Bordetella bronchiseptica gene deletion strain, vaccine prepared from Bordetella bronchiseptica gene deletion strain and application
A technology of gene deletion strain, Bordetella sanguinis, applied in the field of animal bacterial genetic engineering, can solve problems such as deletion, and achieve the effects of stable traits, clear genetic background and good biological safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Construction and identification of Bordetella bronchiseptica LY1aroA gene deletion strain
[0026] 1. Primer design (for gene cloning and molecular detection)
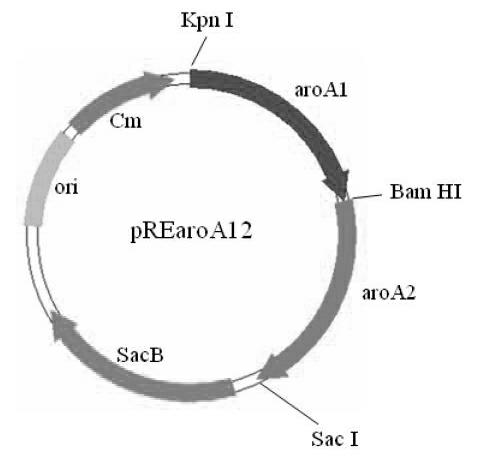
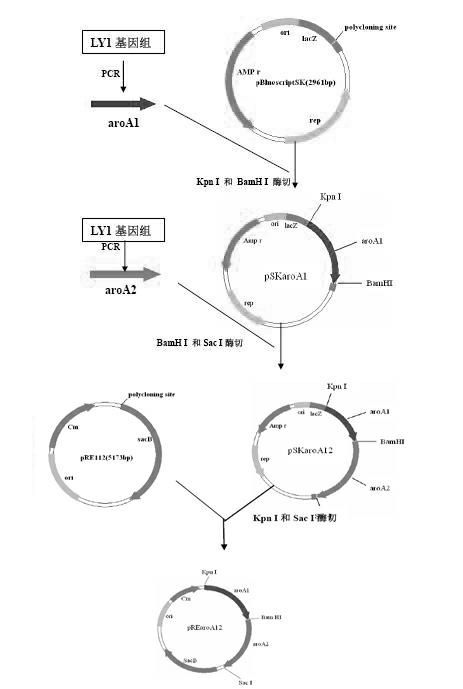
[0027] According to the aroA gene sequence (GenBank No: AF315119) of the reported Bordetella bronchiseptica strain RB50, two pairs of primers (ar1 / ar2 and ar3 / ar4, see Table 1) were designed to obtain the virulent strain of Bordetella bronchiseptica. LY1 (LY1 is a virulent strain of wild-type Bordetella bronchiseptica isolated from pigs, which was deposited in the China Center for Type Culture Collection (CCTCC) on December 23, 2011, and the preservation number: CCTCC NO: M2011478.) in the genome Respectively amplify the upstream and downstream fragments aroA1 (upper arm) and aroA2 (lower arm) of the aroA gene. The sizes of the amplified fragments are 927bp and 887bp respectively. The Kpn I and BamH I restriction sites are introduced into the two ends of the upper arm and the two ends of the lower arm ...
Embodiment 2
[0039] Example 2 Bordetella bronchiseptica gene deletion strain aroA - Analysis of growth characteristics of LY1
[0040] gene deletion strain aroA - LY1 was cultured at 37°C for 36 hours on a TSA plate containing 10% calf serum, and the diameter of the colony was about 0.7mm, which was significantly smaller than that of the parent strain LY1 (1.5mm) (see Figure 5 ). Parent strain LY1 and gene deletion strain aroA - LY1 from 10 6 CFU / mL to start culturing, sampling every 1h, and counting viable bacteria. The results showed that the gene deletion strain aroA - LY1 grew significantly slower than the parental strain LY1 (see Image 6 ), the mean generation interval (Mean generation time) was 38.7min, which was 12.6min longer than that of the parental strain (26.1min), indicating that the growth rate of the gene deletion strain LY1 was significantly slower than that of the parental strain LY1.
Embodiment 3
[0041] Example 3 Gene deletion strain aroA - Genetic stability of LY1
[0042] The gene deletion strain aroA prepared by the present invention - Streak culture of LY1 on a TSA plate containing 10% calf serum, pick a single colony on the TSA plate containing 10% calf serum, culture in a 37°C incubator for 24 hours, and then pick a single colony on the cultured plate Transfer to a TSA plate containing 10% calf serum for 50 consecutive transfers, and observe the colony size. The results showed that the gene deletion strain aroA - The colony diameter of LY1 is still about 0.7mm, which is significantly smaller than that of the parent strain LY1 (about 1.5mm), and still conforms to aroA - The growth rate of the LY1 gene deletion strain was obviously slow. At the same time, use primers ar1 / ar4 (see Table 1) to carry out PCR identification, gene deletion strain aroA - Inheritance of the aroA deletion gene in LY1 see Figure 7 . Figure 7 It shows that the 1, 10, 20, 30, 40 and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com