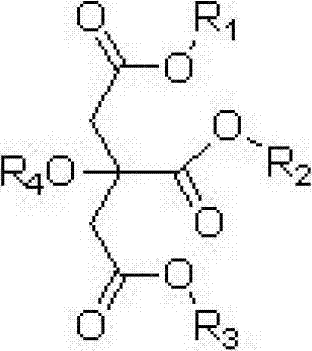
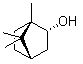
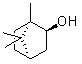
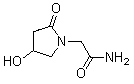
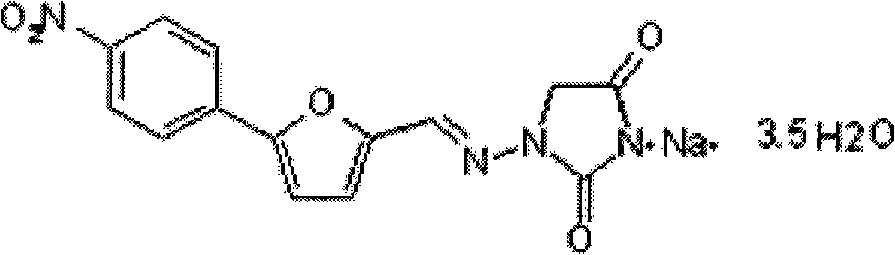
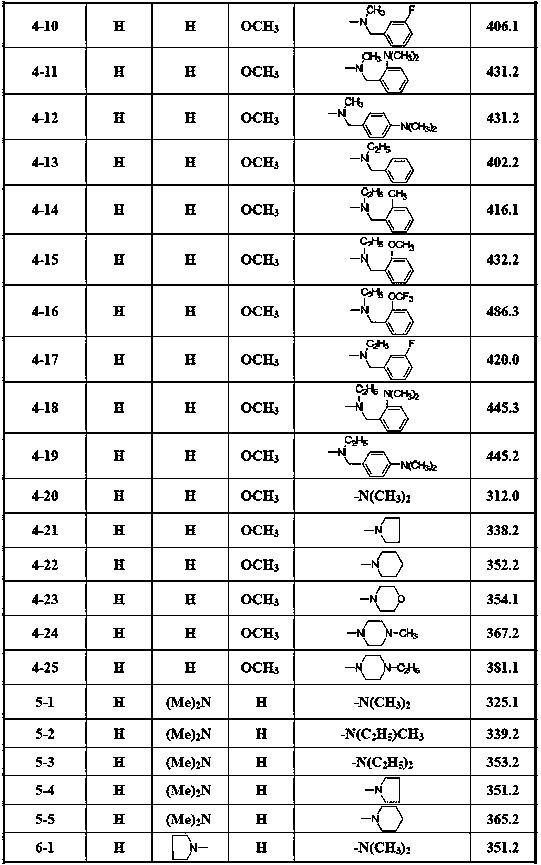
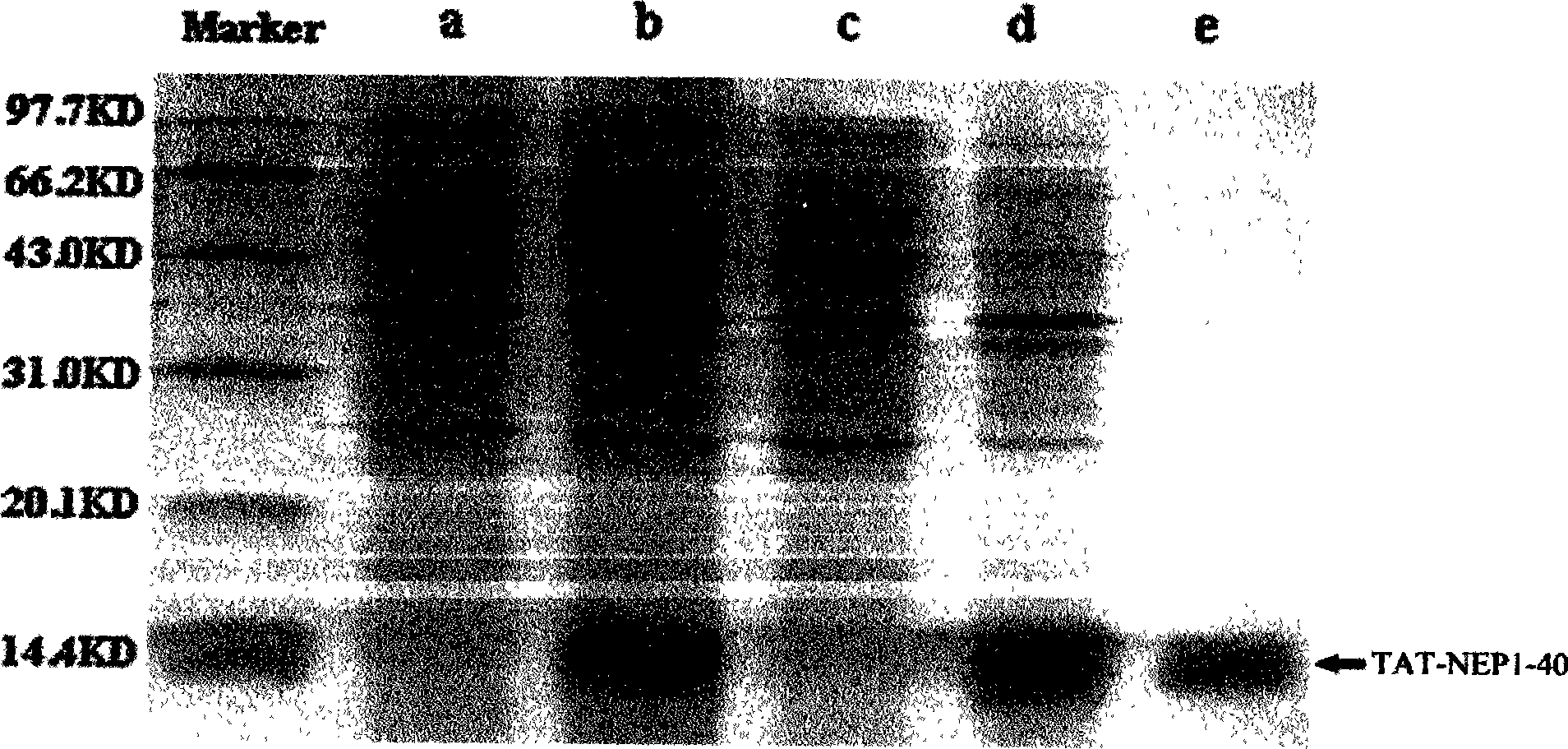Patents
Literature
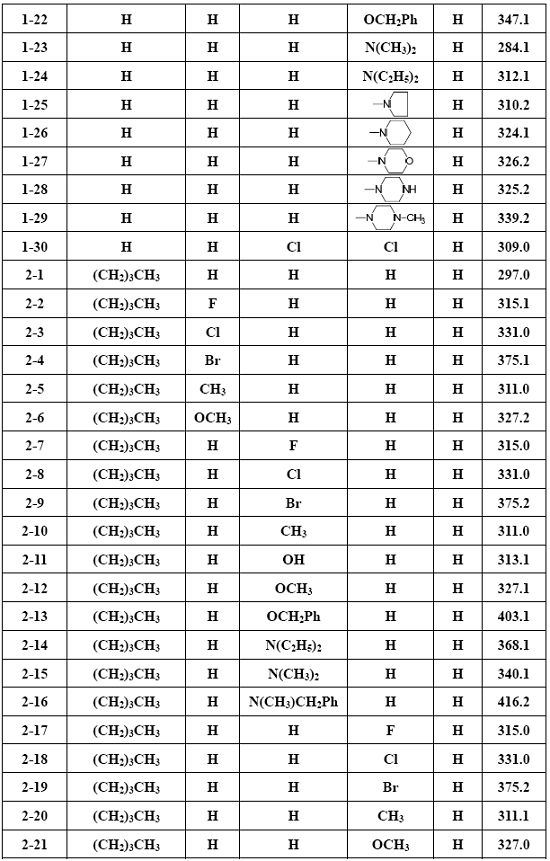
115 results about "Cerebral trauma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In fact, brain injuries are one the leading reasons that children develop cerebral palsy. Traumatic brain injuries and damage are considered a severe medical issue and treatment must begin as soon as possible for the best possible chances of helping the child.
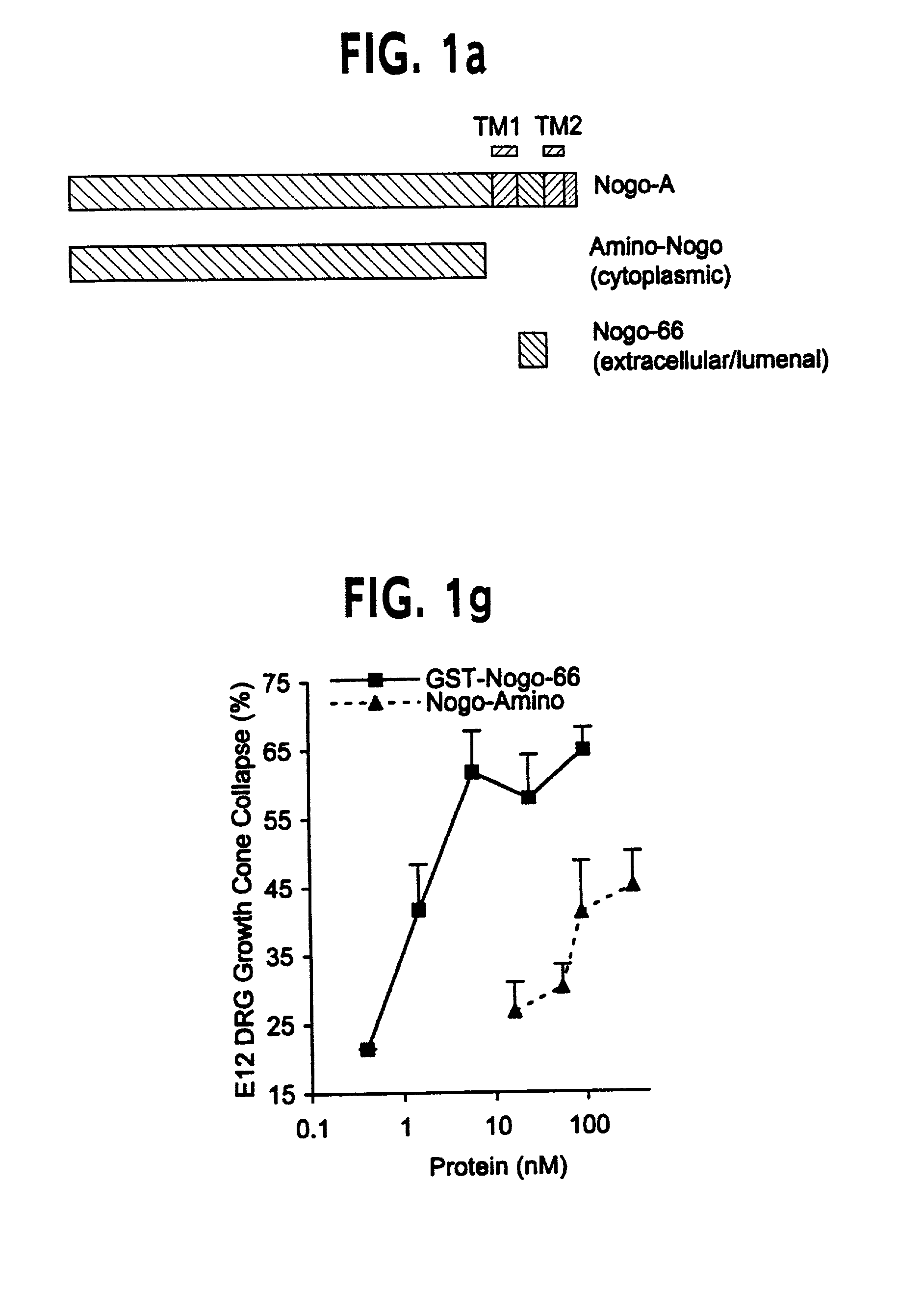
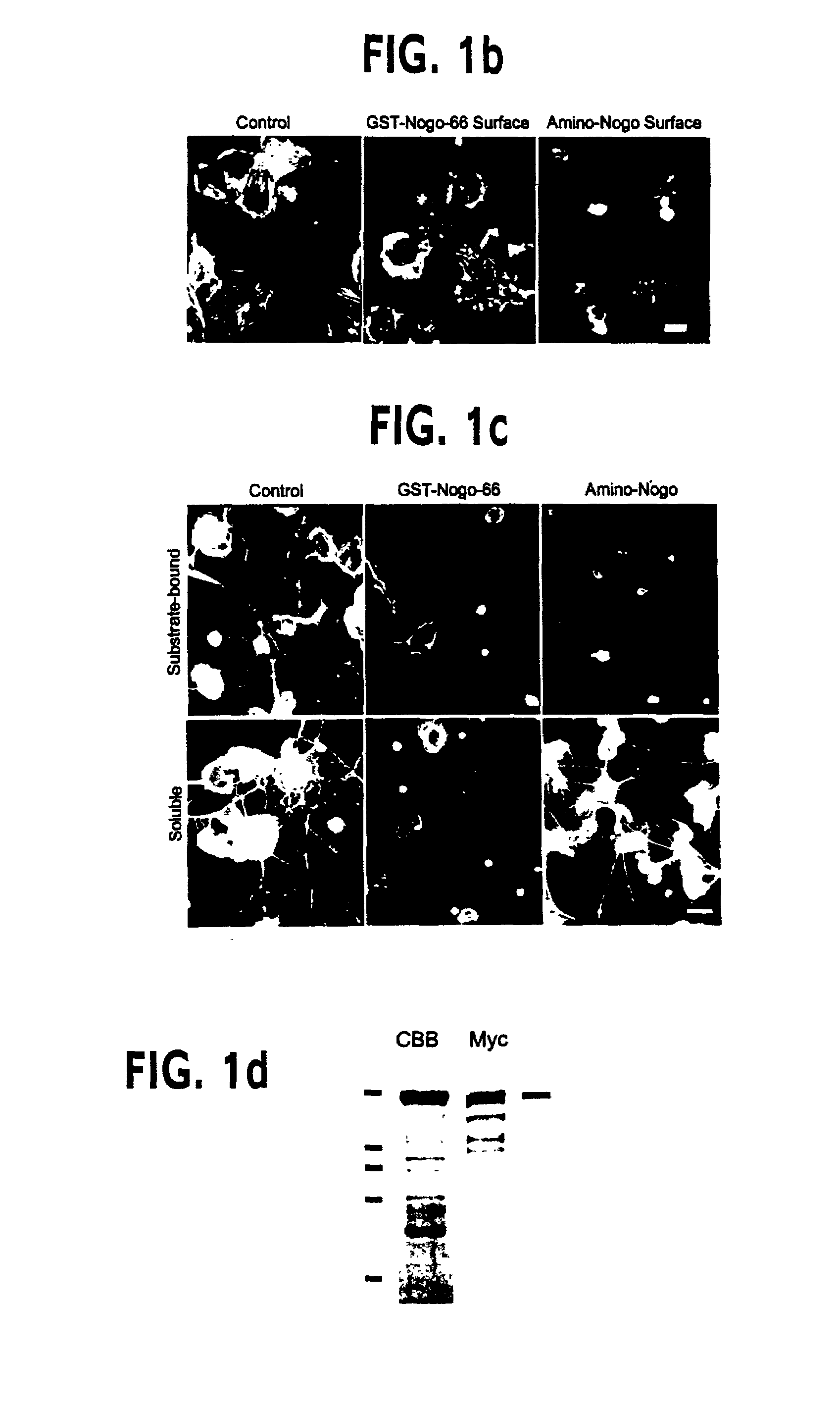
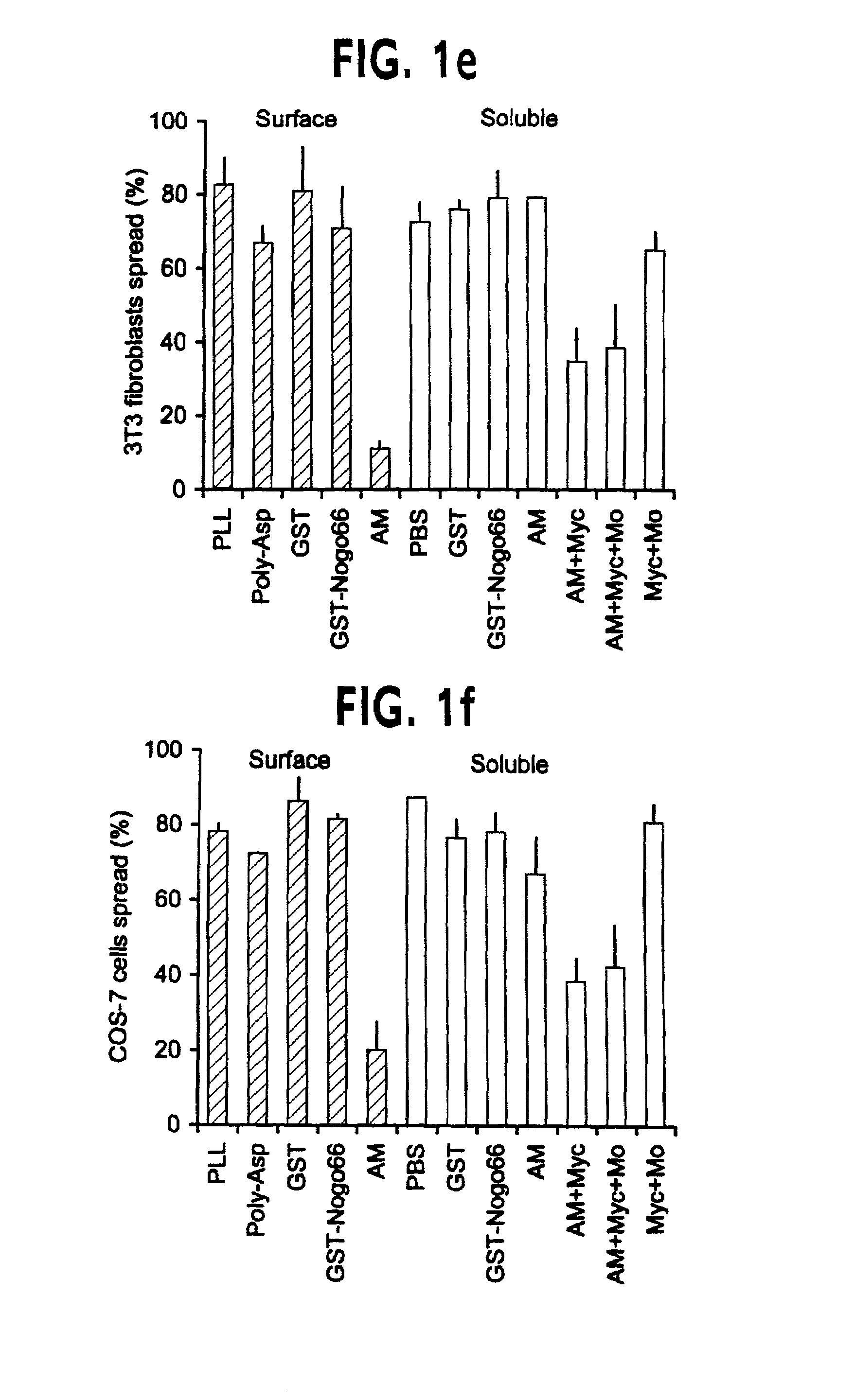
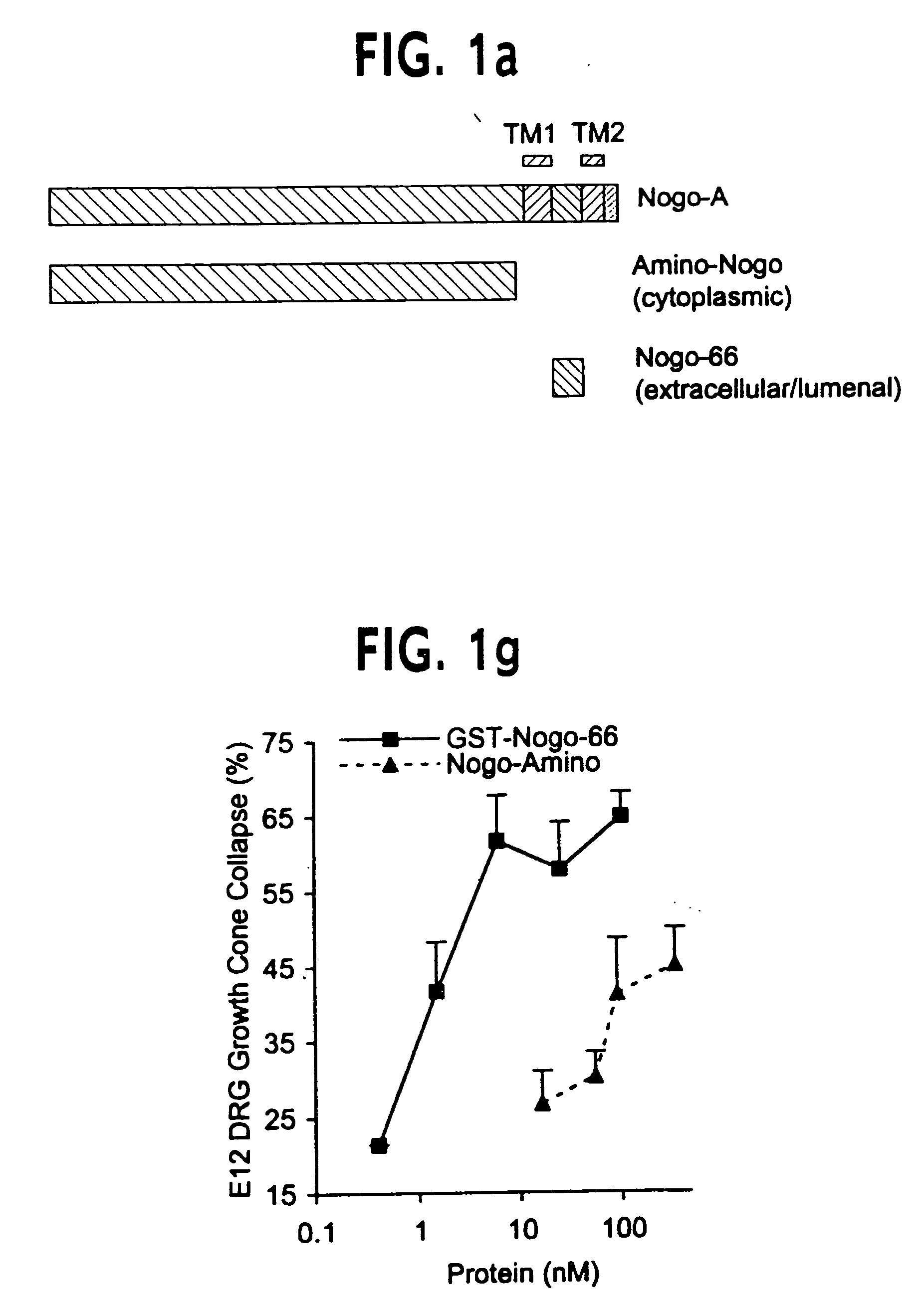
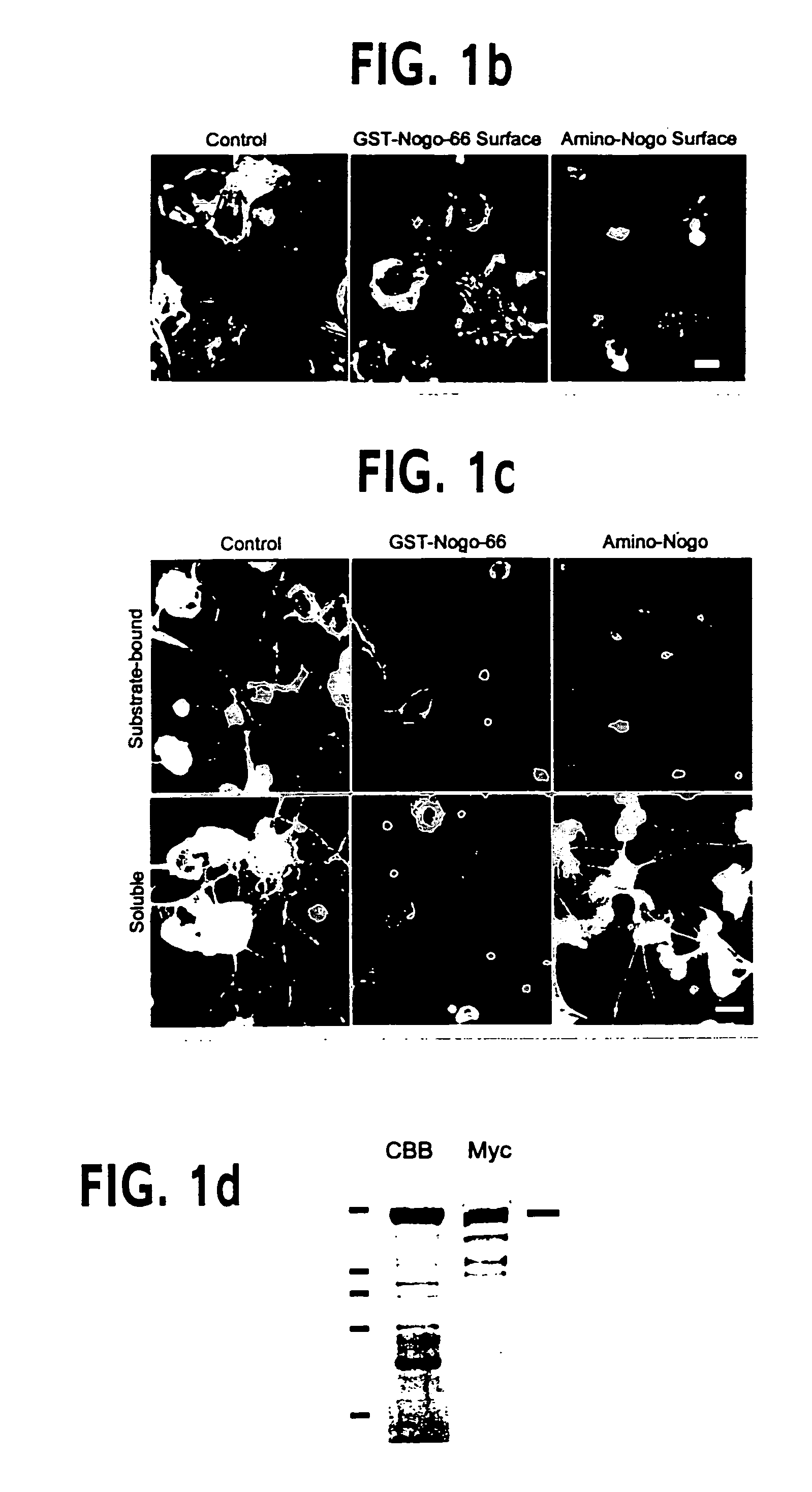
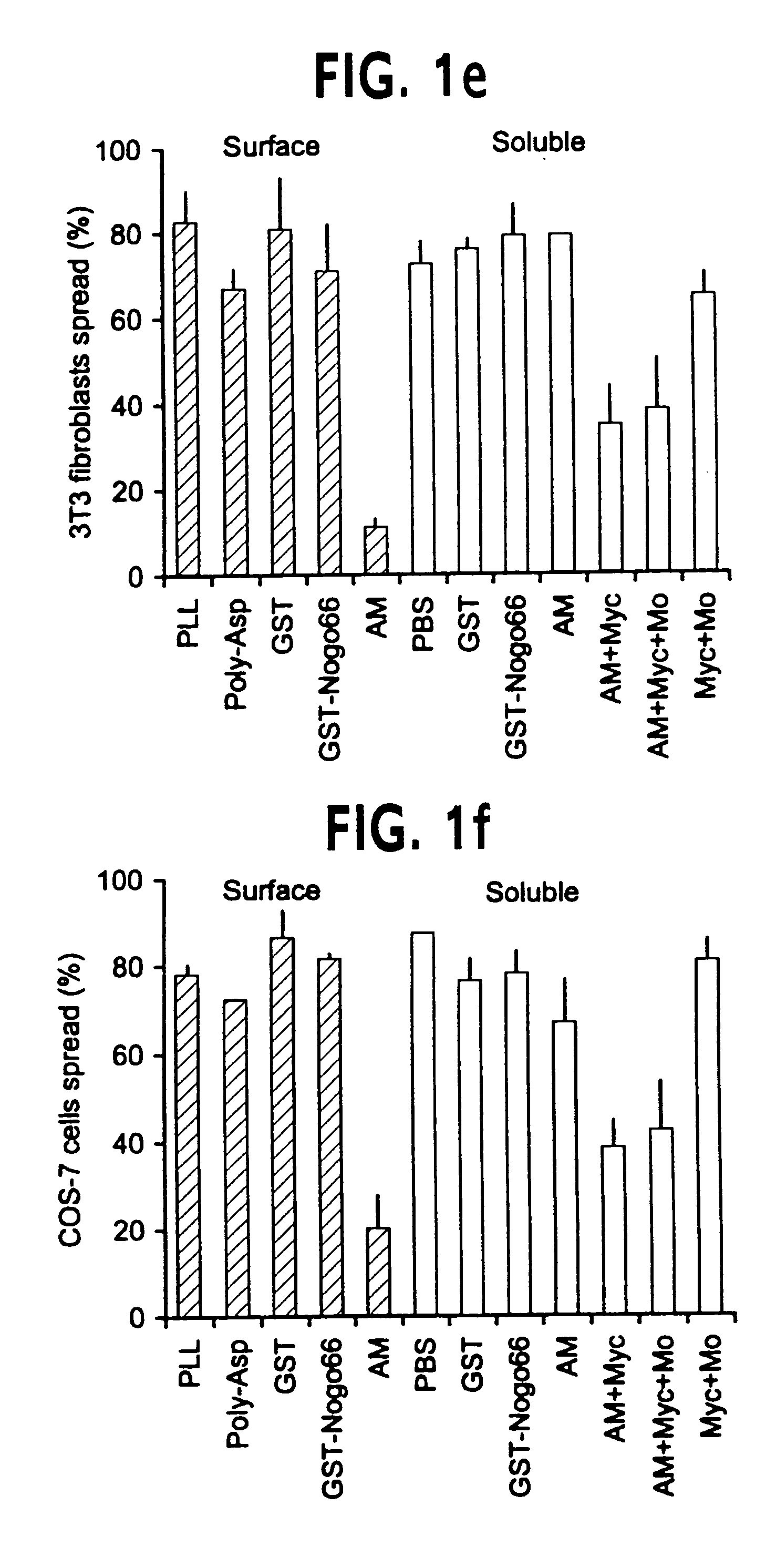
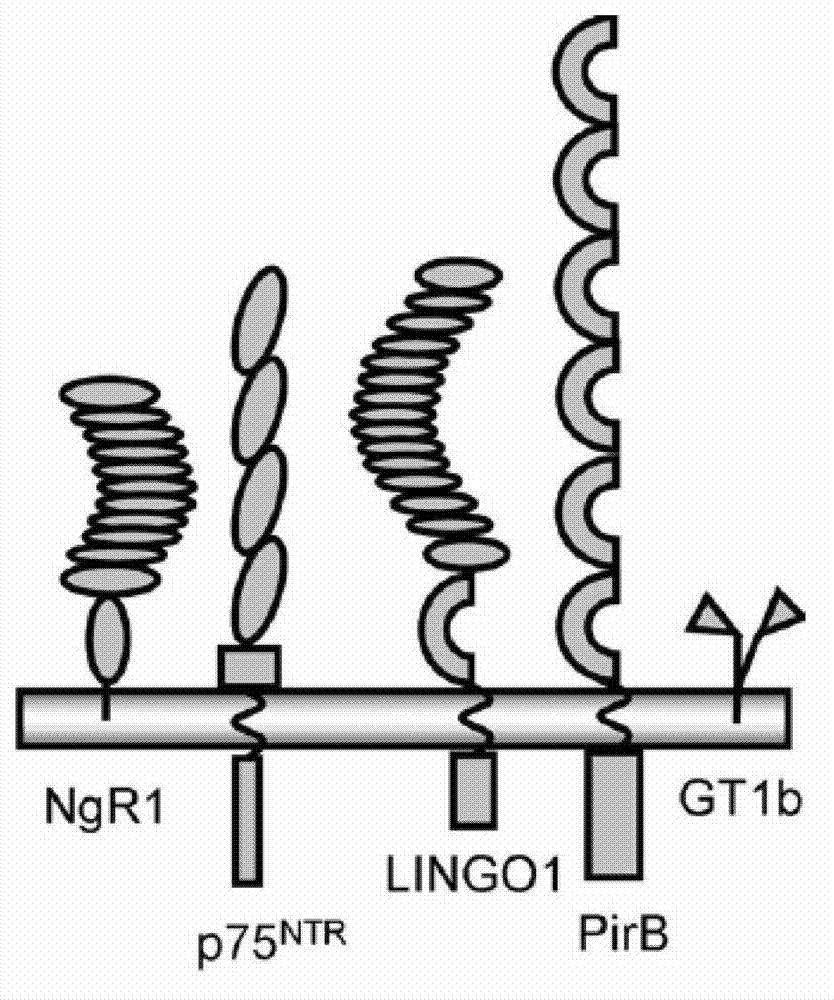
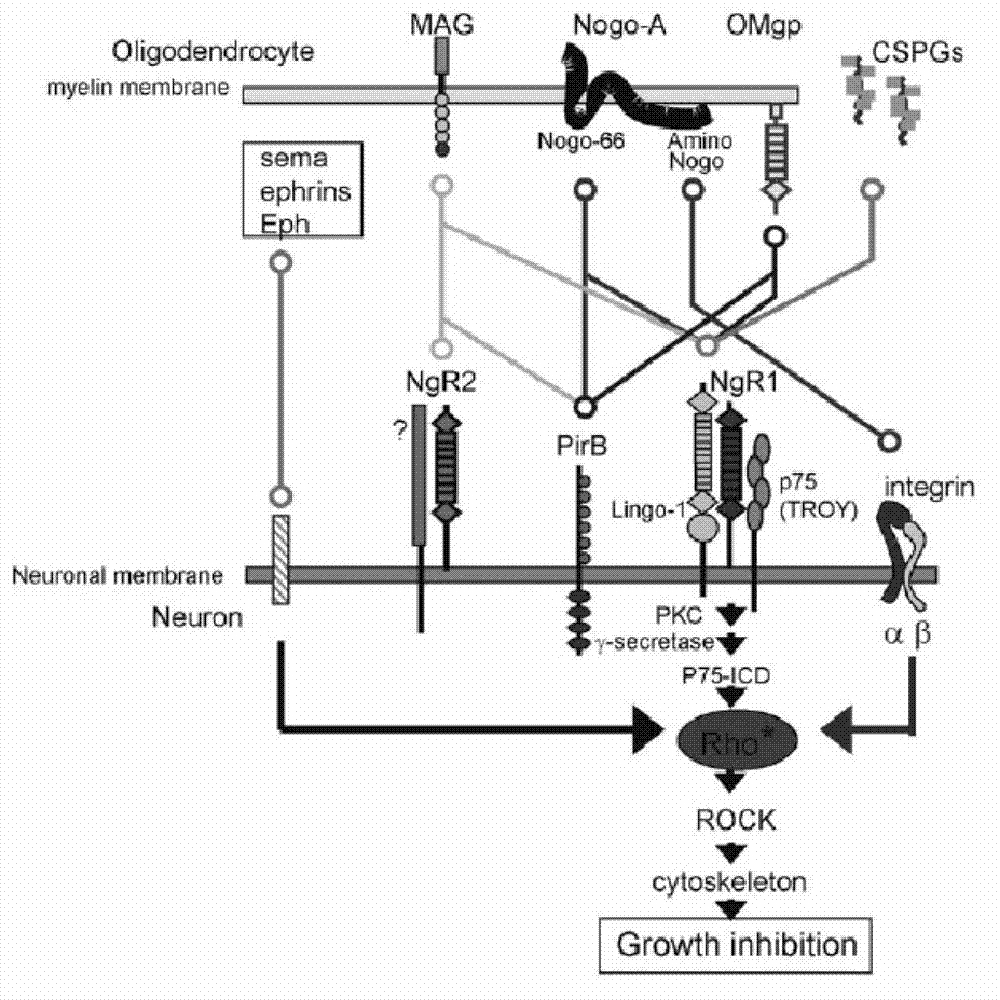
Nogo receptor-mediated blockade of axonal growth
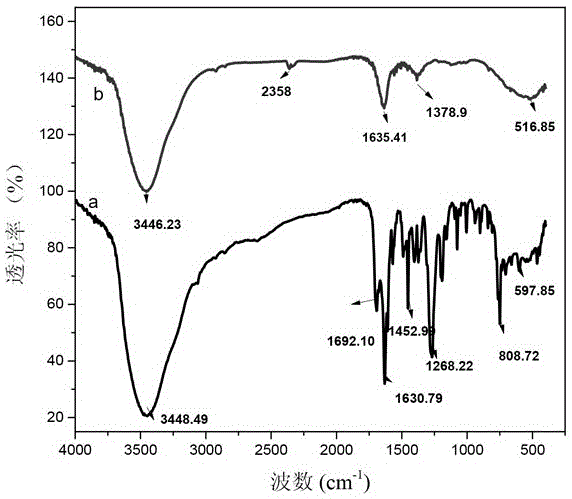
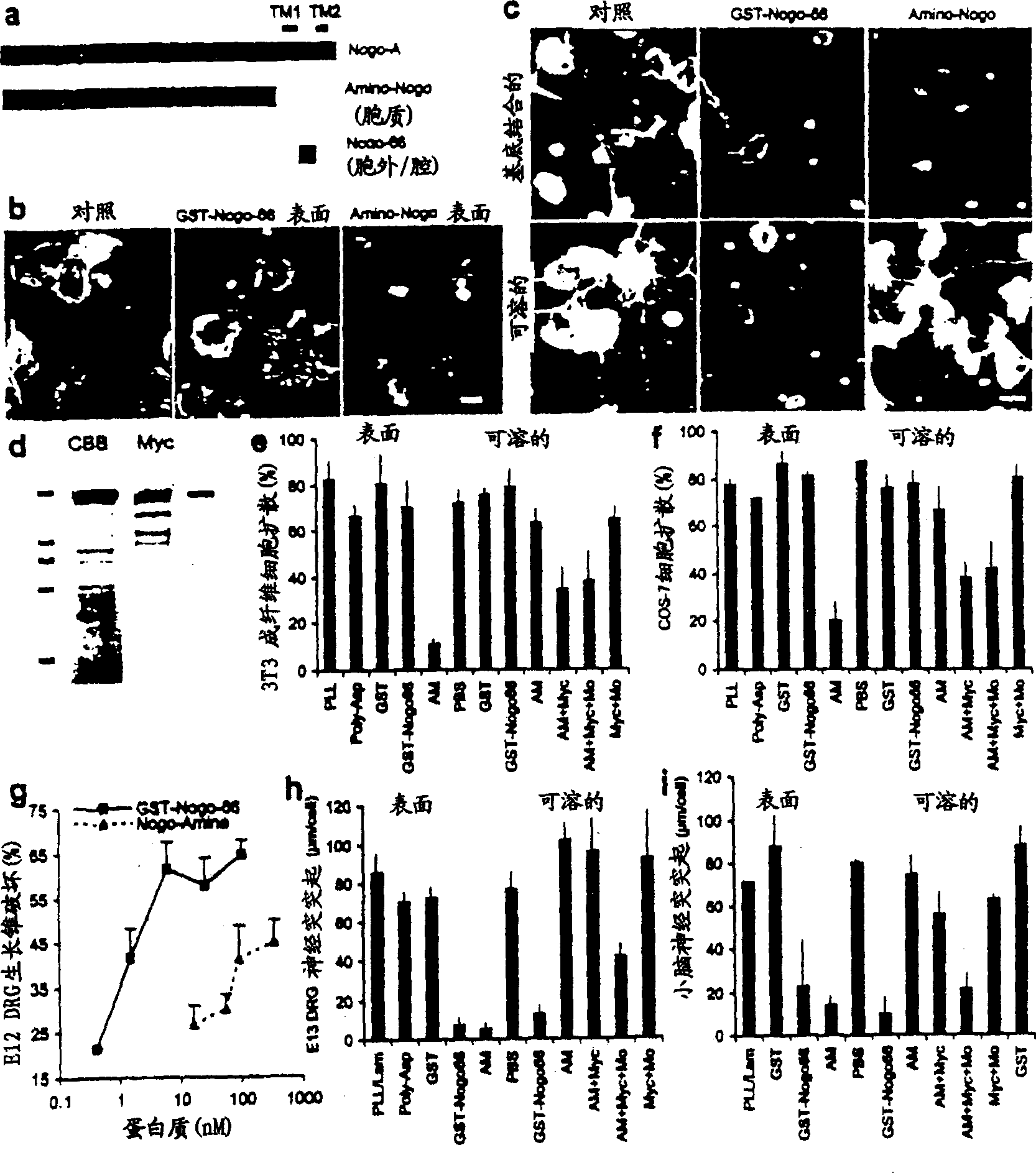
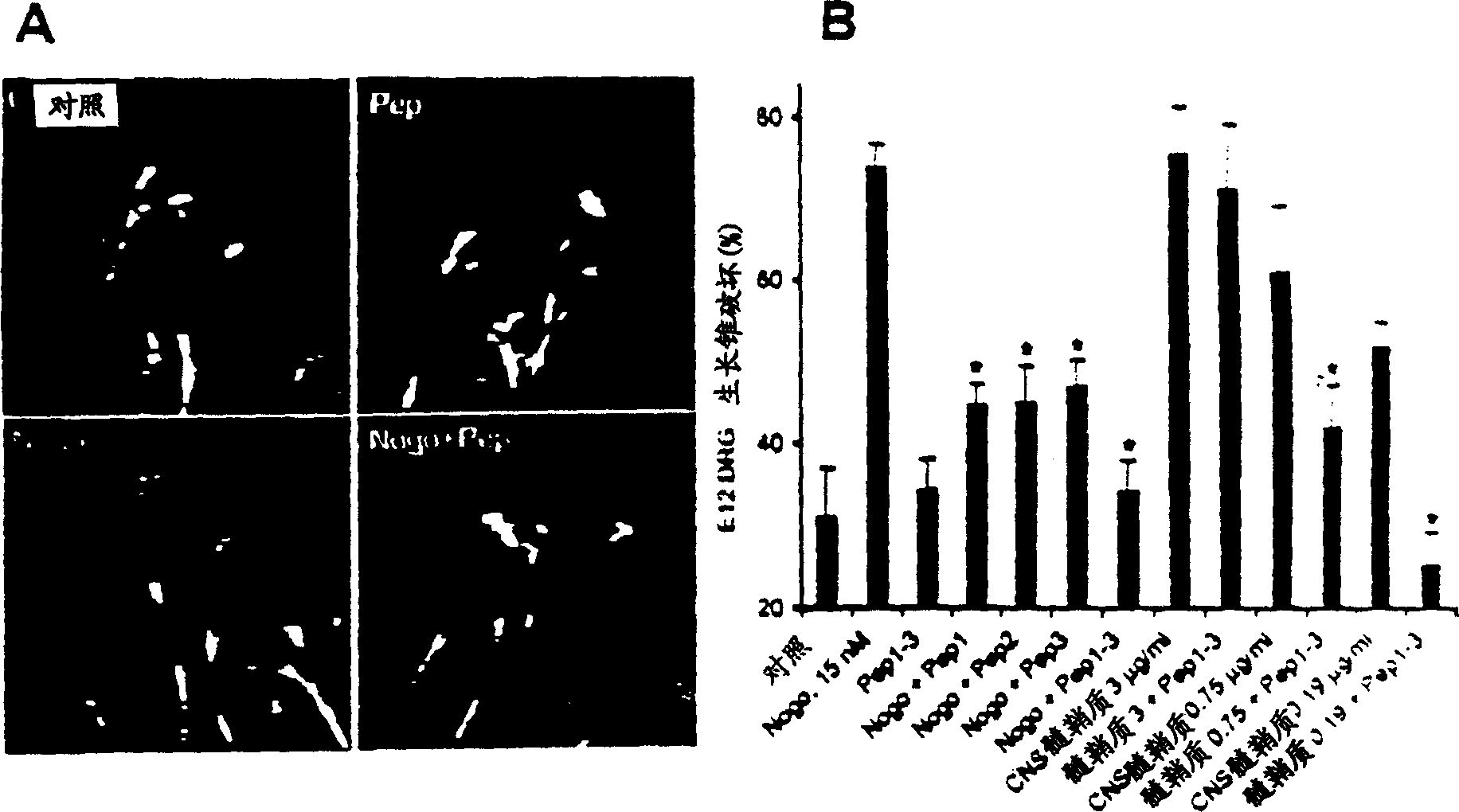
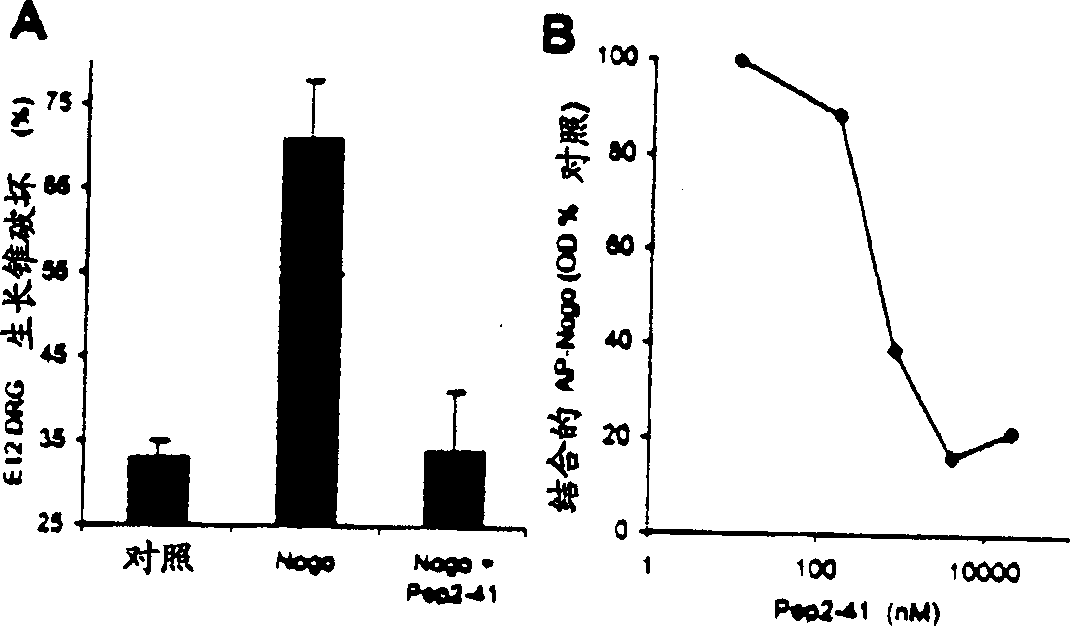
Disclosed are NgR proteins and biologically active Nogo (ligand) protein fragments. Also disclosed are compositions and methods for modulating the expression or activity of the Nogo and NgR protein. Also disclosed are peptides which block Nogo-mediated inhibition of axonal extension. The compositions and methods of the invention are useful in the treatment of cranial or cerebral trauma, spinal cord injury, stroke or a demyelinating disease.
Owner:YALE UNIV
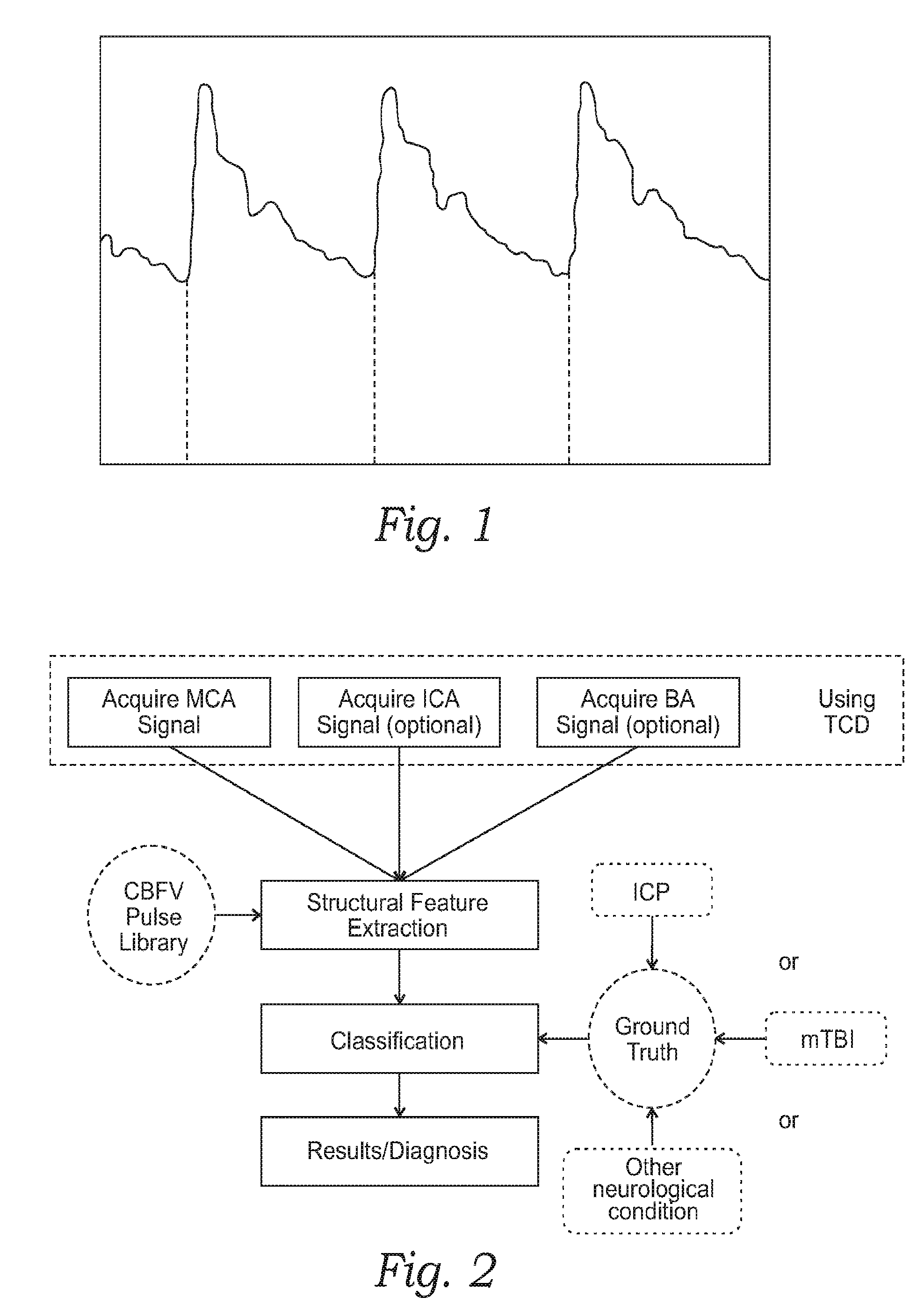
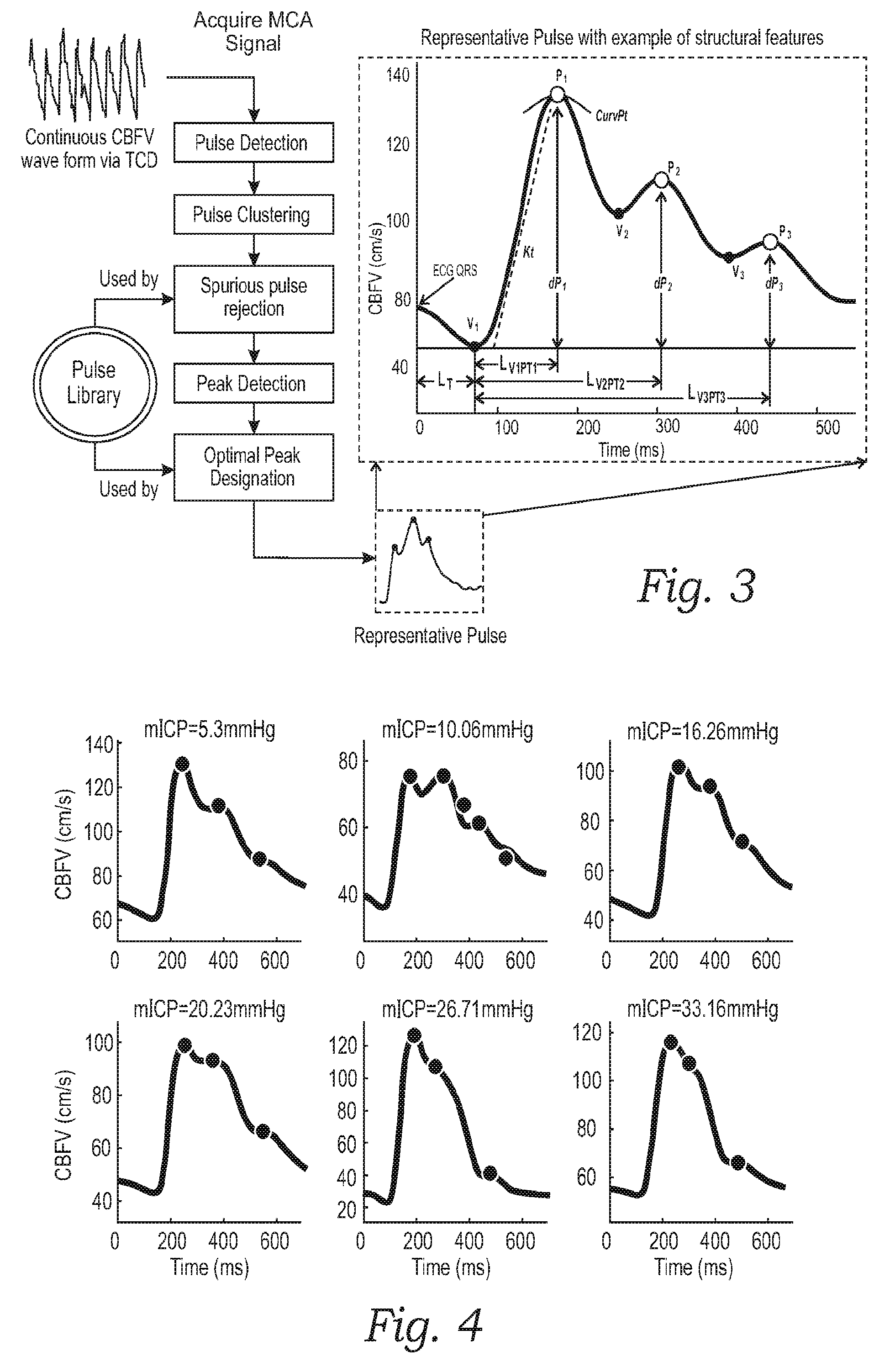
Monitoring structural features of cerebral blood flow velocity for diagnosis of neurological conditions
InactiveUS20160278736A1Quantitative precisionImprove accuracyMedical imagingSurgical instrument detailsDiseaseNervous system
The systems and methods described herein include a non-invasive diagnostic tool for intracranial hypertension (IH) detection and other neurological conditions like mild and moderate TBI that utilizes the transcranial Doppler (TCD) measurement of cerebral blood flow velocity (CBFV) in one or more cerebral vessels. A headset includes a TCD scanner which automatically locates various cerebral arteries and exerts an appropriate pressure on the head to acquire good CBFV signals.
Owner:NEURAL ANALYTICS
Tissue-engineered neural tissues and construction method thereof
InactiveCN102228718AConducive to survivalLarge apertureNervous system cellsProsthesisMatrigelRegenerative medicine
The invention discloses tissue-engineered neural tissues and a construction method thereof, belonging to the technical field of tissue engineering and regeneration medicine. Subcultured and purified neural stem cells serve as seed cells of the tissue-engineered neural tissues, a liquid type I collagen and Matrigel matrix serves as stent materials, and the seed cells and the stent materials are cultured after being compounded in vitro. A stent system adopted in the invention can be used for effectively maintaining the survival and proliferation of the neural stem cells, promoting the differentiation of the neural stem cells, and forming engineered neural tissues consisting of mature neurons, astrocytes and oligodendrocytes. The tissue-engineered neural tissues have an important significance in treating neural system injury through the transplantation of the tissue-engineered neural tissues, and can also serve as an in-vitro model for the psychological research of neurodevelopment, cerebral trauma and neuroelectricity.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
hNT-neuron human neuronal cells to replace ganglion cells
Disclosed herein is the treatment of vision loss in a mammal by transplanting an effective amount of hNT-Neuron cells. The treatment can be accomplished by injecting the cells into the retinal area of the eye. Additionally, the cells can be injected into the visual cortex of the brain. Conditions to be treated are vision loss due to optic nerve damage, including glaucoma, optic nerve sheath meningioma and glioma, Graves' ophthalmopathy, benign or malignant orbital tumors, metastatic lesions, tumors arising from the adjacent paranasal sinuses or middle cranial fossa, giant pituitary adenomas, brain tumors or abscesses, cerebral trauma or hemorrhage, meningitis, arachnoidal adhesions, pseudotumor cerebri, cavernous sinus thrombosis, dural sinus thrombosis, encephalitis, space-occupying brain lesions, severe hypertensive disease or pulmonary emphysema.
Owner:LAYTON BIOSCI
Oral Chinese herbal preparation for treating post-traumatic brain syndrome
InactiveCN102631579AReduce dosageFast absorptionNervous disorderInanimate material medical ingredientsLicorice rootsTherapeutic effect
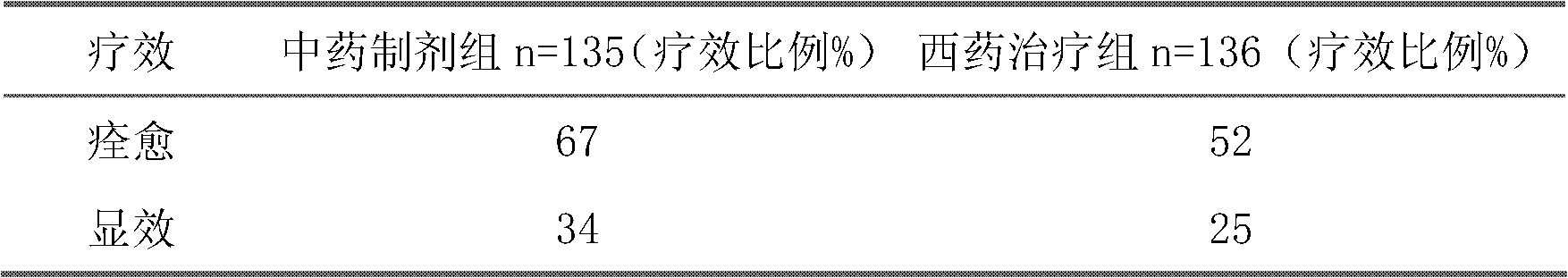
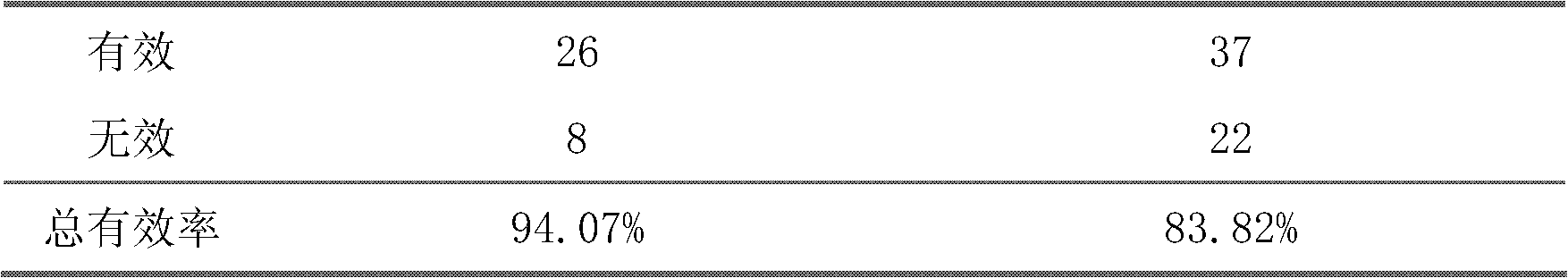
The invention belongs to an oral Chinese herbal preparation for treating post-traumatic brain syndrome, and the oral Chinese herbal preparation is a human medicinal product. The preparation is prepared from the following Chinese herbs in parts by weight: 10+ / -2 parts of Astragalus mongholicus, 10+ / -2 parts of American ginseng, 10+ / -2 parts of Angelica sinensis, 10+ / -2 parts of medlar, 10+ / -2 parts of the root of red-rooted salvia, 10+ / -2 parts of ligusticum wallichii, 10+ / -2 parts of root of common peony, 10+ / -2 parts of peach kernel, 10+ / -2 parts of Poria cocos, 10+ / -2 parts of pericarpium citri reticulatae, 10+ / -2 parts of plantain seed, 10+ / -2 parts of tabasheer, 10+ / -2 parts of rhizoma acori graminei, 10+ / -2 parts of gastrodia elata, 10+ / -2 parts of uncaria, 10+ / -2 parts of spina date seed, 10+ / -2 parts of amber powder and 6+ / -1.2 parts of honey-fried licorice root. Clinical effect observation proves that the total effective rate of the oral Chinese herbal preparation to the oral traditional Chinese medicine preparation is 94.07%, and has better treatment effect (P<0.01) compared with Western medicines such as Rotundine, piracetam, oryzanol and nimodipine for treating post-traumatic brain syndrome. Moreover, the Chinese herbal preparation has no obvious toxic or side effect, and can be taken for a long time. The dose of the preparation is small, and effective medicinal components are easy to release, can be absorbed quickly and can fully play the pharmaceutical effect. The preparation is convenient to carry, easy to take and beneficial to large-scale production.
Owner:HOSPITAL NO 3 CPLA
Medicinal composition for preventing and curing intracranial hypertension
InactiveCN1111405CHeterocyclic compound active ingredientsCardiovascular disorderControlled hypotensionHypotension shock
This invention relates to the medicinal composition for prevention and cure of intracranial hypertension, which contains piracetam (1-acetamido-2ketone-pyrrolidine) and the carrier tolerable to pharmaceutics. It can further contain osmotic diuretic such as mannitol,etc.; or anti-tissue exuedative medicine such as esculin sodium; or the medicine such as nitroglycerin, sodium nitroprusside, etc. used for resulting in controllable hypotension.
Owner:ZHUHAI HEFAN MEDICINE
Nogo receptor-mediated blockade of axonal growth
Owner:YALE UNIV
Achyranthes bidentata polypeptide, preparing process and uses
InactiveCN101270147APromote growthInhibit apoptosisNervous disorderPeptide/protein ingredientsDiseaseMedicinal herbs
The present invention discloses achyranthes bidentata polypeptide, a production method and applications, and the achyranthes bidentata polypeptide is produced with the steps including polypeptide extraction, polypeptide separation, etc. The achyranthes bidentata polypeptide is used to the preparation of drugs and health products for treating peripheral nerve injuries, cerebrovascular accidents, ischemic brain injuries, cerebral traumas, optic nerve injuries, spinal cord injuries and central nervous system injuries, antiaging and preventing neurodegenerative diseases. Experiments prove that the extracted achyranthes bidentata polypeptide (protein) of the present invention has the effects of promoting the growth of nerves, preventing the fading of nerve cells and promoting the regeneration of injured nerves, the source of medicinal materials is single, the preparation method is advanced, the technique is reasonable, the ingredients of preparations are definite, safety is high, and pharmacological action is obvious.
Owner:NANTONG UNIVERSITY
Drug for treating depression
InactiveCN105943618AIdentifying the role of treating depressionGood effectNervous disorderPlant ingredientsReactive DepressionEndogenous depression
The invention discloses a drug for treating depression. The drug is prepared by evenly mixing 0.3-99.7 parts by weight of industrial hemp seed extract and 99.7-0.3 parts by weight of industrial cannabinoid. It is proved through experiments that the drug can significantly shorten the tail suspension immobilization time of mice of a behavioral despair depression model and significantly shorten immobility time of forced swimming, has an obvious anti-depressant effect on a mouse depression model caused by reserpine and can significantly increase the frequency of spontaneous activities of the mice, and the effects are all superior to those of cannabidiol. The drug can be used for preparing drugs for treating various depression symptoms including endogenous depression, reactive depression, masked depression, secondary depression caused by drugs, climacteric or postnatal depression, depression caused by cerebral trauma or cerebral apoplexy, diabetes combined depression and depressive neurosis and used for preparing drugs for treating symptoms such as secondary learning memory decline and anhedonia caused by depression.
Owner:云南瑞酚生物科技有限公司
Application of gastrodiaelata blume parishin extractive in preparation of medicament for protecting brain
The invention relates to an application of gastrodiaelata blume parishin extractive in preparation of a medicament for protecting brain, wherein the medicament is used for treatment of brain injury. Total content of a parishin derivative in the gastrodiaelata blume parishin extractive is more than 50%, preferably more than 75%, more preferably more than 95%. The parishin provides a certain protection effects for cerebral ischemia reperfusion injury, nerve cell injury induced by N-methyl-D-aspartate and closed cerebral trauma. The extractive provided in the invention provides a new selection approach for developing the medicament for protecting brain.
Owner:北京协和制药二厂有限公司
Rhein supramolecular hydrogel as well as preparation method and application thereof
ActiveCN106692037AImprove solubilityRetain biological activityOrganic active ingredientsNervous disorderDiseaseSolubility
The invention discloses a rhein supramolecular hydrogel as well as a preparation method and application thereof. The rhein supramolecular hydrogel comprises rhein and a N3HCO3 solution, wherein the rhein is dissolved into the N3HCO3 solution. The preparation method comprises the following steps: dissolving the rhein into the N3HCO3 solution, and performing ultrasonic dispersion, so as to obtain the rhein supramolecular hydrogel. The rhein supramolecular hydrogel has the characteristics of anti-inflammatory property, antibacterial property and antineoplastic property, can solve the problems of poor solubility and short half-life period of rhein, reserves the biological activity of the rhein, and has obvious treatment effect on the application of medicines for treating microglial cell mediated inflammatory encephalopathy, such as Alzheimer disease, Parkinson disease, cerebral trauma and cerebral edema.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Small volume intravenous injection of gastrodine and its prepn. method
InactiveCN1751691AReduce adverse reactionsImprove product qualityOrganic active ingredientsNervous disorderVeinInner Ear Diseases
A low-capacity intravenous injection of gastrodine for treating neurasthenia, neurasthenia syndrome, cerebral trauma syndrome, dizziness, Meniere's disease, reuralgia, headache, etc is prepared from gastrodine, the water for injection and metallic chelating agent. Its preparing process is also disclosed.
Owner:巴里莫尔制药(通化)有限公司
NOGO receptor-mediated blockade of axonal growth
Disclosed are Nogo receptor proteins and biologically active Nogo (ligand) protein fragments. Also disclosed are compositions and methods for modulating the expression or activity of the Nogo and Nogo receptor protein. Also disclosed are peptides which block Nogo-mediated inhibition of axonal extension. The compositions and methods of the invention are useful in the treatment of cranial or cerebral trauma, spinal cord injury, stroke or a demyelinating disease.
Owner:YALE UNIV
Medicinal composition and application thereof
ActiveCN103417534AImprove traumatic nerve functionImprove memory impairmentNervous disorderHydroxy compound active ingredientsCerebral ischaemiaFunctional disability
The invention relates to a medicinal composition, in particular to a composition comprising 4-hydroxyl-2-oxo-1-pyrrolidine acetamine and 2-borneol, and application of the composition in medicines for preventing and / or treating cognition impairment. The composition provided by the invention can remarkably improve a cerebral trauma neural function of a rat and can remarkably prolong the time of passive activities. The composition provided by the invention plays a role in improving dysmnesia of the rat, caused by scopolamine, and can remarkably improve cognitive disorder of the rat, caused by cerebral ischemia. The composition has a remarkable effect in preventing and / or treating cognition impairment.
Owner:北京茗泽中和药物研究有限公司
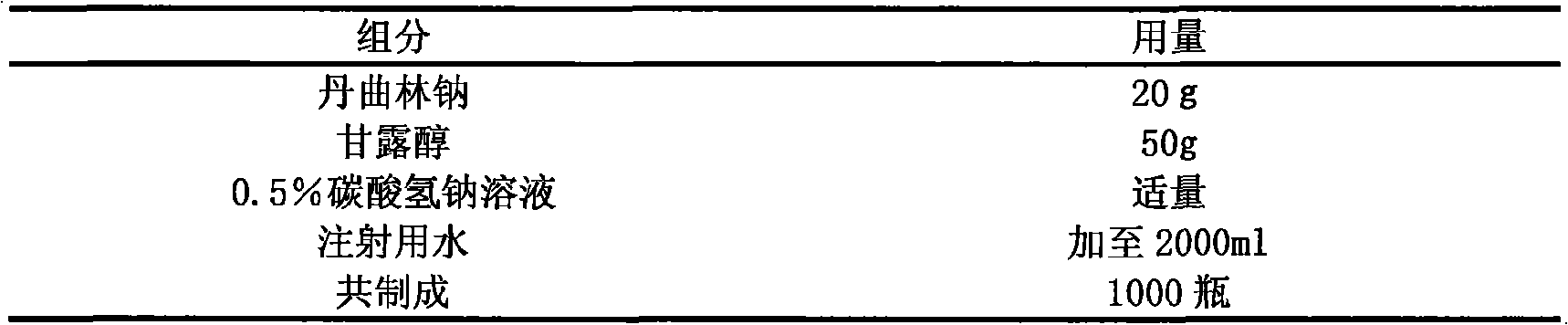
Freeze-dried powder needle preparations taking dantrolene sodium as activity component and preparation technique thereof
The invention relates to lyophilized powder for injection with dantrolene sodium as an active ingredient and a preparation method and uses thereof. The lyophilized powder is a pharmaceutical composition formed by mixing dantrolene sodium as the active ingredient and pharmaceutically-acceptable adjuvant, and is mainly suitable for spastic hypermyotonia state caused by upper motor neuron damage due to various reasons, such as apoplexy, cerebral trauma, spinal injury and multiple sclerosis. A lyophilized powder for intravenous injection can be prepared by using dantrolene sodium as a raw material and adding certain types and proportion of adjuvants according to the technical proposal given in the invention.
Owner:FUKANGREN BIO PHARMA
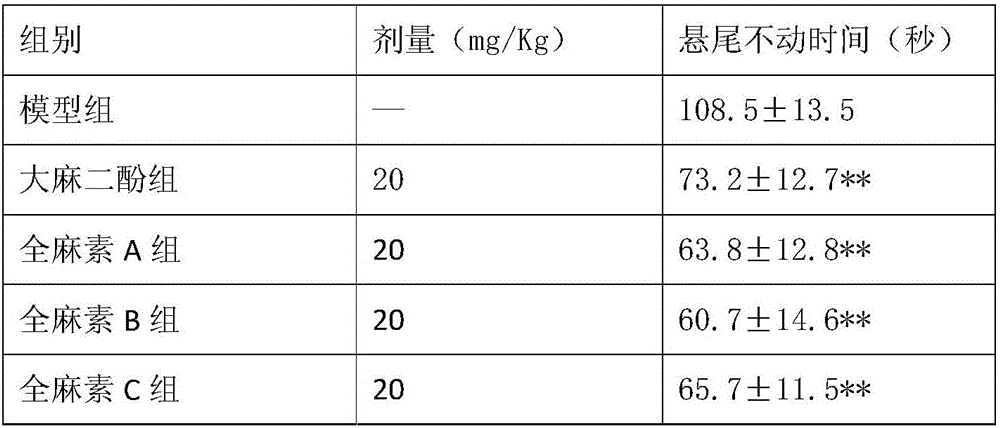
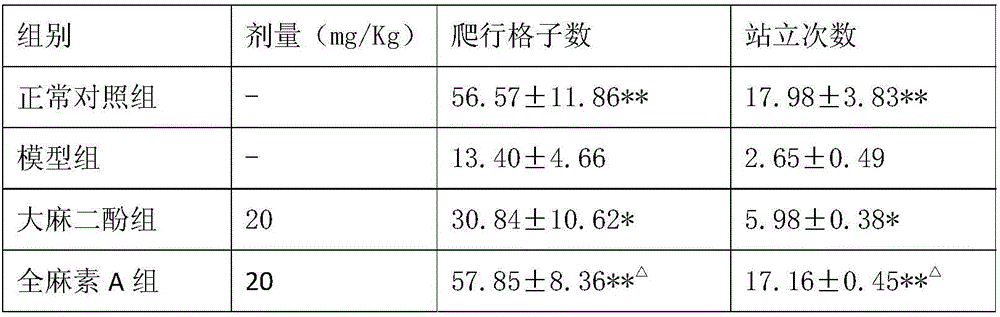
Application of all-cannabinoid in preparation of drugs for treating depression
InactiveCN105963359AShorten immobility timeIncrease the number of voluntary activitiesNervous disorderPlant ingredientsEndogenous depressionReactive Depression
The invention discloses application of all-cannabinoid in preparation of drugs for treating depression. The all-cannabinoid is prepared by uniformly mixing, by weight, 0.3-99.7 parts of an industrial hemp seed extract with 99.7-0.3 parts of industrial cannabinoids. The all-cannabinoid has the obvious relieving effect on the symptoms such as secondary learning memory deterioration of the depression and can be used for preparing the drugs for treating various depression symptoms including endogenous depression, reactive depression, latent depression, secondary depression caused by drugs, climacteric or postnatal depression, depression induced by cerebral trauma or stroke, diabetes-complicated depression and depressive neurosis and used for preparing the drugs for treating the symptoms such as secondary learning memory deterioration and anhedonia which are caused by the depression, and the effect of the all-cannabinoid is better than that of cannabidiol. The all-cannabinoid can be prepared into the clinical drugs of various dosage forms through a conventional preparation method and is convenient to use.
Owner:云南瑞酚生物科技有限公司
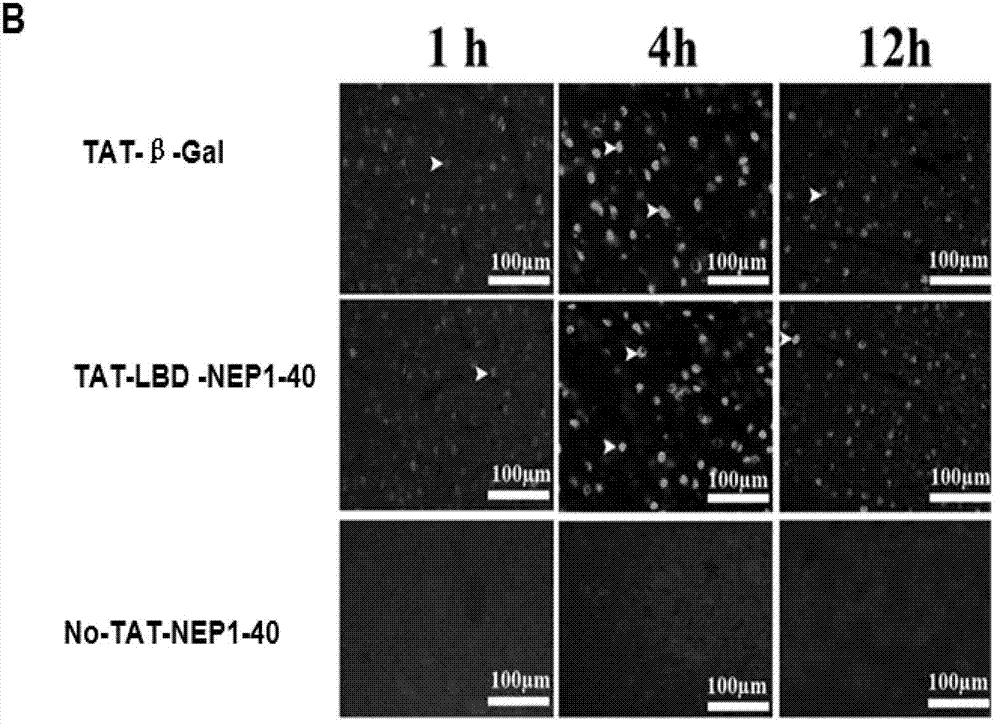
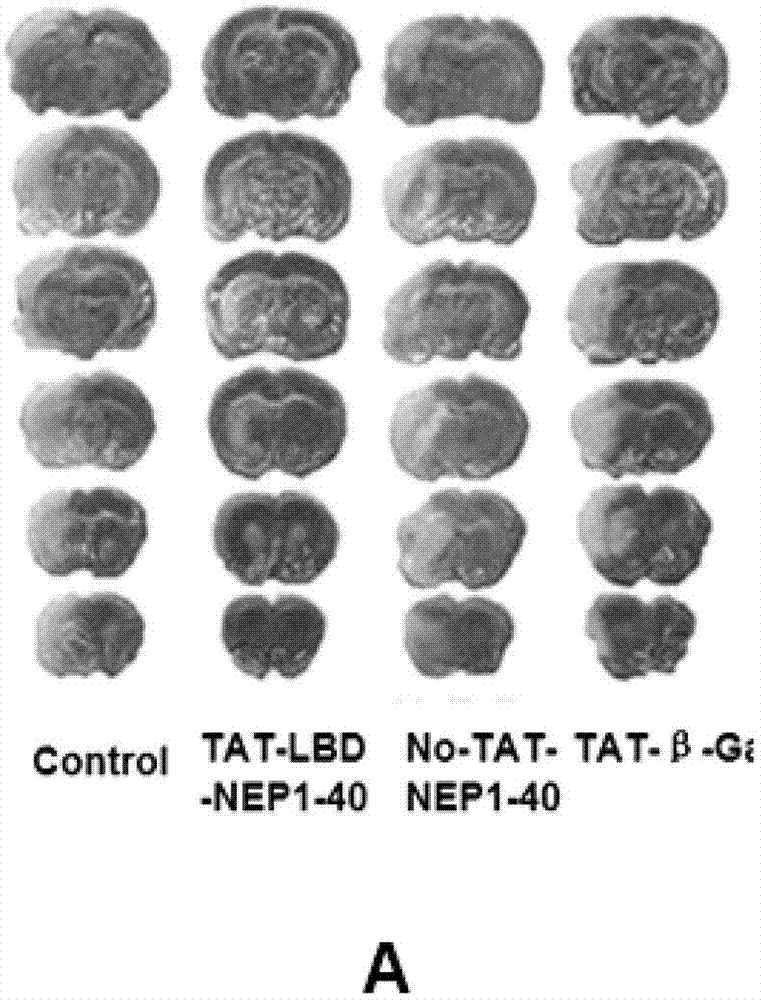
TAT-LBD-NEP1-40 fusion protein, construction method and application thereof
InactiveCN103113473AOvercome limitationsLittle side effectsNervous disorderPeptide/protein ingredientsNervous systemNogo Receptors
The invention discloses a TAT-LBD-NEP1-40 fusion protein, a construction method and an application thereof for treating central lesion. The TAT-LBD-NEP1-40 fusion protein constructed by the invention remains selective antagonistic activity of NEP1-40 to a Nogo receptor and has the advantages of high transduction efficiency and easy transmission of blood and spine barriers and blood brain barrier, so that the limit of NEP1-40 is overcome so as to avoid the deficiency of extra damage to nervous tissues caused by micro-injection of NEP1-40 or micro pump implantation and difficulty for NEP1-40 to pass through blood and spine barriers and blood brain barrier to meet corresponding biological concentration. The infusion protein provided by the invention can be massively prepared, and is low in cost and high in activity. The infusion protein can be used to treat various CNS (Central Nervous System) damages such as cerebral ischemia and anoxia, cerebral hemorrhage, cerebral trauma and spinal cord injury, promote regeneration of nervous tissues and neural functional recovery, and treat CNS damages.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
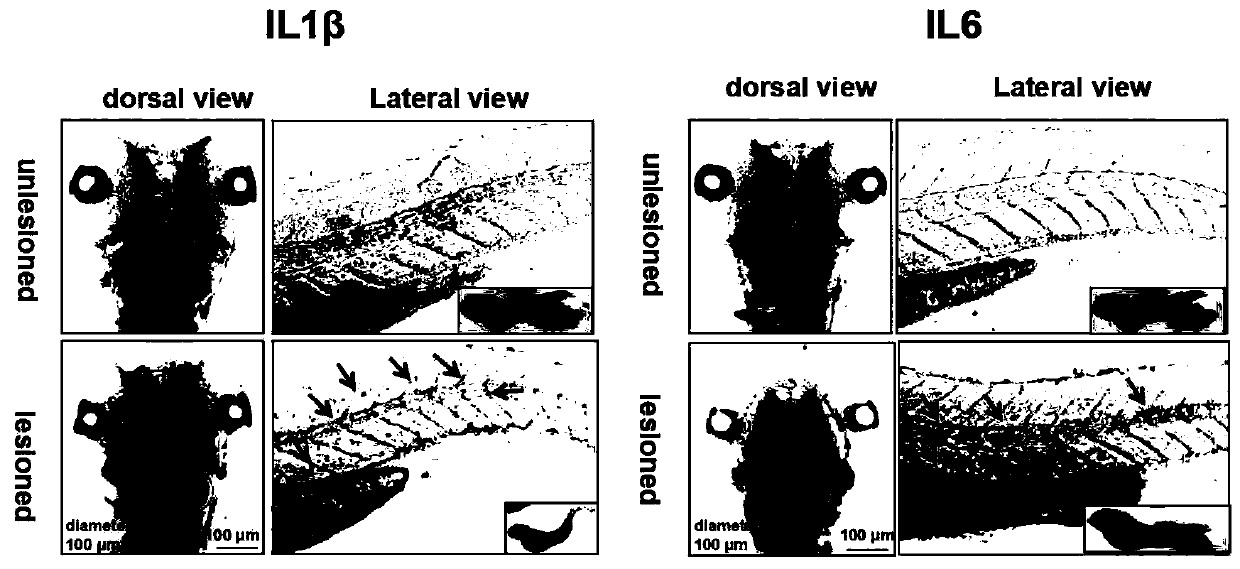
Zebra fish brain trauma model and preparation method and application thereof
ActiveCN110178757APromote repairGood prospectsClimate change adaptationPisciculture and aquariaDiseaseEmbryo
The invention discloses a zebra fish brain trauma model and a preparation method and application thereof. The preparation method of the zebra fish brain trauma model includes the steps that for an embryo in the 3dpf period, a needle is used for puncturing the hindbrain of a zebra fish, and microglial cells of the hindbrain of the zebra fish are labeled with fluorescent protein. After clinical first-line anti-inflammatory drugs are used for intervention, and more microglial cells can be activated to migrate and aggregate towards a wound part, so that injured hindbrain repairing is promoted; themicroglial-cell transgenic zebra fish brain trauma model can be used for rapid screening of drugs, so that an economical, convenient and reliable research model is provided for enterprise drug research and development and for evaluating the curative effect of the anti-inflammatory drugs in clinical brain trauma, encephalitis and other diseases, and the microglial-cell transgenic zebra fish braintrauma model has good application prospects and high application value.
Owner:AFFILIATED HOSPITAL OF GUANGDONG MEDICAL UNIV
Application of nanogold in preparation of reagent for measuring blood-brain barrier permeability
ActiveCN103800920AAccurately reflectSimple ingredientsColor/spectral properties measurementsIn-vivo testing preparationsBlood brain barrier permeabilityMaterials science
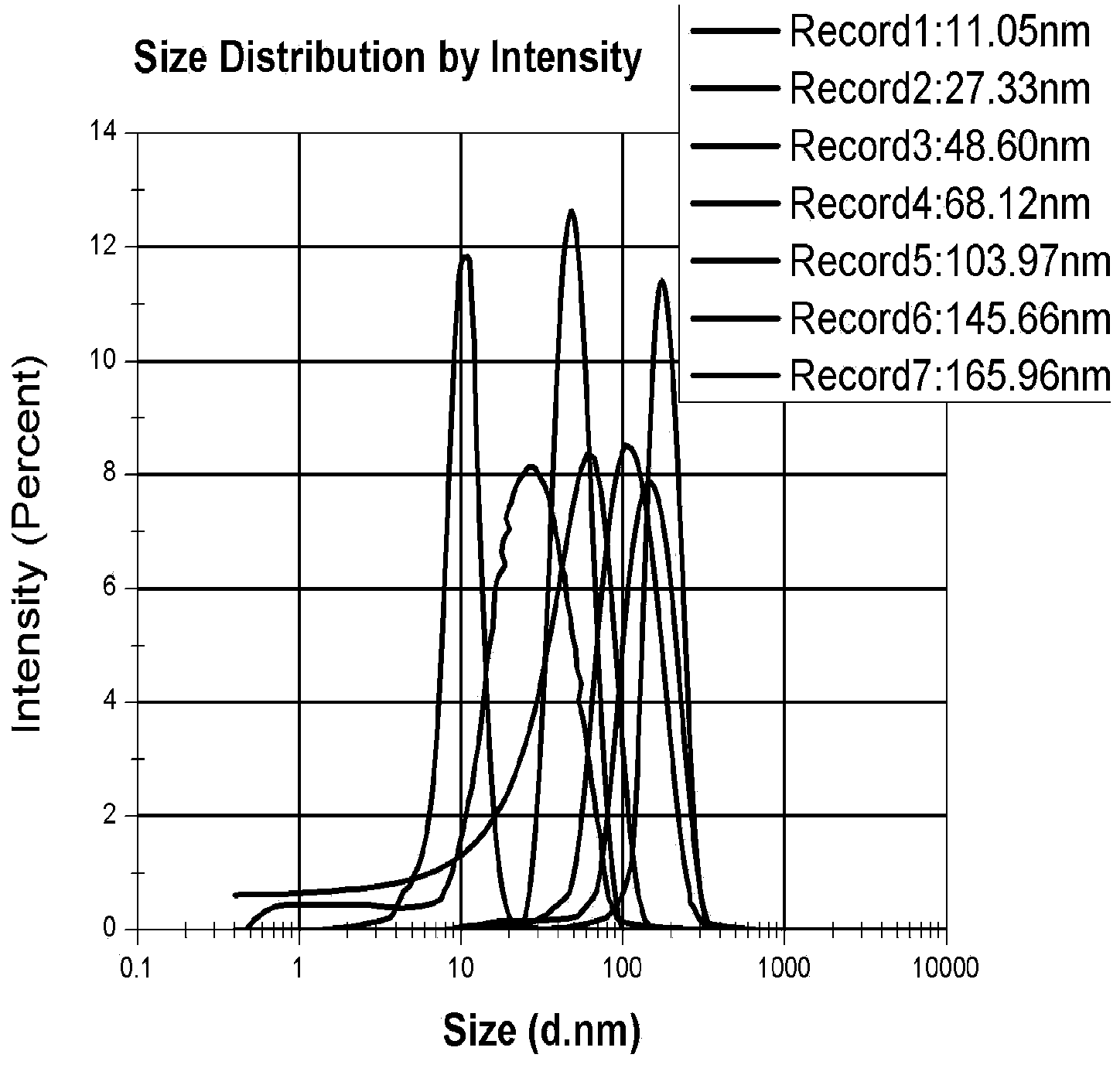
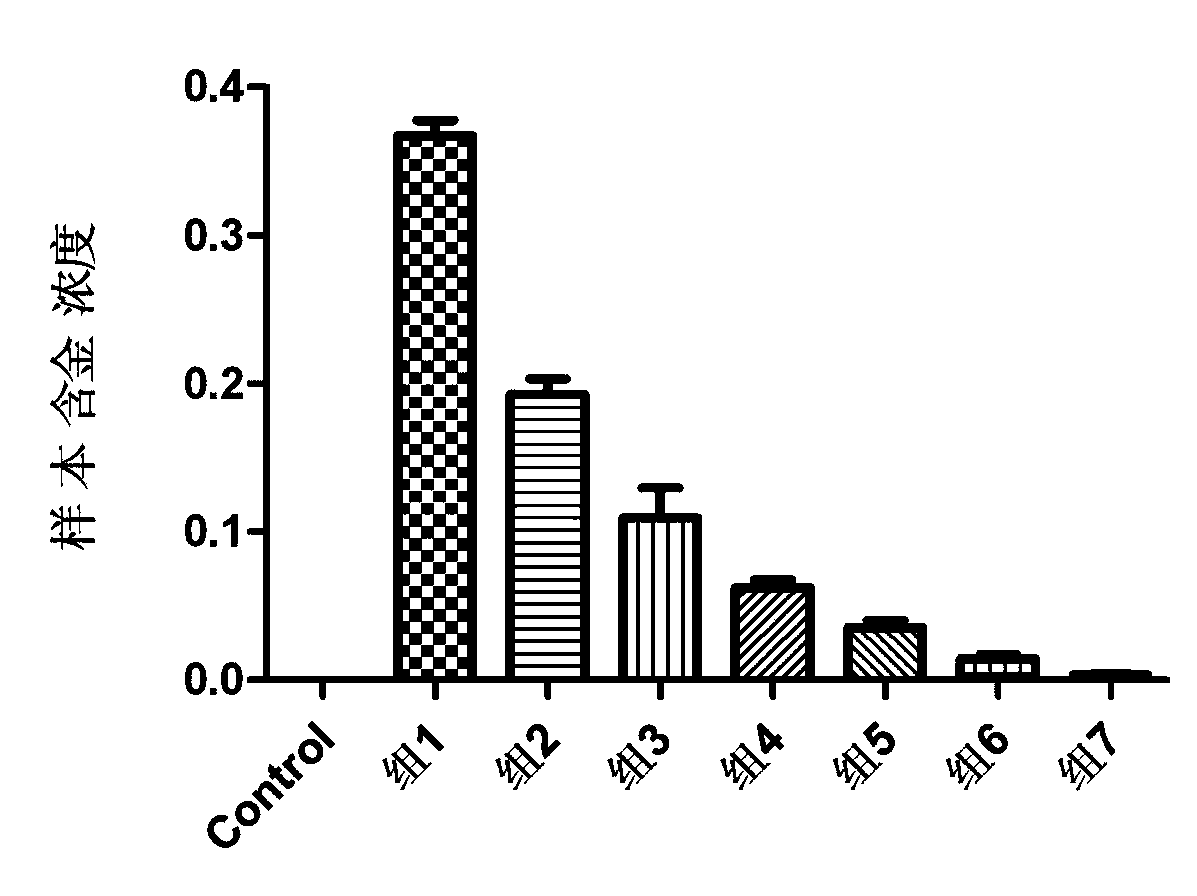
The invention provides the application of nanogold in preparation of a reagent for measuring blood-brain barrier permeability. The nanogold is formed by the following nanogold particles with the particle sizes by weight percent: 1 part of nanogold particles with the particle size range of 9-15nm and the average particle size of 11+ / -0.5nm, 1 part of nanogold particles with the particle size range of 18-31nm and the average particle size of 27.5+ / -0.5nm, 1 part of nanogold particles with the particle size range of 41-63nm and the average particle size of 48.5+ / -0.5nm, 1 part of nanogold particles with the particle size range of 50-76nm and the average particle size of 68+ / -0.5nm, 1 part of nanogold particles with the particle size range of 93-114nm and the average particle size of 104+ / -0.5nm, 1 part of nanogold particles with the particle size range of 134-156nm and the average particle size of 145.5+ / -0.5nm and 1 part of nanogold particles with the particle size range of 151-176nm and the average particle size of 166+ / -0.5nm. According to the application, the quantitative measurement for the blood-brain barrier permeability of animals with cerebral trauma can be completed by the nanogold particles with different particle sizes; the number of nanogold permeating into the blood-brain barrier can be measured, and the largest pore of the opening of the blood-brain barrier can be estimated according to the particle sizes of the nanogold permeating into the blood-brain barrier; therefore, a quantitative measurement standard is provided for various blood-brain barrier damage models in animal experiments.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Pyridine methylamino phthalide compound and preparation method and application thereof
InactiveCN112010837AHigh inhibition rateOrganic active ingredientsSenses disorderHuntingtons choreaNervous system
The invention discloses a novel pyridine methylamino phthalide compound (I) and pharmaceutically acceptable salts, a preparation method and a pharmaceutical composition thereof and application thereofin preparation of medicines for treating and / or preventing nervous system related diseases. The diseases include but are not limited to vascular dementia, Alzheimer's disease, Parkinson's disease, Huntington's disease, HIV related dementia, multiple sclerosis, amyotrophic lateral sclerosis, neuropathic pain, glaucoma, cerebral arterial thrombosis, cerebral hemorrhagic stroke, nerve injury causedby cerebral trauma and the like.
Owner:SICHUAN UNIV
Salicylamide compound preparation method and application thereof
ActiveCN111170884AInhibition of productionThe dose-effect relationship is obviousSenses disorderNervous disorderHuntingtons choreaNervous system
The invention discloses a class of novel salicylamide compounds (I) and a pharmaceutically acceptable salt thereof, a preparation method and a pharmaceutical composition thereof, and applications of the novel salicylamide compounds (I) and the pharmaceutically acceptable salt thereof in preparation of drugs for treating and / or preventing nervous system related diseases, wherein the diseases include but are not limited to vascular dementia, Alzheimer's disease, Parkinson's disease, Huntington's disease, HIV related dementia, multiple sclerosis, amyotrophic lateral sclerosis, neuropathic pain, glaucoma, cerebral arterial thrombosis, cerebral hemorrhagic stroke, nerve injury caused by cerebral trauma and the like.
Owner:SICHUAN UNIV
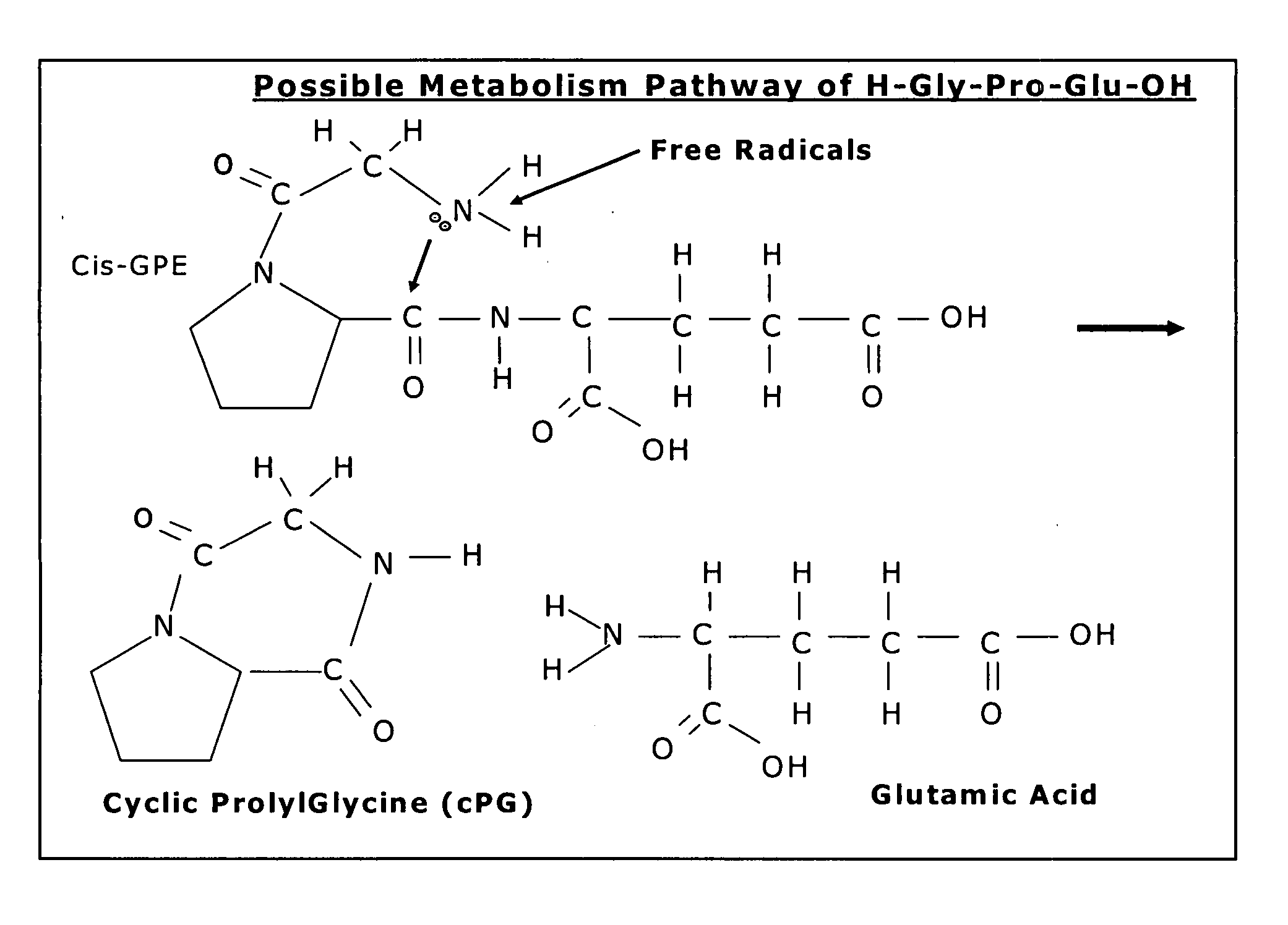
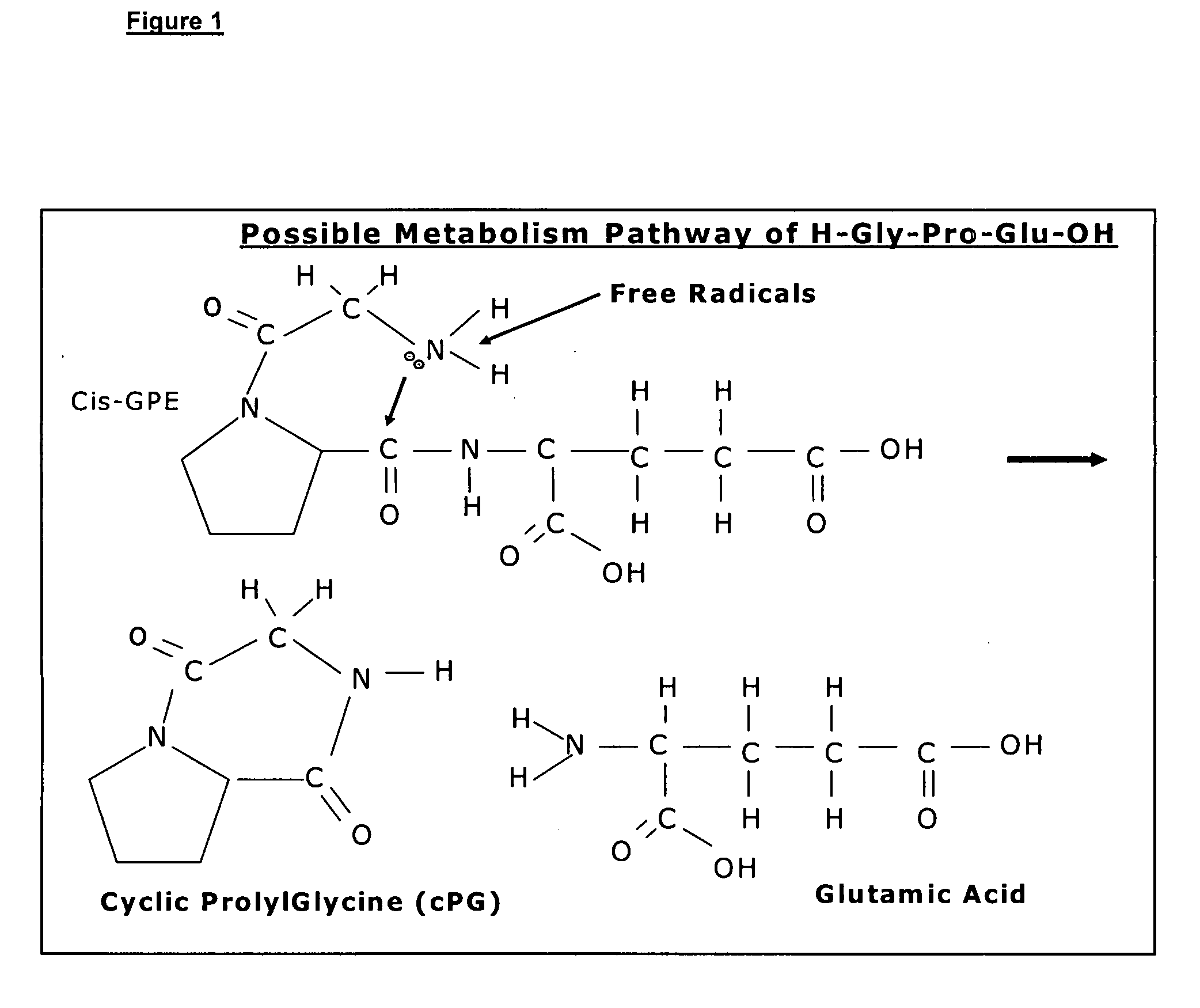
Therapeutic agent composition and method of use
The invention relates to the use of cyclic Prolyl Glycine (“cyclic PG” or “cPG”) and analogs and mimetics thereof, as neuroprotective agents for the treatment and or prevention of neurological disorders including but not limited to cerebral ischemia or cerebral infarction resulting from a range of phenomena, such as thromboembolic or hemorrhagic stroke, cerebral basospasms, hypoglycemia, cardiac arrest, status epilepticus, perinatal asphyxia, anoxia such as from drowning, pulmonary surgery, and cerebral trauma, as well as to the treatment and prevention of chronic neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and Huntington's disease, and as anticonvulsants.
Owner:TRAN LOI H
Application of emodin in preparation of drugs for treating depression
InactiveCN101961325AOrganic active ingredientsNervous disorderEndogenous depressionPost-pregnancy depression
The invention discloses an application of emodin as an active ingredient in the preparation of drugs for treating depression, wherein The emodin is used as an active ingredient to prepare drugs for treating the depression. The invention provides an application of the emodin as an active ingredient in the production of drugs for preventing and treating the depression. Emodin has the characteristic of low toxicity and is used for preparing drugs for preventing or treating various kinds of depression symptoms comprising endogenous depression, reactive depression, secretiveness depression, drug induced secondary depression, climacteric or postpartum depression, cerebral trauma or cerebral apoplexy induced depression and depressive neurosis, and can also be applied to drugs for treating diabetes accompanied by depression and related diseases.
Owner:SOUTHWEST JIAOTONG UNIV
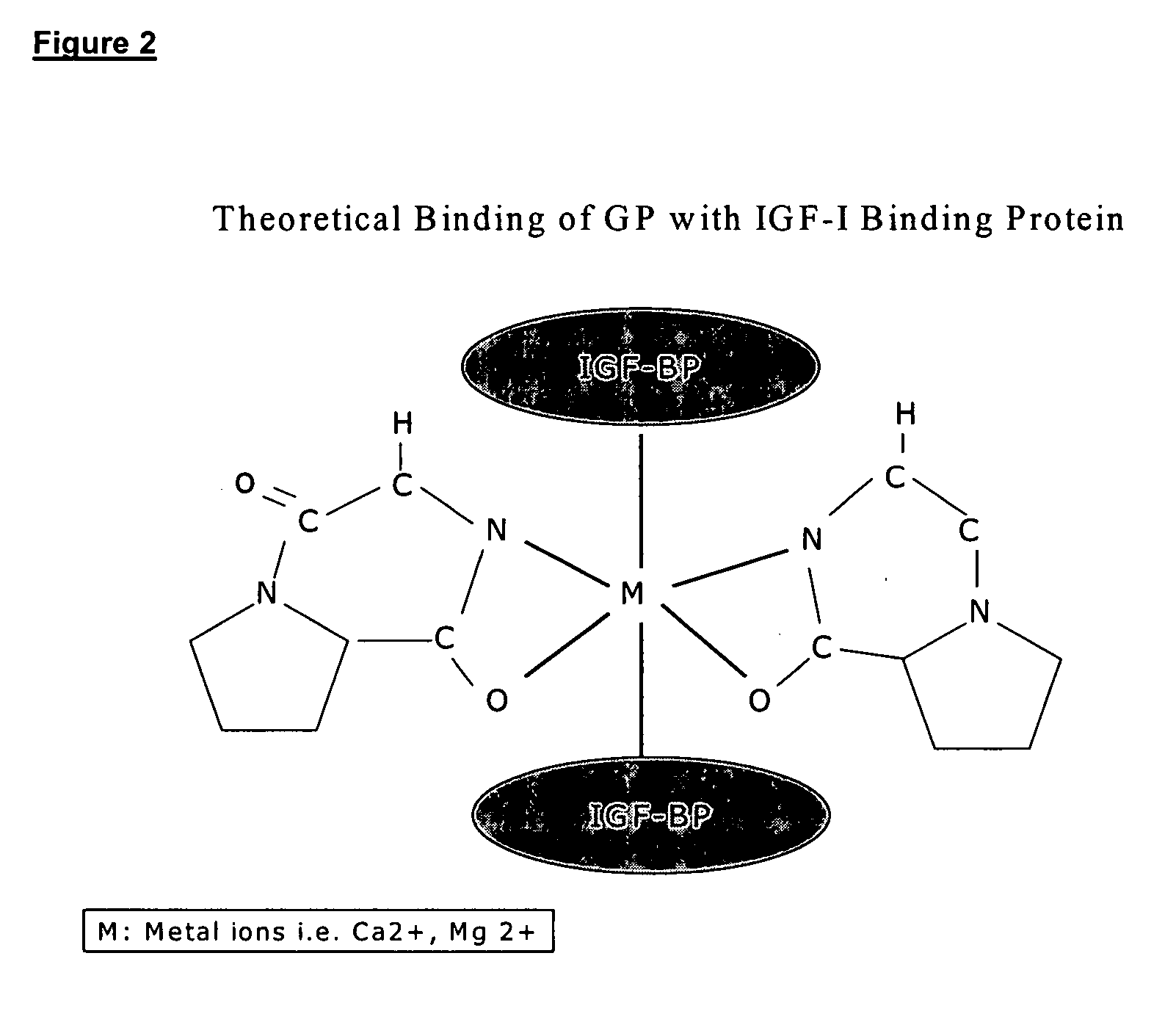
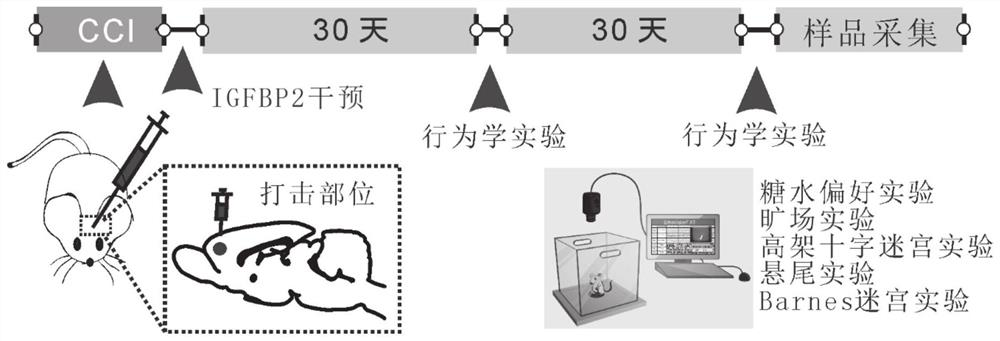
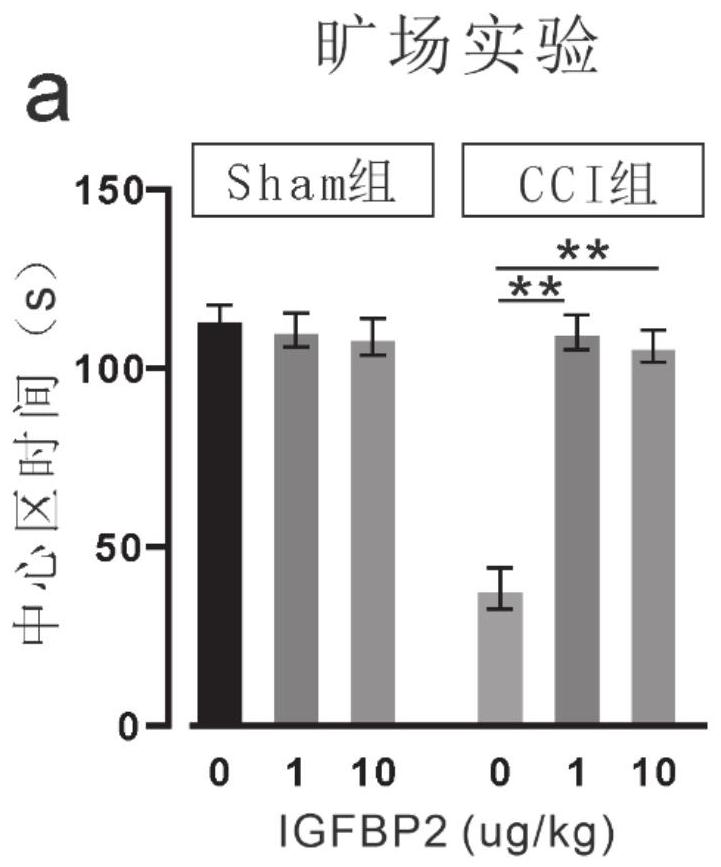
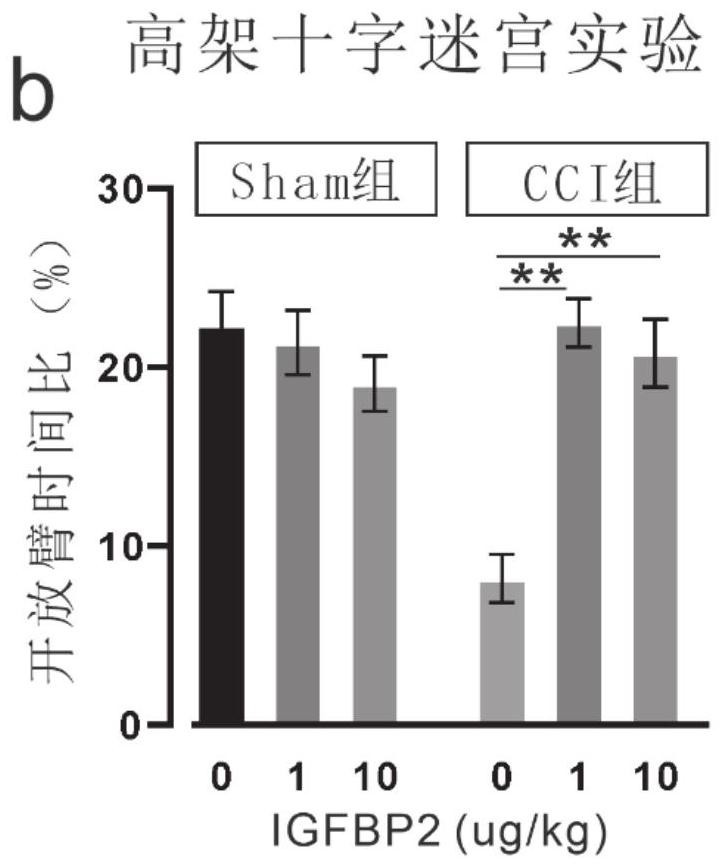
Application of insulin-like growth factor binding protein 2 in preparation of medicine for treating mental disorder caused by cerebral trauma
PendingCN112957453ANervous disorderPeptide/protein ingredientsPharmaceutical drugInsulin-like growth factor-binding protein
The invention relates to application of insulin-like growth factor binding protein 2 in preparation of a medicine for treating mental disorder caused by brain trauma. The IGFBP2 intervention can significantly improve anxiety and depression behaviors caused by brain trauma and cognitive function decline behaviors caused by brain trauma.
Owner:SHANGHAI MENTAL HEALTH CENT (SHANGHAI PSYCHOLOGICAL COUNSELLING TRAINING CENT)
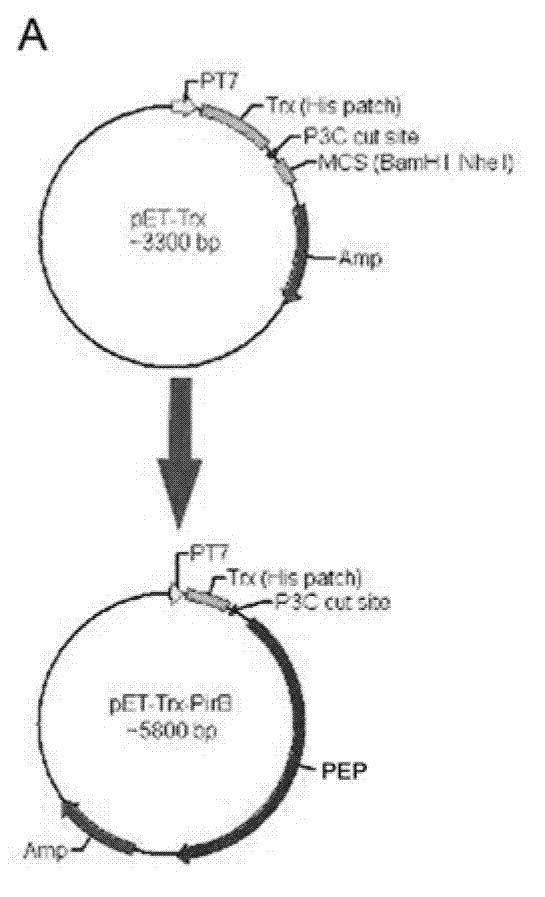
PirB extracellular polypeptide and application
InactiveCN103113467APromote growthNervous disorderCell receptors/surface-antigens/surface-determinantsEscherichia coliDisease
The invention provides PirB extracellular polypeptide which potentially treats central lesion. The polypeptide has an extracellular amino acid sequence of a paired immunoglobin-like receptor. The polypeptide segment can be massively expressed in escherichia coli and combined with myelin inhibiting factors MAG, Nogo-66 and OMgp in high affinity, so as to antagonize the bioactivity and function of PirB. The PEP provided by the invention can be effectively combined with myelin inhibiting factors MAG, Nogo-66 and OMgp so as to promote axon growth. The PEP provided by the invention can be massively prepared, is low in cost and high in activity, can be used for preparing medicines for treating various CNS (Central Nervous System) damages such as cerebral ischemia and anoxia, cerebral hemorrhage, cerebral trauma and spinal cord injury, and can effectively promote the regeneration of nervous tissues and neural functional recovery.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
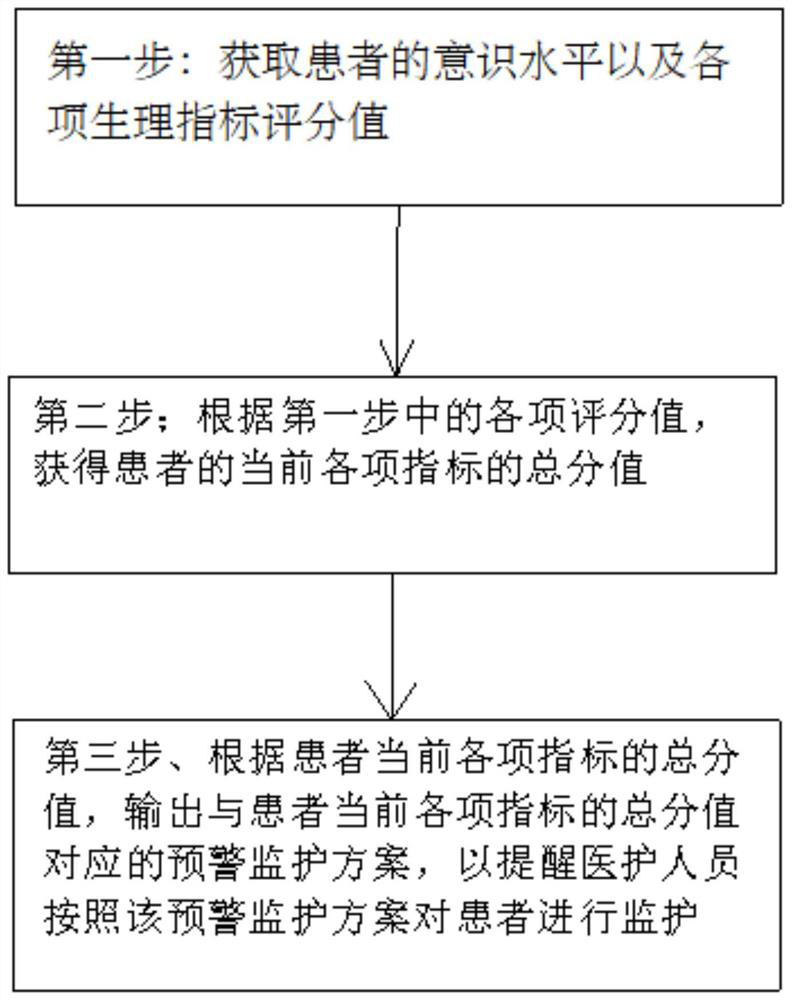
Early warning method for craniocerebral trauma specialized disease and operation terminal
The invention provides an early warning method for a craniocerebral trauma specialized disease. The early warning method comprises the following steps: step 1, acquiring the consciousness level and various physiological index score values of a patient; step 2, obtaining a total score of each current index of the patient according to each score in the step 1; and step 3, outputting an early warning monitoring scheme corresponding to the total score of each current index of the patient according to the total score of each current index of the patient so as to remind the medical staff to monitor the patient according to the early warning monitoring scheme; the defect that medical staff can not achieve the purpose of early warning and judging the illness state of a patient by judging the illness state of the patient by themselves is overcome.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Chinese medicinal composition for treating hemorrhagic brain contusion and laceration
InactiveCN1634249ASignificant effectNervous disorderPharmaceutical delivery mechanismMedicinal herbsBrain Contusion
The invention discloses a Chinese medicinal composition for treating hemorrhagic brain contusion and laceration, which is prepared from ten Chinese medicinal herbs including peach kernels, safflower, Ligusticum wallichii, Chinese angelica root, dried rehmannia root, poria cocos wolf. The composition can be prepared into any of the pharmacologically acceptable dosage forms.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
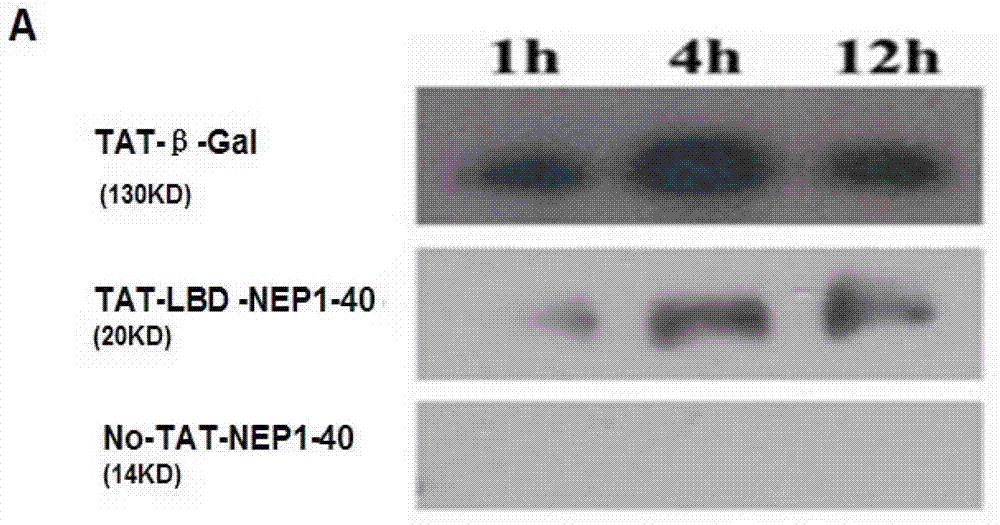
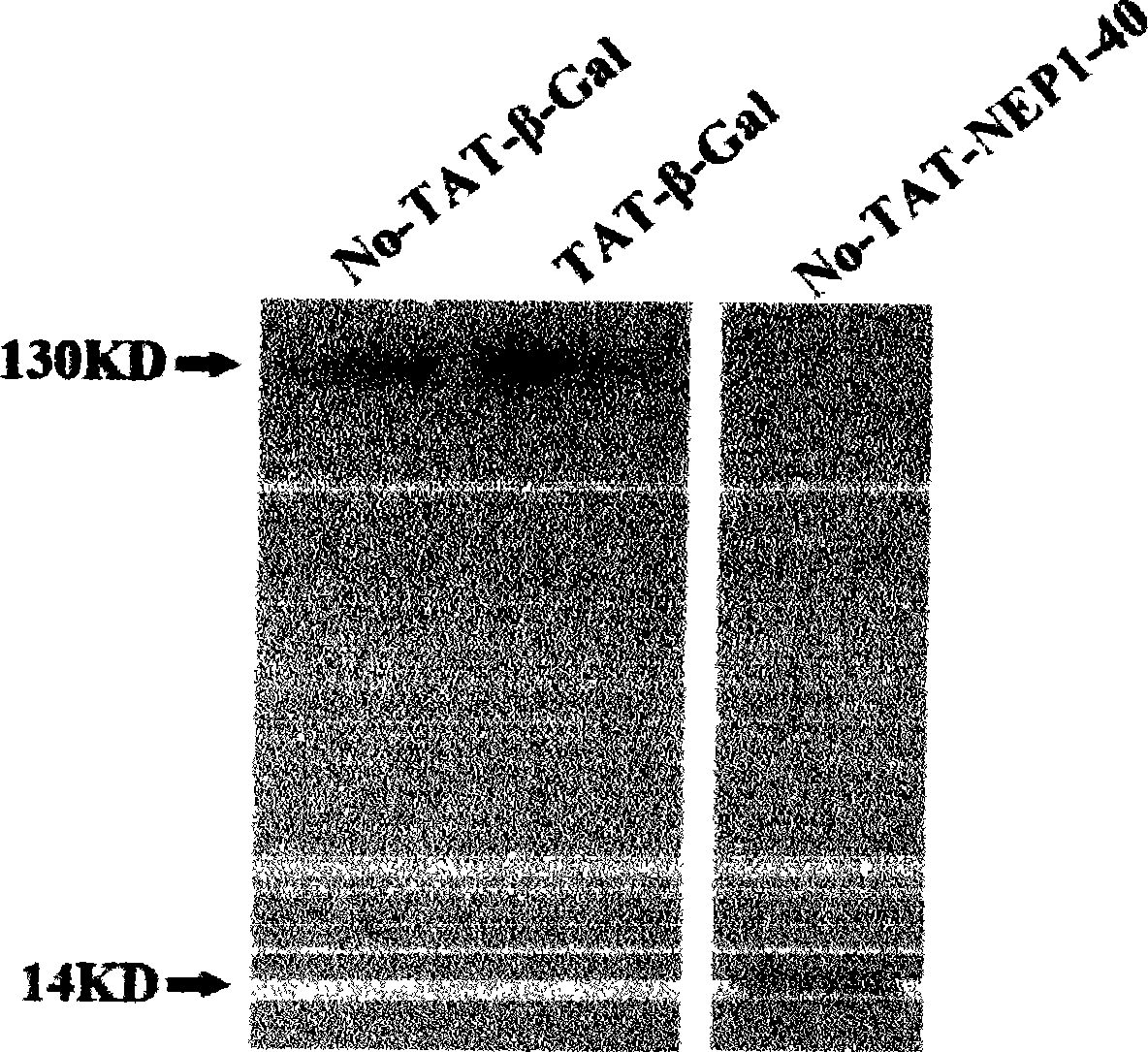
TAT protein transduction modified NEP1-40 fusion protein and use thereof
InactiveCN101418046APreserve penetrationSolve the problem of not being able to cross the blood-brain barrier (BBB)Nervous disorderPeptide/protein ingredientsNervous systemHigh activity
The invention discloses a preparation method for TAT-NEP1-40 fusion protein and application in treating the injuries of a central nervous system, CNS. The TAT-NEP1-40 fusion protein is characterized in that the TAT-NEP1-40 fusion protein comprises the protein transduction domain (PTD) of a transactivating transduction protein TAT of the human immunodeficiency virus (HIV) and Nogo extracellular peptide residues 1-40, NEP1-40. The fusion protein retains the selective antagonistic activity of the NEP1-40 to a Nogo receptor, NgR, and has the advantages of high transduction efficiency and easy penetration of BBB and BCSB, thereby overcoming the limitation of the NEP1-40, avoiding the extra injuries on nervous tissues caused by the mode of injecting the NEP1-40 through microinjection or miniature pump implantation, and overcoming the problem that the NEP1-40 can hardly pass BBB and BCSB to reach corresponding biological concentration. The fusion protein can be prepared in a large quantity, has a low cost and high activity, and can be used for treating a plurality of CNS injuries including cerebral ischemia, cerebral anoxia, cerebral hemorrhage, cerebral trauma, spinal core injury, and the like, and promoting the regeneration and functional rehabilitation of nervous tissues.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
3-hydroxy chalcone Mannich base compound and preparation and application methods thereof
The invention discloses a 3-hydroxy chalcone Mannich base compound (I), medically acceptable salts of the 3-hydroxy chalcone Mannich base compound, a preparation method of the 3-hydroxy chalcone Mannich base compound, a drug combination of the 3-hydroxy chalcone Mannich base compound and application of the 3-hydroxy chalcone Mannich base compound to preparing drugs for treating and / or preventing nervous system-related diseases including but not limited to vascular dementia, Alzheimer's disease, Parkinson's disease, Huntington's disease, HIV-related (human immunodeficiency virus-related) disease, multiple sclerosis , amyotrophic lateral sclerosis, neuropathic pain, glaucoma, cerebral arterial thrombosis and nerve injuries caused by cerebral trauma.
Owner:SICHUAN UNIV
Pharmaceutical composition for treating cerebroma and cerebral concussion, and preparation method thereof
InactiveCN101411857AGood curative effectRelieve painInorganic active ingredientsAntineoplastic agentsCerebral paralysisPost concussion
The invention provides a medicine composition for treating encephaloma and cerebral concussion and a preparation method thereof, belongs to a medicine composition, and in particular relates to a medicine prepared by using plant traditional Chinese medicines as raw materials, and the preparation method thereof. Raw materials for preparing active ingredients of the medicine comprise ginger, realgar, hemlock parsley, rhizoma gastrodiae, leech and the like. The preparation method comprises the following steps: processing mixed materials into fine powder; and preparing the fine powder into capsules, watered pills, tablets or granules. The medicine can treat various headaches, dizziness, paralysis, numbness and the like caused by encephaloma, cerebral trauma, brain haemostasis, concussion of brain sequela, basilar papilledema and cerebral paralysis with high efficacy but without side effects.
Owner:张兴仕
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com