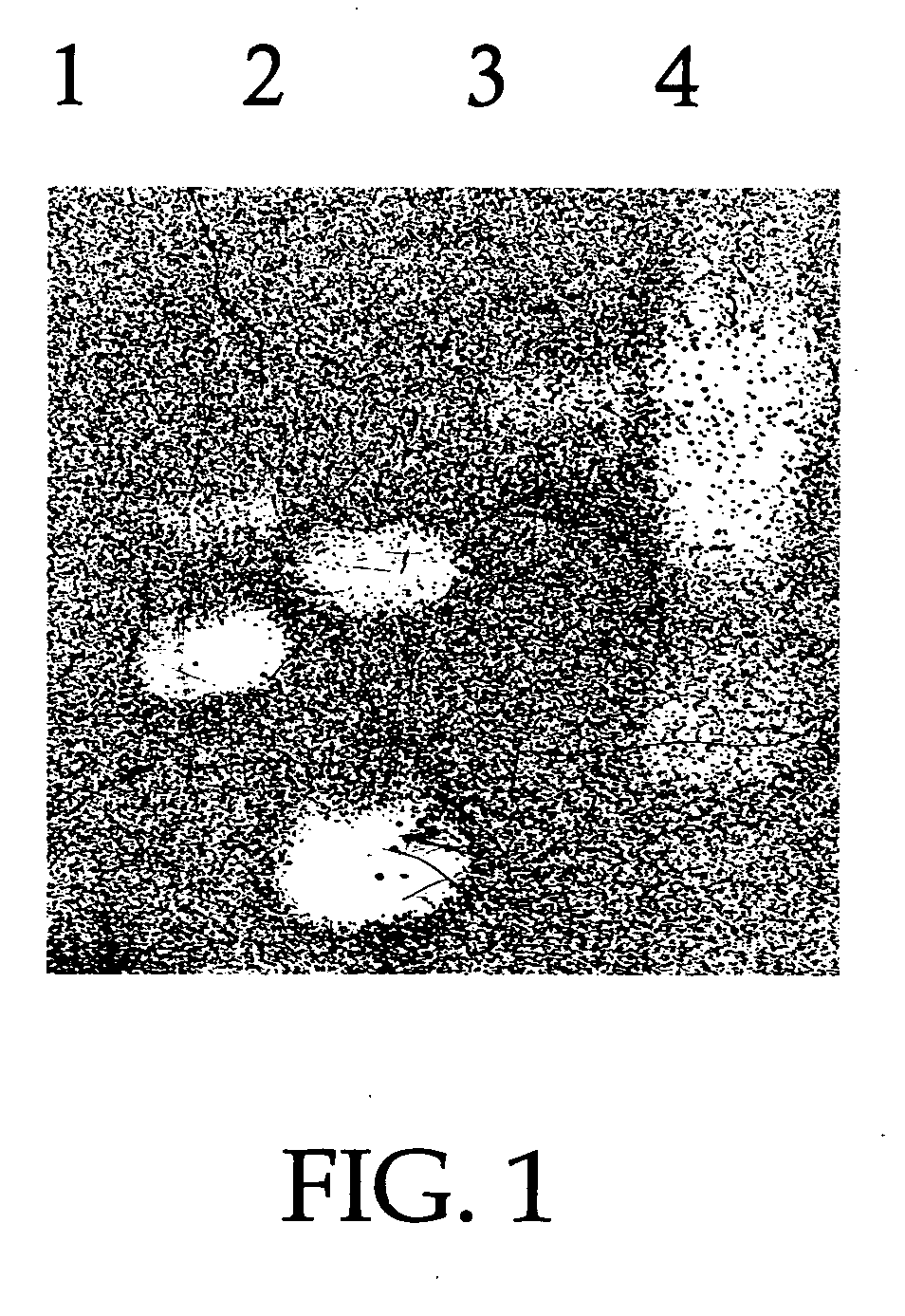
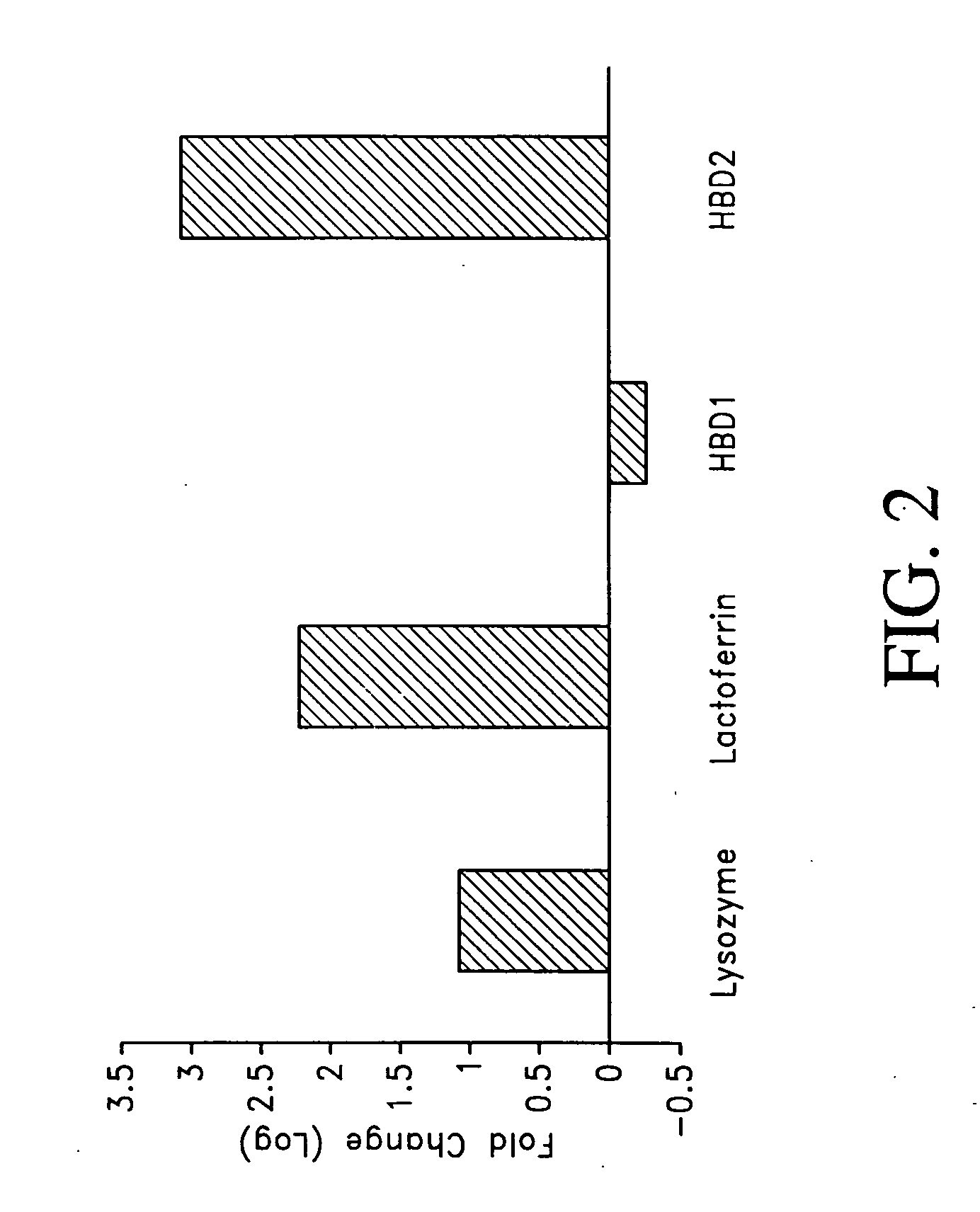
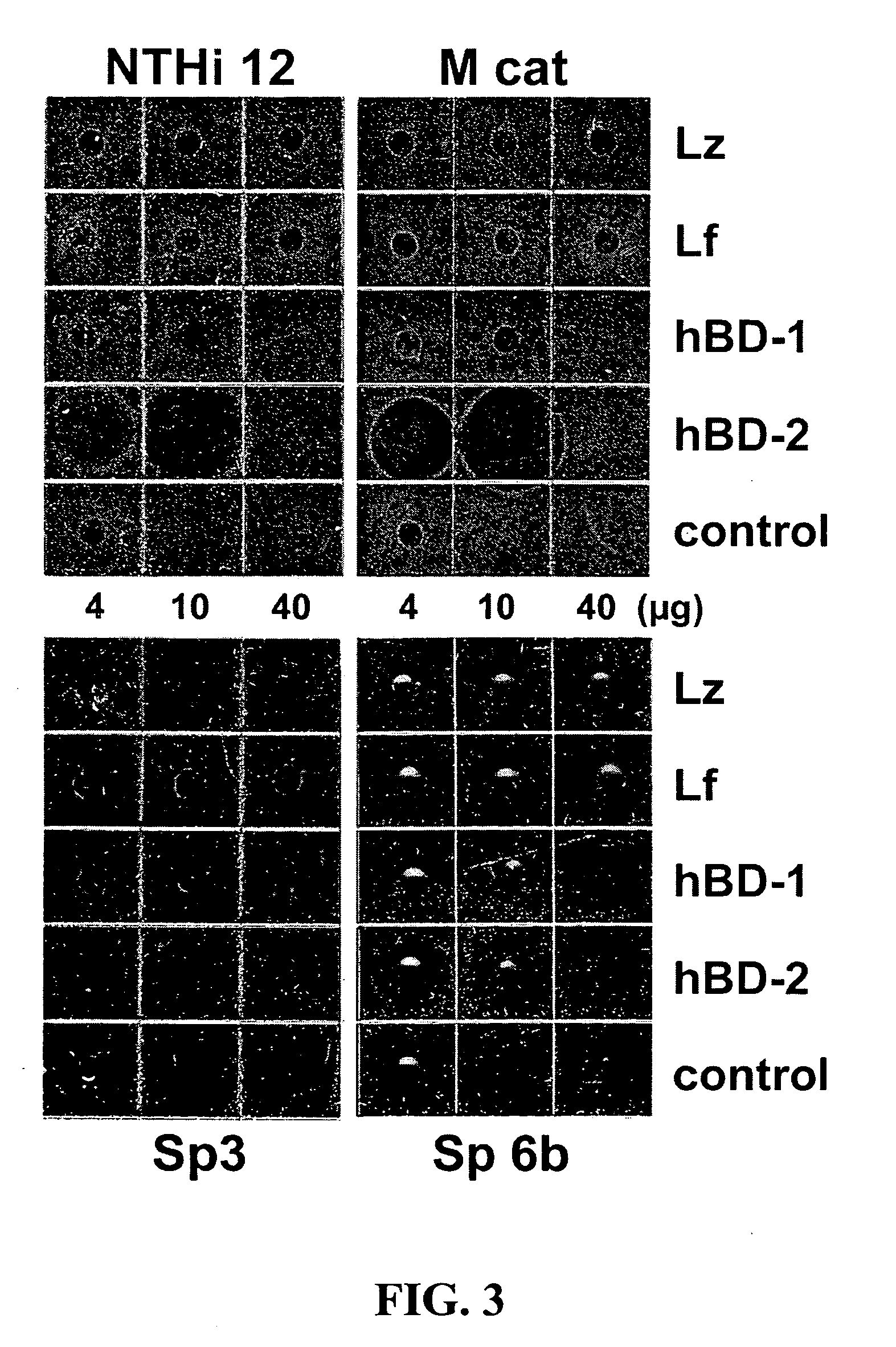
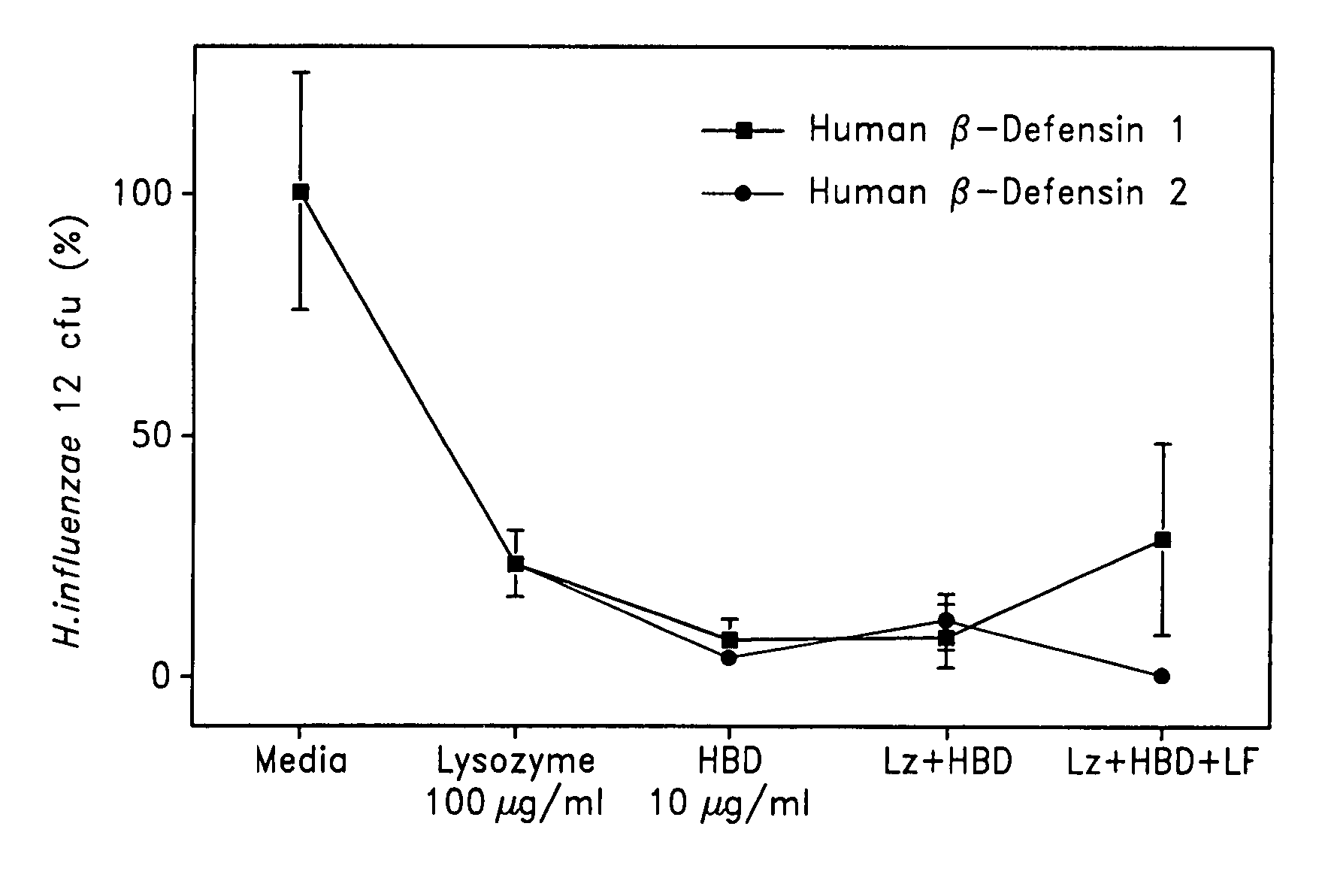
Patents
Literature
212 results about "Meningitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An infection of meninges, protective tissue of the brain.
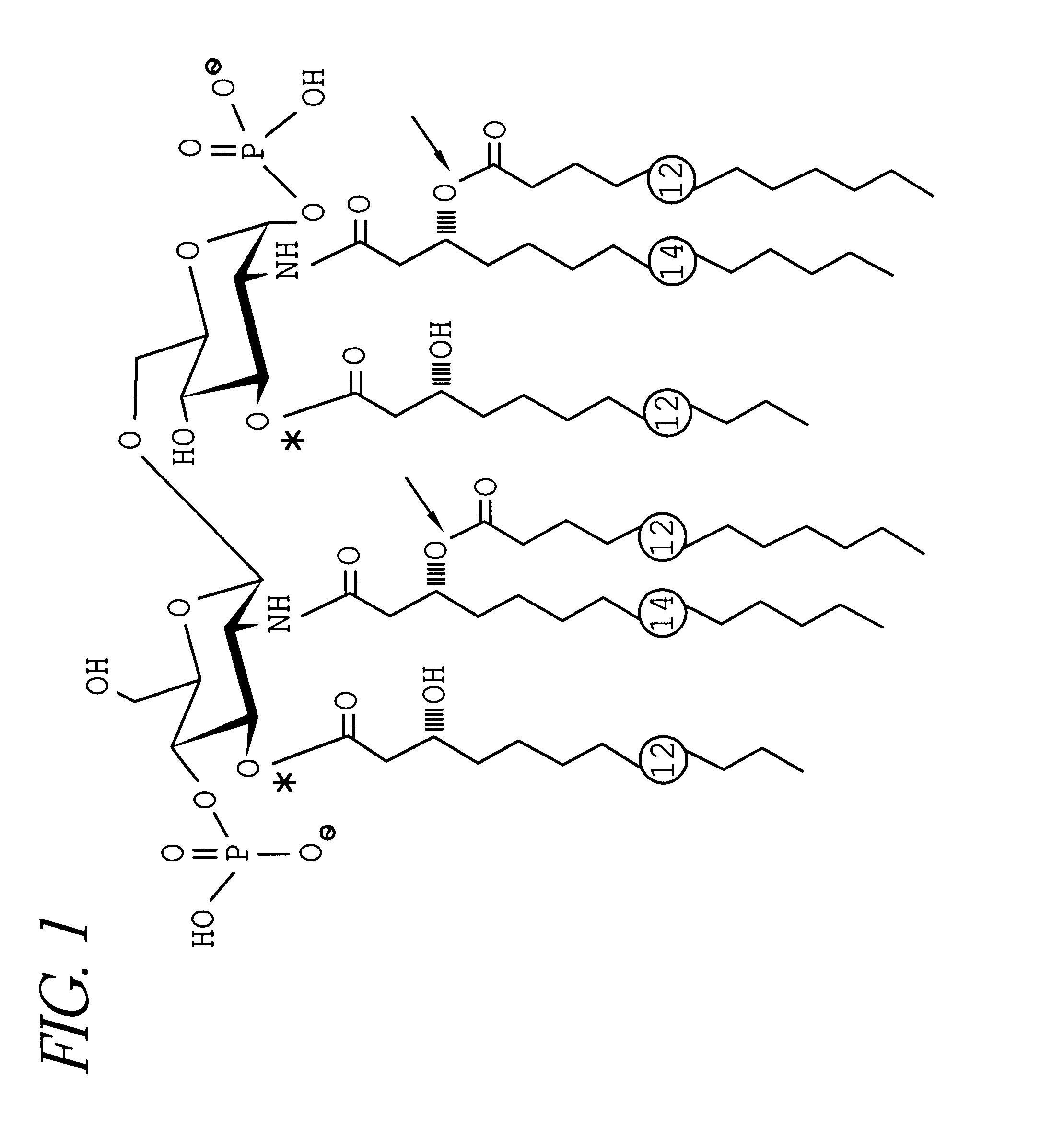
Conjugate vaccine for Neisseria meningitidis
InactiveUS6531131B1Bacterial antigen ingredientsCarrier-bound antigen/hapten ingredientsConjugate vaccineLacto-N-tetraose
A conjugate vaccine for Neisseria meningitidis comprising lipooligosaccharide which does not contain a lacto-N-tetraose antigen from which at least one primary O-linked fatty acid has been removed conjugated to an immunogenic carrier. The vaccine is useful for prevention of meningitis and septic shock in mammals.
Owner:THE GOVERNMENT OF THE UNITED STATES REPRESENTED BY SEC DEPT OF HEALTH & HUMAN SERVICES
Meningococcal antigens
InactiveUS6709660B1Easy to insertEfficient HarvestingAntibacterial agentsOrganic active ingredientsCoccidiaNucleotide
The invention provides proteins from Neisseria meningitidis (strains A & B), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics.
Owner:NOVARTIS AG
Combined parasympathetic stimulation and drug therapy
InactiveUS20080125843A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsMedical devicesNervous systemMyelitis
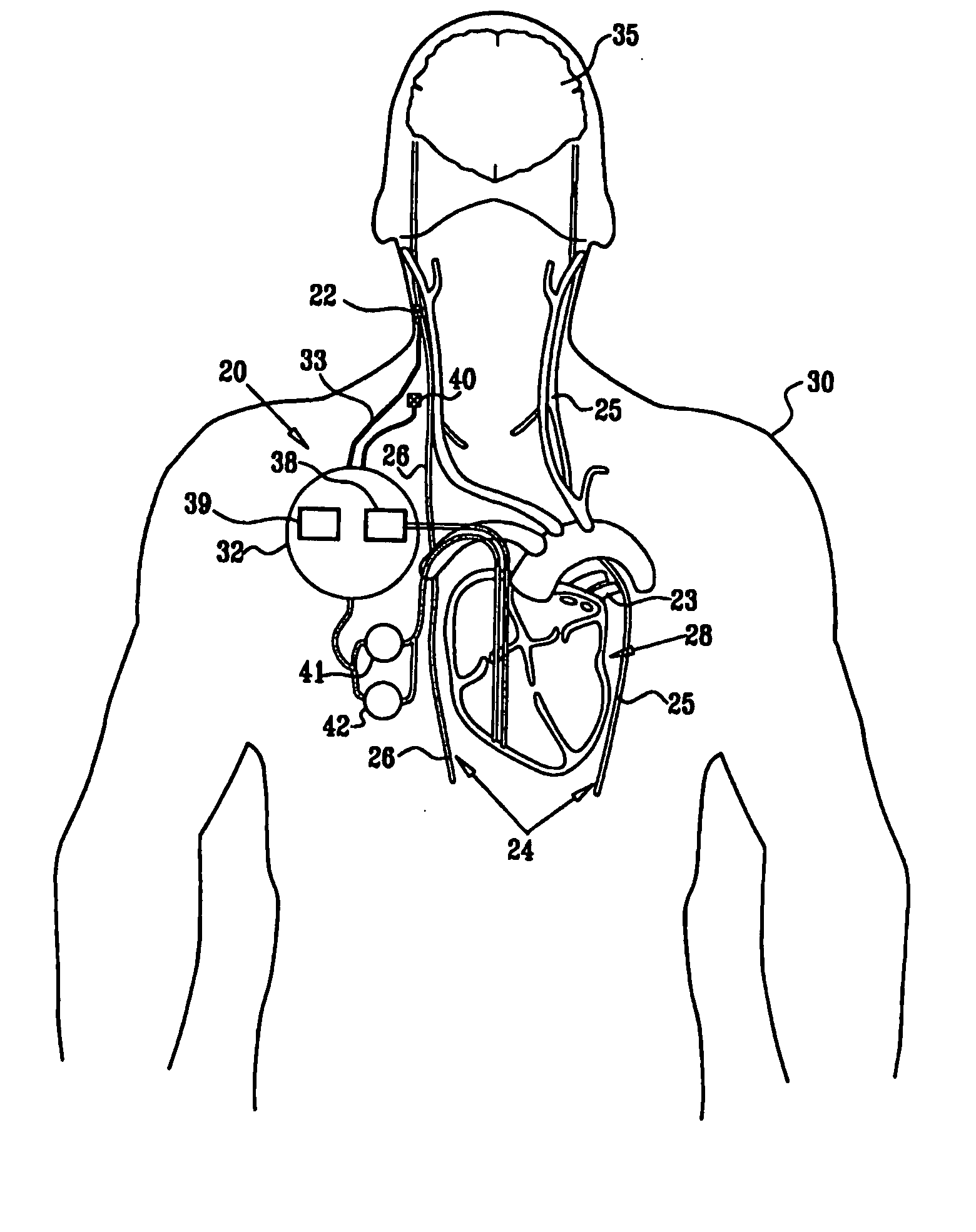
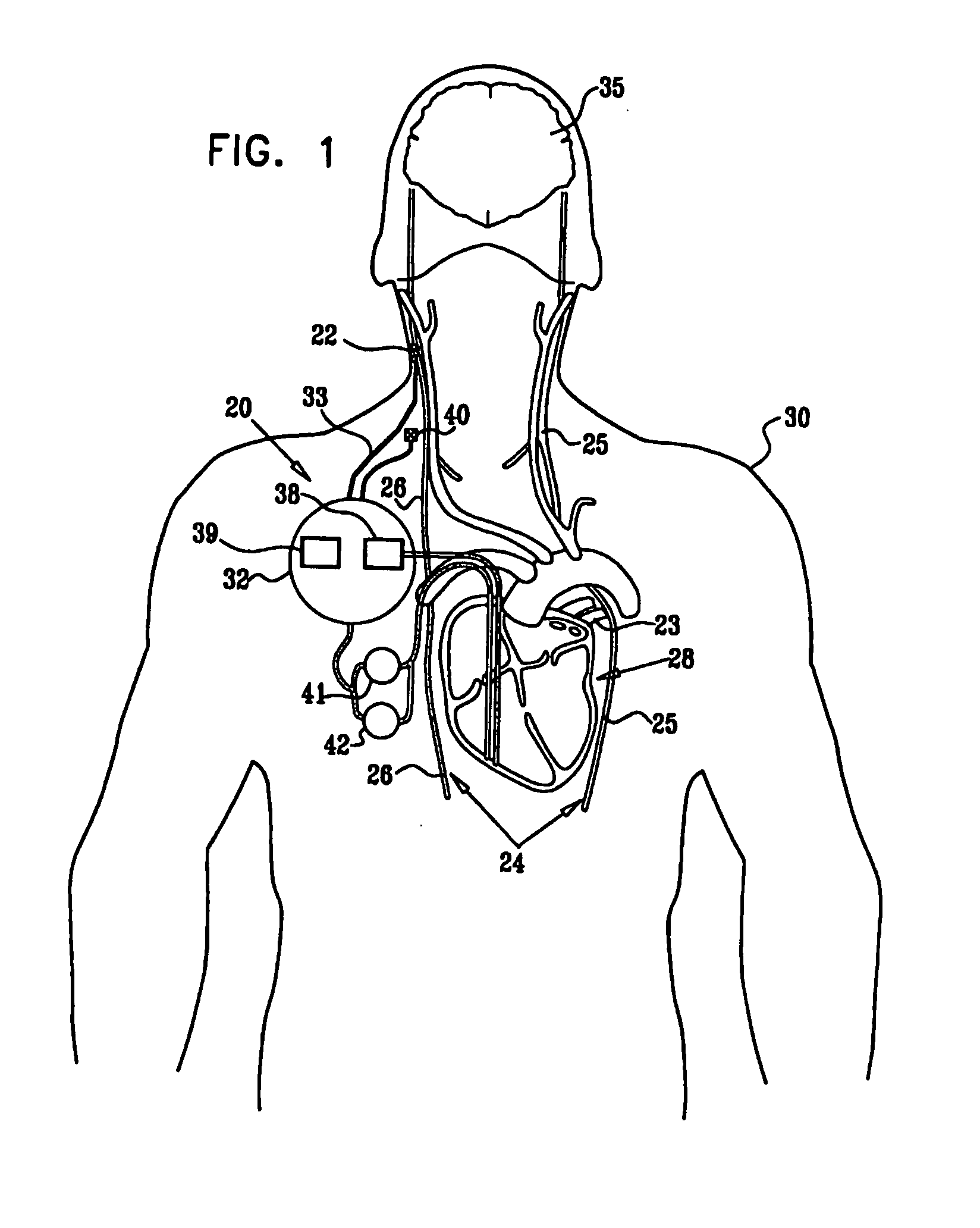
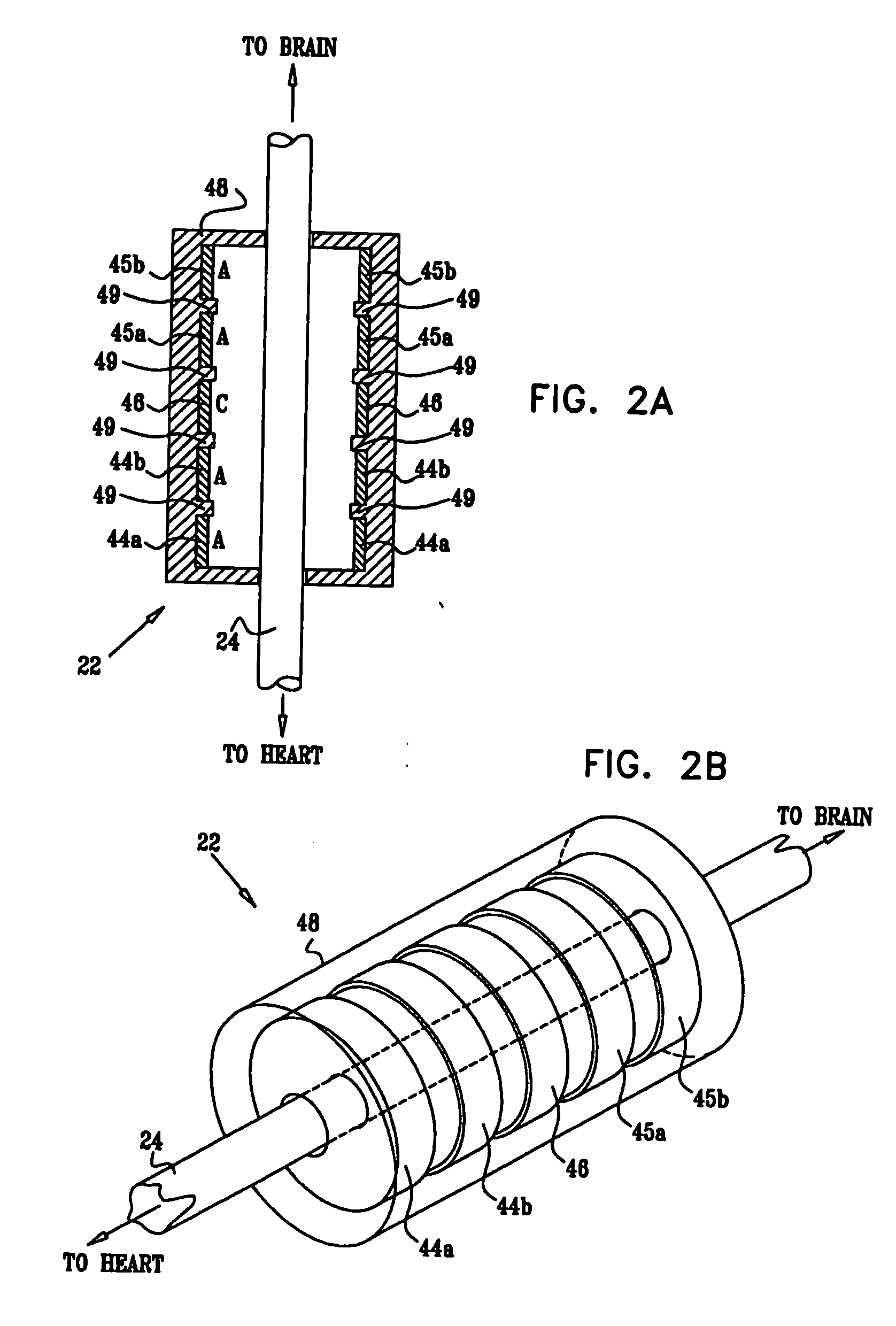
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
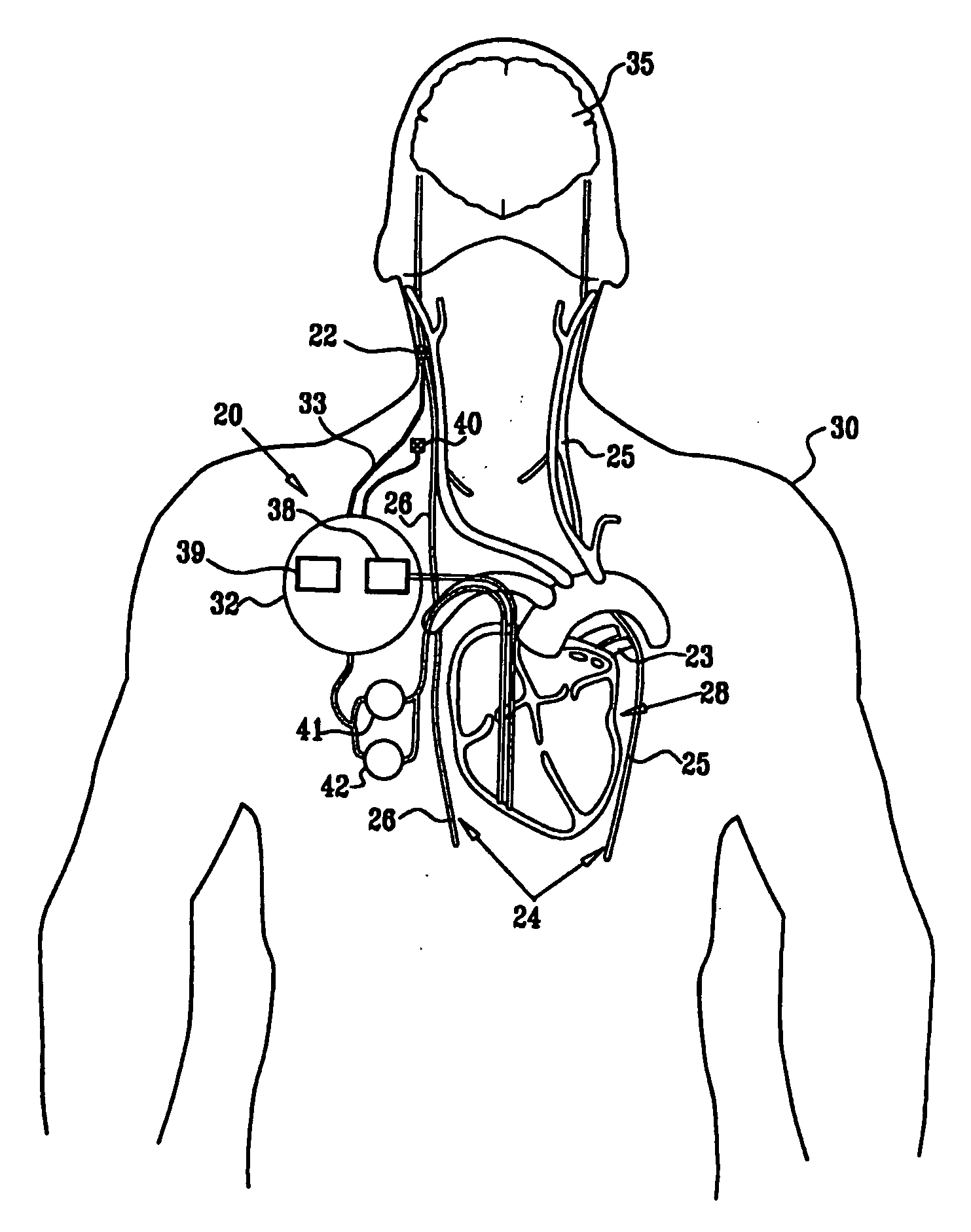
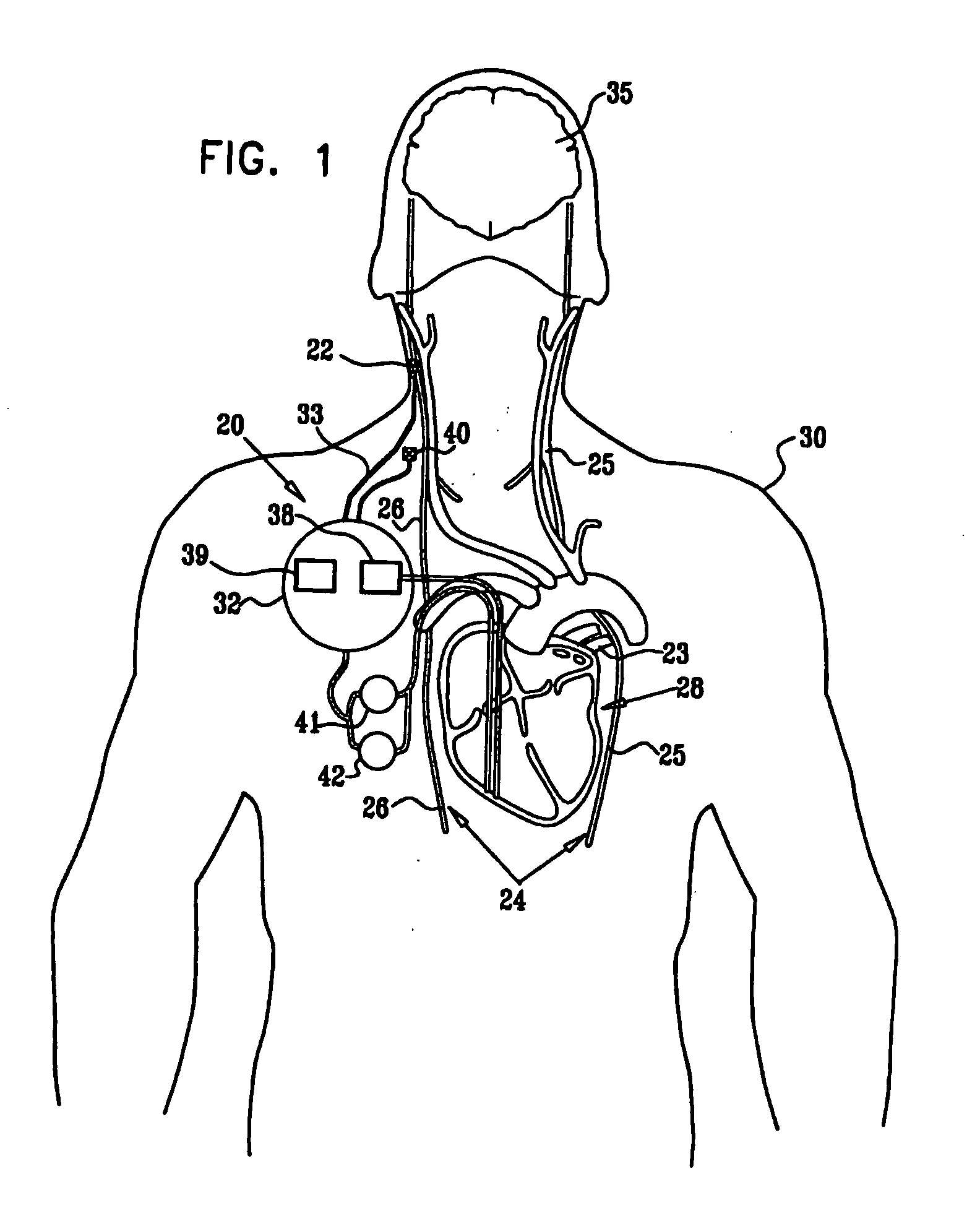
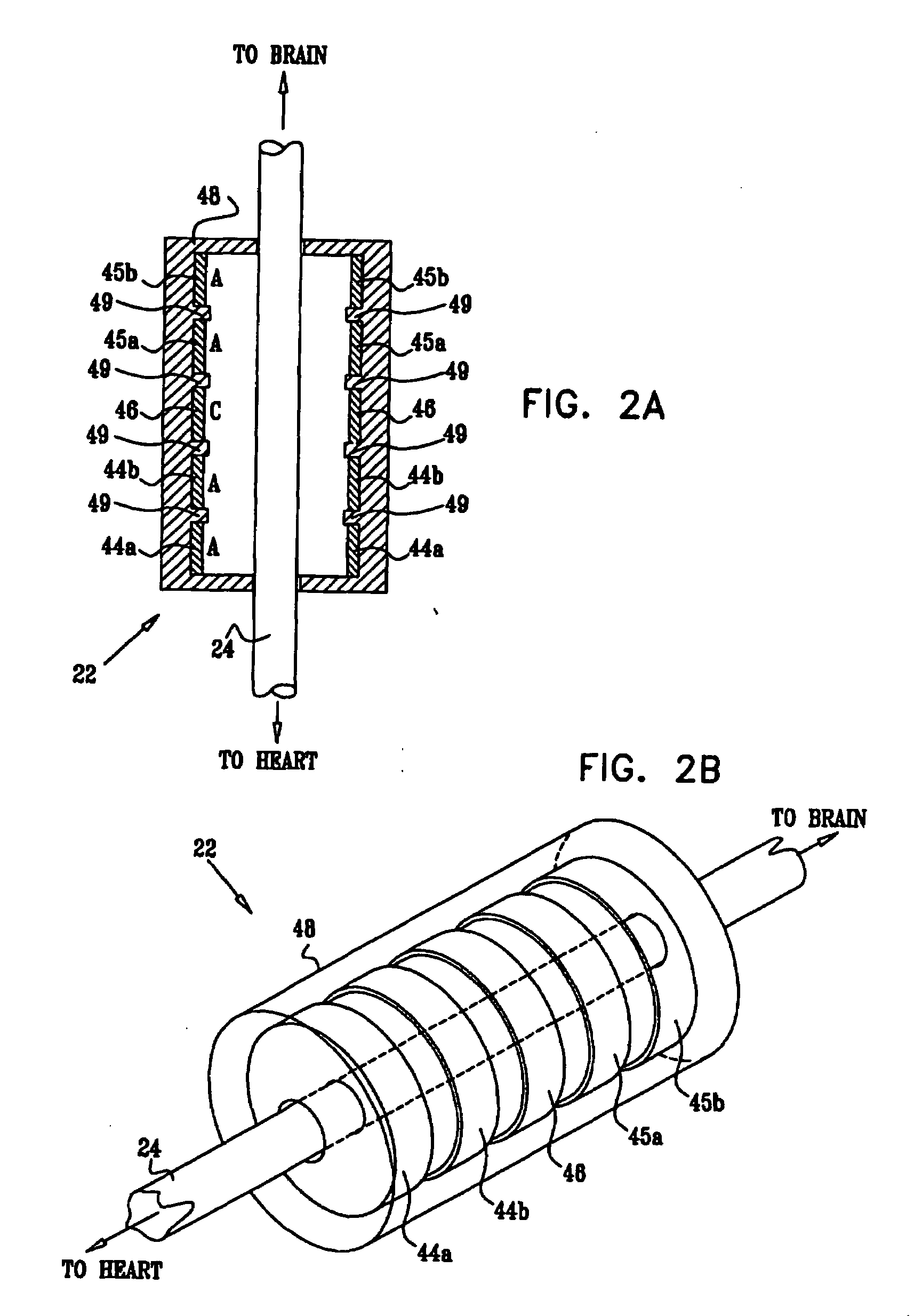
Cerebrospinal Fluid Purification System
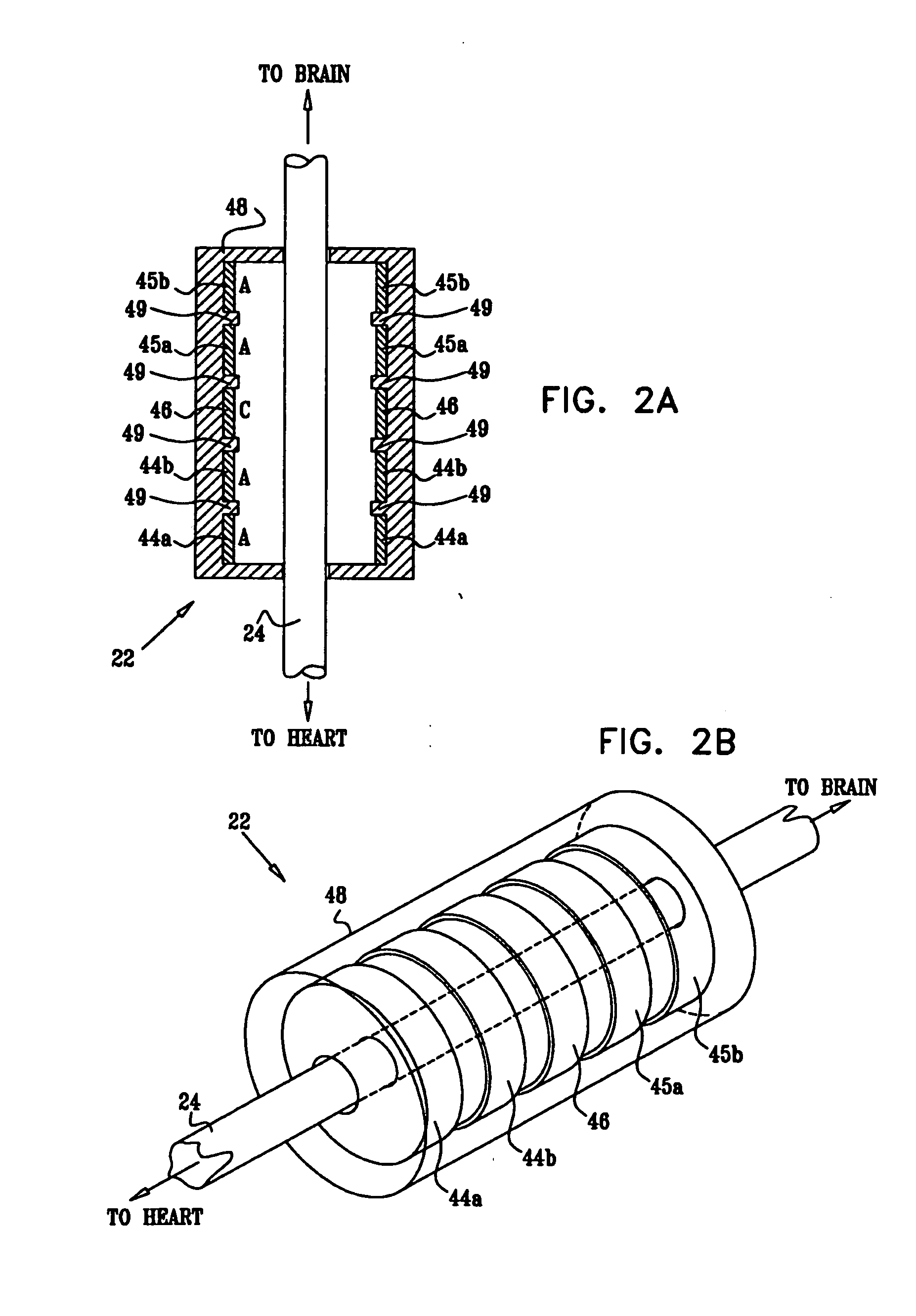
The present invention provides methods and systems for conditioning cerebrospinal fluid (CSF). The methods provide for efficiently removing target compounds from CSF. The systems provide for a multilumen flow path and exchange of a majority volume portion of CSF in the CSF space. The removal and / or delivery of specific compounds can be tailored to the pathology of the specific disease. The removal is targeted and specific, for example, through the use of specific size-exclusion thresholds, antibodies against specific toxins, and other chromatographic techniques, as well as delivery and / or removal of targeted therapeutic agents. The invention finds use as a diagnostic, therapeutic and drug delivery platform for a variety of diseases affecting the CNS by accessing the CSF space. Exemplified disease conditions treatable by the present CSF processing systems and methods include, but are not limited to: Cerebral Vasospasm, Guillain Bane Syndrome, illustrating multi-lumen lumbar approach Alzheimer's, Parkinson's, Huntington's, Multiple Sclerosis, Amyotrophic Lateral Sclerosis, Spinal Cord Injury, Traumatic Brain Injury, Stroke, Cancer affecting the brain or spinal cord, Prion disease, Encephalitis from various causes, Meningitis from various causes, diseases secondary to enzymatic or metabolic imbalances, Biological Warfare, etc. For the first time, the present invention offers patients a disease-modifying, disruptive technology treatment platform that addresses the known disease pathogenesis of a number of neurologic conditions to which there are presently limited and ineffective treatment options.
Owner:NEUROFLUIDICS
Parasympathetic stimulation for heart conditions
InactiveUS20080125819A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsHeart stimulatorsMyelitisNervous system
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Mucosal meningococcal vaccines
InactiveUS20070207090A1Improve securityReduced dosAntibacterial agentsBacterial antigen ingredientsMucosal Immune ResponsesAdjuvant
The invention provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred. The invention also provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The use of chitosan and / or detoxified ADP-ribosylating toxin adjuvants enhances anti-meningococcal mucosal immune responses and can shift the Th1 / Th2 bias of the responses.
Owner:NOVARTIS AG
Minimal-heart-rate reduction parasympathetic stimulation
ActiveUS20080275514A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsInternal electrodesMyelitisNervous system
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Gene knockout carrier and gene knockout method of NLRP1 gene of MH7A cell
InactiveCN106755091AKnockout EfficientConvenient researchCell receptors/surface-antigens/surface-determinantsGenetically modified cellsSequence designInflammatory factors
The invention relates to the molecular biology field and particularly relates to a gene knockout carrier, a construction method and application thereof and a gene knockout method of an NLRP1 gene of an MH7A cell. A CRISPR-Cas9 gene knockout system is utilized for carrying out CRISPR targeting sequence design by taking NLRP1 as a target gene so as to prepare a knockout carrier aiming at the NLRP1 gene, and the knockout carrier is utilized for transfecting the MH7A cell, so that the NLRP1 gene in the MH7A cell can be efficiently knocked out; and therefore, a research platform for rheumatoid arthritis is effectively built, so that the research of the pathogenesis of the rheumatoid arthritis is greatly promoted, and the researches of the interaction of NLRP1 inflammasomes and various inflammatory factors and the disease related molecular mechanisms of the rheumatoid arthritis and meningitis can be promoted.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
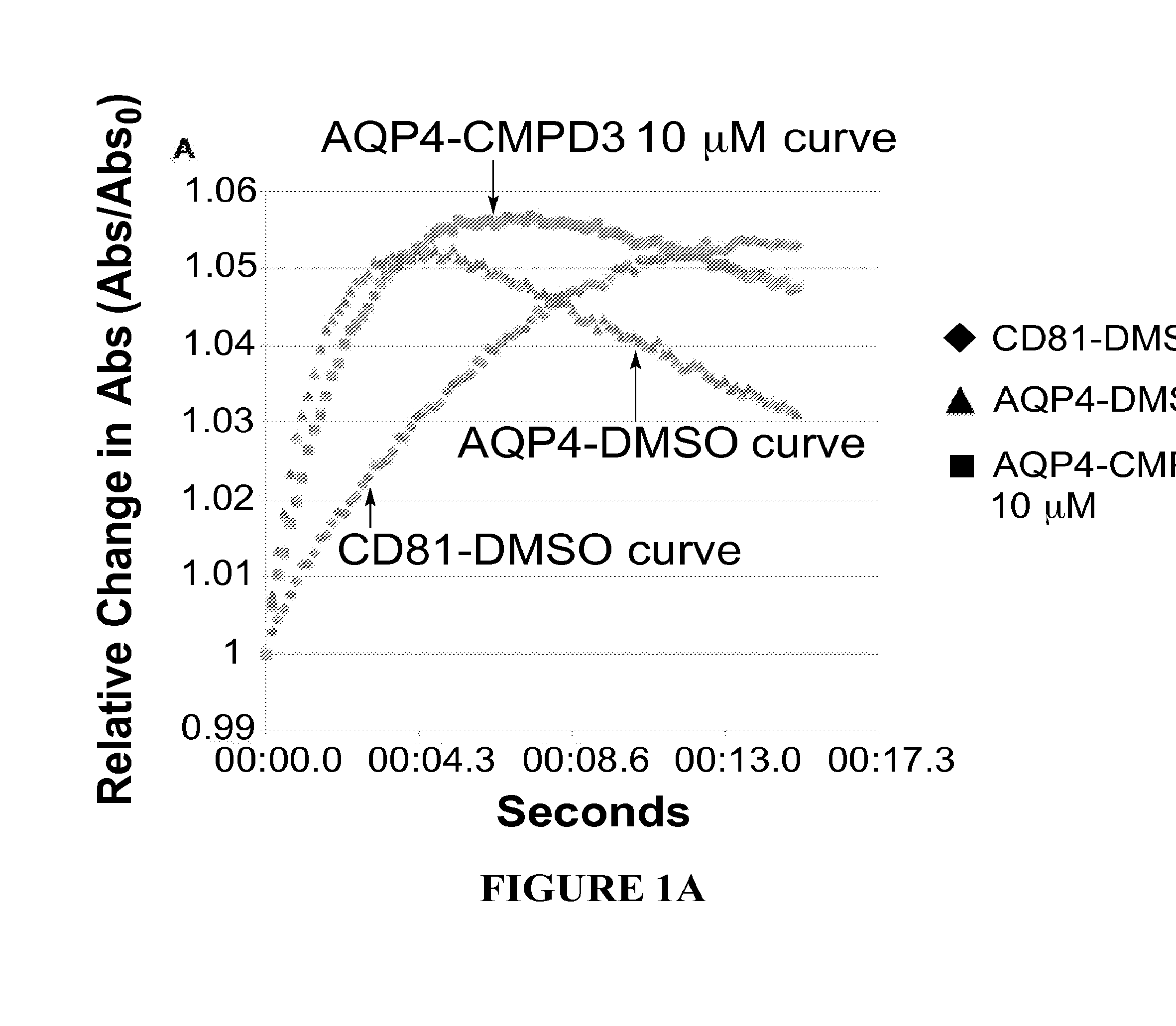
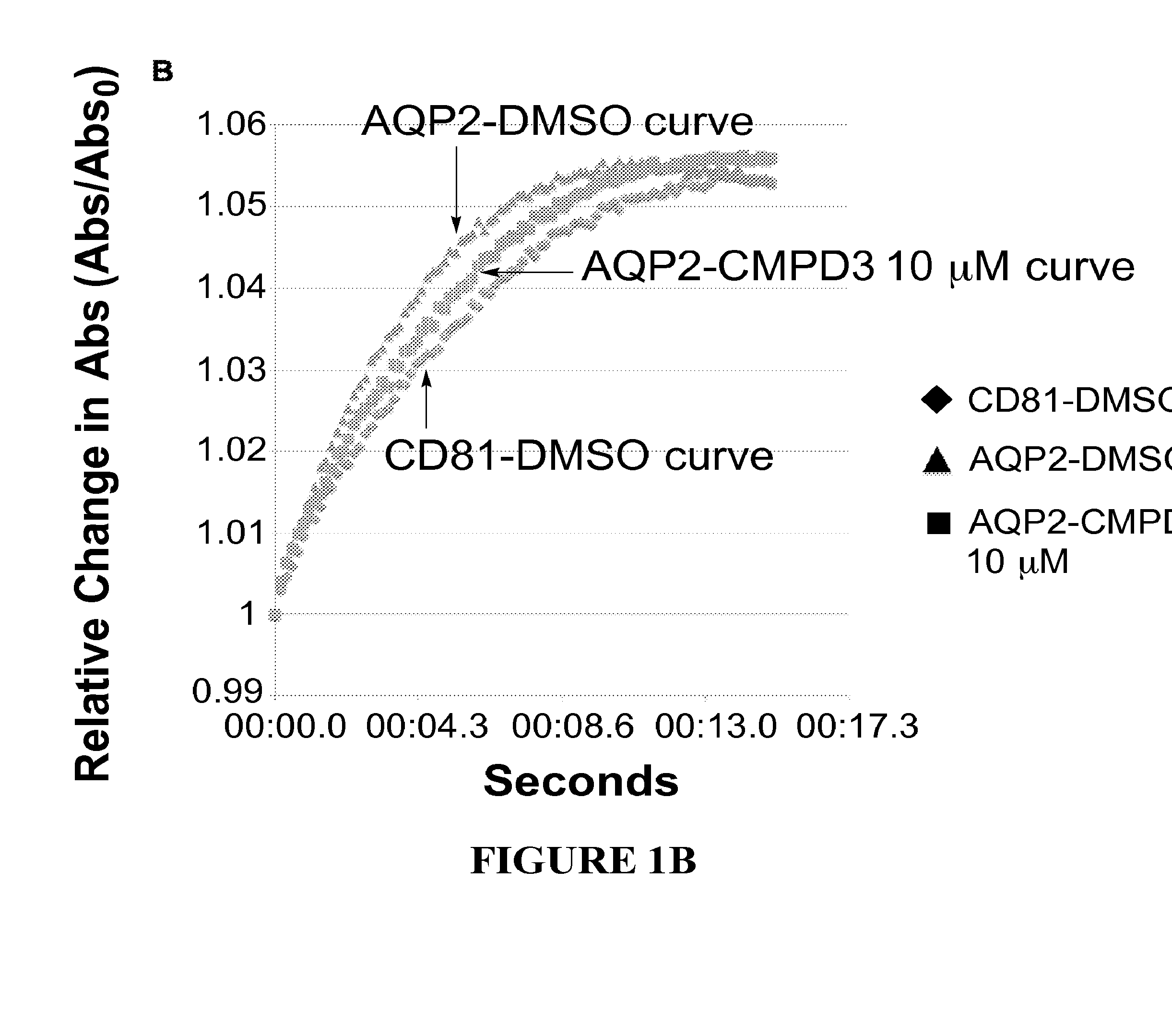
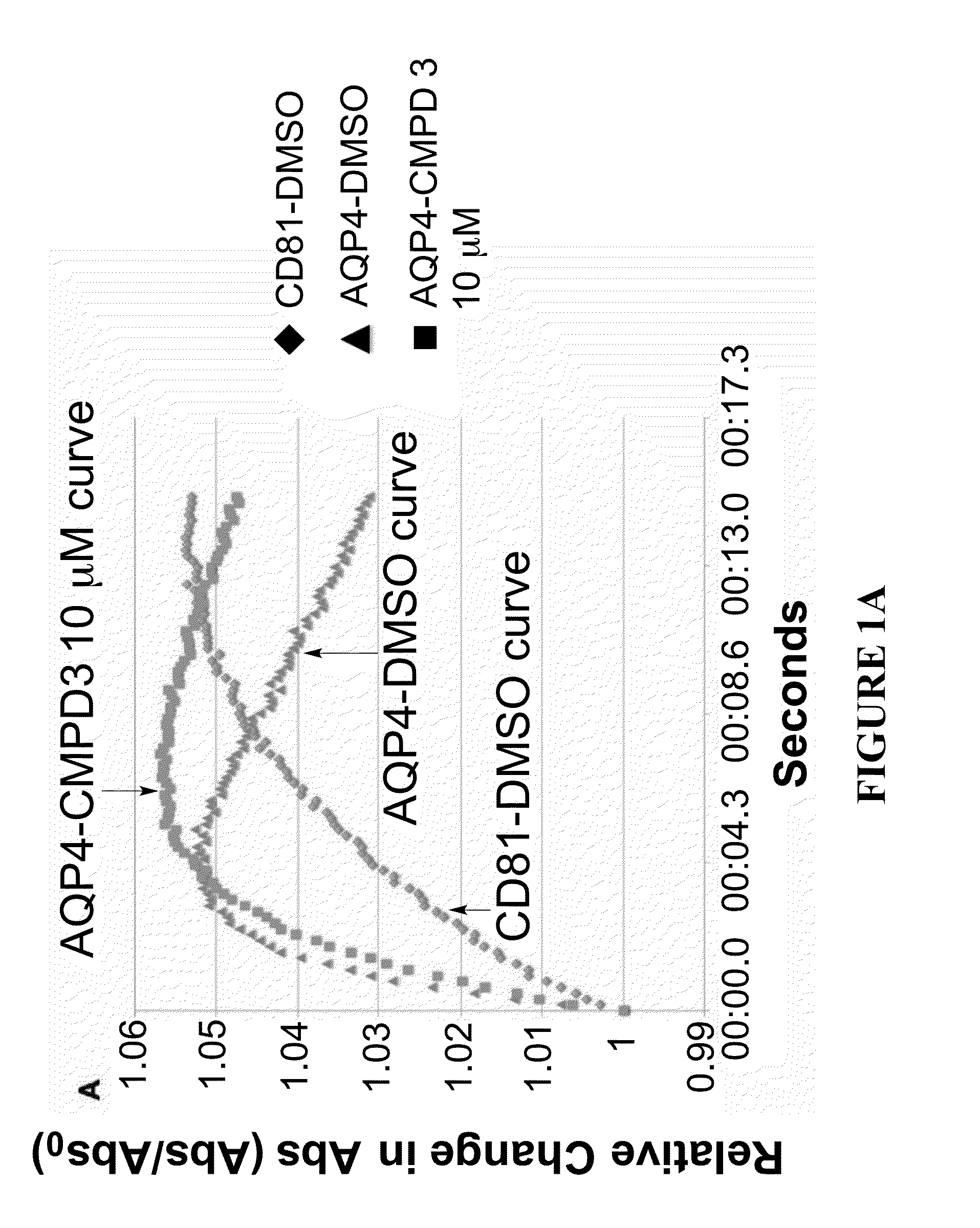
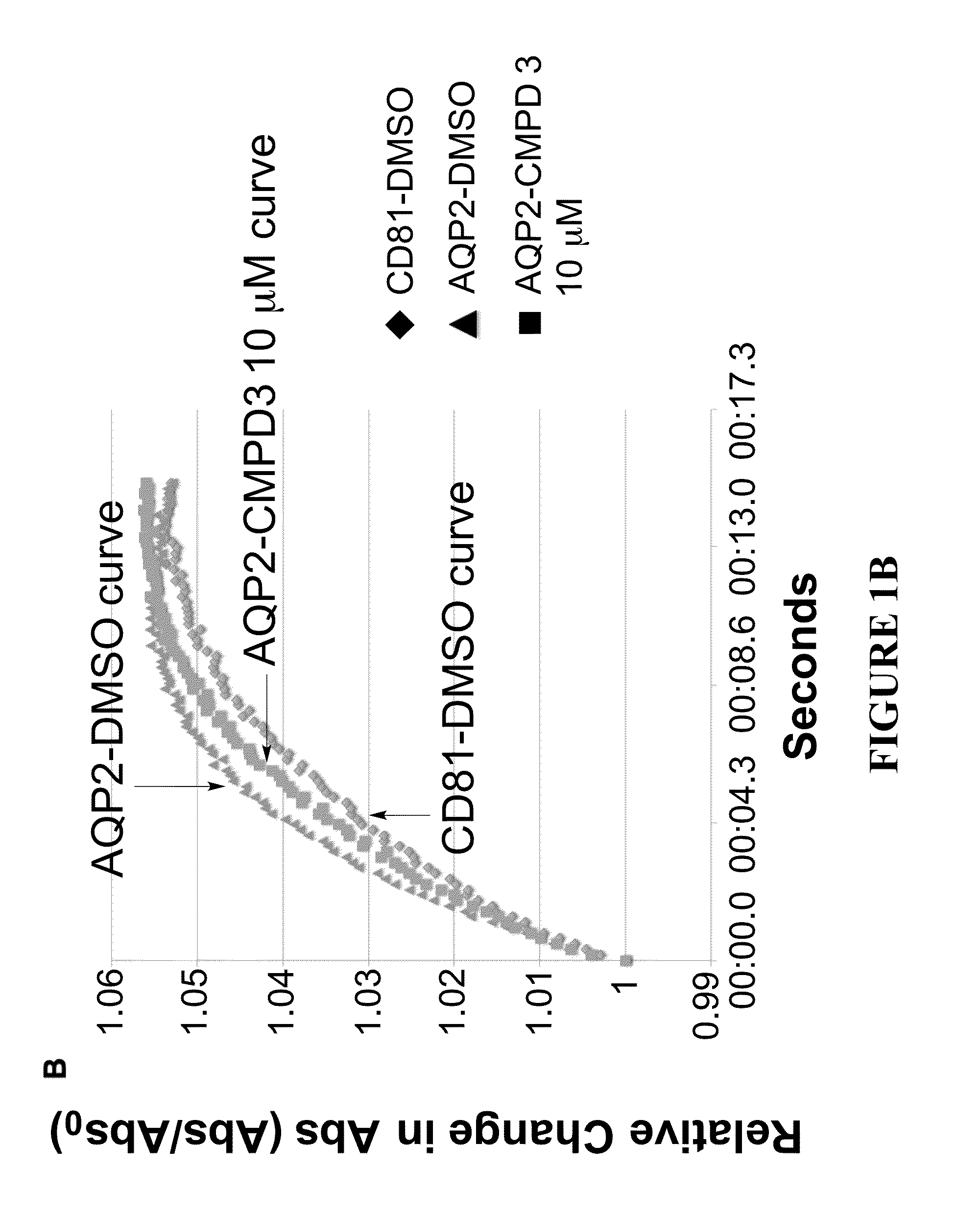
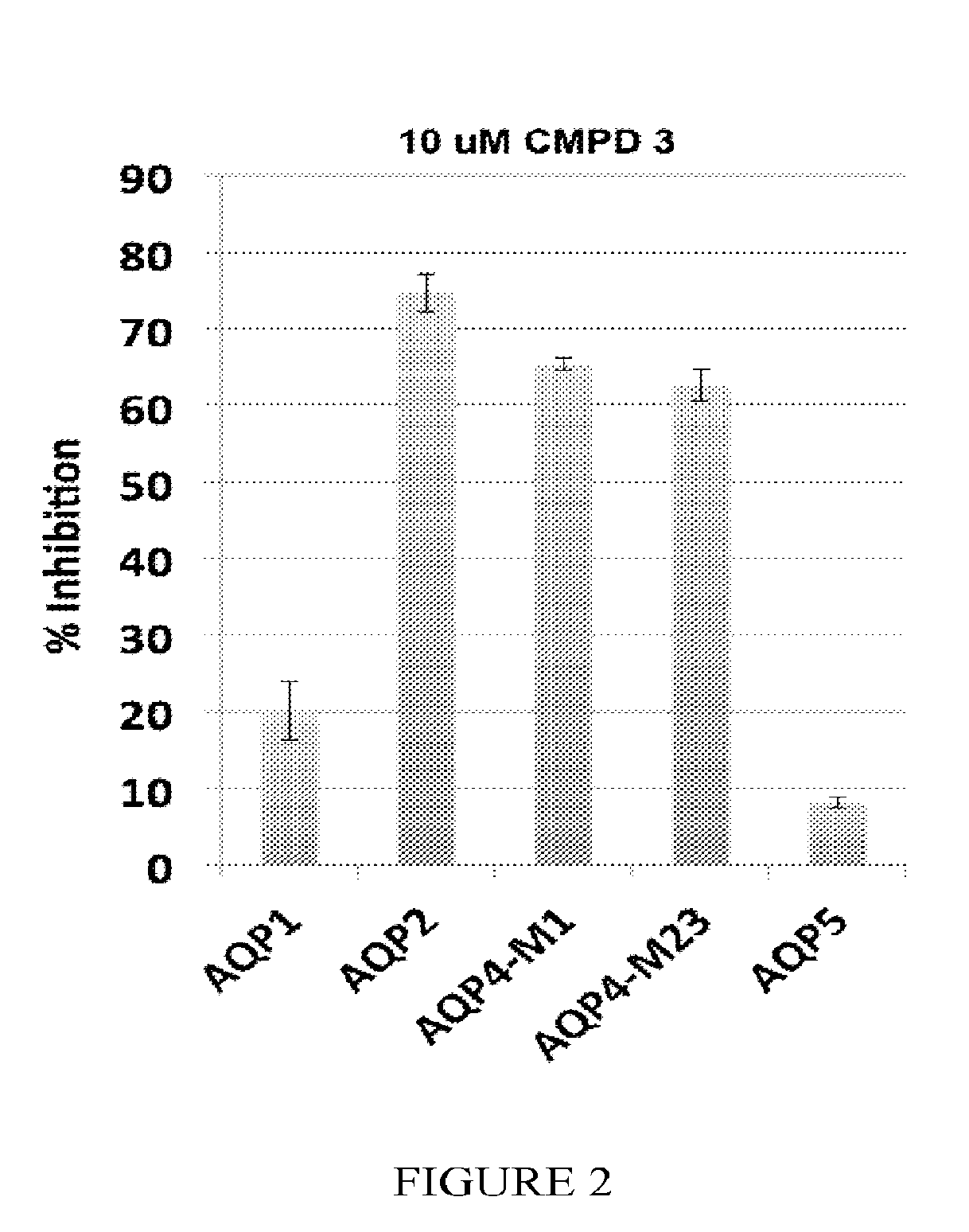
Methods
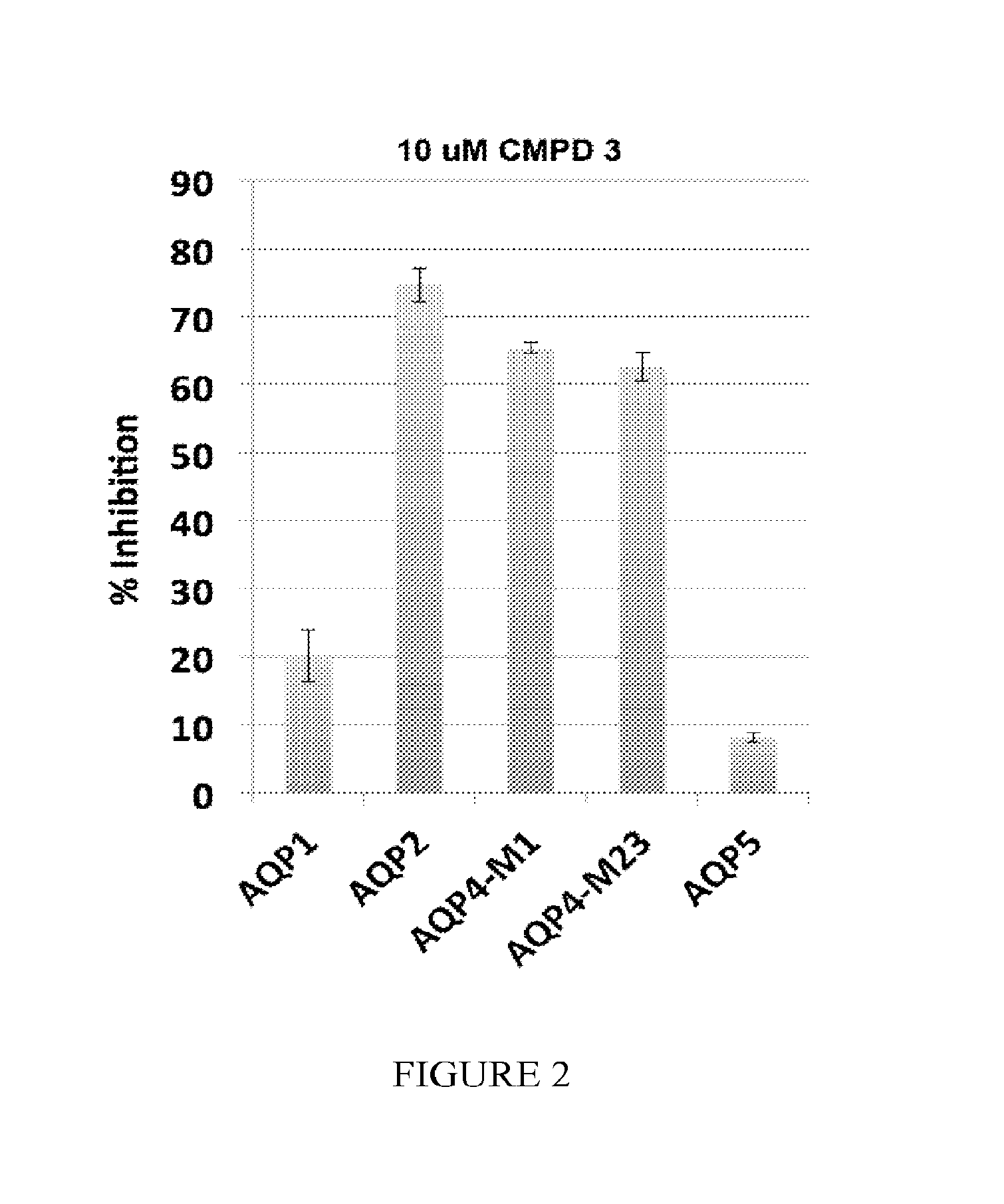
The present invention relates to the use of selective aquaporin inhibitors, e.g., of aquaporin-4 or aquaporin-2, e.g., certain phenylbenzamide compounds, for the prophylaxis, treatment and control of aquaporin-mediated conditions, e.g., diseases of water imbalance, for example edema (particularly edema of the brain and spinal cord, e.g., following trauma or ischemic stroke, as well as the edema associated with glioma, meningitis, acute mountain sickness, epileptic seizures, infections, metabolic disorders, hypoxia, water intoxication, hepatic failure, hepatic encephalopathy, diabetic ketoacidosis, abscess, eclampsia, Creutzfeldt-Jakob disease, and lupus cerebritis, as well as edema consequent to microgravity and / or radiation exposure, as well as edema consequent to invasive central nervous system procedures, e.g., neurosurgery, endovascular clot removal, spinal tap, aneurysm repair, or deep brain stimulation, as well as retinal edema), as well as hyponatremia and excess fluid retention, and diseases such as epilepsy, retinal ischemia and other diseases of the eye associated with abnormalities in intraocular pressure and / or tissue hydration, myocardial ischemia, myocardial ischemia / reperfusion injury, myocardial infarction, myocardial hypoxia, congestive heart failure, sepsis, and neuromyelitis optica, as well as migraines, as well as to novel assays for identifying aquaporin inhibitors.
Owner:AEROMICS
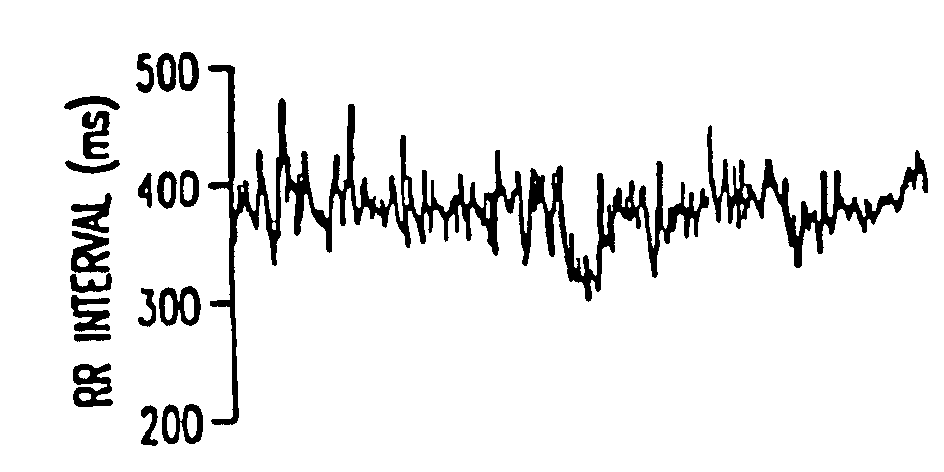
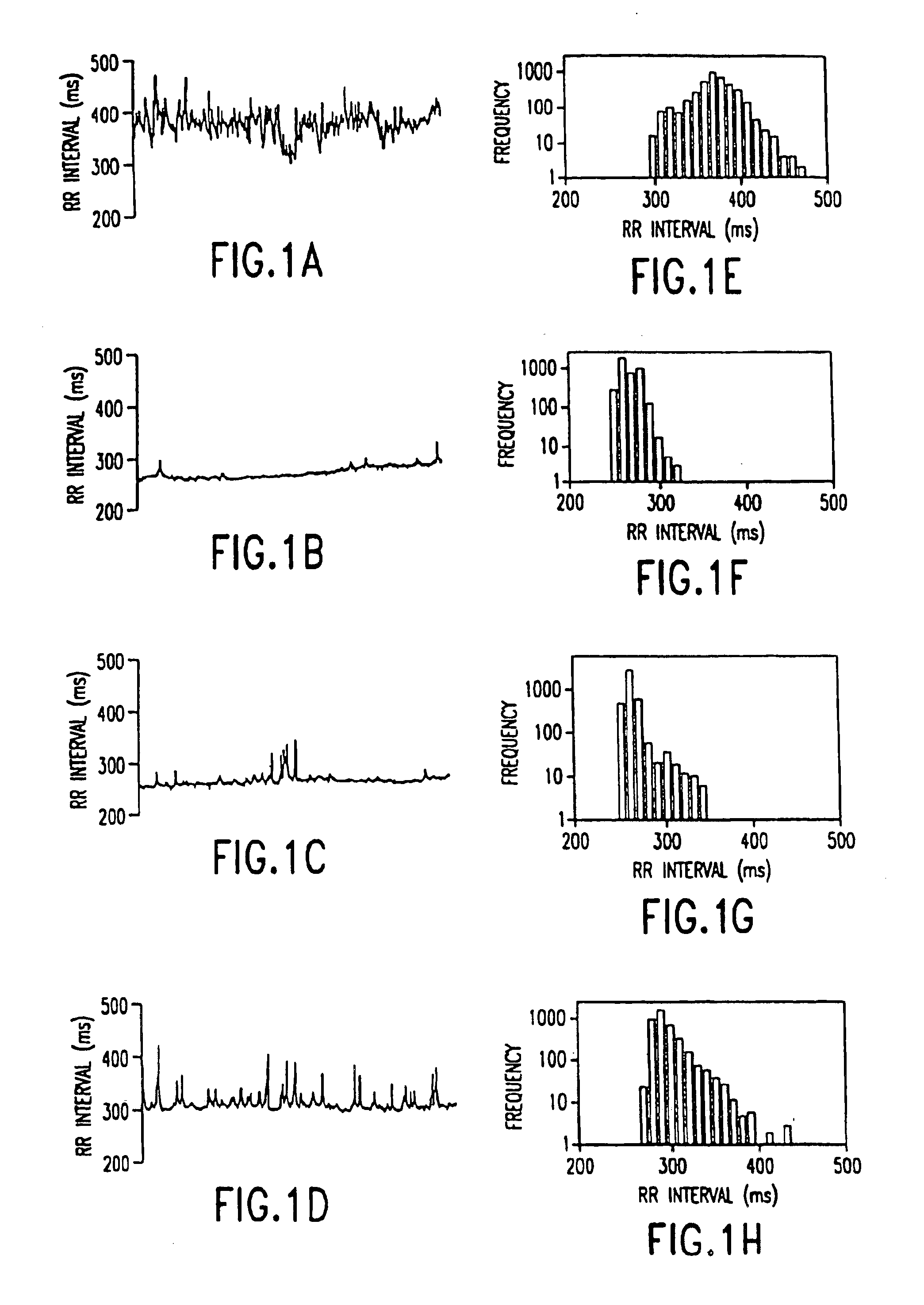
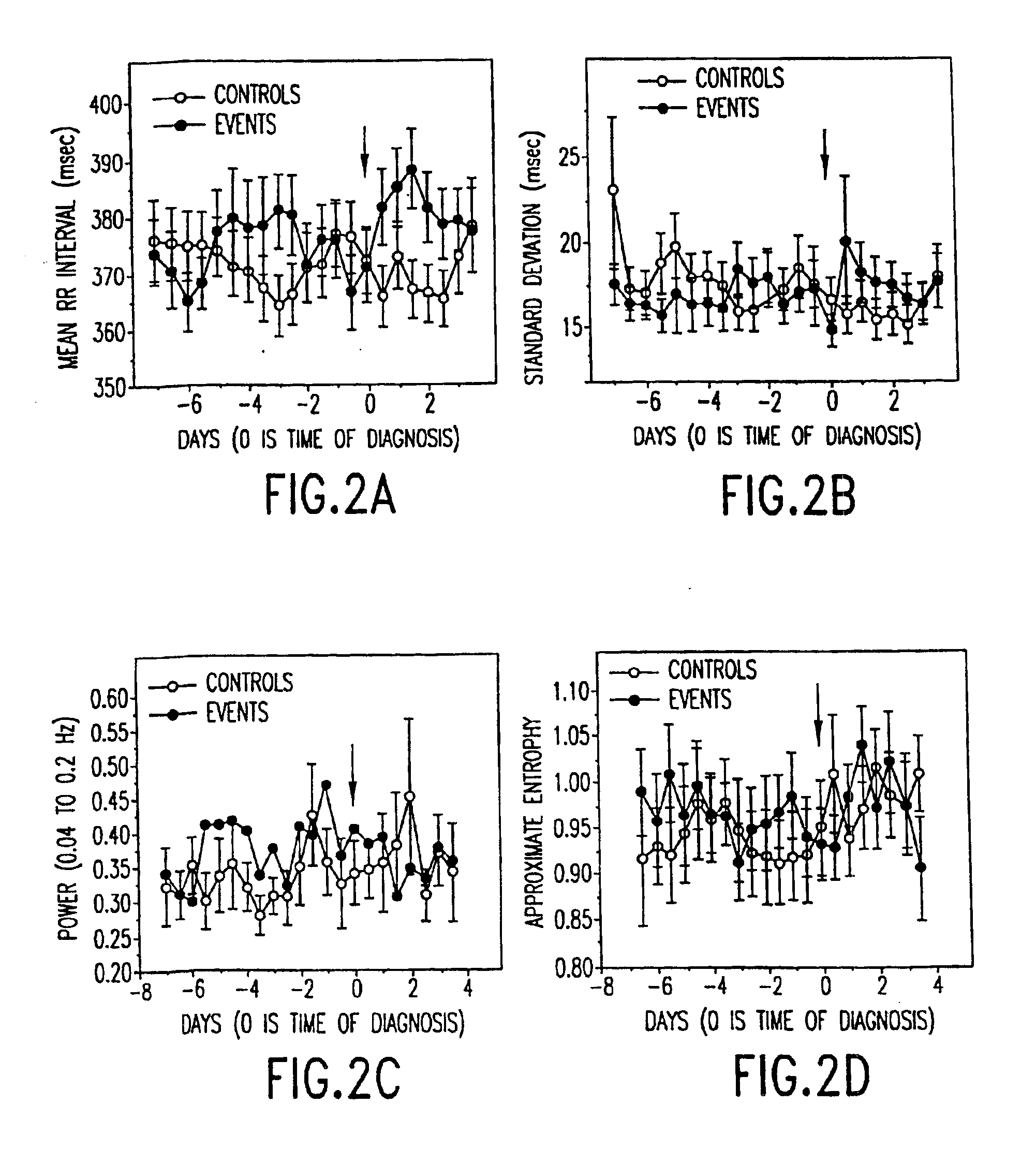
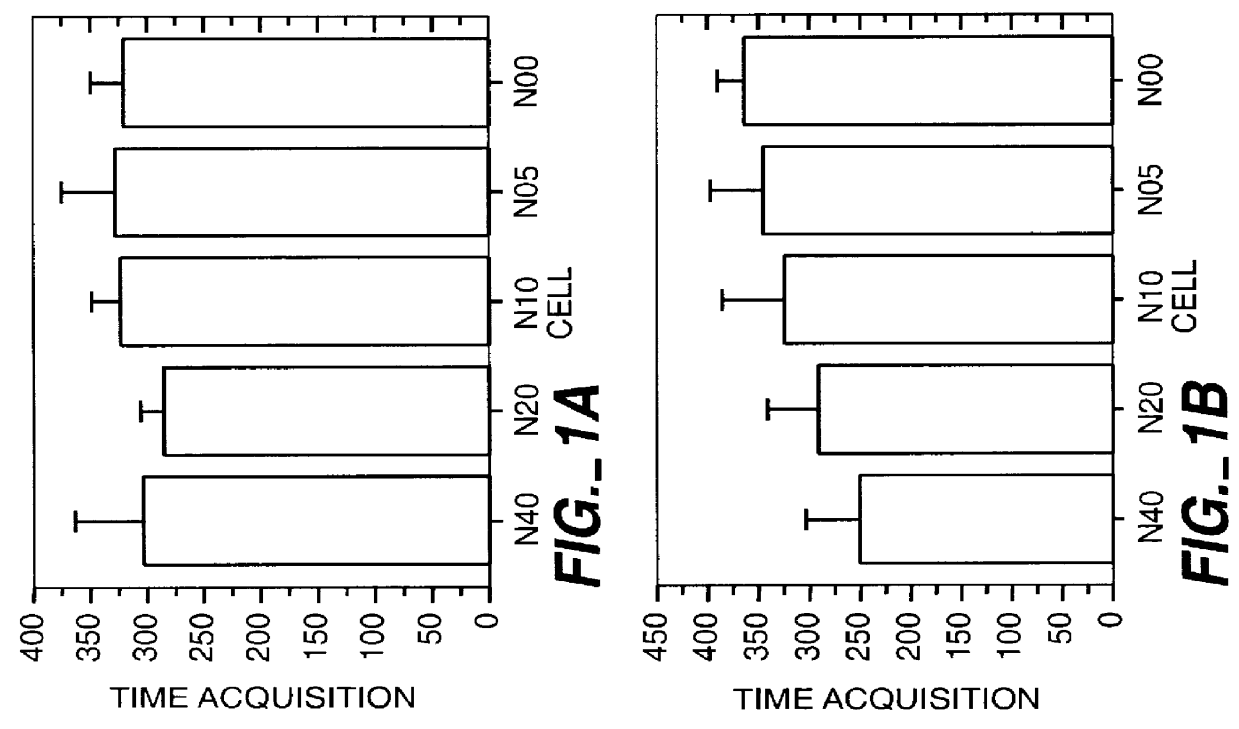
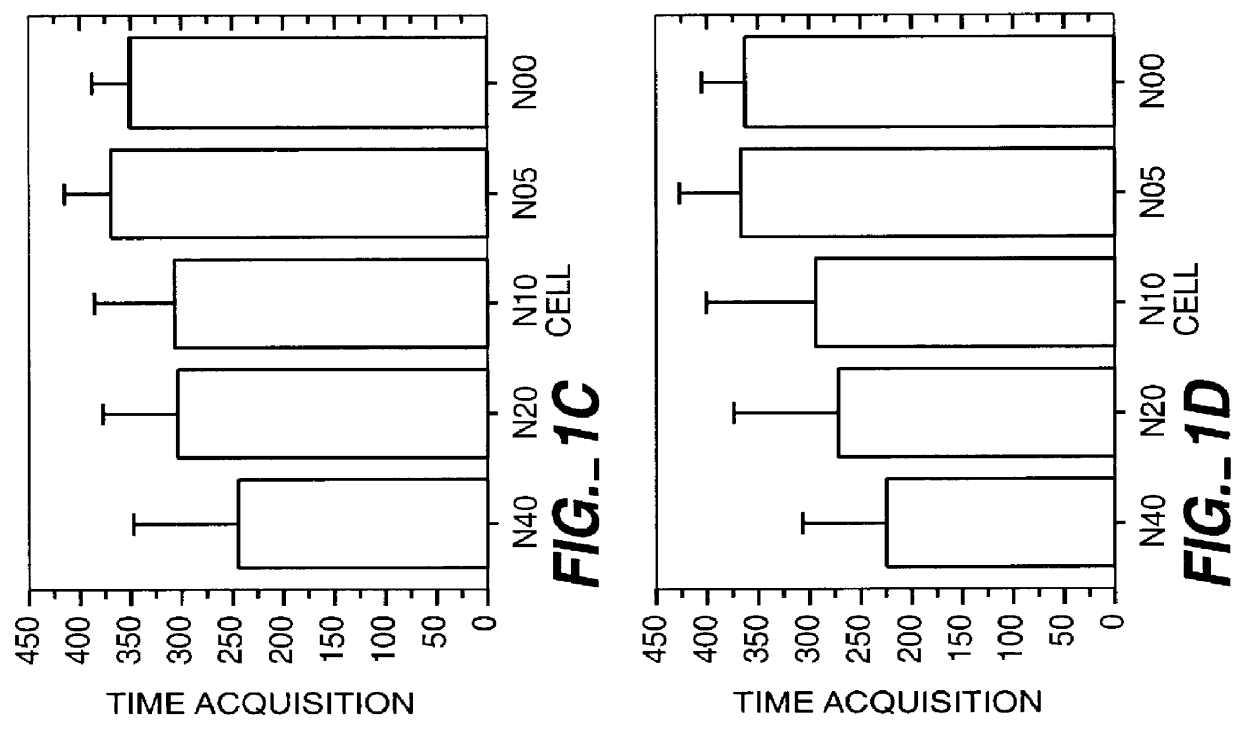
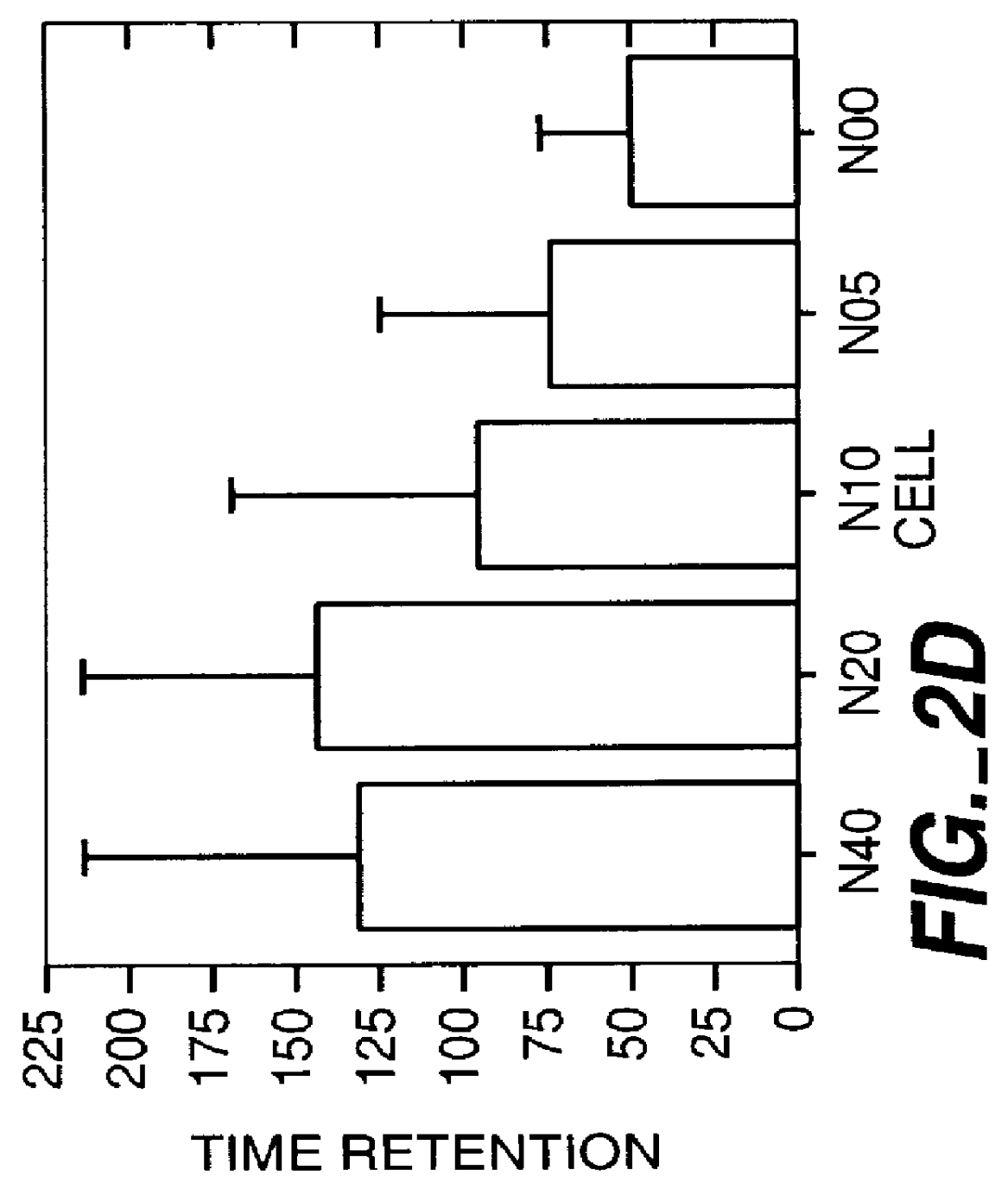
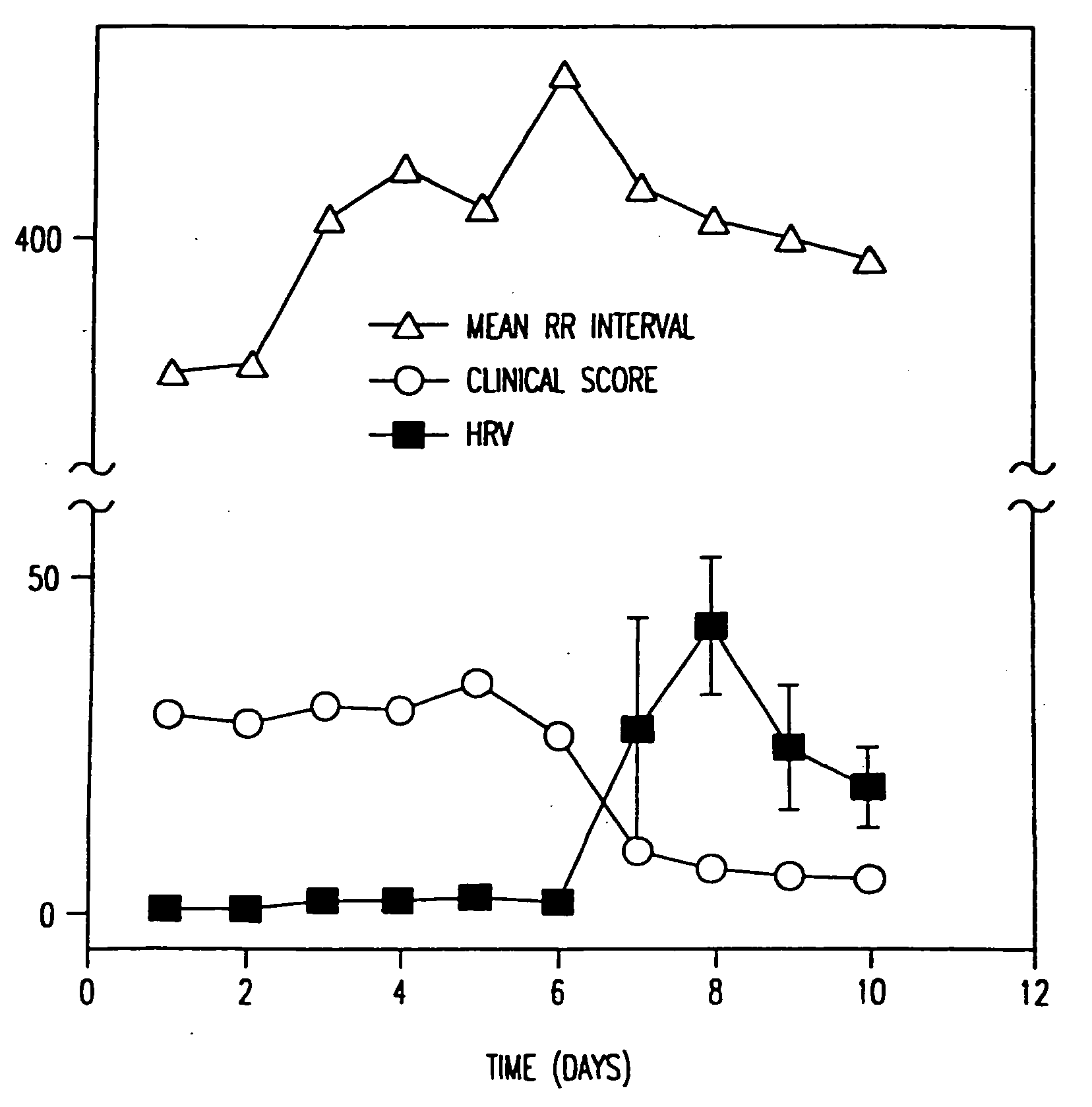
Method for the early diagnosis of subacute, potentially catastrophic illness
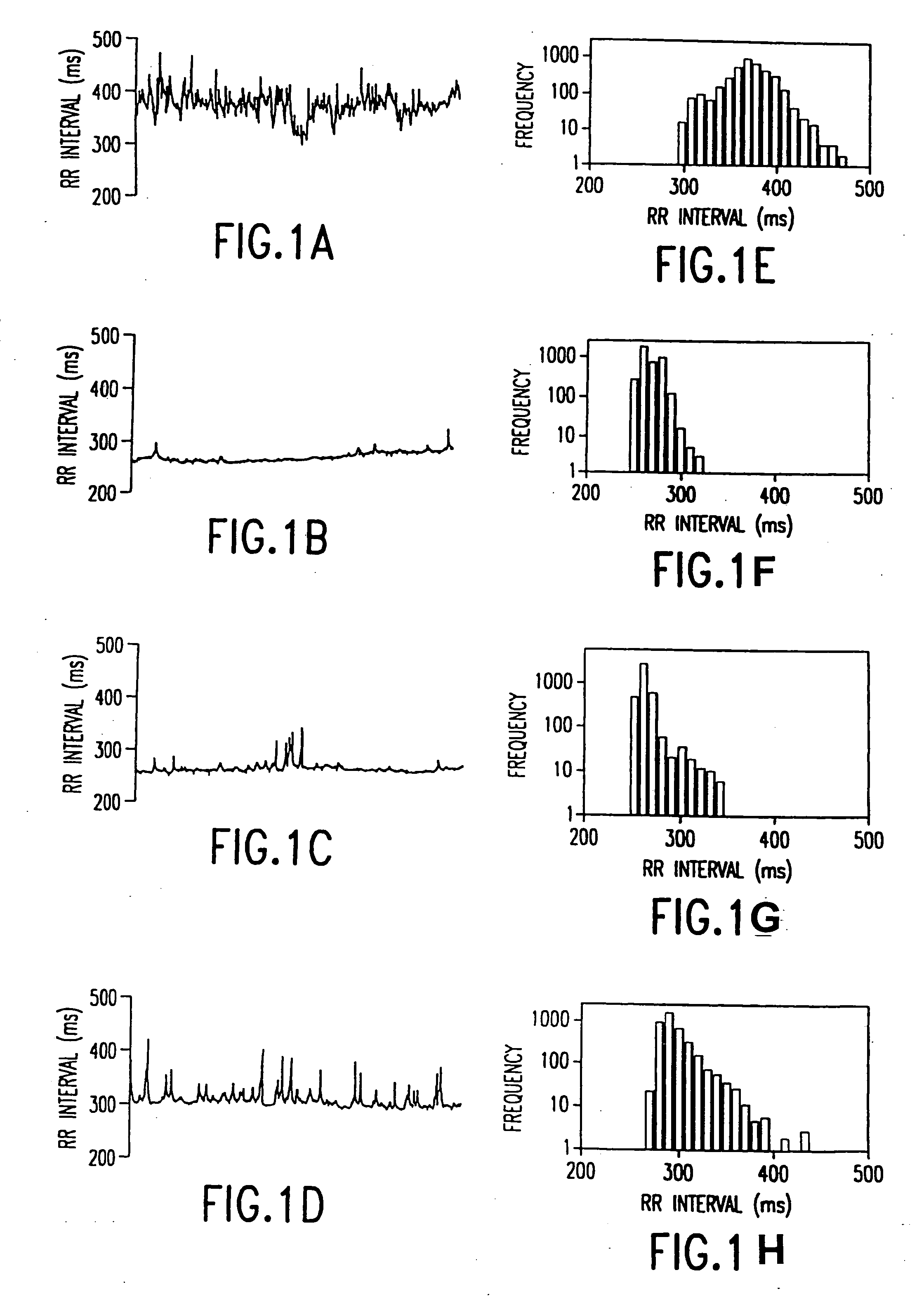
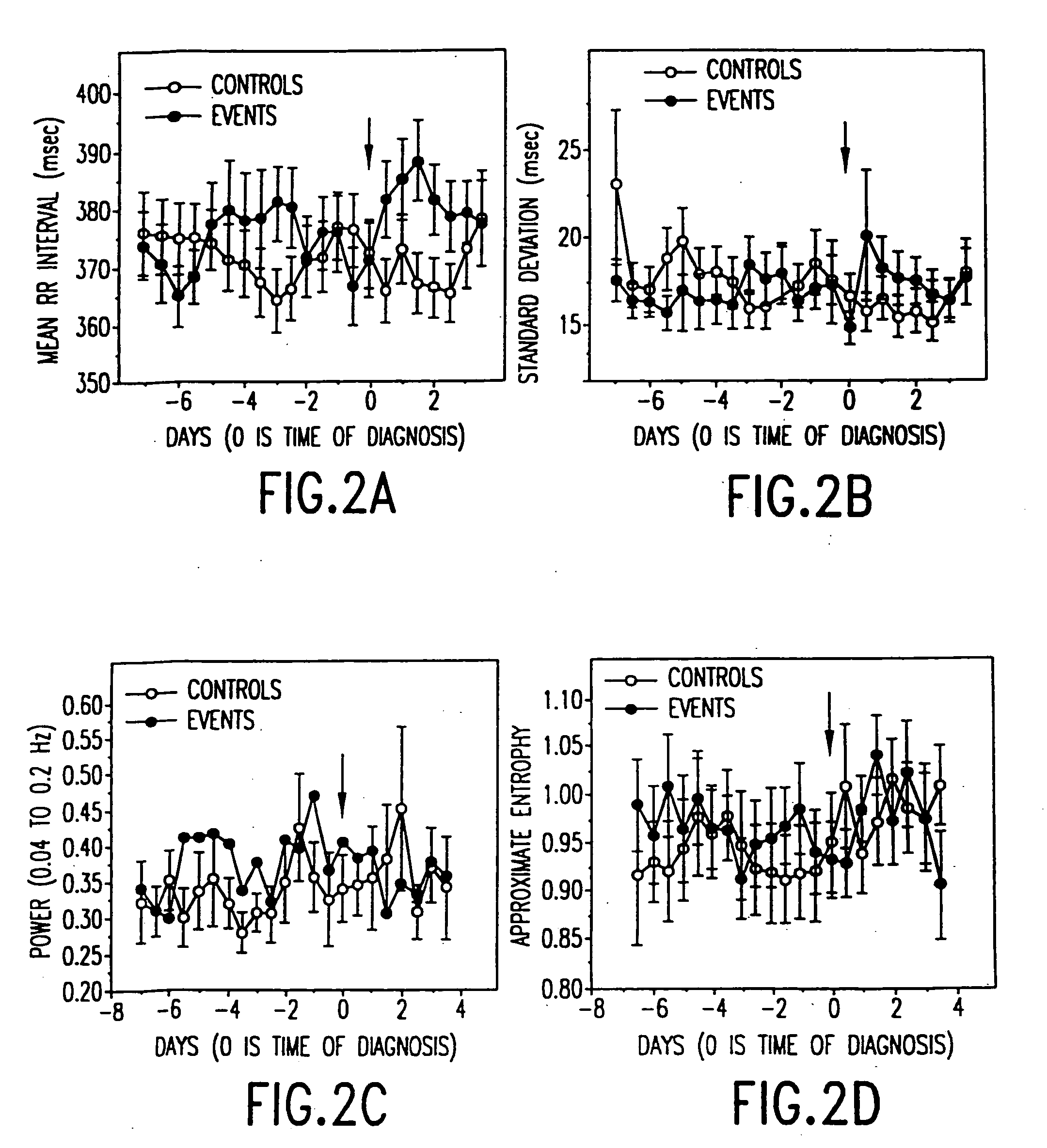
In one aspect of the invention, there is provided a method for early detection of subacute, potentially catastrophic illness in an infant. The method comprises: (a) monitoring heart rate variability in the infant; and (b) identifying at least one characteristic abnormality in the heart rate variability that is associated with the illness. This method can be used to diagnose illnesses such as, but not limited to, sepsis, necrotizing enterocolitis, pneumonia and meningitis. In another aspect of the present invention, there is provided a method for early detection of subacute, potentially catastrophic illness in an infant, which comprises: (a) monitoring the patient's RR intervals; (b) generating a normalized data set of the RR intervals; (c) calculating one or more of (i) moments of the data set selected from the third and higher moments and (ii) percentile values of the data set; and (d) identifying an abnormal heart rate variability associated with the illness based on one or more of the moments and the percentile values.
Owner:VIRGINIA UNIV OF THE +1
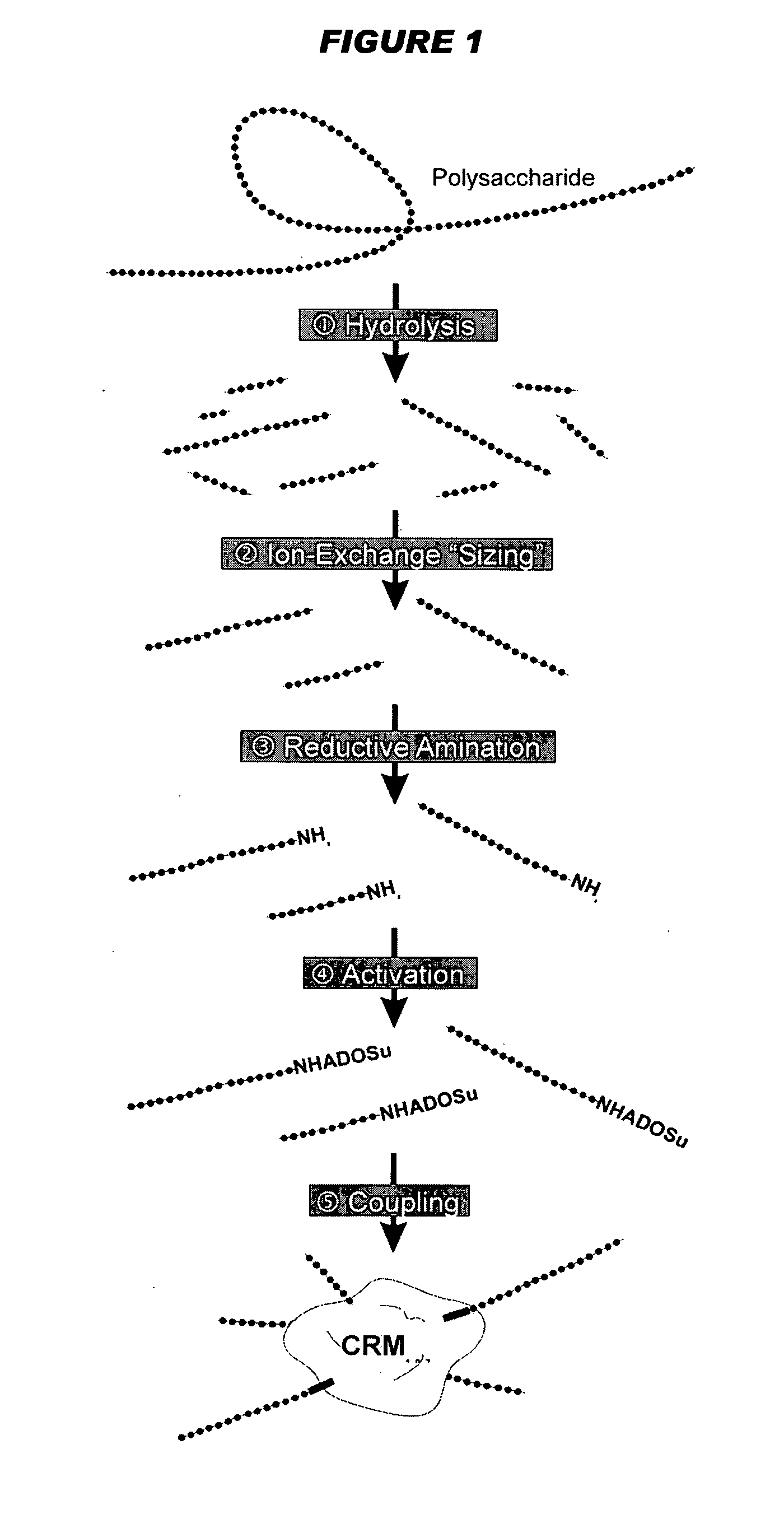
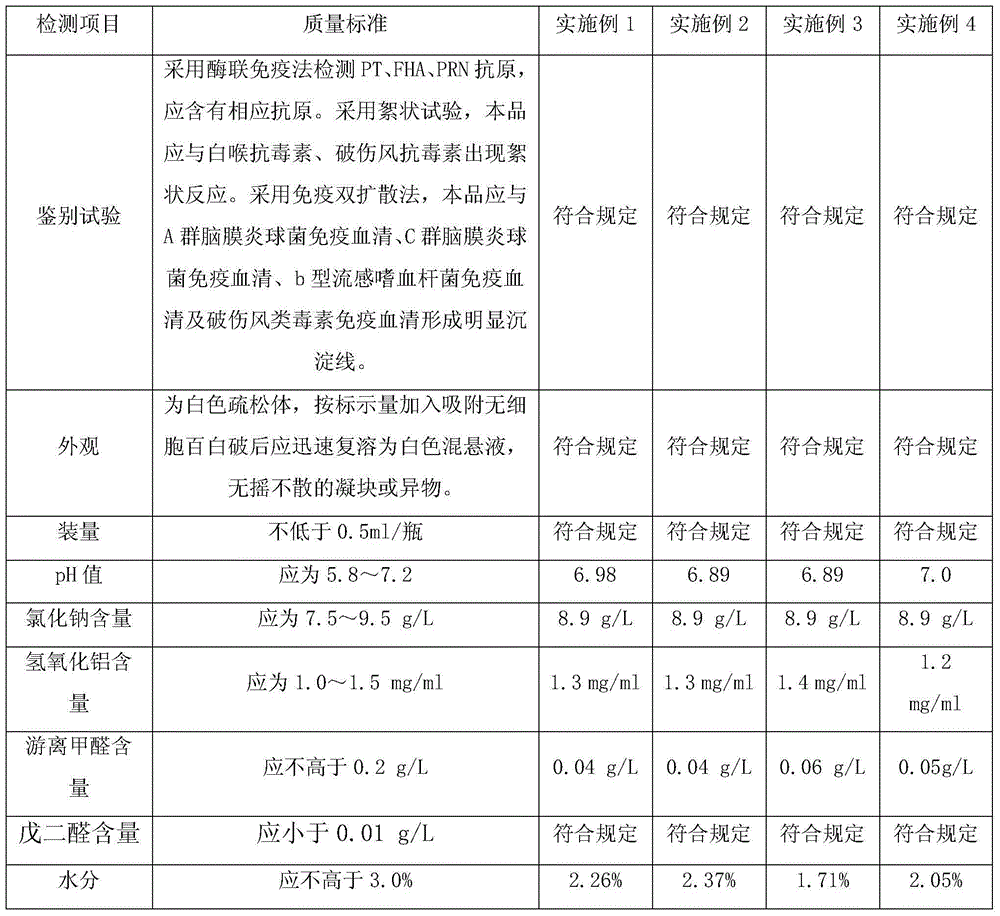
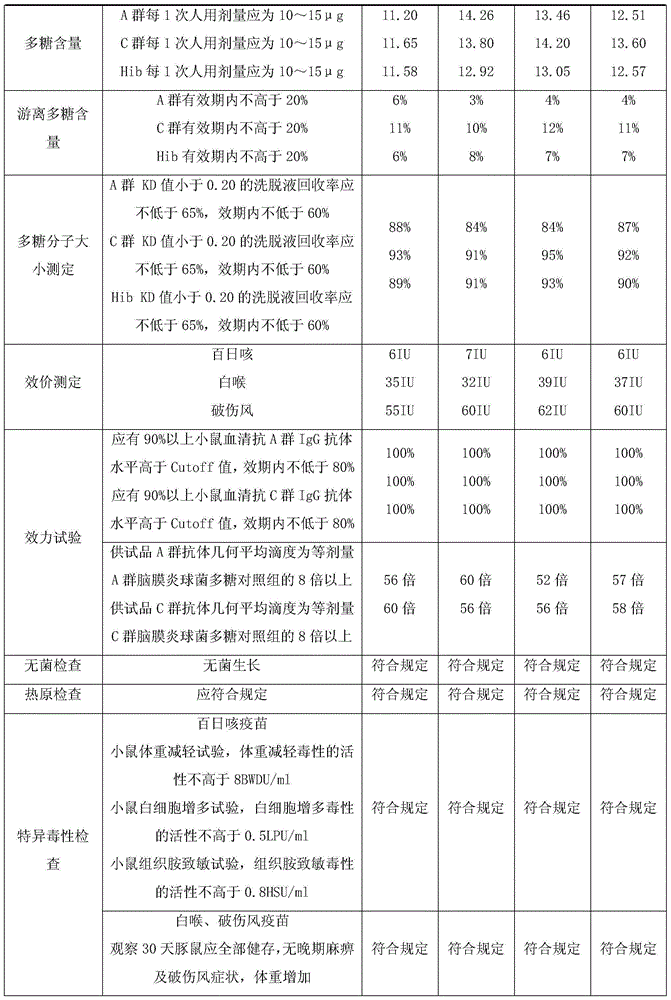
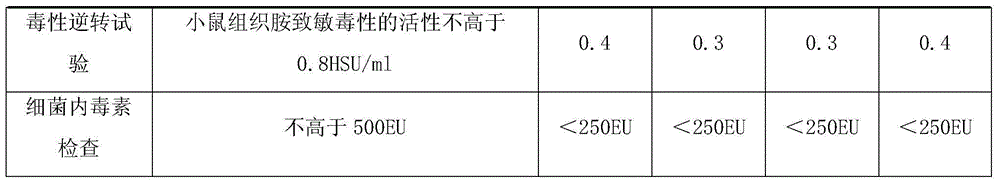
Separated and purified acellular pertussis-diphtheria-tetanus, b-type haemophilus influenzae and A-group and C-group meningococcus combined vaccine and preparation method thereof
InactiveCN104689309ARelieve painReduce the burden onAntibacterial agentsBacterial antigen ingredientsHemagglutininAluminium hydroxide
The invention discloses a combined vaccine and a preparation method thereof. The combined vaccine is formed by a component A and a component B, wherein the component A is a liquid preparation and composed of pertussis toxin, filamentous hemagglutinin, pertussis adhesion, diphtheria toxoid, tetanus toxoid, aluminium hydroxide and sodium chloride; the component B is a freeze-drying preparation and composed of A-group meningitis polysaccharide conjugate, C-group meningitis polysaccharide conjugate, b-type haemophilus influenzae polysaccharide conjugate and lactose. The preparation method of the vaccine includes the steps of preparing of acellular pertussis-diphtheria-tetanus vaccine semi-finished products, A-group and C-group meningococcocci and b-type haemophilus influenzae combined vaccine semi-finished products, split charging and packaging. The vaccine has the characteristics of being safe, effective, controllable and capable of preventing diseases through an injection, the preparation method is easy to operate, preparation is facilitated, cost is low, and the combined vaccine is suitable for industrialized mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
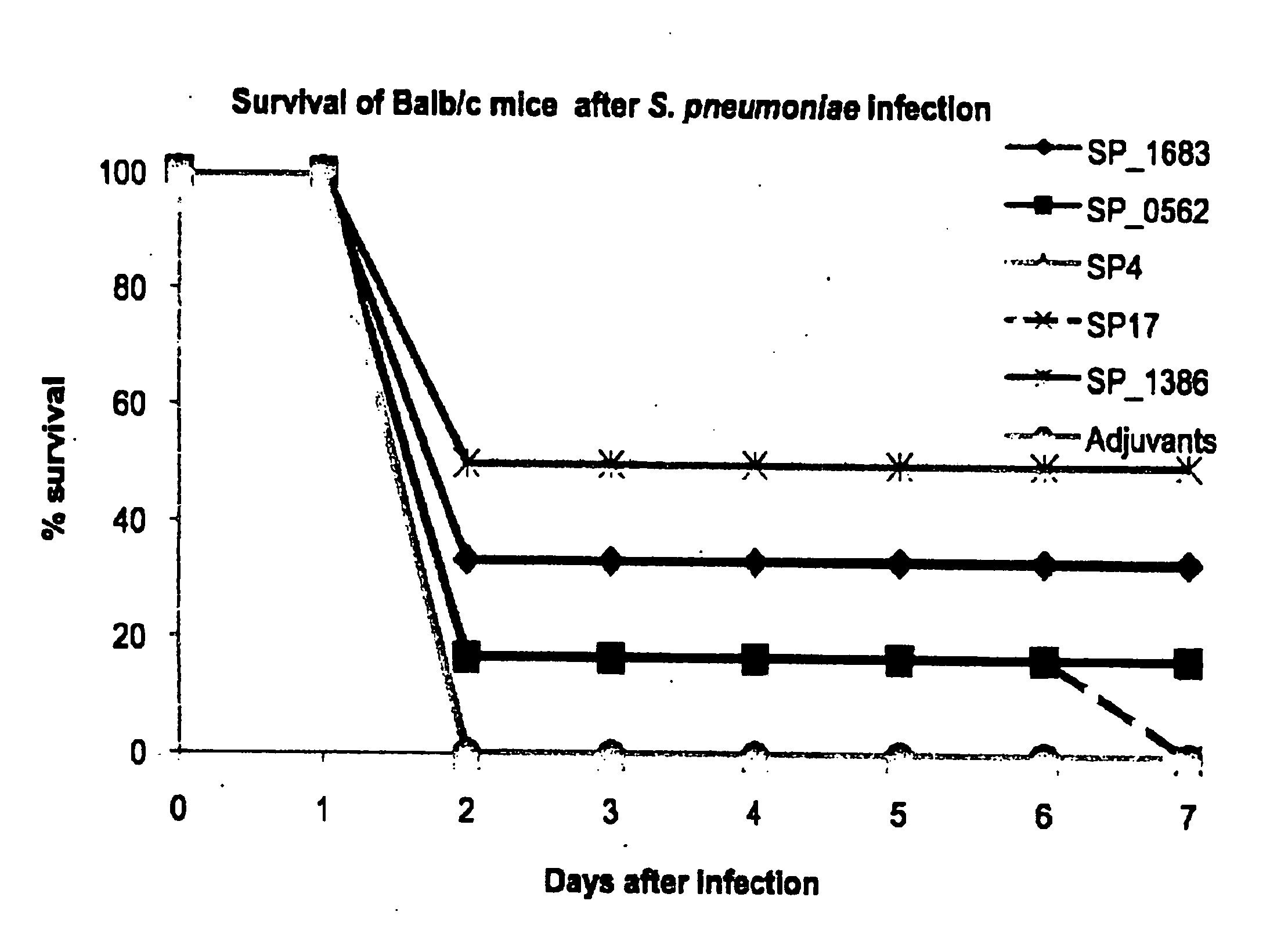
Streptococcus pneumoniae vaccines
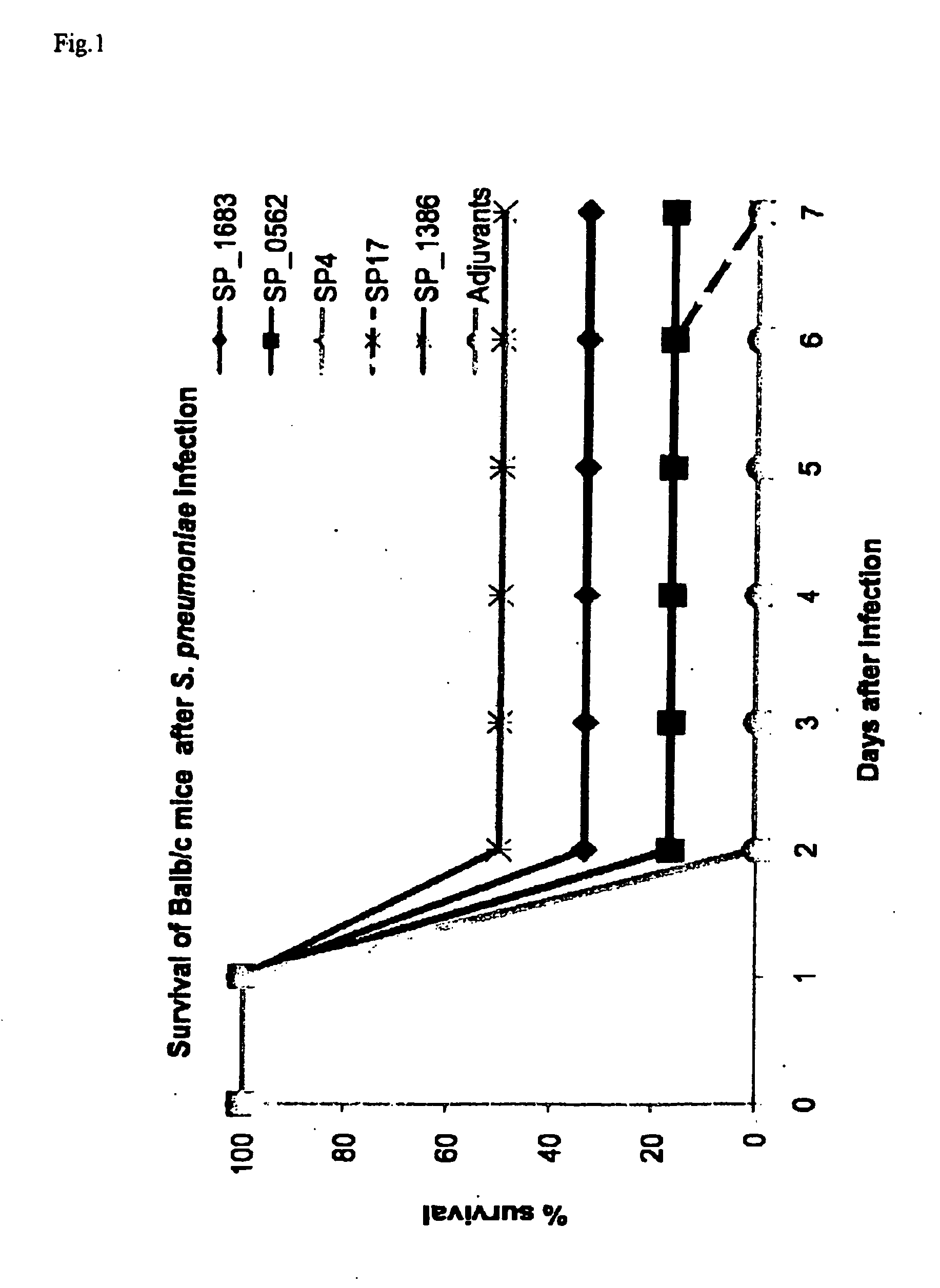
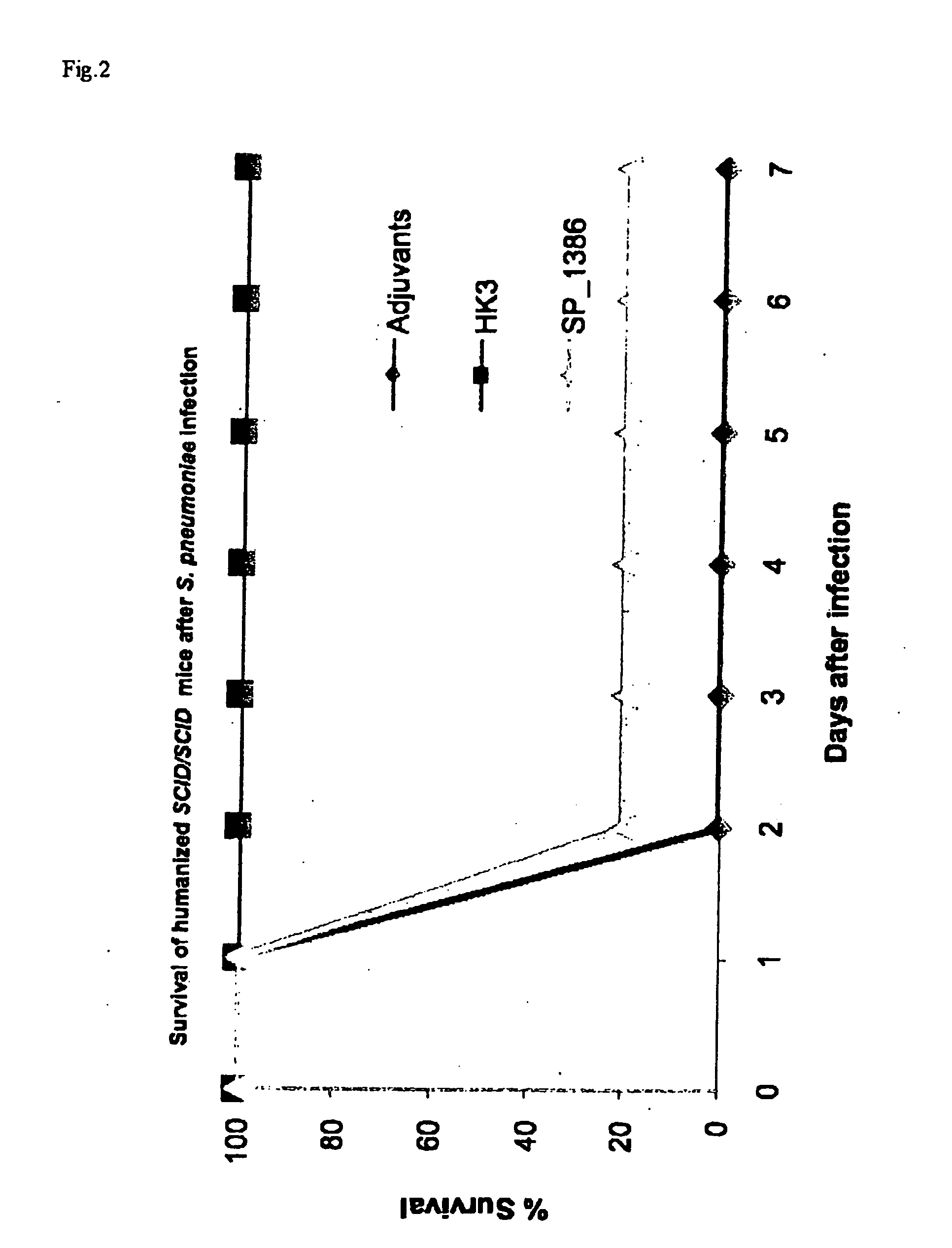
Streptococcus pneumoniae is a major cause of pneumoniae, meningitis, and major cause of morbidity and mortality throughout the world by bacterial otitis media, pneumoniae, meningitis, and bacteraemia. It is an important agent of disease in man especially among infants, the elderly and immunocompromised persons. The present invention provides a solution to this problem by providing a substantially pure or isolated disease related antigen selected from the group consisting of the isolated, recombinant or synthetic S. pneumoniae human immunogenic antigens of SP_0562, SP_0965, SP_0082 (in particular the fragments SP4 and SP 17 of said SP_0082), and a Periplasmic Binding Protein (PBP) (in particular SP_1683 or SP_1386), or a fragments thereof or substantially identical antigen for use in a treatment to induce a immunological memory in a human against S. pneumoniae cells for use in a vaccination treatment of S. pneumoniae disorder in human or against S. pneumoniae in a human. In a particular embodiment, the present invention provides an isolated, recombinant or synthetic S. pneumoniae Periplasmic Binding Protein (PBP) (in particular SP_1683 or SP_1386) as a disease related antigen for use in a treatment to induce a immunological memory in a human against S. pneumoniae cells for use in a vaccination treatment of S. pneumoniae disorder in human or for use in the treatment of an S. pnewnoniae infection in a human. It further provides antibodies that specifically bind to the S. pneumoniae disease related antigens identified herein for use in the treatment of a an S. pneumoniae infection in a human, such as for example in a treatment to induce immunological memory in a human against S. pneumoniae, i.e. in a vaccination treatment of S. pneumoniae. It is also an aspect of the present invention to provide the use of any one of the S. pneumoniae disease related antigens as identified herein, or of the antibodies specific for said antigens in methods to diagnose for a S. pneumoniae disorder in a human.
Owner:KATHOLIEKE UNIV LEUVEN
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS7501134B2Easy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Nucleic acid kit for bacterial pathogen diagnosis and method for using the same
InactiveUS20040010129A1Sugar derivativesMicrobiological testing/measurementBacteroidesNucleic acid sequencing
The present invention relates to a nucleic acid kit for bacterial pathogen diagnosis and method for using the same, which provides with a quick diagnosis for 20 species of bacterial pathogens. The present invention is to align the nucleic acid sequences of each bacterial pathogen, single out the specific region thereof, find out the corresponsive consensus primers, and amplify the specific nucleic acid sequences of each bacterial pathogen, whereafter the nucleic acid kit is acquired. Further, such nucleic acid kit can be utilized as probes to be conjugated on polymers as diagnostic chips for bacterial pathogens (for example, the meningitis chip), and then the detection reaction proceeds as the nucleic acid sequences of pathogen purified from clinical sample and amplified by using the foregoing primers are to react with the bacterial pathogen diagnostic chip of the present invention, with the species of the infecting bacteria thus being determined.
Owner:GENEMASTER LIFESCI
hNT-neuron human neuronal cells to replace ganglion cells
Disclosed herein is the treatment of vision loss in a mammal by transplanting an effective amount of hNT-Neuron cells. The treatment can be accomplished by injecting the cells into the retinal area of the eye. Additionally, the cells can be injected into the visual cortex of the brain. Conditions to be treated are vision loss due to optic nerve damage, including glaucoma, optic nerve sheath meningioma and glioma, Graves' ophthalmopathy, benign or malignant orbital tumors, metastatic lesions, tumors arising from the adjacent paranasal sinuses or middle cranial fossa, giant pituitary adenomas, brain tumors or abscesses, cerebral trauma or hemorrhage, meningitis, arachnoidal adhesions, pseudotumor cerebri, cavernous sinus thrombosis, dural sinus thrombosis, encephalitis, space-occupying brain lesions, severe hypertensive disease or pulmonary emphysema.
Owner:LAYTON BIOSCI
Method and apparatus for the early diagnosis of subacute, potentially catastrophic illness
In one aspect of the invention, there is provided a method and apparatus for early detection of subacute, potentially catastrophic infectious illness in a premature newborn infant. The method comprises: (a) continuously monitoring heart rate variability in the premature newborn infant; and (b) identifying at least one characteristic abnormality in the heart rate variability that is associated with the illness. This method can be use to diagnose illnesses such as, but not limited to, sepsis, necrotizing enterocolitis, pneumonia and meningitis. In another aspect of the present invention, there is provided a method and apparatus for early detection of subacute, potentially catastrophic infectious illness in a patient. The method comprises: (a) continuously monitoring the patient's RR intervals; (b) generating a normalized data set of the RR intervals; (c) calculating one or more of (i) moments of the data set selected from the third and higher moments and (ii) percentile values of the data set; and (d) identifying an abnormal heart rate variability associated with the illness based on one or more of the moments and the percentile values.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS20050220883A1Without usingEasy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Microparticles with absorbed polypeptide-containing molecules formed without the use of surfactant, methods of making such microparticle compositions, and uses thereof, are disclosed. The microparticles comprise a polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, and the like. Preferred polymers are poly(D,L-lactide-co-glycolides), more preferable those having a lactide / glycolide molar ratio ranging from 40:60 to 60:40 and having a molecular weight ranging from 20,000 Daltons to 70,000 Daltons. Preferred polypeptide containing molecules are bacterial and viral antigens (including HIV antigens, meningitis B antigens, streptococcus B antigens, and Influenza A hemagglutinin antigens).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Method and system for detecting at least one particle in a bodily fluid, and associated method for diagnosing meningitis
ActiveUS20170045439A1Efficient use ofLess expensiveBiological particle analysisIndividual particle analysisPhotovoltaic detectorsPhotodetector
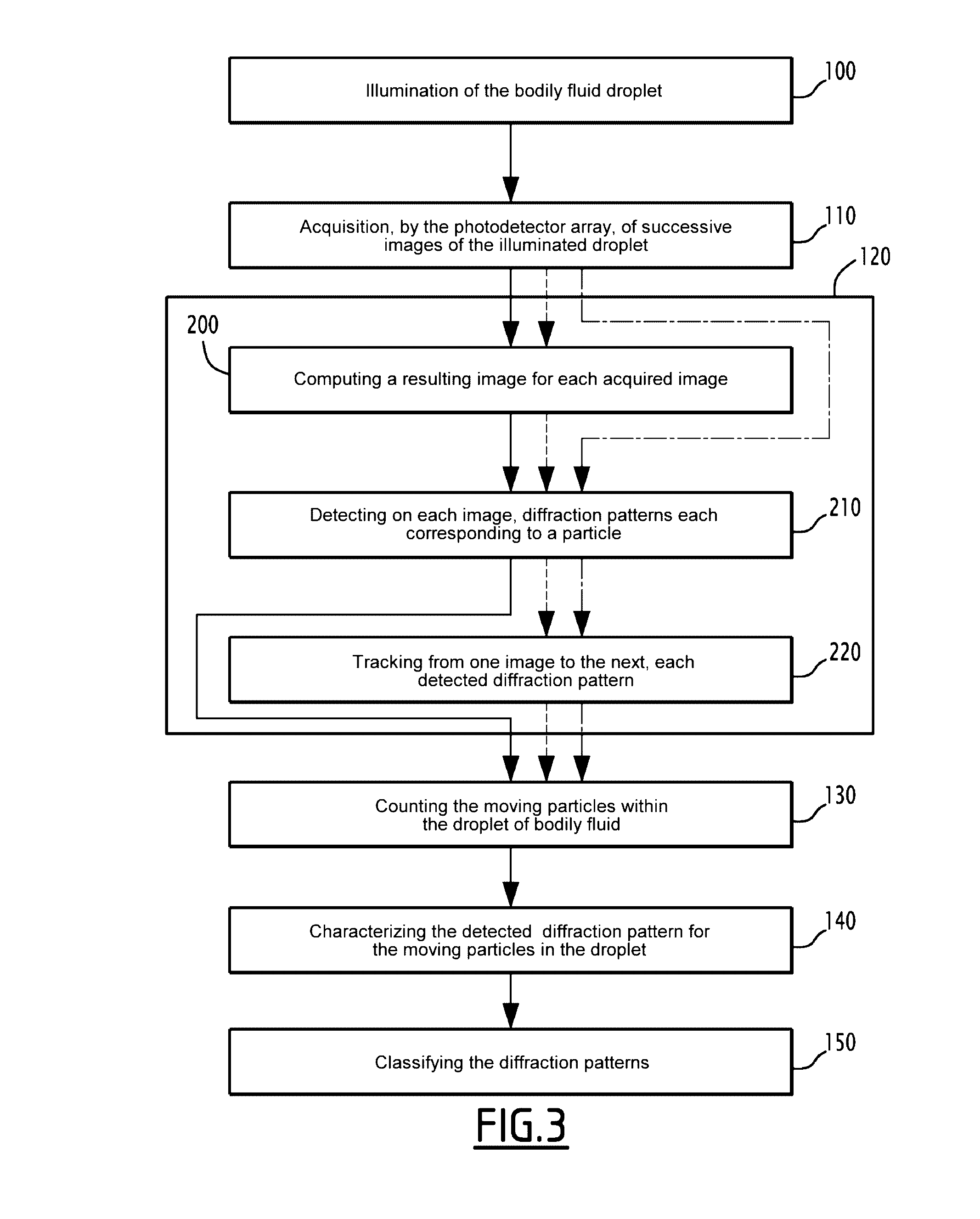
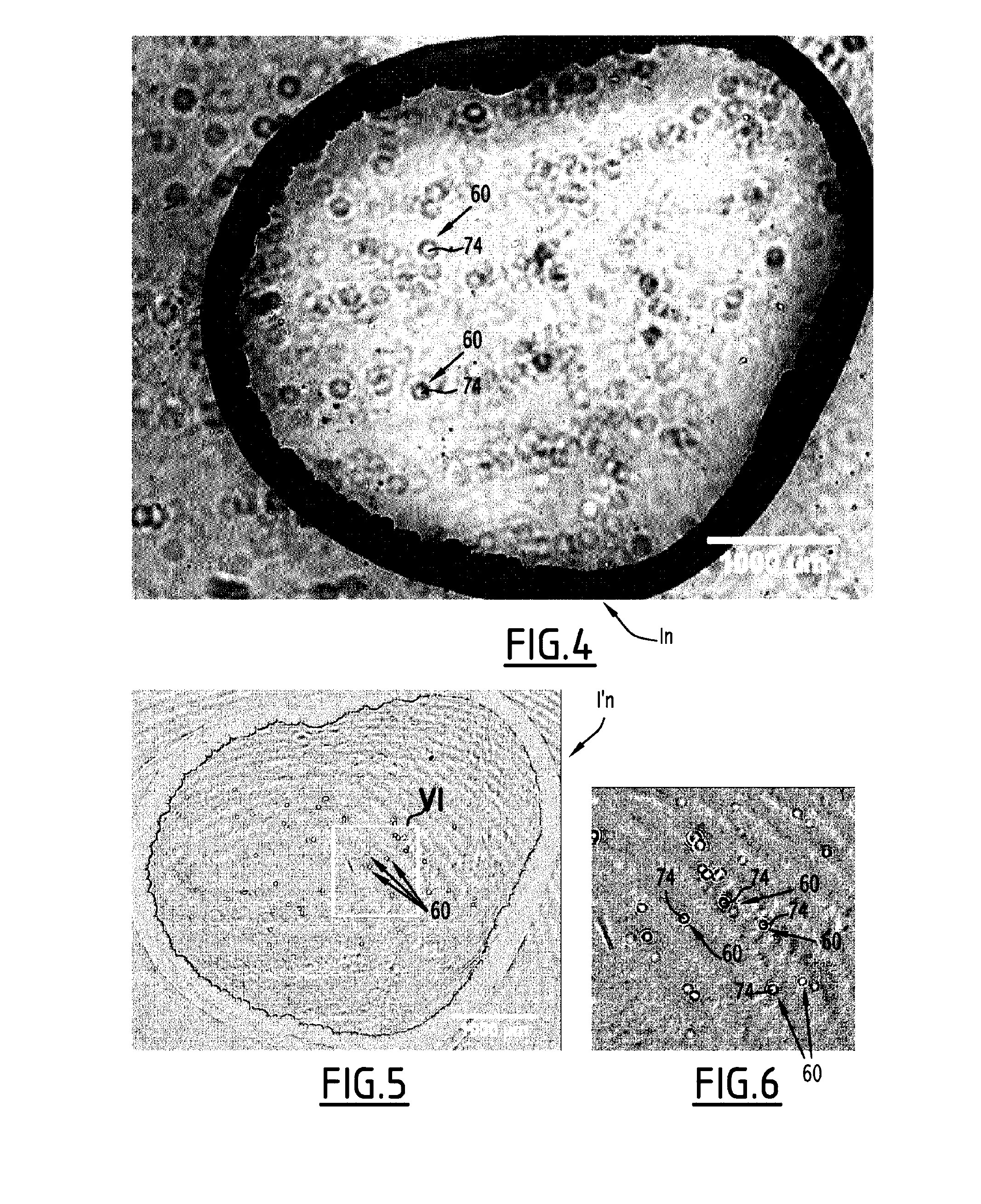
This method for detecting at least one particle in a bodily fluid is carried out via a detection system including a light source, a transparent substrate and a photodetector array, the substrate being positioned between the light source and the photodetector. This method includes the placement of a droplet of bodily fluid on the substrate, the illumination of the droplet via the light source, the acquisition of several successive images of the droplet via the photodetector, each image being formed by radiation transmitted by the illuminated droplet and including at least one elementary diffraction pattern, each elementary diffraction pattern corresponding to waves diffracted by a particle upon illumination of the droplet, the identification, via the acquired images of the mobile elementary diffraction patterns, and the counting of moving particles in the droplet, via the identified mobile elementary diffraction patterns.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Kit for synchronously detecting twenty-three meningitis pathogens and detection method of kit
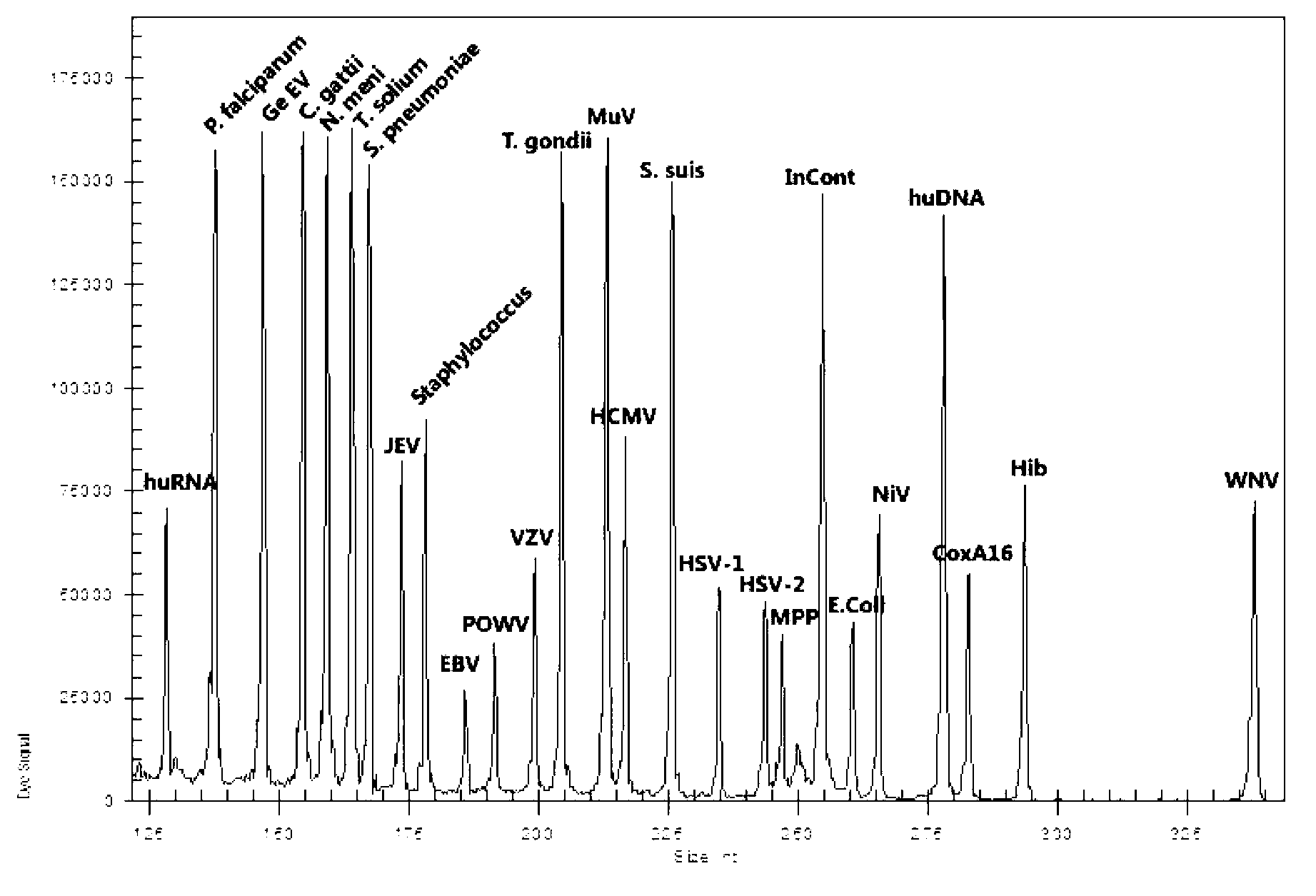
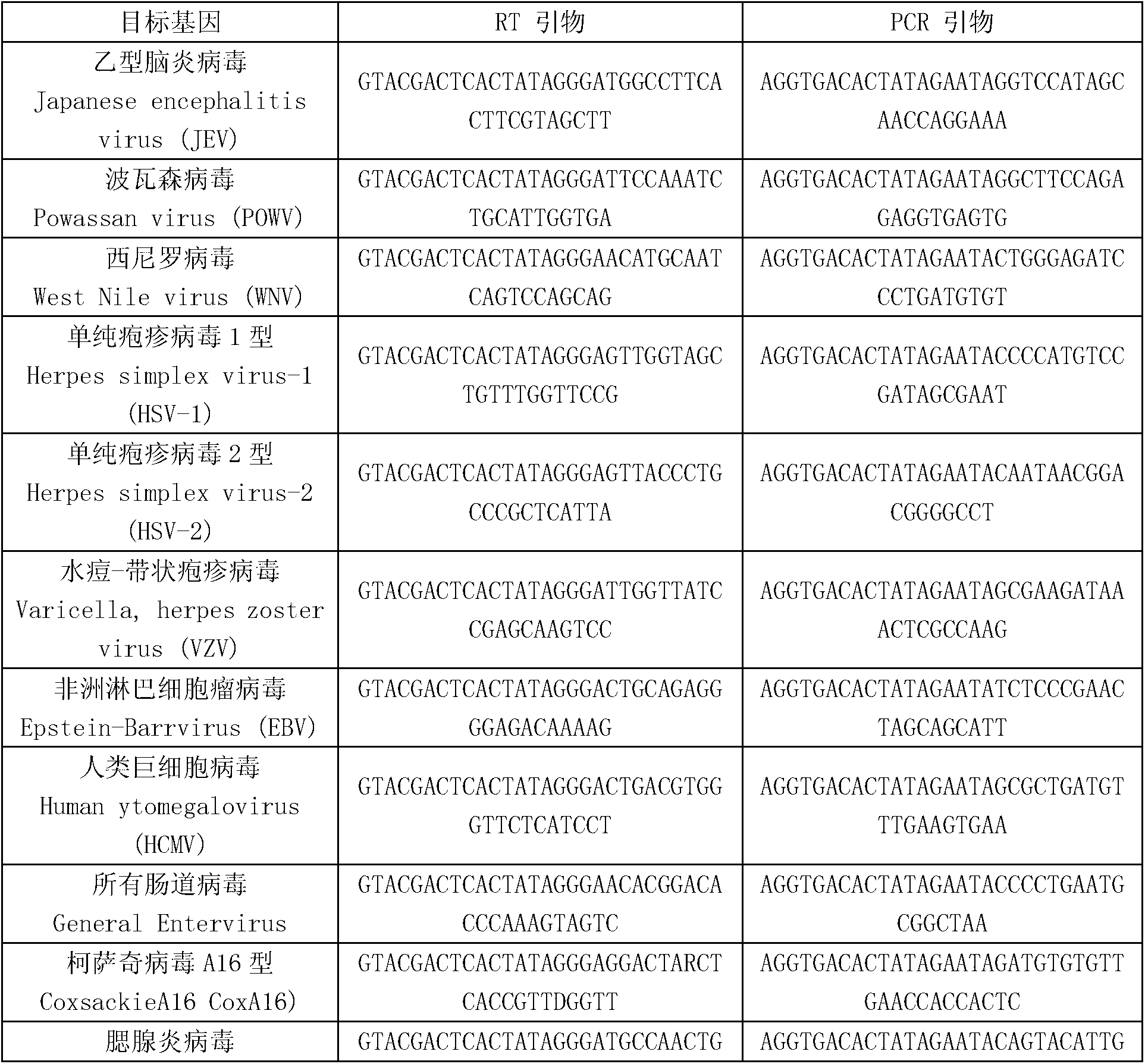
ActiveCN103074448AEnsuring Quality JudgmentsStrong specificityMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-three meningitis pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the twelve meningitis pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-13 (sequence identifier number 1-13), and the PCR primer comprises forward and reverse PCR amplification primers of the rest eleven meningitis pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the twelve meningitis pathogens and the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 14-52. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
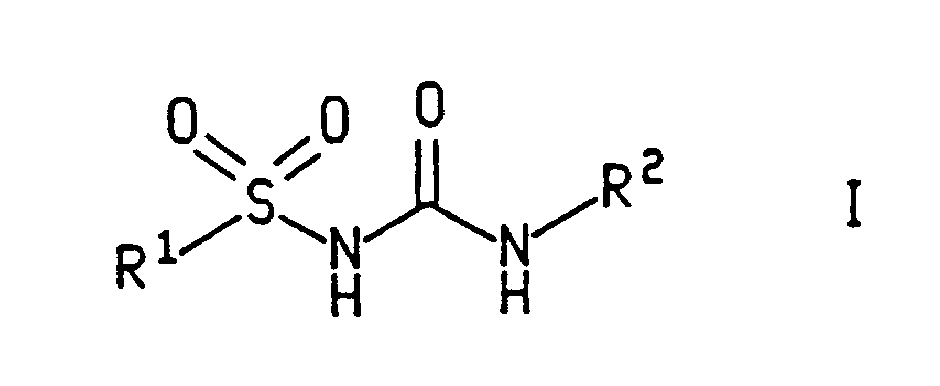
Sulfonyl urea derivatives and their use in control of interleukin-1 activity
A compound of formula (I) wherein R<1> and R<2> are as defined in the description, R<2> being an aromatic group, useful in the treatment and condition selected from the group consisting of the group meningitis and salpingitis, septic shock, disseminated intravascular coagulation, and / or adult respiratory distress syndrome, acute or chronic inflammation, arthritis, cholangitis, colitis, encephalitis, endocarditis, glomerulonephritis, hepatitis, myocarditis, pancreatitis, pericarditis, reperfusion injury, vasculitis, acute and delayed hypersensitivity, graft rejection, and graft-versus-host disease, auto-immune diseases including Type 1 diabetes mellitus and multiple sclerosis, periodonate diseases, interstitial pulmonary fibrosis, cirrhosis, systemic sclerosis, keloid formation tumors which produce IL-1 as an autocrine growth factor, cachexia, Alzeimer's disease, percussion injury, depression, atherosclerosis, osteoporosis in a mammal, including a human.
Owner:PFIZER INC
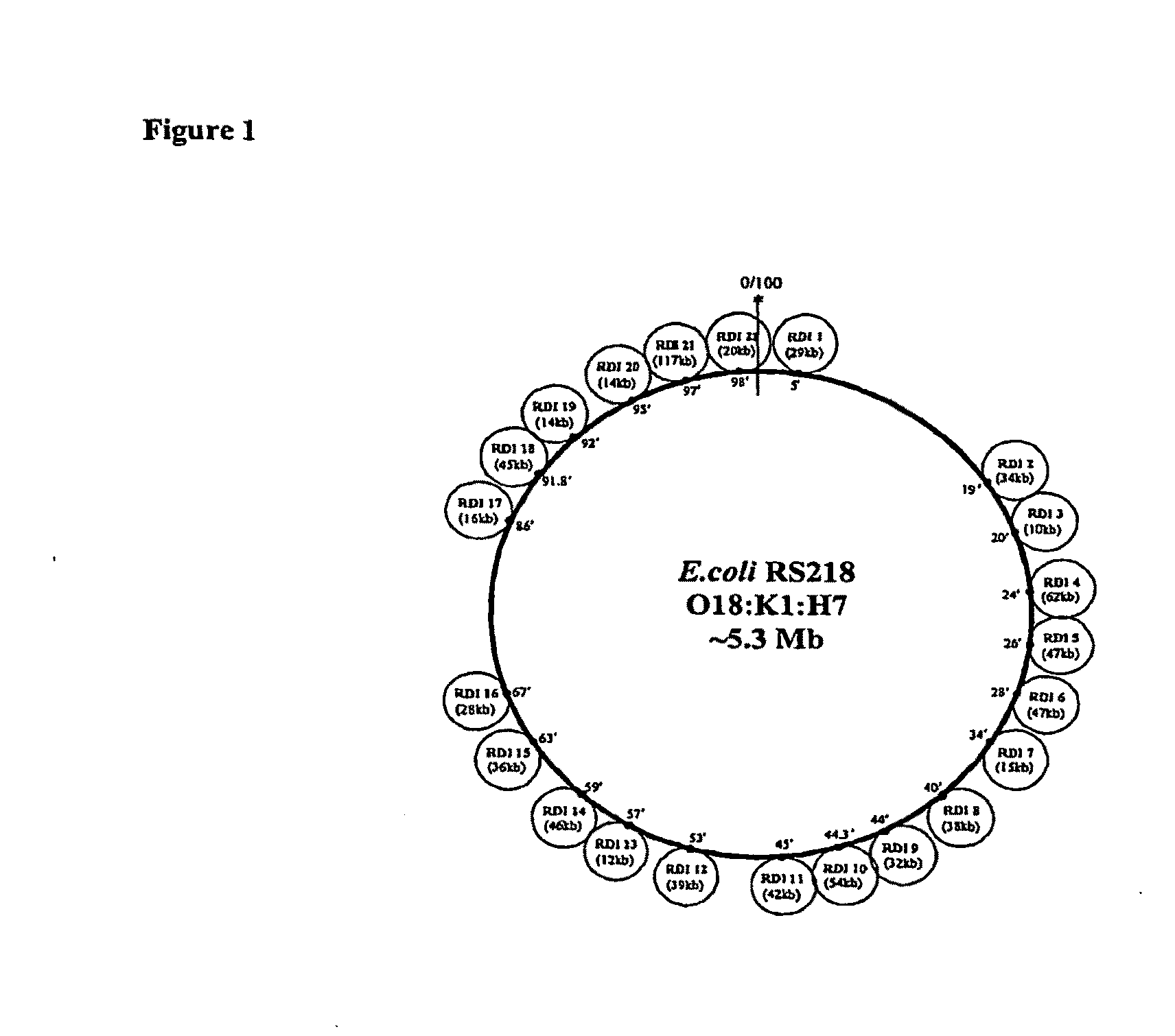
Methods and Compositions for Treating Bacterial Infection
ActiveUS20100136027A1Enhance immune responseStabilizes and reduces symptomAntibacterial agentsBacteriaEscherichia coliBacteremia
The present invention relates to methods and compositions for the treatment of bacterial infection, for example extraintestinal E. coli infection such as E. coli bacteremia, meningitis and sepsis. The invention relates also to methods of diagnosis and prevention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Immunomodulatory agents for treatment of inflammatory diseases
InactiveUS20070123455A1Conferring oxidation resistancePrevent dimerizationBiocideOrganic active ingredientsAutoimmune conditionAutoimmune disease
The present invention provides methods and compositions suitable for treating inflammatory disorders such as allergy, asthma, artherosclerosis, autoimmune disease, infection, injury, meningitis, psoriasis, and transplant rejection. In particular, the present invention provides methods and compositions comprising human S100A8 and / or S100A9 for reducing inflammation.
Owner:RGT UNIV OF CALIFORNIA
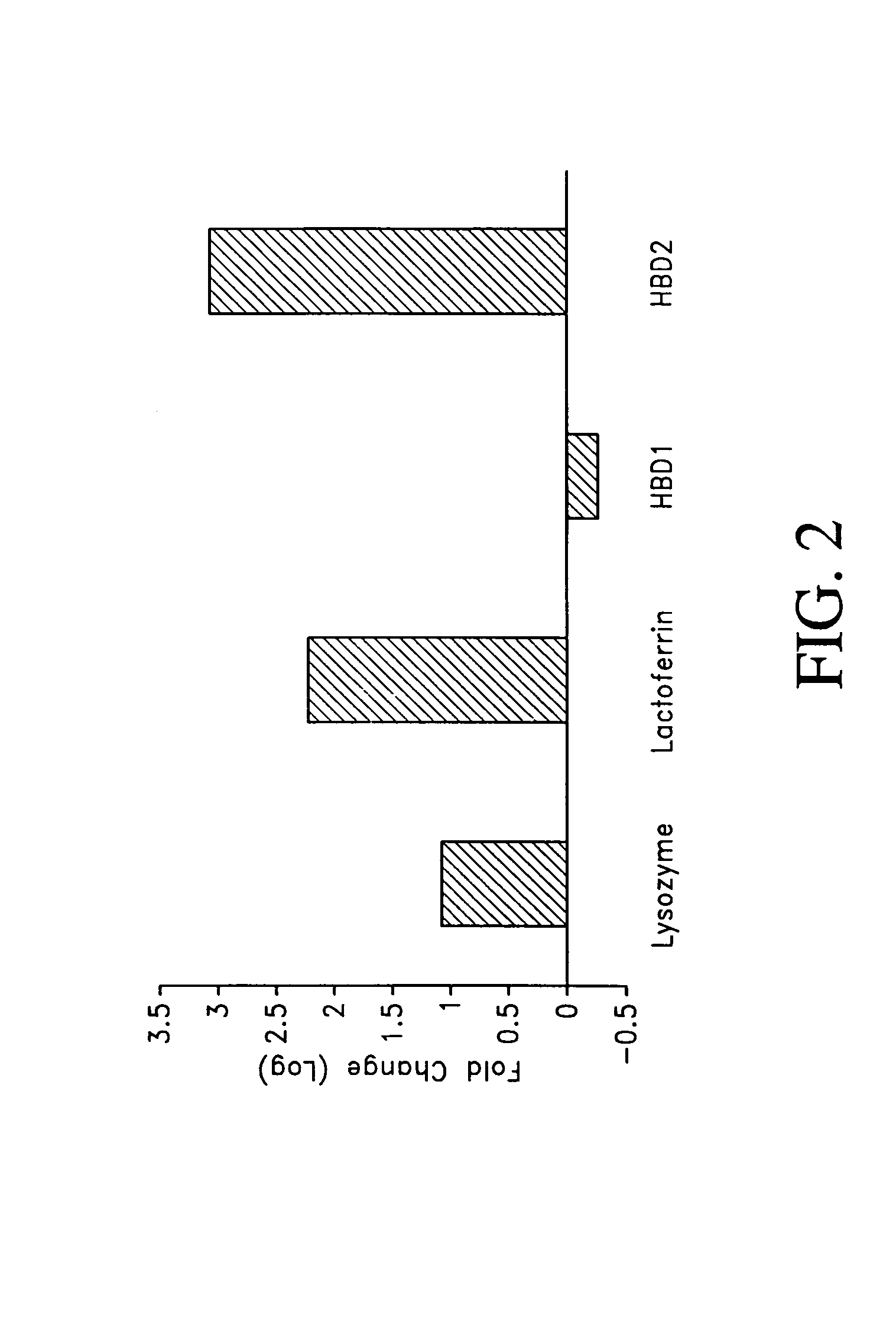
Use of antimicrobial proteins and peptides for the treatment of otitis media and paranasal sinusitis
The pharmaceutical composition and a method of treatment of infectious diseases, such as otitis media, paranasal sinusitis, labyrinthitis and meningitis are described. The composition comprises EP2E or homologues thereof.
Owner:LIM DAVID
Use of antimicrobial proteins and peptides for the treatment of otitis media and paranasal sinusitis
The pharmaceutical composition and a method of treatment of infectious diseases, such as otitis media, paranasal sinusitis, labyrinthitis and meningitis are described. The composition comprises EP2E or homologues thereof.
Owner:LIM DAVID
Methods
The present invention relates to the use of selective aquaporin inhibitors, e.g., of aquaporin-4 or aquaporin-2, e.g., certain phenylbenzamide compounds, for the prophylaxis, treatment and control of aquaporin-mediated conditions, e.g., diseases of water imbalance, for example edema (particularly edema of the brain and spinal cord, e.g., following trauma or ischemic stroke, as well as the edema associated with glioma, meningitis, acute mountain sickness, epileptic seizures, infections, metabolic disorders, hypoxia, water intoxication, hepatic failure, hepatic encephalopathy, diabetic ketoacidosis, abscess, eclampsia, Creutzfeldt-Jakob disease, and lupus cerebritis, as well as edema consequent to microgravity and / or radiation exposure, as well as edema consequent to invasive central nervous system procedures, e.g., neurosurgery, endovascular clot removal, spinal tap, aneurysm repair, or deep brain stimulation, as well as retinal edema), as well as hyponatremia and excess fluid retention, and diseases such as epilepsy, retinal ischemia and other diseases of the eye associated with abnormalities in intraocular pressure and / or tissue hydration, myocardial ischemia, myocardial ischemia / reperfusion injury, myocardial infarction, myocardial hypoxia, congestive heart failure, sepsis, and neuromyelitis optica, as well as migraines, as well as to novel assays for identifying aquaporin inhibitors.
Owner:AEROMICS
Cefathiamidine hydrate, preparation method thereof and application thereof
InactiveCN101768170AReduce humidityEasy to makeAntibacterial agentsOrganic active ingredientsDiseaseJoint infections
The invention relates to a cefathiamidine hydrate, a preparation method thereof and application thereof. The crystalline hydrate has high storage stability, and is applicable in preparing medicaments for treating or preventing gram positive or negative bacteria sensitive bacteria, infections of human or animal respiratory system, liver and biliary system, five sense organs and urogenital system, bone and joint infection and soft skin tissue infection caused by the gram positive or negative bacteria sensitive bacteria, and diseases such as endocarditis, ichorrhemia, meningitis and the like.
Owner:刘力
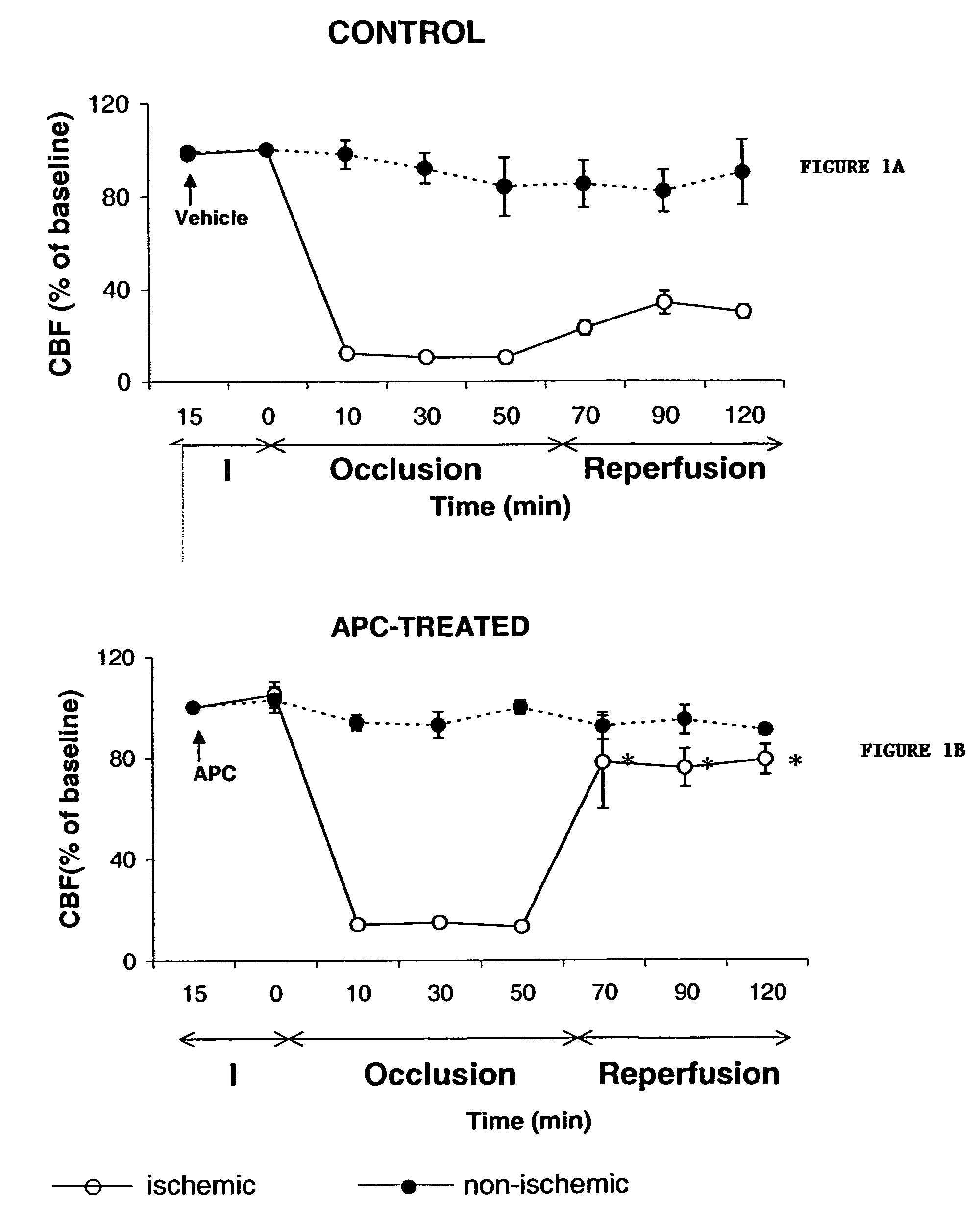
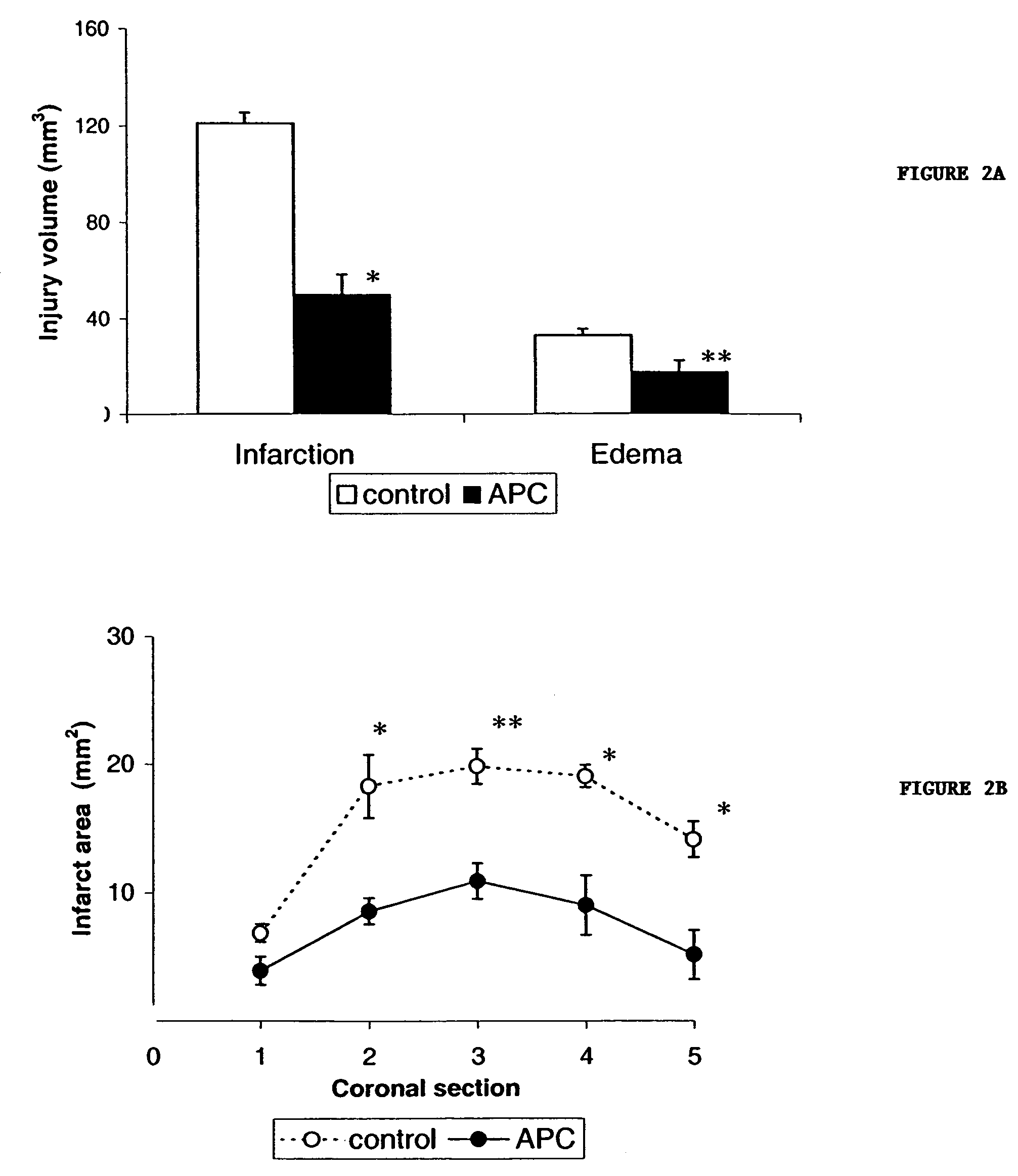
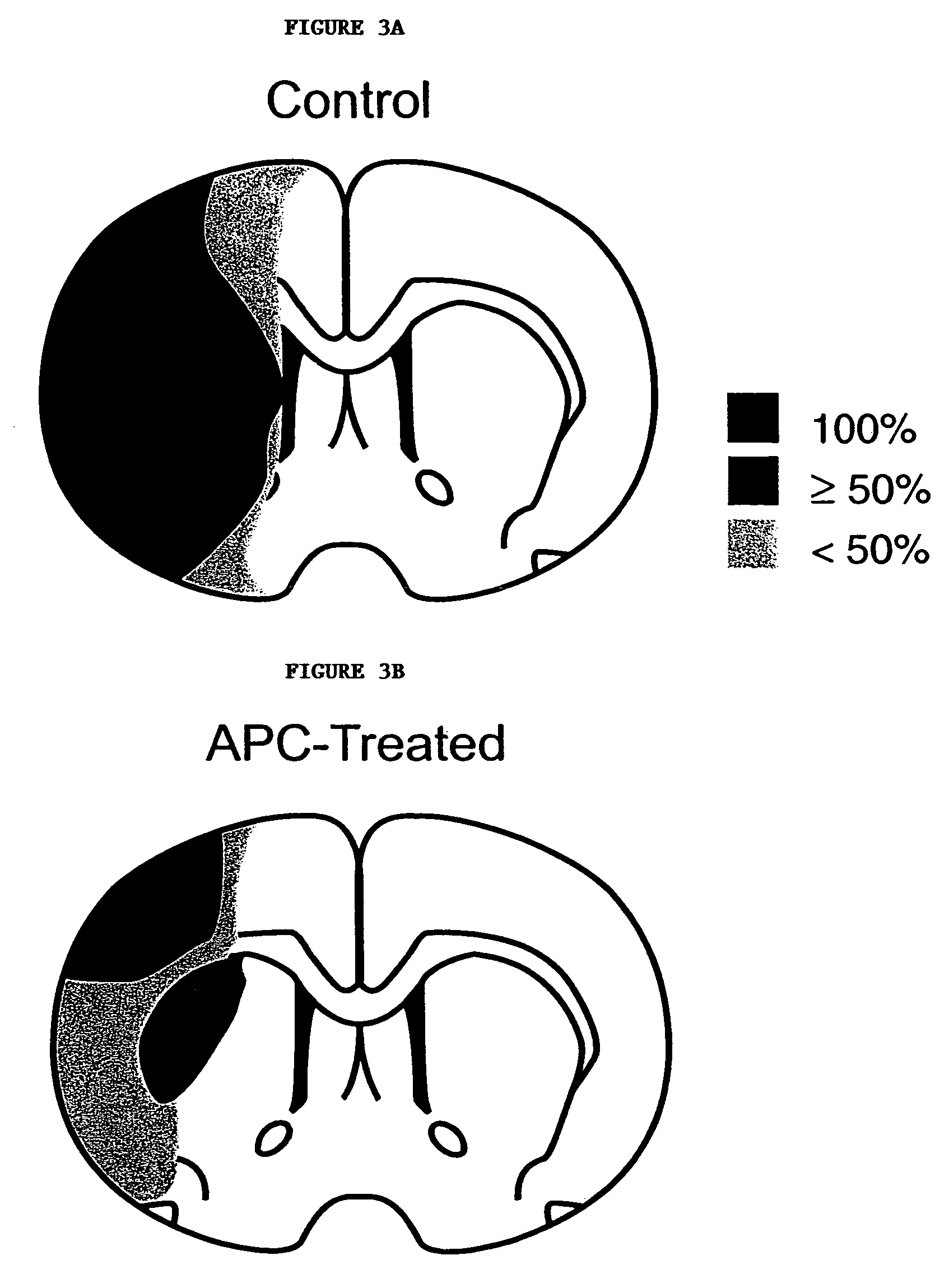
Neuroprotective, antithrombotic and anti-inflammatory uses of activated protein C (APC)
InactiveUS7074402B2Reduce inflammationReduce neuroinflammationBiocideNervous disorderRisk strokeDisease cause
The present invention provides methods for treating subjects having or at risk of having a neuropathological disorder or brain inflammatory diseases with and without vascular involvement, and systemic inflammatory vascular disease by administering a therapeutically effective amount of Activated Protein C (APC) to the subject. Brain disorders and brain inflammatory vascular diseases that can be treated by the invention method include all neurodegenerative diseases with different types of neuronal dysfunction, including stroke, Alzheimer's disease, Parkinson's disease, Huntington disease, neuroimmunological disorders such as multiple scelrosis and Gullian-Barre, encephalitis, meningitis, as well as other peripheral vascular diseases, such as diabetes, hypertension, artheriosclerosis. Also included are methods of treatment using APC in combination with a co-factor, such as Protein S.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Traditional Chinese medicine composition for treating meningitis
InactiveCN102000257ASignificant effectNo side effectsAntibacterial agentsAntimycoticsDiseaseMedicinal herbs
The invention relates to a medicine for treating meningitis, in particular to a traditional Chinese medicine composition for treating meningitis, which is prepared from Chinese medicinal plant herbs as raw materials. The traditional Chinese medicine composition is characterized by comprising the following raw material medicines for preparing effective ingredients in weight: 20-30 g of milkvetch roots, 15-25 g of suberect spatholobus stems, 15-25 g of Szechuan lovage rhizome, 10-20 g of stiff silkworms, 10-20 g of Chinese angelica, 10-20 g of peony tree barks, 5-15 g of grassleaf sweetflag rhizome, 5-15 g of clematis roots, 5-15 g of arisaema cum bile, 4-12 g of straight ladybell roots and 4-12 g of dwarf lilyturf tubers. All the medicinal herbs of the traditional Chinese medicine composition are compounded and mutually supplemented, and the traditional Chinese medicine composition has the efficacies of tonifying Qi, consolidating superficial resistance, invigorating blood circulation, enriching blood, moistening the lungs, reducing phlegm, replenishing yin, clearing away heat, promoting saliva secretion, dispelling the wind, removing dampness, activating meridians, stopping pain, relieving dizziness, arresting convulsion and the like. The traditional Chinese medicine composition has synergistic effect, can be used for treating both principal and secondary aspect of disease, has no side effect and has obvious curative effect on meningitis caused by thermotoxin, going deep into ying blood, invagination pericardium and the like.
Owner:胡海舰
Risk evaluation and management strategy involving patient follow-ups relating to the use or discontinuation of a complement inhibitor
ActiveUS20160140298A1Reduce morbidityData processing applicationsDrug and medicationsComplement InhibitorsMedicine
This invention provides, inter alia, a complement-inhibitor-based treatment plan coupled with a risk evaluation and management strategy (“REMS”) and a safety support program (“SSP”) for reinforcing the REMS. The REMS and SPP are implemented using one or more computer devices with software tools programmed to enforce conditions of the REMS and / or prompt follow-ups by registered nurses enrolled in the SSP. The software tool(s) determines whether a prescriber requesting the complement inhibitor has agreed to abide by the REMS, and can prompt a provider of the complement inhibitor to provide updated educational materials to the prescriber at predetermined times or intervals, to monitor the prescriber for compliance with the REMS, and / or to monitor patients for signs of adverse events. Using exemplary embodiments described herein, a risk of adverse events (especially, but not limited to, meningococcal infections) can be managed and an incidence of the adverse events can be reduced.
Owner:ALEXION PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com