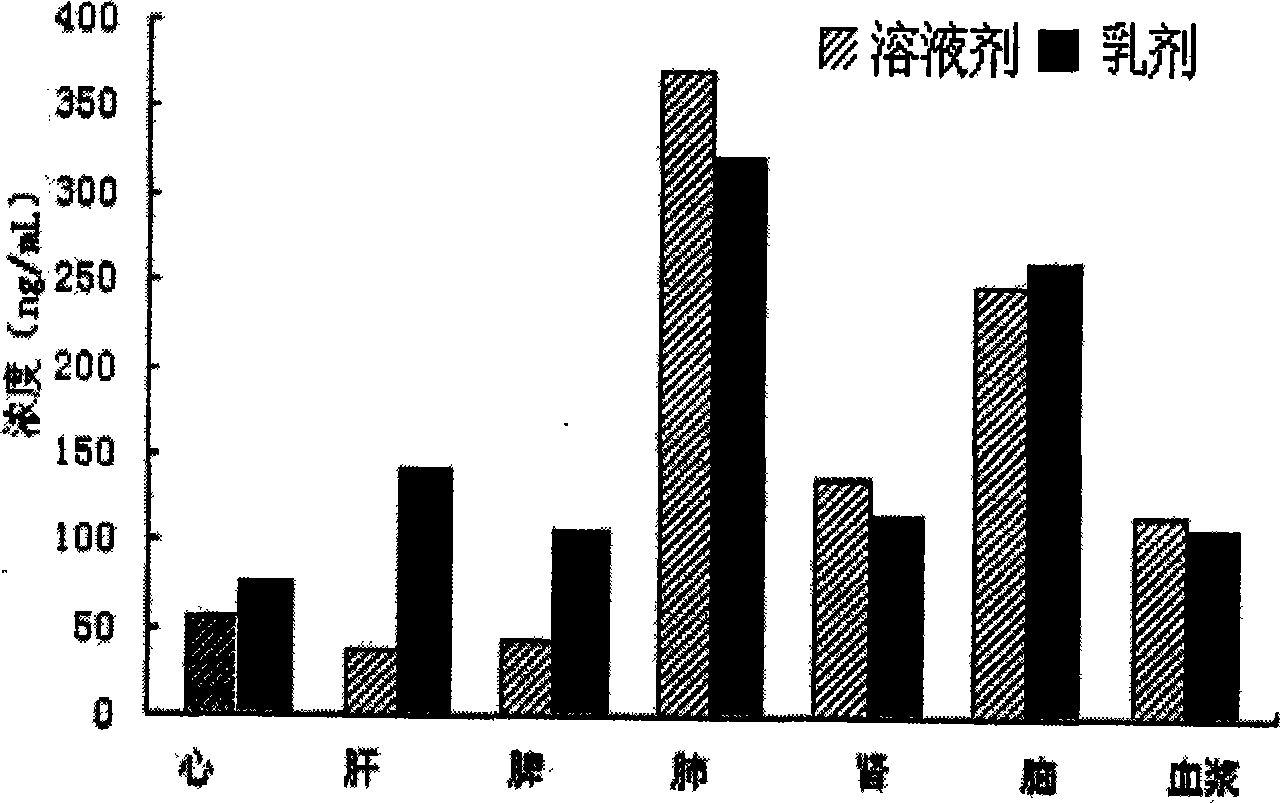
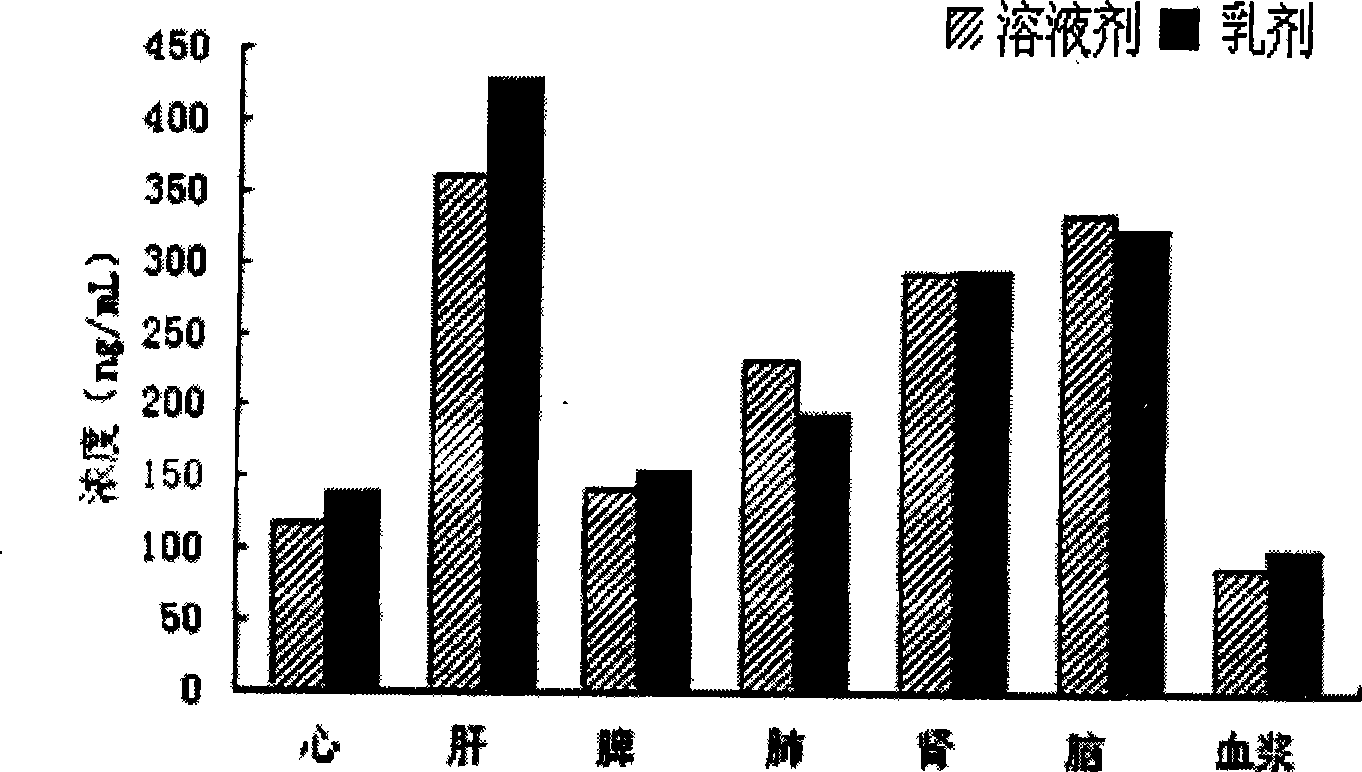
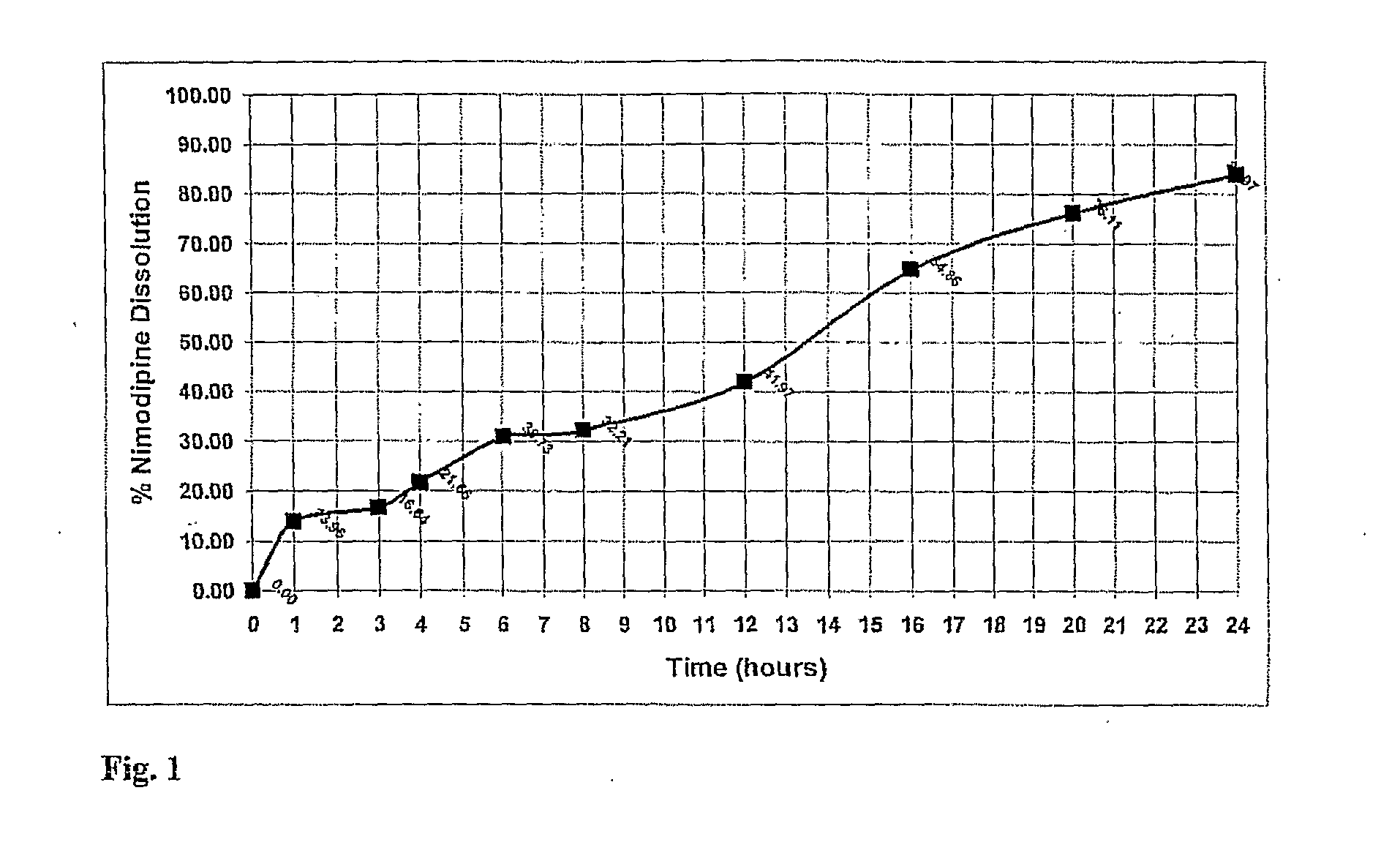
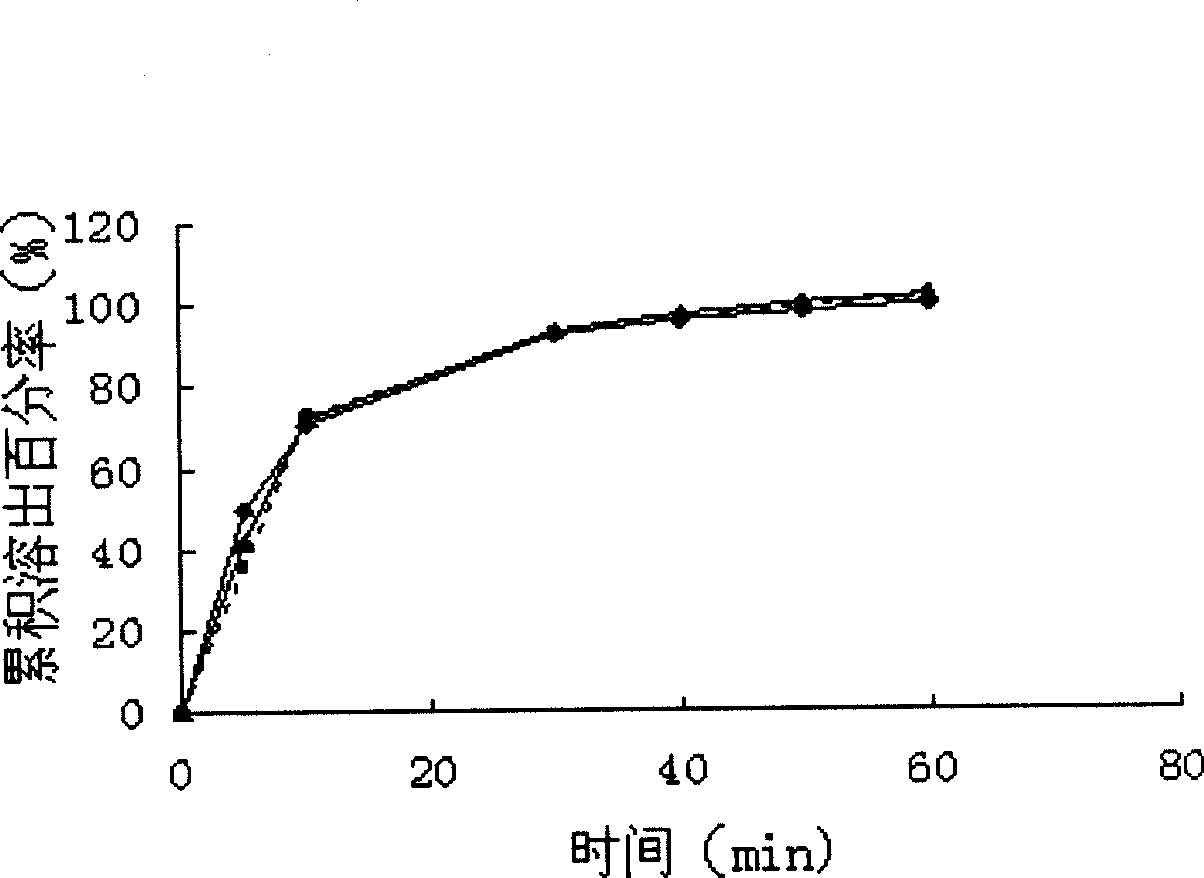
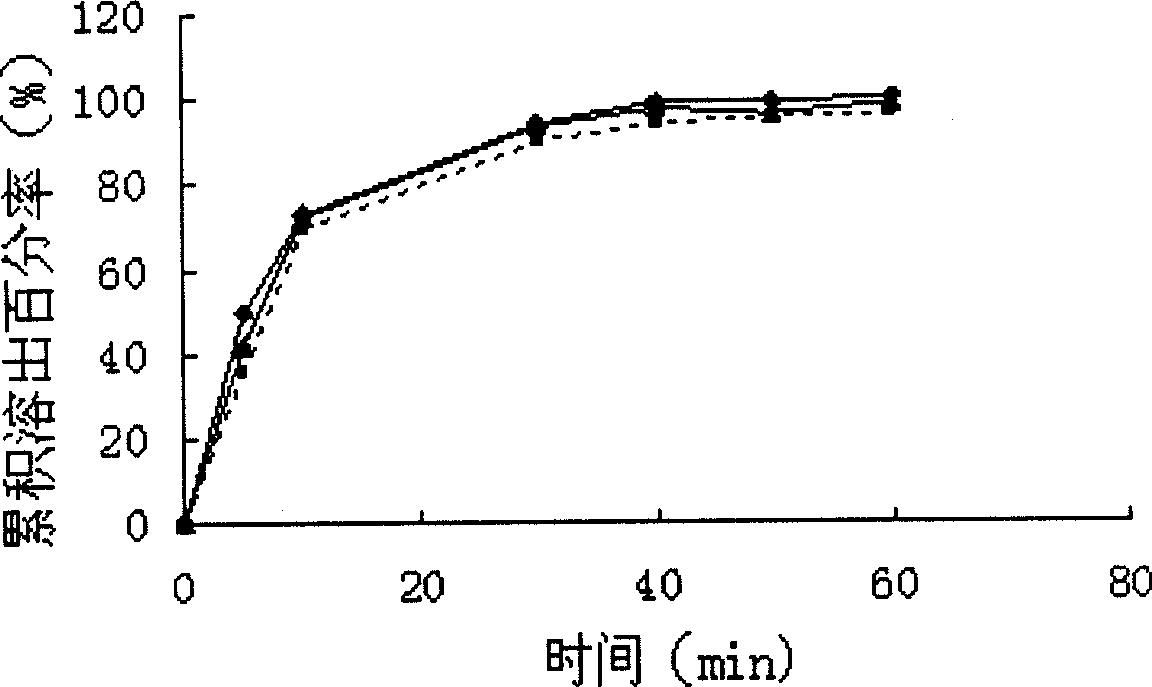
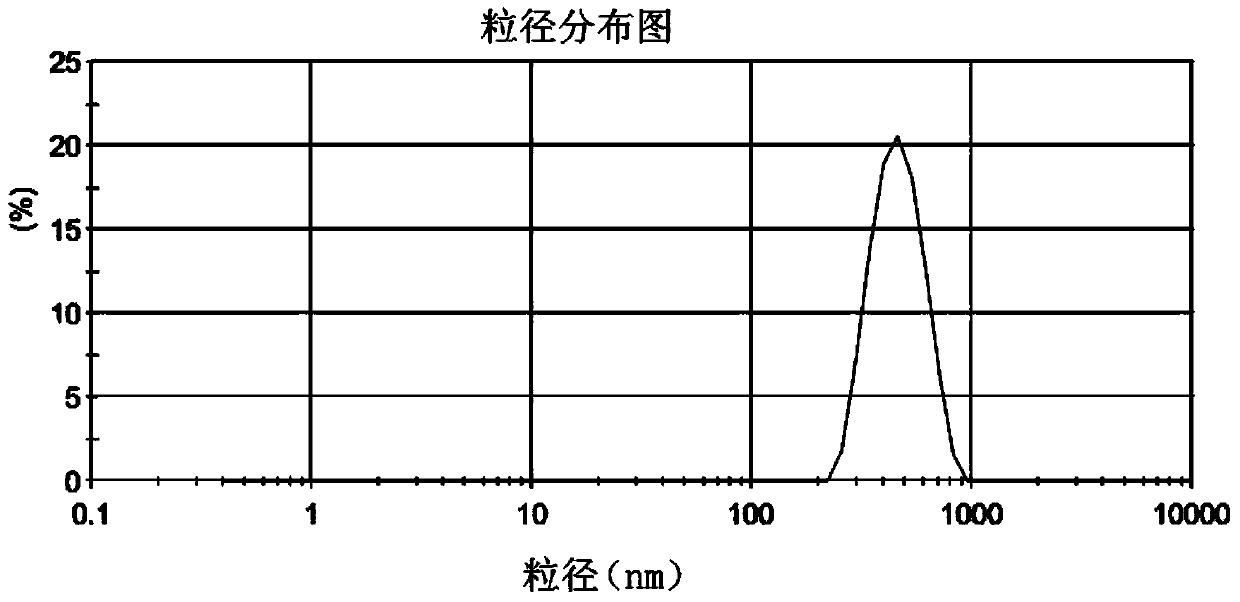
Patents
Literature
162 results about "Nimodipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nimodipine is used to decrease problems due to a certain type of bleeding in the brain (subarachnoid hemorrhage-SAH).
Combination pharmaceutical compositions
InactiveUS20100215737A1Slow changeGood for healthPowder deliveryBiocideControlled releaseImmediate release
A modified release dosage product (5) comprises a plurality of minicapsules or minispheres (1, 2) containing nimodipine, and a plurality of minicapsules or minispheres (3), (4) containing tacrolimus. There are uncoated minicapsules or minispheres (1) encapsulating micronized nimodipine for immediate release and a controlled release polymer coated minicapsule or minisphere (2) encapsulating micronized nimodipine for delayed, sustained, controlled or targeted release. There are uncoated seamless minicapsules (3), the core of which comprises tacrolimus lipid-based formulation for immediate release and a controlled release polymer coated seamless minicapsule (4), the core of which comprises tacrolimus lipid-based formulation for delayed, sustained, controlled release or targeted release. The final dosage form may be a hard gelatin capsule (5).
Owner:COULTER IVAN
Nimodipine pharmaceutical composition and method of producing the same
InactiveCN101129366AReduce the impactStable contentOrganic active ingredientsPharmaceutical non-active ingredientsLipid formationNimodipine
The invention discloses a new nimodiping drug composition and making method, which is made of nimodiping, sorbitol or dehydration sorbitol fatty acid simple lipid surface activator, benign solvent of nimodiping, auxiliary solubilizer and injecting water to form the concentrated solution of injection or oral agent. The invention has big density, good stability, low findings and simple making technique, which can't induce damage for brain tissue due to cerebrovascular spasm.
Owner:沈阳万爱普利德医药科技有限公司 +1
Lyophilized composition of nimodipine
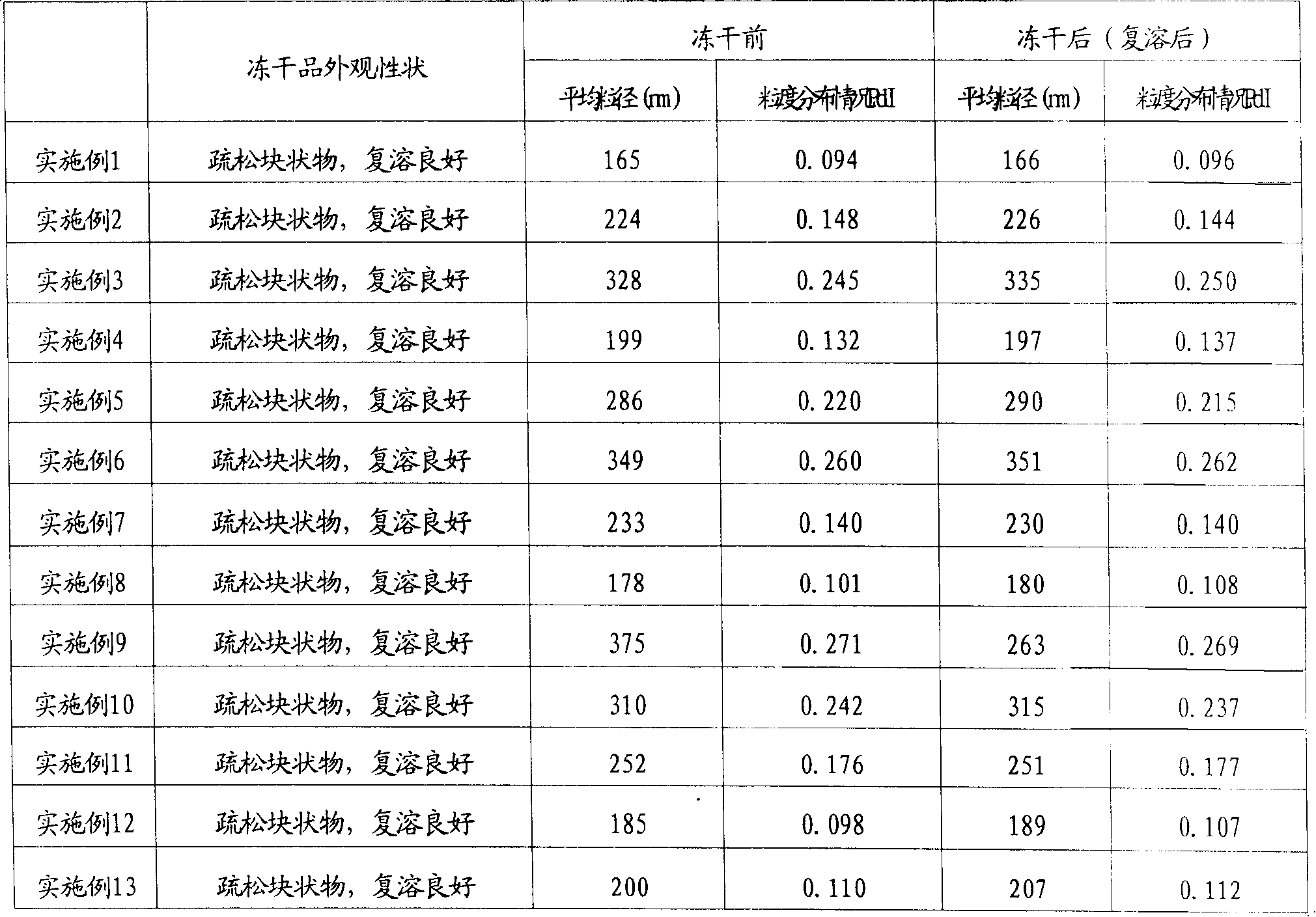
A freeze dried nimodipine composition in the form of injection contains nimodipine, phosphatide, cyclodextrin, and its derivative or surfactant. Its advantages are high solubility, high stability and high safety.
Owner:KUNMING LONGJIN PHARMA
Nimodipine lipid microsphere injection and preparation method thereof
ActiveCN101485632AImprove solubilityImprove stabilityOrganic active ingredientsNervous disorderSolubilityLipid formation
The invention provides a nimodipine lipid microsphere injection, which is prepared from the following components in percentage by weight: 0.08 percent of nimodipine, 0.5 to 2.3 percent of lecithin for injection, 2 to 8 percent of soybean oil for injection, 2 to 8 percent of medium chain fatty acid for injection, 1 to 3 percent of glycerin, 0.1 to 0.2 percent of tween-80, 0.03 to 0.05 percent of sodium oleic acid, and the balance being water for injection. The preparation method comprises steps of preparation of an oil phase, preparation of water phase, preparation of colostrum, homogenization and canning. In the nimodipine lipid microsphere injection, the soybean oil for injection and the medium chain fatty acid for injection are used to prepare the oil phase, the nimodipine is a fat soluble drug and can be better dissolved in the oil phase, the lipid microsphere in which the soybean oil for injection is the main component has solvent characteristics, is non-toxic, and can guide the fat soluble drugs to be dissolved in emulsion particles and perform the metabolism along with lipid oil drops and slowly release, thereby maintaining the effective blood concentration, lowering toxic and side effects of the drugs, increasing the solubility and stability of the nimodipine drug, improving the drug-loading rate and reducing the hydrolysis of the drugs.
Owner:沈阳信康药物研究有限公司
Injection medication combination of nimodipine and its preparing method
InactiveCN1480140ALow non-aqueous phase contentLess irritatingPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
A composite injection of nimodipine in the form of liquid injection or freeze dried powder is prepared from nimodipine and propanediol and tween kind of surfactant. Its advantage is high solubility. Its preparing process is also disclosed.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Pharmaceutical nimodipine compositions
InactiveUS20100239665A1Reduced incidence and severityReduce riskPowder deliveryBiocideImmediate releaseNimodipine
A modified release solid dosage product comprises a plurality of minicapsules or minispheres containing nimodipine, wherein when exposed to a use environment more than 40% of the nimodipine is released within 12 hours and wherein the Tmax is reached within 6 hours. The product may be a capsule 4 having a first population of uncoated minispheres 1 containing nimodipine for immediate release and a second population of coated minispheres 2 containing nimodipine for sustained release. There may be another population of coated minicapsules 3.
Owner:COULTER IVAN
Nimodipine micelle injection and preparation method thereof
ActiveCN102525917AGood water solubilityImprove stabilityOrganic active ingredientsSenses disorderNimodipineOrganosolv
The invention discloses a nimodipine micelle injection and a preparation method thereof. The nimodipine micelle injection is prepared by using active ingredients, i.e. nimodipine and phospholipid / bile salt. According to the nimodipine micelle injection disclosed by the invention, the solubility of the nimodipine is greatly increased, and no crystal precipitation exists when the nimodipine micelle injection is diluted; the biocompatibility of the phospholipid / bile salt is good, and organic solvents with great toxic side effects, such as ethyl alcohol, propylene glycol and the like, are not needed to for solubilizing so that the toxicity of the nimodipine micelle injection is lowered, the vascular stimulation is little, and the adverse reaction of the nimodipine micelle injection is reduced; and the nimodipine micelle injection can be directly used for intravenous injection and can also be used for instilling after the nimodipine micelle injection is diluted to various degrees, and thus, the clinical administration is greatly facilitated.
Owner:SICHUAN BAILI PHARM CO LTD
Nimodipine gel for nasal cavity
InactiveCN1679561AFine and even textureModerate viscosityOrganic active ingredientsNervous disorderNasal cavityNose
A gel of nimodipine for nasal cavity is prepared from nimodipine, hydrophilic gel and pharmacologically acceptable additive (pH regulator, humectant, anticeptic, etc). Its advantages are high biological utilization and no toxin to vibrissae.
Owner:FUDAN UNIV
Medicament microsphere and preparation method thereof
InactiveCN103610649AReduce solubilityHigh encapsulation efficiencyOrganic active ingredientsAntipyreticDipyridamoleMicrosphere
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a medicament microsphere and a preparation method thereof. The medicament microsphere comprises medicinal materials, a high polymer material and a surfactant, wherein the ratio of the medicament to the high polymer material is 1:(1-3); the medicament preferably is celecoxib, ketoprofen, dipyridamole and nimodipine. The medicament microsphere is prepared by adopting an O / W emulsified solvent diffusion-volatilization method. The O / W emulsified solvent diffusion-volatilization method comprises the following steps: respectively weighing the medicinal materials, the high polymer material and a release regulator according to the prescription amount, and then adding the weighted materials to an organic solvent; ultrasonically or mechanically stirring and dissolving as a disperse phase; taking a surfactant solution as a continuous phase; warming and stirring after low-temperature emulsification under an agitation state, so as to remove an organic solvent; and then carrying out solid separation, washing by distilled water, and drying, so as to obtain the medicament microsphere. Thus, the prepared microsphere is high in encapsulation efficiency and high in yield.
Owner:SHENYANG PHARMA UNIVERSITY
Oral Chinese herbal preparation for treating post-traumatic brain syndrome
InactiveCN102631579AReduce dosageFast absorptionNervous disorderInanimate material medical ingredientsLicorice rootsTherapeutic effect
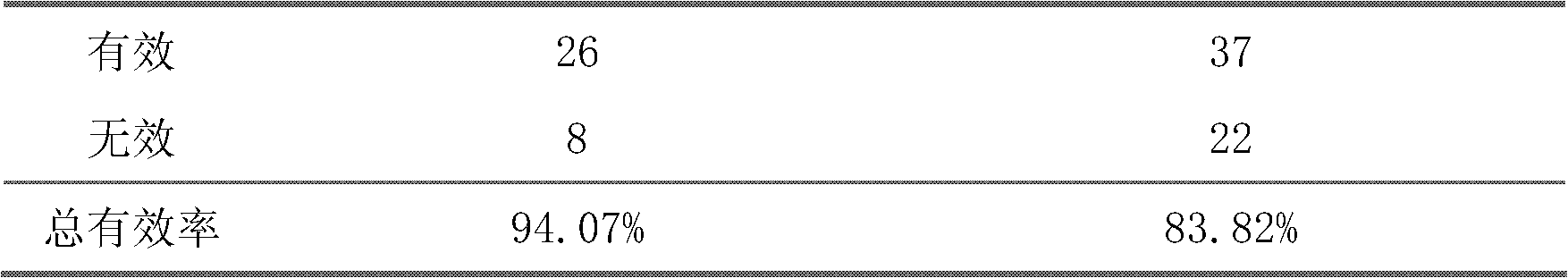
The invention belongs to an oral Chinese herbal preparation for treating post-traumatic brain syndrome, and the oral Chinese herbal preparation is a human medicinal product. The preparation is prepared from the following Chinese herbs in parts by weight: 10+ / -2 parts of Astragalus mongholicus, 10+ / -2 parts of American ginseng, 10+ / -2 parts of Angelica sinensis, 10+ / -2 parts of medlar, 10+ / -2 parts of the root of red-rooted salvia, 10+ / -2 parts of ligusticum wallichii, 10+ / -2 parts of root of common peony, 10+ / -2 parts of peach kernel, 10+ / -2 parts of Poria cocos, 10+ / -2 parts of pericarpium citri reticulatae, 10+ / -2 parts of plantain seed, 10+ / -2 parts of tabasheer, 10+ / -2 parts of rhizoma acori graminei, 10+ / -2 parts of gastrodia elata, 10+ / -2 parts of uncaria, 10+ / -2 parts of spina date seed, 10+ / -2 parts of amber powder and 6+ / -1.2 parts of honey-fried licorice root. Clinical effect observation proves that the total effective rate of the oral Chinese herbal preparation to the oral traditional Chinese medicine preparation is 94.07%, and has better treatment effect (P<0.01) compared with Western medicines such as Rotundine, piracetam, oryzanol and nimodipine for treating post-traumatic brain syndrome. Moreover, the Chinese herbal preparation has no obvious toxic or side effect, and can be taken for a long time. The dose of the preparation is small, and effective medicinal components are easy to release, can be absorbed quickly and can fully play the pharmaceutical effect. The preparation is convenient to carry, easy to take and beneficial to large-scale production.
Owner:HOSPITAL NO 3 CPLA
Monolayer osmotic pump controlled releasing tablets of nimodipine
InactiveCN1552323ALasting effectGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismCellulose acetatePolyethylene glycol
A release controlled nisoldipine tablet with single-layer osmotic pump for treating hypertension, angina pectoris, heart failure, etc contains nisoldipine, the release-controlling auxidiary chosen from sodium chloride, potassium chloride, glyceride behenate, etc, and other auxiliaries. Its advantages are sure and durable curative effect and low toxic by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
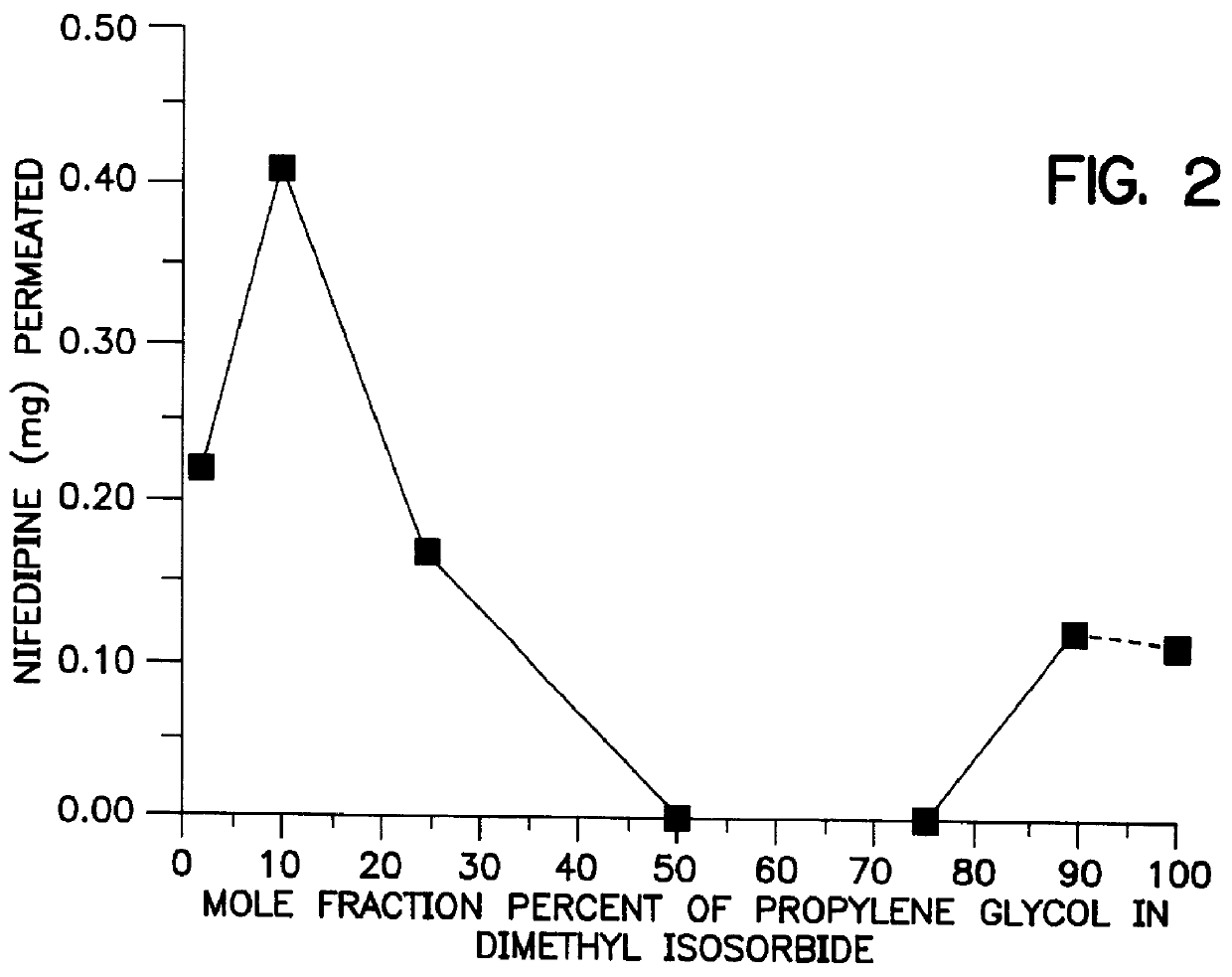
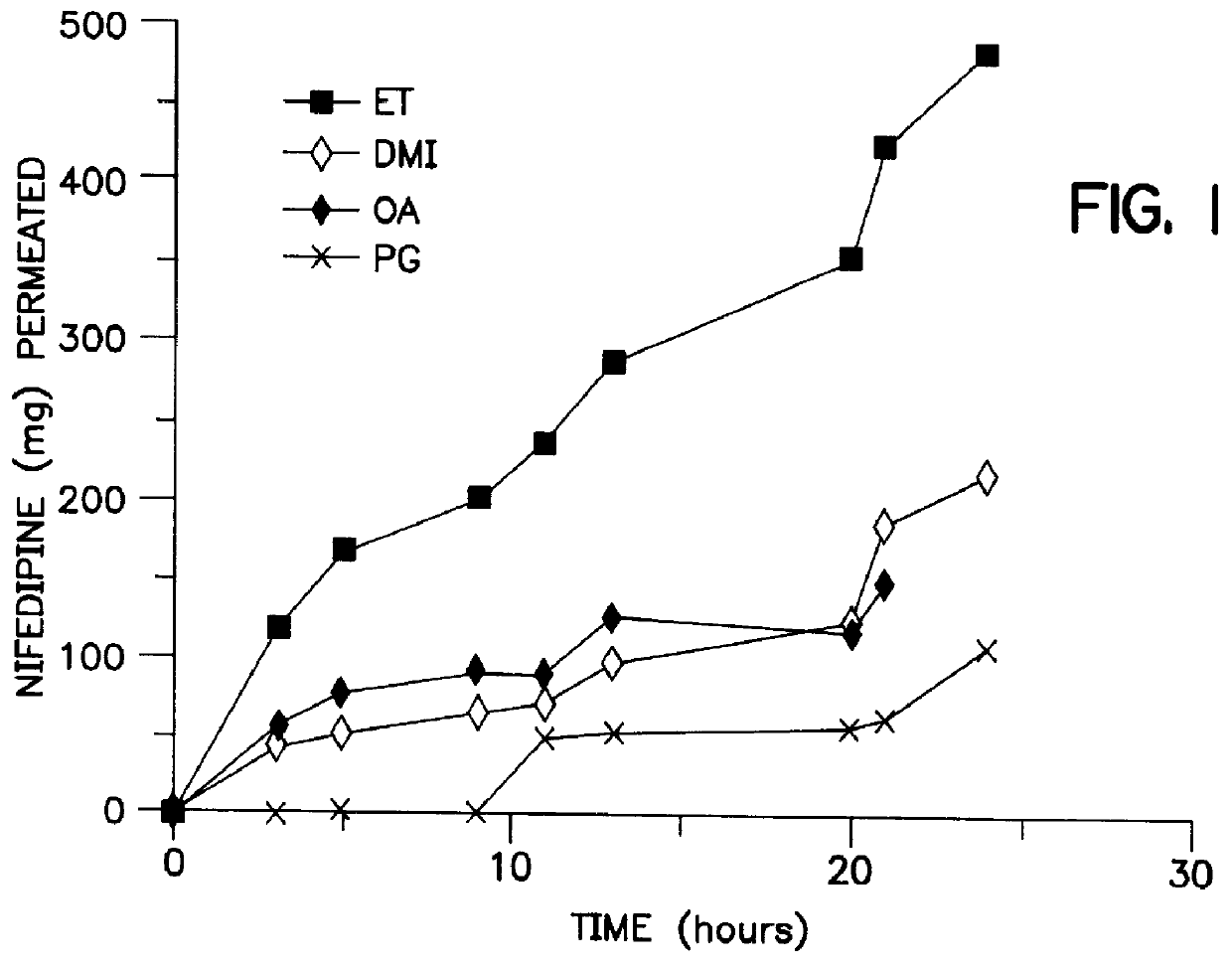
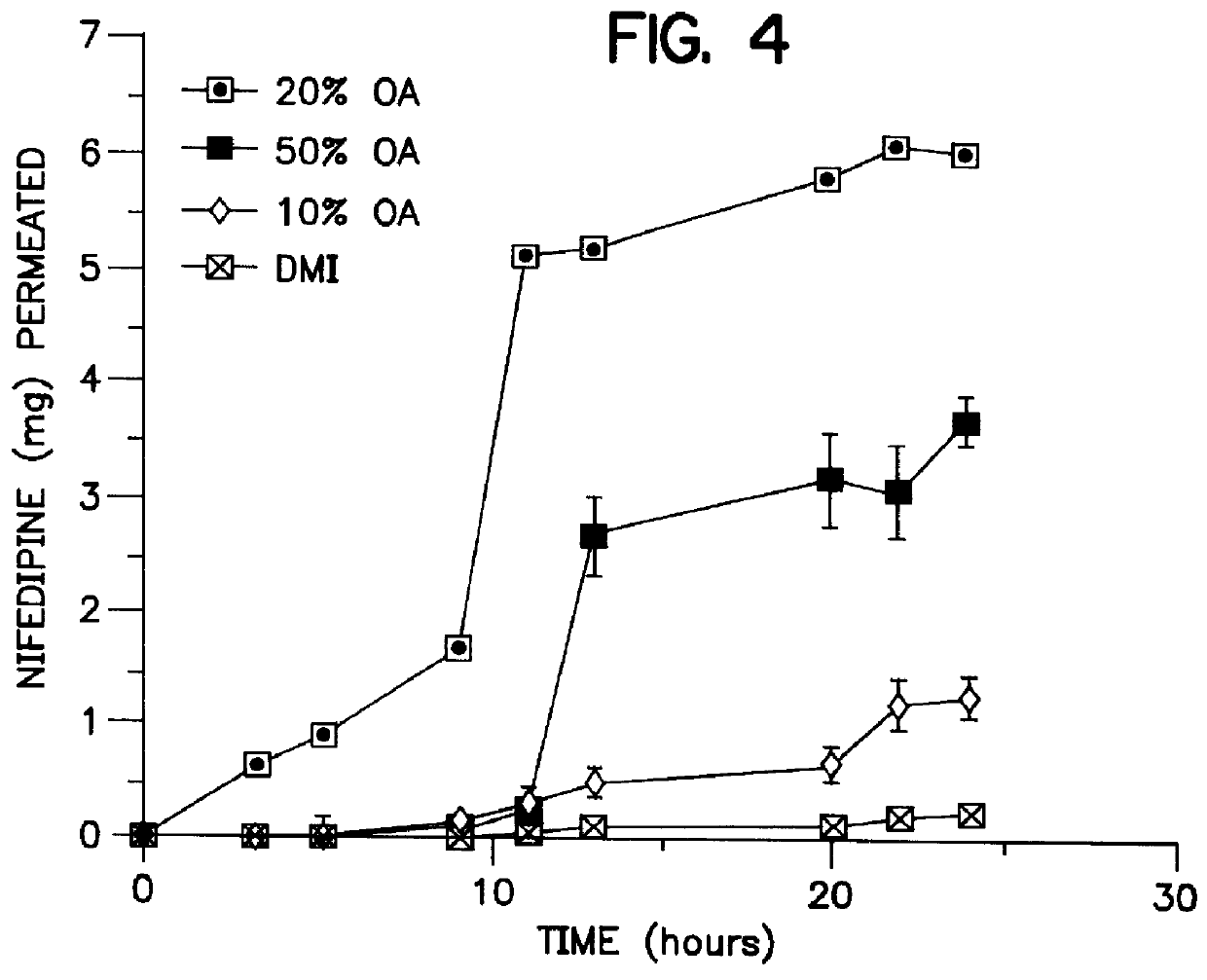
Transdermal delivery of calcium channel blockers, such as nifedipine
InactiveUS6106856AImprove pass rateLittle influenceAdhesive dressingsDrug compositionsDihydropyridinePolypropylene glycol
A transdermal formulation of dihydropyridine calcium antagonists and specifically nifedipine, nimodipine and nitrendipine. The calcium antagonists are dispersed in a mixed liquid. The mixed liquid comprises varying mole fractions of cis-oleic acid and dimethylisosorbide dispersed in a polypropylene glycol base.
Owner:THE BOARD OF GOVERNORS FOR HIGHER EDUCATION STATE OF RHODE ISLAND AND
Nimodipine capsule containing semi-solid combination and preparation
InactiveCN101254180AHigh dissolution rateDissolution rate is fastOrganic active ingredientsNervous disorderHard CapsuleSemi solid
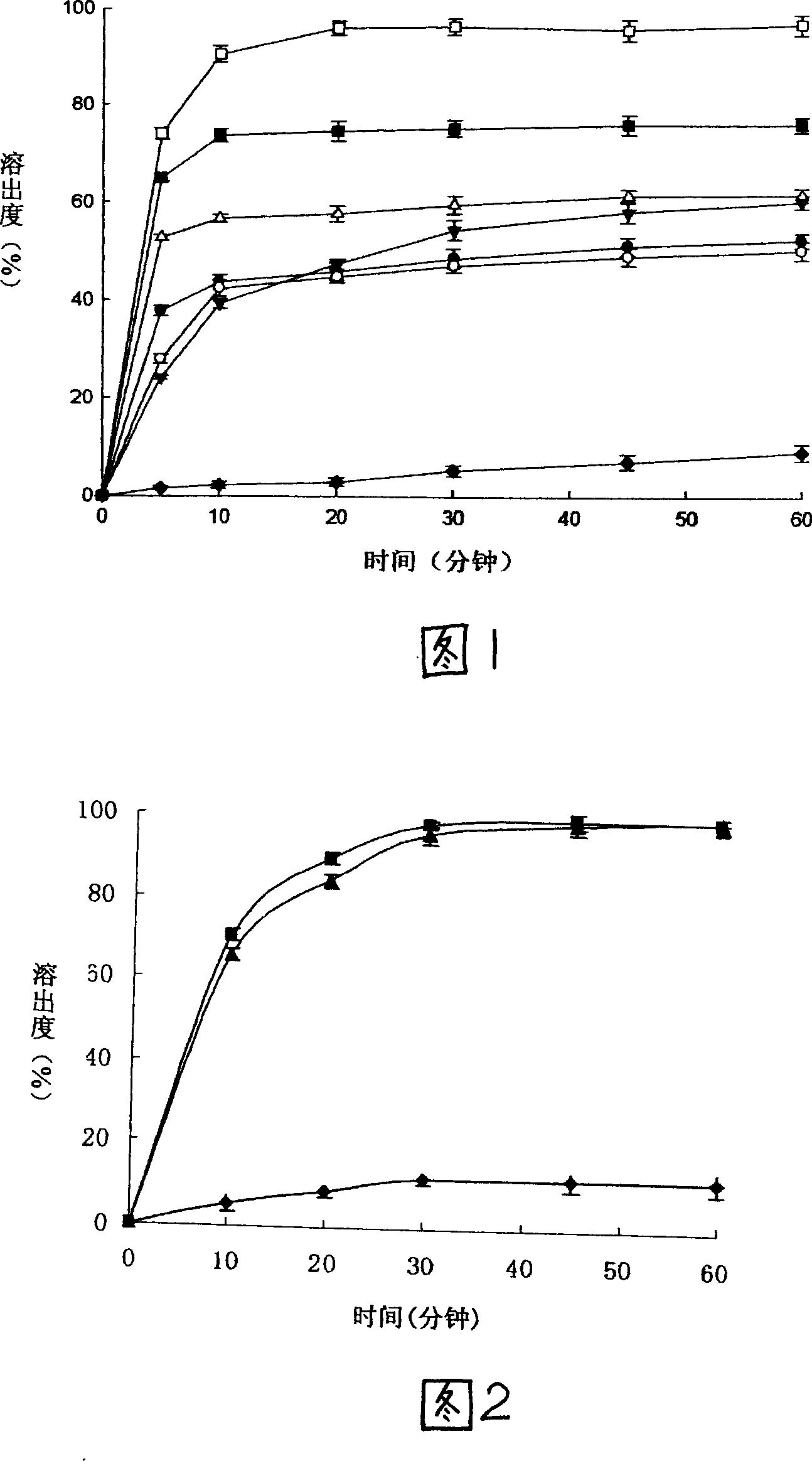
The invention discloses a nimodipine capsule with high dissolution and semi-solid composition as content and a preparation method thereof, and belongs to the pharmaceutical preparation field. The method comprises the following steps of mixing micronized nimodipine and a hydrophilic carrier, preparing into solid dispersion by melting extrusion method, cooling, pulverizing, mixing with semi-solid matrix, surfactant, melting point regulator under heating, and filling the content into hard capsule with a capsule filling machine. The invention combines the melting extrusion technique and semi-solid filling hard capsule technique, and after the prepared novel nimodipine capsule is administered into human body, the nimodipine quickly diffuses in molecular form and saturates metabolic enzymes, so as to improve bioavailability. The inventive nimodipine capsule solves the low dissolution and low utilization ratio problems of prior nimodipine preparation; and has the advantages of high dissolution, good stability, simple preparation method, and applicability to industrial production.
Owner:武汉星福海药业有限公司
Self-micro emulsion solft capsule of dihydropyridine type calcium ion agonist, and its prepn. method
InactiveCN1559407AHigh dissolution rateImprove permeabilityOrganic active ingredientsNervous disorderDihydropyridineEmulsion
A self-microemulsified softcapsule of nimodipine or nitrendipine is prepared from nimodipine or nitrendipine, coemulsifier, self emulsifier, oil and antioxidizing agent through proportionally mixing nimodipine or nitrendipine, coemulsifier and self emulsifier, constant-temp oscillating, adding oil and antioxidizing agent, stirring, cooling, and shaping.
Owner:SHENYANG PHARMA UNIVERSITY
Nimodipine nanometer granule and its prepn. method
InactiveCN1903173AOrganic active ingredientsPharmaceutical delivery mechanismNanoparticleCholesterol
A nanoparticle of nimodipine contains nimodipine, the carrier chosen from poloxamer cholesterol ester of fatty acid and cholesterol ester of fatty acid, stabilizer and water. Its preparing process is also disclosed.
Owner:张文芳
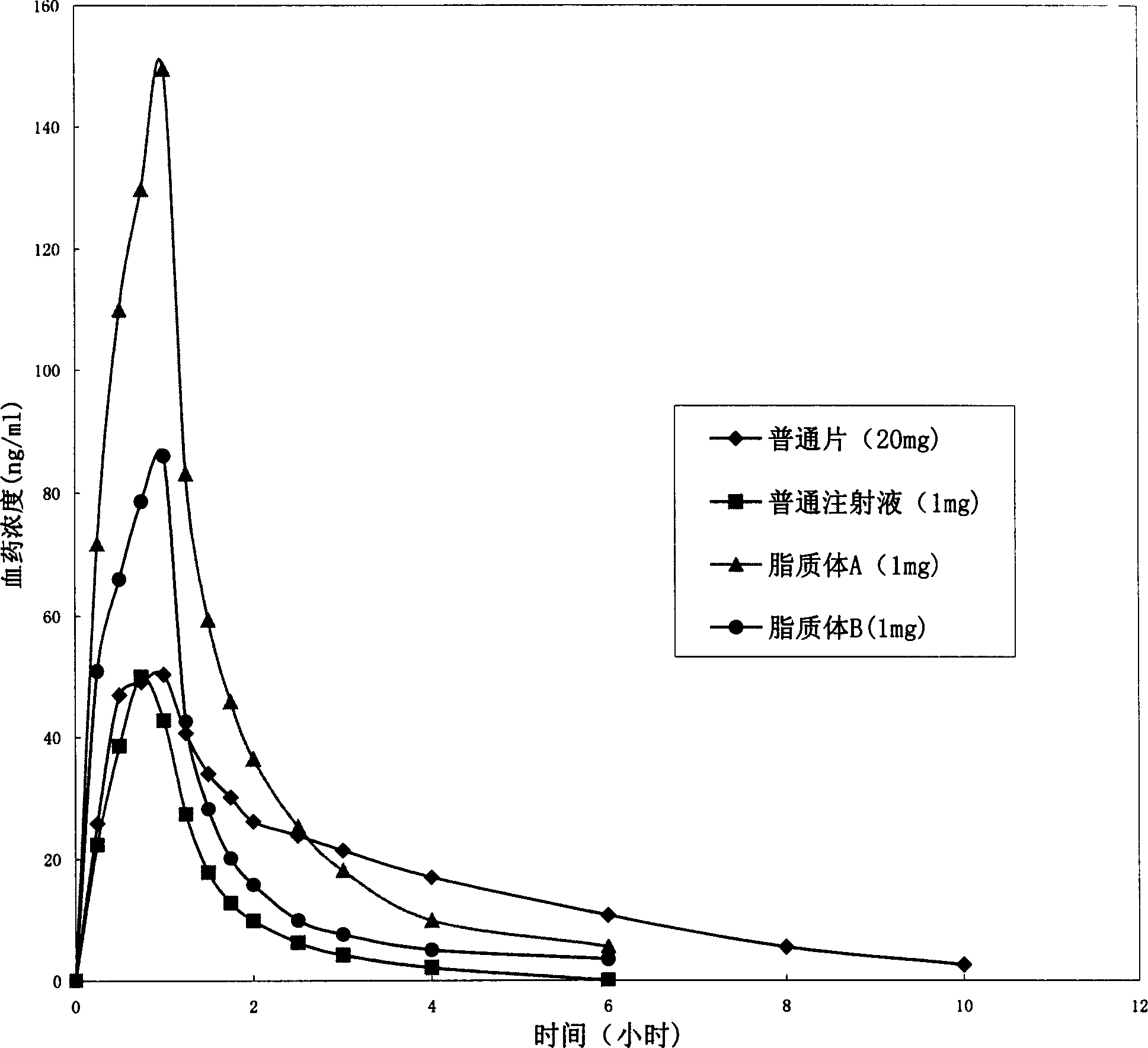
Nimodipine lyophilized emulsion for injection and preparing method thereof
InactiveCN101199522AAvoid stimulationAvoid harmOrganic active ingredientsPowder deliveryFreeze-dryingNimodipine
The invention relates to nimodipine lyophilization dry emulsion for injection. Before freeze-dried or reconstituted, according to percentage concentration per 1000 ml of fat emulsion, the lyophilization dry emulsion contains 0.001 percent to 0.2 percent of nimodipine, 0.5 percent to 30 percent of oiliness solvent, 0.1 percent to 5 percent of emulsifier, 5 percent to 40 percent of the freeze-drying protective agent and 0.1 percent to 10 percent of isotonic regulator. The invention also relates to a preparation method of nimodipine lyophilization dry emulsion. The invention has the advantages that ethanol is avoided to decrease irritation; the product can be mixed with any proportion of water for injection, sodium chloride solution, glucose solution, blank fat emulsion or other aqueous solution without phenomena of precipitation or crystallization; in addition, compared with the fat emulsion, the lyophilization dry emulsion is more helpful to improve the stability of nimodipine and excipient of the nimodipine, thereby lowering the requirements of production, transportation and storage conditions and prolonging the period of validity.
Owner:YAOPHARMA CO LTD +1
Nimoldipine new nano liposome, its precursor freeze dryed matter and its preparing method
InactiveCN1554340AQuality improvementReduce distributionPowder deliveryOrganic active ingredientsFreeze-dryingCholesterol
The present invention relates to pharmaceutical, and is especially new nano Nimoldipine liposome, its freeze dried precursor and preparation process. The nano Nimoldipine liposome consists of Nimoldipine, phosphatide, cholesterol, surfactant and antioxidant. The Nimoldipine liposome has stable quality, small average size and stable wrapping rate as high as 80%.
Owner:CHINA PHARM UNIV
Oral disintegrants of nimodipine and their preparation
InactiveCN1582927ADisintegrates quicklyGreat tasteOrganic active ingredientsPharmaceutical non-active ingredientsSorbentNimodipine
An oral disintegrating tablet of nimodipine is prepared from nimodipine, carrier, adsorbent, disintegrant, filler, soluble polybasic alcohol, and other auxiliaries.
Owner:范敏华
Method for preparing nimodipine dispersible tablet with high dissolution
ActiveCN1951373AImprove oral bioavailabilityDissolution rate is fastOrganic active ingredientsNervous disorderDrug metabolismNimodipine
The invention relates to a method for preparing soluble Dunimodi disperser. Wherein, it comprises that using the Dunimodi material and hydrophilic carrier, via fusion protrusion technique, to prepare their sosoloid; breaking the sosoloid, to be mixed with stuff, lubricant and surface activator into tablet. The invention has simple process while the product has high dissolve speed, the drug can be released as molecule, to improve its utilization.
Owner:徐竹青
Nimodipine lipid nano particle compositions, and its prepn. method
InactiveCN1418626AImprove efficacyEliminate shortcomingsOrganic active ingredientsPowder deliveryLipid formationMicropore Filter
The present invention relates to a nimodipine lipid nano granular composition and its preparation method. It can be made into injection, its composition includes 0.002-0.2% of nimodipine, 0.02-10% ofphospholipid and 0-2% of Poloxamer. Its preparation method includes the following steps: dissolving the above-mentioned materials in ethyl alcohol, adding injection water, making hydration for 1-2 hr. to obtain nimodipine lipid granular solution, high-pressure homogenizing for 10 min. under the condition of nitrogen gas flow, filtering with 0.2 micrometer micropore filtering membrane, adding injection water, filling, sealing, sterilizing so as to obtain the invented product.
Owner:SHENYANG PHARMA UNIVERSITY
Novel nimodipine composition
The invention relates to a novel nimodipine composition, in particular the freeze-drying pharmaceutical composition for injection, wherein the composition Nimodipine, polyethylene glycol 400, Twain-80, Hydroxypropyl-beta-cyclodextrin, and water for injection.
Owner:REYOUNG PHARMA
Nimodipine solid dispersoid and preparation method thereof
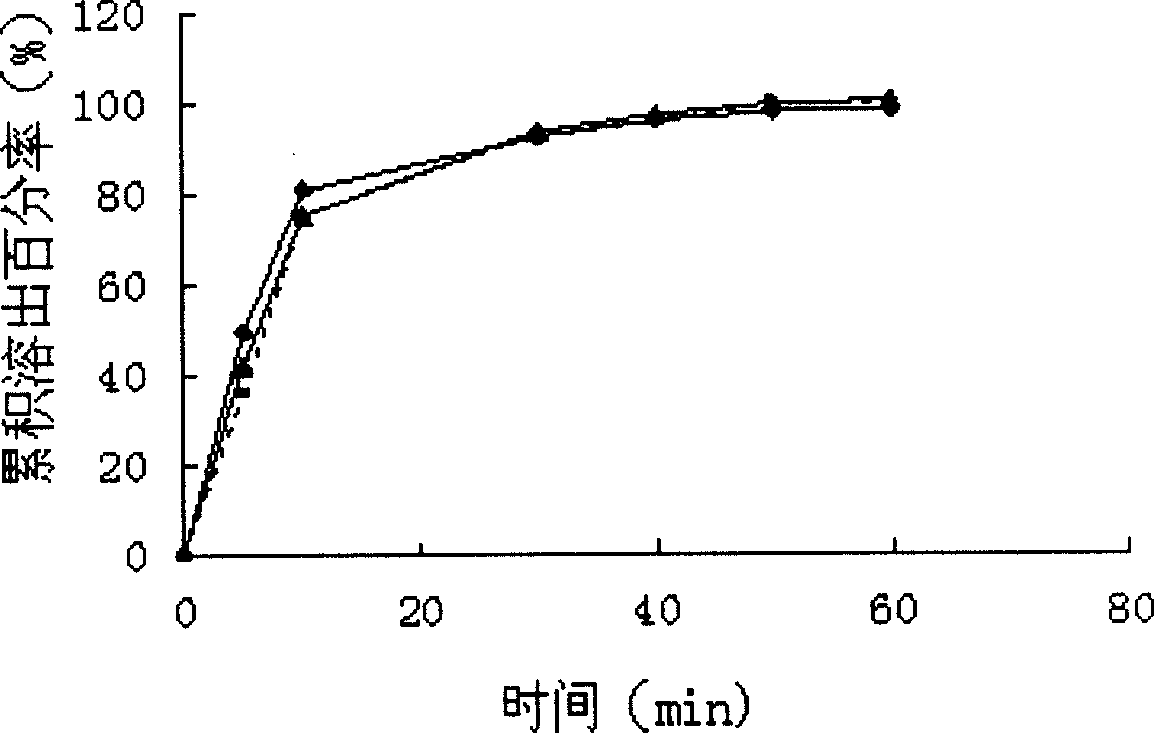
InactiveCN101474177ARapid dissolutionNo significant bioavailabilityOrganic active ingredientsCardiovascular disorderSolubilityNimodipine
The invention relates to the field of medical technology, more particularly to a nimodipine solid dispersoid and a preparation method thereof. The invention employs the nimodipine as an active ingredient and adds a macromolecular carrier material and an antisticking agent to prepare the nimodipine solid dispersoid; and the feeding ratio of the nimodipine, the macromolecular carrier material and the antisticking agent is 1:0.2-10:0.2-10. The nimodipine solid dispersoid is prepared by a melting method, a coprecipitation method, a solvent dispersion method, a solvent melting method or a grinding method respectively. The invention, by preparing the solid dispersoid with the nimodipine and a small amount of carrier material, significantly increases the equilibrium solubility and the degree of in vitro dissolution thereof and prominently enhances bioavailability, thus solving the problems of large dosage of the carrier of the solid dispersoid and severe aging; in addition the invention has the characteristics of small dosage of the carrier, rapid dissolution, high bioavailability and simple preparation method without the aging phenomenon.
Owner:SHENYANG PHARMA UNIVERSITY
Nimodipine/ligustrazine double-load PLGA nanoparticles and preparation method thereof
ActiveCN103494820AReduce releaseLow toxicityOrganic active ingredientsPharmaceutical non-active ingredientsHalf-lifePhosphate
The invention relates to nimodipine / ligustrazine double-load PLGA nanoparticles which are prepared through a method comprising the steps that: nimodipine, ligustrazine phosphate and a polylactic acid-glycolic acid copolymer are precisely weight according to a mass ratio of 1:5-20:40-60; the materials are dissolved into acetone, such that an organic phase is obtained; a PVA water solution with a mass concentration of 0.5-1.0% is adopted as an aqueous phase; under stirring, the organic phase is slowly dropped into the aqueous phase; when dropping is finished, the mixture is continued to be stirred for 2-4h under a constant temperature of 40-50 DEG C, such that the organic solvent is volatilized; centrifugation is carried out; a sediment is washed 2-3 timed by using distilled water, and is lyophilized, such that the nimodipine / ligustrazine double-loading PLGA nanoparticles are obtained. According to the invention, PLGA is adopted as a carrier material, and P-gp inhibitors TMP and NMD are applied in combination, such that NMD / TMP-PLGA-NPs are prepared. Therefore, defects of short drug half-life, easy generation of cytotoxicity, and the like of simple combination are avoided, a P-gp efflux effect is inhibited, and NMD distribution to tissues is promoted. Therefore, the nanoparticles have certain advantages over single-load nanoparticles.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Nimodipine sub micro-emulsion injection and preparation method thereof
InactiveCN101416942AAllergy freeReactivity NoneOrganic active ingredientsEmulsion deliveryWater bathsWater use
The invention provides a nimodipine submicron emulsion injection and a preparation method thereof. The invention adopts nimodipine of effective dose as a drug and contains pharmaceutic adjuvant such as oil phase, an emulsifier, an auxiliary emulsifying agent, a pH regulator, and an iso-osmotic regulator; the nimodipine, the emulsifier; the auxiliary emulsifying agent and oil used for injection are weighed according to the formula amount, stirred and dissolved in a water bath, added with water used for injection and the iso-osmotic regulator and the like, and fully mixed and emulsified; the pH value of the solution is regulated and the solution is added with the water used for injection until the specified cubage; a high-pressure homogenizer is used for carrying out homogenization for a plurality of times until specified granularity is realized; a filtering membrane of 0.22 Mum is used for filtering and sterilizing; and nitrogenization, subpackaging and sterilization are carried out, and the nimodipine submicron emulsion injection is obtained. The nimodipine submicron emulsion injection of the invention does not contain auxiliary cosolvents, such as benzoic alcohol, polyethylene glycol, and the like, and has the advantages of high drug-load rate and packaging efficiency, slow release of drugs, good stability, and the like. The preparation process is simple, and a preparation which does not contain any organic solvent is provided, thus radically eliminating the defects of organic solvents, separating out no drugs, reducing the work load of medical care personnel and eliminating the psychological burden of the medical care personnel and patients; in addition, the nimodipine submicron emulsion injection has low cost and stable quality and is suitable for industrialized large scale production.
Owner:李淑斌
Preparation method of Nimodipine
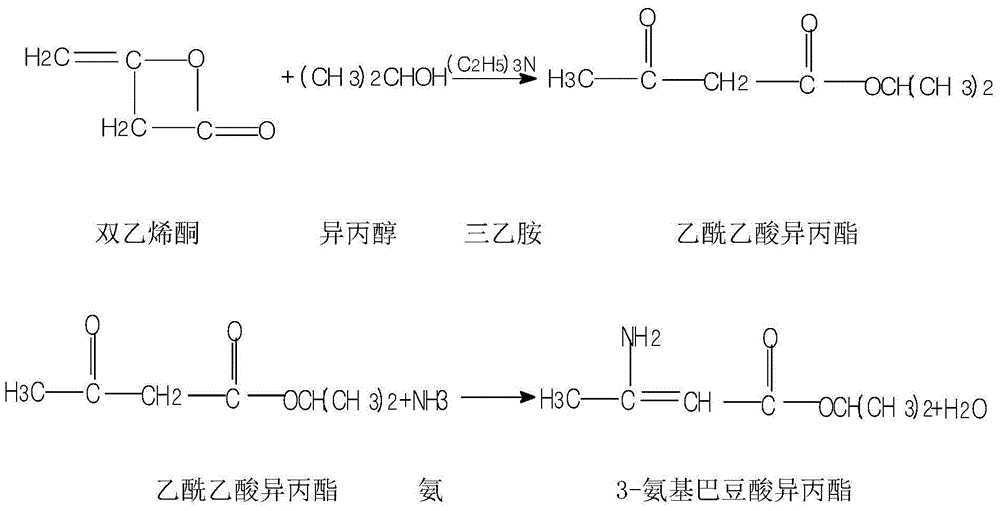
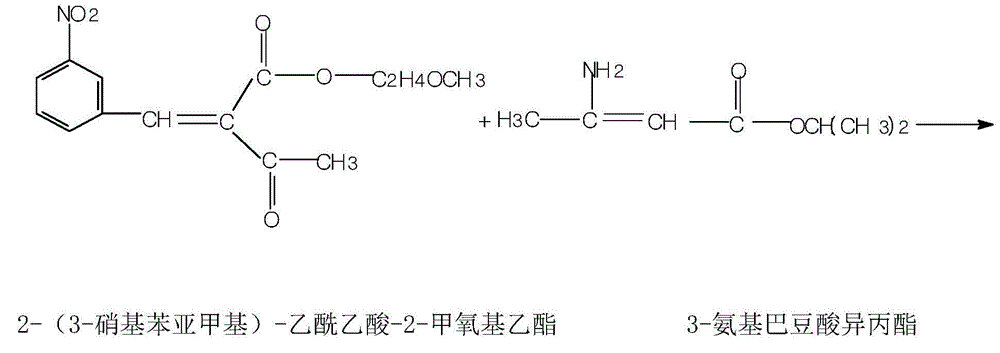
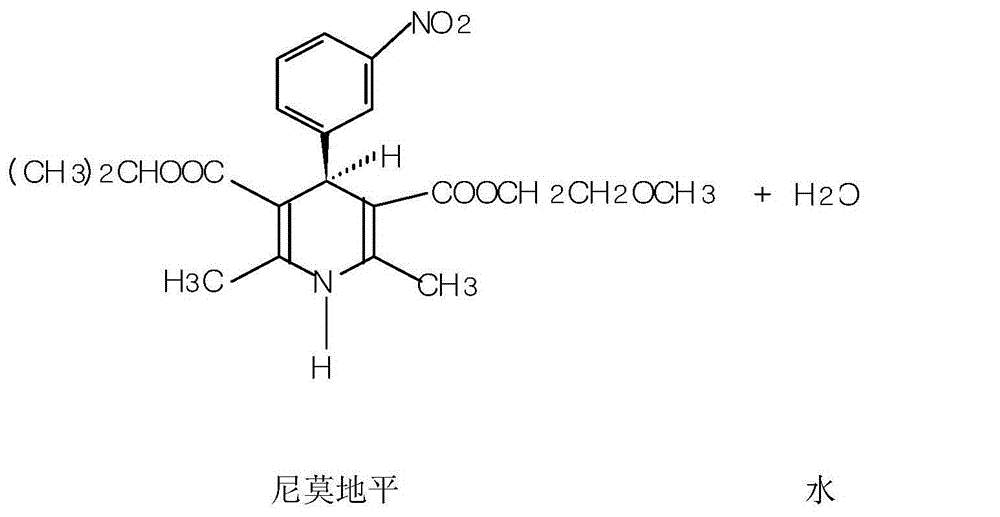
InactiveCN105732484AReduce hydrolysis reactionIncrease contentOrganic chemistryAcetic acidInorganic salts
The invention belongs to the pharmaceutical technical field, and more specifically to a preparation method of Nimodipine. The preparation method includes an ammonia ester reaction and a cyclization reaction. In the ammonia ester reaction, after an ammonia-filling reaction ends, firstly an inorganic salt is added to remove water from the ammonia ester reaction, and then isopropyl 3-aminocrotonate is obtained through rectification. The cyclization reaction is carried out on the isopropyl 3-aminocrotonate and 2-(3-nitrobenzylidene)-acetoacetate-2-methoxyethyl ester, and a fatty alcohol is used as a reaction solvent. The preparation method of the Nimodipine has the advantages that the finished product yield is high, the primary yield reaches 85-89%, the quality is good, the solvent can be recycled and reused, and the raw material cost can be greatly reduced. Total impurities detected by HPLC can decrease to 0.1-0.3% from 0.4-1.0% in the original technology.
Owner:QINGDAO HUANGDAO HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Nimodipine pulse sustained release mircopill preparation and preparing method thereof
ActiveCN101229132AObvious symptomsGood preventive treatmentOrganic active ingredientsNervous disorderSustained release pelletsDisease patient
The invention relates to a pulsed sustained-release pellet comprising nimodipine and a preparation method is also provided. The sustained-release pellet comprises: one of gastric-dissolved and medicines containing sustained-release pellets and enteric-coated and medicines containing sustained-release pellets, or the combination. The preparation method is that: making medicines containing pellets using nimodipine and excipents, coating the medicines containing pellets with the gastric coating or the enteric coating, mixing the sustained-release pellets coated with gastric coating and enteric coating in proportion and putting the sustained-release pellets into capsules or tabletting. The pulsed sustained-release pellet of the invention has a special release behavior and needs to be taken only once per day. The release curve agrees well with the disease circadian rhythm of cerebrovascular disease patients, playing a role of the best prevention and treatment.
Owner:SHENZHEN SOUTH CHINA PHARMA
Nimodipine soft capsule and its prepn
InactiveCN1772301AEasy to makeTo achieve the purpose of sustained releaseOrganic active ingredientsPharmaceutical delivery mechanismNimodipinePharmaceutical formulation
Owner:CHINA PHARM UNIV
Fat emulsion injection containing nimodipine and preparation method thereof
InactiveCN103893119AImprove stabilityImprove securityOrganic active ingredientsSenses disorderPolymer scienceNimodipine
The invention discloses a fat emulsion injection containing nimodipine and a preparation method thereof. The fat emulsion injection containing the nimodipine is prepared from nimodipine, injection oil, injection phospholipid, injection polyethylene glycol hydroxy stearate, an isoosmotic adjusting agent, a pH regulator and injection water. The preparation method comprises the following steps: mixing the nimodipine, the injection oil, the injection phospholipid with the injection polyethylene glycol hydroxy stearate, and agitating to clear and transparent as an oil phase; evenly mixing the isoosmotic adjusting agent with the injection water as a water phase; mixing the oil phase with the water phase; processing emulsion particles into the stated particle size range by adopting a two-step homogenization method; bulking and sterilizing after adjusting the pH value. The injection phospholipid and the polyethylene glycol hydroxy stearate are adopted as a mixed emulsifier, and the prepared fat emulsion injection containing the nimodipine can endure high-pressure moist heat sterilization, and does not need an expensive sterile manufacturing process, so that the production cost is greatly reduced, and industrial production is facilitated.
Owner:GUANGDONG PHARMA UNIV
Nimodipine solid dispersant and tablet and their preparation methods
ActiveCN104739770AImprove bioavailabilityPromote dissolutionPowder deliveryOrganic active ingredientsSolubilityOrganic solvent
The invention discloses a nimodipine solid dispersant and tablet and their preparation methods. The preparation method of the nimodipine solid dispersant comprises the following steps of blending nimodipine, polyvinylpyrrolidone and a volatile organic solvent to obtain a uniform mixed solution, uniformly spraying the mixed solution to a filler by a fluidized bed under the conditions of atomization pressure of 1.2-3.0bar, a liquid spraying rate of 3-12g / min, an inlet air temperature of 20-80 DEG C and an inlet air amount of 20-80m<3> / h, and carrying out granulation and drying, wherein a mass ratio of nimodipine to polyvinylpyrrolidone is 1: (1-6) and a mass ratio of nimodipine to the filler is 1: (2-15). The preparation method of the nimodipine solid tablet comprises carrying out raw material drying, adding accessory materials into the dried raw materials and carrying out tabletting. The preparation methods have simple processes. Preparation processes of the solid dispersant and tablet can be carried out simultaneously. The nimodipine solid dispersant particles have uniform particle size distribution, high solubility, good stability and high bioavailability.
Owner:SHANGHAI CHEMPARTNER CO LTD
Topical pharmaceutical composition comprising a cholinergic agent or a calcium channel blocker
A method and composition are provided for the treatment of an anorectal disorder and for controlling the pain associated therewith. The method comprises administering to a subject in need of such treatment therapeutically effective amounts of a calcium channel blocker either alone or together with a nitric oxide donor. Amlodipine, anipamil, barnidipine, benidipine, bepridil, darodipine, diltiazem, efonidipine, felodipine, isradipine, lacidipine, lercanidipine, lidoflazine, manidipine, mepirodipine, nicardipine, nifedipine, niludipine, nilvadipine, nimodipine, nisoldipine, nitrendipine, perhexiline, tiapamil, verapamil and pharmaceutically acceptable salts thereof, are suitable calcium channel blockers.
Owner:SLA PHARMA AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com