Polyvalent inactivity vaccine for preventing and treating atrophic rhinitis of swine
A technology for atrophic rhinitis and inactivated vaccines, applied in the direction of antibody medical components, medical preparations containing active ingredients, bacterial antigen components, etc., can solve problems such as incomplete absorption, less protective antigen components, and restrictions on the application of PMT. Achieve effective treatment and prevention, high immune protection efficacy, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of the polyvalent inactivated vaccine of embodiment 1 porcine atrophic rhinitis
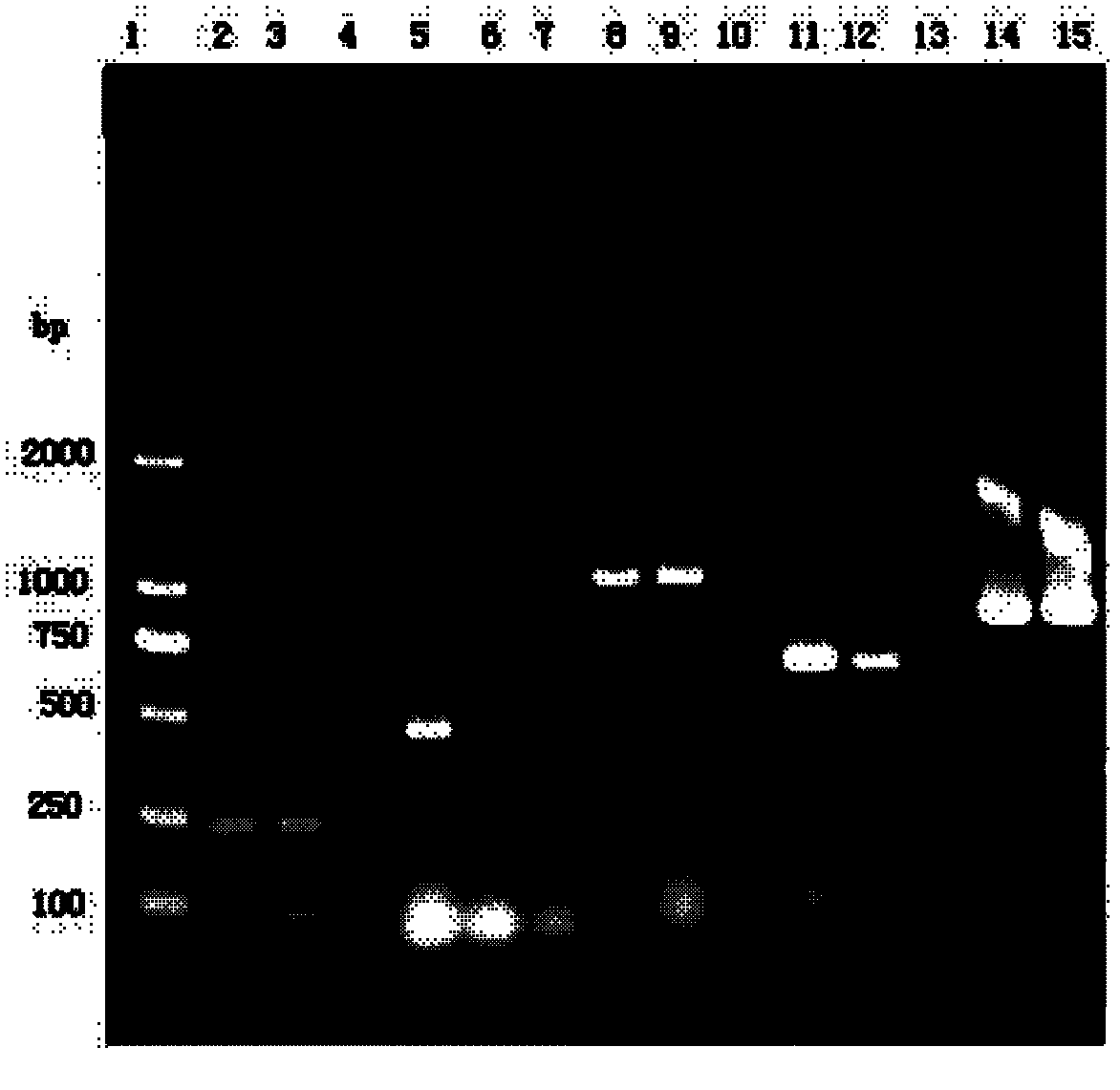
[0032] 1. The source and identification of the bacterial classification of the embodiment of the present invention
[0033] 1.1 The source of strains
[0034] The preservation number of Bordetella bronchiseptica (Bb) HN8 strain is CCTCC NO: M2011223, the preservation number of Pasteurella multocida (Pasteurella multocida) type A HN5 strain is CCTCC NO: M2011222, Pasteurella multocida The deposit number of the D-type HB4 strain is CCTCC NO: M2011221. The strains of the above three strains were isolated and identified by Pulaike Bioengineering Co., Ltd. The vaccine prepared by the above strains has good immunity and strong specificity, and has an irreplaceable role for other strains.
[0035] 1.2 Identification of strains
[0036] 1.2.1 Biochemical identification
[0037] Bronchiseptica Bordetella HN8 strain had hemolysis; catalase, oxidase, urease, citrate, sodium chloride...
Embodiment 2
[0064] The preparation of the polyvalent inactivated vaccine of embodiment 2 porcine atrophic rhinitis 2
[0065] The inactivation of bacterial classification and bacterial classification, the extraction of PMT toxoid and inactivation are the same as embodiment 1.
[0066] See Table 1 below for the ratio of each antigen in Example 2.
Embodiment 3
[0067] The preparation of the polyvalent inactivated vaccine of embodiment 3 porcine atrophic rhinitis 3
[0068] The inactivation of bacterial classification and bacterial classification, the extraction of PMT toxoid and inactivation are the same as embodiment 1.
[0069] See Table 1 below for the ratio of each antigen in Example 3.
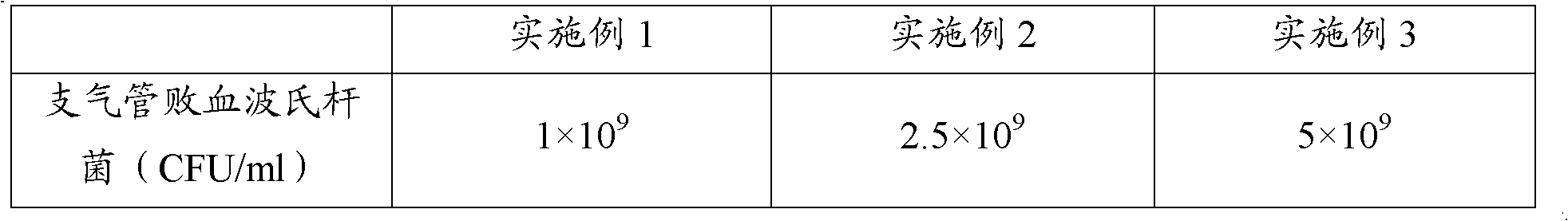
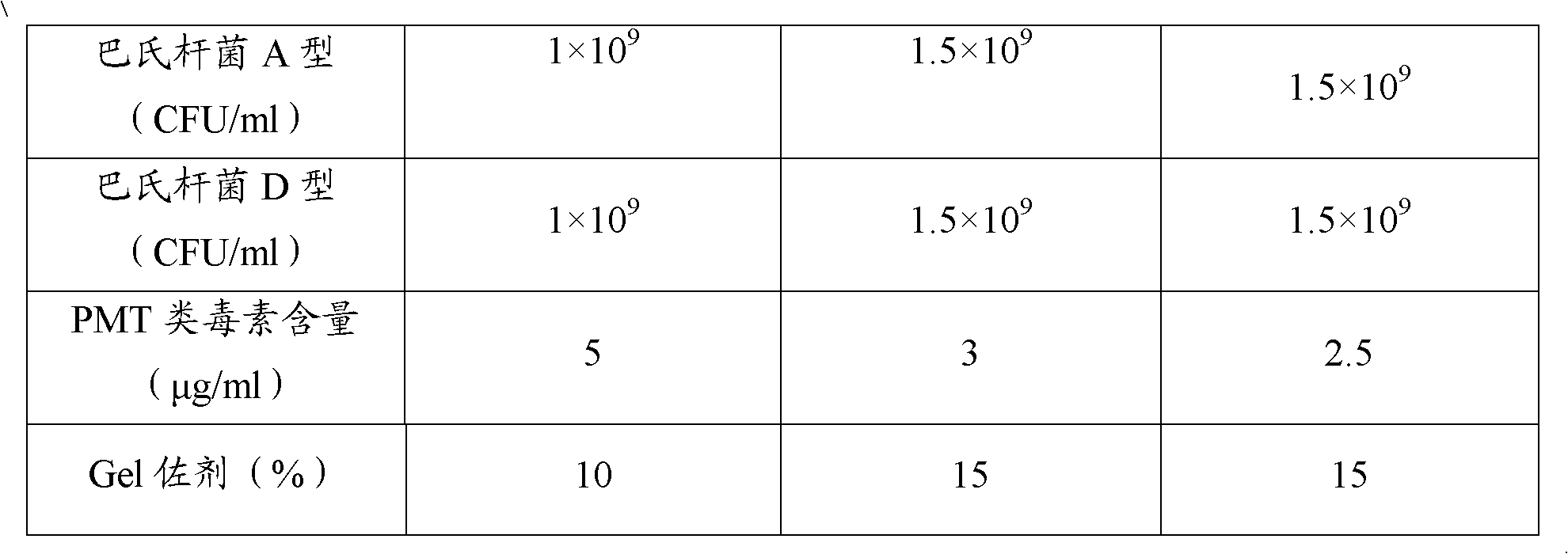
[0070] The concrete proportioning ratio of the vaccine of embodiment 1-3 is shown in Table 1:
[0071] The composition ratio of the polyvalent inactivated vaccine of table 1 porcine atrophic rhinitis
[0072]
[0073]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com