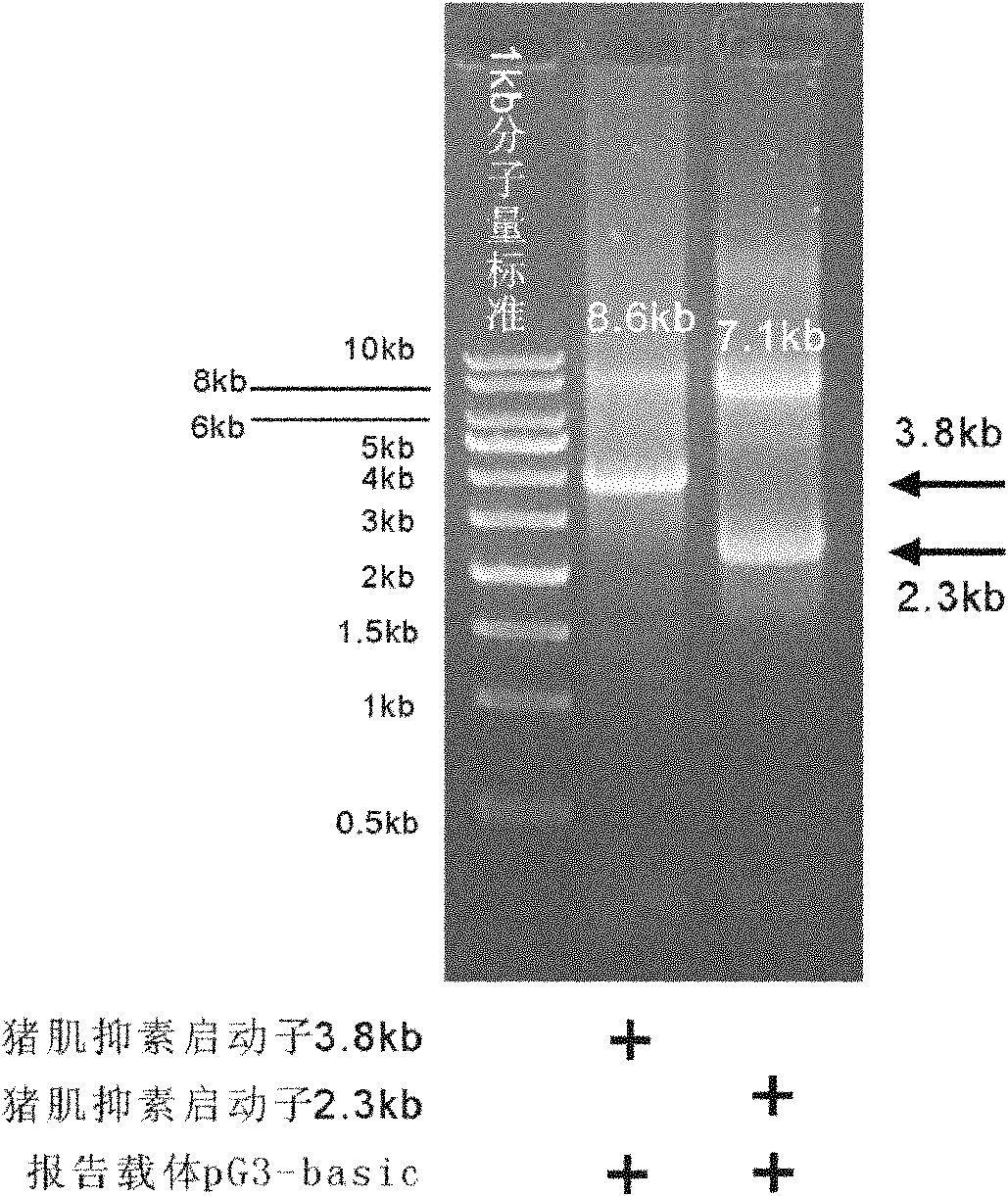
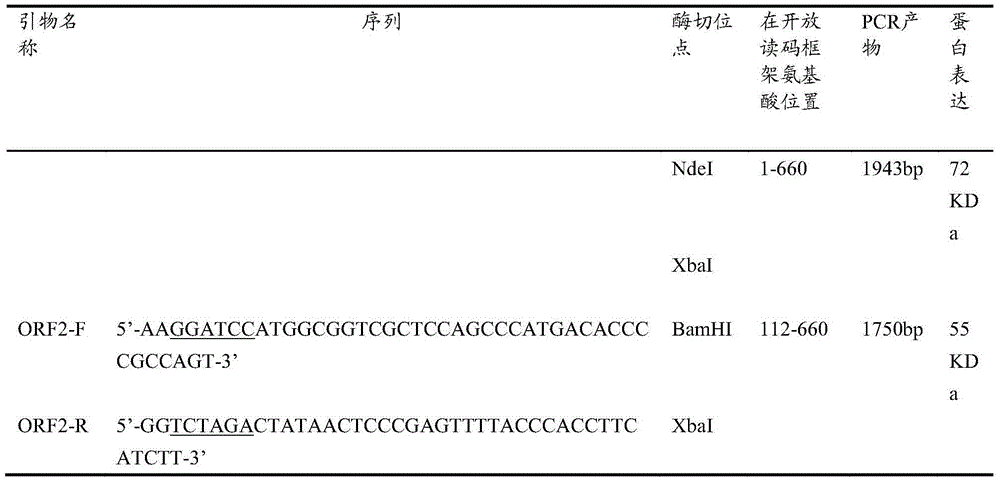
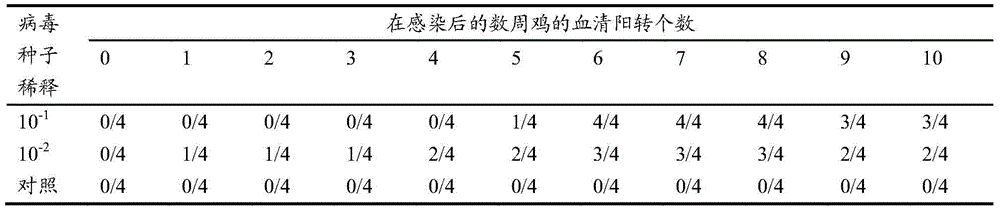
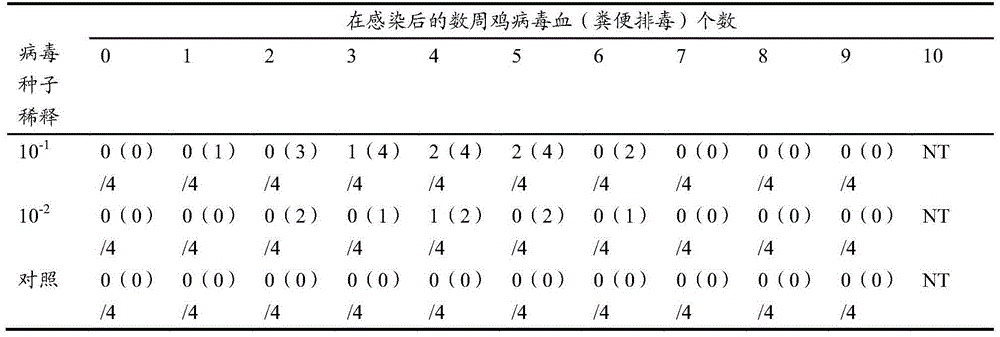
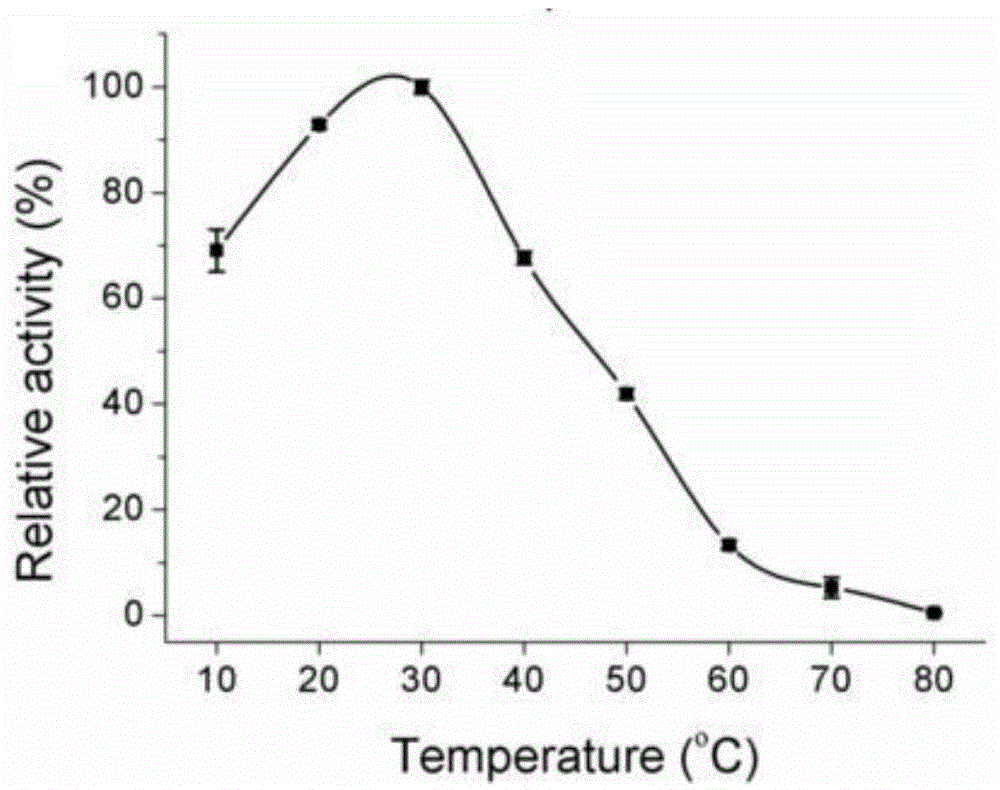
Patents
Literature
234 results about "Coli strain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Each type of E. coli strain varies in its transmission and method of infection. EHEC E. coli strains are the most life threatening due to their shiga-toxin production. ETEC E. coli strains are the most common cause of traveler’s diarrhea when travelling to developing countries.
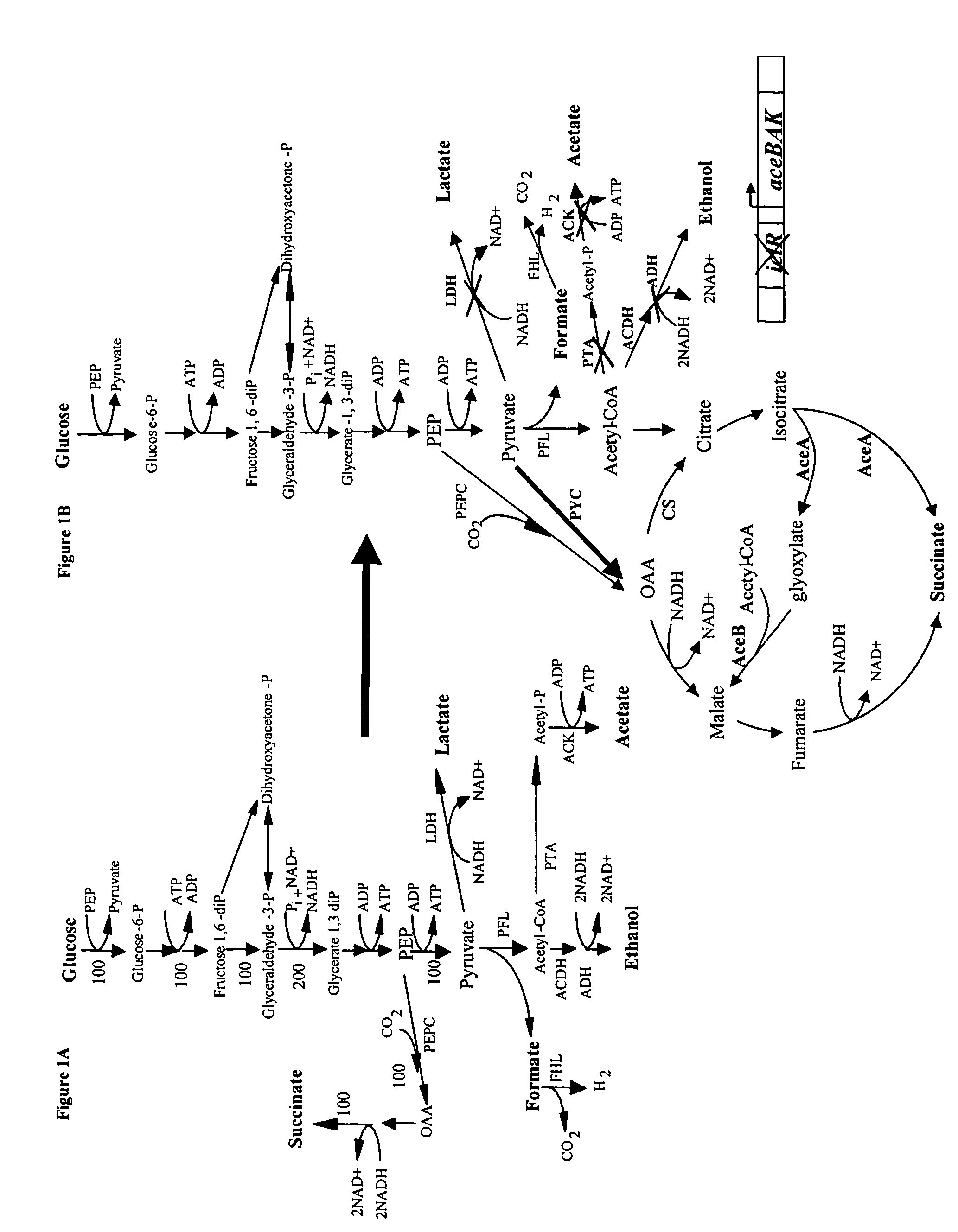
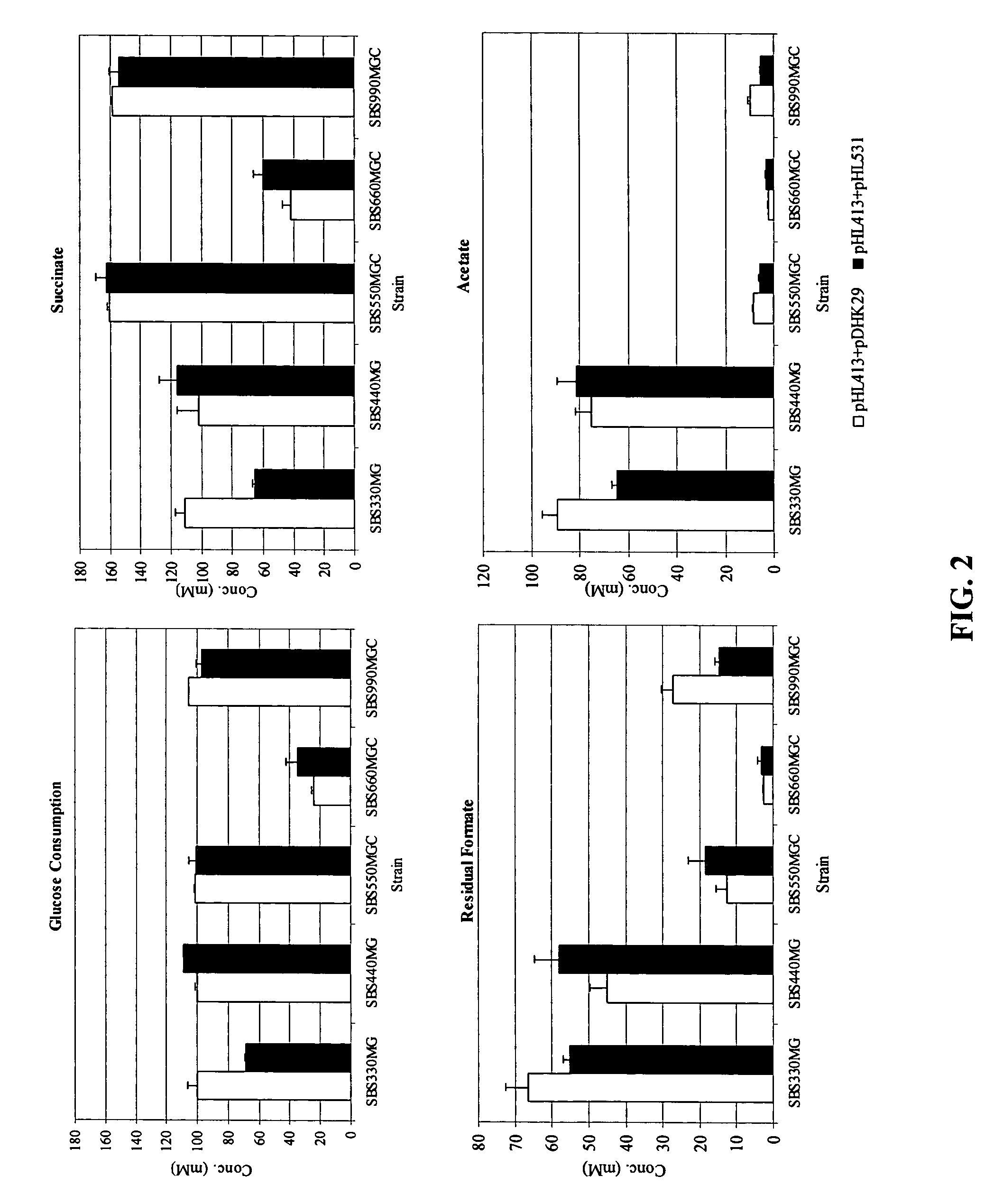
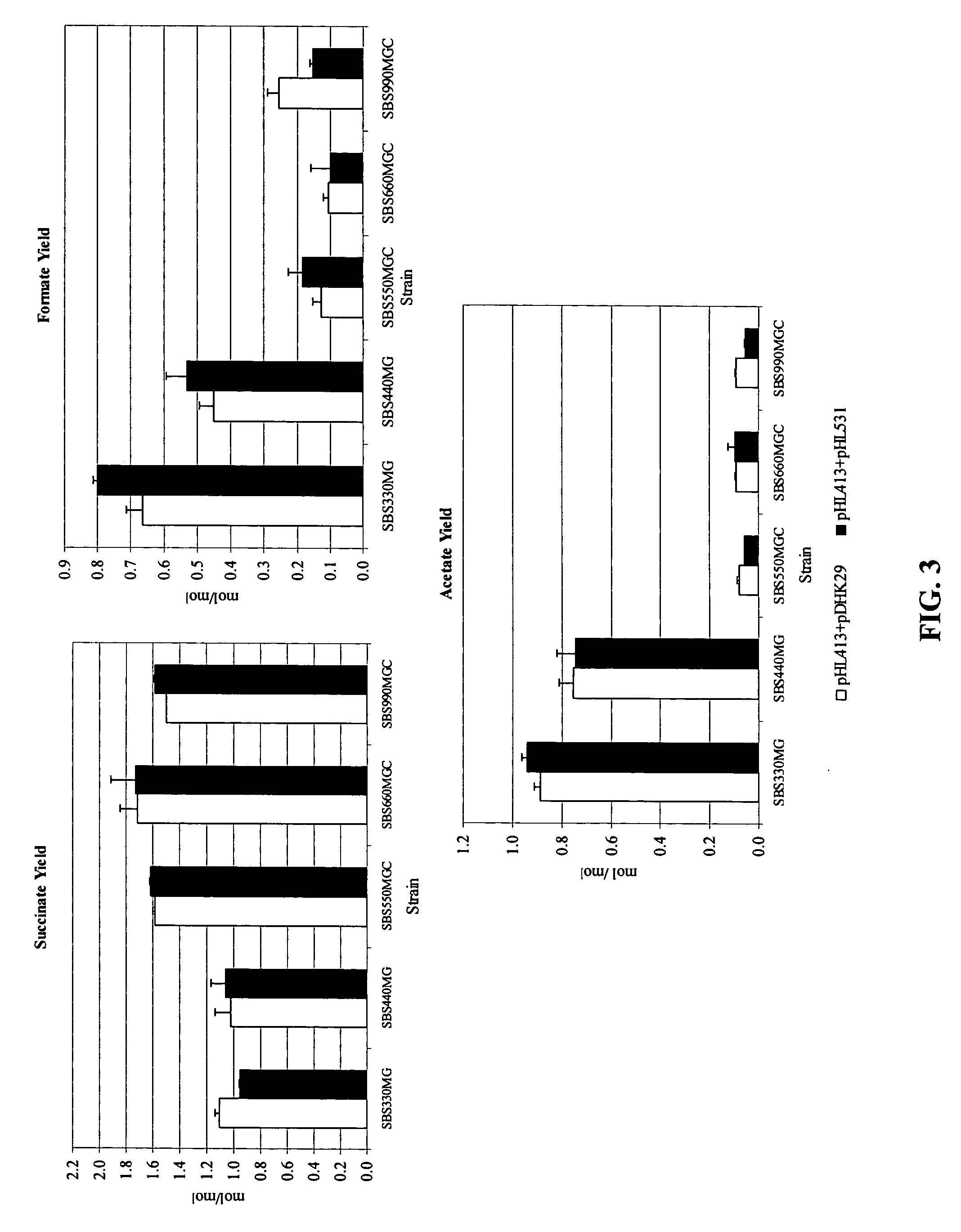
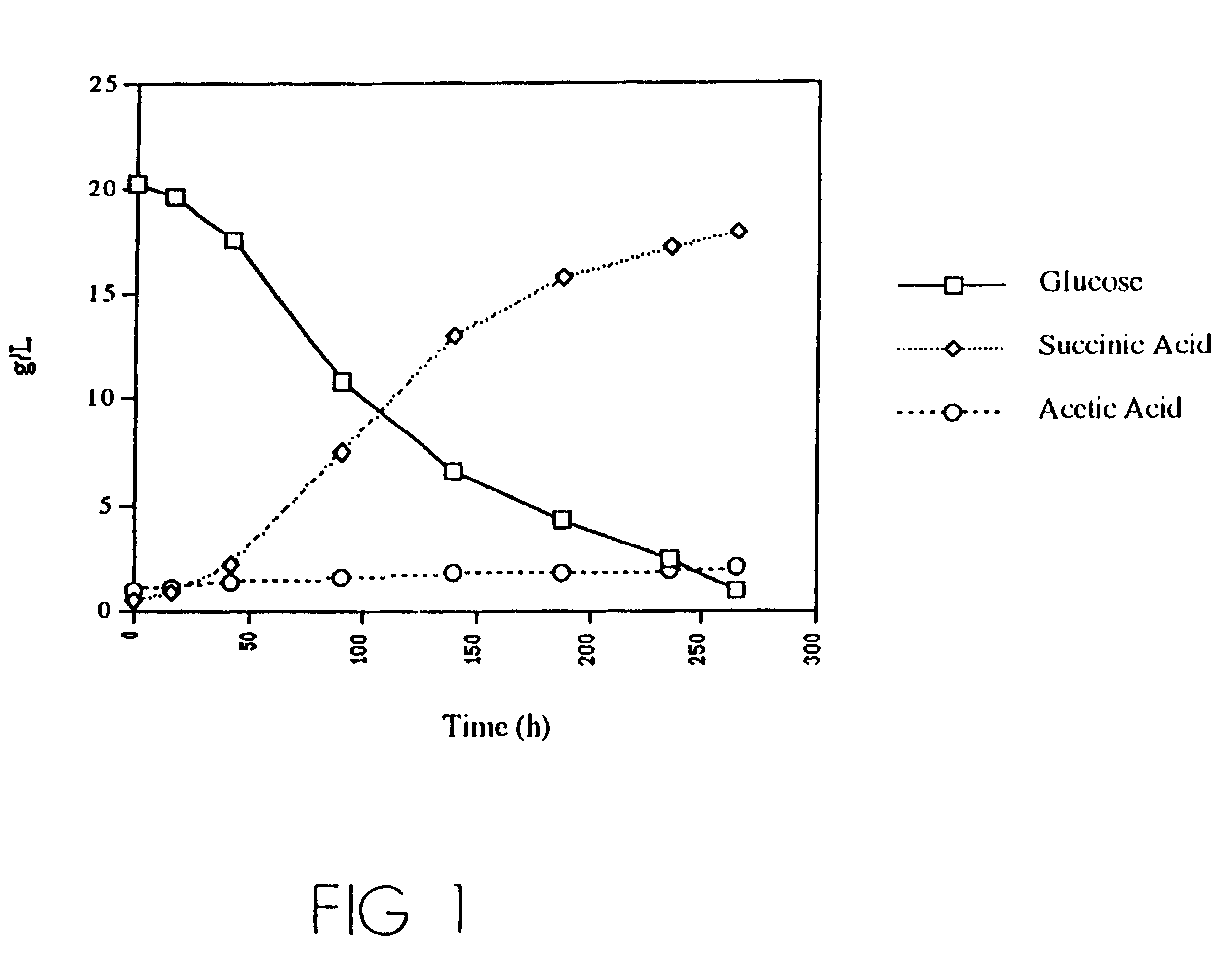
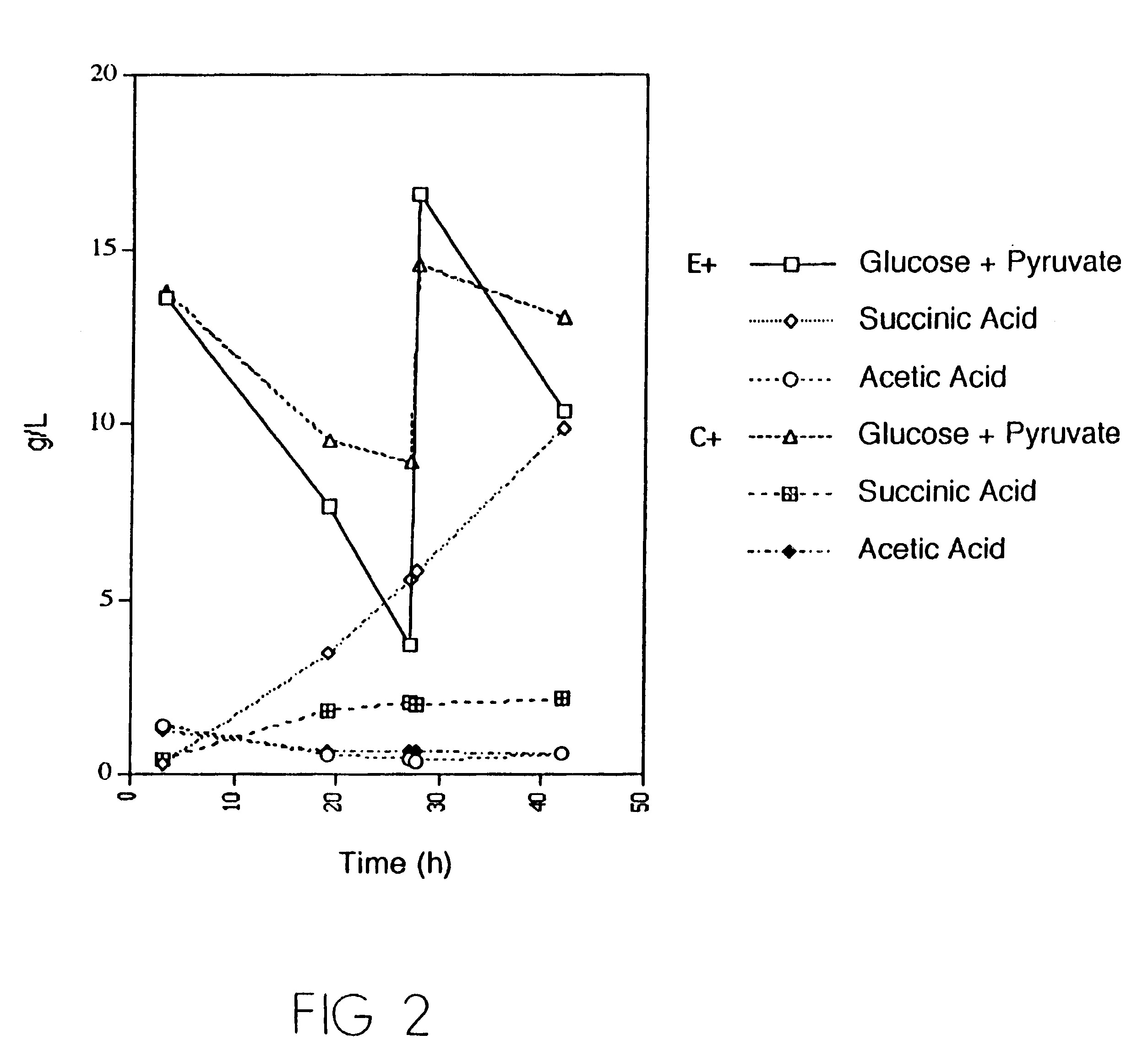
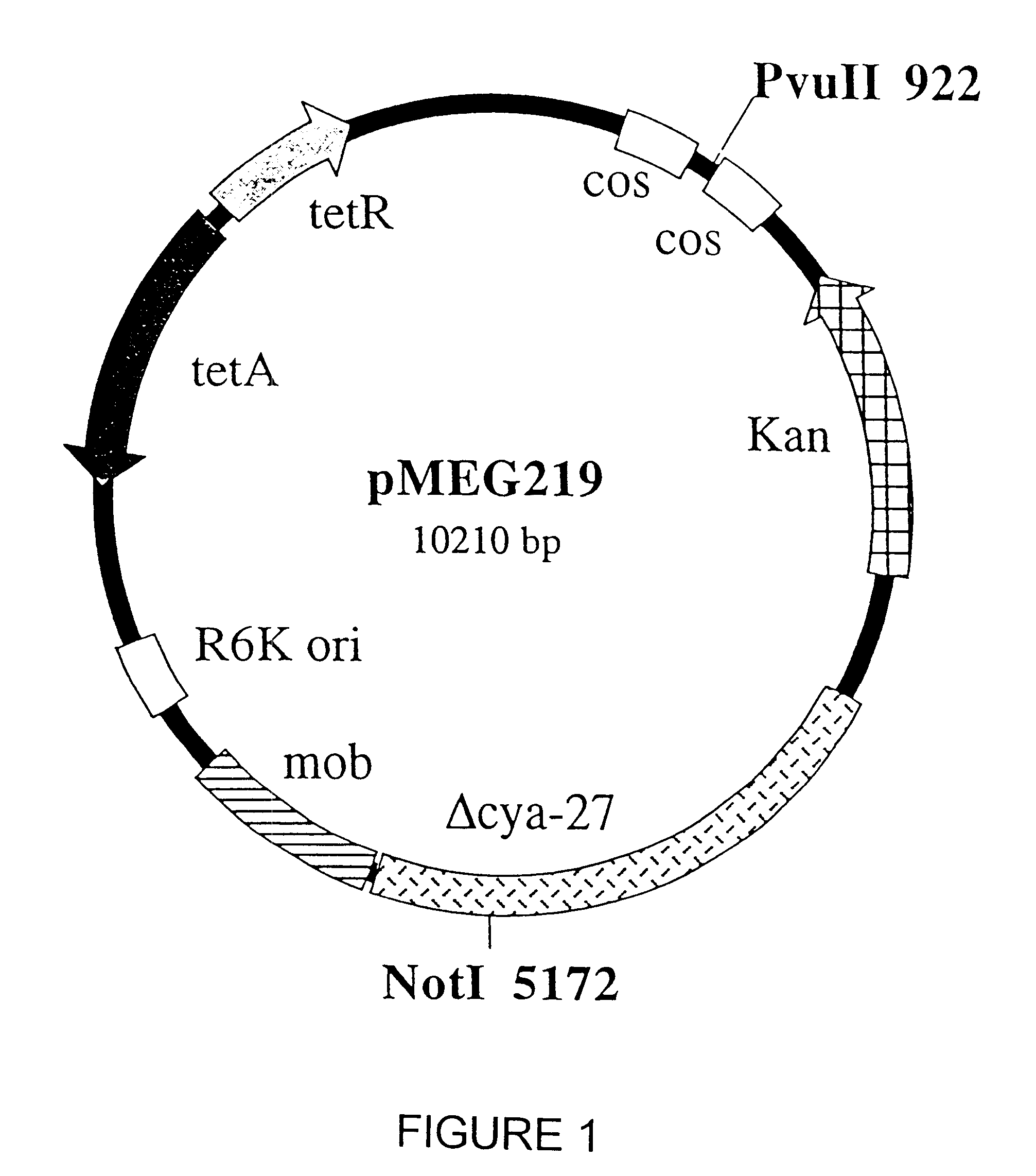
Mutant E. coli strain with increased succinic acid production
The invention relates to a mutant strain of bacteria, which either lacks or contains mutant genes for several key metabolic enzymes, and which produces high amounts of succinic acid under anaerobic conditions.
Owner:RICE UNIV
Mutant E. coli strain with increased succinic acid production
InactiveUSRE37393E1Cheap productionIncrease biomassBacteriaUnicellular algaeLactate dehydrogenaseBiological body
A method for isolating succinic acid producing bacteria is provided comprising increasing the biomass of an organism which lacks the ability to catabolize pyruvate, and then subjecting the biomass to glucose-rich medium in an anaerobic environment to enable pyruvate-catabolizing mutants to grow.The invention also provides for a mutant that produces high amounts of succinic acid, which has been derived from a parent which lacked the genes for pyruvate formate lyase and lactate dehydrogenase, and which belongs to the E.coli Group of Bacteria.
Owner:UNIVERSITY OF CHICAGO
Live attenuated salmonella vaccines to control avian pathogens
A vaccine for protecting birds against infection by avian pathogenic gram negative microbes is disclosed. The vaccine is a recombinant Salmonella strain expressing O-antigen of an avian pathogenic gram negative microbe such as an E. coli strain that is pathogenic in poultry. The recombinant Salmonella strain also does not express Salmonella O-antigen. Methods of using the vaccine to immunize birds are also disclosed.
Owner:AVANT IMMUNOTHERAPEUTICS
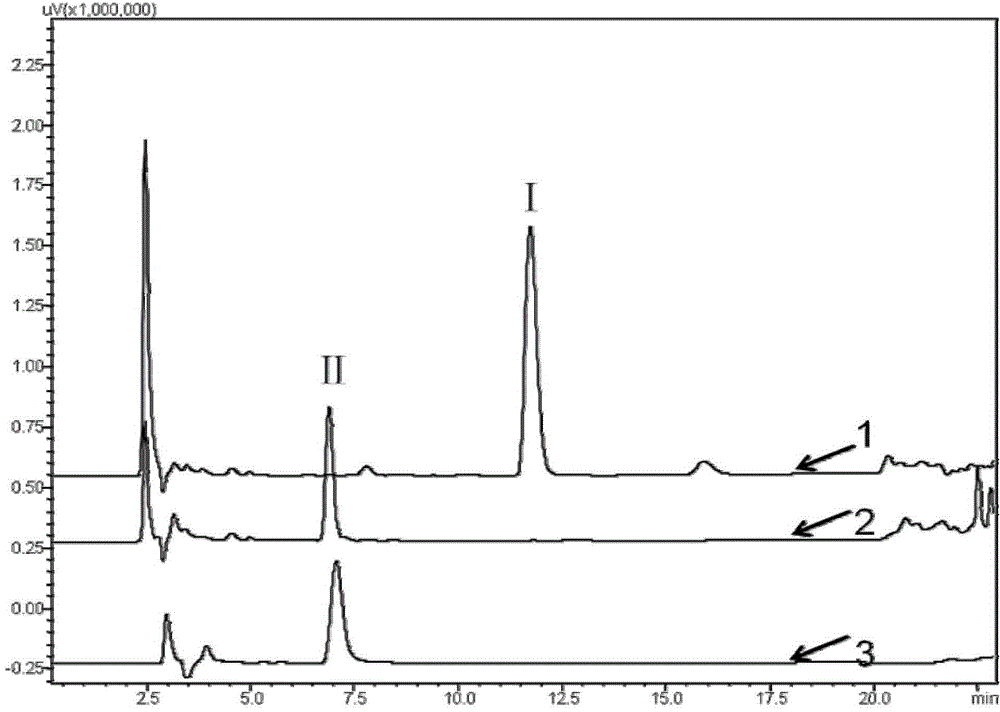
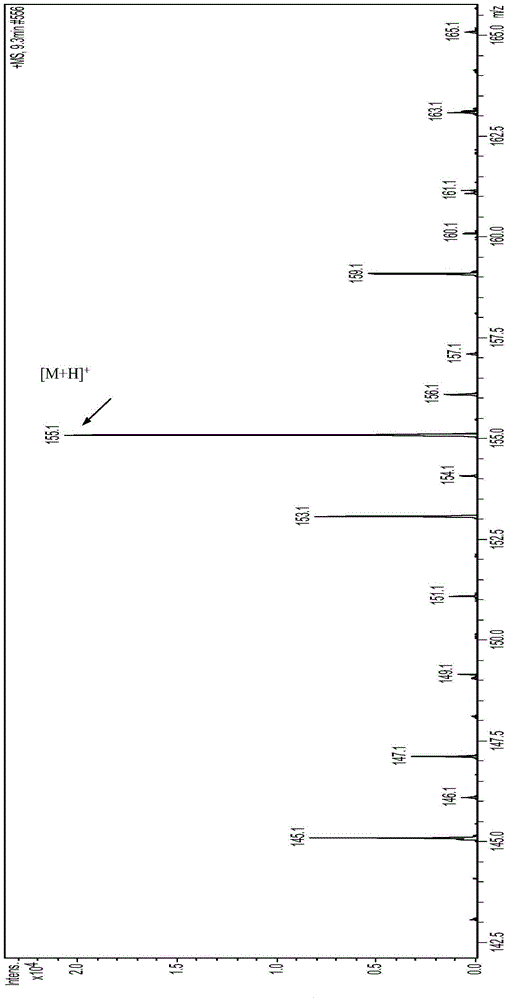
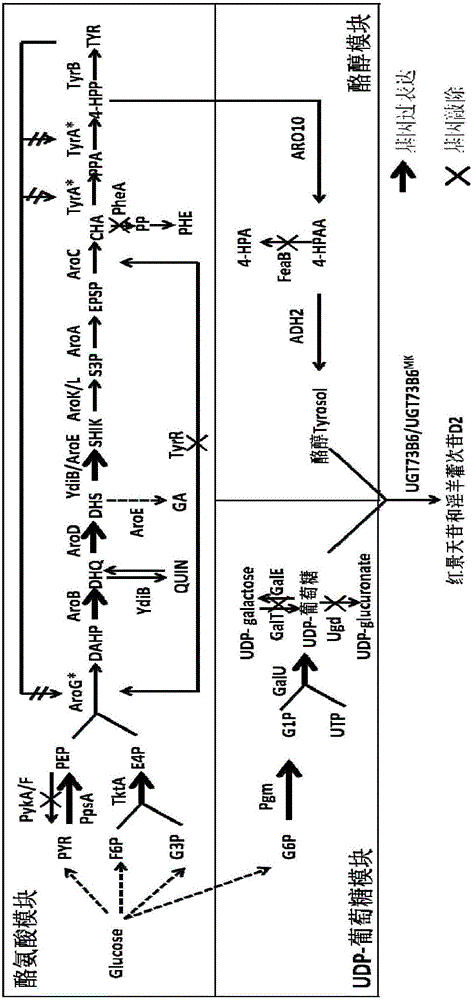
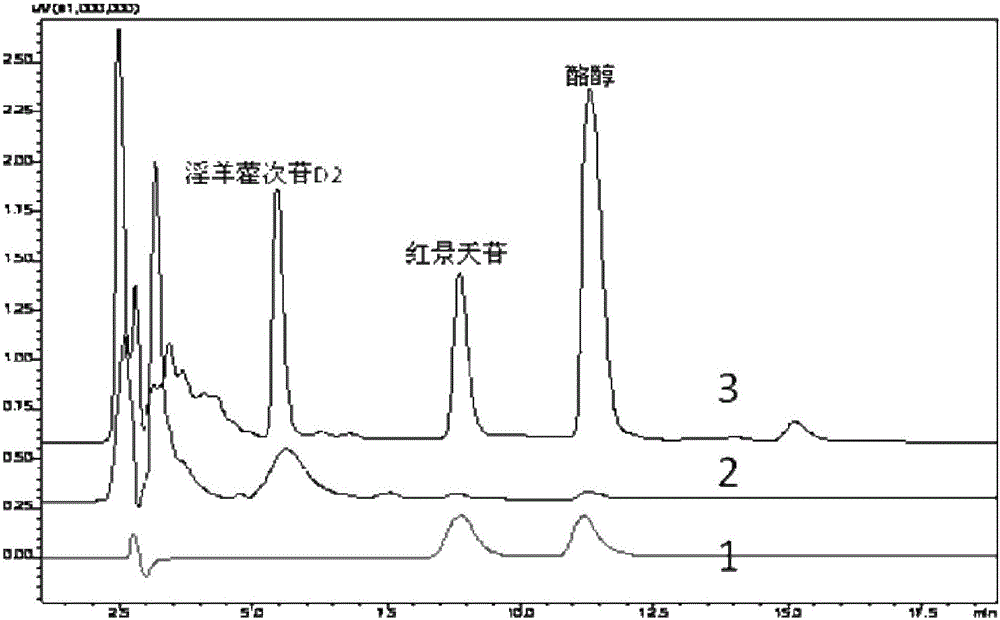
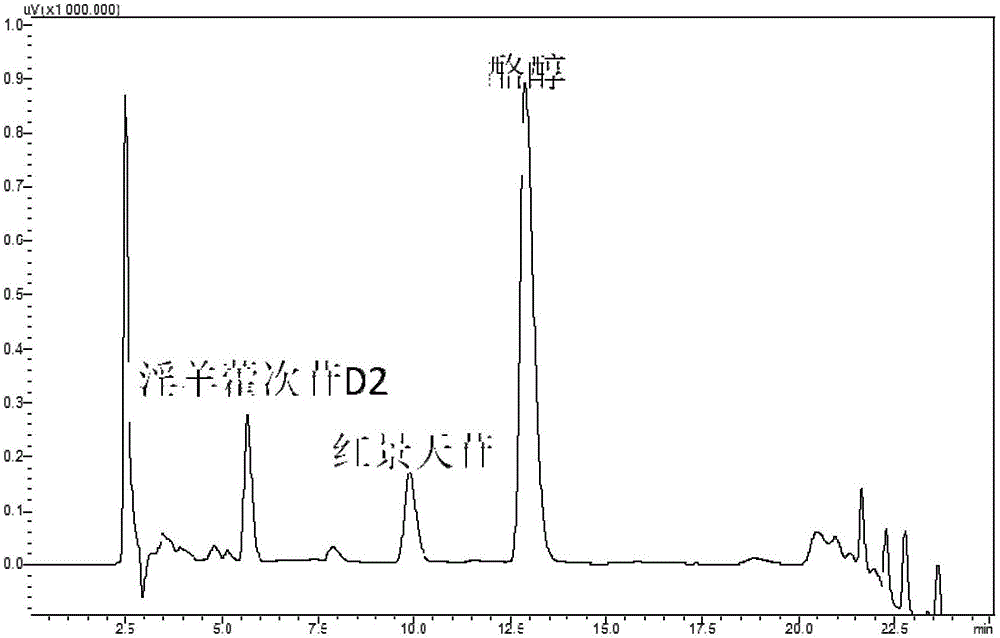
Recombinant escherichia coli using glucose for producing hydroxytyrosol as well as recombination method and application
ActiveCN104805110AIncrease productionBacteriaMicroorganism based processesHydroxytyrosolEscherichia coli
The invention discloses recombinant escherichia coli using glucose for producing hydroxytyrosol as well as a building method and application. The method comprises the following steps that SEQ ID NO.1 and 2 are used as primers; the escherichia coli BW25113 genome DNA (deoxyribonucleic acid) is used as a template; HpaBC gene PCR (polymerase chain reaction) augmentation is carried out; vectors pTrcHisB are connected; middle plasmids are obtained; the middle plasmids are used as templates; the SEQ ID NO.3 and 2 are used as primers; PCR augmentation is carried out, and plasmids pTrcHisB-ARO10 are connected; expression vectors pTrcHisB-ARO10-HpaBC are obtained; in addition, the vectors and the plasmids pBb3 are simultaneously transferred into escherichia coli strains BMGA to obtain the recombinant escherichia coli. The recombinant escherichia coli contains ARO10 genes from saccharomyces cerevisiae, and HpaBC genes from the escherichia coli endogenesis. The yield of the hydroxytyrosol can be obviously improved.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
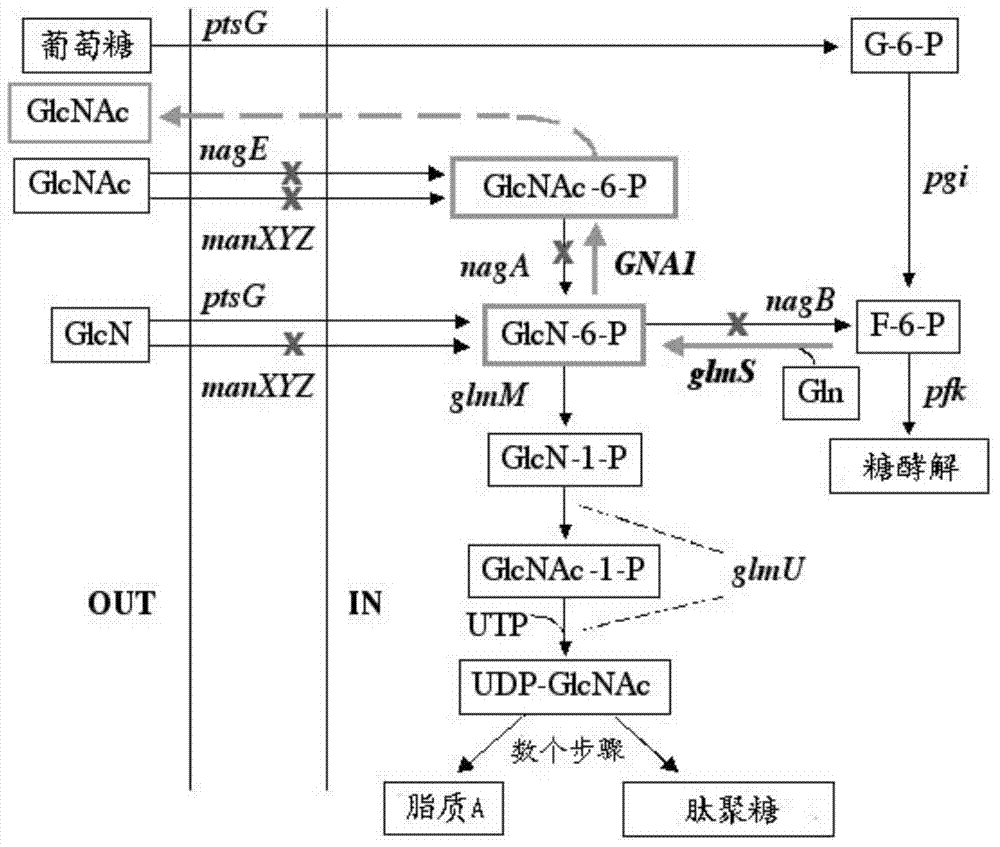
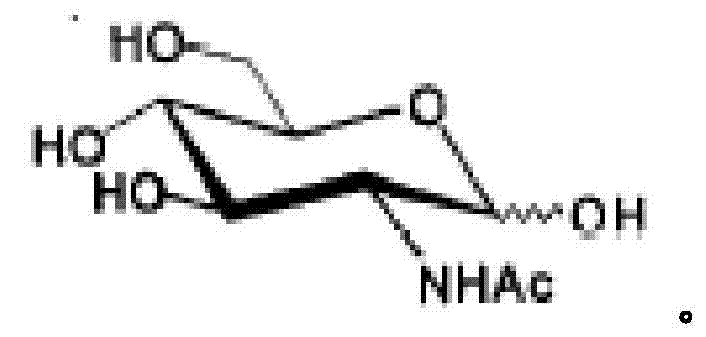
Genetically engineered bacteria for efficiently producing N-acetylglucosamine
InactiveCN104293724AImprove fermentation yieldImprove stabilityBacteriaHydrolasesEscherichia coliBiotechnology
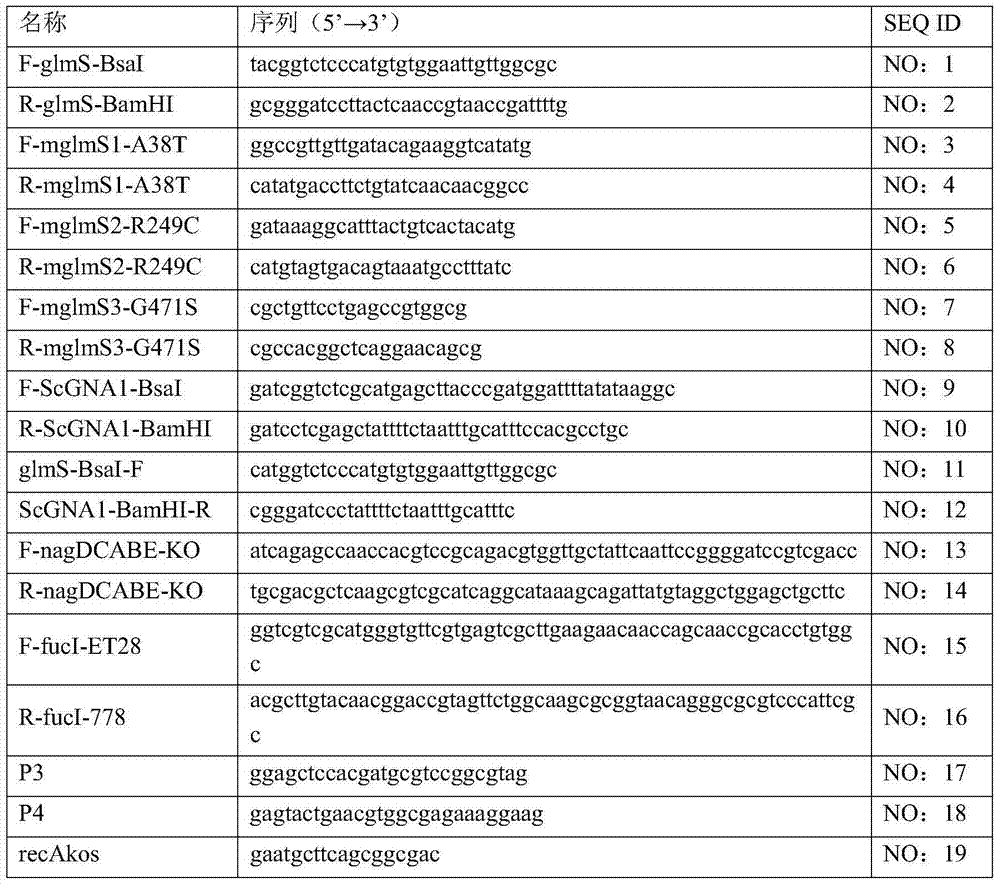
The invention provides genetically engineered bacteria for efficiently producing N-acetylglucosamine. The genetically engineered bacteria are prepared by the following steps: integrating chromosome deficiency nag DCABE gene clusters of Escherichia coli, respectively connecting 6-glucosamine phosphate synthetase mutant genes and N-acetylglucosamine transferase genes which are respectively mediated by a T7 promoter and a Trc promoter with a gene expression cassette in series, wherein the 6-glucosamine phosphate synthetase mutant genes are obtained by mutating wild 6-glucosamine phosphate synthetase genes from an Escherichia coli W3110 strain source into A38T / R249C / G471S mutants. The genetically engineered bacteria constructed by the invention have the advantages of high N-acetylglucosamine fermentation yield and high strain stability and have wide industrial application prospects.
Owner:SHANGHAI RES & DEV CENT OF INDAL BIOTECH +1
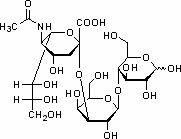
Preparation method of 3'-sialic acid lactose
InactiveCN102154163AChange the status quo of less ingredientsEfficient additionBacteriaMicroorganism based processesEscherichia coliSialic acid
The invention provides a preparation method of 3'-sialic acid lactose, belonging to the technical field of biological engineering. The preparation method comprises the following steps of: taking colibacillus CCTCC (colibacillus China center for type culture collection) NO: M208088 as a starting strain, adding a fermentation medium into a fermentation tank, fermenting for 2-6h, then adding a fed batch culture medium, and adding lactose in a flowing way at the mid-later period of the fermentation; and centrifuging the fermentation liquid to obtain liquid supernatant 1 and a thallus, diluting the centrifuged thallus by adding water and splitting in a heating way, recentrifuging to obtain liquid supernatant 2, merging the liquid supernatant 1 and the liquid supernatant 2, and absorbing, desorbing and vacuum drying by ion exchange resin to obtain the 3'-sialic acid lactose. A mass of the 3'-sialic acid lactose can be prepared by the CCTCC NO: M208088. The 3'-sialic acid lactose can change the current situation that the sialyloligosaccharide component is less in exogenous adding substances in conventional baby milk, and can provide the guarantee to add more efficient sialic acid trophic factors into the baby milk.
Owner:朱莉
TaqMan(TM)-PCR for the detection of pathogenic E. coli strains
InactiveUS6664080B1Addressing slow performanceIncrease valueSugar derivativesMicrobiological testing/measurementPathogenicityOligonucleotide Primer
The present invention relates to a method for the detection of pathogenic E. coli in a sample comprising PCR amplification of DNA isolated from said sample using oligonucleotide primers specific for pathogenic E. coli.
Owner:BAVARIAN NORDIC RES INST AS
Recombinant Escherichia coli producing salidroside, construction method and applications thereof
ActiveCN107435049AMetabolic pathway optimizationIncrease productionBacteriaTransferasesEscherichia coliSalidroside
The invention discloses recombinant Escherichia coli producing salidroside, a construction method and applications thereof. The construction method comprises: (1) using Escherichia coli [delta]A as a starting strain, and carrying out gene knockdown or gene silencing to make the galE gene, the galT gene and the ugd gene on the Escherichia coli chromosome be not expressed to obtain an Escherichia coli strain BMGU; and (2) introducing genes such as ARO10, UGT73B6MK, pgm, galU and T7Polymerase to over-express the genes such as ARO10, UGT73B6MK, pgm, galU and T7Polymerase so as to achieve the heterologous synthesis of salidroside, such that the recombinant Escherichia coli producing salidroside is obtained. According to the present invention, the efficient UDP-glucosyltransferase mutant UGT73B6MK is introduced, and the glucose metabolism pathway is optimized, such that the yield of salidroside is significantly improved.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Preparation method and drug carrying method of escherichia coli outer membrane vesicle, and application of outer membrane vesicle in anti-tumor
InactiveCN107142228AHigh yieldHigh feasibilityBacteriaMicroorganism based processesUltrafiltrationPharmaceutical Substances
The invention discloses a preparation method and a drug carrying method of an escherichia coli outer membrane vesicle, and application of the outer membrane vesicle in anti-tumor. The preparation method of the escherichia coli outer membrane vesicle comprises the following steps of culturing a culture medium using LB in vitro, culturing an escherichia coli BL21 strain, performing centrifugation, filtration and ultrafiltration treatment to obtain an upper layer culture solution free of escherichia coli, and finally centrifuging the upper layer culture solution by a supercentrifuge to prepare the escherichia coli outer membrane vesicle. The particle size of the prepared escherichia coli outer membrane vesicle is uniform, and is about 50nm. Usually, a bacterium outer membrane vesicle is low in yield and difficult in drug carrying. The invention excogitates a novel drug carrying method for the bacterium outer membrane vesicle, so that the carrying efficiency of an anti-tumor drug can be significantly improved; an inhibition effect on multiplication and invasion of a tumor cell is increased; and a good application prospect of the bacterium outer membrane vesicle serving as a novel non-virus drug carrier is presented.
Owner:ZHEJIANG UNIV
Live attenuated i(salmonella) vaccines to control avian pathogens
A vaccine for protecting birds against infection by avian pathogenic gram negative microbes is disclosed. The vaccine is a recombinant Salmonella strain expressing O-antigen of an avian pathogenic gram negative microbe such as an E. coli strain that is pathogenic in poultry. The recombinant Salmonella strain also does not express Salmonella O-antigen. Methods of using the vaccine to immunize birds are also disclosed.
Owner:MEGAN HEALTH
Halogenohydrin dehalogenation enzyme and encoding gene and vector and bacterial strain and application
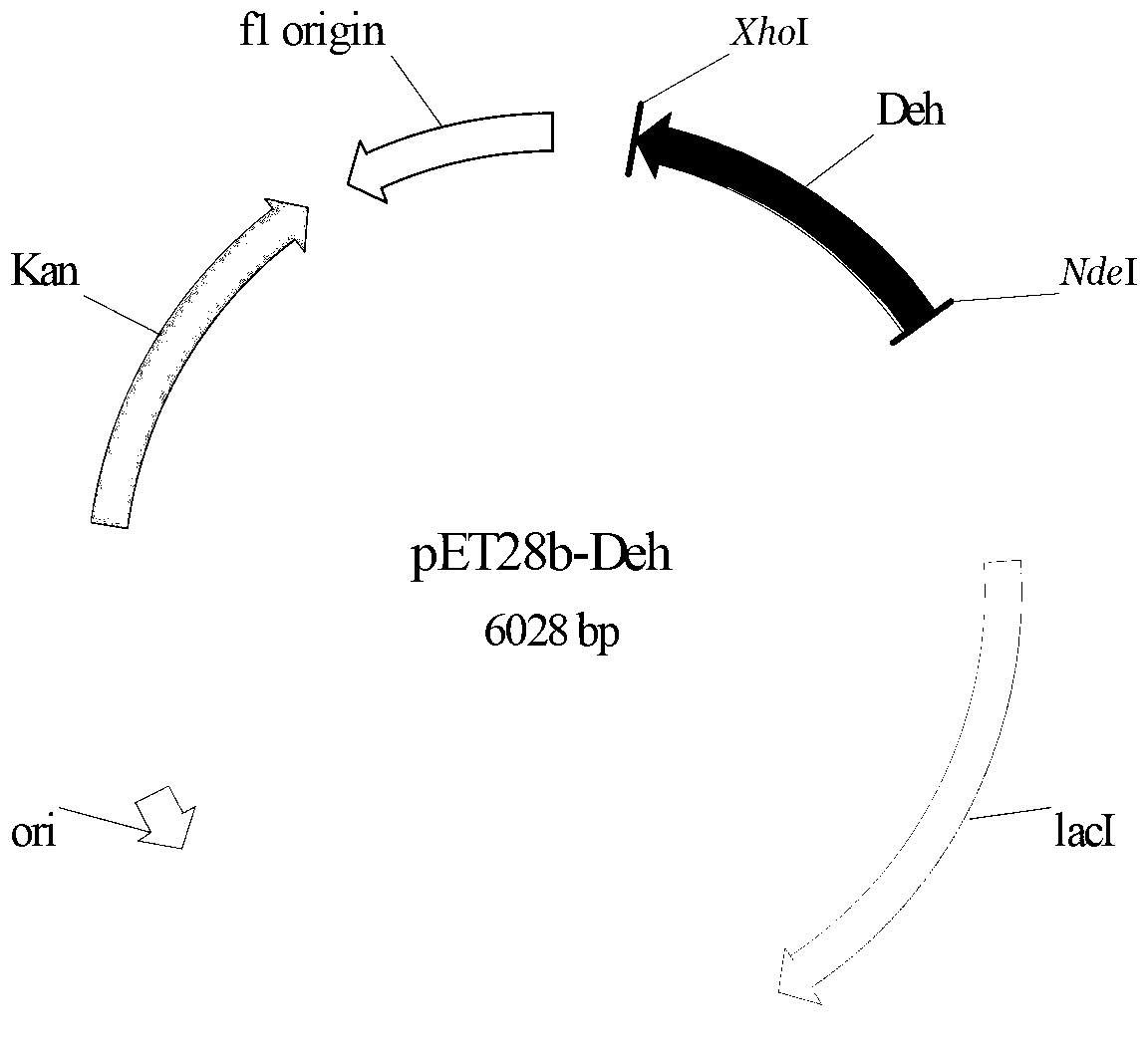
The invention provides a halogenohydrin dehalogenation enzyme originating from Agromyces sp., an encoding gene and a vector, and provides application of the halogenohydrin dehalogenation enzyme, the encoding gene and the vector in the process of preparing epoxy chloropropane and (R)-4- cyano-cn-3- hydroxybutyric acid ethyl ester. The halogenohydrin dehalogenation enzyme amino acid sequence is shown in SEQ ID NO.2, and the encoding gene sequence is shown in SEQ ID NO.1. The halogenohydrin dehalogenation enzyme, the encoding gene, the vector and the application have the advantages that the halogenohydrin dehalogenation enzyme originating from Agromyces sp. CCTCC NO. M 2012299 and the encoding gene of the halogenohydrin dehalogenation enzyme are provided; and the encoding gene of the halogenohydrin dehalogenation enzyme can be connected and constructed with an expression vector to obtain expression recombinant plasmid pET28b-Deh of the encoding gene, then can be transformed to escherichia coli BL21, obtained and transformed to escherichia coli bacterial strain respectively and correspondingly to obtain recombinant escherichia coli, and the recombinant escherichia coli has the halogenohydrin dehalogenation enzyme and can be utilized for carrying out biotransformation and catalysis for an enzyme source.
Owner:ZHEJIANG UNIV OF TECH
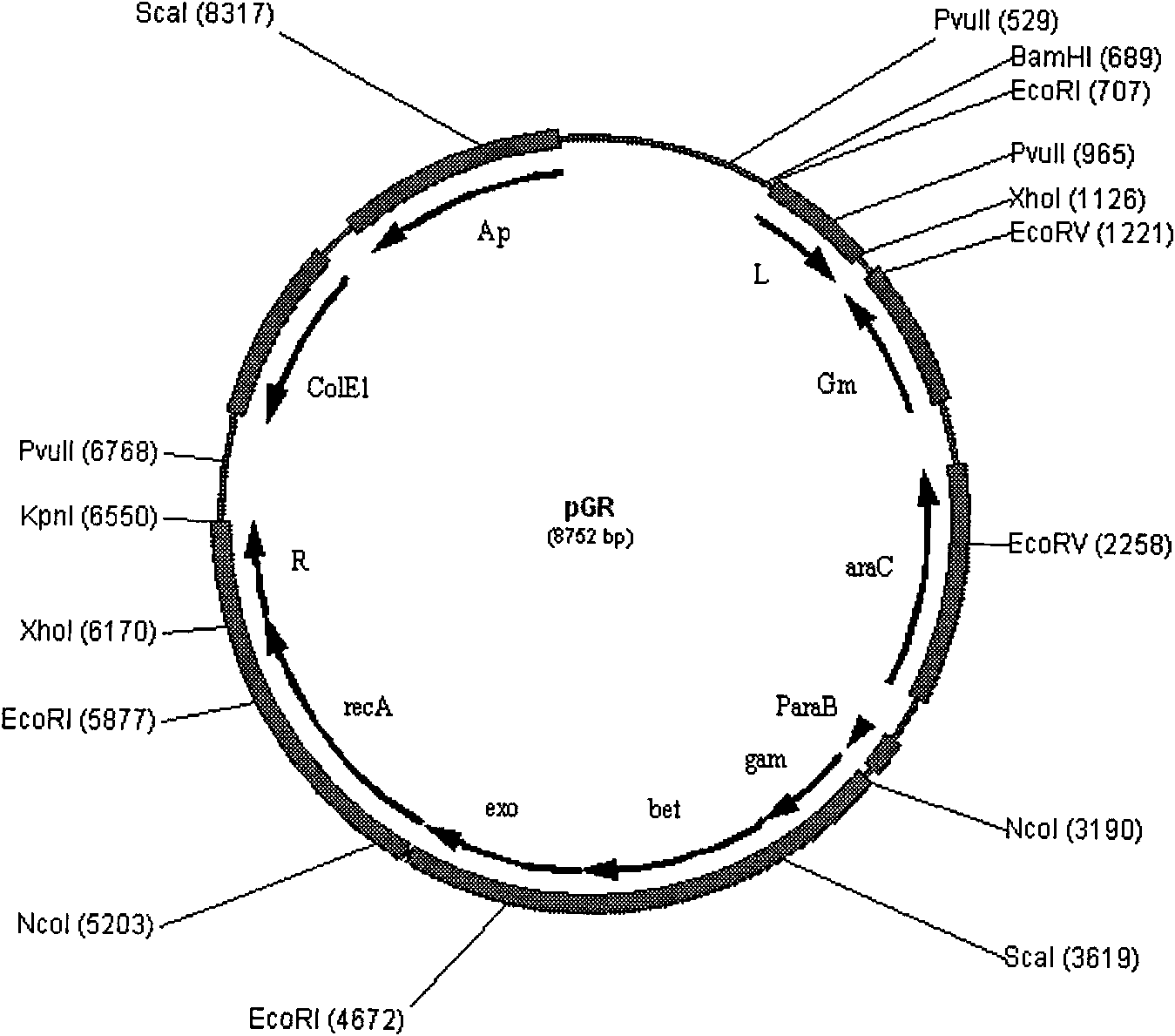
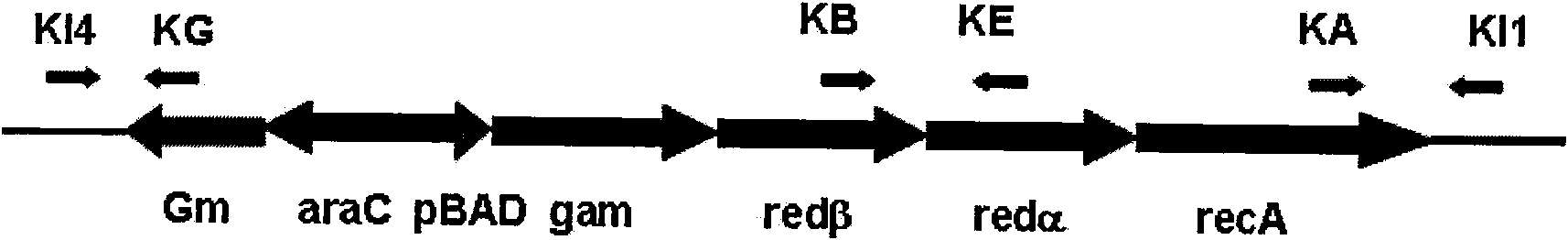
Escherichia coli strain for recombined engineering
InactiveCN101633901AWill not harmNo distractionBacteriaMicroorganism based processesEscherichia coliEnzyme Gene
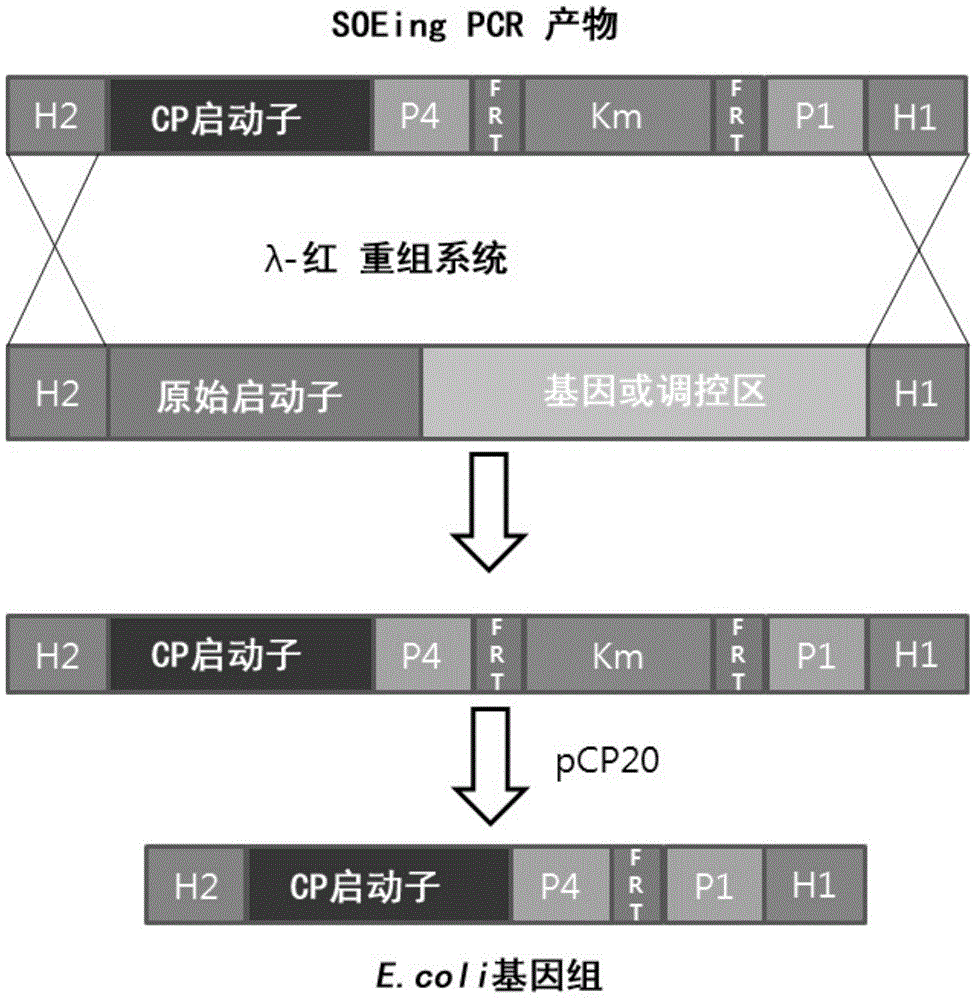
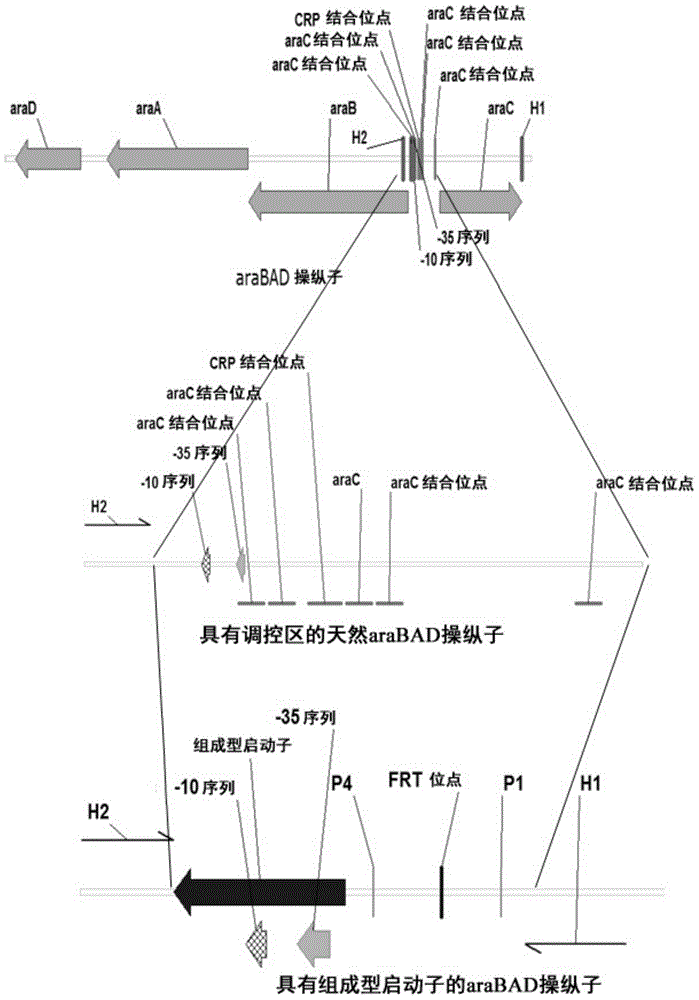
The invention relates to escherichia coli CGMCC NO.3192 for recombined engineering and a variant thereof. The strain is obtained by integrating gene segments subjected to the recombined engineering, i.e. a regulatory gene araC of the Arabinose ara operon from the escherichia coli, a promoter pBAD of the Arabinose ara operon from the escherichia coli, recombined enzyme genes red alpha, red alpha beta and gam from a gamma bacteriophage, a gene recA and gentamicin resistance gene (Gm) from the escherichia coli, into an endA gene area of a gene group of the escherichia coli DH10B, wherein red alpha, red alpha beta, gam and recA are driven by pBAD induced by arabinose. The recombined enzyme can catalyze homologous recombination among DNA short segments to finish the recombined engineering. The length of the DNA segment is about 50 base pairs. The invention has convenient operation of carrying out the recombined engineering by the strain and high recombined efficiency, thereby becoming the universal strain for the recombined engineering.
Owner:NANJING NORMAL UNIVERSITY
Construction method of genetic engineering strain for producing shikimic acid
InactiveCN102304539AReduce the content of intermediate productsIncrease productionMicroorganism based processesVector-based foreign material introductionEnzyme GeneTryptophan
The invention discloses a construction method of a genetic engineering strain for producing shikimic acid. The method comprises the following steps of: 1, constructing an escherichia coli strain of which the aroA gene is knocked out; 2, constructing a recombinant expression plasmid pAR63 containing key enzyme genes aroGFBR, aroE, aroB, aroD, tktA and ppsA in the metabolic pathway of the shikimic acid, so that the genes are subjected to transcriptional control of a tryptophan promoter Ptrp; and 3, transferring the recombinant expression plasmid pAR63 into the escherichia coli strain of which the aroA gene is knocked out to obtain a production strain for expressing the shikimic acid. In the method, the aim of interrupting the metabolism of the shikimic acid is fulfilled by the knock-out of the single gene aroA, a small number of genes are knocked out, the operating difficulty is low, and the expression of the genes is in the state of starting and stopping automatically and is started automatically in the middle and late period without the induction of inducers, so the possibility of adding toxic substances into a culture medium is reduced, and the shikimic acid has high yield.
Owner:河南孟成生物药业股份有限公司
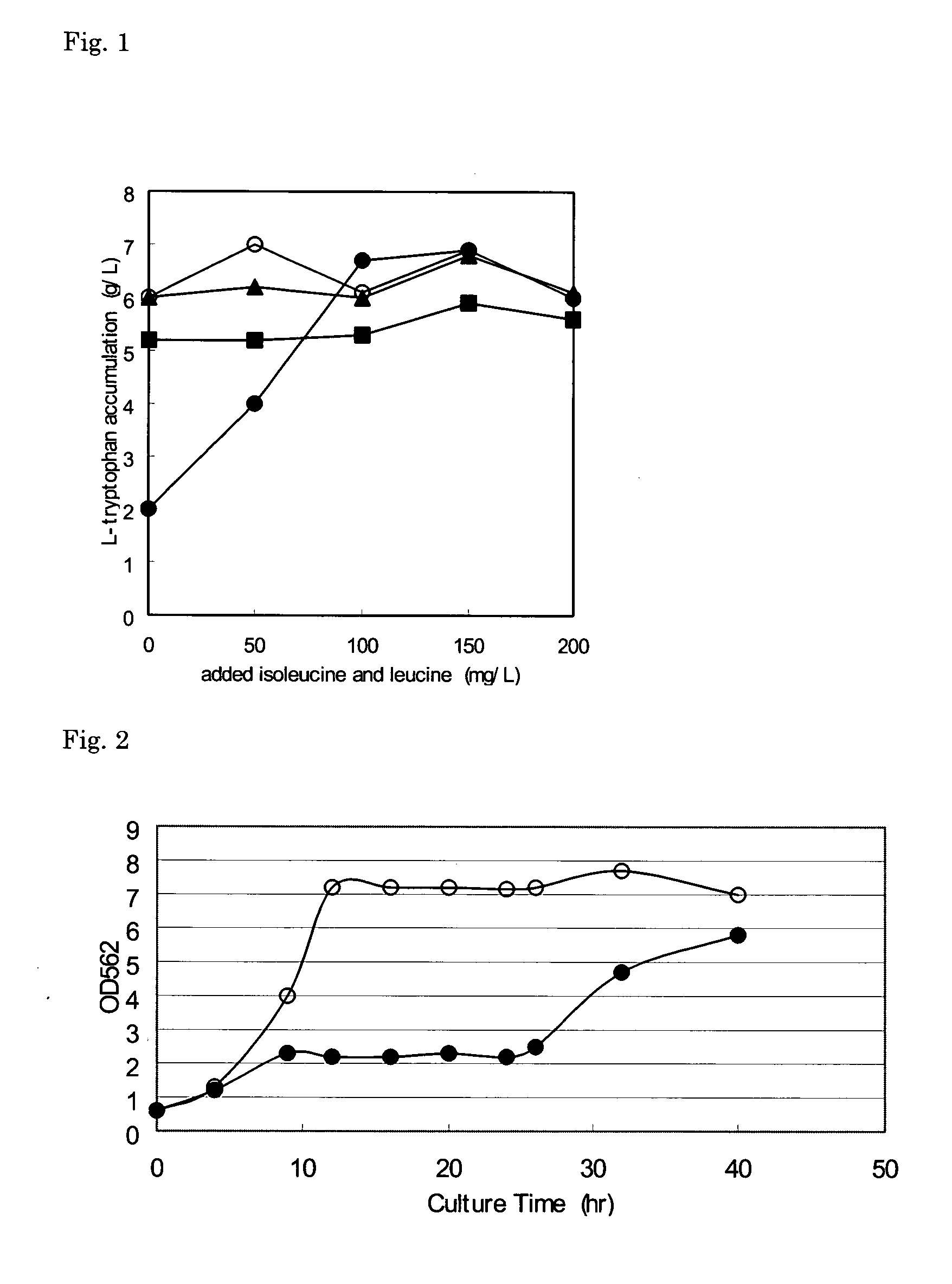
L-amino acid-producing bacterium and a method for producing L-amino acid
ActiveUS20050260720A1Efficient productionImprove abilitiesSugar derivativesBacteriaCysteine thiolateTyrosine
An L-amino acid-producing strain of Escherichia coli is bred by modifying an Escherichia coli K12 strain or a derivative thereof so as to become resistant to L-valine and have an ability to produce one or more L-amino acids selected from the group consisting of L-tryptophan, L-phenylalanine, L-lysine, L-tyrosine, L-glutamic acid, L-histidine, L-cysteine, and L-proline.
Owner:AJINOMOTO CO INC
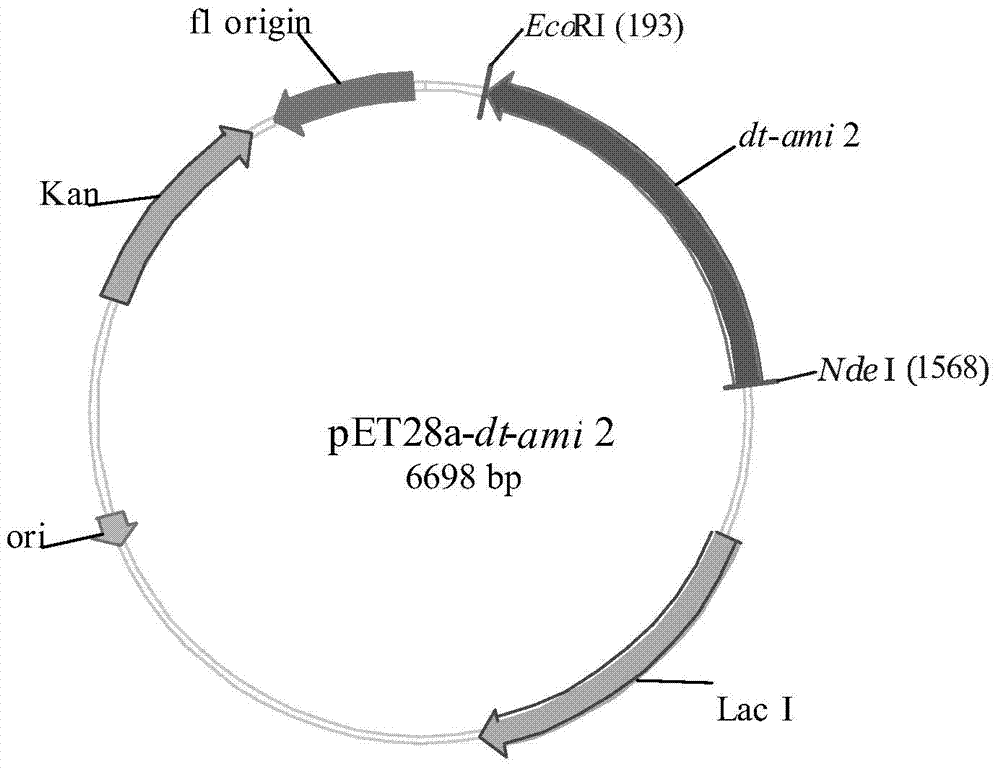
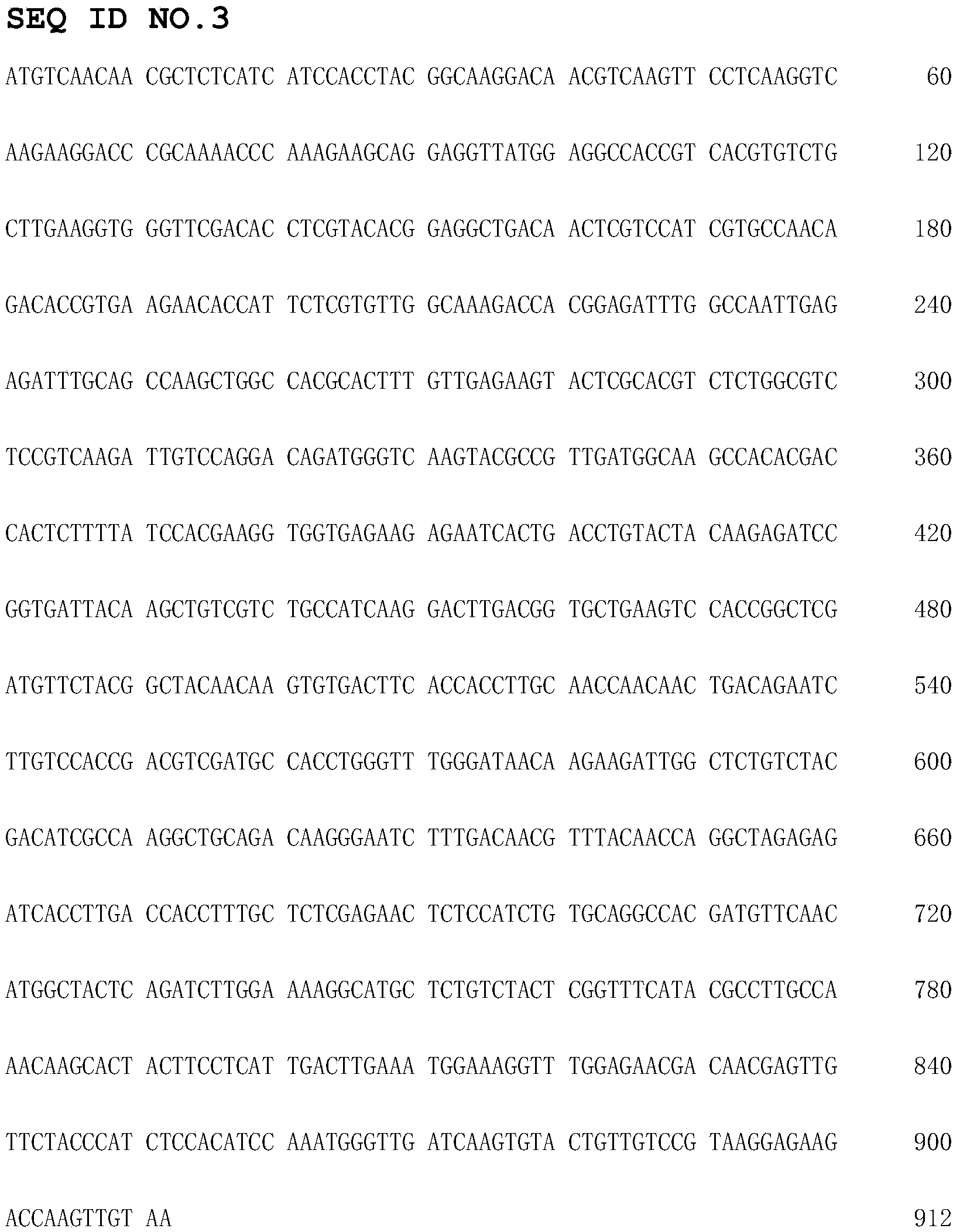
Recombinant amidase Dt-Ami 2, encoding gene, vector, engineering strain and applications of recombinant amidase Dt-Ami 2 and engineering strain
The invention discloses a recombinant amidase Dt-Ami 2, an encoding gene, a vector, an engineering strain and applications of the recombinant amidase Dt-Ami 2 and the engineering strain. The invention provides a Delftia tsuruhatensis (CCTCC No. M 205115)-derived amidase gene, and a recombinant amidase WB encoded by the gene; and the amidase gene can be connected with an expression vector so as to obtain an intracellular expression recombinant plasmid containing the gene, then the intracellular expression recombinant plasmid is transferred to an escherichia coli strain so as to obtain recombinant escherichia coli, the recombinant escherichia coli contains recombinant amidase, and the recombinant amidase can be used as a catalyst for catalyzing the hydrolysis of a series of amide compounds.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of recombinant uricase
The invention provides a nucleotide sequence suitable for secretory expression of recombinant candida utilis uricase (uricase, EC1.7.3.3) by virtue of escherichia coli, a carrier containing the nucleotide sequence, an engineering cell containing the carrier, and a method for purifying the recombinant candida utilis uricase from the engineering cell. On the basis of a natural sequence of a uricase gene, a codon of the uricase gene is optimized, the transformed uricase gene is cloned to a prokaryotic expression vector pET42a(+); an escherichia coli Rossetta strain is transformed; the expression level can reach 50% of soluble protein of the escherichia coli by IPTG induction; the expressed protein is soluble protein; and the biological activity of the expressed protein can be up to that of 37.89U / mg recombinant protein. The recombinant candida utilis uricase product can be efficiently and simply obtained at low cost. The invention also comprises an application of the recombinant candida utilis uricase prepared by the method in preparation of medicines, foods, health care products and cosmetics for treating hyperuricemia-related diseases or pathological symptoms of a human body.
Owner:吉林金梓源生物科技股份有限公司
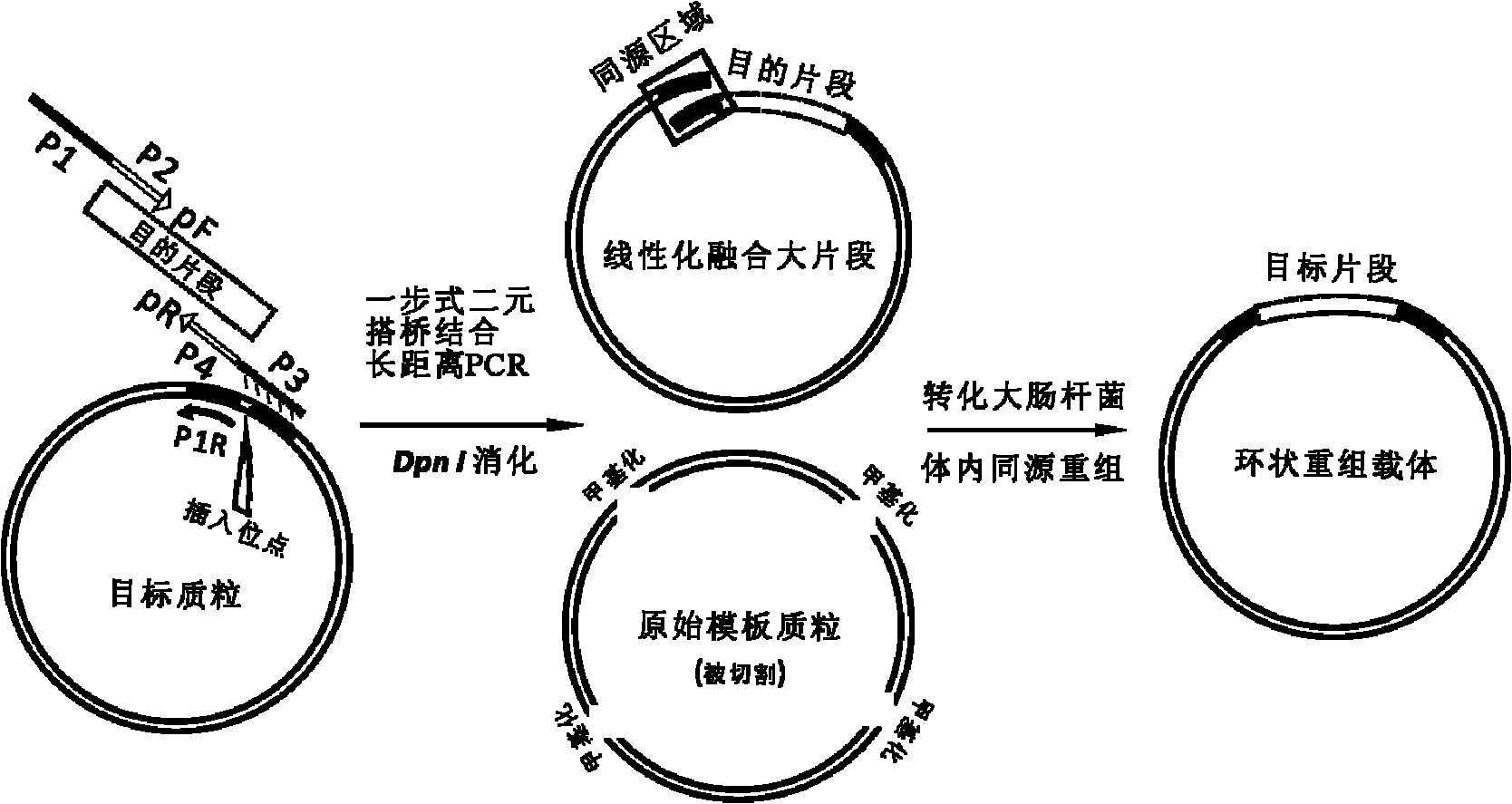
Method for cloning seamless gene
InactiveCN102080075ALow priceThe principle is simpleVector-based foreign material introductionPolymerase chain reactionPlasmid dna
The invention discloses a method for cloning a seamless gene without an artificial basic group, comprising the steps of: (1) designing and synthesizing a chimera primer according to sequences of a target gene and a target vector; (2) carrying out a single-step binary bridging coupling long-distance PCR (Polymerase Chain Reaction) to obtain a fusion linear fragment; (3) digesting PCR products and removing methylated template plasmid DNA (Deoxyribose Nucleic Acid) by utilizing DpnI (Diphosphopyridine nucleotide I); (4) transforming an escherichia coli strain DH5 alpha; (5) selecting monoclonal colonies for sequencing and identifying; and (6) confirming the integration situation of recombinant plasmids by utilizing enzyme digestion. By means of the method, special restriction enzyme and ligase as well as alkaline phosphatase are needless and any target gene can be merged into any position of any target vector. The method has the advantages of simple principle, simpleness and convenience in operation, accurate and controllable locus, high positive rate and wide application range.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Hepatitis E virus-like particle vaccine preparation method and application
ActiveCN104857510AEasy to prepareEasy to zoom inDigestive systemImmunoglobulins against virusesEscherichia coliDisease
The invention discloses a hepatitis E virus-like particle vaccine preparation method, and belongs to the biological medicine technical field. According to the method, hepatitis E virus truncated capsid protein gene codon optimization is performed, prokaryotic expression system escherichia coli strain B834-pRARE2 is used, by fusion of purification tag MBP expression, different cracking agent screening and combination, bacterial membrane extraction, maltose amylose affinity chromatography, molecular sieve chromatography for removal of MBP and non-virus structural protein, and promotion of virus-like particle assembly, the hepatitis E virus-like particle purity is more than 99.0%, and is free of protein label and bioactive (in the form of non-inclusion body, and soluble). The hepatitis E virus-like particle vaccine produces complete protection to pig, dog and chicken HEV infection, cross protective antigens are between different genotype HEV virus, and the hepatitis E virus-like particle vaccine can be used as an animal vaccine to prevent the diseases and infection caused by pig, dog and poultry HEV.
Owner:张澍 +1
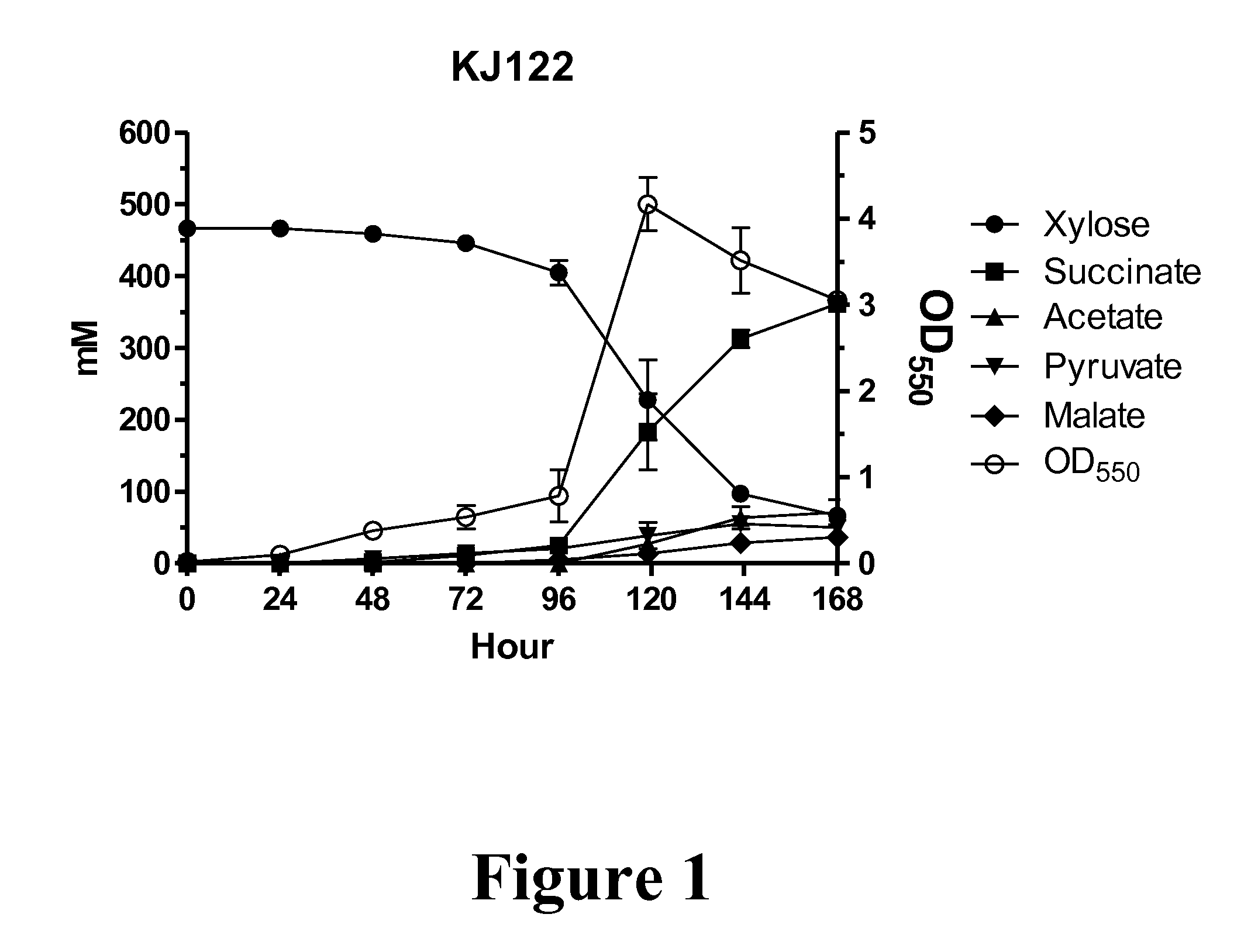
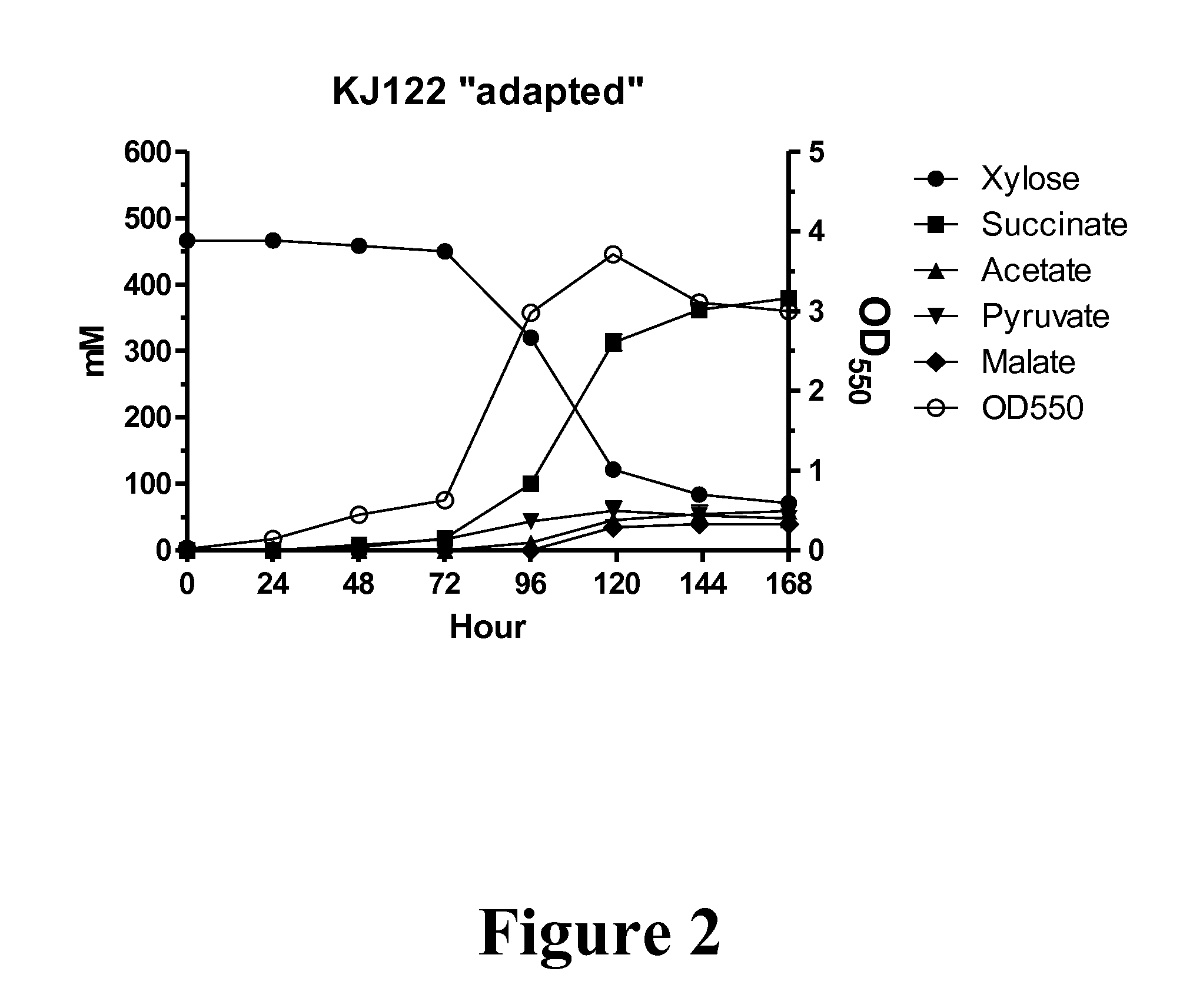
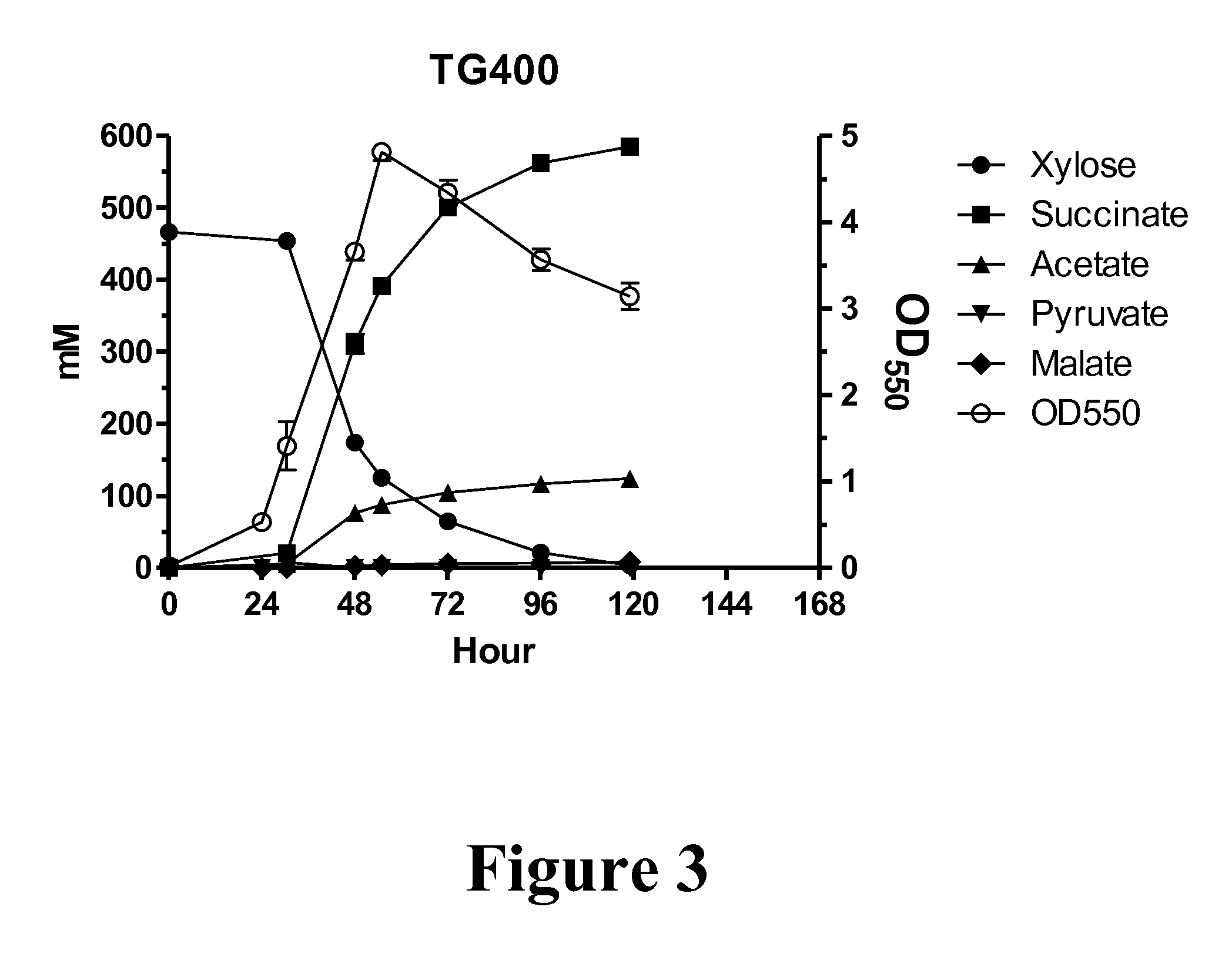
Metabolic evolution of escherichis coli strains that produce organic acids
This invention relates to the metabolic evolution of a microbial organism previously optimized for producing an organic acid in commercially significant quantities under fermentative conditions using a hexose sugar as sole source of carbon in a minimal mineral medium. As a result of this metabolic evolution, the microbial organism acquires the ability to use pentose sugars derived from cellulosic materials for its growth while retaining the original growth kinetics, the rate of organic acid production and the ability to use hexose sugars as a source of carbon. This invention also discloses the genetic change in the microorganism that confers the ability to use both the hexose and pentose sugars simultaneously in the production of commercially significant quantities of organic acids.
Owner:PTT GLOBAL CHEMICAL PUBLIC COMPANY LIMITED
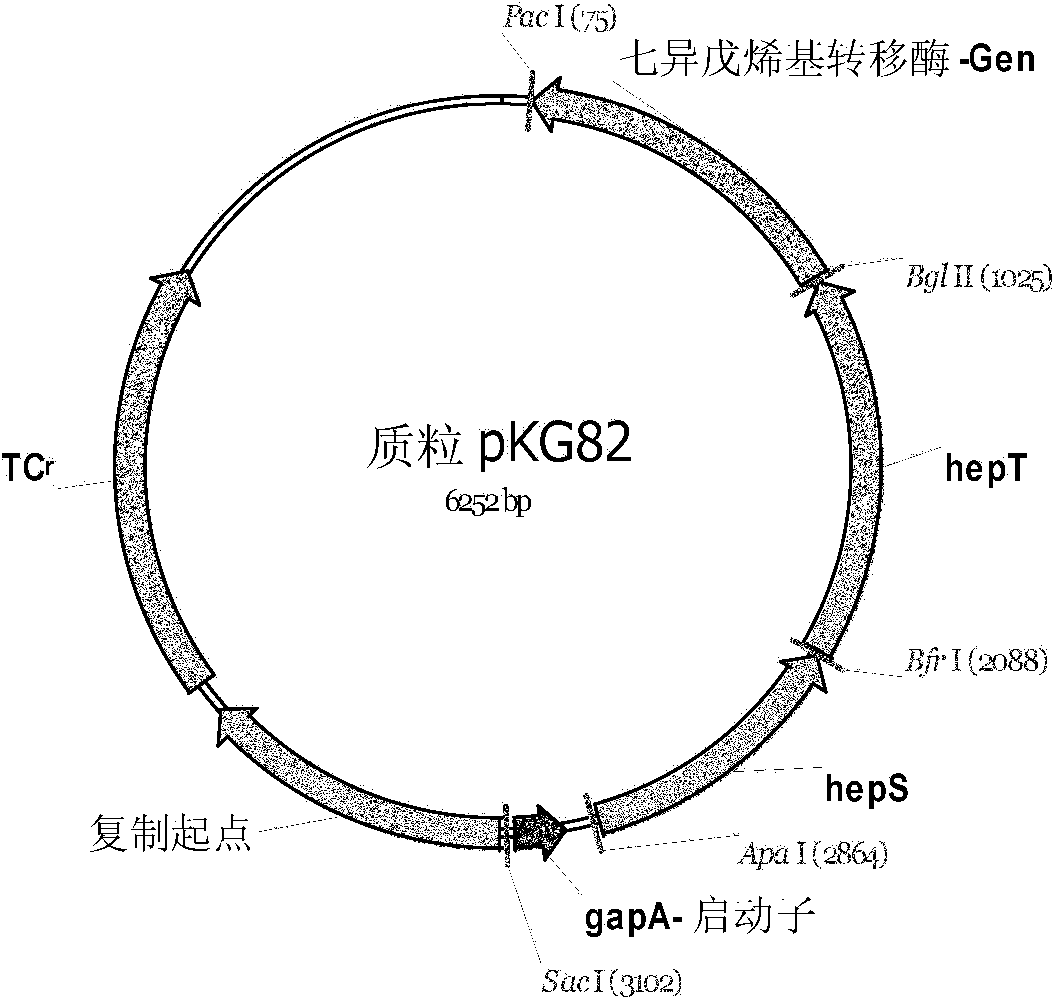
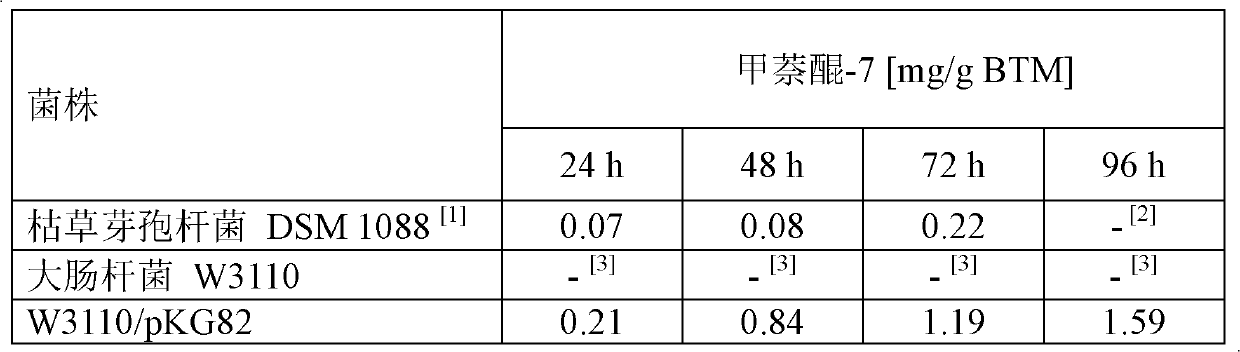
Method for fermentative production of menaquinone-7 using escherichia coli
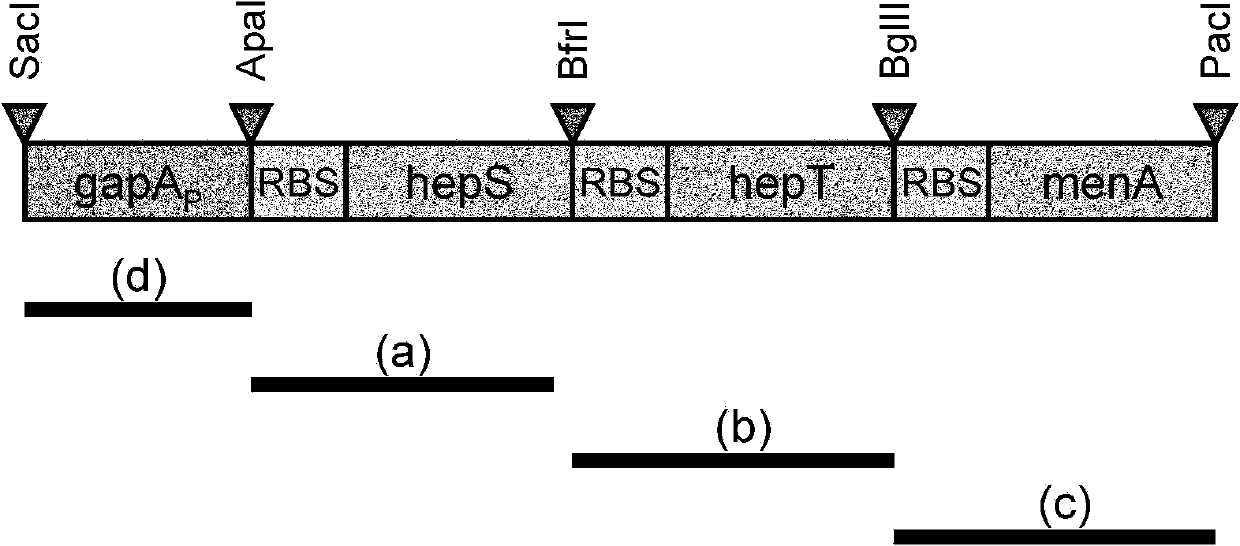
InactiveCN102168116ASuitable for productionBacteriaMicroorganism based processesBiotechnologyFermentation
The invention relates to a method of producing menaquinone-7, characterized in that cells of an E. coli strain comprising the B. subtilis DSM 1088 hepS gene, the B. subtilis DSM 1088 hepT gene and the putative B. subtilis DSM 1088 heptaprenyl transferase gene are fermented in a fermentation medium, with menaquinone-7 being accumulated in the cells of the fermented E. coli strain.
Owner:WACKER CHEM GMBH
Enol dehydrogenase, encoding gene, carrier, engineering bacteria and application of enol dehydrogenase
The invention provides an enol dehydrogenase and an encoding gene thereof, a carrier containing the encoding gene, engineering bacteria and application of the enol dehydrogenase. An amino acid sequence of the enol dehydrogenase is shown in SEQ ID No.1; the encoding gene is shown in SEQ ID No.2. The invention provides a nucleotide sequence of an enol dehydrogenase gene from Yokenella WZY002 (Yokenella sp.WZY002). The enol dehydrogenase gene can be connected with an expression carrier to build an intracellular expression recombinant plasmid containing the gene, and the intracellular expression recombinant plasmid is transformed into an escherichia coli strain, so as to obtain recombinant escherichia coli. The recombinant escherichia coli is subjected to cell disruption, separation and purification, so as to obtain the recombinant enol dehydrogenase. The recombinant enol dehydrogenase has the ability of reducing substrates, such as olefine aldehyde, olefine ketone, aromatic aldehyde, aromatic ketone and the like to generate corresponding alcohol. Oxidation of alcohol and reduction of aldehyde are simultaneously catalyzed by the recombinant escherichia coli or the recombinant enol dehydrogenase as a biological catalyst, and crotonaldehyde, benzyl alcohol, nerol and geraniol can be generated.
Owner:ZHEJIANG UNIV OF TECH
Alginate lyase Alga and coding gene and application thereof
PendingCN105713889AIncrease enzyme activityFermentationVector-based foreign material introductionHeterologousAlginate lyase
The present invention discloses an alginate lyase Alga genetic sequence and a preparation method and application thereof. Alginate lyase Alga is derived from Vibrio alginolyticus 40B. The present invention provides a process for preparing the novel alginate lyase Alga, a genetic engineering method is used, an alginate lyase Alga coding gene is cloned into an E. coli expression vector to obtain a recombinant E. coli strain capable of heterologous expression of the alginate lyase Alga, and the alginate lyase Alga prepared by heterologous expression of the recombinant E. coli strain has the function of degrading sodium alginate to prepare sodium alginate oligosaccharide. The alginate lyase Alga can be widely used in chemical industry, agriculture, food, feed additives, pharmaceuticals and algae genetic engineering and other fields.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
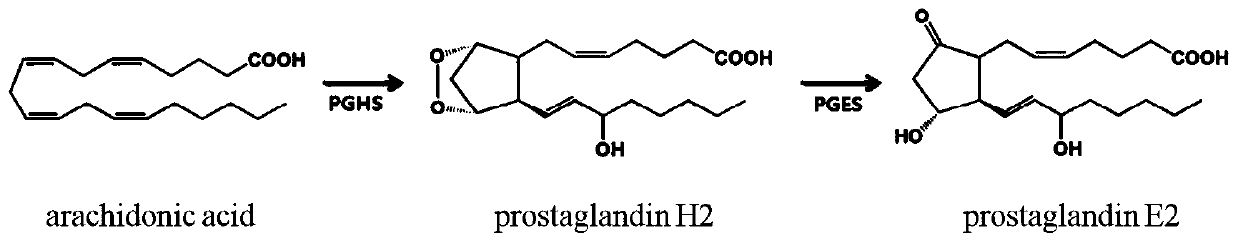
Genetic engineering bacteria and application thereof, method for producing prostaglandin E2
ActiveCN111394289AEasy to prepareImprove efficiencyBacteriaMicroorganism based processesProstaglandin-E SynthasesGenetic engineering
The present invention discloses recombinant escherichia coli. The recombinant escherichia coli over-expresses an encoding gene of prostaglandin H synthase and an encoding gene of prostaglandin E synthase. The present invention also discloses an application of the recombinant escherichia coli in production of prostaglandin E2. The present invention also discloses a method for producing the prostaglandin E2. Arachidonic acid is used as a substrate and the recombinant escherichia coli is used to produce the prostaglandin E2. The escherichia coli strain capable of expressing the prostaglandin H synthase and the prostaglandin E2 synthetase is constructed and the expressed enzymes can directly catalyze conversion of the arachidonic acid into the prostaglandin E2.
Owner:HEFEI KNATURE BIO PHARM CO LTD +1
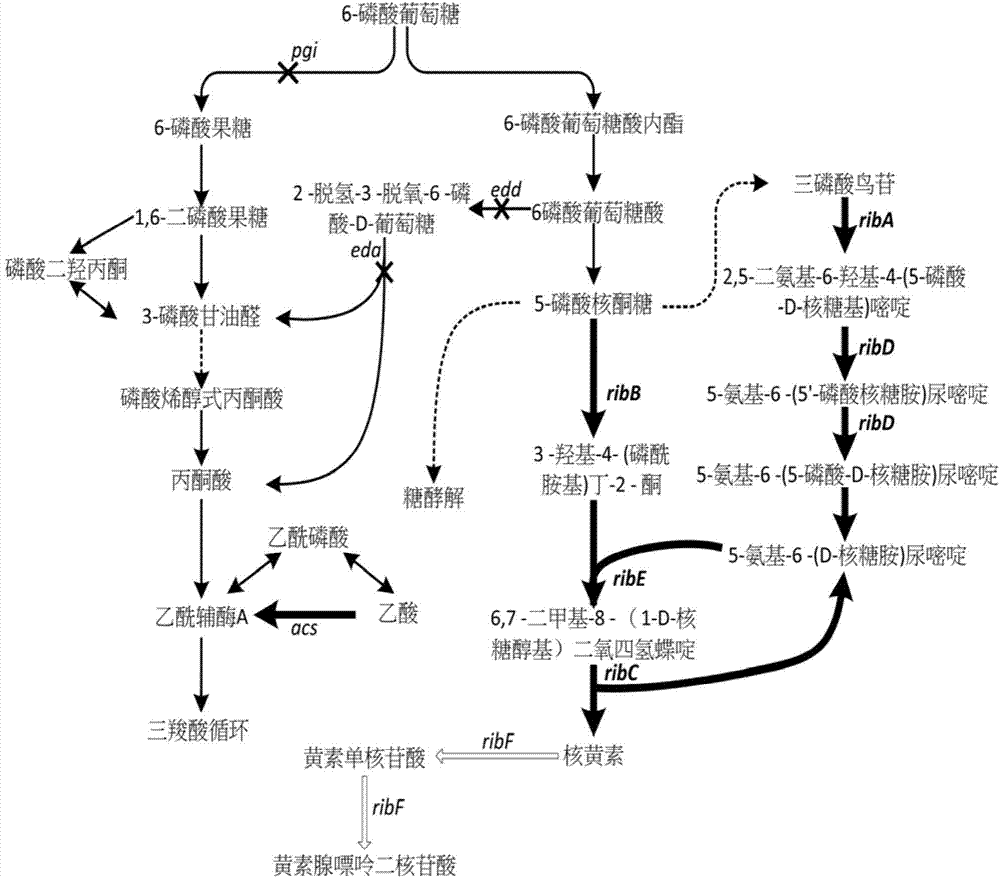
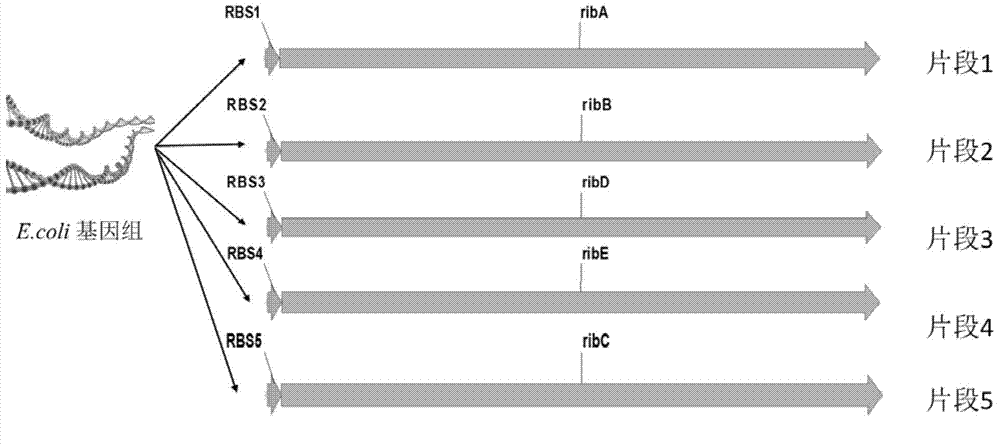
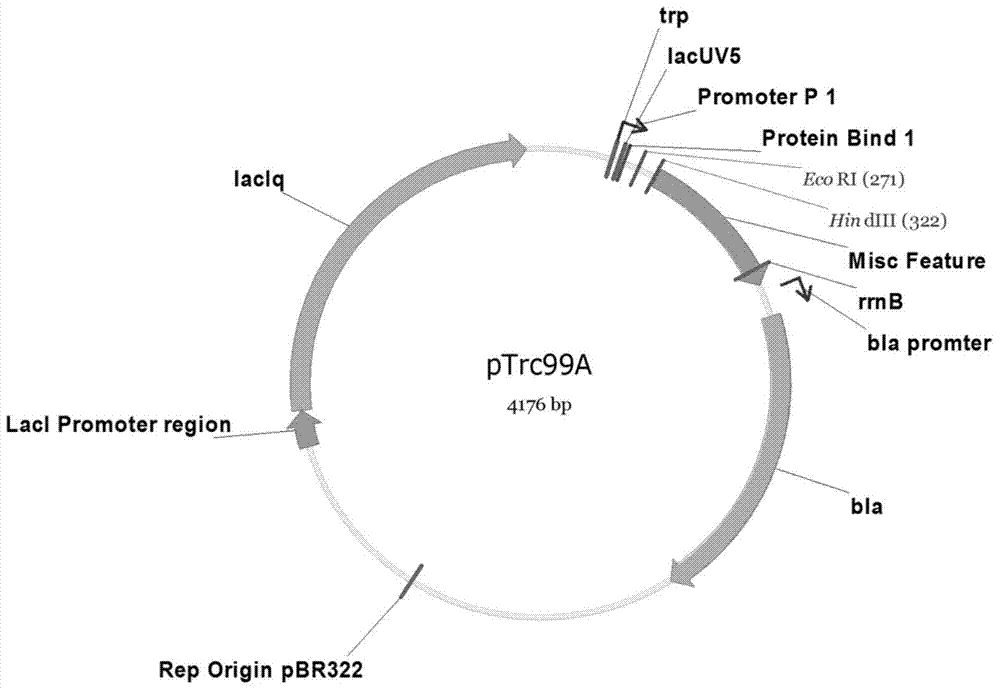
Escherichia coli for producing riboflavin and constructing method and use of Escherichia coli
ActiveCN103865944AIncrease productionHigh yieldBacteriaMicroorganism based processesEscherichia coliRiboflavin Metabolism
The invention discloses an Escherichia coli for producing riboflavin and constructing method and use of Escherichia coli. The preparation method comprises the following steps: designing ribosome bind sites RBS1, RBS2, RBS3, RBS4 and RBS5, respectively connecting the ribosome bind sites with genes ribA, ribB, ribD, ribE and ribC into fragments, splicing the fragments in order and enzyme-cutting and linking to a plasmid pTrc99a to obtain plasmid p20C-EC10, transferring into the Escherichia coli to obtain RF01S strain, transforming related gene of glycolytic pathway and ED pathway of the RF01S strain so as to improve the output of the riboflavin, and transforming the consumption pathway of the riboflavin to decrease the metabolism velocity of the riboflavin so as to further improve the riboflavin accumulation, inserting a Ptrc strong promoter into genome of the strain to obtain the Escherichia coli for producing the riboflavin. The constructed Escherichia coli strain has clear genetic background, and the glucose can be used as substrate to produce the riboflavin, and the shake-flask fermenting result is more than 1g / L, and a foundation is provided for improving the riboflavin output and yield.
Owner:TIANJIN UNIV
Enterohemorrhagic E. coli bacteriophage Esc-CHP-1 and use thereof for inhibiting proliferation of enterohemorrhagic E. coli
ActiveUS10265353B2Strong specificityLess side effectsAntibacterial agentsEnzymologyNucleotideBacteriophage
The present invention relates to a Myoviridae bacteriophage Esc-CHP-1 that is isolated from the nature and can kill specifically enterohemorrhagic E. coli strains, which has a genome represented by the nucleotide sequence of SEQ. ID. NO: 1 (Accession NO: KCTC 12660BP), and a method for preventing and treating the infections of enterohemorrhagic E. coli using the composition comprising said bacteriophage as an active ingredient.
Owner:INTRON BIOTECHNOLOGY INC
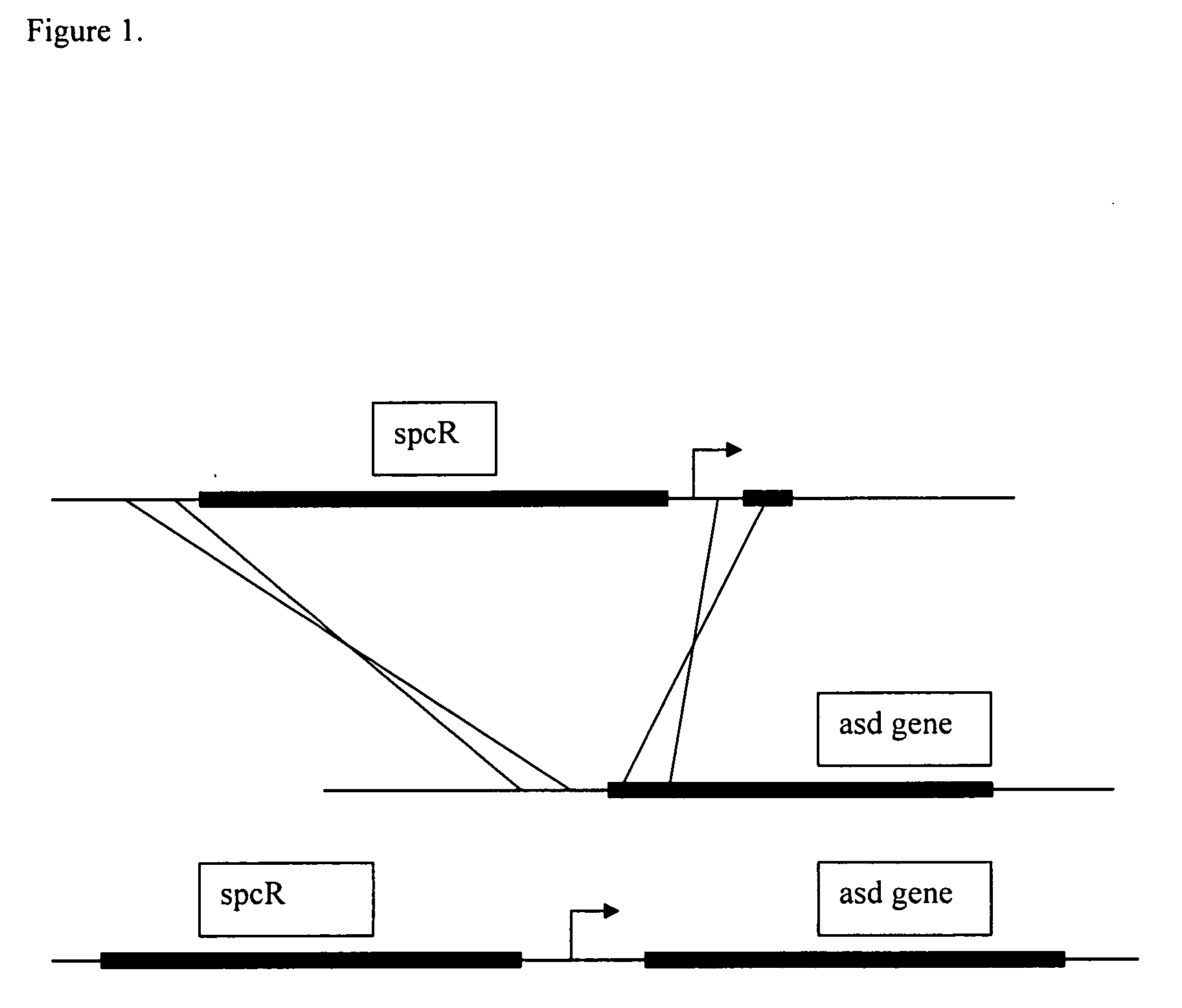
Escherichia coli strains that over-produce L-threonine and processes for their production
InactiveUS20060205044A1Efficiently produce amino acid and amino acidHigh yieldBacteriaOxidoreductasesL-threonineBinding site
The invention relates to novel bacterial strains and constructs as well as methods for production of L-amino acids, including but not limited to L-threonine. Such novel bacterial strains may be characterized by, for instance, Escherichia coli strains in which an aspartate semialdehyde dehydrogenase (asd) gene is operably associated with at least one non-native promoter, non-native ribosome binding site, or both.
Owner:ARCHER DANIELS MIDLAND CO
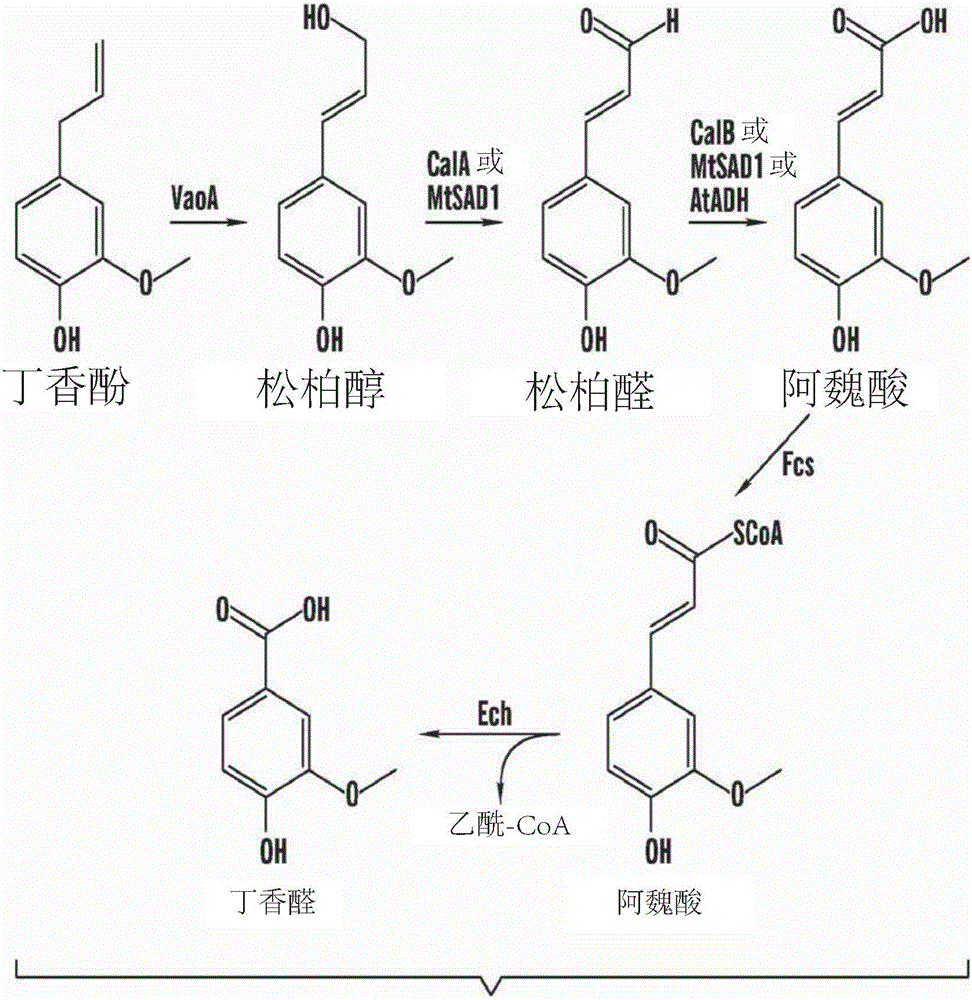
Methods of making vanillin via the microbial fermentation of ferulic acid from eugenol using a plant dehydrogenase.
A bioconversion method of making vanillin including expressing VaoA gene in a mixture, expressing MtSAD1 gene in the mixture, feeding eugenol to the mixture, and converting ferulic acid to vanillin by incubating with a microbial Amycolalopsis sp. strain (Zhp06) and / or a recombinant E.coli strain.
Owner:BGN TECH
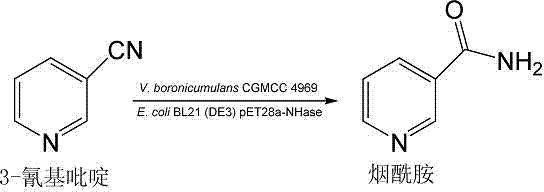
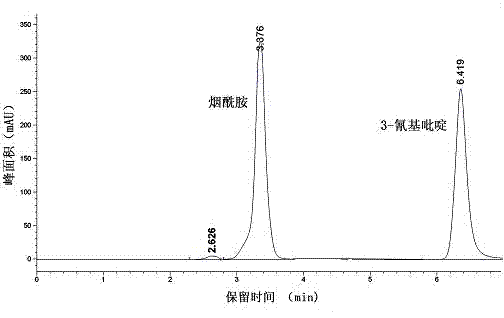
Vaporophage bacterium cgmcc4969 and its application in the biotransformation of 3-cyanopyridine to nicotinamide
The invention discloses the use of variovoraxboronicumulans CGMCC 4969 in bioconversion of 3-cyanopyridine for forming nicotinamide. The nitrile hydratase gene cluster produced by the strain consists of a DNA sequence represented by SEQ ID No.1 in a sequence table; the DNA consisting of the sequence represented by SEQ ID No.1 is recombined onto a pET28a plasmid and can be induced to express in EscherichiacoliBL21(DE3) strain; and the Escherichia coli cells containing expressed proteins and cell extracting solution can convert 3-cyanopyridine into nicotinamide.
Owner:NANJING NORMAL UNIVERSITY
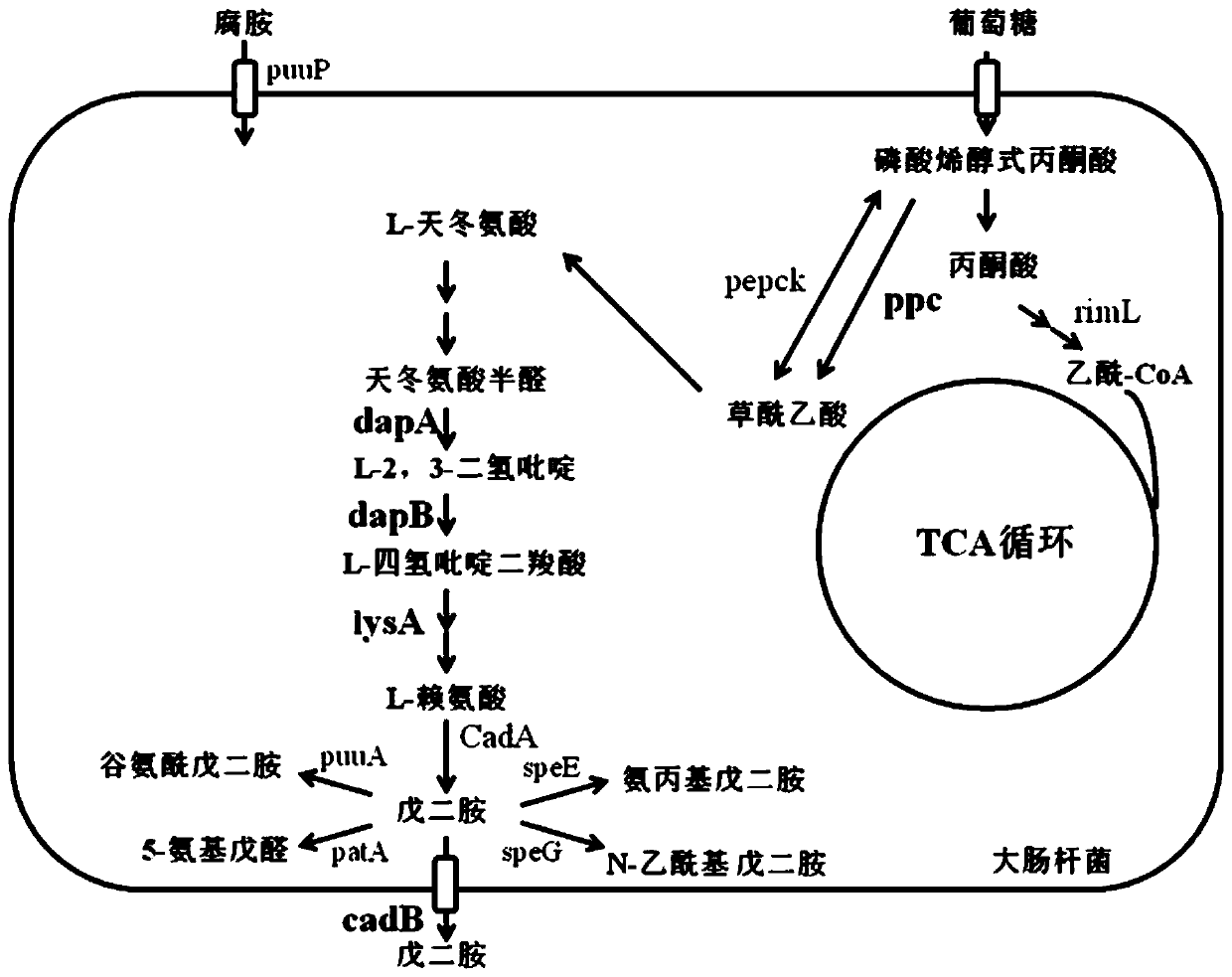
Bioconversion method for producing 1,5-pentanediamine
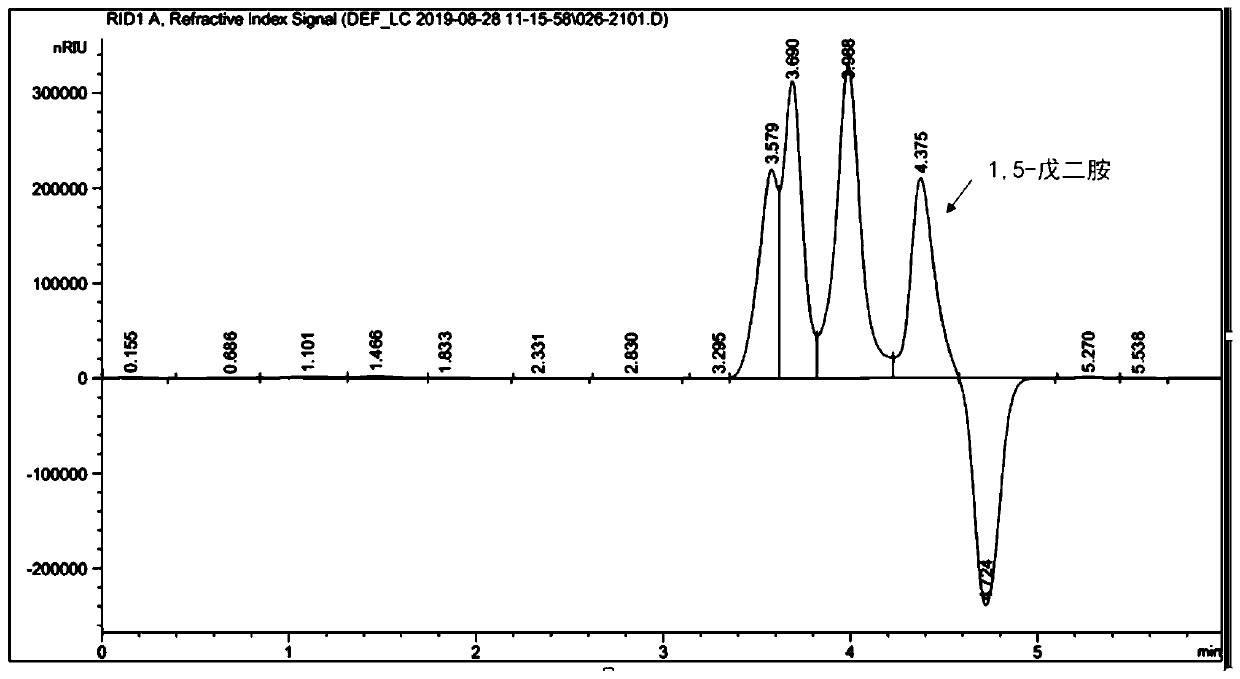
PendingCN110699394AIncrease usageIncrease productionBacteriaMicroorganism based processesBiotechnologyGenetic engineering
The invention belongs to the technical field of genetic engineering, and relates to a bioconversion method for producing 1,5-pentanediamine. The method can increase the yield of 1,5-pentanediamine bymodifying a metabolic pathway of Escherichia coli. The method specifically comprises the following steps: selecting an Escherichia coli strain, firstly, knocking out a 1,5-pentanediamine degradation pathway; secondly, adding a synthetic precursor,namely lysine, of 1,5-pentanediamine; thirdly, overexpressing a transporter gene and finally directly fermenting the Escherichia coli strain DFC1002 to produce 1,5-pentanediamine. After the metabolic pathway of Escherichia coli is modified, themethod can produce 1,5-pentanediamine with high yield.The method is simple and easy to implement, has high yield of 1,5-pentanediamine, few by-products, and good repeatability, and is suitable for industrialization.
Owner:NANJING UNIV OF TECH
Method for preparing mutant escherichia coli capable of simultaneously utilizing glucose and xylose
ActiveCN104371963AShorten the timeIncrease productivityBacteriaMutant preparationChemical industryWild type
The present invention relates to a method for preparing a mutant E. coli strain, capable of simultaneously using glucose and xylose, by genetic engineering and evolutionary adaptation; the mutant E. coli prepared using the same; and a method for producing biofuels, biologically active ingredients, medicinal materials or base chemicals for the chemical industry using the mutant E. coli. Being capable of simultaneously using glucose and xylose, in contrast to wild-type E. coli, the mutant E. coli can be effectively applied to the enzymatic saccharification process of producing biofuels from a biomass.
Owner:UNIST (ULSAN NAT INST OF SCI & TECH)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com