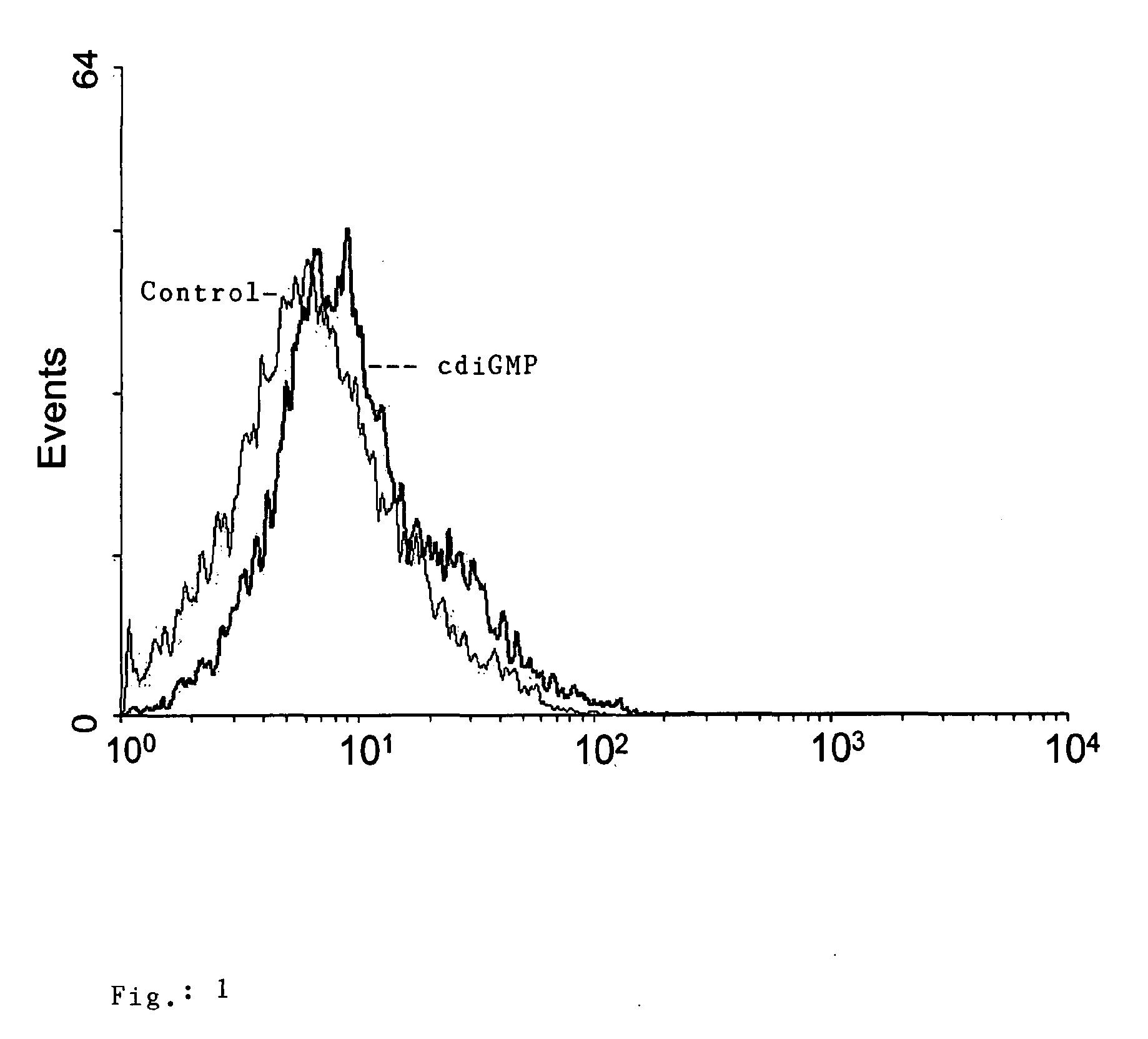
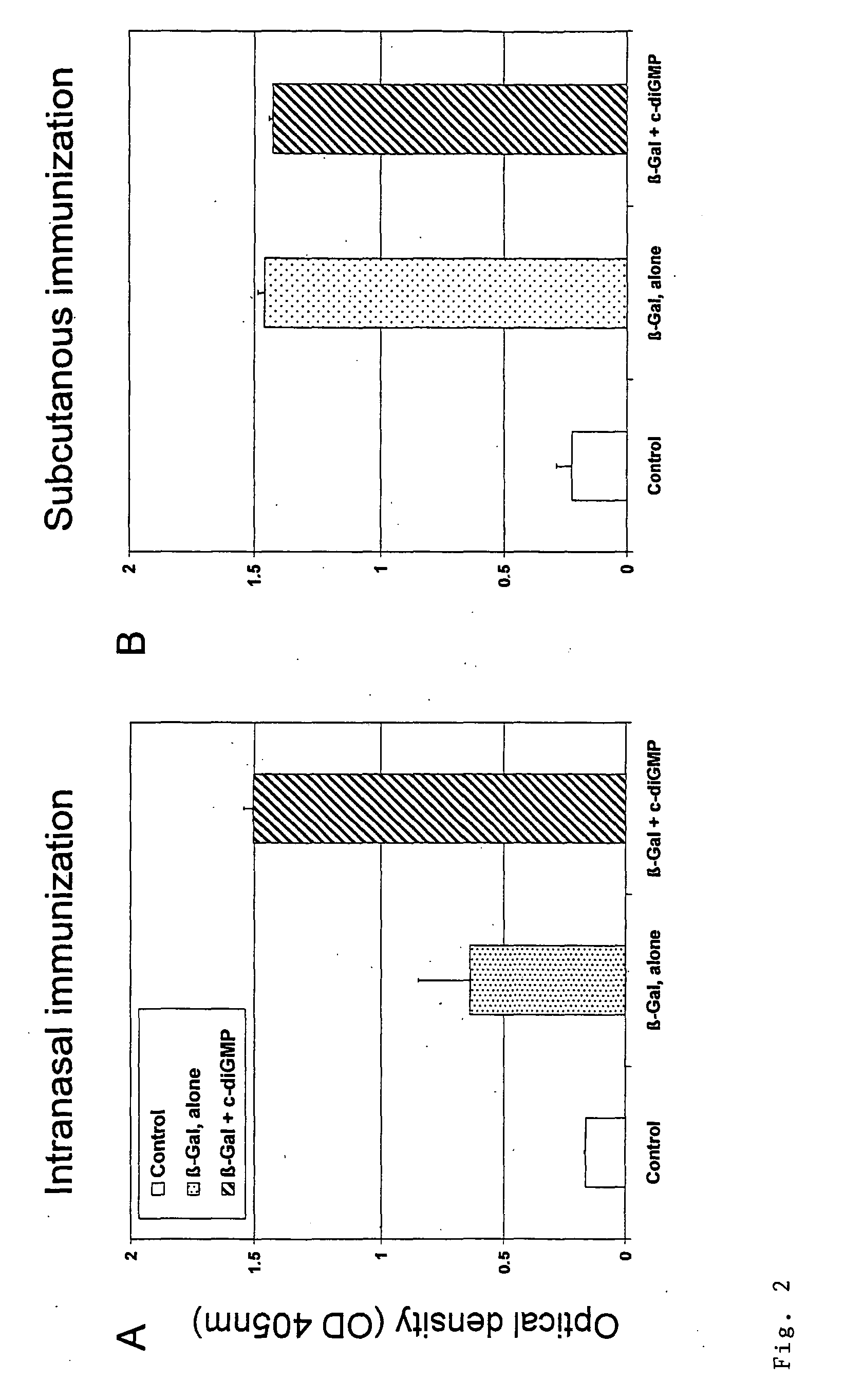
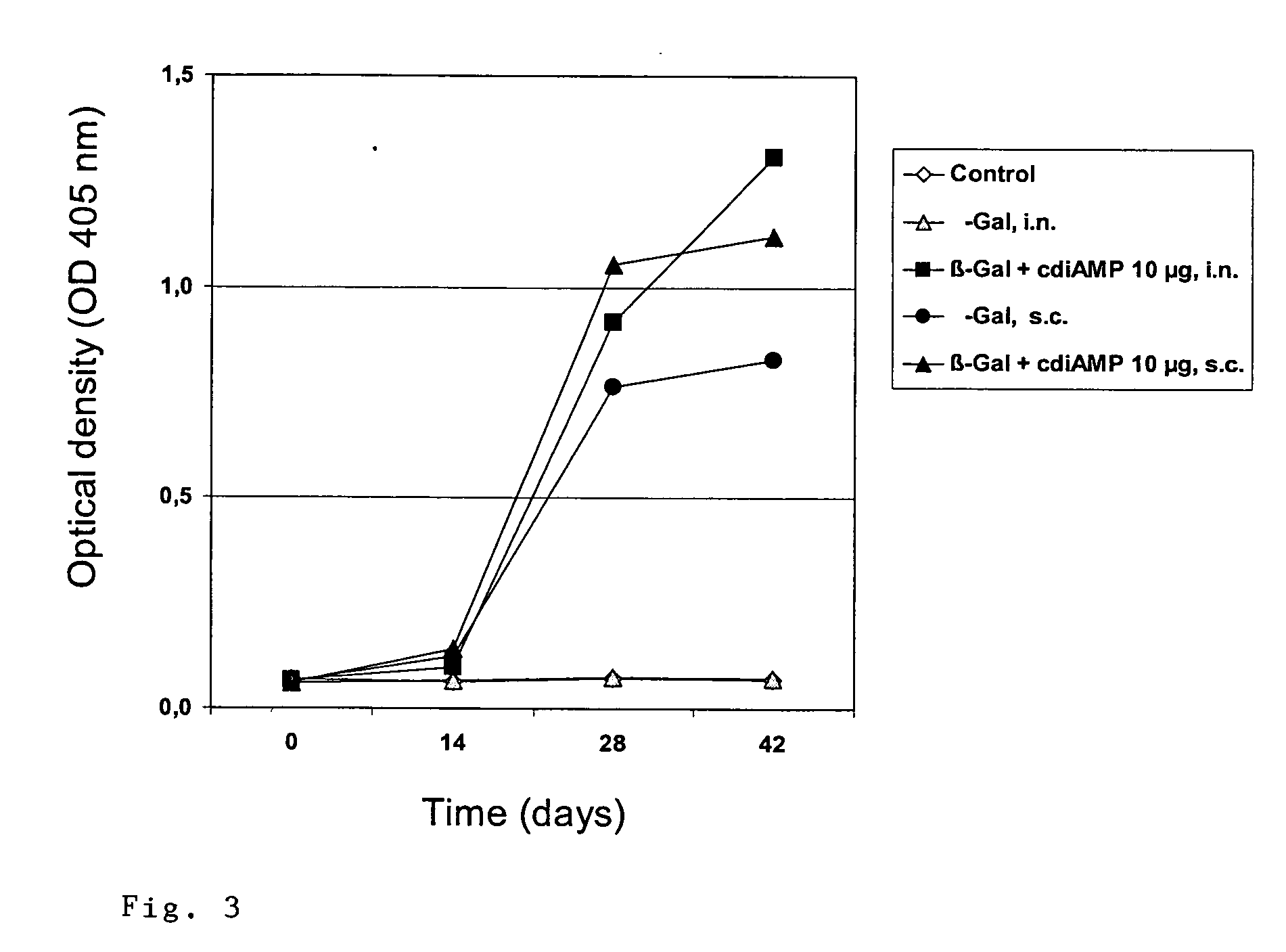
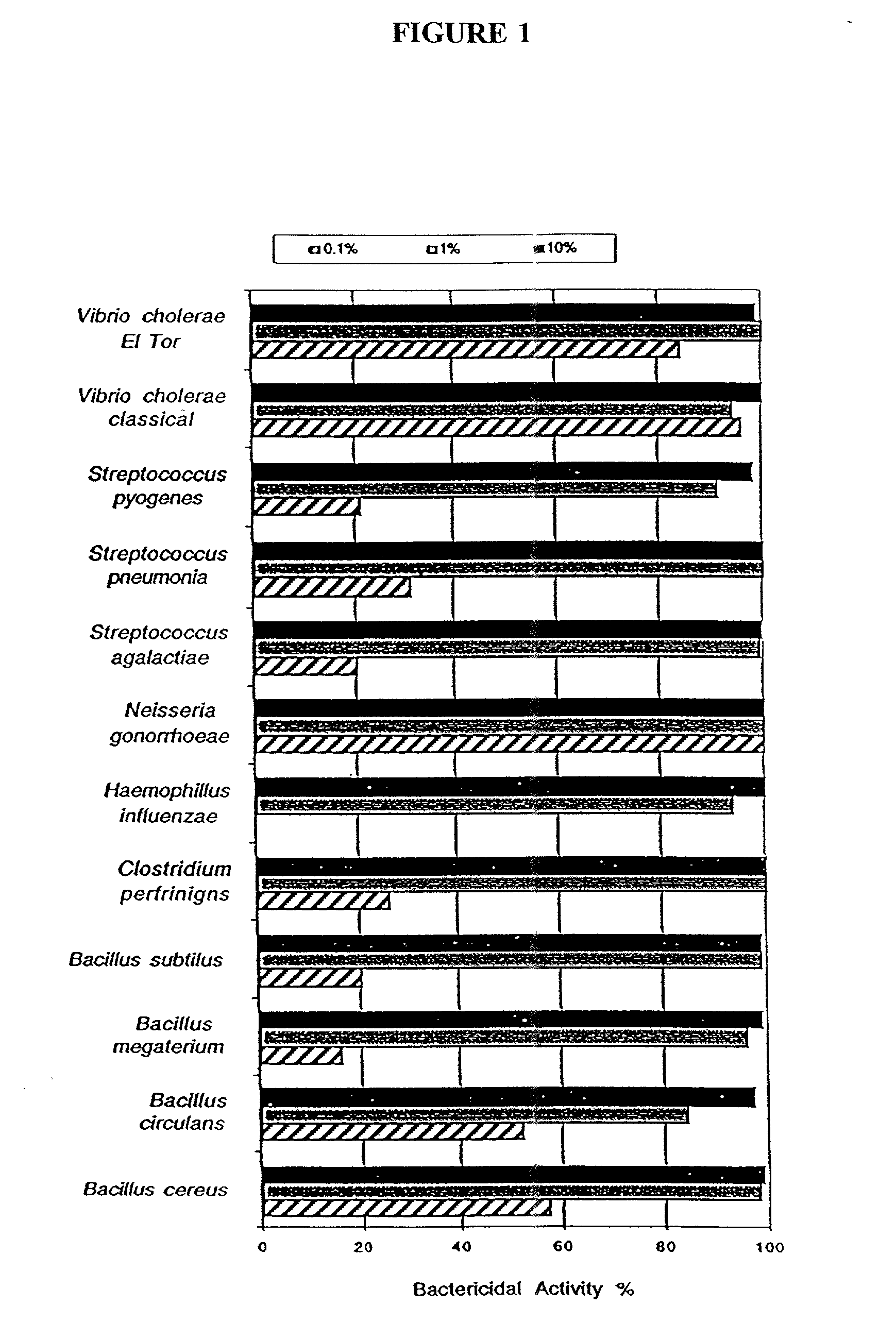
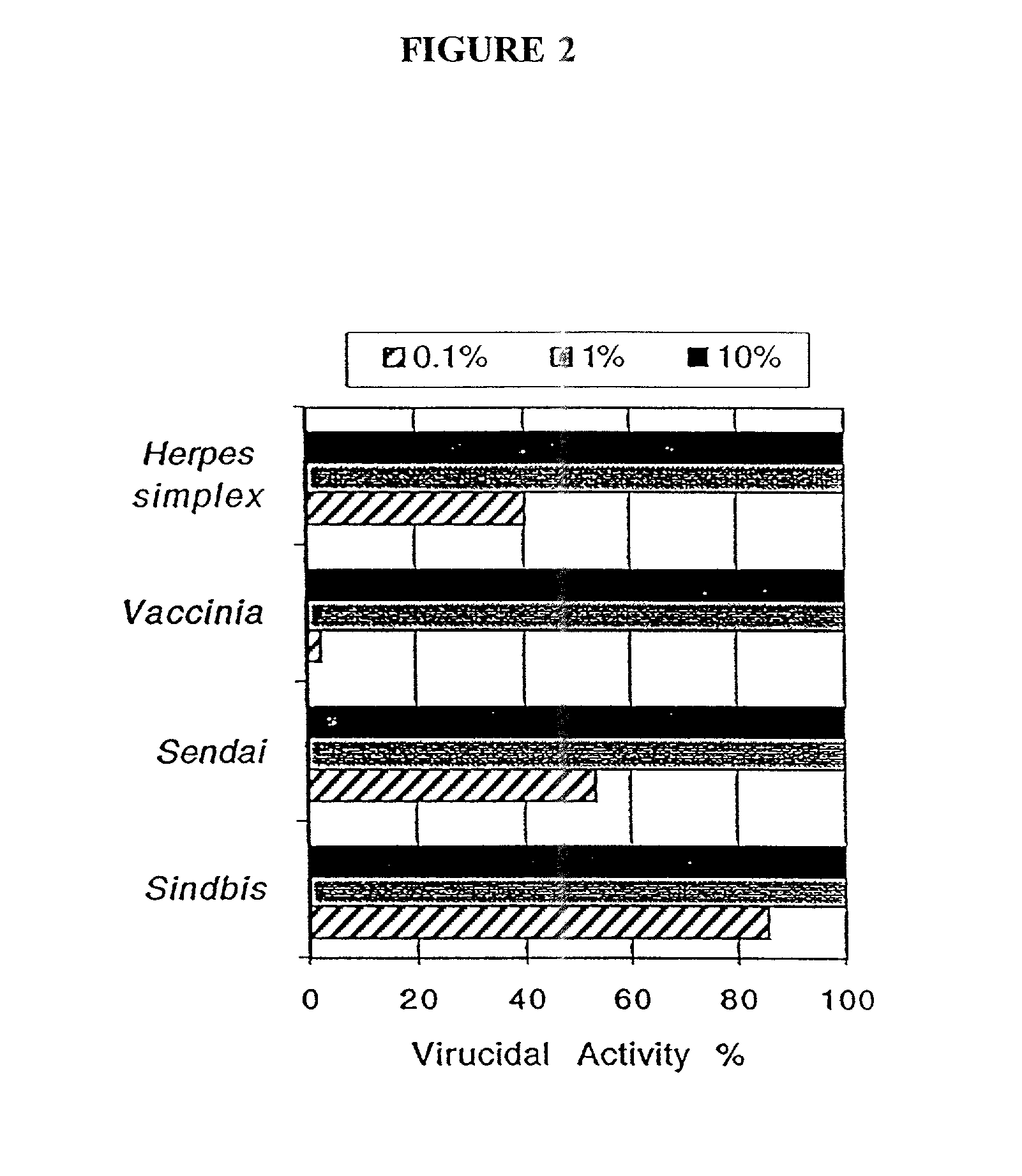
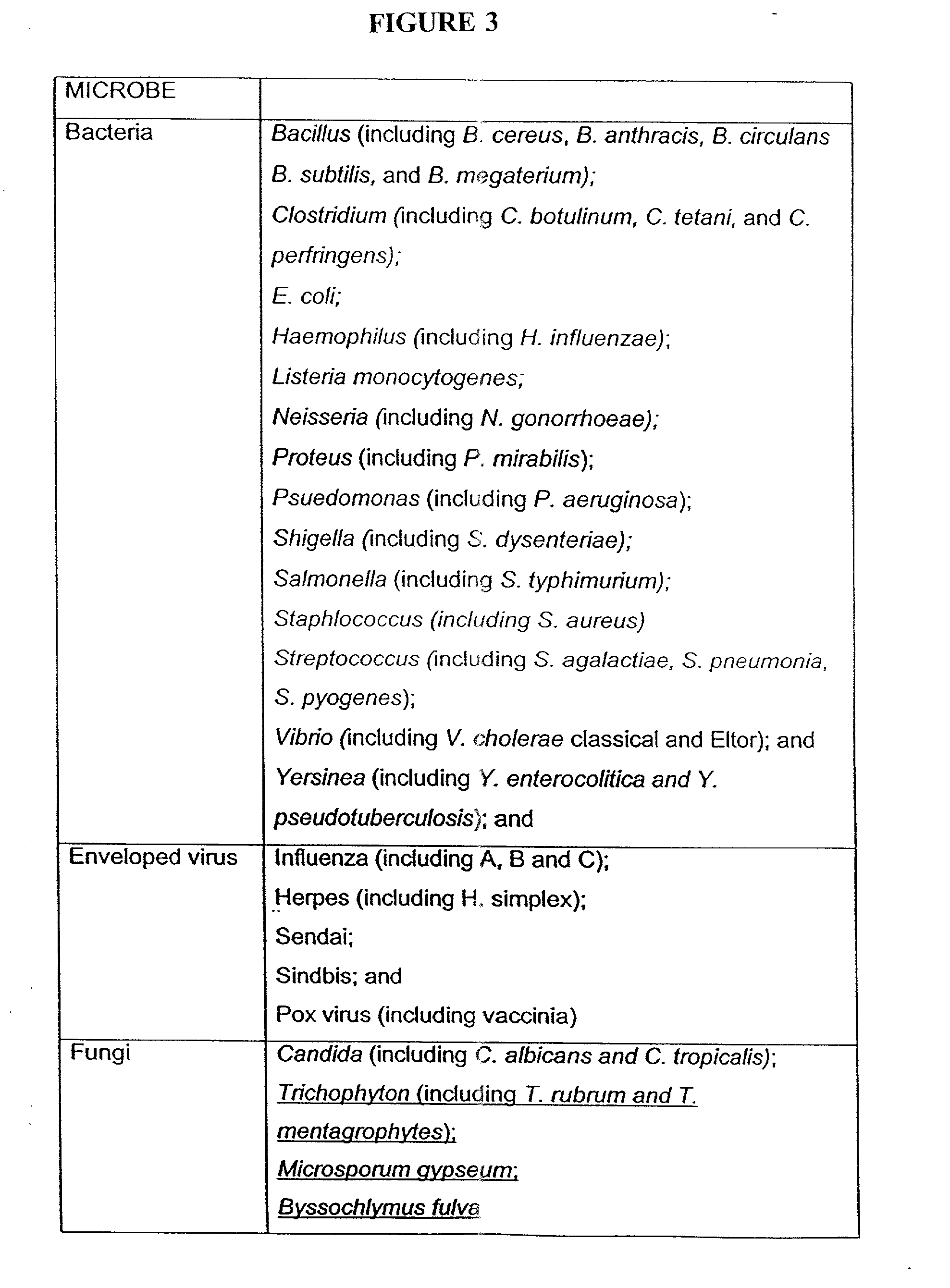
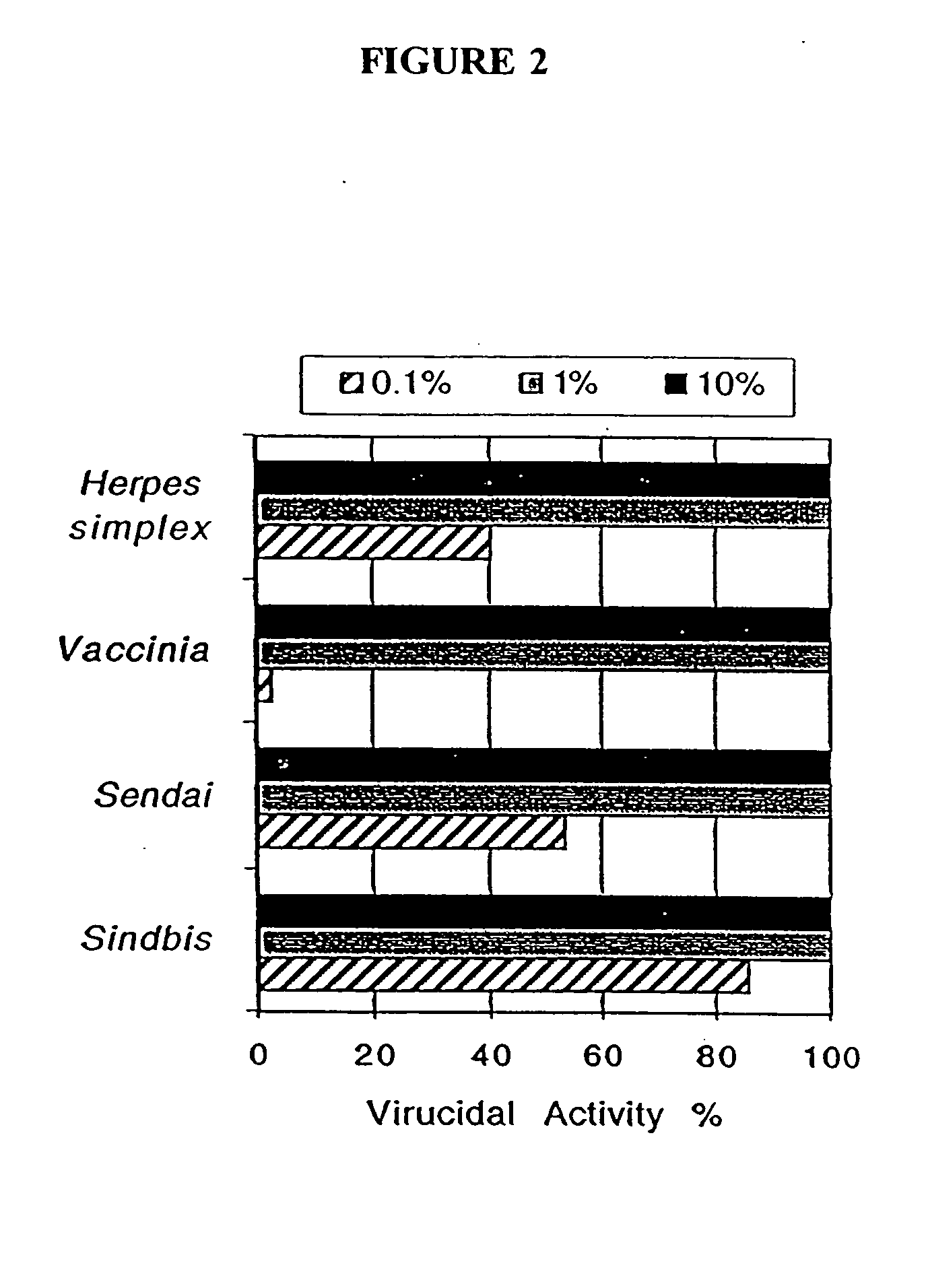
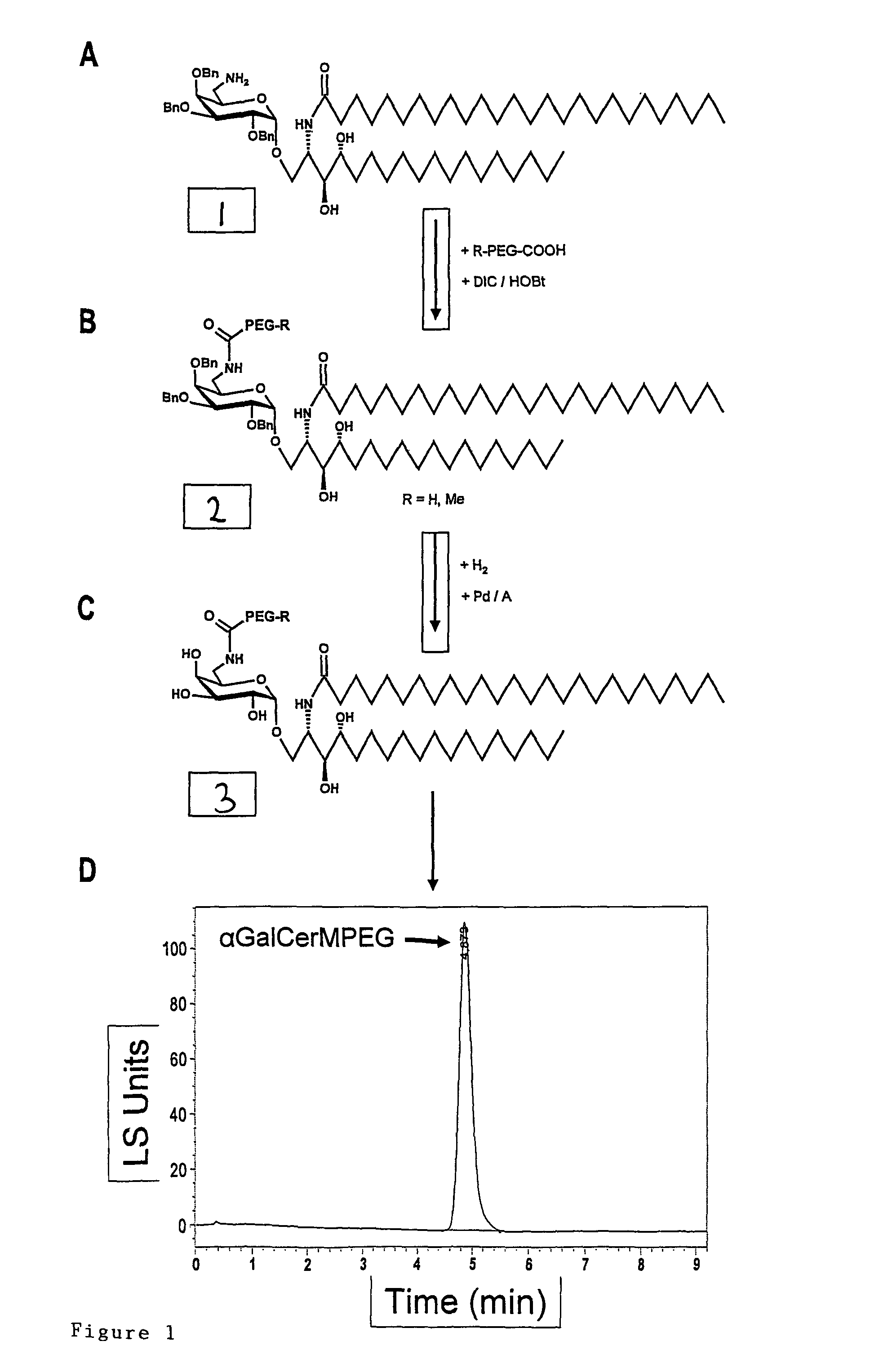
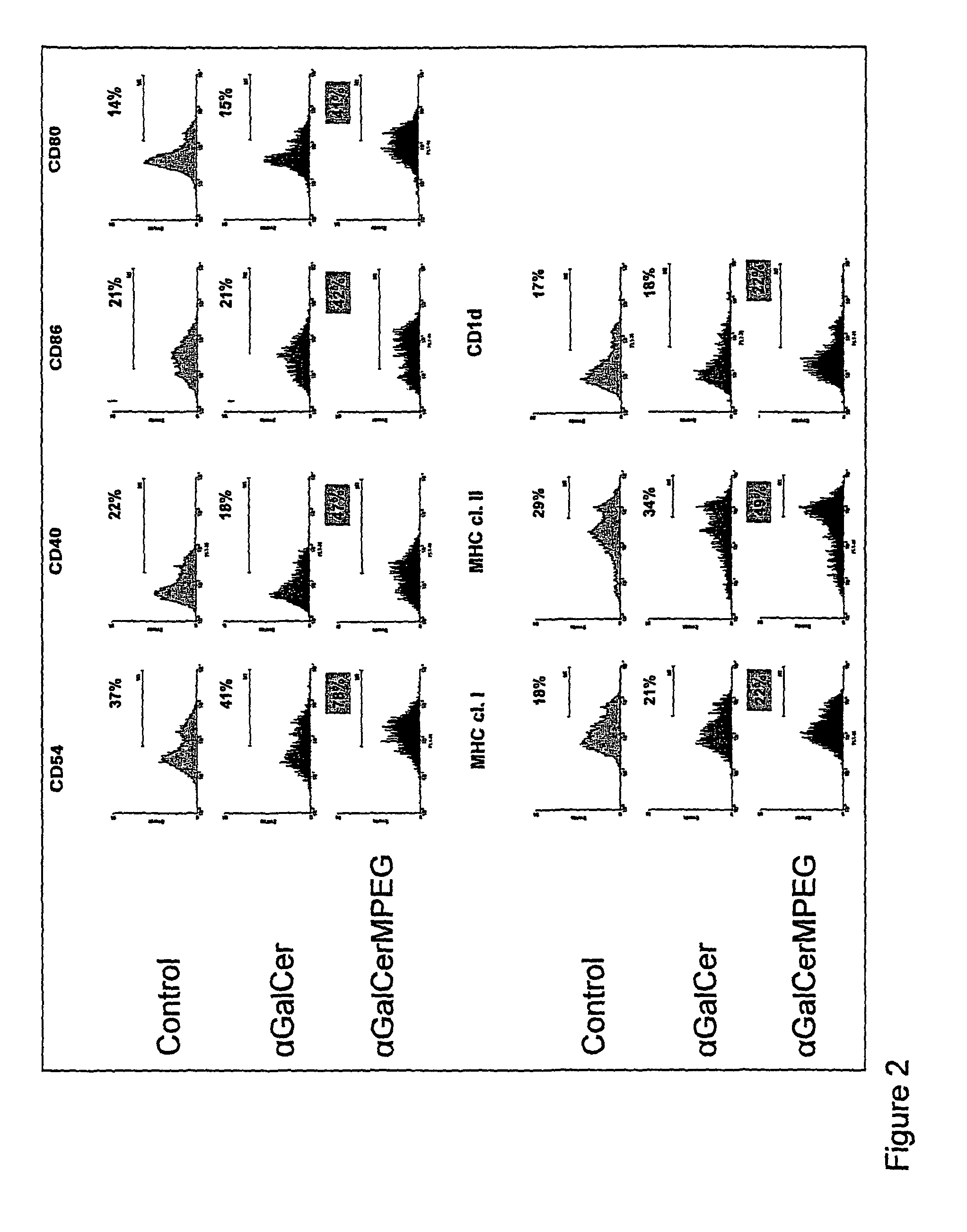
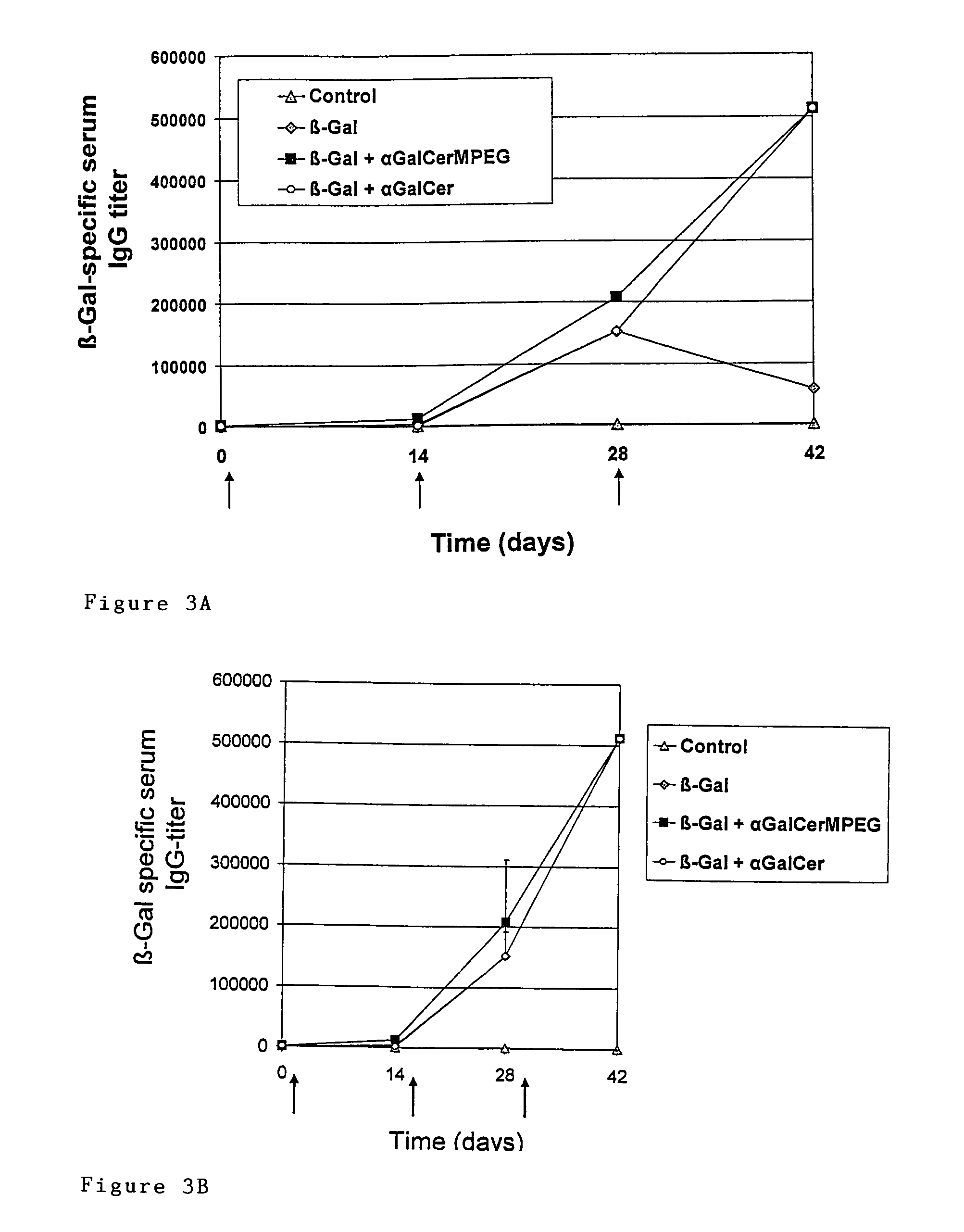
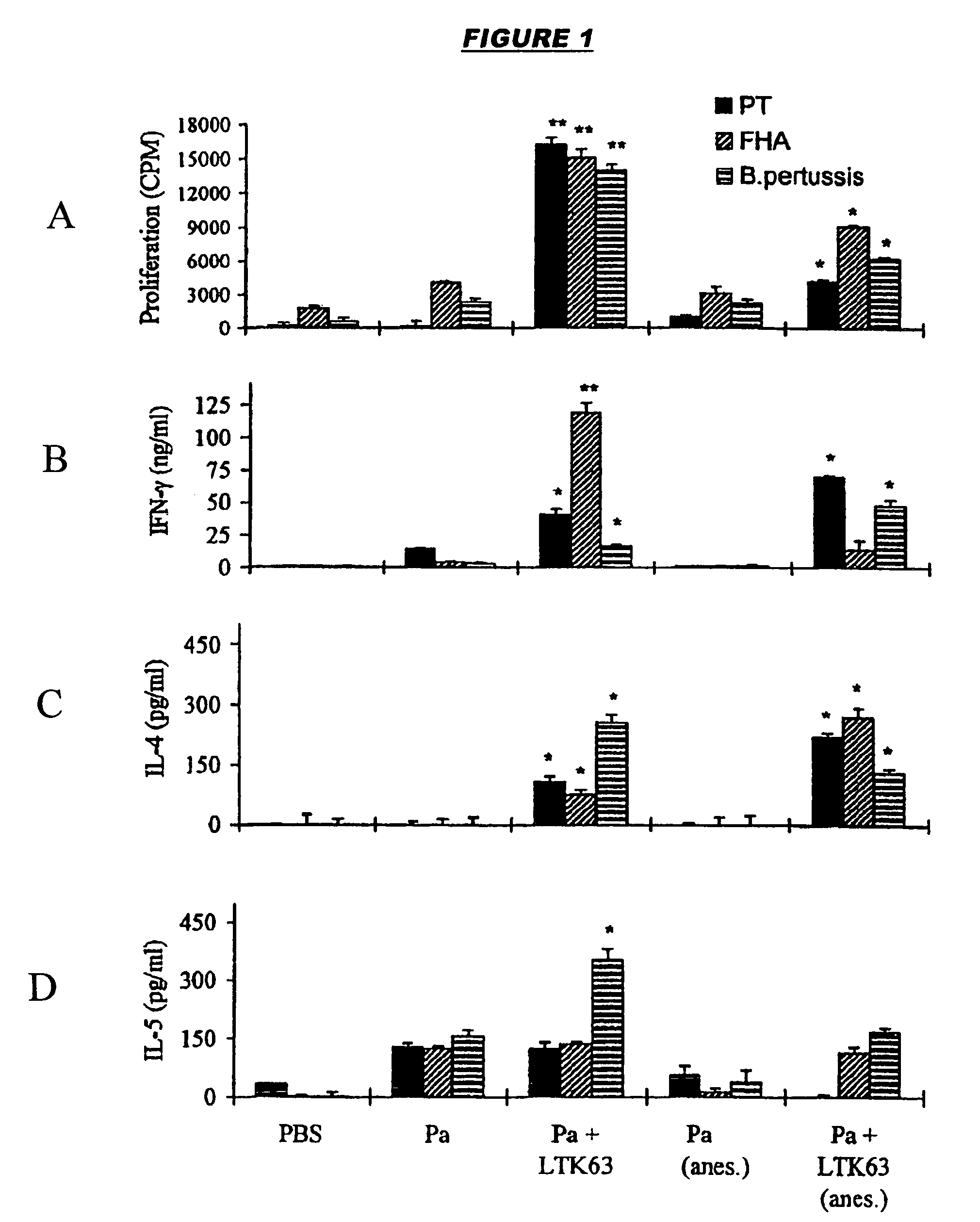
Patents
Literature
67 results about "Mucosal adjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclic-Dinucleotides and Its Conjugates as Adjuvants and Their Uses in Pharmaceutical Compositions
ActiveUS20080286296A1Severe formLess immunomodulatory effectOrganic active ingredientsAntipyreticDiseaseAutoimmune responses
The present invention relates to new adjuvants and the uses in pharmaceutical compositions, like in vaccines. In particular, the present invention provides new compounds useful as adjuvants and / or immunomodulators for prophylactic and / or therapeutic vaccination in the treatment of infectious diseases, inflammatory diseases, autoimmune diseases, tumors, allergies as well as for the control of fertility in human or animal populations. The compounds are particularly useful not only as systemic, but preferably as mucosal adjuvants. In addition, the invention relates to its uses as active ingredients in pharmaceutical compositions.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Nanoemulsion vaccines
InactiveUS20030194412A1Prevented pathological effectReduce riskAntibacterial agentsSsRNA viruses negative-senseMucosal adjuvantImmunity
The present invention provides methods and compositions for the stimulation of immune responses. Specifically, the present invention provides methods and compositions for the use of nanoemulsion compounds as mucosal adjuvants to induce immunity against environmental pathogens. Accordingly, in some embodiments, the present invention provides nanoemulsion vaccines comprising a nanoemulsion and an inactivated pathogen or protein derived from the pathogen. The present invention thus provides improved vaccines against a variety of environmental and human-released pathogens.
Owner:RGT UNIV OF MICHIGAN
Nanoemulsion vaccines
InactiveUS20060257426A1SsRNA viruses negative-senseAntibacterial agentsMucosal adjuvantCompound (substance)
The present invention provides methods and compositions for the stimulation of immune responses. Specifically, the present invention provides methods and compositions for the use of nanoemulsion compounds as mucosal adjuvants to induce immunity against environmental pathogens. Accordingly, in some embodiments, the present invention provides nanoemulsion vaccines comprising a nanoemulsion and an inactivated pathogen or protein derived from the pathogen. The present invention thus provides improved vaccines against a variety of environmental and human-released pathogens.
Owner:RGT UNIV OF MICHIGAN
Novel, non-antigenic, mucosal adjuvant formulation which modulates the effects of substances, including vaccine antigens, in contact with mucosal body surfaces
InactiveUS20030104010A1Antibacterial agentsSsRNA viruses negative-senseMucosal adjuvantVaccine antigen
Adjuvant for mucosal vaccines which modulates the effects of substances, including vaccine antigens in contact with mucosal body surfaces.
Owner:BIOTEC PHARMACON
Nontoxic mucosal adjuvant
InactiveUS7070781B2Improving immunogenicityImprove responseAntibacterial agentsBiocideBiological bodyGynecology
A non-toxic mucosal adjuvant is provided which may be admixed with further antigens to provide a vaccine administrable to mucosal surfaces in organisms including man. Preferably, the non-toxic mucosal adjuvant is a detoxified mutant of a bacterial ADP-ribosylating toxin, optionally comprising one or more amino acid additions, deletions or substitutions.
Owner:CHIRON CORP
Recombinant helicobacter pylori protein vaccine and preparation method thereof
InactiveCN107298716ARetain the infrastructureRetain activityAntibacterial agentsBacterial antigen ingredientsAdjuvantVaccine antigen
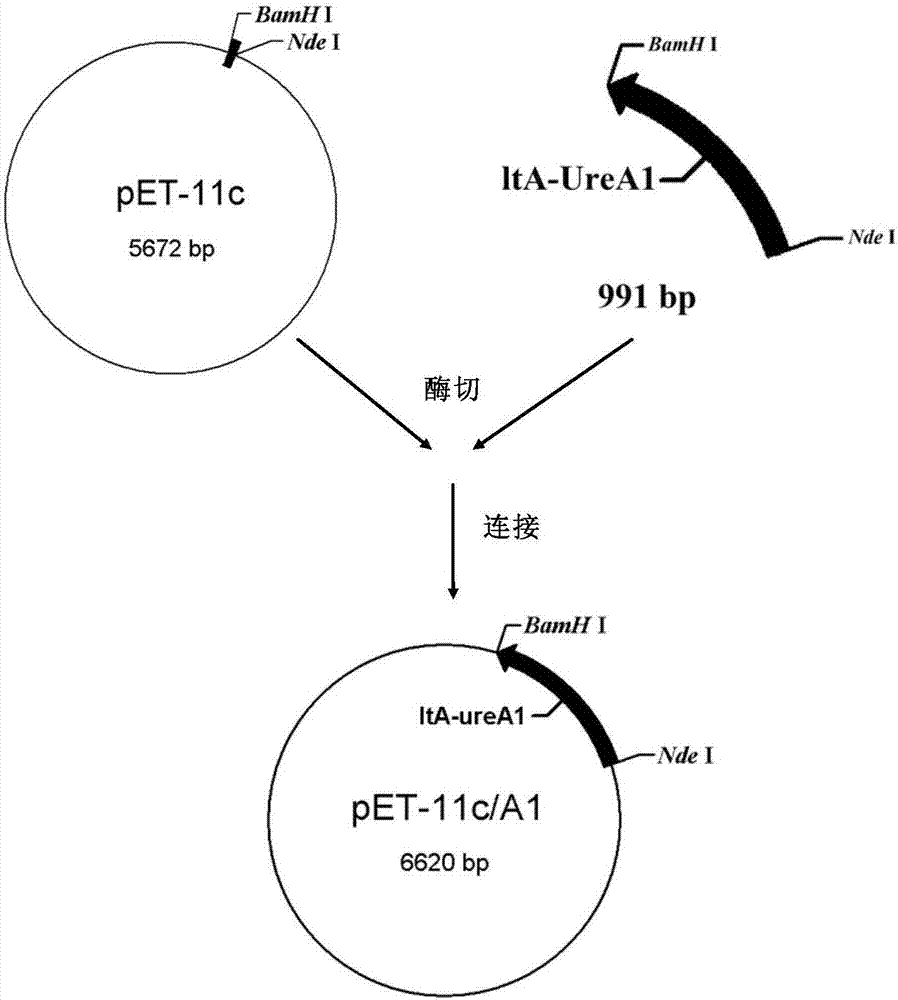
The invention discloses a recombinant helicobacter pylori protein vaccine and a preparation method thereof. The active ingredient recombinant fusion protein of the vaccine consists of recombinant LTAl-Ureal protein and LTB protein, the amino acid sequence of the recombinant LTAl-Ureal protein is shown as Seq ID No.1, the amino acid sequence of the LTB protein is shown as Seq ID No.2. Epitope-containing gene segments of Hp urease A subunit is inserted in an LTA subunit-encoded gene, a toxic part-containing segment is replaced to prepare a recombinant plasmid so as to express and obtain recombinant fusion protein polymer as a vaccine antigen, the fusion antigen and LTB protein pentamer are combined to form a hexamer structure, so that not only can the structure basis and activity of an LT mucosa adjuvant be remained, but also the toxicity can be removed, the immune response of organism muscosa can be effectively induced through immunity of mucosa path, to generate specific IgA antibody. The recombinant helicobacter pylori protein vaccine provides a vaccine manner for preventing and treating infection of helicobacter pylori.
Owner:成都亿妙生物科技有限公司
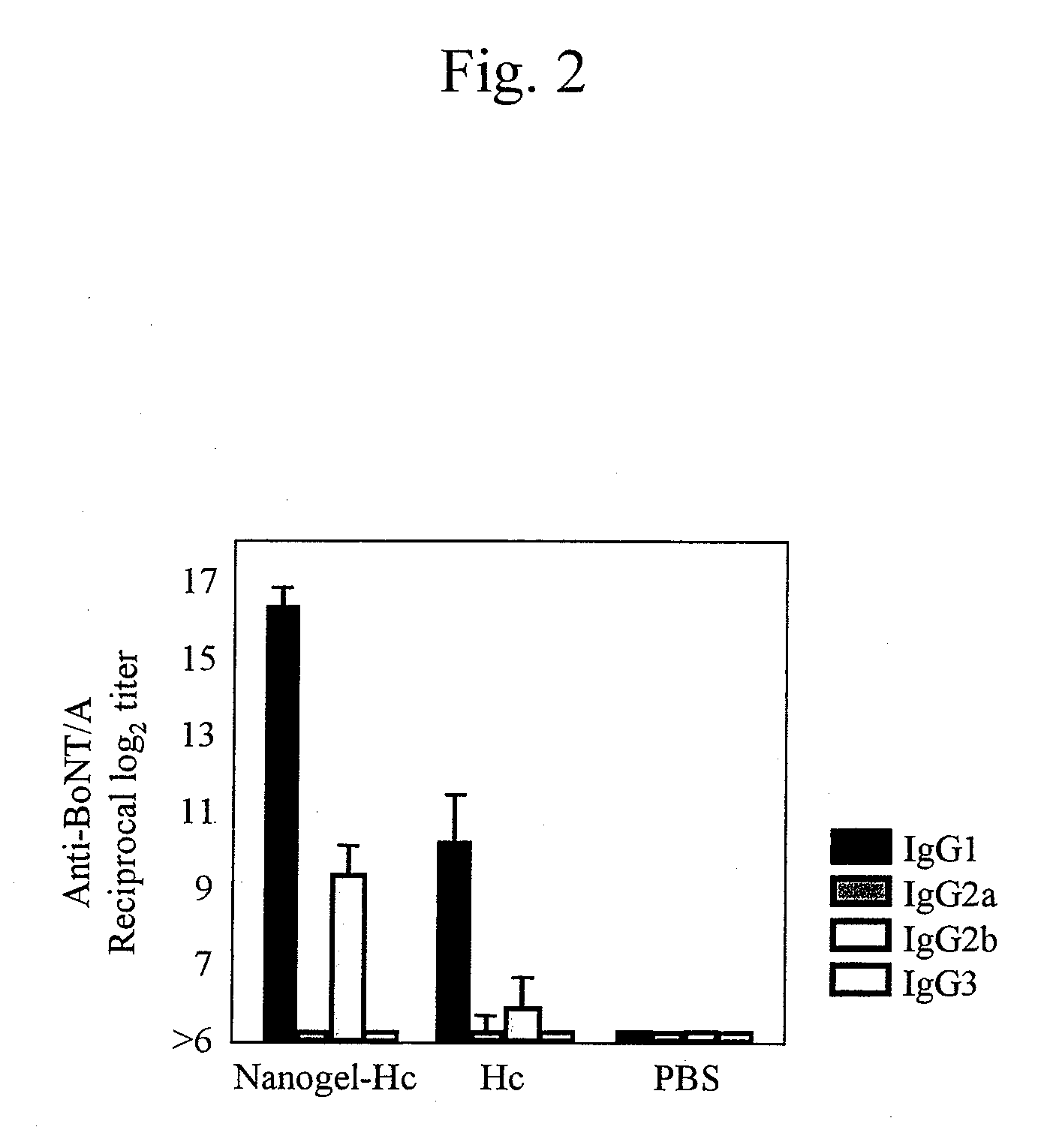
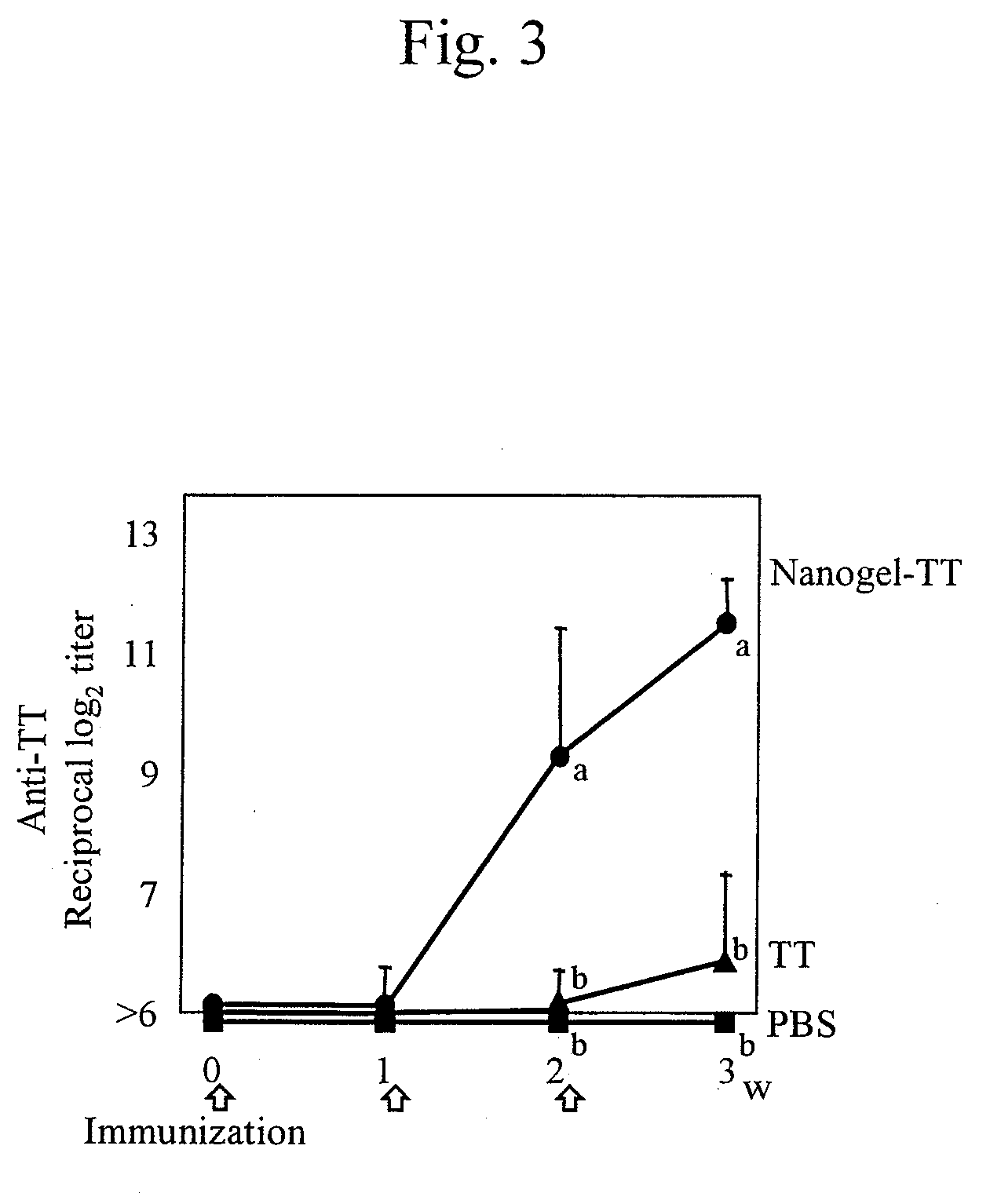
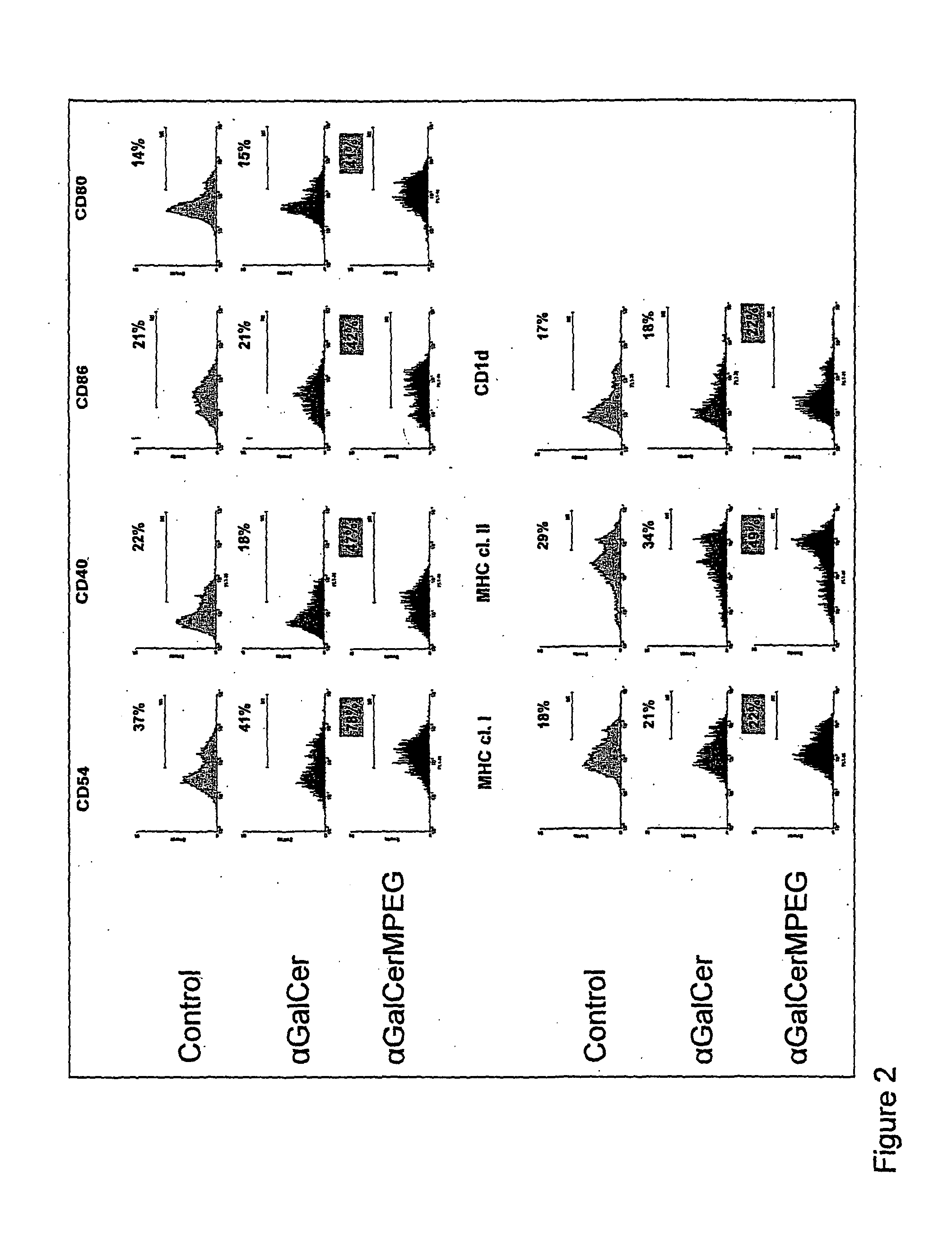
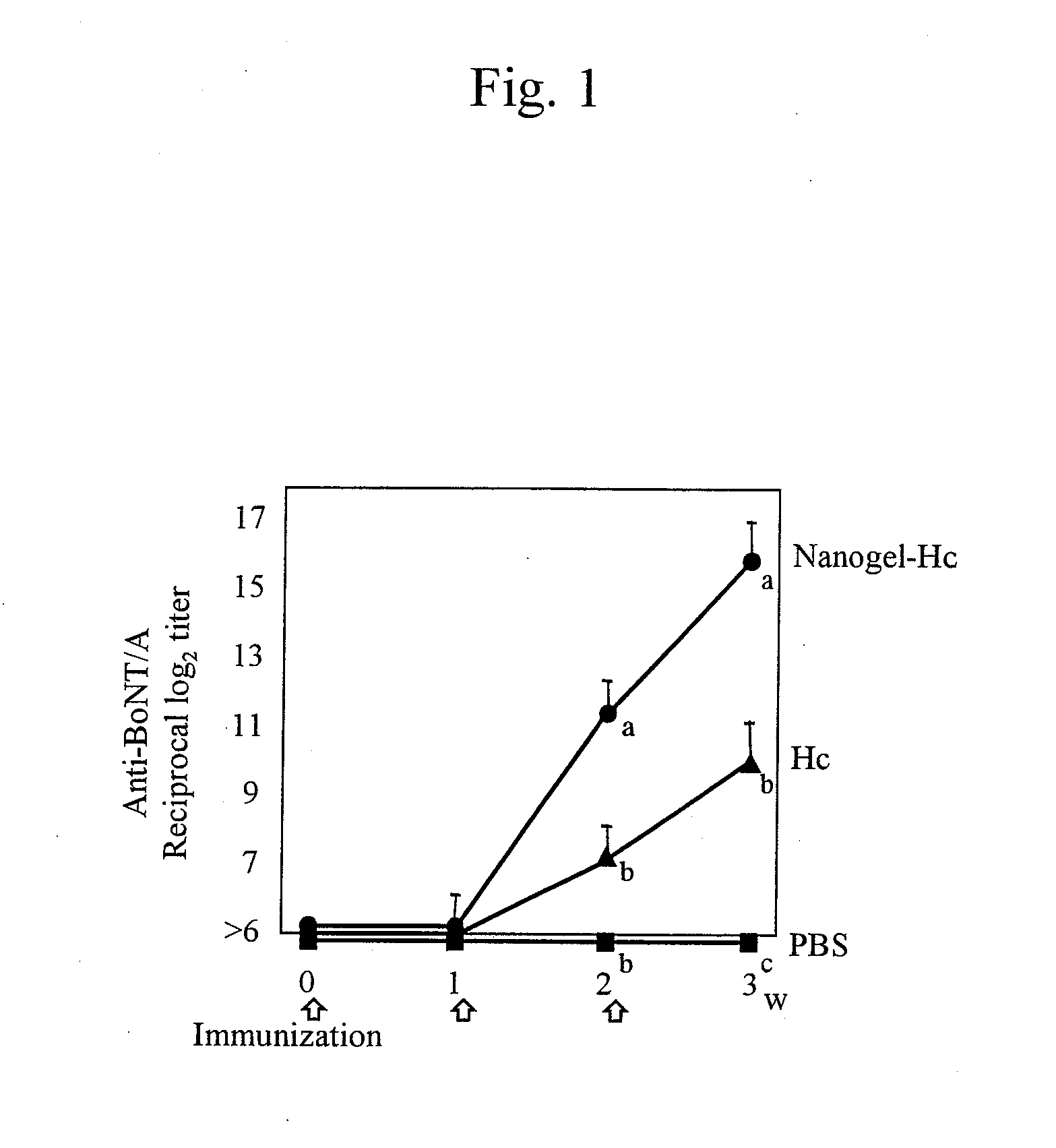
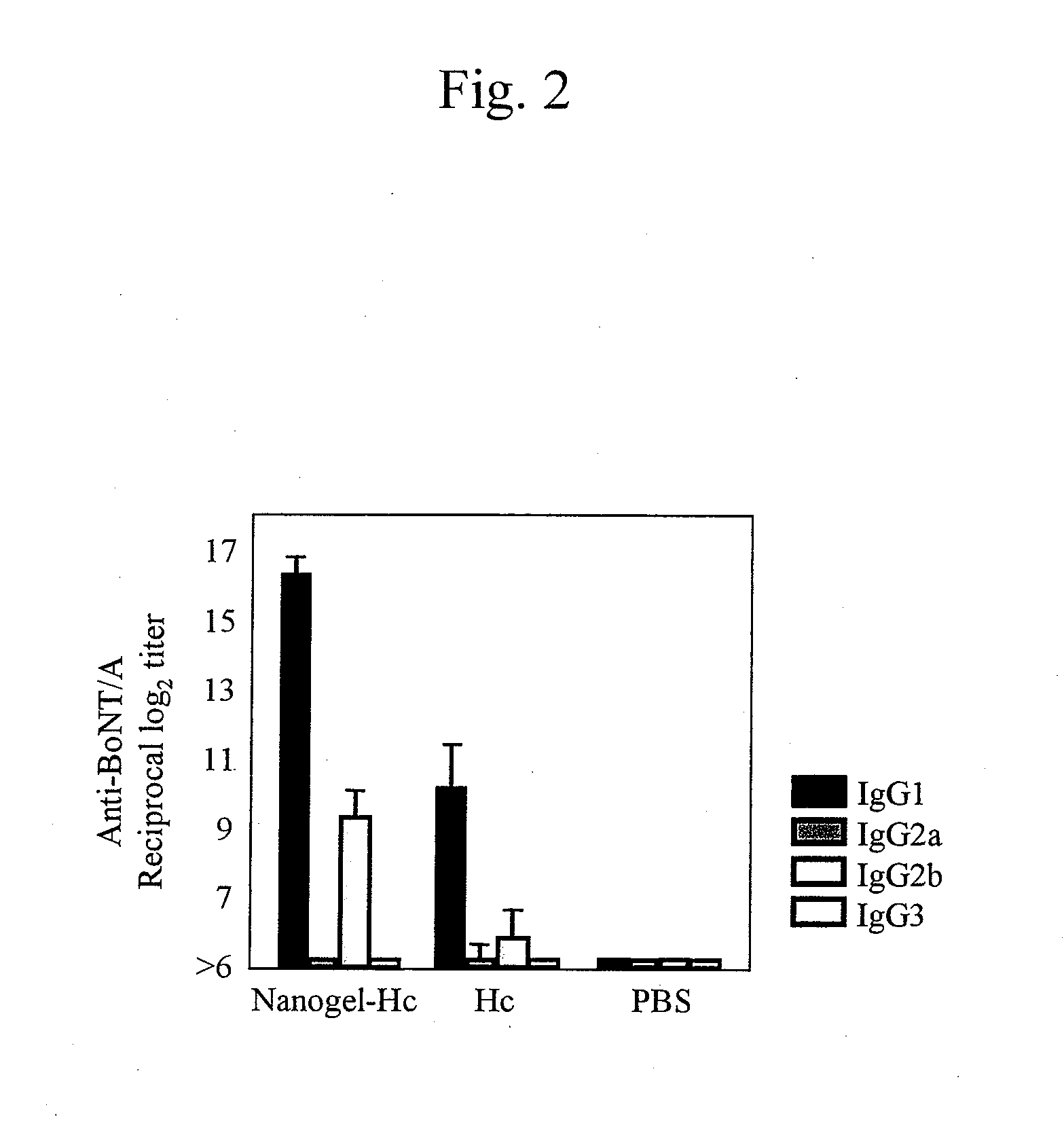
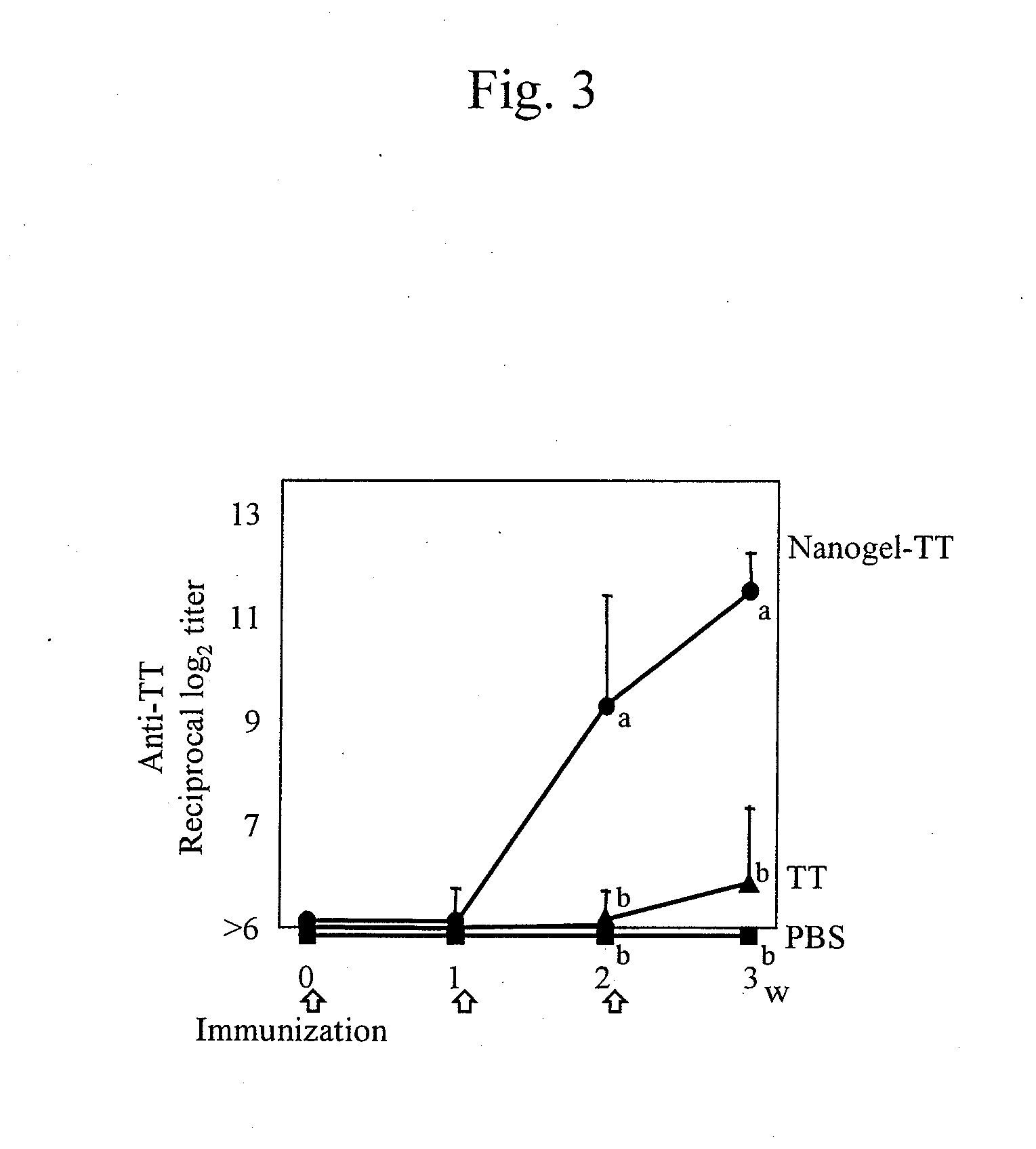
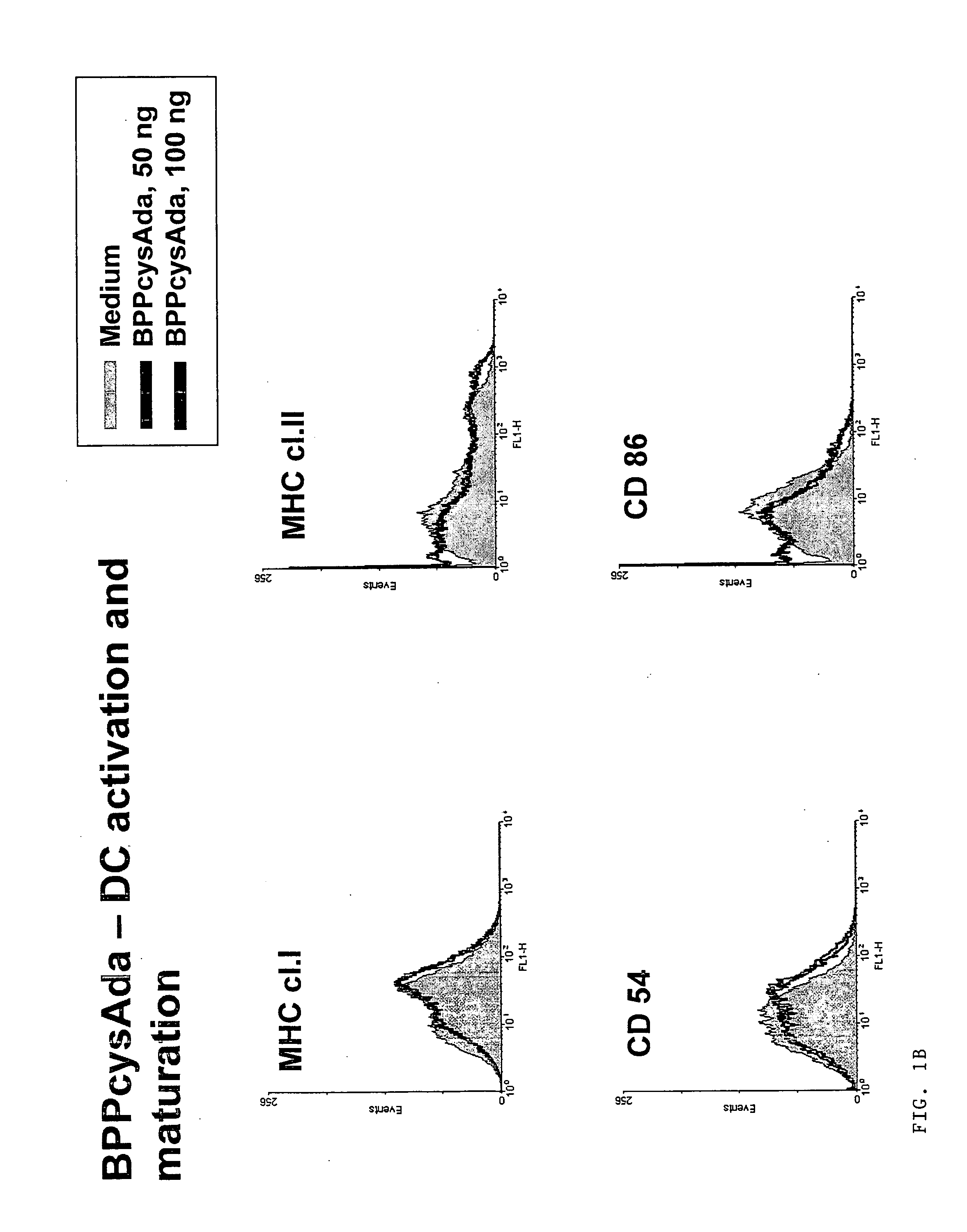
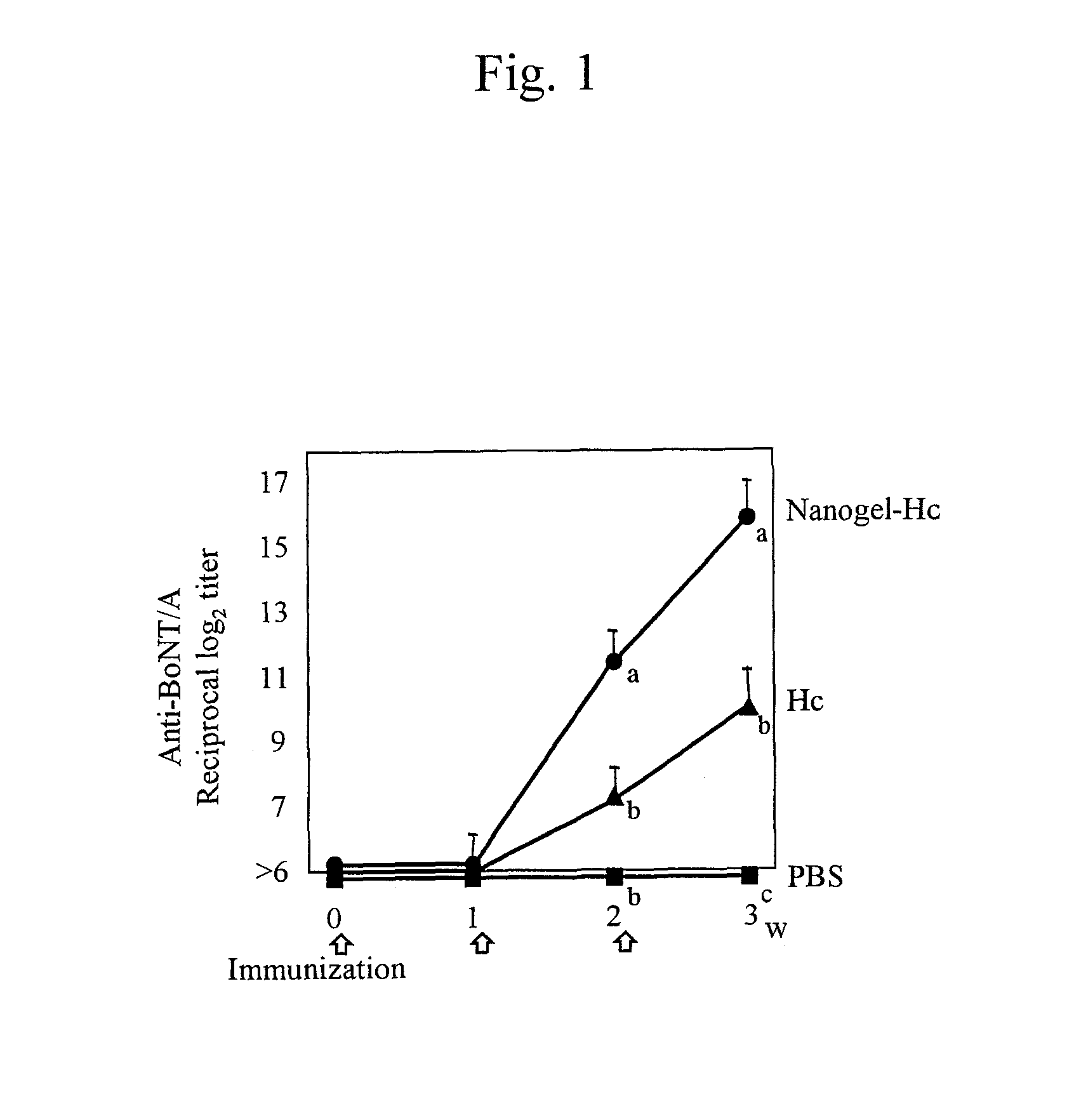
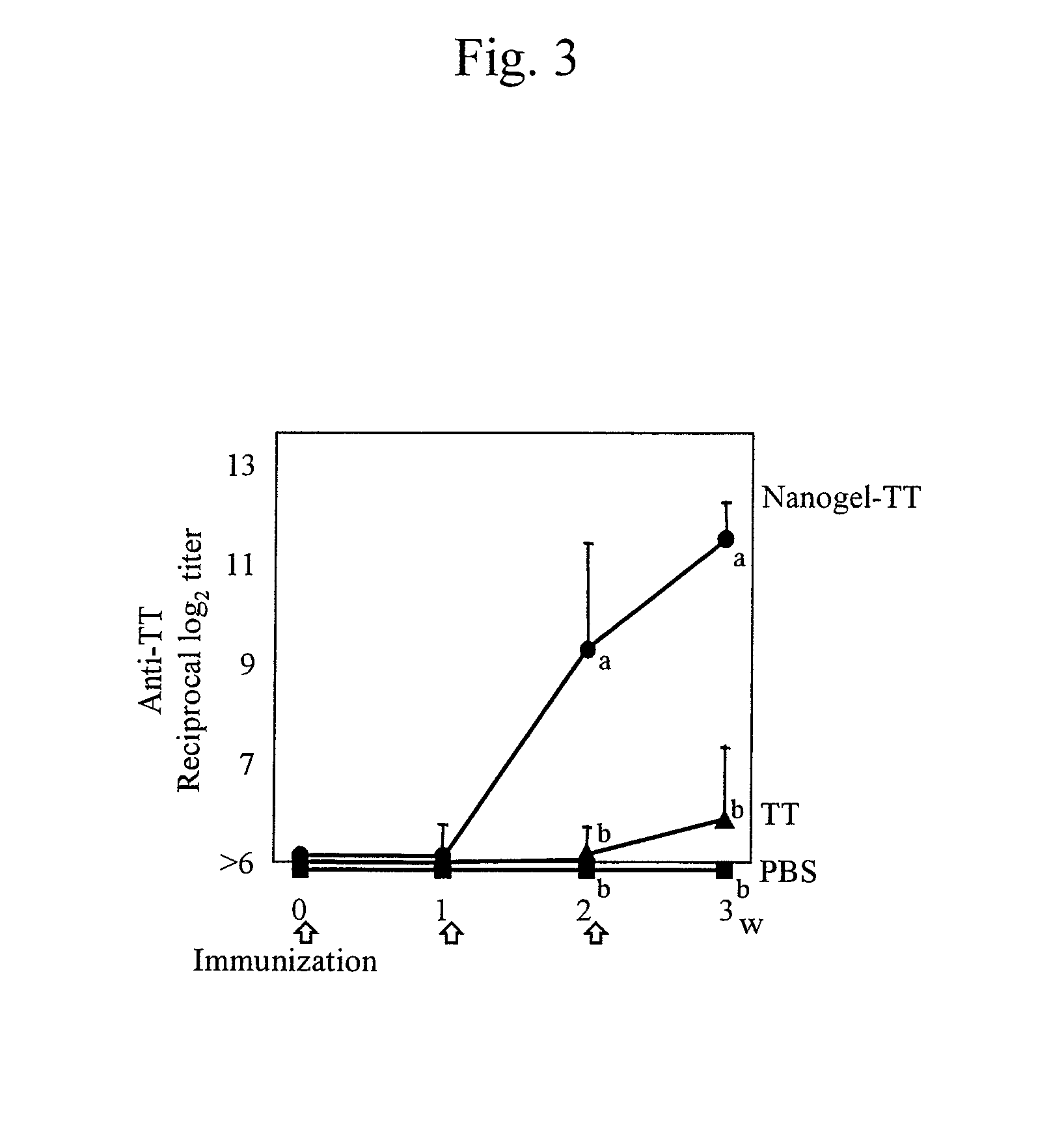
Mucosal vaccine using cationic nanogel
InactiveUS20110206729A1Effectively induces systemic and mucosal immune responseEfficient deliveryAntibacterial agentsBacterial antigen ingredientsMedicineCholesterol
A mucosal vaccine for the prevention or treatment of microbial infections is described that is capable of inducing vaccine antigen-specific immune responses in an organism without the addition of a mucosal adjuvant. The mucosal vaccine comprises a composite of a nanogel comprising a hydrophilic polysaccharide having a cationic functional group and a hydrophobic cholesterol added thereto as a side chain and a vaccine antigen. The vaccine is administered via a mucosal route.
Owner:NAT UNIV CORP TOKYO MEDICAL & DENTAL UNIV
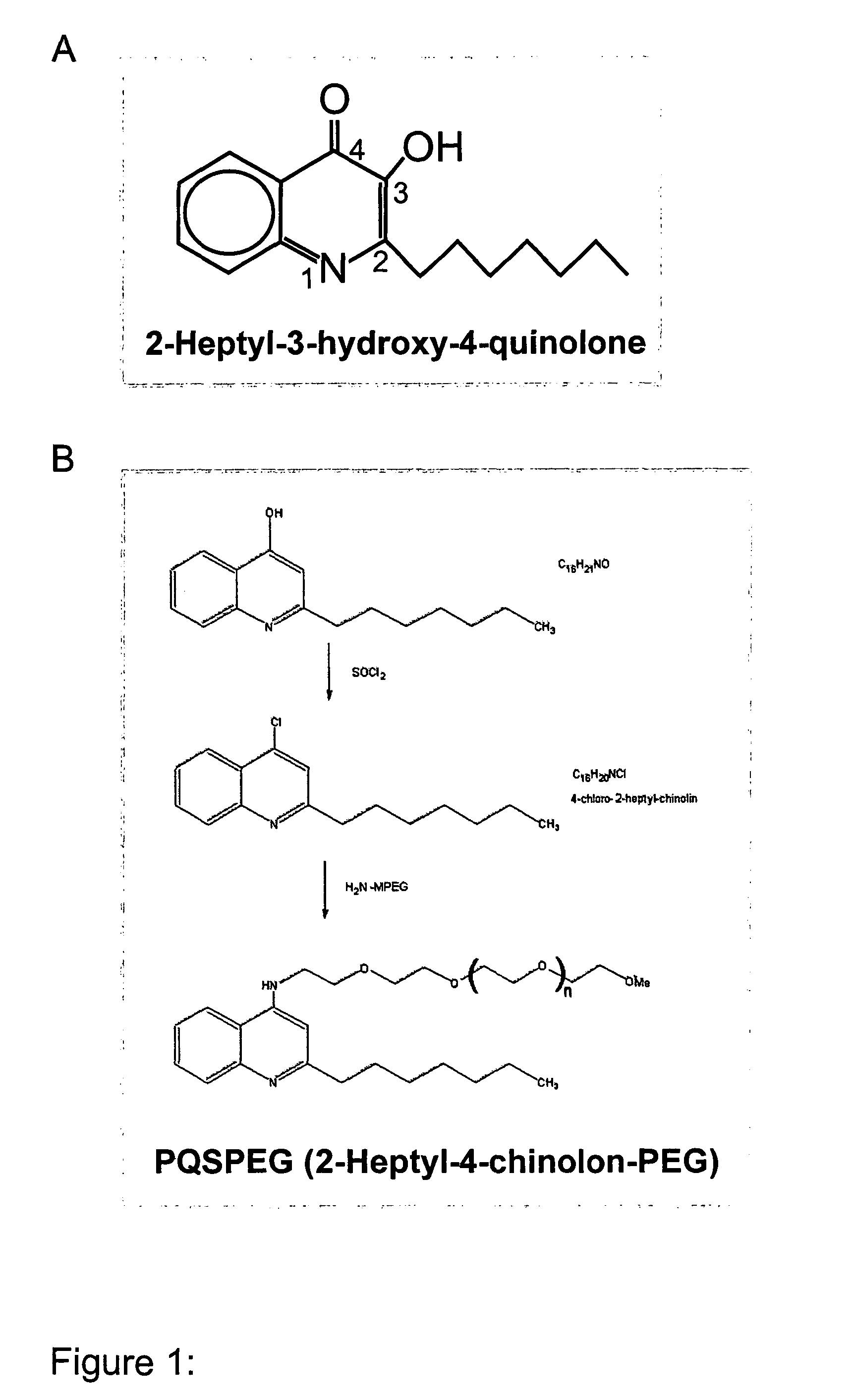
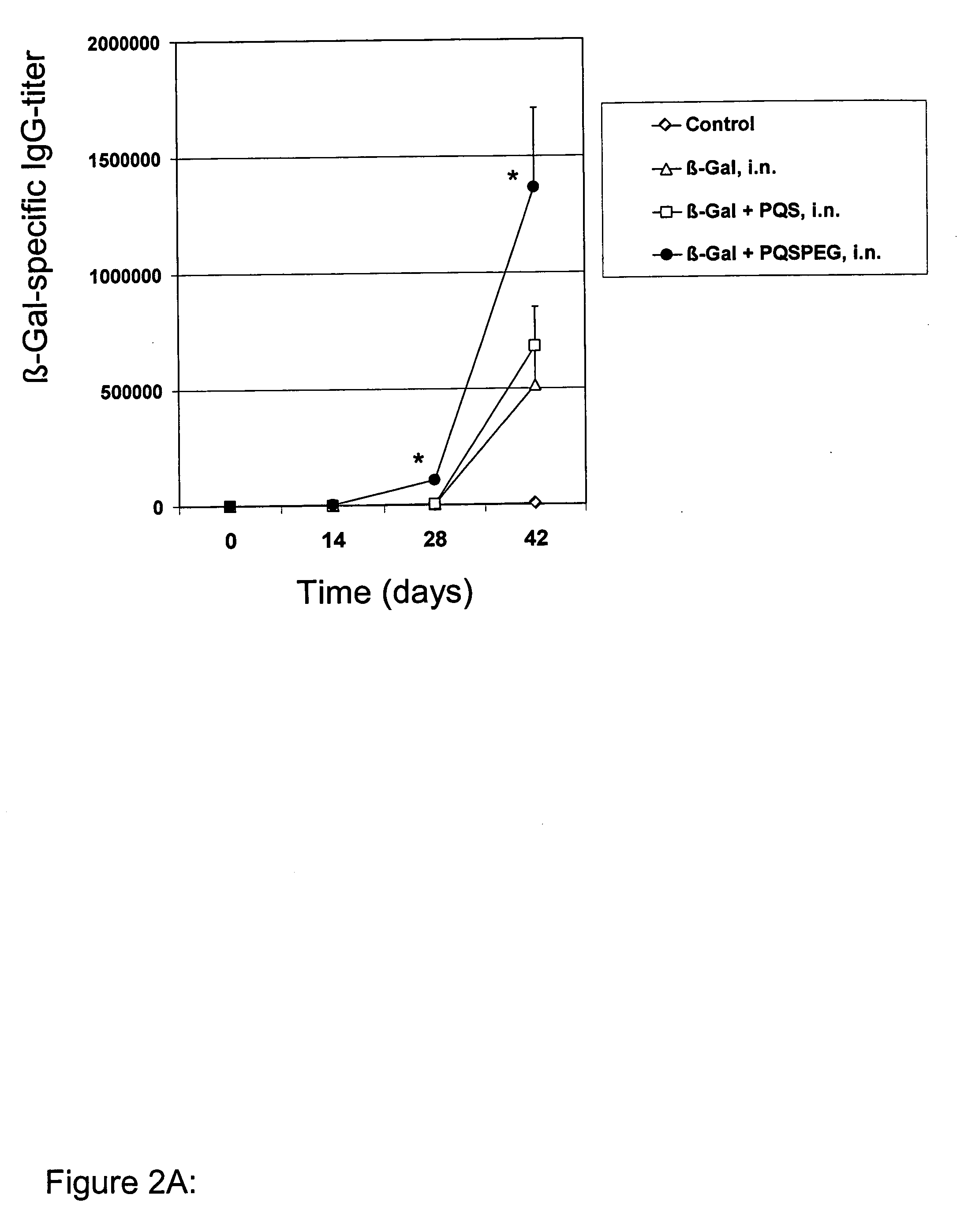
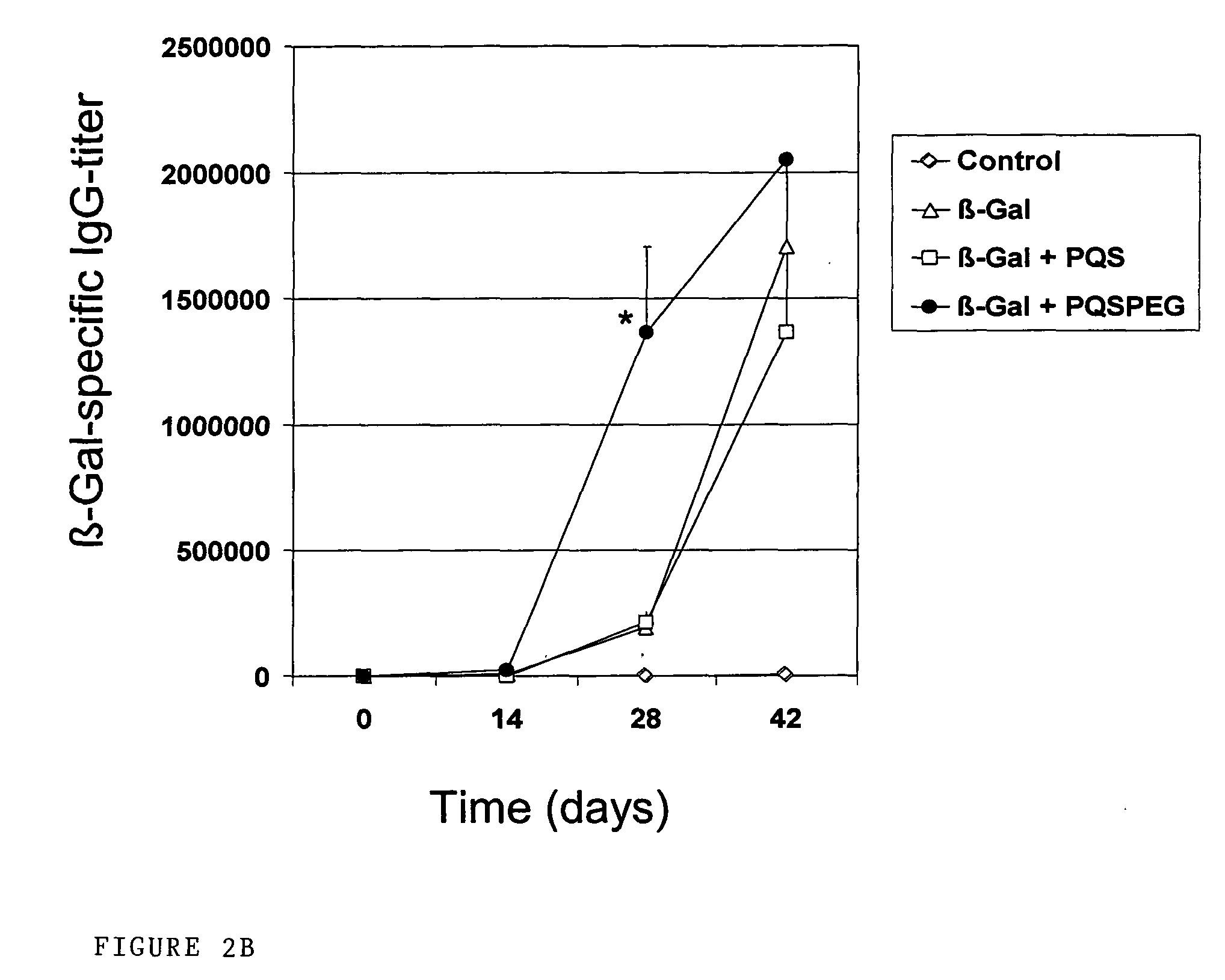
Pqs and its conjugates as adjuvants and their uses in pharmaceutical compositions
InactiveUS20090169609A1Less immunomodulatory effectEffective presentationOrganic active ingredientsOrganic chemistryWhole bodyMucosal adjuvant
The present invention relates to new adjuvants and the uses in pharmaceutical compositions, like in vaccines. In particular, the present invention provides new compounds useful as adjuvants and / or immunomodulators for prophylactic and / or therapeutic vaccination in the treatment of infectious diseases, inflammatory diseases, autoimmune diseases, tumours, allergies as well as for the control of fertility in human or animal populations. The compounds are particularly useful not only as systemic, but preferably as mucosal adjuvants. In addition, the invention relates to its uses as active ingredients in pharmaceutical compositions.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Novel, non-antigenic, mucosal adjuvant formulation which enhances the effects of substances, including vaccine antigens, in contact with mucosal body surfaces
InactiveUS20020009463A1Antibacterial agentsSsRNA viruses negative-senseMucosal adjuvantVaccine antigen
Adjuvant for mucosal vaccines which modulates the effects of substances, including vaccine antigens in contact with mucosal body surfaces.
Owner:BIOTEC PHARMACON
Adjuvants on the basis of bisacyloxypropylcystene conjugates and derivatives and their uses in pharmaceutical compositions
InactiveUS8119689B2Less immunomodulatory effectEffective presentationAntibacterial agentsOrganic active ingredientsImmunologic disordersAutoimmune disease
Bisacyloxycysteine type conjugates have been found to be useful as adjuvants and / or immunomodulators for prophylactic and / or therapeutic vaccination in the treatment of infectious diseases, inflammatory diseases, autoimmune diseases, tumors, and allergies, as well as in the control of fertility in human or animal populations. The compounds can be administered by system or mucosal routes and are particularly useful as mucosal adjuvants. These compounds can function as active ingredients in pharmaceutical compositions.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Swine fever oral attenuated freezing-dry vaccine and preparation method thereof and freeze-drying protective agent
InactiveCN106310250APrevent atrophyAvoid excessively large internal aperturesAntiviralsAntibody medical ingredientsGlycineFreeze-drying
The invention discloses a swine fever oral attenuated freezing-dry vaccine and a preparation method thereof and a freeze-drying protective agent, the swine fever oral attenuated freezing-dry vaccine includes antigen swine fever virus, a mucosal adjuvant and the freeze-drying protective agent, the freeze-drying protective agent includes 1-3% of gelatin, 1-3% of glycine and balance of water, the swine fever oral attenuated freezing-dry vaccine can well protect the activity of CSFV (Classical Swine Fever Virus), reduces live virus loss, can induce higher levels of IgG and IgA antibodies in oral immunization tests of mice, can promote expression of type I interferon in thymus and spleen, has the effect of protecting antigen activity and improving oral swine fever antigen immune efficiency, and can be used as a swine fever oral vaccine protective agent for industry development.
Owner:SHANGHAI ACAD OF AGRI SCI
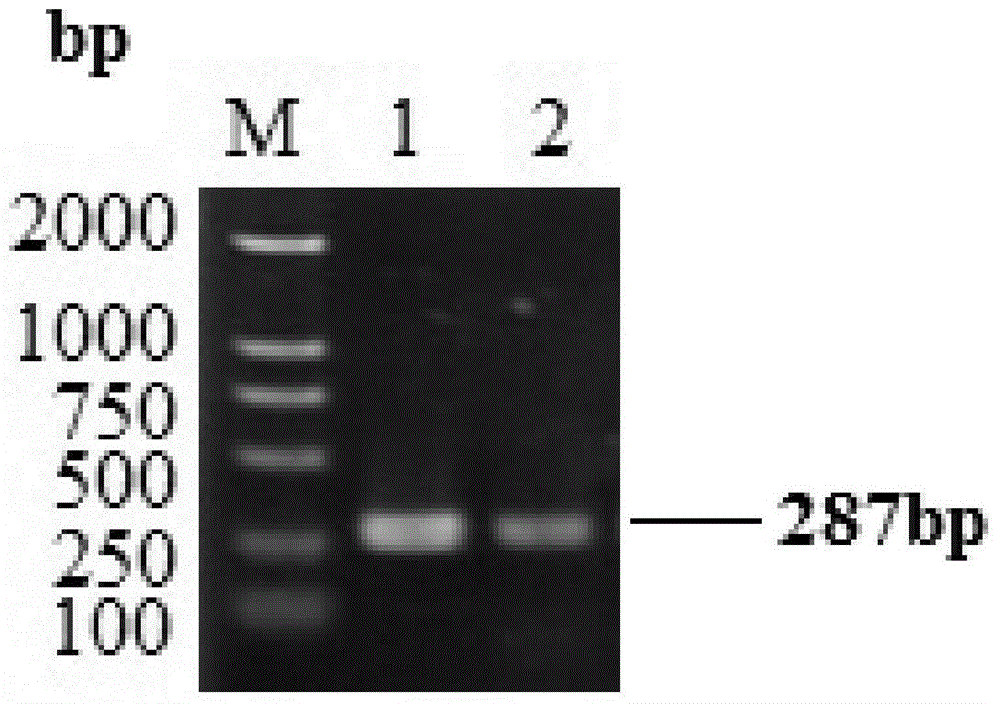
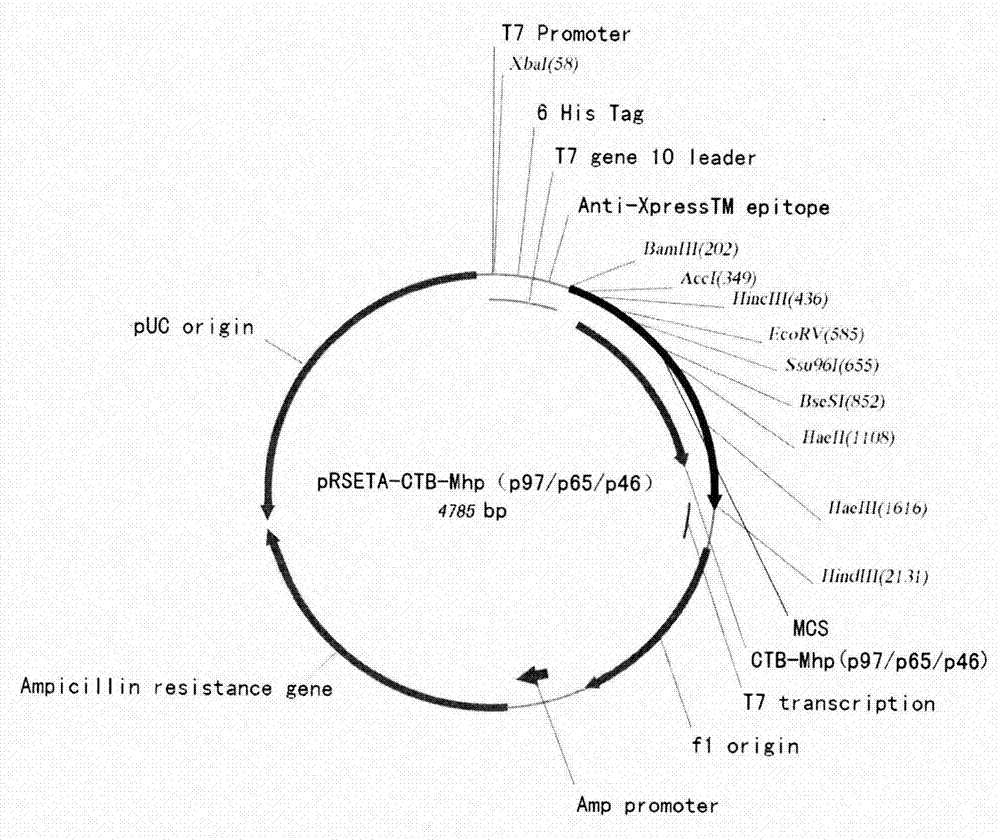
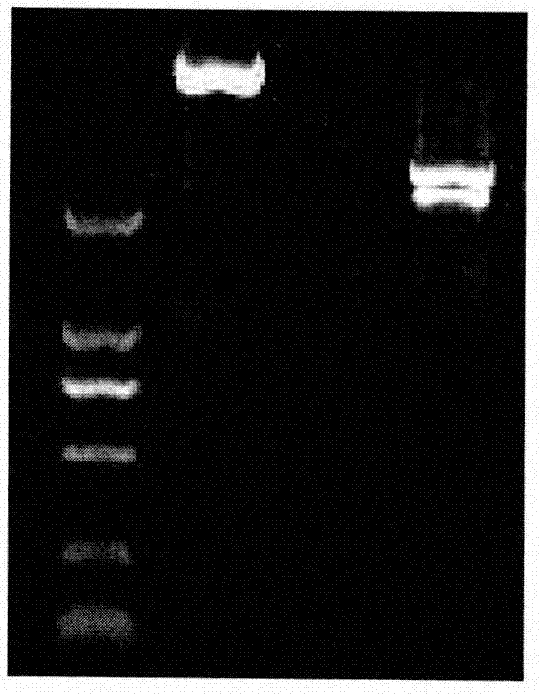
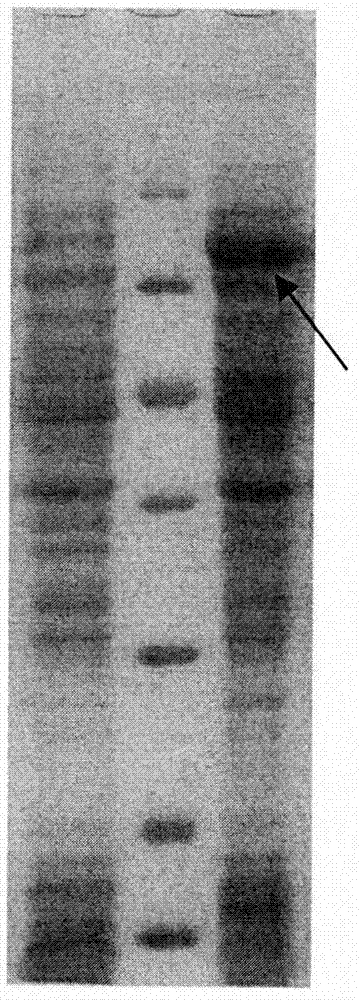
Mycoplasma hyopneumoniae multi-epitope mucosal vaccine
The invention relates to preparation and application of a mycoplasma hyopneumoniae multi-epitope mucosal vaccine. A mycoplasma hyopneumoniae membrane protein, an adhesive protein P97, a lipoprotein P65, a specific membrane protein P46, a B cell epitope, a Th epitope, a CTL epitope and a cholera toxin subunit B are taken as a vaccine frame structure, a pRSETA carrier is cloned in through flexible linker connection, then Escherichia coli is transformed, and fermentation, purification and preparation technologies are carried out, so that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine with ideal immunogenicity is obtained. A self-made mucosal adjuvant is used in a preparation process, so that production and using processes of the vaccine are simpler and more convenient. Animal experiments show that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine not only has good safety but also can stimulate effective mucosal immunity, humoral immunity and cellular immune reactions.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
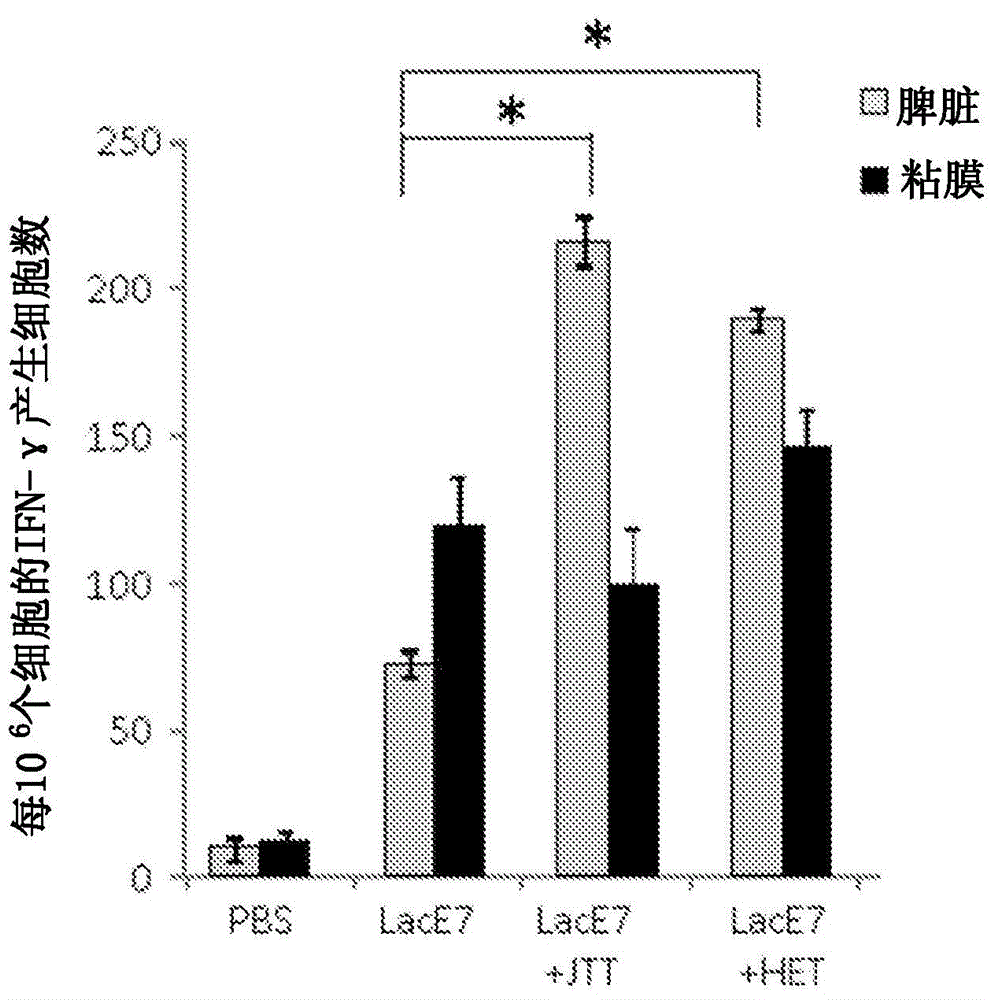
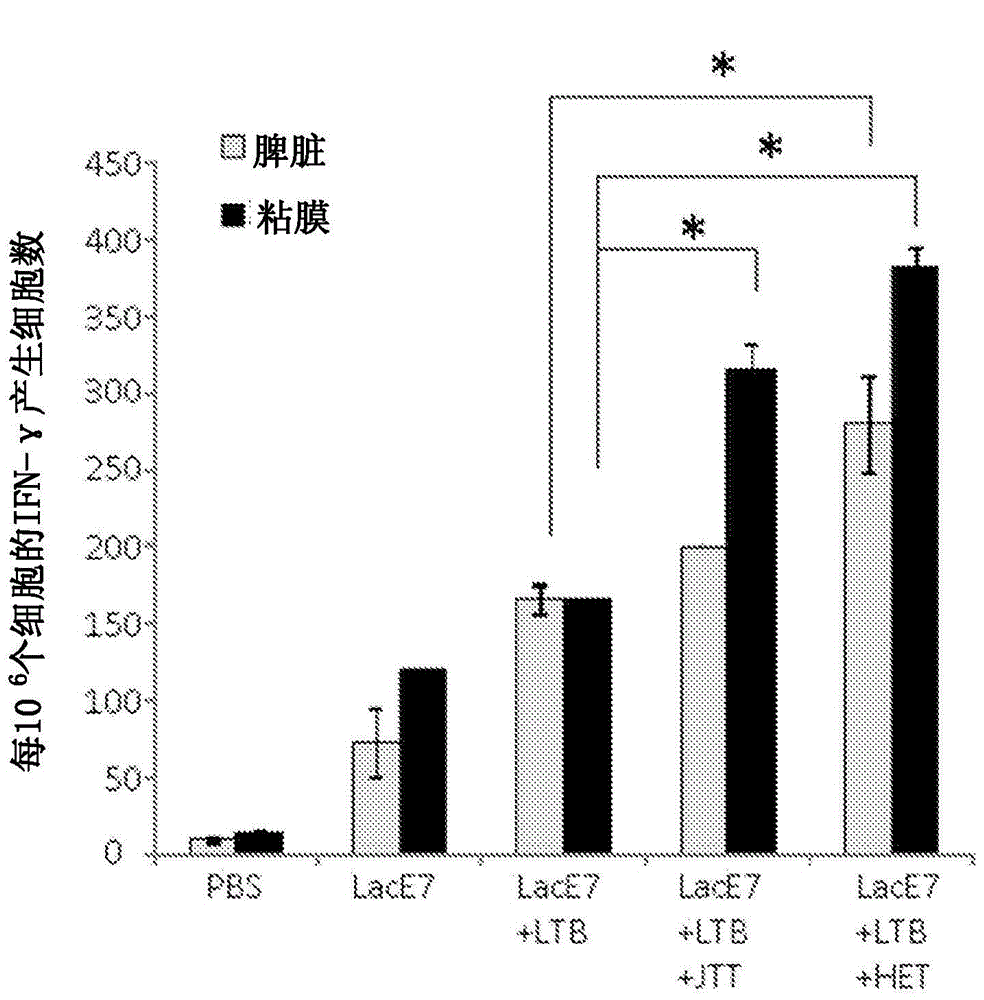
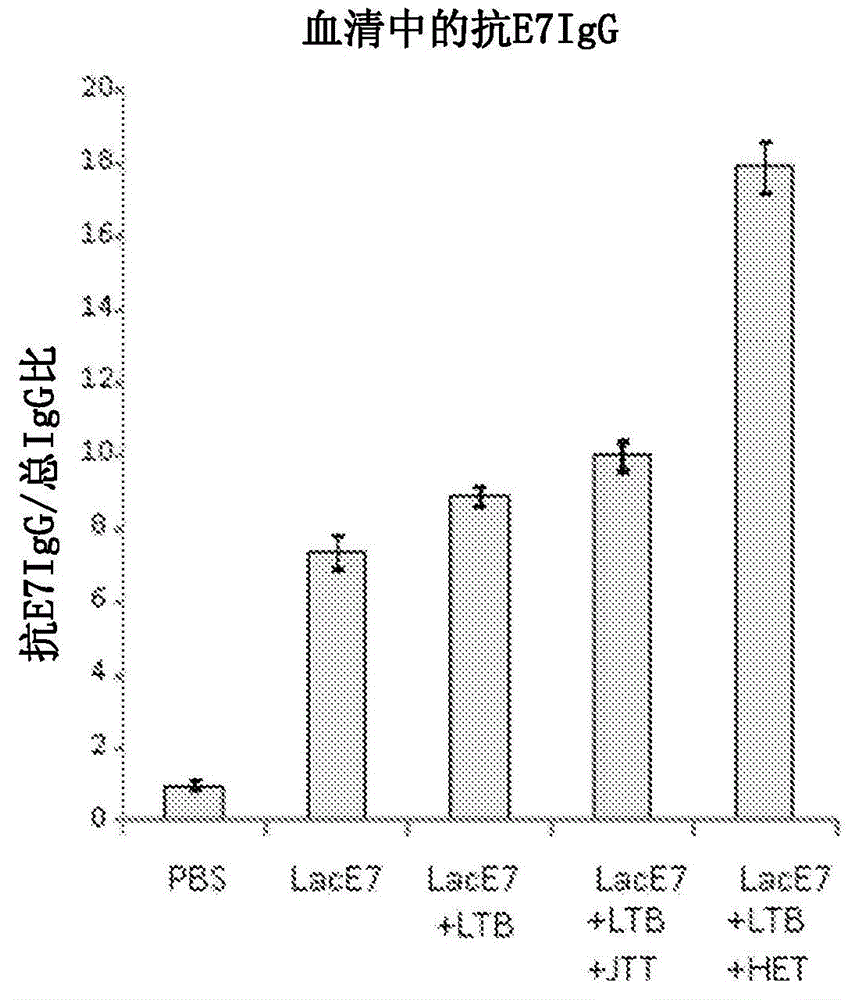
Mucosal immunity-stimulating agent, and oral pharmaceutical composition for treating hpv infection
A mucosal immunity-stimulating agent comprises a Kampo medicine having a revitalizing activity and a mucosal adjuvant. An oral pharmaceutical composition for treating HPV infection comprises at least E7 polypeptide of HPV and a Kampo medicine having a revitalizing activity.
Owner:THE UNIV OF TOKYO
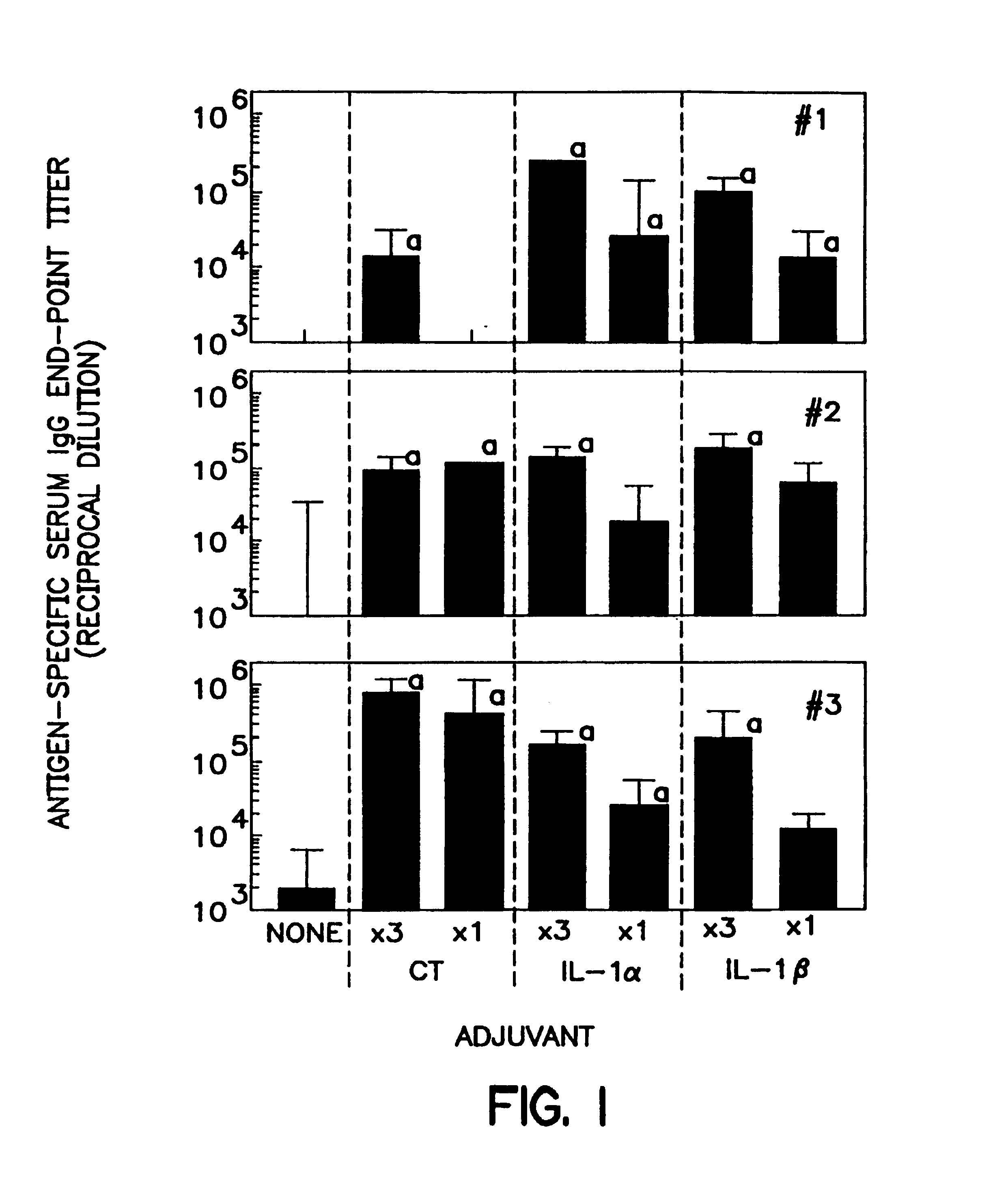
Substantially non-toxic biologically active mucosal adjuvants in vertebrate subjects
InactiveUS7041294B2Address oral toleranceSafe and effectiveBacterial antigen ingredientsProtozoa antigen ingredientsInterleukin 1αWhite blood cell
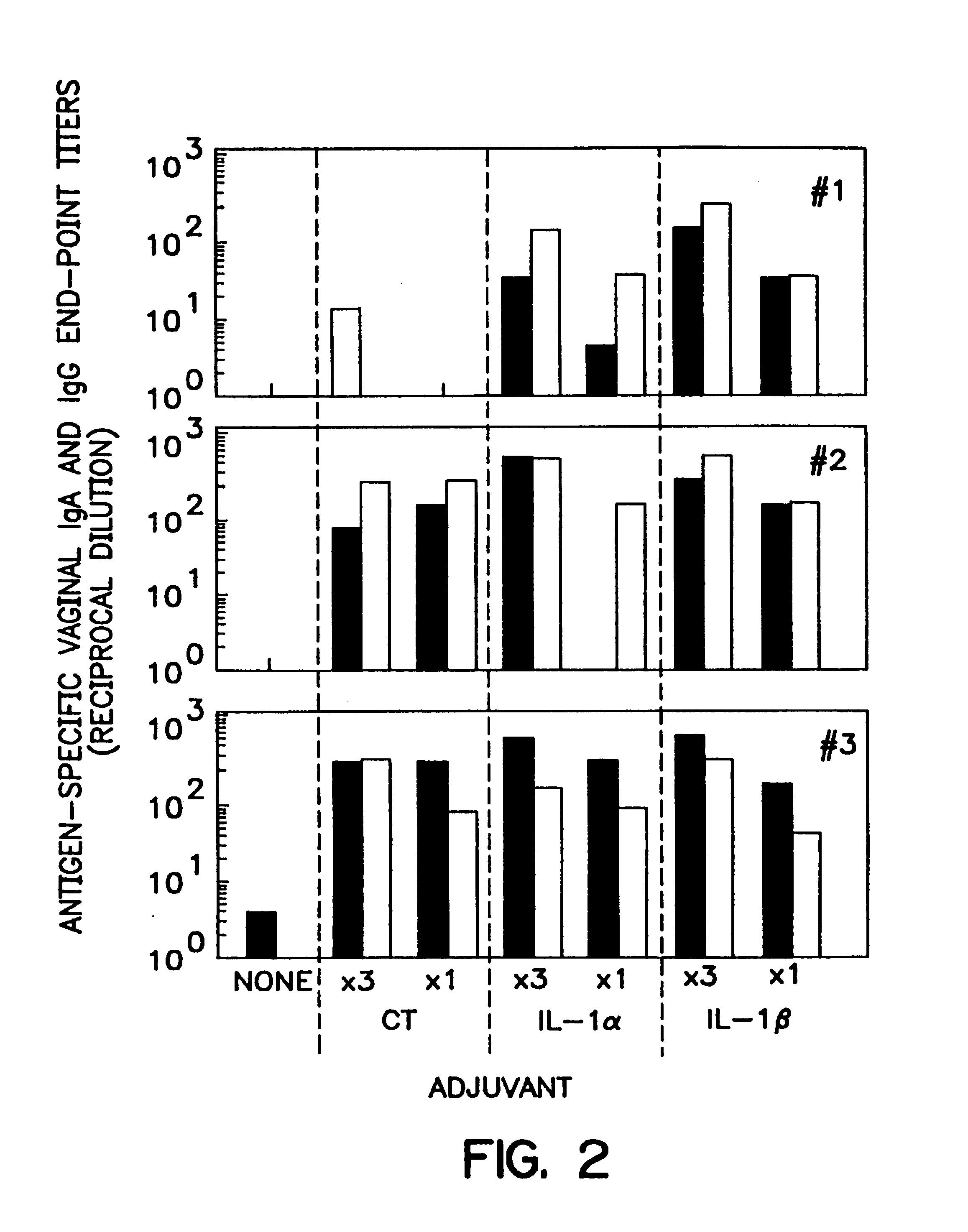
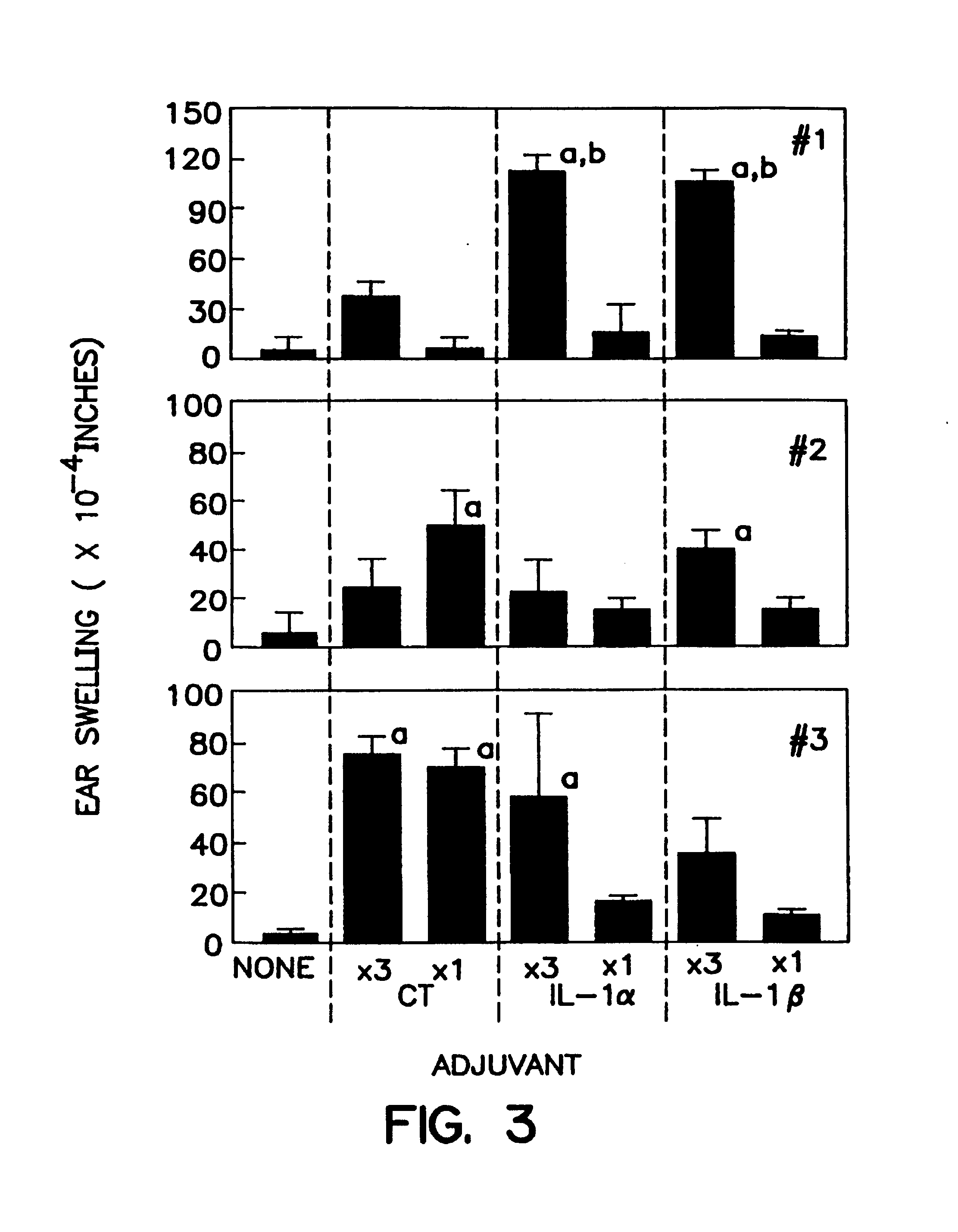
A method of eliciting an immune response against an antigen in a vertebrate subject, the method comprising the steps of providing an antigen-adjuvant composition comprising the antigen and a substantially non-toxic adjuvant molecule having biological activity in mucosal tissues, and administering said antigen-adjuvant composition to the vertebrate subject in a manner such that initial contact occurs in mucosal tissue of the vertebrate subject, whereby an immune response is elicited. Cytokines are preferred adjuvants. Preferred cytokines are interleukin-1α(IL-1α) and interleukin-1β (IL-1β).
Owner:DUKE UNIV
Non-toxic mucosal adjuvant
InactiveUS20060177469A1Improving immunogenicityImprove responseAntibacterial agentsBacterial antigen ingredientsMucosal adjuvantMutant
A non-toxic mucosal adjuvant is provided which may be admixed with further antigens to provide a vaccine administrable to mucosal surfaces in organisms including man. Preferably, the non-toxic mucosal adjuvant is a detoxified mutant of a bacterial ADP-ribosylating toxin, optionally comprising one or more amino acid additions, deletions or substitutions.
Owner:CHIRON CORP
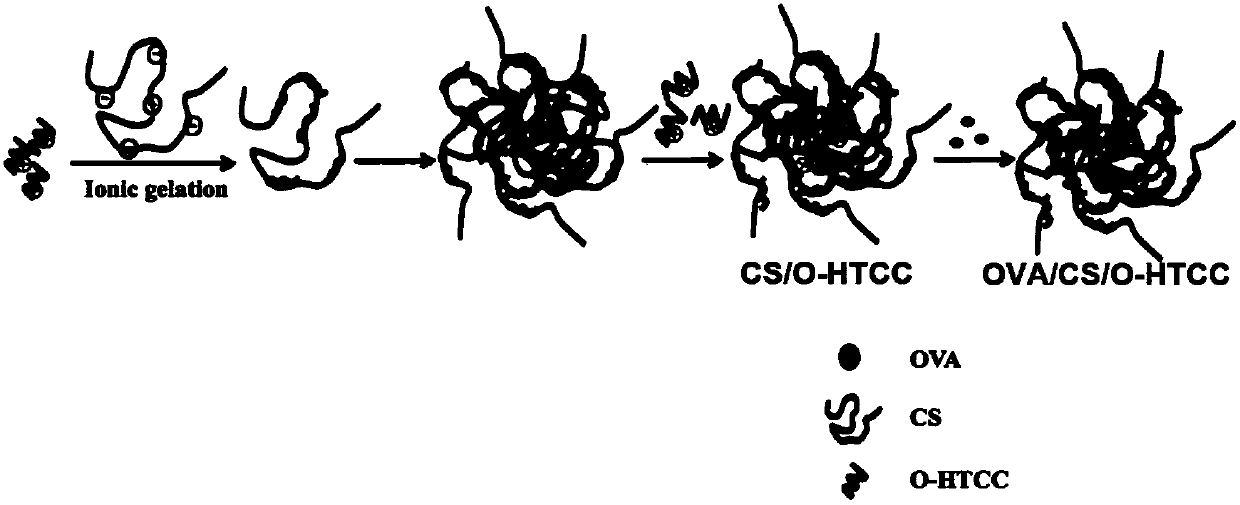
Curdlan sulfate/6-O-quaternized chitosan nanoparticles and application thereof in mucosal vaccines
InactiveCN107625961APromote proliferationPromote activationAntiinfectivesRespiratory disorderEpitheliumMucosal adjuvant
Nanoparticles are prepared from curdlan sulfate and 6-O-quaternized chitosan by an ion gelation method for the first time; the method is simple and convenient, green, safe and pollution-free, and through controlling experimental steps and parameters, the prepared curdlan sulfate / 6-O-quaternized chitosan nanoparticles have uniform size and good stability. Evaluation of the membrane penetrating ability, the immune regulation ability and the mucosal adjuvant effect of the curdlan sulfate / 6-O-quaternized chitosan nanoparticles shows that the positively charged characteristic of the nanoparticles make the nanoparticles more easily act on effective parts through epithelium mucosae, so as to play a role of immune enhancers and help an antigen reach the effective parts to play relatively good immune adjuvant effect; the nanoparticles have great industrial application value.
Owner:SHANDONG UNIV
Delivery system and application of chitosan mucosa compromising mucosal adjuvant
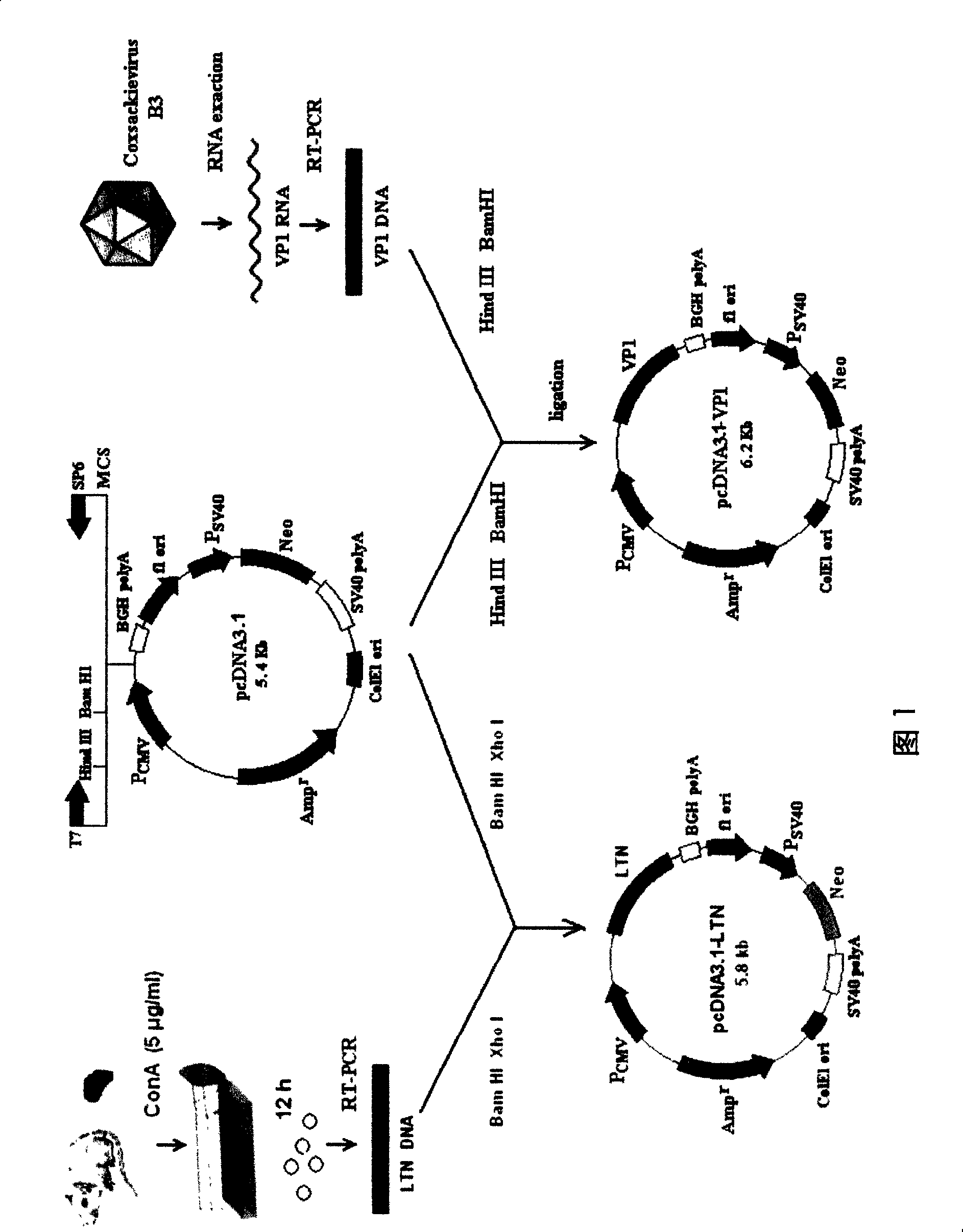
InactiveCN101204364AConvenient inductionOrganic active ingredientsViral antigen ingredientsMucosal adjuvantViral Myocarditis
The invention relates to a chitosan mucosal delivery system, and the invention comprises two components which are the target antigen nanoparticles and the mucosal adjuvant nanoparticles. The target antigen nanoparticle comprises chitosan and target antigen coding plasmid DNA; the mucosal adjuvant nanoparticle comprises chitosan and mucosal adjuvant coding plasmid DNA. The mucosal vaccine which is produced according to the mucosal delivery system comprises two components, a component is the chitosan-VP1 nanoparticle which is formed by the chitosan and the plasmid of the coding CVB3 structural protein VP1 and the other component is the chitosan-VP1 nanoparticle which is formed by the chitosan and the plasmid of the coding lymphocyte chemotactic factor. By using the mucosal delivery system to carry out the mucosal immunity, the secreting of CVB3 specific generalization IgG and intestinal tract SIgA is induced effectively, and the occurrence of the Coxsackie vital myocarditis is effectively prevented.
Owner:FUDAN UNIV
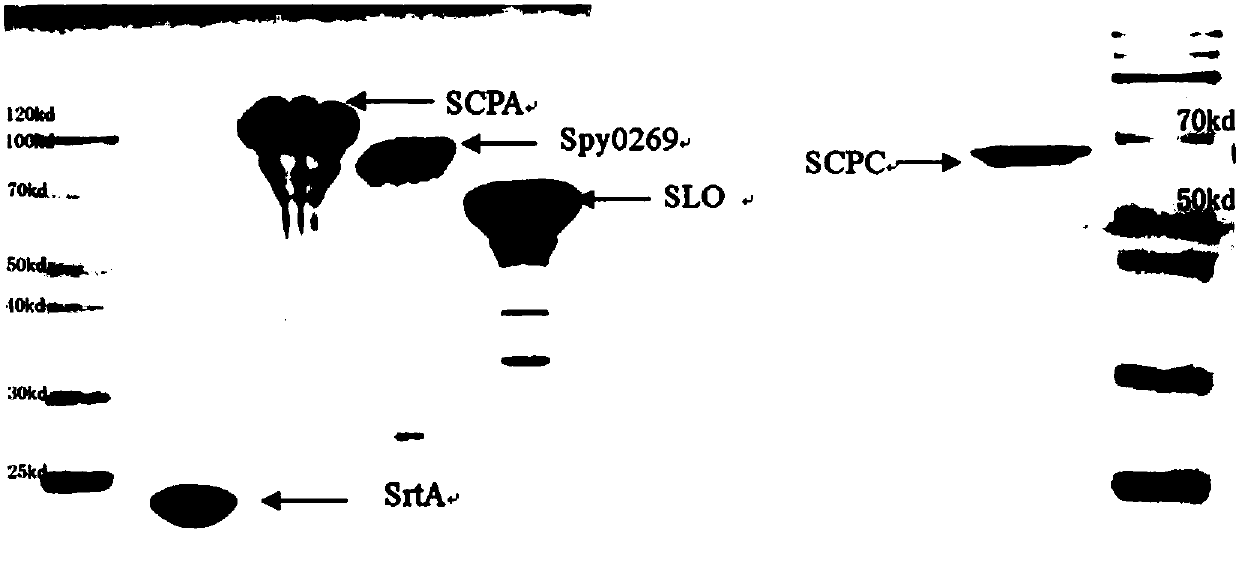
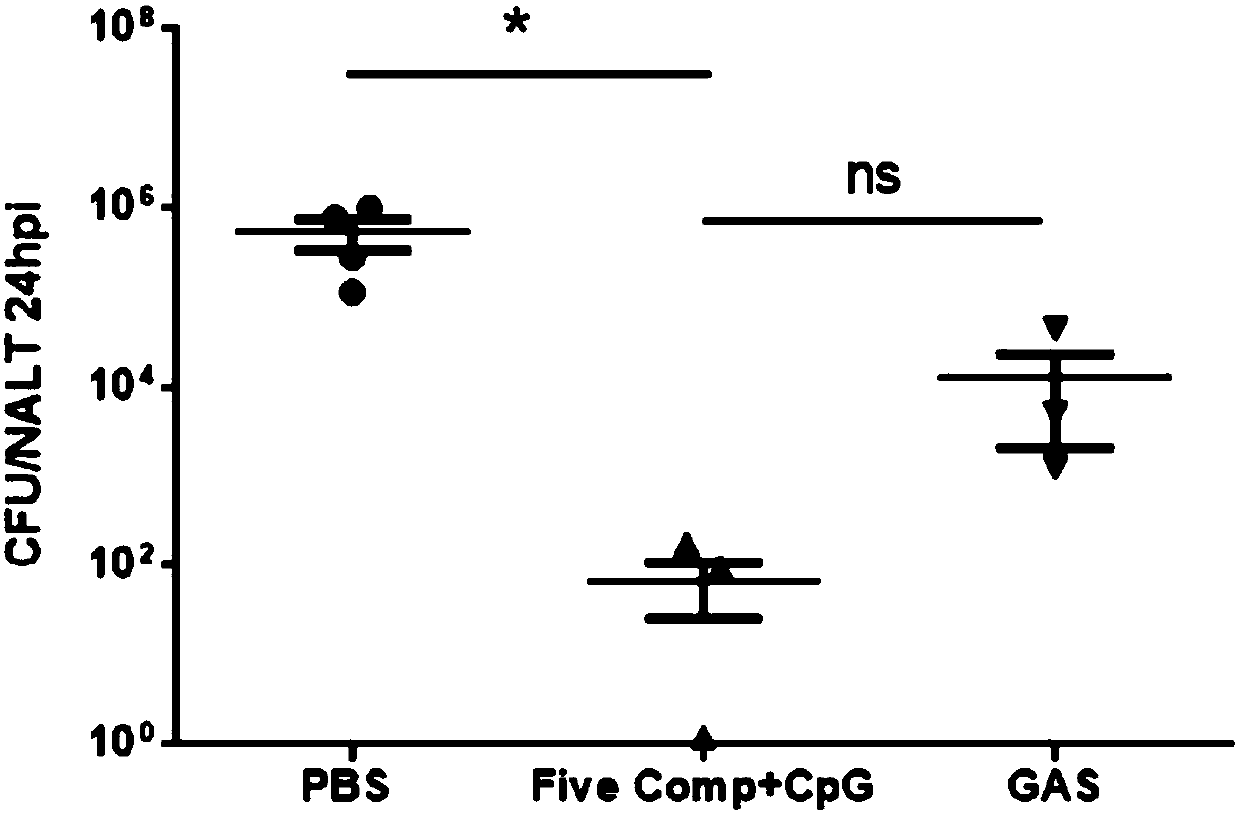
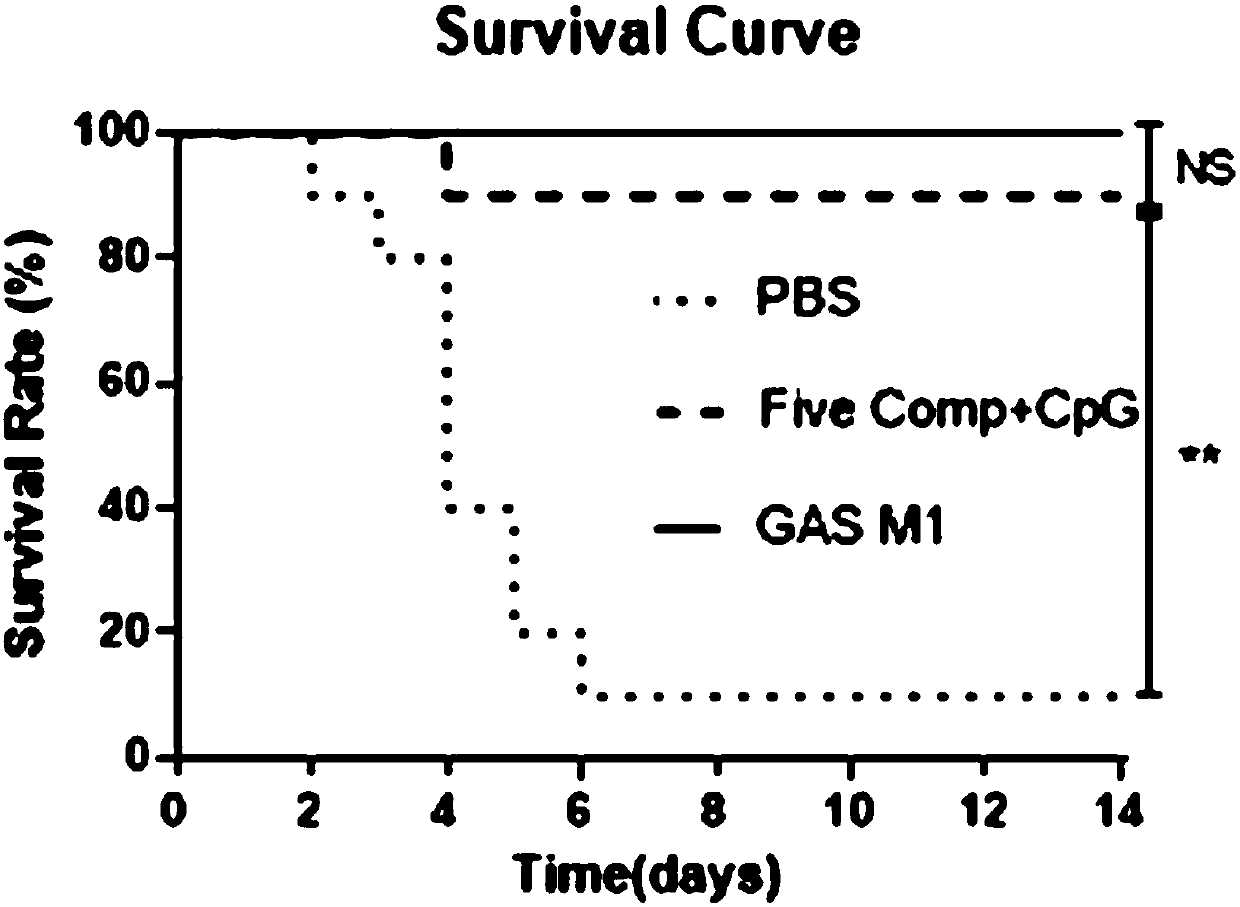
Broad-spectrum multi-subunit vaccine for preventing A type streptococcal infection
ActiveCN107737334ANo tissue damageNo local side effectsAntibacterial agentsBacterial antigen ingredientsSide effectMucosal adjuvant
The invention discloses a vaccine for preventing A type streptococcal infection. The vaccine provided by the invention has the active ingredients such as ingredient A, ingredient B, ingredient C, ingredient D, ingredient E and ingredient F; the ingredient A is sortase or fusion protein with sortase; the ingredient B is SCPA or fusion protein with SCPA; the ingredient C is Spy0269 or fusion proteinwith Spy0269; the ingredient D is SCPC or fusion protein with SCPC; the ingredient E is SLO or fusion protein with SLO; and the ingredient F is adjuvant CpG or other mucosal adjuvants. The vaccine provided by the invention has the advantages of high efficiency, broad spectrum and low cost. Meanwhile, the vaccine provided by the invention adopts the way of mucosal immunity, has the characteristicsof no tissue damage, no local side effect and simple and convenient use, and is easy to popularize and use.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Mucosal adjuvant and its preparation method and use
InactiveCN102688488ASafe and non-toxicReduce releaseAntibacterial agentsGenetic material ingredientsNasal cavityVaccination
The invention provides a mucosal adjuvant. The mucosal adjuvant is prepared by copolymerization crosslinking compounding of oligo-chitosan and lymphotactin encoding plasmids. The invention also discloses a preparation method and a use of the mucosal adjuvant. When the mucosal adjuvant and a gene vaccine composed of chitosan or oligo-chitosan are synchronously dropped into a nasal cavity for immunization, coxsackievirus specific serum antibody and systemic (spleen and lymph glands) Th1-type immune responses are induced and especially, intestinal mucosa partially-reinforced specific-secretion-type sIgA and IFNgama+Th1 responses are induced, and thus a gene vaccine used with the mucosal adjuvant is obviously superior to an exposed gene vaccine and effectively prevents coxsackievirus-caused myocarditis. The mucosal adjuvant can be used as a novel mucosal vaccination adjuvant.
Owner:FUDAN UNIV
Chlamydia vaccines
Vaccine preparations are provided for the prevention of Chlamydia infections comprising a major outer membrane protein from chlamydia and a mucosal adjuvant such as a combination of QS21 and 3D-MPL, or chlorea Toxin or Heat labile enterotoxin. Such preparations provide protection from Chlamydia induced fertility.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Nanoemulsion vaccines
The present invention provides methods and compositions for the stimulation of immune responses. Specifically, the present invention provides methods and compositions for the use of nanoemulsion compounds as mucosal adjuvants to induce immunity against environmental pathogens. Accordingly, in some embodiments, the present invention provides nanoemulsion vaccines comprising a nanoemulsion and an inactivated pathogen or protein derived from the pathogen. The present invention thus provides improved vaccines against a variety of environmental and human-released pathogens.
Owner:RGT UNIV OF MICHIGAN
Immune adjuvant and application thereof
InactiveCN110974953AGood adjuvant effectSsRNA viruses negative-senseViral antigen ingredientsMucosal Immune ResponsesMucosal adjuvant
The invention discloses an immune adjuvant. The immune adjuvant comprises one or more selected from a salt chitosan solution, a quaternized chitosan solution or a hydroxylated chitosan solution, wherein the pH value of the salt chitosan solution is 4.5-5.5, the pH value of the quaternized chitosan solution is 5.8-8, and the pH value of the hydroxylated chitosan solution is 5.8-8. The screened specific chitosan derivatives can enhance humoral immune response and mucosal immune response of different antigens, have a good adjuvant effect, lay a foundation for research on the action mechanism of chitosan derivative adjuvants, and are expected to replace chitosan in development of mucosal adjuvants.
Owner:NAT VACCINE & SERUM INST
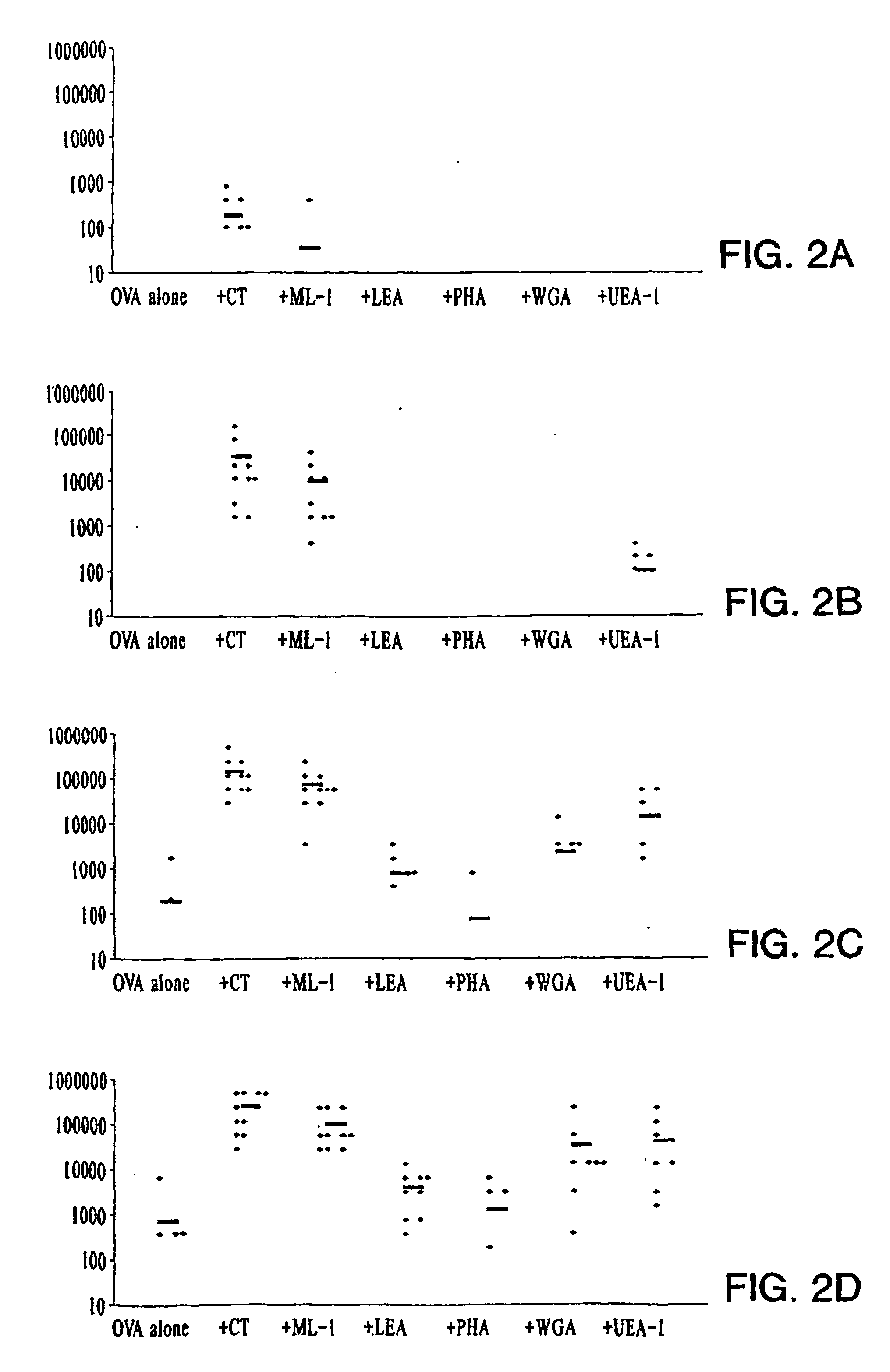
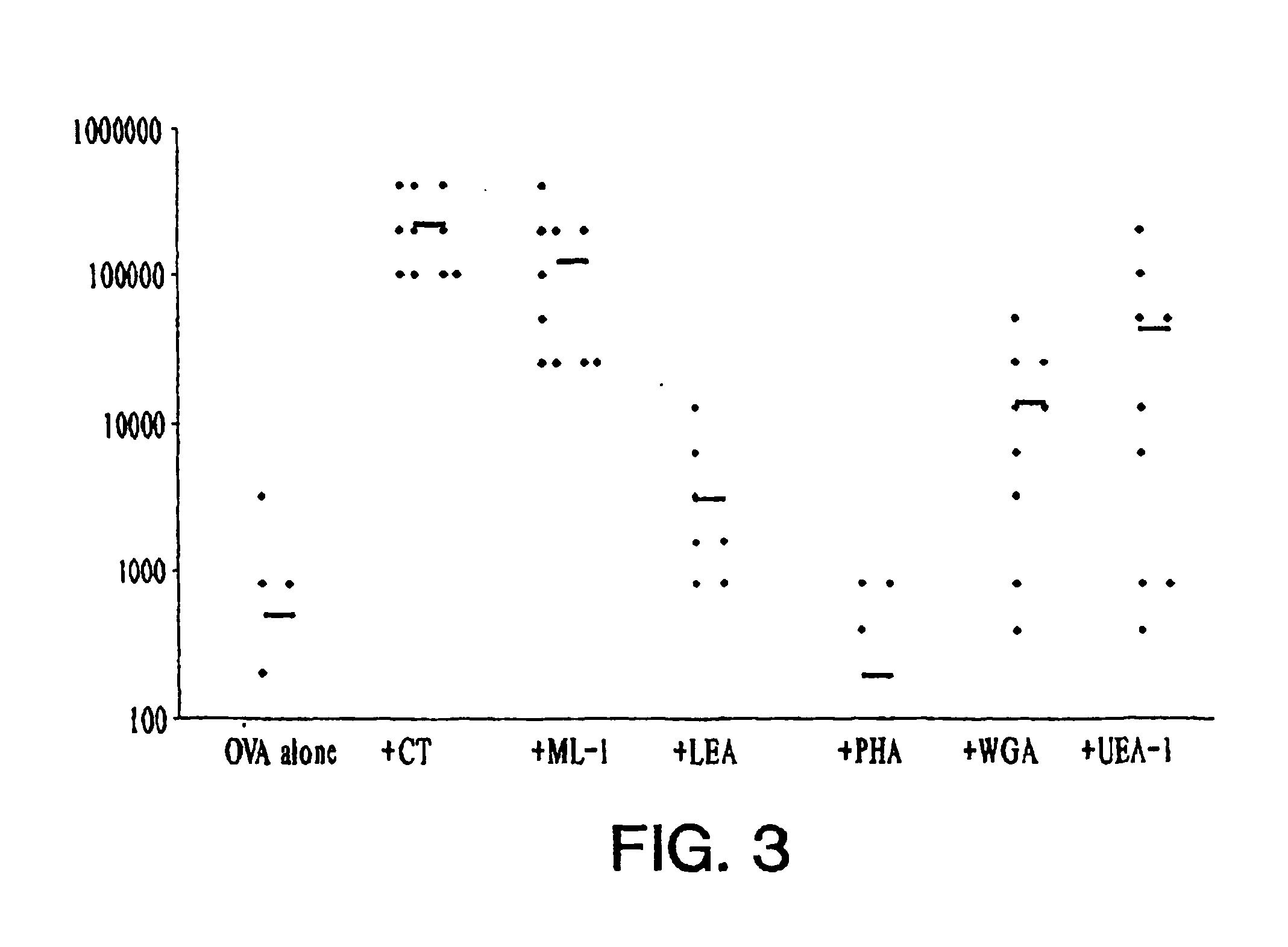
Plant lectins as mucosal adjuvants
InactiveUS6863896B1Enhance immune responseSimple methodPeptide preparation methodsDepsipeptidesPlant LectinsMucosal adjuvant
The invention provides a method of increasing an immune response in a mammal. The method involves administering to the mammal an admixture comprising an immunogen and a plant lectin. The plant lectin acts as an adjuvant to increase an immune response against the immunogen. The method is especially well-suited for mucosal administration to humans and other mammals.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
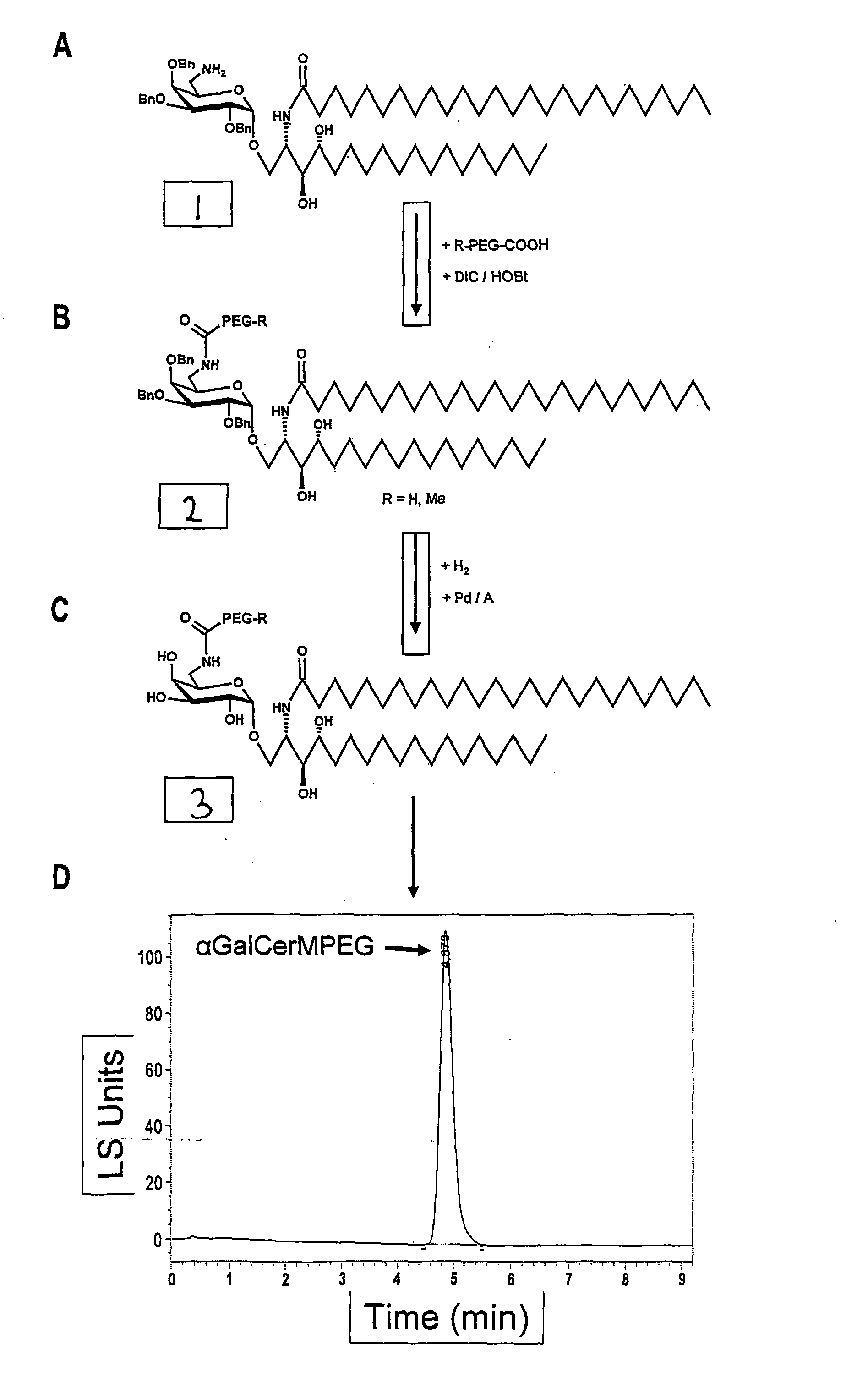
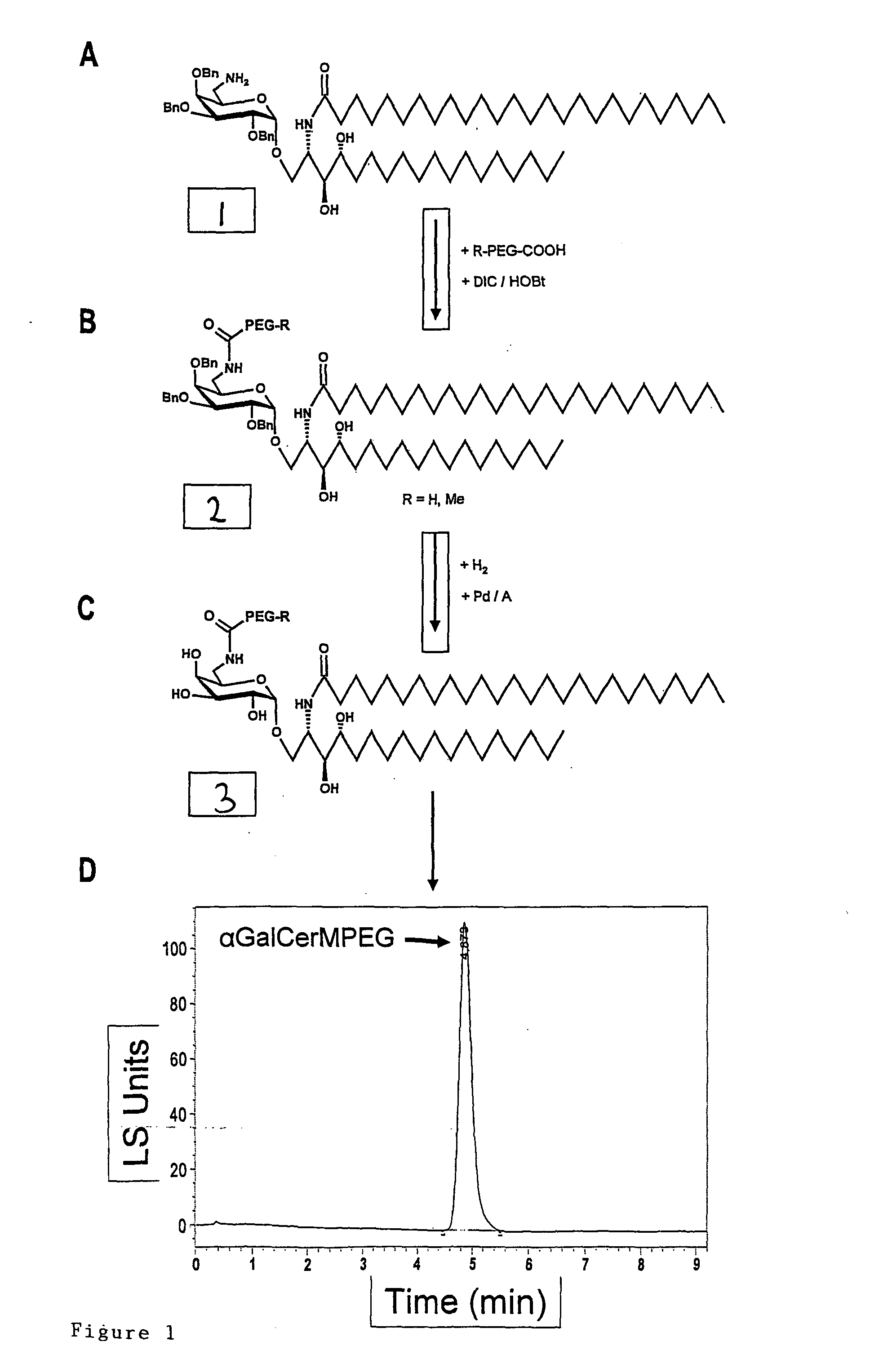
Hexosylceramides as Adjuvants and Their Uses in Pharmaceutical Compositions
ActiveUS20080206319A1Improve solubilityImprove compound stabilityBiocideAntibiotics chemistryWhole bodyAllergy
The present invention relates to new adjuvants and the uses in pharmaceutical compositions, like in vaccines. In particular, the present invention provides new compounds useful as adjuvants for prophylactic and / or therapeutic vaccination in the treatment of infectious diseases, inflammatory diseases, autoimmune diseases, tumours, allergies as well as for the control of fertility in human or animal populations. The compounds are particularly useful not only as systemic, but preferably as mucosal adjuvants. In addition, the invention relates to its uses as active ingredients in pharmaceutical compositions.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Mucosal Vaccine Using Cationic Nanogel
A mucosal vaccine for the prevention or treatment of microbial infections is described that is capable of inducing vaccine antigen-specific immune responses in an organism without the addition of a mucosal adjuvant. The mucosal vaccine comprises a composite of a nanogel comprising a hydrophilic polysaccharide having a cationic functional group and a hydrophobic cholesterol added thereto as a side chain and a vaccine antigen. The vaccine is administered via a mucosal route.
Owner:INTPROP STRATEGY NETWORK INC
Hexosylceramides as adjuvants and their uses in pharmaceutical compositions
ActiveUS8053417B2Less immunomodulatory effectEffective presentationBiocideAntibiotics chemistryWhole bodyAutoimmune disease
The present invention relates to new adjuvants and the uses in pharmaceutical compositions, like in vaccines. In particular, the present invention provides new compounds useful as adjuvants for prophylactic and / or therapeutic vaccination in the treatment of infectious diseases, inflammatory diseases, autoimmune diseases, tumours, allergies as well as for the control of fertility in human or animal populations. The compounds are particularly useful not only as systemic, but preferably as mucosal adjuvants. In addition, the invention relates to its uses as active ingredients in pharmaceutical compositions.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Mucosal DTPa vaccines
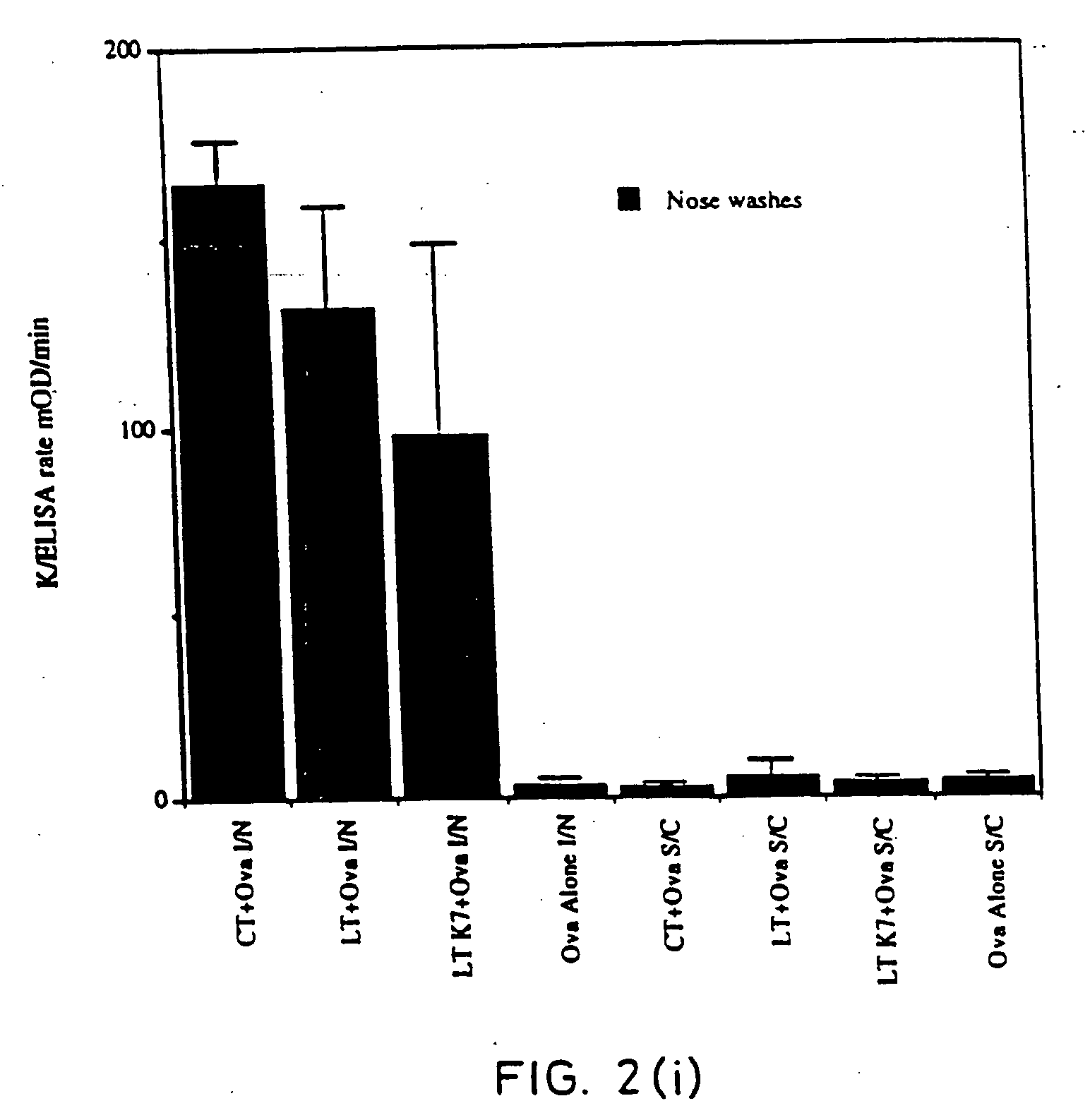
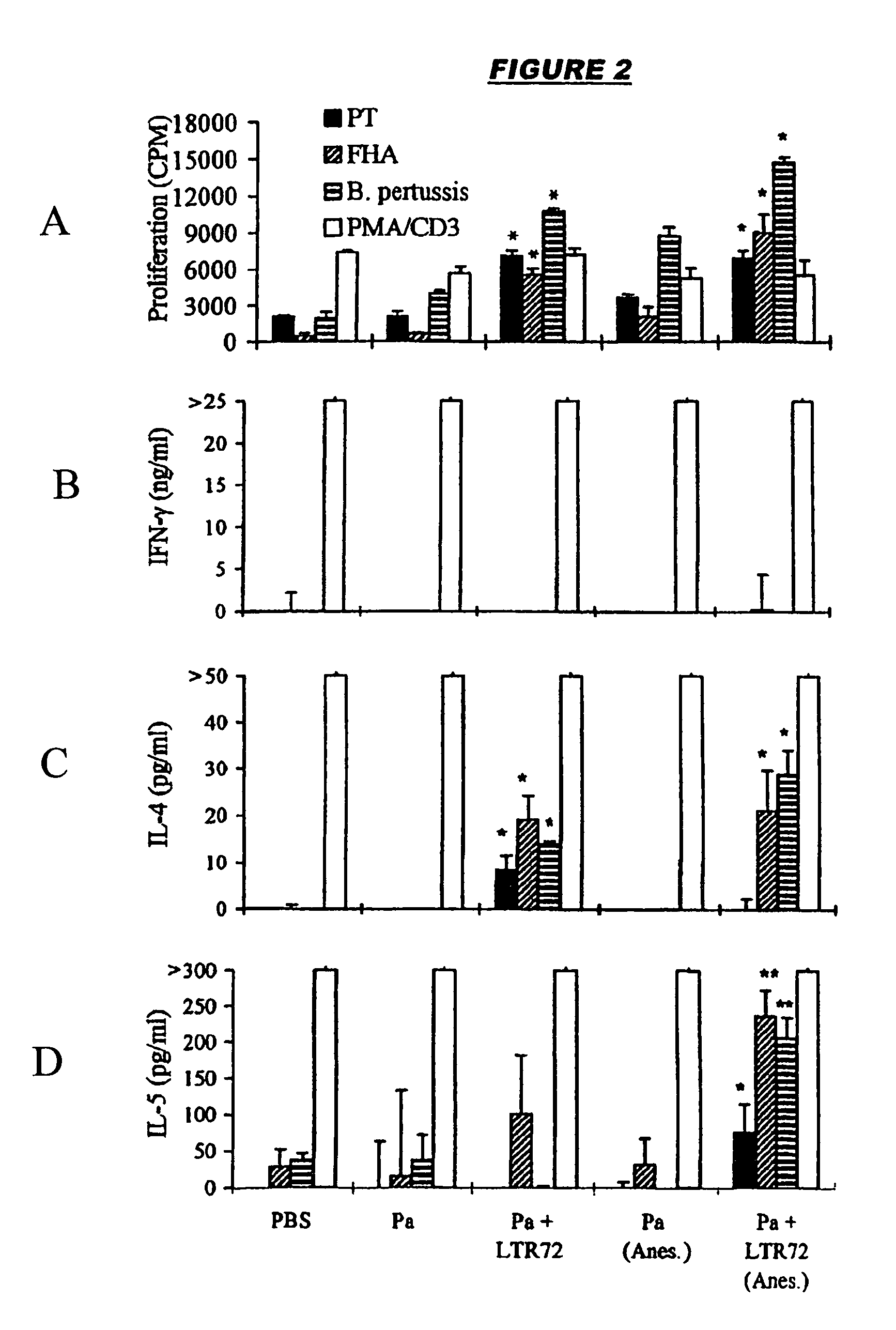
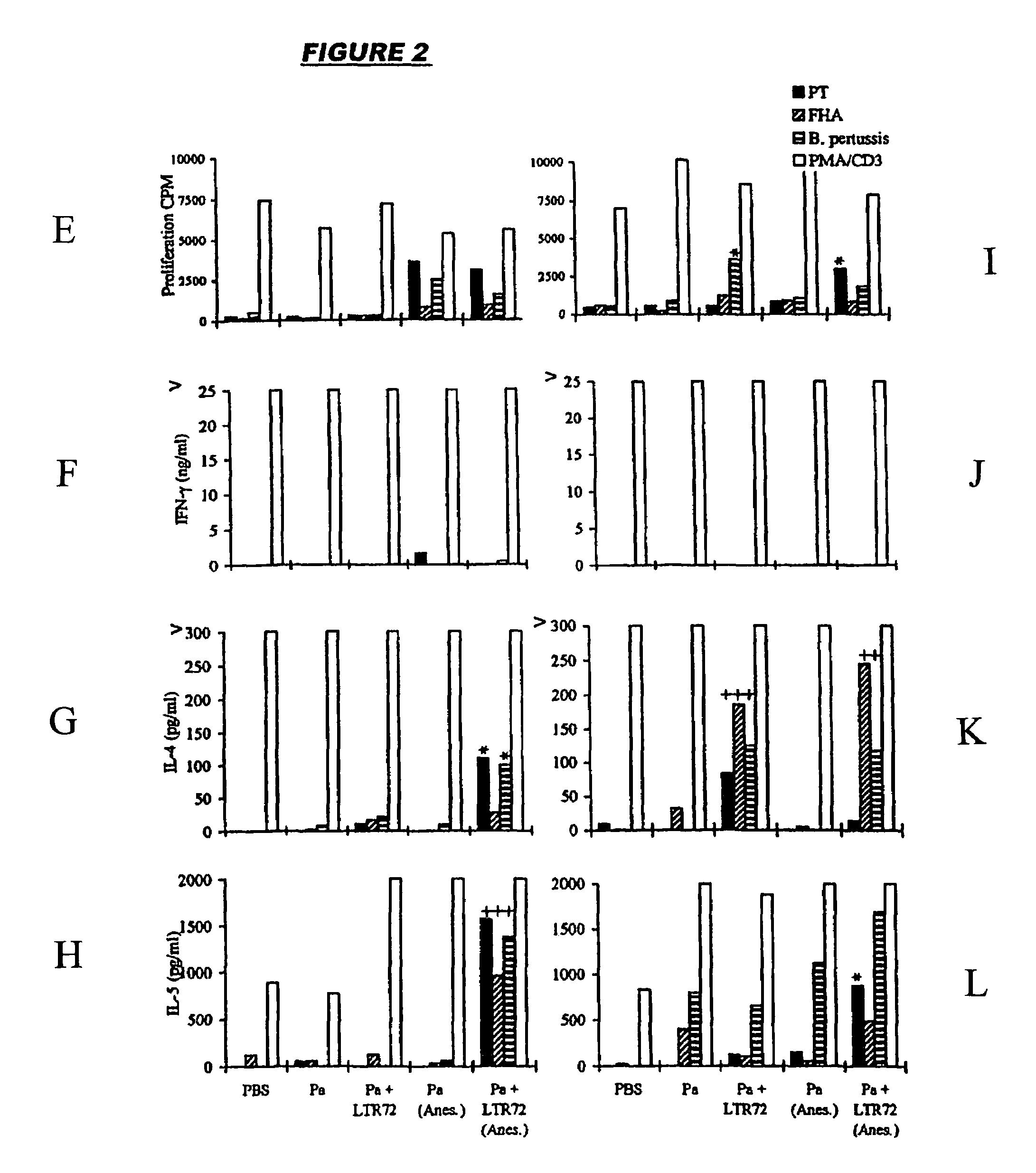
Mucosal DTPa vaccines, especially intranasal vaccines, comprising (a) a diphtheria antigen, a tetanus antigen and an acellular pertussis antigen, and (b) a detoxified mutant of cholera toxin (CT) or E. coli heat labile toxin (LT). Component (b) acts as a mucosal adjuvant. The acellular pertussis antigen preferably comprises pertussis holotoxin (PT) and filamentous haemagglutinin (FHA) and, optionally, pertactin. The mucosally-delivered combined DTPa formulation is capable of generating a level of protection against B. pertussis infection equivalent to that observed by alum-adjuvanted parenteral administration.
Owner:NOVARTIS AG
Adjuvants on the basis of bisacyloxypropylcystene conjugates and derivatives and their uses in pharmaceutical compositions
InactiveUS20090017106A1Enhancing in vitroEnhancing in in vivoAntibacterial agentsBiocideImmunologic disordersWhole body
The present invention relates to new adjuvants and the uses in pharmaceutical compositions, like in vaccines. In particular, the present invention provides new conjugates of the bisacyloxycysteine type useful as adjuvants and / or immunomodulators for prophylactic and / or therapeutic vaccination in the treatment of infectious diseases, inflammatory diseases, autoimmune diseases, tumours, allergies as well as for the control of fertility in human or animal populations. The compounds are particularly useful not only as systemic, but preferably as mucosal adjuvants. In addition, the invention relates to its uses as active ingredients in pharmaceutical compositions.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Mucosal vaccine using cationic nanogel
A mucosal vaccine for the prevention or treatment of microbial infections is described that is capable of inducing vaccine antigen-specific immune responses in an organism without the addition of a mucosal adjuvant. The mucosal vaccine comprises a composite of a nanogel comprising a hydrophilic polysaccharide having a cationic functional group and a hydrophobic cholesterol added thereto as a side chain and a vaccine antigen. The vaccine is administered via a mucosal route.
Owner:INTPROP STRATEGY NETWORK INC
Dental caries vaccine and method for preparing same
InactiveCN105214084AAntibacterial agentsBacterial antigen ingredientsResponse effectVirulent characteristics
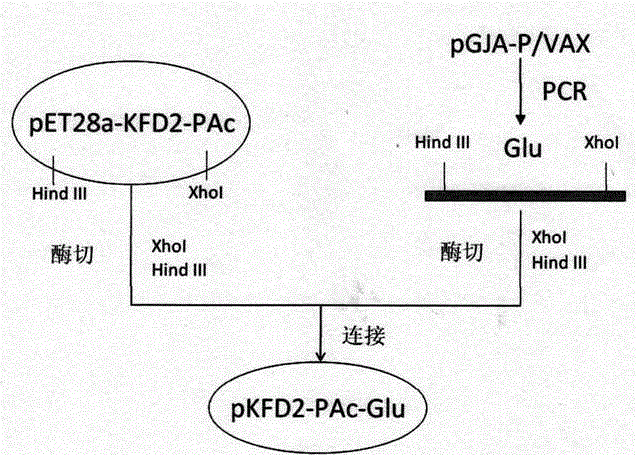
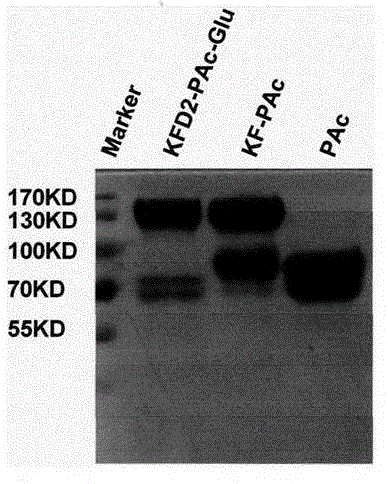
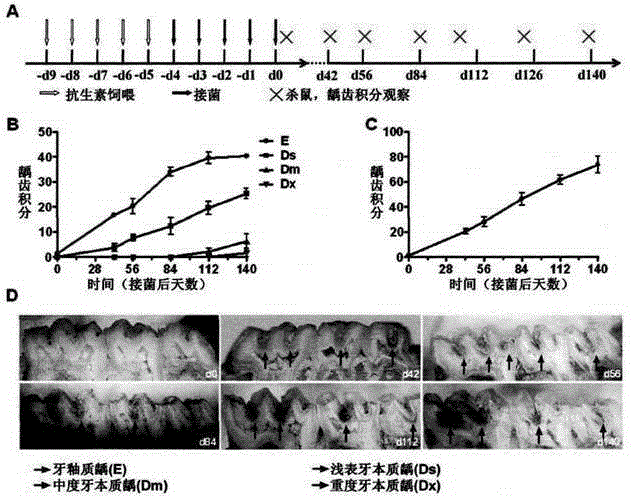
The invention discloses dental caries vaccine. The dental caries vaccine is a recombinant protein. Antigenic DNA (deoxyribonucleic acid) sequences derived from surface proteins PAc of streptococcus mutans are inserted in downstream 3' ends of DNA sequences of adjuvants derived from flagellin or replace all hypermutation regions of the DNA sequences of the adjuvants derived from the flagellin, and the antigenic DNA sequences and antigenic DNA sequences derived from glucosyltransferase of the streptococcus mutans are connected with one another and then are jointly expressed to obtain the recombinant protein. The dental caries vaccine has the advantages that rat models for the therapeutic dental caries vaccine are built, virulence factors PAc and Glu of the streptococcus mutans are used as vaccine antigens, flagellin proteins (KF) of recombinant bacteria are used as mucosal adjuvants, immune response effects and anti-caries protection effects of the PAc, KF-PAc and KFD2-PAc-Glu are systematically studied and compared, the KFD2-PAc-Glu is the relatively optimal therapeutic vaccine as shown by results, and in other words, the dental caries vaccine is the relatively optimal therapeutic vaccine.
Owner:鄢慧民 +4
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com