Mucosal adjuvant and its preparation method and use
A mucosal adjuvant and plasmid technology, applied in the field of genetic engineering, can solve problems such as inability to effectively induce mucosal immune responses, and achieve the effects of preventing viral myocarditis, enhancing Th1 responses, and promoting activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
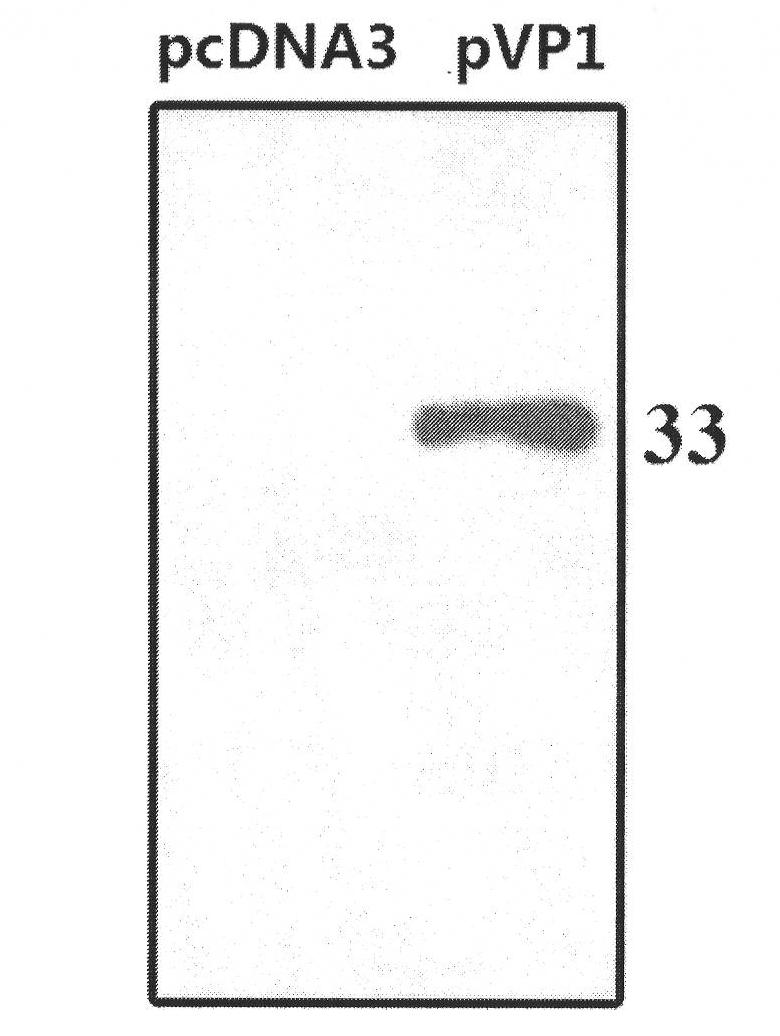
[0061] Example 1 Construction and in vitro expression of pcDNA3.1-pVP1 target antigen gene vaccine
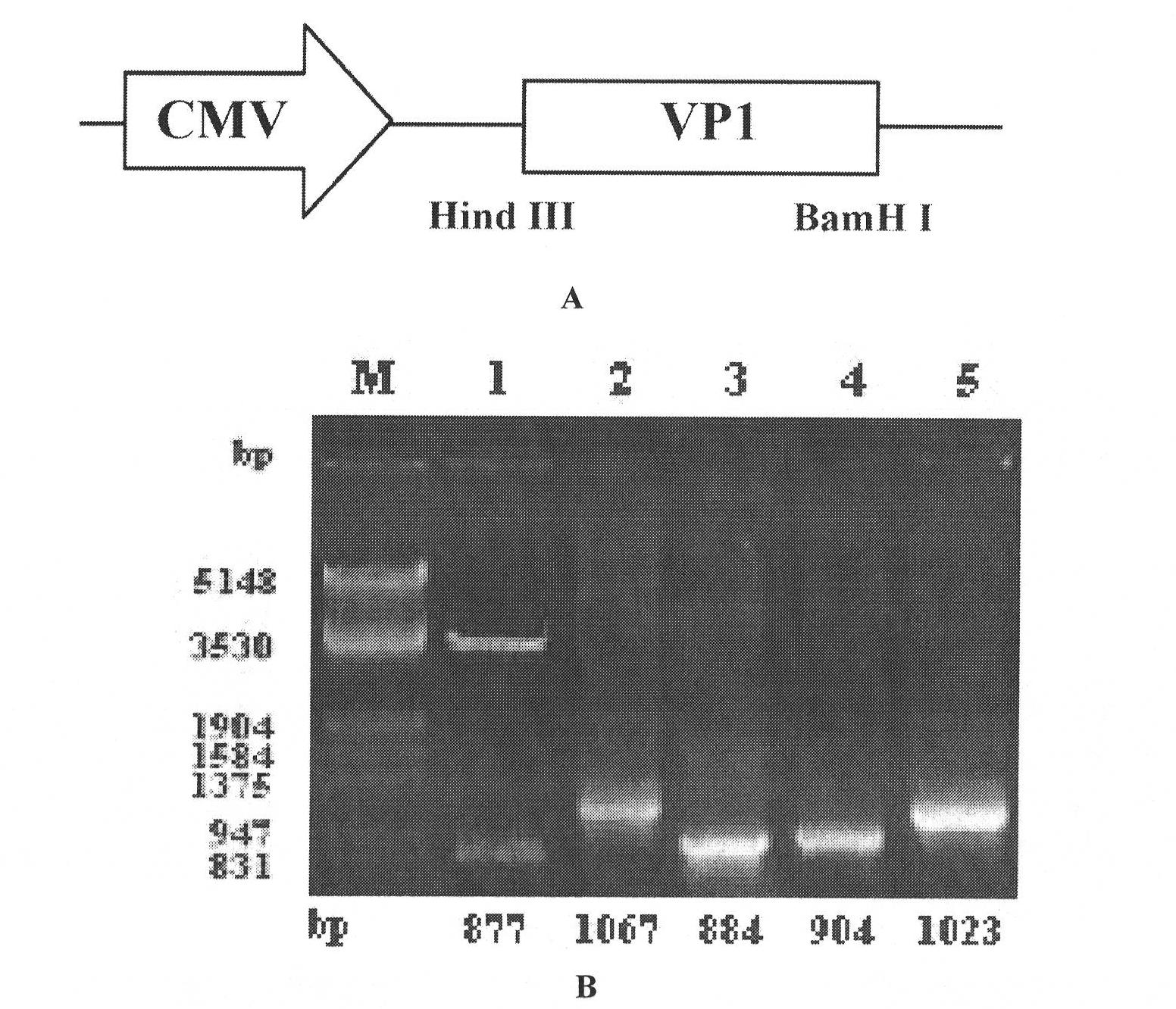
[0062] 1) Obtain CVB3 VP1 gene from freshly cultured CVB3 by RT-PCR. According to the CVB3 Nancy strain VP1 cDNA sequence reported in the literature (Klump WM, et al. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA J Virol, 1990, 64(4): 1573), design VP1 upstream and downstream primers: KV1: 5'- CCCAAGCTTGCCACCATGGGCCCAGTGGAAGACGCG-3'; KV2: 5'-CGGGATCCTTACTAAAATGCGCCCGTATTTGTC-3'. And synthesize the upstream and downstream primers T7-1: 5'-CGATGAAGATCTCT-3'; T7-2: 5'-CGATGAAGATCTCT-3' in the cloning site in the pcDNA3.1 vector. The liquid phase viral RNA was extracted according to the RNAex Reagent&System Kit of Shanghai Huashun Biological Company. The cDNA was obtained by reverse transcription using the 2-N First Strand cDNA Synthesis Kit of Shanghai Sangon Biotechnology Co., Ltd. Carry out specific PCR with KV1, KV2 primer subsequently, the product is VP1...
Embodiment 2
[0064] Example 2 Construction and in vitro expression of pcDNA3.1-LTN mucosal adjuvant plasmid
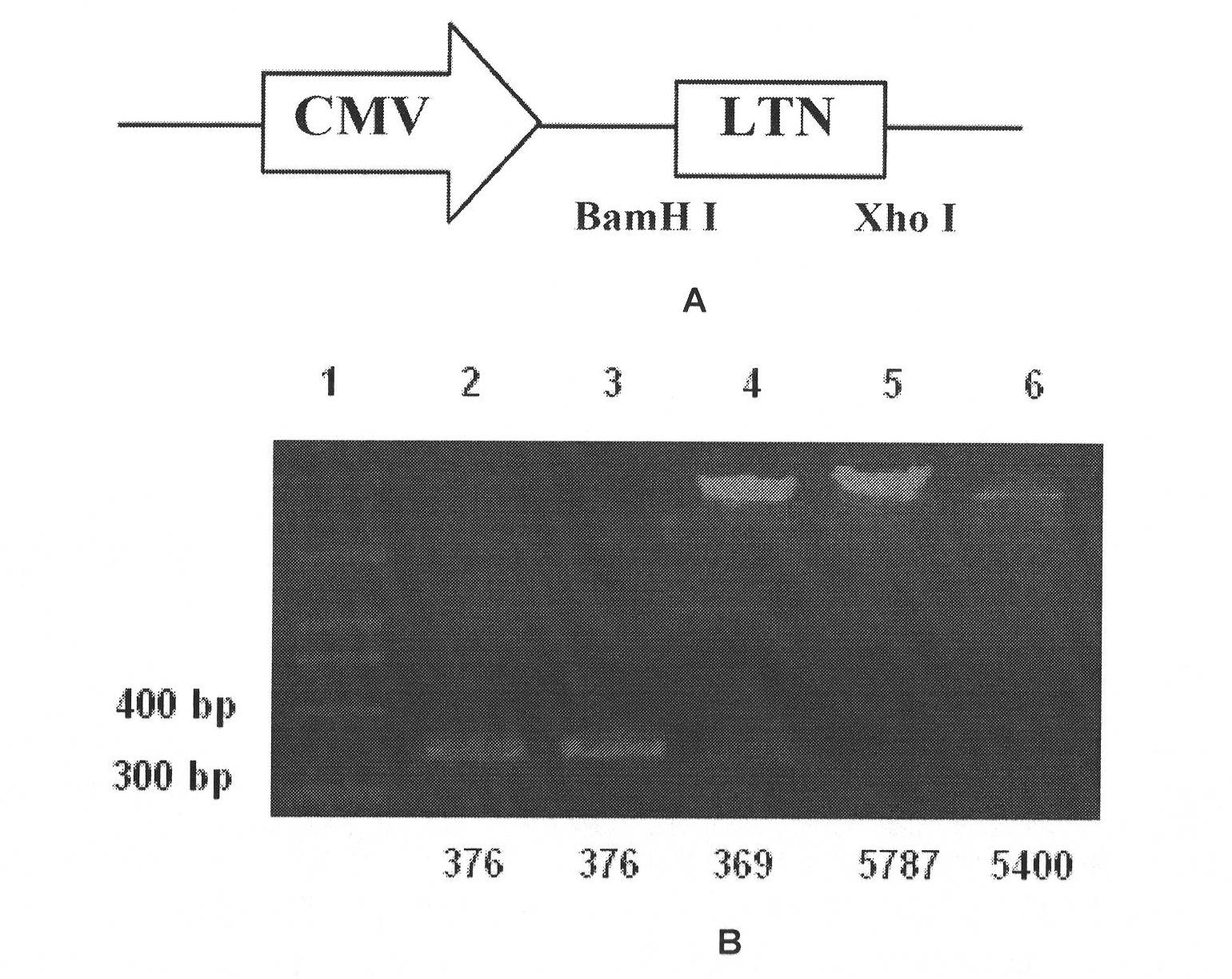
[0065] 1) The LTN gene was obtained from ConA-activated mouse splenocytes by RT-PCR. Take the spleen of normal BALB / c mice 5×10 6 , to extract total cellular RNA. The cDNA was obtained by reverse transcription using the 2-N First Strand cDNA Synthesis Kit of Shanghai Sangon Biotechnology Co., Ltd. According to the gene sequence of LTN in NCBI GeneBank (NM_008510.1), design the upstream and downstream primers of LTN: LTN-1: 5'-5'CG GGATCC GCCACCATGAGACTTCTCCTCCT-3' has a BamH I restriction site added to its 5' end; LTN-2: 5'-CCG CTCGAG GGAGGCTGTTACCCAGT-3' has an Xho I restriction site added to its 3' end. Perform PCR with the above primers to obtain the chemokine Ltn coding gene (376bp) ( image 3 ). The RT-PCR product was purified and recovered with Golden Beads DNA Gel Purification Kit, digested with BamHI and Xho I, and ligated with pcDNA3.1 that had undergone the same d...
Embodiment 3
[0067] Example 3 In vitro chemotactic activity of pcDNA3.1-LTN mucosal adjuvant plasmid expression product
[0068] Concentrate the supernatant of stably transfected pcDNA3.1-LTN by 20-25 times with PEG20000, and add it into a 48-well chemotaxis cell (Neuroprobe, USA); 10 6 / ml) was added to the upper chamber and incubated for 4 hours. Take out the filter membrane, Wright Giemsa staining, count chemotactic cells and calculate the chemotaxis index (CI). The results showed that the supernatant of 293T cells stably transfected with pcDNA3.1-LTN could effectively chemoattract splenocytes to move across the membrane, CI=2.875; while the control pcDNA3.1 plasmid transfected supernatant or normal cell supernatant had no chemotactic activity, CI Figure 5 )
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com