Monoclonal antibodies against tissue factor pathway inhibitor (TFPI)
A monoclonal antibody, tissue factor technology, applied in the direction of protease inhibitors, antibodies, protease inhibitor immunoglobulins with anti-peptide structure, etc., can solve problems such as long duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Example 1: Panning and screening of human antibody library against human TFPI
[0182] Panning Human Antibody Libraries Against TFPI
[0183] Anti-TFPI antibodies were selected by panning the phage display combinatorial human antibody library HuCal Gold (Rothe et al., J. Mol. Biol., 2008, 376: 1182-1200) against human TFPI (American Diagnostica). Briefly, 200 μl of TFPI (5 μg / ml) was coated overnight at 4° C. on 96-well Maxisorp plates, and then the plates were blocked with PBS buffer containing 5% milk. After washing the plates with PBS containing 0.01% Tween-20 (PBST), aliquots of the combinatorial human antibody library were added to the TFPI-coated wells and incubated for 2 hours. Unbound phage were washed away with PBST, and antigen-bound phage were diluted with dithiothreitol, infected, and amplified in E. coli strain TG1. Phage are rescued by helper phage for the next round of panning. A total of three rounds of panning were performed and clones from the first...
Embodiment 2
[0195] Embodiment 2: Expression and purification of anti-TFPI antibody
[0196] Anti-TFPI antibodies were expressed (as Fab fragments) and purified from bacterial strain TG1. Briefly, single clones of bacterial strain TG1 containing antibody expression plasmids were picked and grown overnight in 8 ml 2x YT medium in the presence of 34 μg / ml chloramphenicol and 1% glucose. A volume of 7 ml of culture was transferred to 250 ml of fresh 2x YT medium containing 34 μg / ml chloramphenicol and 1% glucose. After 3 hours of incubation, 0.5 mM IPTG was added to induce Fab expression. The incubation was continued overnight at 25°C. Centrifuge the culture to pellet the bacterial cells. The pellet was then resuspended in Bug Buster lysis buffer (Novagen). After centrifugation, filter the supernatant of the bacterial lysate. Fab fragments were affinity purified by Ni-NTA columns (Qiagen) according to the manufacturer's instructions.
Embodiment 3
[0197] Example 3: EC of Anti-TFPI Antibodies 50 and determination of binding affinity
[0198] Use of purified Fab antibodies to determine the EC of anti-TFPI antibodies against human or mouse TFPI 50 . EC was assessed in an ELISA similar to that described above 50 . Use SoftMax to analyze the results. The binding affinity of anti-TFPI antibodies was determined in a Biacore assay. Briefly, antigens (either human or mouse TFPI) were immobilized on CM5 chips using an amine coupling kit (GE HealthCare) according to the manufacturer's instructions. The amount of immobilized TFPI was adjusted relative to the antigen mass to give approximately 300 RU. Antibody Fabs were analyzed in the mobile phase and at least five different concentrations (0.1, 0.4, 1.6, 6.4 and 25 nM) of purified antibody were used in the Biacore assay. Kinetics and binding affinities were calculated using Biacore T100 evaluation software.
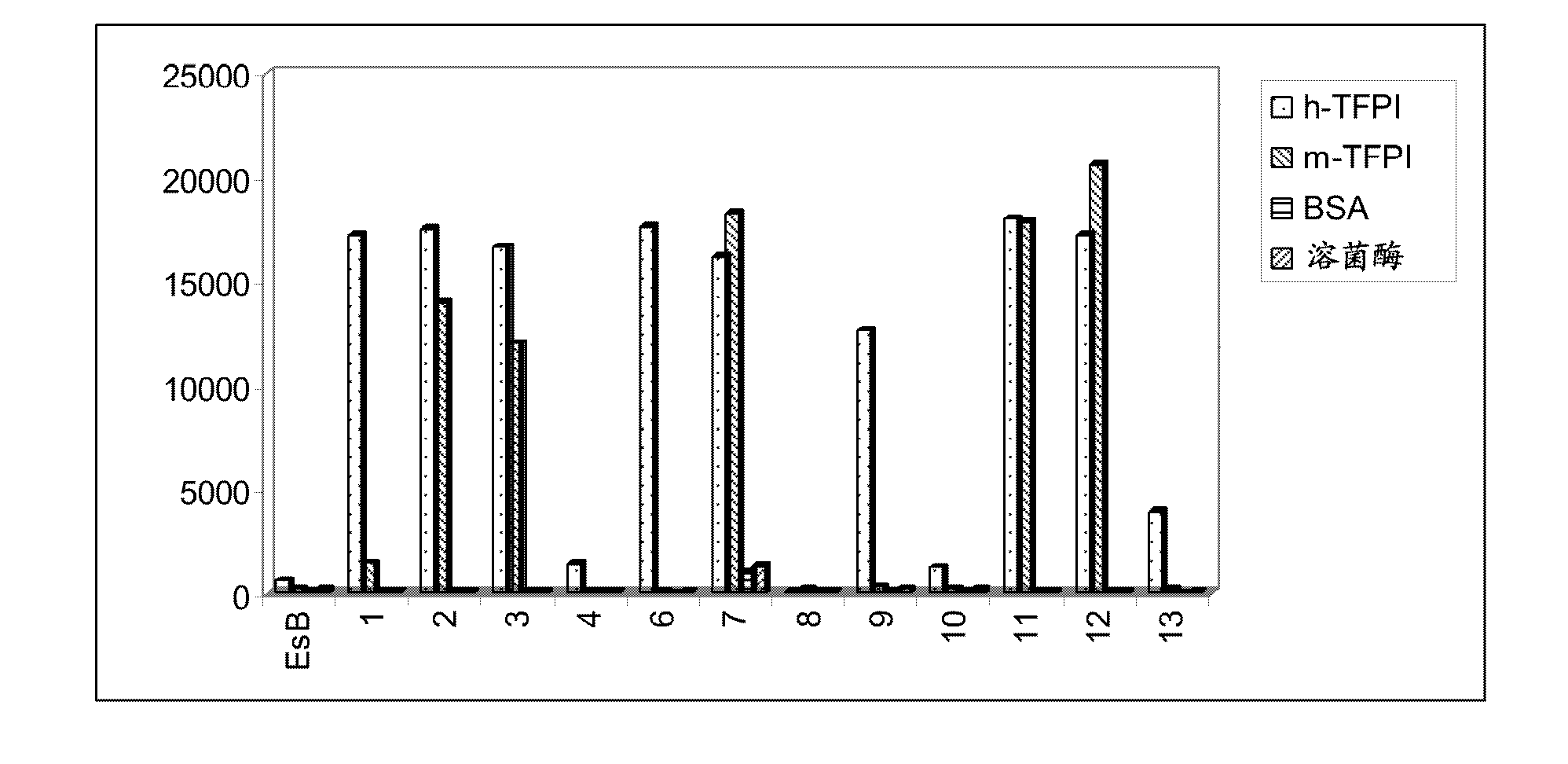
[0199] As shown in Table 3, 24 anti-TFPI Fabs showed various ECs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com