Mesoporous mixed oxide materials as a new class of SO2 resistant catalysts for hydrocarbon oxidation
a technology of mixed oxide materials and hydrocarbon oxidation, which is applied in the direction of catalyst activation/preparation, alkali metal oxides/hydroxides, metal/metal-hydroxide catalysts, etc., can solve the problems of low specific surface area of materials and limited potential applications as catalysts, and achieve high catalyst activity, high specific surface area, and high component dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0047]This example concerns the preparation of mesoporous La—Co—Zr mixed oxide materials with various atomic (La+Co / La+Co+Zr) ratios (designated as Meso LCZ[x] where x=(La+Co) / La+Co+Zr). They are prepared from amorphous La—Co citrate complex precursor as La and Co source, zirconium sulfate as Zr source, and cetyltrimethylammonium bromide (CTAB) as the surfactant.
[0048]The La—Co precursor was prepared according to Baythoum and Sale (Baythoun, M. S. G. and Sale, F. R., J. Mat. Sci., 17, 1982, 2757). In a typical synthesis, for the Meso-LCZ material with x=0.5, the La—Co citratè complex precursor was prepared from 1.65 g of lanthanum nitrate, La(NO3)3.6H2O, 1.45 g of cobalt nitrate, Co(NO3)2.6H2O (atomic Co / La=1), and 1.92 g of citric acid. 7.0 g of CTAB was dissolved in 120 g of distilled water and 30 g of HCl 10%. Then, the La—Co citrate complex precursor and 3.55 g of zirconium sulfate dissolved in 100 g of distilled water were added, giving a clear homogeneous solution.
[0049]The mi...
example 2
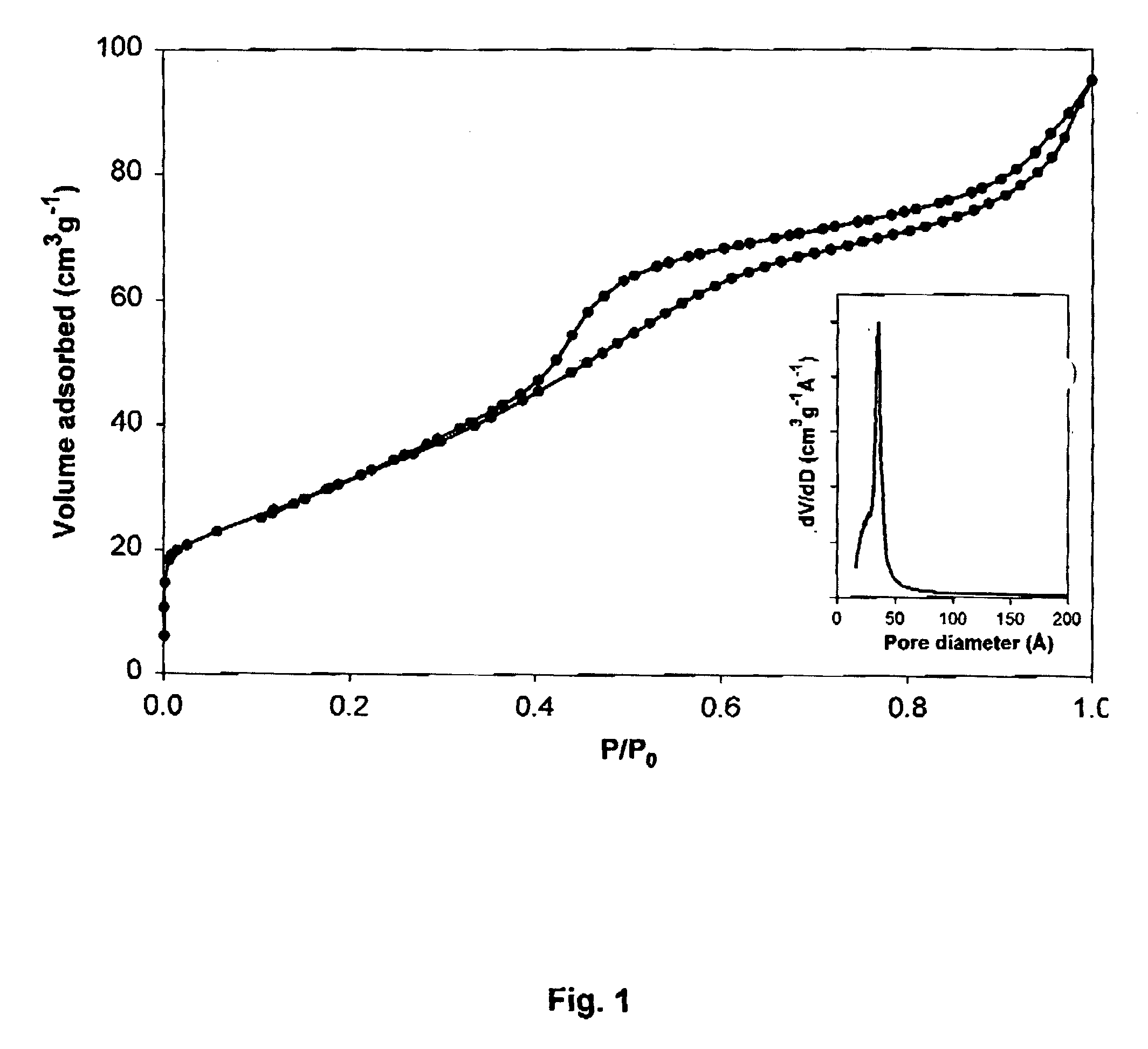
[0053]Preparation of mesoporous La—Mn—Zr mixed oxide materials with an atomic (La+Mn / La+Mn+Zr) ratio of 0.5 (designated as Meso LMZ[0.5]). The synthesis was performed operating under the conditions described in example 1, except that manganese nitrate was used instead of cobalt nitrate for the preparation of an amorphous La—Mn citrate complex precursor. FIG. 6 shows the N2 adsorption / desorption isotherms of the Meso LMZ[0.5] sample after calcination at 400° C. for 8 h. It exhibits the typical behaviour of mesoporous molecular sieves. The specific surface area and pore volume were 205 m2 / g and 0.115 cm3 / g, respectively. The inset in FIG. 6 shows the BJH pore size distribution. The narrow pore size distribution with a pore diameter of about 22.5 Å indicates the textural uniformity of the materials. Table 2 also summarizes the physico-chemical properties of the Meso LMZ[0.5] sample after calcination for 8 h at different temperatures. The pore size diameters are substantially enlarged w...
example 3
[0054]This example illustrates the preparation of mesoporous La—Co—Zr mixed oxide materials with an atomic (La+Co / La+Co+Zr) ratio of 0.5 (namely Meso LCZ[0.5]) using metal acetate precursor as Co and La sources. The synthesis was also performed operating under the conditions described in example 1 except that cobalt acetate and lanthanum acetate were used instead of amorphous La—Co citrate complex precursor and no citric acid was added. The Meso LCZ[0.5] sample was also calcined in air at different temperatures (e.g. 400, 500 and 600° C.) for 8 h. The N2 adsorption / desorption isotherms of these samples were also carried out and the results are summarized in Table 3. Similar trends were observed for this Meso LCZ[0.5] sample as compared to the Meso LMZ[0.5] sample after calcination at different temperatures.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com