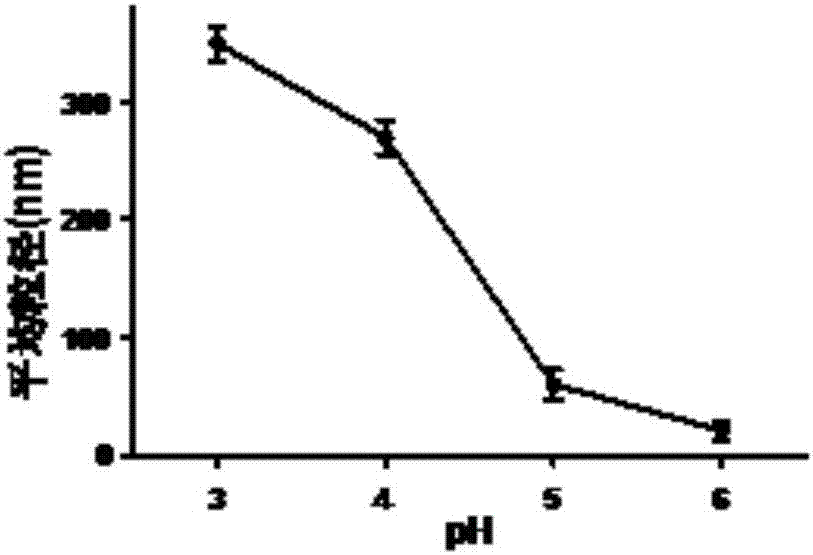
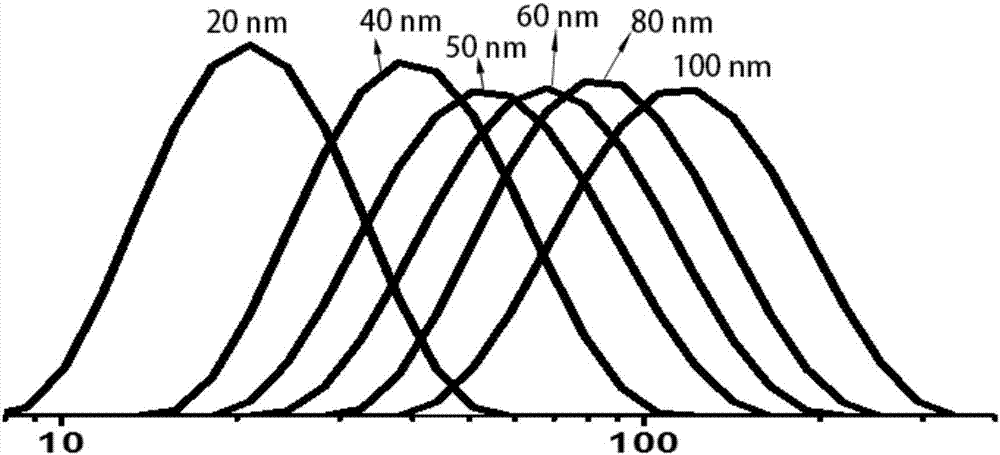
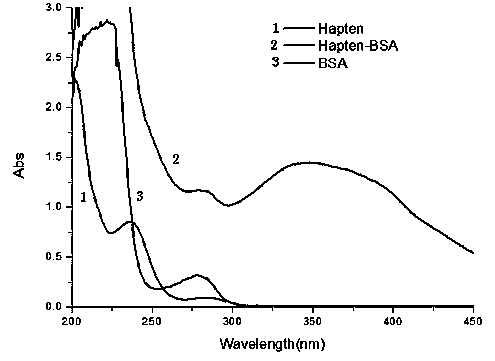
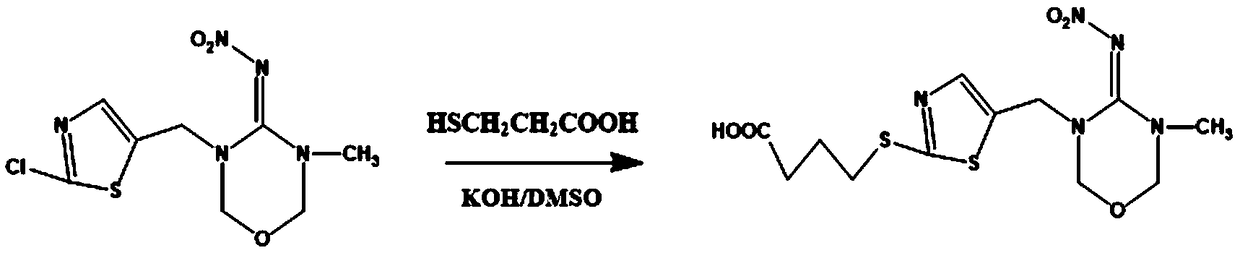
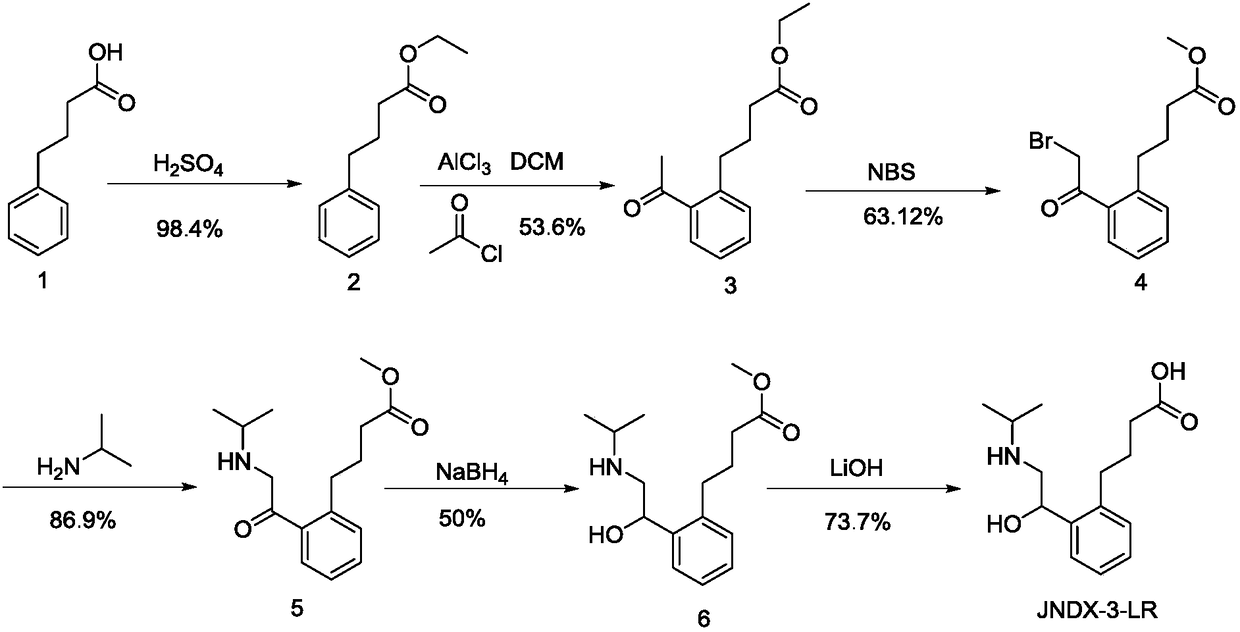
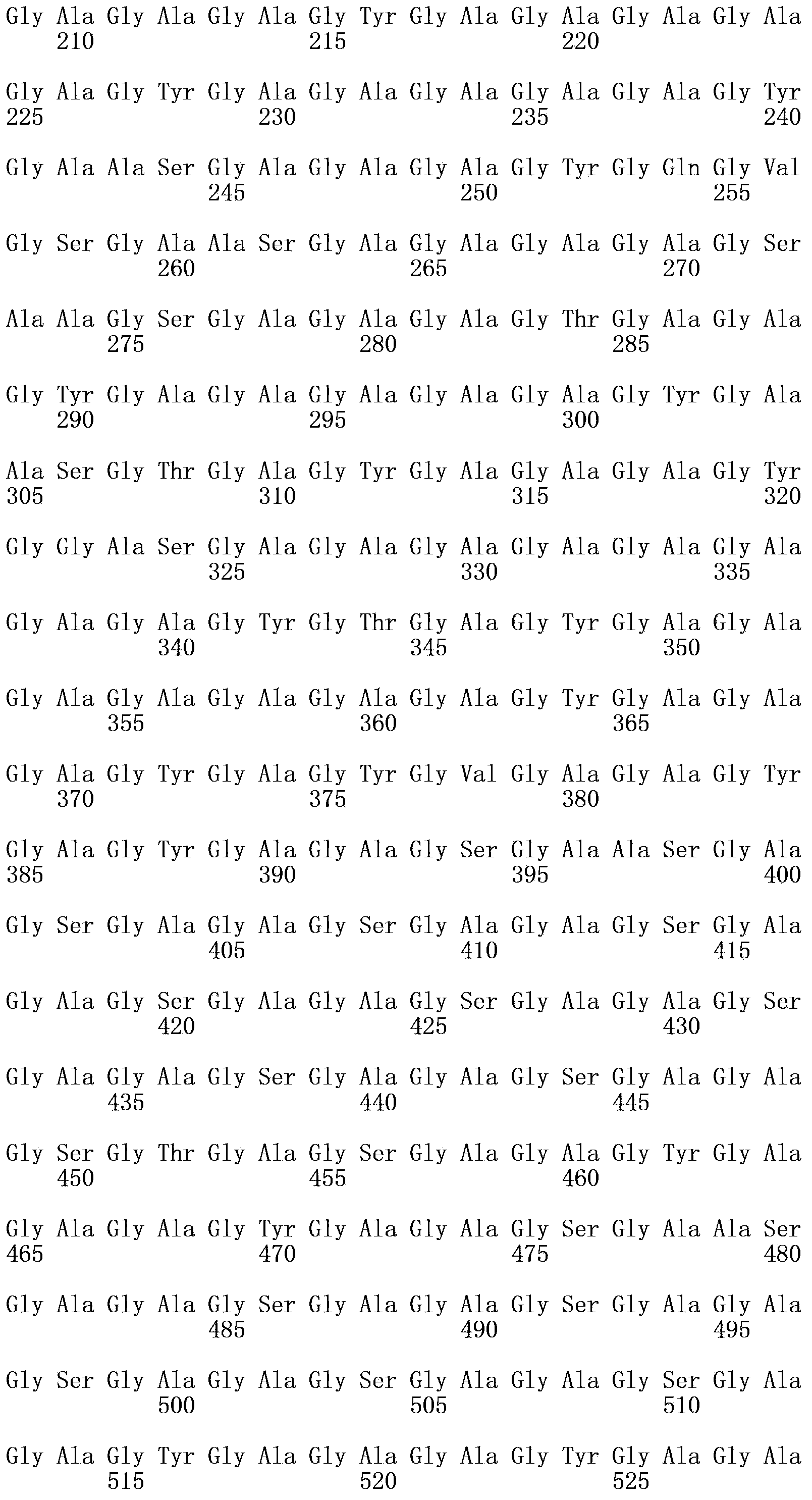Patents
Literature
137 results about "Freund's adjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
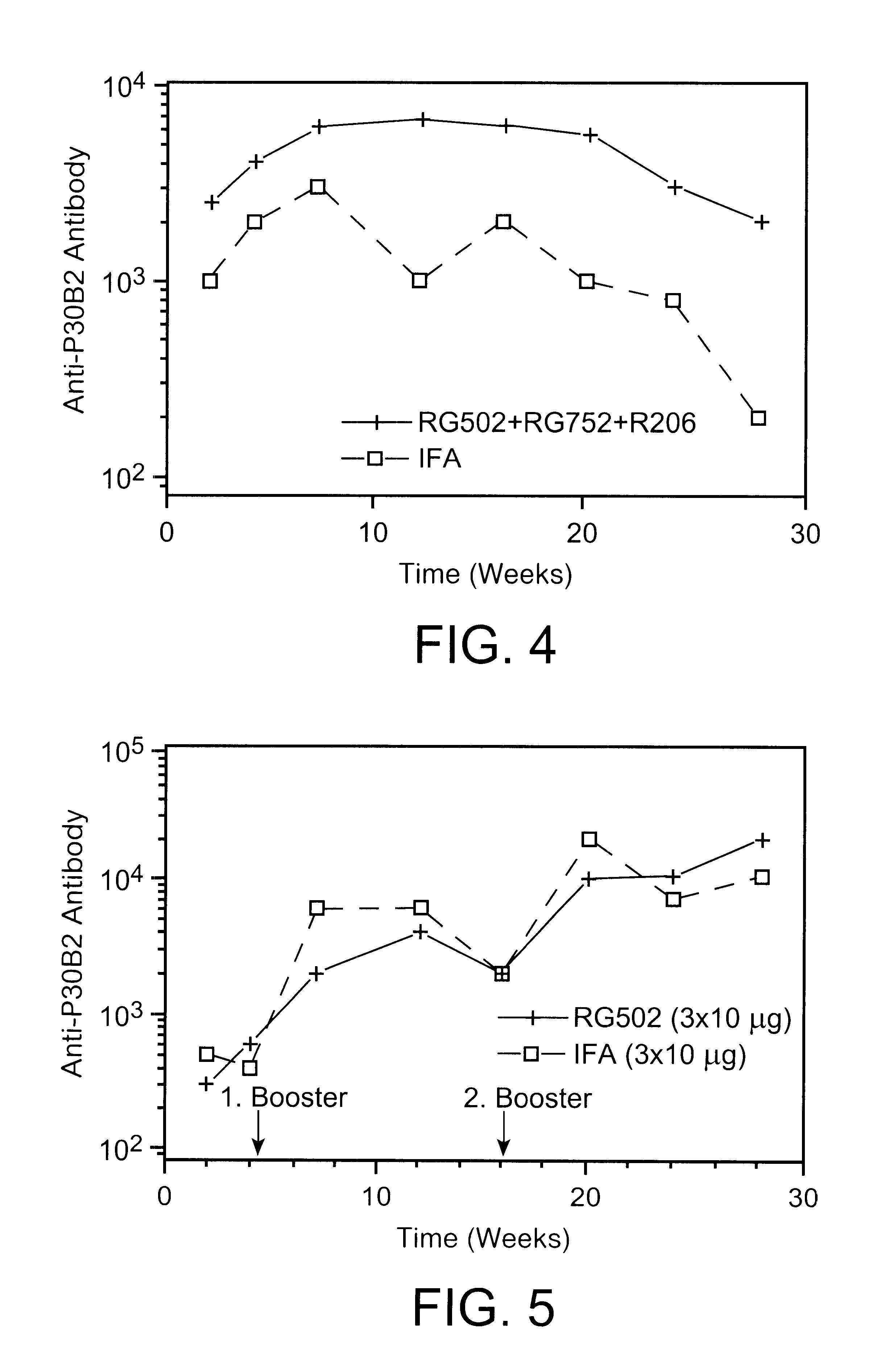
Freund's adjuvant is a solution of antigen emulsified in mineral oil and used as an immunopotentiator (booster). The complete form, Freund's Complete Adjuvant (FCA or CFA) is composed of inactivated and dried mycobacteria (usually M. tuberculosis), whereas the incomplete form (FIA or IFA) lacks the mycobacterial components (hence just the water in oil emulsion). It is named after Jules T. Freund.
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic polypeptide
InactiveCN103509107AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansPenicillinKeyhole-limpet haemocyanin
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic polypeptide. The method comprises the following steps: synthesizing a "CGAGAGSGAGAGS" polypeptide sequence by utilizing an Fmoc method, coupling the polypeptide with keyhole limpet hemocyanin (KLH) through the cysteine on the N terminus of the polypeptide so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain a primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antibody titer in the rabbit blood sample reaches 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate.
Owner:ZHEJIANG UNIV +1
Nonhuman model animal suffering from Guillain-Barré syndrome and/or fisher syndrome
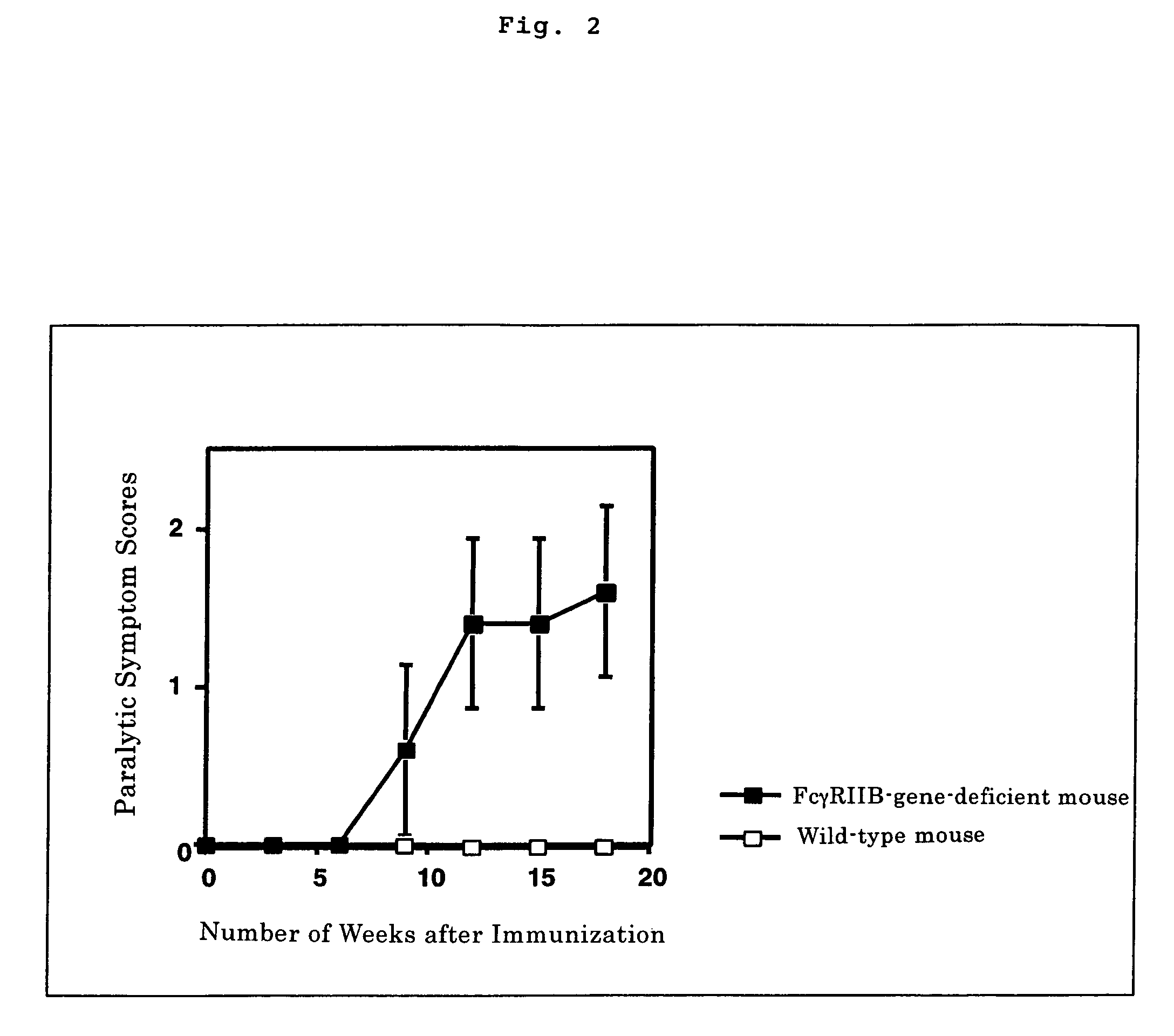
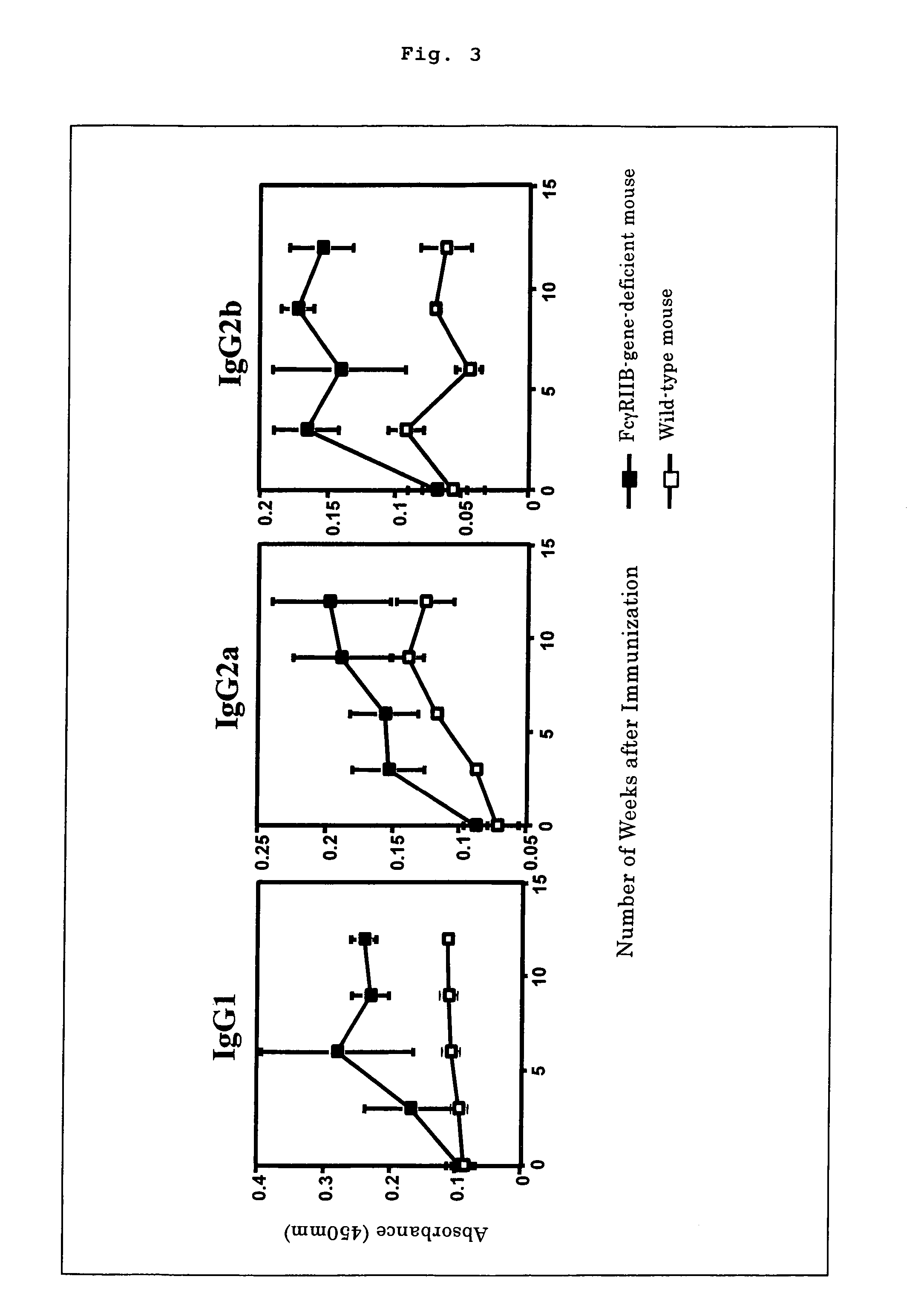
The present invention intends to provide a non-human animal model of Guillain-Barré syndrome, which can be obtained by immunizing FcγRIIB-gene-deficient non-human animal with ganglioside GQ1b, and a screening method of a therapeutic agent for Guillain-Barré syndrome using the non-human animal model. A mouse model of Guillain-Barré syndrome is generated by immunizing FcγRIIB-gene-deficient mice with gangliosides GM1, GM2, GD1a, and GQ1b together with Freund's adjuvant every three weeks four times in total.
Owner:JAPAN SCI & TECH CORP
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic dodecapeptide
InactiveCN103509108AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansKeyhole-limpet haemocyaninPrimary immunization
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic dodecapeptide. The method comprises the following steps: synthesizing a polypeptide with a "CGYGAGAGAGYGA" sequence, coupling the polypeptide with keyhole limpet hemocyanin (KLH) so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, carrying out an emulsion treatment so as to obtain primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then carrying out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antiserum titer of rabbit arrives at 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate. The antibody prepared by the invention has a strong specificity, and can be used for detection and analysis of silk fibroin in textile, and the like.
Owner:ZHEJIANG UNIV +1
Protein self-assembled novel nanovaccine and preparation method thereof
ActiveCN107157933ASimple componentsQuality improvementSsRNA viruses negative-senseAntibacterial agentsCross-linkImmune effects
The invention relates to a protein self-assembled novel nanovaccine and a preparation method thereof. The protein self-assembled novel nanovaccine is prepared on the basis of antigen protein self-assembly; in the process of vaccine preparation, a molecular adjuvant is selectively introduced, the antigen content is higher than or equal to 85%, a high-efficiency immune effect can be triggered without needing assistance of an aluminum adjuvant, a Freund's adjuvant and the like, mercapto groups between protein molecules are exposed by virtue of physical regulation and control, and stable protein nanoparticles mainly based on disulfide bond crosslinking are formed through a mercapto / disulfide bond exchange reaction. The defects that the conventional nanovaccine needs to be introduced with an exogenous carrier or a cross-linking agent and the like are overcome, and the immune effect and the biosafety of the vaccine can be improved at the same time; the obtained vaccine granules are tidy in morphology, high in stability, flexible in regulation and control mode and good in repeatability, and can effectively stimulate dendritic cell maturation; the protein self-assembled novel nanovaccine has relatively strong generality and universality, is verified in a series of antigen proteins, and has a potential significant application value in the fields of novel vaccinating methods and biological pharmacy.
Owner:TONGJI UNIV
Chloramphenicol universal monoclonal antibody hybridoma cell strain and application thereof
ActiveCN104263701AHigh affinityHigh detection sensitivityMicroorganism based processesTissue cultureBALB/c1,3-Propanediol
Owner:JIANGNAN UNIV
Hybridoma cell strain secreting thiamethoxam monoclonal antibody and application thereof
InactiveCN108998422AHigh detection sensitivityImprove featuresMicroorganism based processesDepsipeptidesBALB/cIc50 values
The invention relates to a hybridoma cell strain secreting thiamethoxacin monoclonal antibody and application thereof, belonging to the field of food safety immunodetection. The accession number of the hybridoma cell strain is CGMCC No. 14699. According to the invention, a complete Freund's adjuvant is used for primary immunization of a BALB / c mouse, then an incomplete Freund's adjuvant is used for booster immunization three times, and a thiamethoxam complete antigen containing no adjuvant is used for impact immunization once, so the BALB / c mouse is immunized; and then the high-titer low-IC50spleen cells of the immunized mouse are fused with mouse myeloma cells by using a PEG method, and then the cell strain is obtained through indirect competitive ELISA screening and subcloning three times. The monoclonal antibody secreted by the cell strain has good specificity and detection sensitivity (with an IC50 value of 0.81 ng / mL) to thiamethoxam and can be used for detection of thiamethoxamresidues in food.
Owner:JIANGNAN UNIV +1
Immunological response potentiation process
Owner:RMF DICTAGENE
Preparation method of porcine deltacoronavirus recombinant N protein and preparation method of polyclonal antibody of porcine deltacoronavirus recombinant N protein
The invention discloses a preparation method of a porcine deltacoronavirus (PDCoV) recombinant N protein. The method comprises the steps that the N gene segment of the PDoV is amplified, subjected toprokaryotic expression and purified in sequence, and the recombinant N protein is obtained. On this basis, the invention further provides a preparation method of a polyclonal antibody of the recombinant N protein. The method comprises the steps that the PDoV recombinant N protein and the Freund's adjuvant are emulsified to immunize animals to obtain polyclonal antibody serum. Through experiments,it is shown that the N gene of the PDCoV is successfully amplified, and the amplified N gene is subjected to prokaryotic expression and purified, the immunogenicity of the recombinant N protein is primarily studied, and a basis is laid for developing a diagnostic kit or preparing the polyclonal antibody.
Owner:GUANGXI VETERINARY RES INST
Universal phthalic acid esters monoclonal antibody hybridoma cell strain and application thereof
ActiveCN104004719AHigh affinityHigh detection sensitivityImmunoglobulins against animals/humansMicroorganism based processesBALB/cIndirect elisa
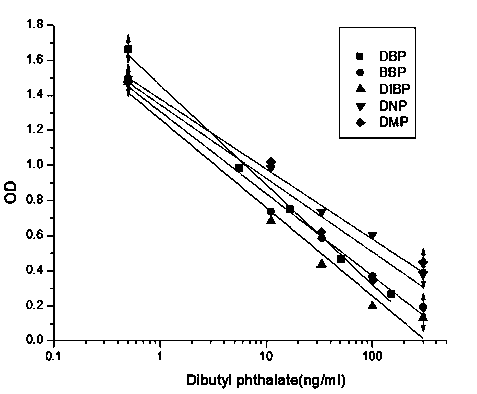
The invention discloses a universal phthalic acid esters monoclonal antibody hybridoma cell strain and application thereof, and belongs to the technical field of food safety immunological detection. Hapten is prepared and is coupled with albumen based on the glutaraldehyde method to obtain phthalic acid dibutyl ester complete antigen, and the phthalic acid dibutyl ester complete antigen and freund's adjuvant are evenly mixed to be injected to immune BALB / c mice in a subcutaneous injection mode; envelope antigen is formed in a synthesis mode based on the diazotization method and is used for screening mouse serum and cell supernatant. The splenocyte of the immune mice is fused with myeloma cells of the mice based on a PEG method, and indirect ELISA, indirect competition ELISA screening and three times of subcloning are carried out to obtain selective group hybridoma cell strain monoclonal cell strain C. The monoclonal cell strain C has certain recognition capability on DEHP and DINP, and the requirement for phthalic acid ester plasticizer immunodetection products in current market can be met.
Owner:无锡迪腾敏生物科技有限公司
Preparations that potentiate immunogenicity in low immunogenic antigens
ActiveUS20020136735A1Antibacterial agentsBacterial antigen ingredientsImmunogenic peptideVaccine Immunogenicity
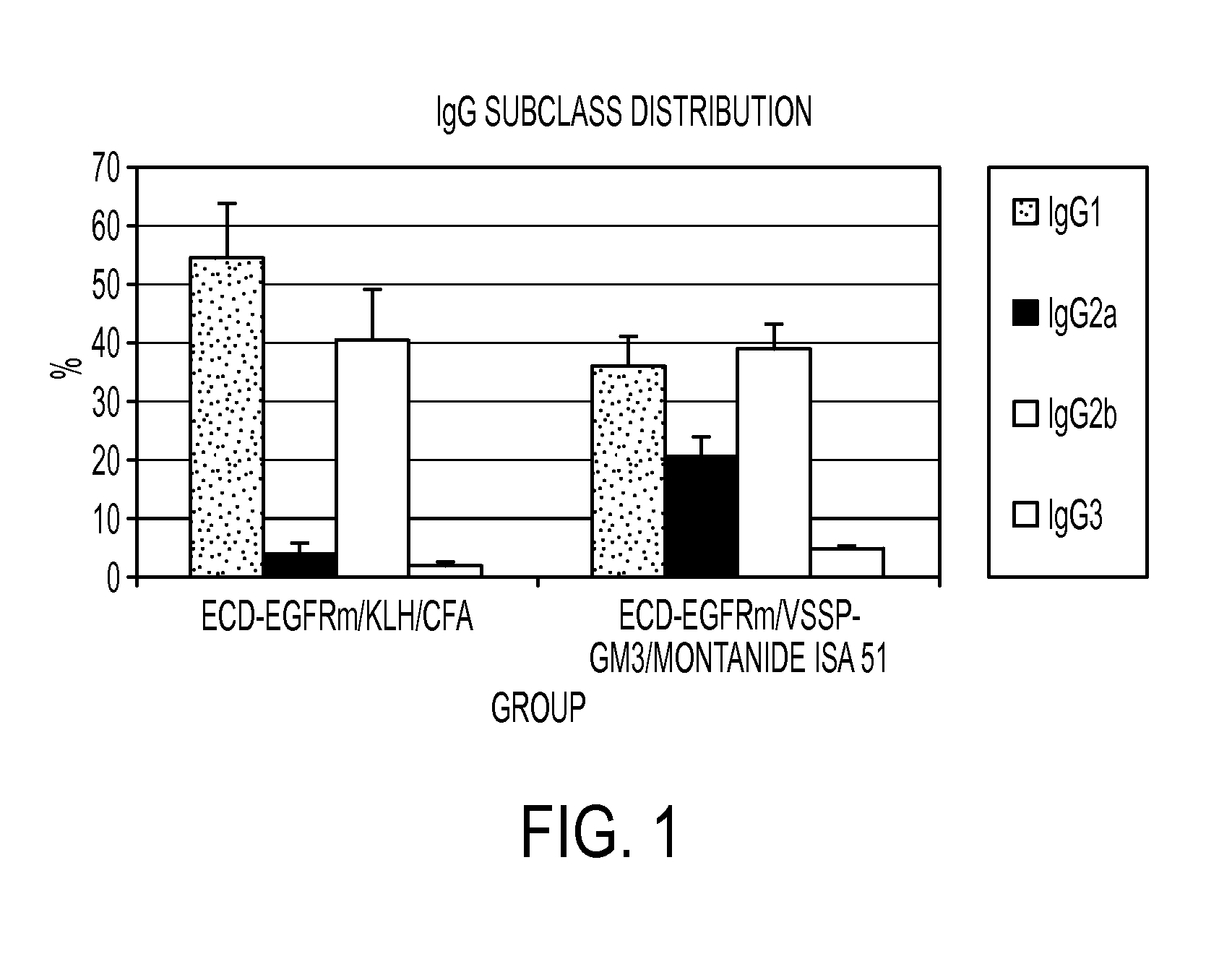
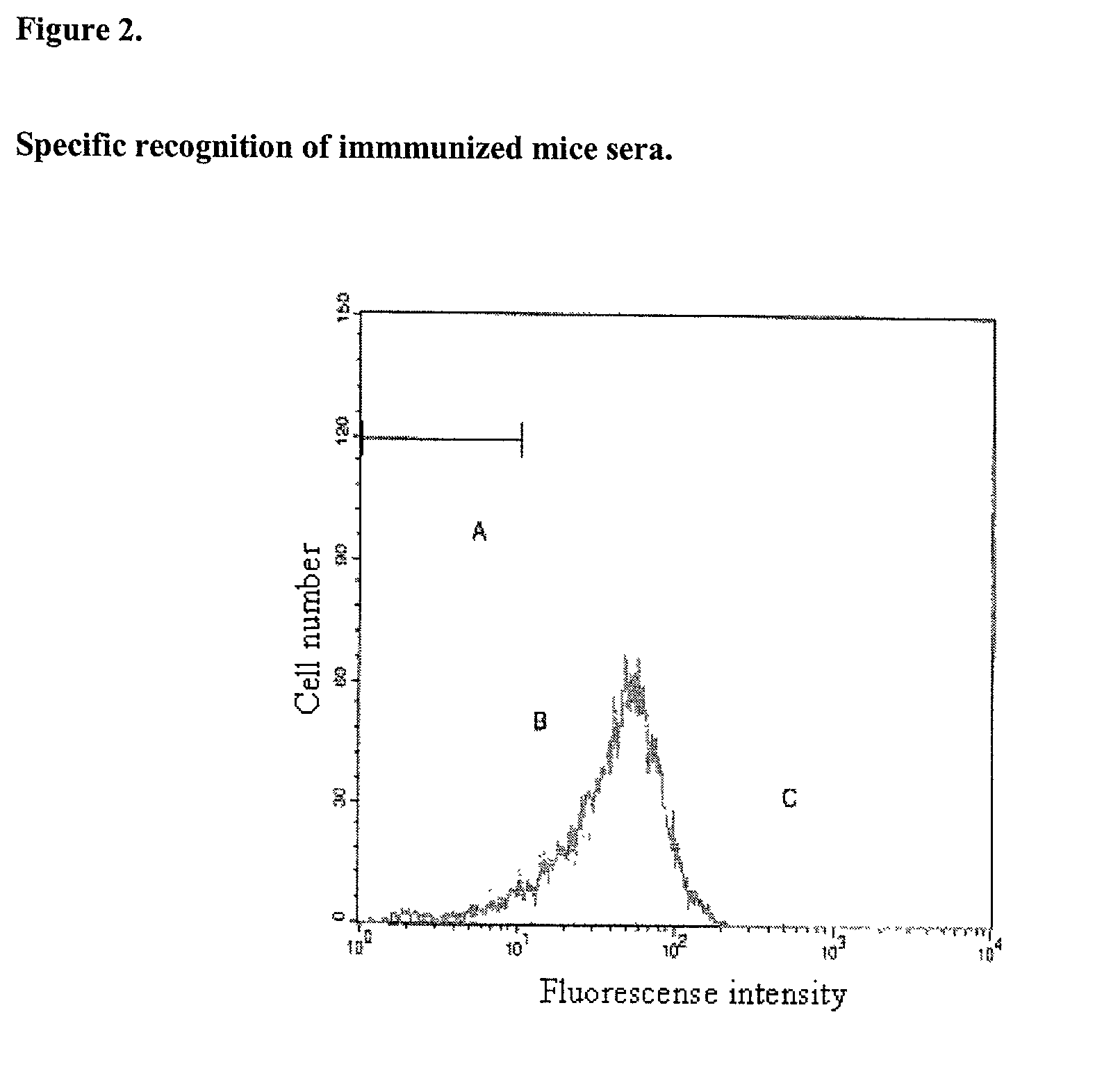
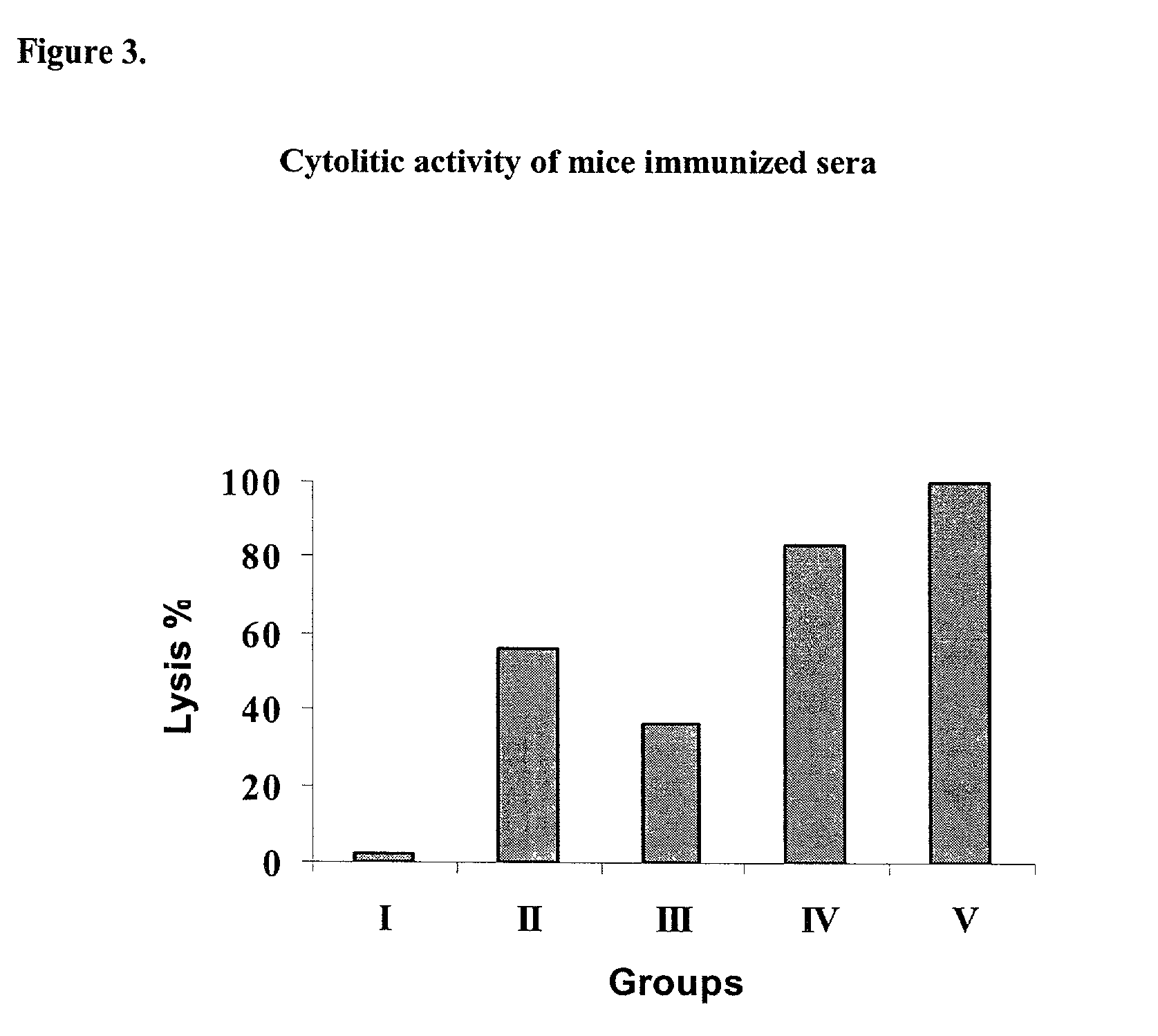
This invention discloses means for obtaining immunogenic peptides, polypeptides, proteins, and their corresponding nucleic acid sequences, target cells with vaccine interest, or lysates thereof, without making structural changes in said antigens, through their association with Very Small Size Proteoliposomes. The object of the invention is to provide immunogenic compositions containing peptides, polypeptides, proteins, their corresponding DNA sequences, cells or their lysates and Very Small Size Proteoliposomes (VSSP), which are formed by binding the Outer Membrane Protein Complex (OMPC) of Neisseria meningitidis with gangliosides, by means of hydrophobic links. Additionally, it is stated that these compositions can be formulated alone or in the form of emulsions with the Incomplete Freund's Adjuvant (IFA), and may also be lyophilized. The essence of the invention consists in describing compositions that triggers immunogenicity in low immunogenic antigens, such as growth factor receptors, without imparting structural changes therein. Particularly, this invention refers to preparation of immuno-stimulating compositions capable of generating antigen-specific immune responses, even in immuno-compromised hosts, such as those suffering form cancer or viral or bacterial chronic infections. In said patients, the administration of the vaccine compositions described in this invention has lead to the reestablishment of the functionality of the immune system. Vaccine compositions of this invention can be used to protect or treat infectious, or auto-immune diseases.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Preparations that potentiate immunogenicity in low immunogenic antigens
ActiveUS7776342B2Reestablishment of functionalityEasy to useAntibacterial agentsSnake antigen ingredientsImmunogenic peptideImmunogenicity
This invention discloses means for obtaining immunogenic peptides, polypeptides, proteins, and their corresponding nucleic acid sequences, target cells with vaccine interest, or lysates thereof, without making structural changes in said antigens, through their association with Very Small Size Proteoliposomes.The object of the invention is to provide immunogenic compositions containing peptides, polypeptides, proteins, their corresponding DNA sequences, cells or their lysates and Very Small Size Proteoliposomes (VSSP), which are formed by binding the Outer Membrane Protein Complex (OMPC) of Neisseria meningitidis with gangliosides, by means of hydrophobic links. Additionally, it is stated that these compositions can be formulated alone or in the form of emulsions with the Incomplete Freund's Adjuvant (IFA), and may also be lyophilized.The essence of the invention consists in describing compositions that triggers immunogenicity in low immunogenic antigens, such as growth factor receptors, without imparting structural changes therein. Particularly, this invention refers to preparation of immuno-stimulating compositions capable of generating antigen-specific immune responses, even in immuno-compromised hosts, such as those suffering form cancer or viral or bacterial chronic infections. In said patients, the administration of the vaccine compositions described in this invention has lead to the reestablishment of the functionality of the immune system. Vaccine compositions of this invention can be used to protect or treat infectious, or auto-immune diseases.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Soft-shelled turtle systemic septicemia spherical virus inactivated vaccine and its preparation method
InactiveCN102626514AEfficient production processLeadingViral antigen ingredientsInactivation/attenuationVirus ProteinTGE VACCINE
The invention discloses a soft-shelled turtle systemic septicemia spherical virus inactivated vaccine and its preparation method, and is characterized in that the vaccine contains the inactivated soft-shelled turtle systemic septicemia spherical virus with the collection number being CGMCCNo.5378. The preparation method comprises the following steps of: cutting internal organ of sick soft-shelledturtles into pieces, adding into a TNE buffer, centrifuging, taking a supernatant, centrifuging, taking a white precipitate, suspending in the TNE buffer, centrifuging the precipitate, taking the supernatant, and centrifuging to obtain a white precipitate, namely the purified virus; diluting the purified virus by the use of aseptic normal saline until the final concentration of viral protein reaches 0.5-1mg / ml, and adding 0.5% (final volume) of formalin for inactivation in a thermostat water bath cauldron; and completely emulsifying inactivated virus and a complete Freund's adjuvant accordingto the volume ratio of 1:1 to obtain the inactivated vaccine. The soft-shelled turtle systemic septicemia spherical virus inactivated vaccine proposed for the first time is safe and effective and hasstrong immunity, and the preparation method is simple and easy to operate.
Owner:NINGBO UNIV
Preparation method of kit for detecting fish livetin source of carp family
The invention discloses the method for preparing the kit for detecting vitellogenin of cyprinid. The first step is to induce, separate and purify the vitellogenin. The prepared solution of lynoral is injected into the stomach cavity of the cyprinid. With a period of time of induction, the blood serum of the fish blood is obtained through the centrifugal procedure. The freeze-dried powder is prepared by using the separated purification of ion exchange column and desalting of dialysis. The next step is to prepare polyclonal antibody of vitellogenn. The weighted freeze-dried power is taken to dissolve in the double steamed water with Freund's adjuvant being added to emulsify. The polyclonal antibody is prepared through the immune giant blanc. The invention can be used for quantificationally detecting vitallogenin in blood, liver and body of cyprinid with accuracy.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Anti-natamycin monoclonal antibody hybridoma cell strain and application thereof
ActiveCN104560886AHigh detection sensitivityImprove featuresTissue cultureImmunoglobulinsBALB/cMonoclonal
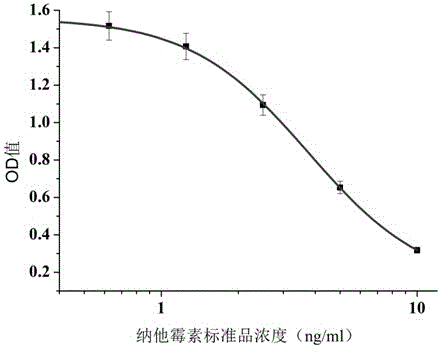
The invention discloses an anti-natamycin monoclonal antibody hybridoma cell strain and application thereof, and belongs to the technical field of food safety immunological detection. The scheme adopted by the invention is as follows: mixing and emulsifying natamycin complete antigen with an equal amount of complete freund's adjuvant, and then immunizing BALB / c mouse through subcutaneous injection on the back of the mouse, wherein the first immunization uses the complete freund's adjuvant, and subsequent immunization uses an incomplete freund's adjuvant; fusing splenocytes of the mouse with a high titre and a low IC50 value and myeloma cells of the mouse through a PEG method; performing indirect competitive ELISA screening and three times of subcloning to obtain a hybridoma (monoclonal) cell strain. Monoclonal antibodies secreted by the hybridoma (monoclonal) cell strain has relatively good specificity and detection sensitivity (the value of IC50 is 2.2 microgram / L) for natamycin, and can be used for detecting residual natamycin in food safety.
Owner:JIANGNAN UNIV
Anti-clorprenaline monoclonal antibody hybridoma cell strain and application thereof
InactiveCN108517317AHigh detection sensitivityImprove featuresOrganic compound preparationCarboxylic acid esters preparationBALB/cCLORPRENALINE
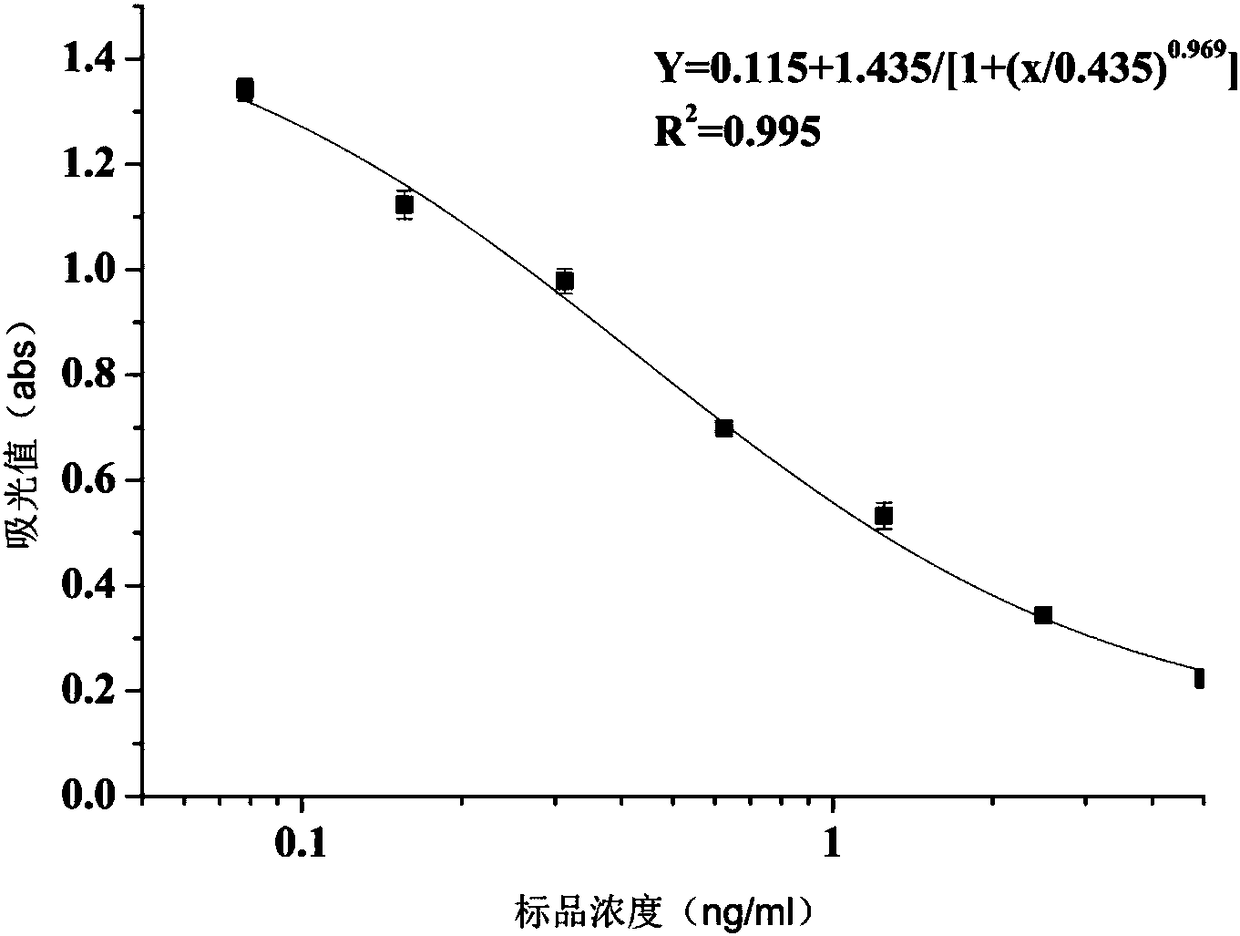
The invention discloses an anti-clorprenaline monoclonal antibody hybridoma cell strain and application thereof, and belongs to the field of food safety immunodetection. A method is characterized in that after the clorprenaline complete antigen is subjected to mixed emulsification with the same quantity of a Freund's adjuvant, the BALB / c mouse immunization is performed through back subcutaneous injection. In the first immunization, the complete Freund's adjuvant is used; then, in-complete Freund's adjuvants are used. The high-valence low-IC50 mouse splenocytes are fused with mouse myeloma cells by a PEG method; through indirect competition ELISA screening and three times of subclone, a hybridoma cell strain is obtained. The monoclonal antibody secreted by the cell strain has good specificity and detection sensitivity; the IC50 value is 0.435ng / ml; the strain can be used for detecting clorprenaline residue in food.
Owner:JIANGNAN UNIV +1
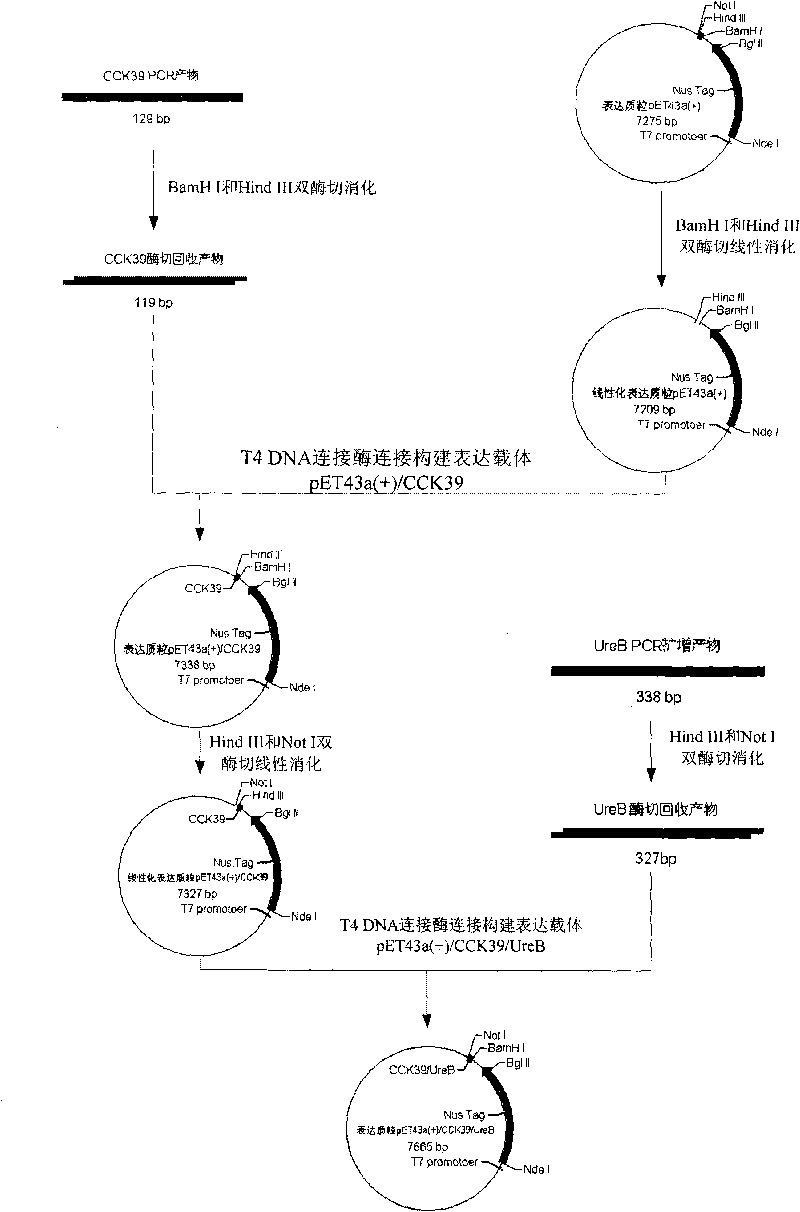
Yolk powder containing double-titer yolk antibody of anti-porcine CCK/Urease, and preparation method thereof
ActiveCN101731458AImprove efficiencyInhibition of urease activityEgg immunoglobulinsAnimal feeding stuffBacteroidesYolk
The invention discloses yolk powder containing double-titer yolk antibody (Immunoglobulin yolk, IgY) of cholecystokinin / intestinal bacterial urease. A preparation method of the yolk powder comprises the following steps: 1) preparing vaccine by mixing purified cholecystokinin 39 peptide (CCK39) / urease B subunit (UreB) fusion protein with Freund's adjuvant well, immunizing healthy laying hens, determining yolk IgY titer through enzyme-linked immunosorbent assay and collecting hyperimmune eggs; and 2) separating yolk from hyperimmune eggs and preparing the yolk into yolk powder. The yolk powder containing double-titer IgY, which is prepared by the method, is added to feed for feeding pigs, and the satiety of the pigs is not produced or delayed due to CCK in the IgY and secreted by porcine intestinal canals so as to increase feed intake. The IgY can also neutralize urease secreted by intestinal bacteria so as to reduce the production of ammonia, protect porcine intestinal canals and respiratory tract, reduce the waste of feed protein and improve culture environment.
Owner:胡文锋
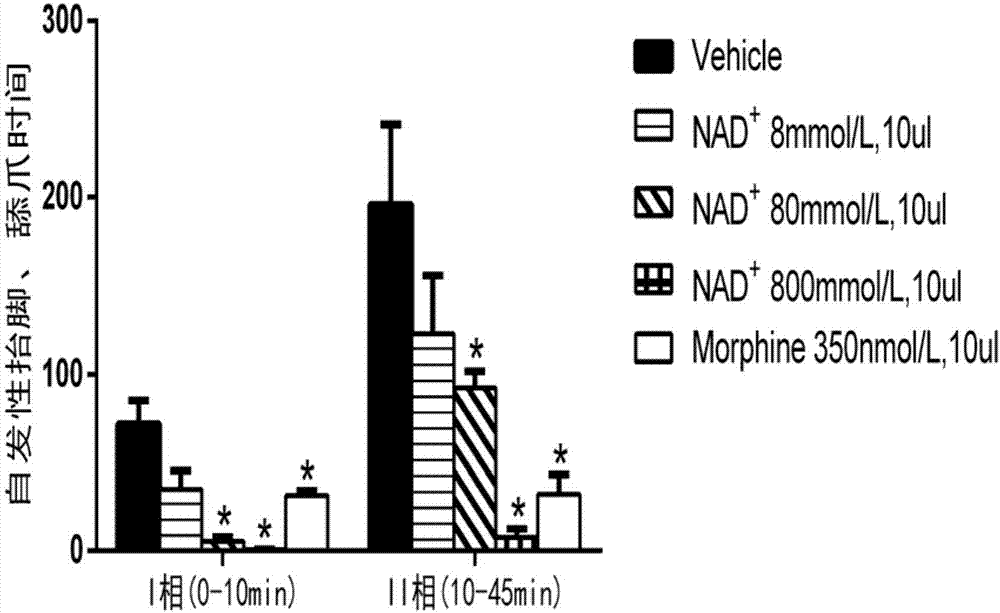
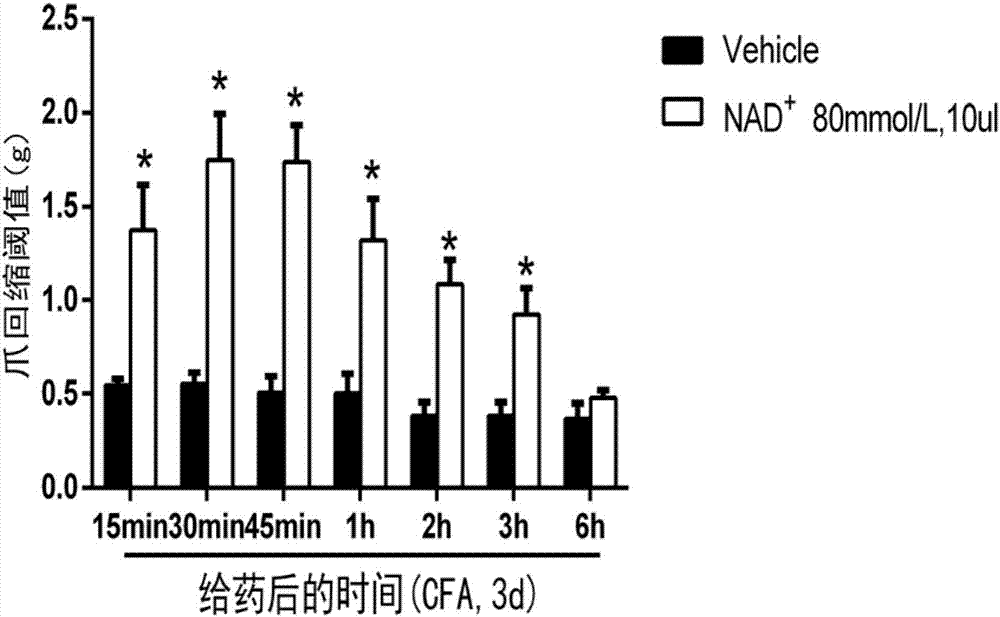
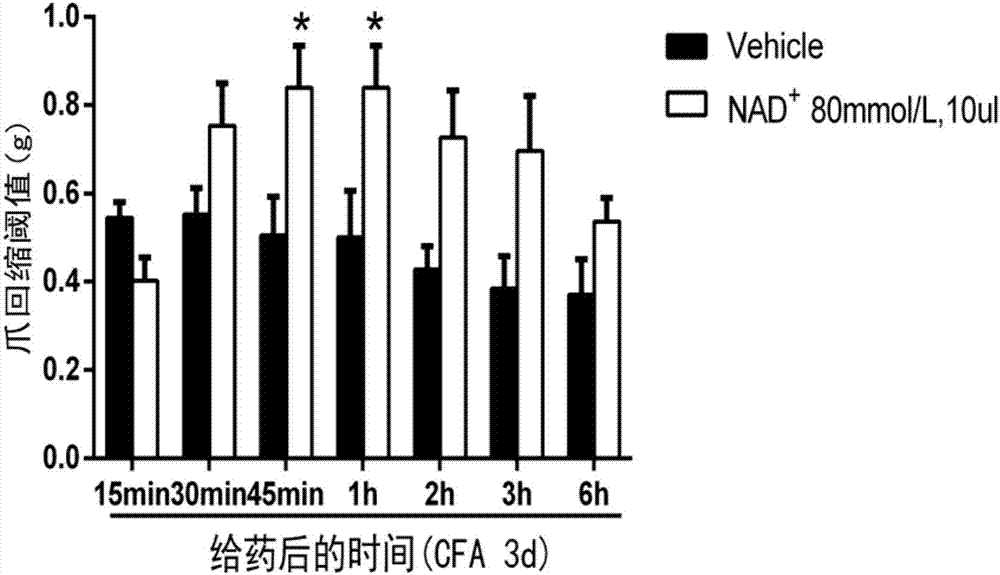
Application of nicotinamide adenine dinucleotide to prepare drug for treating inflammatory pain
InactiveCN107233352AAchieve analgesic effectAnalgesic effect on inflammatory painOrganic active ingredientsAntipyreticFreund's adjuvantAnalgesics effects
The invention relates to an application of nicotinamide adenine dinucleotide to prepare drug for treating inflammatory pain. The modern pharmacological experiment proves that after injecting nicotinamide adenine dinucleotide to an ICR mouse, the nicotinamide adenine dinucleotide has analgesic effect to the inflammatory pain induced by formalin or complete Freund's adjuvant; it indicates that the nicotinamide adenine dinucleotide can be applied to treat inflammatory pain.
Owner:TONGJI UNIV
Anti-chlorothalonil monoclonal antibody hybridoma cell strain and application thereof
InactiveCN104017774AHigh detection sensitivityImprove featuresMicroorganism based processesTissue cultureBALB/cChlorothalonil
The invention relates to an anti-chlorothalonil monoclonal antibody hybridoma cell strain and application thereof, belonging to the technical field of immunologic detection in food safety. The anti-chlorothalonil monoclonal antibody hybridoma cell strain D is collected in China General Microbiological Culture Collection Center, and the collection number is CGMCC No.9304.After the chlorothalonil complete antigen and equivalent Freund's adjuvant are mixed and emulsified, the emulsion is subjected to back subcutaneous injection to immunize a BALB / c mouse. The complete Freund's adjuvant is utilized for the first immunization, and the incomplete Freund's adjuvant is utilized afterwards. The high-titer IC50 mouse splenocyte and mouse myeloma cell are fused by a PEG process, and are subjected to indirect competitive ELISA screening and three-time subcloning to obtain the hybridoma cell strain D. The monoclonal antibody secreted by the cell strain D has favorable specificity and detection sensitivity for chlorothalonil (the IC50 value is 1.5 mu g / L), and can be used for chlorothalonil residue detection in food safety.
Owner:JIANGNAN UNIV
ETEC (enterotoxigenic escherichla coli) yolk antibody powder and preparation method thereof
InactiveCN105713088AHigh potencyImprove high temperature stabilityEgg immunoglobulinsImmunoglobulins against bacteriaEscherichia coliYolk
The invention discloses ETEC (enterotoxigenic escherichla coli) yolk antibody powder and a preparation method thereof. The method comprises following steps: escherichia coli strains are cultured in a solid culture medium at 35-40 DEG C for 18-24 h; after culture, pilin is extracted coarsely from the solid culture medium and is purified; the purified pilin and equal volume of a freund's adjuvant are mixed, an oil-emulsion vaccine is formed and used for immunizing a primiparous hen, and serum and a yolk antibody are collected after immunization; the yolk antibody is subjected to spray drying through a powder sprayer, and the yolk antibody powder is prepared. Analysis of the titer of the immunized yolk antibody with ELISA (enzyme-linked immunosorbent assay) discovers that higher-titer yolk antibody can be obtained by using purified pilin as an antigen for immunization. After the yolk antibody powder is prepared from the yolk antibody through spray drying by the powder sprayer, the antibody titer is only reduced by one degree of multiple proportions, the yolk antibody has very good high-temperature stability, and a power preparation process adopting a spray drying method is applicable to production of the yolk antibody powder.
Owner:FOSHAN UNIVERSITY
Epitope vaccine for resisting A/B subgroup avian leucosis virus infection and preparation method and application of epitope vaccine
ActiveCN104548087ALow costEase of mass productionAntiviralsAntibody medical ingredientsLeucosisNucleotide
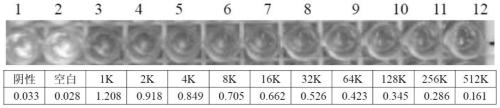
The invention relates to the field of animal virology and immunology, and provides an epitope vaccine for resisting A / B subgroup avian leucosis virus infection. The epitope vaccine is prepared by highly active recombinant protein His-cENV which is obtained through screening and purification after prokaryotic expression and a freund's adjuvant in a united manner, wherein the nucleotide sequence for encoding the recombinant protein His-cENV is shown as SEQ ID NO.1. Through the epitope vaccine, 7-day-old breeding poultry chicks are immune and can produce 1:128000 neutralizing antibodies. Vitro virus neutralization experiments and animal experiments show that the epitope vaccine can neutralize different ALV-A / B isolated strains, so that chicken flocks are effectively protected to resist the infection of ALV-A / B strains. Because the epitope vaccine is based on a multi-epitope antigen gene sequence which is originated form env, the defect of ALV-A / B virus variation is overcome, a new era of the ALV-A / B vaccine is opened, a new way of resisting the ALV-A / B infection is provided, and a technical support for preventing and controlling the ALV-A / B is provided.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Yolk antibody oral preparation for treating piglet PED (porcine epidemic diarrhea) and preparation method thereof
InactiveCN105412923APlay a preventive roleReduce incidenceEgg immunoglobulinsImmunoglobulins against virusesYolkProtective antigen
The invention provides a yolk antibody oral preparation for treating piglet PED (porcine epidemic diarrhea). The yolk antibody oral preparation is characterized by containing a yolk antibody extracted from egg yolks collected after nonimmune laying hens are immunized with PEDV (porcine epidemic diarrhea virus) epidemic strain protective antigen gene COE protein. The invention further provides a preparation method of the oral preparation, comprising the steps: taking a current epidemic strain as a basis, performing prokaryotic expression and purification on PEDV protective antigen gene COE, so as to obtain target protein; preparing immunogen with freund's adjuvant, and then inoculating the nonimmune laying hens; collecting eggs after immunity, and extracting a yolk antibody from yolk; and preparing the safe and efficient yolk antibody oral preparation from the yolk antibody. The yolk antibody oral preparation disclosed by the invention can be used for effectively treating diseased piglets with PED, and meanwhile, the yolk antibody oral preparation can be used for preventing un-infected piglets from getting infected in the same group. The oral preparation is simple in preparation technique, and is suitable for bulk production and clinical use.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
Tiamulin monoclonal antibody hybridoma cell line and application thereof
ActiveCN108330102AImprove featuresHigh detection sensitivityTissue cultureImmunoglobulinsBALB/cSubcutaneous tissue
The invention discloses a Tiamulin monoclonal antibody hybridoma cell line and application thereof, belonging to the field of food security immunity detection. The complete antigen of Tiamulin and equivalent Freund's adjuvant are mixed and emulsified completely, and then are injected into a BALB / c mice through back subcutaneous tissues so as to immunize the BALB / c mice; the first time of immunizing (100 mug / one mice) uses the complete Freund's adjuvant, immunity enhancement (50 mug / one mice) for multiple times uses the incomplete Freund's adjuvant, and finally, the complete antigen of the Tiamulin (20 mug / one mice, not containing adjuvant) is used for impacting immunity. Efficient but low-cost spleen cells of IC50 mice are fused with mice myeloma cells through a PEG method, and the hybridoma cell line is obtained through screening through an indirect cELISA and three times of subcloning. The monoclonal antibody secreted by the cell line has high specificity and detection flexibility (the value for IC50 is 0.73ng / mL) for the Tiamulin, and provides a raw material for immunity detection of residual Tiamulin in food, thereby having practical application value.
Owner:JIANGNAN UNIV +1
Medicinal composition for treating rheumatoid arthritis, and preparation method and application thereof
InactiveCN102579601AGood treatment effectSafe and effective treatmentAntipyreticComponent separationMedicinal herbsSide effect
The invention discloses a medicinal composition for treating rheumatoid arthritis. The medicinal composition is prepared from the following Chinese herbal medicines in part by weight: 2.5 to 10 parts of periploca forrestii schltr, 1 to 4 parts of gentiana macrophylla and 1 to 4 parts of yanhusuo. The invention also discloses a preparation method and a quality detection method for the medicinal composition, and application of the medicinal composition. Through reasonable compatibility of the periploca forrestii schltr, the gentiana macrophylla and the yanhusuo, the medicinal composition has an obvious curative effect on arthritis induced by a complete freund adjuvant, good anti-inflammatory and analgesic effects, a better pharmaceutical effect compared with the simple medicinal material and the medicine which is prepared from other Chinese herbal medicines, and small toxic and side effects and achieves a synergistic effect. The modern pharmological study proves that the medicinal composition can safely and effectively treat rheumatoid arthritis and provides a new choice for clinical treatment.
Owner:SICHUAN NORMAL UNIVERSITY
Streptococcus suis B cell dominant epitope tandem vaccine and preparation method thereof
ActiveCN109180822AIncrease productivityHigh purityAntibacterial agentsBacterial antigen ingredientsProtective antigenGenetic engineering
The invention discloses a streptococcus suis B cell dominant epitope tandem protein, relates to a method for preparing a streptococcus suis B cell dominant epitope tandem vaccine and discloses a use of a B cell dominant epitope tandem gene and a fusion protein in preparation of a vaccine. The method for preparing the vaccine comprises selecting B cell dominant epitopes of streptococcus suis protective antigens GAPDH, MRP and DLDH through software, connecting amino acid sequences as GGGG flexible fragments in series, carrying out prokaryotic expression through the recombinant expression vectorformed from the fragments in series, purifying the expressed fusion protein, and processing the fusion protein and a Freund's adjuvant into a vaccine preparation. The mouse immunogenicity evaluation result shows that the vaccine can effectively protect the streptococcus suis type 2. Compared with the traditional inactivated vaccine and attenuated vaccine, the genetic engineering vaccine provided by the invention has the advantages of convenient and safe preparation process, high production efficiency, high product purity, good stability, high yield and high safety.
Owner:SHANGHAI JIAO TONG UNIV
Aeromonas hydrophila heat shock protein subunit vaccine and preparation method thereof
InactiveCN104001164AAvoid atavismAvoid pollutionAntibacterial agentsBacterial antigen ingredientsVaccine antigenAeromonas hydrophila
Belonging to the technical field of gene engineering and vaccinology, the invention relates to an aeromonas hydrophila heat shock protein subunit vaccine and preparation method thereof. The vaccine antigen contains the amino acid sequence shown as SEQIDNo.1. The preparation method comprises: cloning of heat shock protein gene; construction of plasmid pETDnaK; and inducible expression of plasmid pETDnaK. The aeromonas hydrophila heat shock protein subunit vaccine provided by the invention has the advantages of: 1. safety and no toxicity, thus avoiding atavism of attenuated vaccine and possible water pollution caused by repeated immunization by a lot of inactivated vaccines; 2. high protection rate, with the immunoprotection efficiency of the recombinant subunit vaccine AHDnaK on aeromonas hydrophila up to 81.8%; and 3. simple operation, i.e. being injectable after being mixed with a cheap commercial adjuvant Freund's adjuvant.
Owner:HANGZHOU NORMAL UNIVERSITY
Preparation method of cyanea nozakii kishinouye toxinanti-toxic serum
ActiveCN110078824AHigh potencyHigh puritySerum immunoglobulinsImmunoglobulins against animals/humansSerum igeMedicine
The invention relates to the field of biotechnology, and in particular to a preparation method of cyanea nozakii kishinouye toxin anti-toxic serum. The cyanea nozakii kishinouye toxin is attenuated; and after the attenuating treatment, the cyanea nozakii kishinouye toxin is mixed with a Freund's adjuvant for animal immunization, blood is collected after immunization, and an antiserum is separatedand purified, thereby obtaining the cyanea nozakii kishinouye toxin anti-toxic serum. At the same time, the detoxification experiment in the animal shows that the cyanea nozakii kishinouye toxinanti-toxic serum prepared by the invention has the characteristics of high titer, good purity and strong detoxification effect, and lays an important foundation for the in-depth research and application ofthe cyanea nozakii kishinouye toxinanti-toxic serum and antibody thereof.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Application of miR-218-5p compound as chronic pain marker in preparation of medicine for treating inflammation type chronic pain
InactiveCN104031999ALower heat shock thresholdClear pathogenesisNervous disorderAntipyreticStimulantChronic pain
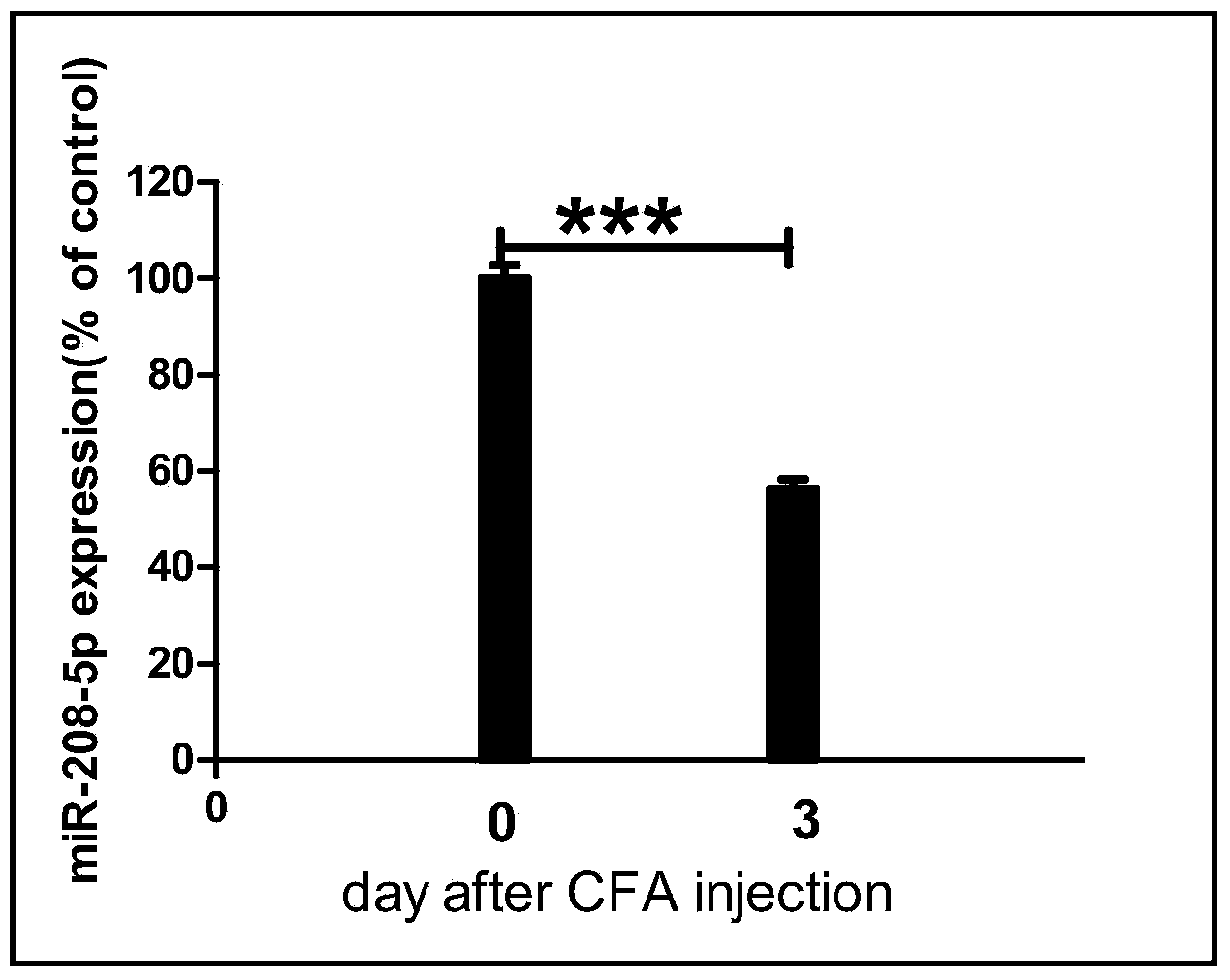
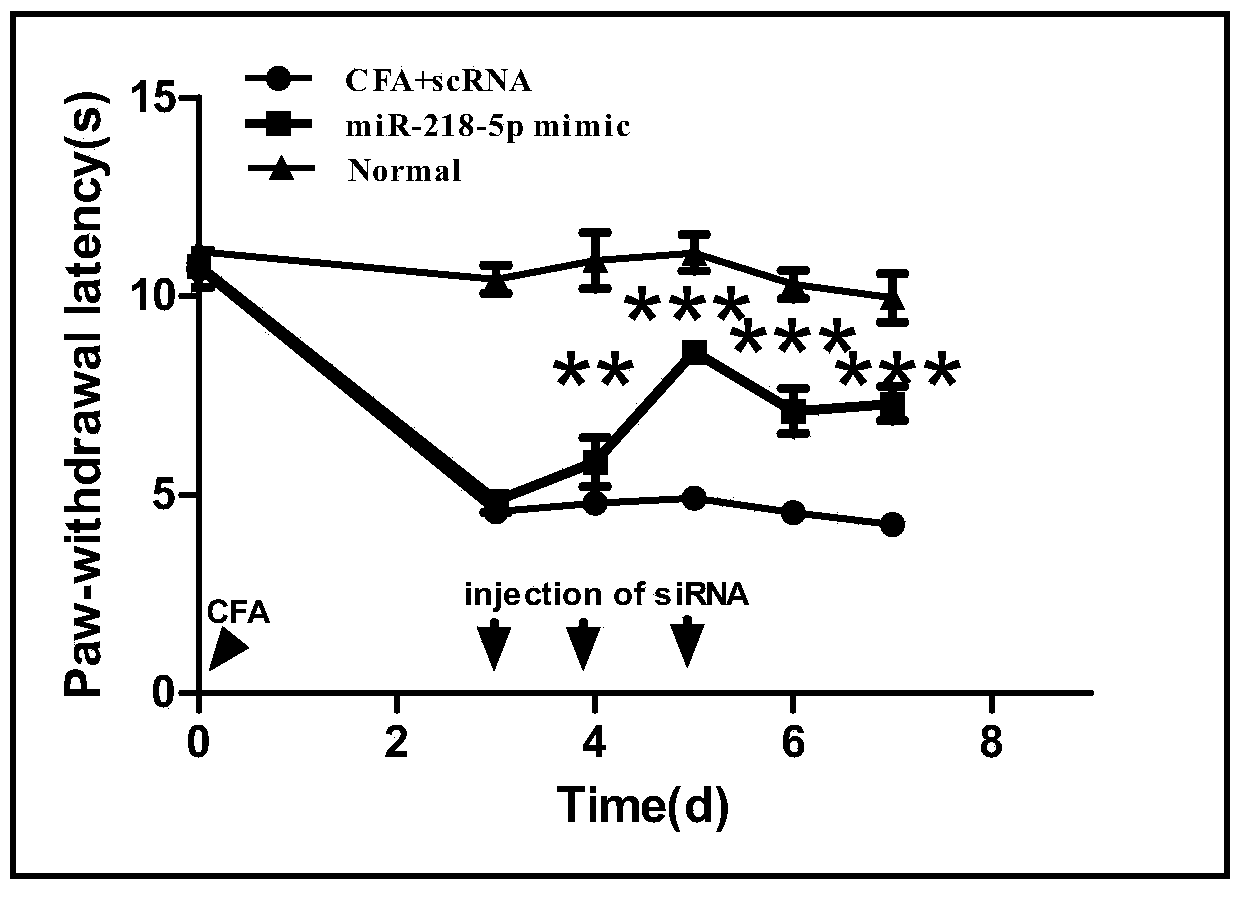
The invention relates to an application of miRNA in preparation of a medicine for treating inflammation type chronic pain, and in particular relates to an application of a miR-218-5p compound. The application comprises the steps: (1) screening a miR-218-5p target gene, verifying the gene through a reporter gene, and culturing an inflammation type chronic pain model mouse by using complete Freund's adjuvant (CFA); (2) increasing the expression quantity of the miRNA in spinal cord of the inflammation type chronic pain model mouse by using a miR-218-5p stimulant; (3) detecting the inhibition function of the miR-218-5p on SYNGR1 protein expression of CFA-resulted inflammation type chronic pain by using a Western blot method; and (4) detecting the expression of a spinal cord nerve cell activity marker c-fos of the miR-218-5p for inhibiting the inflammation type chronic pain state. The process is simple and low in cost, and the inflammation type chronic pain is effectively inhibited by using the miR-218-5p, and an effective interference target is provided for prevention and treatment on chronic pain. The further quantitative analysis on a blood clinical specimen of a patient suffering chronic pain shows that the miR-218-5p can be used as a marker of occurrence of chronic pain.
Owner:XUZHOU MEDICAL COLLEGE
Preparation method and application of Vibrio parahaemolyticus toxoid vaccine
InactiveCN102274494AEasy to operateAntibacterial agentsDepsipeptidesVibrio parahaemolyticusTGE VACCINE
The present invention is a preparation method of Vibrio parahaemolyticus toxoid vaccine, which is characterized in that: first amplifying the target gene tdh; then cloning the target gene fragment tdh into the vector pET-28 to construct the expression vector pET-28-TDH, The pET-28-TDH plasmid was transferred into the expression strain BL21; after culturing and induced expression, the cloned expression product was obtained; the cloned expression product was detected by dot blot reaction, and the Vibrio parahaemolyticus toxoid was obtained; the Vibrio parahaemolyticus toxoid was mixed with An equal volume of complete Freund's adjuvant was mixed evenly to obtain the Vibrio parahaemolyticus toxoid vaccine. The vibrio parahaemolyticus toxoid vaccine prepared by the method of the invention can be used as an immune drug for marine fishes attacked by the vibrio parahaemolyticus, and the immune protection rate can reach about 50%.
Owner:HUAIHAI INST OF TECH
Preparation method of anti-idiotypic egg yolk antibody
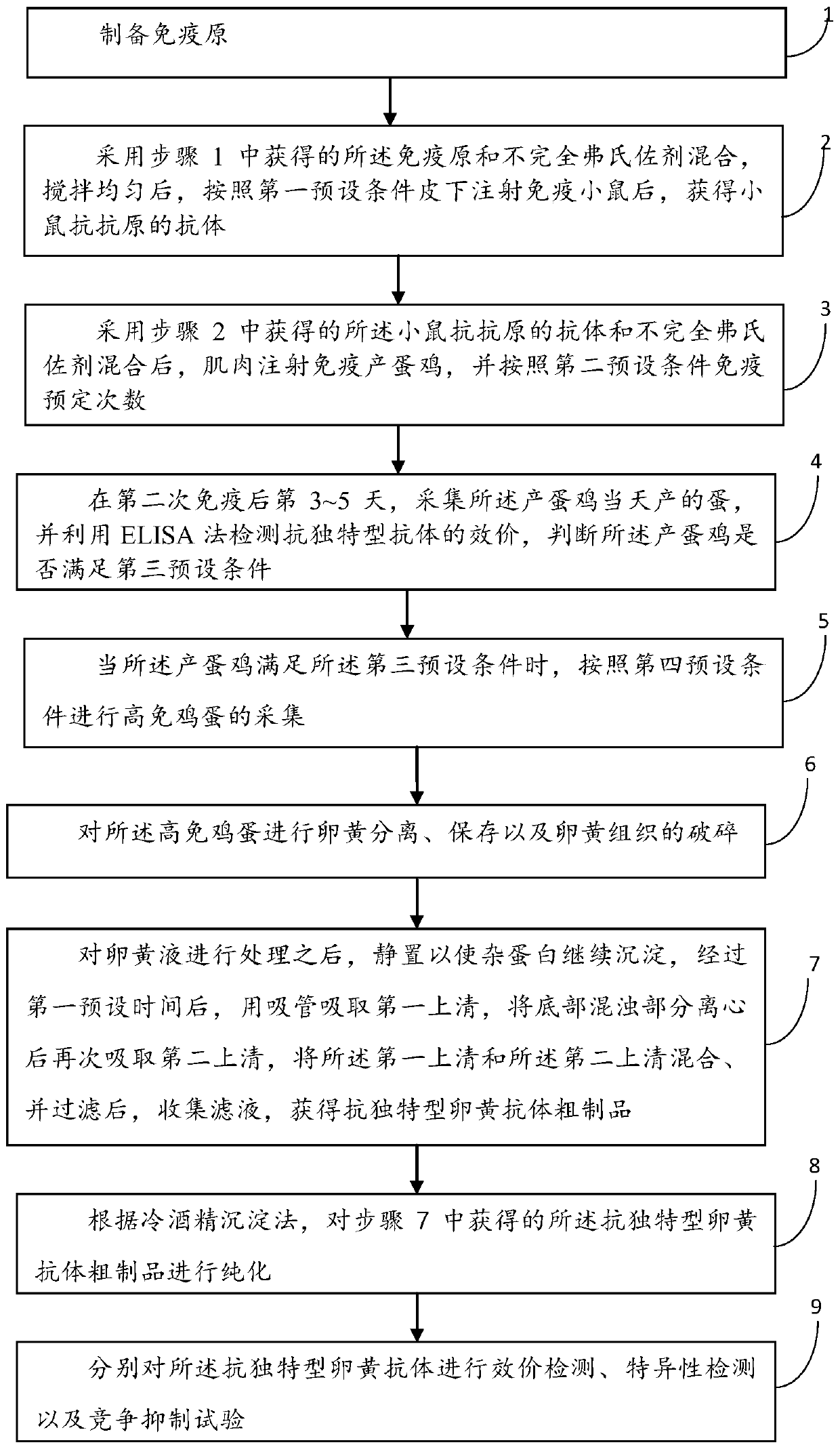
PendingCN110724196AShorten the development cycleShorten the production cycleEgg immunoglobulinsYolkAnimal science
The invention discloses a preparation method of an anti-idiotypic egg yolk antibody and relates to the technical field of antibody preparation. The preparation method comprises the steps as follows: preparing an immunogen; mixing the immunogen with an incomplete Freund's adjuvant, after uniform mixing, and performing subcutaneous injection immunization on a mouse according to a first preset condition to obtain an anti-antigen antibody of the mouse; after mixing the anti-antigen antibody of the mouse with the incomplete Freund's adjuvant, injecting the mixture to immunize laying hens for presettimes according to a second preset condition; acquiring eggs laid by laying hens the same day; collecting high-immunity eggs according to a fourth preset condition; performing yolk separation, preservation and crushing of yolk tissue on high-immunity eggs; after treating yolk liquid, collecting filtrate to obtain a crude product of the anti-idiotypic egg yolk antibody; purifying the crude productof the anti-idiotypic egg yolk antibody; and conducting titer detection, specific detection and competitive inhibition tests on the anti-idiotypic egg yolk antibody separately.
Owner:西安咸辅生物科技有限责任公司
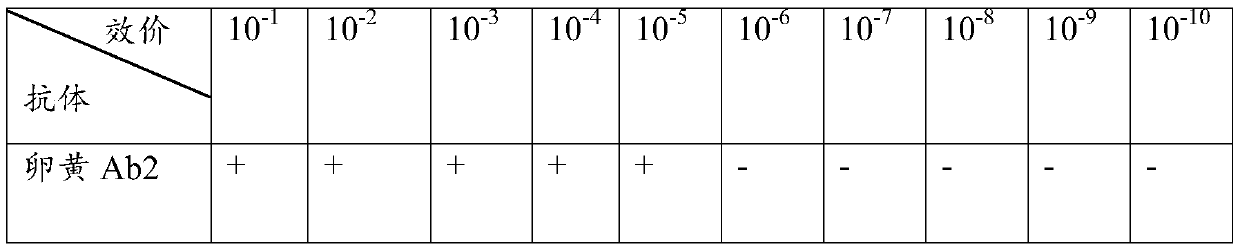
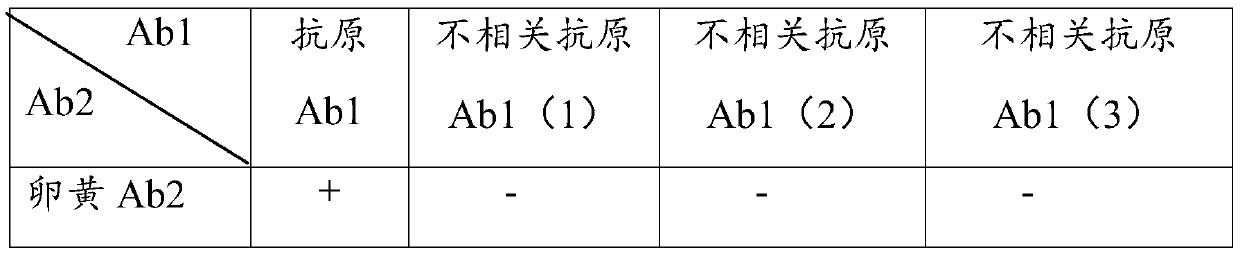
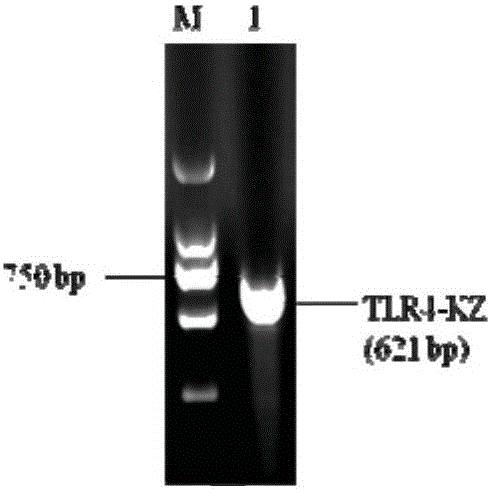
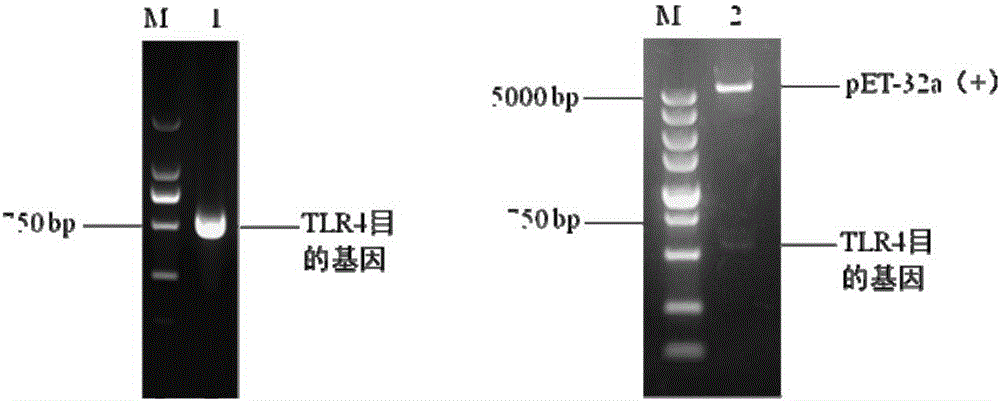
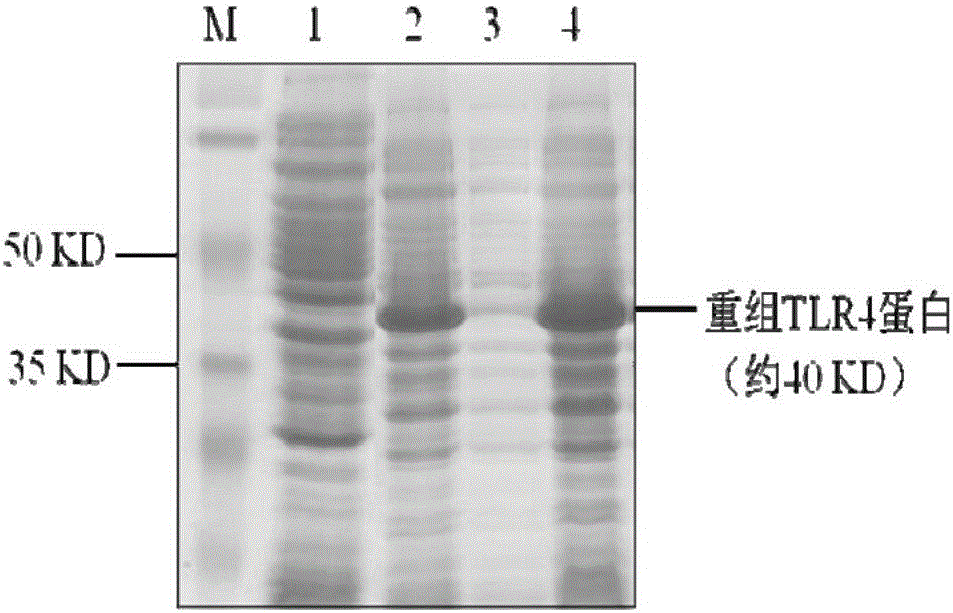
Preparation method and application of porcine TLR4 polyclonal antibody
ActiveCN105924525AImprove efficiencyImprove responseSerum immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseasePurification methods
The invention discloses a preparation method of a porcine TLR4 polyclonal antibody. According to the preparation method, recombinant TLR4 protein (antigen) and freund's adjuvant are emulsified, then an immune animal obtains polyclonal antibody serum, wherein the recombinant TLR4 protein has the amino acid sequence as indicated in the sequence table SEQ. ID. No. 1. It is shown through tests that the antibody prepared through the method is good in reactivity and specificity; due to the fact that a TLR4 gene is a member of a significant pattern recognition receptor Toll-like receptor family targeted to innate immune response, the porcine TLR4 polyclonal antibody can be applied to detection of the porcine TLR4 protein and related research; a good foundation is laid for researching the interaction mechanism between porcine bacterial and viral infection diseases and TLR4 and the virus immunity escape mechanism, and a new target is provided for developing new generation vaccine and medicine. Meanwhile, a prokaryotic expression vector constructed through the method is high in recombinant protein expression efficiency, and the separation and purification method is simple and easy to operate.
Owner:GUANGXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com