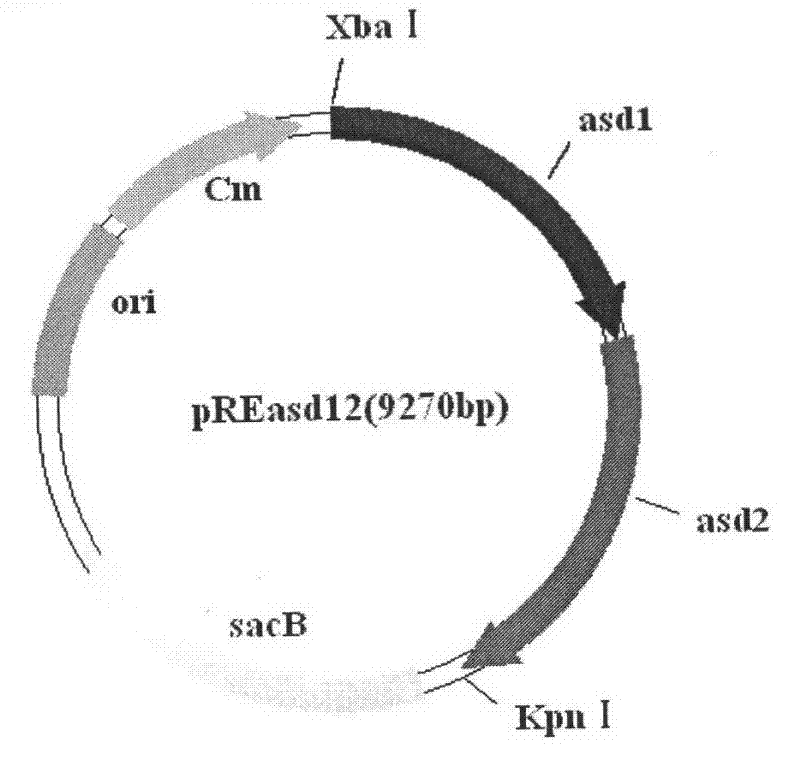
Patents
Literature
53results about How to "Comply with biosafety requirements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
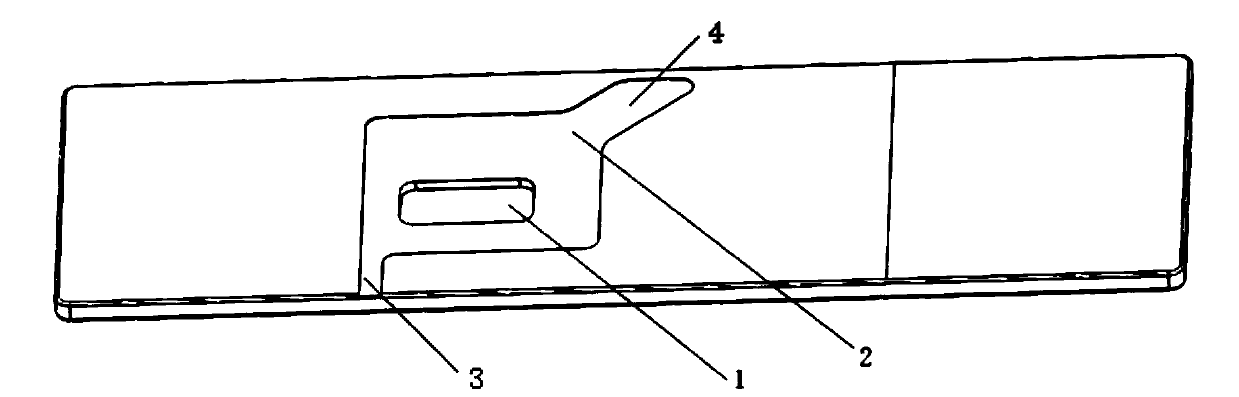
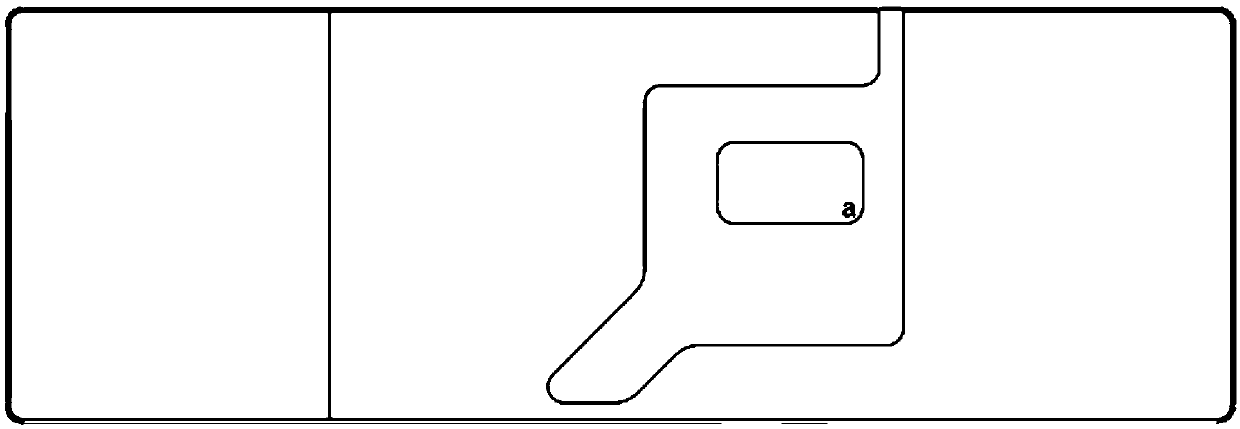
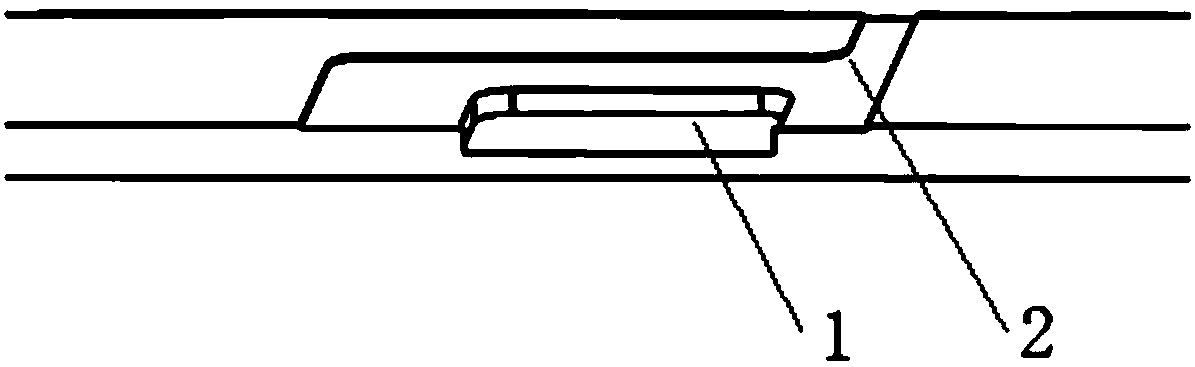
Interlayer cup for collecting worm eggs and test method thereof
PendingCN106769327AAvoid discomfortImprove the detection ratePreparing sample for investigationSludgeEngineering
The invention discloses an interlayer cup for collecting worm eggs and a test method thereof. The interlayer cup comprises a cup body, a filtering screen and a retaining film, wherein the cup body is equipped with a top cover and a bottom cover; the filtering screen and the retaining film are arranged in the cup body so as to separate the cup body into three parts; the filtering screen is arranged close to the top cover; the retaining film is arranged close to the bottom cover. The invention has the beneficial effects that samples are concentrated through the retaining film, the detection rate of the worm eggs can be obviously increased and all the worm eggs in the samples can enter into a reading link; the whole process is performed in a device; the effluent is gathered together; the operation environment is free from sample or odor overflow; the biosafety requirement for the laboratory is met; the discomfort of the experimenter for the odor and perception of the sample test can be overcome; the operation is simple and convenient; the completing time for each sample is within 3 minutes; the interlayer cup is suitable for the parasitic ovum test for the parasitic ovum-containing water and for various samples, such as, sludge and excrements; manual coating is not required; the egg collection and the coating are simultaneously completed; the labor cost can be reduced.
Owner:HUNAN TECH NEW MEDICAL SYST
Mouse-typhus salmonella gene-deletion mutant strain without containing resistance marks, vaccine and application thereof
InactiveCN102140430AReduced toxicityGood securityAntibacterial agentsBacteriaDeletion mutantGenetic engineering
The invention relates to a mouse-typhus salmonella gene-deletion mutant strain without containing resistance marks, a vaccine and application thereof, belonging to the technical field of animal bacterium genetic engineering. The invention utilizes a mediated allelic exchange technique of recombinant suicidal plasmid for obtaining the mouse-typhus salmonella gene-deletion mutant strain (Salmonellatyphimurium SL13441) (the preservation number is CCTCC NO: M2010301) without containing the resistance marks. The mutant strain is deficient in an important toxicity regulating gene-cya gene in mouse-typhus salmonella, so that the toxicity of the mouse-typhus salmonella is obviously weakened after the deficiency of the cya gene, but the mouse-typhus salmonella still has immunogenicity. The invention also discloses the Salmonella gullinarum gene-deletion vaccine prepared by utilizing the gene-deletion mutant strain and an application of the gene-deletion mutant strain in preparing the vaccine, and the prepared vaccine meets the requirement on biological safety. The gene-deletion vaccine prepared in the invention can effectively prevent infection of Salmonella gullinarum and has the advantages of good stability and safety and low cost, is convenient to use and has obvious economic and social benefits.
Owner:HENAN UNIV OF SCI & TECH
Vaccine for differentiating porcine actinobacillus pleuropneumonia serum 7-type double-gene deletion mutant of vaccine immunity and virus infection animal and application thereof
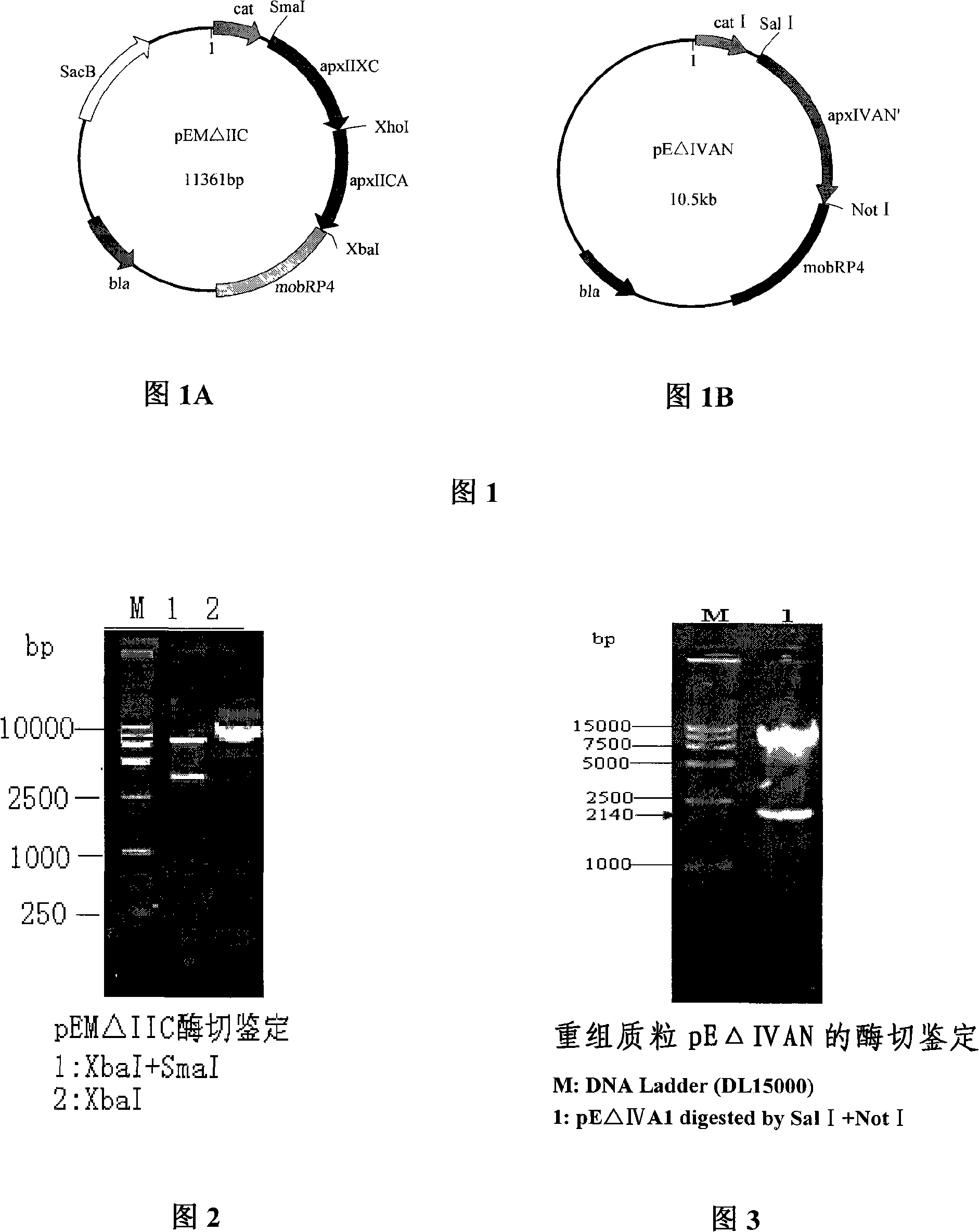
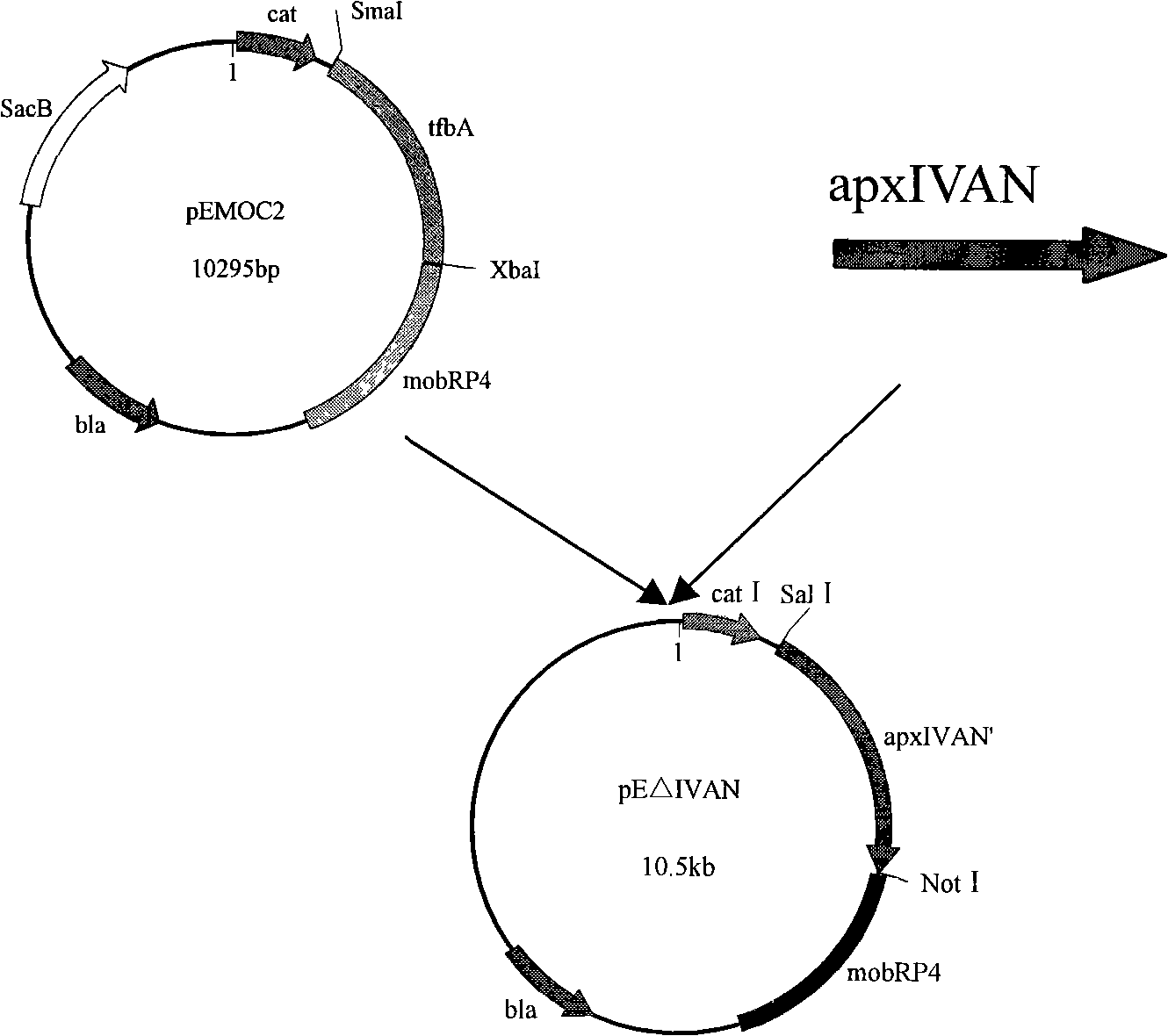
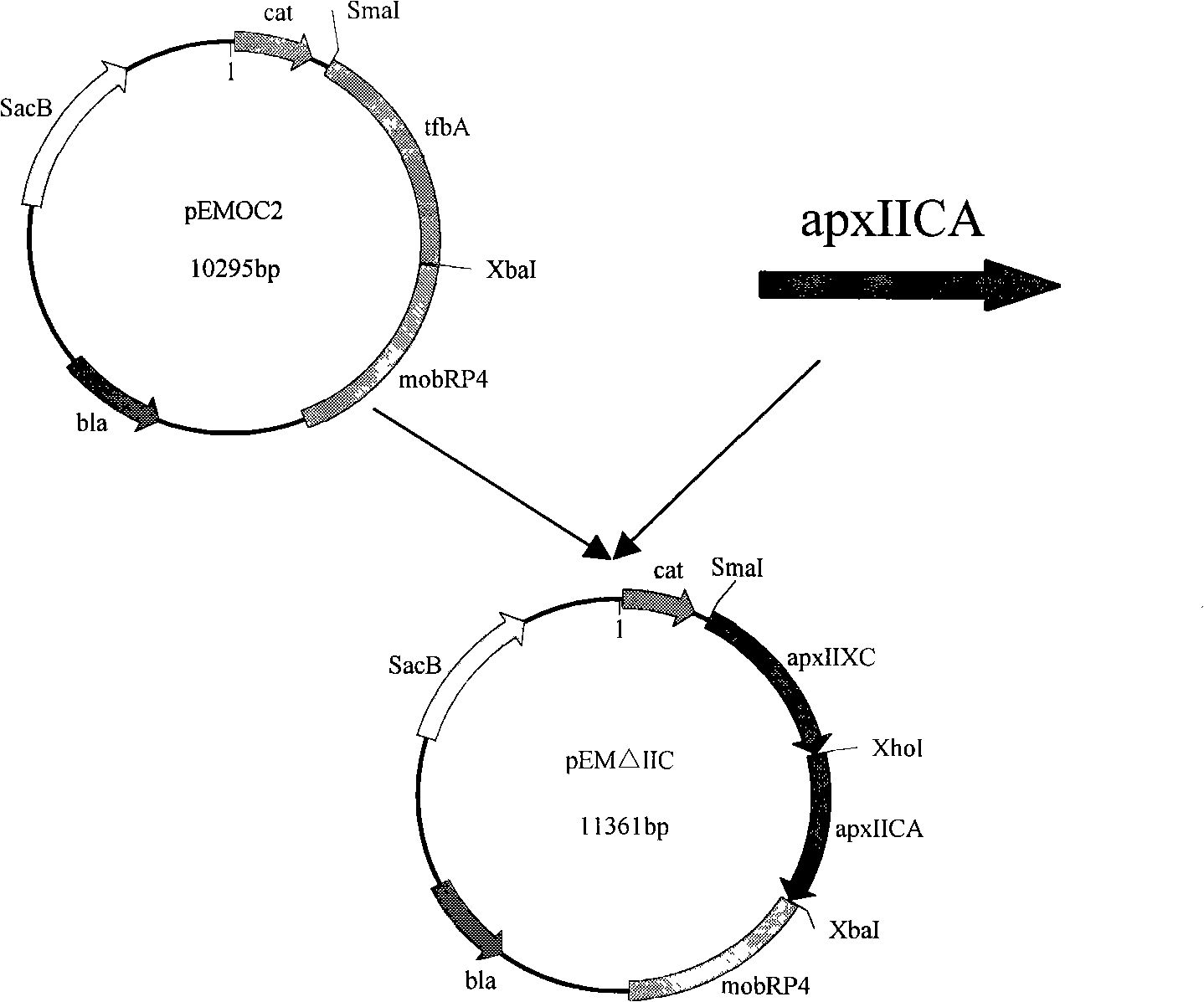
InactiveCN101265457AStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsGenetic engineeringToxin
The invention belongs to the animal bacteria genetic engineering field, and relates particularly to construction of a vaccine for a serotype-7 double gene deletion mutation strain for differentiating actinobacillus pleuropneumoniae of swine of a vaccine immunized animal and a wild virus infected animal, and the application of the strain. In the invention, a serotype-7 double gene deletion mutation strain APP-7-mut01 for differentiating actinobacillus pleuropneumoniae of swine of a vaccine immunized animal and a wild virus infected animal (with accession number of CCTCC NO:M207004) is obtained. The strain deletes two main virulence factors of apxIIC and apxIVAN of APP serotype-7. The mutant strain is safe for swines, and has toxins of ApxIIA and ApxIVAC protein, thereby ensuring the immunity efficacy of the vaccine. The vaccine of the mutant strain is used to immunize animals, so that antibodies aiming at Apx IVAN protein are no longer produced inside the animals, however wild virus infection can produce antibodies aiming at ApxIVAN. The invention further discloses the construction, the vaccine preparation and the application of the double gene deletion mutation strain.
Owner:HUAZHONG AGRI UNIV
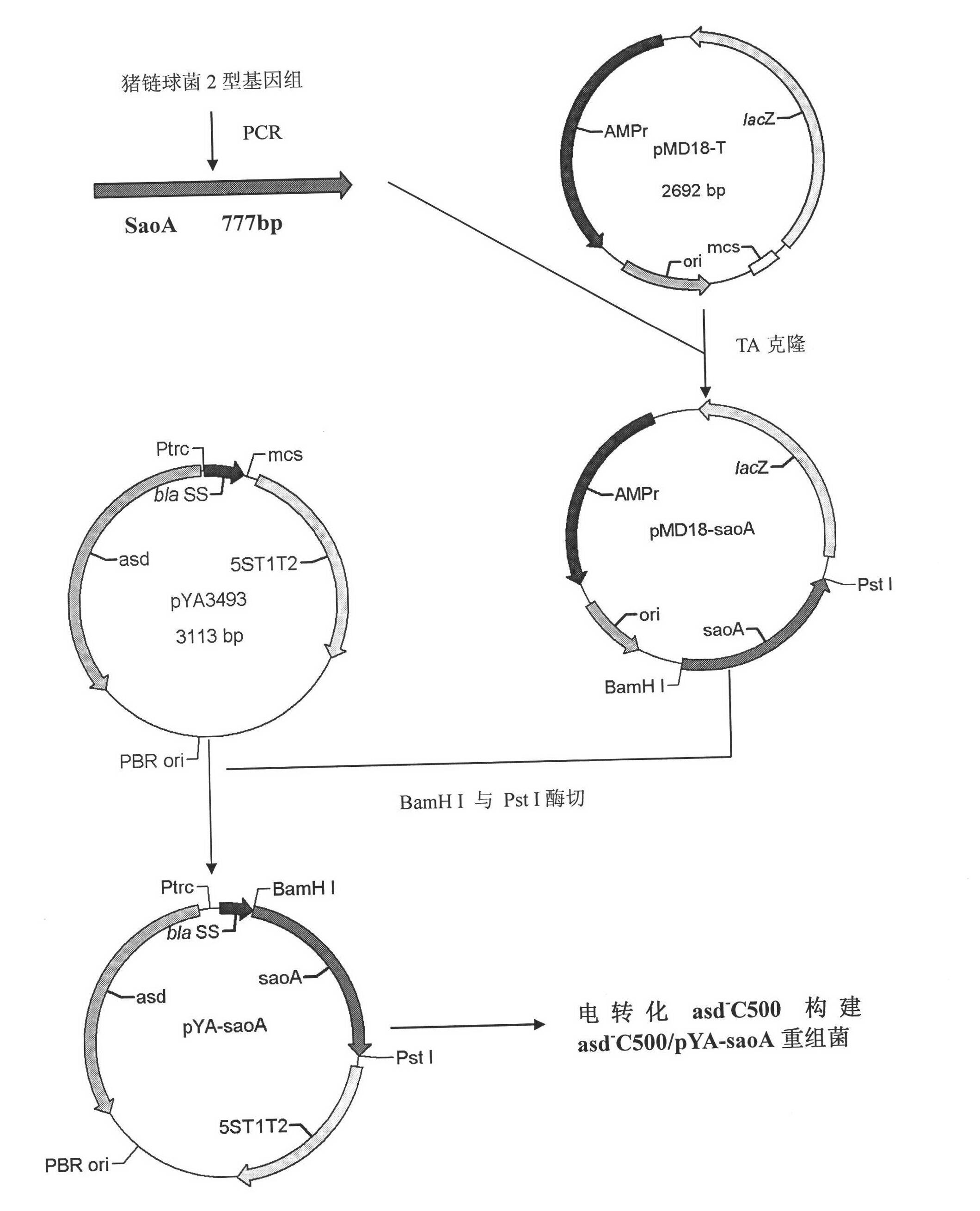
Recombinant Salmonella choleraesuis for expressing surface antigen gene sao of streptococcus suis type 2, vaccine and application
ActiveCN101979501AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesAntigen
The invention belongs to the field of animal bacterium gene engineering, and in particular relates to construction of resistance marker-free recombinant Salmonella choleraesuis for expressing surface antigen sao gene segment of streptococcus suis type 2, preparation of a vaccine and application. The resistance marker-free recombinant Salmonella choleraesuis for expressing the surface antigen sao gene segment of the streptococcus suis type 2, namely asd-C500 / Pya-saoA is obtained, and the collection number is CCTCC NO: M2010156. The asd gene of the Salmonella choleraesuis is deleted in the recombinant strain, and the recombinant strain contains plasmid pYA-saoA capable of expressing the asd and the sao gene segment of the streptococcus suis type 2. The invention also discloses a method for preparing the recombinant strain and the vaccine, and application in preparing Salmonella choleraesuis-streptococcus suis type 2 vaccines.
Owner:HUAZHONG AGRI UNIV +1
Genetic engineering live vaccine of recombinant Salmonella choleraesuis and Porcine epidemic diarrhea virus, preparation and application
ActiveCN103013895APreserve immune efficiencyHigh biosecurityAntibacterial agentsBacteriaBacteroidesBacterial genetics
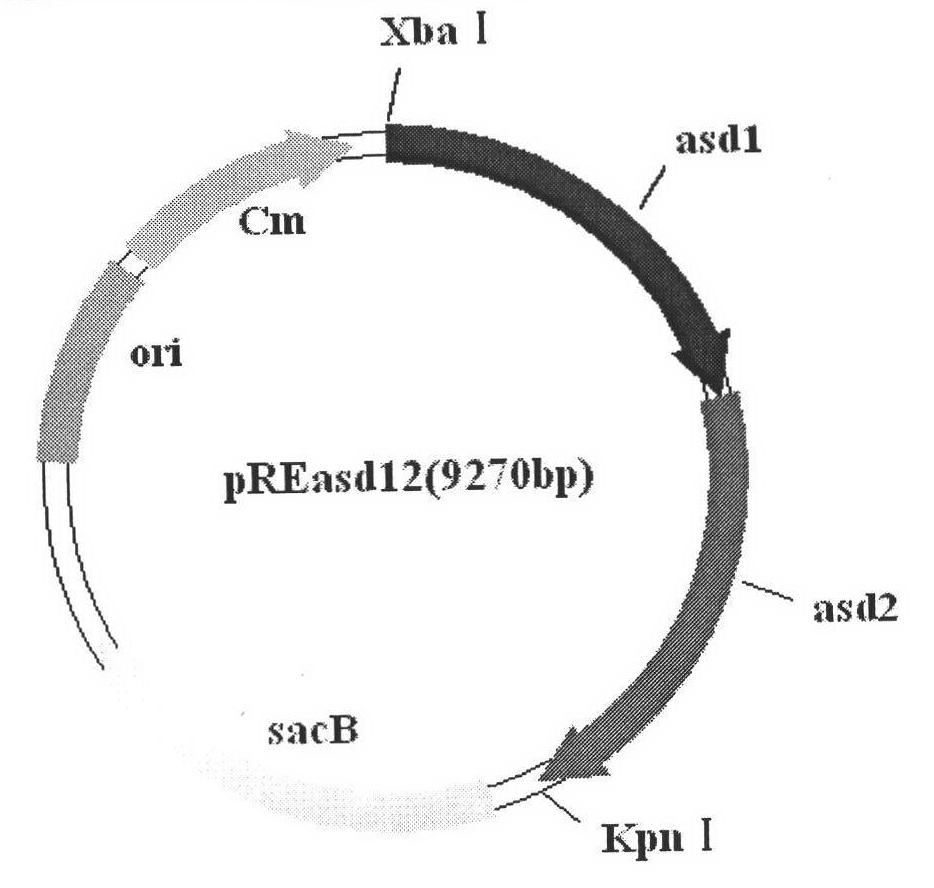
The invention belongs to the technical field of animal bacterial genetic engineering, and concretely relates to a construction of recombinant Salmonella choleraesuis strains C501-Coe and C501-SD with no resistance marker and expressing main antigenic sites of porcine epidemic diarrhea virus, a preparation of a vaccine and an application. The invention obtains the recombinant Salmonella choleraesuis strains C501-Coe and C501-SD with no resistance marker and expressing the main antigenic sites of the porcine epidemic diarrhea virus, and the strains have the following accession number respectively: CCTCC NO: M2011296 and CCTCC NO: M2011297. The two recombinant strains are deleted with an asd gene necessary for growth of the S. choleraesuis, and contain plasmids which can express the asd gene, as well as a COE gene fragment and a SD gene fragment of the Porcine epidemic diarrhea virus in the strains. <{EN3}>The invention further discloses a method and an application by using the recombinant strain to prepare the vaccine of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus. <{EN4}>The vaccine provided by the invention can stimulate swine to generate a protective immunization reaction for resisting the Salmonella choleraesuis and the Porcine epidemic diarrhea virus, and can effectively prevent infection of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus. The invention further discloses a method and an application by using the recombinant strain to prepare the vaccine of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus. The vaccine provided by the invention can stimulate swine to generate a protective immunization reaction for resisting the Salmonella choleraesuis and the Porcine epidemic diarrhea virus, and can effectively prevent infection of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus.
Owner:HUAZHONG AGRI UNIV +1
Recombinant salmonella choleraesuis, bivalent genetic engineering vaccine and application
InactiveCN101880647AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacteriaBacteroidesRecombinant vaccines
The invention belongs to the technical field of bacterial gene engineering of animals, and in particular relates to the construction of a recombinant salmonella choleraesuis strain which does not contain resistance markers and expresses major antigenic loci of porcine transmissible gastroenteritis virus, vaccine preparation and application. In the recombinant salmonella choleraesuis strain C500 (pYA-2SLN) which does not contain the resistance markers and expresses the major antigenic loci of the porcine transmissible gastroenteritis virus, the preservation No. is CCTCC NO: M 209189, and the strain misses asd genes which are necessary for the growth of salmonella choleraesuis and contains plasmid capable of expressing the asd genes, genes of an antigenic locus A, an antigenic locus D and an antigenic locus N321 of the porcine transmissible gastroenteritis virus in the strain. The invention also discloses a method for preparing the salmonella choleraesuis and porcine transmissible gastroenteritis vaccine by utilizing the recombinant strain and application thereof. The prepared recombinant vaccine can stimulate pigs to generate protective immune response for resisting the salmonella choleraesuis and the porcine transmissible gastroenteritis virus, and prevent the infection of the salmonella choleraesuis and the porcine transmissible gastroenteritis virus effectively.
Owner:HUAZHONG AGRI UNIV
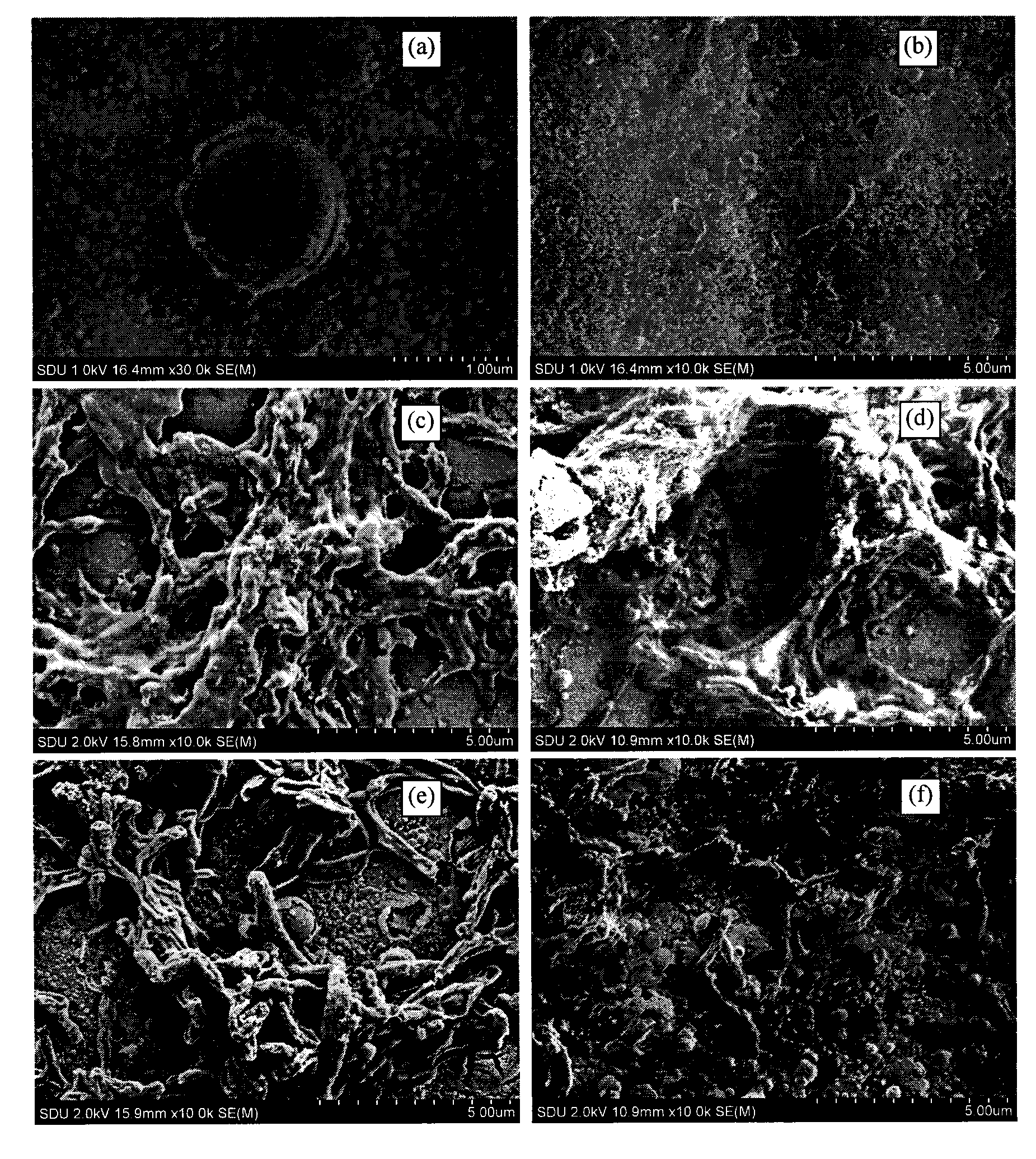
Hydroxylapatite-bioglass film and preparation technology thereof
InactiveCN101549172ANo toxicityGood biocompatibilityVacuum evaporation coatingSputtering coatingApatiteWhole body
The invention discloses a hydroxylapatite-bioglass film, which is prepared by taking hydroxylapatite or bioglass or the mixture of both as a target material and using the following technology of: adopting pulse laser with energy density of 5J-cm to complete the sedimentation of the film in an argon atmosphere with underlayer temperature of 15 DEG C to 600 DEG C and pressure of 5Pa to 45Pa or an oxygen atmosphere with pressure of 1*10-9*10Pa and under the condition with pulse number of 3,000-18,000. The bio-film has no cytotoxic effect and good biocompatibility, causes no hemolytic reaction and acute systemic toxicity effect, imposes no obvious inhibition on the growth and proliferation of cells, generates no toxic action to laboratory animals, and meets the requirements for biological safety.
Owner:SHANDONG UNIV
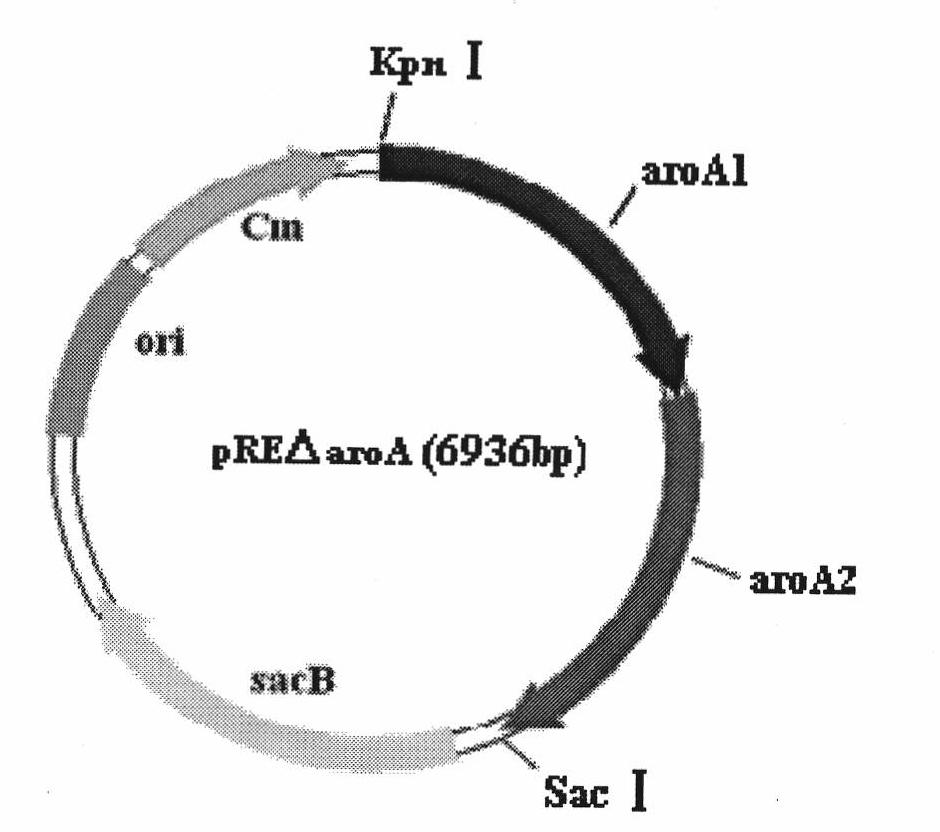
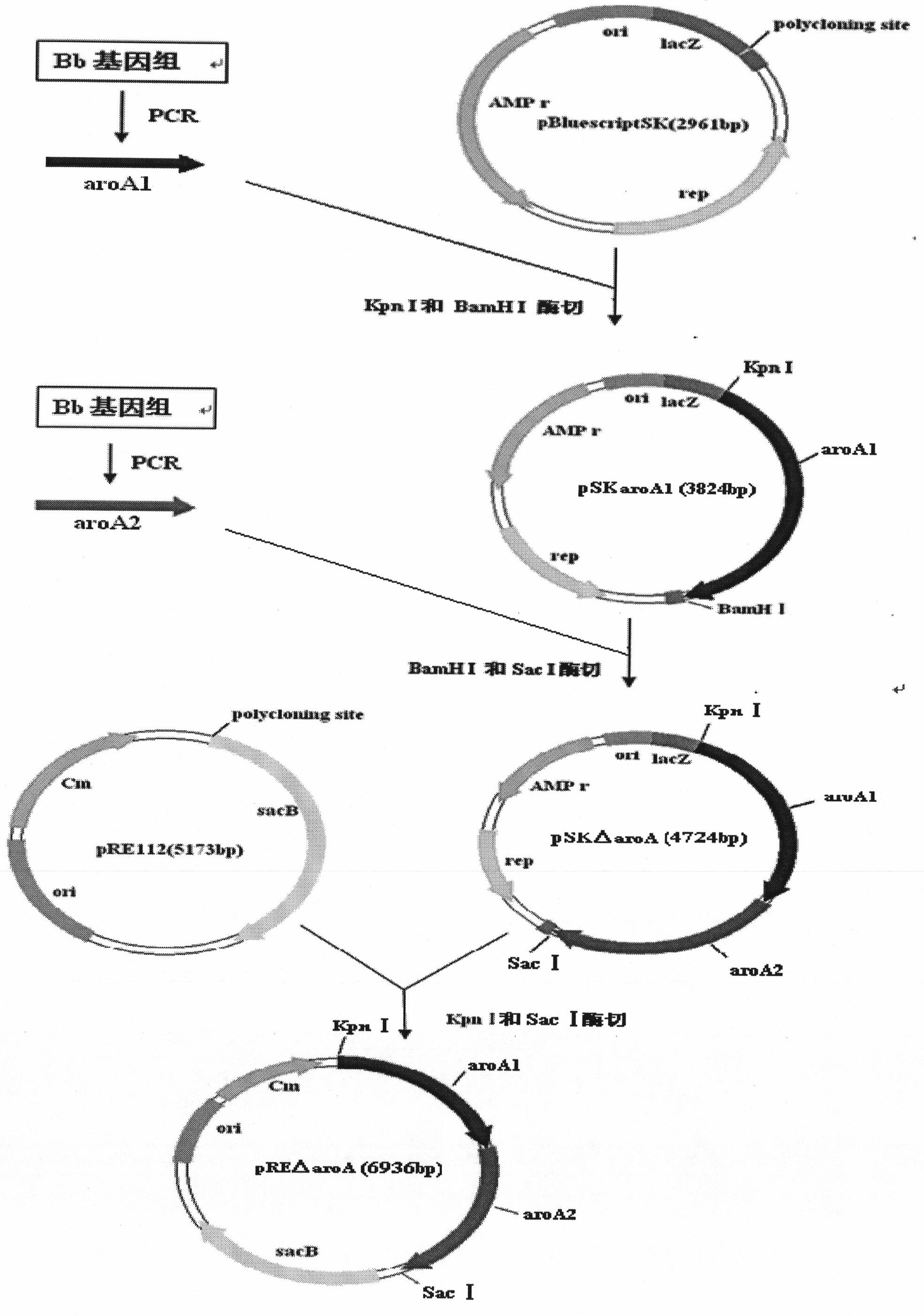
Bordetella bronchiseptica gene deleted vaccine and application
InactiveCN101575586AGood virulence factorGood other immunogenicityAntibacterial agentsBacterial antigen ingredientsBordetella bronchisepticaMicrobiology
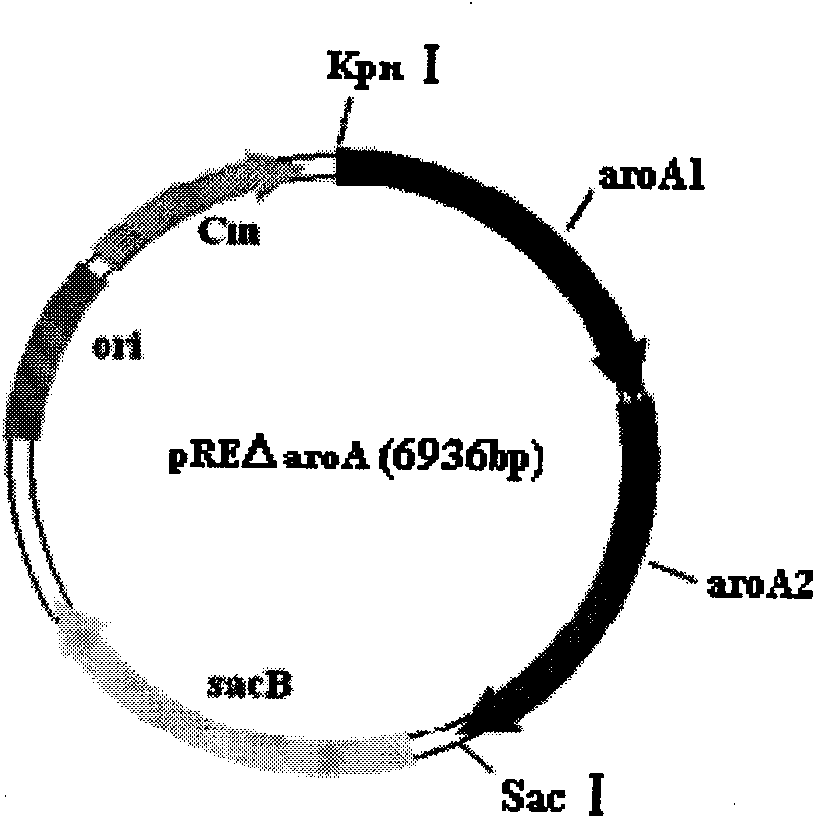
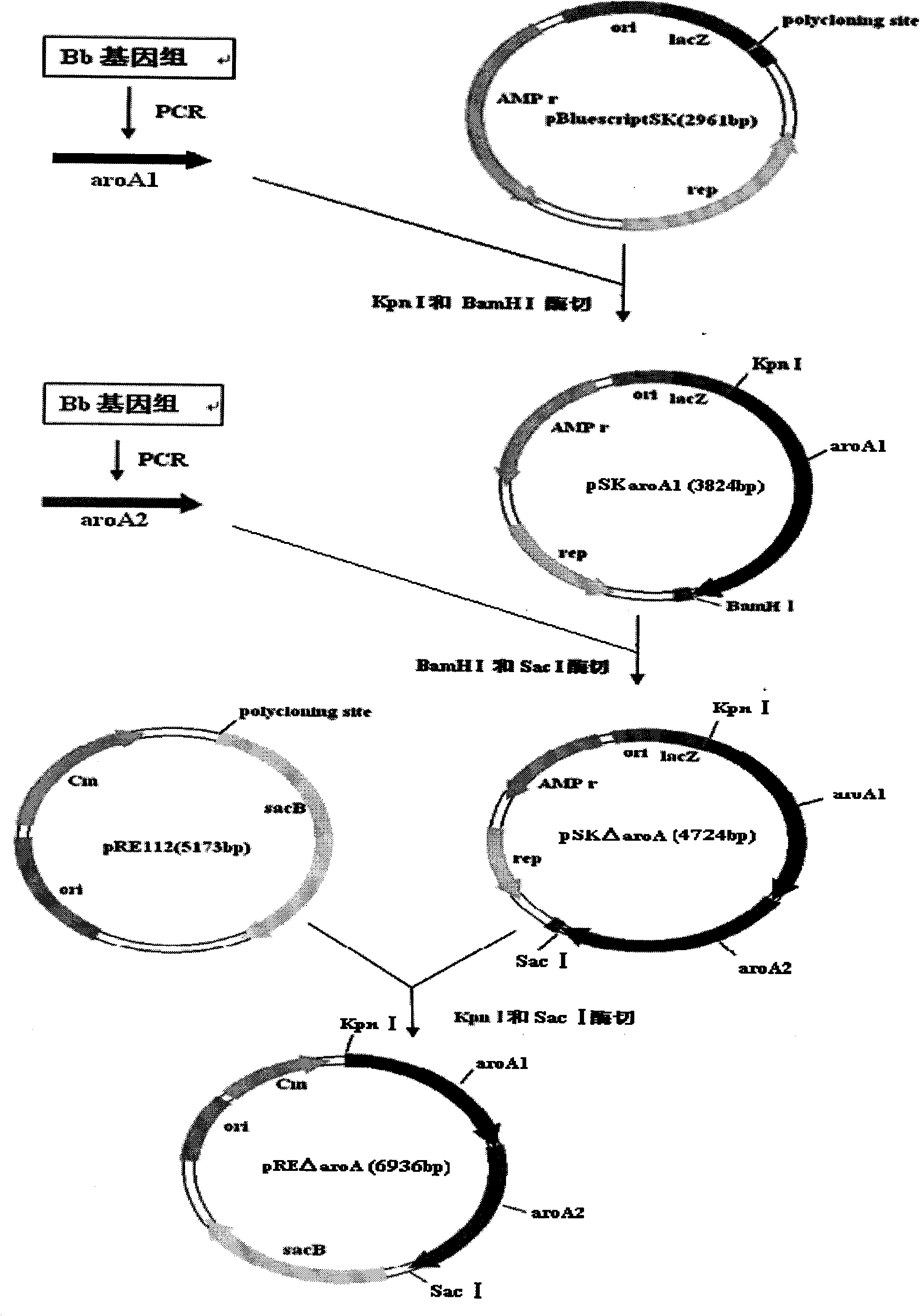
The invention belongs to the technical field of vaccine preparation of animal gene engineering and particularly relates to the construction of a Bordetella bronchiseptica aroA (5-enolpyruvyl-shikimate-3-phosphate synthase) gene deleted bacterial strain, and the preparation and application of a gene deleted vaccine. The Bordetella bronchiseptica gene deleted bacterial strain QH0814DeltaaroA is preserved in the China Center for Type Culture Collection with the collection number of CCTCCNO: M208018. The deletion of 5-enolpyruvyl-shikimate-3-phosphate synthase (aroA) gene with the full length of 1340bp causes an obstacle when the bacterial strain metabolizes aromatic amino acid. The invention prepares the Bordetella bronchiseptica gene deleted vaccine by using the gene deleted bacterial strain. The invention also discloses the application of the gene deleted bacterial strain in preparing the Bordetella bronchiseptica gene deleted vaccine (attenuated live vaccine).
Owner:HUAZHONG AGRI UNIV
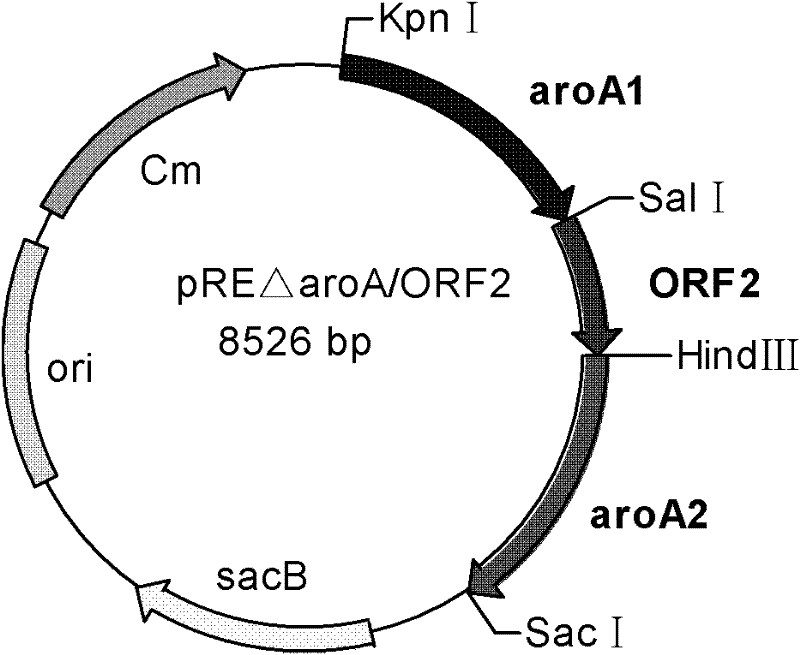
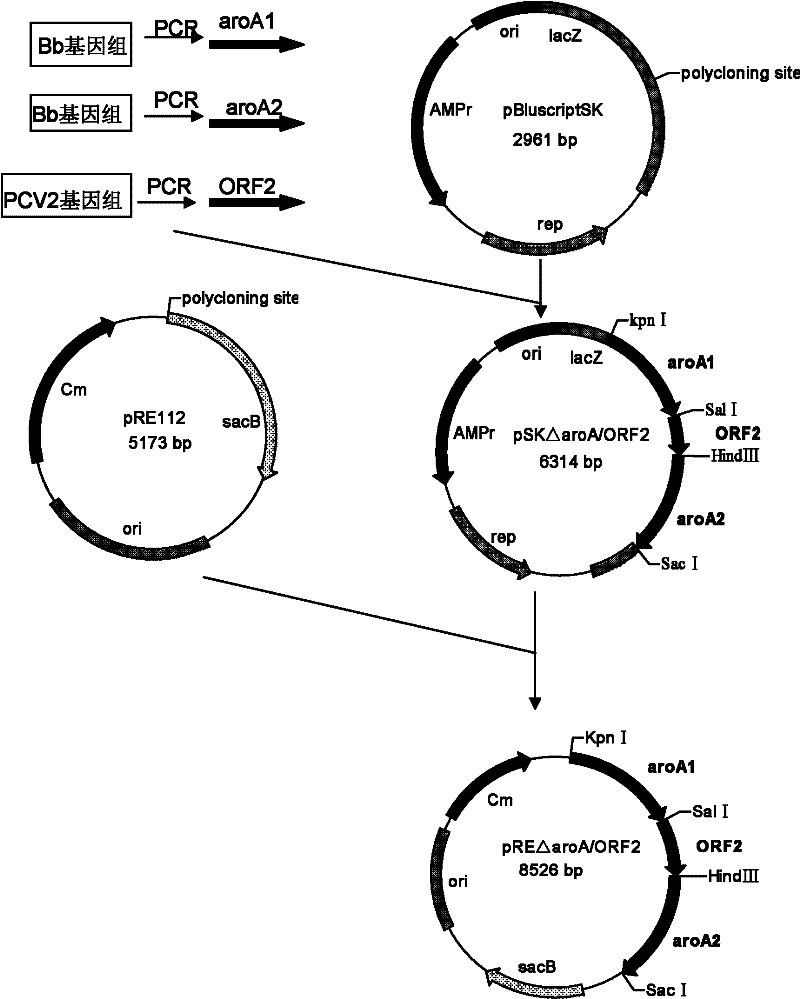
Recombinant Bordetella bronchiseptica strain expressing ORF2 gene fragment of porcine circovirus type 2, vaccine and application
InactiveCN102199571AGood immune protectionImmunization is easy to operateAntibacterial agentsBacterial antigen ingredientsGenetic engineeringOrf2 gene
The invention discloses the construction of a recombinant Bordetella bronchiseptica strain which does not contain a resistance marker and is used for expressing an ORF2 gene fragment of porcine circovirus type 2, the preparation of a vaccine and application, and belongs to the field of genetic engineering. The recombinant Bordetella bronchiseptica strain QH0814delta aroA / ORF2 which does not contain the resistance marker and is used for expressing the ORF2 gene fragment of the porcine circovirus type 2 is obtained. In the strain, a 5-enolpyruvyl-shikimate-3-phosphate synthase (aroA) gene of Bordetella bronchiseptica is deleted; and the strain contains the ORF2 gene fragment of the porcine circovirus type 2. The invention discloses the preparation of the recombinant strain and the vaccine and application of the recombinant strain and the vaccine to the preparation of porcine circovirus type 2 and porcine Bordetella bronchiseptica vaccines. The genetic engineering vaccine can stimulate pigs to produce the protective immune reaction of resisting the porcine circovirus type 2 and porcine Bordetella bronchiseptica wild strains, and can effectively prevent the porcine circovirus type 2 and porcine Bordetella bronchiseptica from infecting the pigs.
Owner:HUAZHONG AGRICULTURAL UNIVERSITY +1
Gene recombined swine cholera salmonella choleraesuis vaccine for blue-ear disease and application thereof
ActiveCN103421729AImprove protectionPreserve immune efficiencyAntibacterial agentsBacteriaBacteroidesDisease
The invention relates to a gene recombined swine cholera salmonella choleraesuis vaccine for a blue-ear disease and application thereof, belongs to the technical field of animal bacteria genetic engineering, and particularly relates to construction of recombined swine cholera salmonella choloraesuis without resistance maker and used for expressing main immunogenicity membrane protein of porcine reproductive and respiratory syndrome virus(PRRSV) and preparation and application of a live vaccine. Recombined swine cholera salmonella choleraesuis C501-GP5m expressing the swine PRRSV GP5m protein is gained and preserved in the China model culture preservation centre, and the preservation number is CCTCC NO: M2012275. The invention further discloses a method for preparing swine cholera salmonella choleraesuis and the porcine reproductive and respiratory syndrome virus vaccine from the recombination strain and application thereof. The prepared recombined live vaccine can stimulate swine to develop the immunity to resist swine cholera salmonella choloraesuis and PRRSV main immunogenicity membrane protein GP5m, and can effectively prevent the infection of paratyphus suum and PRRSV.
Owner:HUAZHONG AGRI UNIV
Recombinant Salmonella enteria serovar Pullorum, (S. Pullorum), as well as preparation method and application thereof
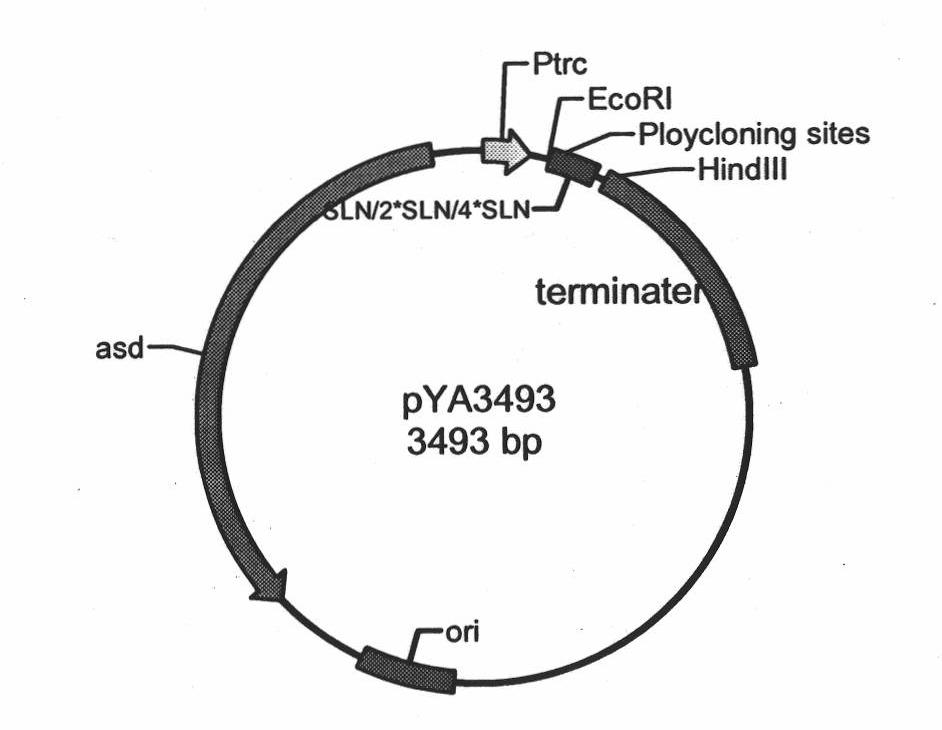
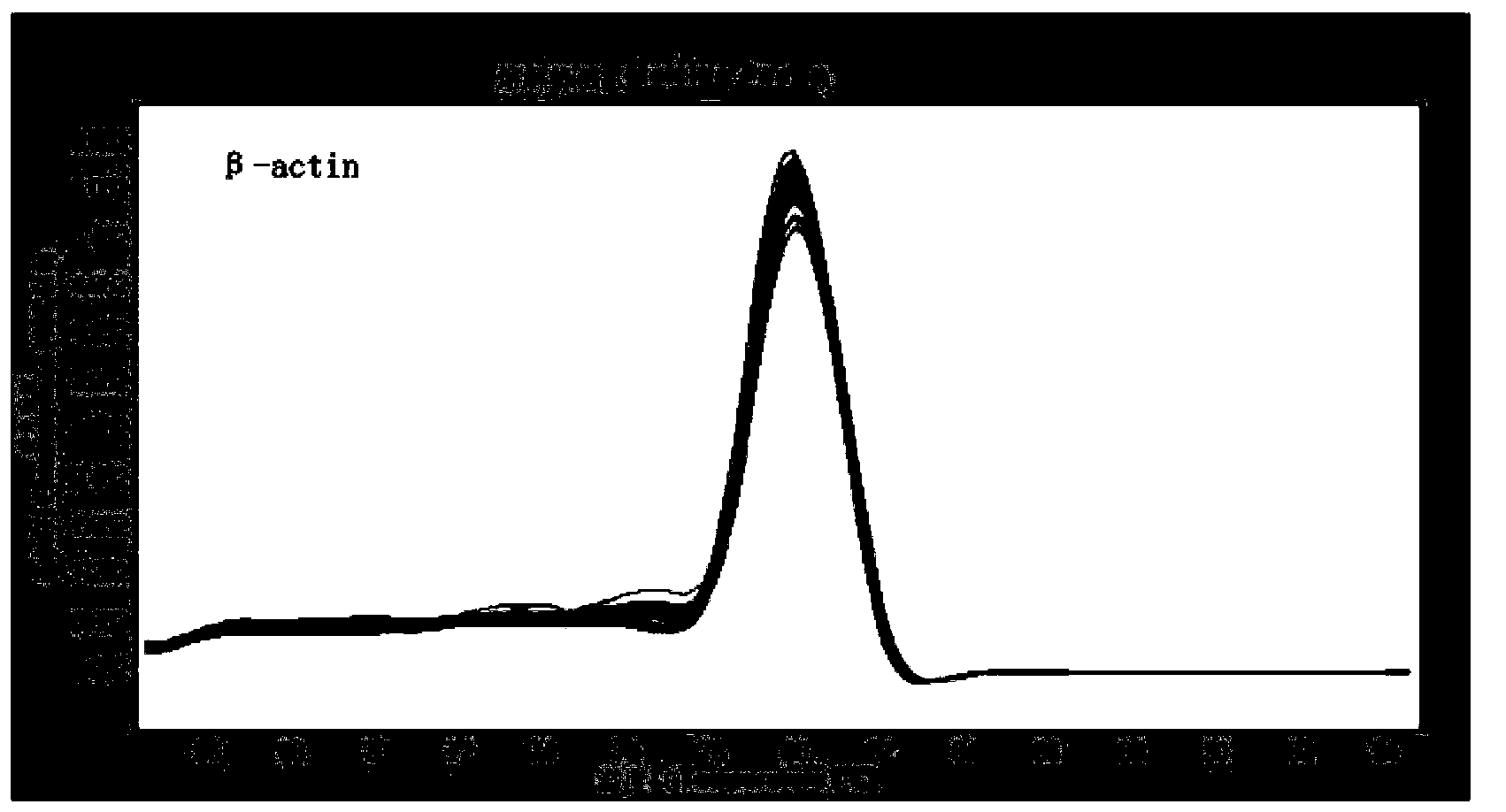
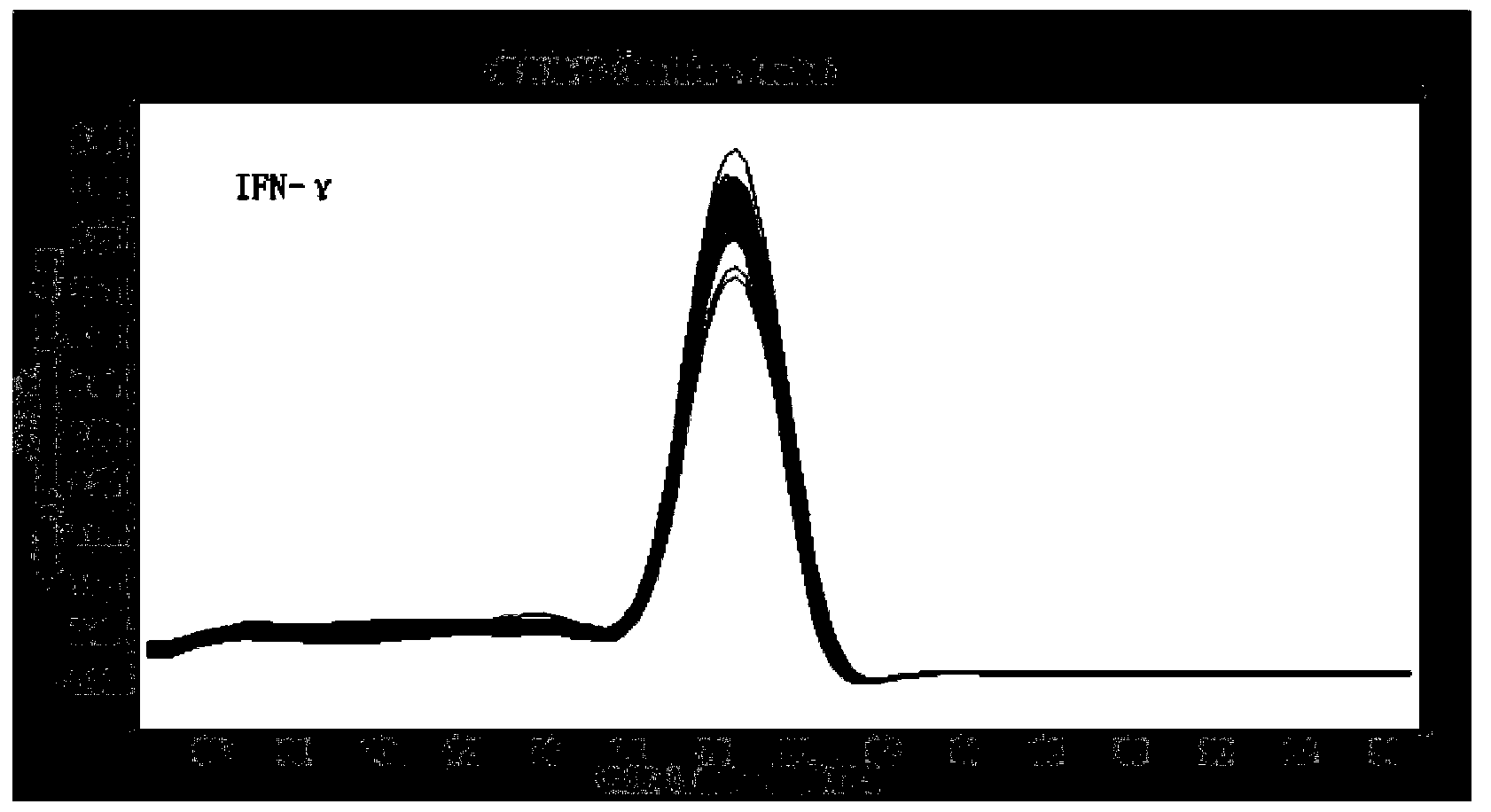
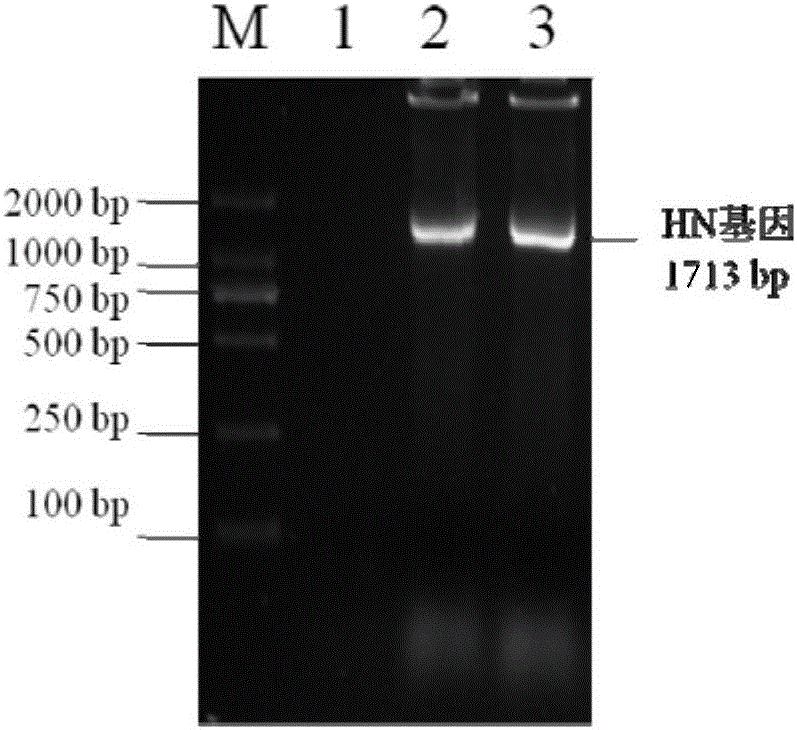
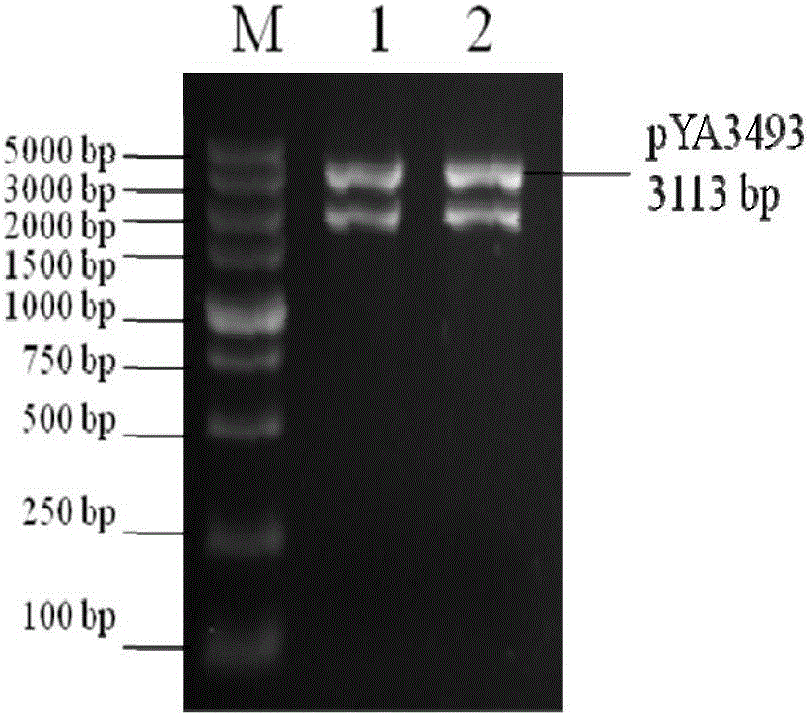
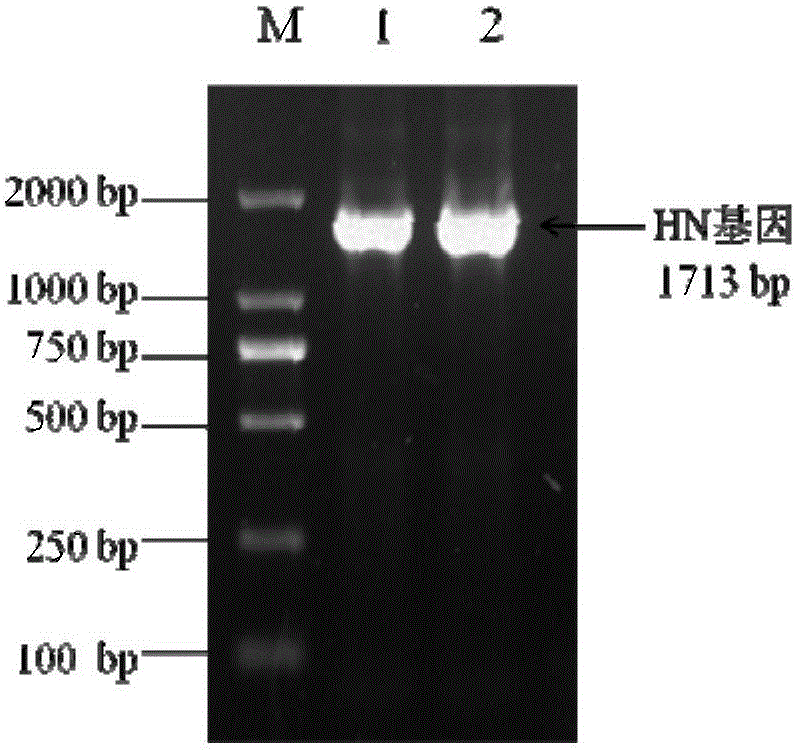
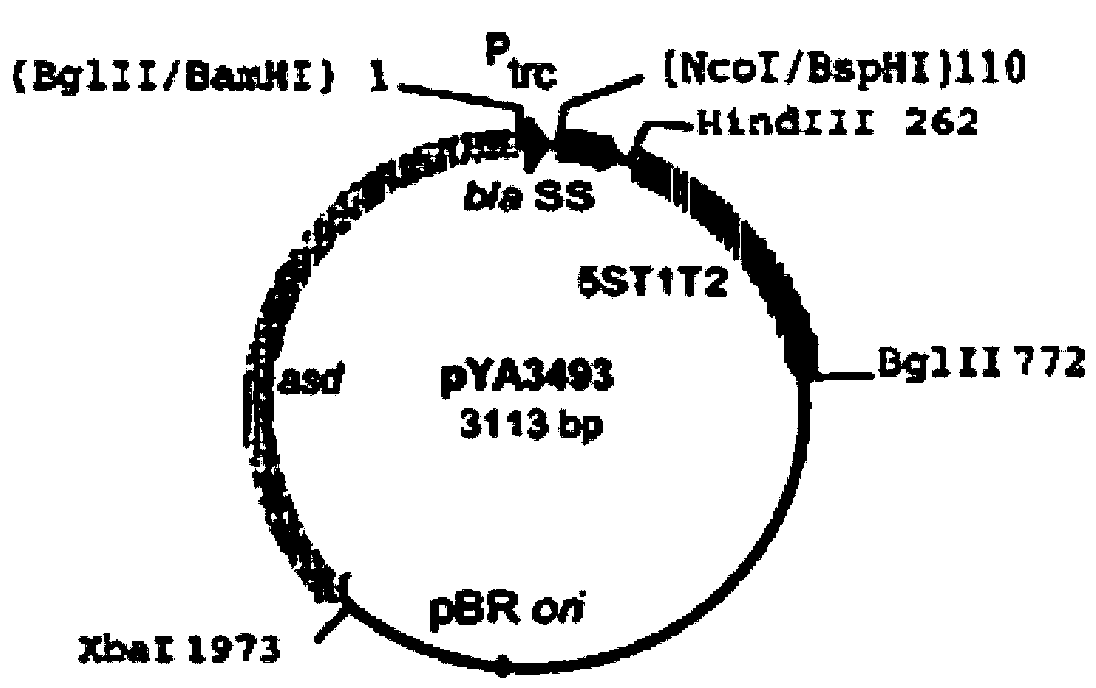
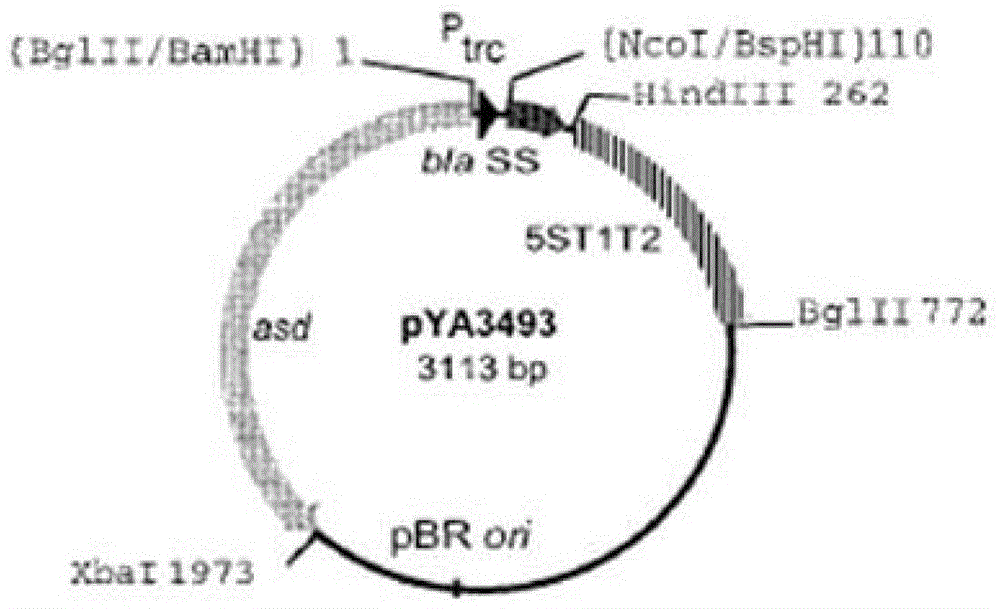
The invention discloses recombinant S. Pullorum, as well as a preparation method and an application thereof, belonging to the technical field of animal bacterial gene engineering. The name of the recombinant S. Pullorum is S. Pullorum C79-13deltacrpdeltaasd(pYA3493-HN), the preservation number is CCTCC NO:M 2015305, the newcastle diseases virus protective antigen gene-HN gene expressed by the recombinant S. Pullorum is the important immunogenic gene segment for the newcastle diseases virus, and has good immune protection. The recombination strain derives from the S. Pullorum standard strain C79-13, completely reserves the immune efficacy of C79-13 for S. Pullorum, has weaker toxicity than that of C79-13, has good bio-safety, and can simultaneously provide protection aiming at S. Pullorum and newcastle disease pathogenes. Meanwhile, the recombinant strain contains no resistance markers, and completely accords with the requirements on the bio-safety of vaccines.
Owner:HENAN UNIV OF SCI & TECH
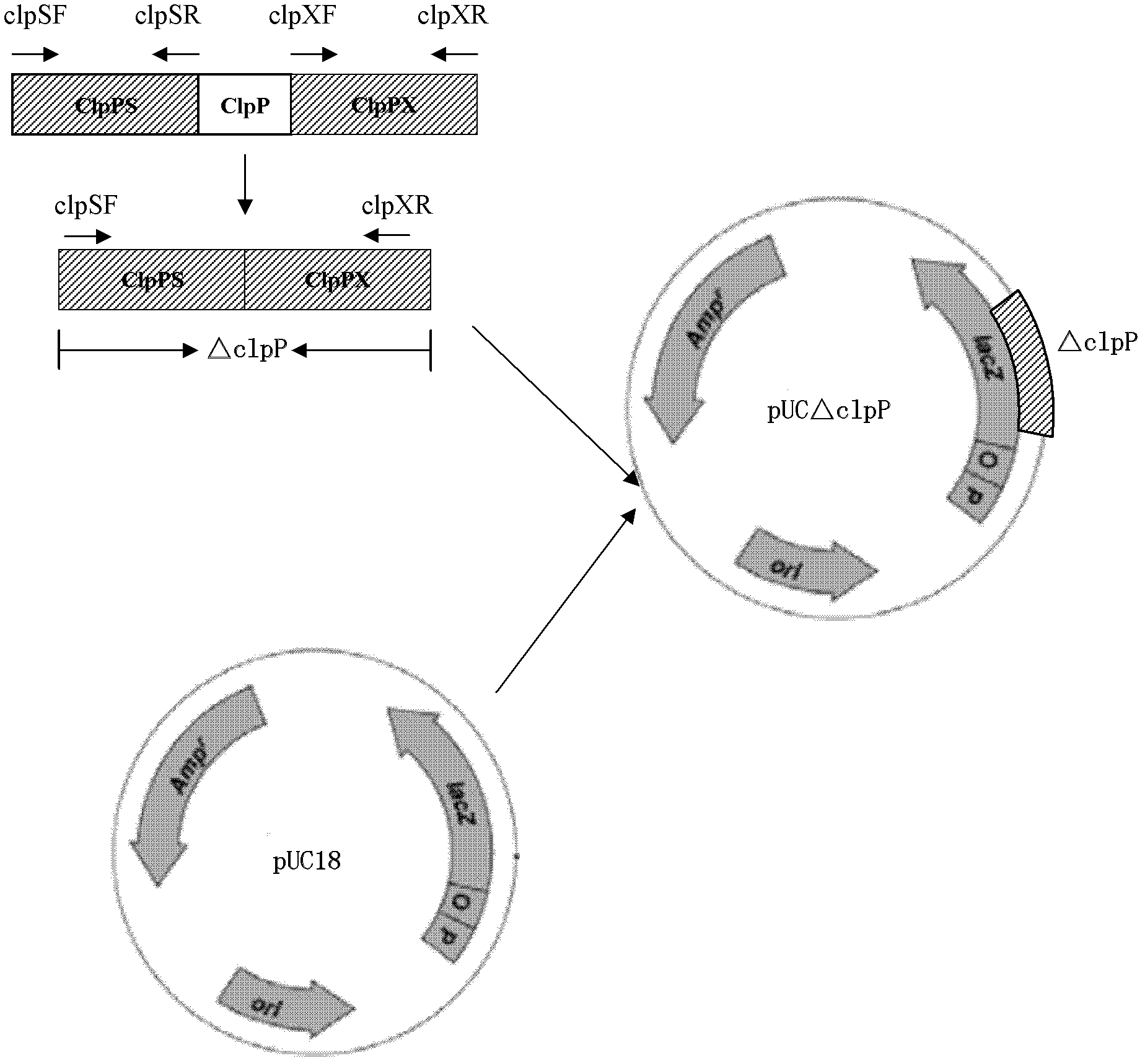
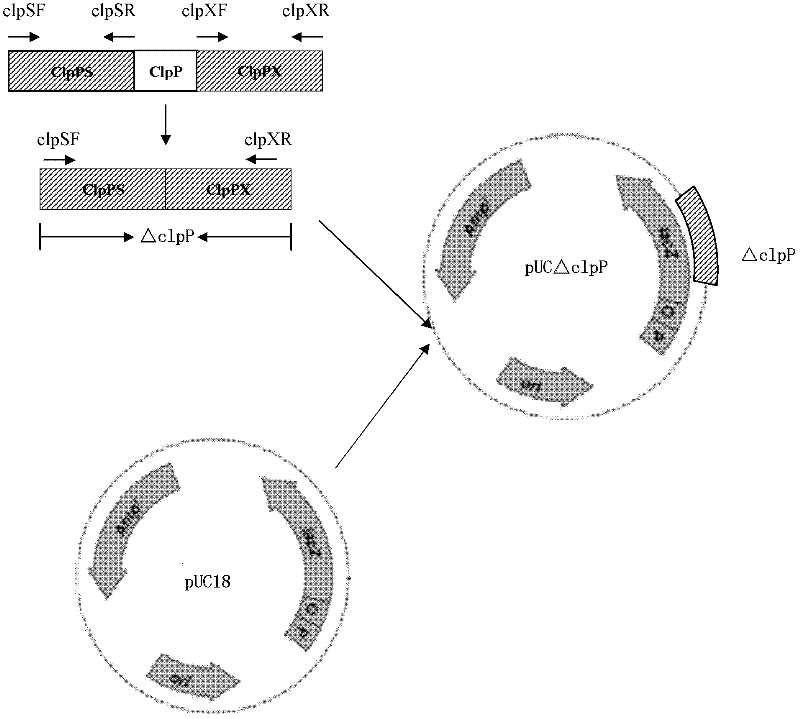
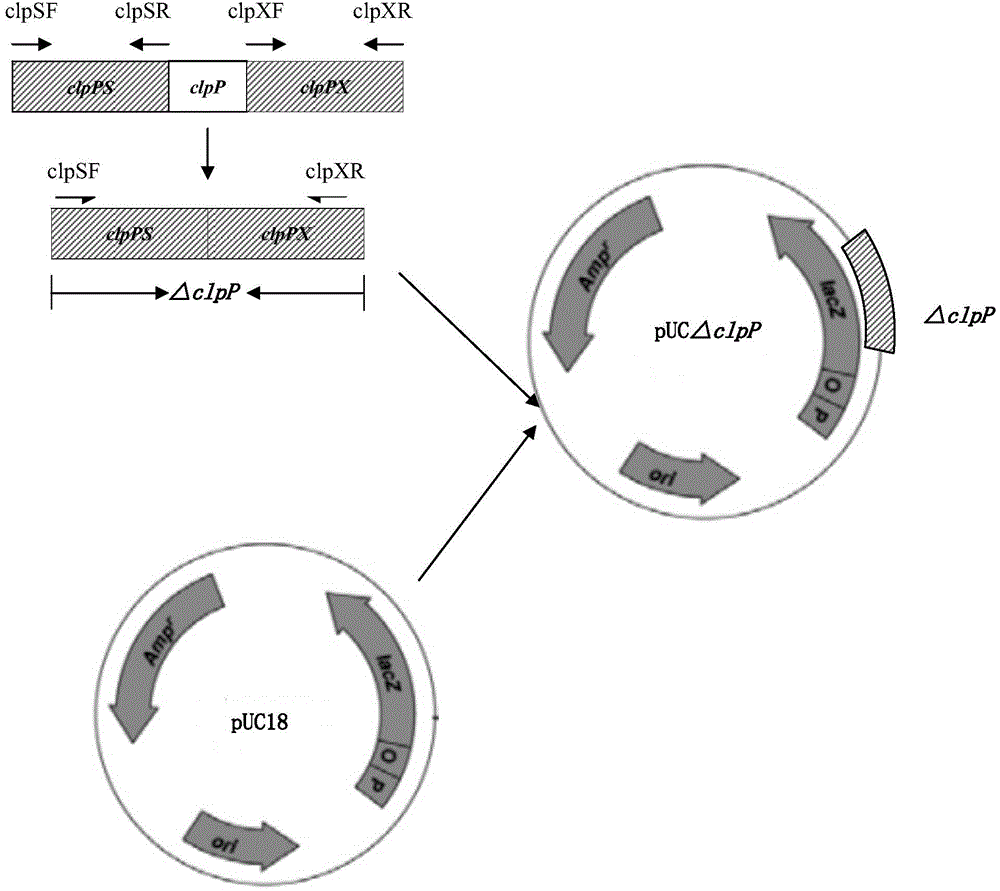
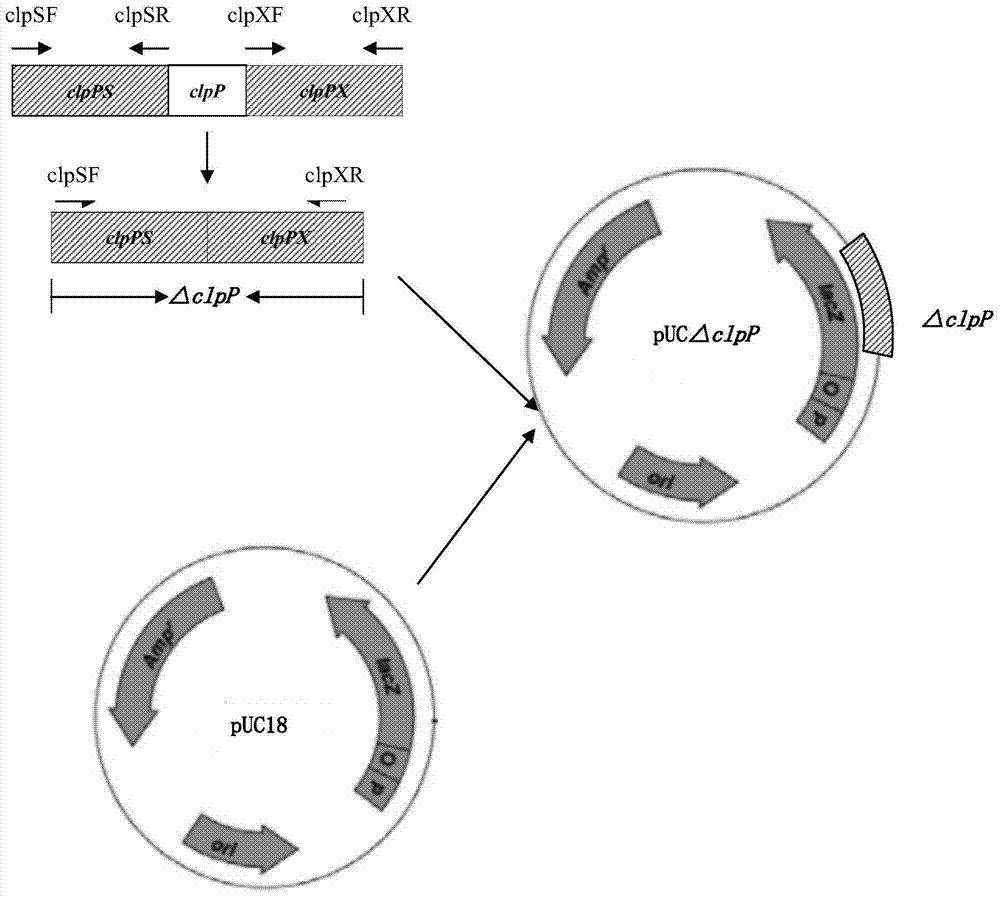
Porcine actinobacillus pleuropeumoniae ClpP protease gene-deleted strain containing no resistance marker, construction method thereof, and application thereof
InactiveCN102443552AStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsBiotechnologyGenetic engineering
The invention discloses a porcine actinobacillus pleuropeumoniae ClpP protease gene-deleted strain containing no resistance marker, and a construction method thereof. The invention belongs to the technical field of bacteria genetic engineering. According to the invention, a recombinant strain APPdeltaclpP of actinobacillus pleuropeumoniae is a strain obtained through coding gene deactivation upon ClpP protease in actinobacillus pleuropeumoniae with an oriented homologous recombination technology, and the expression of the ClpP protease is damaged. With the technology provided by the invention, the virulence of the mutant strain is lower than a parent strain, and the mutant strain is safe to animals. Therefore, an important basis is provided for the modification upon porcine contagious pleuropneumonia vaccines and the development of corresponding differential diagnosis reagents. The strain and the method provided by the invention have important significances in promoting the eradication and purification of porcine contagious pleuropneumonia all around the world.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
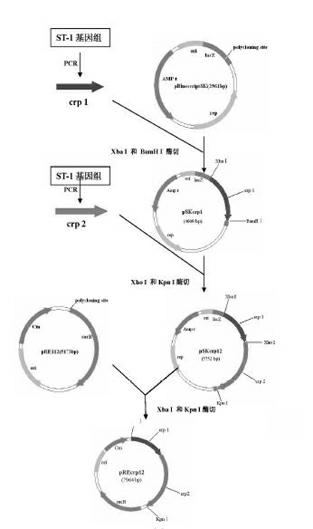
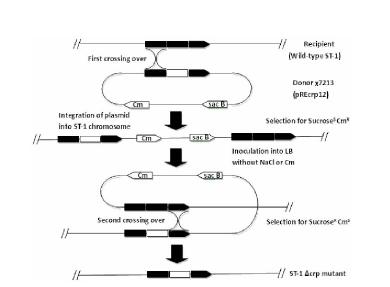
Salmonella typhi gene deletion strain, vaccine prepared from salmonella typhi gene deletion strain and application
ActiveCN102676419AGood immune protectionWeak toxicityAntibacterial agentsBacteriaSide effectSalmonella typhi Infections
The invention relates to a Salmonella typhi gene deletion strain, a vaccine prepared from the Salmonella typhi gene deletion strain and application of the strain. The bacterial strain is Salmonella typhi delta crpST-1 and preserved in the China center for type culture collection, and the preservation number is CCTCC NO: M2011373. The Salmonella typhi gene deletion strain disclosed by the invention has excellent immunizing protection efficacy and weak toxicity without any adverse side effect on immunized pigs and is safer compared with the traditional vaccine. Therefore, the vaccine which is prepared from the gene deletion strain built by a parent strain has wide market application prospect. In addition, the Salmonella typhi gene deletion strain disclosed by the invention retains the immune protective efficacy aiming to salmonella typhi infection, and has clear genetic background and stable characteristics, so that the Salmonella typhi gene deletion strain has better biosecurity. The Salmonella typhi gene deletion strain disclosed by the invention does not contain a resistance tag and totally accords with the biological security requirement of vaccine.
Owner:HENAN UNIV OF SCI & TECH
Bordetella bronchiseptica gene deleted vaccine and application
InactiveCN101575586BGood antigenStrong targetingAntibacterial agentsBacterial antigen ingredientsMicrobiologyBordetella bronchiseptica
The invention belongs to the technical field of vaccine preparation of animal gene engineering and particularly relates to the construction of a Bordetella bronchiseptica aroA (5-enolpyruvyl-shikimate-3-phosphate synthase) gene deleted bacterial strain, and the preparation and application of a gene deleted vaccine. The Bordetella bronchiseptica gene deleted bacterial strain QH0814DeltaaroA is preserved in the China Center for Type Culture Collection with the collection number of CCTCCNO: M208018. The deletion of 5-enolpyruvyl-shikimate-3-phosphate synthase (aroA) gene with the full length of 1340bp causes an obstacle when the bacterial strain metabolizes aromatic amino acid. The invention prepares the Bordetella bronchiseptica gene deleted vaccine by using the gene deleted bacterial strain. The invention also discloses the application of the gene deleted bacterial strain in preparing the Bordetella bronchiseptica gene deleted vaccine (attenuated live vaccine).
Owner:HUAZHONG AGRI UNIV
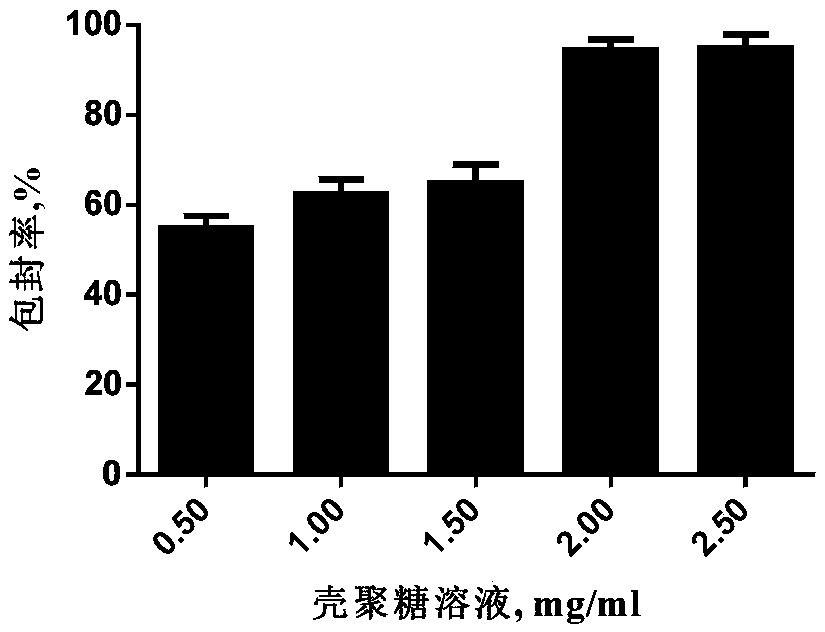
Preparation method and application of attenuated live vaccine chitosan microsphere for porcine epidemic diarrhea virus
ActiveCN109172817AComply with biosafety requirementsHigh clinical safetySsRNA viruses positive-senseViral antigen ingredientsSodium bicarbonateAntigen
The invention discloses a preparation method and application of attenuated live vaccine chitosan microsphere for a porcine epidemic diarrhea virus. The method comprises the following steps: culturinga porcine epidemic diarrhea virus attenuated strain PEDV-YN144, and filtering, so as to obtain virus filtrate; adding a sodium hydrogen carbonate solution into the virus filtrate, slowly dropwide adding a chitosan solution under a magnetic stirring condition; slowly dropwise adding a sodium tripolyphosphate solution; and stirring, so as to obtain mixed liquid; uniformly mixing gelatin with the mixed liquid, and carrying out freeze drying, so as to obtain the attenuated live vaccine chitosan microsphere for the porcine epidemic diarrhea virus. An organic reagent is not added in a packaging process adopted in the method, the antigen activity is preserved, the preparation process is simple, the time is saved, and the attenuated live vaccine chitosan microsphere is easy to store and transport.According to the attenuated live vaccine chitosan microsphere for the porcine epidemic diarrhea virus, the immune effect of a vaccine can be improved, and the action time of the vaccine can be prolonged.
Owner:HUAZHONG AGRI UNIV
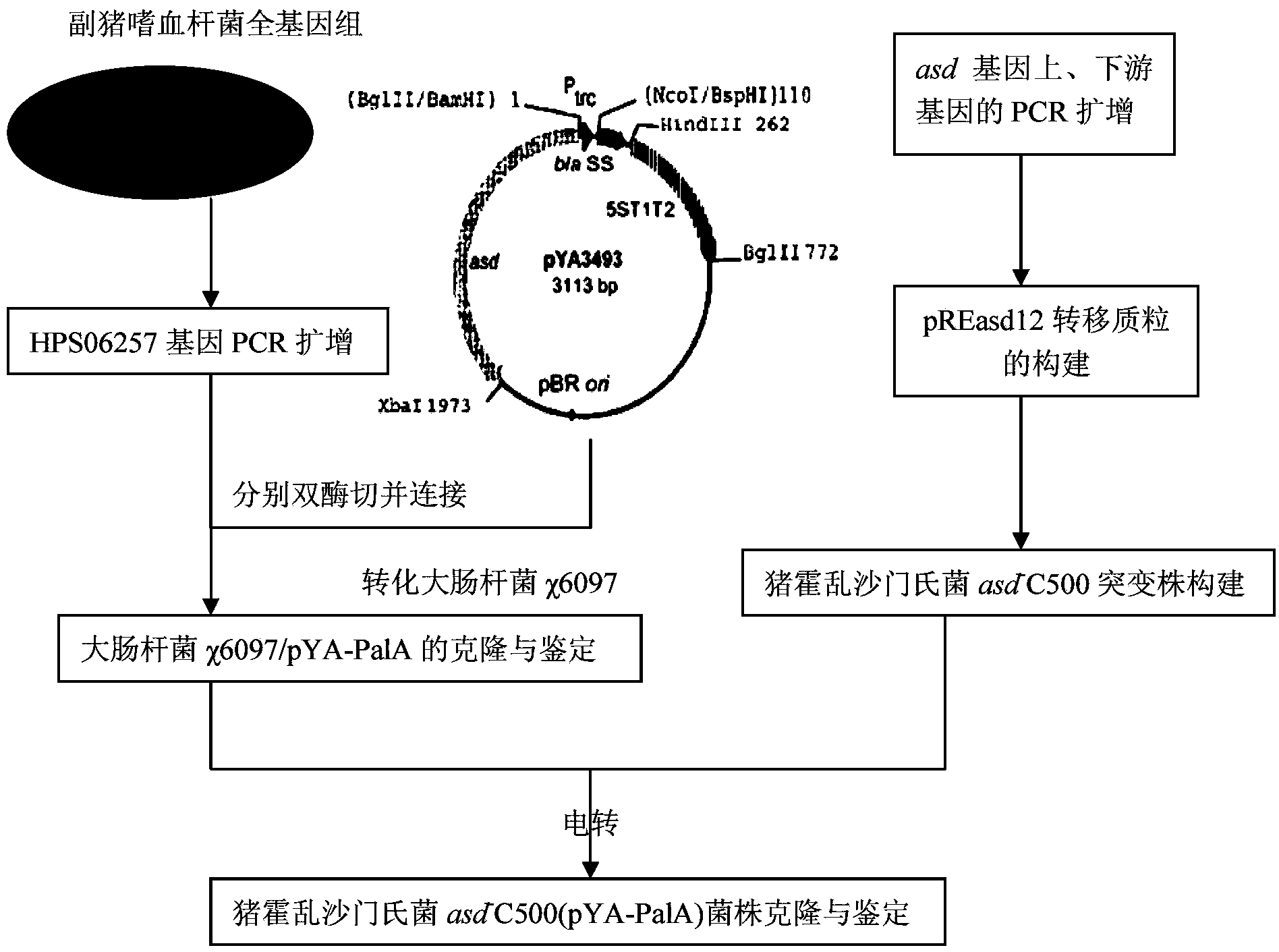
Haemophilus parasuis attenuated salmonella vaccine
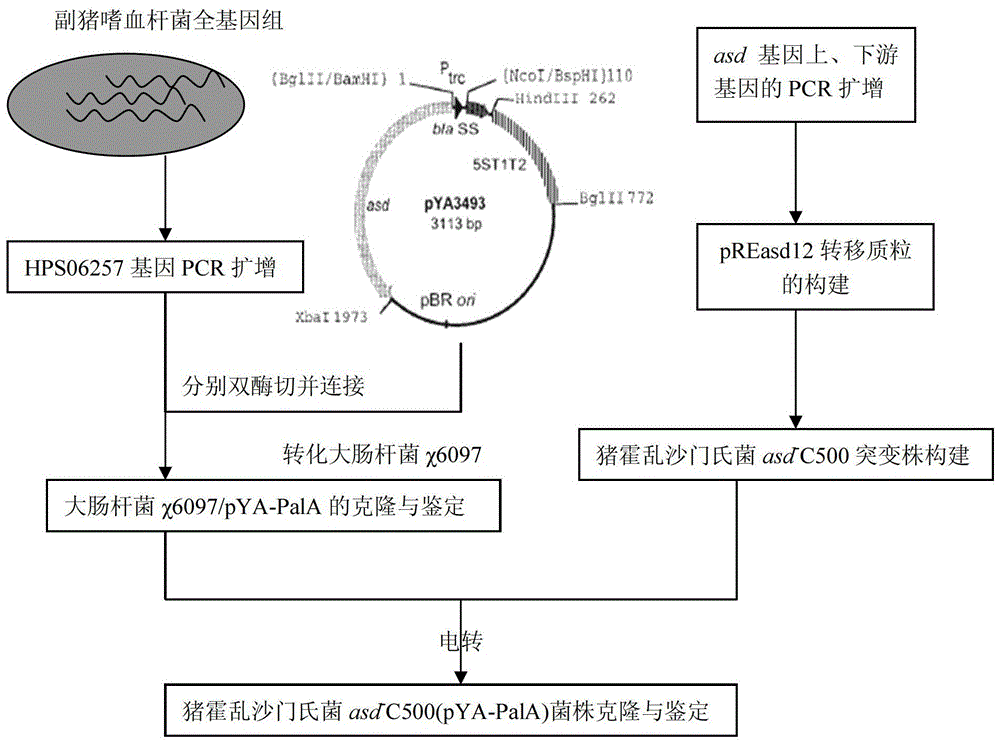
ActiveCN103421731AGood immune protectionBroad market application prospectsAntibacterial agentsBacterial antigen ingredientsAntigenPlasmid
The invention belongs to the technical field of animal bacteriosis gene engineering vaccines, particularly relates to strain construction, vaccine preparation and application of a recombined haemophilus parasuis attenuated salmonella vaccine strain without resistance makers and used for expressing a surface antigen hps 06257 of haemophilus parasuis, and aims to obtain a recombinant salmonella choleraesuis asd-C500 / pYA-06257 (the preservation number is CCTCC NO: M2013054) without resistance makers and used for expressing the surface antigen HPS 06257 of haemophilus parasuis. The recombinant bacteria is lack of an asd gene on a salmonella choleraesuis genome, and contains a recombinant plasmid pYA-06257 which can express the asd gene and an outer membrane antigen hps 06257 gene of haemophilus parasuis on the strain. The invention further discloses a construction method of the recombinant strain asd-C500 / pYA-06257 and a corresponding preparation method of the haemophilus parasuis attenuated salmonella vaccine, as well as application to the preparation of the haemophilus parasuis attenuated salmonella vaccine.
Owner:HUAZHONG AGRI UNIV
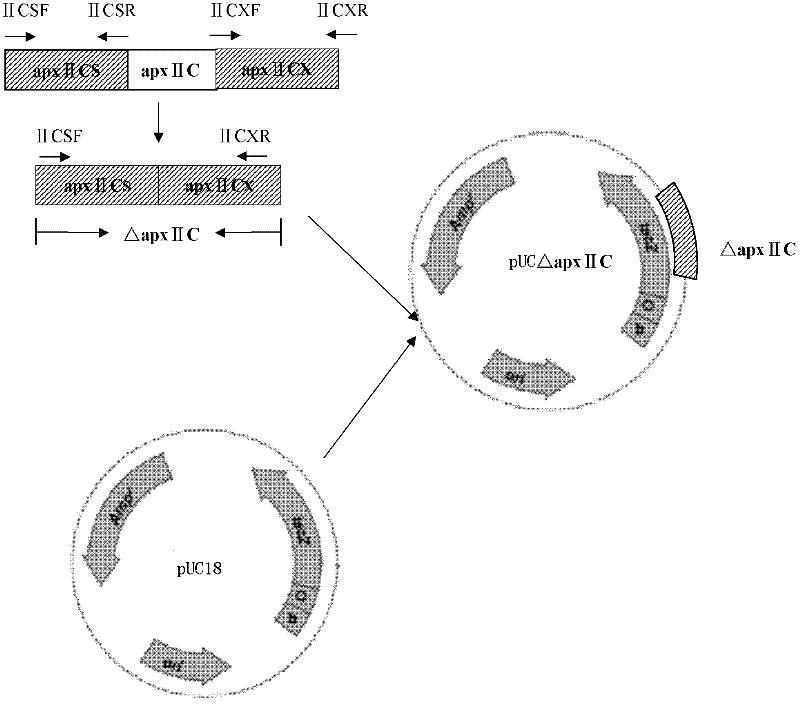
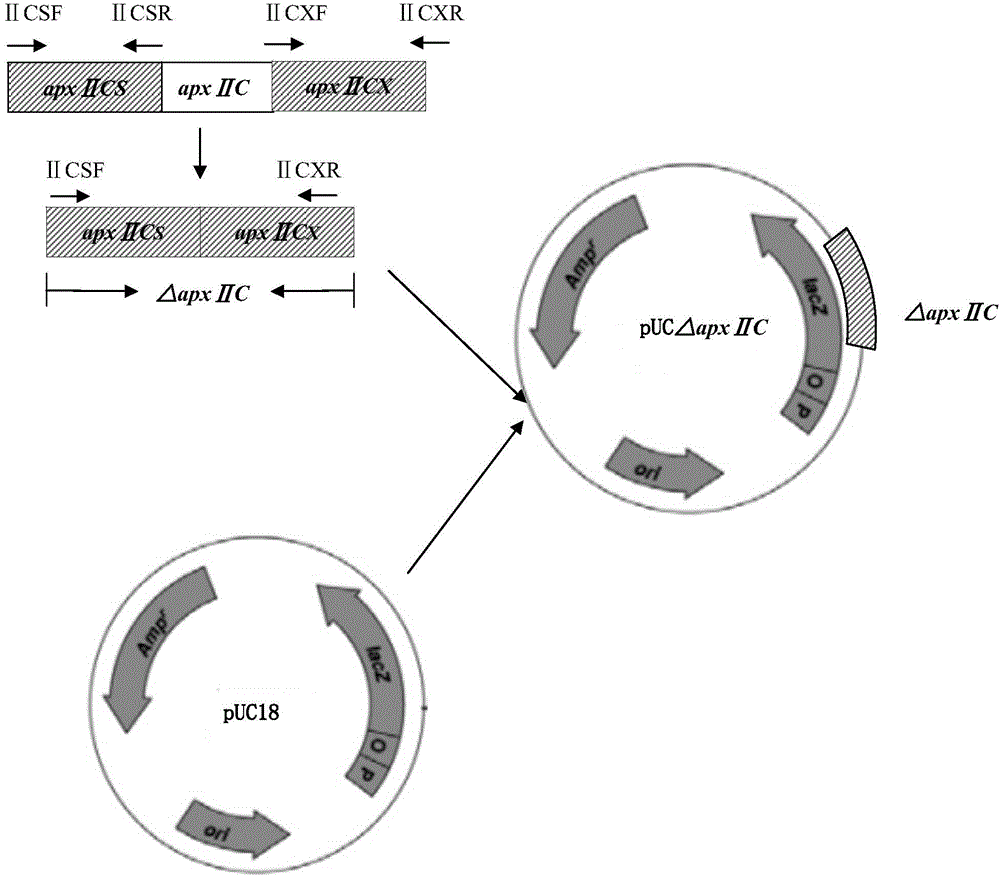
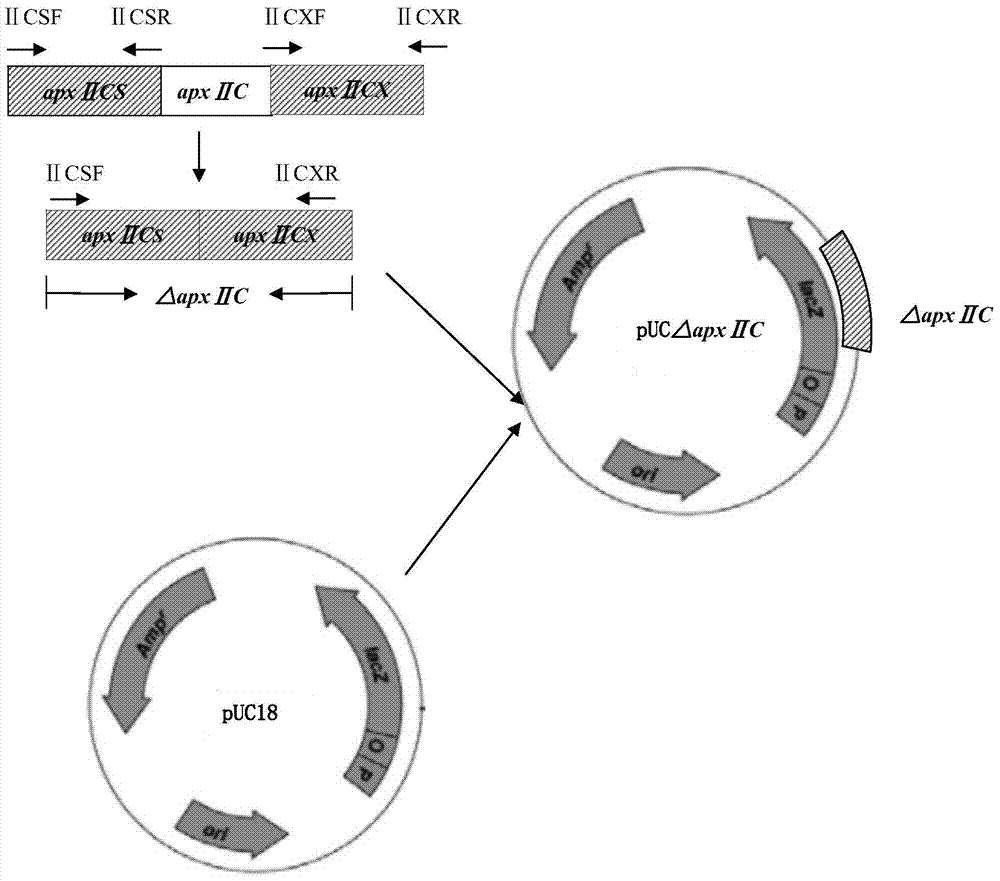
Resistance marker-free porcine actinobacillus pleuropneumoniae double-gene defective strain, construction method and application thereof
InactiveCN102517232AStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsBiotechnologyDifferential diagnosis
The invention discloses a resistance marker-free porcine actinobacillus pleuropneumoniae (APP) serum type 7 double-gene defective strain, and belongs to the technical field of bacterial gene engineering. The recombinant strain APPdeltaclpPdeltaapx II C of APP is obtained by inactivating ClpP protease in the APP and a coded gene of a hemolysin activated factor Apx II C by adopting a directional homologous recombination technology, and expression of the ClpP protease and the Apx II C protein is destroyed. The obtained double-gene defective strain has lower toxicity compared with a parent strain, is safe to animals, provides an important basis for transformation of porcine contagious pleuropneumonia (PCP) vaccines and research of matched identification and diagnosis reagents, and has great significance for promoting elimination and purification of the global PCP.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Improved small culture device for filamentous fungi and culture method thereof
PendingCN109628275AImprove protectionFlat growthBioreactor/fermenter combinationsFungiEngineeringFungi culture
The invention discloses an improved small culture device for filamentous fungi and a culture method thereof. The device comprises a glass slide, a piece of cover glass and a wet box; a central grooveis formed in the middle of the glass slide, the central groove is externally and annularly provided with a peripheral groove, the peripheral groove is provided with a main body groove, a corner located at the main body groove, a first extending groove communicated with the edge of the glass slide and a second extending groove forming an opposite angle with the first extending groove. The device compresses filamentous fungi culture space in the narrow glass slide, the dye liquor flowing speed is limited, growing fungi can be shown in a plane, the morphological structures of the filamentous fungi after dyeing can be better protected, planer imaging is clear, and the effect of observing the morphology of the filamentous fungi under a microscope is better.
Owner:上海皓信生物科技有限公司
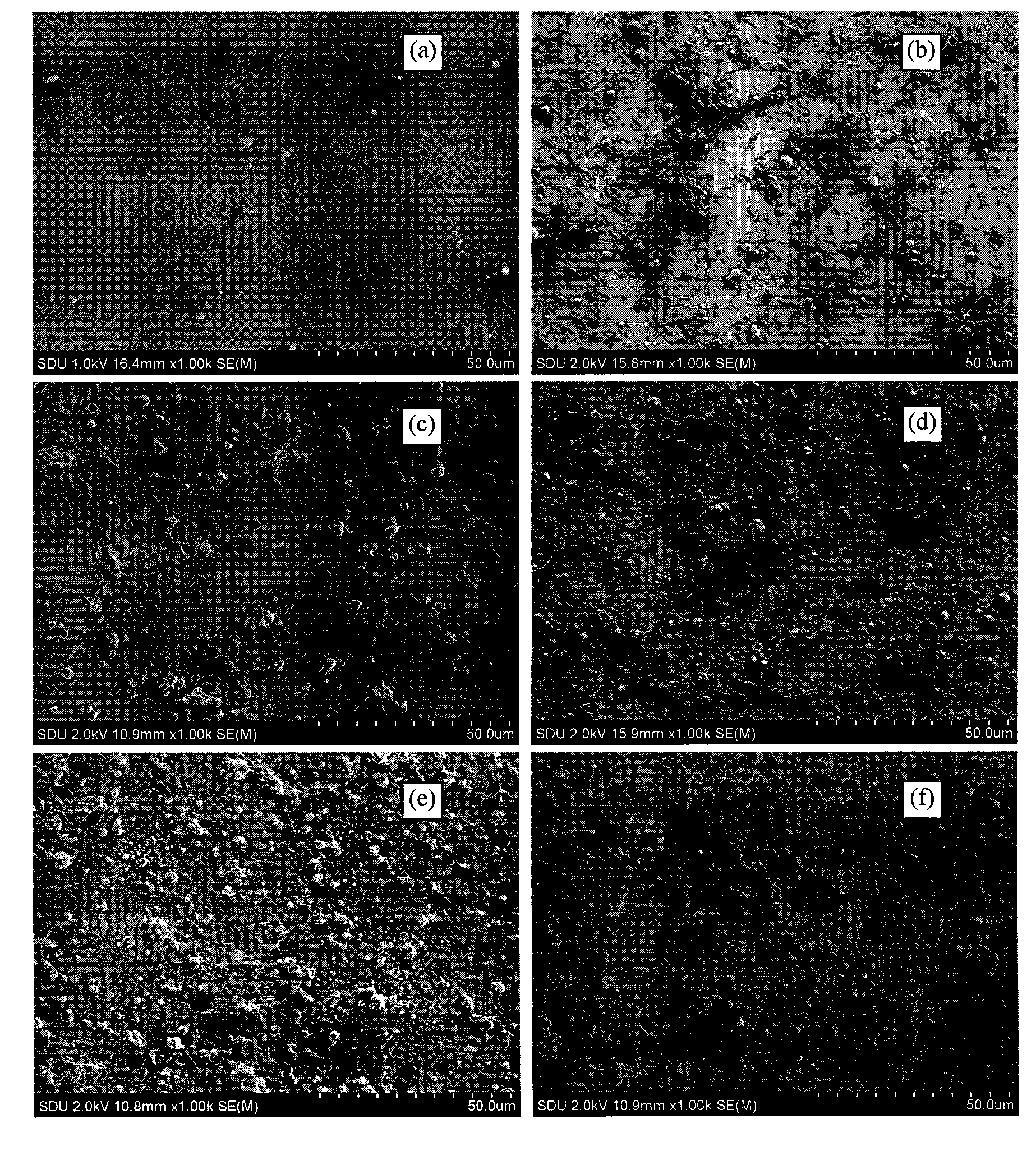
Hydroxylapatite-bioglass film and preparation technology thereof
InactiveCN101549172BNo toxicityGood biocompatibilityVacuum evaporation coatingSputtering coatingArgon atmosphereWhole body
The invention discloses a hydroxylapatite-bioglass film, which is prepared by taking hydroxylapatite or bioglass or the mixture of both as a target material and using the following technology of: adopting pulse laser with energy density of 5J-cm2 to complete the sedimentation of the film in an argon atmosphere with underlayer temperature of 15 DEG C to 600 DEG C and pressure of 5Pa to 45Pa or anoxygen atmosphere with pressure of 1*10-3-9*10-3Pa and under the condition with pulse number of 3,000-18,000. The bio-film has no cytotoxic effect and good biocompatibility, causes no hemolytic reaction and acute systemic toxicity effect, imposes no obvious inhibition on the growth and proliferation of cells, generates no toxic action to laboratory animals, and meets the requirements for biological safety.
Owner:SHANDONG UNIV
Resistance marker-free attenuated live vaccine against porcine contagious pleuropneumonia (PCP) and application thereof
InactiveCN103555645AStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsGenetic engineeringTGE VACCINE
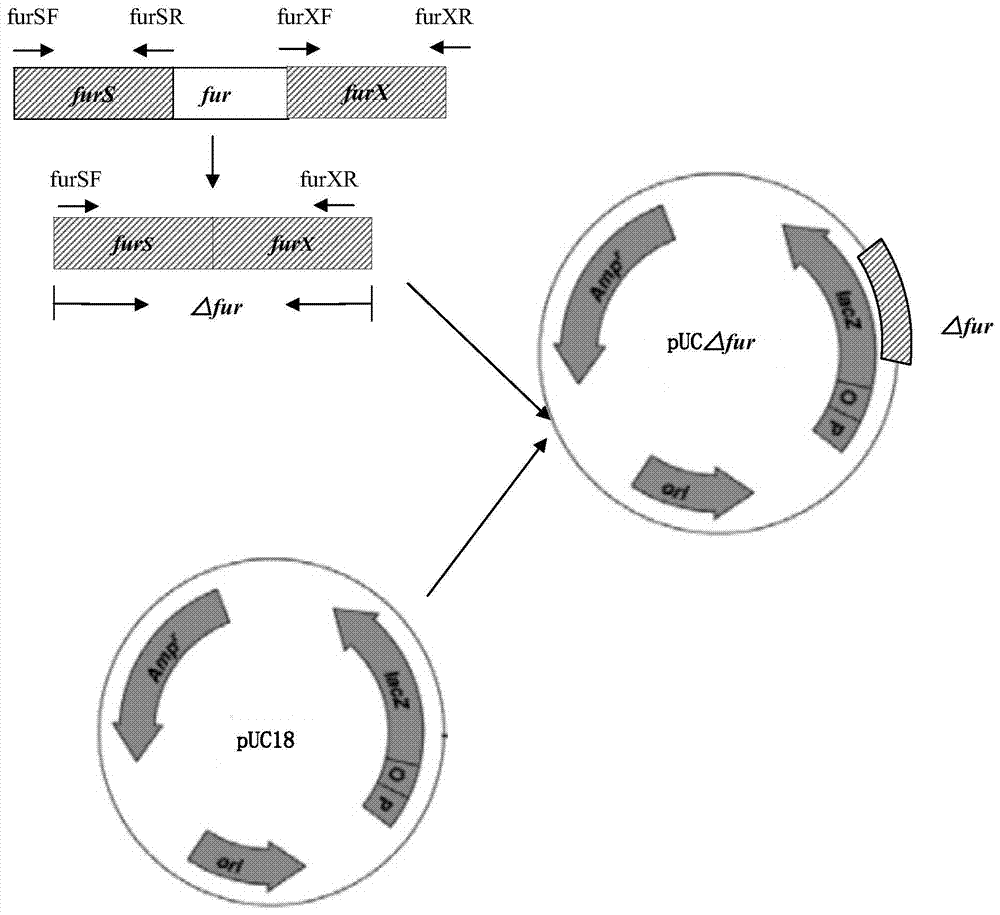
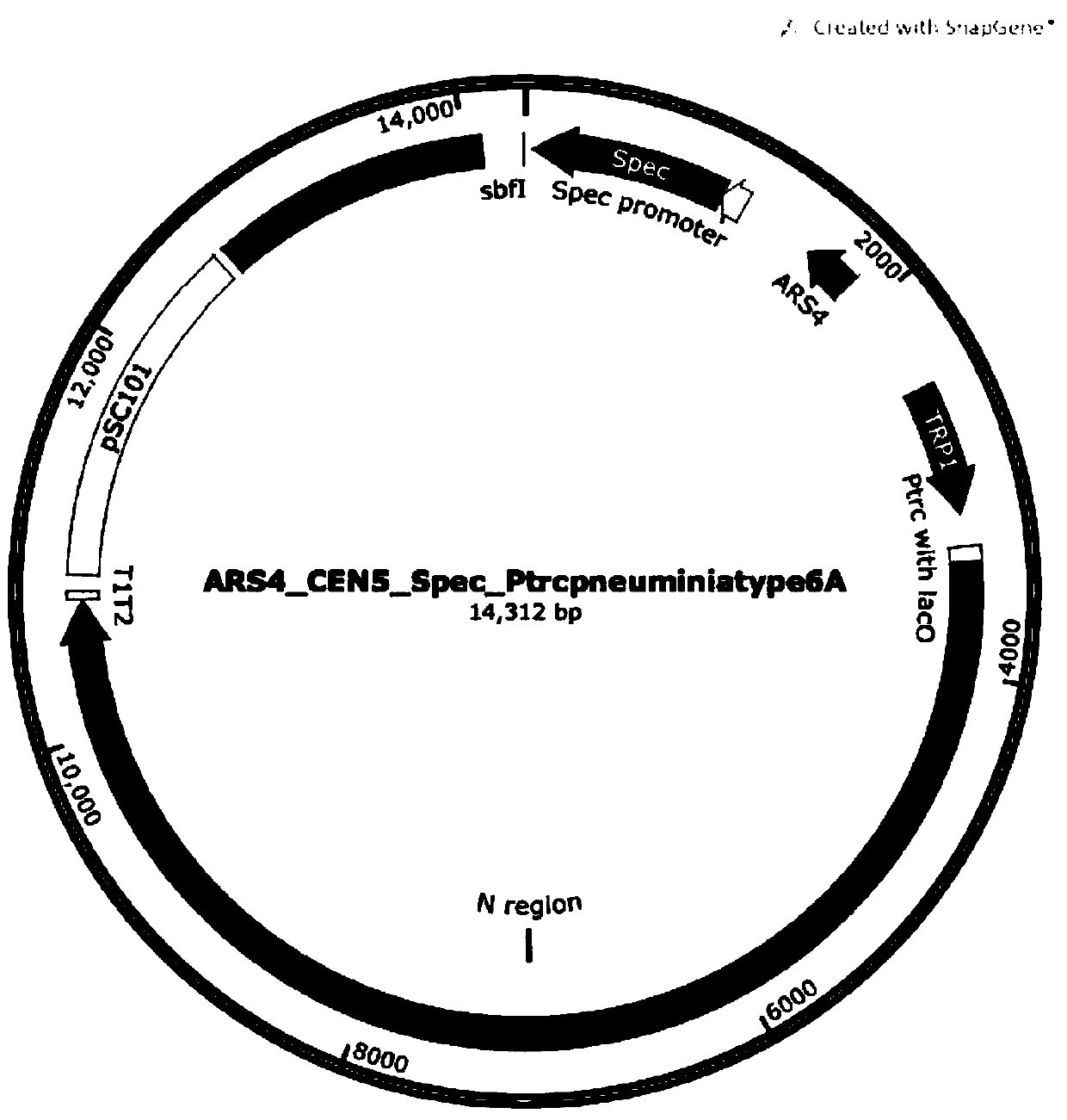
The invention discloses a resistance marker-free attenuated live vaccine against porcine contagious pleuropneumonia (PCP) and application thereof and further discloses a resistance marker-free actinobacillus pleuropneumoniae (APP) serum 7 type three gene deletion strain used for preparing the vaccine, belonging to the field of bacterial gene engineering technology. The resistance marker-free APP serum 7 type three gene deletion strain APP-Delta clpP-Delta apxIIC-Delta fur is a strain obtained by inactivating coding genes of protease ClpP, an ApxII toxin activator ApxIIC and an iron absorption regulatory protein Fur of APP through oriented homologous recombination and destroys expression of ClpP, ApxIIC and the Fur protein. The three gene deletion strain obtained in the invention has smaller virulence, decreased by more than 100 times, compared with that of a parent strain, and is safe to animals; A pig immunized by using the strain is well protected from attacks by different serum type virulent strains of APP, and all the protection rates are greater than 80%; and the three gene deletion strain can be used as the attenuated live vaccine for immunoprophylaxis of PCP.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Salmonella choleraesuis gene deletion mutant without resistant marker and vaccine thereof
InactiveCN101962625AAvoid infectionPreserve immune protection potencyAntibacterial agentsBacteriaAnimal testingVeterinary Drugs
Owner:HENAN UNIV OF SCI & TECH
Engineering yeast strain for preventing porcine circovirus disease
PendingCN105779320ARetain immune adjuvant propertiesImproving immunogenicityFungiAntibody mimetics/scaffoldsDiseaseEscherichia coli
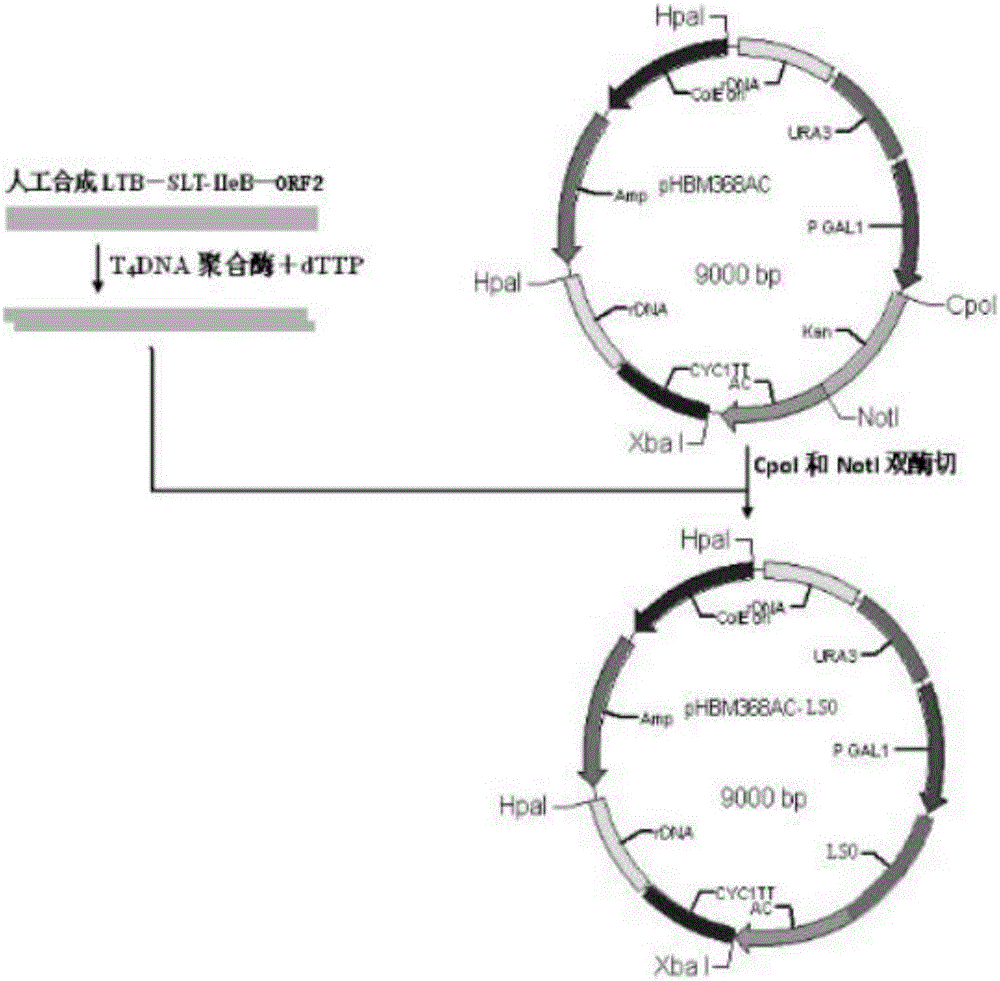
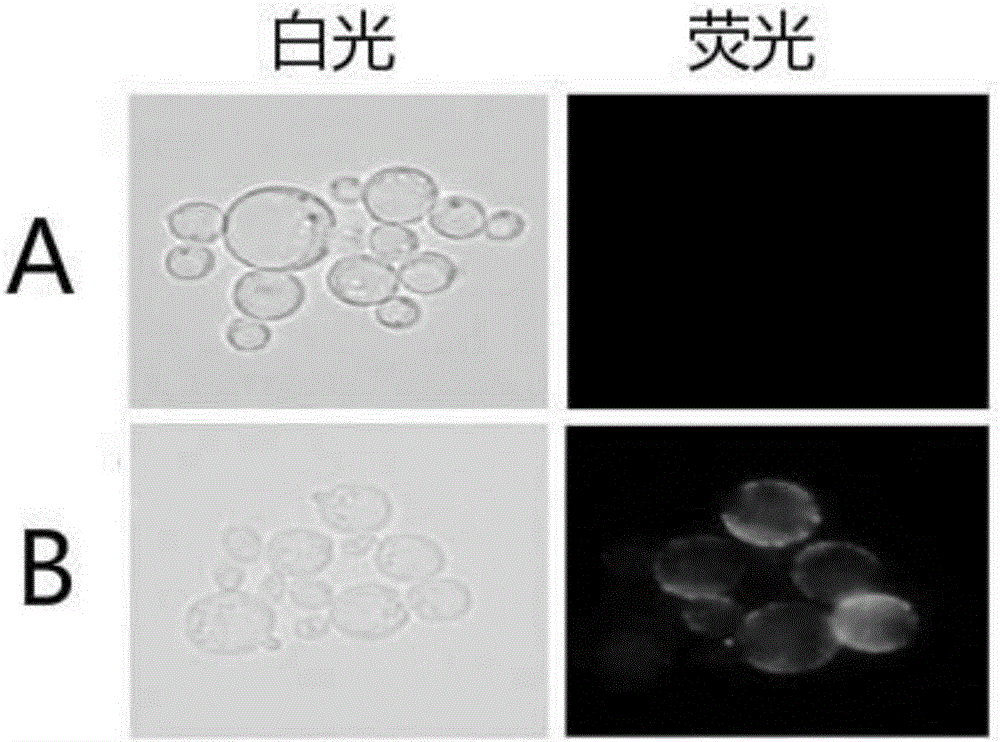
The invention discloses an engineering yeast strain for preventing a porcine circovirus disease. A gene engineering bacteria comprise genes for coding LSO fusion protein; the LSO fusion protein has a sequence shown as SEQ ID NO:1. A high-copying yeast integrating type plasmid surface is used for showing the fusion protein of a multiple-epitope antigen of the porcine circovirus PCV2 and a protective antigen LTB SLT IIeB of pathogenic swine colibacillosis; meanwhile, the recombinant fusion protein LTB SLT IIeB per se has the effect of an immunologic adjuvant, is used as both the antigen and an adjuvant, and achieves the dual functions; the immunogenicity and the immune efficiency of CAP protein coded by ORF2 of the porcine circovirus PCV2 shown by recombinant beer yeast strains are improved. The genetic engineering yeast strain can be applied to the prevention and treatment of the porcine circovirus disease.
Owner:GUANGZHOU WISDOM BIO TECH +1
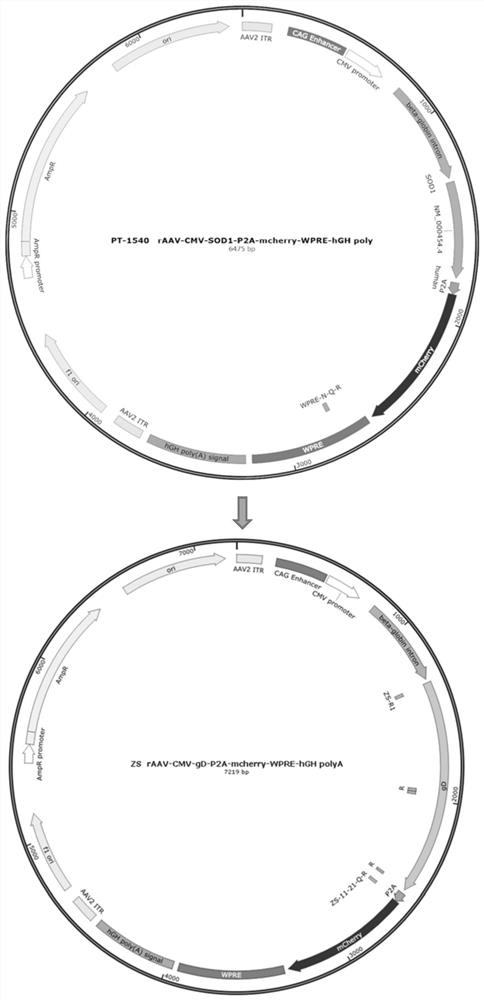
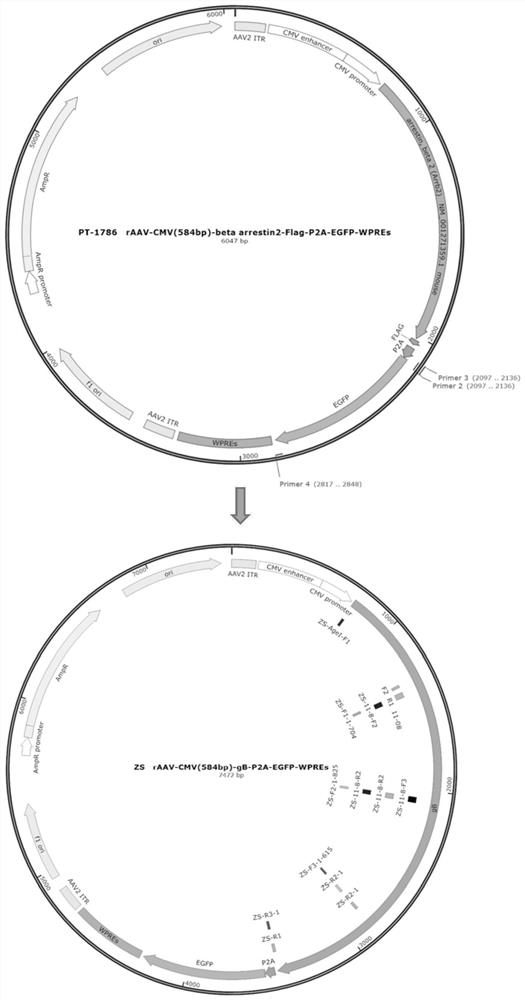
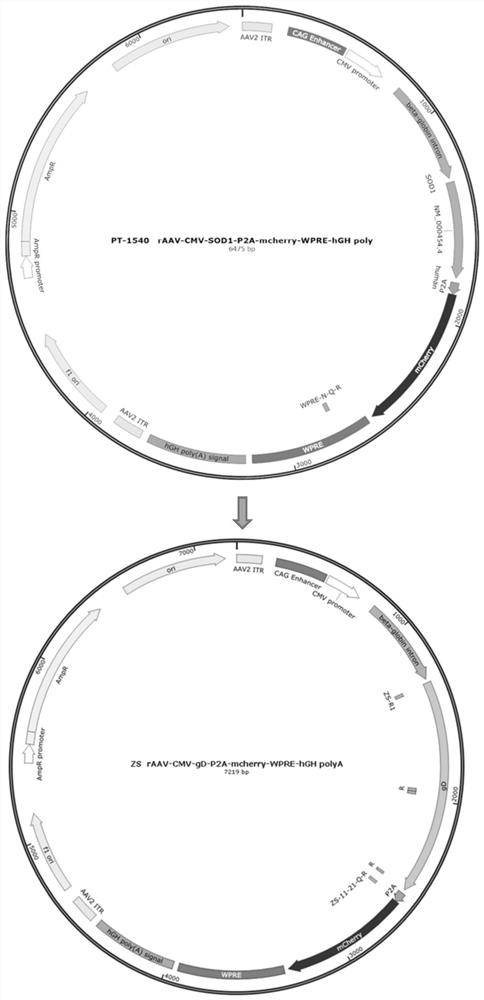
Recombinant adeno-associated virus transfer vector containing gD protein gene of variant porcine pseudorabies virus, virus and preparation and application thereof
The invention belongs to the technical field of gene engineering, and particularly relates to a recombinant adeno-associated virus transfer vector containing a gD protein gene of a variant porcine pseudorabies virus, a recombinant adeno-associated virus, and preparation and application thereof. The recombinant adeno-associated virus (rAAV) is used as a carrier to express the porcine pseudorabies virus gD protein, and the rAAV has the advantages of safety, effectiveness, specificity and the like. The genetically engineered strain prepared by the method expresses the gD immunogenic protein of the epidemic variation porcine pseudorabies virus strain, and has good immunoprotection for multiple epidemic variation porcine pseudorabies virus strains. The mouse is subjected to intramuscular injection of the anterior shin of the right lower limb, then the body can be continuously induced to generate specific neutralizing antibodies and cellular immunity at a high level.
Owner:BRAINVTA (WUHAN) CO LTD +1
Resistance marker-free attenuated live vaccine against porcine contagious pleuropneumonia (PCP) and application thereof
InactiveCN103555645BStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsSerum igePleuronectes pinnifasciatus
The invention discloses a resistance marker-free attenuated live vaccine against porcine contagious pleuropneumonia (PCP) and application thereof and further discloses a resistance marker-free actinobacillus pleuropneumoniae (APP) serum 7 type three gene deletion strain used for preparing the vaccine, belonging to the field of bacterial gene engineering technology. The resistance marker-free APP serum 7 type three gene deletion strain APP-Delta clpP-Delta apxIIC-Delta fur is a strain obtained by inactivating coding genes of protease ClpP, an ApxII toxin activator ApxIIC and an iron absorption regulatory protein Fur of APP through oriented homologous recombination and destroys expression of ClpP, ApxIIC and the Fur protein. The three gene deletion strain obtained in the invention has smaller virulence, decreased by more than 100 times, compared with that of a parent strain, and is safe to animals; A pig immunized by using the strain is well protected from attacks by different serum type virulent strains of APP, and all the protection rates are greater than 80%; and the three gene deletion strain can be used as the attenuated live vaccine for immunoprophylaxis of PCP.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for constructing negative bacteria capable of expressing polysaccharides of positive bacteria and mutants thereof
ActiveCN106191091BPromote research and developmentPromote Applied ResearchBacteriaMicroorganism based processesSalmonella entericaBacterial polysaccharide
The invention provides a method for constructing a gram-negative bacterium capable of expressing a gram-positive bacterium polysaccharide. The method comprises the following steps: transforming a low-copy expression plasmid of the gram-positive bacterium polysaccharide into an asd and rfbP gene-deletion gram-negative bacterium, screening by a balanced lethal system to obtain the gram-negative bacterium capable of producing the gram-positive bacterium polysaccharide. The invention also provides the gram-negative bacterium capable of producing the gram-positive bacterium polysaccharide prepared by the method. The invention also provides a mutant of Salmonella enterica serovar Typhimurium P0005, which is named as Salmonella enterica subsp. enterica serovar Typhimurium P0005, and has the CCTCC NO: M 2016344. The gram-negative bacterium can express the gram-positive bacterium polysaccharide, and the method provided by the invention does not contain any resistance marker and meets the biosafety requirements of subsequent vaccines and other application.
Owner:SICHUAN AGRI UNIV
A kind of attenuated Salmonella vaccine of Haemophilus parasuis
ActiveCN103421731BGood immune protectionEasy to operateAntibacterial agentsBacterial antigen ingredientsAntigenHaemophilus
The invention belongs to the technical field of animal bacteriosis gene engineering vaccines, particularly relates to strain construction, vaccine preparation and application of a recombined haemophilus parasuis attenuated salmonella vaccine strain without resistance makers and used for expressing a surface antigen hps 06257 of haemophilus parasuis, and aims to obtain a recombinant salmonella choleraesuis asd-C500 / pYA-06257 (the preservation number is CCTCC NO: M2013054) without resistance makers and used for expressing the surface antigen HPS 06257 of haemophilus parasuis. The recombinant bacteria is lack of an asd gene on a salmonella choleraesuis genome, and contains a recombinant plasmid pYA-06257 which can express the asd gene and an outer membrane antigen hps 06257 gene of haemophilus parasuis on the strain. The invention further discloses a construction method of the recombinant strain asd-C500 / pYA-06257 and a corresponding preparation method of the haemophilus parasuis attenuated salmonella vaccine, as well as application to the preparation of the haemophilus parasuis attenuated salmonella vaccine.
Owner:HUAZHONG AGRI UNIV
Protective clothing air conditioning unit
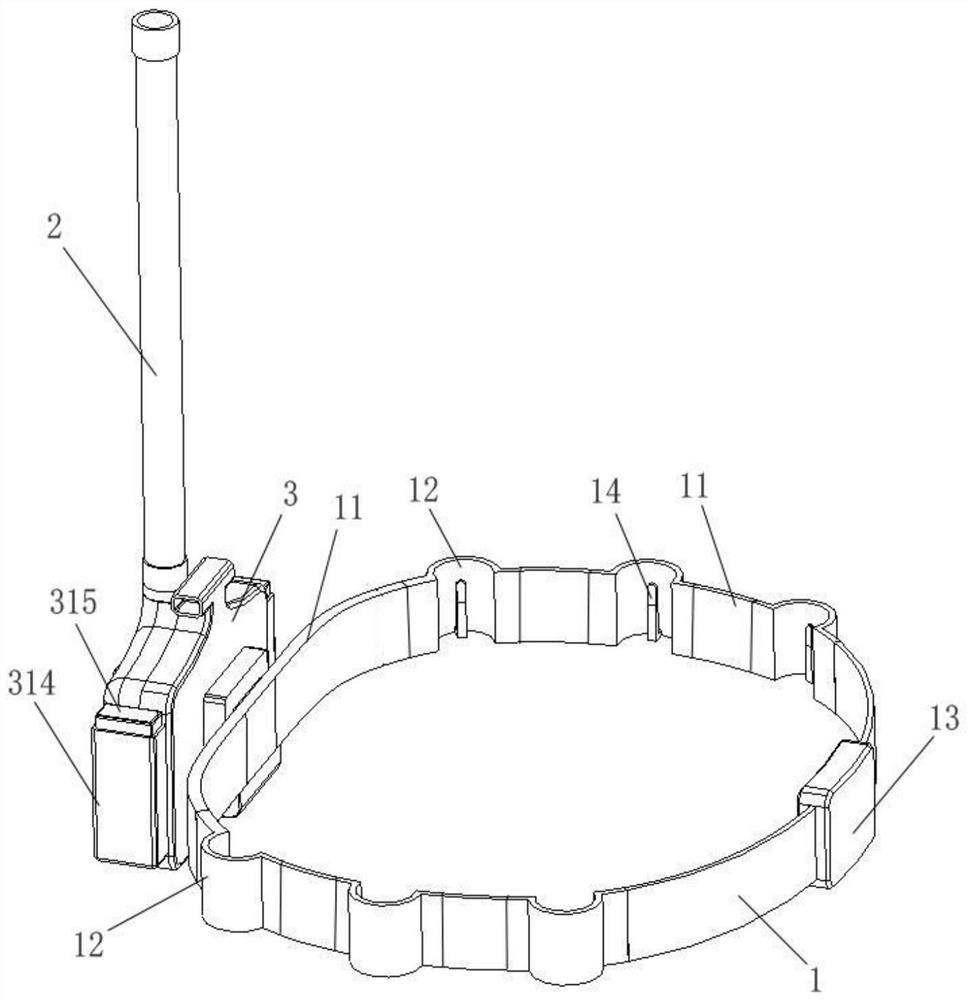
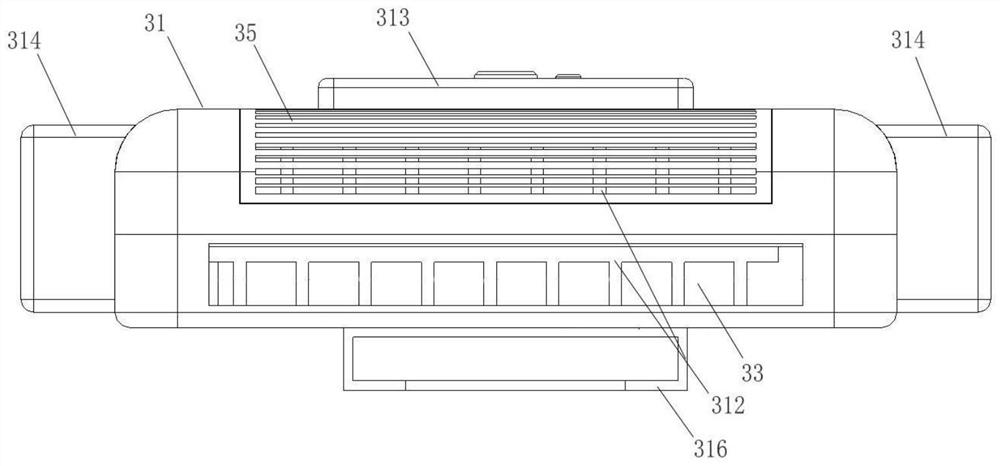
PendingCN114601222AImprove protectionCompact designMachines using electric/magnetic effectsRefrigeration safety arrangementCold airEngineering
The protective clothing air conditioning unit comprises a ventilation waistband, an air conveying hose and an air conditioner body, and the air conditioner body is worn on the back waist of a user through the ventilation waistband; the air conditioner body provides cold air or warm air meeting the biological safety requirement for the interior of the protective suit through the air conveying hose. The air conditioner body adopts the design of the cross-flow fan and the semiconductor chilling plate, and efficient supply of cold air or warm air meeting the biological safety requirement is achieved through reasonable air opening design and internal flow guide layout. The ventilation waistband enables cold air or warm air to penetrate through the waistband and enter the lower half body of the protective suit. The air conditioning unit designed by the invention can provide a comfortable internal environment meeting the biological safety requirement for the protective clothing; the air conditioner body is compact in structure and reasonable in layout design, the air conditioner body is of the design structure that air enters from the outer side and / or the bottom and exhausts from the upper portion, air suction and air exhaust are independent of each other and do not affect each other, and the unit size is effectively reduced while the refrigerating or heating efficiency is improved.
Owner:刘思聪
Recombinant adeno-associated virus transfer vector containing variant porcine pseudorabies virus gd protein gene, virus and preparation and application thereof
The invention belongs to the technical field of genetic engineering, and more particularly relates to a recombinant adeno-associated virus transfer vector containing the gD protein gene of mutated porcine pseudorabies virus, a recombinant adeno-associated virus, and its preparation and application. The recombinant adeno-associated virus (rAAV) prepared by the invention is used as a vector to express the gD protein of porcine pseudorabies virus, and the rAAV has various advantages such as safety, effectiveness and specificity. The genetic engineering strain prepared by the invention expresses the gD immunogenic protein of the circulating mutant porcine pseudorabies virus strain, and has good immune protection against multiple circulating mutant porcine pseudorabies virus strains. A single injection into the right lower limb tibialis anterior muscle in mice can induce sustained high levels of specific neutralizing antibodies and cellular immunity.
Owner:BRAINVTA (WUHAN) CO LTD +1
ExPEC (extraintestinal pathogenic escherichia coli) double-gene deleted strain and vaccine prepared from same
InactiveCN112680392AGood immune protectionClear genetic backgroundAntibacterial agentsBacteriaEscherichia coliExtraintestinal Pathogenic Escherichia coli
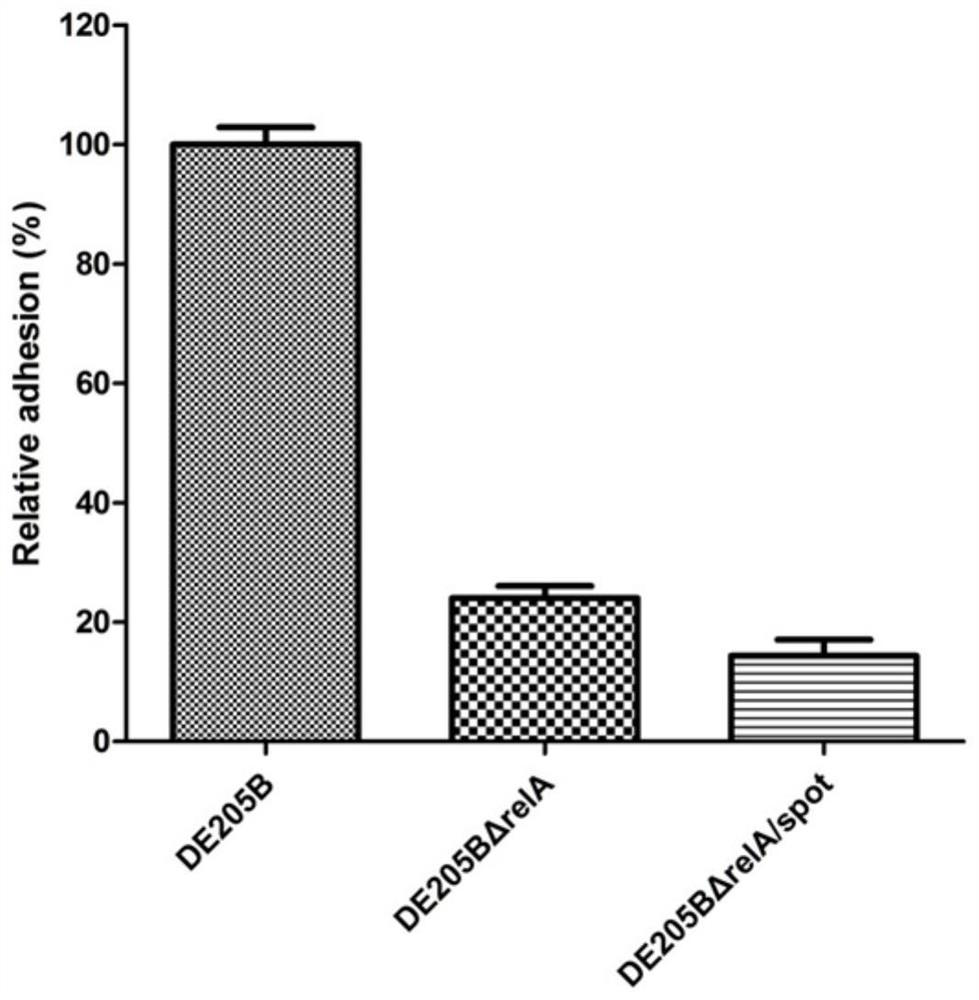
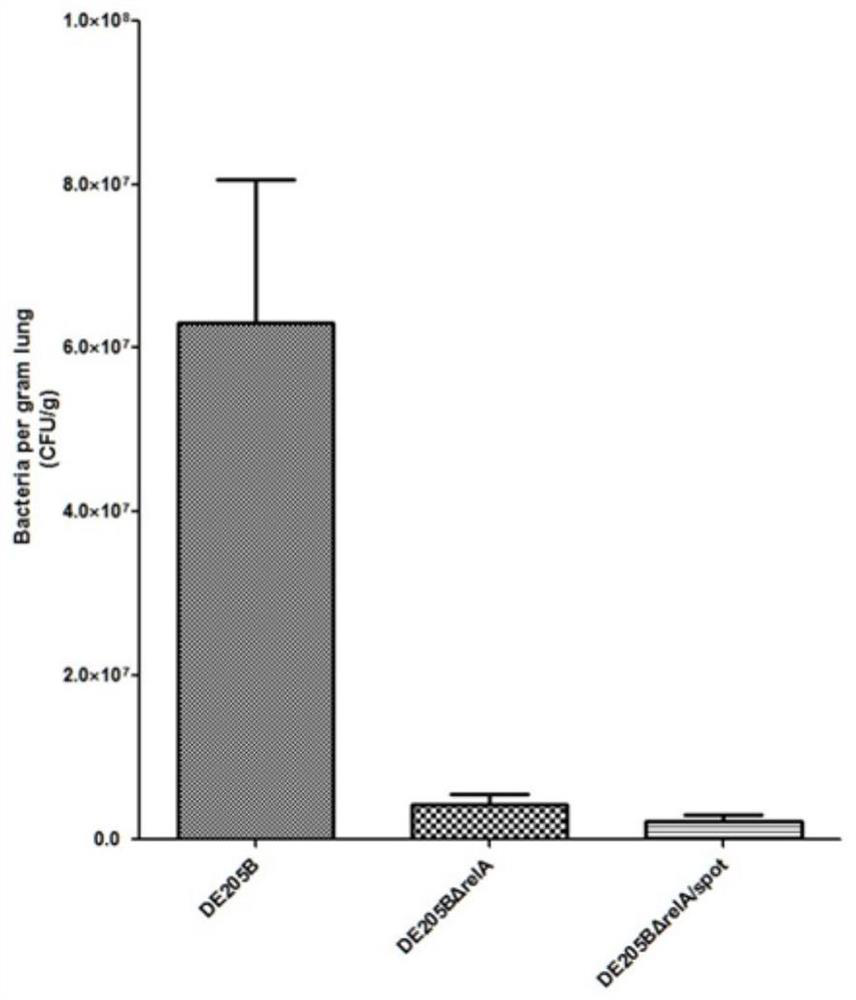
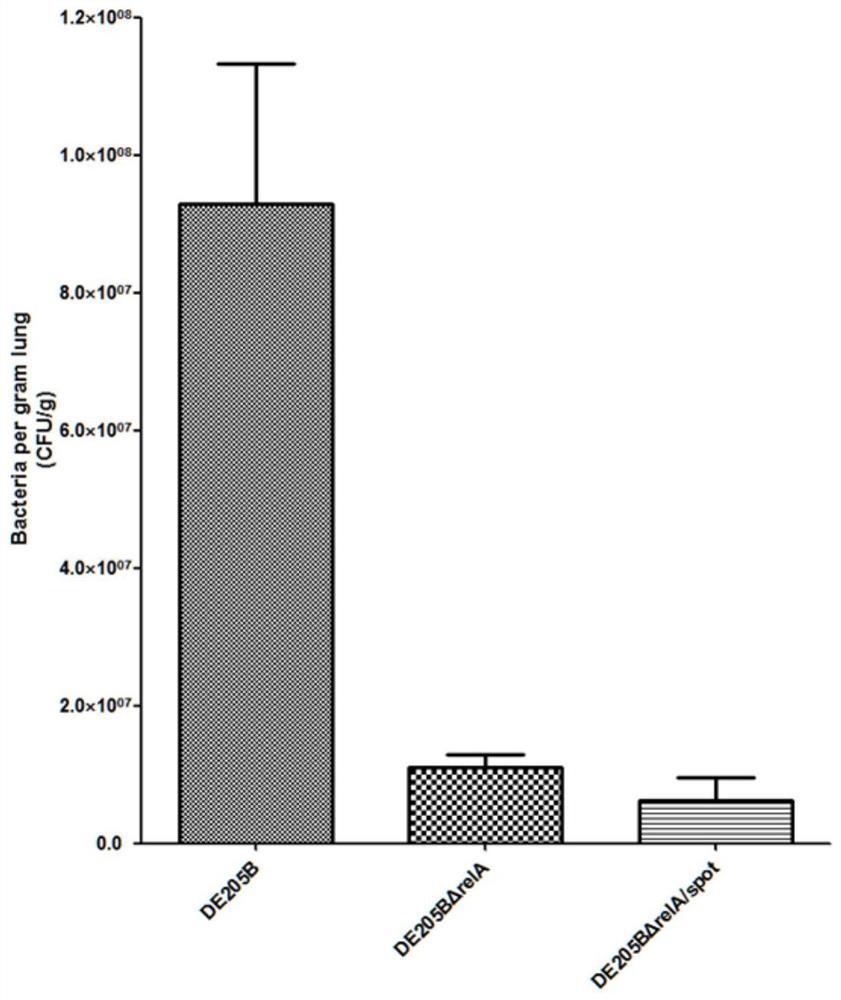
The invention discloses an ExPEC (extraintestinal pathogenic escherichia coli) double-gene deleted strain and a vaccine prepared from the same. A construction method of the ExPEC double-gene deleted strain comprises the following steps: knocking out a relA gene and a spoT gene in an ExPEC virulent strain DE205B by a Red homologous recombination method to obtain an ExPEC relA and SpoT double-gene deleted strain DE205B[delta]relA / spoT, the strain is preserved in the China Center for Type Culture Collection, and the preservation number of the strain is CCTCC NO: M 2020610. The deleted strain has good biological safety and immunogenicity, is relatively weak in toxicity, and can be used for preparing the attenuated vaccine and preventing ExPEC infection. The attenuated vaccine prepared from the ExPEC double-gene deleted strain has an immune protective effect on ExPEC infection, and has a good toxin attacking immune protective effect.
Owner:NANTONG UNIVERSITY
Recombinant Salmonella choleraesuis for expressing surface antigen gene sao of streptococcus suis type 2, vaccine and application
ActiveCN101979501BGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesAntigen
The invention belongs to the field of animal bacterium gene engineering, and in particular relates to construction of resistance marker-free recombinant Salmonella choleraesuis for expressing surface antigen sao gene segment of streptococcus suis type 2, preparation of a vaccine and application. The resistance marker-free recombinant Salmonella choleraesuis for expressing the surface antigen sao gene segment of the streptococcus suis type 2, namely asd-C500 / Pya-saoA is obtained, and the collection number is CCTCC NO: M2010156. The asd gene of the Salmonella choleraesuis is deleted in the recombinant strain, and the recombinant strain contains plasmid pYA-saoA capable of expressing the asd and the sao gene segment of the streptococcus suis type 2. The invention also discloses a method for preparing the recombinant strain and the vaccine, and application in preparing Salmonella choleraesuis-streptococcus suis type 2 vaccines.
Owner:HUAZHONG AGRI UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com