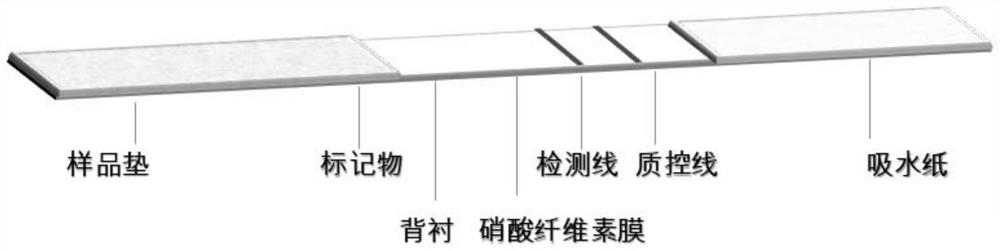
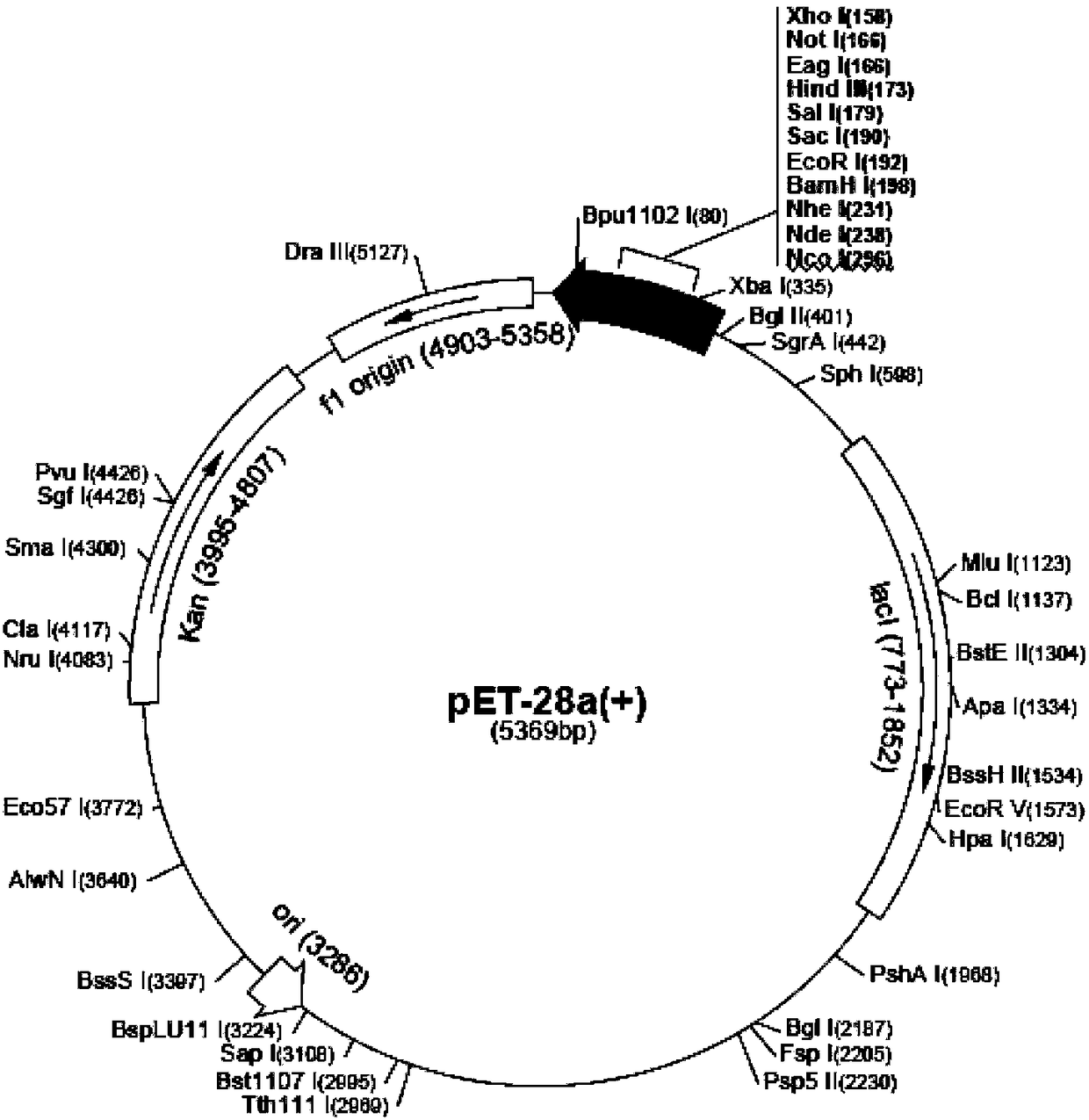
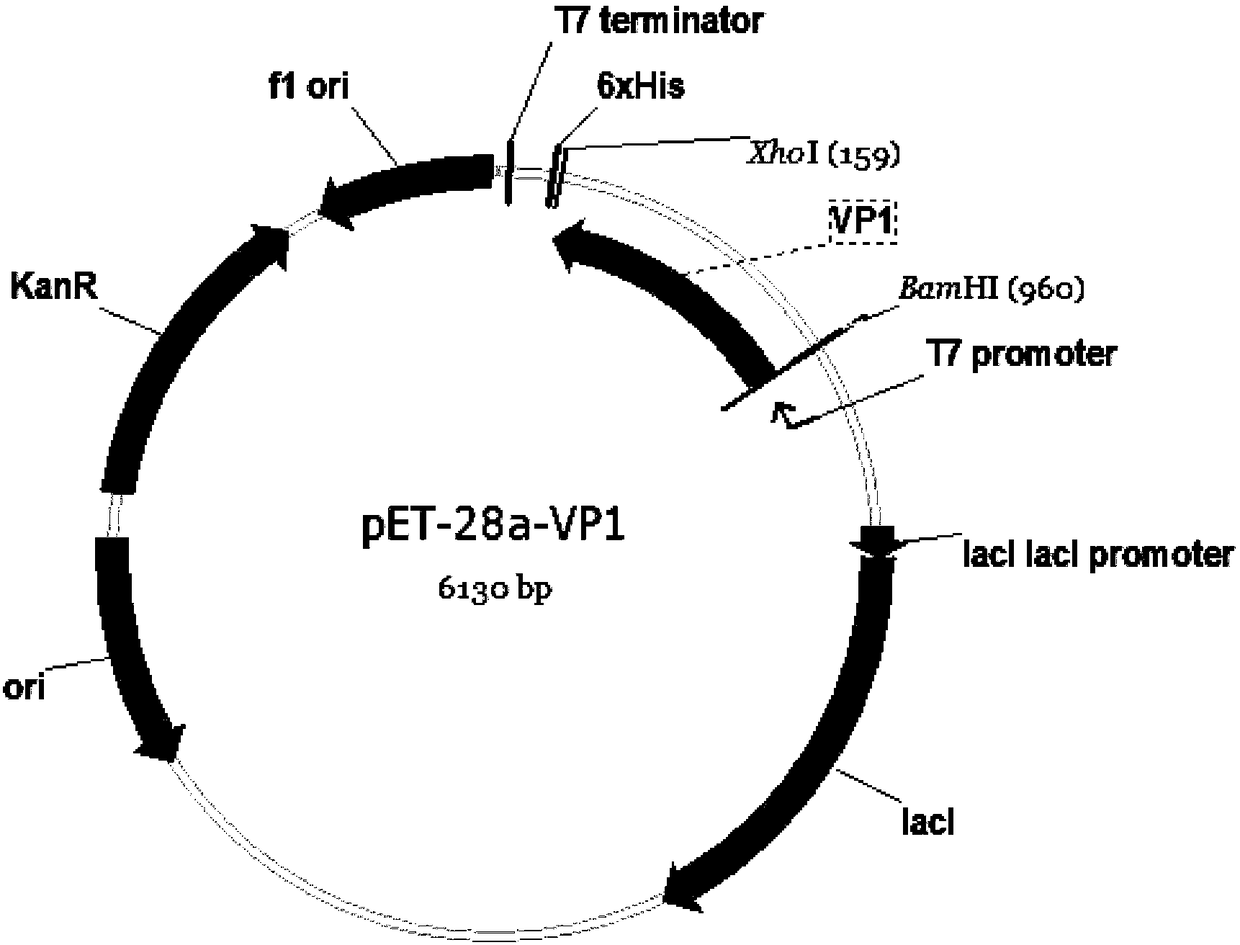
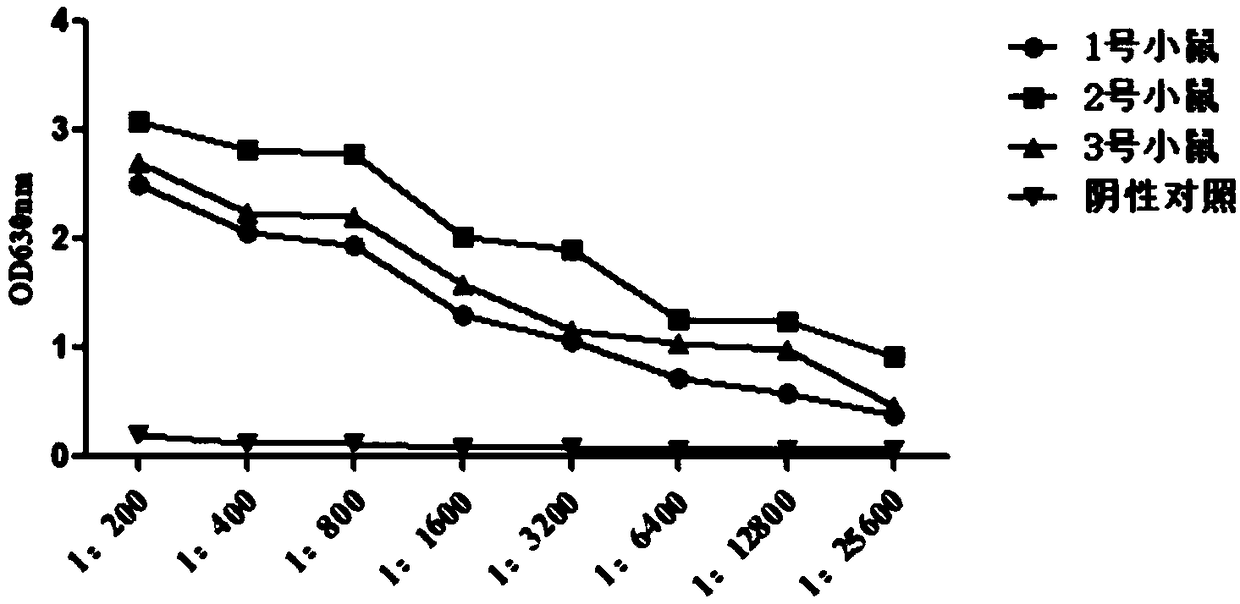
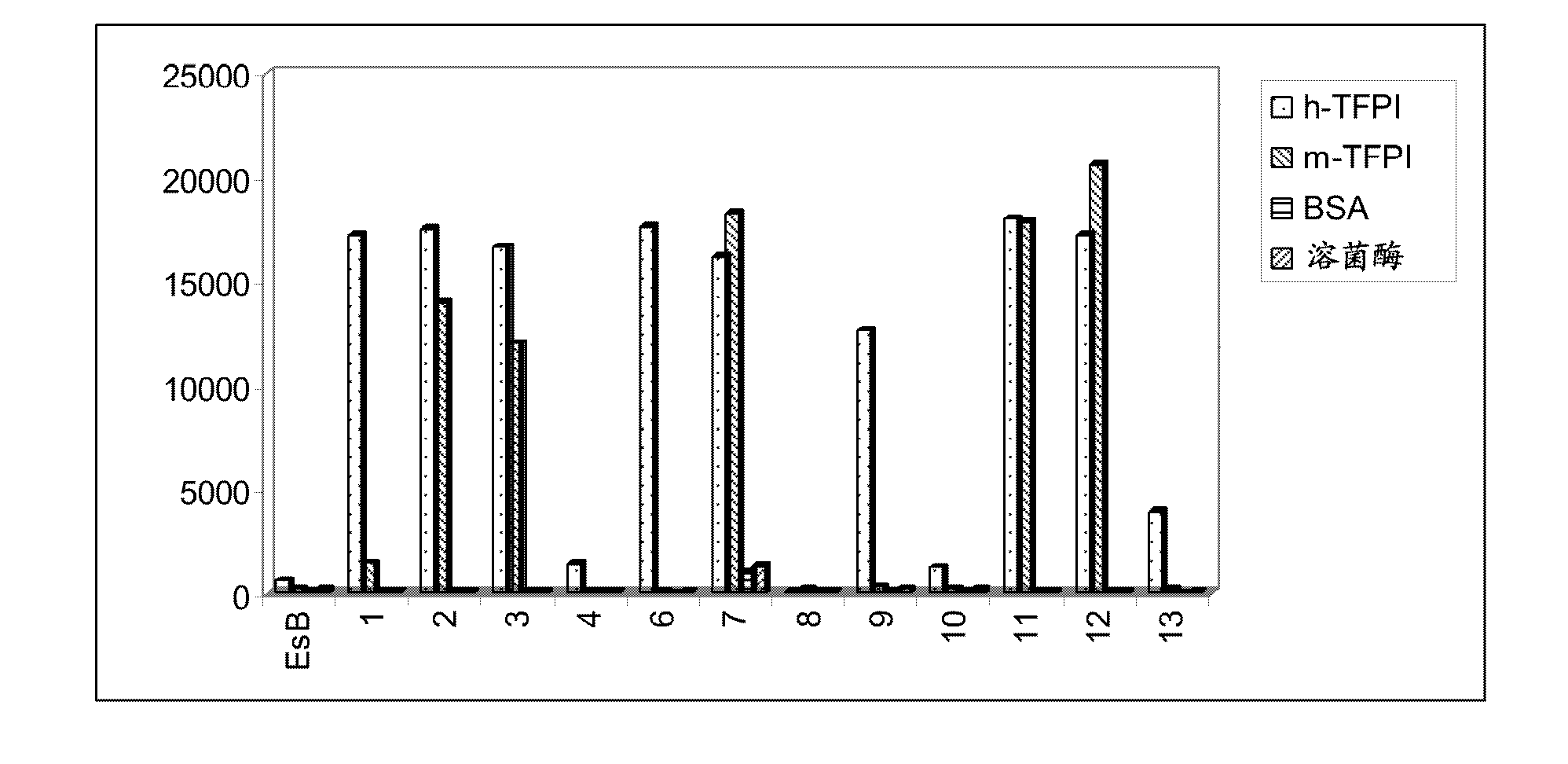
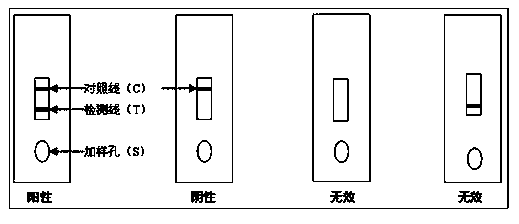
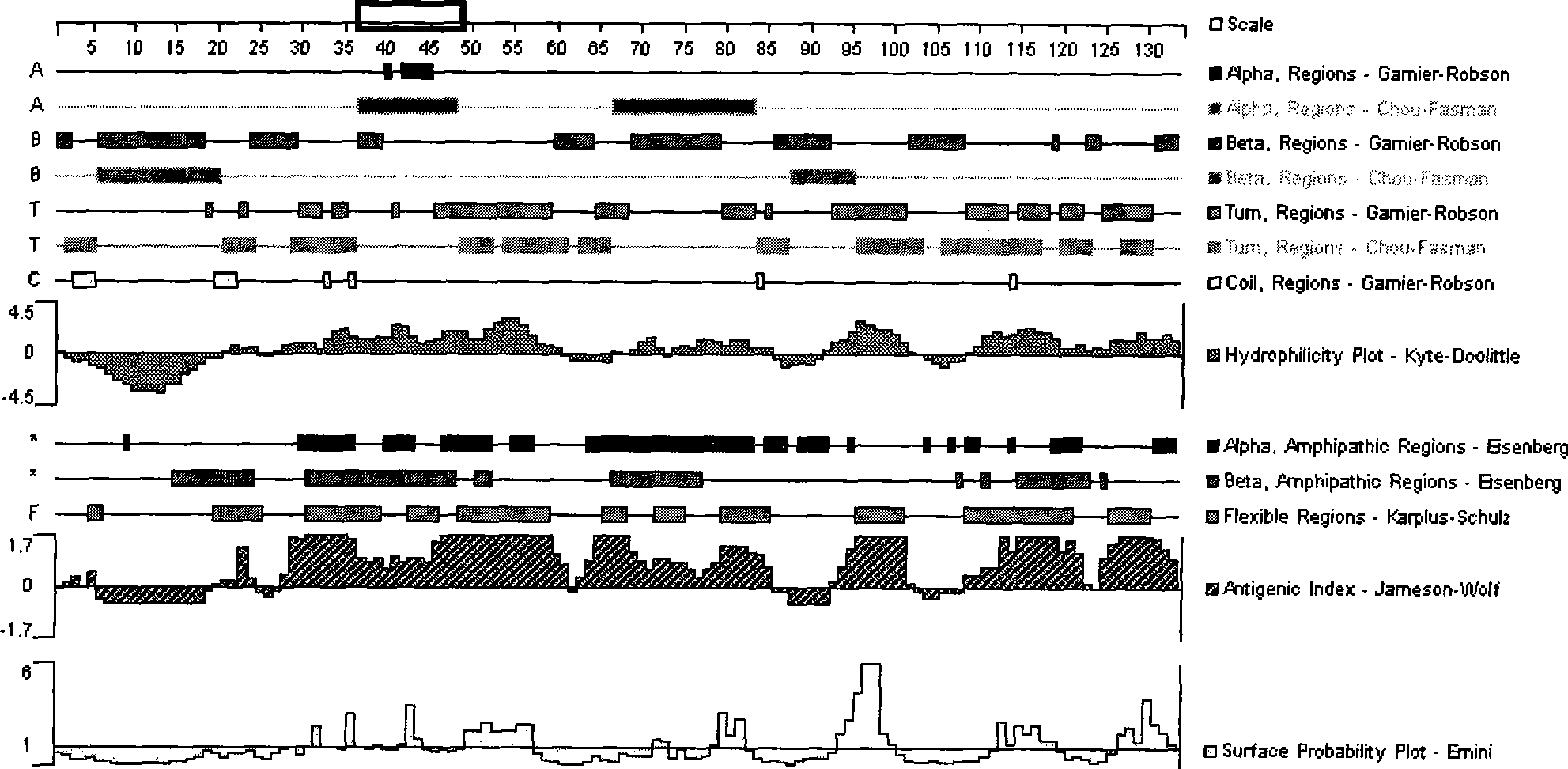
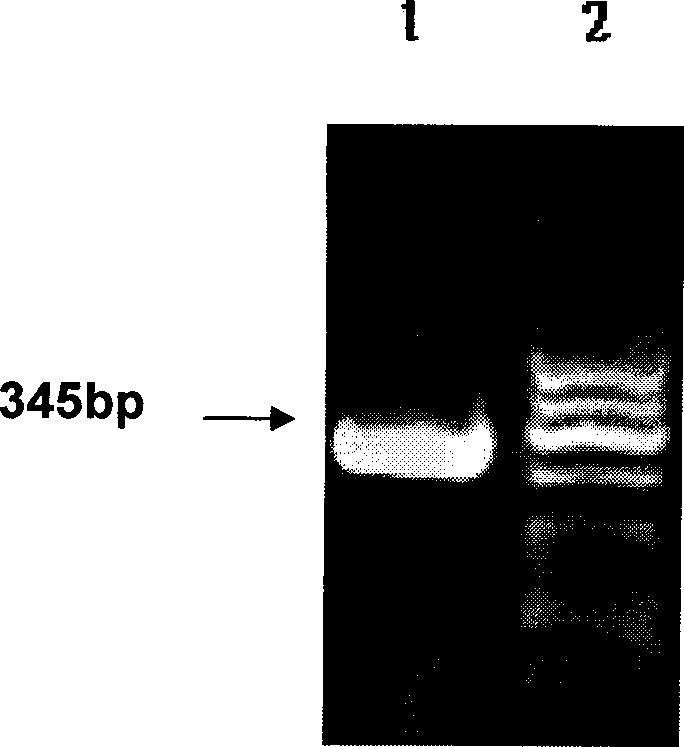
Patents
Literature
202 results about "Antibodies monoclonal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monoclonal antibodies are proteins that are made in a laboratory. These proteins are designed to attach to areas on the surface of cancer cells and interfere with their growth and spread. Monoclonal antibodies are similar to the antibodies your body naturally produces when you are exposed to bacteria or viruses,...
Anthracycline derivative conjugates, process for their preparation and their use as antitumor compounds
The present invention relates to conjugates of therapeutically useful anthracyclines with carriers such as polyclonal and monoclonal antibodies, proteins or peptides of natural or synthetic origin; methods for their preparation, pharmaceutical composition containing them and use thereof in treating certain mammalian tumors.
Owner:GENENTECH INC +1
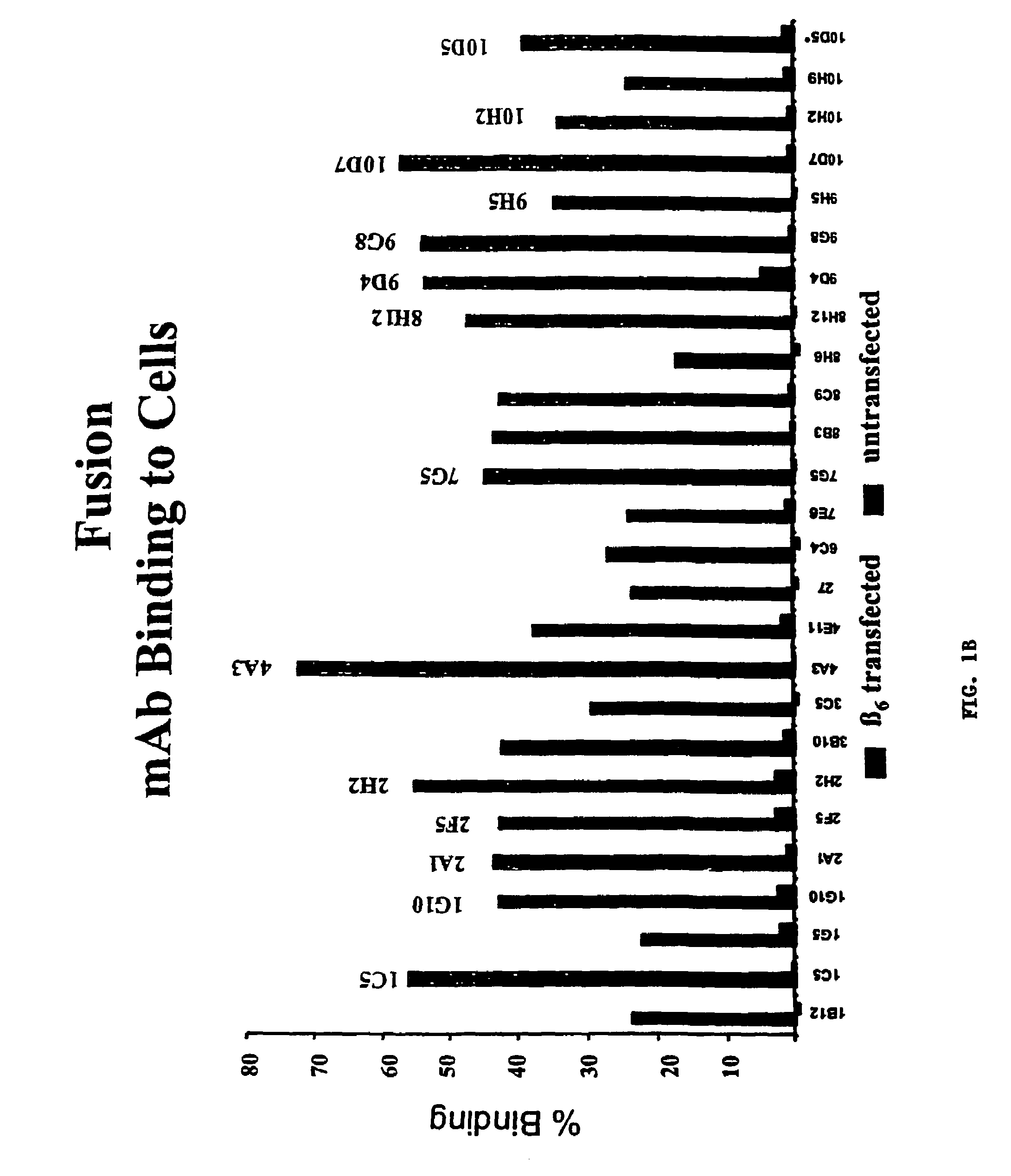
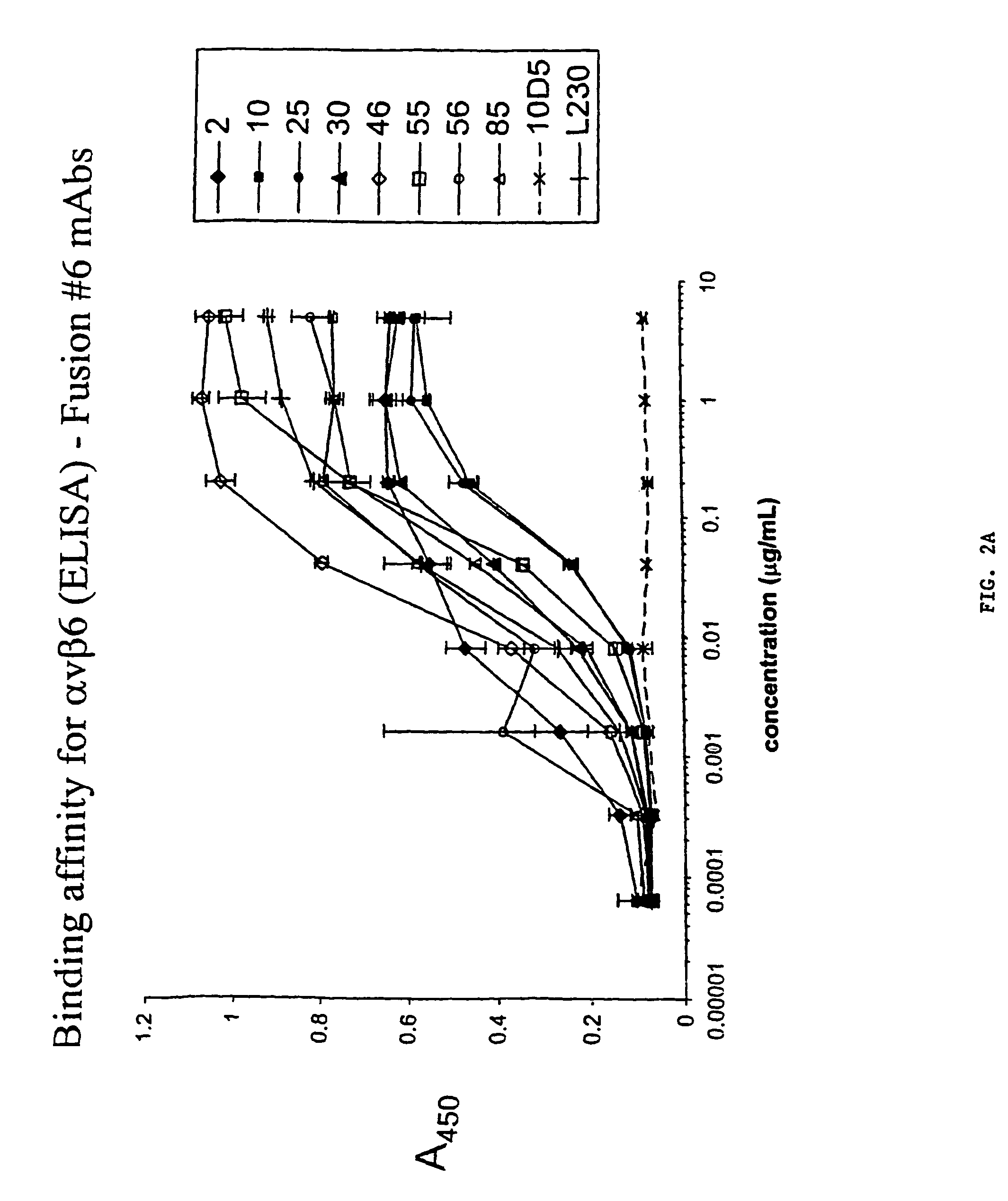
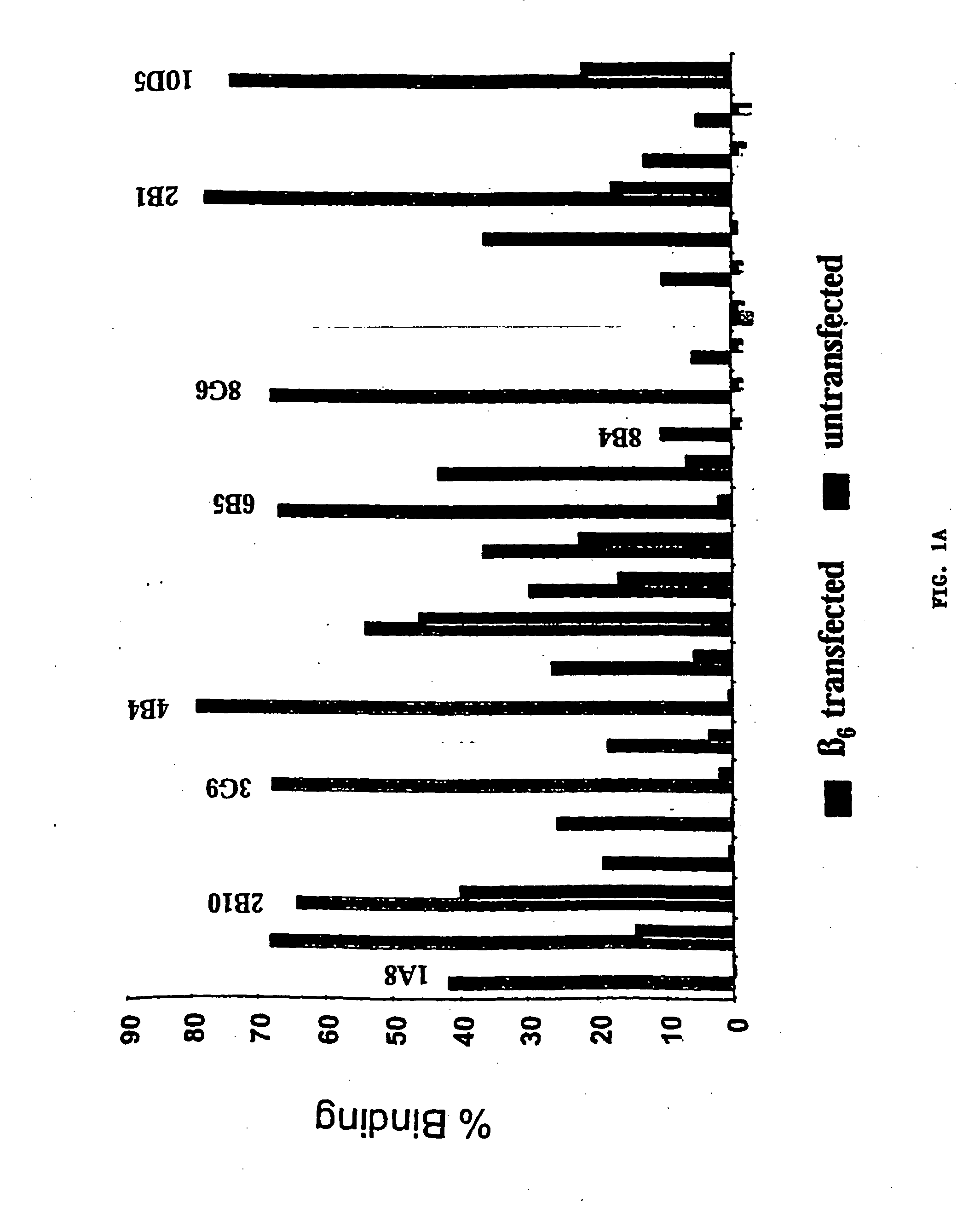
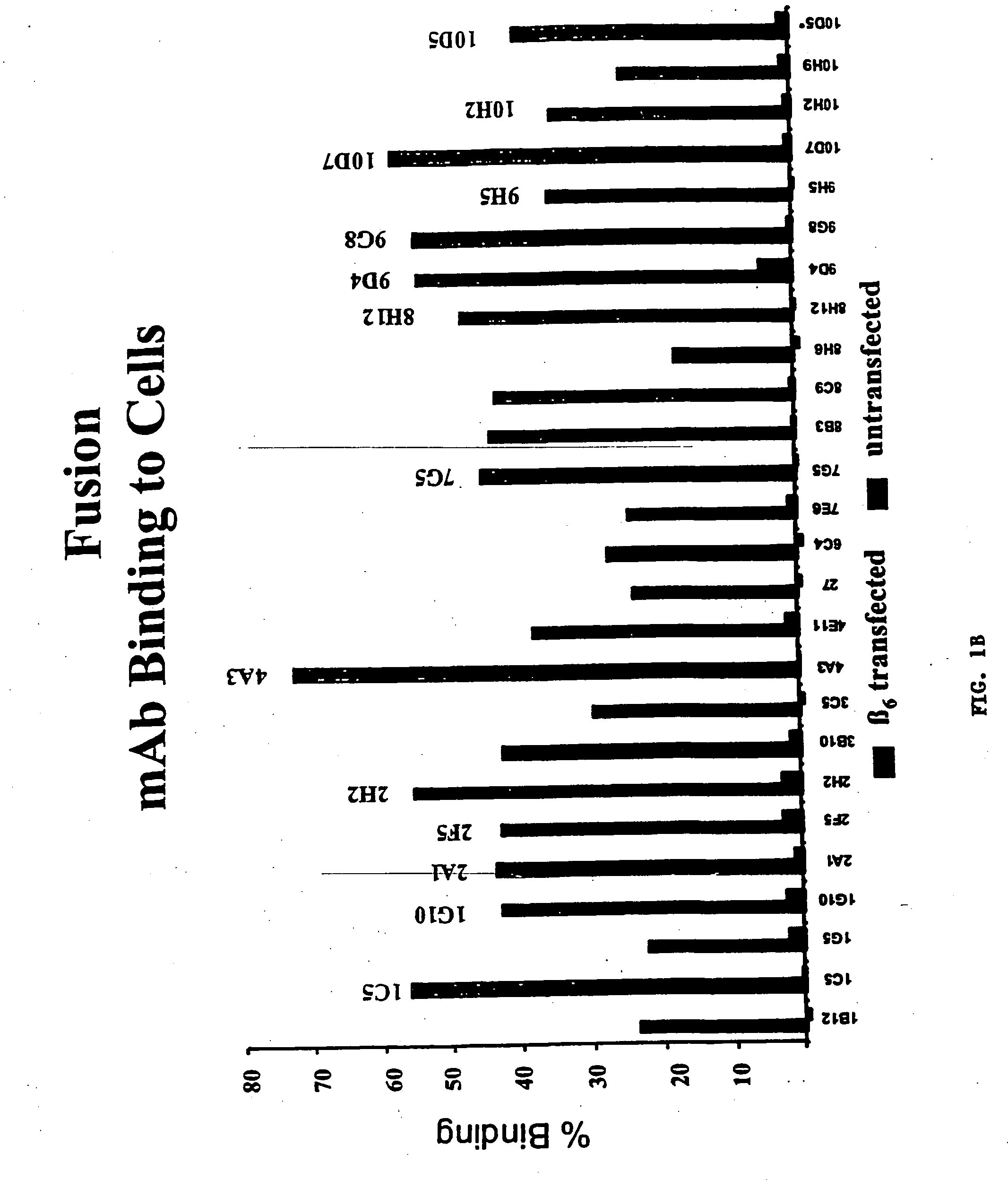
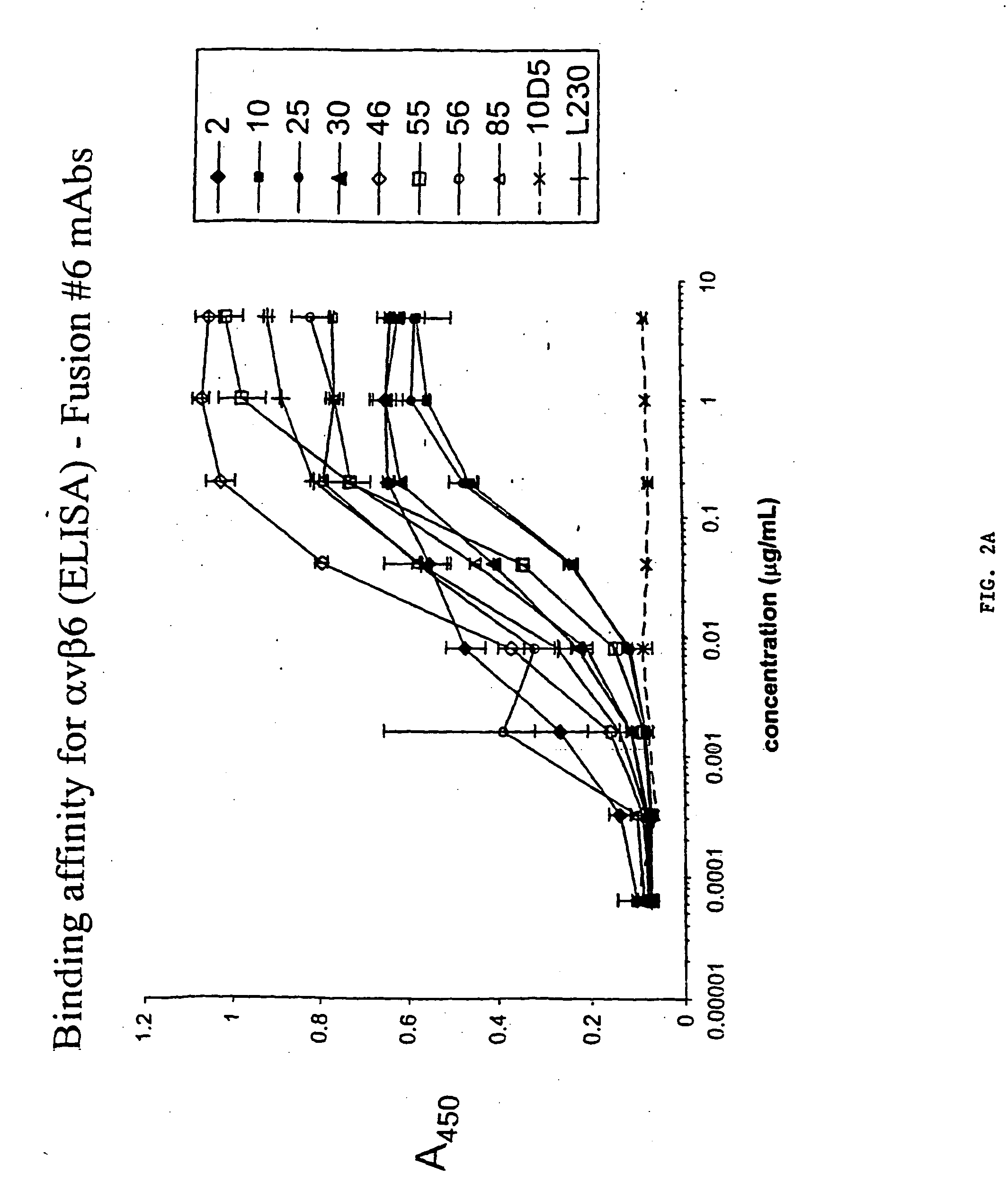
Anti-alphavbeta6 antibodies
InactiveUS7465449B2Reduce functionHigh affinitySkeletal disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntiendomysial antibodies
Monoclonal antibodies that specifically bind to M.96. Also included are methods of using these antibodies to treat mammals having or at risk of having 006-mediated diseases, or to diagnose % Qmediated diseases.
Owner:BIOGEN MA INC +1
Anti-alphavbeta6 antibodies
ActiveUS20050255102A1Reduced effector functionHigh affinitySkeletal disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseMammal
Monoclonal antibodies that specifically bind to M.96. Also included are methods of using these antibodies to treat mammals having or at risk of having 006-mediated diseases, or to diagnose % Qmediated diseases.
Owner:BIOGEN MA INC +1
H5 subtype avian flu virus hemagglutinin protein monoclonal antibody, and its preparing method and use
This invention relates to a monoclonal antibody capable of combining with H5 subtype avian influenza virus HA protein specifically, the hybridoma cell line secreting said antibody and a preparing method. The invention also relates to a serial test kit for testing H5 subtype avian influenza virus by the antibody and a bit of said antibody in the test sample of the virus and its usage in treatment.
Owner:XIAMEN UNIV
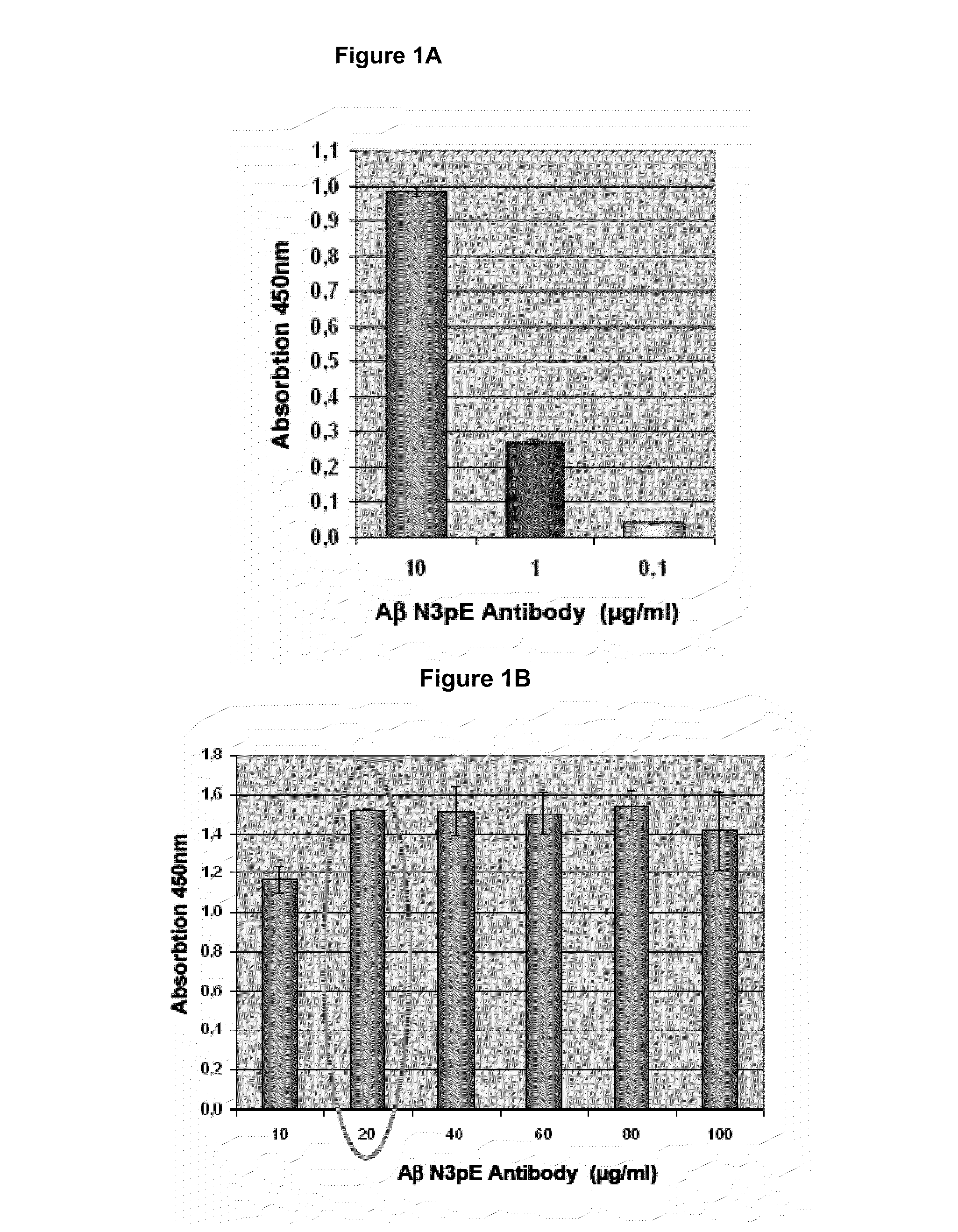
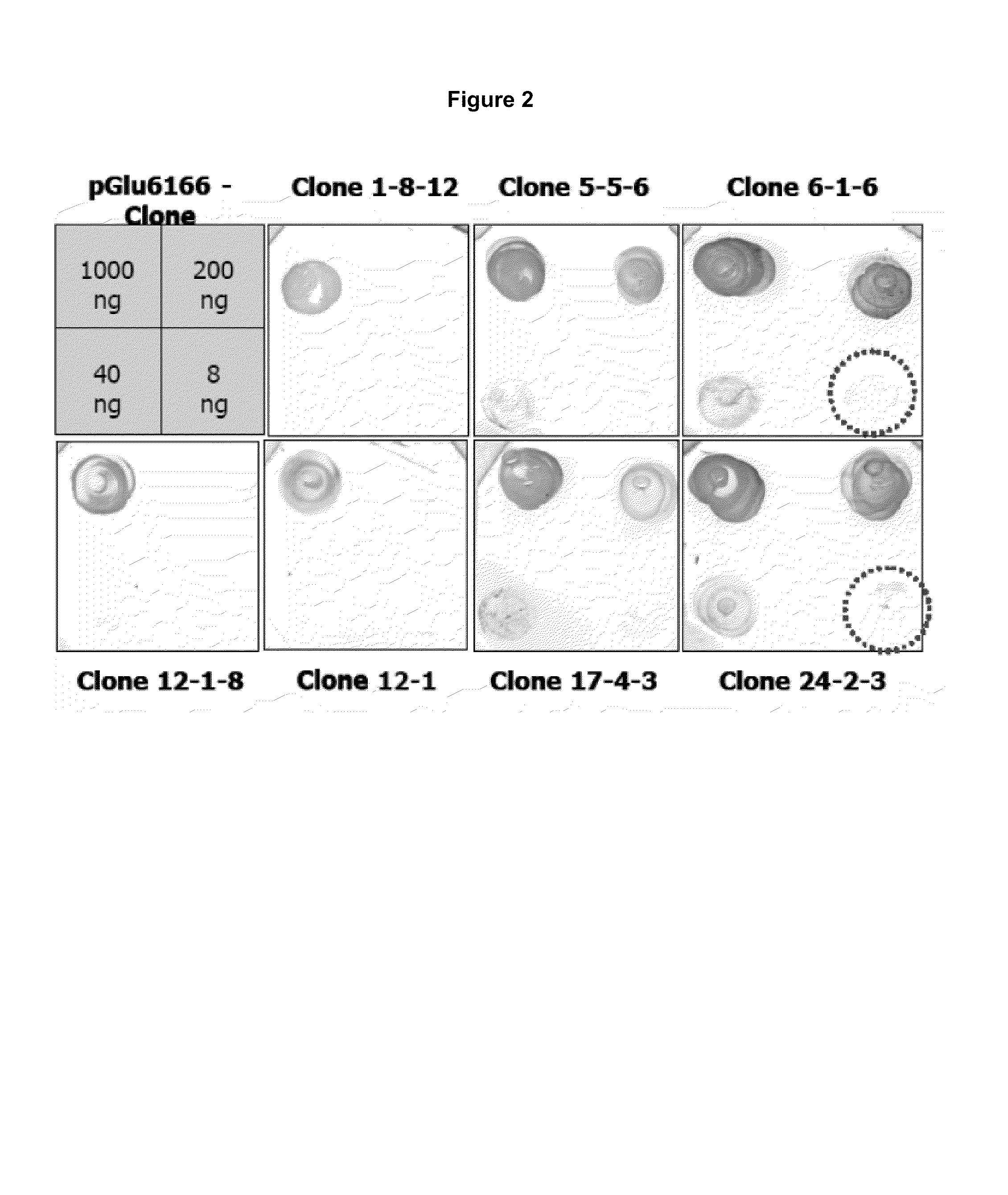
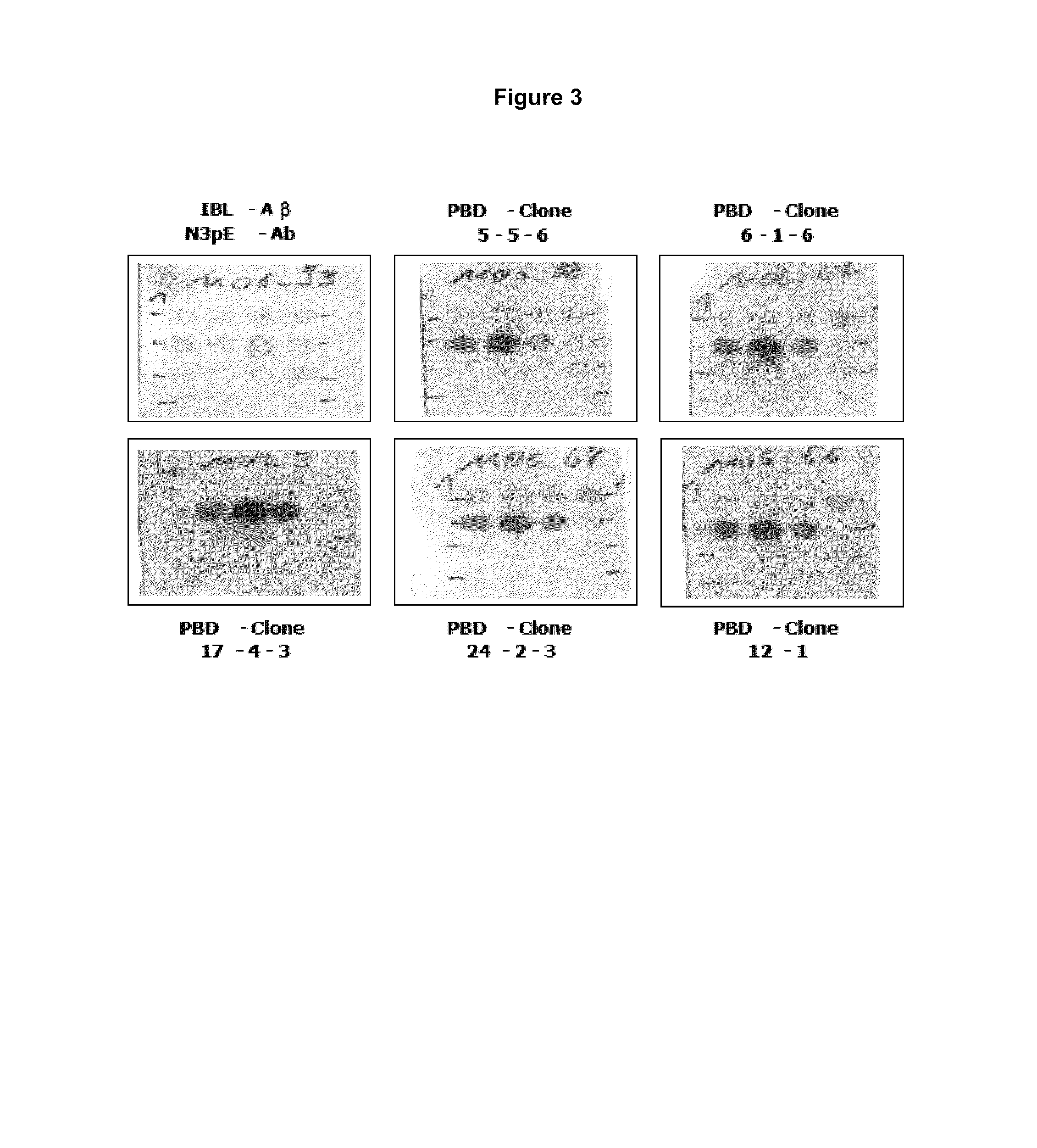
Antibodies specific for N-terminal truncated and pyroglutamate modified amyloid-beta peptides
The present invention pertains to novel diagnostic assays for the diagnosis of amyloidosis, in particular Alzheimer's disease, and related aspects. In particular, monoclonal antibodies and an antibody assay are provided.
Owner:VIVORYON THERAPEUTICS NV
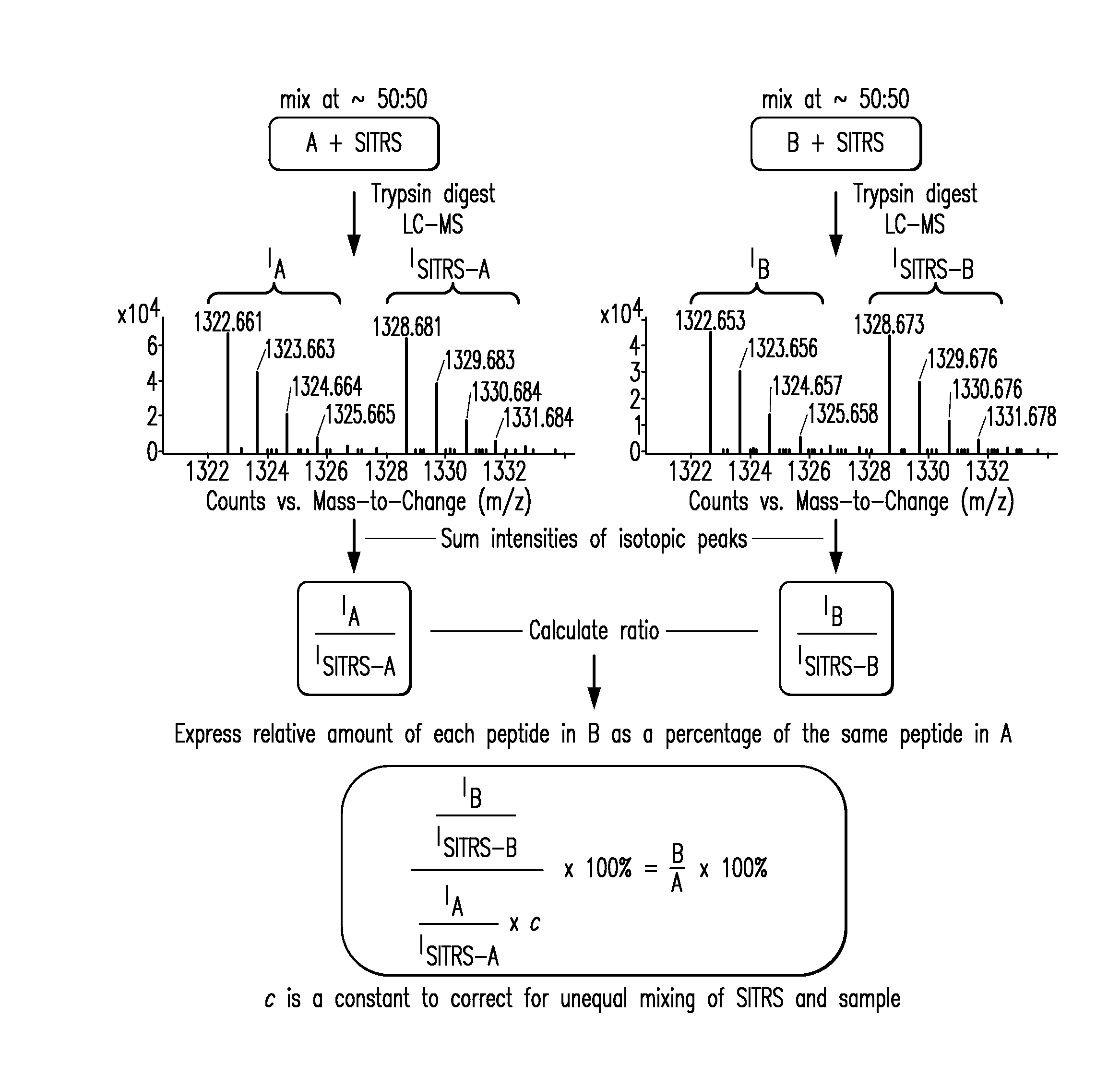
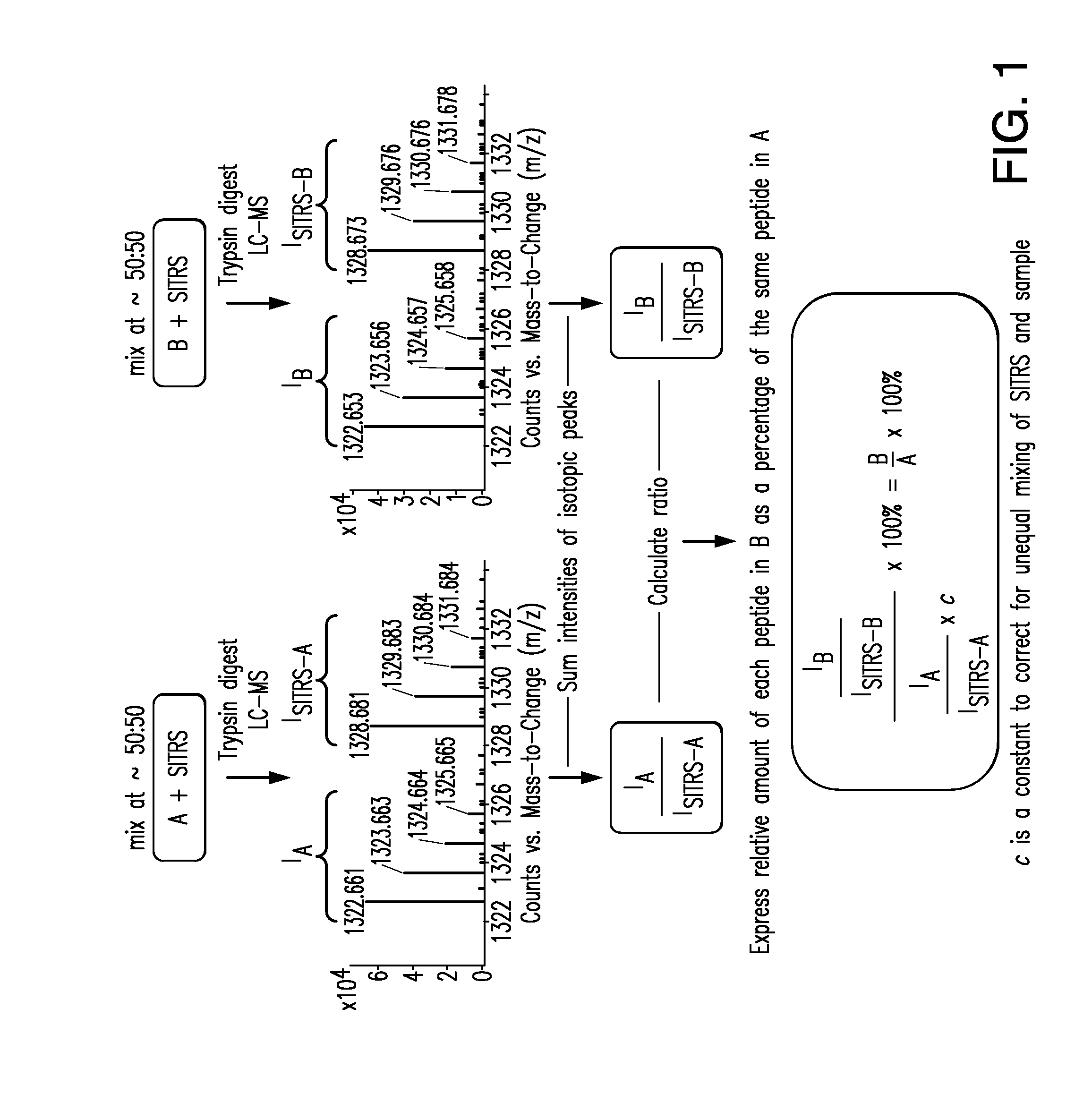
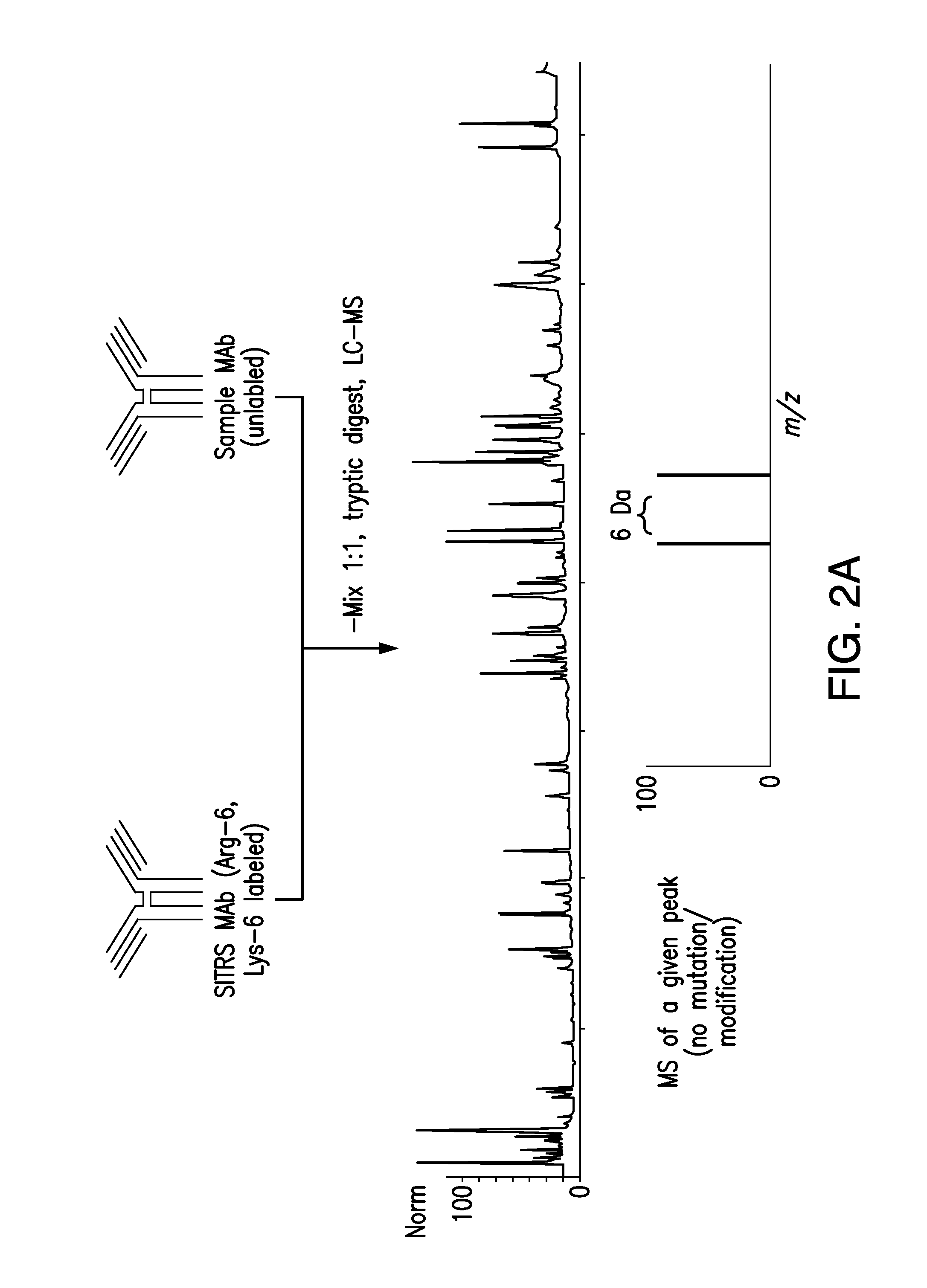
Methods and Systems for the Analysis of Protein Samples
The present invention relates to methods for analyzing protein samples, e.g., monoclonal antibodies, via the SITRS technique, as well as methods and systems that facilitate the processing of data generated by the SITRS technique.
Owner:ABBVIE INC
Anti-rabies virus monoclonal antibody and preparation method and application
InactiveCN101560255AExperimental costs are highEasy to operateImmunoglobulins against virusesTissue cultureImmunoblot AnalysisHybridoma cell
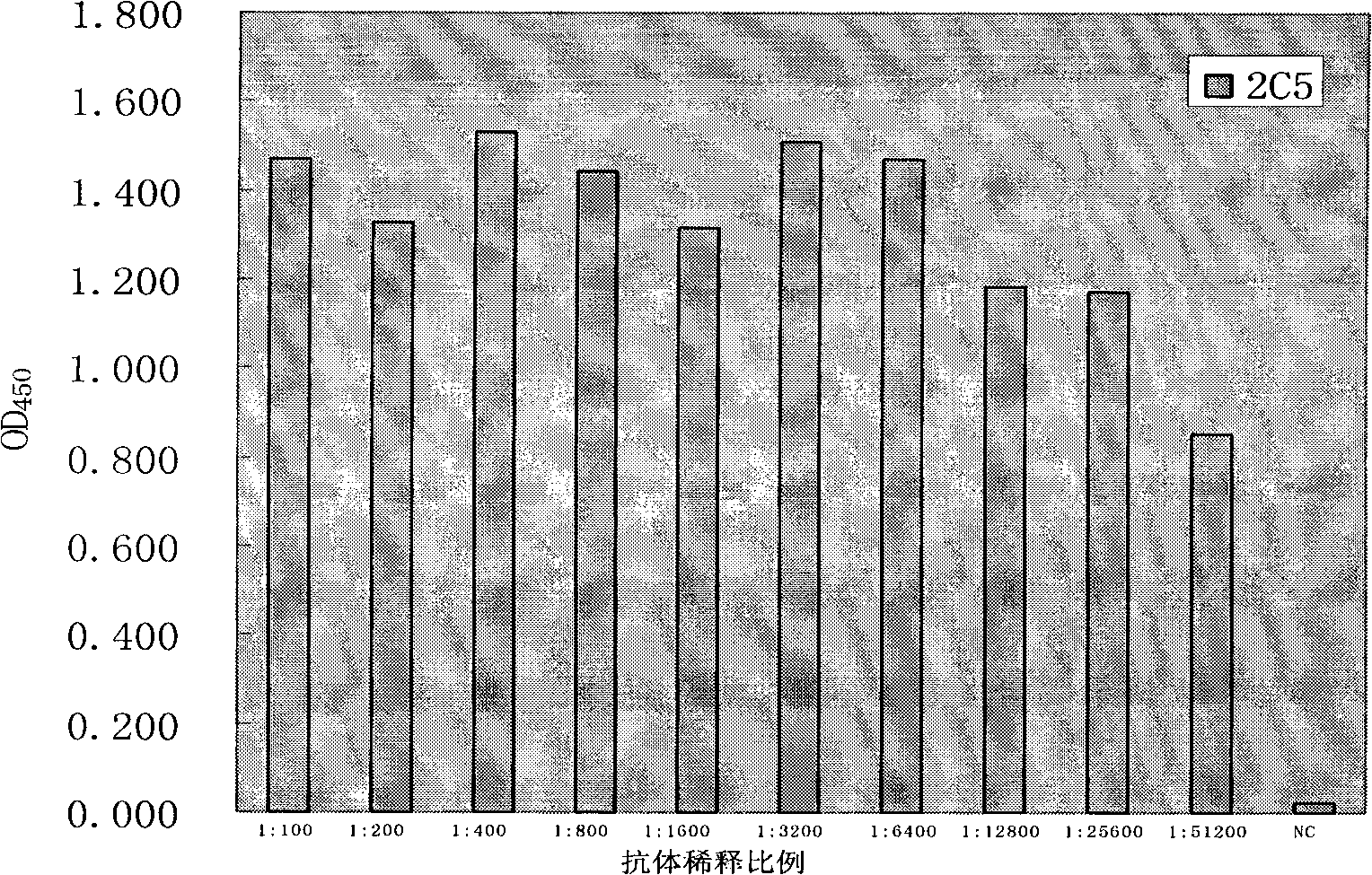
The invention discloses an anti-rabies virus monoclonal antibody and a preparation method and an application, belonging to the field of biomedicine and particularly relating to the preparation of a monoclonal antibody capable of identifying rabies virus and the application. The monoclonal antibody of the invention is screened by indirect Enzyme-linked immunosorbent assay (ELISA), and specificity and affinity thereof combined with antigen are identified by methods such as polyacrylamide gel electrophoresis analysis, speckle ELISA, immunoblot analysis and the like. The anti-rabies virus monoclonal antibody of the invention can be applied in multiple testing methods of antigen of rabies virus and can be also applied in the preparation of rabies virus detecting kit. The anti-rabies virus monoclonal antibody is secreted by anti-rabies virus monoclonal antibody hybridoma cell strain 2C5 with the preservation number being CGMCC No.3014.
Owner:NANJING MEDICAL UNIV +1
Methods for detection or measurement of viruses
InactiveUS20110262892A1Improve automationMicrobiological testing/measurementBiological material analysisViral antibodyActive agent
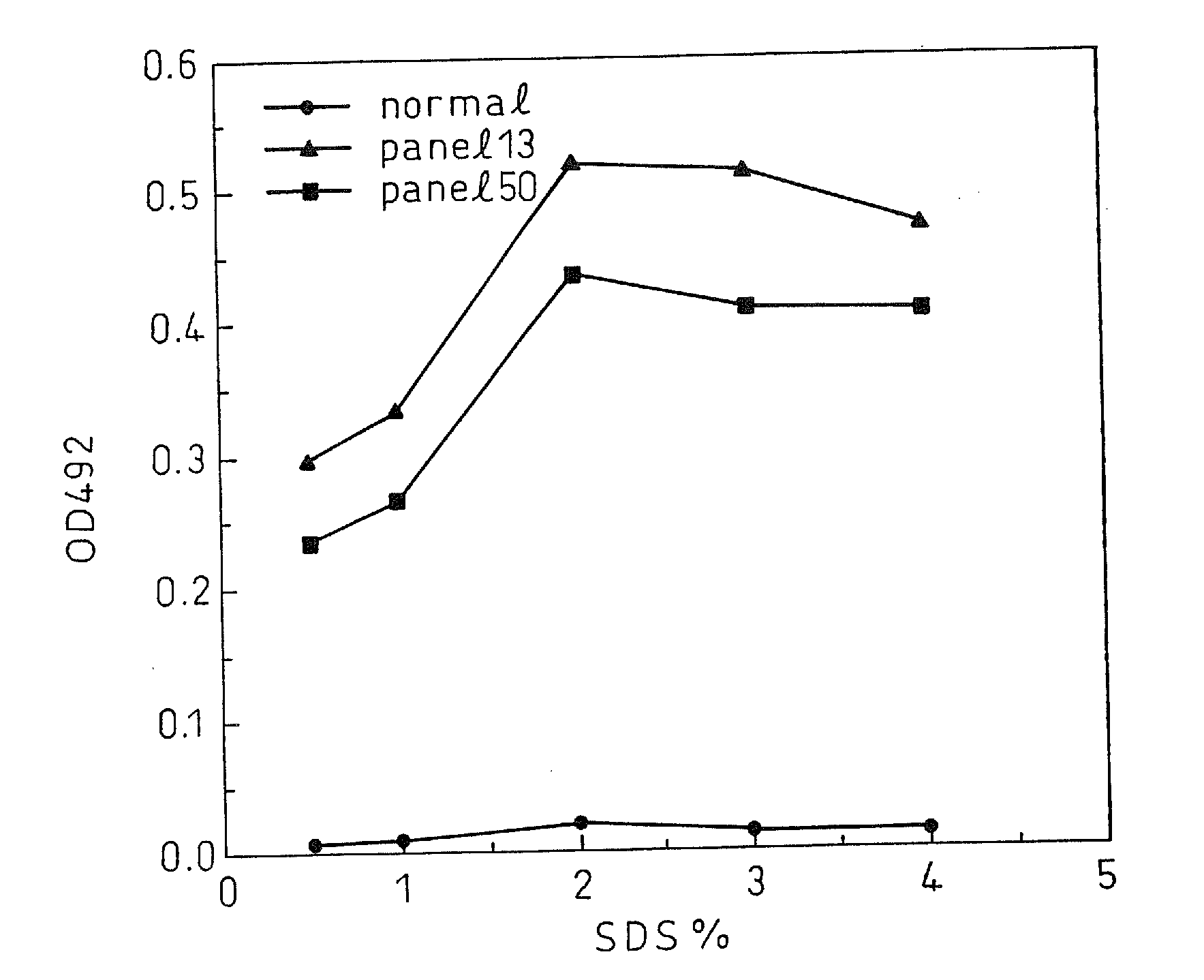
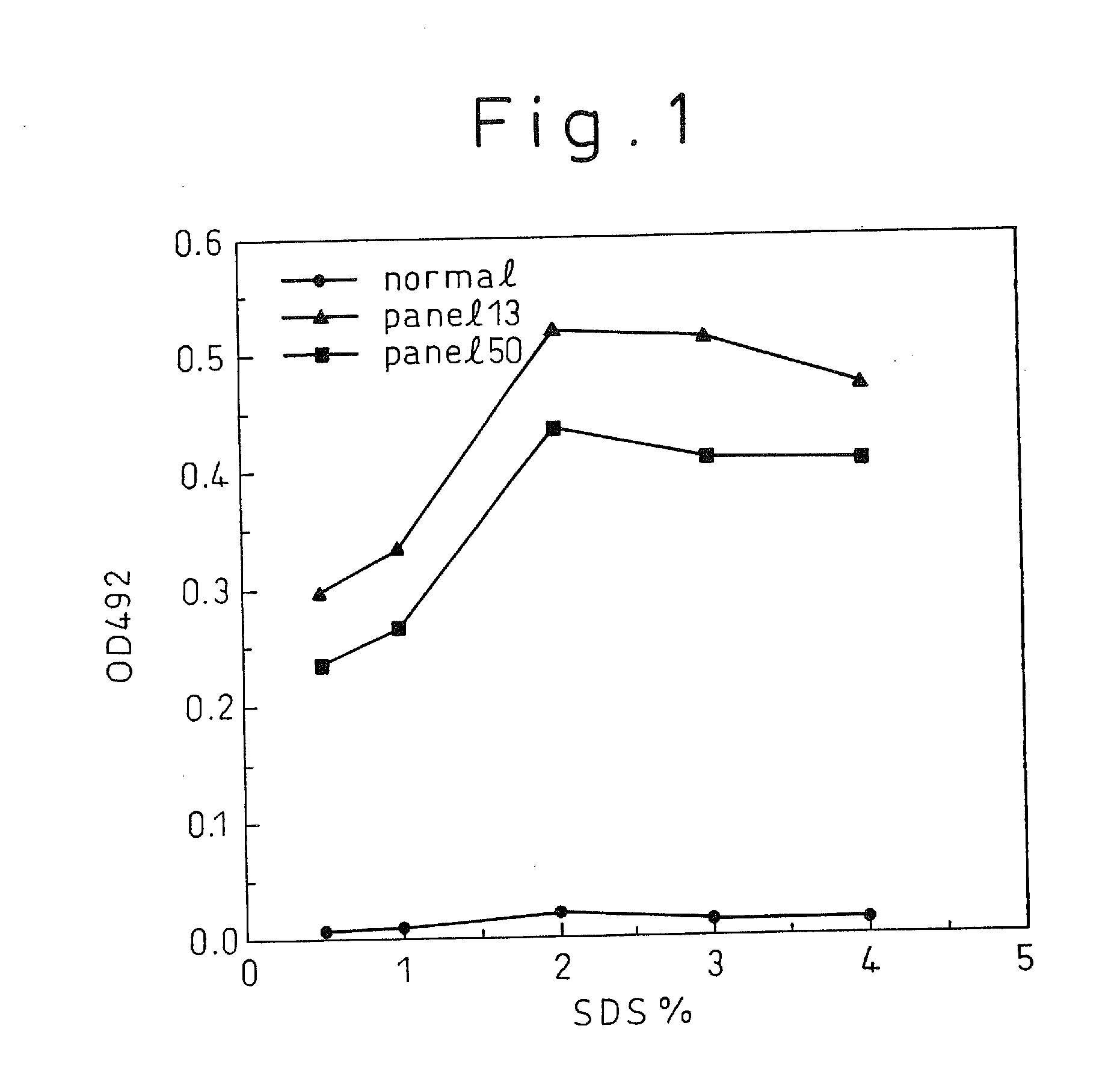
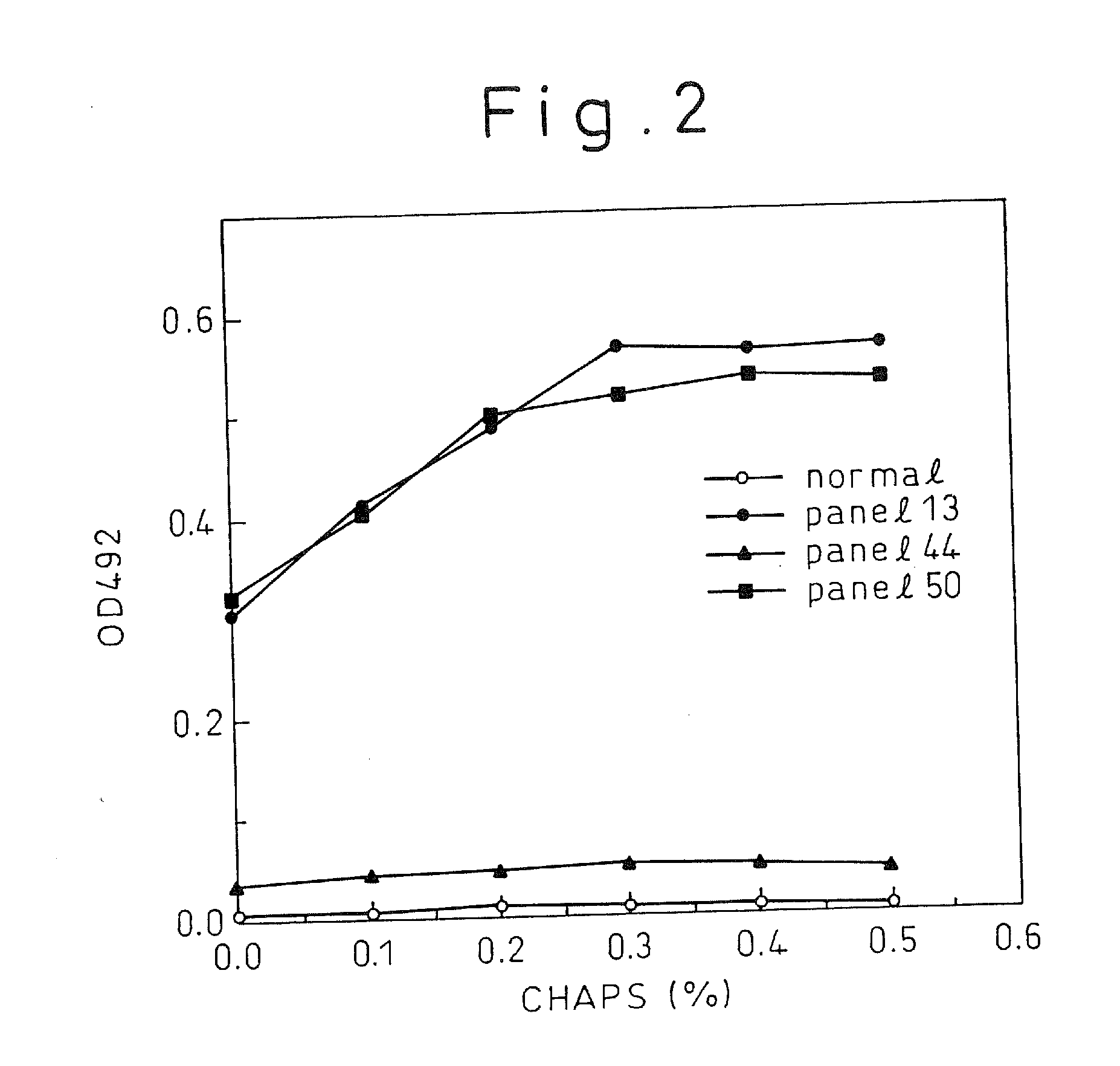
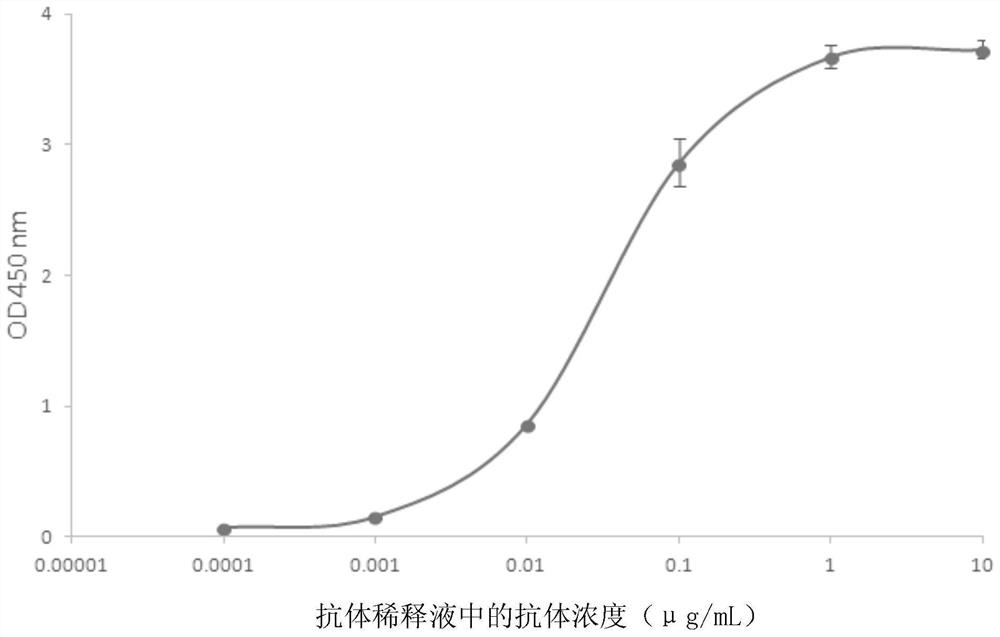
A method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) an anionic surfactant and (2) an amphoteric surfactant, nonionic surfactant or protein denaturant; a virus assay method using said treating method; a method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) a chaotropic ion and (2) an acidifying agent; a virus assay method using said treating method; a virus assay method, characterized in that a virus antigen and a virus antibody are measured based on their binding to their probe in the presence of a surfactant with an alkyl group of 10 or more carbon atoms and a secondary, tertiary or quaternary amine, or a nonionic surfactant, or of both of them; and a monoclonal antibody and a hybridoma producing the same for carrying out said method.
Owner:AOYAGI KATSUMI +4
Hybridoma cell capable of secreting an anti-novel coronavirus N protein monoclonal antibody, onoclonal antibody and application
ActiveCN111733141AQuick monitoringIncreased sensitivityBiological material analysisImmunoglobulins against virusesProtein.monoclonalAntigen testing
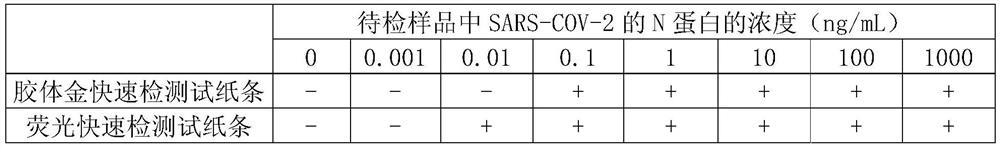
The invention discloses a hybridoma cell capable of secreting an anti-novel coronavirus (SARS-COV-2) N protein monoclonal antibody, a monoclonal antibody and anapplication. The invention provides a hybridoma cell strain N-3G3, wherein the preservation number of strain is CCTCC NO: C202075. The invention also protects the monoclonal antibody secreted by the hybridoma cell strain N-3G3. The antibodywith high sensitivity and high specificity is the key to the development and implementation of an antigen detection technology. The specific antibody of the N protein of the SARS-CoV-2 is obtained onthe basis of a monoclonal antibody technology, and an SARS-COV-2 detection test strip is prepared from the N protein of the SARS-CoV-2. When the test strip provided by the invention is used for detecting the SARS-CoV-2, the operation is simple, the sensitivity is high, the specificity is strong, and the rapid monitoring and prevention of the SARS-COV-2 can be realized.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Monoclonal antibodies reactive with defined regions of the T cell antigen receptor
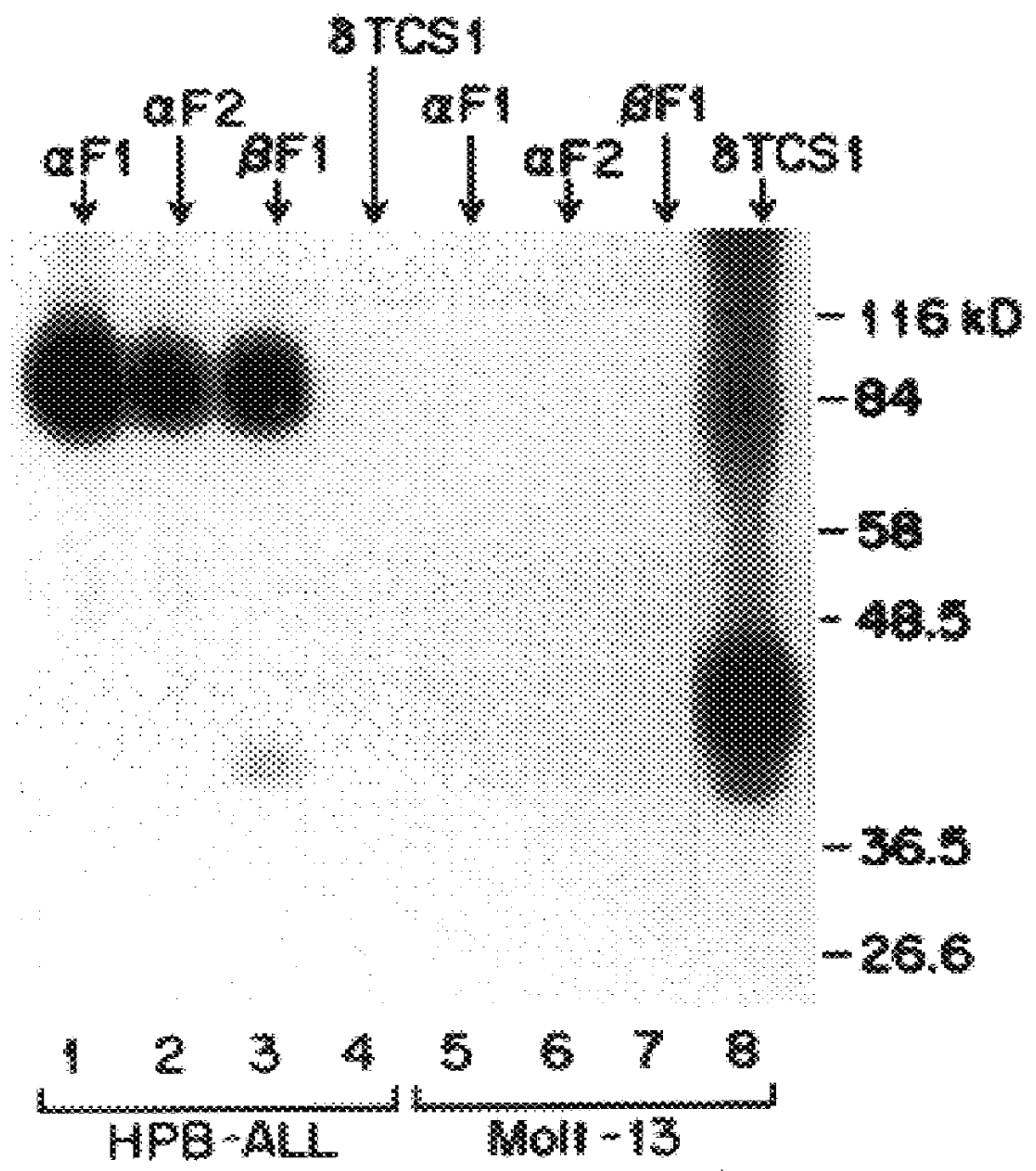
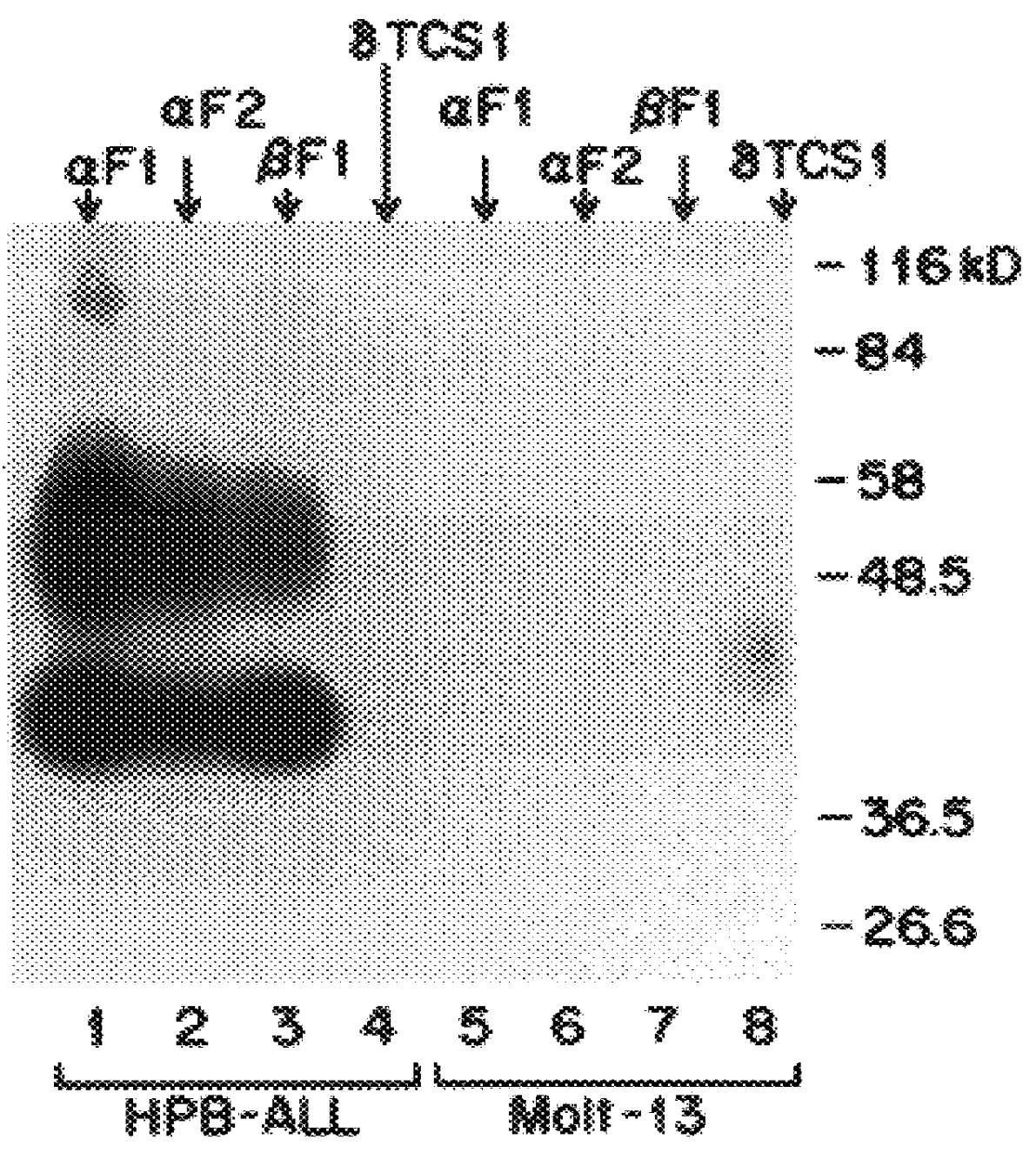
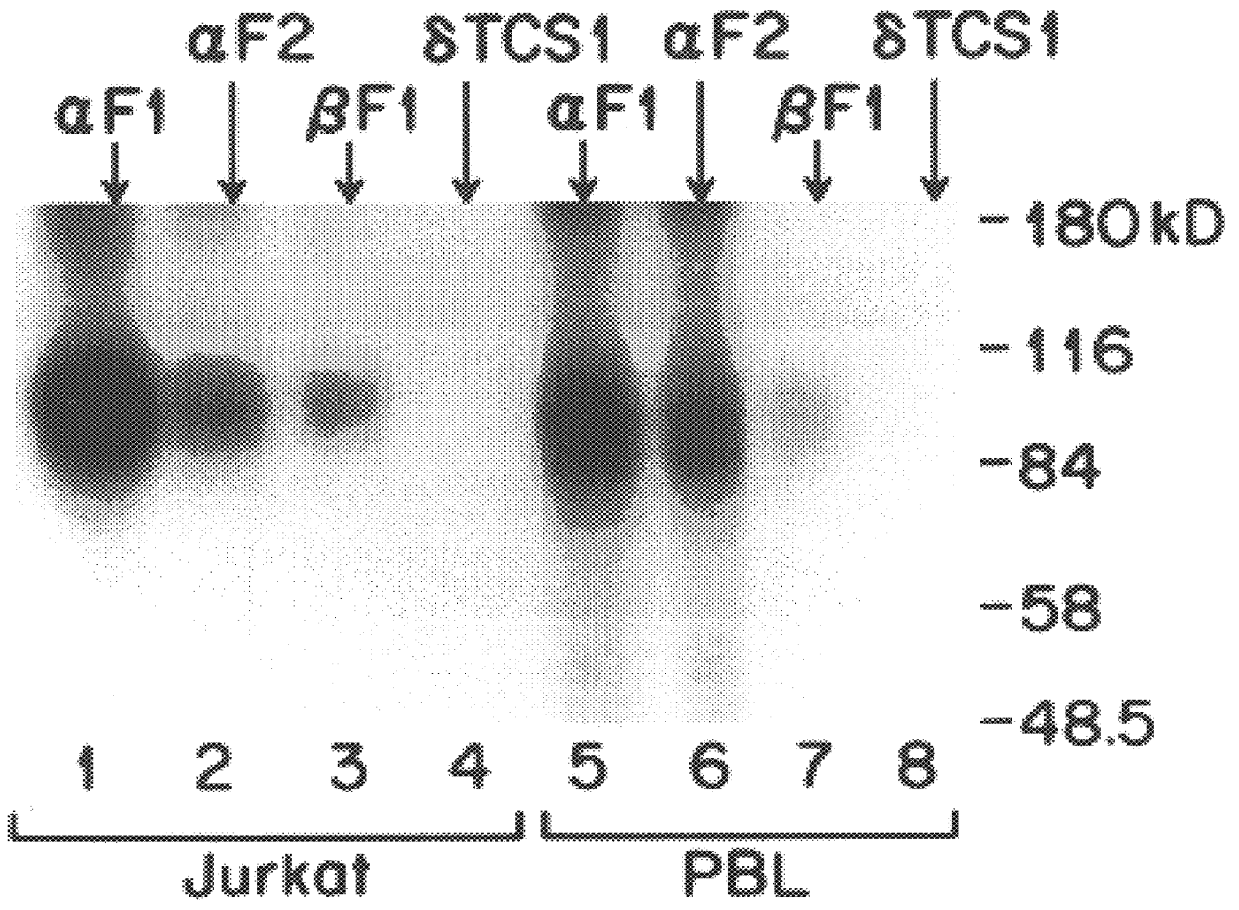
The present invention relates to monoclonal antibodies which recognize defined regions of the T-cell receptor (TCR). In a specific embodiment, the invention provides monoclonal antibodies which are reactive with a constant region of the alpha chain of the TCR. In particular embodiments, the invention relates to two monoclonal antibodies, termed alpha F1 and alpha F2, which react with two different epitopes on the framework region of the alpha monomer of the TCR molecule. In another specific embodiment, the invention is directed to monoclonal antibodies reactive with a variable region of the beta chain of the TCR. In particular, the invention provides two monoclonal antibodies, termed W112 and 2D1, which react with beta chain variable regions V beta 5.3 and V beta 8.1, respectively. In another specific embodiment, the invention is directed to monoclonal antibodies reactive with a variable region of the delta chain of the TCR. In particular, the invention provides monoclonal antibody delta TCS1, isotype IgG2a. The monoclonal antibodies of the invention have value in diagnosis and therapy and are useful tools for study of the immune system.
Owner:ASTRAZENECA AB
Kir3dl2 binding agents
ActiveUS20150232556A1Maintain specificityAvoid competitionAntipyreticAnalgesicsAntibody fragmentsCancer research
The present invention relates to methods for the treatment of cancer and inflammatory disease using antibodies (e.g. monoclonal antibodies), antibody fragments, and derivatives thereof that specifically bind KIR3DL2. The invention also relates to antibodies, cells producing such antibodies; methods of making such antibodies; fragments, variants, and derivatives of the antibodies; pharmaceutical compositions comprising the same.
Owner:INNATE PHARMA SA
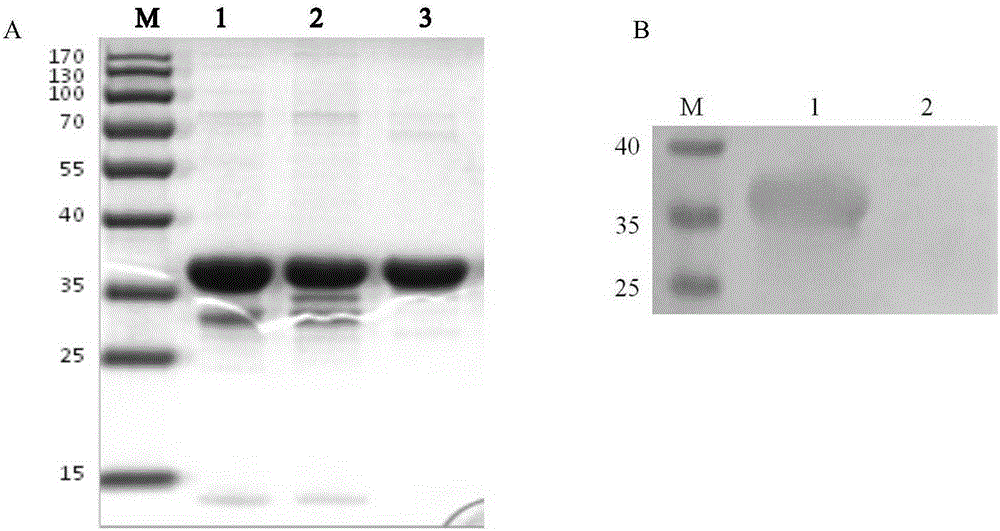
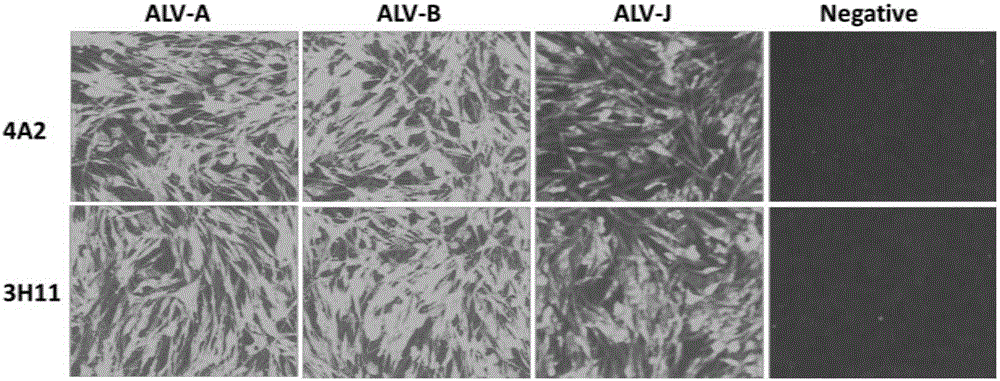
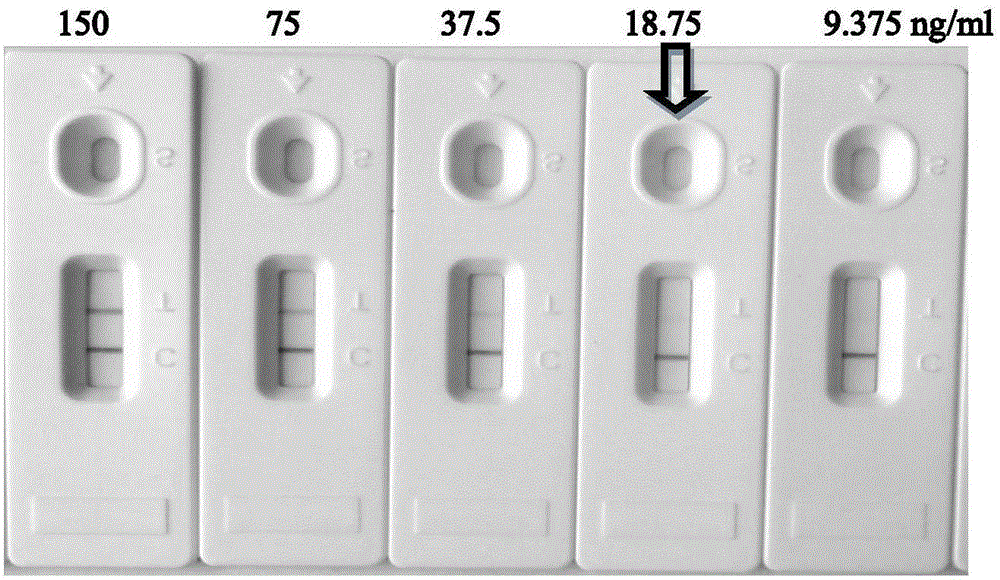
Anti-avian leukosis virus p27 protein monoclonal antibody, gold-colloidal strip containing same and application
ActiveCN105198986AHigh potencyStrong specificityMicroorganism based processesImmunoglobulins against virusesProtein.monoclonalAvian leukosis viruses
The invention discloses an anti-avian leukosis virus p27 protein monoclonal antibody, a gold-colloidal strip containing the same, and application. The anti-avian leukosis virus p27 protein monoclonal antibody is generated by secretion of a hybridoma cell strain with a preservation number of CGMCC NO. 11089 or a hybridoma cell strain with a preservation number of CGMCC NO. 11090. The monoclonal antibody is high in titer to p27 protein and good in specificity, and therefore can be used for preparing a kit and the gold-colloidal strip for detecting an avian leukosis virus. A preparation method of the monoclonal antibody provided by the invention is simple, and an antibody purification process is simple, and the efficiency is high, and the cost is low. As the gold-colloidal strip prepared by the anti-avian leukosis virus p27 protein monoclonal antibody provided by the invention is adopted to detect the anti-avian leukosis virus, the specificity is strong, and the operation is simple, and convenience, speediness and simplicity are realized, besides, a special instrument and equipment are not needed, and professional training is not needed, and a result is clear and easy to recognize; the operation is simple and convenient; the popularization is easy, and the antibody is more suitable for real-time detection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
GITR Antibodies For The Treatment of Cancer
InactiveUS20110212086A1Effective treatmentSatisfies needAntibody ingredientsFused cellsGlucocorticoid-Induced TNFR-Related ProteinCancer cell
The present invention provides compositions and methods for inhibiting the growth of a GITR-expressing cancer cell which cells may include, but are not limited to cells of epithelial origin such as NSCLC, prostate cancer, breast cancer, colon cancer and ovarian cancer and to treat or ameliorate the symptoms associated with the presence of these cells in a subject. Suitable compositions for use in these methods are antibodies that selectively recognize and bind to GITR (Glucocorticoid-induced TNFR-related protein) present on these cancer cells. The antibodies can be either polyclonal or monoclonal antibodies.
Owner:GENZYME CORP
Early infection detection kit of monoclonal antibody-mediated pig pleuropneumoniae
InactiveCN101949934AImmunoglobulins against bacteriaPeptide preparation methodsBiotinAntibodies monoclonal
The invention relates to the research field of biological diagnostic reagents, in particular to an ELISA (Enzyme-Linked Immunosorbent Assay) kit for detecting APP (Actinobacillus Pleuropneumoniae). The kit comprises a washing solution, a colorimetric solution, a stopping solution, a positive control specimen, a negative control specimen and an ELISA plate coating a first antibody as well as a second antibody for detection, wherein the first antibody is a monoclonal protein antibody of the APP rApxIVN, and the second antibody is a biotin-labeled monoclonal protein antibody of the APP rApxIVN. The invention can be applied to detecting APP-infected pig serum and has favorable importance and sensitivity.
Owner:YANGZHOU UNIV
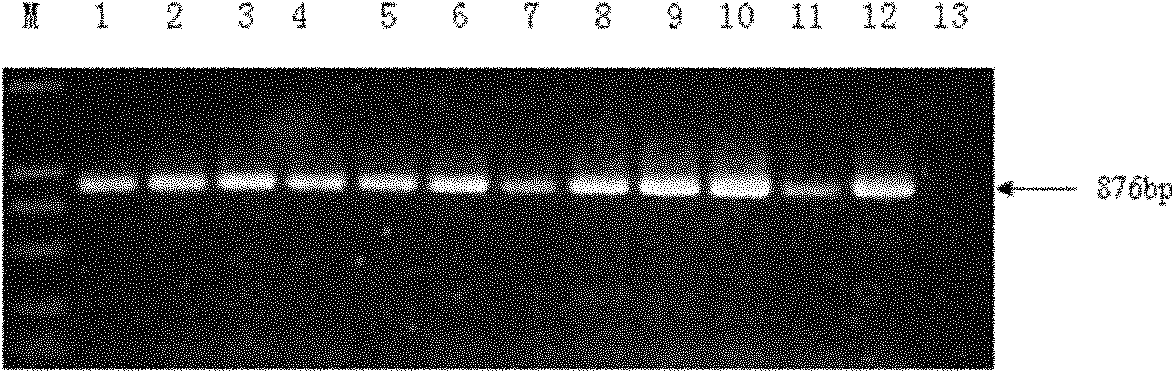
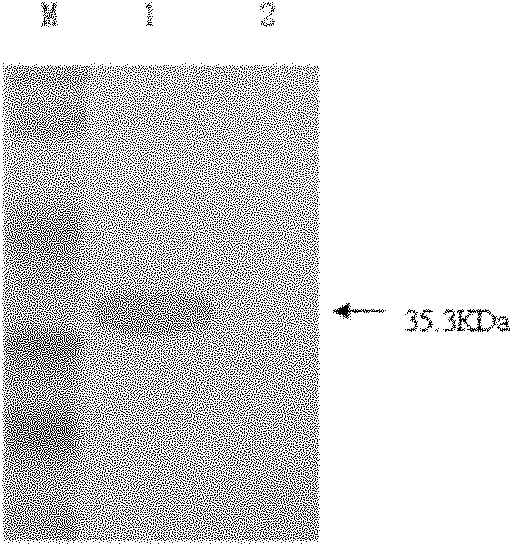
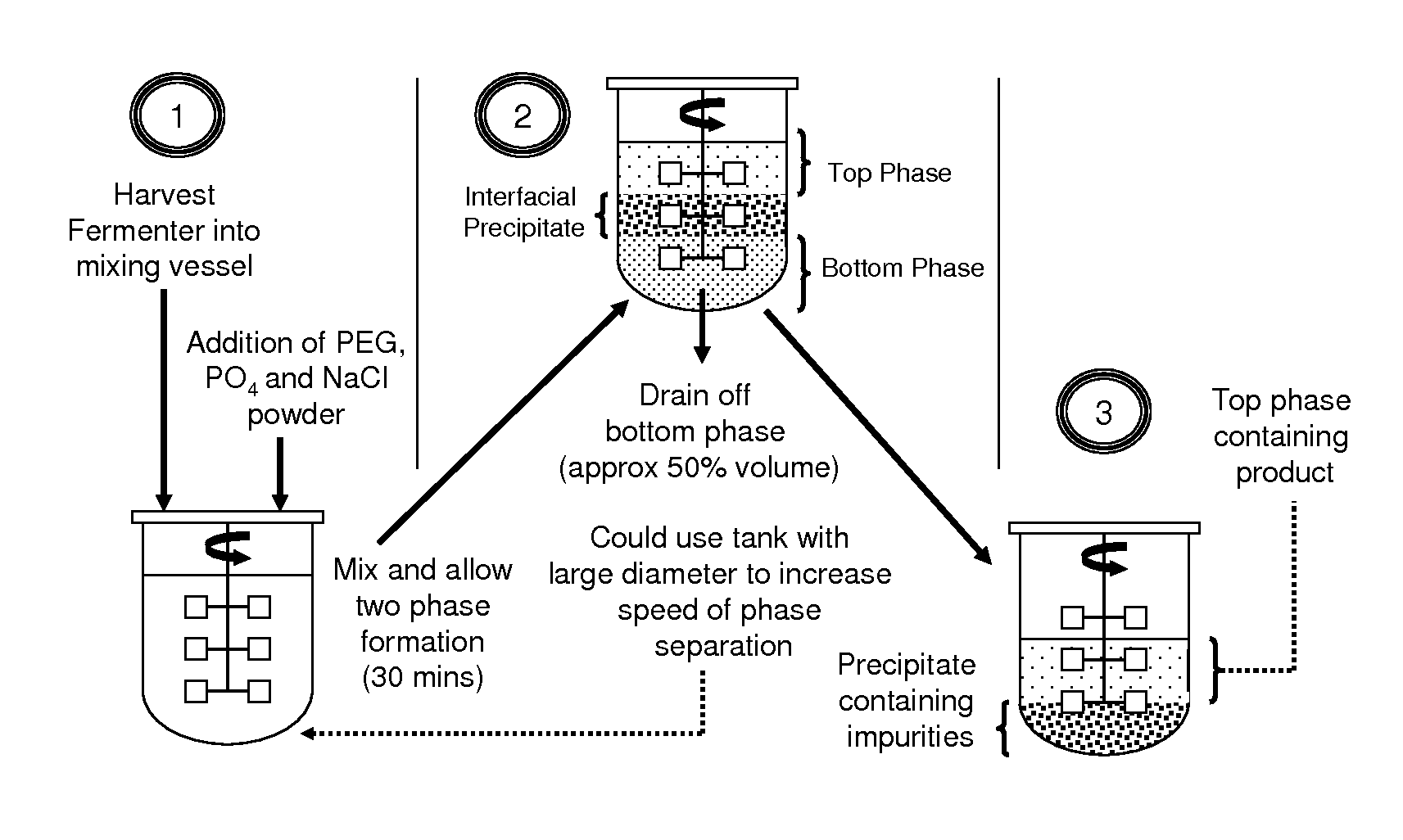
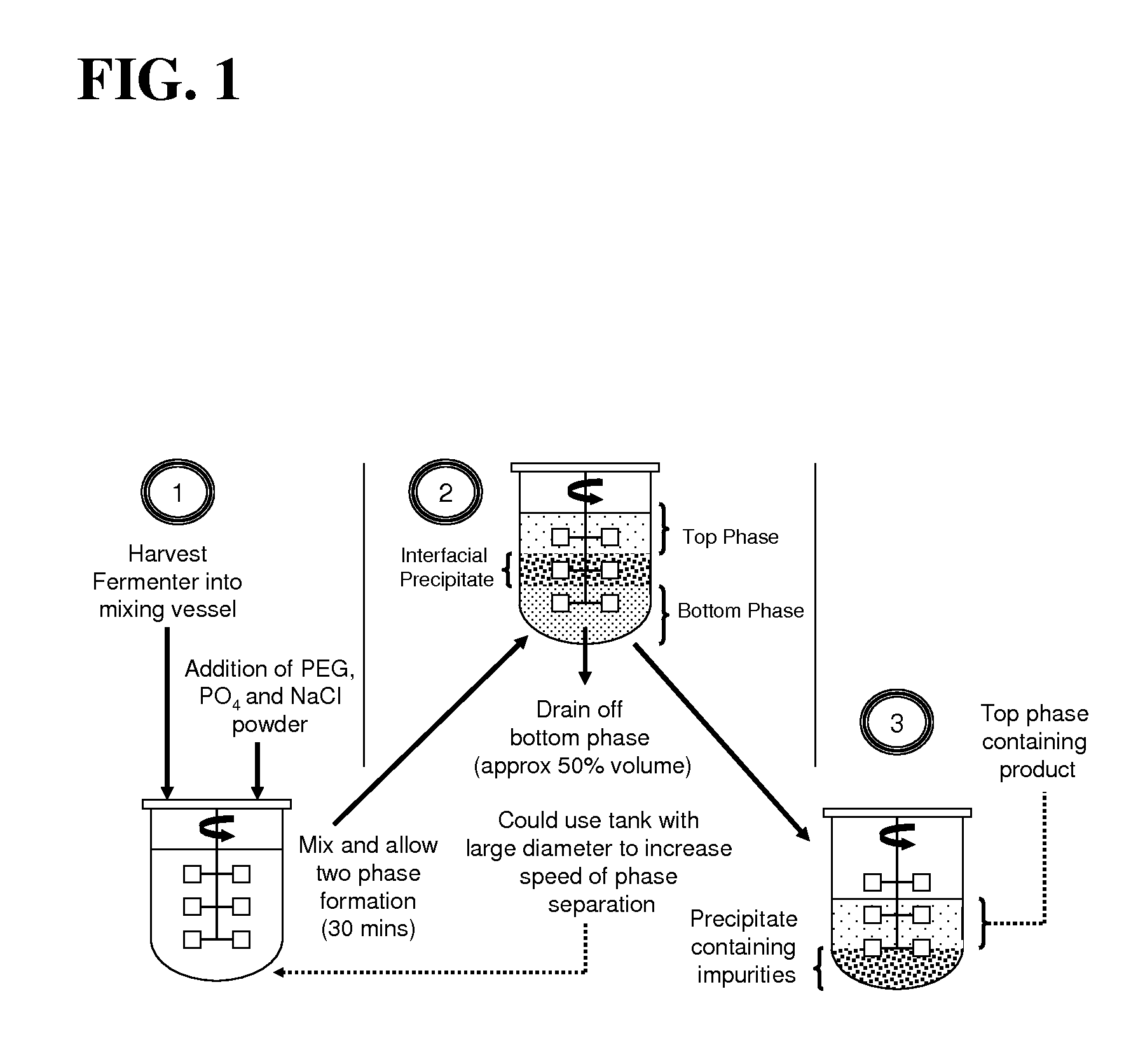
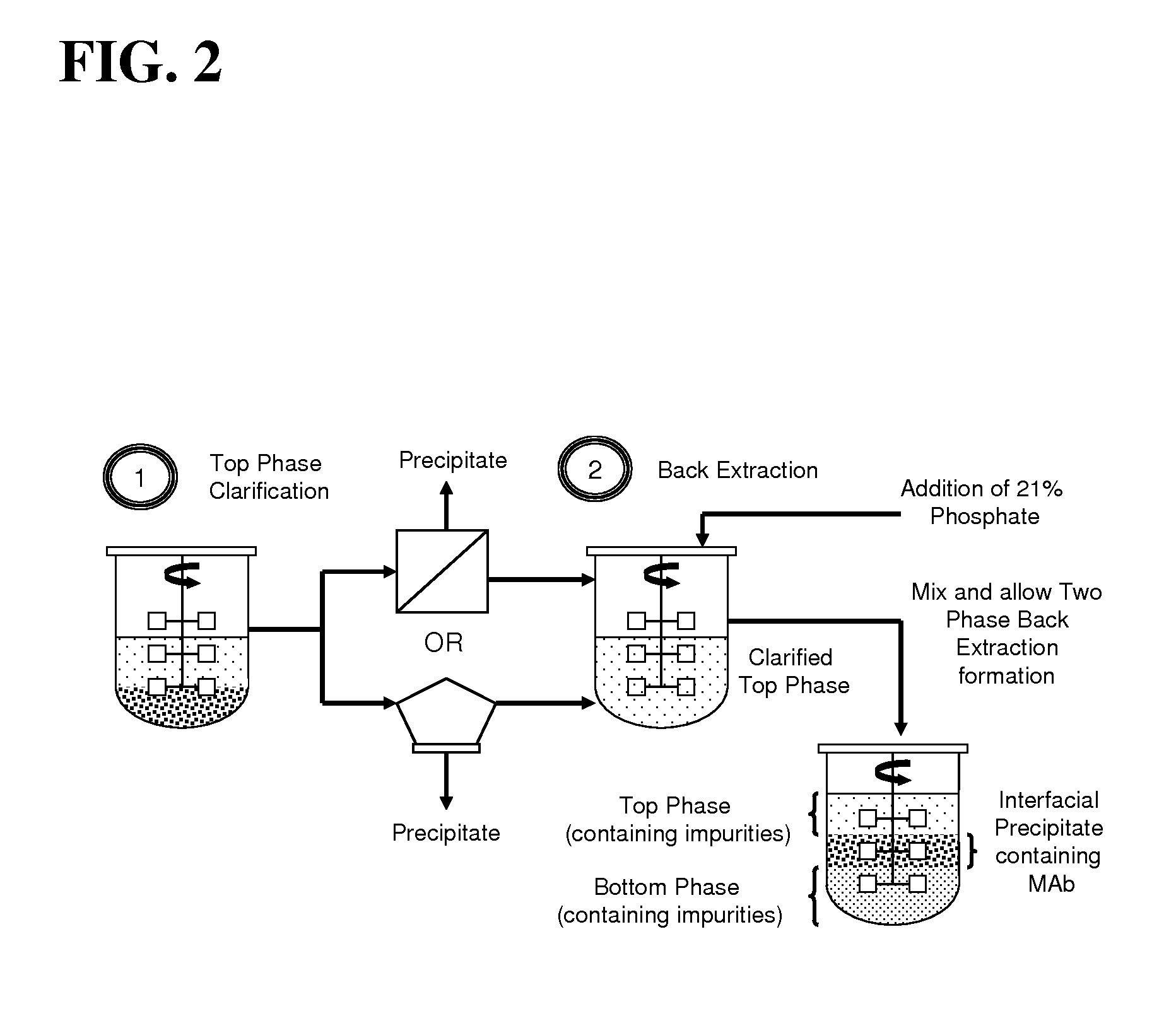
Aqueous two phase extraction augmented precipitation process for purification of therapeutic proteins
InactiveUS20110257378A1Comparable performanceConsiderably robustPeptide preparation methodsDepsipeptidesTherapeutic proteinPhosphate
The invention relates to an aqueous two phase extraction (ATPE) augmented precipitation process, which may be used to recover and also partially purify therapeutic proteins, including monoclonal antibodies from a crude multi-component mixture. The process involves the formation of a forward extraction PEG-Phosphate ATPE system in which the target product is preferentially partitioned to the polymer rich phase. A second ATPE back extraction system is then formed by introducing the polymer rich phase from the forward extraction to a new phosphate salt rich phase, causing the product to precipitate at the interface between the two phases. This precipitate is then recovered and resolubilised in a suitable buffer and may be passed on for further purification.
Owner:GE HEALTHCARE BIO SCI CORP
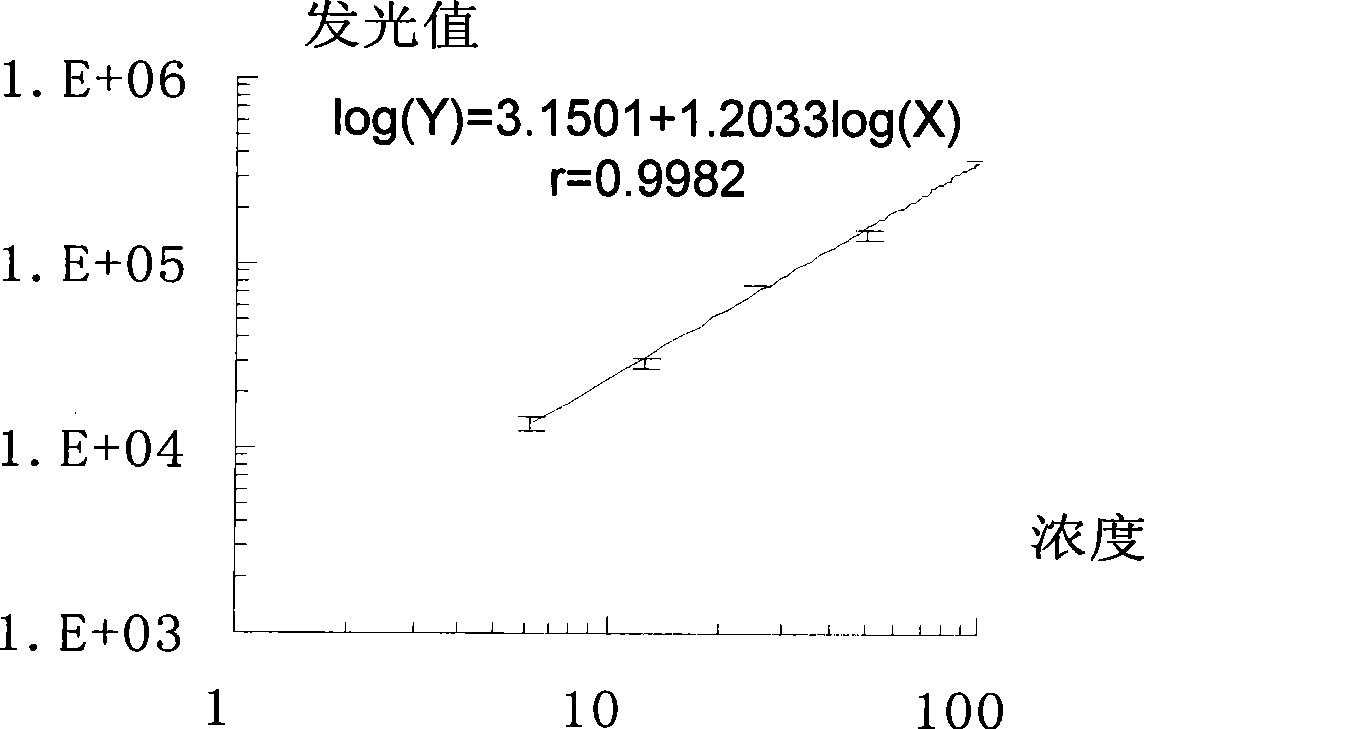
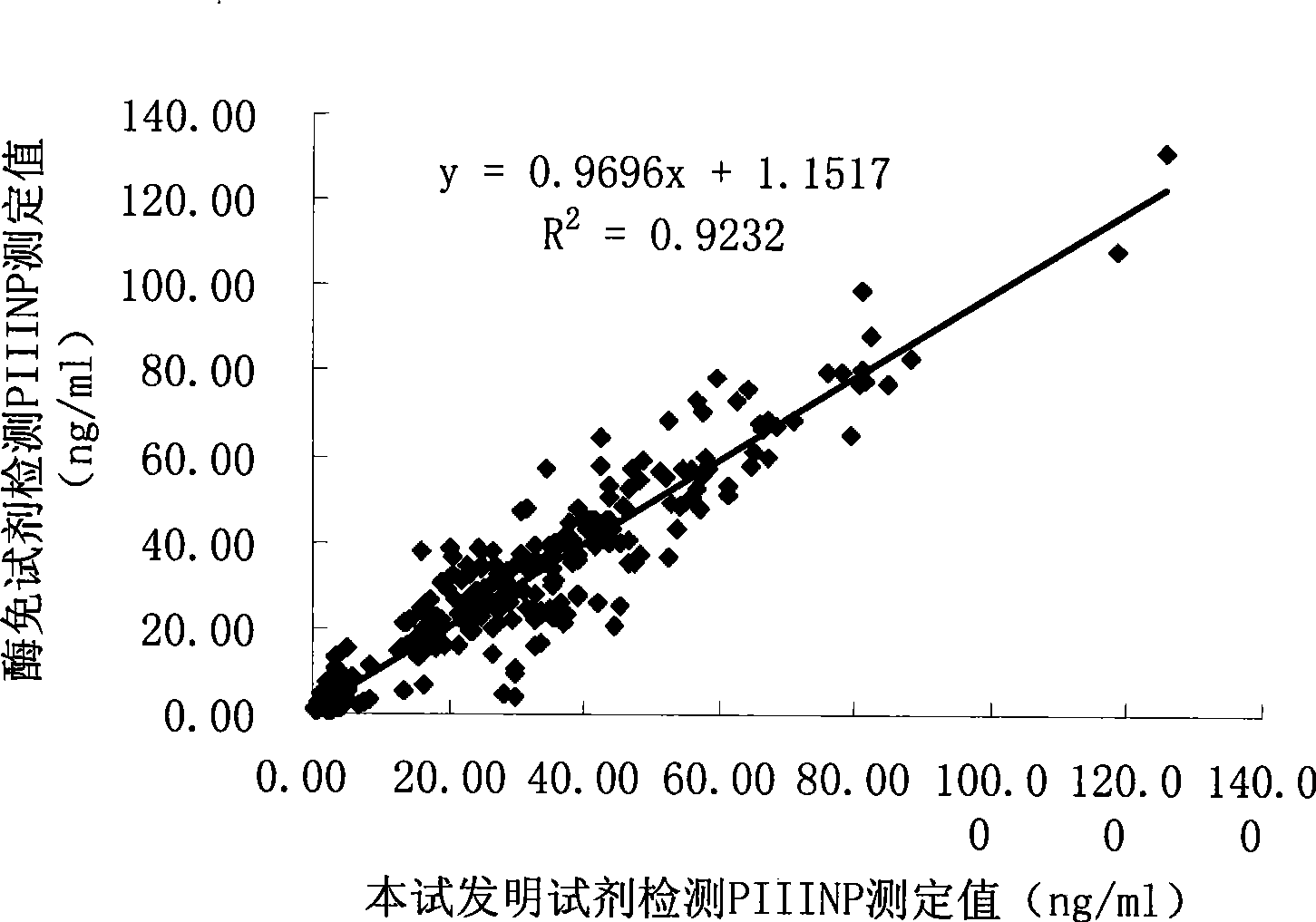
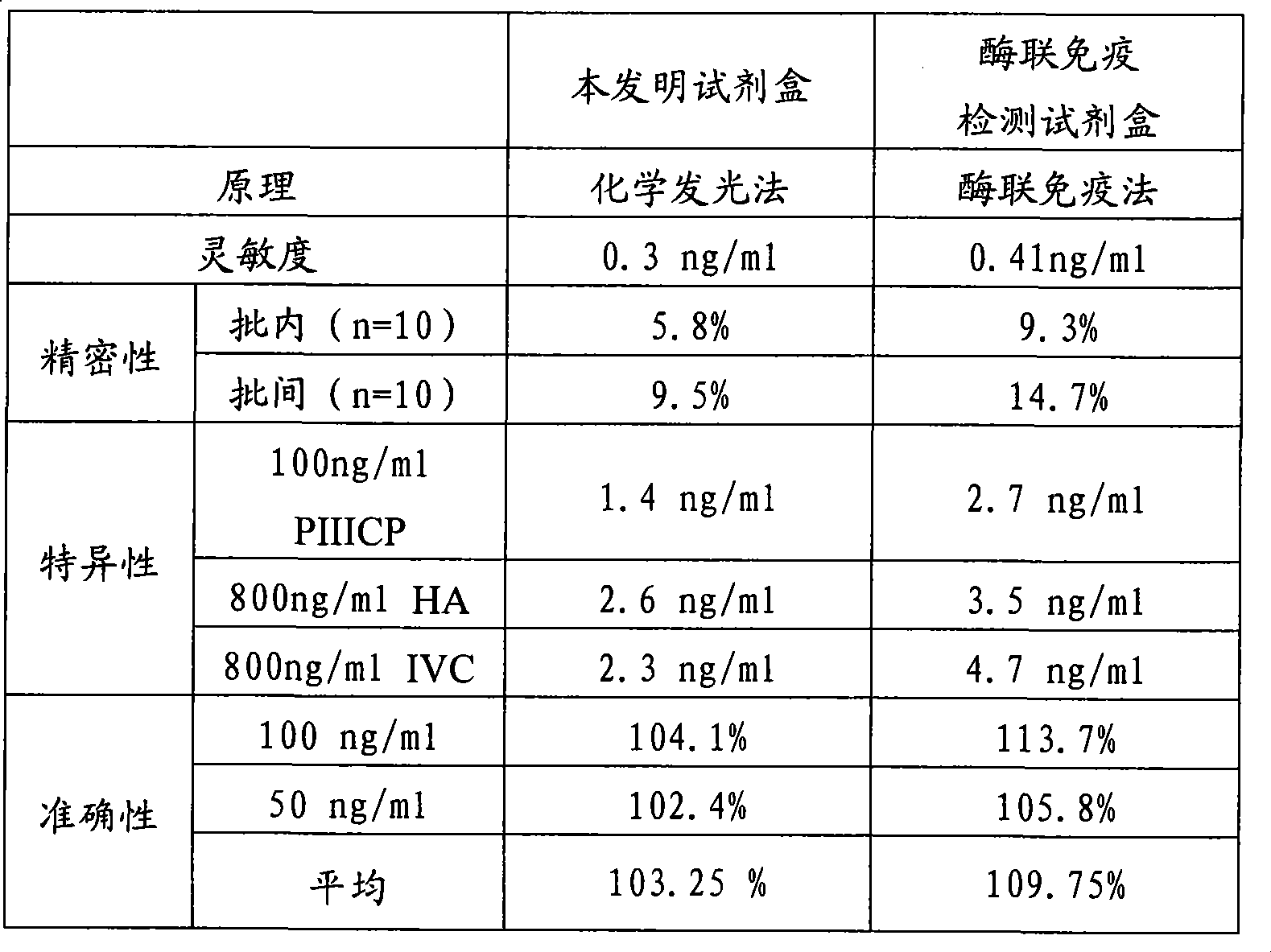
III type precollagen N end peptide chemiluminescence immune analysis quantitative determination reagent kit and preparing method thereof
InactiveCN101377509AGuaranteed sensitivityEasy to operateChemiluminescene/bioluminescenceBiological testingQuantitative determinationMonoclonal antibody
The invention relates to the medical field of immunoassay, more specially, the invention provides a chemiluminescent immunoassay quantitative detection kit for III-type procollagen N-terminal peptide (PIIINP) and a preparation method thereof. The kit of the invention comprises 1) III-type procollagen N-terminal peptide calibrators, 2) solid-phase vectors which are coated with III-type procollagen N-terminal peptide monoclonal antibodies, 3) enzyme markers for III-type procollagen N-terminal peptide monoclonal antibodies, 4) chemiluminescent substrates and 5) concentrated washing solution. Further, the preparation method of the kit according to the invention comprises the following steps: 1) preparing the III-type procollagen N-terminal peptide calibrators with pure III-type procollagen N-terminal peptide, 2) coating the vectors with III-type procollagen N-terminal peptide monoclonal antibodies, 3) marking the III-type procollagen N-terminal peptide monoclonal antibodies with the enzyme, 4) preparing the chemiluminescent substrates, 5) preparing the concentrated washing solution, 6) packaging the III-type procollagen N-terminal peptide calibrators, the enzyme markers, the chemiluminescent substrates and the concentrated washing solution and 7) assembling finished products. The kit of the invention has the advantages of simplicity, convenience, rapidness, sensitivity, stability and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
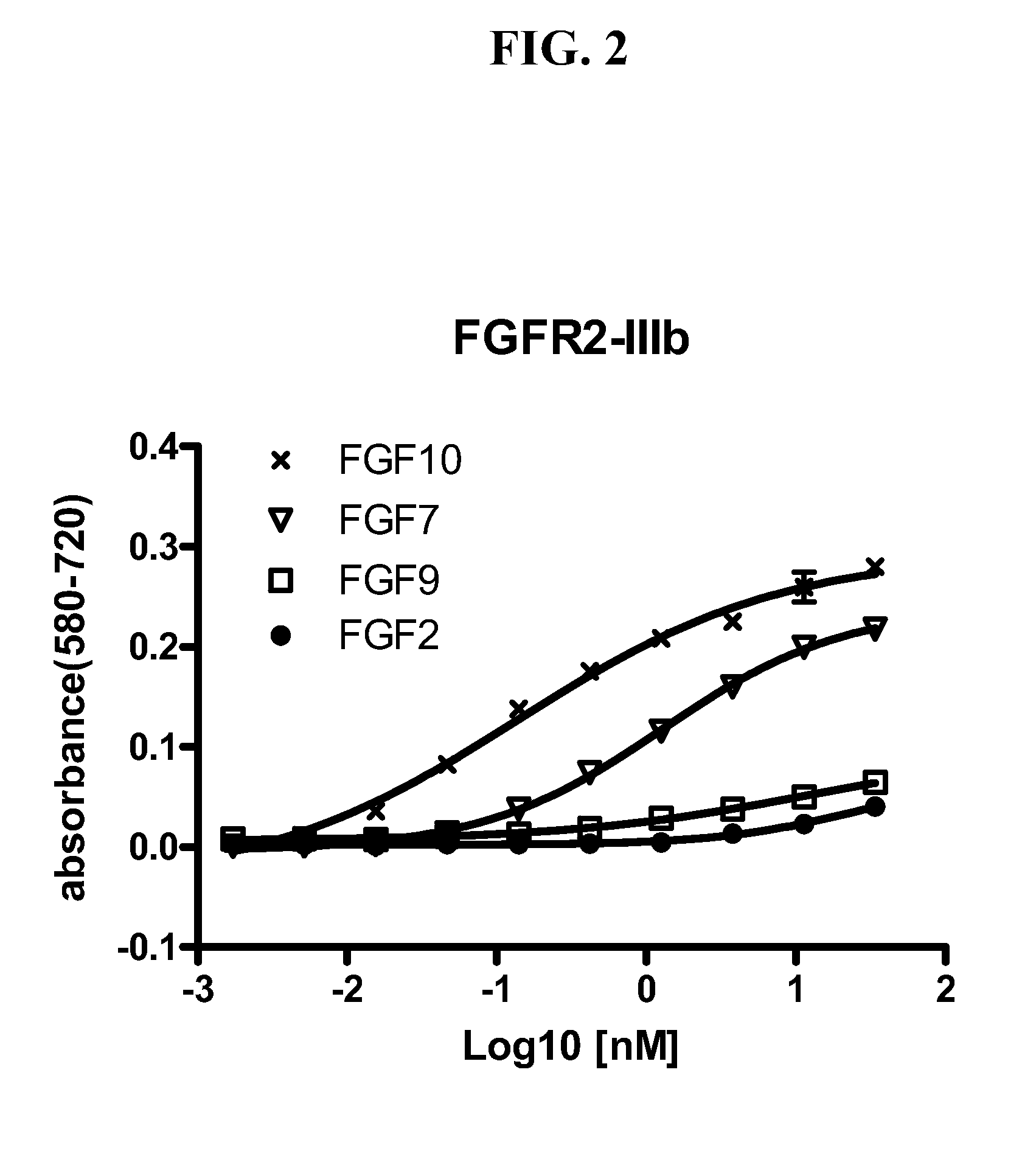
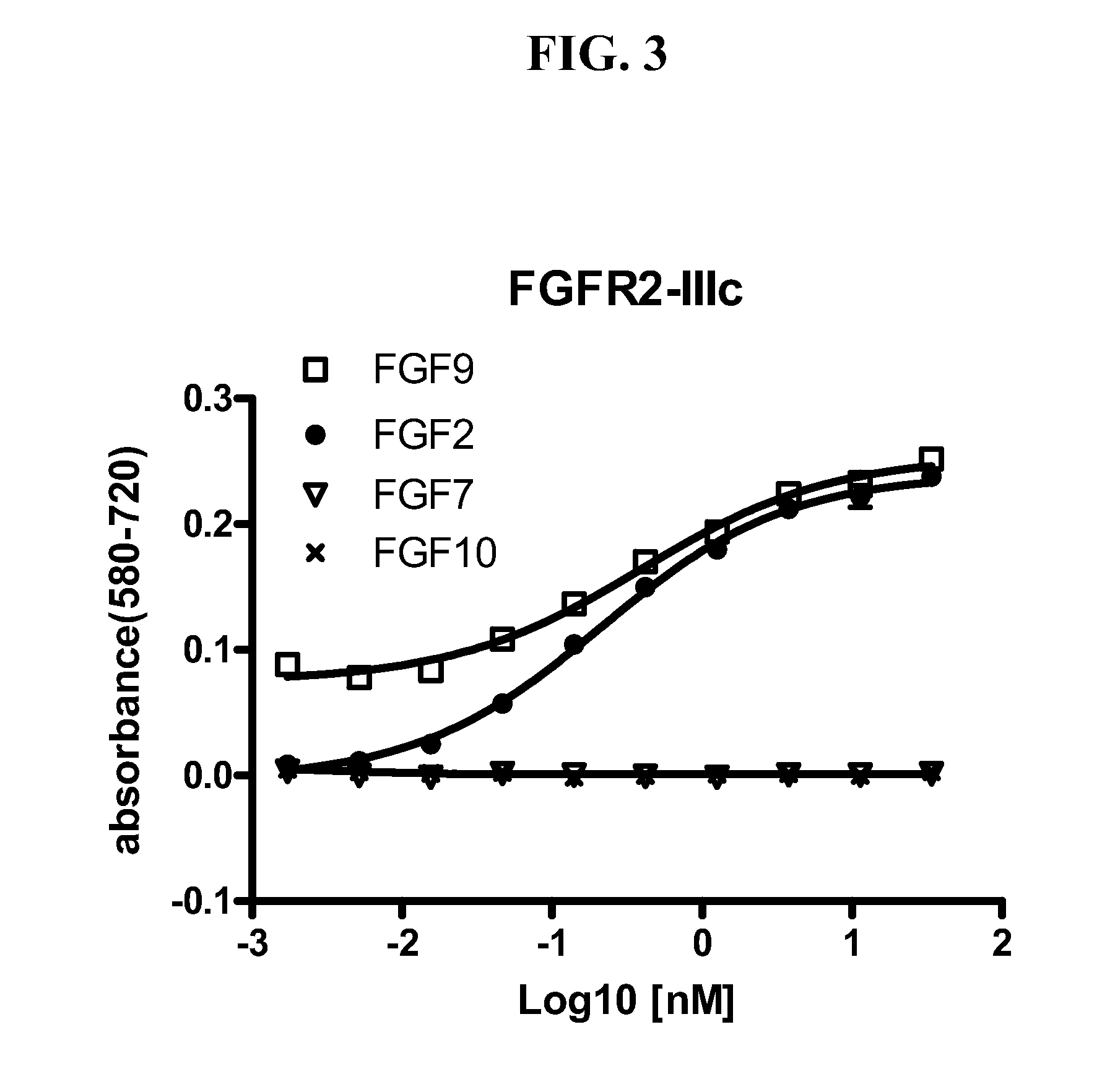
Anti-fgfr2 antibodies
InactiveUS20110305687A1Reduce and eliminate immune responseBacteriaFermentationMonoclonal antibodyBiological activation
Monoclonal antibodies that bind and inhibit biological activities of human FGFR2 are disclosed. The antibodies can be used to treat cell proliferative diseases and disorders, including certain forms of cancer, associated with activation or overexpression of FGFR2.
Owner:AVEO PHARM INC
Fluorescent protein and/or coupled protein monoclonal antibody labeling method and kit thereof
ActiveCN112098640AAchieve directional couplingStrong specificityBiological material analysisFluorescence/phosphorescenceDisulfide bondingProtein.monoclonal
The invention provides a fluorescent protein and / or coupled protein monoclonal antibody labeling method and a kit thereof. The preparation method comprises the following steps: firstly, carrying out desalination treatment on fluorescent protein and / or coupled protein, blocking sulfydryl of the fluorescent protein and / or coupled protein by adopting NEM, and carrying out cross-linking reaction on the fluorescent protein and / or coupled protein NEM and S-SMCC; meanwhile, cross-linking SLCSPDP and the monoclonal antibody, reducing disulfide bonds of the monoclonal antibody through TCEP, and conducting sulfhydrylation on the monoclonal antibody; and finally, carrying out cross-linking reaction on the sulfhydrylated antibody and NEM-fluorescent protein and / or coupled protein-S-SMCC. Directional coupling of the fluorescent protein and / or the coupling protein and the monoclonal antibody is achieved, the cross-linking efficiency of labeling of the fluorescent protein and / or the coupling proteinand the monoclonal antibody is improved, the specificity of labeling of the fluorescent protein and / or the coupling protein and the monoclonal antibody is further improved, and a fluorescence signal is enhanced.
Owner:ZHEJIANG ZHENGXI BIOMEDICAL CO LTD
Human occludin antigen epitope peptide, antigen, antibody, kit and use
ActiveCN108084257AGood antigenicityStrong specificityCell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeOccludin
The present invention relates to a human occludin antigen epitope peptide, a human occludin antigen, a human occludin antibody, a kit and use. An amino acid sequence of the human occludin antigen epitope peptide is as shown in one of sequence table SEQ ID NO. 1 and sequence table SEQ ID NO.2. The human occludin antigen is prepared from the human occludin antigen epitope peptide and a protein carrier by coupling. A human occludin monoclonal or polyclonal antibody is prepared from the human occludin antigen. The human occludin monoclonal or polyclonal antibody is used for the preparation of an occludin in-vitro diagnostic kit. The human occludin antigen epitope peptide has good antigenicity, highly specific monoclonal and polyclonal antibodies can be produced by use of an antigen (immunogen)produced from the human occludin antigen epitope peptide for immunizing an animal, and the human occludin antigen epitope peptide can be applied to human occludin in vitro testing.
Owner:深圳市安群生物工程有限公司
Human heparin binding protein assay kit with high sensitivity and wide detection range
InactiveCN109613259AWide detection rangeGuaranteed SensitivityBiological testingMicrosphereConcentration gradient
The embodiments of the invention relate to the field of immunoassay, and in particular to a human heparin binding protein assay kit with high sensitivity and wide detection range. The human heparin binding protein assay kit comprises: a reagent 1, a reagent 2 and human HBP calibrators with different concentration gradients. The reagent 2 comprises a carboxyl latex microsphere labeled only with human HBP monoclonal antibody and a carboxyl latex microsphere labeled only with human HBP polyclonal antibody. The average particle diameter of the carboxyl latex microsphere labeled only with the humanHBP monoclonal antibody > the average particle diameter of the carboxyl latex microsphere labeled only with the human HBP polyclonal antibody. The human heparin binding protein assay kit provided bythe invention can complete a single sample test within 10 minutes, and indexes of precision, accuracy and anti-interference are excellent. The invention can be used clinically to predict an organ dysfunction caused by sepsis, and can be used as an early diagnostic marker for sepsis, especially serious bacterial infection.
Owner:BEIJING BEIER BIOENG
ESC42 protein, preparation method and use thereof
InactiveCN1840544APeptide/protein ingredientsImmunoglobulins against animals/humansGeneAntibacterial drug
The provided human epididymis specific express protein ESC42 with coded sequence can be prepared by chemical method or genetic engineering technique, and can be used to prepare antibacterial drug or new contraceptive.
Owner:李建远
Seneca Valley virus VP1 protein, coding gene, hybridoma cell line and monoclonal antibody and application thereof
The invention relates to a Seneca valley virus VP1 protein, a coding gene, a hybridoma cell line and a monoclonal antibody and application thereof, which belongs to the technical field of viruses. TheSeneca valley virus Hubei strain VP1 protein is used as a Seneca Valley virus antigen protein in immunodetection. The invention provides a hybridoma cell line 2G6 of a monoclonal antibody capable ofsecreting the Seneca Valley virus Hubei strain VP1 protein according to claim 1, and a preservation number of the hybridoma cell line is CCTCC NO. C2017226. The monoclonal antibody is secreted from the hybridoma cell line 2G6. The invention relates to application of the monoclonal antibody in immune combined Seneca Valley virus Hubei strain VP1 protein. The invention provides application of the monoclonal antibody in a kit for preventing the Seneca Valley virus Hubei strain from infecting the cells.
Owner:HUAZHONG AGRI UNIV
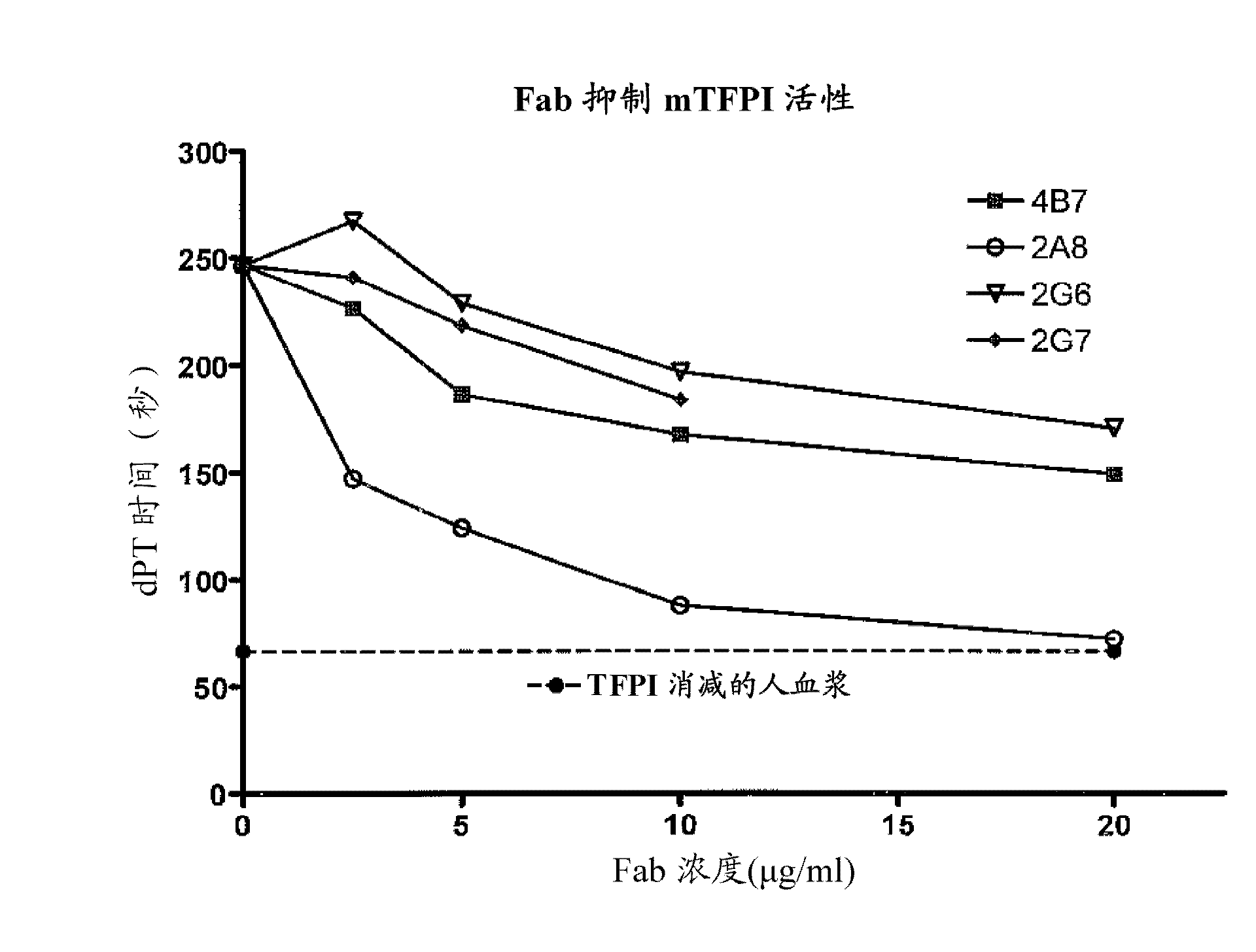
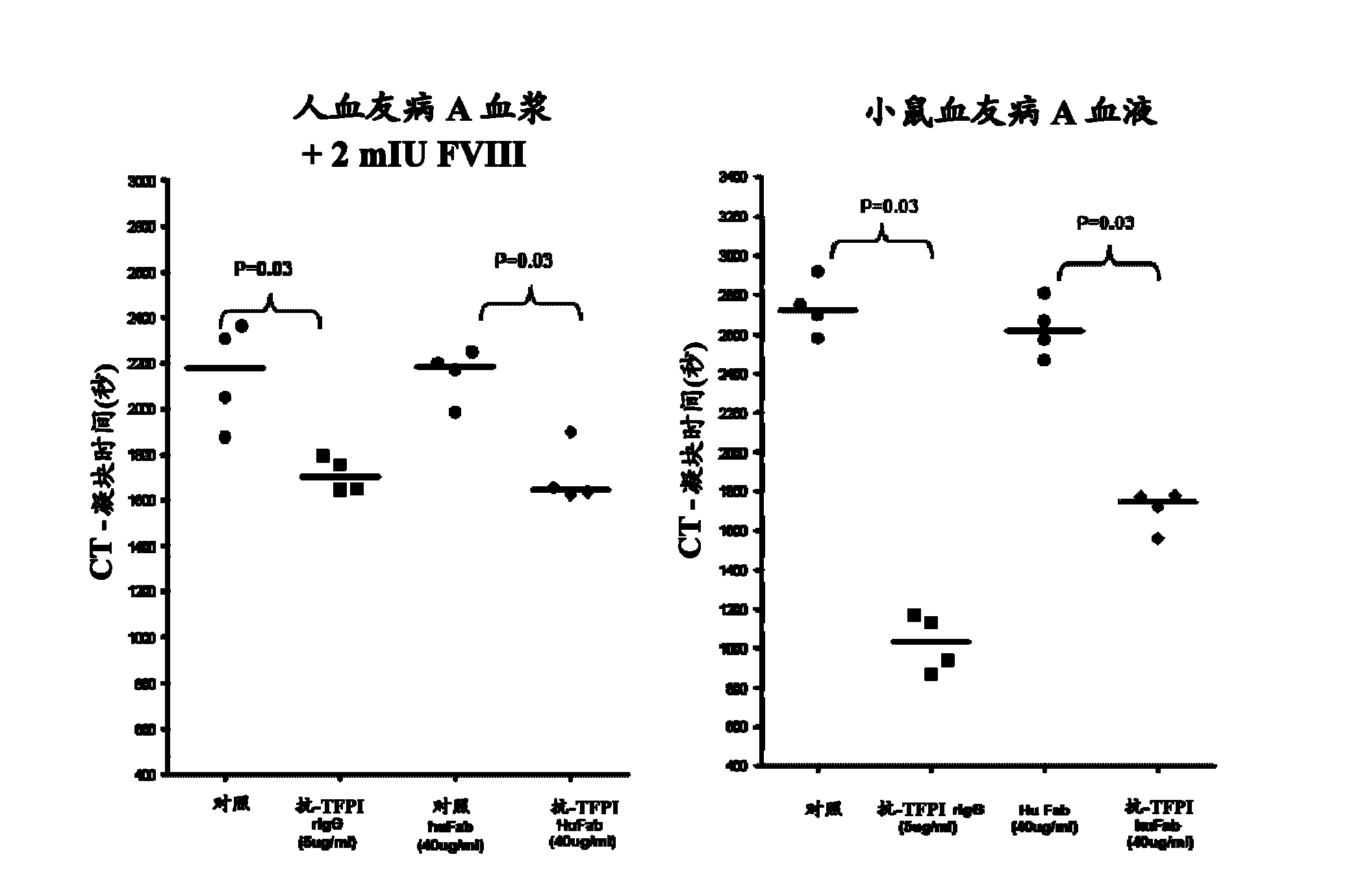
Monoclonal antibodies against tissue factor pathway inhibitor (TFPI)
InactiveCN102143979AShorten bleeding timeImmunoglobulins against blood coagulation factorsAntibody mimetics/scaffoldsMonoclonal antibodyTissue factor pathway inhibitor
Isolated monoclonal antibodies that bind human tissue factor pathway inhibitor (TFPI) and the isolated nucleic acid molecules encoding them are provided. Pharmaceutical compositions comprising the anti-TFPI monoclonal antibodies and methods of treating deficiencies or defects in coagulation by administration of the antibodies are also provided. Methods of producing the antibodies are also provided.
Owner:BAYER HEALTHCARE LLC
Neutralizing monoclonal antibody resisting to tetanus toxin and application thereof
ActiveCN105542004AStrong specificityProlonged deathAntibacterial agentsImmunoglobulins against bacteriaLethal doseComplete antibody
The invention discloses a neutralizing monoclonal antibody resisting to tetanus toxin. Hybridoma cells are obtained from tetanus toxin heavy chain C fragment immune mice, light chain and heavy chain variable region genes of the antibody are taken, human-mouse chimeric complete antibody expression vectors are built, CHO cell transfection is carried out, purification is carried out, and then the antibody is obtained. The antibody has the activity of specific binding to tetanus toxin, the monoclonal antibody can partially protect mice against attack of tetanus toxin, and four antibodies can completely protect mice against a twofold lethal dose of tetanus toxin in combination.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Porcine pseudorabies virus gE IgM antibody colloidal gold immunochromatograohic assay test strip as well as preparation method and application thereof
InactiveCN103983781AImprove detection accuracyImprove featuresBiological material analysisProtein.monoclonalAssay
The invention discloses a porcine pseudorabies virus gE IgM antibody colloidal gold immunochromatograohic assay test strip as well as a preparation method and application thereof. The test strip comprises a sample absorption region, a colloidal gold labeled probe region, a solidifying antibody region, a water absorption region, a bottom plate and a clamping shell, wherein the sample absorption region, the colloidal gold labeled probe region, the solidifying antibody region and the water absorption region are sequentially bonded on the bottom plate and are interlapped in the clamping shell; the sample absorption region is coated with purified porcine pseudorabies virus gE protein; the colloidal gold labeled probe region is coated with colloidal gold labeled porcine pseudorabies virus gE protein monoclonal antibody; the solidifying antibody region is sequentially provided with a detection line T coated with mouse anti-pig IgM monoclonal antibody and a control line C coated with goat anti-mouse IgG. The test strip is simple to operate, good in repeatability, high in sensitivity, rapid and intuitive in result, simple in process and low in cost, can be mass-prepared, and is suitable for mass field test and early diagnosis for porcine pseudorabies virus infection in swinery.
Owner:WUHAN CHOPPER BIOLOGY
Anti-Calponin protein monoclonal antibody and cell strain, preparation method and application thereof
ActiveCN113234155AStrong specificityIncreased sensitivityImmunoglobulins against animals/humansMicroorganism based processesEscherichia coliAntigen
The invention relates to a monoclonal antibody capable of recognizing a human Calponin antigen, a secretory cell strain, a preparation method of the monoclonal antibody and application of the secretory cell strain in immunodetection. According to the technical scheme, amino acids from the first site to the 297th site at the C tail end of the Calponin protein are selected as antigen peptides, codon optimization is carried out, a gene segment suitable for being expressed in escherichia coli BL21 is formed, and finally the obtained recombinant protein comprises a Calponin protein segment and a histidine protein tag. A mouse is immunized by the recombinant protein, and the mouse hybridoma cell strain 24H5 capable of efficiently secreting the anti-Calponin protein monoclonal antibody and the anti-Calponin protein monoclonal antibody secreted by the cell strain are obtained through cell fusion, screening and subcloning. The antibody obtained by the scheme has high specificity and sensitivity, can specifically recognize cells expressing Calponin protein, and is suitable for immunological detection, especially immunohistochemical detection.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
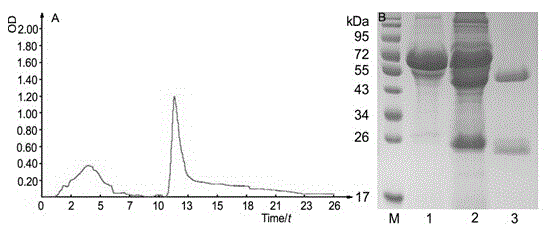
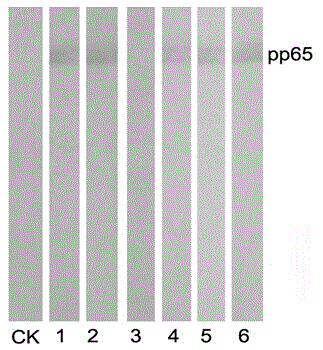
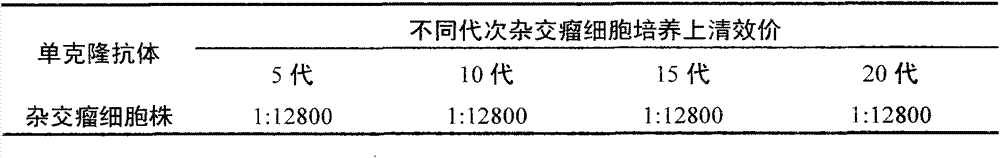
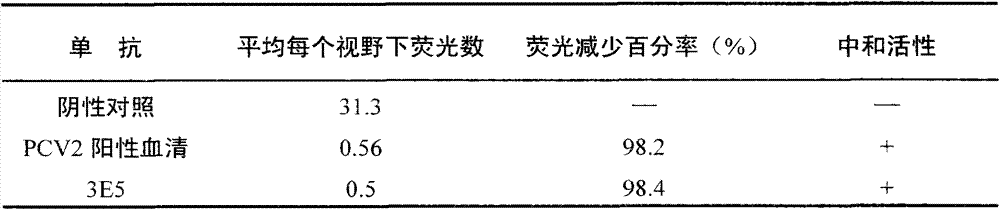
Preparation method and application of anti-HCMV (human cytomegalovirus) Pp65 protein monoclonal antibody
The invention discloses a high-specificity and high-affinity anti-Pp65 monoclonal antibody which is generated by a hybridoma cell strain 8D6 of the anti-Pp65 monoclonal antibody, wherein the cell strain 8D6 is collected in China Center For Type Culture Collection (CCTCC) and has a collection number of CCTCC NO:C201476. The monoclonal antibody secreted by the hybridoma cell strain has good affinity and specificity, and the immunoreactivity and the specificity of the antibody are superior to those of domestic and imported monoclonal antibodies. The anti-Pp65 monoclonal antibody can serve as a key raw material for blood white cell immunofluorescent cytochemical diagnosis and immunofluorescent quantitative PCR (polymerase chain reaction) detection of HCMV (human cytomegalovirus) infection, also can serve as a key raw material for detecting HCMV by using a double antibody sandwich method, has important meaning for establishment of a quick and sensitive clinical diagnosis reagent to HCMV infection, and has important application prospects.
Owner:HUNAN NORMAL UNIVERSITY
Hybridoma cell strain secreting porcine circovirus type 2 Cap protein monoclonal antibody
Owner:NANJING AGRICULTURAL UNIVERSITY
Rapid colloidal gold detection test strip for pig porcine reproductive and respiratory syndrome virus
ActiveCN103995136AEasy to operateMeet the needs of on-site testingBiological material analysisBiological testingCelluloseColloidal au
The invention discloses a rapid colloidal gold detection test strip for a porcine reproductive and respiratory syndrome virus. The test strip comprises a sample absorbing pad, a glass fiber membrane containing a colloidal gold marked M protein monoclonal antibody of the porcine reproductive and respiratory syndrome virus, a nitrocellulose membrane with a detection line T and a control line C, a piece of water absorbing paper and a substrate, wherein the detection line T is wrapped with a GP5 protein monoclonal antibody of the porcine reproductive and respiratory syndrome virus; the control line C is wrapped with a second antibody IgG; the sample absorbing pad, a glass fiber membrane, the nitrocellulose membrane and the water absorbing paper are adhered to the substrate sequentially in a mutual lap joint manner. The test strip adopts a membrane chromatography double-antibody sandwich method for detecting the porcine reproductive and respiratory syndrome virus in a sample, is simple, convenient and rapid to operate, does not need special instruments, equipment or special training, is easy to popularize, is applicable to basic level, is applicable to large-scale on-site detection and epidemiological investigation in emergency, and plays an assistant role in diagnosis on infection of the porcine reproductive and respiratory syndrome virus, wherein the result is clear and easy.
Owner:WUHAN CHOPPER BIOLOGY
Epididymal protease inhibitor protein monoclonal antibody and application thereof
InactiveCN101519448AStrong specificityMicroorganism based processesAntibody ingredientsEpididymal protease inhibitorMale infertility
The invention discloses an epididymal protease inhibitor protein monoclonal antibody and application thereof, which belong to the field of biological engineering. The monoclonal antibody is secreted by hybridoma preserved in China Center for Type Culture Collection (CCTCC) with the preservation number of C200849. The monoclonal antibody can be combined to the epididymal protease inhibitor protein Eppin protein of specific expression in a testis and epididymis tissue by specificity. The monoclonal antibody can be used for preparing male infertility diagnostic reagents, male contraceptives and anti-fertility study biological products.
Owner:NANJING MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com