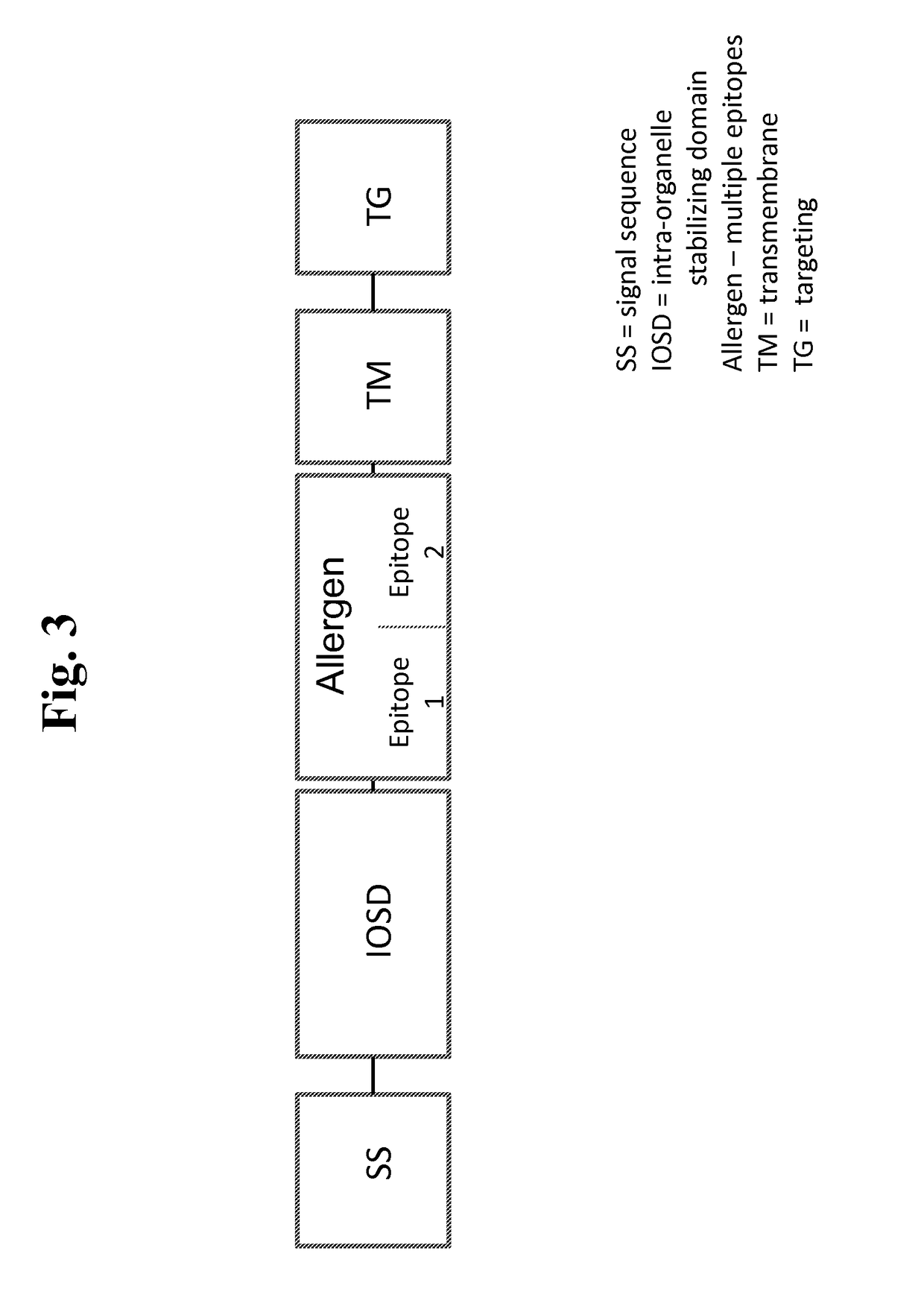
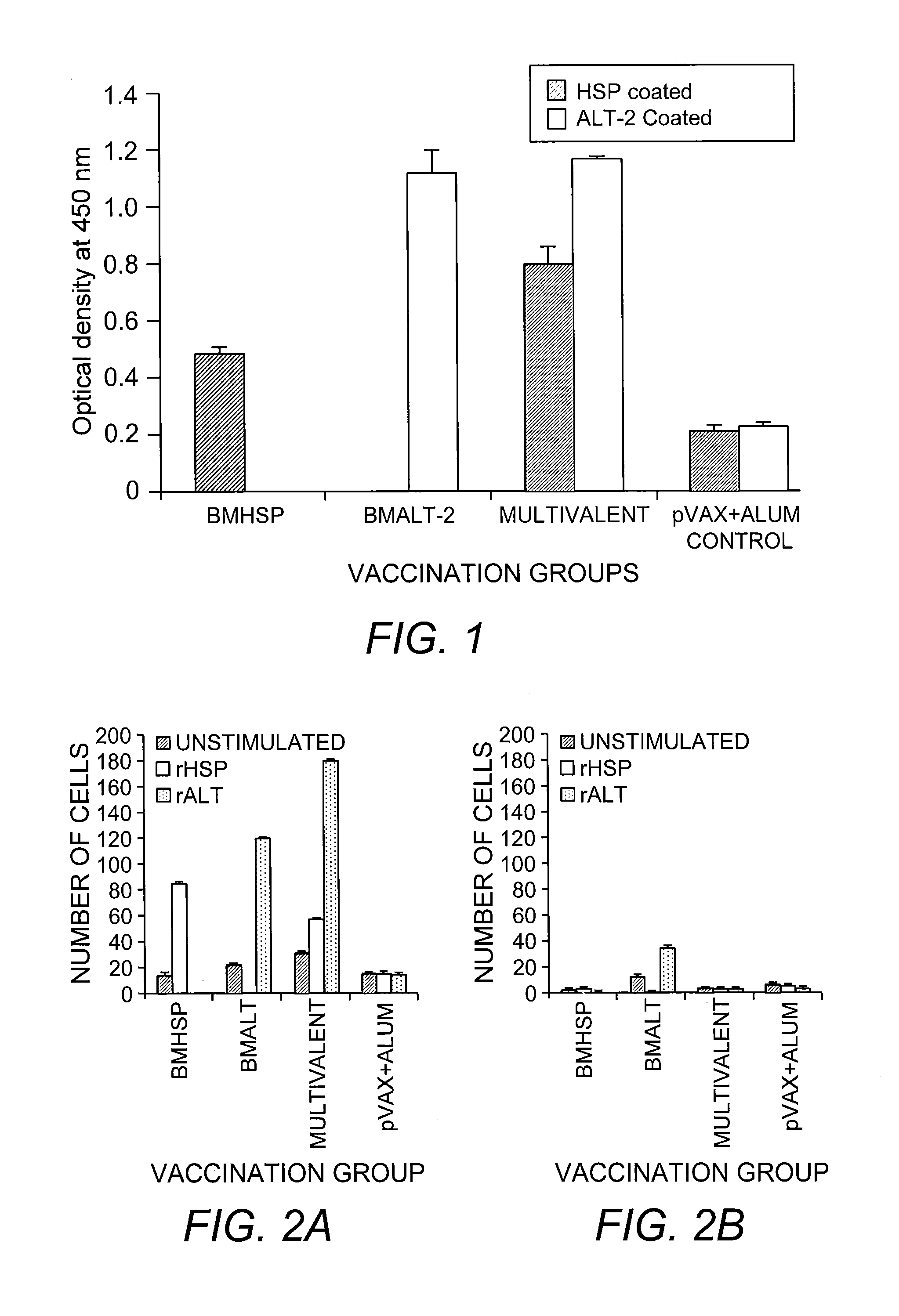
Patents
Literature
133 results about "Multivalent Vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multivalent vaccine. Etymology: L, multus, many, valere, value, vaccinus, cow. a vaccine prepared from several antigenic types within a species. Also called polyvalent vaccine.
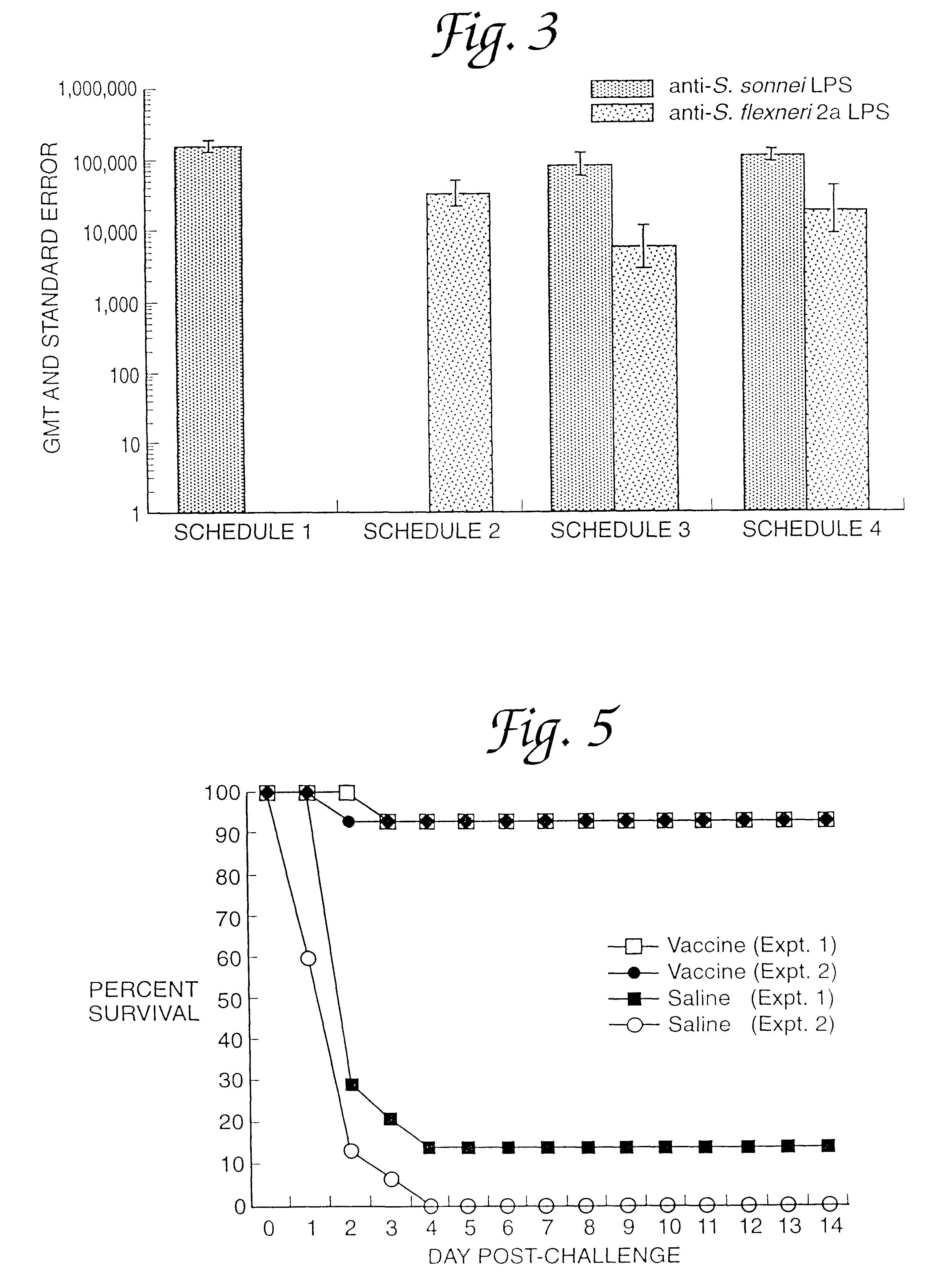
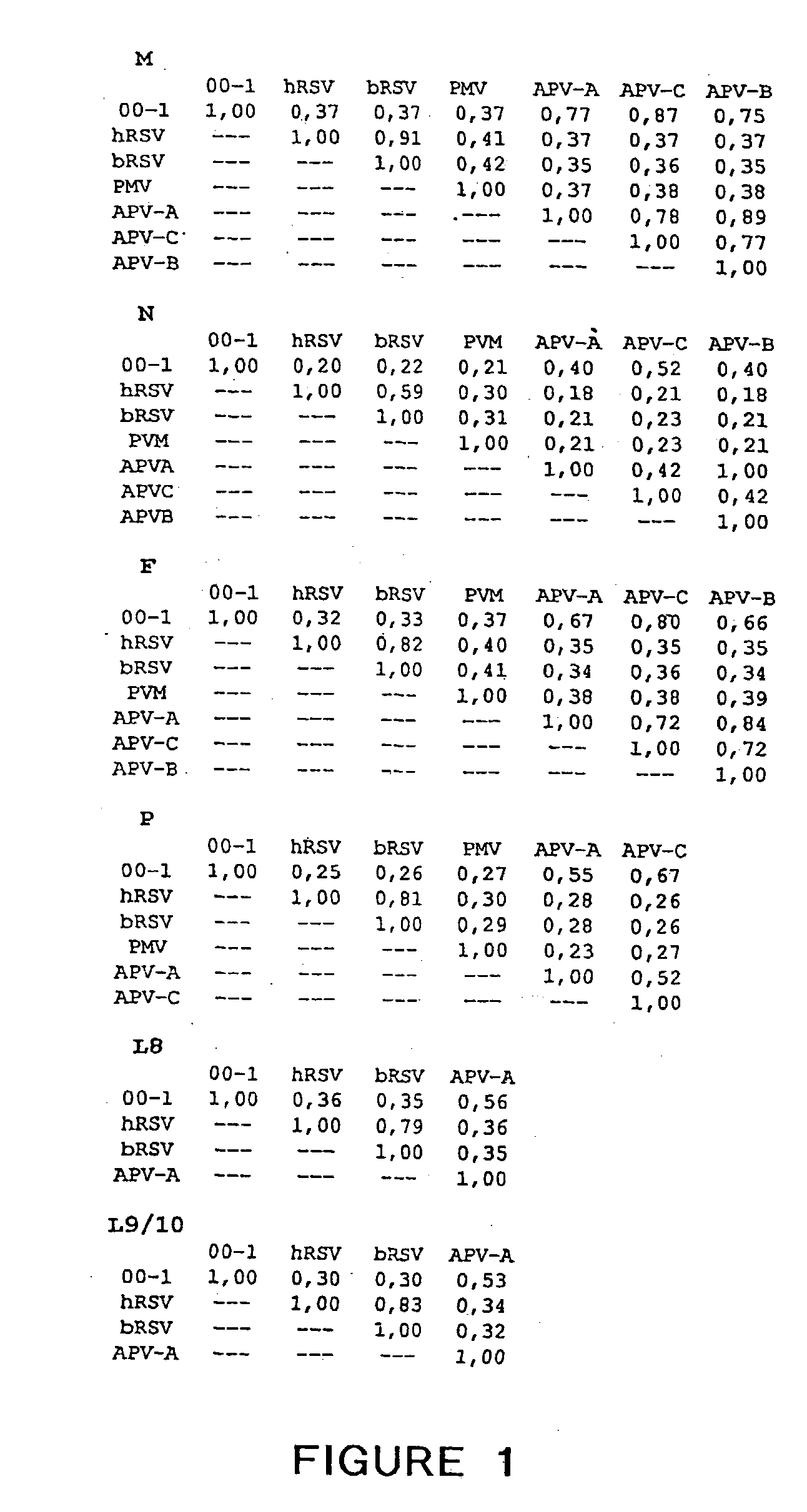
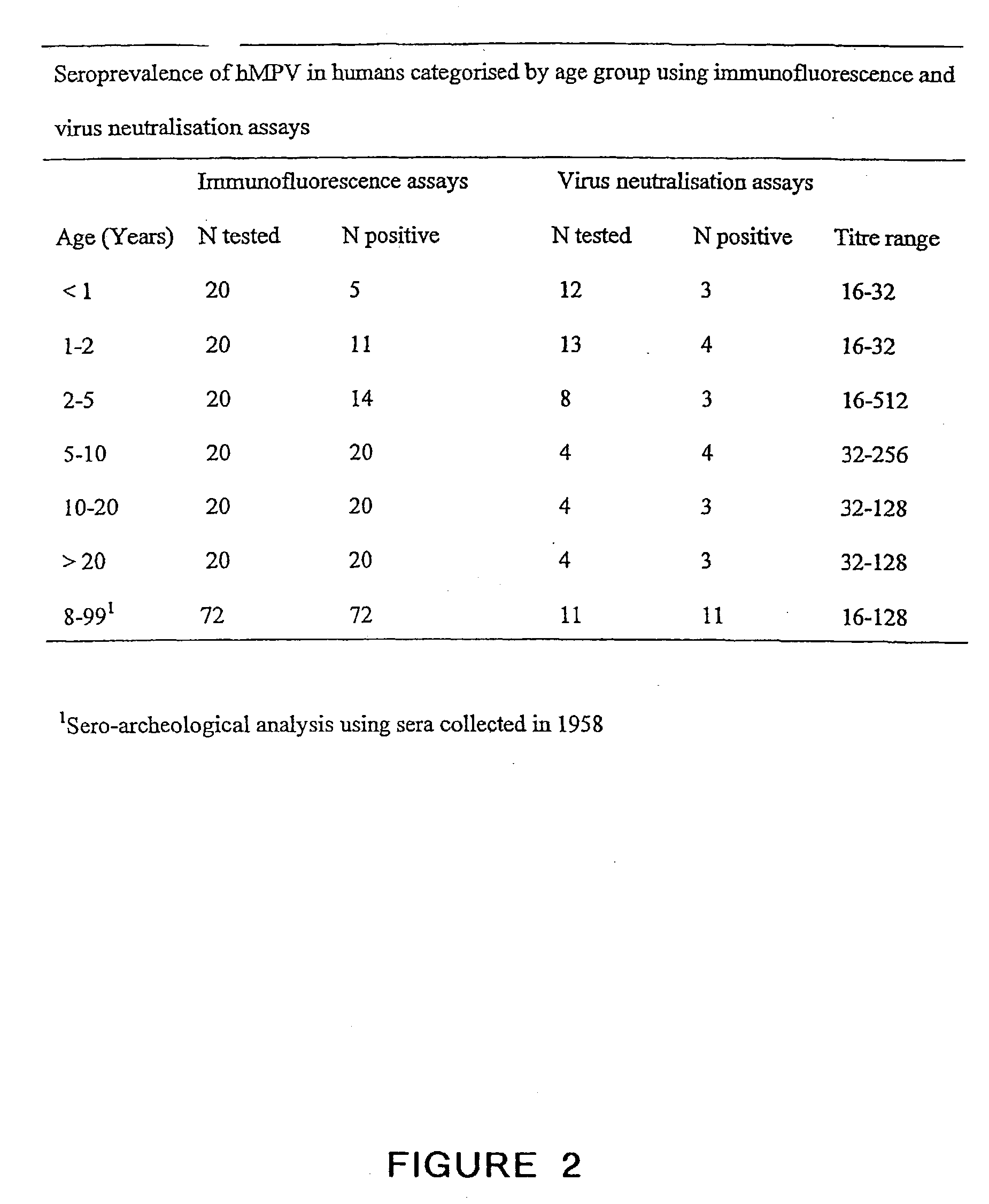
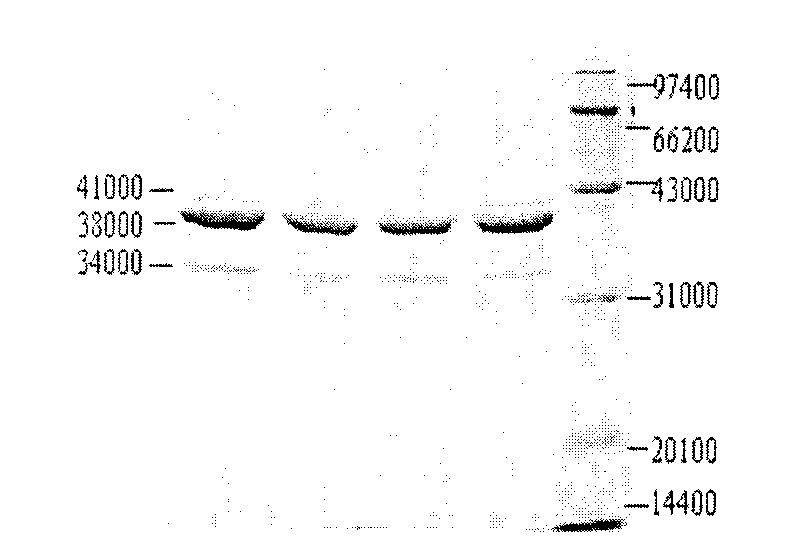
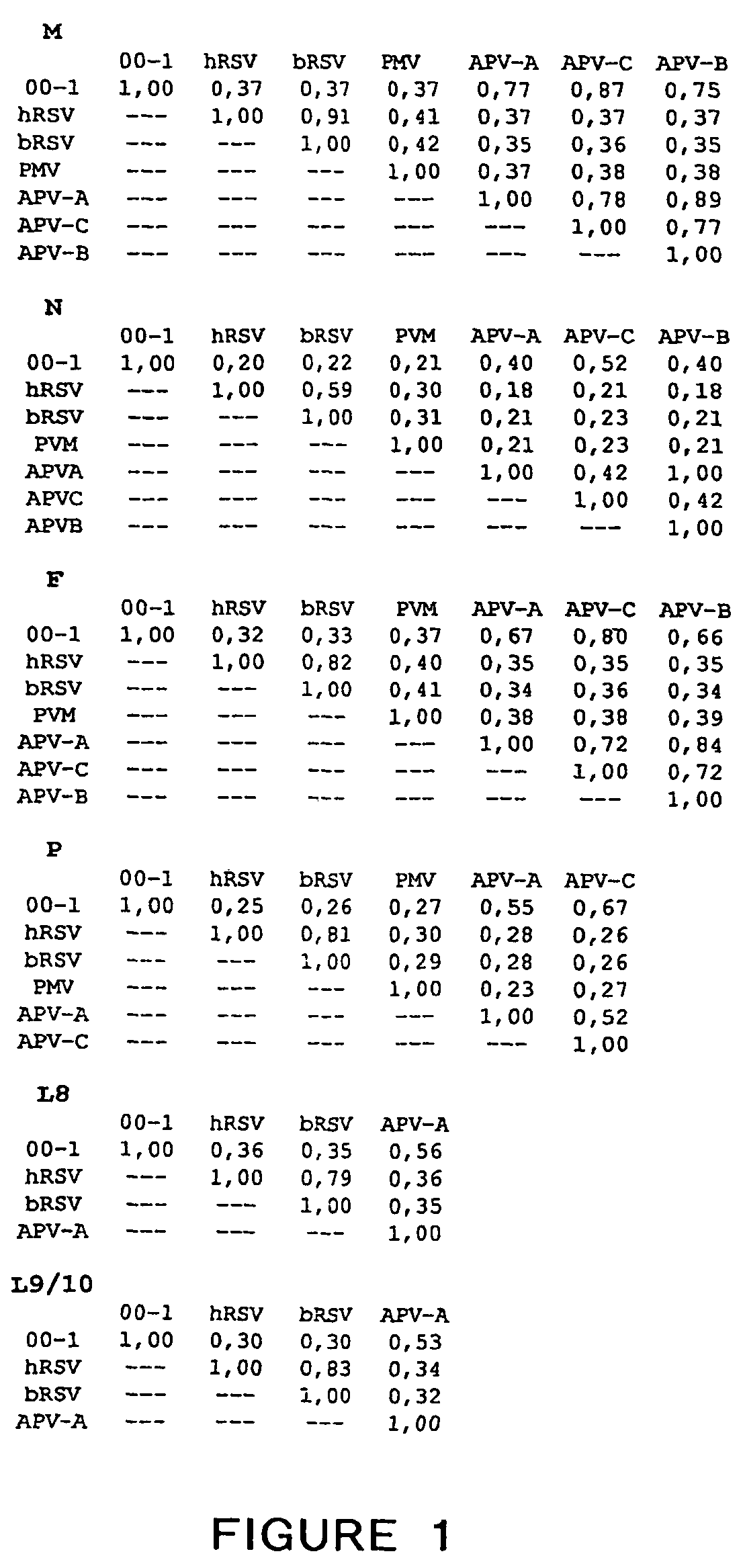
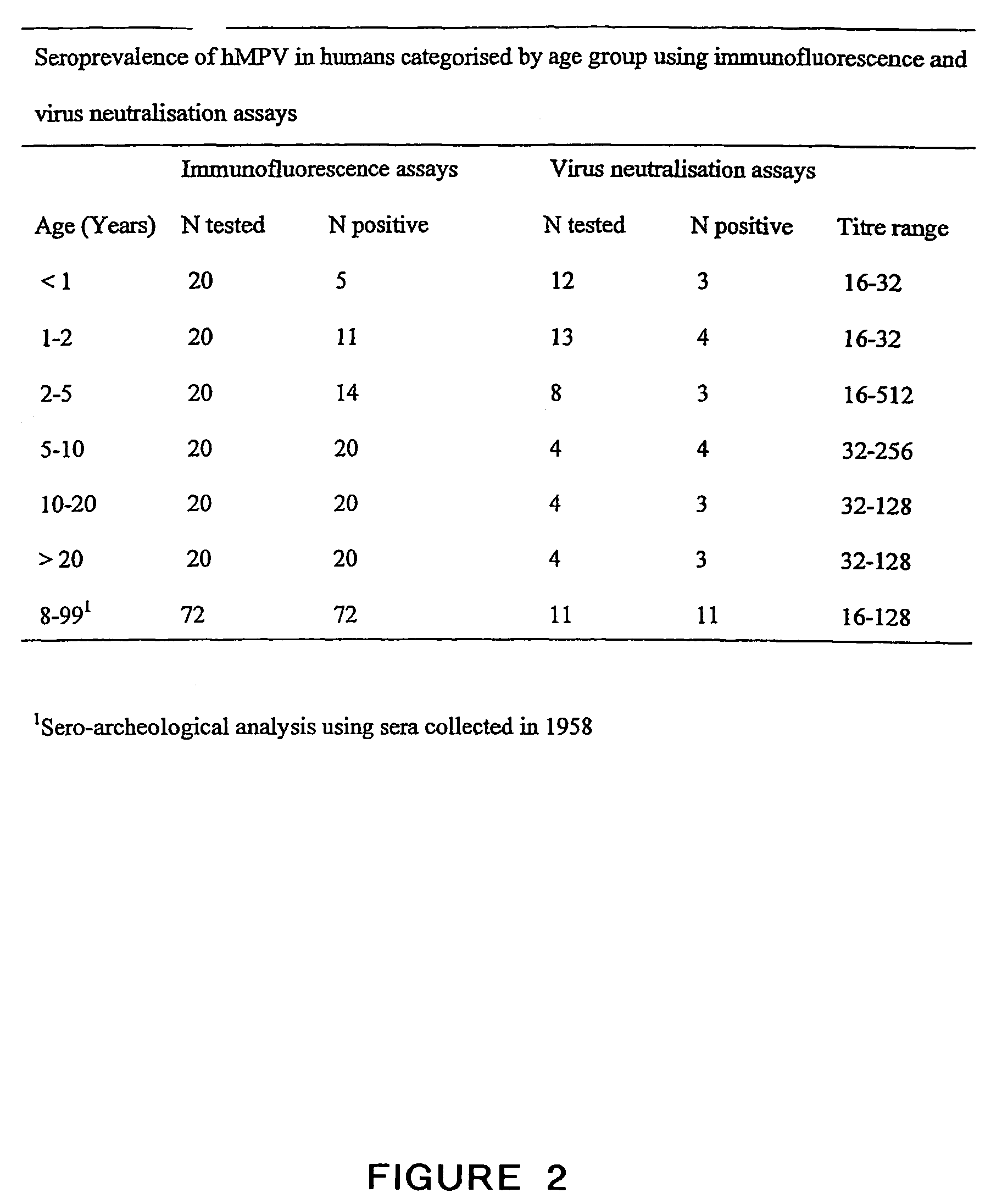
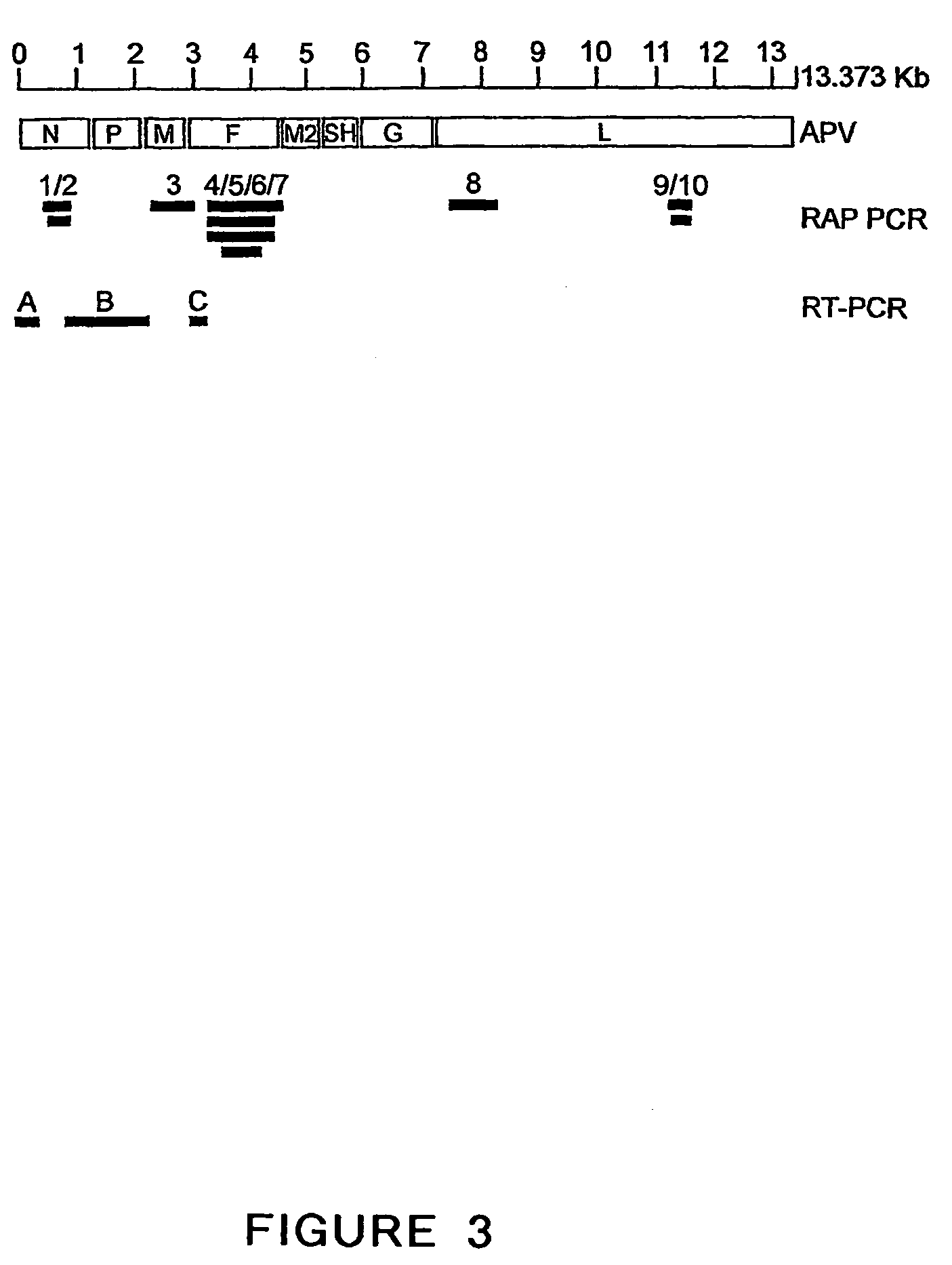
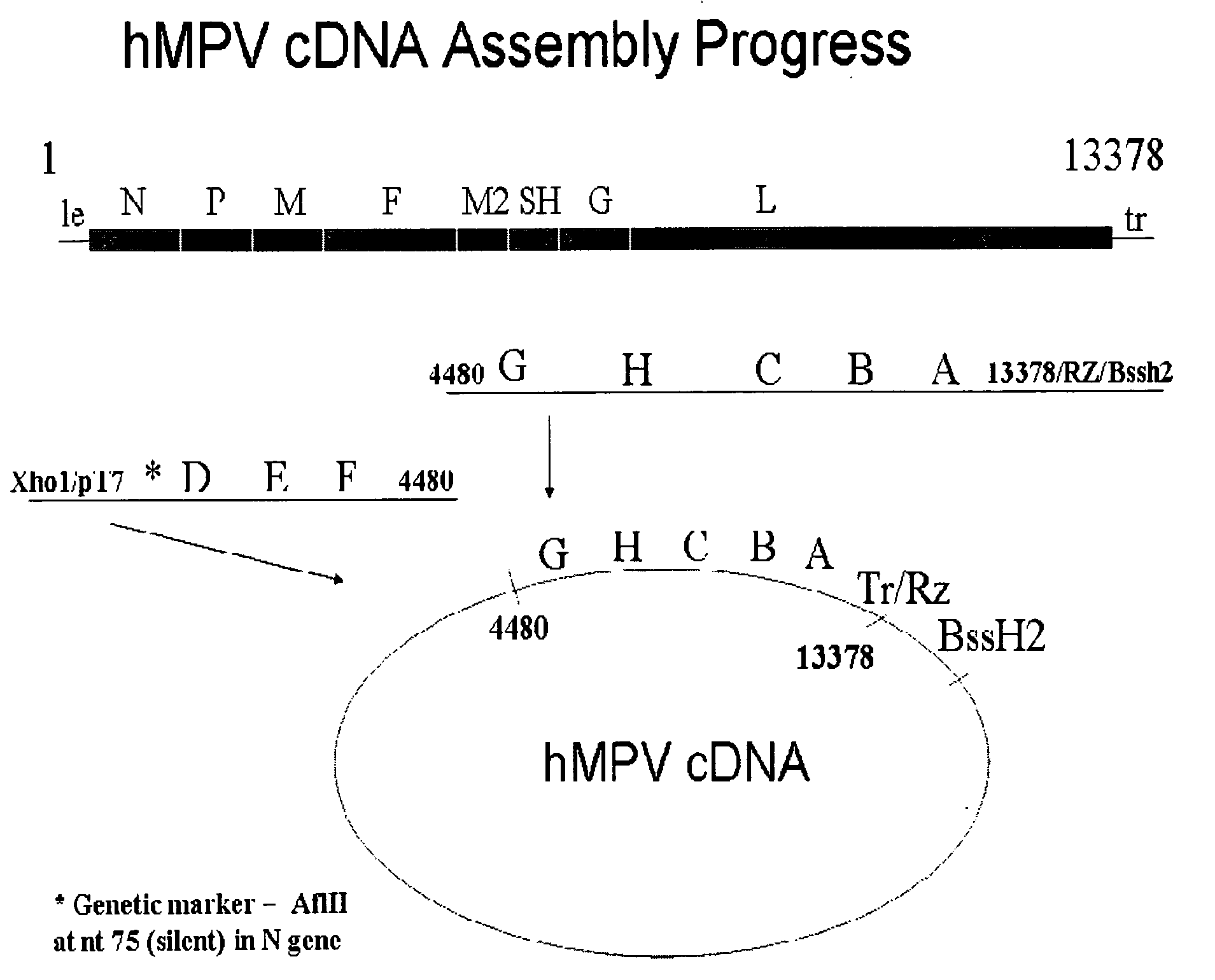
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences and methods for propagating virus
ActiveUS20050019891A1Narrow downSymptoms improvedSsRNA viruses negative-senseVirus peptidesNegative strandHeterologous
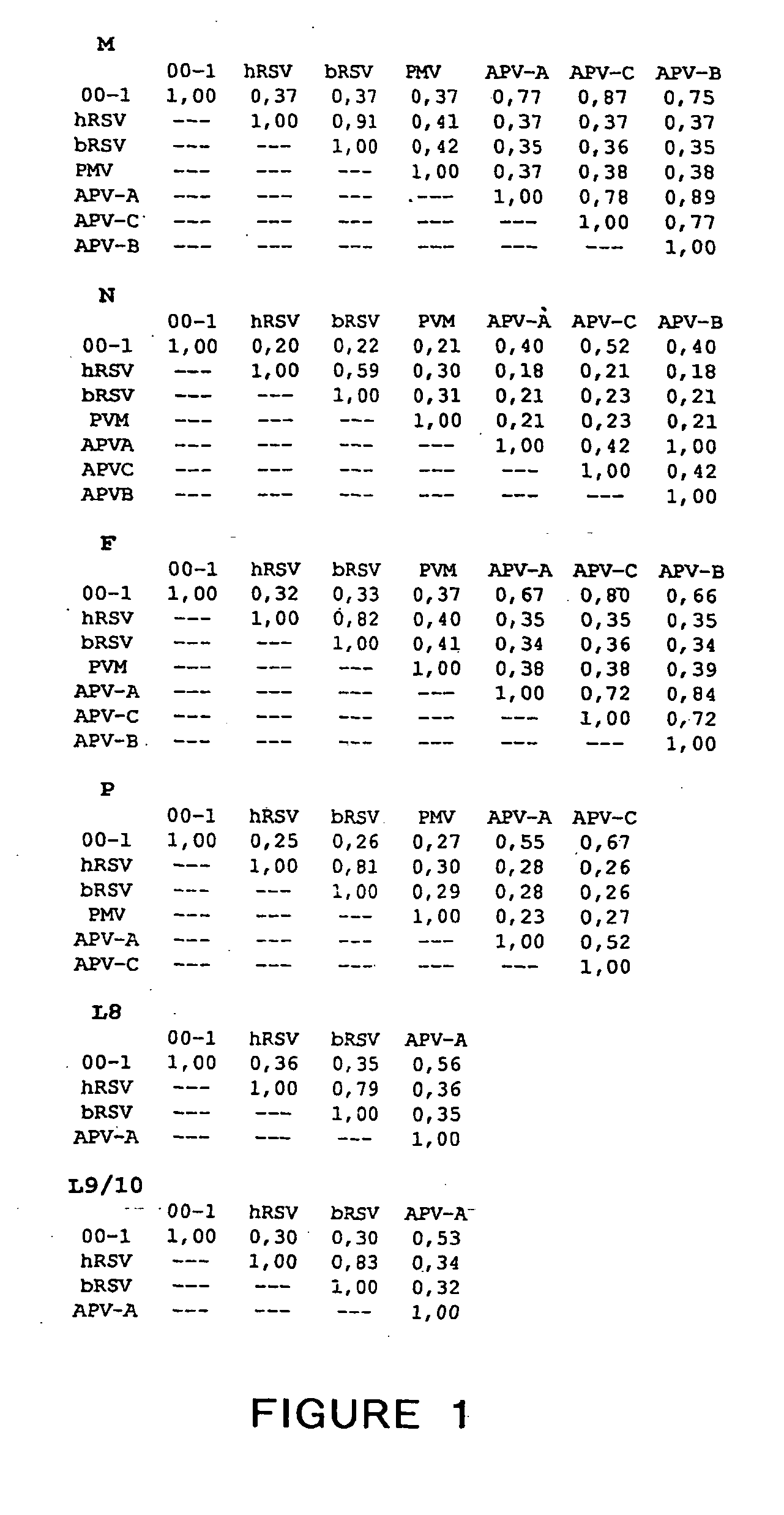
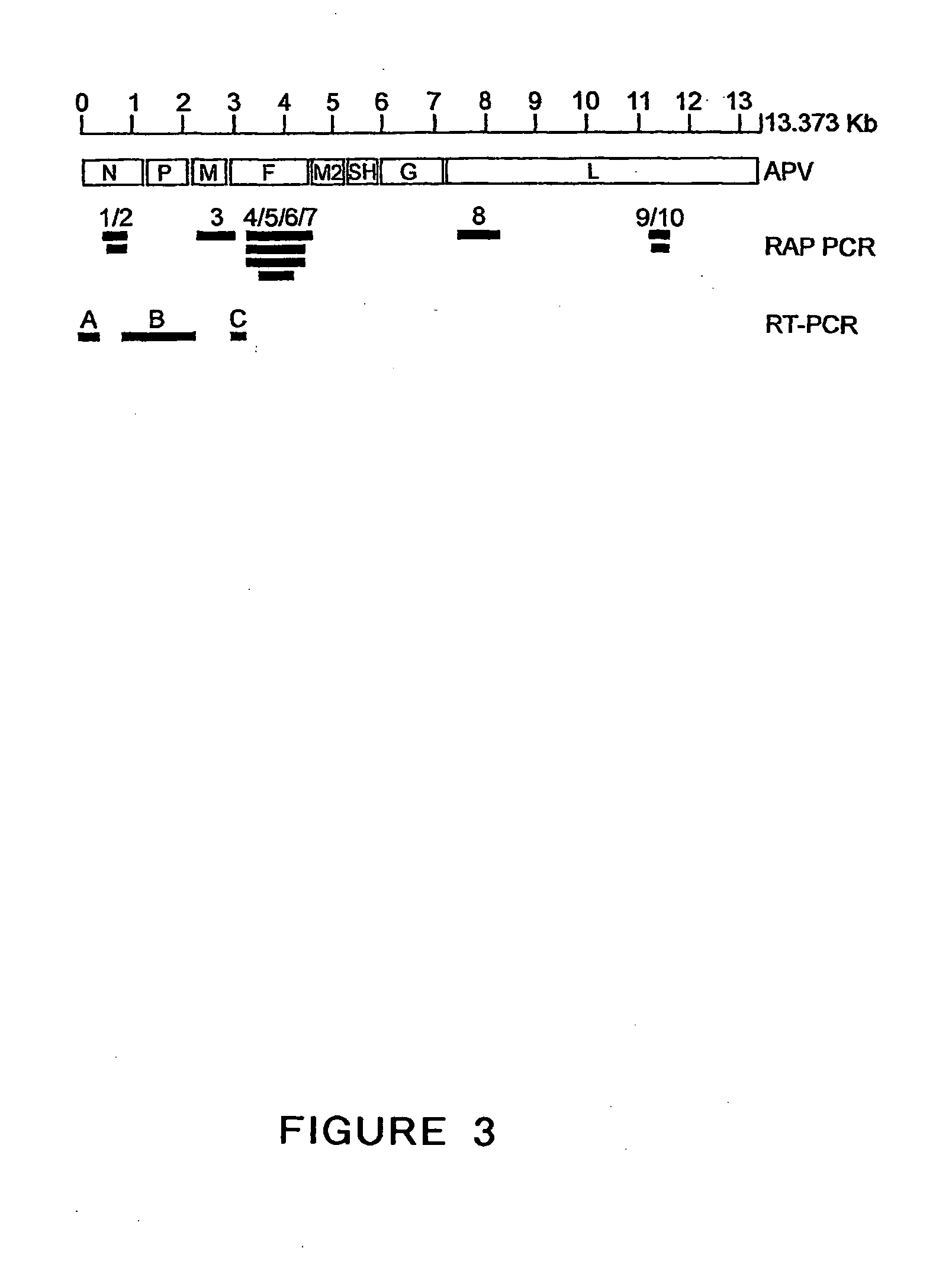
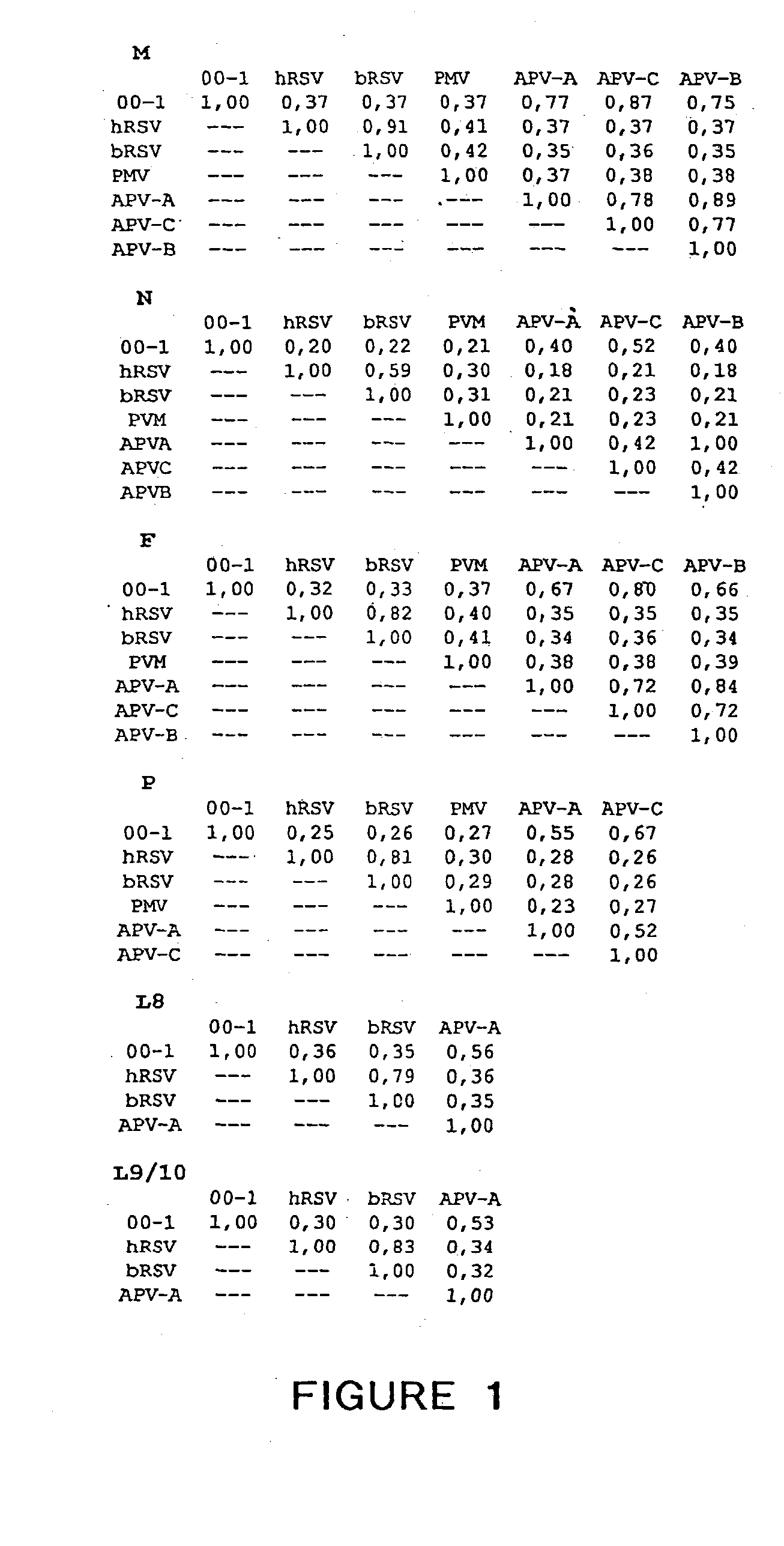
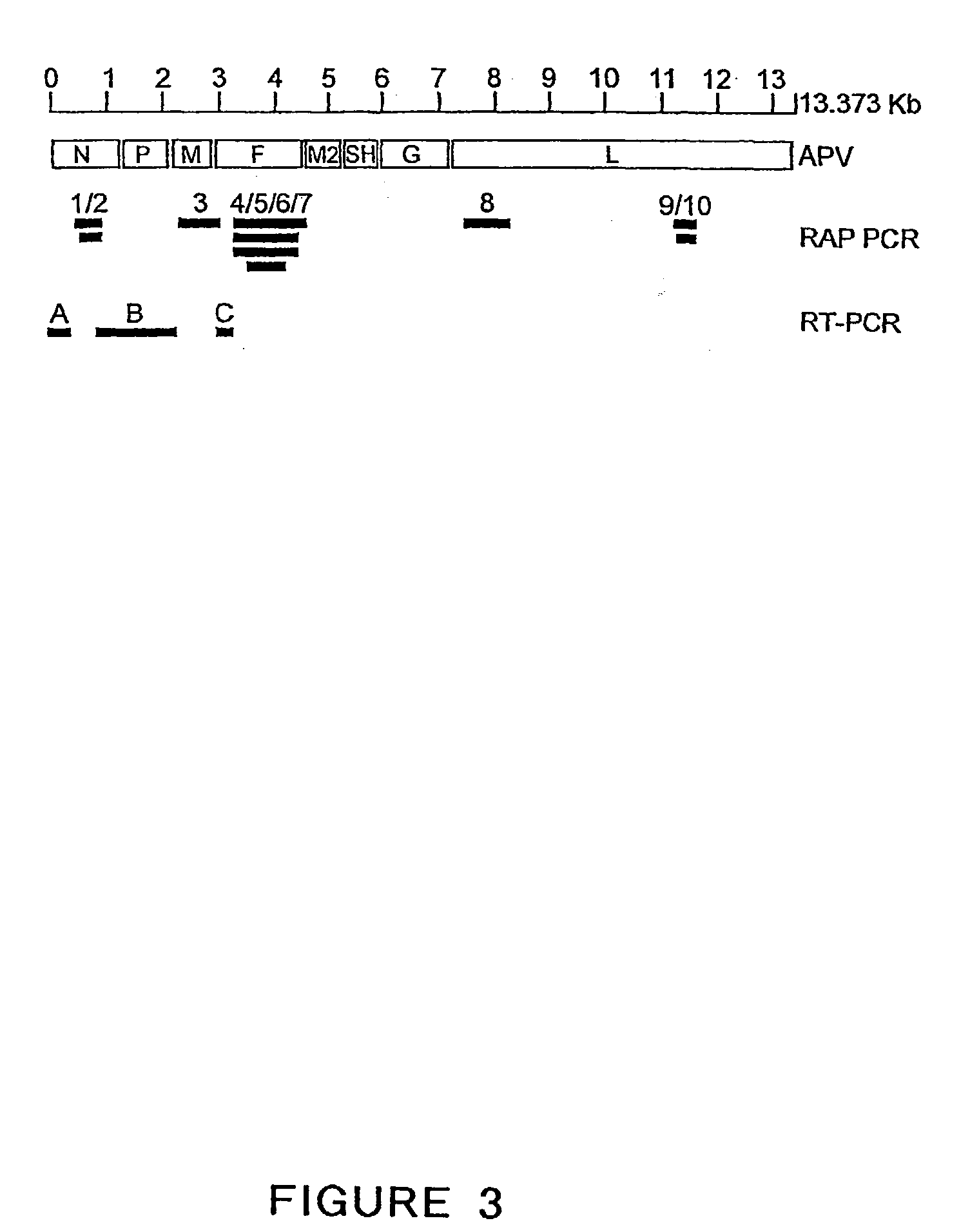
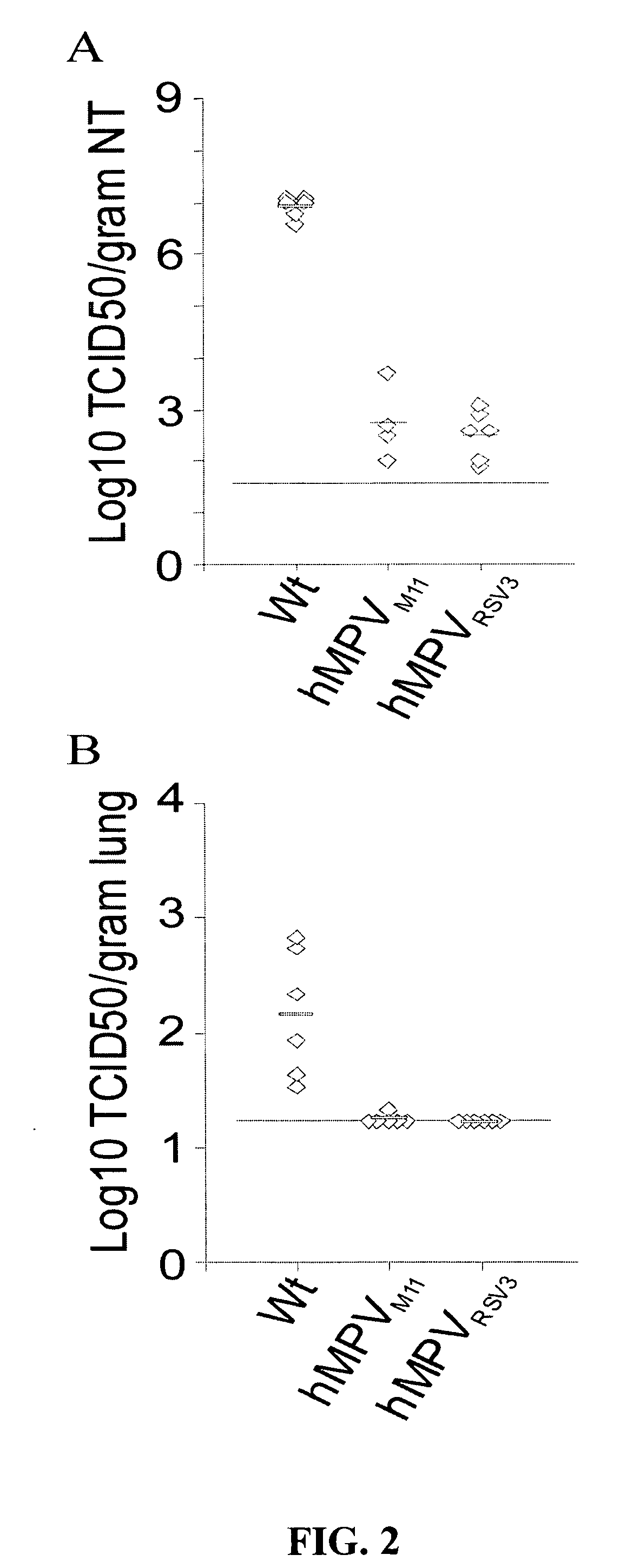
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. The invention also provide methods for propagating virus.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Pcv2 mycoplasma hyopneumoniae immunogenic compositions and methods of producing such compositions
InactiveUS20090317423A1Reduce incidenceEffective immunityViral antigen ingredientsAntiinfectivesDiseaseActive component
Multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of M. hyo infection, preferably M. hyo bacterin, or an immunogenic composition comprising M. hyo bacterin, and at least one immunogenic active component of another disease-causing organism in swine, preferably PCV2 wherein the preferred PCV2 antigen for such a multivalent vaccine is PCV2 ORF 2 protein.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Methods for the production of non-covalently complexed and multivalent proteosome sub-unit vaccines
InactiveUS6476201B1Shorten the timeIncrease temperatureAntibacterial agentsOrganic active ingredientsContinuous monitoringContamination
A continuous method for preparing proteosome-amphiphilic determinant vaccines for parenteral or mucosal administration using diafiltration or ultrafiltration technology. The amphiphilic determinants include lipopolysaccharides from gram negative bacteria, e.g. S. flexneri, P. shigelloides and S. sonnei. Proteosomes are obtained from group B type 2b meningococci. The active proteosome-amphiphilic determinant complexes (non-covalent complexes) of the vaccine are formed using diafiltration or ultrafiltration to remove the detergent under non-static conditions. The use of diafiltration or ultrafiltration decreases processing time and the opportunity for contamination and further permits the use of ambient temperature and efficient scale-up. In addition, the process permits the reliable and continuous monitoring of the dializate which enhances the efficiency of the entire process. The time of dialysis for the production of a lot of vaccine is reduced from 7-10 days to less than 72 hours and usually less than 48 or 24 hours. The use of the process optimizes the presence of each antigenic component in the preparation of multivalent vaccines.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +1
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
ActiveUS20040005544A1Narrow downSymptoms improvedOrganic active ingredientsFungiHeterologousNegative strand
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
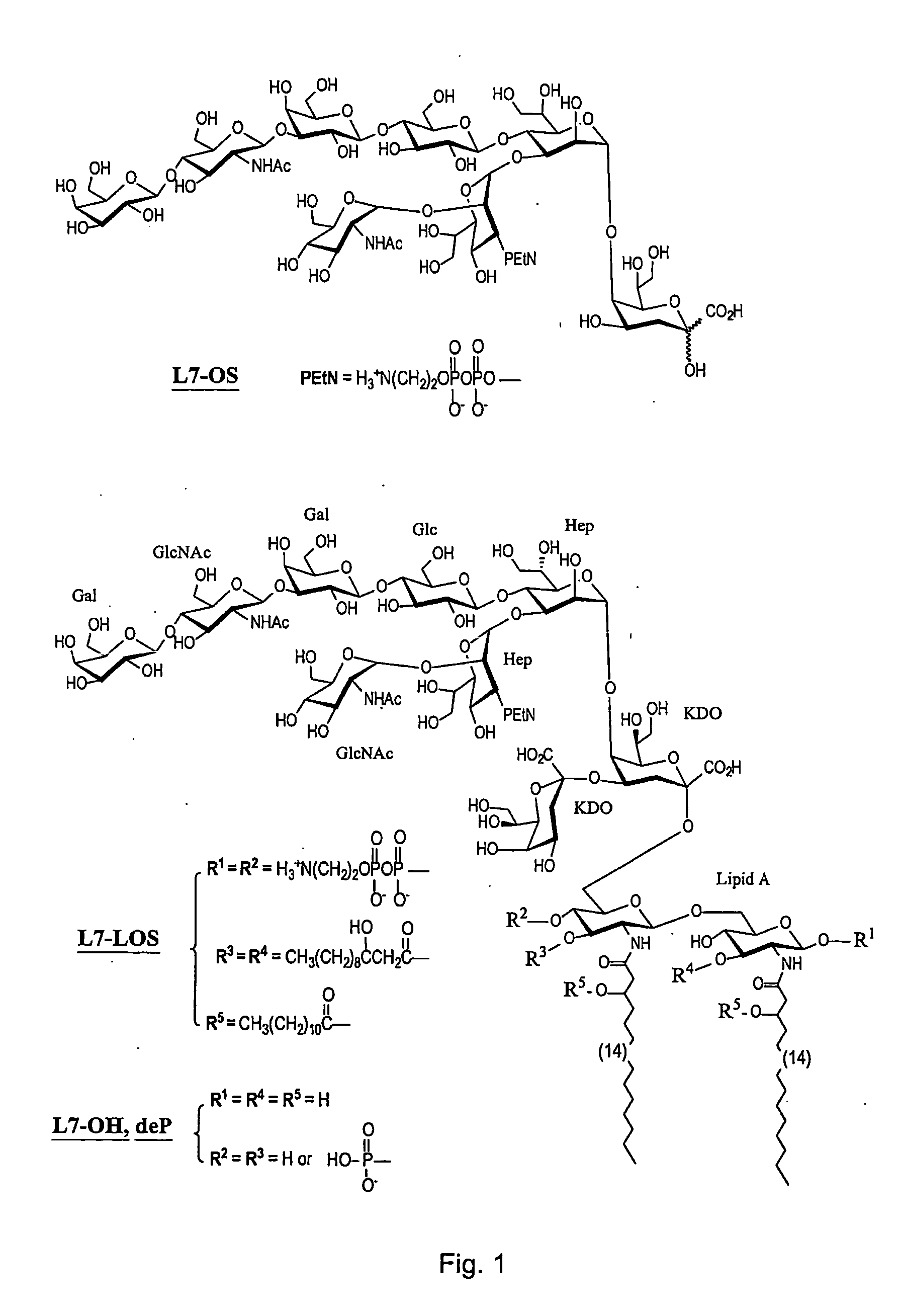
Synthesis of lipopolysaccharide-protein conjugate vaccines via the lipid a region following removal of the glycosidic phosphate residue
InactiveUS20050147624A1Avoid bacterial infectionImproving immunogenicityBacterial antigen ingredientsPeptide preparation methodsConjugate vaccinePhosphorylation
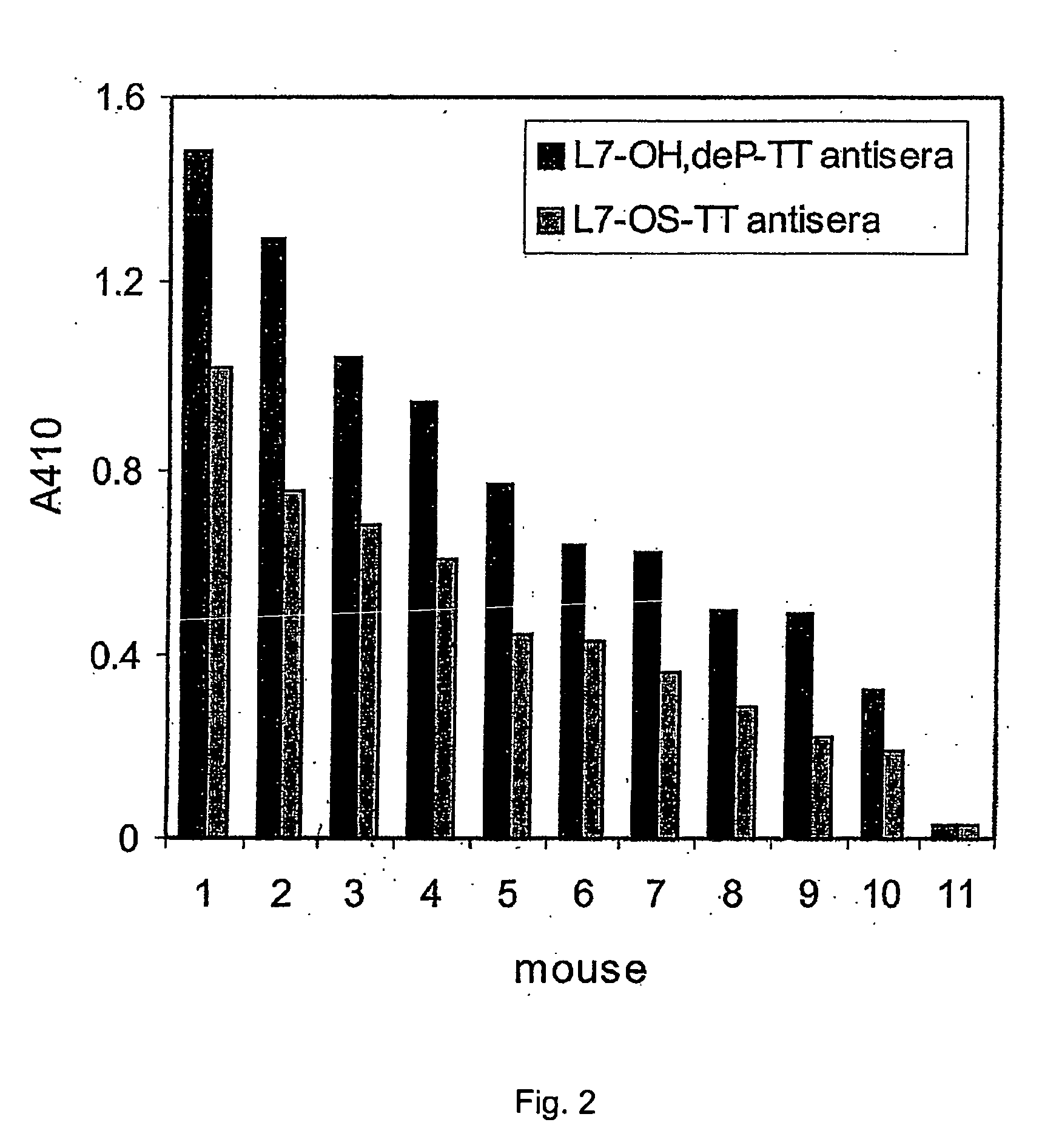
This invention relates to lipopolysaccharide-protein conjugate vaccines with appropriate presentation of conserved inner core oligosaccharide epitopes having improved immunogenic properties. These are based upon antigenic, detoxified bacterial lipopolysaccharides which optimally present an inner core oligosaccharide epitope following removal of at least a glycosidic phosphate of the lipid A region. These partially or completely dephosphorylated antigenic, detoxified bacterial lipopolysaccharides are linkable to an immunologically acceptable carrier and can be used in polyvalent or multivalent vaccines.
Owner:THE CHANCELLOR MASTERS & SCHOLARS OF THE UNIV OF OXFORD +1
Hemorrhagic feline calicivirus, calicivirus vaccine and method for preventing calicivirus infection or disease
The present invention relates to a novel, isolated and purified hemorrhagic feline calicivirus FCV-DD1. The invention further embraces monovalent and multivalent vaccines containing the new FCV-DD1 strain. In addition, the invention encompasses methods of protecting felines against infection or preventing disease caused by feline calicivirus alone or in addition to other pathogens that comprises administering to the felines an immunologically effective amount of the monovalent and multivalent vaccines described herein. Also, the invention concerns methods for diagnosing or detecting the hemorrhagic feline calicivirus in a susceptible host, asymptomatic carrier and the like by detecting the presence of feline calicivirus FCV-DD1 or antibodies raised or produced against feline calicivirus FCV-DD1 antigen.
Owner:ELANCO US INC
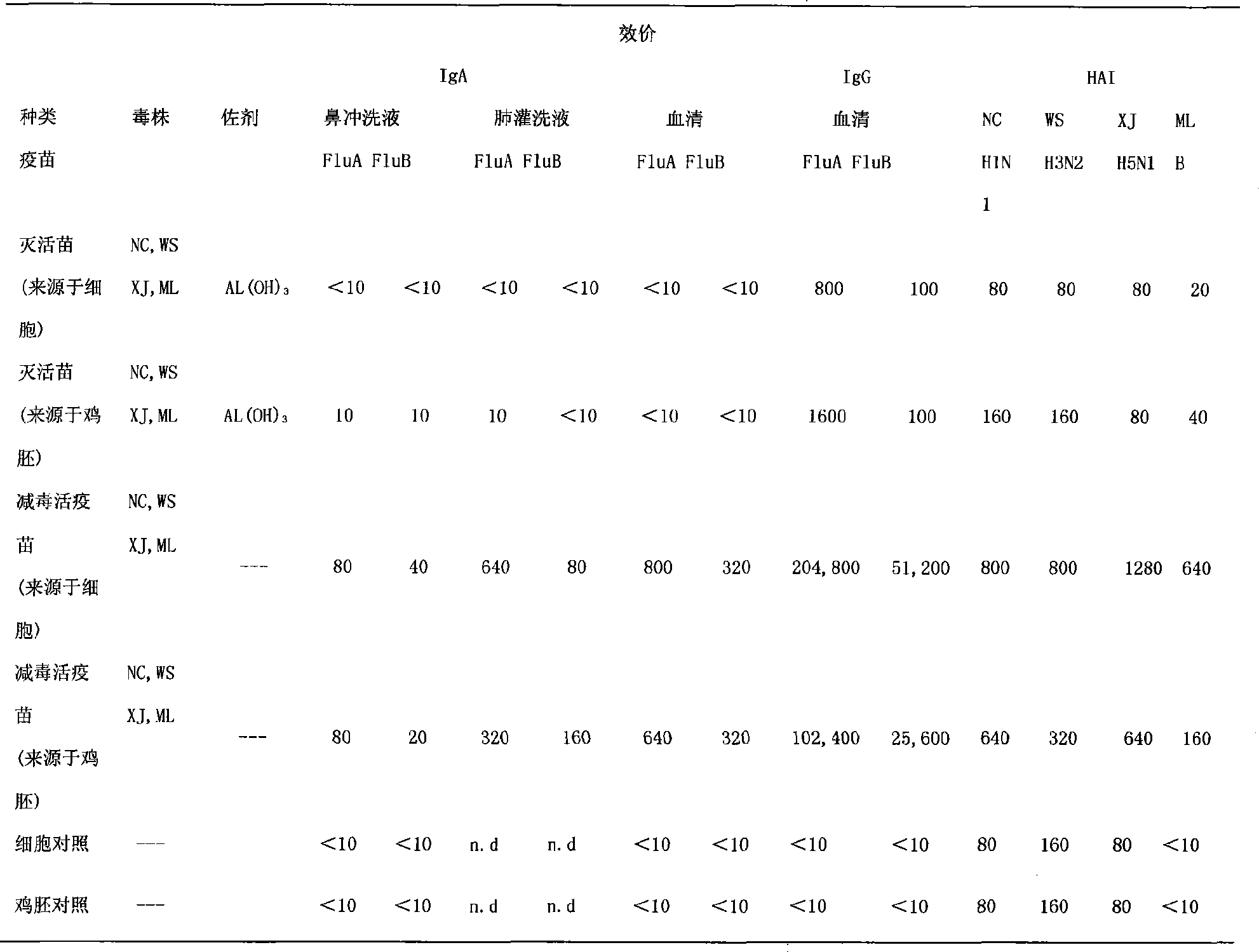
Preparation of nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and application thereof
InactiveCN101732711ANo side effectsAntiviralsViruses/bacteriophagesVirus-like particleMultivalent Vaccine
The invention discloses a nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and preparation method thereof. The vaccine is inactivated vaccine antigen of totivirus, lytic virus, viron or virus-like particles, flue multivalent vaccine antigen is flue pentavalent, namely H1N1, H3N2, B, H5N1 and A (H1N1) or multivalent vaccine antigen combined on the basis at will, or flue multivalent vaccine antigen obtained by containing all the combination of the HA selecting from H1, H2, H3, H4, H5, H6, H7, H8, H9, H10, H11, H12, H13, H14, H15 and H16 and the NA selecting from N1, N2, N3, N4, N5, N6, N7, N8 and N9 subtypes on the basis. The content of flu multivalent inactivated vaccine antigen HA in the vaccine of the invention is 1.0-15.0 Mug / 0.2ml / per person, and the vaccine of the invention can effectively prevent routine human flue, high pathogenicity H5N1 avian-human flu, influenza A (H1N1) and infection of other subtype influenza viruses.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences and methods for propagating virus
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. The invention also provide methods for propagating virus.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
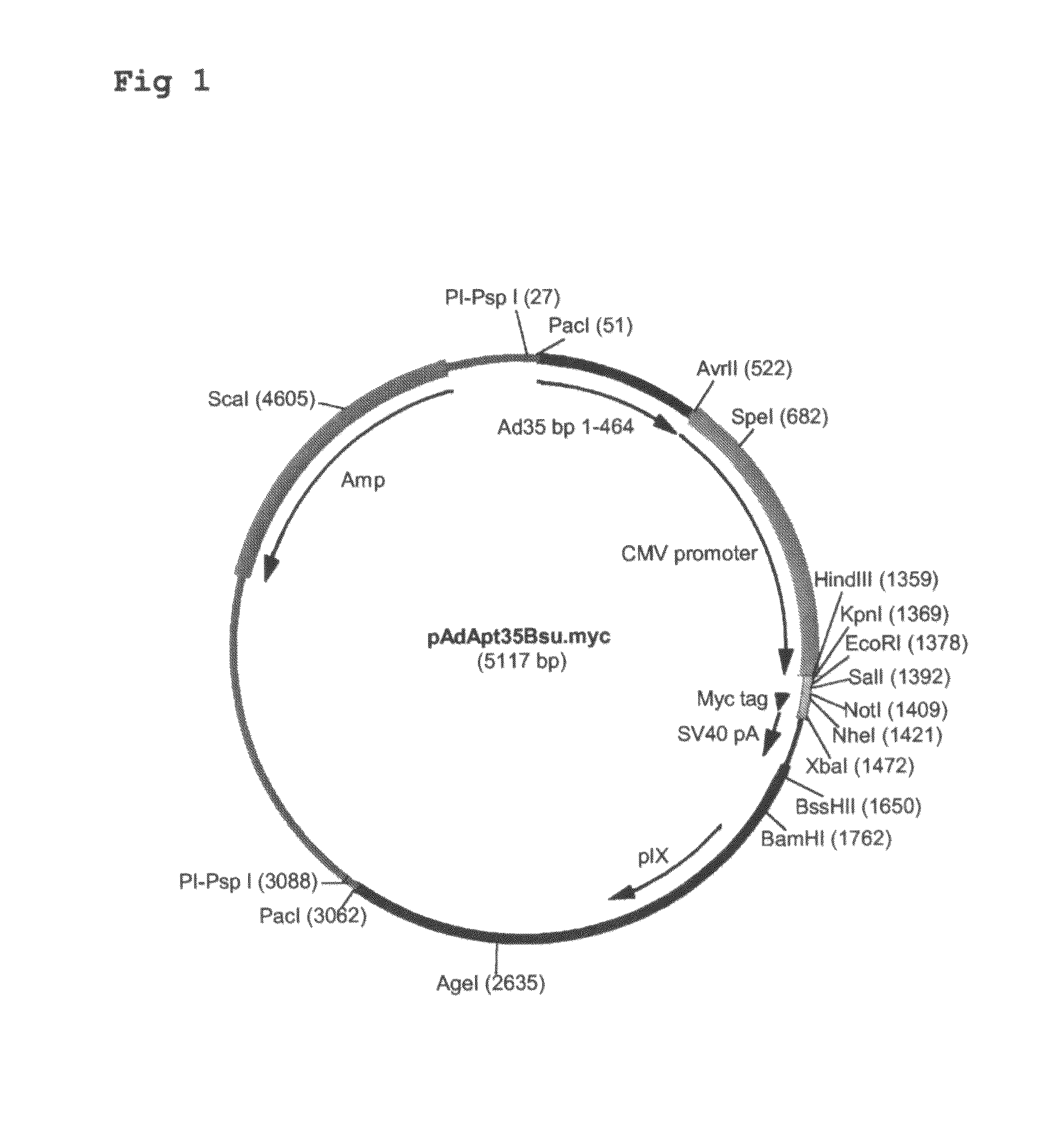
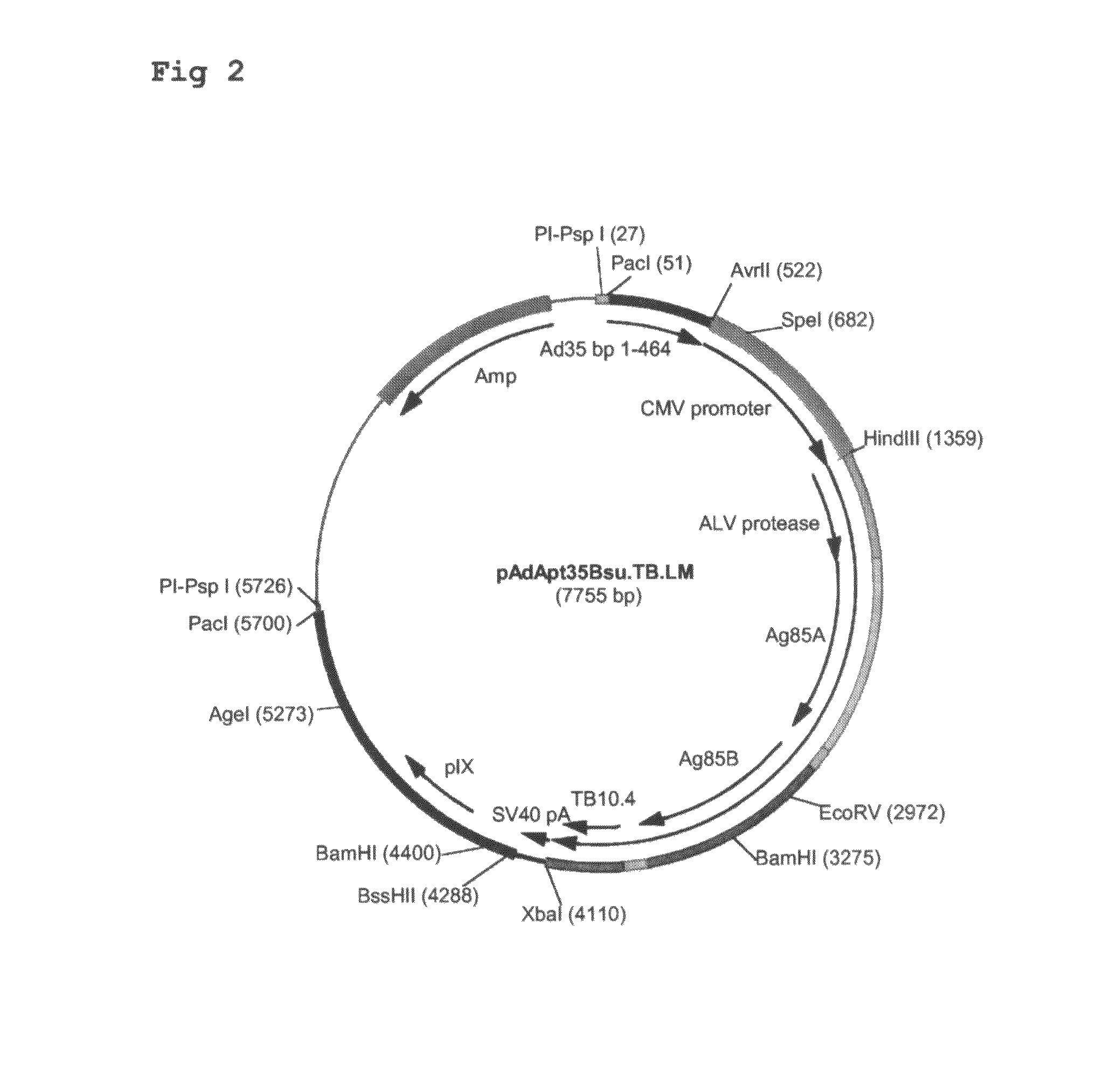
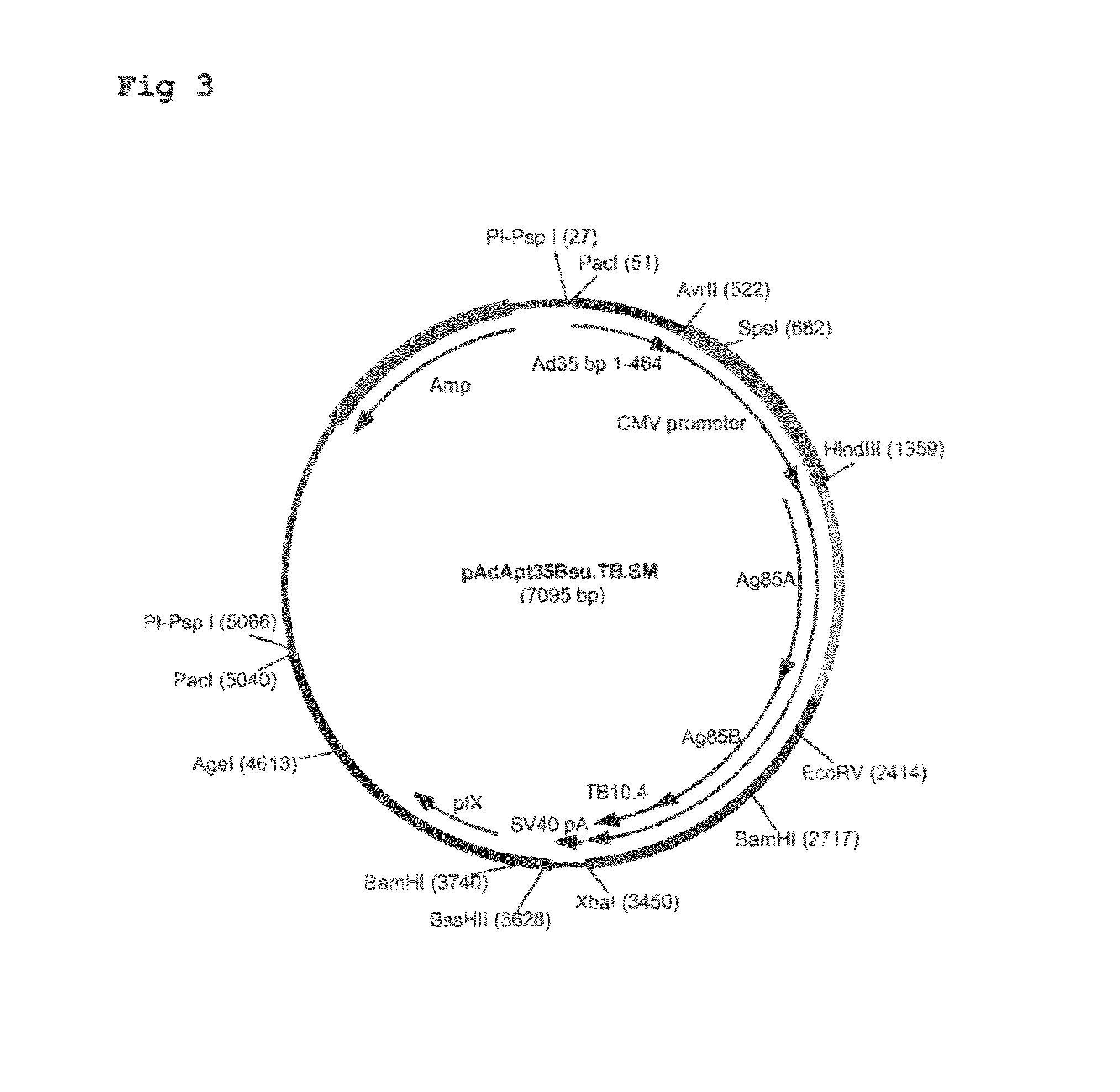
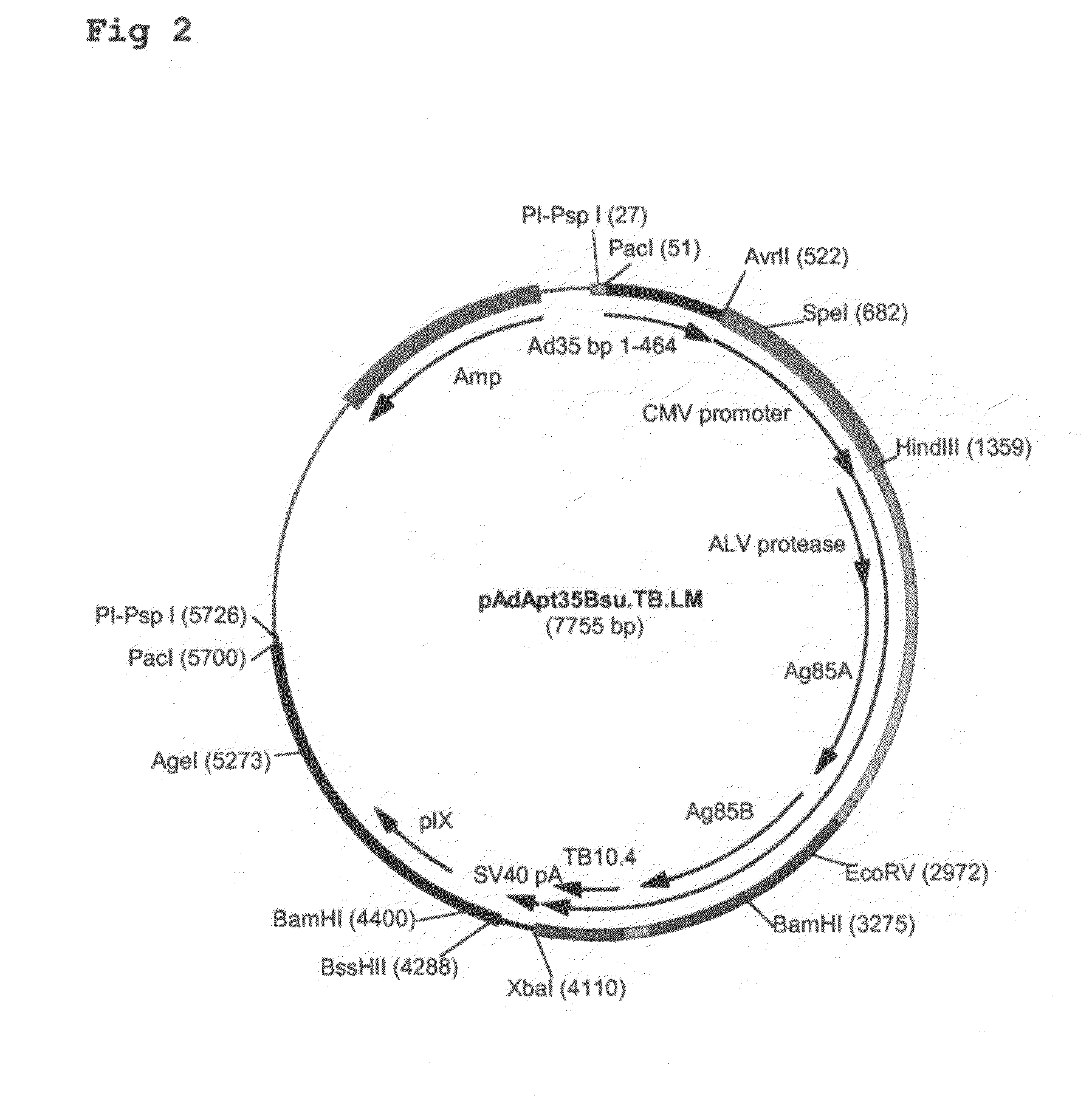
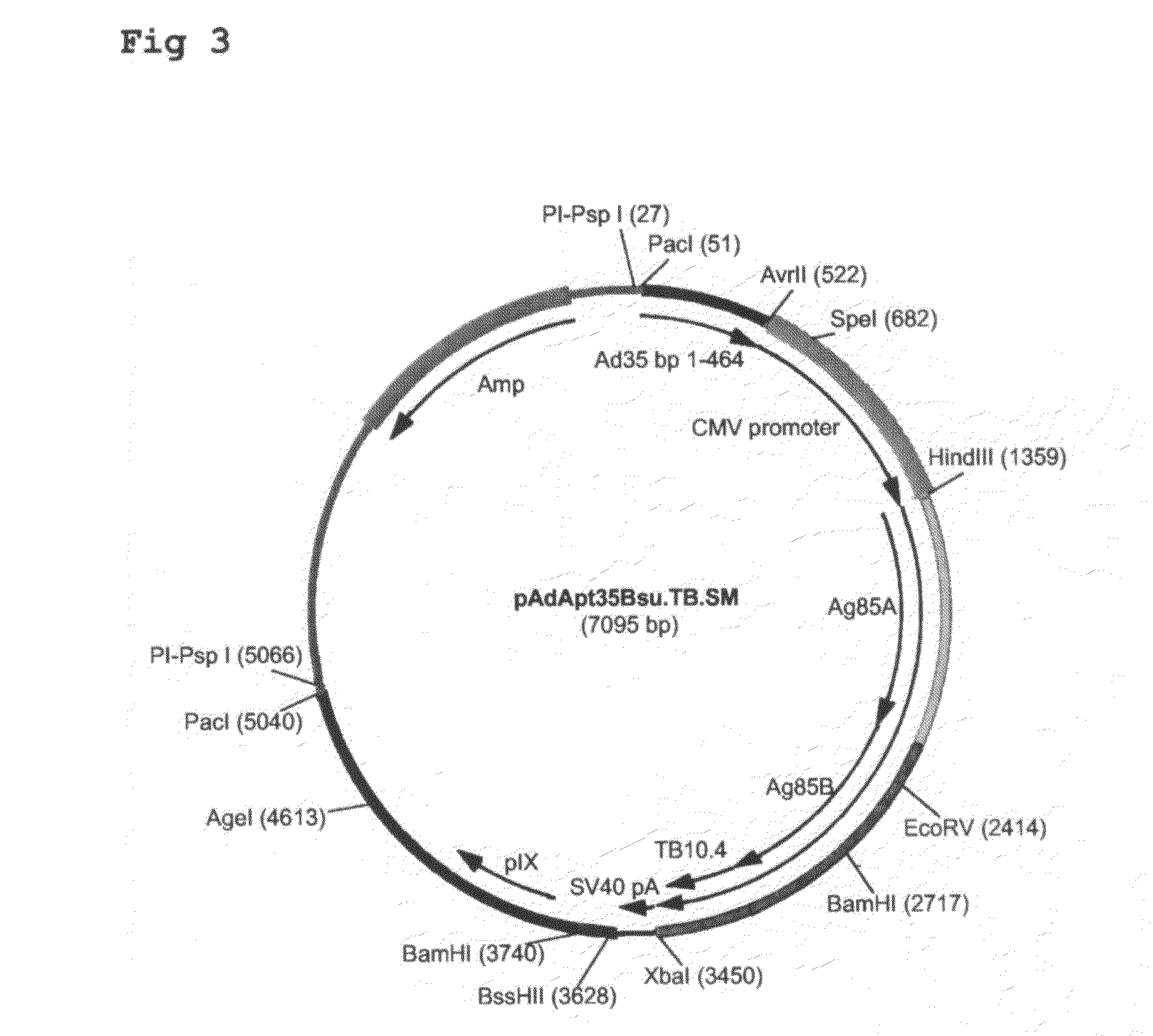
Multivalent vaccines comprising recombinant viral vectors
The invention relates to vaccines comprising recombinant vectors, such as recombinant adenoviruses. The vectors comprise heterologous nucleic acids encoding for at least two antigens from one or more tuberculosis-causing bacilli. The invention also relates to the use of specific protease recognition sites linking antigens through which the encoded antigens are separated upon cleavage. After cleavage, the antigens contribute to the immune response in a separate manner. The recombinant vectors may comprise a nucleic acid encoding the protease cleaving the linkers and separating the antigens. The invention furthermore relates to the use of genetic adjuvants encoded by the recombinant vectors, wherein such genetic adjuvants may also be cleaved through the presence of the cleavable linkers and the specific protease.
Owner:AERAS GLOBAL TB VACCINE FOUND +1
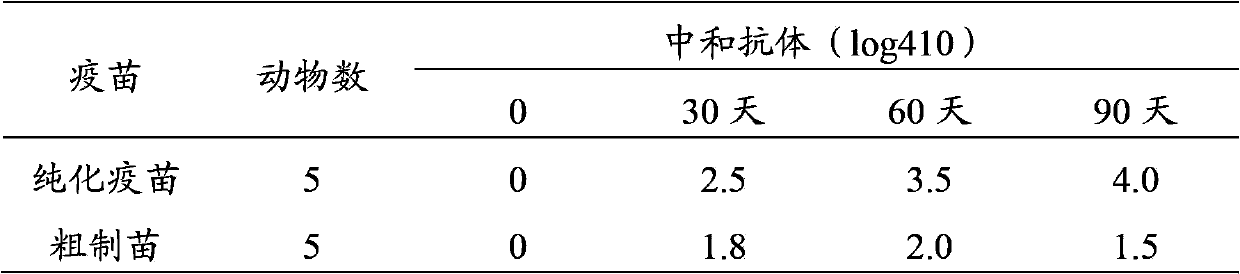
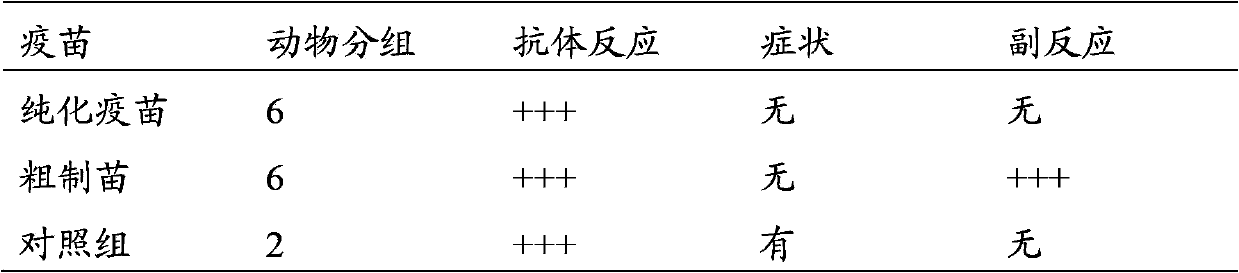
Method for preparing purified foot-and-mouth disease vaccine
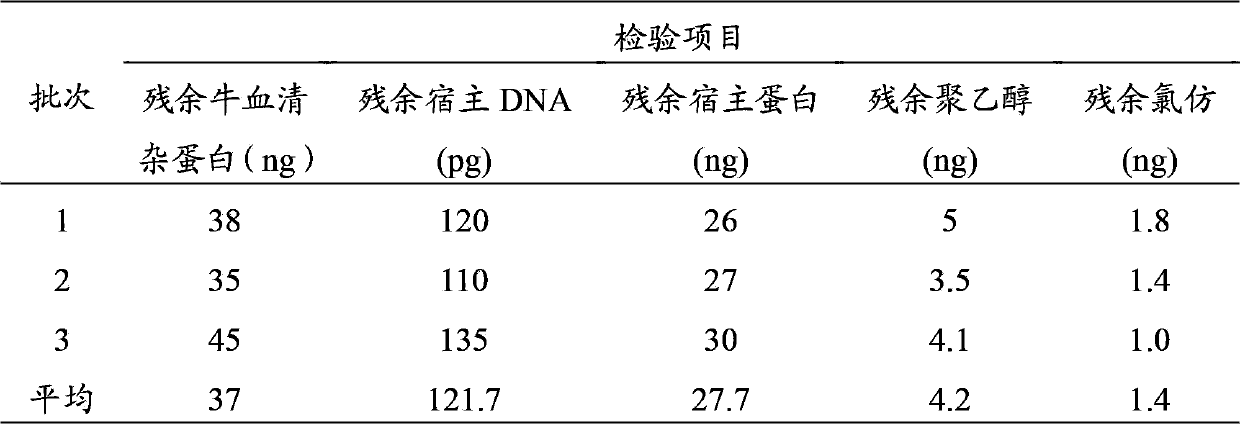
InactiveCN103374547ARule out emergency responseReduced risk of contamination with exogenous agentsAntiviralsVertebrate cellsContinuous flow centrifugationSaccharum
The invention discloses a method for preparing a purified foot-and-mouth disease vaccine, a serum or animal-derived ingredient free culture medium and an application of the serum or animal-derived ingredient free culture medium to the preparation of the foot-and-mouth disease vaccine, belonging to the filed of biotechnology. The method for preparing the purified foot-and-mouth disease vaccine comprises the following steps of: culturing a foot-and-mouth disease virus by using the serum or animal-derived ingredient free culture medium, purifying an obtained virus solution to obtain a purified antigen, subjecting a cell strain BHK-21 or BSR to the multiple-generation acclimatization culture and the suspension culture by 300L of a microcarrier through the serum-free culture medium, inoculating the cell strain BHK-21 or BSR against the foot-and-mouth disease vaccine, stirring at the rotating speed of 30-50rpm, microfiltrating, ultrafiltrating, concentrating 50-200 times, carrying out chromatography with a Sephawse6FF molecular sieve or density gradient zonal centrifugation with a continuous flow, and inactivating with beta-propiolactone to obtain the serotype univalent or multivalent vaccine for cattle, sheep and pigs.
Owner:北京必威安泰科技有限公司 +1
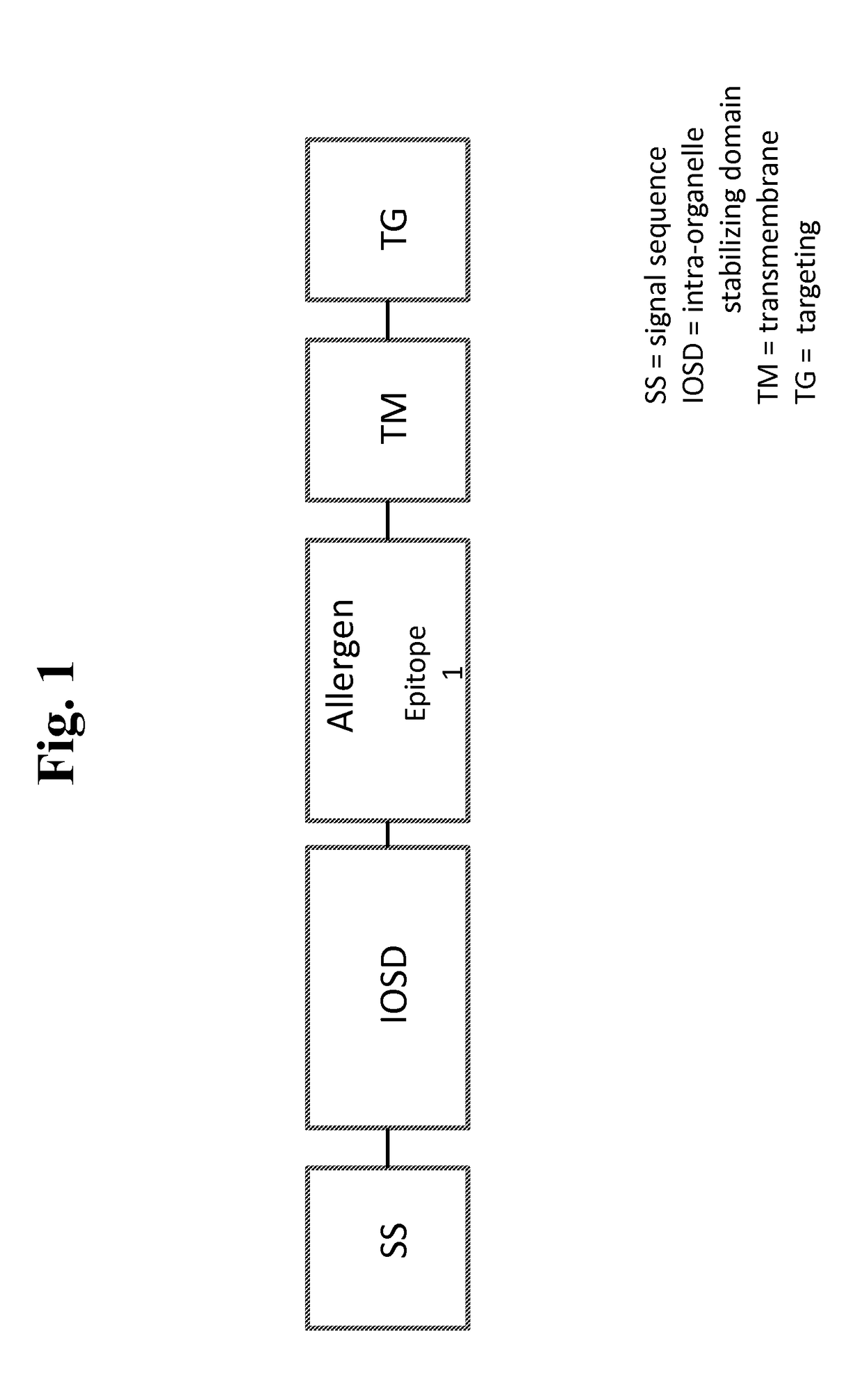
Nucleic acids for treatment of allergies
ActiveUS9744230B2Genetic material ingredientsFusions for enhanced expression stability/foldingComplete proteinEpitope
Owner:IMMUNOMIC THERAPEUTICS
Pcv2 mycoplasma hyopneumoniae immunogenic compositions and methods of producing such compositions
ActiveUS20150174233A1Reduce incidenceReduce severityBacterial antigen ingredientsViral antigen ingredientsDiseaseMultivalent Vaccine
Multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of M. hyo infection, preferably M. hyo bacterin, or an immunogenic composition comprising M. hyo bacterin, and at least one immunogenic active component of another disease-causing organism in swine, preferably PCV2 wherein the preferred PCV2 antigen for such a multivalent vaccine is PCV2 ORF 2 protein.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
HPV CD8+ T-cell epitopes
InactiveUS7153659B2Improving immunogenicitySugar derivativesPeptide/protein ingredientsEpitopeHPV vaccines
The present invention provides means to identify functional CD8+ T-cell epitopes in any protein of interest. The present invention further provides CD8+ T-cell epitopes of various proteins. In additional embodiments, the present invention provides epitopes suitable for use in prophylactic and / or therapeutic vaccines. In particularly preferred embodiments, the present invention provides modified epitopes suitable for use in prophylactic and / or therapeutic vaccines.In some preferred embodiments, the present invention provides means for the development of HPV vaccines, in particular multivalent vaccines for the prevention of infection with high-risk HPV strains. In particular, the present invention provides means to identify CD8+ T-cell epitopes in HPV strains such as HPV 16 and HPV 18. In additional embodiments, the present invention provides means for the development of therapeutic vaccines against high-risk HPV types that prevent the development of benign and / or malignant tumors in infected individuals. The present invention further provides epitopes suitable for use in prophylactic and therapeutic vaccines.
Owner:GENENCOR INT INC
Large-scale full-suspension culture method of porcine circovirus type 2
ActiveCN107312746AReduce dosageImprove immunityViral antigen ingredientsMicroorganism based processesEngineeringPorcine kidney
The invention discloses a large-scale full-suspension production method of a porcine circovirus type 2. The inventors of the invention domesticate a porcine kidney cell adaptable to large-scale serum-free full-suspension culture, which is named as sPK15-YP, is collected in the China General Microbiological Culture Collection Center and has the culture collection number of CGMCC NO.13846. The sPK15-YP cell adaptable to full-suspension culture is used for achieving serum-free large-scale culture of the porcine circovirus type 2 (PCV2), so that the conventional spinner bottle culture technology is replaced, the human resource is reduced, the product quality is improved, and the bottleneck that the virus content is low during PCV2 full-virus culture is solved; by a full-suspension sPK15-YP cell technology, a high-potency PCV2 semifinished product is prepared; by a hollow fiber method, a PCV2 virus culture solution is concentrated and purified to obtain a more pure PCV2 virus concentrated antigen. By the large-scale full-suspension production method, a solid foundation is laid for studying a PCV2 multivalent vaccine, the dosage of the vaccine is reduced, the stress of a swine herd is reduced and the immune level of the swine herd is enhanced.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
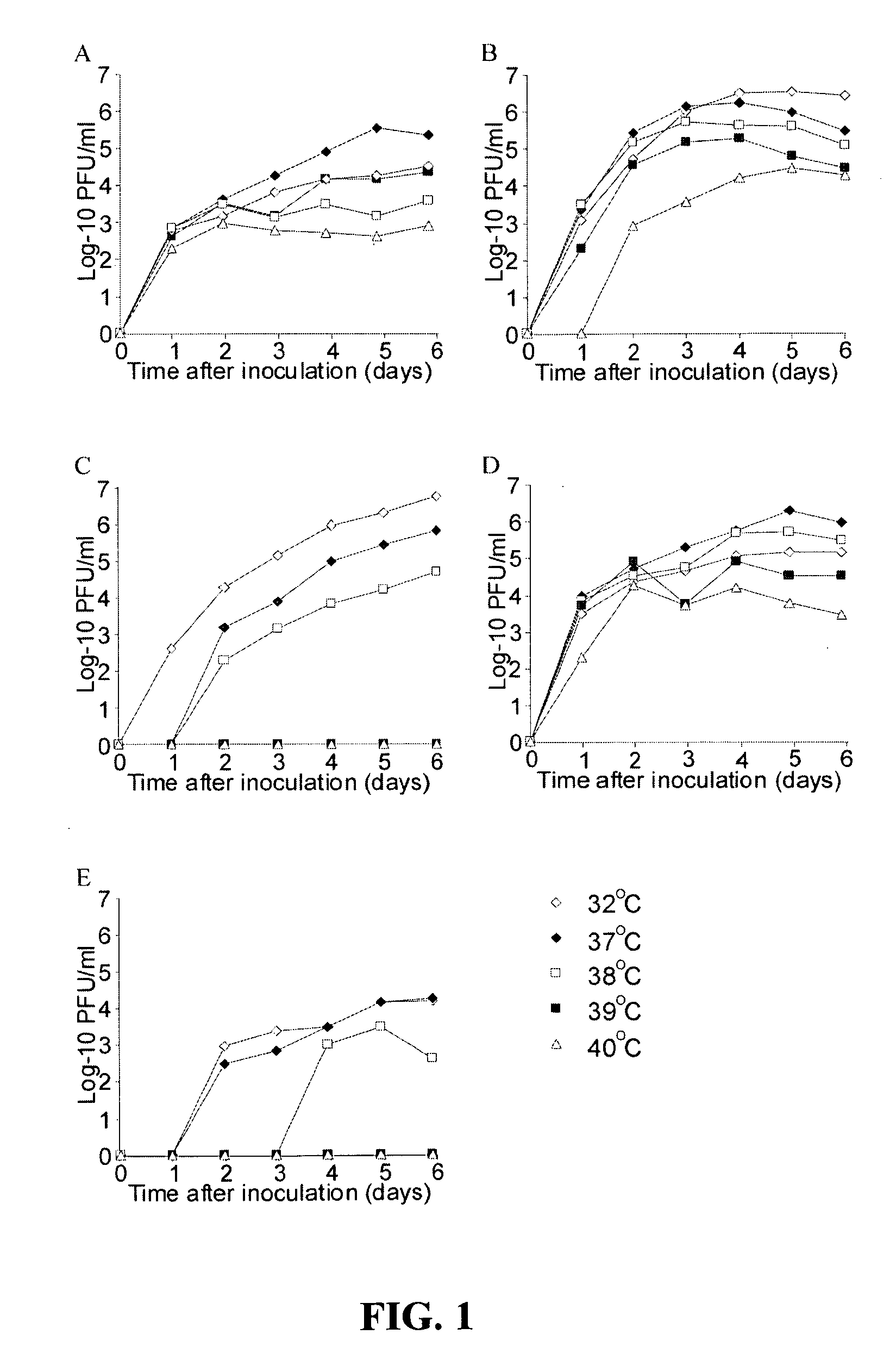
Live attenuated metapneumovirus strains and their use in vaccine formulations and chimeric metapneumovirus strains
The invention relates to an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae with one or more genetic modifications. The present invention also relates to the mutant components, i.e., nucleic acids and proteins, of these mutant mammalian MPVs. These mutant mMPV can be attenuated. These mutant mMPVs can encode non-native sequences. The invention further relates to vaccine formulations comprising the mMPV, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. In addition, the invention relates to chimeric viral RNA polymerase complex and assays using these chimeric RNA polymerase complexes. The chimeric RNA polymerase complexes of the invention are composed of different RNA polymerase components from different viruses of the family of paramyxoviridae.
Owner:VIRONOVATIVE
Multivalent vaccine for filariasis
ActiveCN103298485AProtozoa antigen ingredientsPeptide/protein ingredientsWAS PROTEINMultivalent Vaccine
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Virus-like particle vaccines
InactiveUS20160185826A1Easy to foldStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMultivalent VaccineViral structural protein
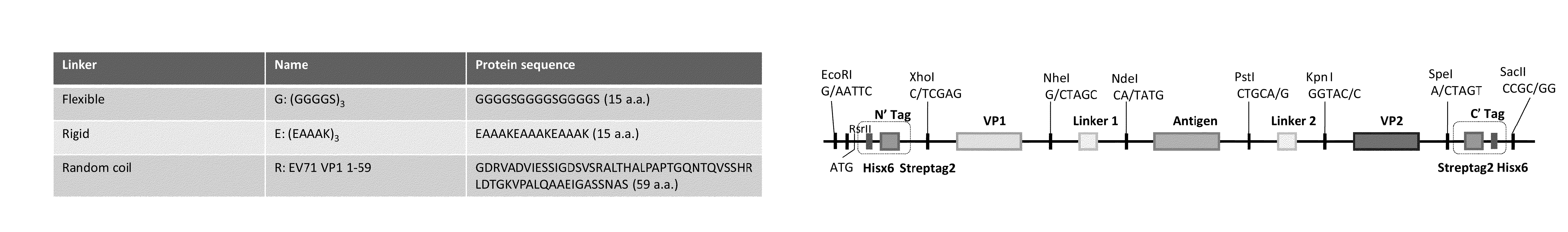
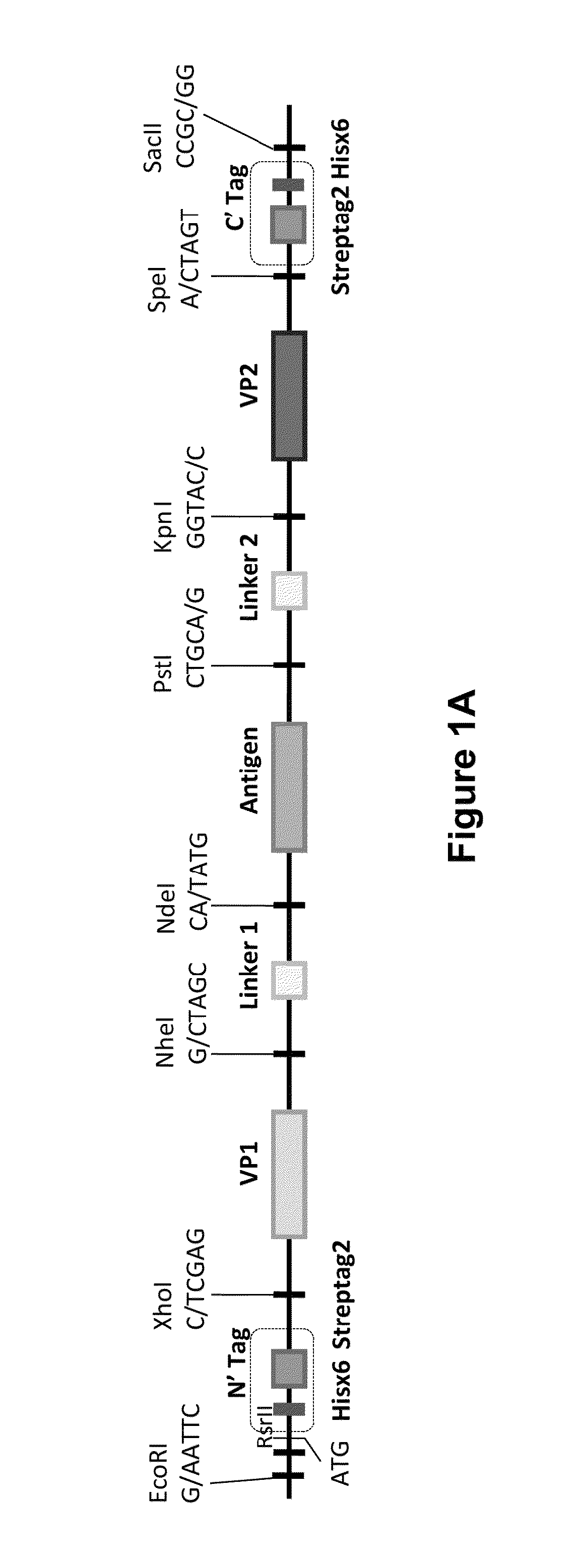
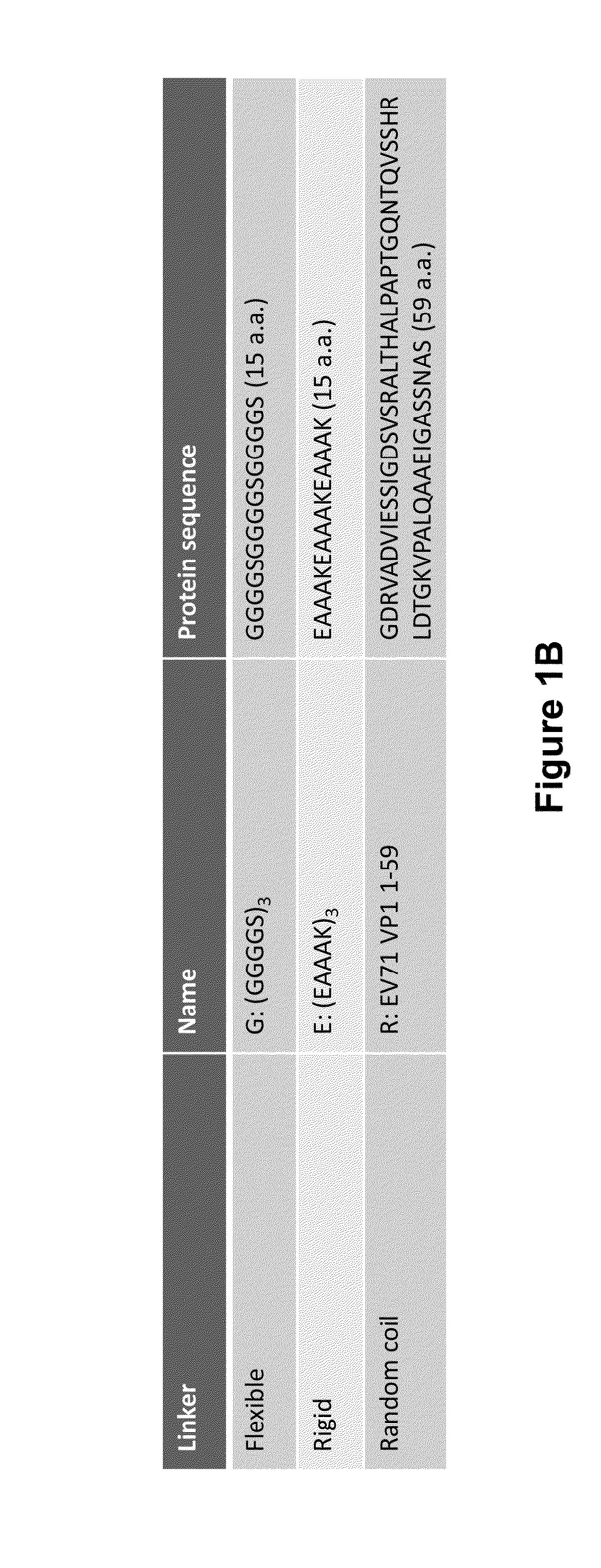
The invention is directed to dimeric fusion proteins and virus-like particles comprising such dimeric fusion proteins. These dimeric fusion proteins comprise an antigen or antigenic fragment carried between two viral structural proteins or fragments thereof, with or without linkers, in a manner that, relative to traditional monomeric platforms, minimizes steric hindrance among the antigen or antigenic fragment and the viral structural proteins or fragments thereof. This novel design provides for multivalent vaccines and enhanced immunogenicity. The invention also relates to nucleic acids encoding such dimeric fusion proteins and host cells comprising such nucleic acids. The invention further relates to pharmaceutical compositions comprising the dimeric fusion proteins and / or virus-like particles of the invention, and methods of prevention or treatment using such compositions.
Owner:MEDIGEN BIOTECH
Multivalent Vaccines Comprising Recombinant Viral Vectors
The invention relates to vaccines comprising recombinant vectors, such as recombinant adenoviruses. The vectors comprise heterologous nucleic acids encoding for at least two antigens from one or more tuberculosis-causing bacilli. The invention also relates to the use of specific protease recognition sites linking antigens through which the encoded antigens are separated upon cleavage. After cleavage, the antigens contribute to the immune response in a separate manner. The recombinant vectors may comprise a nucleic acid encoding the protease cleaving the linkers and separating the antigens. The invention furthermore relates to the use of genetic adjuvants encoded by the recombinant vectors, wherein such genetic adjuvants may also be cleaved through the presence of the cleavable linkers and the specific protease.
Owner:AERAS GLOBAL TB VACCINE FOUND +1
Compositions comprising streptococcus pneumoniae polysaccharide-protein conjugates and methods of use thereof
ActiveUS20190192648A1Improve stabilityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseCarrier protein
The invention is related to multivalent immunogenic compositions comprising more than one S. pneumoniae polysaccharide protein conjugates, wherein each of the conjugates comprises a polysaccharide from an S. pneumoniae serotype conjugated to a carrier protein, wherein the serotypes of S. pneumoniae are as defined herein. In some embodiments, at least one of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. In further embodiments, each of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. Also provided are methods for inducing a protective immune response in a human patient comprising administering the multivalent immunogenic compositions of the invention to the patient. The multivalent immunogenic compositions are useful for providing protection against S. pneumoniae infection and diseases caused by S. pneumoniae. The compositions of the invention are also useful as part of treatment regimes that provide complementary protection for patients that have been vaccinated with a multivalent vaccine indicated for the prevention of pneumococcal disease.
Owner:MERCK SHARP & DOHME LLC
Prevention, treatment and detection of pig progressive atrophic rhinitis
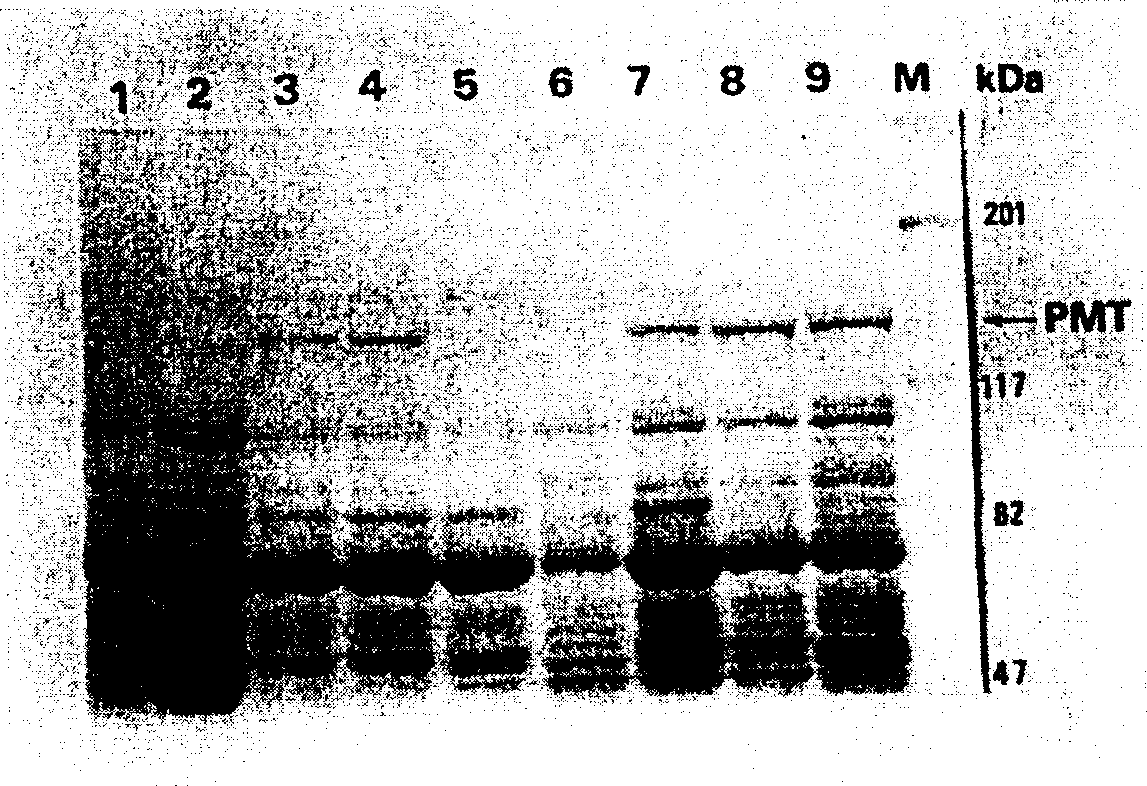
Disclosed is the animal vaccine for the prevention, treatment and detection of pig progressive atrophic rhinitis, which comprises three polypeptides generated by antibody against Pasteurella multocida related to progressive atrophic rhinitis, these polypeptides all have a amino acid sequence, which mainly corresponds to the 2-486, 486-986 or 986-1281 amino acid residue of the PMT protein. The invention also discloses the multivalent vaccine for the prevention and treatment of at least PAR for animals.
Owner:简茂盛
ExPEC glycoconjugate vaccine formulations
ActiveUS10159751B2Improve stabilityImproved stability against thermal stressInorganic non-active ingredientsCarrier-bound antigen/hapten ingredientsPseudomonas exotoxinEscherichia coli
Compositions and methods for inducing an immune response against extra-intestinal pathogenic Escherichia coli (ExPEC) are described. In particular, multivalent vaccines containing E. coli antigen polysaccharide covalently bound to a exotoxin A of Pseudomonas aeruginosa (EPA) carrier protein that can withstand multiple environmental stresses are described.
Owner:JANSSEN PHARMA INC
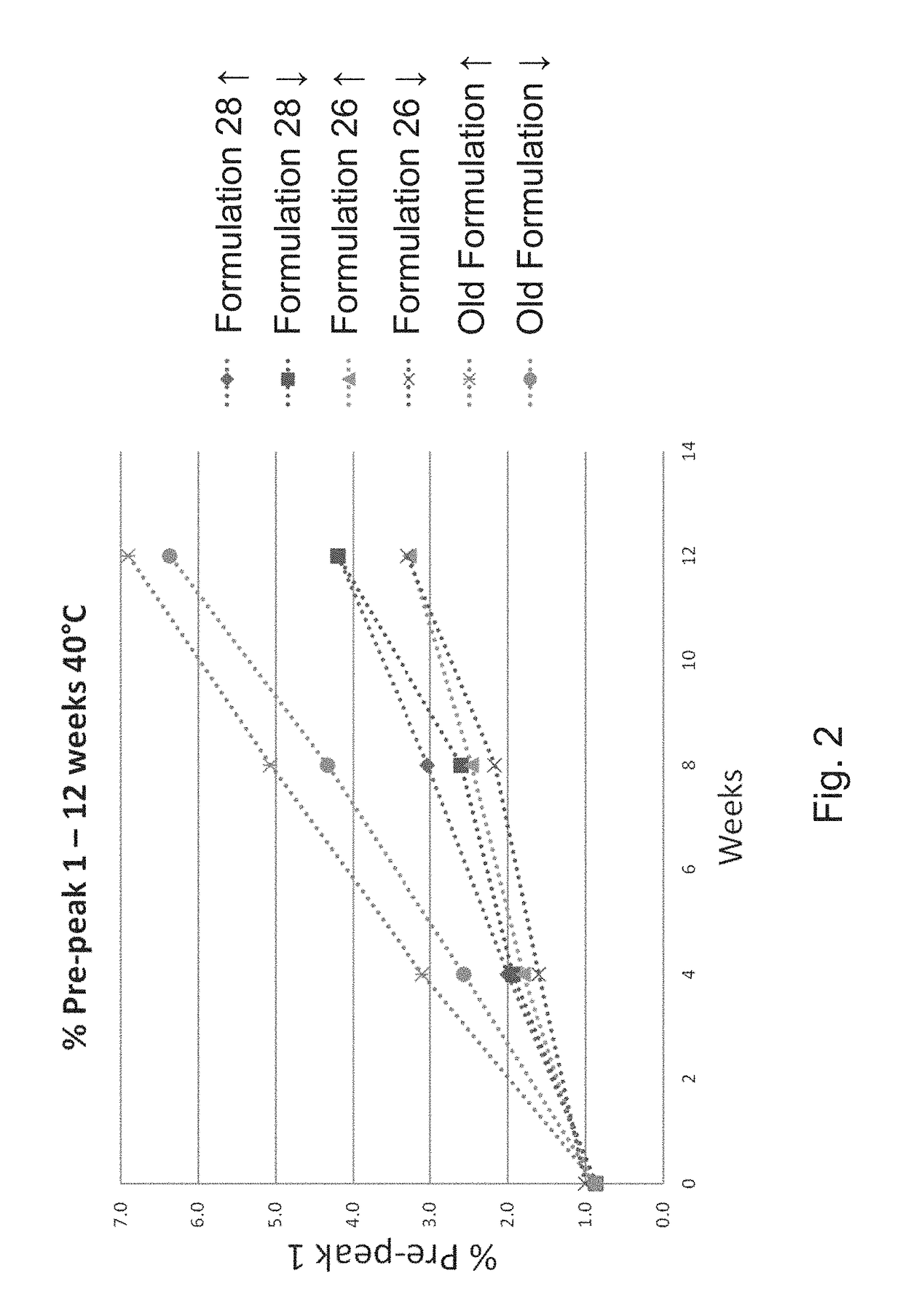
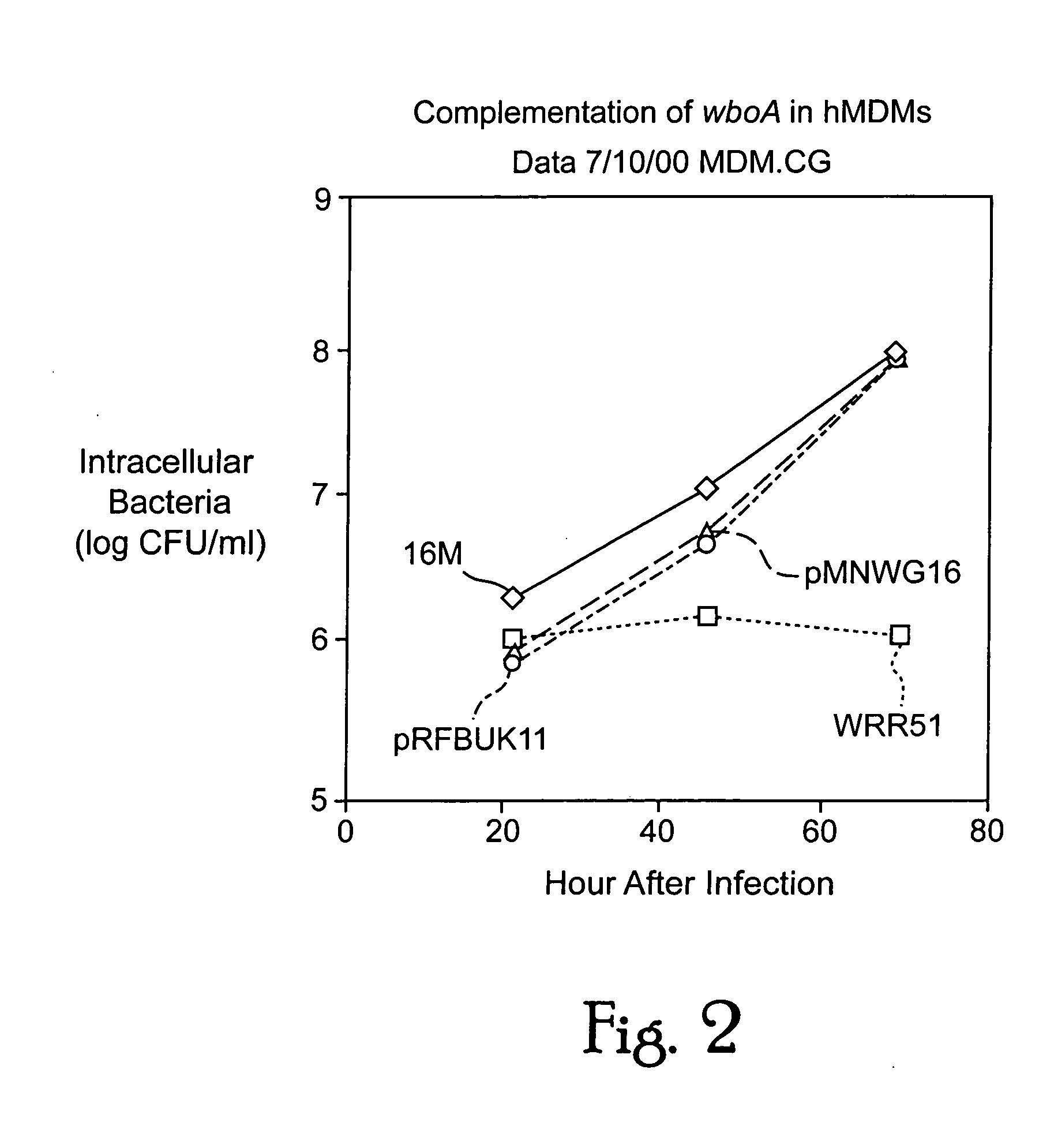
Immunogenic compositions including rough phenotype Brucella host strains and complementation DNA fragments
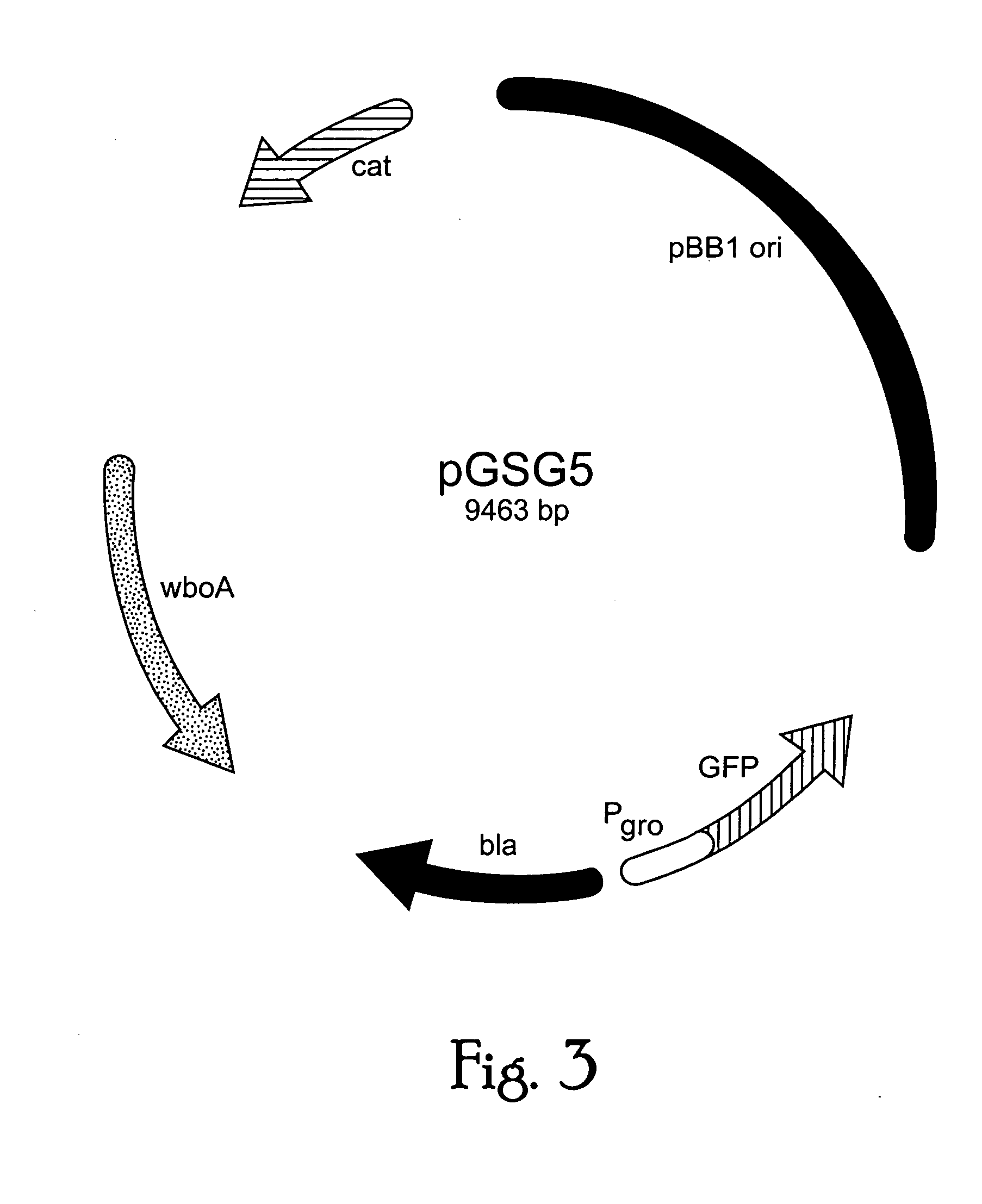
InactiveUS20050142151A1Sufficient attenuationEasy to operateBiocideBacterial antigen ingredientsHeterologousHeterologous Antigens
Live attenuated vaccines against brucellosis and infection by other diseases are described. It has been discovered that trans complementation of the Brucella wboA gene can be used to maintain an expression vector in an attenuated Brucella host cell in a vaccinee. Further, heterologous antigens can be expressed using this Brucella platform, thus effecting a multivalent vaccine against Brucella and the disease corresponding to the heterologous antigen.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
A high-tilter coxsackievirus A10 domesticated strain and applications thereof
InactiveCN107746832APrevent and/or treat diseaseFree from harmSsRNA viruses positive-senseViral antigen ingredientsHep 2 cellCoxsackie meningitis
A high-tilter coxsackievirus A10 domesticated strain TA151R-1 stable in passage is disclosed. The virus strain can infect RD cells, HEK293 cells, Vero cells, MRC-5 cells, Hep-2 cells, WI-38 cells, andother cell lines, and can be used for preparing a monovalent or polyvalent vaccine. The prepared vaccine can protect bodies from being harmed by coxsackievirus, can completely protect the body from attack by heterogenous viruses, can effectively prevent and / or treat diseases caused by coxsackievirus infection, and has a wide application prospect.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Nasal-spraying immune influenza multivalent vaccine and preparation method thereof
InactiveCN101450208AEasy to useLess antigenPowder deliveryAerosol deliveryHighly Pathogenic Avian Influenza VirusInfluenza a
The invention discloses a nasal spray immunity flu polyvalent vaccine and a preparing method. Flu quadrivalence (H1N1, H3N2, H5N1, B type) or polyvalent attenuated live vaccine is used for immunoprophylaxis through nose spraying, popular flu and highly pathogenic avian influenza can be prevented safely and efficiently.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Protein complex system for increased immunogenicity and functionality, and methods making and use
ActiveUS20140017269A1Improving immunogenicityImprove functionalitySsRNA viruses negative-senseSsRNA viruses positive-senseEscherichia coliRotavirus RNA
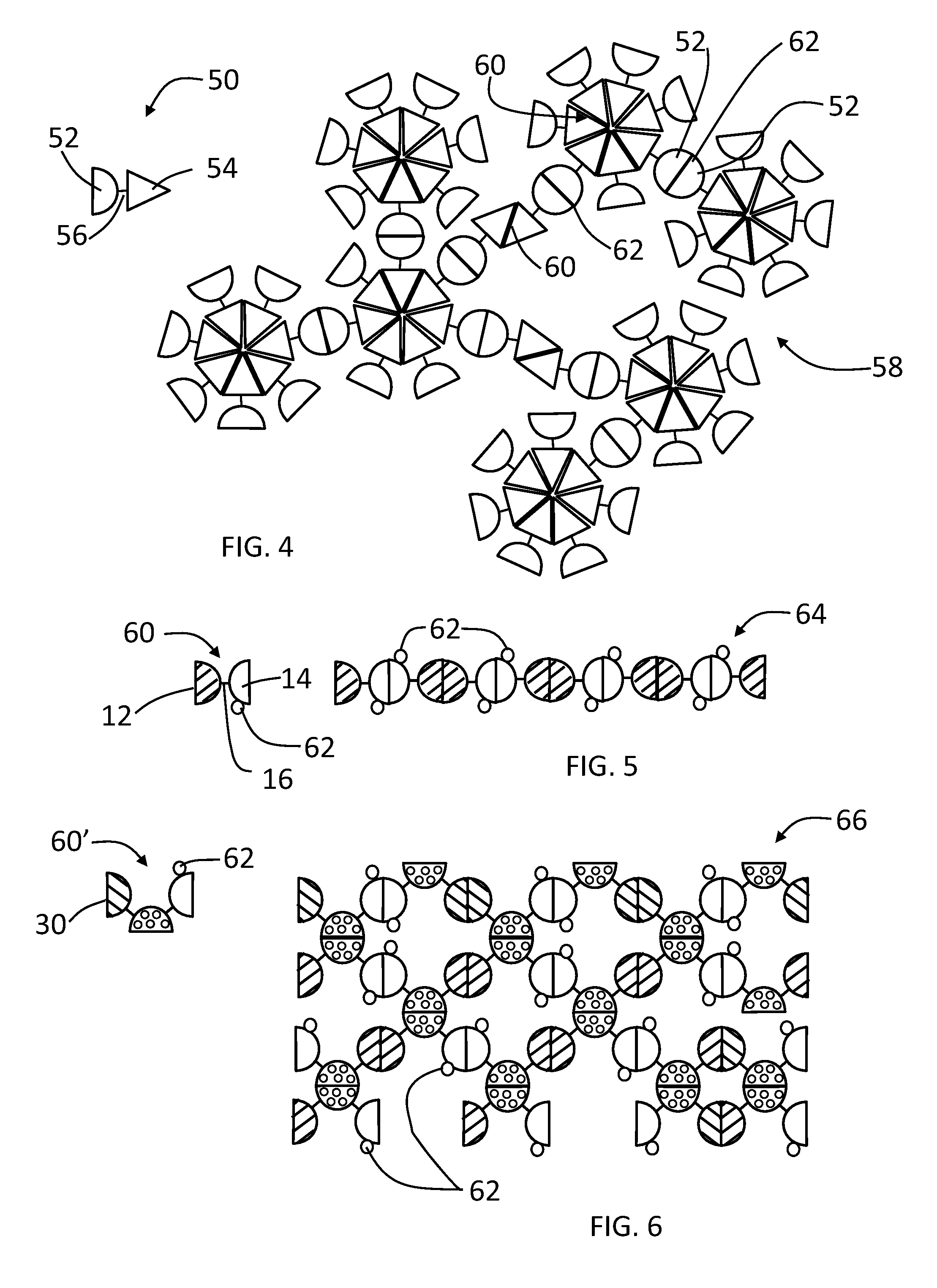
Genes for proteins which spontaneously form dimers and / or oligomers can be recombinantly linked together, which upon expression in E. coli produces stable dimeric fusion proteins that spontaneously self-assemble into enormous, polyvalent complexes having increased immunogenicity and functionality. Linear, network and agglomerate complexes with enormous sizes and polyvalences are constructed using glutathione S-transferase, Norovirus P domains (NoV P− and NoV+), the protruding (P) domain of hepatitis E virus (HEV P), the astrovirus P domain (AstV), a monomeric peptide epitope (M2e of influenza virus), and / or a protein antigen (VP8* of rotavirus) fused in different combinations. The resulting complexes can contain hundreds to thousands NoV P-protein, HEV, AstV, M2e and / or VP8* copies and exhibit higher immunogenicity than the individual proteins alone. The large size and multivalent nature of the complexes are candidates as a bivalent or multivalent vaccines against Norovirus and other pathogens, and for generation of antibodies for diagnosis and research purposes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Influenza viruses with mutant PB2 gene segment as live attenuated vaccines
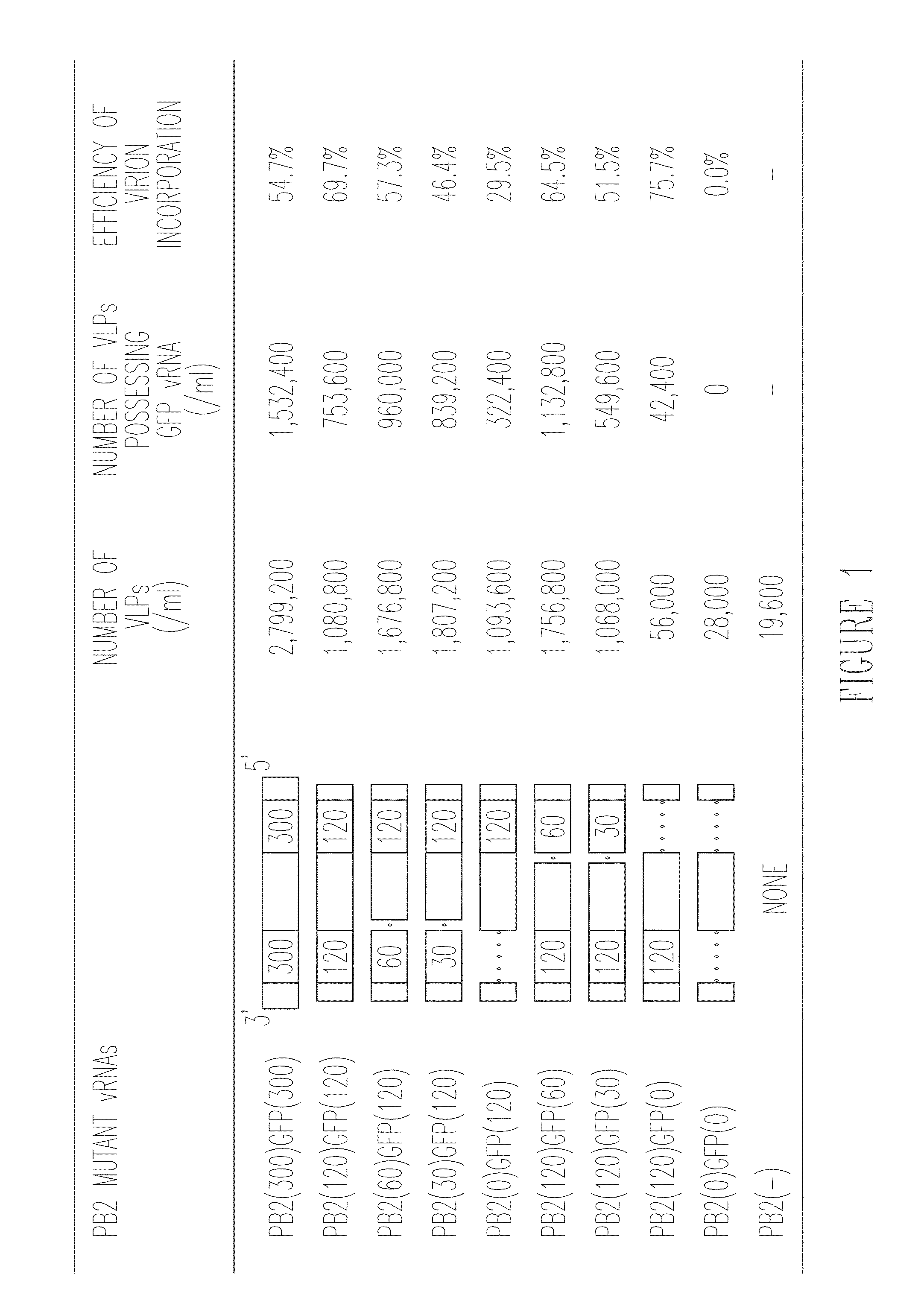
ActiveUS9101653B2Effective trackingProcess safetySsRNA viruses negative-senseViral antigen ingredientsMultivalent VaccineTGE VACCINE
The invention provides a recombinant biologically contained influenza virus that is a PB2 knockout virus, e.g., one that is useful to generate a multivalent vaccine, and methods of making and using that virus.
Owner:WISCONSIN ALUMNI RES FOUND
Prevention, treatment and detection of progressive atrophic rhinitis of pig
The invention discloses a method for manufacturing a vaccine for animals with progressive atrophic rhinitis (PAR), which comprises the following steps that: nucleic acid fragments of polypeptides with three respective amino acid sequences encoded by which are 2-486, 486-986 or 986-1281 amino acid residues corresponding to Pasteurella multocida toxin (PMT) protein are expressed in Escherichia coli in a large amount respectively, and the polypeptides are separated and used for preparing a vaccine which can excite the generation of an antibody against Pasteurella multocida (related to the progressive atrophic rhinitis). The invention also discloses a method for manufacturing a multivalent vaccine capable of at least preventing the PAR for the animals, which comprises the following steps that: the multivalent vaccine is obtained by mixing the three polypeptides for the PAR prepared by the method and at least one pathogenic antigen related with other animal diseases or an epitope thereof.
Owner:简茂盛
Multivalent Vaccine for Filariasis
The present invention is a multivalent vaccine for immunizing an animal against filariasis. In some embodiments, the antigens of the multivalent vaccine are protein-based, DNA-based, or a combination thereof.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Compositions comprising streptococcus pneumoniae polysaccharide-protein conjugates and methods of use thereof
The invention is related to multivalent immunogenic compositions comprising more than one S. pneumoniae polysaccharide protein conjugates, wherein each of the conjugates comprises a polysaccharide from an S. pneumoniae serotype conjugated to a carrier protein, wherein the serotypes of S. pneumoniae are as defined herein. In some embodiments, at least one of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. In further embodiments, each of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. Also provided are methods for inducing a protective immune response in a human patient comprising administering the multivalent immunogenic compositions of the invention to the patient. The multivalent immunogenic compositions are useful for providing protection against S. pneumoniae infection and / or pneumococcal diseases caused by S. pneumoniae. The compositions of the invention are also useful as part of treatment regimes that provide complementary protection for patients that have been vaccinated with a multivalent vaccine indicated for the prevention of pneumococcal disease.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com