Patents
Literature
112 results about "Soft gelatin capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
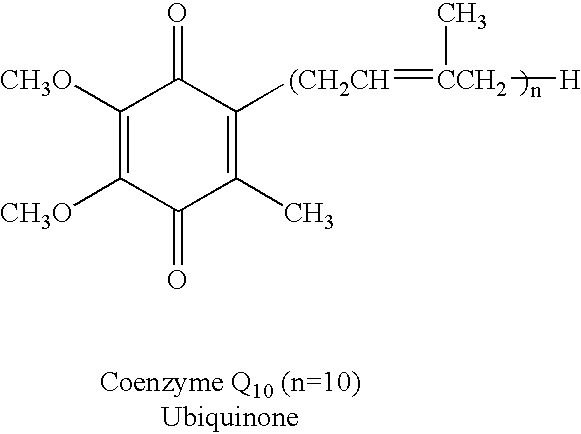
Reduced form of Coenzyme Q in high bioavailability stable oral dosage form
InactiveUS6740338B1Improve bioavailabilityReduced form requirementsBiocideEther/acetal active ingredientsOral medicationBioavailability
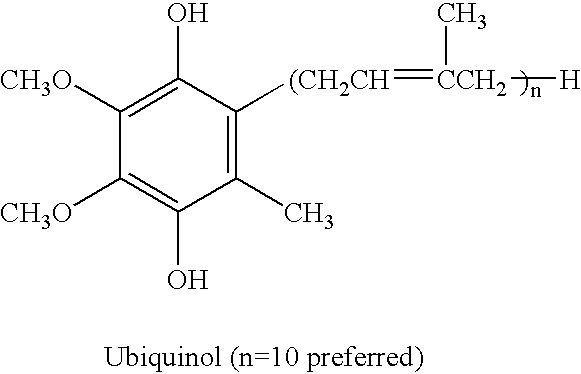
The present invention relates to a reduced form of Coenzyme Q also known as ubiquinol in oral dosage form such as a gelatin capsule, preferably a soft gelatin capsule. Compositions according to the present invention include storage stable compositions comprising effective amounts of ubiquinol in combination with an amount of a reducing agent effective to maintain ubiquinol in its reduced state when formulated in capsules, tablets and other orally administrable form. Methods of use are also disclosed.
Owner:QUTEN RES INST LLC
Non-ionic non-aqueous vehicles for topical and oral administration of carrier-complexed active agents
InactiveUS20070036843A1Control releaseLow costOrganic active ingredientsPharmaceutical non-active ingredientsActive agentNon ionic
An improved controlled release composition for non-parenteral administration of active agents and other therapeutics, particularly for oral or topical administration, has been developed. The composition is made by dispersing a complex formed of an active agent bound to an ion-exchange resin or to another form of resin or carrier, in a non-ionic non-aqueous (“NINA”) vehicle. The complexes are optionally coated with one or more layers of coating material to provide a controlled pattern of release of active agent from the carrier. Replacing the usual aqueous vehicle with a NINA vehicle, such as an oil or an ointment, allows the active agent-carrier complexes, with or without coatings, to be both orally and topically administered. The compositions can be formulated as powders, liquids, liquid suspensions, gels, capsules, soft gelatin capsules, tablets, chewable tablets, topical ointments, lotions, pourable or pumpable fluids, semisolid, crushable tablets, and unit-of-use sachets or capsules for reconstitution or direct application. The combination of multiple active agents is possible with this system, in which one or more active agents are bound to particles and one or more active agents are dissolved or dispersed in the NINA vehicle. This allows the combination of two or more active agents, which are otherwise incompatible, into a single dosage form.
Owner:COLLEGIUM PHARMA INC
Non-gelatin soft capsule system
ActiveUS20060099246A1Improve stabilityImprove solubilityBiocidePharmaceutical non-active ingredientsHigh resistanceCarrageenan
A non-gelatin encapsulation system for liquid filled soft capsules, by nature of the carrier, the cationic-ionic balance of the carrier and the active ingredients, or the concentration of the active ingredients and excipients, are difficult or impossible to commercially encapsulate in gelatin capsules. In particular, the system is adapted for the encapsulation of highly basic, or alkaline, fills. The system provides for a predominantly starch and gelling carrageenan based shell, which displays high resistance to both concentrated fills and to alkaline fills, in particular, to those fills which contain the salt or salts of weak acids and strong bases.
Owner:R P SCHERER TECH INC
Chewable soft gelatin capsules
InactiveUS20070292501A1Improve overall utilizationOrganic active ingredientsCapsule deliveryMedicineActive agent
The present invention is directed to compositions and methods of delivery of fill materials containing active agents, optionally dissolved or suspended in a suitable carrier, encapsulated in a chewable soft gelatin capsule.
Owner:SOFT GEL TECHNOLGIES
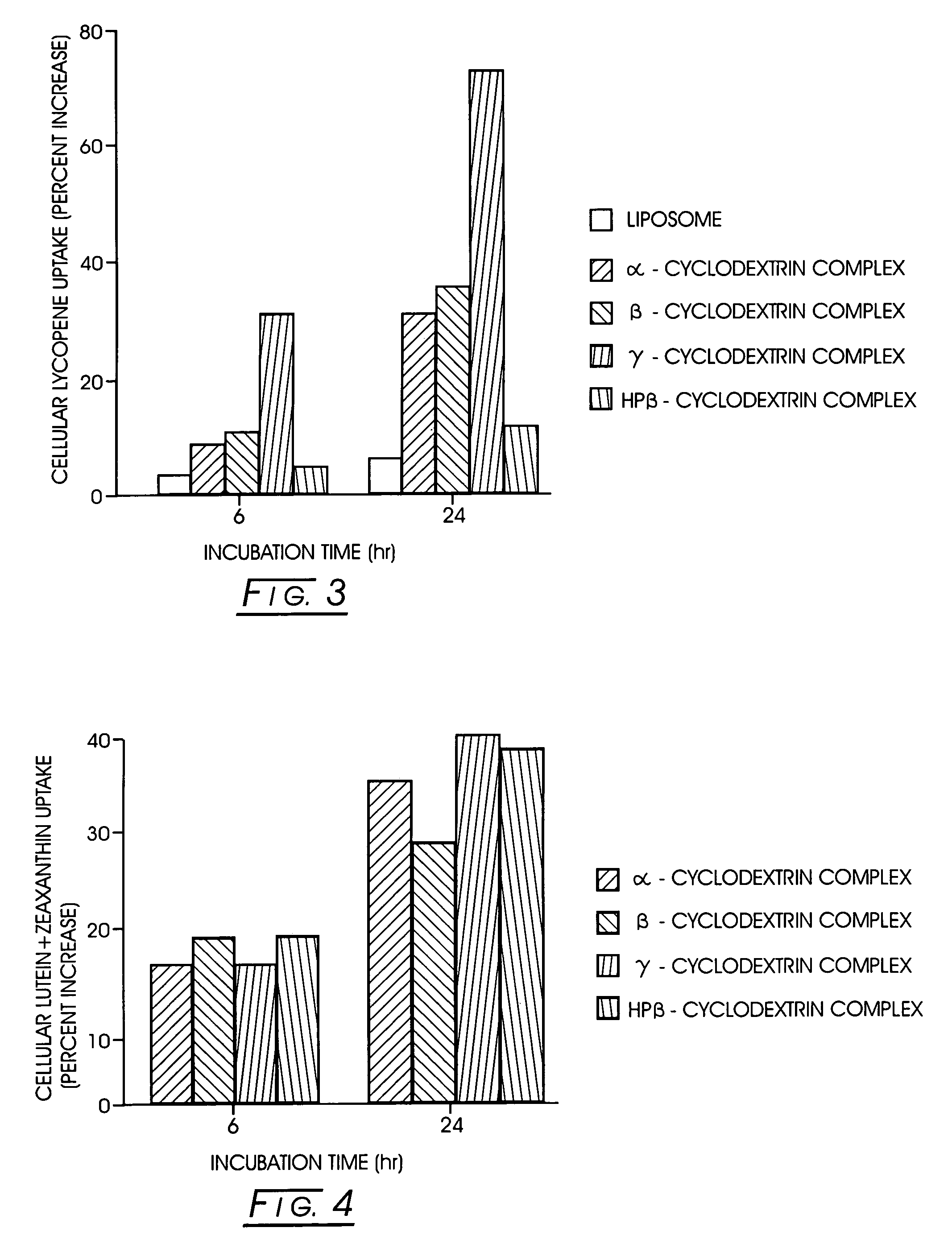
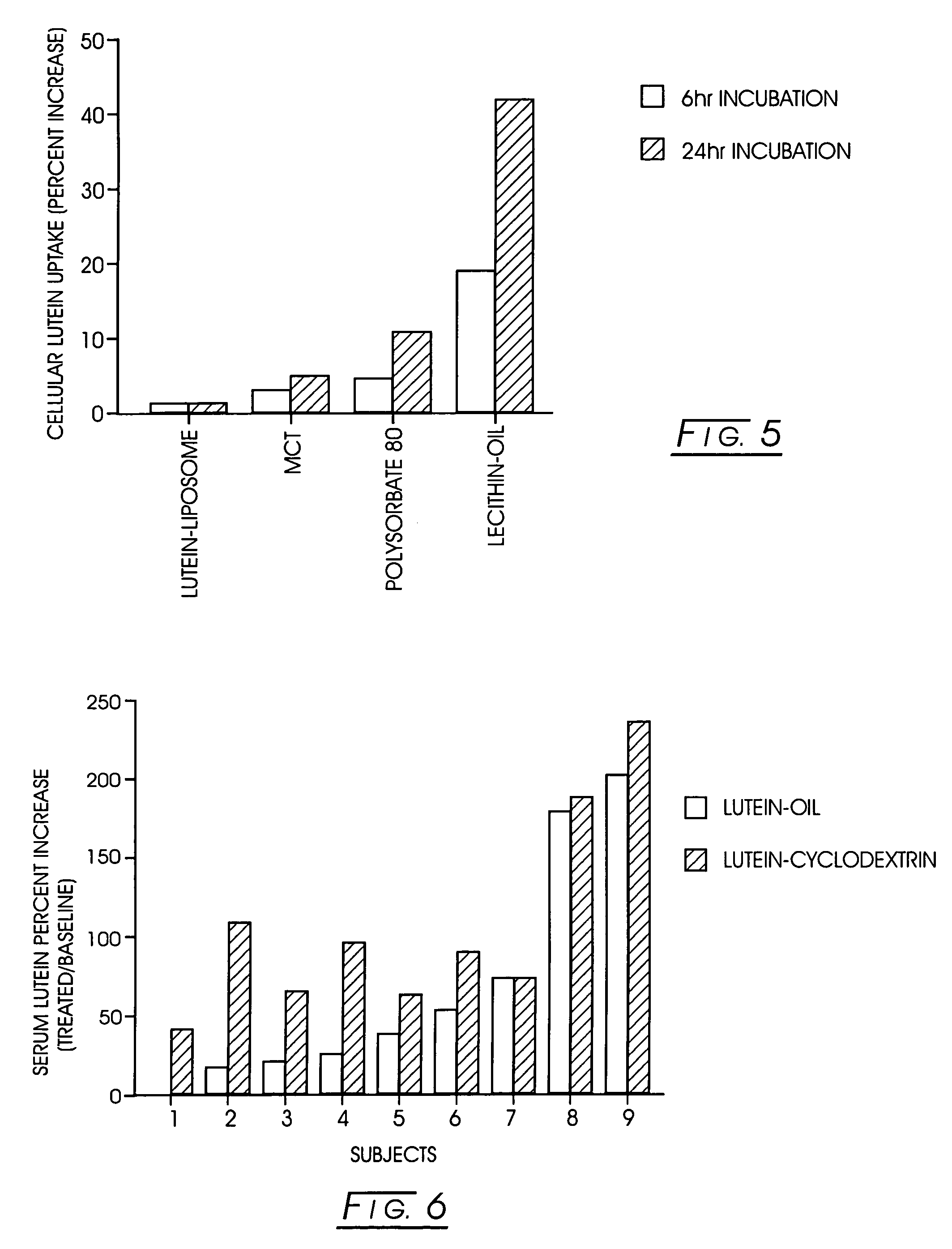
Bioavailable carotenoid-cyclodextrin formulations for soft-gels and other encapsulation systems
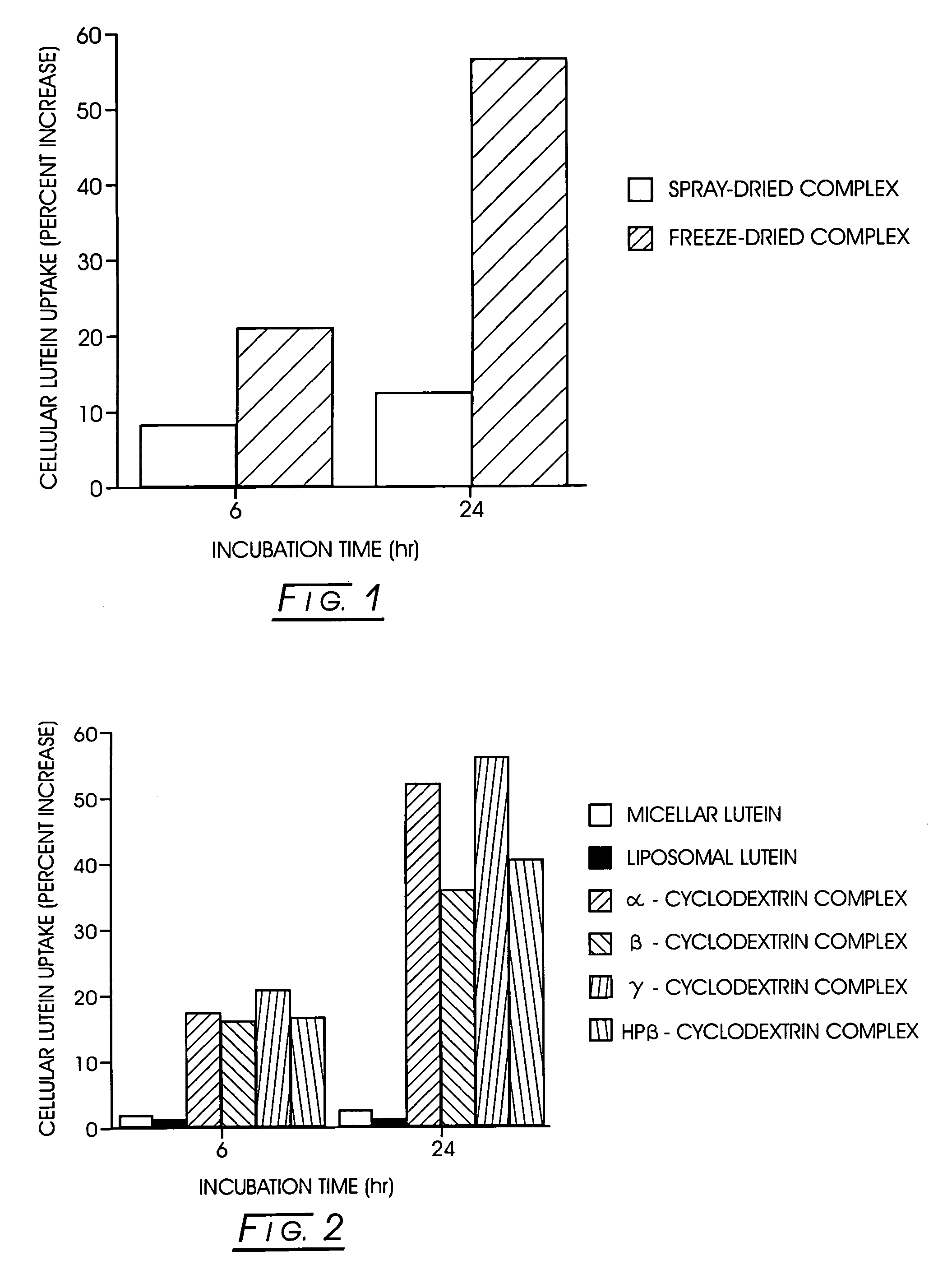
The present invention describes an improved commercial process for the production of carotenoid-cyclodextrin complexes and formulation of the complex for human ingestion. Complexation with cyclodextrins (e.g., α-cyclodextrin, β-cyclodextrin, γ-cyclodextrin, or HP-β-cyclodextrin) significantly improves the uptake of carotenoids (e.g., lycopene, lutein, or zeaxanthin) and their mixtures in vitro. The method for making such complexes includes forming a carotenoid / cyclodextrin complex; freezing drying said carotenoid / cyclodextrin complex; and blending said freeze-dried carotenoid / cyclodextrin complex with a mixture of lecithin and a vegetable oil or a vegetable oil suitable for soft gelatin capsules. The cyclodextrin / carotenoid complex can be formed in a molar ratio of between about 0.5:1.0 and 10:1. In vivo, in a human study, the lutein / γ-cyclodextrin complex formulated in lecithin-oil or oil showed a better absorption of lutein, as compared to the free lutein-oil formulation.
Owner:BIOACTIVES
Novel soft-gelatin capsule comprising S-adenosylmethionine and a method for producing the same
InactiveUS20020164369A1Easy to handleReadily availableBiocideSugar derivativesS-Adenosyl methionineGelatin film
The invention provides a novel soft gelatin capsule comprising a fill material consisting essentially of S-adenosylmethionine (SAMe) salt disposed within an enteric coated soft gelatin film.
Owner:ORCHID CHEM & PHARM LTD
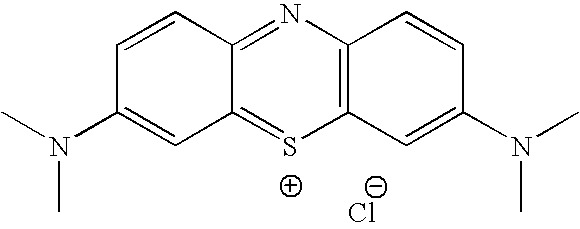
Methylene Blue Derivatives
InactiveUS20070116757A1Facilitated releaseRapid uptakeOrganic chemistryPill deliveryImmediate releaseFatty acid
Pharmaceutical compositions comprising a fatty acid salt, a dicarboxylic acid salt, an alkyl sulfate salt, an aryl sulfate salt or an alkyl aryl sulfonate salt of methylene blue or a derivative of methylene blue are described herein. The compositions are preferably administered orally and can be administered as tablets, soft or hard shell capsules (e.g. soft gelatin capsules), suspensions or solutions. The composition can also be formulated as a suppository or enema or rectal administration. The compositions further comprise a pharmaceutically acceptable carrier and optionally one or more pharmaceutically acceptable excipients. Suitable excipients include diluents, binders, plasticizers, lubricants, disintegrants, colorants, stabilizers, surfactants, and combinations thereof. The fatty acid salts, alkyl sulate salts, aryl sulfate salts or alkyl aryl sulfonate salts can be co-mixed or co-melted with one or more fatty acids to make more hydrophobic compositions, which may result in less staining formulations. The compositions can be formulated for immediate release, controlled release such as extended release, delayed release, and pulsatile release, or combinations thereof. In one embodiment, the derivative of methylene blue is methylene dodecylsulfate.
Owner:COLLEGIUM PHARMA INC
Soft gelatin capsules containing particulate material
Disclosed are suspensions suitable for encapsulation in gelatin capsules, comprising a solid phase consisting of solid particles having a mean diameter of at least about 149 mum, and a liquid phase capable of suspending the solid phase, the suspension having a predetermined rheology at a temperature suitable for encapsulation into gelatin capsules.
Owner:CATALENT USA WOODSTOCK INC +3
Non-gelatin soft capsule system
ActiveUS8231896B2Increase resistanceLarge doseBiocidePharmaceutical non-active ingredientsCarrageenanSoftgel
A non-gelatin encapsulation system for liquid filled soft capsules, by nature of the carrier, the cationic-ionic balance of the carrier and the active ingredients, or the concentration of the active ingredients and excipients, are difficult or impossible to commercially encapsulate in gelatin capsules. In particular, the system is adapted for the encapsulation of highly basic, or alkaline, fills. The system provides for a predominantly starch and gelling carrageenan based shell, which displays high resistance to both concentrated fills and to alkaline fills, in particular, to those fills which contain the salt or salts of weak acids and strong bases.
Owner:R P SCHERER TECH INC
Method for preparing alginate soft capsule
ActiveCN101564667AAvoid eccentricityShrinkage is smallPharmaceutical non-active ingredientsCapsule deliveryLiquid stateOil water
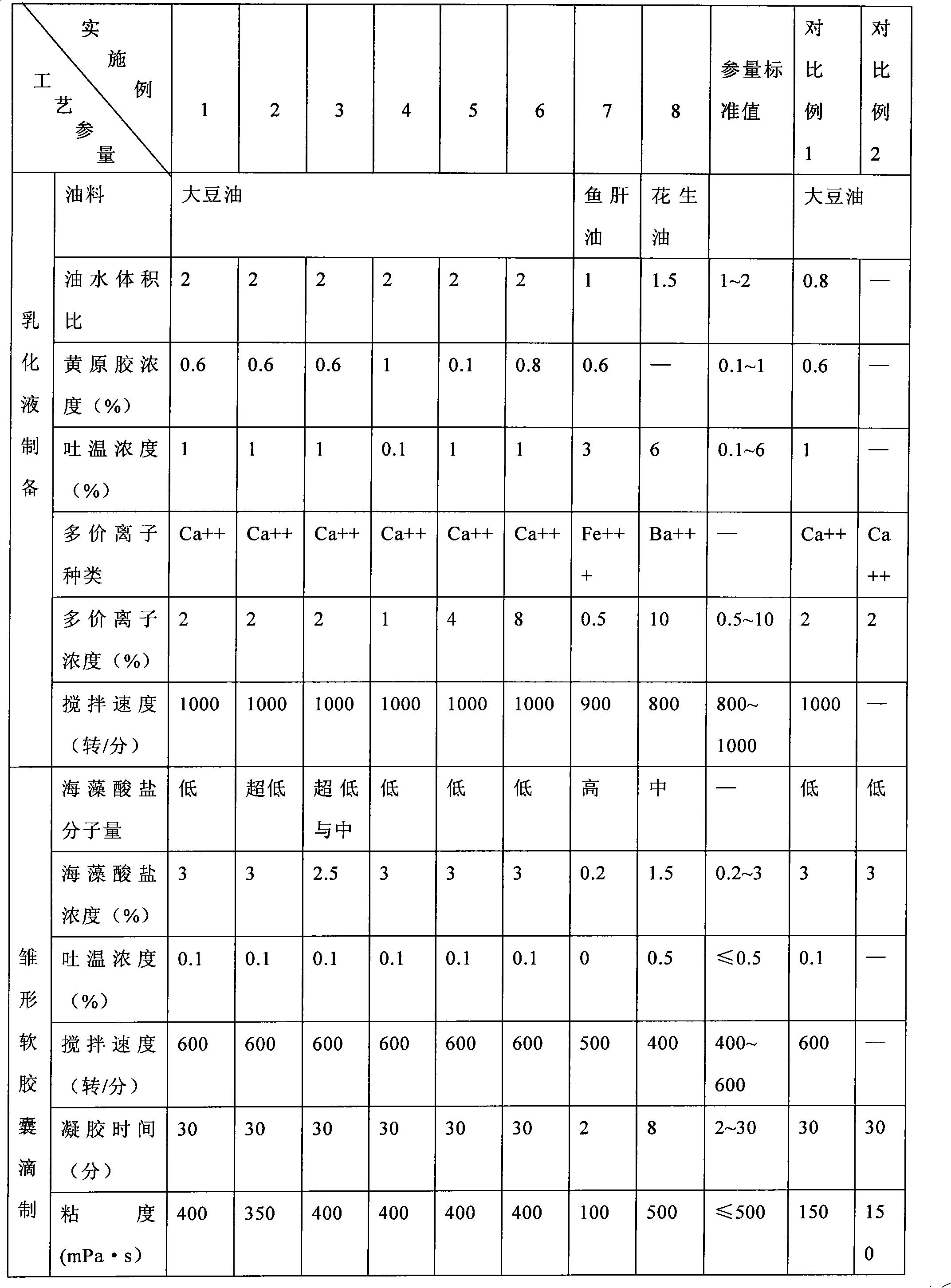
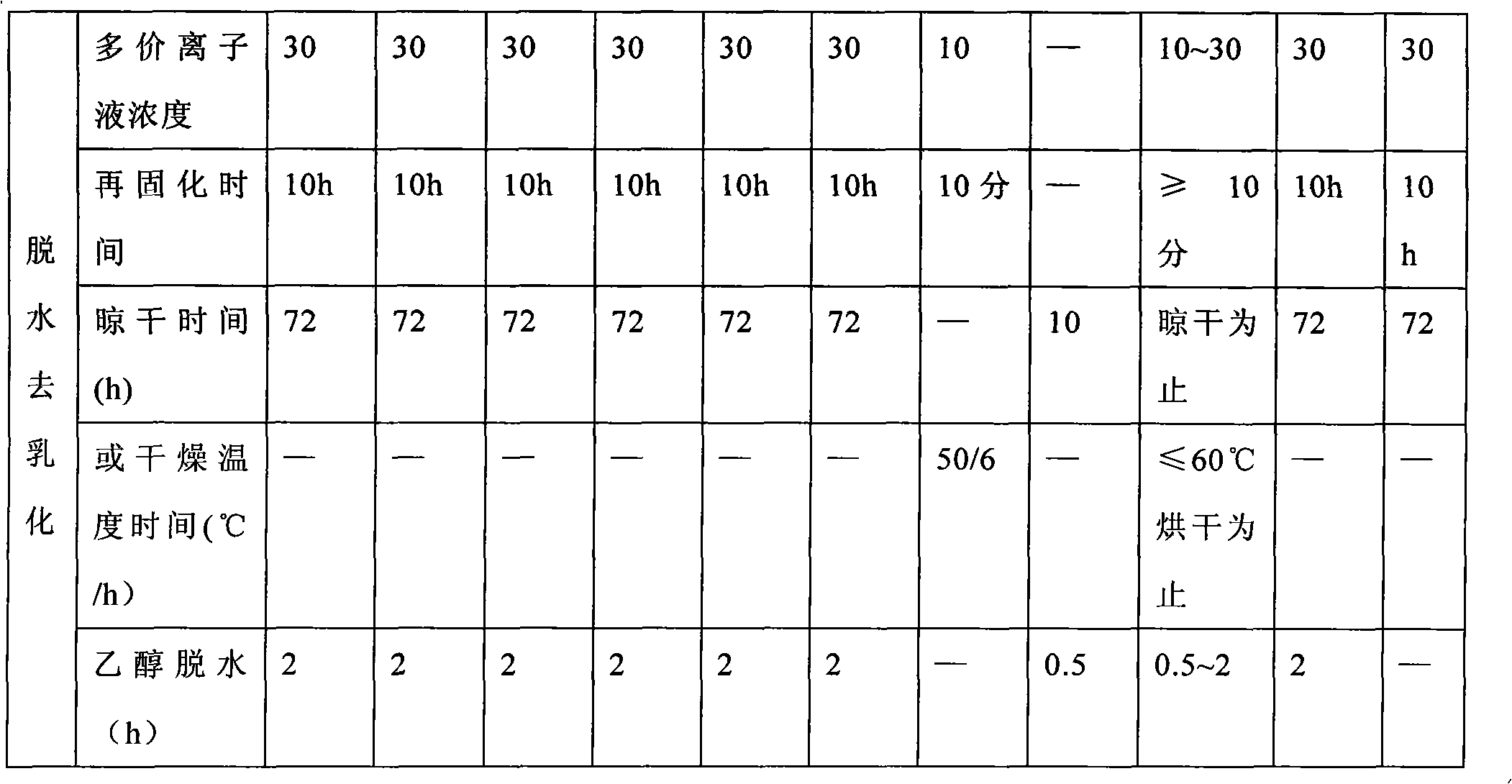
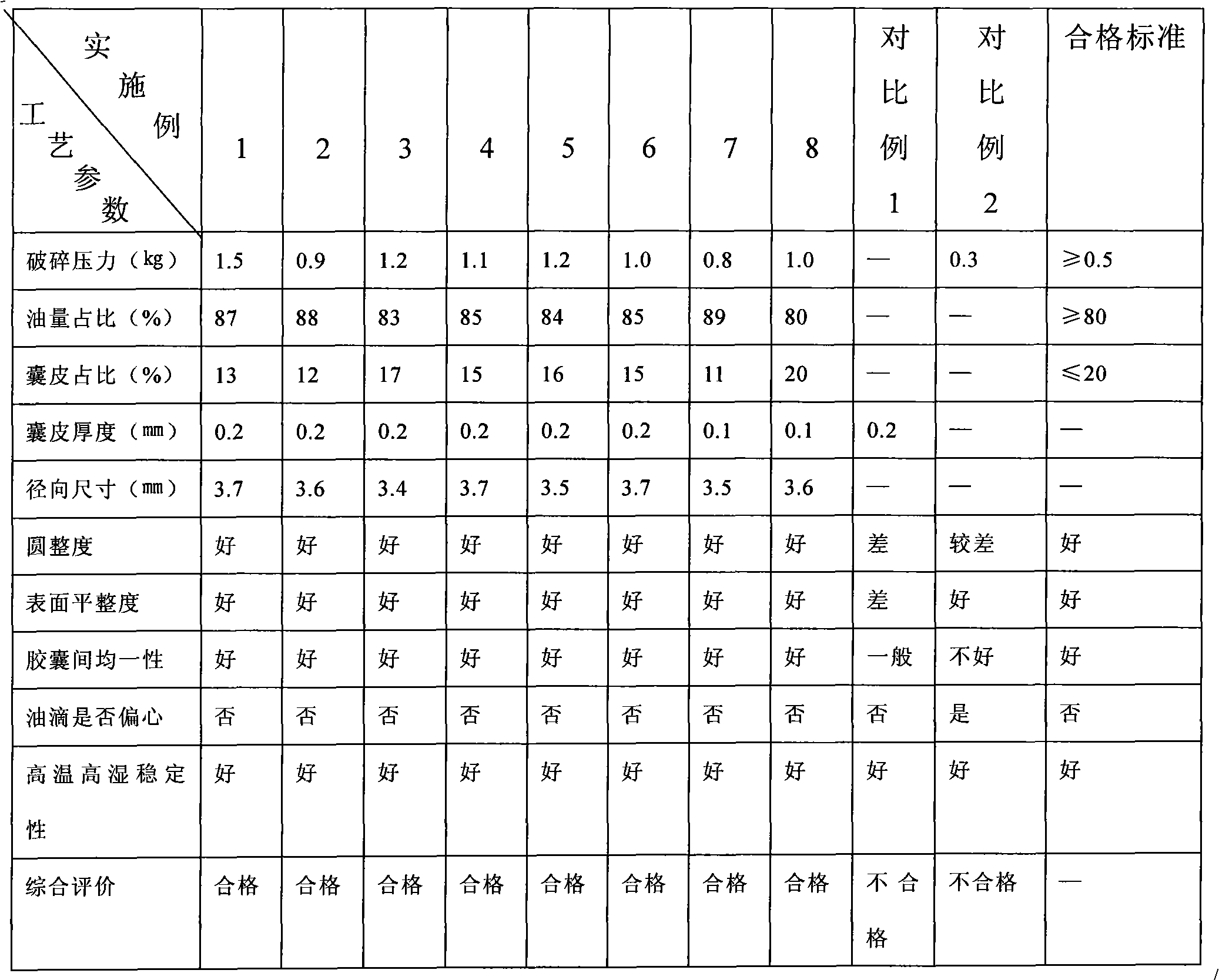
The invention relates to a method for preparing alginate soft capsule. By adopting a mode of emulsifying / de-emulsifying and emulsion dropping, oil-in-water emulsion containing polyvalent metal ions with high oil-water ratio is dropped into univalent soluble alginate solution, and then the polyvalent metal ions react with the univalent alginate solution to form insoluble alginate gel so as to obtain a primary soft capsule form of which outer layer is wrapped with the alginate gel; then, the primary soft capsule form is subjected to steps of drying and dehydrating so that the emulsion inside the soft capsule is de-emulsified; and finally, a circular soft capsule is obtained, wherein the interior of the circular soft capsule has oily liquid substances, the exterior of the circular soft capsule has an alginate gel wrapping layer, the radial size of the circular soft capsule is in millimeter scale, and the circular soft capsule can be directly taken orally. The soft capsule obtained by the method has the advantages of good mechanical property, even and round appearance, non-eccentric oil drops and high oil content; and the preparation method is simple and has low cost. Under the condition that the internal oily liquid substances are the same, the soft capsule which is prepared by the method and can be directly taken orally can be used for replacing gelatin soft capsules which are directly taken orally.
Owner:南京健辉生物科技有限公司
Film forming composition for producing soft starch material capsule and preparation method of film forming composition
ActiveCN103893771AComponent simplificationReduce interactionCosmetic preparationsToilet preparationsPolymer chemistryDirect production
The invention relates to a film forming composition for producing a soft starch material capsule and a preparation method of the film forming composition, and the soft starch material capsule prepared from the film forming composition. The composition is formed by hydroxypropyl starch and water which are in a certain proportion, wherein optionally, proper auxiliary materials such as an opacifying agent, a colorant and a flavouring agent which are used for improving the performance of the soft capsule can be added. The preparation method comprises the following steps: mixing the materials according to a certain sequence; and controllably heating the materials in a temperature rising manner so as to obtain the film forming composition. The film forming composition can be directly produced so as to obtain the soft starch material capsule by indiscriminately using a production method of the traditional soft gelatin capsule.
Owner:HUNAN ER KANG PHARMA
Personal lubricant compositions and kits for providing personal lubrication
This invention relates to body orifice moisturizing compositions and kits and methods of their use. More particularly, it relates to vaginal moisturizing compositions and kits and methods of their use. The vaginal moisturizing compositions of this invention are preferably in the form of a soft gelatin capsule containing therein a lubricating fluid, said soft gelatin capsule being capable of insertion into a body orifice. It will provide sustained lubrication to the body orifice.
Owner:LIN SHUN Y +2
Stable solutions of sparingly soluble actives
InactiveUS20110020440A1Stable pharmaceutical compositionComposition is stableBiocideAmide active ingredientsDepressantCombinatorial chemistry
The present invention relates to a stable pharmaceutical composition comprising soft gelatin capsules containing at least one sparingly soluble active drug (singly or in combination with sparingly soluble and / or soluble drugs) and a solvent system, wherein the solvent system comprises of solvent, co-solvent, solubilizer(s), surfactant, aqueous solution of alkali and crystal growth inhibitor. The present invention further relates to process for preparing a stable pharmaceutical composition of sparingly soluble active drug(s) in soft gelatin capsules.
Owner:CADILA PHARMA
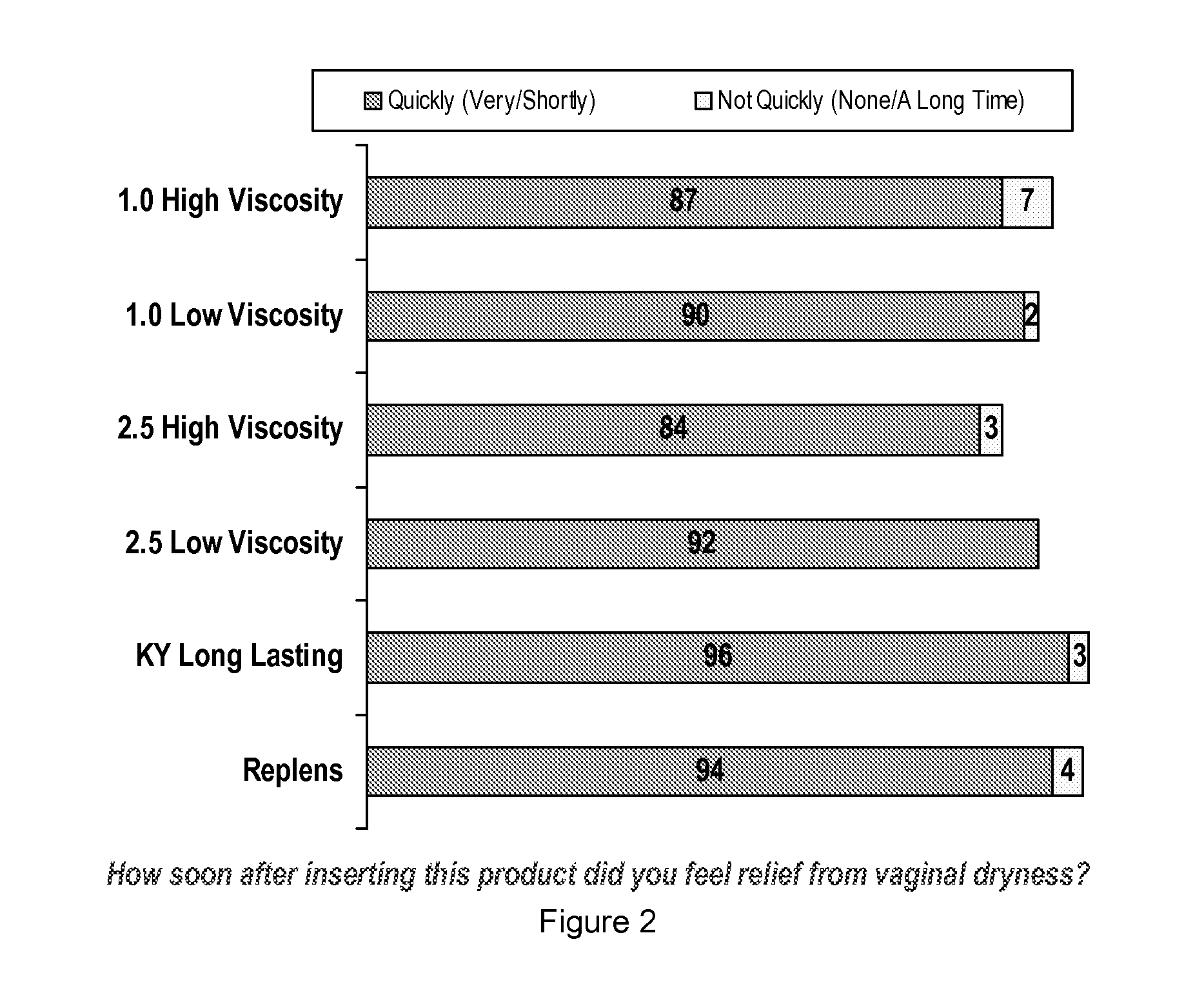
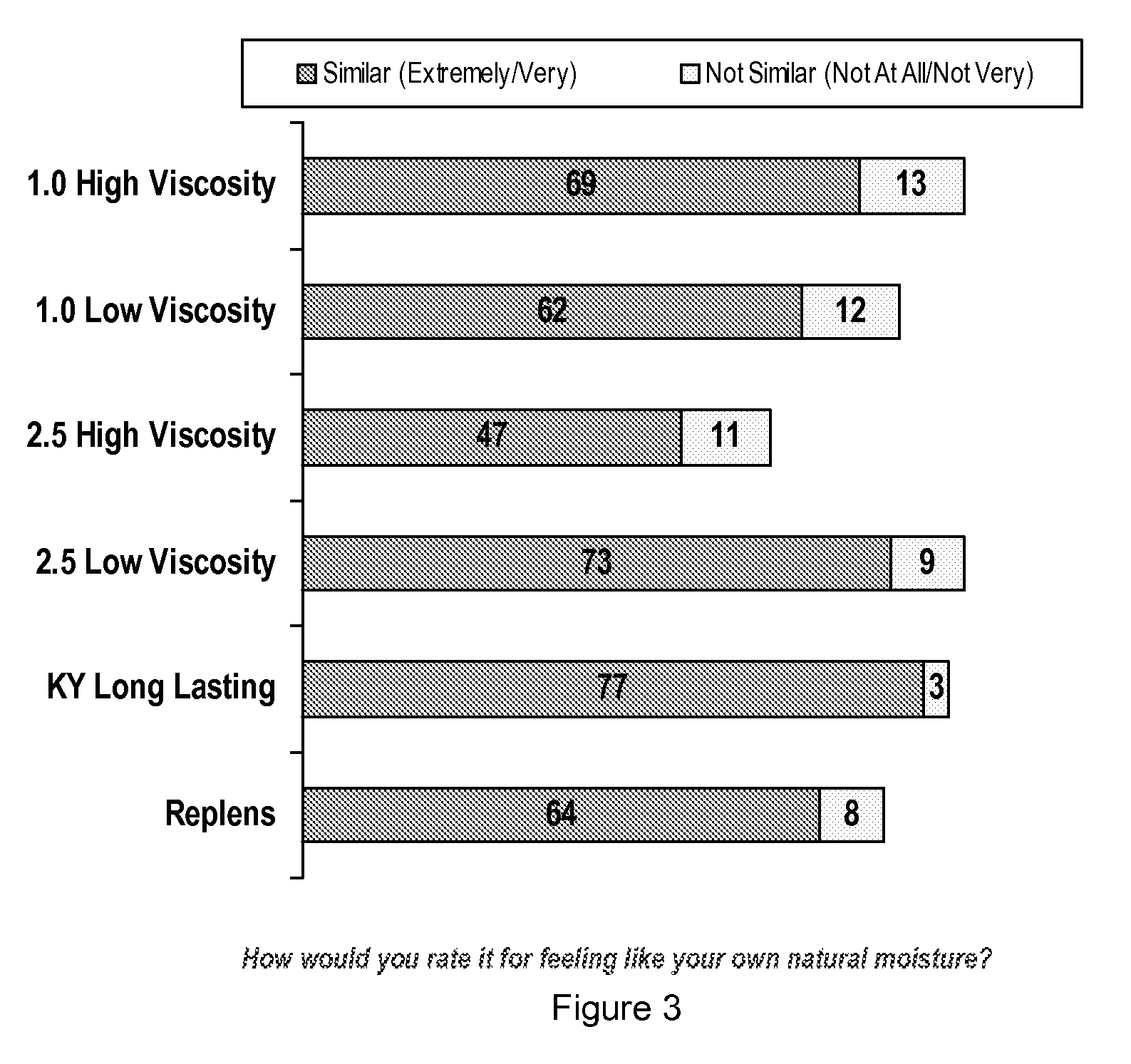
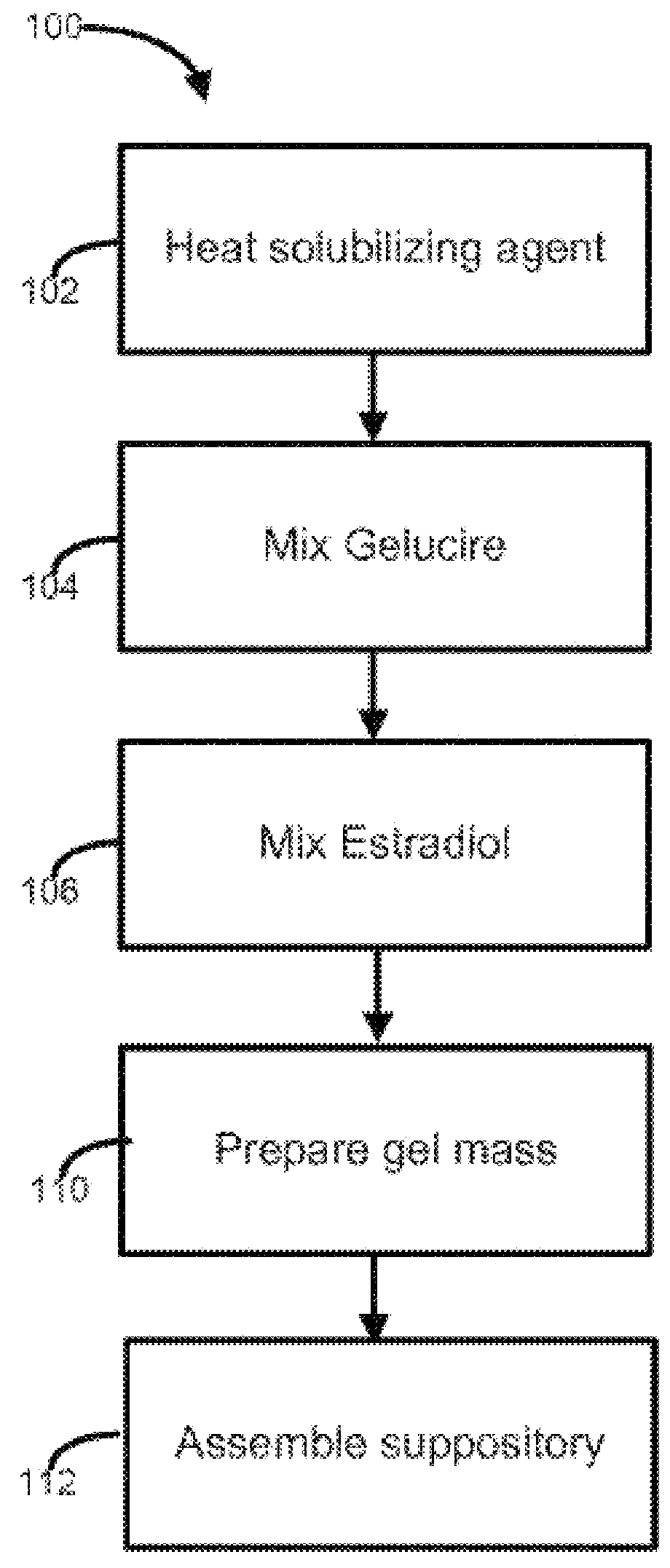
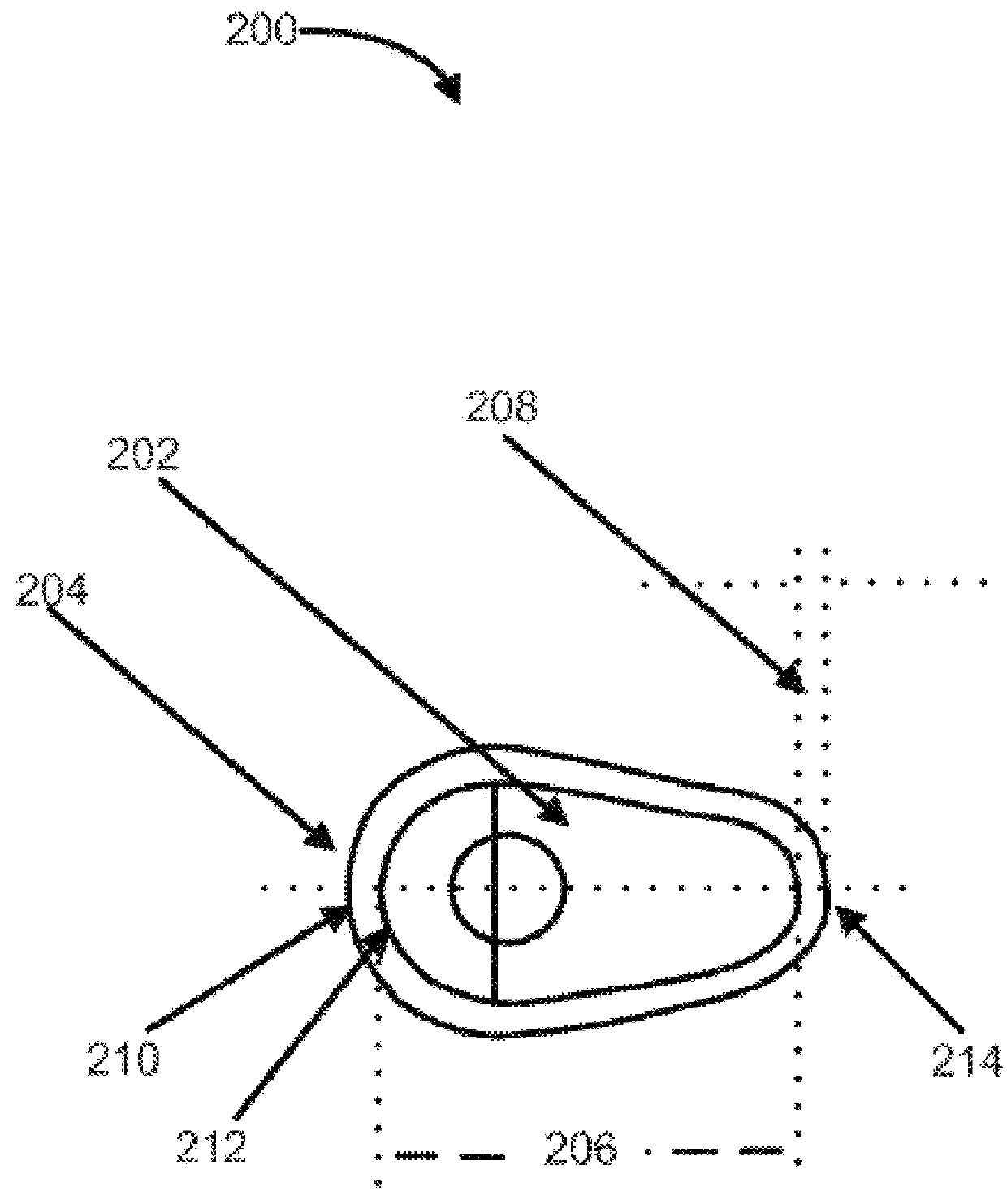
Vaginal inserted estradiol pharmaceutical compositions and methods
ActiveUS20180161345A1Reduce in quantityIncrease the number ofOrganic active ingredientsPharmaceutical delivery mechanismVaginal atrophyVagina
In one aspect, pharmaceutical compositions and methods for the treatment of vulvovaginal atrophy (VVA) are provided. In one embodiment, the method comprises digitally inserting into the lower third of the vagina of a subject having VVA a soft gelatin capsule containing a liquid pharmaceutical composition.
Owner:THERAPEUTICSMD
High Concentration Self-Microemulsifying Coenzyme Q10 Preparations For Nutritional Use
InactiveUS20090060891A1Promote dissolutionPeptide/protein ingredientsMetabolism disorderHigh concentrationPolyethylene glycol
A method and composition are presented for enhancing the dissolution and bioavailable properties of CoQ10 nutritional supplements and / or therapeutic agents for a human being and other mammals. The method includes preparing an anhydrous self-microemulsifying base composition by combining: CoQ10, a water-immiscible, and a non-ionic surfactant, containing polyethylene glycol. For an orally administered CoQ10 nutritional supplement in a capsule formulation, a unit dosage from the composition is added to a dissolvable capsule, preferably a soft gelatin capsule, in order to form the nutritional supplement. When a capsule containing the self-microemulsifying composition enters the digestive tract, the temperature of the body's digestive juices warms the composition, causing any of the CoQ10 that may have re-crystallized out of the composition to become re-dissolved into the composition before the capsule dissolves. The re-dissolution of CoQ10 is bioavailable when the capsule dissolves. Upon dissolution of the capsule, the self-microemulsifying composition comes into contact with the digestive juices and naturally forms micellar-type bioavaible microemulsions, consisting of micelles containing CoQ10. In addition to the capsule formulation, the invention includes parenteral, liquid, topical and ophthalmic formulations.
Owner:BIOAVAILABILITY INC
Soft-gelatin capsule comprising S-adenosylmethionine and a method for producing the same
InactiveUS6759395B2Easy to swallowGood optionBiocideSugar derivativesS-Adenosyl methionineGelatin film
The invention provides a novel soft gelatin capsule comprising a fill material consisting essentially of S-adenosylmethionine (SAMe) salt disposed within an enteric coated soft gelatin film.
Owner:ORCHID CHEM & PHARM LTD
Gelatine enteric capsule shell material
InactiveCN101461792AOvercome the secondary dipping molding preparationCapsule deliveryMacromolecular non-active ingredientsProduction lineOrganic solvent
The invention provides a gelatin enteric-coated capsule shell material, which contains the following components by weight portion: (A) 10 to 25 weight portions of gelatin; (B) 10 to 30 weight portions of enteric coating material; (C) 13 to 30 weight portions of water; and (D) 0.01 to 20 weight portions of pH regulator, wherein the material basically does not contain an organic solvent. The invention provides the enteric-coated capsule shell material which has good performance, does not need the organic solvent, and is also suitable for producing on the prior hard and soft gelatin capsule production line.
Owner:吴国庆
Preparation method of soft capsule without gelatin
InactiveCN102670563AImprove the level of science and technologyKeep healthyPharmaceutical non-active ingredientsCapsule deliveryGellan gumPullulan
A preparation method of a soft capsule without gelatin belongs to the technical filed of processing of soft capsules. The preparation method is that pullulan taken as major ingredient is respectively mixed with xanthan gum, carrageenan or gellan gum and other microbial polysaccharide gum or seaweed polysaccharide gum, the mixture is subjected to such procedures as sol, degassing, drooling and drying to form a gum skin film, and the gum skin film is pressed through a soft capsule pressing machine via a film rotating method to form the soft capsule. According to the invention, the soft capsule only adopts the pullulan, xanthan gum or carrageenan and other microbial polysaccharide gum or seaweed polysaccharide gum as the raw materials, and does not use the gelatin, adopts two processes of drooling to form the film and soft capsule pressing through the film rotating method; and meanwhile, during pressing, any oil-soluble drugs or health care food can be installed, so that the soft capsule without gelatin can be made.
Owner:JIANGNAN UNIV
Enteric valproic acid
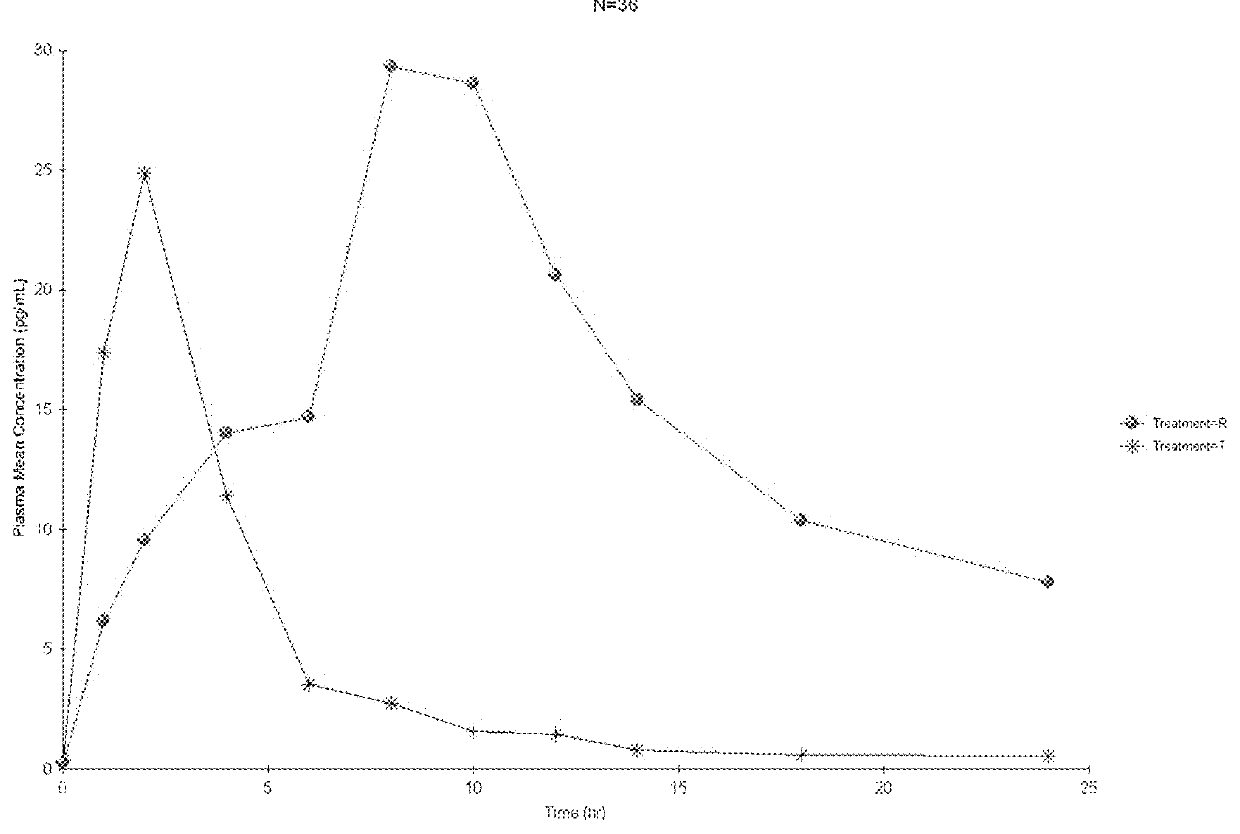
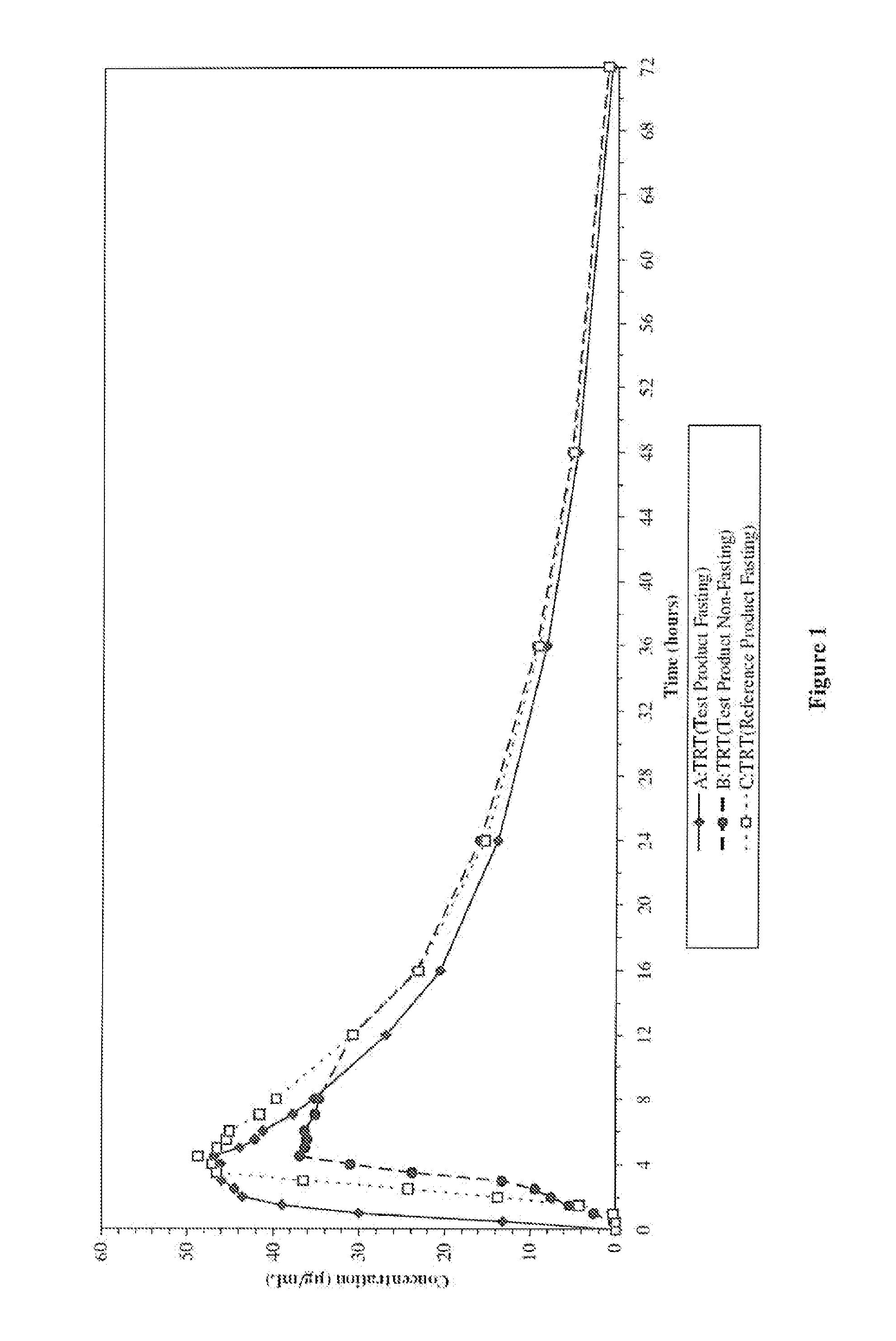
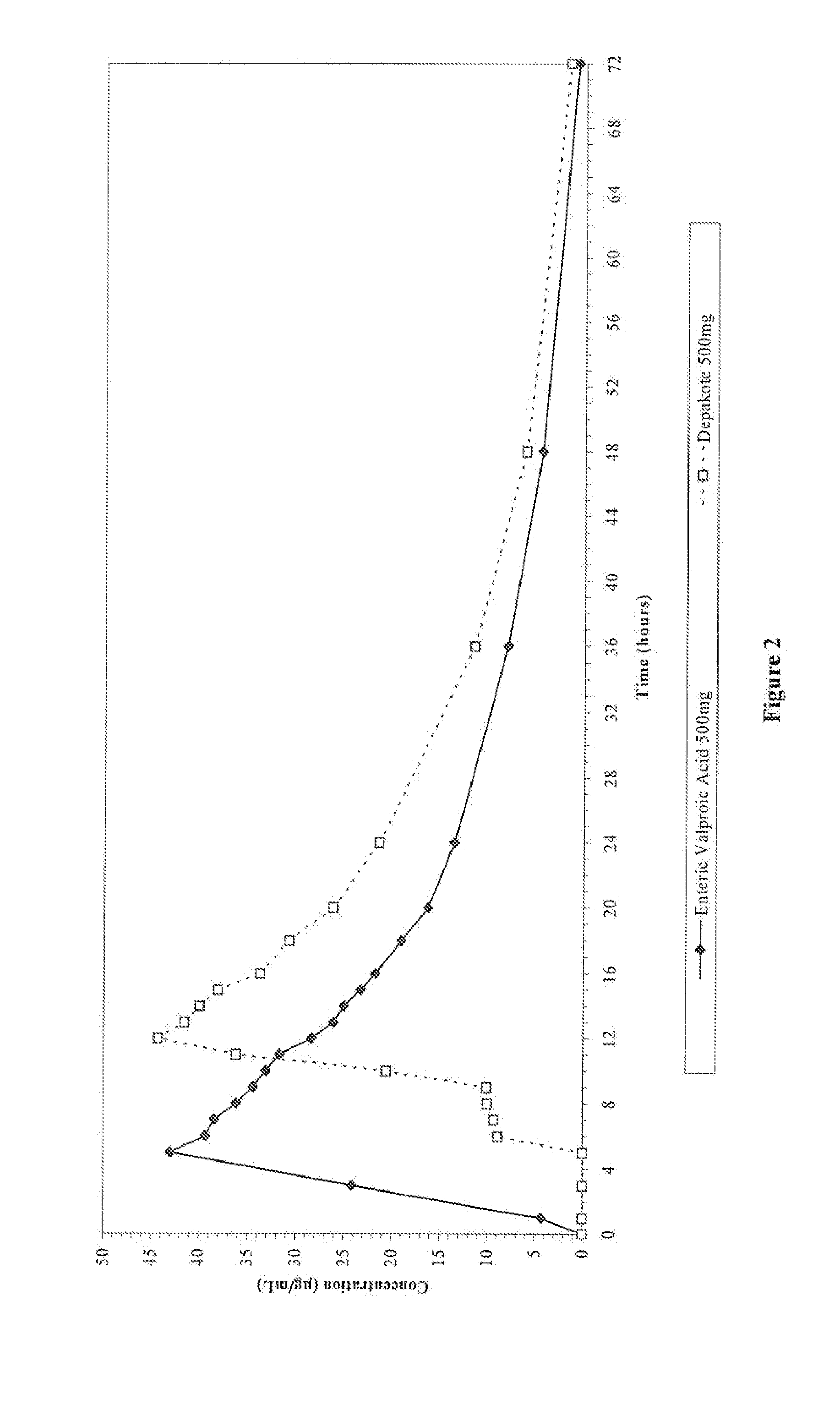
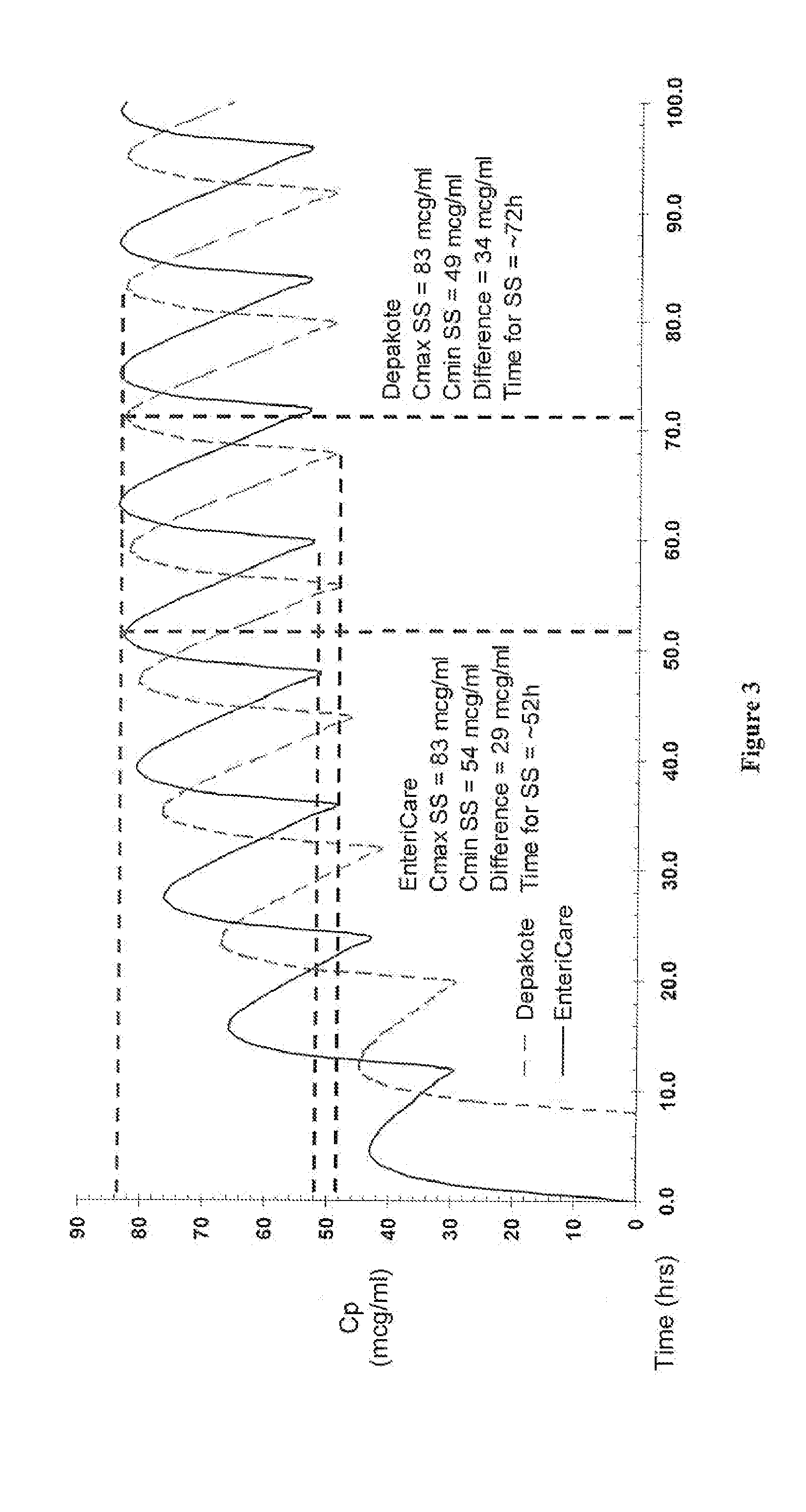
An enteric valproic acid soft gelatin capsule, in which the enteric polymer is a component of the capsule shell rather than a coating, has been developed. The fill material comprises valproic acid or divalproex sodium and, optionally, one or more pharmaceutically acceptable excipients such as corn oil. The capsule shell is prepared from a mass comprising a film-forming polymer, an acid insoluble polymer, an aqueous solvent, and optionally a plasticizer. Suitable film-forming polymers include gelatin. Suitable acid-insoluble polymers include acrylic-acid / methacrylic acid copolymers. The acid-insoluble polymer is present in an amount from about 8% to about 20% by weight of the wet gel mass. The weight ratio of acid-insoluble polymer to film-forming polymer is from about 25% to about 50%. The aqueous solvent is water or an aqueous solution of alkalis such as ammonia or diethylene amine or hydroalcoholic solutions of the same. Suitable plasticizers include glycerin and triethylcitrate. The enteric soft gelatin capsule does not require an enteric coating and thus is not susceptible to the processing problems associated with enteric coated dosage forms. Enteric valproic acid soft gelatin capsules may be smaller in size and thus easier to swallow than currently available enteric coated tablets due to the presence of fewer ingredients, as well as smaller amounts of ingredients in the capsule shell.
Owner:PATHEON SOFTGELS INC
Stabilized feverfew formulations
InactiveUS20050186269A1Avoid problemsSustained effectOrganic active ingredientsBiocideMedicineHeadache severe
The present invention is directed to compositions, methods of delivery and packaged nutraceuticals of a stabilized Feverfew Extract. More specifically, the stabilized Feverfew extract contains at least about 4% parthenolide. In a particular embodiment, the stabilized Feverfew extract is provided in a soft gelatin capsule, at a concentration effective to provide relief of the identified affliction, such as a migraine headache.
Owner:SOFT GEL TECHNOLGIES
Dermatologic soft gel compositions
InactiveUS20050158377A1Improve bioavailabilityTreatment safetyAntibacterial agentsCapsule deliveryOral medicationDermatology
Orally administrable softgels or soft gelatin capsules and fill compositions therefore for use in treating various dermatological conditions. These compositions are also particularly useful for treating children or patients of at least 55 years of age.
Owner:STIEFEL LABORATORIES
Formula of non-gelatin soft capsule shell
InactiveCN104337793AEasy to shrinkFacilitated releasePharmaceutical non-active ingredientsCapsule deliveryAntioxidantPlasticizer
The invention discloses a formula of a non-gelatin soft capsule shell. The soft capsule shell is prepared from the following components in percentage by weight: 25-75 percent of modified starch, 10-30 percent of carrageenan, 10-25 percent of a plasticizer, 40-50 percent of water, 2-12 percent of a thickening agent and the balance of pigment which can be added according to the required color of the soft capsule pill. According to the formula, the main component gelatin of a traditional capsule shell material is replaced by modified starch and carrageenan, so that multiple defects of gelatin can be overcome, the modified starch has the characteristics of film performance, air resistance, adhesion and the like, has high safety performance and good elasticity, and has the dissolving speed superior to that of gelatin; the soft capsule prepared from modified starch and carrageenan has stable property, and cannot easily perform a crosslinking phenomenon; and the soft capsule prepared by using gelatin replacing materials does not have the problem of oxidation crosslinking, and an antioxidant does not need to be added.
Owner:ZHEJIANG CHUNBAO CAPSULES
Orally administrable pharmaceutical formulation
InactiveUS20060029661A1Low extractabilityMinimize abuse potentialOrganic active ingredientsBiocideOral medicationMedicine
The present invention relates to pharmaceutical formulations for oral administration through a soft gelatin capsule, wherein the pharmaceutical dosage form has pseudoephedrine hydrochloride as the active pharmaceutical ingredient. The active pharmaceutical ingredient, pseudoephedrine hydrochloride as an active is embedded in a suitable matrix, wherein said matrix composition is characterized by reducing the extractability of the pseudoephedrine hydrochloride.
Owner:M S STRIDES
Novel herbal formulation as brain tonic
InactiveUS20080286394A1Treating and preventing amnesiaEnhance memoryBiocideNervous disorderHerbal preparationsAmnesia
The invention provides a novel herbal formulation used to improve the memory and in treatment of amnesia as a brain tonic. Formulation(s) comprises of oleaginous oil of Sesamum indicum and the alcoholic extract of Centella asiatica. Conventionally used as emulsion or as a soft gelatin capsule for oral dosage forms. Sesamum indicum used in paralysis, aphrodisiac and dysmenorrhoea. The plant of Centella asiatica is considered as a useful alternative and tonic in diseases of the skin, nerves and blood.
Owner:COUNCIL OF SCI & IND RES
Multi-panax healthcare food and preparation method thereof
The invention discloses a multi-panax healthcare food and a preparation method thereof. A brain-invigorating and intelligence-developing healthcare food is made from two or more varieties of the main raw materials such as panax ginseng, panax quinquefolius, radix pseudostellariae and root of pilose asiabell, effective ingredients generated from separation and extraction of the main raw materials, and effective parts made through emendation of the main raw materials or chemical monomers as the main ingredients; the preparation method of the multi-panax healthcare food disclosed by the invention comprises the steps of separation, extraction, emendation and refinement of the main raw materials, namely panax ginseng, panax quinquefolius, radix pseudostellariae and root of pilose asiabell; and dosage forms of the product include but are not limited to pulvis, granules, tablet, hard gelatin capsule, soft gelatin capsule, drop pill, micro-pill, injection medicine, spray medicine, oral liquid and infusion solution. The product can also be researched and developed into a medicament with brain invigoration, intelligence development and memory improving functions according to the relevant regulations of China.
Owner:吉林绿波中药药业有限公司
Crystallization inhibitor and its use in gelatin capsules
The present invention describes soft gelatin capsules that encapsulate a water-insoluble active ingredient and an excipient composed of a crystallization inhibitor that stabilizes the water-insoluble inhibitor. The crystallization inhibitor being at least one mononacylglycerol compound whose acyl group is a fatty acid residue of 6-18 carbon atoms. The capsule contents are more resistant to turbidity, forming a coarse emulsion, and crystallization of the active ingredient compared with compositions absent the crystallization inhibitor.
Owner:IVAX PHARMA
Reduction of cross-linking gelatin in gelatin capsules
InactiveUS8895059B2Improve permeabilityPrevent/reduce gel cross-linkingBiocideOrganic active ingredientsCross-linkCarboxylic acid
The invention relates to compositions and methods for reducing cross-linking in the gelatin shell of gelatin capsules by incorporation of free amino acid into the capsule shell and by inclusion of an ester of carboxylic acid either into the capsule filling, and / or into the capsule shell and / or into the lubrication agent, or in combinations thereof. Described are soft gelatin capsules characterized by improved stability as compared with gelatin capsules that do not contain amino acid in the shell and carboxylic acid ester in the filling, shell or in the lubrication agent, or in combinations thereof.
Owner:IVAX PHARMA
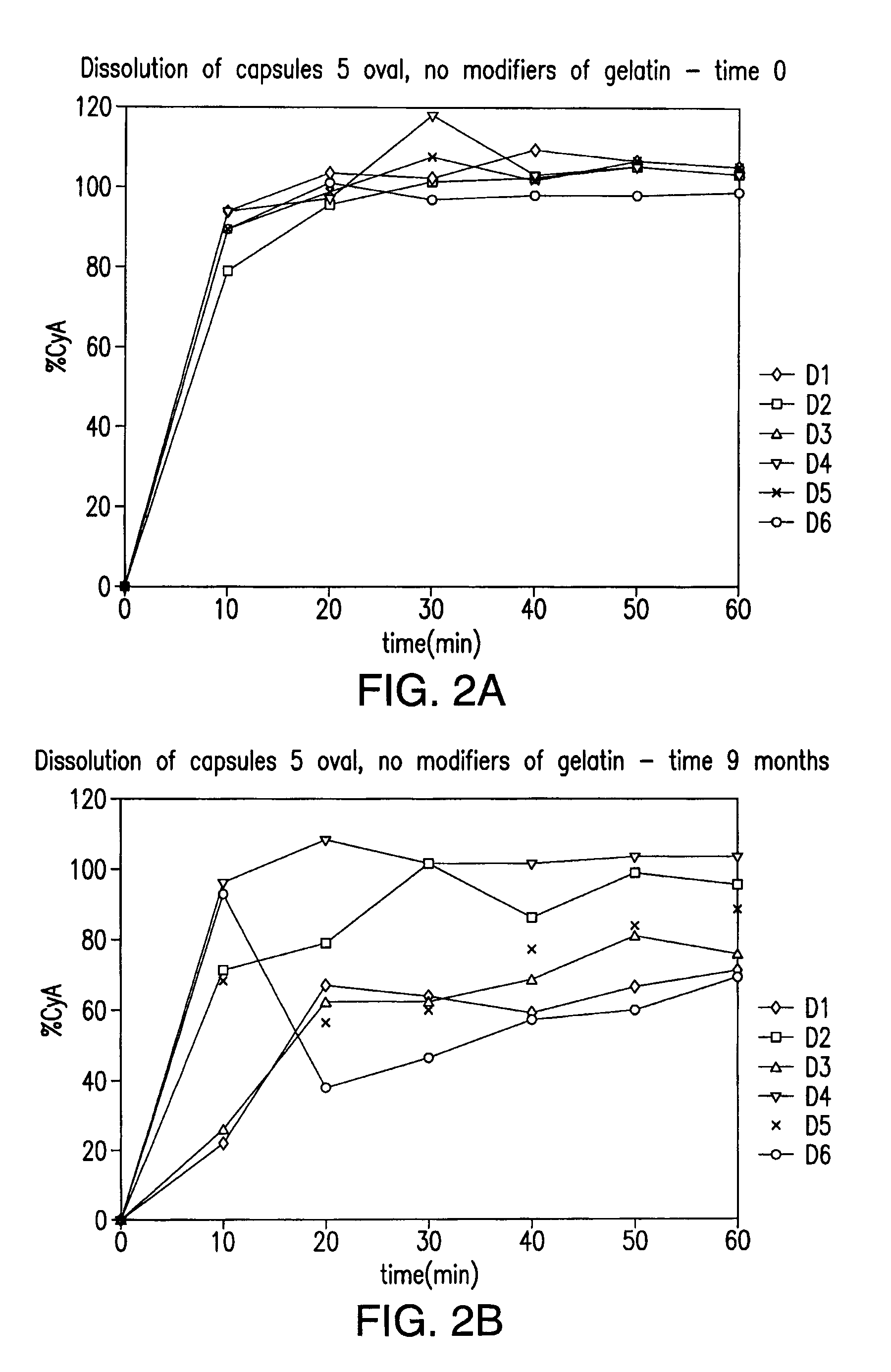
Soft-gelatin capsule formulation
ActiveUS8026393B2Increased shelf stabilityImprove stabilityBiocideOrganic chemistryPlasticizerSoft gelatin capsule
Owner:R TECH UENO
Highly concentrated pourable aqueous solutions of potassium ibuprofen, their preparation and their uses
Concentrated pourable potassium ibuprofen liquid compositions and their preparation are described. They are comprised of (i) potassium ibuprofen, (ii) water; and (iii) methanol, ethanol, 1-propanol, 2-propanol, 1,1-dimethylethanol, or a mixture of any two or more of them. The amount of dissolved potassium ibuprofen in the composition is in the range of about 60 to about 90 wt %. These compositions are suitable for use in the preparation of pharmaceutical dosage forms such as liquid-filled soft gelatin capsules, syrups, elixirs, suspensions; solid dosage forms such as tablets or caplets; and topically-applied products such as lotions, creams or ointments.
Owner:ALBEMARLE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com