Nucleic acid fluorescent PCR detection kit for coxsacki evirus A16 and human enter ovirus 71
A technology for coxsackie virus and detection kit, which is applied in the direction of determination/inspection of microorganisms, microorganisms, biochemical equipment and methods, etc., to achieve the effects of easy purification, high purity and yield, and improved detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The coxsackievirus type A16 and enterovirus type 71 nucleic acid fluorescent PCR detection kits provided in this example include the following components: RNA extraction solution, RNA eluent, internal standard, PCR reaction solution, CA16 / EV71 enzyme Mixed solution, CA16 / EV71 positive control substance, CA16\EV71 negative control substance, upstream and downstream primers CA16-F, EV71-F and CA16-R, EV71-R for target polynucleotide amplification Probes CA16-P and EV71-P for acid detection, upstream and downstream primers IC-F and IC-R for detecting internal standards, and probe IC-P for detecting internal standards.
[0058] The internal standard is a recombinant of a 90 base pair artificially synthesized DNA sequence inserted into the pUC18T vector, and its concentration is 1.00E+03copies / ml~1.00E+06copies / ml; the 90 base pair sequence for:
[0059] 5'-CAGCTTGTTGTAACAAAGCATCCGCTCCCCCATTCATGTTGCTGGGTACAGACAGTTACCTTCCACTAGGCAAATCTCAACAGGATCAG-3'.
[0060] The RNA extrac...
Embodiment 2
[0103] This embodiment provides a Coxsackievirus A16 / Enterovirus 71 nucleic acid fluorescent PCR detection kit, the composition of which is the same as in Example 1, and is used to detect CA16-RNA and EV71-RNA in throat swab samples , detection method and step are identical with embodiment 1.
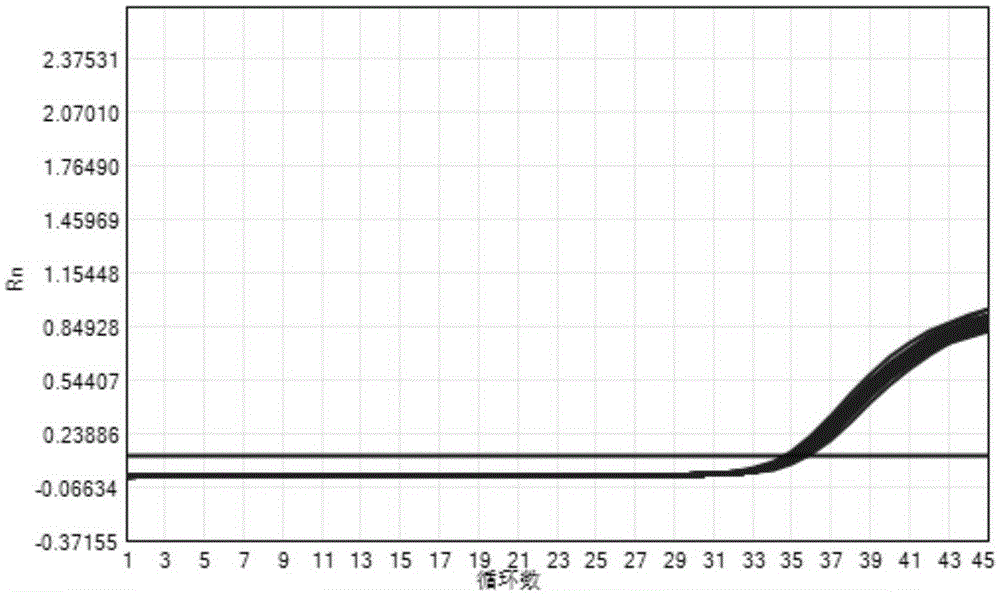
[0104] Among them, the concentration gradient of CA16 and EV71 is 4.0E+08copies / ml~4.0E+02copies / ml, and the results of CA16 gradient sample detection (CA16 channel) are as follows image 3 As shown, the EV71 gradient sample detection (EV71 channel) results are as follows Figure 4 shown. It can be seen from the analysis in the figure that the target channels of the CA16 and EV71 gradient samples detected by this detection kit are all positive, indicating that the detection range of the kit for CA16 and EV71 samples is 4.0E+08copies / ml~4.0E+02copies / ml.
Embodiment 3
[0106] This example provides the detection limit of CA16-RNA and EV71-RNA in the sample of a Coxsackievirus A16 / Enterovirus 71 nucleic acid fluorescent PCR detection kit sample. The composition of the kit is the same as in Example 1, and the detection method and steps are the same as in Example 1.
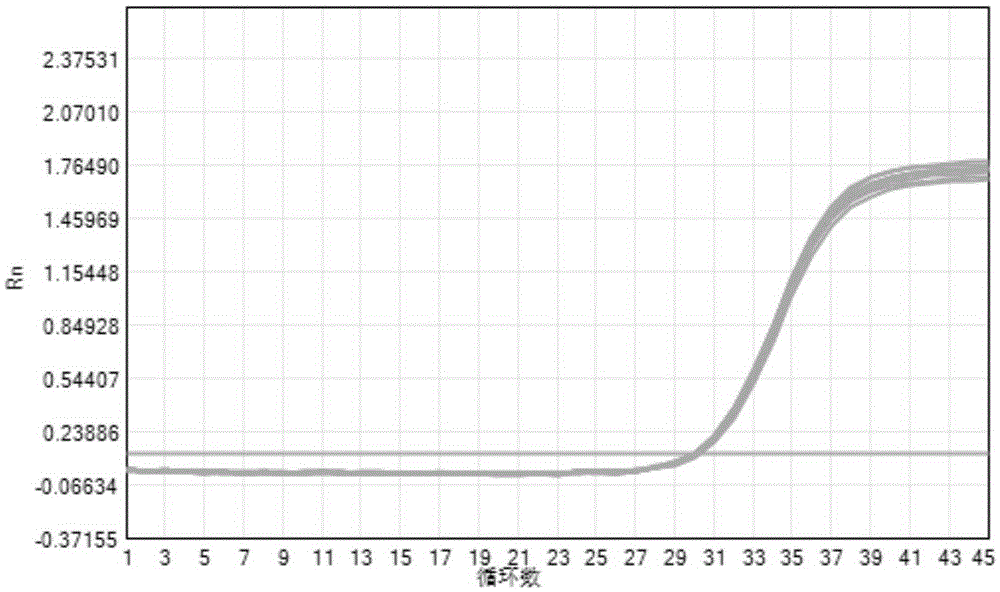
[0107] Among them, the CA16 reference product amplification curve (CA16 channel) results are as follows Figure 5 As shown, the EV71 reference product amplification curve (EV71 channel) results are as follows Image 6 As shown, the concentration of CA16 reference substance and EV71 reference substance is 400copies / ml. From the analysis in the figure, it can be known that the detection kit detects CA16 and EV71 detection sensitivity reference products 20 times are all positive, indicating that the detection sensitivity of the kit to CA16 and EV71 is 400copies / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com