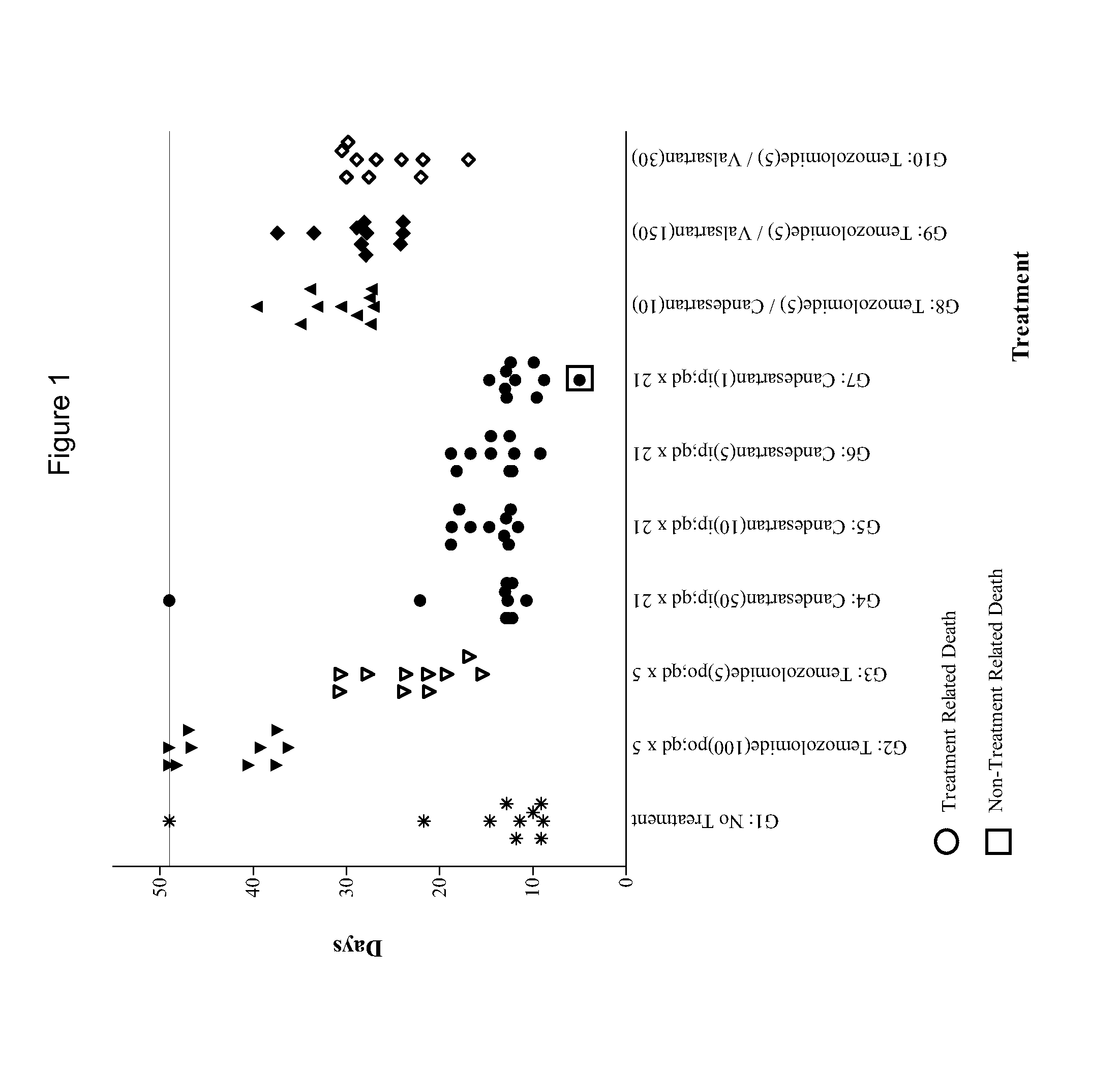
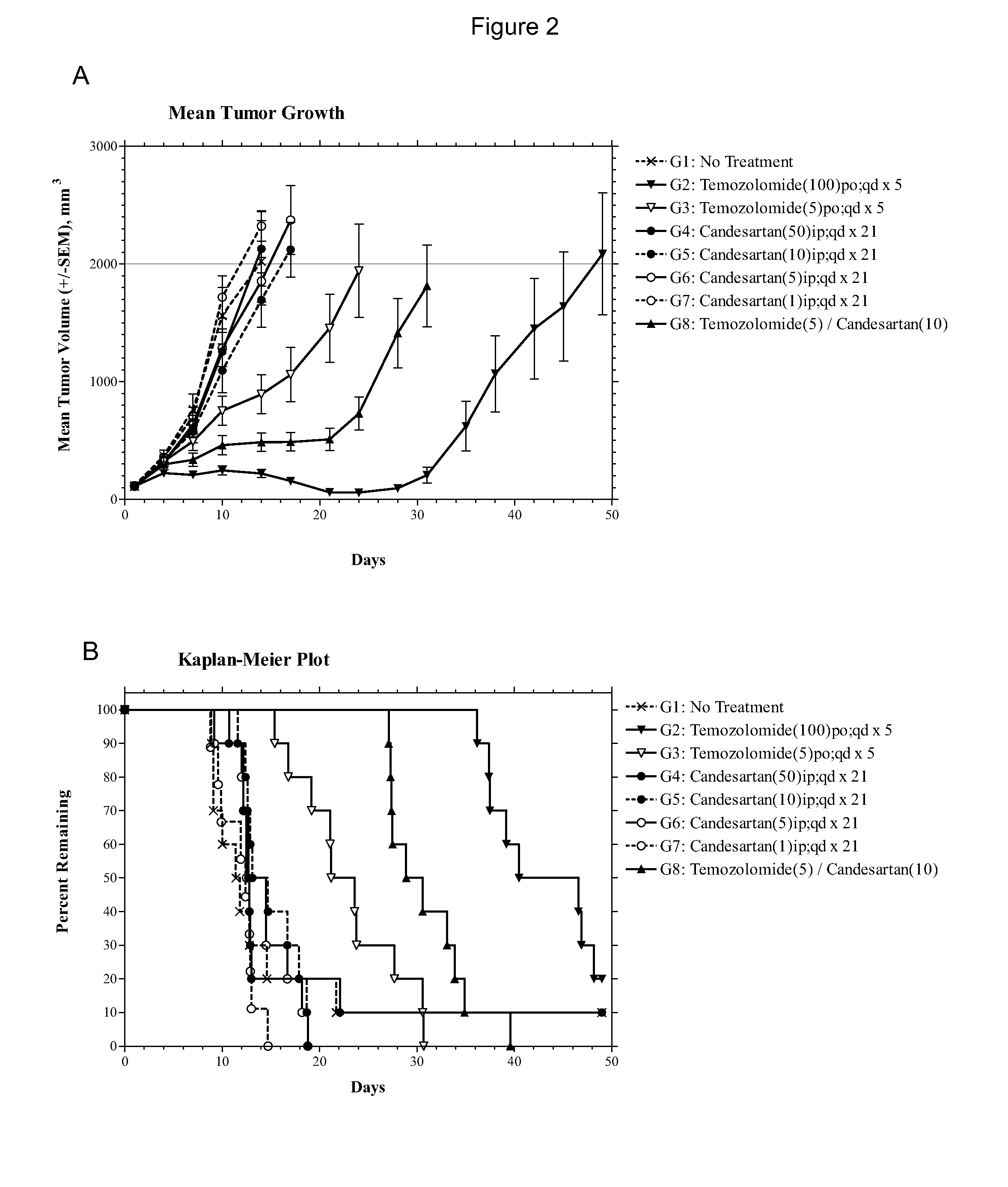
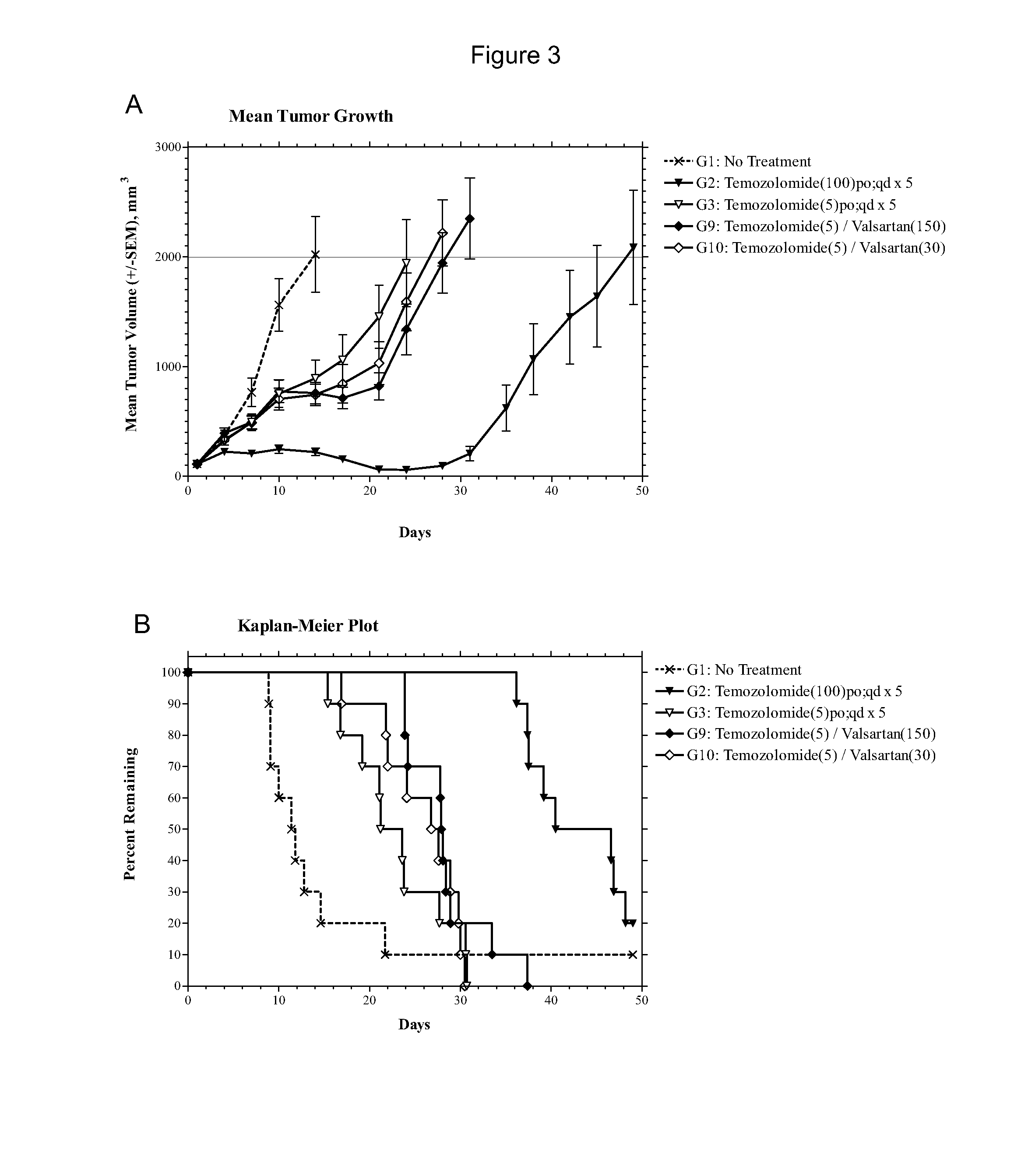
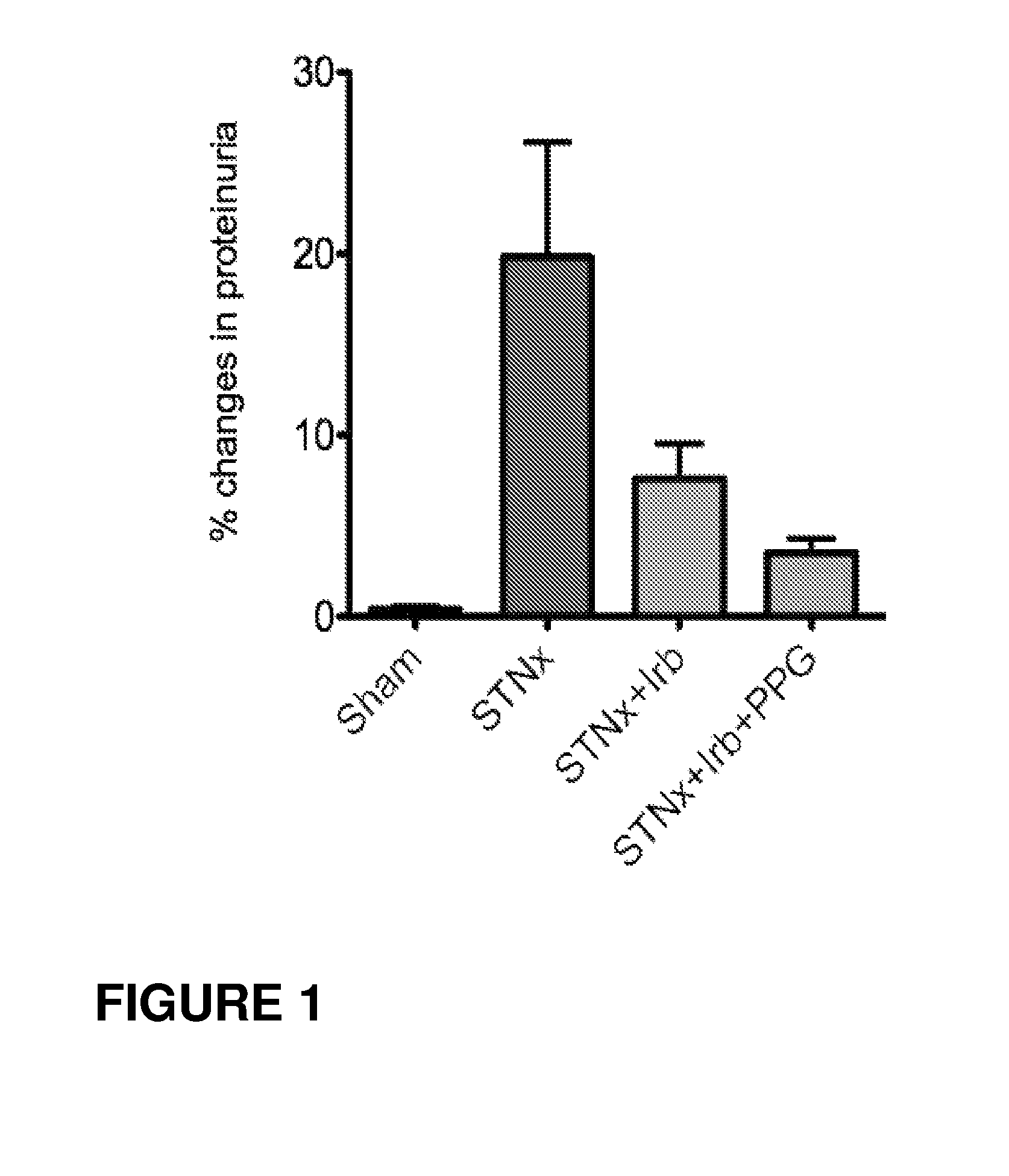
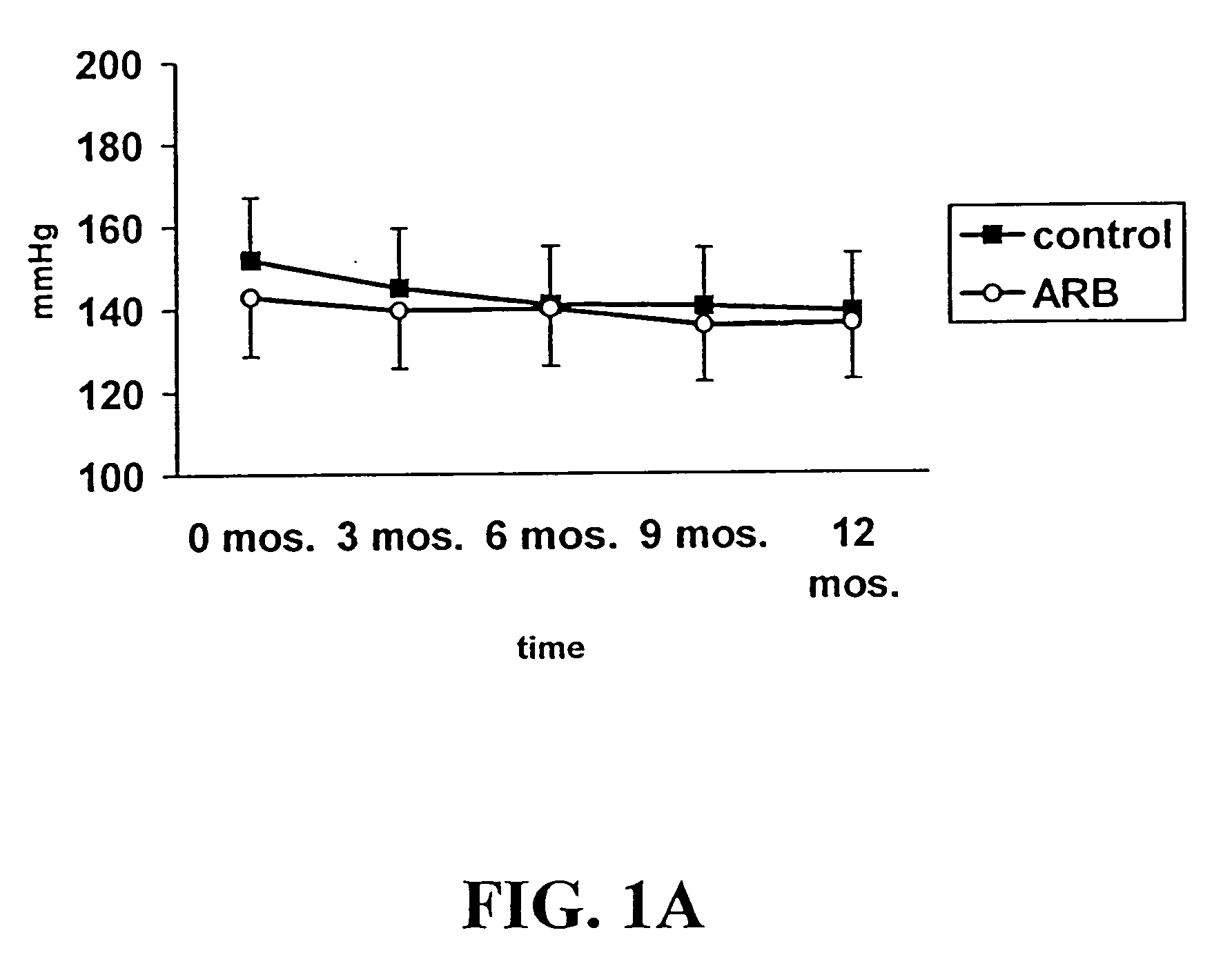
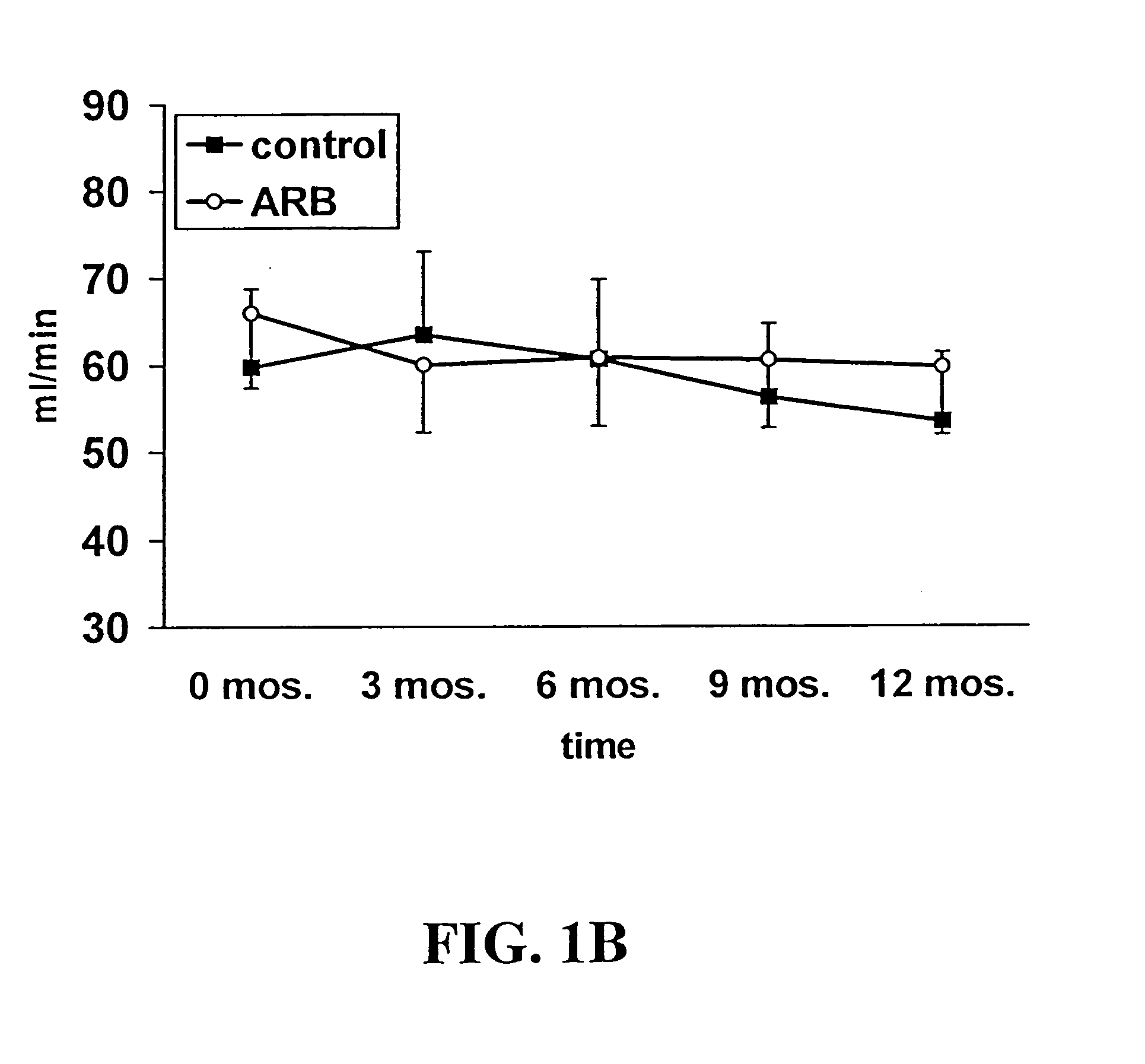
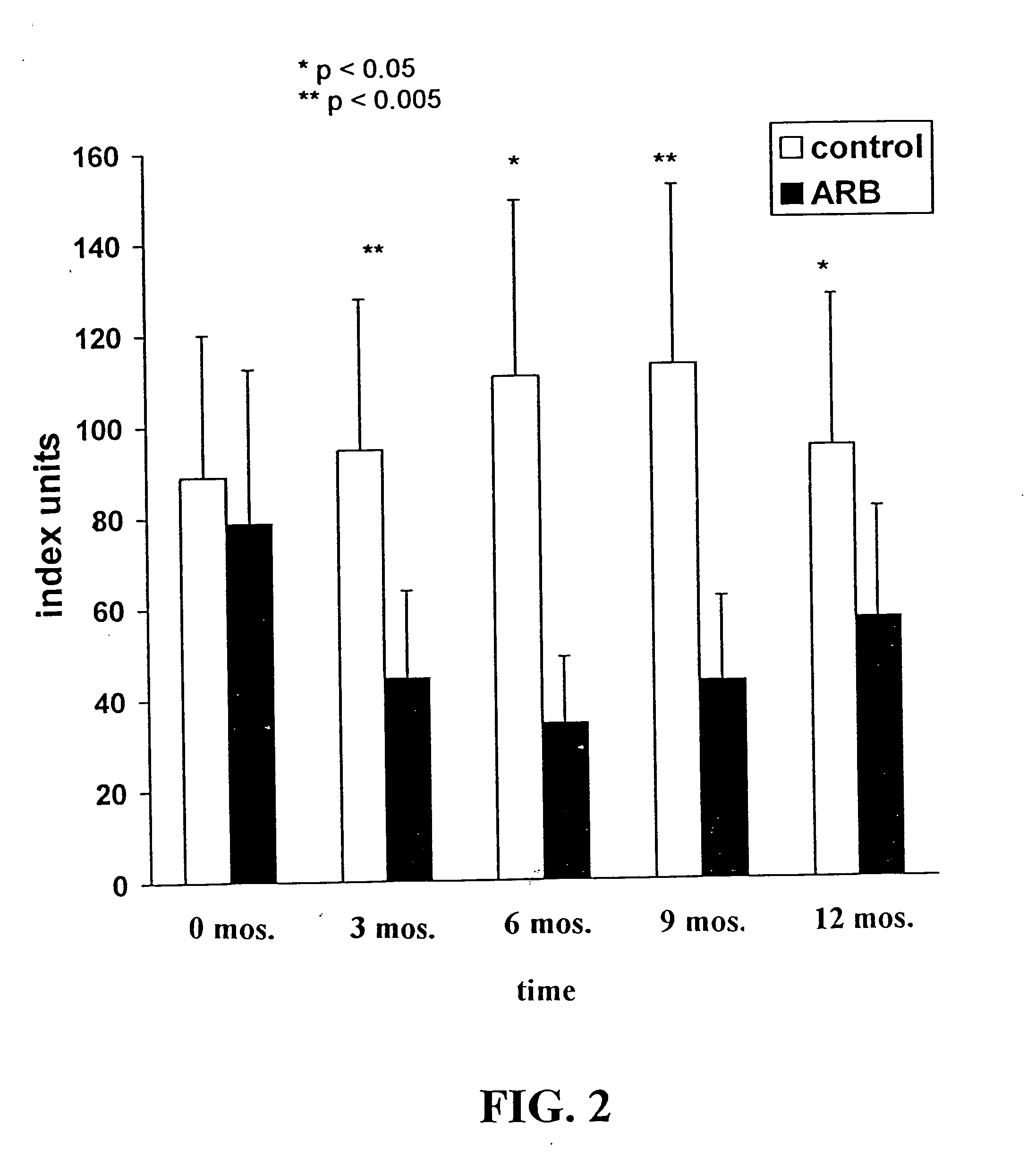
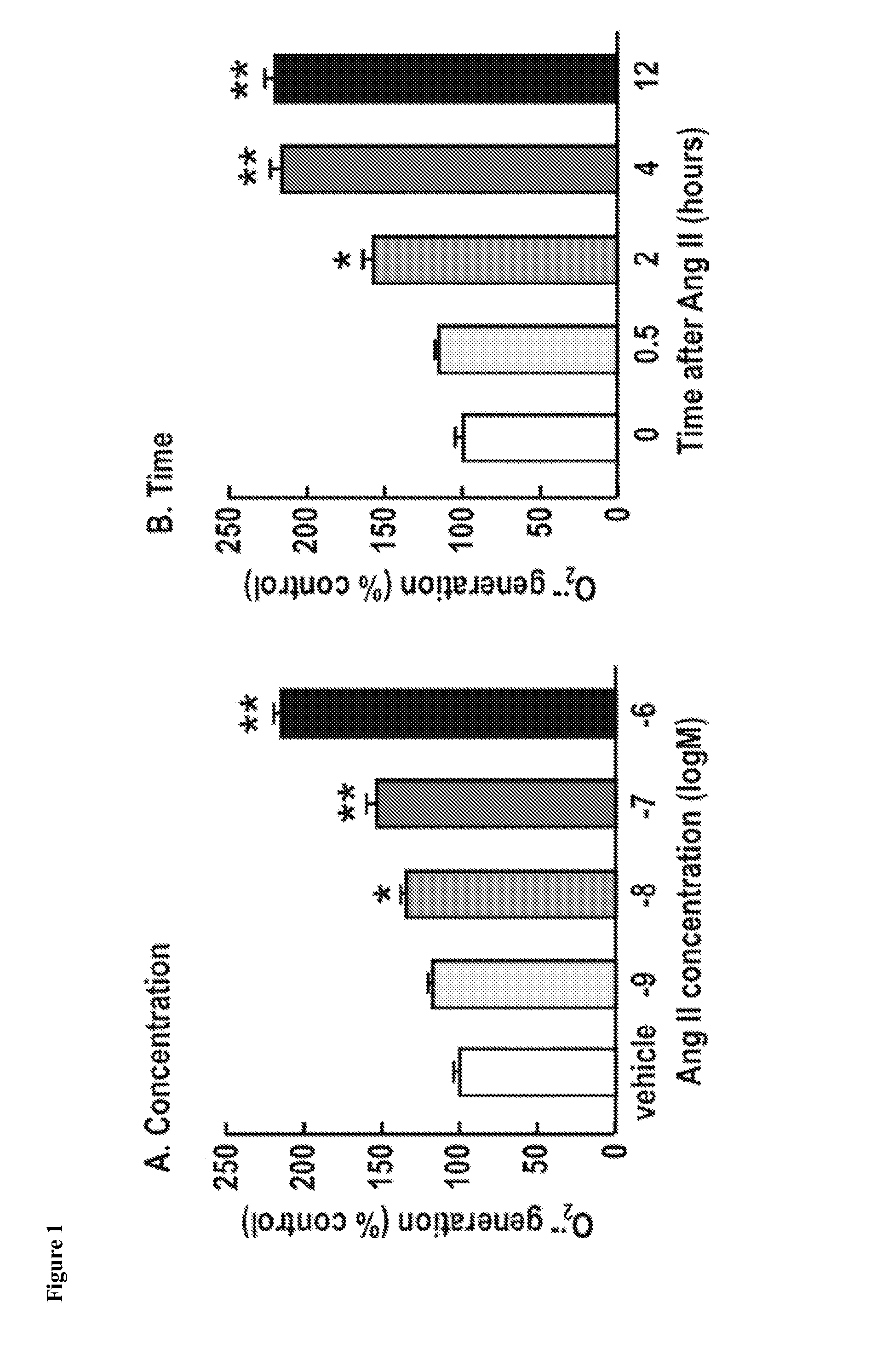
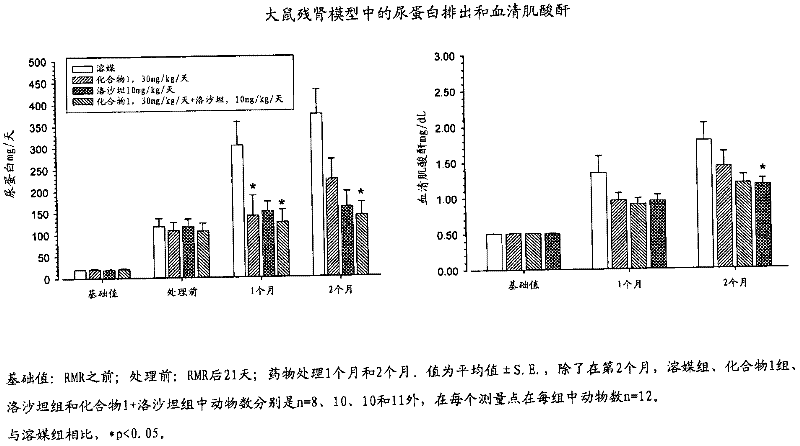
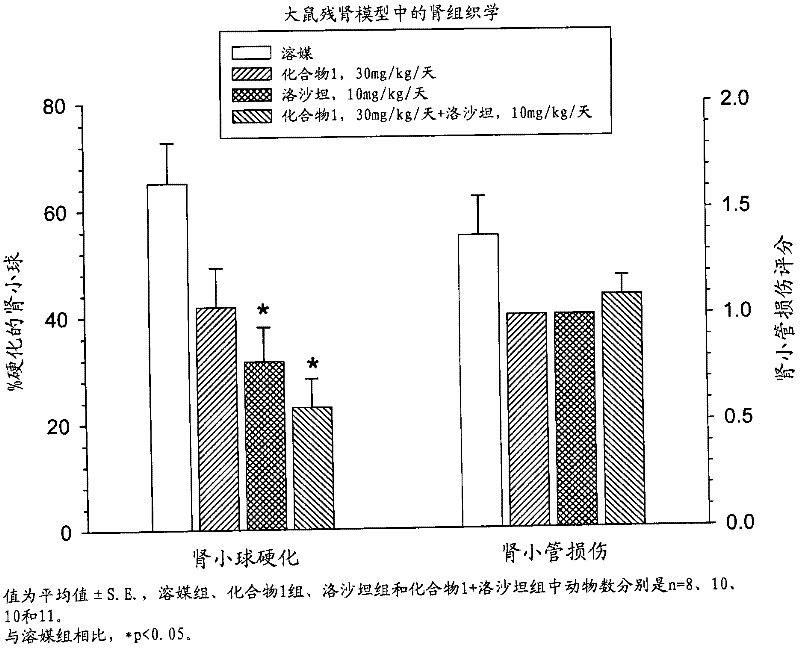
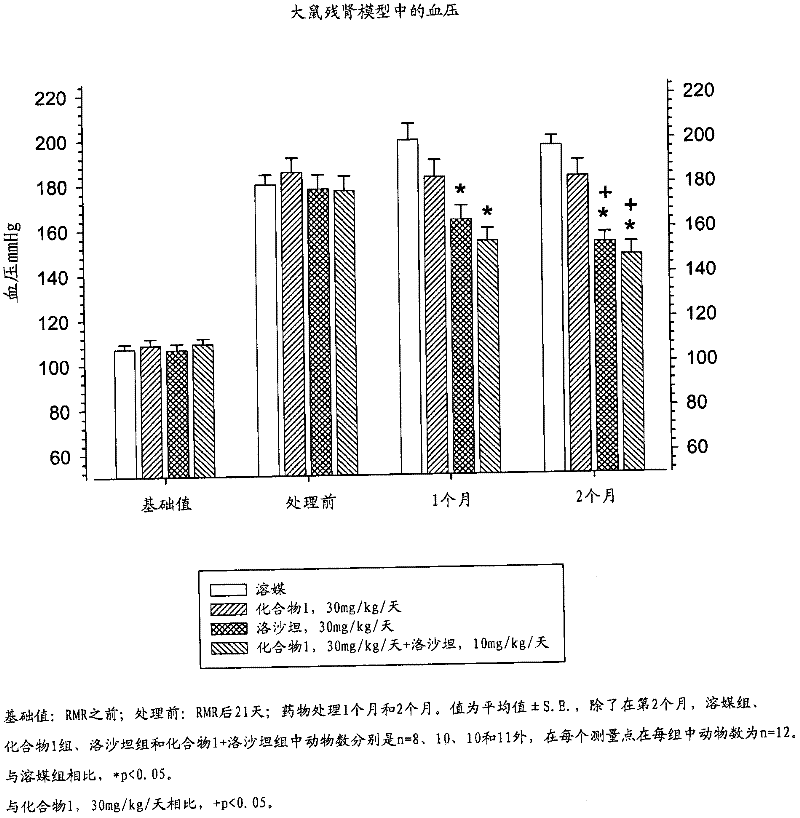
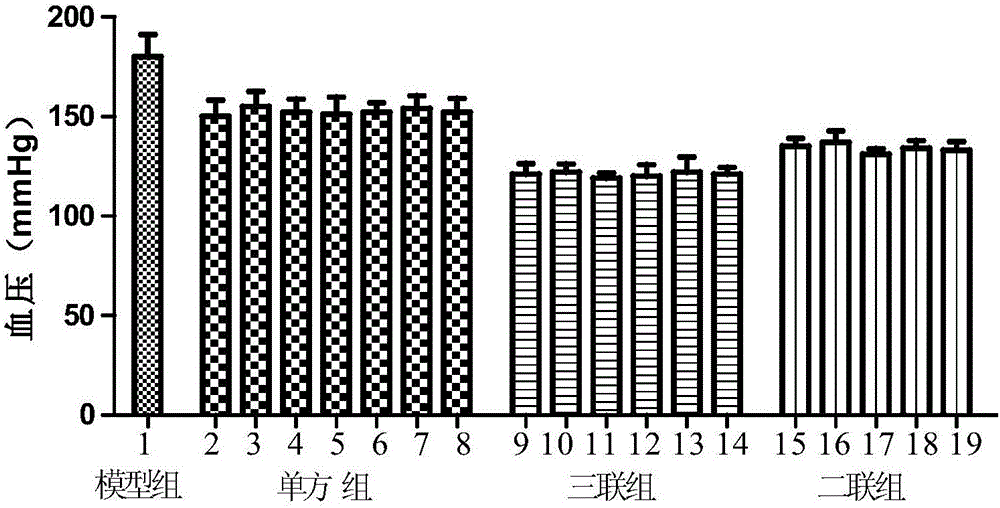
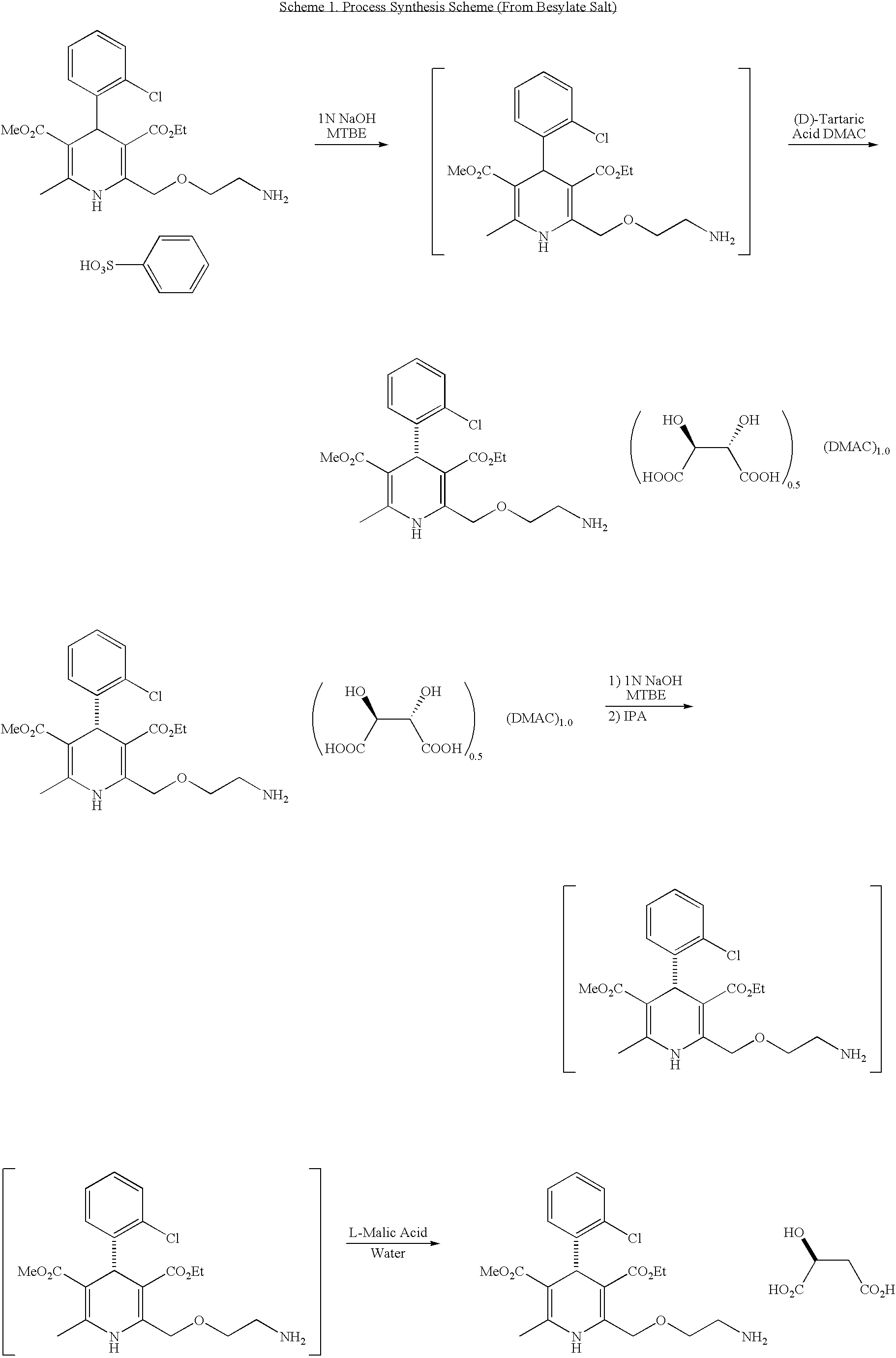Patents
Literature
40 results about "Angiotensin receptor ii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
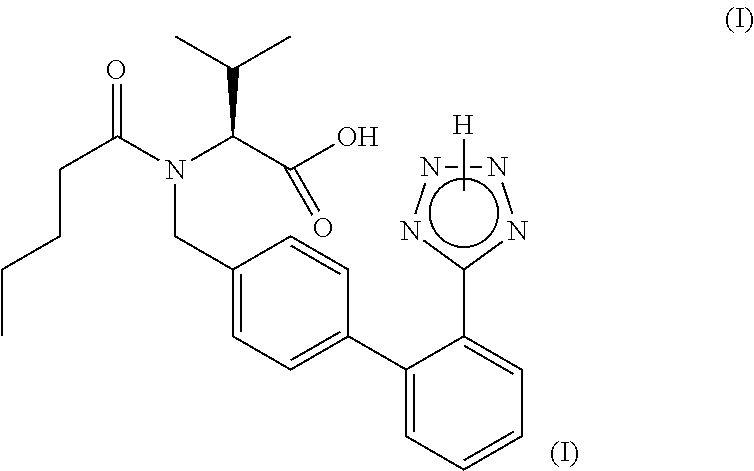
Angiotensin II receptor blockers (ARBs), also known as angiotensin II receptor antagonists, AT1 receptor antagonists or sartans, are a group of pharmaceuticals that modulate the renin–angiotensin system.
Inhalable formulations for treating pulmonary hypertension and methods of using same
InactiveUS20040265238A1Many symptomBiocideDispersion deliveryAngiotensin-converting enzymeBeta blocker
The present invention is directed to an inhalable formulation for the treatment of pulmonary hypertension in a mammal (e.g., humans), wherein the formulation comprises at least one hypertension reducing agent, including but not limited to an angiotensin converting enzyme inhibitor, angiotensin receptor blocker, beta-blocker, calcium-channel blocker or vasodilator, or any combination thereof. The formulations of the present invention may be a solution or suspension, and preferably are suitable for administration via nebulization. The present invention is also directed to a method and kit for treating a mammal suffering from pulmonary hypertension.
Owner:DEY
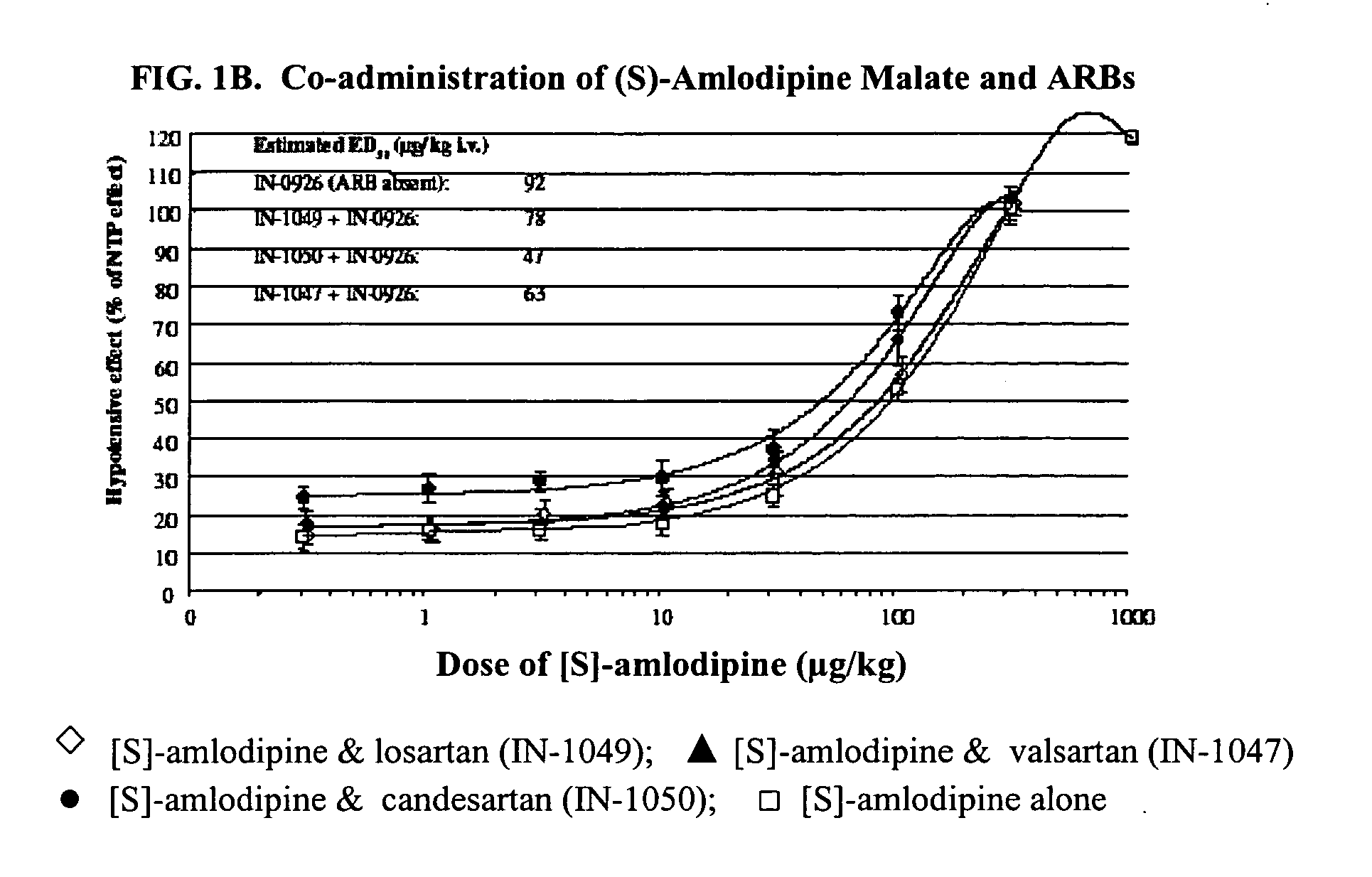
Compositions comprising (S)-amlodipine and an angiotensin receptor blocker and methods of their use
A pharmaceutical composition comprising enantiomerically pure (S)-amlodipine, an ARB and optional other active agents, and methods of treating, preventing and managing cardiovascular diseases and disorders, and symptoms thereof, using the composition, are disclosed.
Owner:SEPACOR INC
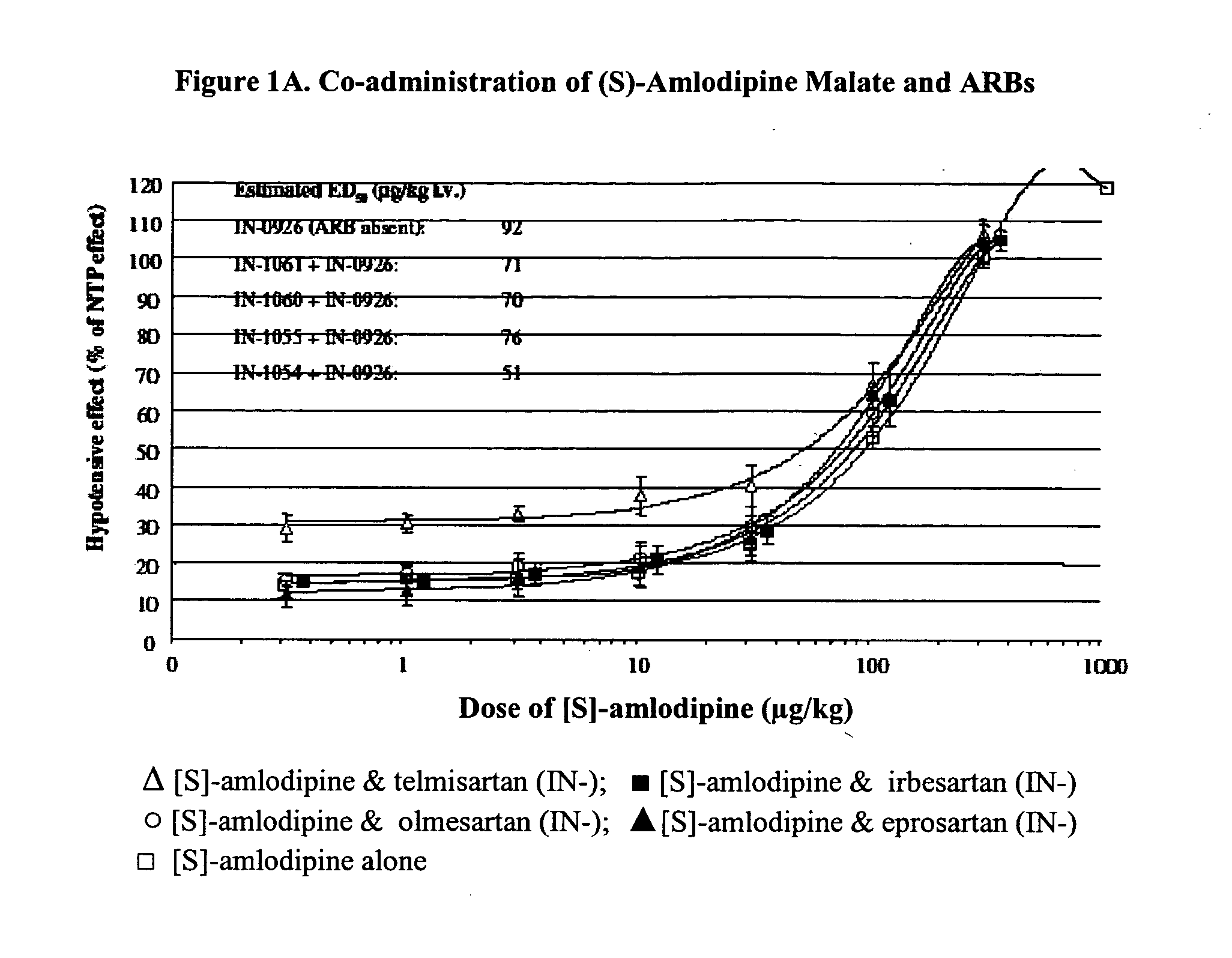
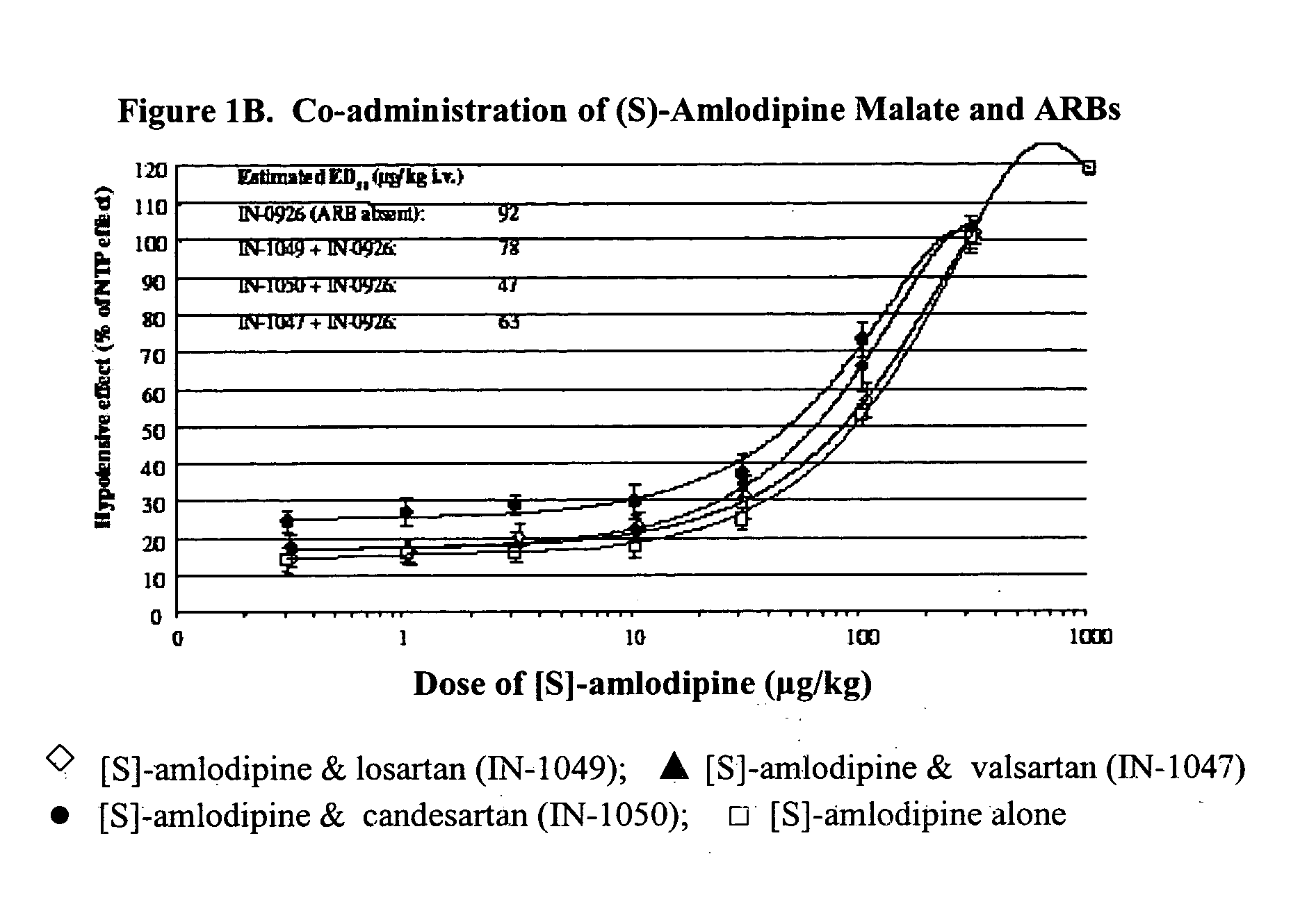
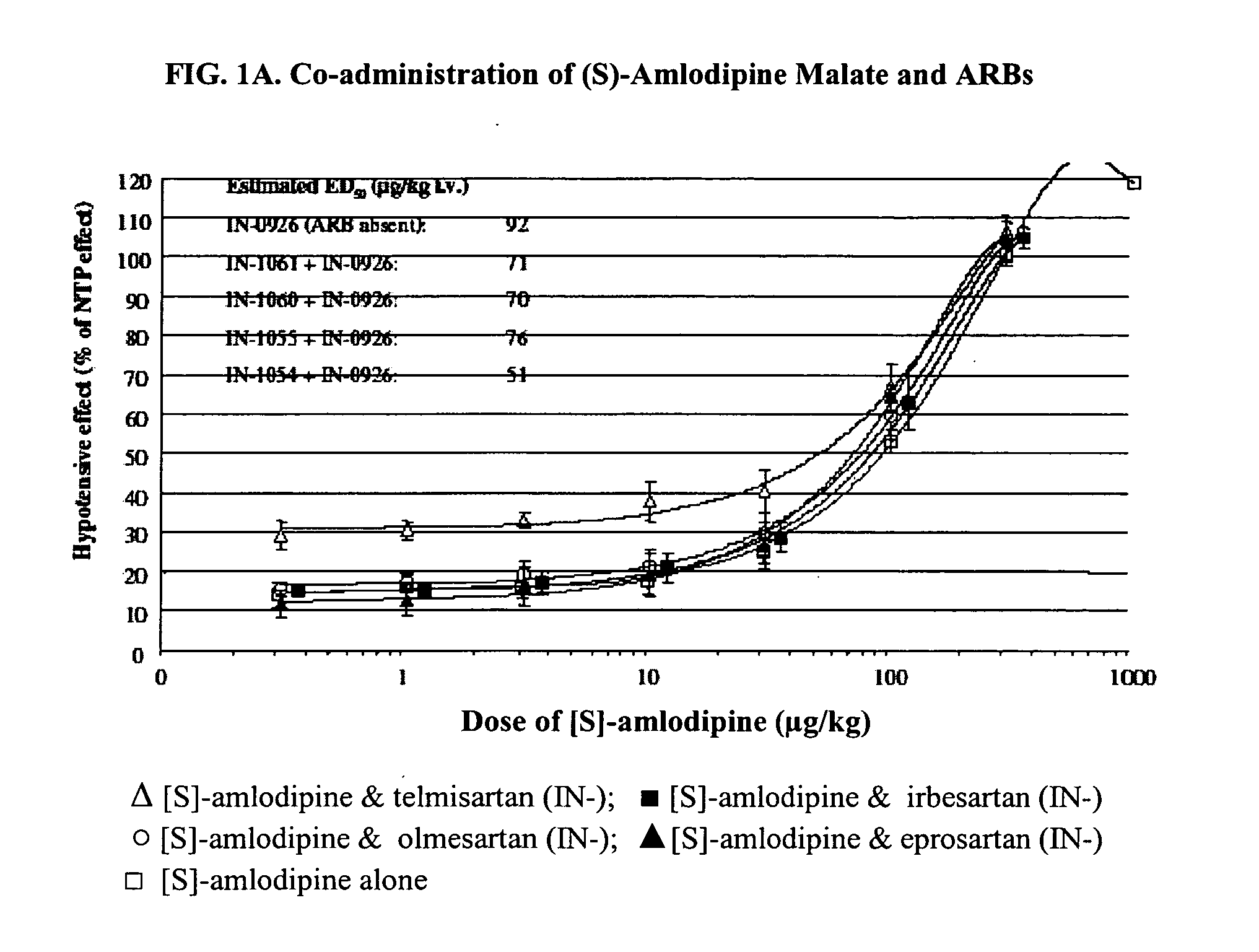
Compositions comprising (S)-amlodipine malate and an angiotensin receptor blocker and methods of their use
A pharmaceutical composition comprising enantiomerically pure (S)-amlodipine malate, an ARB and optional other active agents, and methods of treating, preventing and managing cardiovascular diseases and disorders, and symptoms thereof, using the composition, are disclosed.
Owner:SEPACOR INC
Inhalable formulations for treating pulmonary hypertension and methods of using same
The present invention is directed to an inhalable formulation for the treatment of pulmonary hypertension in a mammal (e.g., humans), wherein the formulation comprises at least one hypertension reducing agent, including but not limited to an angiotensin converting enzyme inhibitor, angiotensin receptor blocker, beta-blocker, calcium-channel blocker or vasodilator, or any combination thereof. The formulations of the present invention may be a solution or suspension, and preferably are suitable for administration via nebulization. The present invention is also directed to a method and kit for treating a mammal suffering from pulmonary hypertension.
Owner:MYLAN SPECIALTY
Methods and compositions for treating diseases associated with excesses in ACE
Numerous common diseases are associated with the ACE D / D genotype and will respond to an adequate tissue-inhibitory dose of ACE inhibitors such as quinapril. Detailed genotype studies establish the association and several of these diseases are successfully treated using higher than normal dosages of ACE inhibitors, especially hydrophobic ACE inhibitors. ACE inhibitors have also been found to be useful in inhibiting apoptosis and aging in general. Formulations containing a second active agent such as a diuretic, or a compound such as furosemide 20 mg / day (for creatinine<2.5 mg / dl) or furosemide 40 mg / day (for creatinine>2.5 mg / dl), are used to prevent fluid retention and congestive heart failure in patients with renal failure. The ACE inhibitors can also be combined with an angiotensin receptor blocker.
Owner:GENOMED
Compositions and Methods for Treating Hypertension and Inflammation
InactiveUS20080014187A1Reducing hypertensionReduce inflammationBiocidePeptide/protein ingredientsInterleukin 6Weakness
The present invention relates to pharmaceutical compositions for reducing essential hypertension and systemic inflammation. While many drugs have been found to treat hypertension, the currently available drugs often do not maintain reduced blood pressure at the preferred norm of 115 / 75 mm Hg or less throughout a 24 hour period. The current invention provides compositions comprising at least one hypertensive drug combined with the natural product Coenzyme Q10 (ubiquinone or CoQ10), which synergizes with the antihypertensive drugs to maintain low blood pressure throughout the day and night while generating other positive effects on the risks of cardiovascular disease, renal failure, and stroke. CoQ10 also counteracts some of the side effects of some hypertensive drugs such as tiredness, weakness, and / or liver toxicity. The invention further describes therapeutically effective methods for reducing systemic inflammation in hypertensive mammals comprising treatment with an antihypertensive composition that includes at least one angiotensin-converting enzyme inhibitor or angiotensin receptor blocker and CoQ10 (ubiquinone). The invention metrics for reducing systemic inflammation comprise the reduction of serum levels of high sensitivity C-reactive protein (CRP), Interleukin 6 (IL-6), and / or tumor necrosis factor-alpha (TNF-alpha). These antihypertensive-CoQ10 combinations will synergistically reduce both hypertension and systemic inflammation.
Owner:VILLEPONTEAU BRYANT RICHARD
Compositions and methods of treating gliomas
The present invention provides, inter alia, methods for treating or ameliorating the effects of a glioma. Methods of this invention include administering to a subject in need thereof an effective amount of a first active agent, such as e.g., an angiotensin receptor blocker, an antifungal agent, a bisphosphonate, an oxytocin inhibitor, an interleukin-1 (IL-1) inhibitor, a cyclooxygenase inhibitor, an α2δ voltage-dependent calcium channel (VDCC) inhibitor, a dihydroorotate dehydrogenase inhibitor, a calcium channel blocker, a renal sodium-chloride symporter inhibitor, an a2 adrenergic agonist, a phenothiazine antipsychotic, a calcineurin inhibitor, a 5-HT agonist, an angiotensin-converting enzyme (ACE) inhibitor, a direct rennin inhibitor, or combinations thereof, and a second active agent, which is a chemotherapeutic agent. Compositions for treating or ameliorating the effects of a glioma are also provided.
Owner:BIOMED VALLEY DISCOVERIES INC
Methods and compositions for treating diseases associated with excesses in ACE
InactiveUS20060111397A1Prevent fluid retentionPrevent heart failureBiocideSenses disorderCreatinine riseActive agent
Over 40 common diseases, in addition to congestive heart failure (CHF) due to hypertension (HTN) or non-insulin dependent diabetes mellitus (type II diabetes mellitus) (NIDDM), atherosclerotic peripheral vascular disease (ASPVD) due to HTN or NIDDM, and chronic obstructive pulmonary disease; emphysema (COPD), are associated with the ACE D / D genotype and should also respond to an adequate tissue-inhibitory dose of ACE inhibitors such as quinapril. Several of these diseases have now been successfully treated using higher than normal dosages of ACE inhibitors, especially hydrophobic ACE inhibitors, with good outcomes. ACE inhibitors have also been found to be useful in inhibiting apoptosis and aging in general. Dosages that have been utilized are typically greater than quinapril at a dose of 40 to 80 mg / day, i.e. up to 1 mg / kg per day for a “typical” 80 kg patient. New formulations of ACE inhibitors have been developed for these higher dosages, including 80 mg tablets, controlled and / or sustained release formulations, and formulations containing a second active agent such as a diuretic, or a compound such as furosemide 20 mg / day (for creatinine <2.5 mg / dl) or furosemide 40 mg / day (for creatinine >2.5 mg / dl), to prevent fluid retention and congestive heart failure in patients with renal failure. The ACE inhibitors can also be combined with an angiotensin receptor blocker.
Owner:MOSKOWITZ DAVID W
Combination therapy
The invention relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof, and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof. The invention also relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof; and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof which inhibits a component of the chemokine receptor pathway other than the chemokine receptor. Oral sustained release pharmaceutical compositions comprising the pharmaceutical composition, as well as injectable sustained release pharmaceutical compositions comprising the pharmaceutical composition are described. The invention further relates to tablets, capsules, injectable suspensions, and compositions for pulmonary or nasal delivery comprising the pharmaceutical composition. Also described are: methods for assessing the efficacy of the pharmaceutical composition; methods for assessing the inhibition or partial inhibition activity of the pharmaceutical composition; methods for the treatment, amelioration or prevention of a condition or disease comprising administering to a subject a therapeutically effective amount of the pharmaceutical composition; and the use of the pharmaceutical composition for the manufacture of a dosage form for the treatment of a disease.
Owner:DIMERIX BIOSCIENCES PTY LTD
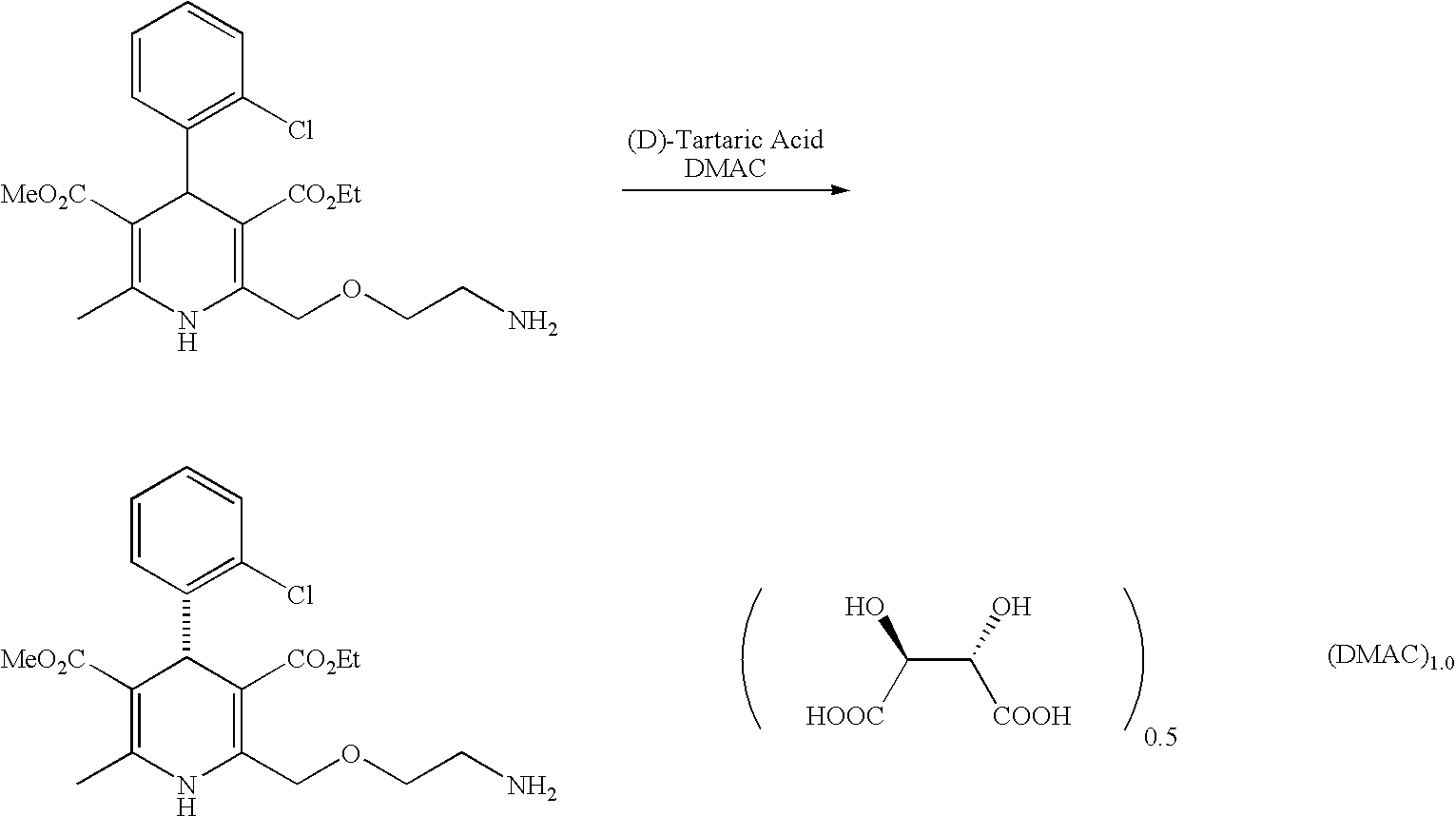
Process for the manufacture of organic compounds
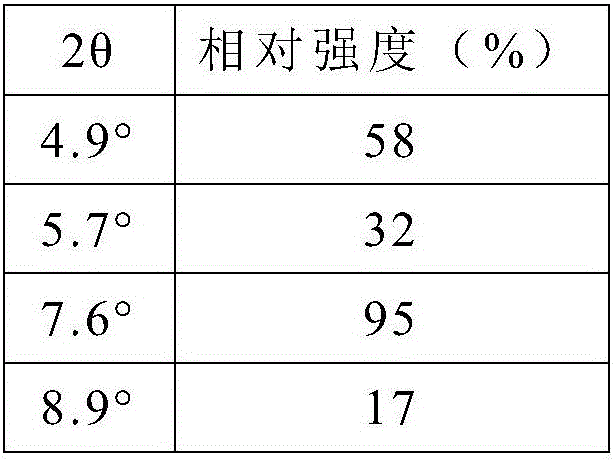
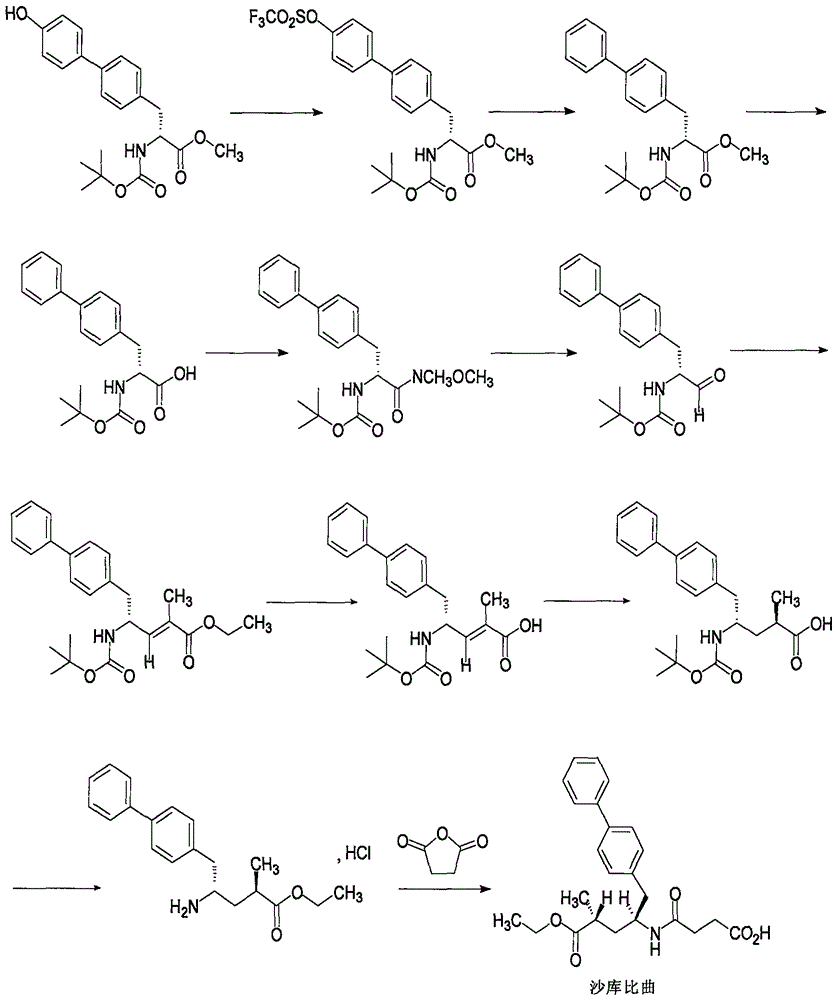
InactiveCN102596899AOrganic compound preparationCardiovascular disorderAngiotensin receptor iiOrganic compound
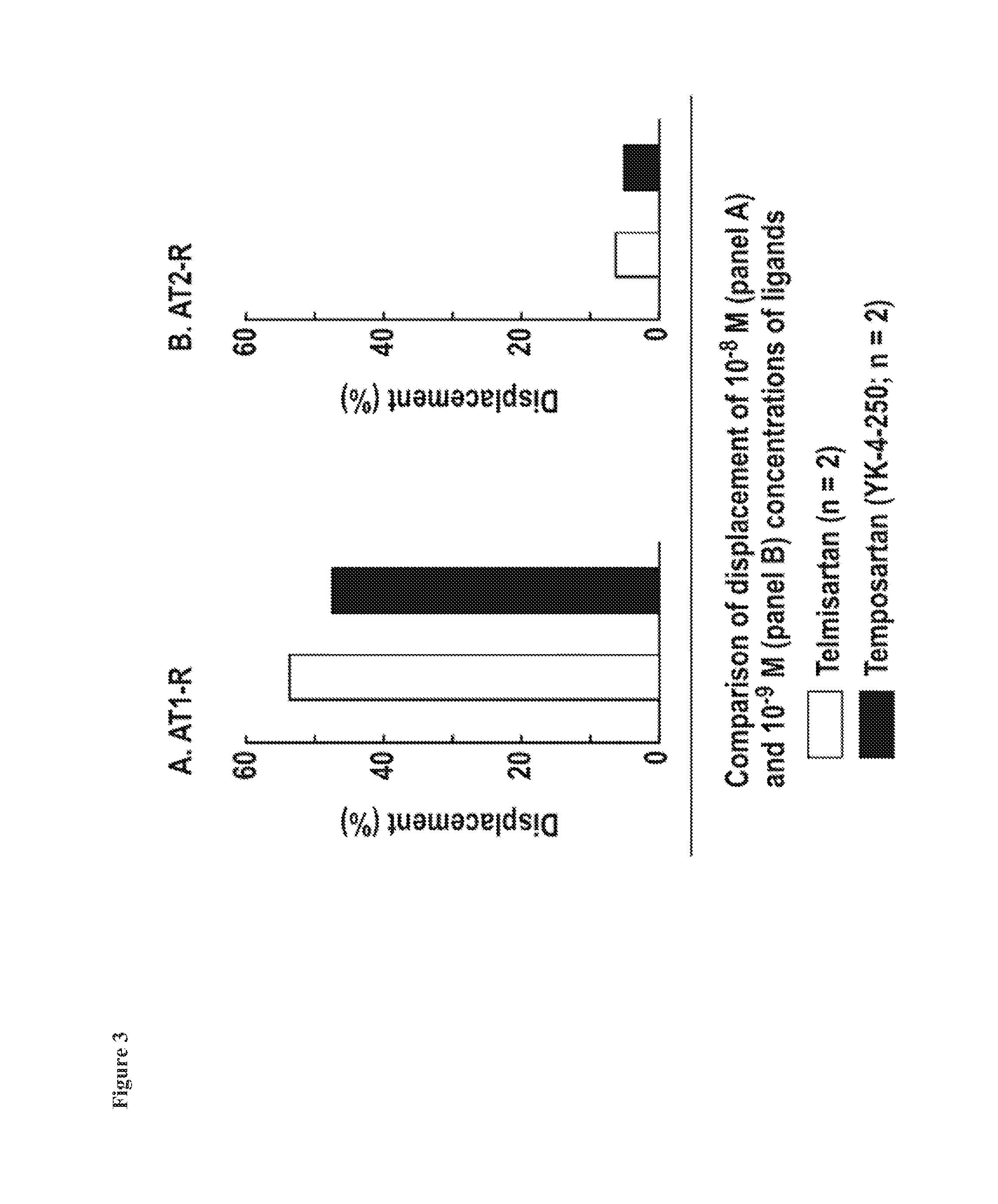
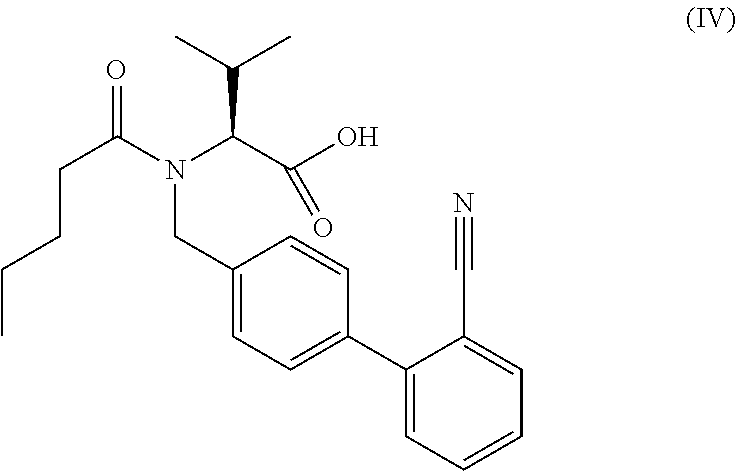
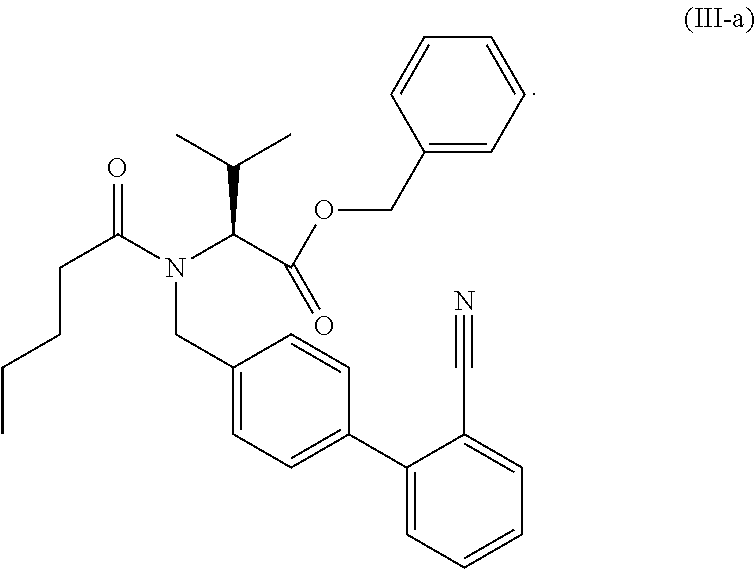
The present invention relates to processes for the manufacture of an angiotensin receptor blocker (ARB; also called angiotension II receptor antagonist or AT1 receptor antagonist) and salts thereof, to novel intermediates and process steps.
Owner:NOVARTIS AG
New Use
InactiveUS20160206597A1Reduced glomerular filtration rateProvide protectionBiocideUrinary disorderDiseaseAngiotensin receptor
The present invention relates to methods and pharmaceutical compositions for renal protection in a mammal in need thereof, such as a mammal having a disease manifested by atrial enlargement and / or remodeling or suffering from hypertension or heart failure or being prone to suffering from hypertension and / or heart failure, comprising administration of a therapeutically effective amount, or a prophylactically effective amount, of an Angiotensin Receptor Neprilysin inhibitor (ARNi) or of a combination of an Angiotensin Receptor Blocker (ARB) with a Neutral Endopeptidase inhibitor (NEPi) or with a NEPi pro-drug to said mammal.
Owner:NOVARTIS AG
Method of decreasing inflammation in kidney transplantion using angiotensin receptor blockers
InactiveUS20050282877A1Reduce in quantityInhibition of activationBiocideAnimal repellantsAngiotensin Receptor BlockersAngiotensin receptor
Disclosed is a method of inhibiting production of IFN-Γ in patients having a transplanted organ. The method involves administering to the patient an amount of an angiotensin receptor-blocking compound, the amount being effective to inhibit production of IFN-Γ by T cells. The method can be used to treat inflammation involving an allograft, to treat chronic allograft nephropathy, and to treat other pathologies associated with allograft rejection.
Owner:WISCONSIN ALUMNI RES FOUND
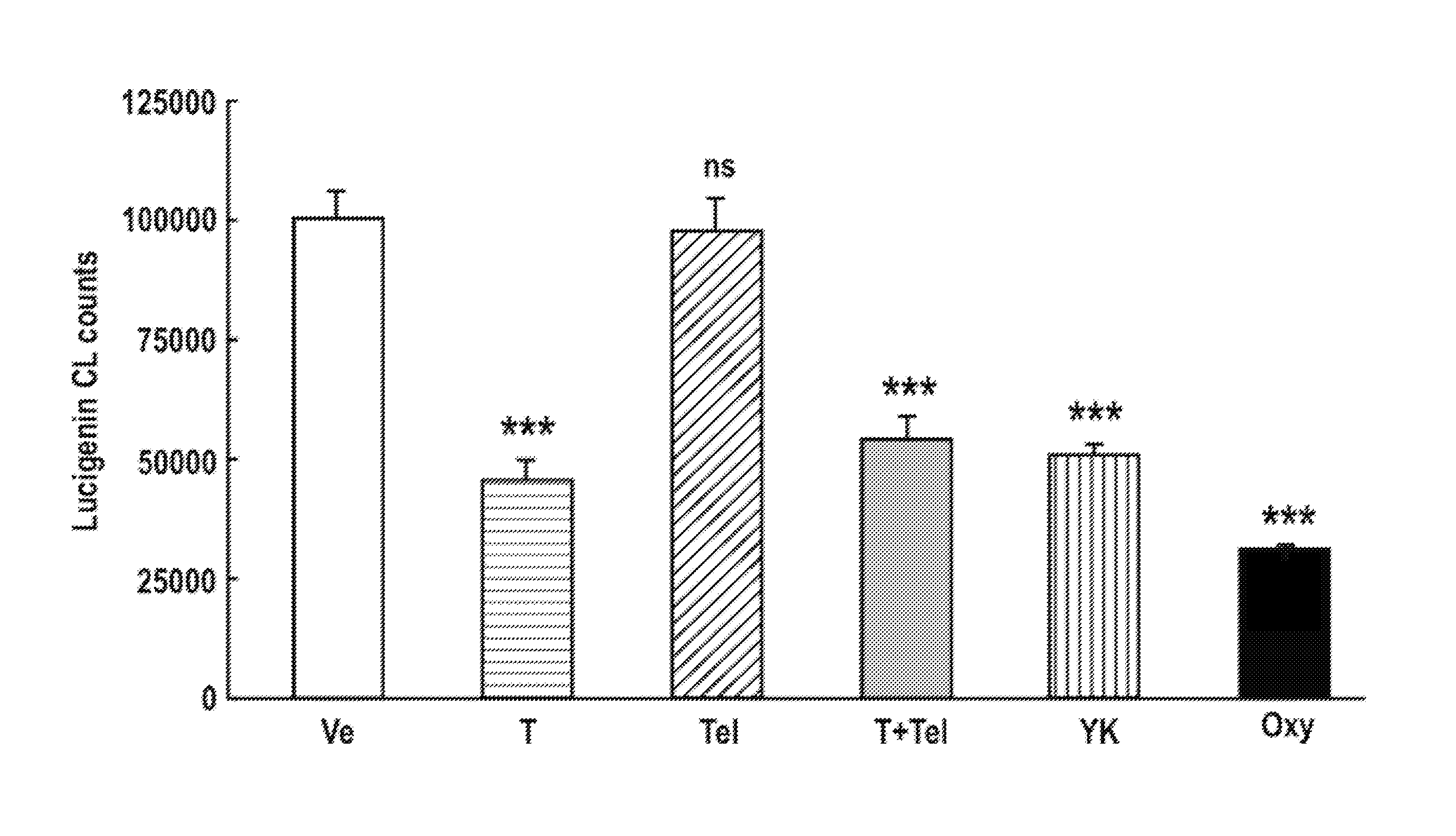
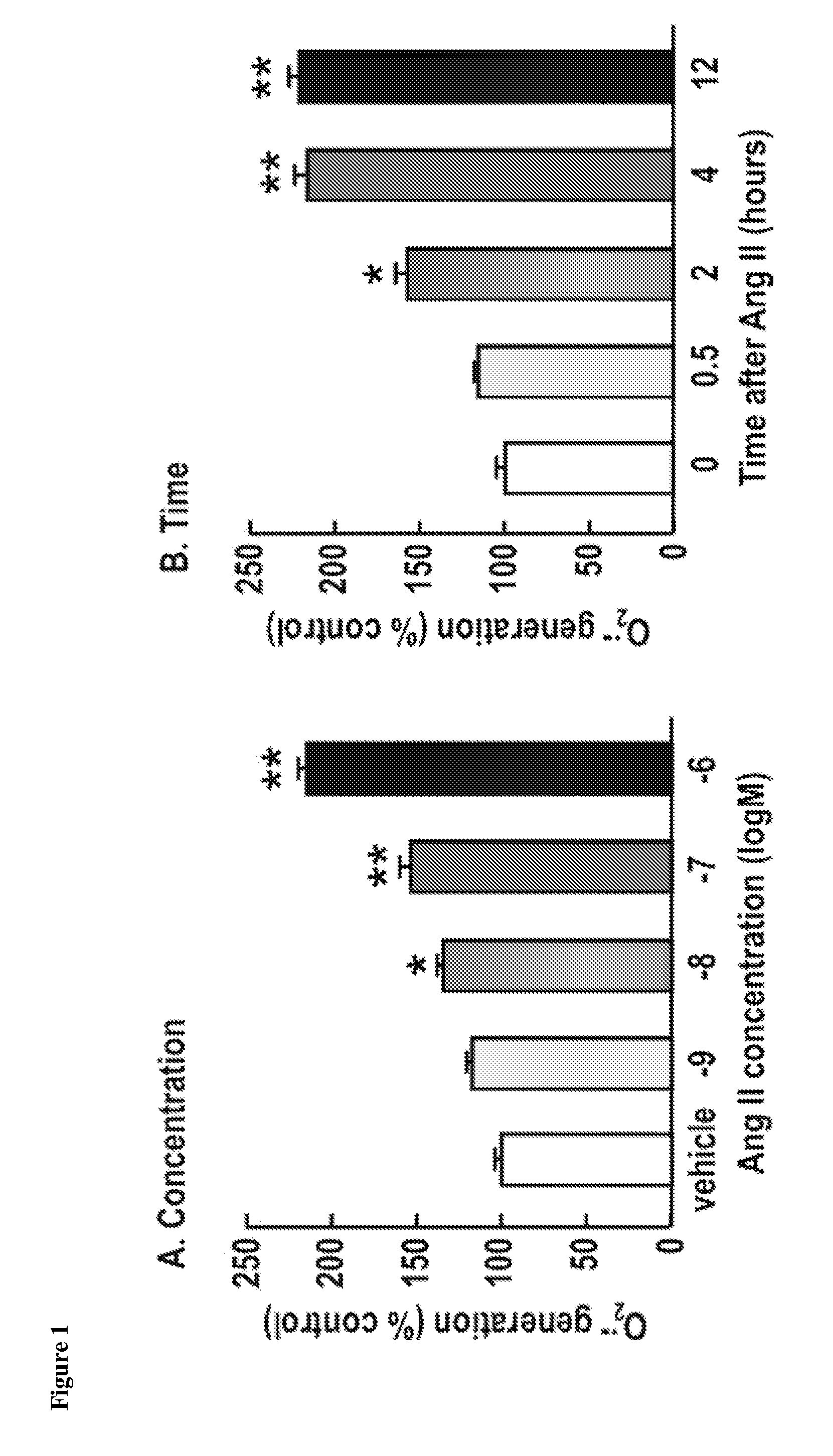
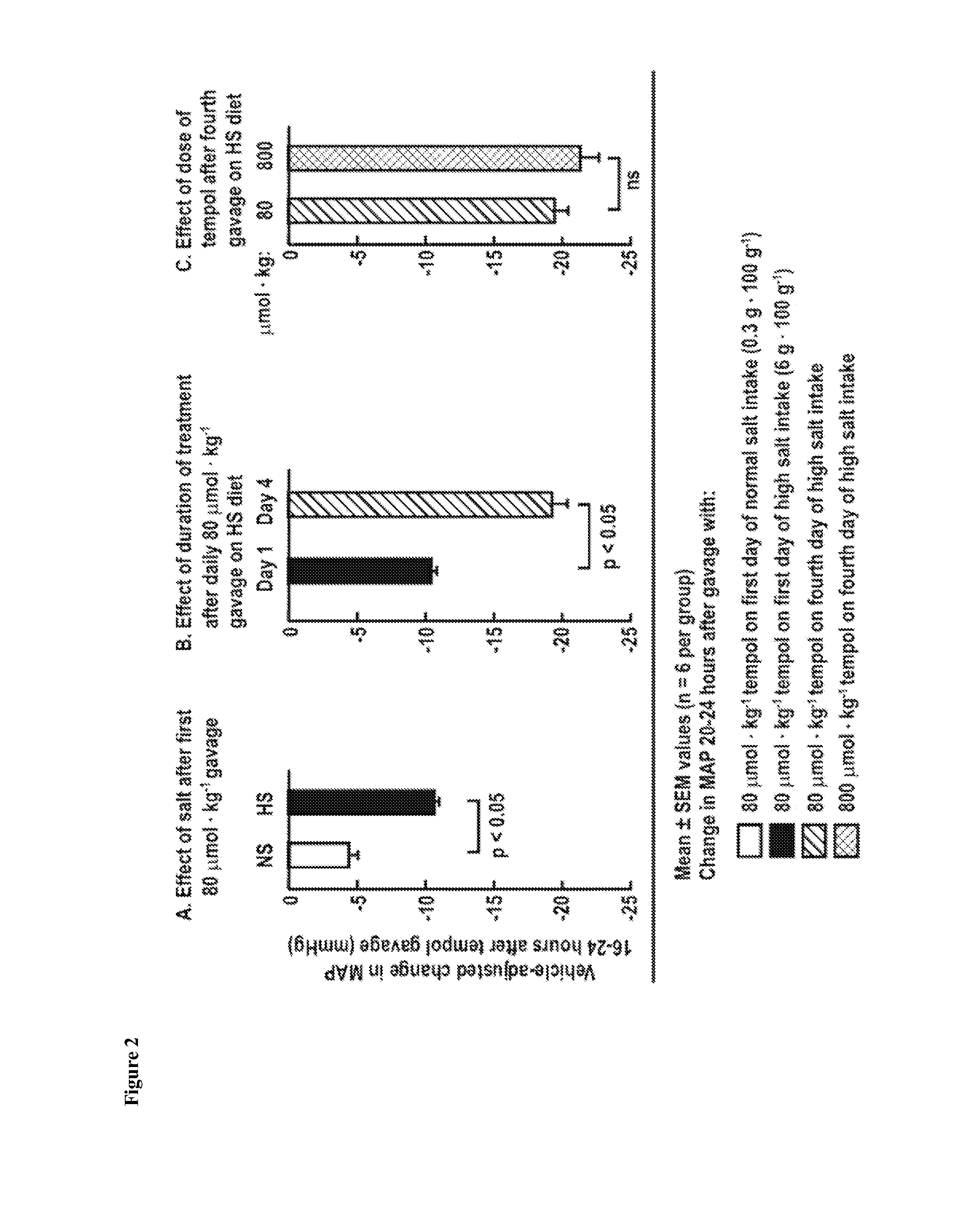
Treatment for Oxidative Stress and/or Hypertension
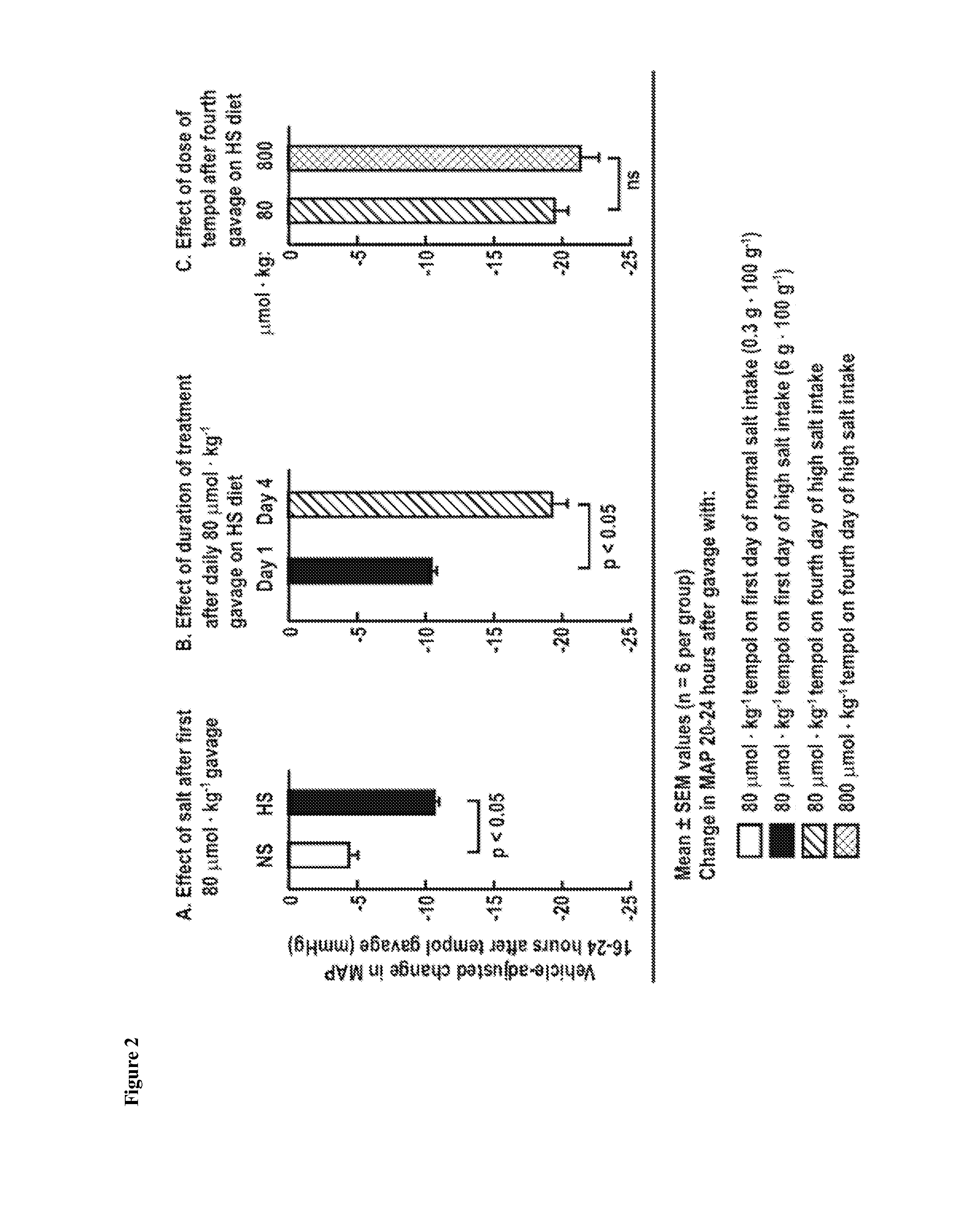
One aspect of the invention relates to compounds, compositions and methods for treating oxidative stress and / or hypertension. In certain embodiments, the invention relates to a mixture of tempol and an angiotensin receptor blocker (ARB) and the use of said mixture to treat oxidative stress and / or hypertension. In certain embodiments, the invention relates to a tempol / ARB adduct and the use of said adduct to treat oxidative stress and / or hypertension.
Owner:GEORGETOWN UNIV
New use
InactiveUS20160213646A1Reduced ejection fractionPharmaceutical delivery mechanismCardiovascular disorderAngiotensin receptorAngiotensin receptor ii
The present invention relates to methods and pharmaceutical compositions for the prevention or in delaying time to first occurrence of mortality, in particular cardiovascular death, and / or of cardiovascular hospitalizations in a patient suffering from chronic systolic heart failure, comprising administration of a therapeutically effective amount, or a prophylactically effective amount, of an Angiotensin Receptor Neprilysin inhibitor (ARNi) or of a combination of an Angiotensin Receptor Blocker (ARB) with a Neutral Endopeptidase inhibitor (NEPi) or with a NEPi pro-drug to said patient.
Owner:NOVARTIS AG
Combination therapy
ActiveUS20130289084A1Inhibits and partially inhibits inositol phosphate productionAvoid adjustmentBiocidePill deliveryDiseaseAngiotensin receptor
The invention relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof, and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof. The invention also relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof; and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof which inhibits a component of the chemokine receptor pathway other than the chemokine receptor. Oral sustained release pharmaceutical compositions comprising the pharmaceutical composition, as well as injectable sustained release pharmaceutical compositions comprising the pharmaceutical composition are described. The invention further relates to tablets, capsules, injectable suspensions, and compositions for pulmonary or nasal delivery comprising the pharmaceutical composition. Also described are: methods for assessing the efficacy of the pharmaceutical composition; methods for assessing the inhibition or partial inhibition activity of the pharmaceutical composition; methods for the treatment, amelioration or prevention of a condition or disease comprising administering to a subject a therapeutically effective amount of the pharmaceutical composition; and the use of the pharmaceutical composition for the manufacture of a dosage form for the treatment of a disease.
Owner:DIMERIX BIOSCIENCES PTY LTD
Treatment for oxidative stress and/or hypertension
One aspect of the invention relates to compounds, compositions and methods for treating oxidative stress and / or hypertension. In certain embodiments, the invention relates to a mixture of tempol and an angiotensin receptor blocker (ARB) and the use of said mixture to treat oxidative stress and / or hypertension. In certain embodiments, the invention relates to a tempol / ARB adduct and the use of said adduct to treat oxidative stress and / or hypertension.
Owner:GEORGETOWN UNIV
Establishment of stress cardiomyopathy animal model
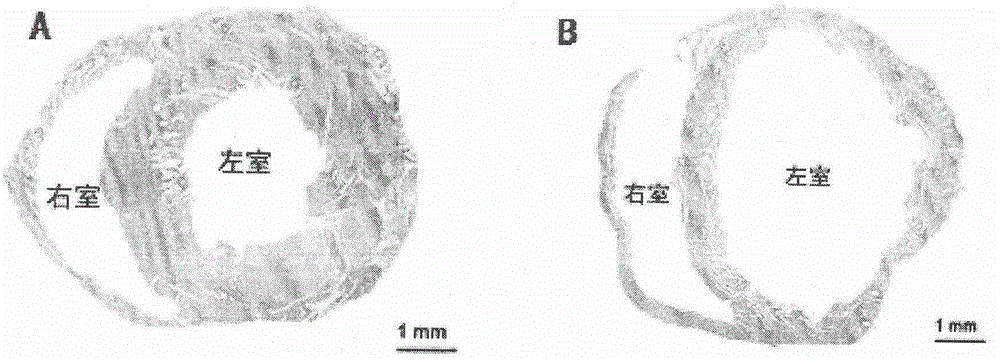
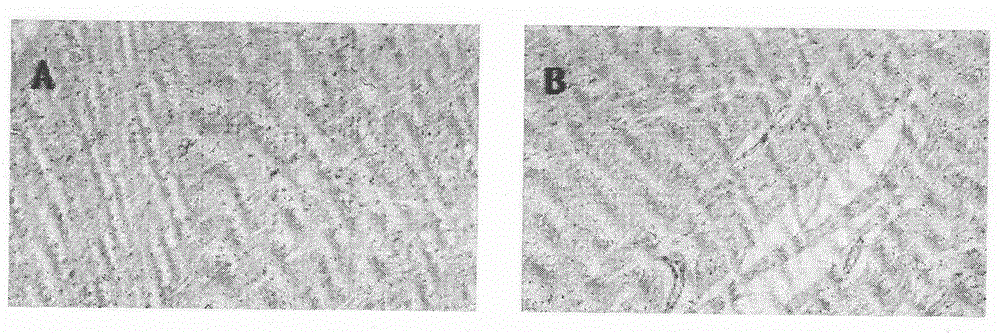
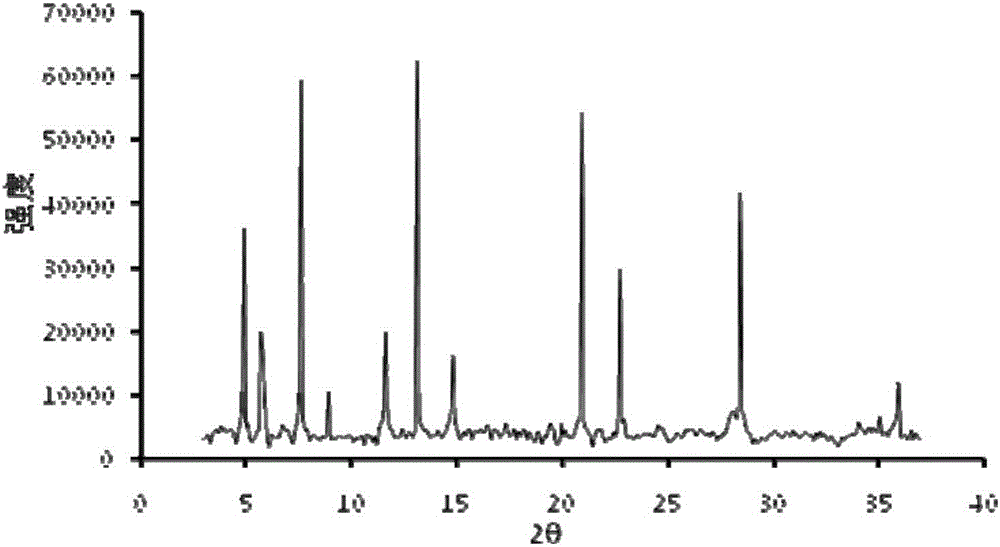
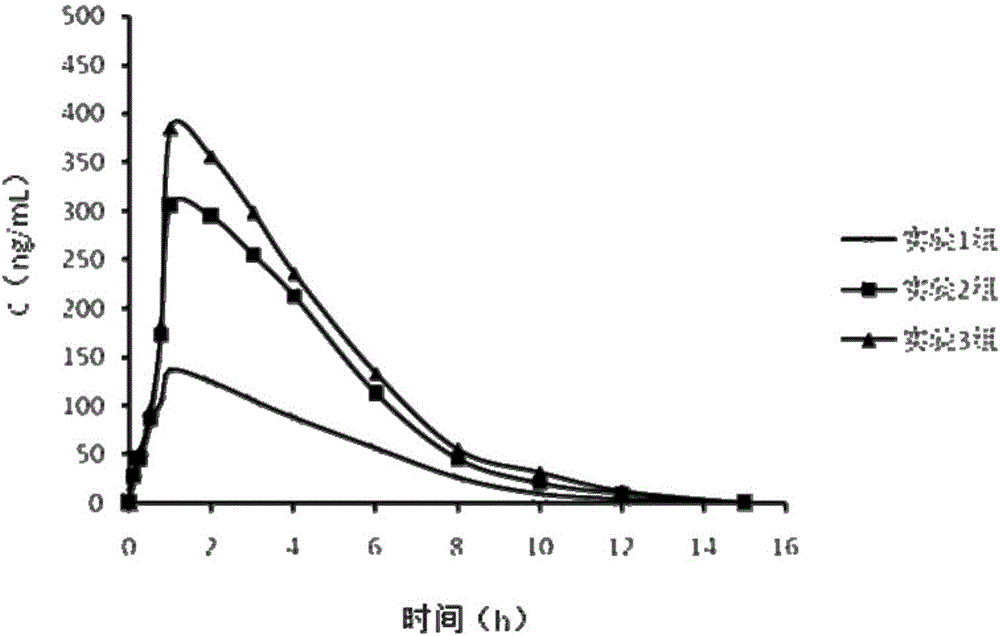
The invention relates to the field of animal model establishment, and discloses a method for establishing an animal model of stress cardiomyopathy, including preoperative preparation, operation, vagus nerve stimulation and postoperative suturing, by studying the blank group, experimental group, ARB class Drug interference, ACEI drugs, studied the induction of cardiomyopathy under drug-induced and electrical stimulation, and performed pathological characterization to verify the successful establishment of animal models to solve the problem that there is currently no suitable animal model of stress cardiomyopathy available problem in research.
Owner:郭瑞威
Compound of angiotensin receptor blocker and levosimendan and use of compound
ActiveCN106214680AGood treatment effectConvenient treatmentMetabolism disorderHeterocyclic compound active ingredientsAngiotensin receptorMedicine
The invention provides a compound of an angiotensin receptor blocker and levosimendan and use of the compound. The compound is composed of the angiotensin receptor blocker and levosimendan, which can be mixed directly or be connected indirectly through a hydrogen bond, wherein a co-crystallized sodium hydrate formed by connection through hydrogen bond is more stable in property; and the pharmacokinetic properties are improved significantly; the action mechanisms of the angiotensin receptor blocker and the levosimendan are different, but the formed compound has an unexpected synergistic effect and has positive application prospects in the anti-heart failure and anti-hypertensive treatment field.
Owner:赛隆药业集团股份有限公司(长沙)医药研发中心
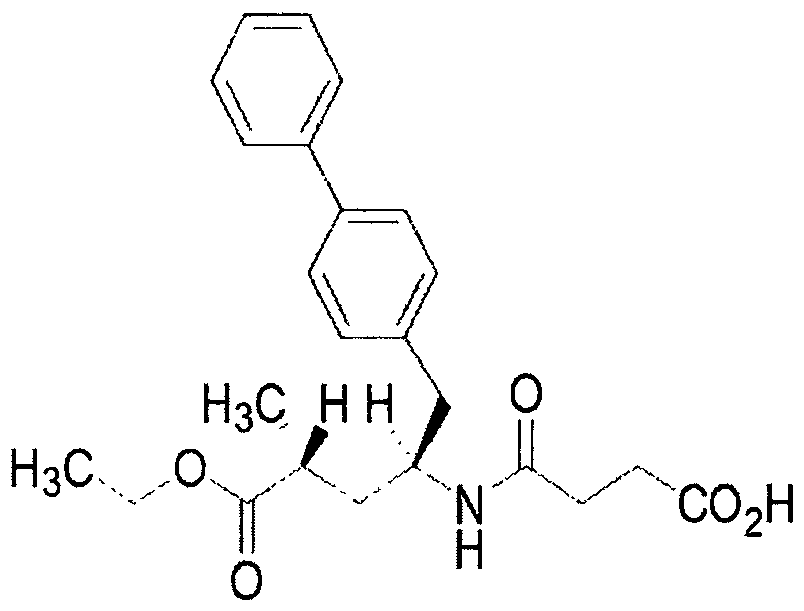
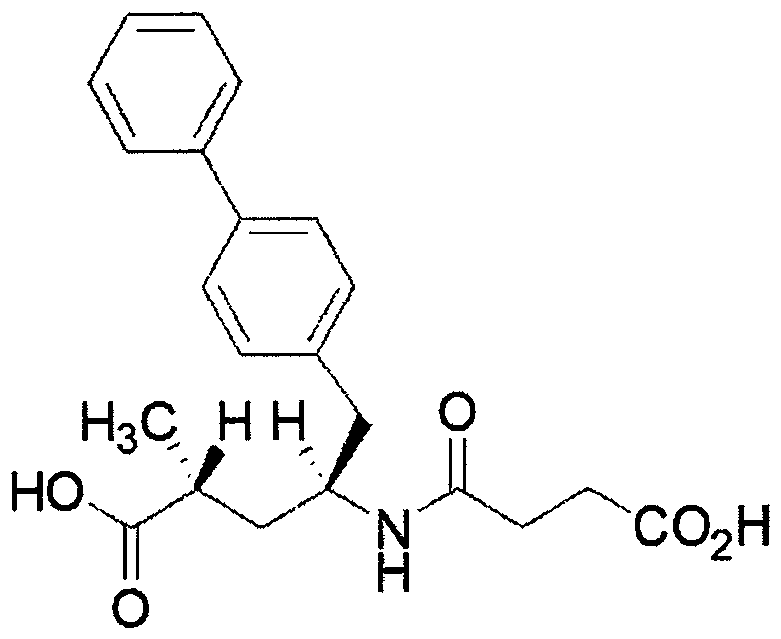
Eutectic drug for treating heart failure
ActiveCN106674206AOrganic active ingredientsOrganic chemistry methodsFimasartanAngiotensin receptor ii
The invention discloses a eutectic drug for treating heart failure and relates to a eutectic formed by sacubitril and an angiotensin-receptor blocker, wherein the angiotensin-receptor blocker is selected from: drugs such as fimasartan and the like.
Owner:顾国明 +1
Methods for prevention and treatment of fibromyalgia by contact vasodilators
PendingUS20220241264A1Prevent and treat fibromyalgiaOrganic active ingredientsNervous disorderBeta blockerAngiotensin Receptor Blockers
Methods are provided to prevent and to treat fibromyalgia by using a vasodilator such as an angiotensin receptor blocker, ACE inhibitor, calcium channel blocker, phosphodiesterase inhibitor, alpha blocker, or beta blocker. More particularly, the methods do not employ orally administered vasodilators, but instead vasodilators that are administered through contact with the epidermis, e.g., in topical or other form suitable for contact with tissues to be treated. The compositions promote neo capillary formation, whereby symptoms of fibromyalgia are treated, reduced, or ameliorated.
Owner:WEINBERG ASSA
Methods for prevention and treatment of urogenital atrophy of menopause by contact vasodilators
InactiveUS20210069102A1Inhibition formationPowder deliveryAerosol deliveryAngiotensin Receptor BlockersAngiotensin receptor ii
Methods are provided to prevent and to treat urogenital (e.g., urovaginal) atrophy syndrome of menopause by using a vasodilator such as an angiotensin receptor blocker, ACE inhibitor, or calcium channel blocker. More particularly, the methods do not employ orally administered vasodilators, but instead vasodilators that are administered through contact with the epidermis, e.g., in topical or other form suitable for contact with tissues to be treated.
Owner:WEINBERG ASSA
Process for the manufacture of organic compounds
InactiveUS20120238761A1Reduce laborShorten the timeOrganic compound preparationCardiovascular disorderSimple Organic CompoundsAngiotensin receptor ii
The present invention relates to processes for the manufacture of an angiotensin receptor blocker (ARB; also called angiotension II receptor antagonist or AT1 receptor antagonist) and salts thereof, to novel intermediates and process steps.
Owner:NOVARTIS AG
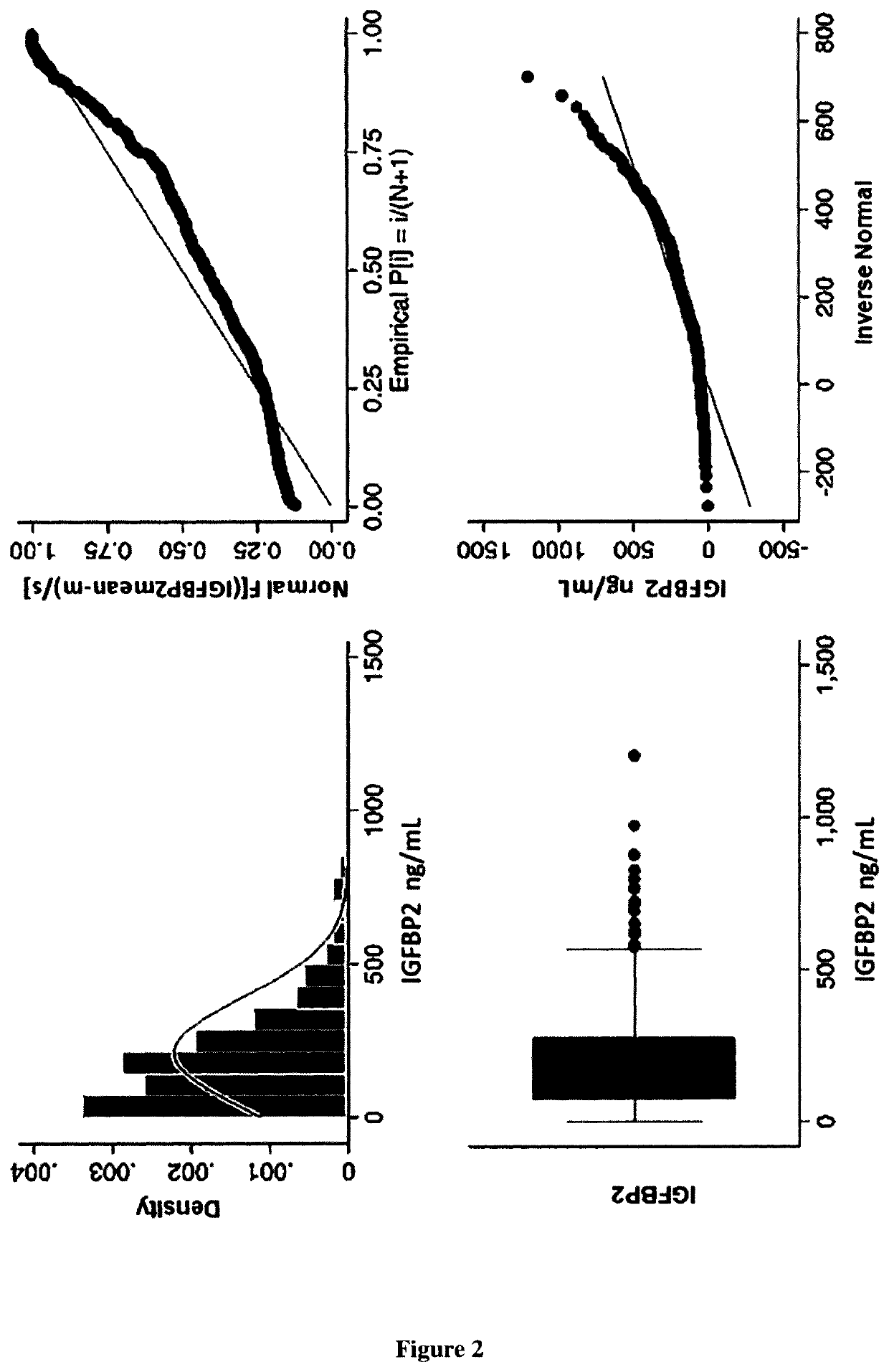
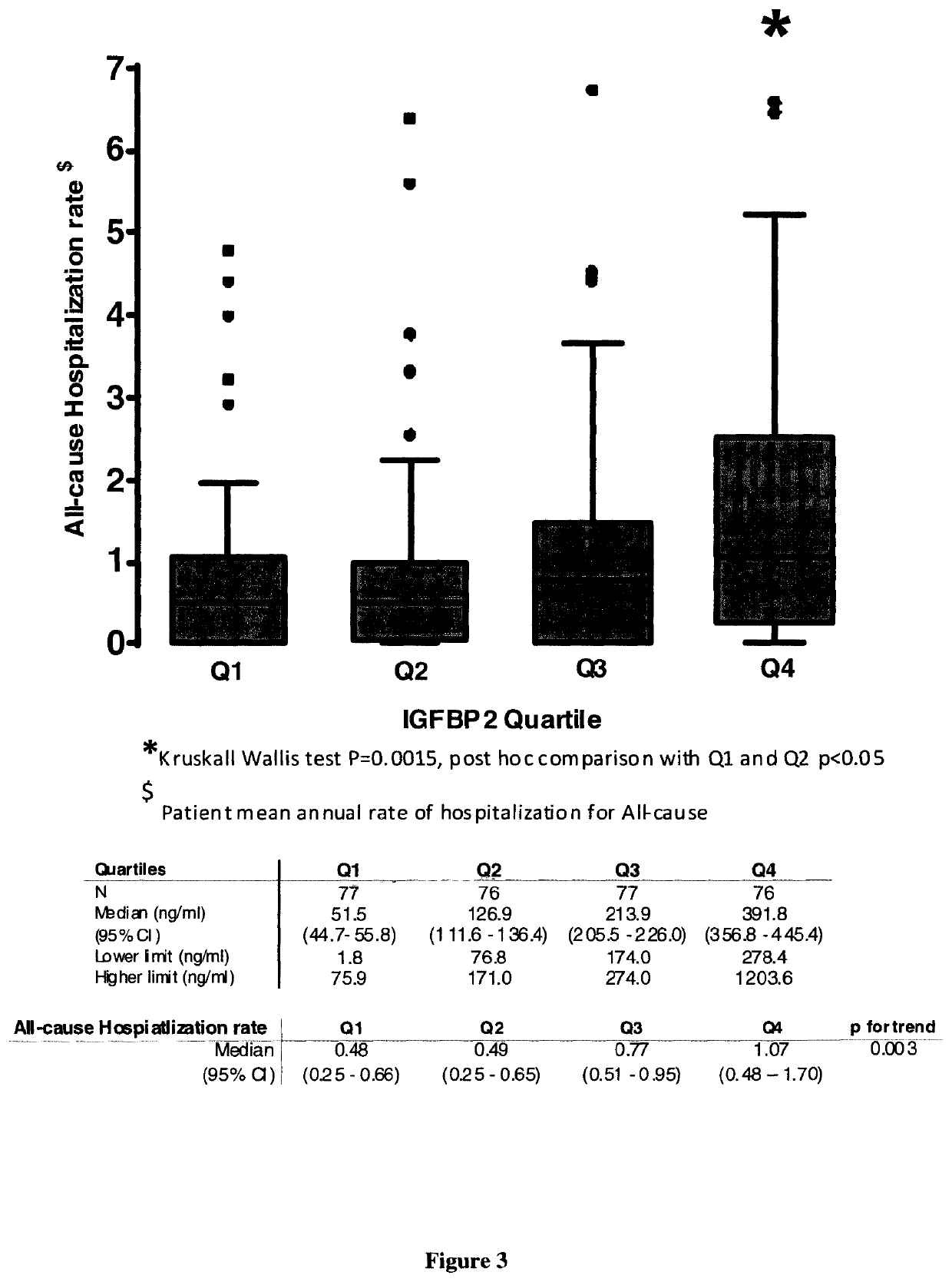
Biomarker of rehospitalization after heart failure
The invention is a method for treating a heart failure patient with the steps of measuring the concentration of IGFBP2 in a blood, plasma or urine sample obtained from the heart failure patient and comparing the patient's IGFBP2 level to a threshold value derived from IGFBP2 measured in samples taken from a group of patients with heart failure selected from the group consisting of stage I, stage stage III and stage IV heart failure, according to New York Heart Association (NYHA) classification system. A patient with an IGFBP2 level that exceeds the threshold value is admitted or readmitted to a hospital and treated with at least one treatment selected from the group consisting of administration of a beta-blocker, an angiotensin-converting enzyme inhibitor, an angiotensin receptor blocker, an aldosterone antagonist, an implantable cardiac defibrillator, a cardiac resynchronization therapy, an implantable left ventricular assist device and a heart transplant.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
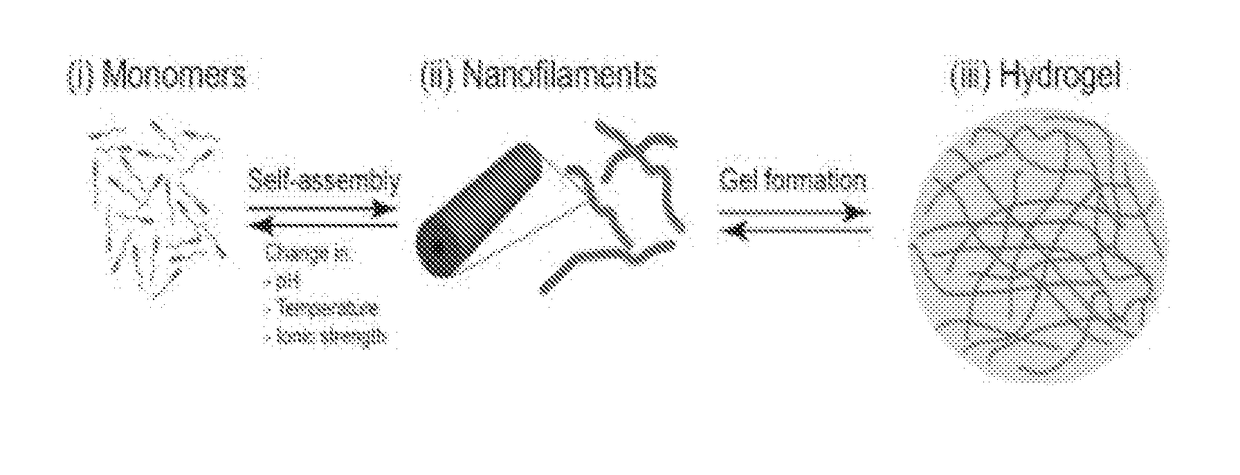
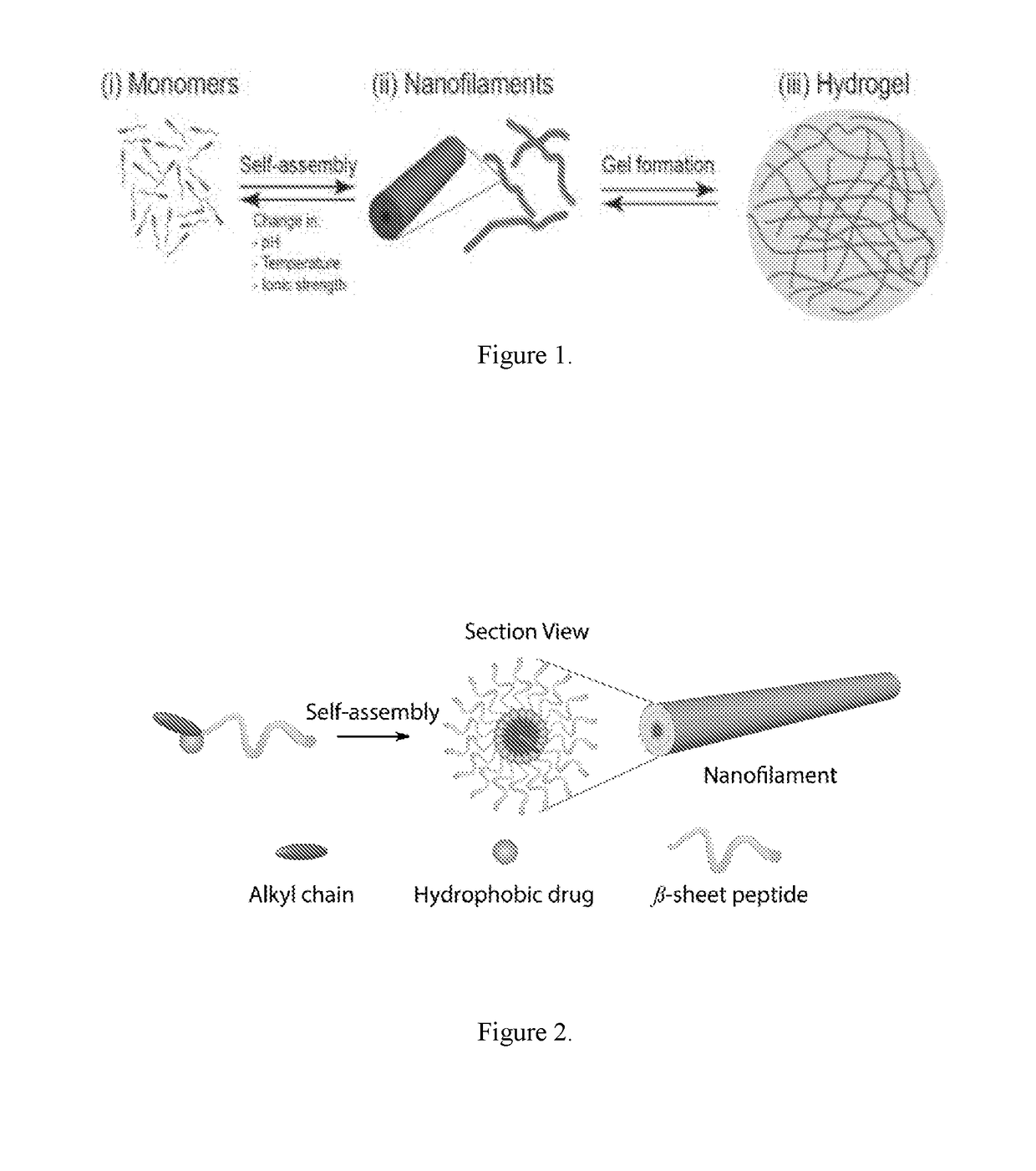
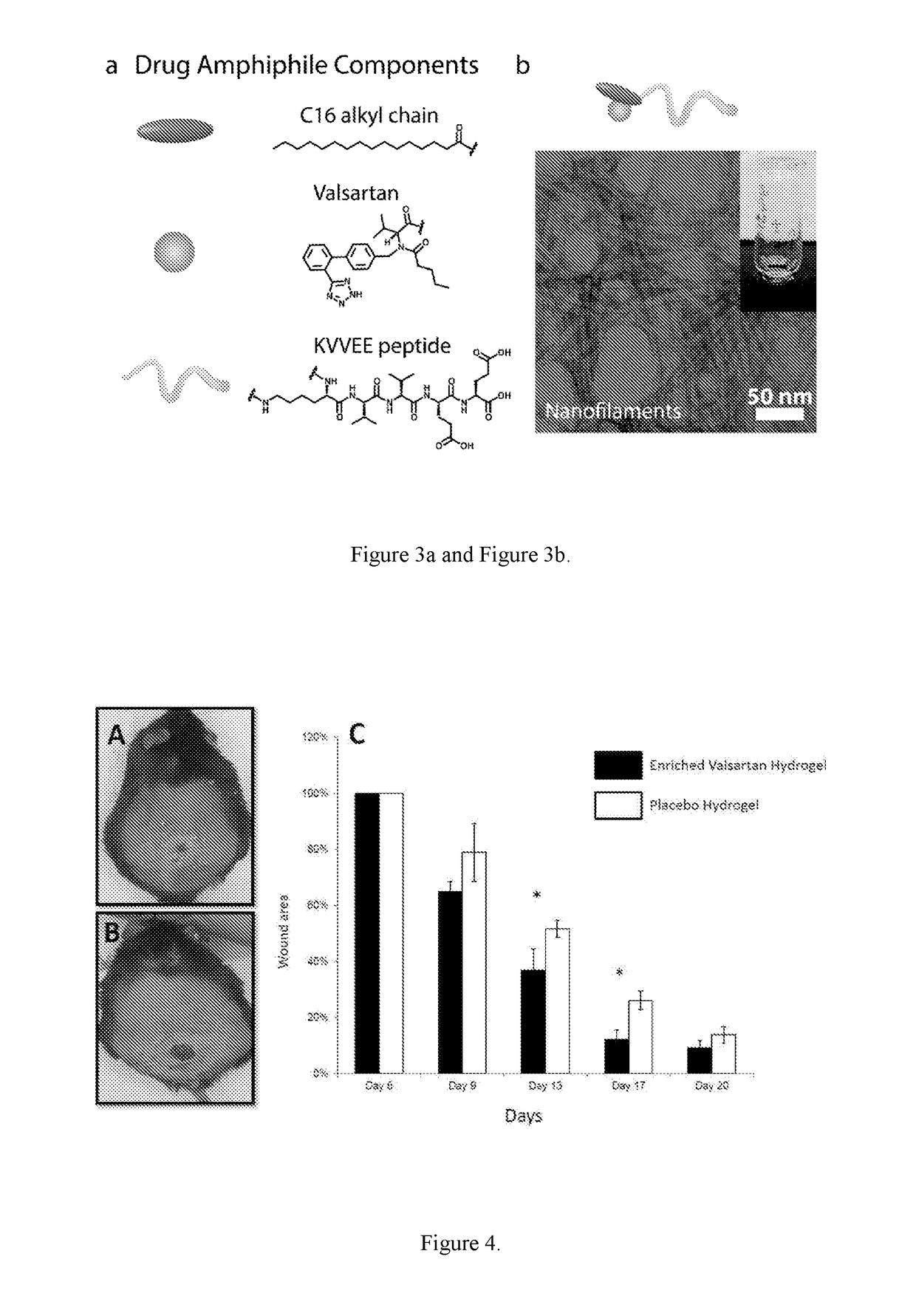
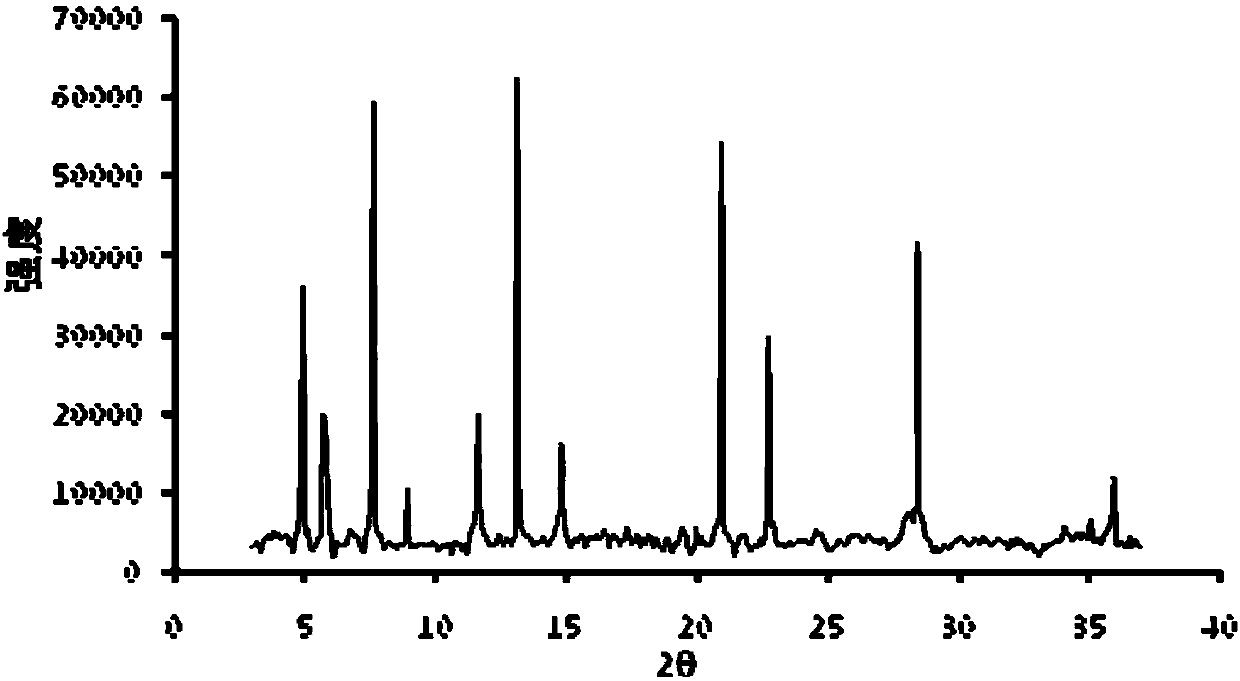
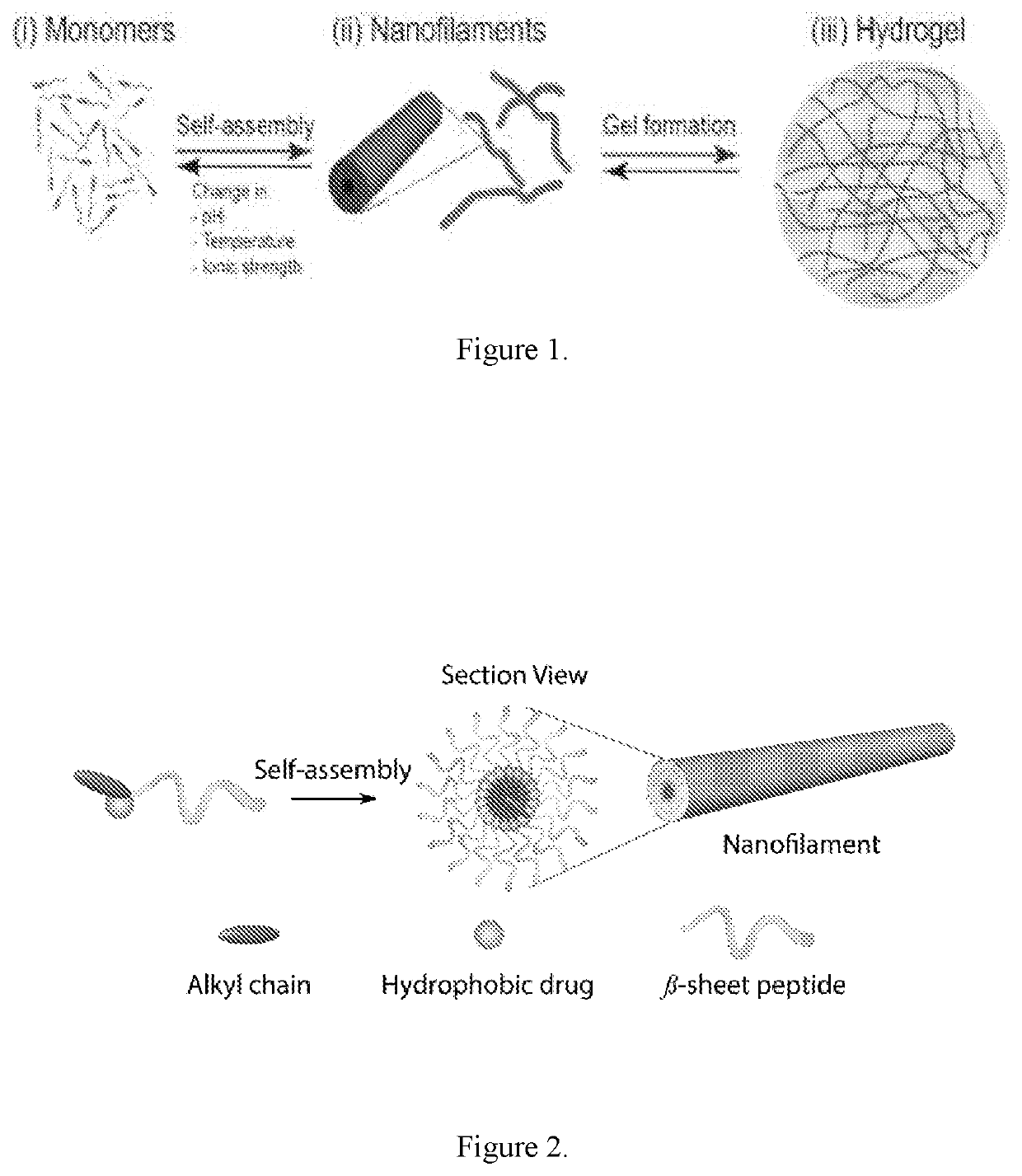
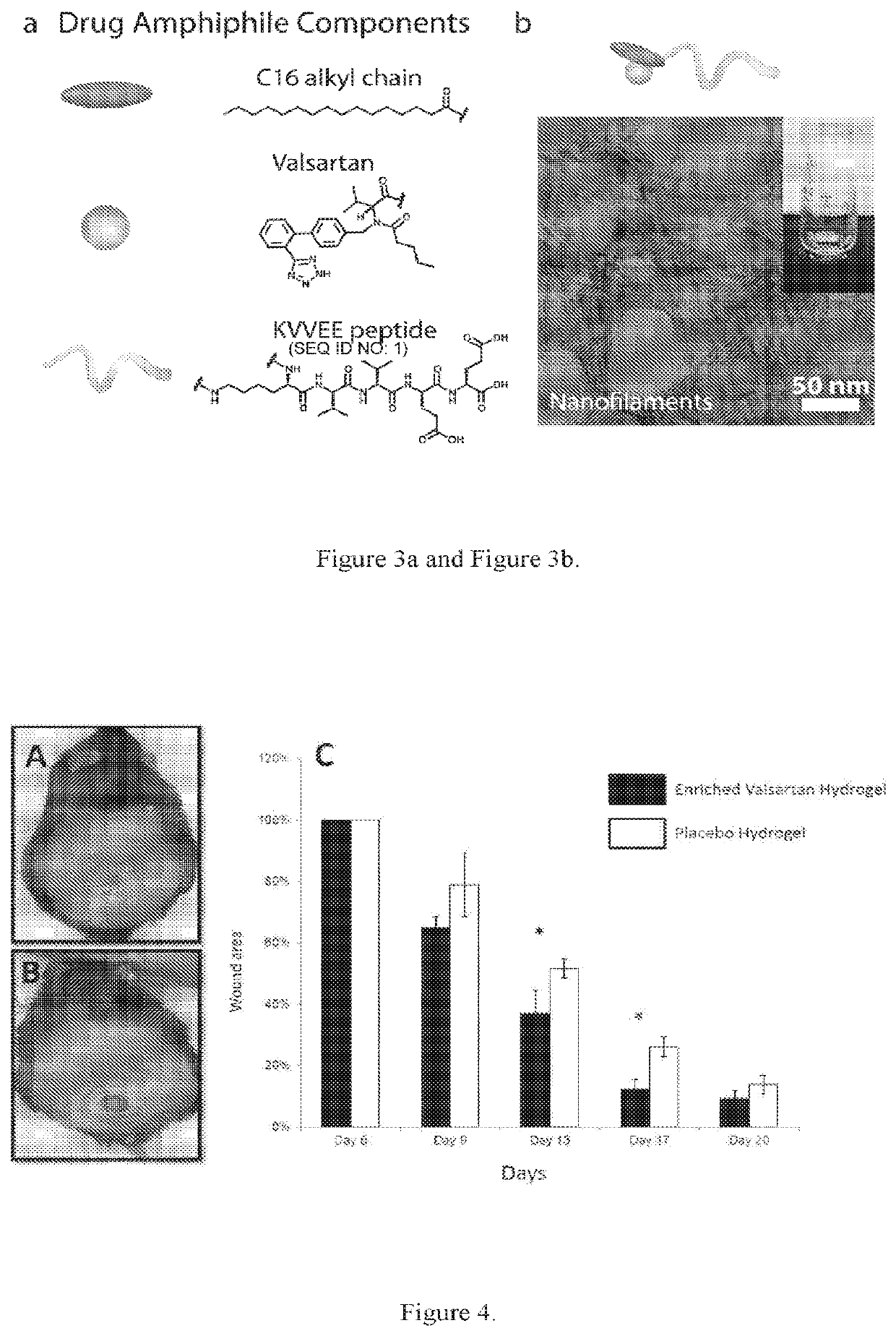
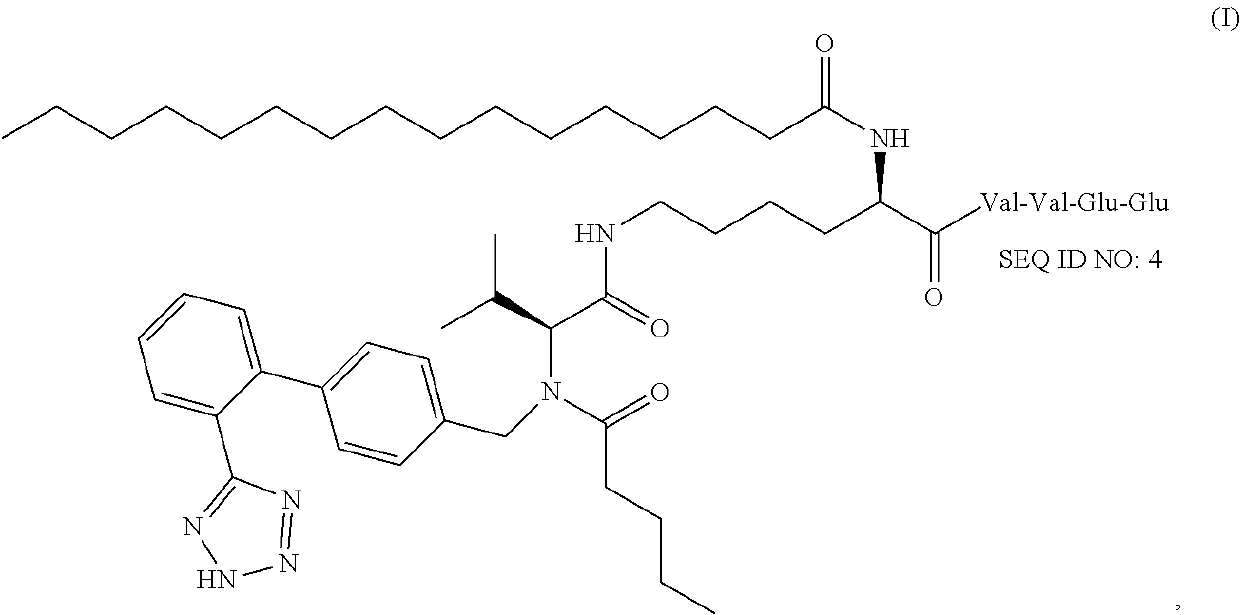
Supramolecular hydrogels containing angiotensin receptor blockers for targeted treatment of diabetic wounds
ActiveUS20180289831A1Aerosol deliveryOintment deliveryAngiotensin receptorAngiotensin Receptor Blockers
The invention described herein provides novel ampiphilic compounds that self-assemble into a hydrogel composition useful for treating wounds, including chronic wounds and diabetic wounds. The compounds of the invention have structural characteristics, such as hydrophilic and hydrophobic moieties, that enable self-assembly into discrete nanostructures, which then entangle to form the hydrogel. Also provided are methods for treating wounds.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method to prevent and treat macular degeneration by calcium channel blockers, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers
ActiveUS20210290607A1Avoid damageAvoid symptomsSenses disorderNitro compound active ingredientsCapillary networkAngiotensin Receptor Blockers
A method is provided to prevent and to treat Macular Degeneration by using Calcium Channel Blockers, Angiotensin-Converting Enzyme (ACE) Inhibitors, or Angiotensin Receptor Blockers (ARB), and more particularly, to a method to prevent and treat Macular Degeneration by using Calcium Channel Blockers, Angiotensin-Converting Enzyme Inhibitors, or Angiotensin Receptor Blockers that are not taken orally, but administered by ophthalmic preparation directly onto or into the eye where Macular Degeneration is formed, to increase the capillary network and blood supply to the retinal macula.
Owner:WEINBERG ASSA
A compound of angiotensin receptor antagonist and levosimendan and its use
ActiveCN106214680BGood treatment effectConvenient treatmentMetabolism disorderHeterocyclic compound active ingredientsAngiotensin receptor iiAnti hypertension
The invention provides a compound of an angiotensin receptor blocker and levosimendan and use of the compound. The compound is composed of the angiotensin receptor blocker and levosimendan, which can be mixed directly or be connected indirectly through a hydrogen bond, wherein a co-crystallized sodium hydrate formed by connection through hydrogen bond is more stable in property; and the pharmacokinetic properties are improved significantly; the action mechanisms of the angiotensin receptor blocker and the levosimendan are different, but the formed compound has an unexpected synergistic effect and has positive application prospects in the anti-heart failure and anti-hypertensive treatment field.
Owner:赛隆药业集团股份有限公司(长沙)医药研发中心
Combination therapy comprising angiotensin receptor blockers and vasopressin receptor antagonists
The present invention relates to certain pharmaceutical compositions containing at least one vasopressin receptor antagonist and at least one angiotensin receptor blocker (ARB) and methods for preparing these compounds, compositions, intermediates and derivatives thereof and for the treatment of vasopressin and / or angiotensin-mediated disorders.
Owner:JANSSEN PHARMA NV
Compound antihypertensive preparation and application thereof
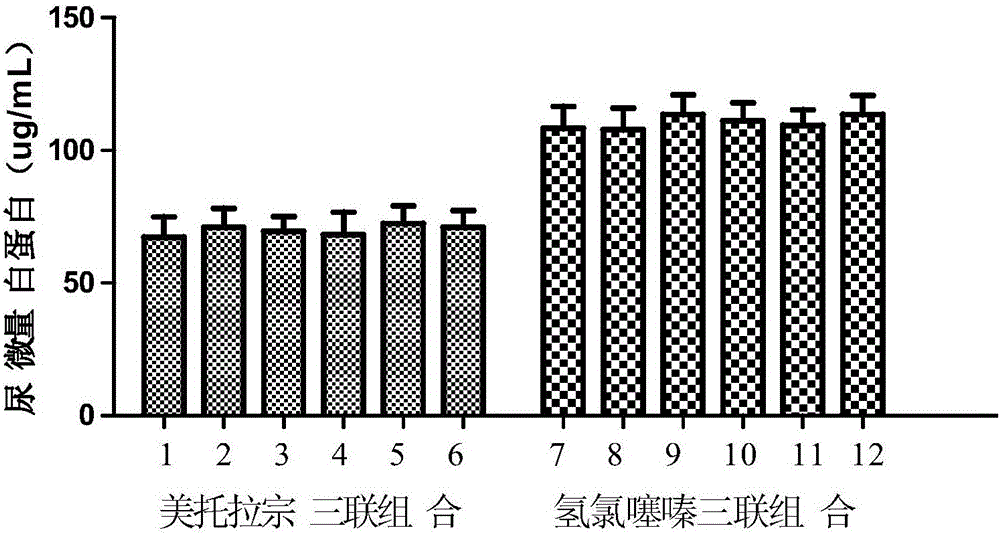
InactiveCN105106962AImprove complianceStrengthen the synergistic antihypertensive effectOrganic active ingredientsDrageesAdjuvantAngiotensin receptor ii
The invention provides a pharmaceutical composition for treating hypertension. The pharmaceutical composition is characterized by comprising (1) angiotensin II receptor blocker, (2) diuretic metolazone, (3) calcium channel blocker and (4) pharmaceutically acceptable adjuvant materials, wherein the weight ratio among the angiotensin receptor blocker, the metolazone and the calcium channel blocker is 2-200:0.5-5:2-50. The pharmaceutical composition for treating hypertension has the advantages that the pharmaceutical composition combines the angiotensin receptor blocker, the metolazone and the calcium channel blocker, and accordingly, synergetic antihypertensive effect is enhanced, adverse reaction is reduced and compliance of patients is improved; the pharmaceutical composition is wide in application range and can be also used for patients suffering from severe renal damage; the pharmaceutical composition is applicable to mild and moderate essential hypertension, particularly secondary hypertension caused by renal damage.
Owner:XIAN LIBANG ZHAOXIN BIOTECH CO LTD
Supramolecular hydrogels containing angiotensin receptor blockers for targeted treatment of diabetic wounds
ActiveUS10835615B2Aerosol deliveryOintment deliveryAngiotensin Receptor BlockersAngiotensin receptor ii
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method to prevent and treat osteoarthritis by calcium channel blockers, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers
InactiveUS20210290606A1Prevent relapsePreventing osteoarthritisOrganic active ingredientsNervous disorderAngiotensin Receptor BlockersAngiotensin receptor ii
A method is provided to prevent and to treat Osteoarthritis syndrome by using Calcium Channel Blockers, Angiotensin-Converting Enzyme (ACE) Inhibitors, or Angiotensin Receptor Blockers (ARB), and more particularly, to a method to prevent and treat Osteoarthritis syndrome by using Calcium Channel Blockers, Angiotensin-Converting Enzyme Inhibitors, or Angiotensin Receptor Blockers that are not taken orally, but administered by intra-articular injection into the affected joint or administered by topical application to the skin in a region of the affected joint, so as to increase the capillary network and articular blood supply to the joint or to increase hyaluronic acid production to the articular cartilage, bones, articular ligaments, synovia tissue and other soft tissues.
Owner:WEINBERG ASSA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com