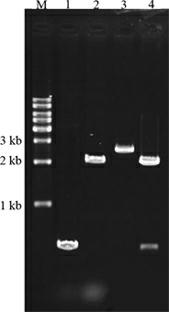
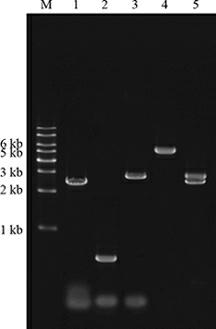
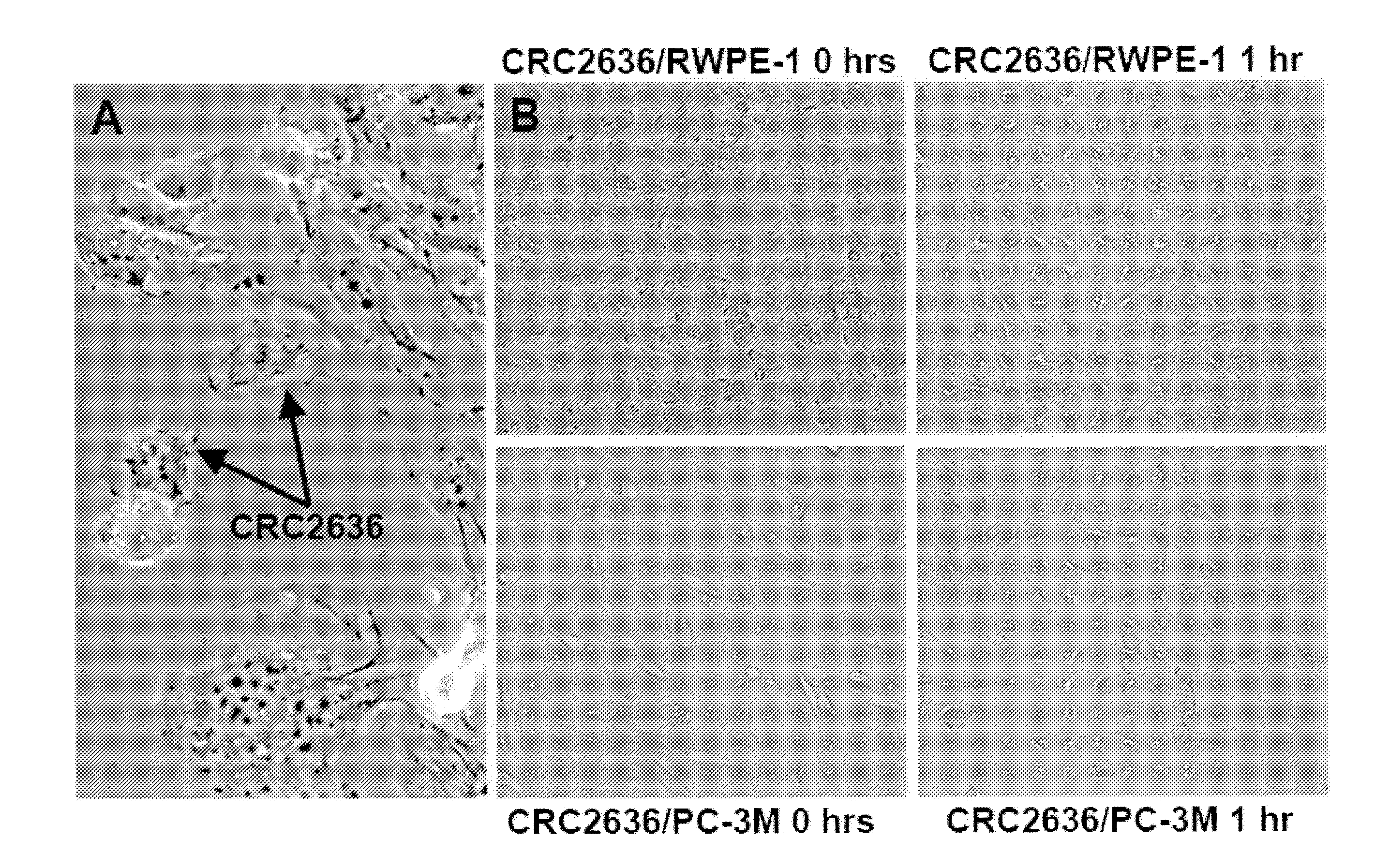
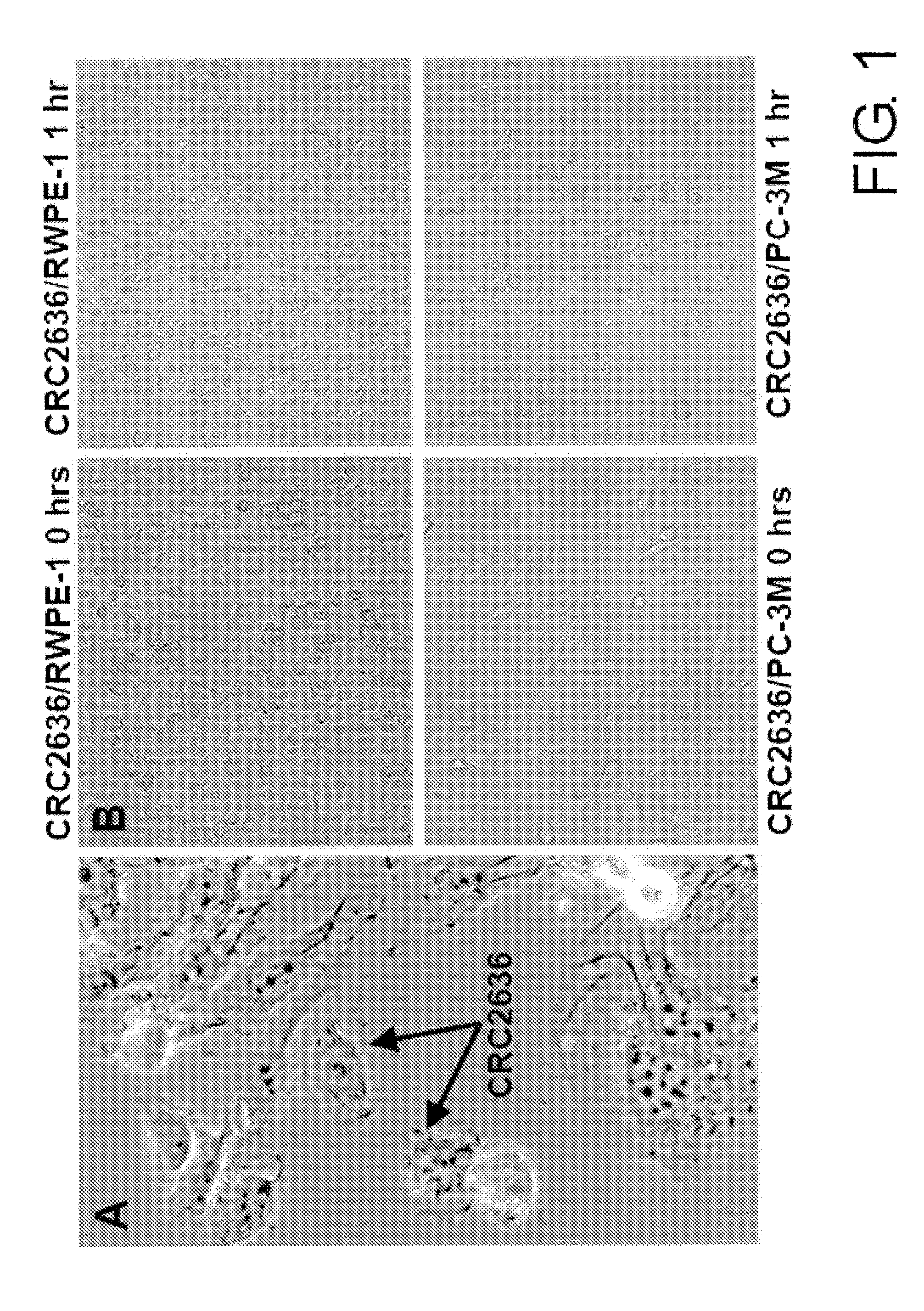
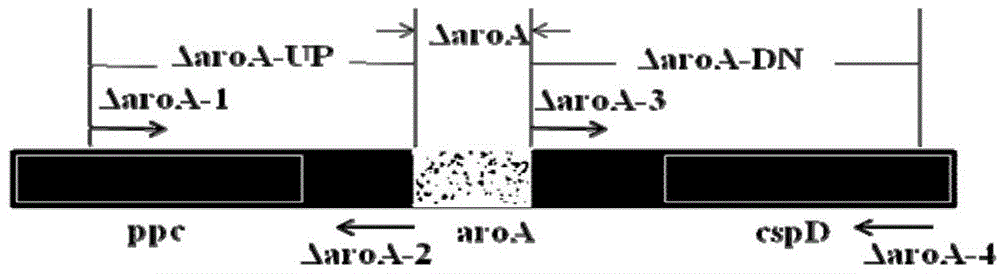
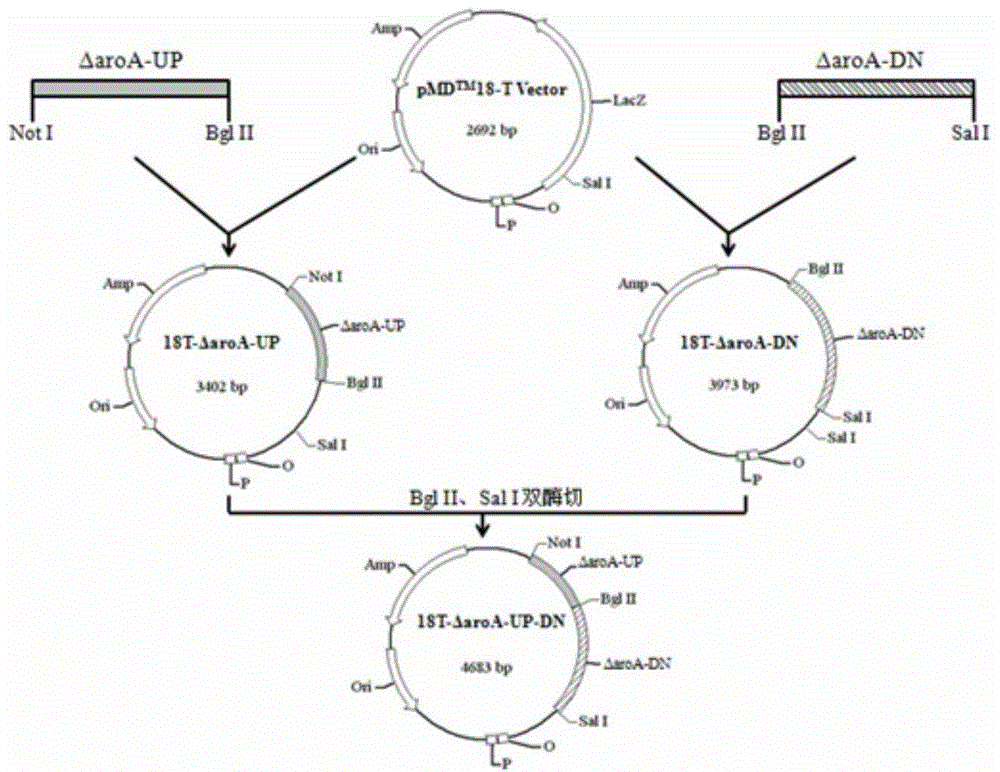
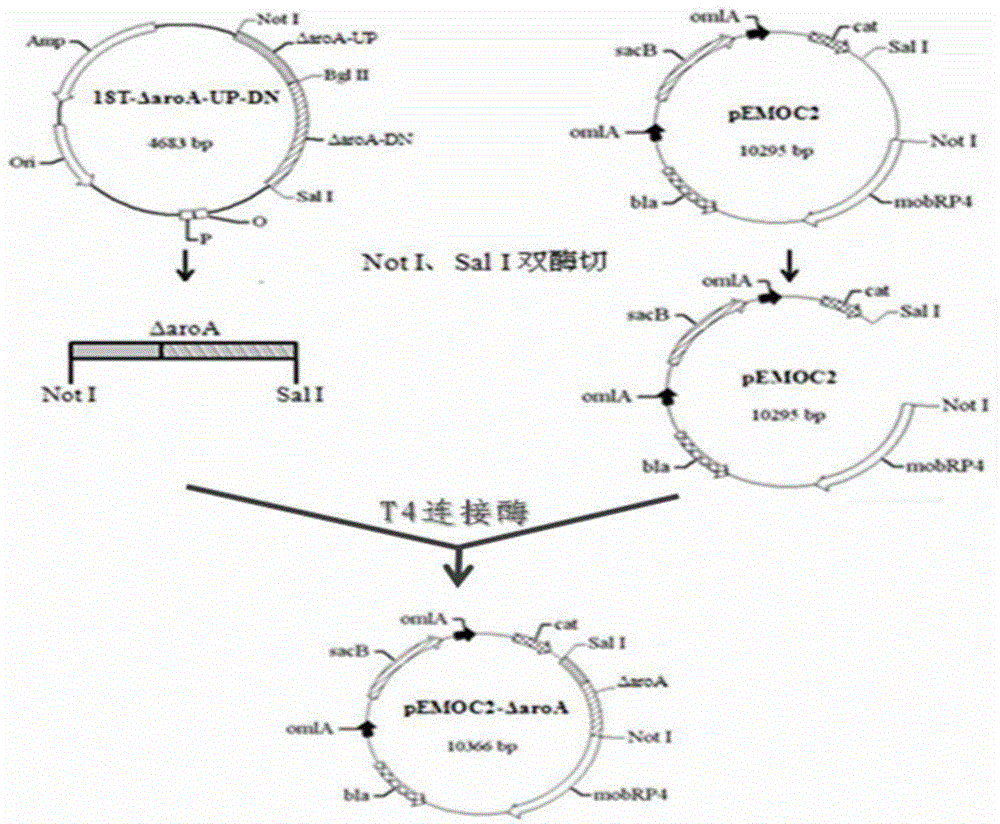
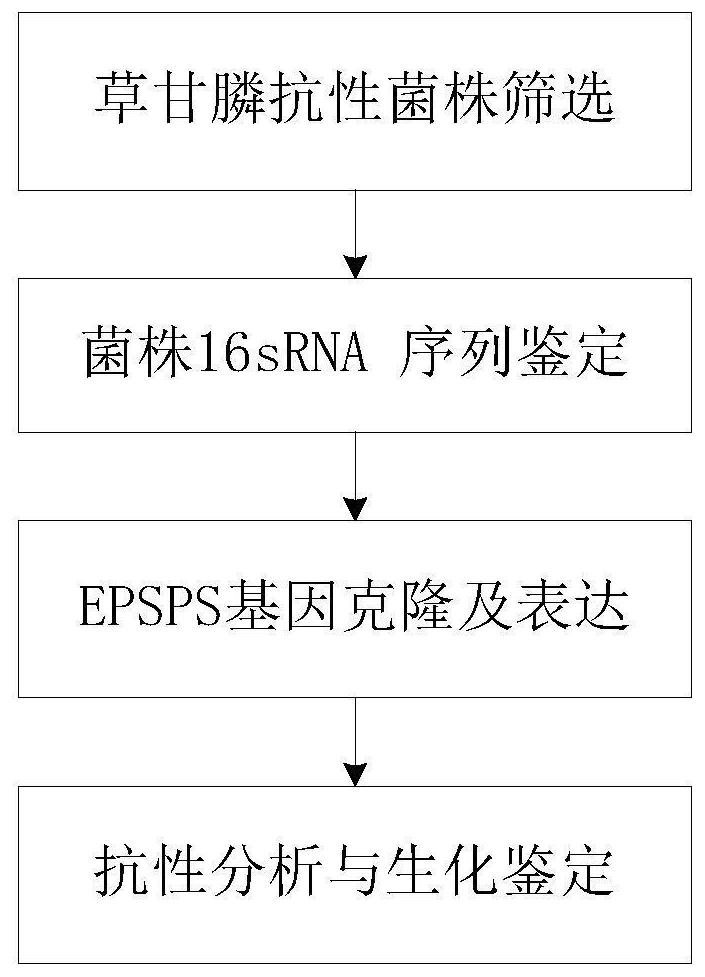
Patents
Literature
48 results about "Aroa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aroa is a genus of moths in the subfamily Lymantriinae first described by Francis Walker in 1855. Species are distributed in South Africa, China, throughout India, Sri Lanka, Myanmar, and Java.
Attenuated Pasteurella piscicida vaccine for fish
Live-attenuated vaccines against Edwardsiella ictaluri or against Pasteurella piscicida are disclosed. Both vaccines are incapable of reversion to virulence, because both are made by deletion mutations in the aroA gene, the purA gene, or both. These vaccines may be used not only to vaccinate fish against Edwardsiella ictaluri or Pasteurela piscicida, but also to serve as vectors to present antigens from other pathogens to the fish, thereby serving as vaccines against other pathogens as well, with no risk of infection by reversion to the virulent form of the pathogen in which the antigen occurs naturally.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Recombinant DNA (deoxyribonucleic acid) fragment containing roundup ready gene and application thereof
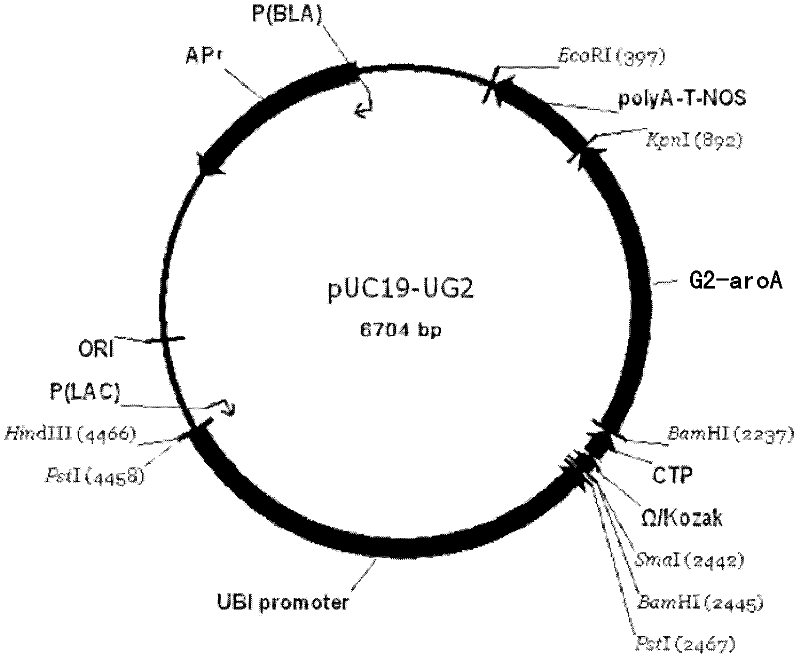
The invention discloses a recombinant DNA (deoxyribonucleic acid) fragment containing a roundup ready gene and application thereof. The recombinant DNA fragment provided by the invention comprises the following components from 1) to 3): 1) a promoter; 2) the roundup ready gene which is promoted by the promoter to be transcribed, the nucleotide sequence of the roundup ready gene is a) or b), wherein a) represents a sequence 2 in a sequence table, and b) represents a protein which has at least 98% of identity with the sequence 2 in the sequence table and is shown by a coding sequence 9; and 3) tanscription termination sequence. Compared with the transgenic corn which contains the recombinant DNA fragment in which prokaryote roundup ready gene G2-aroA is transplanted, the transgenic corn which is transplanted with the recombinant DNA fragment provided by the invention is obviously higher in the expression quantity of G2-aroA protein; and the transgenic corn is also obviously higher in the glyphosate tolerance.
Owner:BEIJING ORIGIN SEED TECH
Construction method of genetic engineering strain for producing shikimic acid
InactiveCN102304539AReduce the content of intermediate productsIncrease productionMicroorganism based processesVector-based foreign material introductionEnzyme GeneTryptophan
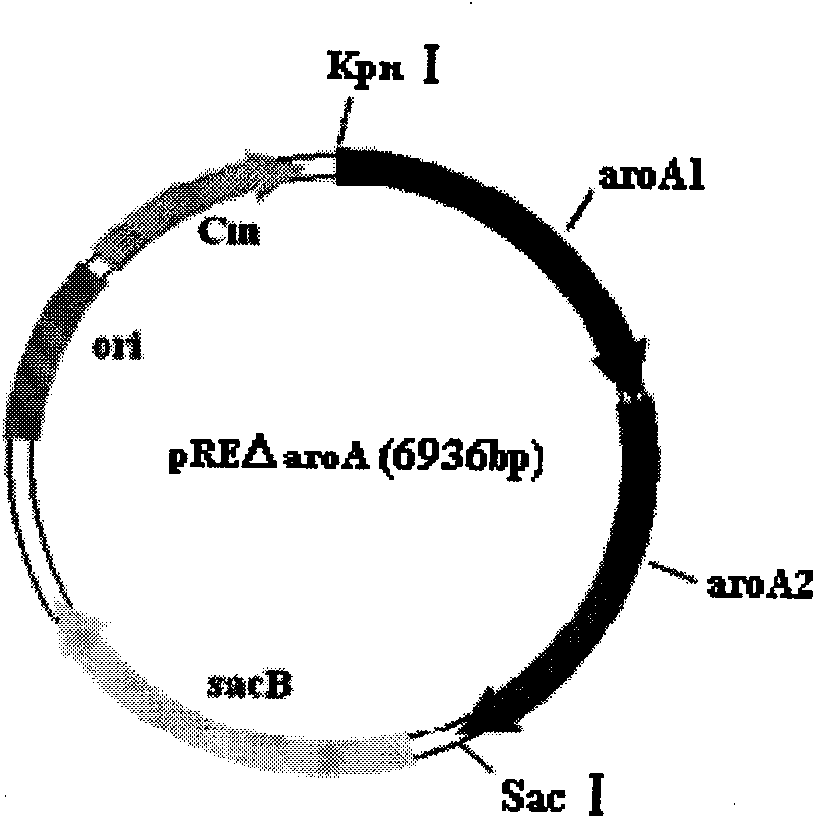
The invention discloses a construction method of a genetic engineering strain for producing shikimic acid. The method comprises the following steps of: 1, constructing an escherichia coli strain of which the aroA gene is knocked out; 2, constructing a recombinant expression plasmid pAR63 containing key enzyme genes aroGFBR, aroE, aroB, aroD, tktA and ppsA in the metabolic pathway of the shikimic acid, so that the genes are subjected to transcriptional control of a tryptophan promoter Ptrp; and 3, transferring the recombinant expression plasmid pAR63 into the escherichia coli strain of which the aroA gene is knocked out to obtain a production strain for expressing the shikimic acid. In the method, the aim of interrupting the metabolism of the shikimic acid is fulfilled by the knock-out of the single gene aroA, a small number of genes are knocked out, the operating difficulty is low, and the expression of the genes is in the state of starting and stopping automatically and is started automatically in the middle and late period without the induction of inducers, so the possibility of adding toxic substances into a culture medium is reduced, and the shikimic acid has high yield.
Owner:河南孟成生物药业股份有限公司
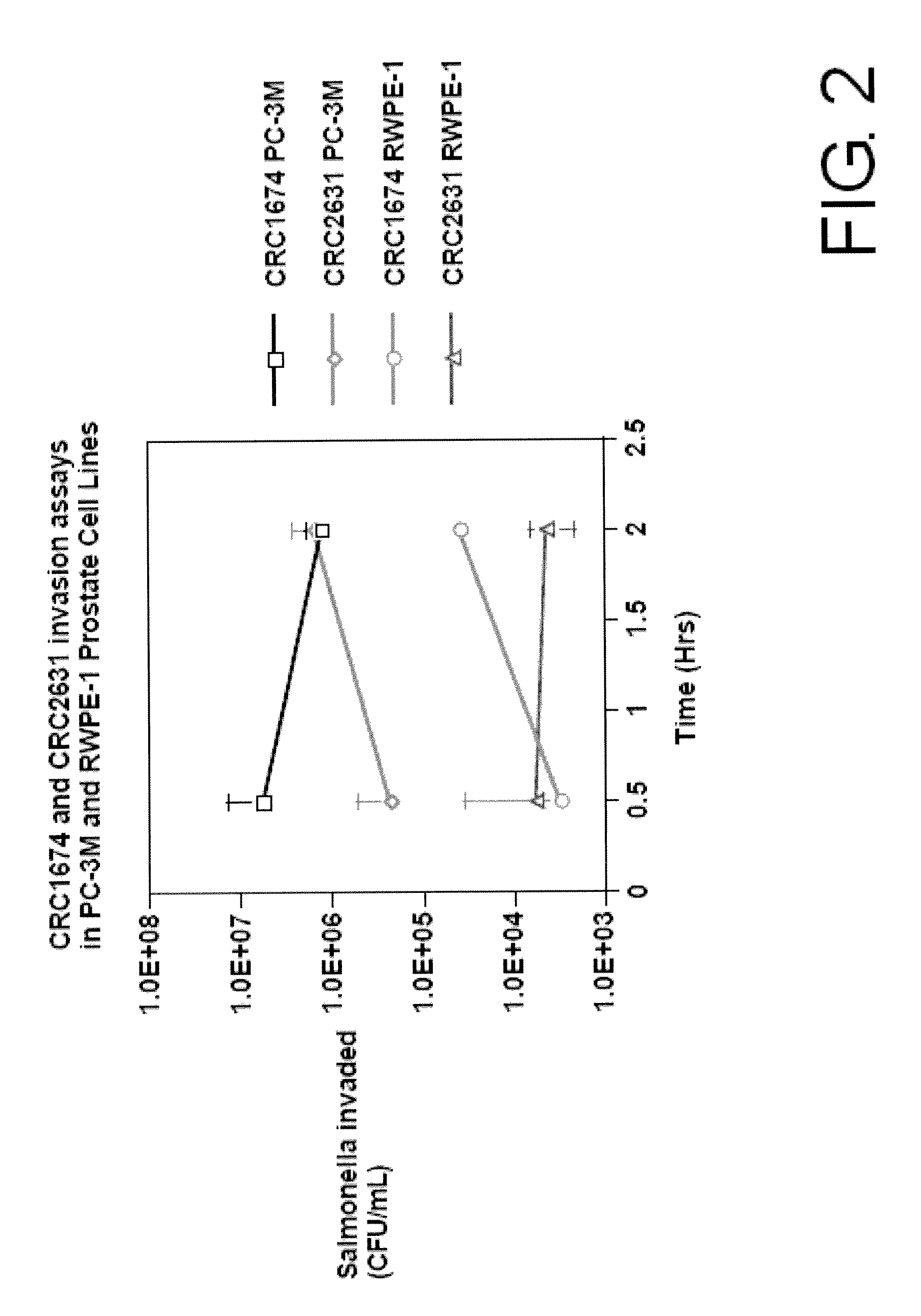
Microorganism Strain CRC2631 of Salmonella typhimurium and its Use as a Cancer Therapeutic
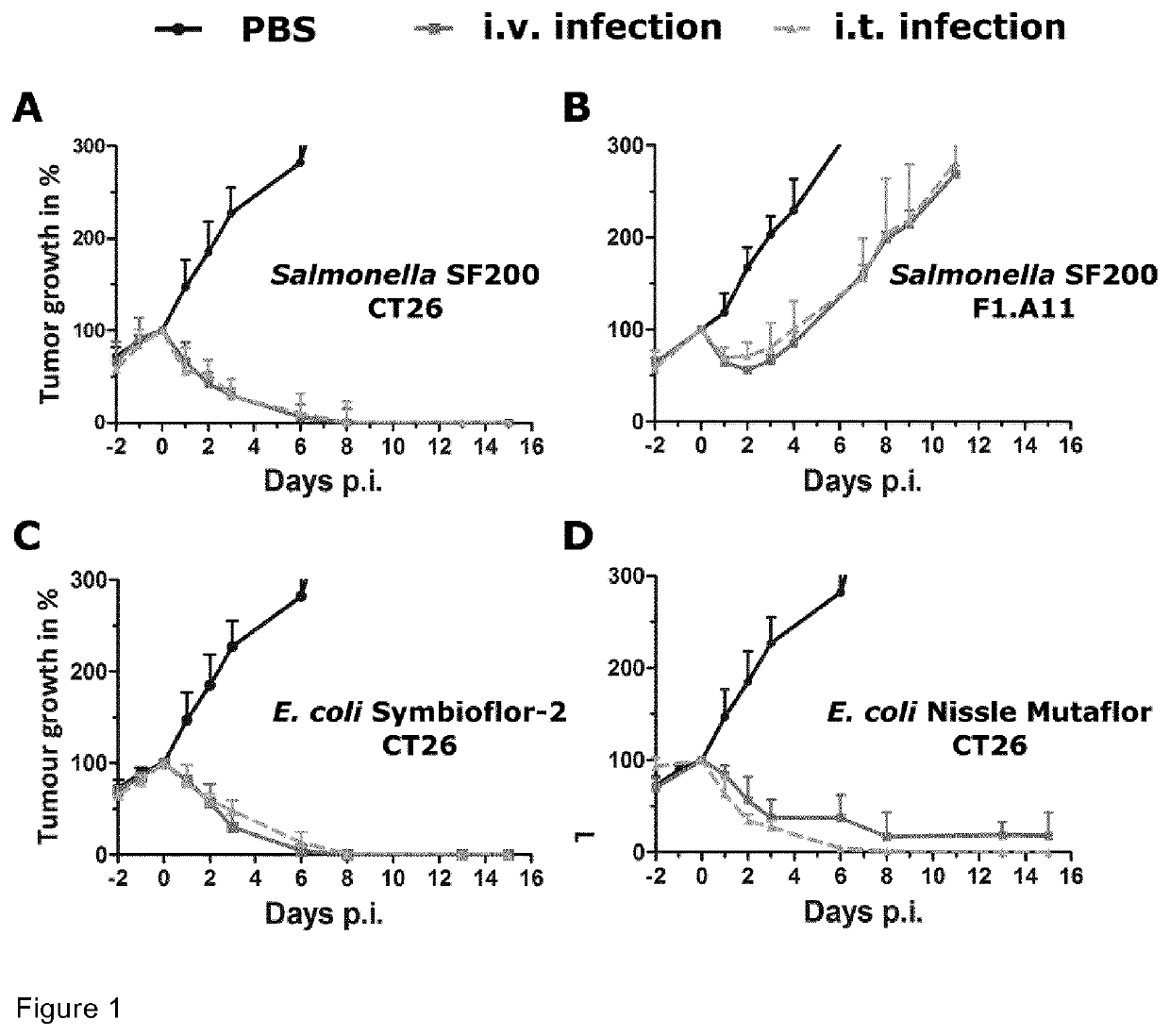
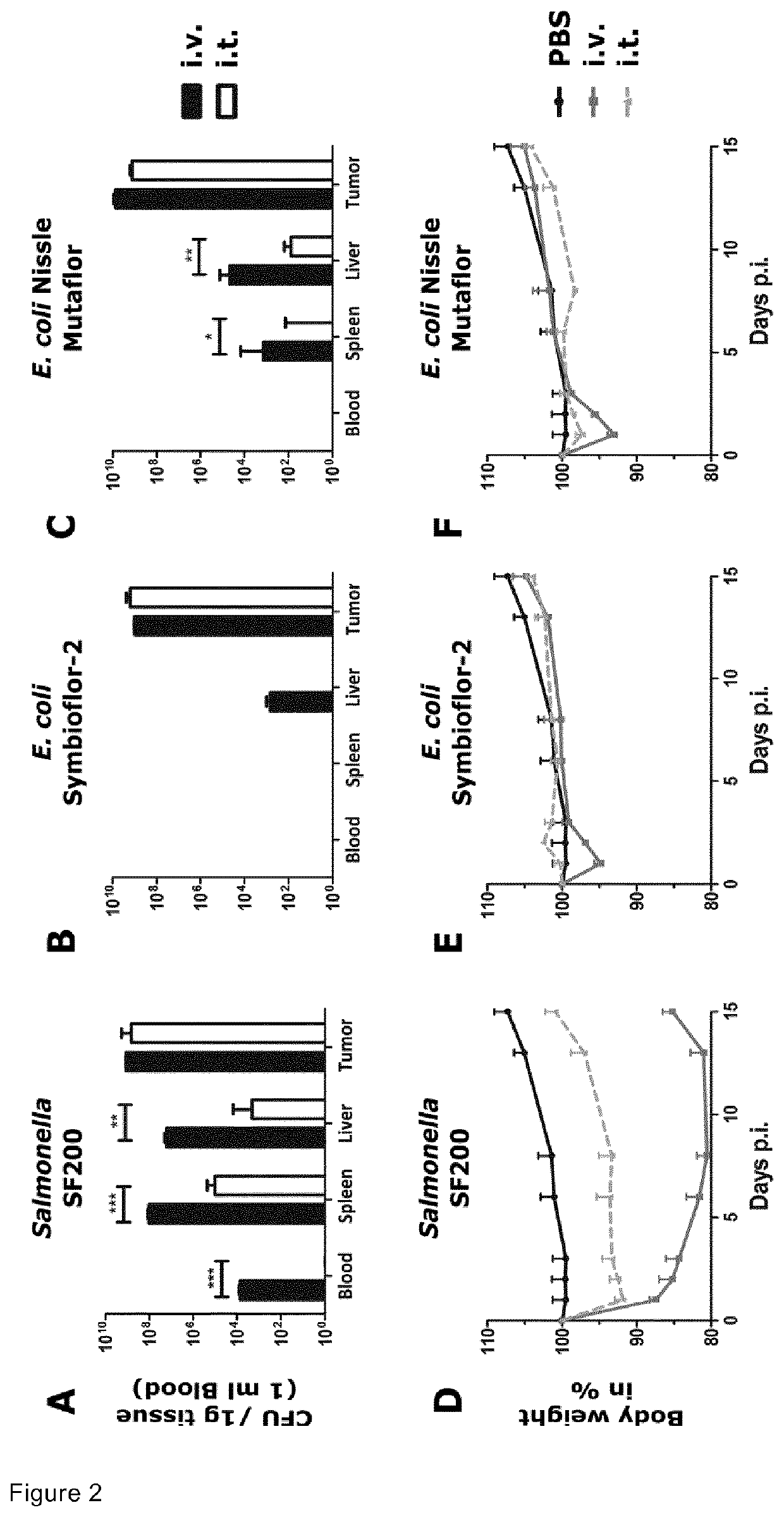
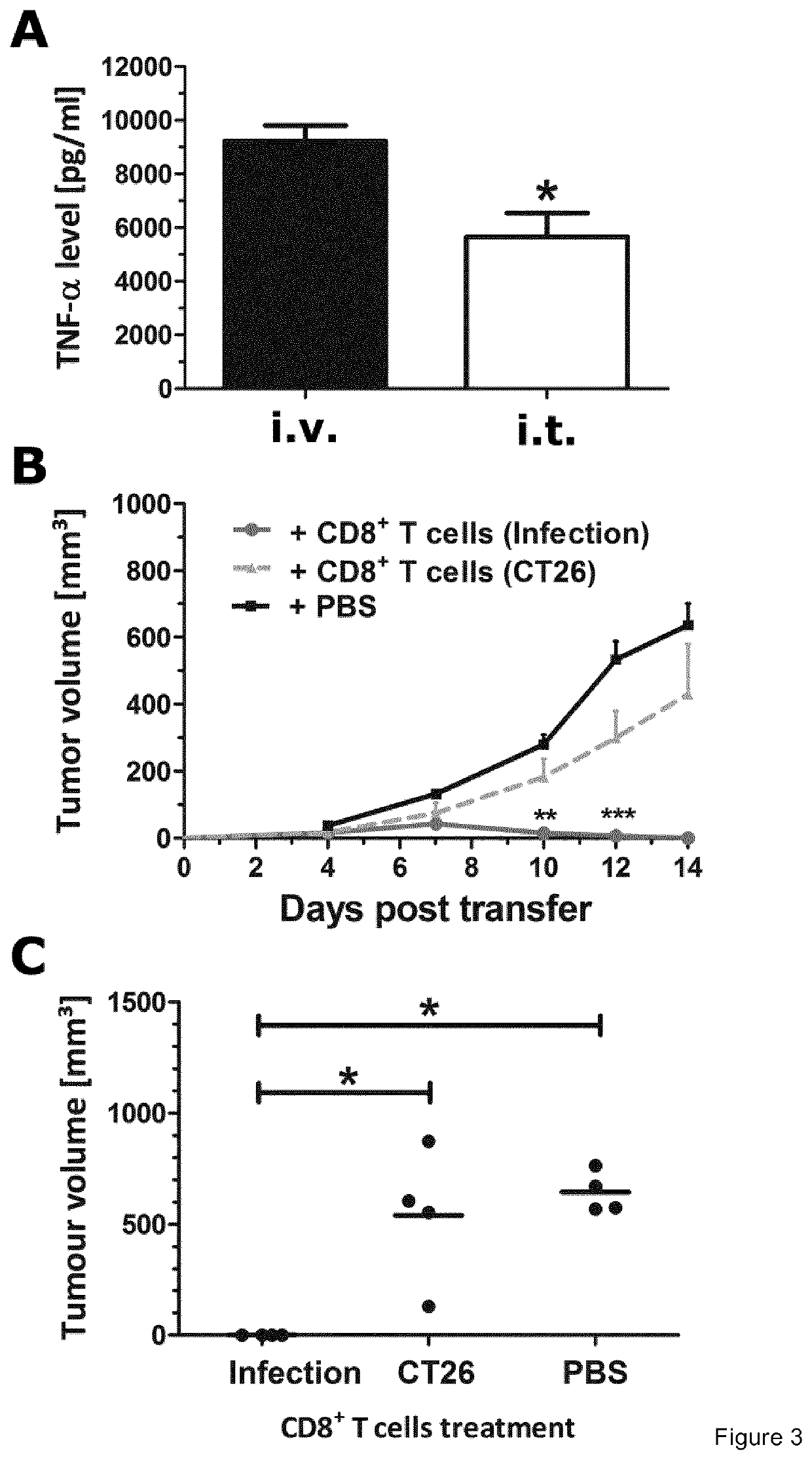
The present invention provides a biologically pure isolate of the genus Salmonella having a disruption of at least one gene selected from the group consisting of aroA, rfaH, and thyA, as well as a method of treating cancer including the step of administering such a Salmonella to a subject in need thereof.
Owner:CANCER RES CENT
Avian pathogenic escherichia coli strain and application thereof in vaccine preparation
The invention belongs to the field of biotechnology, and provides an avian pathogenic escherichia coli strain, which is an avian pathogenic escherichia coli luxS and aroA double-gene-deleted strain with the preservation numbr of CGMCC10601. The avian pathogenic escherichia coli strain provided by the invention can be used for preparing avian pathogenic escherichia coli inactivation and attenuated vaccine and bacterial ghost vaccine, and preventing avian pathogenic escherichia coli infection.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Method for detecting roundup ready protein G2-aroA and special enzyme linked immune kit
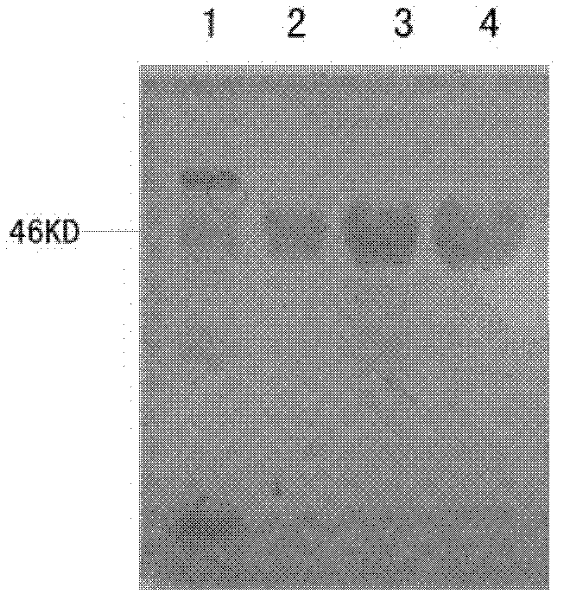
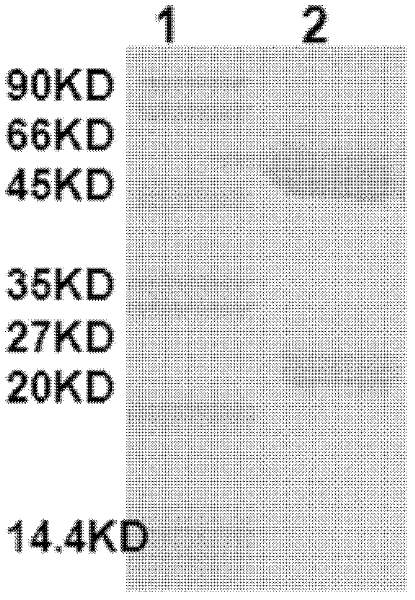
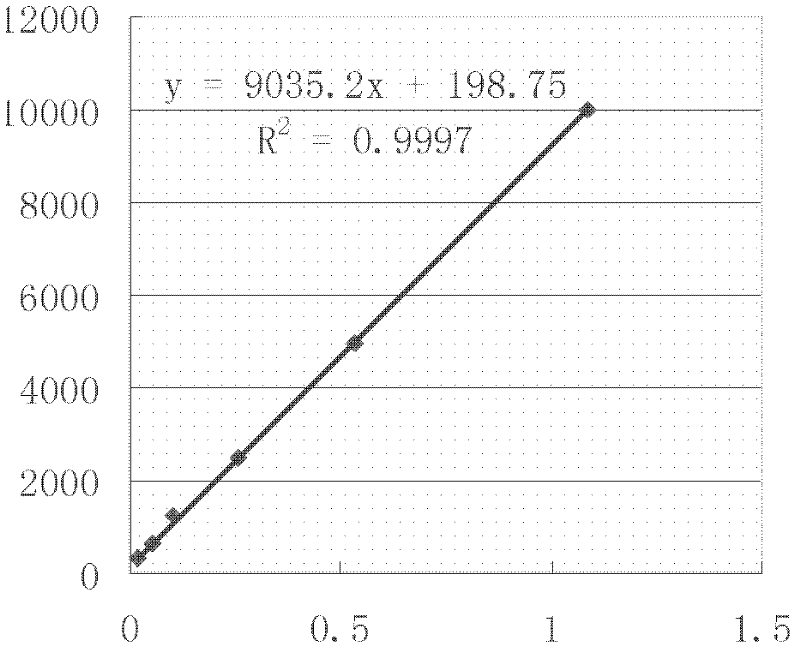
The invention discloses a method for detecting a roundup ready protein G2-aroA and a special enzyme linked immune kit. The enzyme linked immune kit contains independently packaged anti-G2-aroA protein monoclonal antibody; the amino acid sequence of the G2-aroA protein refers to sequence 9 in a sequence list; and the monoclonal antibody is generated by a hybridoma cell strain AntiG2-5F11 CGMCC No.5772. The enzyme linked immune kit provided by the invention can be used for qualitatively or quantitatively detecting the expression of the roundup ready protein G2-aroA in transgenic plants such as corn, and has the advantages of rapidness, sensitiveness and strong specificity.
Owner:BEIJING ORIGIN SEED TECH
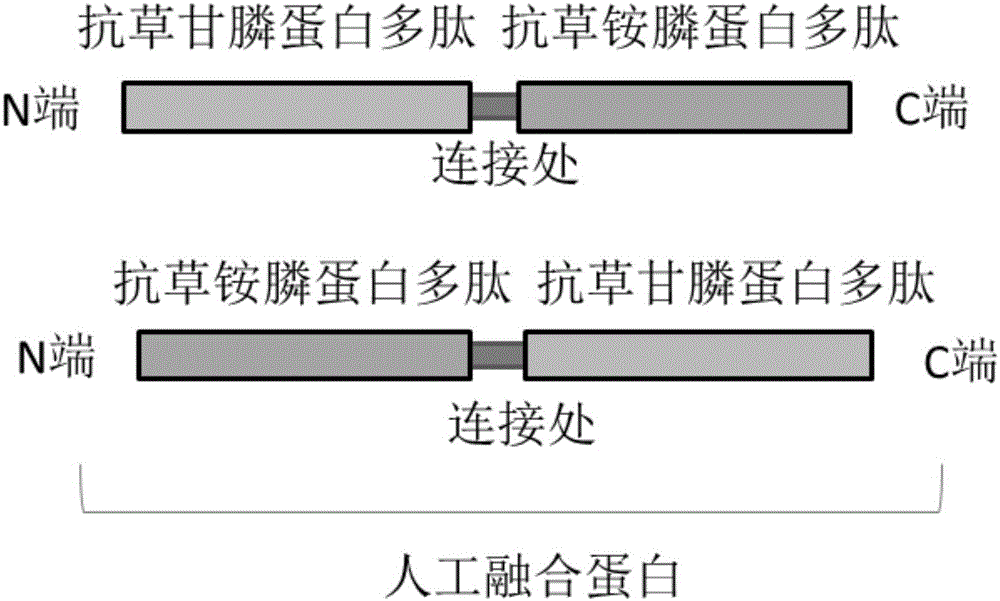
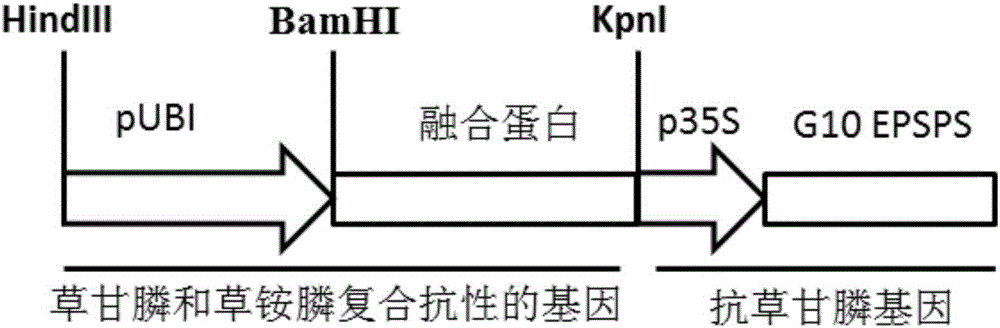
Fusion gene of compound antibody type with glyphosate and glufosinate, encoded protein and application thereof
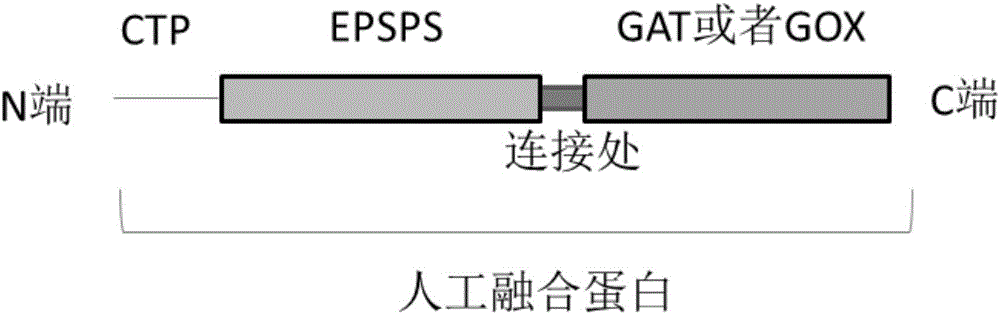
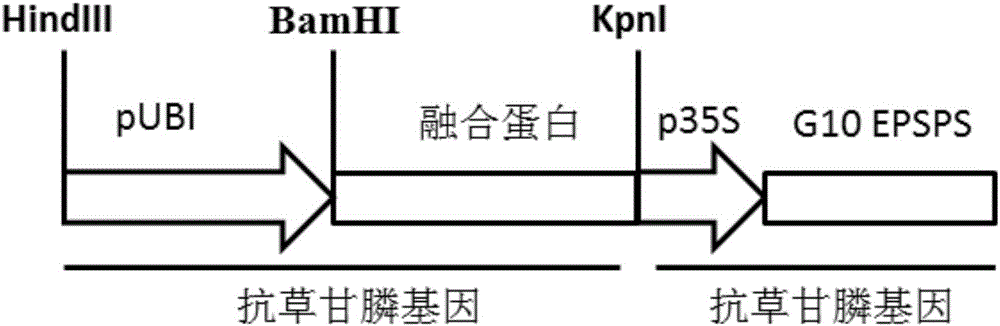
The present invention discloses a fusion gene of compound antibody type with glyphosate and glufosinate, encoded protein and application thereof, The fusion gene composes of a glyphosate-tolerant encoded-protein gene and a glufosinate-tolerant encoded-protein gene. The glyphosate encoded gene is one of the following: CP4, aroA, G7, G10, GOX or GAT; the glufosinate encoded gene is bar or pat. The compound protein can give compound antibodies to the plants, glyphosate and glufosinate; it can be used to produce transgene plants with the antibody property of glyphosate and glufosinate. The provided compound protein with antibody properties of glyphosate and glufosinate can be used in herbicides for monocotyledonous plants and dicotyleons; it is mainly used in herbicides for insect resistant corn, rice, soybean, wheat and rape.
Owner:ZHEJIANG UNIV
Bivalent expression carrier for culturing anti-glyphosate plants
ActiveCN101100676AAvoid negative effectsImprove resistance to glyphosateFermentationVector-based foreign material introductionTransfer geneAcetyltransferase
A bivalent plant expression carrier for culturing high anti-roundup transfer gene plant contains G2-aroA and gat. It has better anti-roundup performance and can be used to culture various high anti-roundup transfer gene plants.
Owner:LONGPING BIOTECHNOLOGY (HAINAN) CO LTD
Glyphosate-resistant Fusion Gene, Encoding Protein and Application
InactiveCN106350532AHigh resistance to glyphosateAntibody mimetics/scaffoldsTransferasesGlyphosateTransgene
The invention discloses a glyphosate-resistant fusion gene, coding protein and application thereof, the fusion gene consists of the EPSPS protein encoding gene and the glyphosate N-acetyltransferase or glyphosate oxidase encoding gene; the EPSPS protein is one of the following: CP4, aroA, G7 or G10; glyphosate N-acetyltransferase encoding gene is GAT, and glyphosate oxidase encoding gene is GOX. Compared with glyphosate-resistant protein, the glyphosate-resistant fusion gene and the coding protein have the following advantages: the glyphosate-resistant fusion gene and the coding proteincan be resistant to glyphosate by two different tolerance mechanisms; two glyphosate tolerance mechanisms and high glyphosate resistance of transgenic plants can be conferred by one gene. The glyphosate-resistant fusion protein can be used in the anti-glyphosate of monocotyledonous and dicotyledonous plants, and is mainly used for herbicide-resistant insect-resistant maize, rice, soybean, wheat and rape.
Owner:ZHEJIANG UNIV
Bordetella bronchiseptica gene deleted vaccine and application
InactiveCN101575586AGood virulence factorGood other immunogenicityAntibacterial agentsBacterial antigen ingredientsBordetella bronchisepticaMicrobiology
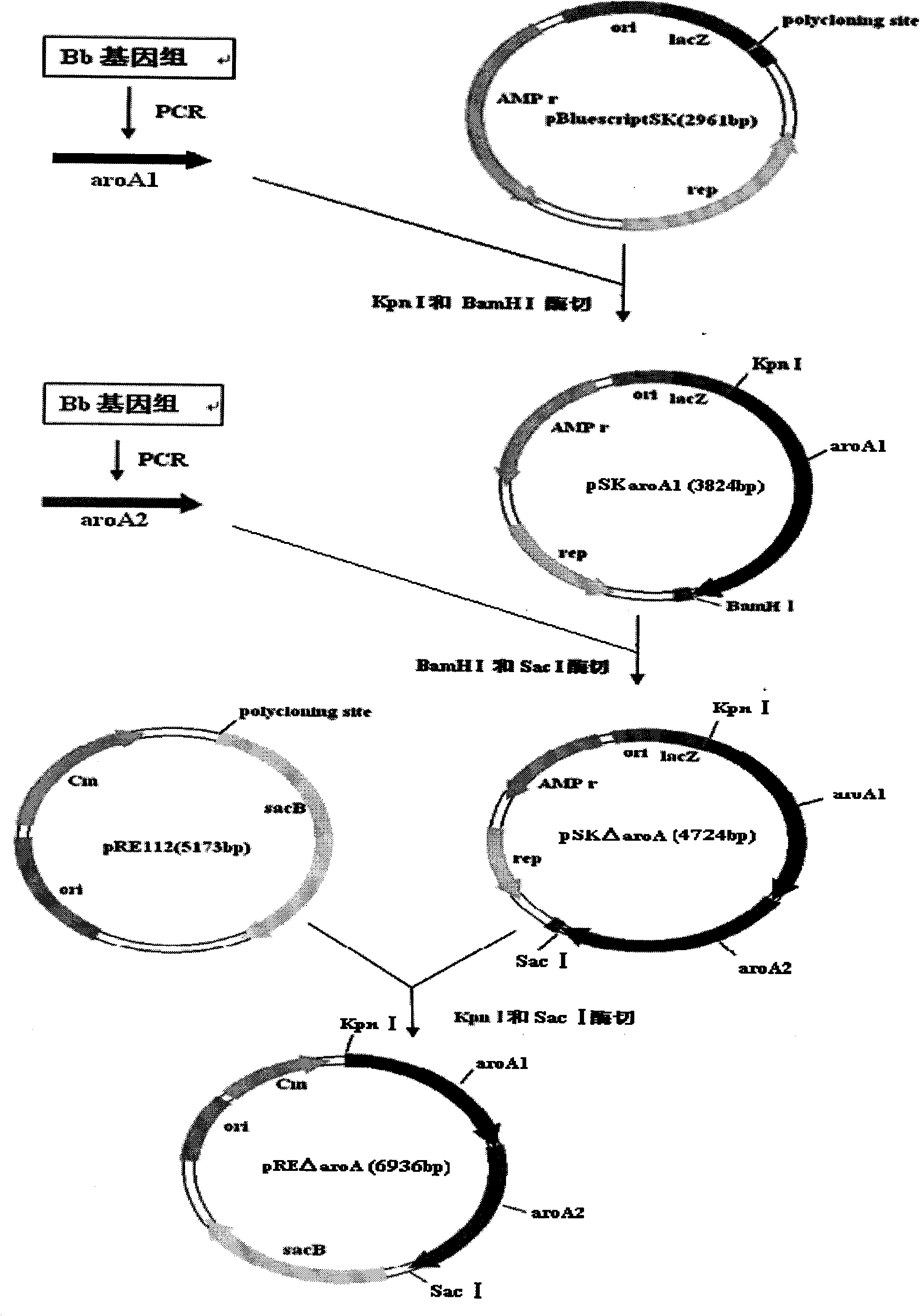
The invention belongs to the technical field of vaccine preparation of animal gene engineering and particularly relates to the construction of a Bordetella bronchiseptica aroA (5-enolpyruvyl-shikimate-3-phosphate synthase) gene deleted bacterial strain, and the preparation and application of a gene deleted vaccine. The Bordetella bronchiseptica gene deleted bacterial strain QH0814DeltaaroA is preserved in the China Center for Type Culture Collection with the collection number of CCTCCNO: M208018. The deletion of 5-enolpyruvyl-shikimate-3-phosphate synthase (aroA) gene with the full length of 1340bp causes an obstacle when the bacterial strain metabolizes aromatic amino acid. The invention prepares the Bordetella bronchiseptica gene deleted vaccine by using the gene deleted bacterial strain. The invention also discloses the application of the gene deleted bacterial strain in preparing the Bordetella bronchiseptica gene deleted vaccine (attenuated live vaccine).
Owner:HUAZHONG AGRI UNIV
Recombinant bordetella bronchiseptica strain, vaccine and use
ActiveCN103421728AGood immune protectionImmunization is easy to operateAntibacterial agentsBacterial antigen ingredientsBiotechnologyBordetella bronchiseptica
The invention belongs to the technical field of animal bacteriology and vaccine genetic engineering preparation, and relates to a resistance mark-free recombinant bordetella bronchiseptica strain for expression of a pig toxaigenic pasteurella multocida toxA gene fragment, and its vaccine containing the resistance mark-free recombinant bordetella bronchiseptica strain, a preparation method and a use. The resistance mark-free recombinant bordetella bronchiseptica strain QH0814 delta aroA / toxA-N for expression of the pig toxaigenic pasteurella multocida toxA gene fragment does not contain a 5-enolpyrul-shikimate-3-phosphate synthase aroA gene of bordetella bronchiseptica, and contains the pig toxaigenic pasteurella multocida toxA gene fragment. The invention also discloses the preparation method of the resistance mark-free recombinant bordetella bronchiseptica strain and the vaccine use. The genetic engineering vaccine can stimulate a pig to produce a protective immune response for resisting pig toxaigenic pasteurella multocida and a wild strain of pig bordetella bronchiseptica, and can effectively prevent the double infection caused by pig toxaigenic pasteurella multocida and pig bordetella bronchiseptica.
Owner:HUAZHONG AGRI UNIV
Bordetella bronchiseptica gene deletion strain, vaccine prepared from Bordetella bronchiseptica gene deletion strain and application
ActiveCN102676421AGood immune protectionWeak toxicityAntibacterial agentsBacterial antigen ingredientsBordetella bronchisepticaMicrobiology
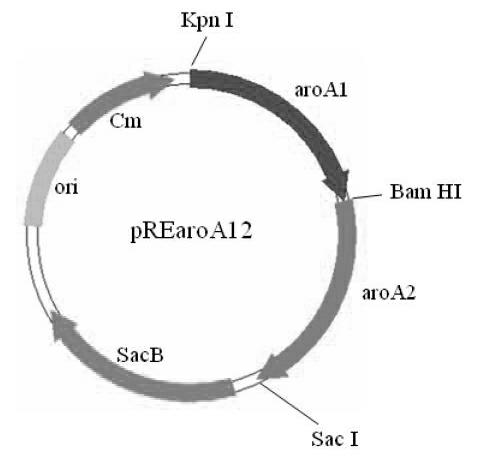
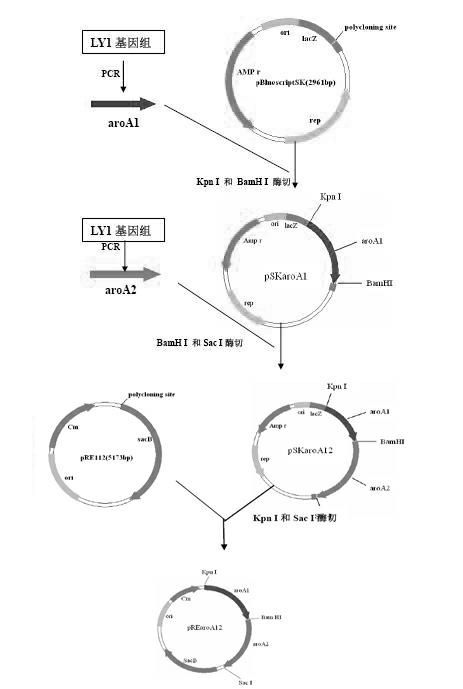
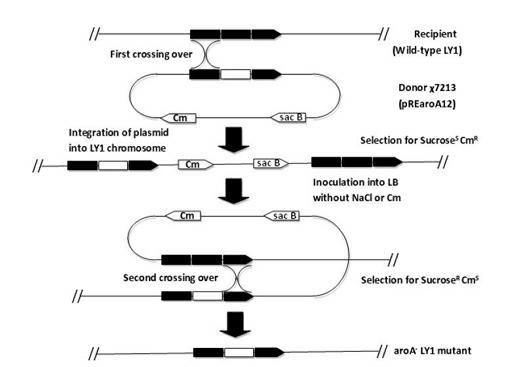
The invention relates to a Bordetella bronchiseptica gene deletion strain, vaccine prepared from the Bordetella bronchiseptica gene deletion strain and application of the strain. The aroA gene deletion strain, namely aroA-LY1 (Bordetella bronchiseptica), is preserved in the China Center for Type Culture Collection (CCTCC) on 23 December 2011, and the preservation number is CCTCCNO: M 2011479. The Bordetella bronchiseptica gene deletion strain disclosed by the invention has excellent immune protection efficacy and weak toxicity without any adverse side effect on immunized pigs and is safer compared with the traditional vaccine. Therefore, the vaccine which is prepared from the gene deletion strain built by a parent strain has wide market application prospect. In addition, the Bordetella bronchiseptica gene deletion strain disclosed by the invention retains the immune protective efficacy aiming at Bordetella bronchiseptica infection, and has clear genetic background and stable characteristics, so that the Bordetella bronchiseptica gene deletion strain has better biosecurity. The Bordetella bronchiseptica gene deletion strain disclosed by the invention does not contain a resistance tag and totally accords with the biological security requirement of vaccine.
Owner:HENAN UNIV OF SCI & TECH
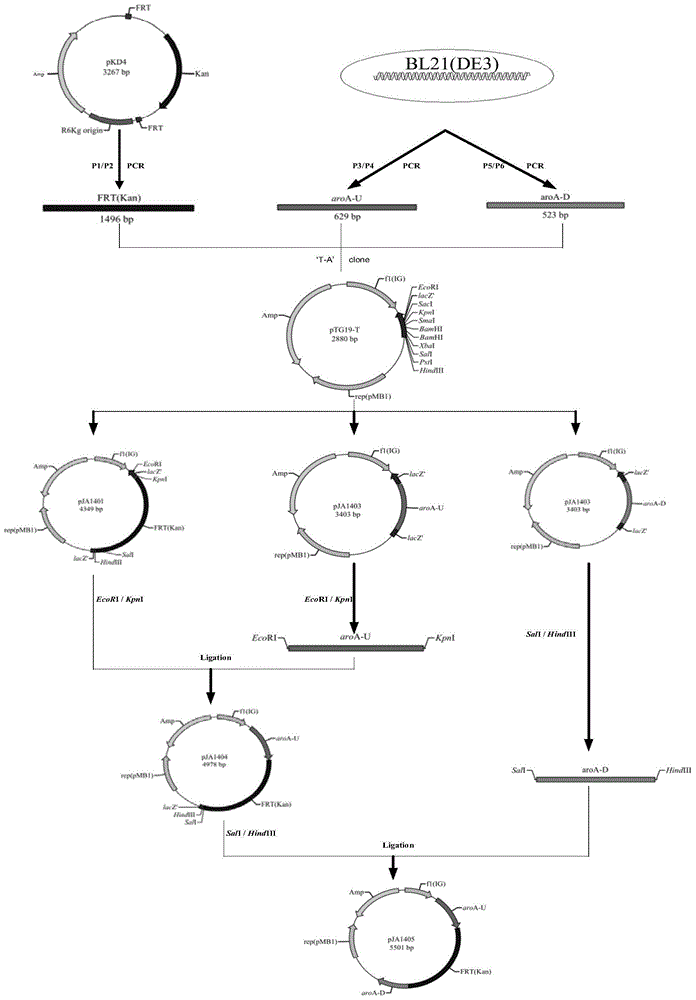
Construction method and application of BL21(DE3)delta aroA strain
ActiveCN104099363AAchieve the effect of killing two birds with one stoneBacteriaMicroorganism based processesPlasmidBiotechnology
The invention relates to the field of biological carriers, in particular to a construction method and an application of a BL21(DE3)delta aroA strain. The construction method comprises the following steps: an aroA gene knockout targeting segment is constructed; pKD46 plasmid is transformed into a BL21(DE3) strain, and a BL21(DE3) / pKD46 strain is obtained; and the aroA gene knockout targeting segment is transformed into the strain, and the BL21(DE3)delta aroA strain is obtained. According to the provided construction method of the BL21(DE3)delta aroA strain, homologous flanking sequences larger than 500 bp are adopted, and a Red homologous recombination technology is used for knocking out the aroA gene of the BL21(DE3) strain successfully; the strain can be used for EPSPS functional complementation verification and further achieves strong expression of recombinant foreign protein of a pET system, and dual purposes are achieved.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
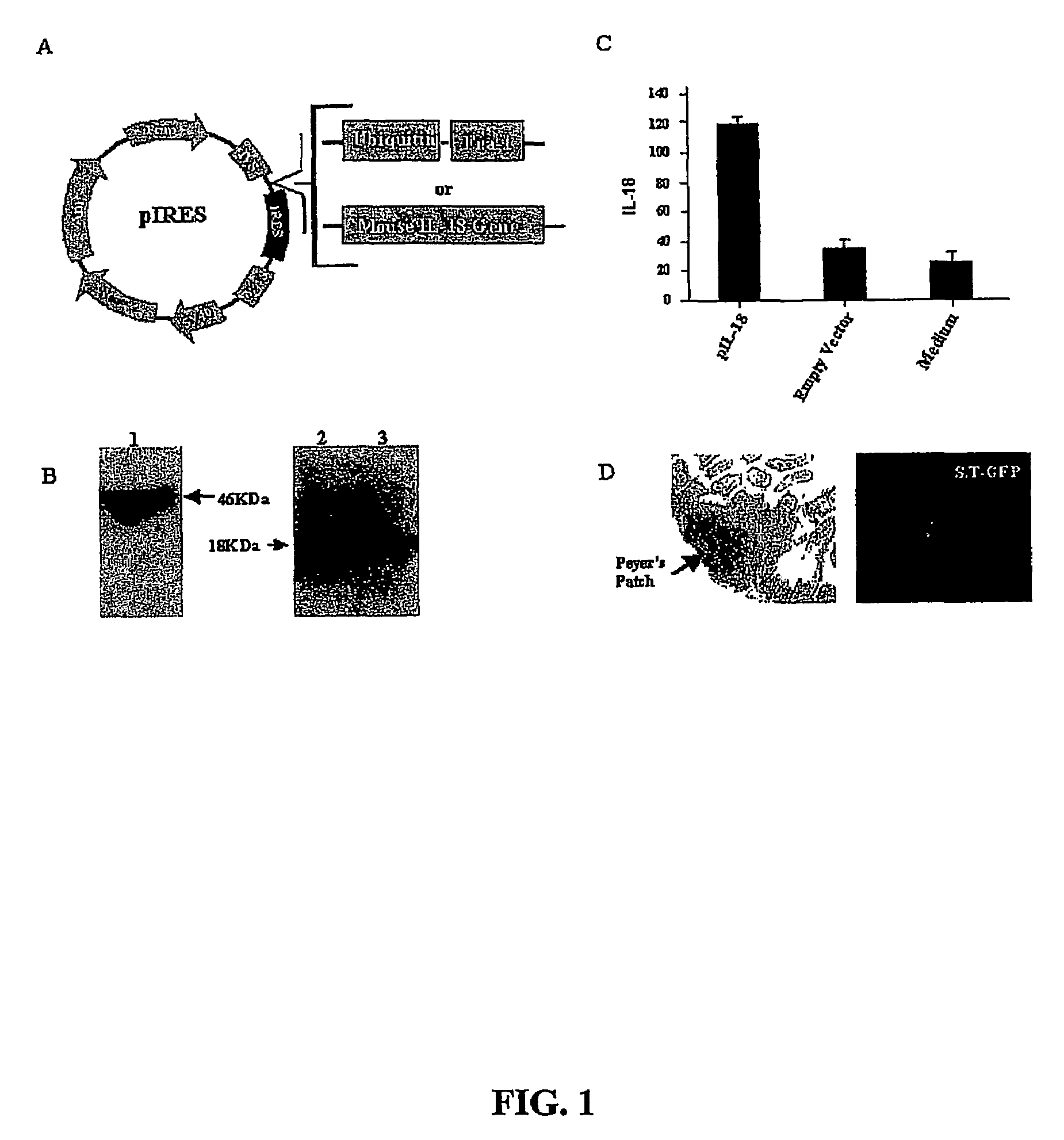
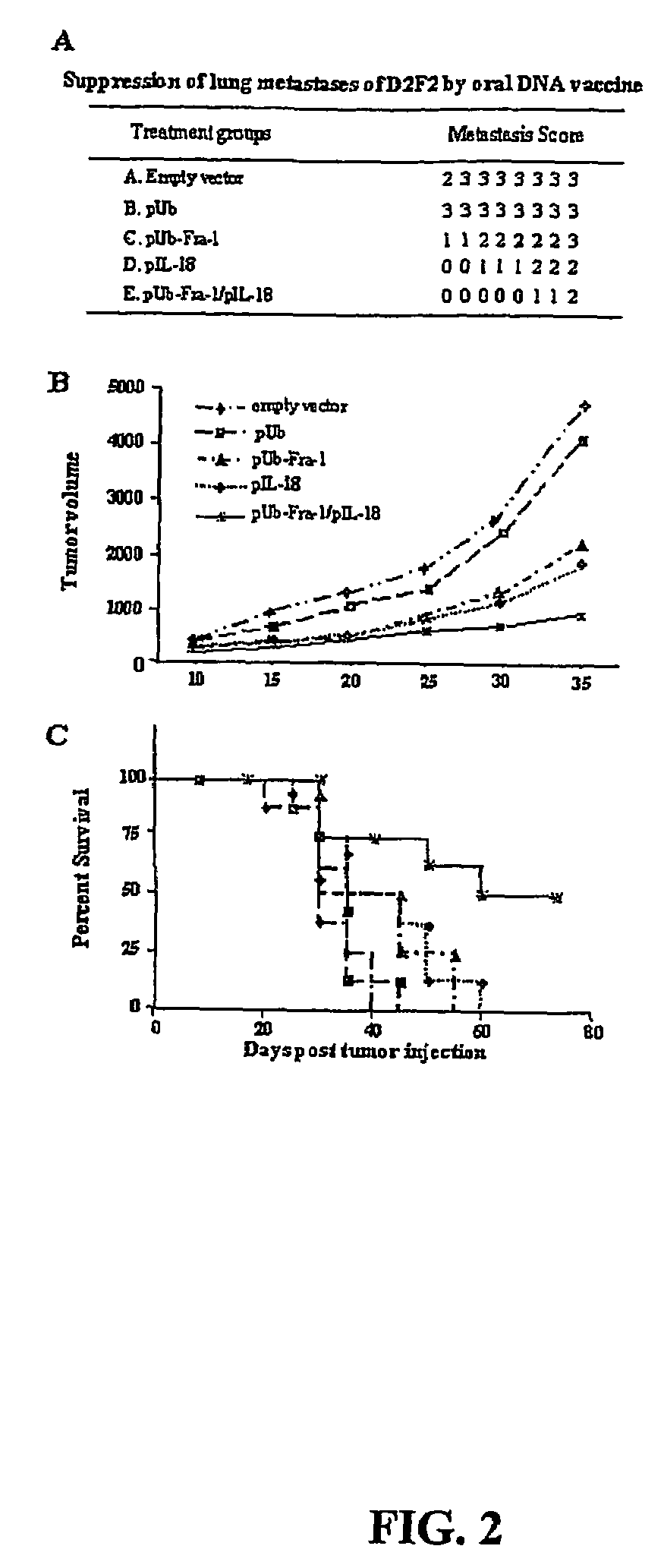
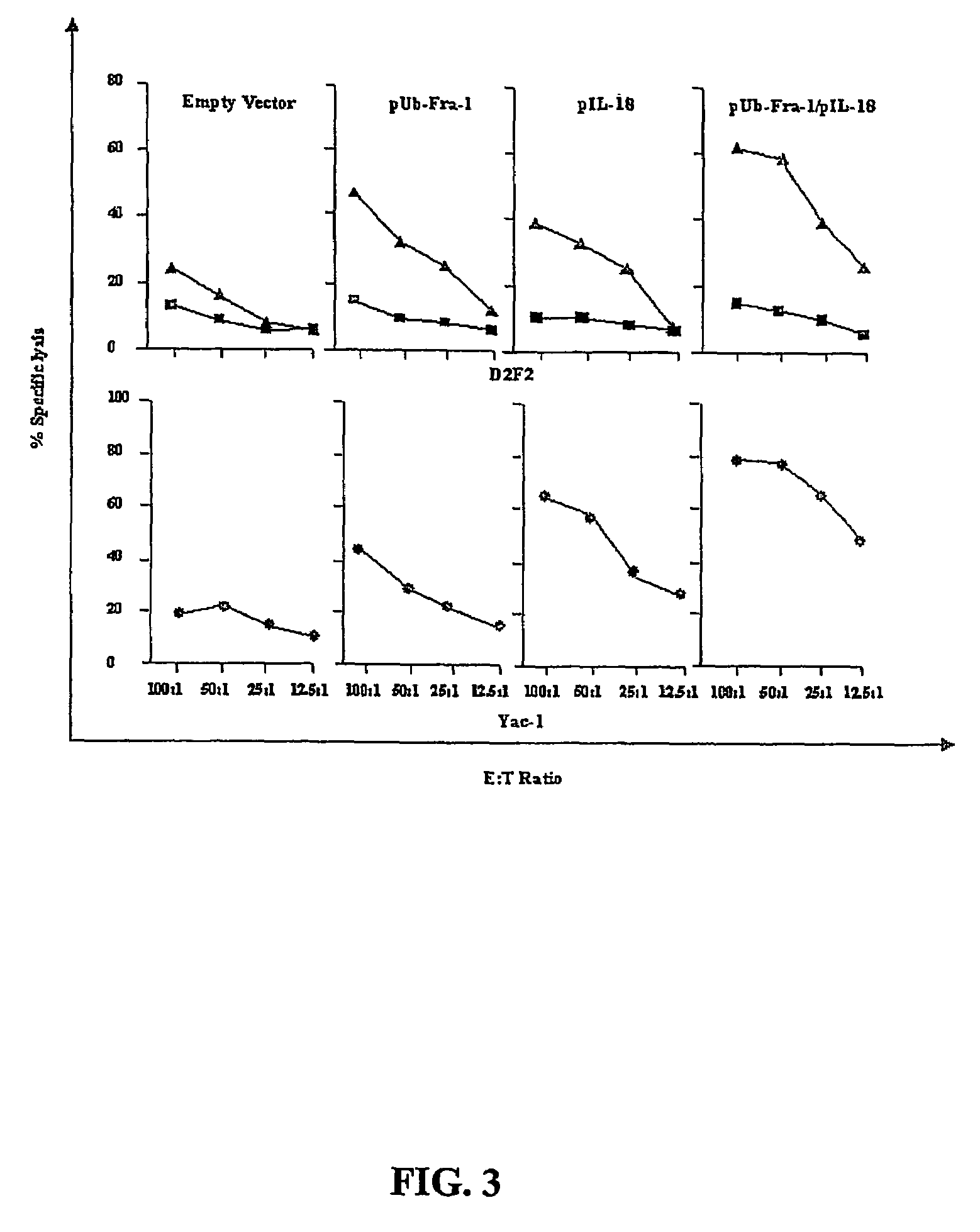
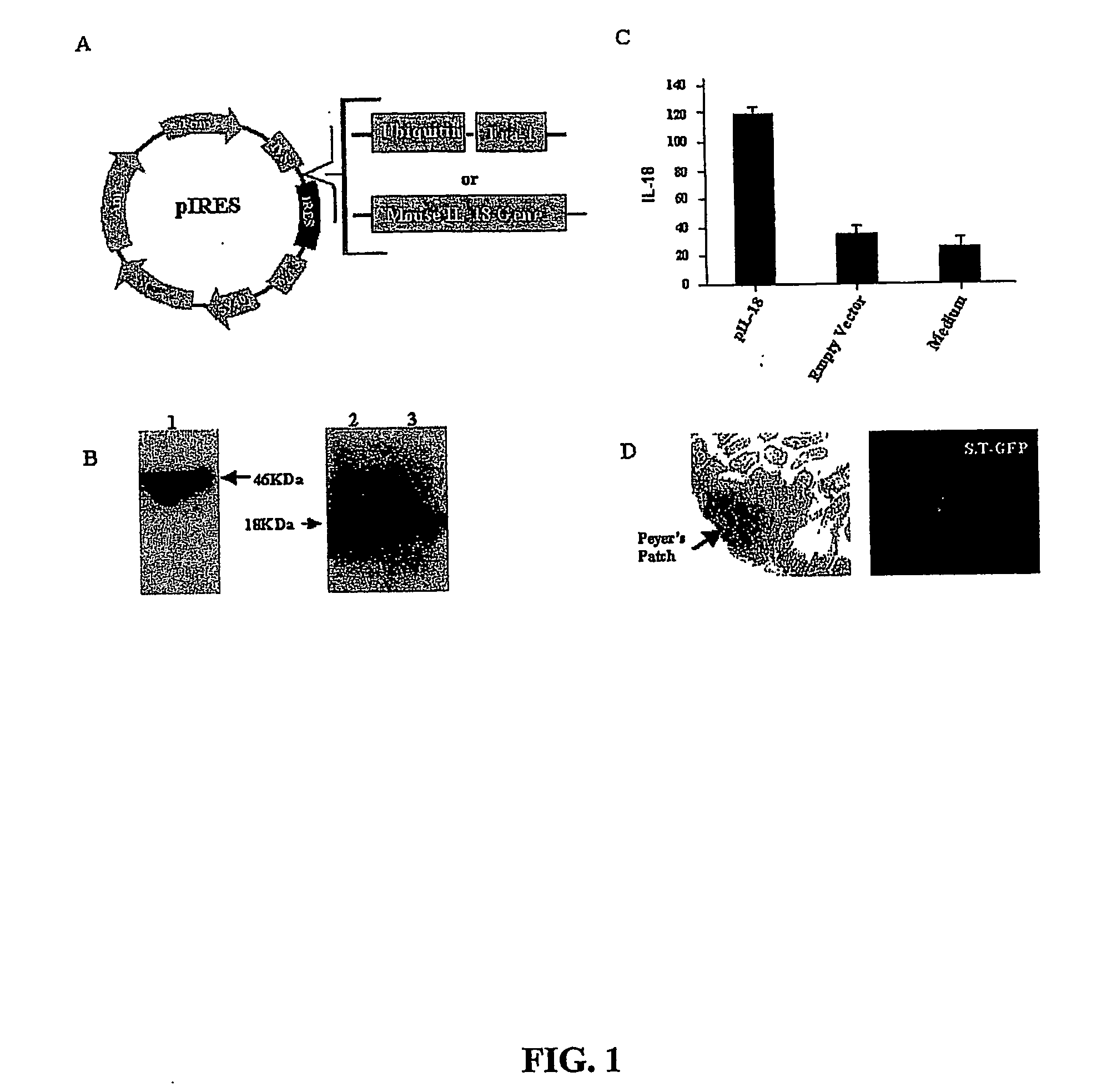
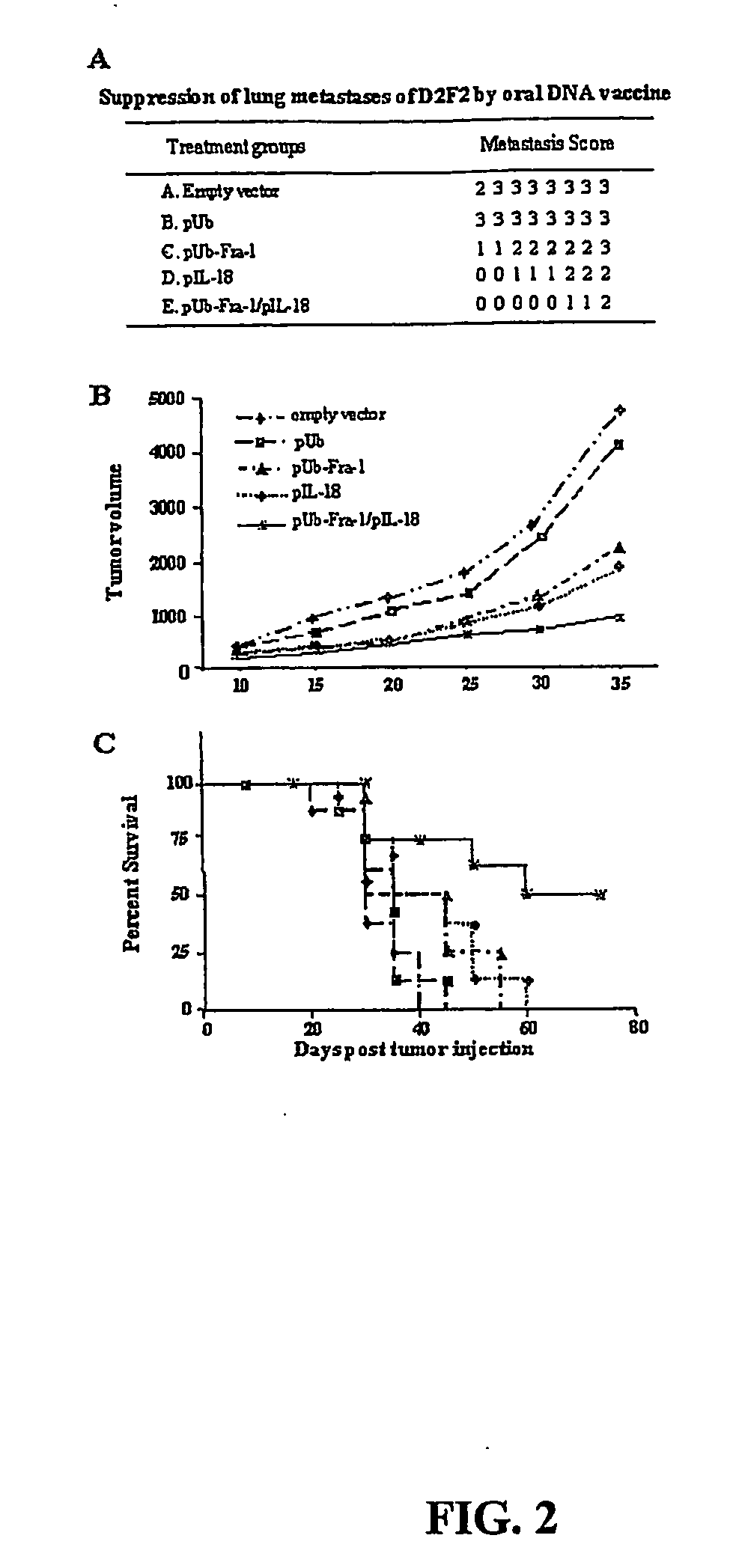
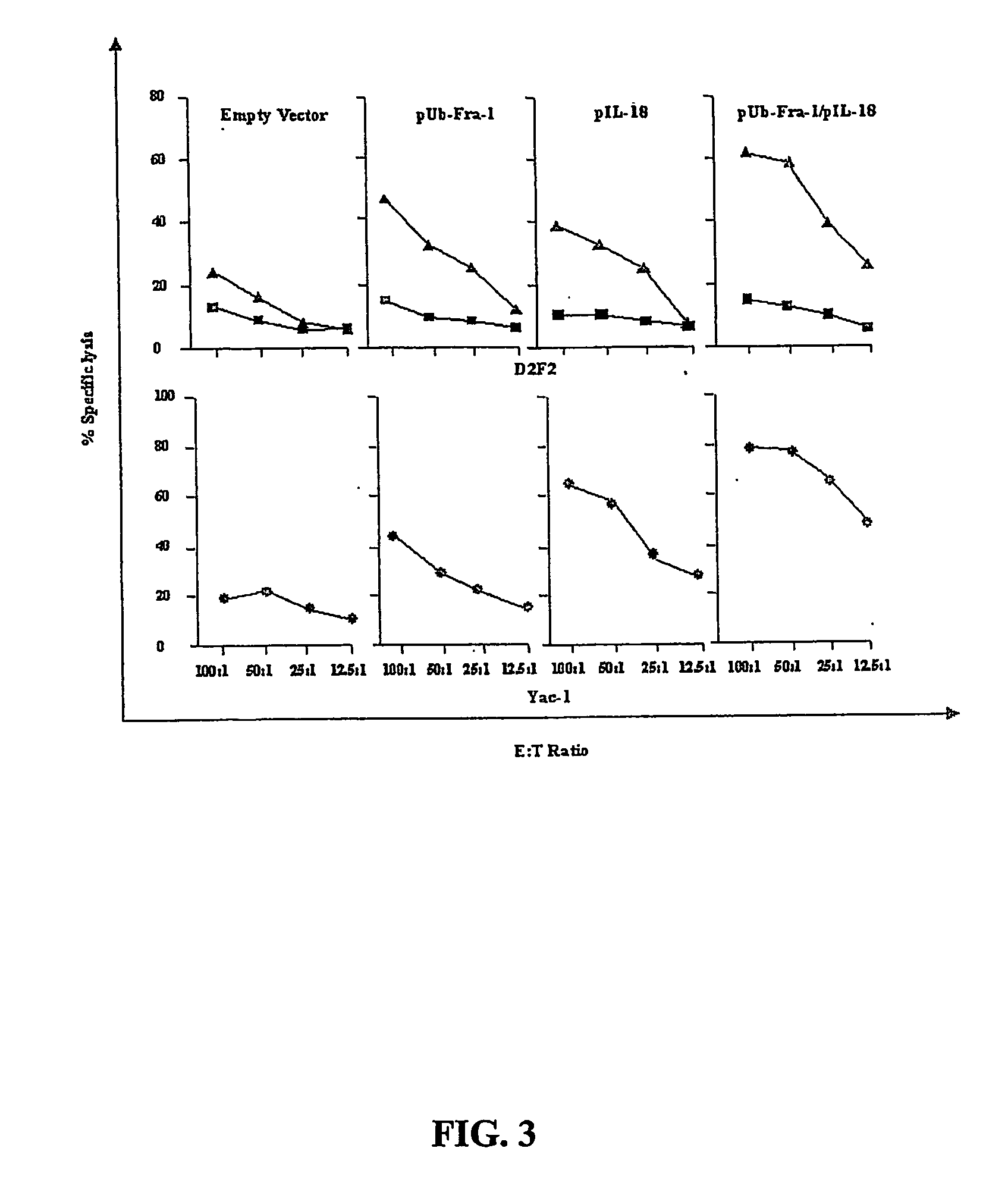
DNA vaccines against tumor growth and methods of use therof
A DNA vaccine suitable for eliciting an immune response against cancer cells comprises a polynucleotide construct operably encoding an a Fra-1 protein, such as a polyubiquitinated human Fra-1 protein, and IL-18, such as human IL-18, in a pharmaceutically acceptable carrier. In a preferred embodiment, the polynucleotide construct is operably incorporated in an attenuated bacterial vector, such as an attenuated Salmonella typhimurium, particularly a doubly attenuated aroA− dam− S. typhimurium. Transformed host cells, methods of inhibiting tumor growth, of vaccinating a patient against cancer, and of delivering genetic material to a mammalian cell in vivo are also described.
Owner:THE SCRIPPS RES INST
Dna vaccines against tumor growth and methods of use thereof
InactiveUS20070110717A1Provide protectionAvoid long termBiocideWhole-cell/virus/DNA/RNA ingredientsCancer cellNucleotide
A DNA vaccine suitable for eliciting an immune response against cancer cells comprises a polynucleotide construct operably encoding an a Fra-1 protein, such as a polyubiquitinated human Fra-1 protein, and IL-18, such as human IL-18, in a pharmaceutically acceptable carrier. In a preferred embodiment, the polynucleotide construct is operably incorporated in an attenuated bacterial vector, such as an attenuated Salmonella typhimurium, particularly a doubly attenuated aroA- dam- S. typhimurium. Transformed host cells, methods of inhibiting tumor growth, of vaccinating a patient against cancer, and of delivering genetic material to a mammalian cell in vivo are also described.
Owner:THE SCRIPPS RES INST
Primer pair for detecting G2-aroA gene-modified herbicide-tolerant corn G11105E-823C
ActiveCN104017881AMicrobiological testing/measurementDNA/RNA fragmentationAgricultural sciencePcr method
The invention discloses a primer pair for detecting G2-aroA gene-modified herbicide-tolerant corn G11105E-823C. The primer pair for detecting the G2-aroA gene-modified herbicide-tolerant corn G11105E-823C provided by the invention is concretely composed of two DNA molecule single chains represented by sequence 1 and sequence 2 in a sequence table. Experiments show whether corns to be detected are the G2-aroA gene-modified herbicide-tolerant corn G11105E-823C can be detected through a PCR method by using the primer pair, and the method is high in accuracy rate, strong in specificity and high in sensibility.
Owner:BEIJING ORIGIN SEED TECH
Gene aroA for encoding 5-enolpyruvyl-shikimate-3-phosphate synthase and application thereof
InactiveCN102766643AStrong resistanceGood glyphosate resistanceFermentationGenetic engineeringGene defectResistant genes
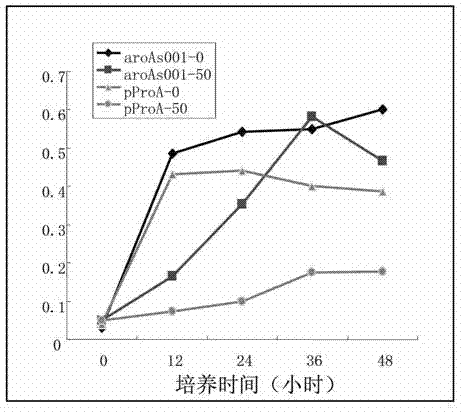
The invention discloses a gene aroAS001 for encoding 5-enolpyruvyl-shikimate-3-phosphate synthase, and an application of the gene, wherein the base sequence of the gene is represented by SEQ ID No.3; the gene is suitable for enhancing glyphosate resistance of crops; specifically, gene aroAS001, and an expression vector or a cloning vector containing gene aroAS001, or strains containing the expression vector or cloning vector are transferred in the crops. The gene provided by the invention is an excellent glyphosate resistant gene, the aroA gene defect type strains transferred with the gene can grow in a culture medium containing lower than 400mM glyphosate; compared with other known EPSP (enolpyruvyl-shikimate-3-phosphate) synthase genes, the gene provided by the invention has significant glyphosate resistance and brings about unexpected beneficial effects.
Owner:PLANT PROTECTION RES INST OF GUANGDONG ACADEMY OF AGRI SCI
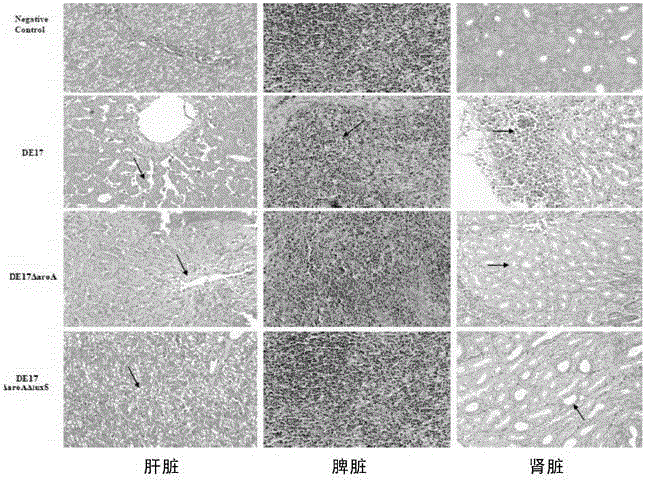
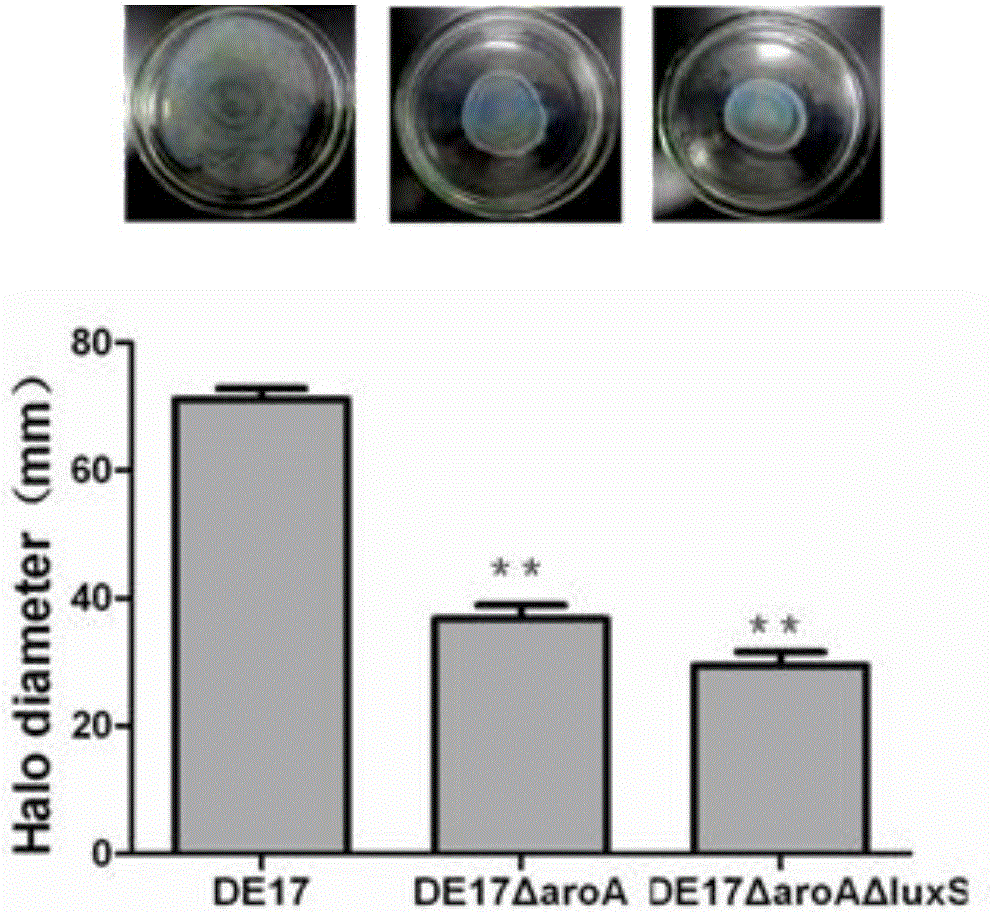
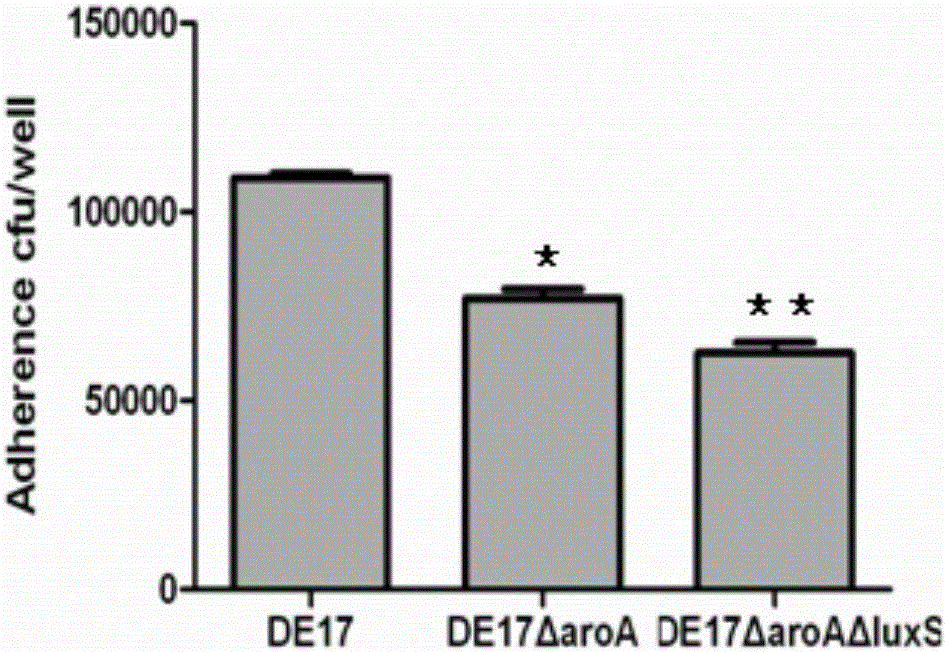
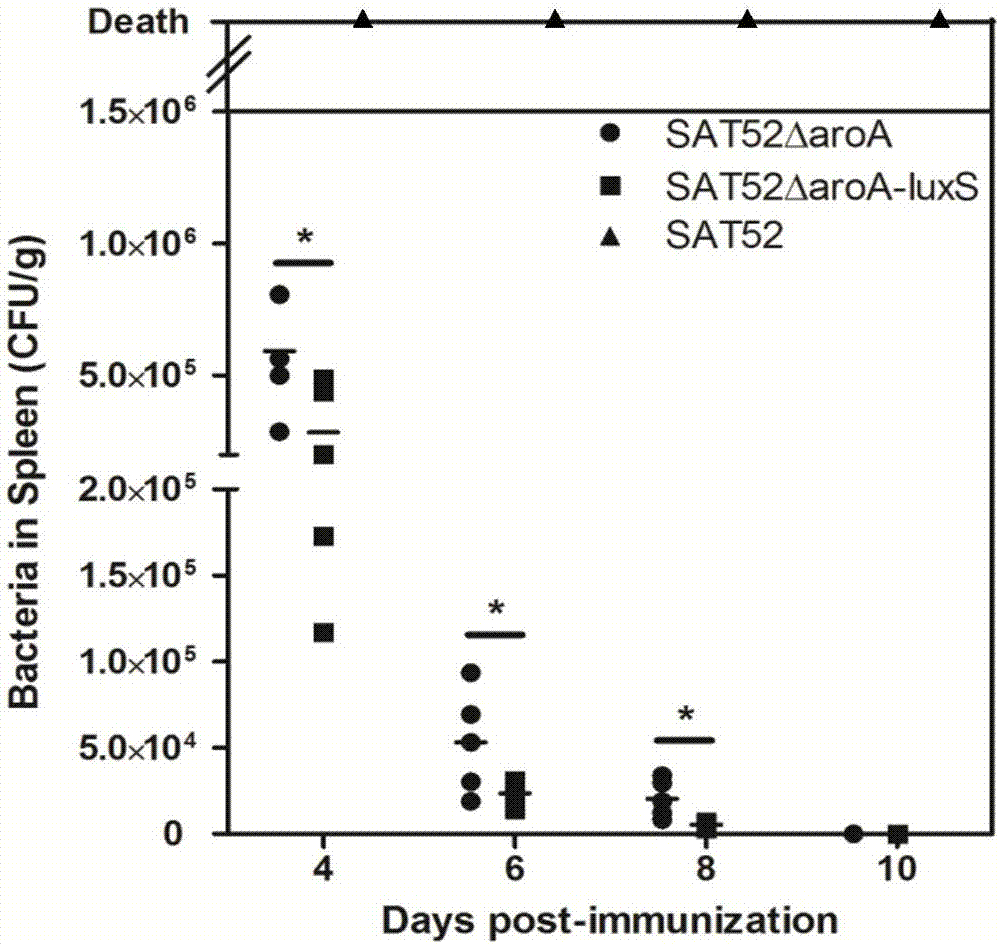
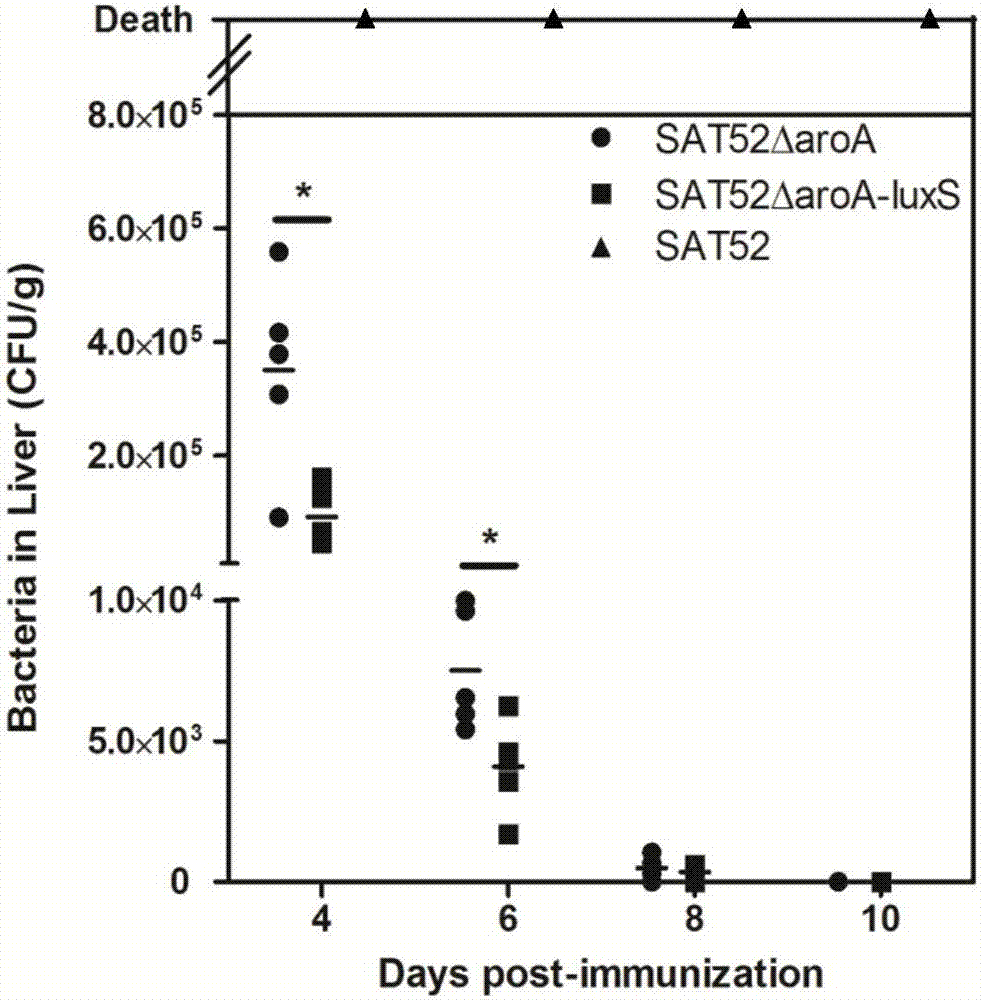
Salmonella typhimurium aroA and luxS double-gene-deleted strain and attenuated vaccine prepared by same
ActiveCN107034160ALow toxicityImmunity remains goodAntibacterial agentsCarbon-sulfur lyasesMicroorganismAttenuated vaccine
The invention provides a salmonella typhimurium aroA and luxS double-gene-deleted strain and an attenuated vaccine prepared by the same. The salmonella typhimurium aroA and luxS double-gene-deleted strain is collected at China General Microbiological Culture Collection Center, and the collection number of the strain is CGMCC NO. 13736. The double-gene-deleted strain is obtained by deleting aroA and luxS genes by a Red homologous recombination method, so that toxicity is remarkably reduced, the prepared salmonella typhimurium double-gene-deleted attenuated vaccine is good in immunity maintenance, and the strain can be used for preparing the salmonella typhimurium double-gene-deleted attenuated vaccine for preventing and treating salmonella typhimurium infection.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Cold-adapted I-type 5-enolpyruvoyl shikimic acid-3-phosphate synthase gene
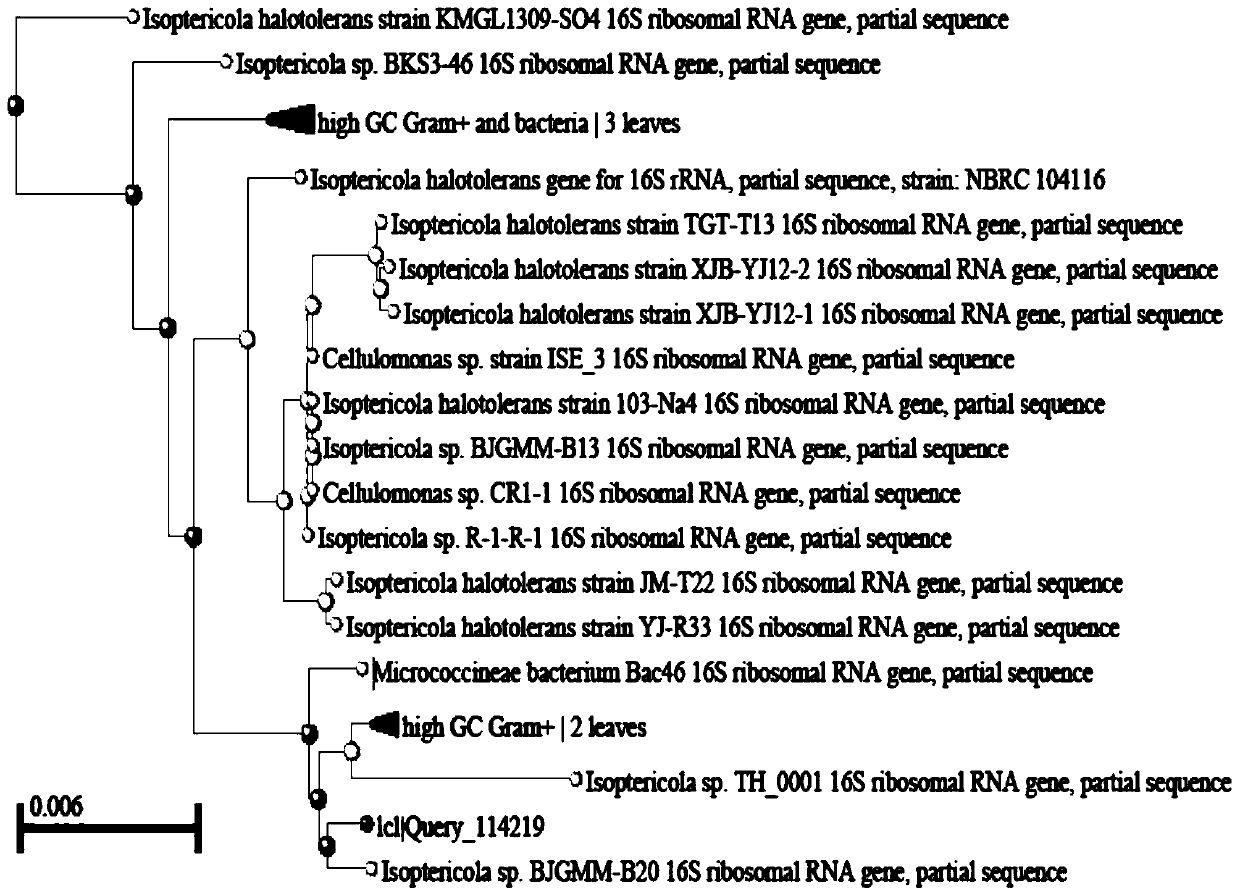
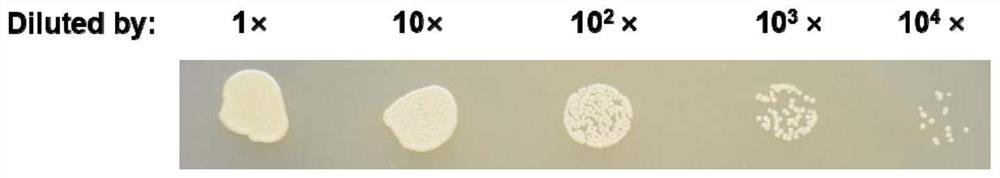
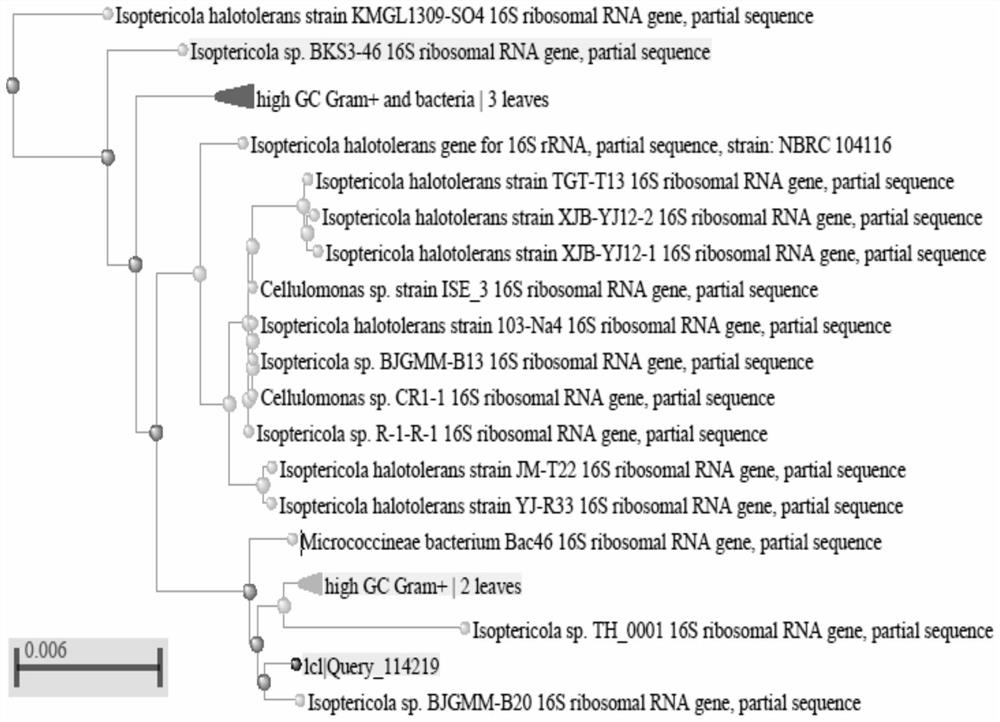
The invention belongs to the technical field of microbial genetic engineering, and particularly relates to a cold-adapted I-type 5-enolpyruvoyl shikimic acid-3-phosphate synthase gene. The nucleotidesequence of the I-type 5-enolpyruvylshikimic acid-3-phosphate synthase (EPSPS) gene is as represented by SEQ ID NO: 1 in a sequence table, and the sequence of a protein encoded by the gene is as represented by SEQ ID NO: 2. The cold-adapted I-type 5-enolpyruvoyl shikimic acid-3-phosphate synthase gene is obtained by cloning according to an aroA gene conserved sequence of Isoptericola sp. GR1TH_0001 reported in Genbank. Biological tests prove that the enzyme has high tolerance to glyphosate and has unique low-temperature adaptability.
Owner:HUAZHONG AGRI UNIV
Treatment of mycobacterium tuberculosis with antisense oligonucleotides
InactiveUS20060183676A1Reduced synthetase activityReduce the amount requiredAntibacterial agentsBiocideAntigenNucleotide
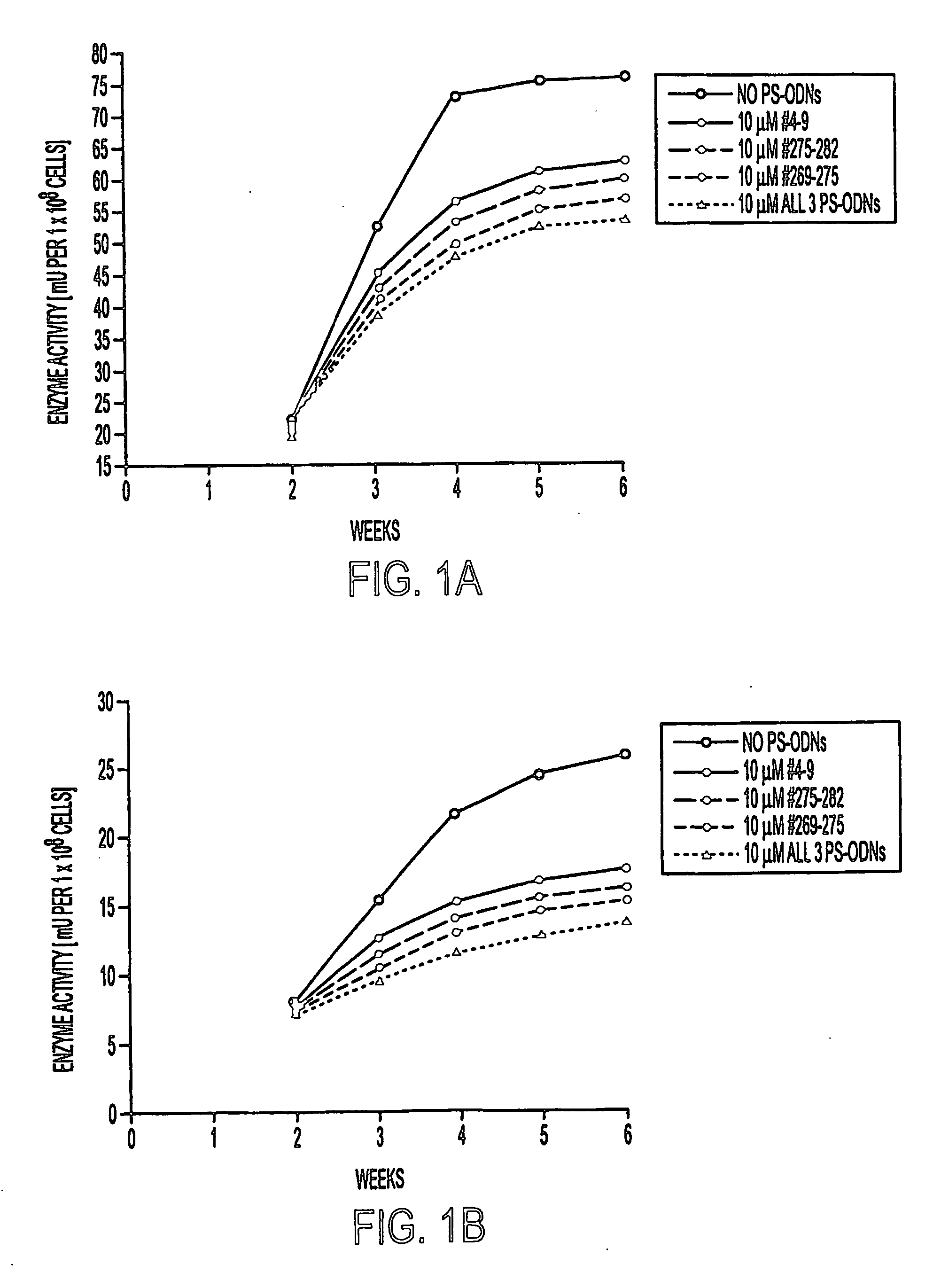
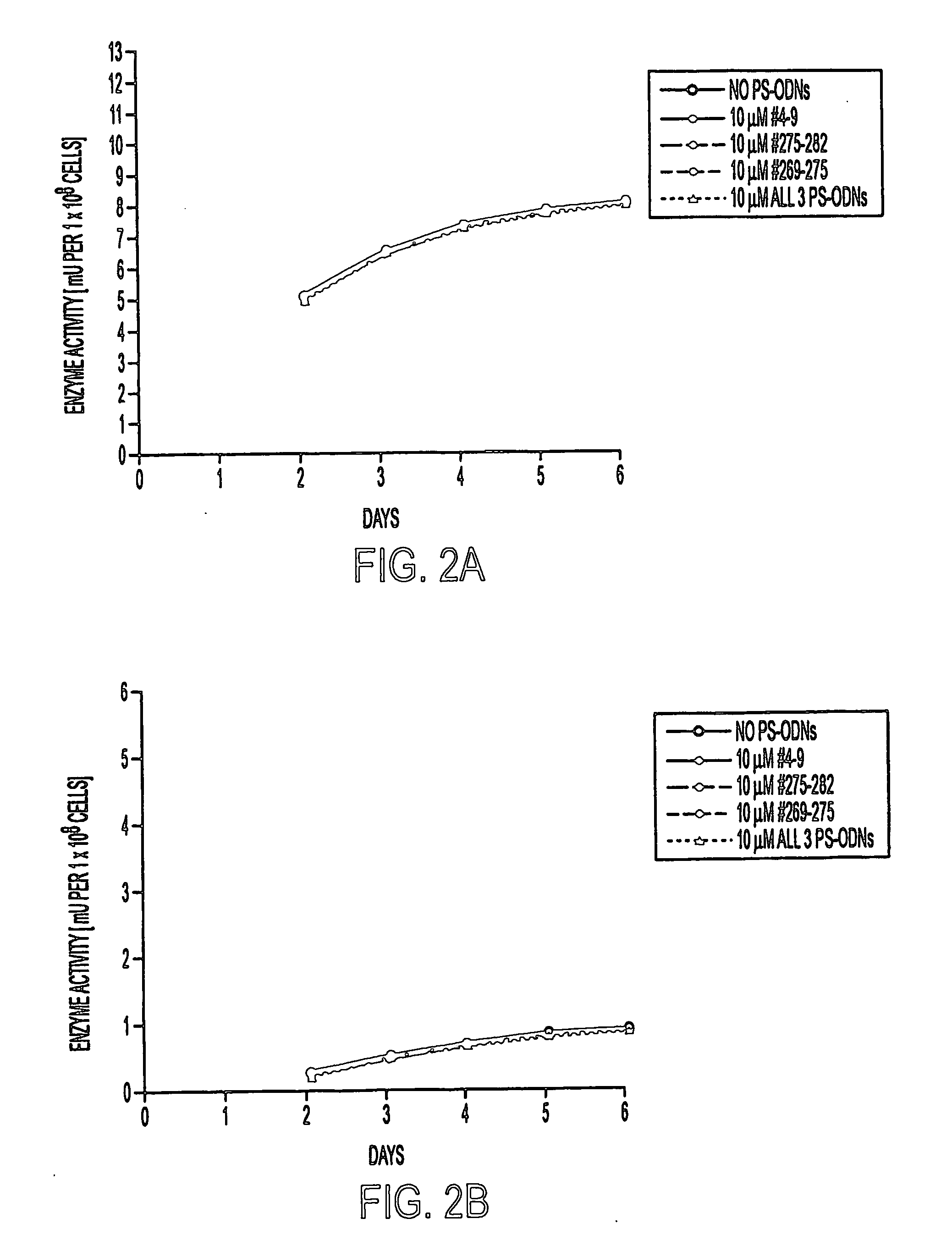
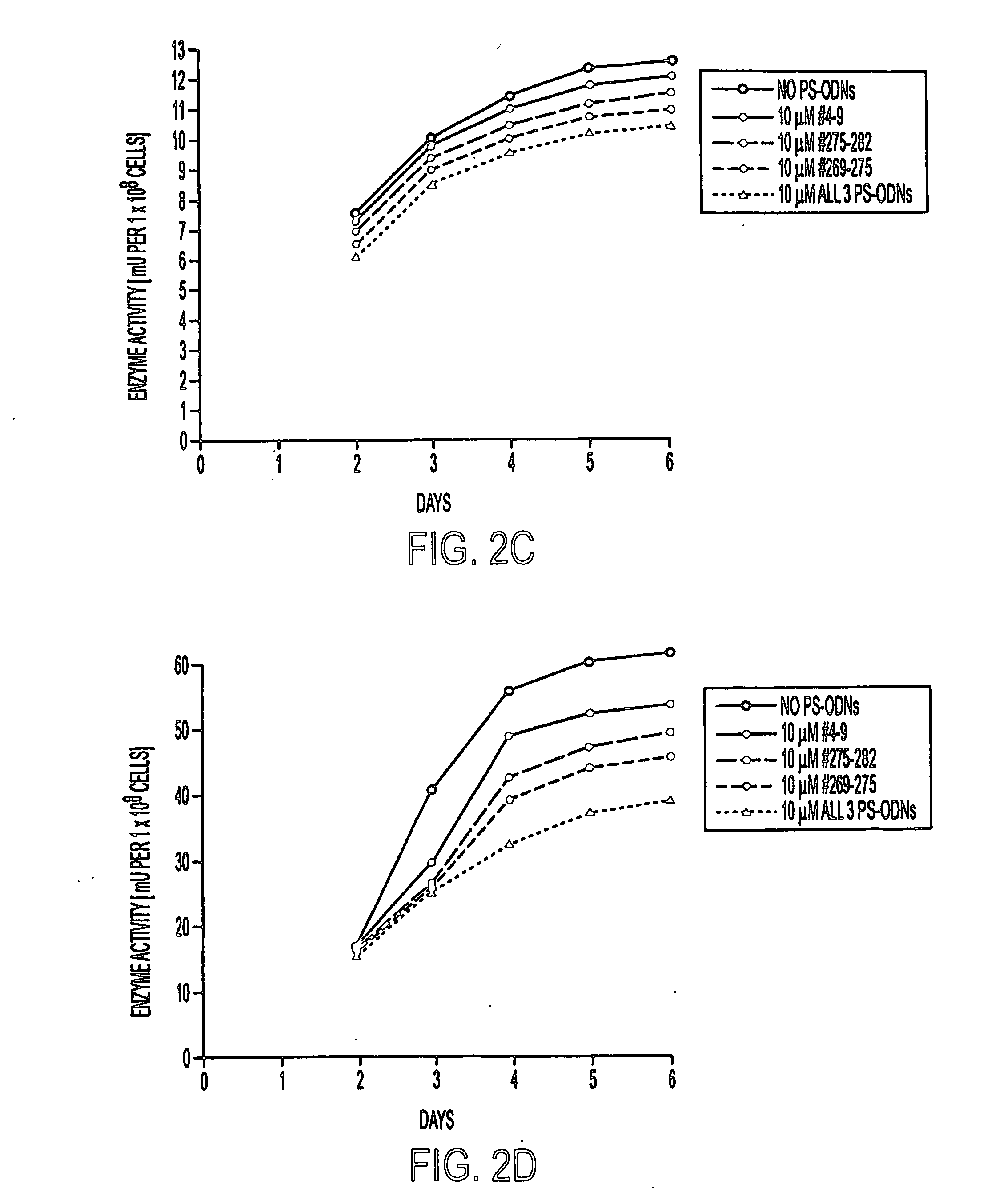
Methods of inhibiting the proliferation of Mycobacterium tuberculosis comprising contacting Mycobacterium tuberculosis with an effective amount of a polynucleotide complementary to an mRNA transcript expressed by Mycobacterium tuberculosis are provided. Typical methods of the invention utilize phosphorothioate modified antisense polynucleotides (PS-ODNs) against the mRNA of M. tuberculosis genes such as glutamine synthetase, aroA, ask, groES, and the genes of the Antigen 85 complex. Optionally, the methods employ multiple antisense polynucleotides targeting different Mycobacterium tuberculosis transcripts. In preferred embodiments of the invention, the antisense polynucleotides are complementary to the 5′ regions of the Mycobacterium tuberculosis transcripts.
Owner:RGT UNIV OF CALIFORNIA
Avian E. coli vaccine for protection against colibacillosis
ActiveUS7357935B2Prevention and amelioration of colibacillosisImprove responseAntibacterial agentsBacterial antigen ingredientsEscherichia coliDisease
A genetic deletion mutant live E. coli vaccine suitable for mass application to poultry, including chickens, is provided. Also provided is a safe and effective method to protect poultry against the ravages of Escherichia coli bacillosis infection and disease in which a live mutant aroA-gene deleted E. coli immunogen is administered to chickens, turkeys and the like via mass application routes such as coarse sprays and drinking water.
Owner:ZOETIS SERVICE LLC
Salmonella and immunogenic compostition containing the same as we as its use
ActiveUS20200055904A1Reduce the impactSuitable for transportationBacteria material medical ingredientsDepsipeptidesPharmaceutical medicineFlagellin
In a first aspect, the present invention relates to a mutated Salmonella strain comprising mutations in flagellin II genes, like the fliF gene, in particular, in addition the aroA gene, the IpxR gene, the pagP gene, the pagL gene, the ydiV gene and optionally the eptA gene and further optionally, the arnT gene. In a further aspect, immunogenic compositions comprising said Salmonella strain are provided optionally together with a pharmaceutically accepted carrier, diluent or effluent. Moreover, a method for producing outer membrane vesicles of Salmonella is provided, said method comprises the steps of cultivating the Salmonella strain according to the present invention and isolating the outer membrane vesicles accordingly. The present invention provides the bacteria or the outer membrane vesicles (OMVs) obtainable by the methods according to the present invention and its use as a transport moiety or as an immunogenic composition, like a vaccine or immunotherapy platform espedaily for therapeutic treatment of cancer of tissue or blood.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Haemophilus parasuis with double-gene deletion and construction method thereof
ActiveCN103865834BExcellent immune efficiencyAntibacterial agentsBacteriaBiological propertyVirulent characteristics
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST
A cold-adapted type I 5-enolpyruvylshikimate-3-phosphate synthase gene
The invention belongs to the technical field of microbial genetic engineering, and in particular relates to a cold-adapted type I 5-enolpyruvylshikimate-3-phosphate synthase gene. The nucleotide sequence of the type I 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene is as described in the sequence listing SEQ ID NO: 1, and the protein sequence encoded by the gene is as SEQ ID NO: 2 mentioned. The cold-adapted type I 5-enolpyruvyl shikimic acid-3-phosphate synthase gene was cloned according to the conserved sequence of the aroA gene of the halophilic termite fungus Isoptericola sp.GR1TH_0001 reported in Genbank, and verified by biological experiments , proving that the enzyme has high tolerance to glyphosate and has unique low temperature adaptability.
Owner:HUAZHONG AGRI UNIV
Method and primer pair for detecting transgenic G2-aroA gene herbicide-resisting corn G1105E-823C
The invention discloses a method and a primer pair for detecting transgenic G2-aroA gene herbicide-resisting corn G1105E-823C. The primer pair is specifically a primer pair group for detection or auxiliary detection on whether corn to be detected is transgenic G2-aroA gene herbicide-resisting corn G1105E-823C or not; the primer pair consists of a primer pair 1 and a primer pair 2; the primer pair 1 consists of two single-chain DNA molecules as shown in a sequence 1 and a sequence 2 in a sequence list; the primer pair 2 consists of two single-chain DNA molecules as shown in a sequence 3 and a sequence 4 in the sequence list. Experiment shows that whether the corn to be detected is transgenic G2-aroA gene herbicide-resisting corn G1105E-823C or not can be detected through the primer pair 1 and the primer pair 2 by using a PCR method, and the method is high in accuracy rate, high in specificity and high in sensitivity.
Owner:BEIJING ORIGIN SEED TECH
Avian Escherichia coli vaccine strain
ActiveCN112442473AMaintain immunogenicityAntibacterial agentsBacteriaESCHERICHIA COLI ANTIGENIntramuscular injection
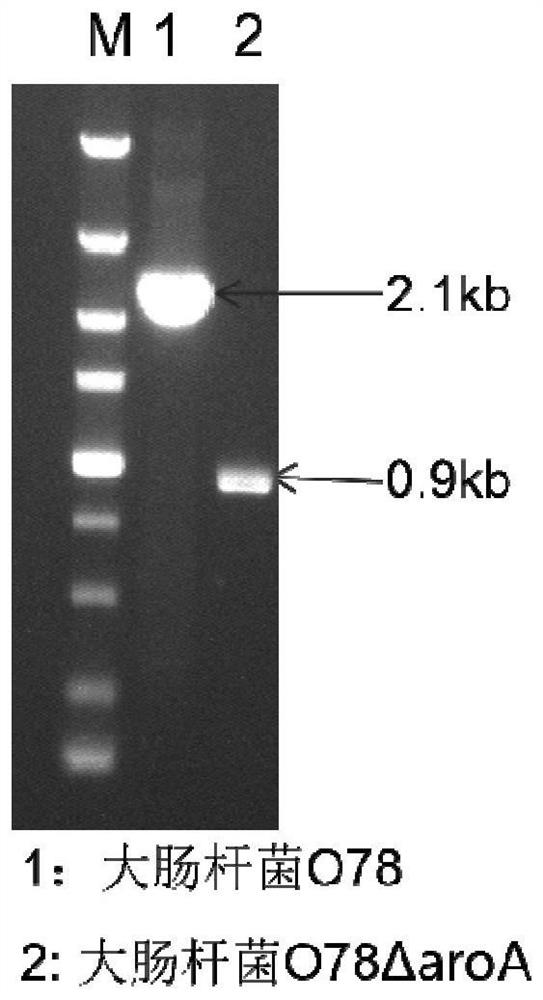
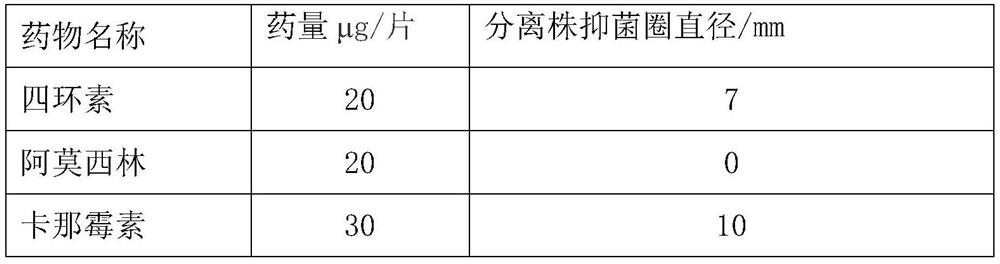
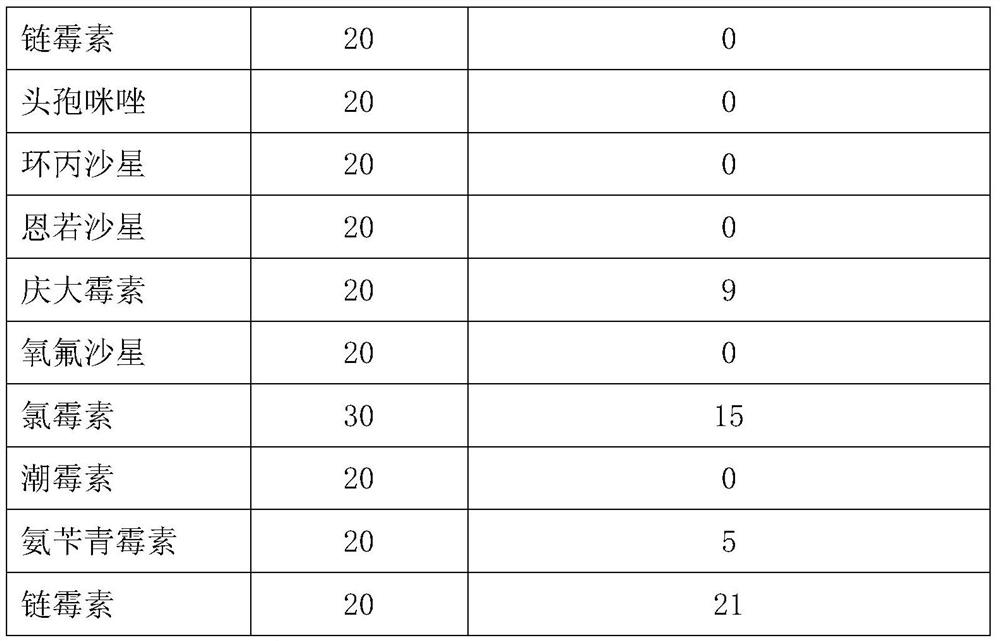
The invention provides an avian Escherichia coli vaccine strain. The avian Escherichia coli vaccine strain is a low virulent strain prepared by removing aroA genes on the basis of screening to obtainnovel avian pathogenic Escherichia coli O78. The avian Escherichia coli vaccine strain provided by the invention is prepared by deleting the aroA genes of an avian pathogenic Escherichia coli YBO78 strain with the preservation number of CCTCC M 2020382. The Escherichia coli vaccine YBO78-1 strain provided by the invention can be used by a conventional method, for example, the strain is applied ona large scale by economical and easy-to-operate spray and drinking water, and can also be applied by intramuscular injection or other modes.
Owner:YEBIO BIOENG OF QINGDAO
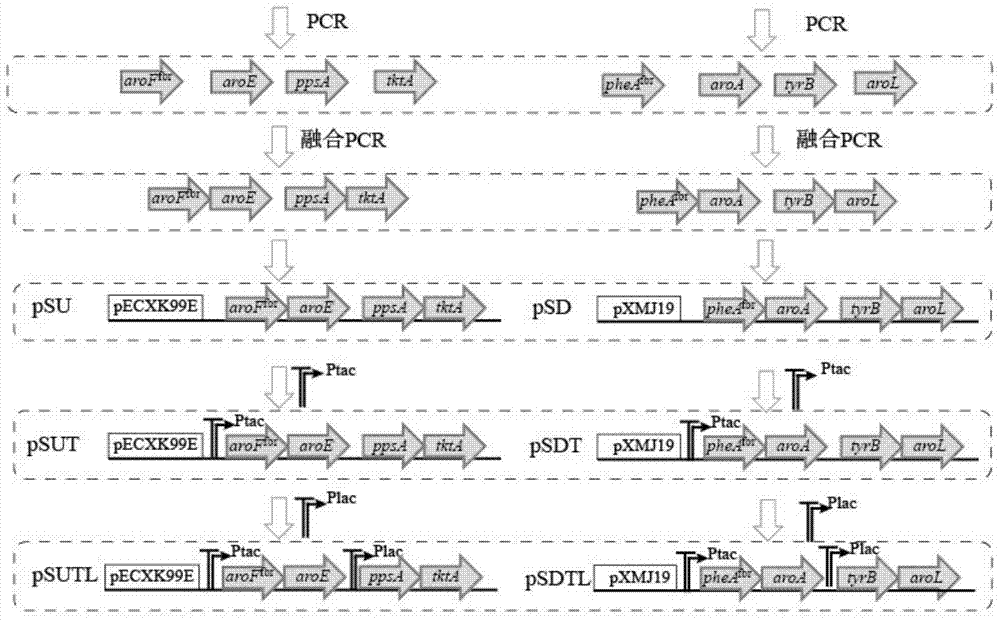
A recombinant Corynebacterium glutamicum producing l-phenylalanine and its construction and application
InactiveCN104531597BIncrease productionBacteriaTransferasesEscherichia coliCorynebacterium efficiens
The invention discloses a recombined Corynebacterium glutamicum for producing L-Phe and a constructing method and the application thereof, and belongs to the field of metabolic engineering. In Corynebacterium glutamicum ATCC 13032, two shuttle expression vectors pEC-XK99E and pXMJ19 of the Corynebacterium glutamicum and escherichia coli are used for expressing eight key enzyme genes comprising aroF<fbr>, tktA, ppsA, aroL, pheA<fbr>, aroE, aroA and tyrB in a L-Phe synthetic route, two promoters Ptac and Plac of different intensities are used for carrying out combinational expression on the eight genes to improve the L-Phe yield, the L-Phe yield can reach as high as 5.59+ / -0.11 g / l, and accumulated shikimic acid is 0.31+ / -0.11 g / L. According to the method, over expression is achieved for the L-Phe shikimic acid key enzyme genes, and the yield of L-Phe produced by the Corynebacterium glutamicum in a fermentation mode is improved.
Owner:JIANGNAN UNIV +1
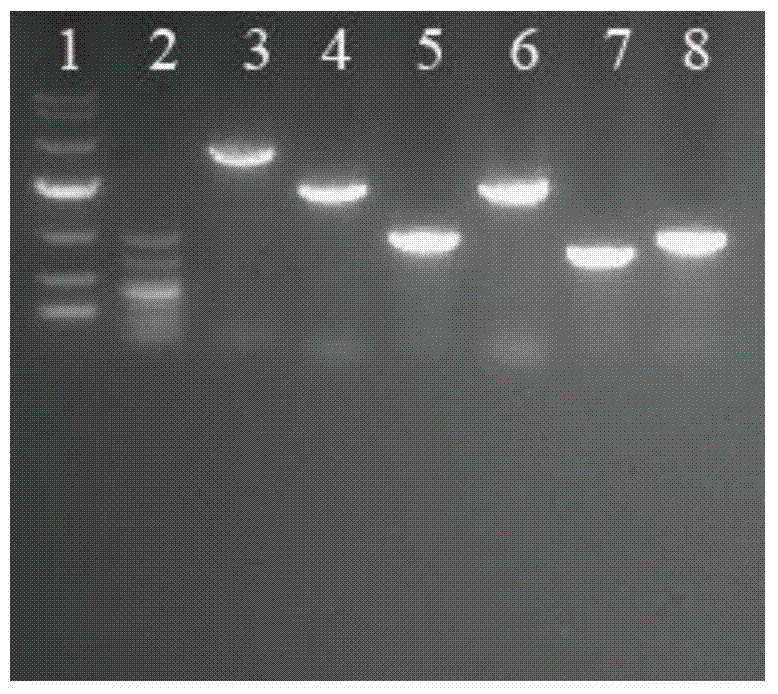
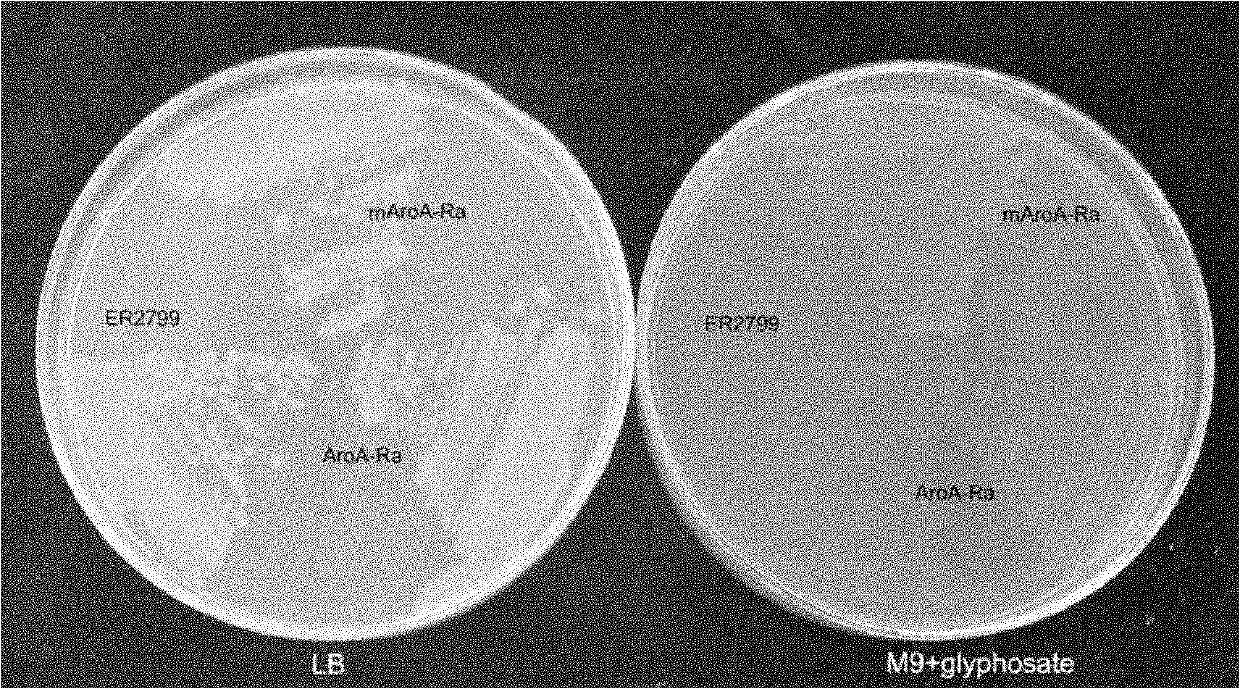
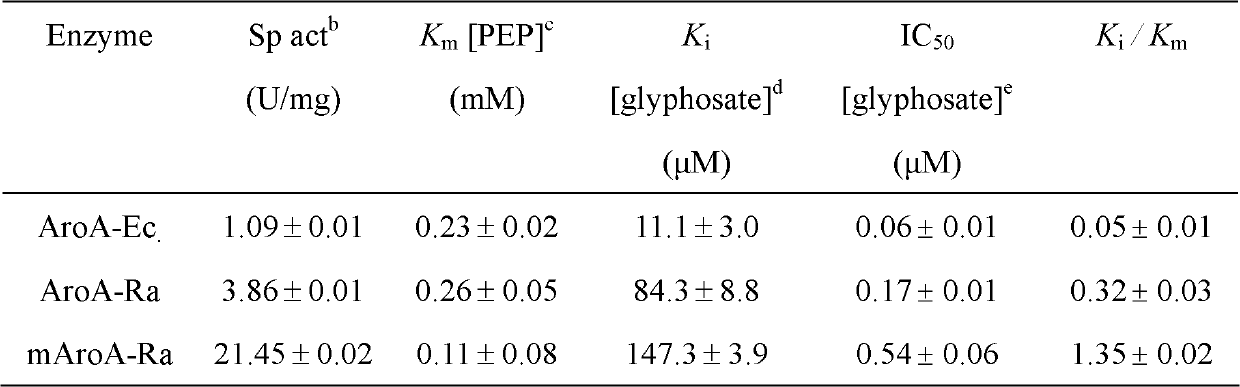
EPSP synthase AroA-Ra multisite mutant of rahnella aquatilis
InactiveCN102559708BIncrease resistanceHigh affinityDNA/RNA fragmentationNucleotideMolecular rearrangement
The invention relates to an EPSP synthase AroA-Ra multisite mutant of a rahnella aquatilis, particularly to the multisite mutant improved from the gene coded as 5- enol acetone shikimic acid-3-EPSP synthase of the rahnella aquatilis through DNA molecular rearrangement method and function complementation method, and the resistance of the improved multisite mutant to the glyphosate is greatly improved. The nucleotide sequence of the multisite mutant is as indicated by SEQ ID No1, the encoded amino acid sequence is as indicated by the SEQ ID No2, and the mutant has 6 different mutational sites under the same molecule. The multisite mutant not only retains the high affinity of the original EPSP synthase toward the PEP, but also reduces the affinity of the EPSP synthase toward the glyphosate herbicide, so that the glyphosate resistance of the mutant is greatly improved.
Owner:SHANGHAI ACAD OF AGRI SCI +1
Gene site-directed mutation method and glyphosate-resistant gene obtained by said method and its expression vector and trans foring factor
InactiveCN1226411CHigh activityReduce sensitivityFermentationPlant genotype modificationBase JEnzyme Gene
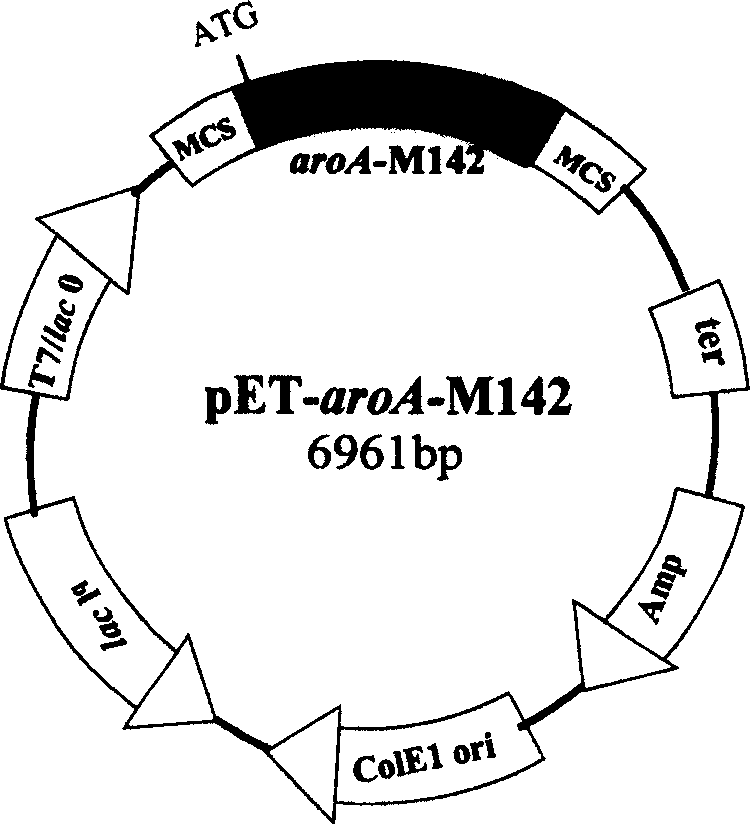
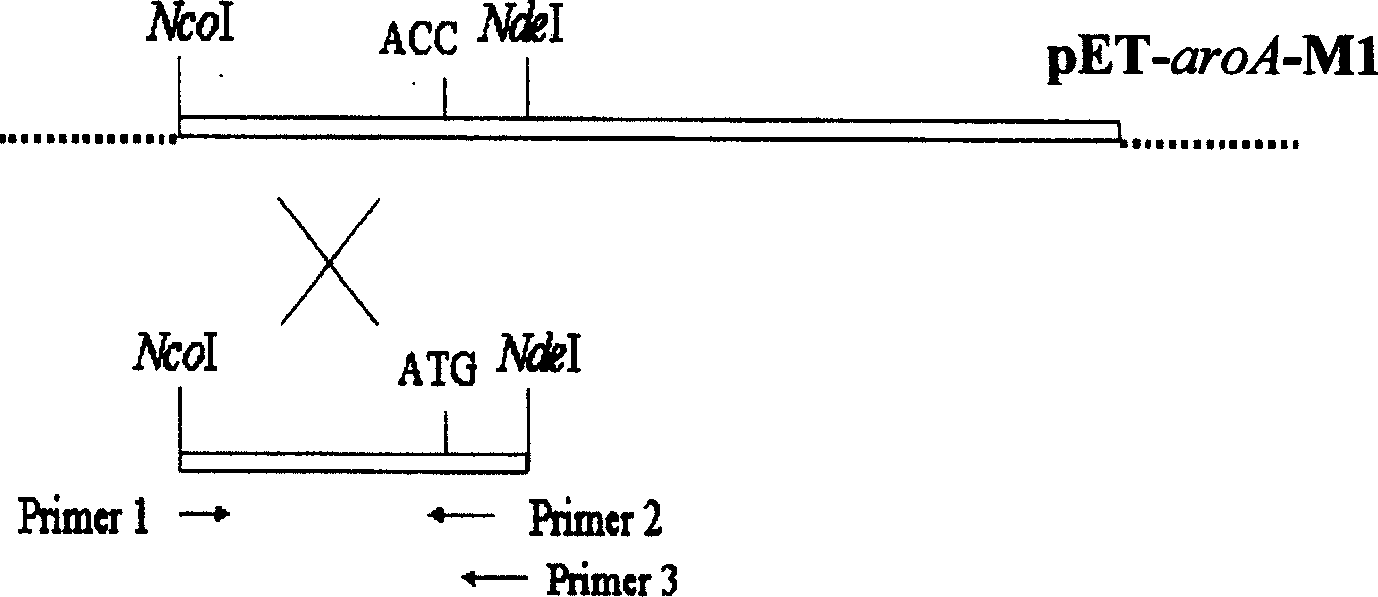
The invention refers to a gene-pointing mutation method, reformed aroA gene and carrier and transformer containing said gene. The method selects DNA, sequence that the distance between the point to be mutated and the monozyme pitch in point is 10-20 bases as source mould, according to sequence character designs the corresponding one 5'-end solicitatino and two 3'-end solicitation, and adopts two-step PCR expanding reaction to obtain pad-in point mutation gene segment. The invention makes further improvement on the gene aroA-M1 to obtain EPSP in order to compound enzyme gene aroA-M142 which makes further improvement on enzyme character.
Owner:SUN YAT SEN UNIV
Method for detecting roundup ready protein G2-aroA and special enzyme linked immune kit
The invention discloses a method for detecting a roundup ready protein G2-aroA and a special enzyme linked immune kit. The enzyme linked immune kit contains independently packaged anti-G2-aroA protein monoclonal antibody; the amino acid sequence of the G2-aroA protein refers to sequence 9 in a sequence list; and the monoclonal antibody is generated by a hybridoma cell strain AntiG2-5F11 CGMCC No.5772. The enzyme linked immune kit provided by the invention can be used for qualitatively or quantitatively detecting the expression of the roundup ready protein G2-aroA in transgenic plants such as corn, and has the advantages of rapidness, sensitiveness and strong specificity.
Owner:BEIJING ORIGIN SEED TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com