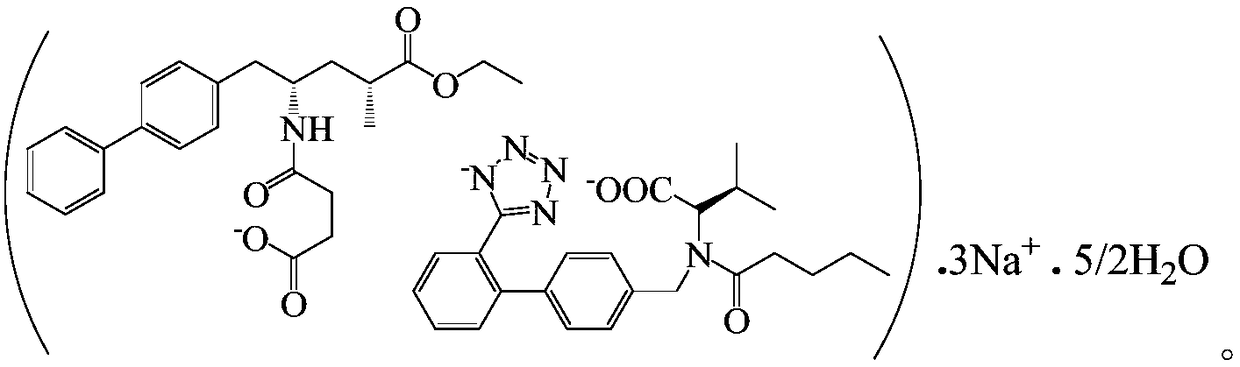
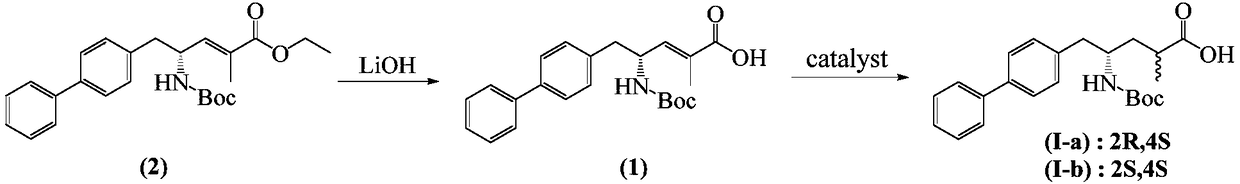
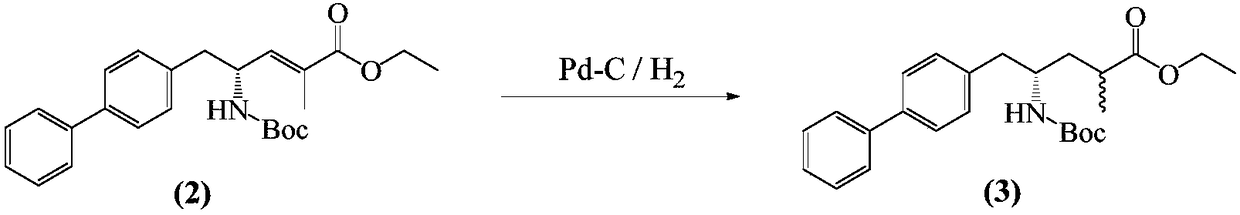Method for separation and purification of LCZ696 intermediate diastereoisomer
A technology for separation and purification of diastereomers, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., and can solve problems such as excessive product impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of (R, E)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpent-2-enoic acid (1), reaction formula as follows:
[0031]
[0032] According to Example 1 of patent CN101516831, ethyl (R,E)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpent-2-enoate (2) Hydrolysis with lithium hydroxide in ethanol gave white solid (R,E)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentane -2-enoic acid (1).
Embodiment 2
[0033] Embodiment 2 prepares diastereomer formula (I-a) and formula (I-b) mixture, and reaction formula is as follows:
[0034]
[0035] Add (R,E)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpent-2-enoic acid 10g into 150ml ethanol, and then add 10 % palladium carbon 1g, hydrogenation at 40-50°C for 6 hours at a pressure of 0.5 MPa. The catalyst was filtered off, and the solvent was removed from the filtrate under reduced pressure to obtain a mixture of diastereoisomers of formula (I-a) and formula (I-b). As determined by HPLC, (I-a):(I-b) was about 80:20.
Embodiment 3
[0036] Example 3 Preparation of (2R, 4S)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentanoic acid (formula I-a)
[0037] Add 5 g of the mixture of formula (I-a) and formula (I-b) obtained in Example 2 into 10 ml of tetrahydrofuran, heat to reflux to dissolve completely, cool to below 40 ° C, add 100 ml of n-heptane, continue cooling to below 10 ° C, filter, filter The cake was washed with 20 ml of tetrahydrofuran / n-heptane (5:1), and dried to obtain a white powder. Dissolve the white powder with 8ml of tetrahydrofuran under reflux, cool to below 40°C, add 80ml of n-heptane, continue cooling to below 10°C, filter, wash the filter cake with 10ml of tetrahydrofuran / n-heptane (5:1), and dry A white powder was obtained.
[0038] As determined by HPLC, the formula (I-a): formula (I-b) in the white powder is higher than 95:5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com