Patents
Literature
520 results about "Biosafety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biosafety is the prevention of large-scale loss of biological integrity, focusing both on ecology and human health. These prevention mechanisms include conduction of regular reviews of the biosafety in laboratory settings, as well as strict guidelines to follow. Biosafety is used to protect from harmful incidents. Many laboratories handling biohazards employ an ongoing risk management assessment and enforcement process for biosafety. Failures to follow such protocols can lead to increased risk of exposure to biohazards or pathogens. Human error and poor technique contribute to unnecessary exposure and compromise the best safeguards set into place for protection.
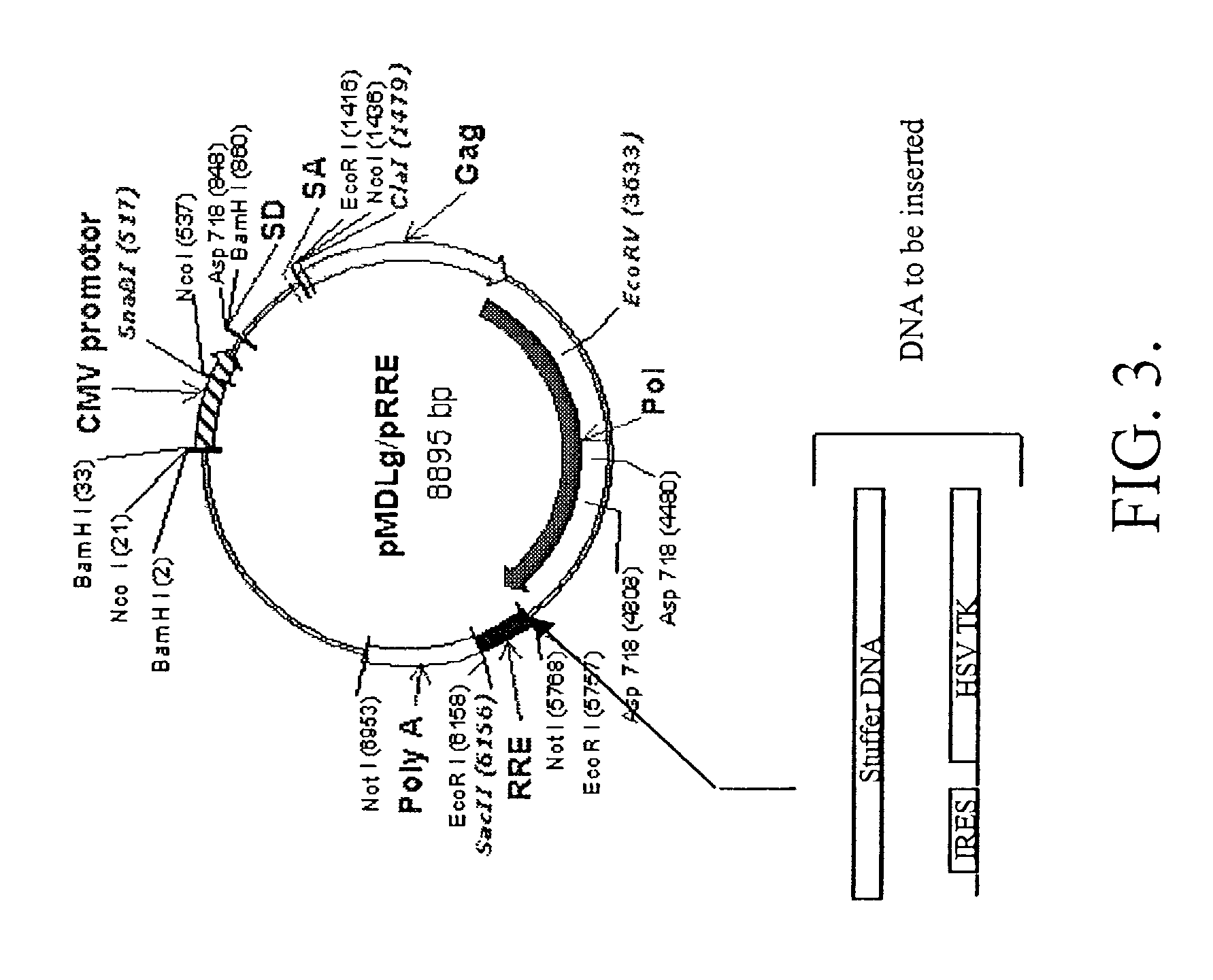
Methods and compositions relating to improved lentiviral vector production systems
ActiveUS7629153B2Increasing its biosafetySafe transfection and transductionVectorsGenetic material ingredientsTranscriptional Regulatory ElementsTransgene
The present invention provides HIV-derived lentivectors which are multiply modified to create highly safe, efficient, and potent vectors for expressing transgenes for gene therapy. The lentiviral vectors comprise various combinations of an inactive central polypurine tract, a stuffer sequence, which may encode drug susceptibility genes, and a mutated hairpin in the 5′ leader sequence that substantially abolishes replication. These elements are provided in conjunction with other features of lentiviral vectors, such as a self-inactivating configuration for biosafety and promoters such as the EF1α promoter as one example. Additional promoters are also described. The vectors can also comprise additional transcription enhancing elements such as the wood chuck hepatitis virus post-transcriptional regulatory element. These vectors therefore provide useful tools for genetic treatments for inherited and acquired disorders, gene-therapies for cancers and other disease, the creation of industrial and experimental production systems utilizing transformed cells, as well as for the study of basic cellular and genetic processes.
Owner:RES DEVMENT FOUND
Method and system for assessing and managing biosafety and biosecurity risks
Methods and systems for evaluating, mitigating, and managing biosafety and biosecurity risks. The methods and systems establish biosafety and biosecurity risk management procedures and systems, and facilitate auditing of facilities and testing individuals and companies to evaluate compliance with such procedures and systems. Systems and business methods for establishing biosafety and biosecurity risk management procedures and systems, auditing and certifying companies, facilities, systems and individuals for compliance, and analyzing biosafety and biosecurity risks for making investment decisions and for pricing and underwriting such risks in the insurance industry.
Owner:SHAHI GURINDER S +1
Coronavirus rapid detection kit based on S protein ligand and ACE2 receptor competitive chromatography
ActiveCN111273016AImmunochromatographic fastEasy immunochromatographyCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsReceptorBlood plasma
Owner:浙江诺迦生物科技有限公司 +1
Collagen sponge and preparation method thereof
InactiveCN102068714APromote proliferationPromote tissue repairAbsorbent padsBandagesCross-linkPorosity
The invention discloses collagen sponge and a preparation method thereof. The collagen sponge does not contain a chemical cross-linking agent, has the porosity of over 90 percent, and is prepared by performing radiation cross-linking on aqueous solution of collagen and then freeze-drying the aqueous solution of collagen; and sterilization is performed during cross-linking so that the biosafety problem caused during preparation is solved. The collagen sponge prepared by the method has no residue of chemical reagents, better biocompatibility and structural uniformity, and adjustable degradation rate and mechanical strength, and can be used as biomedical materials such as dressing, tissue engineering stents, artificial tissue components, tissue fillers, embolic agents and the like.
Owner:PEKING UNIV
Methods and kits for ascertaining biosafety of an agent
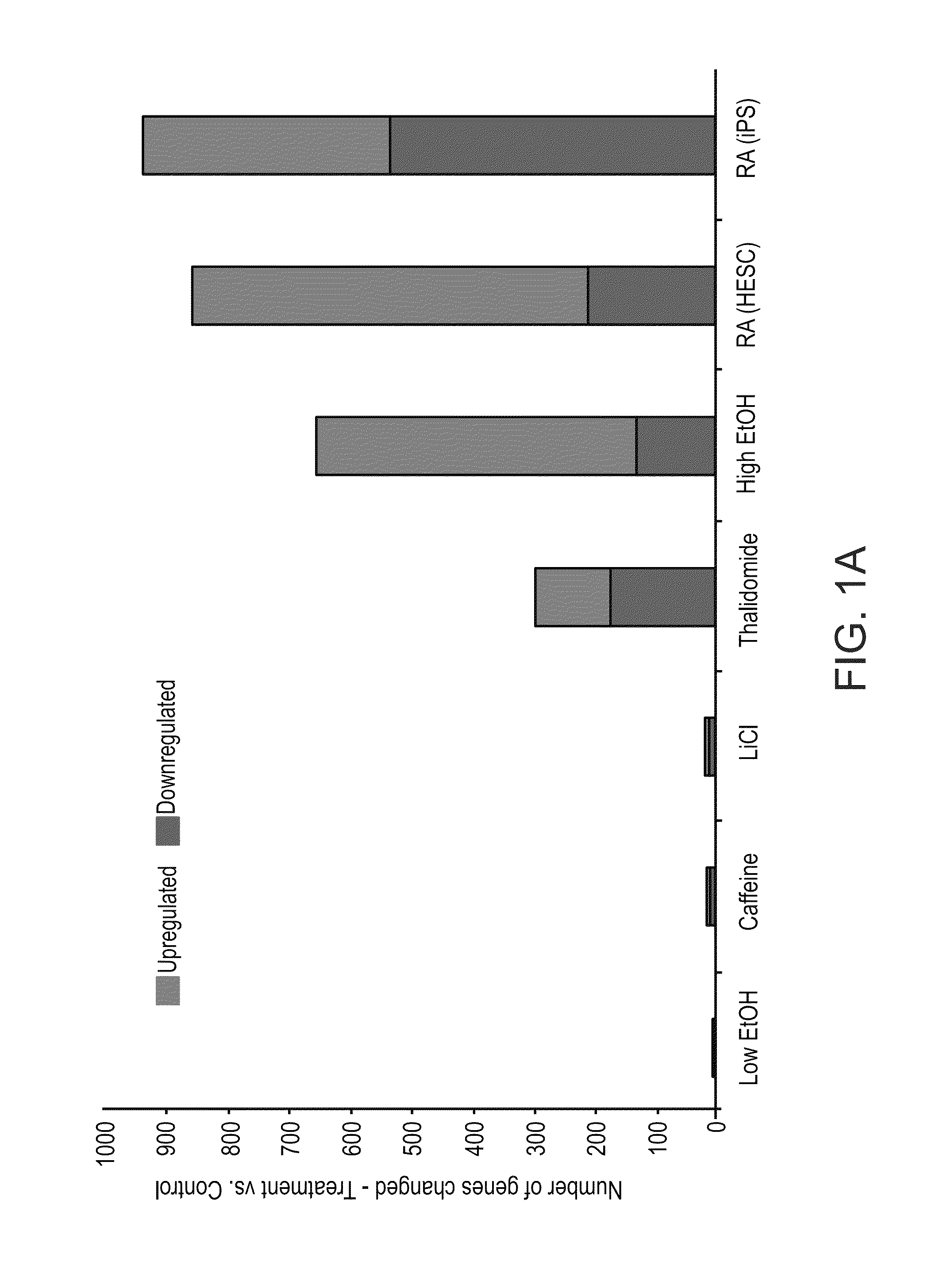
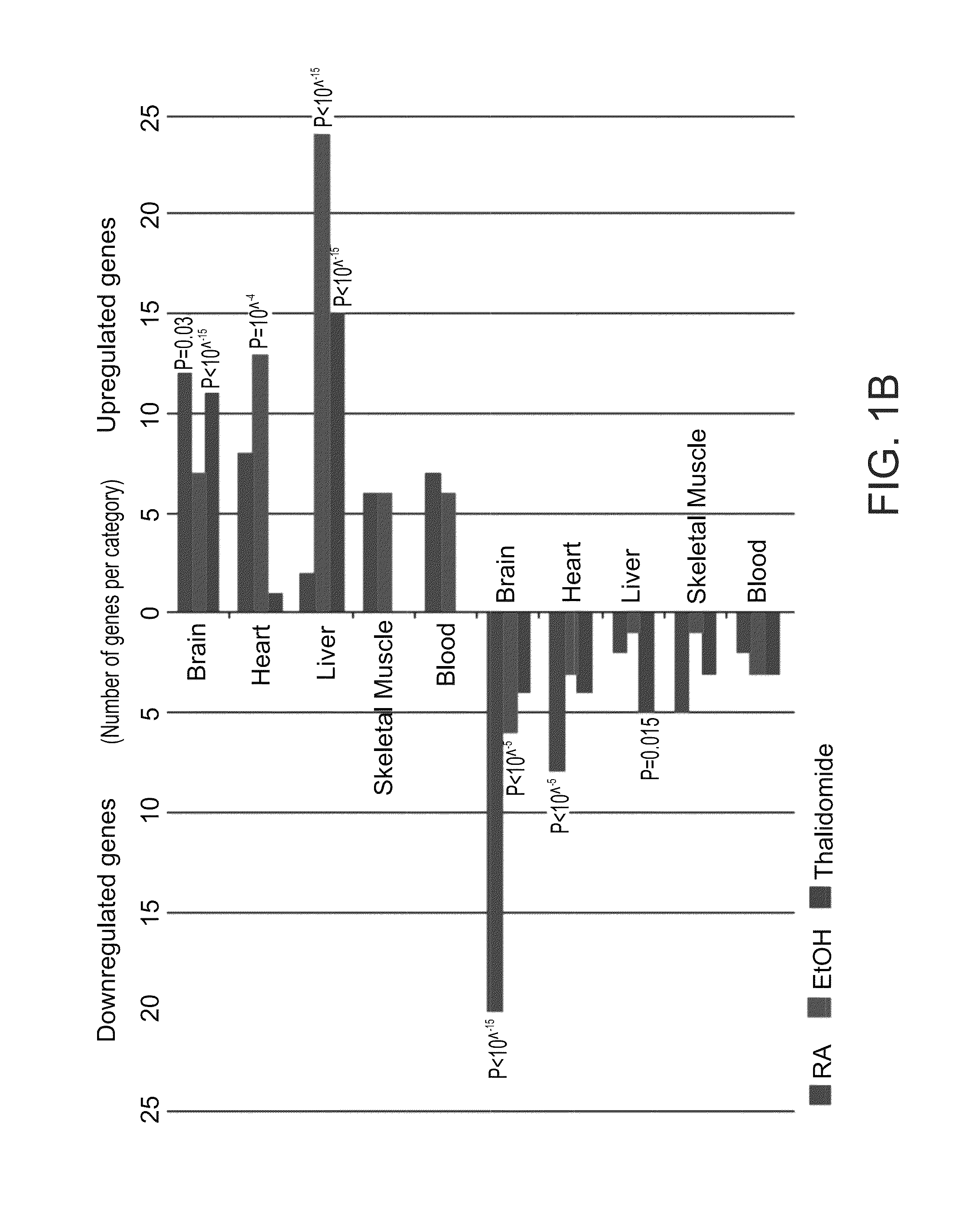
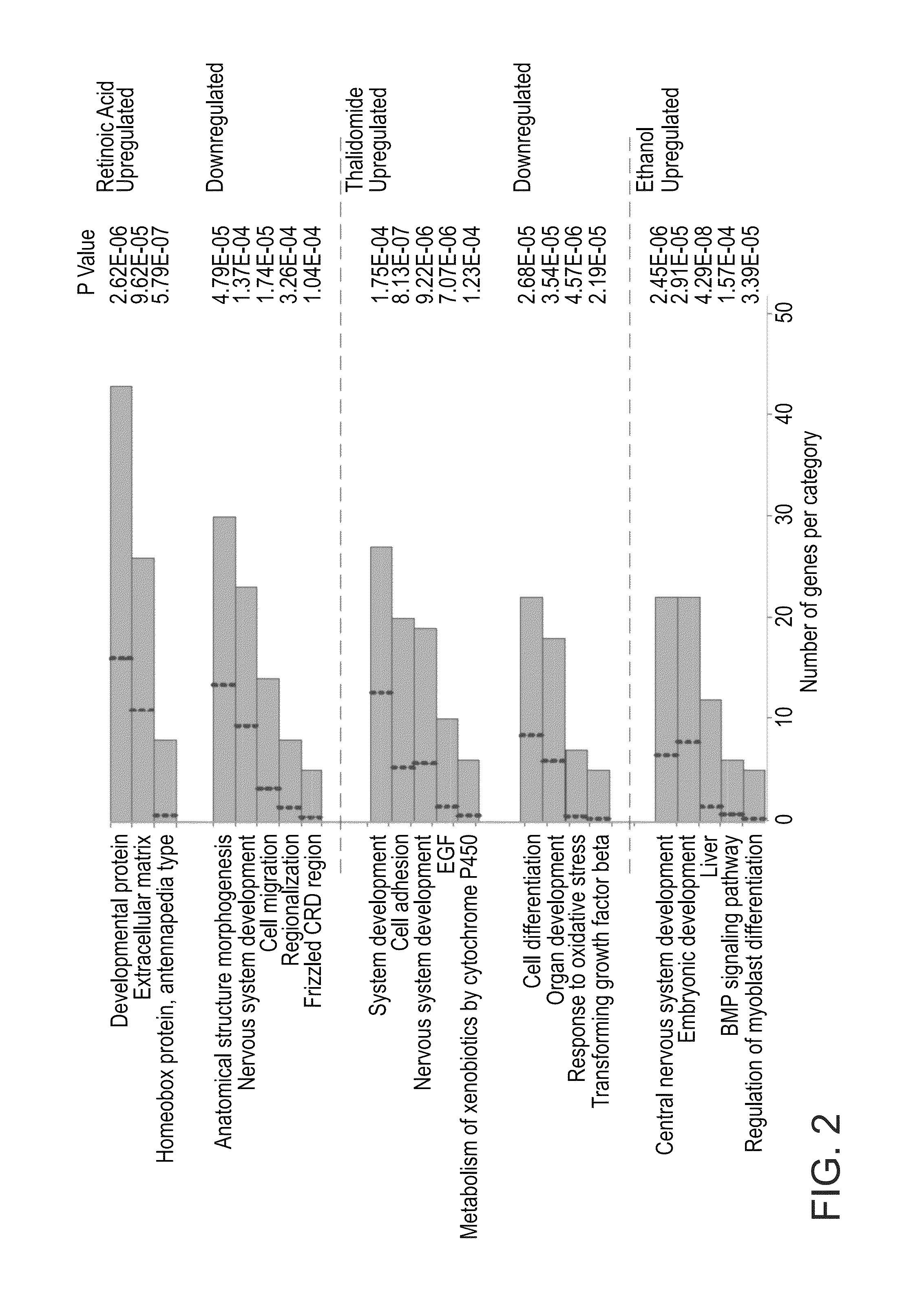
ActiveUS20110287974A1Nucleotide librariesMicrobiological testing/measurementTissue specific geneTissue specific
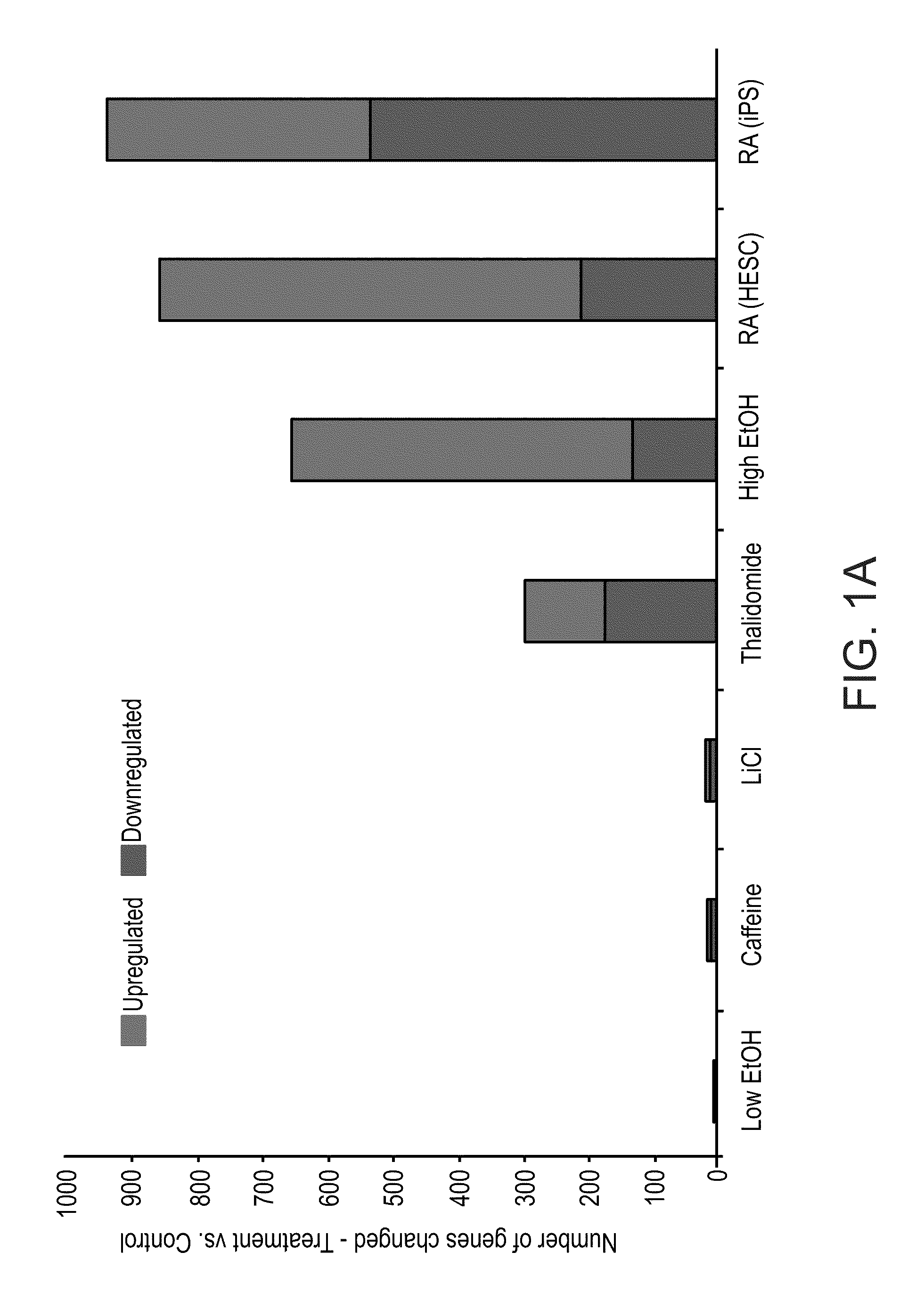
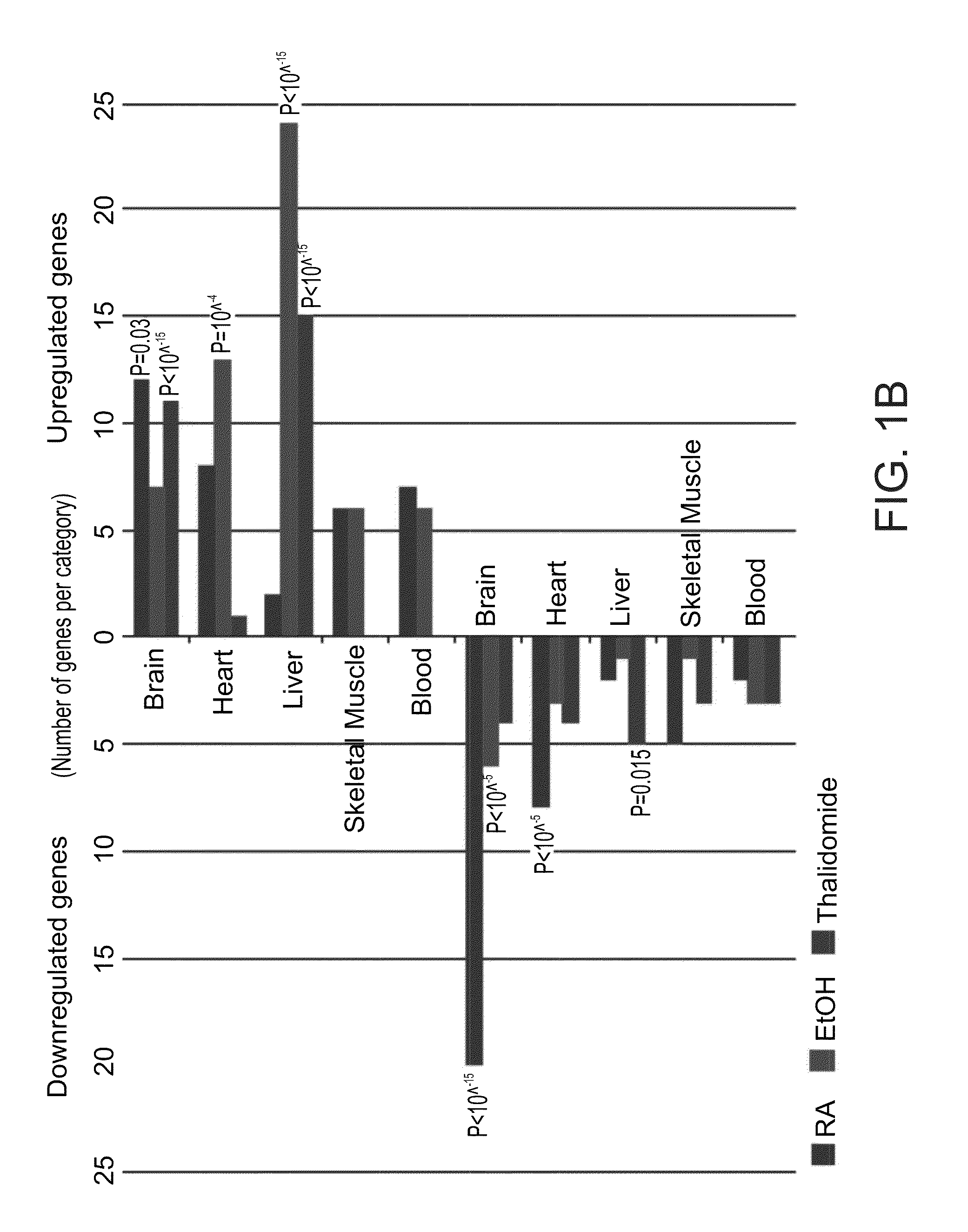
A method of ascertaining the bio-safety of an agent is disclosed. The method comprises:(a) contacting the agent with differentiating human pluripotent stem cells;(b) analyzing a level of gene expression of a plurality of genes in the differentiating human pluripotent stem cells, wherein the agent is qualified as being safe if at least one of the following qualification parameters are fulfilled:(i) the agent causes a difference in the level of gene expression below a predetermined number of genes as compared to control differentiating human pluripotent stem cells that have not been contacted with the agent;(ii) the agent causes a difference in gene expression below a predetermined number of tissue-specific genes of a tissue as compared to control differentiating human pluripotent stem cells that have not been contacted with the agent; or(iii) the agent causes a difference in gene expression below a predetermined number of genes involved in fetal development as compared to control differentiating human pluripotent stem cells that have not been contacted with the agent.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562369AGood repeatabilityStrong specificityImmunoassaysImmunodiagnosticsProtein s antigen
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, an S protein antigen of SARS-CoV-2 and acompetitive substance. The competitive substance is marked with a signal substance and can be specifically combined with the new coronavirus S protein antigen. Whether a tested person is infected bythe new coronavirus or not and whether infection risks exist or not are judged by detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
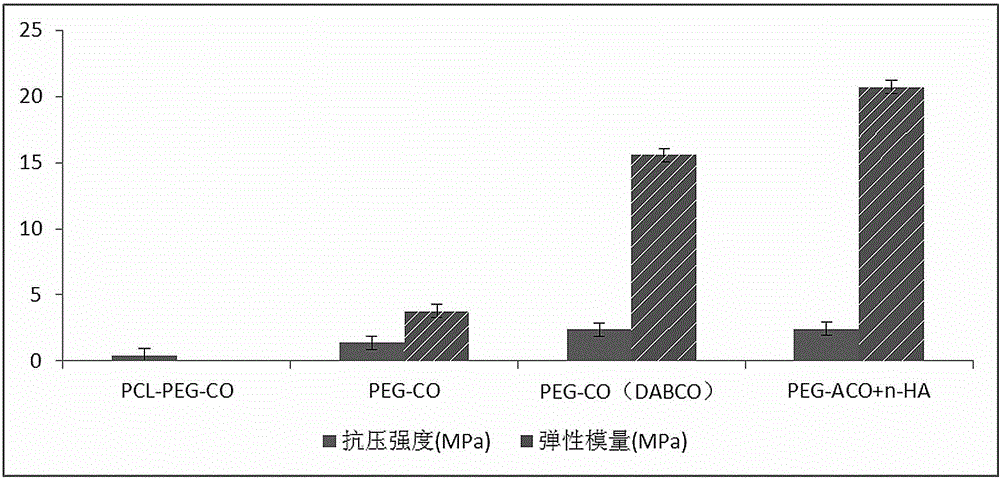
Fluorescent graft degradable block polyurethane, bone repair material and preparation method thereof
InactiveCN104356345APromote growthGood biocompatibilityPharmaceutical delivery mechanismTissue regenerationPolymer scienceBiocompatibility
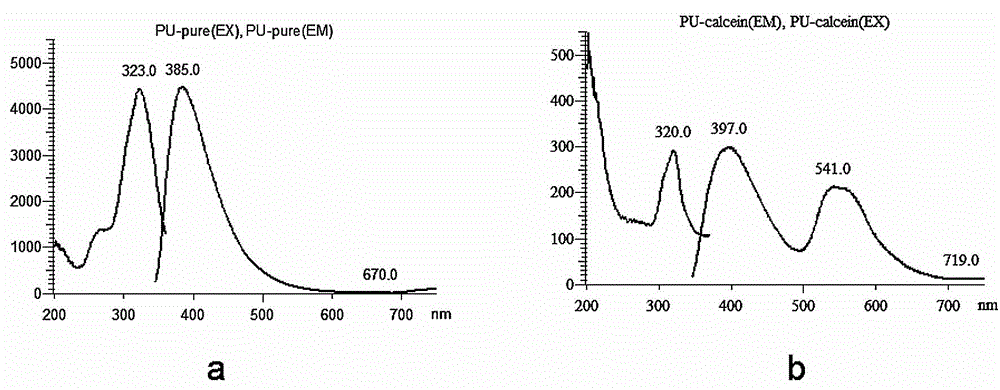
The invention relates to a fluorescent graft degradable block polyurethane, a bone repair material and a preparation method thereof. The polyurethane is formed by grafting a pharmaceutically acceptable fluorescent component into a structure formed by polymerizing a hard segment of aliphatic diisocyanate and a soft segment of a degradable polymer chain segment of a hydroxyl-terminated polymer or block copolymer. The bone repair material is composed of the polyurethane and nano hydroxyapatite powder. The polyurethane and corresponding bone repair material have favorable biocompatibility and degradability, have the fluorescent characteristic, can be used for performing tracing evaluation on the degradation process of the block polyurethane, analyzing the mechanism of degradation and inspecting the influence of the degradation rate on the mechanical properties of the material and the tissue regeneration and reconstruction process, and provide a new visual angle and means for evaluating the biosafety of the high-polymer degradable material. The preparation technique is simple, is easy to control and operate, and has favorable application prospects in the field of biomedicine.
Owner:SICHUAN UNIV
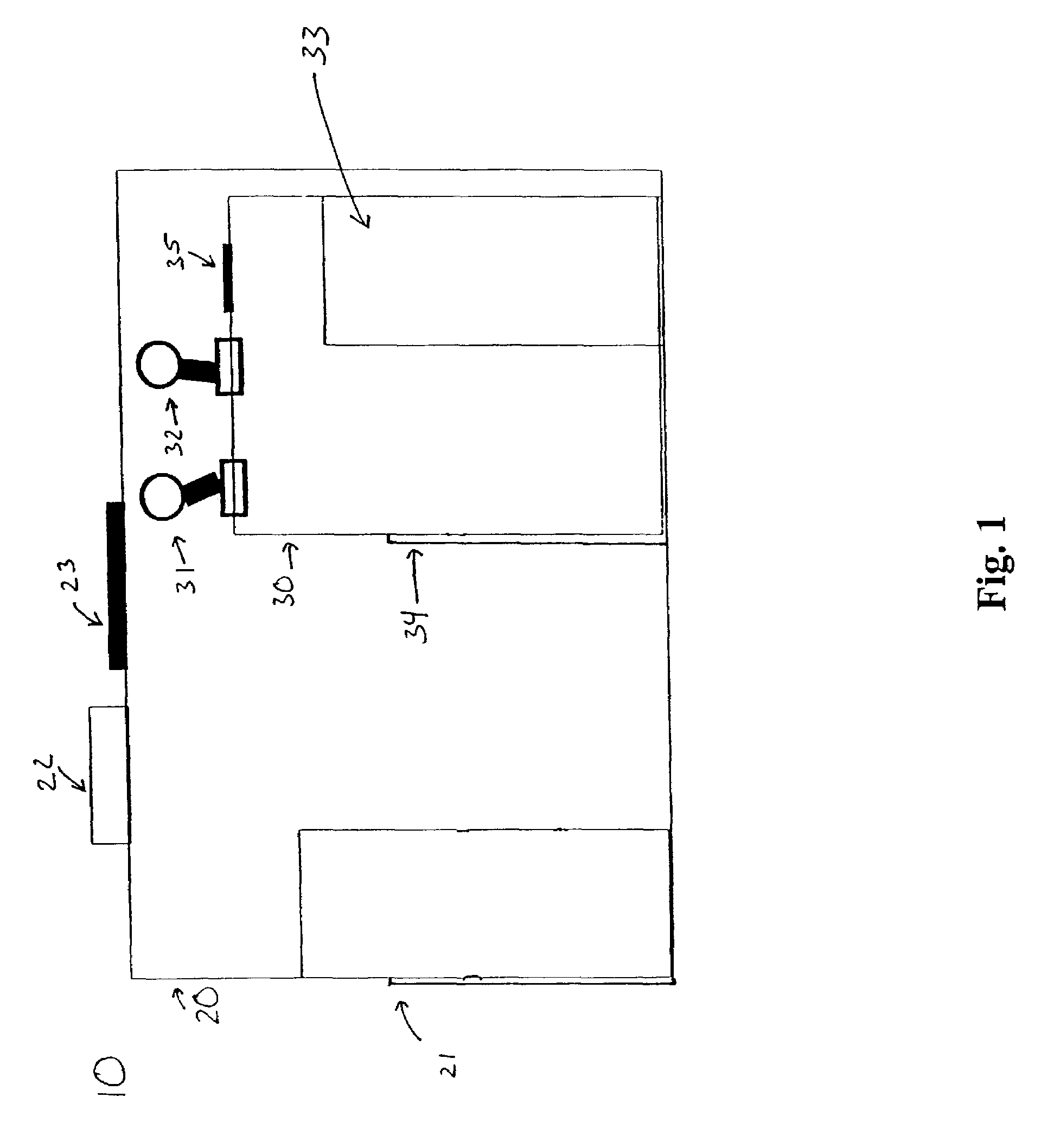
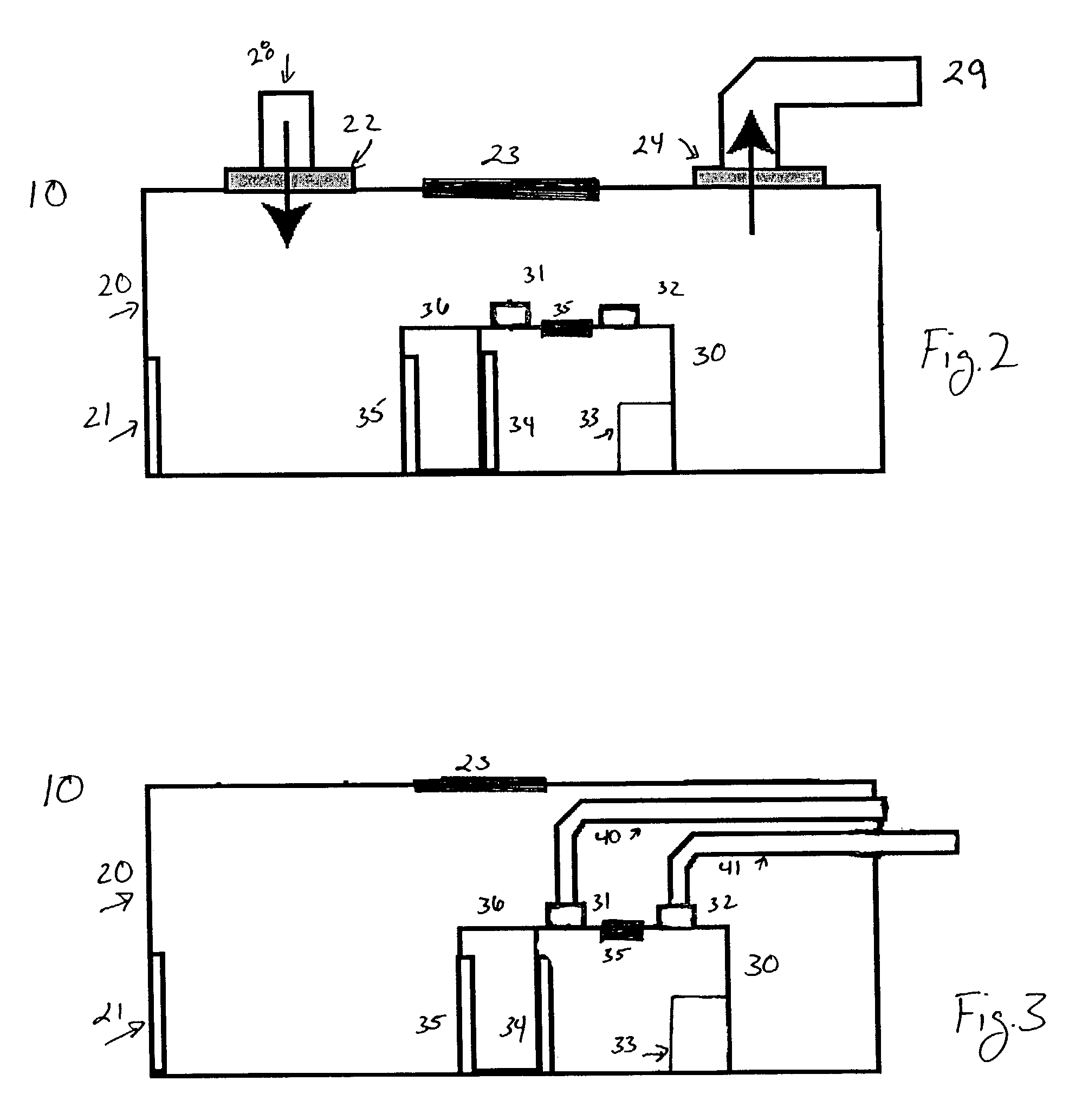
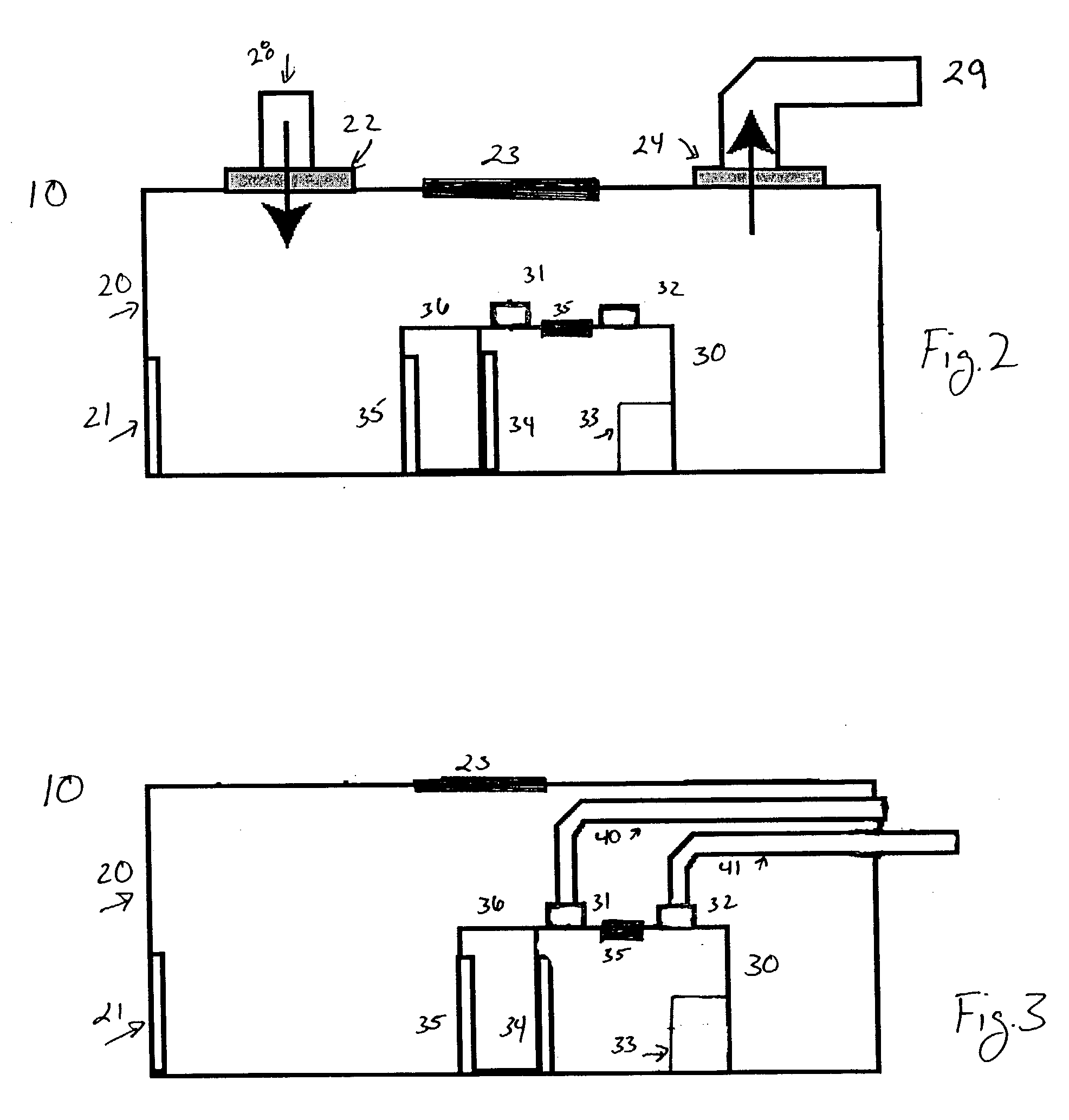
Modular biosafety containment apparatus and system
InactiveUS7335243B2Rapid deploymentNegative pressurizationCombination devicesDispersed particle filtrationModularityEngineering
Owner:HOMAN JANE +1
Modular biosafety containment apparatus and system
InactiveUS20060107635A1Rapid deploymentNegative pressurizationCombination devicesBreathing protectionModularityEngineering
Owner:HOMAN JANE +1
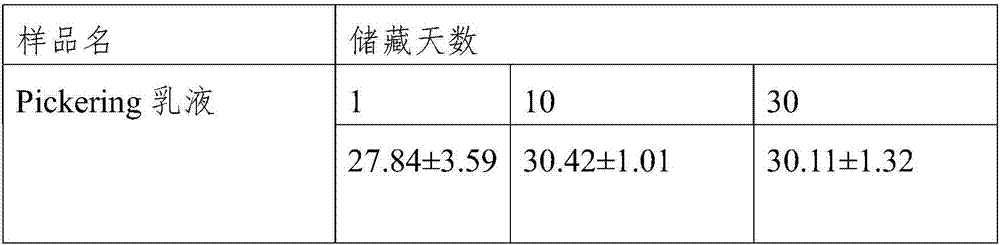
Pickering emulsion prepared from peanut protein isolate and preparation method thereof
ActiveCN107455550ALow costSimple and fast operationProtein composition from vegetable seedsVegetable proteins working-upProtein isolatePickering emulsion
The invention relates to a method for preparing a Pickering emulsion from peanut protein isolate, and the method comprises the following steps: preparing a peanut protein dispersion solution by taking a peanut protein isolate solution as a raw material; preparing a proteoglycan mixed dispersion solution from a polysaccharide solution and the peanut protein isolate dispersion solution; adding transglutaminase to the proteoglycan mixed dispersion solution, and preparing gel blocks by crosslinking reaction; preparing a microgel particle dispersion solution by taking the gel blocks as raw materials; and then adding the microgel particle dispersion solution to edible oil for preparing the Pickering emulsion. The method disclosed by the invention adopts high-speed shearing equipment, high-pressure homogenizing equipment and other common equipment for conducting granulation, and the Pickering emulsion prepared by high-speed shearing is not added with any inorganic materials in the preparation process, and has good biosafety and strong biocompatibility. The prepared Pickering can be stable at room temperature for more than 30 days, and can be used as a delivery system of fat-soluble and photosensitive active substances.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Preparation method of tissue repair material
ActiveCN104524634AIncreasing the thicknessAffect regenerationProsthesisTissue repairAutologous tissue
The invention relates to a preparation method of a tissue repair material. The preparation method comprises the following steps: carrying out pre-treatment; vibrating in an organic solvent to degrease; deactivating a virus with an ethanol solution; removing cells by high and low osmotic solutions; swelling in an acidic or alkaline liquid; and quickly lyophilizing and sterilizing. The thickness of the tissue repair material is increased by 2-4 times, and the material is loose and porous, small in change of mechanical property and degradation property and good in cell removal effect and has relatively high biosafety and biocompatibility. Due to the structural characteristics of proper thickness and looseness and porosity, the material is suitable for damage repair for filling damaged parts required by repair such as cartilage injury, skin and soft tissue defect, gingival recession and the like. Animal experiments verify that the repaired region after eight weeks has no depressed phenomena and is fully replaced by regenerated tissues. Moreover, the regenerated tissue and autologous tissue are healed well and are free of separating phenomenon, which verifies that the material can provide sufficient three-dimensional space for cell proliferation and crawl, so that the repaired region has good integration degree and filling degree, thereby realizing tissue repair and regeneration.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Method for preparing insect feed with organic waste and insect feed
ActiveCN103504151ARealize resource processingValuable protein resourceFood processingAnimal feeding stuffFermentationNutrients substances
The invention relates to the technical field of insect feed processing, in particular to a method for preparing protein insect feed with organic waste and the prepared insect feed. The method comprises the steps of directly crushing the organic waste into powder, mixing with beneficial microorganisms for fermentation, and adding necessary nutrition additives to form the insect feed. The raw material is not required to be sterilized and dried at high temperature; the production cycle is shortened; the production cost is lowered; simultaneously, macromolecular substances of the organic waste are converted to small peptides, organic acid and sugar by physiologic metabolism of the beneficial microorganisms; various nutrient substances such as bio-enzymes and vitamins which have a prebiotic effect on high protein insects and are easier to absorb are produced; and the purpose of recycling is achieved. Fermentation heat energy produced during fermentation of the microorganisms is effectively utilized to kill pathogenic microorganisms possibly existing in the organic waste, and the biosafety of the insect feed is ensured.
Owner:GUANGZHOU UNIQUE BIOTECH CO LTD +1
Method for preparing carbon nanotube composite conductive hydrogel coating modified electrode
InactiveCN103196965AImprove electrochemical activityGood biocompatibilityMaterial electrochemical variablesMetal electrodesPolymer chemistry
The invention relates to the field related to biomedical materials and medical apparatuses and in particular relates to a method for preparing a carbon nanotube composite conductive hydrogel coating modified electrode by adopting an electrophoretic deposition method. The method comprises the following steps of: 1. preparing carbon nanotube dispersion liquid; 2. preparing a carbon nanotube composite polymer sol body; 3. pretreating a metal electrode; 4. preparing a composite conductive hydrogel coating through electrophoretic deposition; and 5. crosslinking the composite coating and finally forming the carbon nanotube composite conductive hydrogel coating modified electrode, wherein the prepared electrode is put in phosphate buffered solution for standby use. The method has the advantages that various carbon nanotube surface treatment methods and various sol-gel phase transformation or crosslinking methods are adopted, thus solving the problems that the carbon nanotube and the composite material thereof are difficult to disperse in the water solution systems, are easy to agglomerate and have potential biosafety risks and the like.
Owner:UNIV OF SCI & TECH BEIJING
Chemical crosslinking type glucan hydrogel and preparation method thereof
The invention relates to chemical crosslinking type glucan hydrogel and a preparation method thereof, and belongs to the technical field of biomedical functional polymer materials. The preparation method comprises the steps that glucan is dissolved in water at room temperature and then placed in an ice-water bath, and sodium periodate is added to obtain a mixed solution; the mixed solution is stirred for reaction for 2-6 h on the room temperature and dark conditions; the stirred solution is dialyzed with a regenerated cellulose dialysis bag in deionized water, and after dialysis is completed, freeze drying is performed to obtain partial hydroformylation glucan solids with the hydroformylation degree of 40-60 percent; the obtained partial hydroformylation glucan solids are dissolved in the deionized water to obtain an aqueous hydroformylation glucan solution, a multi-amino crosslinking agent is added in the aqueous hydroformylation glucan solution to be mixed evenly at the room temperature, and then the glucan hydrogel is prepared. The chemical crosslinking type glucan hydrogel is prepared by taking natural glucan macromolecules with the good biosafety as a material basis through chemical crosslinking of the partial hydroformylation glucan and the multi-amino crosslinking agent.
Owner:KUNMING UNIV OF SCI & TECH
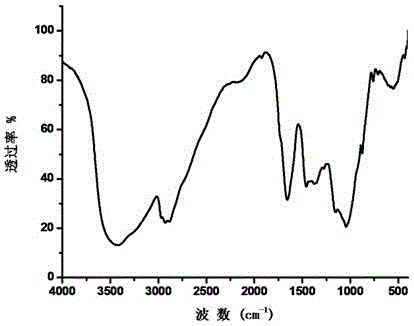
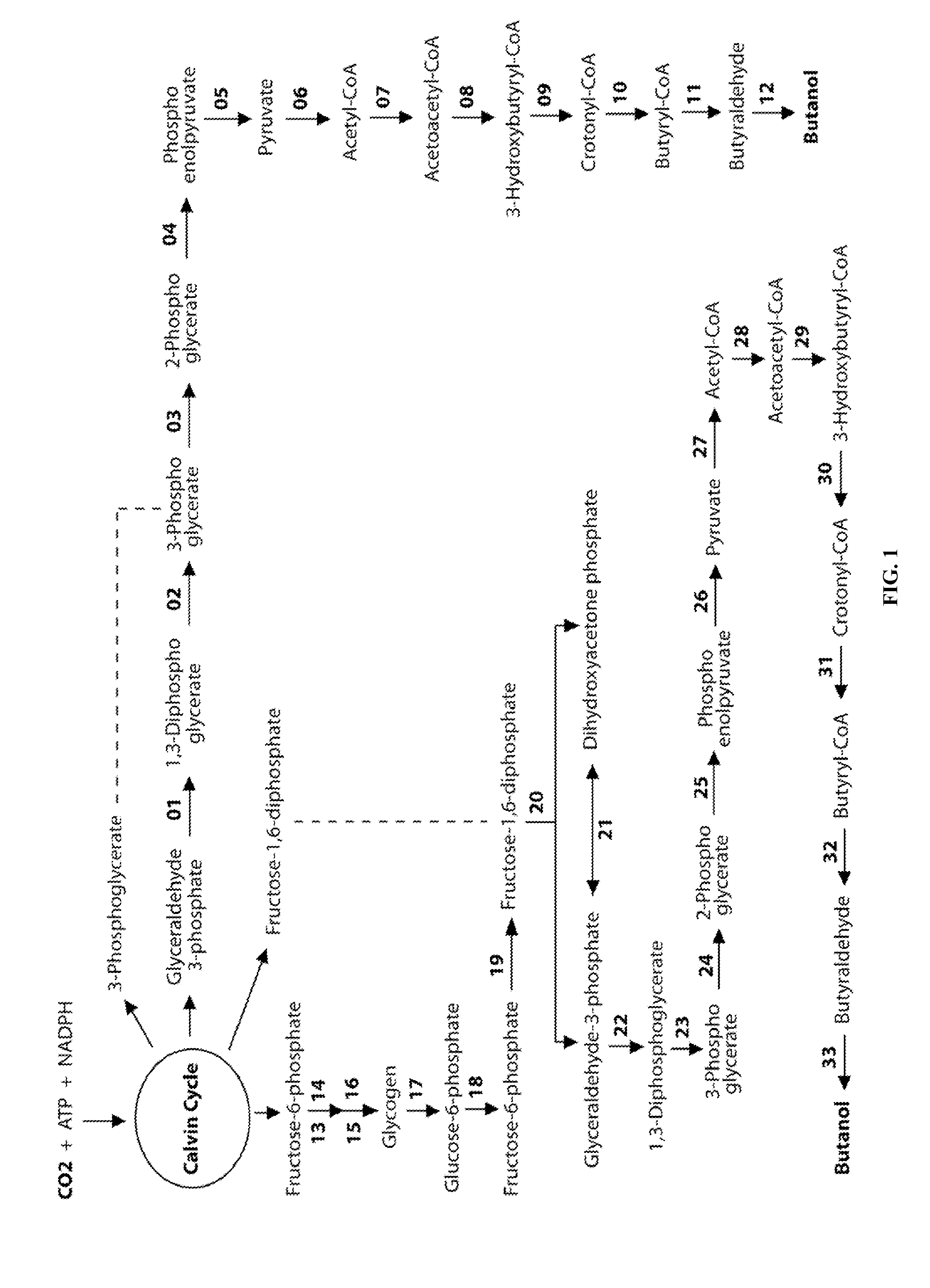
Designer Organisms for Photobiological Butanol Production from Carbon Dioxide and Water
ActiveUS20100330637A1Weaken energyReduce total powerBryophytesAlgae productsCellulosePhylum Cyanobacteria
The present invention provides a biosafety-guarded photobiological butanol production technology based on designer transgenic plants, designer algae, designer blue-green algae (cyanobacteria and oxychlorobacteria), or designer plant cells. The designer photosynthetic organisms are created such that the endogenous photobiological regulation mechanism is tamed, and the reducing power (NADPH) and energy (ATP) acquired from the photosynthetic process are used for synthesis of butanol (CH3CH2CH2CH2OH) directly from carbon dioxide (CO2) and water (H2O). The butanol production methods of the present invention completely eliminate the problem of recalcitrant lignocellulosics by bypassing the bottleneck problem of the biomass technology. The photobiological butanol-production technology of the present invention is expected to have a much higher solar-to-butanol energy-conversion efficiency than the current technology and could also help protect the Earth's environment from the dangerous accumulation of CO2 in the atmosphere.
Owner:LEE JAMES WEIFU
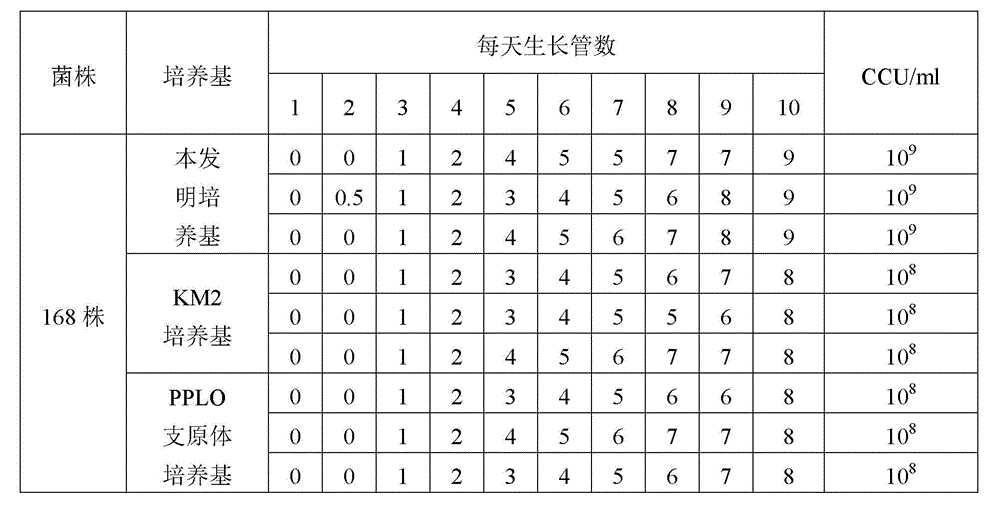
Low serum efficiency mycoplasma gallisepticam attenuated strain culture medium and preparation method thereof
ActiveCN103074246AIncrease the titer of live bacteriaReduce allergic reactionsBacteriaMicroorganism based processesAntigenCulture mediums
The present invention relates to a low serum efficiency mycoplasma gallisepticam attenuated strain culture medium and a preparation method thereof, and belongs to the technical field of veterinary biology. The culture medium comprises: (1) a base culture medium; and (2) an auxiliary culture medium, wherein the auxiliary culture medium mainly comprises MEM, yeast extract powder, tryptone, glucose, an inorganic salt, and the like, and growth, high titer and stability of the semi-finished product can be ensured with the auxiliary culture medium. According to the present invention, a titer of the semi-finished product bacterial liquid prepared by using the preparation method is up to 10<11> CCU / ml; and the culture medium adopts reduced pig serum to culture mycoplasma gallisepticam so as to reduce allergic stress reactions on chicken by heterologous pig serum, consider animal biosafety, improve antigen titer, and reduce production cost.
Owner:兆丰华生物科技(南京)有限公司
On-line biosafety pre-alarming method for water quality
InactiveCN101059493ARealize safety warningReal-time overall qualityMicrobiological testing/measurementTesting waterSystem qualityFiber
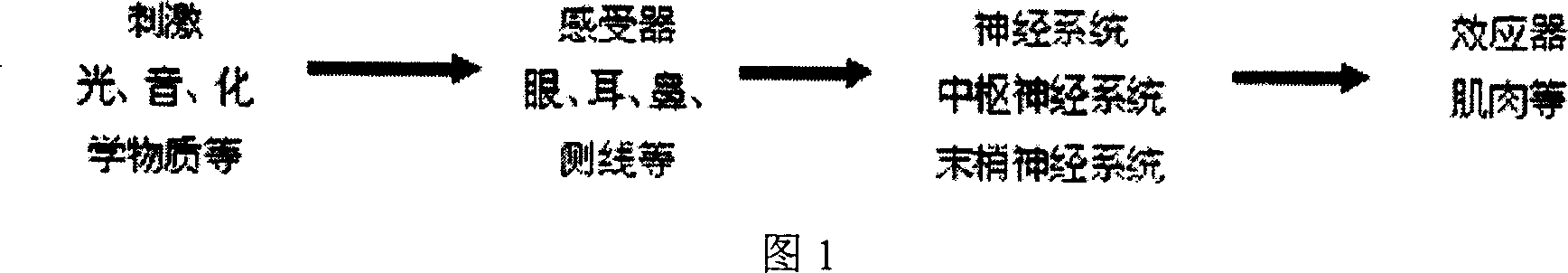
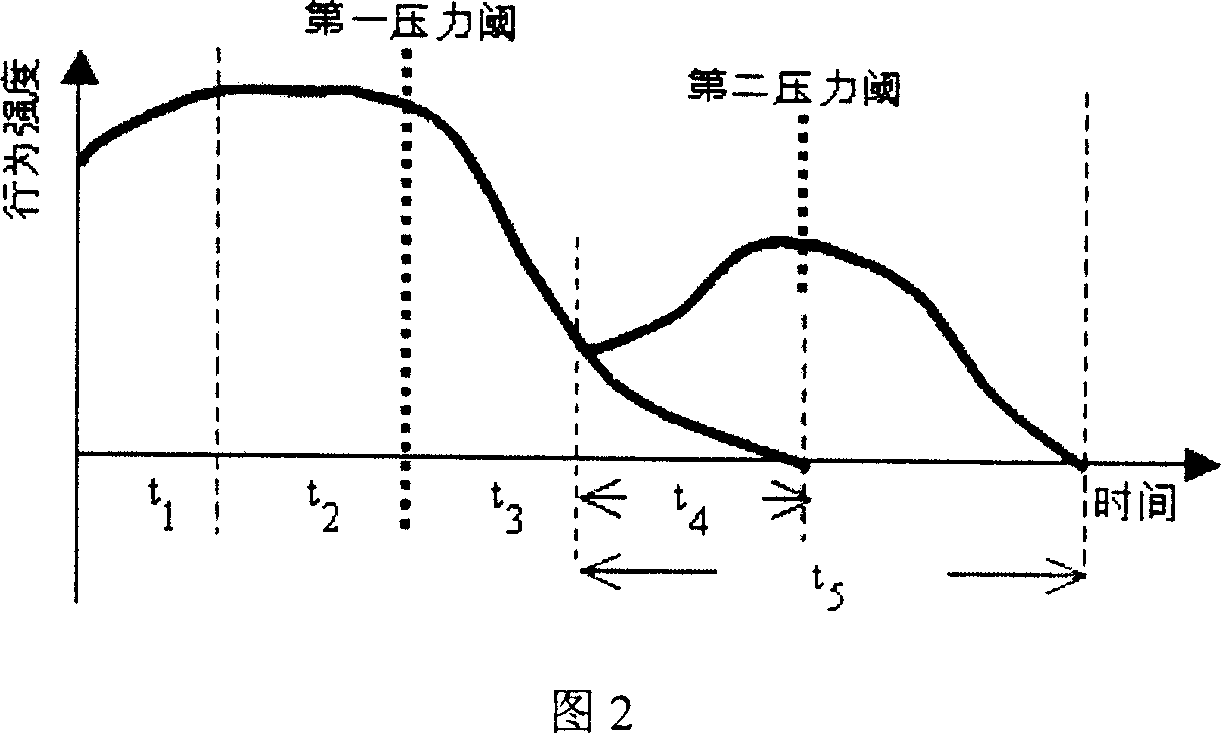
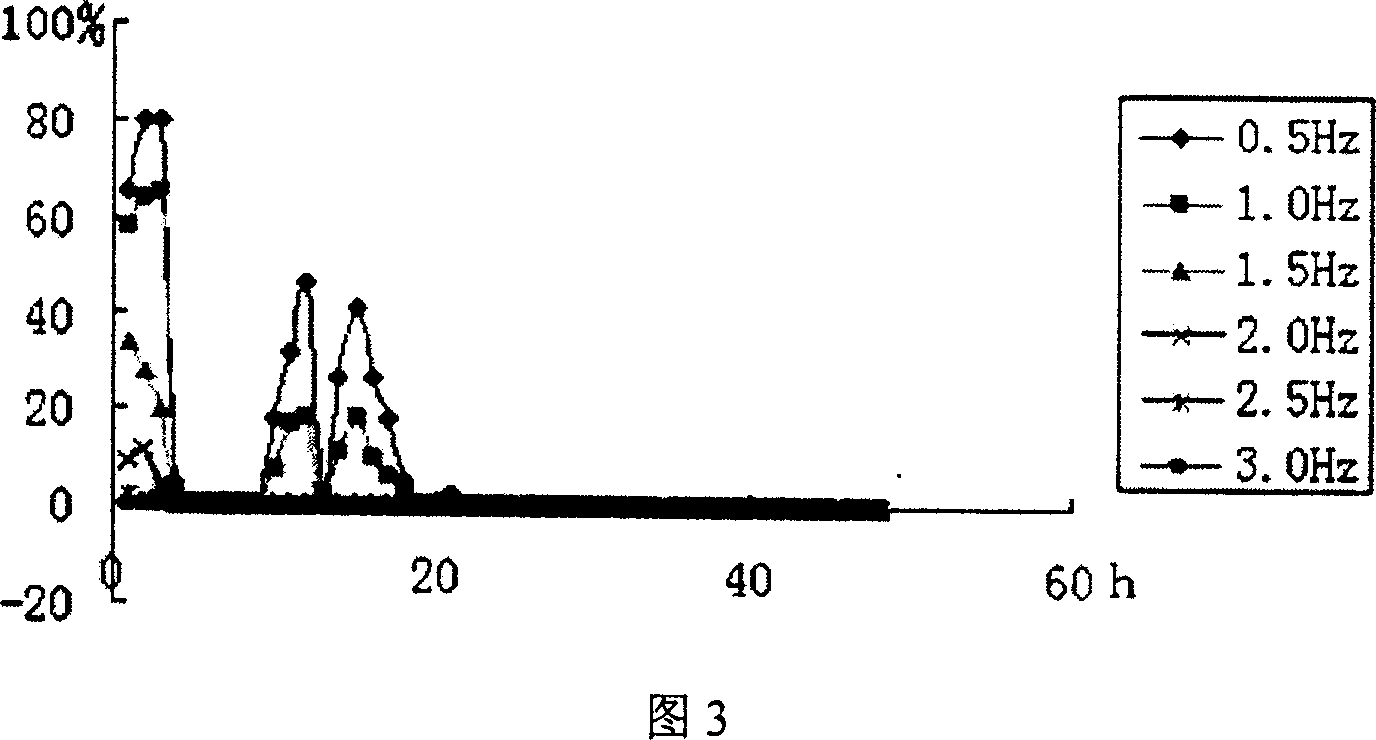
The invention relates to a water quality online biological safety alarm method, comprising that using the object aquatic life with multiply motions as instructor, using differential fiber and three-dimension spatial technique to online detect the motion change of aquatic life before reaching a first pressure threshold (t1+t2), to evaluate the water condition biological system quality, to realize water quality safety biological safety alarm, that using the motion change or physiological reaction of the aquatic life to represent water quality. The inventive method can overcome the defects of traditional water quality online detect method which can only periodically detect at key point and fail to real-time detect water, therefore, the invention is based on differential filter and three-dimension spatial technique to detect the motion change of aquatic life, to real-time, online and on-site detect quality.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
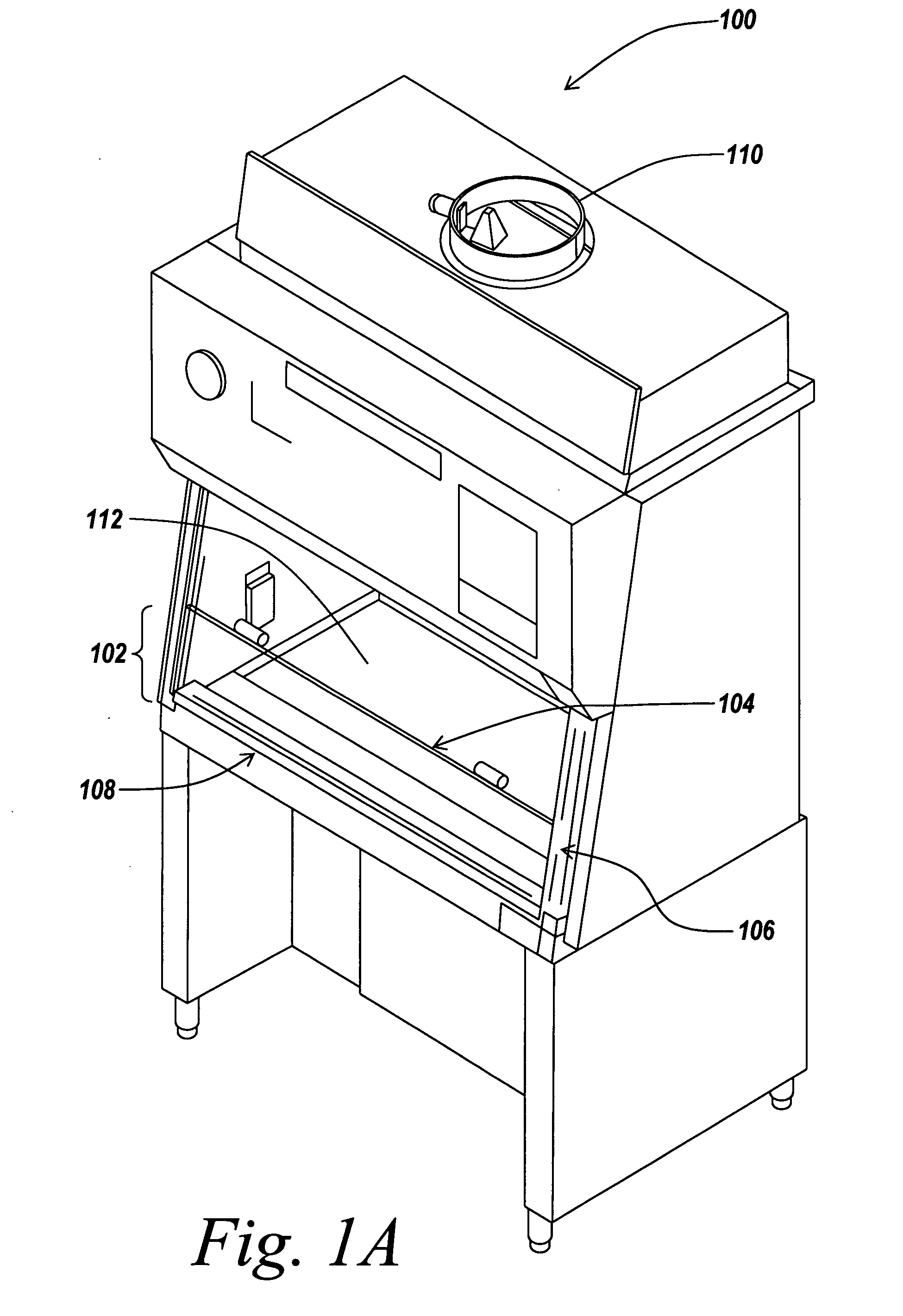
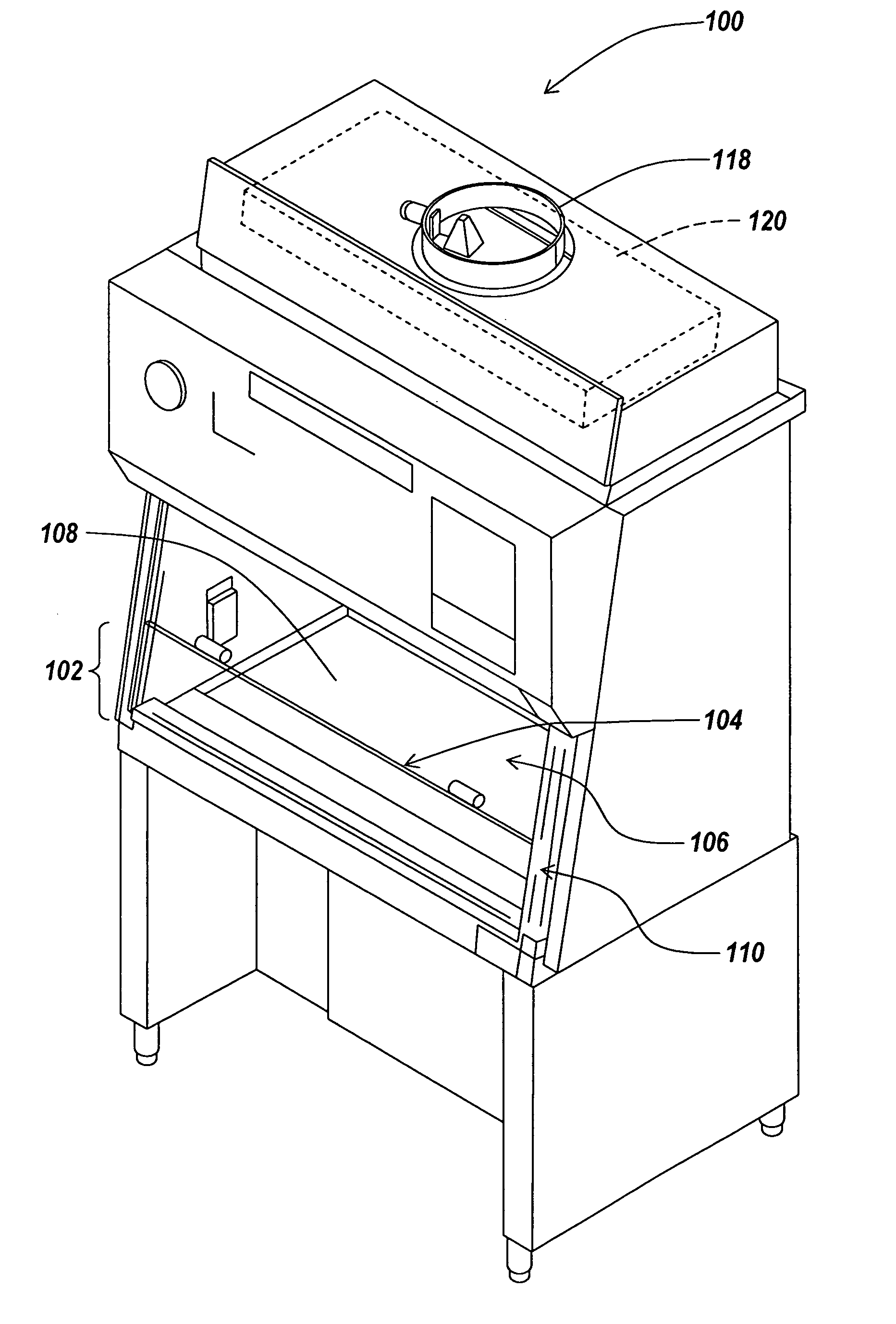
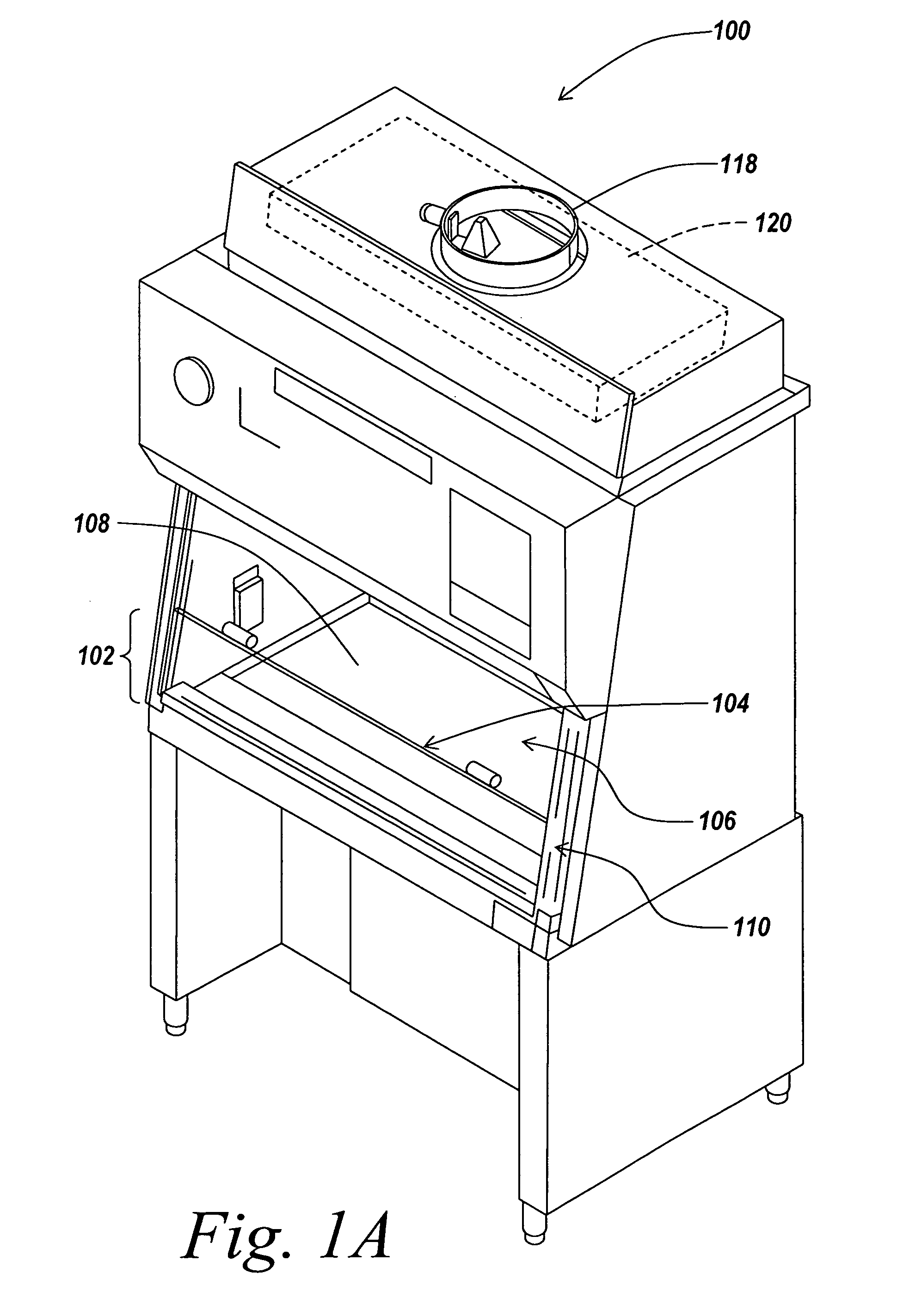
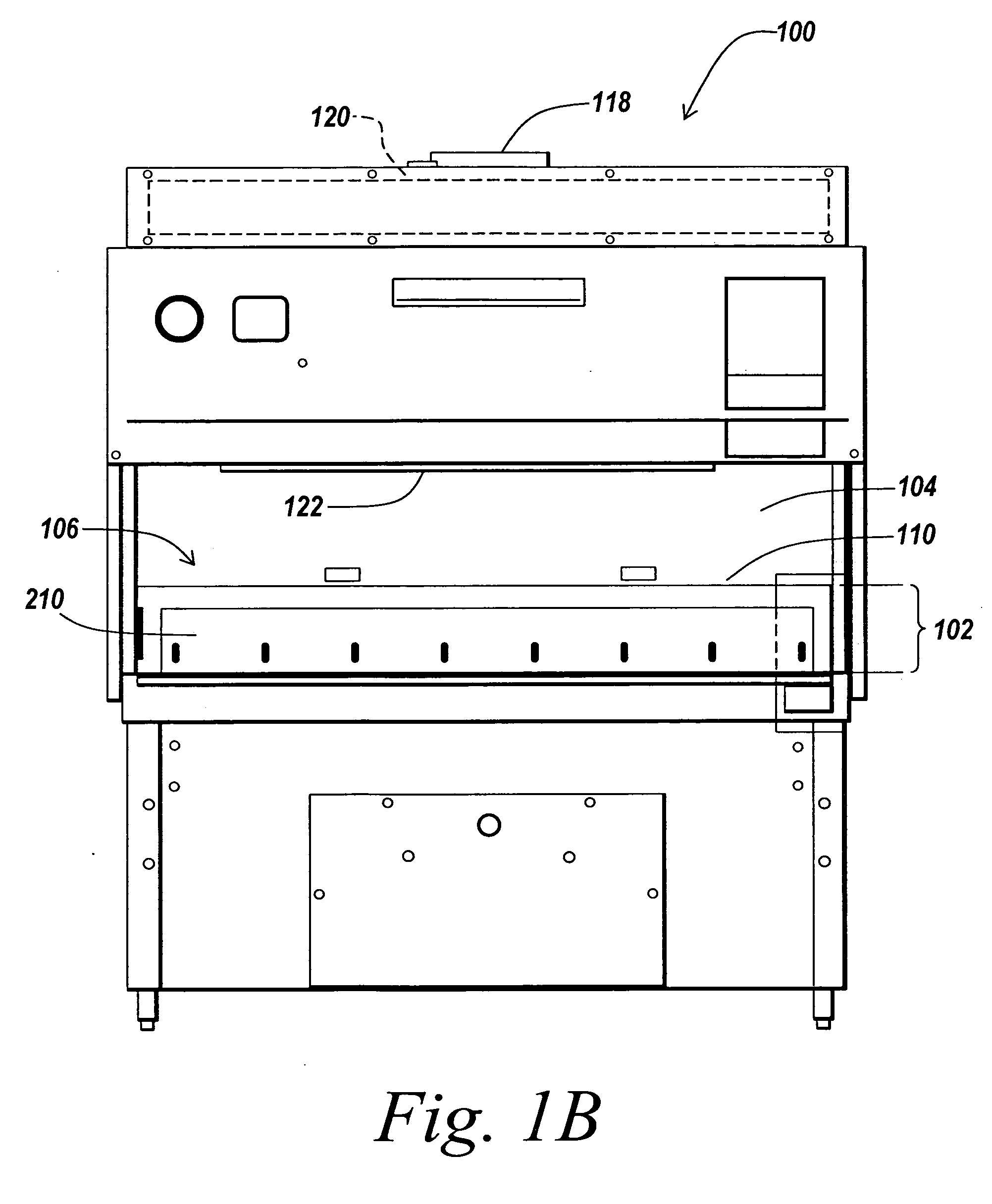
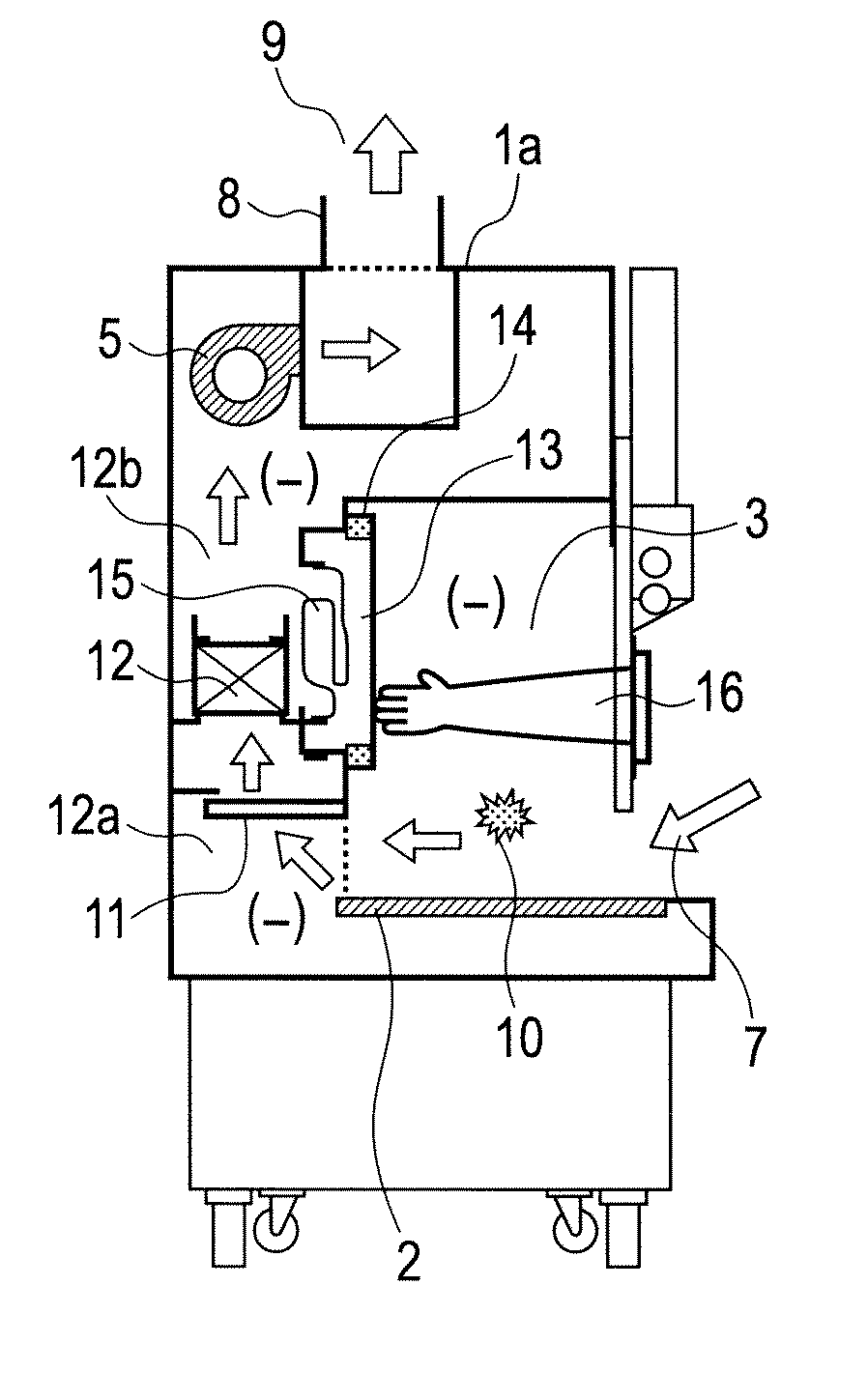
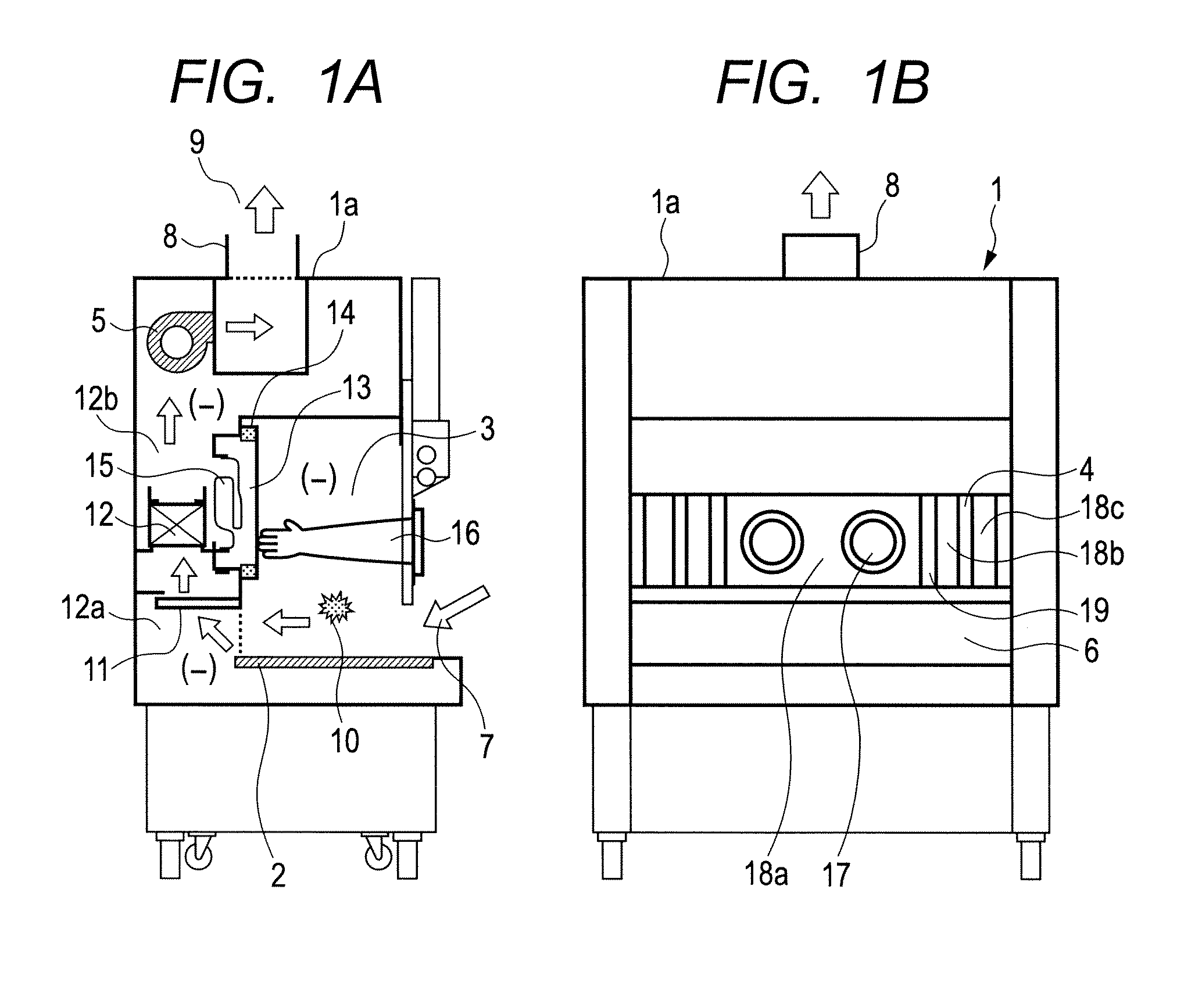
Air bypass system for biosafety cabinets
InactiveUS20080278040A1Reduce static pressureReduce air noiseMechanical apparatusDomestic stoves or rangesEngineeringBiosafety cabinet
A biosafety cabinet has an air bypass system. The air bypass system reduces air noise and static pressure in the biosafety cabinet, and continues a supply of air to the blower, when the view screen or door is fully closed by providing an alternate path for the air entering the cabinet. The air bypass system further includes an armrest provided on the door sill. The armrest may have perforations on the front and rear surfaces of the armrest to allow the air to travel under the armrest, through an air inlet. The air bypass system additionally blocks germicidal light generated inside the biosafety cabinet from escaping when the view screen or door is fully closed.
Owner:BAKER COMPANY THE
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562368AHigh sensitivityEasy to operateBiological material analysisImmunodiagnosticsNeutralising antibody
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, a first antigen and a second antigen ofS protein of SARS-CoV-2, and the second antigen is marked with a signal substance. Whether a tested person is infected by the new coronavirus or not and whether infection risks exist or not are judgedby detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
High-efficiency air filtering unit
ActiveCN101711935ARealize on-site scanning leak detectionRealize functionDispersed particle filtrationAir filtrationAir filter
The invention discloses a high-efficiency air filtering unit which can detect leakages on site and disinfect in situ. The high-efficiency air filtering unit comprises a detection gasoloid generating section, a mixing section, an upstream sampling section, a high-efficiency filter pressing device, a scanning and leakage detecting device, a disinfecting device, a filter resistance monitoring device, and the like, wherein the filter pressing device adopts an eccentric pressing mechanism, and a filter replacement mode is a bag-in and bag-out mode; the scanning and leakage detecting device is an automatic scanning and leakage detecting device; and a line scanning detecting method is used as a detecting technology. The high-efficiency air filtering unit has the characteristics of good box body tightness, disinfectant corrosion resistance and safe and convenient filter replacement. The high-efficiency air filtering unit meets the requirement for biosafety, and is suitable for blowing-in and exhaust handling systems of high-grade biosafety laboratories.
Owner:SANITARY EQUIP INST ACAD OF MILITARY MEDICAL SCI PLA +1
Low-serum culture medium for efficiently culturing mycoplasma hyopneumoniae and preparation method thereof
ActiveCN103060220AIncrease the titer of live bacteriaReduce allergic reactionsAntibacterial agentsBacterial antigen ingredientsMycoplasma cultureOrganism
The invention relates to an efficient mycoplasma hyopneumoniae culture medium and a preparation method thereof, and belongs to the technical field of veterinary biology. The efficient mycoplasma hyopneumoniae culture medium comprises an A liquid and a B liquid mainly consisting of MEM, yeast leaching powder, tryptone, glucose, inorganic salt and the like. The prepared culture medium of the invention has the main advantages of low serum content which is only 10%-15%, while the serum content in common culture medium is 20% even more. The culture medium prepared by the low serum relieves the pig allergic to the stress reaction, meanwhile gives consideration to the biosafety of animals. Besides the valence of the semi-finished bacterial solution prepared by the method is up to 109CCU / ml, which is much higher than the culture medium prepared by the common technology.
Owner:兆丰华生物科技(南京)有限公司 +1
Cellulose/black phosphorus nanosheet composite hydrogel and preparation method thereof
The invention provides a cellulose / black phosphorus nanosheet composite hydrogel. The composite hydrogel comprises a cellulose three-dimensional network structure and black phosphorus nanosheets loaded in the cellulose three-dimensional network structure. The black phosphorus nanosheets can be stably loaded in the composite hydrogel system, and are not prone to agglomerate, and the composite hydrogel has the advantages of uniform, stable and high photothermal conversion efficiency, good compatibility with biological fluids, complete biodegradability and high biosafety, and can be used in biomedical field. The invention also provides a preparation method of the composite hydrogel.
Owner:SHENZHEN UNIV
Natural glycan-based multifunctional microspheres, preparation method and application
The invention discloses natural glycan-based multifunctional microspheres, a preparation method and an application. The preparation method of the microspheres comprises steps as follows: a water dispersion liquid is prepared from natural glycan, carboxymethyl saccharides and a material with a wound healing promoting function are added, the mixture is stirred uniformly, and a water phase is obtained; an emulsifier is dissolved in a fat-soluble solvent, an oil phase is obtained, and the water phase is dropwise added to the oil phase and stirred; a crosslinking agent is dropwise added and stirred; the mixture is left to stand, a first polar solvent is used for cleaning, the oil phase is removed, a water layer is cleaned with a second polar solvent, microspheres are settled, the water layer isextracted, the microspheres are dried, and the natural glycan-based multifunctional microspheres are obtained. The microspheres can effectively block the bleeding area and promote hemostasis, do notcontain any crosslinking agent or other aids, are high in biosafety, has no hemolysis phenomenon and can be degraded and absorbed in short time. The microspheres have the efficacy of preventing breeding of bacteria and promoting rapid healing of wound while stopping bleeding of the wound.
Owner:安徽中科迈德医疗科技有限公司
Biosafety cabinets with air filters accessible through the work chamber
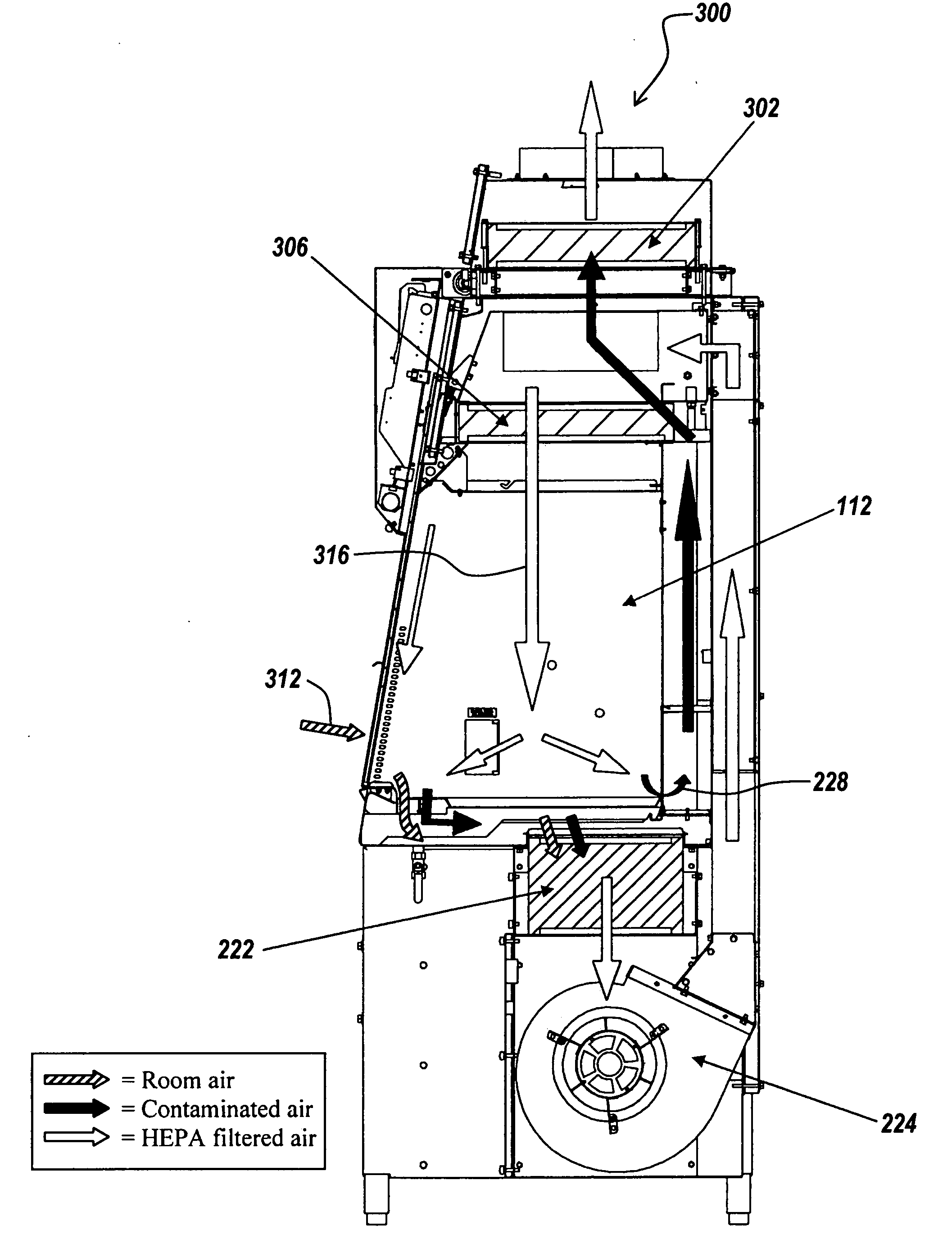
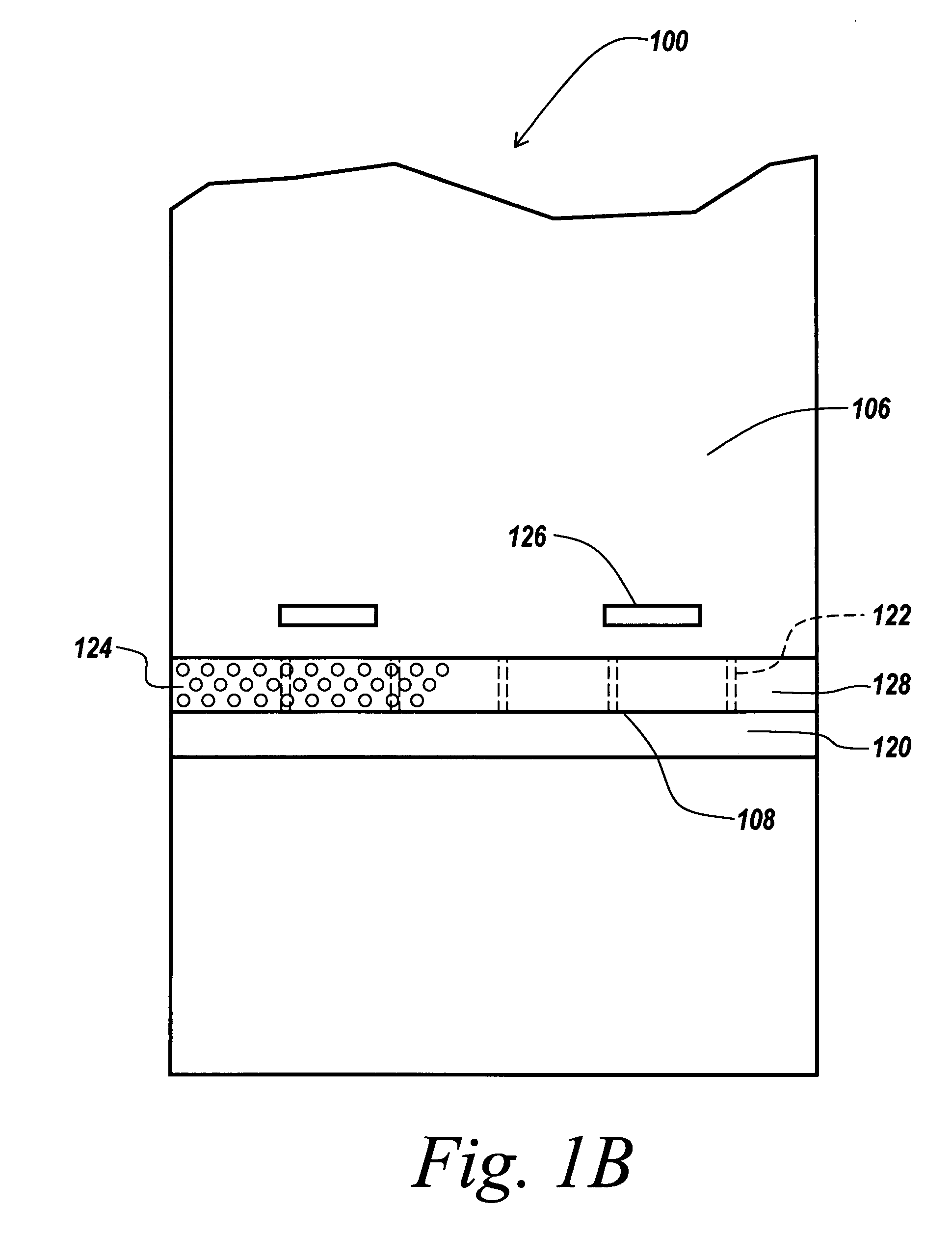
ActiveUS20080278042A1Prevent materialAvoid passingCombination devicesDomestic stoves or rangesParticulatesAir filter
A biosafety cabinet includes one or more air filters below the work surface and a system for holding, sealing, replacing and disposing the air filters through the work access opening of the biosafety cabinet. The air filters under the work surface of the biosafety cabinet capture particulates entering the cabinet from the exterior environment and the particulates within the cabinet's work chamber. Instead of a mechanical clamp, the perimeter of the air filters are sealed using a gasket and a tape, eliminating the accumulation of the contaminants around mechanical clamps. The air filters may be accessed through the work access opening of the biosafety cabinet, packaged for disposal and removed from the biosafety cabinet without being exposed to the exterior environment. A filter cover with an adhesive surface is used to cover and lift the used, contaminated air filter within the biosafety cabinet.
Owner:BAKER
Methods and kits for ascertaining biosafety of an agent
ActiveUS8945847B2Nucleotide librariesMicrobiological testing/measurementTissue specific geneExpression gene
A method of ascertaining the bio-safety of an agent is disclosed. The method comprises:(a) contacting the agent with differentiating human pluripotent stem cells;(b) analyzing a level of gene expression of a plurality of genes in the differentiating human pluripotent stem cells, wherein the agent is qualified as being safe if at least one of the following qualification parameters are fulfilled:(i) the agent causes a difference in the level of gene expression below a predetermined number of genes as compared to control differentiating human pluripotent stem cells that have not been contacted with the agent;(ii) the agent causes a difference in gene expression below a predetermined number of tissue-specific genes of a tissue as compared to control differentiating human pluripotent stem cells that have not been contacted with the agent; or(iii) the agent causes a difference in gene expression below a predetermined number of genes involved in fetal development as compared to control differentiating human pluripotent stem cells that have not been contacted with the agent.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
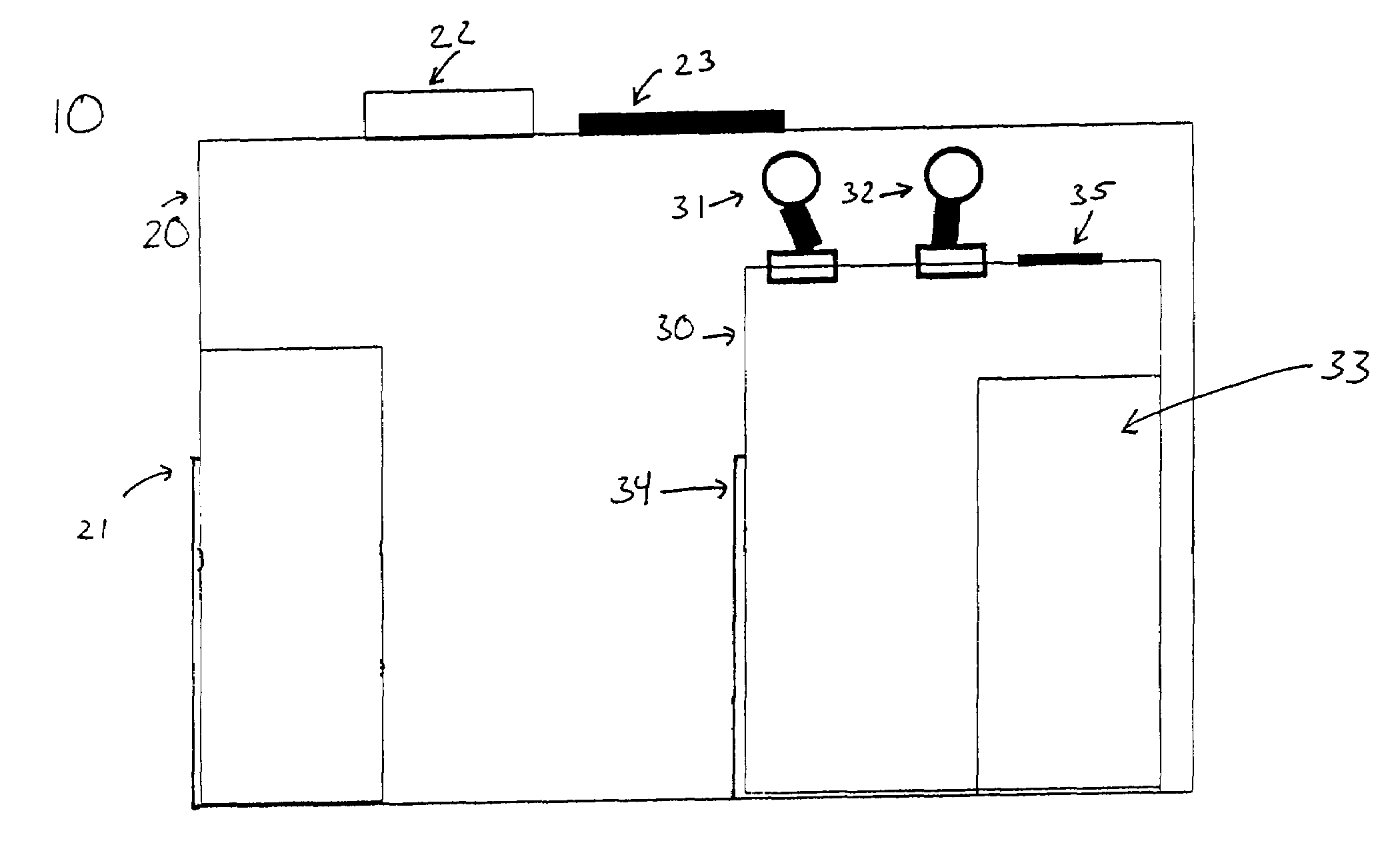
Biosafety cabinetry
ActiveUS20120019110A1Avoid biological contaminationImprove workabilityBaby-incubatorsManipulatorWorkspaceEngineering
The workability of operation to be performed, while preventing chemical or biological contamination, using a biosafety cabinet and gloves attached to the cabinet is improved. A biosafety cabinet includes a workspace formed in a main body cabinet thereof. Air in the workspace is discharged to outside via filters. A vertically movable front shutter is disposed on a front of the workspace. A front opening is formed below the front shutter to be at a front of the workspace. The front shutter includes plural laterally movable small windows which are overlapped like layered sliding doors. One of the plural small windows is provided, on the workspace side thereof, with a pair of glove ports to which a pair of gloves for use in operation in the workspace can be air-tightly attached.
Owner:HITACHI IND EQUIP SYST CO LTD
Avian influenza virus inactivated vaccine and preparation method thereof
InactiveCN102600464AAchieve mass productionIncrease volumeAnimal cellsAntiviralsAdjuvantVaccine Production
The invention discloses an avian influenza virus inactivated vaccine and a preparation method thereof. Adaptive avian influenza viruses are adaptive to screening and domestication of a continuous cell line, and after the viruses are adaptive to cells, the number of the viruses is continuously increased until the viruses are inoculated into a cell culture bottle or a bioreactor to culture. After the obtained culture is inactivated, the obtained culture and mineral oil adjuvant are mixed and emulsified so as to prepare the vaccine. A virus reproduction method for producing an avian vaccine by means of utilizing a large quantity of chicken embryo is omitted, resource consumption is reduced, environmental pollution is decreased, biosafety is guaranteed, cost is lowered, and production efficiency is improved. By the aid of the preparation method, high-potency viruses can be produced to prepare corresponding vaccines, and requirements of fast and high-efficient vaccine production are met.
Owner:SINOPHARM YANGZHOU VAC BIOLOGICAL ENG CO LTD +1
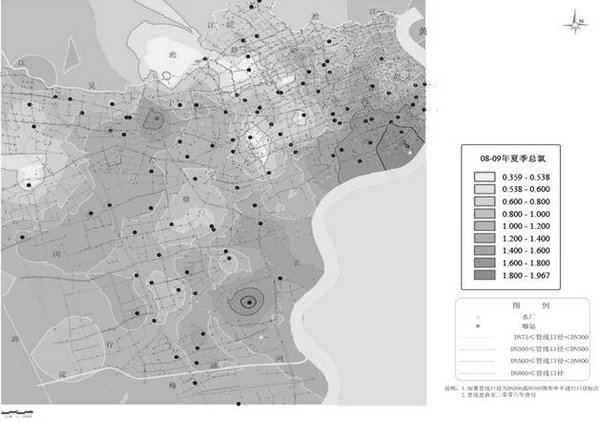
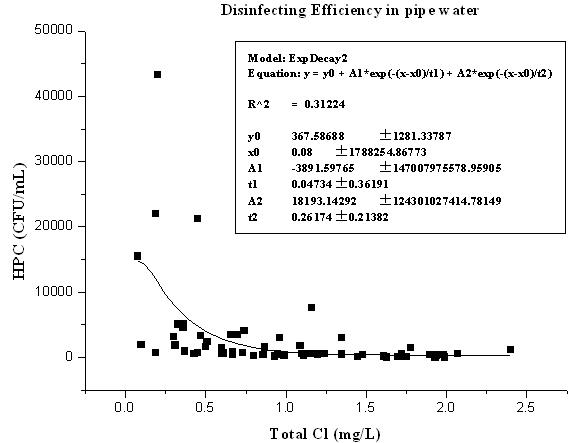
Joint debugging and joint control method for guaranteeing biosafety of pipe network water quality based on real-time ArcGis
ActiveCN102306021AAvoid the shortcomings of poor applicability and low accuracyTotal factory controlProgramme total factory controlUltrasound attenuationDisinfection by-product
The invention relates to a joint debugging and joint control method for guaranteeing biosafety of pipe network water quality based on real-time ArcGis. The method comprises the following steps: (1) importing the residual chlorine data of each water quality monitoring point of a water supply pipe network into a geographic information system; (2) according to the distribution of water works,, secondary pressurized pump stations and transmission and distribution pipe networks, analyzing the spatial variation rule of disinfectant attenuation in the water of current transmission and distribution pipe network to regulate and control residual chlorine of the pipe network; (3) performing secondary analysis on the distribution of residual chlorine of the pipe network, observing whether the most disadvantageous point of the pipe network transmission and distribution satisfy the minimum value of the residual chlorine required for model calculation to control a microbial indicator to be qualified; and (4) if the most disadvantageous point of the pipe network transmission and distribution cannot satisfy the minimum value required for the model, repeating the step (2) until the residual chlorine distribution of the pipe network basically satisfies the microbial indicator control condition at the most disadvantageous point in the pipe network transmission and distribution. Through the invention, the microbial indicator of the pipe network reaches the standard, the spatial distribution of the residual chlorine of the pipe network is more uniform, and the addition of disinfectant and the amount of disinfection byproduct are reduced.
Owner:SHANGHAI JIAO TONG UNIV
Protein self-assembled novel nanovaccine and preparation method thereof
ActiveCN107157933ASimple componentsQuality improvementSsRNA viruses negative-senseAntibacterial agentsCross-linkImmune effects
The invention relates to a protein self-assembled novel nanovaccine and a preparation method thereof. The protein self-assembled novel nanovaccine is prepared on the basis of antigen protein self-assembly; in the process of vaccine preparation, a molecular adjuvant is selectively introduced, the antigen content is higher than or equal to 85%, a high-efficiency immune effect can be triggered without needing assistance of an aluminum adjuvant, a Freund's adjuvant and the like, mercapto groups between protein molecules are exposed by virtue of physical regulation and control, and stable protein nanoparticles mainly based on disulfide bond crosslinking are formed through a mercapto / disulfide bond exchange reaction. The defects that the conventional nanovaccine needs to be introduced with an exogenous carrier or a cross-linking agent and the like are overcome, and the immune effect and the biosafety of the vaccine can be improved at the same time; the obtained vaccine granules are tidy in morphology, high in stability, flexible in regulation and control mode and good in repeatability, and can effectively stimulate dendritic cell maturation; the protein self-assembled novel nanovaccine has relatively strong generality and universality, is verified in a series of antigen proteins, and has a potential significant application value in the fields of novel vaccinating methods and biological pharmacy.
Owner:TONGJI UNIV
Artificially synthesized Bt insecticidal gene FLIa as well as preparation method and application thereof
The invention provides an artificially synthesized Bt insecticidal gene FLIa as well as a preparation method and application thereof, belonging to the technical field of insecticidal protein and biosafety. The nucleotide sequence of the Bt insecticidal gene FLIa is shown as SEQ ID NO: 1, and the nucleotide sequence of encoded protein of the Bt insecticidal gene FLIa is shown as SEQ ID NO: 2 in a sequence table. The preparation method of the Bt insecticidal gene FLIa comprises the steps of exchanging and fusing Domain I and Domain II of a insecticidal gene CrylAb and Domian III of a insecticidal gene CrylIa to obtain a recombinant insecticidal gene MCrylI; on the basis of the recombinant insecticidal gene MCrylI, connecting the carbon terminal of a insecticidal gene CrylJal at a 3minute terminal to obtain a recombinant insecticidal gene FLMCrylIa; performing codon reconstruction on the recombinant insecticidal gene FLMCrylIa to obtain the Bt insecticidal gene FLIa. The Bt insecticidal gene FLIa can be stably inherited and expressed in transformation plants, and is high in expression quantity, and obtained transgenic plants are good in insect resistance. The gene is used for transforming crops of corn, cotton, rice, vegetables and the like, and enabling the same to have corresponding insect-resistant activity, so that the using amount of pesticides is reduced and environment pollution and production cost are reduced.
Owner:JILIN ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com