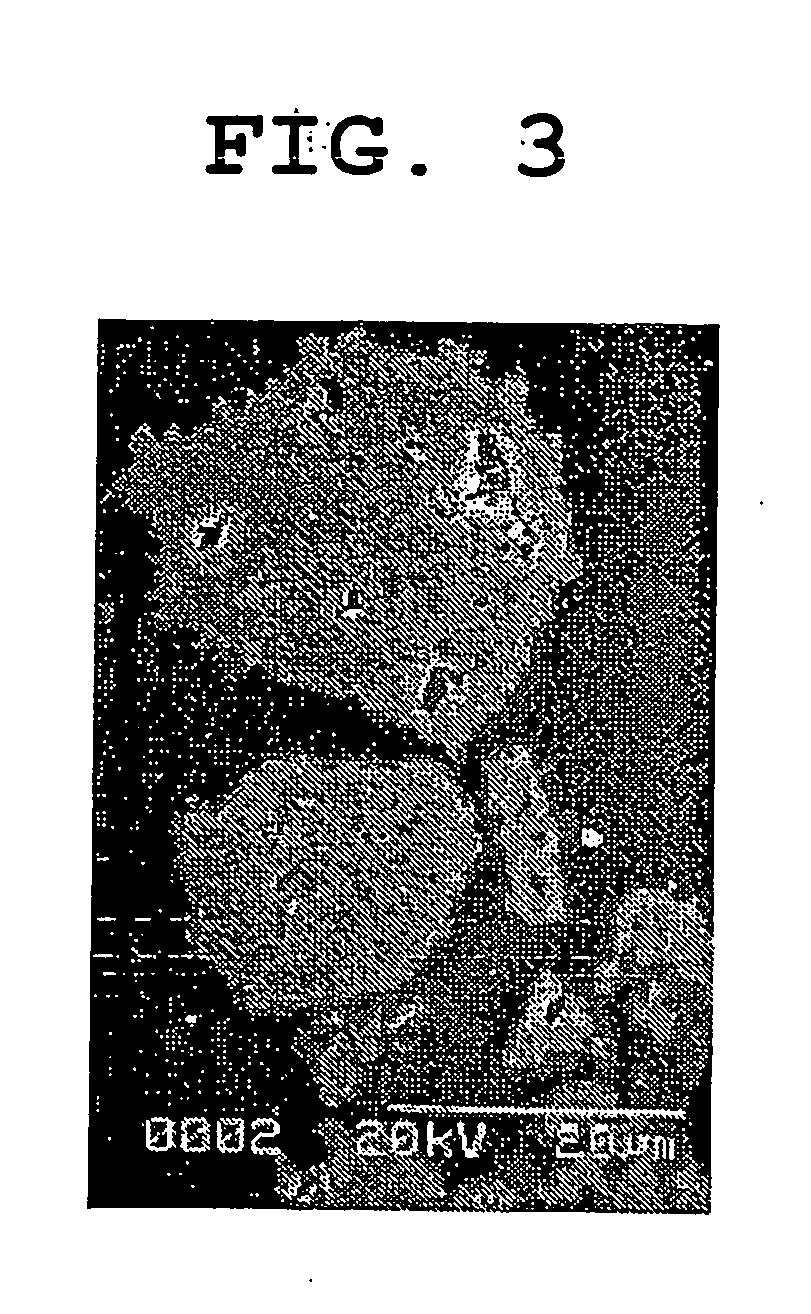
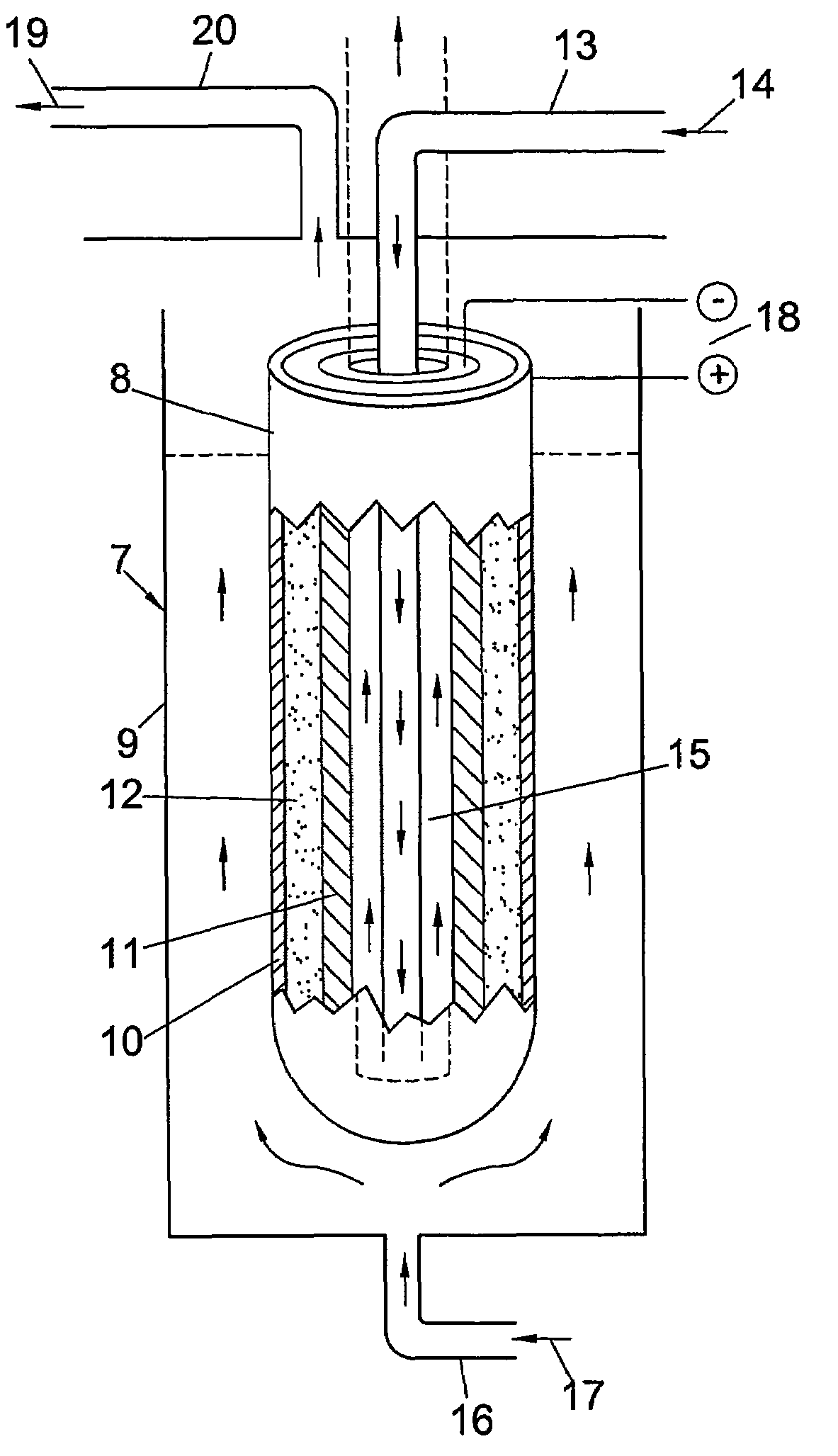
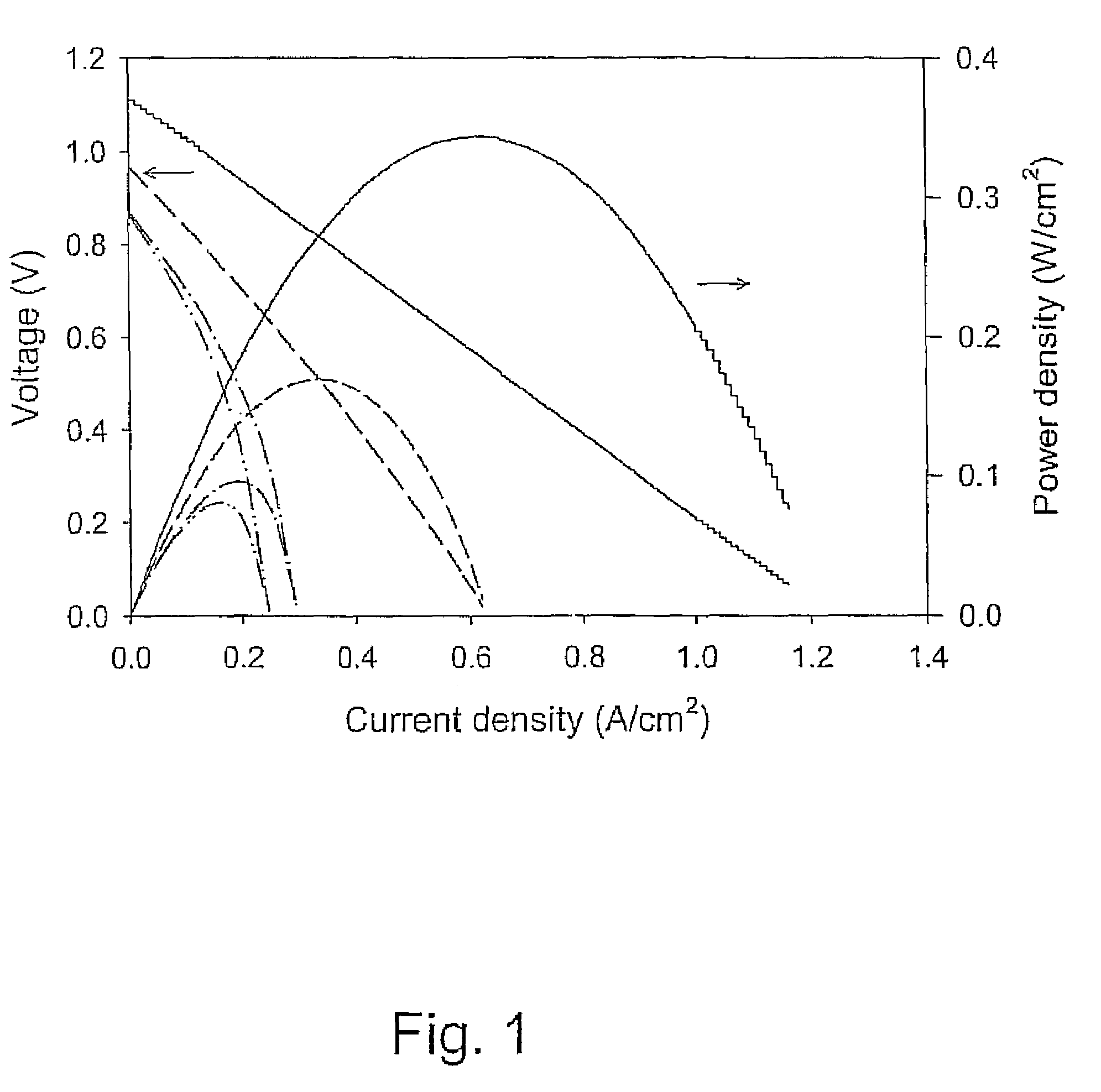
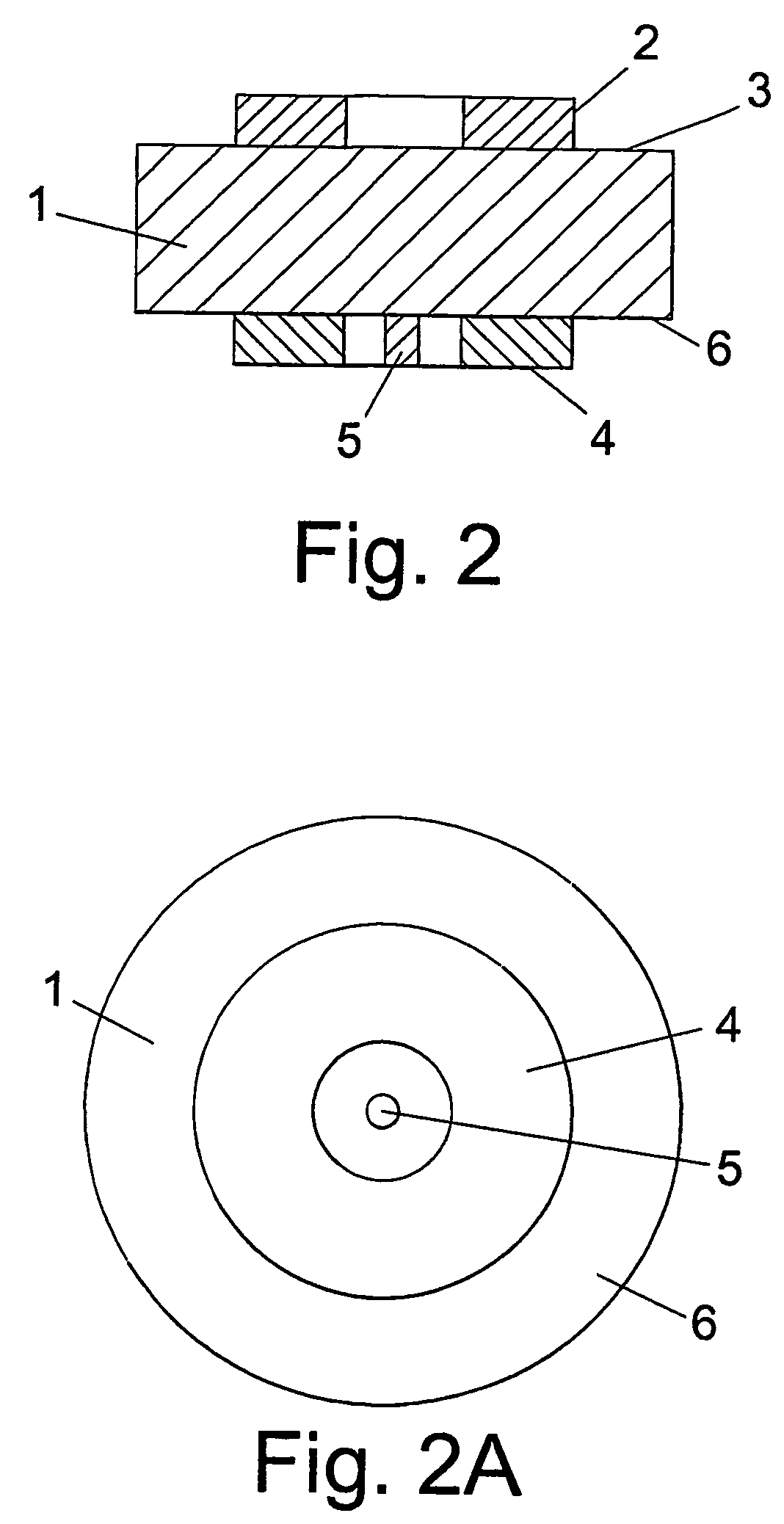
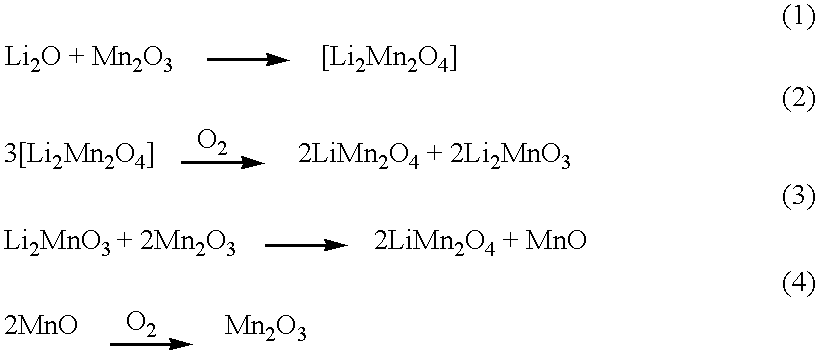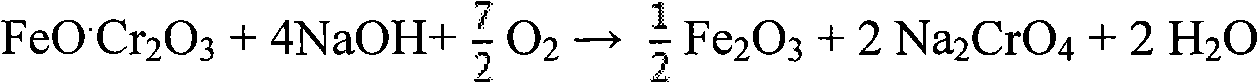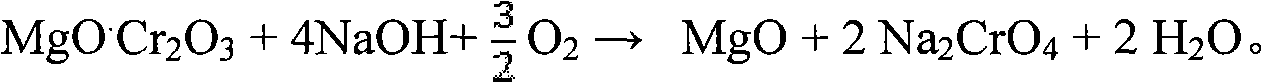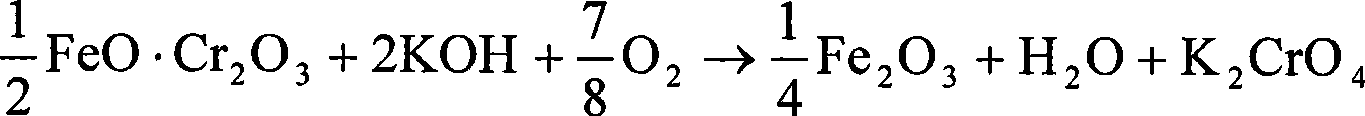Patents
Literature
489results about "Chromates/bichromates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
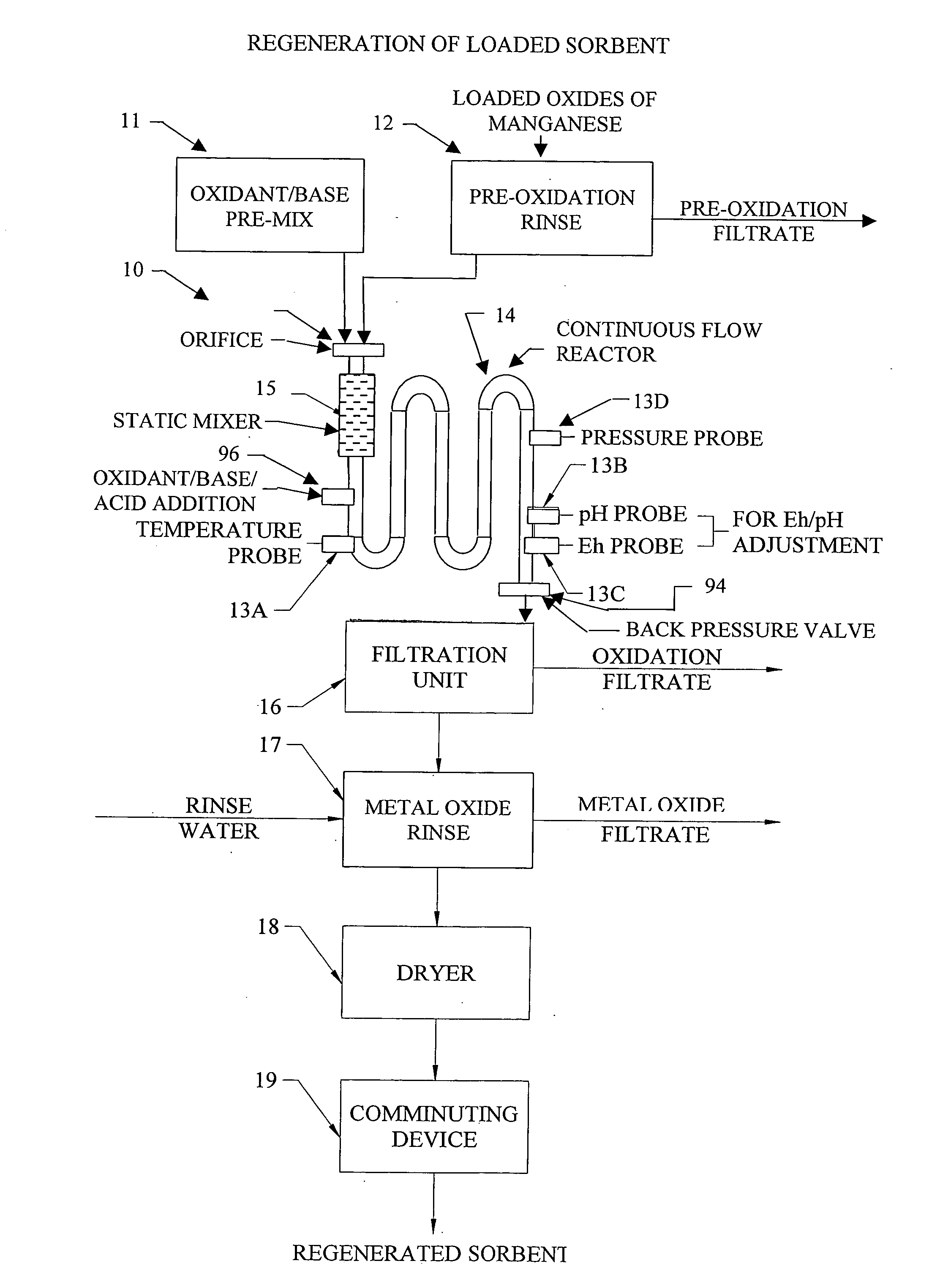
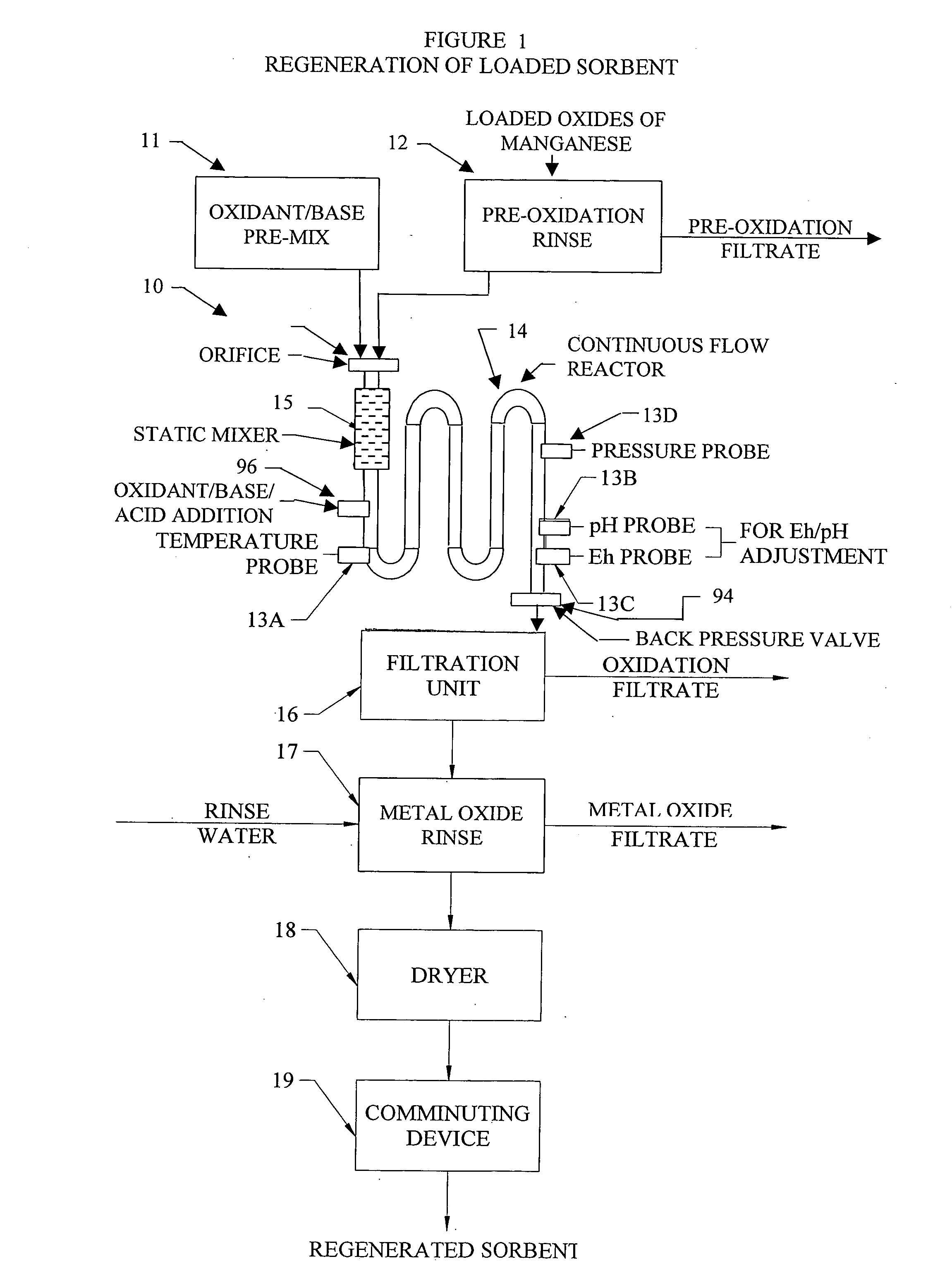
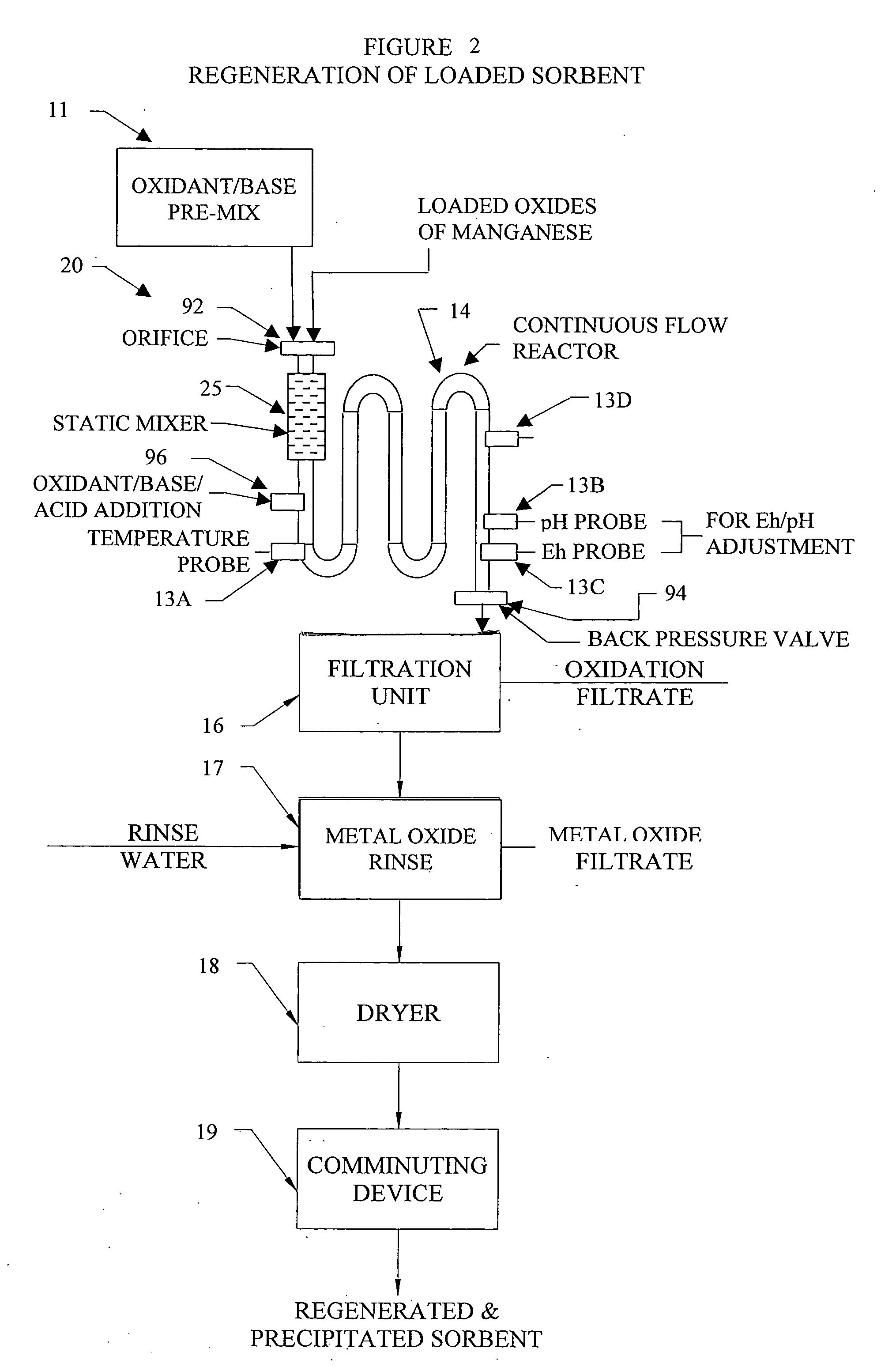
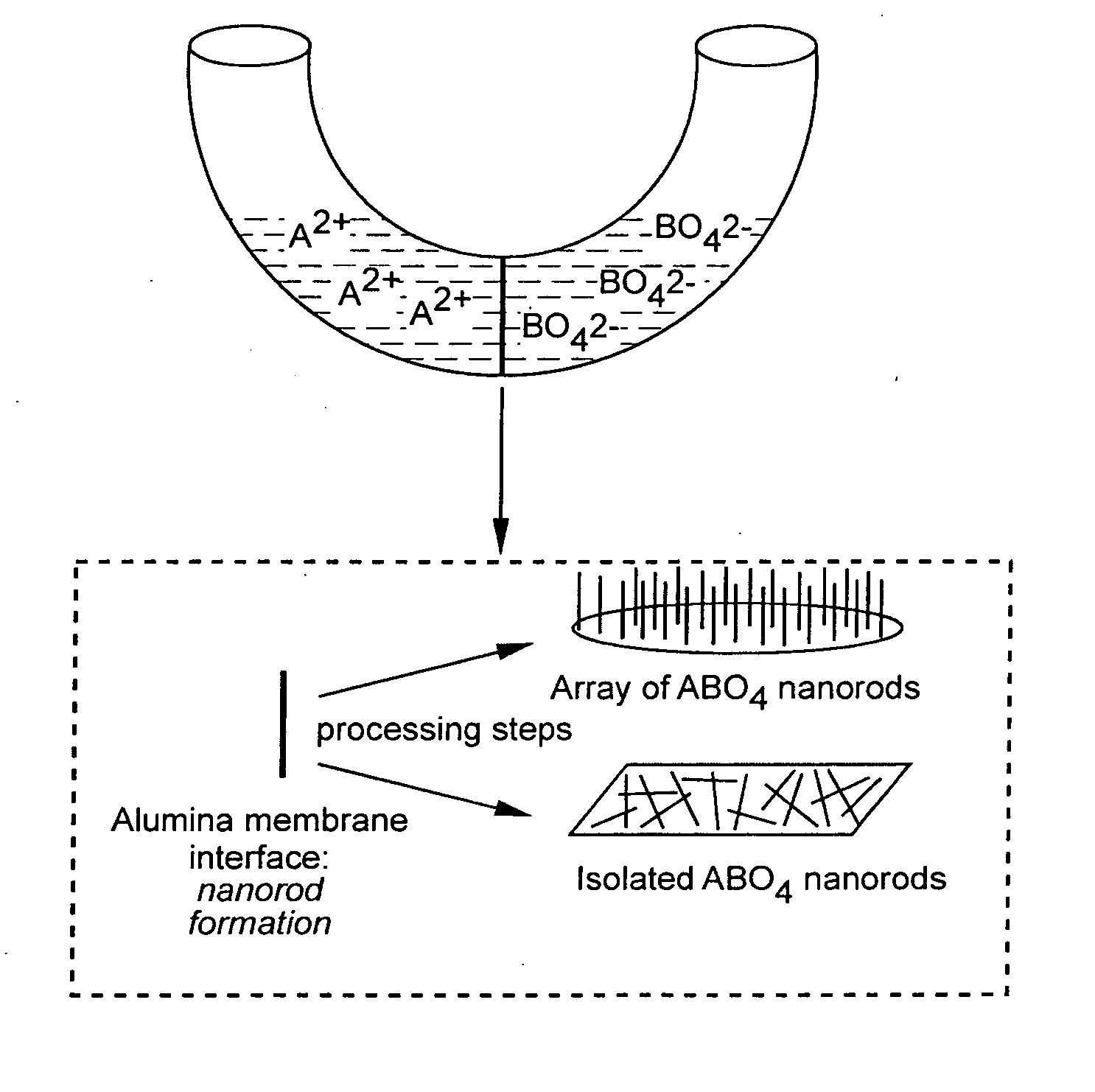
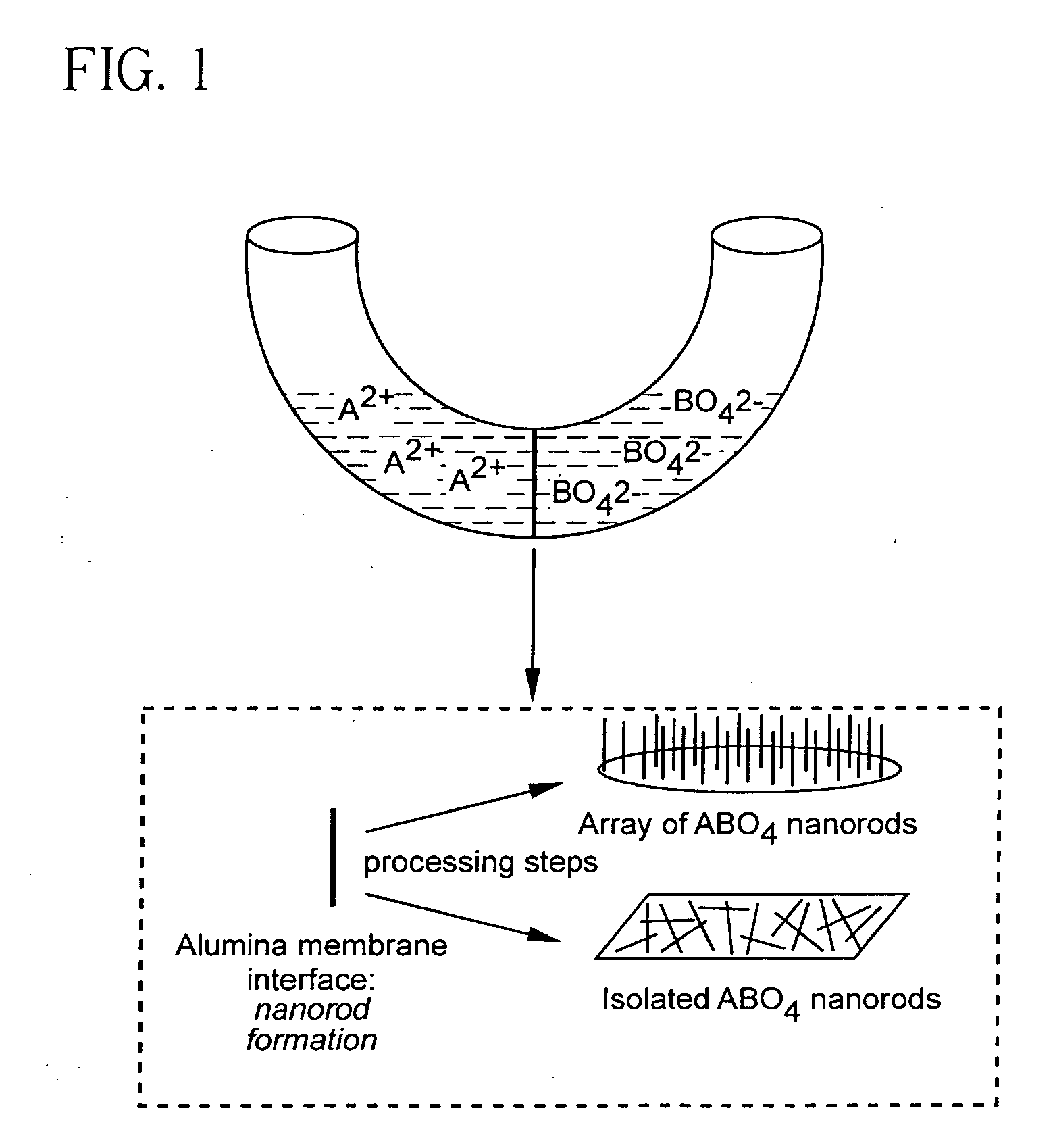
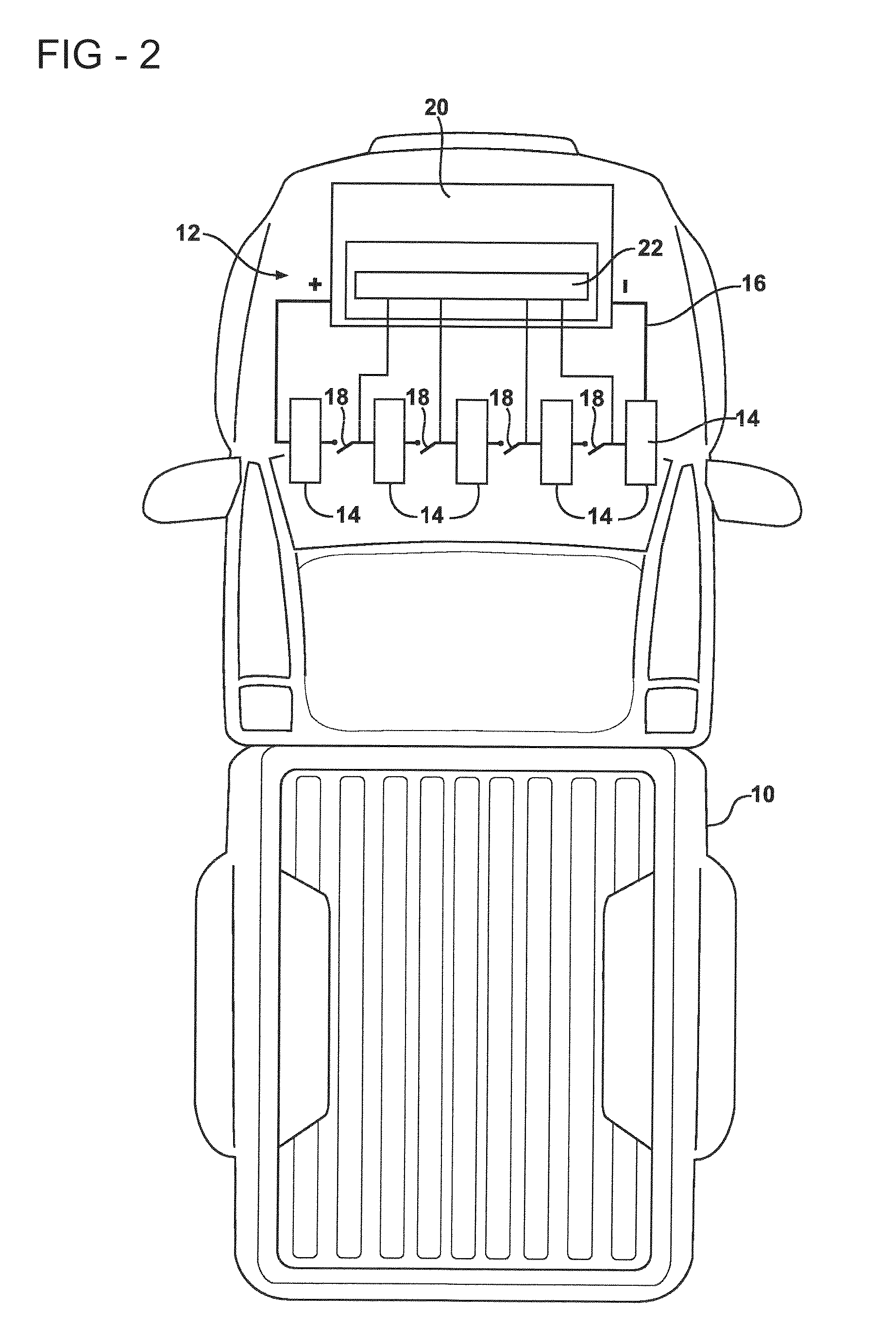
Metal oxide processing methods and systems
InactiveUS20050074380A1Move quicklyIncrease load capacityCombination devicesTemperatue controlIndustrial gasBatch processing
Methods and systems for processing metal oxides from metal containing solutions. Metal containing solutions are mixed with heated aqueous oxidizing solutions and processed in a continuous process reactor or batch processing system. Combinations of temperature, pressure, molarity, Eh value, and pH value of the mixed solution are monitored and adjusted so as to maintain solution conditions within a desired stability area during processing. This results in metal oxides having high or increased pollutant loading capacities and / or oxidation states. These metal oxides may be processed according to the invention to produce co-precipitated oxides of two or more metals, metal oxides incorporating foreign cations, metal oxides precipitated on active and inactive substrates, or combinations of any or all of these forms. Metal oxides thus produced are, amongst other uses; suitable for use as a sorbent for capturing or removing target pollutants from industrial gas streams or drinking water or aqueous streams or for personal protective respirators.
Owner:ENVIROSCRUB TECH CORP
Cathode intercalation compositions, production methods and rechargeable lithium batteries containing the same
InactiveUS6248477B1Easy to adaptOxygen/ozone/oxide/hydroxideElectrode thermal treatmentMetalSpinel group
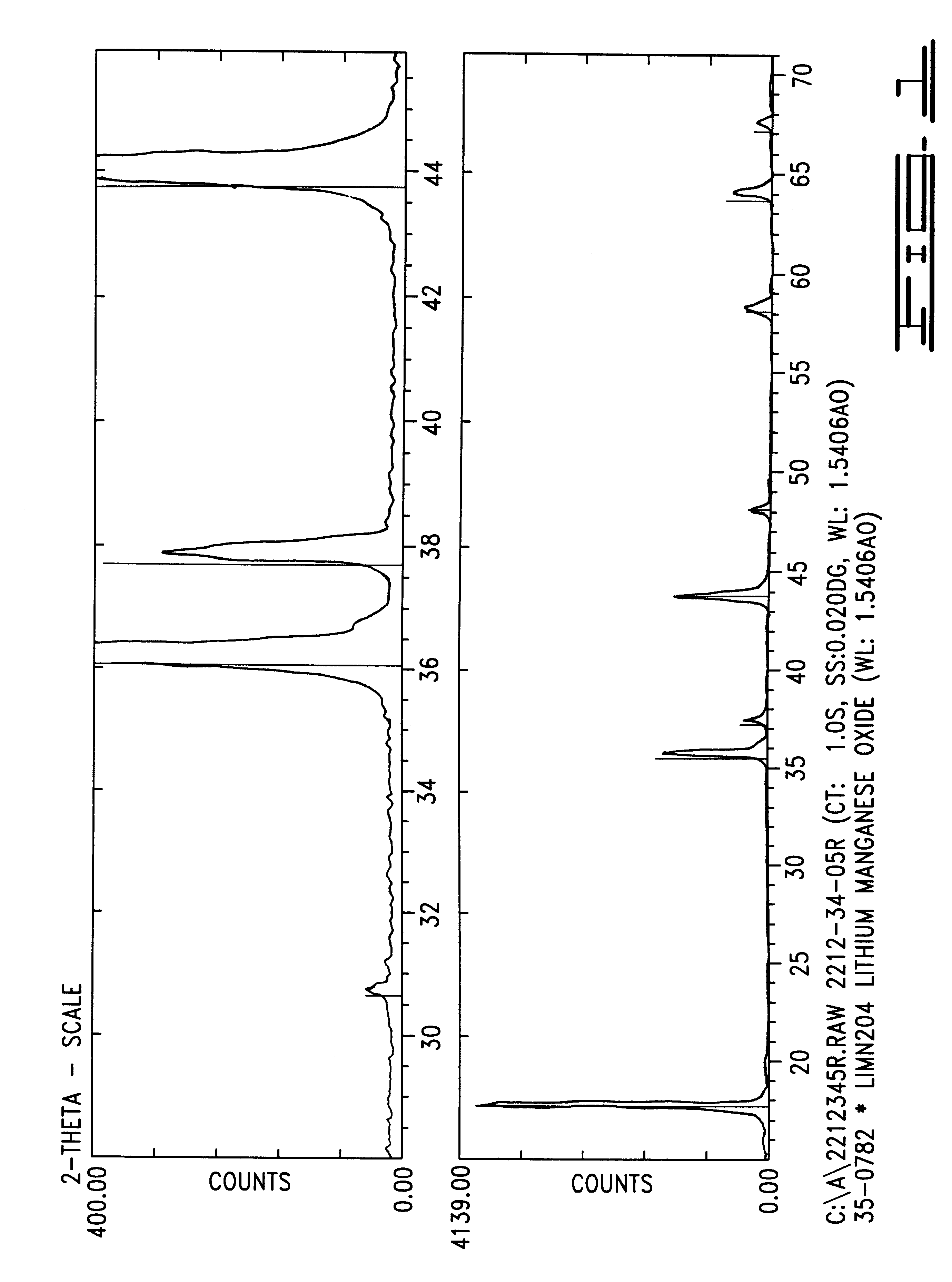
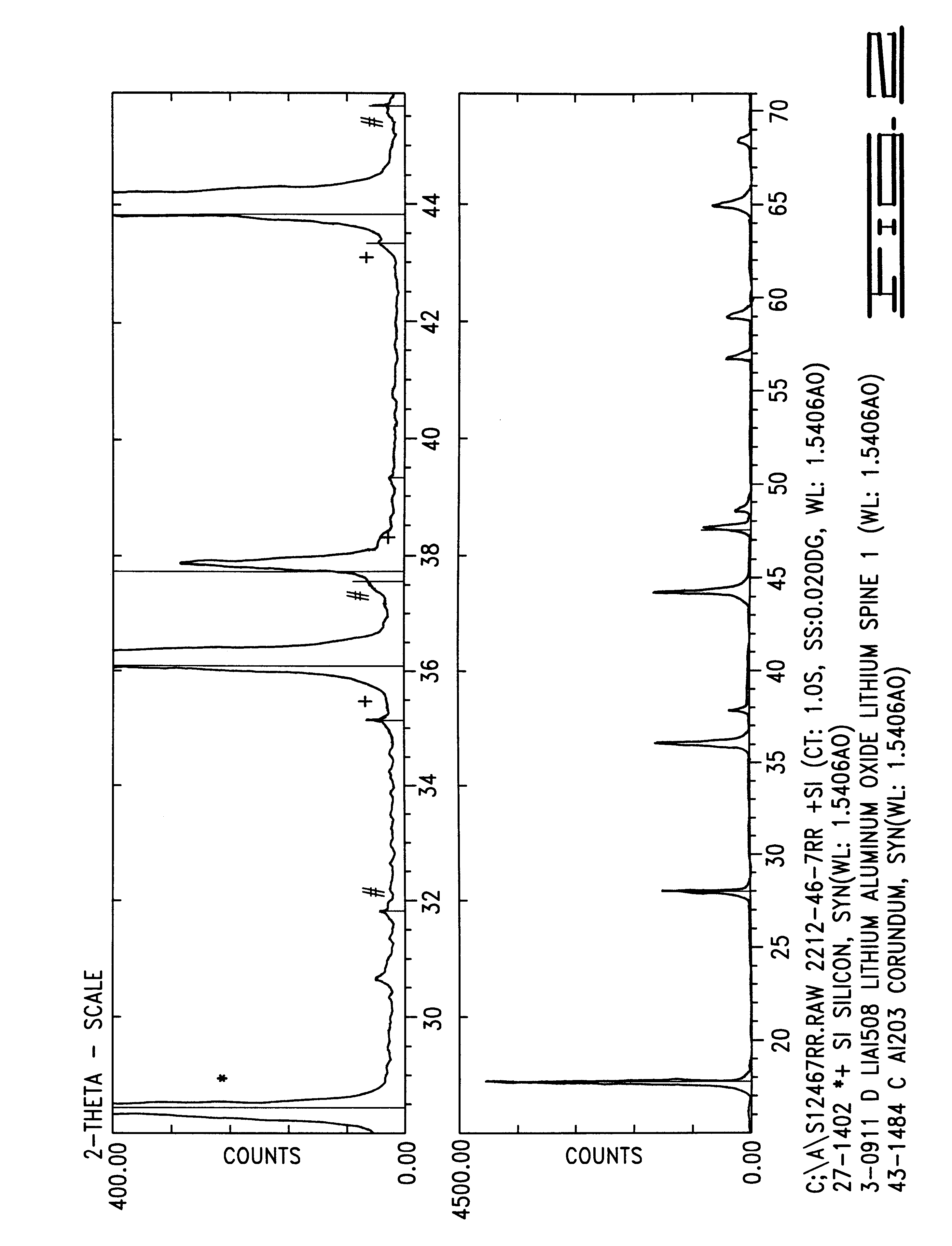
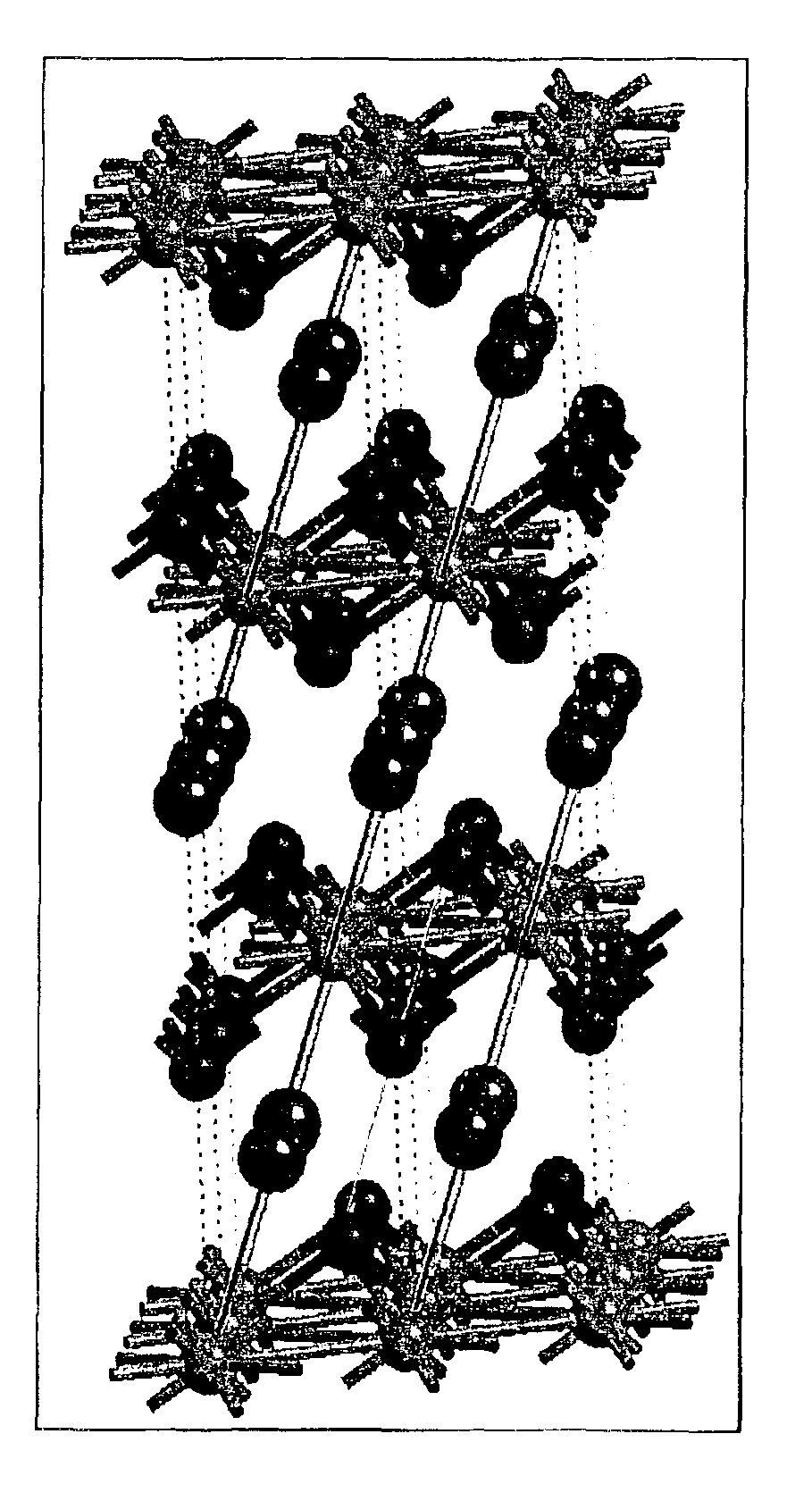
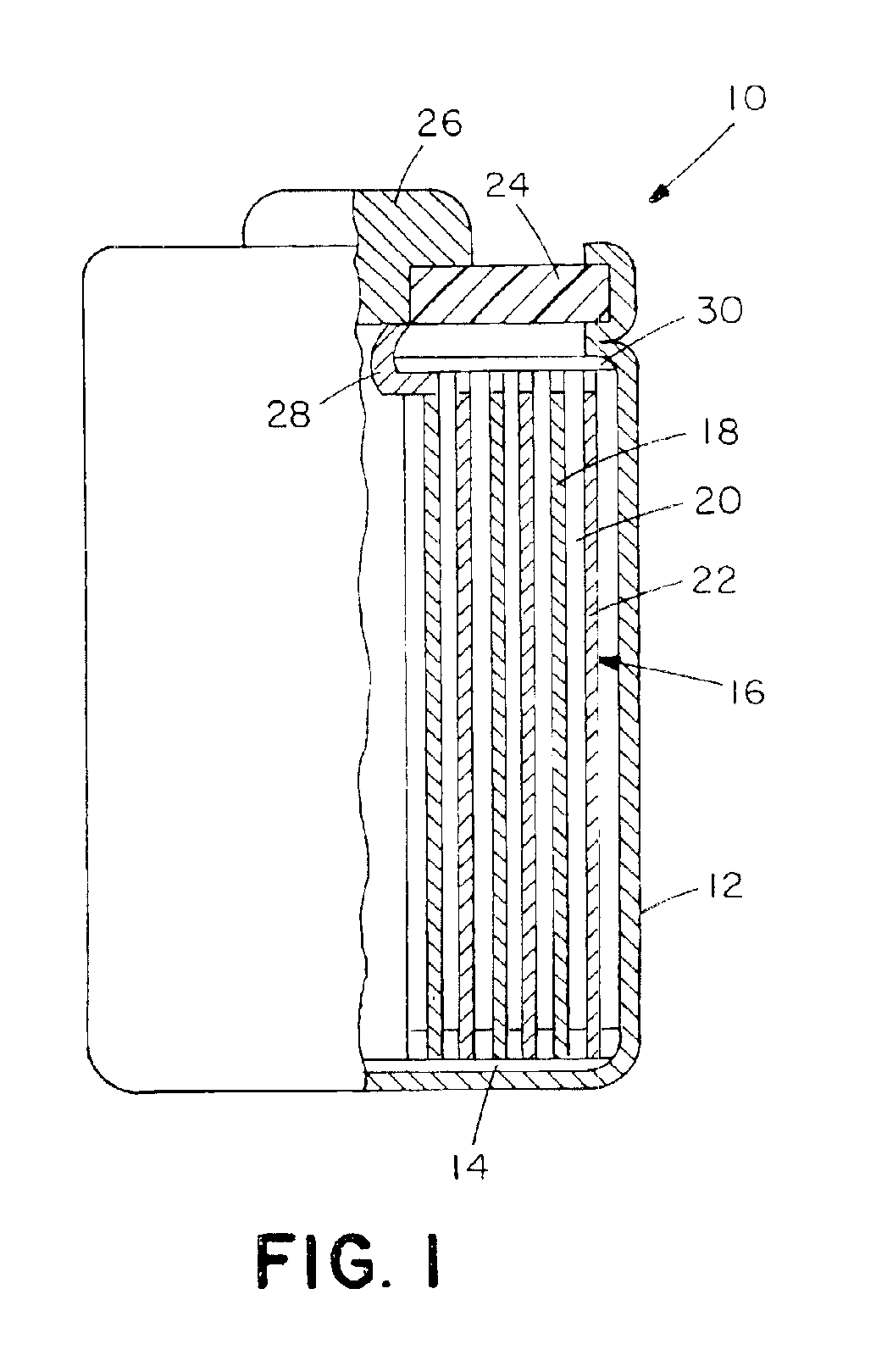
Intercalation compositions having spinel structures with crystallites of metal oxides (M2O3) dispersed throughout the structure are provided having the general formula Li1+xMyMn2-x-yO4. Methods of producing the intercalation compositions and rechargeable lithium batteries containing the compositions are also provided.
Owner:EMD ACQUISITION LLC
Gradient cathode material for lithium rechargeable batteries
InactiveUS6921609B2Increase capacityImproved cyclabilityAluminium compoundsAlkaline accumulatorsManganesePotassium
A composition suitable for use as a cathode material of a lithium battery includes a core material having an empirical formula LixM′zNi1−yM″yO2. “x” is equal to or greater than about 0.1 and equal to or less than about 1.3. “y” is greater than about 0.0 and equal to or less than about 0.5. “z” is greater than about 0.0 and equal to or less than about 0.2. M′ is at least one member of the group consisting of sodium, potassium, nickel, calcium, magnesium and strontium. M″ is at least one member of the group consisting of cobalt, iron, manganese, chromium, vanadium, titanium, magnesium, silicon, boron, aluminum and gallium. A coating on the core has a greater ratio of cobalt to nickel than the core. The coating and, optionally, the core can be a material having an empirical formula Lix1Ax2Ni1−y1−z1Coy1Bz1Oa. “x1” is greater than about 0.1 a equal to or less than about 1.3. “x2,”“y1” and “z1” each is greater than about 0.0 and equal to or less than about 0.2. “a” is greater than 1.5 and less than about 2.1. “A” is at least one element selected from the group consisting of barium, magnesium, calcium and strontium. “B” is at least one element selected from the group consisting of boron, aluminum, gallium, manganese, titanium, vanadium and zirconium.
Owner:TIAX LLC
Method of hydrothermal liquid phase sintering of ceramic materials and products derived therefrom
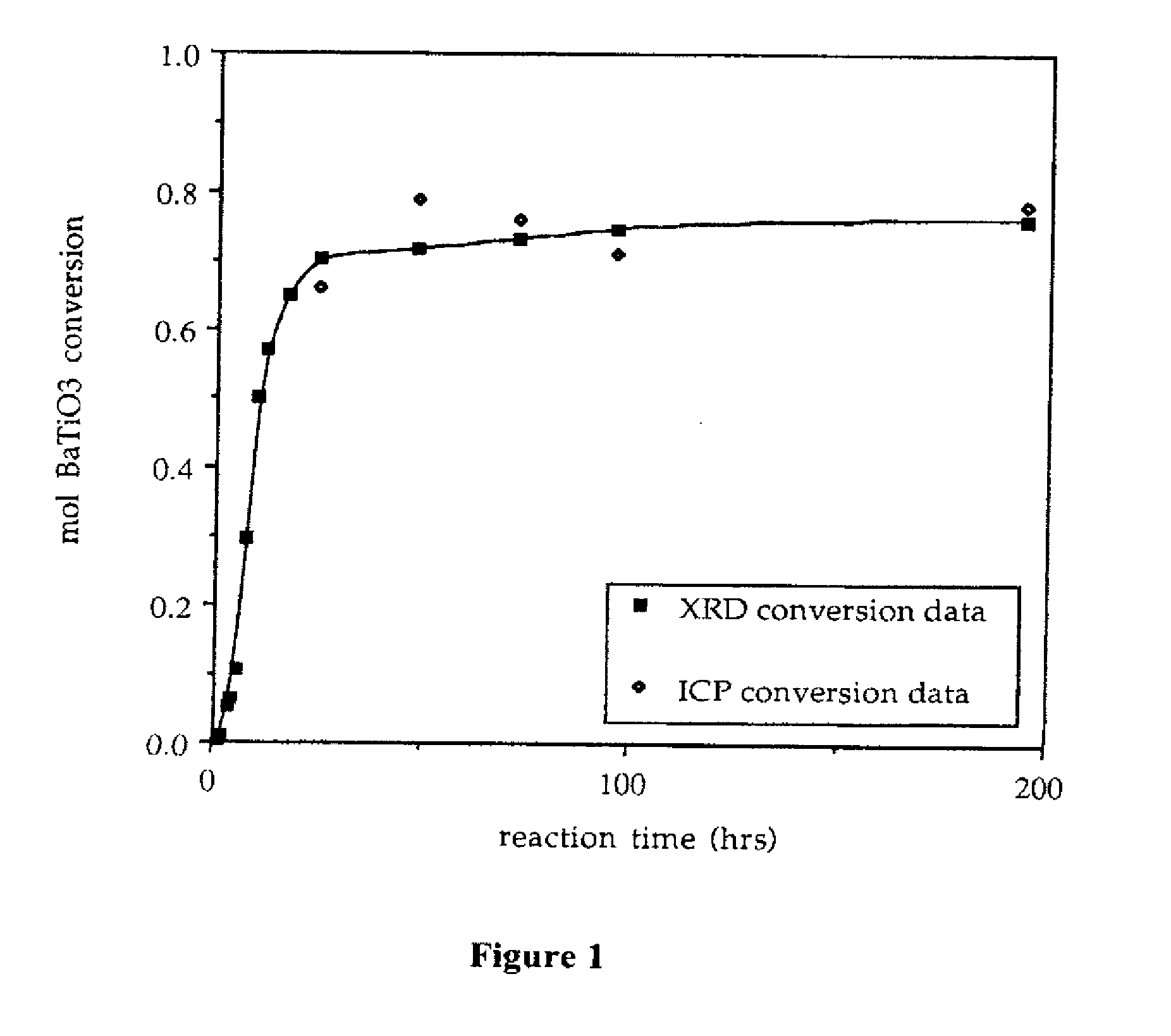
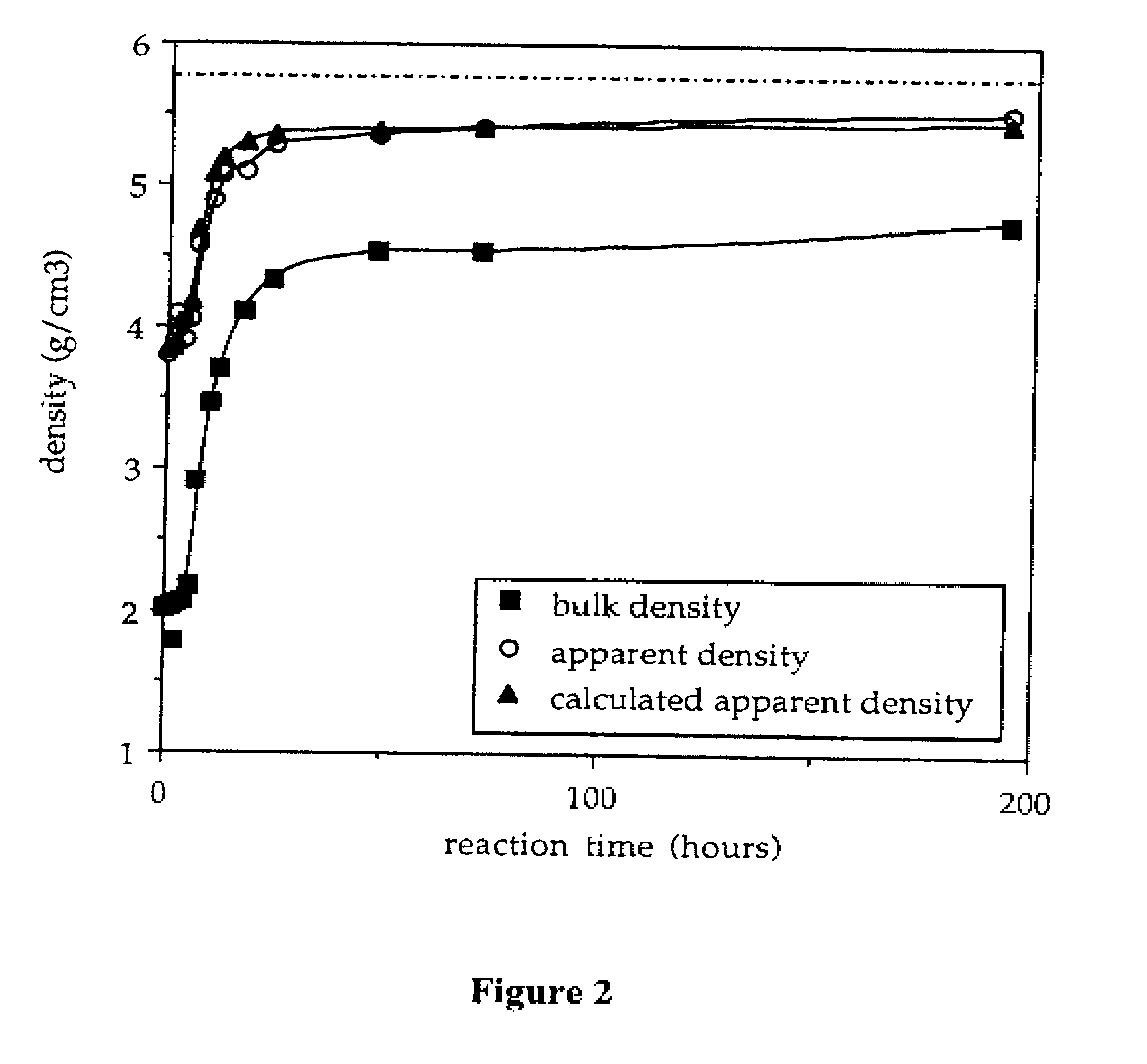
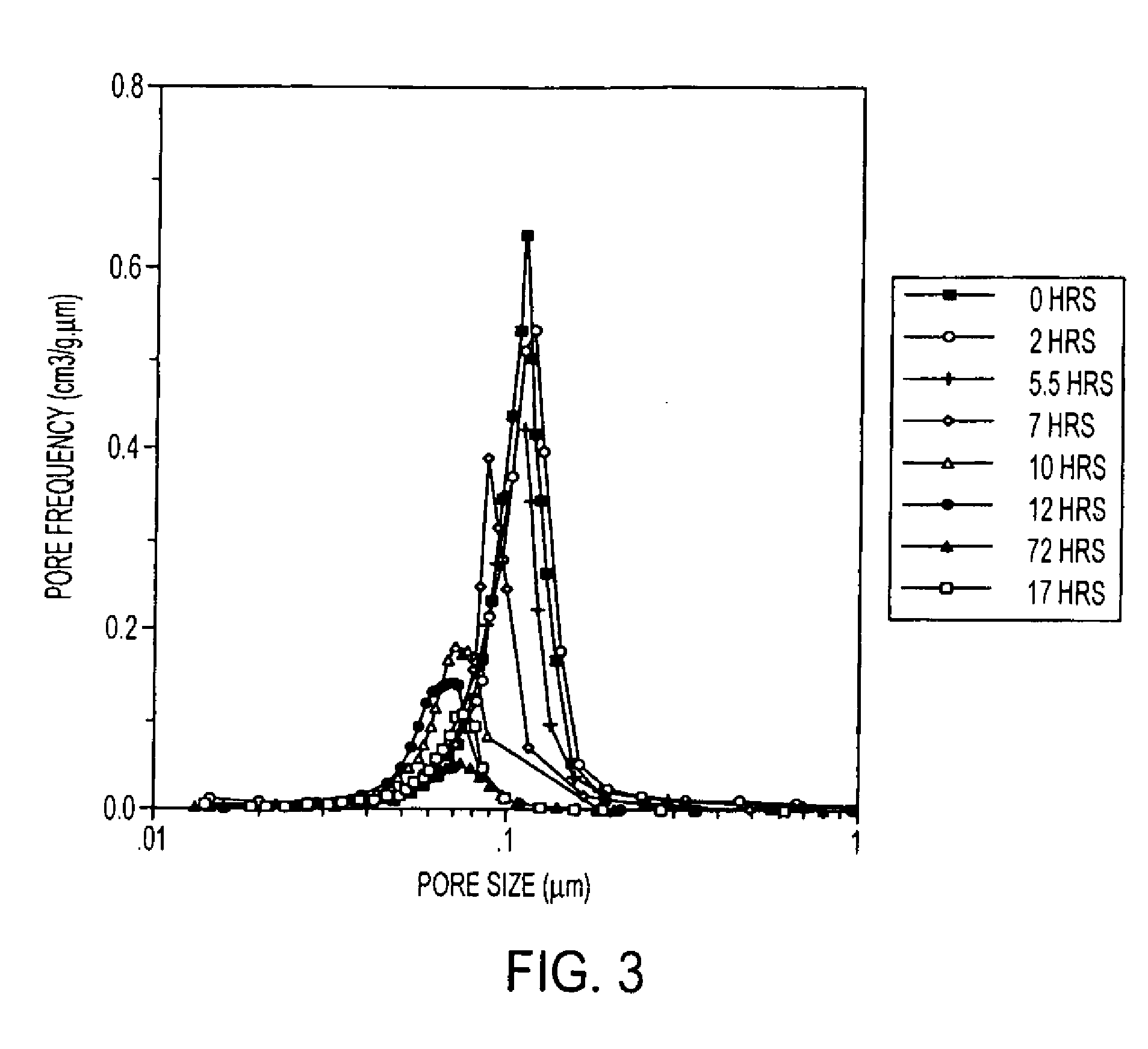
Provided here is a method of producing a monolithic body from a porous matrix, comprising: (i) providing a porous matrix having interstitial spaces and comprising at least a first reactant; (ii) contacting the porous matrix with an infiltrating medium that carries at least a second reactant; (iii) allowing the infiltrating medium to infiltrate at least a portion of the interstitial spaces of the porous matrix under conditions that promote a reaction between the at least first reactant and the at least second reactant to provide at least a first product; and (iv) allowing the at least first product to form and fill at least a portion of the interstitial spaces of the porous matrix, thereby producing a monolithic body, wherein the monolithic body does not comprise barium titanate.
Owner:RUTGERS THE STATE UNIV
Cathode active material and nonaqueous secondary battery containing the same
InactiveUS6103422ASuppress deterioration of electrode performanceReduce charge and dischargeAluminium compoundsActive material electrodesManganeseCobalt
PCT No. PCT / JP96 / 03738 Sec. 371 Date Jun. 24, 1998 Sec. 102(e) Date Jun. 24, 1998 PCT Filed Dec. 20, 1996 PCT Pub. No. WO97 / 23918 PCT Pub. Date Jul. 3, 1997The powdery cathode active material of the present invention for use in a nonaqueous electrolyte secondary battery comprises as the active ingredient a lithium manganese composite oxide and at least one nonmanganese metal element selected from the group consisting of aluminum, magnesium, vanadium, chromium, iron, cobalt, nickel, and zinc, provided that the nonmanganese metal element exists in the surface portions of fine particles constituting the powdery cathode active material. A nonaqueous electrolyte secondary battery comprising such a cathode active material, an anode active material and a nonaqueous electrolytic solution containing a lithium salt has a large charge-and-discharge capacity and a stable charge-and-discharge cycle durability, and can be prepared inexpensively.
Owner:KAO CORP
Method for producing mixed metal oxides and metal oxide compounds
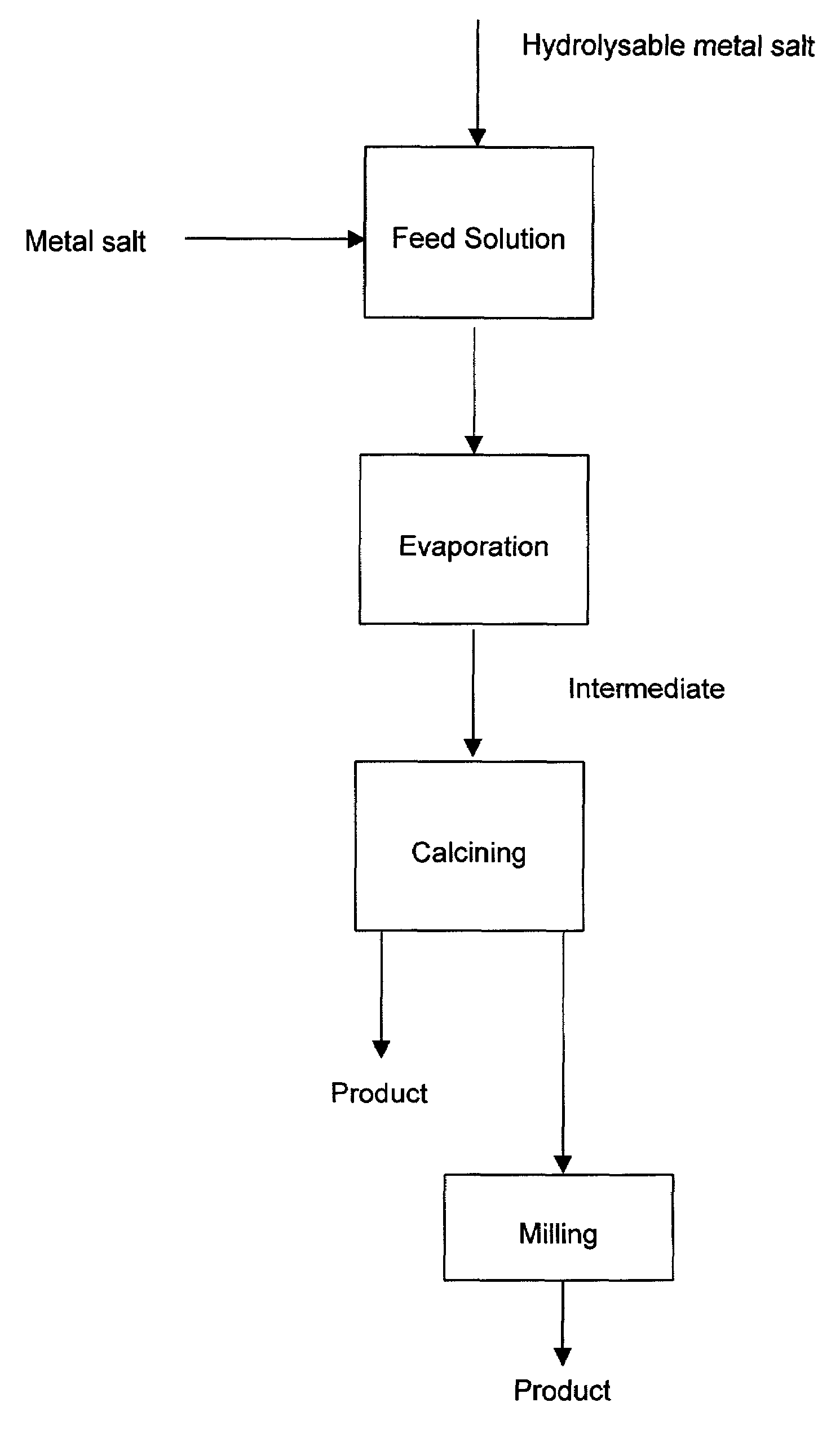
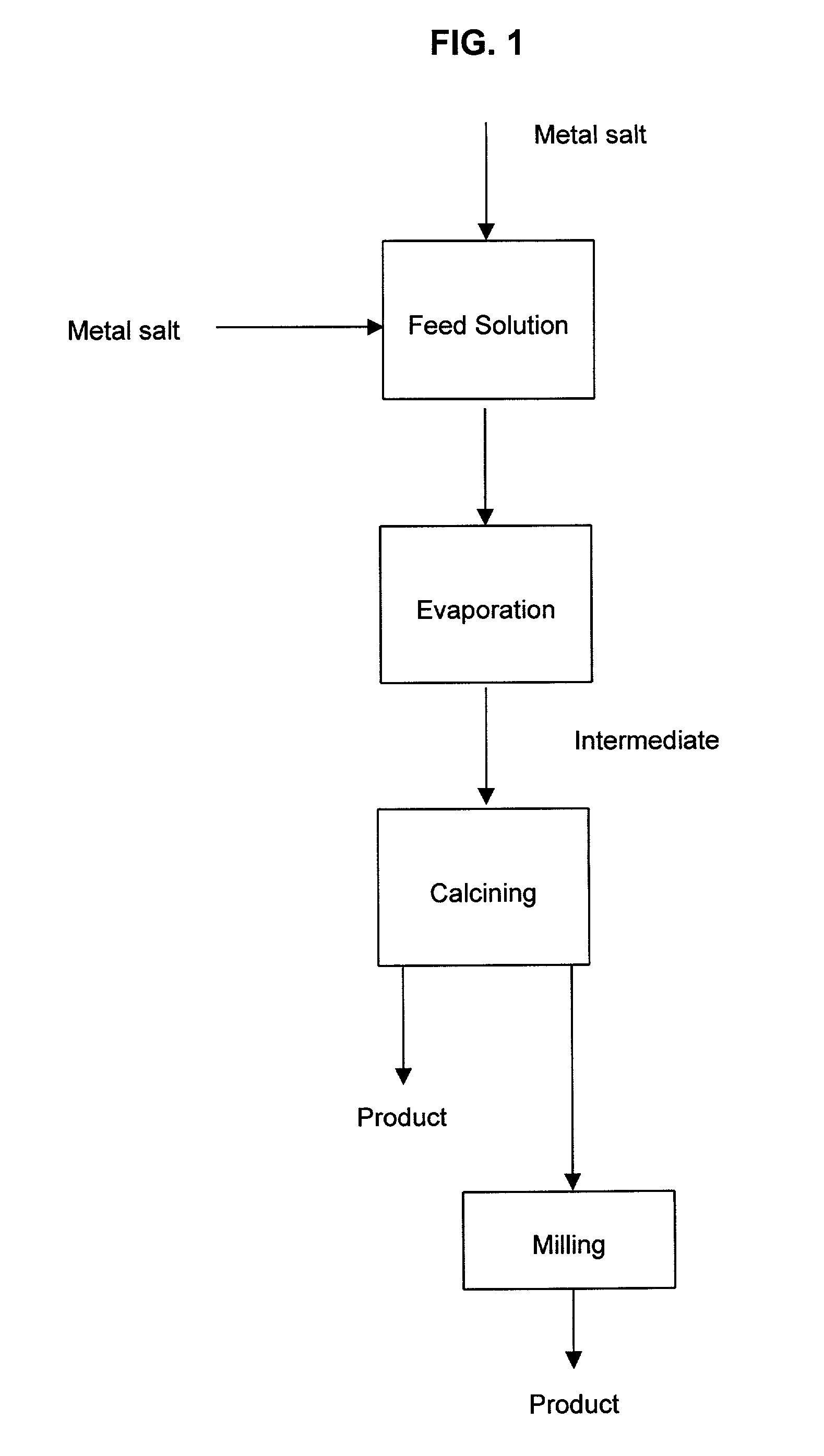
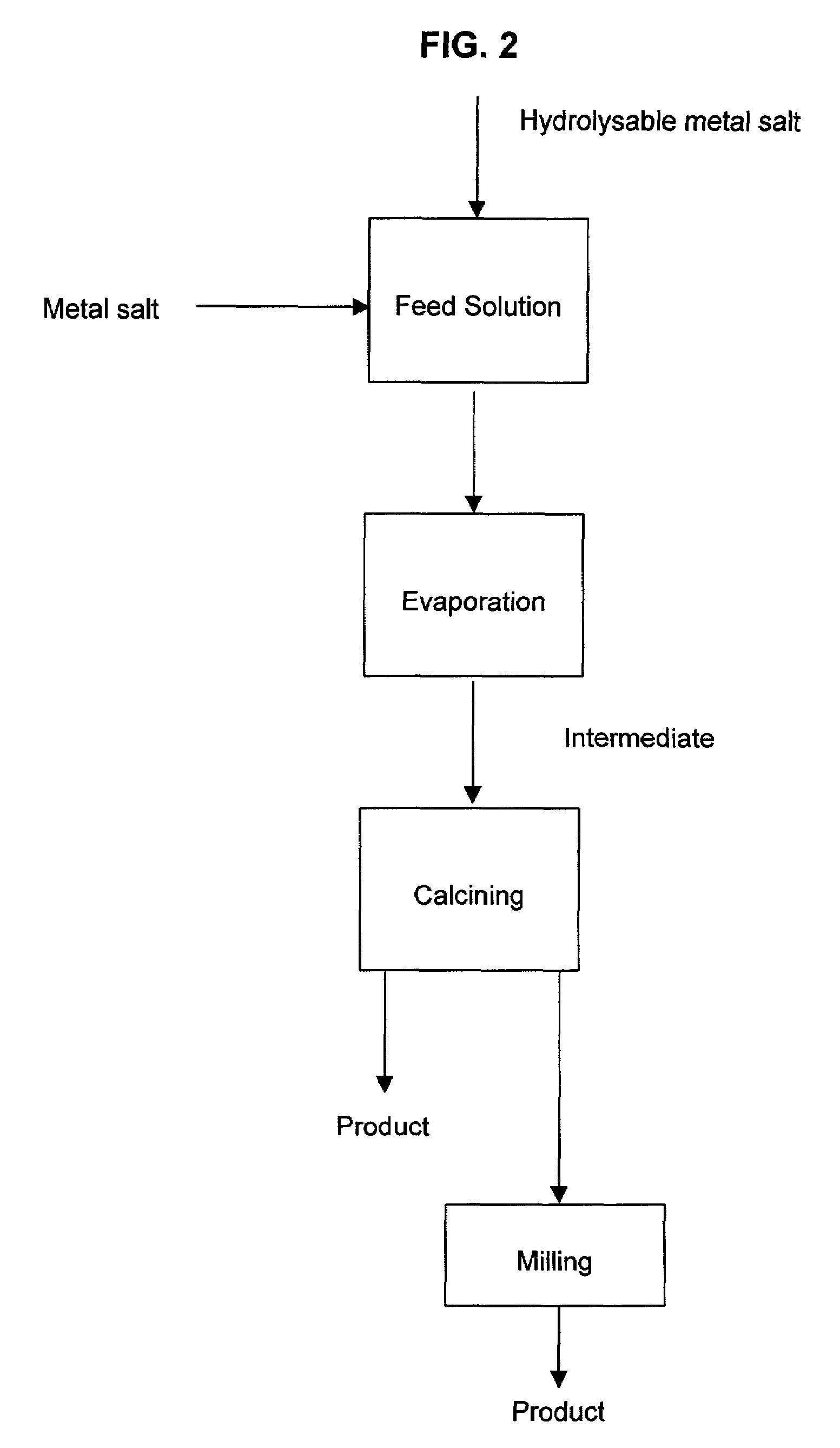
A process to produce mixed metal oxides and metal oxide compounds. The process includes evaporating a feed solution that contains at least two metal salts to form an intermediate. The evaporation is conducted at a temperature above the boiling point of the feed solution but below the temperature where there is significant crystal growth or below the calcination temperature of the intermediate. The intermediate is calcined, optionally in the presence of an oxidizing agent, to form the desired oxides. The calcined material can be milled and dispersed to yield individual particles of controllable size and narrow size distribution.
Owner:ALTAIR NANOMATERIALS INC
Method for purifying electroplating wastewater and comprehensively utilizing resources
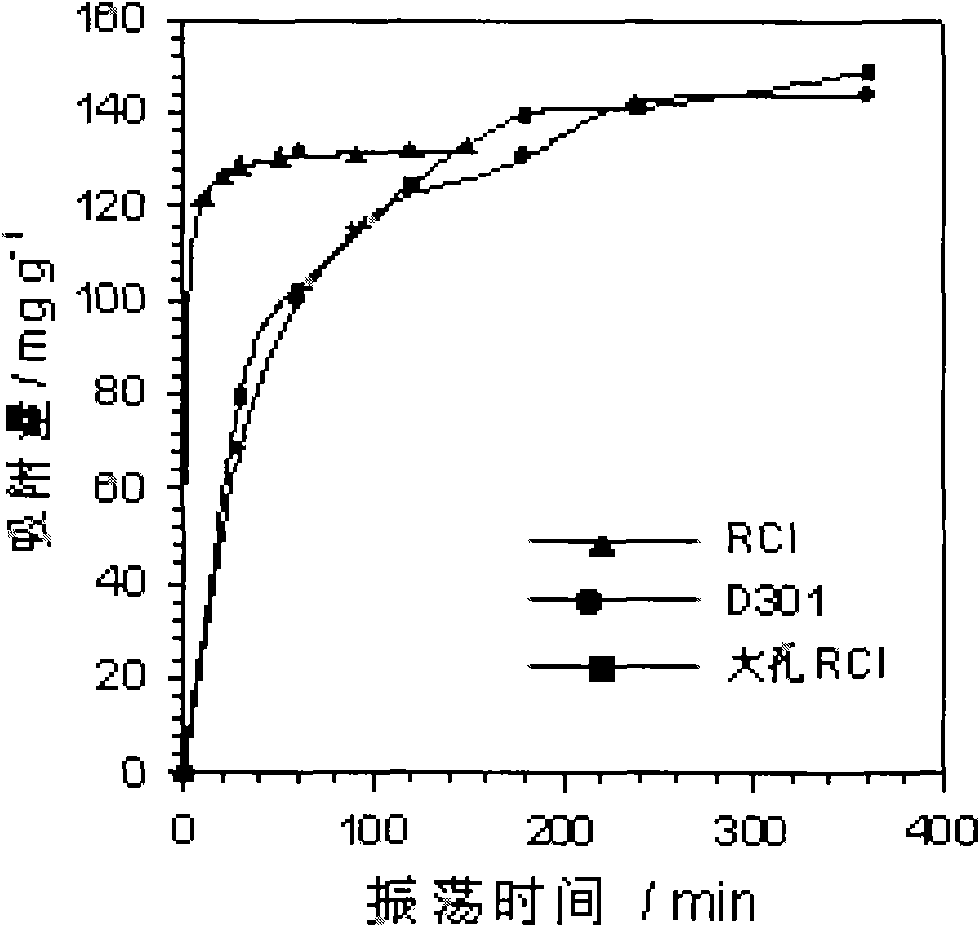
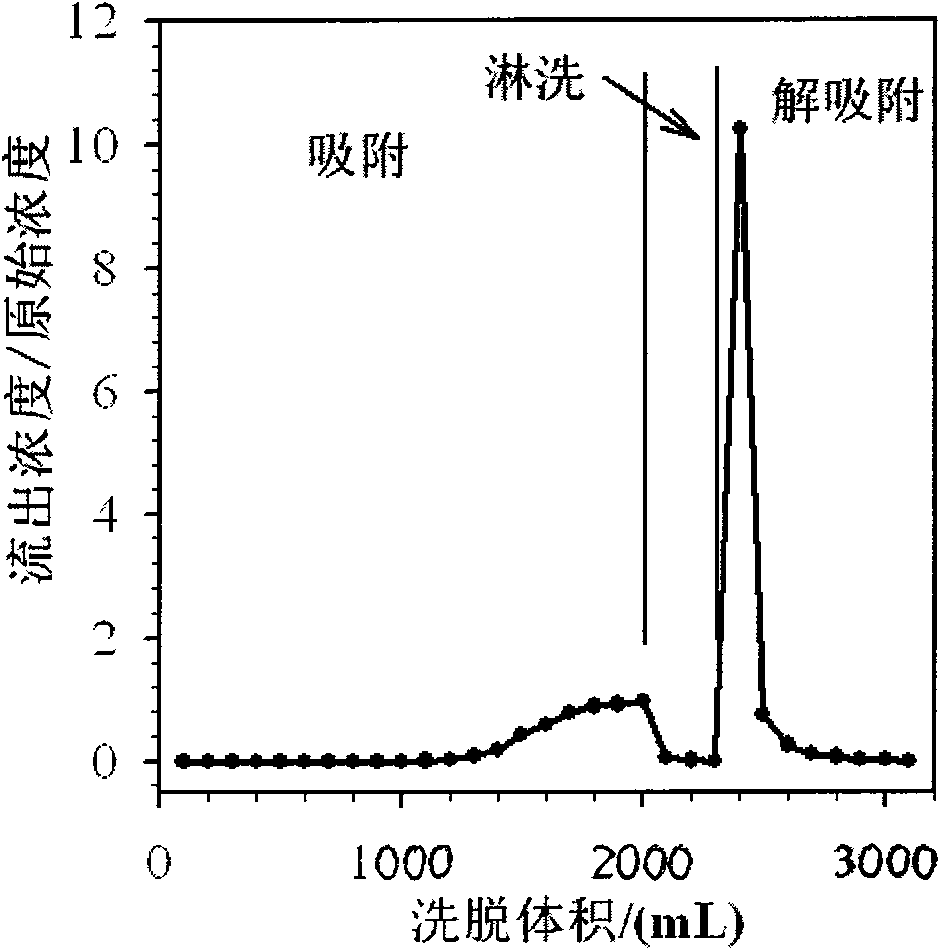
ActiveCN101570372AImprove adsorption capacityEasy to separateWater contaminantsWater/sewage treatment by ion-exchangeChemical reactionWater quality
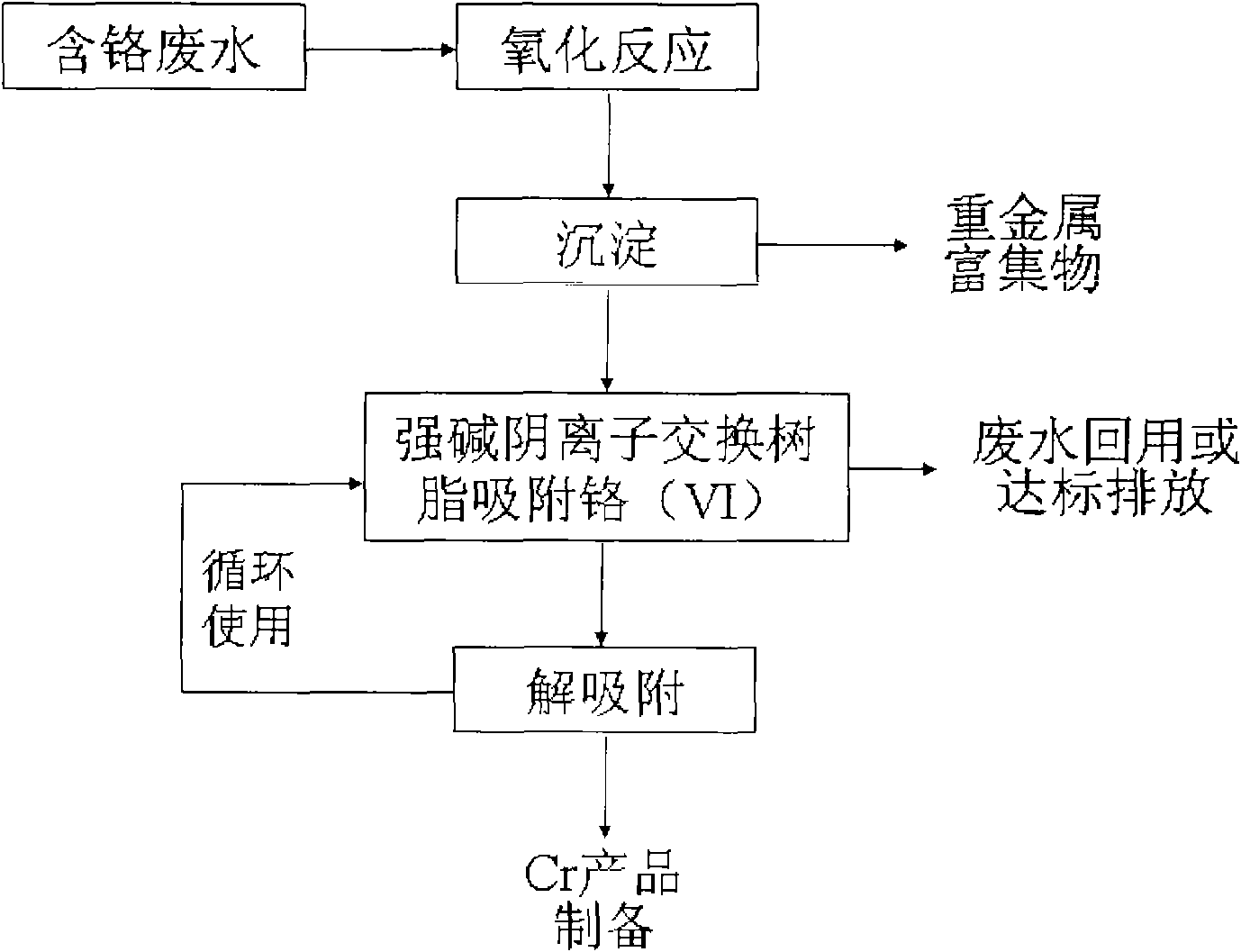
The invention relates to a method for purifying electroplating wastewater and comprehensively utilizing resources, which is to adopt low-cost strong basic anion-exchange resin containing macropores and imidazole structure to reclaim valuable resources in the electroplating wastewater by coupling technology based on a chemical oxidation-reduction method, a precipitation method and an ion exchange method. The method comprises the following steps: firstly, Cr(III) is oxidized into Cr(VI) under basic condition through a chemical reaction; then, Zn, Cu, Ni and other heavy metal elements in the wastewater are transformed into precipitation of hydroxide; and finally, the Cr(VI) in the wastewater is adsorbed by the strong basic anion-exchange resin, the purified water quality achieves the requirement of electroplating pollutant discharge standard and recycle, and the Cr and other heavy metal resources in the electroplating wastewater can be comprehensively reclaimed and utilized. The method overcomes the defects that the prior regenerating process of porous weak-basic resin requires acidification, and the method has the advantages of simple process, low treatment cost, large treatment quantity and acid and base consumption conservation, and is the electroplating wastewater treatment method with green environmental protection and efficient utilization of resources.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
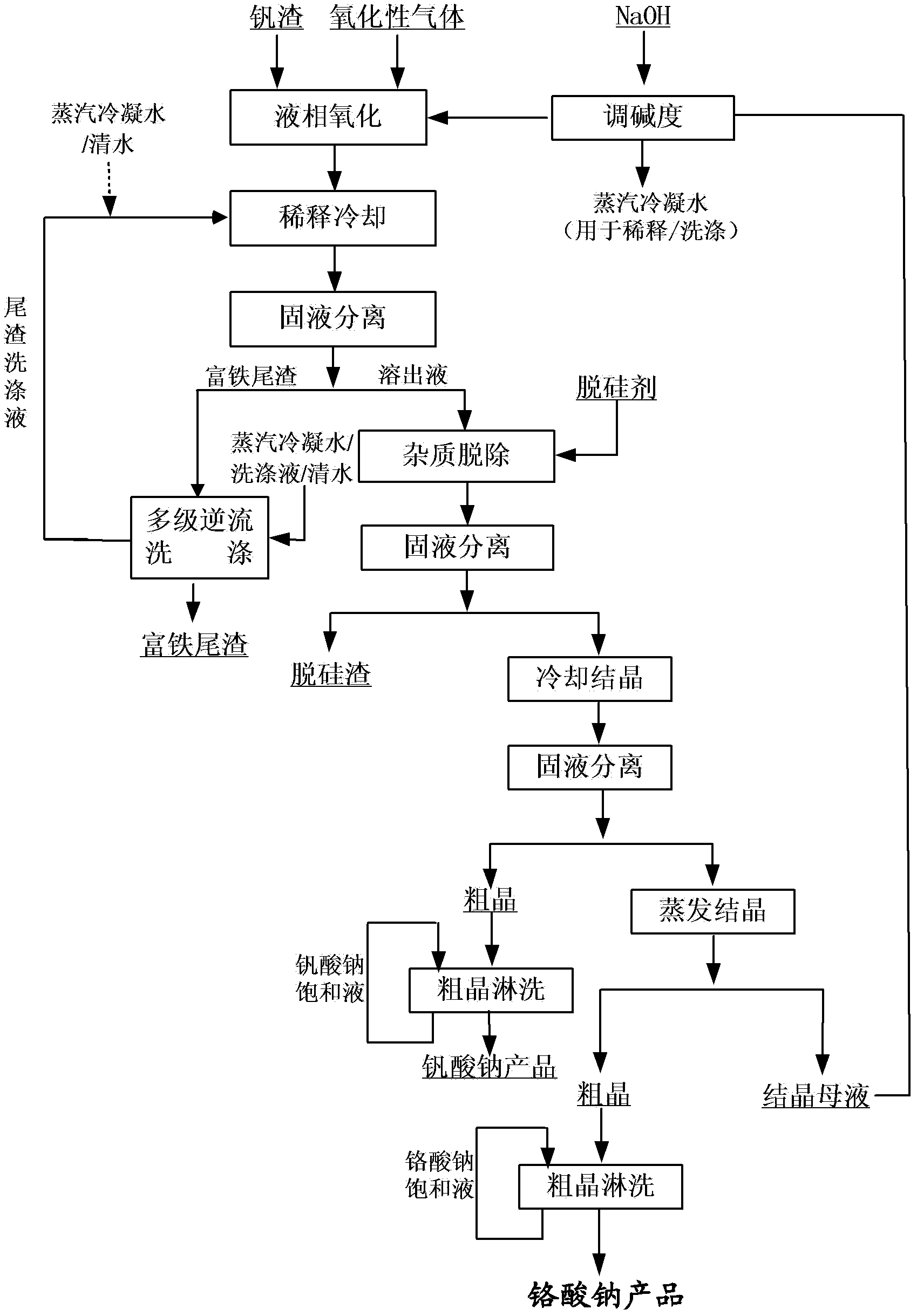
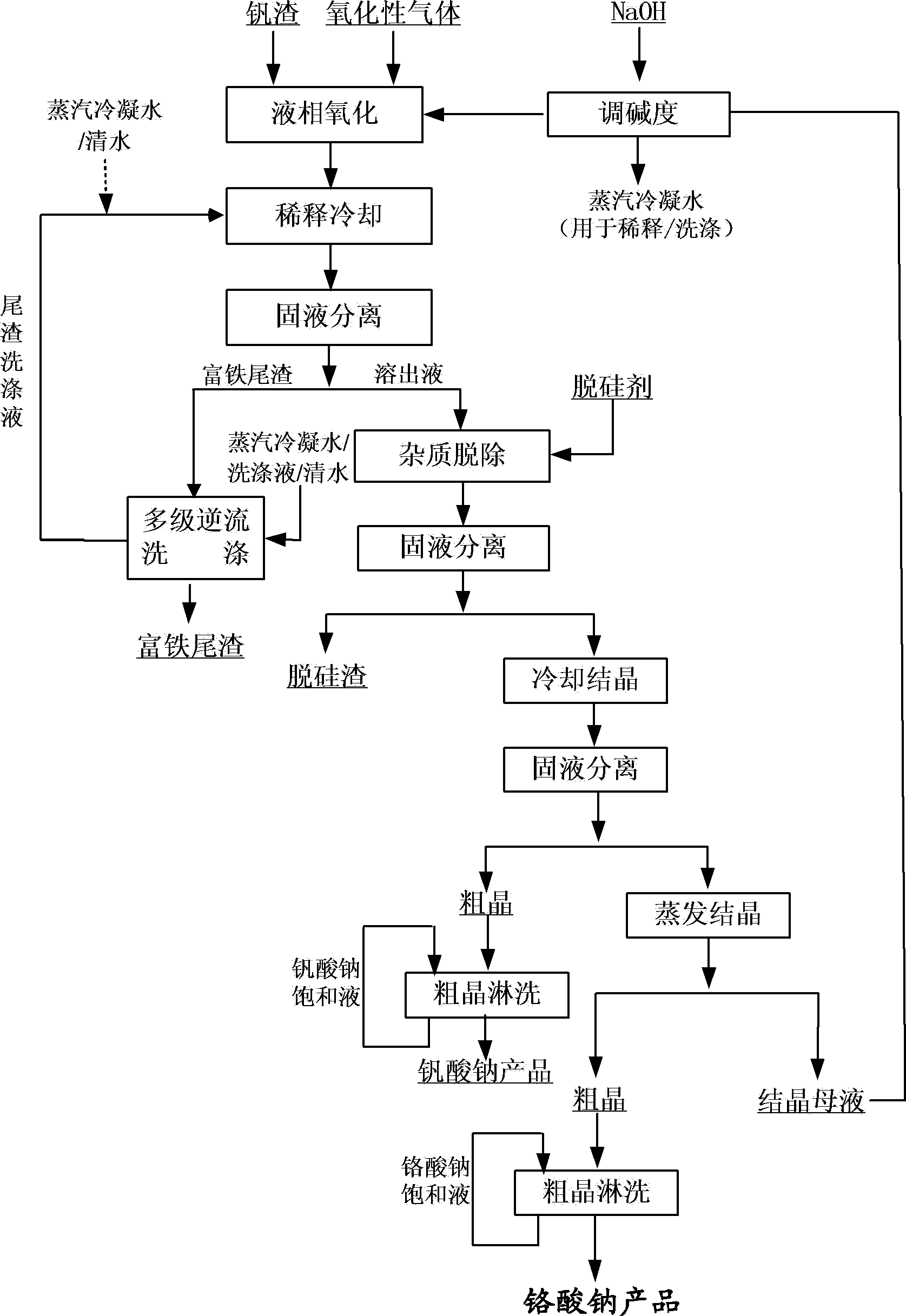
Method for cleaner production of sodium vanadate and sodium chromate by pressure leaching of vanadium slag
ActiveCN102531056ASimple ingredientsAchieve separationChromates/bichromatesVanadium compoundsSlagSlurry
The invention relates to a method for cleaner production of sodium vanadate and sodium chromate by pressure leaching of vanadium slag. The method comprises the following steps of: (1) mixing materials, namely mixing the vanadium slag and a solution of NaOH to obtain a reaction material; (2) reacting, namely performing oxidization reaction on the vanadium slag and oxidizing gas in the solution of NaOH under high pressure to obtain solid-liquid mixed slurry of a solution containing NaOH, Na3VO4, Na2CrO4 and water-soluble impurity components, and iron-rich tailings; (3) performing solid-liquid separation; (4) removing impurities; (5) crystallizing sodium vanadate; and (6) crystallizing sodium chromate. The method is easy to operate and is high in safety; and the operating temperature is greatly lower than the temperature of the traditional vanadium extraction process, energy consumption is low, the high-efficiency co-extraction of vanadium and chromium is realized, and the extraction rate of vanadium and chromium is over 95 percent.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
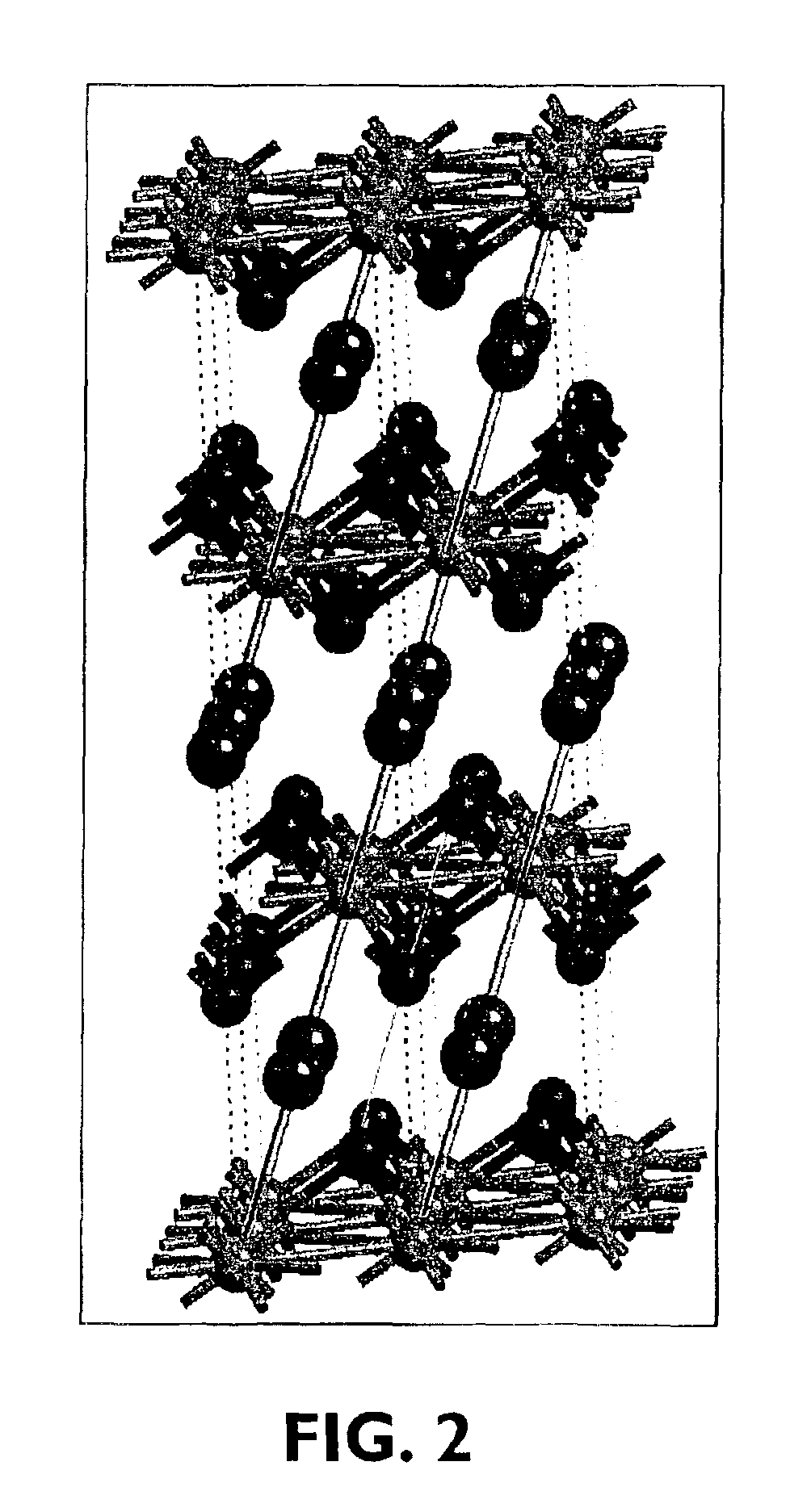
Hydrothermal synthesis of perovskite nanotubes
ActiveUS20050036939A1Reduce the amount requiredThe instrumentation is simpleDigital storageGermanium dioxideStrontium titanateBarium titanate
A low-temperature hydrothermal reaction is provided to generate crystalline perovskite nanotubes such as barium titanate (BaTiO3) and strontium titanate (SrTiO3) that have an outer diameter from about 1 nm to about 500 nm and a length from about 10 nm to about 10 micron. The low-temperature hydrothermal reaction includes the use of a metal oxide nanotube structural template, i.e., precursor. These titanate nanotubes have been characterized by means of X-ray diffraction and transmission electron microscopy, coupled with energy dispersive X-ray analysis and selected area electron diffraction (SAED).
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Method of forming inorganic pigments
The present invention provides a method of forming inorganic pigments using one or more metal alloys. Metal alloys used in the method of the invention are preferably milled to a mean particle size of less than about 10 microns, may be mixed with other metal oxides, and calcined in the presence of oxygen in a rotary kiln. Inorganic pigments formed in accordance with the method of the invention can be used in a wide variety of applications, including the coloration of glass matrixes, ceramic bodies, polymers, and paints.
Owner:FERRO CORP
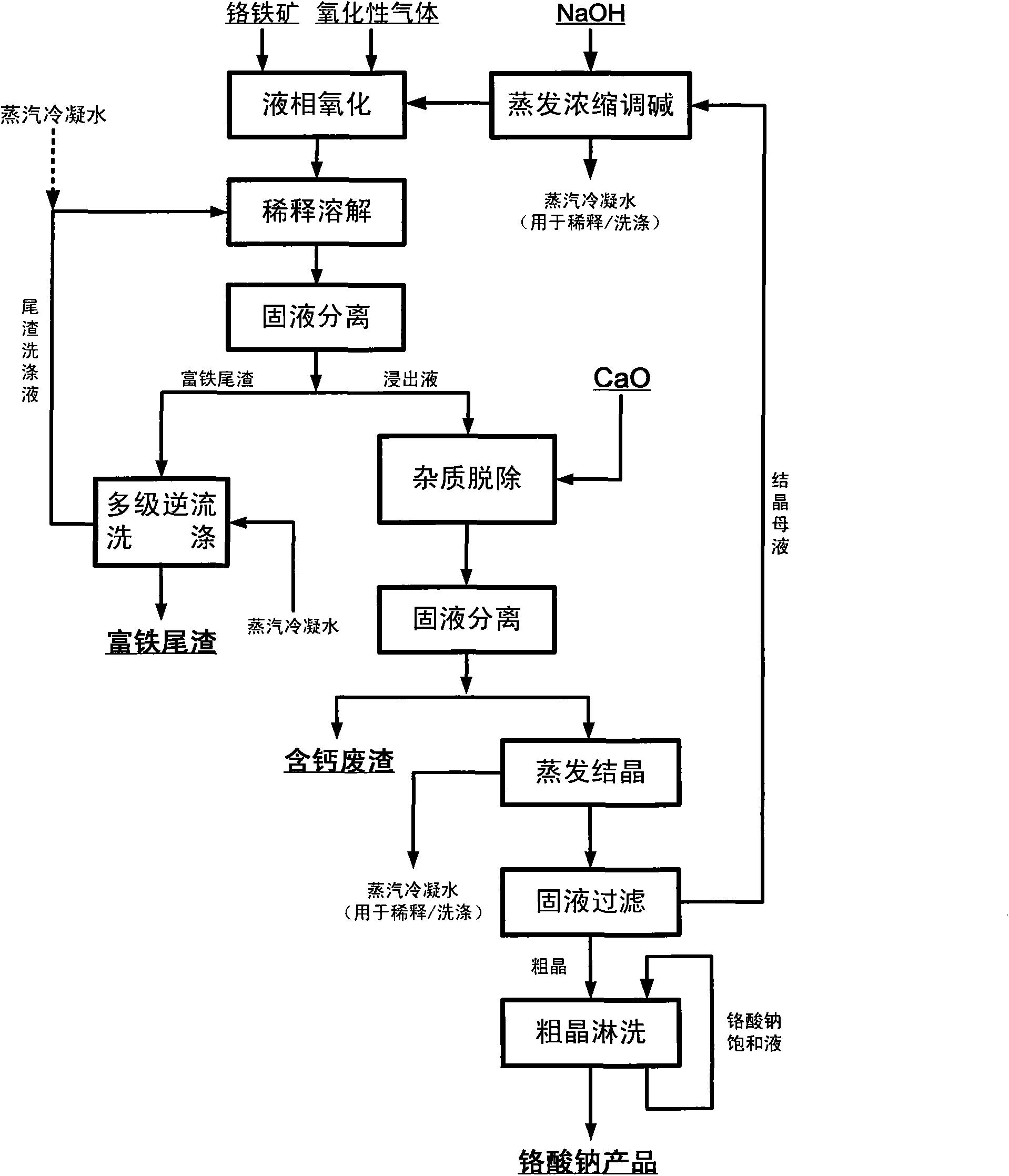
Clean production method for preparing sodium chromate from chromite
InactiveCN101659444AObvious superiorityLow reaction temperatureChromates/bichromatesSlagSodium nitrate
The invention relates to a clean production method for preparing sodium chromate from chromite, which comprises the following steps of: reacting the chromite with an oxidizing gas in a medium of NaOH-NaNO3-H2O, wherein sodium nitrate serves as a catalytic medium and is not consumed in the reaction; obtaining a mixed reaction product of an alkali liquor, the sodium chromate and iron slag after thereaction; leaching the mixed reaction product, performing liquid-solid separation of the alkali liquor and a crystal slag mixture (a mixture of a sodium chromate crystal and the iron slag), dissolvingthe mixture of the iron slag and the sodium chromate crystal, performing liquid-solid separation of the solution of the iron slag and the sodium chromate crystal, performing evaporative crystallization of the sodium chromate solution, rinsing and drying the obtained sodium chromate solution, obtaining qualified sodium chromate products; and circulating the crystallized mother liquor and the alkali liquor together to decompose the chromite. In the method, the chromium conversion ratio is above 99 percent and the chromium content ratio in the slag is less than 0.5 percent.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
High-energy-density Ni-Co-based lithium ion positive electrode material and preparation method thereof
The invention discloses a high-energy-density Ni-Co-based lithium ion positive electrode material. The chemical general formula of a base material is LipNixCo1-xMmO2, M is a doping agent, and a clad material is an active material N; a positive electrode material of a lithium ion secondary battery is composed of second particles formed by gathering of primary particles, or primary particles, or mixed particles of primary particles and second particles. A preparation method of the high-energy-density Ni-Co-based lithium ion positive electrode material comprises the steps of preparation of a precursor of the lithium ion secondary battery positive electrode material and preparation of the lithium ion secondary battery positive electrode material. The Ni-Co binary precursor of the positive electrode material is subjected to a continuous coprecipitation reaction, elements are evenly mixed, the reaction is sufficient, and morphology control is facilitated; the cation mixing phenomenon is reduced through doping of proper elements in a binary high-nickel material, the structure is stabilized, safety and high-temperature performance of the battery material are improved, and the cladding active material improves first-time charge and discharge efficiency and the energy density of the material to a certain extent.
Owner:NANTONG RESHINE NEW MATERIAL
Metal oxide and metal fluoride nanostructures and methods of making same
InactiveUS20070113779A1Easy to controlOvercomes shortcomingMercury oxidesAlkali metal oxides/hydroxidesSingle crystalNanostructure
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Hydrothermal synthesis of perovskite nanotubes
ActiveUS7147834B2The instrumentation is simpleReduce the amount requiredNanoinformaticsDigital storageStrontium titanateBarium titanate
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Lithium titanate cell with reduced gassing
InactiveUS20110067230A1Sufficient durationAvoid decompositionAlkali titanatesFinal product manufactureElectrolyteOperating voltage
A method of manufacturing a lithium cell is disclosed. The method can include providing a lithium cell having an operating voltage range, where the lithium cell includes a negative electrode, a positive electrode, and an electrolyte in contact with, and between, the negative electrode and the positive electrode. The negative electrode can include lithium titanate and the electrolyte can include an additive. The method can also include reducing the additive to form a coating on a surface of the negative electrode in contact with the electrolyte. The reducing step can include overcharging the lithium cell to a voltage greater than an upper limit of the operating voltage range and dropping a voltage of the negative electrode to 0.2-1V vs. lithium.
Owner:ENERDEL
Method for pollution-free production of sodium chromate by pressure leaching of chromite
ActiveCN101817561AObvious superioritySimple ingredientsChromates/bichromatesReaction temperatureHydrometallurgy
The invention belongs to the field of chromite hydrometallurgy and chromium chemical industry, and in particular relates to a method for the pollution-free production of sodium chromate by pressure leaching of chromite. The method comprises the following steps of: 1) reacting the chromite with oxidizing gas in solution of NaOH; 2) diluting the product obtained by the step 1) and making subcrystalline sodium chromate to fully enter a liquid phase; 3) performing solid-liquid separation on the solid-liquid mixed slurry obtained by the step 2); 4) adding calcium oxide into the obtained diluent for removing impurities; and 5) evaporating and crystallizing the obtained solution without the impurities to obtain a sodium chromate crystal and crystallization mother solution; after the solid-liquid separation, rinsing the sodium chromate crystal by using saturated solution of sodium chromate; and drying to obtain a qualified sodium chromate product. The method has the advantages of simple reaction system component, no difficultly separated phase introduced in the system, contribution to high-efficiency separation of the sodium chromate, great reduction in reaction temperature, low energy consumption, effective reduction in production cost of the sodium chromate, and high chromium leaching yield.
Owner:HUBEI ZHENHUA CHEMICAL CO LTD
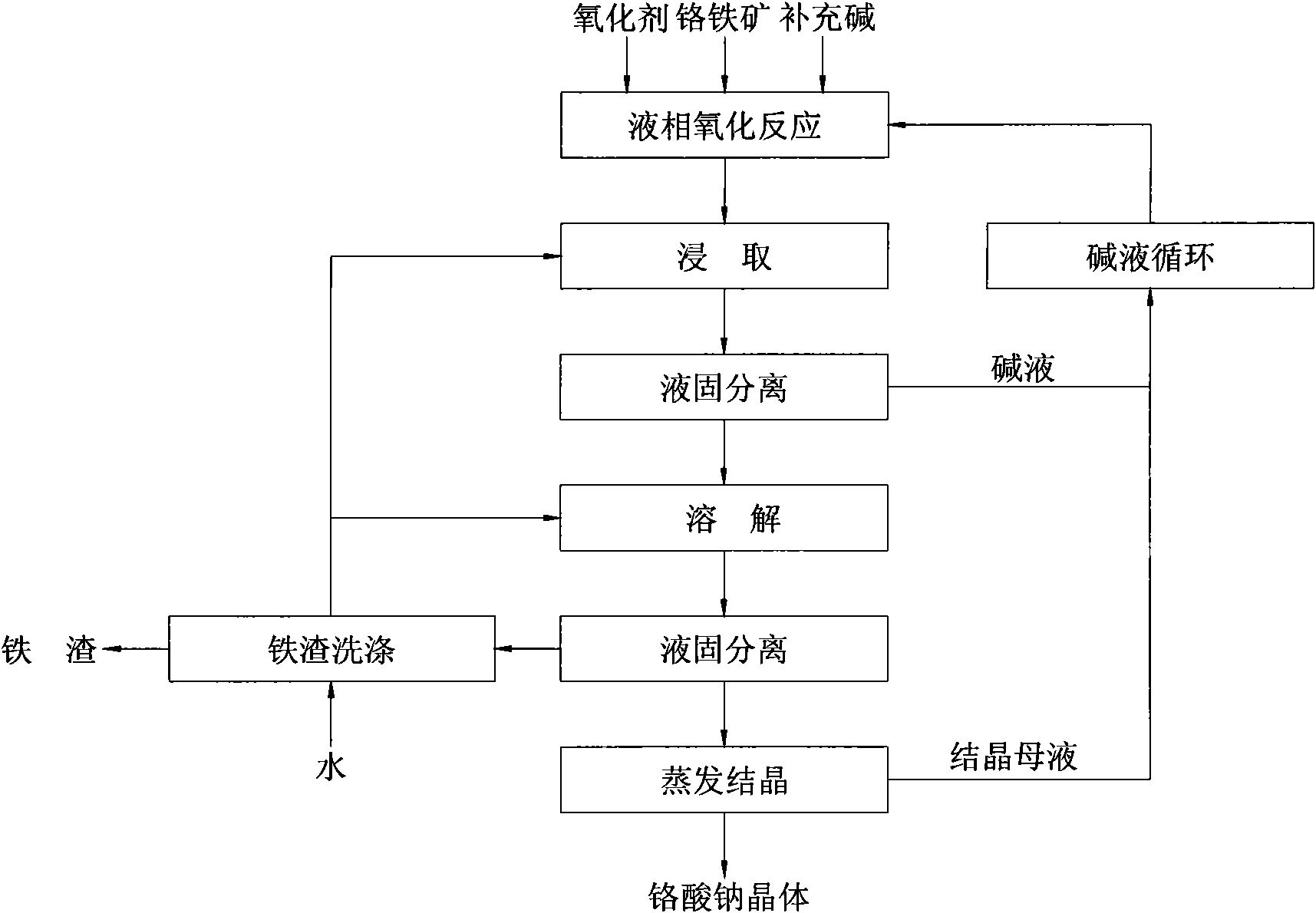
High-efficiency, energy-conservation and clean method for producing chromate
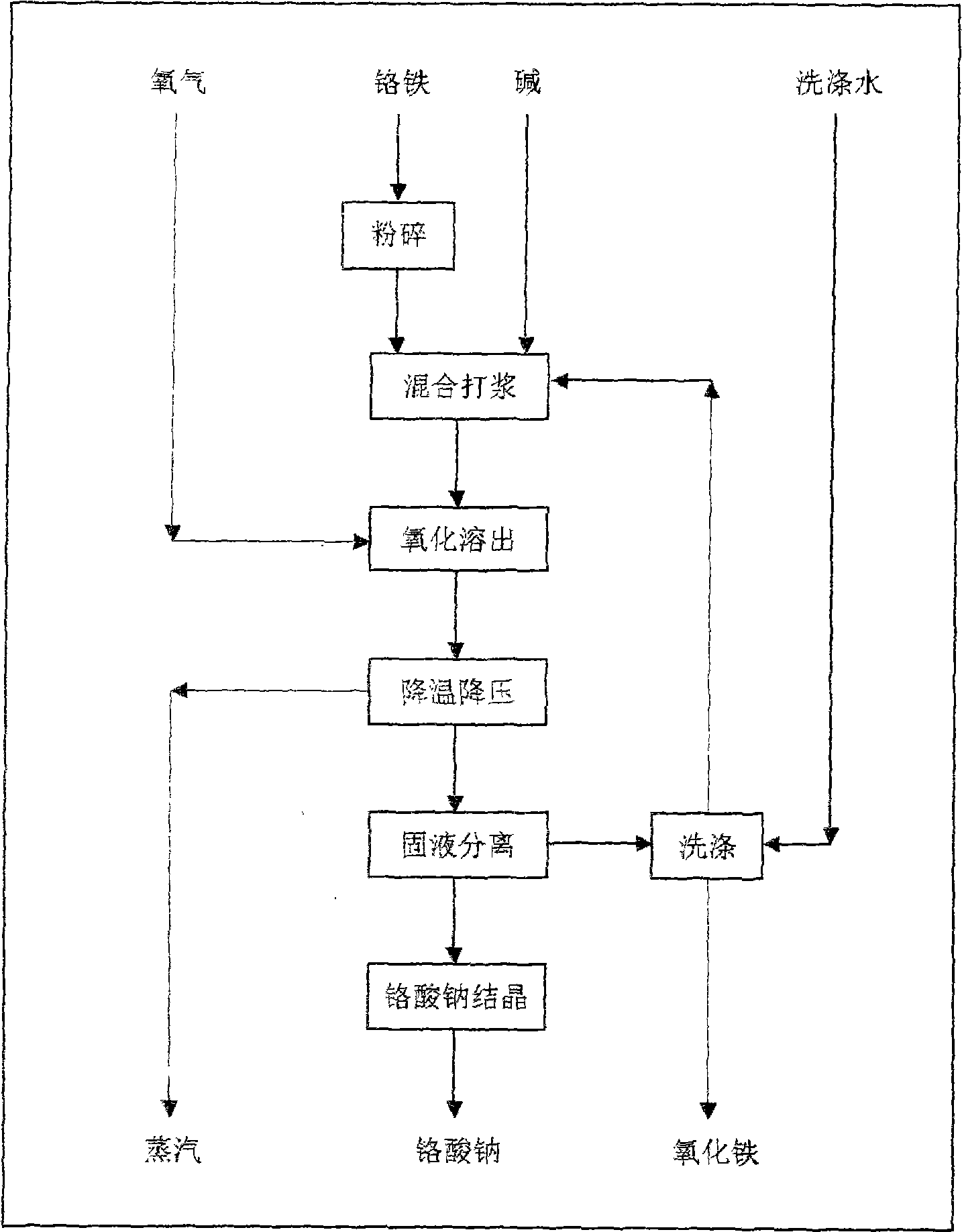
ActiveCN101508466ASolve pollutionAchieving the purpose of cleaner productionChromates/bichromatesWastewaterCleaning methods
The invention provides a method for producing chromate with high efficiency, energy conservation and cleanness. The method uses chromium iron as a raw material, and carries out oxidation dissolution in high-temperature high-pressure aqueous alkali to separate solid and liquid after temperature and pressure reduction so as to obtain a chromate solution, water and ferric oxide or mixture of the water, ferric oxide and chrome oxide. A high-temperature high-pressure reactor is used as the reactor of the method so as to greatly improve equipment efficiency and production efficiency. The method realizes autothermal reaction by system reaction heat to achieve the aim of energy conservation, and is an energy-saving process of chromate production. The method does not need external fuel to supply heating and does not generate waste gas, and the reaction precipitate is the water and the ferric oxide or the mixture of the water, the ferric oxide and the chrome oxide, wherein the water and the ferric oxide or the mixture of the water, the ferric oxide and the chrome oxide can be used for preparing chromium iron paint without generating waste residue; and washing of the precipitate adopts countercurrent washing, and washing water is used for preparing a reaction initial solution without generating waste water. The method does not generate the three wastes, and is a clean process for producing the chromate.
Owner:青海省博鸿化工科技股份有限公司
Process for simultaneous recovery of chromium and iron from Chromite Ore Processing Residue
InactiveUS20040086438A1Simple eco-friendlySolvent extractionMolybdeum compoundsIron saltsChromate salt
The present invention relates to a process for simultaneous recovery of chromium and iron from Chromite Ore Processing Residue (COPR) and more particularly, the present invention relates to an economical and environment-friendly process for recovering chromium as a chromate salt and iron as an iron salt from non-leachable Chromite Ore Processing Residue and avoids landfilling of toxic metals.
Owner:COUNCIL OF SCI & IND RES
Conversion of alkanes to oxygenates
Owner:MARATHON OIL CO
Titania fiber, method for producing the fiber and method for using the fiber
InactiveUS6191067B1Improve spinning stabilityHigh mechanical strengthAluminium compoundsOther chemical processesFiberPore diameter
A continuous fiber of titania are made having an average diameter per a monofilament of from 5 to 50 mum, which has a BET specific surface area of 10 m2 / g or more, a pore volume of 0.05 cc / g or more, a volume of pores having a pore diameter of not less than 10 angstroms being 0.02 cc / g or more and an average tensile strength per a monofilament of 0.1 GPa or more, or which has an average tensile strength per a monofilament of 0.5 GPa or more.
Owner:SUMITOMO CHEM CO LTD
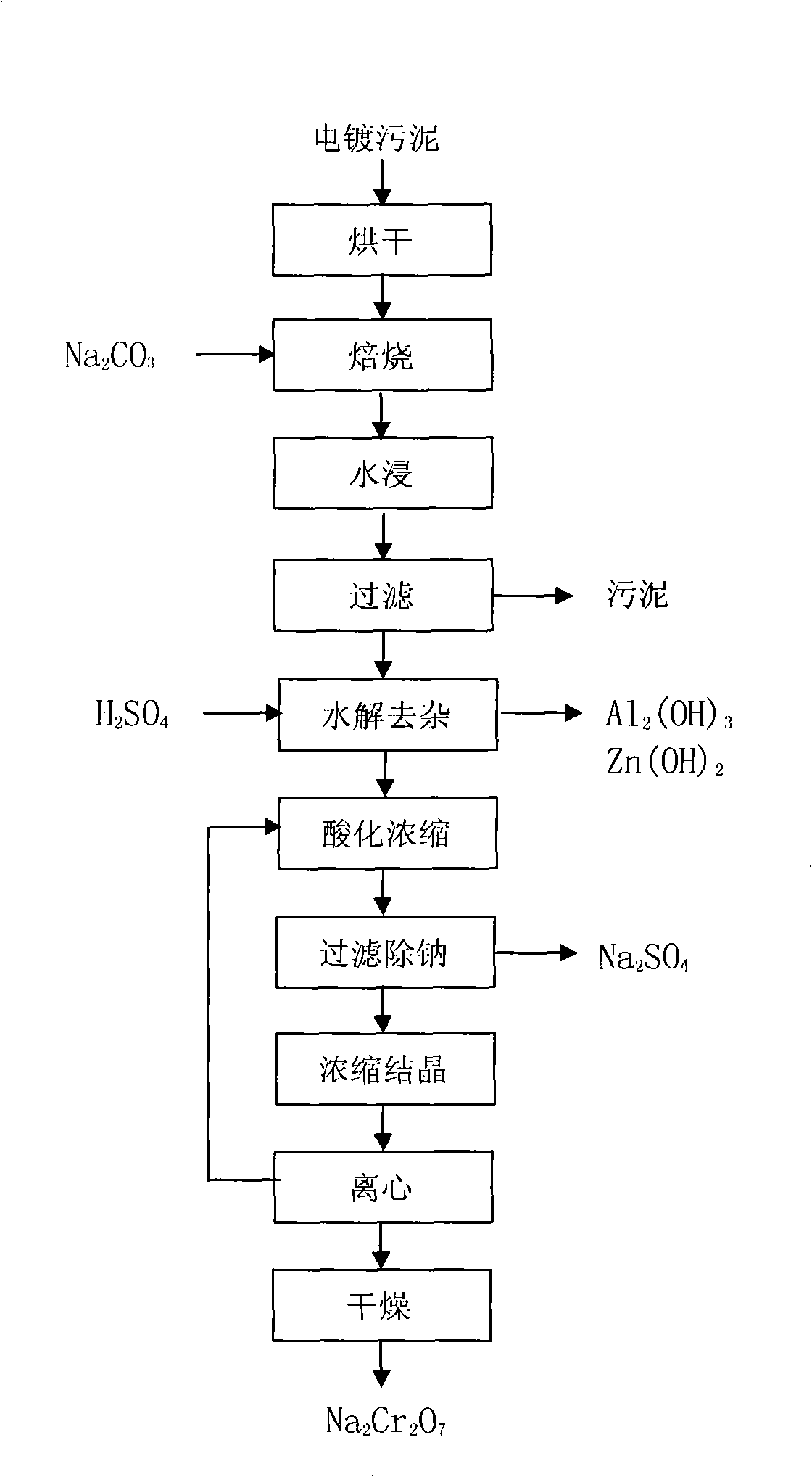
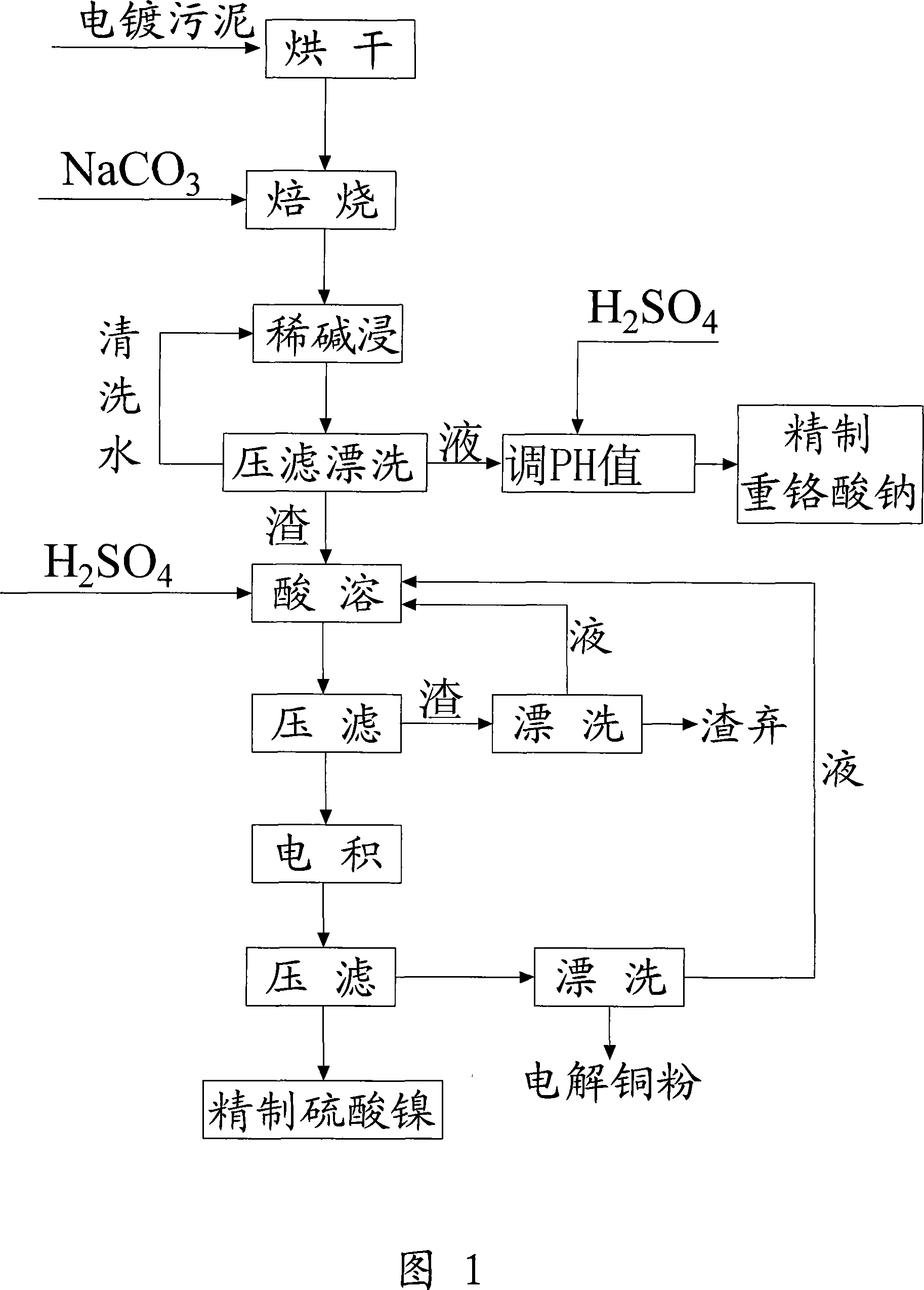
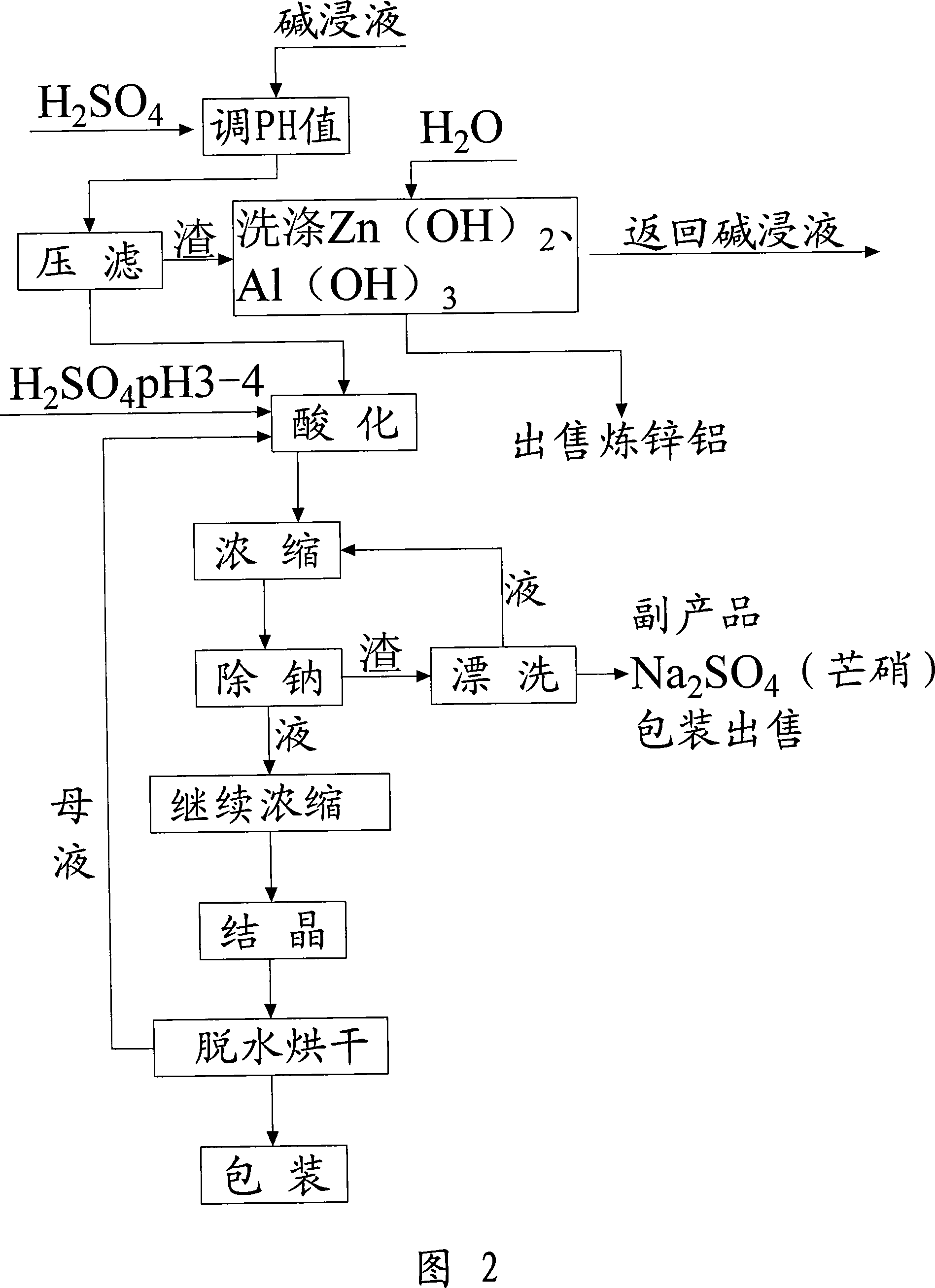
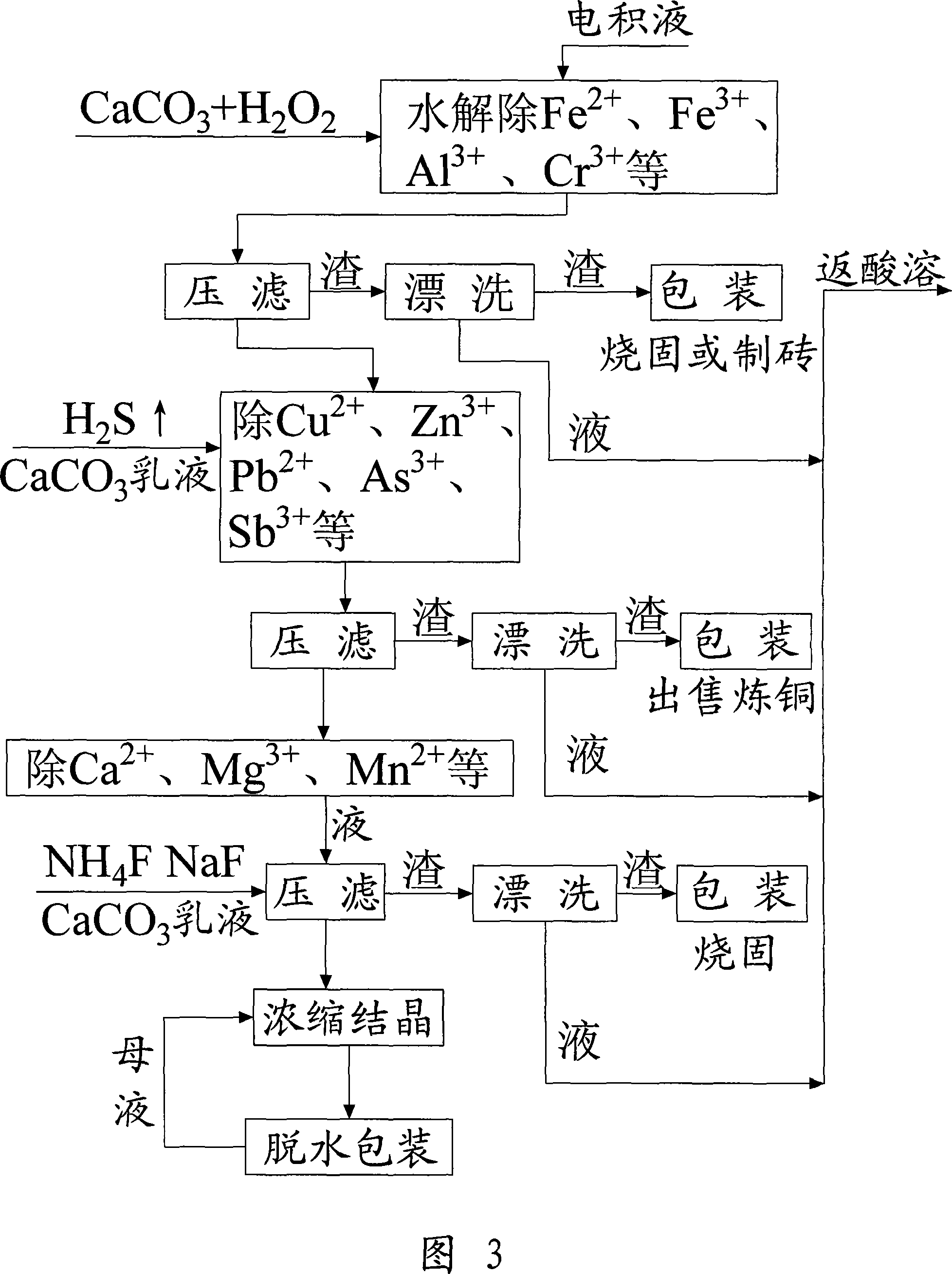
Method for recovering sodium dichromate form chromium-containing electroplating sludge
InactiveCN101333007AImprove recycling efficiencyOvercome the disadvantages of secondary pollutionSludge treatmentChromates/bichromatesResource utilizationSludge
The invention discloses a method for recovering sodium acid chromate from chrome-containing electroplating sludge. The electroplating sludge is blended with Na2CO3 according to a certain proportion and is baked at a certain temperature to obtain Na2CrO4, Cr<3+> is oxidized into Cr<6+> chrome and aluminum and zinc are taken as corresponding oxides; Cr, Al and Zn are dissolved in liquid by water soaking to generate respective salt, and the solid containing the metals such as Ni, Cu, Fe, Ca, Mg, and the like, is filtered and separated; the Na2CrO4 solution is transformed by hydrolysis acidification and Al2(OH)3 and Zn(OH)2 are removed to realize the separation of Zn, Al and Cr; the chrome-containing solution is acidated into sodium acid chromate (Na2Cr2O7), cooled after being concentrated to a certain volume, and filtered to remove sodium sulfate; the sodium acid chromate solution is processed by condensation, crystallization, centrifugation and drying to obtain sodium acid chromate finished product. By adopting sodium treatment and oxidation process, the rate of recovering chrome from chrome-containing electroplating sludge can exceed 90 %, and the resource utilization of electroplating sludge can be realized.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
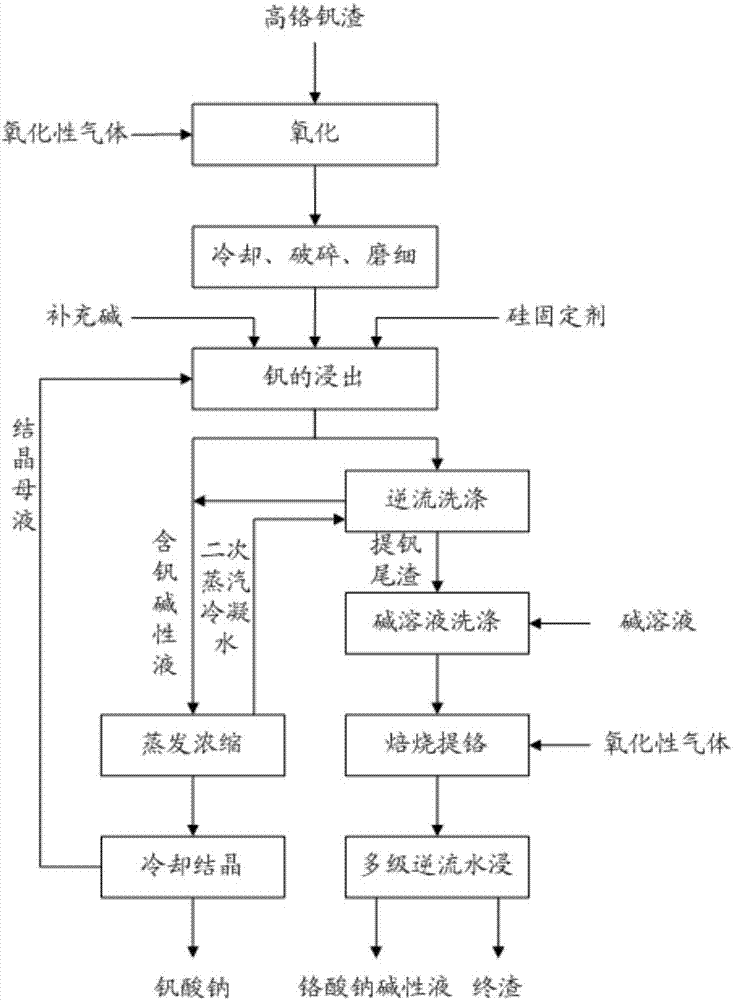
Cleaning process for producing sodium vanadate and sodium chromate alkali solution by high chromium vanadium slag
ActiveCN103757425AImprove resource utilizationImprove separation rateChromates/bichromatesVanadium compoundsSlagSilicon
The invention relates to a cleaning process for producing sodium vanadate and sodium chromate alkali solution by high chromium vanadium slag. The method provided by the invention comprises the main steps of: introducing oxidizing gas into the high-temperature high chromium vanadium slag separated from molten iron to oxidize vanadium into pentavalent vanadium; leaching vanadium out of the high chromium vanadium slag with an alkali solution, adding a silicon fixing agent for synchronized fixing of silicon, evaporating and concentrating the alkali leaching solution, cooling and crystallizing to prepare sodium vanadate, and reusing the cooling crystallization mother liquor; and conducting immersion cleaning and mixing on the vanadium-extracted tailings subjected to multistage countercurrent washing with alkali solution with a certain concentration, carrying out a roasting reaction in roasting equipment, and immersing reaction clinker with water and filtering to obtain an alkali solution of sodium chromate and the final slag. The method has the advantages of simple process, strong operability, recovery rate of vanadium greater than 98%, recovery rate of chromium more than 95% and separation rate of vanadium and chromium greater than 99%, thereby achieving efficient extraction and separation of vanadium and chromium.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
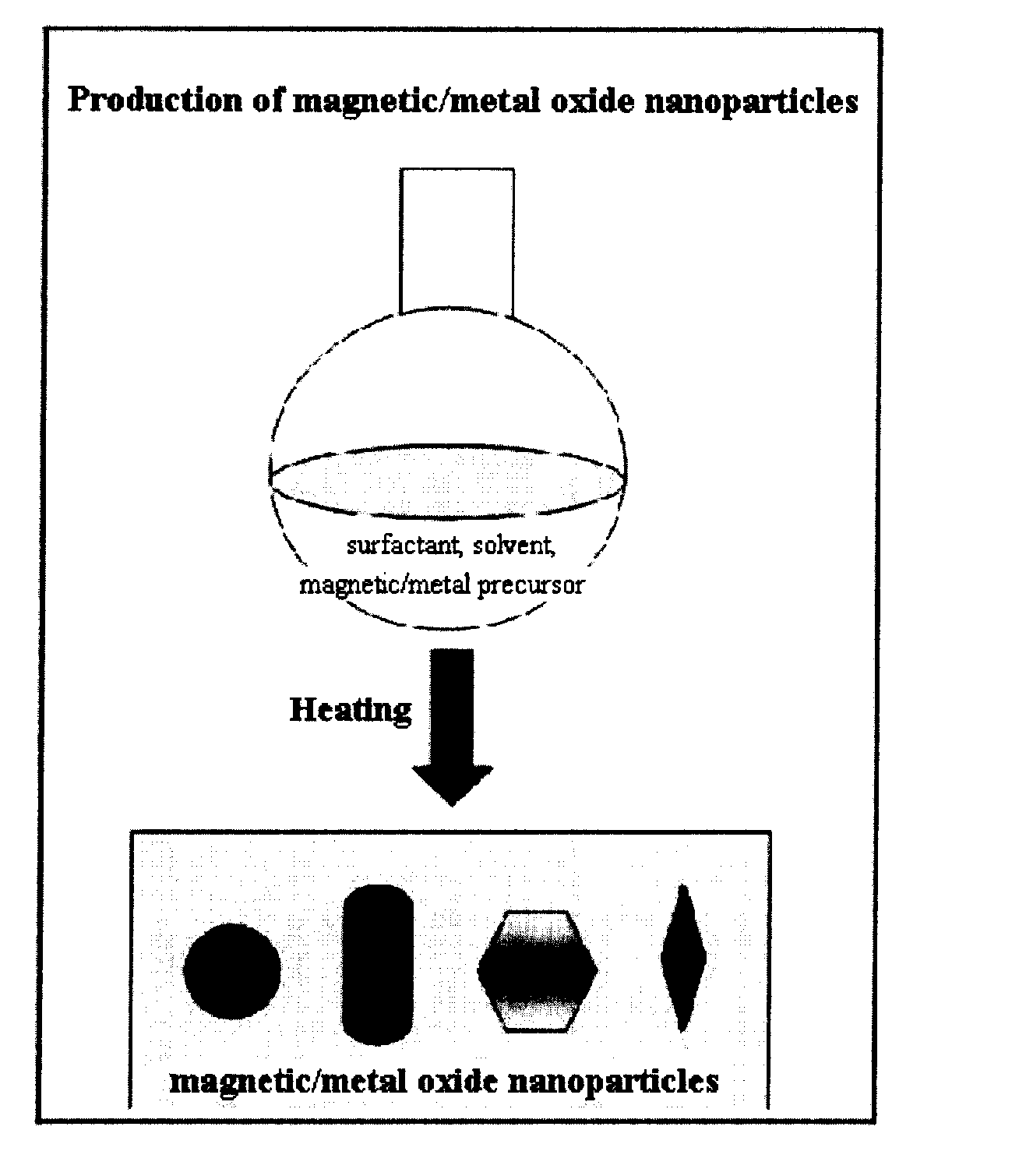
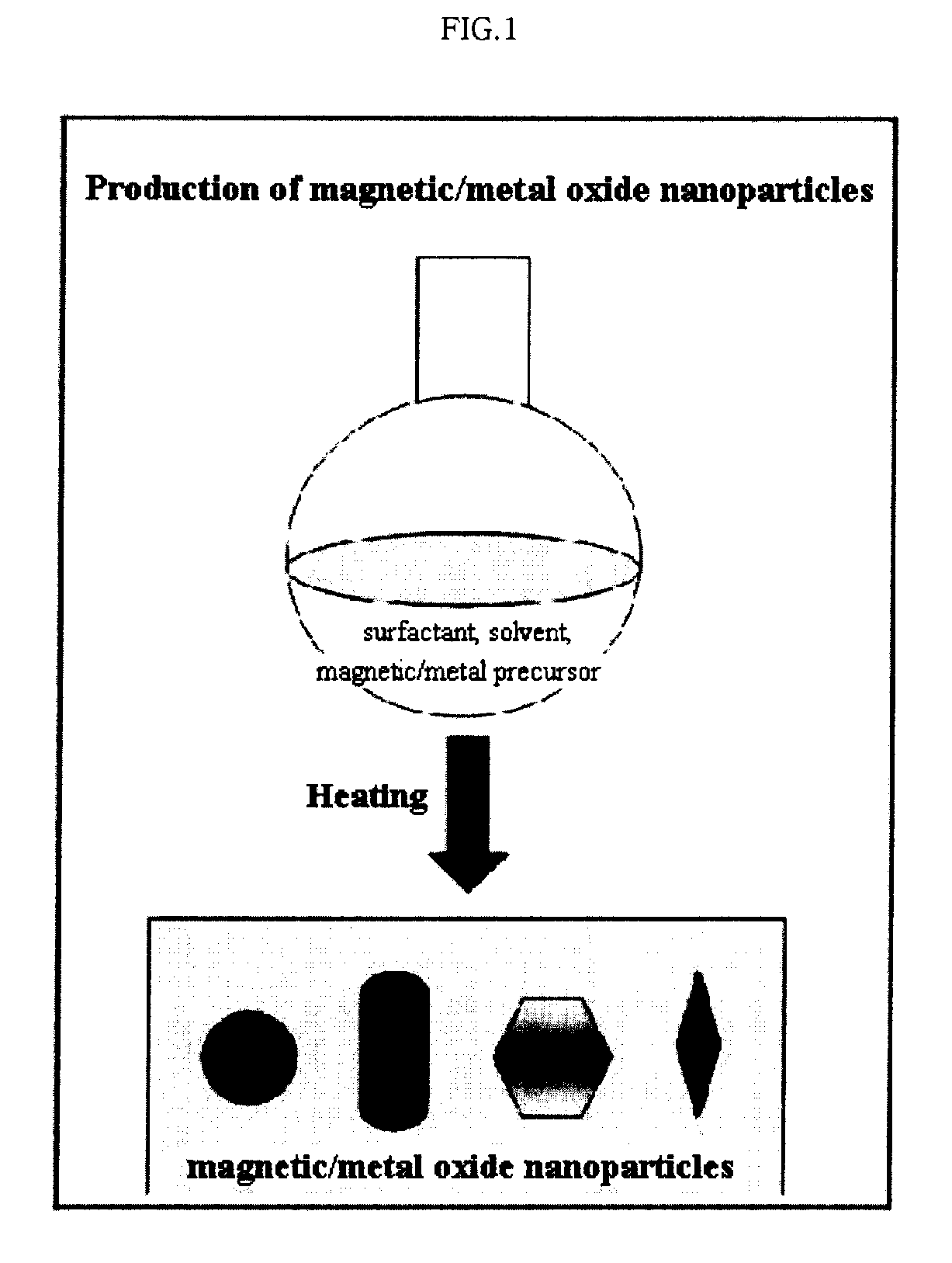
Preparation Method of Magnetic and Metal Oxide Nanoparticles
ActiveUS20080003159A1Efficient mass productionUniform shapeCopper oxides/halidesManganese oxides/hydroxidesMetal oxide nanoparticlesMagnetic oxide
This invention relates, in general, to a method of producing magnetic oxide nanoparticles or metal oxide nanoparticles and, more particularly, to a method of producing magnetic or metal oxide nanoparticles, which comprises (1) adding a magnetic or metal precursor to a surfactant or a solvent containing the surfactant to produce a mixed solution, (2) heating the mixed solution to 50-6001 C to decompose the magnetic or metal precursor by heating so as to form the magnetic or metal oxide nanoparticles, and (3) separating the magnetic or metal oxide nanoparticles. Since the method is achieved through a simple process without using an oxidizing agent or a reducing agent, it is possible to simply mass-produce uniform magnetic or metal oxide nanoparticles having desired sizes compared to the conventional method.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Lithium-manganese composite oxide granular secondary particle, method for production thereof and use thereof
ActiveUS20050123832A1Improve output characteristicsMaintain good propertiesAluminium compoundsLithium compoundsMicrometerCompound (substance)
The invention provides granular secondary particles of a lithium-manganese composite oxide which are granular secondary particles made up of aggregated crystalline primary particles of a lithium-manganese composite oxide and have many micrometer-size open voids therein, the open voids having an average diameter in the range of from 0.5 to 3 μm and the total volume of the open voids being in the range of from 3 to 20 vol. % on average based on the total volume of the granules. These particles are suitable for use as a constituent material for non-aqueous electrolyte secondary batteries showing high-output characteristics. The invention further provides a process for producing the granular secondary particles of a lithium-manganese composite oxide which includes spray-drying a slurry prepared by dispersing a fine powder of a manganese oxide, a lithium source, an optional compound containing at least one element selected from Al, Co, Ni, Cr, Fe, and Mg, and an agent for open-void formation to thereby granulate the slurry and then calcining the granules at a temperature of from 700 to 900° C.
Owner:TOSOH CORP
Perovskite-based fuel cell electrode and membrane
InactiveUS7504172B2Lower cost of capitalReduce energy costsCell electrodesFinal product manufactureSyngasFuel cells
The present invention provides a material suitable for use in a solid oxide fuel cell, wherein the material is of an, optionally doped, double perovskite oxide material having the general formula (I): (LnaXb)e(Z1cZ2d)fOg (I) wherein Ln is selected from Y, La and a Lanthanide series element, or a combination of these and X also represents an element occupying the A site of a perovskite oxide and is selected from Sr, Ca and Ba, and Z1 and Z2 represent different elements occupying the B site of a perovskite oxide and are selected from Cr, Mn, Mg and Fe, and wherein a has a value from 0 to 1, preferably 0.7 to 1.0, b has a value of from 1 to 0, preferably 0.3 to 0, and each of c and d has a value of from 0.25 to 0.75, provided that a+b has a value of 1, and c+d, has a value of 1, and wherein e has a value of from 0.8 to 1, wherein f has a value of from 0.8 to 1, and g has a value of from 2.5 to 3.2. Also provided are SOFCs having an electrode or functional layer of a material or containing a material of the invention, as well as mixed ionic / electronic conducting membranes suitable for use in a syngas reactor or oxygen separator, comprising a layer of a double perovskite material of the invention, and a method of oxidising a fuel in an SOFC having an anode of a double perovskite material of the invention.
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
Electroplating sludge treating and utilizing process
InactiveCN101066827ASimple and easy to purifyEasy easy yieldSludge treatment by de-watering/drying/thickeningWaste water treatment from metallurgical processNickel saltSlag
The electroplating sludge treating and utilizing process includes the following steps: 1. adding alkali to stoved electroplating sludge, roasting, leaching out soluble metal in dilute alkali solution, and filtering to obtain alkali leached slag and alkali leached liquid; 2. adding acid to the alkali leached liquid, filtering to obtain hydrolyzed slag and hydrolyzed liquid, and preparing bichromate with the hydrolyzed liquid; and 3. dissolving the alkali leached slag in acid solution, filtering, electrodepositing the filtrate and filtering to obtain electrodeposited solution, depurating the electrodeposited solution and crystallizing to obtain nickel salt. The process has low power consumption and low cost, and can convert most of Cr, Cu and Ni in electroplating sludge into bichromate, nickel salt, copper powder and other side product.
Owner:台州盛世环境工程有限公司
Clean production method for preparing potassium chromate from chromic iron
InactiveCN101481144AImprove liquidityReduced corrosion resistance requirementsChromates/bichromatesForeign matterPotassium nitrate
The invention belongs to the field of a method for producing chromic salt, in particular relates to a method for producing and cleaning potassium chromate. The method comprises the steps that chromite is reacted with oxidant with a chemical dose inside KOH-KNO3-H2O medium to obtain a mixed resultant of reaction containing alkali liquor, potassium chromate and slag after reaction, wherein the mass ratio of KOH and chromite is 1:1 to 2:1, and the mass ratio of KNO3 and chromite is 0.5:1 to 2:1. Through the leaching of the mixed resultant of reaction, the phases of potassium chromate macrocrystal-alkali liquor containing potassium nitrate-slag are separated, foreign matters in the solution are removed, the mixture is evaporated and crystallized, the obtained potassium chromate crystal is eluted and the pure product of potassium chromate is obtained. The percent conversion of chromium is higher than 99 percent and the rate of containing chromium in slag is less than 0.2 percent.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Mesoporous mixed oxide materials as a new class of SO2 resistant catalysts for hydrocarbon oxidation
InactiveUS7132093B2Large specific surface areaGood dispersionGold compoundsMercury oxidesCeriumMesoporous silica
The oxide materials are of the class of ternary mesoporous mixed oxide materials including lanthanum, a metal M selected from the group consisting of Cr, Mn, Fe, Co, Ni, Cu and Zn, and zirconium or cerium such a mesoporous La—Co—Zr mixed oxide material designated as Meso LCZ[x] where x is the atomic ratio (La+Co) / La+Co+Zr. They are useful as catalysts since they show high activities for hydrocarbon oxidation and good resistance against poisoning agents. These highly ordered mesoporous mixed oxides are synthesized by: preparing an amorphous solution of a La-M precursor and adding a salt of zirconium or cerium thereto; acidifying the amorphous solution in the presence of a surfactant under conditions to obtain a clear homogeneous solution; adjusting pH of the solution under conditions to form a solid precipitate; separating the solution and surfactant from the precipitate; and calcinating the precipitate.
Owner:UNIV LAVAL
Process for roasting chromite resources in ring kiln through pure oxygen by using low-temperature method and harmlessly and deeply utilizing chromium residue
InactiveCN101824530AImprove resource conversion rateMagnesium carbonatesChromium trioxideSodium bicarbonateSlag
The invention belongs to the field of metallurgy and chemical engineering. The process comprises the following steps of: firstly, crushing chromite, adding sodium hydroxide and a catalyst to be oxidized and roasted by using a low-temperature pure oxygen method; diluting, cooling, extracting and filtering to obtain a sodium chromate crystal and ferrum-magnesium slag; adding an alkali washing solution into a sodium hydroxide solution to back extract to obtain the sodium hydroxide solution for recycling; adding water into the sodium chromate crystal and ferrum-magnesium slag to be dissolved and feeding filtrate into a carbonizer to decompose to extract aluminum; carbonizing, evaporating, condensing and crystallizing the extracted solution to obtain sodium chromate; and carbonizing ferrum-magnesium filter slag to generate sodium bicarbonate, reacting to generate a magnesium hydrogen carbonate solution, heating and cracking to generate a magnesium carbonate product and drying a filter cake to obtain ore refined powder; and secondly, crushing chromium residue, adding sodium bicarbonate in the ration of 1:8, adding a catalyst for calcination, cooling and adding water to soak; adding an aluminum hydroxide crystal into supernatant liquid, carbonizing and decomposing to remove aluminum in a reaction tank; filtering and washing an aluminum hydroxide product; adding a reducing agent into the filtrate to reduce hexavalent chromium to generate anhydrous chromium hydroxide and drying and roasting to obtain chromium sesquioxide; and returning the filtrate to a system for mixing after pyrolyzing and extracting to remove magnesium.
Owner:白向南 +2
Roasting method for producing sodium chromate using chrome iron ore as raw material
InactiveCN1418823AAvoid scaringSolve the pollution of the environmentChromates/bichromatesBrucitePericlase
The method for preparing sodium chromate by calcining chromite includes the following steps: pulverizing chromite and sieving with 200 mesh sieve, adding sodium carbonate whose added amount is 80-150% of chromite amount, and adding magnesite, baudisserite, periclase, brucite or synthetic magnesium oxide, magnesium hydroxide and magnesium carbonate and calcining them at 1000-1200 deg.c for 10-60 min. under the condition of oxidation atmosphere, then extracting clinker and dissolving sodium chromate so as to obtain the goal of preparing sodium chromate. The conversion rate of chromite can be upto 97-99.5%.
Owner:TIANJIN CHEM RES & DESIGN INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com