Transdermal immune influenza multivalent vaccine and preparation method thereof
A multivalent vaccine and influenza technology, applied in the field of vaccines, can solve problems such as pain, reduce side effects, avoid cross-infection, and simplify the process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Chicken embryo culture of influenza virus adopts traditional culture method. Type A influenza virus strains include (subtypes such as H1, H3, H5) and type B influenza virus strains are provided by China CDC and Hualan Biotechnology Co., Ltd.
[0043] For influenza virus culture, inoculate the allantoic cavity of 9-11-day-old SPF chicken embryos, culture at 33-35°C for 48-72h (the culture time varies slightly depending on the virus subtype), freeze the chicken embryos at 4°C overnight, and harvest the chicken embryos The allantoic fluid acquires a large amount of virus.
[0044] Mammalian cells (such as Vero cells, MDCK cells, diploid cells, etc.) culture influenza virus, type A influenza virus strains include (H1, H3, H5 and other subtypes) and type B influenza virus strains are produced by China CDC and Hualan Bio Provided by Technologies Inc.
[0045] Use a microcarrier system (such as Cytodex 1, 3, etc.) to culture in a 100ml WAVE reactor, gradually transition to a...
Embodiment 2
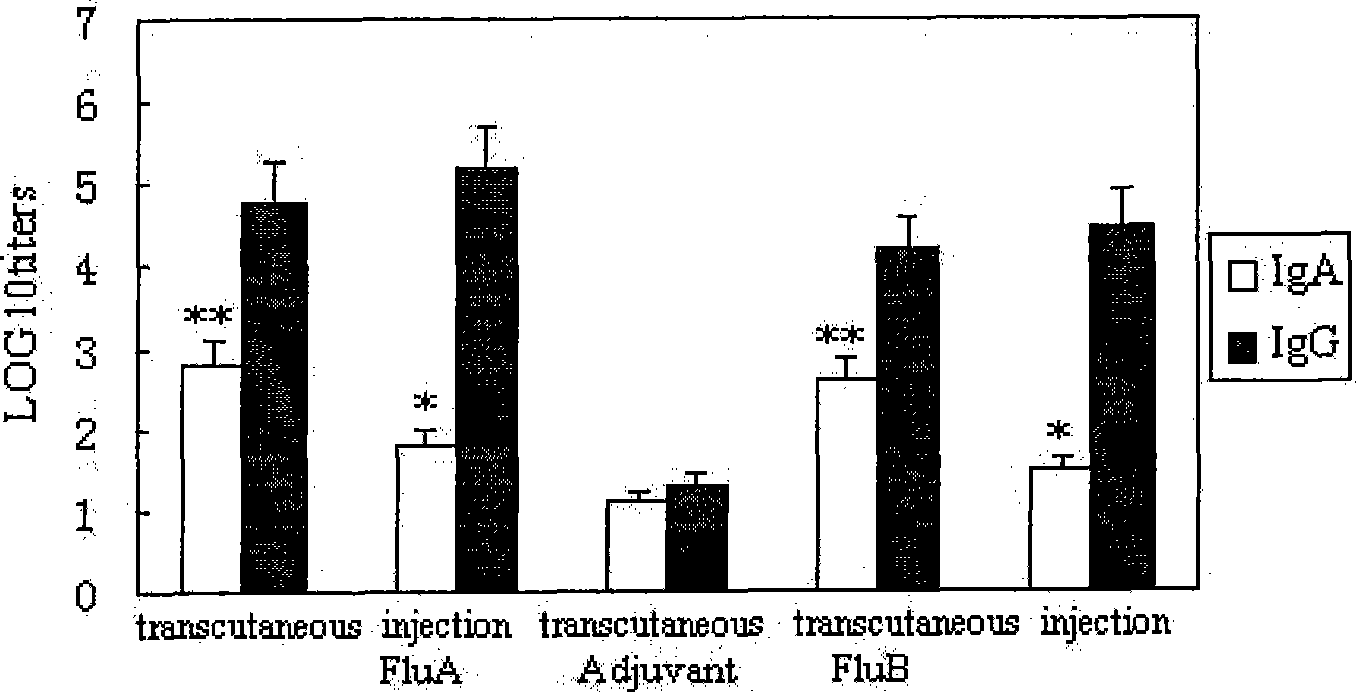
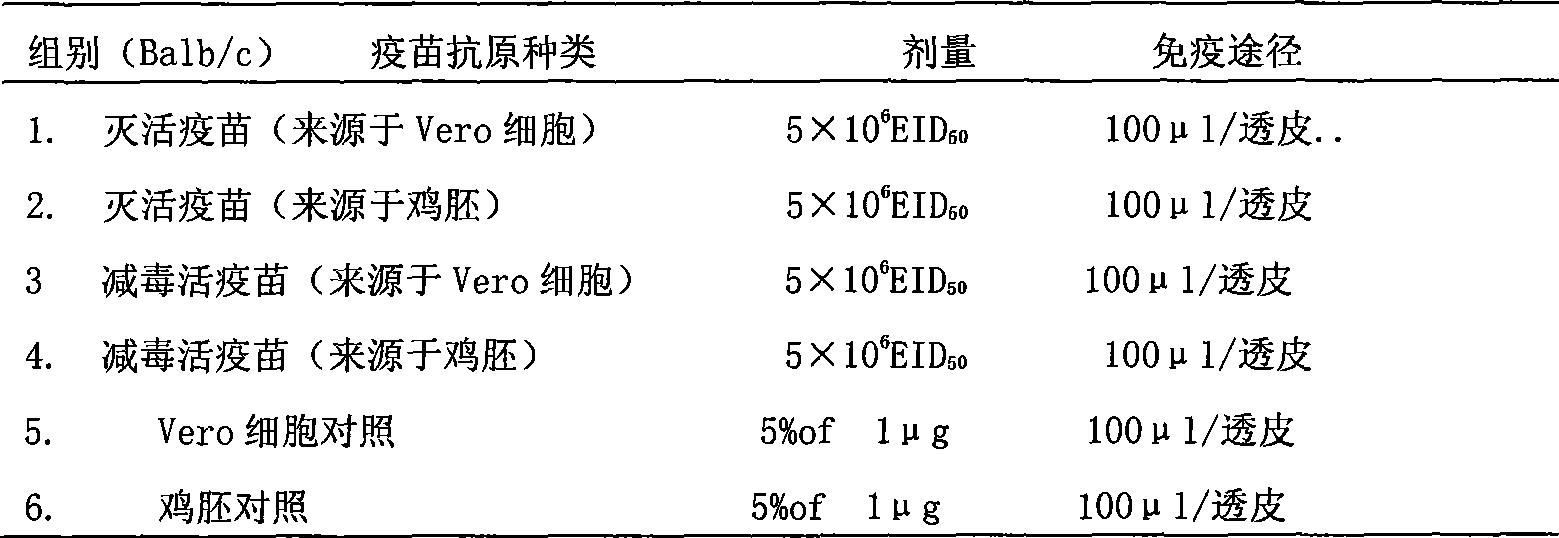
[0074] Example 2: In vivo effect observation of influenza multivalent inactivated / attenuated live vaccine in mice
[0075] 1. Immunization plan, use different dosage forms (transdermal, injection immunization as control) influenza virus vaccine to immunize mice
[0076]① Shave 6-8 week old Balb / c mice with 40# scissors. The shaving process should not cause any damage to the skin. The shaving ranges from the middle of the chest to close to the lower part of the strong back. Let the mice rest for 24h. Mice had previously been tagged for identification and pre-bleeded to obtain pre-immune sera.
[0077] ②Then immunize the mice as follows. Mice were anesthetized with 0.03-0.06 ml of 20 mg / ml xylazine solution and 0.5 ml of 100 mg / ml ketamine; this dose of anesthesia made the mice immobile for about 1 h.
[0078] ③Add the immune solution to the skin of the mouse's hair-free back in the following way: gently place a 1.2cm×1.6cm hollow polystyrene plate on the back of the mouse,...
Embodiment 3
[0096] Example 3: In vivo effect observation of multivalent influenza inactivated / attenuated live vaccine in ferrets
[0097] 1. Immunization plan, using different dosage forms of influenza virus vaccine to immunize ferrets
[0098] ① According to the method of preparing the adjuvant / antigen mixture in Example 1, prepare a dosage form suitable for transdermal immunization of ferrets. The solution can be appropriately diluted with PBS for LT.
[0099] ② Male and female ferrets aged 6-10 weeks, with a body weight of 1-2 kg, were selected for this experiment, and all these animals were seronegative before the experiment. 14 days after immunization, blood was drawn from the jugular vein of ferrets to determine the HI titer.
[0100] ③ 6 groups of ferrets received transdermal immunization with vaccines of different dosage forms, each with 1ml of antigen solution containing adjuvants such as LT, CT or CpG, and the patch was used on the back of the ear or outside the upper limbs, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com