Preparation method of antisense RNA polyvalent vaccine for CoViD-19
A technology of multivalent vaccine and RNA interference, which is applied in the field of preparation of CoViD-19 antisense RNA multivalent vaccine, to achieve the effect of convenient vaccination, safe use and quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of shRNA Adenovirus Vector Vaccine shRNA / Ad5
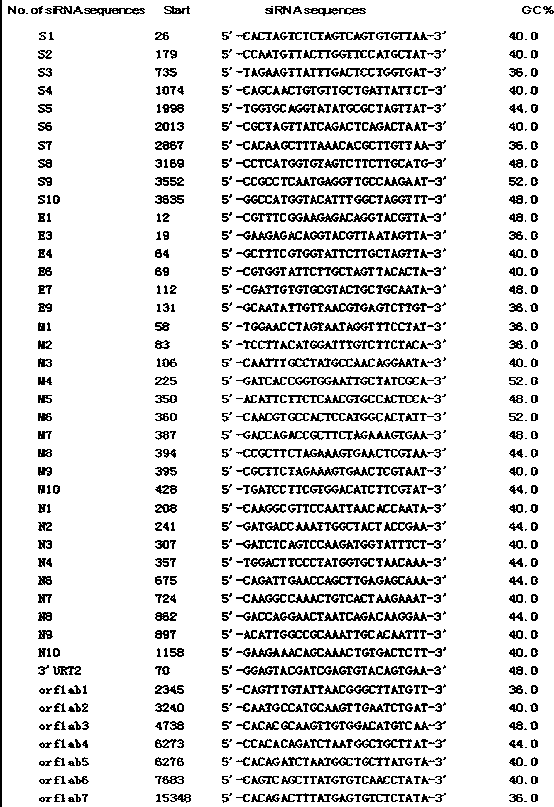
[0035] Screen targeted interference gene shRNA from ORF1ab, S, E, M, N genes of SARS-CoV-2, construct interference vector pSilencer-shRNA (pSilencer-ORF1ab / S / E / M / N), and transfer shRNA expression in it Construct the adenovirus shuttle plasmid pDC312-shRNA, and co-transfect HEK293 cells with the adenovirus backbone plasmid pBHGloxAEl, obtain recombinant adenovirus shRNA / Ad5 through homologous recombination, and prepare CoViD by multiple amplification and purification of HEK293 cells -19 shRNA / Ad5 vaccine, after spray vaccination, shRNA is introduced into respiratory epithelial cells by recombinant adenovirus vector (Ad5), and dsRNA, siRNA and antisense RNA are sequentially generated in the cells, and finally SARS-CoV-2 RNA is specifically induced and / or mRNA degradation.
[0036] This example relates to a preparation method of CoViD-19 vaccine, involving but not limited to ORF1ab, S, E, M, N genes and primers th...
Embodiment 2
[0153] Preparation of siRNA / LHNPs for siRNA lipid nanoparticle vaccine
[0154] According to the SARS-CoV-2 genome sequence, using shRNA design software to predict, multiple RNA interference target gene siRNA sequences can be obtained, from which 1 to 3 conserved siRNA sequences with high silencing efficiency and no homology to the human genome can be screened , using a nucleic acid synthesis method to synthesize siRNA from a 4'-C-methyl-modified mononucleotide (AUCG), using a ring-opening reaction to synthesize a lipid-like epoxyalkylamine derivative EAAD, and using a nanoprecipitation method to prepare EAAD Lipid complex nanoparticle carrier siRNA / LHNPs loaded with siRNA, the inner layer is polylactic acid glycolic acid PLGA containing EAAD and nCoV2019 siRNA conjugates, the outer layer is wrapped with liposomes that can improve the biocompatibility of the carrier, and the outermost layer is PEG, which can improve the structural stability of the carrier, and then formulate s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com