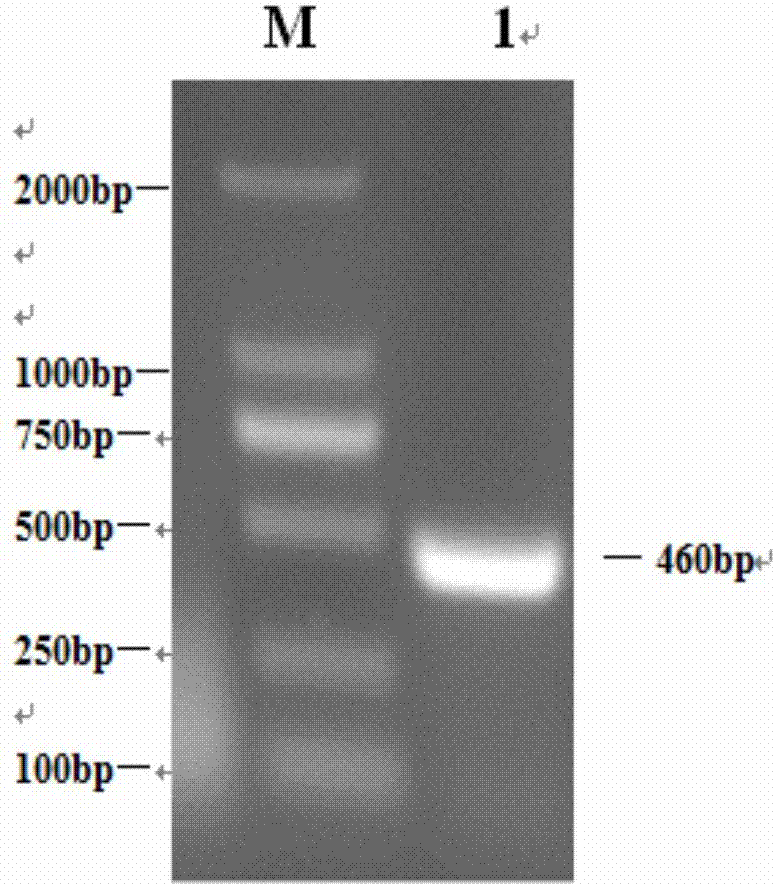
Patents
Literature
68 results about "Seneca Valley virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
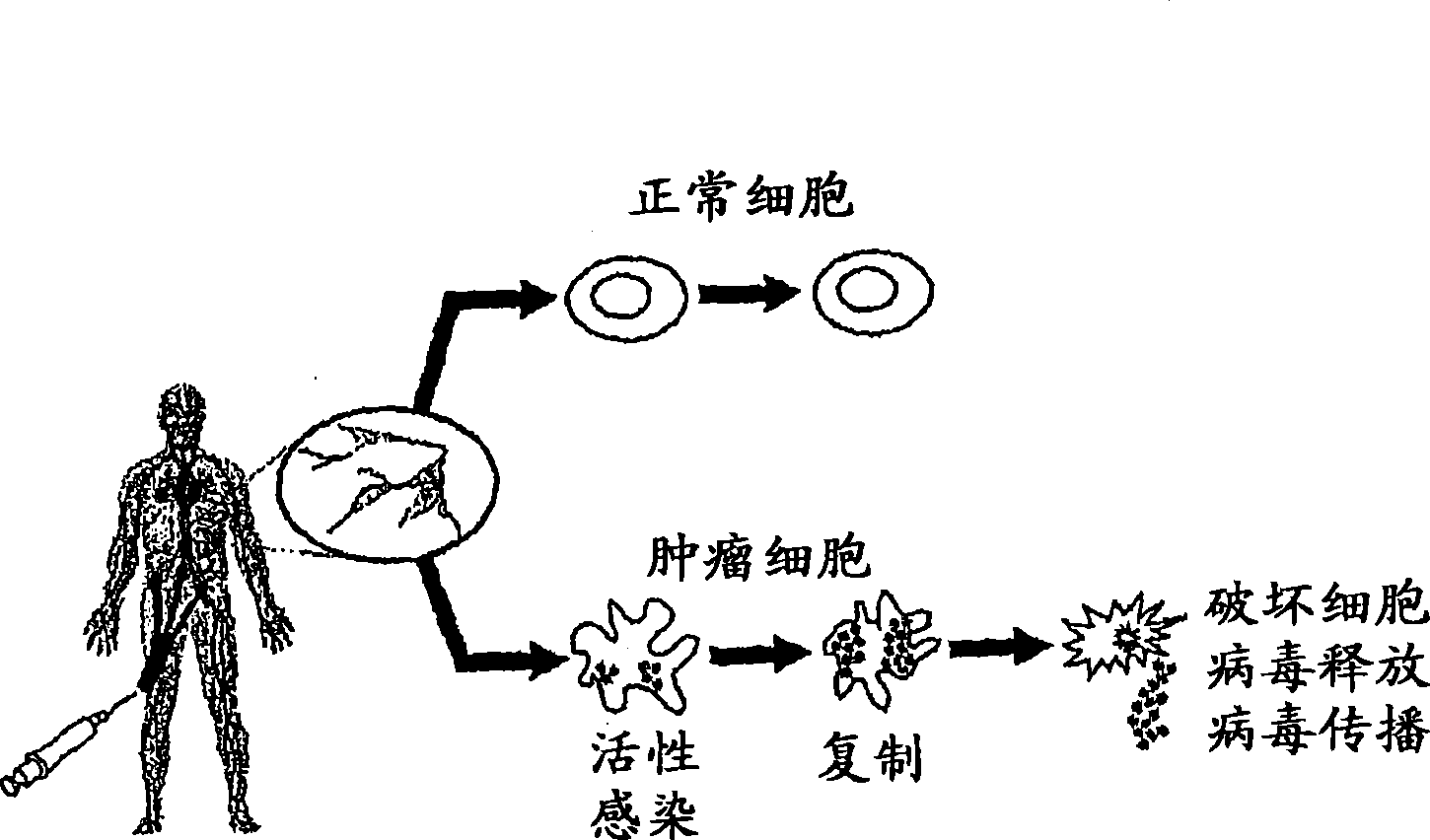
Seneca Valley virus (SVV) is a small non-enveloped virus with a positive-sense, single-stranded RNA genome of approximately 7.2 kilobases.
Enzyme-linked immunoassay kit of structural protein antibody for seneca valley virus
ActiveCN107253978AIncreased sensitivityStrong specificitySsRNA viruses positive-senseVirus peptidesChemical synthesisPositive control
The invention discloses an enzyme-linked immunoassay kit of a structural protein antibody for a seneca valley virus. The kit comprises an elisa plate, positive control serum, negative control serum, an HRP-conjugated antibody, a sample diluent, a 20-fold concentrated detergent, a substrate solution A, a substrate solution B and a stop solution, wherein the elisa plate is coated with a structural protein epitope polypeptide composition for the seneca valley virus. The epitope polypeptide composition is one or any combination of more than two of a polypeptide as shown in a sequence 1, a polypeptide as shown in a sequence 2, a polypeptide as shown in a sequence 3 or a polypeptide as shown in a sequence 4 in the sequence table. The elisa plate is coated with a chemical synthetic antigen peptide, so that the kit is low in antigen dosage and high in sensitivity and specificity, and whether the structural protein antibody is infected by the seneca valley virus or not can be efficiently detected. The kit is high in sensitivity, good in specificity, convenient in operation, and has a good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Seneca valley virus strain and application thereof
ActiveCN109182278AImproving immunogenicityDistant relationshipSsRNA viruses positive-senseViral antigen ingredientsDiseaseVaccine Immunogenicity
The invention discloses a Seneca valley virus strain and an application thereof, wherein the Seneca valley virus SVV-HeNXX / swine / 2017 is deposited in China Center For Type Culture Collection with theserial number of CCTCCNO: V201767. The Seneca valley virus SVV-HeNXX / swine / 2017 has good immunogenicity. It can induce immune animals to produce higher level of immune protection and can be used for preparing vaccine against Seneca Valley Virus Disease. The vaccine has high safety and no detoxification after immunization, and can induce animals to produce higher level of antibody quickly, so as toachieve high-efficient prevention of Porcine Seneca Valley Virus Disease, and has good popularization and application value.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
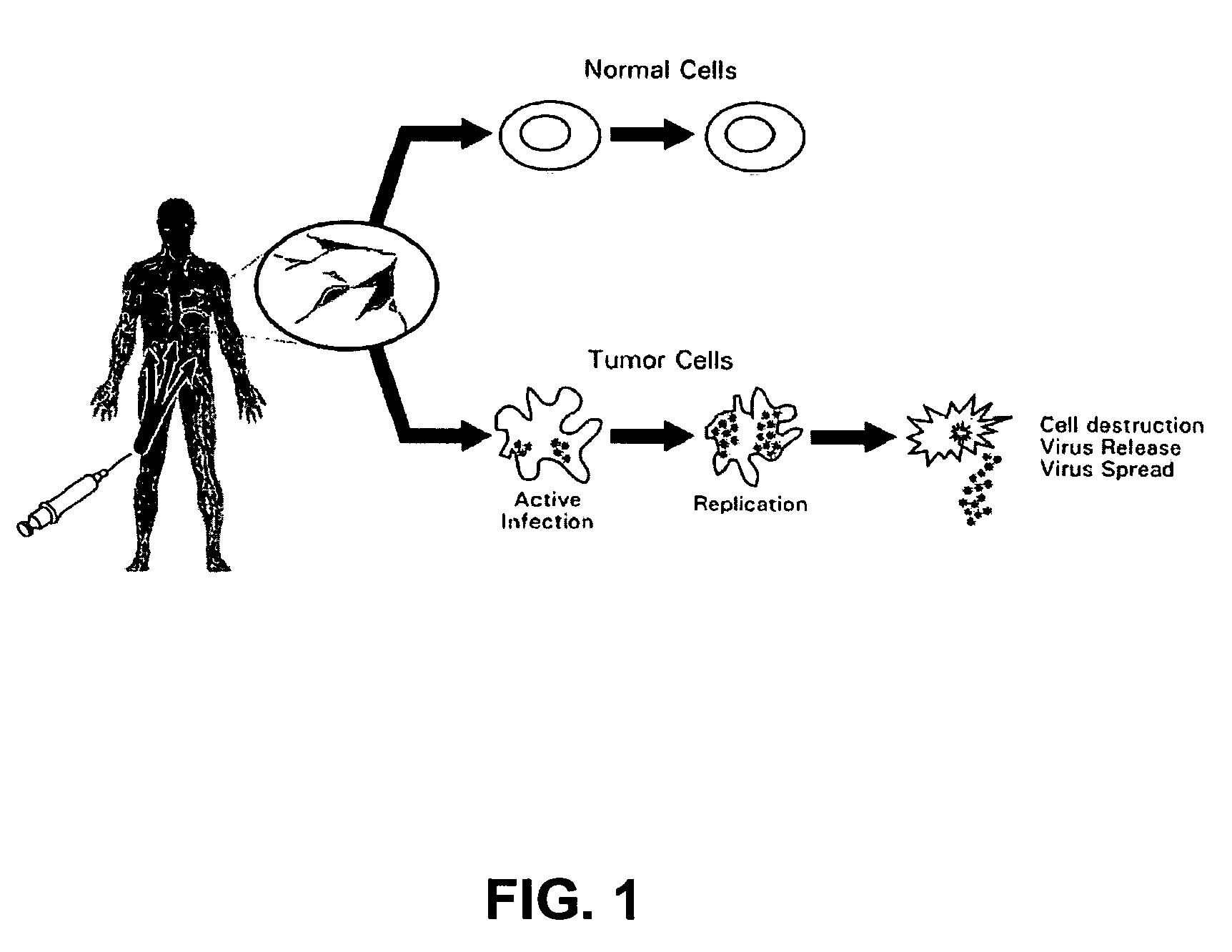
Seneca valley virus based compositions and methods for treating disease
ActiveUS20060159659A1High therapeutic indexSafe and effective and new lineBiocideSsRNA viruses positive-senseAbnormal tissue growthProtein detection
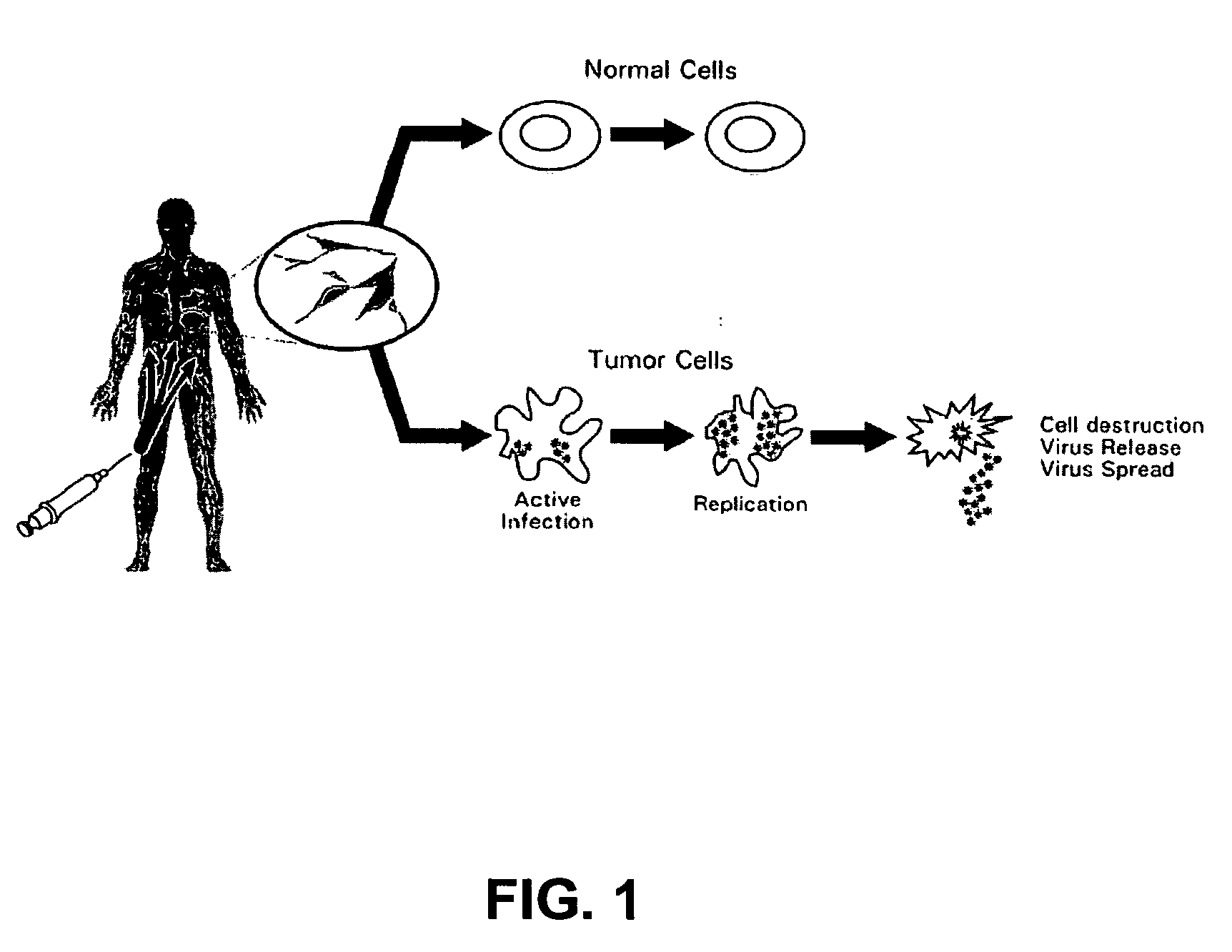
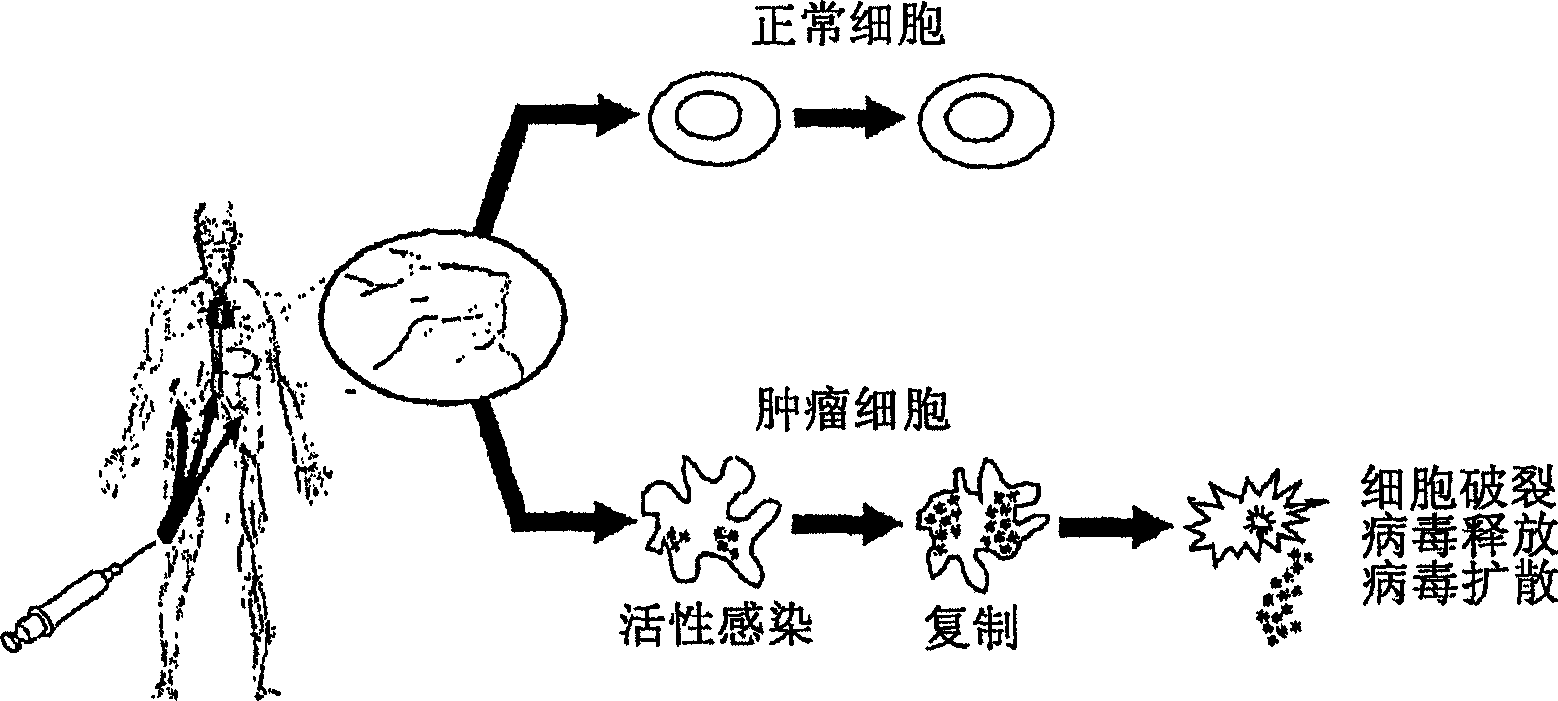
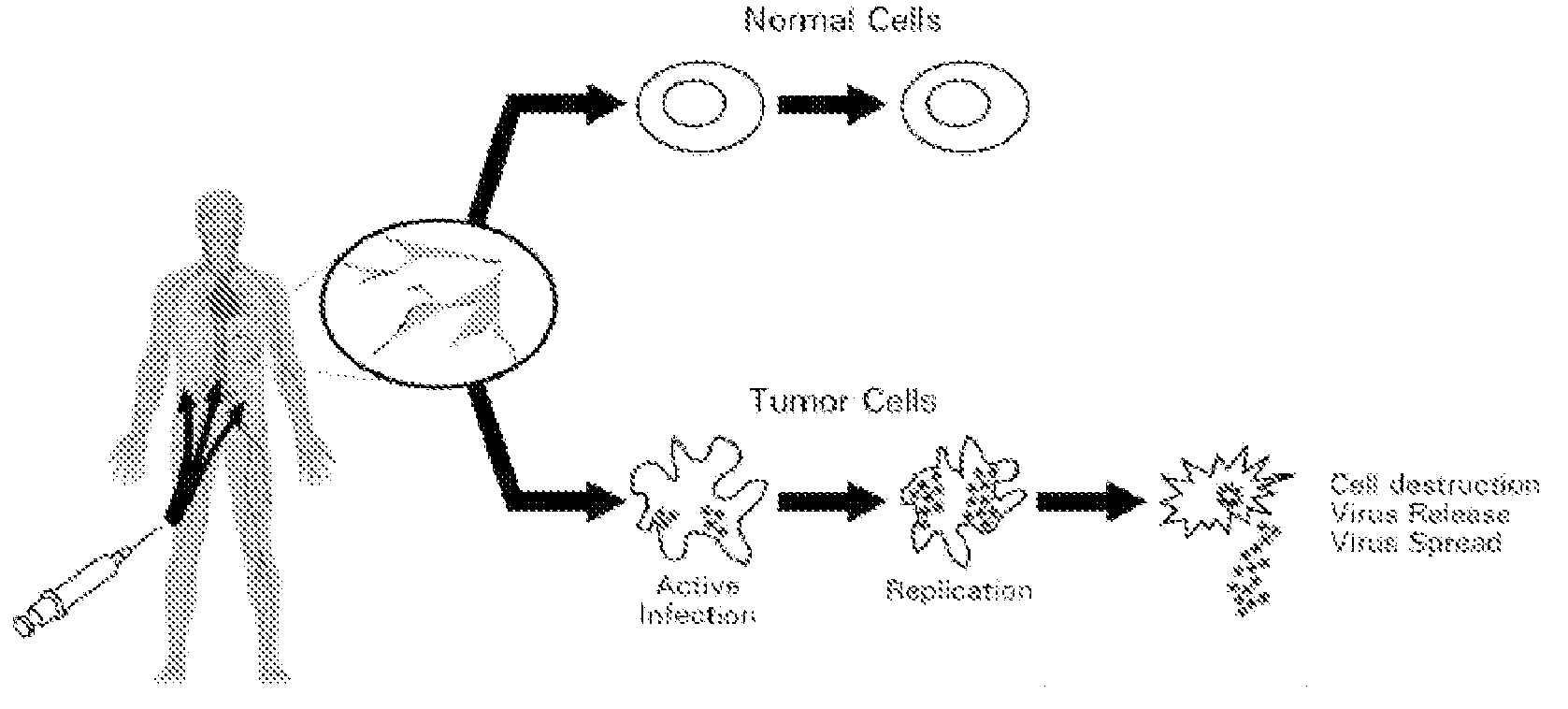
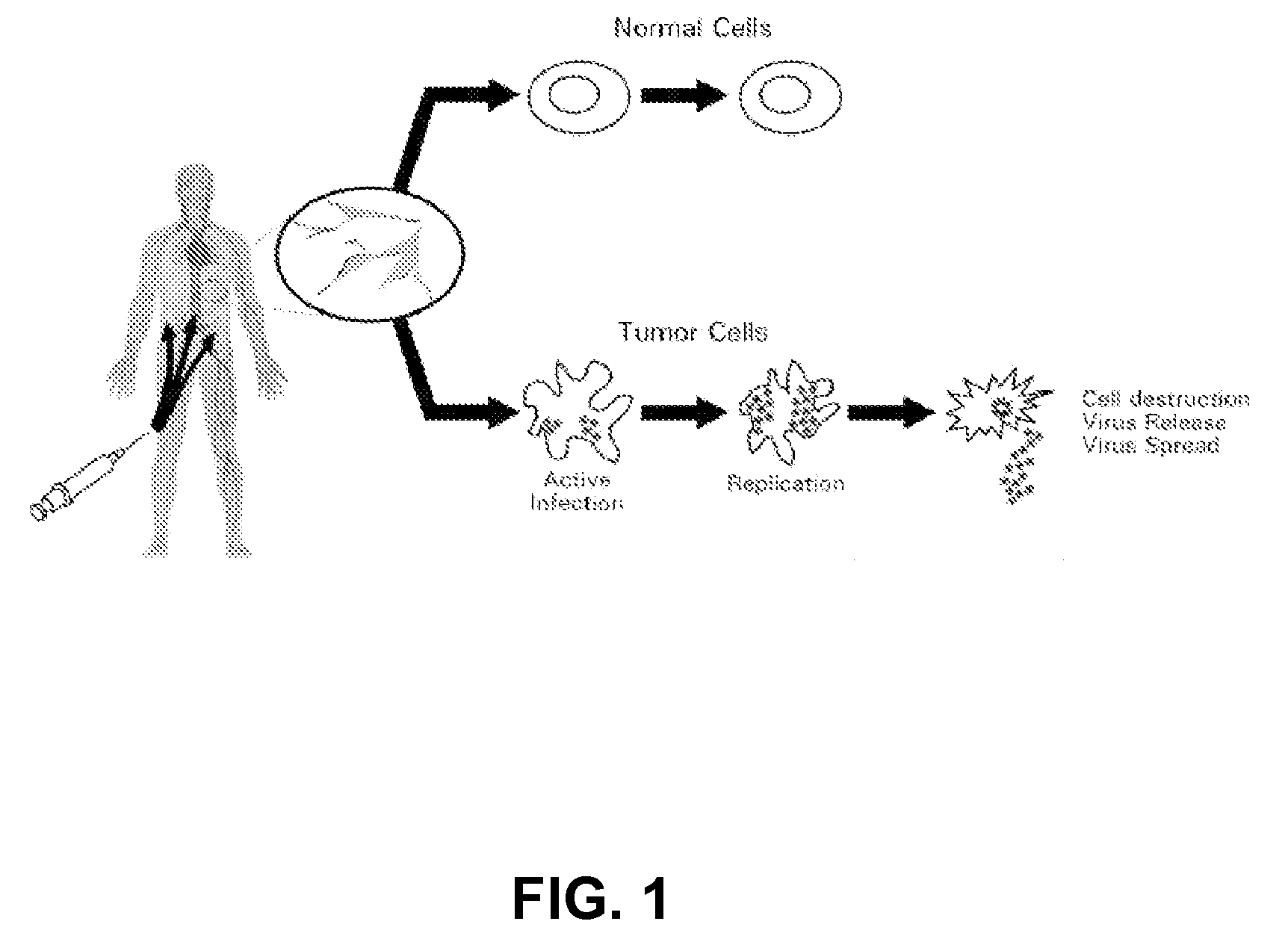
The present invention relates to a novel RNA picornavirus that is called Seneca Valley virus (“SVV”). The invention provides isolated SVV nucleic acids and proteins encoded by these nucleic acids. Further, the invention provides antibodies that are raised against the SVV proteins. Because SVV has the ability to selectively kill some types of tumors, the invention provides methods of using SVV and SVV polypeptides to treat cancer. Because SVV specifically targets certain tumors, the invention provides methods of using SVV nucleic acids and proteins to detect cancer. Additionally, due to the information provided by the tumor-specific mechanisms of SVV, the invention provides methods of making new oncolytic virus derivatives and of altering viruses to have tumor-specific tropisms.
Owner:PERCEIVER PHARMA +1
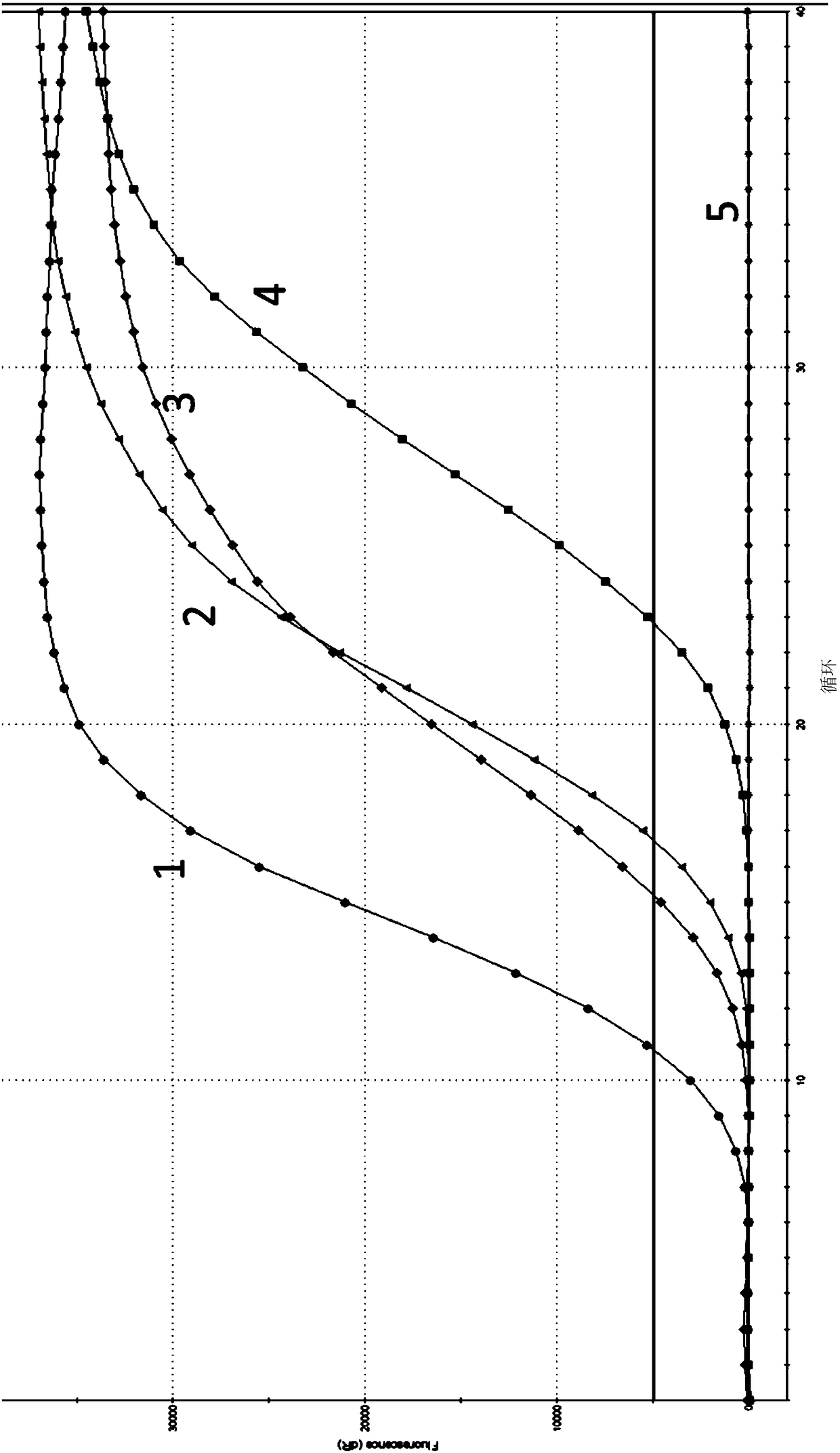
Dual real-time fluorescence quantitative PCR detection kit for foot-and-mouth disease and Seneca valley virus
InactiveCN107326100ARapid differential testImprove throughputMicrobiological testing/measurementMicroorganism based processesFluorescenceRapid identification
The invention provides a combination of a primer and a probe used for dual real-time fluorescence quantitative PCR detection of the foot-and-mouth disease virus and the Seneca valley virus and a detection kit. The sequences of the combination of the primer and the probe are respectively shown in the SEQ ID NO:1-7. The invention further provides a non-diagnostic-purpose dual real-time fluorescence quantitative PCR detection method for the foot-and-mouth disease virus and the Seneca valley virus. The three serotype foot-and-mouth disease viruses of O, A and AsiaI and the Seneca valley virus are rapidly identified and detected at the same time in the same reaction, the detection is finished within two hours, and the primer, the probe and the detection method have the advantages of rapidness, specificity, sensitivity and high throughput, and meet the requirements on large-batch rapid identification and detection of the three serotype foot-and-mouth disease viruses of O, A and AsiaI and the Seneca valley virus.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Porcine seneca valley virus strain and application thereof
ActiveCN107513524AGood strain backgroundLay the material foundationSsRNA viruses positive-senseViral antigen ingredientsPig farmsDisease
The invention discloses a porcine seneca valley virus strain and an application thereof. In the invention, the seneca valley virus strain CH-FJZZ-2017 is isolated from pathological materials of porcine idiopathic vesicular disease in a pig farm in Fujian Province, with the preservation number of CGMCC No. 12160. The isolated porcine seneca valley virus CH-FJZZ-2017 in the invention is isolated from swinery newly suffering from the epidemic disease within Chinese territory, represents the current epidemic predominant virus strain in China, has a good virus strain background, and can be used as an inactivated vaccine production virus strain and a virus seed for testing, thereby providing a material for subsequent relevant experimental study, and laying a material foundation.
Owner:CHINA ANIMAL HUSBANDRY IND
Primer for detecting RT-PCR of Seneca Valley viruses and RT-PCR detecting method
InactiveCN107034313ASpecific detectionGood primer specificityMicrobiological testing/measurementMicroorganism based processesNucleotideNucleotide sequencing
The invention relates to the technical field of detection of pig viruses, in particular to a primer for detecting RT-PCR of Seneca Valley viruses and a detecting method. Nucleotide sequences of primers are as shown in SEQ ID NO: 1 and SEQ ID NO: 2. The RT-PCR detecting method comprises the following steps: designing and synthesizing the primers; extracting a sample RNA and storing for subsequent use; taking the obtained sample RNA as a template RNA, and carrying out RT-PCR reaction by using the primers; and judging and reading a detecting result. According to the scheme, the technical means of virus detection is enriched, specificity and sensitivity are good, the detection speed is high, and the application value is also high.
Owner:WENS FOOD GRP CO LTD
HRM detecting primers and method for distinguishing foot-mouth disease virus and Seneca Valley virus
ActiveCN105695628AStrong specificityGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceGene type
The invention belongs to the technical field of biological detection, and discloses HRM detecting primers and a method for distinguishing the foot-mouth disease virus (FMDV) and the Seneca Valley virus (SVV). The primers have the sequences shown in SEQ ID NO:1 and SEQ ID NO:2 and are high in specificity. By means of the primers, PCR amplification is conducted on the FMDV and the SVV, then fluorescent data is collected by monitoring the combination situation of double-chain DNA fluorescent dyes and PCR amplification products in the temperature rise process in real time, and the FMDV and the SVV are distinguished according to the difference of two dissolution curves; the two gene types can be distinguished after PCR amplification is conducted through the primers, it takes people only 3 hours for the whole operation process, no virus cell culture is needed, and the type distinguishing time is greatly shortened; expanses are low, no specific probe is needed, and fluorescent saturated dyes are low in price and easy to obtain; accuracy, specificity and repeatability are high, analysis can be accurately and rapidly conducted at high throughput, and the primers and method are easy to apply and popularize in clinical practices.
Owner:SOUTH CHINA AGRI UNIV
Seneca valley virus based compositions and methods for treating disease
The present invention relates to a novel RNA picornavirus that is called Seneca Valley virus ('SVV'). The invention provides isolated SVV nucleic acids and proteins encoded by these nucleic acids. Further, the invention provides antibodies that are raised against the SVV proteins. Because SVV has the ability to selectively kill some types of tumors, the invention provides methods of using SVV and SVV polypeptides to treat cancer. Because SVV specifically targets certain tumors, the invention provides methods of using SVV nucleic acids and proteins to detect cancer. Additionally, due to the information provided by the tumor-specific mechanisms of SVV, the invention provides methods of making new oncolytic virus derivatives and of altering viruses to have tumor-specific tropisms.
Owner:NOVARTIS PHARM AG
Seneca valley virus based compositions and methods for treating disease
InactiveCN101448526ASimple and fast life cycleEasy to operateSugar derivativesGenetic material ingredientsViral nucleic acidTropism
The present invention relates to a novel RNA picornavirus that is called Seneca Valley virus ('SVV'). The invention provides isolated SW nucleic acids and proteins encoded by these nucleic acids. Further, the invention provides antibodies that are raised against the SVV proteins. Because SVV has the ability to selectively kill some types of rumors, the invention provides methods of using SVV and SVV polypeptides to treat cancer. Because SVV specifically targets certain tumors, the invention provides methods of using SW nucleic acids and proteins to detect cancer. Additionally, due to the information provided by the tumor-specific mechanisms of SVV, the invention provides methods of making new oncolytic virus derivatives and of altering viruses to have tumor-specific tropisms.
Owner:纽特罗佩克斯公司 +1
TaqMan-MGB fluorescent quantitative polymerase chain reaction (PCR) detection primer, TaqMan-MGB fluorescent quantitative PCR detection probe and TaqMan-MGB fluorescent quantitative PCR detection method for seneca valley virus (SVV)
InactiveCN106916910ASimple designImproved conservatismMicrobiological testing/measurementDNA/RNA fragmentationMgb probeBiology
The invention relates to the technical field of molecular biology detection, in particular to a TaqMan-MGB fluorescent quantitative polymerase chain reaction (PCR) detection specific primer (as shown in SEQ ID No. 1 and SEQ ID No. 2), a TaqMan-MGB fluorescent quantitative PCR detection probe as shown in SEQ ID No. 3) and a TaqMan-MGB fluorescent quantitative PCR detection method for seneca valley virus (SVV). The method comprises the steps of drawing a standard curve; extracting ribonucleic acid (RNA) of a sample virus; carrying out reverse transcription on the RNA of the sample virus; enabling the product of the reverse transcription to have a TaqMan-MGB fluorescent quantitative PCR, and reading the result. The primer provided by the invention has better specificity and sensitivity; the MGB probe provided by the invention is shorter and is beneficial to probe design, and the Tm value difference between a paired template and a non-paired template is improved, so that the experimental result is more stable and accurate; the method provided by the invention has the advantages of being simple and rapid, easy to operate, visual in results, high in sensitivity, good in stability, real-time quantitative, and the like, and shortens the reaction time.
Owner:WENS FOOD GRP CO LTD
Seneca valley virus real-time fluorescence quantification PCR detection primer and kit
ActiveCN105925728AStrong specificityAccurate identificationMicrobiological testing/measurementMicroorganism based processesAgricultural scienceFluorescence
The invention belongs to the technical field of biological detection, and discloses a Seneca valley virus real-time fluorescence quantification PCR detection primer and a kit. Firstly, the Seneca valley virus real-time fluorescence quantification PCR detection primer and a probe are obtained through design and screening, the sequences of an upstream primer, a downstream primer and the probe are shown as SEQ ID NO: 1-3; the primer and the probe are used to specifically amplify a Seneca valley virus, real-time fluorescence quantification monitors the condition of combination of a double-stranded DNA fluorescent dye with a PCR amplification product in the PCR process in real time, then fluorescent data are acquired, and the SVV (Seneca Valley Virus) is identified according to a CT value; the SVV is identified after the method is used to carry out PCR amplification, the accuracy is high, the specificity and the repeatability are good, the SVV can be identified accurately, rapidly and efficiently, and the popularization and the application in clinical practice benefits are facilitated.
Owner:SOUTH CHINA AGRI UNIV
Solid phase competition ELISA kit for detecting Seneca valley virus antibody, and applications thereof,
InactiveCN107894508AAvoid cross reactionShorten detection timeBiological testingElisa kitSenecavirus
The invention discloses a solid phase competition ELISA kit for detecting Seneca valley virus antibody, and applications thereof, wherein the kit comprises Seneca valley virus inactivated antigen-coated enzyme label plate and HRP-labeled Seneca valley virus rabbit anti-IgG. According to the present invention, the primary antibody and the secondary antibody in the traditional ELISA are replaced with the HRP-labeled rabbit anti-IgG so as to simplify the operation step; by changing the coating stabilization process, the surface of the solid phase carrier is coated with the SVV inactivated antigen, such that the enzyme-labeled antibody diluent preparation process is changed, the enzyme-labeled antibody working liquid can be stably stored without the change of the activity and the titer, and the solid phase competition ELISA kit for the specific detection of the SVV antibody, and the detection method thereof are established; and with the kit, the blank in the SVV ELISA antibody detection ismade up, the problems of low repeatability, low sensitivity and cumbersome operation procedures in the SVV antibody detection in the existing VNT detection are overcome, and the effective technical means is provided for the SVV antibody detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation method and application of full-length infectious clone of porcine SVV (Seneca Valley Virus)
ActiveCN109810976ANo gene deletions were foundMicrobiological testing/measurementViruses/bacteriophagesSenecavirusViral infection
The invention relates to a preparation method and an application of a full-length infectious clone of a porcine SVV (Seneca Valley Virus), comprising the following steps: (1) amplification of a full sequence of an SVV / GD05 strain genome; (2) construction of infectious clone plasmid of a pSVV-GD05 virus; (3) construction of pSVV-GD05-iLOV recombinant plasmid; and (4) rescue of a parental virus (SVV-GD05) and a recombinant virus (SVV-GD05-iLOV). The method provided by the invention utilizes a strain of the SVV isolated from a Chinese pig herd to construct a viral infectious clone pSVV-GD05 withbacterial plasmid as a skeleton, and can successfully rescue the virus. At the same time, a reporter gene iLOV is inserted into an SVV infectious clone virus genome, and the recombinant SVV virus capable of expressing the reporter gene is successfully rescued. The preparation method and application of the invention provide an effective platform for deep and basic research and application of SVV, and have important scientific application value.
Owner:YANGZHOU UNIV
Seneca valley virus SVV-ZM-201801 and application thereof
ActiveCN109554352APromote rapid proliferationHigh titerSsRNA viruses positive-senseViral antigen ingredientsCytopathic effectCulture cell
The invention provides a Seneca valley virus SVV-ZM-201801 and an application thereof. Separation is performed from pig tissues, and sub-culture and plaque purification are performed, so that the Seneca valley virus is obtained. The separated strain can be stably proliferated on sub-culture cells, typical cytopathic effects are formed, and the virus titer is as high as 108.5-1010.5TCID50 / mL. The separated strain does not have obvious pathogenicity on piggies. The Seneca valley virus separated strain has good proliferating properties, and is good in immunogenicity. Vaccines prepared from the separated strain can induce the piggies to generate high-level neutralizing antibodies, and powerful technical support is provided for effective prevention and control of the Seneca valley virus.
Owner:CHINA ANIMAL HUSBANDRY IND
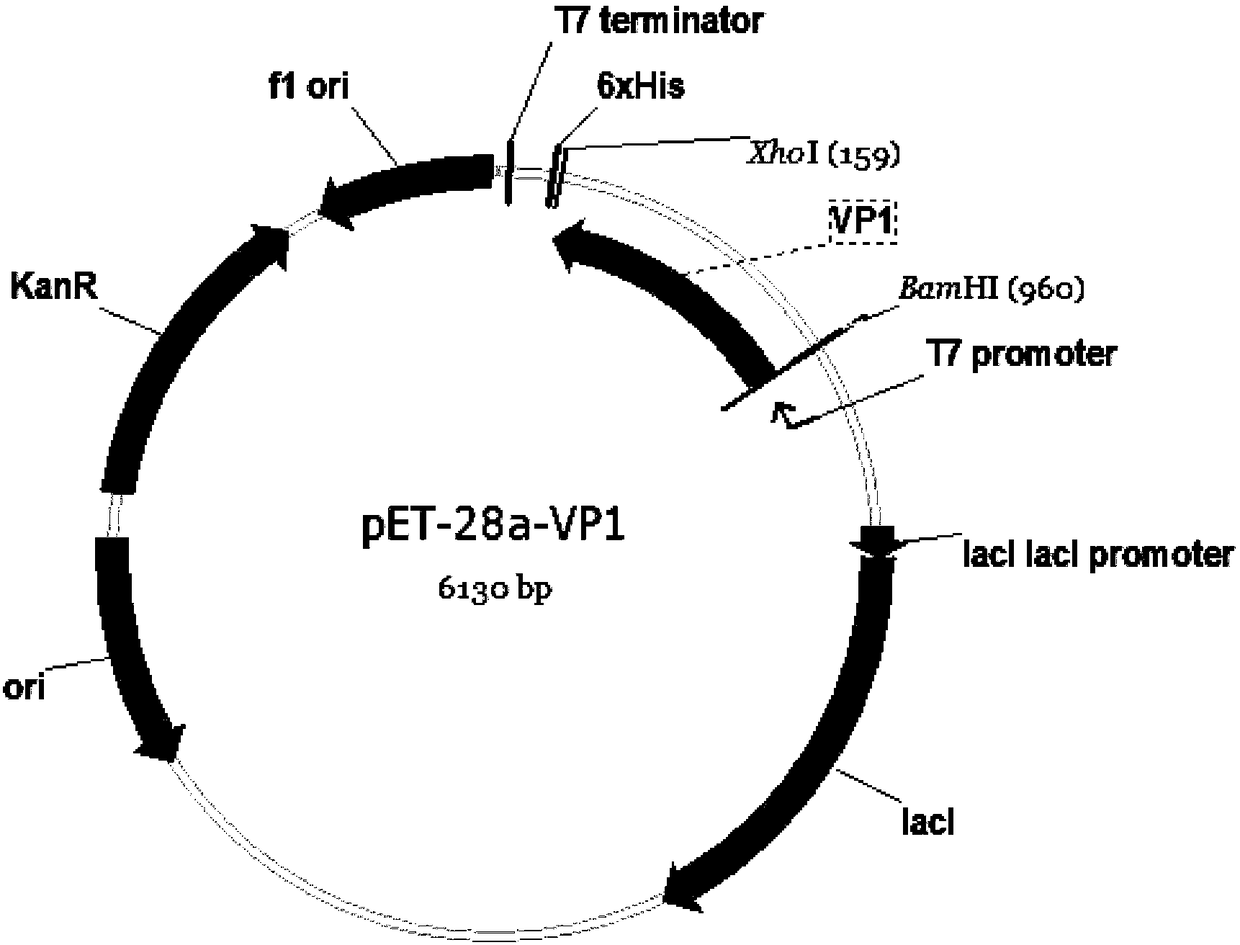
Seneca Valley virus VP1 protein, coding gene, hybridoma cell line and monoclonal antibody and application thereof
The invention relates to a Seneca valley virus VP1 protein, a coding gene, a hybridoma cell line and a monoclonal antibody and application thereof, which belongs to the technical field of viruses. TheSeneca valley virus Hubei strain VP1 protein is used as a Seneca Valley virus antigen protein in immunodetection. The invention provides a hybridoma cell line 2G6 of a monoclonal antibody capable ofsecreting the Seneca Valley virus Hubei strain VP1 protein according to claim 1, and a preservation number of the hybridoma cell line is CCTCC NO. C2017226. The monoclonal antibody is secreted from the hybridoma cell line 2G6. The invention relates to application of the monoclonal antibody in immune combined Seneca Valley virus Hubei strain VP1 protein. The invention provides application of the monoclonal antibody in a kit for preventing the Seneca Valley virus Hubei strain from infecting the cells.
Owner:HUAZHONG AGRI UNIV
Seneca valley virus structural protein epitope polypeptide and application thereof
ActiveCN109627293AIncreased sensitivityStrong specificitySsRNA viruses positive-senseVirus peptidesChemical synthesisEpitope
The invention discloses a Seneca valley virus structural protein epitope polypeptide and application thereof. The polypeptide is the polypeptide represented by a sequence 1 in a sequence table, a sequence 2 in the sequence table, a sequence 3 in the sequence table or a sequence 4 in the sequence table. The Seneca valley virus structural protein epitope polypeptide is used for preparing a chemically synthesized antigen peptide coated elisa plate used for a kit, is low in antigen dosage and high in sensitivity and specificity, can effectively detect whether a structural protein antibody infectedwith a Seneca valley virus exists or not, is high in sensitivity, good in specificity and fast and convenient to operate and has a good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Chemiluminescence immunoassay kit for Seneca valley virus nonstructural protein 3ABC antibody detection
InactiveCN108872576AIncrease productionHigh detection sensitivityChemiluminescene/bioluminescenceBiological material analysisSwine vesicular diseasePositive control
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Seneca valley virus, preparation method of seneca valley virus inactivated vaccine, seneca valley virus inactivated vaccine and application thereof
ActiveCN109679927AImprove securityImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsDiseaseVaccine Immunogenicity
The invention provides a seneca valley virus A / ZJ / 2015, a preparation method of a seneca valley virus inactivated vaccine, the seneca valley virus inactivated vaccine and an application thereof, and belongs to the technical field of veterinary biological products. The Latin name of the seneca valley virus is seneca valley, and the preservation number is CGMCC No.15034. According to the invention,the seneca valley virus is cultured to prepare the inactivated vaccine for preventing seneca valley virus diseases. The vaccine has good immunogenicity to healthy susceptible one month old pigs, and the protection rate after inoculation is 90% or above.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
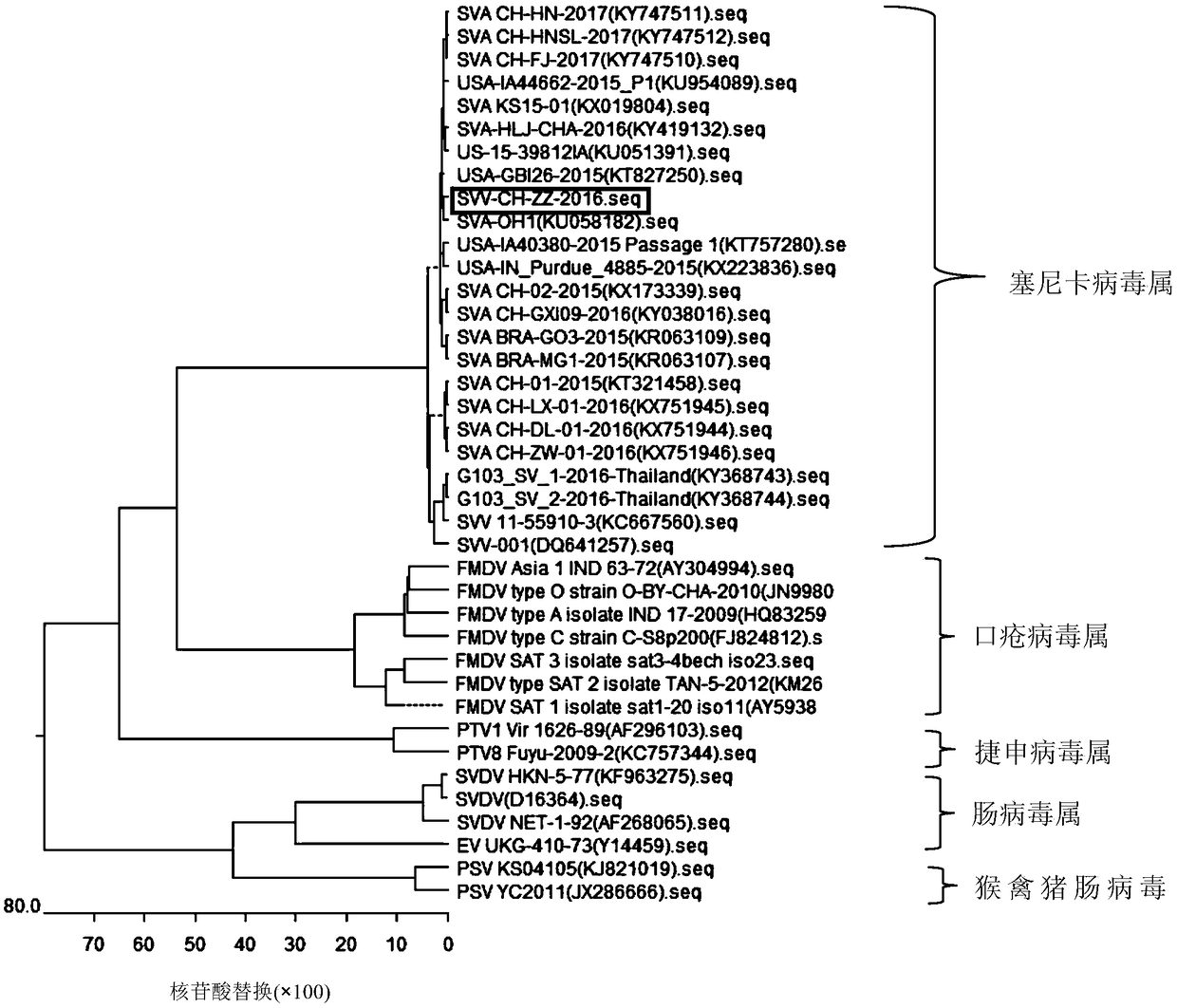
Whole genome sequence of Seneca valley virus SVV/CH/ZZ/2016 and amplification primer thereof
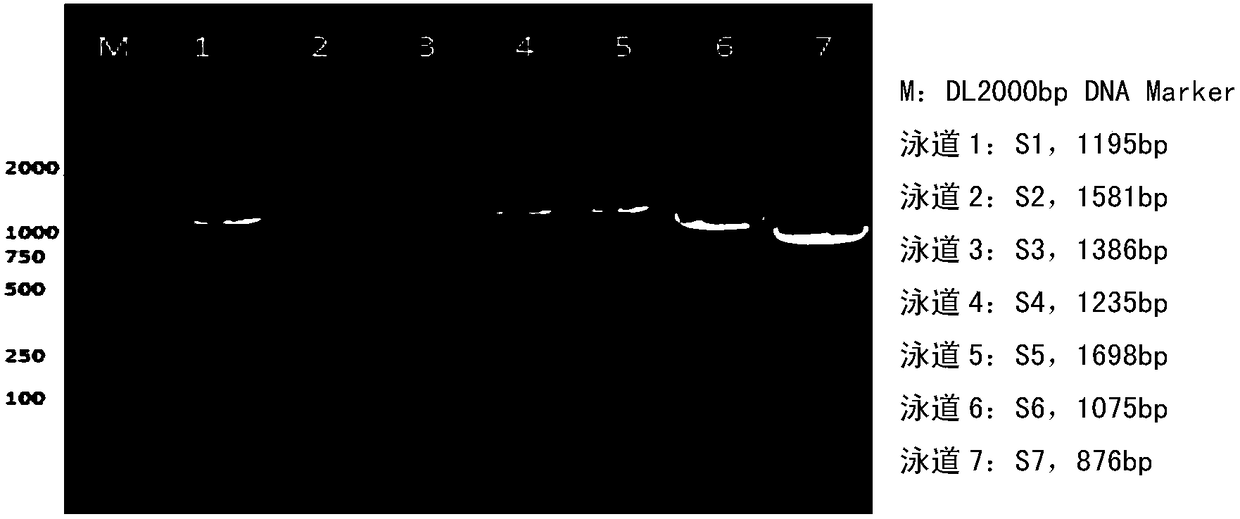
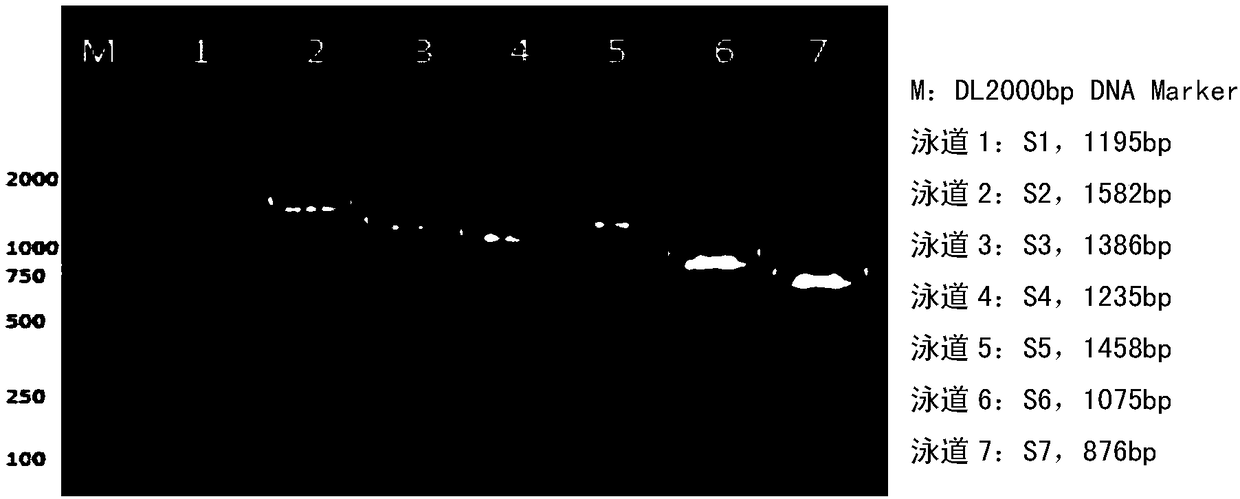
The invention discloses a whole genome sequence of Seneca valley virus SVV / CH / ZZ / 2016 and an amplification primer thereof. The whole genome sequence of Seneca valley virus SVV / CH / ZZ / 2016 is obtained by the following steps: amplifying 7 nucleotide sequence fragments (S1, S2, S3, S4, S5, S6 and S7) of the strain of SVV / CH / ZZ / 2016 by a one-step RT-PCR method; performing clone sequencing on the 7 nucleotide sequence fragments; sequentially splicing, editing and correcting the DNA sequences of the 7 nucleotide sequence fragments, to finally obtain the whole genome sequence of Seneca valley virus SVV / CH / ZZ / 2016. The whole genome sequence of Seneca valley virus SVV / CH / ZZ / 2016 obtained in the invention is favorable for further studying the pathogenic mechanism, molecular epidemiology, reverse genetics and the like of the Seneca valley virus, and provides important data support and theoretical basis for the diagnostic reagent development, vaccine development and the like of the Seneca valley virus.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Seneca Valley virus (SVV)/CH/ZZ/2016
The invention discloses a Seneca Valley virus (SVV) / CH / ZZ / 2016 strain with the specific physical and chemical characteristics. The Seneca Valley virus (SVV) / CH / ZZ / 2016 strain is a small non-envelopedRNA virus, and belongs to the small RNA virus family. The virus is resistant to organic solvents, protease and acid, but sensitive to alkali and shows sensitivity to high temperature. The physical andchemical characteristics of the Seneca Valley virus(SVV) / CH / ZZ / 2016 strain are of great significance for the research on how to prevent and control SVV epidemic and SVV vaccine development.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Chemiluminescence immunoassay kit for Seneca valley virus structural protein VP1 antibody detection
InactiveCN108872574AIncrease productionHigh detection sensitivityChemiluminescene/bioluminescenceBiological material analysisPositive controlStructural protein
The invention discloses a chemiluminescence immunoassay kit for Seneca valley virus structural protein VP1 antibody detection. The kit comprises a Seneca valley virus structural protein VP1 antigen coated chemiluminescence immunoassay plate, positive control serum, negative control serum, an enzyme-labeled antibody, chemiluminescence substrate liquid, a luminescence enhancing agent and concentrated washing liquid, wherein the amino acid sequence of the Seneca valley virus structural protein VP1 is shown as SEQ ID NO.2. The kit provided by the invention uses structural protein VP1 antigen expressed by a prokaryotic expression system; the protein yield is high; the antigen purity reaches 90 percent or higher. An enzymatic chemiluminescence reaction system is used for judging the result; thedetection sensitivity is improved. The kit provided by the invention has the advantages that the operation is simple; the detection time is short; the sensitivity and the specificity are high, and thelike. The kit can be used for Seneca valley virus detection and epidemiological investigation of a great number of samples, and has good market prospects.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Real-time fluorescence quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for detection of foot and mouth disease virus and seneca valley virus and application
InactiveCN108384893AEasy to operateSimple resultMicrobiological testing/measurementMicroorganism based processesPositive controlFluorescence
The invention discloses a real-time fluorescence quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for detection of foot and mouth disease virus and seneca valley virus and application. The kit comprises primers and probes for detecting the foot and mouth disease virus and the seneca valley virus and preferably further comprises nucleic acid extract liquid, 2*Direct qRT-PCRMix, enzyme mixed liquid, negative control and positive control. By adoption of the kit for detecting the foot and mouth disease virus and the seneca valley virus, high specificity, high sensitivity,high stability, simplicity and convenience in operation and the like are achieved. Without extra extraction of virus RNA and reverse transcription, a user only needs to add a to-be-tested sample intoa reaction tube, then performs quantitative analysis on a start template according to fluorescence signal changes and a Ct value and standard curve relation and finally calculates a copy number of the to-be-tested sample. The kit is not only applicable to quantitative analysis in research and development institutions but also suitable for pathogen detection and analysis in all levels of prevention and control institutions, basic veterinary stations, large and medium sized farms and the like, thereby having a promising application prospect.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Seneca Valley virus based compositions and methods for treating disease
ActiveUS7638318B2Small and easily manipulatable genomeSimple and fast lifecycleBiocideSsRNA viruses positive-senseDiseaseTropism
The present invention relates to a novel RNA picornavirus that is called Seneca Valley virus (“SVV”). The invention provides isolated SVV nucleic acids and proteins encoded by these nucleic acids. Further, the invention provides antibodies that are raised against the SVV proteins. Because SVV has the ability to selectively kill some types of tumors, the invention provides methods of using SVV and SVV polypeptides to treat cancer. Because SVV specifically targets certain tumors, the invention provides methods of using SVV nucleic acids and proteins to detect cancer. Additionally, due to the information provided by the tumor-specific mechanisms of SVV, the invention provides methods of making new oncolytic virus derivatives and of altering viruses to have tumor-specific tropisms.
Owner:PERCEIVER PHARMA +1
RT-LAMP (Reverse Transcription-Loop-Mediated Isothermal Amplification) primer group for detecting Seneca valley virus as well as kit and application
ActiveCN108467904AQuick checkStrong specificityMicrobiological testing/measurementMicroorganism based processesPolymerase LQuarantine
The invention relates to the technical field of biotechnologies and in particular relates to an RT-LAMP (Reverse Transcription-Loop-Mediated Isothermal Amplification) primer group for detecting a Seneca valley virus as well as a kit and application. The RT-LAMP primer group for detecting the Seneca valley virus has nucleotide sequences shown as SEQ ID NO. 1-6. The invention further provides a kitcomprising the primer group. The kit adopts a reverse transcription-loop-mediated isothermal amplification technology, depends on primers capable of identifying six specific regions on a target sequence and BstDNA polymerase which has an unwinding function and enables the target sequence to be in loop-mediated isothermal amplification, and is capable of efficiently, rapidly and specifically amplifying the target sequence under isothermal conditions. The primer group and the kit disclosed by the invention are applicable to export quarantine, food hygiene and field detection of livestock farms and are convenient for wide-range popularization and application.
Owner:SOUTH CHINA AGRI UNIV
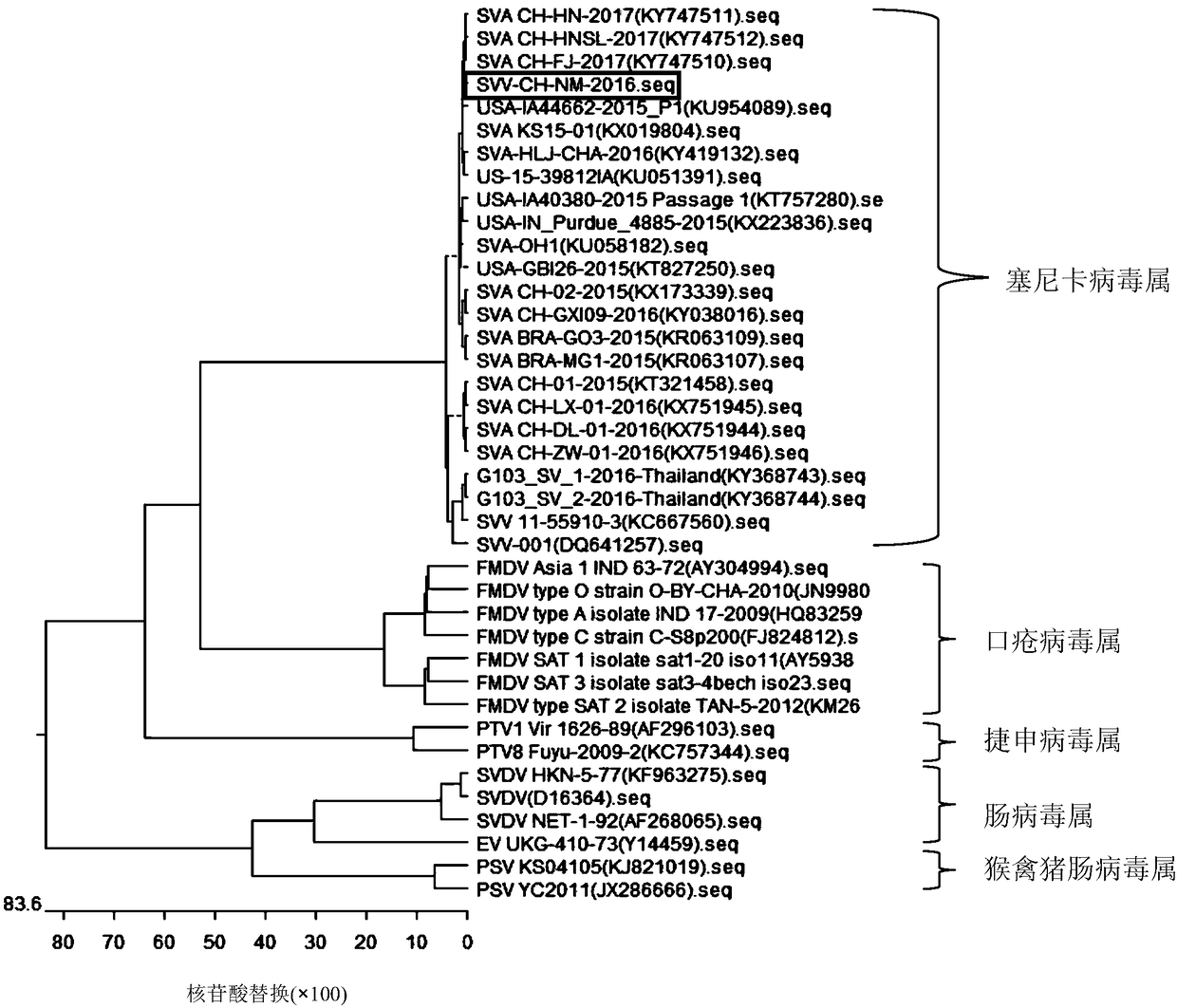
Complete genome sequence and amplification primer of Seneca valley virus SVV/CH/NM/2016
ActiveCN108085323ASsRNA viruses positive-senseMicrobiological testing/measurementNucleotideTGE VACCINE
The invention discloses a complete genome sequence and an amplification primer of a Seneca valley virus SVV / CH / NM / 2016. A one-step RT-PCR (Reverse Transcription-Polymerase Chain Reaction) method is firstly adopted for amplifying seven nucleotide sequence fragments (S1, S2, S3, S4, S5, S6, and S7) of an SVV / CH / NM / 2016 strain, then the seven nucleotide sequence fragments are cloned and sequenced, and then, the DNA sequences of the seven nucleotide sequence fragments are sequentially spliced, edited and corrected to finally obtain the complete genome sequence of the Seneca valley virus SVV / CH / NM / 2016. The obtained complete genome sequence of the Seneca valley virus SVV / CH / NM / 2016 is favorable for further research of the pathogenic mechanism of the Seneca valley virus, molecular epidemiology,reverse genetics and the like, thereby establishing important data support and theoretical basis for diagnostic reagent development, vaccine development and the like of the Seneca valley virus.
Owner:JINYUBAOLING BIO PHARMA CO LTD +1
Seneca valley virus based compositions and methods for treating disease
ActiveUS20100129325A1Small and easily manipulatable genomeSimple and fast lifecycleBiocideSsRNA viruses positive-senseDiseaseTropism
Owner:NOVARTIS AG +1
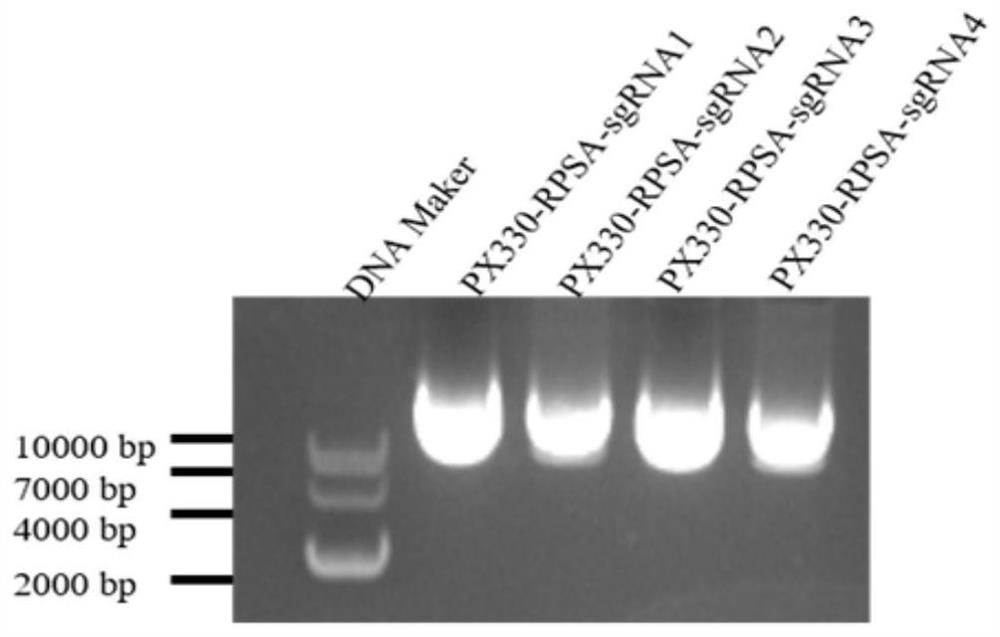
SgRNA for targeted knockout of RPSA gene and construction method of RPSA gene knockout cell line
ActiveCN111849979AInhibition of replicationEasy to copySsRNA viruses positive-senseGenetically modified cellsAntigenPathogenic microorganism
The invention belongs to the field of gene engineering, and particularly relates to SgRNA for targeted knockout of an RPSA gene and a construction method of an RPSA gene knockout cell line. Accordingto the sgRNA of a specific targeted RPSA gene, the sgRNA can specifically target an RPSA gene, the complete knockout of the RPSA gene in a host cell is realized by applying a CRISPR-Cas9 technology, and the knockout efficiency is high; the invention also provides a method for transfecting the sgRNA into a host cell by virtue of a CRISPR-Cas9 technology to construct an RPSA gene knockout cell line.Knocking out the RPSA gene in the host cell not only can promote replication of the FMDV virus, but also can be used for improving the production quantity and antigen expression quantity of the FMDVvaccine; in addition, it is accidentally found that by knocking out the RPSA gene in the host cell, replication of the Seneca valley virus in the cell can be remarkably inhibited; a research tool anda material are provided for further researching a molecular mechanism of the RPSA gene for regulating and controlling pathogenic microorganism replication in cells.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
LAMP (Loop-Mediated Isothermal Amplification) detection primer pair for Seneca valley virus and detection method of Seneca valley virus
ActiveCN108707697AStrong specificityNo cross reactionMicrobiological testing/measurementMicroorganism based processesLoop-mediated isothermal amplificationBiology
The invention relates to the technical field of virus detection, in particular to an LAMP (Loop-Mediated Isothermal Amplification) detection primer pair for a Seneca valley virus and also relates to adetection method using the primer pair. The method is high in specificity, has the sensibility consistent with fluorescent quantitative PCR, is rapid and convenient, can be used for detection of SVVand has a better application prospect.
Owner:NANJING AGRICULTURAL UNIVERSITY
Kit for detecting and identifying Seneca valley virus and foot-and-mouth disease virus type O, A and Asial, and primers and probes thereof
ActiveCN110205405AEasy to operateAvoid pollutionMicrobiological testing/measurementMicroorganism based processesVirologyFoot-and-mouth disease virus
The invention discloses a kit for detecting and identifying a Seneca valley virus and foot-and-mouth disease virus type O, A and Asial, and primers and probes thereof. The kit is used for detecting and identifying a Seneca valley virus, a foot-and-mouth disease virus type O, a foot-and-mouth disease virus type A and a foot-and-mouth disease virus type Asial; and the sequences of the primers and probes are as follows: the sequences of the detection primers and probes of the Seneca valley virus are SEQ1, SEQ2 and SEQ3; the sequences of the detection primers and probes of the foot-and-mouth disease virus type O are SEQ4, SEQ5 and SEQ6; the sequences of the detection primers and probes of the foot-and-mouth disease virus type A are SEQ7, SEQ8 and SEQ9; and the sequences of the detection primers and probes of the foot-and-mouth disease virus type Asial are SEQ10, SEQ11 and SEQ12. The kit can simultaneously perform the detection and discriminating on the Seneca valley virus and the foot-and-mouth disease virus type O, A and Asial, so that operation steps can be simplified, detection time can be shortened, and detection accuracy can be enhanced.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for preparing Seneca valley virus by utilizing suspension cell line
ActiveCN110628698AIncrease production capacityIncrease virus titerSsRNA viruses positive-senseArtificial cell constructsSenecavirusVirology
The invention provides a method for preparing Seneca valley virus by utilizing a suspension cell line. The method for preparing the Seneca valley virus by utilizing the suspension cell line comprisesthe following steps: (1) domesticating a non-suspension BHK-21 cell line into a suspension BHK-21-S cell line; and (2) culturing the Seneca valley virus by using the suspension BHK-21-S cell line obtained in the step (1). By culturing the Seneca valley virus by using the suspension BHK-21-S cell line obtained by performing domestication, yield of the Seneca valley virus can be effectively increased; and thus, foundation is laid for large-scale production of vaccines to the Seneca valley virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com