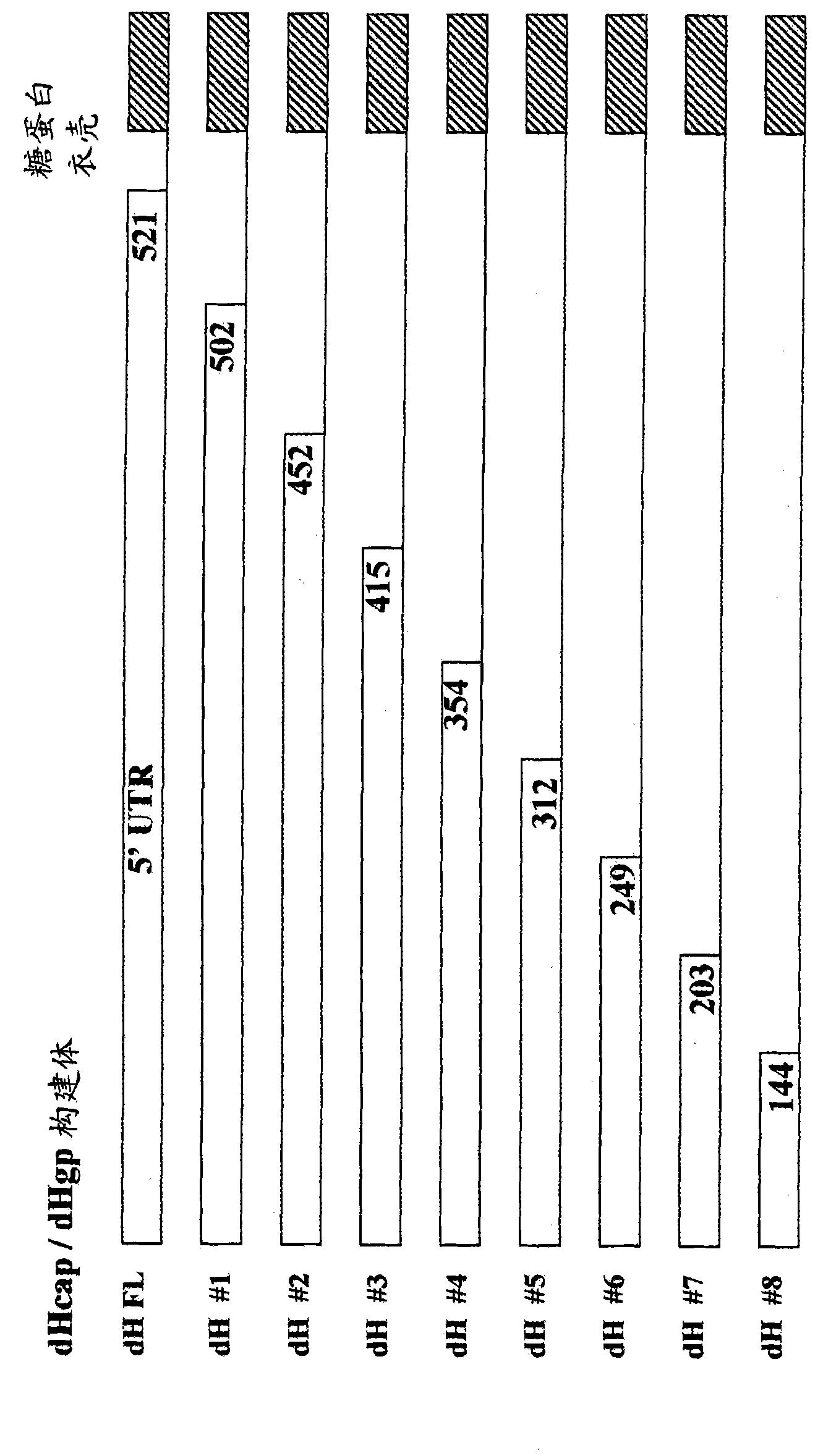
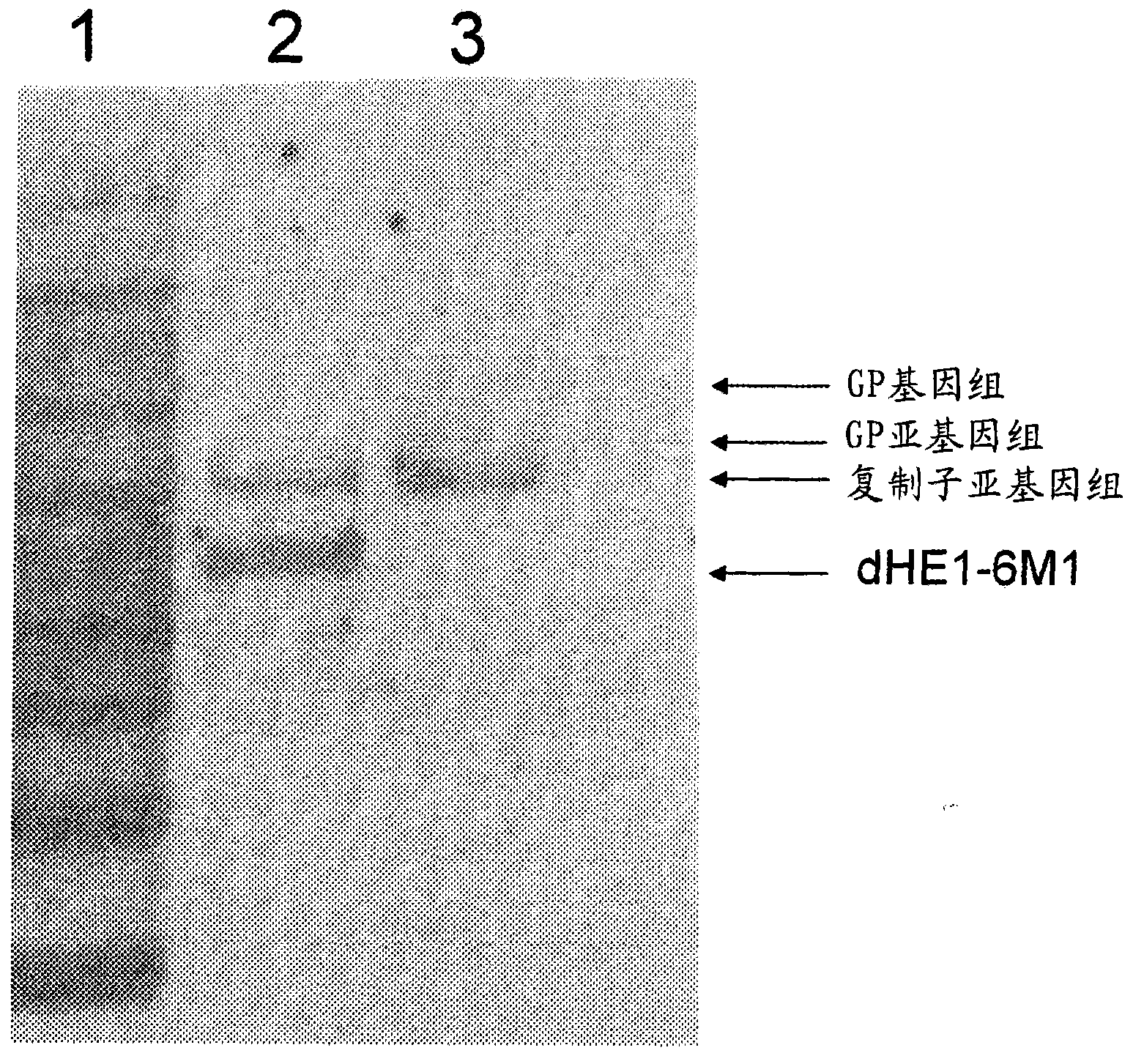
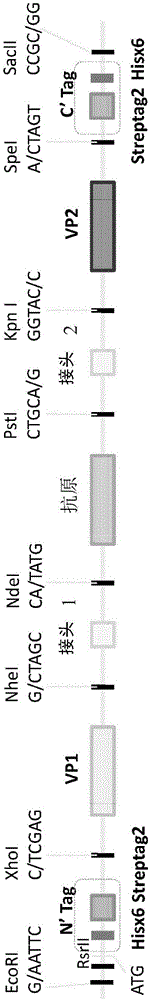
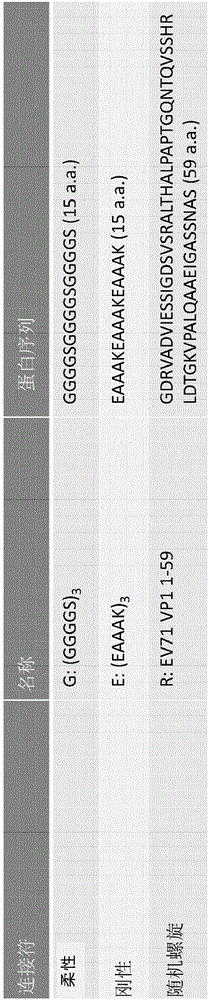
Patents
Literature
67 results about "Viral structural protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A viral structural protein is a viral protein that is a structural component of the mature virus. Examples include the SARS coronavirus 3a and 7a accessory proteins.
Capsid-modified recombinant adenovirus and methods of use
InactiveUS6955808B2Low yieldEfficiencyBiocideAntibody mimetics/scaffoldsSingle-Chain AntibodiesNoninvasive imaging
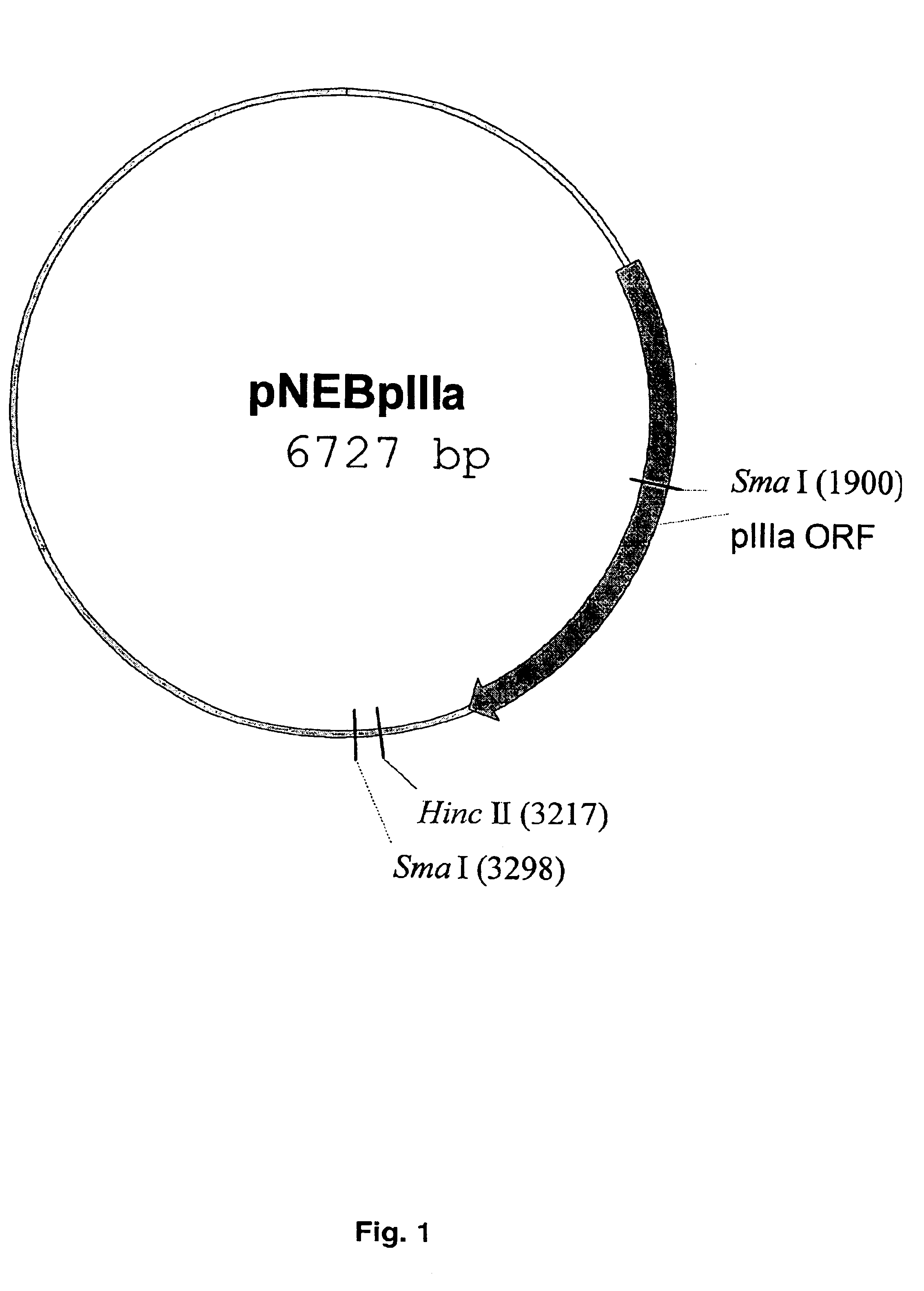
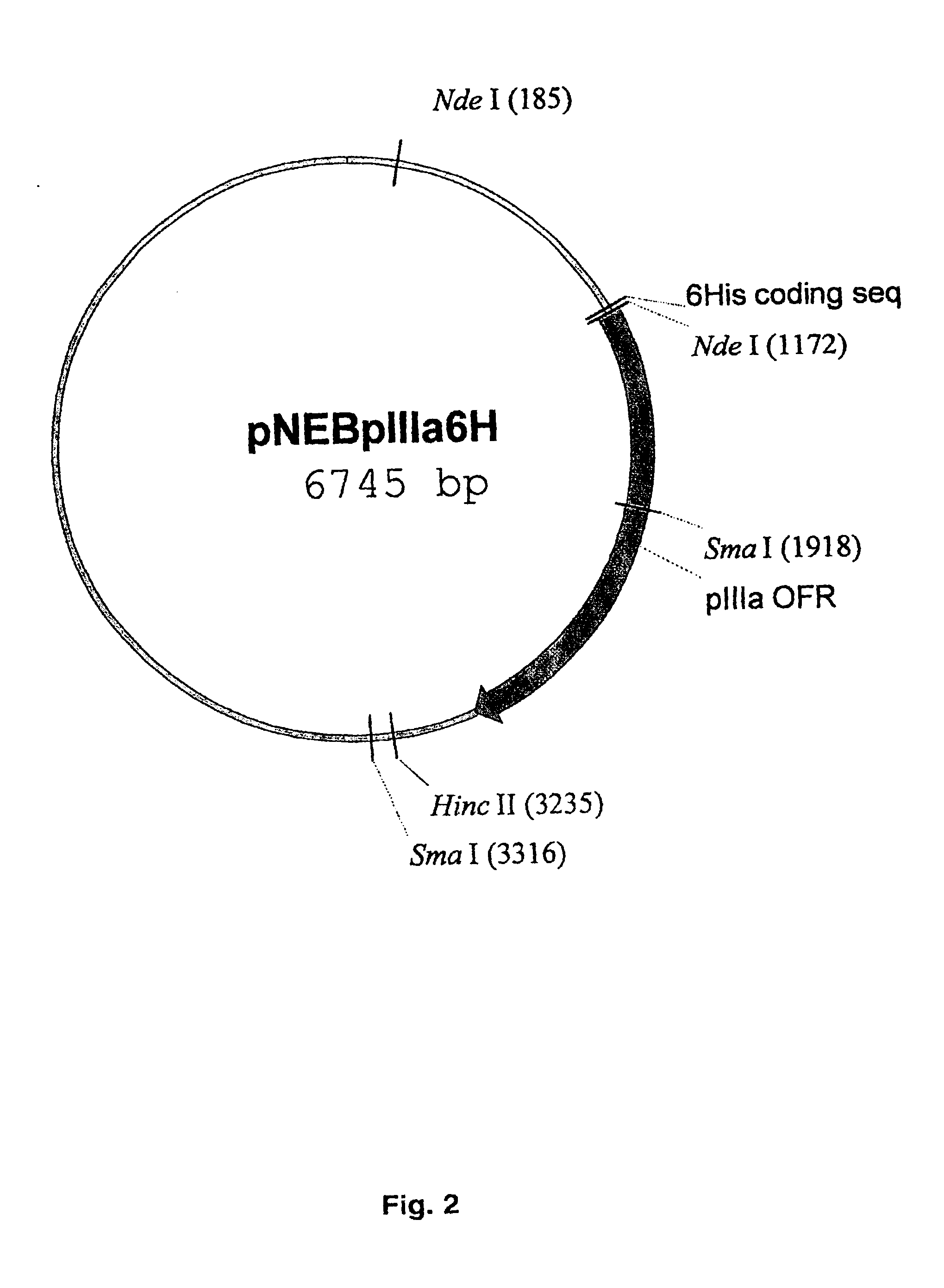
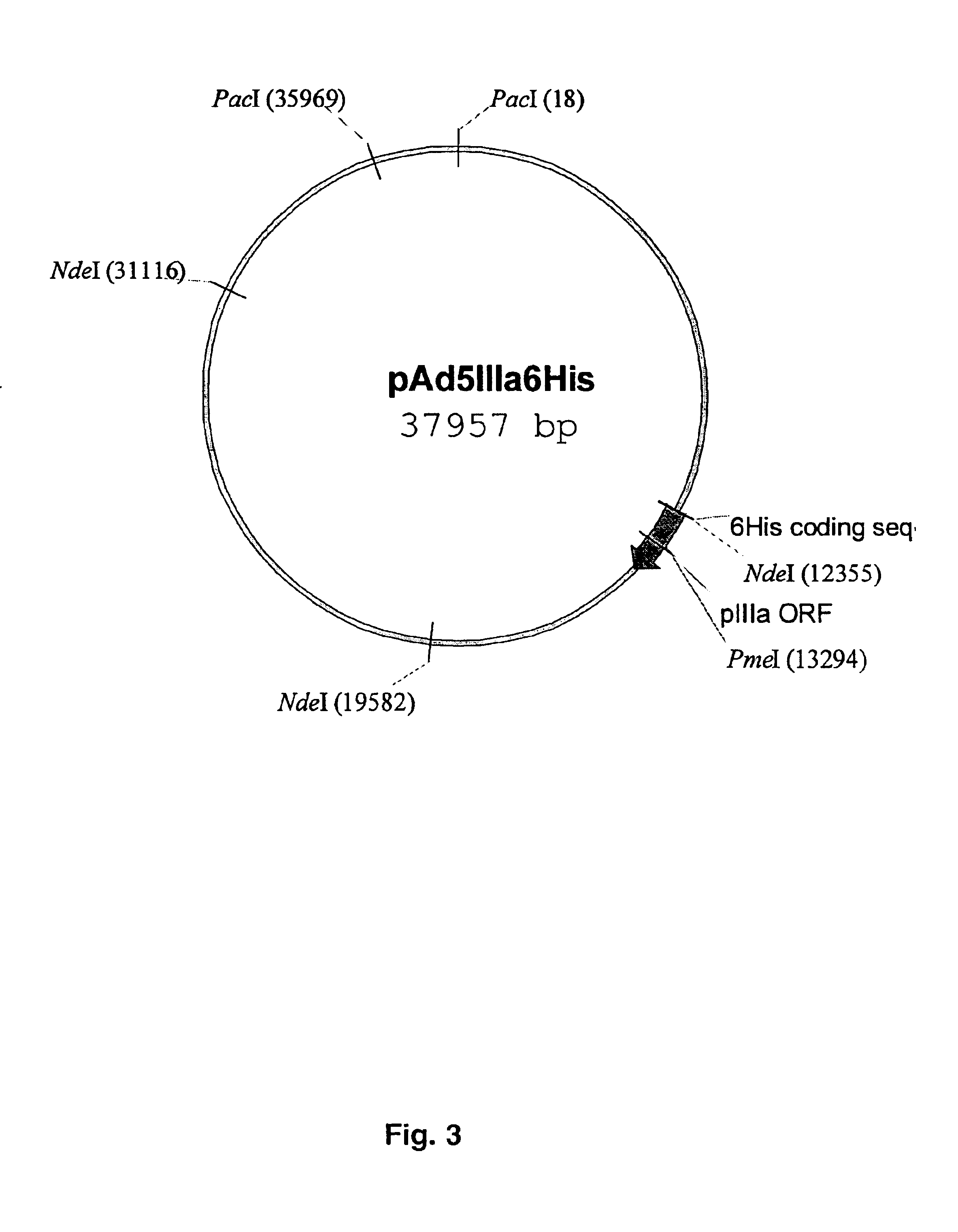
The present invention describes recombinant adenoviral vectors modified by incorporating targeting ligands or label into viral capsid or structural proteins. In one embodiment, single-chain antibody was introduced into the minor capsid proteins pIIIa or pIX so that the adenoviral vector can be targeted to a particular cell type. In another embodiment, there is provided a noninvasive imaging strategy useful for monitoring the replication and spread of conditionally replicative adenoviral vectors. Viral structural proteins such as pIX capsid protein, core proteins mu, V and VII were expressed as fusion protein with a fluorescent label. Once incorporated into the virions, detection of the structural fusion protein label would indicate the localization of the disseminated viral progeny. The detected fluorescent signals also closely correlate with the level of viral replication and progeny production.
Owner:UAB RES FOUND
Virus like particle comprising pd-1 antigen or pd-1 ligand antigen
ActiveUS20150017194A1SsRNA viruses positive-senseAntibody mimetics/scaffoldsVirus Structural ProteinsStructural protein
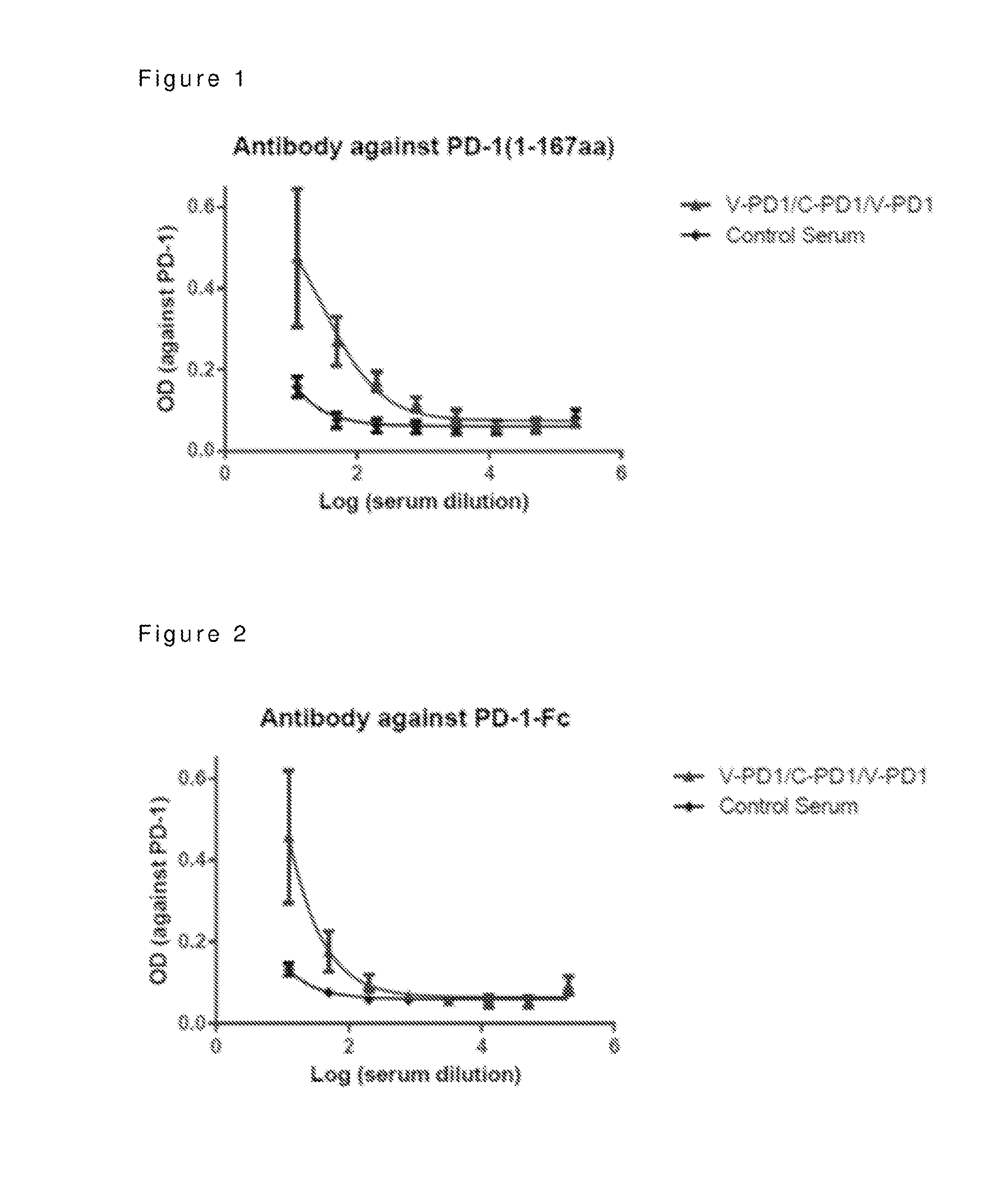
The present invention provides a virus like particle comprising a virus structural protein and an antigen derived from PD-1 or a ligand of PD-1, and a composition or kit comprising thereof, its use in immune response etc.
Owner:VLP THERAPEUTICS LLC
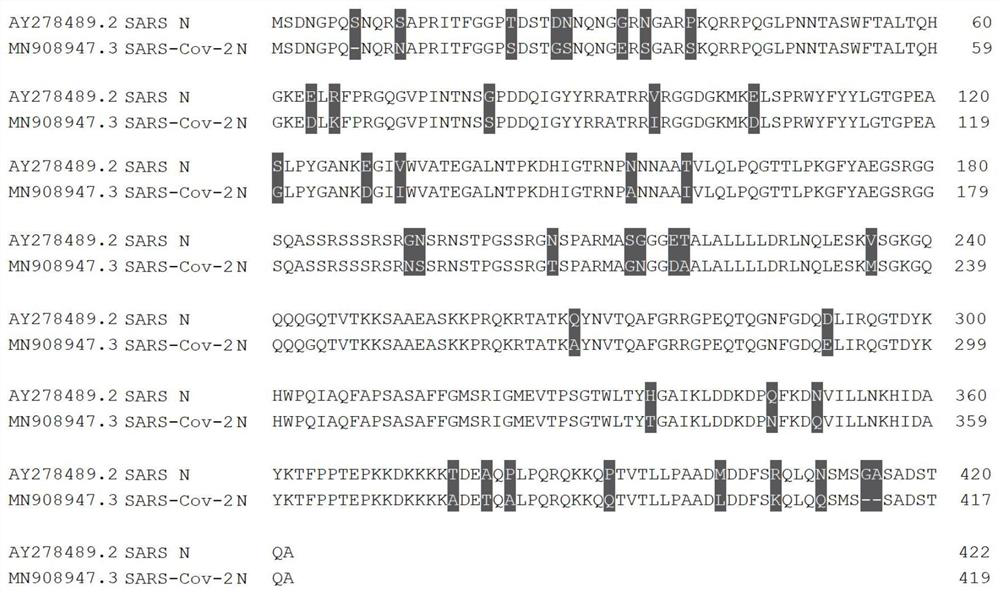
Monoclonal antibody for detecting SARS-CoV-2 virus nucleocapsid protein (N protein) and application thereof
PendingCN112079920AStrong specificityBiological material analysisImmunoglobulins against virusesCapsidGene clone
The invention discloses a monoclonal antibody 64360-52D1 capable of specifically combining with SARS-Cov-2 virus structural nucleocapsid protein (N protein), a preparation method thereof, and 6 complementarity-determining regions (CDR) of heavy chain and light chain variable regions; and more specifically, the monoclonal antibody is secreted by a hybridoma strain 64360#52D1, and can specifically recognize the SARS-Cov-2 virus N protein rather than SARS virus N protein. Thus, the monoclonal antibody can be used for identifying the two coronaviruses with high similarity. The invention further provides an enzyme-linked immunosorbent assay (ELISA) and an immune colloidal gold test strip detection method for specifically detecting the SARS-Cov-2 virus N protein by preparing the 64360#52D1 antibody. The antigen of the antibody is SARS-Cov-2 virus N protein subjected to heat treatment and expressed in mammalian cells; the finally obtained antibody belongs to an IgG1 subtype; and a sequence for encoding the variable region of the antibody is obtained in a gene cloning mode.
Owner:BEIJING PROTEIN INNOVATION
Agents for the inhibition of virus replication through regulation of protein folding
The invention concerns agents for the treatment of acute and chronic infections with human and animal pathogenic viruses which assemble along the cell membrane and are released through budding on the surface of the cell. Hereunto count especially causative agents of infectious diseases such as AIDS, hepatitis, hemorrhagic fever, SARS, smallpox, measles, polio or the flu. The subjects of the invention are agents that contain inhibitors of the protein folding as active components. Hereunto count inhibitors of cellular folding enzymes (the enzymatic chaperones) as well as substances that disturb the folding of proteins through chemical chaperones. The following substance classes and their derivates belong thereunto: Geldanamycin, Deoxyspergualin, 4-PBA or Herbimycin A. Due to these agents the highly organised processes of the assembly and the proteolytical maturation of virus structure proteins is disturbed. As a result the release and production of infectious decendent viruses is prevented.
Owner:VIROLOGIK GMBH
Fusion proteins of recombinant sars coronavirus structural proteins, their production and uses
InactiveUS20100150923A1SsRNA viruses positive-senseAntibody mimetics/scaffoldsSARS coronavirusStructural protein
Fusion proteins of recombinant SARS coronavirus structural proteins, their production and uses are provided. An optimized SARS coronavirus S protein gene which can be highly expressed in the mammalian cell strains and SARS coronavirus S protein variants comprising deletion, modification or mutation amino acids 318-510 corresponding to SARS coronavirus S protein are also provided.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Direct Attachment of Polypeptides to Virus Like Particles
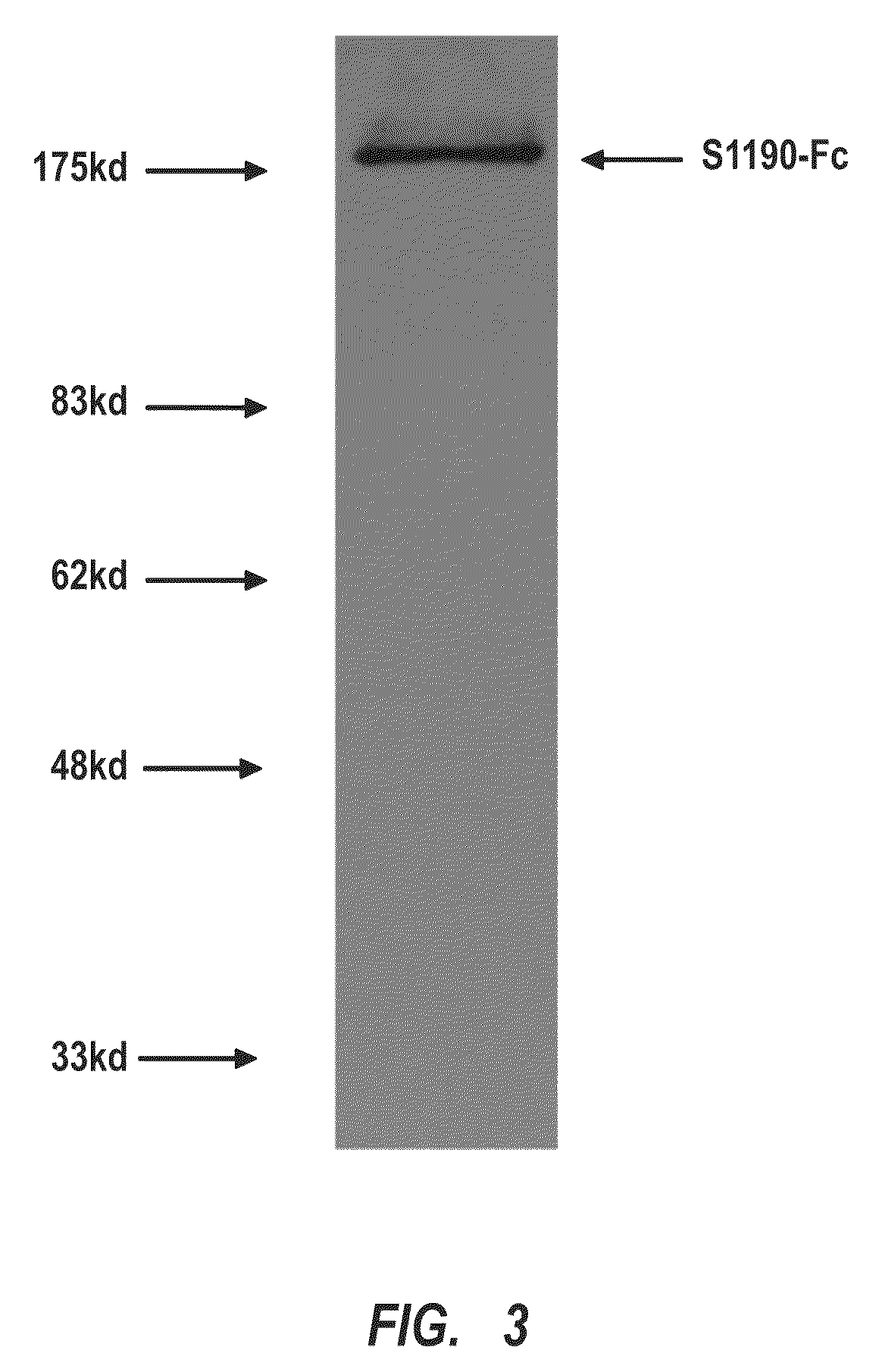
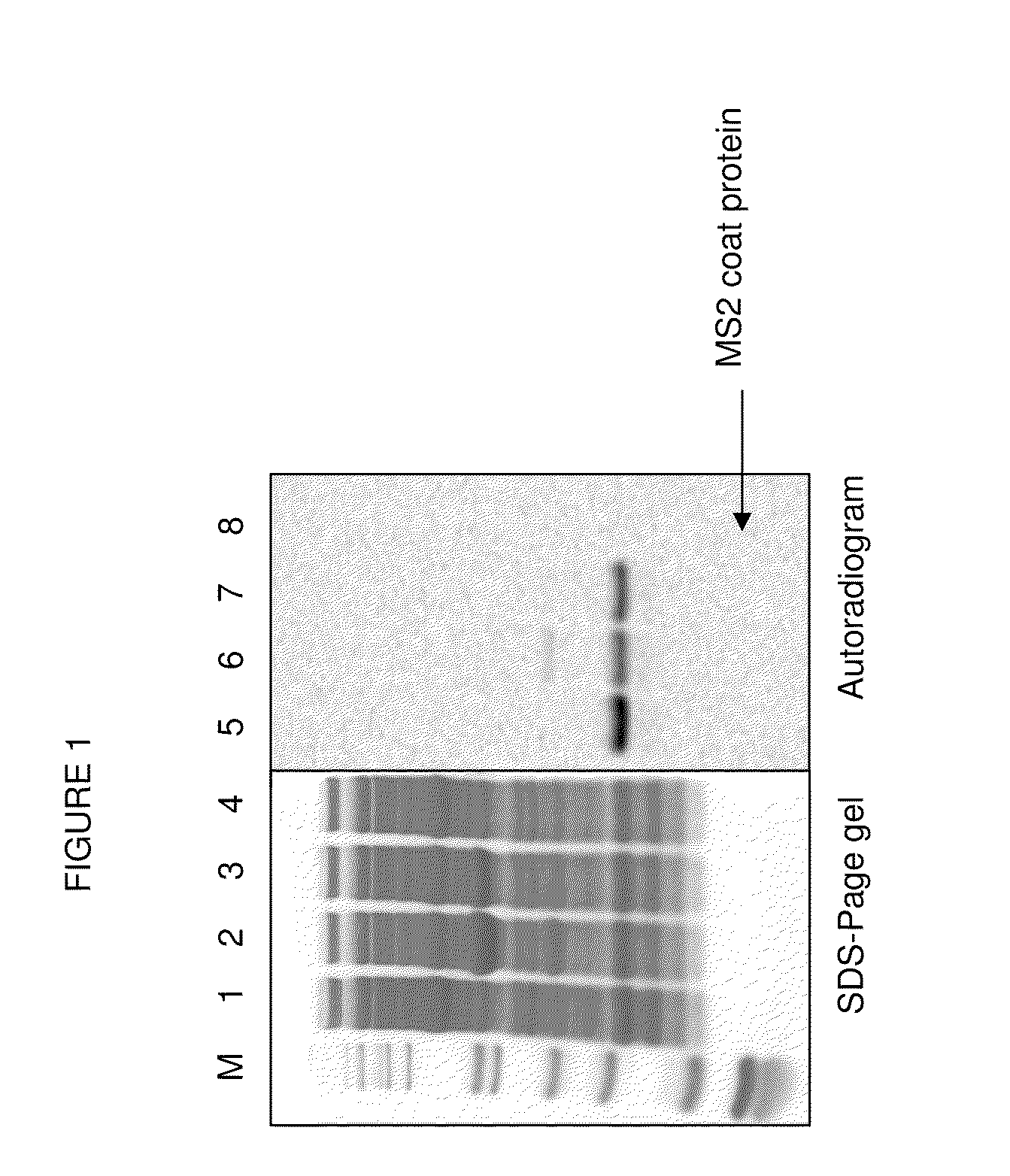
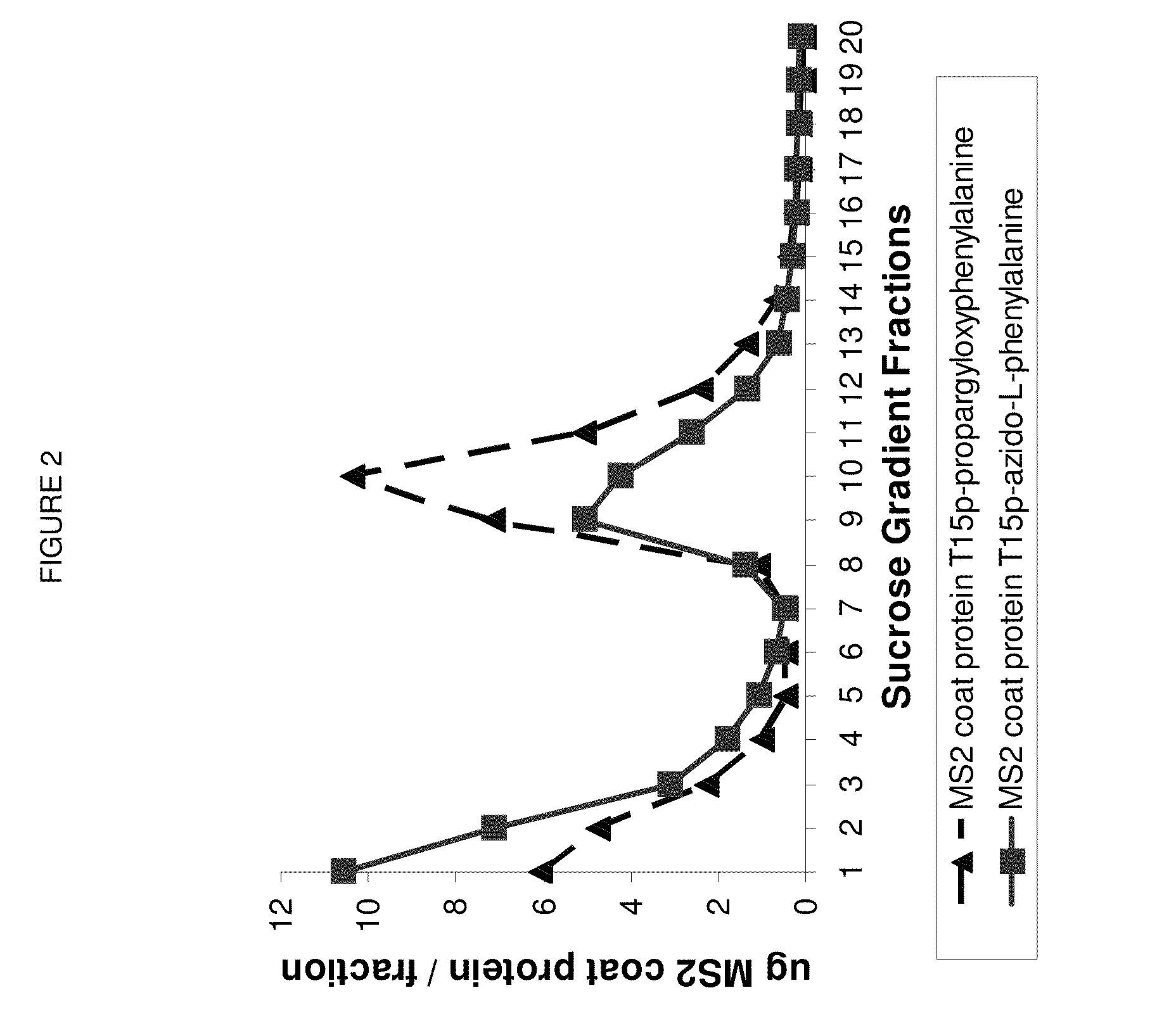
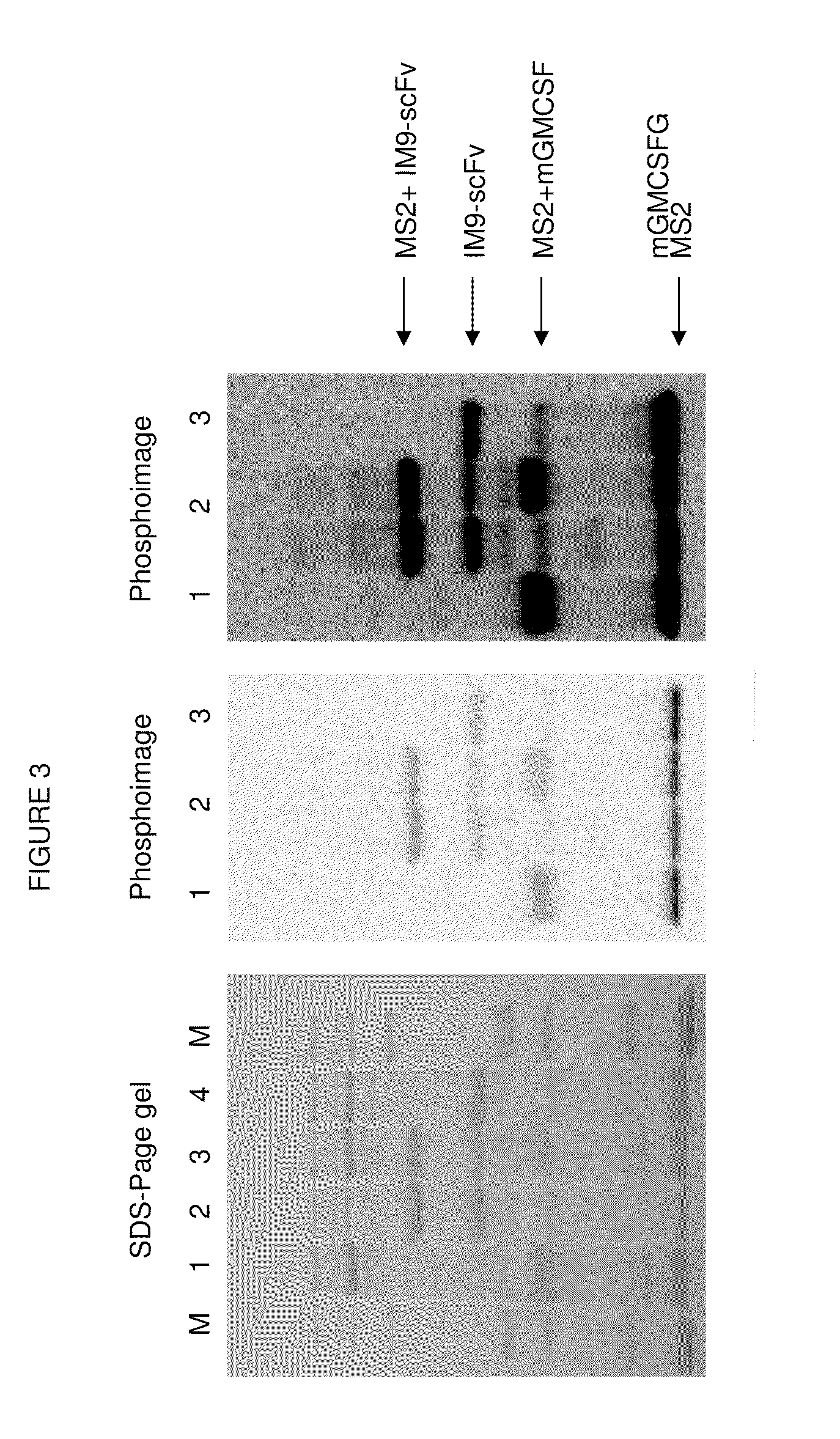
Compositions and methods are provided for the control of direct protein attachment to virus like particles where virus structural proteins that have been modified to comprise an unnatural amino acid at a pre-determined site are reacted with one or more “display” polypeptides that also comprise an unnatural amino acid at a pre-determined site in a one step reaction. The compositions of the invention are useful for various purposes where it is desirable to efficiently and directly attach multiple polypeptides to a single carrier entity, particularly where two or more different polypeptides are attached to a single carrier.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Virus like particle comprising modified envelope protein e3
ActiveUS20160200775A1Increase productionSsRNA viruses positive-senseAntibody mimetics/scaffoldsVirus-like particleChikungunya
A virus like particle comprising a viral structural protein which comprises modified envelope protein E3. The viral structural protein may be that derived from or alphavirus or flavivirus. Especially, the viral structural protein may be derived from Chikungunya virus or Venezuelan equine encephalitis virus.
Owner:VLP THERAPEUTICS LLC
Virus-like particle vaccines
InactiveUS20160185826A1Easy to foldStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMultivalent VaccineViral structural protein
The invention is directed to dimeric fusion proteins and virus-like particles comprising such dimeric fusion proteins. These dimeric fusion proteins comprise an antigen or antigenic fragment carried between two viral structural proteins or fragments thereof, with or without linkers, in a manner that, relative to traditional monomeric platforms, minimizes steric hindrance among the antigen or antigenic fragment and the viral structural proteins or fragments thereof. This novel design provides for multivalent vaccines and enhanced immunogenicity. The invention also relates to nucleic acids encoding such dimeric fusion proteins and host cells comprising such nucleic acids. The invention further relates to pharmaceutical compositions comprising the dimeric fusion proteins and / or virus-like particles of the invention, and methods of prevention or treatment using such compositions.
Owner:MEDIGEN BIOTECH
Alphavirus RNA replicon systems
InactiveUS7235235B2Reduced virulenceLow toxicityBiocideSsRNA viruses positive-senseStructural proteinVirology
The present invention provides a helper cell for expressing an infectious, replication defective, alphavirus particle in an alphavirus-permissive cell. The helper cell includes (a) a first helper RNA encoding (i) at least one alphavirus structural protein, and (ii) not encoding at least one alphavirus structural protein; and (b) a second helper RNA separate from the first helper RNA, the second helper RNA (i) not encoding the alphavirus structural protein encoded by the first helper RNA, and (ii) encoding the at least alphavirus one structural protein not encoded by the first helper RNA, such that all of the alphavirus structural proteins assemble together into alphavirus particles in the cell. Preferably, the helper cell also includes a replicon RNA encoding an alphavirus packaging sequence and an inserted heterogeneous RNA.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Hepatitis E virus-like particle vaccine preparation method and application
ActiveCN104857510AEasy to prepareEasy to zoom inDigestive systemImmunoglobulins against virusesEscherichia coliDisease
The invention discloses a hepatitis E virus-like particle vaccine preparation method, and belongs to the biological medicine technical field. According to the method, hepatitis E virus truncated capsid protein gene codon optimization is performed, prokaryotic expression system escherichia coli strain B834-pRARE2 is used, by fusion of purification tag MBP expression, different cracking agent screening and combination, bacterial membrane extraction, maltose amylose affinity chromatography, molecular sieve chromatography for removal of MBP and non-virus structural protein, and promotion of virus-like particle assembly, the hepatitis E virus-like particle purity is more than 99.0%, and is free of protein label and bioactive (in the form of non-inclusion body, and soluble). The hepatitis E virus-like particle vaccine produces complete protection to pig, dog and chicken HEV infection, cross protective antigens are between different genotype HEV virus, and the hepatitis E virus-like particle vaccine can be used as an animal vaccine to prevent the diseases and infection caused by pig, dog and poultry HEV.
Owner:张澍 +1
Lipoparticles comprising proteins, methods of making, and using the same
ActiveUS8574590B2VirusesMicrobiological testing/measurementViral structural proteinCell Membrane Proteins
The present invention relates to lipoparticles. The invention also relates to producing lipoparticles. The invention further relates to lipoparticles comprising a viral structural protein. The invention further relates to a lipoparticle comprising a membrane protein, and the lipoparticle can be attached to a sensor surface. The invention further relates to methods of producing and using the lipoparticle to, inter alia, assess protein binding interactions.
Owner:INTEGRAL MOLECULAR
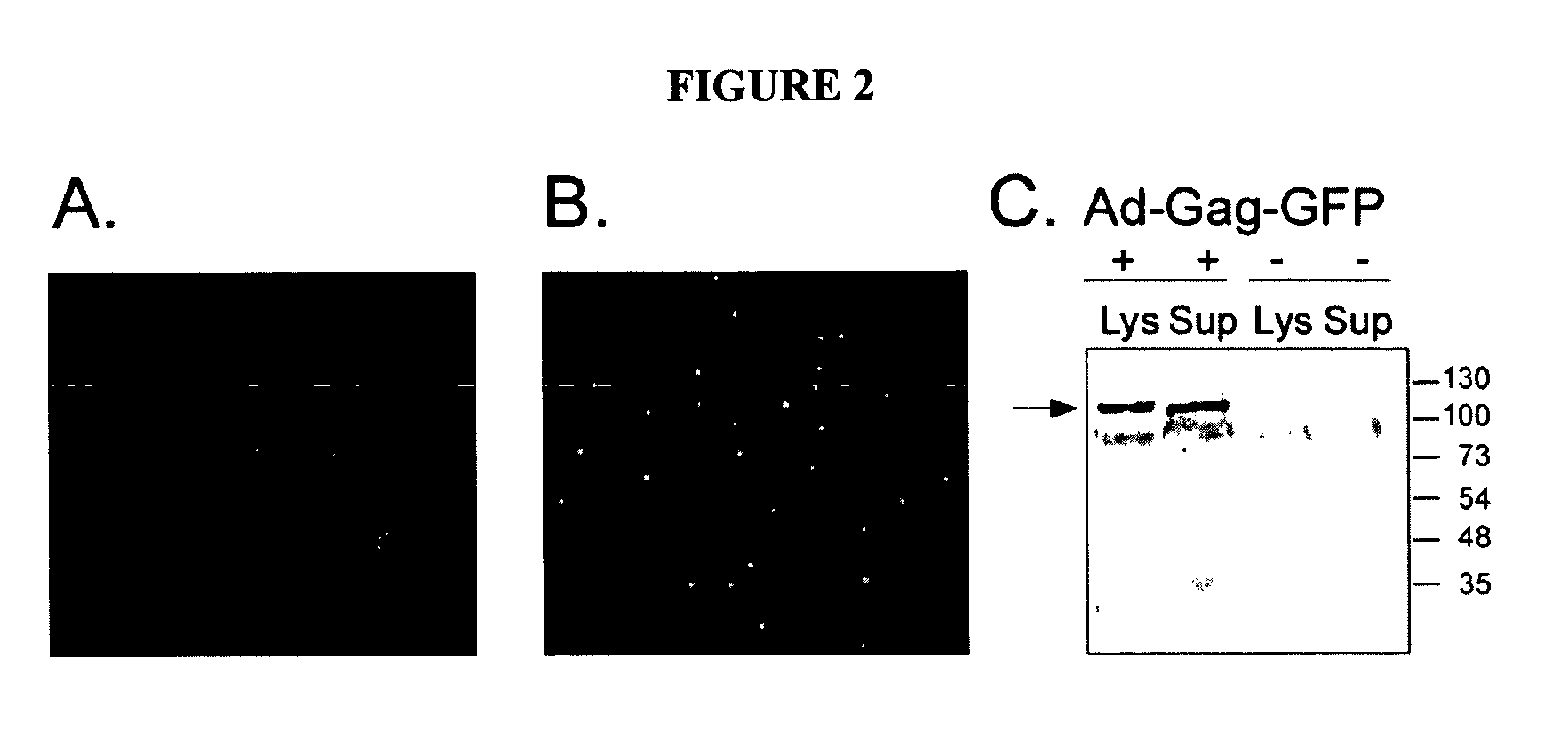
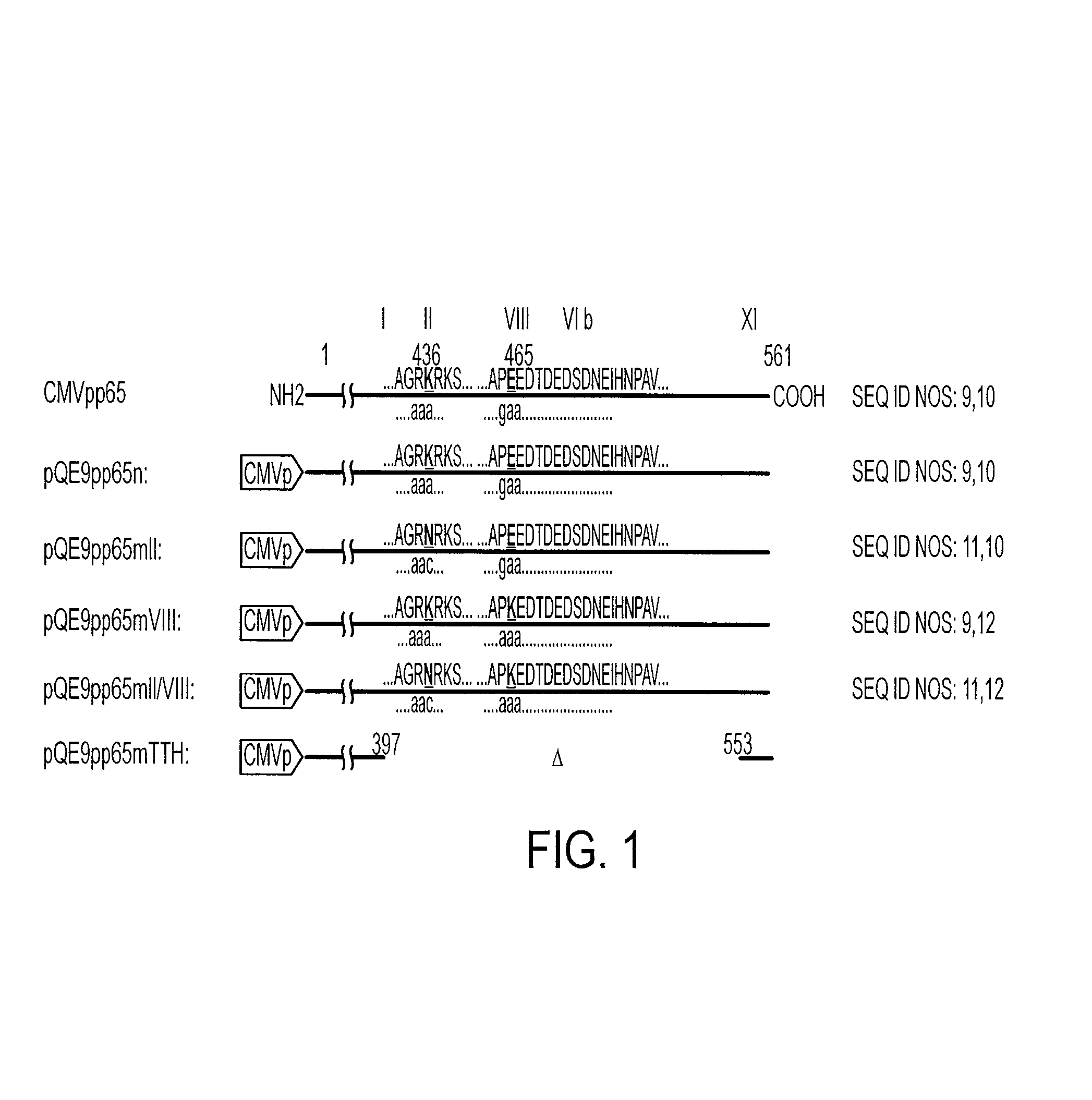
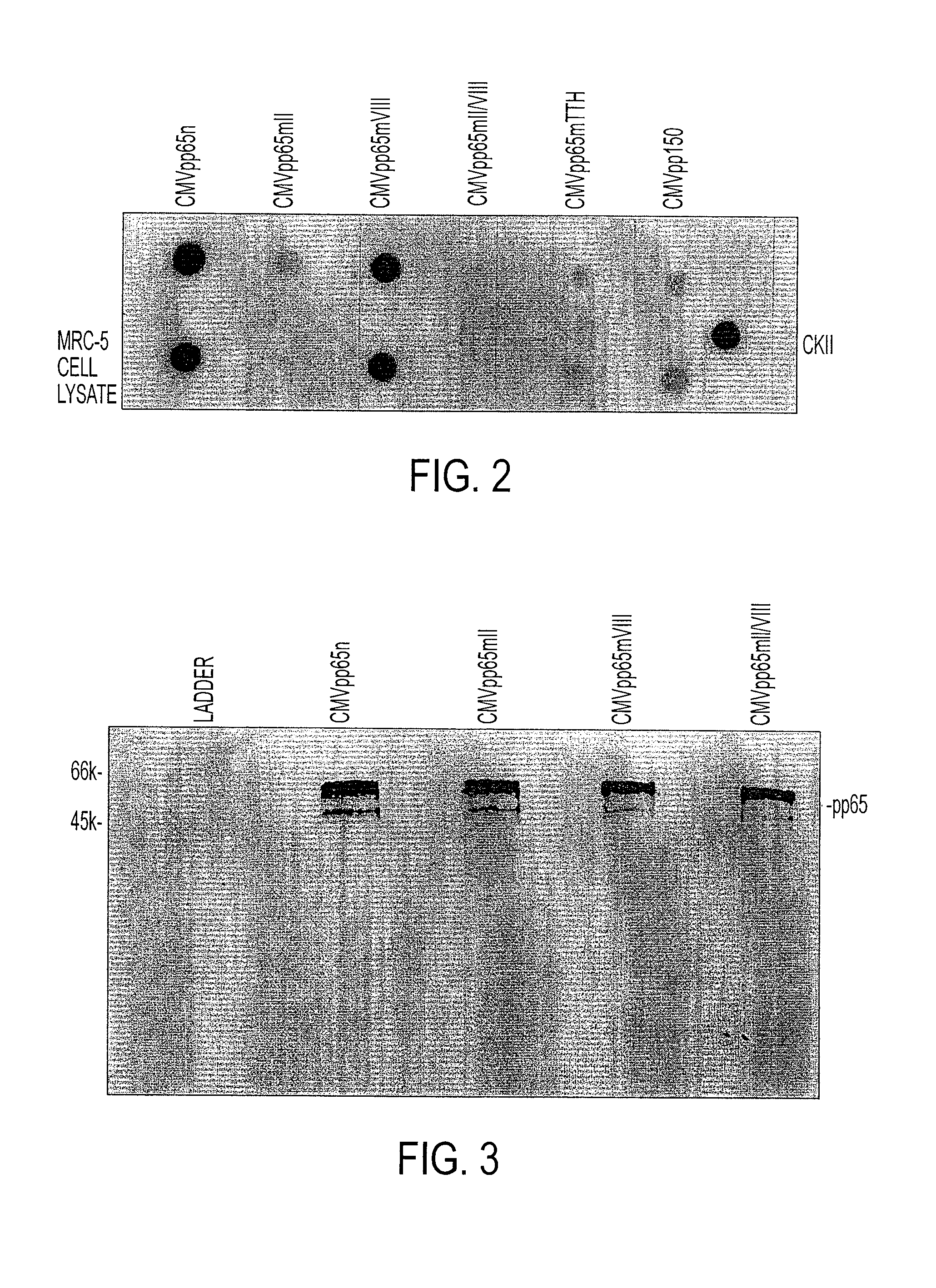
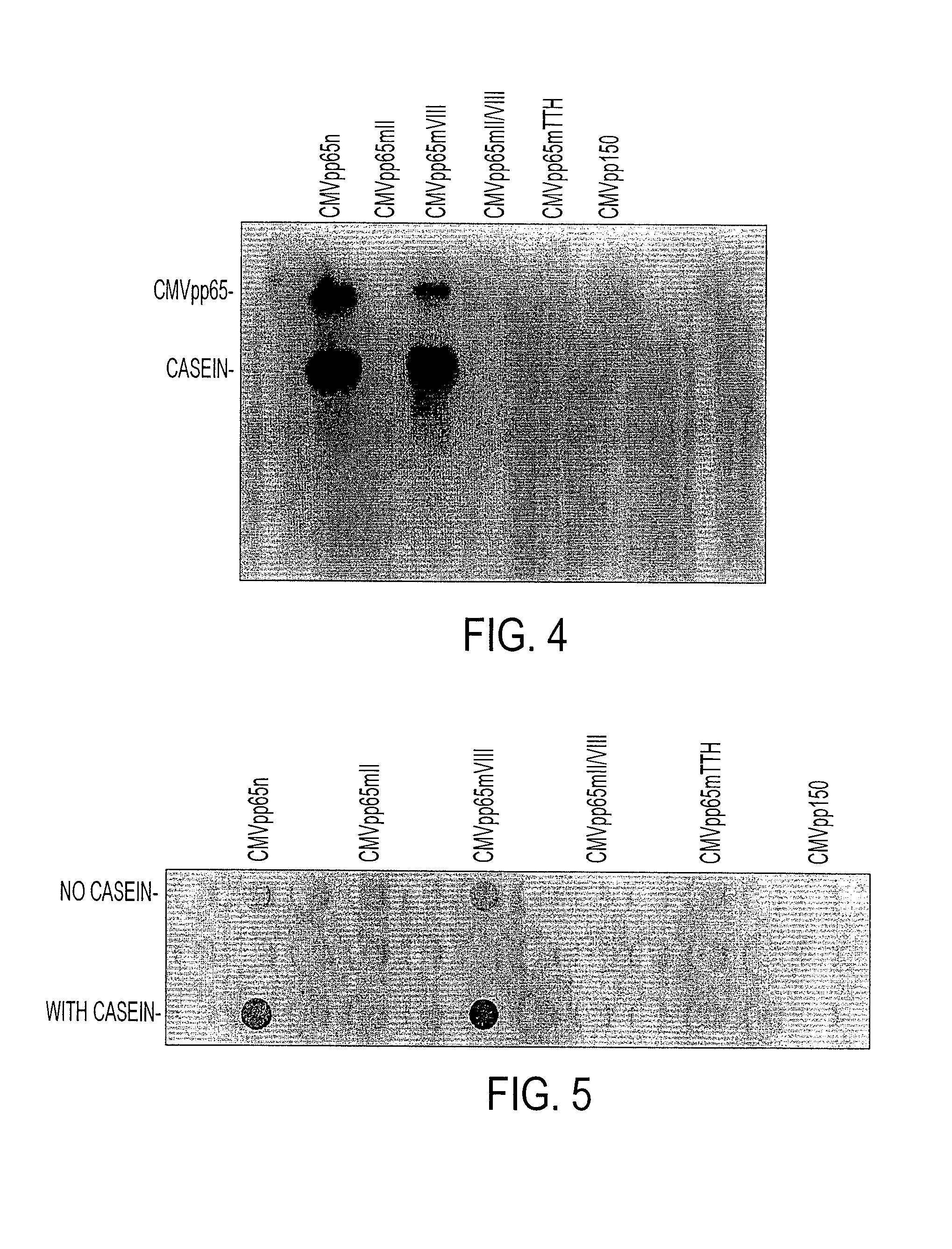
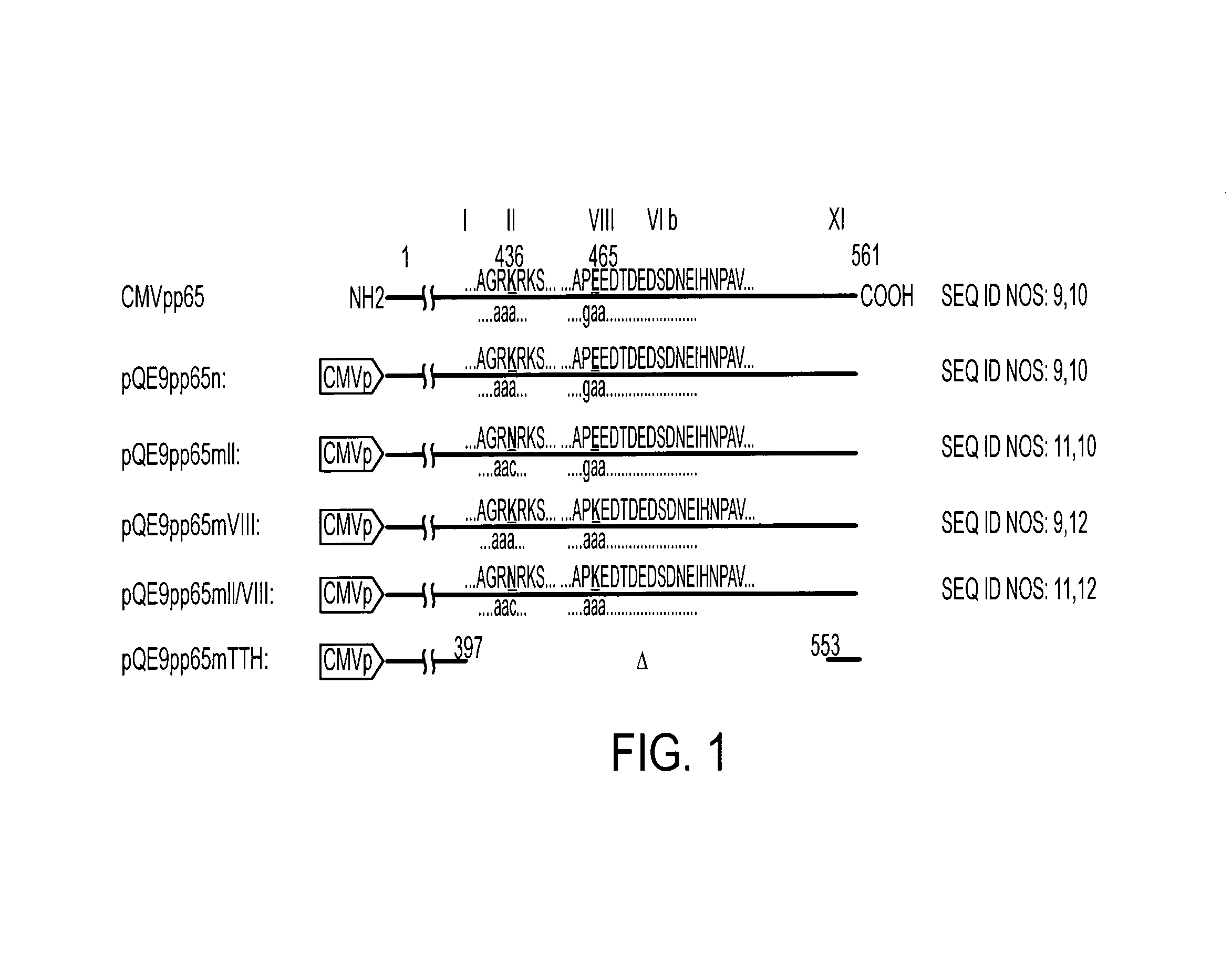
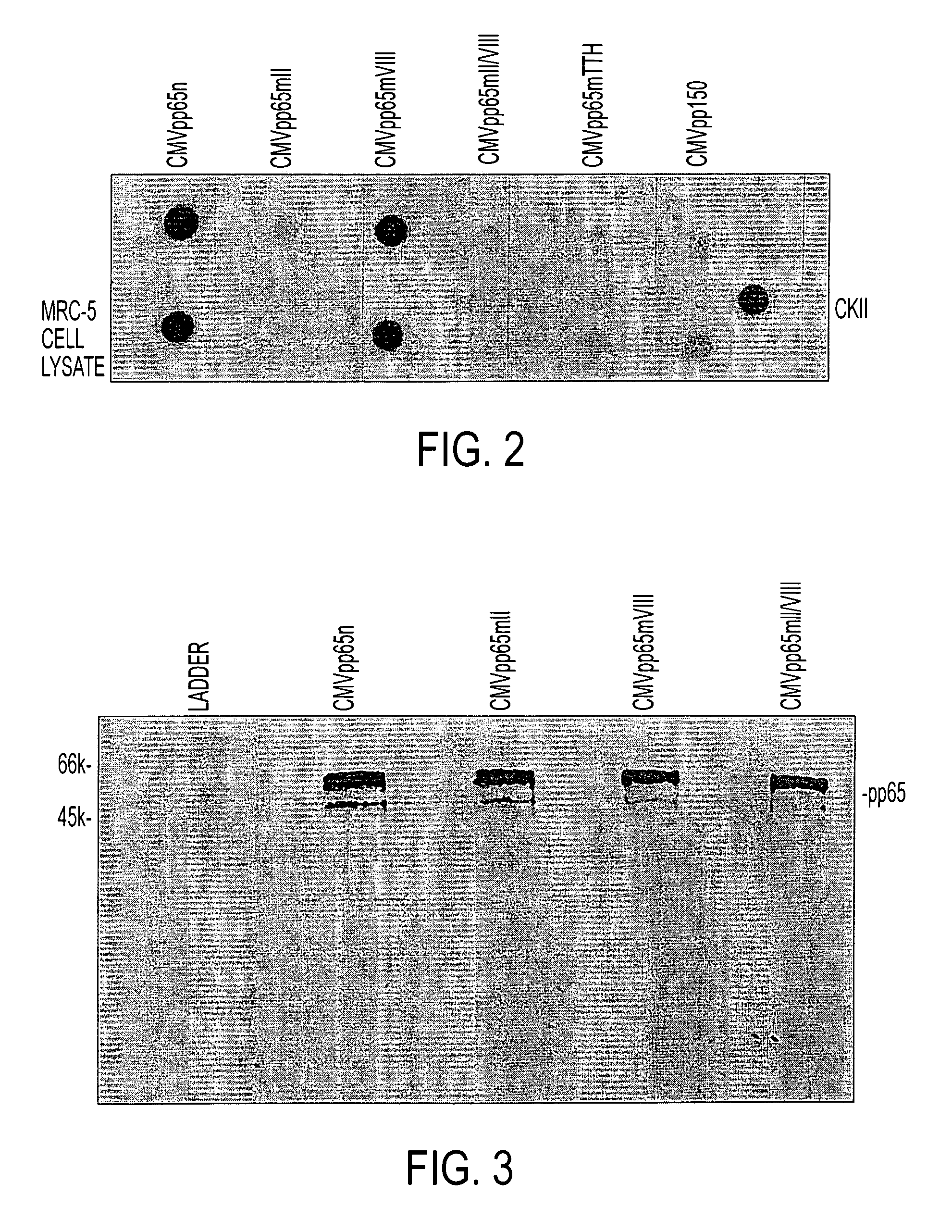
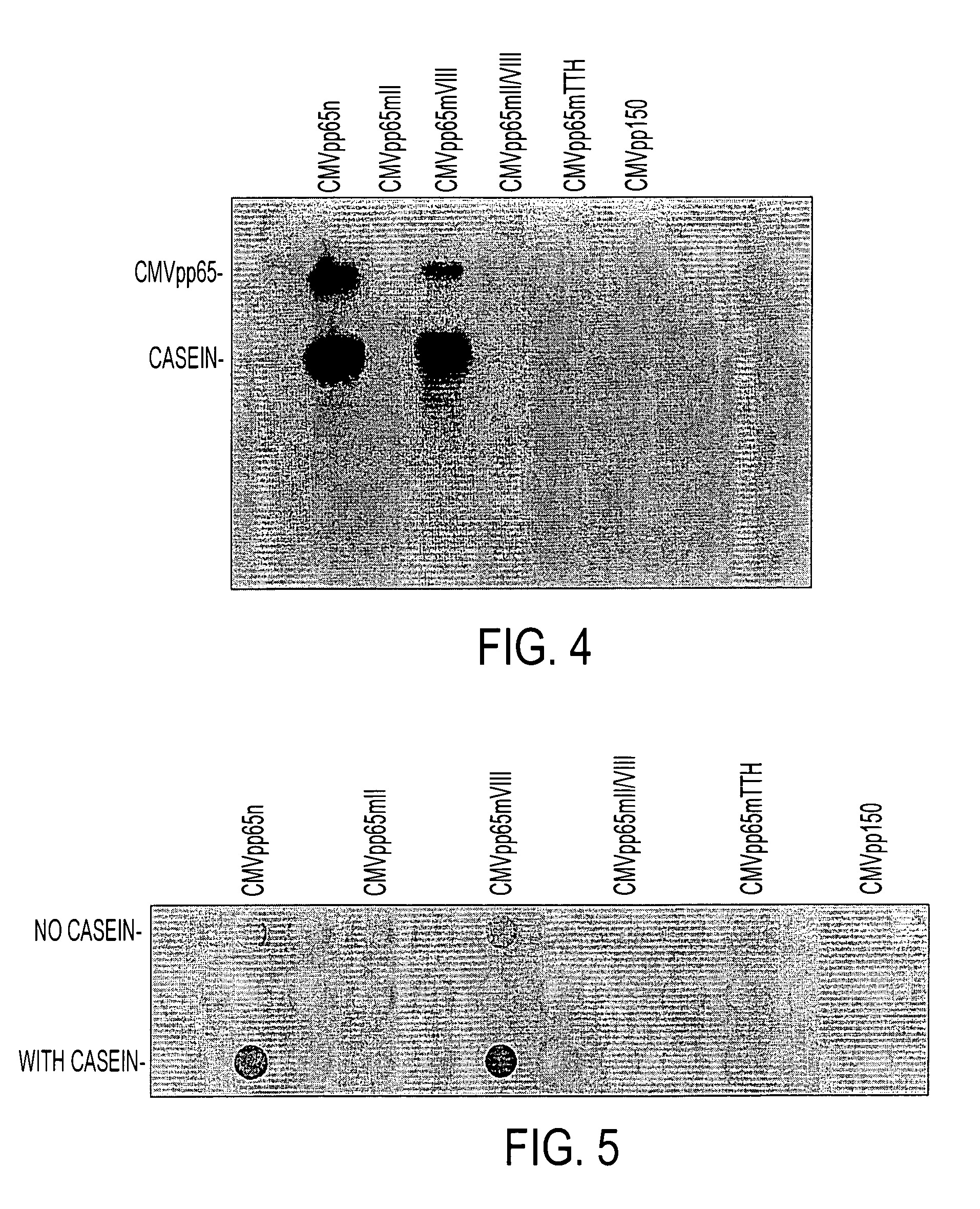
Protein kinase deficient, immunologically active CMVpp65 mutants
InactiveUS7387782B2Viral antigen ingredientsMicrobiological testing/measurementCmv infectionsADAMTS Proteins
This invention relates to mutated CMVpp65, a viral structural protein which activates cell mediated immunity in humans infected with CMV. The mutations remove undesirable protein kinase activity naturally present in the protein and make it suitable for the production of both DNA and protein vaccines. Therefore, the invention provides proteins and DNAS, as well as vaccines comprising the proteins and DNAs, including cellular vaccines and vectors. Other embodiments of the invention relate to methods of enhancing immune response and vaccinating against CMV, including gene therapy methods and vectors.
Owner:CITY OF HOPE
O type foot and mouth disease virus DNA vaccine and its preparing method
The present invention discloses type-One foot and mouth disease virus DNA vaccine and its preparation process, and is especially DNA vaccine including foot and mouth disease structure protein P1 gene and non-structure protein 2A, 2B and 3C gene as well as non-essential 3D gene and its preparation process.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Seneca valley virus structural protein epitope polypeptide and application thereof
ActiveCN109627293AIncreased sensitivityStrong specificitySsRNA viruses positive-senseVirus peptidesChemical synthesisEpitope
The invention discloses a Seneca valley virus structural protein epitope polypeptide and application thereof. The polypeptide is the polypeptide represented by a sequence 1 in a sequence table, a sequence 2 in the sequence table, a sequence 3 in the sequence table or a sequence 4 in the sequence table. The Seneca valley virus structural protein epitope polypeptide is used for preparing a chemically synthesized antigen peptide coated elisa plate used for a kit, is low in antigen dosage and high in sensitivity and specificity, can effectively detect whether a structural protein antibody infectedwith a Seneca valley virus exists or not, is high in sensitivity, good in specificity and fast and convenient to operate and has a good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
High-yield transgenic mammalian expression system for generating virus-like particles
InactiveUS20080063664A1Improving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsMammalVirus-like particle
The present invention provides a method utilizing mammalian expression system for generating virus-like particles (VLPs) of mammalian-hosted viruses, particularly SARS-CoV. The method of the present invention involves expression of viral structural proteins in Vero cells and thereby obtaining recombinant VLPs in the culture medium. SARS-VLPs generated by the method of the present invention are highly immunogenic and can elicit not only humoral but also cellular immune responses in a mammal.
Owner:ACAD SINIC
Virus like particle comprising modified envelope protein E3
ActiveUS9969986B2Increase productionSsRNA viruses positive-senseNervous disorderEncephalitisVirus-like particle
A virus like particle comprising a viral structural protein which comprises modified envelope protein E3. The viral structural protein may be that derived from or alphavirus or flavivirus. Especially, the viral structural protein may be derived from Chikungunya virus or Venezuelan equine encephalitis virus.
Owner:VLP THERAPEUTICS LLC
FRET-based fusion protein, and fluorescent nanoparticle and application thereof
ActiveCN111018997AGuaranteed FRET effectImprove stabilityAntibody mimetics/scaffoldsVirus peptidesFluoProbesVirus-like particle
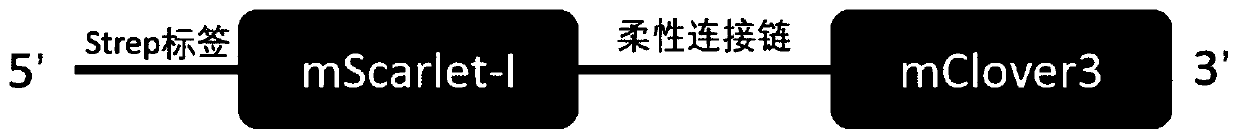
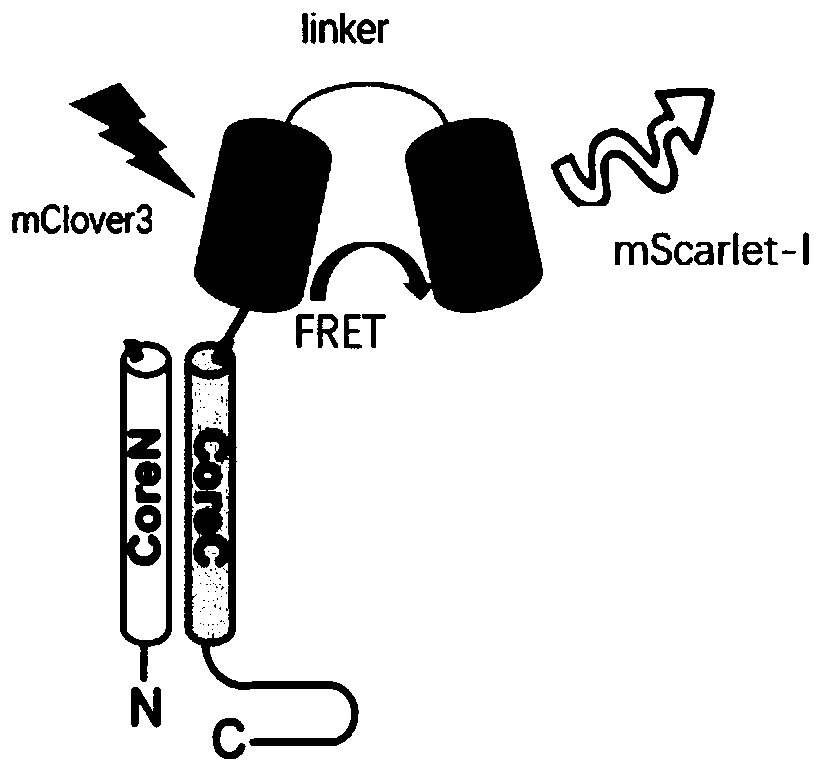
The invention belongs to the technical field of biology, and particularly relates to a fusion protein based on FRET, and a fluorescent nanoparticle and application thereof. The fusion protein comprises: viral structural protein which can be self-assembled into a virus-like particle; and a donor-acceptor pair capable of achieving fluorescence resonance energy transfer. The invention further provides a virus-like fluorescent nanoparticle which is obtained through self-assembling of the fusion protein. The fluorescent nanoparticle improves FRET efficiency, greatly increases a fluorescence signaloutput quantity when the fluorescent nanoparticle serves as a fluorescent probe, improves detection sensitivity and stability, reduces background value and increases a signal-to-noise ratio. In application of the virus-like fluorescent nanoparticle, the virus-like fluorescent nanoparticle can be directly connected with a targeting ligand, can guarantee the FRET effect of a fluorescent protein pair, is good in stability and high in sensitivity, and allows experimental result errors to be reduced and experimental results to be closer to reality.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Vaccine
ActiveUS20160022803A1Avoid infectionSsRNA viruses positive-sensePeptide/protein ingredientsDiseaseRNA Virus Infections
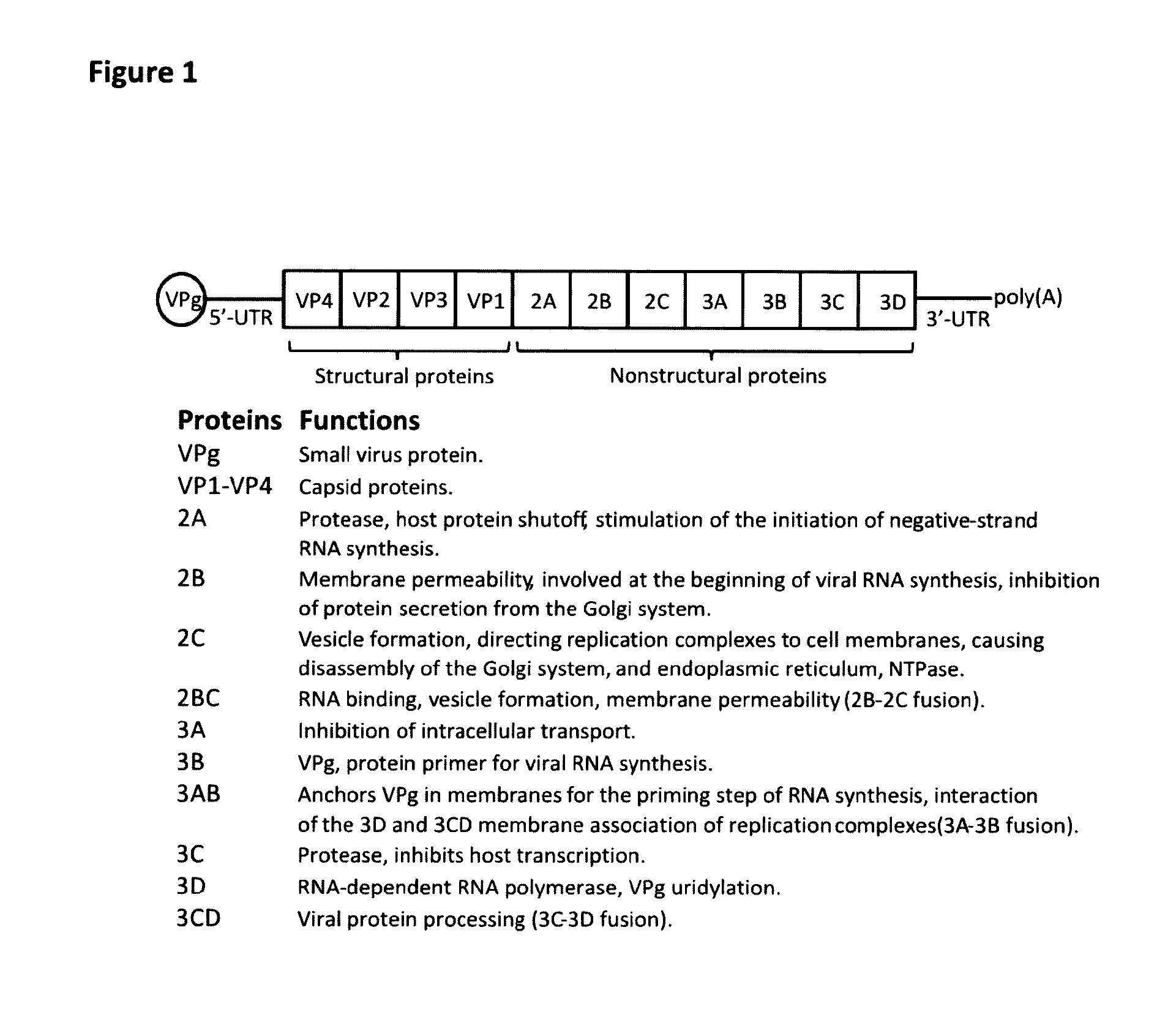
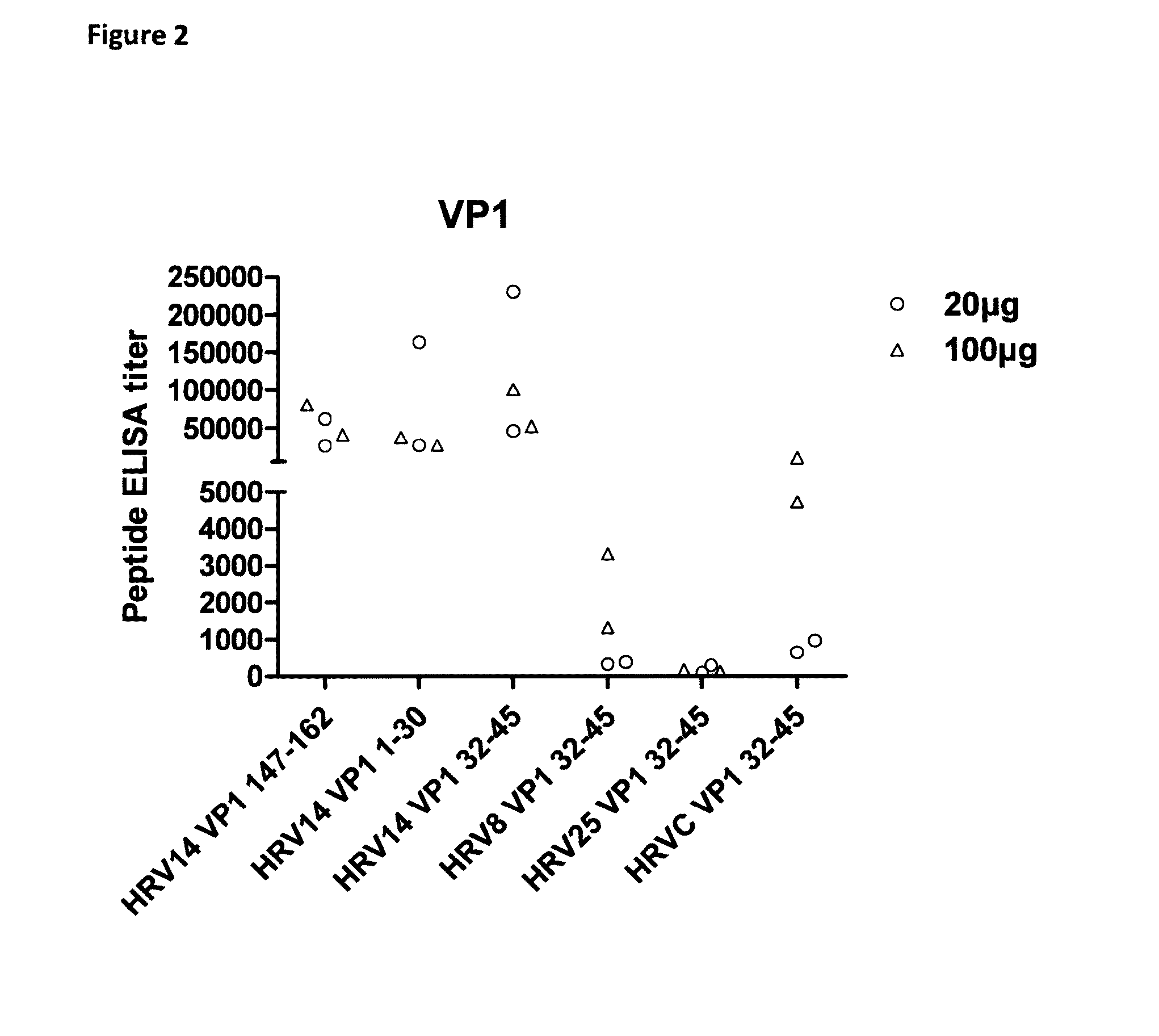
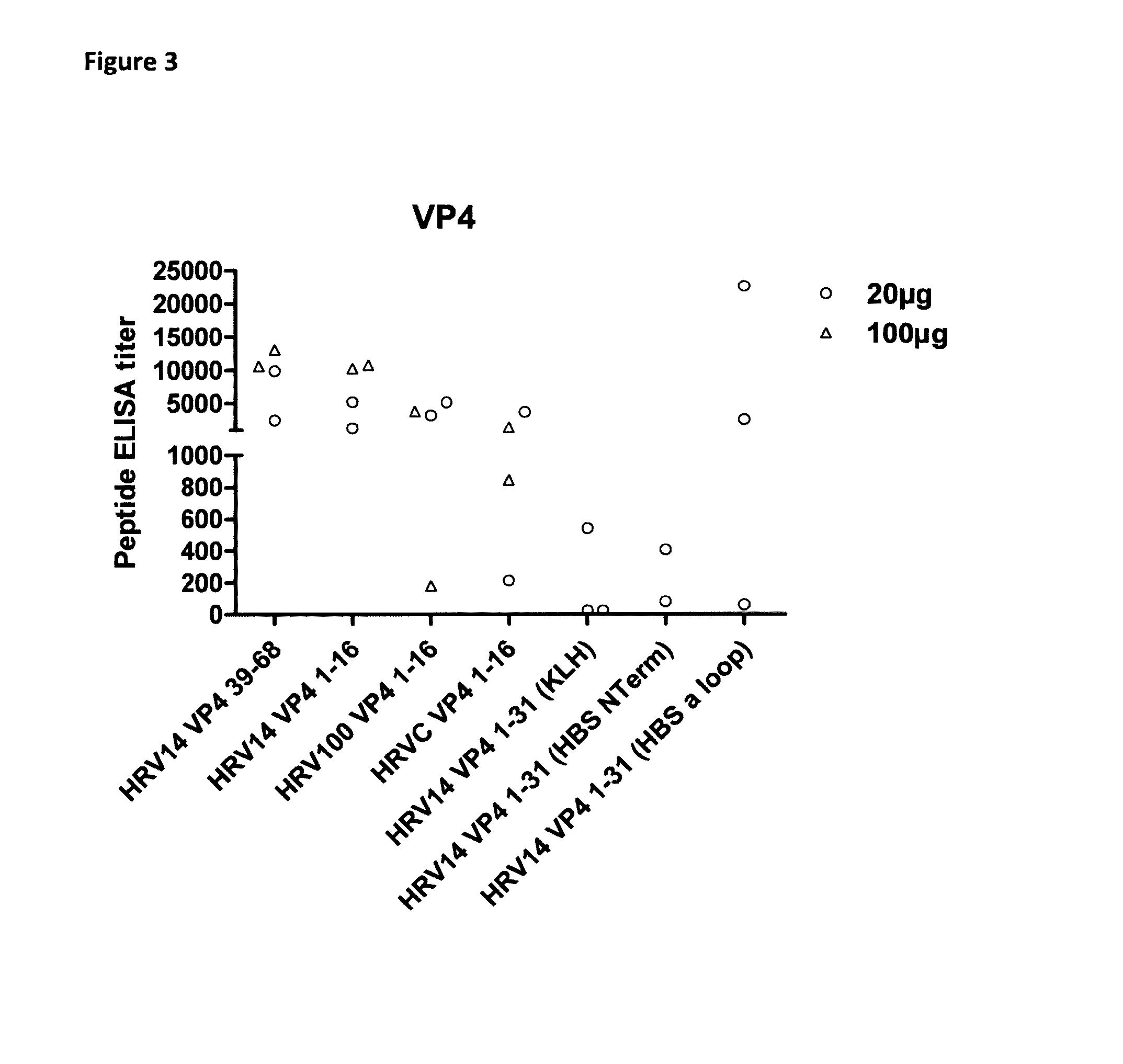
The disclosure provides immunogenic compositions comprising human picornavirus peptides derived from structural proteins of the virus, constructs comprising the peptides, the peptides themselves and their use in the prevention of picornavirus infection and disease. Particular peptides from VP4 and VP1 are disclosed.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Protein delivery system
InactiveCN101146522ASsRNA viruses negative-sensePeptide/protein ingredientsProtein targetVirus-like particle
The present invention relates to a virus-like particle (VLP) having a plasma membrane-derived lipid bilayer envelope, said VLP further comprising a viral structural protein, or fragment or derivative thereof, capable of forming an enveloped VLP, a fusiogenic protein and a recombinant target protein; methods for the delivery of recombinant target proteins to cells using said VLP, therapeutic methods using said VLP, compositions and kits comprising said VLP, methods of producing said VLP, and vectors and host cells for producing said VLP are also described.
Owner:BIOACTIVE PROTEIN DELIVERY THERAPEUTICS
Hepatitis c viral-like particle purification
InactiveUS20050272029A1SsRNA viruses positive-senseViral antigen ingredientsLipid formationHepatitis c viral
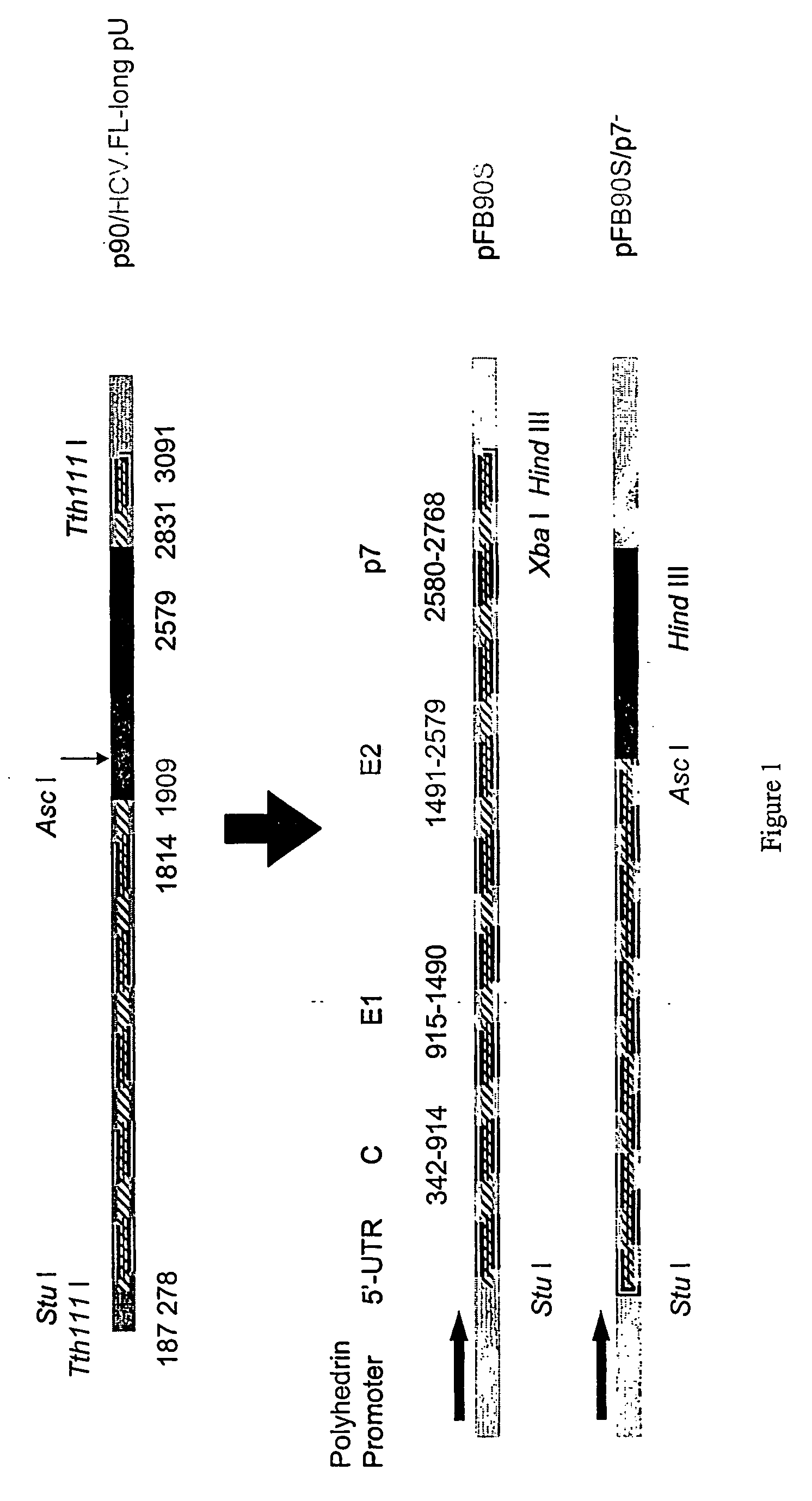
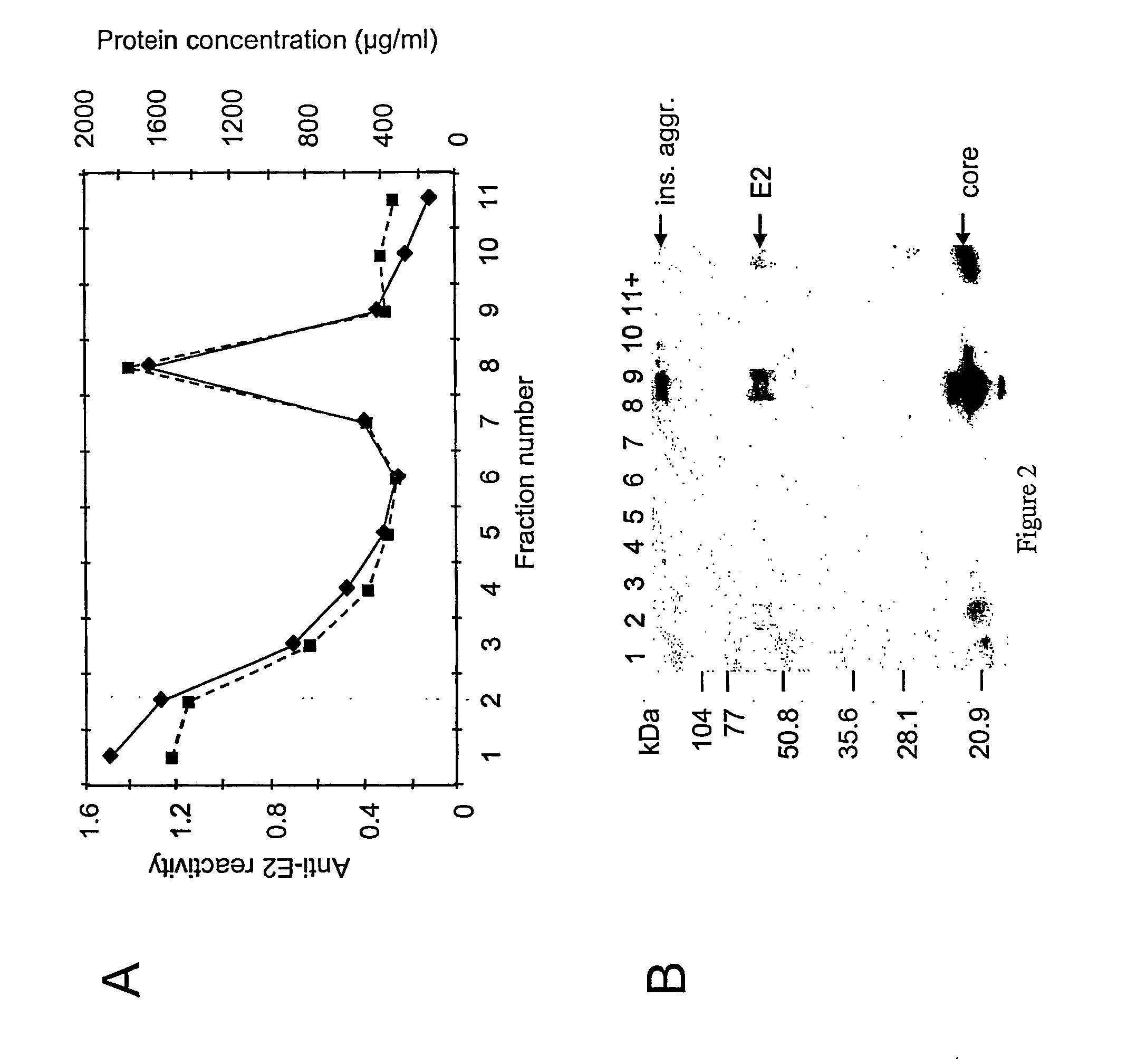
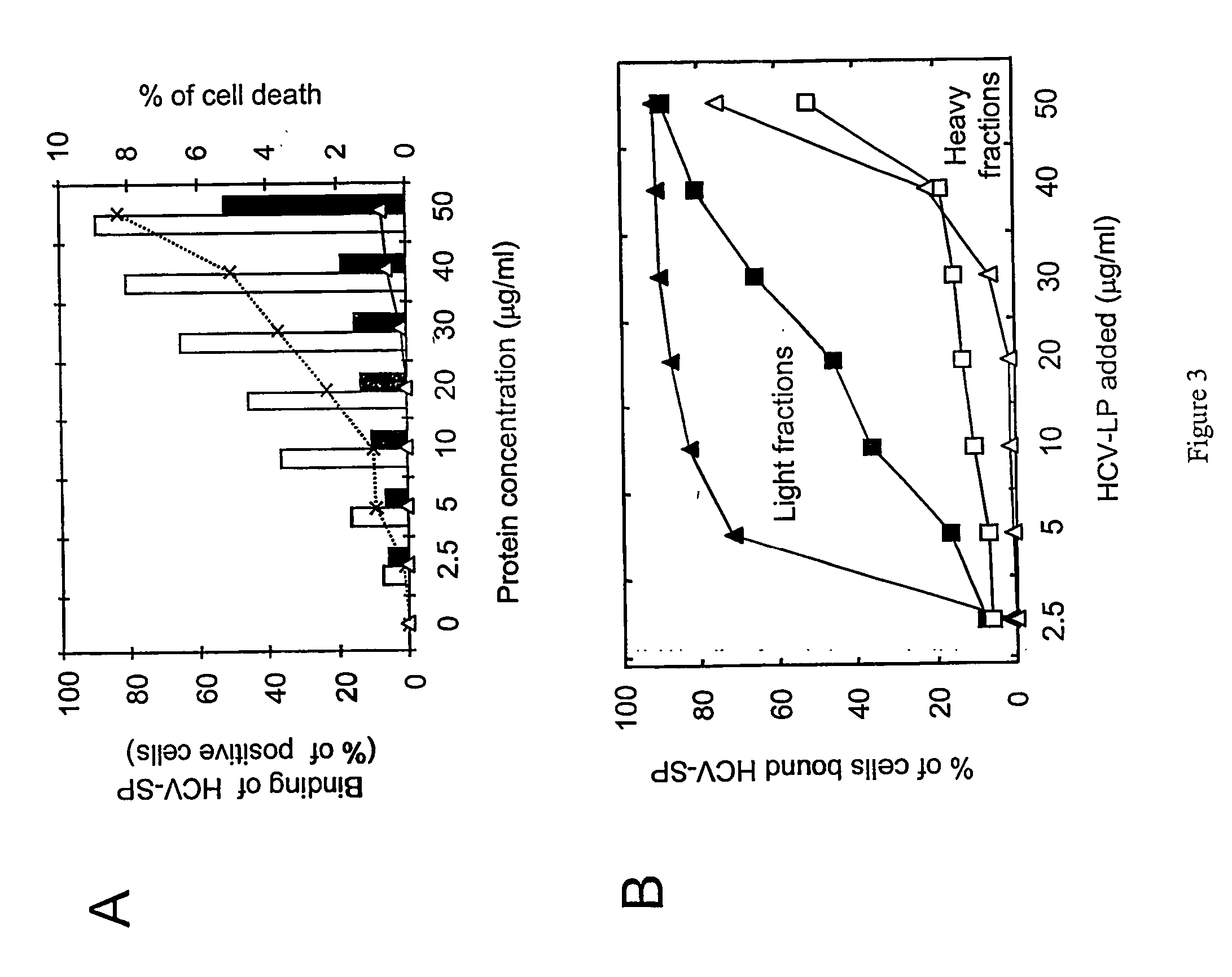
Methods for obtaining HCV complexes and HCV-like particles comprising HCV structural genes are provided. In one method, cells containing HCV-like particles are lysed with digitonin in the presence of protease inhibitors. Polyethylene glycol is slowly added to the lysate, to provide a precipitate that comprises complexes of the HCV structural proteins associated with lipid vesicles or micelles and complexes comprising viral structural proteins in the form of insoluble aggregates. In another method, the lysate is centrifuged through a sucrose cushion. Preferably, the pellet is then subjected to equilibrium ultracentrifugation, to provide a preparation of HCV-like particles that are heterogenous in size. The third method comprises subjecting the infected cells to hypertonic / hypotonic shock, and lysing the cells with digitonin in the presence of protease inhibitors. The lysate is pelleted and fractionated to provide a population of HCV-like particles that are substantially homogenous and have an average diameter of about 50 nm. As used herein the term “substantially homogenous” means that the shape of the particles are similar and that the size of the particles vary by 10% or less. Methods of using the HCV complexes and HCV-Iike particles as screening tools, diagnostic tools, and immunogenic compositions are also provided. Methods of treating patients exhibiting symptoms of HCV infection with compounds or substances that interfere with binding or internalization of the present HCV-like particles to asialoglycoprotein receptors are also provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE +2
Promoterless cassettes for expression of alphavirus structural proteins
ActiveCN101802199ASsRNA viruses positive-senseCell receptors/surface-antigens/surface-determinantsStart codonNucleotide
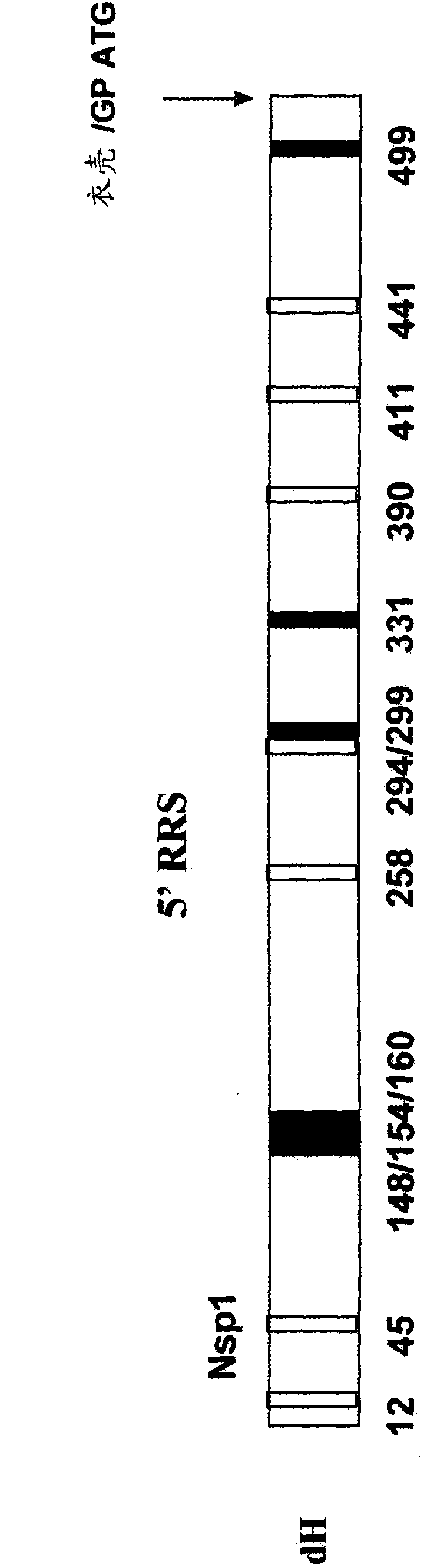
The present invention provides an isolated RNA molecule comprising: a) an alphavirus 5' replication recognition sequence, wherein at least one initiation codon has been removed from the 5' replication recognition sequence; b) a nucleotide sequence encoding an alphavirus structural protein; and c) an alphavirus 3' replication recognition sequence, with the proviso that the RNA molecule does not contain a promoter that directs transcription of the nucleotide sequence of (b), and wherein the alphavirus 5' and 3' replication recognition sequences of (a) and (c) direct replication of the RNA molecule in the presence of alphavirus nonstructural proteins.
Owner:ALPHAVAX INC
Virus-like particle vaccines
InactiveCN106573988ASsRNA viruses negative-senseSsRNA viruses positive-senseMultivalent VaccineViral structural protein
Owner:MEDIGEN BIOTECH
Viral structural protein genetic complement based coronavirus cell model
PendingCN112746059ASsRNA viruses positive-senseViral antigen ingredientsStructural proteinViral structural protein
The present invention relates to recombinant coronavirus and application thereof to preparation of vaccines. A genome of the recombinant coronavirus has deficiency in a gene encoding structural protein, which results in that the recombinant virus produced solely from the genome of the recombinant virus lacks the structural protein. The invention also relates to a preparation method of the recombinant coronavirus. The preparation method comprises the following steps of (1) carrying out in-vitro amplification on virus genome segments and carrying out in-vitro splicing; (2) performing in-vitro transcription to prepare genomic RNA of the recombinant virus, wherein the genomic RNA has a mutation in the gene encoding the structural protein, which results in that the recombinant virus produced solely from the genome of the recombinant virus lacks the structural protein; (3) preparing a packaging cell line, wherein the packaging cell line stably expresses the structural protein which is lacked by the recombinant virus; and (4) transfecting the genome RNA of the recombinant virus into the packaging cell line to generate the recombinant virus. The invention also relates to the packaging cell line for preparing the recombinant coronavirus.
Owner:TSINGHUA UNIV
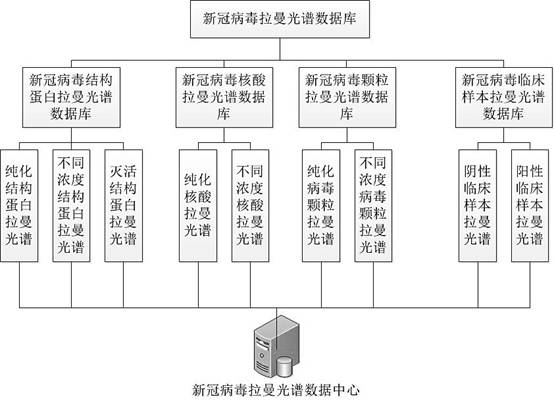
Construction method of novel coronavirus Raman spectrum data center
The invention discloses a construction method of a novel coronavirus Raman spectrum data center. The method comprises the following steps: S1, constructing a novel coronavirus structural protein Ramanspectrum database; S2, constructing a novel coronavirus nucleic acid Raman spectrum database; S3, constructing a novel coronavirus particle Raman spectrum database; S4, constructing a Raman spectrumdatabase of a novel coronavirus clinical detection sample; and storing each novel coronavirus Raman spectrum database into a novel coronavirus Raman spectrum detection server to form a novel coronavirus Raman spectrum data center. According to the invention, a set of complete novel coronavirus Raman spectrum database is effectively established, reliable standard data support is provided for the novel coronavirus Raman detection technology, and the accuracy and confidence of a detection result are effectively improved.
Owner:LASER FUSION RES CENT CHINA ACAD OF ENG PHYSICS
Replication-defective A virus expression vector system and vaccine preparation method
InactiveCN102443603AReduce the risk of useAvoid not being able to expand cultureViral antigen ingredientsAntiviralsNegative strandViral Vaccine
The invention discloses a replication-defective A virus expression vector system and a vaccine preparation method. The vector system consists of a replication-defective A virus vector of a deleted structural gene and minus strand complementary plasmids or cells containing a controllable virus A structural gene. In the preparation method, an exogenous gene target is introduced by constructing the replication-defective A virus vector of a deleted virus structural protein; and the replication-recombinant A virus can be continuously produced in a large scale by combining the minus strand complementary plasmids or cells containing the controllable A virus structural gene, so that the problem of low virus yield of a conventional A virus system can be solved, a target of preparing recombinant virus in a large scale is realized, and exogenous protein and virus sample particles can be expressed in scale. The replication-defective A virus expression vector system disclosed by the invention is suitable for large-scale production of DNA (deoxyribonucleic acid) vaccine, vector virus vaccine and exogenous protein expression.
Owner:SOUTH CHINA UNITED VACCINE INST
Pestivirus replicons providing an RNA-based viral vector system
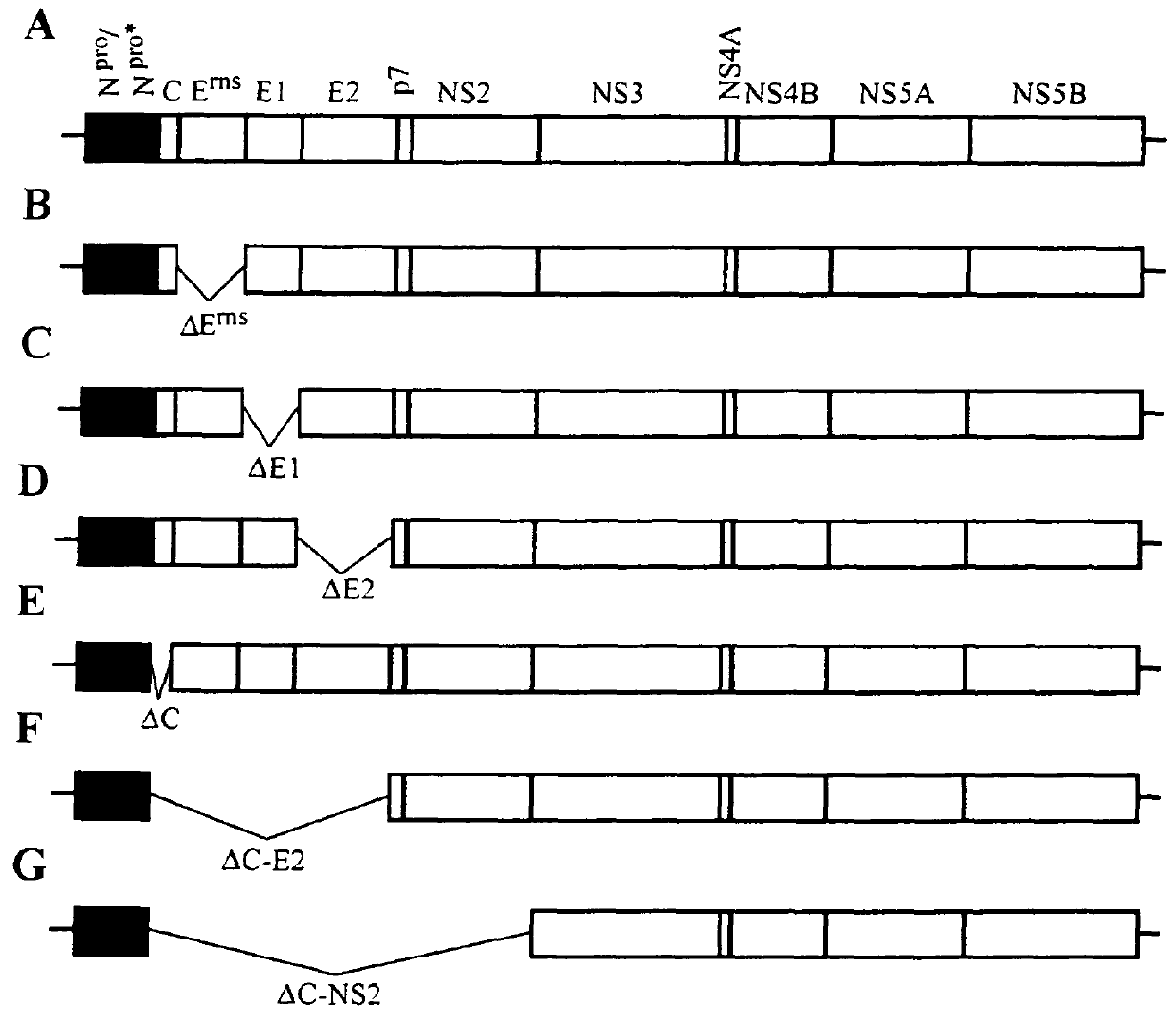
The present invention concerns replicons of pestiviruses, in particular replicons of swine fever virus, engineered to have a defective replication thereby having lost infectivity, and further containing a foreign gene. A replicon of the invention contains all the genetic information required for its replication, but lacks essential codons or all codons of at least one of the genes encoding the viral structural proteins E1, E2, Ems or C protein, and consequently cannot generate infectious virus particles. Particular replicons are generated with a mutated gene encoding a modified Npro protein that no longer controls the cell interferon-induction pathway. Another particular replicon lacks the genes encoding all the structural proteins, the p7 protein and the NS2 protein, and has cytopathogenic properties in transduced cells. The replicons of the invention provide a new vector system that can be used for vaccination, gene delivery and gene therapy applications in mammals, including humans, as naked RNA or packaged into any form of delivery vehicle.
Owner:INST FUER VIROLOGIE & IMMUNOLOGIE IVI
Protein kinase deficient, immunologically active CMVpp65 mutants
This invention relates to mutated CMVpp65, a viral structural protein which activates cell mediated immunity in humans infected with CMV. The mutations remove undesirable protein kinase activity naturally present in the protein and make it suitable for the production of both DNA and protein vaccines. Therefore, the invention provides proteins and DNAs, as well as vaccines comprising the proteins and DNAs, including cellular vaccines and vectors. Other embodiments of the invention relate to methods of enhancing immune response and vaccinating against CMV, including gene therapy methods and vectors.
Owner:CITY OF HOPE
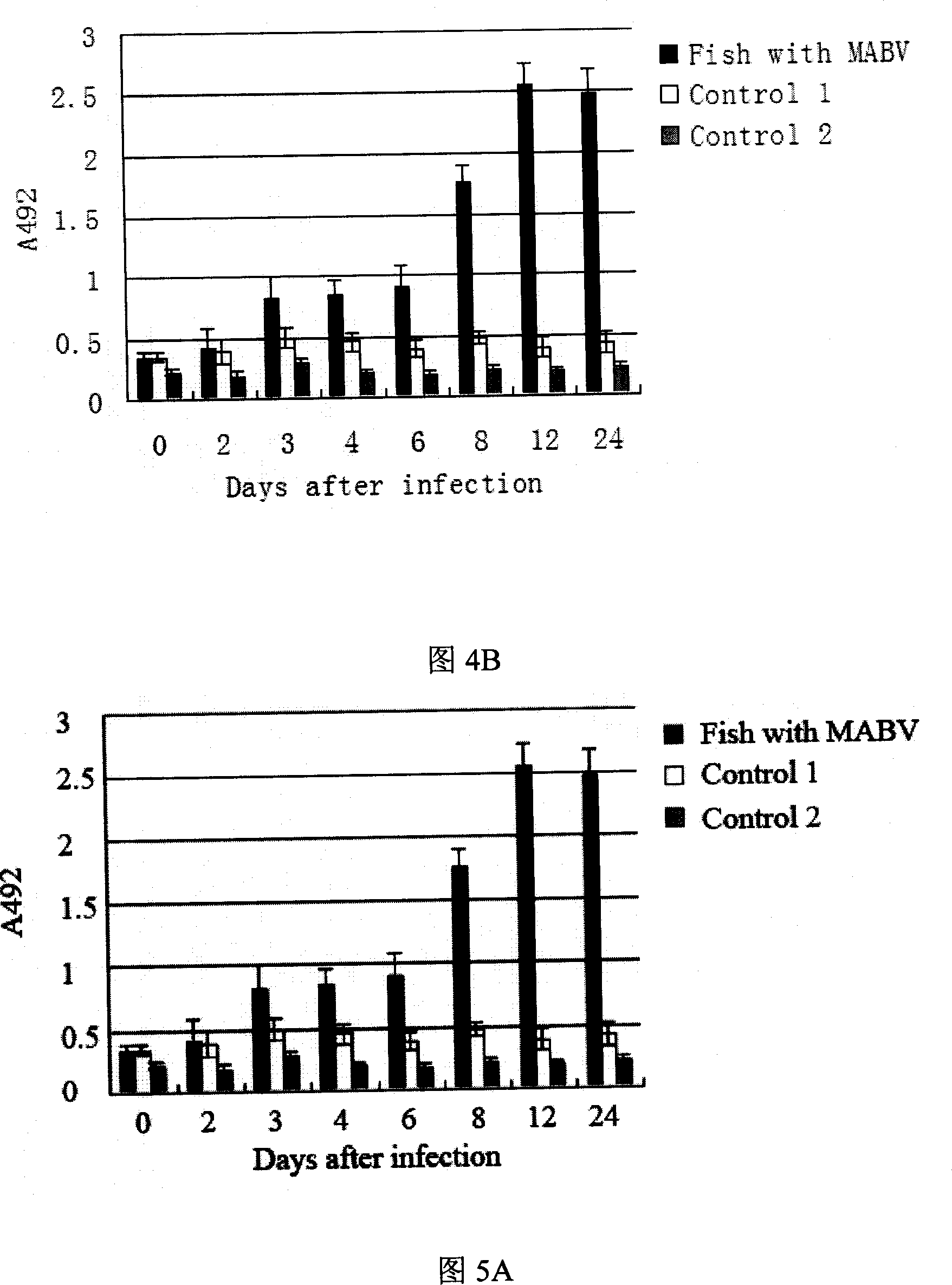
Method of preparing ocean double RNA virus MABV recombination albumen and application thereof
InactiveCN101016547AIncrease credibilityBacteriaViral antigen ingredientsPolyclonal antibodiesWestern blot
The invention discloses a preparing method of restructuring protein with ocean double RNA virus MABV structural protein gene, which is characterized by the following: the restructuring protein includes VP2 fusing protein or VP3 fusing protein and VP2 or VP3 protein; the VP2 fusing protein coded sequence possesses SEQ ID NO.1 nucleic acid sequence in sequence table; the VP3 fusing protein coded sequence possesses SEQ ID NO.2 nucleic acid sequence in sequence table; the polyclonal antibody produced by reconstructing protein increases confidence level during testing ocean double RNA virus MABV; it uses ELISA and western blot to test and is simpler than other method (RT-PCR, original position and so on). To set VP2 and VP3 as vaccine is safer than attenuated vaccine.
Owner:ZHEJIANG UNIV
Production of a parvovirus vaccine in plants as viral coat protein fusions
InactiveUS7270825B2Highly efficient and inexpensive productionEasily and highly purifiedSsRNA viruses positive-sensePeptide/protein ingredientsFeline parvovirusVaccine antigen
The present invention relates to foreign peptide sequences fused to recombinant plant viral structural proteins and a method of their production. Fusion proteins are economically synthesized in plants at high levels by biologically contained tobamoviruses. The fusion proteins of the invention have are useful as antigens for inducing the production of antibodies having desired binding properties, e.g., protective antibodies, or for use as vaccine antigens for the induction of protective immunity against the parvovirus. Feline parvovirus epitopes were fused to the N-terminus of the TMV coat protein, expressed in Nicotiana plants, extracted, purified, characterized and administered to animals, resulting in protective immunity.
Owner:KENTUCKY BIOPROCESSING
Virus-like particle as well as preparation method and application thereof
PendingCN114317571AImproving immunogenicityEnsure structural stabilityAntiviralsAntibody medical ingredientsSwine vesicular diseaseDisease
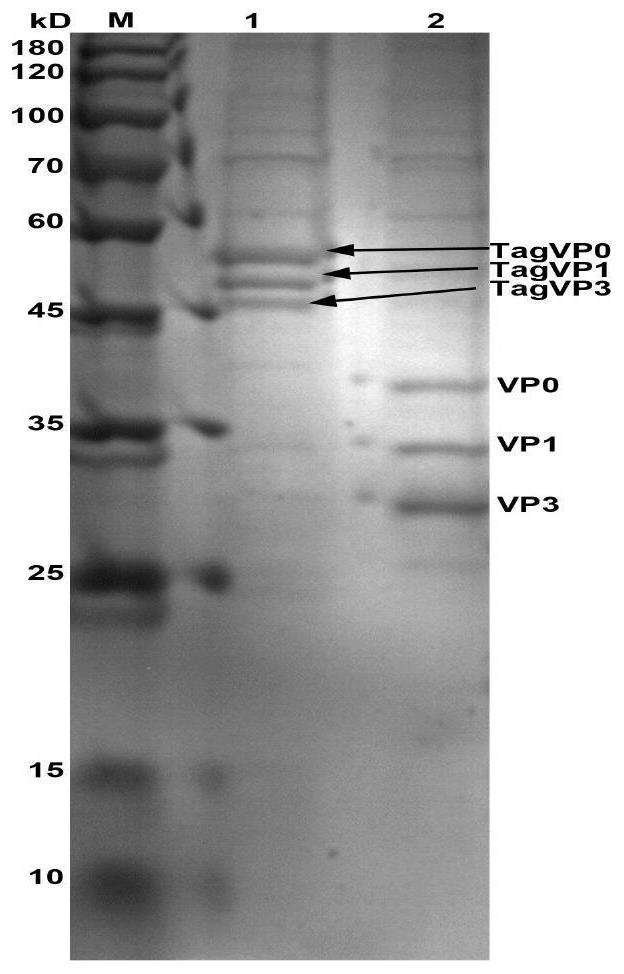
The invention provides a virus-like particle as well as a preparation method and application thereof, and belongs to the technical field of agricultural science, animal husbandry and veterinary science. Small ubiquitin-like modified proteins are used as tag proteins to promote correct folding of virus structural proteins VP0, VP1 and VP3, meanwhile, the structural stability of the proteins is guaranteed, mass expression of the structural proteins is achieved, and after in-vitro self-assembly of the three structural proteins, a large number of virus-like particles similar to natural viruses in performance are obtained. Immunogenicity detection shows that the prepared virus-like particles have high immunogenicity and can be used as reserve vaccines to be applied to prevention and control of swine vesicular disease or foot-and-mouth disease virus transmission.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com