Protein self-assembled novel nanovaccine and preparation method thereof
A nano-vaccine and self-assembly technology, applied in biochemical equipment and methods, drug combinations, pharmaceutical formulations, etc., can solve problems such as high synthesis cost, low antigen density, and complex synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: Nano-vaccine preparation of tumor model antigen
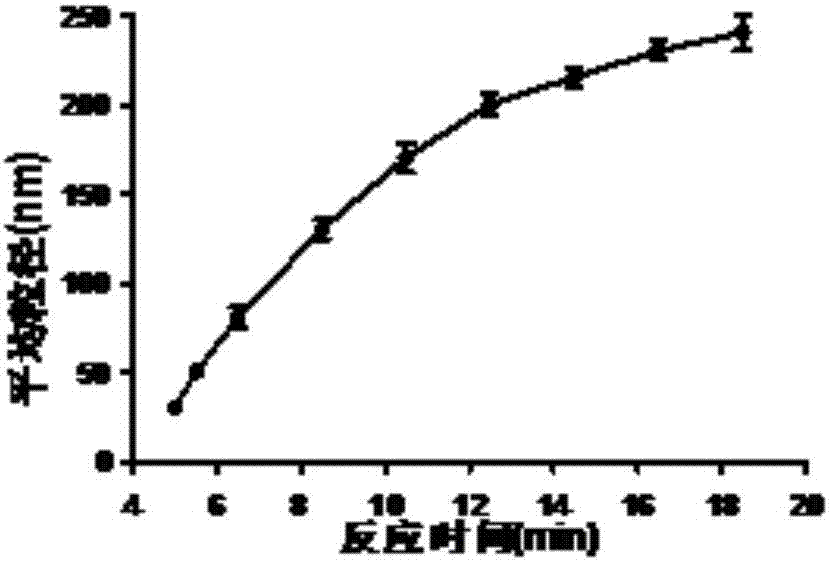
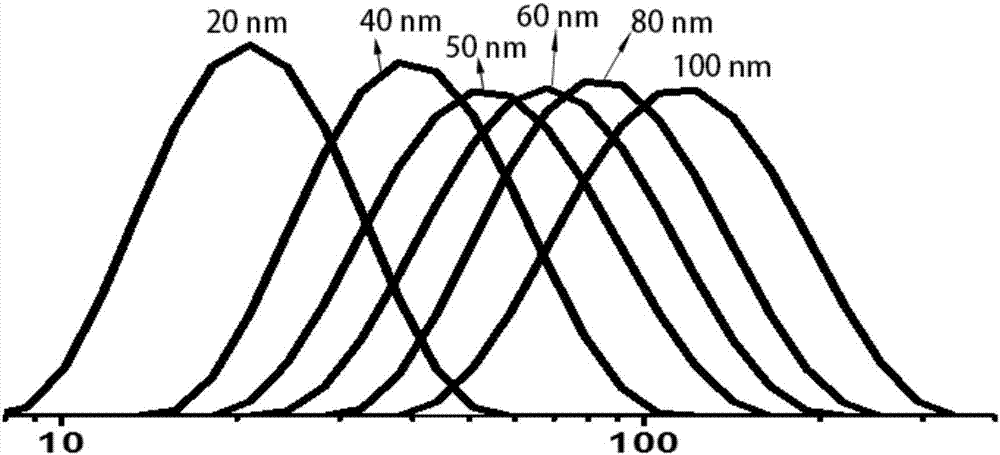
[0075] Dissolve 6mg of OVA powder in 2mL of 0.05M MES buffer at pH 3.5, filter or centrifuge to remove flocculent insoluble matter. Add 50 microliters of 6% sodium dodecyl sulfate aqueous solution to the antigenic protein system, stir for 2 minutes, and mix well. Pour the reaction solution into a screw-top glass bottle and tighten it, place it in an oil bath at 90°C for heating, and stir it on a magnetic stirrer at a speed of 750 rpm, and the reaction time is 4-30 minutes. With different reaction times, nanoparticles with different degrees of opalescence and uniform particle size can be obtained (such as figure 1 As shown, with the difference of the reaction time, the nano-vaccine with good quality, strong controllability and different particle sizes was obtained). After the reaction was completed, the reaction solution was placed in an ice-water bath for 1-2 min to terminate the reaction quickly. Finally, ...
Embodiment 2
[0077] Embodiment 2: Nano vaccine preparation of tumor model antigen
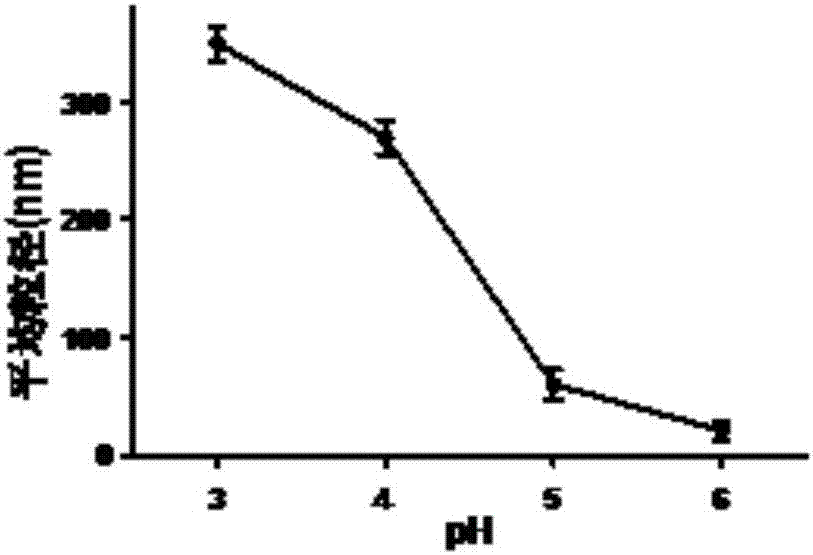
[0078] Dissolve 6mg of OVA powder in 2mL buffer solution with pH of 3.0, 4.0, 5.0, 6.0, filter or centrifuge to remove flocculent insoluble matter. Add 50 microliters of 6% sodium dodecyl sulfate aqueous solution to the antigenic protein system, stir for 2 minutes, and mix well. The reaction solution was poured into a screw-top glass bottle and screwed tightly, heated in an oil bath at 90°C, stirred on a magnetic stirrer at a speed of 750 rpm, and the reaction time was 25 minutes. With different reaction times, nanoparticles with different degrees of opalescence and uniform particle size can be obtained (such as figure 1 shown). After the reaction was completed, the reaction solution was placed in an ice-water bath for 1-2 min to terminate the reaction quickly. Finally, use a 3000 molecular weight ultrafiltration tube to centrifuge, or use a 3000 molecular weight dialysis bag to dialyze for 48 hours to r...
Embodiment 3
[0082] Nano vaccine preparation of embodiment 3 tumor model antigen
[0083] Dissolve 6mg of OVA powder in 2mL of 0.05M MES buffer at pH 3.5, filter or centrifuge to remove flocculent insoluble matter. Add the denaturant to the antigenic protein system, stir for 2 minutes, and mix well. The reaction solution was poured into a screw-top glass bottle and tightened, heated in an oil bath under a temperature gradient of 50°C-120°C, stirred at a speed of 750 rpm on a magnetic stirrer, and the reaction time was 10 minutes. With different reaction times, nanoparticles with different degrees of opalescence and uniform particle size can be obtained (such as figure 1 shown). After the reaction was completed, the reaction solution was placed in an ice-water bath for 1-2 min to terminate the reaction quickly. Finally, use a 3000 molecular weight ultrafiltration tube to centrifuge, or use a 3000 molecular weight dialysis bag to dialyze for 48 hours to remove impurities and unreacted com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com