Genetic engineering live vaccine of recombinant Salmonella choleraesuis and Porcine epidemic diarrhea virus, preparation and application
A technique for porcine epidemic diarrhea and salmonella
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1: Prepare the gene fragment of the porcine epidemic diarrhea protective antigen COE and SD protein capable of prokaryotic expression
[0087] (1) Design of primers required for preparation of prokaryotic expression plasmids pET-32a-COE and pET-32a-SD, the sequences of which are described in Table 1.
[0088] Table 1 The primer sequences required for the preparation of prokaryotic expression plasmids pET-32a-COE and pET-32a-SD
[0089]
[0090]
[0091] The underlined parts of the primers in Table 1 are restriction sites.
[0092] (2) Preparation of COE and SD fragments capable of prokaryotic expression
[0093] Using the multiplex RT-PCR detection method of porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine group A rotavirus reported by Zhang Kun (Zhang Kun, 2010), the clinical disease materials submitted for inspection were detected. Genomic RNA was extracted from porcine epidemic diarrhea virus-infected positiv...
Embodiment 2
[0097] Example 2: Construction and Identification of Recombinant Plasmids pYA-COE and pYA-SD
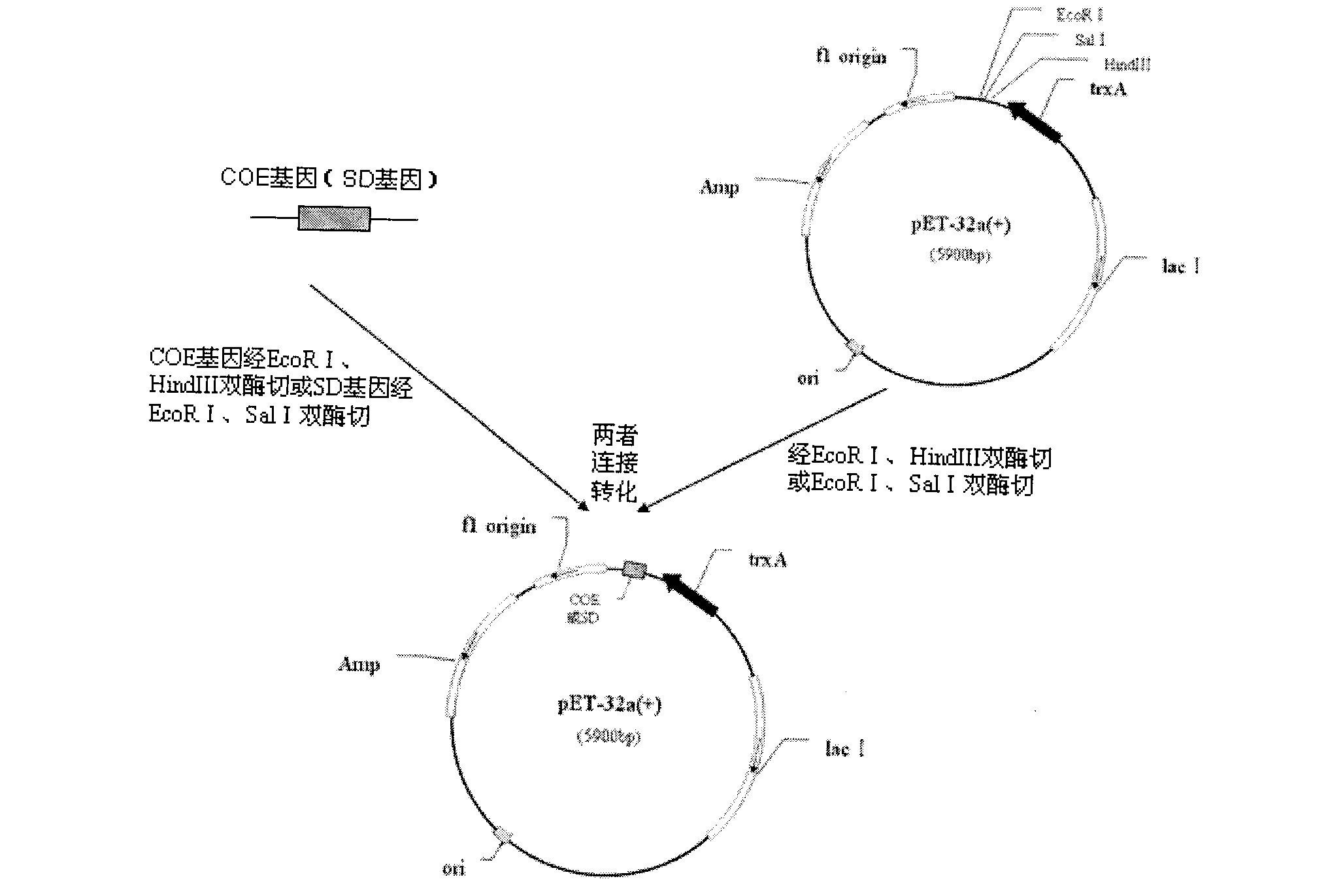
[0098] Recombinant plasmid pET-32a-COE with EcoR I and HindIII restriction sites and pET-32a-SD with ECOR I and Sal I restriction sites were subjected to double digestion and recovery, and then subjected to the same double digestion Recovered shuttle plasmid pYA3493 (plasmid construction map see image 3 ) ligation, the ligation product was transformed into Escherichia coli x6097 with asd gene deletion (source: a gift from Dr. Roy Curtiss III, Washington University, USA), positive clones were screened, the plasmid was extracted, and the recombinant plasmid pYA with the correct construction was obtained after identification by PCR and double enzyme digestion -COE and pYA-SD (eg Figure 4 and Figure 5 shown). Wherein, the PCR amplification system and amplification conditions are as described in Example 1, (2).
Embodiment 3
[0099] Example 3: Construction and identification of recombinant Salmonella strains C501-COE and C501-SD expressing porcine epidemic diarrhea virus COE and SD fusion protein
[0100] (1) identify the design of the primers required for the recombined Salmonella C501-COE and C501-SD, its DNA sequence is as shown in table 2:
[0101] Table 2 identifies the DNA sequence of the primers required for the recombined Salmonella C501-COE and C501-SD
[0102]
[0103] (2) Construction and identification method of recombinant Salmonella C501-COE and C501-SD
[0104] The correctly identified recombinant plasmids pYA-COE and pYA-SD (see Example 2 for sources) were electrotransformed into asd-deleted C500-competent cells respectively, and the parameters of the electroporator (Bio-Rad GenePulserII) were set to: voltage 2.2Kv, Capacitance 25μF, pulse resistance 200Ω and time 4ms, construction flow chart see Figure 6 . On the DAP negative plate, pick a single colony and culture it in TSB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com