Sacubitril intermediate and preparation method thereof
The technology of a compound and an alkyl group, which is applied in the field of Sacubitril intermediates and their preparation, can solve the problems such as difficulty in obtaining raw materials and complicated reactions, and achieve the effects of easy availability of raw materials and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
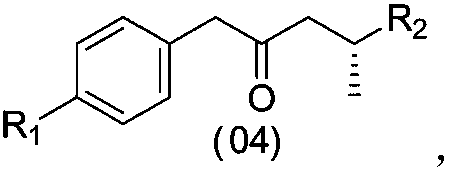
[0079] b) The compound represented by formula (03) is subjected to a deprotection reaction to prepare the compound represented by formula (04). In some embodiments, the preparation method of the compound represented by the formula (04) of the present invention comprises the following steps,
[0080]
[0081] where the R 1 and R 2 can be a group as previously described,
[0082] a) using the compound represented by formula (00) as the starting material, and preparing the compound represented by formula (01) through condensation reaction;
[0083]
[0084] in,
[0085] The R 1 and R 5 may be a group as previously described;
[0086] b) a compound represented by formula (01) and a compound represented by formula (02) undergo a substitution reaction to introduce a chiral methyl group to prepare a compound represented by formula (03),
[0087]
[0088] in,
[0089] The R 1 , R 2 , R 5 and R 6 may be a group as previously described;
[0090] c) The compound rep...
specific Embodiment approach
[0157] In order to enable those skilled in the art to better understand the technical solutions of the present invention, the present invention provides some examples of preparation examples, and some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0158] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0159] In the present invention, mmol means millimole, mol / L and N means mole / liter, h means hour, g means gram, ml means milliliter, L means liter, °C means degree Celsius, ESI-MS means electrospray ionization mass spectrometry, TLC means TLC.
Embodiment 1-a
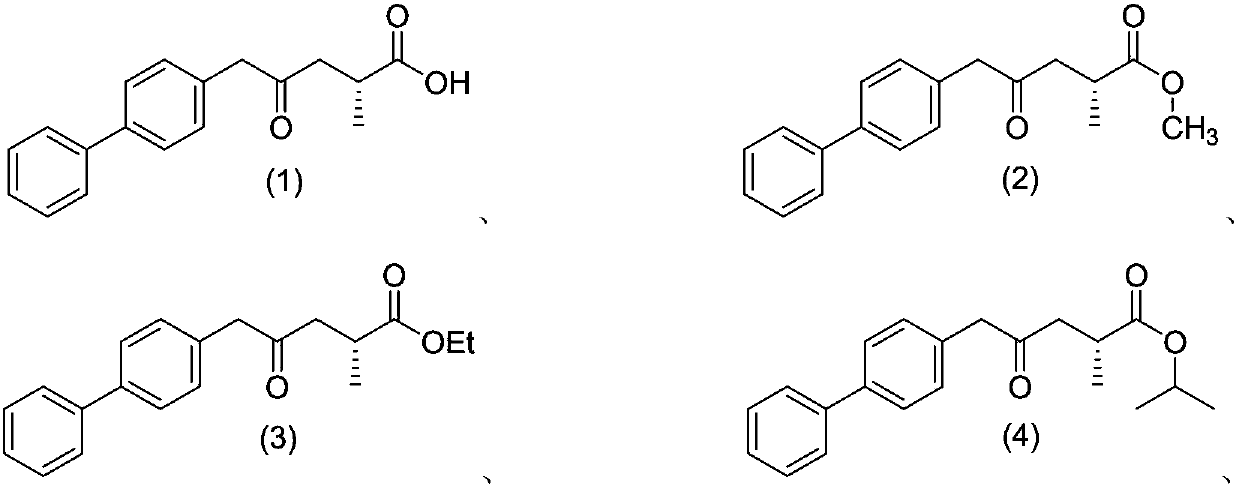
[0161] Example 1-a: Preparation of ethyl 4-([1,1'-biphenyl]-4-yl)-3-oxobutanoate
[0162]
[0163] Add 200mL of ethyl acetate and 28.64g of N,N'-carbonyldiimidazole to a 500mL three-necked reaction flask A at room temperature, and add 25.00g of 4-biphenylacetic acid into reaction flask A in batches to control the gas generation rate. After the addition, the temperature of the reaction solution was raised to 45°C for 3 hours, then cooled to 20-30°C, reduced and vacuumed for 1 hour, and set aside. Add 280mL ethyl acetate, 28.06g EtO to 1L reaction flask B at room temperature 2 CCH 2 COOK and 15.70g magnesium chloride (MgCl 2 ), the temperature was controlled at 20-30°C, and 19.07 g of triethylamine (TEA) was slowly added dropwise, and stirring was continued for 1 h after the dropwise addition was completed. Add the materials in the reaction bottle A to the reaction bottle B at room temperature, raise the temperature to 45° C., and keep the reaction for 16 hours. Cool the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com