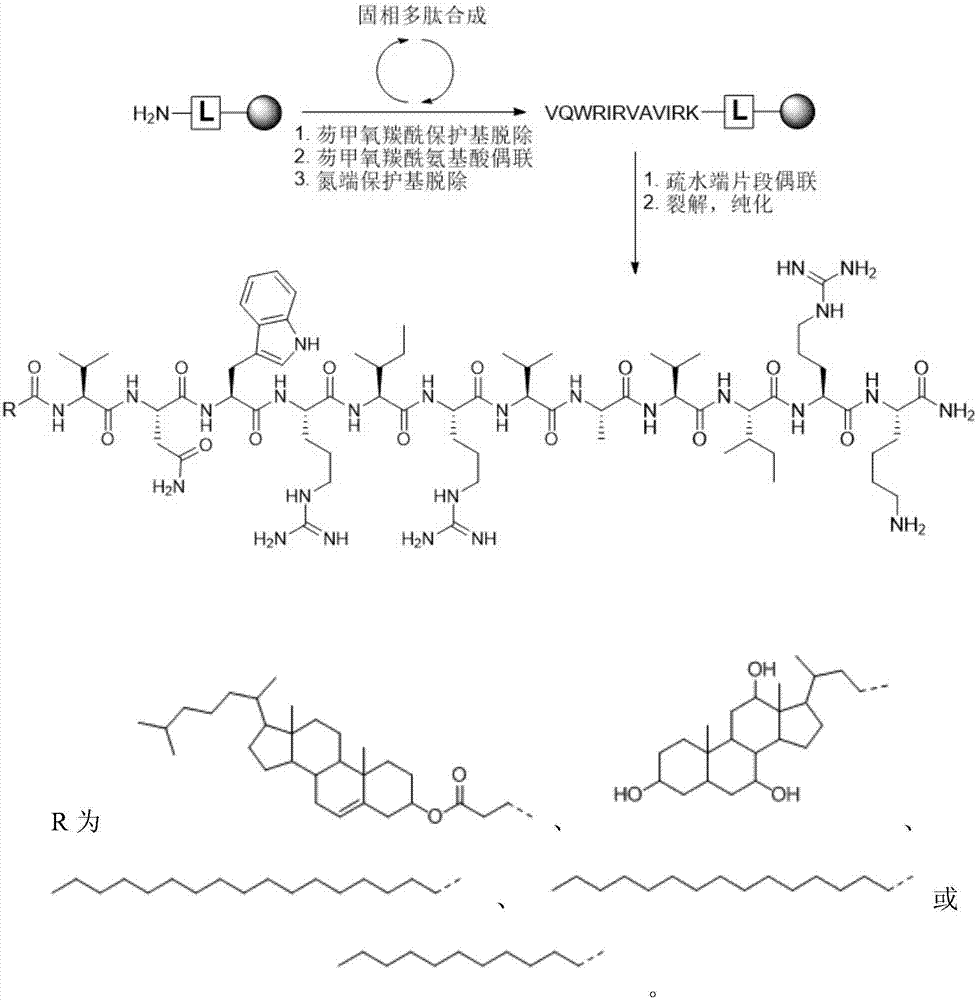
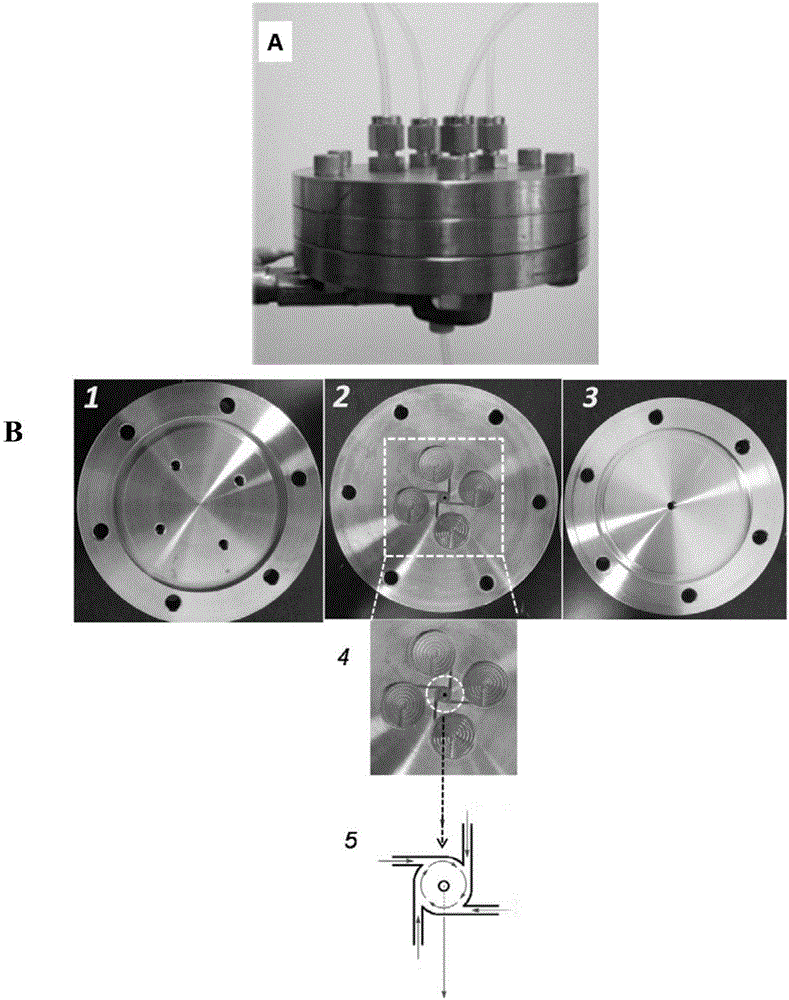
Patents
Literature
344 results about "Immunologic adjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In immunology, an adjuvant is a substance that potentiates and/or modulates the immune responses to an antigen to improve them. The word "adjuvant" comes from the Latin word adiuvare, meaning to help or aid. "An immunologic adjuvant is defined as any substance that acts to accelerate, prolong, or enhance antigen-specific immune responses when used in combination with specific vaccine antigens."
Composition and method of cancer antigen immunotherapy
InactiveUS6406699B1Snake antigen ingredientsPharmaceutical delivery mechanismVaccinationCancer antigen
A cancer immunotherapy method and composition for treating cancer in a patient comprised of vaccinating a patient with a vaccine comprised of the patient's own malignancy and an immunologic adjuvant, removing primed peripheral blood T lymphocytes from the patient, stimulating the primed T lymphocytes to differentiate into effector lymphocytes in vitro, stimulating the effector T lymphocytes to proliferate in vitro, and infusing the effector T lymphocytes back into the patient.
Owner:TVAX BIOMEDICAL LLC
Iscom preparation and use thereof
InactiveUS20060121065A1Low toxicityToxic reductionAntibacterial agentsAntiviralsMonoclonal antibodySoapbark Tree
The invention relates to a composition comprising a mixture of at least two iscom complexes each complex comprising essentially one saponin fraction from Quillaja Saponaria Molina. The complexes may be iscom complexes or iscom matrix complexes. The invention also pertains to the use of such a mixture for the preparation of an immunomodulating pharmaceutical, and adjuvant, formulations for immunsation e.g. for production of monoclonal antibodies and a vaccine. Kits of pars comprising at least two parts, wherein each part comprises one iscom complex or one iscom matrix complex according to the invention are also embraced.
Owner:ISKONOVA AB
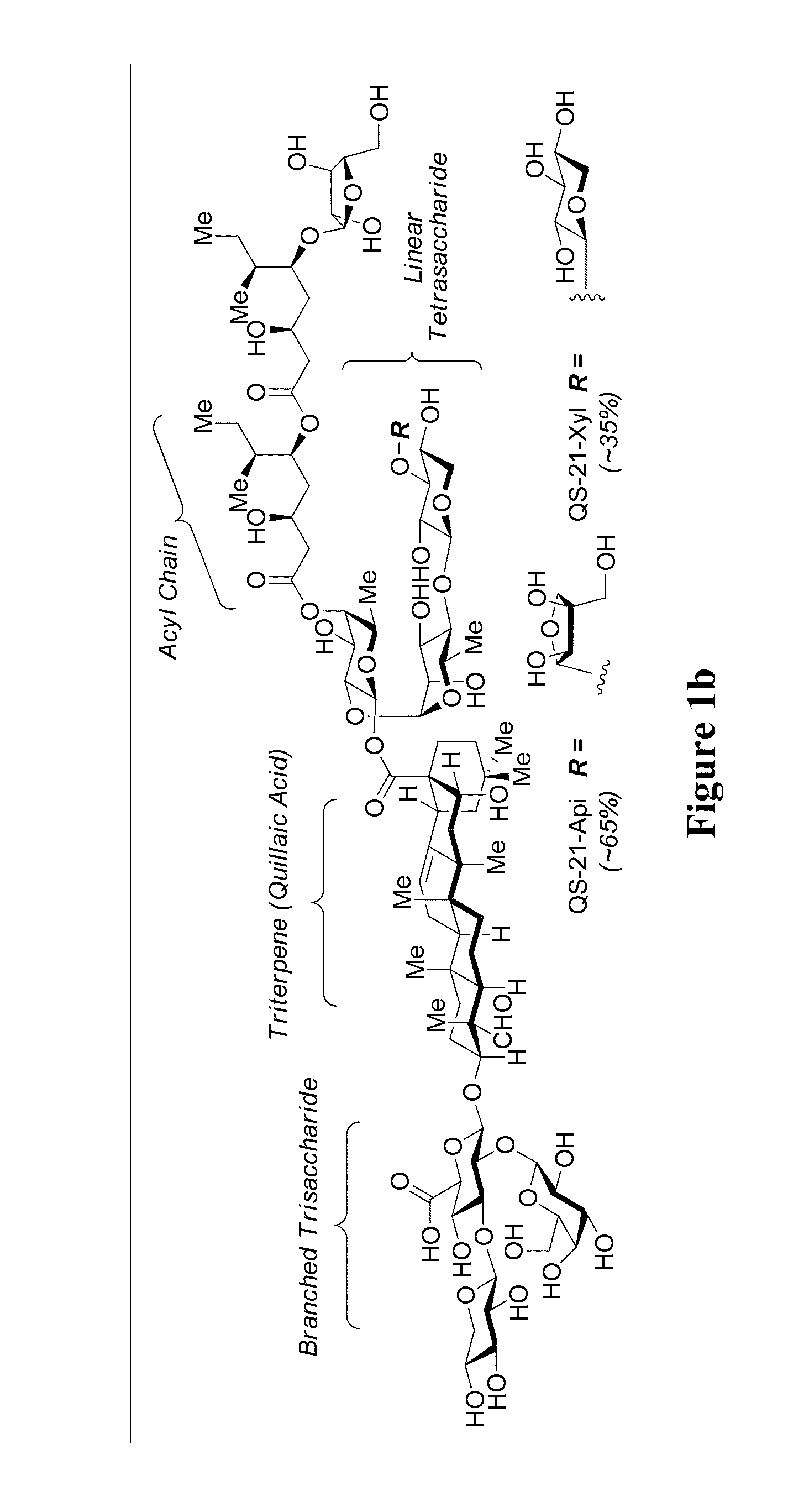
Triterpene saponins, methods of synthesis, and uses thereof
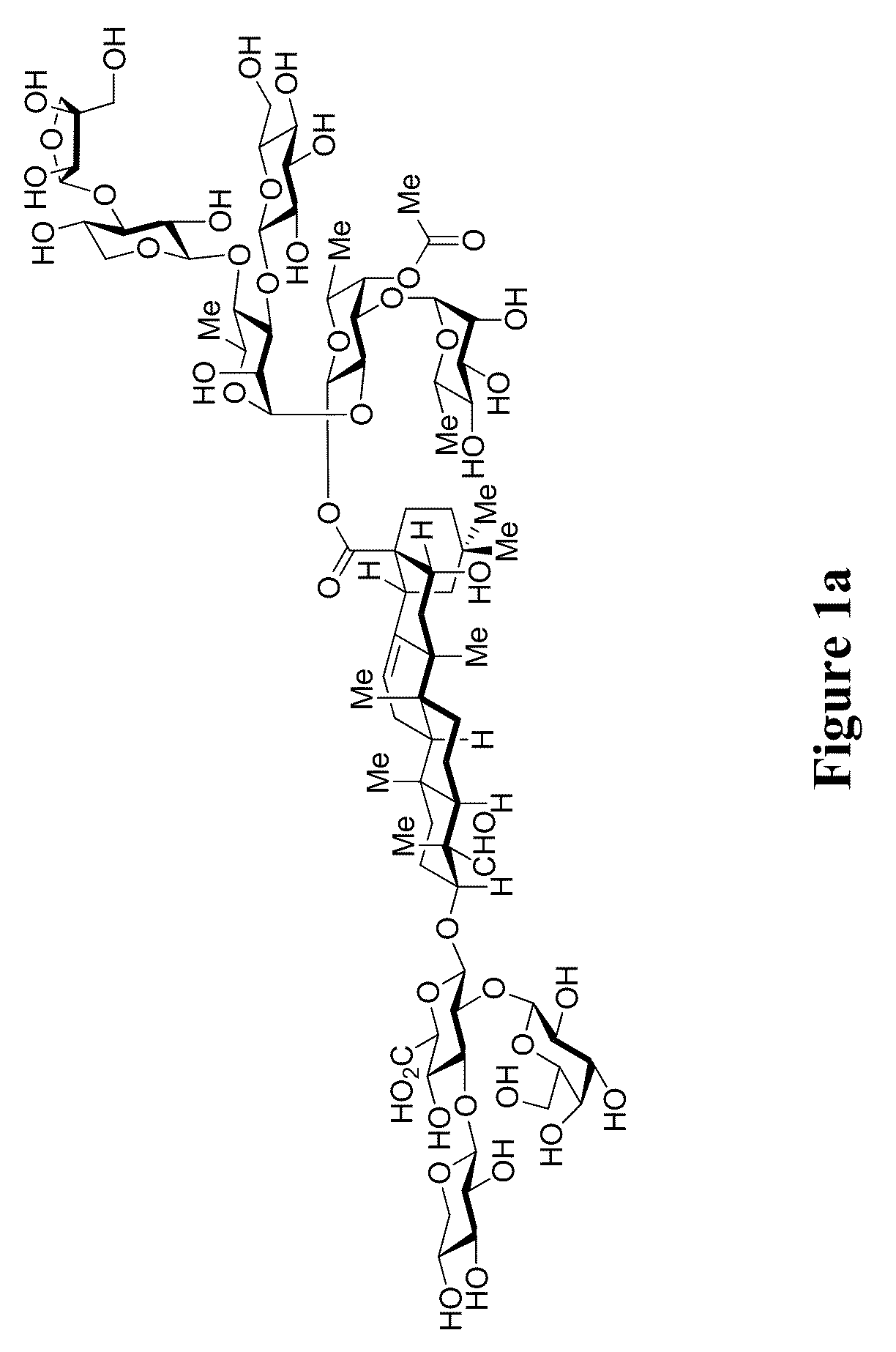
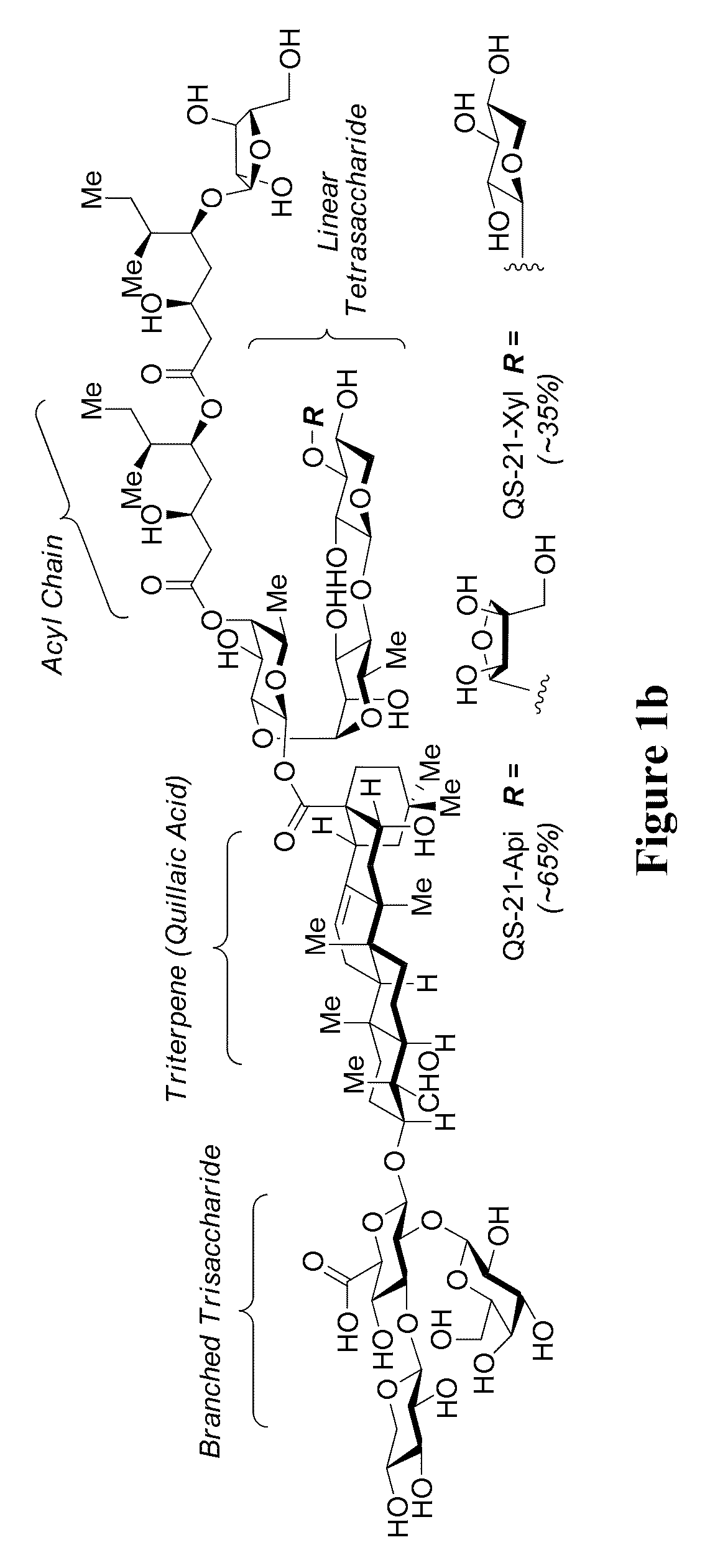
The present invention relates to triterpene glycoside saponin-derived adjuvants, syntheses thereof, intermediates thereto, and uses thereof. QS-7 is a potent immuno-adjuvant that is significantly less toxic than QS-21, a related saponin that is currently the favored adjuvant in anticancer and antiviral vaccines. Tedious isolation and purification protocols have hindered the clinical development of QS-7. A novel semi-synthetic method is provided wherein a hydrolyzed prosapogenin mixture is used to synthesize QS-7, QS-21, and related analogs, greatly facilitating access to QS-7 and QS-21 analogs for preclinical and clinical evaluation.
Owner:SLOAN KETTERING INST FOR CANCER RES
Rhodococcus ruber and application of same as immunologic adjuvant in preparing vaccine
ActiveCN109576180AWill not cause accidental infectionReduce pollutionBacteriaMicroorganism based processesSide effectShort terms
The invention discloses rhodococcus ruber and application of same as an immunologic adjuvant in preparing vaccine. The rhodococcus ruber is also called rhodococcus ruber RDC-01, and the preservation number is CGMCC (China General Microbiological Culture Collection Center) NO. 16640. The rhodococcus ruber disclosed by the invention has the function of increasing and regulating the body immunity andis capable of nonspecifically enhancing the activity of TB (Tuberculosis) lymphocyte, macrophagocyte and NK cells and inducing multiple cell factors such as interferon, and the rhodococcus ruber canbe used as the immunologic adjuvant after being inactivated so as to be added in an oil-adjuvant inactive vaccine, so that generation of an animal antibody induced by the vaccine can be obviously promoted; compared with single use of the oil-adjuvant inactive vaccine, a high-titre antibody can be generated, the use is safe, long-term and short-term toxic and side effects are not generated, and anapplication prospect in the field of preparation of vaccines for animals is good.
Owner:北京利昂盛生物技术有限公司
Antibacterial peptide derivate and application thereof
ActiveCN107446019ASmall molecular weightGood monodispersityAntibacterial agentsOrganic active ingredientsNucleic acid transportChemical synthesis
The invention belongs to the field of biological medicines, mainly relates to an antibacterial peptide derivate and the application thereof, in particular to a hydrophobic modified antibacterial peptide DP7 derivate and the application thereof. The hydrophobic modified antibacterial peptide is characterized in that a hydrophobic fragment is coupled to nitrogen terminal of the antibacterial peptide to realize the hydrophobic modification. The invention also provides a micelle which is prepared through the hydrophobic modified antibacterial peptide, and the application of the hydrophobic modified antibacterial peptide and the micelle in preparation of antibacterial medicines, preparation of a nucleic acid transporter and the preparation of an immunologic adjuvant. The antibacterial peptide is small in molecular weight and can be conveniently synthesized through an Fmoc solid-phase multi-peptide synthesis method; the hydrophobic fragment based chemical synthesizing coupling method is simple and easy to carry out.
Owner:SICHUAN UNIV
Polymer nano carrier preparation as well as preparation method and application thereof
ActiveCN103372217AEasy to manufactureSimple processGenetic material ingredientsPharmaceutical non-active ingredientsDendritic cellNanocarriers
The invention relates to a polymer nano carrier preparation as well as a preparation method and an application thereof. The nano carrier preparation employs a nano carrier micelle of a three-layer structure to carry DNA (Deoxyribonucleic Acid) antigen, protein antigen, polypeptide antigen and immunologic adjuvant. The polymer nano carrier preparation is good in stability and immunogenicity, and can not only prompt ingestion of antigen presentation cells such as dendritic cells for the antigen, but also remarkably induce organisms to make specific immune response.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Triterpene saponins, methods of synthesis, and uses thereof
The present invention relates to triterpene glycoside saponin-derived adjuvants, syntheses thereof, intermediates thereto, and uses thereof. QS-7 is a potent immuno-adjuvant that is significantly less toxic than QS-21, a related saponin that is currently the favored adjuvant in anticancer and antiviral vaccines. Tedious isolation and purification protocols have hindered the clinical development of QS-7. A novel semi-synthetic method is provided wherein a hydrolyzed prosapogenin mixture is used to synthesize QS-7, QS-21, and related analogs, greatly facilitating access to QS-7 and QS-21 analogs for preclinical and clinical evaluation.
Owner:SLOAN KETTERING INST FOR CANCER RES
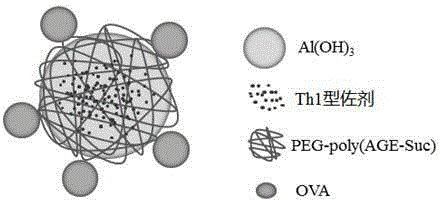
Vaccine vector based on aluminum hydoxide nano-particles
ActiveCN104587464AReduce aluminum contentNo inflammationPharmaceutical non-active ingredientsImmunological disordersDendritic cellTGE VACCINE
The invention provides an aluminum adjuvant used as a vaccine vector. The adjuvant is characterized in that a PEG derivative bio-material and aluminum are compounded to form nano-particles, so that the property of strong Th2 body fluid immunologic adjuvant of aluminum salt is maintained, and the adjuvant can be efficiently transferred to draining lymph nodes in the body and can be easily ingested by dendritic cells to perform effective cross-presentation and induce cellular immunologic response. The aluminum adjuvant has strong Th1 immunologic response.
Owner:SICHUAN UNIV
Toxicity T cell position vaccine of the cell for treating Hepatitis B and the preparing method
The invention relates to bio-pharmaceutical engineer technology domain. At present, the therapeutic drugs for HBV infection mainly depend on IFN-Alpha and nucleotide analog, which can not kill virus thoroughly with worse remote therapeutic effect. The invention provides a cytotoxic T cell epitope vaccine for hepatitis B, comprising a fused polypeptide formed by connecting 21 CTL epitopes selected from CTL epitopes data of HBV antigen and two universal epitopes of auxiliary T lymphocyte. Saccharomyces Serevisiae is selected to express the fused antigen and mixes with immunologic adjvant. The said vaccine can activate and enhance the cellular immune response of patient with chronic HBV infection. The vaccine can also promote the immune elimination of HBV, and can be used for treatment of chronic hepatitis B.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Ginseng total saponin or monomer saponin RbI vaccine immunological adjuvant application
InactiveCN1367022ALong validity periodShort validity periodAntibody medical ingredientsSide effectAluminium hydroxide
The present invention discloses an application of ginseng total saponin or monomer saponin Rb1 as vaccine immunologic adjuvant. The ginseng total saponin or monomer saponin Rb1 and aluminium hydroxide are mixed and used as vaccine immunologic adjuvant, and as compared with traditional aluminium hydroxide adjuvant said invention possesses the following advantages: (1) its side effect is less; (2) said vaccine can be stored under the condition of frozen low-temp. and it can prolong effective period of vaccine; (3) its method is simple and convenient, its quality is easy to be controlled; and (4) it can induce body to produce higher humoral immunity response.
Owner:ZHEJIANG UNIV
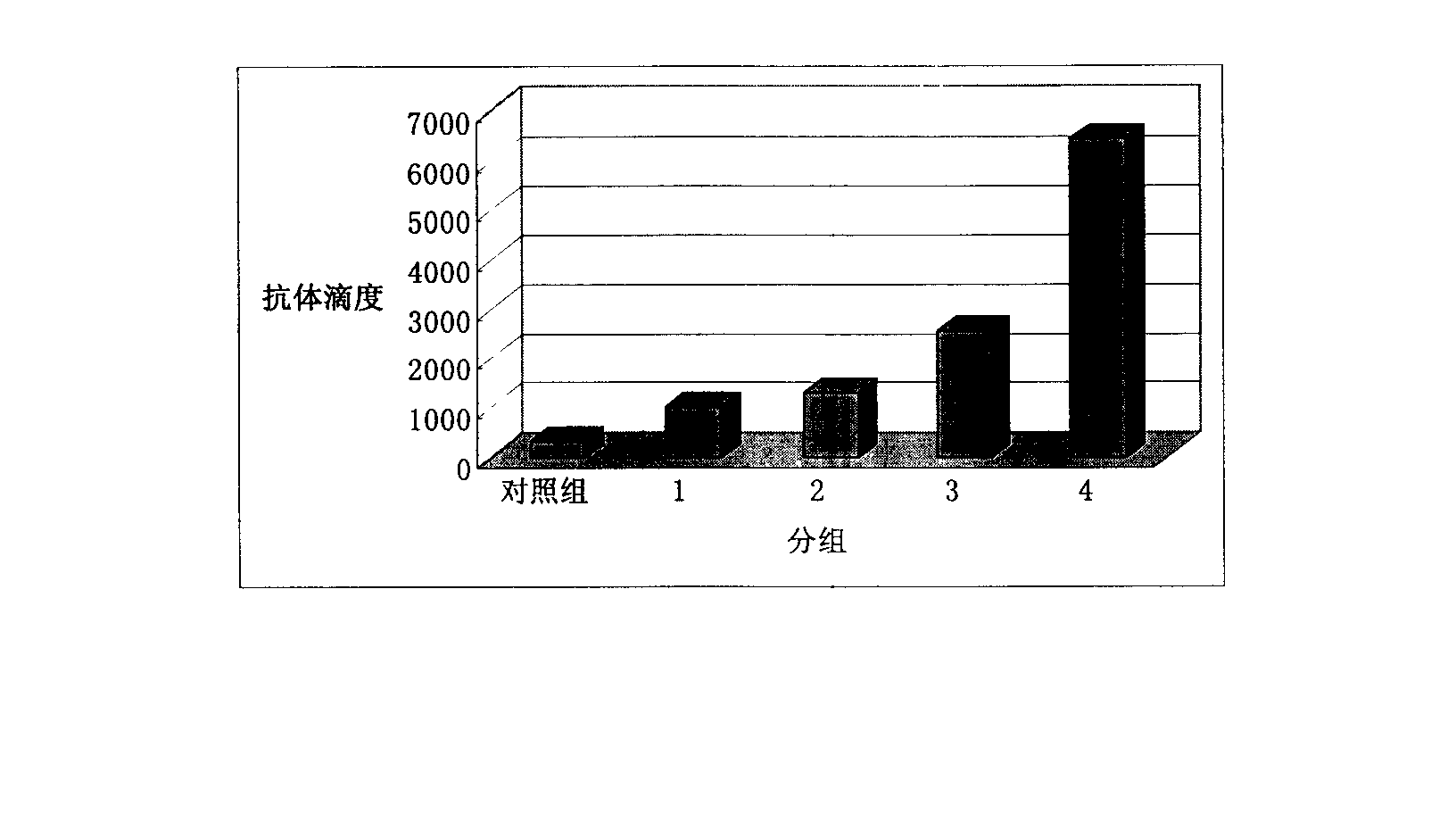
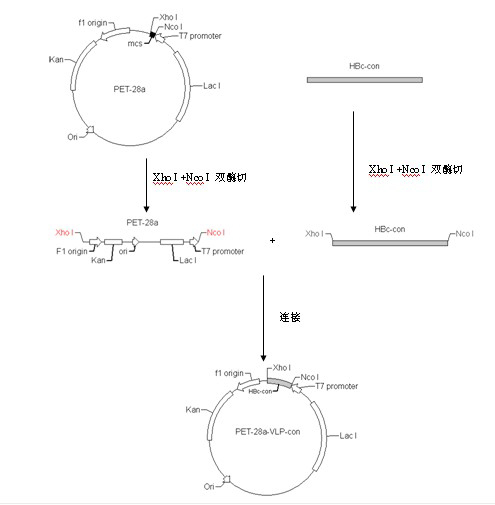
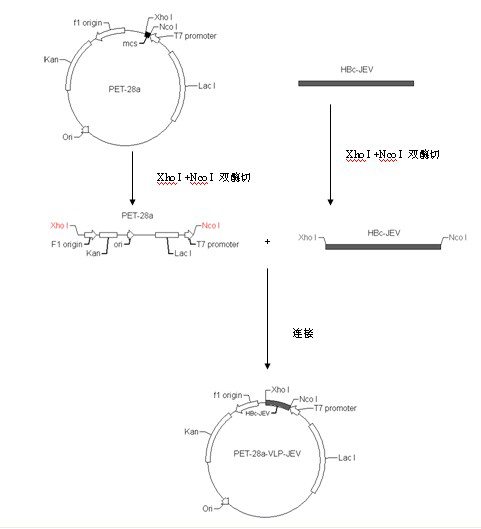
Japanese encephalitis particle vaccine and preparation method and application thereof
InactiveCN102127554AImprove the level ofStrong immune memoryViral antigen ingredientsVirus peptidesHepatitis B virus core AntigenEscherichia coli
The invention belongs to the field of biotechnology, and relates to a vaccine embedded with virus-like particles expressing multi-epitope antigen for Japanese encephalitis, and a preparation method and application thereof. The antigen of the vaccine is virus-like particles formed through spontaneous assembly of hepatitis B virus core antigen embedded with neutralizing antigen epitope expressing Japanese encephalitis virus and cytotoxic lymphocyte (CTL) antigen epitope, and is prepared through soluble expression of escherichia coli and purification. The Japanese encephalitis virus-like particle antigen is properly diluted with physiological saline, or is compatible with immunologic adjuvants to be prepared into the Japanese encephalitis particle vaccine. Animal experiments show that: the vaccine is safe and high-efficiency; mice inoculate with the vaccine generate high-level neutralizing antigen for the Japanese encephalitis virus to protect the mice against the attack of strong Japanese encephalitis virus by 100 percent.
Owner:NANJING AGRICULTURAL UNIVERSITY
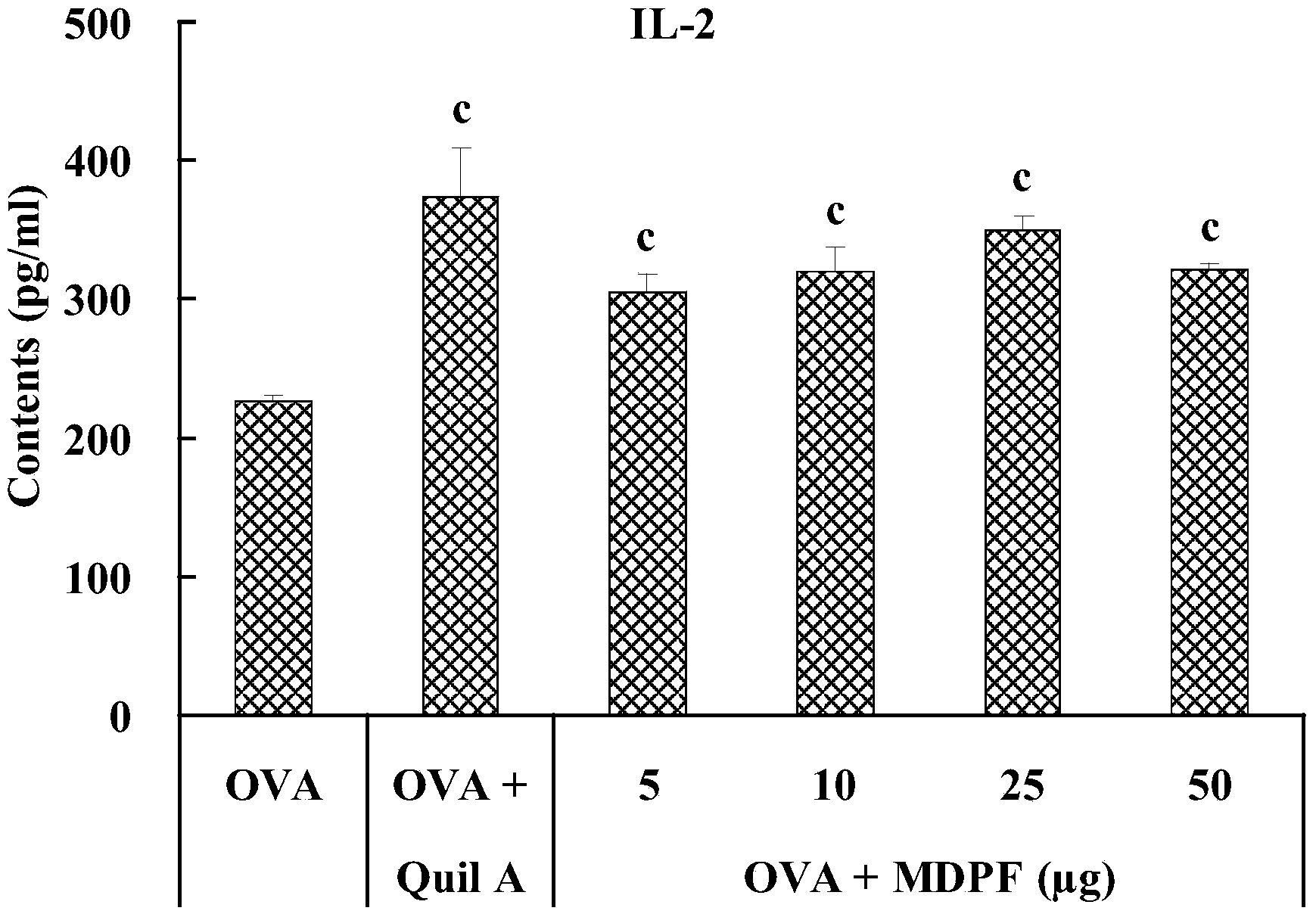
Aluminum hydroxide gel-polysaccharide composite immunologic adjuvant and preparation method and application thereof
ActiveCN102526724AAntigen releaseRelease stabilityDigestive systemAntiviralsImmune effectsImmunocompetence
The invention belongs to the field of biomedicine, and particularly relates to an aluminum hydroxide gel-polysaccharide composite immunologic adjuvant and a preparation method and application thereof. The invention aims to provide a new immunologic adjuvant with good performance. The technical scheme is to provide a composite immunologic adjuvant. The composite immunologic adjuvant mainly contains aluminum hydroxide gel and polysaccharide serving as bacteria source. The aluminum hydroxide gel-polysaccharide composite immunologic adjuvant provided by the invention has the advantages of strong immunocompetence, high clinical safety and the like, and is an excellent composite immunologic adjuvant aiming at various antigens. The hepatitis vaccine and tumor vaccine prepared by taking the aluminum hydroxide gel-polysaccharide composite provided by the invention as adjuvant has stronger immune effect and anti-cancer effect, and thus new selection is provided for the development and application of vaccine.
Owner:WEST VAC BIOPHARMA CO LTD
Transdermal immune influenza multivalent vaccine and preparation method thereof
InactiveCN101450209AEnhanced antigen presentation functionFacilitated DiffusionPowder deliveryAntiviralsBALB/cAdjuvant
The invention provides a transdermal immunity flu polyvalent vaccine and a preparing method. The transdermal immunity flu polyvalent vaccine comprises transdermal immunity adjuvant, flu polyvalent vaccine antigen, permeation agent and medical dressing. The flu polyvalent inactivation or attenuated live vaccine is differ from prior vaccine immunity approach and adjuvant. A result through permeation purpose immunity to Balb / c mouse, ferret, monkey and human body improves that the transdermal immunity flu polyvalent vaccine can generate IgA and IgG antibody with high valency, namely, can induce immune system and mucosal immune simultaneously, also can be used for immunoprophylaxis to popular flu and highly pathogenic avian influenza.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Anti-aflatoxin general type monoclonal antibody hybridoma cell line and application thereof
ActiveCN104004717AHigh detection sensitivityHigh affinityMicroorganism based processesTissue cultureBALB/cPolyethylene glycol
Owner:无锡迪腾敏生物科技有限公司 +1
Use of cyclic dinucleotide cGAMP and its derivatives as potential immunologic adjuvants
The invention belongs to the technical field of medicine and relates to a use of cyclic dinucleotide cGAMP and its derivatives as immunologic adjuvants. The study result shows that cGAMP and its derivatives can stimulate the innate immunity of the human body, are effective immunomodulators, can significantly improve the immune response and antibody titer, can stimulate expression of immature dendritic cell surface histocompatible complex MHCII antigens and an enhancement molecule CD80 / CD86, can increase secretion of cytokines and chemokines (such as interleukin and interferon), and can activate and enhance dendritic cells and T cells. Therefore, cGAMP and its derivatives can be used as potential immunologic adjuvants and play an important role in preparation of vaccines.
Owner:LIAOCHENG CITY ORIENT BIOMEDICAL TECH CO LTD
Bots antibacterial peptide adjuvant and preparation method thereof, and vaccine formulation containing adjuvant and use thereof
ActiveCN103908668ASimple preparation processSimple methodPeptide preparation methodsAntiinfectivesImmune effectsMaggot
The invention relates to a bots antibacterial peptide adjuvant with an immune adjuvant role and a preparation method thereof, and a vaccine formulation containing the antibacterial peptide adjuvant and use thereof in preparation of the vaccine formulation. The antibacterial peptide is a polypeptide component extracted and separated from fly maggots, the cellular immunity and humoral immune response of immunized mice can be facilitated, the organism is induced to generate balanced Th1 type and Th2 type immunoreactions simultaneously, and the adjuvant characteristics better than those of the known aluminum plastic adjuvant in the prior art are displayed. The bots antibacterial peptide adjuvant can be used as an immunologic adjuvant for a plurality of vaccines to play an ideal immune effect. The vaccine of taking the antibacterial peptide as the adjuvant is simple in preparation process, simple and convenient in method, easy to control quality, convenient to use, and good in safety, and can be frozen to store.
Owner:ZHEJIANG UNIV
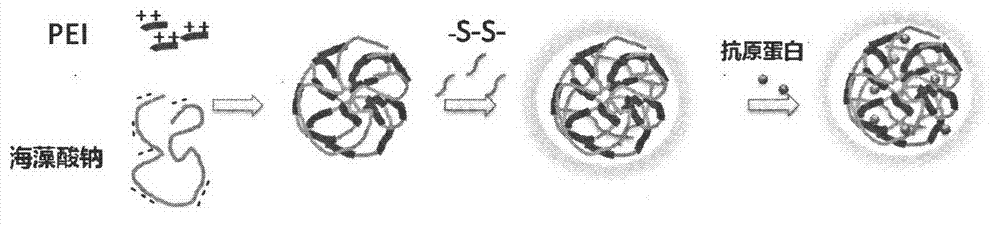
Polysaccharide/PEI nanogel with reduction responsiveness, preparation, and preparation method of polysaccharide/PEI nanogel
ActiveCN103588998AFast releaseEasy to wrapAntibody medical ingredientsAdditive ingredientAqueous solution
The invention discloses a polysaccharide / PEI nanogel with reduction responsiveness, and a preparation method of the polysaccharide / PEI nanogel. The preparation method comprises following steps: a gel is prepared from a polysaccharide and PEI via electrostatic interaction; and then a disulfide cross-linking agent is added for crosslinking, and the polysaccharide / PEI nanogel with reduction responsiveness is obtained. The polysaccharide / PEI nanogel processes following ingredients, by weight, 1 to 100 parts of the polysaccharide, 1 to 100 parts of PEI, and 10 to 2000 parts of the disulfide cross-linking agent. The polysaccharide / PEI nanogel possesses low toxicity; is capable of encapsulating genes or proteins preferably, and protecting substances encapsulated by the polysaccharide / PEI nanogel from degradation effect of lysozyme, and from cytophagy by immune cells; is capable of realizing response on reducing conditions in cells, and increasing release rate of the substances encapsulated by the polysaccharide / PEI nanogel; and can be used as a nanogel carrier to load proteins, polypeptides or nucleic acids. The polysaccharide / PEI nanogel also possesses effects of an immunologic adjuvant. All processes of the preparation method are performed in water, and no environmental pollution is caused.
Owner:珠海中科先进科技产业有限公司
Method for preparing nano aluminum hydroxide gel adjuvant
ActiveCN102988982AImprove immune efficiencyHigh purityAntibody medical ingredientsAluminium oxides/hydroxidesAluminium hydroxideSalt solution
The invention relates to a method for preparing a nano aluminum hydroxide gel adjuvant, belonging to the technical field of processes for preparing immunologic adjuvant of biological products for animals. The method comprises the following steps: mixing an aluminum salt solution and a soda solution which serve as raw materials, and adding a certain amount of stabilizing agent during mixing; shearing by a high-shearing emulsifying machine to obtain the nano aluminum hydroxide solution preliminarily; precipitating, washing and re-precipitating to obtain nano aluminum hydroxide gel; preparing a solution from the nano aluminum hydroxide; and compounding the solution with an inactivated bacterial solution to obtain an inactivated vaccine. The nano aluminum hydroxide gel adjuvant prepared by adopting the method is uniform in particle size distribution, has high purity, strong adsorbability and no side effect and has a good application prospect in the aspect of preparation of immunologic adjuvant.
Owner:山东滨州沃华生物工程有限公司
Astragalus polysaccharide adjuvant and uses in type I riemerella anatipestifer inactivated vaccine
InactiveCN101401935AImprove immunityImprove securityAntibacterial agentsBacterial antigen ingredientsAstragalus polysaccharideRiemerella anatipestifer
The invention relates to a vaccine immunologic adjuvant and a vaccine, in particular to an astragalus polyose adjuvant and application thereof in an I-riemerella anatipestifer inactivated vaccine. The vaccine adjuvant is an astragalus polyose solution with the concentration of 30 milligrams per milliliter. An astragalus polyose inactivated vaccine for the I-riemerella anatipestifer is prepared from a riemerella anatipestifer RA-1 inactivated bacteria liquid as the vaccine and the astragalus polyose solution as the adjuvant through mixing. The astragalus polyose adjuvant improves the immune efficacy of the prior inactivated vaccine, effectively prevents and controls infectious serositis of duck, and provides a basis for the research of a novel immunologic adjuvant. The vaccine has good safety and immunogenicity, and can effectively prevent the infectious serositis of duck.
Owner:武汉市畜牧兽医科学研究所
Nanoparticle containing EV71VP1 protein and preparation method of nanoparticle
ActiveCN106421770AMorphological rulesRound shapeSsRNA viruses positive-senseViral antigen ingredientsNanoparticleAnionic polymers
The invention belongs to the technical field of medicine, and relates to a nanoparticle containing EV71VP1 protein and a preparation method of the nanoparticle. Specifically, the invention relates to a nanoparticle containing EV71 VP1 protein, an immunologic adjuvant, a cationic polymer and an anionic polymer, wherein the immunologic adjuvant is selected from one or more of TNF-alpha, CpG, bcg vaccine and flagellin. The invention further relates to a method for preparing the nanoparticle including immunogenic composition (such as vaccine) of the nanoparticle. The nanoparticle is used for preparing the immunogenic composition (such as vaccine) and for the nanoparticle or the immunogenic composition (such as vaccine) to initiate or enhance the immune response of subjects to EV71.
Owner:GUANGZHOU LIDE BIOMEDICINE TECH CO LTD
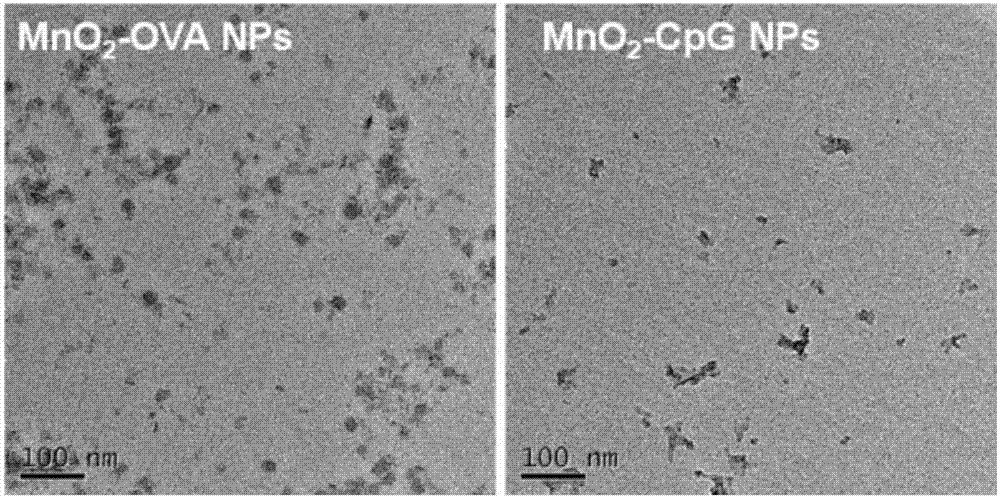
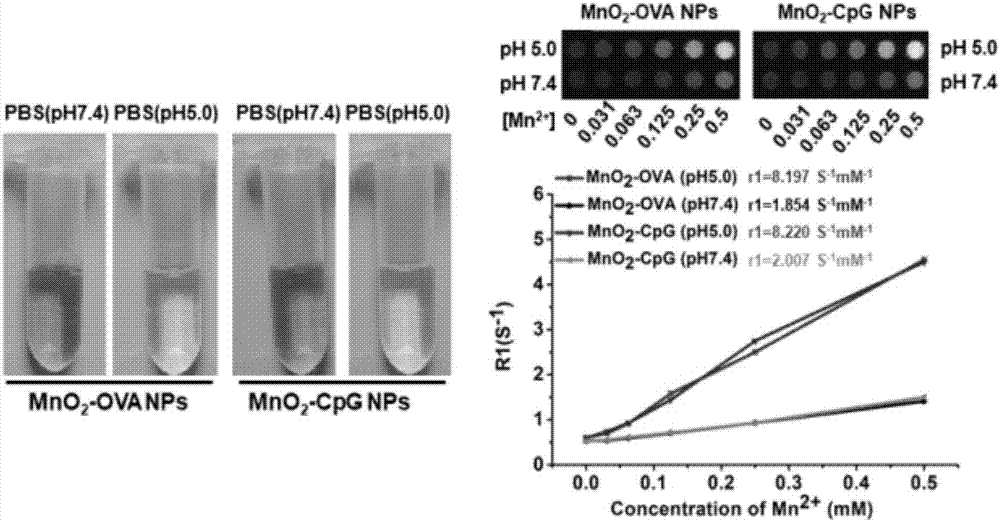
Manganese dioxide nanometer adjuvant and preparation method and application thereof
PendingCN107456575AIncrease intakeImproving immunogenicityAntibody medical ingredientsAgainst vector-borne diseasesStimulantLysosome
The invention provides a manganese dioxide nanometer material. The manganese dioxide nanometer material comprises manganese dioxide nanoparticles and carried matter, wherein the carried matter is compounded onto the surface of the manganese dioxide nanoparticle; the carried matter comprises oligonucleotide CpG and / or antigen; the antigen comprises protein antigen and / or polypeptide antigen. The provided manganese dioxide nanometer material has the advantages that the immunostimulation function of an immunologic adjuvant CpG is greatly improved, the immunity protection function of vaccine is improved, and the inherent defect of effective improving of immune reaction by a large amount of CpG because the oligonucleotide CpG is used as an immunologic stimulant and cannot easily enter the body lymph glands is solved; the manganese dioxide nanometer material is used as a vaccine adjuvant, and can carry the antigen, especially the protein antigen, so that the intake on the antigen by the immune cells in the lymph glands is effectively improved, and the immunogenicity of the antigen is improved; the nanometer adjuvant can be biologically degraded, and can be gradually degraded into manganese ions (Mn<2+>) under the weak acid environment, such as lysosome, and the manganese ions are finally discharged out of the human body.
Owner:SUZHOU INNOVATIVE BIOMATERIALS & PHARM CO LTD
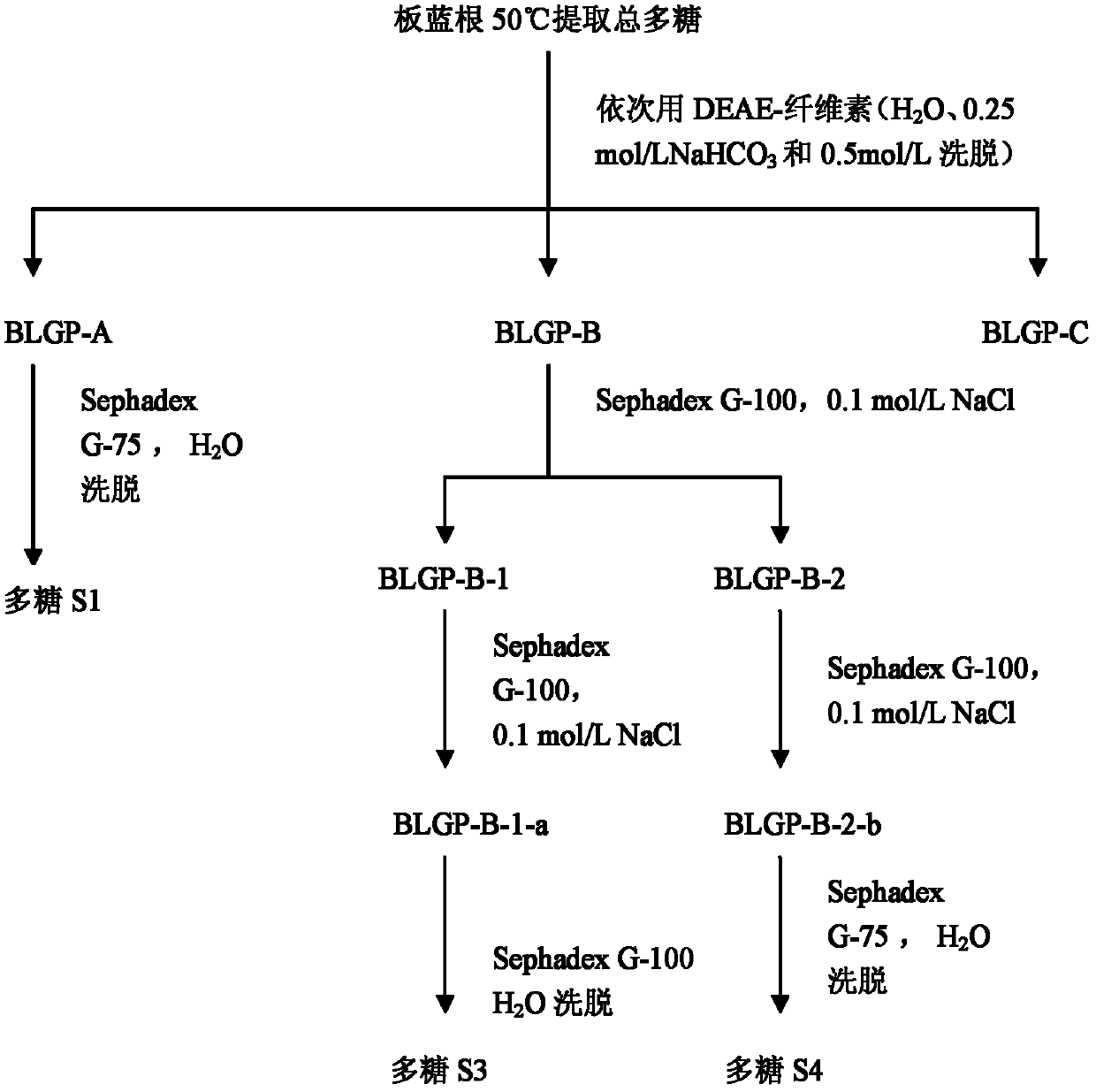
Isatis root polysaccharides, and preparation method and use thereof
The invention belongs to the technical field of medicine, relates to polysaccharide components extracted from isatis roots, and in particular relates to polysaccharide components S1, S3 and S4 extracted from isatis roots. The invention also relates to a preparation method of the polysaccharide components S1, S3 and S4, and use thereof as a vaccine adjuvant or an immunomodulator or for preparing a vaccine composition. The results of activity experiments indicate that the separated polysaccharide components S1, S3 and S4 provided by the invention have vaccine adjuvant activity and immunoregulation effec, thereby providing a new option for immunologic adjuvants.
Owner:BEIJING ZHONGAN ADJUVANT BIOTECH
Poultry IL-2 and newcastle disease virus HN gene recombination fusion protein and application thereof
InactiveCN101870733AEnhance body fluidsEnhance cellular immune responseFungiViral antigen ingredientsSide effectHN Protein
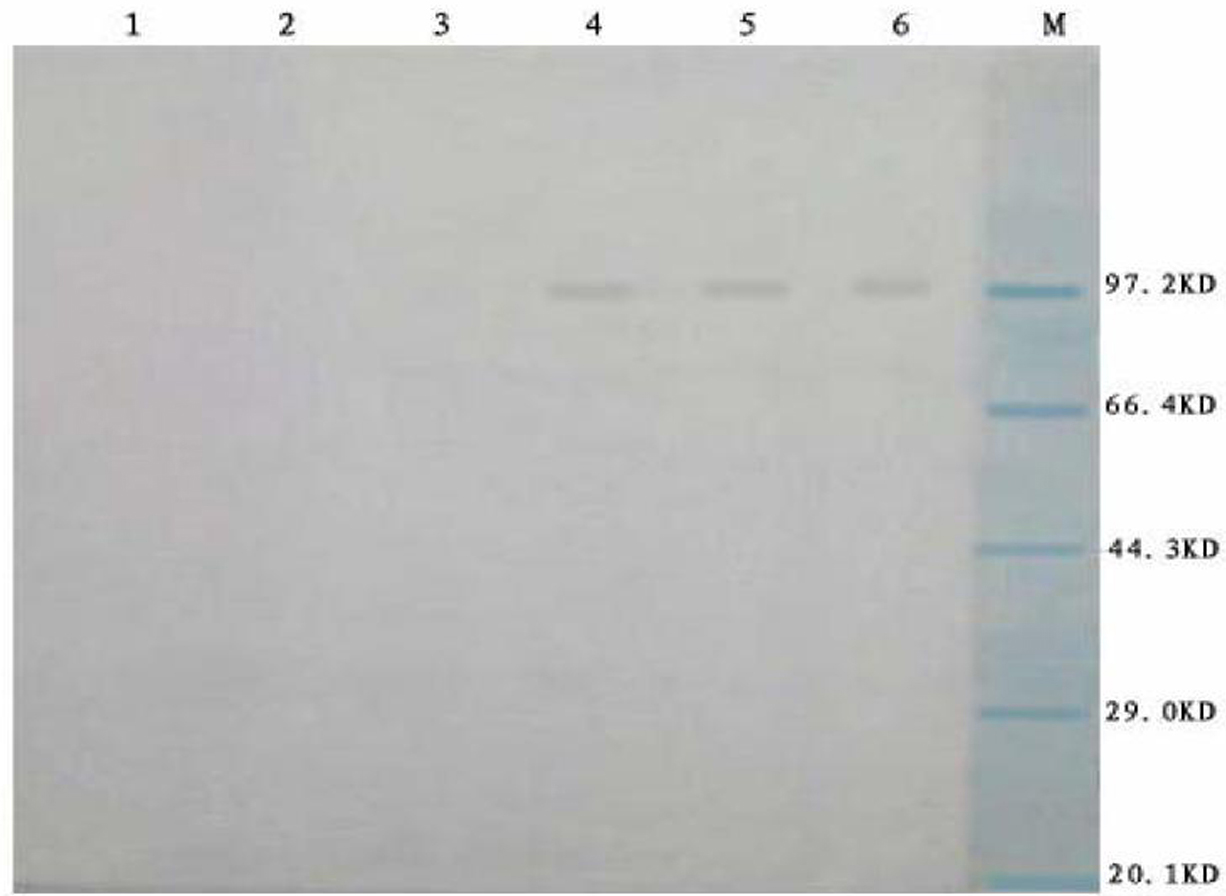
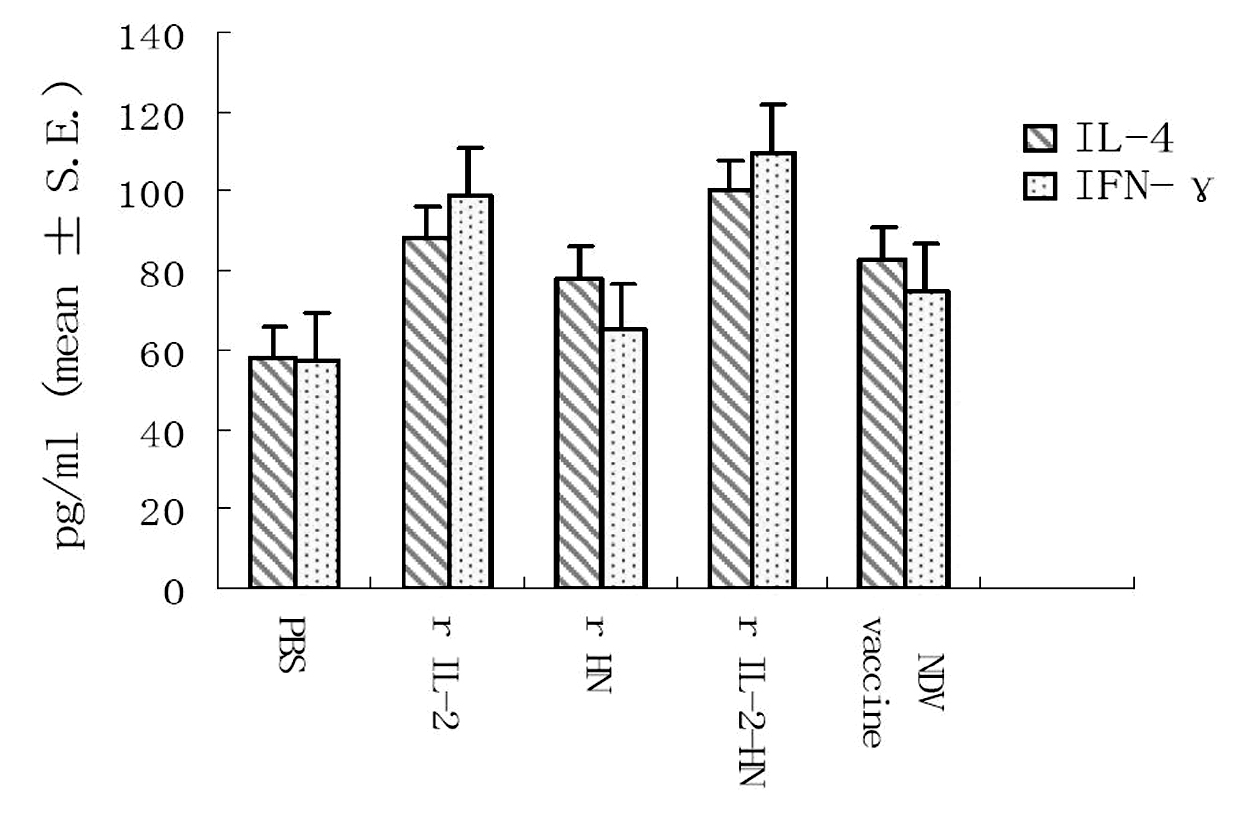
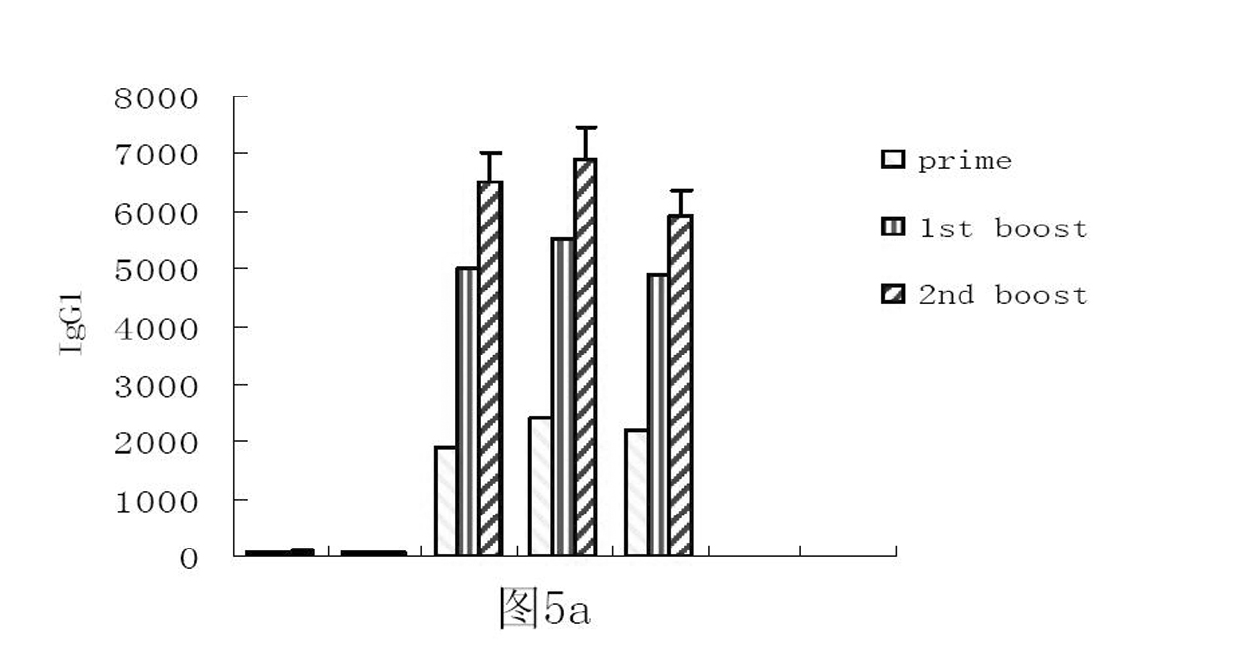
The invention relates to a preparation method and application of fusion protein (chIL-2-HN fusion protein) of recombining poultry interleukin-2 (IL-2) and newcastle disease virus hemagglutinin-neuraminidase (HN), belonging to the technical field of biological engineering. The fusion protein is fused by poultry IL-2 protein and newcastle disease virus HN protein by flexible Linker peptide; a DNA sequence for coding the fusion protein is inserted into an expression vector pPICZ alpha A; saccharomycete X-33 is electrically converted to obtain a genetically engineered microorganism for efficiently expressing recombination chIL-2-HN fusion protein which is prepared by liquid culture and purification; the recombination fusion protein can serve as the novel genetic engineering vaccine and can serve as a novel immunologic adjuvant for newcastle disease common vaccine. The chIL-2-HN fusion protein of the invention has favourable safety and no toxic or side effect, which is verified by experiments of animal immunoassay. In addition, the chIL-2-HN fusion protein can effectively strengthen the level of organism cellular immunity and humoral immunity and has wide application prospect.
Owner:HENAN UNIV OF SCI & TECH
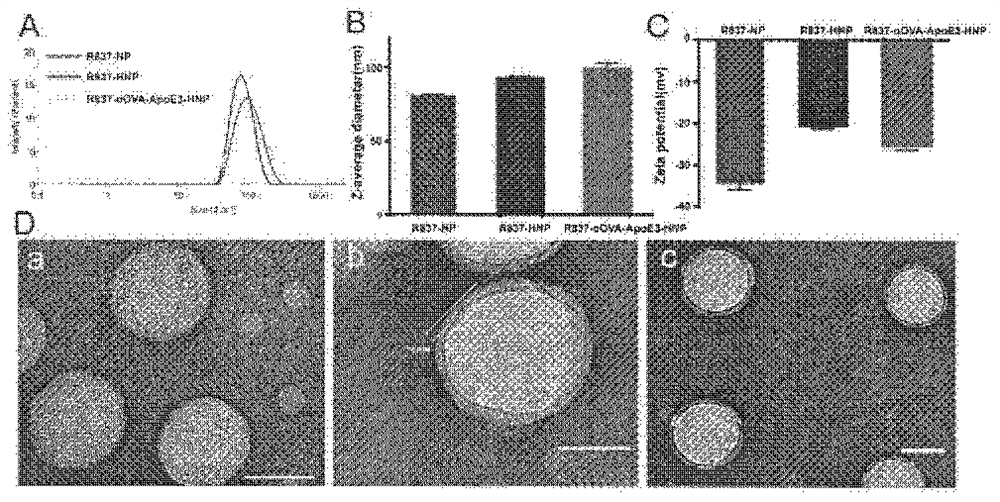
Apolipoprotein modified bionic nano tumor vaccine as well as preparation method and application thereof
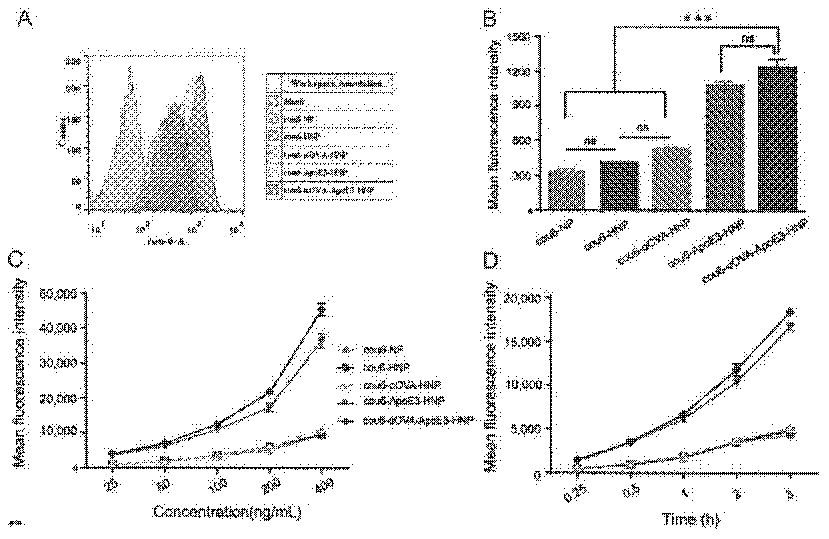
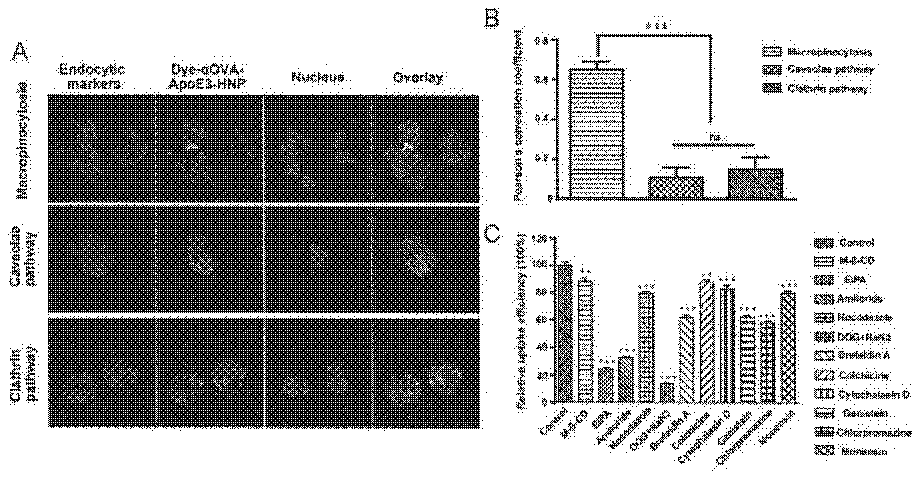
InactiveCN112569207AAvoid degradationImprove intake efficiencyCancer antigen ingredientsMacromolecular non-active ingredientsDendritic cellPotocytosis
The invention belongs to the technical field of biological pharmacy, and relates to an apolipoprotein modified bionic nano tumor vaccine as well as a preparation method and an application thereof. Based on the characteristics of dendritic cell (DC) phagocytic antigen, the bionic nano tumor vaccine is prepared, and the bionic nano tumor vaccine utilizes a macropinocytosis approach to increase the intake of DC to the vaccine, promote the maturation and antigen presentation of DC and improve the immune response effect in vivo. According to the nano vaccine, a biodegradable material is used as a nano core to encapsulate a hydrophobic immunologic adjuvant, lipid is used as a shell to load tumor-associated antigen peptides, and apolipoprotein is modified on the lipid, so that DC is efficiently activated to phagocytize the nano vaccine by utilizing a macropinocytosis pathway. The nanoformulation vaccine is suitable for preventing or treating tumors against one or more antigens or adjuvants.
Owner:FUDAN UNIV
Preparation method of high-activity antigen-loaded dendritic cell
ActiveCN103013915AEnhance killing activityIncreased ability to process tumor antigensBlood/immune system cellsDendritic cellTumor antigen
The invention discloses a preparation method of a high-activity antigen-loaded dendritic cell. The method comprises the following steps of a, collecting and separating single karyocyte from peripheral blood; b, adding the single karyocyte obtained in the step a into DC (dendritic cell) serum-free medium, adding cytokines IL-4 and GM-CSF into the medium, putting in a 5 percent CO2 culture tank at 37 DEG C to culture, and collecting the dendritic cell after 6 days; and c, adding corresponding antigen and mycobacterium tuberculosis purified protein derivative (PPD) into the medium containing the dendritic cell collected in the step b, and keeping on culturing to obtain the high-activity antigen-loaded dendritic cell. According to the preparation method, PPD is added when tumor antigen is loaded to have an effect similar to immunologic adjuvant, so that the capability of DC in treating tumor antigen can be improved, and the killing activity of CTL (cytotoxic lymphocyte) generated by stimulation of the cells to tumor cells can be indirectly improved.
Owner:HANGZHOU CONVERD CO LTD
Japanese encephalitis (JE) vaccine composition and preparing method thereof
InactiveCN105688202AImproving immunogenicityImprove protectionSsRNA viruses positive-senseViral antigen ingredientsWater basedJapanese encephalitis
The invention provides Japanese encephalitis (JE) vaccine composition. The JE vaccine composition comprises a JE virus antigen of immunization effective quantity and water based vaccine adjuvant. The invention further provides a preparing method and application of the JE vaccine composition. The vaccine composition has good immunogenicity and immunizing protection. The immune effect of the JE vaccine composition is remarkably superior to that of single pig JE live vaccine and other live vaccine diluted by immunologic adjuvant. The clinical application prospects are good.
Owner:SICHUAN AGRI UNIV
Self-assembly composite nano granule tumor vaccine and method for preparing same
InactiveCN1927401ARealize three-dimensional attackAchieve inhibitionPowder deliveryGenetic material ingredientsCancer cellProtein molecules
The invention relates to a self-combine compound nanometer cancer vaccine, and relative production. Wherein, said vaccine is formed by cancer cell membrane, protein molecule and DNA particle; the invention builds the cancer antigen molecule and immunologic adjuvant molecule on the eucaryon expression carrier, to anchor and express on the surface of cancer cell; then mixing the cells, to extract cell membrane, and decompose the cell membrane into the membrane liposome with anchored protein molecule, to be mixed with the DNA particles of cancer antigen molecule, immunity adjusting molecule, in certain buffer condition, to form the nanometer cancer vaccine. The invention can be used to treat several carcinomas, contagion, and non-contagion.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Horse anti-EBOV (Ebola virus) immune globulin F (ab')2 and preparation method thereof
InactiveCN105254755AImprove survival rateStable and efficient sourceSerum immunoglobulinsImmunoglobulins against virusesFreeze-dryingIon exchange
The invention provides horse anti-EBOV (Ebola virus) immune globulin F (ab')2 and a preparation method thereof. The preparation method comprises steps as follows: virus-like particles of a GP gene and a VP40 gene expressing EBOV-Z simultaneously are inactivated and purified and then are mixed with an immunologic adjuvant to be taken as immunogens, highly immunized plasma is obtained through repeated immunization of healthy horses, IgG protein is separated and purified, then the IgG protein is subjected to pepsase digestion, an enzyme-digested product is processed by a DEAE ion-exchange chromatographic column, an eluent is collected and subjected to desalination and freeze-drying, and the horse anti-EBOV immune globulin F (ab')2 is obtained. The horse anti-EBOV immune globulin F (ab')2 is an effective drug for preventing and treating Ebola hemorrhagic fever caused by EBOV and has remarkable curative effects for critical patients infected with the EBOV; the preparation method is controlled by adopting unique technological parameters and is suitable for industrial production, and the product is safe and reliable.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Application of streptococcus pneumoniae protein to resisting infection of S. pneumoniae
ActiveCN109456393AIncrease infectionReduced Colonization Protection ExperimentAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaStreptococcus mitis
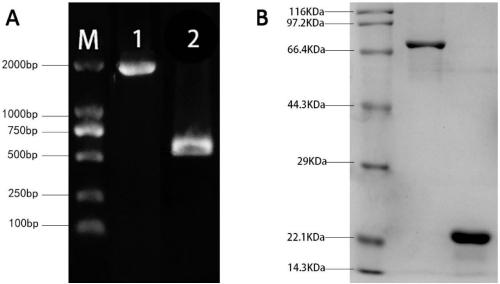
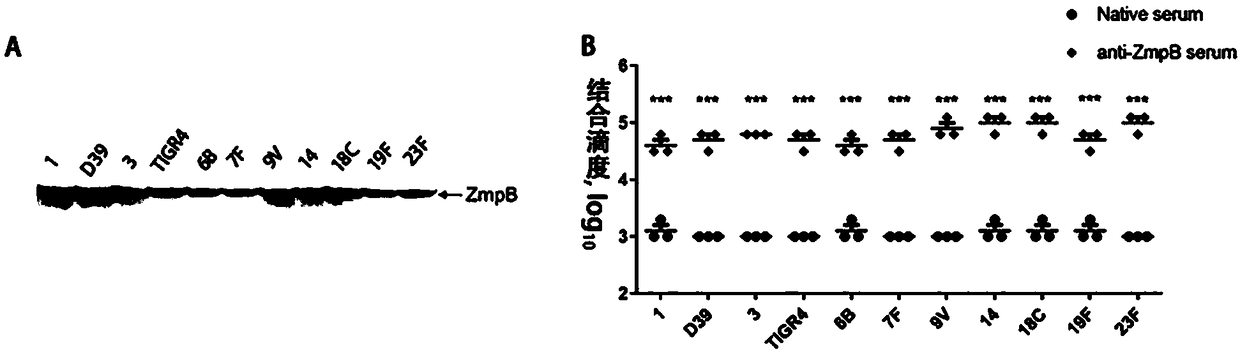
The invention provides application of S. pneumoniae protein to resisting infection of S. pneumoniae. The endopeptidase O (PepO) of S. pneumoniae is a subcutaneous immunologic adjuvant, and the prepared S. pneumoniae protein vaccines have the good protection effects on resisting infection of S. pneumoniae through mixing and fusing expression of the subcutaneous immunologic adjuvant and 673rd to 863rd amino acid peptide fragment of zinc metal protease B (ZmpB).
Owner:CHONGQING MEDICAL UNIVERSITY
Exosome coupled with coronavirus S protein on surface and preparation method for exosome and application of exosome
InactiveCN111647557AImprove securityAvoid the risk of infectionSsRNA viruses positive-senseViral antigen ingredientsCD63Receptor
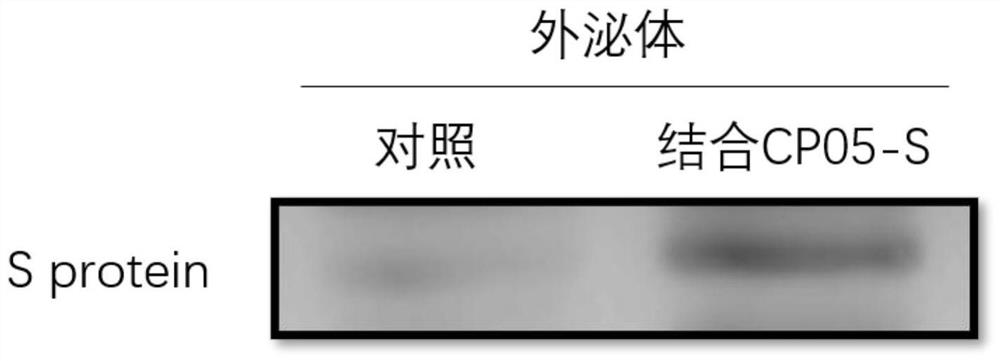
The invention provides an exosome coupled with coronavirus S protein on the surface and a preparation method for the exosome and application of the exosome, and belongs to the technical field of biological pharmacy. A stable cell line is obtained through transient transfection of human cells or infection with a recombinant lentivirus to achieve recombinant expression of aptamer CP05-S protein, andthe aptamer CP05-S protein is mixed with human plasma for incubation, and the exosome is bound to an aptamer CP05 through surface CD63 membrane molecules to form the exosome coupled with the S protein on the surface. The exosome provided by the invention is employed to present antigen S protein, so that a prepared vaccine avoids the toxicity of an immunologic adjuvant and the risk of virus vaccine infection, and loading the S protein to the surface of the exosome to prepare the vaccine is an ideal vaccine development strategy, and is also an ideal strategy for blocking a virus invasion route;and meanwhile, the S protein is delivered through the exosome, and thus, competitive inhibition can be performed on binding of the S protein of the novel coronavirus to a receptor, the blocking action is exerted, and invasion of the novel coronavirus is effectively blocked, and accordingly, as an inhibitor for novel coronavirus infection, the exosome coupled with the S protein can be used for treatment of acute novel coronavirus pneumonia.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com