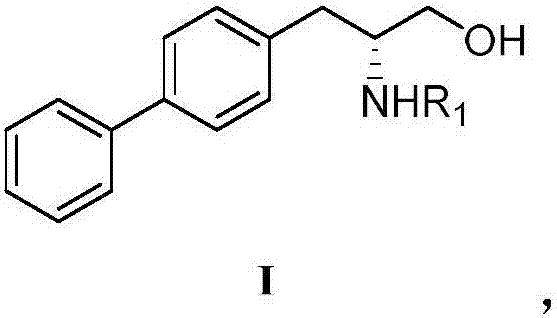
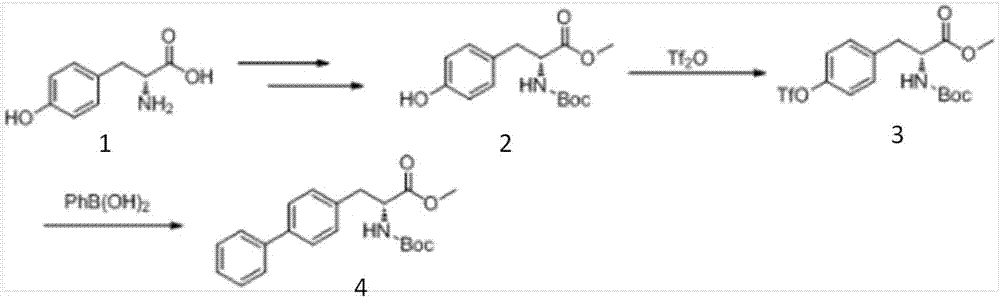
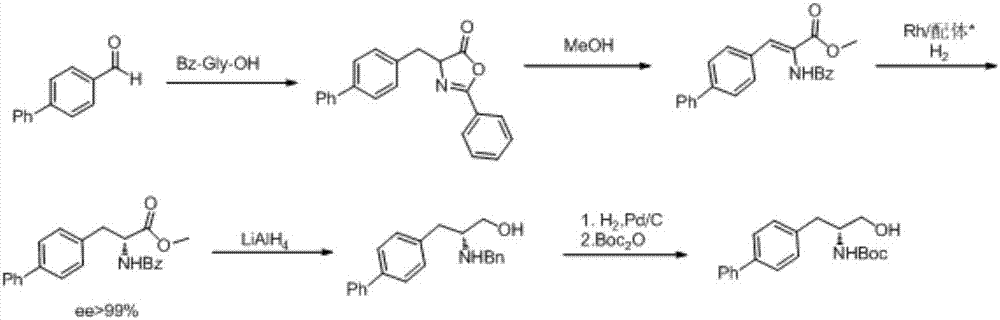
Preparation method of R-diphenyl alaninol
A coupling reaction, amino protecting group technology, applied in the field of medicine and chemical industry, can solve the problems of high cost, long route and high operation requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Embodiment 1 synthetic compound III
[0103] Weigh 20 g of D-Boc tyrosine methyl ester into the reaction flask, add 60 mL of anhydrous tetrahydrofuran as a solvent, add 21.6 mL of triethylamine under ice cooling, then slowly add 12.7 g of benzenesulfonyl chloride dropwise into the reaction flask, and immediately A large amount of white solid formed. After the dropwise addition, the ice bath was removed, and the reaction was carried out at room temperature for 5 hours. The solvent was evaporated to dryness, 80 mL of ethyl acetate was added thereto, and the organic phase was washed successively with saturated aqueous sodium carbonate, saturated aqueous ammonium chloride, and saturated brine. After drying and evaporating to dryness, 29.3 g of yellow oil compound III (4-benzenesulfonyl-D-Boc tyrosine methyl ester) was obtained, with a yield of 99.4%.
Embodiment 2
[0104] Embodiment 2 is synthesized compound Ⅱ by compound Ⅲ
[0105] Weigh 10 g of compound III (4-benzenesulfonyl-D-Boc tyrosine methyl ester) in the reaction flask, add 50 mL of methyl tert-butyl ether to it to dissolve, slowly add 3.49 g of lithium aluminum tetrahydrogen in an ice bath, and add After completion, remove the ice bath and react at room temperature for 1 h. After slow dropwise addition of ethanol to quench, filter. After the filtrate was spin-dried, 50 mL of ethyl acetate was added, and the organic phase was washed successively with saturated aqueous ammonium chloride solution, water, and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure, and column chromatography (PE:EA=5 : 1) 8.5 g of yellow oil compound II (4-benzenesulfonyl-D-Boc phenylalaninol) was obtained, with a yield of 90.8%.
Embodiment 3
[0106] Embodiment 3 is synthesized compound I by compound II
[0107] Add compound II (4-benzenesulfonyl-D-Boc phenylalaninol) 50mg, 0.123mmol) to the reaction flask, phenylboronic acid (60mg, 0.491mmol), potassium carbonate (68mg, 0.491mmol) and [(IMes)Pd (Py)Cl 2 ] (5mol%), add 1 mL of solvent morpholine, and react at 120° C. for 24 h under an argon atmosphere to obtain R biphenylalaninol. Yield: 62.5%, e.e. value>99%, [α]D 25 =+21.9 (c=0.01g / mL, CHCl 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com