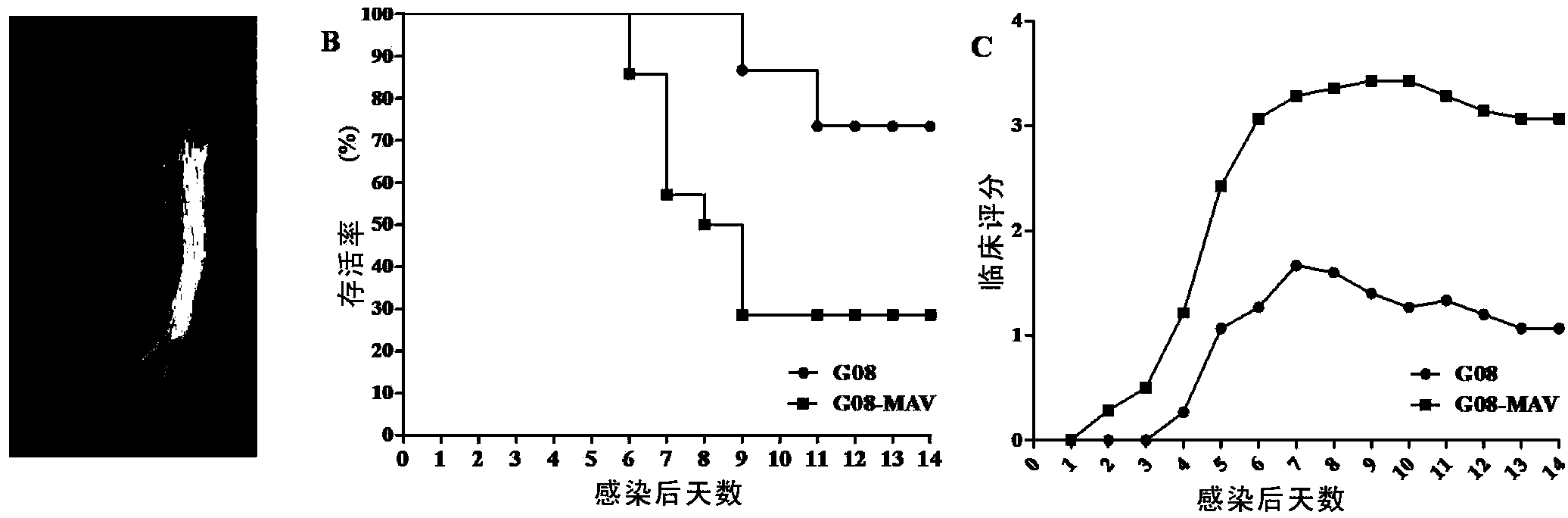
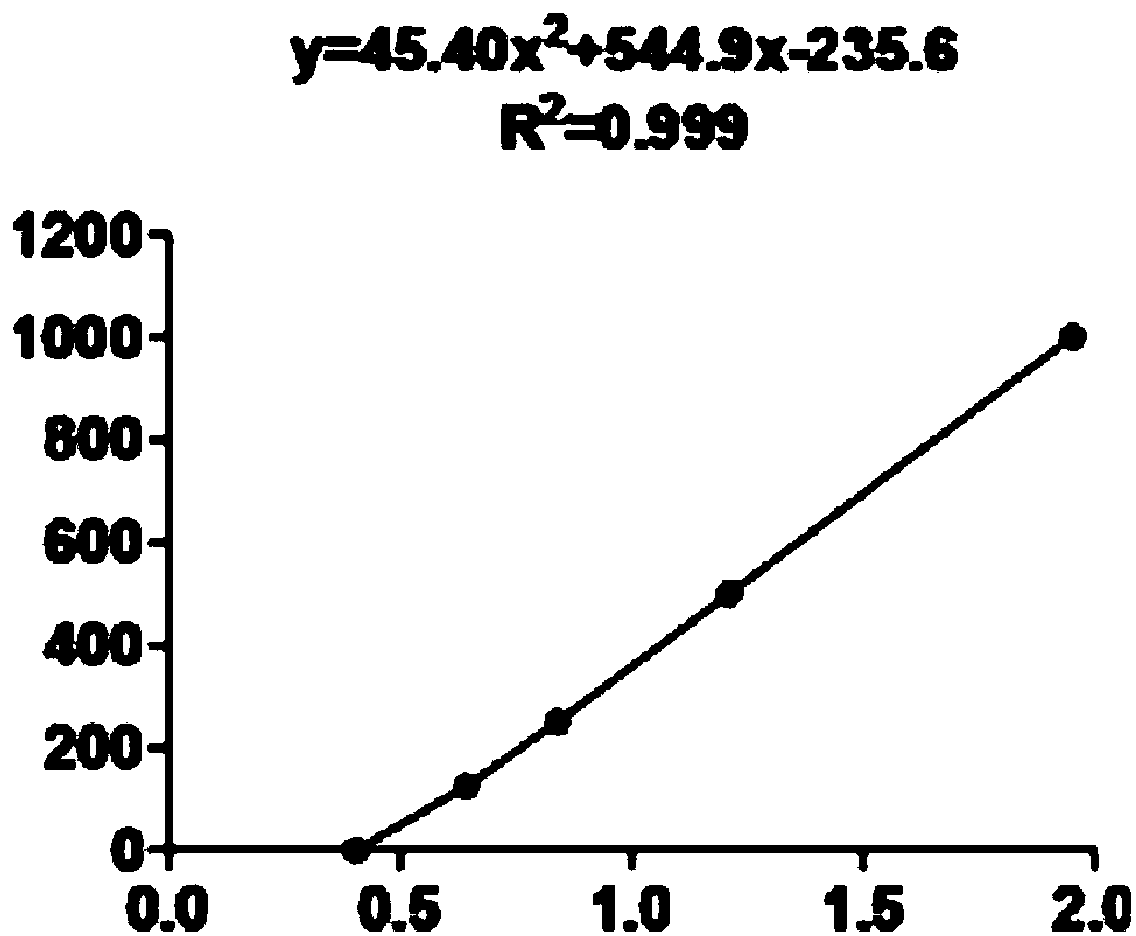
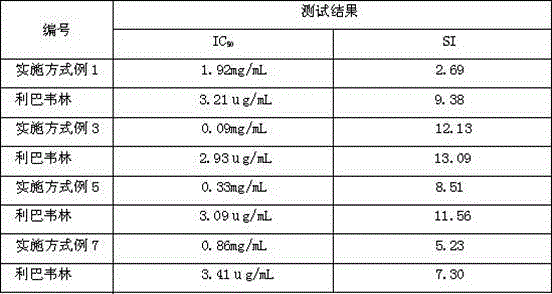
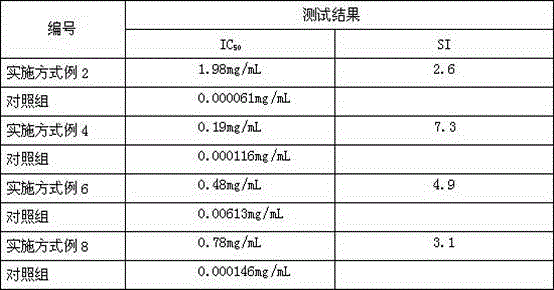
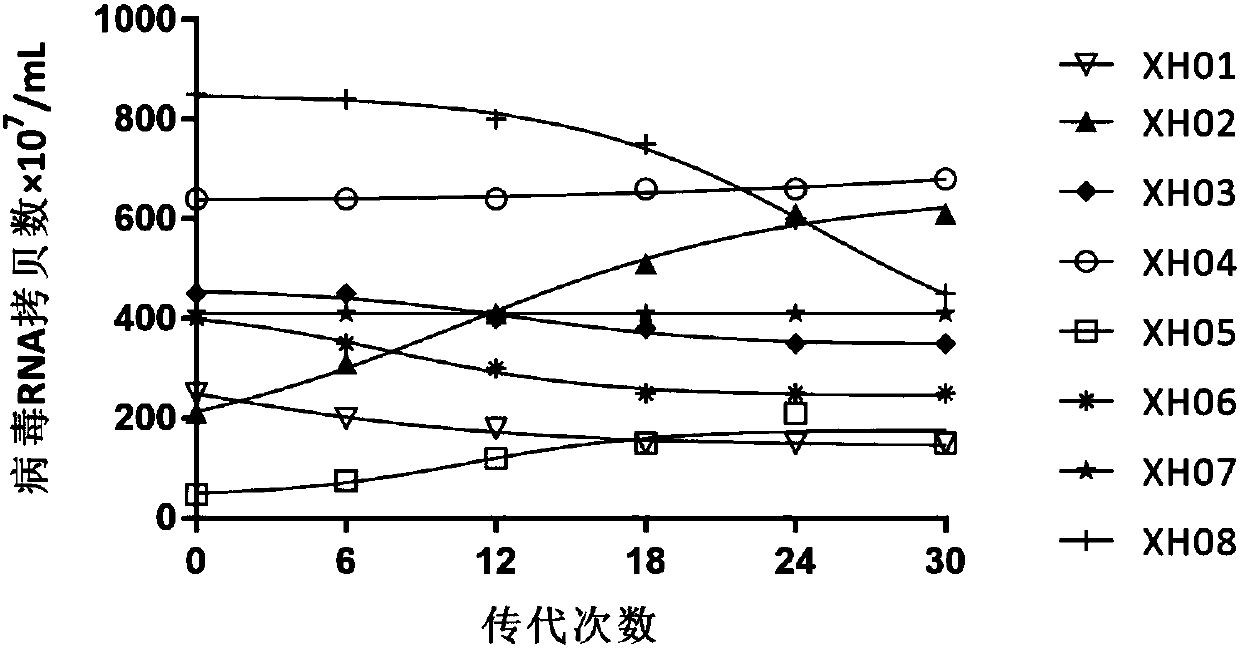
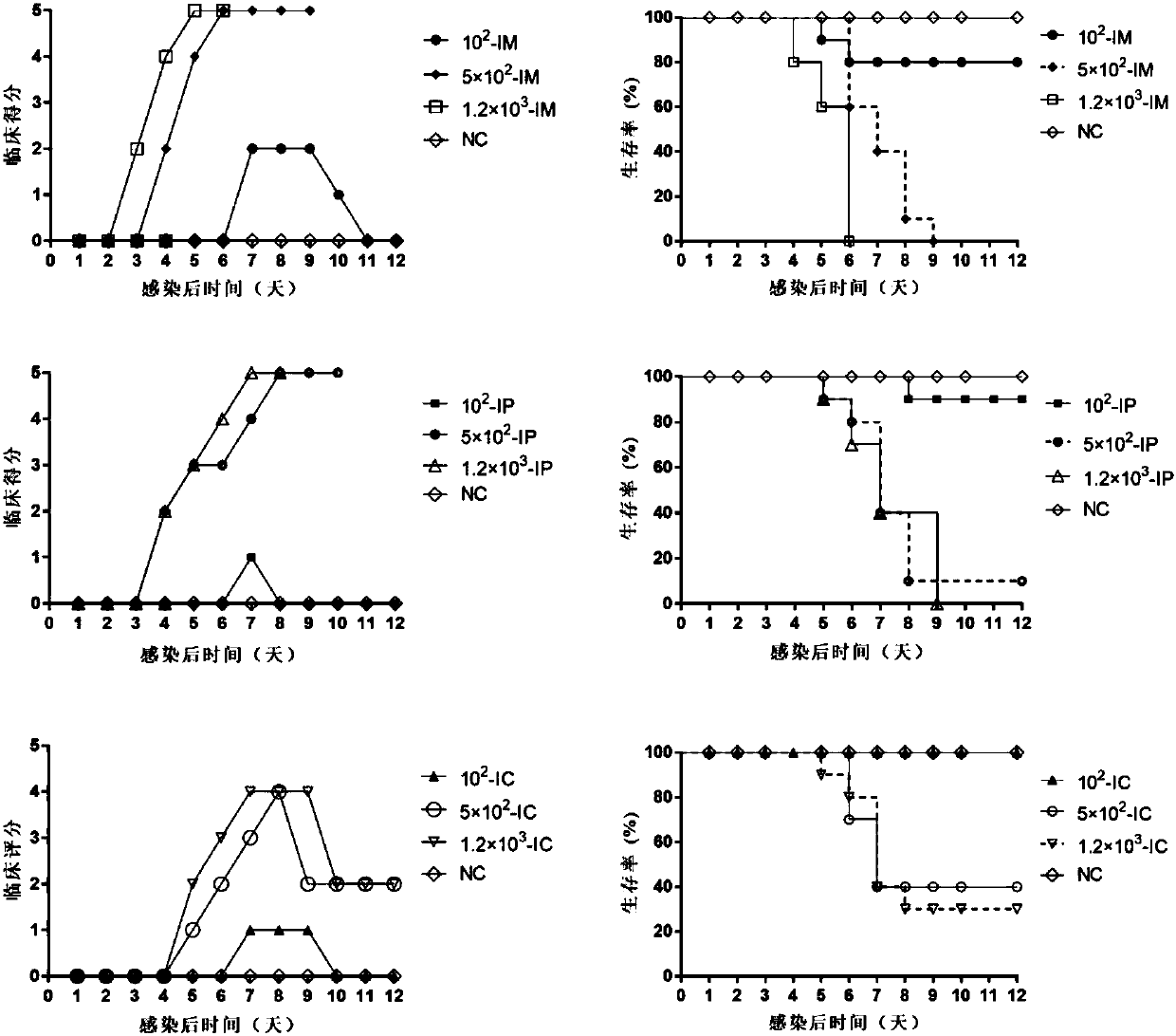
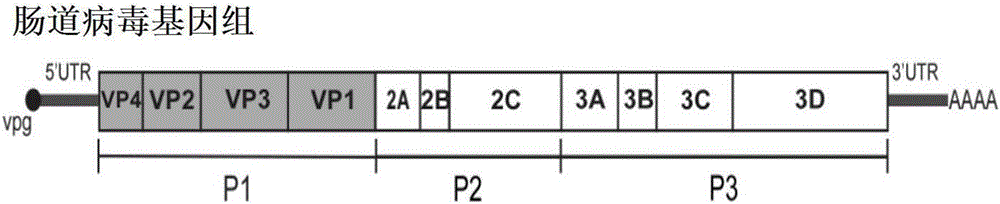
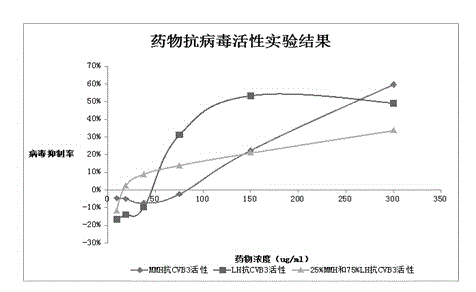
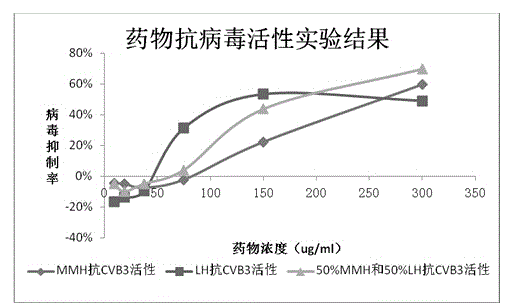
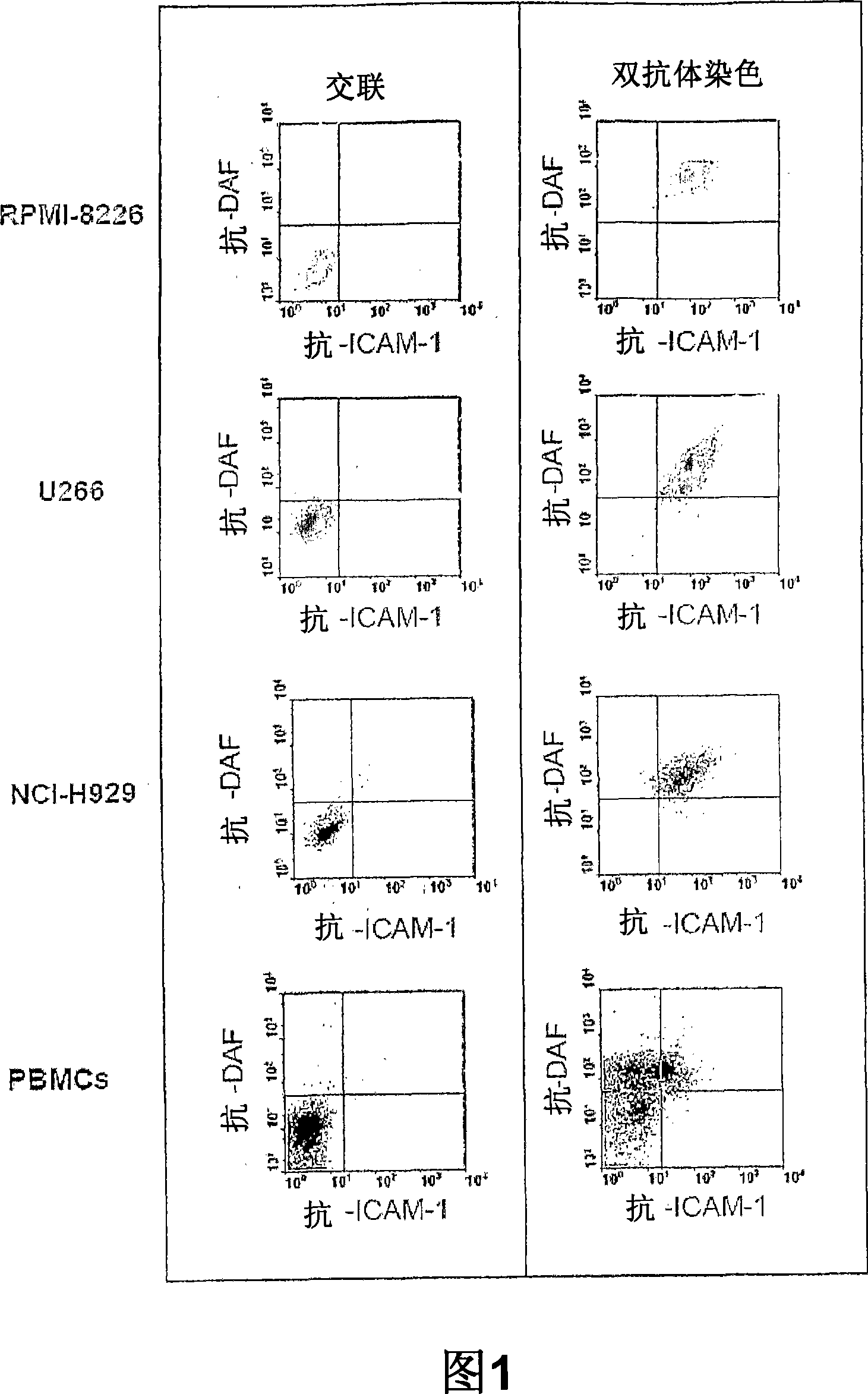
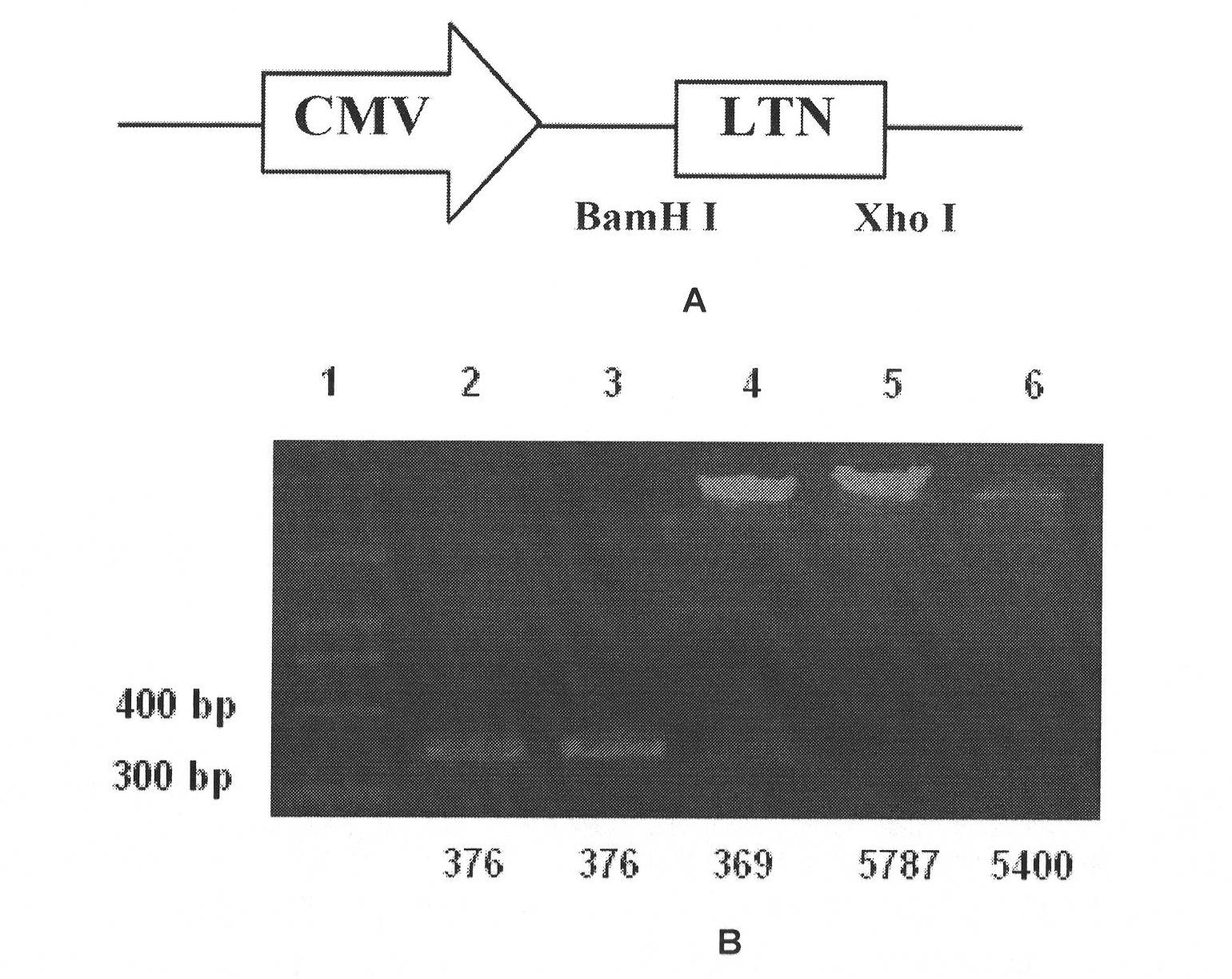
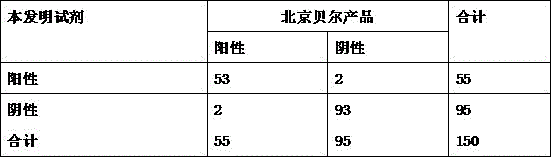
Patents
Literature
82 results about "Coxsackievirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Coxsackievirus is a virus that belongs to a family of nonenveloped, linear, positive-sense single-stranded RNA viruses, Picornaviridae and the genus Enterovirus, which also includes poliovirus and echovirus. Enteroviruses are among the most common and important human pathogens, and ordinarily its members are transmitted by the fecal-oral route. Coxsackieviruses share many characteristics with poliovirus. With control of poliovirus infections in much of the world, more attention has been focused on understanding the nonpolio enteroviruses such as coxsackievirus.
Adenoviral vectors and uses thereof
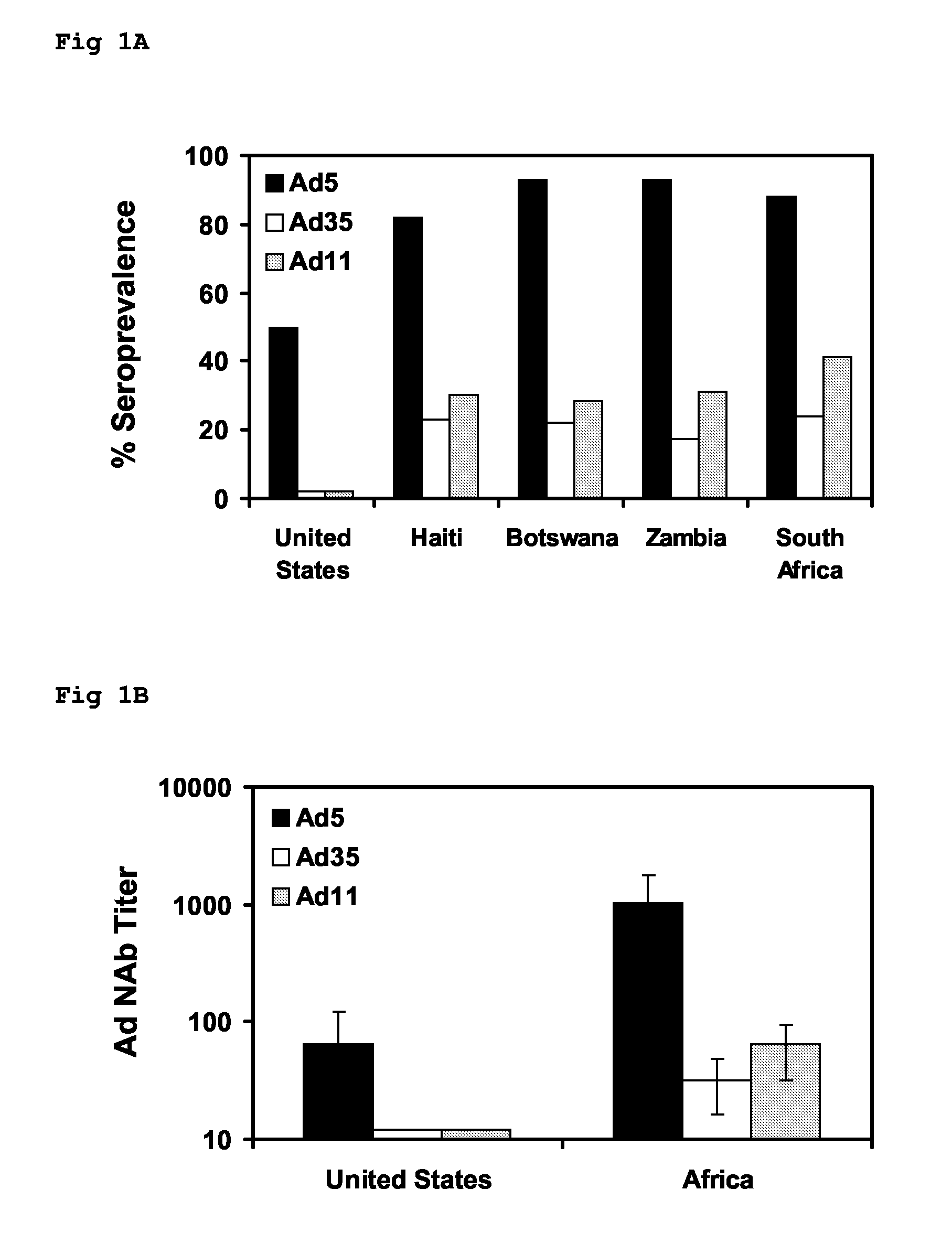
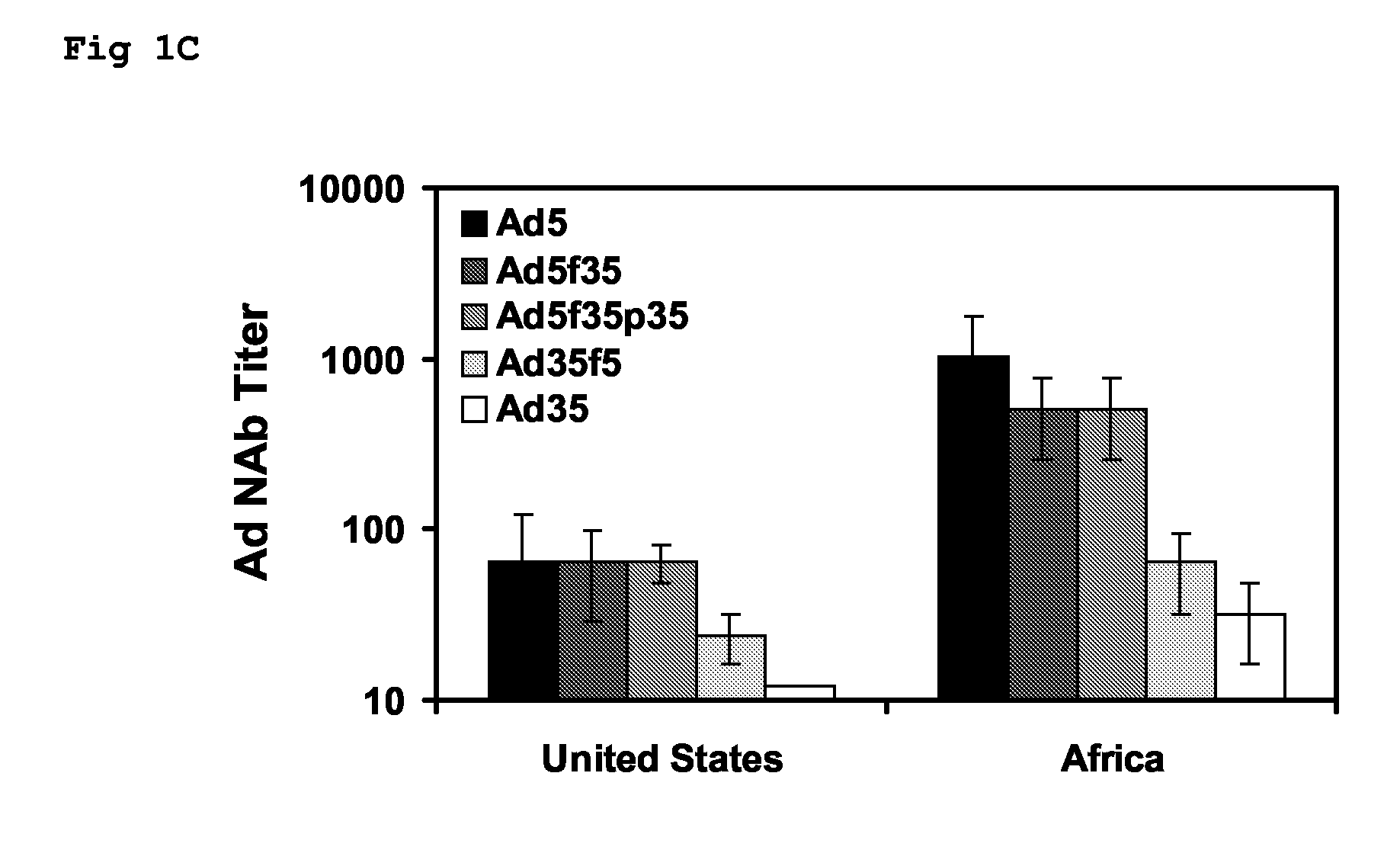
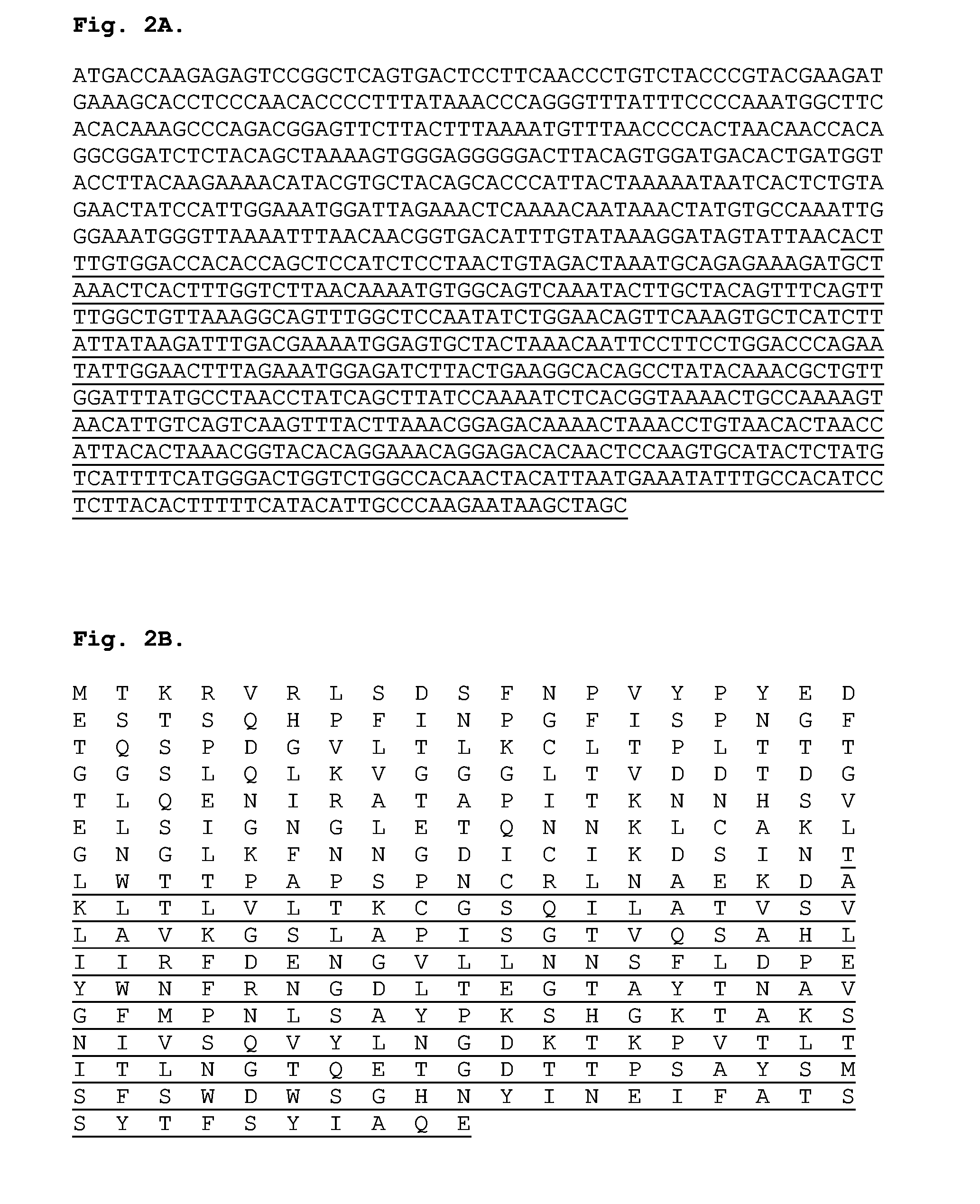
The present invention relates to recombinant adenoviral vectors based on adenoviruses that encounter pre-existing immunity in a minority of the human population and which harbor a chimeric capsid. The chimeric capsid comprises fiber proteins that have at least the knob domain of a human adenovirus that binds to the Coxsackievirus and Adenovirus Receptor (CAR) and a hexon protein from an adenovirus serotype that encounters pre-existing immunity in a low percentage of the human population.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Improved adenoviral vectors and uses thereof
The present invention relates to recombinant adenoviral vectors based on adenoviruses that encounter pre-existing immunity in a minority of the human population and which harbour a chimeric capsid. The chimeric capsid comprises fiber proteins that have at least the knob domain of a human adenovirus that binds to the Coxsackievirus and Adenovirus Receptor (CAR) and a hexon protein from an adenovirus serotype that encounters pre-existing immunity in a low percentage of the human population.
Owner:JANSSEN VACCINES & PREVENTION BV +1
Coxsackie B virus and type 1 diabetes
InactiveUS20100047273A1Slow and delay and progressionSlow and delay symptomSsRNA viruses positive-senseSugar derivativesCOXSACKIE A VIRUSAutoimmune responses
Type 1 diabetes mellitus is characterized by loss of pancreatic insulin-producing beta cells, resulting in insulin deficiency. The usual cause of this beta cell loss is autoimmune destruction. Coxsackie virus has been detected in human pancreatic beta cells and causes insulitis. This non-destructive islet inflammation does not itself cause diabetes, but this disease will occur if viral infection is followed by a separate autoimmune response. The insulitis is mediated mainly by natural killer cells. Islets from coxsackie virus positive samples displayed reduced insulin secretion in response to glucose and other secretagogues. Virus extracted from positive islets was able to infect beta cells from human islets of non-diabetic donors, causing viral inclusions and signs of pyknosis.
Owner:RAPPUOLI RINO +2
Method of using adenoviral vectors with increased immunogenicity in vivo
The invention provides a method of inducing an immune response in a mammal. The method comprises administering to the mammal an adenoviral vector comprising (a) a subgroup C fiber protein wherein a native coxsackievirus and adenovirus receptor (CAR)-binding site is disrupted, (b) a subgroup C penton base protein wherein a native integrin-binding site is disrupted, and (c) a nucleic acid sequence encoding at least one antigen derived from an infectious agent other than an adenovirus which is expressed in the mammal to induce an immune response.
Owner:UNITED STATES OF AMERICA +1
Anti-viral compound
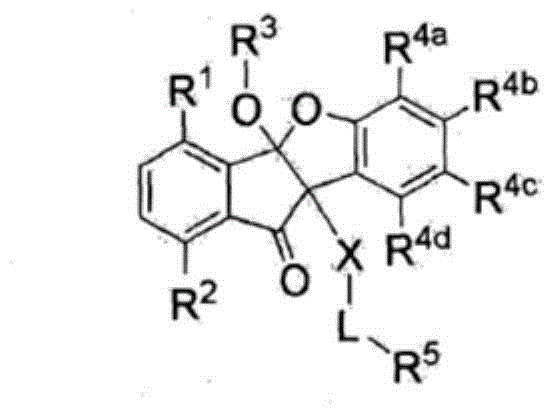
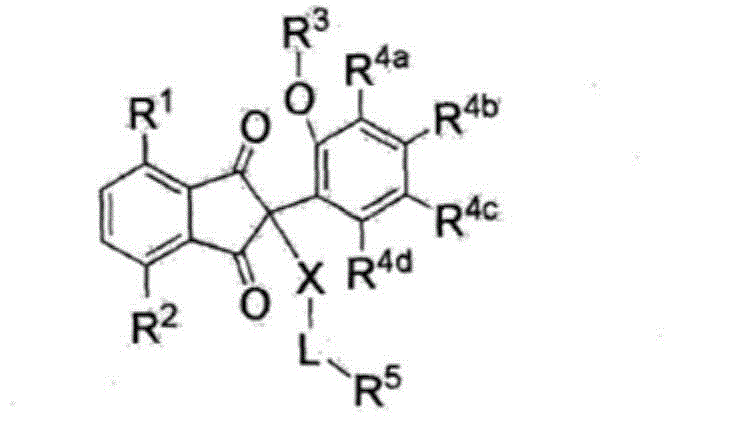
The present invention relates to compounds of Formula (I), which inhibit the growth of picornaviruses, Hepatitus viruses, enteroviruses, cardioviruses, polioviruses, coxsackieviruses of the A and B groups, echo virus and Mengo virus. In said Formula, A is phenyl, pyridyl, substituted phenyl, substituted pyridyl, or benzyl; R is hydrogen, COR4 or COCF; X is N-OH, O or CHR1 R1 is hydrogen, halo, CN, C -C alkyl -C≡ CH, CO(C -C alkyl), CO (C -C alkyl), or CONR2R3 R2 and R3 are independently hydrogen or C -C alkyl; A' is hydrogen, halo, C -C alkyl, benzyl, naphthyl, thienyl, furyl, pyridyl, pyrollyl, COR4 S(O)nR4 or a group of formula (II); R4 is C -C alkyl, phenyl, or substituted phenyl; n is 0,1, or 2; R5 is independently at each occurance hydrogen or halo; m is 1,2,3, or 4; and R6 is hydrogen, halo, CF, OH, CO H, NH, NO, CONHOCH, C -C alkyl, or CO (C -C alkyl), C -C alkoxy; or pharmaceutically acceptable salts thereof.
Owner:ELI LILLY & CO
Coxsackie A16 type virus mutant strain capable of effectively infecting mice
ActiveCN103834618AEffective infectionMicroorganism based processesViruses/bacteriophagesAntiviral drugCoxsackievirus
The invention relates to a Coxsackie A16 type virus mutant strain capable of effectively infecting mice. In the invention, a mouse-adaptive mutant virus strain is unexpectedly obtained through continuous passage of CA16 virus by using the mouse cells and mouse; the mouse-adaptive strain has very strong mouse infection ability and can be used for preparing a mouse model of Coxsackie virus infection so as to study the pathogenic mechanism of the virus, evaluate the antiviral drugs, etc.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Viral particles as immunogens against enterovirus infection and production thereof
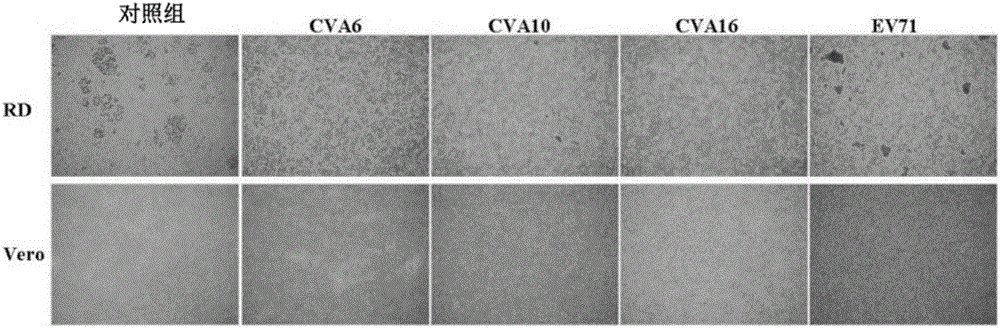
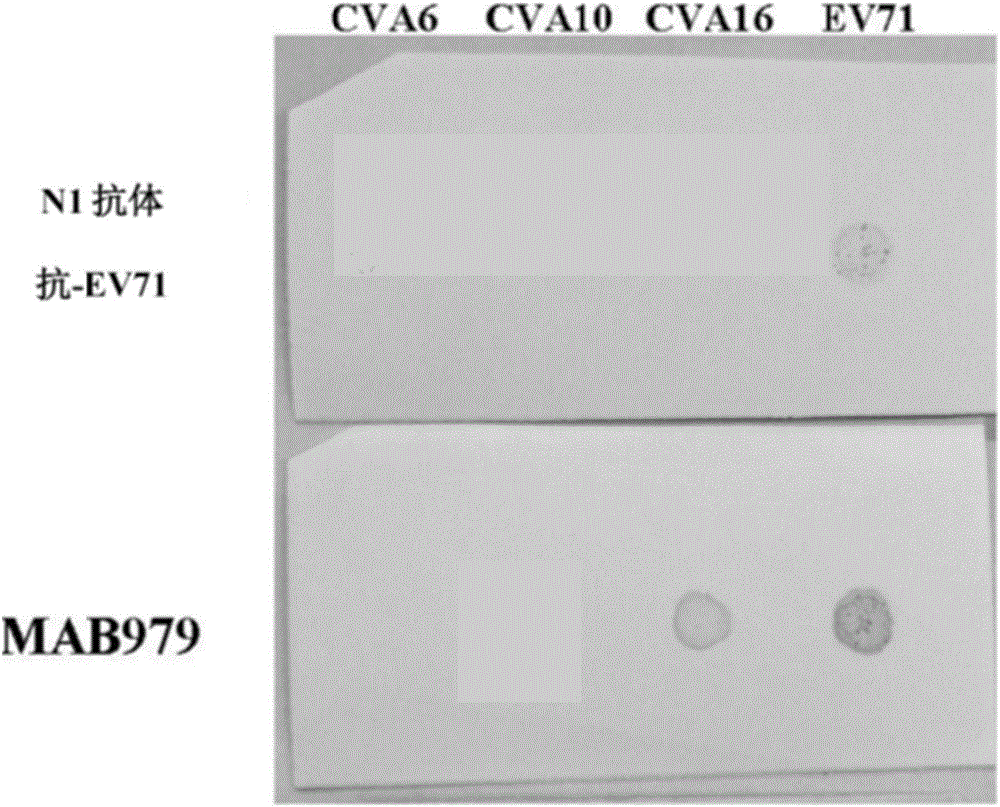
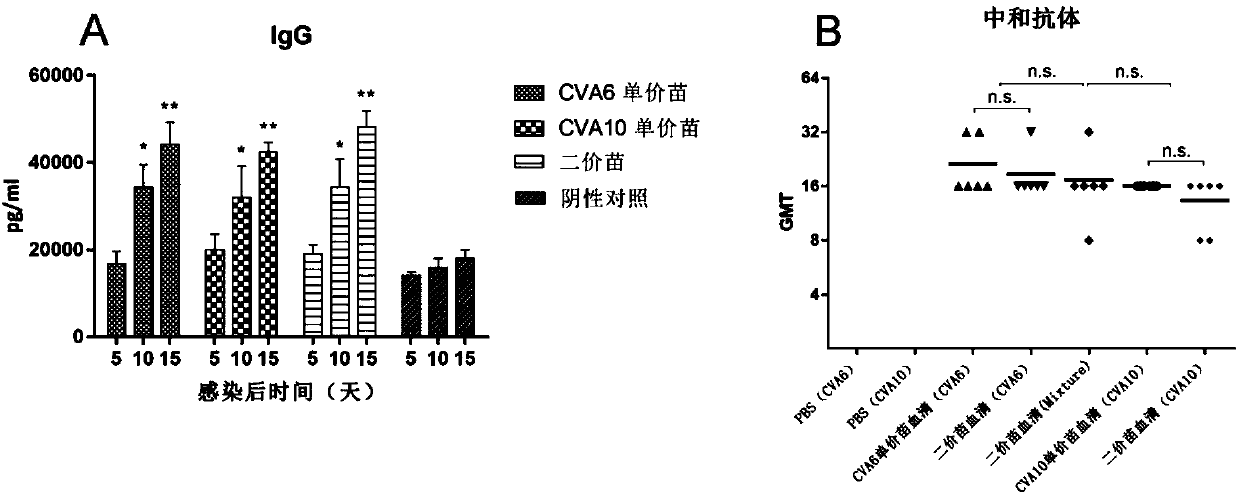
The present invention relates to viral particles as immunogens against enterovirus infection and a method of producing the same. Specifically, the present invention features that human embryo kidney 293 (HEK 293) cells are used to produce viral particles of Enterovirus A, particularly Coxsackievirus A6 (CVA6) particles or Coxsackievirus A10 (CVA10) particles or both and optionally additional viral particles of other Enterovirus A e.g. Coxsackievirus A16 (CVA16) and / or Enterovirus A71 (EV71). The yield of the viral particles in HEK 293 cells is unexpectedly high and effective to induce an immune response against enterovirus infection, especially CVA6 and CVA10. The present invention also relates to an immunogenic composition against enterovirus infection for human use comprising the viral particles as described herein and a method of preventing enterovirus infection or a disease as caused, particularly Hand-Foot-Mouth diseases (HFMD), by administering the immunogenic composition to a subject in need thereof.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH +1
Swine vesicular disease virus and mutant strains and preparation process and use thereof
InactiveUS6200576B1SsRNA viruses positive-senseSugar derivativesSwine vesicular diseaseCoxsackievirus
The present invention relates to a gene of swine vesicular disease virus (SVDV) and the mutant strains of the gene, and the expression plasmids, the preparation process thereof. The invention also relates to a vaccine for use in the prophylaxis of swine vesicular disease composition containing the mutant strains. Furthermore, the invention provides a process for differentiating mutant strains of SVDV from the wild type strain of SVDV, coxsackievirus and foot-and-mouth disease virus by polymerase chain reaction.
Owner:DEV CENT FOR BIOTECHNOLOGY
Medication for anti virus of respiratory tract and application
InactiveCN1687033AEasy to useLow toxicityOrganic active ingredientsOrganic chemistryAnti virusCoxsackievirus
The present invention discloses a medicine with obvious antiviral effect for resisting coxsackie virus, adenovirus Ad7, parafinflueza virus and rhinovirus. Said invention also provides the general formula of said medicine.
Owner:WUHAN UNIV
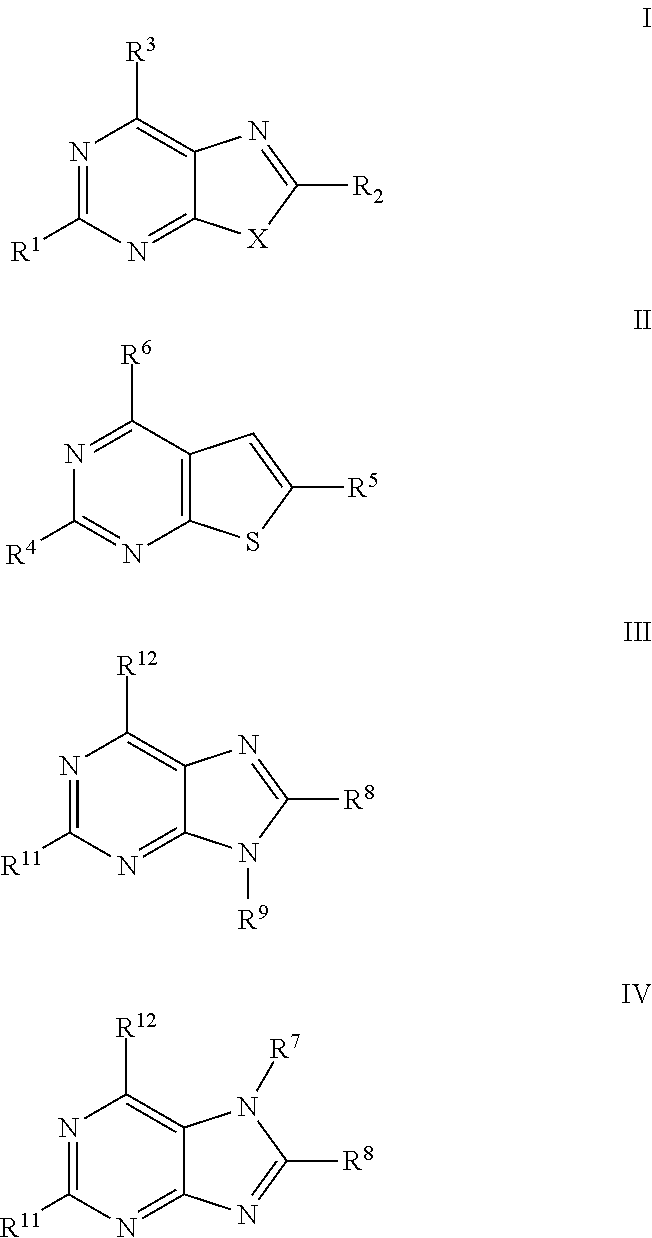
Antiviral Activity of Novel Bicyclic Heterocycles
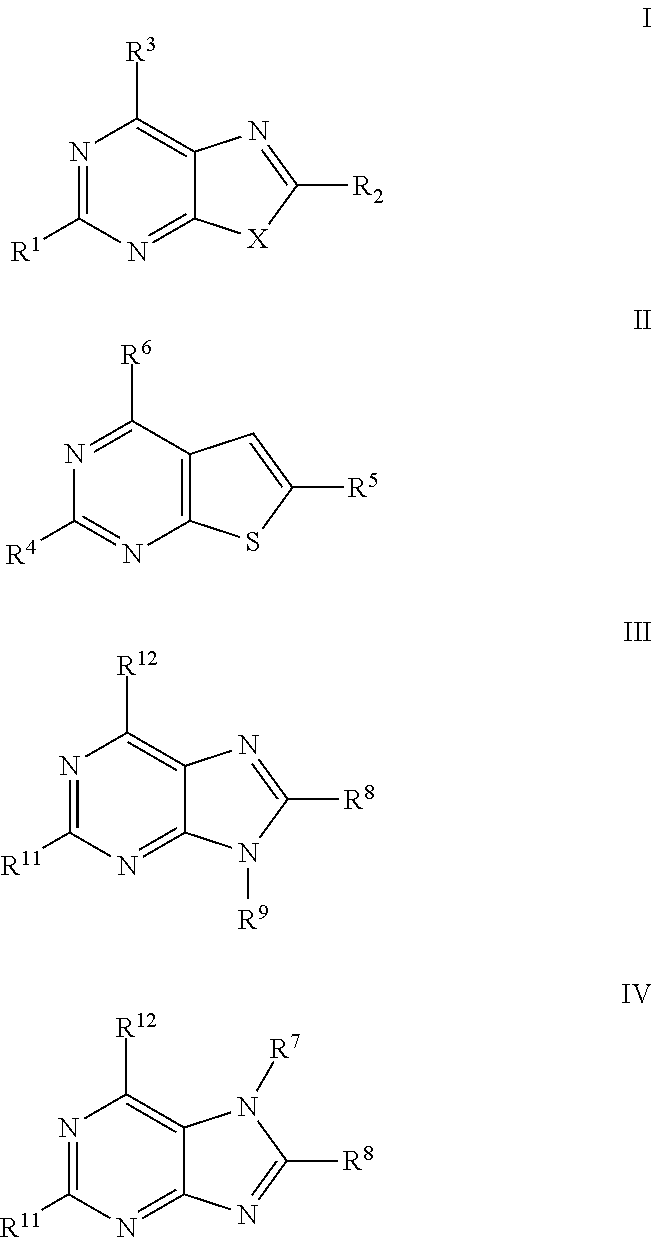
The present invention relates to compound of Formula I, II, III, or IV, and / or a pharmaceutical acceptable addition salt thereof and / or a stereoisomer thereof and / or a solvate thereof, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R11, and R12 are as defined in the claim 1 or as described in detail in the description of the invention, and to the use of said compounds to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections, particularly infections with RNA-viruses belonging to the family of the Retroviridae, the family of the Flaviviridae and the family of the Picornaviridae and more preferably infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus. The present invention also relates to pharmaceutical compositions of said compounds and the use of said pharmaceutical compositions to treat or prevent viral infections. The present invention further relates to the use of said compounds as biologically active ingredients, more specifically as medicaments for the treatment of viral disorders and pathologic conditions such as, but not limited to, viral infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus.
Owner:KATHOLIEKE UNIV LEUVEN
Mucosa M-cell targeted viral myocarditis gene vaccine and preparation method thereof
ActiveCN103341179AValid responseEfficiently induces a responseGenetic material ingredientsAntiviralsWhole bodyViral Myocarditis
The invention discloses a mucosa M-cell targeted viral myocarditis gene vaccine. The vaccine is prepared by compounding a mucosa delivery system capable of targeting mucosa M-cells and B3-type coxsackie virus antigen encoding plasmids through cross-linking copolymerization. The invention further discloses a preparation method of the gene vaccine. The invention further discloses a preparation method of the mucosa delivery system capable of targeting the mucosa M-cells, and the mucosa delivery system is acquired after stable amide-ester bonds are formed by carboxyl groups and amino groups in deacetylated chitosan, wherein the carboxyl groups in peptide fragment CPE30 are targeted by M-cells which are activated by using EDC (Dichloroethane) and NHS (N-Hydroxysuccinimide). By nasally dropping the gene vaccine onto immunized mice, the gene vaccine is proved to be capable of effectively inducing the response between specific antigen serum and mucoantibody, obviously enhancing the local specific T-cell killing ability of the whole body and gastrointestinal mucosa and significantly improving the ability of mice for resisting B3-type coxsackie virus, thereby being an excellent prophylactic vaccine for viral myocarditis.
Owner:SUZHOU UNIV
Traditional Chinese medicine composition for treating viral respiratory tract infection and preparation method thereof
The invention discloses a traditional Chinese medicine composition for treating viral respiratory tract infection and a preparation method thereof. The composition comprises the following raw medicinal materials in parts by weight: 3-20 parts of honey-fried ephedra, 3-20 parts of flos farfarae, 5-25 parts of schizonepeta spike, 5-20 parts of radix asteris, 5-15 parts of apricot kernel, 5-20 parts of roasted perilla seed, 3-15 parts of rhizoma anemarrhenae, 3-15 parts of bulbus fritillariae thunbergii, 5-25 parts of fructus arctii, and 2-15 parts of liquorice. The raw medicinal materials are matched with auxiliary materials and a flavoring agent to be directly or indirectly made into pharmaceutically acceptable capsules, oral liquid, granules, syrup, tablets, effervescent particles, dropping pills, aerosol, spraying agent, gargle, mouthwash and a variety of dosage forms via conventional processes. The traditional Chinese medicine composition can be clinically used for treating or preventing the respiratory tract viral infectious diseases caused by influenza A virus, influenza B virus, rhino virus, coronavirus, respiratory syncytial virus, hand, foot and mouth virus, adenovirus, parainfluenza virus, echovirus, coxsackievirus and the like.
Owner:SHENZHEN TRADITIONAL CHINESE MEDICINE HOSPITAL
Coxsackie virus A10 domestication strain containing virus composition and application of virus composition
InactiveCN107739731AFree from harmProtection against virusesSsRNA viruses positive-senseViral antigen ingredientsDiseaseHEK 293 cells
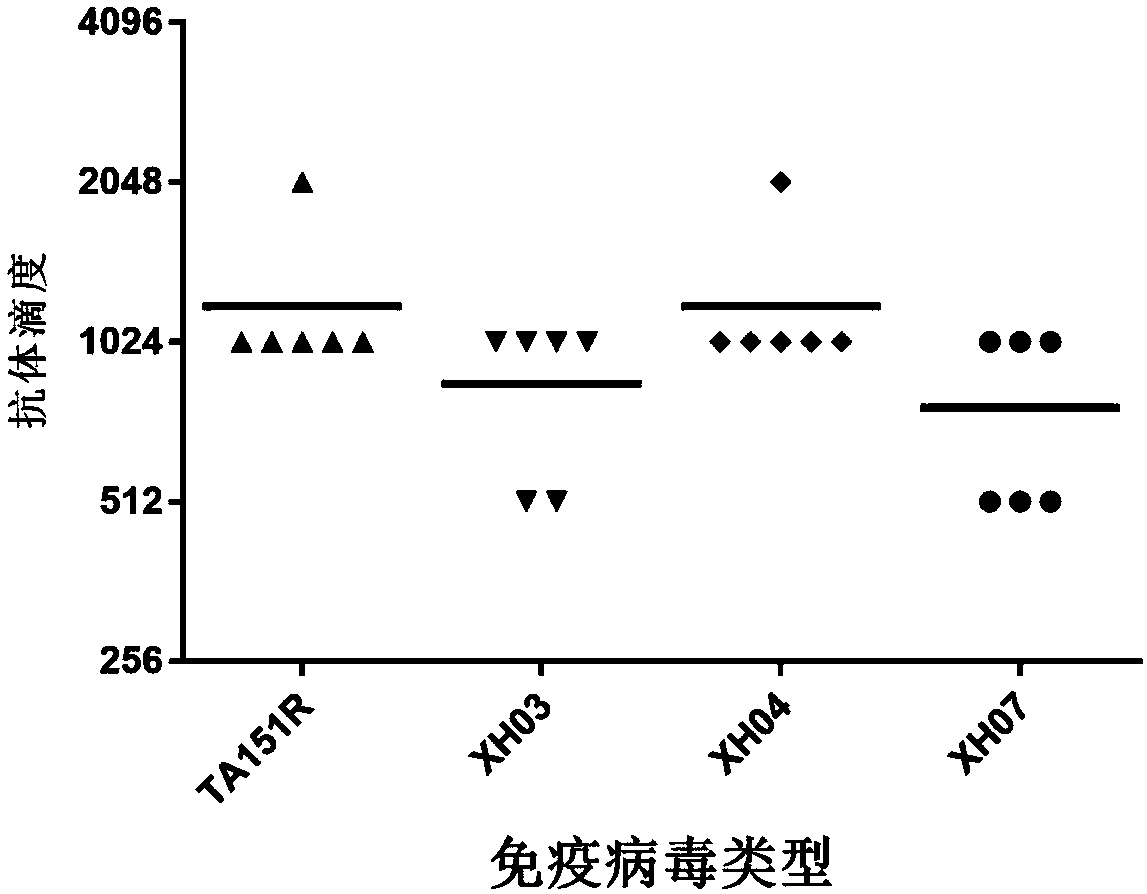
The invention discloses a Coxsackie virus A10 domestication strain TA151R-1 with high titer and stable passage. The virus strain can infect various cell strains including RD cells, HEK 293 cells, Verocells, MRC-5 cells, Hep-2 cells, WI-38 cells and the like and can also be used for preparing a monovalent vaccine or a polyvalent vaccine; the prepared vaccine can protect an organism from damages from Coxsackie virus, can also completely prevent the attacks from other heterologous viruses, can effectively prevent and / or treat diseases caused by infection of the Coxsackie virus and has a wide application prospect.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Building and evaluation of an animal model infected with a coxsackievirus A10 domesticated strain TA151R-1
InactiveCN107744530AImprove replication efficiencyImprove efficiencyCompounds screening/testingViral/bacteriophage medical ingredientsCoxsackievirusPolyvalent Vaccine
A coxsackievirus A10 domesticated strain TA151R-1 high in tilter and stable in passage is disclosed. The virus strain can infect RD cells, HEK293 cells, Vero cells, MRC-5 cells, Hep-2 cells, WI-38 cells, and other cell lines, and can be used for preparing a univalent vaccine or a polyvalent vaccine. The prepared vaccine can protect a body from being harmed by the coxsackievirus, and can completelyprotect the body from attack by heterogenous viruses. An infected animal model can be built efficiently.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Recombinant expression plasmids used for packaging coxsackievirus B5 (CV-B5) pseudovirus, pseudovirus, kit and method
PendingCN106884017ADetection securityQuick checkViruses/bacteriophagesFermentationCoxsackievirusStructural protein
The invention relates to recombinant expression plasmids used for packaging a coxsackievirus B5 (CV-B5) pseudovirus, the pseudovirus, a kit and a method. The recombinant expression plasmids used for packaging the coxsackievirus B5 pseudovirus are respectively named as the pEGFP-CV-B5 (417) plasmid and the pCVB3-replicon, the CV-B5 structural protein expressed by the pEGFP-CV-B5 (417) plasmid can be used for packaging CV-B3 subgenome RNA transcribed by the pCVB3-replicon in the cell, thus the CV-B5 pseudovirus is generated, the pseudovirus can be used for detecting the neutralizing antibody, and since the pseudovirus with single-cycle infection is adopted, the safety problem caused when the live virus is used is avoided. After a plurality of experiments, the result shows that the invention provides the method for detecting the CV-B5 neutralizing antibody which is safe, sensitive, rapid, specific, simple and convenient, and is low in cost. Based on the abovementioned features, the method is particularly suitable for the experiment for rapidly detecting the neutralizing antibody in large scale, and thus the method has the significant application value in developing viral vaccines and detecting the level of the CV-B5 specific neutralizing antibody of individual and group patients.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Nucleic acid composition for detecting four enteroviruses simultaneously and kit and detection method
InactiveCN109504805AMicrobiological testing/measurementMicroorganism based processesCoxsackievirusMicrobiology
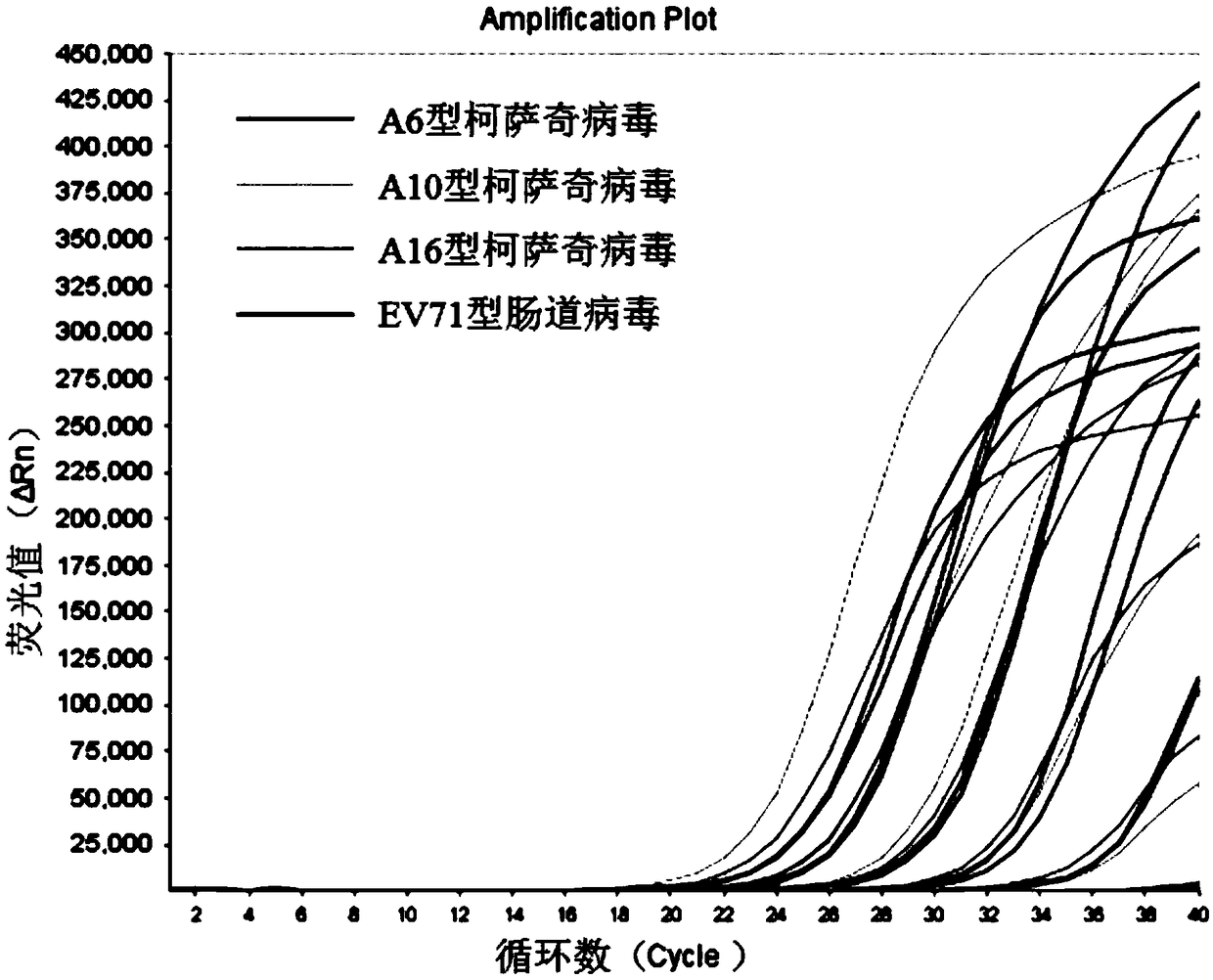
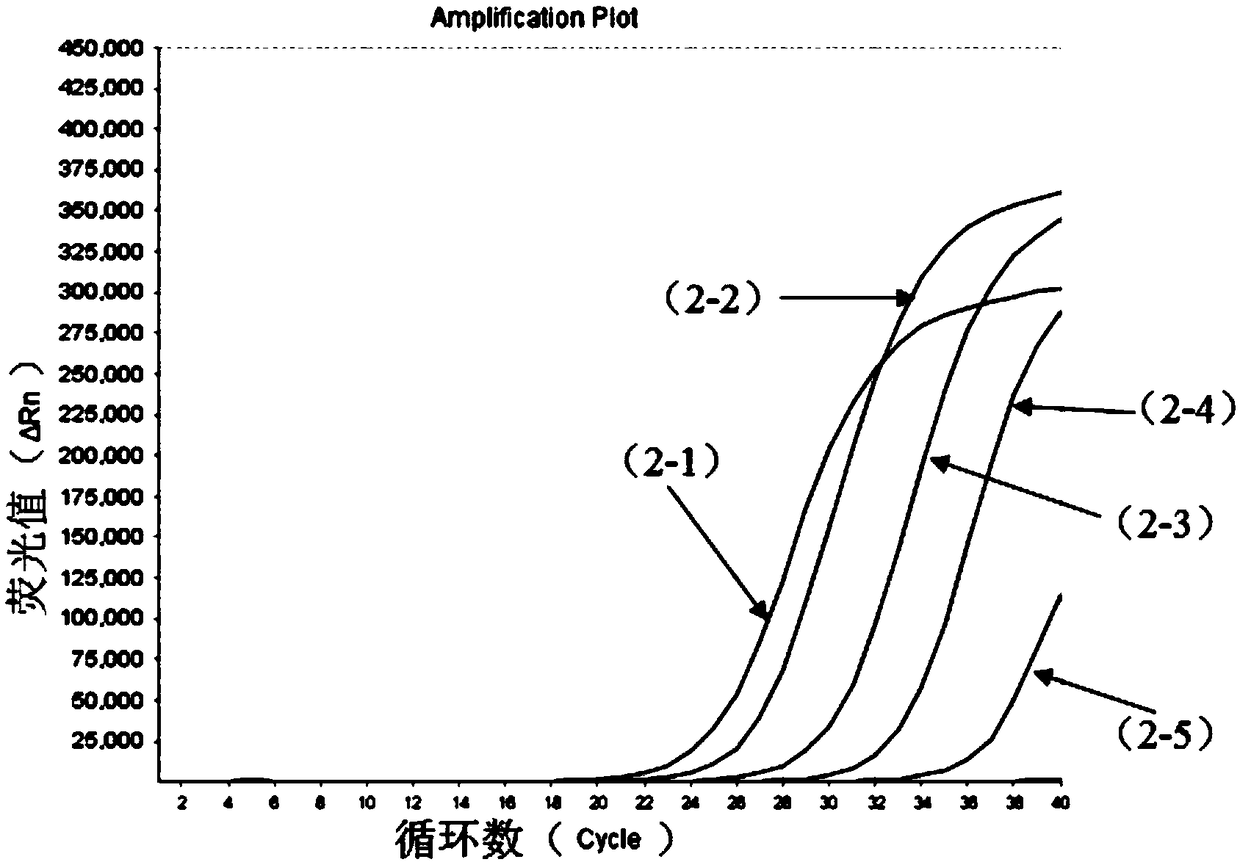
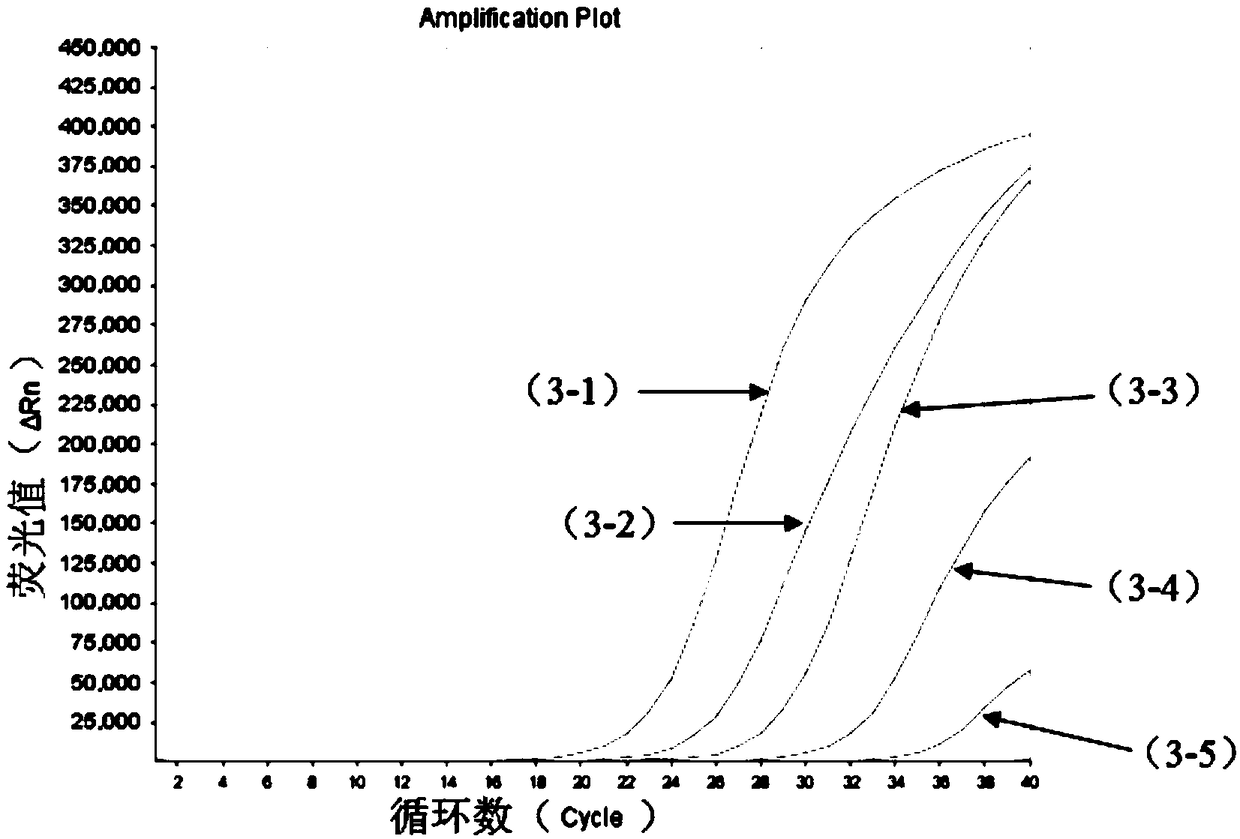
The invention relates to a nucleic acid composition for detecting four enteroviruses simultaneously and a kit and a detection method. The nucleic acid composition comprises type A6 coxsackievirus amplification primer pairs as shown in SEQ ID No.1 and SEQ ID No.2, type A10 coxsackievirus amplification primer pairs as shown in SEQ ID No.3 and SEQ ID No.4, type A16 coxsackievirus amplification primerpairs as shown in SEQ ID No.5 and SEQ ID No.6 and type EV71 enterovirus amplification primer pairs as shown in SEQ ID No.7 and SEQ ID No.8. The nucleic acid composition can detect type A6, type A10 and type A16 coxsackieviruses and type EV71 enterovirus simultaneously.
Owner:深圳市艾伟迪生物科技有限公司
Drug for resisting Coxsackie virus and preparing method and application thereof
The invention discloses a drug for resisting the Coxsackie virus and the preparing method and application thereof. The drug can resist the Coxsackie virus, has a remarkable effect, and is free of toxic and side effects, easy to produce and broad in market development prospect. Specifically, the drug combination for resisting the Coxsackie virus has a synergistic interaction effect after optimization, and the antiviral effect is far better than that of single callicarpa nudiflora or horsetail beefwood.
Owner:海南制药厂有限公司
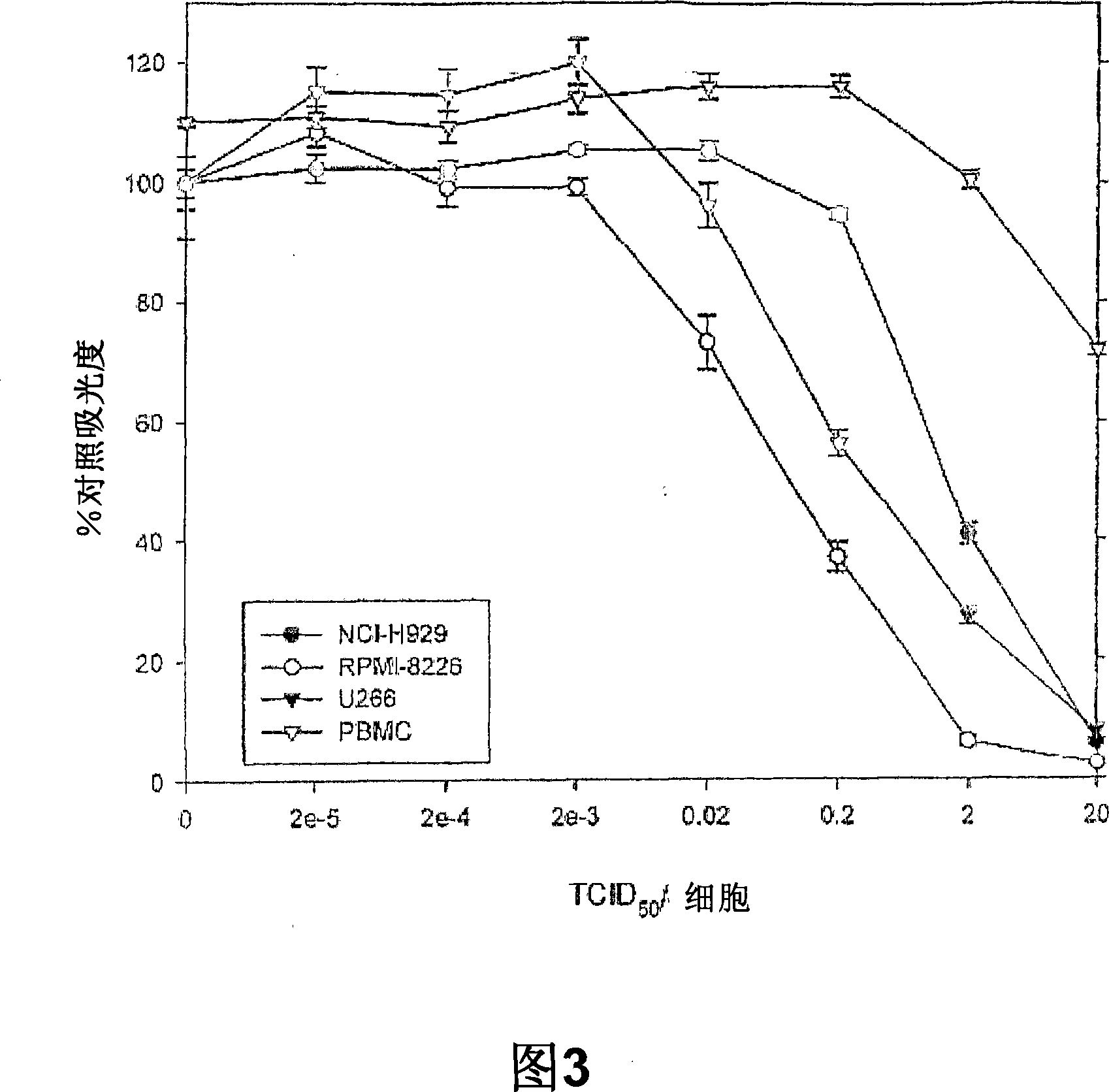
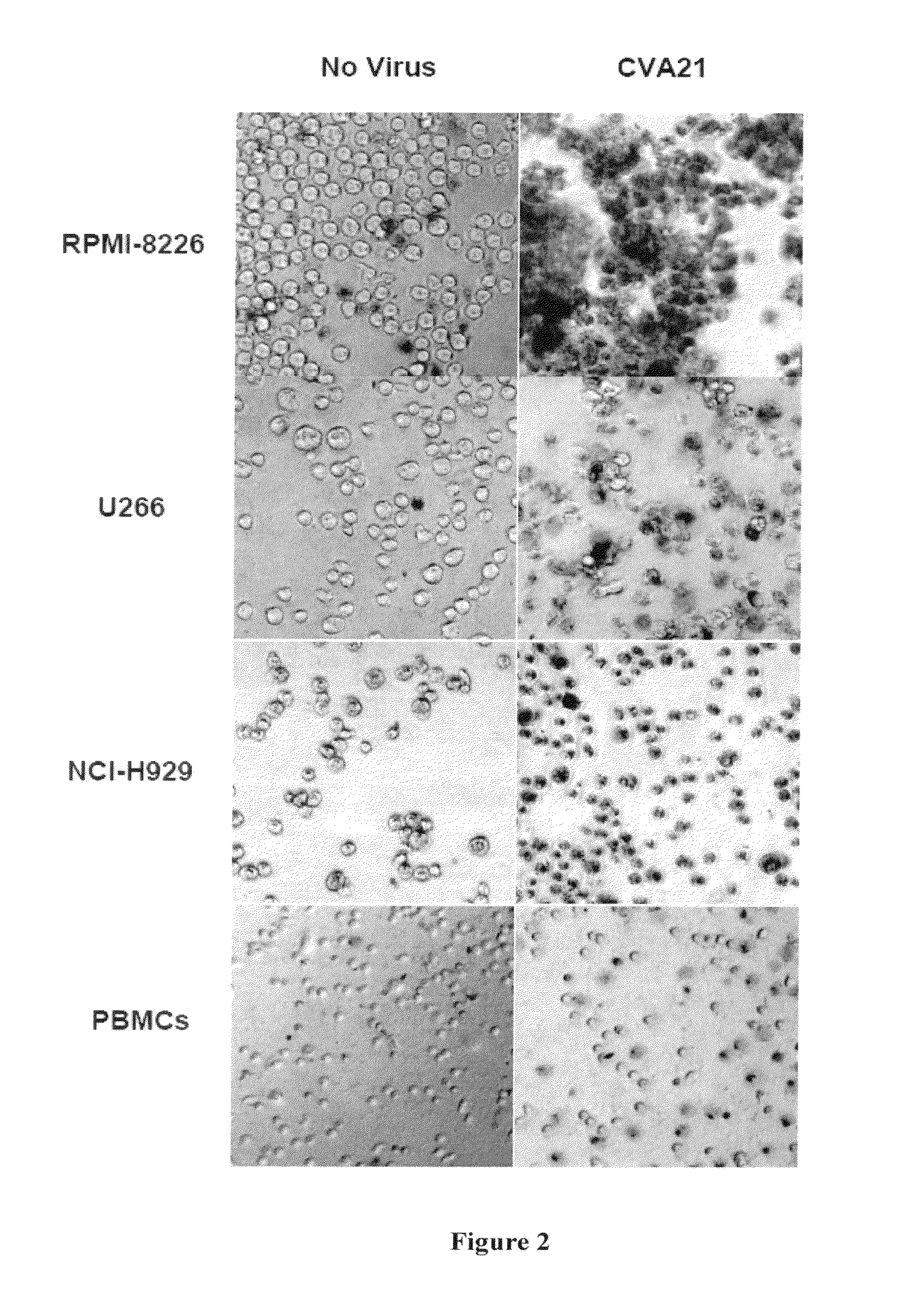
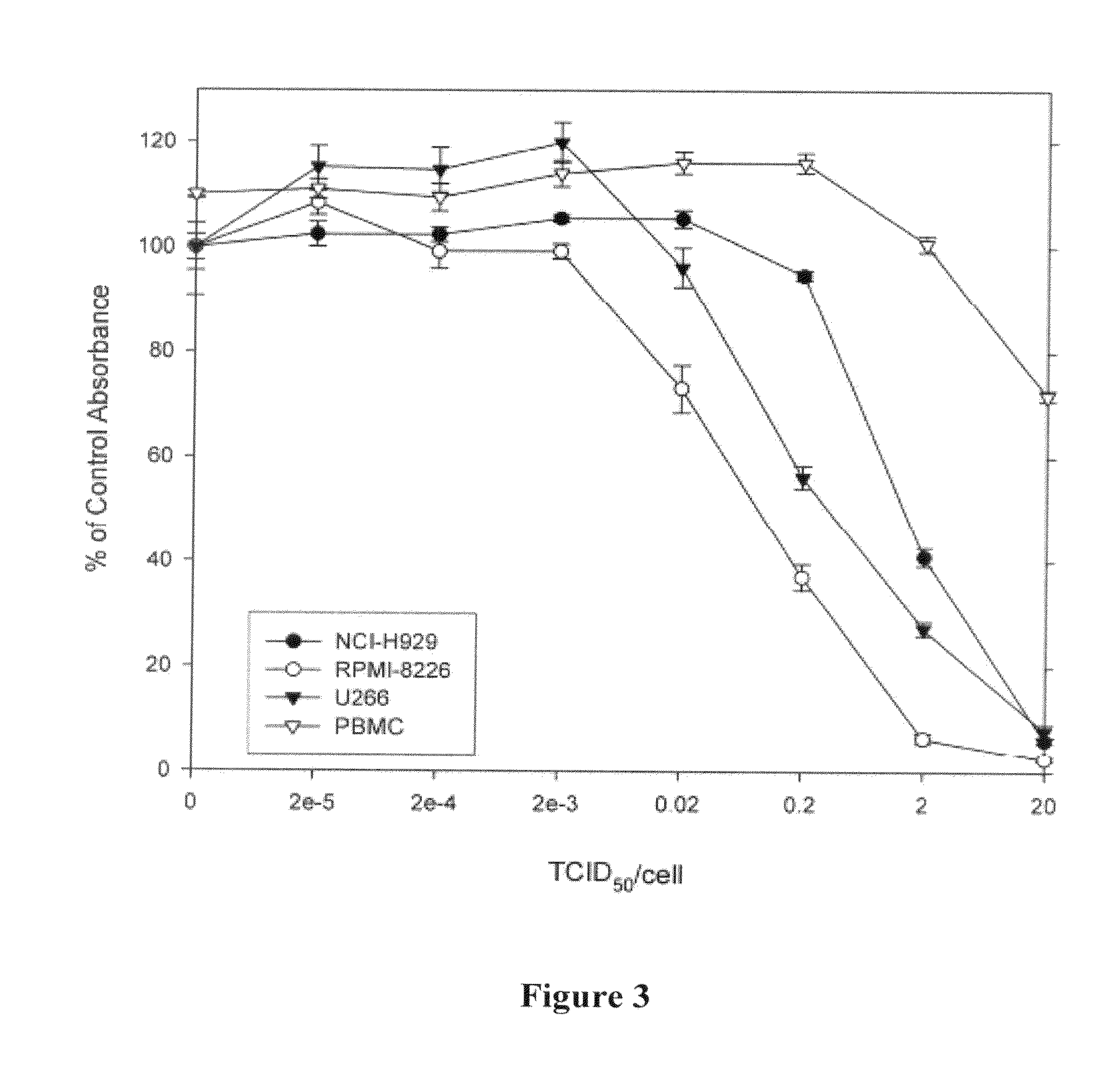
Methods and compositions for treatment of hematologic cancers
The present invention relates to oncolytic picornaviruses and methods and compositions for treating subjects having hematologic cancers. These include methods and compositions for treatment of myeloma, using disclosed Picornavirus such as Coxsackievirus, in methods of direct or indirect administration to subjects and ex vivo purging of malignant cells within auto grafts prior to transplantation.
Owner:MERCK & CO INC
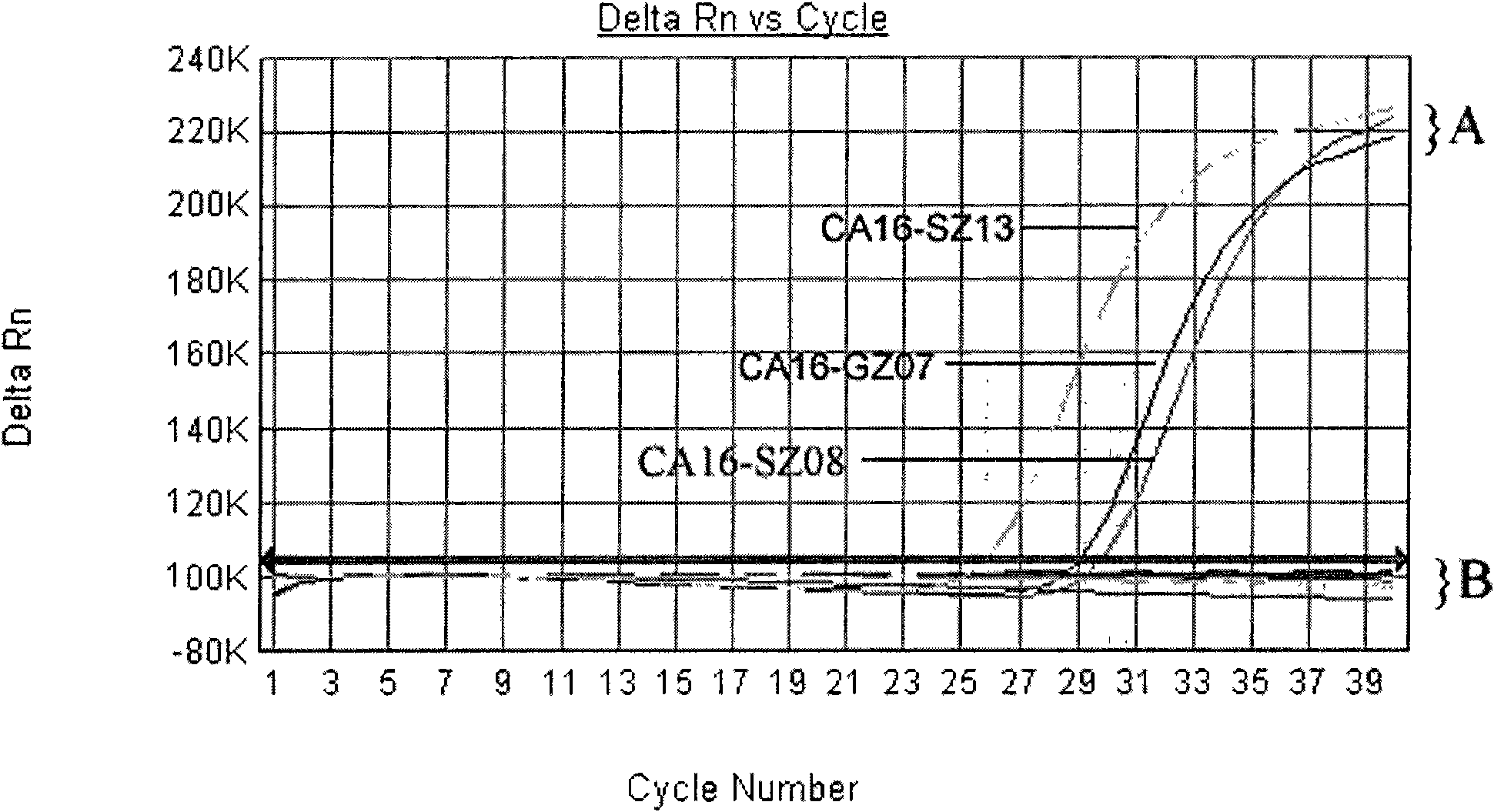
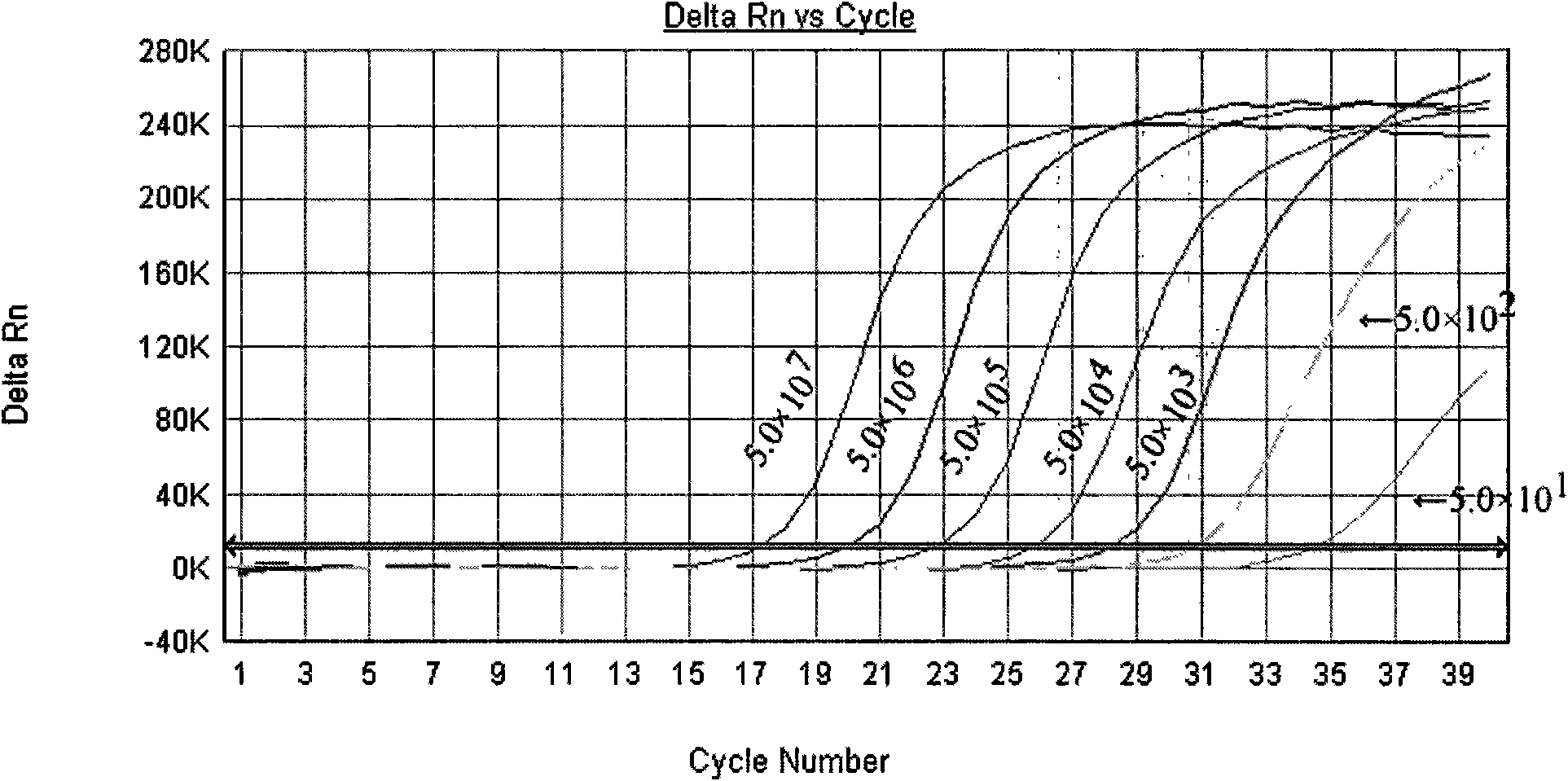
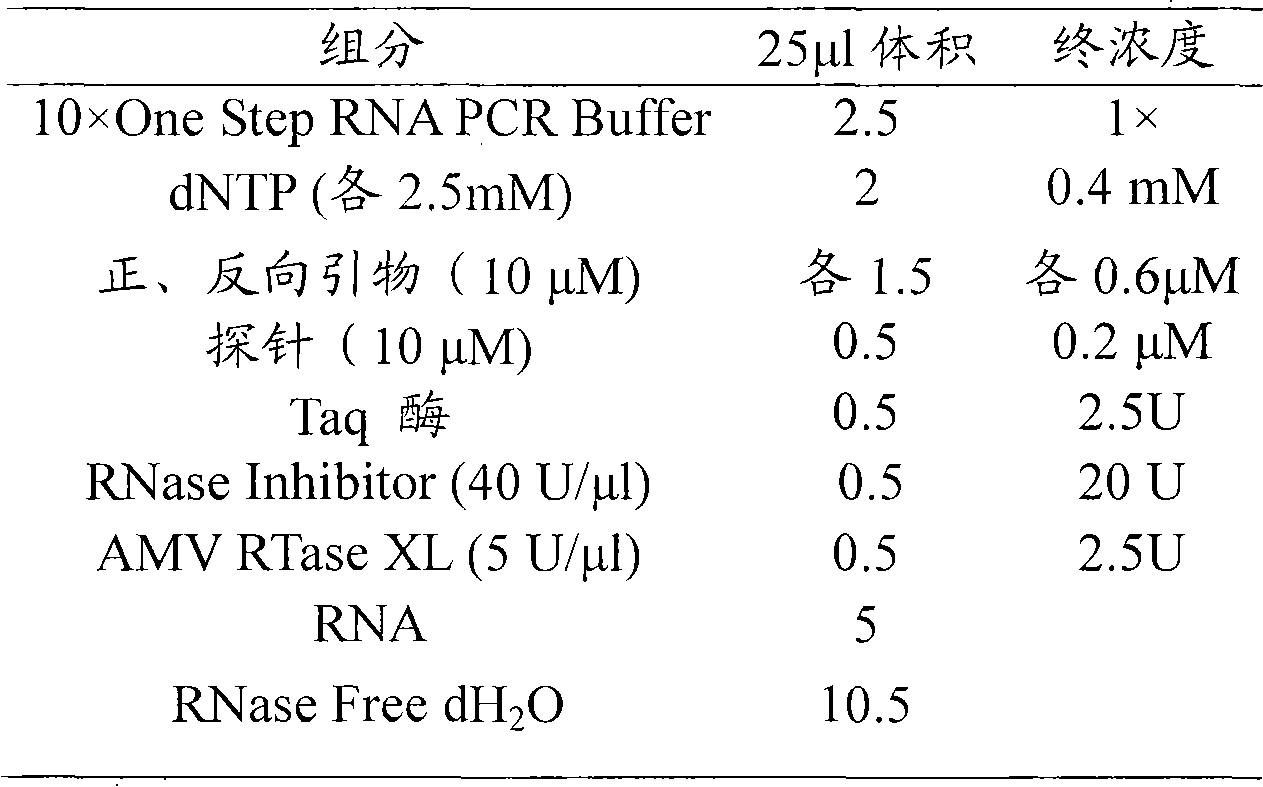
Primer for coxsackie virus A16 nucleic acid detection, probe and kit
InactiveCN101676406AHigh sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationForward primerCoxsackievirus a16
The invention relates to a primer for coxsackie virus A16 nucleic acid detection, probe and kit, wherein the nucleotide sequence of the primer is that: forward primer 5'-GAACCATCACTCCACACAGGAG-3'; backward primer 5'-GTACCCGTGGTGGGCATTG-3' and the nucleotide sequence of the probe is 5'-CAGCCATTGGGAATTTCTTTAGCCGTG-3'. The invention also provides a method for detecting coxsackie virus A16 nucleic acid. Sample RNA is used as template and the above primer and probe are subjected to real-time fluorescence RT-PCR amplification and the result is predicated based on the amplification curve after each circulation. The primer specificity is good, the detection method is quick and simple, the accuracy and the sensitivity is high and the invention provides scientific reference for etiologic diagnosis and differential diagnosis of hand-foot-mouth disease and relative diseases.
Owner:何雅青 +1
Fusion protein and fusion protein expression vector thereof
ActiveCN102816240AImprove bindingAccelerated phagocytosisFungiPeptide/protein ingredientsFusion Protein ExpressionCoxsackie meningitis
The invention provides a fusion protein and a fusion protein expression vector thereof. The fusion protein comprises human-derived Coxsackie virus-adenovirus receptor extracellular region and Fc fragment of human IgG1. The gene sequence of the fusion protein is subjected to codon optimization. The fusion protein expression vector comprises an optimized gene sequence of the fusion protein, and a pPIC3.5K plasmid gene sequence. The fusion protein can be combined with high affinity with Coxsackie virus / adenovirus, and can prevent the Coxsackie virus / adenovirus from infecting body cells especially cells expressing CAR, such as myocardial cells. The fusion protein provided by the invention can be used in treatments of Coxsackie virus and / or adenovirus infectious diseases.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Primer and kit for detecting coxsackievirus A6 type RT-LAMP (Reverse Transcription Loop-mediated Isothermal Amplification) nucleic acid
InactiveCN102816870ALow costStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceHand-foot-and-mouth diseaseSocial benefits
The invention relates to the application field of biological detection technologies, and in particular relates to a primer pair and a kit for coxsackievirus A6 type RT-LAMP (Reverse Transcription Loop-mediated Isothermal Amplification) nucleic acid detection. The primer pair comprises two outer primers F3 and B3, two inner primers FIP and BIP and two loop primers LF and LB; and the kit comprises the primer pair. The CA6RT-LAMP has the characteristics of strong specificity, high sensitivity, simplicity in operation methods, quick detection, easiness in result judgment, low cost and the like, can better satisfy the requirement of on-site quick detection, is easy to popularize and apply in grassroots units, can be used for early rapid diagnosis of epidemic outbreaks, such as hand-foot-and-mouth diseases and the like and clinical cases in disease prevention and control organizations, hospitals, kindergarten units, and has broad market prospect and enormous economic and social benefits.
Owner:何雅青 +2
Mucosal adjuvant and its preparation method and use
InactiveCN102688488ASafe and non-toxicReduce releaseAntibacterial agentsGenetic material ingredientsNasal cavityVaccination
The invention provides a mucosal adjuvant. The mucosal adjuvant is prepared by copolymerization crosslinking compounding of oligo-chitosan and lymphotactin encoding plasmids. The invention also discloses a preparation method and a use of the mucosal adjuvant. When the mucosal adjuvant and a gene vaccine composed of chitosan or oligo-chitosan are synchronously dropped into a nasal cavity for immunization, coxsackievirus specific serum antibody and systemic (spleen and lymph glands) Th1-type immune responses are induced and especially, intestinal mucosa partially-reinforced specific-secretion-type sIgA and IFNgama+Th1 responses are induced, and thus a gene vaccine used with the mucosal adjuvant is obviously superior to an exposed gene vaccine and effectively prevents coxsackievirus-caused myocarditis. The mucosal adjuvant can be used as a novel mucosal vaccination adjuvant.
Owner:FUDAN UNIV
Preparation method of coxsackievirus antigen and rapid detection kit prepared by utilizing antigen and used for detecting coxsackievirus antibody
InactiveCN106046124AHigh antigen yieldImproving immunogenicitySsRNA viruses positive-senseVirus peptidesEscherichia coliInclusion bodies
The invention relates to an artificial genetic engineering-expressed coxsackievirus antigen, a method for preparing the antigen, a method for rapidly detecting a coxsackievirus antibody and a rapid detection kit used for detecting the coxsackievirus antibody. The method for preparing the antigen comprises the following steps: artificially synthesizing optimized coxsackievirus VP1 protein antigen gene sequences, constructing a prokaryotic expression vector and an escherichia coli-expressed coxsackievirus VP1 protein antigen, and renaturing an inclusion body by adopting a dialysis method, a gradient dilution method and a gel chromatography to obtain a recombinant coxsackievirus VP1 protein antigen with a three-dimensional structure and immunocompetence. The method for rapidly detecting the coxsackievirus antibody comprises the step of applying the coxsackievirus VP1 protein antigen. The rapid detection kit used for detecting the coxsackievirus antibody comprises the coxsackievirus VP1 protein antigen which can be directly used for whole blood detection. The kit comprises a rheumatoid factor processing pad which can remove rheumatoid factors in a sample and directly detect IgM in the sample. The coxsackievirus antigen provided by the invention has high specificity. The invention provides the method for preparing the antigen, the method for rapidly detecting the coxsackievirus antibody and the kit for rapidly detecting the coxsackievirus antibody.
Owner:LANZHOU YAHUA BIOTECH
Anti-respiratory virus medicine and use
InactiveCN101084892AEasy to useLow toxicityOrganic active ingredientsAntiviralsCOXSACKIE A VIRUSChemical compound
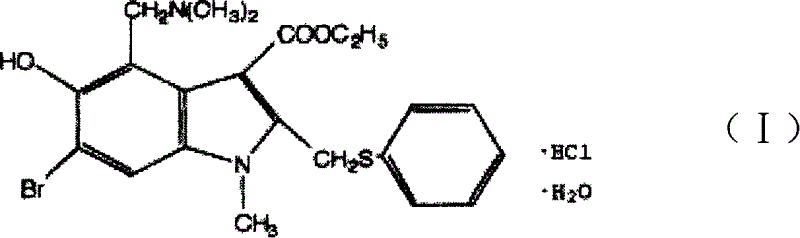
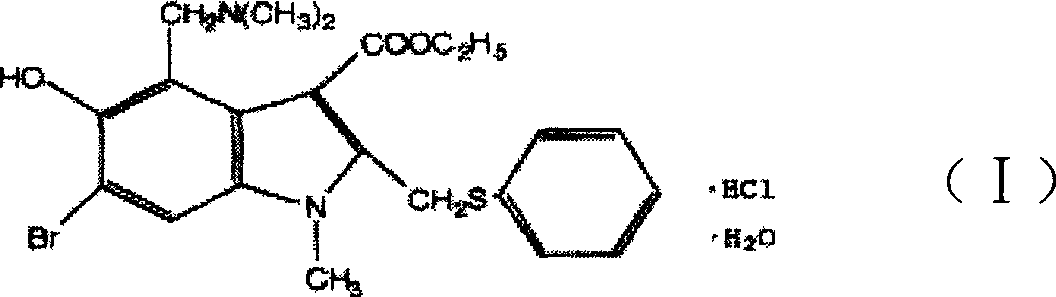
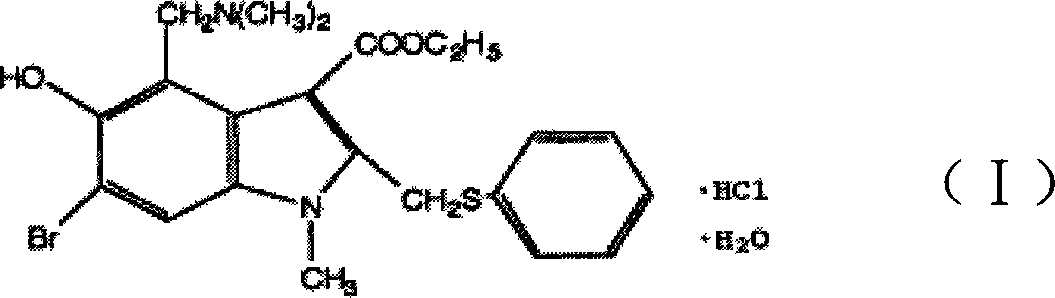
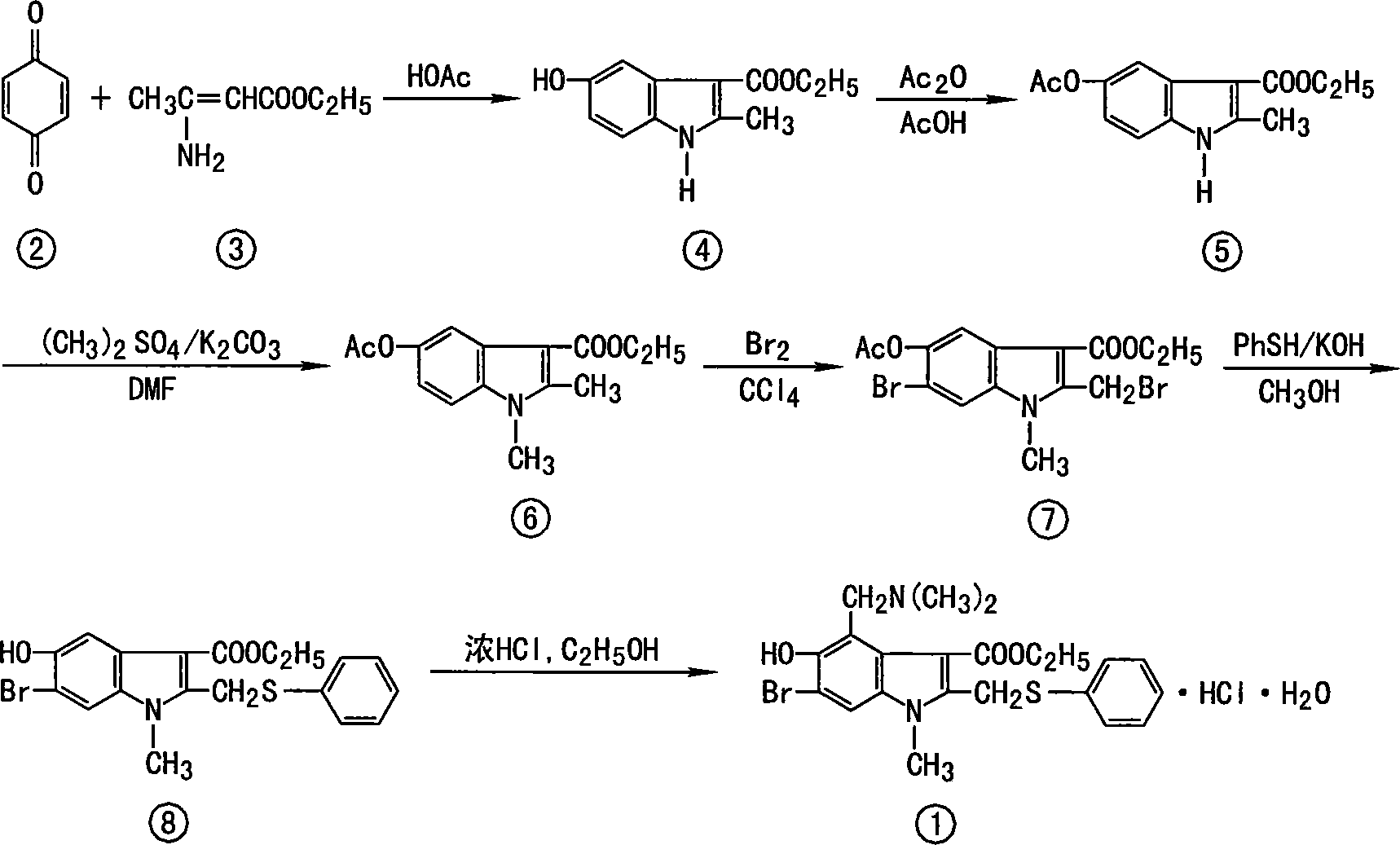
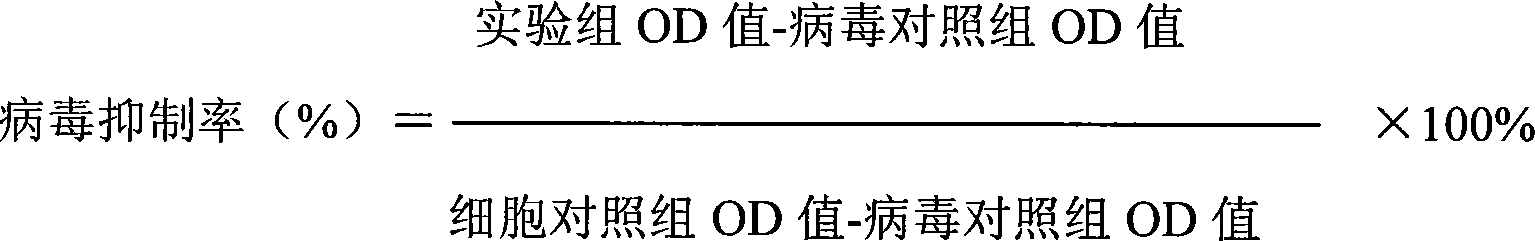
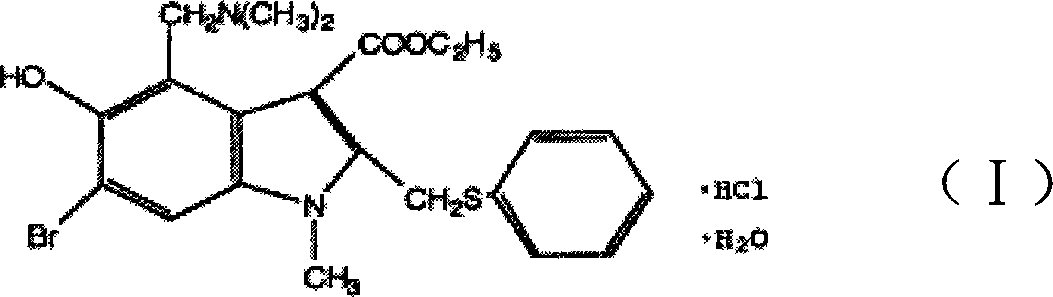
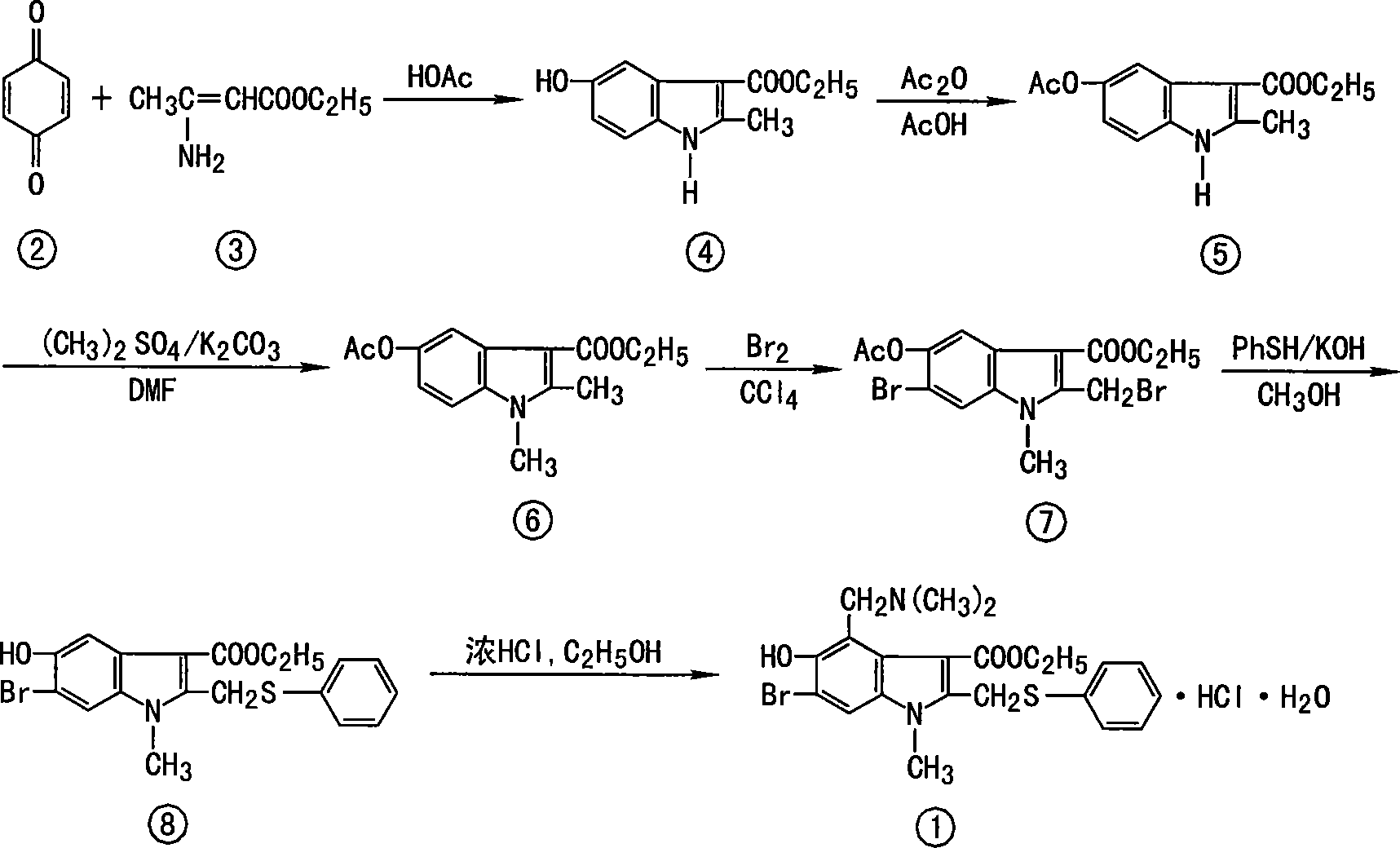
The invention discloses a chemical compound in general formula (I), wherein HO- is hydroxy, Br- is bromine, CH3- is methyl, CH2N(CH3)2- is dimethylaminomethyl, and COOC2H5- is acetoacetate.Medicinal composition with the chemical compound as active ingredient has evident antivirus action against Coxsackie b3 virus, adenovirus Ad 7, parainfluenza virus and rhinovirus. The drug has good antiviral activity against Coxsackie virus, adenovirus Ad 7, parainfluenza virus, and rhinovirus.
Owner:HUBEI QIANJIANG PHARMA
Methods and compositions for treatment of hematologic cancers
The present invention relates to oncolytic Picornaviruses and methods and compositions for treating subjects having hematologic cancers. These include methods and compositions for treatment of myeloma, using disclosed Picornavirus such as Coxsackievirus, in methods of direct or indirect administration to subjects and ex vivo purging of malignant cells within auto grafts prior to transplantation.
Owner:MERCK SHARP & DOHME LLC
Tumor-targeting coxsackie virus/adenovirus mimic peptide and application thereof
ActiveCN110357945AEfficient combinationTo achieve the effect of tracing tumor cellsAntibody mimetics/scaffoldsRadioactive preparation carriersCOXSACKIE A VIRUSTumor target
Owner:CHINA PHARM UNIV
Detection method of coxsackievirus A10 in mixed infection of hand-foot-mouth disease
InactiveCN107815510ALow costShorten the timeMicrobiological testing/measurementHand-foot-and-mouth diseaseAgricultural science
The invention discloses a detection method of coxsackievirus A10 (CV-A10) in mixed infection of hand-foot-mouth disease. The method is as follows: extracting viral RNA, using enterovirus universal primer 224 and 222 for RT-PCR amplification, then using primers AN89 and AN88 for nested PCR amplification, if the virus is non-CV-A10 serotype after comparison, designing specific CV-A10 upstream primerCVA10TF and downstream primer CVA10TR according to the CV-A10 sequence, using the RT-PCR product as a template for nested PCR amplification, and taking the PCR product for 1% agarose gel electrophoresis to identify. The method has the advantages of simple operation, rapid detection, high accuracy, low cost, and the like, and is particularly suitable for large-scale molecular epidemiological investigation.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Novel compound, pharmaceutically acceptable salt or optical isomer thereof, method for preparing same, and pharmaceutical composition for prevention or treatment of viral diseases containing same as active ingredient
ActiveCN105121433ALow cytotoxicityGood antiviral activityOrganic active ingredientsSenses disorderDisease causeHepatitis A
The present invention relates to a novel compound, to a pharmaceutically acceptable salt or optical isomer thereof, to a method for preparing same, and to a pharmaceutical composition for the prevention or treatment of viral diseases containing same as an active ingredient. The novel compound according to the present invention not only has low cytotoxicity but also has excellent antiviral activity against picornavirus such as coxsackievirus, enterovirus, echovirus, poliovirus and rhinovirus, and thus can be effectively used as a pharmaceutical composition for the prevention or treatment of viral diseases such as infantile paralysis, acute hemorrhagic conjunctivitis, viral meningitis, hand-foot-and-mouth disease, vesicular disease, hepatitis A, myitis, myocarditis, pancreatitis, diabetes, epidemic myalgia, encephalitis, cold, herpangina, foot-and-mouth disease, asthma, chronic obstructive pulmonary disease, pneumonia, sinus infection, or otitis media.
Owner:KOREA RES INST OF CHEM TECH +1
Reagent strip for jointly detecting coxsackievirus A6 type and A10 type IgM antibody and preparation method of reagent strip
The invention provides a reagent strip for jointly detecting a coxsackievirus A6 type and A10 type IgM antibody and a preparation method of the reagent strip. A first sample cushion layer is a blood filtering membrane; the surface of the blood filtering membrane is uneven; the blood filtering membrane retains red blood cells by means of physical adsorption; and a sample passes through a second sample cushion layer, a rabbit anti-human red blood cell polyclonal antibody is added into the pretreatment liquid of the second sample cushion layer on the basis of a PBS system, and can be specificallybound to the human red blood cells, and therefore, the red blood cells in the whole blood sample can be further eliminated, and the detection of the whole blood sample can be realized.
Owner:山东康华生物医疗科技股份有限公司
Anti-respiratory virus medicine and use
InactiveCN101084893AEasy to useLow toxicityOrganic active ingredientsAntiviralsCOXSACKIE A VIRUSCoxsackievirus
The invention discloses a chemical compound in general formula (I), wherein HO- is hydroxy, Br- is bromine, CH3- is methyl, CH2N(CH3)2- is dimethylaminomethyl, and COOC2H5- is acetoacetate. Medicinal composition with the chemical compound as active ingredient has evident antivirus action against Coxsackie b3 virus, adenovirus Ad 7, parainfluenza virus and rhinovirus. The drug has good antiviral activity against Coxsackie virus, adenovirus Ad 7, parainfluenza virus, and rhinovirus.
Owner:WUHAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com