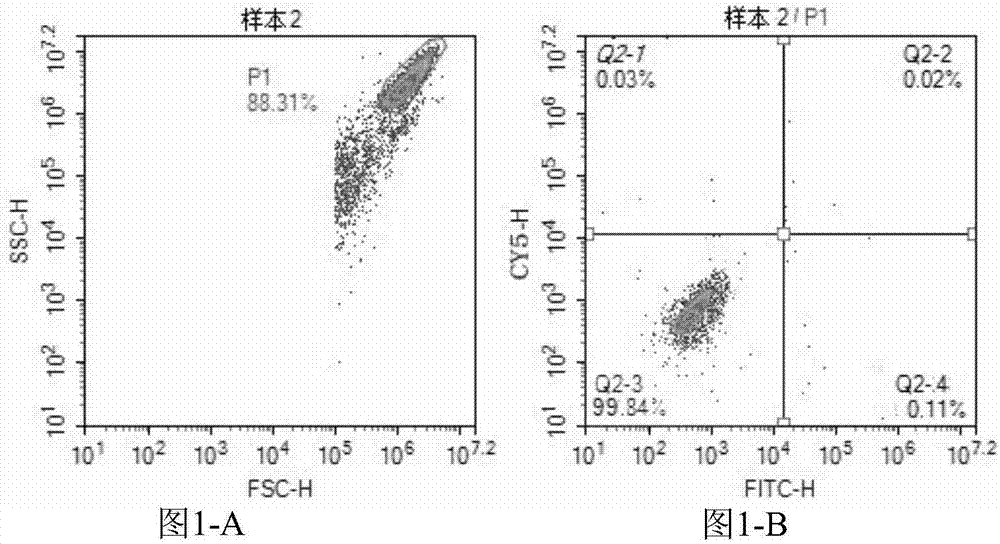
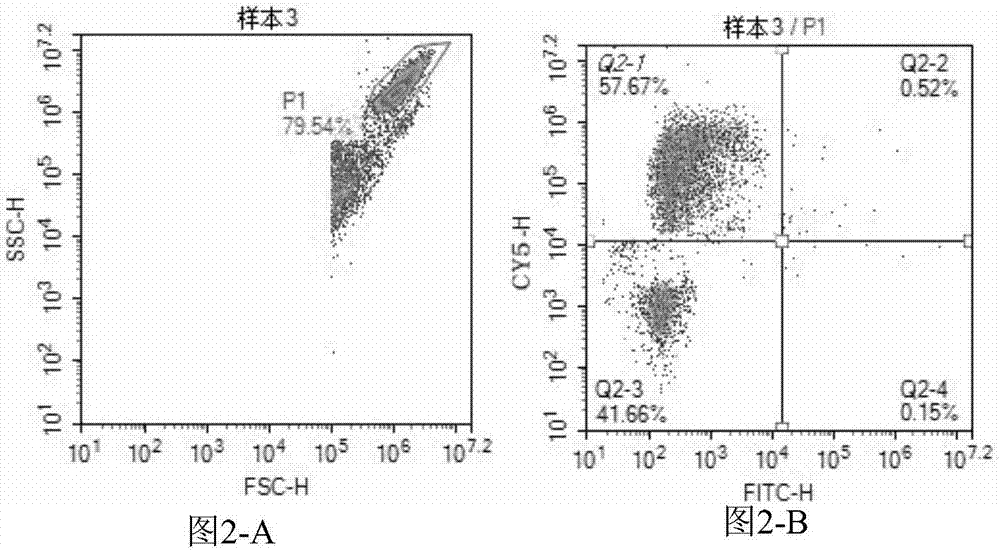
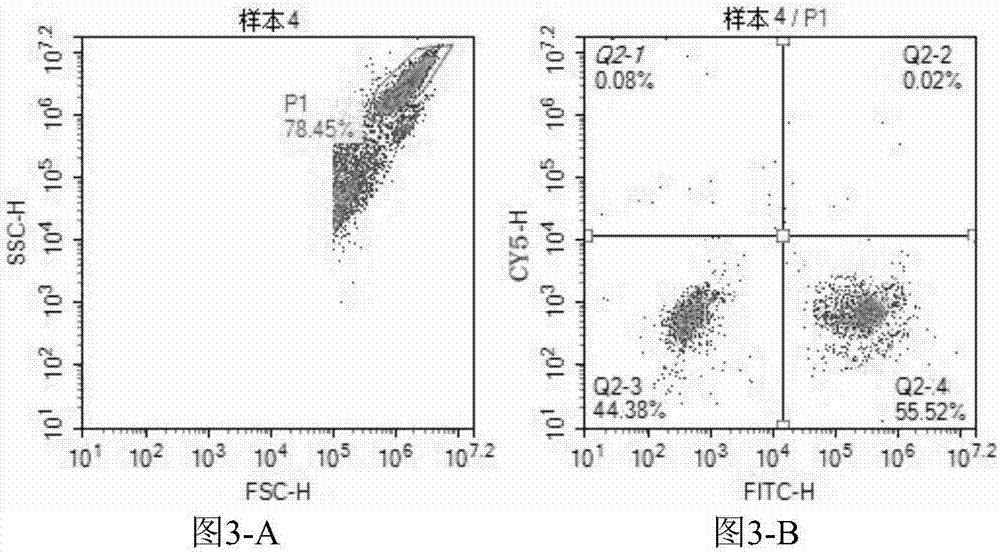
Kit for performing direct immunofluorescent detection on enterovirus 71 and coxsackievirus A16 and application thereof
InactiveCN107121547AQuick checkSensitive detectionBiological material analysisCoxsackievirus a16Immunofluorescence
The invention provides a kit for performing direct immunofluorescent detection on enterovirus 71 and coxsackievirus A16 and application thereof. The kit provided by the invention contains a fluorescein-labeled antibody, a fluorescent dye Evanslan, a slide sealing agent, a slide, a quality control slide, a cell fixing solution, a PBS solution, a PBST solution, 1% Triton-X100, a cell reference substance, a preservative sodium azide and an antibody stabilizer BSA. Through application of the kit provided by the invention, the enterovirus 71 and the coxsackievirus A16 in a sample cell can be directly detected. The kit provided by the invention takes full advantages of the direct immunofluorescence method in aspects of automation and high throughput, can achieve rapid, sensitive and efficient detection on the viruses, has an important significance in rapid and accurate clinical diagnosis and can meet different needs of large hospitals and primary hospitals.
Owner:GUANGDONG HECIN SCI INC
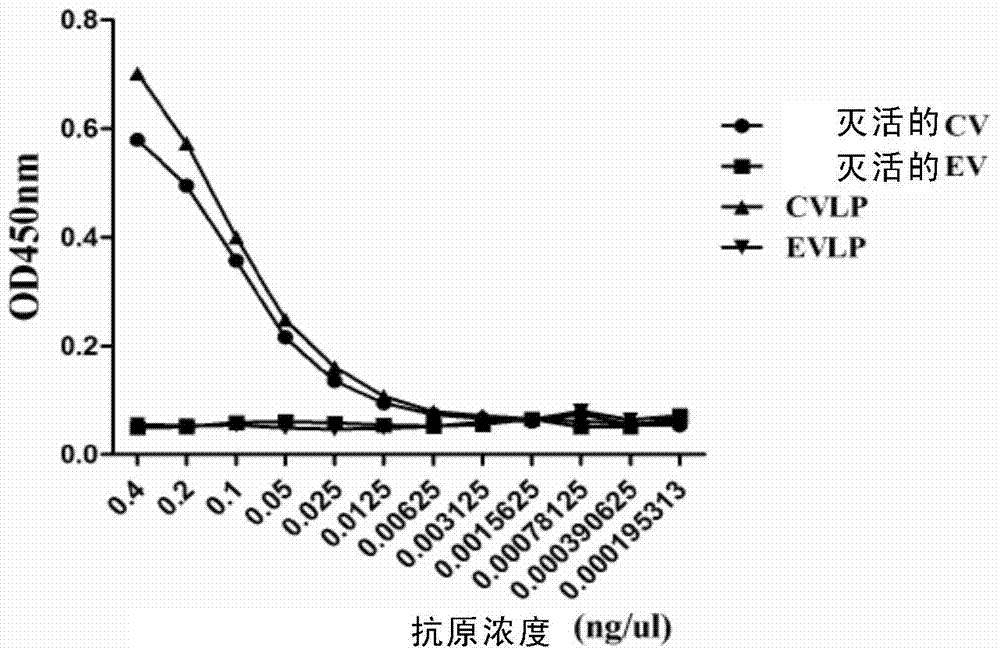
Nucleic acid construct and method for preparing coxsackievirus A16 virus-like particles
ActiveCN108624609AHigh titerHigh expressionSsRNA viruses positive-senseAntibody mimetics/scaffoldsEscherichia coliPurification methods
The invention belongs to the fields of molecular biology, virology and immunology, and relates to a nucleic acid construct and a method for preparing coxsackievirus A16 virus-like particles. The invention also relates to recombinant vectors and recombinant host cells comprising the nucleic acid construct. The invention further relates to a method and a purification method for co-expressing coxsackievirus A16 type capsid proteins VP1, VP2, VP3 and VP4 in series, a method for preparing the coxsackievirus A16 type virus-like particles, and the like. Further, the invention relates to the coxsackievirus A16 type virus-like particles and a pharmaceutical composition containing the same, such as a vaccine. By adopting the nucleic acid construct and the method, soluble co-expression of the capsidproteins of a human hand-foot-and-mouth disease virus (coxsackievirus A16 type) in escherichia coli is realized, and the expression quantity is relatively high; finally obtained interest proteins account for about 10% of the total soluble proteins in bacteria; and the coxsackievirus A16 virus-like particles comprising the interest proteins are highly similar to native coxsackievirus A16 conformation.
Owner:SA BIOTECH (SUZHOU) PTE LTD
Preparation method of hand-foot-and-mouth disease etiology colloidal gold diagnosis detection test paper box
InactiveCN105785018AImprove accuracyHigh detection sensitivityMaterial analysisEtiologyType specific
The invention relates to a preparation method of a hand-foot-and-mouth disease etiology colloidal gold diagnosis detection test paper box, and the method is capable of effectively solving the problems of low detection sensitiveness, low accuracy, high detection leakage possibility and difficult specimen collection in the prior art. The method comprises the following steps of: firstly adhering a reaction film to the center of a backlining; spraying a detection line and a control line on the reaction film; pressing a water absorption cushion to one side, which is adjacent to the tail end of the backlining, of the reaction film, pressing a colloidal gold cushion to the other side of the reaction film, pressing a sample cushion to the other side of the colloidal gold cushion; fixing the sample cushion, the colloidal gold cushion, the reaction film and the water absorption cushion to the backlining; cutting into 4mm-width strips along the cross section direction of the backlining by use of a slitter; assembling testpaper into a testpaper shell, and forming a sample hole and a detection result region on the test paper shell. The high-sensitivity hand-foot-and-mouth disease etiology rapid detection test paper prepared by anti-enterovirus 71 type specific monoclonal antibodies and anti-coxsackievirus A16 type specific monoclonal antibodies is good in detection sensitivity, high in accuracy and low in detection leakage possibility.
Owner:开封市疾病预防控制中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com