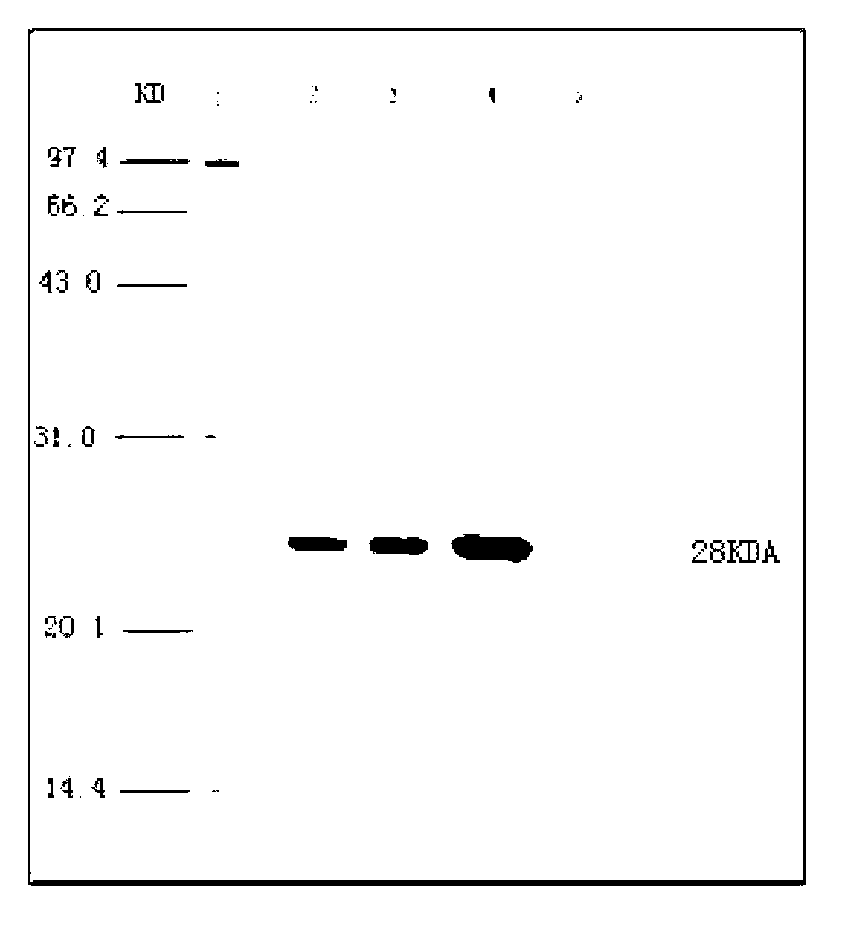
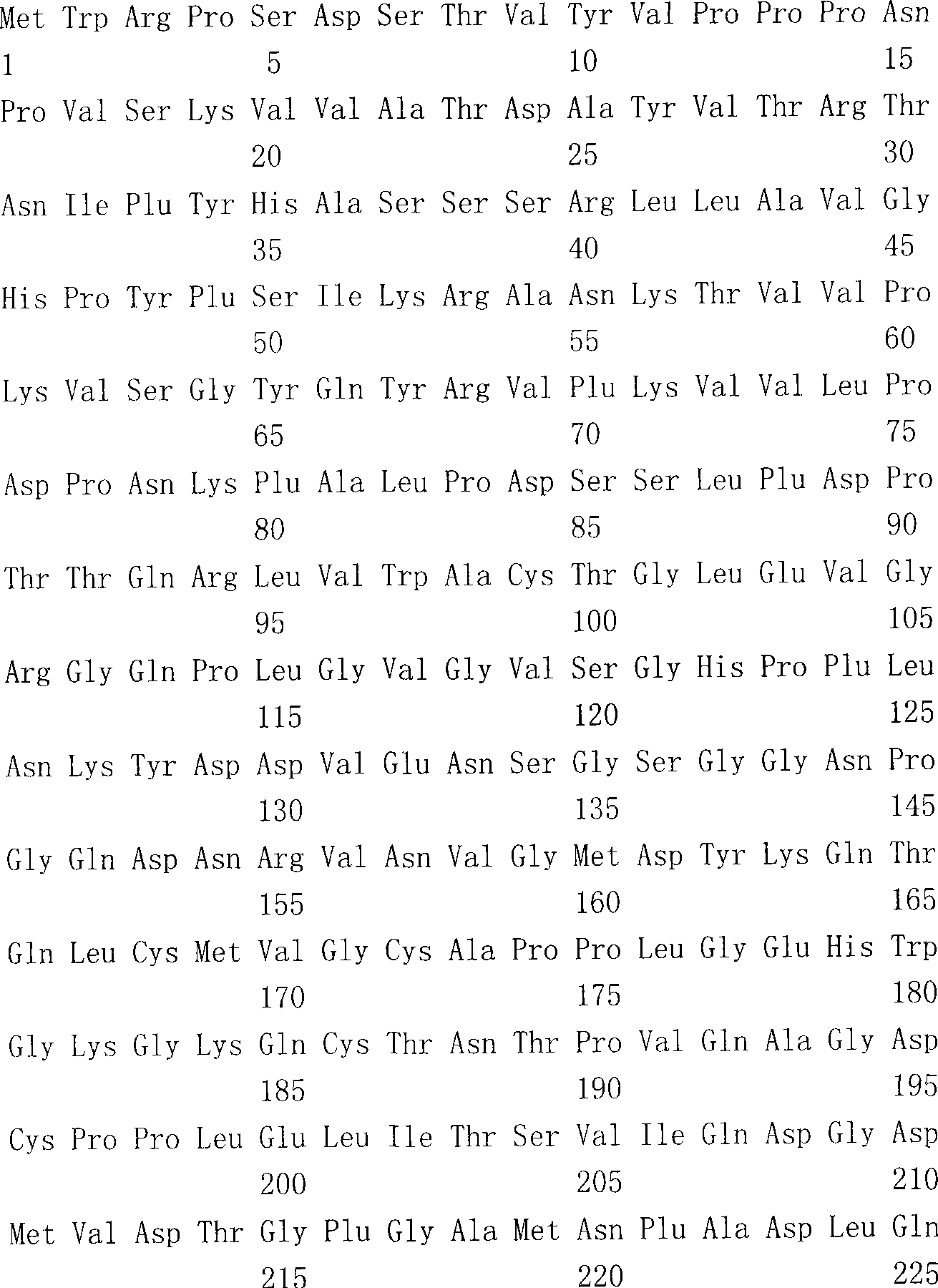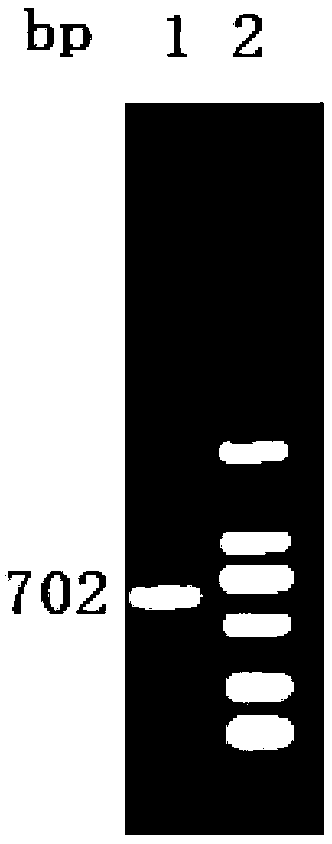Patents
Literature
98 results about "Polyvalent Vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A vaccine designed to elicit an immune response either to more than one infectious agent or to several different antigenic determinants of a single agent.
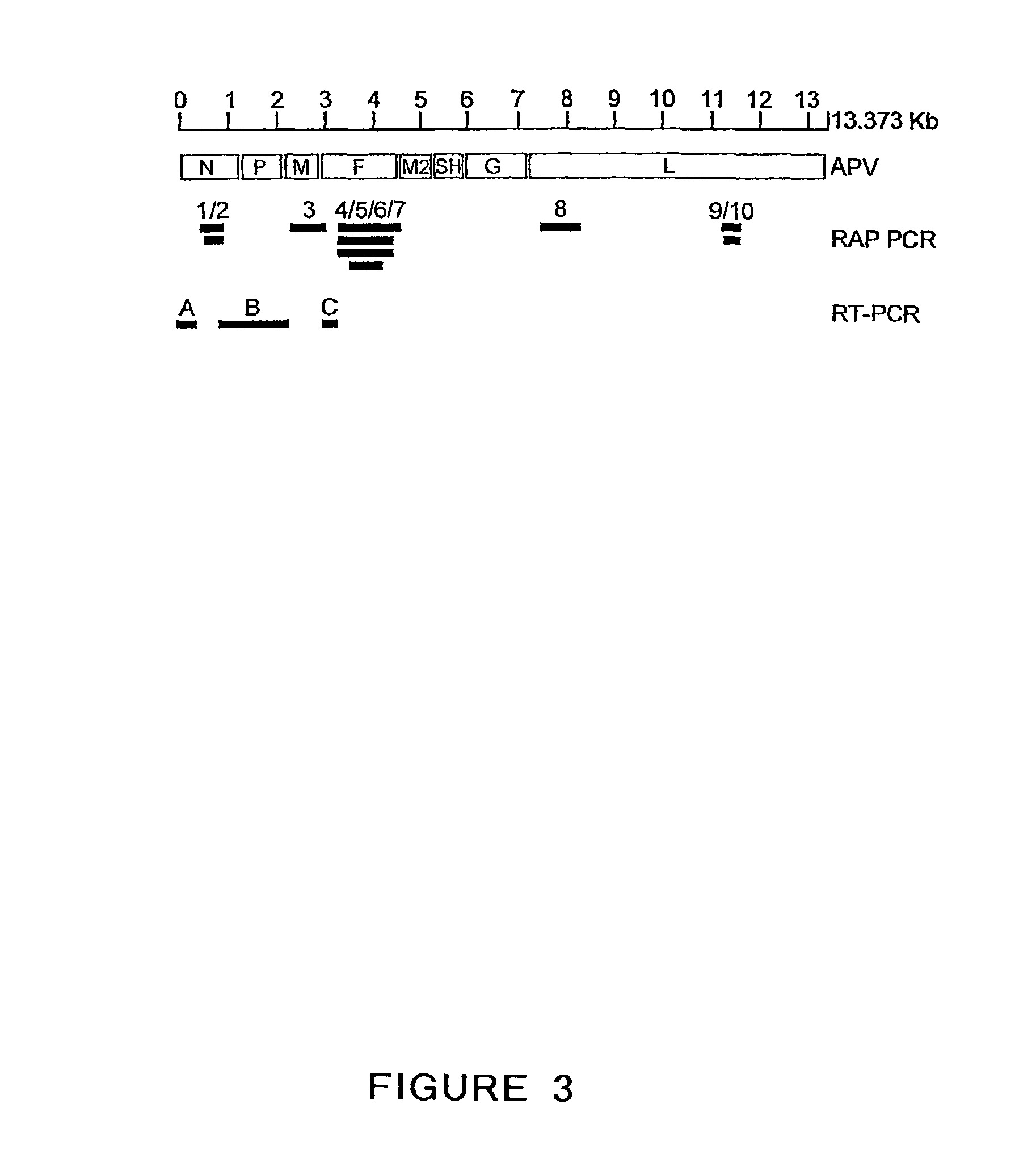
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
ActiveUS20040005544A1Narrow downSymptoms improvedOrganic active ingredientsFungiHeterologousNegative strand
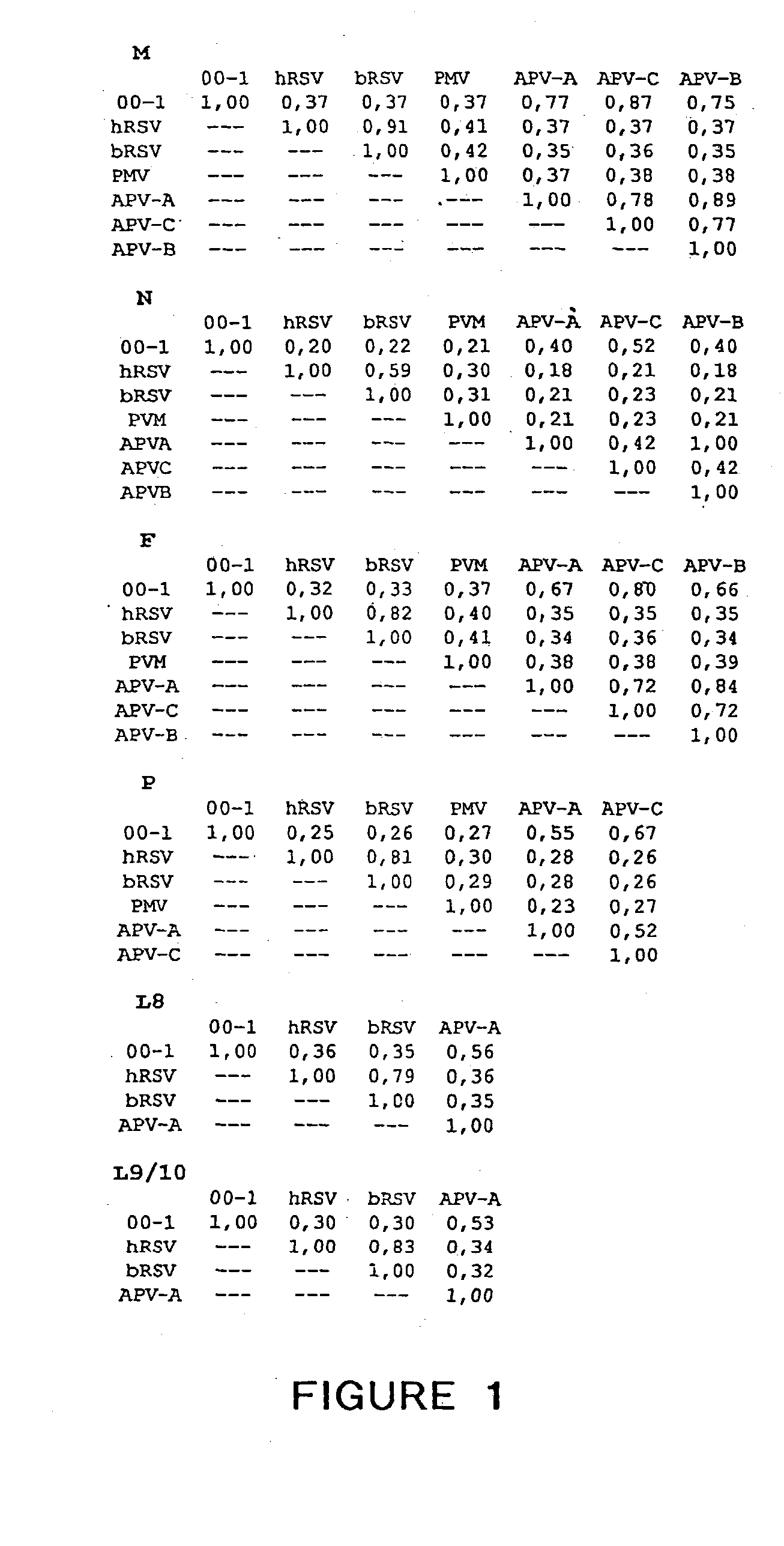
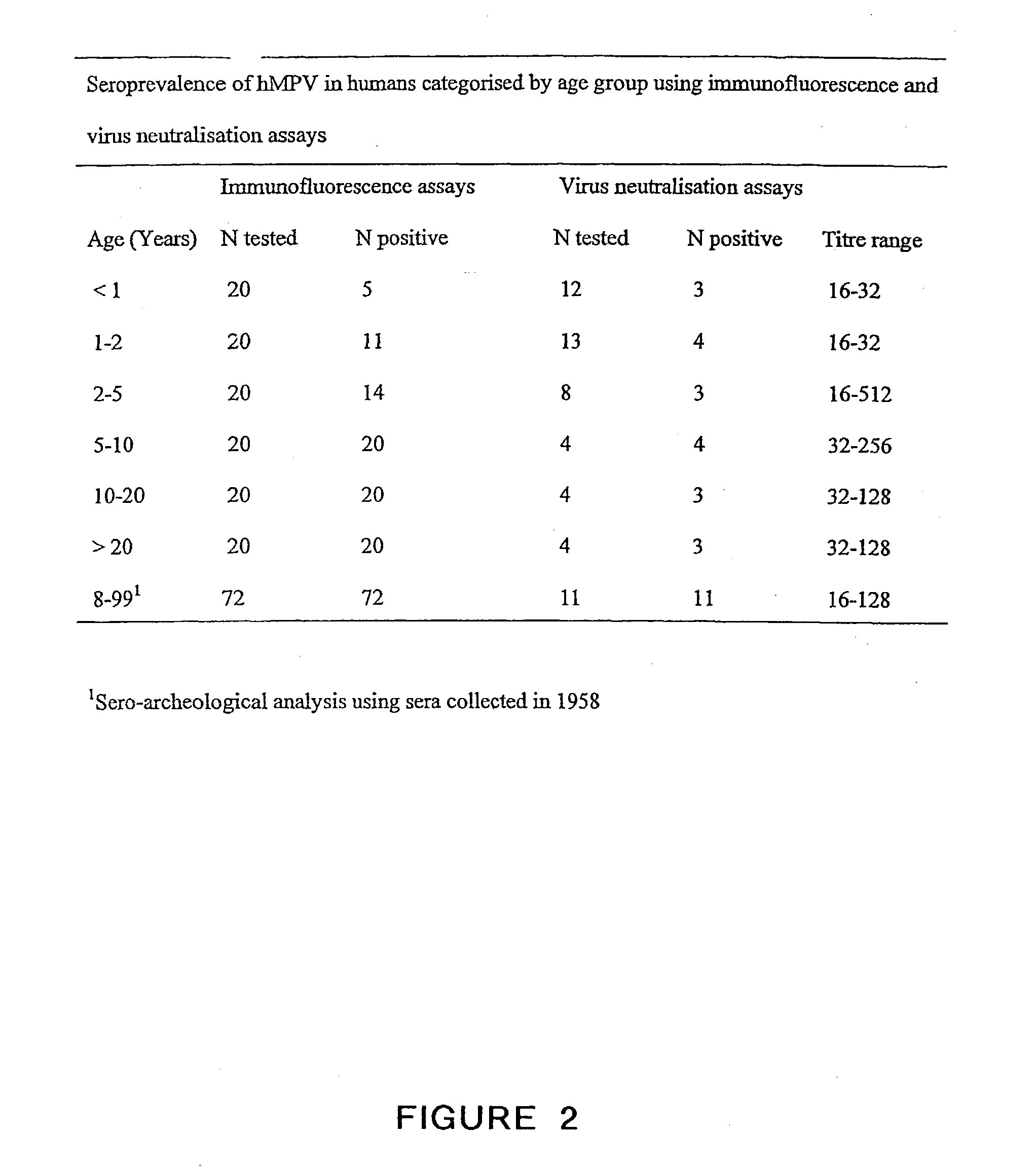
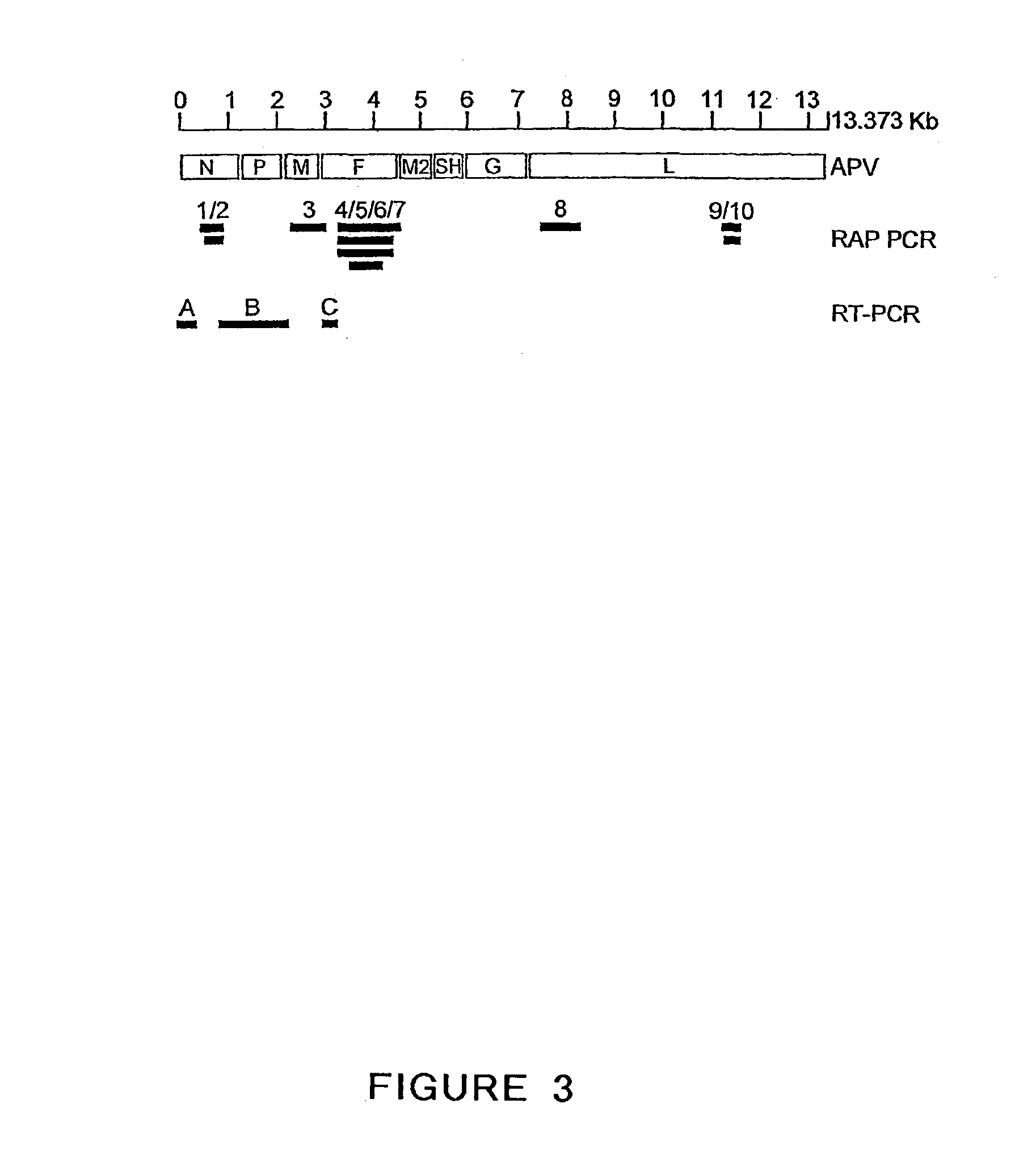
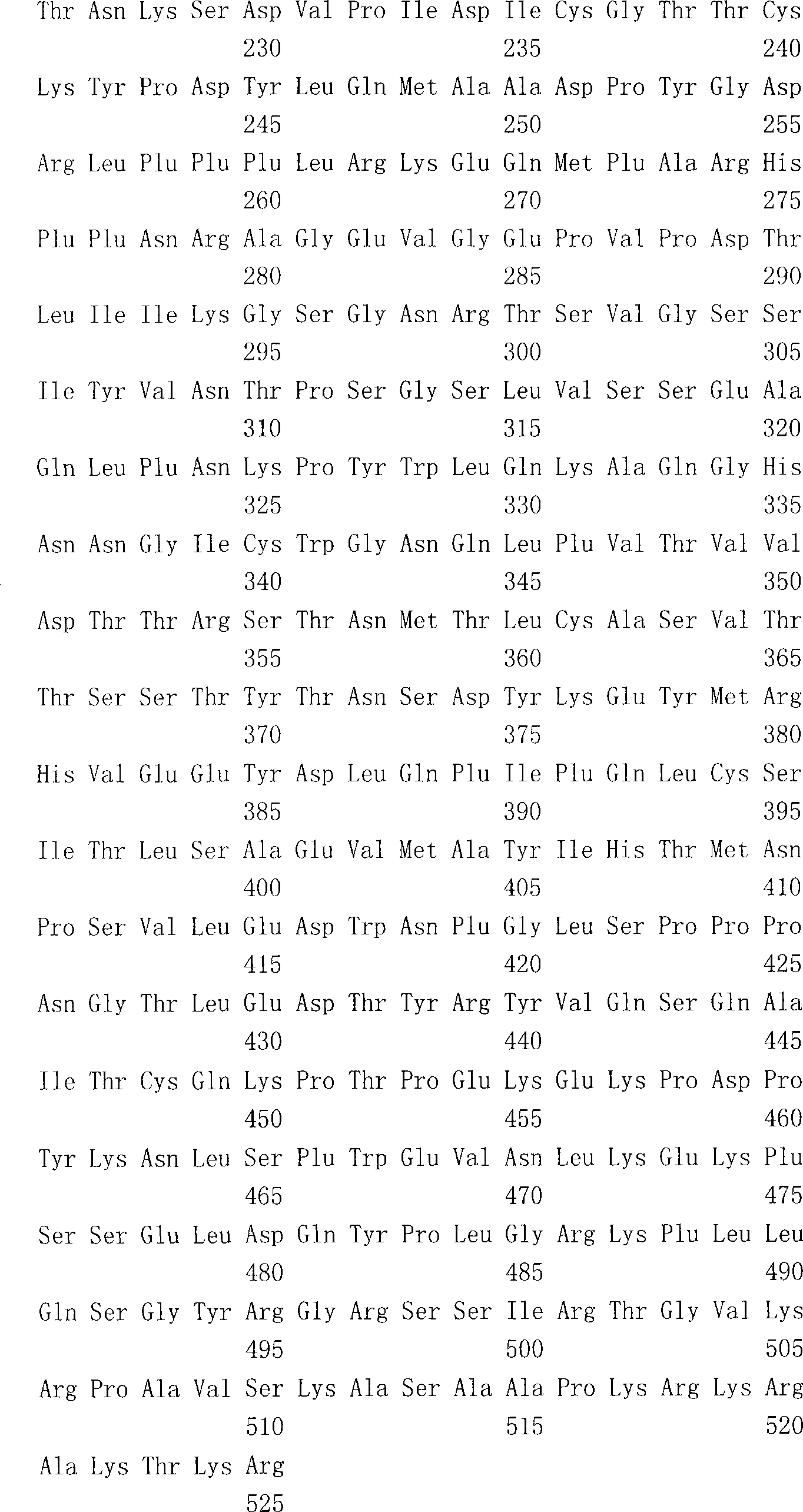
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Human papilloma virus preventative vaccine, construction method and application
InactiveCN101518647AAvoid conversionAchieve broad-spectrum immune effectPeptide/protein ingredientsViral antigen ingredientsPolyvalent VaccineHuman papilloma virus
The invention provides a construction method of a human papilloma virus resistant HPV polyvalent vaccine and application thereof.
Owner:江阴艾托金生物技术有限公司
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae , of the family Paramyxoviridae . The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
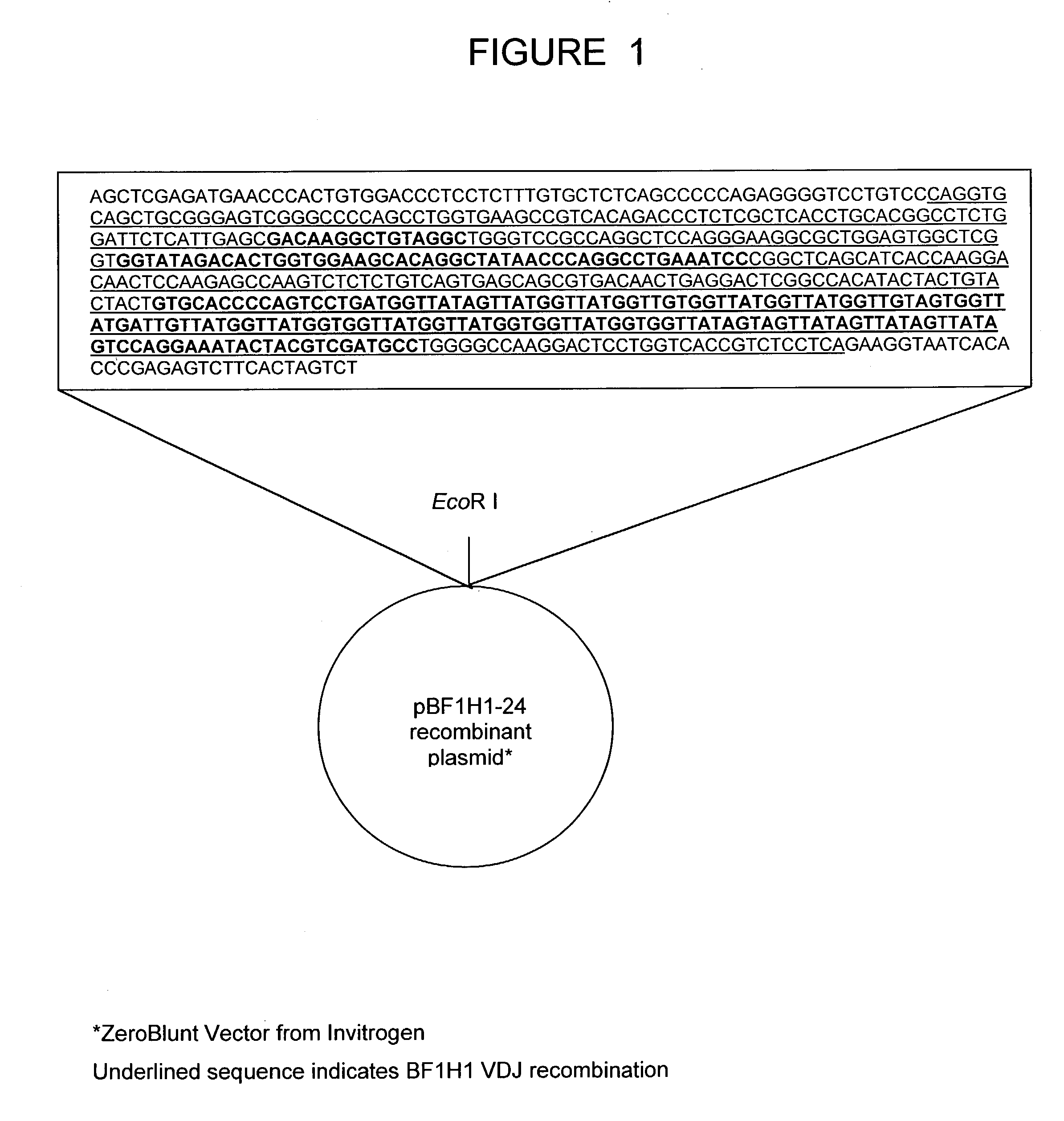
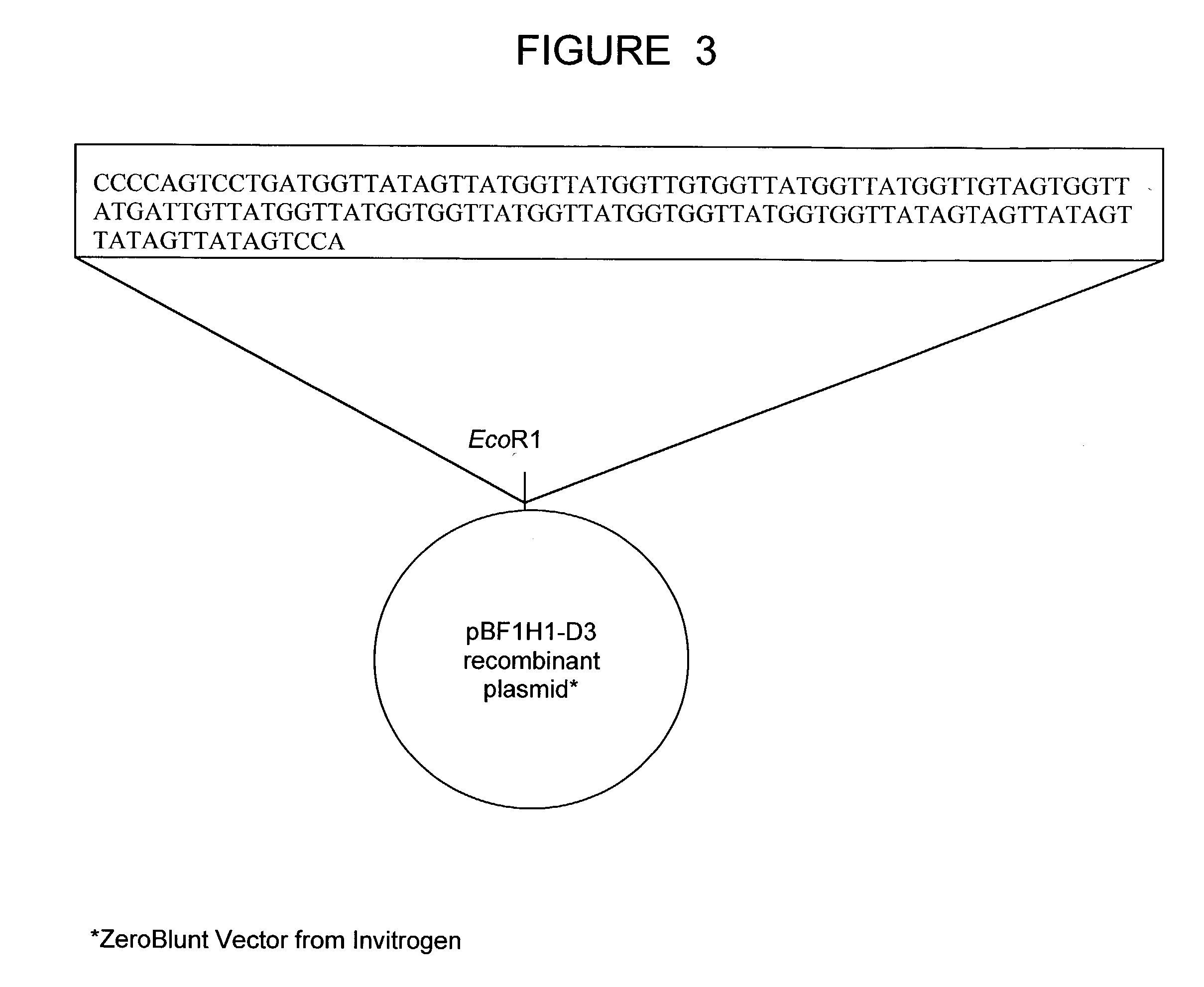
Bovine germline D-genes and their application
ActiveUS7196185B2Large capacitySugar derivativesMicrobiological testing/measurementDiseaseImmunocompetence
The present invention relates to a bovine VDJ cassette (BF1H1) that provides the novel ability to develop chimeric immunoglobulin molecule capable of incorporating both linear T cell epitope(s) (CDR1H and CDR2H) as well as conformational B cell epitope(s) (exceptionally long CDR3H). Further, multiple epitopes can be incorporated for development of multivalent vaccine by replacing at least a portion of an immunoglobulin molecule with the desired epitope such that functional ability of both epitope(s) and parent VDJ rearrangement is retained. The antigenized immunoglobulin incorporating both T and B epitopes of interest is especially useful for development of oral vaccines for use in humans apart from other species including cattle. The long CDR3H in BF1H1 VDJ rearrangement originates from long germline D-genes. The novel bovine germline D-genes provide unique molecular genetic marker for sustaining the D-gene pool in cattle essential for immunocompetence via selective breeding. D-gene specific DNA probe permits typing and selection of breeding cattle stock for maximum gemline D gene pool for better health and disease prevention. The bovine D-genes are unique to cattle and, therefore, provide sensitive and specific forensic analytical tool using molecular biology techniques to determine tissues suspected of bovine origin.
Owner:KAUSHIK AZAD KUMAR +2
Preparation method and application of multiple target complex antigen-loaded CD8<+> cytotoxic T lymphocyte
ActiveCN104926944AImprove the ability to secrete IFN-γBlood/immune system cellsAntibody medical ingredientsEpitopePolyvalent Vaccine
The invention provides a multiple target complex antigen, namely a combined cytotoxic T lymphocyte (CTL) antigen epitope peptide, a polyvalent vaccine with multiple target positions for a tumor cell surface, a multiple target complex antigen-loaded CD8<+> CTL, a preparation method of the CTL, and an application of the lymphocyte to preparation of a drug for treating a cancer. The invention also provides an epitope-induced special CTL effect cell.
Owner:BEIJING BIOHEALTHCARE BIOTECH
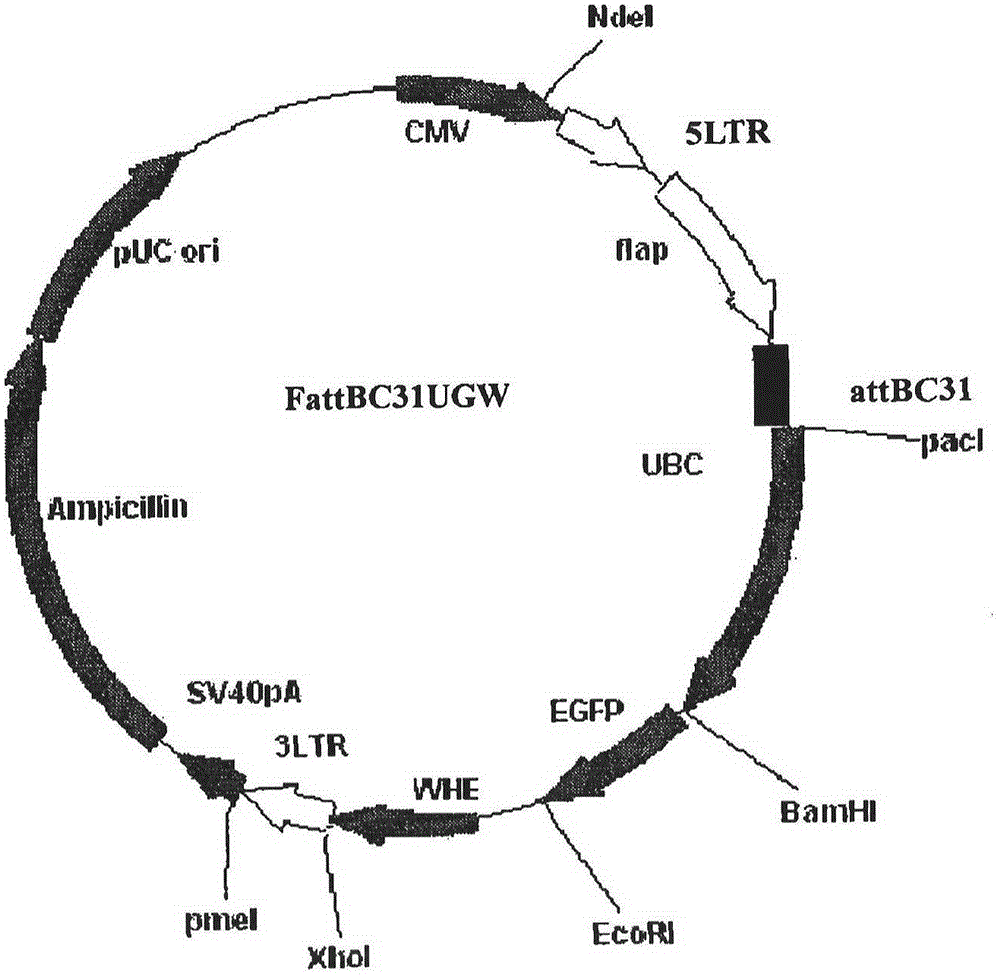
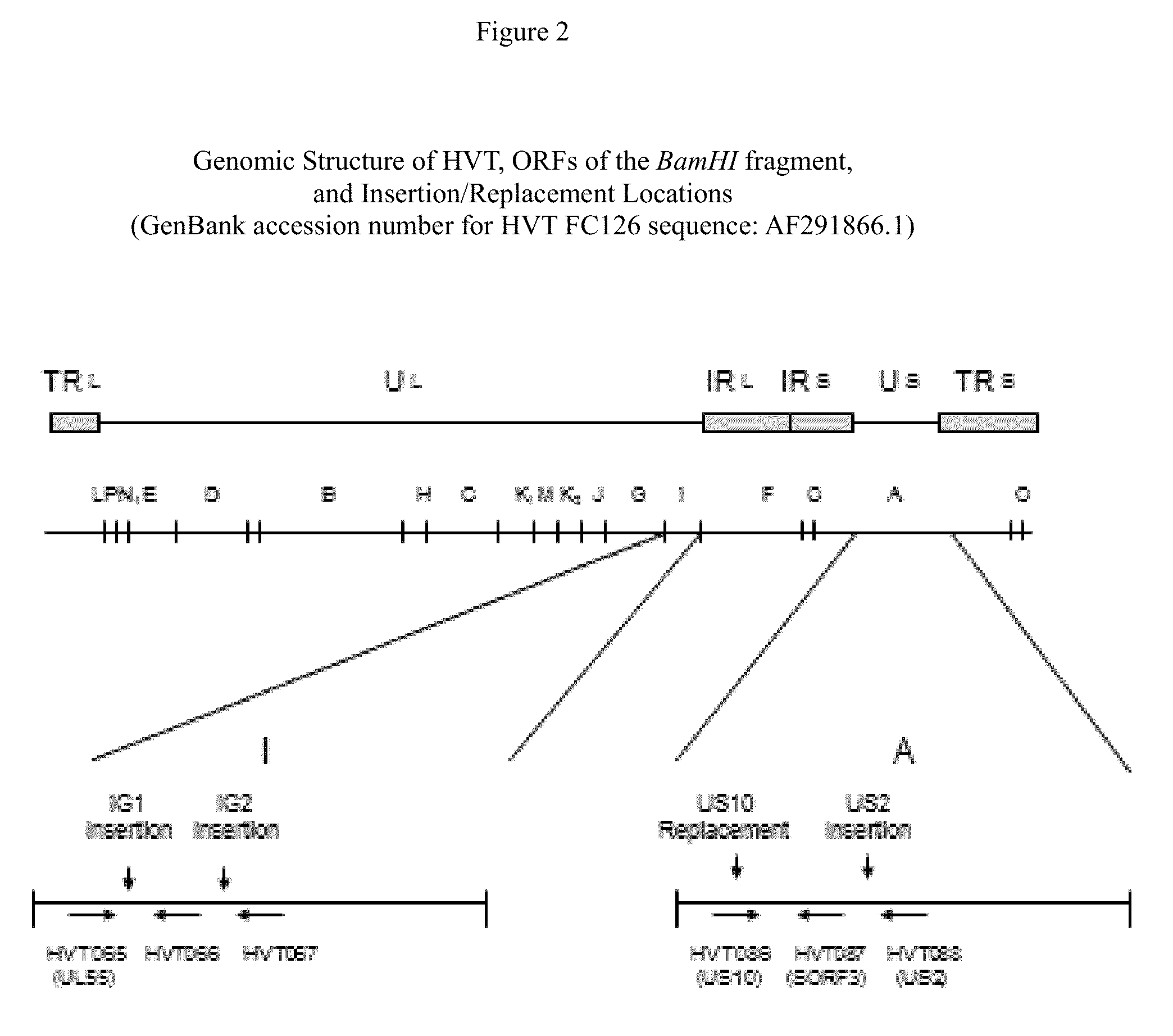
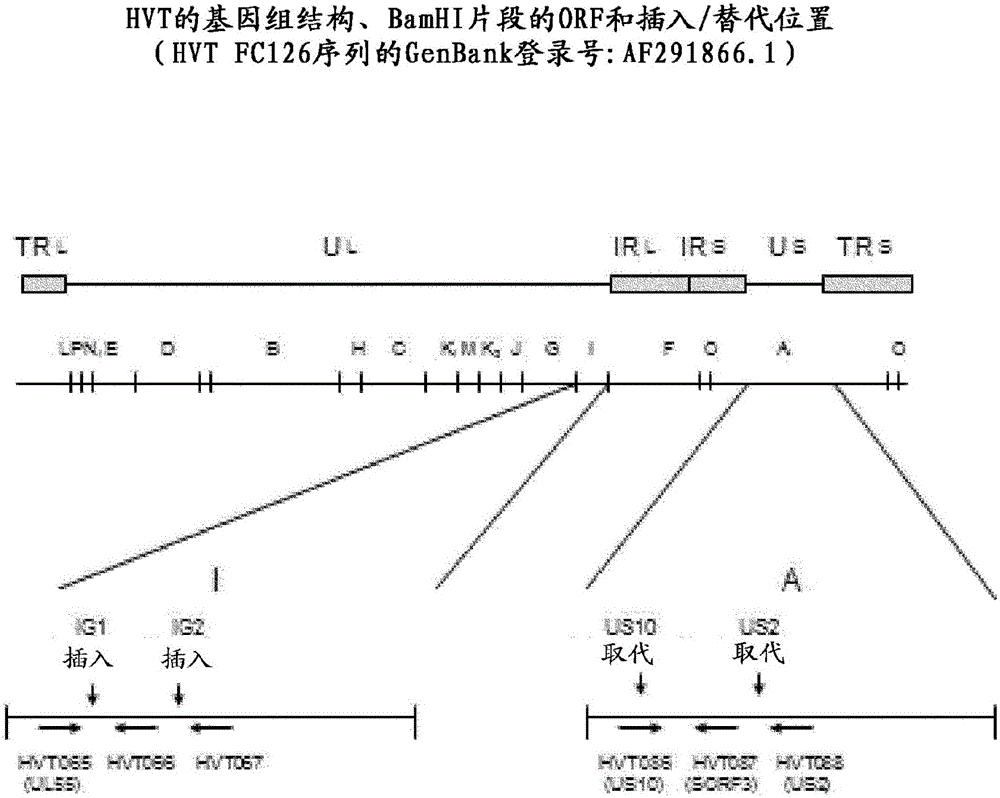
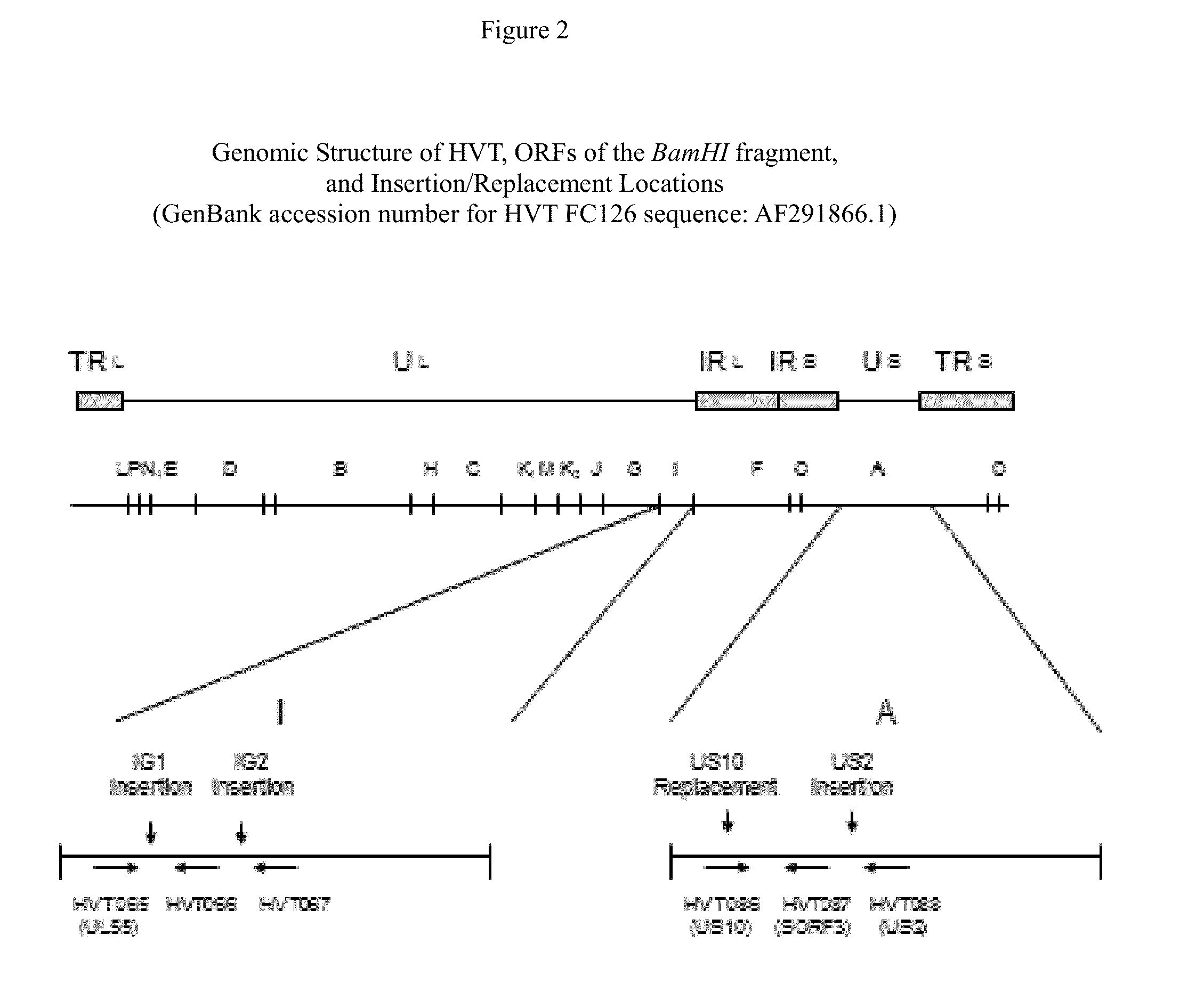
Recombinant HVT vectors expressing antigens of avian pathogens and uses thereof
ActiveUS9114108B2Effective protectionSsRNA viruses negative-senseViral antigen ingredientsAntigenFowl
The present invention provides recombinant herpesvirus of turkeys (HVT) vectors that contain and express antigens of avian pathogens, compositions comprising the recombinant HVT vectors, polyvalent vaccines comprising the recombinant HVT vectors and one or more wild type viruses or recombinant vectors. The present invention further provides methods of vaccination against a variety of avian pathogens and method of producing the recombinant HVT vectors.
Owner:MERIAL INC
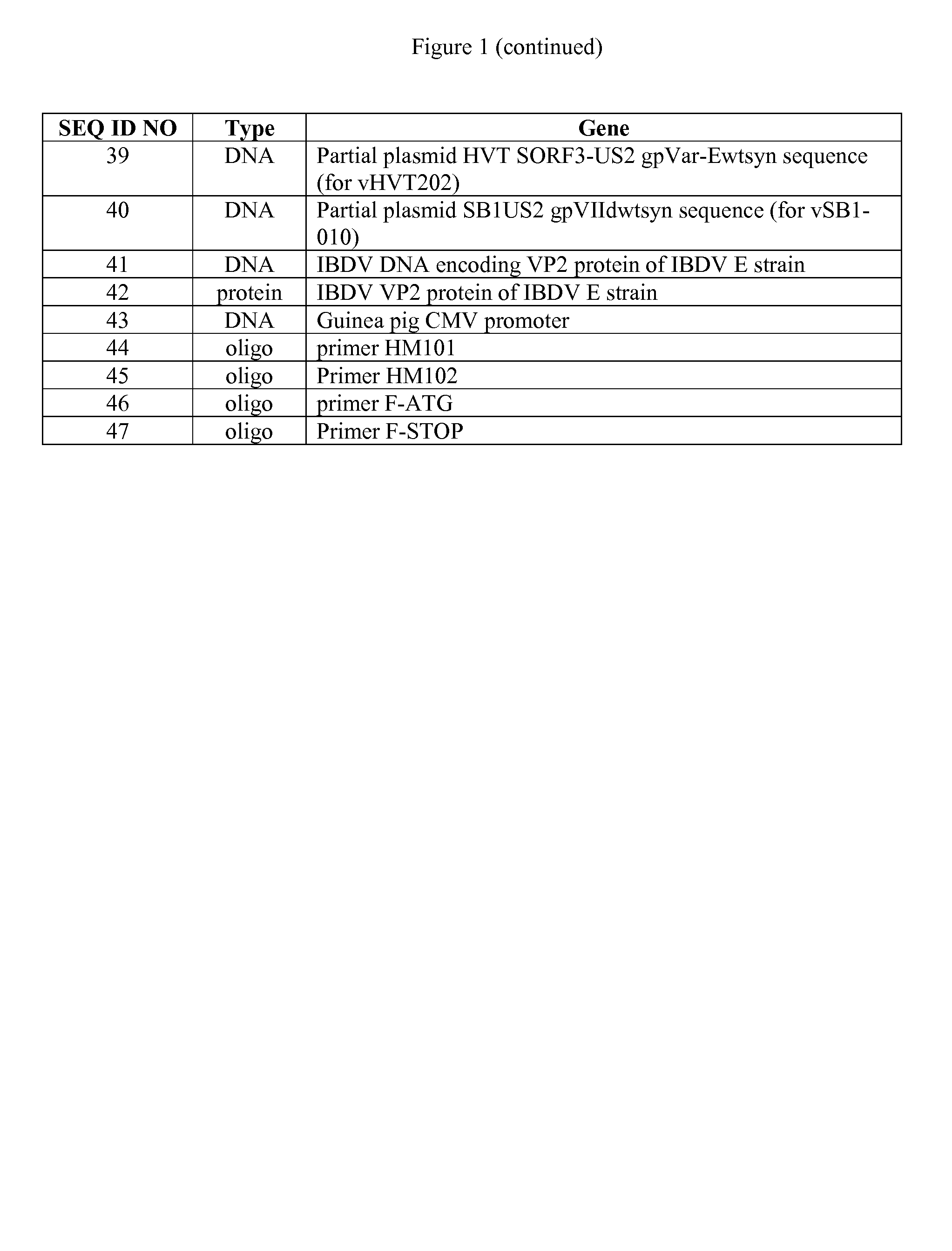
Polyvalent inactivity vaccine for preventing and treating atrophic rhinitis of swine
ActiveCN102302771AEffective therapeuticEffective preventionAntibacterial agentsBacterial antigen ingredientsPasteurella multocida toxinImmune effects
The invention provides a polyvalent inactivity vaccine for preventing and treating atrophic rhinitis of a swine and a preparation method thereof. The polyvalent inactivity vaccine contains inactivated Bordetella bronchiseptica, Pasteurella multocida A, Pasteurella multocida D and PMT (Pasteurella Multocida Toxin) anatoxin. The invention further provides a novel method for culturing and extractingPMT. Compared with the traditional atrophic rhinitis of the swine, the polyvalent inactivity vaccine for the atrophic rhinitis of the swine, provided by the invention, can be used for more generally and effectively treating and preventing the atrophic rhinitis of the swine by comprehensive antigen protection. Finally, in the polyvalent vaccine provided by the invention, the vaccine with a plurality of antigens in a reasonable proportion can be used for solving the problem that the plurality of the antigens interfere each other, thereby improving an immune effect. Furthermore, the inventor provides a water adjuvant by which defects such as incomplete absorption, large side reaction and the like after the traditional alumina gel adjuvant, Freund adjuvant and water-in-oil adjuvant are injected into the water adjuvant can be overcome.
Owner:PU LIKE BIO ENG
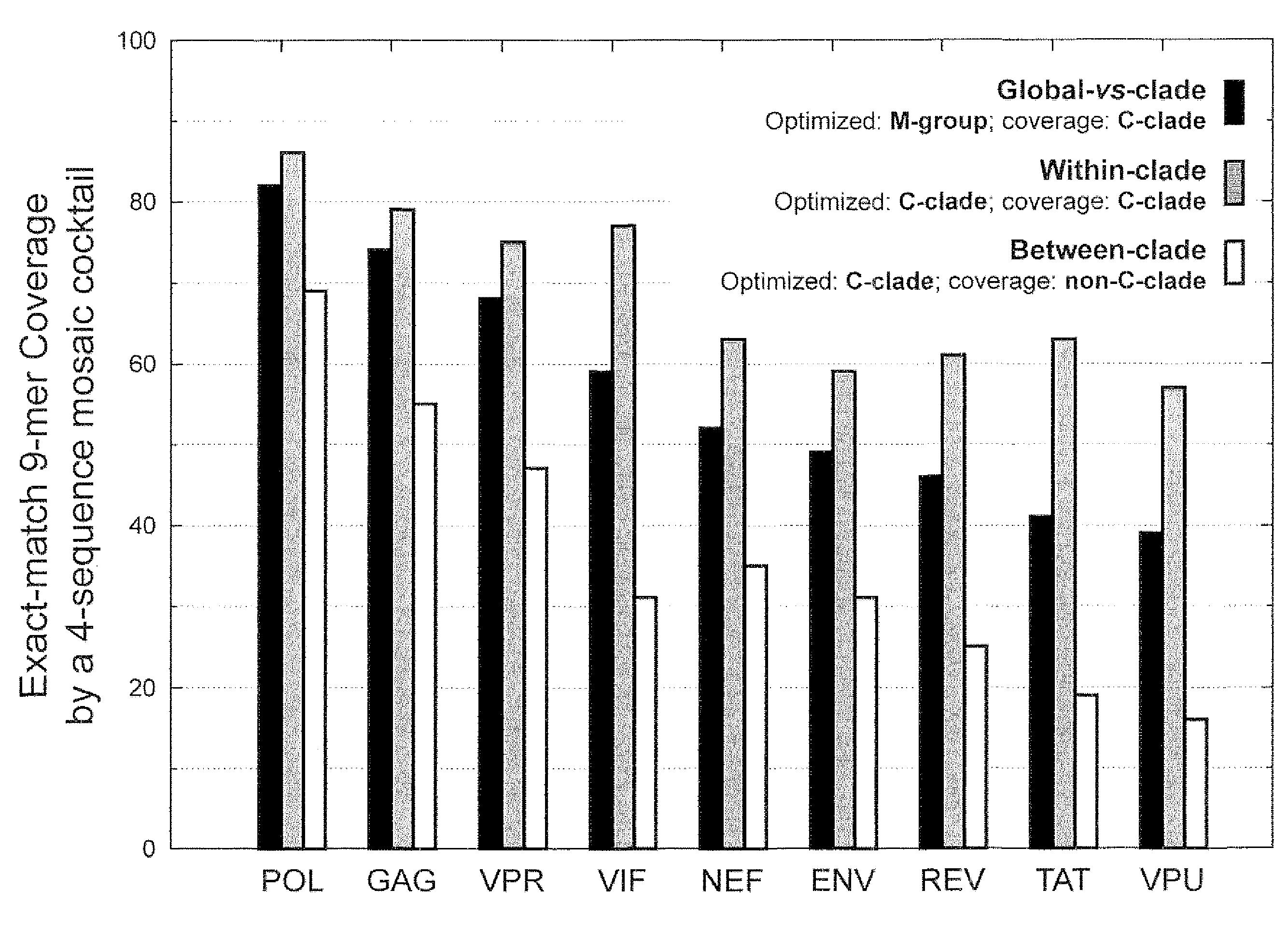
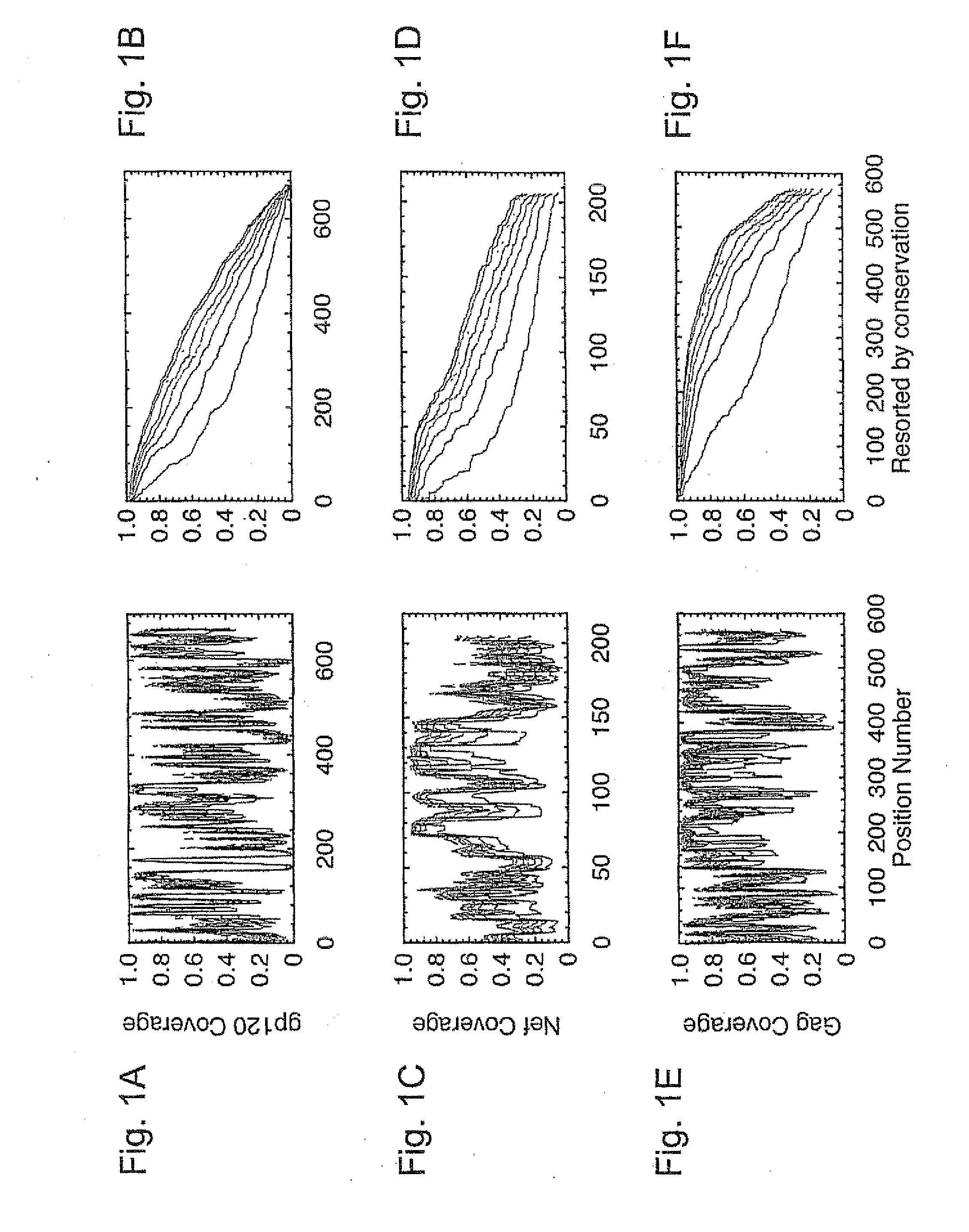
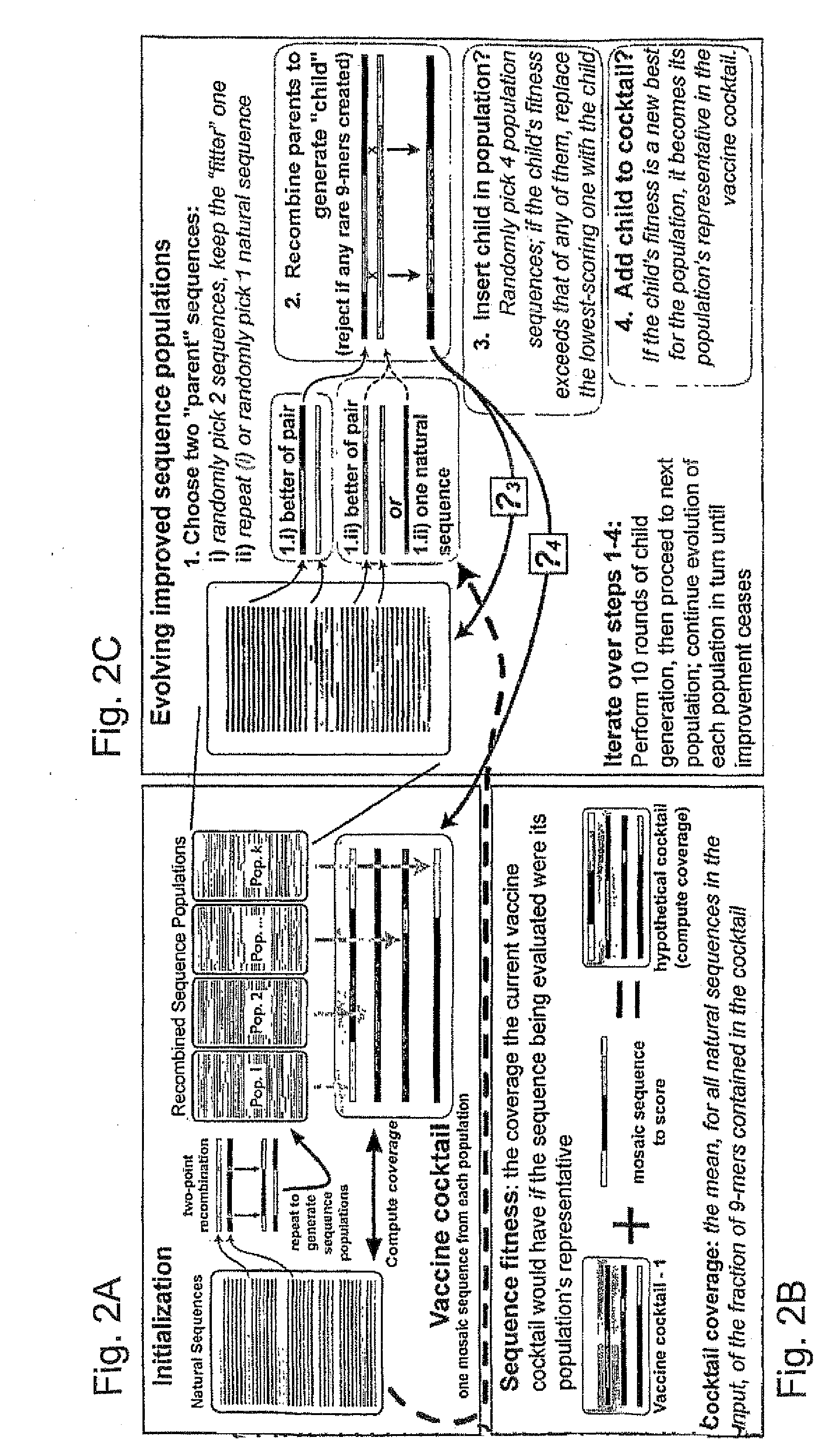
Polyvalent Vaccine
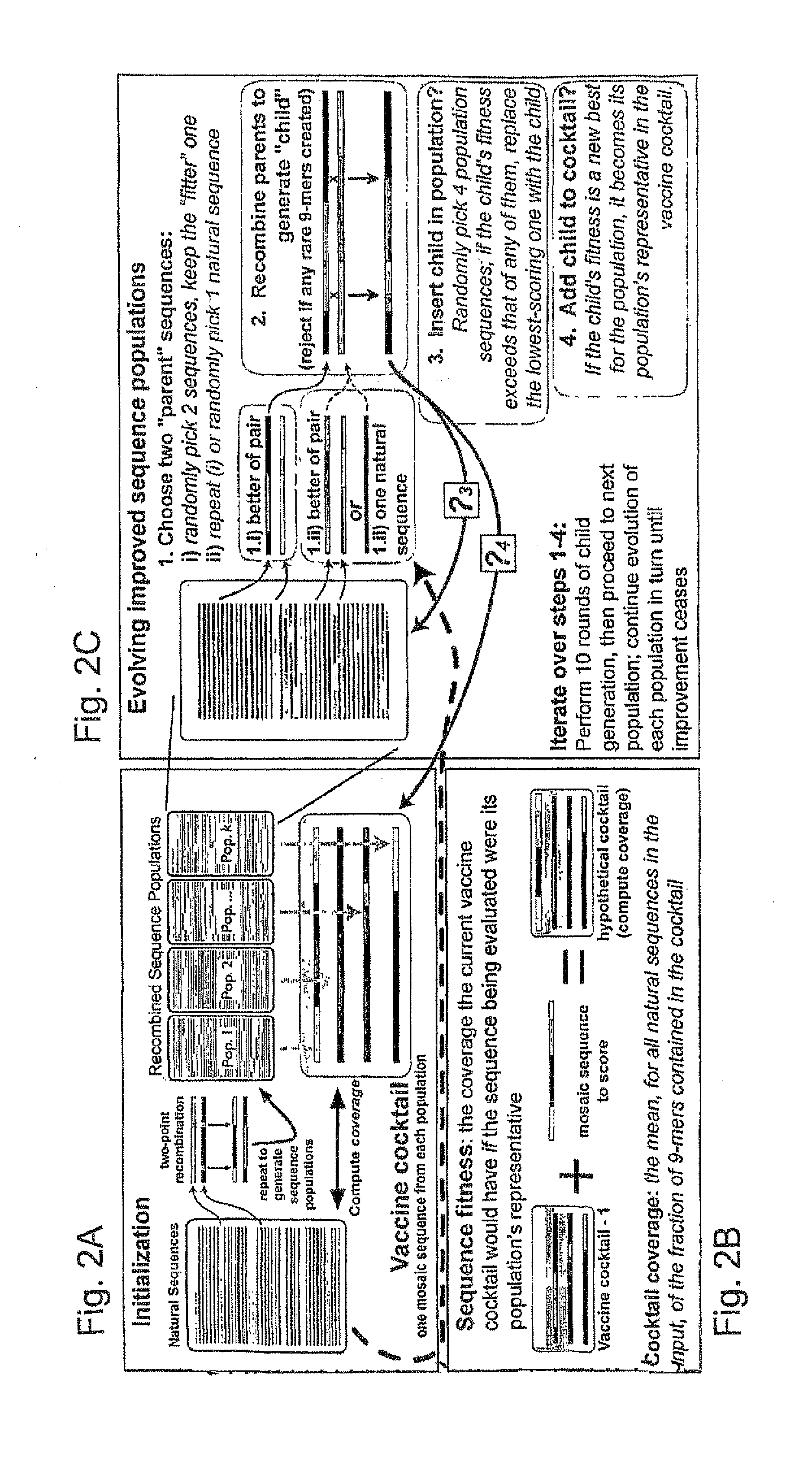
ActiveUS20090324631A1Organic active ingredientsPeptide/protein ingredientsVaccinationGenetic algorithm
The present invention relates, in general, to an immunogenic composition (e.g., a vaccine) and, in particular, to a polyvalent immunogenic composition, such as a polyvalent HIV vaccine, and to methods of using same. The invention further relates to methods that use a genetic algorithm to create sets of polyvalent antigens suitable for use, for example, in vaccination strategies.
Owner:DUKE UNIV +3
Vaccine composition containing porcine circovirus type 2 antigen and haemophilus parasuis antigen, as well as preparation method and application thereof
ActiveCN102988978AChange cognitive biasImprove immunityAntibacterial agentsBacteriaAntigenHaemophilus
The invention relates to a polyvalent vaccine composition for porcine, and in particular relates to a vaccine composition capable of resisting infection of porcine circovirus type 2 (PCV 2) and haemophilus parasuis (HPS) at the same time. The vaccine composition comprises at least one PCV2 antigen, at least one HPS, as well as vector, an excipient and an adjuvant available in the field of veterinary medicine. The vaccine composition can be used for preventing and treating PCV2 related diseases and HPS diseases.
Owner:PU LIKE BIO ENG
Recombinant gallid herpesvirus 3 (mdv serotype 2) vectors expressing antigens of avian pathogens and uses thereof
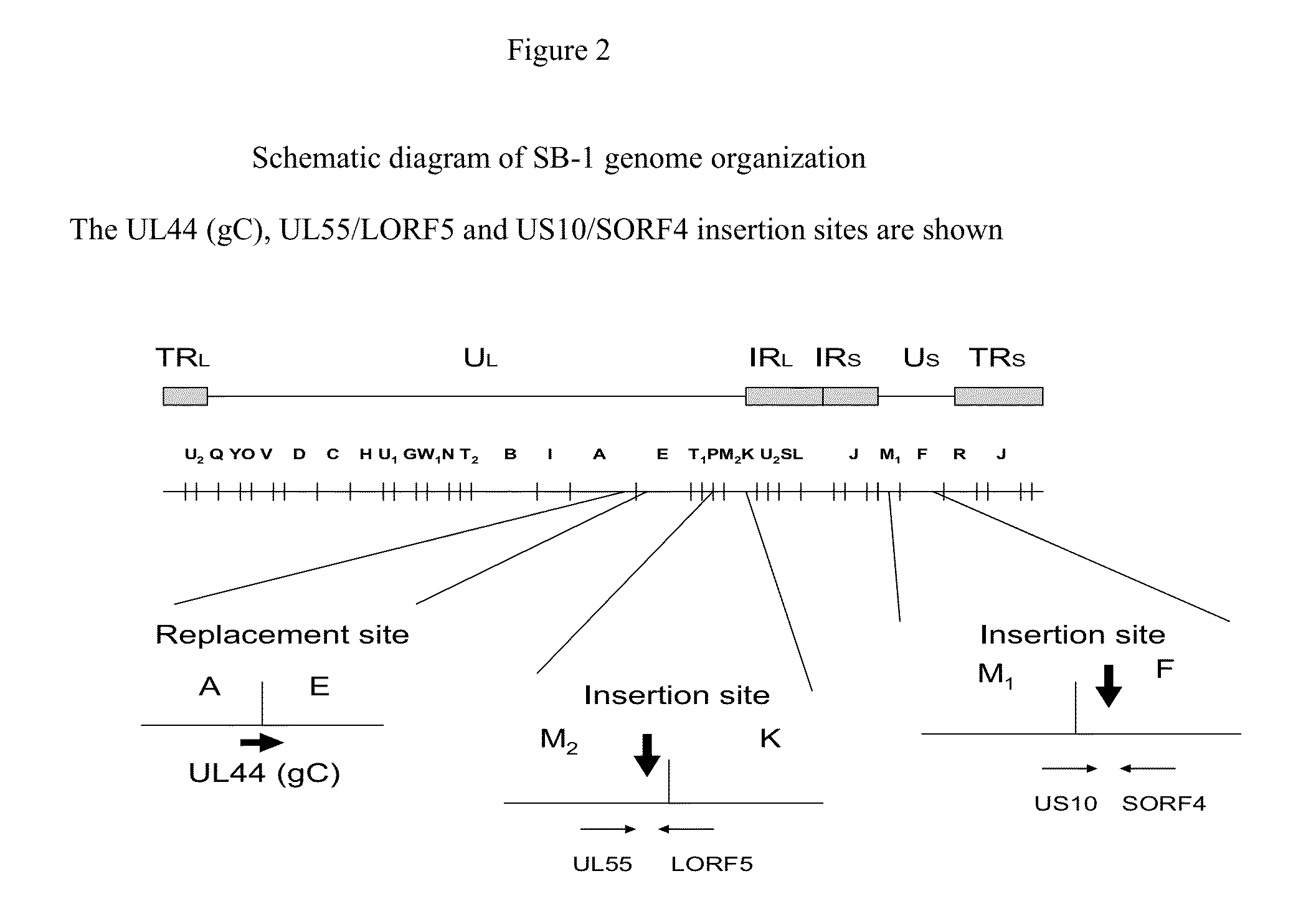
The present invention provides recombinant Gallid herpesvirus 3 (MDV-2) vectors that contain and express antigens of avian pathogens, recombinant Gallid herpesvirus 3 (MDV-2) vectors that contain a mutated gC gene, compositions comprising the recombinant Gallid herpesvirus 3 (MDV-2) vectors, polyvalent vaccines comprising the recombinant Gallid herpesvirus 3 (MDV-2) vectors and one or more wild type viruses or recombinant vectors. The present invention further provides methods of vaccination against a variety of avian pathogens and method of producing the recombinant Gallid herpesvirus 3 (MDV-2) vectors.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH +1
Polyvalent vaccine
The present invention relates, in general, to an immunogenic composition (e.g., a vaccine) and, in particular, to a polyvalent immunogenic composition, such as a polyvalent HIV vaccine, and to methods of using same. The invention further relates to methods that use a genetic algorithm to create sets of polyvalent antigens suitable for use, for example, in vaccination strategies.
Owner:LOS ALAMOS NATIONAL SECURITY +3
Polyvalent vaccine
The present invention relates, in general, to an immunogenic composition (e.g., a vaccine) and, in particular, to a polyvalent immunogenic composition, such as a polyvalent HIV vaccine, and to methods of using same. The invention further relates to methods that use a genetic algorithm to create sets of polyvalent antigens suitable for use, for example, in vaccination strategies.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +3
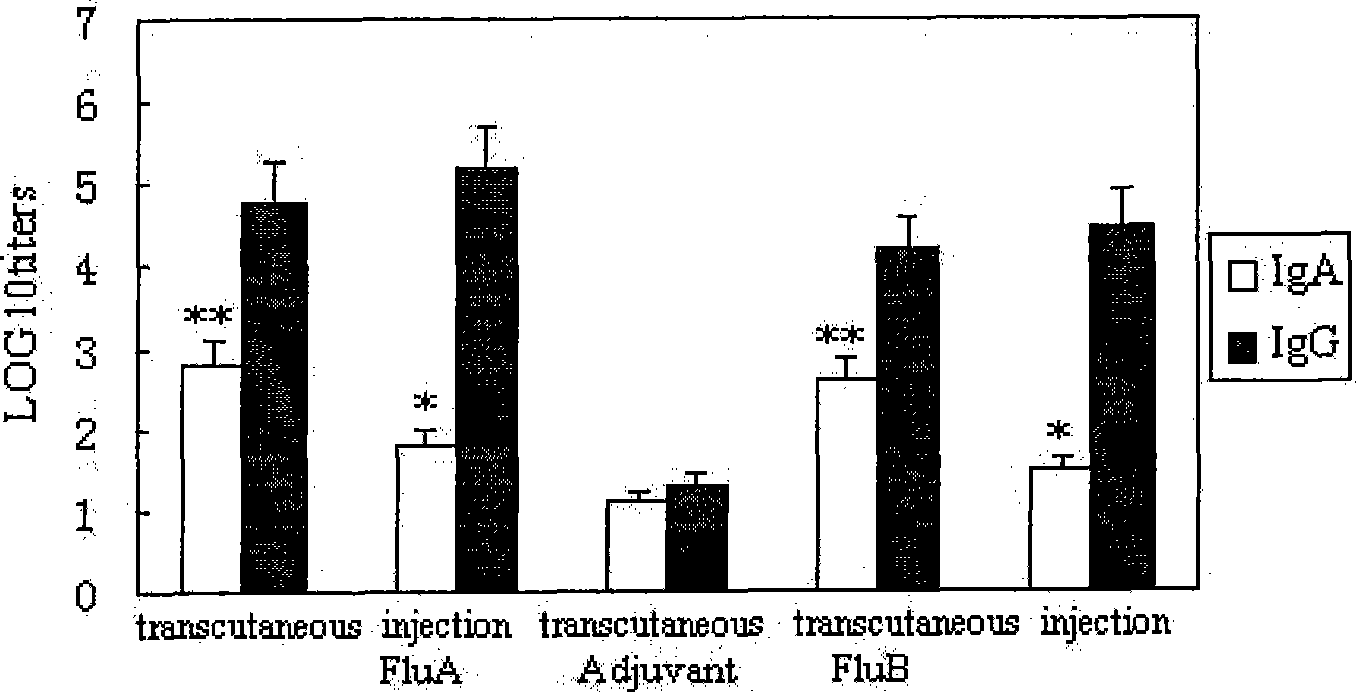
Transdermal immune influenza multivalent vaccine and preparation method thereof
InactiveCN101450209AEnhanced antigen presentation functionFacilitated DiffusionPowder deliveryAntiviralsBALB/cAdjuvant
The invention provides a transdermal immunity flu polyvalent vaccine and a preparing method. The transdermal immunity flu polyvalent vaccine comprises transdermal immunity adjuvant, flu polyvalent vaccine antigen, permeation agent and medical dressing. The flu polyvalent inactivation or attenuated live vaccine is differ from prior vaccine immunity approach and adjuvant. A result through permeation purpose immunity to Balb / c mouse, ferret, monkey and human body improves that the transdermal immunity flu polyvalent vaccine can generate IgA and IgG antibody with high valency, namely, can induce immune system and mucosal immune simultaneously, also can be used for immunoprophylaxis to popular flu and highly pathogenic avian influenza.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Preparation method of antisense RNA polyvalent vaccine for CoViD-19
PendingCN111330003AQuick resultsGood effectAntibacterial agentsSsRNA viruses positive-senseAntisense RNANucleotide
The invention relates to a preparation method of an antisense RNA polyvalent vaccine for CoViD-19. The preparation method comprises the steps of (1) designing shRNA of 2019-nCoV, constructing pSilencer-shRNA and pDC312-shRNA, performing homologous recombination on the pDC312-shRNA and pBHGloxAEl to obtain shRNA / Ad5 capable of generating dsRNA, siRNA and antisense RNA; (2) synthetizing chemically-modified mononucleotide into the siRNA of 2019-nCoV, and further preparing lipid nanoparticles siRNA / LHNPs loading siRNA; (3) compounding the antisense RNA polyvalent vaccine for pulmonary administration from the shRNA / Ad5, the siRNA / LHNPs and a pressurized spray, wherein the shRNA / Ad5 can degrade target genes after continuously outputting siRNA, siRNA / LHNPs can directly and immediately degrade thetarget genes, when the shRNA / Ad5 and the siRNA / LHNPs are used in a compounding ratio, synergistic effects can be achieved, and immunization failure of a univalent vaccine can be avoided.
Owner:翁炳焕
Mage-3 and NY-ESO-1 Based Polyvalent Vaccine for Cancer Immunotherapy
InactiveUS20070243196A1Good effectStimulate immune responseSnake antigen ingredientsCancer antigen ingredientsAntigenAdjuvant
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Methods of treating lymphoma and leukemia
InactiveUS7208146B2Shorten the timeEffective isolationAntibody mimetics/scaffoldsNucleic acid vectorCell tumorPolyvalent Vaccine
The present invention provides multivalent vaccines for the treatment of B-cell malignancies (e.g., lymphomas and leukemias). The present invention also provides methods for the production of custom vaccines, including multivalent vaccines for the treatment of immune cell tumors malignancies as well as methods of treating immune cell tumors using custom vaccines.
Owner:GENITOPE CORP
Human papillomavirus type bivalent virus-like particle mixed protein antigen and construction method
InactiveCN1683010AImproving immunogenicityImprove responseAntiviralsRecombinant DNA-technologyVaccine ImmunogenicityIndividual animal
The present invention relates to human papillomavirus type bivalent virus-like particle mixture, and human papillomavirus type bivalent virus-like particle mixing protein antigen and its construction method. The present invention includes one kind of cervical carcinoma virus HPV16 L1 antigen and one kind of condyloma acuminata virus HPV11 L1 antigen. The HPV11 L1 gene is first cloned from condyloma acuminata tissue through molecular geological method, and both HPV16 L1 gene cloned from cervical carcinoma tissue and the HPV11 L1 gene are then expressed in the insect cell expression system of baculovirus, so as to constitute recombinant virus strain Bacmid / HPV16-HPV11L1. Animal immunizing test shows that the constituted recombinant protein has excellent immunogenicity and immunoreactivity, and may be used as candidate antigen of gene engineering multivalent vaccine for preventing condyloma acuminata and cervical carcinoma.
Owner:HARBIN MEDICAL UNIVERSITY
Cd4+ human papillomavirus (hpv) epitopes
InactiveUS20070037151A1Improving immunogenicityViral antigen ingredientsMicrobiological testing/measurementAbnormal tissue growthHPV vaccines
The present invention provides CD4+ T-cell epitopes in E6, E7 and E2 proteins from various strains of human papillomavirus (HPV). In some preferred embodiments, the present invention provides means for the development of HPV vaccines, in particular multivalent vaccines for the prevention of infection with high-risk HPV strains. In additional embodiments, the present invention provides means for the development of therapeutic vaccines against high-risk HPV types that prevent the development of benign and / or malignant tumors in infected individuals. The present invention further provides epitopes suitable for use in prophylactic and therapeutic vaccines.
Owner:GENENCOR INT INC
Vectors based on recombinant defective viral genomes, and their use in the formulation of vaccines
The vectors comprise a recombinant defective viral genome expressing at least one antigen suitable for the induction of systemic and secretory immune responses or an antibody conferring protection against an infectious agent. The defective viral genome comprises the genome of a parental virus having the viral replicase recognition signals located on ends 3′ and 5′, further comprising internal deletions, and wherein said defective viral genome depends on a helper virus for its replication and encapsidation. These vectors are suitable for the forming of a recombinant system comprising the aforesaid expression vector, and a helper virus. The system is suitable for the manufacture of mono- and polyvalent vaccines against infectious agents of different animal species, especially pigs, dogs and cats, and as expression vehicles for antibodies protective against infectious agents.
Owner:CYANAMID IBERICA SA
Recombinant multivalent vaccine
InactiveUS20100119550A1Improve accuracySecuring and ensuring effectivenessAntibacterial agentsSsRNA viruses negative-sensePolyvalent VaccineVaricella zoster virus
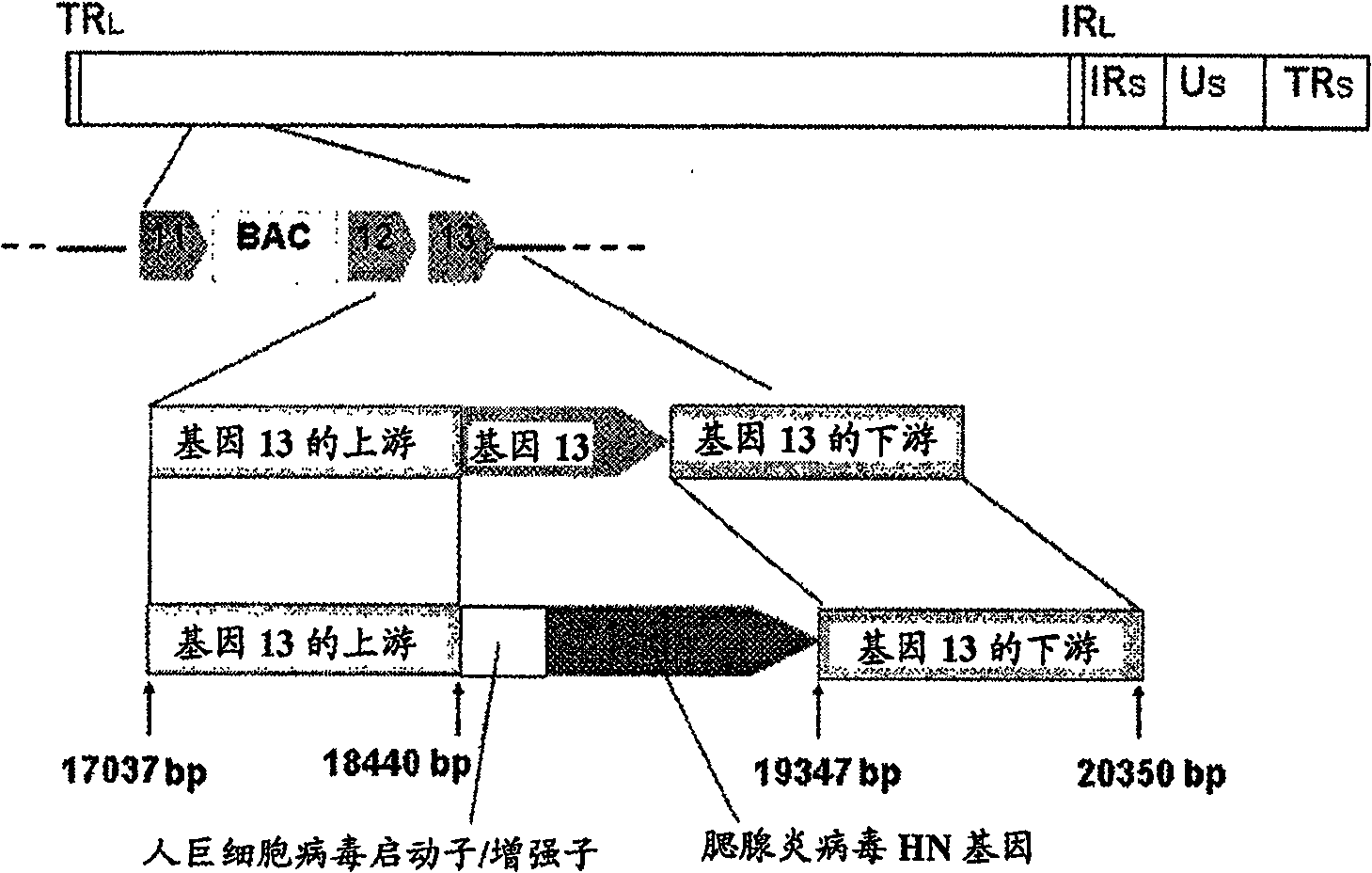
The problems to be solved by the present invention are to provide: a recombinant varicella-zoster virus; a process for producing the same; a pharmacological composition containing a recombinant varicella-zoster virus; a vector containing a BAC vector sequence in the specific gene of a genomic gene of varicella-zoster virus; cells containing such a vector; a fragment capable of homologous recombination with a genome of varicella-zoster virus; a nucleic acid cassette containing the BAC vector sequence; and a multivalent vaccine. The above problems were solved by developing a process for producing a recombinant varicella-zoster virus, wherein the BAC vector sequence is inserted into a specific virus gene.
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV +1
Recombinant Hvt Vectors Expressing Antigens Of Avian Pathogens And Uses Thereof
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Virus-like particles for pseudorabies virus and preparation method for same
ActiveCN102533680AAvoid quality risksEasy to design and manufactureInactivation/attenuationAntiviralsRabiesGlycoprotein G
The invention provides virus-like particles for pseudorabies virus. The virus-like particles consist of pseudorabies virus glycoprotein G and pseudorabies virus matrix protein M, and have stable and homogenous spatial structures; and the outer membrane protein of the virus-like particles contains all or partial fragments of the pseudorabies virus glycoprotein G, and can induce an organism to generate protective-level cellular immunity and humoral immunity. The virus-like particles do not comprise any nucleic acid component of chromosome of the pseudorabies virus, and avoid quality risks caused by inactivator addition. The virus-like particles can be conveniently purified by the conventional purification technology, and the quality risks possibly caused by inactivator addition in the traditional inactivated vaccines are avoided on principle. Meanwhile, due to a simple and quick construction and preparation mode, novel vaccines aiming at new strains can be conveniently and quickly designed and prepared. Based on the principle, novel polyvalent vaccines and multi-vaccines are easily formed on the basis of the particles; and the particles can widely replace related critical raw materials in the conventional practical technologies such as related antigen and antibody detection, functional protein vectors and the like.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Adenovirus-vectored multivalent vaccine
InactiveUS20190307879A1Rapid responseViral antigen ingredientsPharmaceutical delivery mechanismAdjuvantPolyvalent Vaccine
The invention pertains to a vaccine comprising an immunologically effective amount of a novel live-vectored multivalent vaccine formulation that affords immunization to multiple antigens of a pathogen that is relatively impervious to vaccine development by providing multiple virus-expressed antigens and a pharmaceutically acceptable carrier and / or an adjuvant. Further, a method of immunizing a subject against an exposure to a pathogen that is relatively impervious to vaccine development is provided, wherein the method comprising the steps of administering the vaccine to a subject to induce an immune response against antigenic proteins or fragments thereof.
Owner:TEXAS A&M UNIVERSITY
Recombinant multivalent vaccine
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV +1
Preparation method of triple inactivated vaccine for pigs
InactiveCN104998256AImprove immunityHighlight immune functionAntibacterial agentsBacterial antigen ingredientsAntigenProtective antigen
The invention provides a preparation method of a triple inactivated vaccine for pigs. The method determines an antigen composition with excellent immunization effects by selection of the antigen. The prepared polyvalent vaccine has outstanding immunization effects. The prepared vaccine contains a PCP immunization protective antigen exotoxin (Aps), has cross immunization protection effects better than those of a whole cell inactivated vaccine, greatly reduces side reaction, and can simultaneously prevent haemophilus parasuis, swine streptococcosis and actinobacillus pleuropneumonia by combined immunization with inactivated haemophilus parasuis and streptococcus suis. Compared with haemophilus parasuis and streptococcus suis inactivated vaccines sold on the market, the triple inactivated vaccine has the same corresponding pathogen immune protection force. Compared with the actinobacillus pleuropneumonia inactivated vaccine sold on the market, the triple inactivated vaccine has a cross immunization protecting force on diseased pigs with different serotypes and realizes multiple protection purposes.
Owner:TIANJIN RINGPU BIO TECH
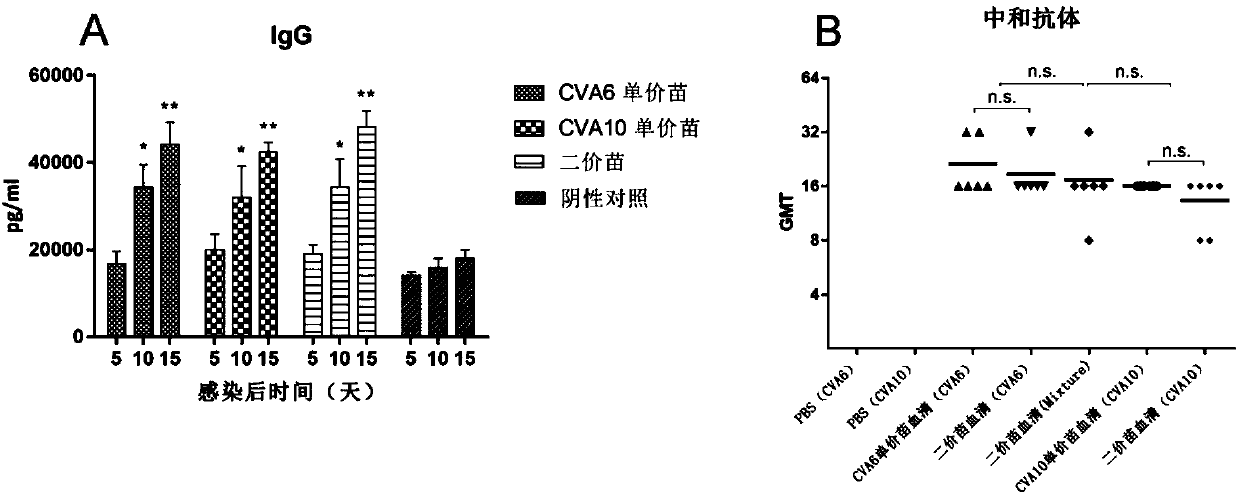
Coxsackie virus A10 domestication strain containing virus composition and application of virus composition
InactiveCN107739731AFree from harmProtection against virusesSsRNA viruses positive-senseViral antigen ingredientsDiseaseHEK 293 cells
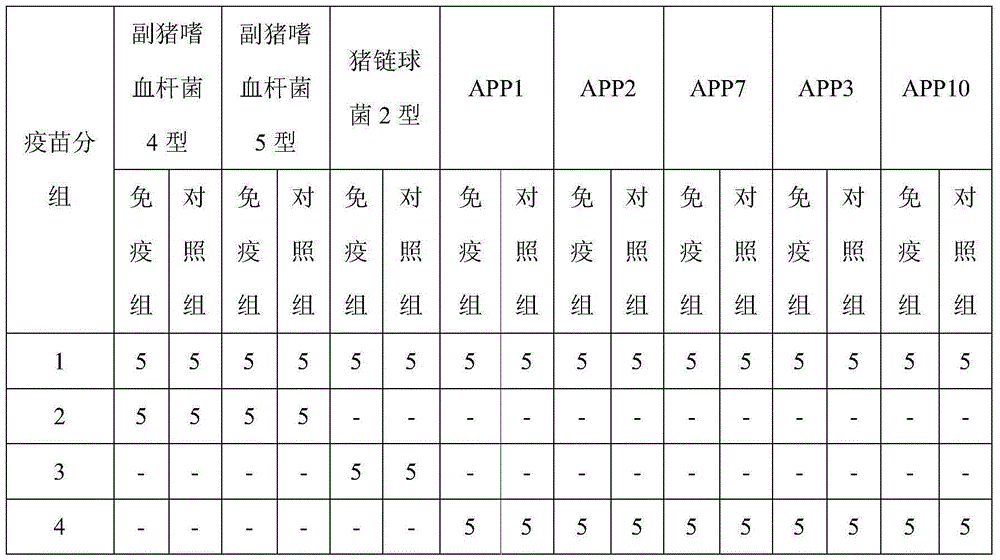
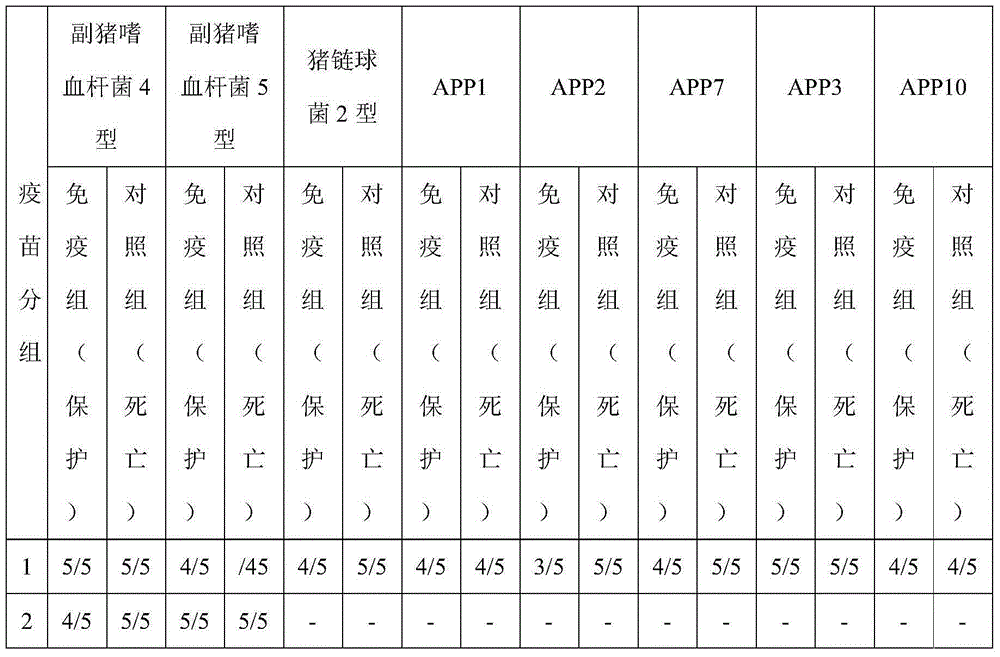
The invention discloses a Coxsackie virus A10 domestication strain TA151R-1 with high titer and stable passage. The virus strain can infect various cell strains including RD cells, HEK 293 cells, Verocells, MRC-5 cells, Hep-2 cells, WI-38 cells and the like and can also be used for preparing a monovalent vaccine or a polyvalent vaccine; the prepared vaccine can protect an organism from damages from Coxsackie virus, can also completely prevent the attacks from other heterologous viruses, can effectively prevent and / or treat diseases caused by infection of the Coxsackie virus and has a wide application prospect.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
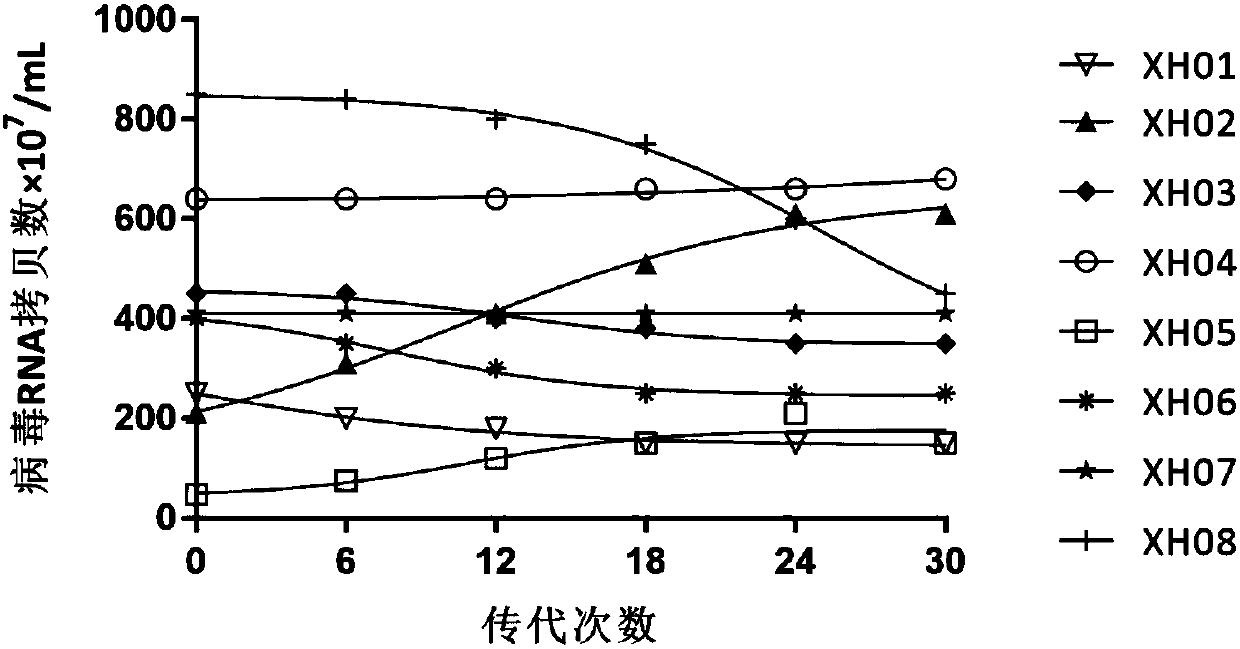
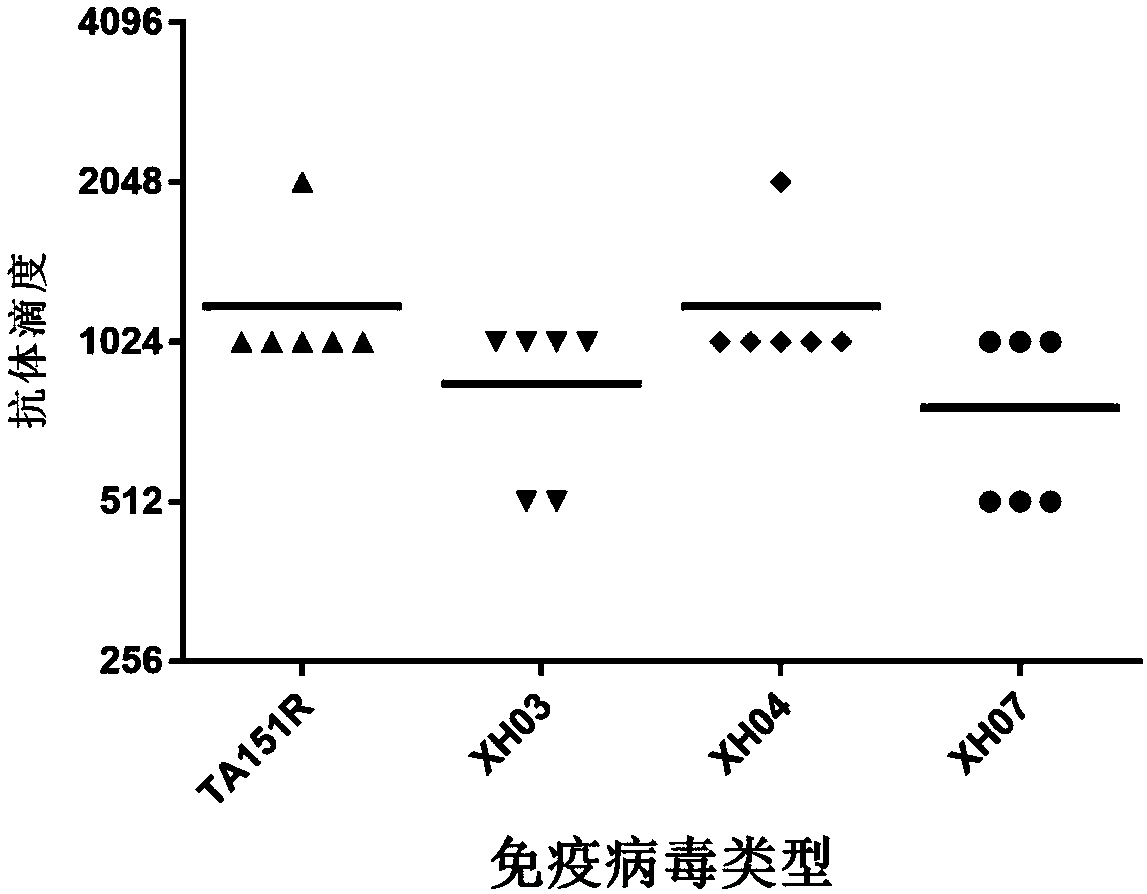
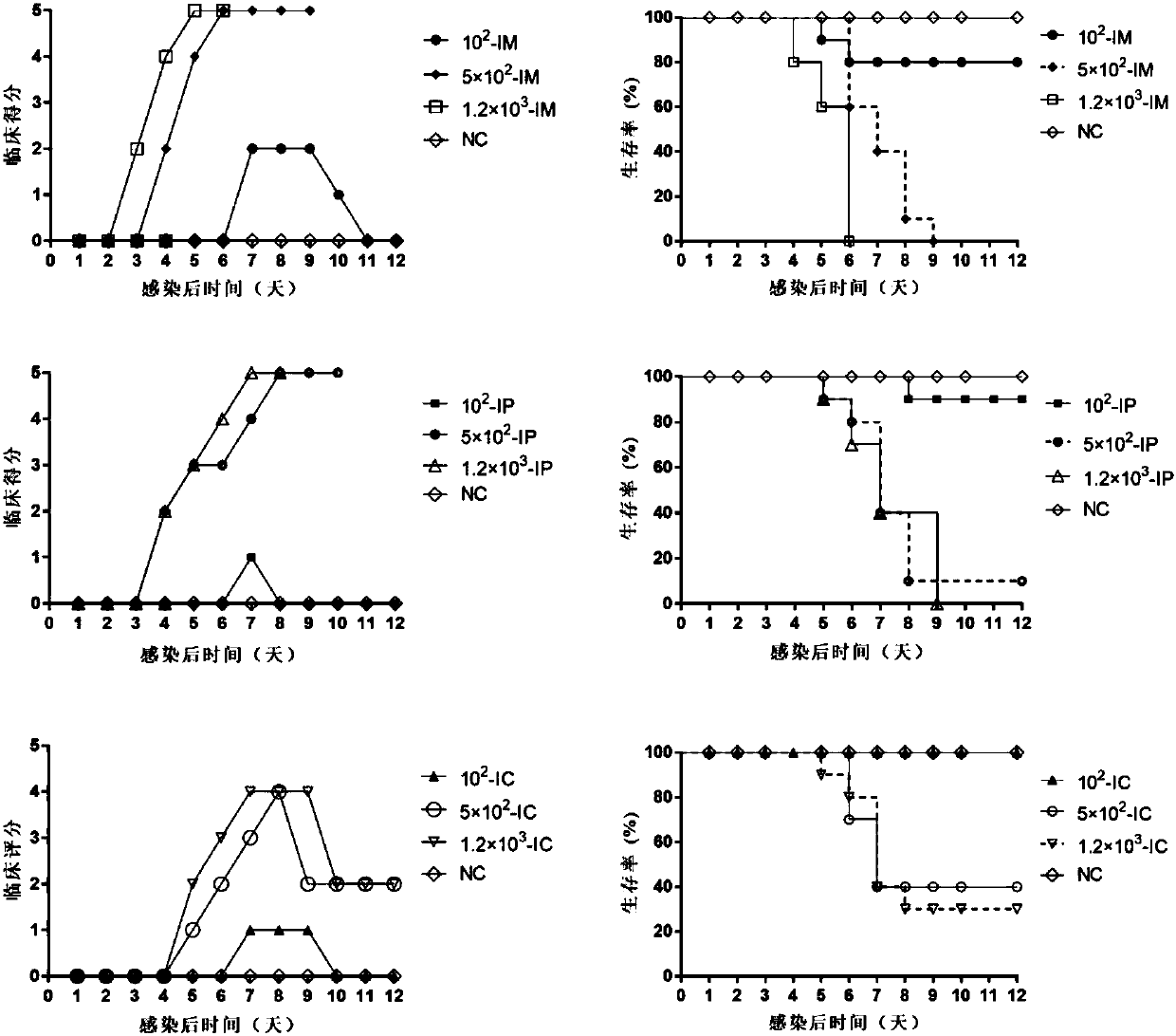
Building and evaluation of an animal model infected with a coxsackievirus A10 domesticated strain TA151R-1
InactiveCN107744530AImprove replication efficiencyImprove efficiencyCompounds screening/testingViral/bacteriophage medical ingredientsCoxsackievirusPolyvalent Vaccine
A coxsackievirus A10 domesticated strain TA151R-1 high in tilter and stable in passage is disclosed. The virus strain can infect RD cells, HEK293 cells, Vero cells, MRC-5 cells, Hep-2 cells, WI-38 cells, and other cell lines, and can be used for preparing a univalent vaccine or a polyvalent vaccine. The prepared vaccine can protect a body from being harmed by the coxsackievirus, and can completelyprotect the body from attack by heterogenous viruses. An infected animal model can be built efficiently.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Bivalent inactivated vaccine of porcine circovirus type 2 and porcine parvovirus and preparation method thereof
InactiveCN102961742AHigh titer contentImmunization is convenient and fastViral antigen ingredientsAntiviralsAntigenAdjuvant
The invention relates to polyvalent vaccines for prevention and treatment of porcine infectious diseases, especially to a combined vaccine for treatment and prevention of porcine circovirus type 2 (PCV2) and porcine parvovirus. By selecting PCV2 and porcine parvovirus, the preparation method provided in the invention consists of: culturing PCV2, and conducting inactivation and concentration; culturing a porcine parvovirus, and performing inactivation and concentration; mixing the two antigenic components in proportion, and supplement an adjuvant to prepare the vaccine. The bivalent vaccine prepared in the invention is easy to use, more secure, and has an immune effect superior to that of combined use of two single vaccines.
Owner:PU LIKE BIO ENG
Preparation method of spleen byproducts for producing homology anti-serum blood and transfer factor from fox, raccoon dog, mink
InactiveCN101422485AEasy to manufactureControl spreadAntibacterial agentsPeptide/protein ingredientsDiseaseBlood collection
The invention relates to a preparation method for obtaining blood used for preparing homologous antiserums and a spleen byproduct used for preparing transfer factors from the bodies of fur bearing animals such as foxes, raccoon dogs and minks; the furs of the foxes, the raccoon dogs and the minks are taken concentratedly in December and confirmed according to the fur-taking dates of different areas; healthy individuals are picked up 45 days before a predicated fur-taking date; the booster immunization of univalent vaccines or polyvalent vaccines of canine distemper, Canine parvovirus enteritis, adenovirus disease, corona virus laxness, parainfluenza, and pasteurellosis is carried out for 3 times by purified and condensed antigens on the foundation of carrying out immunization for once in summer; when the furs are taken, aseptic anticoagulation blood collection is carried out to a heart to improve the blood serum yield; and the spleens of the animals are collected for extracting specific or common transfer factors simultaneously. The blood can be conveniently prepared into the blood serums which resist communicable diseases, and the transfer factors can be extracted the spleen, thus providing guarantee for the healthy development of the breeding in the next year; the invention is used for curing the corresponding communicable diseases of the wild animals of the same species; and when a small amount of communicable disease cases appear in a breeding crowd, the corresponding pathogeny hyper-immune serums and transfer factors are mainly injected to a supposed healthy crowd after the pathogeny is confirmed so as to cure the affected animals in the early period of disease and control the spreading of the epidemic situations.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Recombinant hvt vectors expressing antigens of avian pathogens and uses thereof
ActiveUS20140147457A1Effective protectionSsRNA viruses negative-senseViral antigen ingredientsAntigenVaccination
The present invention provides recombinant herpesvirus of turkeys (HVT) vectors that contain and express antigens of avian pathogens, compositions comprising the recombinant HVT vectors, polyvalent vaccines comprising the recombinant HVT vectors and one or more wild type viruses or recombinant vectors. The present invention further provides methods of vaccination against a variety of avian pathogens and method of producing the recombinant HVT vectors.
Owner:MERIAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com