Mycoplasma hyopneumoniae multi-epitope mucosal vaccine
A technology of mycoplasma pneumonia and mucosal vaccines for swine, applied in the field of biotechnology genetic engineering, can solve the problems of ineffective suppression of MHP transmission, unsatisfactory, antigenic changes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
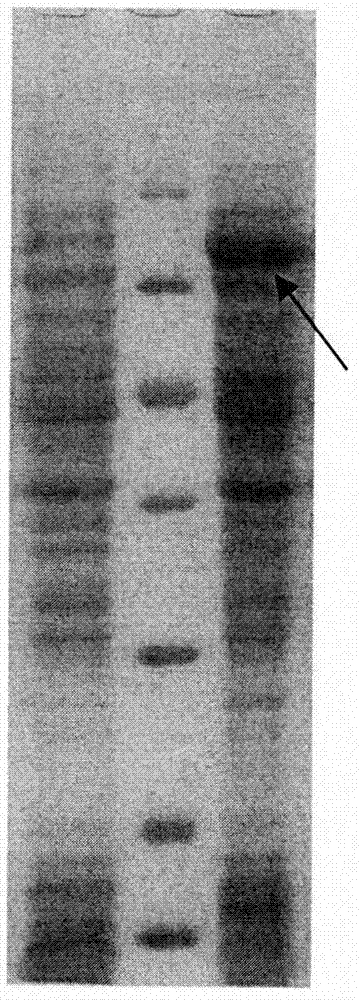
[0052] Example 1 Design Idea of Multi-epitope Mucosal Vaccine Protein of Mycoplasma Porcine Pneumonia
[0053] According to the amino acid sequences of the main membrane proteins in the genome of the main epidemic strains of mycoplasma pneumoniae in China: adhesion protein (P97), lipoprotein (P65), and specific membrane protein (P46), the present invention utilizes relevant bioinformatics software to perform Th expression on it. Epitope, CTL epitope and B cell epitope analysis. The designed epitopes were concatenated and expressed in Escherichia coli, and after fermentation, purification, preparation and other processes, a multi-epitope mucosal vaccine of Mycoplasma suis pneumonia with ideal immunogenicity was obtained. The vaccine prepared by the invention can effectively prevent swine mycoplasma pneumonia.
[0054] Comprehensively analyzed the genome sequence, antigen structure, and epidemiological research progress of domestic Mycoplasma pneumonia epidemic strains in Chi...
Embodiment 2
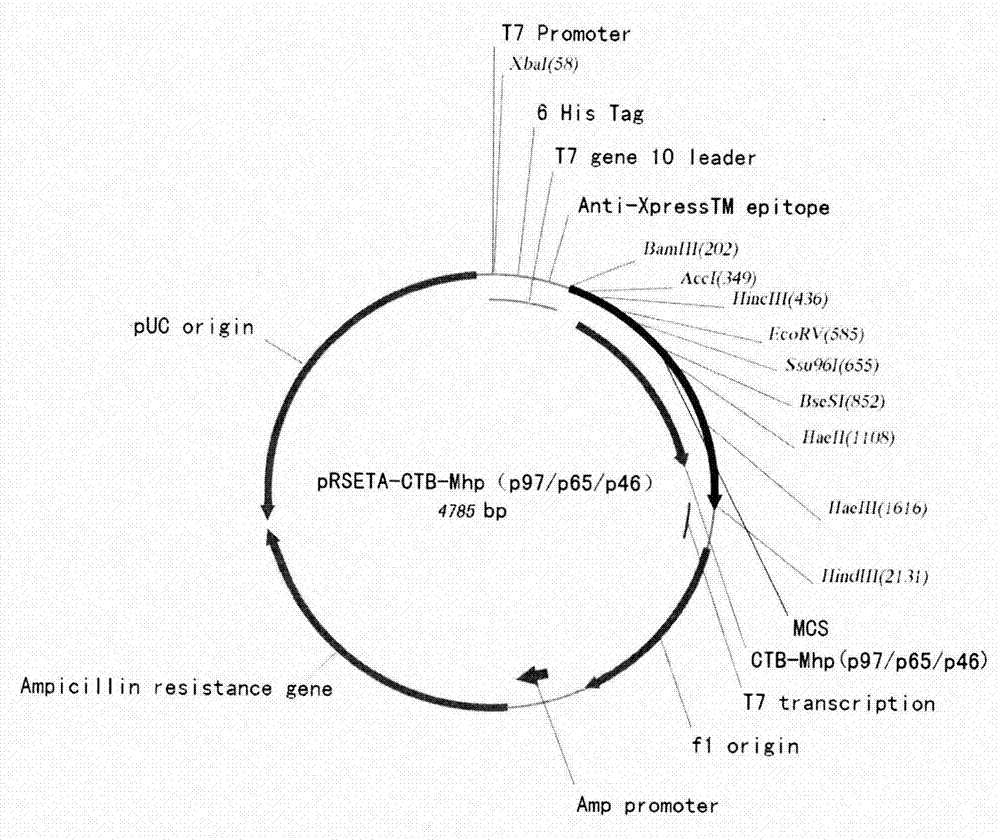
[0055] Embodiment two Escherichia coli expression vector and the construction of expression bacterial strain
[0056] 1 Send the designed polypeptide encoding nucleotides to Shanghai Handsome Biotechnology Co., Ltd. for synthesis. BamHI (5' end) and HindIII (3' end) restriction enzyme sites are designed at both ends of the nucleotide fragment respectively. After synthesis, they were respectively cloned into the pMD18T vector, and sequence determination confirmed that the inserted gene fragment was consistent with the designed sequence (see the sequence list). The recombinant plasmids were named pMD18T-CTB-Mhp (P97 / P65 / P46). Digest the plasmid with the corresponding restriction endonuclease. The expression vector of E. coli is the pRSETA plasmid from Invitrogen Company, which is also treated with the same restriction endonuclease. Digestion conditions: 10 μl reaction system, add 2 μl plasmid into the system , 5 activity units of restriction endonuclease (New England Biolabs), ...
Embodiment 3
[0060] Fermentation, purification and preparation of embodiment three engineering bacteria
[0061] 1 Fermentation Take the production strain pRSETA-CTB-Mhp(P97 / P65 / P46) / BL21(DE3, Plys), inoculate it in 2mL LB liquid medium (containing 100μg / mL ampicillin), and cultivate it with shaking at 200rpm for 12 hours at 37°C Activate the bacteria. Then inoculate the shake flask with an inoculation amount of 1:100, shake and culture at 37°C until OD600=3, and then inoculate it into a fermenter at a ratio of 10%. The fermentation medium is a semi-synthetic medium prepared with distilled water. Calibrate the dissolved oxygen and pH electrodes, start the tank to stir, the rotation speed is 300rpm, and sterilize the tank on-line. When the temperature of the culture solution in the tank drops to 37.0°C, calibrate the pH and dissolved oxygen (OD) zero point. The fermentation temperature was 37.0±0.1°C, the dissolved oxygen was controlled at about 40%, and the pH was controlled at 7.0. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com