Patents
Literature
54 results about "Vaginal atrophy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inflammation of the vagina due to thinning and shrinking of the tissues.
Pharmaceutical compositions
ActiveUS8268806B2Achieve beneficial effectAvoid their undesirable side effectOrganic active ingredientsMuscular disorderEstrogenic EffectsInsulin resistance
Owner:MYRIEL PHARM LLC
Methods for prevention and treatment of conditions arising from local estrogen deficiency
InactiveUS20070238713A1Effective treatmentBiocideOrganic active ingredientsVaginal atrophyVaginal burning
The present invention relates to methods for the prevention and treatment of conditions arising from local estrogen deficiency, such as dyspareunia, vulvar atrophy, vaginal atrophy, vaginal dryness, vulvar itching, vaginal itching, vulvar burning, vaginal burning, vulvar dystrophy, atrophic vaginitis or menopausal sexual dysfunction. In some embodiments, the methods include systemic, for example oral, administration of an estrogen, such as conjugated estrogens, and a progestagen, such as MPA, contemporaneously with local administration of an estrogen, for example conjugated estrogens. In some embodiments, the methods include the oral administration of conjugated estrogens and MPA, and the vulvar, vaginal, or vulvar and vaginal administration of conjugated estrogens, for example in a cream.
Owner:WYETH
Vaginal inserted estradiol pharmaceutical compositions and methods
ActiveUS20150133421A1Reduce in quantityIncrease the number ofOrganic active ingredientsPharmaceutical non-active ingredientsGynecologyVaginal atrophy
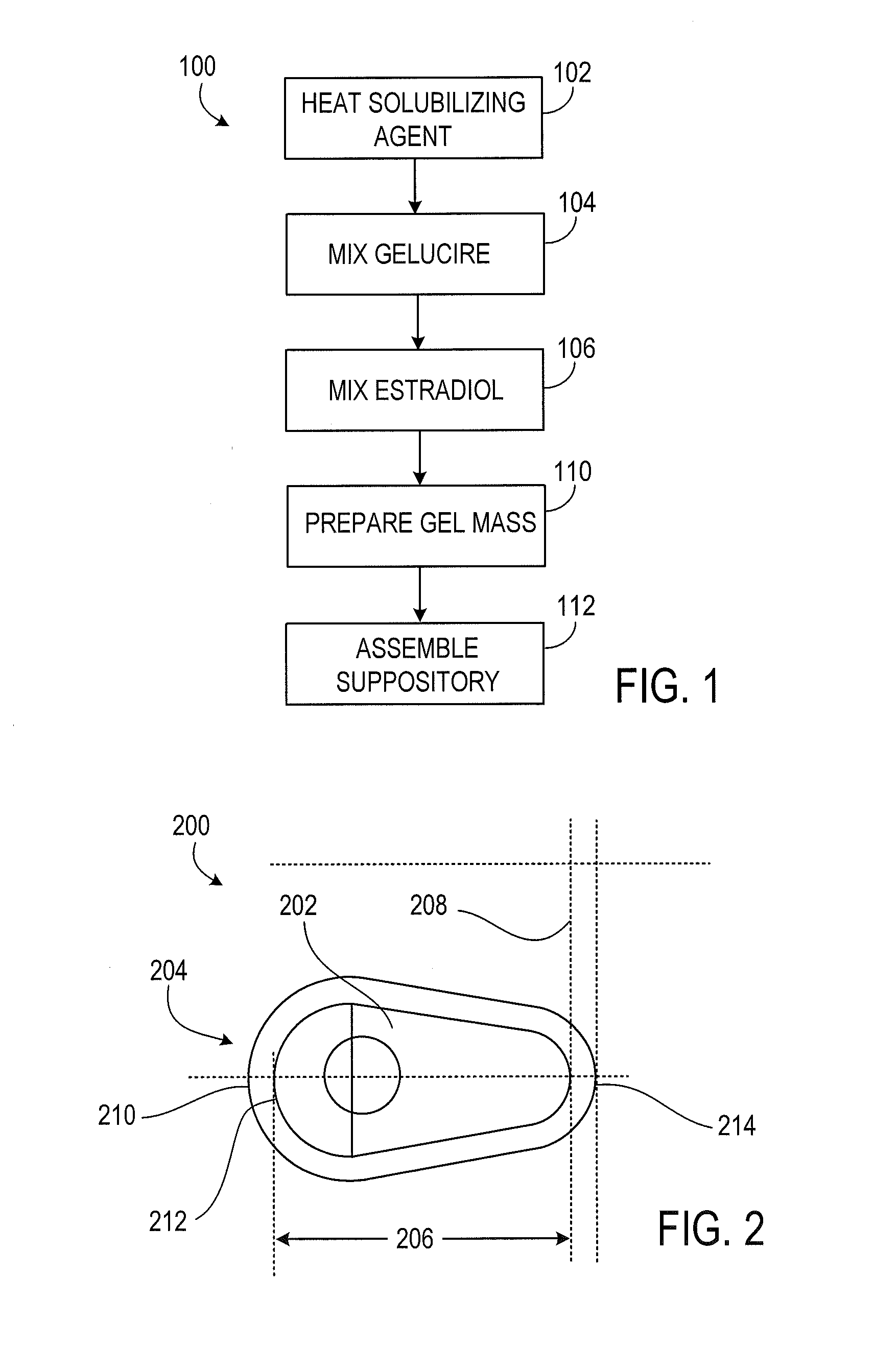
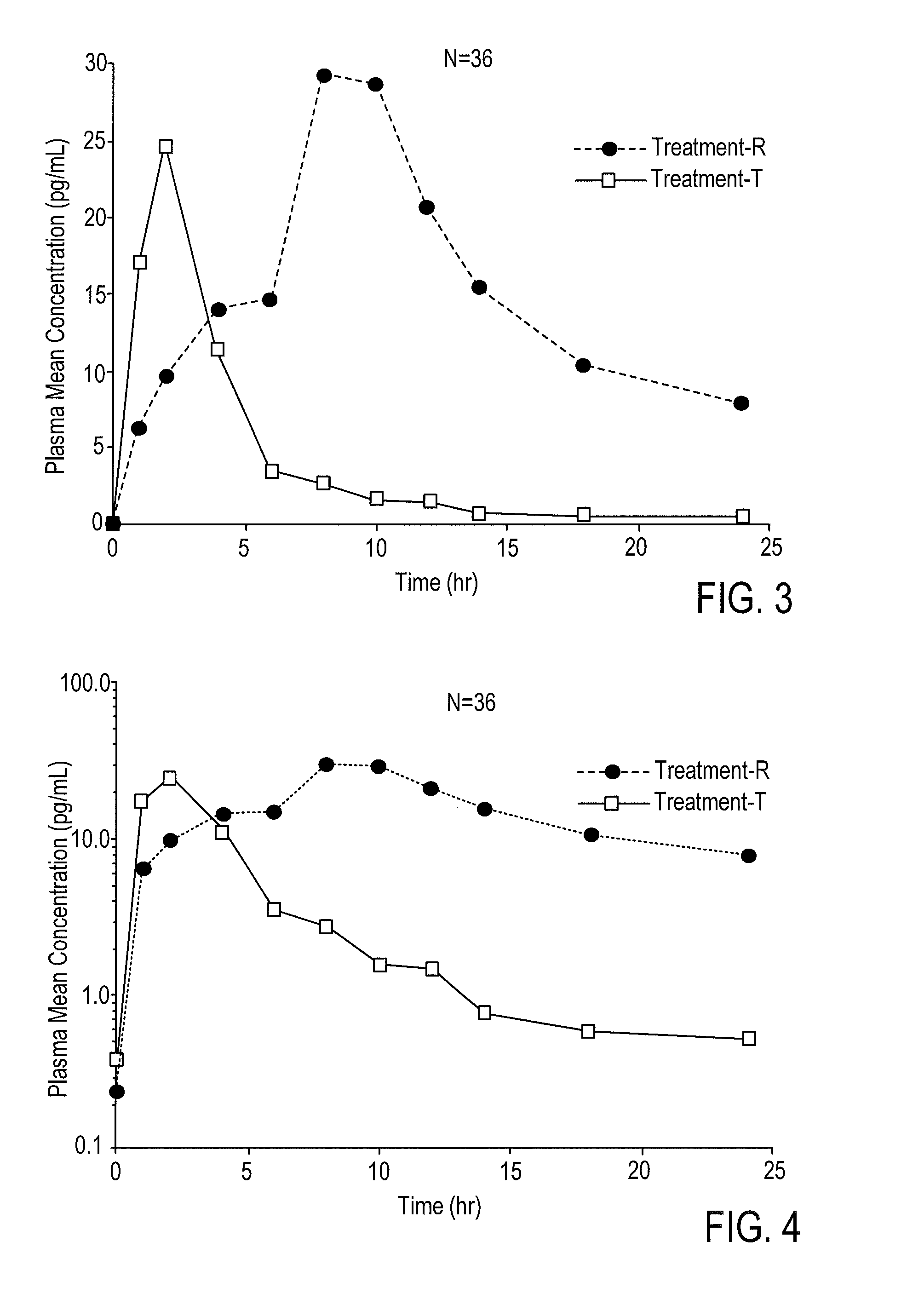
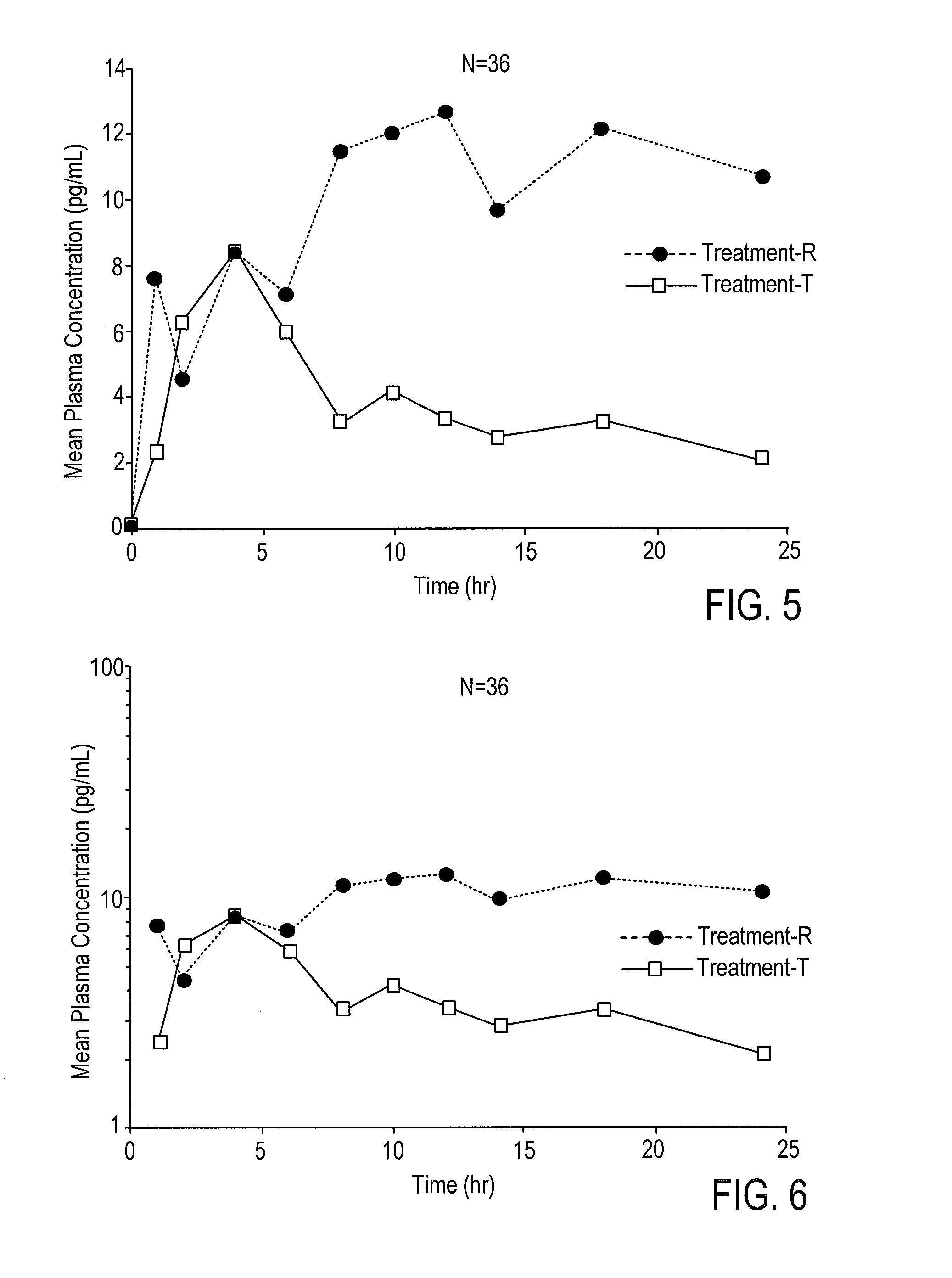
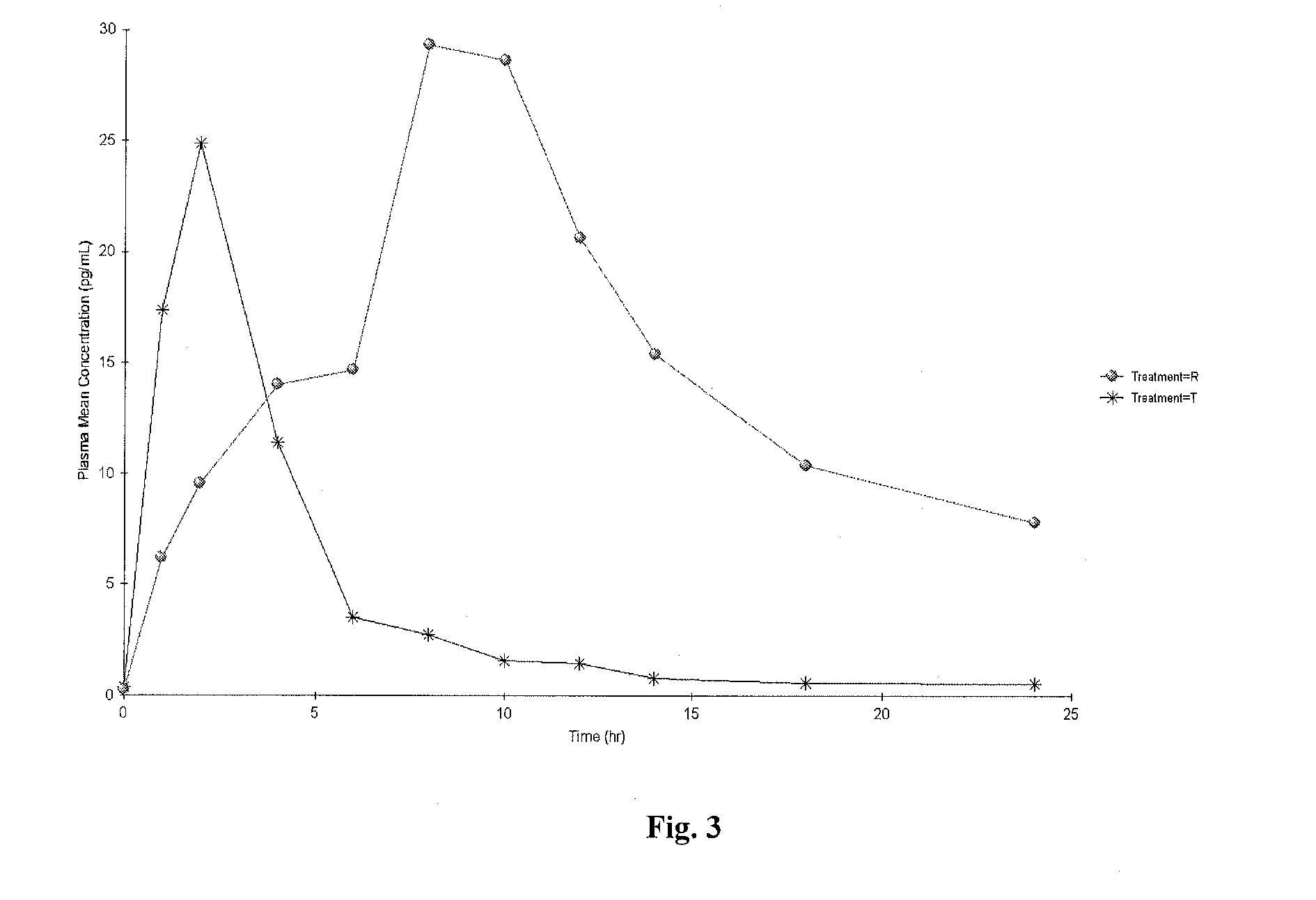
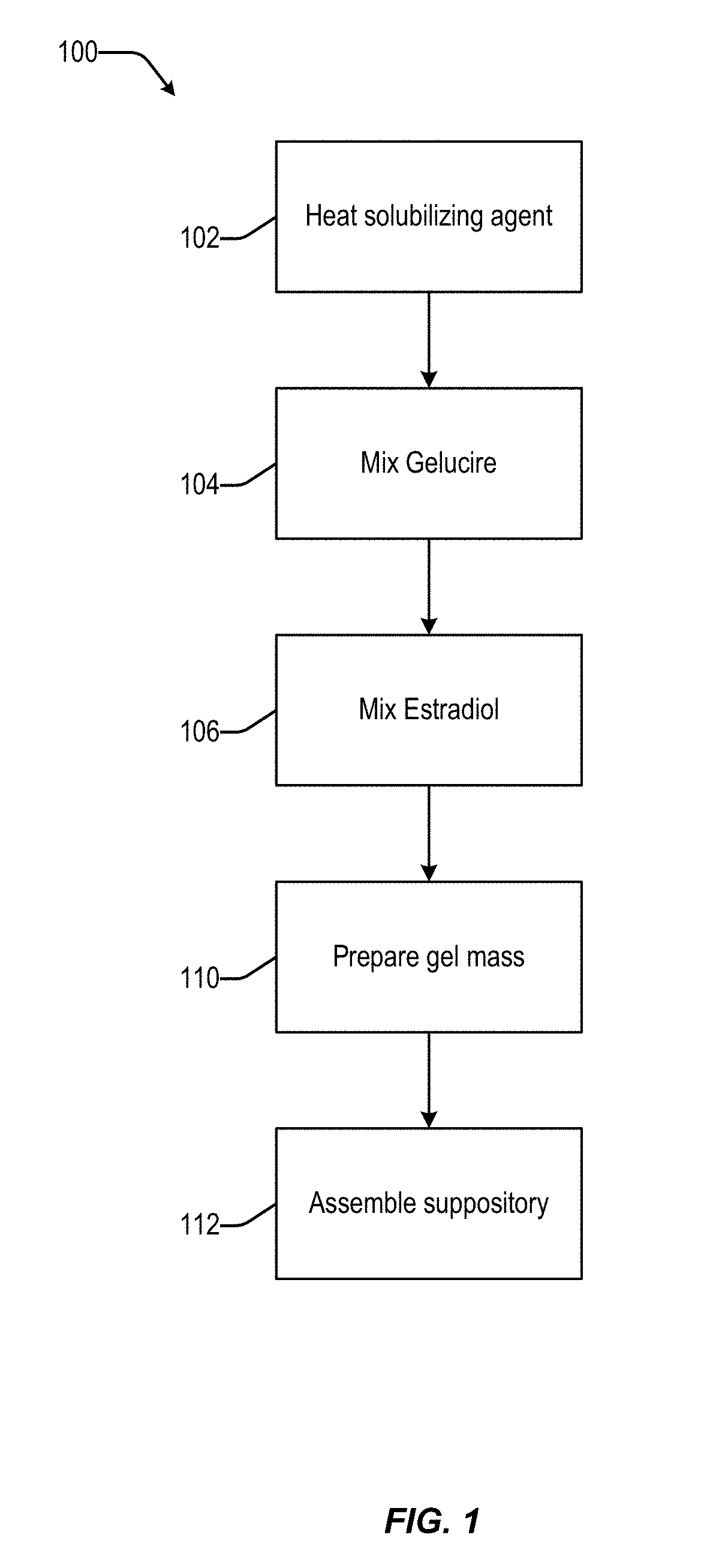
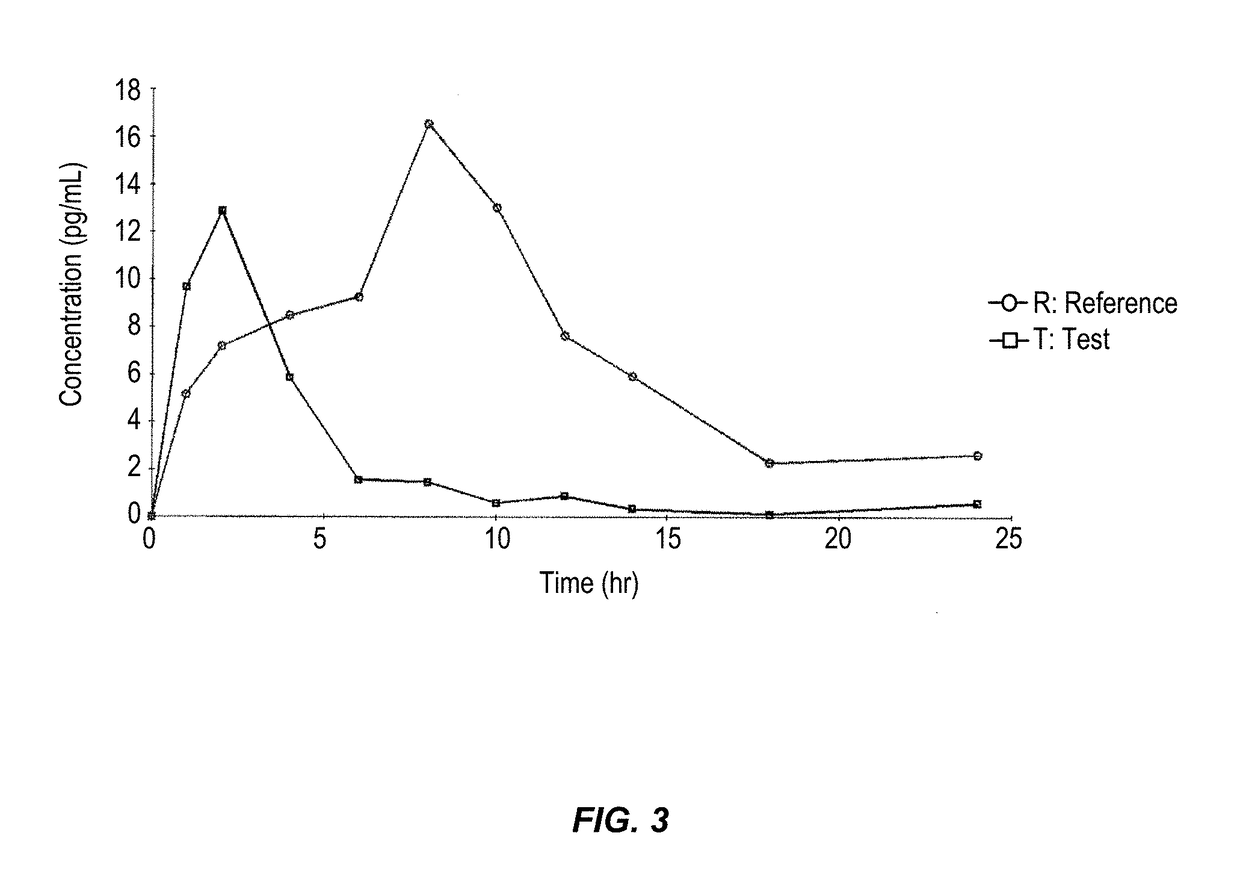
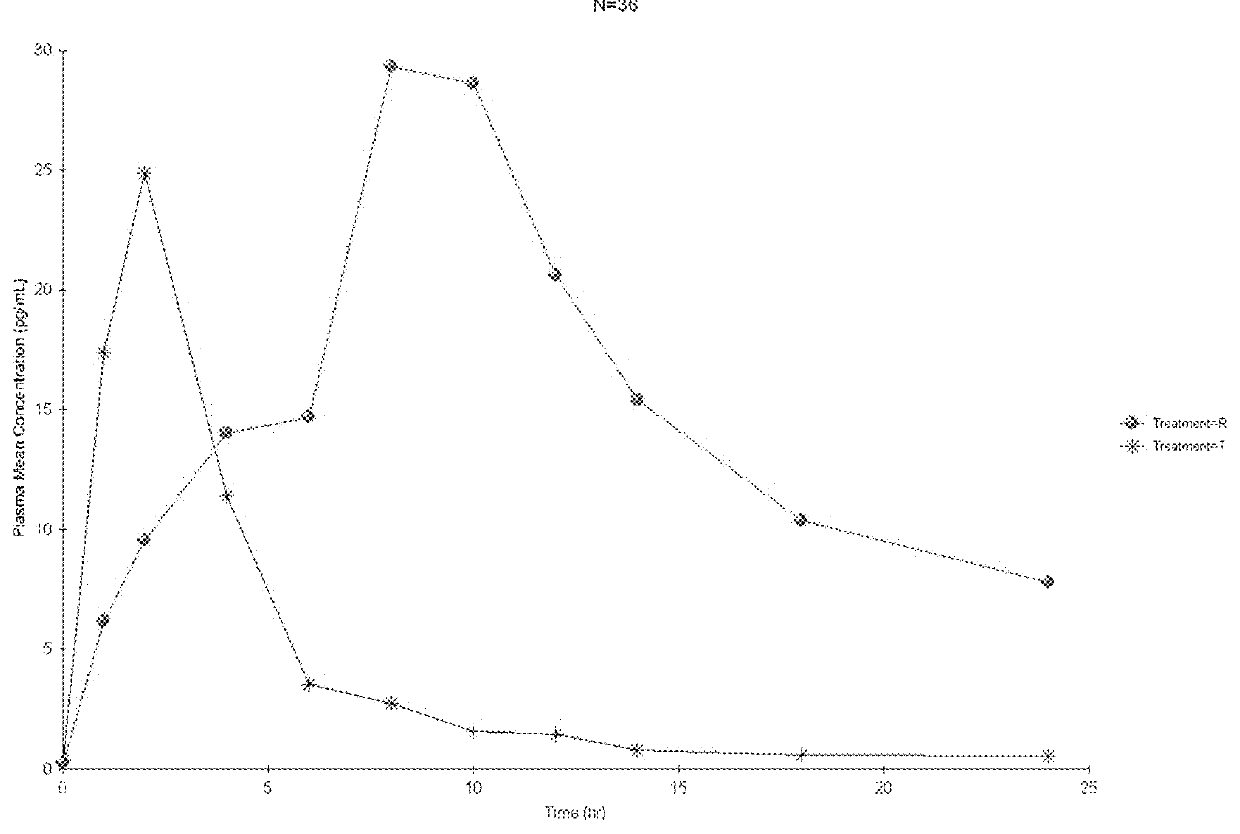
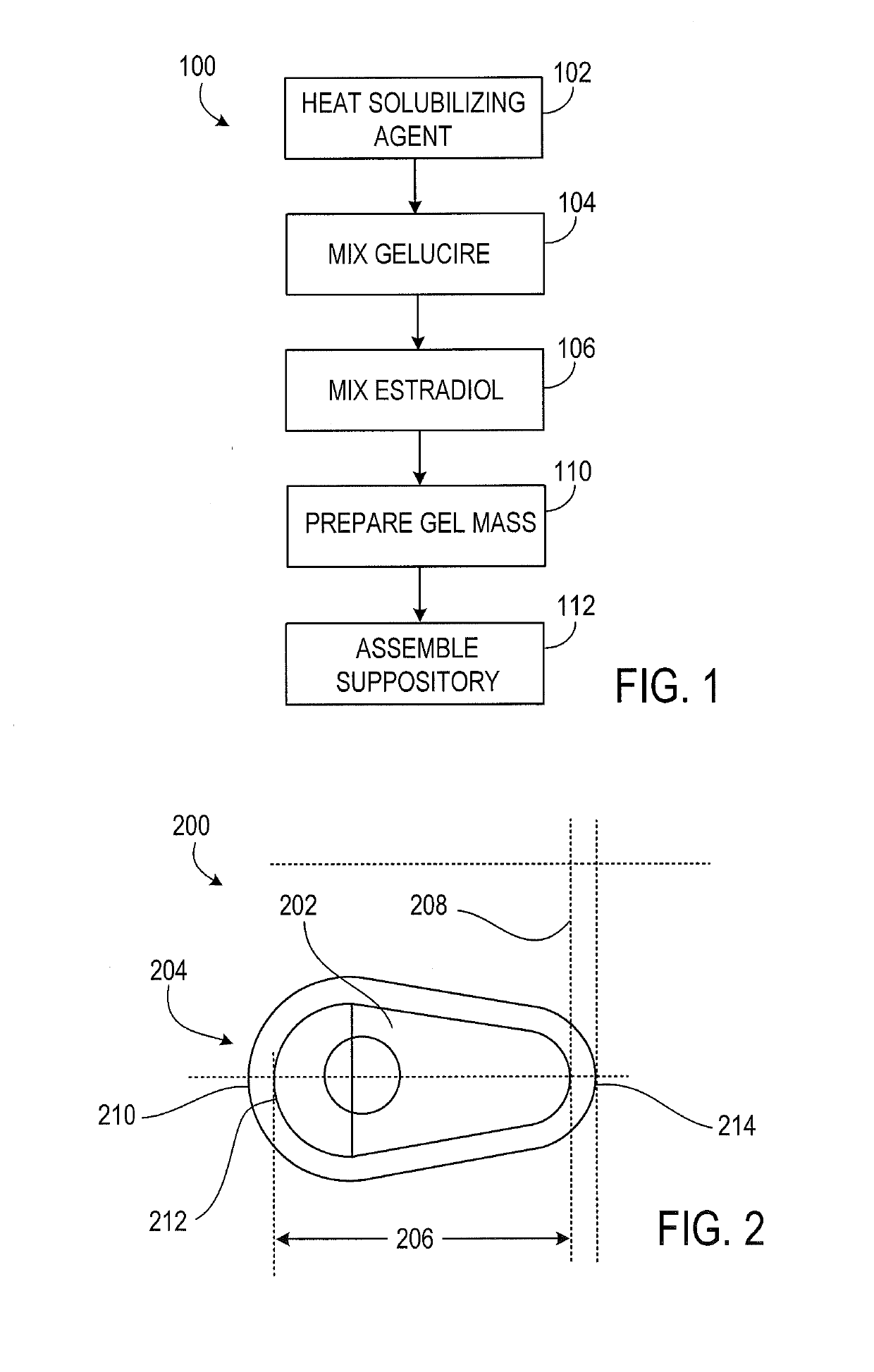
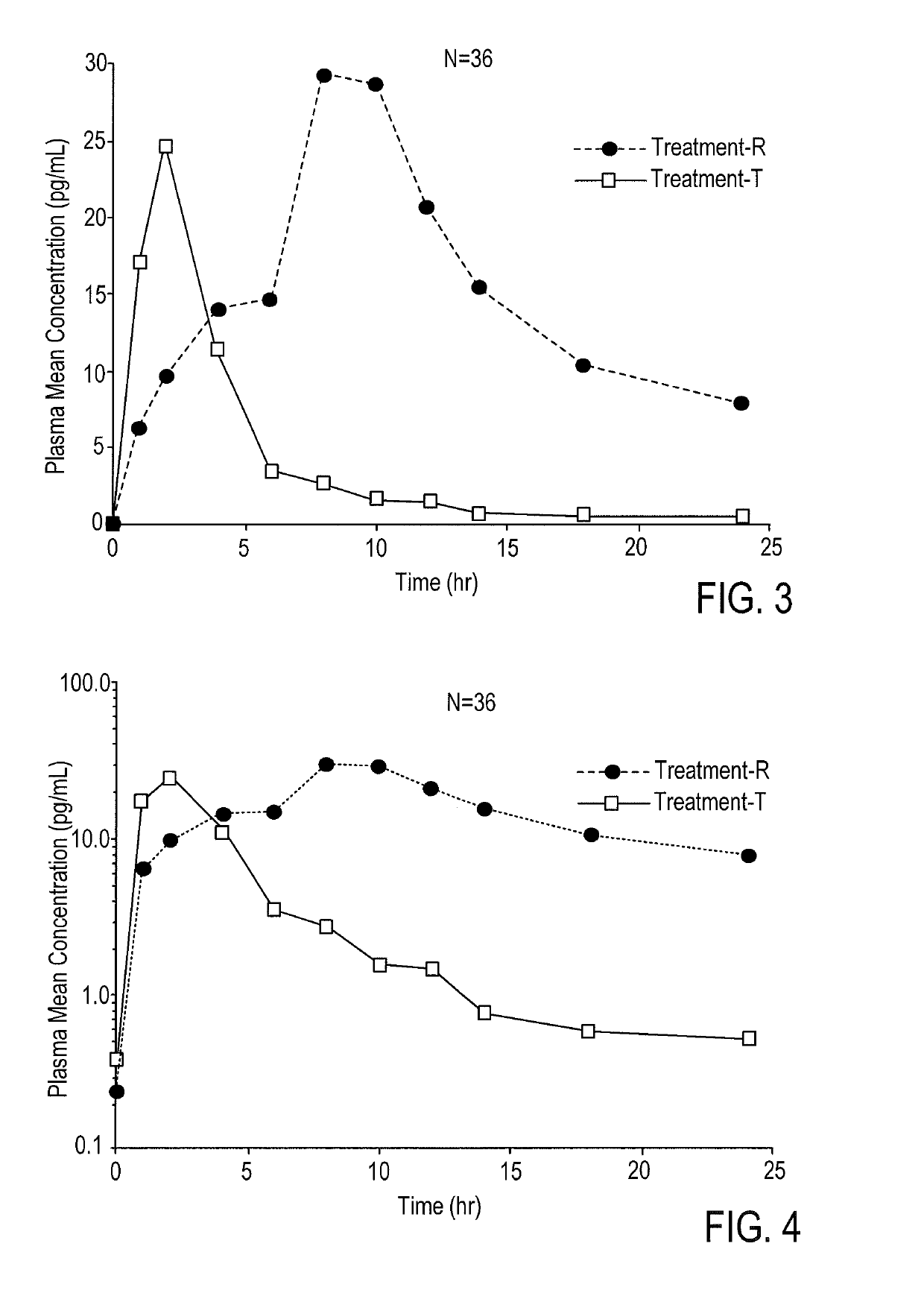
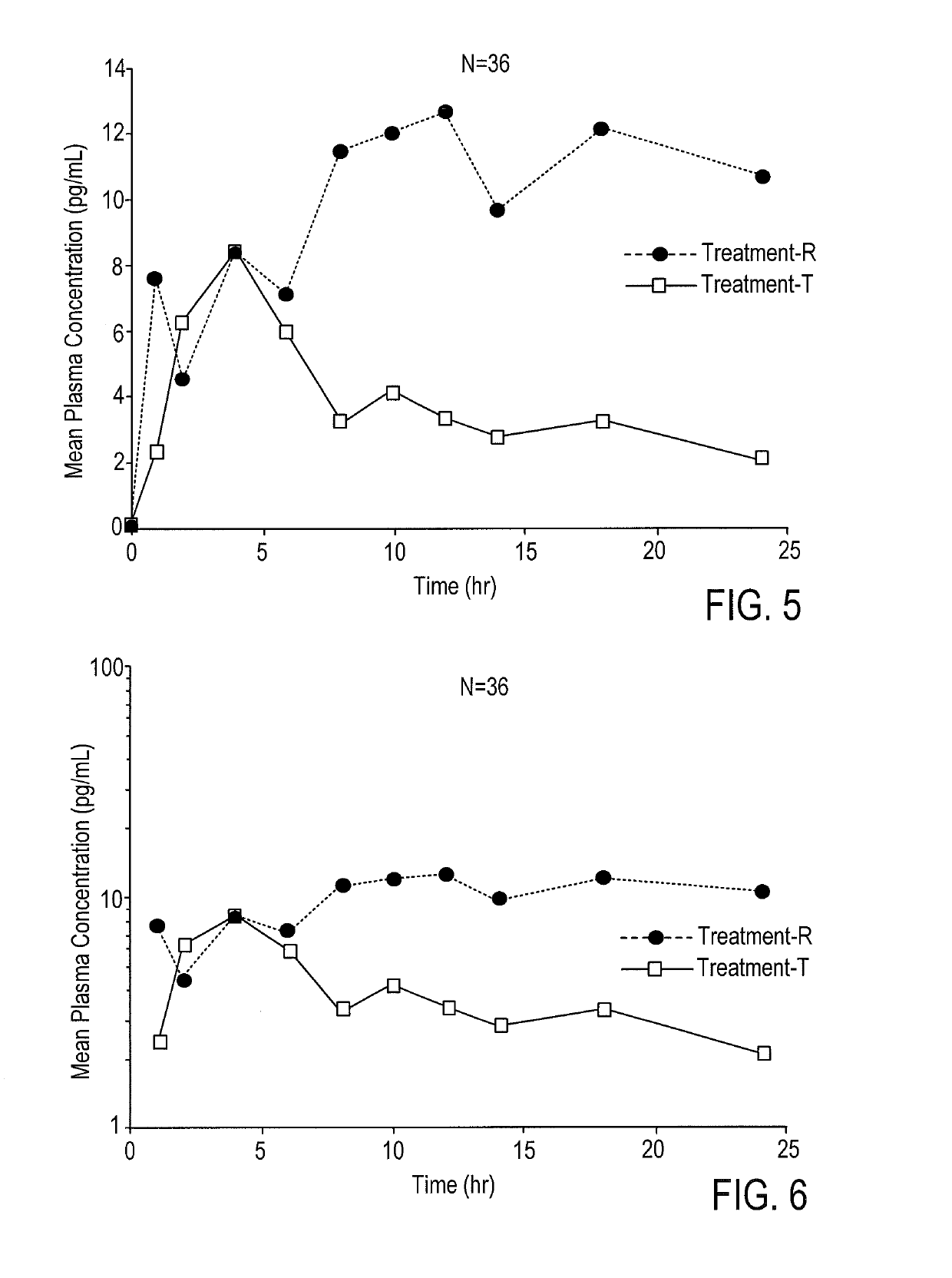
According to various embodiments of this disclosure, pharmaceutical compositions comprising solubilized estradiol are provided. In various embodiments, such compositions are encapsulated in soft capsules which may be vaginally inserted for the treatment of vulvovaginal atrophy.
Owner:THERAPEUTICSMD INC
Vaginal inserted estradiol pharmaceutical compositons and methods
ActiveUS20150045335A1Reduce in quantityIncrease the number ofOrganic active ingredientsOintment deliveryVaginal atrophyVagina
According to various embodiments of this disclosure, pharmaceutical compositions comprising solubilized estradiol are provided. In various embodiments, such compositions are encapsulated in soft capsules which may be vaginally inserted for the treatment of vulvovaginal atrophy.
Owner:THERAPEUTICSMD
Vaginal inserted estradiol pharmaceutical compositions and methods
ActiveUS20170216310A1Reduce in quantityIncrease the number ofOrganic active ingredientsSuppositories deliveryVaginal atrophyVagina
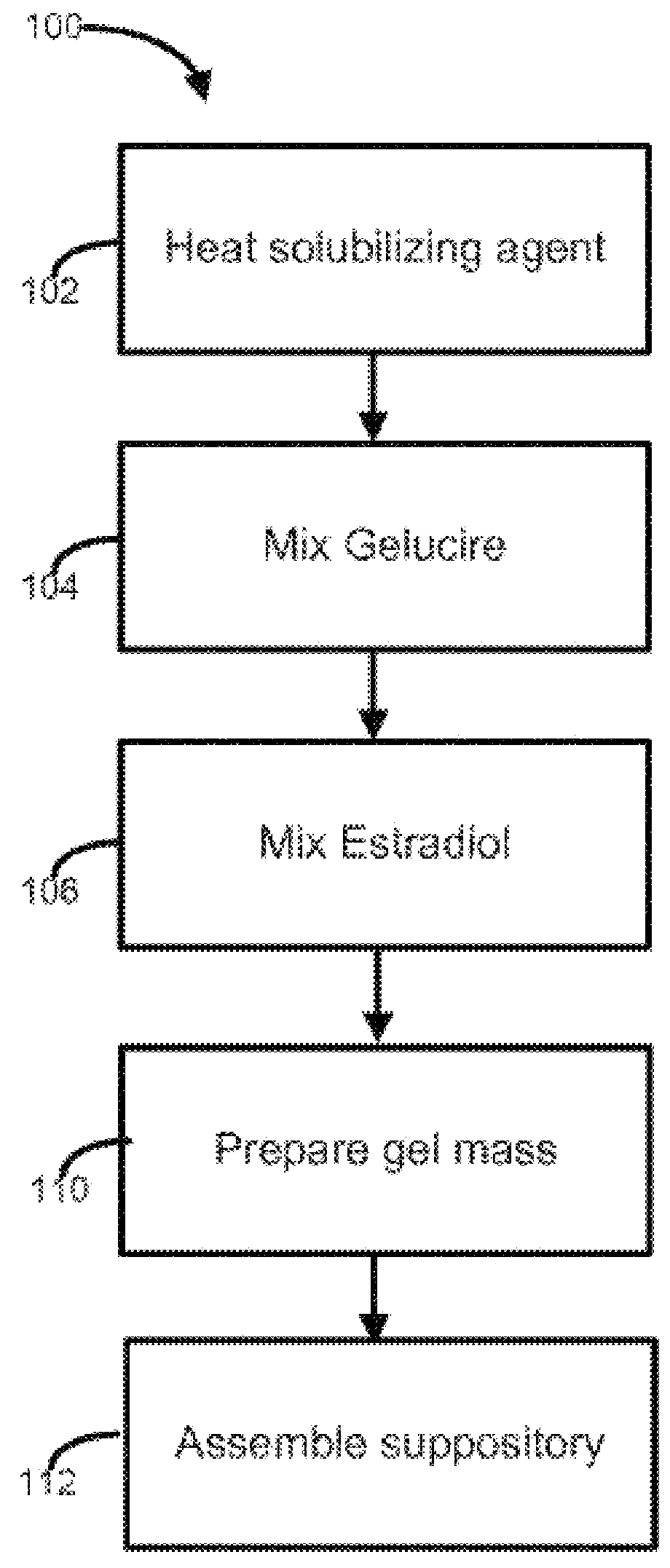
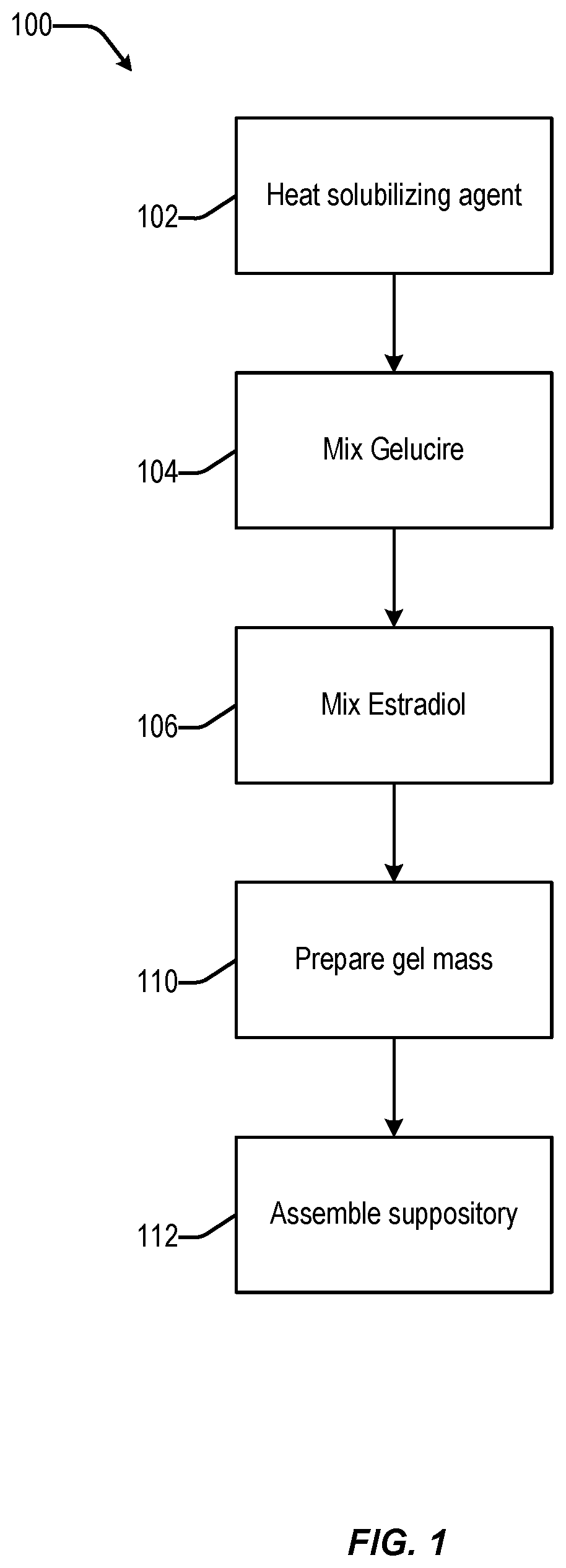
Disclosed herein is, among other things, a soft gel vaginal pharmaceutical composition and dosage form containing solubilized estradiol for the treatment of vulvovaginal atrophy (VVA) and female sexual dysfunction (FSD).
Owner:THERAPEUTICSMD
Vaginal inserted estradiol pharmaceutical compositions and methods
ActiveUS20180161345A1Reduce in quantityIncrease the number ofOrganic active ingredientsPharmaceutical delivery mechanismVaginal atrophyVagina
In one aspect, pharmaceutical compositions and methods for the treatment of vulvovaginal atrophy (VVA) are provided. In one embodiment, the method comprises digitally inserting into the lower third of the vagina of a subject having VVA a soft gelatin capsule containing a liquid pharmaceutical composition.
Owner:THERAPEUTICSMD
Vaginal inserted estradiol pharmaceutical compositions and methods
ActiveUS20190358243A1Vaginal administrationImprove securityOrganic active ingredientsNervous disorderVaginaVulvovaginal atrophy
Disclosed herein is, among other things, a soft gel vaginal pharmaceutical composition and dosage form containing solubilized estradiol for the treatment of vulvovaginal atrophy (VVA) and female sexual dysfunction (FSD).
Owner:THERAPEUTICSMD INC
Pharmaceutical compositions
ActiveUS20090054383A1Achieve beneficial effectPrevent adverse side effectsBiocideOrganic active ingredientsDiseaseEstrogenic Effects
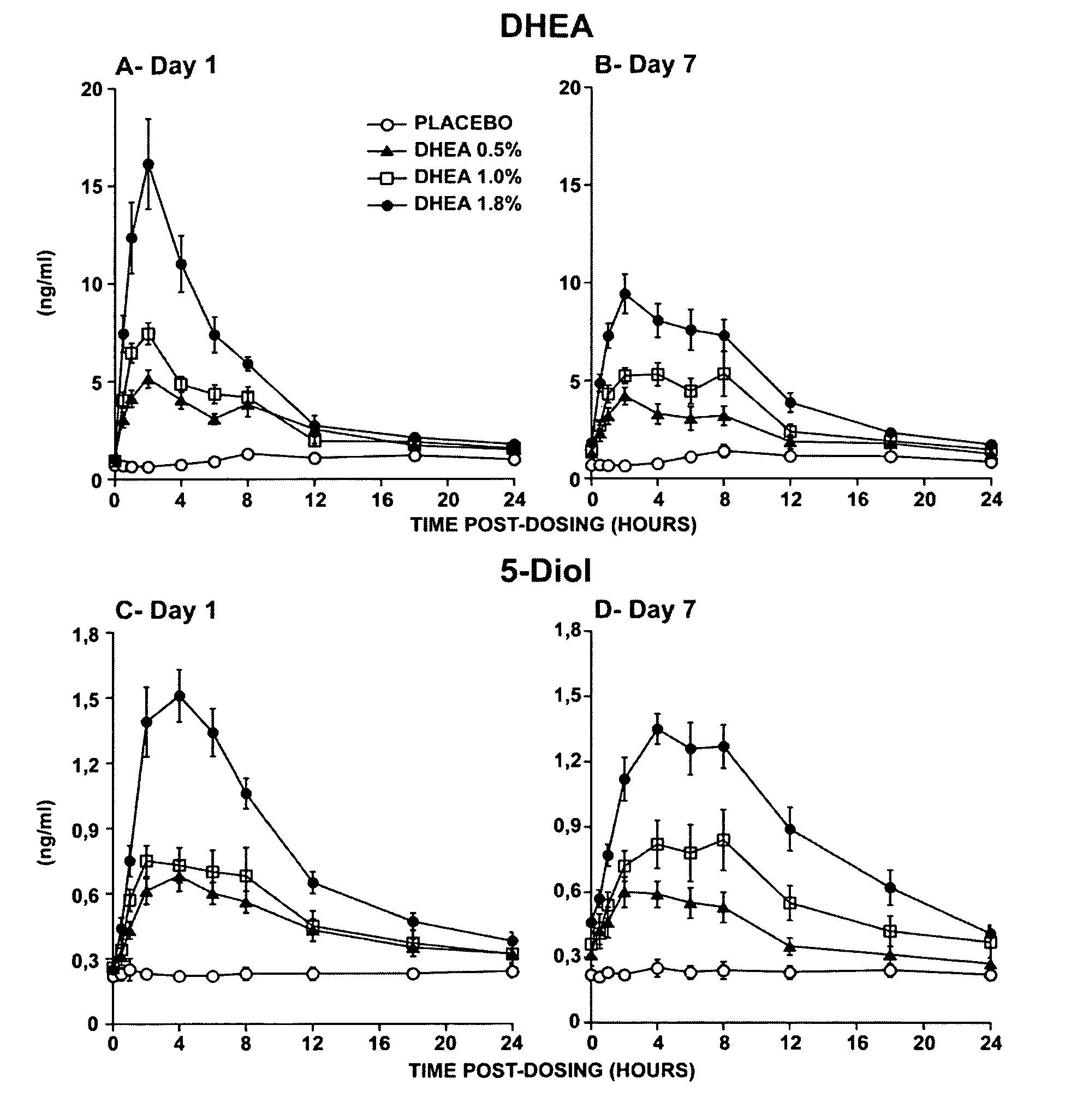
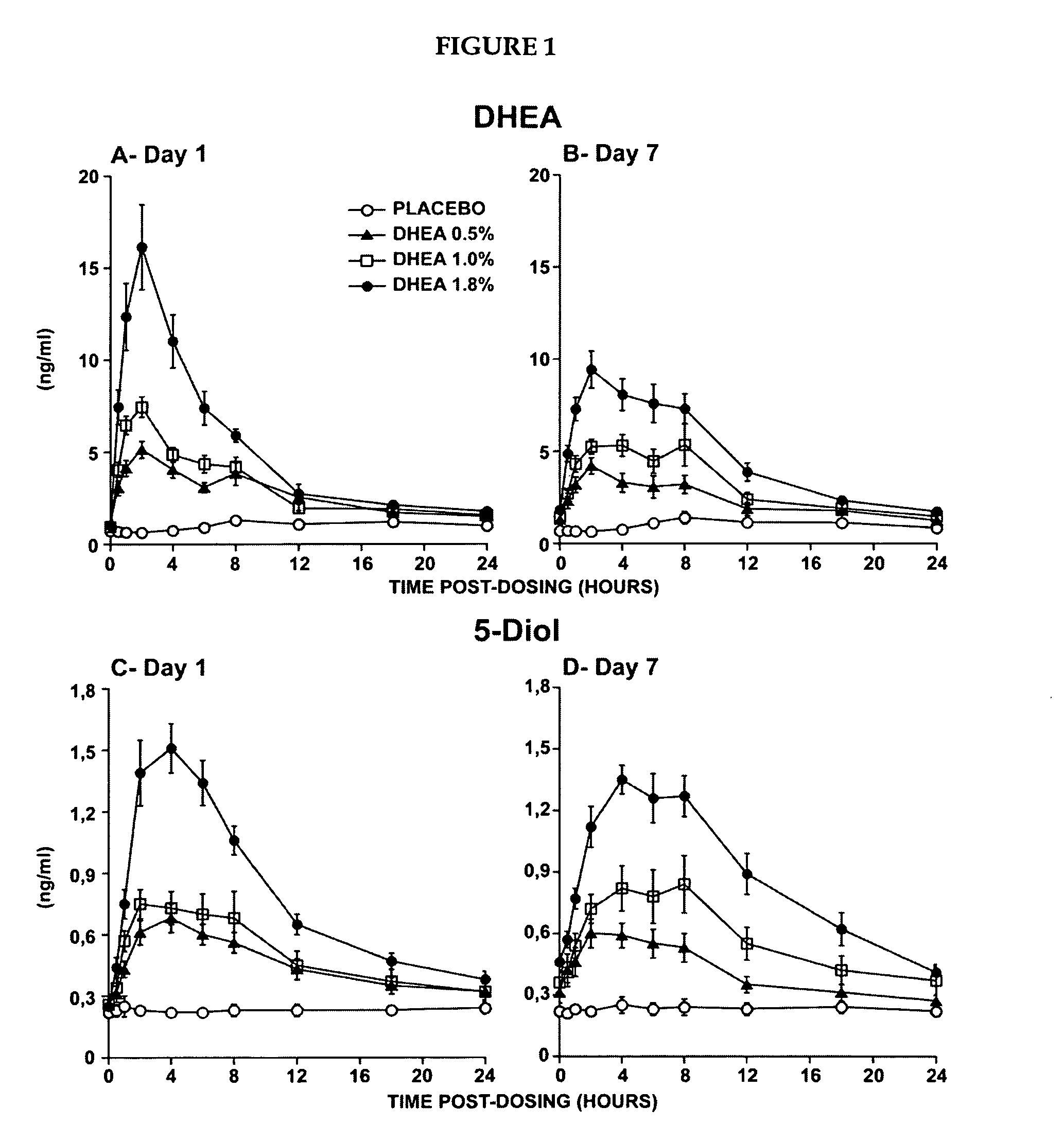
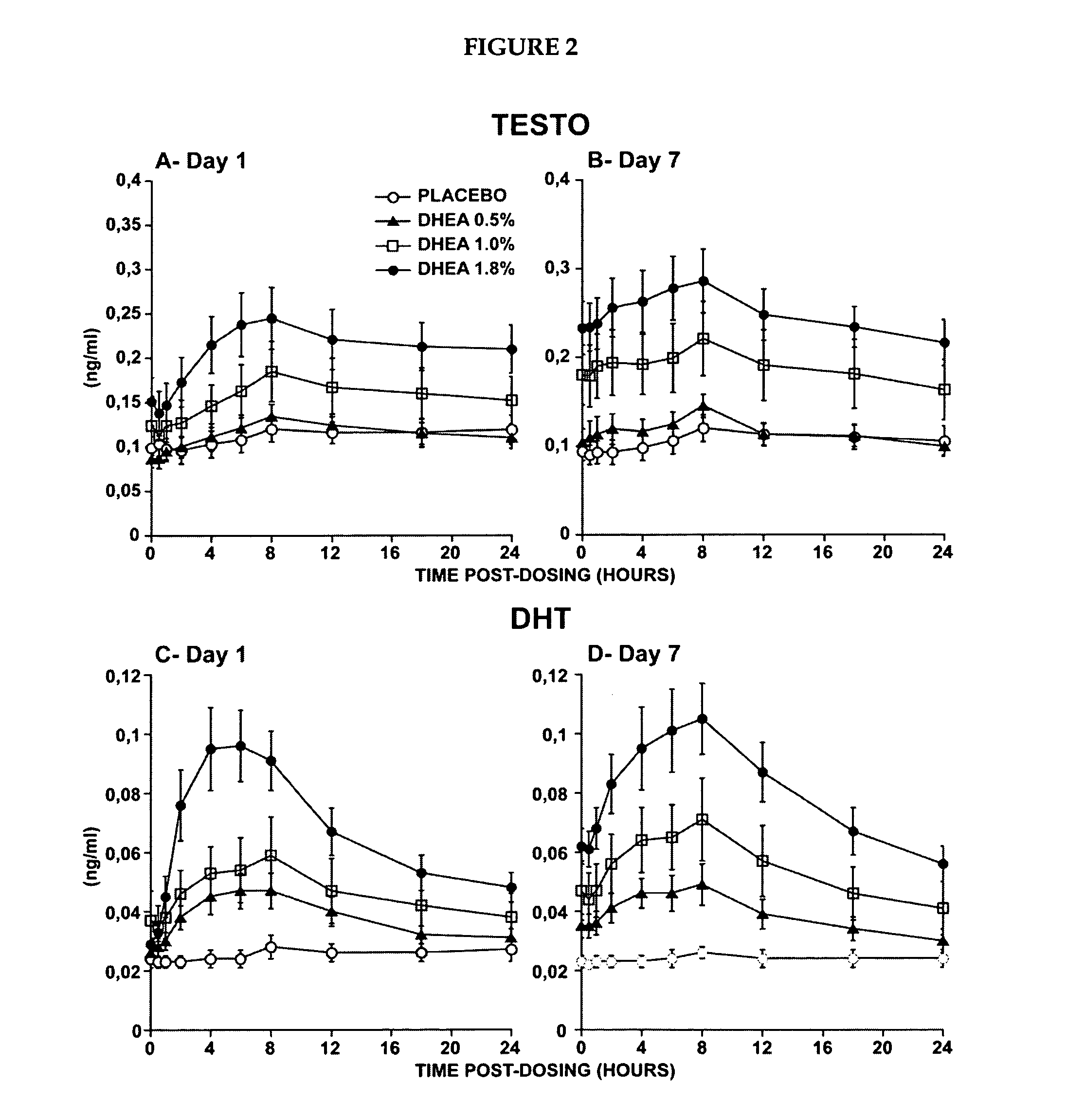
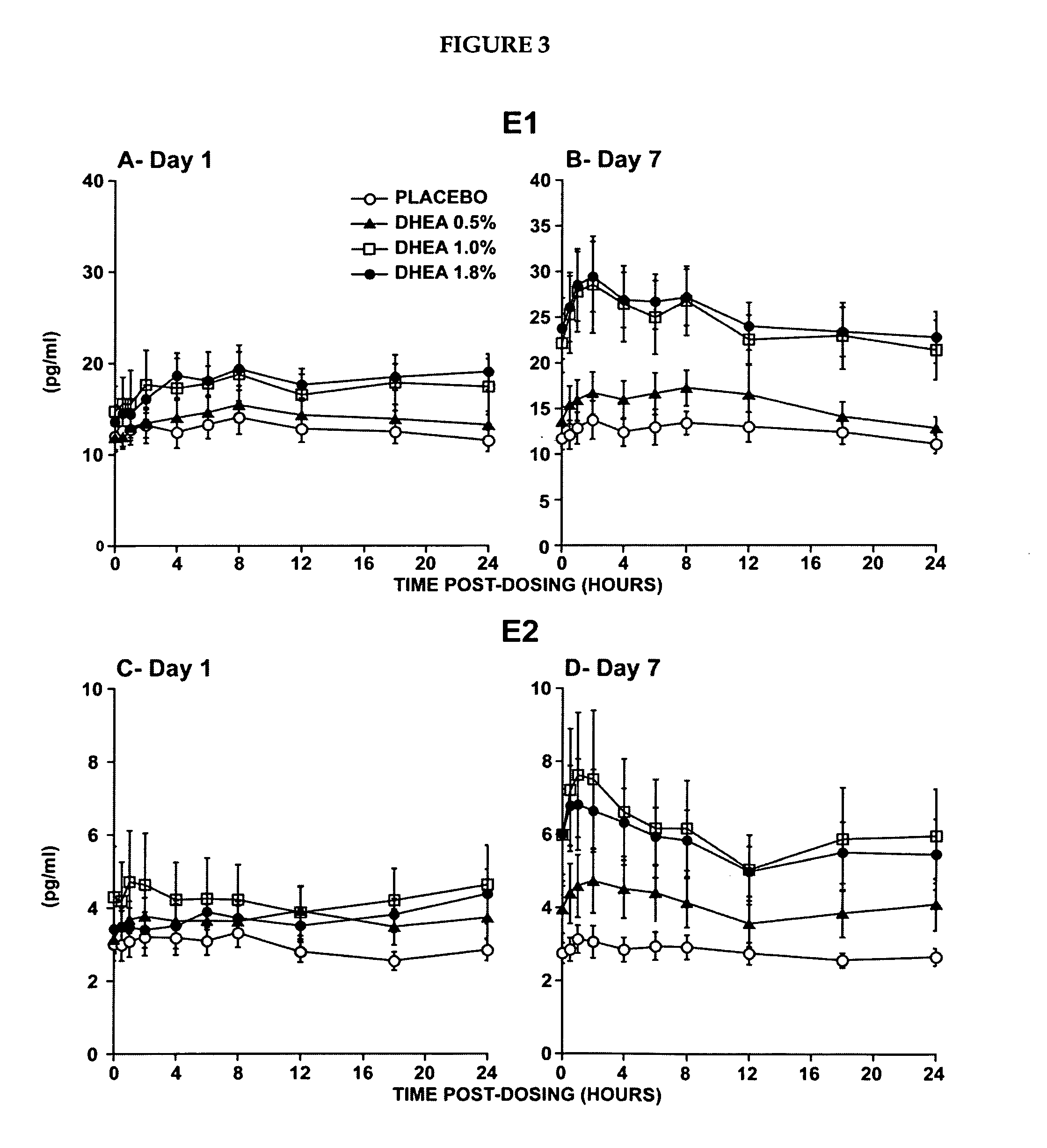
Novel methods for treating or reducing the likelihood of acquiring symptoms or diseases due to the menopause, in postmenopausal women, particularly osteoporosis, vaginal atrophy and dryness, hypogonadism, diminished libido, skin atrophy, connective tissue disease, urinary incontinence, breast, endometrial, ovarian and uterine cancers, hot flashes, loss of muscle mass, insulin resistance, fatigue, loss of energy, aging, physical symptoms of menopause, in susceptible warm-blooded animals including humans involving administration of a sex steroid precursor are disclosed. Said method comprising novel ways of administering and dosing dehydroepiandrosterone (DHEA) in order to take advantage of positive androgenic effects in the vaginal layers lamina propia and / or the layer muscularis, without undesirably causing systemic estrogenic effects in order to avoid the risk of breast and uterine cancer. Pharmaceutical compositions for delivery of active ingredient(s) useful to the invention are also disclosed.
Owner:MYRIEL PHARM LLC
Recombinant human collagen gel and preparation method thereof
InactiveCN105169374AMoisturizing, isolatingImprove atrophyOrganic active ingredientsPeptide/protein ingredientsGlycerolButanediol
The invention relates to the technical field of medical gels, and in particular relates to a recombinant human collagen gel and a preparation method thereof. The recombinant human collagen gel consists of the following raw materials in percentage by weight: 1-3% of small-molecule collagen, 1.5-2.5% of glycerol, 1-2% of chitosan quaternary ammonium salt, 0.5-1.5% of sodium hyaluronate, 0.5-1.5% of epsilon-polylysine, 0.3-0.7% of borneol, 0.3-0.7% of poloxamer, 0.3-0.7% of coenzyme Q10, 0.3-0.7% of butanediol and the balance of purified water. The recombinant human collagen gel provided by the invention has effects of moisture retention and isolation, can improve vaginal atrophy, increase vaginal elasticity and relieve dyspareunia, and also can improve the wettability and the dry state of a vagina, relieve dry and coarse stabbing pain symptoms of cunnus, and ease itching, calor, skin mucous membrane fissure and diffuse superficial bleeding caused by vaginal dryness.
Owner:DONGGUAN DAQING MEDICAL DEVICES CO LTD +2
Recombinant human-derived collagen vagina gel for vaginal dryness and preparation method thereof
ActiveCN104548071AIncrease elasticityImprove drynessOrganic active ingredientsPeptide/protein ingredientsGlycerolSodium methylparaben
The invention relates to an ordinary treatment medicine for vagina dryness, and in particular relates to recombinant human-derived collagen vagina gel for vaginal dryness. The recombinant human-derived collagen vagina gel comprises the following components in percentage by mass: 0.05-1% of recombinant human-derived collagen, 10-30% of poloxamer 407, 0.5-6% of poloxamer 188, 0.1-2% of sodium hyaluronate, 1-4% of glycerinum, an antibacterial preservative which comprises 0.1-1% of phenoxyethanol, 0.05-3% of sodium methylparaben, 0.01-2% of propylparaben, and the balance of water. By adopting the recombinant human-derived collagen vagina gel, the vagina elasticity can be improved, vaginal atrophy can be alleviated, dyspareunia can be relieved, and the sex satisfaction can be improved; by increasing secreta, improving the vagina wetness and alleviating the dryness of vaginas, pruritus, hotness and chapped skin caused by dryness can be remedied, and the symptoms of dryness and stabbing pain of vulvovagina can be alleviated. In addition, the product cannot affect the pH value of vaginas when used in the vaginas, is free of adverse reaction, and is worthy of clinical popularization and application.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Compounds and forms of treatment for female sexual disorders
ActiveUS20160271102A1Effective treatmentIncrease intensityOrganic active ingredientsAerosol deliveryFemale Orgasmic DisorderVaginal atrophy
Owner:GTO PHARMA LLC
Vaginal inserted estradiol pharmaceutical compositions and methods
ActiveUS10537581B2Reduce in quantityIncrease the number ofOrganic active ingredientsOintment deliveryVaginal atrophyPharmaceutical drug
According to various embodiments of this disclosure, pharmaceutical compositions comprising solubilized estradiol are provided. In various embodiments, such compositions are encapsulated in soft capsules which may be vaginally inserted for the treatment of vulvovaginal atrophy.
Owner:THERAPEUTICSMD INC
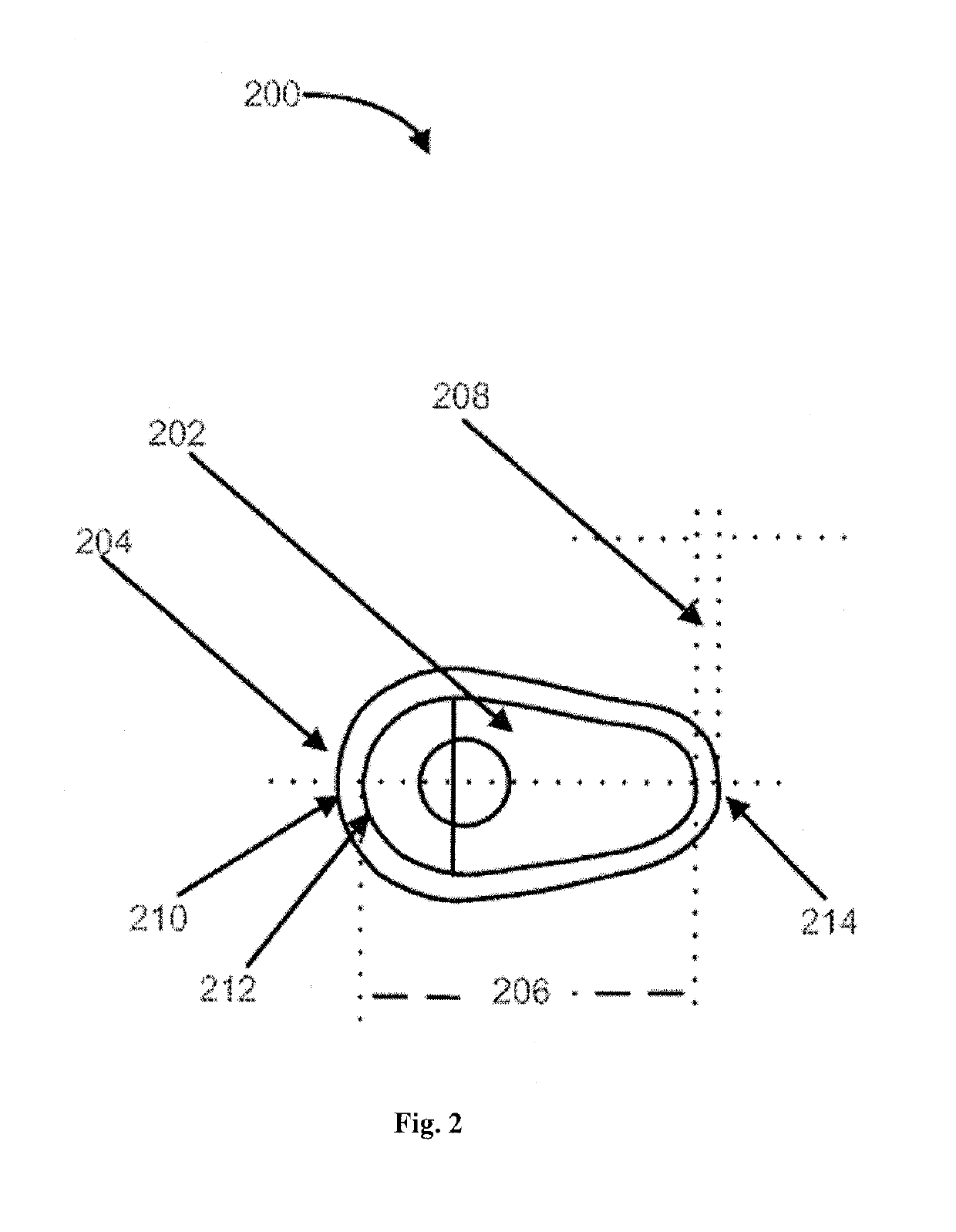
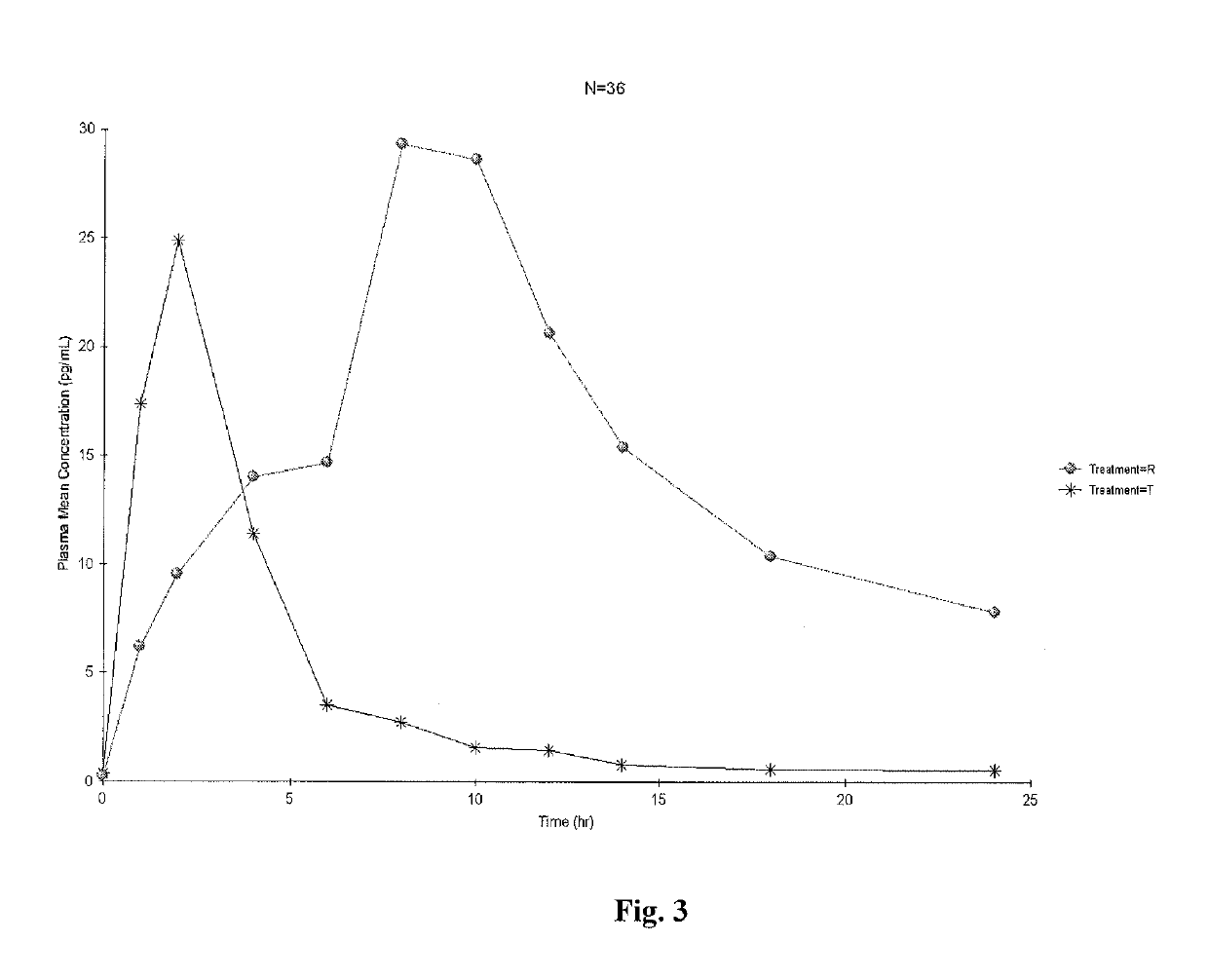
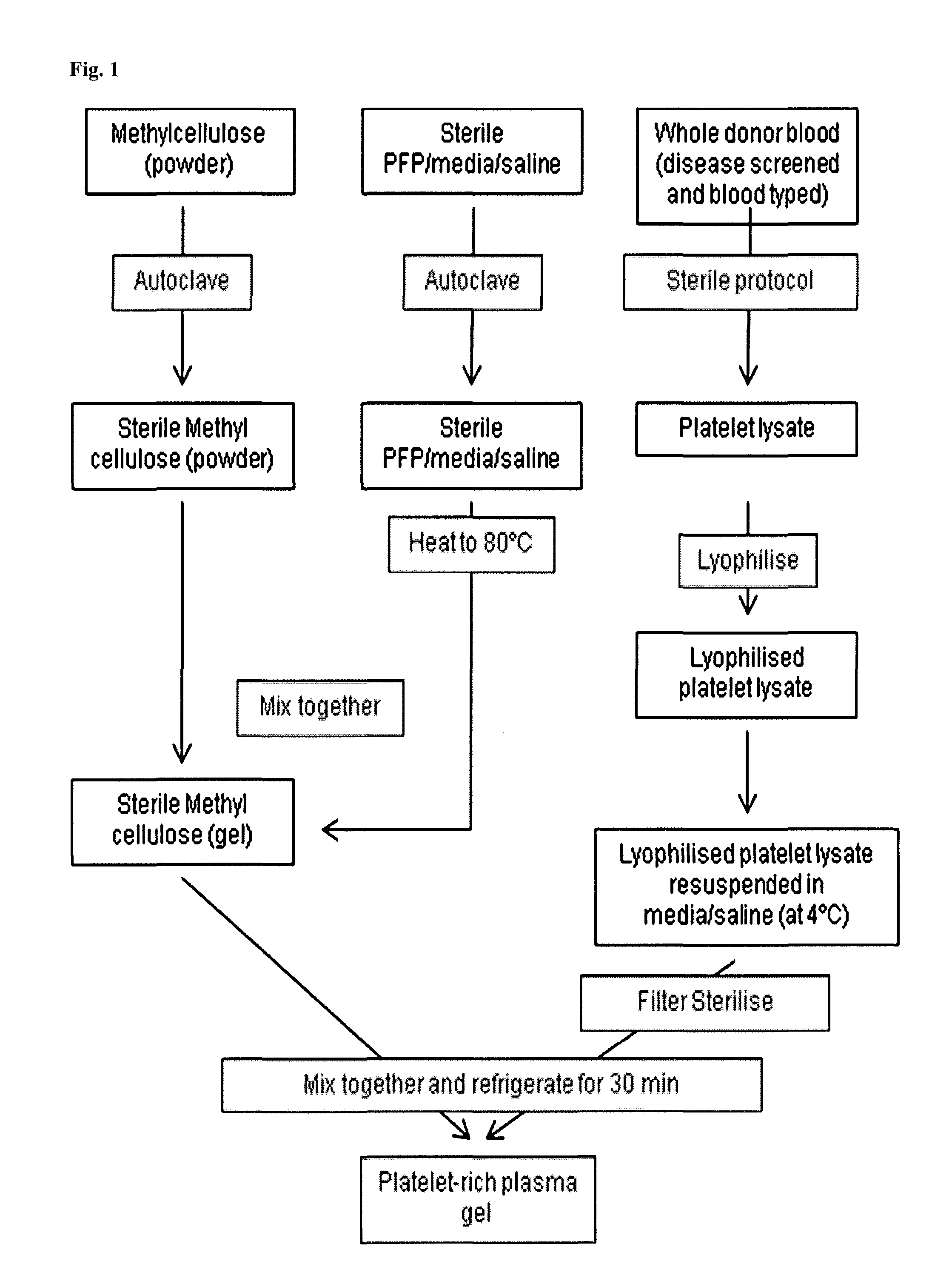
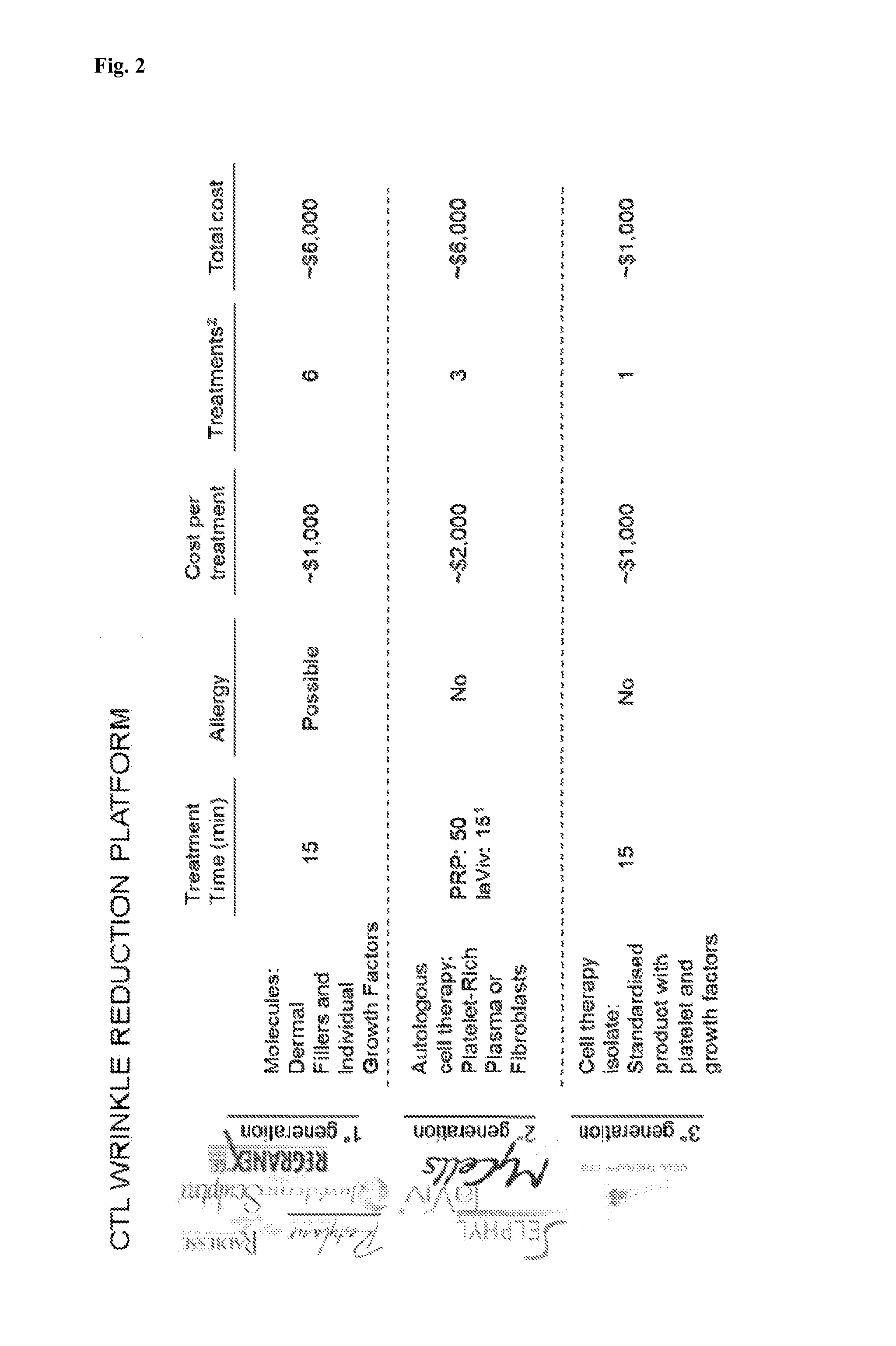
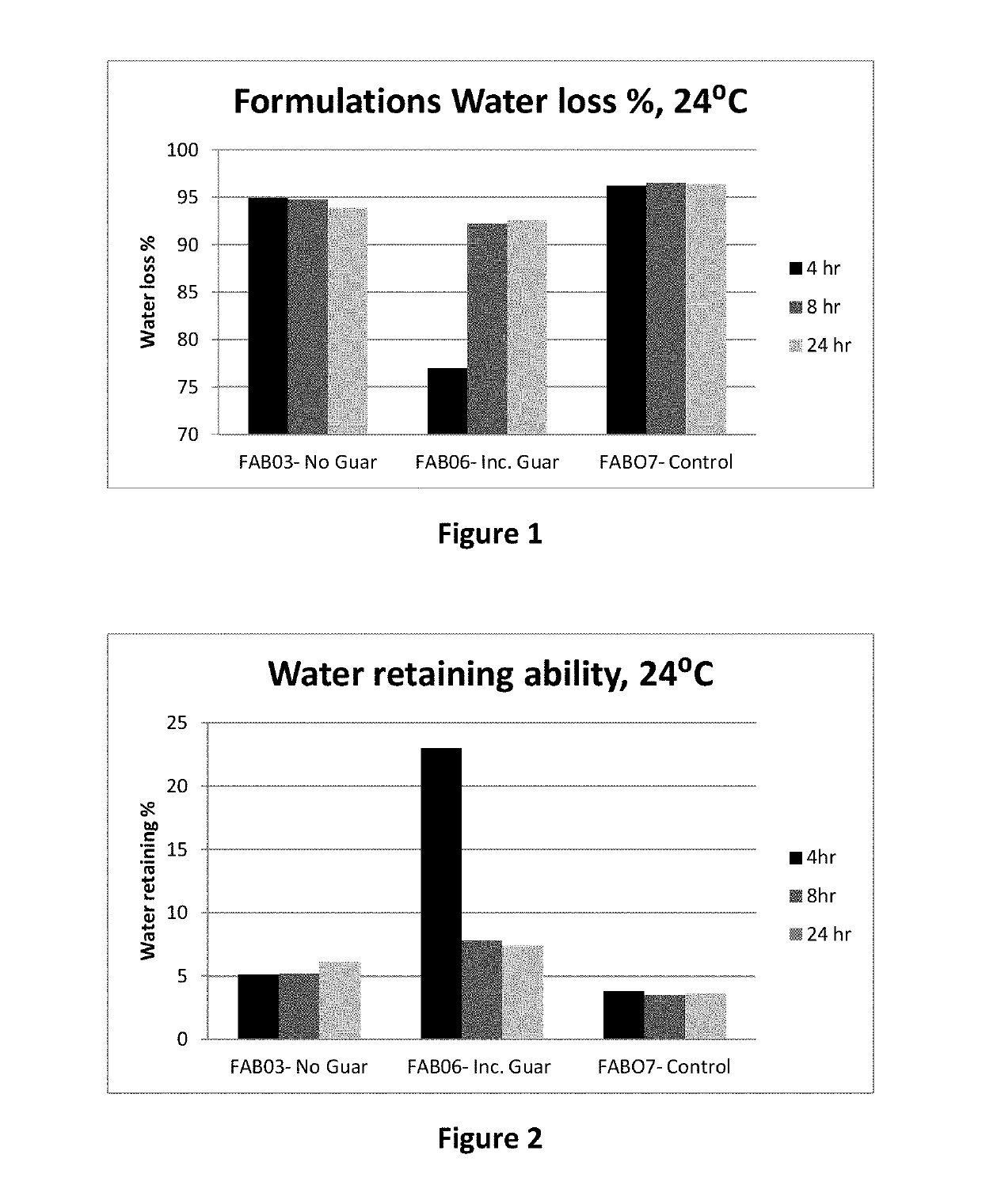
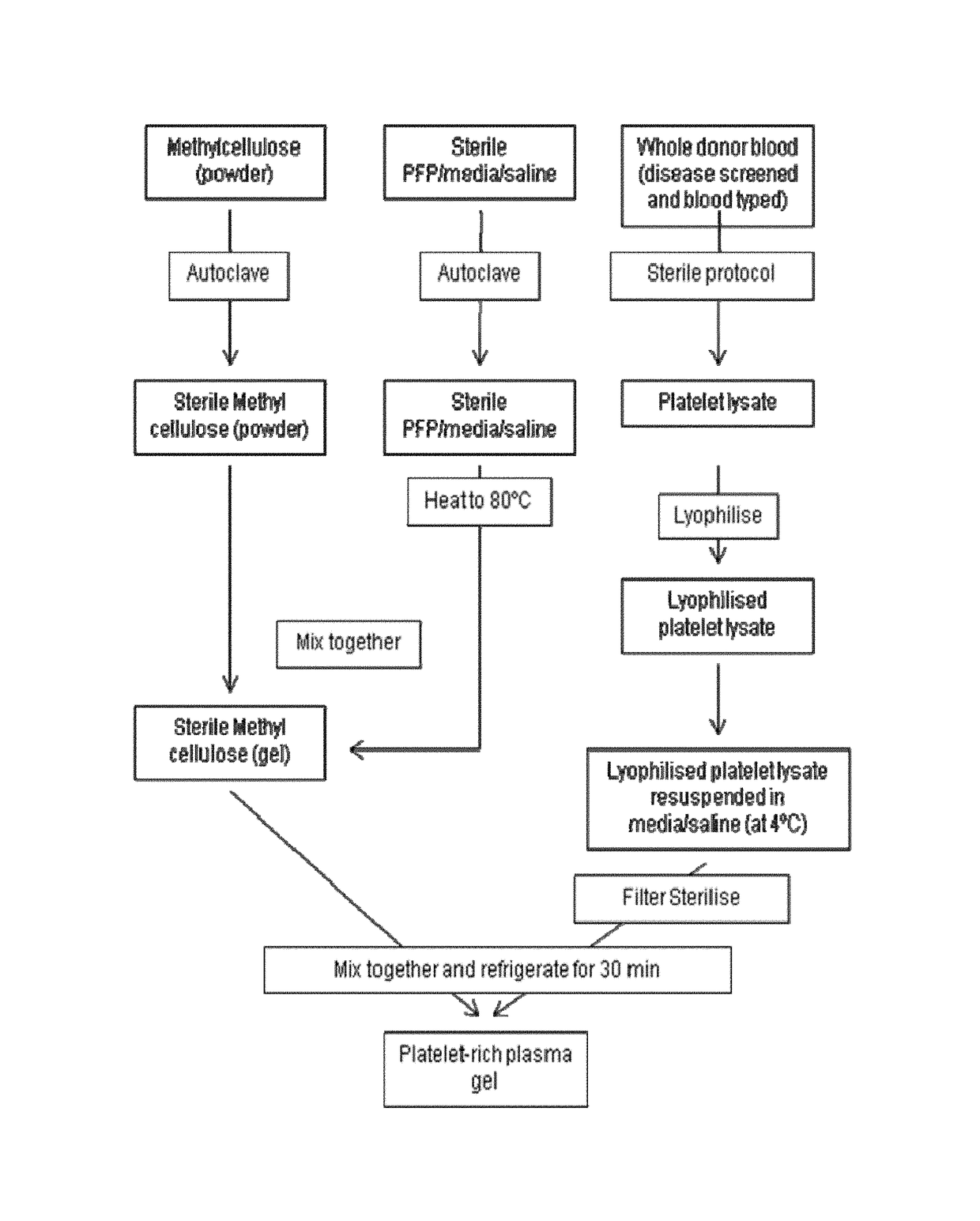
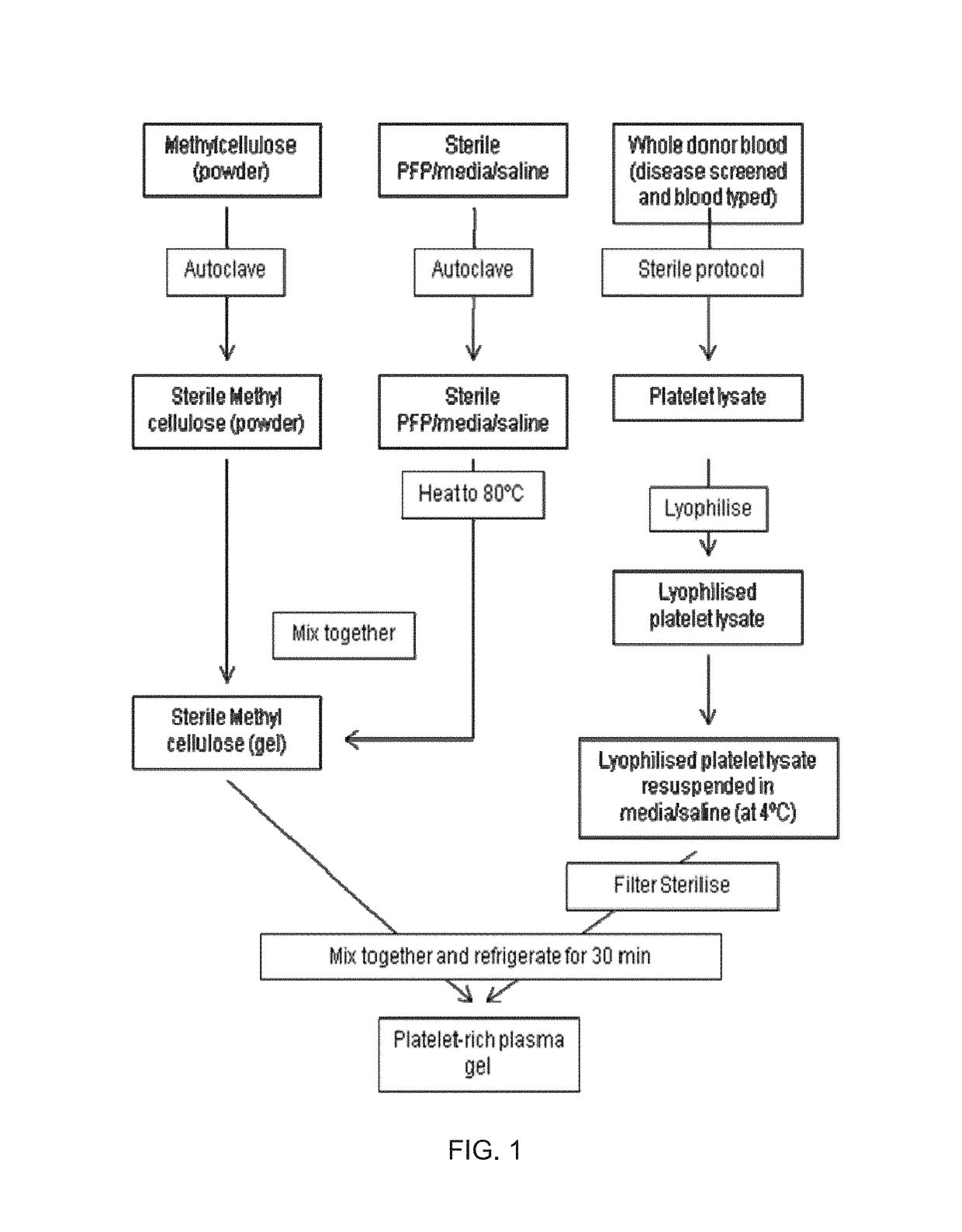
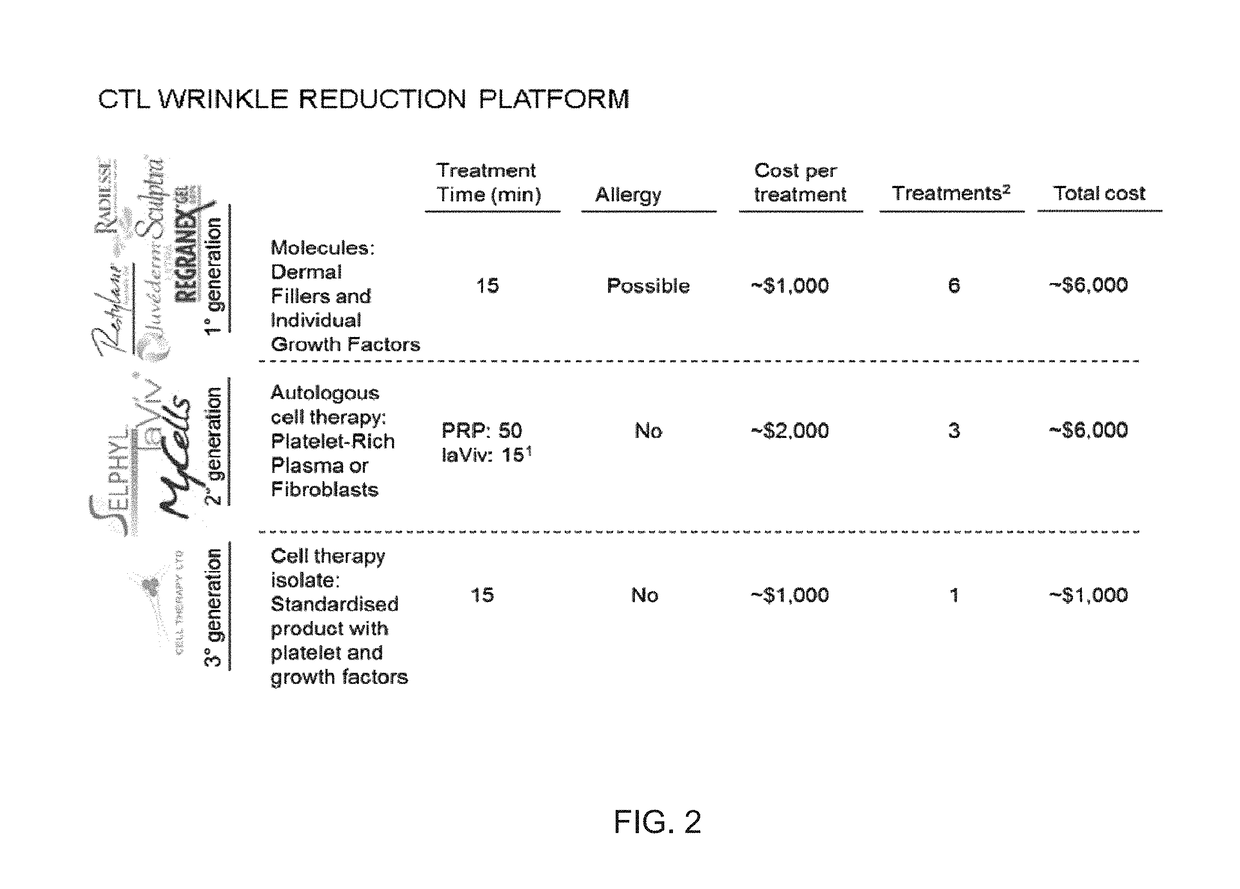
Platelet lysate gel
InactiveUS20140335195A1Easy to adaptCosmetic preparationsToilet preparationsWrinkle skinVaginal atrophy
The invention concerns a pharmaceutical composition comprising a platelet lysate and its use to treat a wound, an anal fissure, vaginal atrophy or a wrinkle.
Owner:CELL THERAPY LTD
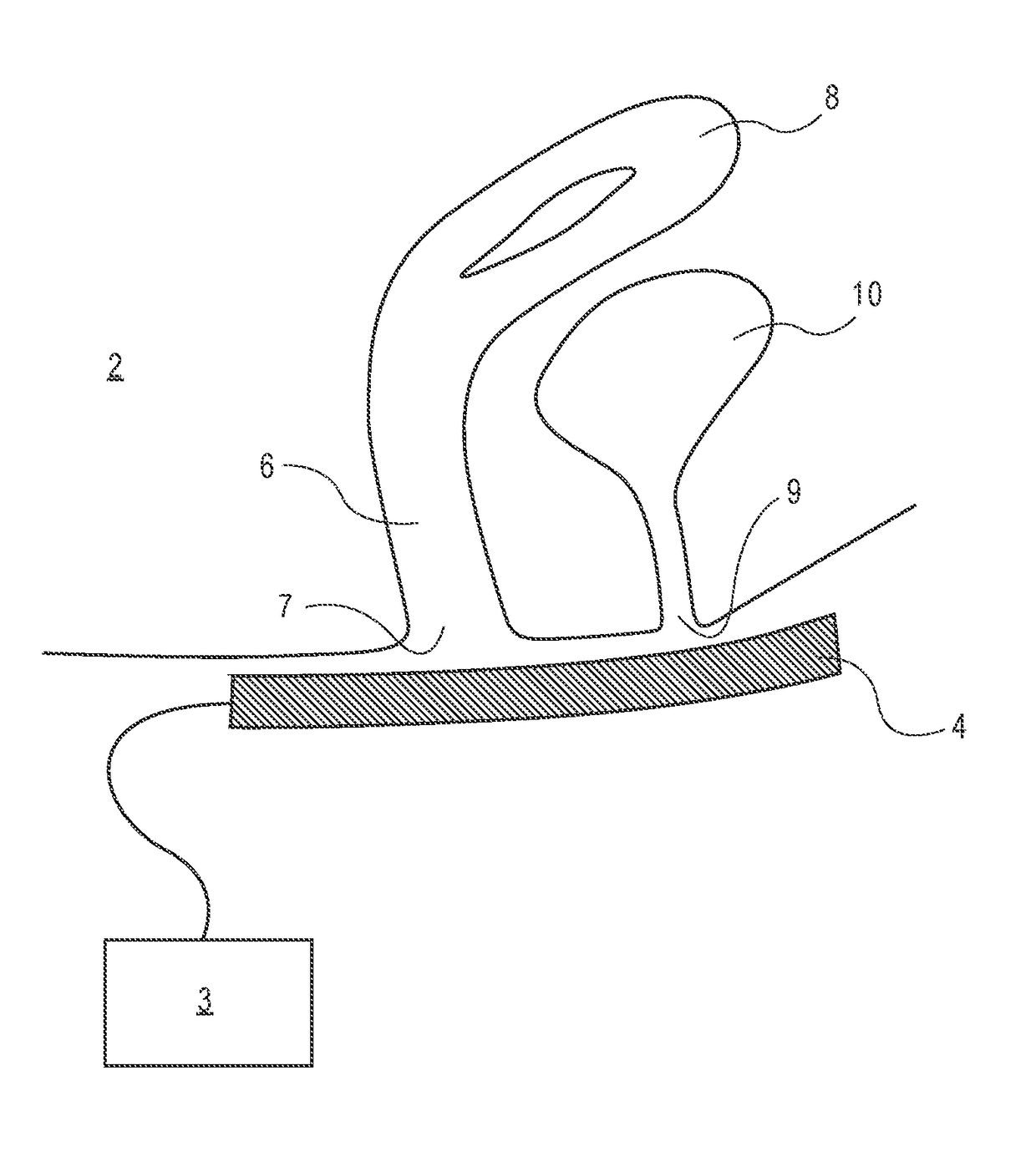
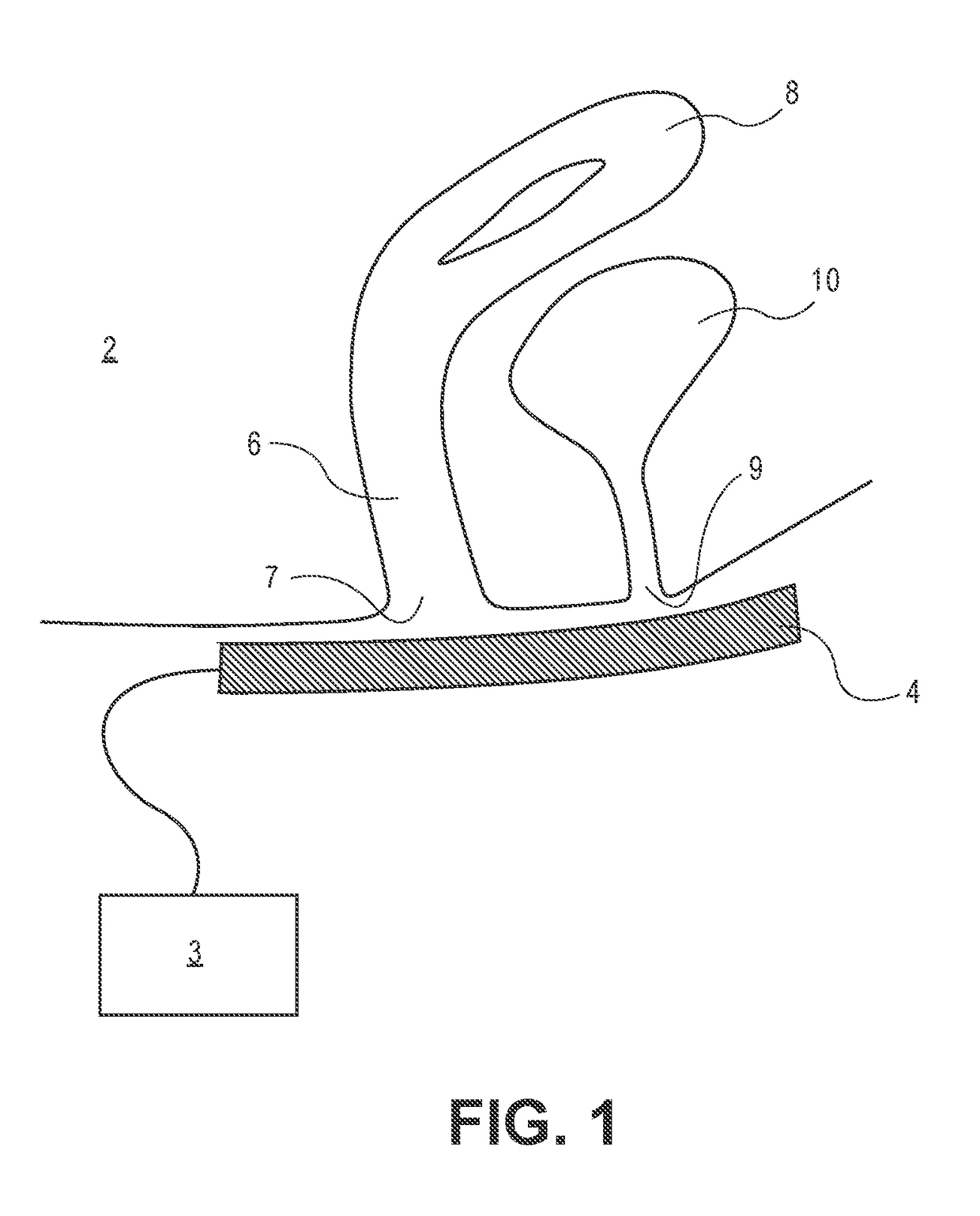
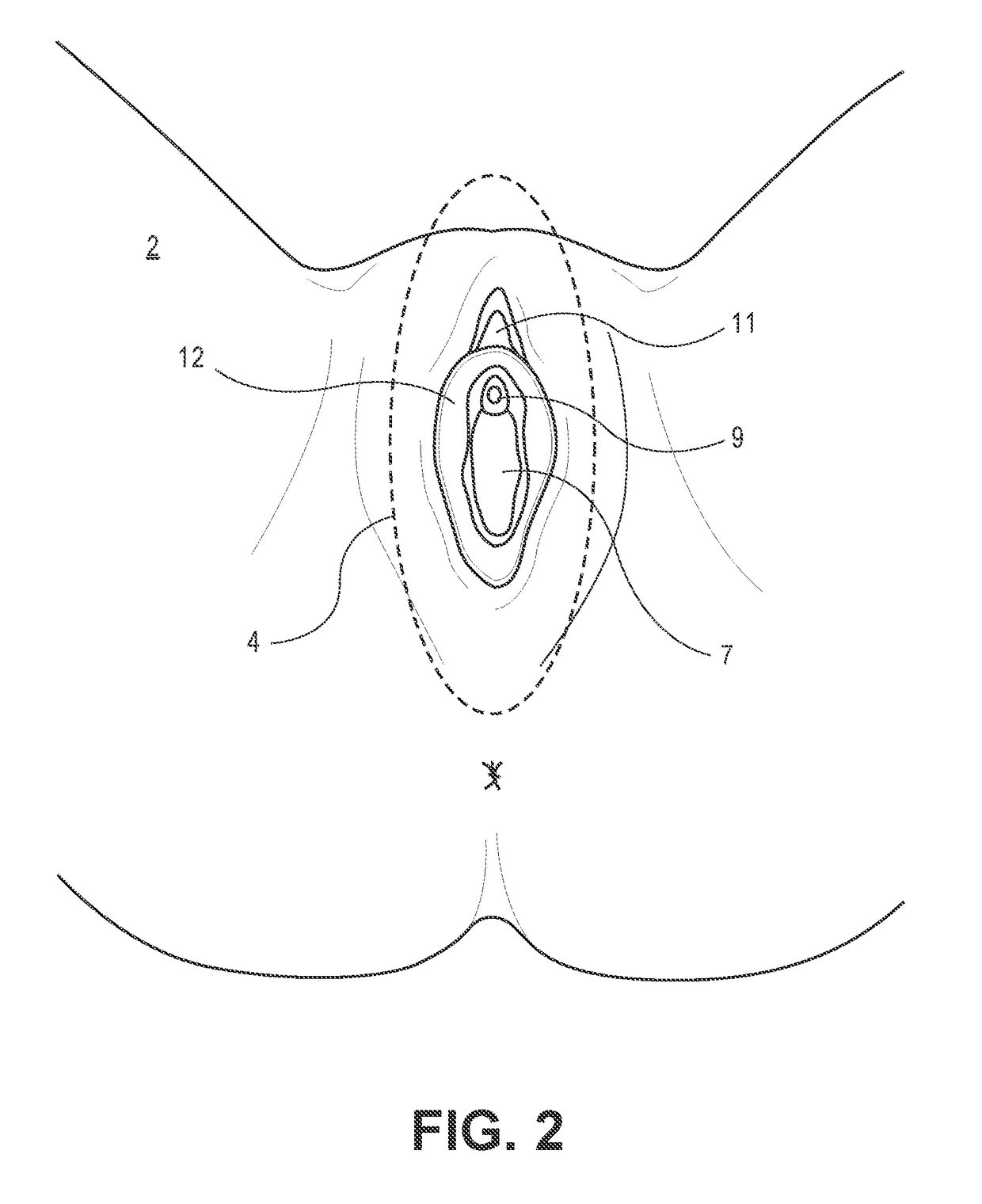
Device and method to treat vaginal atrophy
ActiveUS20160346568A1Good for healthIncreasing vaginal blood flowUltrasound therapySurgeryVaginal atrophyVaginal tissue
A method of treating vaginal tissue atrophy in a female subject, the method including the steps of engaging an energy delivery element with tissue in or around the subject's vagina; applying energy to the tissue from the energy delivery element; and increasing blood flow to internal vaginal tissue to an increased level above a baseline level of blood flow to the internal vaginal tissue, the increased level of blood flow to the internal vaginal tissue persisting after the applying step ceases. The invention also provides devices for performing this therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Device and method to treat vaginal atrophy
A method of treating vaginal tissue atrophy in a female subject, the method including the steps of engaging an energy delivery element with tissue in or around the subject's vagina; applying energy to the tissue from the energy delivery element; and increasing blood flow to internal vaginal tissue to an increased level above a baseline level of blood flow to the internal vaginal tissue, the increased level of blood flow to the internal vaginal tissue persisting after the applying step ceases. The invention also provides devices for performing this therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Compounds and forms of treatment for female sexual disorders
ActiveUS9750716B2Effective treatmentIncrease intensityOrganic active ingredientsPharmaceutical delivery mechanismFemale Orgasmic DisorderVaginal atrophy
Owner:GTO PHARMA LLC
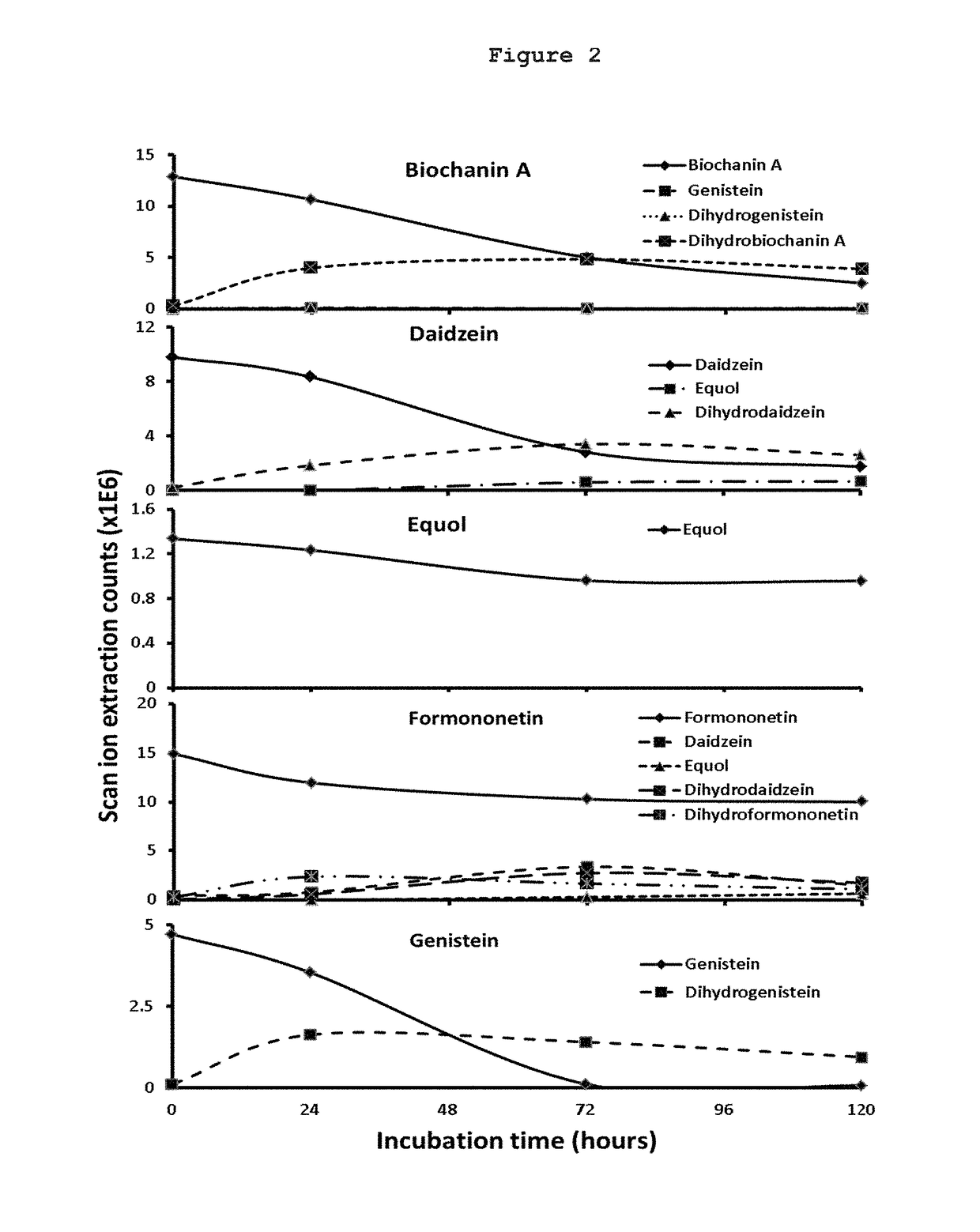
Phytoestrogen product of red clover and pharmaceutical uses thereof
ActiveUS20170266246A1Avoid symptomsChemical property predictionMedical simulationMedicineVaginal atrophy
The present invention provides compositions comprising optimized ratios of Red clover phytoestrogens as determined by a proprietary physiologically based pharmacokinetic and pharmacodynamic model. The compositions are useful for modulating, preventing or treating postmenopausal or climacteric symptoms, which include but are not limited to bone loss, bone remodeling, hot flushes and vaginal atrophy. The present invention also provides methods for modulating, preventing or treating postmenopausal or climacteric symptoms using the compositions disclosed herein.
Owner:MAIN HARBOUR BIOTECH INT LTD
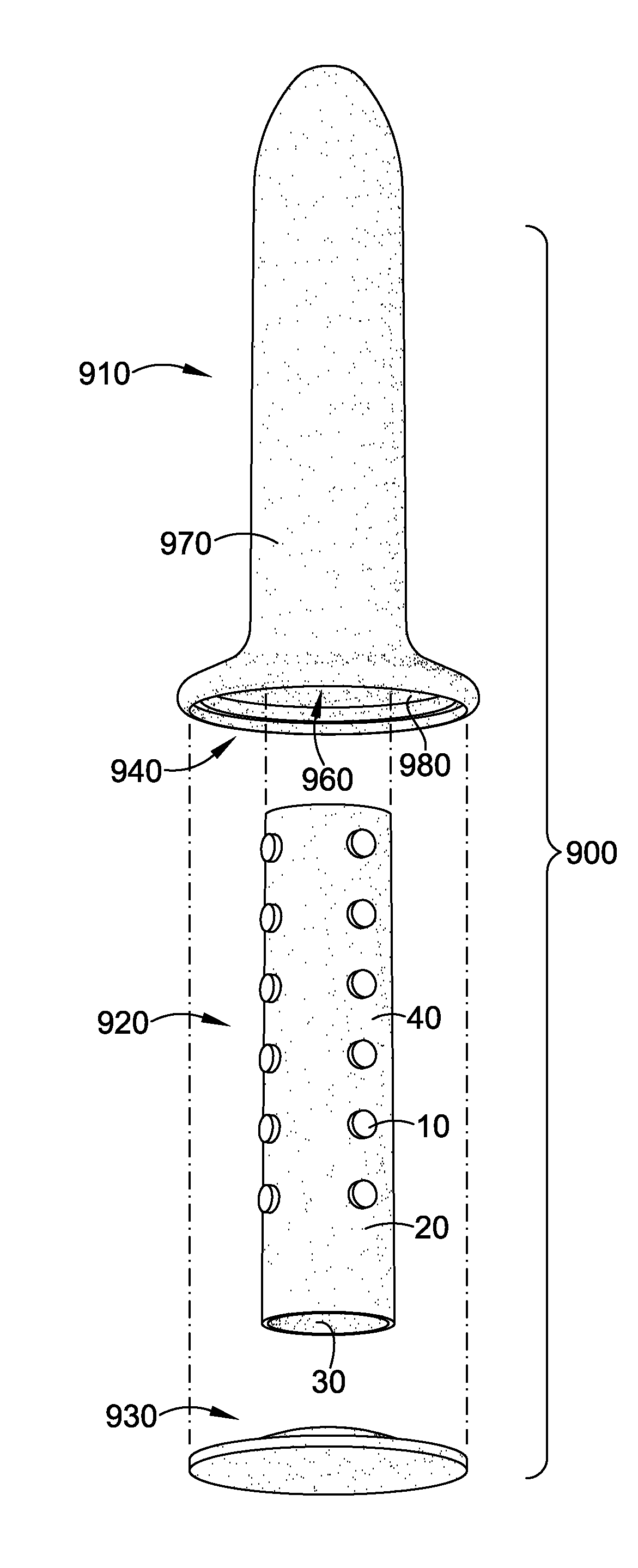
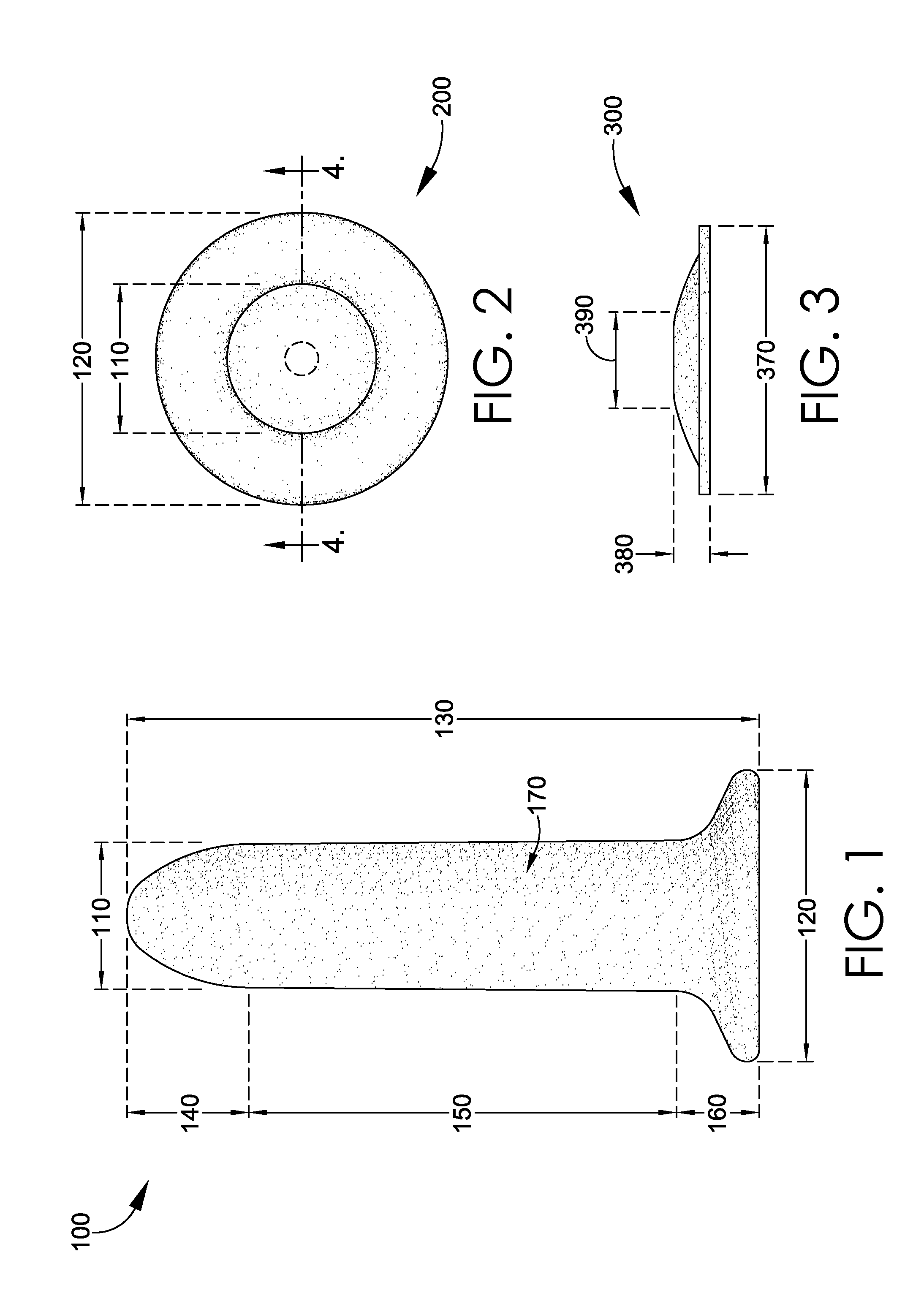
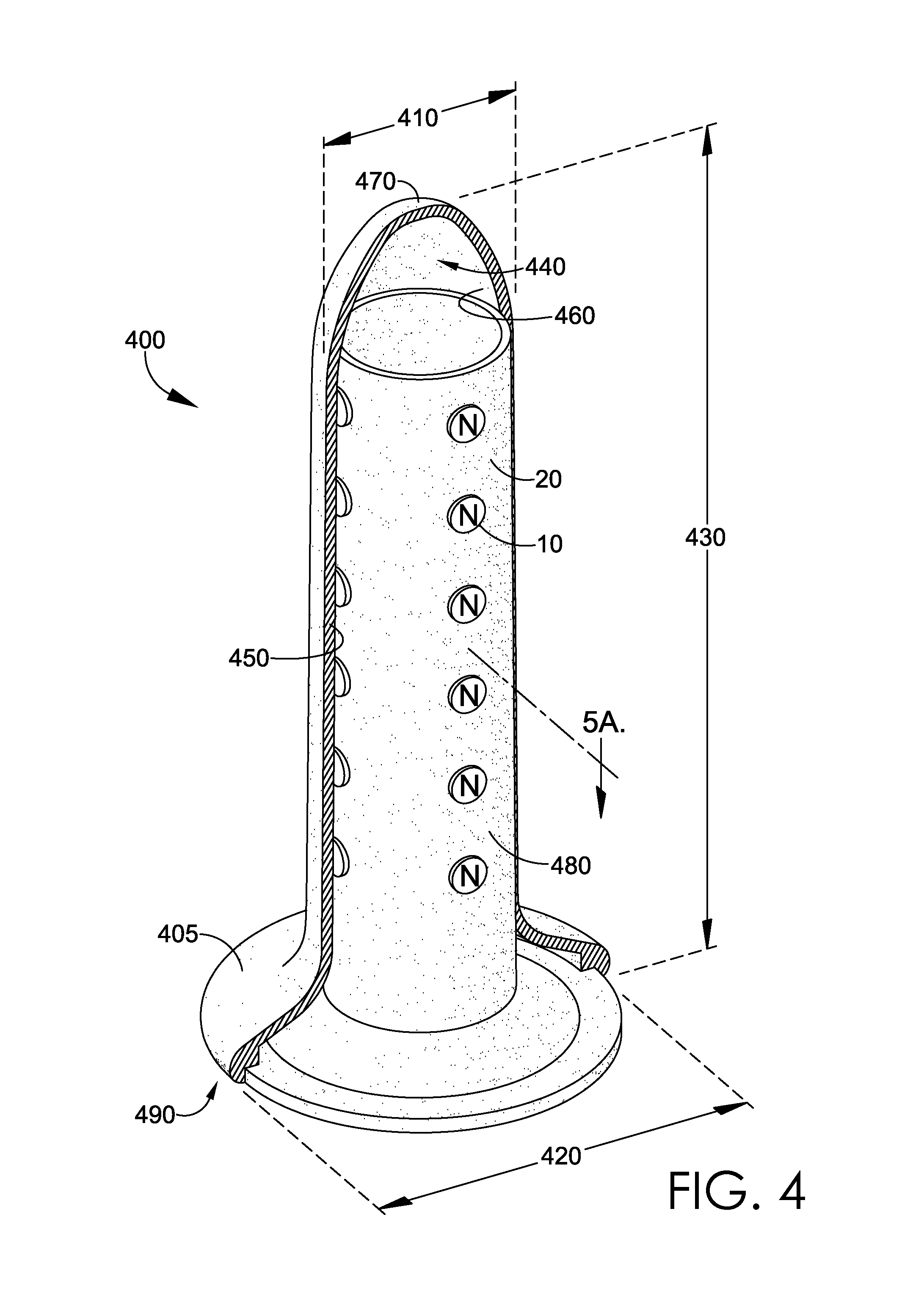
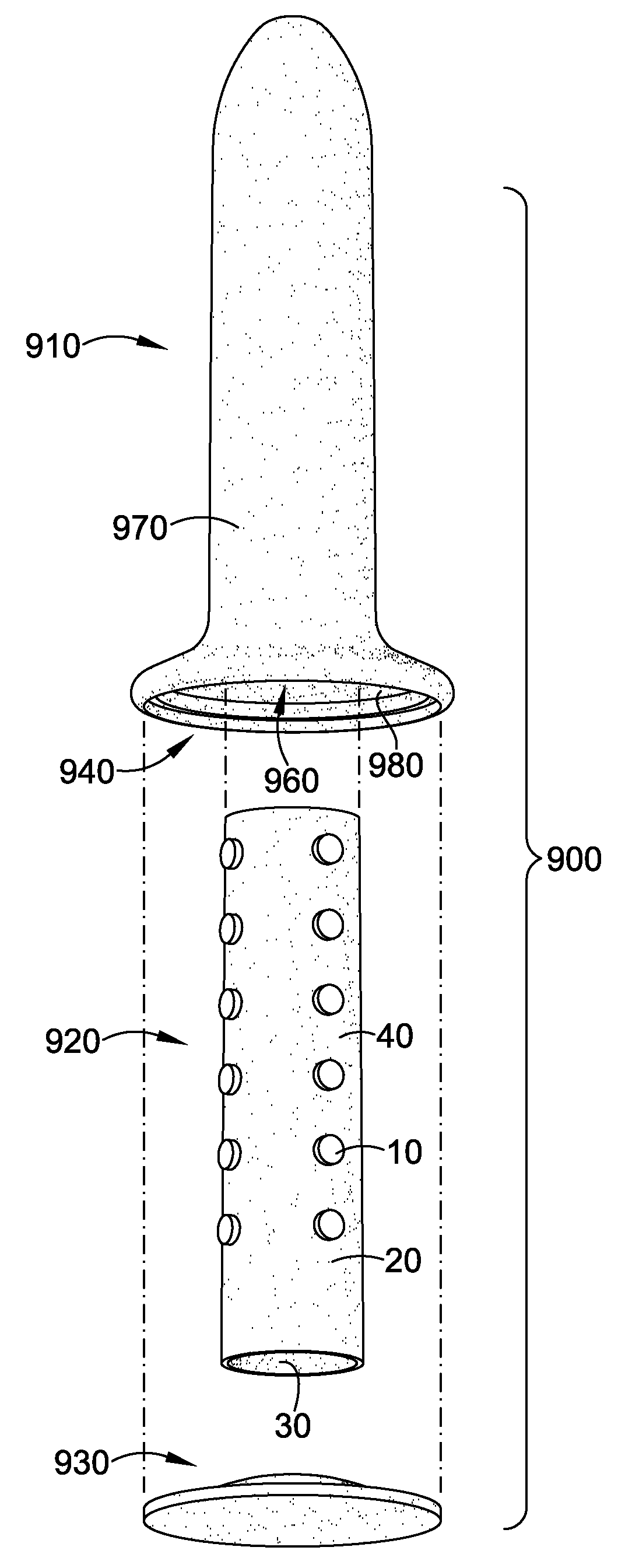
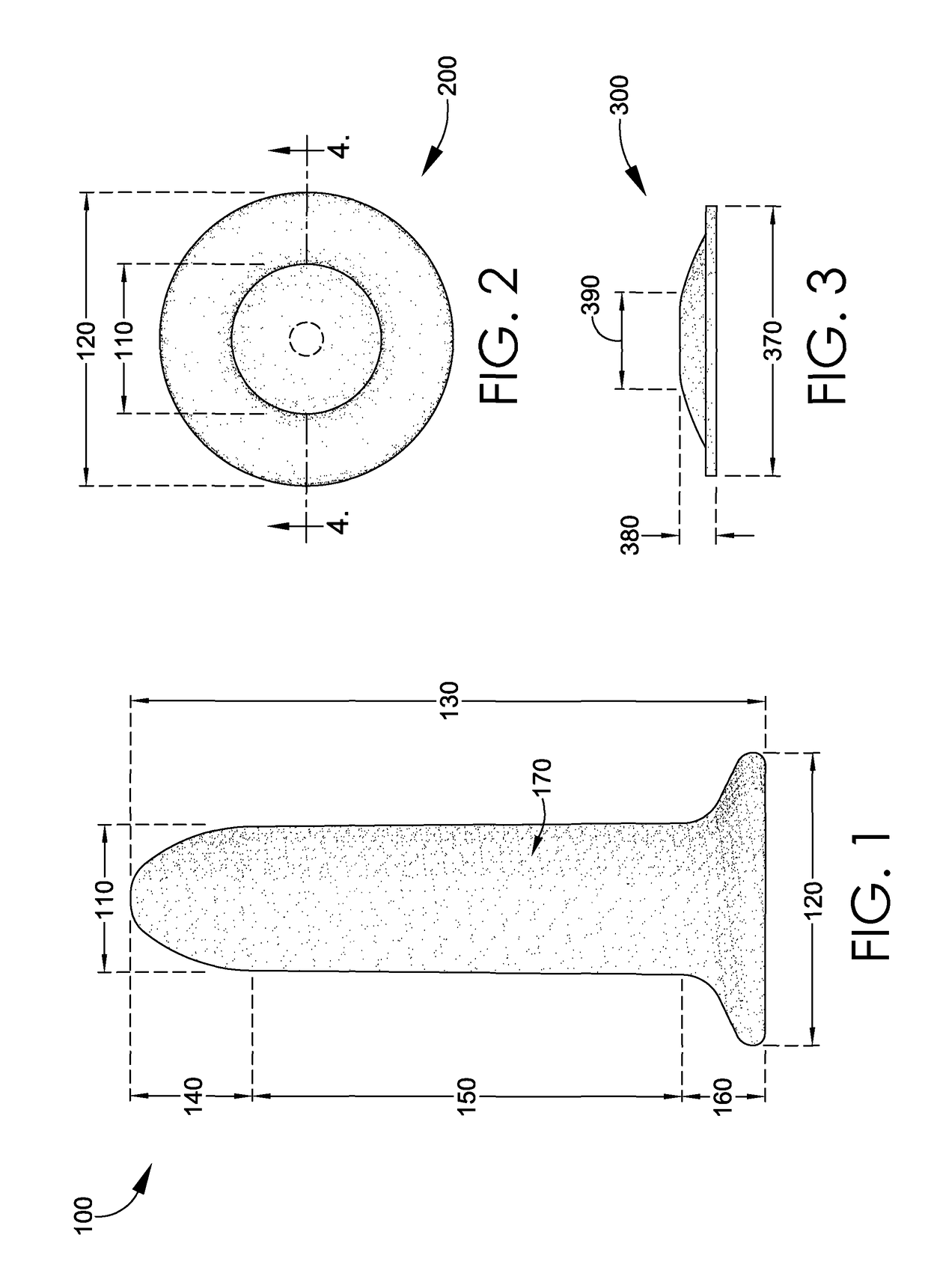
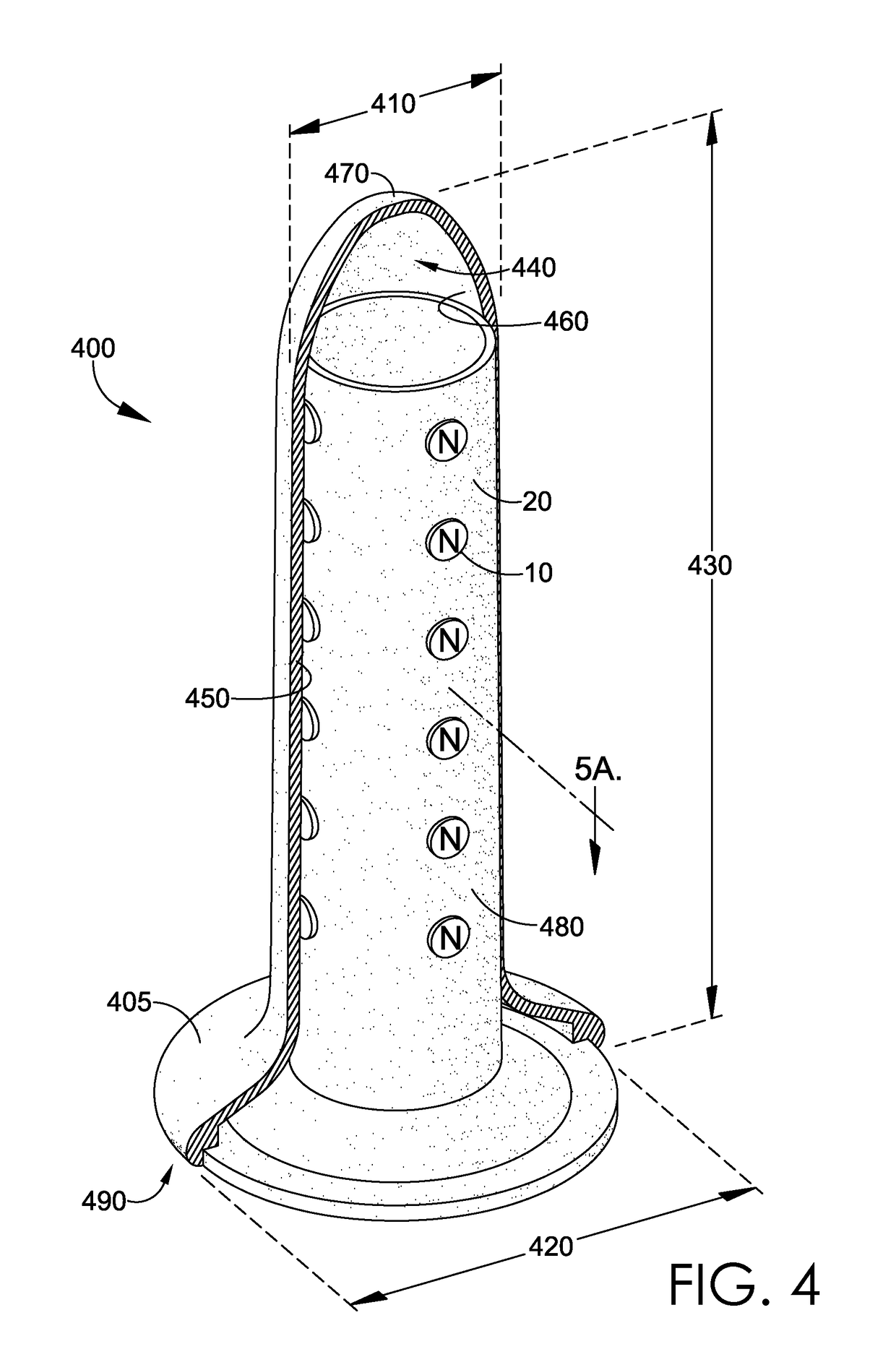
Magnetic vaginal dilator
Aspects of the present invention are related to magnetic medical devices for the treatment of chronic medical conditions such as Vulvodynia, Vaginismus, Vaginal Stenosis, Vaginal Atrophy, among others. The magnetic medical device in accordance with the present invention is of a generally elongated shape having an ogive top end, a middle portion of an active diameter, and a bottom end of a passive diameter. The magnetic medical device has an array of magnets within, where the magnets generate a negative magnetic field adjacent to an external surface of the magnetic medical device.
Owner:VUVATECH
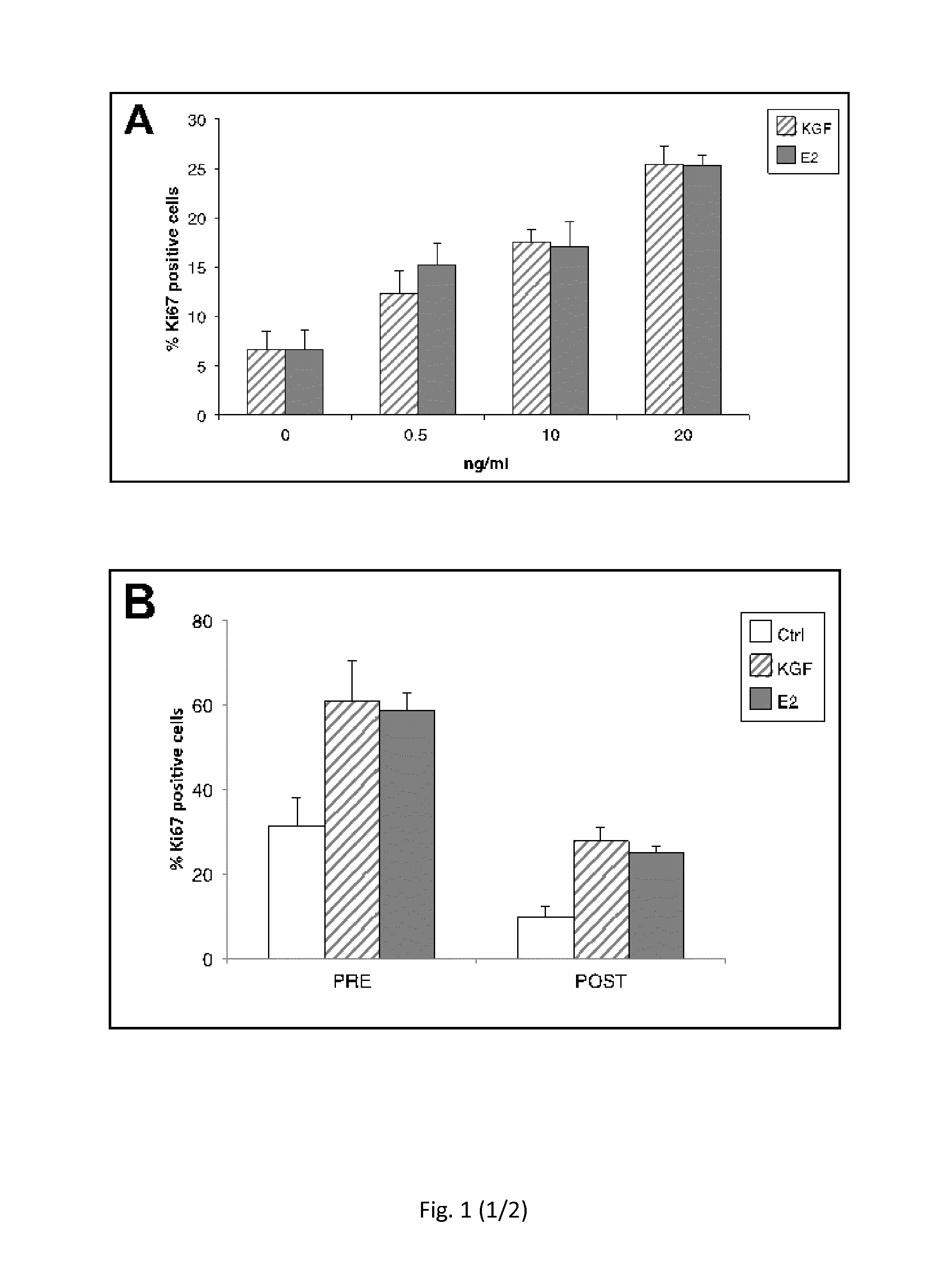
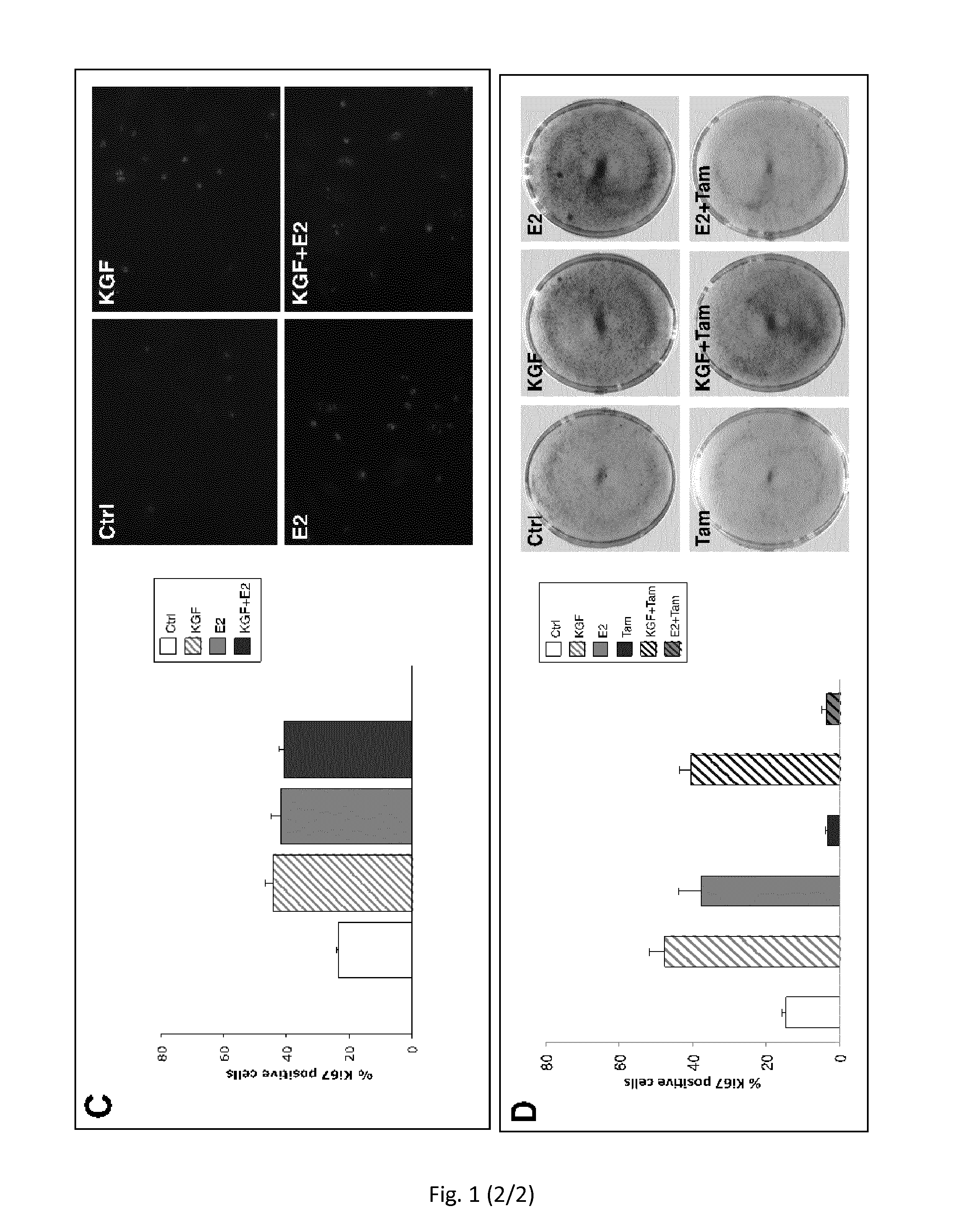
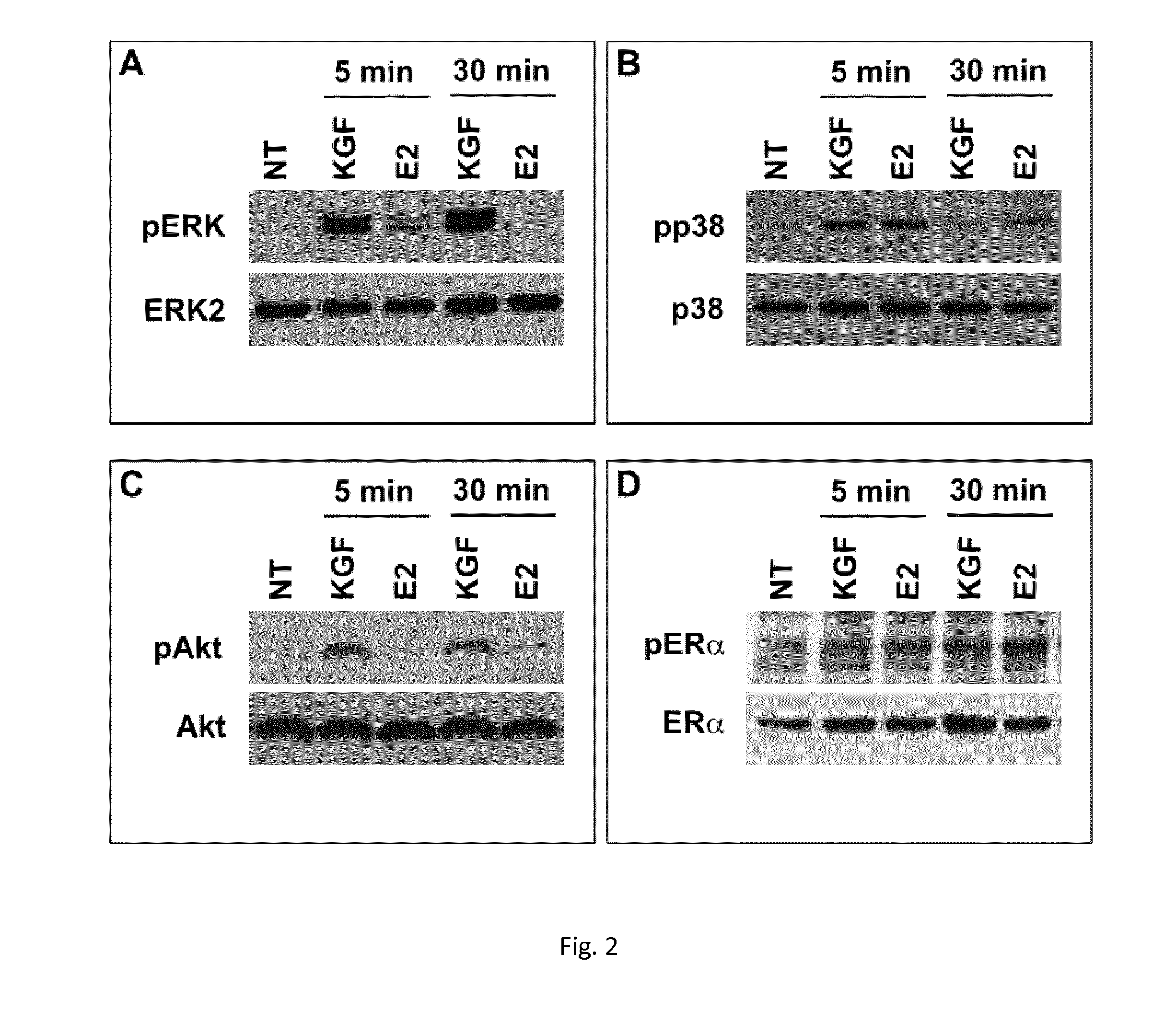
Use of kgf in the treatment of menopausal disorders
ActiveUS20150224172A1Less elasticMore fragileBiocidePeptide/protein ingredientsVaginal PainRadical radiotherapy
The present invention relates to the use of keratinocyte growth factor (KGF / FGF7) and pharmaceutical compositions thereof in the treatment of vaginal atrophy, dysuria, vaginal pain and / or vaginal dryness induced by a post-menopausal state, by surgical intervention, by illness and / or by chemotherapy or radiotherapy treatments.
Owner:UNIV DEGLI STUDI DI ROMA LA SAPIENZA
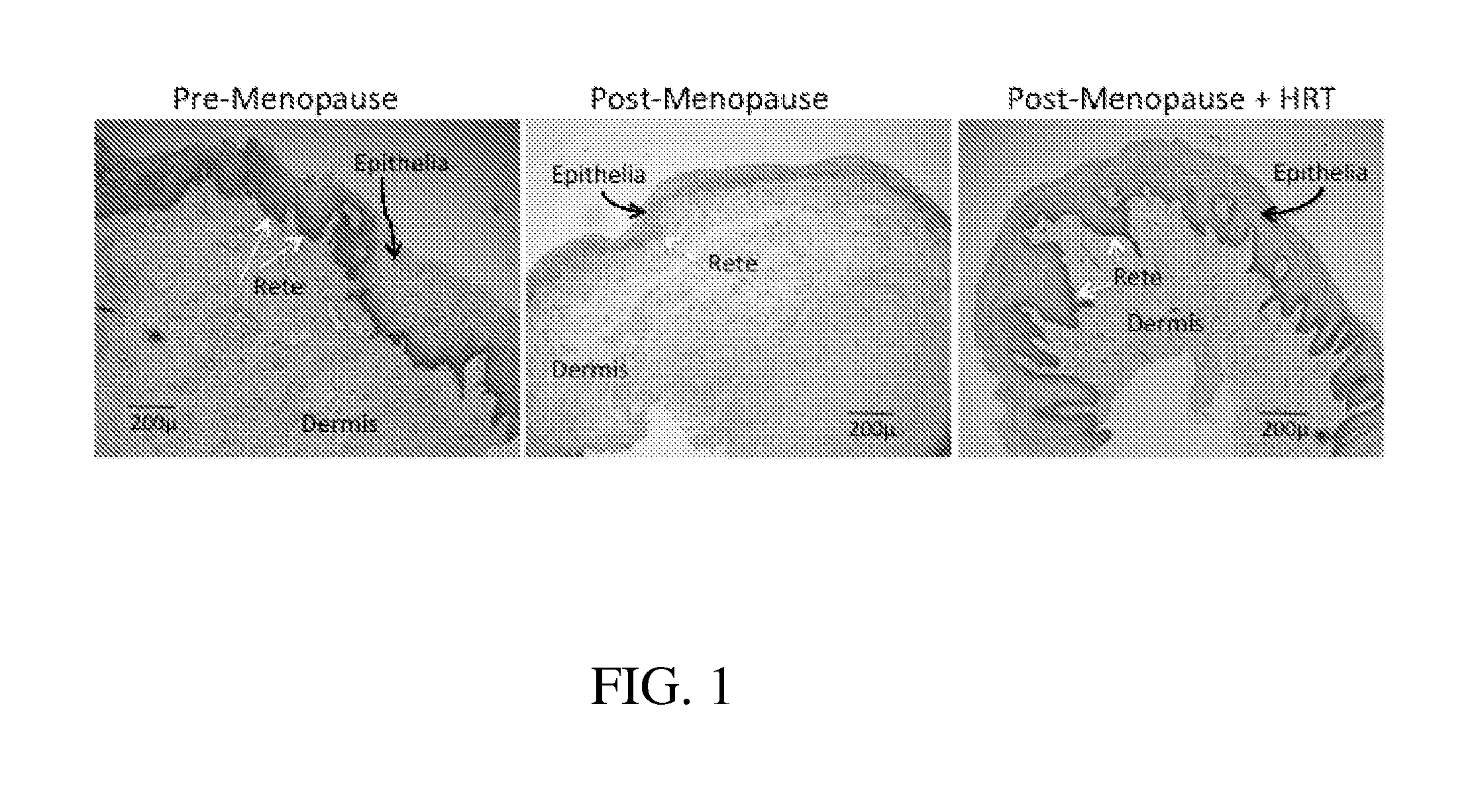
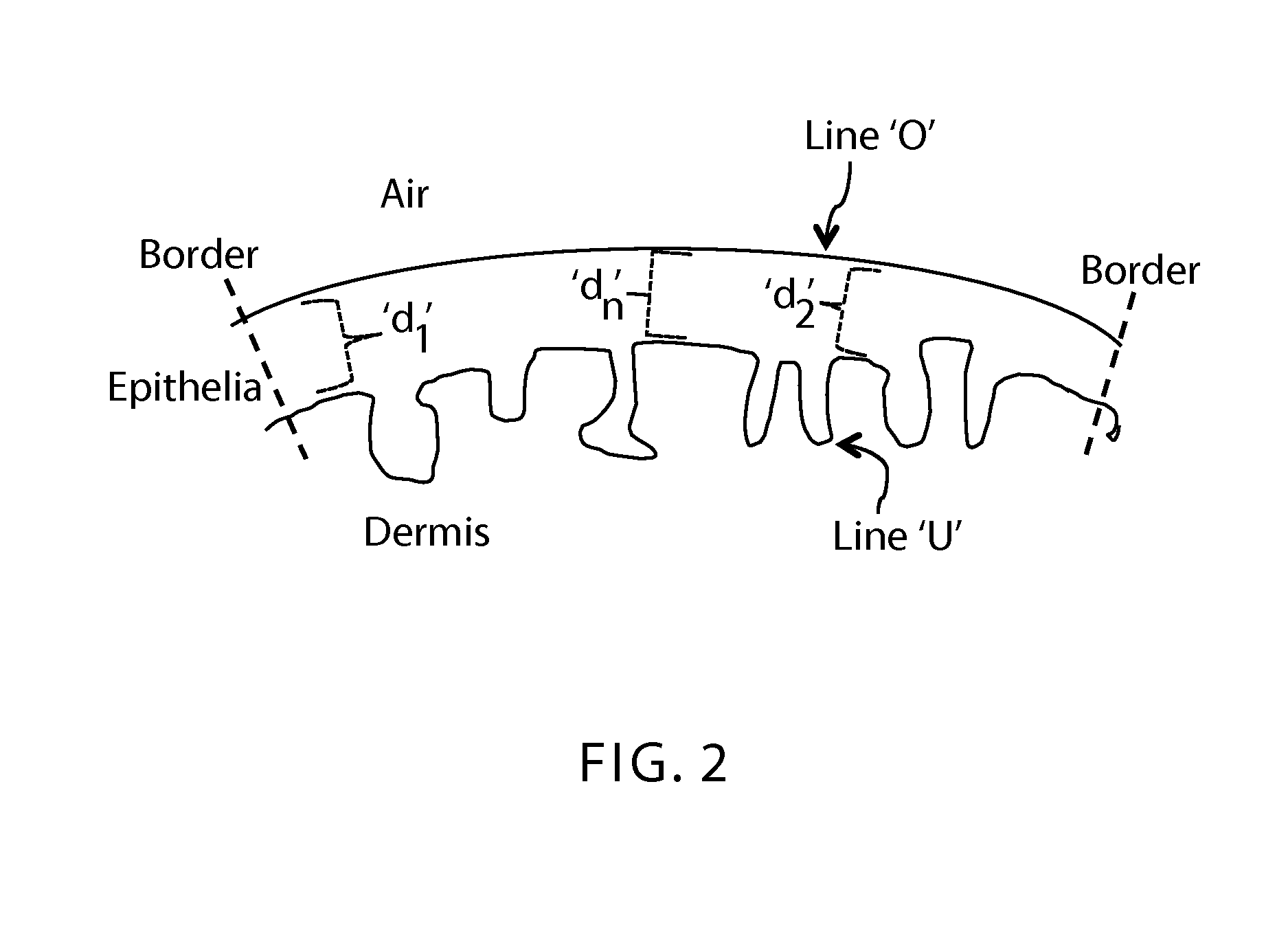
Methods for assessing vaginal atrophy
InactiveUS20160007906A1Organic active ingredientsMicrobiological testing/measurementVaginal atrophyVagina
An array of methods for assessing vaginal atrophy are disclosed. The methods may be used alone or in combination with a treatment or as part of a kit.
Owner:THE PROCTER & GAMBLE COMPANY
Methods for prevention and treatment of urogenital atrophy of menopause by contact vasodilators
InactiveUS20210069102A1Inhibition formationPowder deliveryAerosol deliveryAngiotensin Receptor BlockersAngiotensin receptor ii
Methods are provided to prevent and to treat urogenital (e.g., urovaginal) atrophy syndrome of menopause by using a vasodilator such as an angiotensin receptor blocker, ACE inhibitor, or calcium channel blocker. More particularly, the methods do not employ orally administered vasodilators, but instead vasodilators that are administered through contact with the epidermis, e.g., in topical or other form suitable for contact with tissues to be treated.
Owner:WEINBERG ASSA
Magnetic vaginal dilator
Aspects of the present invention are related to magnetic medical devices for the treatment of chronic medical conditions such as Vulvodynia, Vaginismus, Vaginal Stenosis, Vaginal Atrophy, among others. The magnetic medical device in accordance with the present invention is of a generally elongated shape having an ogive top end, a middle portion of an active diameter, and a bottom end of a passive diameter. The magnetic medical device has an array of magnets within, where the magnets generate a negative magnetic field adjacent to an external surface of the magnetic medical device.
Owner:VUVATECH
Compounds and forms of treatment for female sexual disorders
ActiveUS20170340602A1Effective treatmentIncrease intensityOrganic active ingredientsPharmaceutical delivery mechanismFemale Orgasmic DisorderVaginal atrophy
Owner:GTO PHARMA LLC
Methods and Compositions for Treating Urogenital Disorders
Glycerophosphate salts have been found to drastically improve the treatment of urogenital disorders such as urethral strictures and vaginal atrophy. Methods, devices and kits are described for treating urogenital disorders using a composition comprising an effective amount of a glycerophosphate salt.
Owner:PRELIEF
Compositions comprising sulfated polysaccharides and uses thereof
Owner:ALGAMED THERAPEUTICS A M T
Methods and kits for treating vaginal and vulvar vestibule mucosa disorders
A method of treating a vaginal or vulvar vestibule mucosa disorder involves administering a composition comprising a hyaluronic acid or a salt thereof to the vagina, vulvar or vulvar vestibule of an individual that has the disorder to treat the disorder. The method may be used to treat vaginal atrophy or vulvar vestibulitis syndrome, among other vulvovaginal mucosa disorders.
Owner:LABES VIVACY
Platelet lysate gel
ActiveUS20170326177A1Easy to adaptCosmetic preparationsToilet preparationsWrinkle skinVaginal atrophy
The invention concerns a pharmaceutical composition comprising a platelet lysate and its use to treat a wound, an anal fissure, vaginal atrophy or a wrinkle.
Owner:CELL THERAPY LTD
Vaginal inserted estradiol pharmaceutical compositions and methods
ActiveUS20200206242A9Reduce in quantityIncrease the number ofOrganic active ingredientsPharmaceutical non-active ingredientsVaginal atrophyPharmaceutical drug
According to various embodiments of this disclosure, pharmaceutical compositions comprising solubilized estradiol are provided. In various embodiments, such compositions are encapsulated in soft capsules which may be vaginally inserted for the treatment of vulvovaginal atrophy.
Owner:THERAPEUTICSMD INC
Methods for assessing vaginal atrophy
An array of methods for assessing vaginal atrophy are disclosed. The methods may be used alone or in combination with a treatment or as part of a kit.
Owner:THE PROCTER & GAMBLE COMPANY
Treatment of vaginal atrophy in women with cardiovascular pathology risk
ActiveUS8835414B2Reduce absorptionReversed vaginal atrophyOrganic active ingredientsPharmaceutical delivery mechanismSelf limitingEstriol
The present invention relates to the use of estriol in the preparation of a pharmaceutical formulation for vaginal administration with the capacity to self-limit the absorption of estriol, for the prevention and / or the treatment of urogenital atrophy in women said women having a high probability of suffering from a cardiovascular pathology or suffering or having suffered from a cardiovascular pathology.
Owner:ITF RISECH FARMA S L U
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com