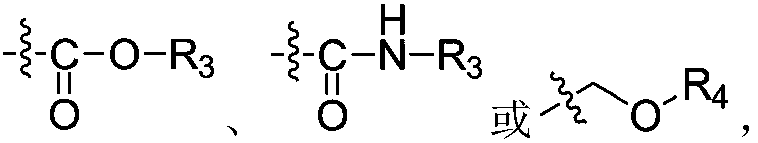
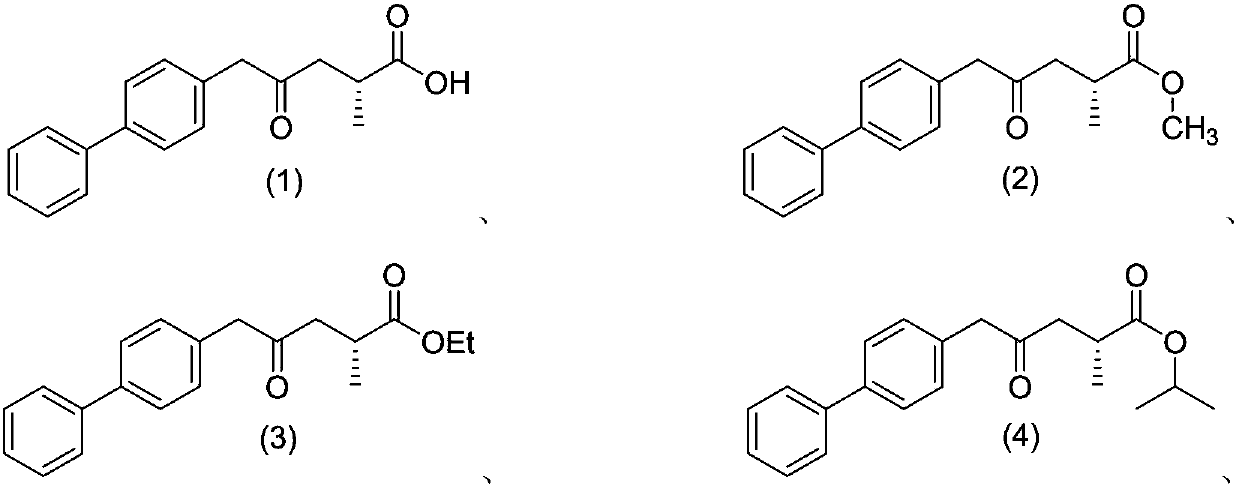
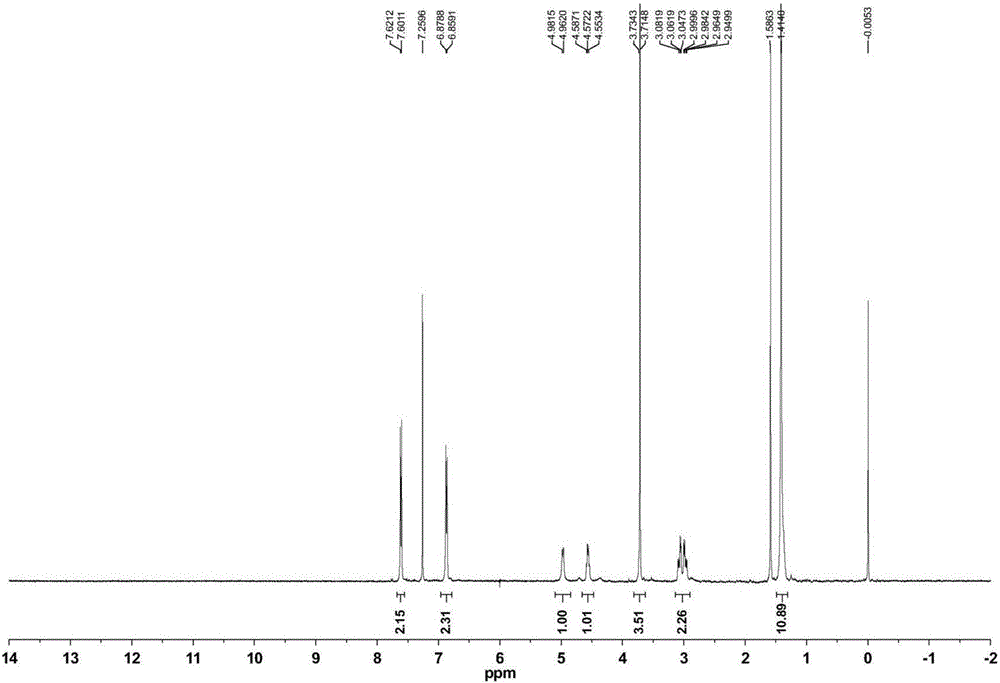
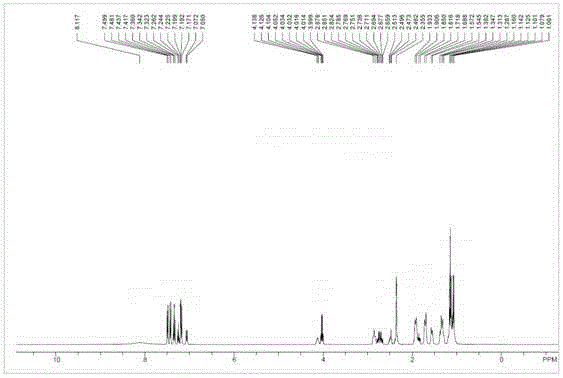
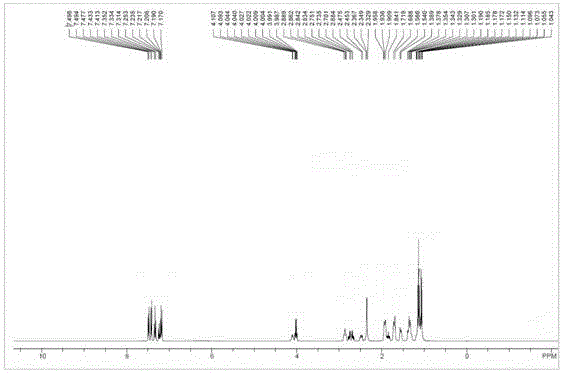
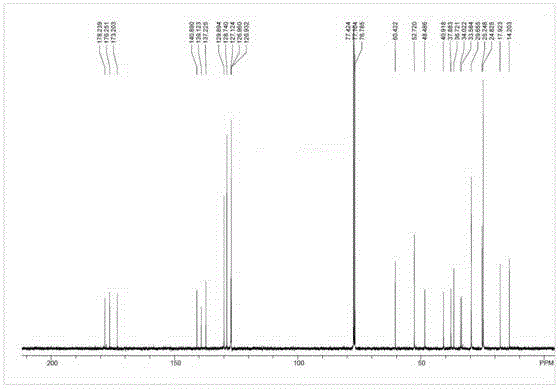
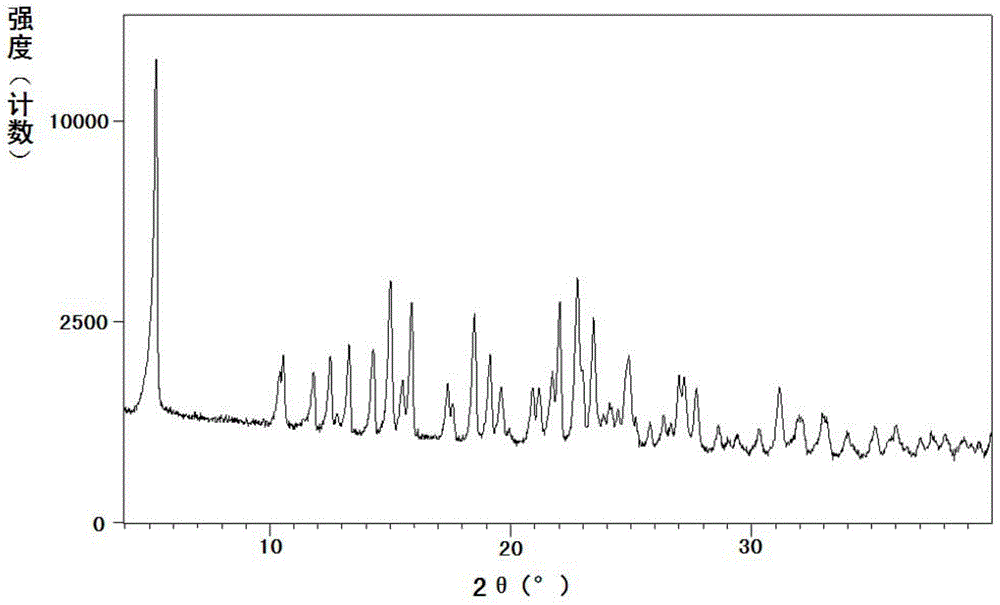
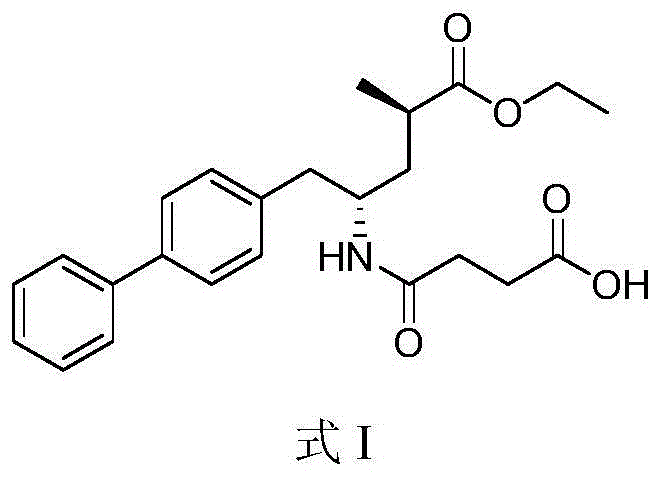
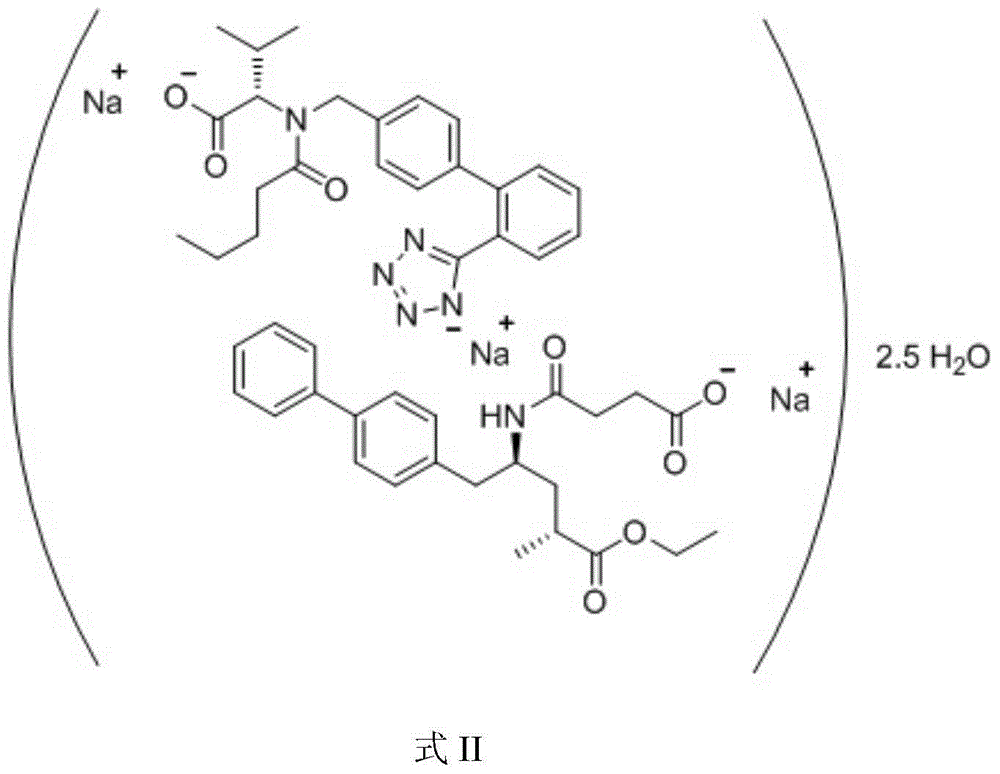
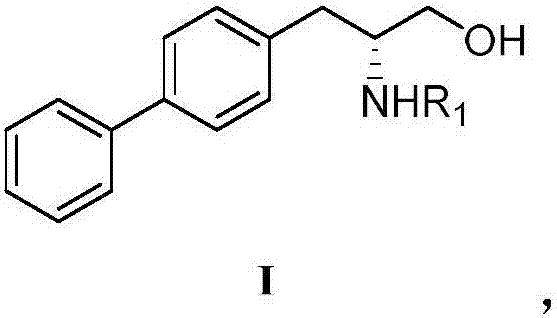
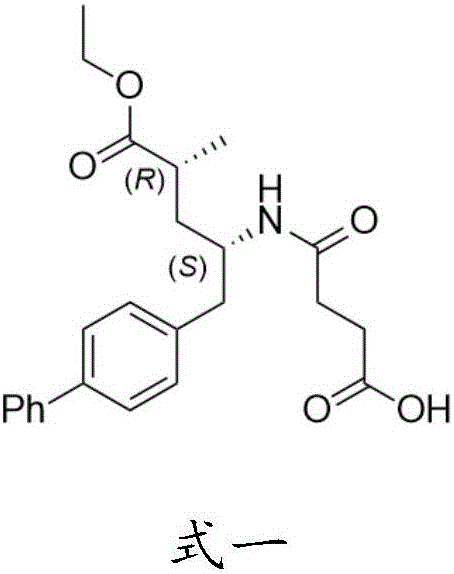
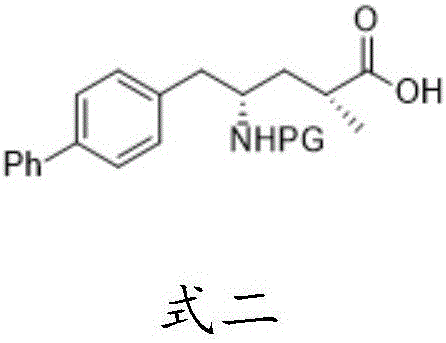
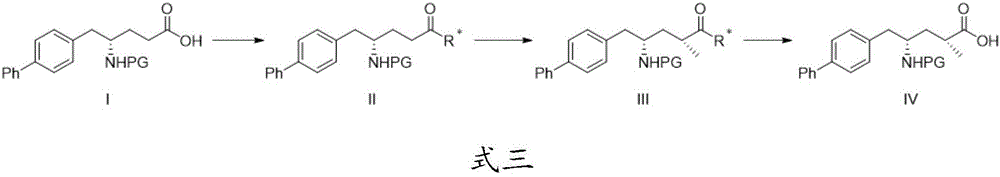
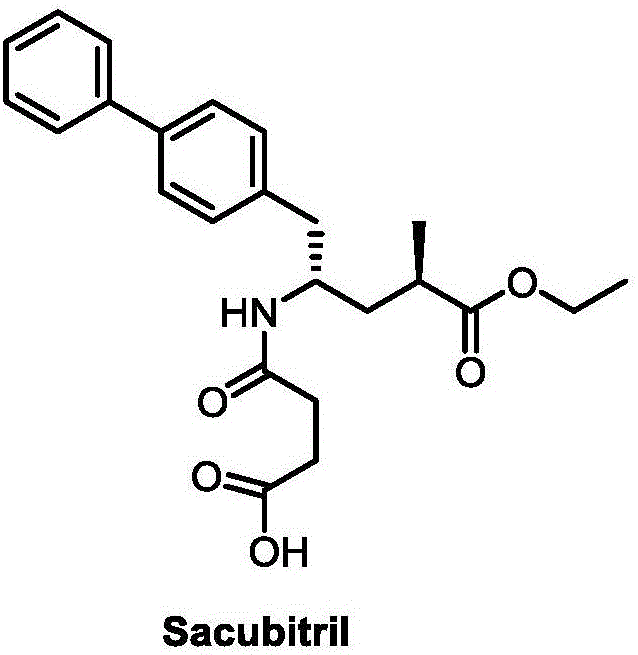
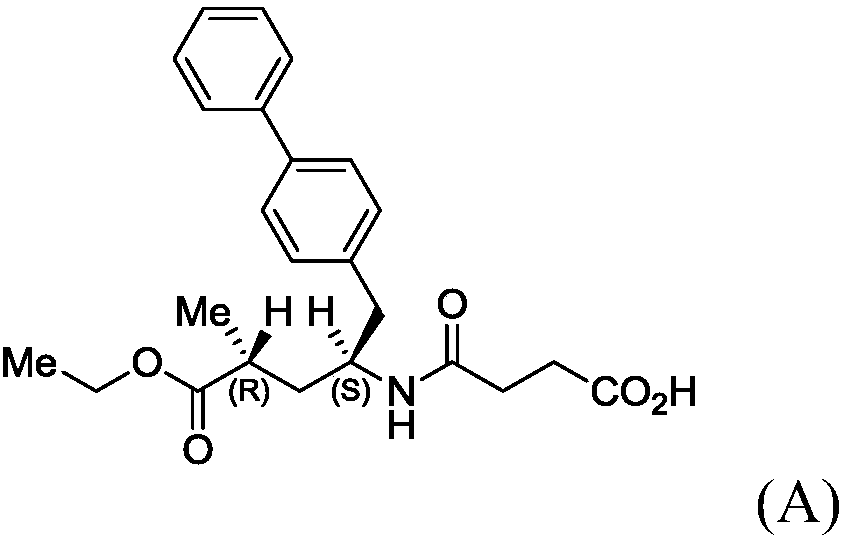
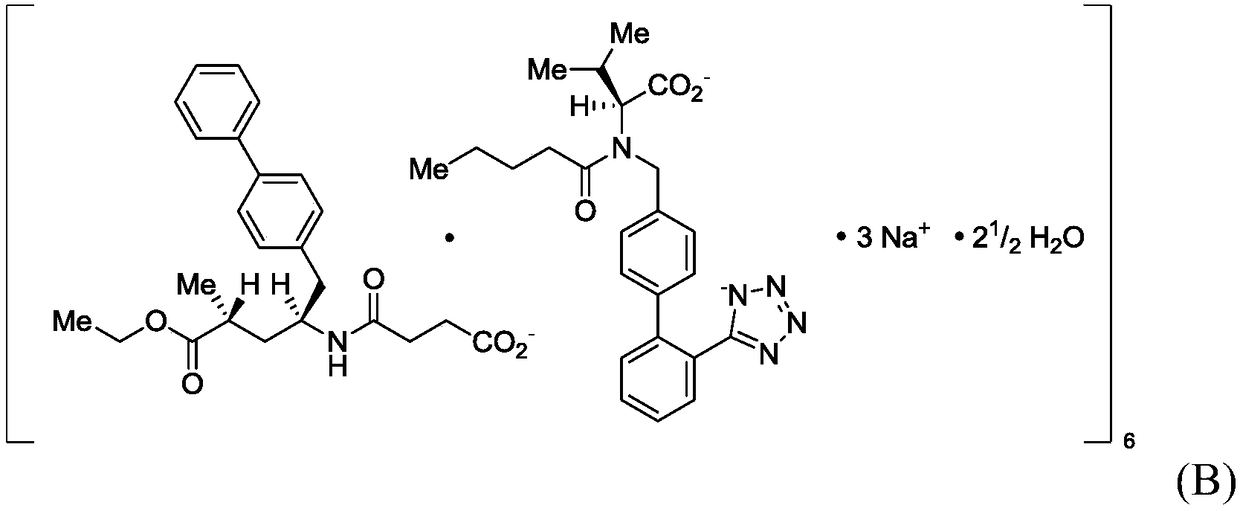
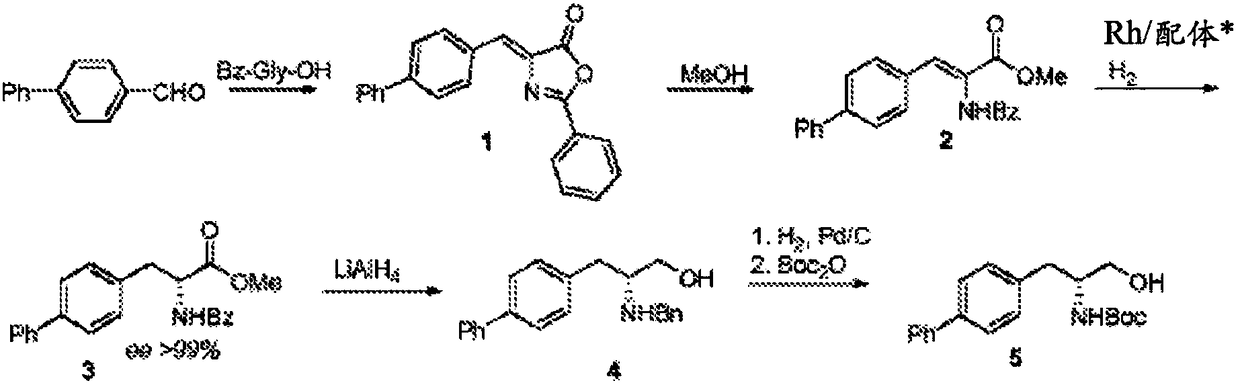
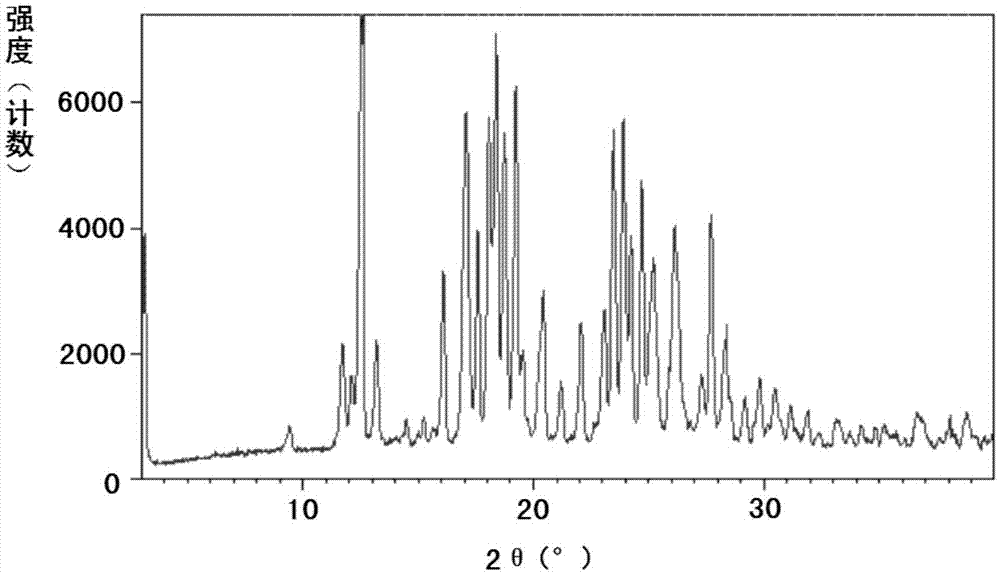
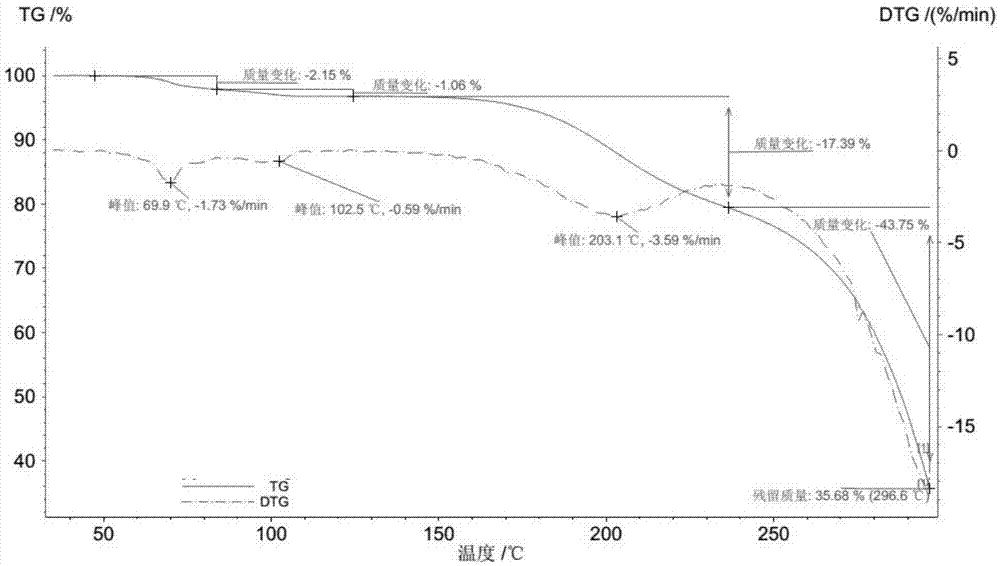
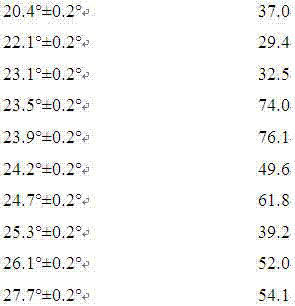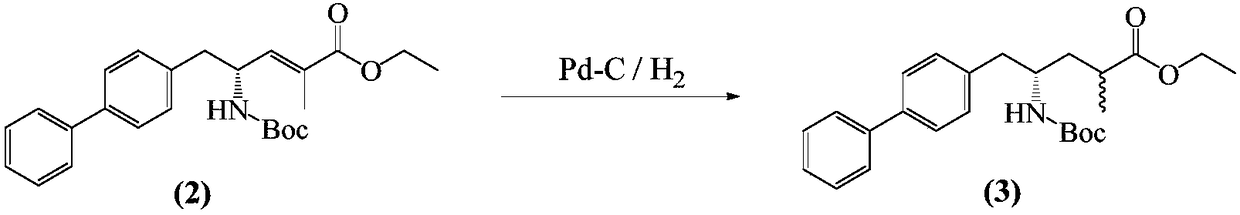Patents
Literature
103 results about "Sacubitril" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
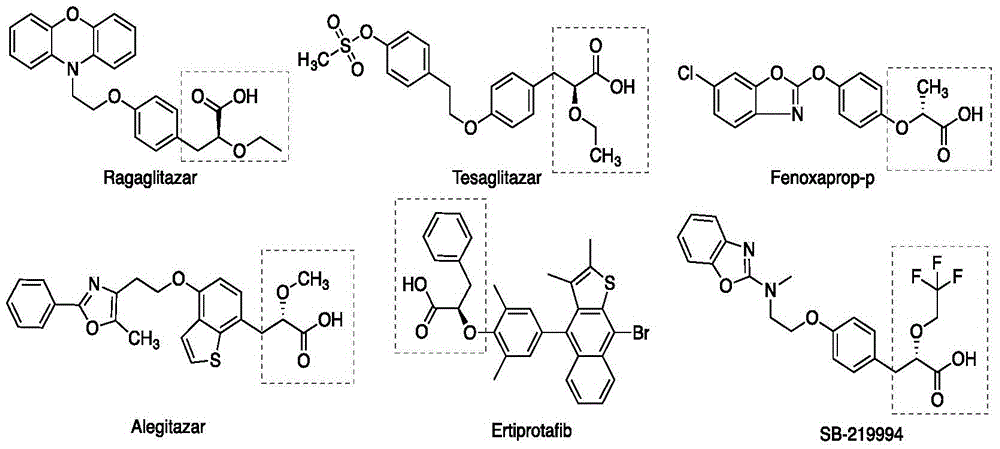
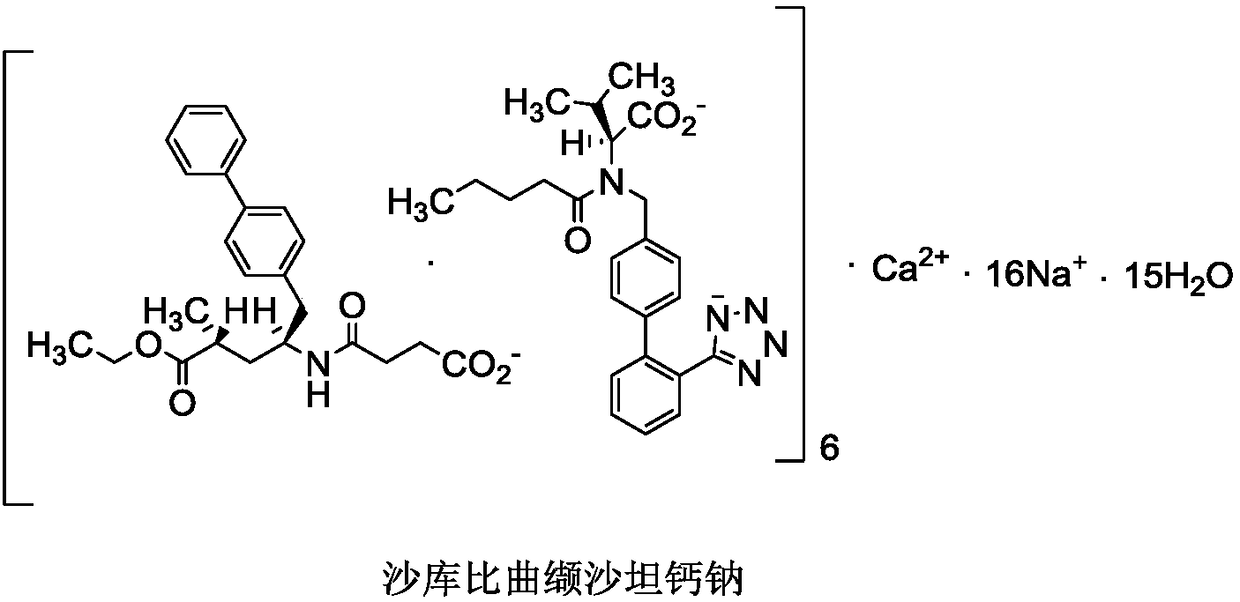
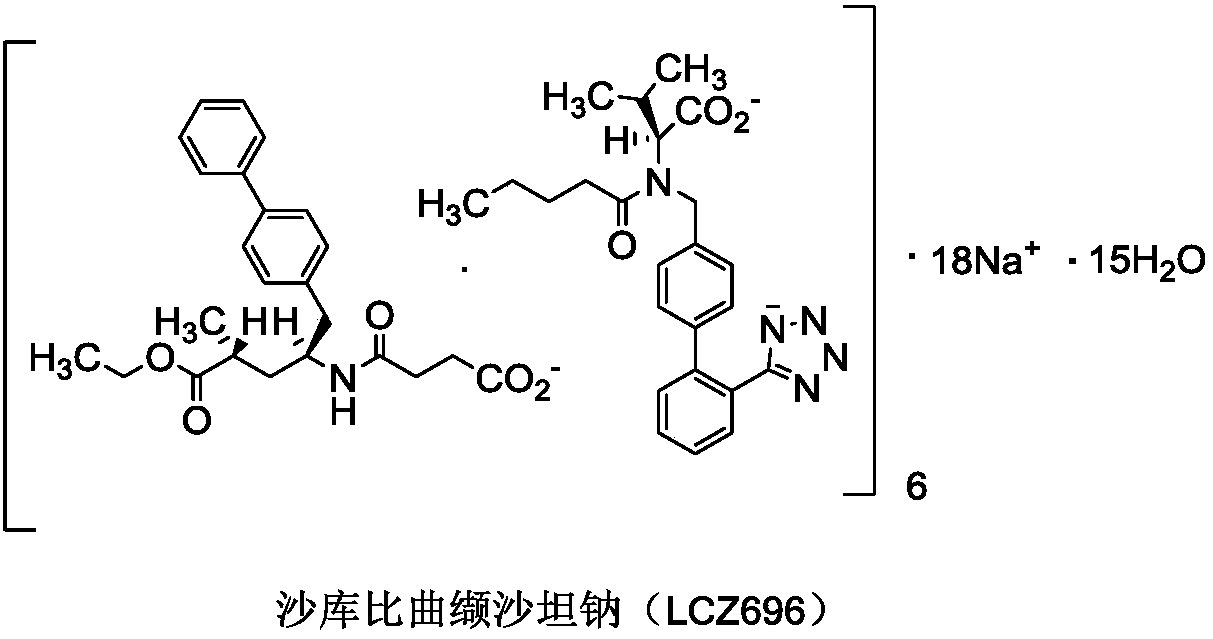
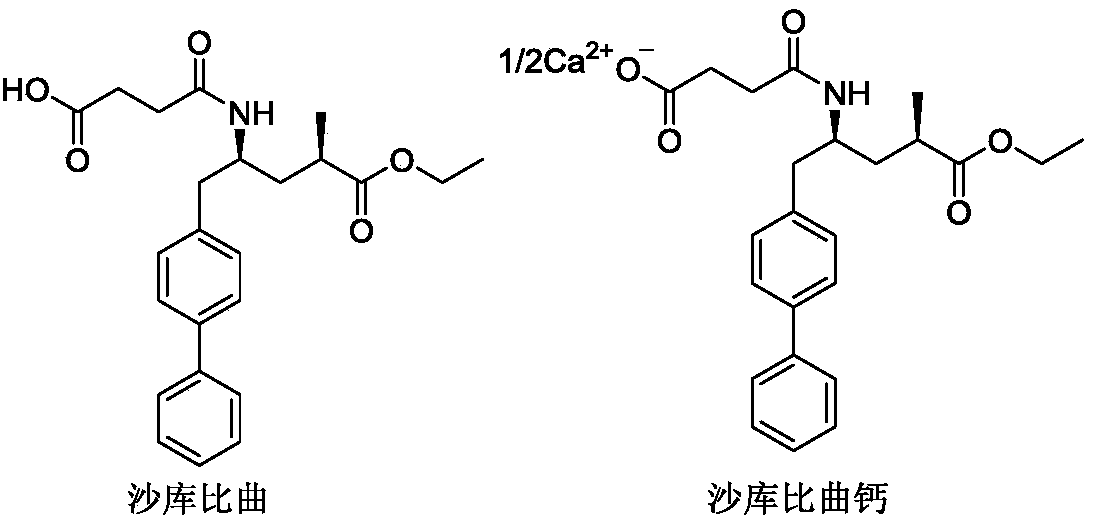
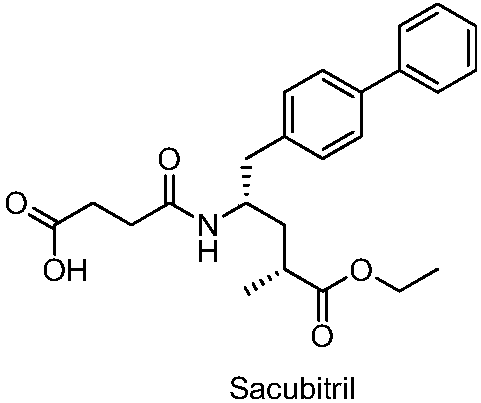
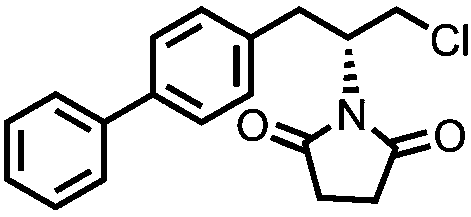
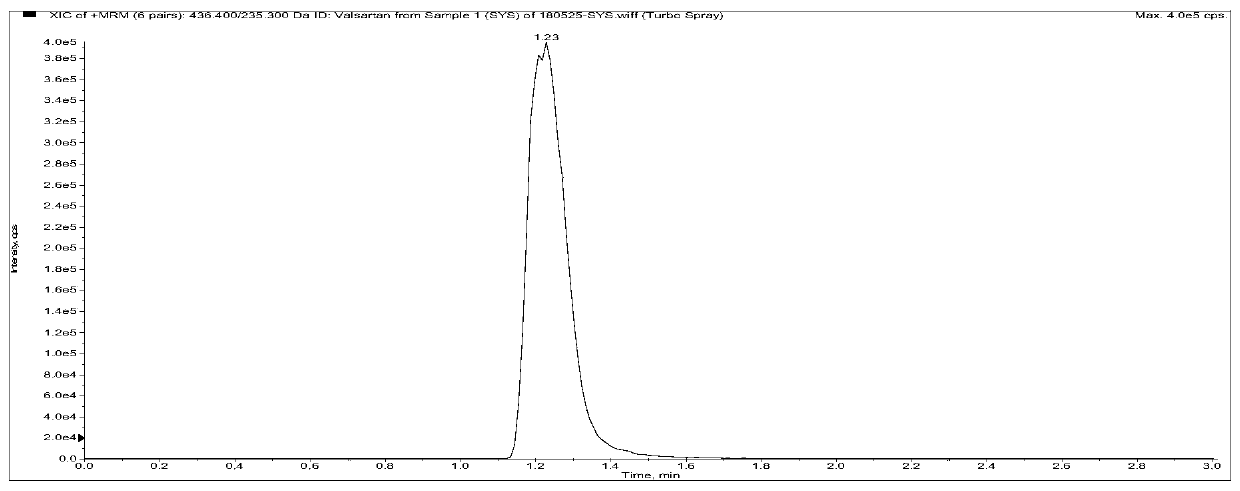
Sacubitril (/səˈkjuːbɪtrɪl/; INN) is an antihypertensive drug used in combination with valsartan. The combination drug sacubitril/valsartan, known during trials as LCZ696 and marketed under the brand name Entresto, is a treatment for heart failure. It was approved under the FDA's priority review process for use in heart failure on July 7, 2015.
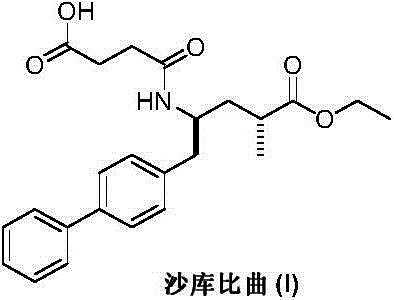
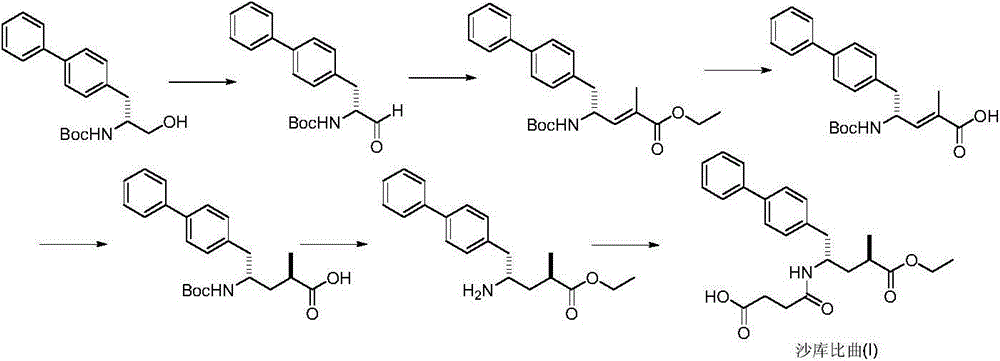
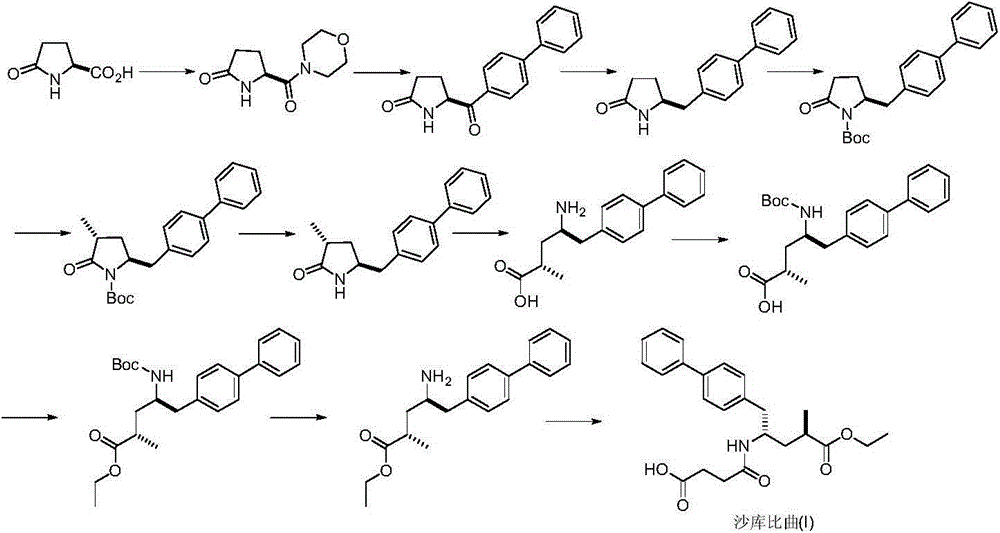
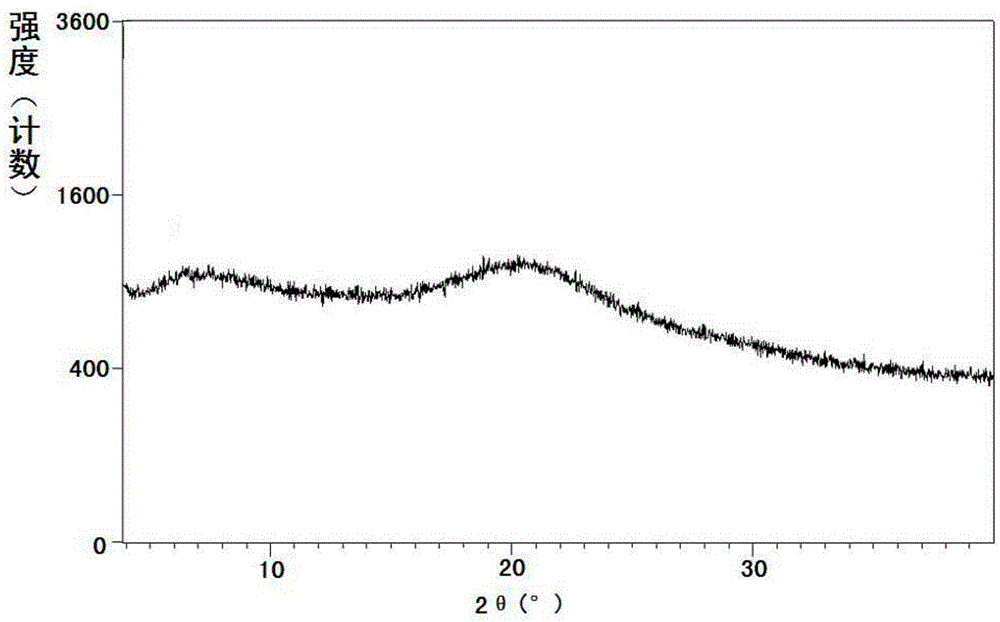
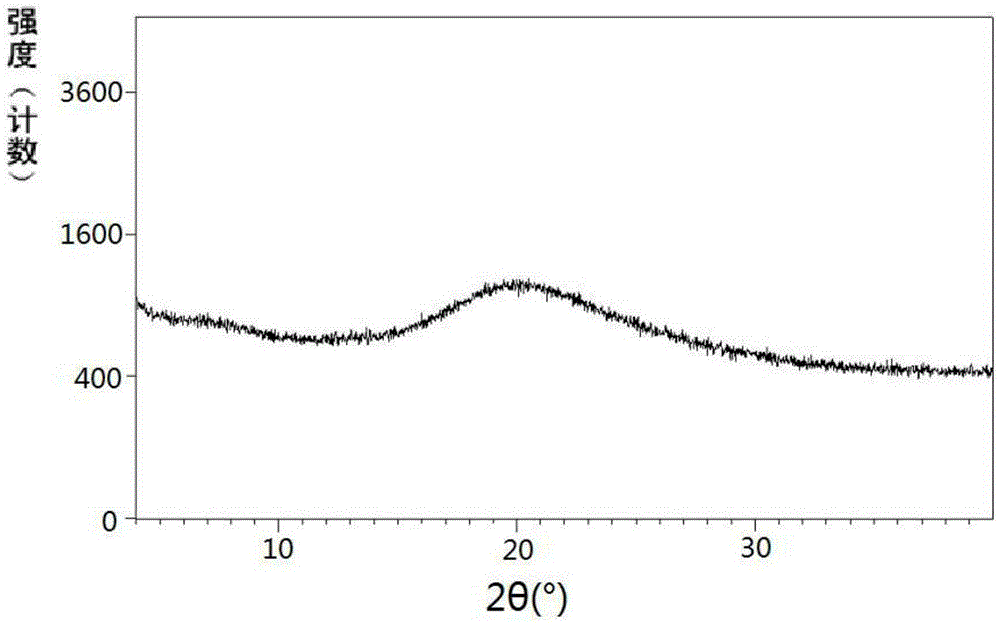
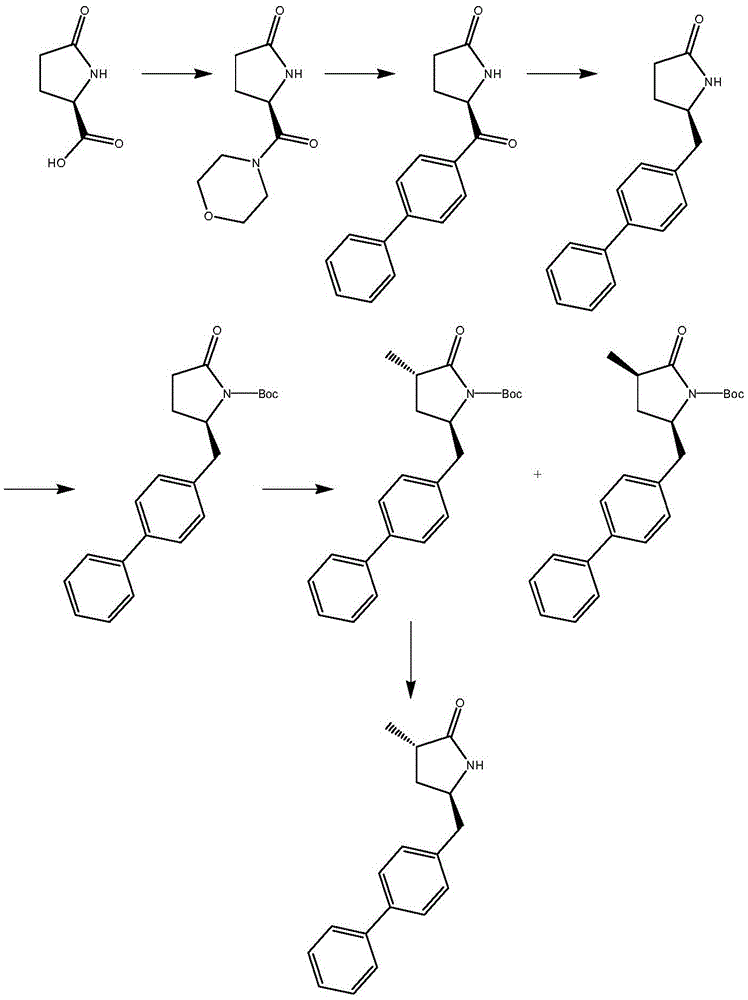
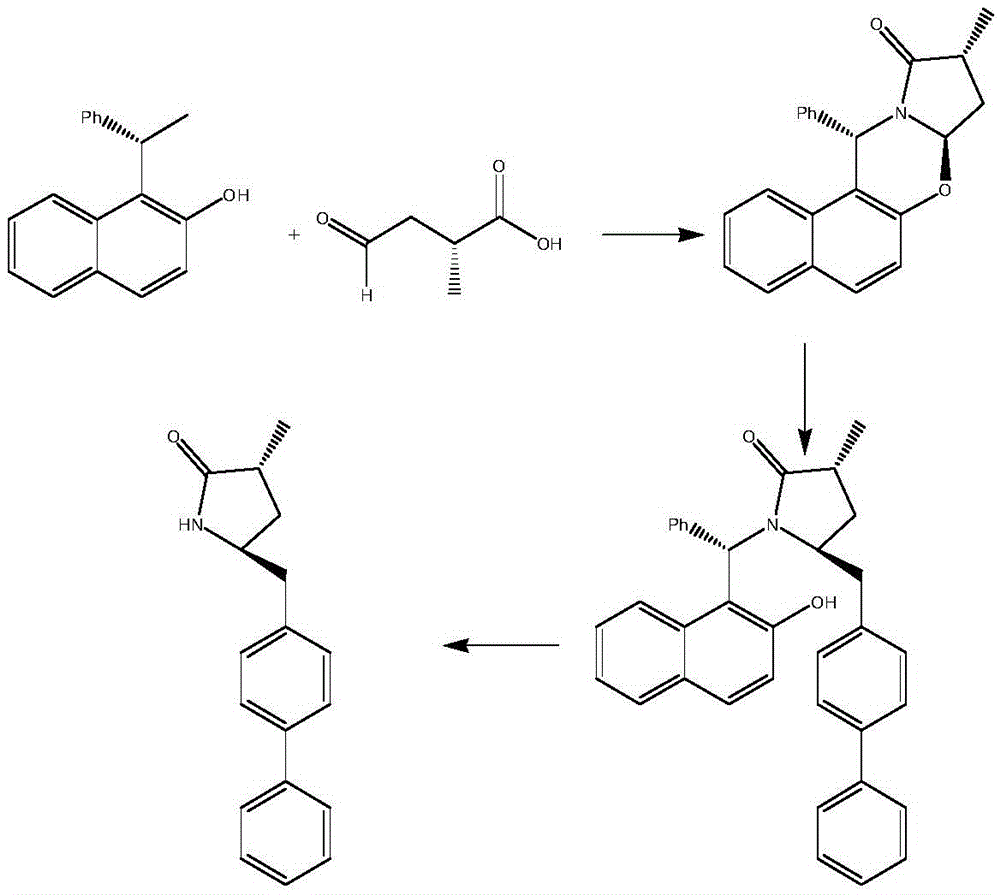
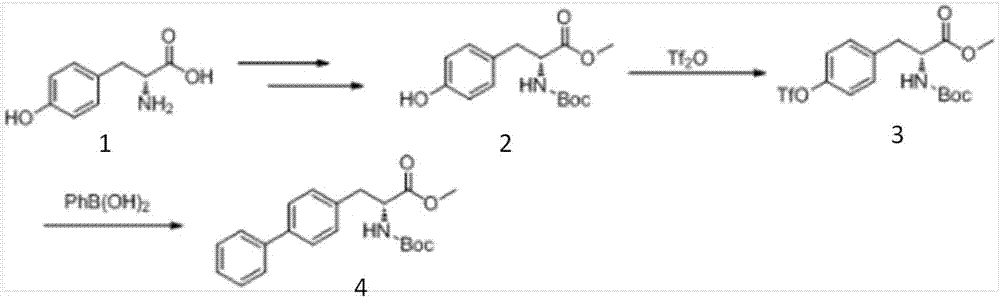
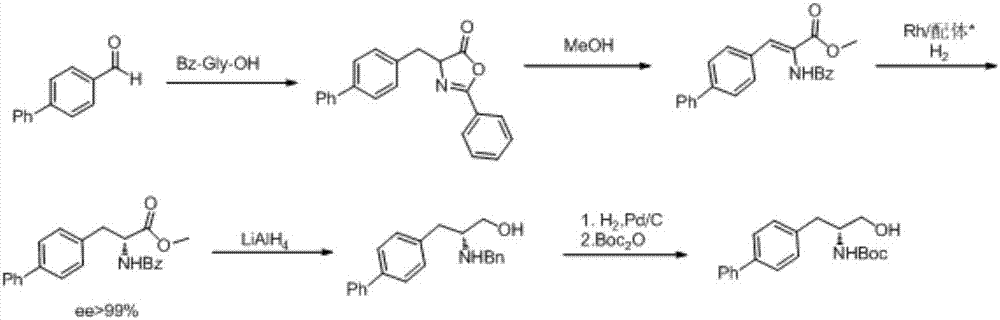
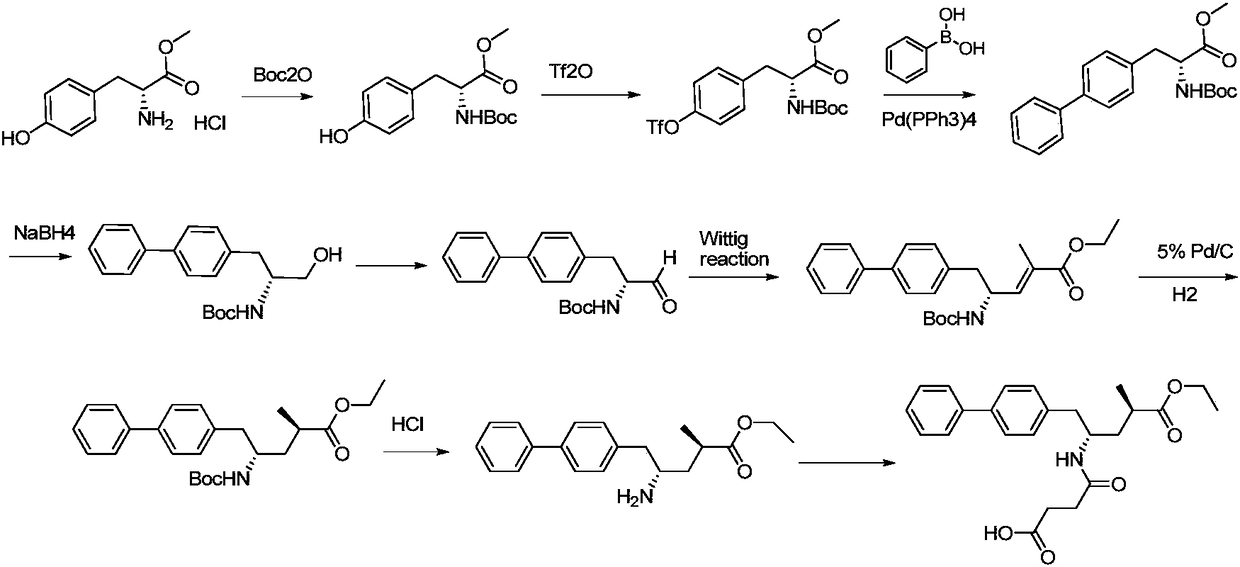
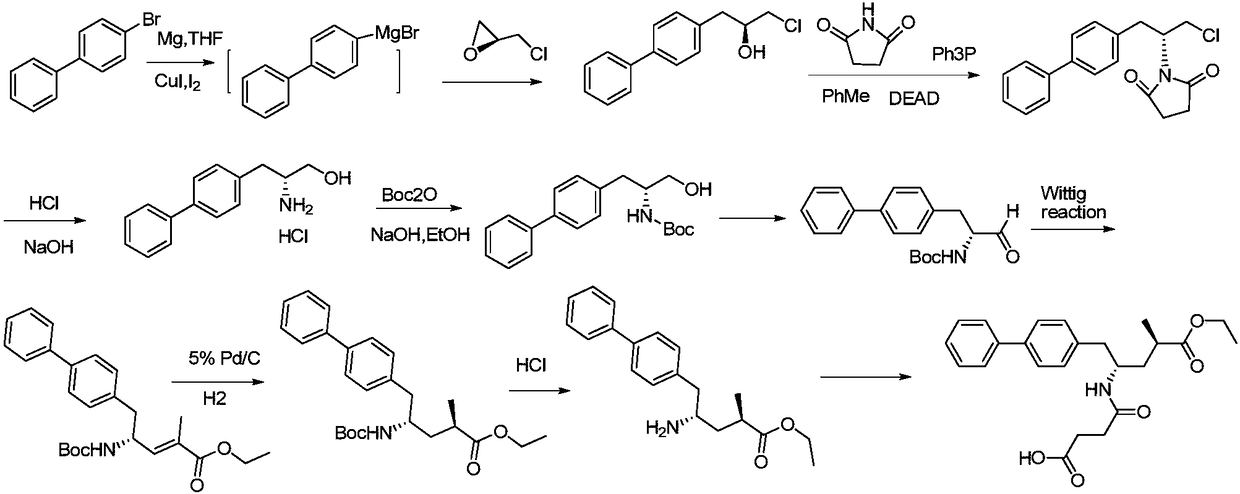
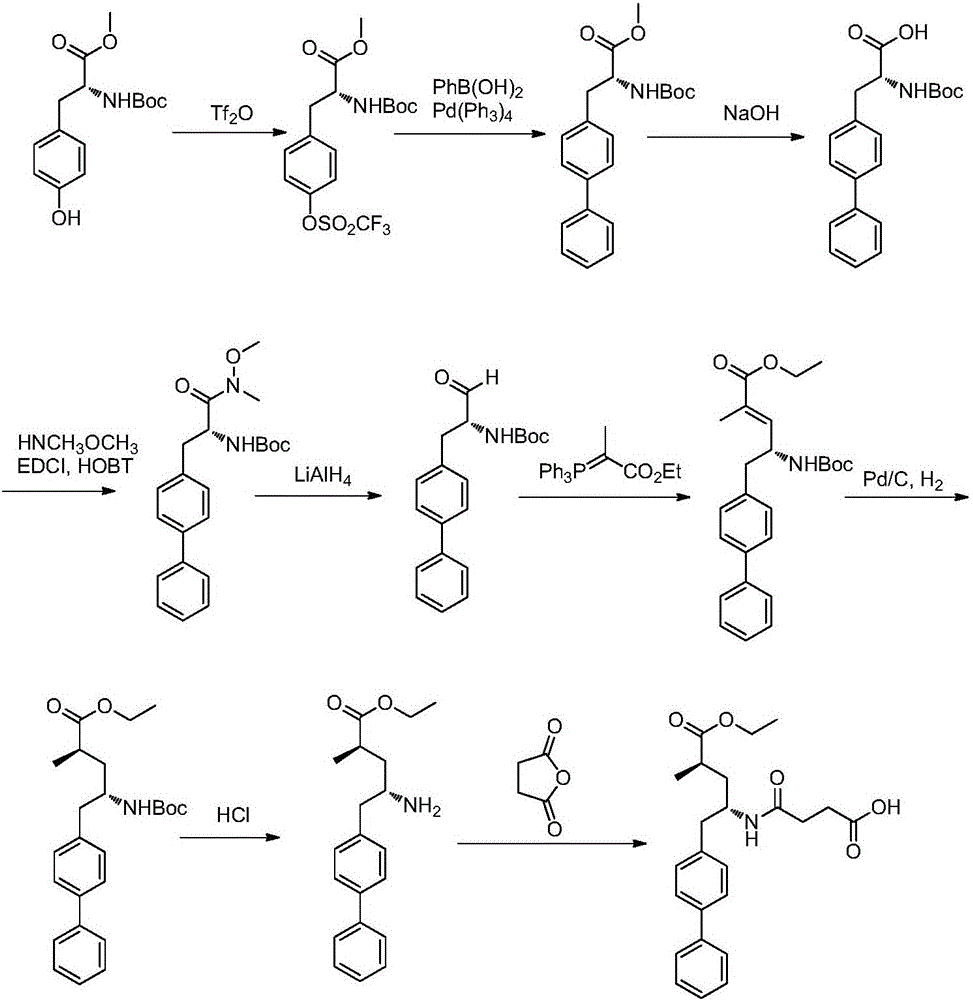
Preparation method of sacubitril
ActiveCN104557600AEase of industrial productionRaw materials are easy to getOrganic compound preparationCarboxylic acid amide separation/purificationSacubitril2-Naphthol
The invention discloses a preparation method of one of components of a novel antihypertensive drug LCZ696, namely, sacubitril (sacubitril, AHU-377, I). The method comprises the following preparation steps: carrying out cyclization, addition, debenzylation, ring opening, esterification, amidation and the like on a chiral induciton reagent (S)-1-(alpha-aminobenzyl)-2-naphthol (S-betti Base) and 2R-methyl-4-oxo-butyric acid to prepare the sacubitril (I). The preparation method is available in raw materials, concise in process, economic and environmentally friendly, and is suitable for industrial production.
Owner:广州博览咨询服务有限公司
Sacubitril derivatives and medicine compositions, preparation methods and application thereof
ActiveCN105693543AEasy to prepareCrystal form controllableAmino compound purification/separationOrganic compound preparationEthylenediamineArginine
The invention provides sacubitril derivatives and medicine compositions, preparation methods and application thereof and belongs to the fields of medicine compounds and preparation thereof. The sacubitril derivatives comprise sacubitril lithium salt, sacubitril kali salt, sacubitril magnesium salt, sacubitril calcium salt, sacubitril strontium salt, sacubitril zinc salt, sacubitril ferric salt, sacubitril ammonium salt, sacubitril diethylamine salt, sacubitril ethylenediamine salt, sacubitril piperazine salt, sacubitril N-(2-ethoxyl)-pyrrolidine salt, sacubitril choline salt, sacubitril cholamine salt, sacubitril diethanol amine salt, sacubitril triethanolamine salt, sacubitril tromethamine salt, sacubitril meglumine salt, sacubitril diisopropylamine salt, sacubitril tert-butylamine salt, sacubitril N, N'-bis-benzyl ethylenediamine salt, sacubitril L-lysine salt, sacubitril L-arginine salt or sacubitril L-histidine salt.
Owner:SICHUAN HAISCO PHARMA CO LTD
Sacubitril intermediate and preparation method of sacubitril intermediate and sacubitril
ActiveCN105924355AImprove qualityHigh yieldOrganic compound preparationCarboxylic acid esters preparationChemical reactionOrganic synthesis
The invention discloses a sacubitril intermediate and a preparation method of the sacubitril intermediate and sacubitril, and belongs to the technical field of organic synthesis of drugs. On one hand, the invention discloses a novel compound-sacubitril intermediate compound B and a preparation method thereof, and on the other hand, the invention discloses a novel preparation method of the sacubitril. The sacubitril intermediate and the preparation method of the sacubitril intermediate and the sacubitril have the following advantages that the synthesizing route is short, the product can be prepared only through four steps of chemical reactions, and the steps of existing patent routes all exceed nine steps; the dicarbonyl compound is prepared through Reformasky condensation supplied by the method, and the cost is low; a first chiral center is introduced by fully utilizing a chiral compound L-(S)-ethyl lactate which is low in cost and easy to obtain in the natural world, and the cost is low; a second chiral center is constructed through a biological enzyme catalysis technique, the optical selectivity reaches up to 99.9%, the quality is good, and the cost is low. By means of the technological means, the total yield of the prepared sacubitril is increased, and the quality of the sacubitril is improved.
Owner:ZHEJIANG HONGYUAN PHARMA
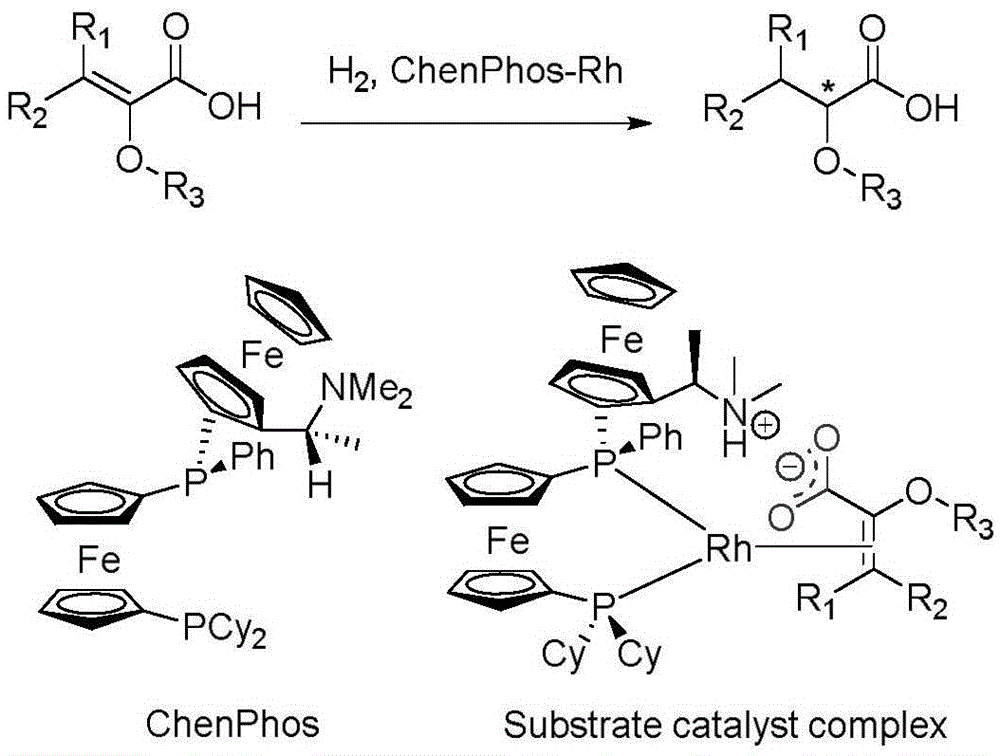
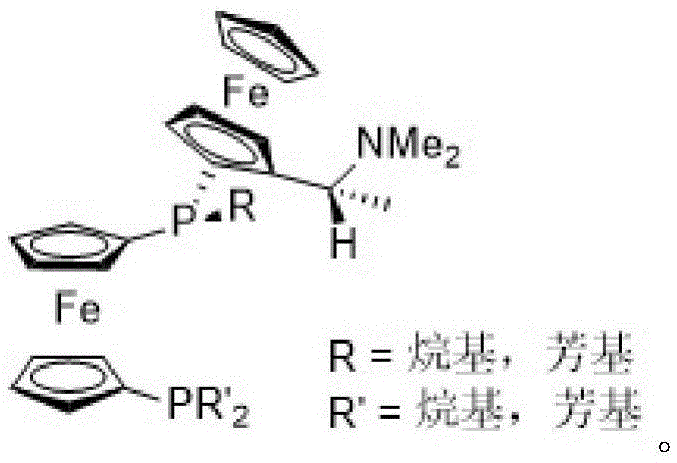
Asymmetric hydrogenation method of alpha-oxo-alpha, beta-unsaturated carboxylic acid
InactiveCN105481622AHigh catalytic activityHigh enantioselectivityOrganic reductionOrganic compound preparationEnkephalinase inhibitorAsymmetric hydrogenation
The invention relates to an asymmetric hydrogenation method of alpha-oxo-alpha, beta-unsaturated carboxylic acid. A metal complex containing ChenPhos chiral ligand is a catalyst high in conversion efficiency, and particularly, the catalyst can be used for synthesizing a core framework in enkephalinase inhibitor Sacubitril through asymmetric hydrogenation. The inhibitor is one of components of medicine LCZ 696 approved by American Food and Drug Administration. The asymmetric hydrogenation method of the alpha-oxo-alpha, beta-unsaturated carboxylic acid is efficient, and the application range of substrate is wide.
Owner:WUHAN CATALYS TECH CO LTD
Improved preparation method of sacubitril intermediate
InactiveCN106397273ACarbamic acid derivatives preparationOrganic compound preparationPalladium catalystSacubitril
The invention relates to an improved preparation method of an important intermediate (2R, 4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methyl pentanoic acid (that is a compound shown in the formula III) for preparing sacubitril which is a neutral endopeptidase (NEP) inhibitor and of an analog or salt of the intermediate. The method mainly comprises that in the presence of a palladium catalyst, hydrogenation is carried out to make the compound shown in the formula III and having high optical purity and the analog or salt of the compound. The improved method is available in raw materials, mild in reaction condition, and simple and convenient to operate. The product quality is excellent and the cost is low, so that the method is suitable for industrial mass production.
Owner:SICHUAN HAISCO PHARMA CO LTD
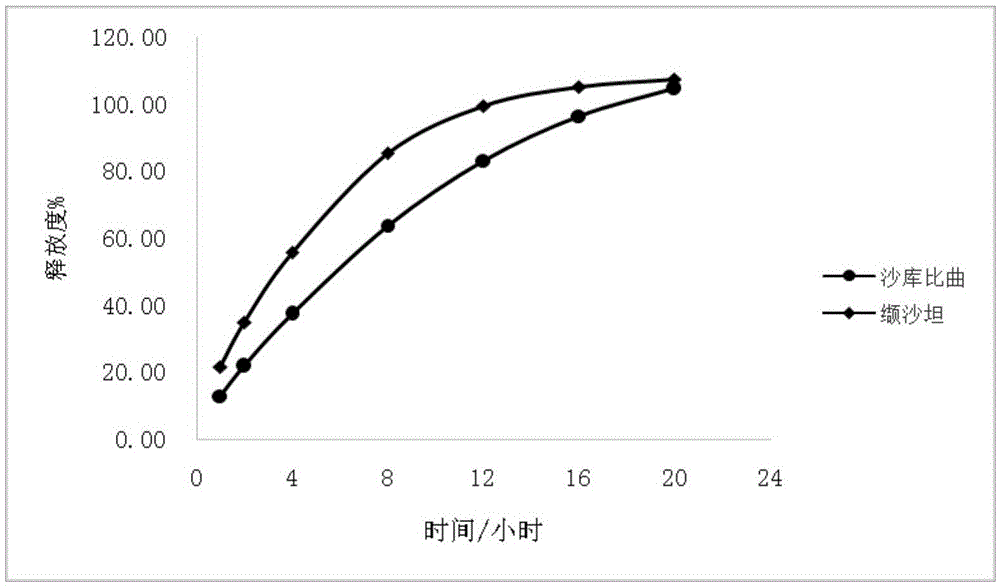
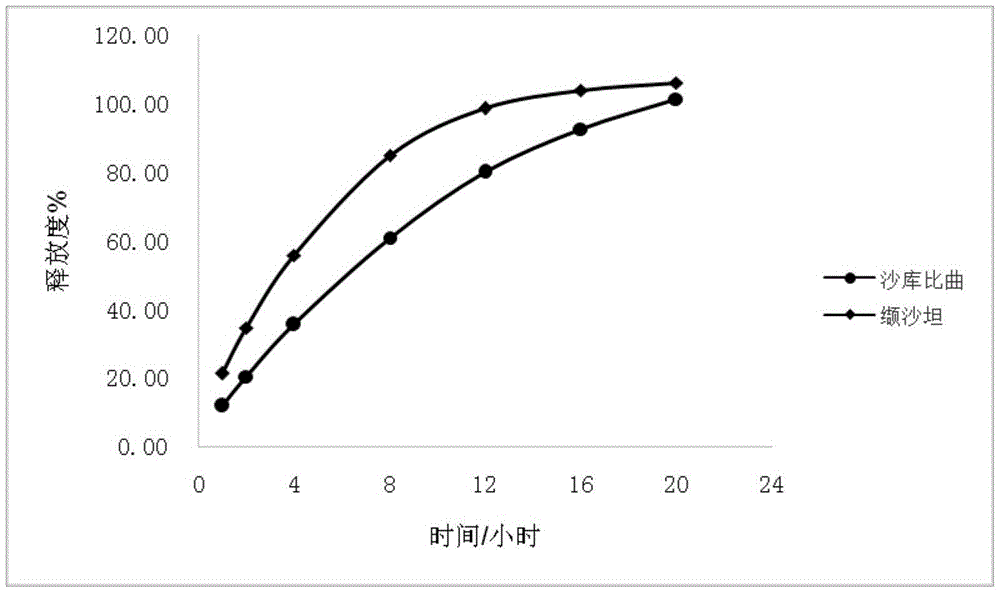
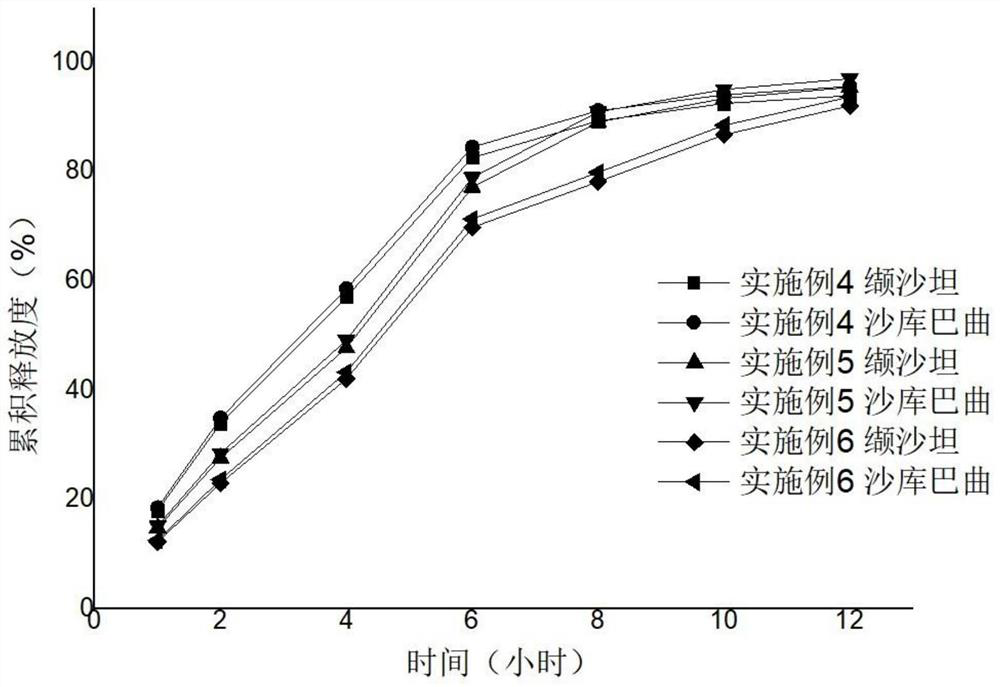
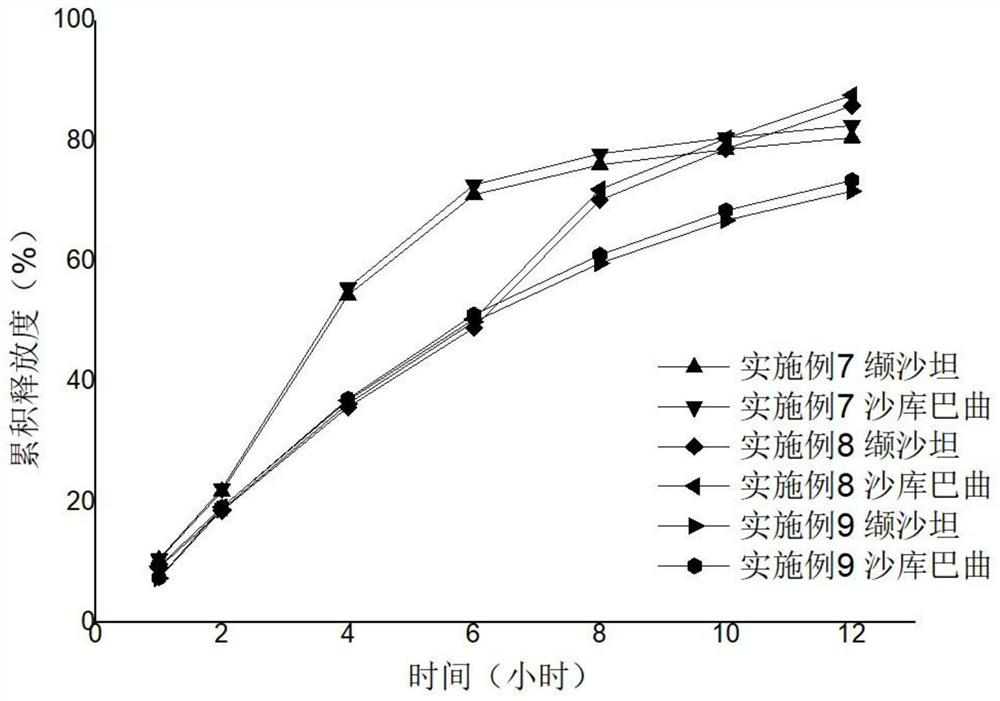
Sacubitril / valsartan sustained release agent and preparation method thereof
ActiveCN105935358ALong duration of actionExtended release characteristicsPharmaceutical non-active ingredientsCoatingsSustained Release TabletIn vitro test
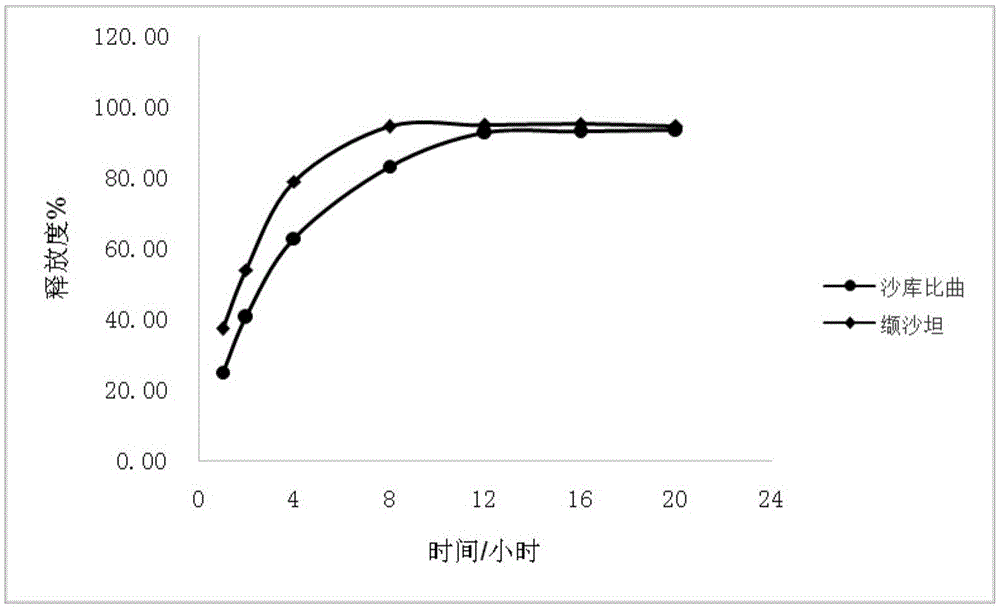
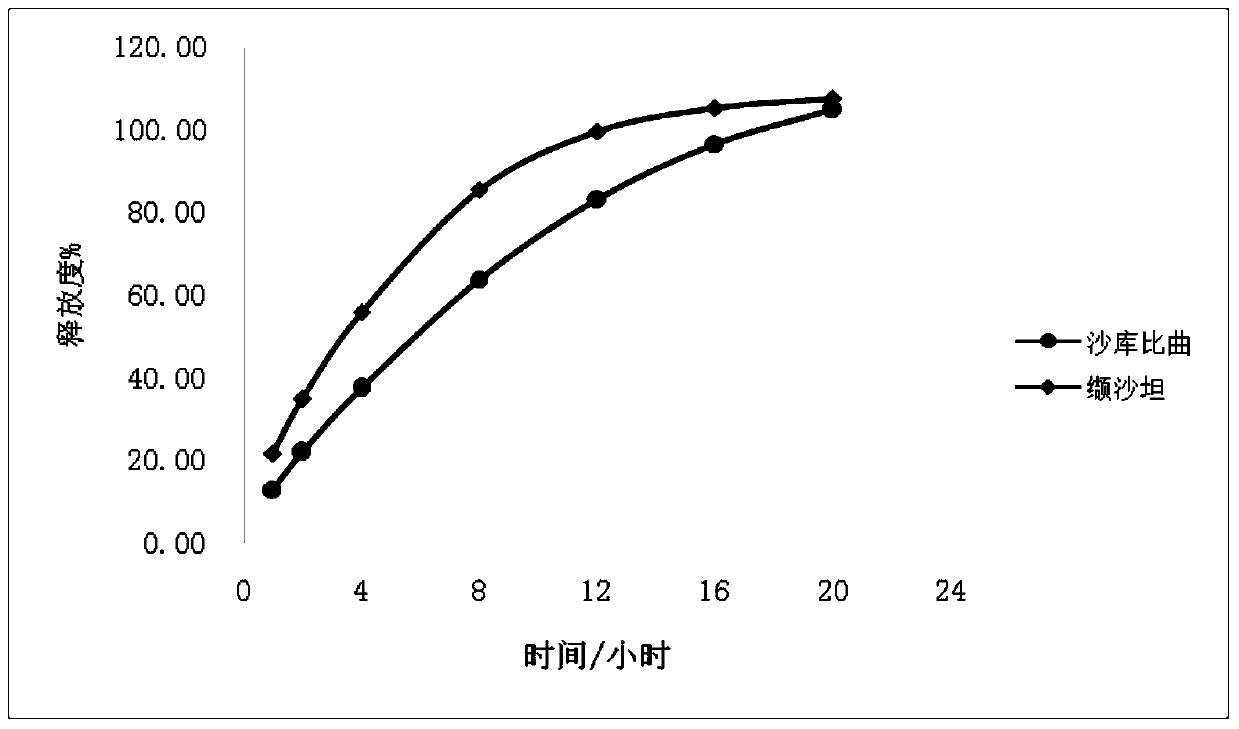
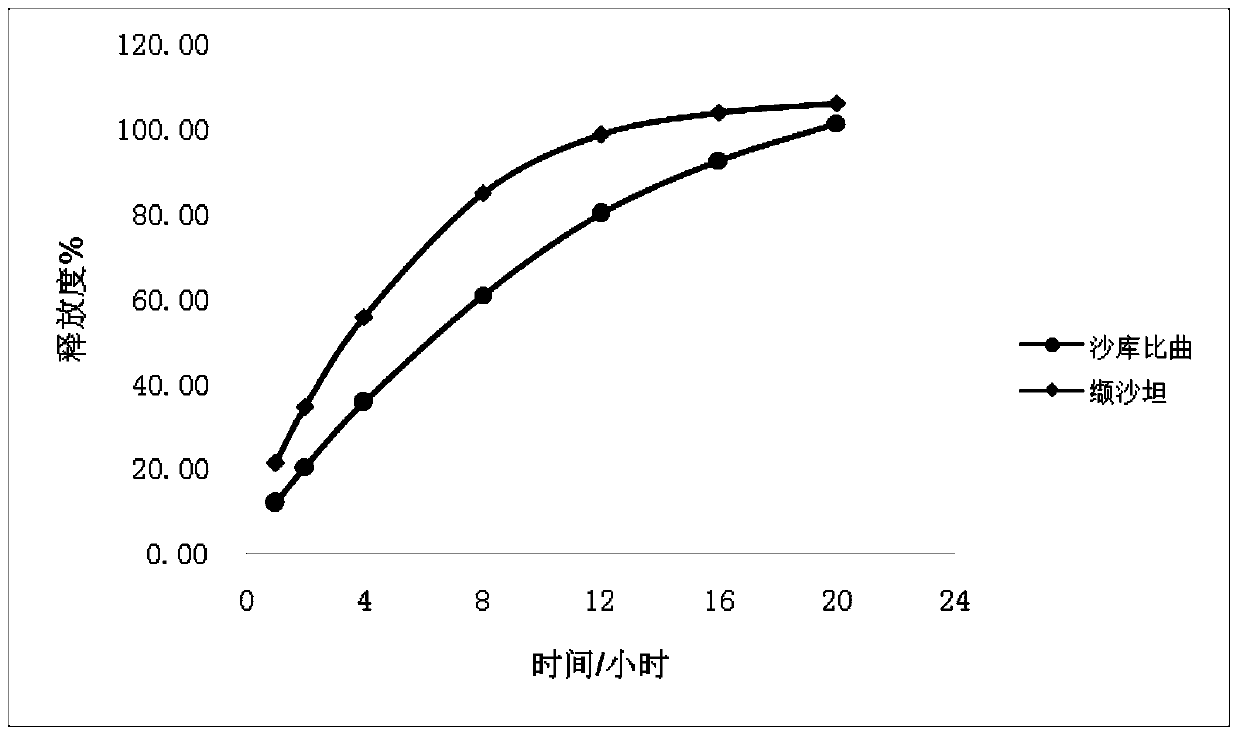
The invention provides a sacubitril / valsartan sustained release agent, including 10wt%-70wt% of sacubitril / valsartan, 10wt%-50wt% of hydrophilic gel framework material, 0-80 wt% of a diluent and 0.1wt%-10wt% of a lubricant, wherein the total content of the above components is 100%. In the study of in vitro releasing rate of intestinal juice, the sacubitril / valsartan sustained release tablet reaches release of sacubitril about 80% to 12 h, release of valsartan about 80% to 8, achieves slow release of the two active ingredients, and shows slow release characteristics in vitro test.
Owner:LIANGJIANG MEDICINE CO LTD
Preparation method for Sacubitril intermediate
InactiveCN105566194AHigh purityHigh yieldOrganic chemistry methodsPhenylmagnesium bromideMethyl methanesulfonate
The invention relates to a preparation method for a Sacubitril intermediate. The preparation method comprises the following steps that (3R,5S)-5-(hydroxymethyl)-3-methyl-2-pyrrolidone is esterified with toluene sulfochloride or methanesulfonyl chloride to obtain (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone; (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone is coupled with 4-diphenylmagnesium bromide or 4-diphenylmagnesium chloride to obtain (3R,5S)-3-methyl-5-(1,1'-diphenyl-4-yl-methyl)-2-pyrrolidinone. According to the preparation method, the method is novel, the raw materials are easy to obtain, the technology is simple, and the purity and yield of the product are both very high.
Owner:张伯引
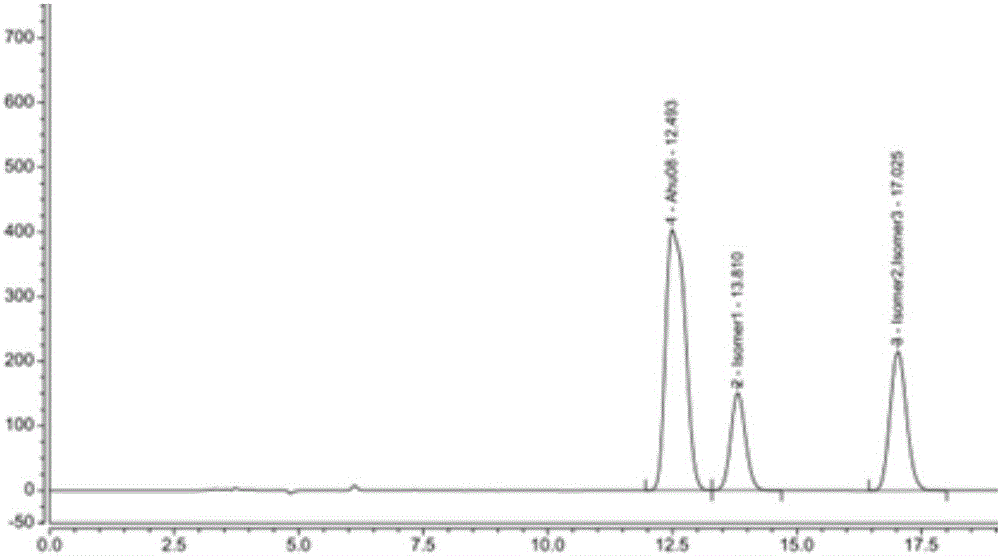
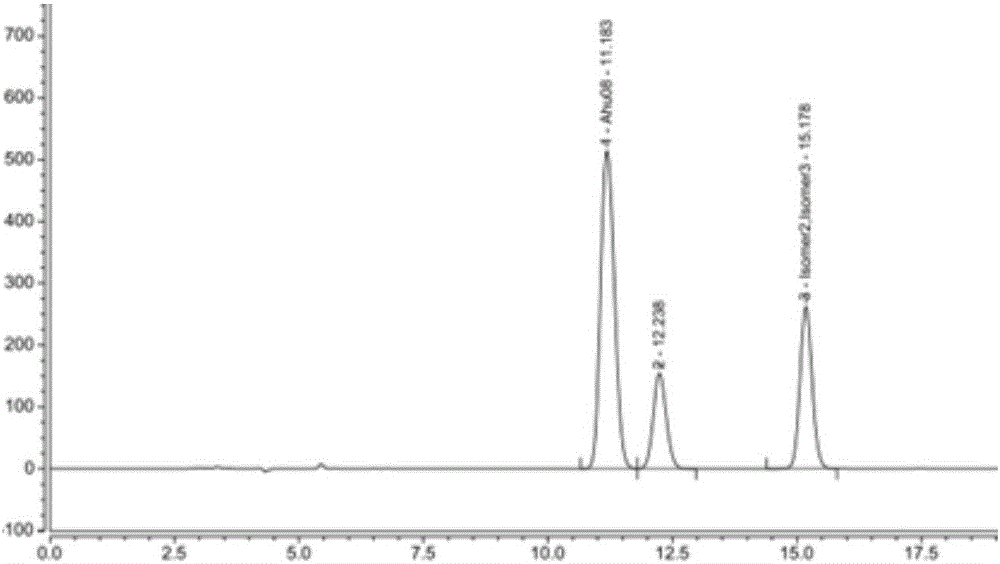
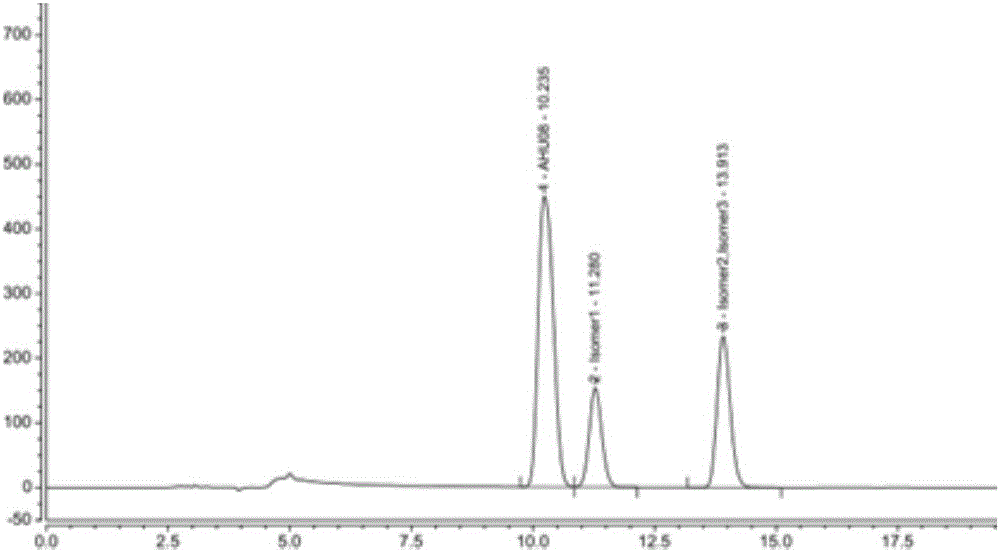
High performance liquid chromatography-based method for separating and detecting sacubitril intermediate and optical isomers thereof
InactiveCN106770855AEfficient separationPrecise content controlComponent separationStationary phaseSacubitril
The invention belongs to the technical field of pharmaceutical analysis, and relates to a high performance liquid chromatography-based method for separating and detecting a sacubitril intermediate and optical isomers thereof. The separation and detection method which adopts chiral column using silica gel coated with an amylose derivative as a stationary phase and adopts a non-polar mixed solvent as a mobile phase, realizes simple, rapid and accurate separation and detection of AHU08 and the optical isomers (impurities) thereof, and solves the separation and detection problem of raw materials and preparations containing the AHU08 and the optical isomers thereof in order to ensure the quality controllability of sacubitril and compositions or preparations containing the sacubitril.
Owner:江苏开元医药有限公司 +1
Sacubitril intermediate and preparation method thereof
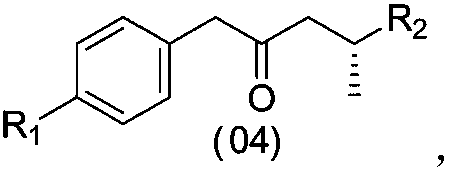
ActiveCN106977415AEconomical and environmentally friendlyRaw materials are easy to getGroup 4/14 element organic compoundsOrganic compound preparationEnvironmental resistanceSacubitril
The invention relates to a sacubitril intermediate and a preparation method thereof, the sacubitril intermediate is a compound shown as formula (04), and the sacubitril intermediate is prepared by deprotection reaction of a compound shown as formula (03). Further, the invention also provides the preparation method of the compound shown as the formula (04) as the intermediate. The method has the advantages of easy availability of raw materials, simple process, economy and environmental protection, and the like, and is very suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Method for preparing Sacubitril intermediate of anti-heart-failure medicine
InactiveCN105753741AHigh reaction yieldEasy to purifyCarbamic acid derivatives preparationOrganic compound preparationWittig reactionSacubitril
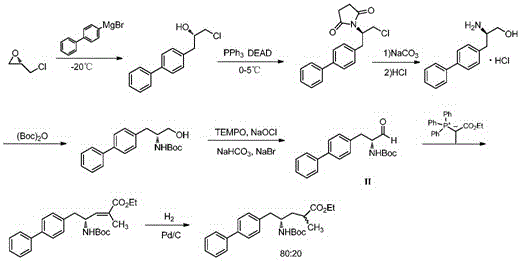
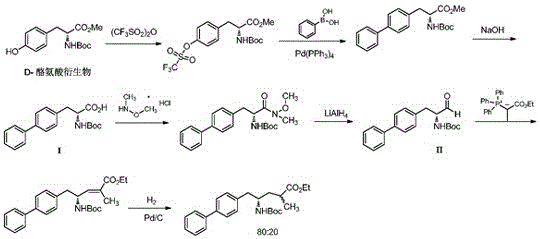
The invention discloses a method for preparing a Sacubitril intermediate of anti-heart-failure medicine as indicated in the formula (VII).The method comprises the following steps of taking D-phenylalanine which is low in price and easy to obtain as the raw material, and conducting an iodination reaction, an esterification reaction, a Boc protection reaction, a negishi coupling reaction, a DIBAL-H reduction reaction and a wittig reaction, so that the Sacubitril intermediate is obtained through preparation.The method for preparing the Sacubitril intermediate is mild in reaction condition and environmentally friendly, compared with existing preparation methods, the yield is higher, and the method is economical, effective and suitable for large-scale industrialized production.
Owner:CHANGZHOU PHARMA FACTORY
Preparing method for anti-heart-failure medicine LCZ696
ActiveCN105348209ASimple manufacturing processIncrease productivityOrganic compound preparationOrganic chemistry methodsOrganic solventAqueous sodium hydroxide
The invention discloses a preparing method for an anti-heart-failure medicine LCZ696. The preparing method comprises the following steps that in an organic solvent, sacubitril dicyclohexyl amine salt and diovan are reacted under the effect of a sodium hydroxide aqueous solution, and the anti-heart-failure medicine LCZ696 is obtained. The preparing method is simple in process, the procedures of ion exchange to calcium salt from sodium salt and hydrochloric acid dissociation in the existing production process are omitted, residues of calcium ions are avoided, and the production efficiency is effectively improved. The formula of sacubitril dicyclohexyl amine salt and the formula of diovan can be seen in the specification.
Owner:ZHEJIANG TIANYU PHARMA +1
Preparation method of sacubitril intermediate having low triphenylphosphine oxide content
InactiveCN106946742AReduce contentCarbamic acid derivatives preparationOrganic compound preparationSodium bicarbonateCarbamate
The invention relates to a preparation method of a sacubitril intermediate having low triphenylphosphine oxide content. The preparation method comprises that water, isopropyl acetate, sodium bromide, sodium bicarbonate and tetramethylpiperidine oxide into (R)-tert-butyl(1-([1, 1'-biphenyl]-4-yl)-3-hydroxypropane-2-yl)carbamate, adding a sodium hypochlorite solution into the mixture drop by drop for a reaction, after the reaction, carrying out layering, taking an organic layer, adding ethoxyformylethylidenetriphenyl phosphorane into the organic layer, after a reaction, concentrating the reaction product, removing isopropyl acetate, adding ethanol, water and lithium hydroxide into the mixture, carrying out heating until reflux, carrying out concentration until drying, adding water and activated carclazyte into the product, carrying out stirring at the room temperature, filtering the mixture, adding ethanol and acetic acid into the filtrate, carrying out heating until reflux, and carrying out cooling and stirring to precipitate solids which are the sacubitril intermediate finished products. The preparation method can reduce triphenylphosphine oxide content of the (R)-tert-butyl(1-([1, 1'-biphenyl]-4-yl)-3-hydroxypropane-2-yl)carbamate.
Owner:常州沃腾化工科技有限公司
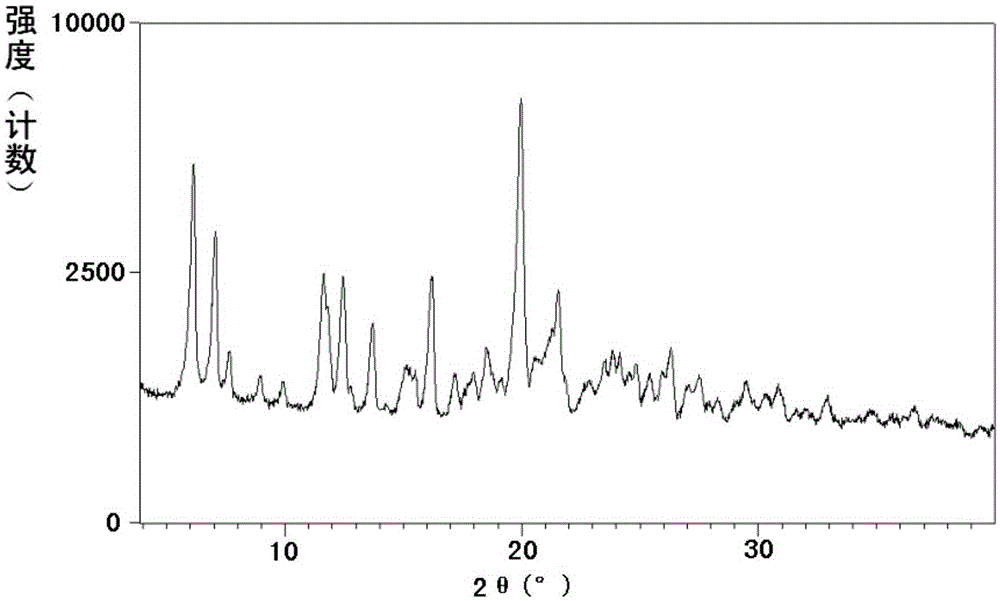
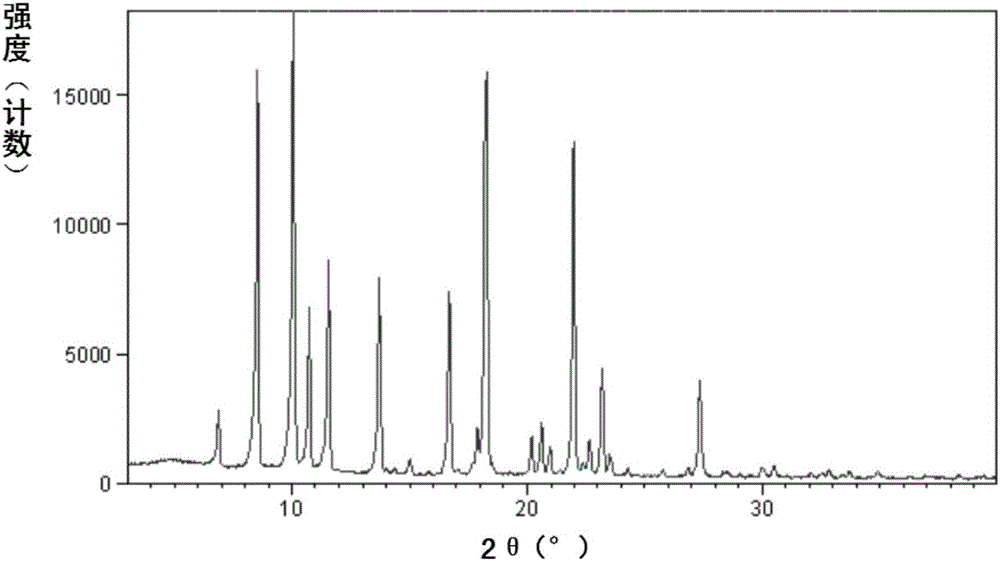
New crystal of sacubitril potassium salt, and preparing method and use thereof
InactiveCN106146333AEasy to prepareHigh purityOrganic active ingredientsOrganic compound preparationSacubitrilSingle crystal
The invention relates to a sacubitril potassium salt crystal form B. The crystal form has the advantages of small hygroscopicity, simple preparation method, easy control of the crystal form, and good stability, and is suitable for preparing various preparations; the invention also relates to the preparing method of the sacubitril potassium salt crystal form B, a pharmaceutical composition containing the sacubitril potassium salt crystal form B, and a use of the sacubitril potassium salt crystal form B in preparing drugs for prevention or treatment of chronic heart failure or hypertension.
Owner:SICHUAN HAISCO PHARMA CO LTD
Preparation method of R-diphenyl alaninol
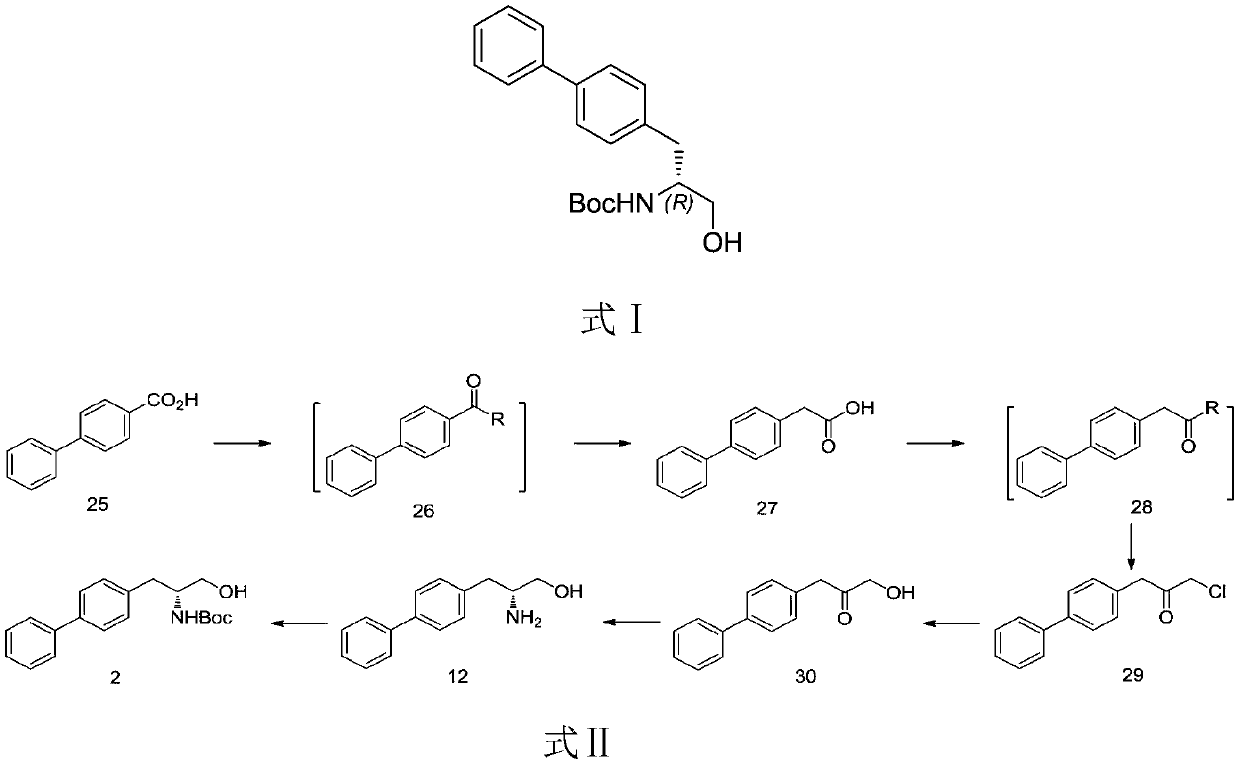
ActiveCN107540574AEasy to operateSafe, stable and reliable productionCarbamic acid derivatives preparationOrganic compound preparationSulfonyl chlorideTyrosine
The invention relates to a preparation method of a sacubitril intermediate, in particular to a preparation method of R-diphenyl alaninol. The preparation method comprises the following steps: performing a reaction on a D-tyrosine derivative serving as a raw material and different substituted sulfonyl chloride (or anhydride) to obtain a compound as shown in a formula III, and preparing the R-diphenyl alaninol by using the compound as shown in the formula III through two routes. The route I is that the compound as shown in the formula III is reduced into a compound as shown in a formula II and the compound as shown in a formula II is coupled to obtain the R-diphenyl alaninol; and the route II is that the compound as shown in the formula III is coupled to obtain a compound as shown in a formula V and reduction is performed to obtain the R-diphenyl alaninol. The compound R-diphenyl alaninol prepared by the method is a key intermediate of one component sacubitril (AHU-377) of a novel pressure-reducing medicine LCZ696 (Entresto). The method is simple to operate, short in route and suitable for industrialized production. (The formulas are as shown in the description.).
Owner:XILING LAB CO LTD
Preparation method of sacubitril-valsartan compound and/or eutectic key intermediate sacubitril calcium
ActiveCN108373423AHigh purityLow costCarbamic acid derivatives preparationOrganic compound preparationTert-Butyloxycarbonyl protecting groupSacubitril, Valsartan
The invention relates to a preparation method of a sacubitril-valsartan compound and / or eutectic key intermediate sacubitril calcium. The provided preparation method of sacubitril calcium adopts (R,E)-5-([1,1'-biphenyl]-4-yl)-4-((t-butyloxycarboryl)amino)-2-methyl-pentyl-2-olfine acid, and hydrogenation, esterification and acylation reactions are carried out, so that sacubitril calcium is obtained. The provided preparation method is simple to operate, low in cost and applicable to industrial production.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Sacubitril intermediate and preparation method and application thereof
ActiveCN109206419AOrganic active ingredientsOrganic compound preparationChemical structureSacubitril
The invention discloses a sacubitril intermediate and a preparation method and application thereof. The sacubitril intermediate has the chemical structure as shown in the formula III and / or formula IV, wherein R is phenyl, benzyl and isopropyl. The application of the intermediate for synthesis of sacubitril has advantages as follows: the preparation process is simple; the reaction condition is mild; and the environment is protected. The invention has great significance and use value for low-cost, large-sale and high-efficiency preparation of high-purity sacubitril.
Owner:SHANGHAI DESANO CHEM PHARMA +3
Sacubitril and preparation method of midbody of sacubitril
ActiveCN106699604AIncrease productivityAchieve asymmetric methylationCarbamic acid derivatives preparationOrganic compound preparationSacubitrilAcylation
The invention discloses sacubitril and a preparation method of midbody of the sacubitril, and relates to the field of pharmaceutical synthesis. According to preparation method of the sacubitril midbody, a compound I of a first chiral center is taken as a starting material, and is subjected to acylation reaction prothetic group adding, asymmetric methylation reaction and hydrolysis prothetic group removal, so that the sacubitril midbody is obtained. Asymmetric methylation in the alpha-position of the carbanyl group is effectively and selectively is realized through the adding of chiral prothetic group and the cooperative control of the chiral prothetic group and the first chiral center, so as to construct a second chiral center. The preparation method of the sacubitril midbody has the advantages that the raw materials are easy to get, the method is simple to operate, separation and purification are convenient, the yield and diastereoselectivity are high, and the dose is convenient to magnify to realize industrial production. The obtained sacubitril midbody is high in chiral purity, a tedious step for separating a diastereoisomer is canceled, and the production efficiency is improved.
Owner:SICHUAN TONGSHENG BIOTECH
Purification method for midbody of sacubitril
InactiveCN106905192AReduce contentCarbamic acid derivatives preparationOrganic compound preparationAcetic acidPurification methods
The invention relates to a purification method for a midbody of sacubitril. The method is characterized by comprising adding (R)-tertiary butyl (1-([1.1'-biphenyl]-4-yl)-3-hydroxy-2-yl)carbamate including S-type isomer impurities and active carbon into solvent, wherein the solvent is mixed solution of n-heptane and ethyl acetate, then heating until flowing back for 8-15min, filtering when being hot to remove the active carbon, flushing the active carbon with the ethyl acetate, combining all scrubbing solution with filtrate, further heating the combined solution until flowing back, cooling to devitrifying, and performing suction filtration, flushing a filter cake with the n-heptane and drying; therefore, the purified finished midbody of sacubitril, the (R)-tertiary butyl (1-([1.1'-biphenyl]-4-yl)-3-hydroxy-2-yl)carbamate is acquired. The purification method has the advantages that content of the S-type isomer impurities in the midbody, the finished (R)-tertiary butyl (1-([1.1'-biphenyl]-4-yl)-3-hydroxy-2-yl)carbamate is less than 0.05wt%.
Owner:常州沃腾化工科技有限公司
Synthetic method for Sacubitril
InactiveCN106380421AHigh reaction yieldLow priceOrganic compound preparationOrganic chemistry methodsSynthonSacubitril
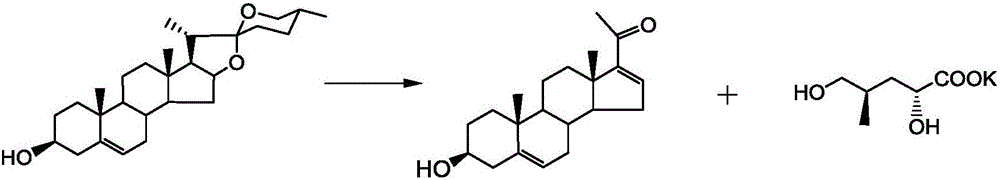
The invention provides a preparation method for Sacubitril as one component of a novel hypotensive drug Entresto. According to the method, chiral synthons, obtained in the oxydative degradation wastes of steroid sapogenin, are adopted as raw materials. The raw materials are subjected to five steps of reactions, and then the high-yield synthesis of Sacubitril is realized. The raw materials of the method are simple, easily available and low in cost. Meanwhile, the synthetic method is characterized by being mild in condition, simple in operation, high in yield and few in by-product. The method can be applied to the industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Preparation method of Sacubitril intermediate
ActiveCN110183357AEasy to synthesizeEfficient synthesisCarbamic acid derivatives preparationPreparation from carboxylic acid halideCarbamateSacubitril
The invention discloses a preparation method of an intermediate compound (R)-tert-butyl (1-((1,1'-diphenyl)-4-yl)-3-hydroxypropane-2-yl) carbamate. The preparation method is represented as the formulashown in the description. According to the preparation method, the steps are simple and short, the reaction yield is increased, reaction conditions are mild, most of the intermediates obtained in thereaction process are not required to be purified and can be subjected to the next reaction directly, large-batch synthesis is facilitated, chiral amine with stereospecificity is efficiently synthesized with aminotransferase, and the preparation method is more suitable for industrial production.
Owner:甘肃皓天医药科技有限责任公司
Preparation method and application of sacubitril intermediate
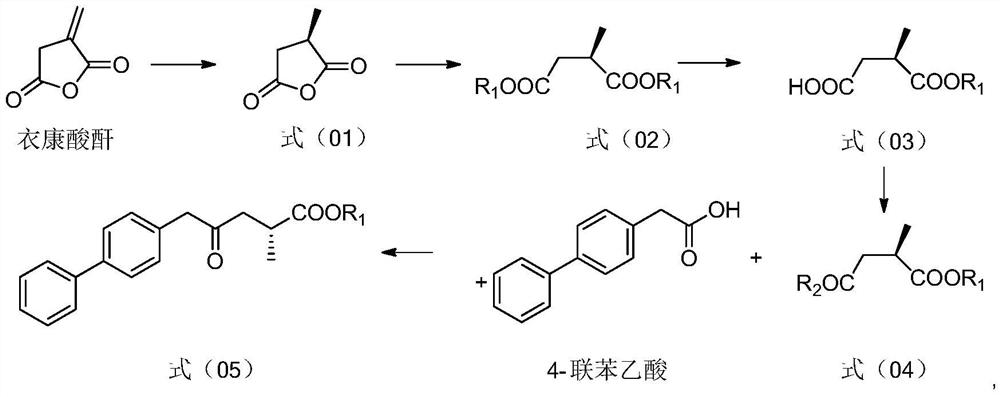
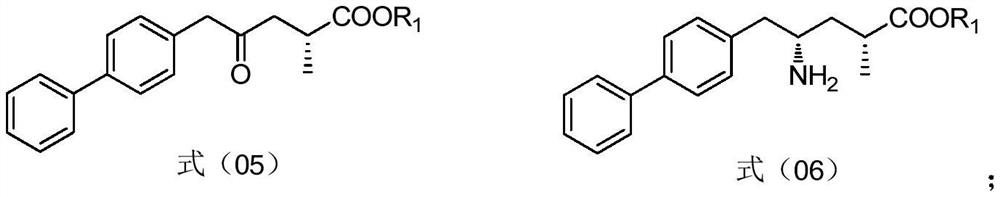
ActiveCN113121342AEase of industrial productionRaw materials are cheap and easy to getOrganic compound preparationCarboxylic acid amides preparationCarboxyl radicalBiochemical engineering
The invention discloses a preparation method and application of a sacubitril intermediate. The sacubitril intermediate disclosed by the invention is obtained by taking itaconic anhydride as a raw material, performing chiral reduction, esterification, selective hydrolysis and carboxyl activation, and finally conducting coupling with 4-biphenylacetic acid. The invention also provides a method for preparing sacubitril by using the sacubitril intermediate. The preparation method provided by the invention has the advantages of easily available raw materials, simple process, economy, environmental protection and the like, and is more suitable for industrial production compared with other routes.
Owner:浙江来益生物技术有限公司
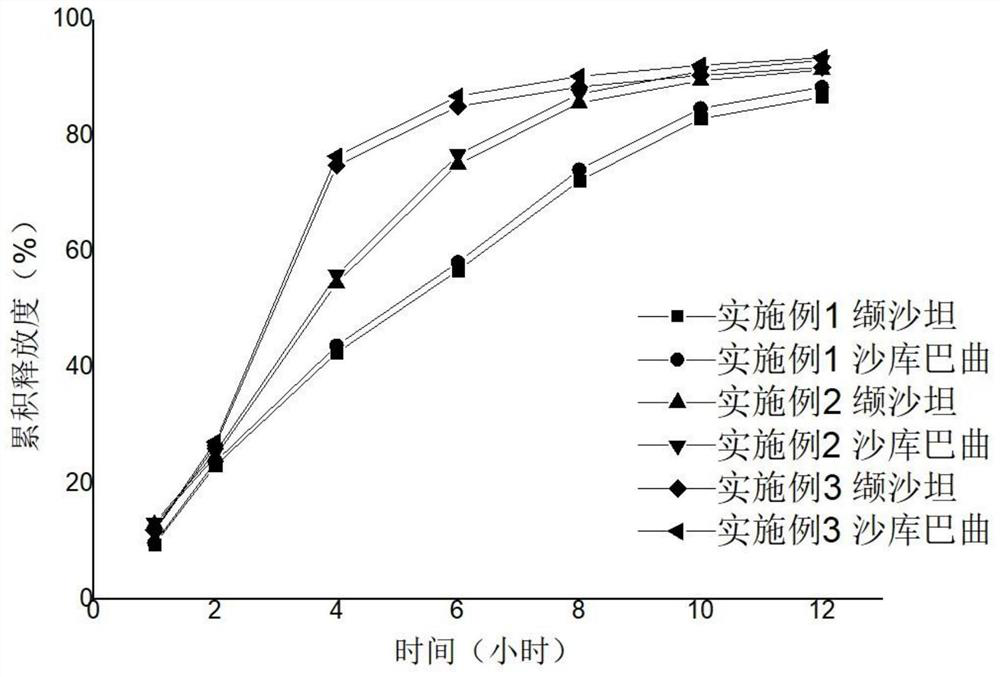
Sacubitril and valsartan sodium single-layer osmotic pump controlled release tablet and preparation method thereof
ActiveCN113456607AFacilitated releaseExhibit controlled release propertiesPharmaceutical non-active ingredientsCoatingsDrug releaseBlood drug concentration
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a sacubitril and valsartan sodium single-layer osmotic pump controlled release tablet and a preparation method thereof. The single-layer osmotic pump controlled release tablet comprises a tablet core, a semi-permeable coating membrane and small drug release holes; the tablet core comprises the following components in percentage by weight: 53-92% of sacubitril valsartan sodium, 2-33% of suspending aid, 0-33% of penetration enhancer and 0.5-3% of lubricant; the semi-permeable coating membrane comprises 60-99% of a semi-permeable membrane forming material and 1-40% of a pore-foaming agent, and the weight of the coating is 3-10% of the weight of the tablet core; and the aperture of the small drug release holes is 0.6mm-1.5 mm. The sacubitril and valsartan sodium single-layer osmotic pump controlled release tablet is orally taken once a day, has stable blood concentration, and is not easily influenced by gastrointestinal tract environment.
Owner:南京康川济医药科技有限公司
Channelization synthesis method for key intermediate of sacubitril
ActiveCN109336804AReduce backmixingNo backmixingOrganic chemistrySynthesis methodsReaction temperature
The invention discloses a channelization synthesis method for a key intermediate of sacubitril. The method comprises the following steps: (S)-1-([1,1'-biphenyl]-4-yl)-3-chloropropan-2-ol, succinimide,triphenylphosphine, ethyl azodicarboxylate and an organic solvent are mixed, the mixed solution is fed continuously into a channelization reactor for reaction, a feed liquid obtained after the reaction is fed into a quenching kettle containing an quenching liquid, a quenching reaction is performed with the quenching liquid, (R)-1-(1-([1,1'-biphenyl]-4-yl)-3-chloropropan-2-yl)pyrrolidin-2,5-dioneis prepared, and the key intermediate of sacubitril is obtained. According to the reaction process, reaction temperature, time and material proportion can be controlled precisely; the reactor has theadvantages of large specific surface area and high mass and heat transfer efficiency, precise proportion moment of the materials can be determined, heat transfer efficiency is high, and safety of a production process is guaranteed.
Owner:ZHEJIANG UNIV OF TECH
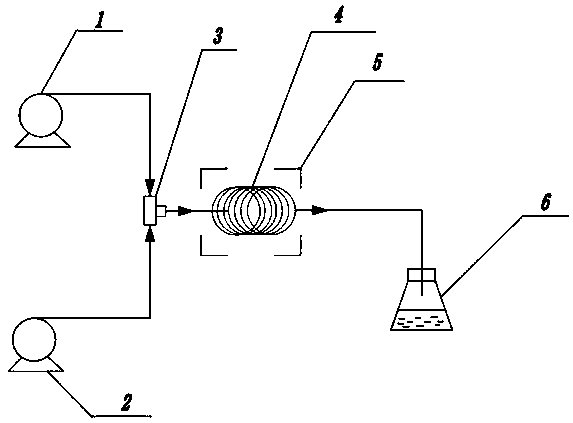
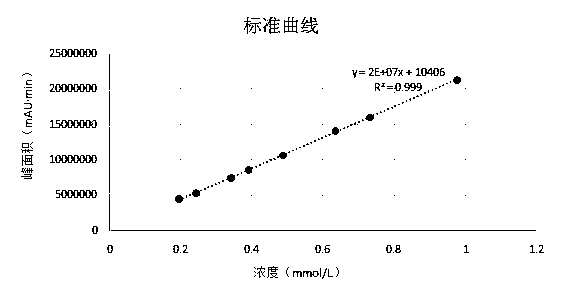
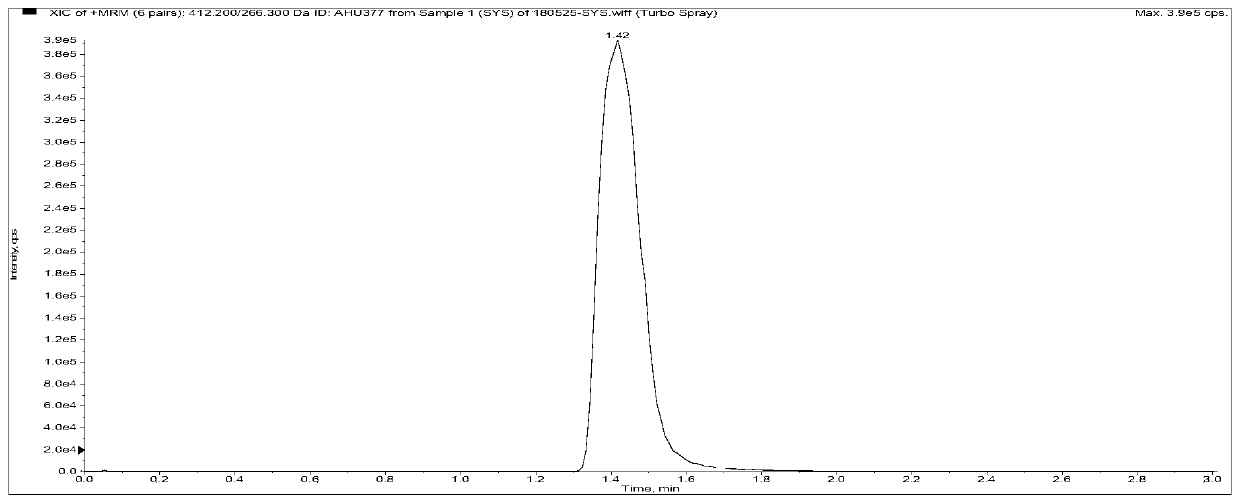
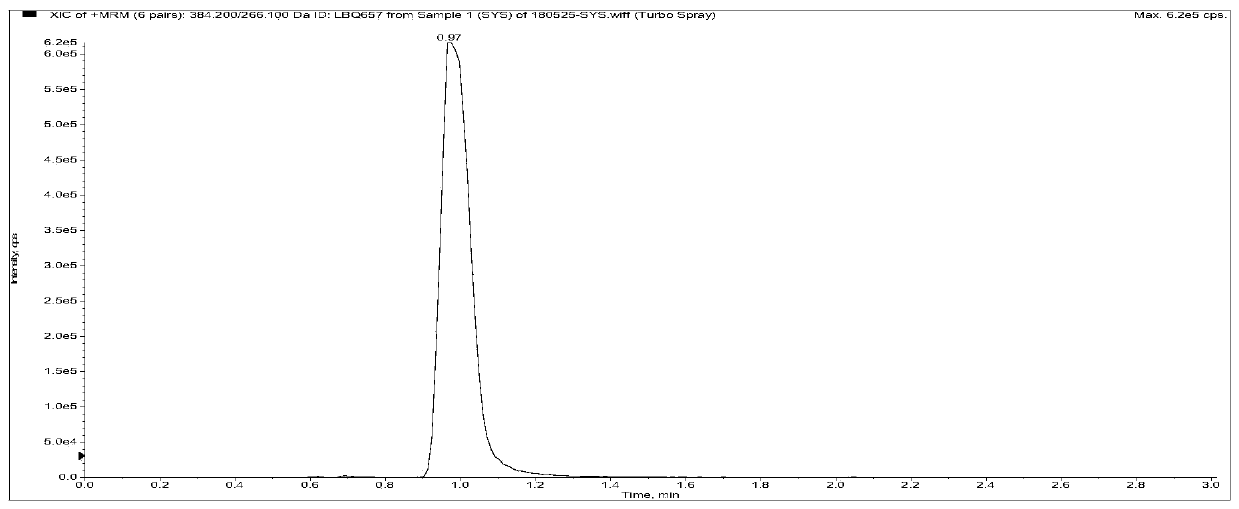
Method for measuring concentrations of sacubitril, desethyl sacubitril and valsartan in human plasma
The invention relates to a method for detecting the concentrations of drugs in human plasma. According to the method, a sample is pretreated by using a methanol protein precipitation method and then further diluted; and detection is carried out by using an ultra-high performance liquid chromatography-tandem mass spectrometry. The method provided by the invention is capable of detecting three substances such as sacubitril, desethyl sacubitril and valsartan at the same time, so that less damage is caused to chromatographic columns and instruments.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Novel process for early sacubitril intermediates
ActiveCN109415308AHigh yieldHigh stereoselectivityCarbamic acid derivatives preparationOrganic compound preparationAklanonic acidCombinatorial chemistry
Owner:NOVARTIS AG
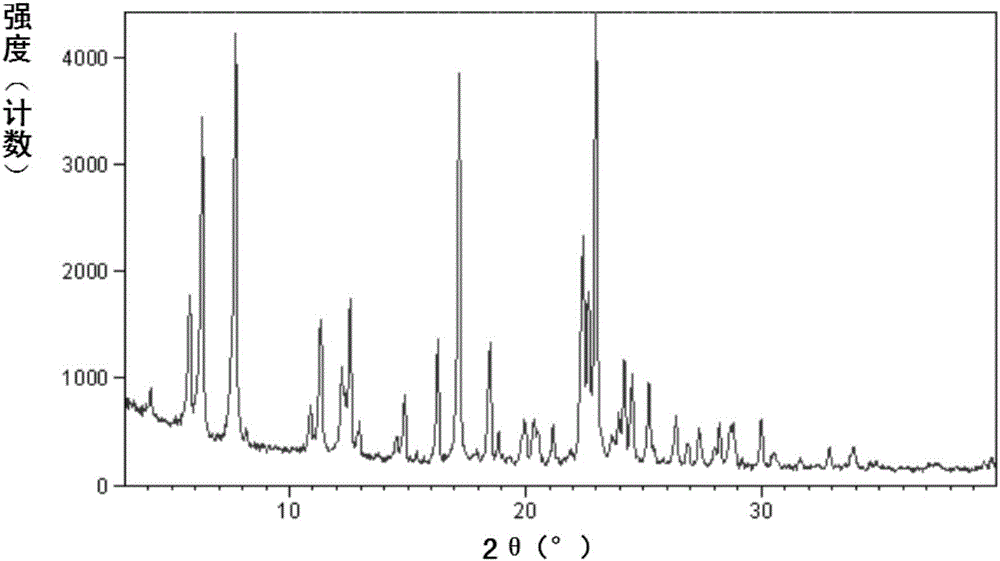
Novel crystal form of sacubitril tromethamine salt, and preparation method and applications thereof
InactiveCN106854164AHigh purityLow hygroscopicityOrganic compound preparationOrganic chemistry methodsSolubilitySacubitril
The present invention relates to a new crystal form of sacubi trimethamine salt, which has high purity, low hygroscopicity and good solubility, and is suitable for preparing various preparations; the present invention also relates to the preparation of the new crystal form The method, the pharmaceutical composition containing the new crystal form, and the use of the new crystal form in the preparation of drugs for preventing or treating chronic heart failure or hypertension.
Owner:SICHUAN HAISCO PHARMA CO LTD
Sacubitril-valsartan dosage regimen for treating heart failure
ActiveUS20180125820A1Safely reachSafely reachedCardiovascular disorderHeterocyclic compound active ingredientsHeart failureRegimen
The present invention relates to sacubitril-valsartan dosage regimens for the treatment of heart failure in a patient.
Owner:NOVARTIS PHARM CORP
A sacubitril valsartan sustained-release agent and preparation method thereof
ActiveCN105935358BLong duration of actionExtended release characteristicsPharmaceutical non-active ingredientsCoatingsSustained Release TabletAdditive ingredient
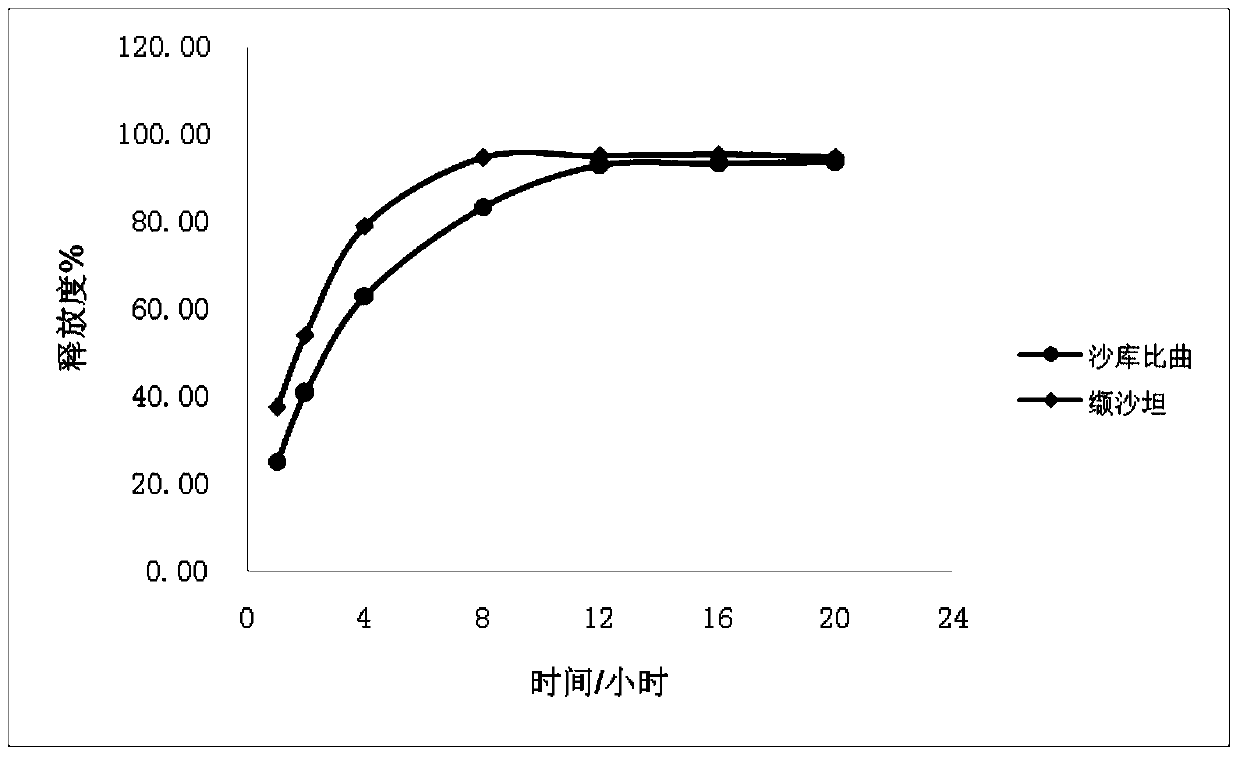
The invention provides a sacubitril / valsartan sustained release agent, including 10wt%-70wt% of sacubitril / valsartan, 10wt%-50wt% of hydrophilic gel framework material, 0-80 wt% of a diluent and 0.1wt%-10wt% of a lubricant, wherein the total content of the above components is 100%. In the study of in vitro releasing rate of intestinal juice, the sacubitril / valsartan sustained release tablet reaches release of sacubitril about 80% to 12 h, release of valsartan about 80% to 8, achieves slow release of the two active ingredients, and shows slow release characteristics in vitro test.
Owner:LIANGJIANG MEDICINE CO LTD
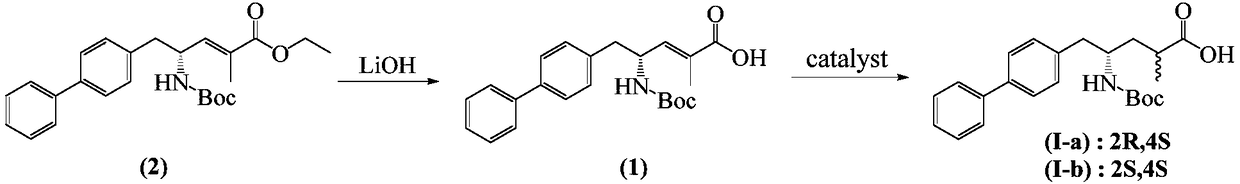
Method for separation and purification of LCZ696 intermediate diastereoisomer
InactiveCN109400504AAchieve recyclingHigh solubility at low temperatureCarbamic acid derivatives preparationOrganic compound preparationOrganic solventSacubitril
A method for separation and purification of LCZ696 intermediate diastereoisomer includes the following steps: (1) placing the mixture of the diastereoisomer (I-a) and (I-b) in an organic solvent untilthe mixture is completely dissolved, cooling to 0-10 DEG C, recrystallizing until the crystal is stable, filtering, taking a filter cake, and drying to obtain a product mixture, and (2) taking the filtrate, removing the solvent under reduced pressure, and drying the solute to obtain an impurity mixture. The method for separation and purification can efficiently and conveniently separate and purify the diastereomers (I-a) and(I-b) and the purity of the resulting product mixture (I-a) is as high as 95 percent, which can be directly used for preparing a high-purity Sacubitril intermediate to finally synthesize LCZ696 so as to ensure the product quality.
Owner:CHONGQING SANSHENG IND CO LTD
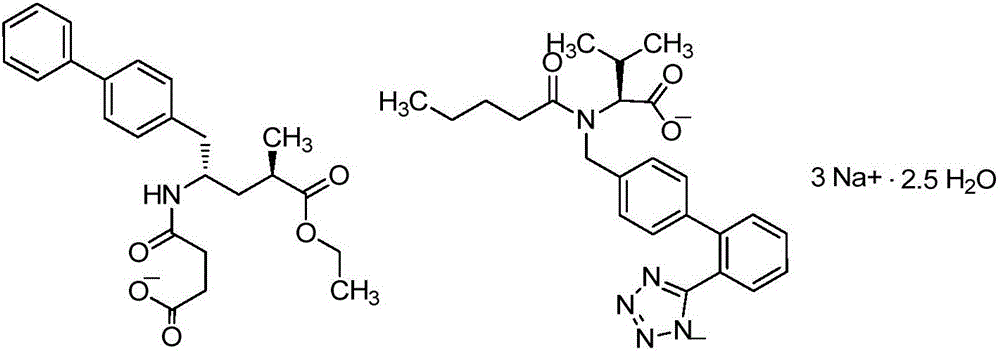
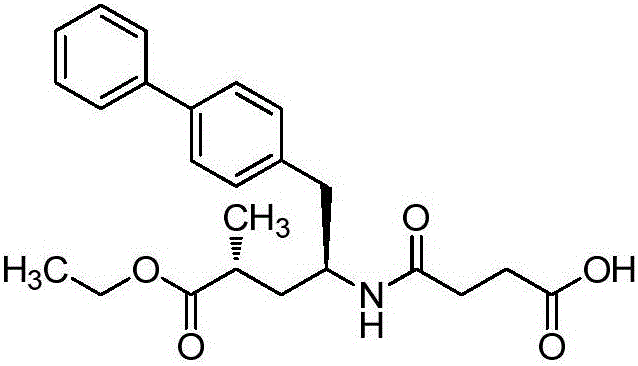
Novel synthesis method of key component Sacubitril of novel anti-heart-failure drug
InactiveCN106496055AReduce usageMethod route shortCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsSacubitril
The invention discloses a novel synthesis method of key component Sacubitril of a novel anti-heart-failure drug. The method includes transforming a starting material compound 1 to a compound 2; coupling the compound 2 with (1'1)-4-biphenylmagnesium bromide to obtain a compound 3; subjecting the compound 3 to hydrolysis reaction by loading a Boc protecting group, and preparing a compound 5 by means of one-pot reaction; subjecting the compound 5 to Boc group removal and esterification reaction to obtain a compound 6, and reacting the compound 6 with succinic anhydride without separation to obtain a compound 7, namely Sacubitril, wherein the route is as following.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com