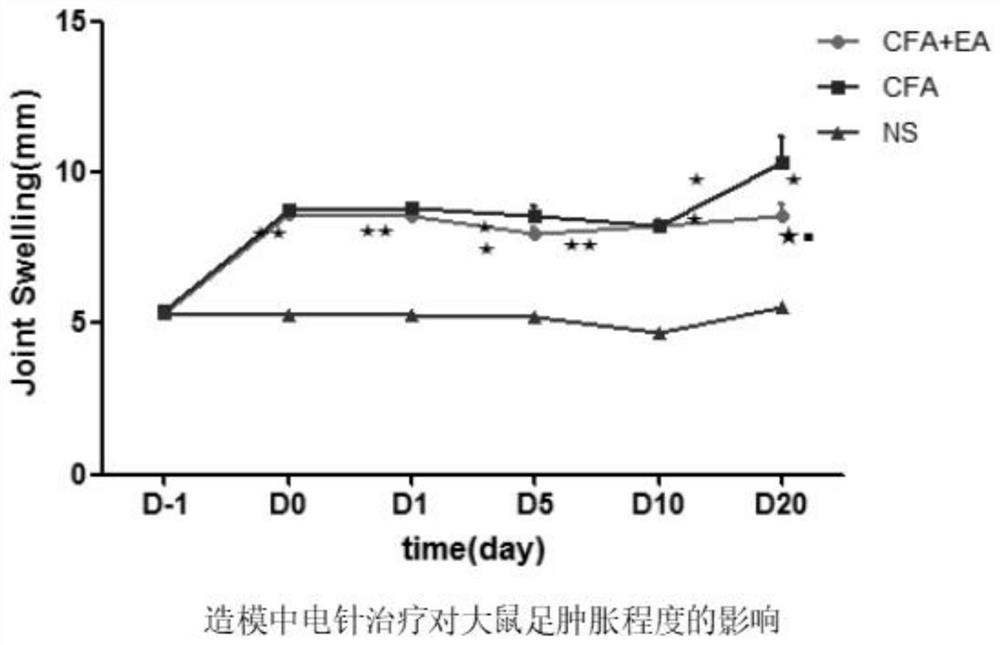
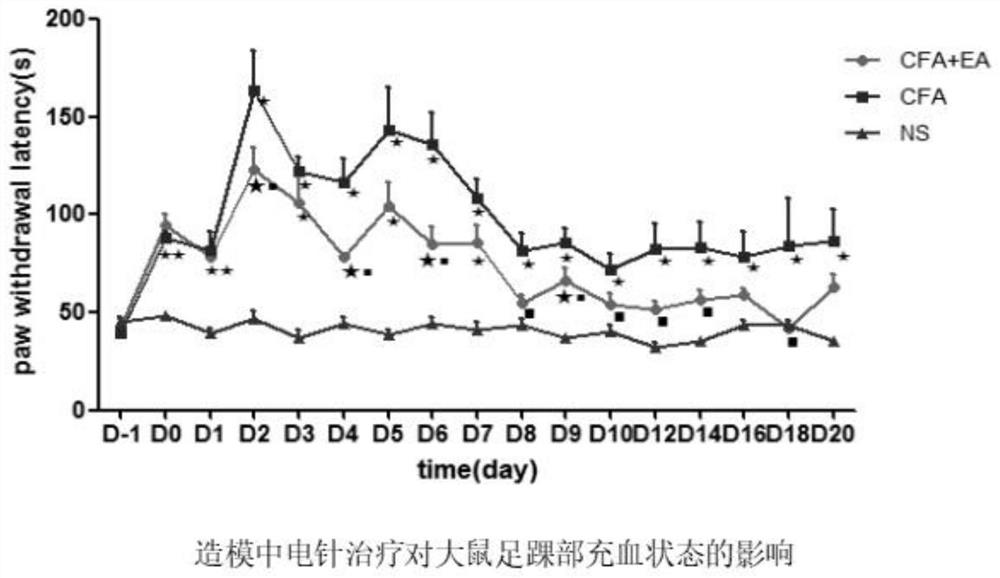
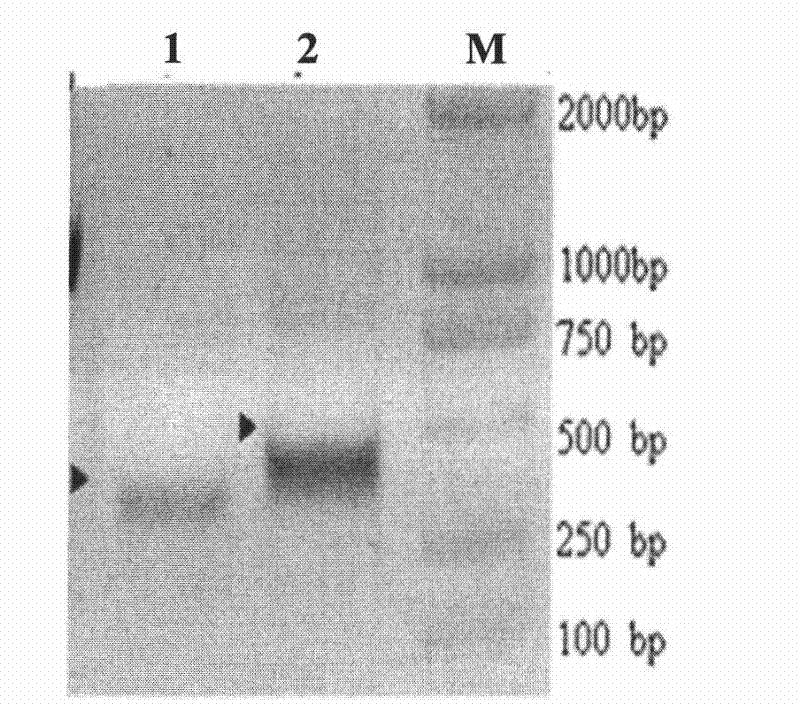
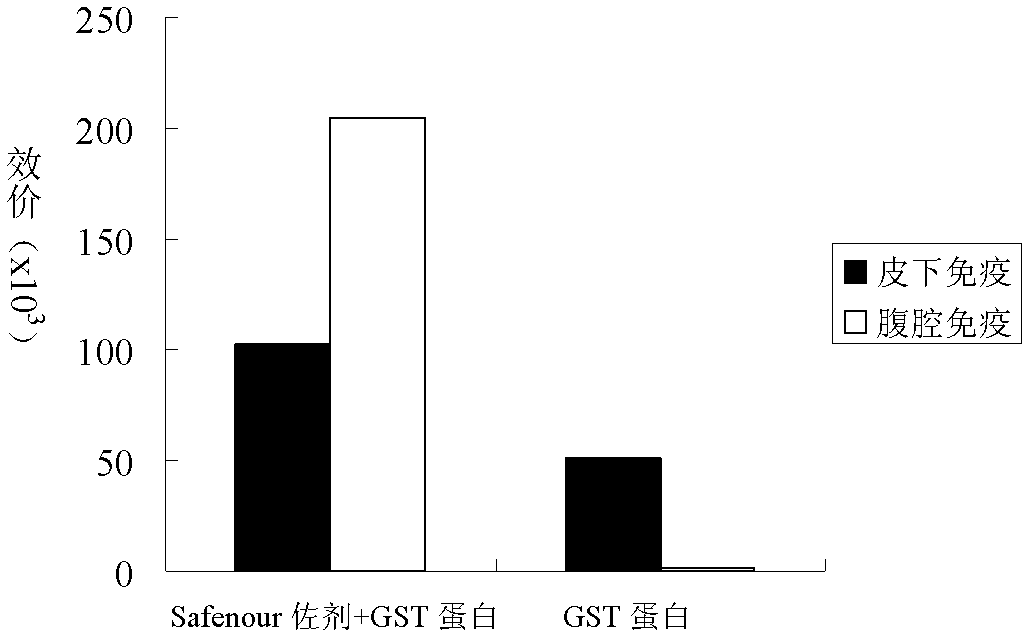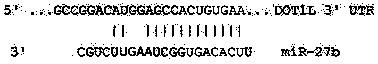Patents
Literature
36 results about "Freund adjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polyvalent inactivity vaccine for preventing and treating atrophic rhinitis of swine
ActiveCN102302771AEffective therapeuticEffective preventionAntibacterial agentsBacterial antigen ingredientsPasteurella multocida toxinImmune effects
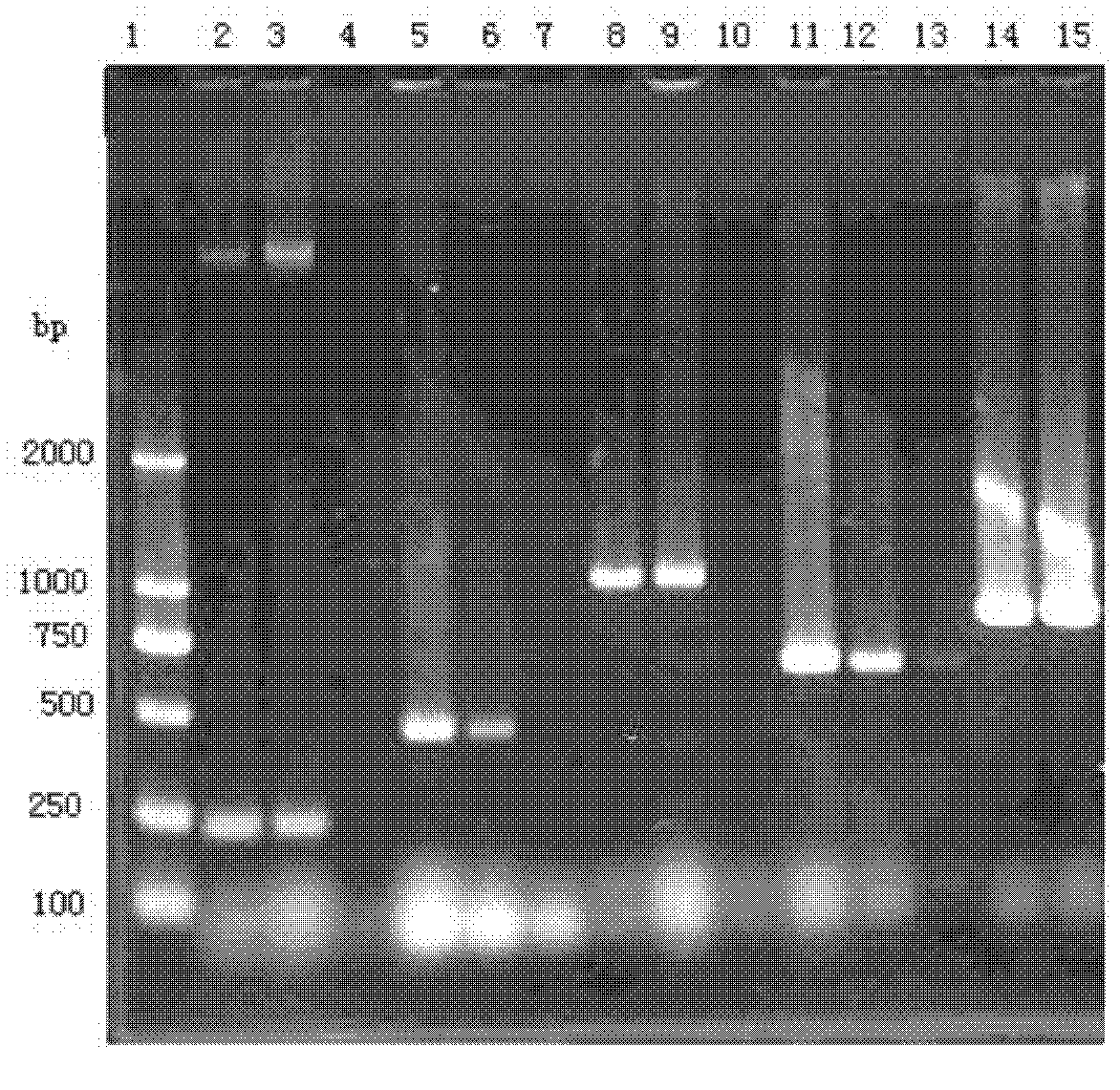
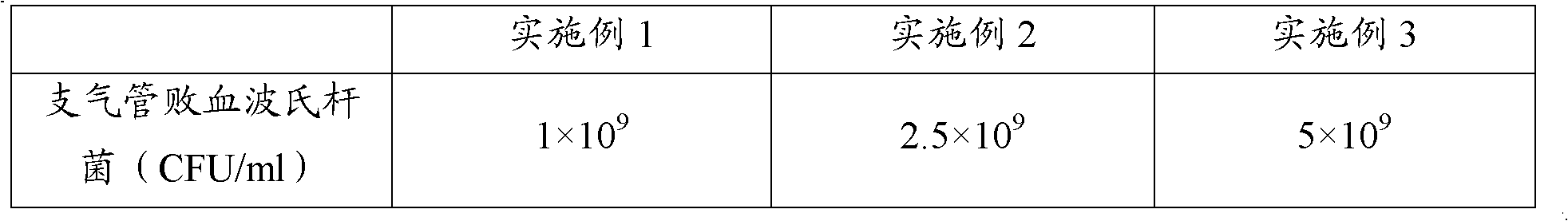
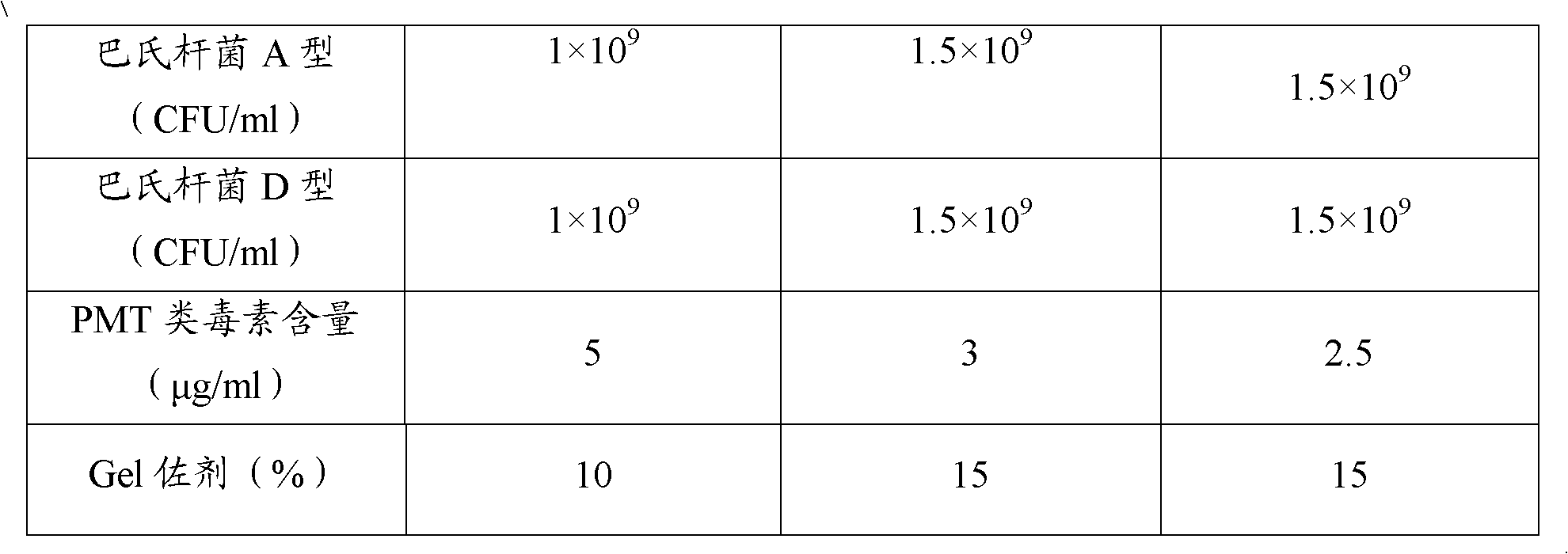
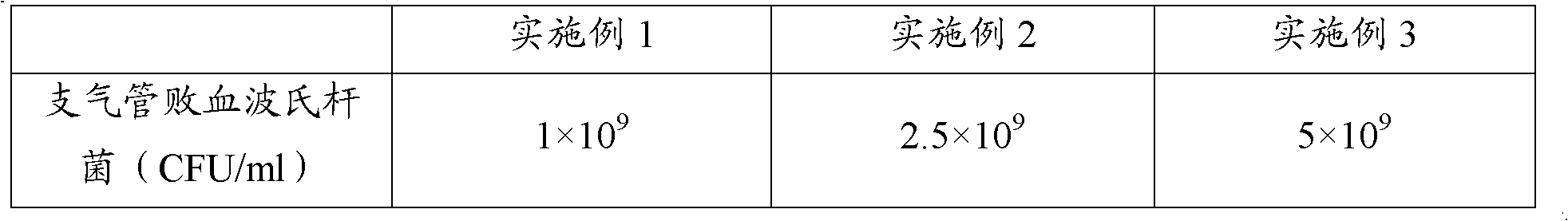
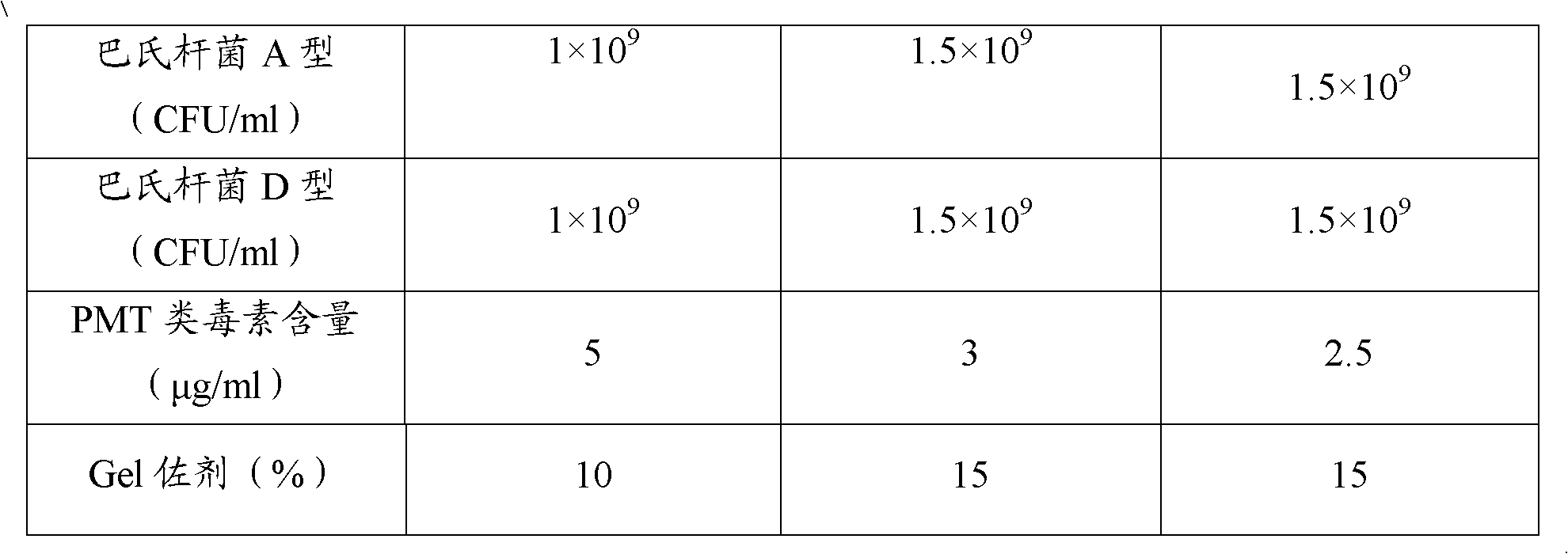
The invention provides a polyvalent inactivity vaccine for preventing and treating atrophic rhinitis of a swine and a preparation method thereof. The polyvalent inactivity vaccine contains inactivated Bordetella bronchiseptica, Pasteurella multocida A, Pasteurella multocida D and PMT (Pasteurella Multocida Toxin) anatoxin. The invention further provides a novel method for culturing and extractingPMT. Compared with the traditional atrophic rhinitis of the swine, the polyvalent inactivity vaccine for the atrophic rhinitis of the swine, provided by the invention, can be used for more generally and effectively treating and preventing the atrophic rhinitis of the swine by comprehensive antigen protection. Finally, in the polyvalent vaccine provided by the invention, the vaccine with a plurality of antigens in a reasonable proportion can be used for solving the problem that the plurality of the antigens interfere each other, thereby improving an immune effect. Furthermore, the inventor provides a water adjuvant by which defects such as incomplete absorption, large side reaction and the like after the traditional alumina gel adjuvant, Freund adjuvant and water-in-oil adjuvant are injected into the water adjuvant can be overcome.
Owner:PU LIKE BIO ENG
A kind of bacillus amyloliquefaciens wh3 and its preparation method and application
InactiveCN102286408AOral lowLow injection toxicityBacteriaMicroorganism based processesFreund adjuvantSclerotinia
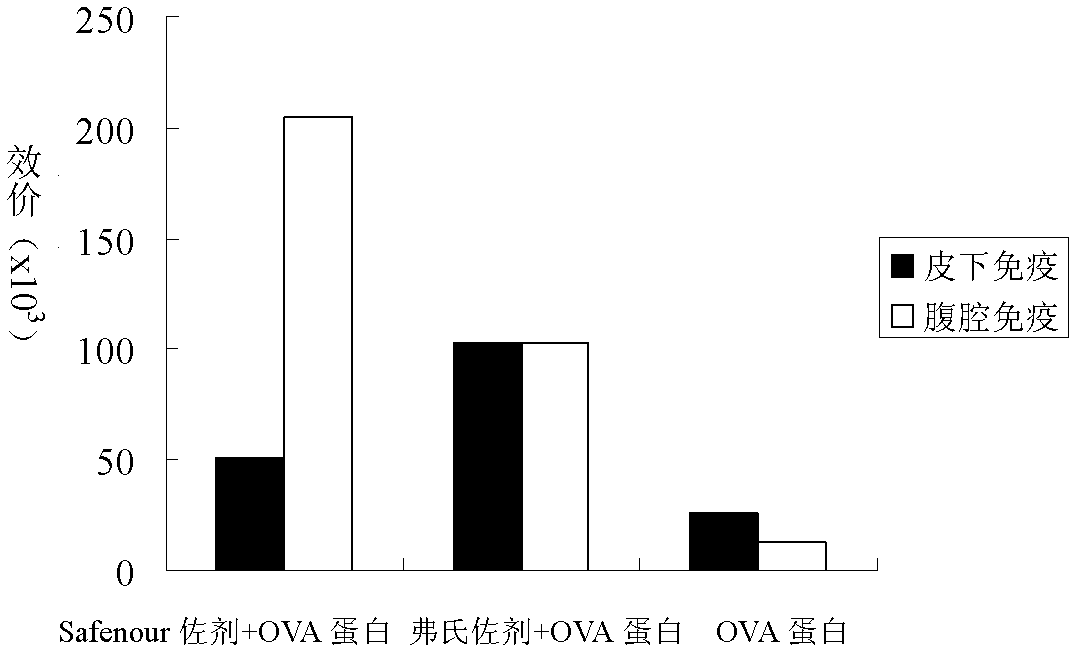
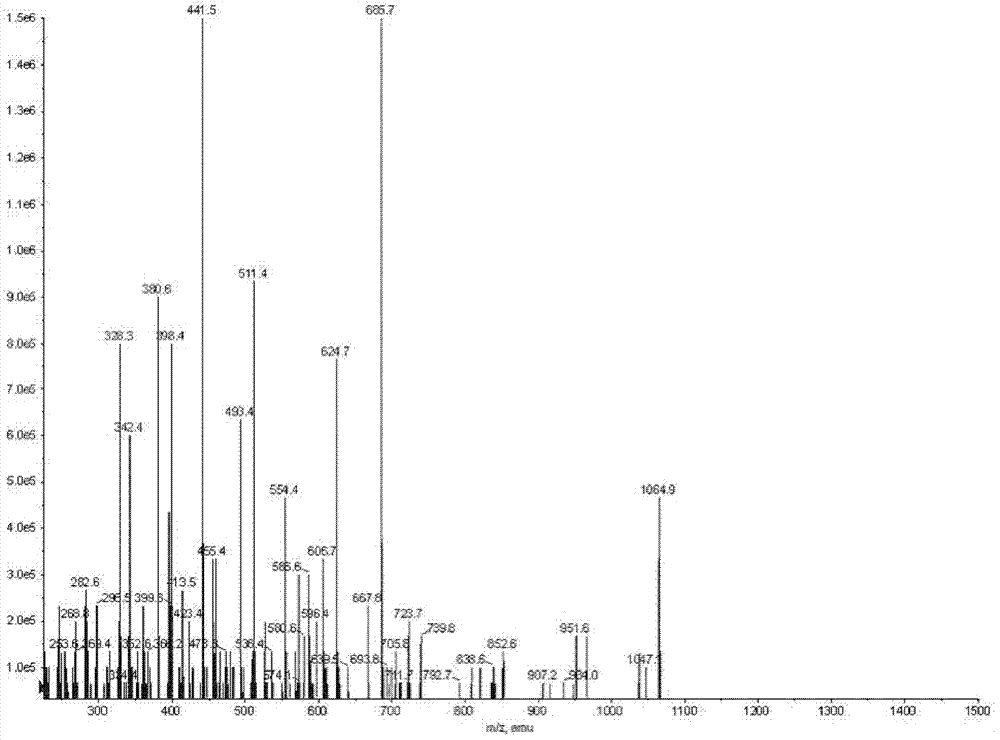
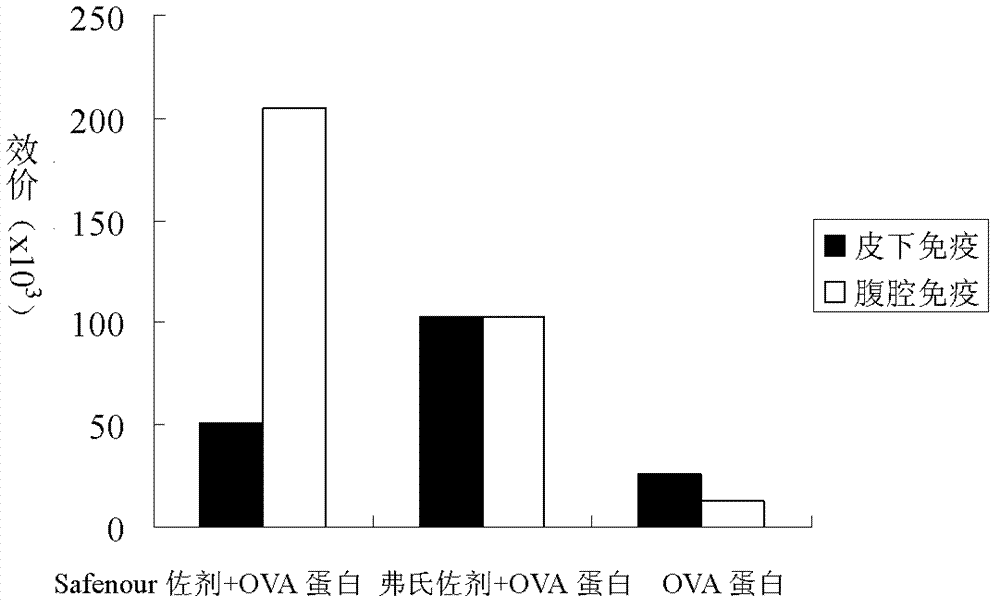
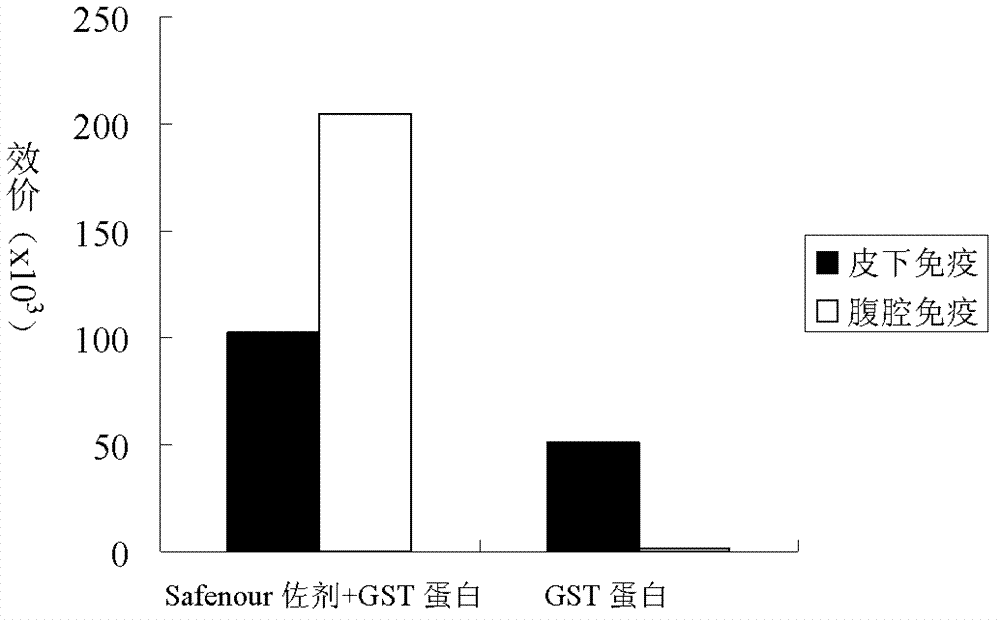
The invention discloses a Bacillus amyloliquefaciens WH3 strain, and a preparation method and application thereof. The preparation method comprises the following steps: 1, separation and identification of bacteria: separating bacteria resistant to rape sclerotinia rot from rape seedlings, and carrying out 16SrDNA and morphological identification to determine that the WH3 strain obtained by separation is Bacillus amyloliquefaciens; 2, separation, purification and identification of an antifungal active substance Safenour: fermenting the WH3 strain, extracting the antifungal active substance, separating and purifying through a sephadex column, and carrying out MALDI-TOF (matrix-assisted laser desorption / ionization-time of flight ) mass spectrometry on the active antifungal substance to inferthat the substance is a ring type polypeptide; and 3, application of the strain in the preparation of vaccines and immunological adjuvants. After the Safenour used as the adjuvant is mixed with a protein antigen and mice are respectively immunized through oral administration and injection of the mixture, effective body fluid and cellular immune response can be activated, and a high-titer specificantibody can be detected in the blood serum. The Safenour has low production cost and high stability, does not need to be emulsified when mixed with the antigen, and has a better immunoenhancement effect in comparison with Freund adjuvants and cholera toxin B subunits.
Owner:武汉光谷世傲生物科技有限公司
Cell inactivated vaccine, egg yoke antibody and injection and freeze-dried powder containing same
InactiveCN102861327AReduce lossesImproving immunogenicityPowder deliveryEgg immunoglobulinsDiseaseHighly pathogenic
The invention discloses cell inactivated vaccine which simultaneously contains porcine circovirus epitopes and goose circovirus epitopes. The cell inactivated vaccine is prepared by the method including screening high-pathogenicity strains from porcine circoviruses; selecting a plurality of virus epitopes; connecting the epitopes with one another; then inserting the connected epitopes into genomes of goose circoviruses to proliferate; and adding Freund adjuvants to formaldehyde for inactivation so as to obtain the cell inactivated vaccine. The invention simultaneously discloses an egg yolk antibody prepared from the cell inactivated vaccine, injection and freeze-dried powder containing the egg yolk antibody. Genes of the porcine circovirus are inserted into the genomes of the goose circoviruses, the immunogenicity of the goose circoviruses is strengthened, immune systems of goose bodies are excited, so that the titer of generated antibodies is higher than that of egg yolk antibodies generated by porcine circovirus vaccine used singularly, a clinical application effect is good, and generated circovirus antibodies have an auxiliary treatment effect for porcine circovirus diseases.
Owner:河南后羿生物工程股份有限公司
Acute monocytic leukemia-associated antigen MLAA-34 epitope polypeptide, and vaccine and pharmaceutical application of acute monocytic leukemia-associated antigen MLAA-34 epitope polypeptide
The invention discloses an acute monocytic leukemia-associated antigen MLAA-34 epitope polypeptide and an application of the acute monocytic leukemia-associated antigen MLAA-34 epitope polypeptide, and belongs to the technical field of biology. The acute monocytic leukemia-associated antigen MLAA-34 epitope polypeptide disclosed by the invention can induce specific CTL, and simultaneously can express tumor cells of MLAA-34 and HLA-A*0201, and T2 cells of loaded peptide in vitro to generate the determined specific killing effect; the epitope polypeptide CP701 is applied to preparation of a polypeptide vaccine aiming at acute monocytic leukemia; and the polypeptide vaccine composed of an MLAA-34 epitope peptide CP701 (236ILDRHNFAI244), a T auxiliary epitope and an incomplete Freund adjuvant can inhibit hu-PBL-SCID amplification of tumors in mice loading human monocytic leukemia cells THP-1, prolongs the survival life, and can induce the specific CTL killing activity and NK cytotoxic activity in vivo.
Owner:XI AN JIAOTONG UNIV
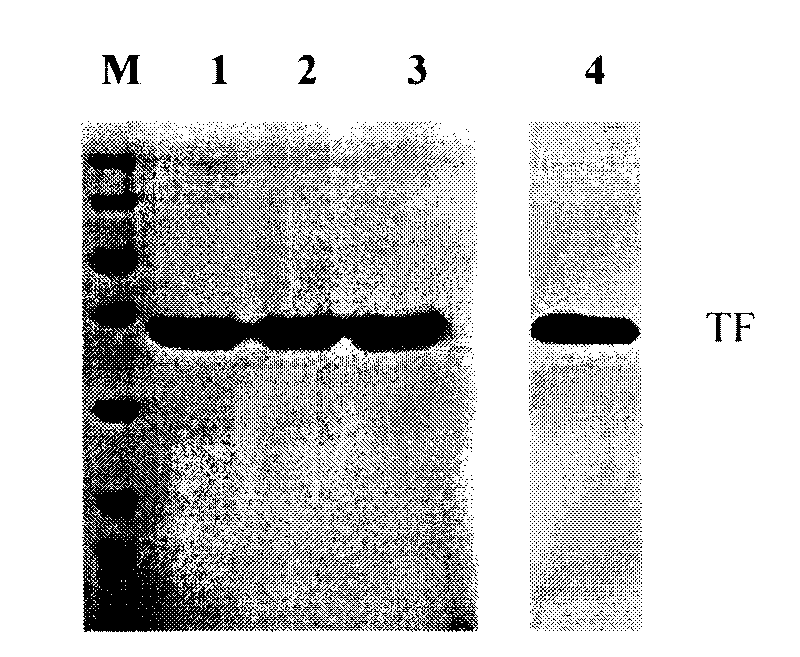
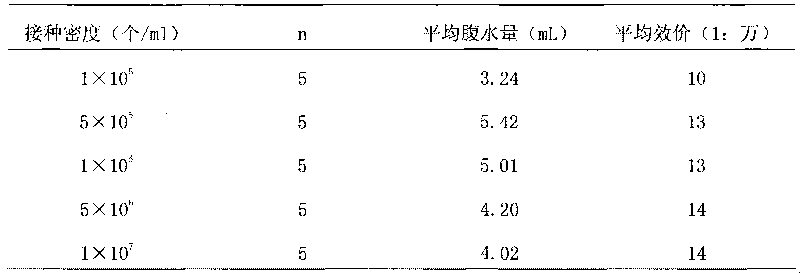
Method for preparing antihuman recombinant tissue factor monoclonal antibody
ActiveCN101717447AStrong specificityEasy to prepareImmunoglobulins against cell receptors/antigens/surface-determinantsFreund adjuvantElisa method
The invention discloses a method for preparing an antihuman recombinant tissue factor monoclonal antibody. The method comprises the following steps: combining a truncated human recombinant tissue factor obtained by purification after prokaryotic expression with a freund adjuvant immunized mouse; fusing mouse spleen cells with myeloma cells; selectively cultivating and screening an HAT selective medium; after the subcloning cultivation is subjected to limiting dilution, selecting target hybridoma cells by an ELISA method, performing massive proliferation, and injecting into an abdominal cavity of a syngeneic mouse to prepare ascites; and obtaining the antihuman recombinant tissue factor monoclonal antibody which has excellent potency and higher yield. The antihuman recombinant tissue factor monoclonal antibody prepared by the method of the invention has the advantages of high sensitivity and strong specificity, can be used for immunoaffinity purification of the human recombinant tissue factor, and also can be used for research and development of anticoagulant medicaments and medicaments for treating tumors.
Owner:山西省生物研究院有限公司 +1
Amyloid protein intra-membrane segment for treating Alzheimer disease and application thereof
InactiveCN102229651AImprove cognitive functionOvercoming the disadvantages of subacute meningitisNervous disorderPeptide/protein ingredientsAcute meningococcaemiaSide effect
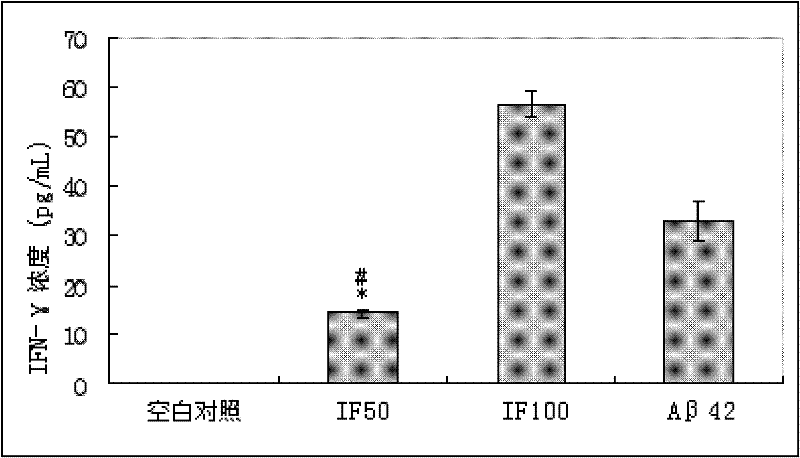
The invention relates to an amyloid protein intra-membrane segment for treating Alzheimer disease and an application thereof, in particular to the application of the amyloid protein intra-membrane segment in the preparation of a vaccine medicament for treating Alzheimer disease. The problem of treating Alzheimer disease can be efficiently solved. The technical scheme provided by the invention is to choose an intra-membrane segment (IF-A beta segment), which is called as AIIGLMVGGVVIA for short, from amino acid sequences of amyloid proteins (A beta42) of 42 amino acids. In a use process, after the synthesized IF-A beta and a Freund adjuvant are mixed and injected to an immunized AD (Alzheimer Disease) model mouse, the A beta42 plaques can be efficiently eliminated, the cognitive function of the AD mouse can be improved and the defect of the sub-acute meningitis caused by using A beta42 as an immunogen can be overcome, thereby developing a therapeutic medicament for efficiently treating AD without side effect.
Owner:CENT SOUTH UNIV
Metformin monoclonal antibody hybridoma cell line and application thereof
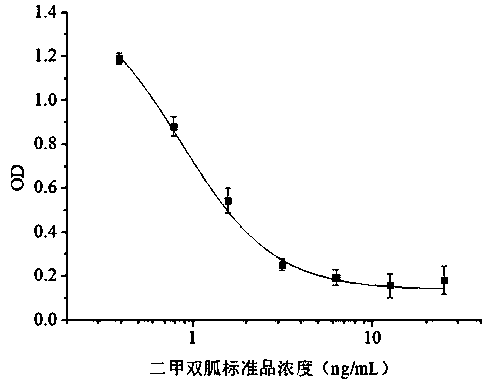
ActiveCN110616195AImprove featuresHigh detection sensitivitySerum albuminBiological material analysisBALB/cFreund adjuvant
The invention discloses a metformin monoclonal antibody hybridoma cell line and application thereof, and belongs to the field of food safety immunoassay. The prepared metformin monoclonal antibody hybridoma cell line Tcf 1A6 is deposited in the general microbiology center of the china microbial species collection management committee, the deposit date is March 7, 2019, and the preservation numberis CGMCC No. 17399. According to the metformin monoclonal antibody hybridoma cell line and application thereof, the complete antigen of the metformin is mixed and emulsified with an equivalent amountof Freund adjuvant, and BALB / c mice is immunized through multi-point subcutaneous injections of neck and back parts to screen hybrid cells after fusion of two kinds of cells; and then cells are screened through indirect competitive enzyme-linked immunosorbent assay and subcloned for three times, and the monoclonal antibody hybridoma cell line Tcf 1A6 is finally obtained. The provided monoclonal antibody secreted by the cell line Tcf 1A6 has better specificity and detection sensitivity to MET(the IC<50> value is 1ng / mL), the residual amount of MET in chicken, chicken liver, and feed can be detected, raw materials are provided for the immunoassay of the MET residues in food, and the practical applicational value is obtained.
Owner:JIANGNAN UNIV
Listeria monocytogenes monoclonal antibody hybridoma cell strain and application thereof
ActiveCN104178458AImmunoglobulins against bacteriaMicroorganism based processesBALB/cFreund adjuvant
The invention relates to a Listeria monocytogenes monoclonal antibody hybridoma cell strain and an application thereof and belongs to the technical field of food safety immunological detection. According to the invention, a complete antigen of a specific polypeptide of Listeria monocytogenes is uniformly mixed with an equal volume of Freund adjuvant (Sigma), BALB / c mice are immunized by subcutaneous injection, after the BALB / c mice are immunized for four times, spleen cells of immunized mice are fused with mice myeloma cells of immunized mice by virtue of a PEG method, an indirect ELISA screening and three times of subcloning are carried out to obtain a monoclonal cell strain A. The monoclonal antibody secreted by virtue of the monoclonal cell strain A has cross reaction with different Listeria monocytogenes culture supernatants and no cross reaction with listeria except Listeria monocytogenes, E. coliO 157, Salmonella and Campylobacter jejuni and thus the Listeria monocytogenes monoclonal antibody hybridoma cell strain can be used in the specific detection of Listeria monocytogenes in a food.
Owner:无锡迪腾敏生物科技有限公司 +1
Medicinal composition for treating rheumatoid arthritis, and preparation method and application thereof
InactiveCN102579601AGood treatment effectSafe and effective treatmentAntipyreticComponent separationMedicinal herbsSide effect
The invention discloses a medicinal composition for treating rheumatoid arthritis. The medicinal composition is prepared from the following Chinese herbal medicines in part by weight: 2.5 to 10 parts of periploca forrestii schltr, 1 to 4 parts of gentiana macrophylla and 1 to 4 parts of yanhusuo. The invention also discloses a preparation method and a quality detection method for the medicinal composition, and application of the medicinal composition. Through reasonable compatibility of the periploca forrestii schltr, the gentiana macrophylla and the yanhusuo, the medicinal composition has an obvious curative effect on arthritis induced by a complete freund adjuvant, good anti-inflammatory and analgesic effects, a better pharmaceutical effect compared with the simple medicinal material and the medicine which is prepared from other Chinese herbal medicines, and small toxic and side effects and achieves a synergistic effect. The modern pharmological study proves that the medicinal composition can safely and effectively treat rheumatoid arthritis and provides a new choice for clinical treatment.
Owner:SICHUAN NORMAL UNIVERSITY
Application of krait venom antibacterial peptide in preparation of medicine for resisting inflammatory factor
InactiveCN103316327AEnhanced inhibitory effectApparent scavenging activityPeptide/protein ingredientsAntipyreticInflammatory factorsDPPH
The invention relates to an application of krait venom antibacterial peptide in the preparation of a medicine for resisting inflammatory factor and belongs to the field of biomedical technology. A lot of researches show that the krait antibacterial peptide has an obvious effect of inhibiting inflammatory factor secretion induced by lipopolysaccharide in vitro and inhibits inflammatory factor secretion through inhibiting MAPK signal channels; the krait venom antibacterial peptide has a remarkable scavenging activity of DPPH and 2,2-azine-bis(3-ethylbenzothiazole-6-sulfoacid)diammonium salt (ABTS+)radical in vitro and has an obvious effect of inhibiting mice inflammation induced by a complete Freund adjuvant in vivo; and the krait venom antibacterial peptide can be applied in the preparation of a medicine for resisting inflammatory factor.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Immunolatex microsphere for detecting CpTI and preparation method thereof
InactiveCN102109521ASmall particlesUniform particle sizeCarrier-bound/immobilised peptidesMaterial analysisRestriction enzyme digestionFreund adjuvant
The invention relates to an immunolatex microsphere for detecting a cowpea trypsin inhibitor (CpTI) and a preparation method thereof. The immunolatex microsphere is coated with a CpTI-resistant protein polyclonal antibody and has the particle size of 200nm. The preparation method of the immunolatex microsphere comprises the following steps of: designing a full-length CpTI gene sequence; inserting a CpTI gene fragment into a glutathione-S-transferase (GST) gene fusion expression vector through restriction enzyme digestion to construct a bacteria fusion expression vector; purifying by using an affinity chromatographic column to obtain GST-CpTI fusion protein; fusing a GST-CpTI fusion protein antigen into a Freund adjuvant to immunize a mouse, and separating and purifying to obtain an anti-mouse CpTI polyclonal antibody; and coupling the antibody with the activated microsphere by a chemical coupling method to obtain the immunolatex microsphere for detecting the CpTI protein. The invention has the advantages that: the preparation method is high in sensitivity and repeatability and easy to operate; and the prepared immunolatex microsphere has small and uniform particle size, the lowest detectable limit of 0.2mu g / ml of the CpTI fusion protein, and the related coefficient R2 of 0.67.
Owner:CHINESE RES ACAD OF ENVIRONMENTAL SCI
Application of xanthiazone compound in preparing drug for preventing or treating rheumatoid arthritis
InactiveCN108283643ASuppress generationGood inhibitory effectOrganic active ingredientsSugar derivativesFibroblast-like synoviocyteFreund adjuvant
The invention relates to the technical field of medicines, in particular to an application of an xanthiazone compound in preparing a drug for preventing or treating rheumatoid arthritis. A formula ofthe xanthiazone compound is shown as follows as shown in the specification. The compound significantly inhibits NO generation for RAW264.7 inflammatory cells and fibroblast-like synoviocytes stimulated by LPS (lipopolysaccharide), and has an obvious inhibiting effect on mouse ear swelling caused by dimethylbenzene and the rheumatoid arthritis induced by collagen, and an obvious swelling diminishing and anti-inflammatory effect on rat toe swelling due to inflammation caused by a Freund adjuvant; and therefore, the compound can be used for preventing and treating the rheumatoid arthritis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
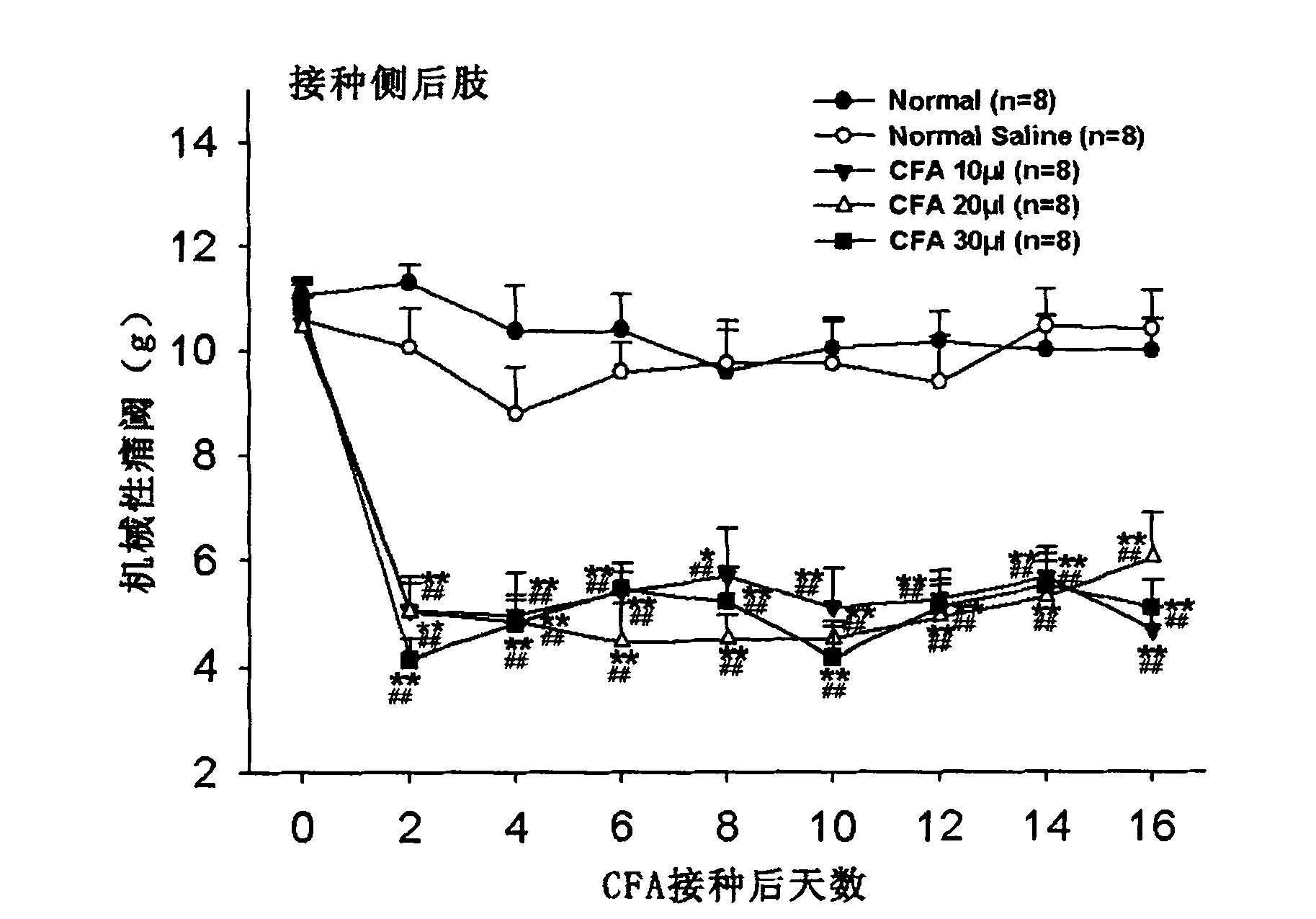
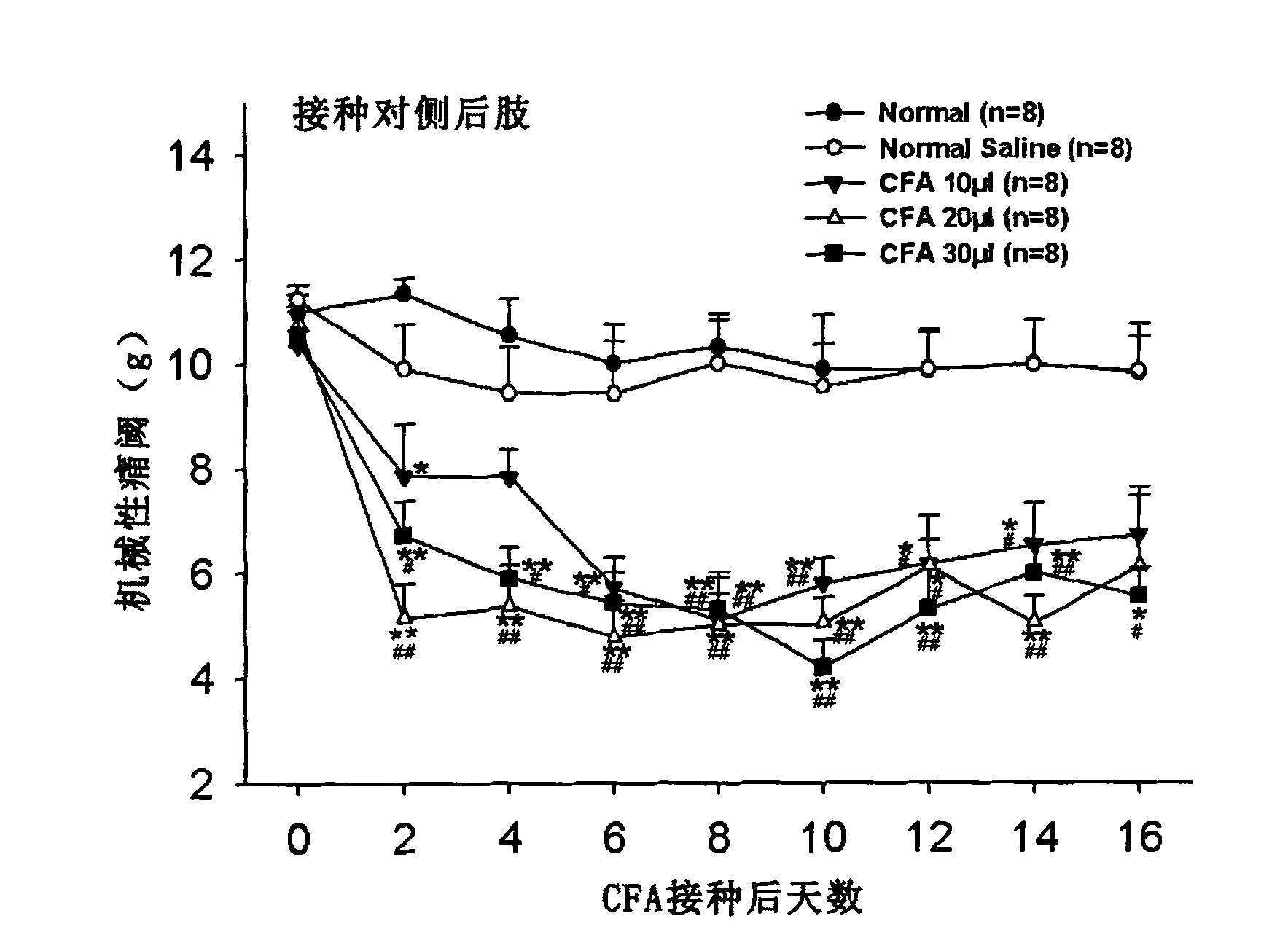
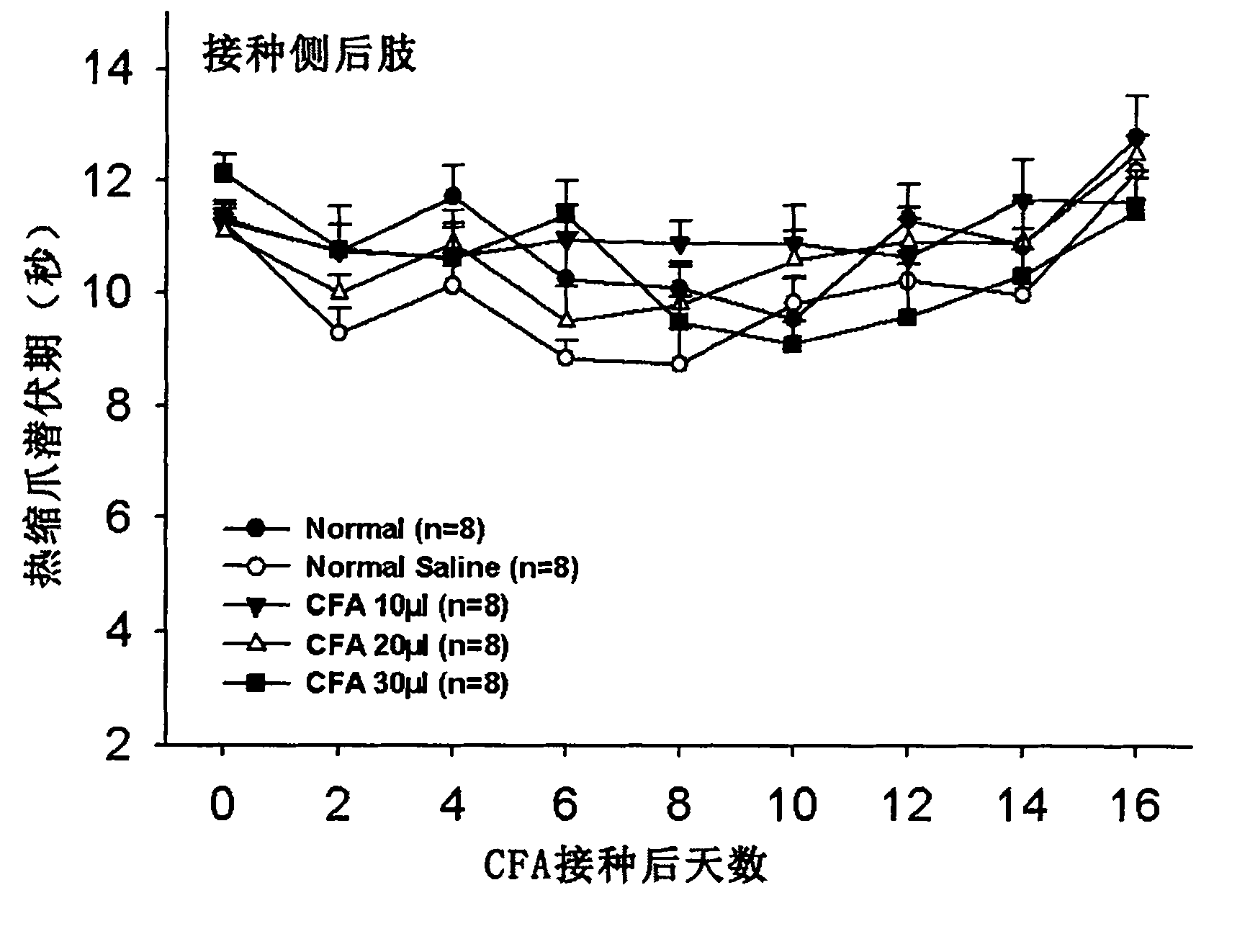
Application of complete Freund adjuvant (CFA) to preparation of tibia inflammatory pain model
Owner:FUDAN UNIV
Method for producing animal model of essential thrombocytopenia
InactiveCN101480493AIncreased sensitivityEasy to operateIn-vivo testing preparationsAnimal husbandryWater bathsBALB/c
The invention relates to a preparation method which comprises the following steps: taking a BALB / C mouse, extracting eye balls or sampling blood from an orbitall vein, carrying out EDTA-Na2 anticoagulation, separating a blood platelet in a gradient centrifugation manner and washing, and adjusting the concentration into 1 to 2*10 / L; taking the suspension liquid of the blood platelet to be respectively mixed with equivalent complete freund adjuvant and incomplete freund adjuvant to form a milk white oil pocket water-shaped admixture which is used as antigen liquid; taking the antigen liquid containing the complete freund adjuvant to be respectively injected into the rear foot palm, the back and the groin of a guinea pig for four times, wherein each injection contains at least 5 points and the injection amount of each point is 100 microlitres; taking the whole blood of the guinea pig at the sixth week and taking blood serum for standby after the whole blood is centrifuged; taking the antiserum from -20 DEG C and placing the antiserum after being melted completely into water bath of 56 DEG C for 30 min; injecting the antiserum to the abdominal cavity of the BALB / C mouse on the first, the third, the fifth, the seventh, the ninth, the eleventh and the thirteen days and taking out on the fifteenth days, wherein the injection amount of each time is 100 microlitres. The invention solves the problem of shorter maintenance time of the blood platelet. The stable and controllable animal model of the idiopathic thrombocytopenic purpura of the mouse can be prepared.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
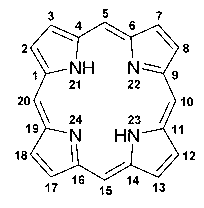
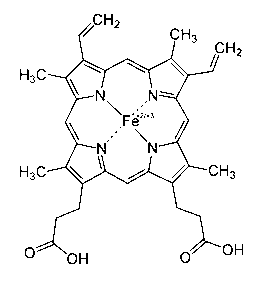
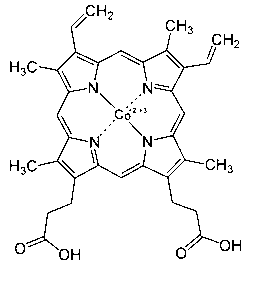
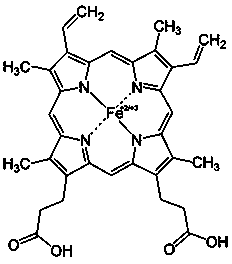
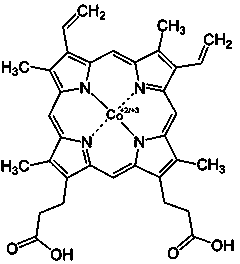
Application of porphyrin pigment serving as immunologic adjuvant and vaccine
InactiveCN103127500AEfficient stimulationQuick clearAntiviralsImmunological disordersFreund adjuvantPorphyrin
The invention discloses application of a porphyrin pigment serving as immunologic adjuvant and further discloses application of the porphyrin pigment serving as a vaccine. The porphyrin pigment is a biological pigment, tissues outside an animal cardiovascular system can be combined with a pathogeny recognition receptor TLR4, and tissue damage and danger signals are simulated to build efficient stimulation to an immune system. The porphyrin pigment is also an ingredient of an animal and a human body, internal metabolic pathways are clear, uncertainty in the safety aspect does not exist, and the porphyrin pigment is suitable for preparation of the immunologic adjuvant and the vaccine for the animal, especially for human. The porphyrin pigment serving as the immunologic adjuvant can remarkably strengthen the humoral immunity effect of antigen or the vaccine and can reach the level of complete Freund adjuvant.
Owner:SUN YAT SEN UNIV
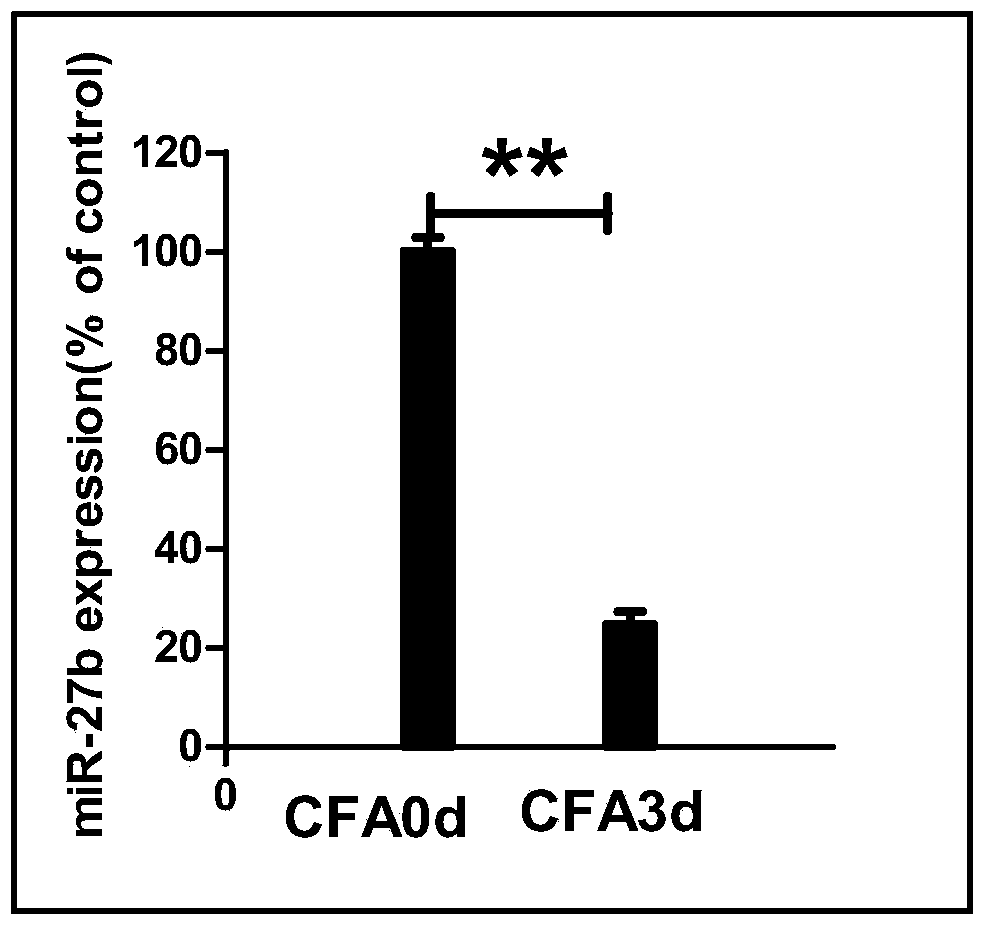
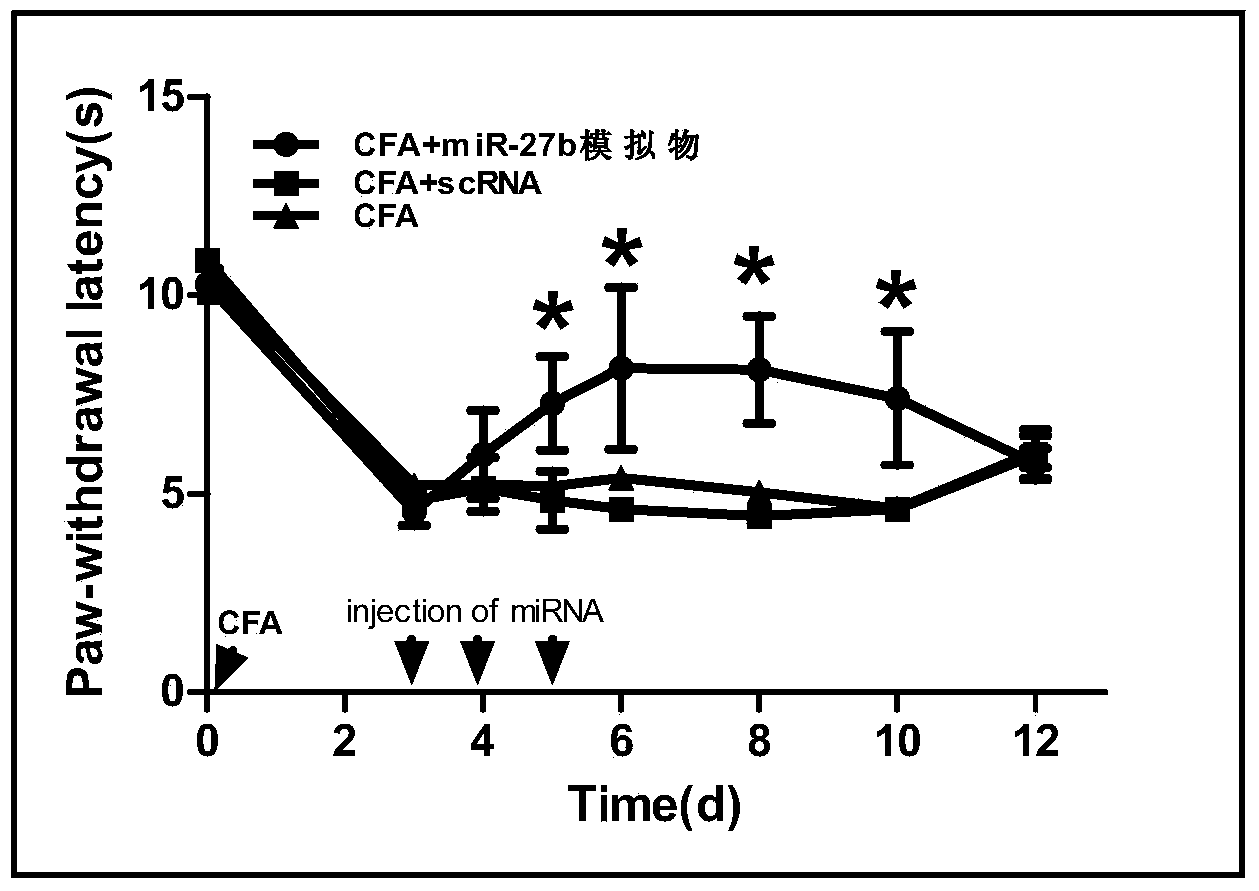
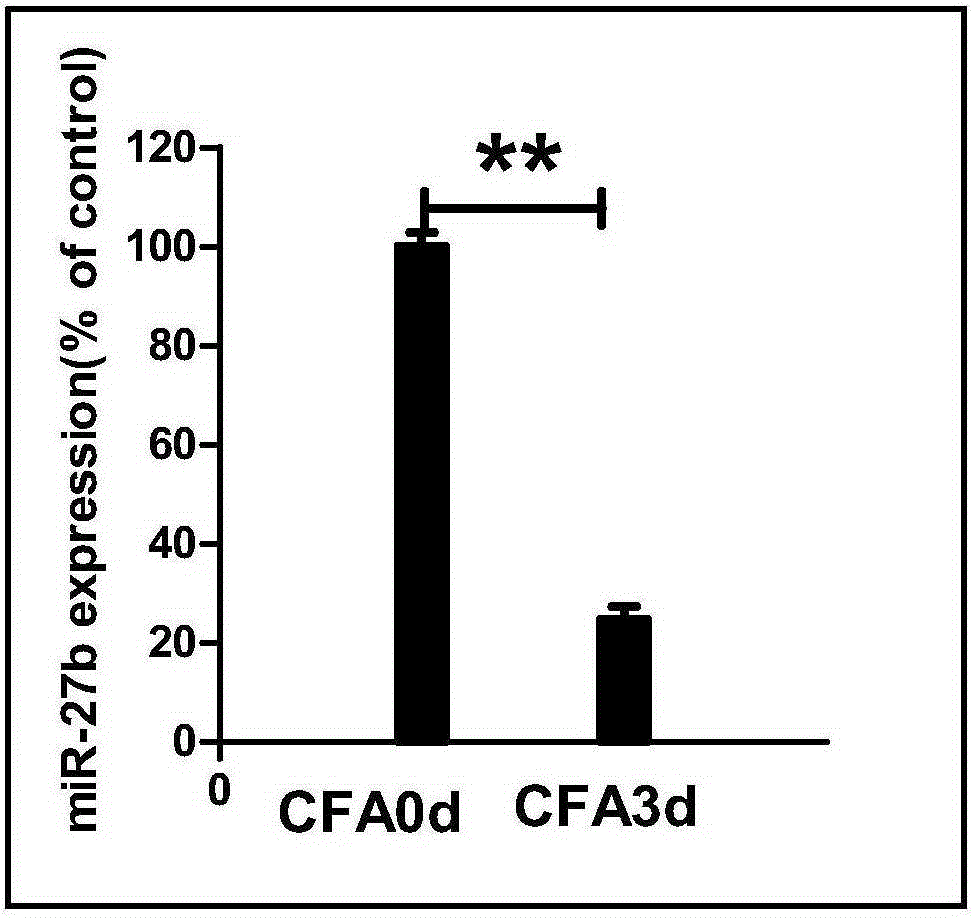
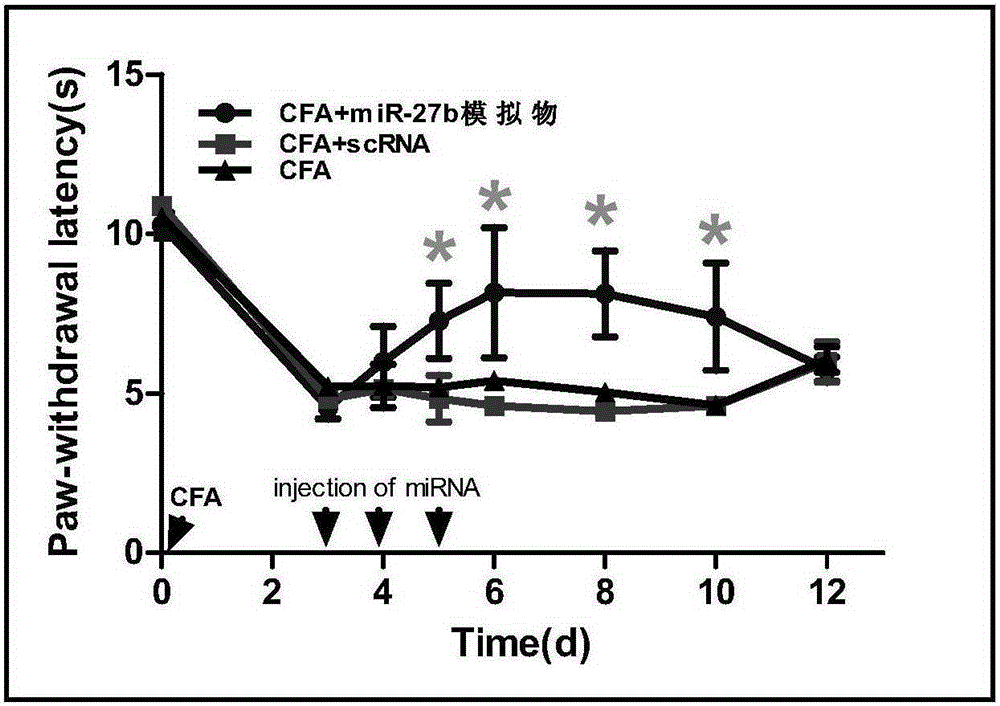
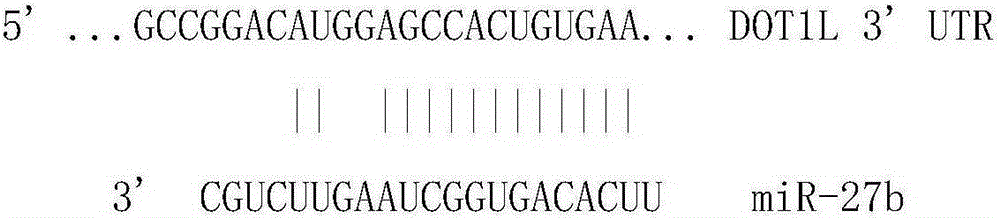
Application of miR-27b compound used as chronic pain marker and application of miR-27b compound in preparing medicament for treating chronic pain
InactiveCN104046688ALower heat shock thresholdClear pathogenesisNervous disorderAntipyreticActivation methodPain behavior
The invention relates to application of a miR-27b compound used as a chronic pain marker and application of the miR-27b compound in preparing a medicament for treating chronic pain. The application comprises the following steps: (1) screening miR-27b target genes, verifying by use of a reporter gene, and making an inflammatory chronic pain model mouse by utilizing a CFA (Complete Freund Adjuvant); (2) increasing the expression quantity of miRNA in the spinal cord of the inflammatory chronic pain model mouse by utilizing a miR-27b simulant; (3) detecting the inhibiting action of miR-27b on the DOT1L protein expression of the CFA causing inflammatory chronic pain by utilizing a Western blot method; (4) detecting a pain behavior by utilizing a heat activation method. The process is simple and the cost is low; the inflammatory chronic pain is effectively inhibited by adopting the miR-27b and an effective intervention medicament for preventing and treating the chronic pain is provided. The invention particularly relates to the application of the miR-27b compound used as the chronic pain marker and the application of the miR-27b compound in preparing the medicament for treating the chronic pain. The application disclosed by the invetnion indicates that the miR-27b can be taken as the marker of chronic pain generation by further carrying out quantitative analysis on the clinical blood specimen of a chronic pain patient.
Owner:XUZHOU MEDICAL COLLEGE
Method for gradient production of goat anti-Cys-C multiple resistant serum by means of amount of antigens
InactiveCN104211806AImprove efficiencyConvenient medical diagnosisSerum immunoglobulinsImmunoglobulins against animals/humansFreund adjuvantAntiserum
The invention discloses a method for gradient production of goat anti-Cys-C multiple resistant serum by means of amount of antigens. The method comprises the following steps: preparation of an immunogen emulsion: mixing and emulsifying 0.1ml of serum containing 4mg of the Cys-C antigen, 1.9ml of sterile PBS and 2ml of freund adjuvant to obtain an immunogen emulsion; first injection for goal immunity: injecting in two regions of each groin of front and rear groins of a goat, totaling 12 injection points; carrying out subcutaneous injection: performing average injecting at two injection points on two sides of the neck; injecting for the second time every 20 days, injecting for 3-8 doses every 10 days sequentially, and sampling blood from the artery of the immune goat every 4 days; putting the blood in a water tank for 1 hour at 40 DEG C; and after serum is fully separated out, centrifugalizing and precipitating to separate antiserum. The Cys-C antigen is subcutaneously injected to the groins of the goat for booster immunization for many times, so that the generated high-efficient goat anti-Cys-C antibody valence can reach over 1:16.
Owner:ZHENJIANG WANSHAN HONGBIAN AGRI PARK
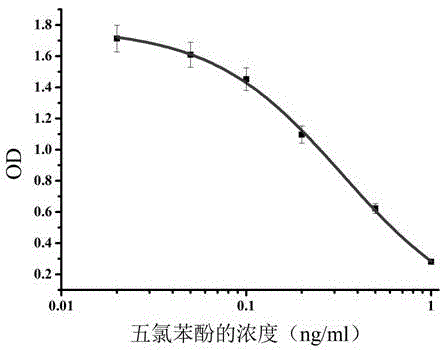
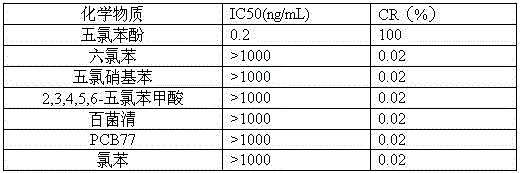
Pentachlorophenol monoclonal antibody hybridoma cell strain 2C3 and application thereof
ActiveCN105462933AHigh detection sensitivityImprove featuresFused cellsImmunoglobulinsBALB/cPentachlorophenol
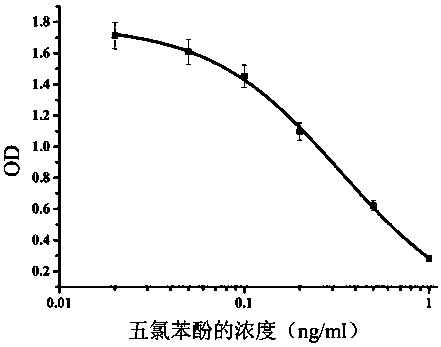
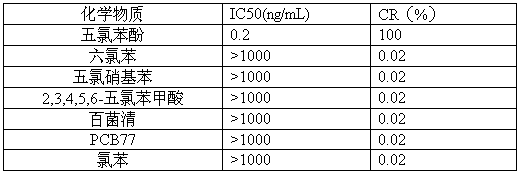
The invention discloses a pentachlorophenol monoclonal antibody hybridoma cell strain 2C3 and application thereof, and belongs to the field of food safety immunological detection. The pentachlorophenol monoclonal antibody hybridoma cell strain 2C3 is collected in the China General Microbiological Culture Collection Center (CGMCC) with a collection number CGMCC No.10871. A preparation method of the hybridoma cell strain 2C3 comprises the following steps: mixing a pentachlorophenol complete antigen and an equivalent Freund's adjuvant, and emulsifying the mixture, and immunizing a BALB / c mouse through back subcutaneous injection, wherein a complete Freund's adjuvant is used in a first immunizing process, and an incomplete Freund adjuvant is used afterwards; fusing spleen cells of the efficient and low-cost IC50 (Half Maximal Inhibitory Concentration) mouse with myeloma cells of the mouse through a PEG (Polyethylene Glycol) method, and performing indirect-competitive ELISA (Enzyme-Linked Immuno Sorbent Assay) screening and three times of sub-cloning to obtain a hybridoma cell strain 2C3. A monoclonal antibody secreted by the cell stain has high specificity and detection sensitivity (the IC50 value is 0.2mumg / L) specific to pentachlorophenol, and can be used for detecting residual pentachlorophenol in food safety.
Owner:JIANGNAN UNIV
Application of porphyrin pigment serving as immunologic adjuvant and vaccine
InactiveCN103127500BEfficient stimulationQuick clearAntiviralsImmunological disordersFreund adjuvantPorphyrin
The invention discloses application of a porphyrin pigment serving as immunologic adjuvant and further discloses application of the porphyrin pigment serving as a vaccine. The porphyrin pigment is a biological pigment, tissues outside an animal cardiovascular system can be combined with a pathogeny recognition receptor TLR4, and tissue damage and danger signals are simulated to build efficient stimulation to an immune system. The porphyrin pigment is also an ingredient of an animal and a human body, internal metabolic pathways are clear, uncertainty in the safety aspect does not exist, and the porphyrin pigment is suitable for preparation of the immunologic adjuvant and the vaccine for the animal, especially for human. The porphyrin pigment serving as the immunologic adjuvant can remarkably strengthen the humoral immunity effect of antigen or the vaccine and can reach the level of complete Freund adjuvant.
Owner:SUN YAT SEN UNIV
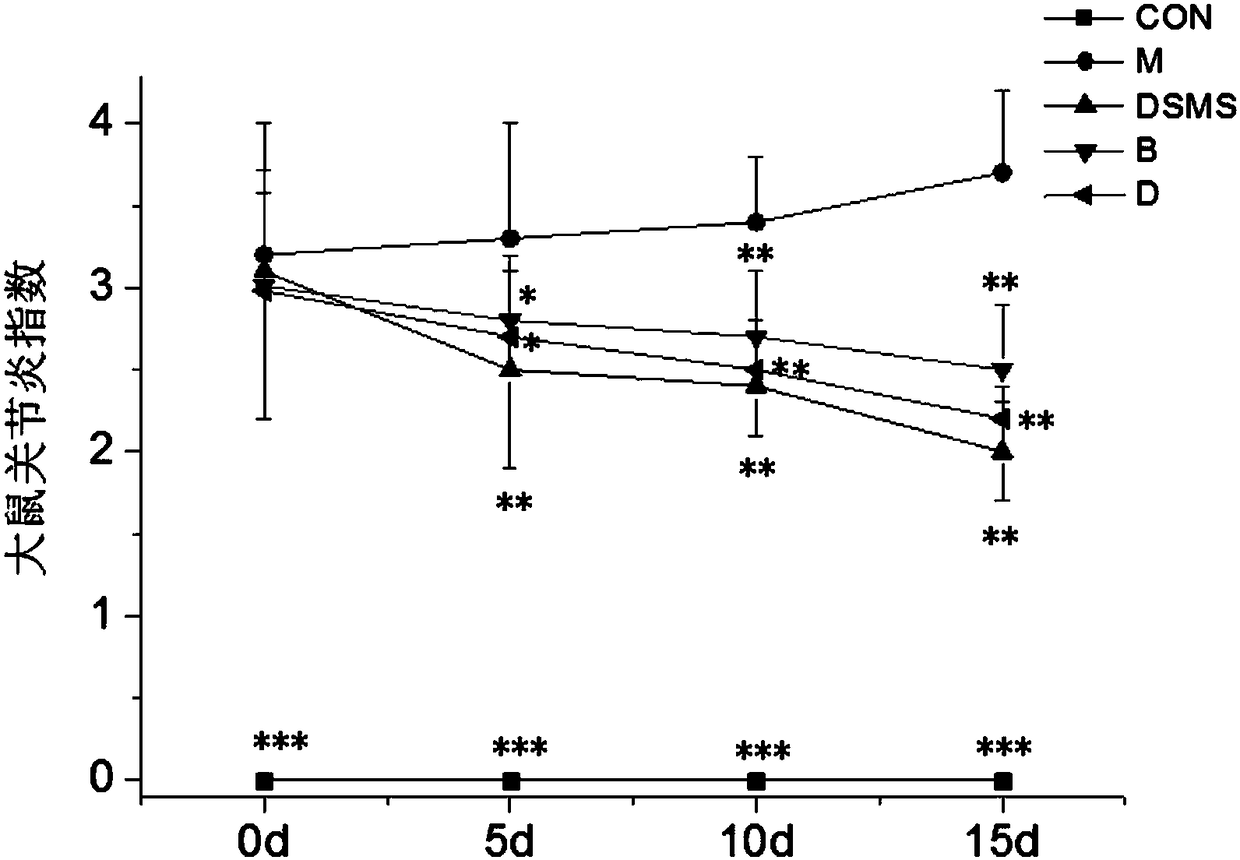
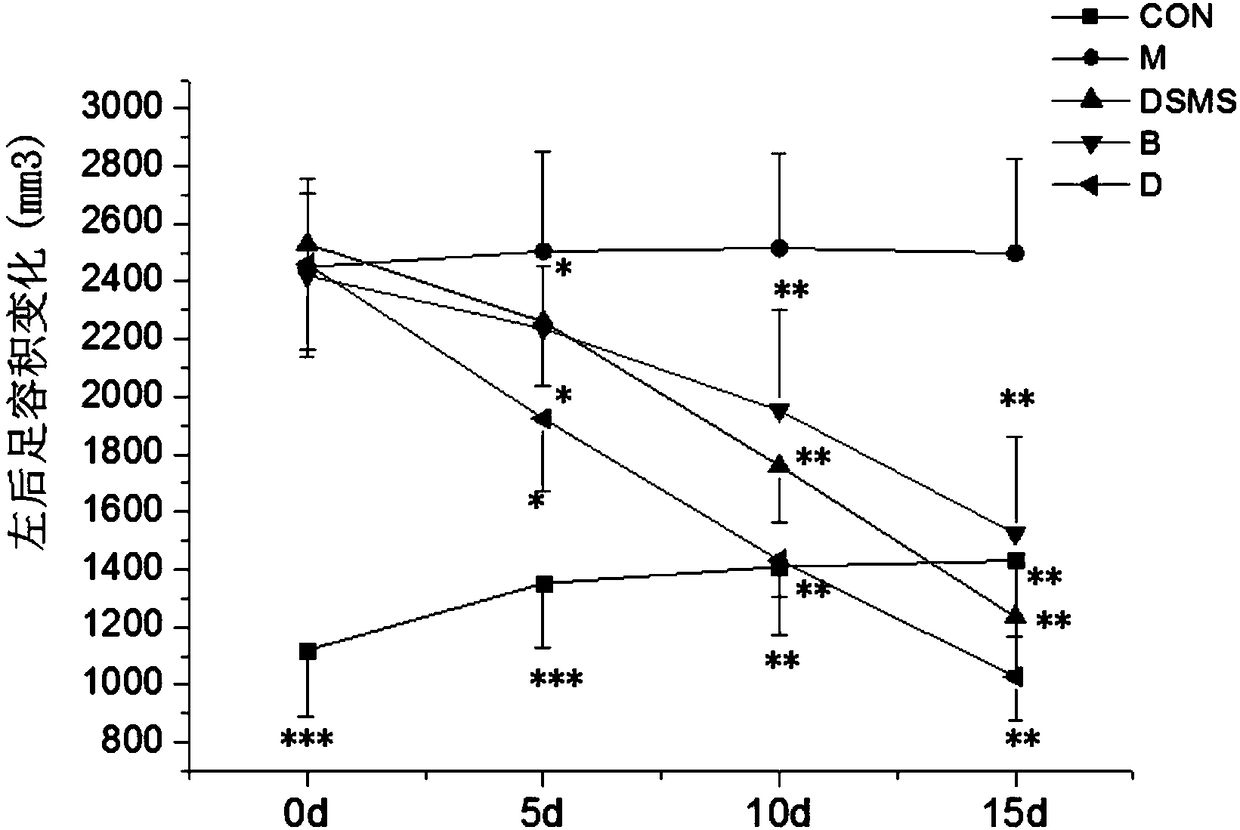
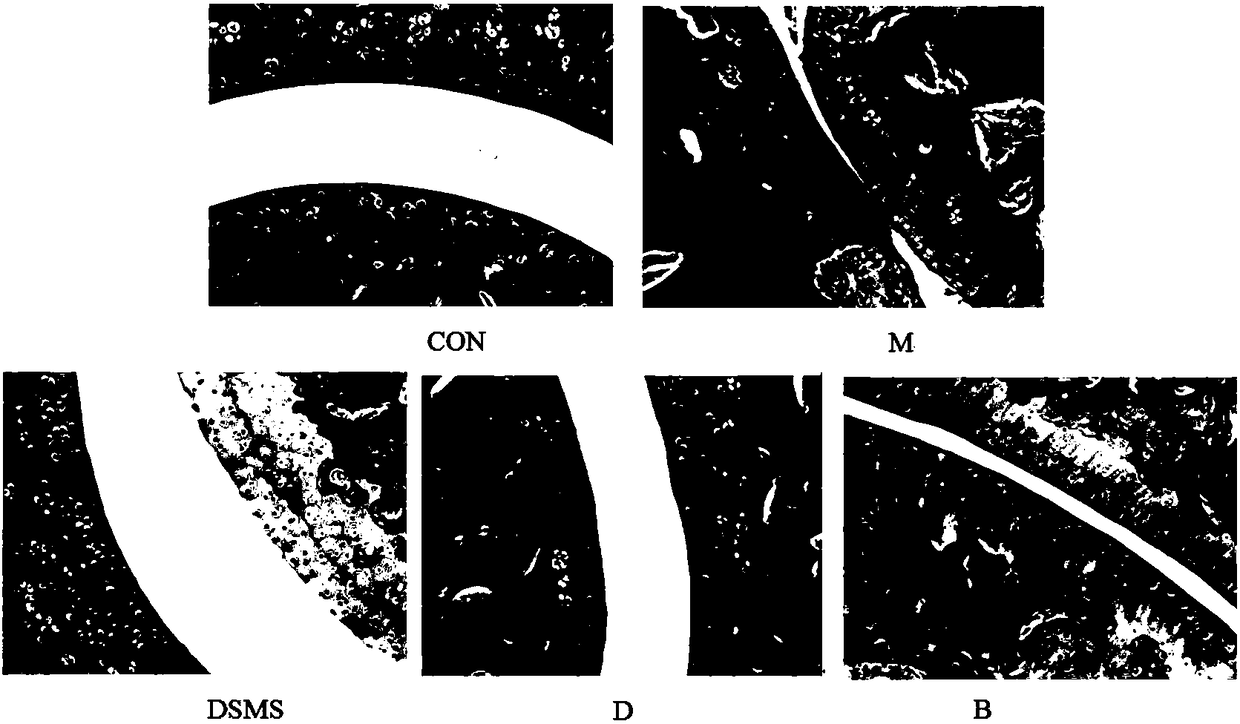
An inducer for establishing an animal model of rheumatoid arthritis and its preparation and application
ActiveCN103830721BEnhance immune responseEasy to operatePeptide/protein ingredientsEmulsion deliveryAcetic acidFreund adjuvant
Owner:四川普莱美行之生物科技有限公司
A pentachlorophenol monoclonal antibody hybridoma cell line 2c3 and its application
ActiveCN105462933BHigh detection sensitivityImprove featuresFused cellsImmunoglobulinsPentachlorophenolBALB/c
The invention discloses a pentachlorophenol monoclonal antibody hybridoma cell strain 2C3 and application thereof, and belongs to the field of food safety immunological detection. The pentachlorophenol monoclonal antibody hybridoma cell strain 2C3 is collected in the China General Microbiological Culture Collection Center (CGMCC) with a collection number CGMCC No.10871. A preparation method of the hybridoma cell strain 2C3 comprises the following steps: mixing a pentachlorophenol complete antigen and an equivalent Freund's adjuvant, and emulsifying the mixture, and immunizing a BALB / c mouse through back subcutaneous injection, wherein a complete Freund's adjuvant is used in a first immunizing process, and an incomplete Freund adjuvant is used afterwards; fusing spleen cells of the efficient and low-cost IC50 (Half Maximal Inhibitory Concentration) mouse with myeloma cells of the mouse through a PEG (Polyethylene Glycol) method, and performing indirect-competitive ELISA (Enzyme-Linked Immuno Sorbent Assay) screening and three times of sub-cloning to obtain a hybridoma cell strain 2C3. A monoclonal antibody secreted by the cell stain has high specificity and detection sensitivity (the IC50 value is 0.2mumg / L) specific to pentachlorophenol, and can be used for detecting residual pentachlorophenol in food safety.
Owner:JIANGNAN UNIV
Acupuncture serum exosome as well as preparation method and application thereof
InactiveCN113528435AEffectively exert the "acupuncture-like" effectProduces analgesic and anti-inflammatory effectsCell dissociation methodsAntipyreticAdjuvantAcupuncture treatment
The invention belongs to the technical field of biology, and discloses an acupuncture serum exosome and a preparation method and application thereof. The preparation method comprises the steps that S1, a rheumatoid arthritis model is established, specifically, a complete Freund adjuvant is injected to the sole of the right posterior foot of a rat to cause inflammation, after 24 hours, interventional electro-acupuncture treatment is conducted on the rat, the sole pain threshold of the rat is measured by using a full-automatic thermal radiation stimulator, and whether the rheumatoid arthritis model is successfully molded or not according to a pain threshold measurement result is judged; S2, serum extraction is conducted, specifically, abdominal aorta blood sample collection is conducted on the successfully molded rat, the blood sample is allowed to stand for 2 hours, centrifuging is conducted for 10 minutes in a centrifugal machine with the temperature of 4 DEG C and the speed of 2000rpm, after centrifugation, supernate is taken as serum and subpackaged in EP tubes, 500 microliters of serum is subpackaged in each EP tube, and all the EP tubes are put into a refrigerator at the temperature of -80 DEG C to be cryopreserved for use; and S3, exosome extraction is conducted, specifically, exosome is extracted from the serum extracted in the step S2 to obtain the acupuncture-like serum exosome capable of being used for treating rheumatoid arthritis.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
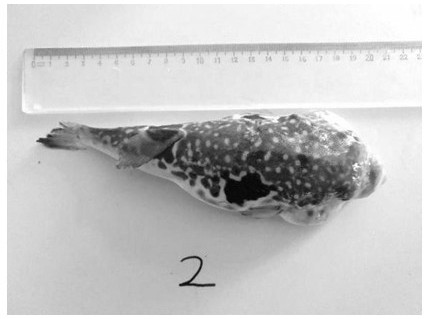
Preparation method of marine filariasis vaccine of redfin puffer
ActiveCN108743930BGood prevention effectReduce economic lossProtozoa antigen ingredientsAntiparasitic agentsIntraperitoneal routeAdjuvant
The invention discloses a method for preparing a marine filariasis vaccine of redfin puffer. A single marine filariasis is picked and inoculated in L-15 complete medium for purification and cultivation, and then enlarged and cultivated, and 100 μl / L of Foreria is added. Marin killed the larvae, centrifuged at 4000rpm in a low-speed centrifuge for 10 minutes to collect the precipitate, added L‑15 medium to prepare an inactivated larvae liquid with a density of 2 million / ml, collected in a 1.5ml centrifuge tube, and stored at ‑80°C. Before immunization, thaw the dead larvae on ice, crush them with electromagnetic waves, mix them with the same amount of Freund's adjuvant or Freund's incomplete adjuvant, and shake the mixture until it is in the form of emulsion, which is to prepare the marine tail silk of puffer puffer Insect vaccines. Intraperitoneal injection can improve the immunity of pufferfish against marine filariasis.
Owner:DALIAN OCEAN UNIV
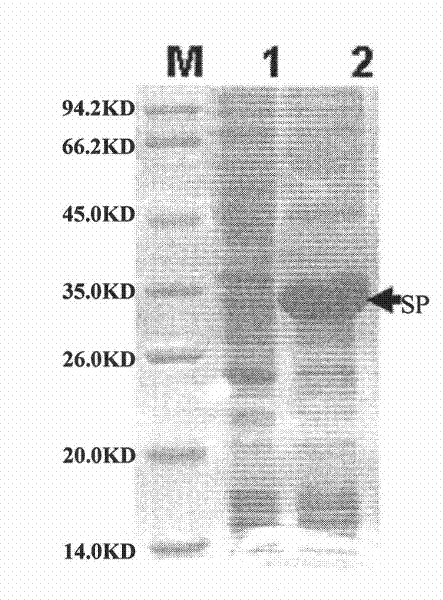
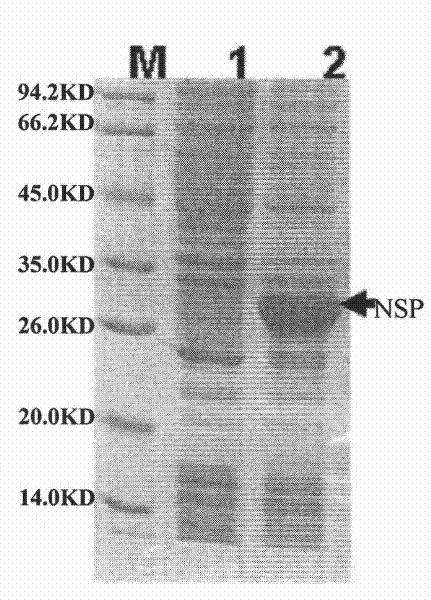
Vaccine for preventing porcine reproductive and respiratory syndrome
The invention discloses a vaccine for preventing porcine reproductive and respiratory syndrome. The vaccine provided by the invention comprises active ingredients consisting of NSP polypeptide and SP polypeptide, wherein an amino acid sequence of the NSP polypeptide is shown in the sequence 3 in a sequence table; and the amino acid sequence of the SP polypeptide is shown in the sequence 1 in the sequence table. The vaccine is prepared by uniformly emulsifying the NSP polypeptide, the SP polypeptide and a Freund adjuvant, and has the characteristics of application to the prevention of the porcine reproductive and respiratory syndrome, security, effectiveness, low cost, easy preparation and suitability for large-scale popularization and application.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Use of mir-27b compound as marker of chronic pain and use in preparation of medicine for treating chronic pain
InactiveCN104046688BLower heat shock thresholdClear pathogenesisNervous disorderAntipyreticActivation methodPain behavior
The invention relates to application of a miR-27b compound used as a chronic pain marker and application of the miR-27b compound in preparing a medicament for treating chronic pain. The application comprises the following steps: (1) screening miR-27b target genes, verifying by use of a reporter gene, and making an inflammatory chronic pain model mouse by utilizing a CFA (Complete Freund Adjuvant); (2) increasing the expression quantity of miRNA in the spinal cord of the inflammatory chronic pain model mouse by utilizing a miR-27b simulant; (3) detecting the inhibiting action of miR-27b on the DOT1L protein expression of the CFA causing inflammatory chronic pain by utilizing a Western blot method; (4) detecting a pain behavior by utilizing a heat activation method. The process is simple and the cost is low; the inflammatory chronic pain is effectively inhibited by adopting the miR-27b and an effective intervention medicament for preventing and treating the chronic pain is provided. The invention particularly relates to the application of the miR-27b compound used as the chronic pain marker and the application of the miR-27b compound in preparing the medicament for treating the chronic pain. The application disclosed by the invetnion indicates that the miR-27b can be taken as the marker of chronic pain generation by further carrying out quantitative analysis on the clinical blood specimen of a chronic pain patient.
Owner:XUZHOU MEDICAL COLLEGE
Polyvalent inactivity vaccine for preventing and treating atrophic rhinitis of swine
ActiveCN102302771BImprove protectionSimple processAntibacterial agentsBacterial antigen ingredientsPasteurella multocida toxinImmune effects
Owner:PU LIKE BIO ENG
A kind of acute monocytic leukemia-associated antigen mla-34 epitope polypeptide and its vaccine and pharmaceutical application
ActiveCN104262459BImprove survivalPossess anti-acute monocytic leukemia effectPeptide/protein ingredientsPeptidesAntigen epitopeAdjuvant
The invention discloses an acute monocytic leukemia-associated antigen MLAA-34 epitope polypeptide and an application of the acute monocytic leukemia-associated antigen MLAA-34 epitope polypeptide, and belongs to the technical field of biology. The acute monocytic leukemia-associated antigen MLAA-34 epitope polypeptide disclosed by the invention can induce specific CTL, and simultaneously can express tumor cells of MLAA-34 and HLA-A*0201, and T2 cells of loaded peptide in vitro to generate the determined specific killing effect; the epitope polypeptide CP701 is applied to preparation of a polypeptide vaccine aiming at acute monocytic leukemia; and the polypeptide vaccine composed of an MLAA-34 epitope peptide CP701 (236ILDRHNFAI244), a T auxiliary epitope and an incomplete Freund adjuvant can inhibit hu-PBL-SCID amplification of tumors in mice loading human monocytic leukemia cells THP-1, prolongs the survival life, and can induce the specific CTL killing activity and NK cytotoxic activity in vivo.
Owner:XI AN JIAOTONG UNIV
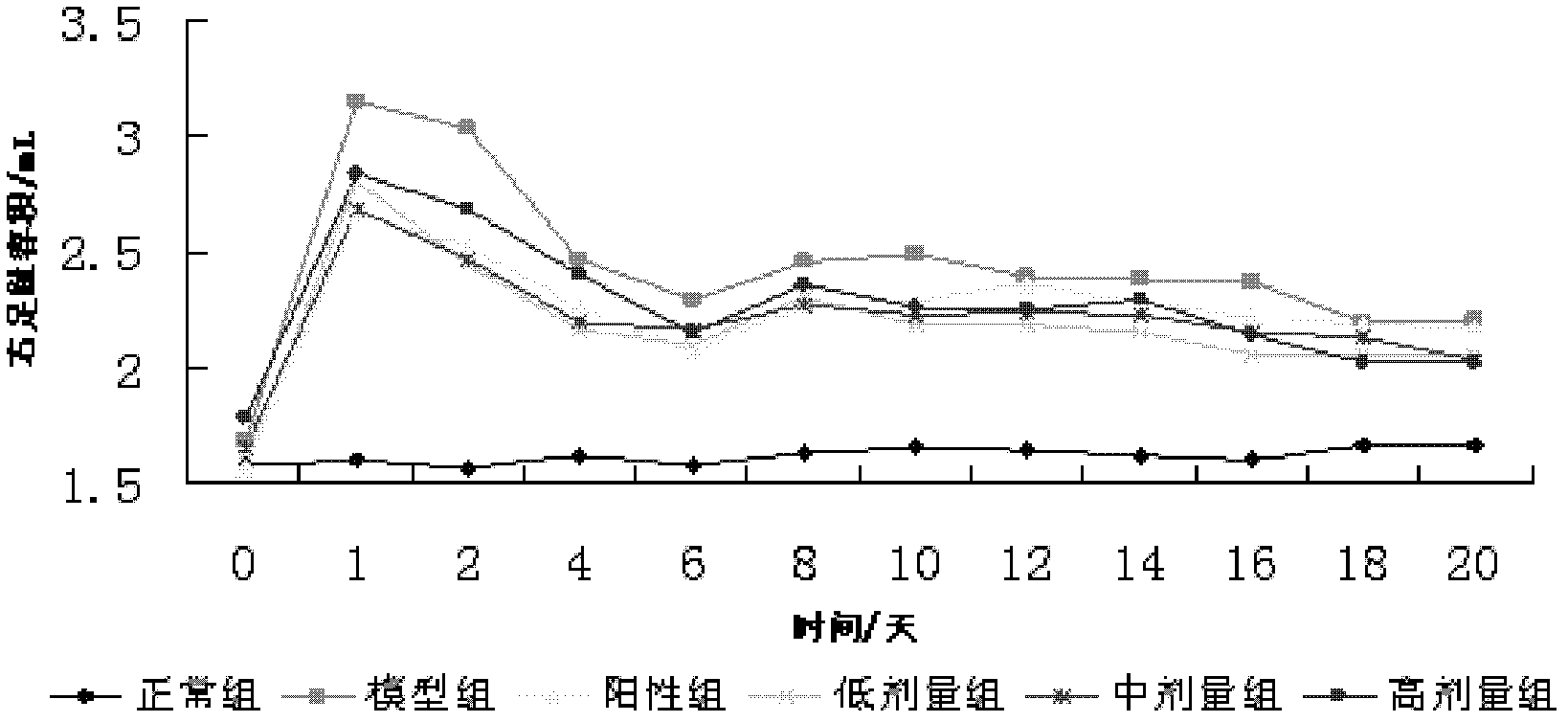
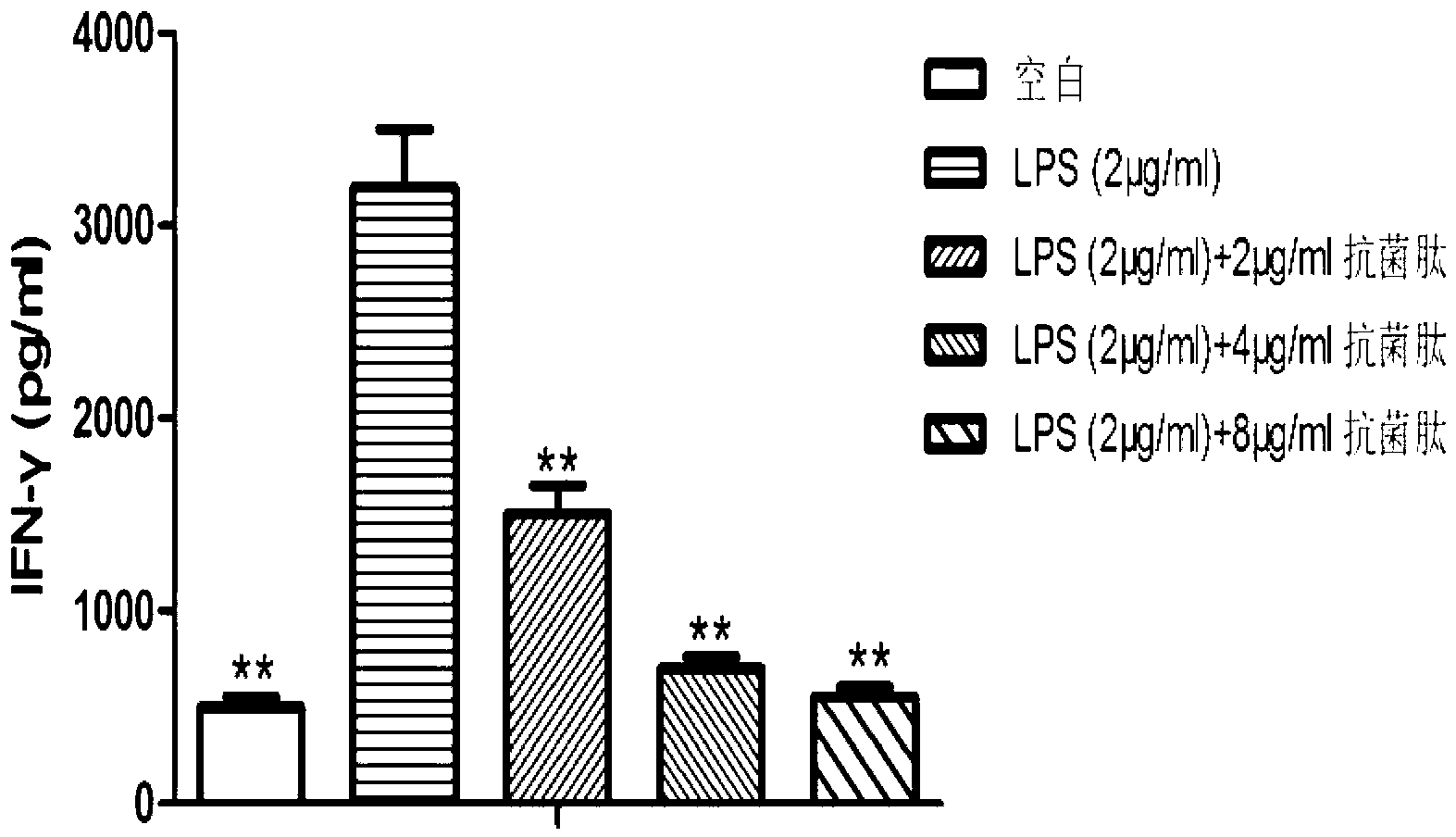
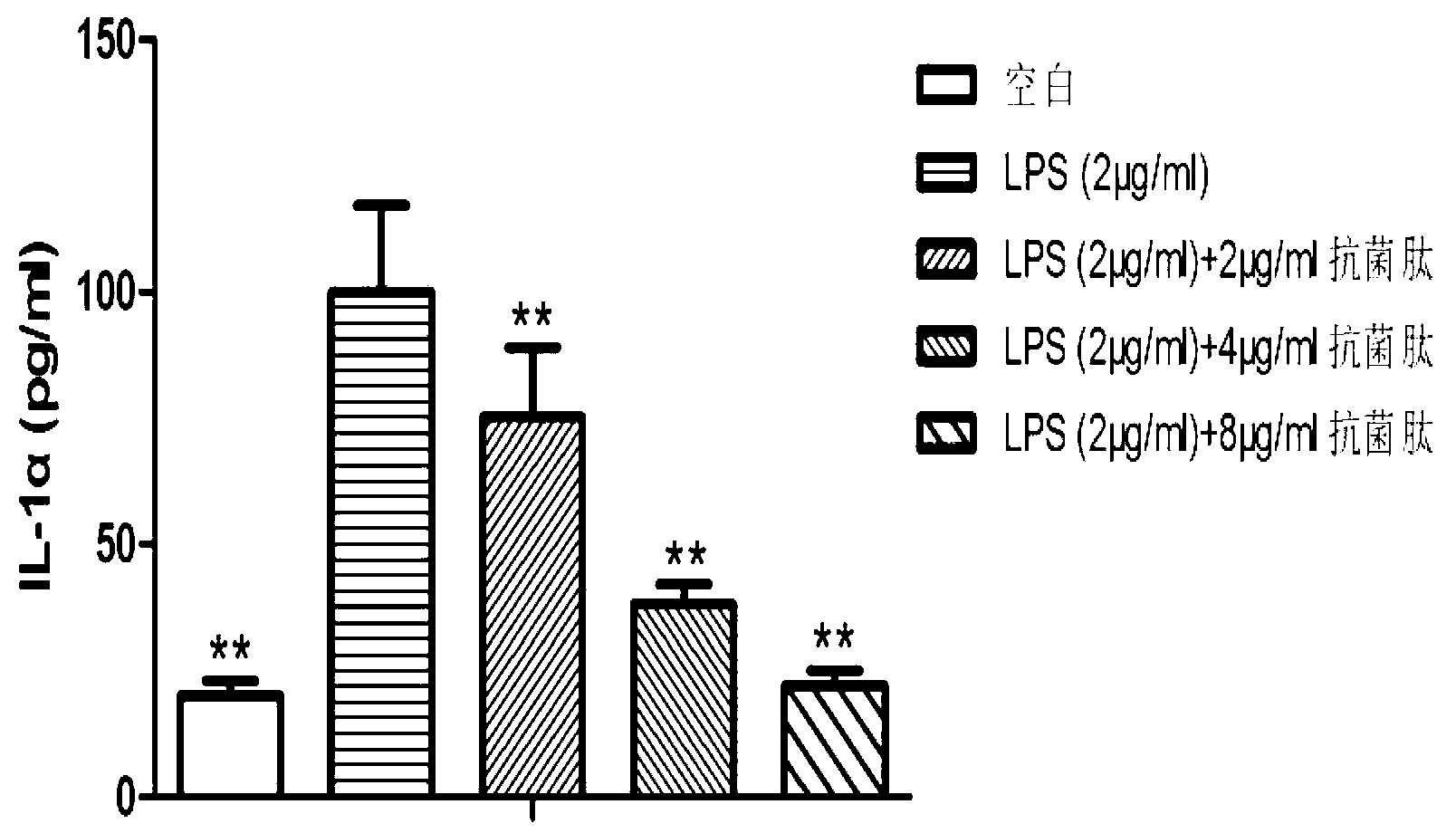
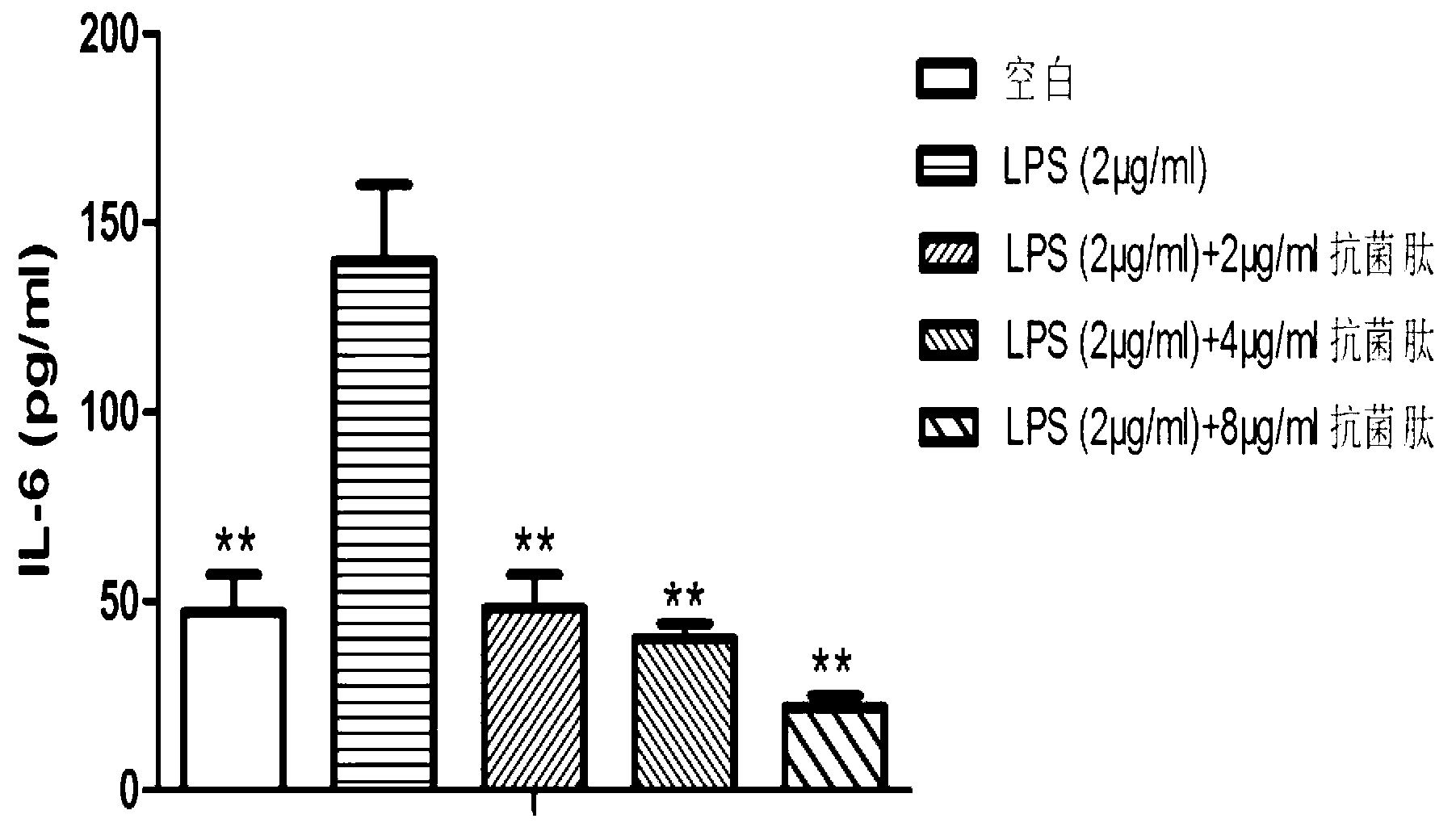
Application of natural antibacterial peptide QHA in preparation of immunologic adjuvant
ActiveCN111420047AImprove immune titerHigh titerPeptide/protein ingredientsAntibody ingredientsAntiendomysial antibodiesFreund adjuvant
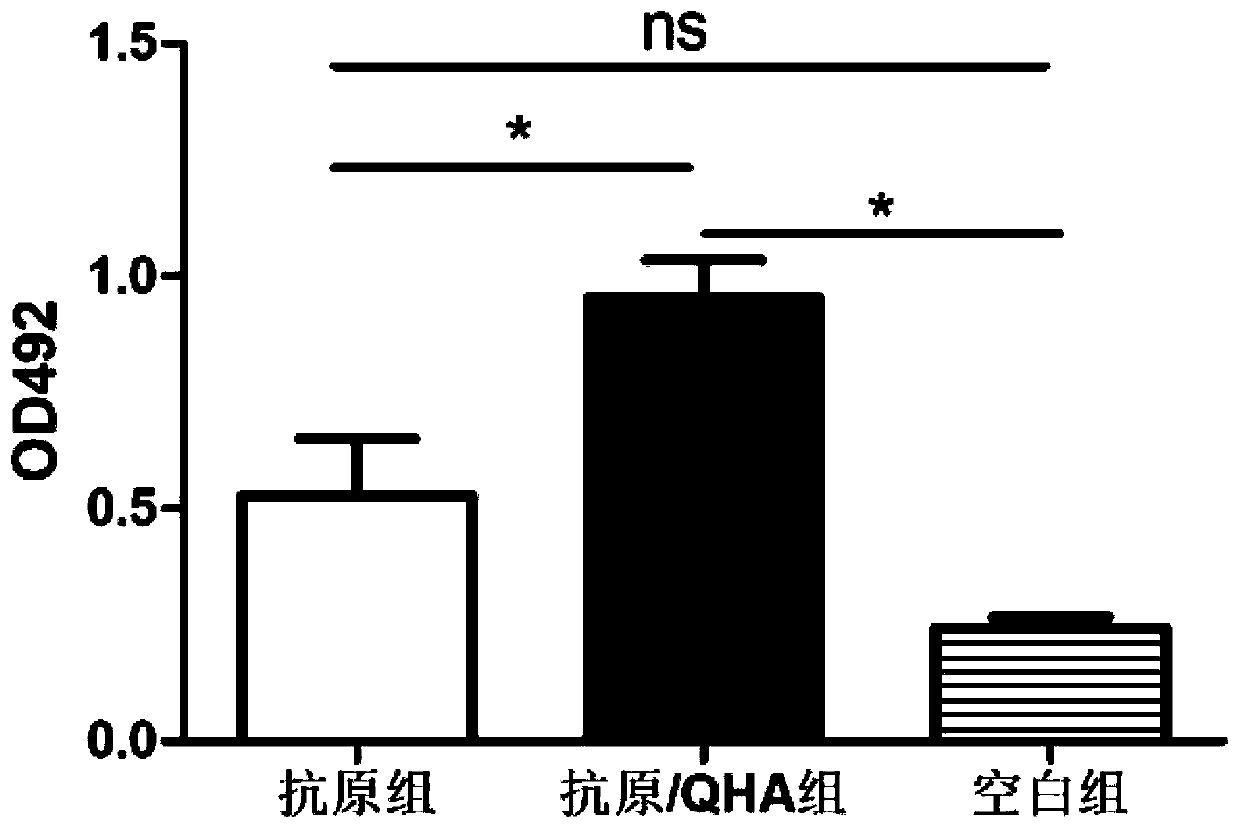
The invention relates to an application of natural antibacterial peptide QHA in preparation of an immunologic adjuvant. The immunologic adjuvant is used for improving the immune titer of an antibody.In addition, the QHA and a Freund adjuvant have a synergistic effect when being combined for use. The invention discloses the novel application of the natural antibacterial peptide QHA. The natural antibacterial peptide QHA can be used for preparing the immunologic adjuvant for improving the immune titer of the antibody. The QHA as immunologic adjuvant for improving the immune titer of the antibody has wide application prospects in the fields of preparation of antibodies, vaccines, immunotherapy drugs, tumor vaccines or immune activators and the like.
Owner:SUZHOU UNIV
A fusion protein contraception vaccine with molecular adjuvant and its preparation
InactiveCN1569227AHas inhibitory effectNo antagonistic effectAntibody medical ingredientsSexual disorderCross-linkAdjuvant
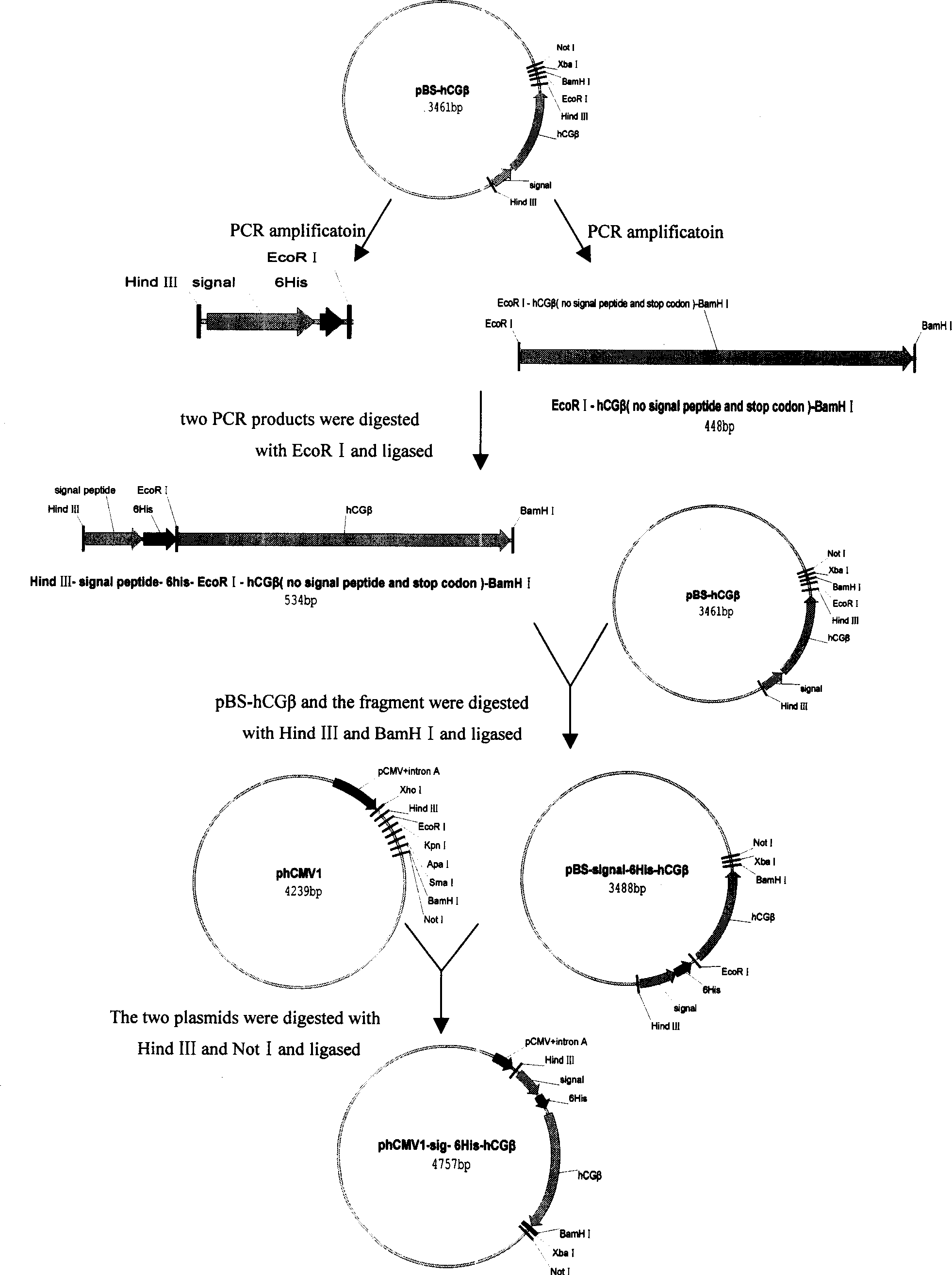
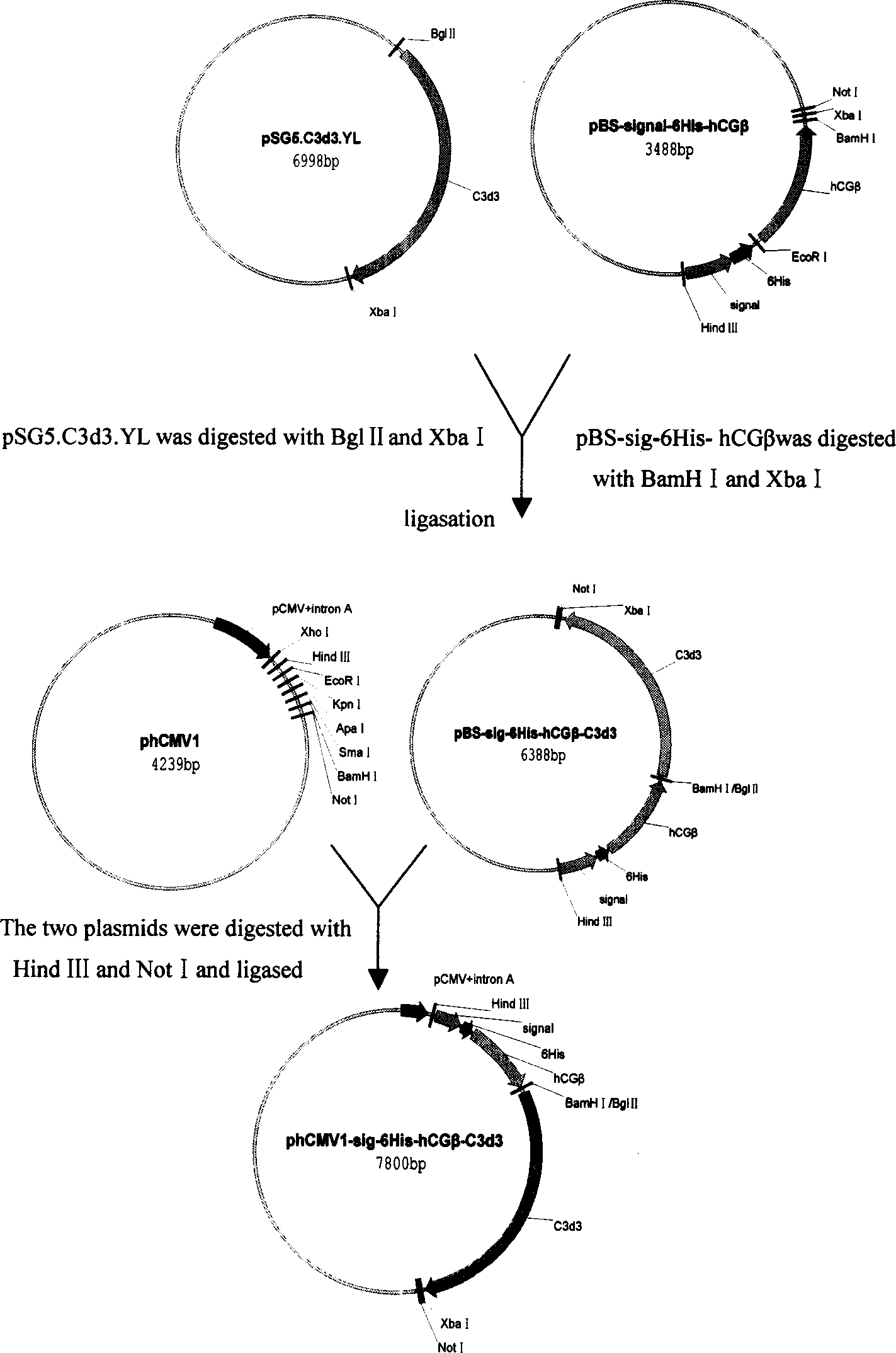
The invention belongs to biological pharmaceutical field. It relates to a contraception vaccine cross linked with the molecule adjuvant, in particular to a hCGª‰-C3d3 confluence protein contraception vaccine cross linked with the molecule adjuvant C3d3. The invention selects eucaryon to express carrier phCMV1, constructs separately of the hCGª‰-C3d3 confluence protein with six histidine purification signals and eucaryon expressed plasmid phCMV1-6His-hCGª‰-C3d3 and phCMV1-6His-hCGª‰ of the hCGª‰protein, in vitro expresses, identifies and purifies hCGª‰-C3d3 confluence protein and hCGª‰protein, applies freunds adjuvant to immune mouse in different species. It is proved that hCGª‰-C3d3 confluence protein vaccine has comparative strong anti generating potent function by detection of the antibody level, biological activity of antiserum and neutralization of the hCG and excretion level of the cell factor by the spleen cells.
Owner:THE OBSTETRICS & GYNECOLOGY HOSPITAL OF FUDAN UNIV
Bacillus amyloliquefaciens WH3, and preparation method and application thereof
InactiveCN102286408BHigh biosecurityReduce manufacturing costBacteriaMicroorganism based processesSclerotiniaFreund adjuvant
The invention discloses a Bacillus amyloliquefaciens WH3 strain, and a preparation method and application thereof. The preparation method comprises the following steps: 1, separation and identification of bacteria: separating bacteria resistant to rape sclerotinia rot from rape seedlings, and carrying out 16SrDNA and morphological identification to determine that the WH3 strain obtained by separation is Bacillus amyloliquefaciens; 2, separation, purification and identification of an antifungal active substance Safenour: fermenting the WH3 strain, extracting the antifungal active substance, separating and purifying through a sephadex column, and carrying out MALDI-TOF (matrix-assisted laser desorption / ionization-time of flight ) mass spectrometry on the active antifungal substance to inferthat the substance is a ring type polypeptide; and 3, application of the strain in the preparation of vaccines and immunological adjuvants. After the Safenour used as the adjuvant is mixed with a protein antigen and mice are respectively immunized through oral administration and injection of the mixture, effective body fluid and cellular immune response can be activated, and a high-titer specificantibody can be detected in the blood serum. The Safenour has low production cost and high stability, does not need to be emulsified when mixed with the antigen, and has a better immunoenhancement effect in comparison with Freund adjuvants and cholera toxin B subunits.
Owner:武汉光谷世傲生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com