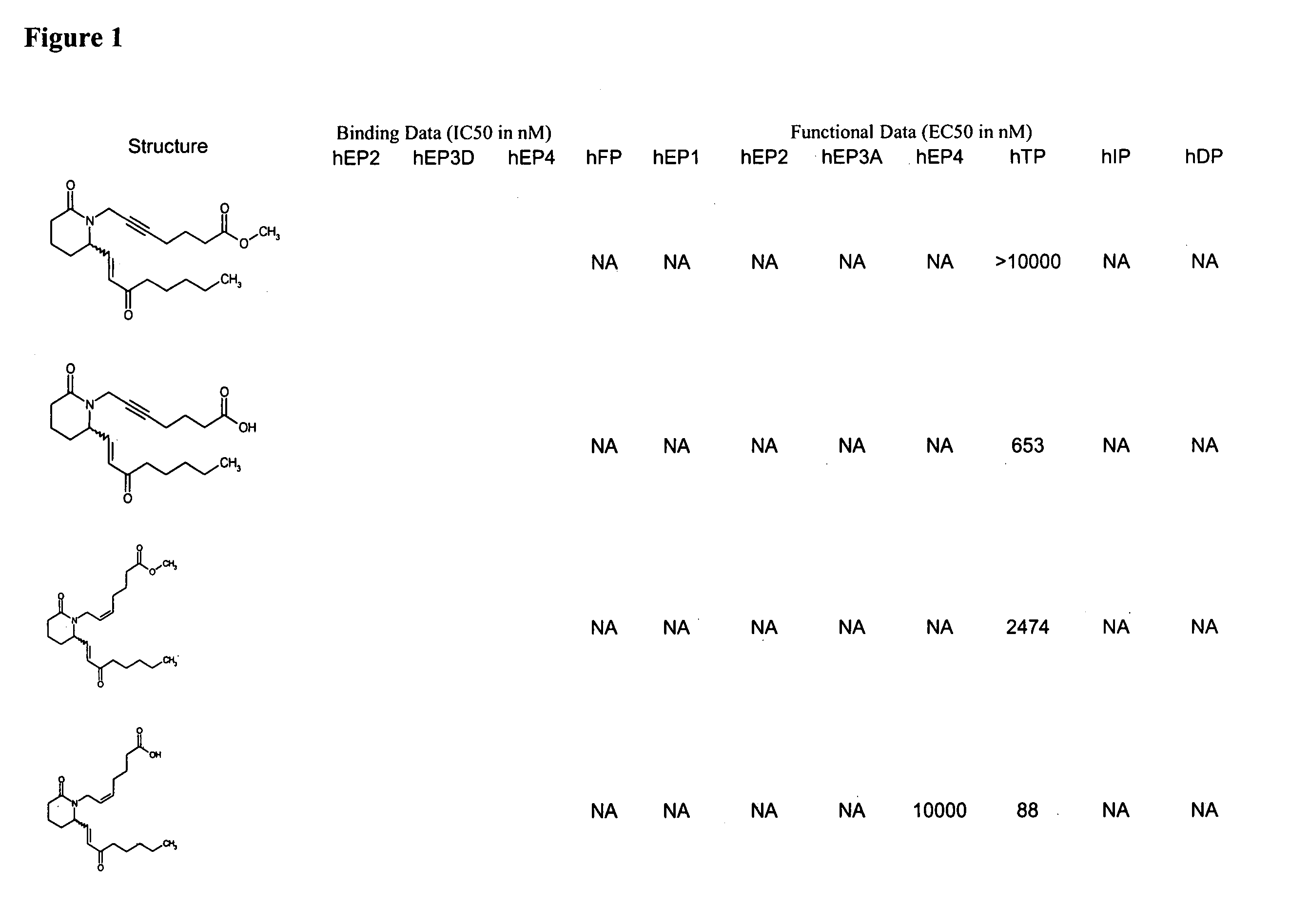
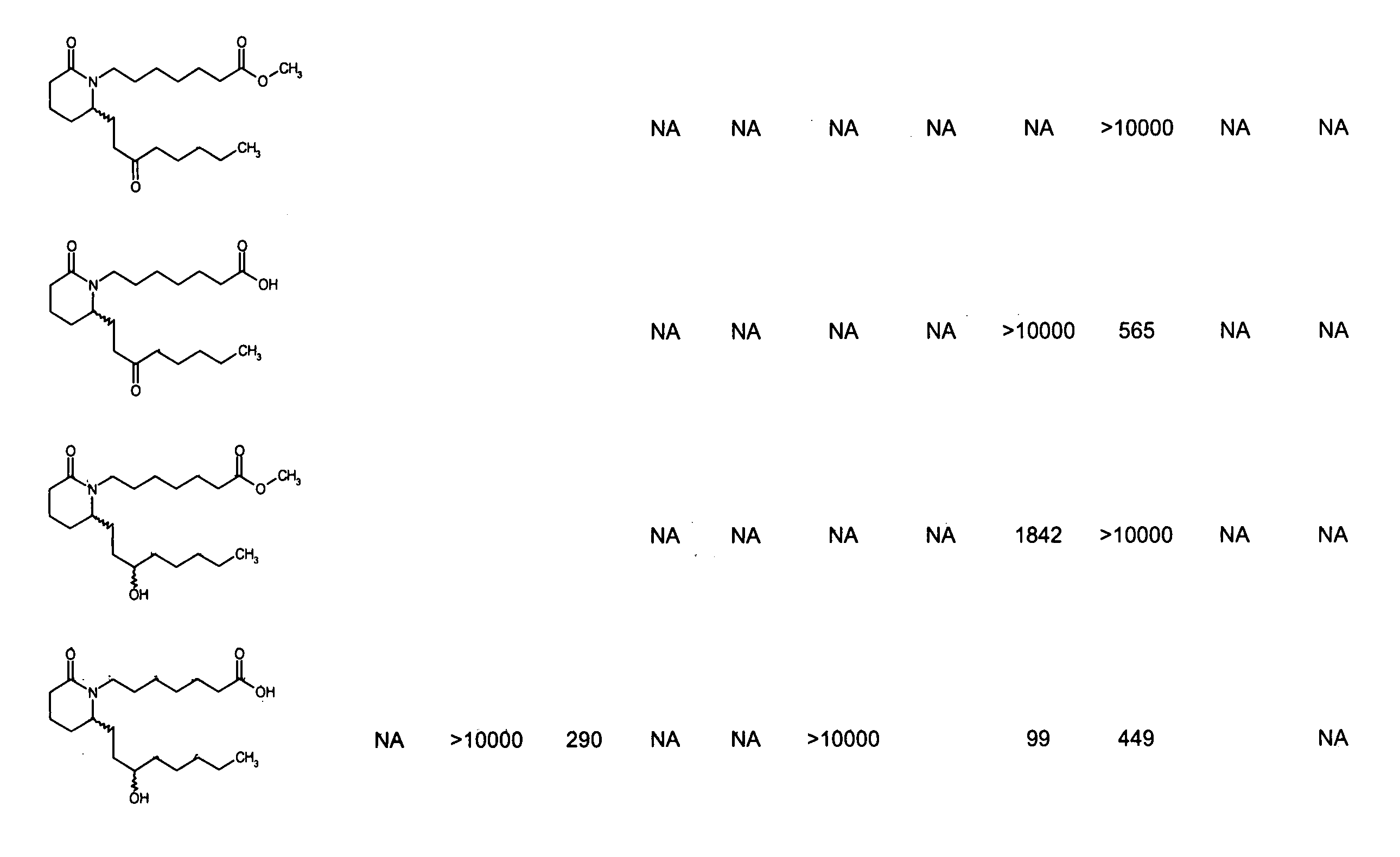
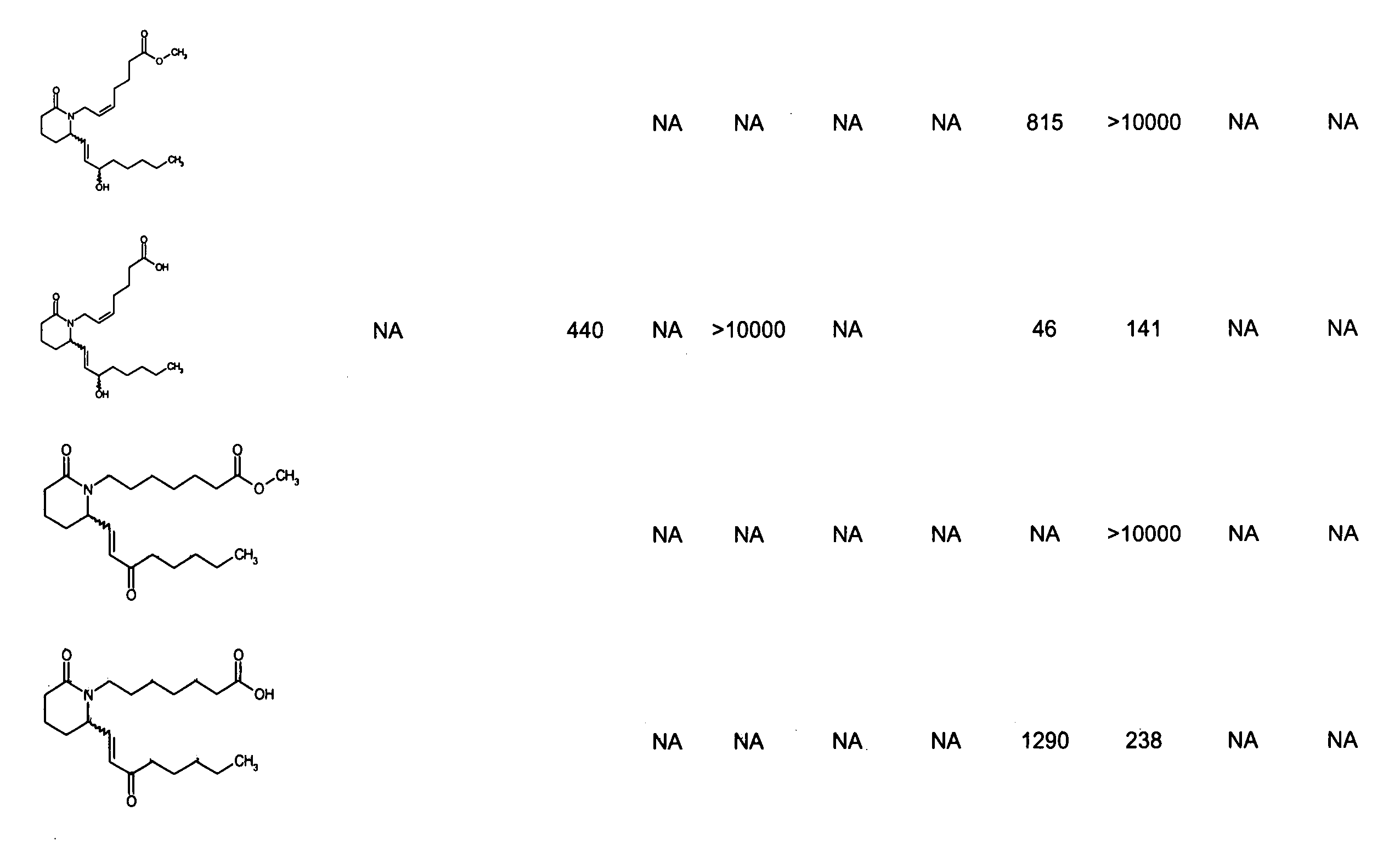
Patents
Literature
111 results about "Prostaglandin E" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prostaglandin E is a family of naturally occurring prostaglandins that are used as medications.
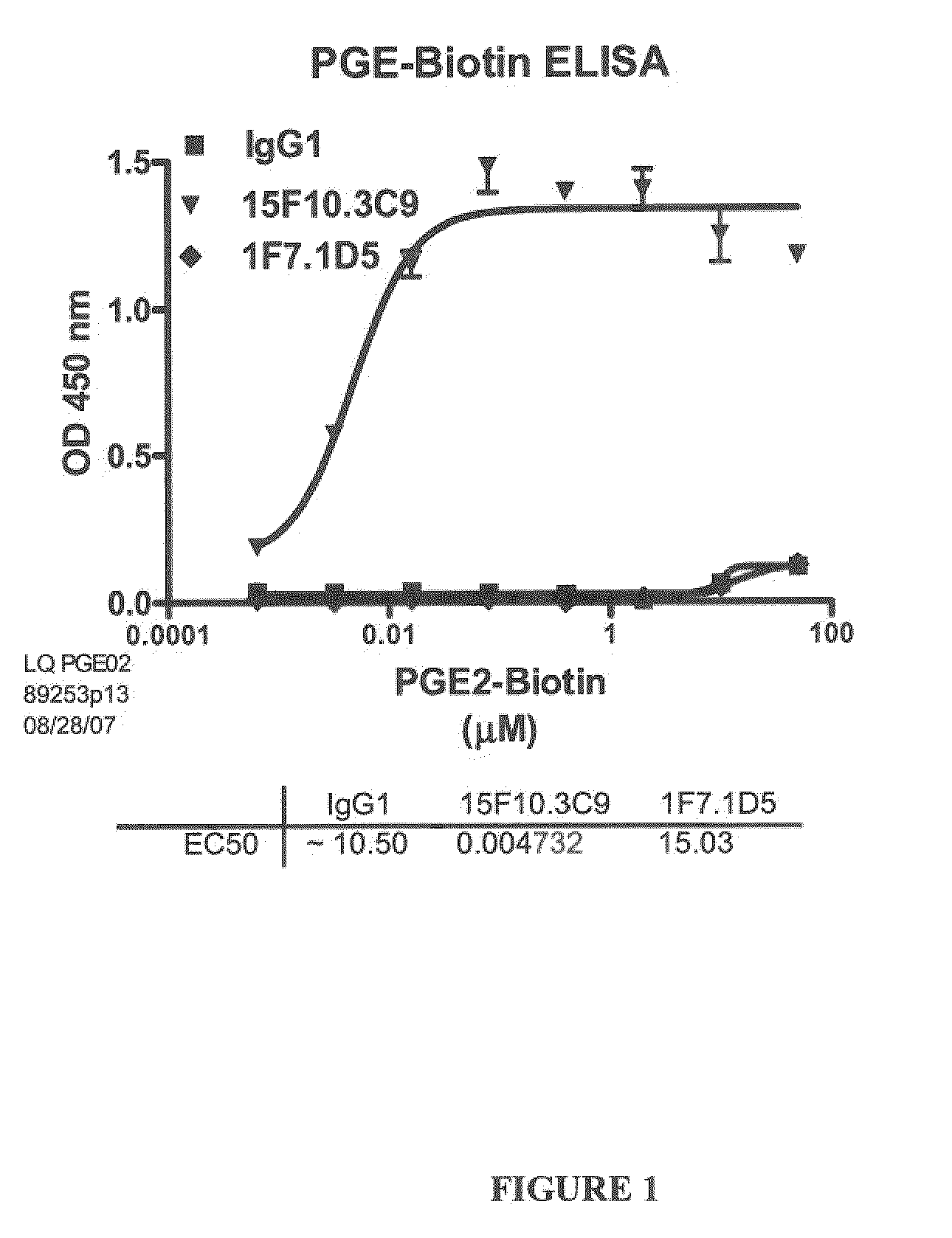
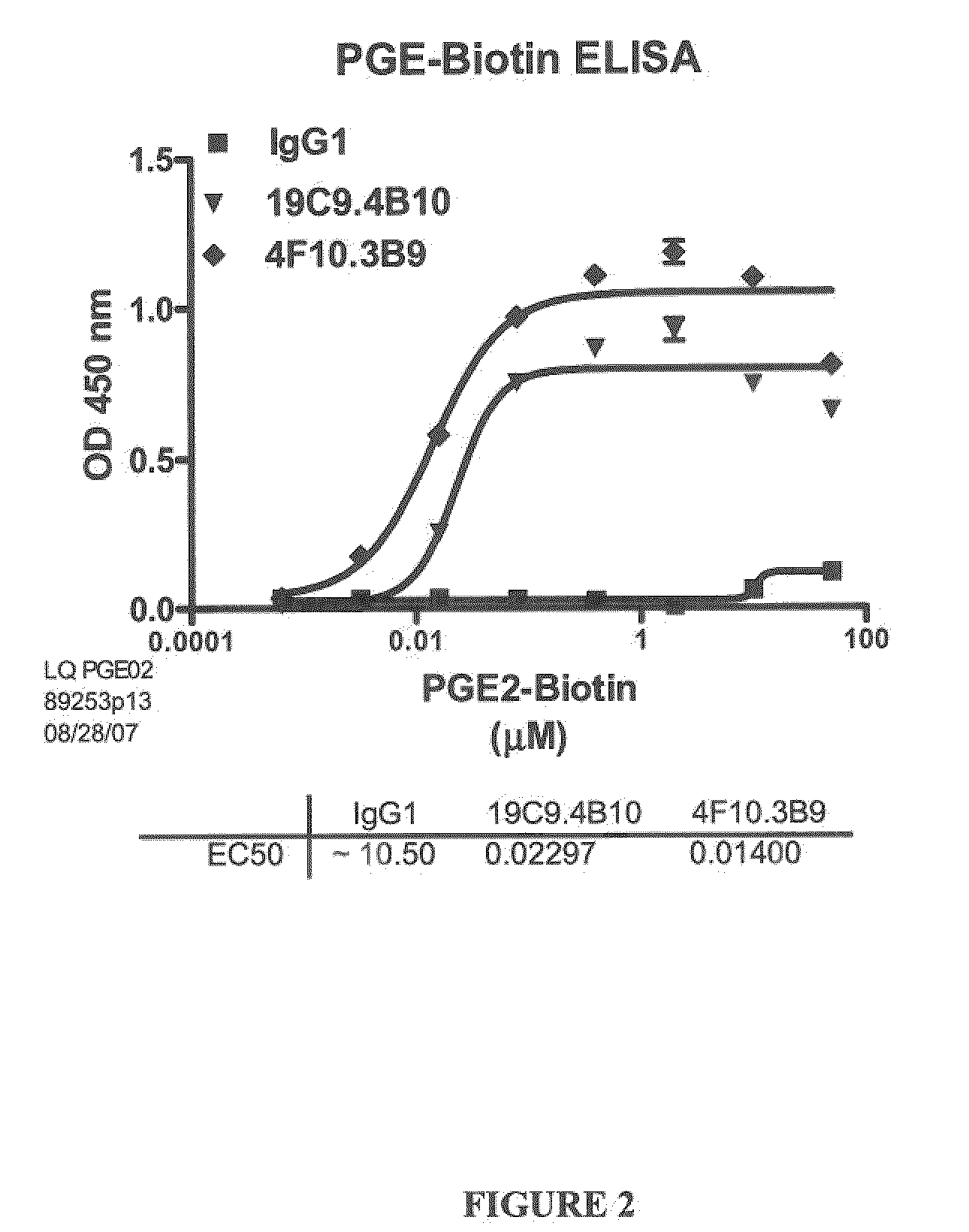
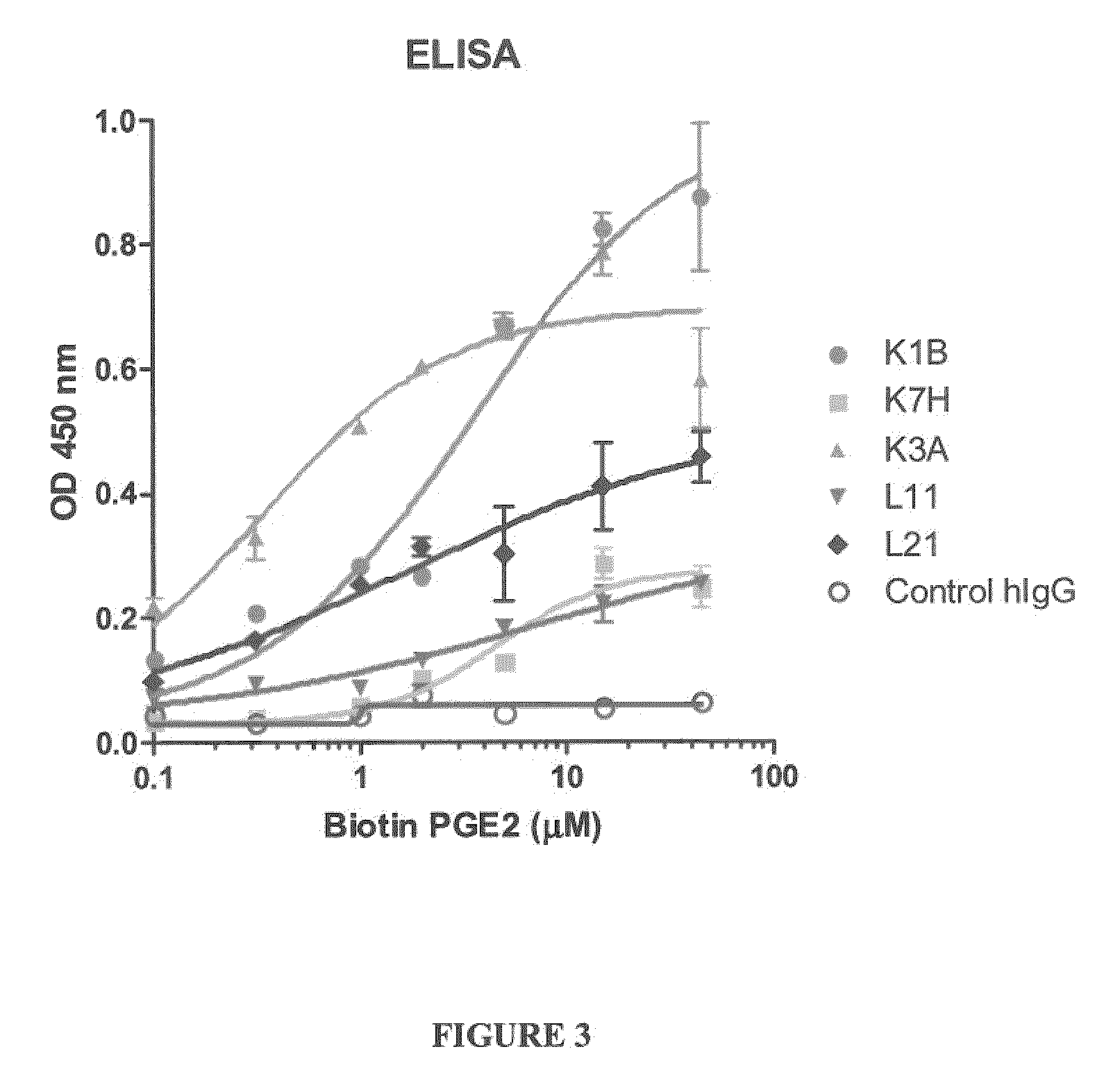
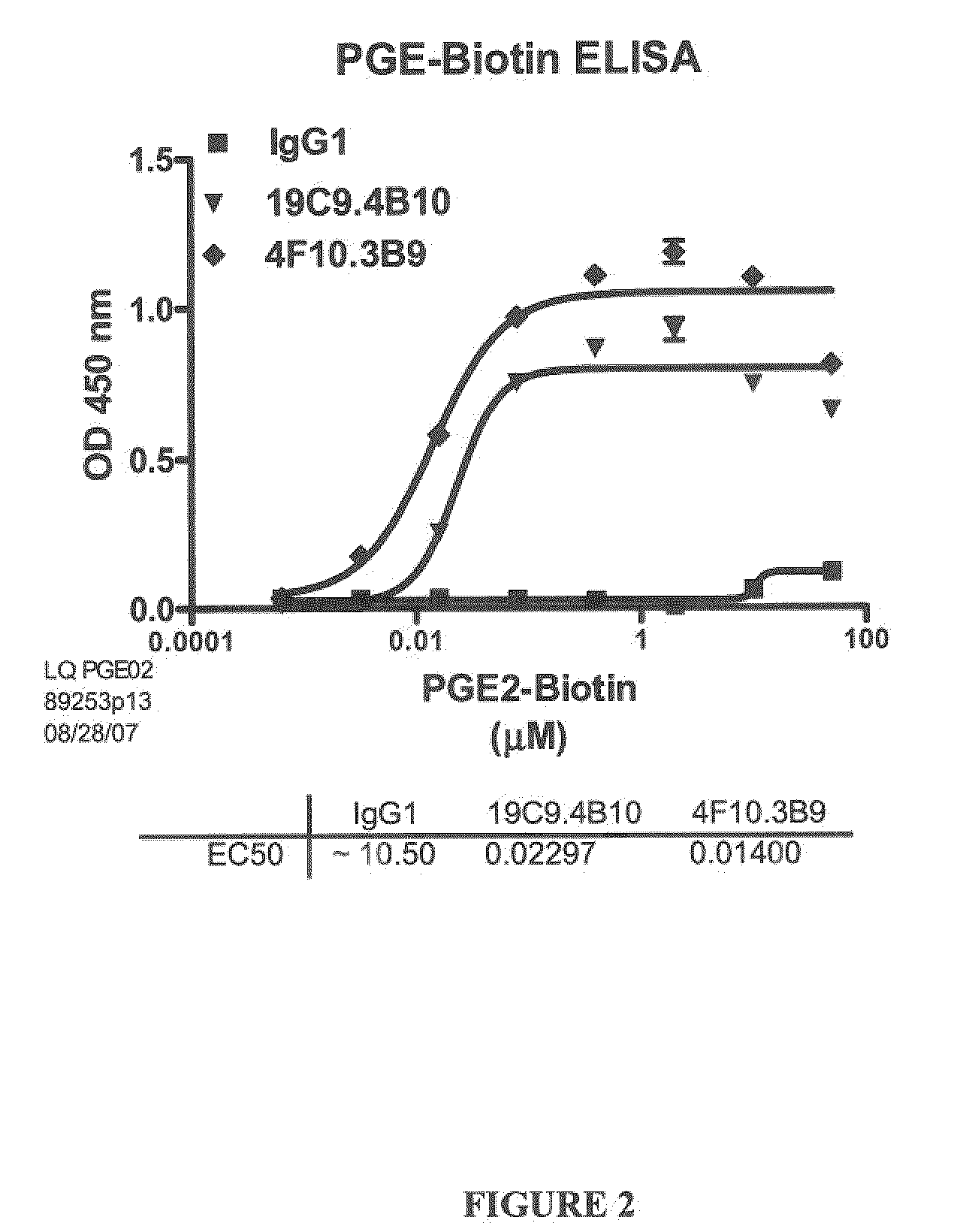
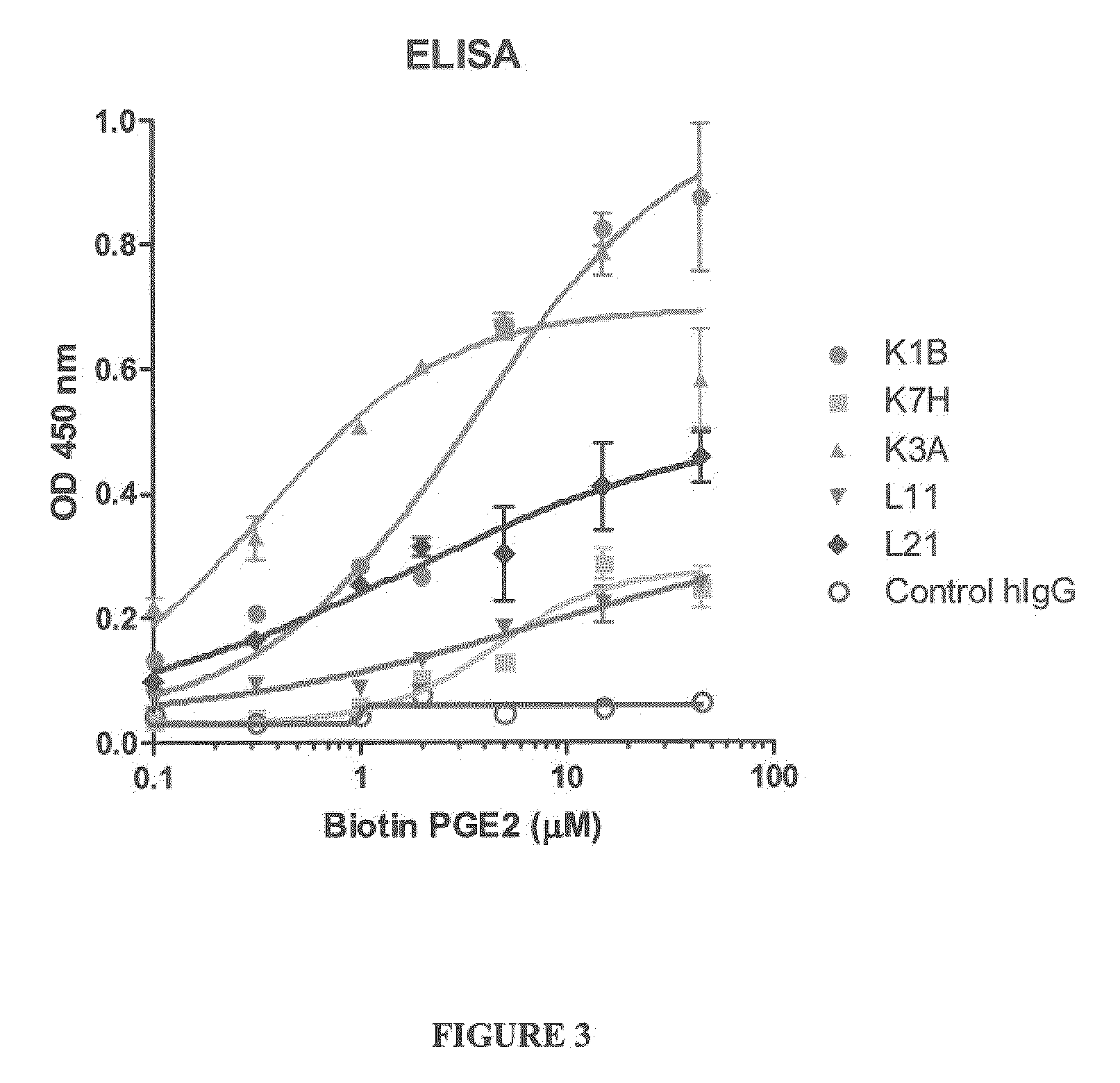
Prostaglandin E2 Binding Proteins and Uses Thereof
ActiveUS20100040537A1Prevent and more symptomAntibacterial agentsOrganic active ingredientsWild typeAntigen binding
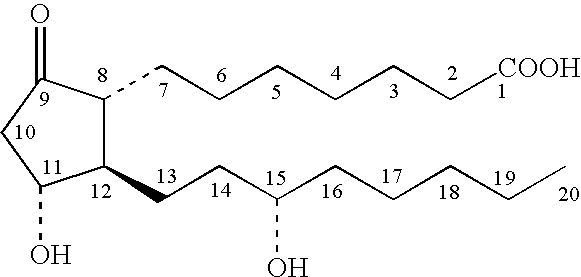
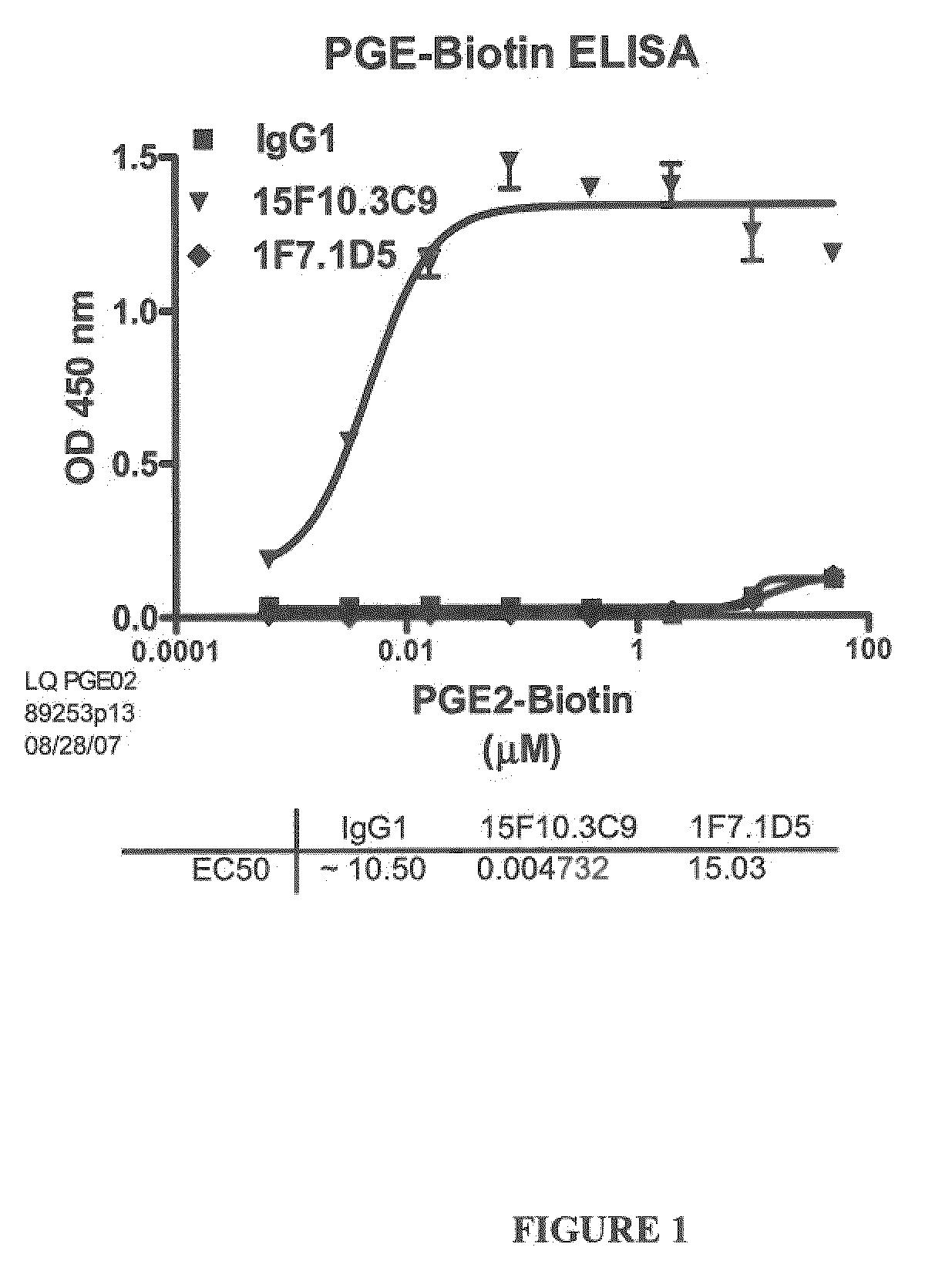
The present invention encompasses prostaglandin E2 (PGE2) binding proteins. The invention relates to antibodies that are wild-type, chimeric, CDR grafted and humanized. Preferred antibodies have high affinity for prostaglandin E2 and neutralize prostaglandin E2 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody, or an antigen-binding portion thereof. Methods of making and methods of using the antibodies of the invention are also provided. The antibodies, or antigen-binding portions, of the invention are useful for detecting prostaglandin E2 and for inhibiting prostaglandin E2 activity, e.g., in a human subject suffering from a disorder in which prostaglandin E2 activity is detrimental.
Owner:ABBVIE INC
Drug preparations for treating sexual dysfunction
Topical gelled compositions comprising a drug which causes vasodilation, and optionally prostaglandin E1, dispersed within a polymer matrix, and methods of treating sexual dysfunction, including both male and female sexual dysfunction, using said compositions.
Owner:L A M PHARMA +1
Remedies for urinary frequency
InactiveUS20060100195A1Suppress pollakiuriaEffective preventionBiocidePeptide/protein ingredientsEccentric hypertrophyUnstable bladder
The present invention relates to an agent for the treatment and / or prevention of pollakiuria comprising a compound having an antagonism to an EP1 receptor which is a prostaglandin E2 receptor subtype. A compound having an antagonism to an EP1 receptor antagonistically acts on an EP1 receptor which is a prostaglandin PGE2 receptor subtype and significantly shows a suppressive activity for urination frequency in models where pollakiuria is induced. Therefore, it is effective for the treatment and / or prevention of pollakiuria (that which is due to nurogenic bladder, nervous bladder, stimulated bladder, unstable bladder, benign prostatic hypertrophy, etc.).
Owner:ONO PHARMA CO LTD
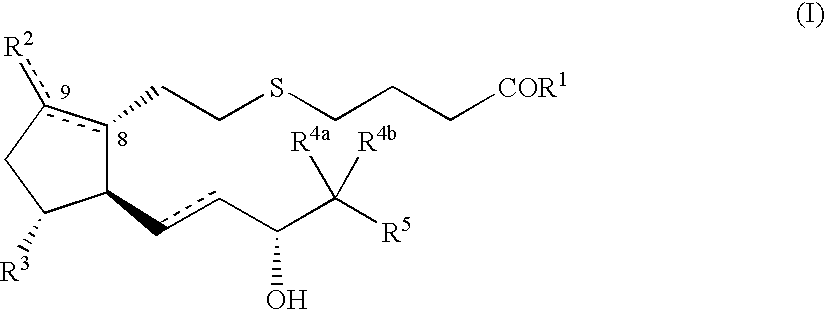
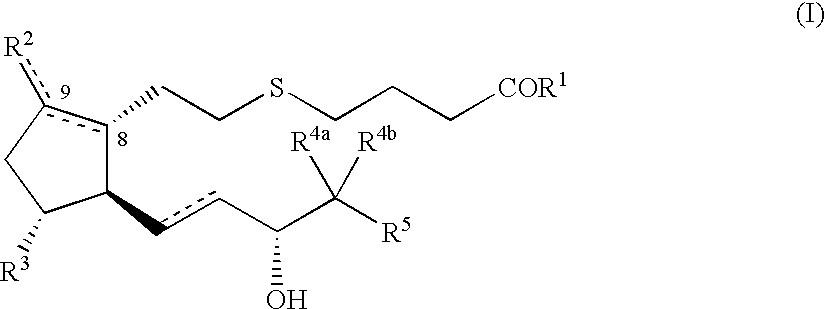
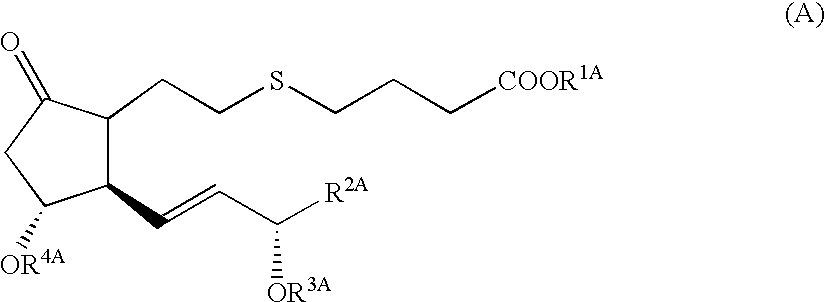
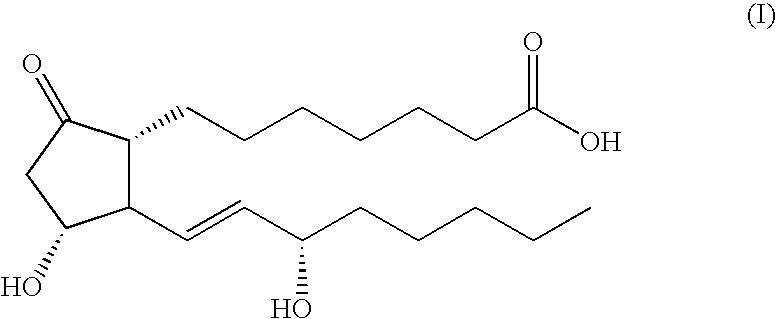
5-thia-omega-substituted phenyl-prostaglandin E derivatives, process for producing the same and drugs containing the same as the active ingredient
The present invention relates to 5-thia-omega-substituted phenylprostaglandin E derivatives of the formula (I)(wherein, all the symbols are as defined in the specification), process for producing them and pharmaceutical compositions comprising them as active ingredient.The compounds of the formula (I) can bind to PGE2 receptors (especially, subtype EP4) strongly, so they are expected to be useful for prevention and / or treatment of immunological diseases (autoimmune diseases such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Sjoegren's syndrome, chronic rheumarthrosis and systemic lupus erythematosus etc., and rejection after organ transplantation etc.), asthma, abnormal bone formation, neuronal cell death, lung failure, liver damage, acute hepatitis, nephritis, renal insufficiency, hypertension, myocardiac ischemia, systemic inflammatory response syndrome, ambustion pain, sepsis, hemophagous syndrome, macrophage activation syndrome, Still's disease, Kawasaki disease, burn, systemic granulomatosis, ulcerative colitis, Crohn's disease, hypercytokinemia at dialysis, multiple organ failure, and shock etc. Further, it is thought that EP4 subtype receptor relates to sleeping disorder and blood platelet aggregation, so the compounds of the present invention are expected to be useful for the prevention and / or treatment of such diseases.
Owner:ONO PHARMA CO LTD
Nitrosated and nitrosylated prostaglandins, compositions and methods of use
The invention describes novel nitrosated and / or nitrosylated prostaglandins, and novel compositions comprising at least one nitrosated and / or nitrosylated prostaglandin, and, optionally, at least one compound that donates, transfers or releases nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor, stimulates endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase, and / or at least one vasoactive agent. The invention also provides novel compositions comprising at least one prostaglandin and at least one S-nitrosothiol compound, and, optionally, at least one vasoactive agent. The prostaglandin is preferably a prostaglandin E1 compound, more preferably alprostadil, and the S-nitrosothiol compound is preferably S-nitrosoglutathione. The invention also provides methods for treating or preventing sexual dysfunctions in males and females, for enhancing sexual responses in males and females, and for treating or preventing cerebrovascular disorders, cardiovascular disorders, benign prostatic hyperplasia (BPH), glaucoma, peptic ulcers or for inducing abortions.
Owner:NITROMED
Topical stabilized prostaglandin E compound dosage forms
InactiveUS6841574B2Improve sexual dysfunctionBiocideCosmetic preparationsProstaglandin EProstaglandin PGE
Compounds of prostaglandin E group (PGE compounds) are stabilized as non-aqueous compositions that include the compound together with a bulking agent that can be a non-aqueous liquid or a solid in a sheet, film or powder form. The composition can optionally include a skin penetration enhancer. A non-aqueous, solid dosage form comprises a PGE compound substantially uniformly distributed in a carrier sheet or film.
Owner:NEXMED HLDG INC
Prostaglandin E2 binding proteins and uses thereof
The present invention encompasses prostaglandin E2 (PGE2) binding proteins. The invention relates to antibodies that are wild-type, chimeric, CDR grafted and humanized. Preferred antibodies have high affinity for prostaglandin E2 and neutralize prostaglandin E2 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody, or an antigen-binding portion thereof. Methods of making and methods of using the antibodies of the invention are also provided. The antibodies, or antigen-binding portions, of the invention are useful for detecting prostaglandin E2 and for inhibiting prostaglandin E2 activity, e.g., in a human subject suffering from a disorder in which prostaglandin E2 activity is detrimental.
Owner:ABBVIE INC
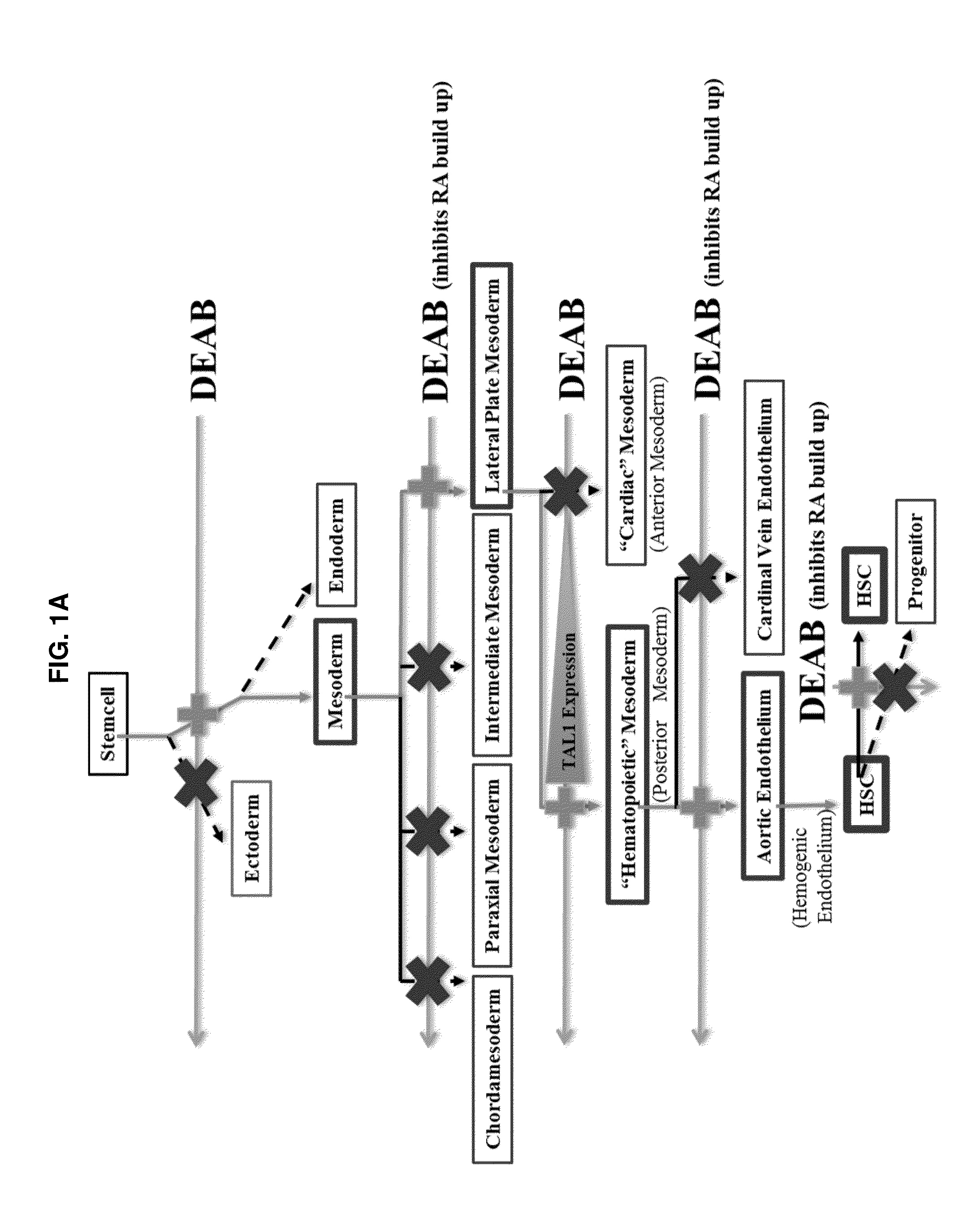
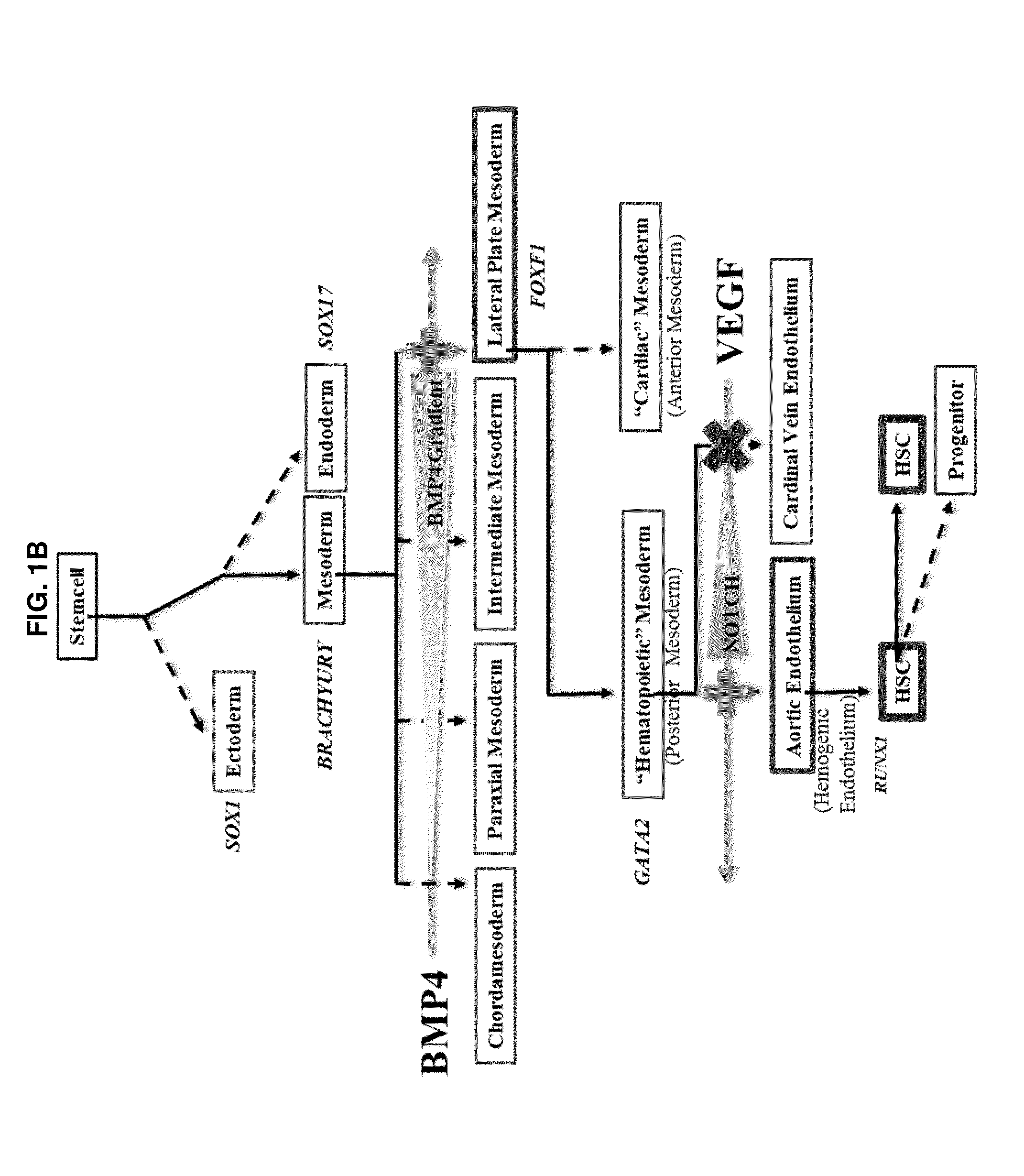
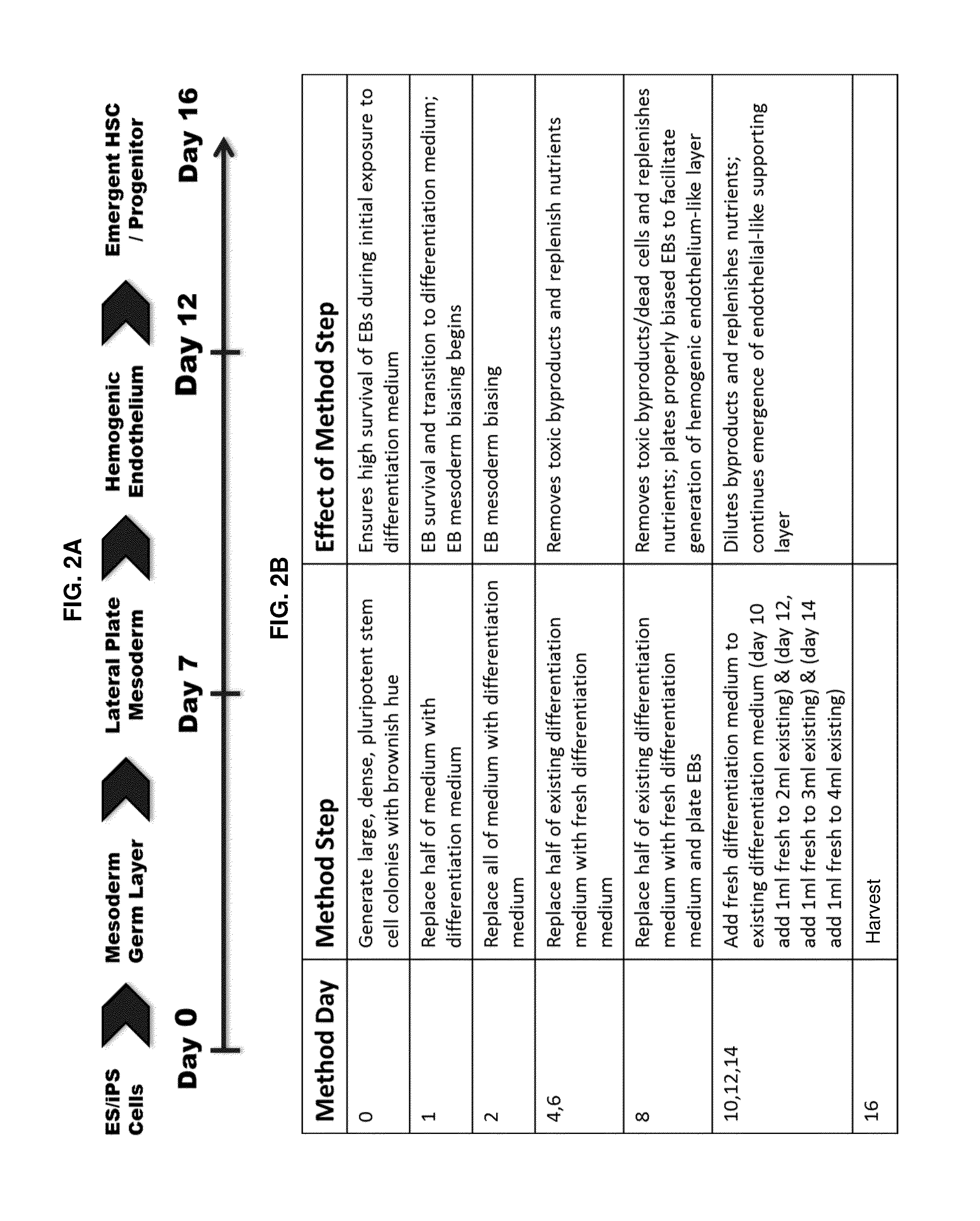
Compositions and methods for differentiating pluripotent stem cells into primitive blood cells and uses thereof
InactiveUS20130171110A1High expressionAccelerate self-renewalBiocideCulture processInduced pluripotent stem cellAntioxidant
Compositions and methods that employ various combinations of such factors as retinoic acid signaling inhibitors, antioxidants, BMP4, VEGF, prostaglandin E2 pathway stimulants, TPO, SCF, FLT-3, EPO, TGFβ1, p38 MAPK inhibitors, beta adrenergic receptor agonists, cell cycle inhibitors, RXR agonists, Cripto, and chromatin remodelers to drive differentiation of pluripotent stem cells towards primitive blood cells. Uses of such primitive blood cells are provided.
Owner:NUCLEUS BIOLOGICS LLC
Emulsion composition comprising prostaglandin e1
An emulsion composition includes prostaglandin E1 (PGE1), a phospholipid with a high purity and a non-proton-providing surfactant that improves stability of PGE1. Embodiments of the emulsion composition include an effective amount of PGE1, about 1% to about 30% (w / w) of a pharmaceutically acceptable oil as an oil base based on the weight of the emulsion composition, about 1% to about 30% (w / w) of a phospholipid with a high purity based on the weight of the oil base, about 1.6% to about 40% (w / w) of a non-proton-providing surfactant based on the weight of the oil base, and the balance of the emulsion composition being water.
Owner:TAIWAN LIPOSOME CO LTD +1
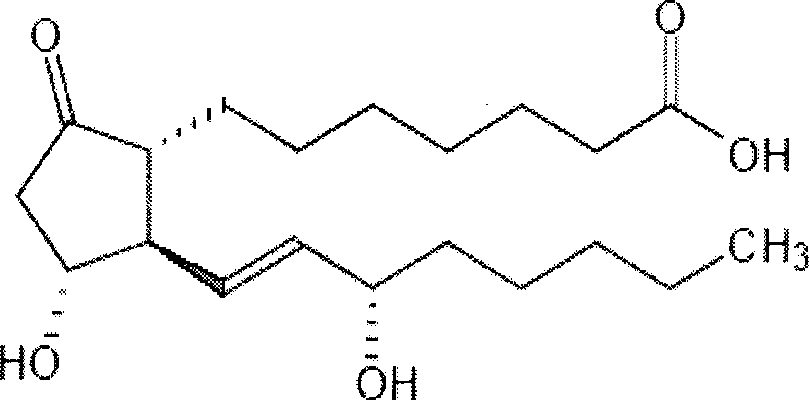
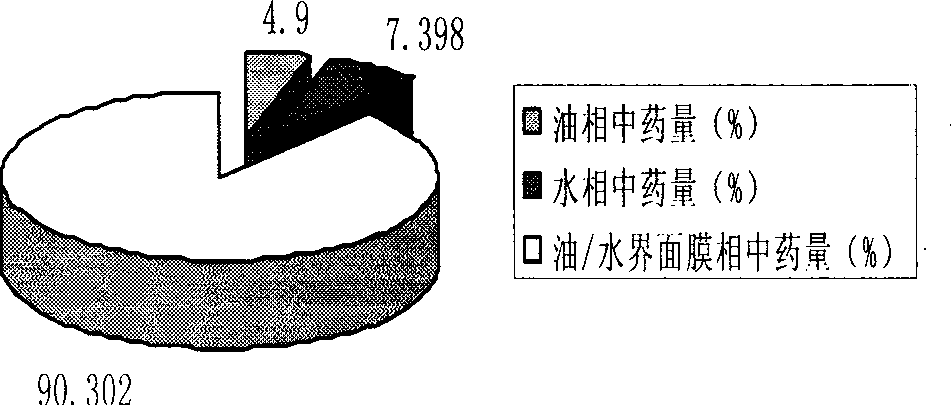
Prostaglandin E1 lipid microsphere injection with charge effect and preparation method thereof
InactiveCN101496787AClinical application safetyEnsure safetyOrganic active ingredientsDigestive systemChemical structureLipid formation
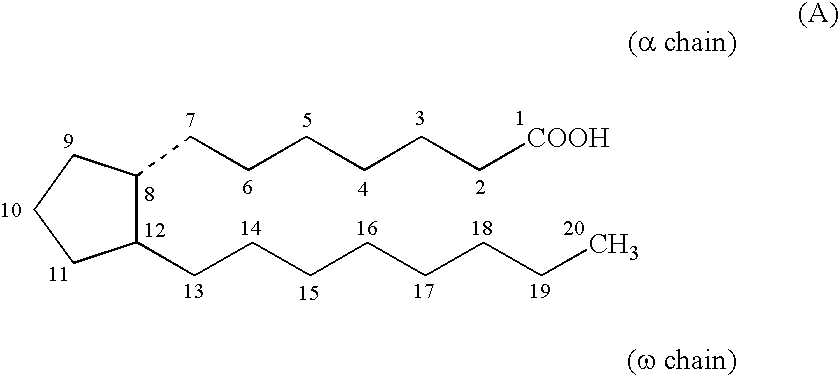
The invention relates to a method for preparing a prostaglandin E1 lipid microsphere injection of a charging non-homogeneous phase (comprising a water phase, an oil / water interfacial film phase and an oil phase) dispersion system, of which the surface of the lipid microsphere can be charged with positive electricity or negative electricity. The prostaglandin E1 is alprostadil, of which the chemical structure comprises a basic skeleton of 20-carbon fatty acid with a 5-carbon ring and two side chains, wherein one side chain is provided with a hydrophilic carboxylic acid group, so that the prostaglandin E1 has the characteristic of light surface activity action. By utilizing the characteristic, and according to the formula and the preparation process provided in the invention, the prostaglandin E1 has an unique drug-carrying mode in a solution of lipid microsphere with the non-homogeneous phase dispersion system, and the prepared lipid microsphere injection is fundamentally different from an alprostadil injection(Kaishi, and is prepared by adopting the technology of the Japanese business corporation LTT Bio-Pharma Co., Ltd. already sold in markets, and the difference lies in that the drug-carrying mode is completely different, the content of degradation products in the preparation such as impurities is more than 50 percent lower than that of in the Kaishi, so that the prostaglandin E1 lipid microsphere injection and the alprostadil injection are fundamentally different. The invention relates to a method for preparing the prostaglandin E1 lipid microsphere injection and the drug-carrying characteristics thereof in a three-phase system; in the formula, 0.0001 to 0.1 weight portion of prostaglandin E1 is used as a drug, the prostaglandin E1 is added with auxiliary materials for medical purpose to prepare the prostaglandin E1 lipid microsphere injection, and the auxiliary materials for medical purpose comprises the following materials in portion by weight: 5 to 20.
Owner:李淑斌
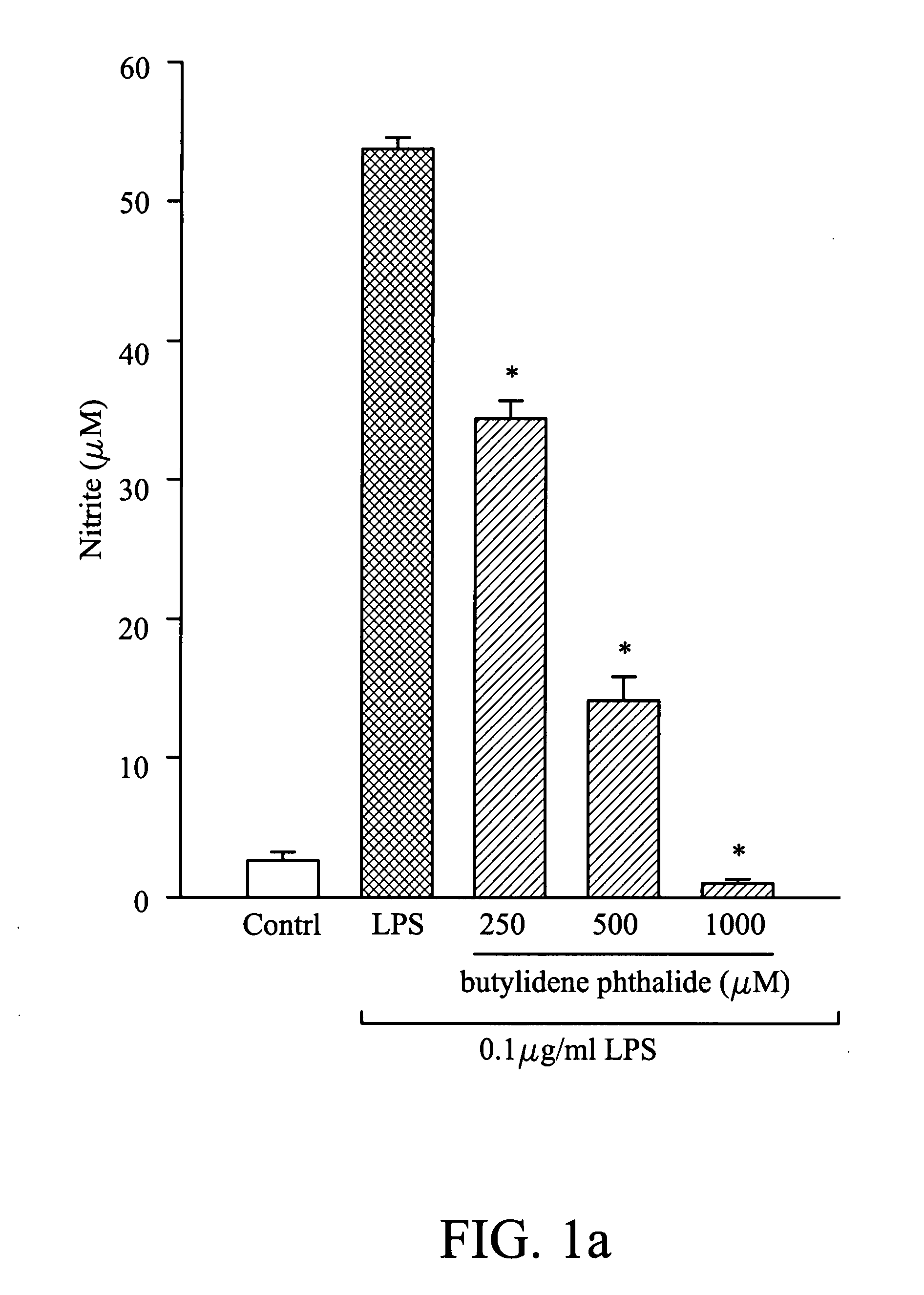
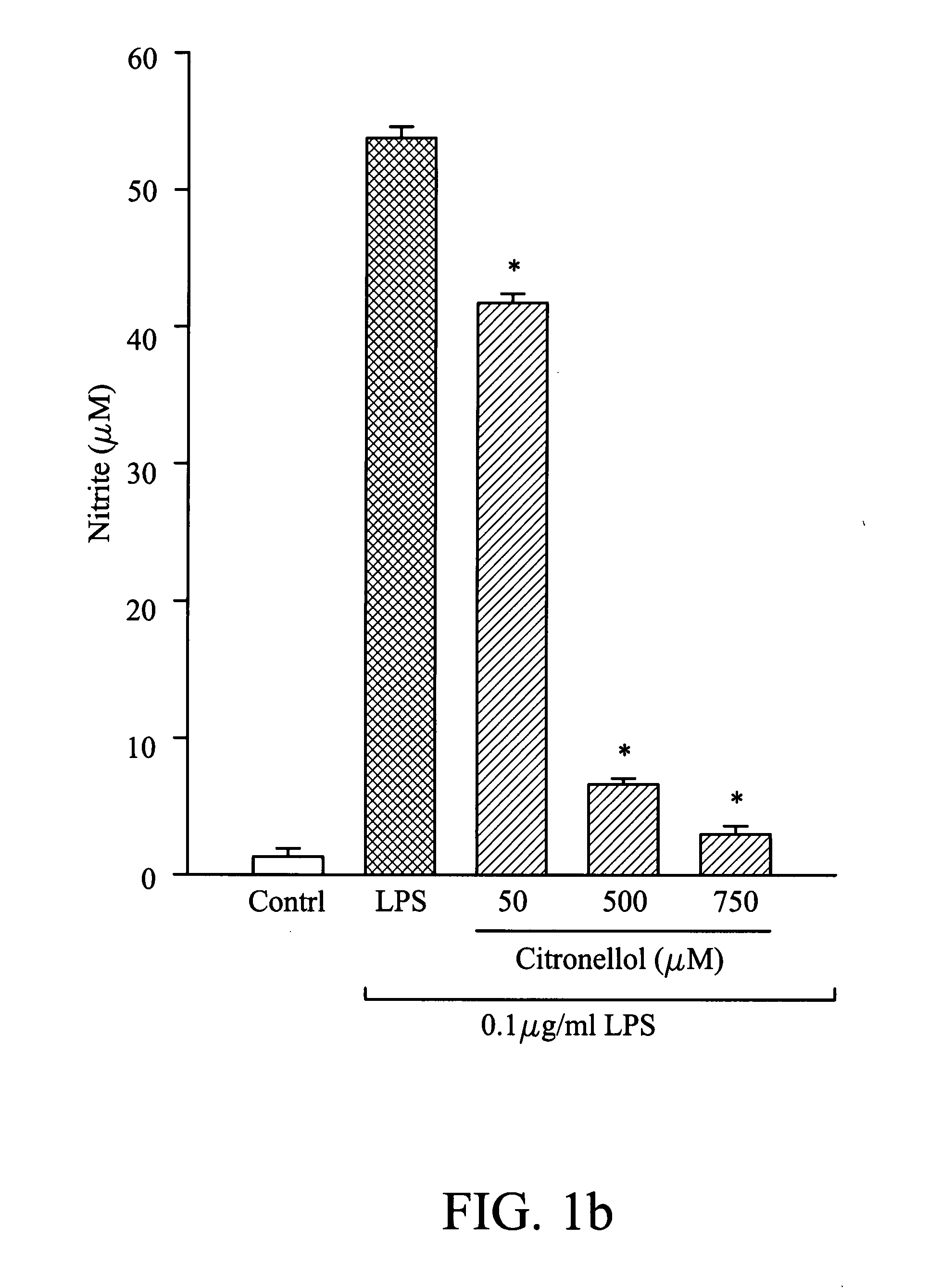
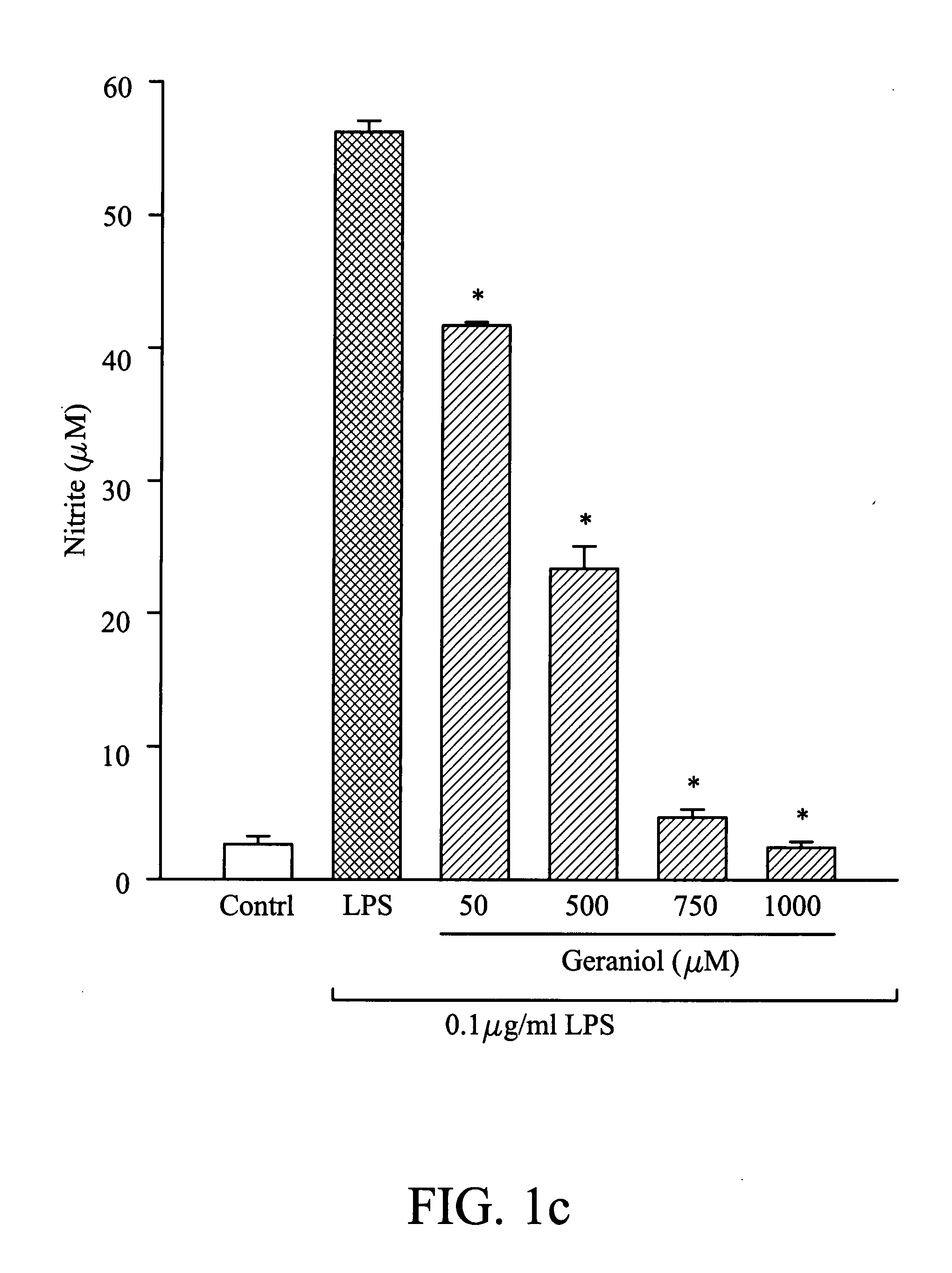
Composition for inhibiting nitric oxide and/or prostaglandin E2 synthesis and method for inhibiting inflammation
InactiveUS20080268078A1Reduce and relieve syndromeBiocideHydroxy compound active ingredientsNitric oxideProstaglandin E
A method for inhibiting nitric oxide and / or prostaglandin E2 synthesis. The method comprises administering a composition to a subject, wherein the composition comprises an effective amount of butylidene phthalide, citronellol, geraniol or combinations thereof, which can be used to reduce or relieve the syndromes of the inflammation.
Owner:YANGSEN BIOTECH
Methods for diagnosing irritable bowel syndrome
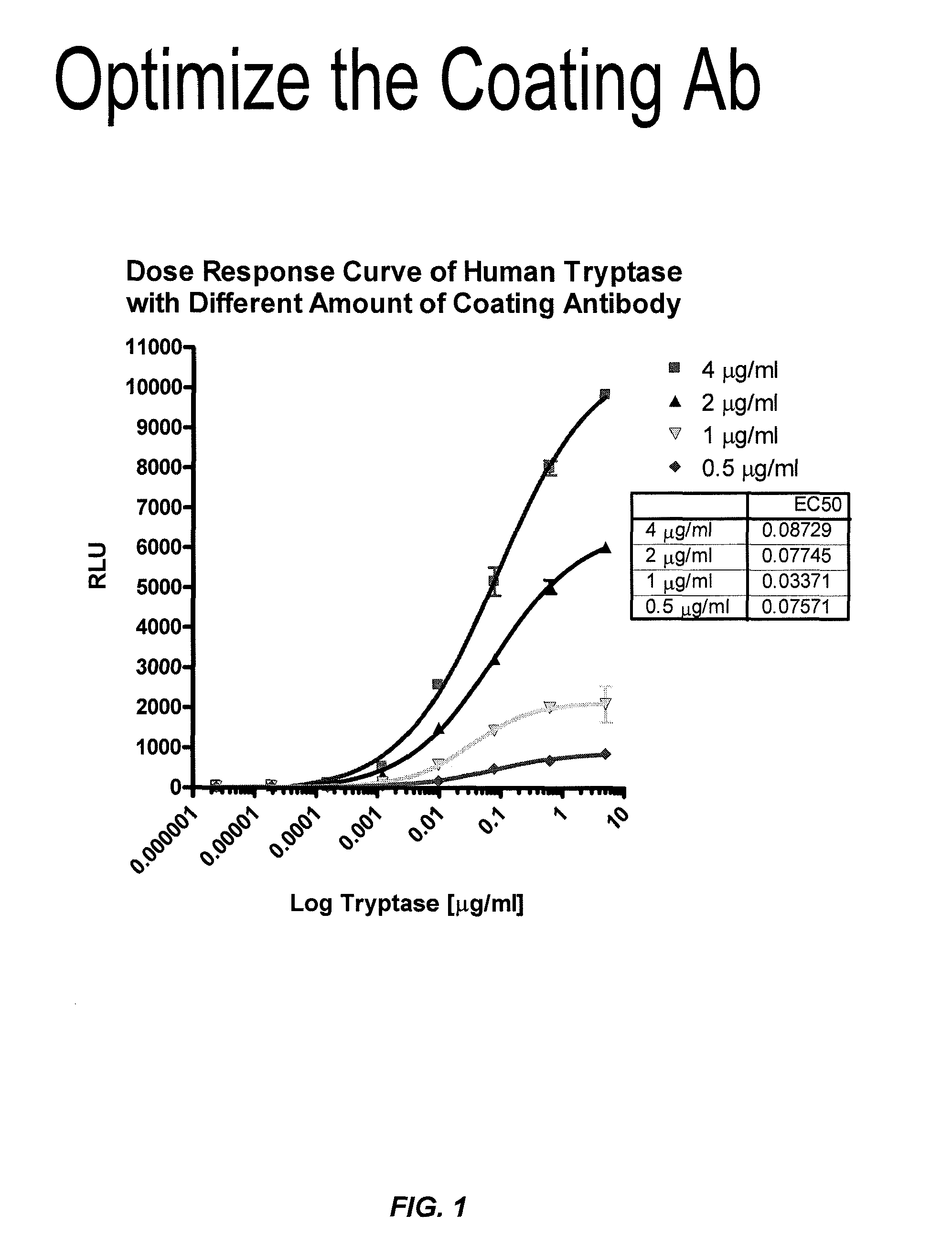
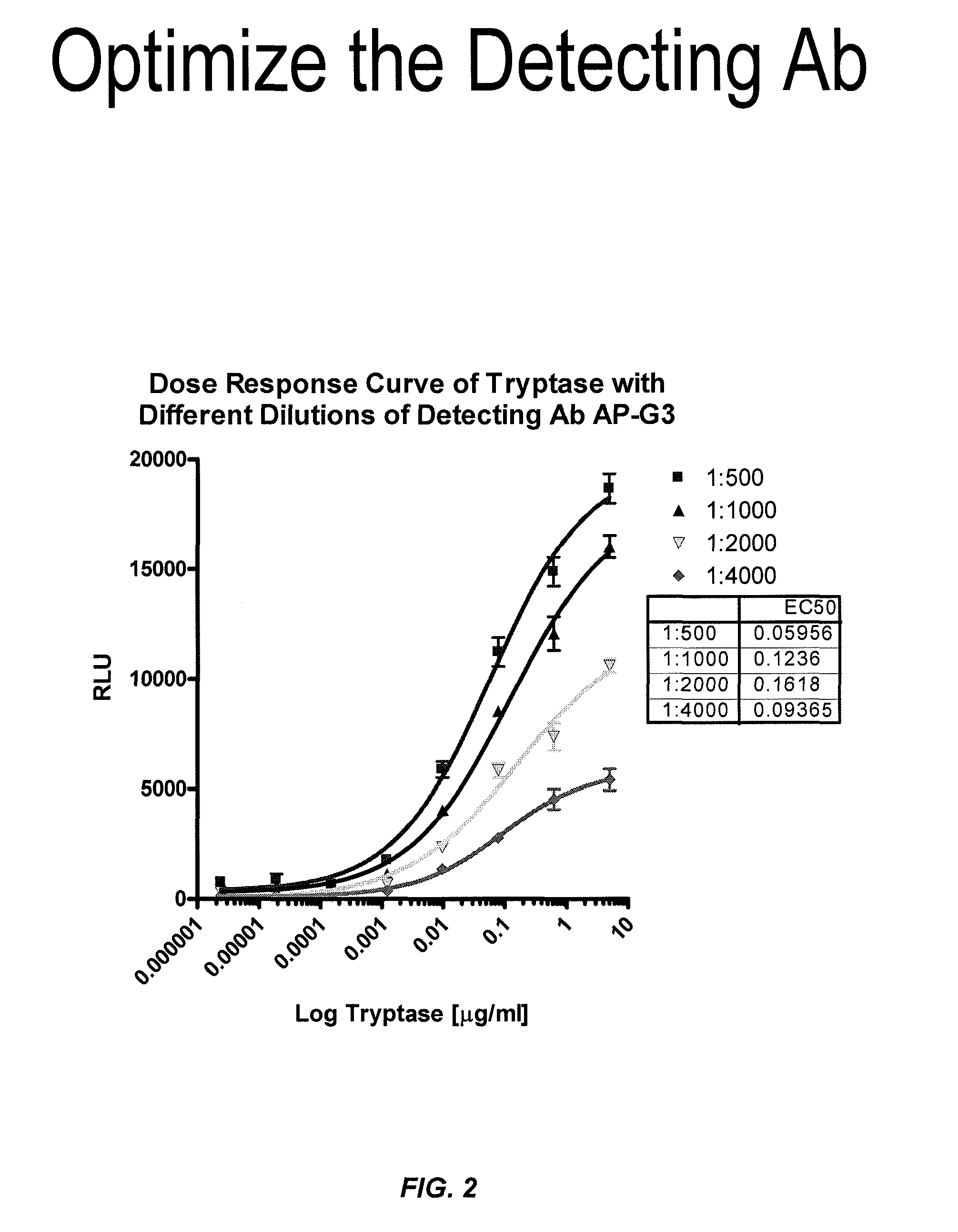
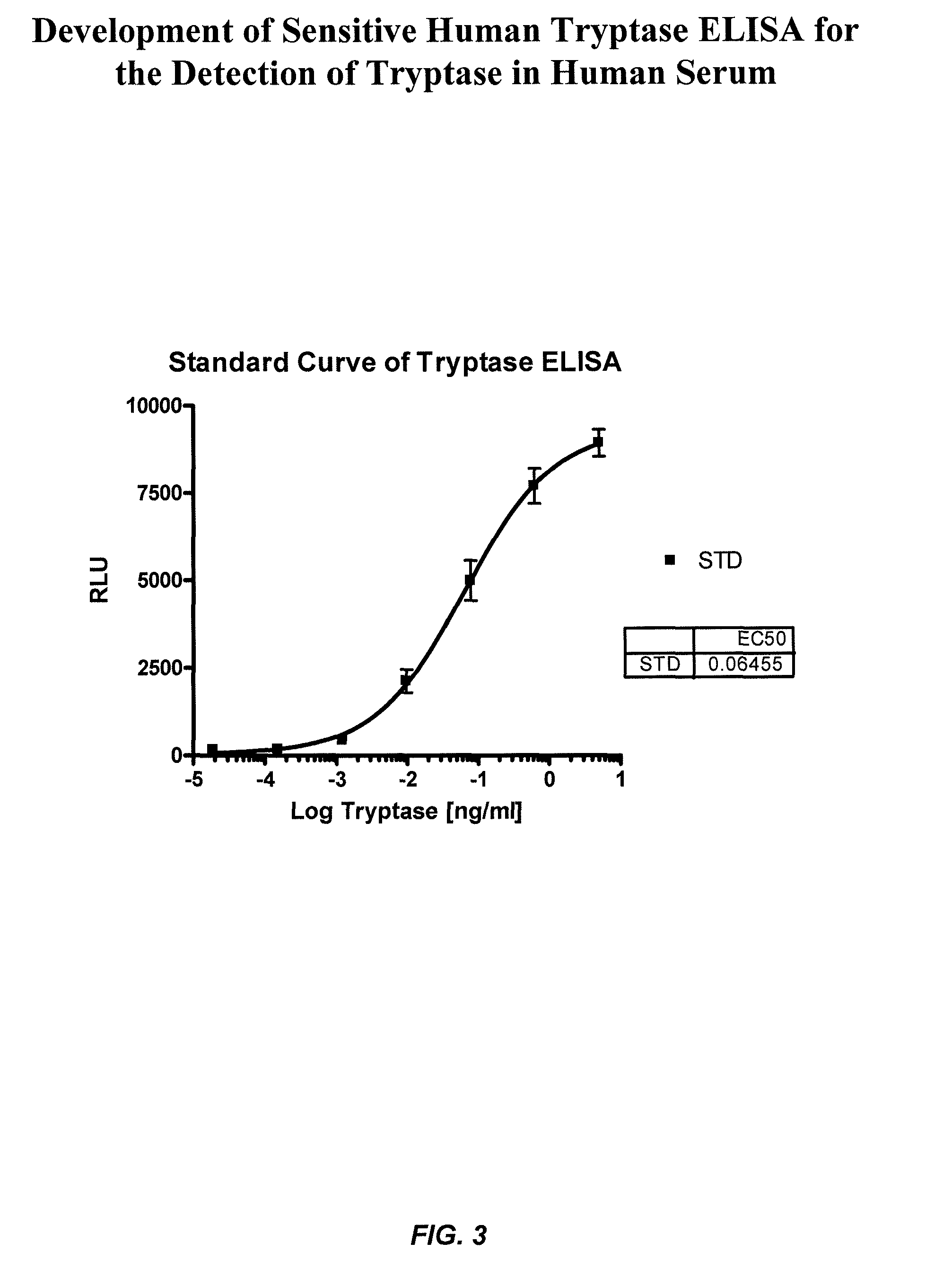
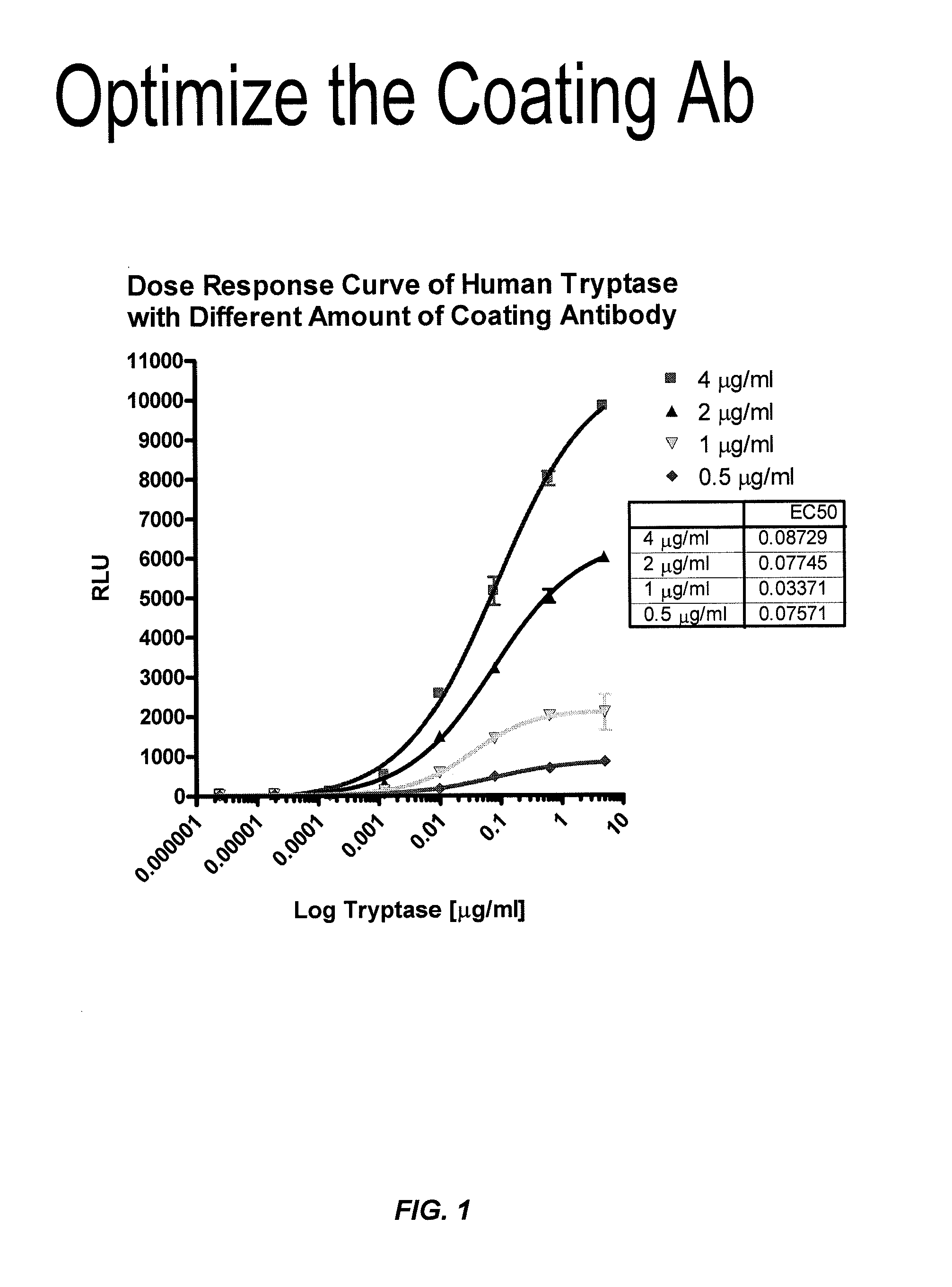
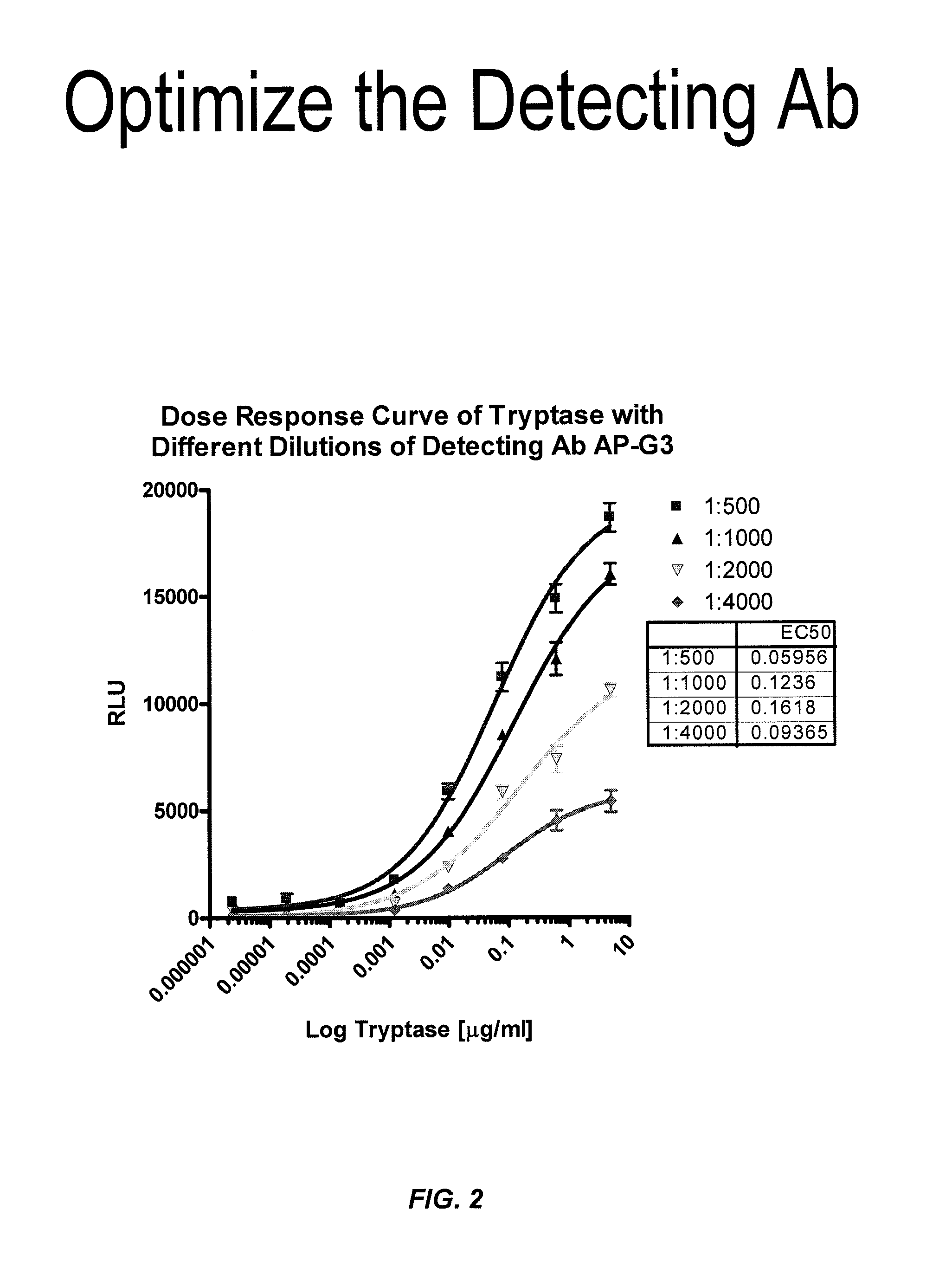
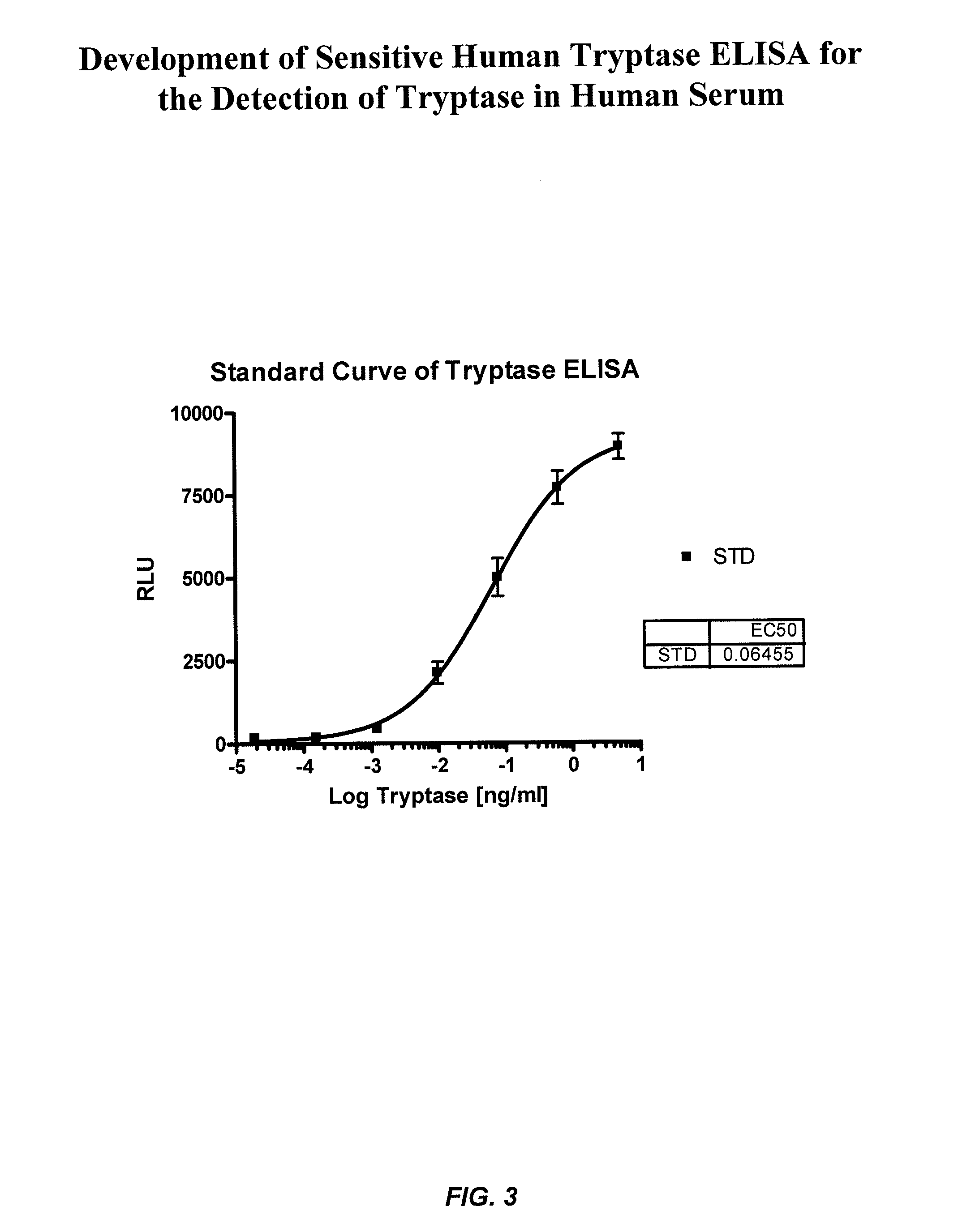
ActiveUS8114616B2Accurate diagnostic predictionBioreactor/fermenter combinationsBiological substance pretreatmentsPhysiologyCapture antibody
The invention provides an ELISA assay for the determination of serum mast cell β-tryptase levels using rabbit anti-tryptase as the capture antibody and alkaline phosphatase conjugated G3 as the detecting antibody. Luminescent substrate CPSD was used to enhance the assay sensitivity. Also provided are methods for aiding in the diagnosis of irritable bowel syndrome by detecting the serum level of β-tryptase, histamine and / or prostaglandin E2.
Owner:PROMETHEUS BIOSCIENCES INC
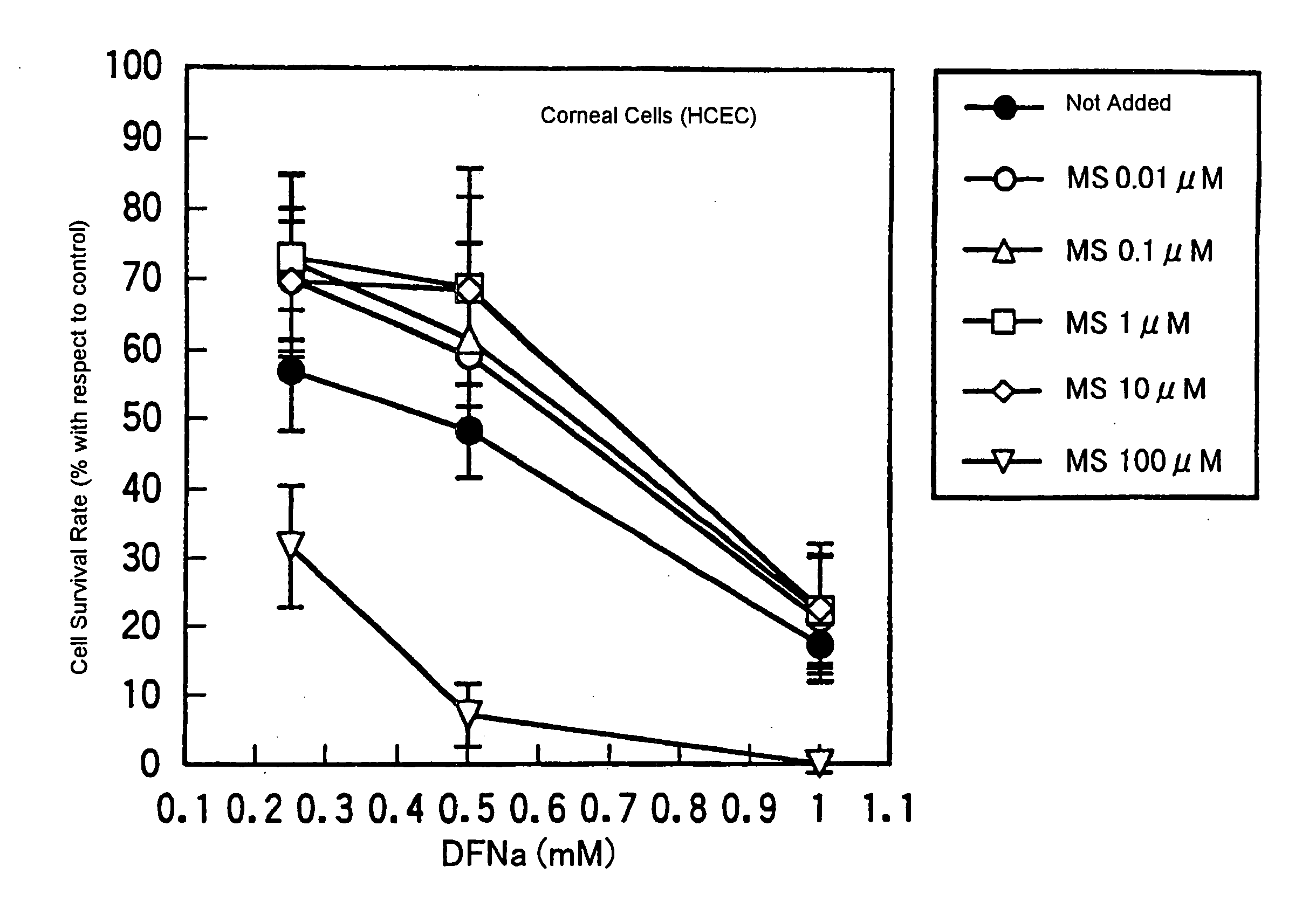
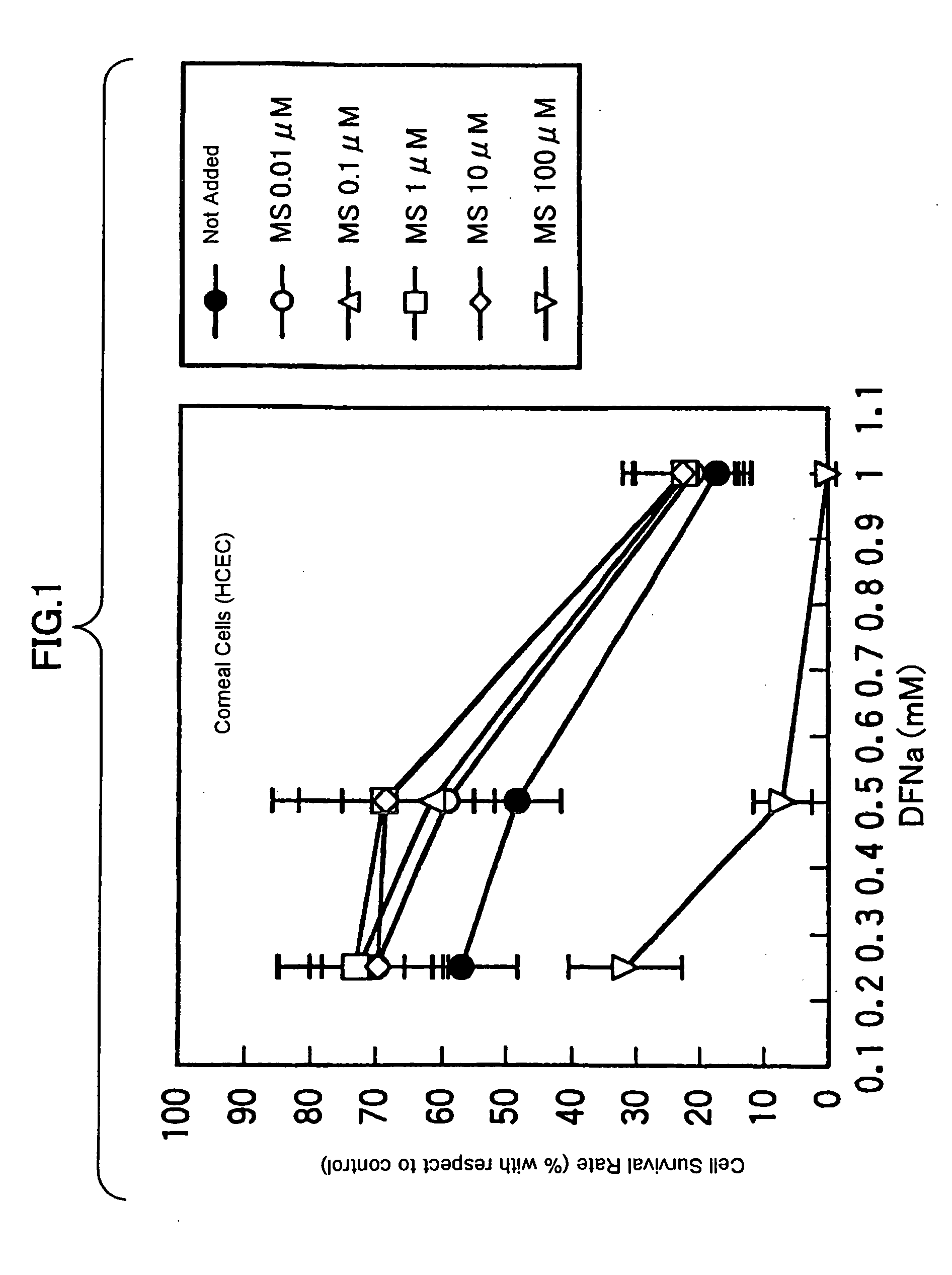
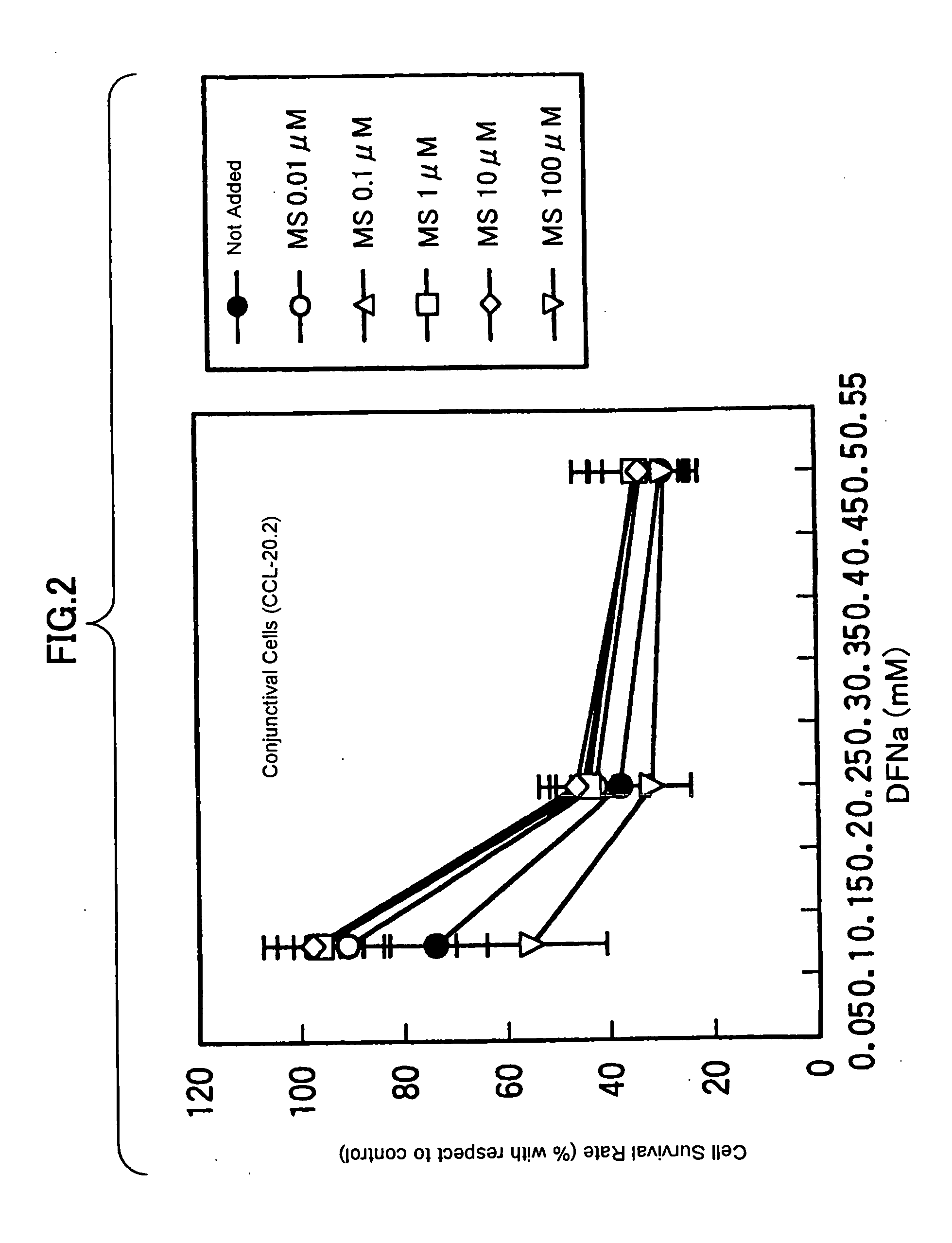
Drugs for treating or preventing disorders of corneal and/or conjunctival epithelial cells
Owner:WAKAMOTO PHARMA
Methods for diagnosing irritable bowel syndrome
ActiveUS20110159521A1Accurate classificationAccurate predictionBioreactor/fermenter combinationsBiological substance pretreatmentsCapture antibodyProstaglandin E2
The invention provides an ELISA assay for the determination of serum mast cell β-tryptase levels using rabbit anti-tryptase as the capture antibody and alkaline phosphatase conjugated G3 as the detecting antibody. Luminescent substrate CPSD was used to enhance the assay sensitivity. Also provided are methods for aiding in the diagnosis of irritable bowel syndrome by detecting the serum level of β-tryptase, histamine and / or prostaglandin E2.
Owner:PROMETHEUS BIOSCI INC
Novel compounds
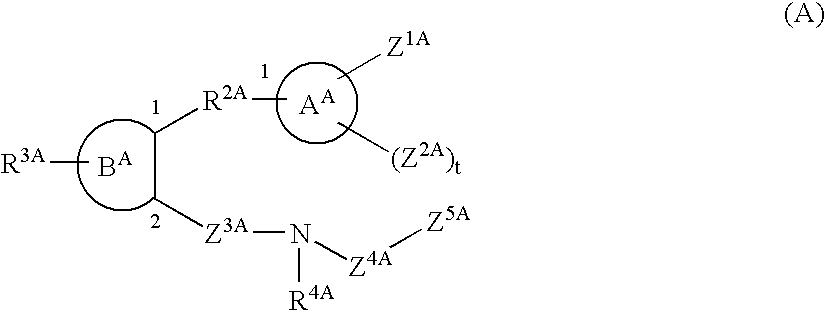
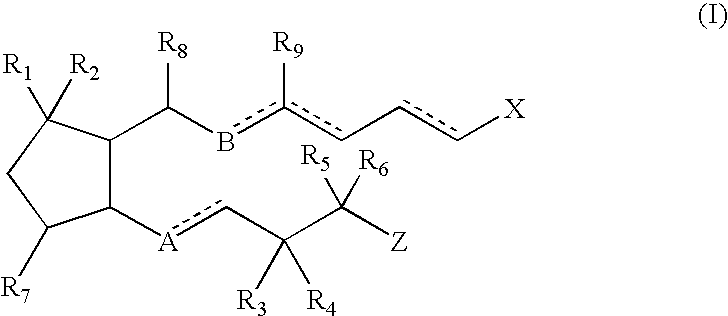
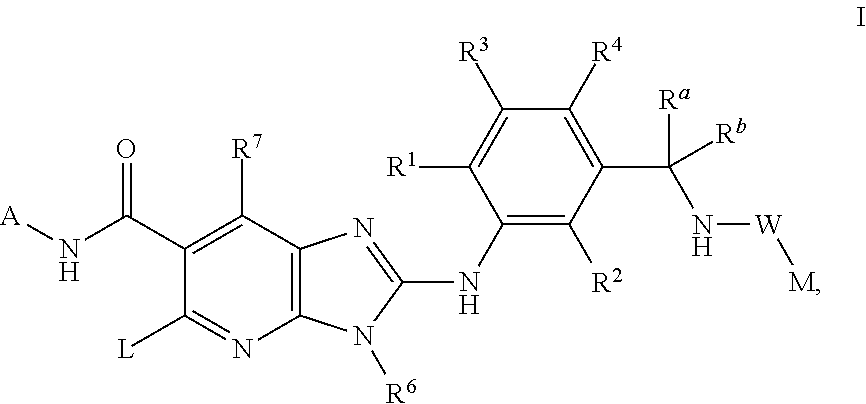
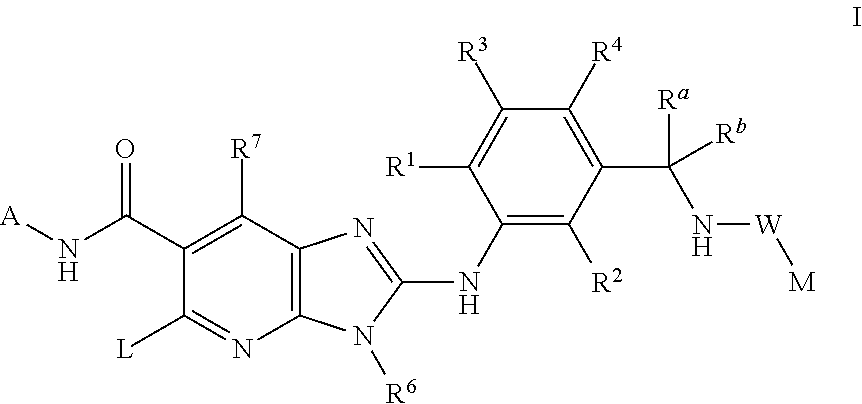
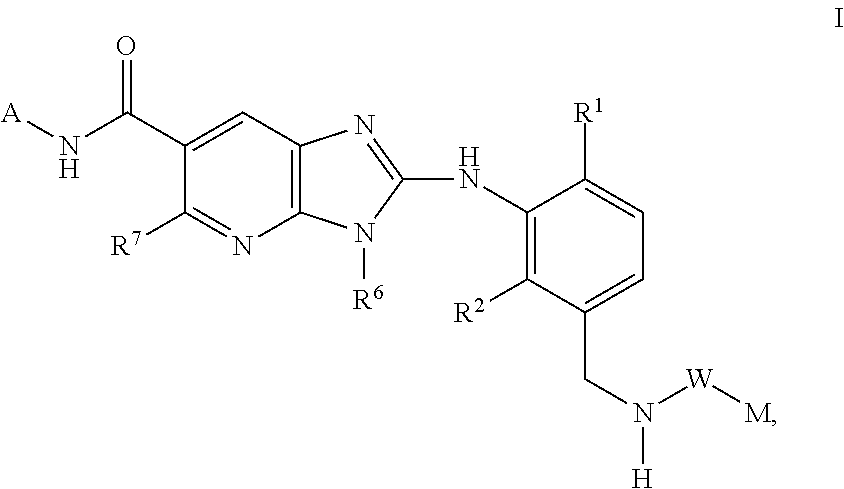
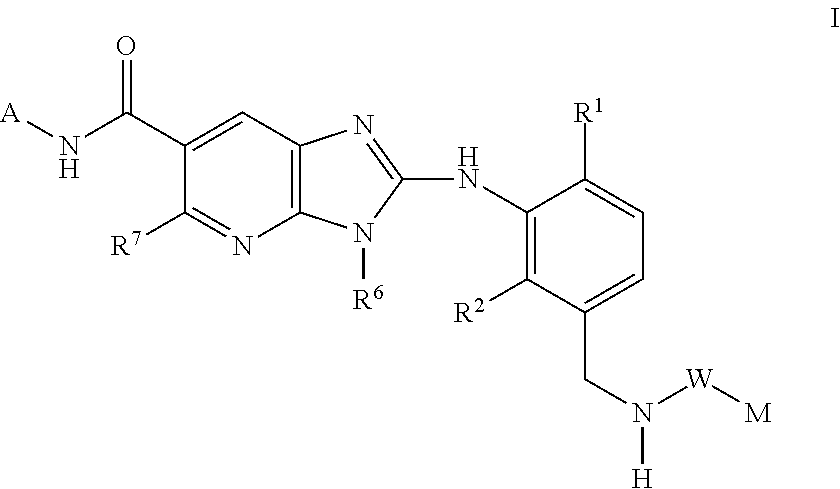
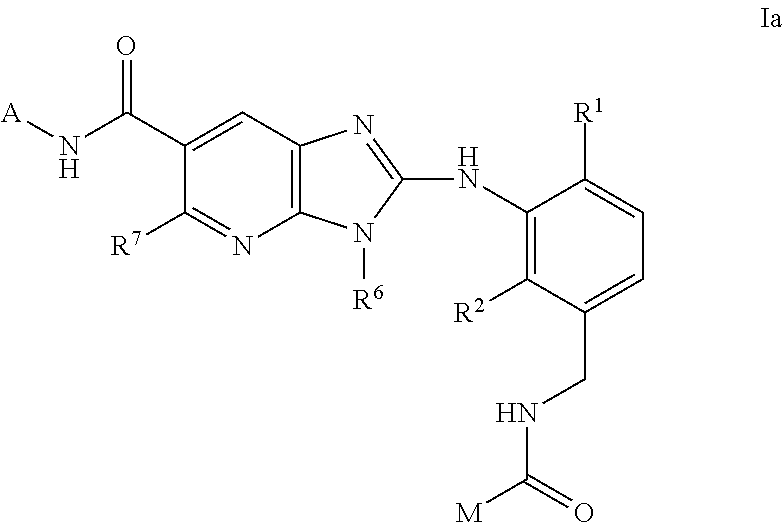
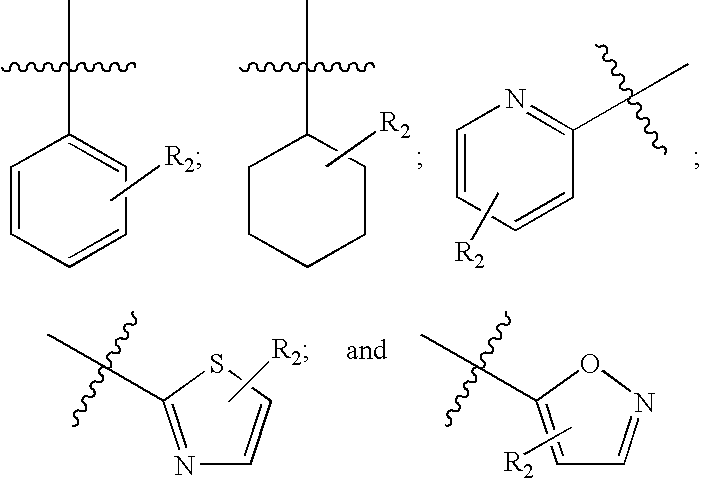
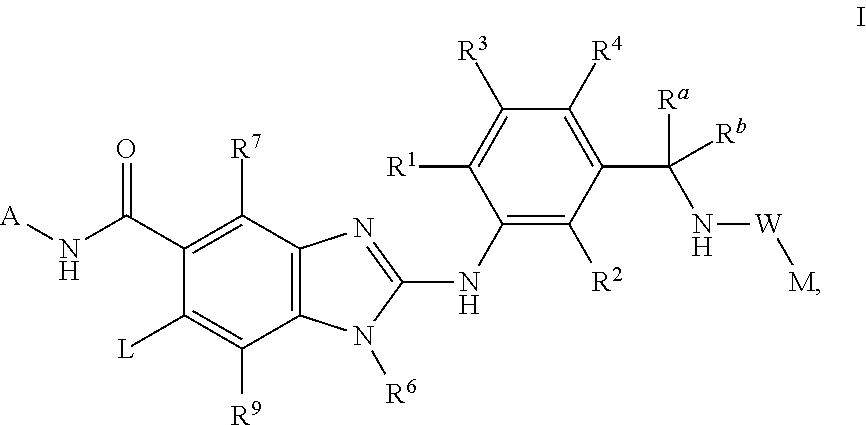
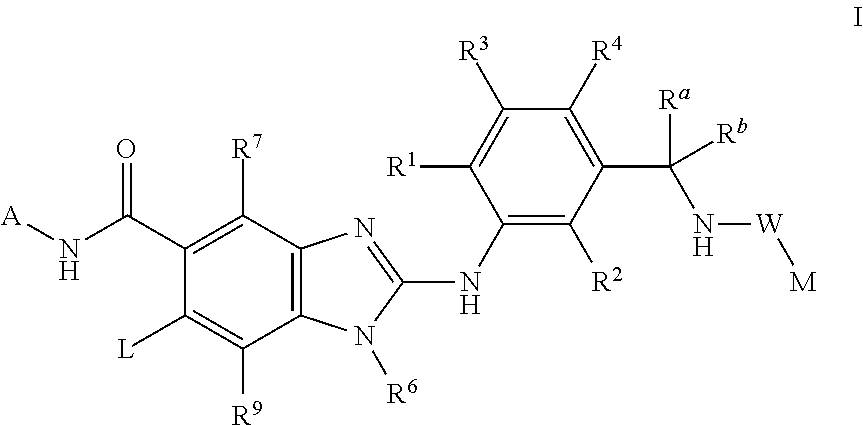
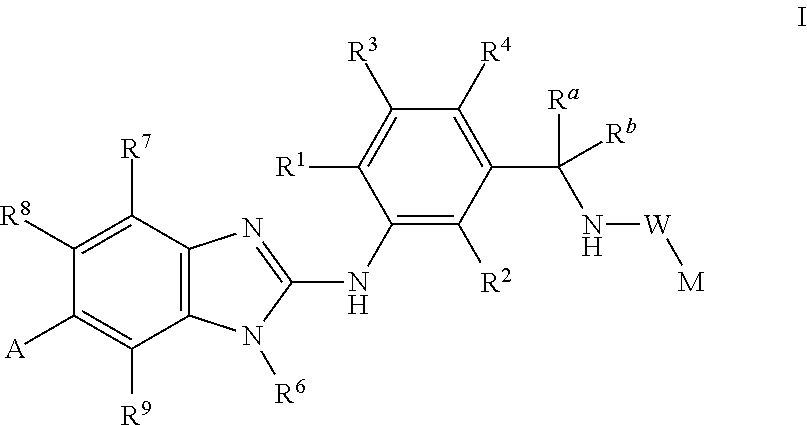
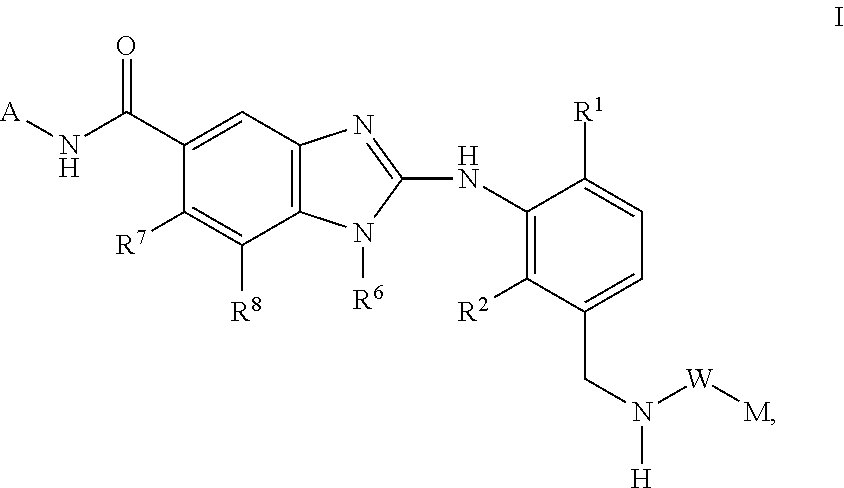
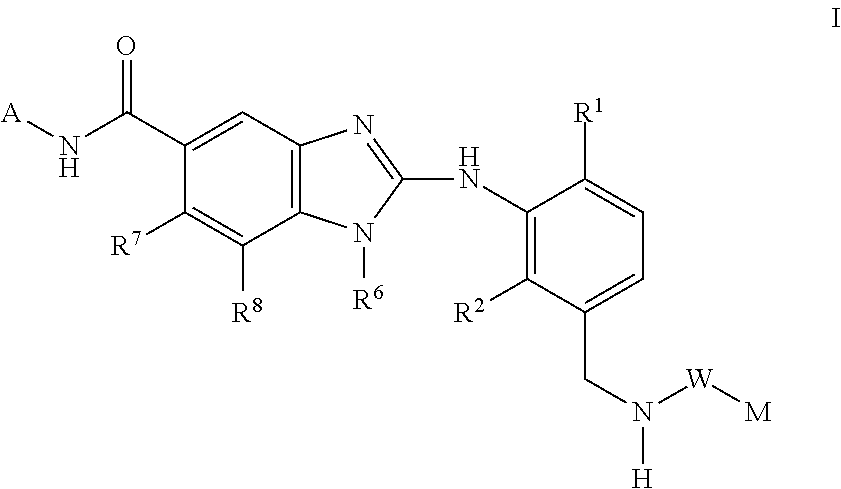
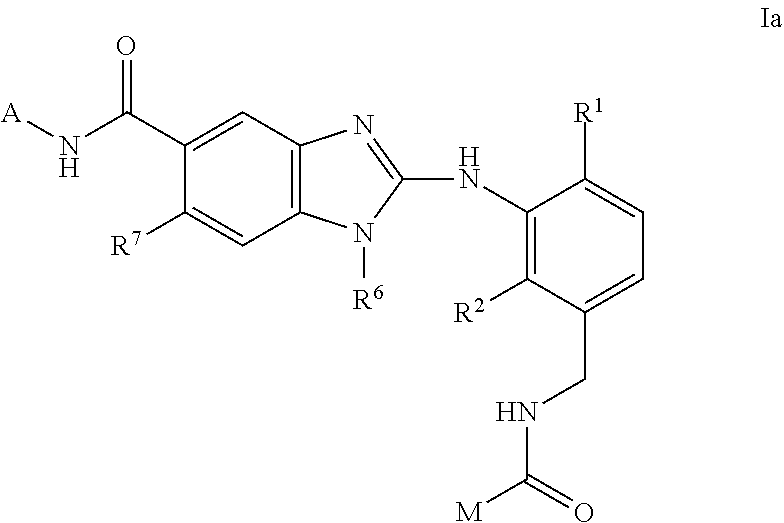
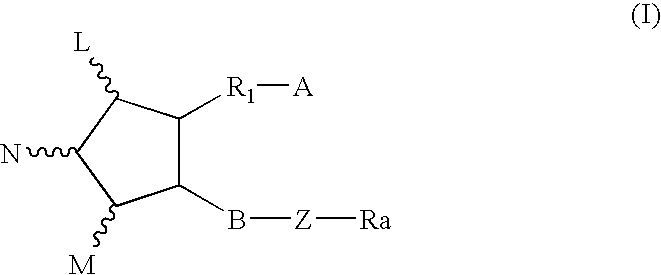
This invention relates to compounds of formula Itheir use as inhibitors of the microsomal prostaglandin E2 synthase-1 (mPGES-1), pharmaceutical compositions containing them, and their use as medicaments for the treatment and / or prevention of inflammatory diseases and associated conditions. A, L, M, W, R1, R2, R3, R4, R6, R7, Ra, Rb have meanings given in the description.
Owner:BOEHRINGER INGELHEIM INT GMBH
Stabilized prostaglandin E composition
A prostaglandin E composition comprises are substantially free from C1-C4 alcohols and include the prostaglandin E compound together with a (C1-C4)-alkyl (C8-C22) carboxylic ester (e.g., ethyl laurate), an N,N-di(C1-C8) alkylamino substituted, (C4-C18) alkyl (C2-C18) carboxylic ester and / or a pharmaceutically acceptable addition salt thereof, and optionally, a viscosity enhancing agent such as guar gum. The prostaglandin E composition can be combined with an aqueous alcoholic diluent to form a pharmaceutical composition for topical application to a patient, for example, to treat sexual dysfunction. The prostaglandin E compositions are stable for prolonged periods of storage at room temperature.
Owner:NEXMED HLDG INC
New compounds
This invention relates to compounds of formula Itheir use as inhibitors of the microsomal prostaglandin E2 synthase-1 (mPGES-1), pharmaceutical compositions containing them, and their use as medicaments for the treatment and / or prevention of inflammatory diseases and associated conditions. A, M, W, R1, R2, R6, R7 have meanings given in the description.
Owner:BOEHRINGER INGELHEIM INT GMBH
Remedies for depression containing EP1 antagonist as the active ingredient
InactiveUS7335776B2Shorten immobility timeInhibition effectBiocideNervous disorderEndogenous depressionReactive Depression
A pharmaceutical composition for the treatment and / or prevention of depression comprising a compound having an antagonistic activity for EP1 receptor which a prostaglandin E2 receptor subtype.EP1 antagonist is useful for the treatment of depression, for example, endogenous depression, reactive depression, weatherability depression, neurological depressed state, the depressed state of brain organic mental disorder.
Owner:ONO PHARMA CO LTD
EP4 Receptor Agonist, Compositions and Methods Thereof
InactiveUS20090270395A1Elevated intraocular pressureOrganic active ingredientsSenses disorderOsteoblastElevated intraocular pressure
This invention relates to potent selective agonists of the EP4 subtype of prostaglandin E2 receptors, their use or a formulation thereof in the treatment of glaucoma and other conditions, which are related to elevated intraocular pressure in the eye of a patient. This invention further relates to the use of the compounds of this invention for mediating the bone modeling and remodeling processes of the osteoblasts and osteoclasts. The compounds of the present invention are the compounds of Formula (I).
Owner:MERCK & CO INC
Drug preparations for treating sexual dysfunction
Topical gelled compositions comprising a drug which causes vasodilation, and optionally prostaglandin E1, dispersed within a polymer matrix, and methods of treating sexual dysfunction, including both male and female sexual dysfunction, using said compositions.
Owner:GLYCOBIOSCI +1
Drug preparations for treating sexual dysfunction
InactiveUS20030138494A1Powder deliveryOrganic active ingredientsSexual dysfunctionDrugs preparations
Topical gelled compositions comprising a drug which causes vasodilation, and optionally prostaglandin E1, dispersed within a polymer matrix, and, methods of treating sexual dysfunction, including both male and female sexual dysfunction, using said compositions.
Owner:L A M PHARMA
Novel compounds
This invention relates to compounds of formula Itheir use as inhibitors of the microsomal prostaglandin E2 synthase-1 (mPGES-1), pharmaceutical compositions containing them, and their use as medicaments for the treatment and / or prevention of inflammatory diseases and associated conditions. A, L, M, W, R1, R2, R3, R4, R6, R7, R9, Ra, Rb have meanings given in the description.
Owner:BOEHRINGER INGELHEIM INT GMBH
Compound recipe formula containing kurarinone prostaglandin E1 and aspirin, its preparation method and application
InactiveCN1415301ANew formulaExact therapeutic effectSalicyclic acid active ingredientsDigestive systemIsoprostaglandin E1Freeze-drying
A compound medicine containing kurarinol, prostaglandic E1 and aspirin is prepared through including the kurarinol and prostaglandin E1 by 6-0-malto-beta-cyclodextrin, mixing, adding others, and preparing the freeze dried powder injection. It can be used for treating cancers, cardiovascular and cerebrovascular diseases and hepatitis. Its advantages are sure curative effect, and no toxic by-effect.
Owner:蔡海德
Novel compounds
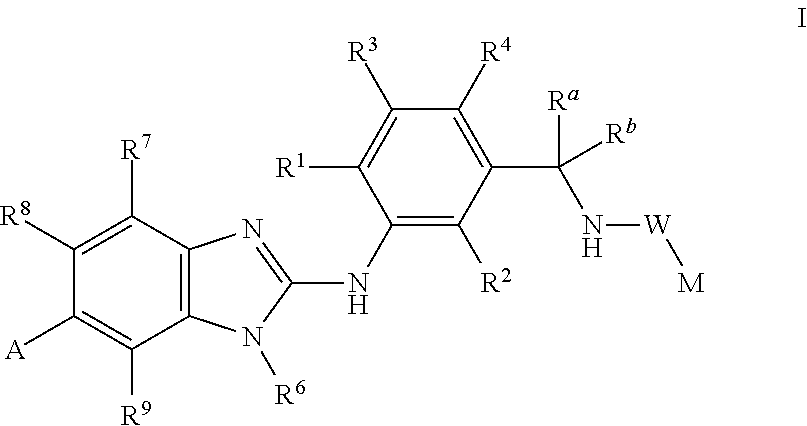
This invention relates to compounds of formula Itheir use as inhibitors of the microsomal prostaglandin E2 synthase-1 (mPGES-1), to pharmaceutical compositions containing them, and their use as medicaments for the treatment and / or prevention of inflammatory diseases and associated conditions. A, M, W, R1, R2, R3, R4, R6, R2, R7, R8, R9, Ra, Rb have meanings given in the description.
Owner:BOEHRINGER INGELHEIM INT GMBH
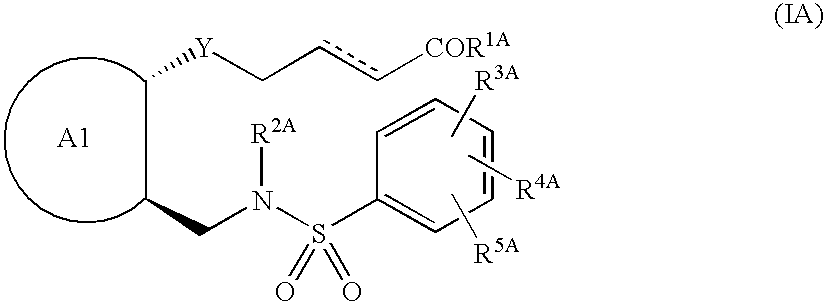
New compounds
This invention relates to compounds of formula Itheir use as inhibitors of the microsomal prostaglandin E2 synthase-1 (mPGES-1), pharmaceutical compositions containing them, and their use as medicaments for the treatment and / or prevention of inflammatory diseases and associated conditions. A, M, W, R1, R2, R6, R7, R8 have meanings given in the description.
Owner:GESYNTA PHARMA AB
Anti-inflammatory and anti-HIV compositions and methods of use
InactiveUS7854946B1Reduces HIV infectionReduce synthesisBiocideAnimal repellantsDiseaseCyclooxygenase
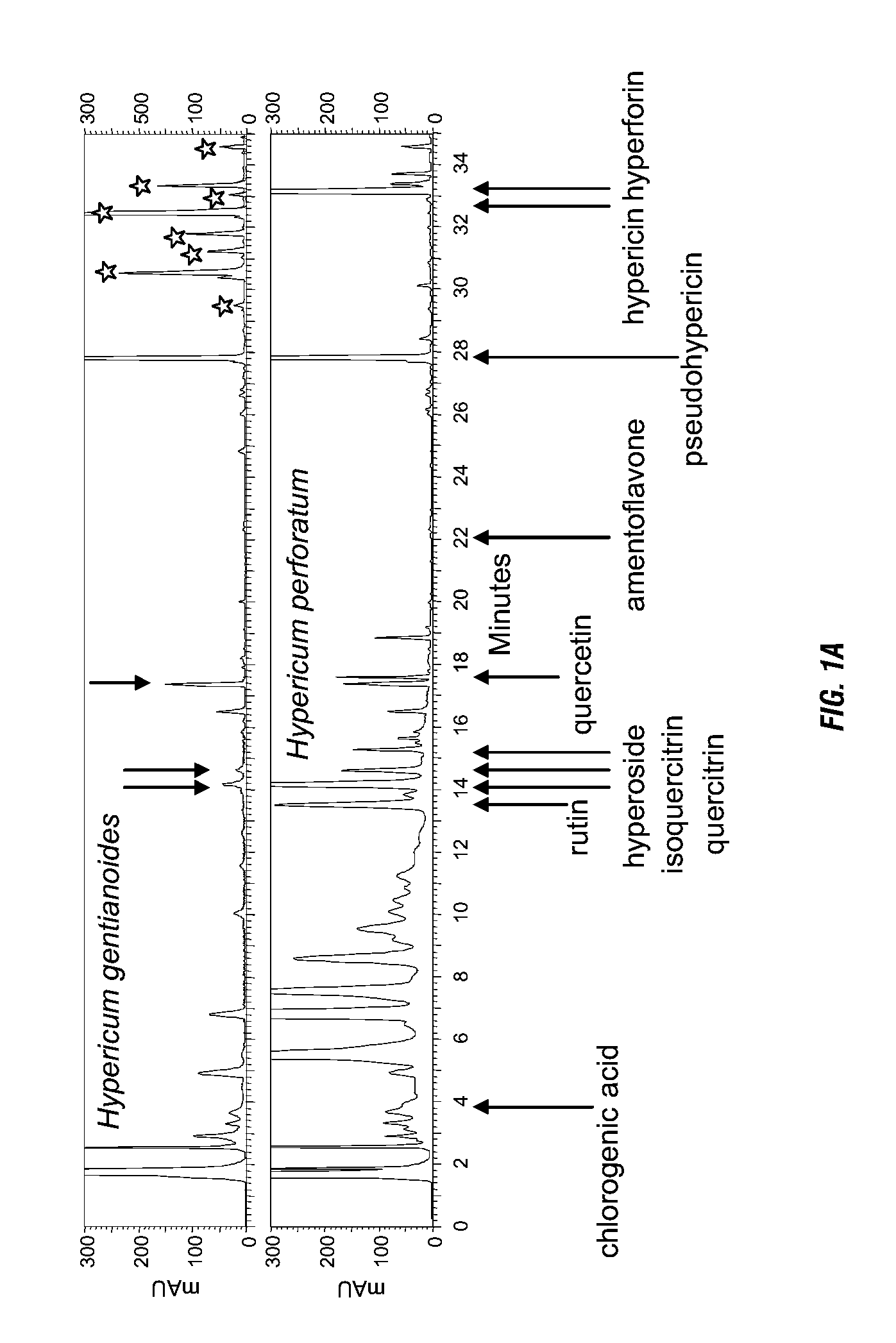
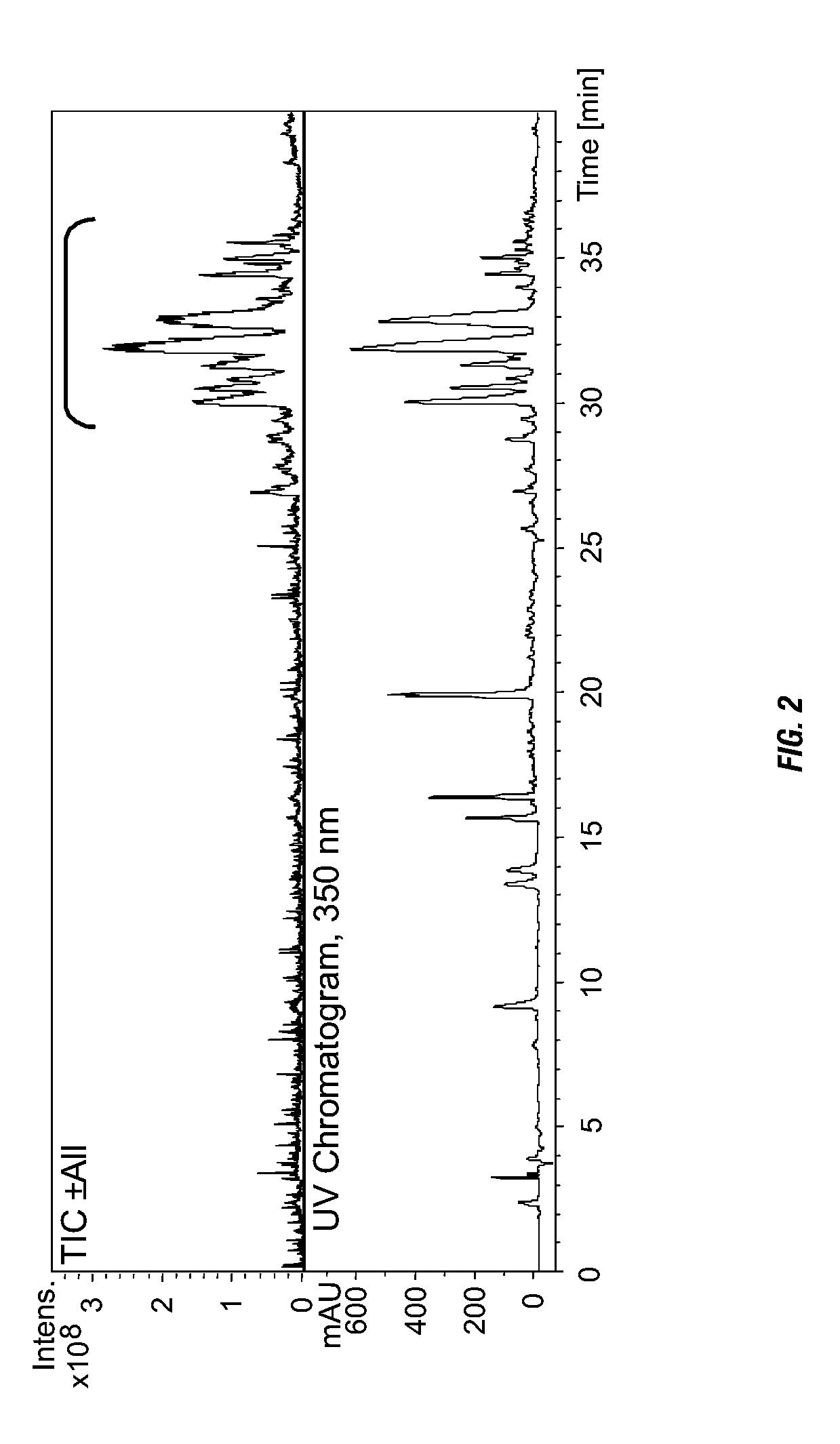
The metabolic fingerprint and anti-inflammatory activity and anti-HIV activity of H. gentianoides is disclosed. High performance liquid chromatography (HPLC) analysis shows that H. gentianoides contains a family of compounds, including some not previously observed in other Hypericum species. H. gentianoides extracts and fractions from these extracts reduce prostaglandin E2 synthesis in mammalian macrophages and inhibit HIV in infected HeLa cells. The present invention provides extracts and fractions thereof from H. gentianoides for use in pharmaceutical compositions and methods for the treatment or inhibition of inflammation, prostaglandin E-mediated disease, disorder or condition, a cyclooxygenase-mediated disease, disorder or condition, or an HIV infection.
Owner:IOWA STATE UNIV RES FOUND
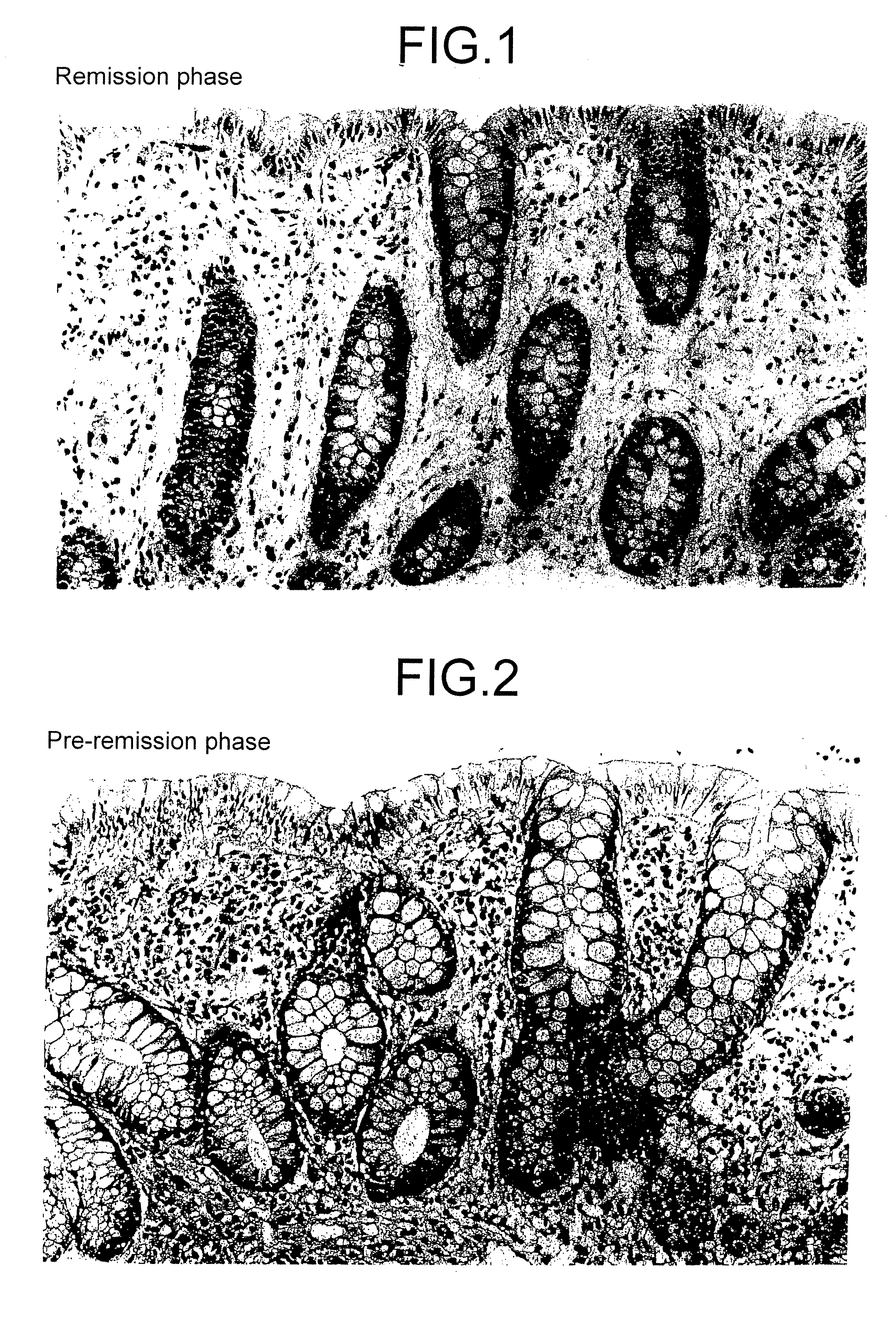
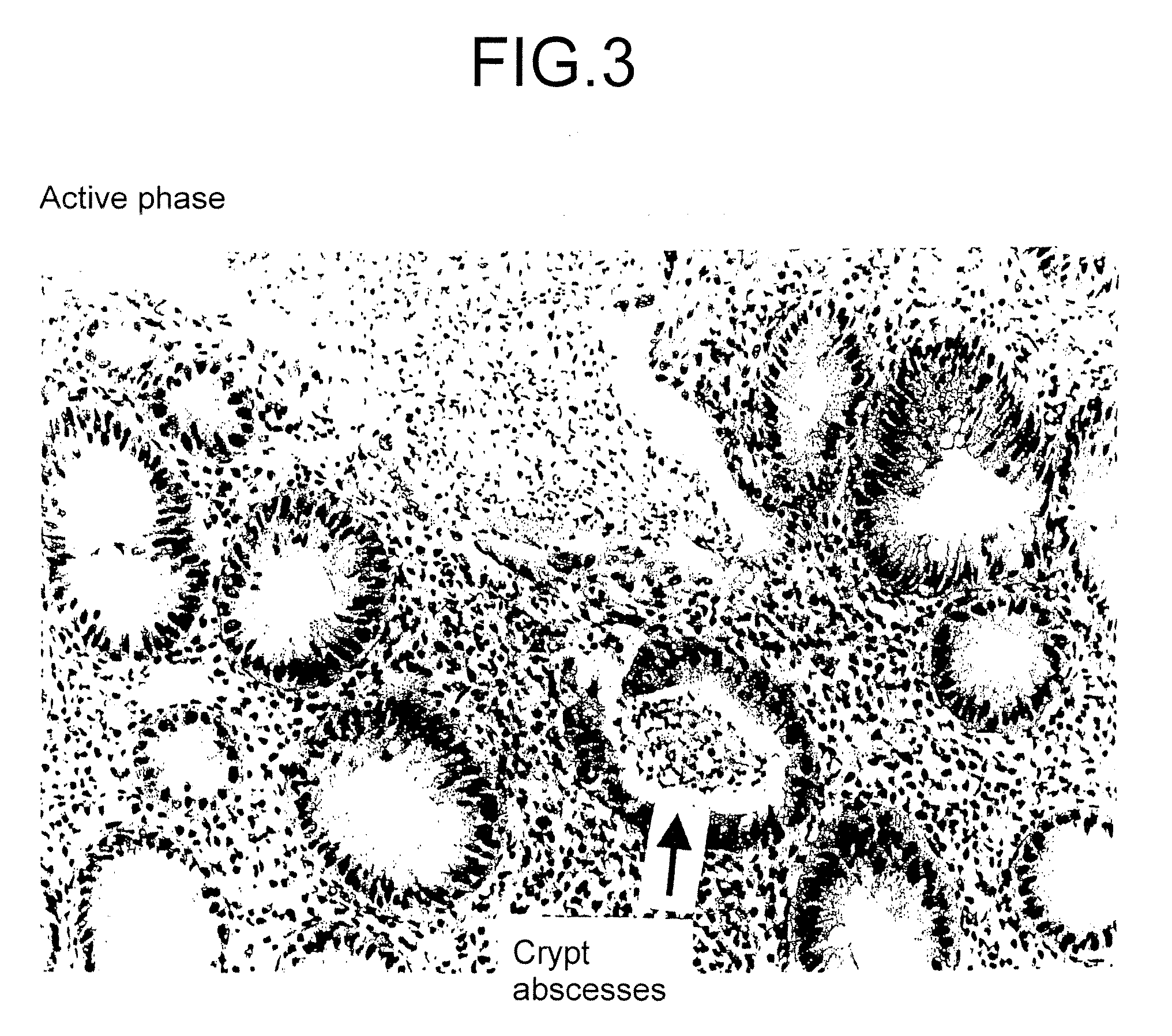
Method for clinical staging of ulcerative colitis or interstitial pneumonia and reagent kit for the same
ActiveUS20090130775A1Simple discriminationImprove the quality of lifeDisease diagnosisBiological testingMetaboliteFlexible endoscope
The invention provides a method capable of readily discriminating pathologic conditions and judging selection of a therapeutic drug, the degree of the therapeutic effect, discontinuation of medication, etc., wherein stages quantitatively judged by digitizing substances contained in urine, which is different from conventional methods for judging stages of an ulcerative colitis and an interstitial pneumonitis which are performed by observation of mucous lesions with endoscopy requiring the skill or by analysis of histological samples collected from the living body.The method measures the value of main metabolites of prostaglandin E (PGE-MUM) concentration in urine and judges stages between the pre-remission phase of and the remission phase of ulcerative colitis.The method also measures the value of the PGE-MUM concentration in urine and judges stages between the active phase and the non-active phase of interstitial pneumonitis.
Owner:MUTSUNORI FUJIWARA +3
Method for treating abdominal discomfort
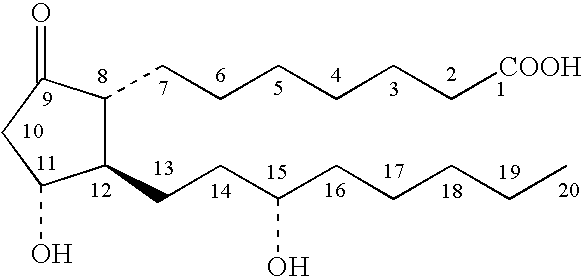
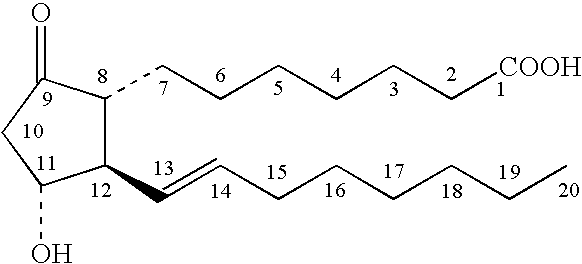
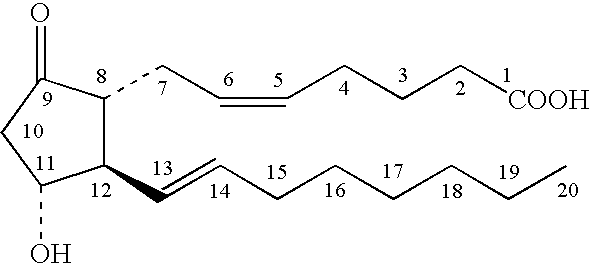
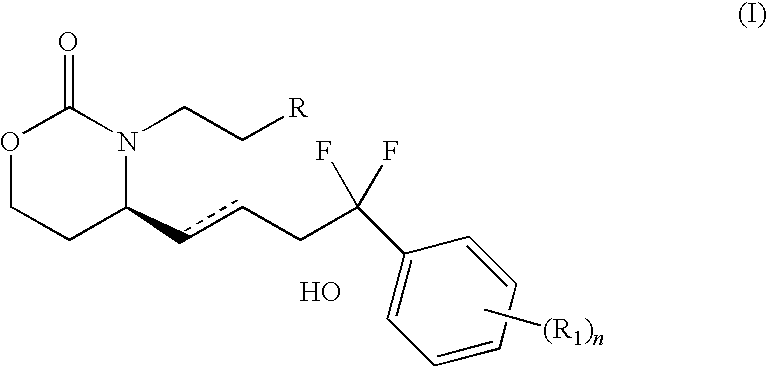
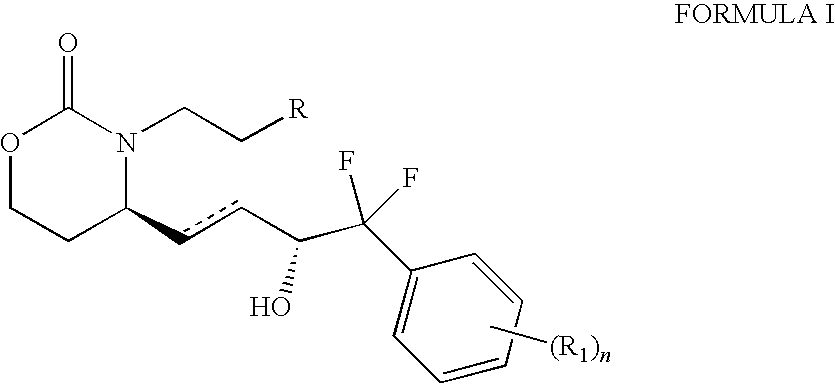
A method for treating irritable bowel syndrome in a mammalian subject includes administering an effective amount of 13,14-dihydro-15-keto-16,16-difluoro-18-methyl-prostaglandin E1 or 13,14-dihydro-15-keto-16,16-difluoro-prostaglandin E1, or a salt, ether, ester or amide thereof, to the subject. A method for treating abdominal discomfort associated with irritable bowel syndrome in a mammalian subject includes administering an effective amount of 13,14-dihydro-15-keto-16,16-difluoro-18-methyl-prostaglandin E1 or 13,14-dihydro-15-keto-16,16-difluoro-prostaglandin E1, or a salt, ether, ester or amide thereof, to the subject.
Owner:SUCAMPO
Method(s) of preventing, arresting, reversing and treatment of atherosclerosis
InactiveUS20080279925A1Increase contentPrevention arrestBacteriaPeptide/protein ingredientsGene deliveryLipid formation
A method of preventing, arresting, reversing and treatment of atherosclerosis by enhancing the activities of Δ6 and Δ5 desaturases such that the cell, tissue, and plasma levels of various polyunsaturated fatty acids (PUFAs) and, in particular that of endothelial cells lining the blood vessels will increase. More particularly, the invention is directed to enhance the activities of Δ6 and Δ5 desaturases that, in turn, will lead to increased production of products of polyunsaturated fatty acids such as prostacyclin (PGI2), PGI3, lipoxins, resolvins, protecting, PGE1 (prostaglandin E1), nitric oxide, and nitrolipids such that atherosclerotic process will be prevented, arrested, reversed and this will lead to efficient arrest, regression, prevention and / or treatment of even established atherosclerosis and its associated conditions. More particularly, the invention is directed to the delivery of proteins, peptides, lipids, lipoproteins, glycolipids, synthetic chemicals such as statins and their derivatives, troglitazones and their derivatives, and other compounds (synthetic or natural) that have the ability to enhance the activities of Δ6 and Δ5 desaturases in vivo, also provides methods of efficiently delivering cDNA clones of Δ6 and Δ5 desaturases and genes of Δ6 and Δ5 desaturases to endothelial cells and other target cells to prevent, arrest, reverse and treat atherosclerosis.
Owner:DAS UNDURTI N
Piperidinyl prostaglandin E analogs
The present invention provides a method of treating ocular hypertension or glaucoma which comprises administering to an animal having ocular hypertension or glaucoma therapeutically effective amount of a compound represented by the general formula I;wherein X, Y, Z, D and R3 are as defined in the specification.Also disclosed are compounds comprisingor a pharmaceutically acceptable salt or a prodrug thereof;wherein A, X, J, and R3 are as defined in the specification.Also disclosed are compounds having an α and an ω chain comprisingor derivatives thereof,as defined in the specificationor pharmaceutically acceptable salts or prodrugs thereof.
Owner:ALLERGAN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com