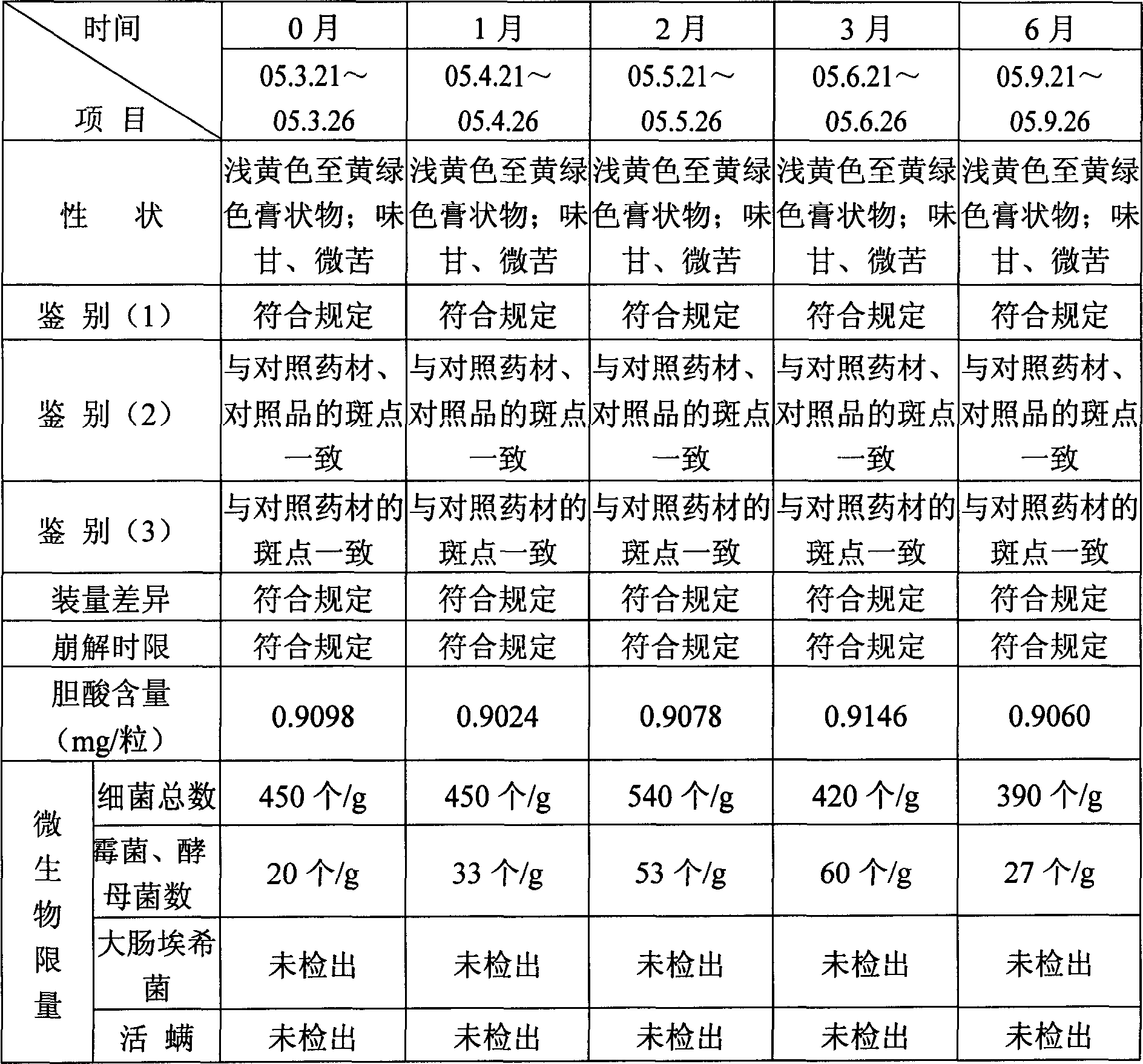
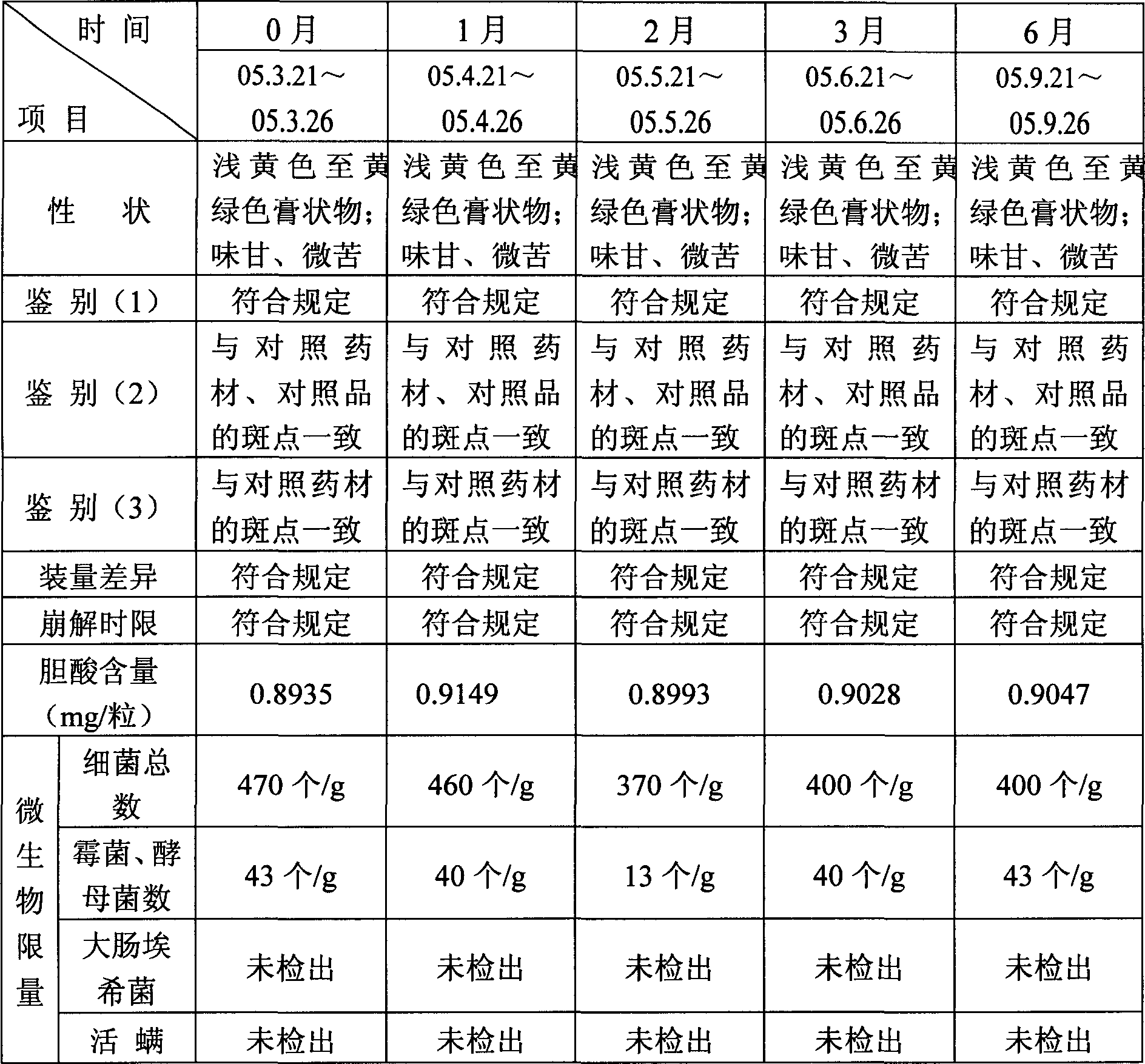
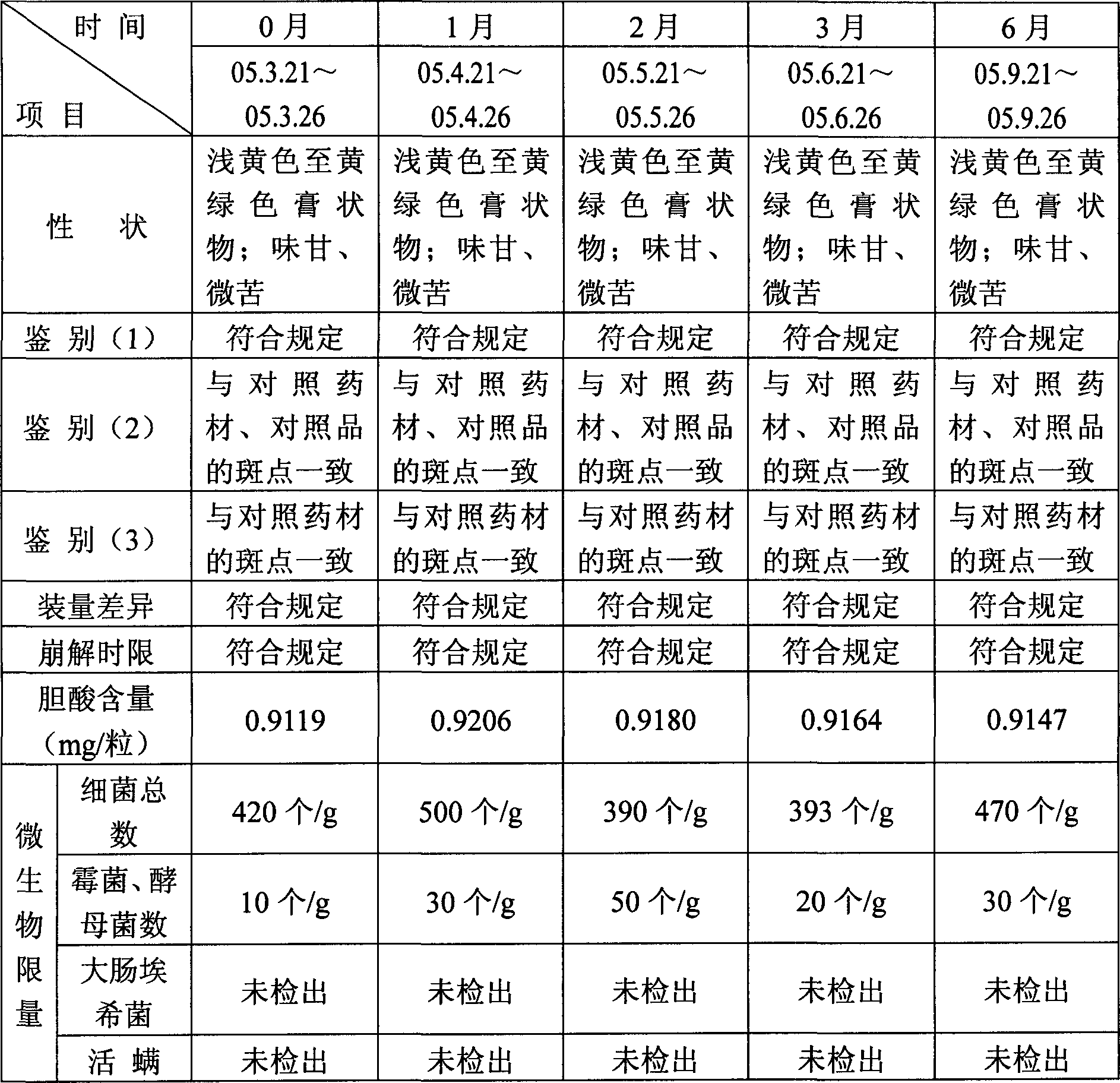
Patents
Literature
87 results about "Hard gelatin capsules" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Combination pharmaceutical compositions
InactiveUS20100215737A1Slow changeGood for healthPowder deliveryBiocideControlled releaseImmediate release
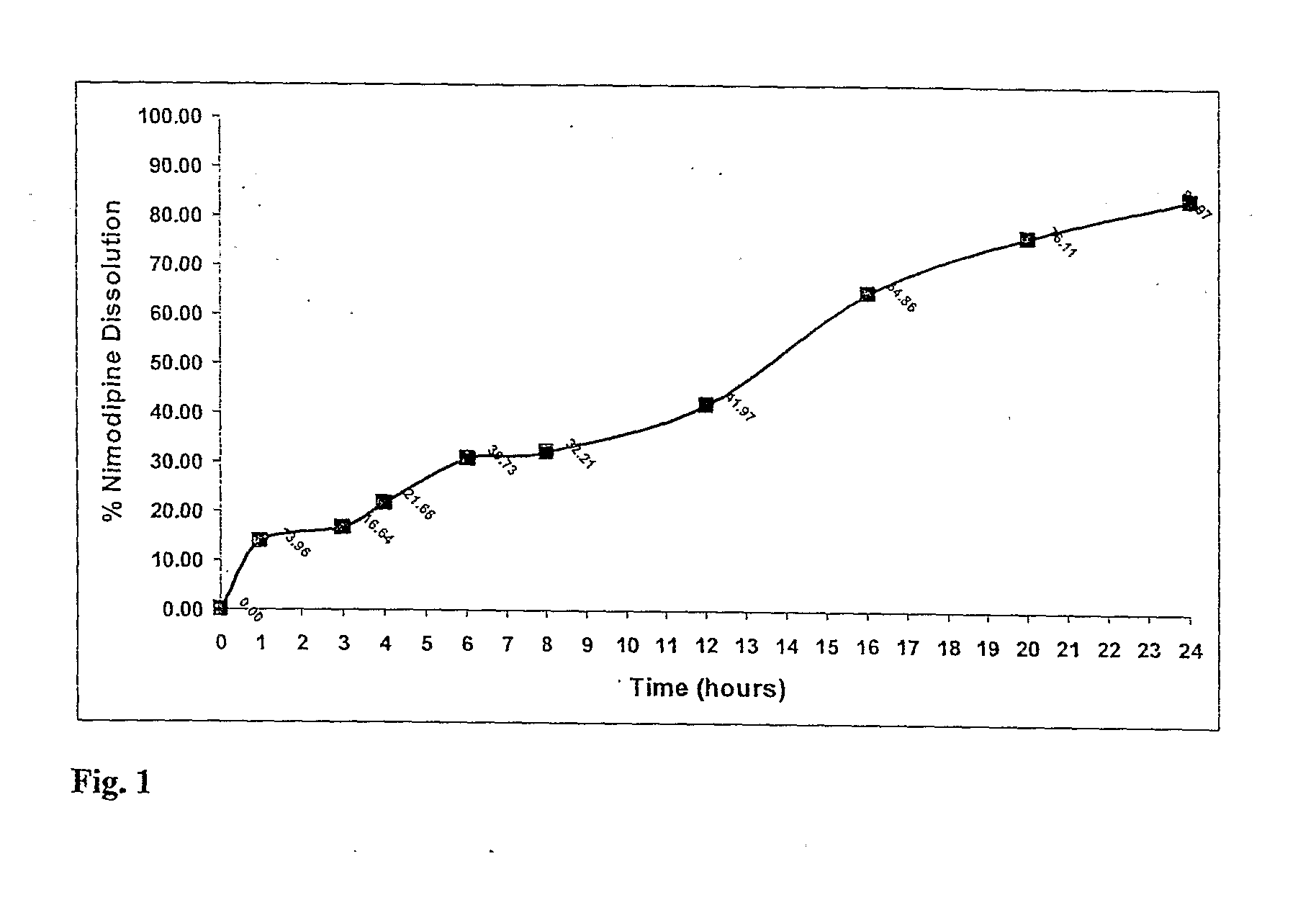
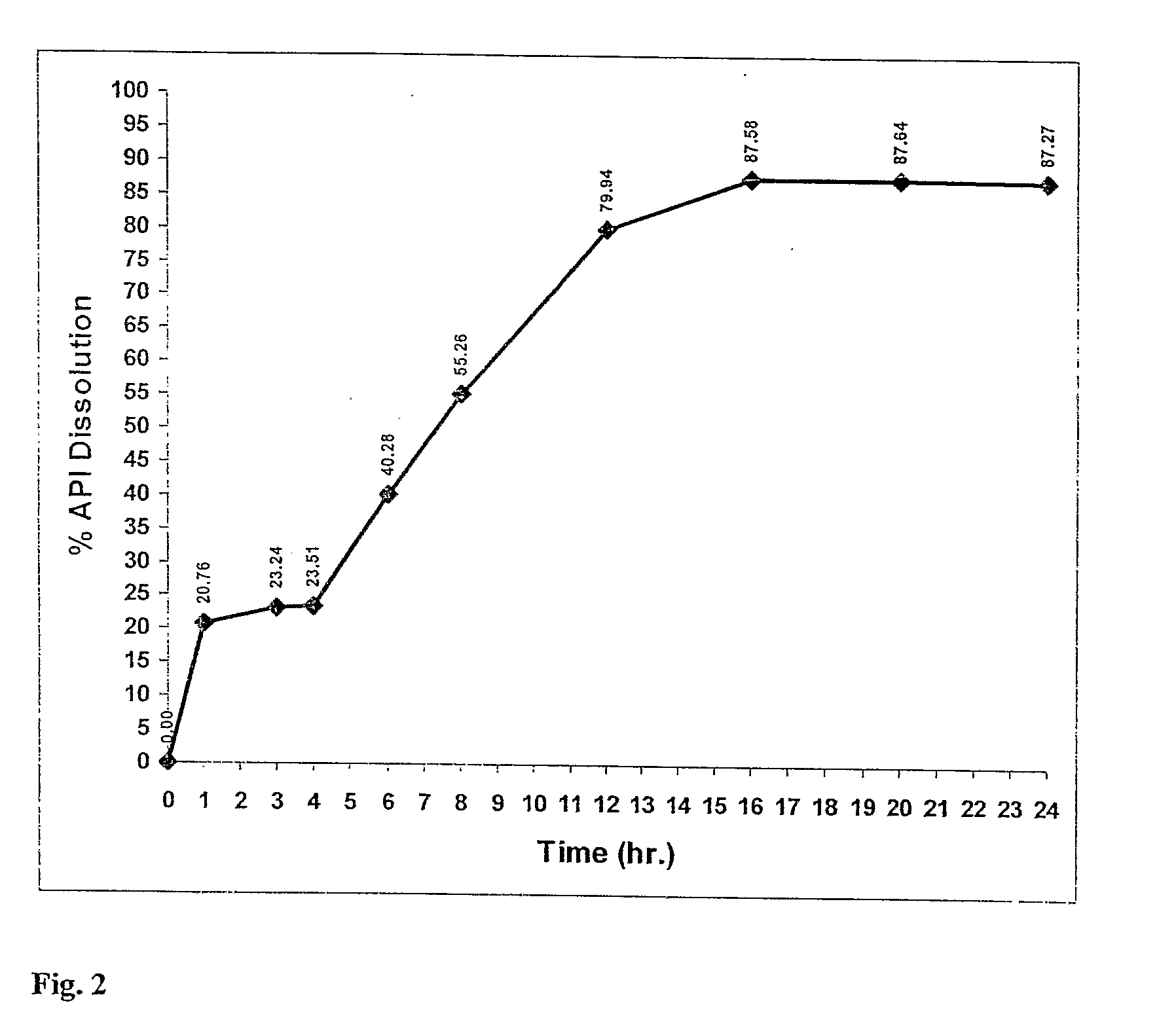
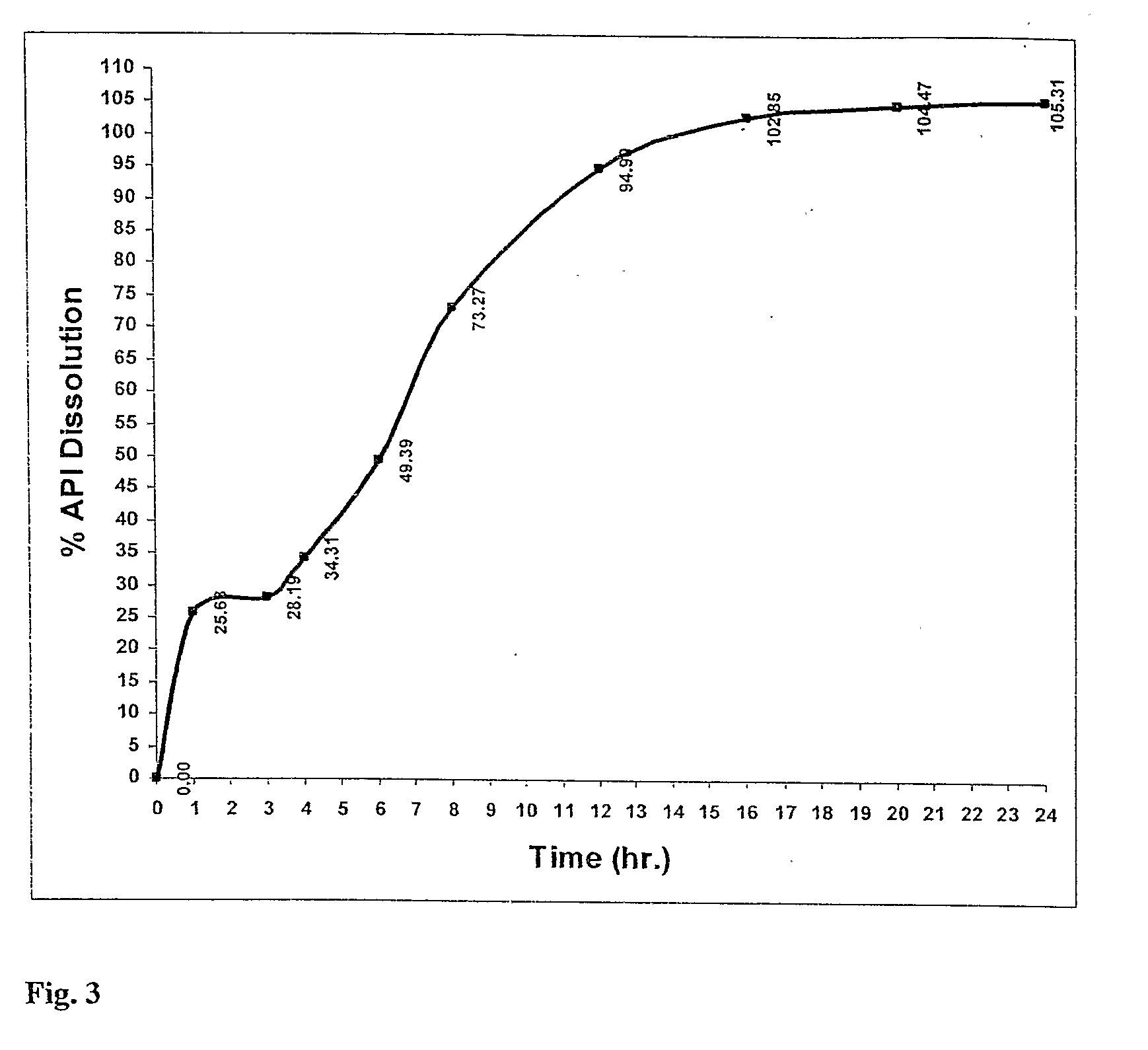
A modified release dosage product (5) comprises a plurality of minicapsules or minispheres (1, 2) containing nimodipine, and a plurality of minicapsules or minispheres (3), (4) containing tacrolimus. There are uncoated minicapsules or minispheres (1) encapsulating micronized nimodipine for immediate release and a controlled release polymer coated minicapsule or minisphere (2) encapsulating micronized nimodipine for delayed, sustained, controlled or targeted release. There are uncoated seamless minicapsules (3), the core of which comprises tacrolimus lipid-based formulation for immediate release and a controlled release polymer coated seamless minicapsule (4), the core of which comprises tacrolimus lipid-based formulation for delayed, sustained, controlled release or targeted release. The final dosage form may be a hard gelatin capsule (5).
Owner:COULTER IVAN
Apparatus for metering and dispensing powder into hard gelatin capsules or the like
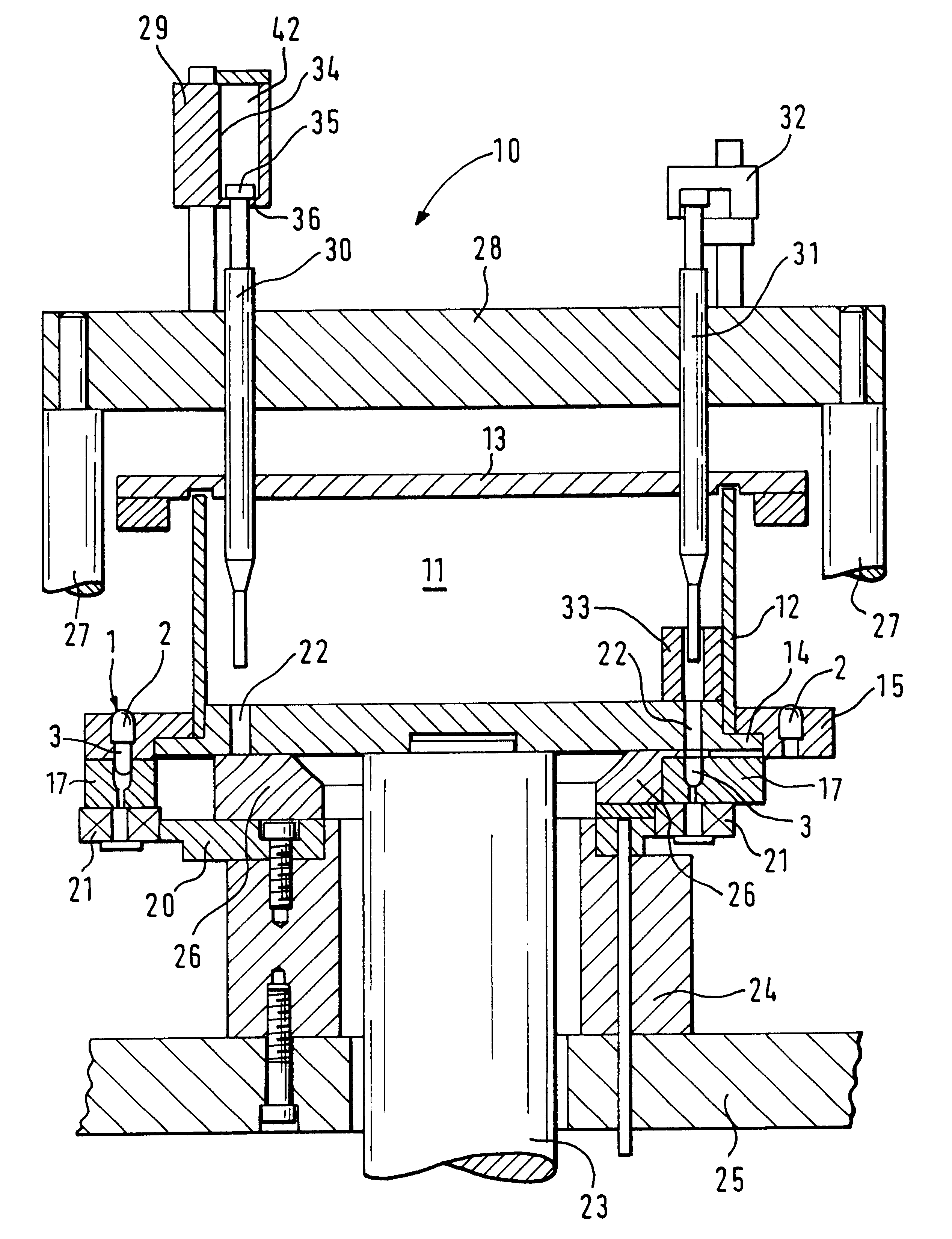
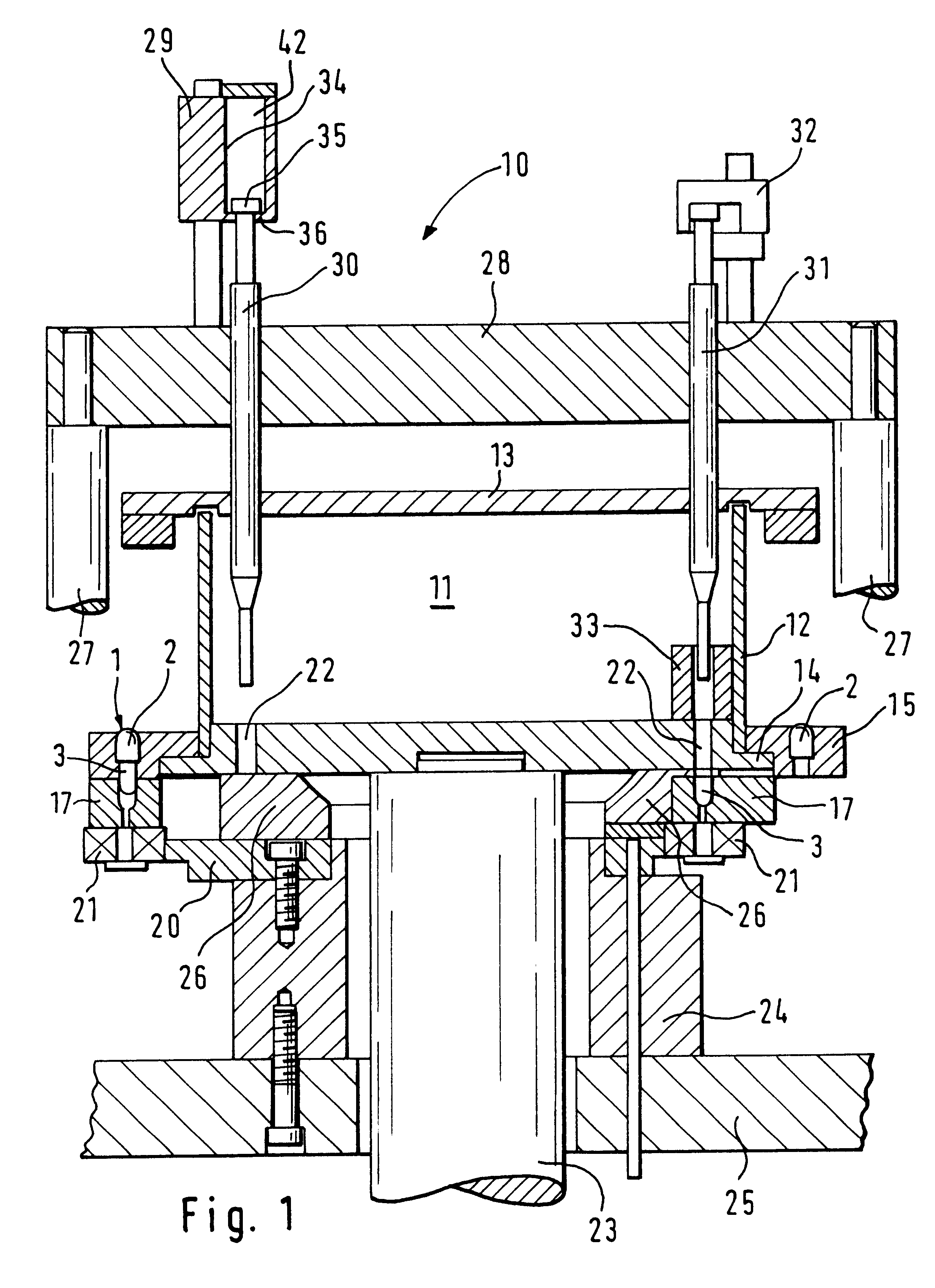
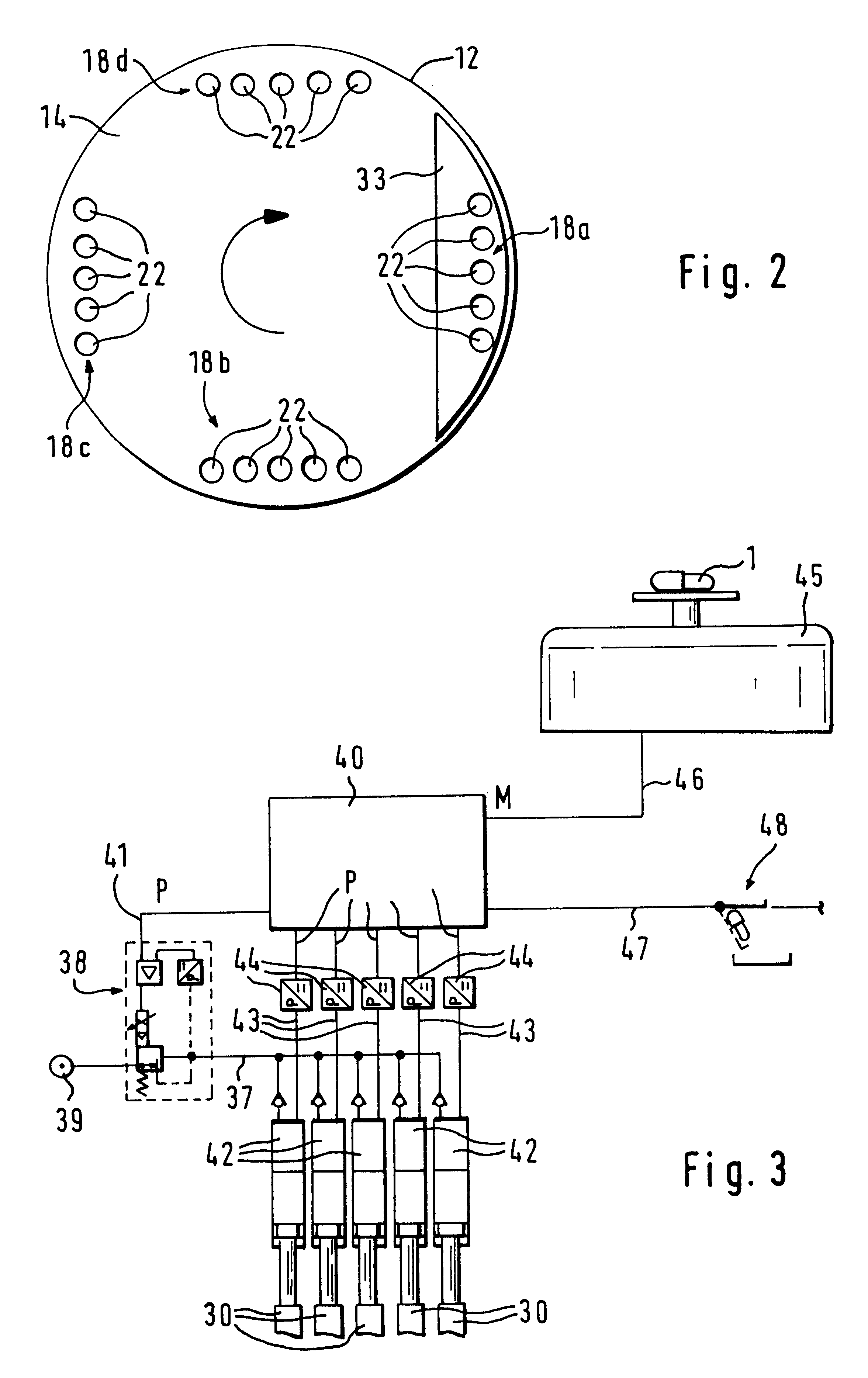
InactiveUS6098675AIncrease productionImprove metering accuracyMovable measuring chambersSolid materialEngineeringProduct Containers
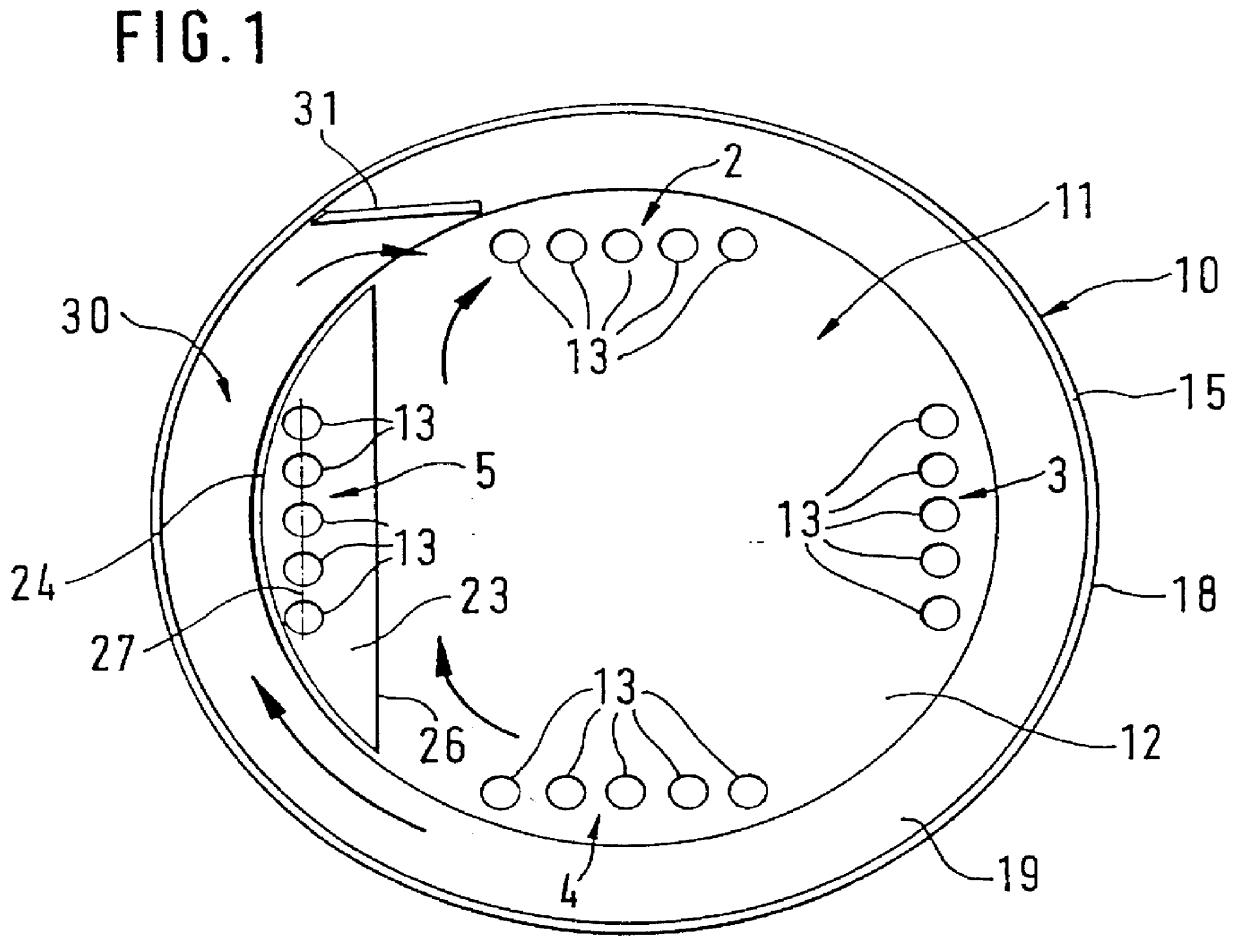
PCT No. PCT / DE97 / 01943 Sec. 371 Date Aug. 10, 1998 Sec. 102(e) Date Aug. 10, 1998 PCT Filed Sep. 4, 1997 PCT Pub. No. WO98 / 25823 PCT Pub. Date Jun. 18, 1998An apparatus for metering and dispensing powder into hard gelatin capsules or the like including an incrementally rotated product container. Bores that cooperate with stuffing dies and transfer dies are formed on the bottom of the product container. A repelling element is disposed in the region of the transfer die. Because of the special shape of the product container, an interstice is formed between the repelling element and a wall portion of the product container; the effect of the interstice is that the bores following the repelling element are adequately supplied with powder. The apparatus according to the invention has high metering accuracy and high output.
Owner:ROBERT BOSCH GMBH
Apparatus for metering and dispensing powder into hard gelatin capsules or the like
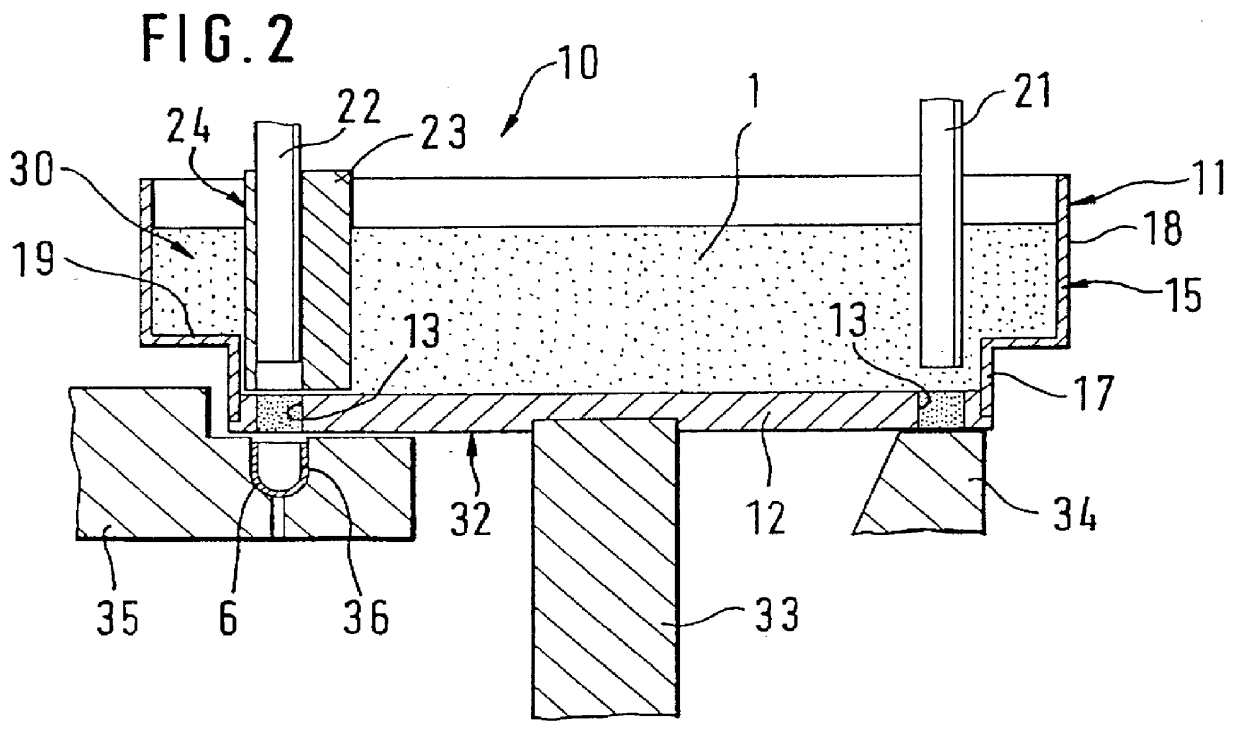
InactiveUS20010035431A1Simple format adaptationControl performanceLiquid transferring devicesSolid materialEngineeringMechanical engineering
An apparatus for metering and dispensing powder into hard gelatin capsules or the like. The apparatus has a metering disk that rotates in advancing steps, with bores disposed in its base. The bores which cooperate with tamping plungers that move up and down. The tamping plungers are disposed on a common tamping plunger support and when inserted into the bores, compress the powder into compressed pellets. In order to detect breakage of the springs and in order to be able to make a statement as to the mass of the compressed pellets, means are provided which detect the spring path of the tamping plungers immediately preceding the ejection plungers.
Owner:SYNTEGON TECHNOLOGY GMBH
Apparatus for metering and dispensing powder into hard gelatin capsules or the like
InactiveUS6390330B2Simple format adaptationControl performanceLiquid transferring devicesSolid materialEngineeringMechanical engineering
An apparatus for metering and dispensing powder into hard gelatin capsules or the like. The apparatus has a metering disk that rotates in advancing steps, with bores disposed in its base. The bores which cooperate with tamping plungers that move up and down. The tamping plungers are disposed on a common tamping plunger support and when inserted into the bores, compress the powder into compressed pellets. In order to detect breakage of the springs and in order to be able to make a statement as to the mass of the compressed pellets, means are provided which detect the spring path of the tamping plungers immediately preceding the ejection plungers.
Owner:SYNTEGON TECHNOLOGY GMBH
Solid dispersion pharamaceutical formulations
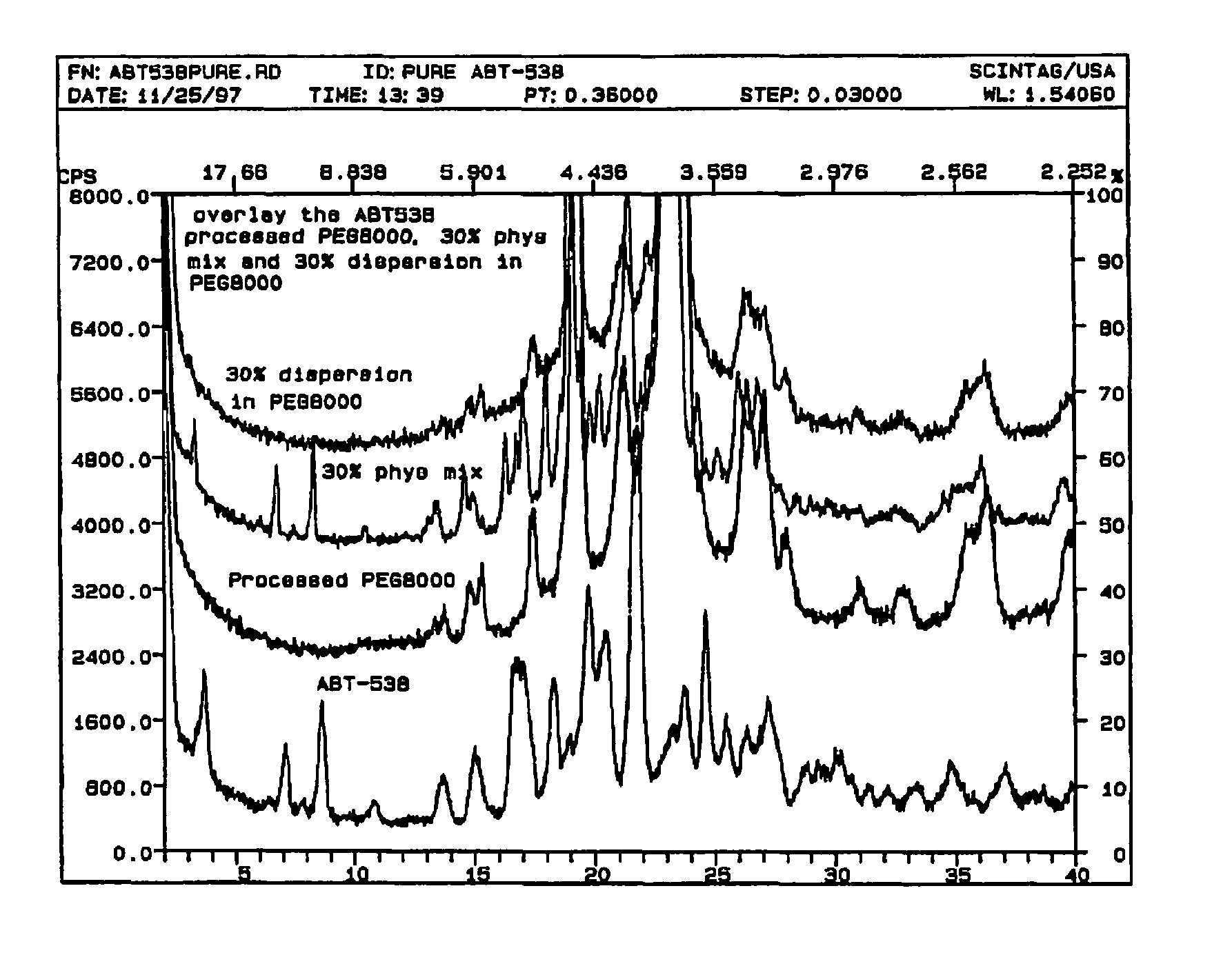
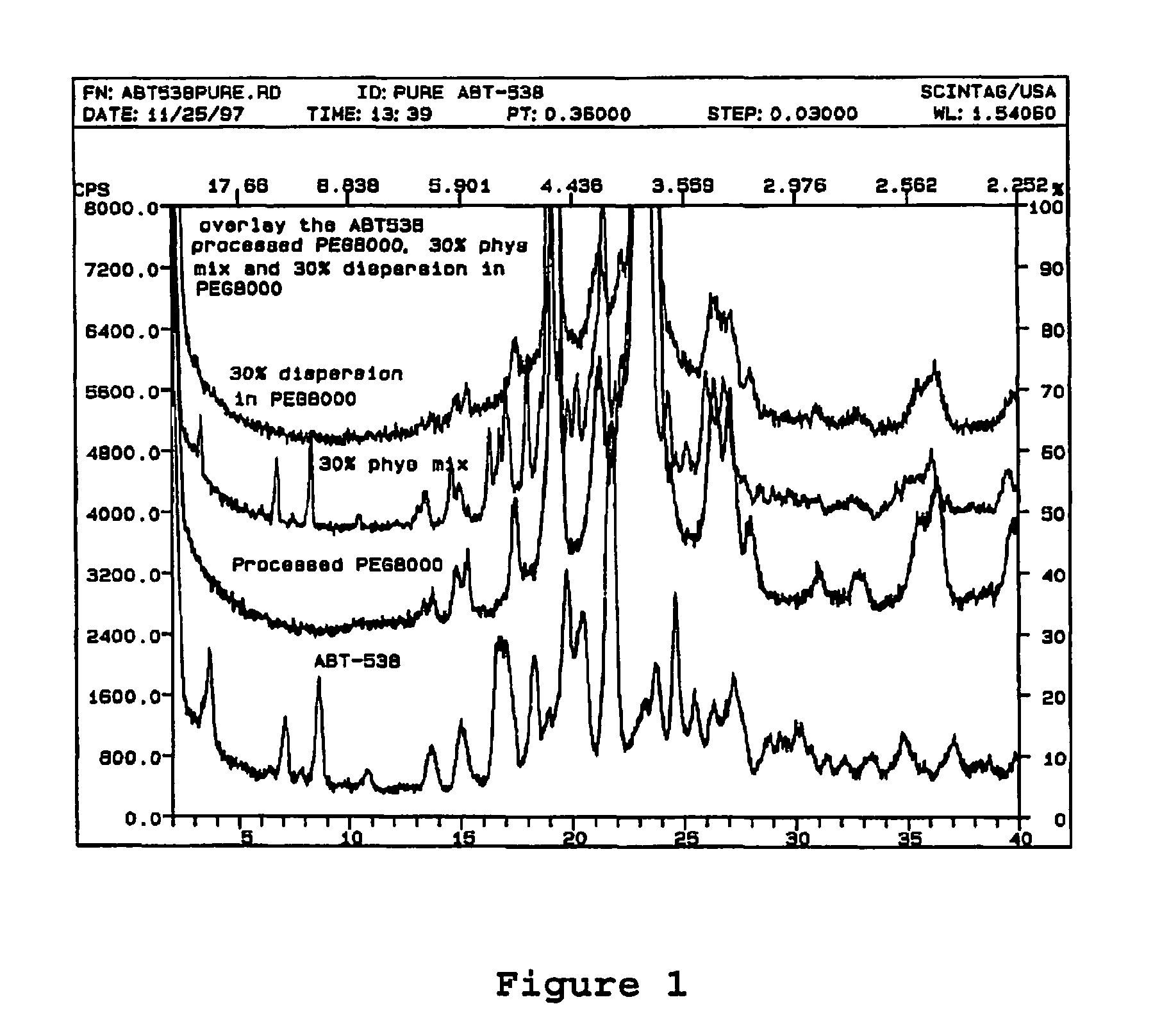
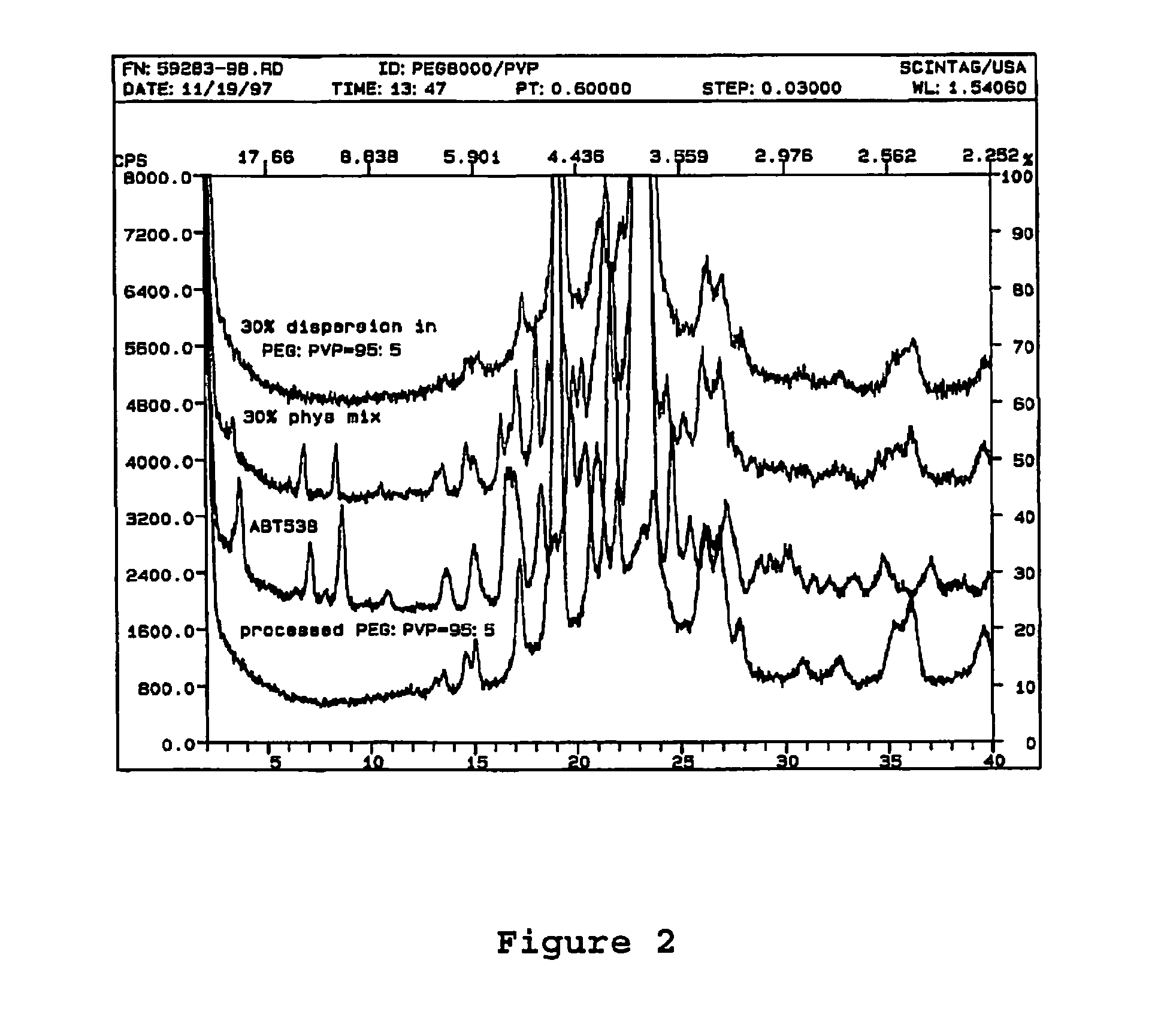
A pharmaceutical composition is disclosed which comprises a solid dispersion of an HIV protease inhibitor in a water soluble carrier, such as PEG, having enhanced bioavailability and improved dissolution properties. The solid dispersion may optionally be encapsulated in hard gelatin capsules, compressed into a tablet, or may be granulated with a pharmaceutically acceptable granulating agent. Also disclosed are methods of making said solid dispersion and methods of treating an HIV infection employing said solid dispersion.
Owner:ABBVIE INC
Full automatic hard gelatin capsule liquid and soft-body filler
ActiveCN1607161ALow quality stability requirementsIncrease productivityCapsule deliveryLiquid materialHard CapsuleSoft materials
A hard capsule filling machine for low or intermediate viscosity liquid and soft material consists of the first and second rail, pouring apparatus, gluing device for gluing inner joining area of capsule cap, negative pressure sleeve join device, upper and lower mold block circular moving in rail, every join mold can hold multiple capsule simultaneously, capsule can be joined under vacuum condition. Said invention has higher productivity and yield. When defect capsule occurred and contaminated mold, the cleaned or new mold can be replaced at any position in the first rail.
Owner:广东强基药业有限公司
Formulation of plant source hard capsule case and preparing method thereof
InactiveCN1927186AHigh transparencyImprove stabilityPharmaceutical non-active ingredientsCapsule deliveryCarrageenanLocust bean gum
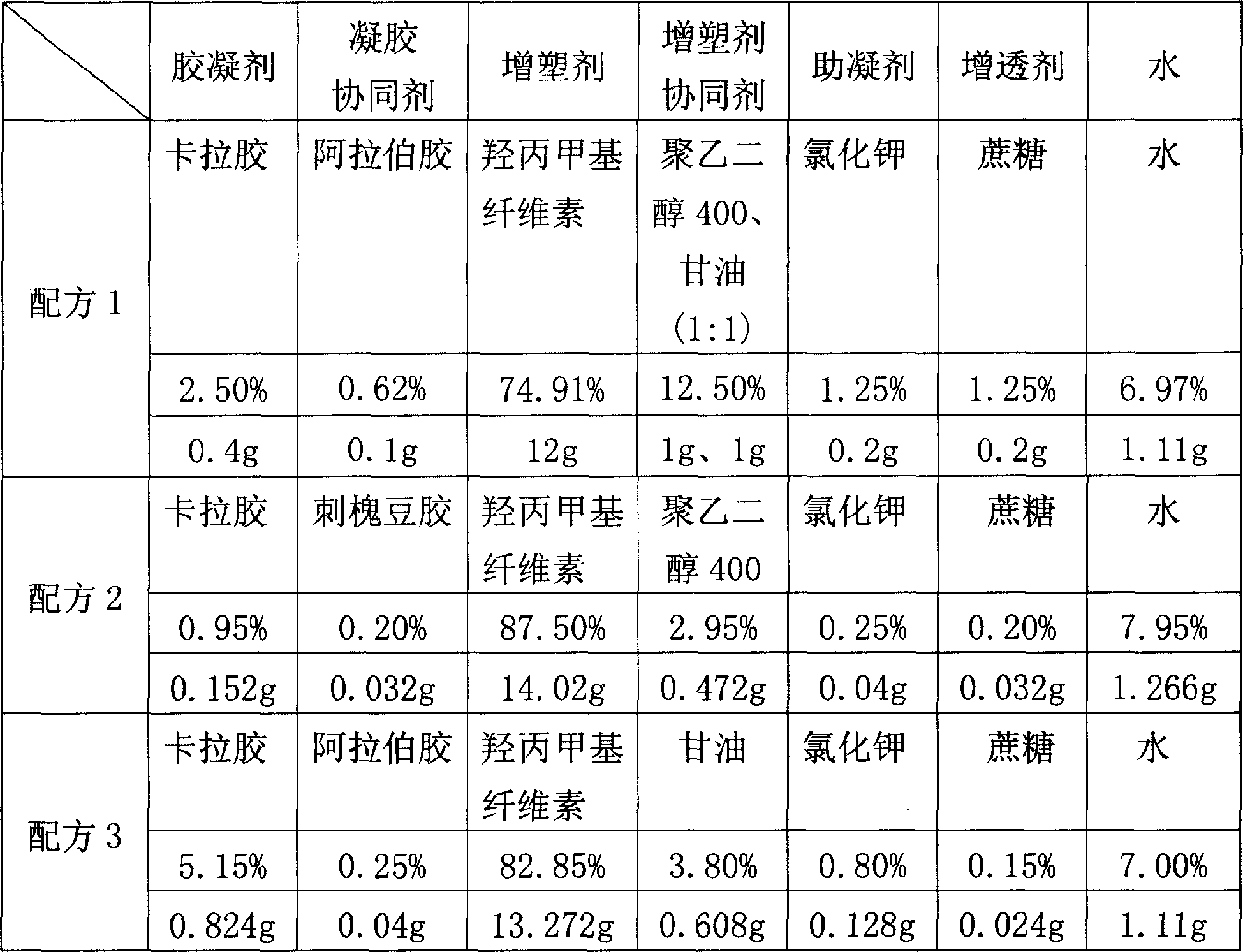
The invention relates to a formulation of plant source hard gelatin capsule shells and process for preparation, wherein the formulation includes (by weight ratio) 0.01-1.00% of potassium bromide, 0.02-0.80% of sucrose, 0.01-0.80% of acacia gum or locust bean glue, 0.10-2.00% of carrageenan, 0.50-5.20% of plasticizer synergistic agent, 70-90% of distilled water, 9.00-29.00% of methyl hydroxypropylcellulose.
Owner:QINGDAO UNIV
Inhibitors of crystallization in a solid dispersion
A pharmaceutical composition is disclosed which comprises a solid dispersion of a pharmaceutical compound in a water soluble carrier, such as polyethylene glycol (PEG), and a crystallization inhibitor, such as polyvinylpyrrolidone or hydroxypropylmethylcellulose. The solid dispersion may optionally be encapsulated in hard gelatin capsules, compressed into a tablet, or may be granulated with a pharmaceutically acceptable granulating agent. Also disclosed are methods of making said solid dispersion and methods of treatment employing said solid dispersion.
Owner:ABBVIE INC
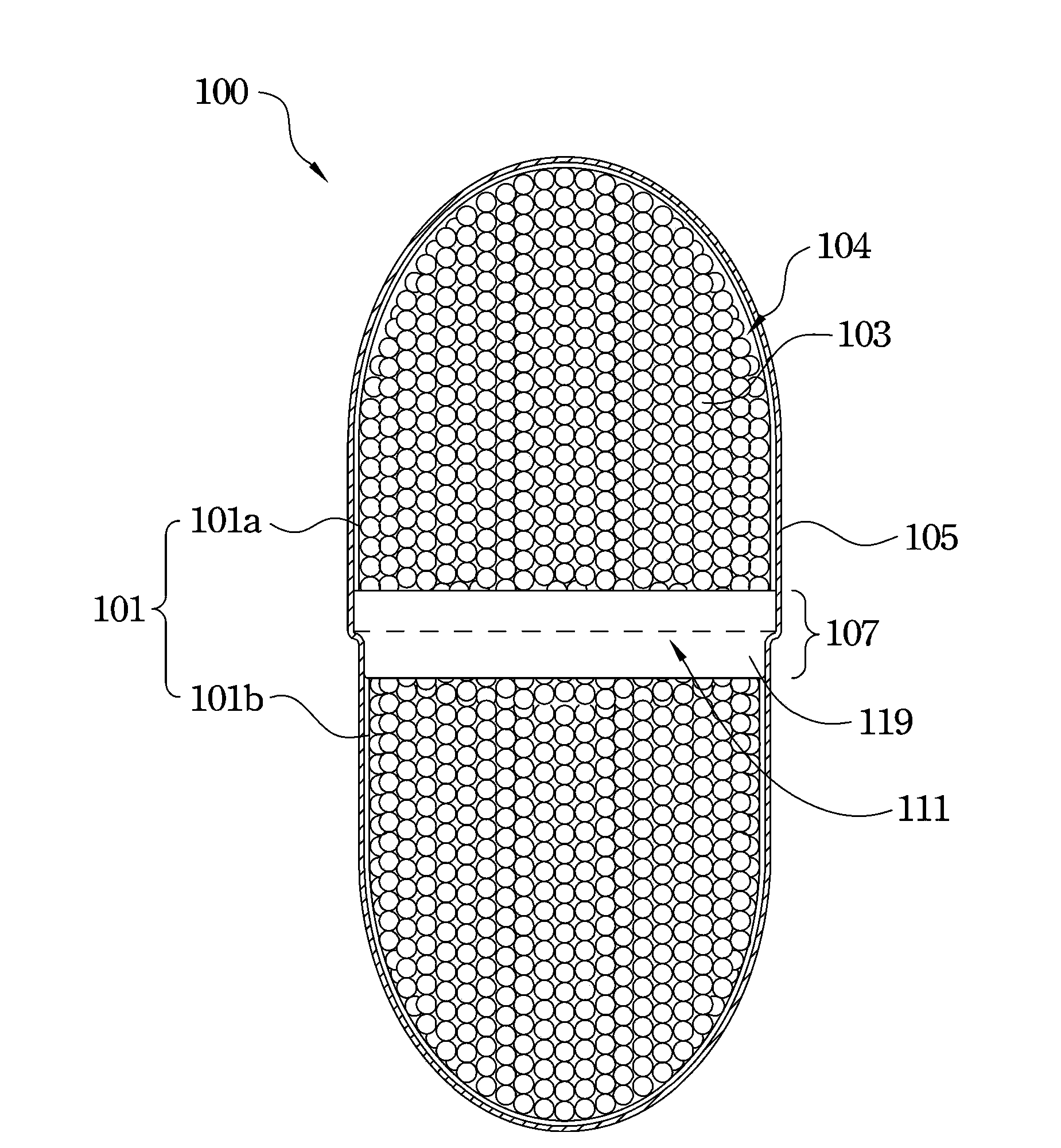
Capsule filling machine and method for producing hard gelatin capsules
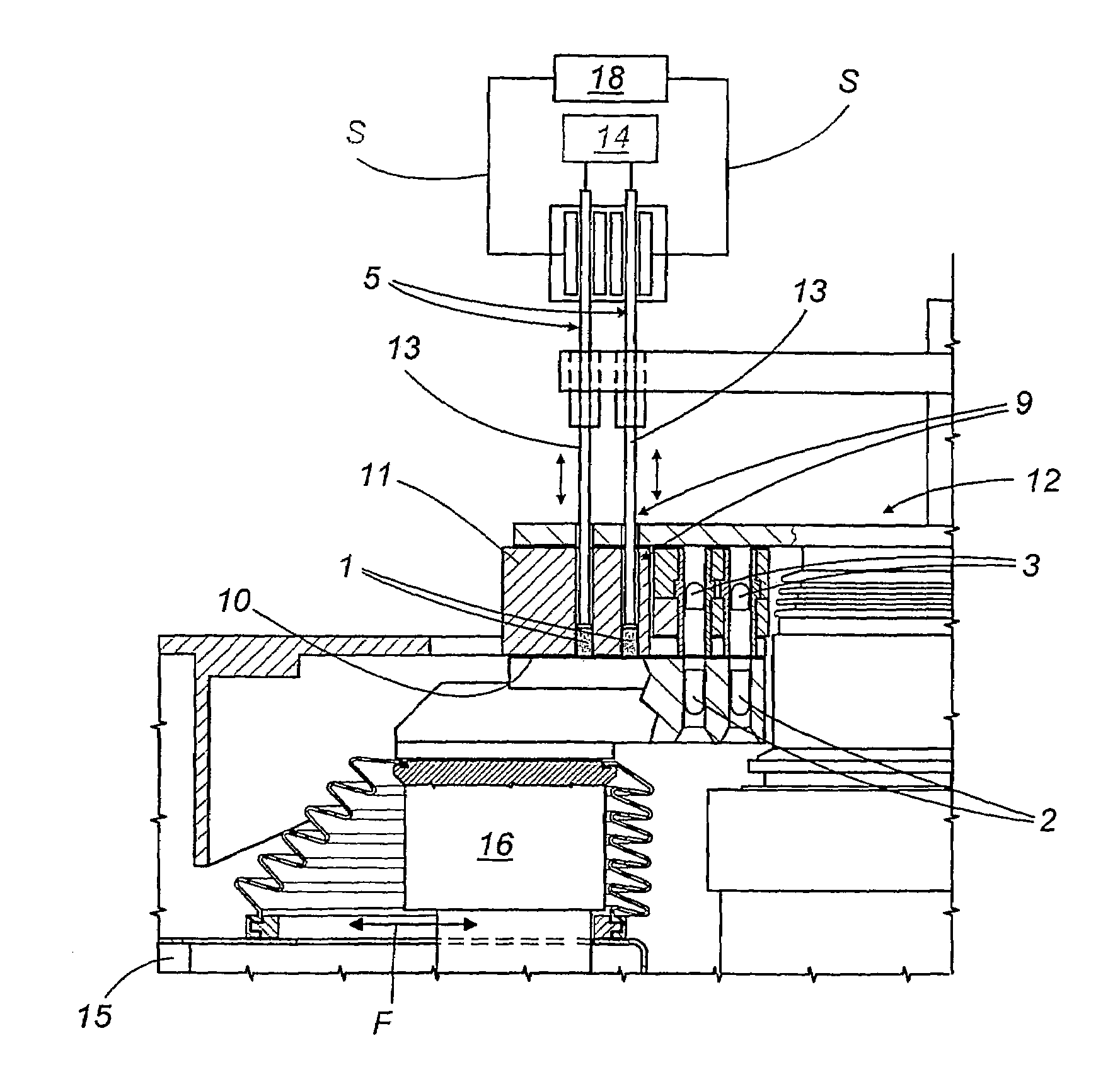
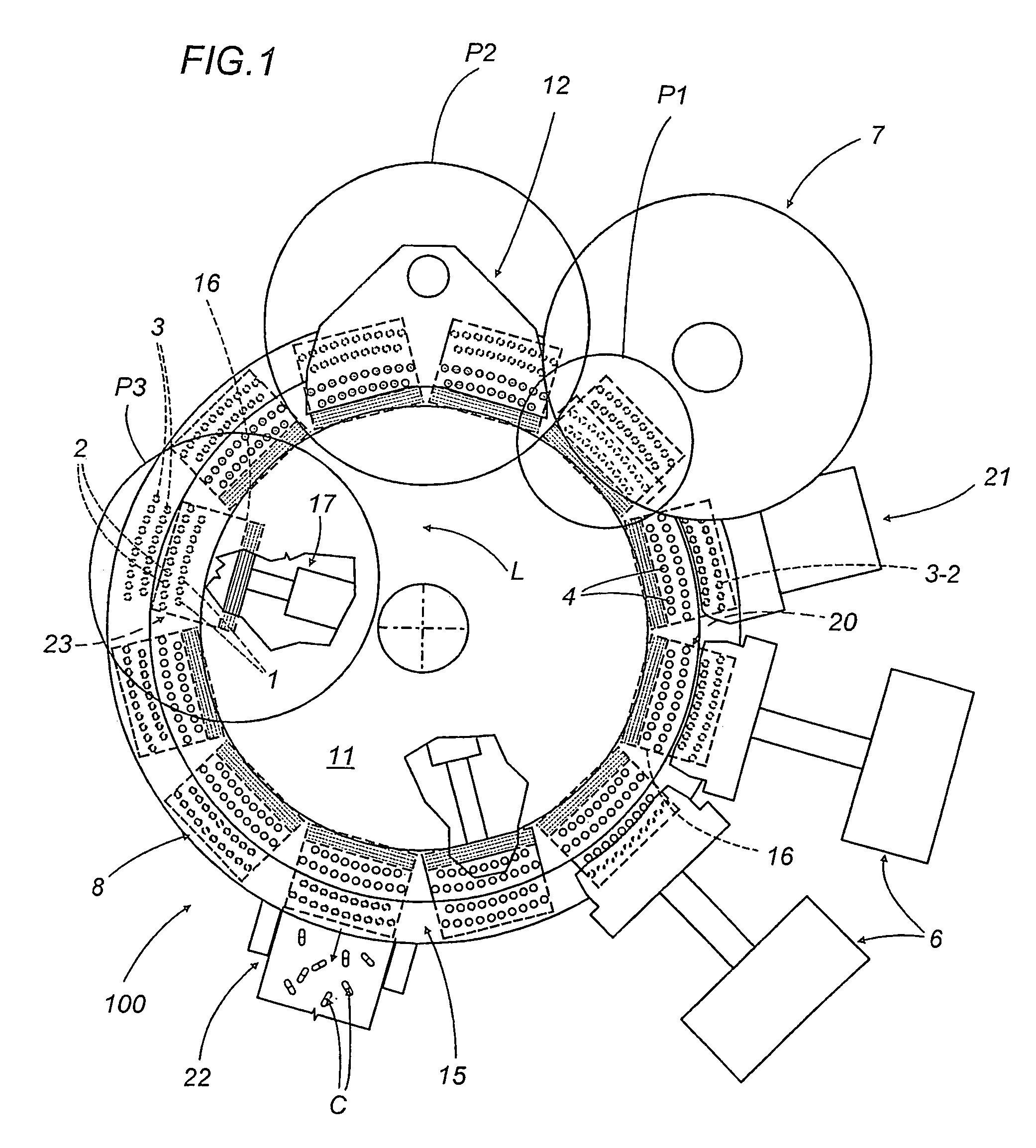
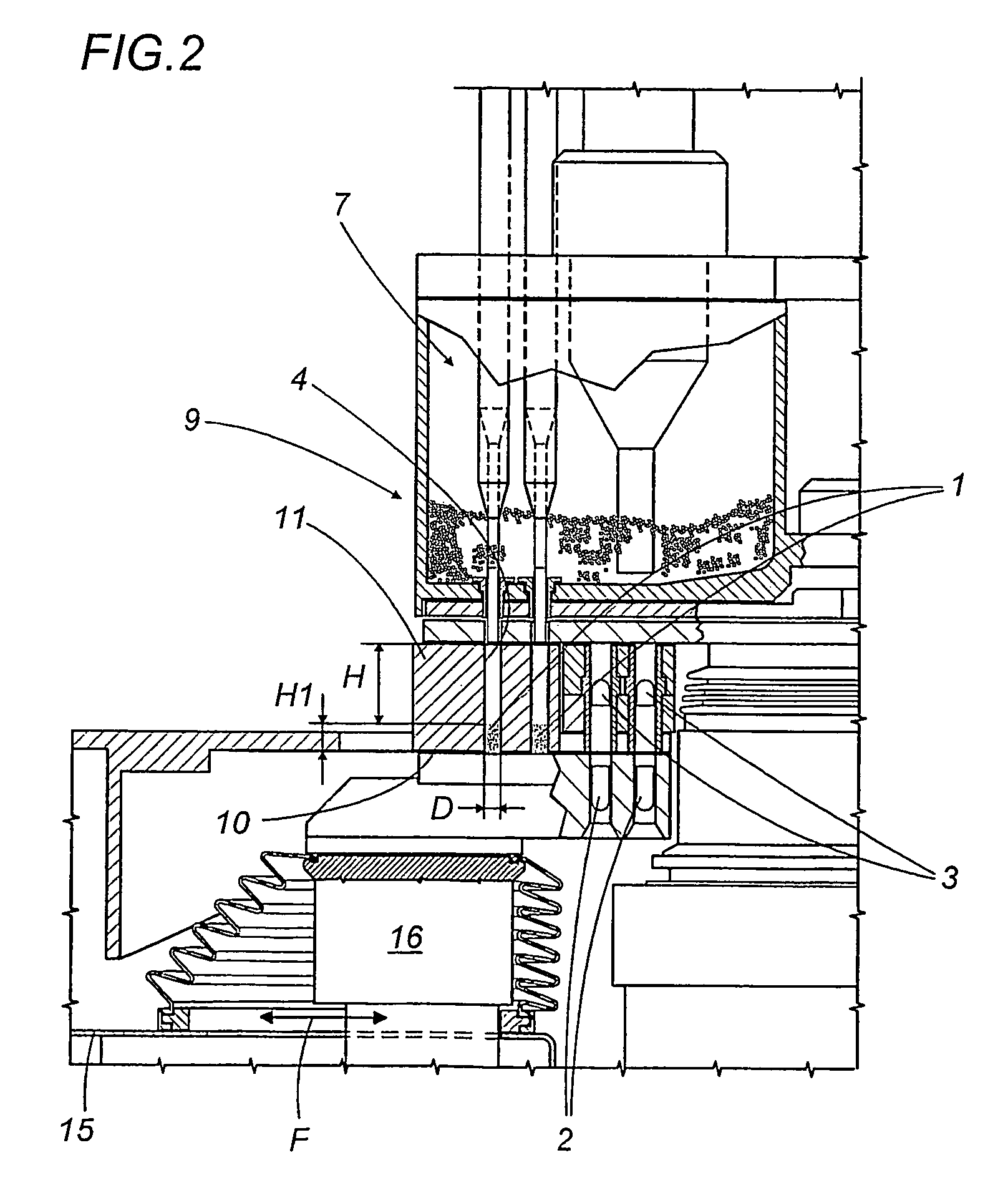
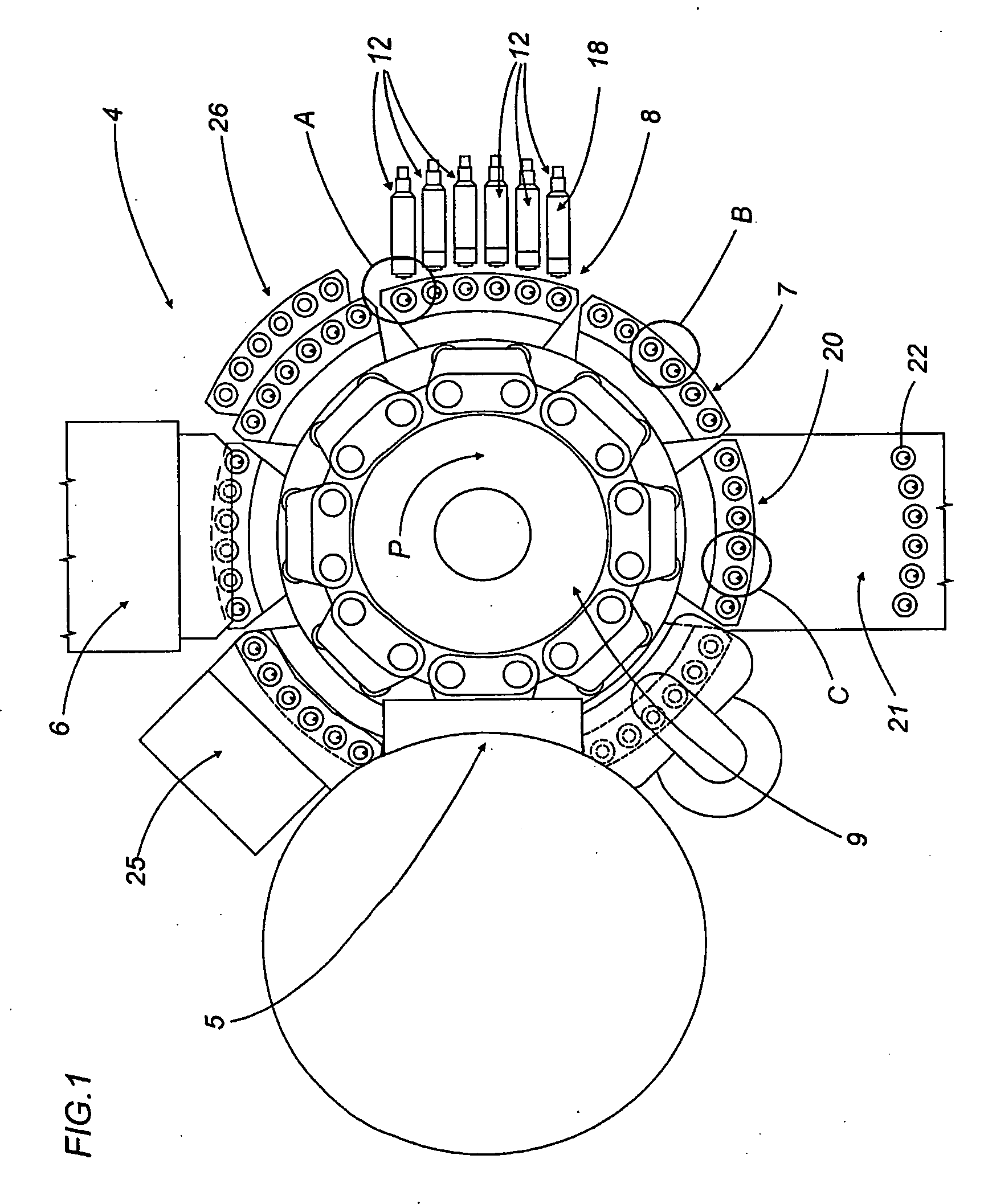
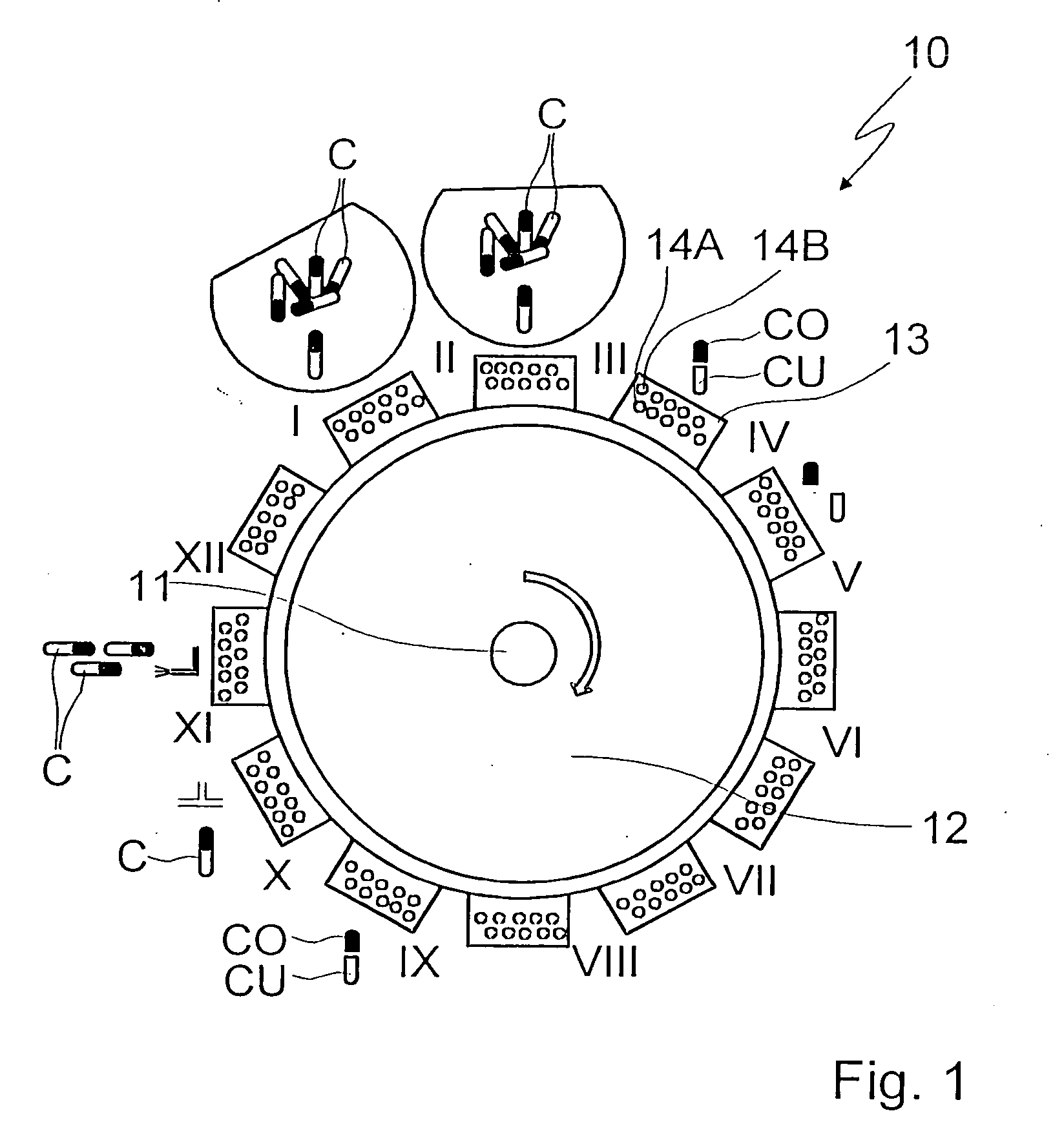
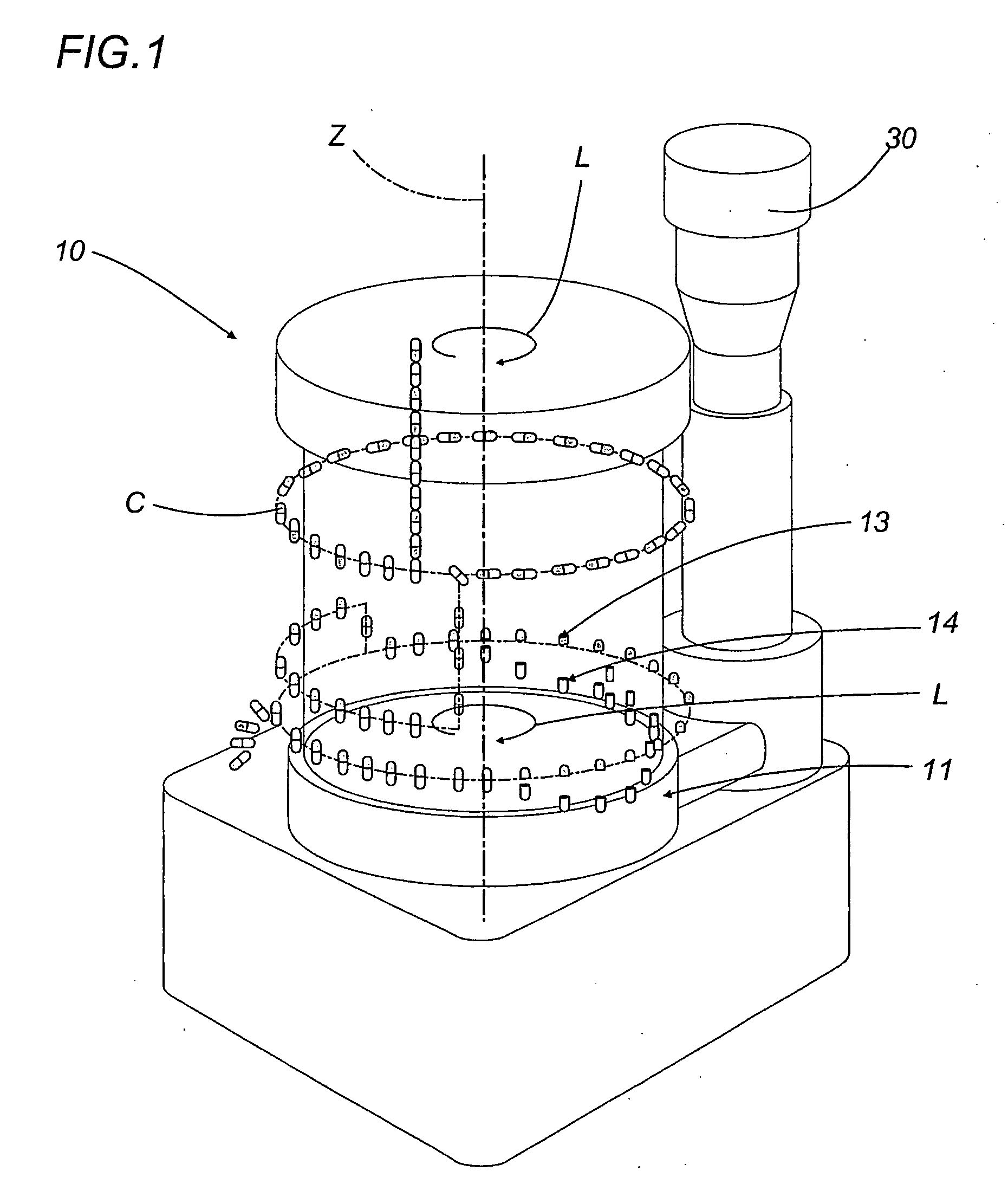
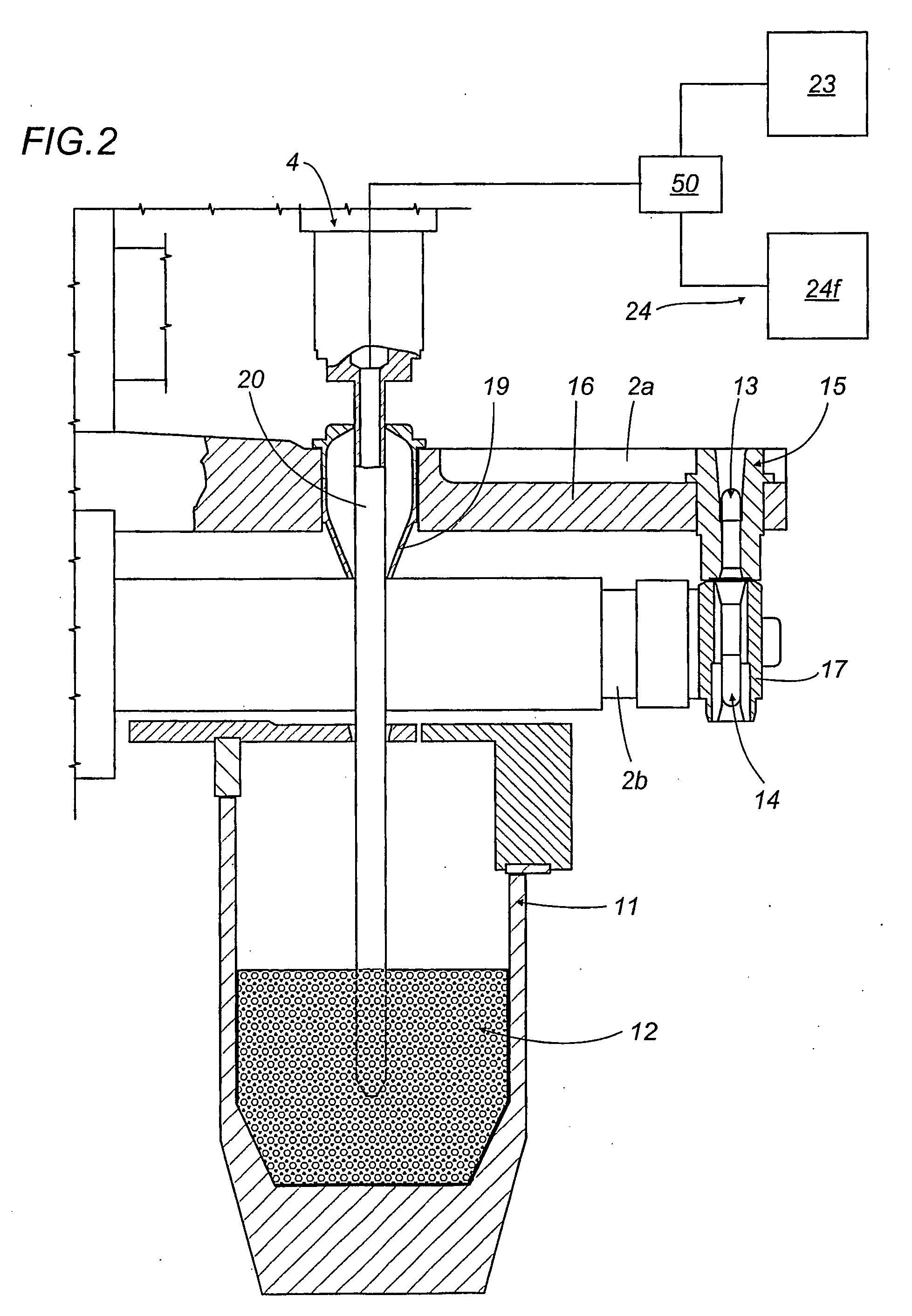
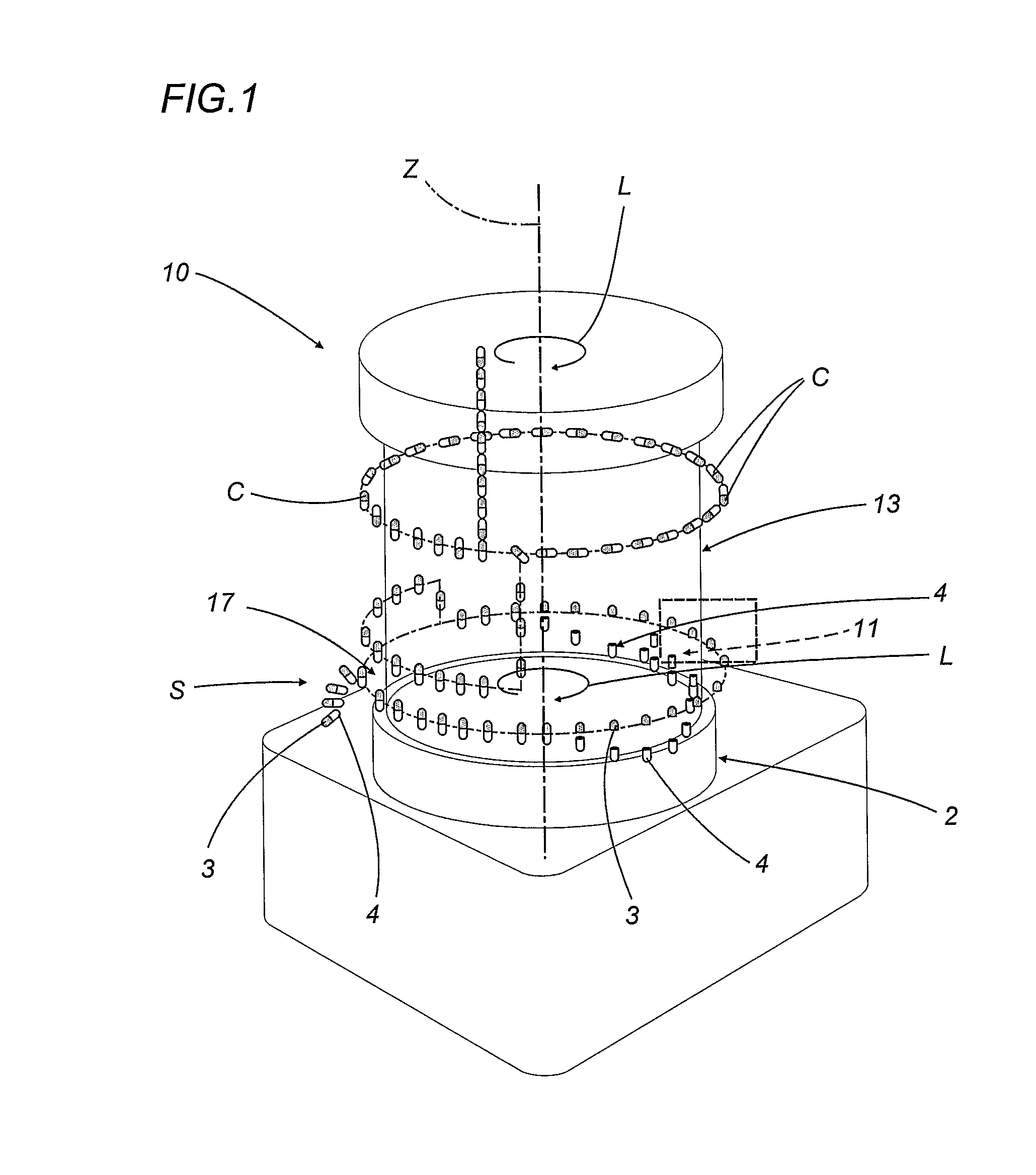
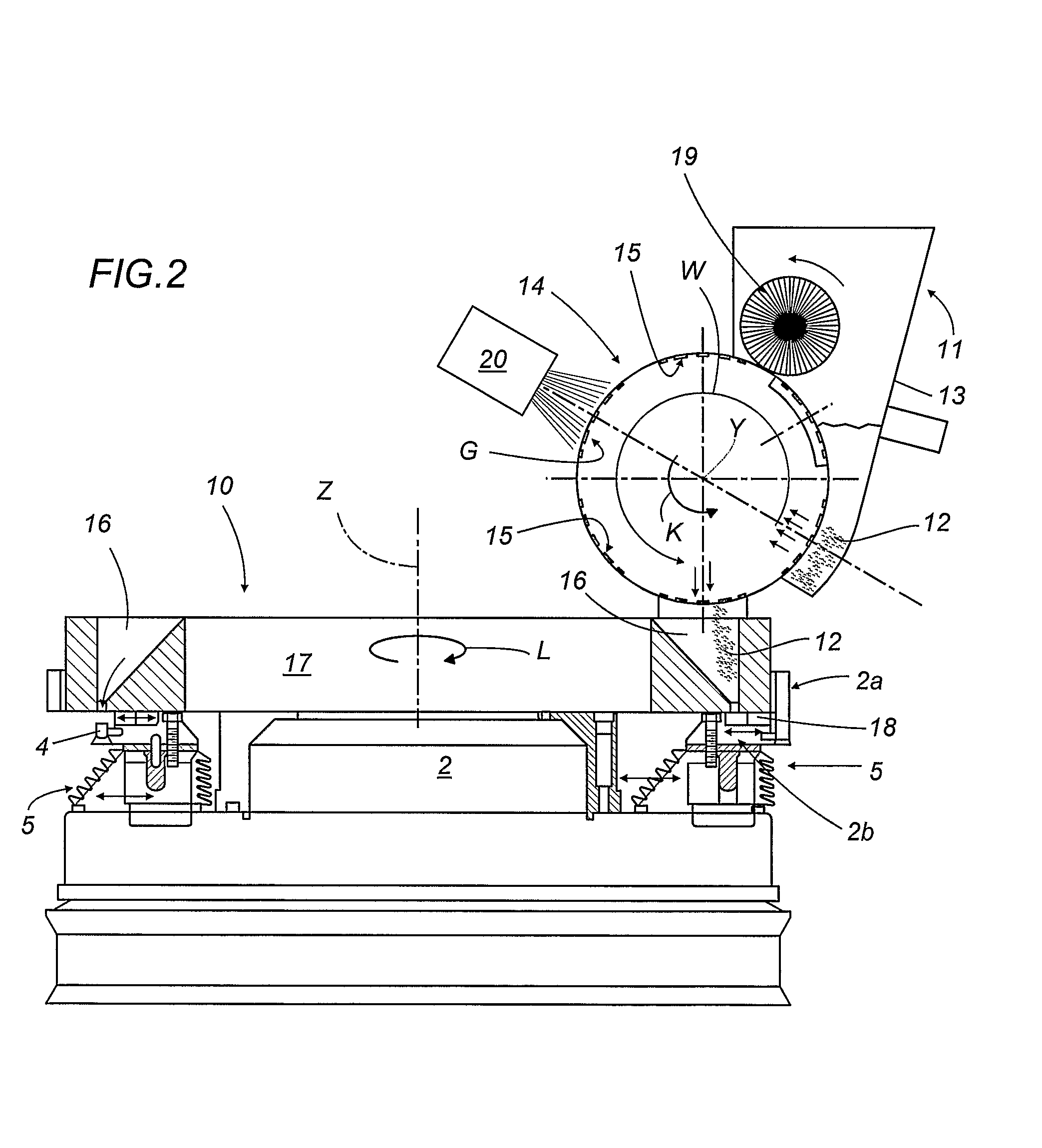
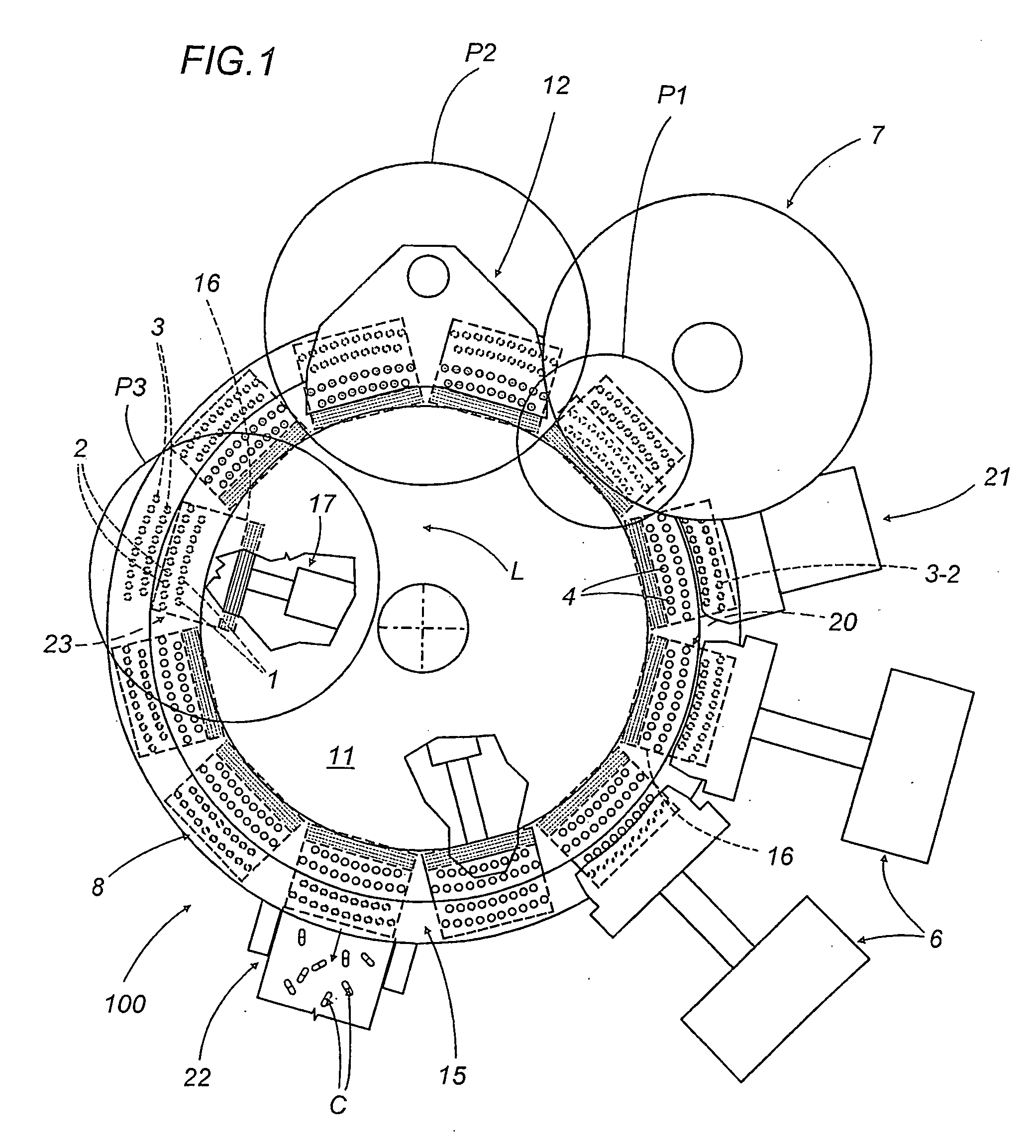
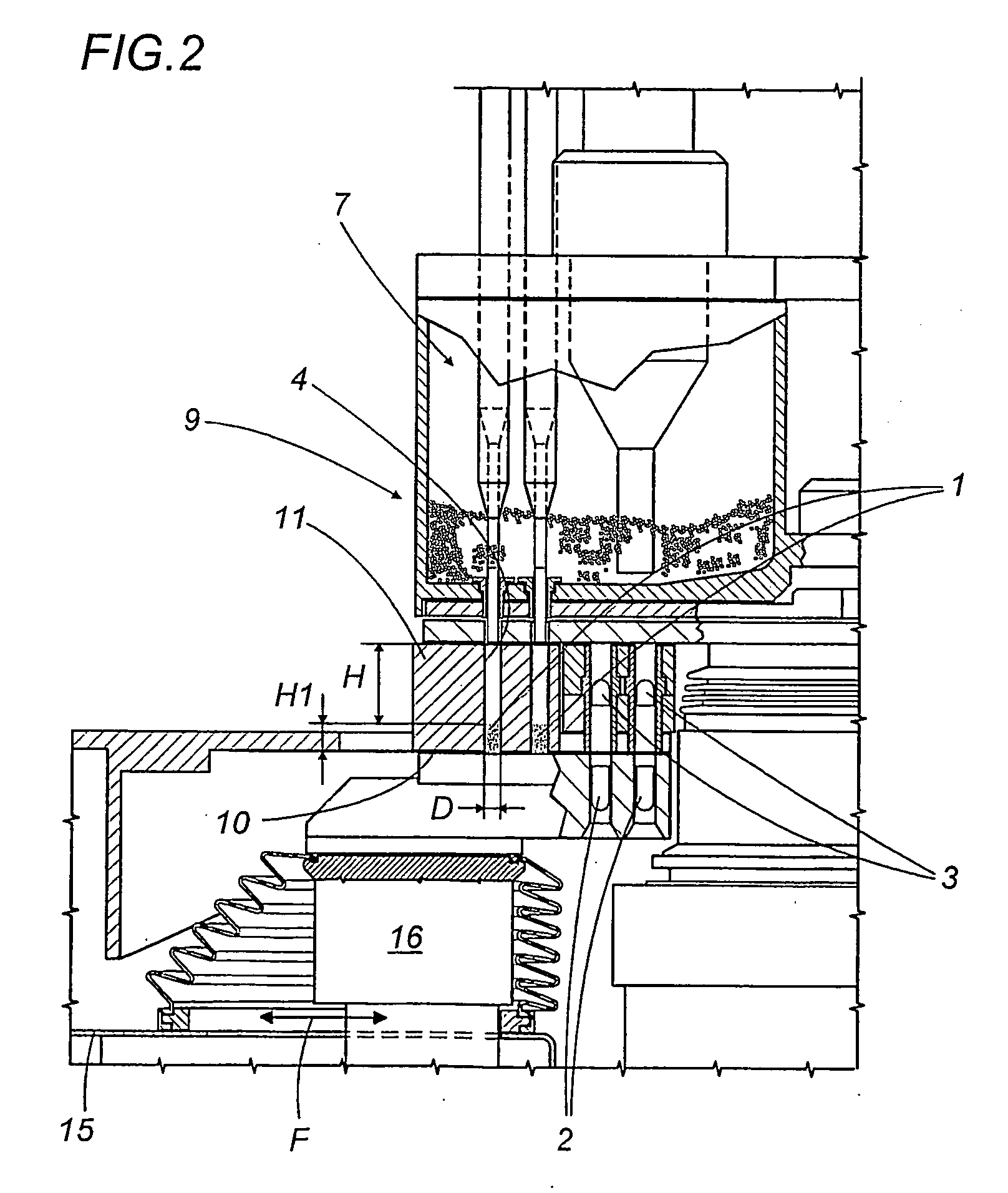
A capsule filling machine (100) for the production of hard gelatin capsules (C) of the type with lid (3) and body (2) containing a quantity (1) of pharmaceutical material comprises a rotary turret or carousel (15) which defines at least one capsule (C) handling line (L) and on which the following are positioned, one after the other: at least one station (6) for feeding empty capsules (C); at least one opening station (20) where the capsule bodies (2) are separated from the lids (3) to form two separate rows of capsule bodies (2) and lids (3); at least one station (7) for feeding and dosing the quantities (1) of pharmaceutical material to be filled into the capsule bodies (2); and at least one station (8) for closing the capsules (C) by placing a lid (3) over each respective body (2); the machine (100) also comprises means (9) for detecting and volumetrically checking the quantity (1) of pharmaceutical material filled into each capsule body (2), the detecting and checking means (9) comprise transducer means (5) for measuring the volume of said quantities (1) before they are filled into the capsule bodies (2).
Owner:IMA IND MASCH AUTOMATICHE SPA
Capsule Filling Machine and Method For Producing Sealed Capsules
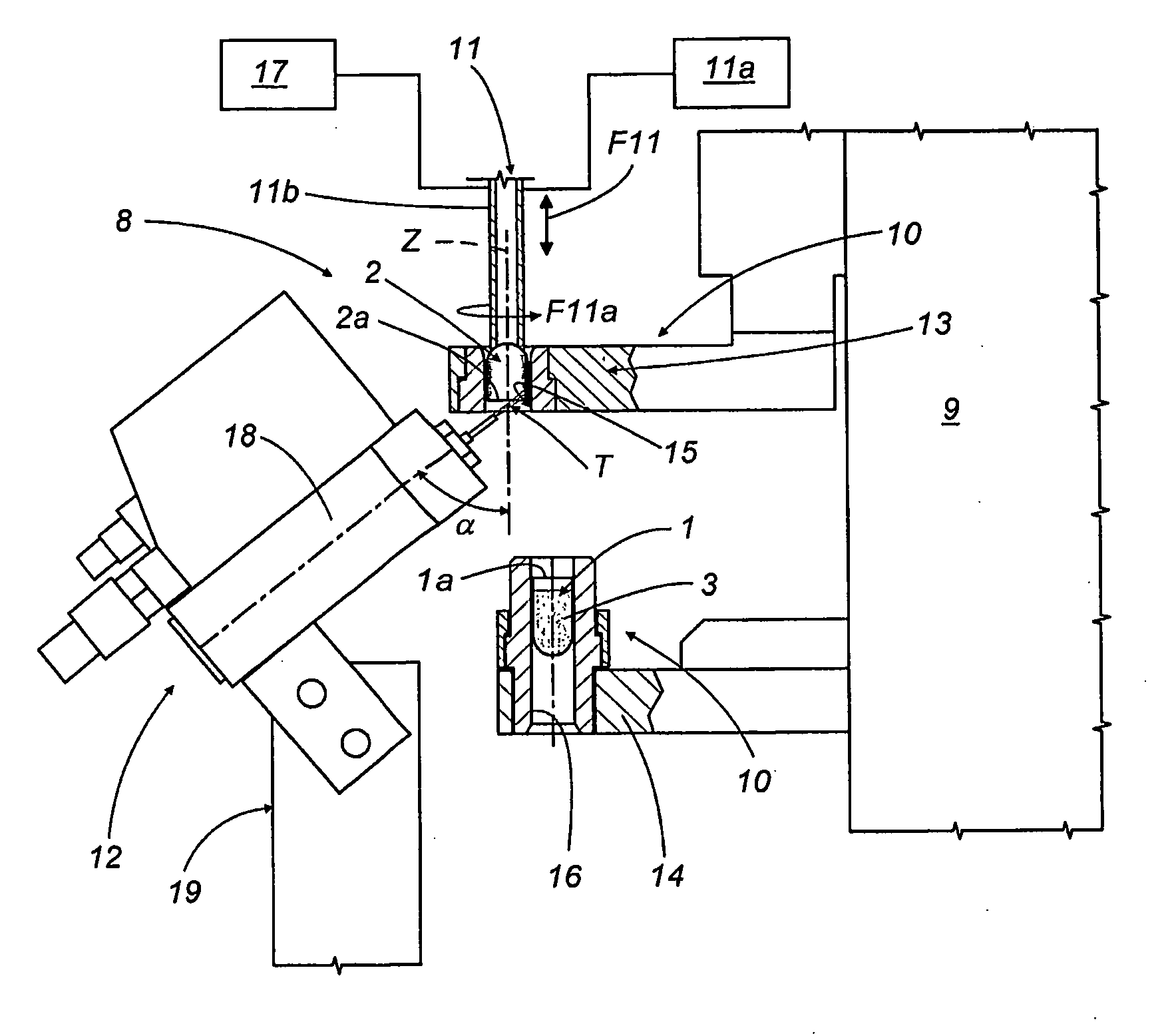
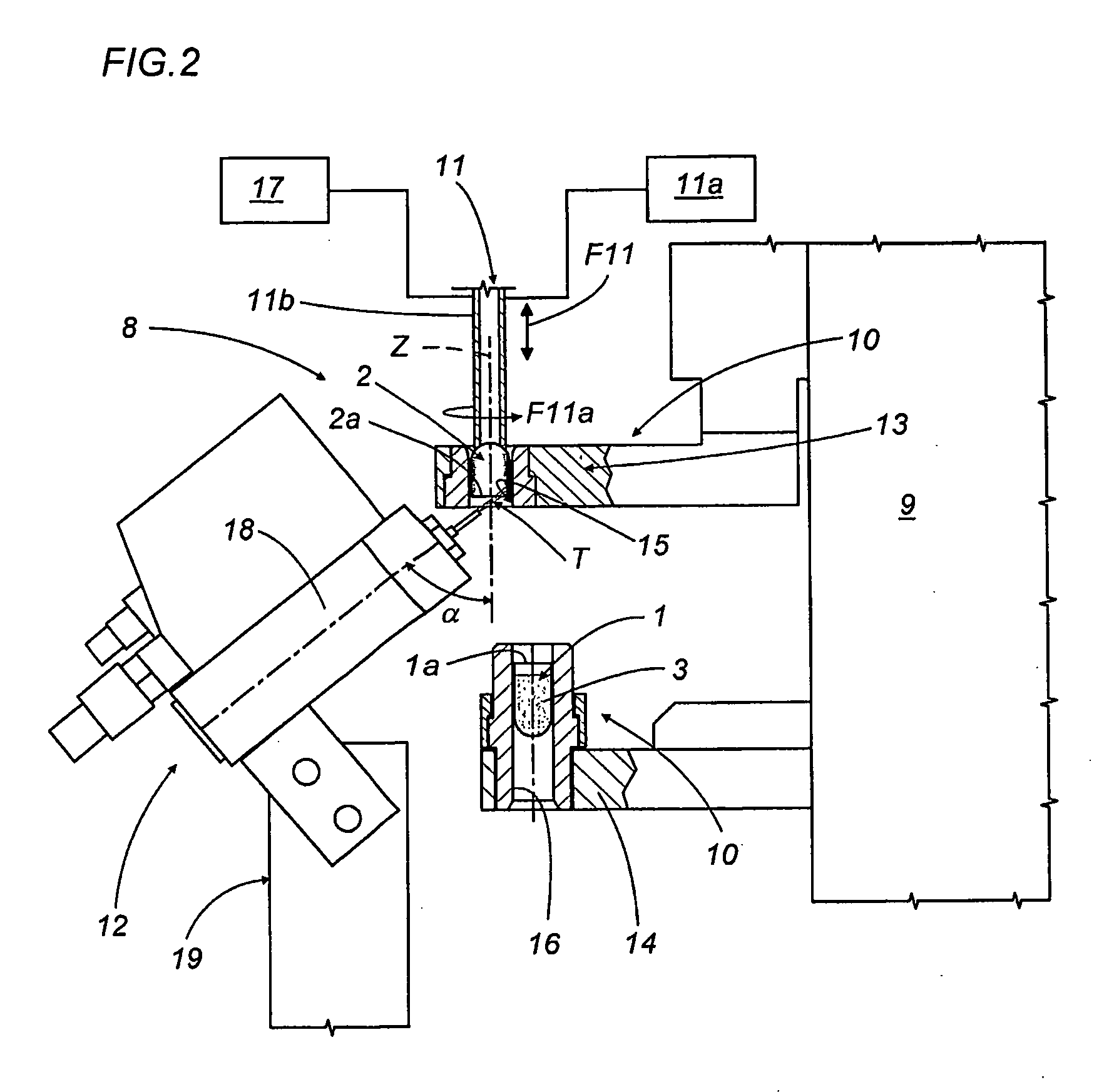
A capsule filling machine (4) for the production of hard gelatin capsules (C) of the type with lid (2) and body (1) containing pharmaceutical material, the machine (4) being of the type comprising a station (5) for feeding the capsule bodies (1) and lids (2); a dosing station (6) for filling a dose of the material into each capsule body (1); and a station (7) for closing the capsules (C) by placing each lid (2) over the respective body (1) so that their respective annular ends (2a, 1a) overlap. Between the dosing station (6) and the closing station (7) there is at least one intermediate operating station (8) for applying a sealing substance to the capsule lid and bodies in the vicinity of their ends (1a, 2a).
Owner:IMA IND MASCH AUTOMATICHE SPA
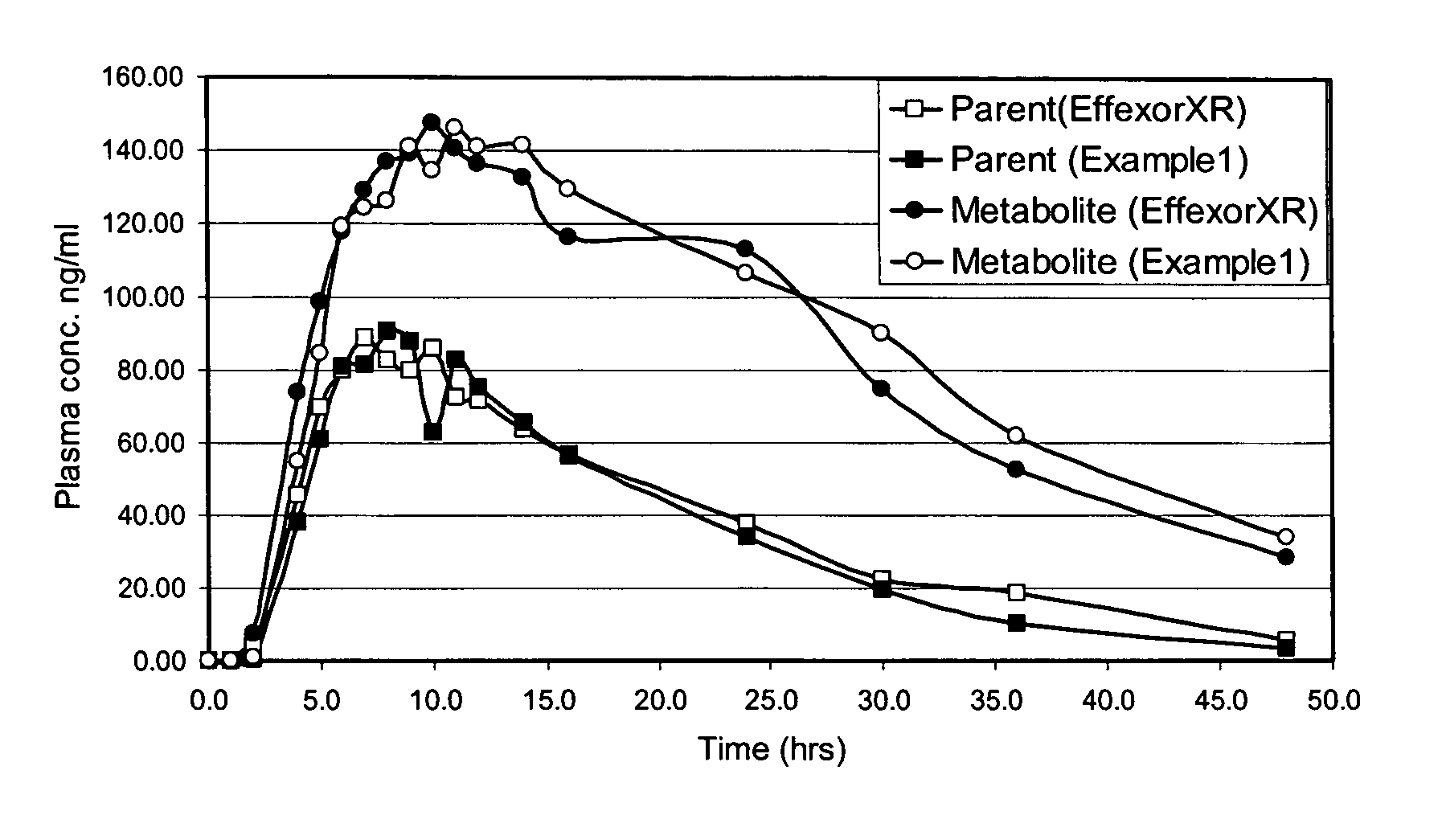
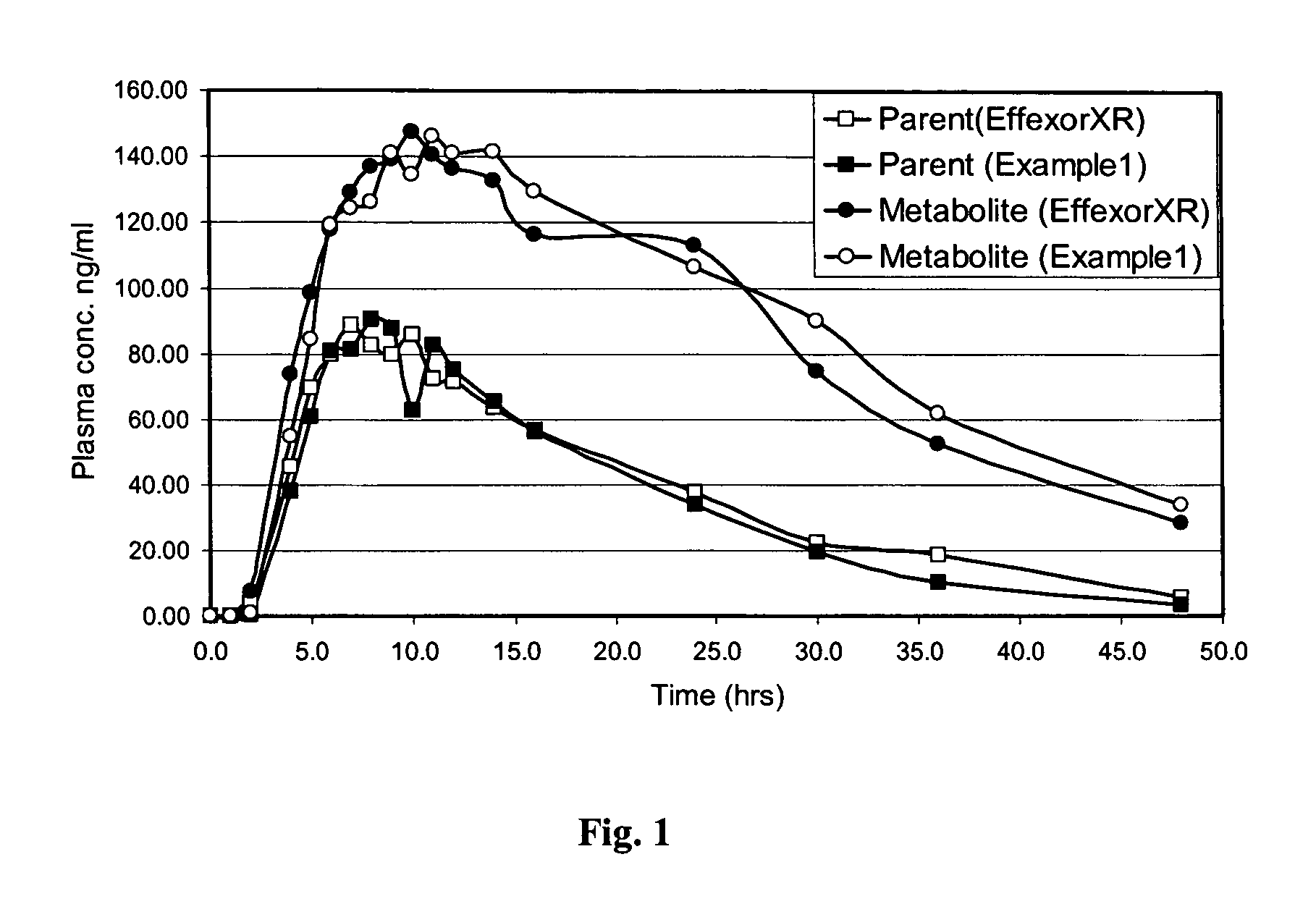
Extended release formulation of venlafaxine hydrochloride
InactiveUS20050169985A1Facilitated releaseReduce processing timeOrganic active ingredientsNervous disorderMini tabletsPharmaceutical formulation
The present invention relates to an extended release once daily pharmaceutical formulation comprising venlafaxine hydrochloride and pharmaceutically acceptable excipients. More particularly, the present invention relates to an extended release composition in the form of mini-tablets which are incorporated in hard gelatin capsules.
Owner:ALEMBIC LTD
Sustained releasing minipills of diltiazem hydrochloride and its preparation
ActiveCN1546039AUnleash behavioral influencePrevent and reduce seizuresOrganic active ingredientsGranular deliveryPharmaceutical formulationDisease cure
The invention discloses a Diltiazem Hydrochloride slow release type micro-drop pill and the process for preparation, which comprises mixing two or more Diltiazem Hydrochloride micro-drop pills by a finite proportion, packing into hard gelatin capsule or pressing into tablet. The preparation can be used for the prevention and cure of hypertension and stenocardia diseases triggered by blood pressure and heart rate rise within several hours after getting up.
Owner:KAMP PHARMA
Production of pharmaceutical formulations for treatment of edema and venous disorders
InactiveUS6077534AImprove securityImprove bioavailabilityPowder deliveryGranular deliveryDiseaseAdemetionine
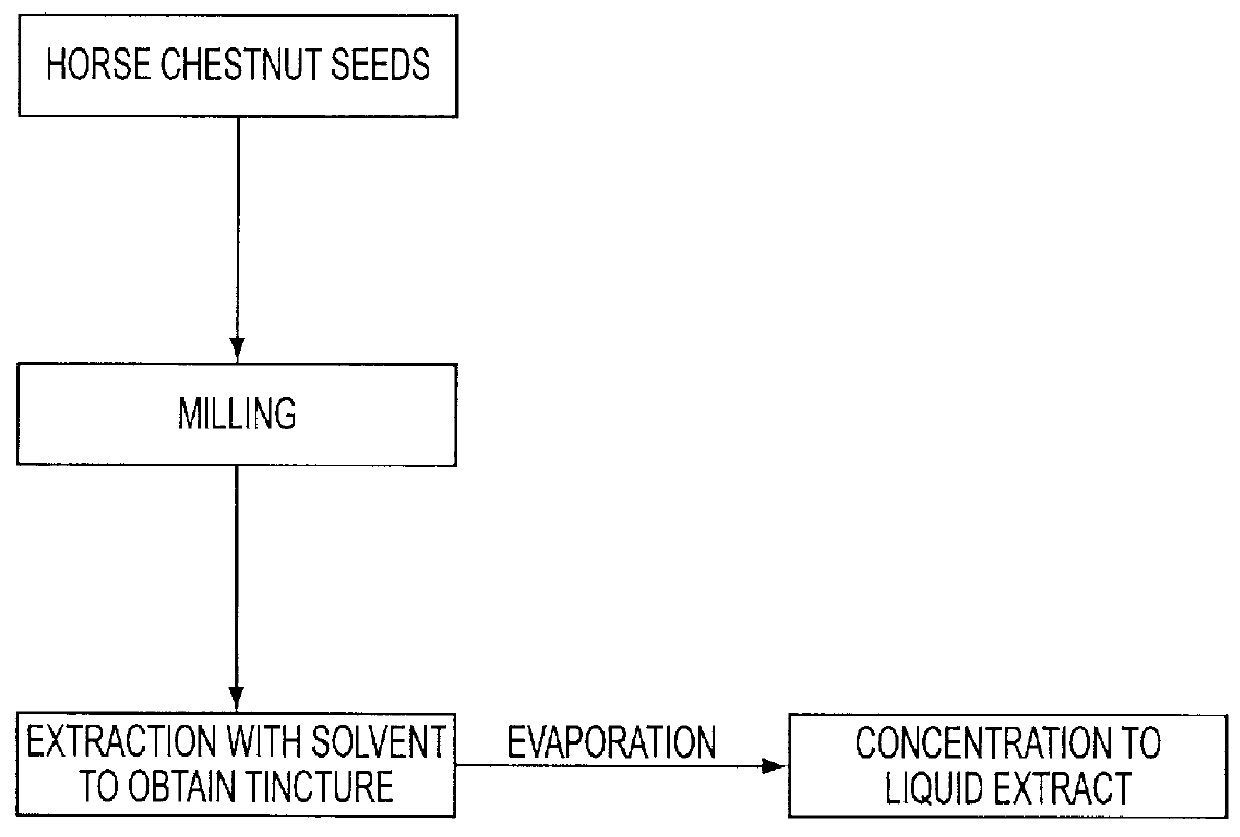
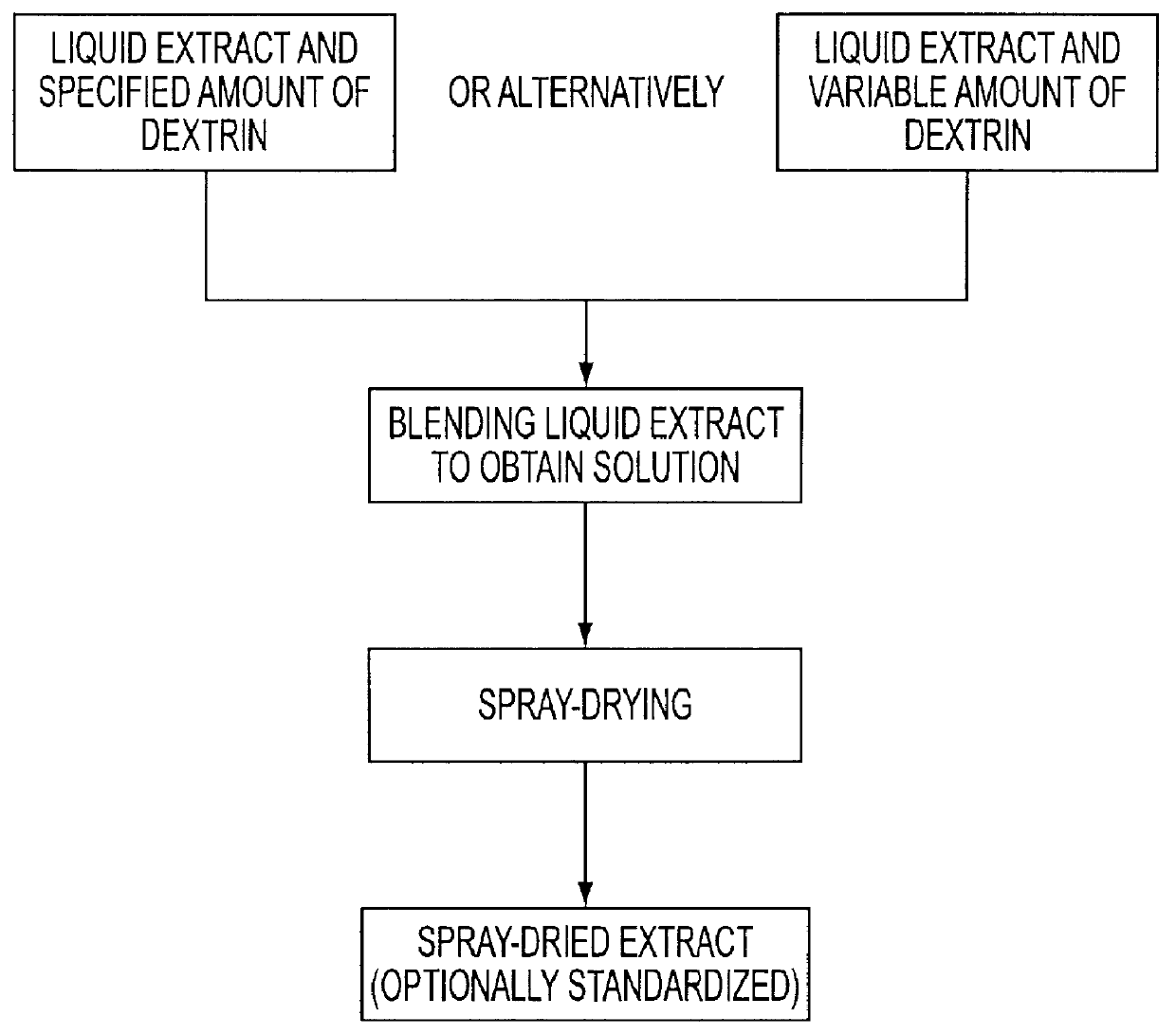
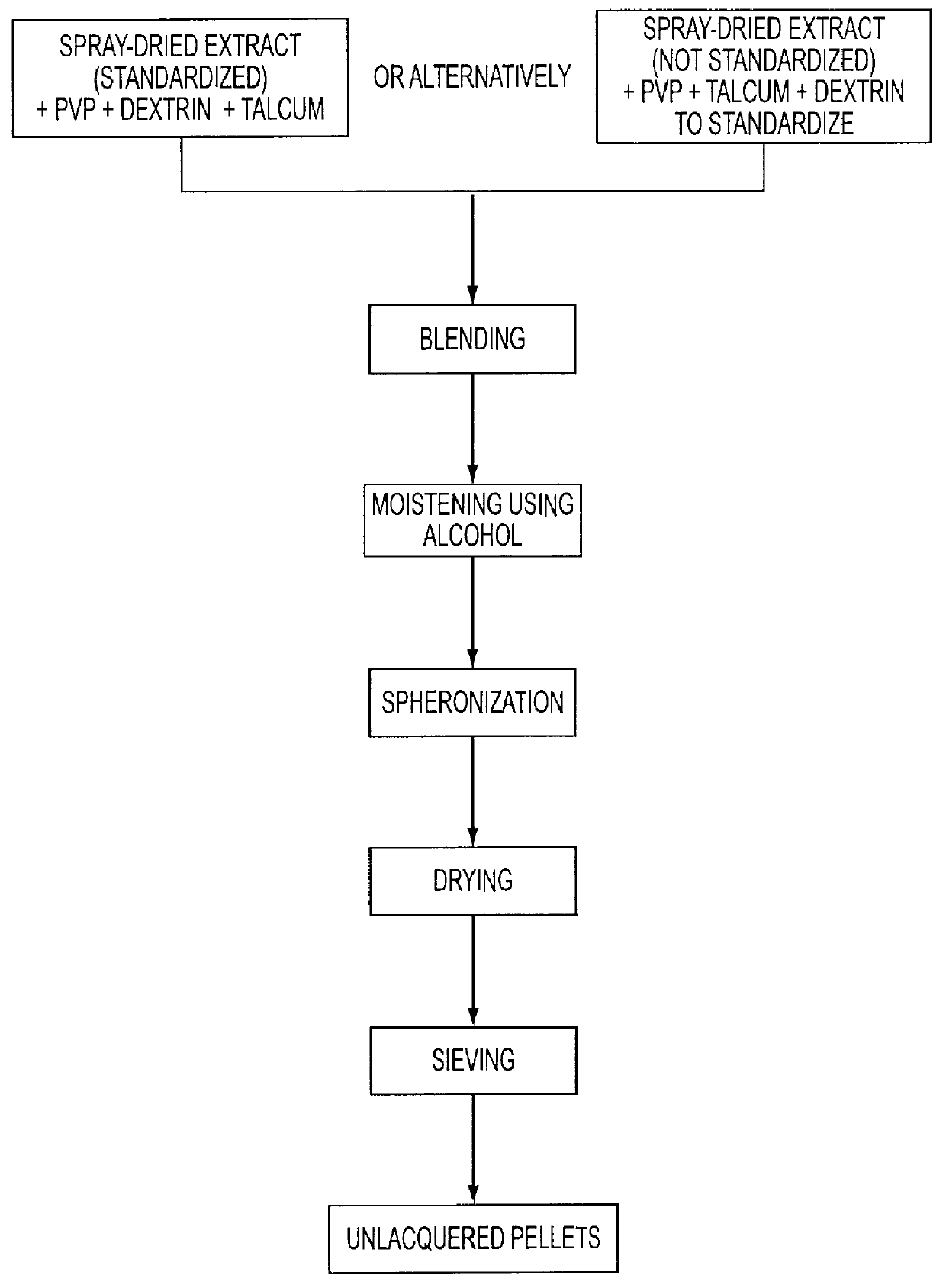
The invention relates to a medicament containing a standardized dry extract of horse chestnut seeds which is active against various types of edema and diseases of the venous circulatory system and to a process for manufacturing this medicament. The dry seed extract is processed to the form of pellets which can be coated to obtain sustained release of the agent. In this way satisfying therapeutical blood levels of the triterpene glycosides as agent are achieved. The finished medicament according to the invention can best be provided as hard gelatin capsule or matrix tablet containing the extract pellets.
Owner:TEMMLER WERKE
Multi-panax healthcare food and preparation method thereof
The invention discloses a multi-panax healthcare food and a preparation method thereof. A brain-invigorating and intelligence-developing healthcare food is made from two or more varieties of the main raw materials such as panax ginseng, panax quinquefolius, radix pseudostellariae and root of pilose asiabell, effective ingredients generated from separation and extraction of the main raw materials, and effective parts made through emendation of the main raw materials or chemical monomers as the main ingredients; the preparation method of the multi-panax healthcare food disclosed by the invention comprises the steps of separation, extraction, emendation and refinement of the main raw materials, namely panax ginseng, panax quinquefolius, radix pseudostellariae and root of pilose asiabell; and dosage forms of the product include but are not limited to pulvis, granules, tablet, hard gelatin capsule, soft gelatin capsule, drop pill, micro-pill, injection medicine, spray medicine, oral liquid and infusion solution. The product can also be researched and developed into a medicament with brain invigoration, intelligence development and memory improving functions according to the relevant regulations of China.
Owner:吉林绿波中药药业有限公司
Sustained release beadlets containing stavudine
InactiveUS7135465B2Sufficient amountPowder deliveryCosmetic preparationsBlood levelRetroviral infection
Extended dosage forms of stavudine are provided comprising beadlets formed by extrusion-spheronization and coated with a seal coating. The beadlets are also coated with a modified release coating such that a hard gelatin capsule containing such beadlets will provide blood levels of stavudine over approximately 24 hours. The beadlets are prepared from a dry blend of stavudine, a spheronizing agent, a suitable diluent and a stabilizing amount of magnesium stearate. The magnesium stearate, in contrast to other similar pharmaceutical adjuncts, has been found to stabilize stavudine against degradation due to hydrolysis in the presence of the limited amount of water necessary for the extrusion-spheronization process. Also included in the scope of the invention are hard gelatin capsules containing, in addition to the stavudine beadlets, similar beadlets containing other therapeutic agents utilized to treat retroviral infections.
Owner:BRISTOL MYERS SQUIBB CO
Room-temperature stable dronabinol formulations
ActiveUS8628796B2Improve stabilityLow oxygen permeabilityBiocideDigestive systemMedicineRoom temperature
Owner:BENUVIA OPERATIONS LLC
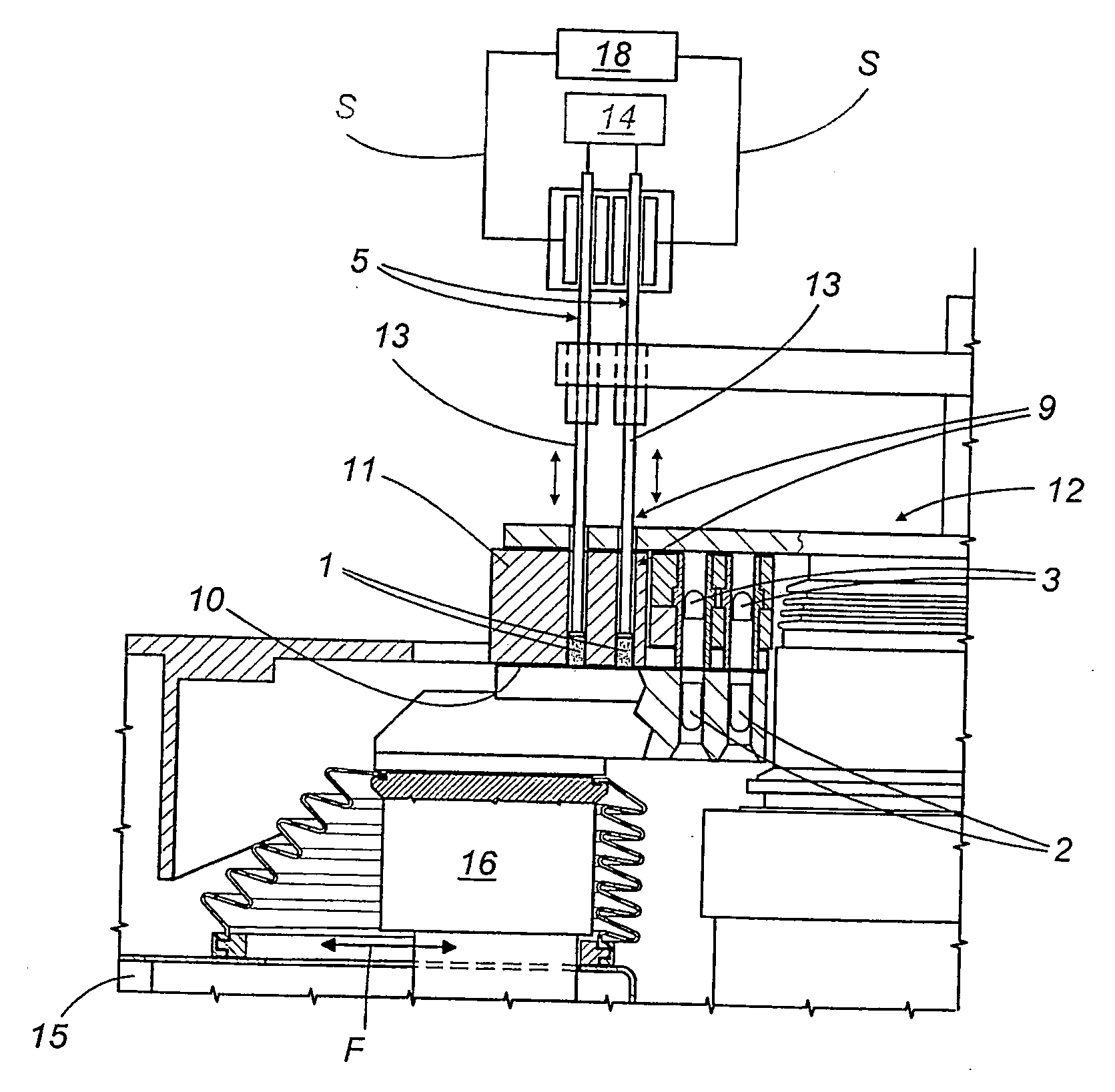
Machine for filling and sealing two-part capsules
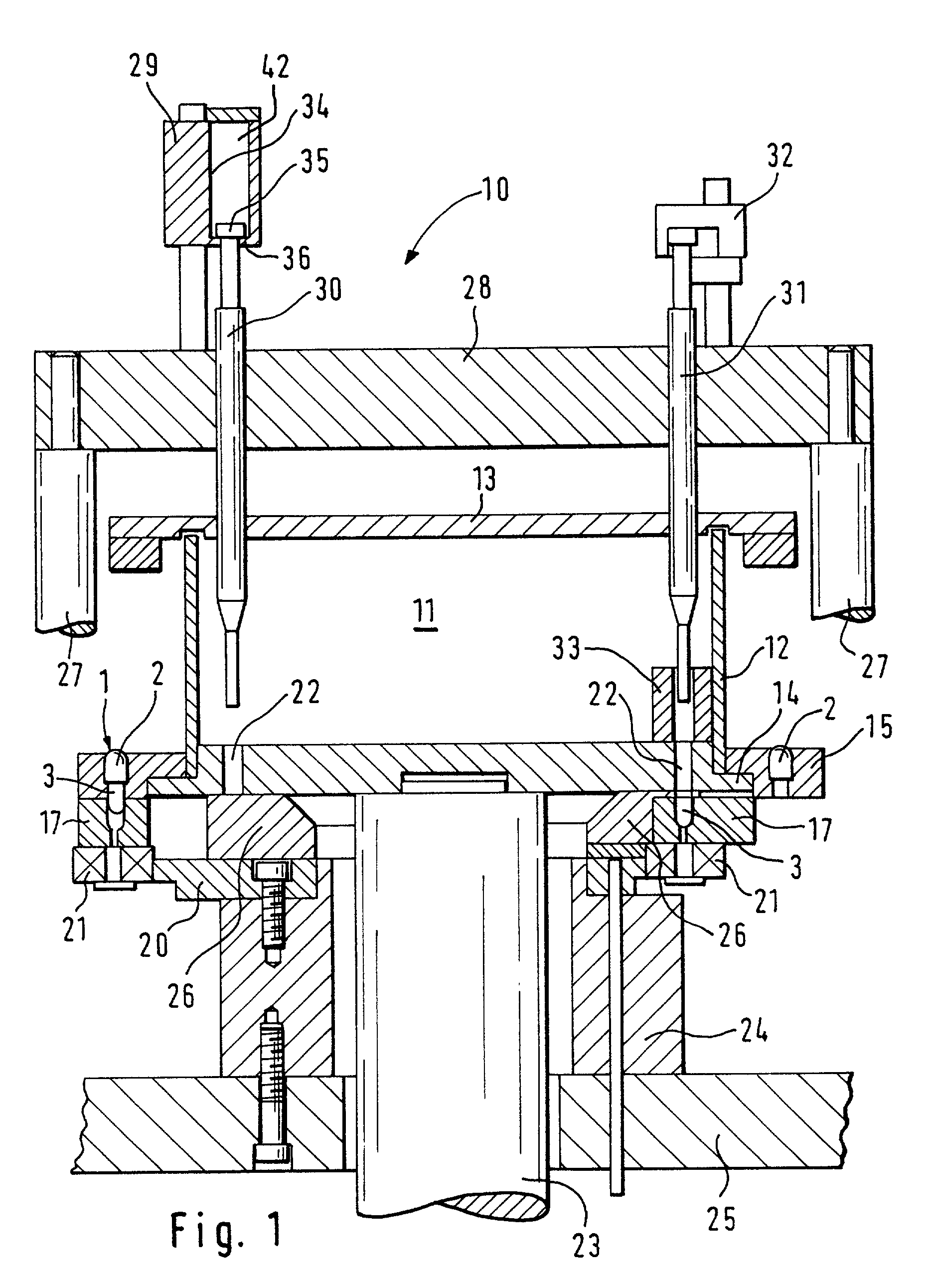
InactiveUS20070044433A1Easy to produceComplicated to manufactureSolid materialCapsule deliveryEngineeringBiomedical engineering
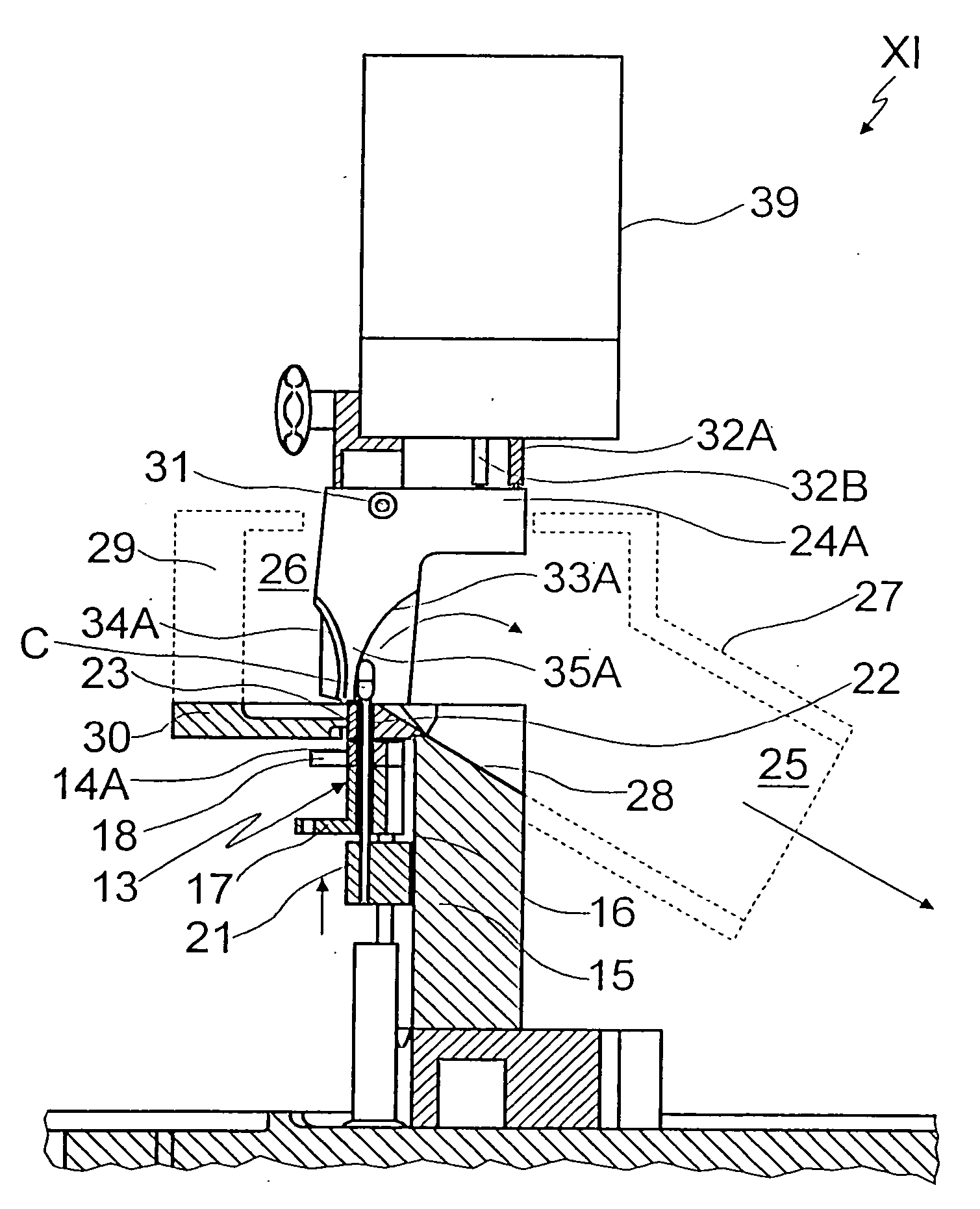
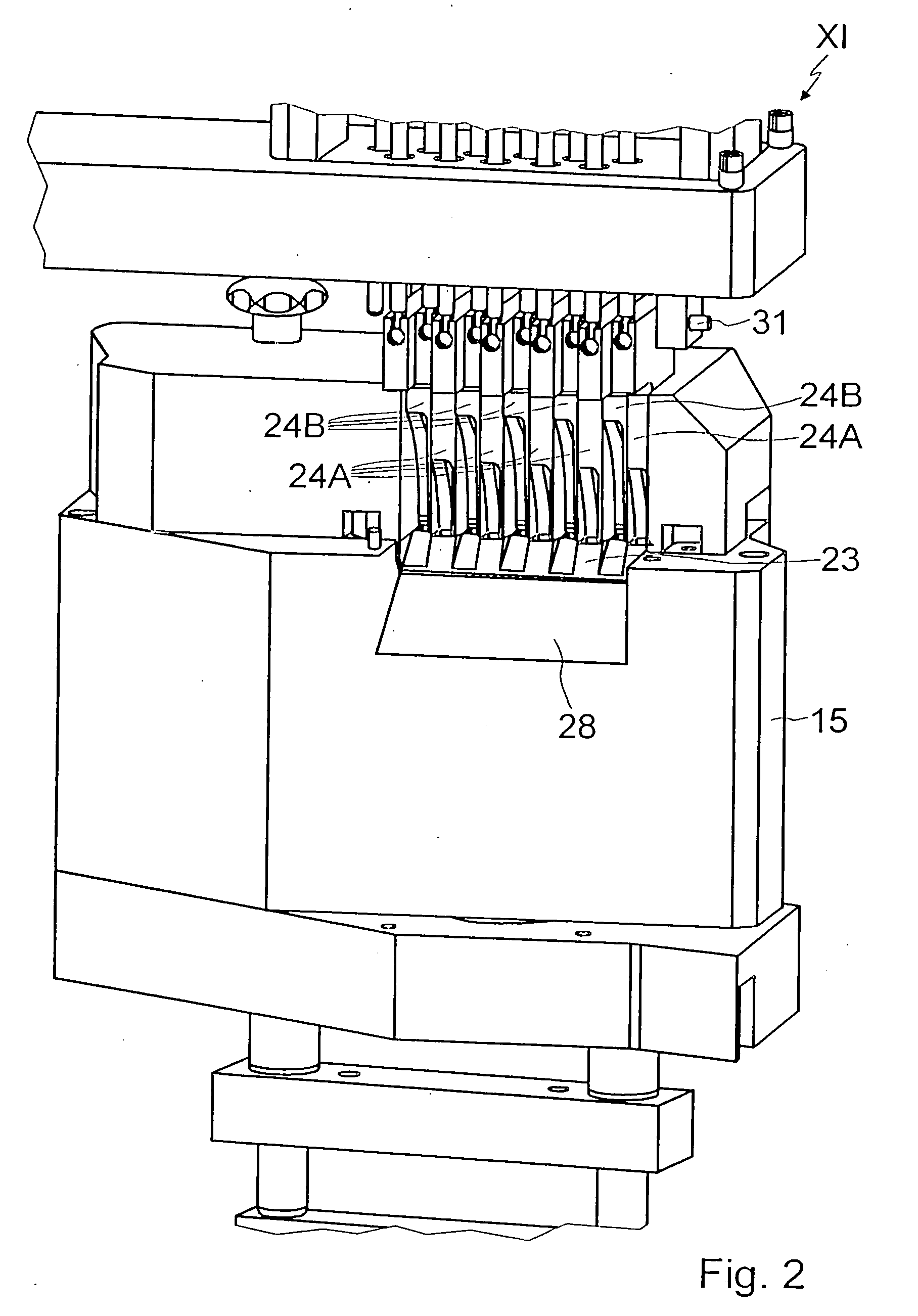
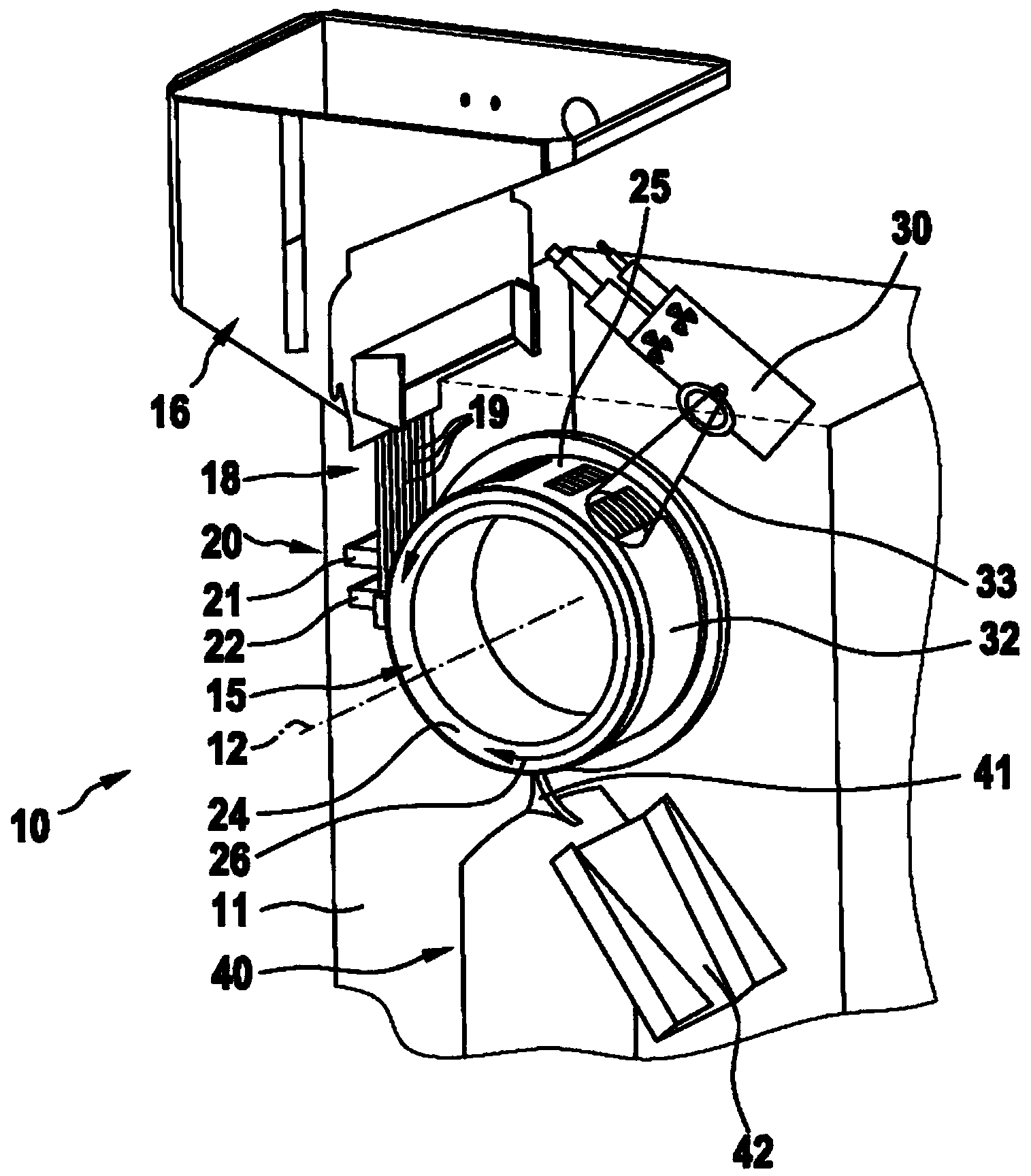
A machine for filling and sealing two-part capsules (C), in particular hard gelatin capsules, is proposed. The machine includes a capsule delivery device, which has receptacles (14A), each for one capsule (C). The further includes at least one capsule expulsion station (XI), which has a capsule expulsion device (19) for axially expelling the capsules (C) from the respective receptacle (14A); guide flaps (24A, 24B) which are individually controllable by means of an actuating device (39) and are pivotable relative to a pivot shaft (31) and are each assigned to one capsule receptacle (14A) and which each have two guideways (33A, 34A), triggerable by means of the actuating device (39), for the respective associated capsules (A); and partitions, which separate the guideways (33A, 34A) of adjacent guide flaps (24A, 24B) from one another. The partitions are each an integrated component of one guide flap (24A, 24B) (FIG. 3).
Owner:ROBERT BOSCH GMBH
Capsule filling machine
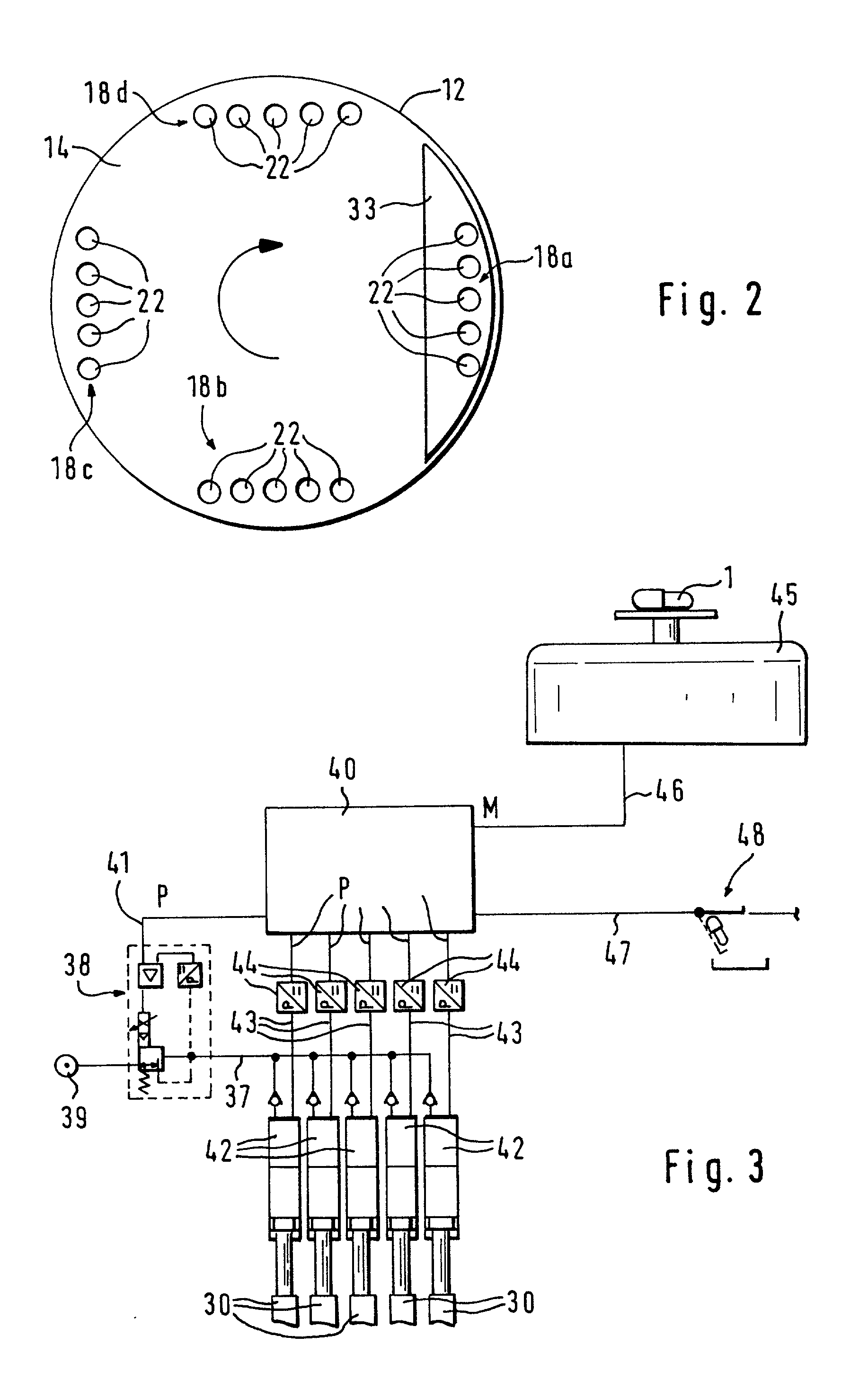
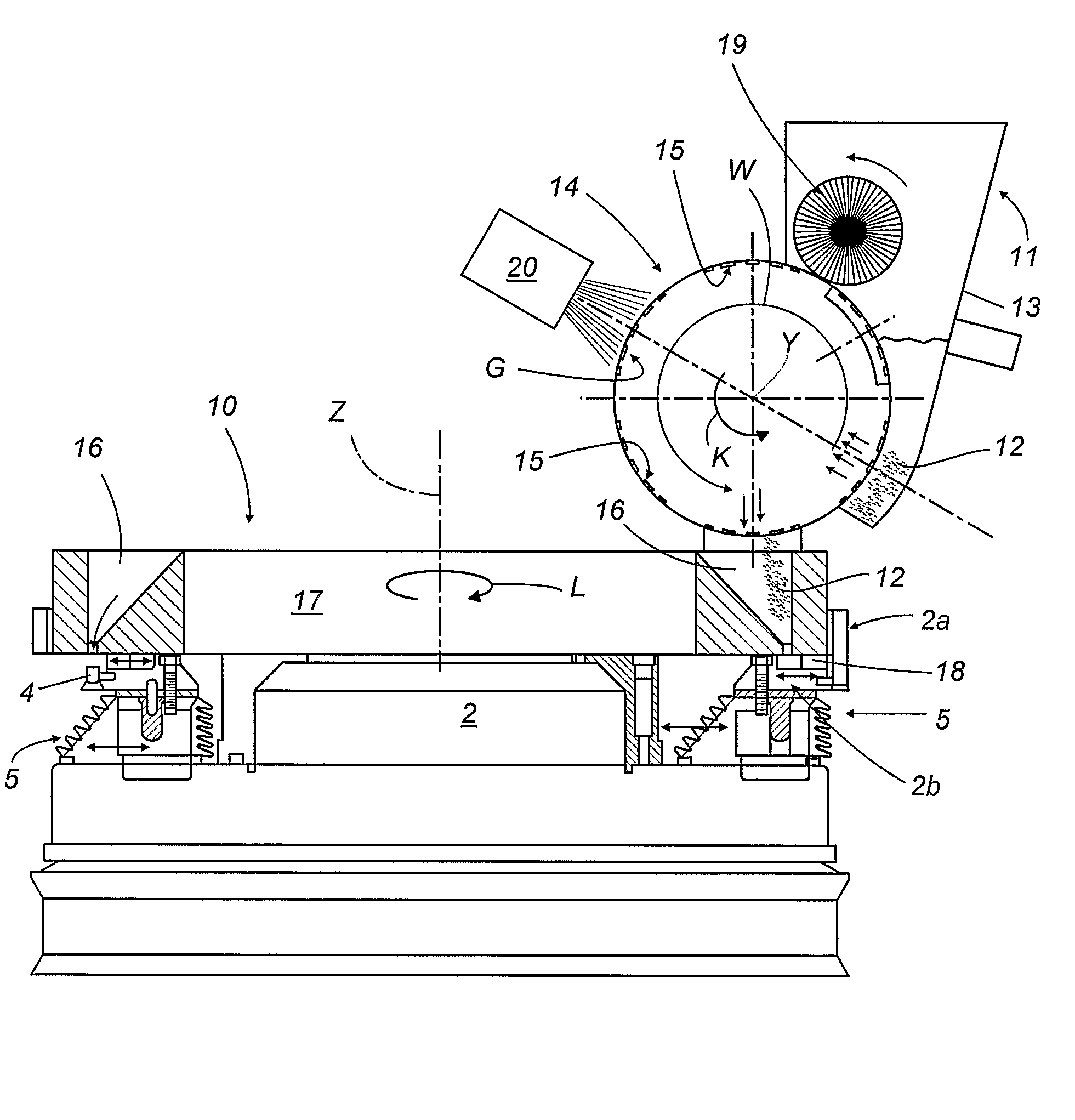
InactiveUS20060064943A1High precision dosingProduction speedCapsule deliveryPackagingMedicinePharmaceutical drug
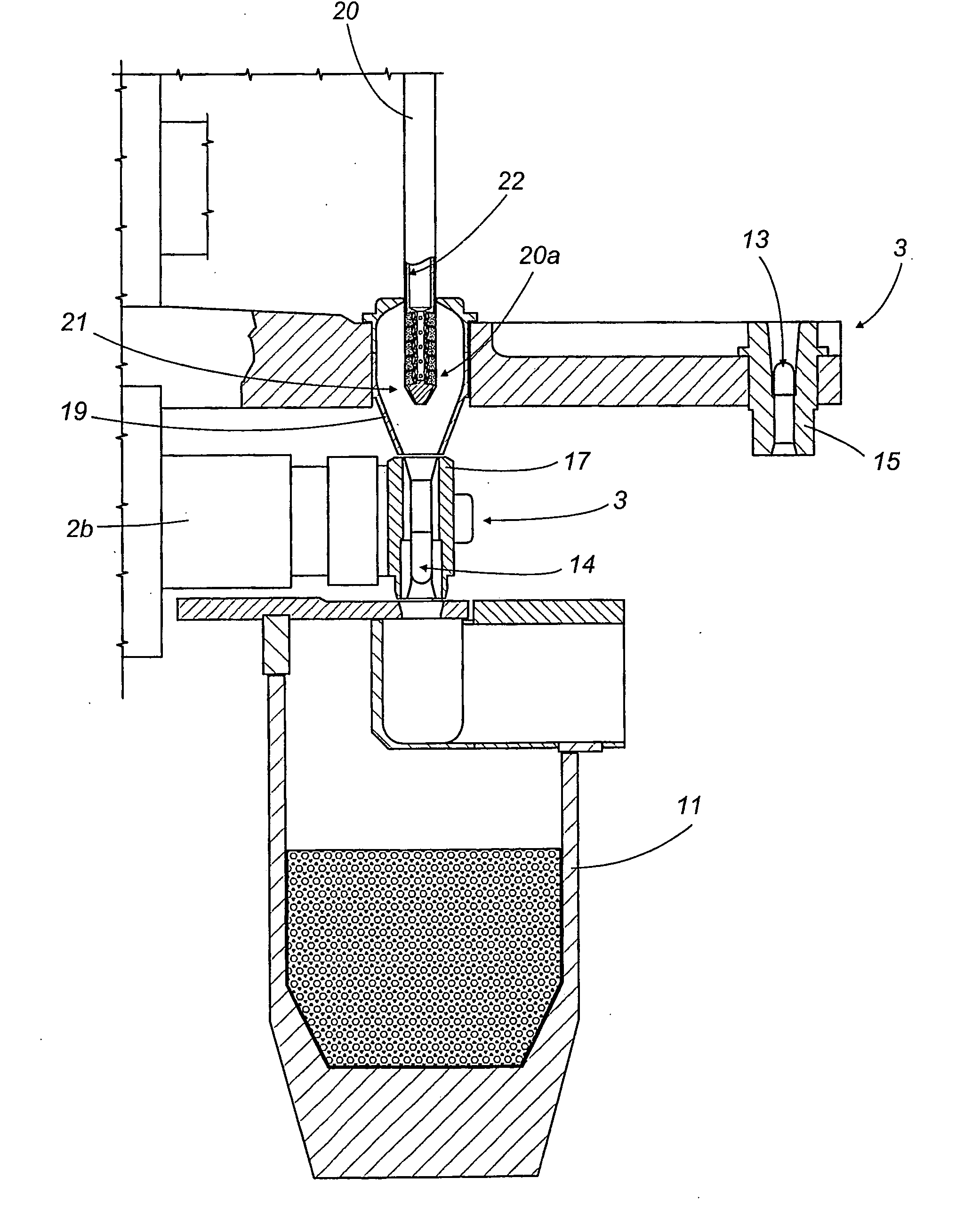
A capsule filling machine (10) for the production of hard gelatin capsules (C) of the type with a capsule lid and a capsule body (13, 14) containing pharmaceutical micro-tablets (12) comprises a first rotary carrousel (2) supporting a plurality of slide units (3) for picking up and handling capsules (C) in order to open then close the capsules (C) by separating then joining the capsule lids (13) and the capsule bodies (14); a second carrousel (4), which rotates in such a way that it is synchronized with the first carousel (2), having a plurality of reciprocating doser means (21) moving between a first operating position in which the doser means (21) are designed to pick up particulate material (12) from a tank (11) containing the material which is attached to the machine (10) and a second operating position in which they release the material into the capsule (C) bodies (14). The doser means (21) each comprise a hollow nozzle (22) with a plurality of seats (25) on its edge for picking up and holding the particulate pharmaceutical material (12), each seat (25) communicating with pneumatic means (24,24a,24b), the pneumatic means (24,24a,24b) comprising pneumatic vacuum means (24a) which, in the first operating position, suck up and hold individual particles (12) of the material in respective seats (25) of the nozzle (22), and pressurised pneumatic means (24b) which generate a flow that discharges the particles (12) from the seats (25) in the second operating position to release the material into the capsule (C) bodies (14).
Owner:IMA IND MASCH AUTOMATICHE SPA
Intermittent Motion Capsule Filling Machine
InactiveUS20080209858A1High dosing precisionGuaranteed productivityCapsDecorative coversIntermittent motionHard gelatin capsules
A capsule filling machine (10) for producing hard gelatin capsules (C) of the type with lid and body (3, 4) containing particles (12) of pharmaceutical material, in particular microtablets (12) or pellets comprises a rotary carousel (2) mounting a plurality of slide units (5) for holding and handling the capsules (C) in order to open and then close the capsules (C) by first separating and then pairing the capsule lids (3) and bodies (4); and means (11) for feeding the particles (12) to the carousel (2) for filling doses of the particles (12) into the respective capsule bodies (4); the feed means (11) comprise at least one hopper (13) containing a mass of these particles (12), and roller means (14) partly immersed in said mass of particles (12) in the hopper (13); the roller means (14) having a plurality of suction recesses (15) for accommodating and retaining a predetermined number of the particles (12) drawn from the hopper (13) and then releasing the particles (12) into a series of hollow conduits (16) mounted on the carousel (2).
Owner:IMA IND MASCH AUTOMATICHE SPA
Polyvinyl alcohol compositions
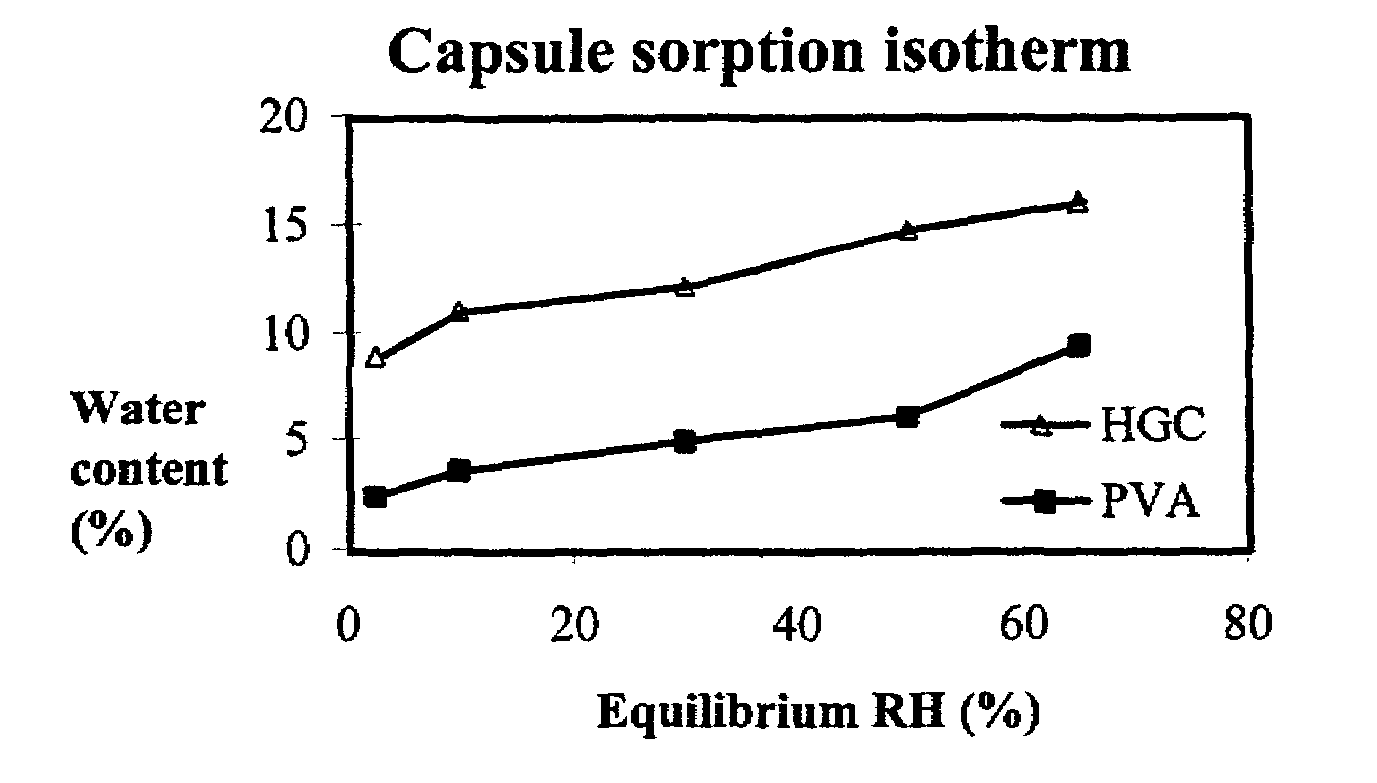
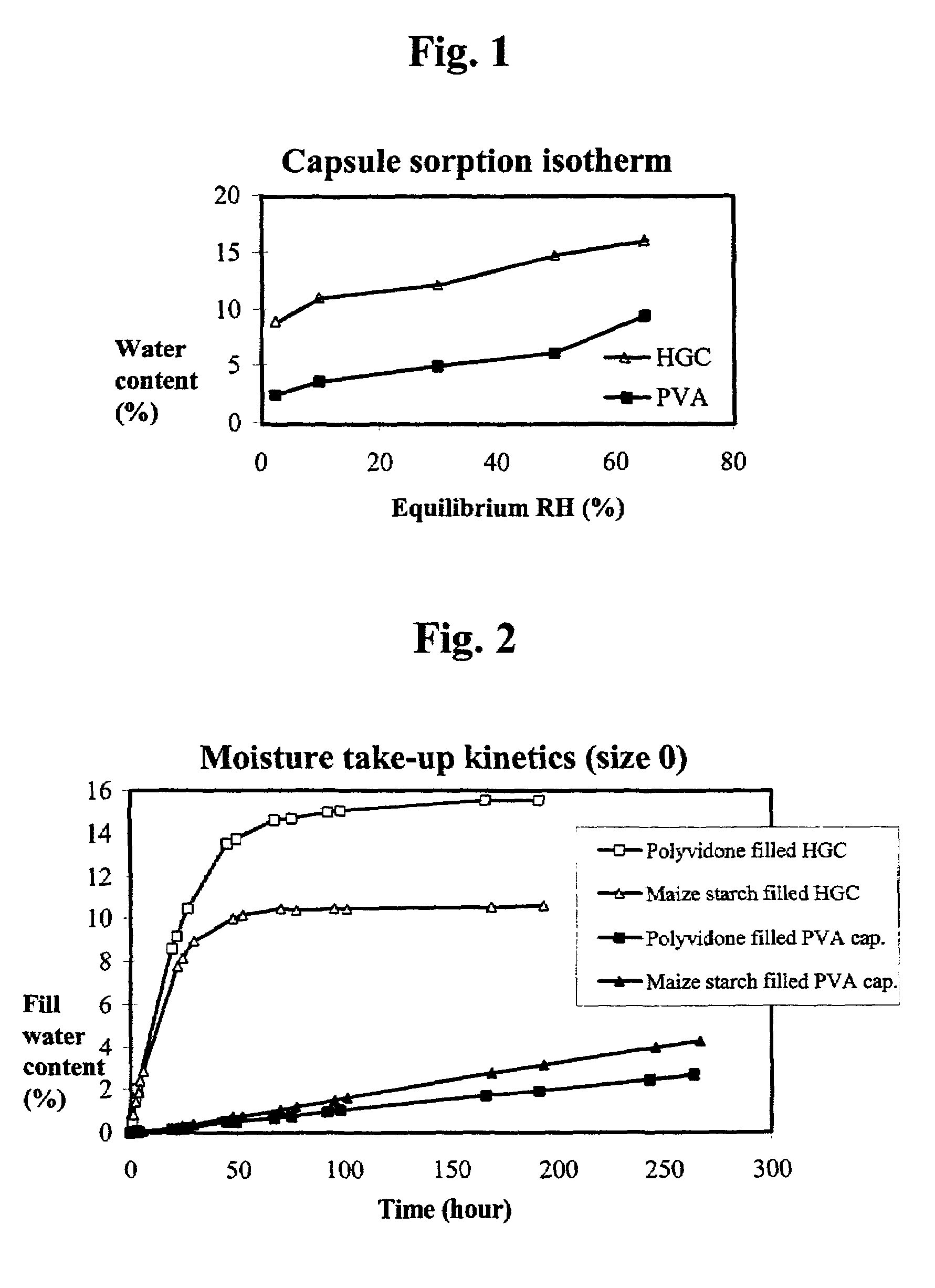
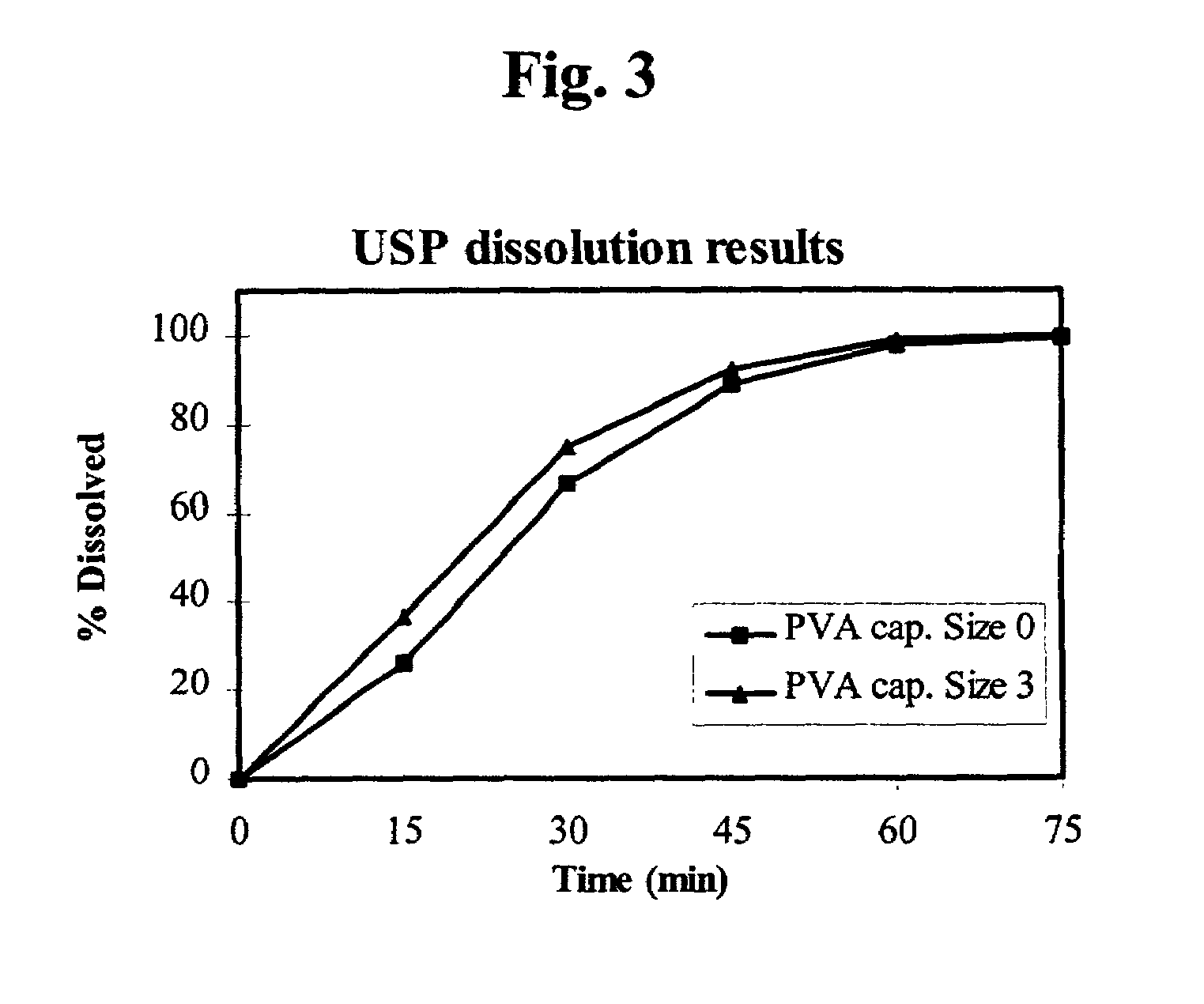
The invention concerns polyvinyl alcohol (PVA) compositions for the use in pharmaceutical, veterinary, food, cosmetic or other products like films for wrapping food, aspics or jellies, preferably for predosed formulations like soft or hard capsules. Compared with hard gelatine capsules (HGC) capsule films consisting of PVA have extremely low water vapour permeability and much lower water content.
Owner:CAPSUGEL BELGIUM NV
Extended release formulation of venlafaxine hydrochloride
InactiveUS7807195B2Facilitated releaseReduce processing timeOrganic active ingredientsNervous disorderMini tabletsPharmaceutical formulation
The present invention relates to an extended release once daily pharmaceutical formulation comprising venlafaxine hydrochloride and pharmaceutically acceptable excipients. More particularly, the present invention relates to an extended release composition in the form of mini-tablets which are incorporated in hard gelatin capsules.
Owner:ALEMBIC LTD
Compound Chinese medicine preparation of breviscapine
InactiveCN101015592AHigh degree of automationQuality controllableOrganic active ingredientsPill deliveryConvalescenceOrally disintegrating tablet
This invention relates to a Breviscapine compound Chinese medicinal preparation for treating cardio-cerebrovascular diseases. The preparation is prepared from Breviscapine, ginkgo leaf extract, Ginesenoside, schisandra propinqua extract and medicinal auxiliary materials by making into oral preparation including orally disintegrating tablet, dispersible tablet, dripping pill, soft capsule, hard gelatin capsule, and pelletized granule. Product has effects in promoting flow of qi and blood circulation, dissipating blood stasis, and smoothing collaterals, and health protection and theraupetic effect for treating cardio-cerebrovascular disease such as convalescence of apoplexy, coronary heart disease, and angina pectoris, and aneuria and senile dementia.
Owner:KPC PHARM INC
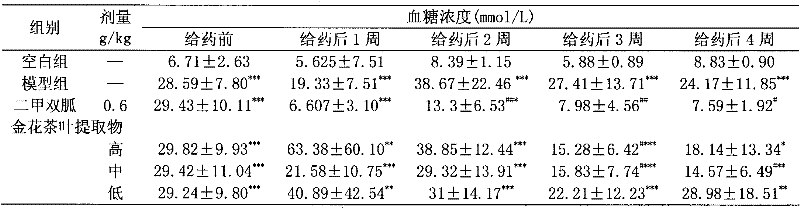
A kind of Jinhua tea capsule for auxiliary hypoglycemic and preparation method thereof
InactiveCN102293866ADefinite curative effectQuality improvementMetabolism disorderCapsule deliveryAcute hyperglycaemiaTraditional medicine
The invention relates to the activity of golden camellia tea for assisting in lowering blood sugar, the capsule of golden camellia for assisting in lowering blood sugar and a preparation method thereof. The prescription of golden camellia tea auxiliary hypoglycemic capsule is: 33%-66% of golden camellia tea extract, 67%-34% of medicinal excipients. The preparation method is as follows: taking golden camellia tea extract and medicinal auxiliary materials according to the weight percentage of the prescription, and processing them into hard capsules. Among them, golden camellia tea extract refers to the extract obtained by soaking, extracting, and concentrating the golden camellia tea leaves with an appropriate solvent; pharmaceutical excipients refer to medicinal starch or dextrin or a mixture of the two; solvent refers to water or ethanol or a mixture of both. Jinhua tea capsules have a significant hypoglycemic effect on mice with hyperglycemia induced by alloxan. The golden camellia tea capsule of the present invention and its function of assisting in lowering blood sugar. There is no report before this, and this is found by the inventor of this patent through experiments, therefore, special application for patent protection.
Owner:FANGCHENGGANG WANJING FORESTRY
Capsule Filling Machine and Method For Producing Hard Gelatin Capsules
A capsule filling machine (100) for the production of hard gelatin capsules (C) of the type with lid (3) and body (2) containing a quantity (1) of pharmaceutical material comprises a rotary turret or carousel (15) which defines at least one capsule (C) handling line (L) and on which the following are positioned, one after the other: at least one station (6) for feeding empty capsules (C); at least one opening station (20) where the capsule bodies (2) are separated from the lids (3) to form two separate rows of capsule bodies (2) and lids (3); at least one station (7) for feeding and dosing the quantities (1) of pharmaceutical material to be filled into the capsule bodies (2); and at least one station (8) for closing the capsules (C) by placing a lid (3) over each respective body (2); the machine (100) also comprises means (9) for detecting and volumetrically checking the quantity (1) of pharmaceutical material filled into each capsule body (2), the detecting and checking means (9) comprise transducer means (5) for measuring the volume of said quantities (1) before they are filled into the capsule bodies (2).
Owner:IMA IND MASCH AUTOMATICHE SPA
Device for checking pharmaceutical products, in particular hard gelatin capsules
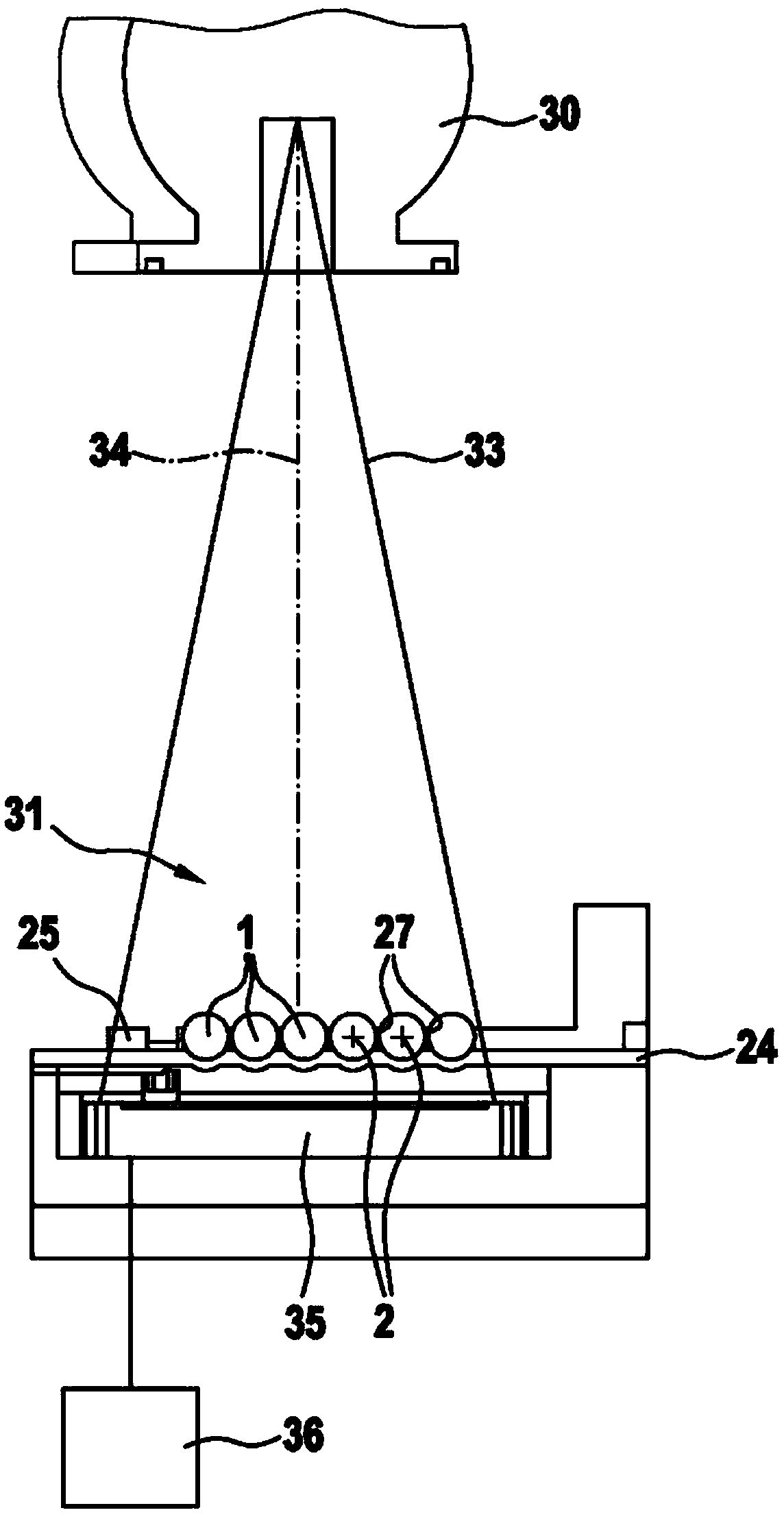
ActiveCN103459254AReduce processing costsImprove efficiencyRadiation measurementPackaging under vacuum/special atmosphereX-rayPhysics
The invention relates to a device (10; 10a; 10b; 10c; 50) for checking pharmaceutical products (1), in particular hard gelatin capsules, by means of at least one radiation source (30; 60) preferably embodied as an X-ray source, and a conveying device which conveys the products (1) in a clocked manner in a radiation area (31) of the radiation source (30; 60). The radiation emitted by the radiation source (30; 60) penetrating the products (1) preferably perpendicular to the longitudinal axes thereof (2), and the radiation is captured on the side of the products (1) opposite the radiation source (30) by means of at least one sensor element (35) which is coupled to an evaluation device (36). The invention is characterised in that the conveyor device is embodied as a conveyor wheel (15; 15a; 51) which can rotate in a stepped manner about an axis (12; 52), and the products (1) are arranged, whilst being conveyed in the radiation area (31), in receiving areas (28; 37; 56) of the conveyor wheel (15; 5a; 51).
Owner:SYNTEGON TECHNOLOGY GMBH
Prescription of liquid status of Vitamin K1 and its preparation
InactiveCN1593393ASolve the real problemDisperse fastOrganic active ingredientsDigestive systemWater solubleSolvent
The invention discloses a liquid type composition of vitamin K1 and its preparation and method for making same, wherein the composition comprises vitamin K1 and solvent, the liquid type vitamin K1 can be prepared into the dosage forms of capsule, liquid type hard gelatin capsule and drop. The preparation process is also disclosed by the invention.
Owner:SHENYANG PHARMA UNIVERSITY
Sustained release beadlets conty. stavudine
InactiveCN1420774AContinuous combination therapyPowder deliveryAntiviralsBlood levelRetroviral infection
Extended dosage forms of stavudine are provided comprising beadlets formed by extrusion-spheronization and coated with a seal coating. The beadlets are also coated with a modified release coating such that a hard gelatin capsule containing such beadlets will provide blood levels of stavudine over approximately 24 hours. The beadlets are prepared from a dry blend of stavudine, a spheronizing agent, a suitable diluent and a stabilizing amount of magnesium stearate. The magnesium stearate, in contrast to other similar pharmaceutical adjuncts, has been found to stabilize stavudine against degradation due to hydrolysis in the presence of the limited amount of water necessary for the extrusion-spheronization process. Also included in the scope of the invention are hard gelatin capsules containing, in addition to the stavudine beadlets, similar beadlets containing other therapeutic agents utilized to treat retroviral infections.
Owner:BRISTOL MYERS SQUIBB CO
Pharmaceutical hard capsule containing inorganic substance
InactiveUS20050169984A1Prevent degradationPrevent degeneration of coatingInorganic non-active ingredientsMacromolecular non-active ingredientsHard CapsuleAluminum silicate
The objective of the present invention is to improve the preservation stability of drugs filled within the hard capsule. Especially, the objective of the present invention is to improve the preservation stability of drugs filled within the hard gelatin capsule which base material is gelatin. The present invention provides a capsule which improvement is that inorganic substance is comprised within the capsule. The present invention also provides the hard capsule wherein the inorganic substance comprised therein is any one or more than one selected from the group consisting hydrated aluminum silicate, synthetic aluminum silicate, light anhydrous silicic acid, hydrated silicon dioxide and magnesium aluminometasilicate.
Owner:ASUBIO PHARMA
A liquid capsule of 'She Dan Chuan Bei' and preparation method thereof
InactiveCN1951471ALow costDisintegrates quicklyReptile material medical ingredientsCapsule deliveryPEG 400Bile Juice
Disclosed is a liquid capsule for treating wind-heat and cough and its preparing process, wherein the capsule comprises hard gelatin capsule shell and content prepared from the following raw materials (by weight portions): snake bile 44-54 parts, Sichuan fritillary bulb 265-325 parts, and polyethylene glycols 400 378-462 parts. The preparing process comprises the steps of disintegrating Sichuan fritillary bulb into fines, mixing with snake bile juice homogenously, drying, disintegrating into fines, charging right amount of polyethylene glycol 400 and water.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Hard capsules with enteric film coating
ActiveCN103417380ADelay the time of deterioration of renal functionReduce poisonDigestive systemCarbon active ingredientsDiseaseHard Capsule
The present invention provides a hard capsule with enteric film coating, which is a particular advantage of the sealing structure and the enteric film coating composition coated hard gelatin capsules, hard gelatin capsules so that the enteric film coating in the stomach and small intestine with complete shape, but may disintegrate in the large intestine and the release point of the hard capsule shell activated carbon, thereby selectively adsorbing the pro-protein small molecule compounds such as indole, indoxyl sulfate, p-cresol, hippuric acid and the like. Further, the resultant film-coated hard gelatin capsules may be enteric prevention or treatment of a liver disease or kidney disease a pharmaceutical composition.
Owner:CHENHO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com