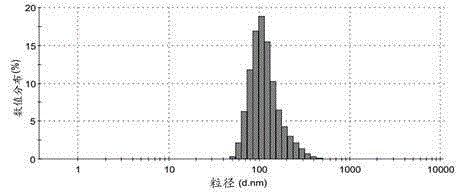
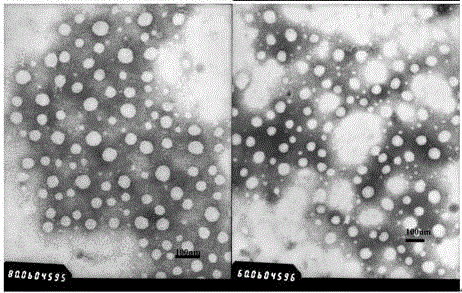
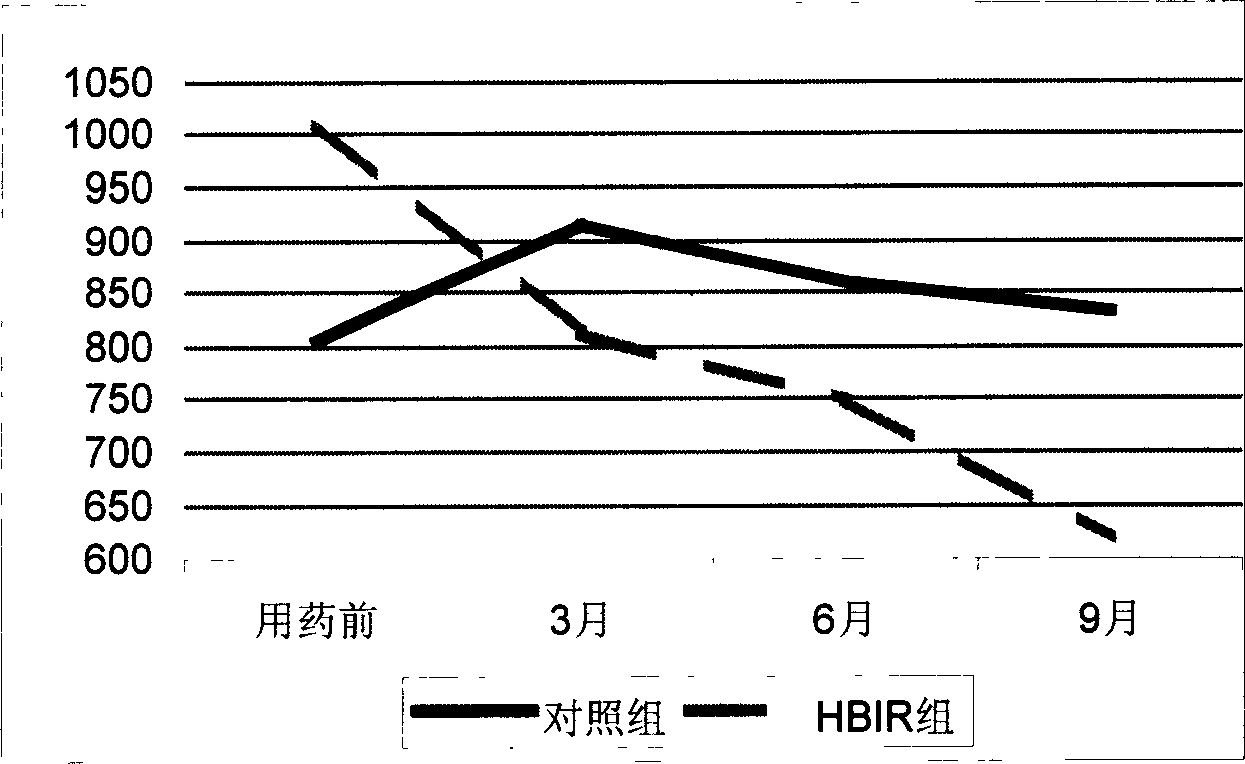
Patents
Literature
322 results about "Cell immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An immune response is a two-way assault on a pathogen – the cell mediated immune response and the humoral immune response. Cell mediated immune response is carried out by the T-cells or T lymphocytes (Fig. 11). So, it is also called T-cell immunity. This type of immune response is to defend against pathogens that may invade host cells.
High-yield Transgenic Mammalian Expression System for Generating Virus-like Particles
ActiveUS20100166769A1Improving immunogenicityStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMammalVirus-like particle
Virus-like particles (VLPs) of mammalian-hosted viruses, such as SARS-CoV and influenza viruses, have been recombinantly produced from Vero cells. The VLPs closely emulate the exterior of authentic virus particles and are highly immunogenic. They can elicit not only humoral but also cellular immune responses in a mammal. Compositions and methods related to the VLPs are also described.
Owner:ACAD SINIC
Immunogenic compositions and methods of using the compositions for inducing humoral and cellular immune responses
ActiveUS9044420B2Reduce the possibilityAntibacterial agentsAntimycoticsImmunogenicityInfective disorder
Compositions and methods are provided herein for improved dual immunization strategies that induce in a subject an immune response that includes a humoral immune response and cellular immune response, both CD4 and CD8 T lymphocyte immune responses, thereby providing a complete adaptive immune response to one or more antigens. The methods described are therefore useful for treating and / or preventing (i.e., reducing the likelihood or risk of occurrence) different diseases, disorders, and conditions such as cancers and infectious diseases for which induction of both a humoral immune response and cellular immune response is desired and beneficial.
Owner:IMMUNE DESIGN CORP
Methods and compositions for increasing CD4lymphocyte immune responsiveness
InactiveUS20050124645A1Increase and restore immune responsivenessInhibition of activationBiocidePeptide/protein ingredientsCell immune responseMicrobiology
Owner:FINKEL TERRI
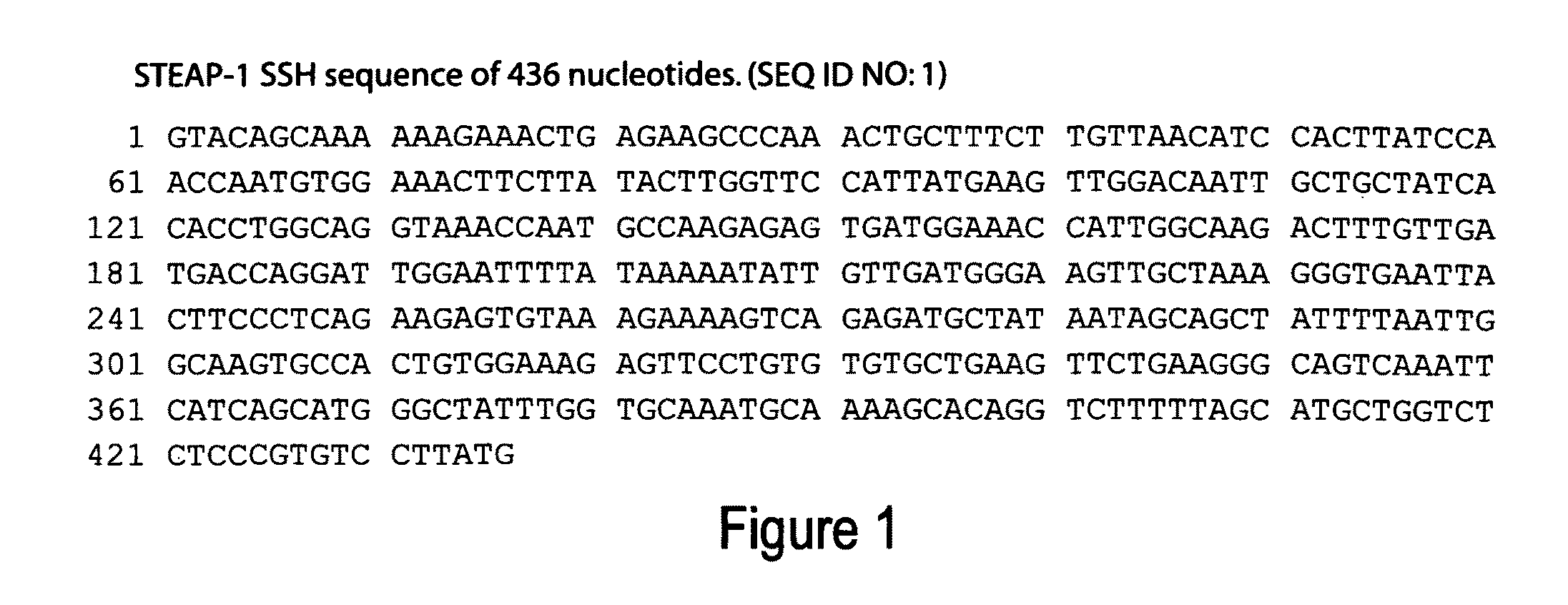
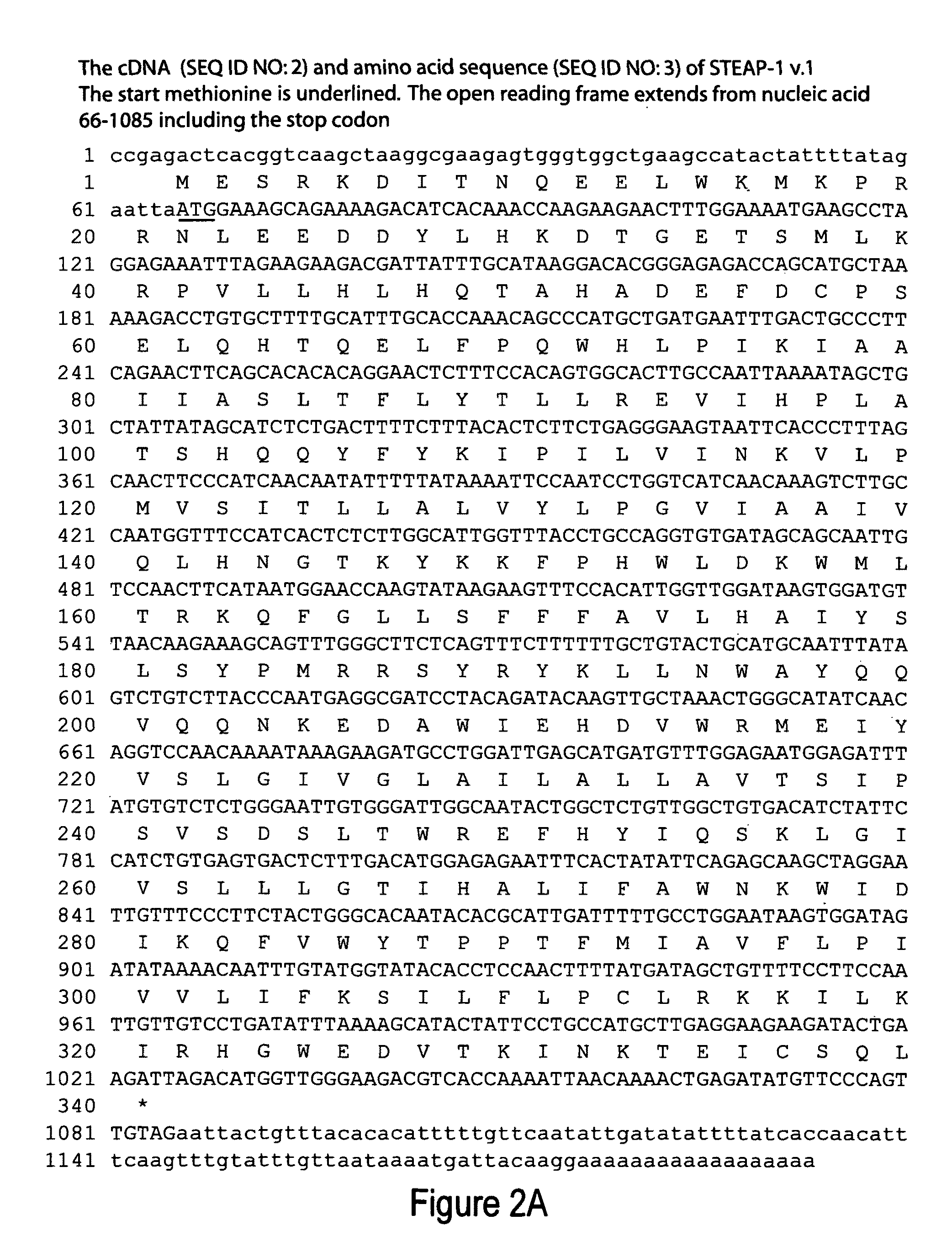
Antibodies and molecules derived therefrom that bind to STEAP-1 proteins
Antibodies and molecules derived therefrom that bind to novel STEAP-1 protein, and variants thereof, are described wherein STEAP-1 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, STEAP-1 provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The STEAP-1 gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with STEAP-1 can be used in active or passive immunization.
Owner:AGENSYS
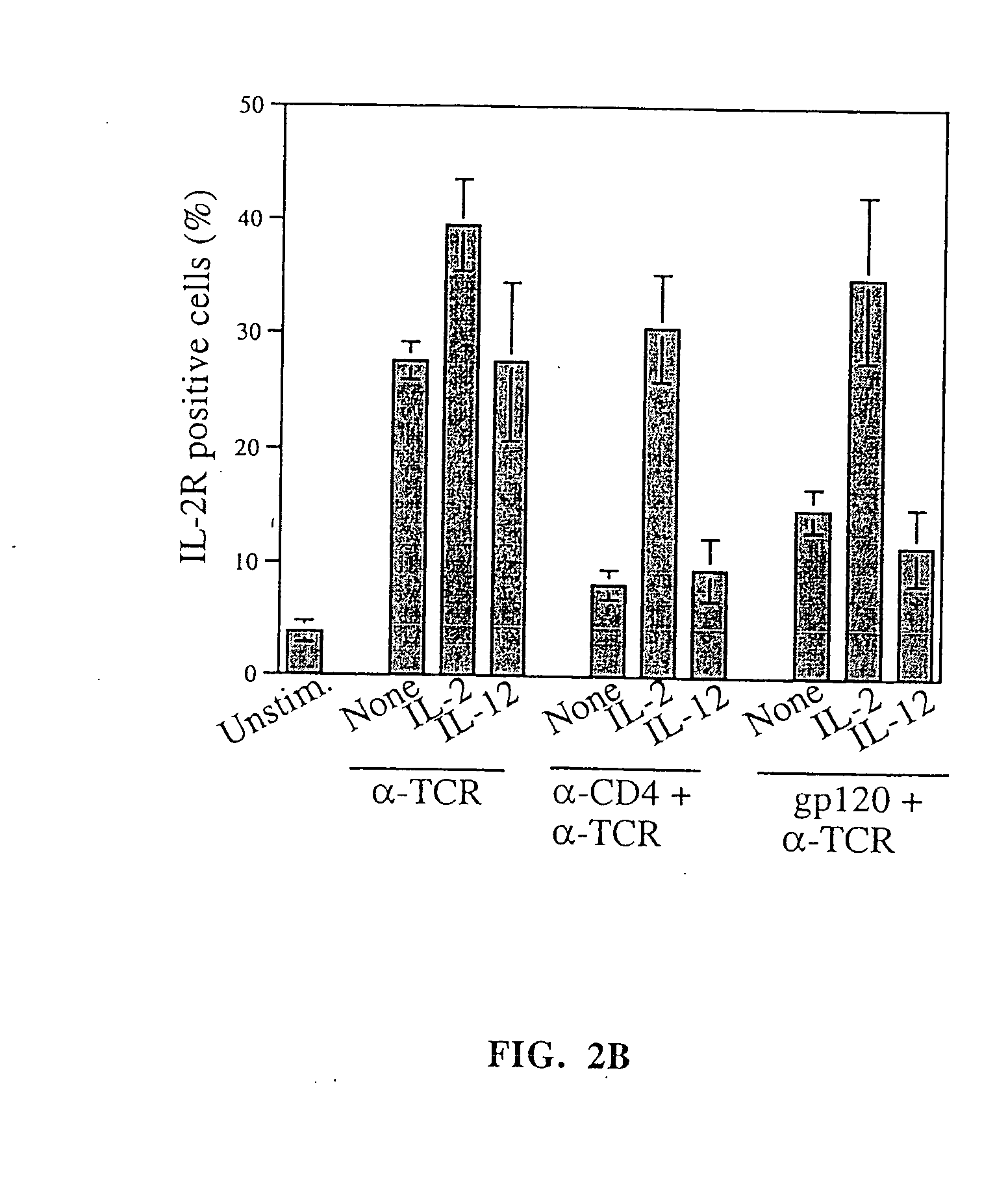
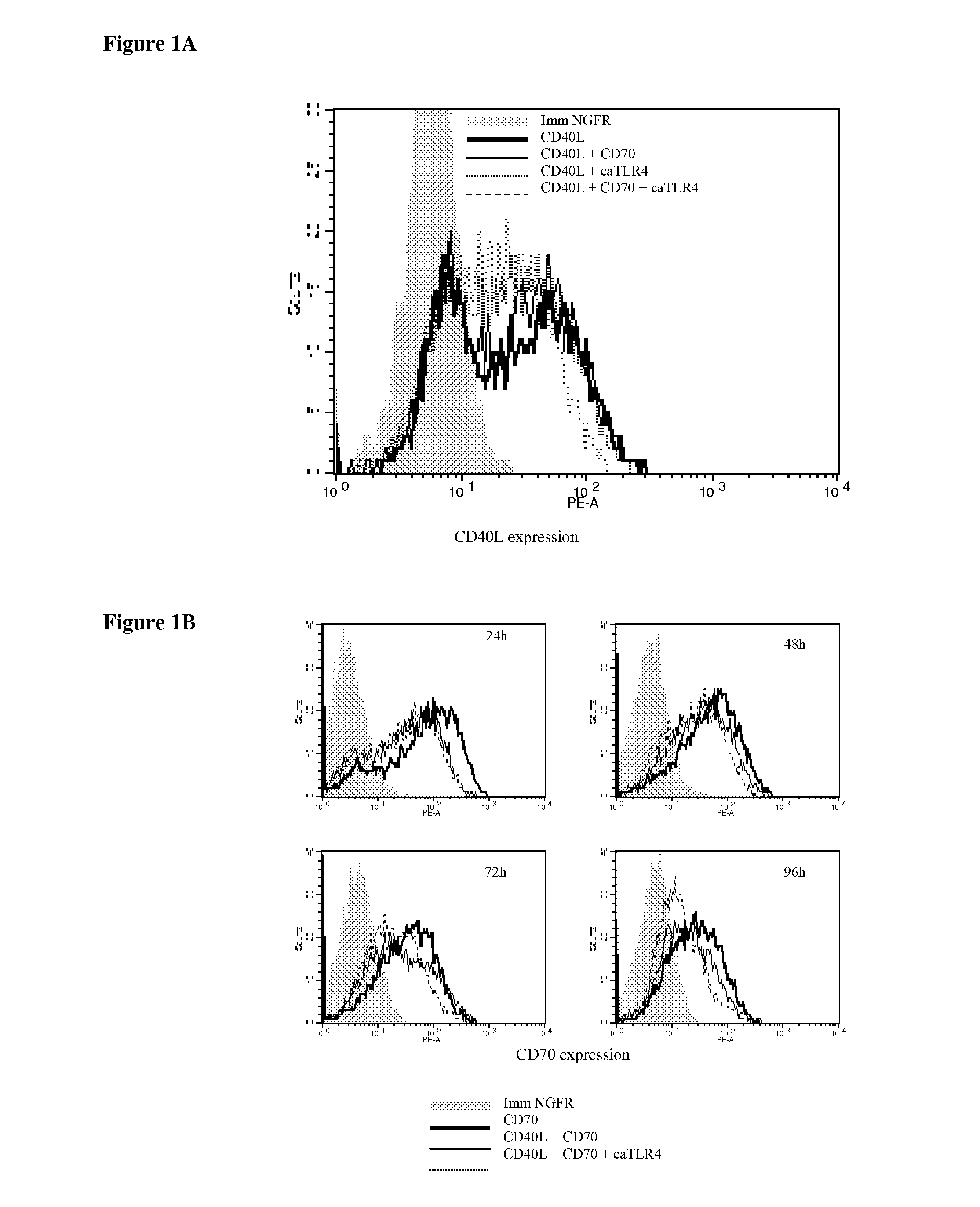
Enhancing the t-cells stimulatory capacity of human antigen presenting cells and their use in vaccination
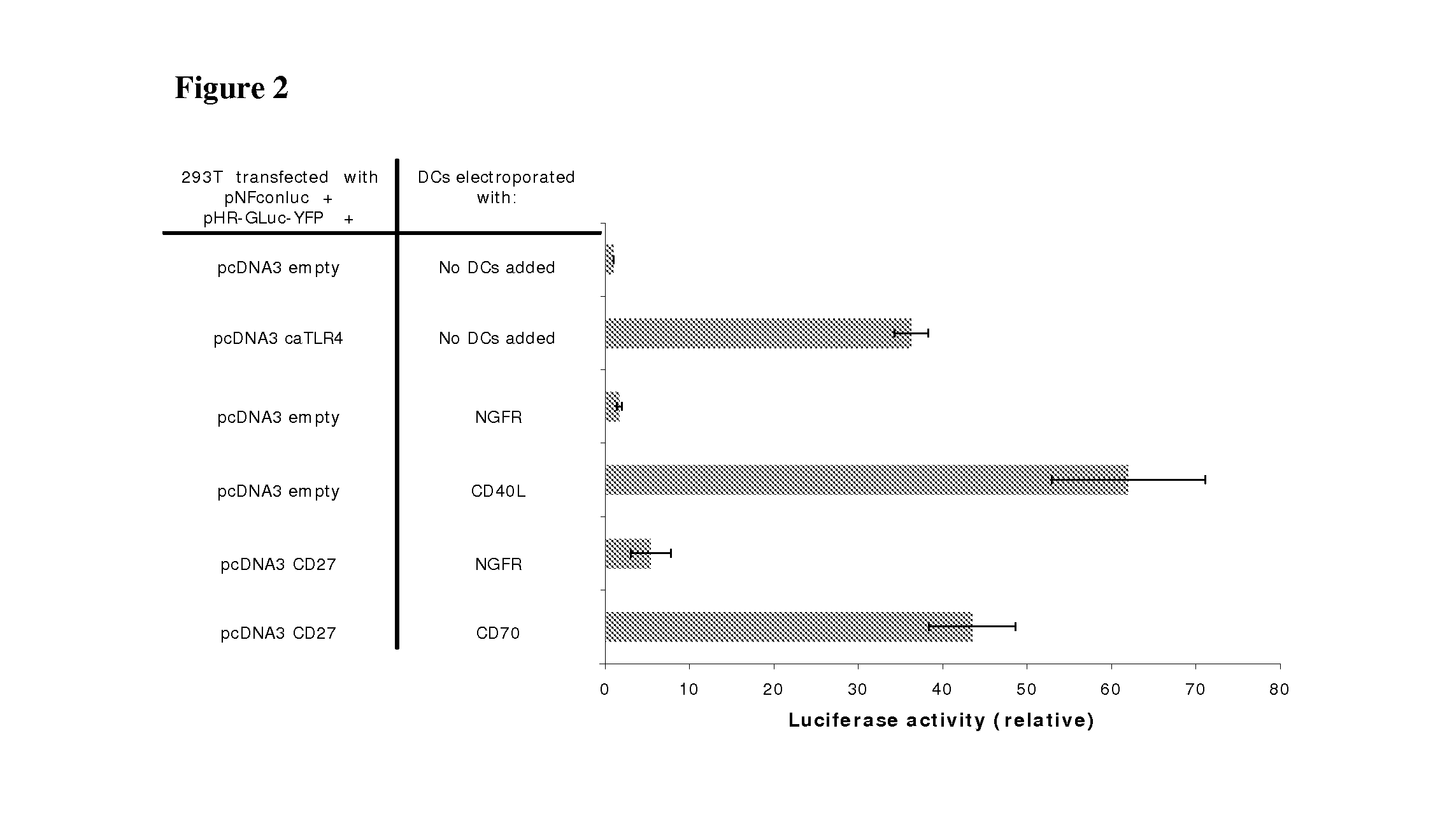
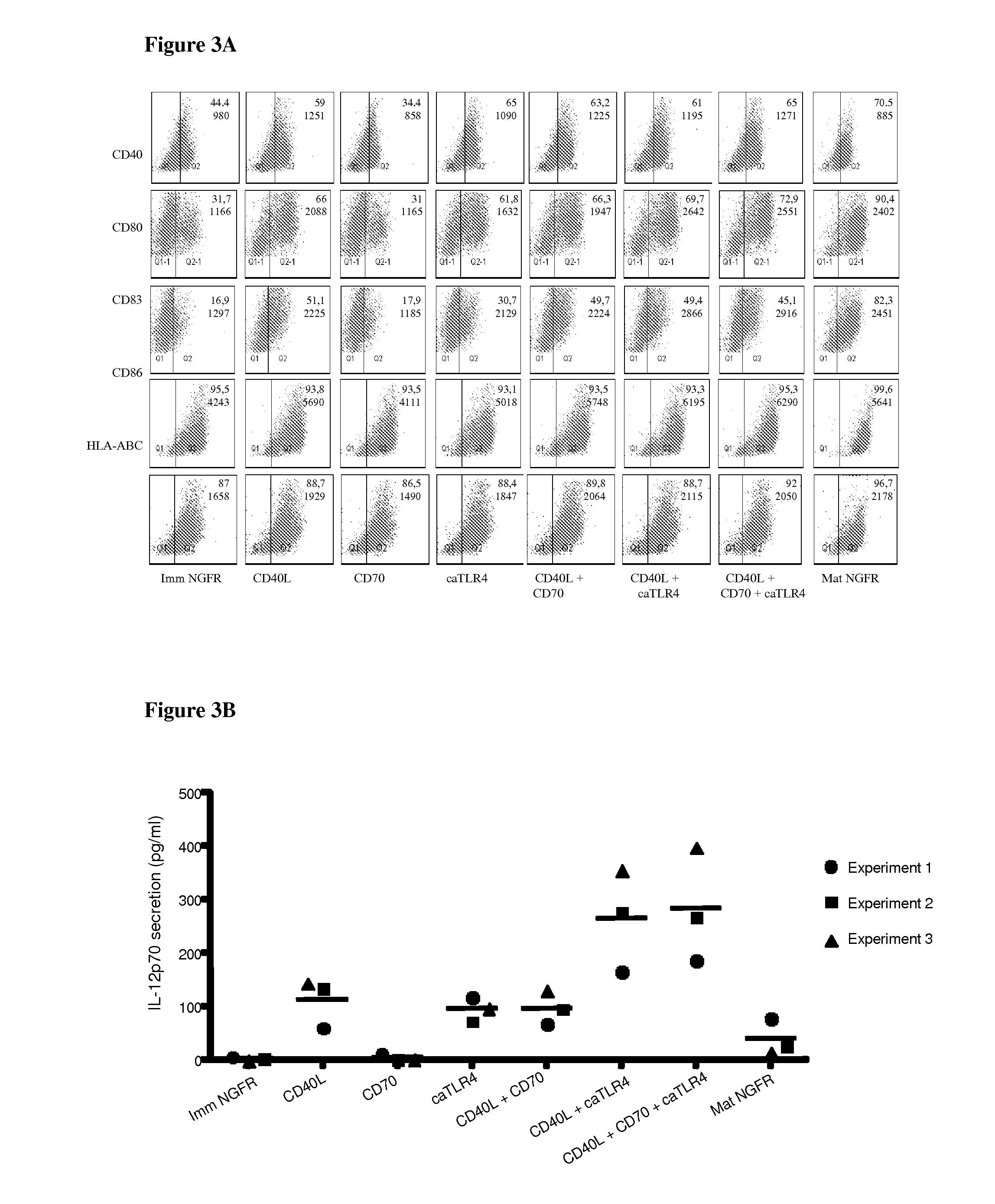
With the current invention, we provide new methods of enhancing the T-cell stimulatory capacity of human dendritic cells (DCs) and their use in cancer vaccination. The method comprises the introduction of different molecular adjuvants to human DCs through transfection with at least two mRNA or DNA molecules encoding markers selected from the group of: CD40L, CD70, constitutively active TLR4 (caTLR4), IL-12p70, EL-selectin, CCR7 and / or 4-1 BBL; or in combination with inhibition of SOCS, A20, PD-L1 and / or STAT3 expression, for example through siRNA transfection. We could show a clear increase in the immunostimulatory capacity of DCs obtained in this way, enabling them to elicit an unexpectedly high T-cell immune response in vitro. Introduction of at least two of the above molecules, in combination with a tumor-specific antigen enables the DCs to elicit a significant host-mediated T-cell immune response in vivo against the tumor antigen and thus makes them very attractive in the manufacturing of anti-cancer vaccines.
Owner:VRIJE UNIV BRUSSEL
Bursa of Fabricius heptapeptide with immune regulation effect
InactiveCN101830968ASynthetic technology is matureImprove efficiencyPeptide preparation methodsAntibody medical ingredientsSide effectImmunologic Competence
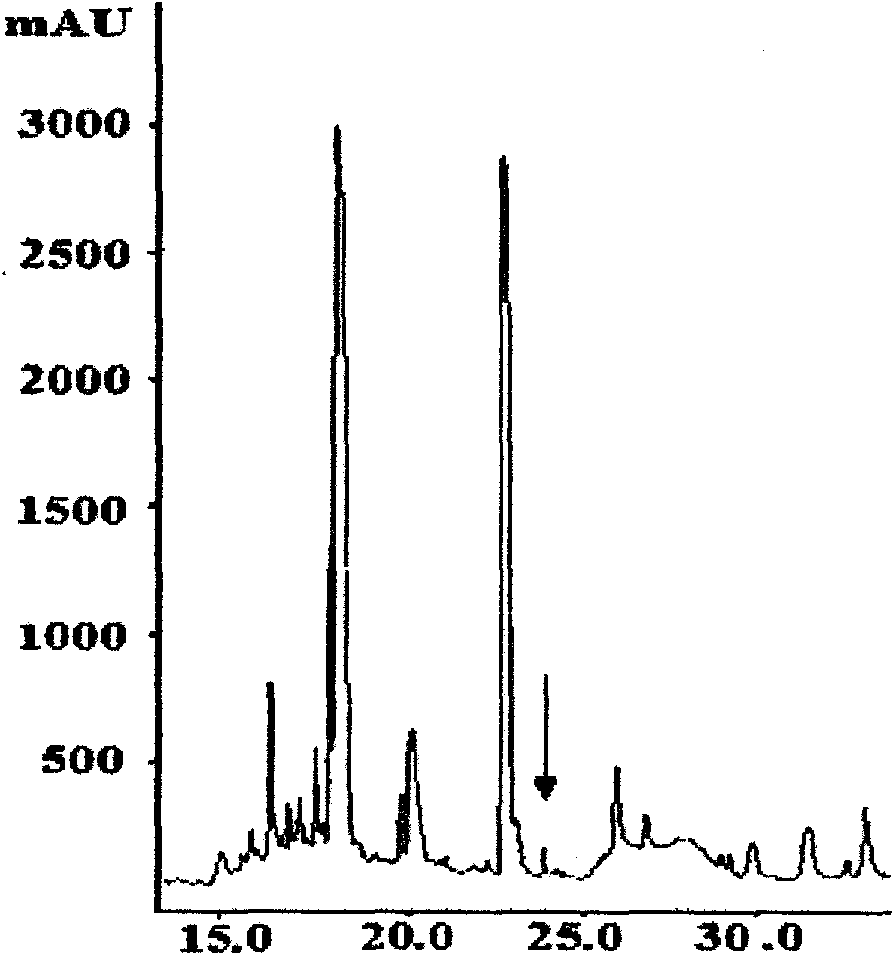
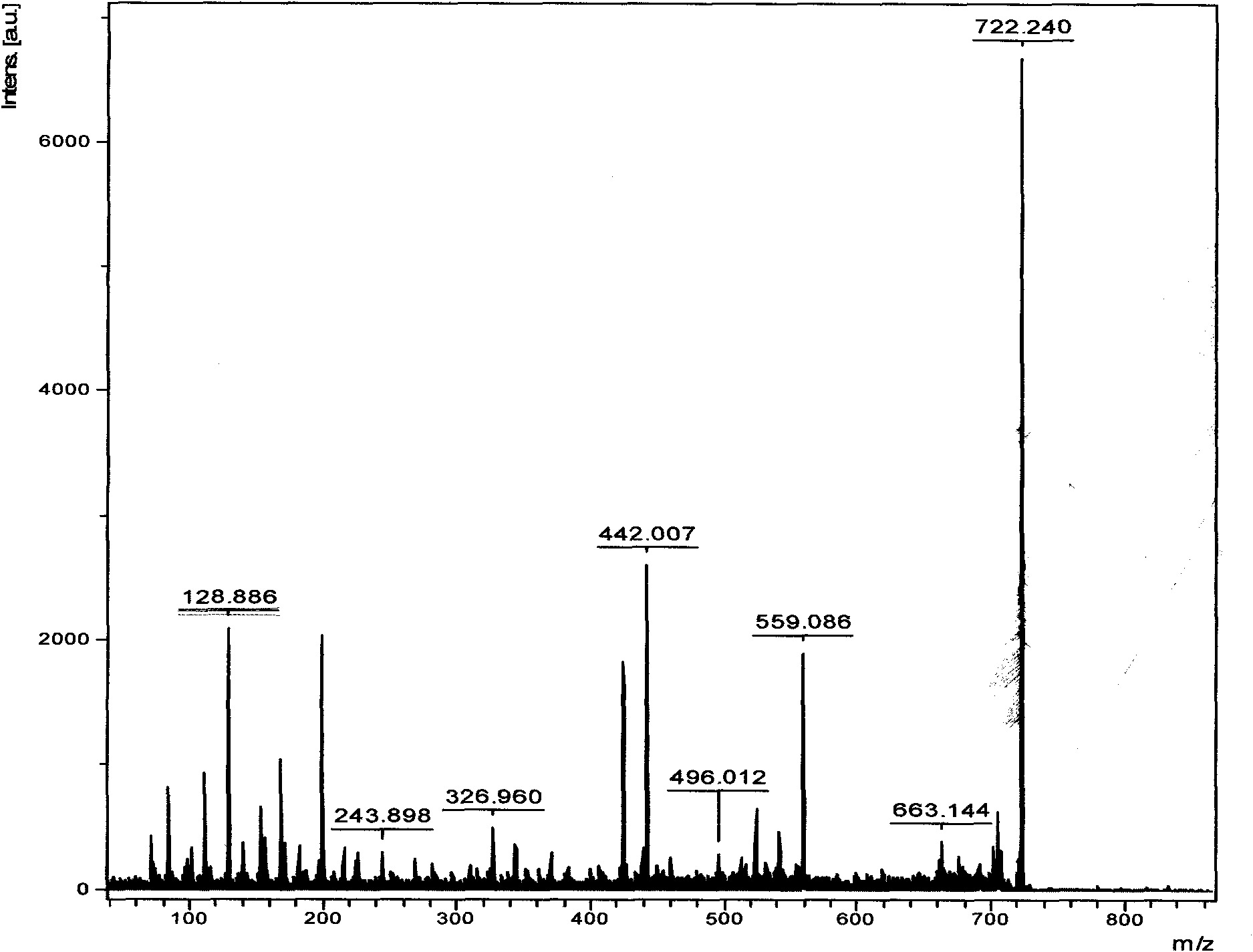
The invention relates to bursa of Fabricius heptapeptide with immune regulation effect and application thereof in immunity (in improving the immune capability of animals, improving the immune effect of vaccines and affecting the activity of tumor cells), and belongs to the field of immunology. The molecular weight of the separated heptapeptide is 722.240, the amino acid sequence is EPASGMM, and the heptapeptide has a simple structure, no toxic or side effect and extremely weak immunogenic property. The heptapeptide can be separated and extracted from bursa of Fabricius, also can be chemically synthesized, has low cost, and can be massively produced. The bursa of Fabricius heptapeptide has induction effect on the production of antibody and subtype thereof, and meanwhile can regulate the production of cell factors, partition of T lymphocytes and proliferation of spleen cells, and promote the immune reaction of the cells. The bursa of Fabricius heptapeptide is an immune regulation factor on functions, has wide application prospect on the aspects of immune regulation, immune therapy and the like, and can be applied in the fields of basic immune research, clinical application research and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
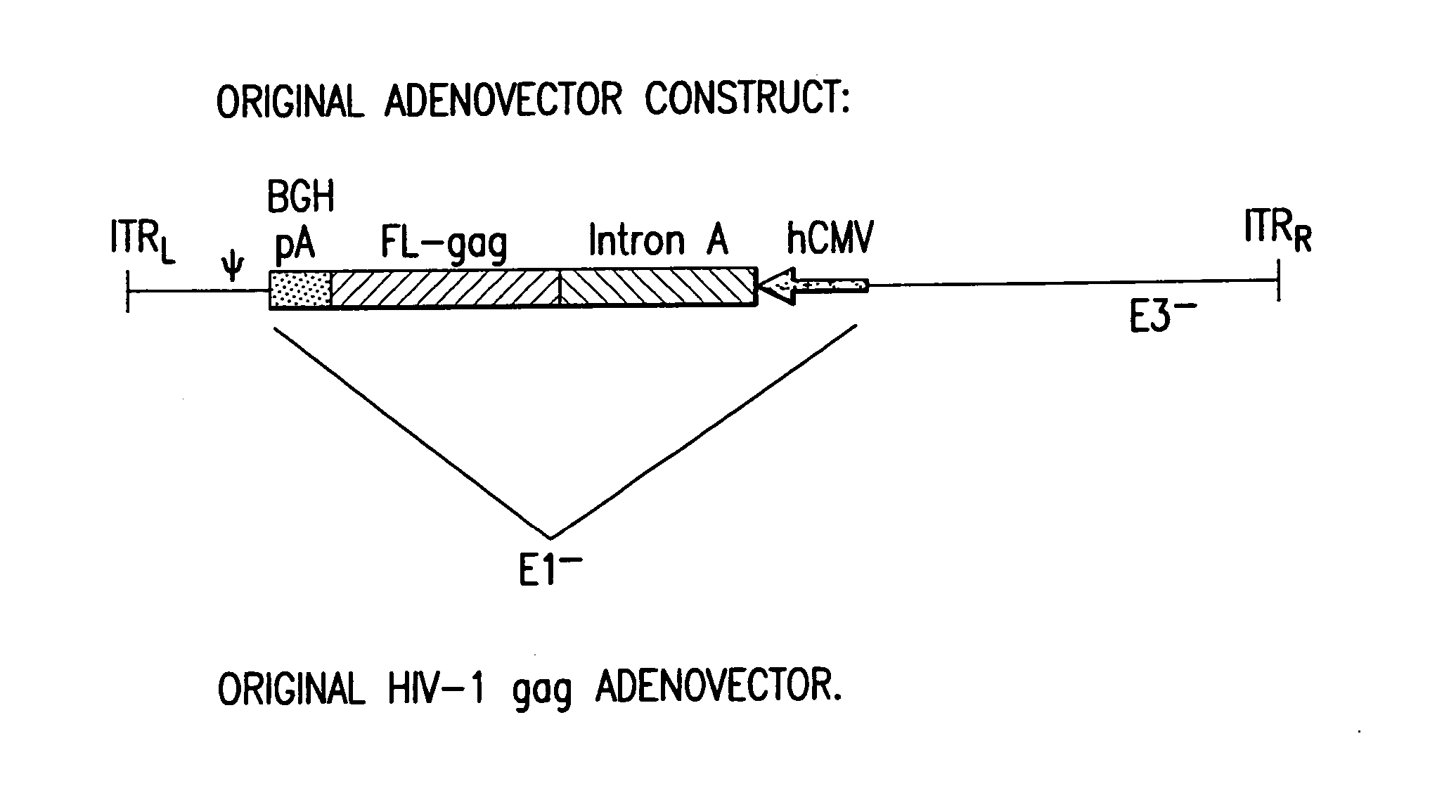
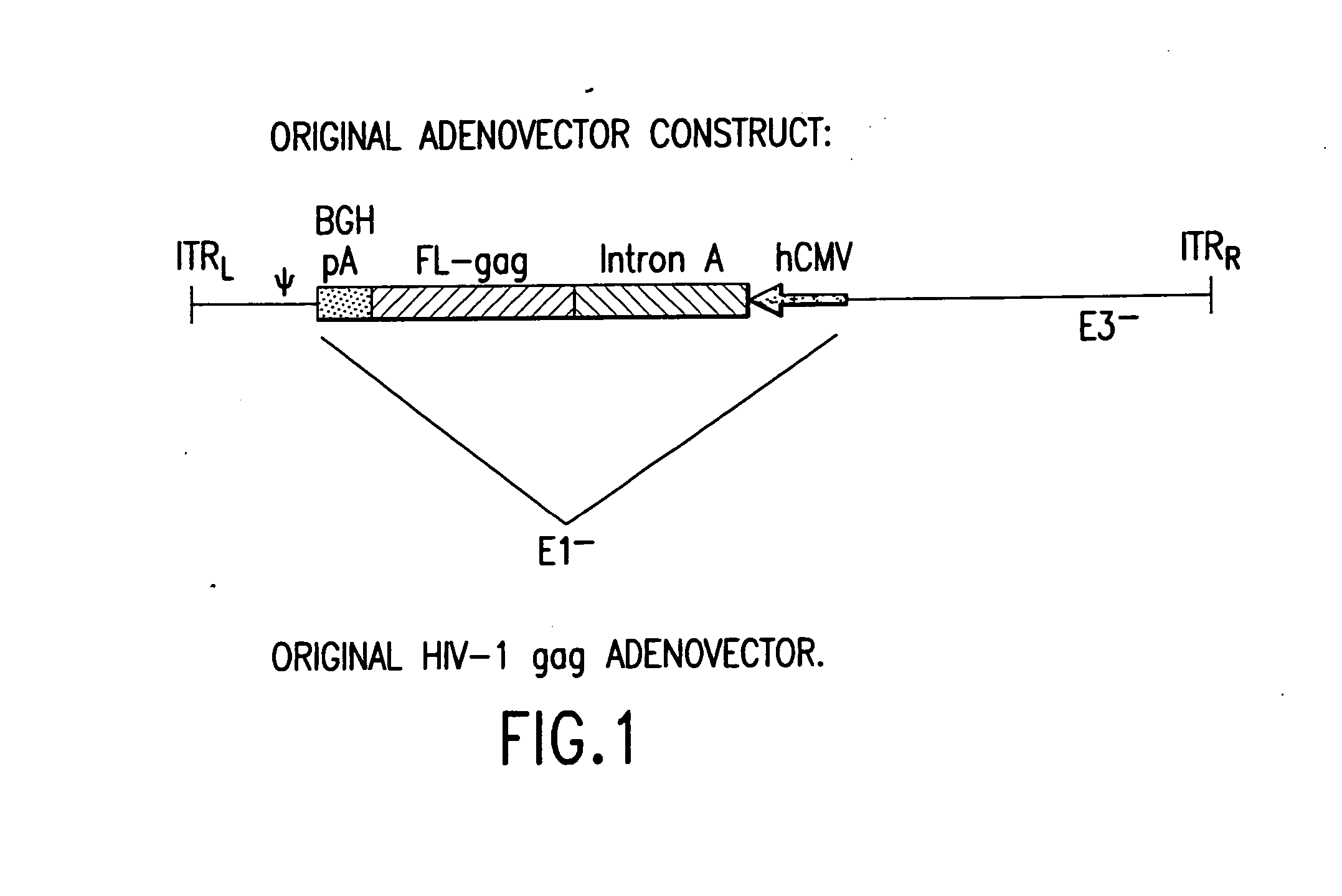
Enhanced first generation adenovirus vaccines expressing codon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070054395A1Genetically stableImprove featuresVectorsViral antigen ingredientsMammalReverse transcriptase
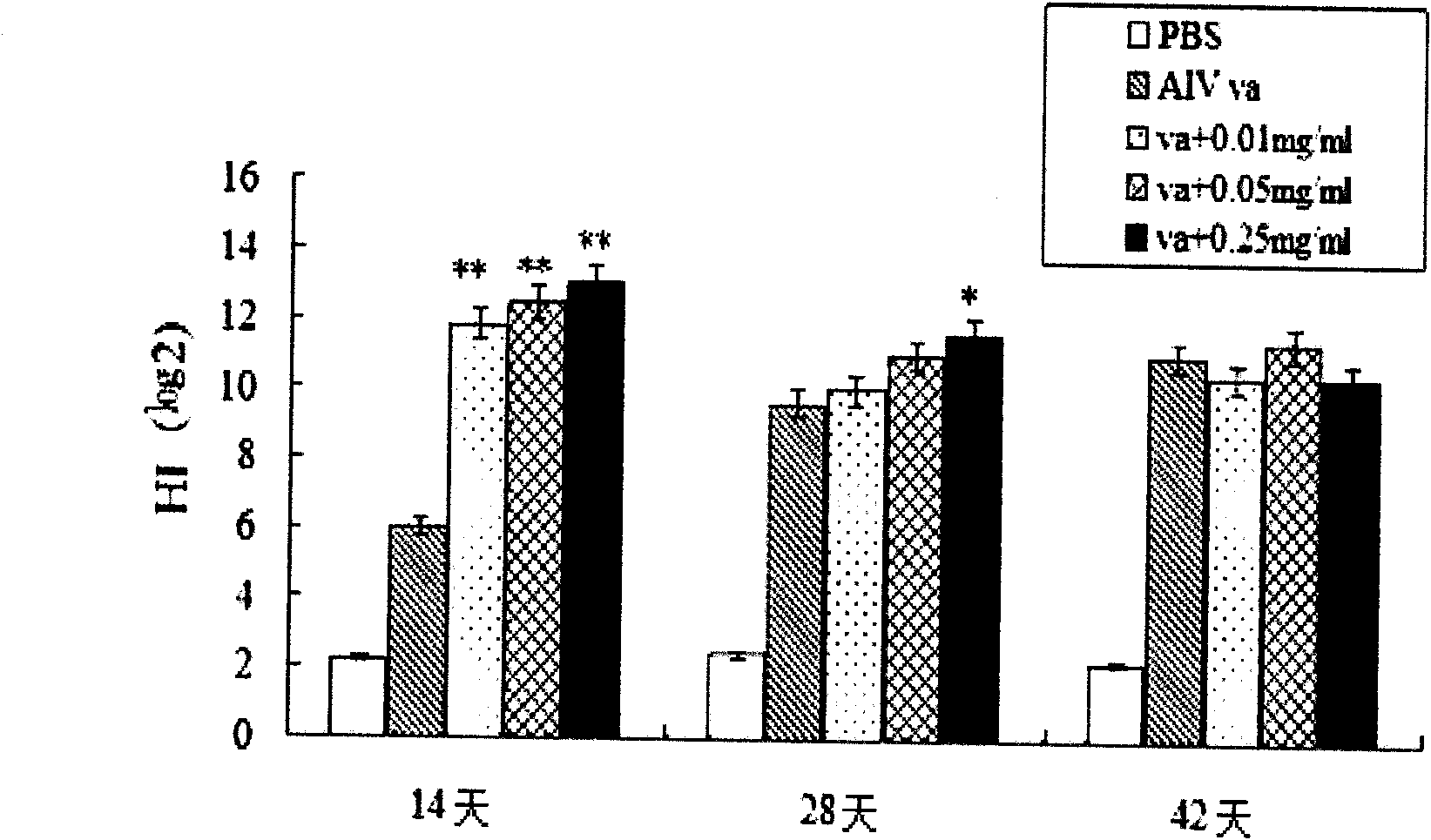
First generation adenoviral vectors and associated recombinant adenovirus-based HIV vaccines which show enhanced stability and growth properties and greater cellular-mediated immunity are described within this specification. These adenoviral vectors are utilized to generate and produce through cell culture various adenoviral-based HIV-1 vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof. These adenovirus vaccines, when directly introduced into living vertebrate tissue, preferably a mammalian host such as a human or a non-human mammal of commercial or domestic veterinary importance, express the HIV1-Gag, Pol and / or Nef protein or biologically modification thereof, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, encoding codon optimized HIV-1 Pol, derivatives of optimized HIV-1 Pol (including constructs wherein protease, reverse transcriptase, RNAse H and integrase activity of HIV-1 Pol is inactivated), HIV-1 Nef and derivatives of optimized HIV-1 Nef, including nef mutants which effect wild type characteristics of Nef, such as myristylation and down regulation of host CD4. The adenoviral vaccines of the present invention, when administered alone or in a combined modality regime, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
Vaccine vector based on aluminum hydoxide nano-particles
ActiveCN104587464AReduce aluminum contentNo inflammationPharmaceutical non-active ingredientsImmunological disordersDendritic cellTGE VACCINE
The invention provides an aluminum adjuvant used as a vaccine vector. The adjuvant is characterized in that a PEG derivative bio-material and aluminum are compounded to form nano-particles, so that the property of strong Th2 body fluid immunologic adjuvant of aluminum salt is maintained, and the adjuvant can be efficiently transferred to draining lymph nodes in the body and can be easily ingested by dendritic cells to perform effective cross-presentation and induce cellular immunologic response. The aluminum adjuvant has strong Th1 immunologic response.
Owner:SICHUAN UNIV
Methods and compositions for enhancing vaccine immune responses
ActiveUS20150202272A1Increase cytolytic activityIncrease immune responseSsRNA viruses negative-senseViral antigen ingredientsImmunogenicityT cell immunity
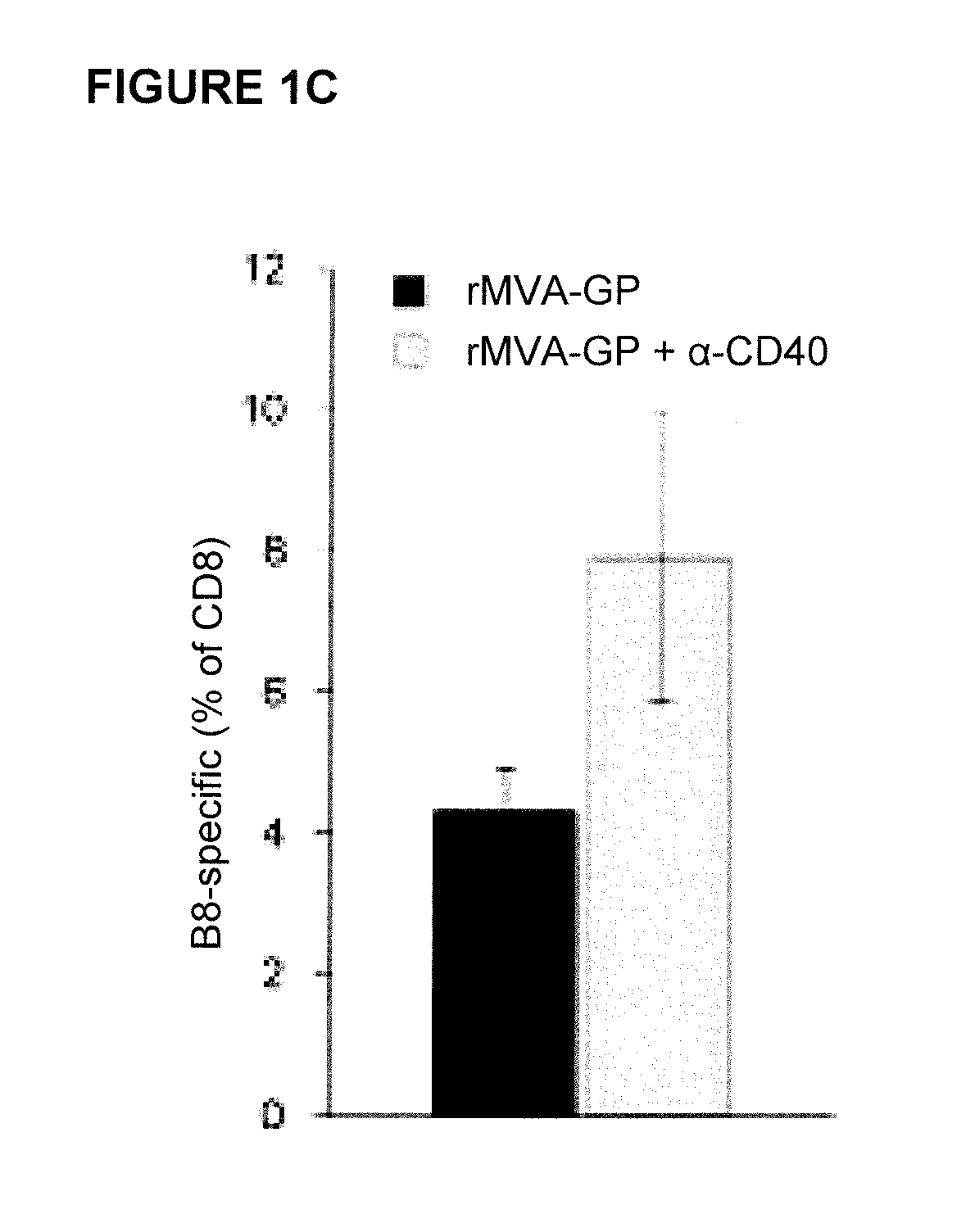
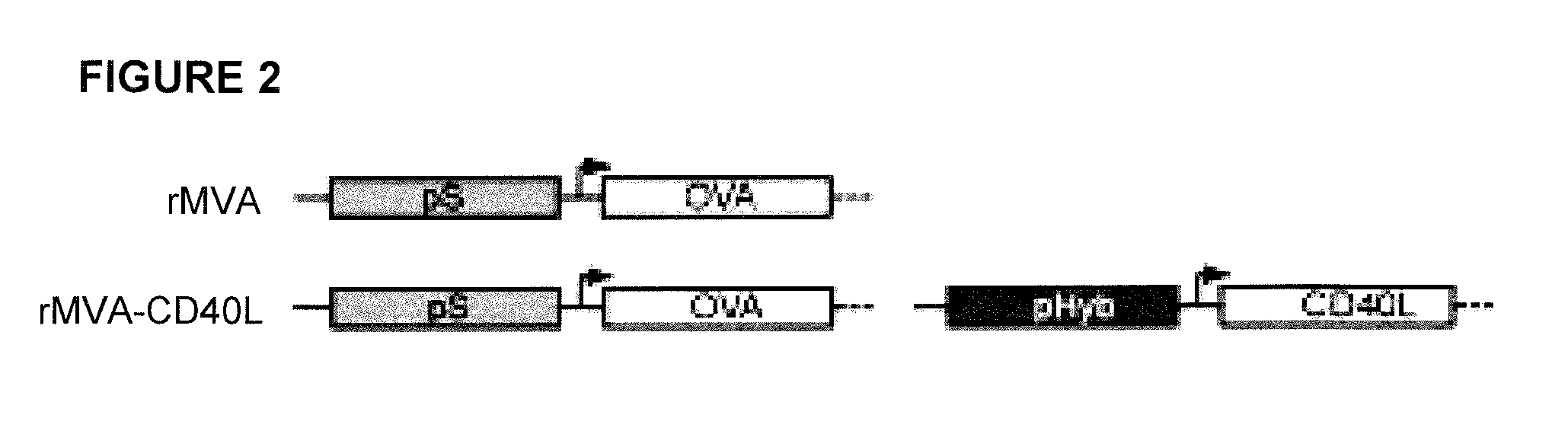
Provided herein are immunogenic compositions comprising a recombinant modified vaccinia virus Ankara (MVA) comprising a nucleic acid sequence encoding a CD40 ligand (CD40L) and a nucleic acid sequence encoding a heterologous disease-associated antigen, wherein the immunogenic composition induces increases T-cell immune responses specific for the heterologous disease-associated antigen when administered to a human host, and related methods and uses.
Owner:BAVARIAN NORDIC AS
Toxicity T cell position vaccine of the cell for treating Hepatitis B and the preparing method
The invention relates to bio-pharmaceutical engineer technology domain. At present, the therapeutic drugs for HBV infection mainly depend on IFN-Alpha and nucleotide analog, which can not kill virus thoroughly with worse remote therapeutic effect. The invention provides a cytotoxic T cell epitope vaccine for hepatitis B, comprising a fused polypeptide formed by connecting 21 CTL epitopes selected from CTL epitopes data of HBV antigen and two universal epitopes of auxiliary T lymphocyte. Saccharomyces Serevisiae is selected to express the fused antigen and mixes with immunologic adjvant. The said vaccine can activate and enhance the cellular immune response of patient with chronic HBV infection. The vaccine can also promote the immune elimination of HBV, and can be used for treatment of chronic hepatitis B.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Saponin with immunoadjuvant function, preparation method, vaccine preparation containing the saponin as adjuvant and uses thereof
InactiveCN101402666ASimple preparation processSimple methodAntiinfectivesSteroidsDiseaseCell immune response
The invention relates to a saponin with immune adjuvant function and a preparation method thereof, a vaccine preparation containing the saponin as an adjuvant, and applications of the saponin and the vaccine preparation in the prevention and treatment of infectious diseases and cancers of human and animals. The saponin is platycodin D, platycodin D2, or a total-saponin containing the two saponin compounds. The platycodin D and platycodin D2 are both extracted and separated from balloonflower, a Chinese medicine. The saponin can induce an organism to generate Th1-type and Th2-type immune responses, show the capability of inducing the organism to generate stronger cell immune response and humoral immune response to a vaccine than the alhydrogel adjuvant known in the prior art, and can be taken as the immune adjuvant for a plurality of vaccines and achieve an ideal immunity effect. The vaccine which takes the saponin as the adjuvant has simple preparation technology and simple and convenient method, and the quality is easy to control and the saponin can be reserved by freezing.
Owner:ZHEJIANG UNIV
Preparation method of allogenic blood vessel decellularised scaffold
InactiveCN101011604ALower immune responseGood tissue compatibilityCatheterAcellular scaffoldCell membrane
The invention relates to a method for preparing the conspecific allo vessel de-cell support, which comprises that using the abdominal aorta of rabbit of New Zealand, using surface activator Triton X-100 to extract the liposome of cell membrane, and digesting the parenzyme, using nuclease DNAse and RNAse to degrade the DNA and RNA of cell, to obtain the support. The invention uses the similar structures of conspecific allo materials, with better histocompatibility, to support healing after surgery, with simple preparation and high efficiency. And the invention can be transplanted with low caused immunity reaction of host, to supply physical strength and tension. The invention can be used to replace blood vessel.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and preparation method thereof
ActiveCN101775399AImproving immunogenicityFacilitate presentationGenetic material ingredientsVirus peptidesAdjuvantRecombinant vaccines
The invention discloses an Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and a preparation method thereof. The recombinant vaccine comprises the following components: proteins coded by foot-and-mouth disease virus multi-epitope genes and the fusion genes of carrier proteins, and foot-and-mouth disease virus 3D proteins. The preparation method comprises the following steps: diluting the proteins expressed by the foot-and-mouth disease virus multi-epitope genes and the fusion genes of the carrier proteins and the foot-and-mouth disease virus 3D proteins, mixing the diluted proteins uniformly, adding an adjuvant into the mixture to emulsify the mixture. Animal models and animal immune effect experiments show that the bovine Asia1 epitope recombinant vaccine can make comprehensive immune protective response, can induce injected and immunized bovine and guinea pigs to generate high level neutralizing antibodies, and can also induce cell immune response, so the recombinant vaccine can effectively protect animals against the virulent attack of the foot-and-mouth disease viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Zika virus disease vaccine taking human Ad5 replication-defective adenovirus as vector
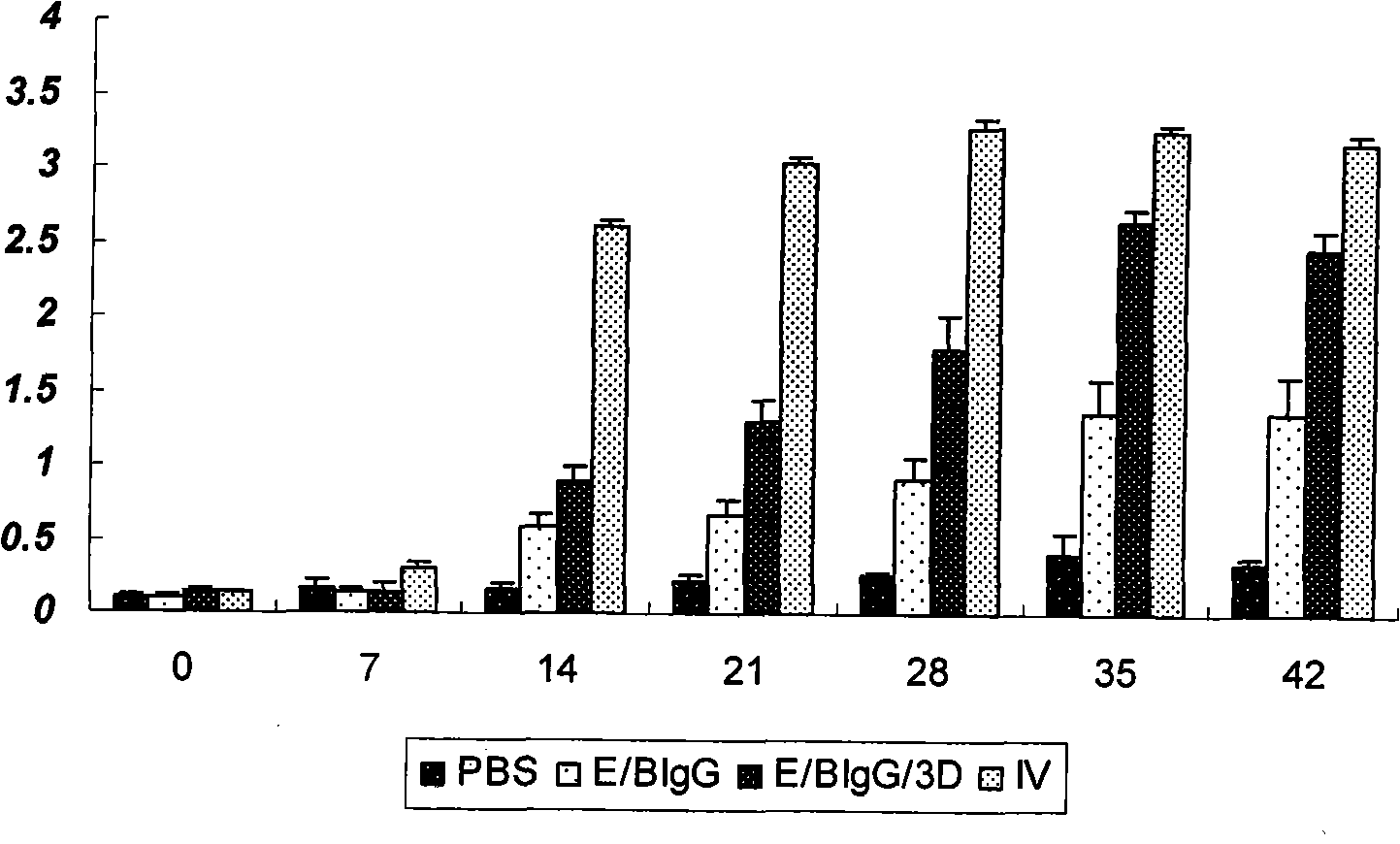
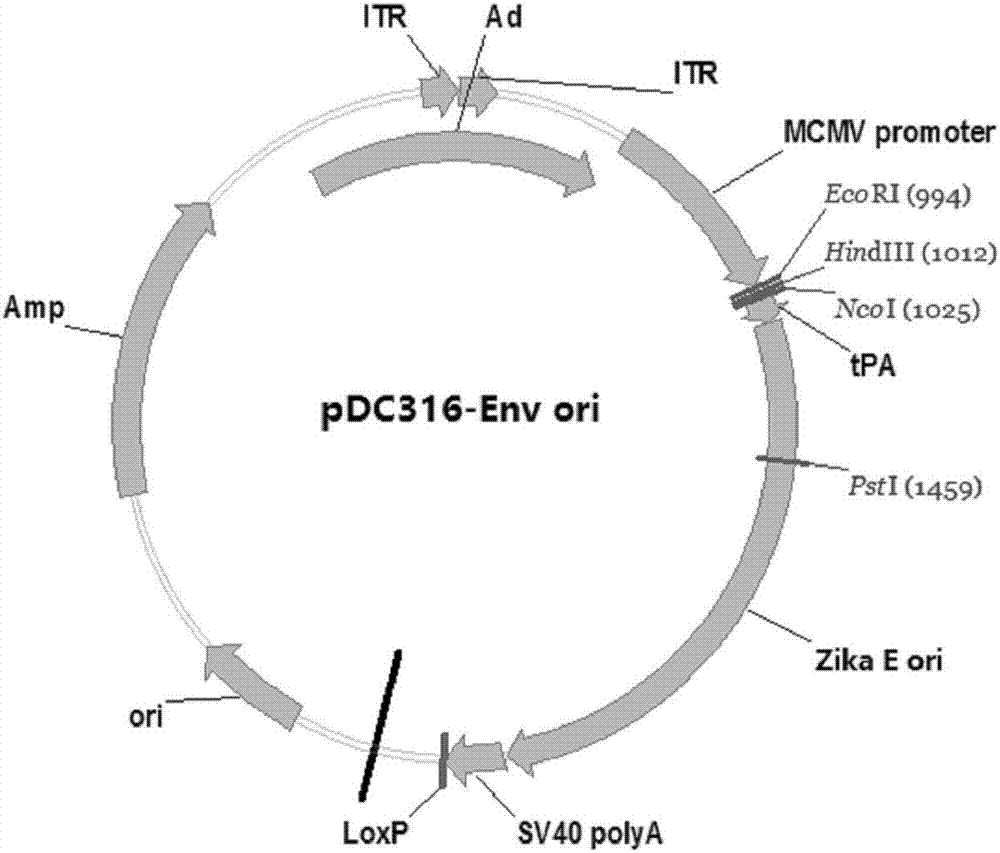
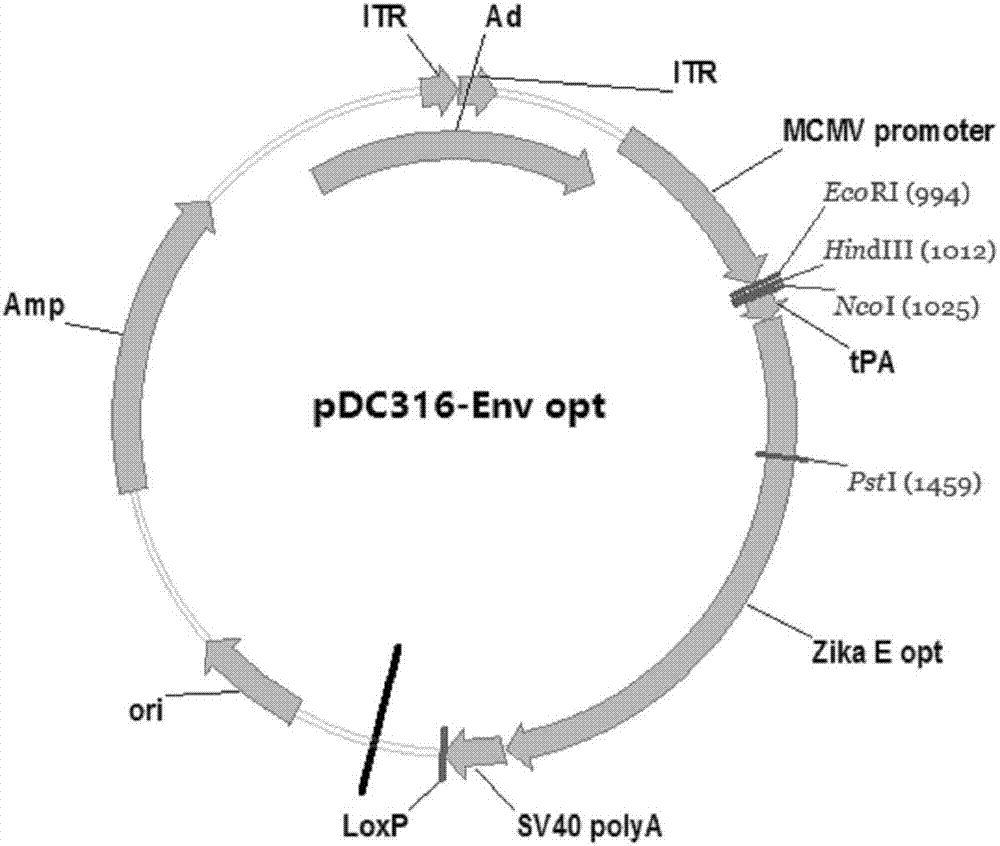
ActiveCN107190013ARapid responseRapid immune responseSsRNA viruses positive-senseViral antigen ingredientsInfected cellShuttle vector
The invention discloses a codon-optimized nucleotide sequence capable of expressing an Env protein of a zika virus. The sequence can be fused with a protein prM and a protein prM endogenous signal peptide of a codon-optimized zika virus; after the sequence is inserted into a shuttle vector pDC316, the sequence and an auxiliary vector pBHGlox_E1, 3Cre realize cotransfection of a cell HEK293, so as to package an E1 and E3 combined missing-recombinant adenovirus taking a replication-defective human type-5 adenovirus as a vector; the recombinant adenovirus vector can efficiently express an envelope protein of the zika virus in an infected cell. After the nucleotide sequence-inserted recombinant adenovirus serving as a vaccine is immune to an animal for single time, strong humoral immune and cellular immune responses can be quickly induced. A zika vaccine taking the recombinant adenovirus as the vector is suitable for large-scale and rapid preparation and can be used for emergent vaccination for a large scale of people in a zika outbreak and prophylactic immunization for people at ordinary times.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Nucleotide sequence for encoding novel coronavirus antigen and application of nucleotide sequence
ActiveCN112626090AIncrease GC contentRaise the ratioSsRNA viruses positive-senseViral antigen ingredientsWild typeCell immune response
The invention provides a nucleotide sequence for encoding a novel coronavirus antigen and application of the nucleotide sequence. According to the nucleotide sequence, a wild-type DNA sequence for encoding SARS-CoV-2 virus surface protein Spike is optimized, a wild-type gene signal peptide is optimized and then inserted into an eukaryotic expression vector, the eukaryotic expression vector is introduced into a host cell, a virus Spike antigen is efficiently expressed in the host cell, and an antiviral humoral immune response and a cellular immune response are systematically activated after antigen presentation. The antibody generated by the activated humoral immune response can prevent invasion of the SARS-CoV-2 virus, and the activated cellular immune response can further remove cells infected by the virus and adjust adverse reactions caused by potential side effects of ADE.
Owner:ADVACCINE SUZHOU BIOPHARMACEUTICALS CO LTD
Method for detecting pathogenic microorganism by using antigen-stimulated cellular immune response and test pen
InactiveCN102033129AImprove featuresHigh sensitivityChemiluminescene/bioluminescenceBiological material analysisPathogenic microorganismAntigen
The invention discloses a method for detecting a pathogenic microorganism by using antigen-stimulated cellular immune response. In the method, immune cells, which have been exposed to the microorganism, in blood are stimulated by specific antigen of the pathogenic microorganism or a functional segment of the pathogenic microorganism, so a cell factor, such as interferon gamma (IFN-gamma), is produced by the immune cells; and then whether the pathogenic microorganism exists in animals is judged by detecting the amount of the IFN-gamma. In the invention, the IFN-gamma in the blood is detected by a colloidal gold-labeled immunochromatographic assay. In addition, the invention also discloses a colloidal gold immunochromatographic assay test pen for detecting the pathogenic microorganism. By the method, the pathogenic microorganism in an incubation period or early morbidity can be detected quickly and effectively.
Owner:ABBOTT DIAGNOSTICS (SHANGHAI) CO LTD
Fusion protein of SVV and FMDV, encoding gene of fusion protein, expression vector, cell line, engineering bacteria, vaccine and application
The invention relates to the technical field of biomedicine, and particularly provides a fusion protein of an SVV and an FMDV, an encoding gene of the fusion protein, an expression vector, a cell line, engineering bacteria, a vaccine and application. The fusion protein is obtained by replacing an epitope capable of inducing the body to produce a neutralizing antibody with a decoy epitope and comprises SVV VP1 protein fragments, FMDV VP1 protein fragments and complement C3d protein fragments. The fusion protein combines antigens for inducing the body to produce the neutralizing antibody, of twopathogens of SVV and FMDV, and the safety of the antigens is improved while the antigenic epitopes of the SVV and the FMDV are reserved. The complement C3d molecules can excite nonspecific body fluidand cellular immune reactions in the body, and play an important role in increasing the antibody titer of the vaccine, and activating the cellular immune response of the body. The vaccine prepared bymeans of the fusion protein is safe and effective, and hydroa and foot-and-mouth diseases can be effectively prevented.
Owner:天康生物制药有限公司
Protein recombinant lactococcus lactis for secretory expression of core antigen COE of PEDV (Porcine Epidemic Diarrhea Virus) as well as preparation method and application of protein recombinant lactococcus lactis
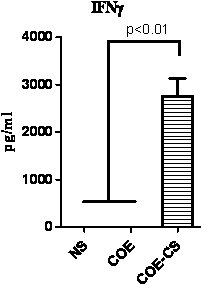
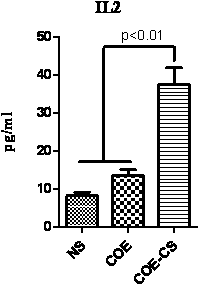
ActiveCN104120142AStrong immune responseGood industry prospectsBacteriaMicroorganism based processesStaphylococcus lactisTGE VACCINE
The invention belongs to the field of animal biomedicine engineering, and in particular discloses protein recombinant lactococcus lactis for secretory expression of a core antigen COE of a PEDV (Porcine Epidemic Diarrhea Virus) as well as a preparation method and an application of the protein recombinant lactococcus lactis. The method comprises the following steps: connecting a signal peptide and other sequences onto a gene of the core antigen COE of the PEDV via an overlap extension PCR method by designing multiple primers, connecting the gene to a lactococcus lactis expression vector pNZ8048 and introducing the vector with the gene into a cell of the lactococcus lactis NZ9000 in an electrotransformation manner so as to obtain recombinant bacteria; and inducing the recombinant bacteria with nisin so as to obtain an expression, and directly taking all induced cultures as oral vaccines capable of stimulating mice and inducing strong cellular immune responses. Thus, the protein recombinant lactococcus lactis can serve as a novel oral vaccine product with a good industrial prospect and has a positive effect for reducing harms of the PEDV to pig industry, thereby playing a great practical significance for promoting the healthy development of the pig industry.
Owner:SOUTH CHINA AGRI UNIV
Hepatitis B nucleic acid vaccine with optimized codon
InactiveCN101502650AHigh protein expressionImprove responseDigestive systemAntiviralsMammalDigestion
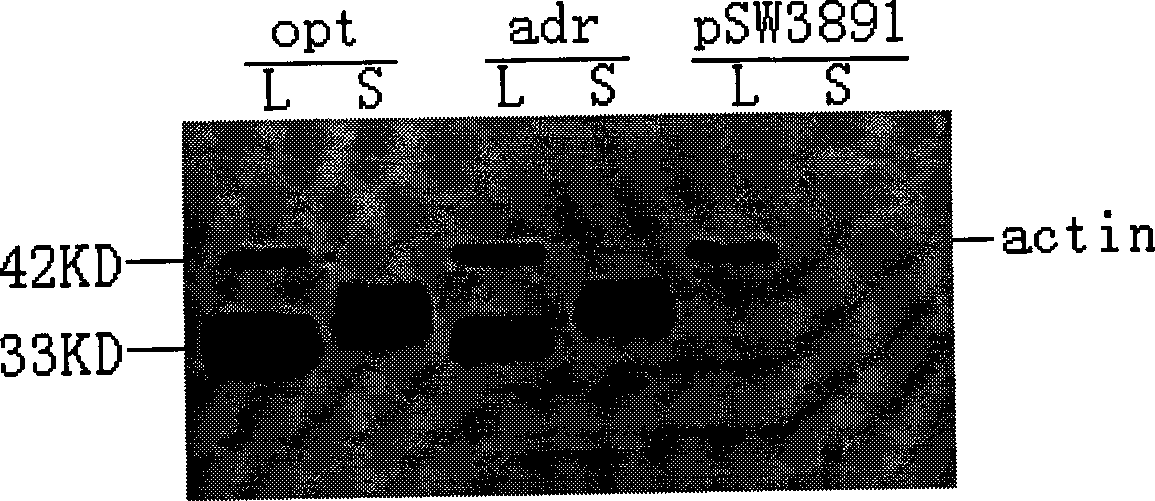
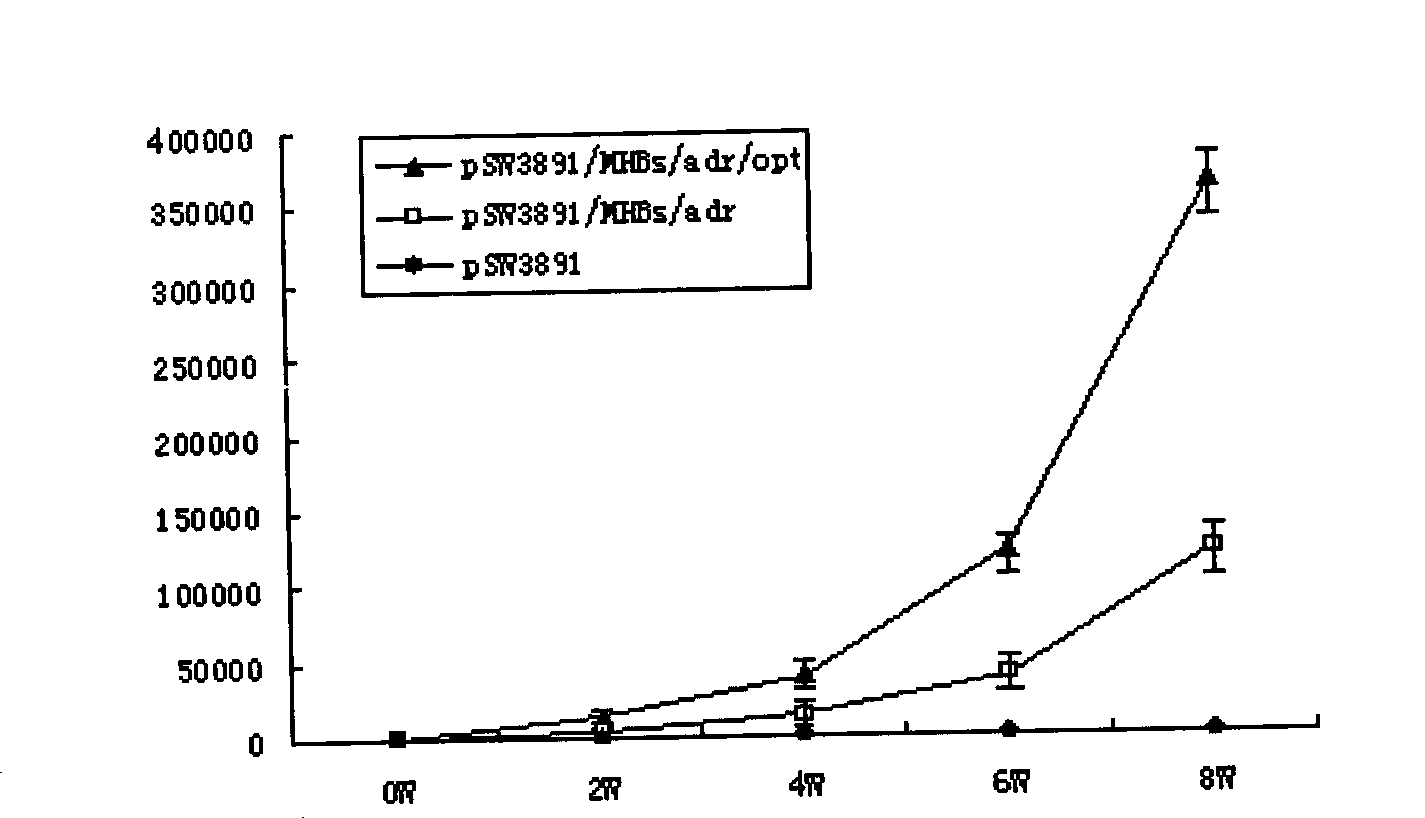
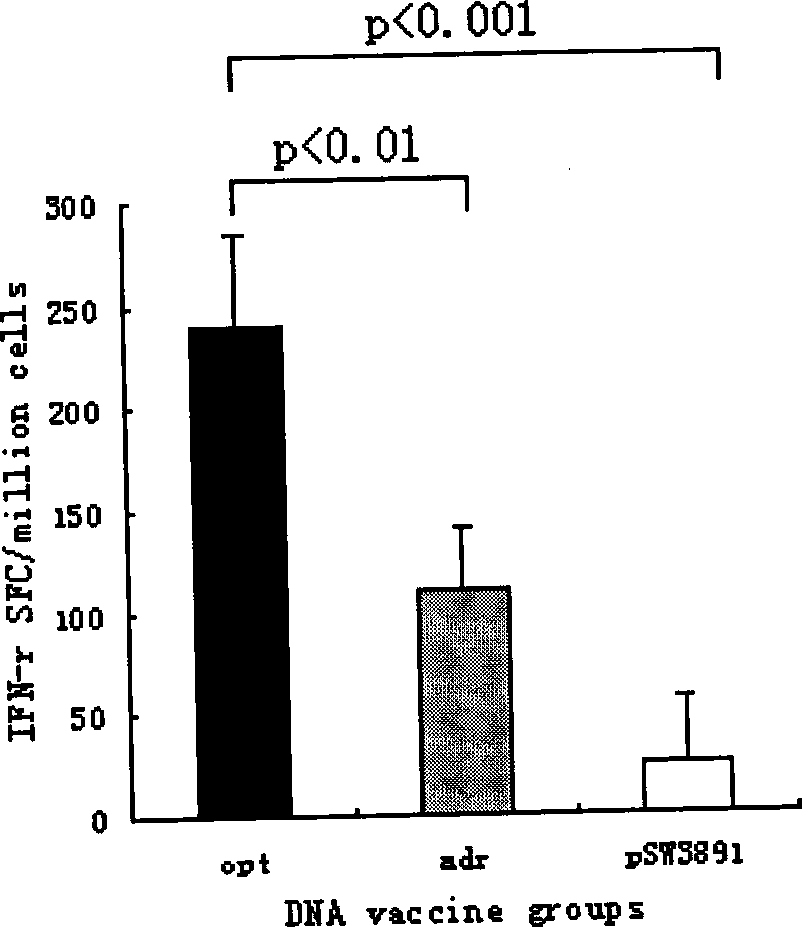
The invention relates to a hepatitis B virus nucleic acid vaccine optimized by codon. In the invention, hepatitis B surface antigen (HBs) gene order (adr subtype) is analyzed to find codon locus which tells the differences between the codon preferences of the gene order and the codon preferences of the mammal; the codon of the HBs gene order is replaced to obtain the surface antigen gene; the gene order is combined and expanded to obtain MHBs, Pst I, BamH I, double digestion MHBs gene and carrier pSW3891 plasmid optimized by the codon; 10ul connection system is configured to obtain a middle protein gene. The nucleic acid vaccine of the invention overcomes the defects that the differences between prokaryote and eukaryote in terms of codon preferences cause that the foreign gene can not be expressed effectively in mammal reservoir and can not generate relatively good immune sheltering effect; in addition, the invention remarkably improve protein expression of the foreign gene in the mammal reservoir, effectively stimulates immune system of the reservoir to generate relatively good immunological reaction of human body fluids and cellular immune response.
Owner:邢益平 +1
Preparation method of oral subunit vaccine for porcine epidemic diarrhea virus
ActiveCN104353069AEasy to degradeStrong humoral immune responseAntiviralsPharmaceutical non-active ingredientsImmune effectsTGE VACCINE
The invention belongs to the field of biological medicine engineering, and particularly discloses a preparation method of an oral subunit vaccine for a porcine epidemic diarrhea virus. The prepared subunit vaccine can be directly used as an oral vaccine to immune a mouse, and the mouse can be stimulated to generate stronger humoral immune response and cellular immune response. The vaccine is not degraded easily in the stomach and intestines, the immune effect is remarkable, the preparation process is simple, and the oral subunit vaccine plays an active role in relieving harm caused by the porcine epidemic diarrhea virus to the pig industry and has important practical significance in promoting healthy development of the pig industry.
Owner:SOUTH CHINA AGRI UNIV
Staphylococcus aureus fusion protein of fibronectin and bindin, its preparation method and uses
InactiveCN1884302AImprove protectionImproving immunogenicityAntibacterial agentsSkeletal disorderStaphylococcus cohniiMicrococcus pyogenes
The invention discloses a fusion protein for Micrococcus pyogenes surface protein, and also discloses the method for preparing the same as well as its usage. The fusion protein is fused by Micrococcus pyogenes fnb and thioredoxin Trx on expression carrier, and the process comprises preparation of Micrococcus pyogenes surface protein fnb coding gene, colony of fnb coding gene into expression carrier and protein fusion. The fusion protein is soluble protein and retains immunity of natural protein. It is detected that said fusion protein is characterized by good response for humoral immunity and cell immunity, enlargement of protection range of protein vaccine and prevention of infection caused by most of Micrococcus pyogenes.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Application of HBcAg (hepatitis B core antigen) virus-like particle serving as cancer therapeutic vaccine carrier
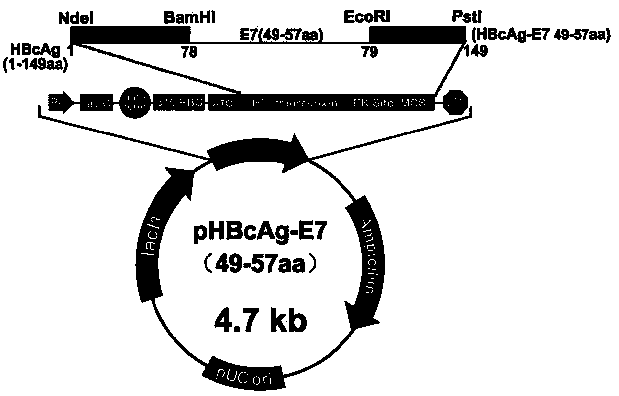
InactiveCN105497886AImprove the level ofViral antigen ingredientsAntineoplastic agentsHepatitis B virus core AntigenEscherichia coli
The invention relates to the field of molecular biology and immunology, in particular to an application of an HBcAg (hepatitis B core antigen) virus-like particle serving as a cervical cancer therapeutic vaccine carrier. A preparation method comprises steps as follows: an HPV16 E749-57CTLs epitope peptide fragment is selected, a DNA (deoxyribose nucleic acid ) fragment of the HPV16 E749-57CTLs epitope peptide fragment is inserted between 78 and 79 amino acids of the HBcAg through genetic recombination, an obtained recombinant plasmid pHBcAg-E749-57 is converted into Escherichia coli DH5alpha, and an HBcAg virus-like particle vaccine presenting E749-57 is obtained after induction expression and purification. After a tumor-bearing mouse is immunized with the virus-like particle vaccine, the body of the mouse can be induced to generate a higher HPV16E7 specific cellular immunologic response, and growth of tumors is remarkably inhibited.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
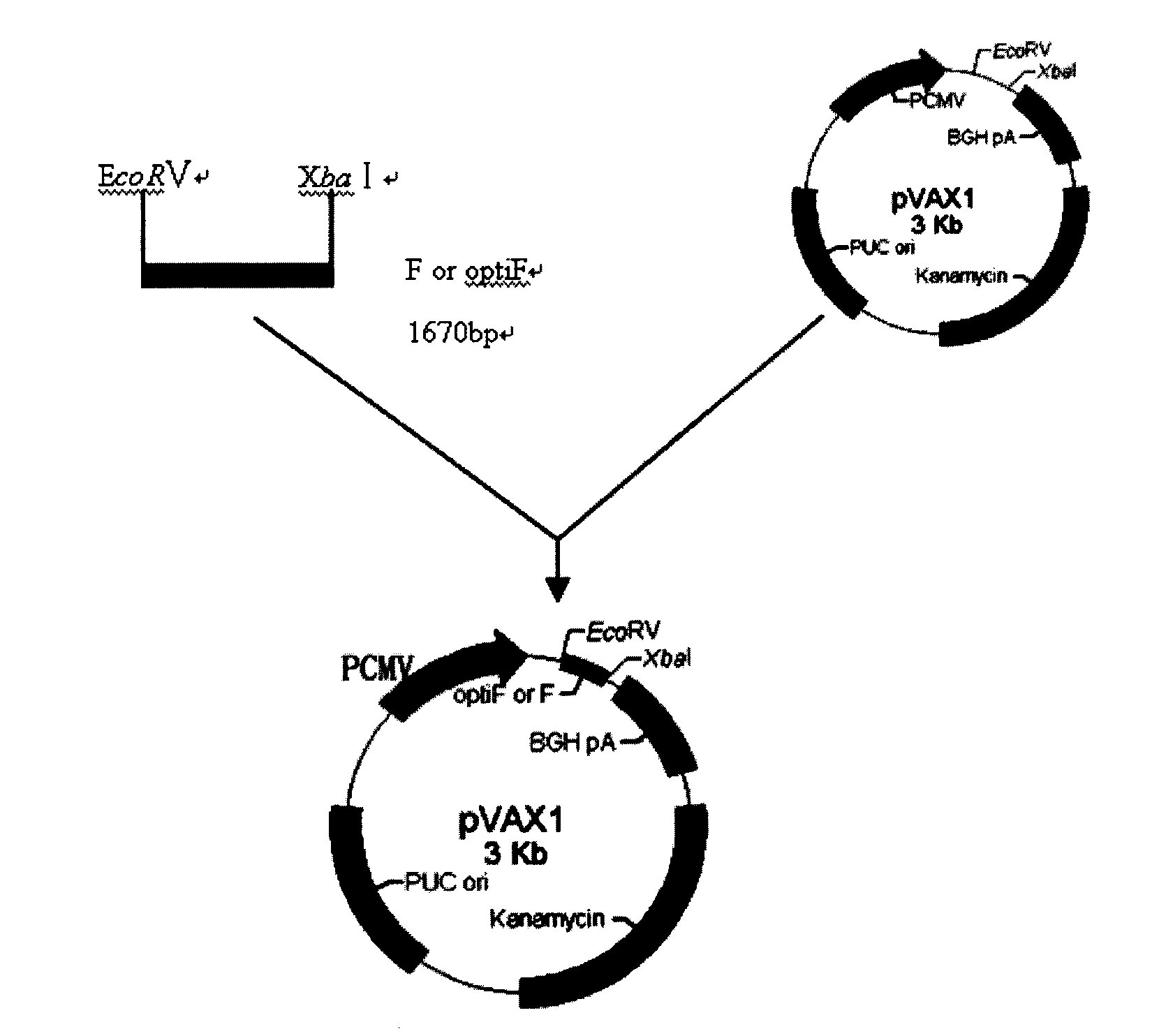
Manually-combined Newcastle diseases virus F gene and recombining expression vector and application thereof
InactiveCN101629178APrevent proliferationEffective controlViral antigen ingredientsGenetic material ingredientsWild typeRestriction site
The invention discloses a manually-combined Newcastle diseases virus F gene and a recombining expression vector and an application thereof. Codons of NDVF gene OFR are all replaced into chicken bias codons, EcoR V restriction sites and Kozak sequences are added upstream, Xbal I restriction sites and TGA stop codons are added downstream, and downstream bases A of the stop codons are changed into G, obtained genes (SEQ, ID NO: 2) can be efficiently expressed in eukaryotic cells, and the expression efficiency of the obtained genes is higher than that of wild type genes. Expressed genes have immunogenicity and biologic activity of natural protein of Newcastle diseases virus, can stimulate chicken to generate body fluid stronger immunologic response and stronger cell immunologic response than the wild F gene DNA vaccine so as to enable immunized chicken to obtain 100 percent of resistance to fatal attack of high-pathogenicity Newcastle strains, and can effectively inhibit the propagation of the virus in the chicken.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Fabricius bursa undecapeptide capable of promoting immunity
ActiveCN104829690APeptide/protein ingredientsImmunoglobulins against animals/humansB cells differentiationTissue fluid
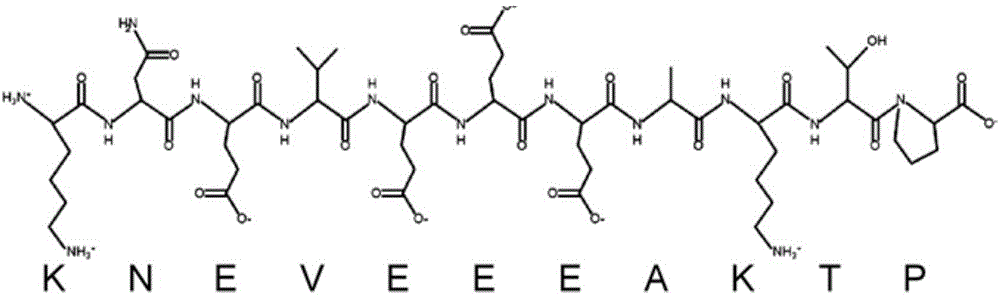
The invention provides Fabricius bursa undecapeptide capable of promoting the immunity. The Fabricius bursa undecapeptide is a novel polypeptide separated from Fabricius bursa tissue fluid, and the amino acid sequence of the Fabricius bursa undecapeptide is represented by the SEQ ID No.1. The provided polypeptide has an inductive effect on the generation of antibodies, and at the same time can modulate the cell factors, proliferate the lymphocyte, and promote the cellular immune response. Meanwhile, the B cell differentiation is modulated in a dose-dependent way, and the Fabricius bursa undecapeptide has a wide application prospect and can be applied to the fields such as immune regulation, immune treatment, and the like.
Owner:QINGDAO AGRI UNIV
Mouthwash capable of clearing lichen planus and Candida albicans of oral cavity
InactiveCN101843862AGood treatment effectImprove recovery rateAntimycoticsDigestive systemOral lichen planusCandida famata
The invention relates to mouthwash prepared in a Chinese medical formula, and has the functions of replenishing qi, strengthening the spleen, tonifying the kidney, soothing the nerves, clearing the heat, detoxicating, clearing the damp, reducing the cellular immune response, the capillary permeability and the inflammatory exudates, and improving the immunity of the organism. The invention has the advantages of good treatment effect, high cure rate and no recurrence fundamentally; and the oral drug not only has good preventive effect, but also has good therapeutic effect.
Owner:邱宏亮
Compositions and methods for increasing immunogenicity of glycoprotein vaccines
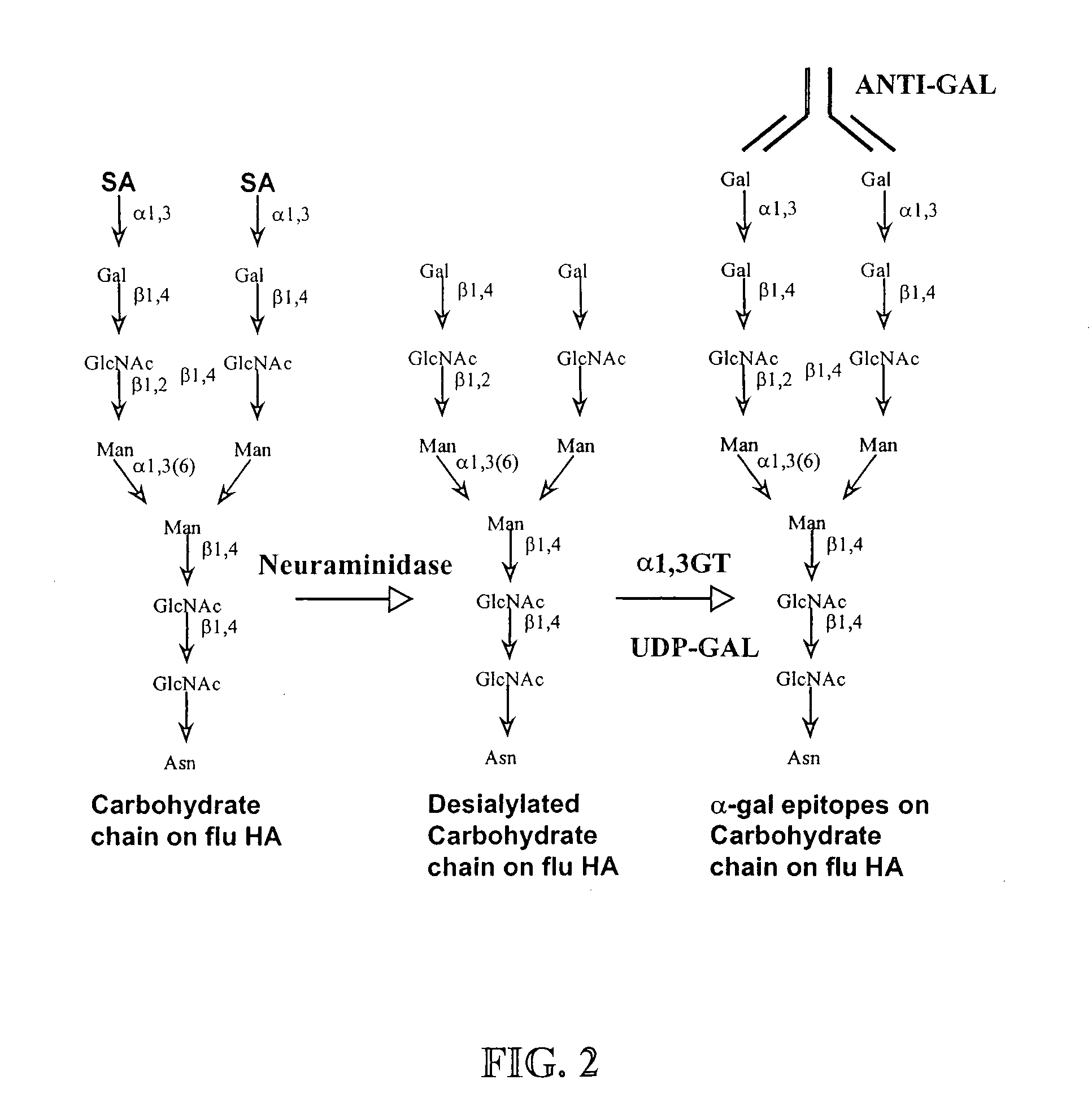
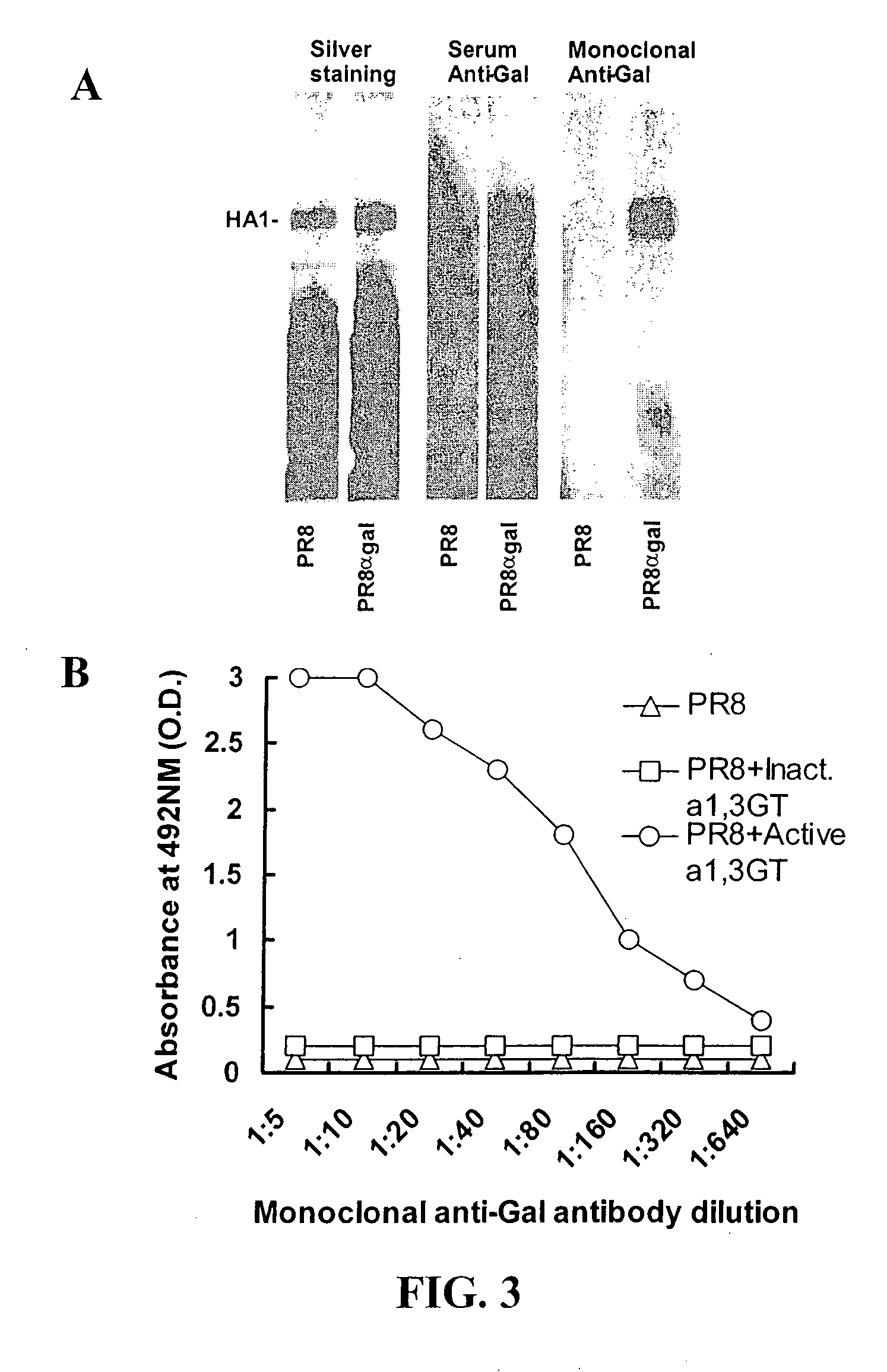
ActiveUS20100145015A1Improve targetingHeightened humoral and cellular immune responseSsRNA viruses negative-senseFusion with post-translational modification motifHemagglutininInfluenza virus A hemagglutinin
The present invention relates to the microbial immunogens engineered to bear α-gal epitope(s) for induction of potent humoral and cellular immune responses when administered to subjects having anti-Gal antibodies. In one embodiment, the present invention provides compositions and methods for propagating influenza virus in human, ape, Old World monkey or bird cells that have been engineered to express an α1,3galactosyltransferase (α 1,3GT) gene to produce virions bearing hemagglutinin molecules containing α-gal epitopes, to increase the immunogenicity of the influenza virus. In another embodiment, the present invention provides fusion proteins between influenza virus hemagglutinin and a microbial peptide or protein of interest, and enzymatic processing of this fusion protein to carry α-gal epitopes, to increase the immunogenicity of the microbial peptide or protein of interest.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
The DNA vaccine of an anti-infection of hepatitis C virus
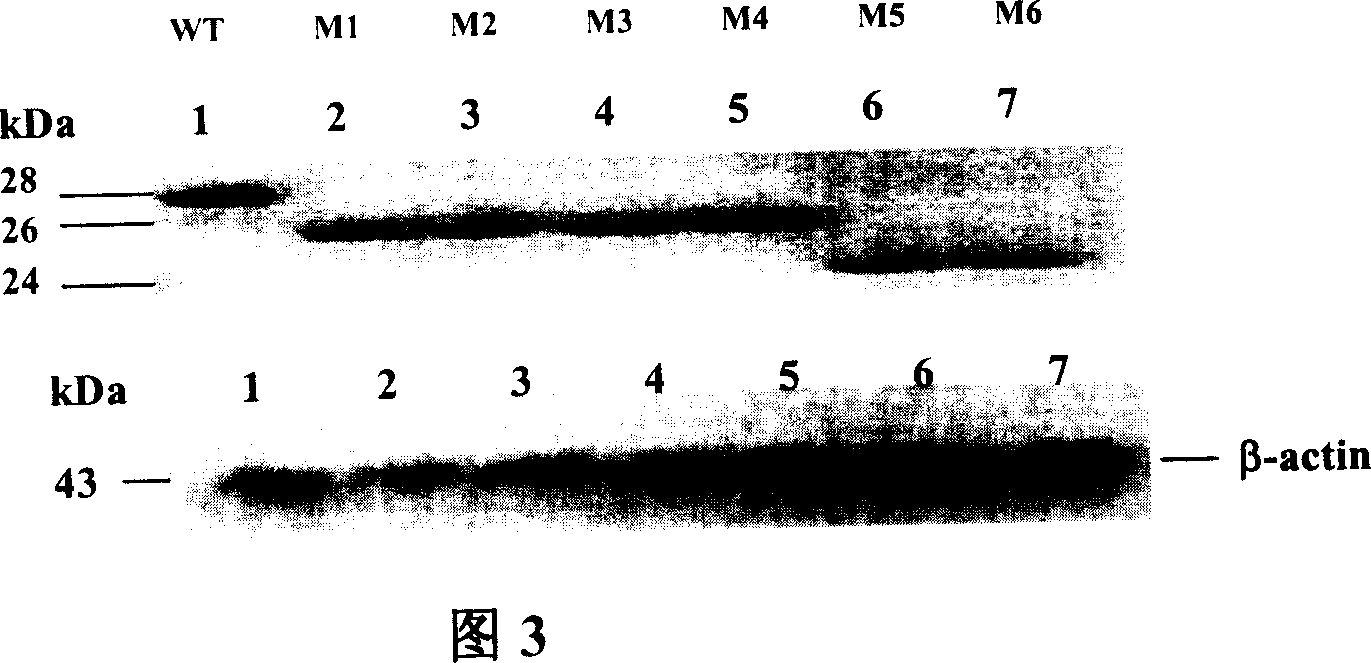
InactiveCN1943789AEasy to makeGood effectGenetic material ingredientsDigestive systemImmune recognitionMutant
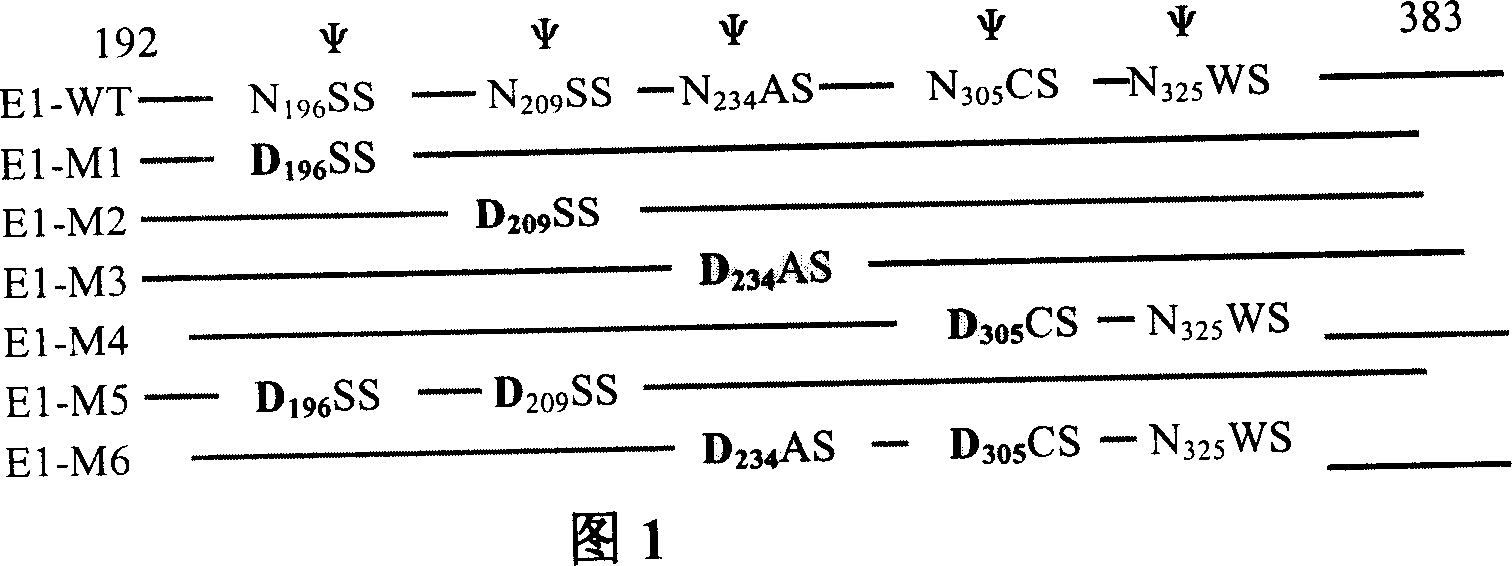
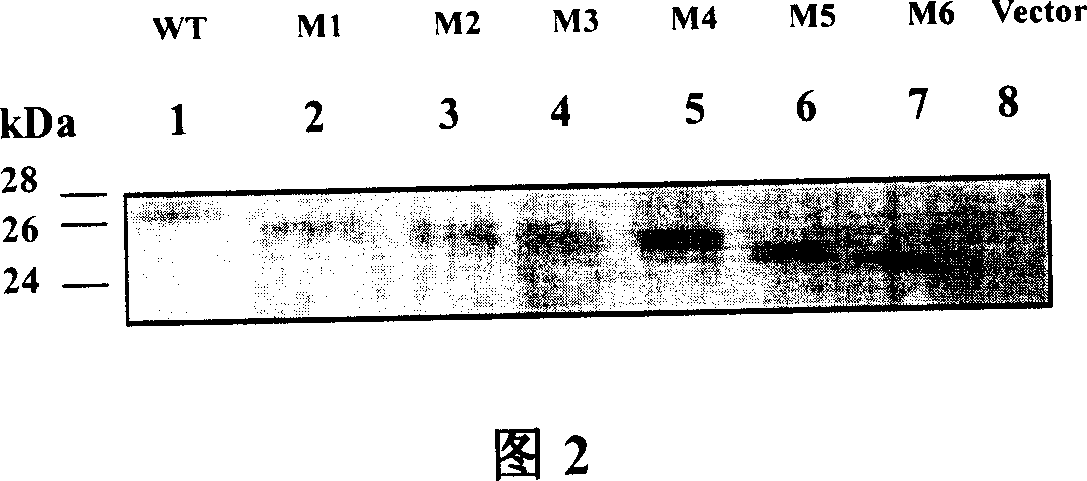
The invention relates to preparation and application of DNA vaccine that can resist to the infection of hepatitis C virus. The invention constructed a recombinant DNA vaccine that can express HCV enveloped glycoprotein E1 with molecular cloning techniques, on this basis constructed six N-glycosylation mutants M1-M6 of the E1 and select N-glycosylation mutant M2 as DNA vaccine that resist to infection of hepatitis C virus and application in the preparation of DNA vaccine that prevent the HCV virus infection. The advantages of the invention are : experiments reveal that N-sugar chain of HCV E1 enveloped glycoprotein can limit the host's cellular immune responses, its glycosylation may shield the T and B cell epitope of virus, thereby enable the virus to evade immune recognition; with E1 glycosylate mutants M2 may be used as DNA vaccines that are HCV therapeutic and preventive and enhance the immunogenicity, besides preparation of mutants M2 E1 glycosylate is simple, convenient and effective, and has good application prospect.
Owner:WUHAN UNIV
Aminoacid mimic epitope of human B lymphocyte stimulating factor receptor and use thereof
InactiveCN101348521APromote proliferationBiological carrier is goodPeptide/protein ingredientsPeptidesAdjuvantA-DNA
The invention relates to a mimic eptitope peptide of BAFF-R and a DNA coding the peptide and the application of the mimic eptitope peptide in the preparation of antitumor bacterins and drugs. The mimic eptitope of 7 amino acids of BAF-R is a mimic eptitope of molecule BAFF-R with high affinity with a monoclonal antibody of BAFF-R, and is obtained through selection from a phage random display 7-peptide bank with a monoclonal antibody of BAFF-R as the antigen, wherein the sequence of the amino acids is Gly Tyr Thr Arg Trp Gly Cys. The 7-amino-acid mimic eptitope can also be used to construct a poly-peptide vaccine. The clonal inhibition rates of the phage display peptide provided by the invention are all more than 50 percent, and can specifically inhibit the combination between antibody and antigen for a larger extent, greatly improve the proliferation of mouse spleen lymphocytes without adjuvant, the mimic antigen is good in immunogenicity, as illustrated by the induced cell immune response after mice are vaccinated with the mimic antigen.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
CTL (Cytotoxic T Lymphocyte) epitope peptide of foot-and-mouth disease virus type O and screening method of CTL epitope peptide
ActiveCN103864905AImprove bindingConvenient researchSsRNA viruses positive-senseVirus peptidesCtl epitopeDisease
The invention discloses a CTL (Cytotoxic T Lymphocyte) epitope peptide of a foot-and-mouth disease virus type O as well as a screening method and application of the CTL epitope peptide. The CTL epitope peptide is composed of nine amino acid residues, and the amino acid sequence of the CTL epitope peptide is as follows: Ala-Thr-Arg-Val-Thr-Glu-Leu-Leu-Tyr. The epitope peptide has relatively strong combining capacity with SLA (Swineleukocyteantigen)-I proteins from various strains of swine and can induce cytotoxic immune response so as to be suitable for preparing vaccines for preventing and controlling foot-and-mouth disease viruses of various strains of swine and wide in application range. According to the invention, a CTL simulated epitope peptide of a foot-and-mouth disease virus is combined with a single-chain molecule of SLA-I of six strains of constructed swine in vitro, thus a polypeptide which can be combined with a complex can be screened through mass spectrum measurement; in addition, a simulated epitope peptide which can be induced to generate the immune response capacity of T cells is determined through ELISPOT (Enzyme-Linked Immunospot Assay) detection. The invention provides a method for screening and authenticating the CTL epitope of the foot-and-mouth disease virus in a large scale, and lays the foundation for researching and preparing a multi-epitope vaccine of a foot-and-mouth disease.
Owner:DALIAN UNIV
Hepatitis B treating vaccine prepn and its prepn process and use
InactiveCN1548156AIncrease concentrationEnhance immune functionDigestive systemAntiviralsBALB/cYeast
The hepatitis B treating vaccine preparation includes recombinant hepatitis B vaccine 40-80 microgram / ml and aluminum 0.5 mg / ml. The preparation process, use and composition of the hepatitis B treating vaccine are also disclosed. The preparation can induce cell immune response for body to eliminate HBV, increase the cell immunity of Balb / c mouse, promote the high level expression of Th1 cell factor, raise T proliferation level and raise specific CTL activity.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com