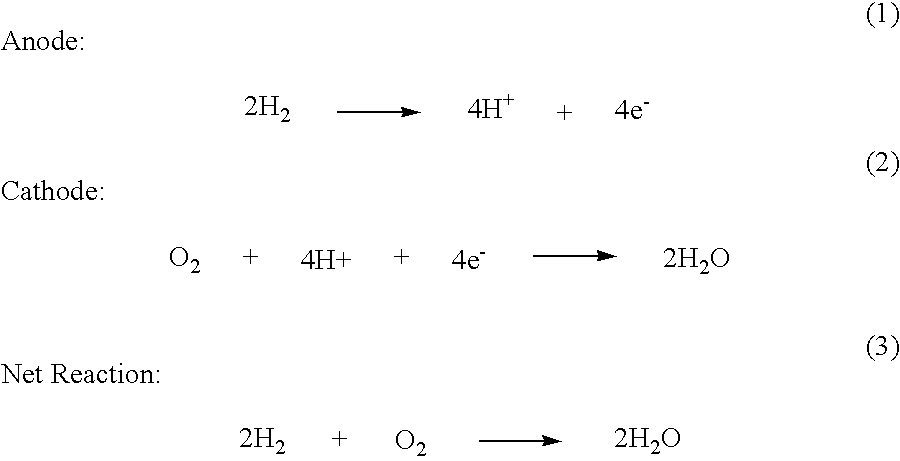Patents
Literature
565results about "Gold compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
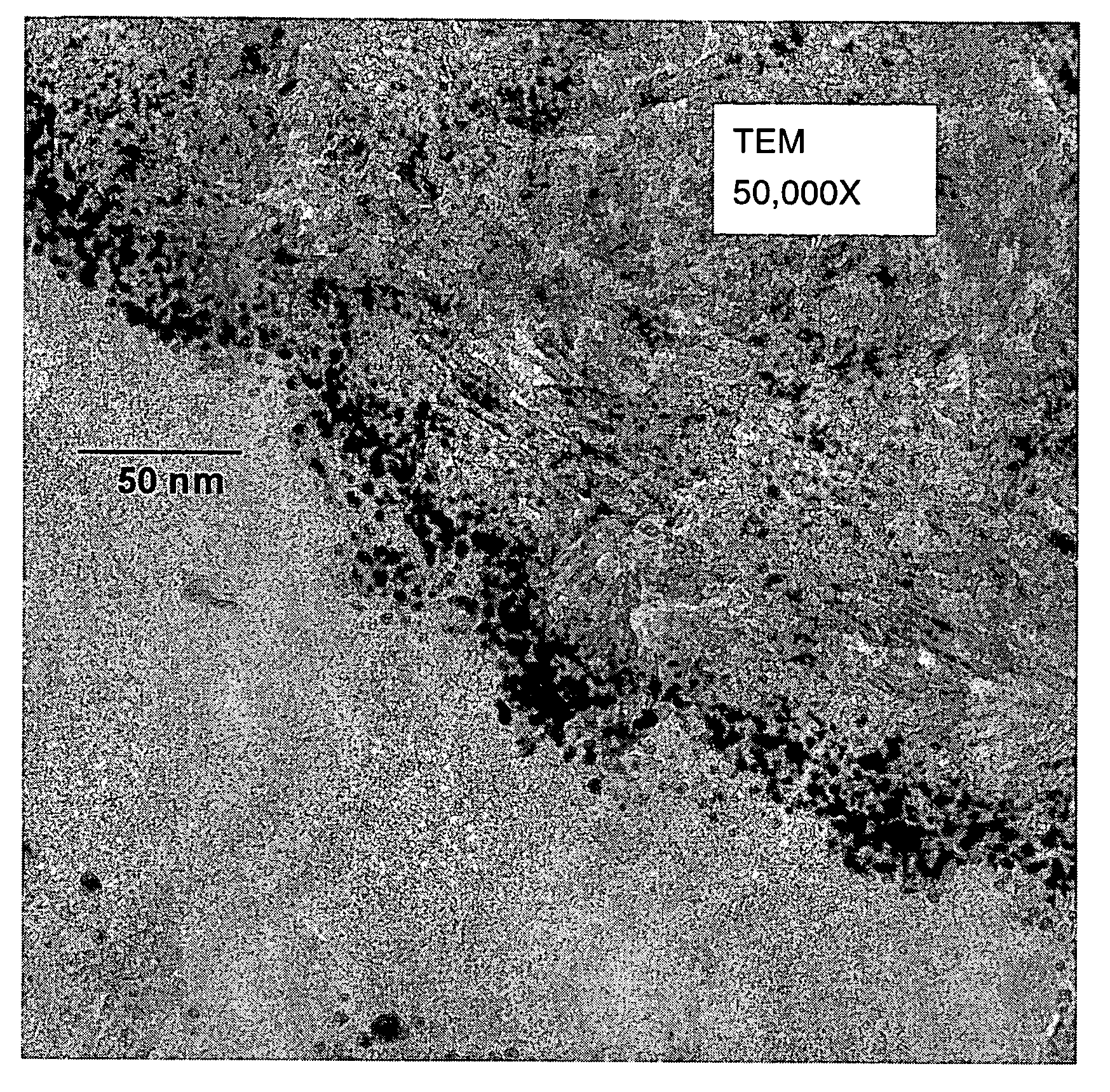
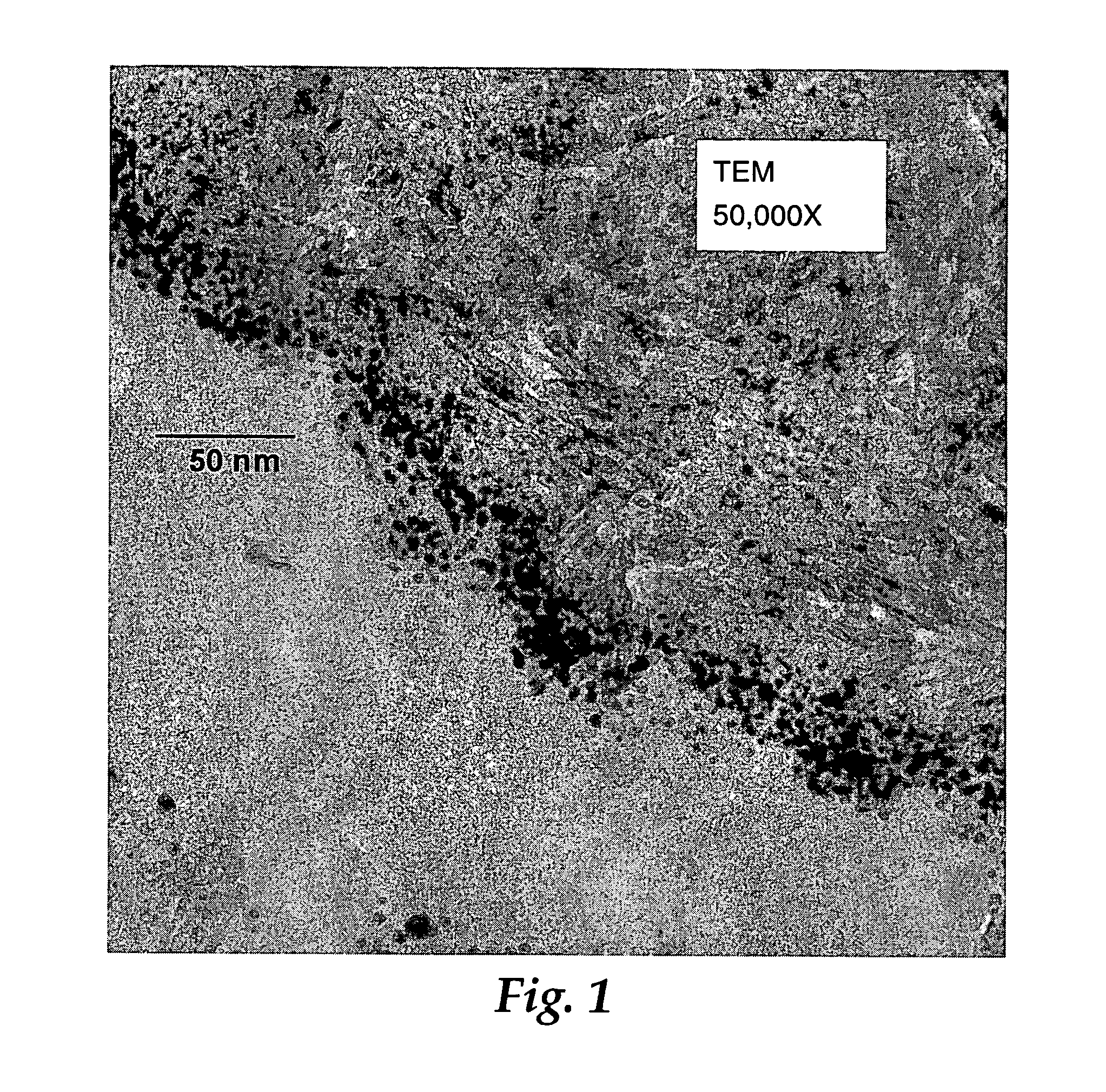
Catalysts, activating agents, support media, and related methodologies useful for making catalyst systems especially when the catalyst is deposited onto the support media using physical vapor deposition
InactiveUS20050095189A1Improve performanceEasy to useMaterial nanotechnologyInternal combustion piston enginesGas phaseAdditive ingredient
Use of physical vapor deposition methodologies to deposit nanoscale gold on activating support media makes the use of catalytically active gold dramatically easier and opens the door to significant improvements associated with developing, making, and using gold-based, catalytic systems. The present invention, therefore, relates to novel features, ingredients, and formulations of gold-based, heterogeneous catalyst systems generally comprising nanoscale gold deposited onto a nanoporous support.
Owner:3M INNOVATIVE PROPERTIES CO
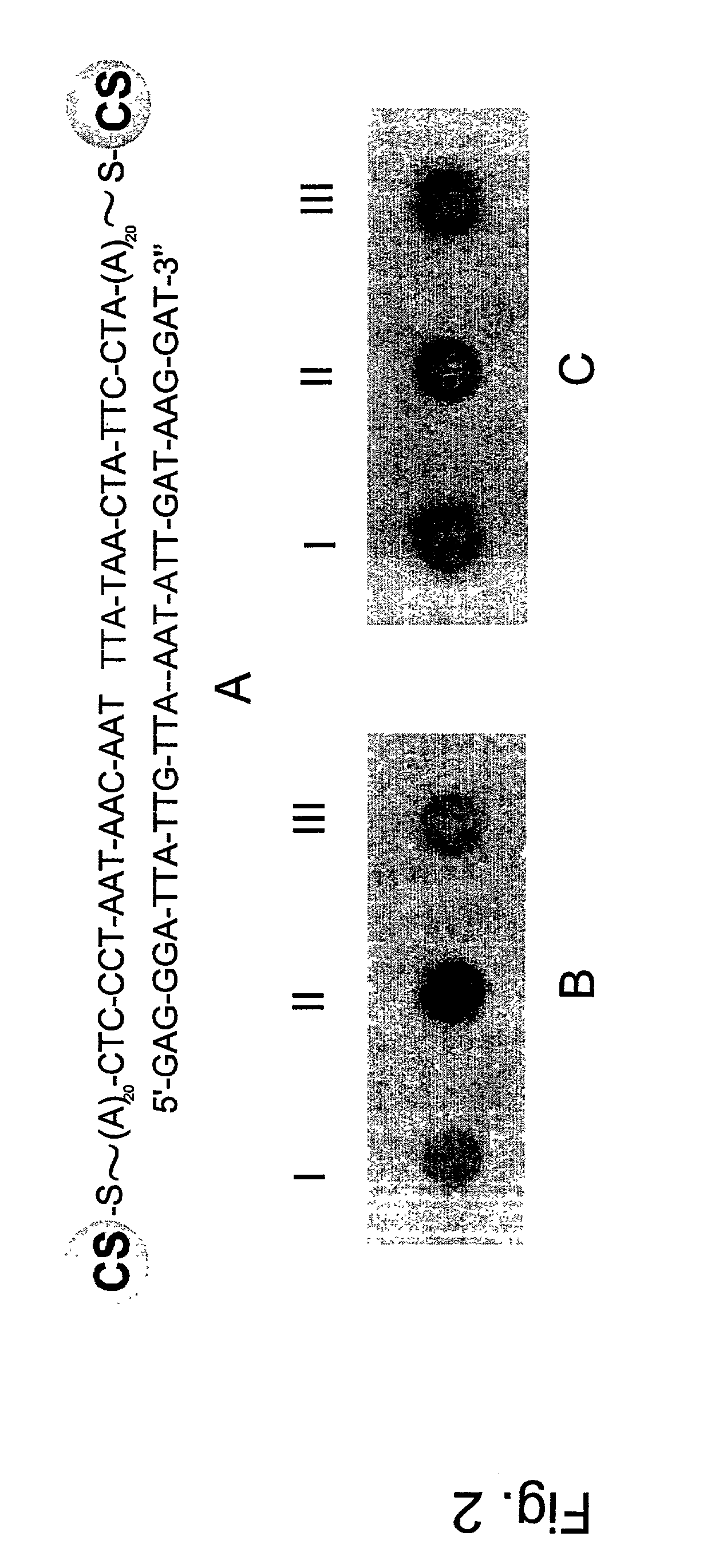
Non-alloying core shell nanoparticles
The present invention relates composite core / shell nanoparticles and a two-step method for their preparation. The present invention further relates to biomolecule-core / shell nanoparticle conjugates and methods for their preparation. The invention also relates to methods of detection of biomolecules comprising the biomolecule or specific binding substance-core / shell nanoparticle conjugates.
Owner:NORTHWESTERN UNIV
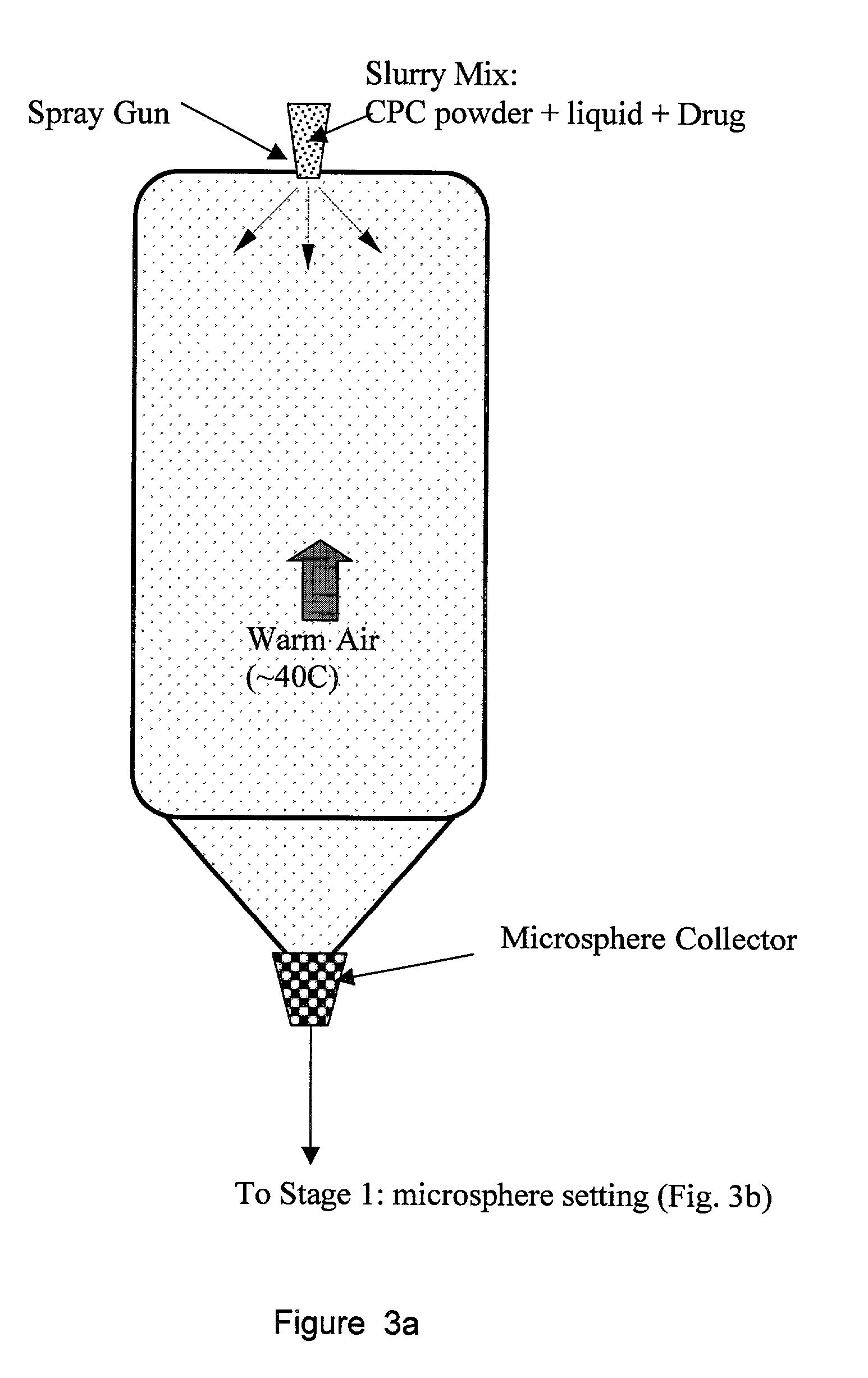
Biofunctional hydroxyapatite coatings and microspheres for in-situ drug encapsulation
InactiveUS20020155144A1Improve relationshipImprove interface strengthPowder deliveryOrganic active ingredientsGene deliverySide effect
Owner:THE UNIV OF BRITISH COLUMBIA
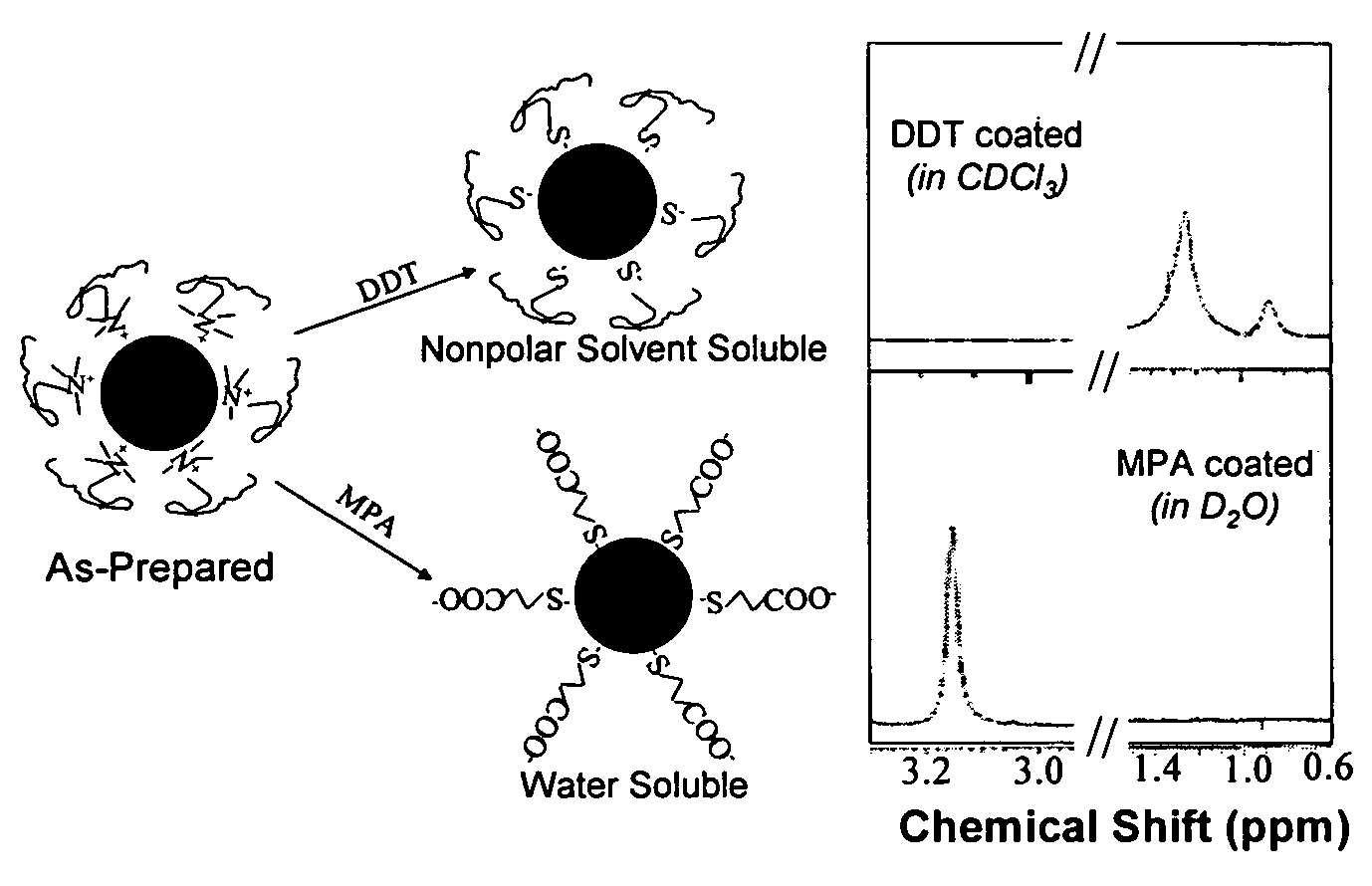
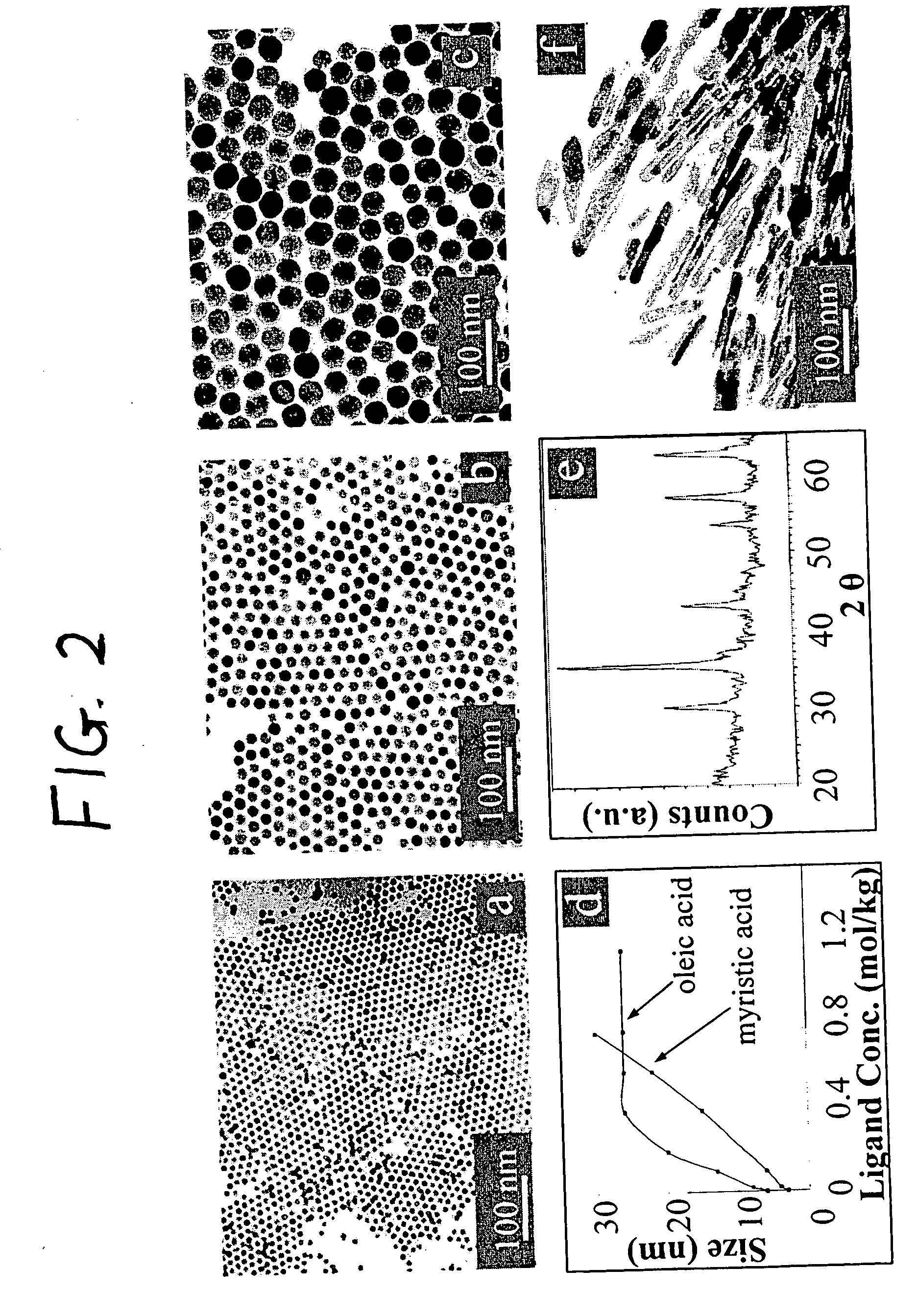
Monodisperse noble metal nanocrystals
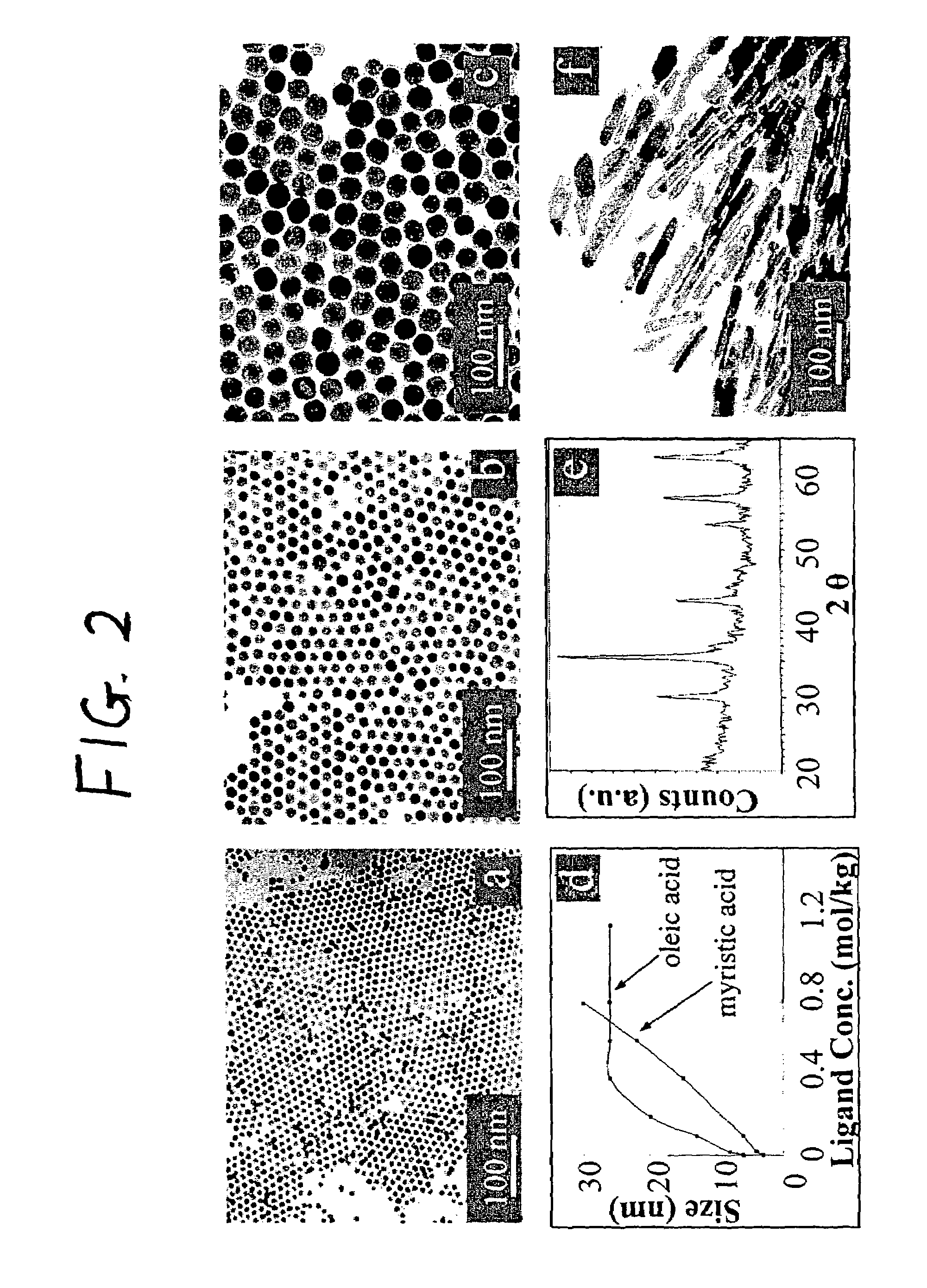
Nanoparticle compositions of noble metals, and methods of making them, are described. The nanoparticle compositions are made by reacting a salt or complex of a noble metal, such as Au, Ag, Cu or Pt, with a weak ligand, and a reducing agent, in a single liquid phase. The noble metal is typically provided as a halide or carboxylate. The ligand is preferably a fatty acid or aliphatic amine. The reducing agent is preferably a borohydride reagent, hydrazine, or a mixture thereof. Nanocrystals in the size range of 1 nm to 20 nm are produced, and can be made in substantially monodisperse form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Catalysts, activating agents, support media, and related methodologies useful for making catalyst systems especially when the catalyst is deposited onto the support media using physical vapor deposition
InactiveUS7727931B2High catalytic activityTendency increaseMaterial nanotechnologyInternal combustion piston enginesGas phasePhysical chemistry
Use of physical vapor deposition methodologies to deposit nanoscale gold on activating support media makes the use of catalytically active gold dramatically easier and opens the door to significant improvements associated with developing, making, and using gold-based, catalytic systems. The present invention, therefore, relates to novel features, ingredients, and formulations of gold-based, heterogeneous catalyst systems generally comprising nanoscale gold deposited onto a nanoporous support.
Owner:3M INNOVATIVE PROPERTIES CO
Spin-coatable liquid for formation of high purity nanotube films
ActiveUS20050058590A1Lower Level RequirementsPigmenting treatmentMaterial nanotechnologyLiquid mediumSpins
Certain spin-coatable liquids and application techniques are described, which can be used to form nanotube films or fabrics of controlled properties. A spin-coatable liquid for formation of a nanotube film includes a liquid medium containing a controlled concentration of purified nanotubes, wherein the controlled concentration is sufficient to form a nanotube fabric or film of preselected density and uniformity, and wherein the spin-coatable liquid comprises less than 1×1018 atoms / cm3 of metal impurities. The spin-coatable liquid is substantially free of particle impurities having a diameter of greater than about 500 nm.
Owner:ZEON CORP
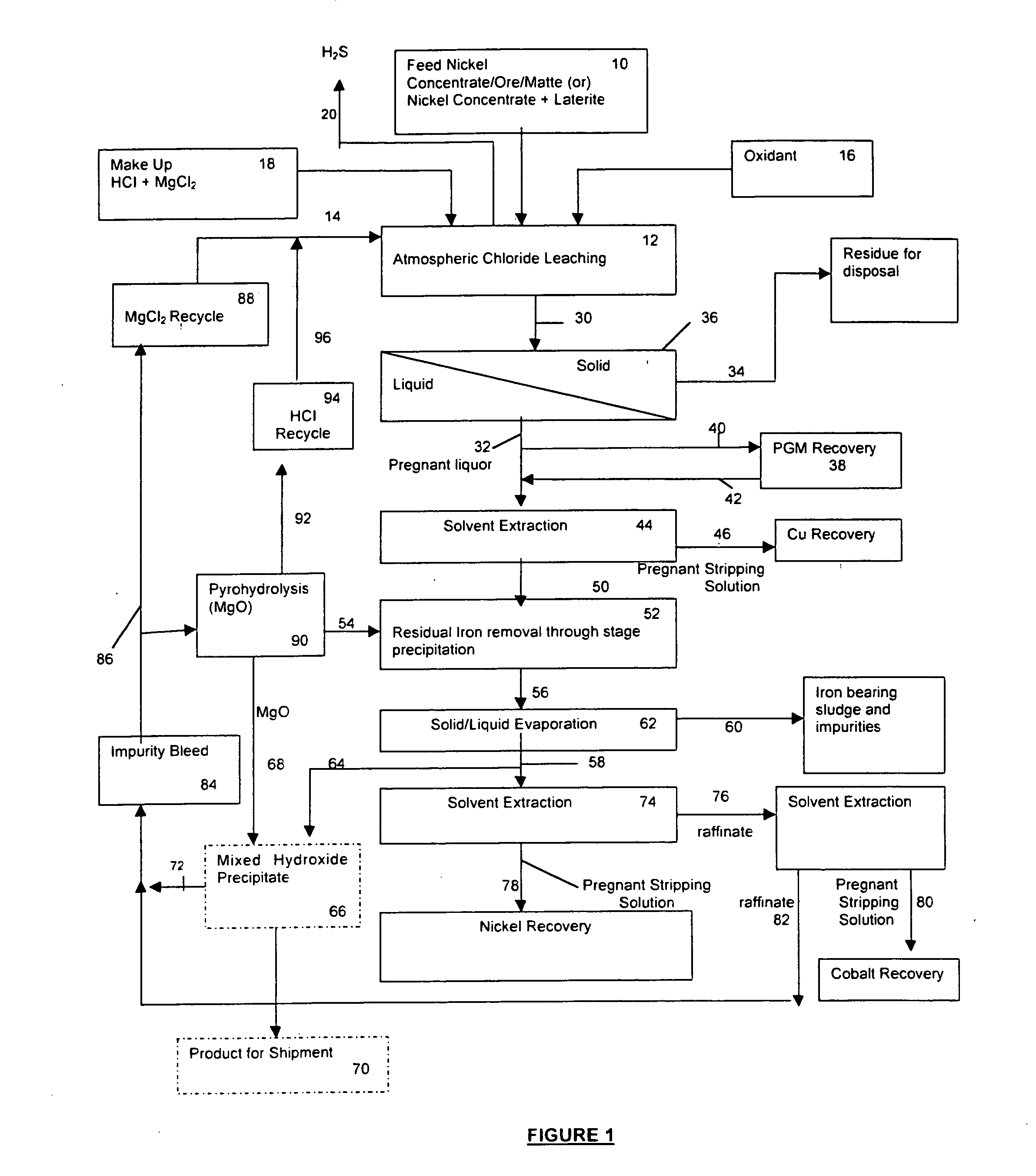
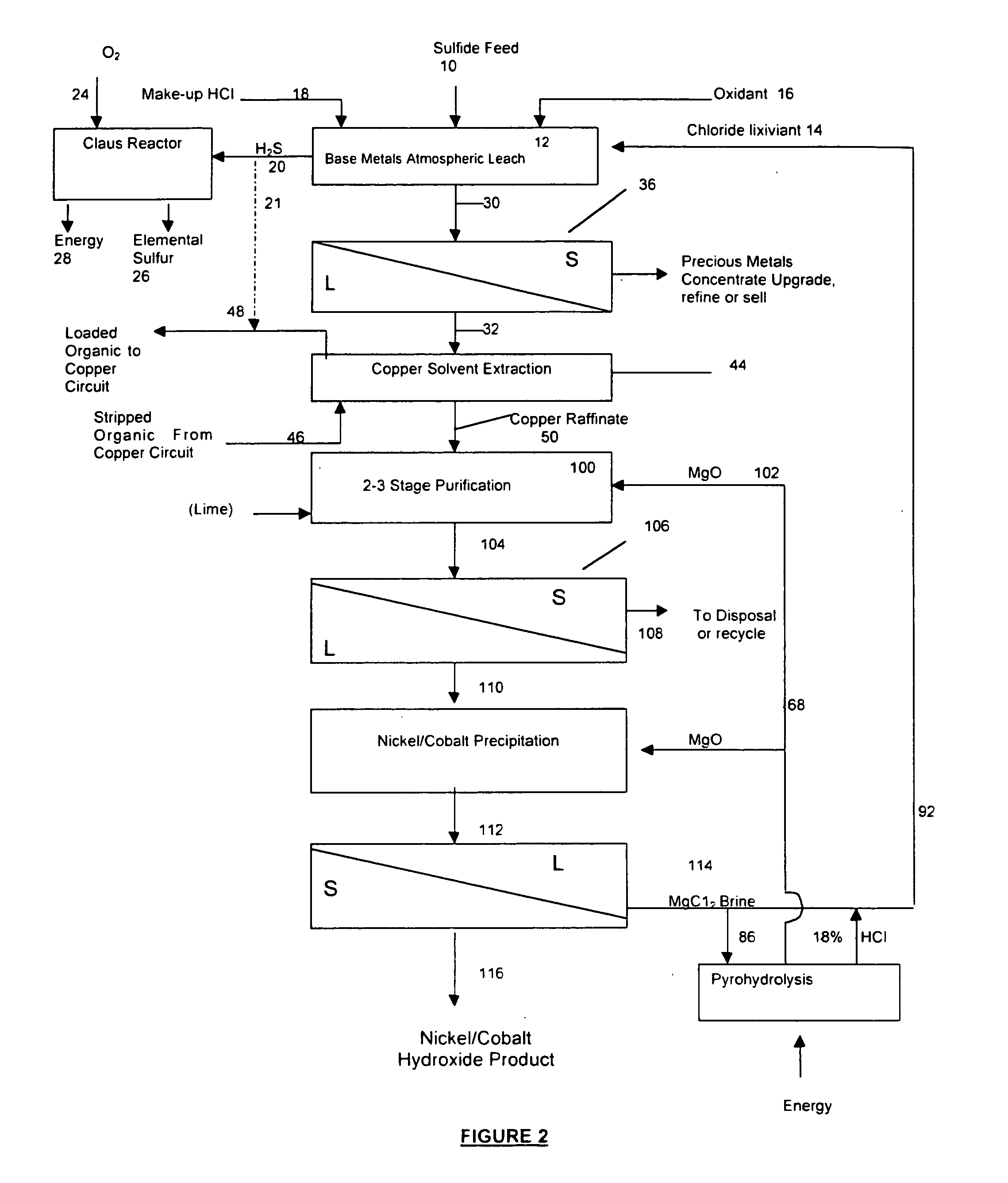
Process for the recovery of value metals from base metal sulfide ores
InactiveUS20050118081A1Simple gas/liquidReduce the amount requiredSulfur compoundsSolid sorbent liquid separationSulfideDissolution
A process for leaching a value metal from a base metal sulfide ore, comprising the step of leaching the ore with a lixiviant comprising a chloride, an oxidant and hydrochloric acid is disclosed. The leaching is controlled, by use of low concentrations of hydrochloric acid and a redox potential, to effect formation of hydrogen sulfide from the base metal sulfide ore. The hydrogen sulfide is stripped from the leach solution, thereby reducing the amount of sulfate generated in the leach to very low levels. The leaching may also be conducted to limit the co-dissolution of platinum group metals and gold with the base value metals. The leach forms a value metal-rich leachate and a solids residue. The solids residue may be subsequently leached to recover the platinum group metals and gold. The value metal-rich leachate can be is oxidized and neutralized to recover the value base metals. In an embodiment, the chloride is magnesium chloride and lixiviant solution is regenerated.
Owner:JAGUAR NICKEL INC
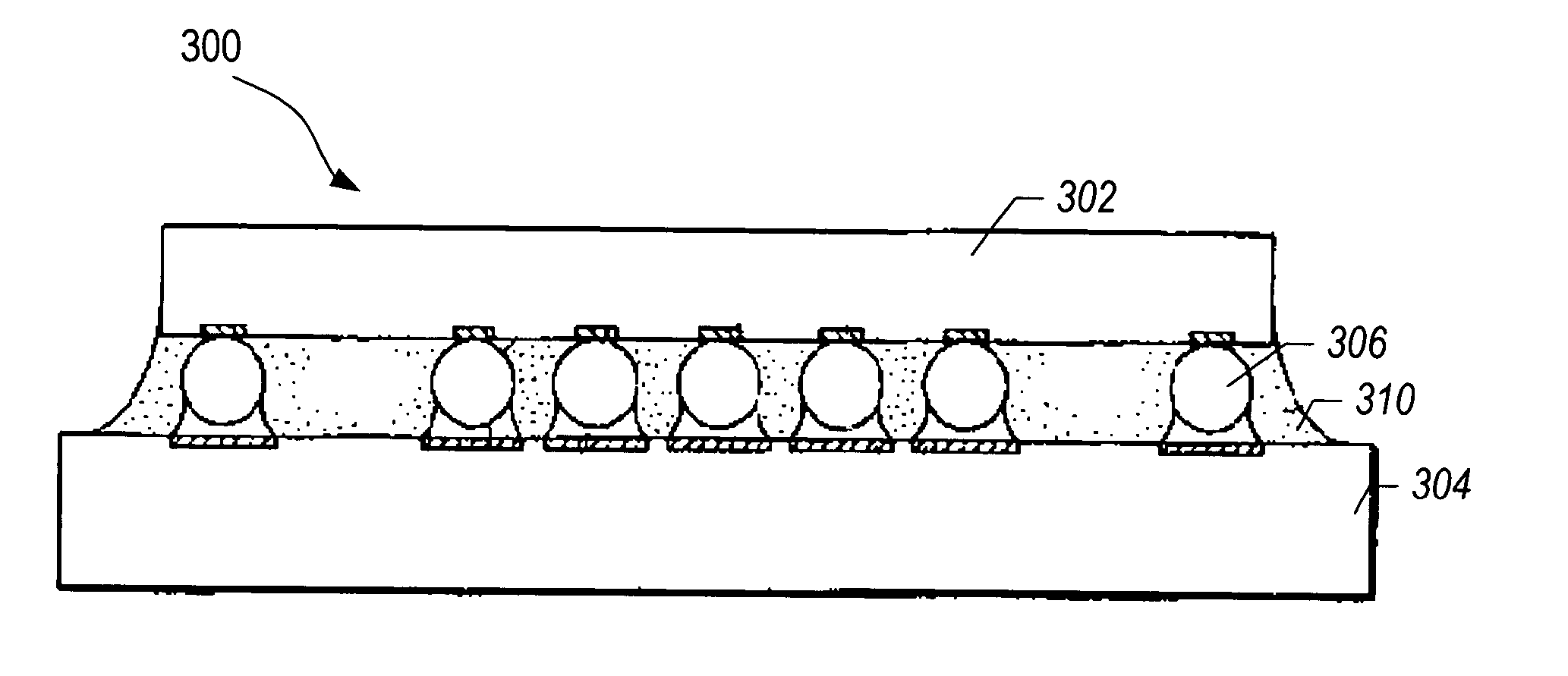
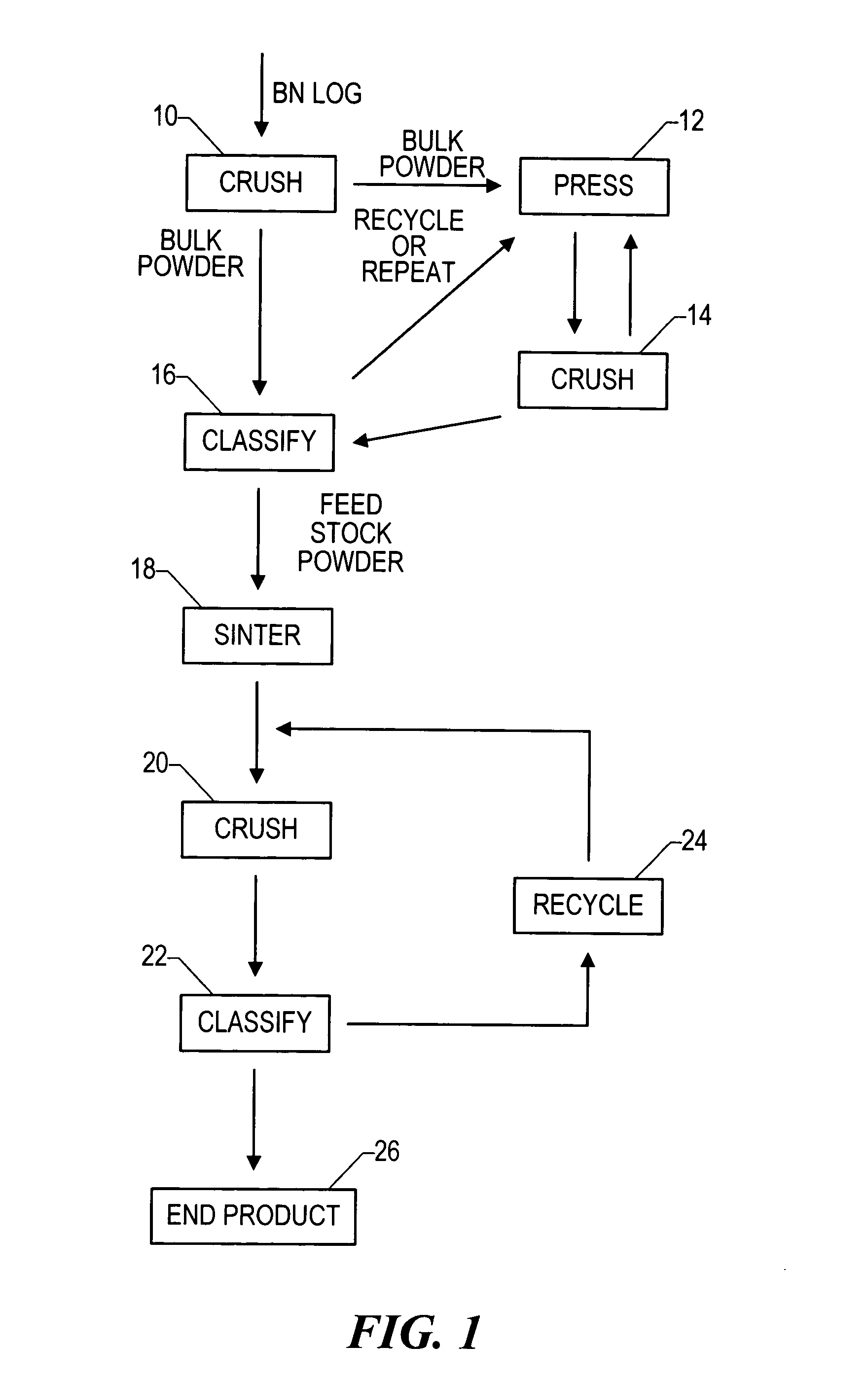
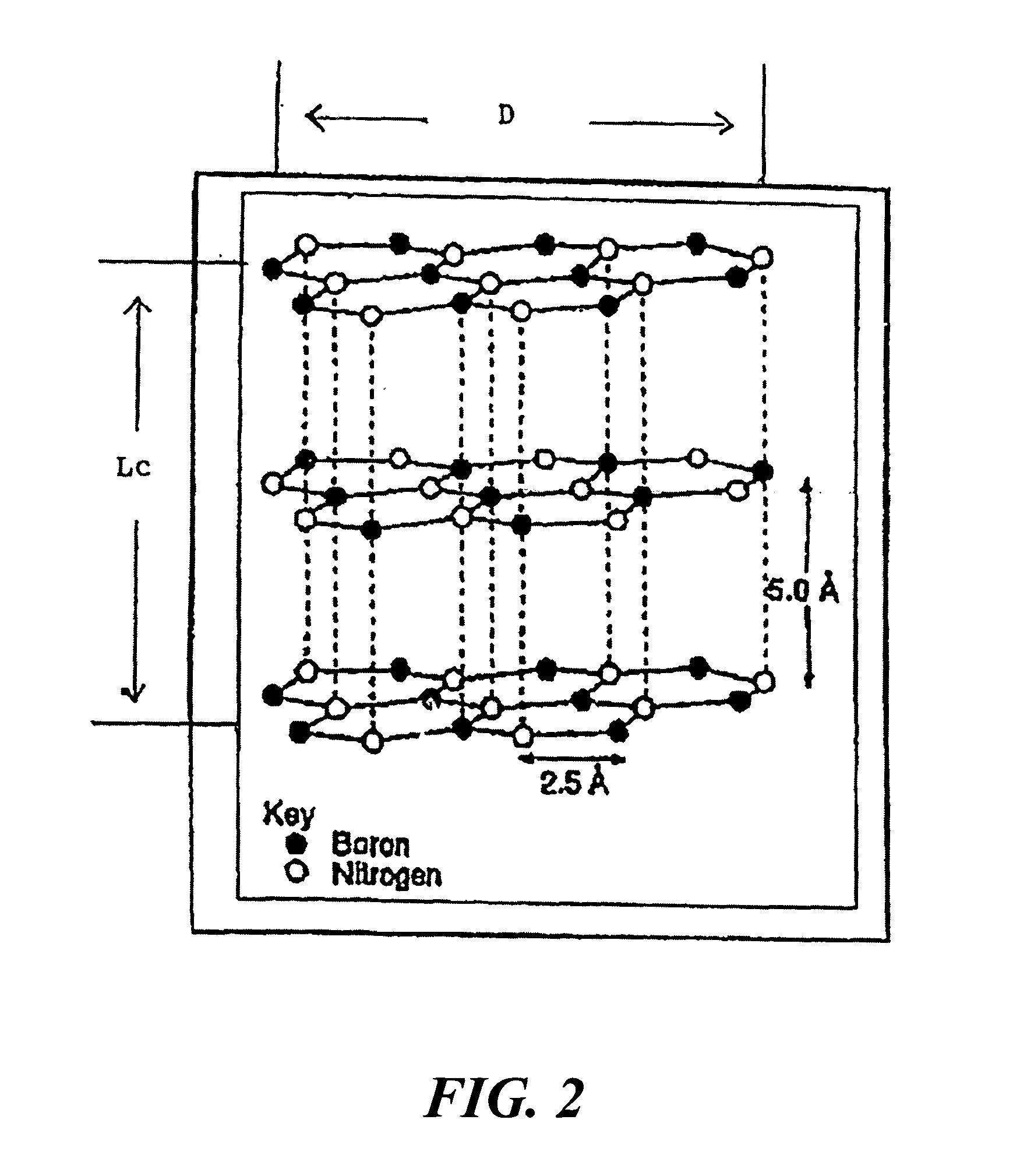
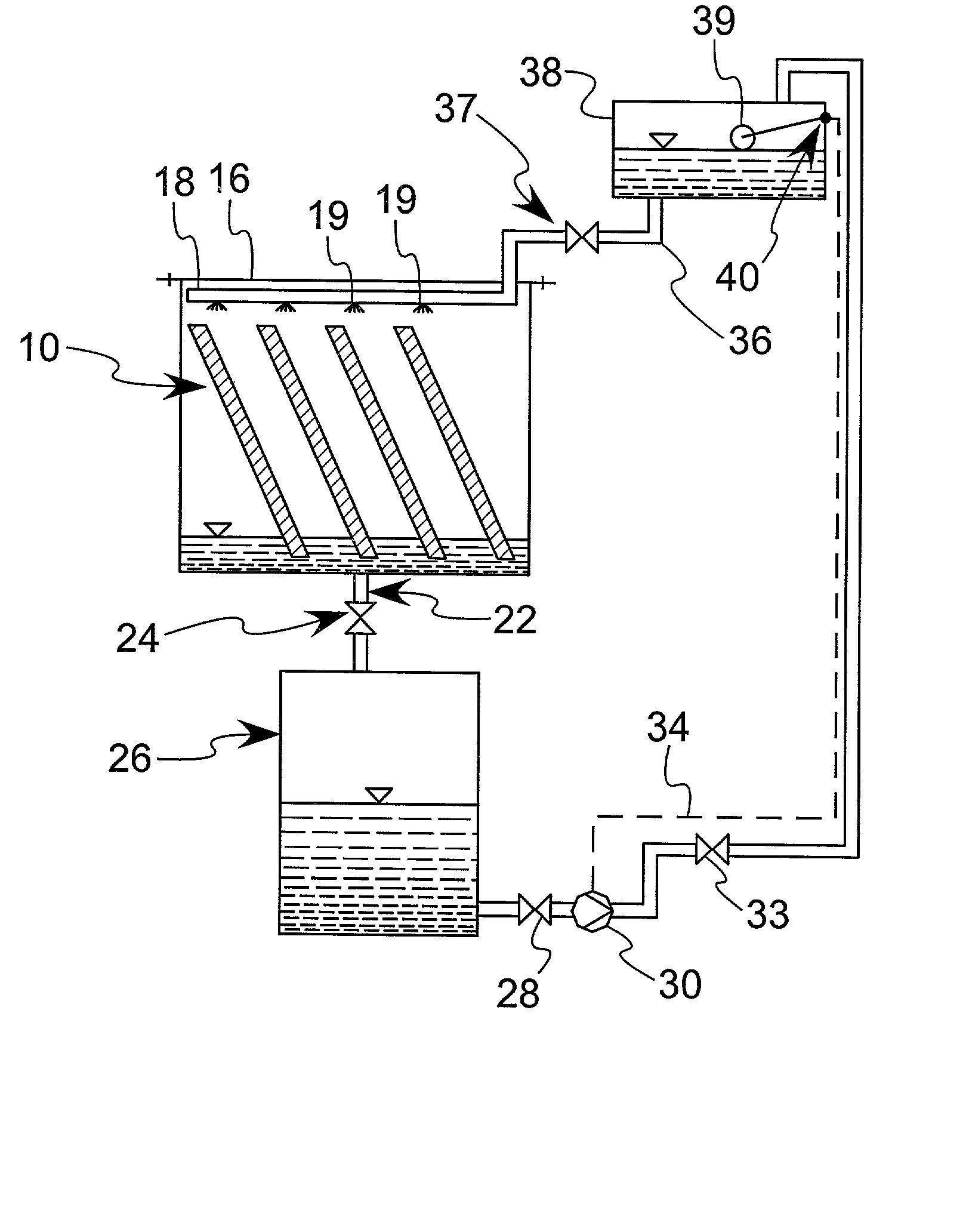
Boron nitride agglomerated powder
Novel boron nitride agglomerated powders are provided having controlled density and fracture strength features. In addition methods for producing same are provided. One method calls for providing a feedstock powder including boron nitride agglomerates, and heat treating the feedstock powder to form a heat treated boron nitride agglomerated powder. In one embodiment the feedstock powder has a controlled crystal size. In another, the feedstock powder is derived from a bulk source.
Owner:SAINT GOBAIN CERAMICS & PLASTICS INC
Enhanced practical photosynthetic CO2 mitigation
InactiveUS20020072109A1Increase the amount of waterBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismCyanobacteria
This process is unique in photosynthetic carbon sequestration. An on-site biological sequestration system directly decreases the concentration of carbon-containing compounds in the emissions of fossil generation units. In this process, photosynthetic microbes are attached to a growth surface arranged in a containment chamber that is lit by solar photons. A harvesting system ensures maximum organism growth and rate of CO2 uptake. Soluble carbon and nitrogen concentrations delivered to the cyanobacteria are enhanced, further increasing growth rate and carbon utilization.
Owner:OHIO UNIV
Non-alloying core shell nanoparticles
The present invention relates composite core / shell nanoparticles and a two-step method for their preparation. The present invention further relates to biomolecule-core / shell nanoparticle conjugates and methods for their preparation. The invention also relates to methods of detection of biomolecules comprising the biomolecule or specific binding substance-core / shell nanoparticle conjugates.
Owner:NORTHWESTERN UNIV
Conductive paste
InactiveUS20060145125A1Pigmenting treatmentGroup 1/11 element organic compoundsConductive pasteAdhesive
The present invention provides an electroconductive paste that can contain a high proportion of an electroconductive powder, has excellent electroconductivity reliability and migration resistance, has a highly competitive price due to a reduced amount of silver plating, and is suitable for use in solder electrode formation, an electroconductive adhesive, etc. The electroconductive paste of the present invention comprises a binder and an electroconductive powder containing 80 to 97 wt % of a substantially spherical silver-coated copper powder in which the surface of a copper powder is coated with silver and the surface thereof is further coated with 0.02 to 0.5 wt % relative to the copper powder of a fatty acid, and 3 to 20 wt % of a flat-shaped silver-coated copper powder in which the surface of a copper powder is coated with silver and the surface thereof is further coated with 0.02 to 1.2 wt % relative to the copper powder of a fatty acid.
Owner:HITACHI CHEM CO LTD
Non-alloying core shell nanoparticles
The present invention relates composite core / shell nanoparticles and a two-step method for their preparation. The present invention further relates to biomolecule-core / shell nanoparticle conjugates and methods for their preparation. The invention also relates to methods of detection of biomolecules comprising the biomolecule-core / shell nanoparticle conjugates.
Owner:NORTHWESTERN UNIV
Precipitated aragonite and a process for producing it
InactiveUS20030213937A1Less expensiveEfficient and less-expensiveCalcium/strontium/barium carbonatesInorganic/elemental detergent compounding agentsParticulatesAragonite
Disclosed is a novel form of particulate precipitated aragonite, and a novel process for producing it.
Owner:3P TECH
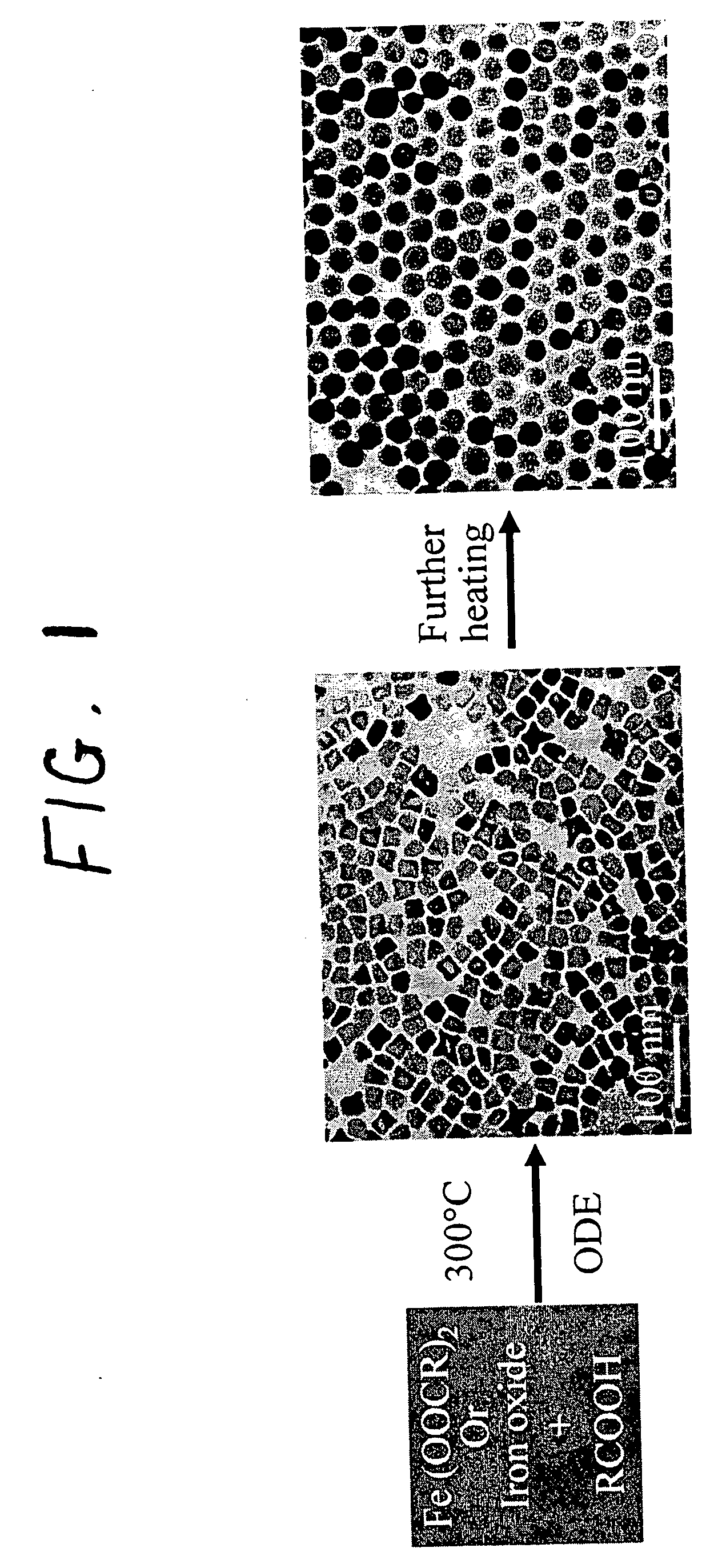
Synthetic control of metal oxide nanocrystal sizes and shapes
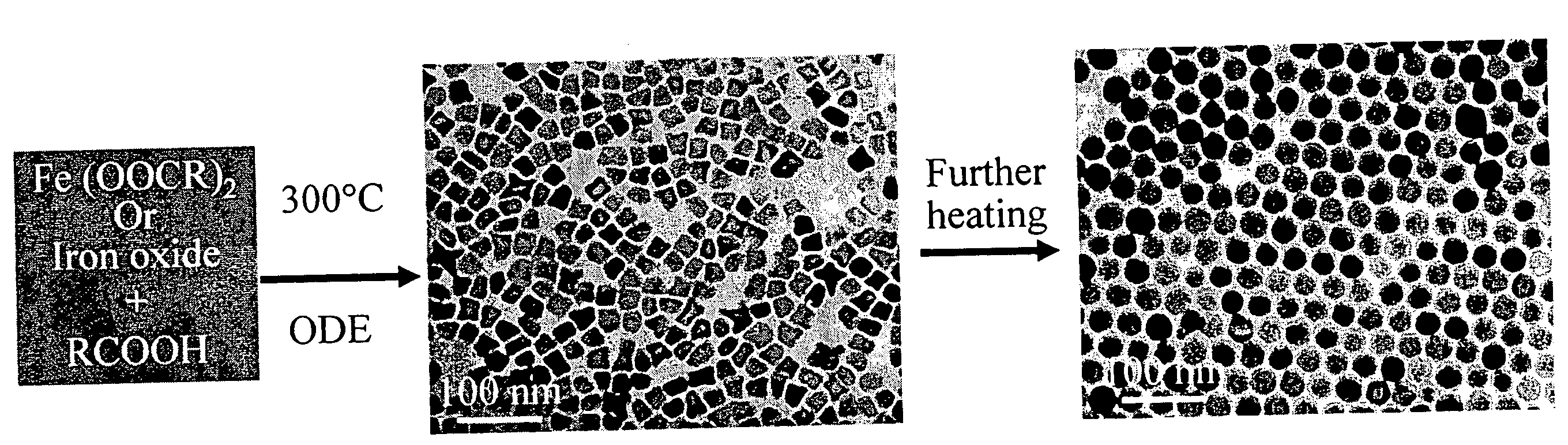
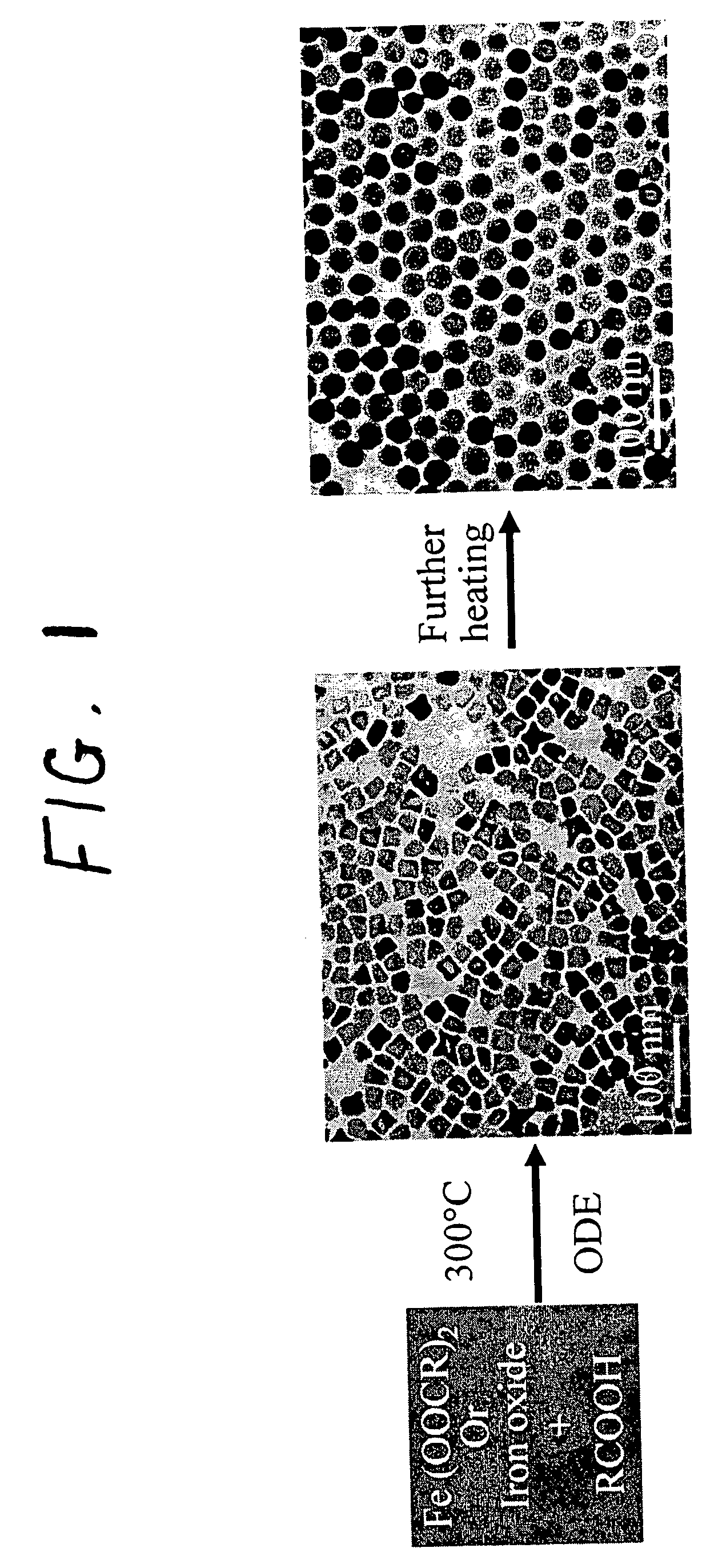
A general, reproducible, and simple synthetic method that employs readily available chemicals permits control of the size, shape, and size distribution of metal oxide nanocrystals. The synthesis entails reacting a metal fatty acid salt, the corresponding fatty acid, and a hydrocarbon solvent, with the reaction product being pyrolyzed to the metal oxide. Nearly monodisperse oxide nanocrystals of Fe3O4, Cr2O3, MnO, Co3O4, NiO, ZnO, SnO2, and In2O3, in a large size range (3-50 nm), are described. Size and shape control of the nanocrystals is achieved by varying the reactivity and concentration of the precursors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Methods for removing heavy metals from water using chemical precipitation and field separation methods
InactiveUS6896815B2Small sizeChemical cost reductionSolid sorbent liquid separationGold compoundsWater useSludge
A two-step chemical precipitation process involving hydroxide precipitation and sulfide precipitation combined with “field separation” technology such as magnetic separation, dissolved air flotation, vortex separation or expanded plastics flotation, effectively removes chelated and non-chelated heavy metal precipitates and other fine particles from water. In the first-step, the non-chelated heavy metals are precipitated as hydroxides and removed from the water by a conventional liquid / solids separator such as an inclined plate clarifier to remove a large percentage of the dissolved heavy metals. The cleaned water is then treated in a second precipitation step to remove the residual heavy metals to meet discharge limits. In the second precipitation step, any metal precipitant more effective than hydroxide for metal precipitation can be used. The invention improves metal removal, lowers cost because fewer chemicals are used, produces less sludge, and reduces the discharge of toxic metals and metal precipitants to the environment.
Owner:CORT STEVEN L
Methods of Making Binary Metal Oxide Nanostructures and Methods of Controlling Morphology of Same
ActiveUS20100278720A1Reduce crystallinityControl dimensionalityCopper oxides/halidesManganese oxides/hydroxidesPorous membraneNanostructure
The present invention includes a method of producing a crystalline metal oxide nanostructure. The method comprises providing a metal salt solution and providing a basic solution; placing a porous membrane between the metal salt solution and the basic solution, wherein metal cations of the metal salt solution and hydroxide ions of the basic solution react, thereby producing a crystalline metal oxide nanostructure.
Owner:WONG STANISLAUS S +1
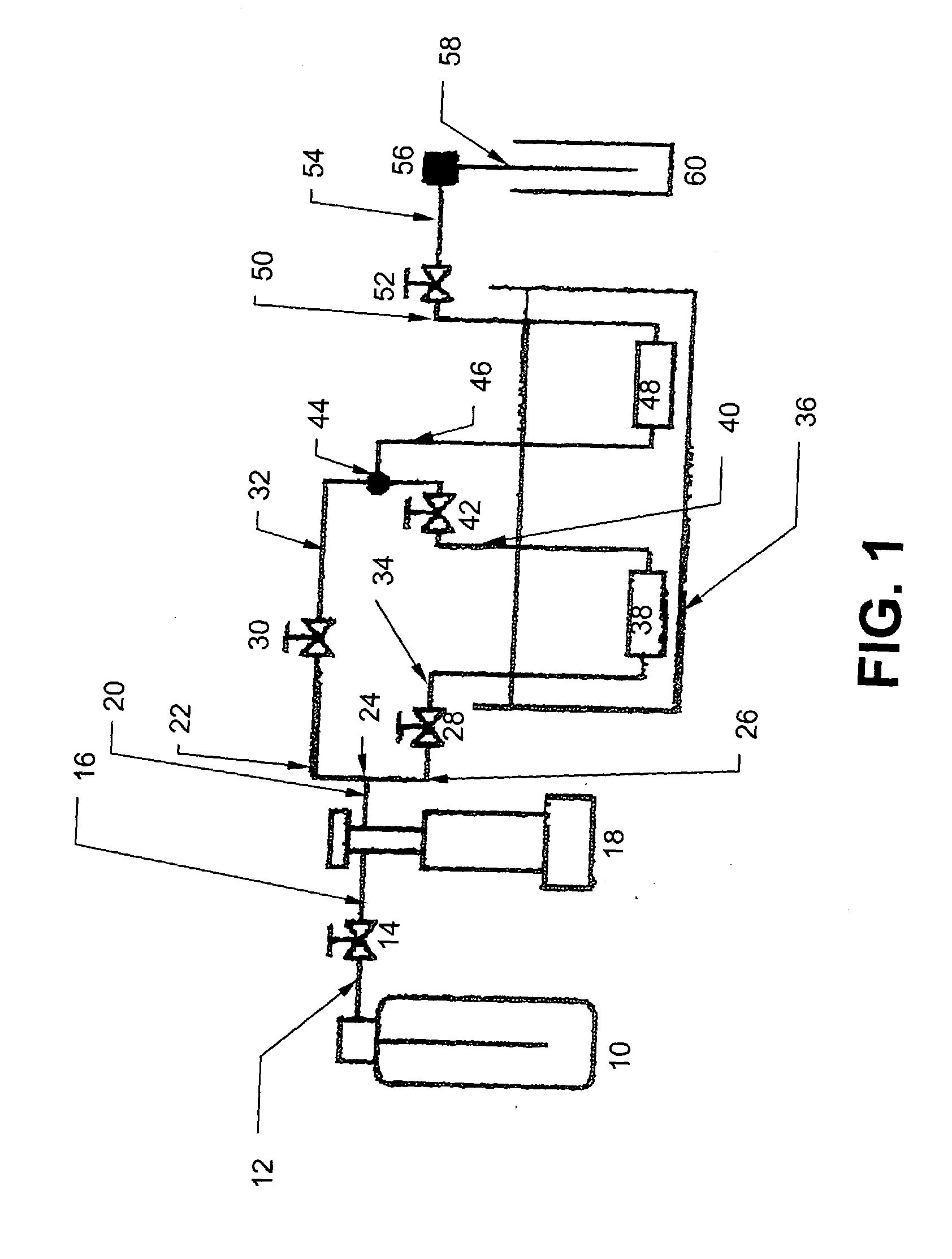
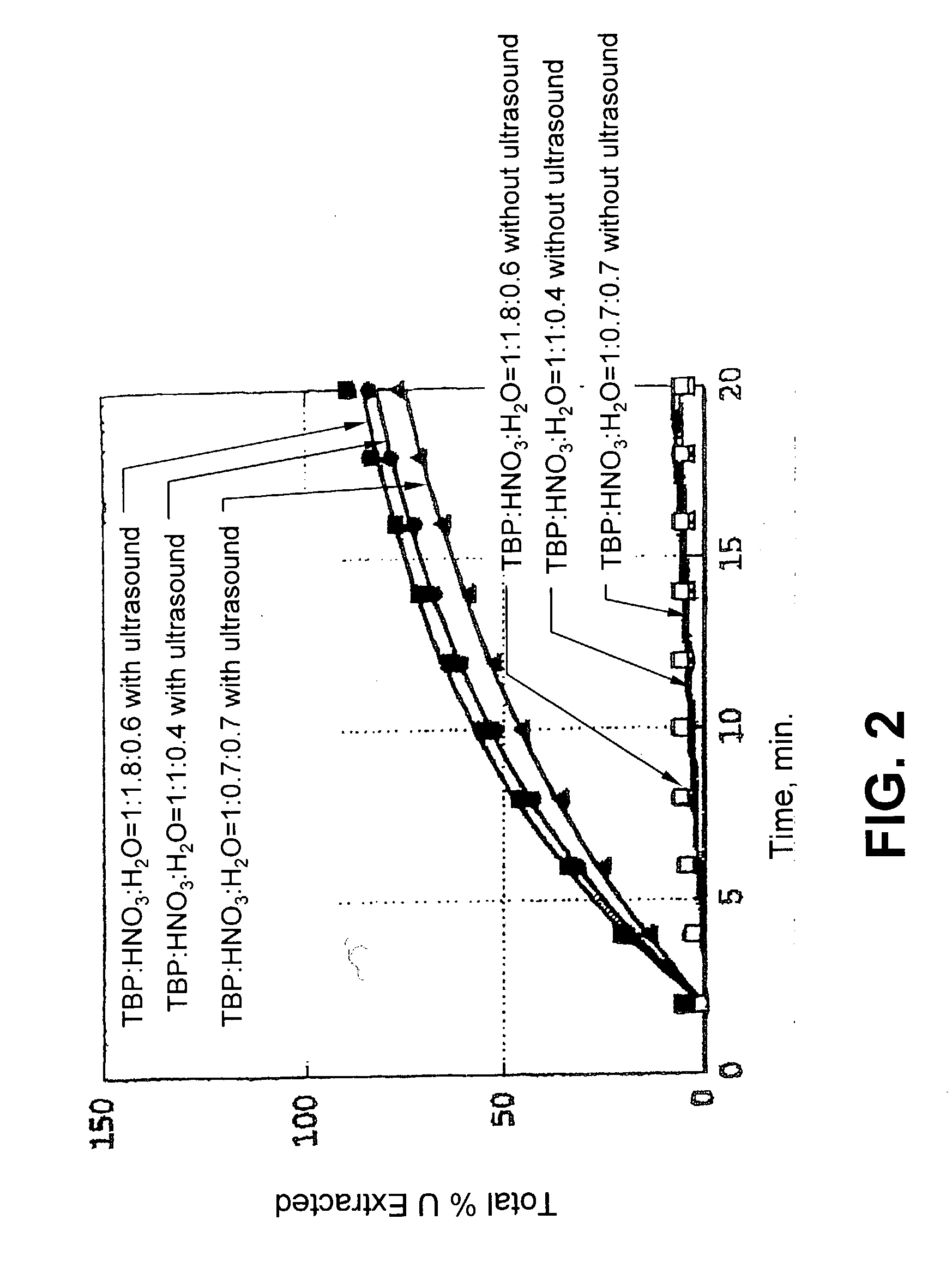
Ultrasound enhanced process for extracting metal species in supercritical fluids
InactiveUS20030183043A1Enhances rate and efficiencyReduce probabilitySolid sorbent liquid separationGold compoundsUranium oxidePresent method
Improved methods for the extraction or dissolution of metals, metalloids or their oxides, especially lanthanides, actinides, uranium or their oxides, into supercritical solvents containing an extractant are disclosed. The disclosed embodiments specifically include enhancing the extraction or dissolution efficiency with ultrasound. The present methods allow the direct, efficient dissolution of UO2 or other uranium oxides without generating any waste stream or by-products.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST +1
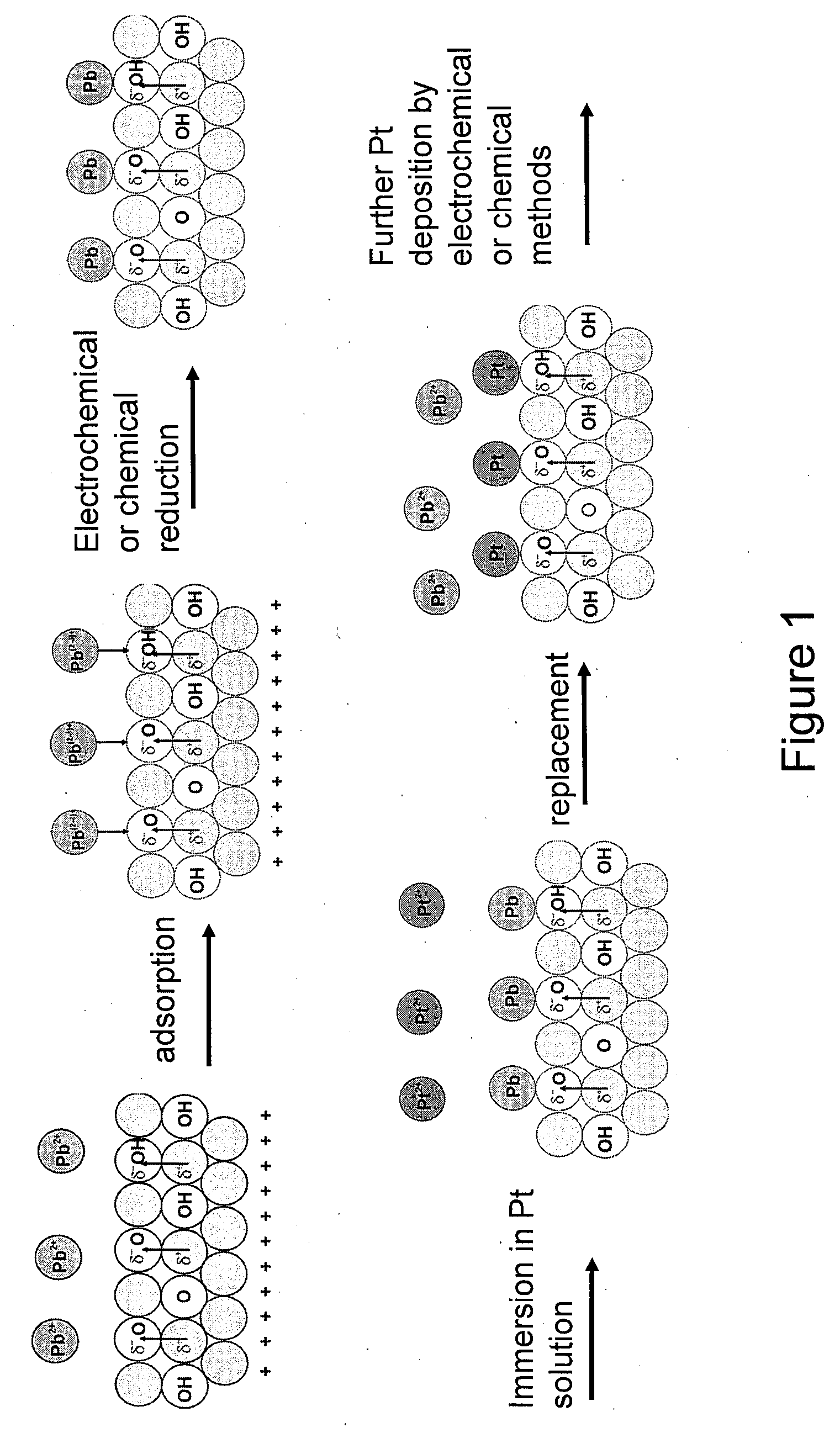
Synthesis of Metal-Metal Oxide Catalysts and Electrocatalysts Using a Metal Cation Adsorption/Reduction and Adatom Replacement by More Noble Ones
InactiveUS20070264189A1Improve stabilityReducing and preventing oxidationCell electrodesGold compoundsHydrogenFuel cells
The invention relates to platinum-metal oxide composite particles and their use as electrocatalysts in oxygen-reducing cathodes and fuel cells. The invention particularly relates to methods for preventing the oxidation of the platinum electrocatalyst in the cathodes of fuel cells by use of these platinum-metal oxide composite particles. The invention additionally relates to methods for producing electrical energy by supplying such a fuel cell with an oxidant, such as oxygen, and a fuel source, such as hydrogen. The invention also relates to methods of making the metal-metal oxide composites.
Owner:BROOKHAVEN SCI ASSOCS
Apparatus and method for preparing cerium oxide nanoparticles
InactiveUS20050031517A1Quick responseReaction time is thus limitedMaterial nanotechnologyMixing methodsCerium nitrateNanoparticle
This invention provides a method for preparing cerium oxide nanoparticles with a narrow size distribution. The cerium oxide nanoparticles obtained by the method of the invention are nearly all crystalline. The method comprises providing a first aqueous solution comprising cerium nitrate and providing a second aqueous solution comprising hexamethylenetetramine. The first and second aqueous solutions are mixed to form a mixture, and the mixture is maintained at a temperature no higher than about 320° K to form nanoparticles. The nanoparticles that are formed are then separated from the mixture. A further aspect of the present invention is an apparatus for preparing cerium oxide nanoparticles. The apparatus comprises a mixing vessel having a first compartment for holding a first aqueous solution comprising cerium nitrate and a second compartment for holding a second aqueous solution comprising hexamethylenetetramine. The mixing vessel has a retractable partition separating the first and second compartments. When the retractable partition is retracted, rapid mixing of the first aqueous solution with the second aqueous solution takes place to form a mixture, and the mixture is maintained at a temperature no higher than about 320° K to form nanoparticles therein.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Non-alloying core shell nanoparticles
InactiveUS7147687B2Useful propertyMaterial nanotechnologyPowder deliveryCore shell nanoparticlesNanometre
The present invention relates composite core / shell nanoparticles and a two-step method for their preparation. The present invention further relates to biomolecule-core / shell nanoparticle conjugates and methods for their preparation. The invention also relates to methods of detection of biomolecules comprising the biomolecule-core / shell nanoparticle conjugates.
Owner:NORTHWESTERN UNIV
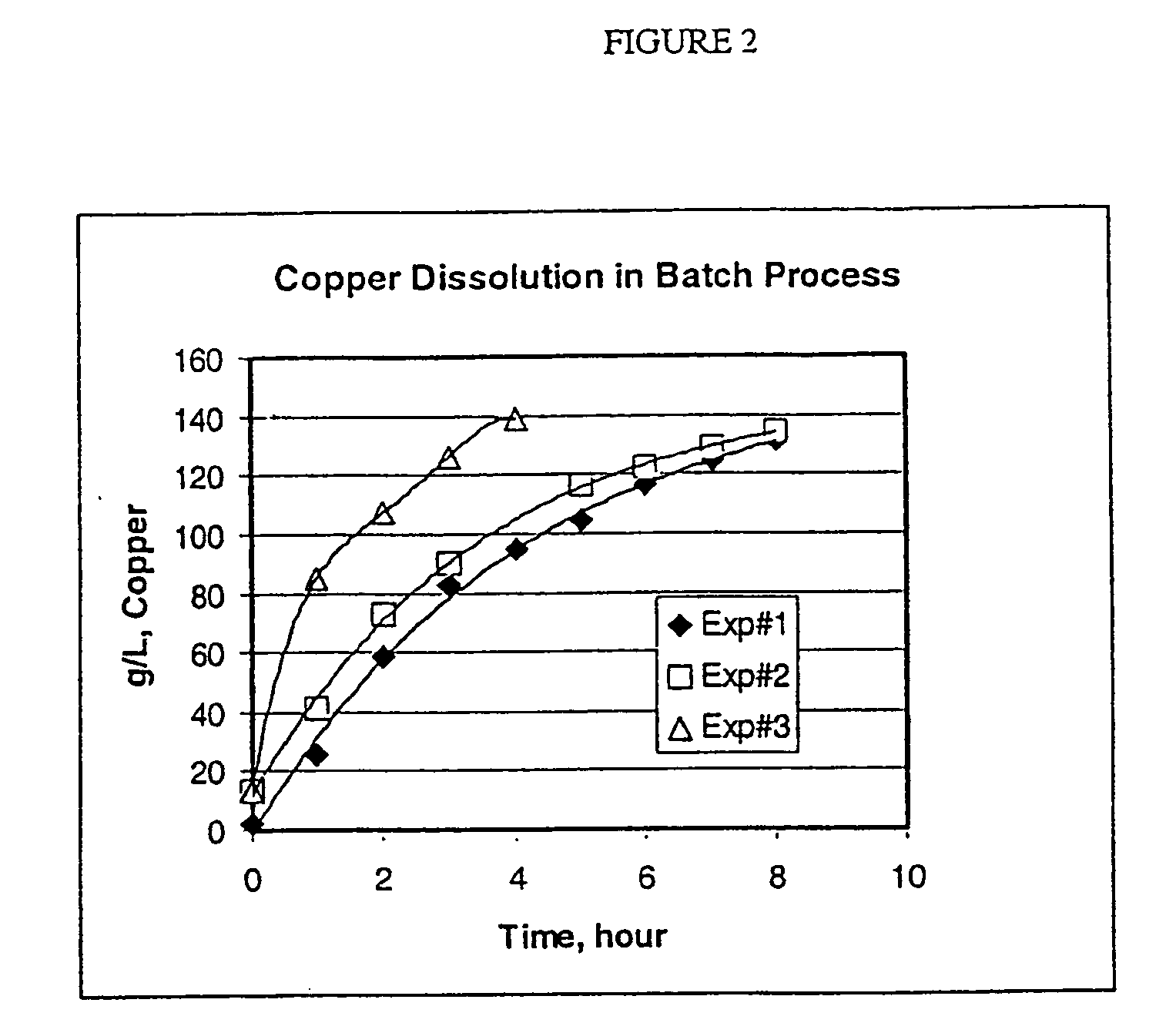
Process for the dissolution of copper metal
ActiveUS20050130866A1High initial dissolution rateShorten the timeGroup 1/11 element organic compoundsSolvent extractionGramEquivalent weight
Process for producing a copper-containing aqueous solution, in which a copper mass is dissolved in the presence of air in an aqueous leach liquor containing monoethanolamine and an acid, wherein the amount of acid equivalents is between 0.05 and about 0.7 times the equivalents of monoethanolamine, and wherein the rate of copper dissolution into the aqueous leach liquor is greater than about 4.3 grams of copper per liter of leach liquor per hour until a product having at least about 80 grams per liter is obtained.
Owner:KOPPERS PERFORMANCE CHEM
Synthetic control of metal oxide nanocrystal sizes and shapes
A general, reproducible, and simple synthetic method that employs readily available chemicals permits control of the size, shape, and size distribution of metal oxide nanocrystals. The synthesis entails reacting a metal fatty acid salt, the corresponding fatty acid, and a hydrocarbon solvent, with the reaction product being pyrolyzed to the metal oxide. Nearly monodisperse oxide nanocrystals of Fe3O4, Cr2O3, MnO, Co3O4, NiO, ZnO, SnO2, and In2O3, in a large size range (3-50 nm), are described. Size and shape control of the nanocrystals is achieved by varying the reactivity and concentration of the precursors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
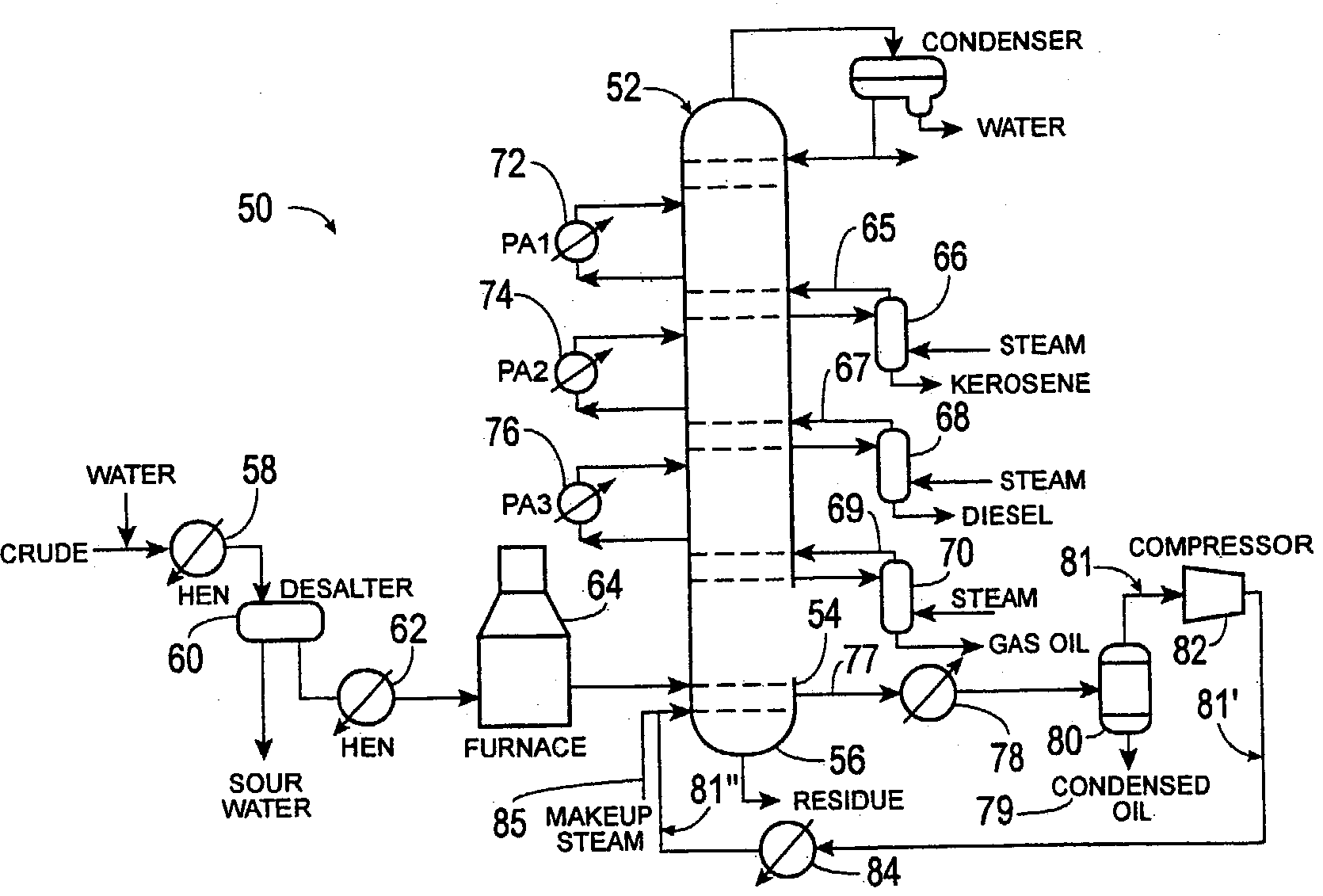
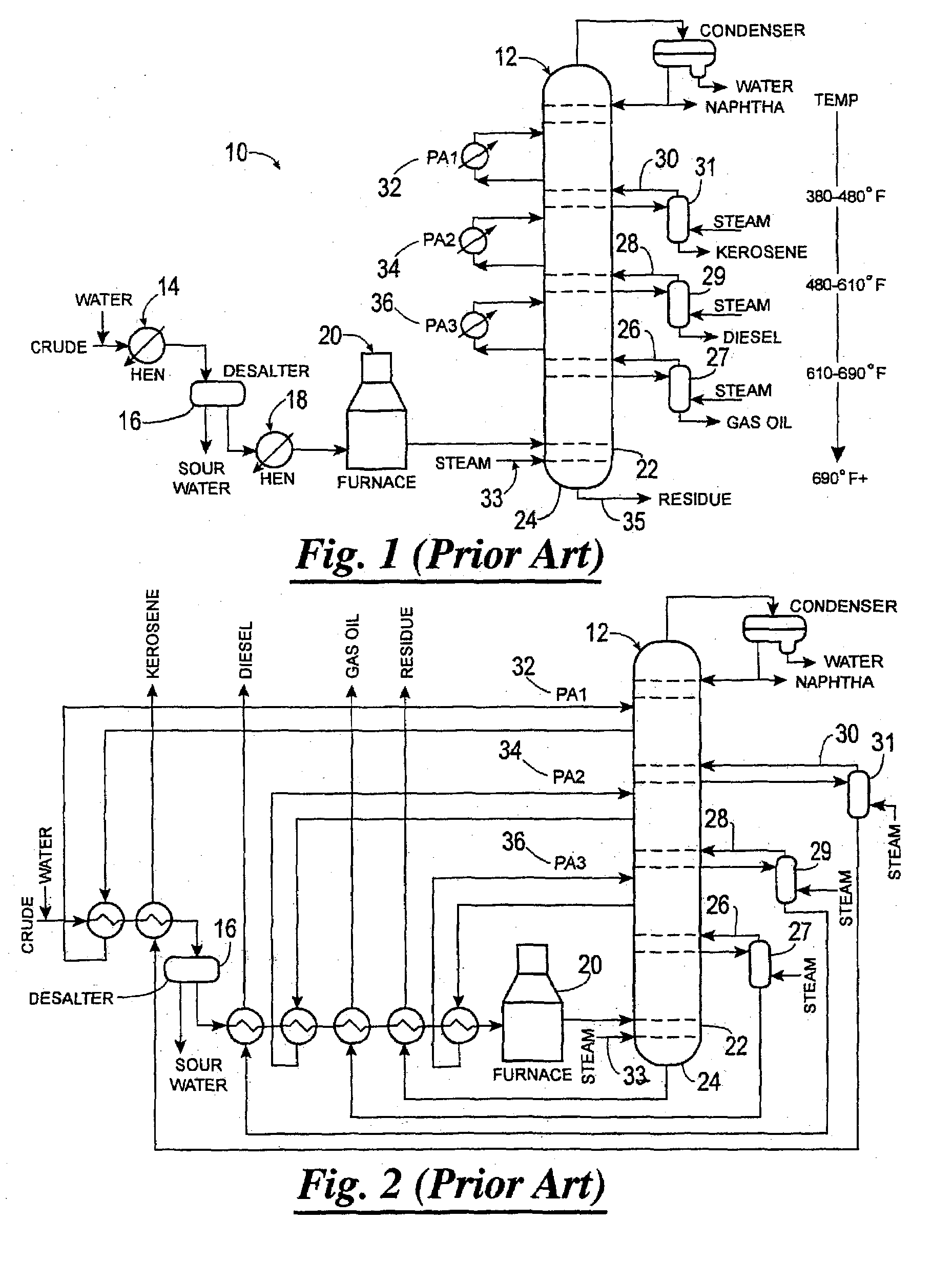
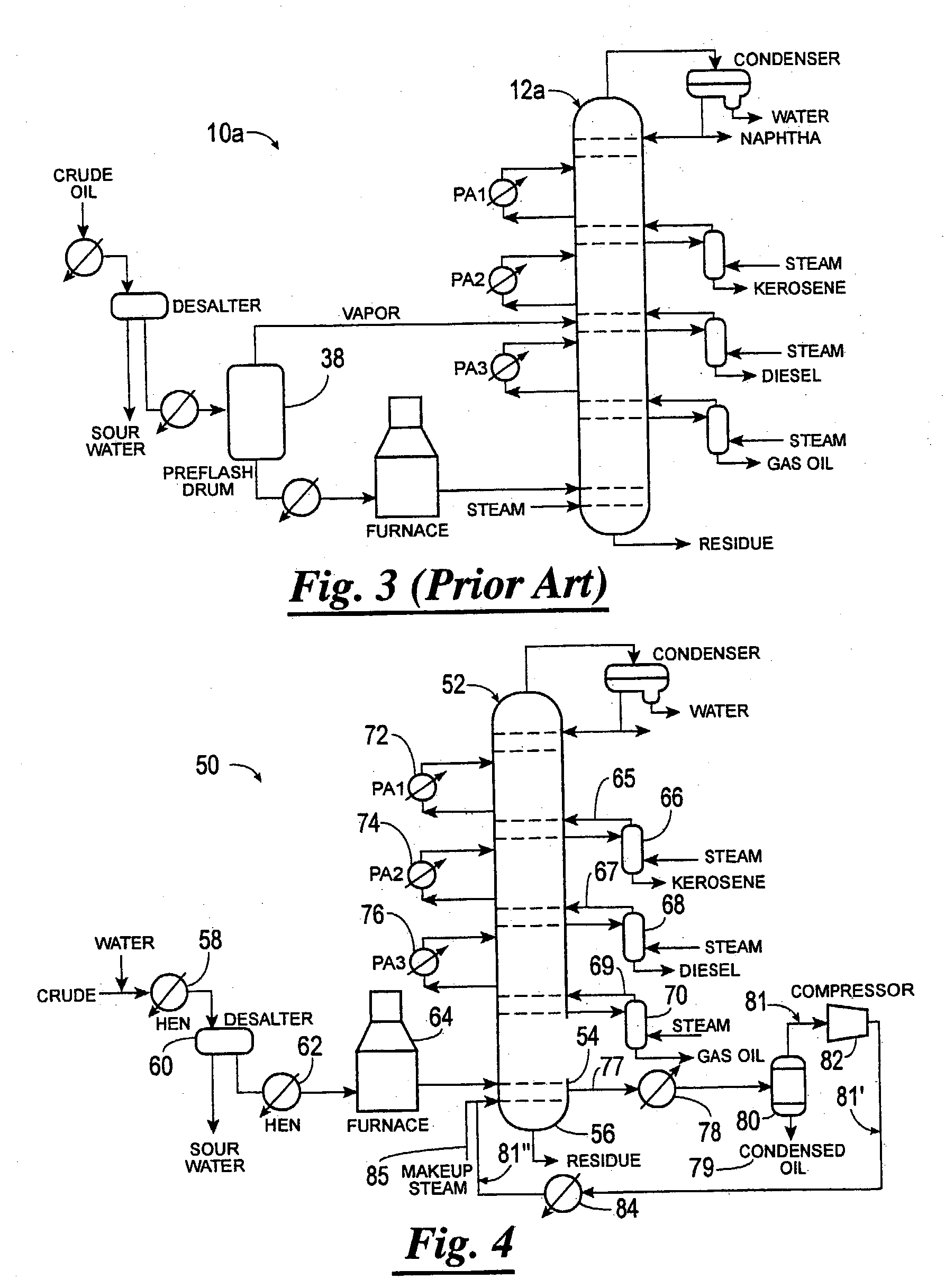
Method of increasing distillates yield in crude oil distillation
Methods of separating components of a mixture, such as crude oil, are disclosed which increase the yield of individual components while decreasing the yield of residue. In one method, a heated mixture is fed to a column, a vapor stream is withdrawn from the column and separated, and a portion of the vapor stream is recycled back to the column. In another method, a mixture is separated into streams composed substantially of components having light, intermediate or heavy molecular weight and / or low, intermediate or high boiling point, respectively, and the streams are fed into the column at different positions. In both methods, individual light, intermediate and / or heavy molecular weight and / or low, intermediate and / or high boiling point component streams are then selectively withdrawn from the column.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
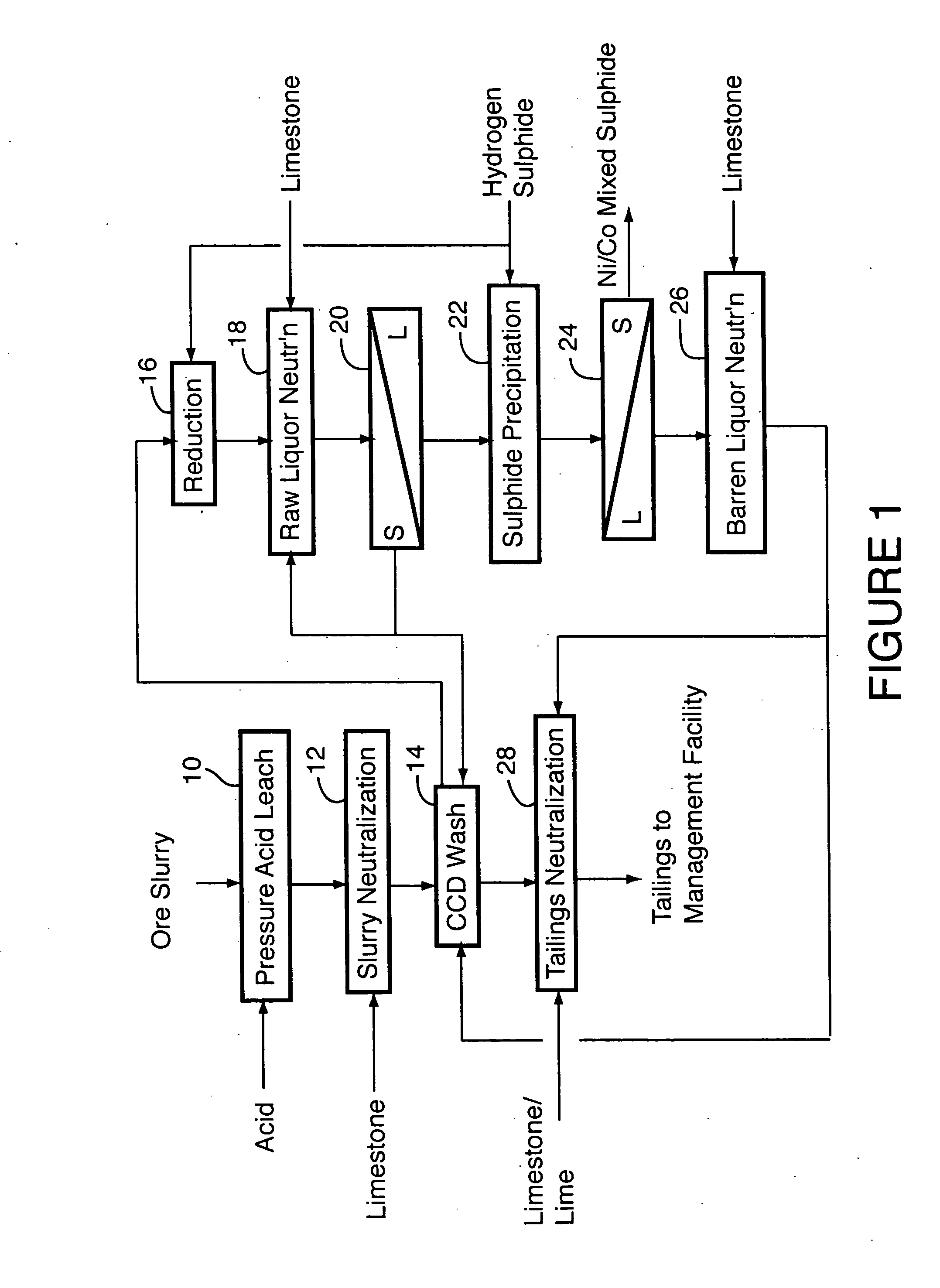
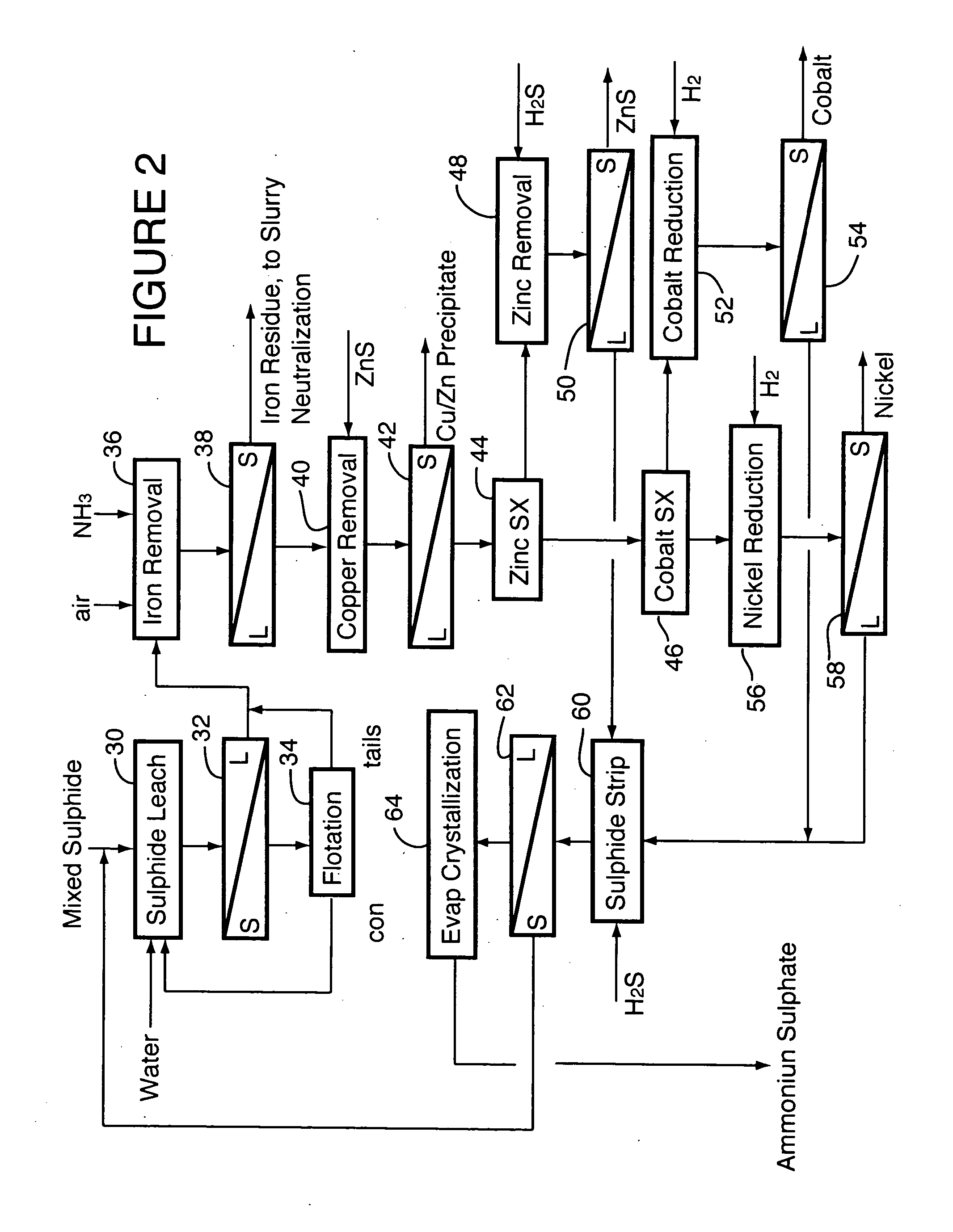
Process for recovery of nickel and cobalt from laterite ore
ActiveUS20060228279A1Efficient separation and recoveryHigh purityCobalt sulfidesSolvent extractionFree solutionSlurry
A process for recovering nickel and cobalt values from nickel- and cobalt-containing laterite ores as an enriched mixed nickel and cobalt sulphide intermediate and for producing nickel and cobalt metal from the nickel and cobalt sulphide intermediate. The laterite ore is leached as a slurry in a pressure acid leach containing an excess of aqueous sulphuric acid at high pressure and temperature, excess free acid in the leach slurry is partially neutralized to a range of 5 to 10 g / L residual free H2SO4 and washed to yield a nickel- and cobalt-containing product liquor, the product liquor is subjected to a reductant to reduce any Cr(VI) in solution to Cr(III), the reduced product liquor is neutralized to precipitate ferric iron and silicon at a pH of about 3.5 to 4.0, and the neutralized and reduced product liquor is contacted with hydrogen sulphide gas to precipitate nickel and cobalt sulphides. The precipitated nickel and cobalt sulphides can be leached in a water slurry in a pressure oxidation leach, the leach solution subjected to iron hydrolysis and precipitation, the iron-free solution contacted with zinc sulphide to precipitate copper, the iron- and copper-free solution subjected to zinc and cobalt extraction by solvent extraction to produce a nickel raffinate, the nickel raffinate contacted with hydrogen gas to produce nickel powder and the cobalt strip solution from the solvent extraction step contacted with hydrogen gas to produce cobalt powder.
Owner:SHERRITT INTERNATIONAL
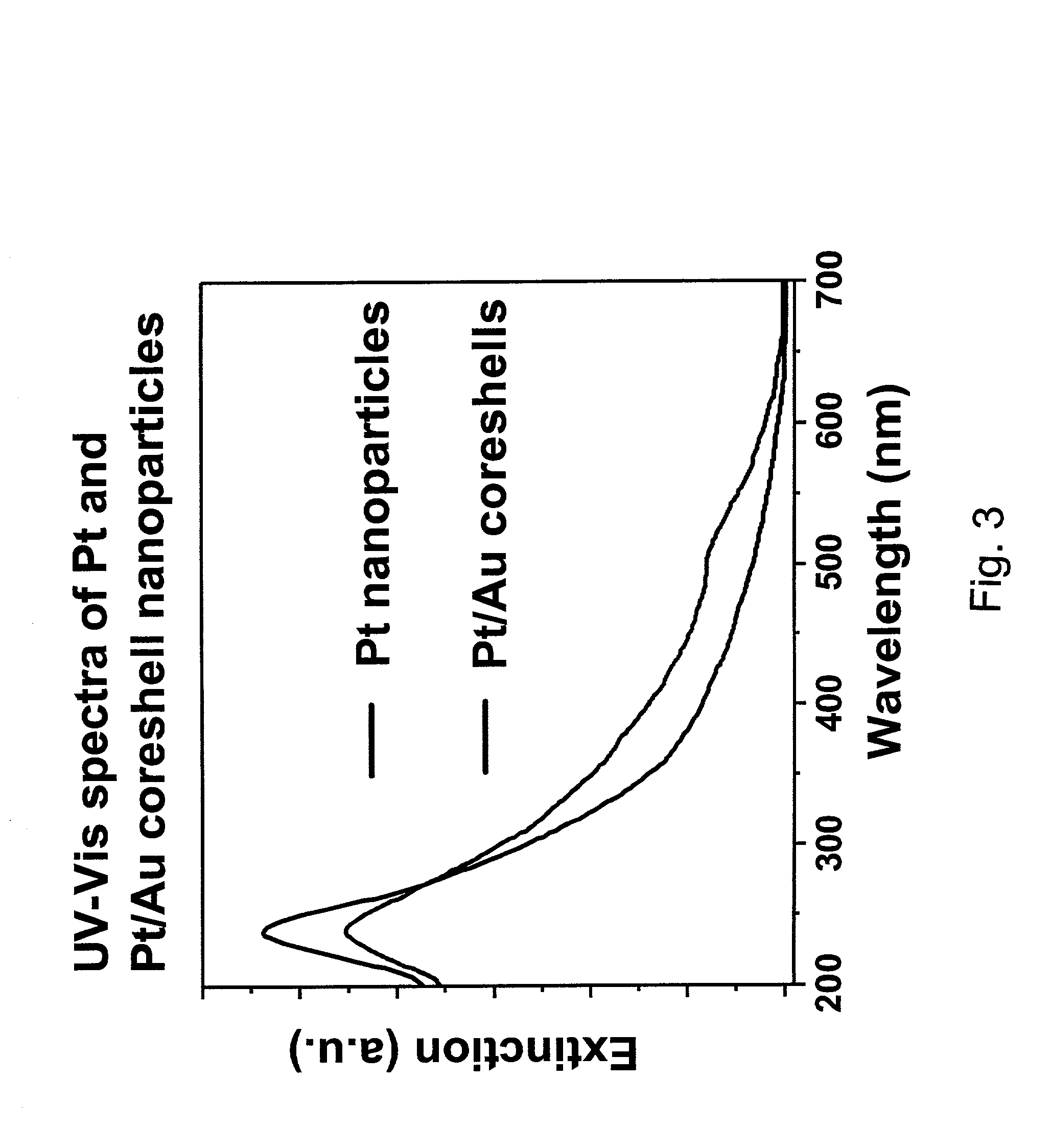
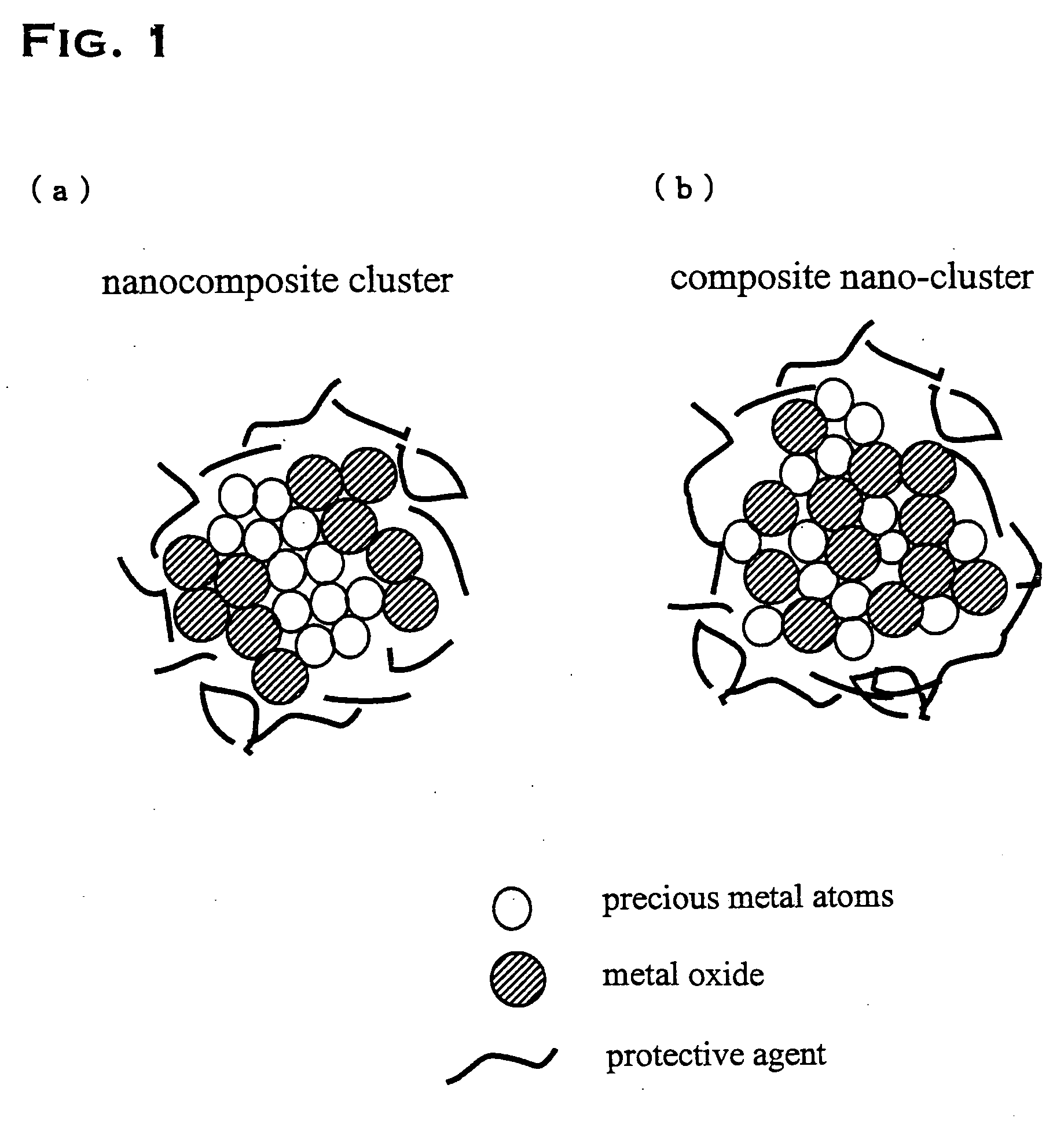
Precious metal - metal oxide composite cluster
InactiveUS20050065026A1Increase the effective surface areaSmall granularityMaterial nanotechnologyTransportation and packagingOxide compositeMaterials science
The present invention provides a precious metal—metal oxide composite cluster, wherein said cluster is formed as a single particle by combining a precious metal portion comprising a single atom or an aggregate of a plurality of atoms consisting of one or more precious metals, and a metal oxide portion comprising a single molecule or an aggregate of a plurality of molecules consisting of one or more metal oxides, and wherein said particle has a particle size between 1 and 100 nm.
Owner:TANAKA PRECIOUS METAL IND
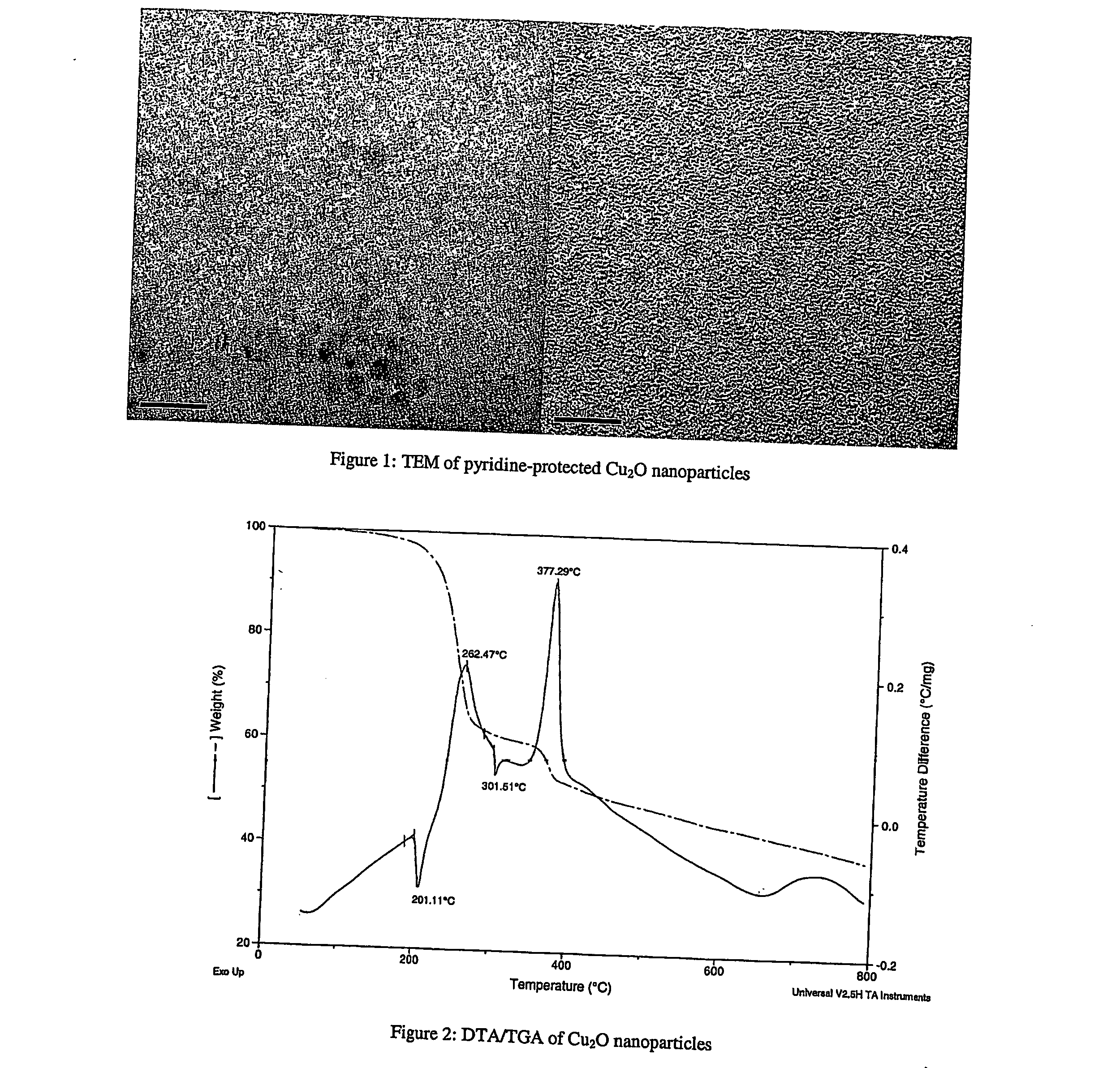
Metal oxide-containing nanoparticles
InactiveUS20060084278A1Easy to modifyRaise the ratioMaterial nanotechnologyOxide/hydroxide preparationNanoparticleCopper oxide nanoparticles
The present invention provides a copper oxide-containing composition that includes copper oxide nanoparticles and one or more heteroatom donor ligands bonded to the surface of the nanoparticles, where x and y are numbers having a ratio that is equal to the ratio of the average number of M atoms to the average number of 0 atoms in the nanoparticles. The nanoparticles are stabilized by the one or more heteroatom donor ligands which act as a protective layer that cap the surface of the nanoparticles. The present invention also provides a solution of the copper oxide nanoparticles that may be applied to a substrate and then subsequently reduced to copper metal. Finally, the invention provides a method of preparing the copper oxide nanoparticles.
Owner:WAYNE STATE UNIV
Process for the rapid leaching of chalcopyrite in the absence of catalysts
InactiveUS6277341B1Increase surface areaImprove misalignmentSolvent extractionGold compoundsPregnant leach solutionChalcopyrite
Owner:MINTEK
Process for preparing nano-sized metal oxide particles
InactiveUS20050260122A1Efficiently provideNanosized metal oxide particles more efficientlyNanostructure manufactureGold compoundsHigh concentrationAlcohol
The present invention is directed to novel sol-gel methods in which metal oxide precursor and an alcohol-based solution are mixed to form a reaction mixture that is then allowed to react to produce nanosized metal oxide particles. The methods of the present invention are more suitable for preparing nanosized metal oxide than are previously-described sol-gel methods. The present invention can provide for nanosized metal oxide particles more efficiently than the previously-described sol-gel methods by permitting higher concentrations of metal oxide precursor to be employed in the reaction mixture. The foregoing is provided by careful control of the pH conditions during synthesis and by ensuring that the pH is maintained at a value of about 7 or higher.
Owner:KANEKA CORP +1
Pure metal and ceramic nanofibers
InactiveUS20140332733A1Few voidFew defectOrganic active ingredientsGold compoundsFiberElectrospinning
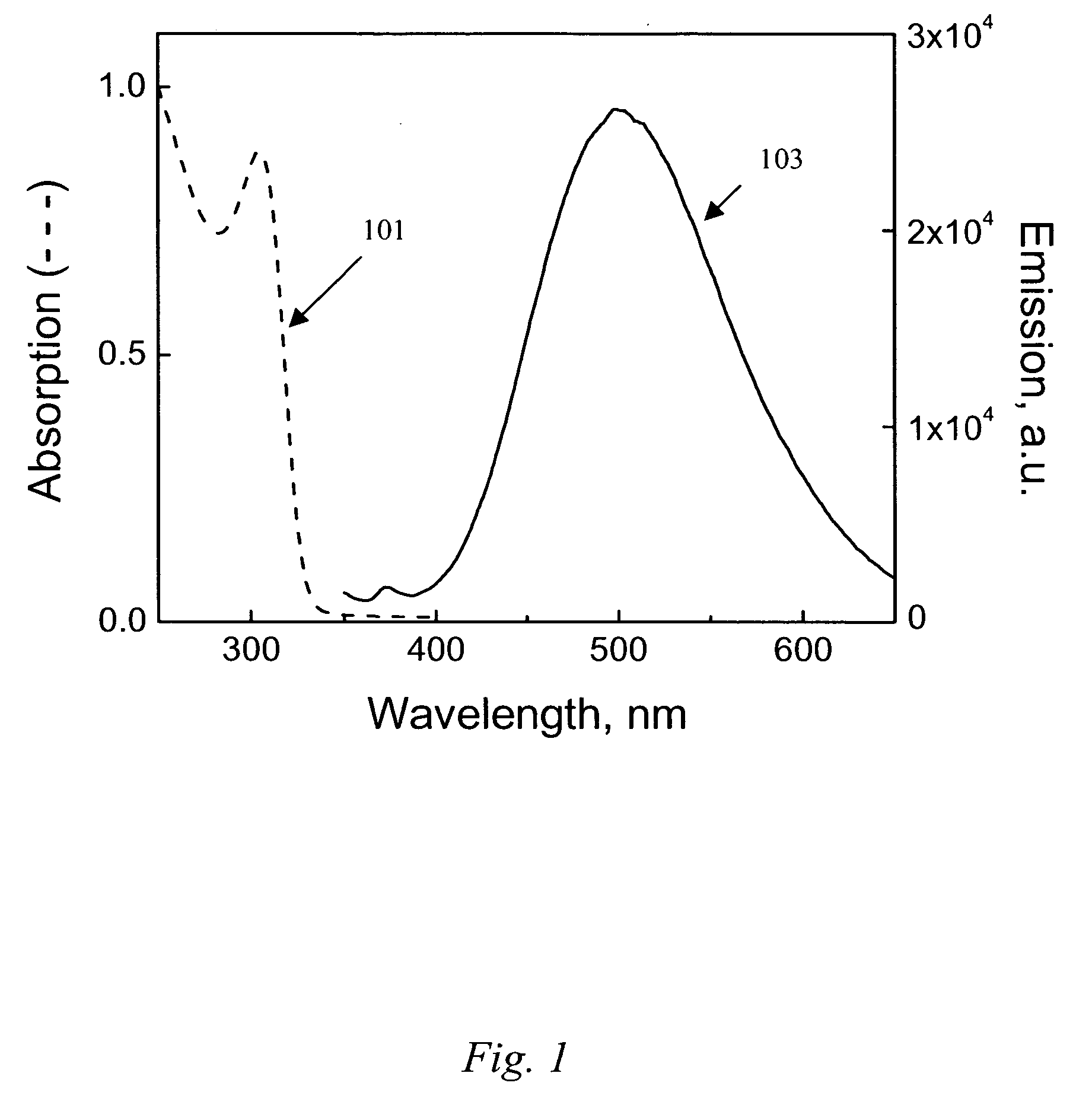
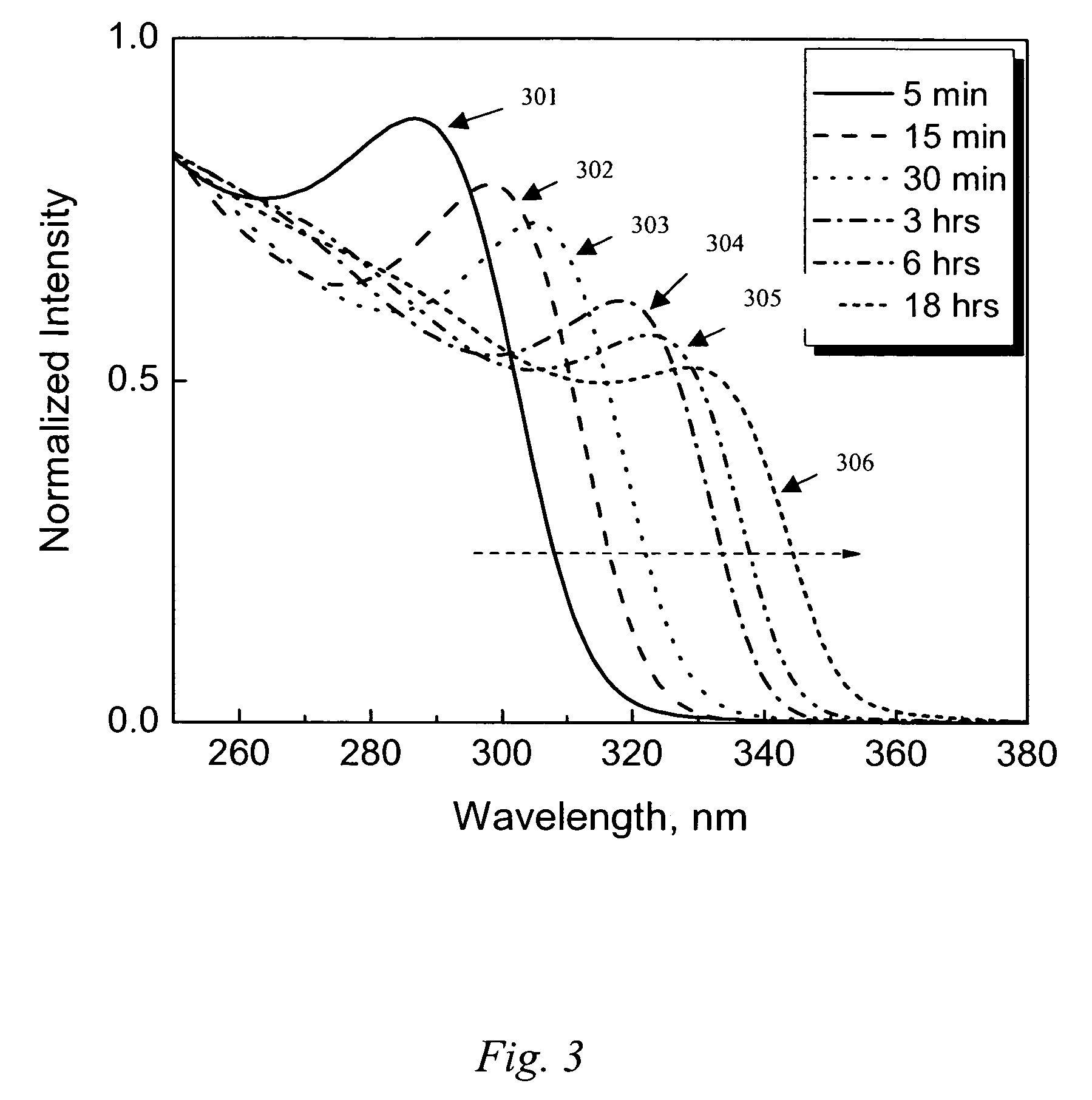
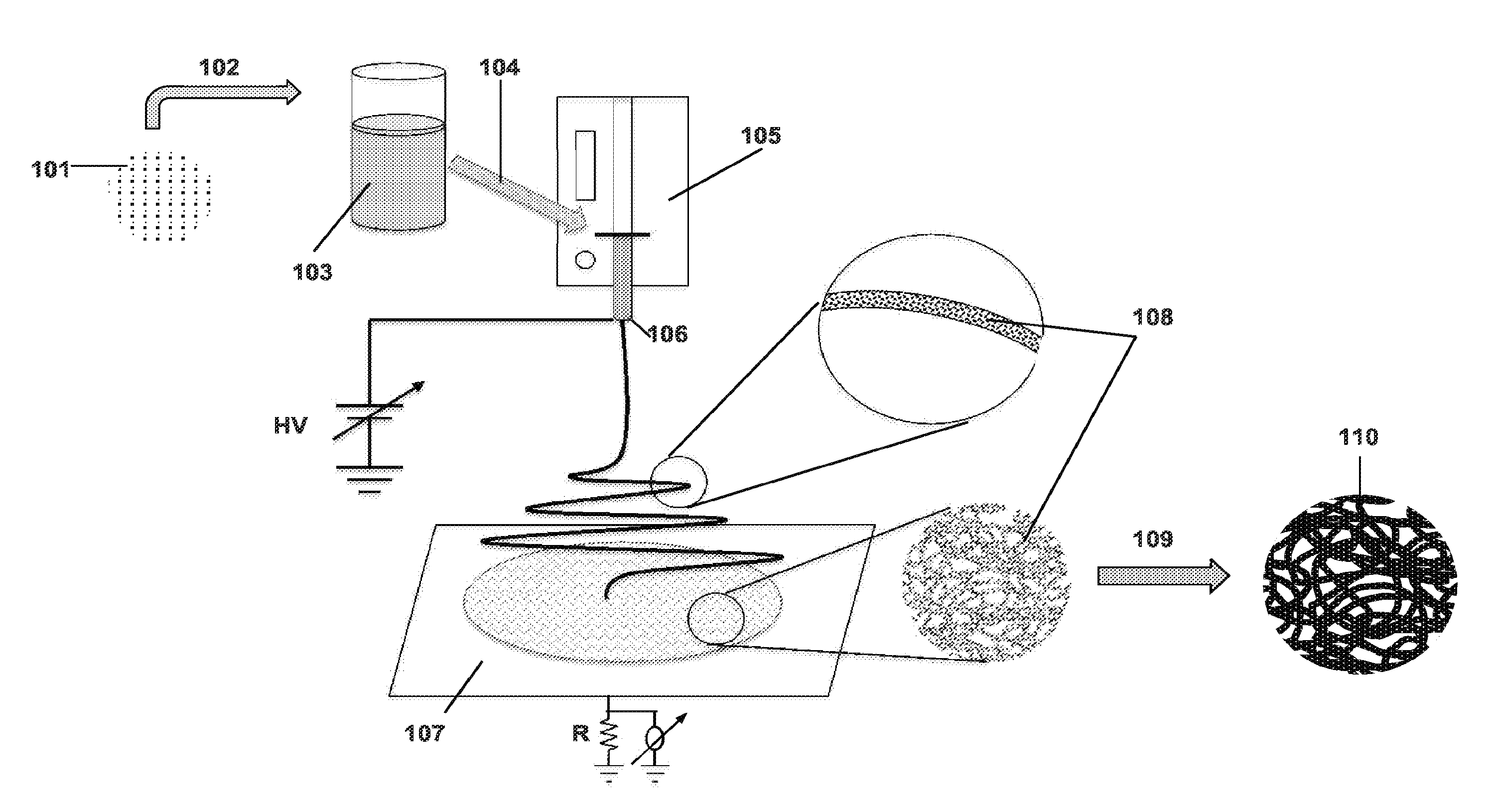
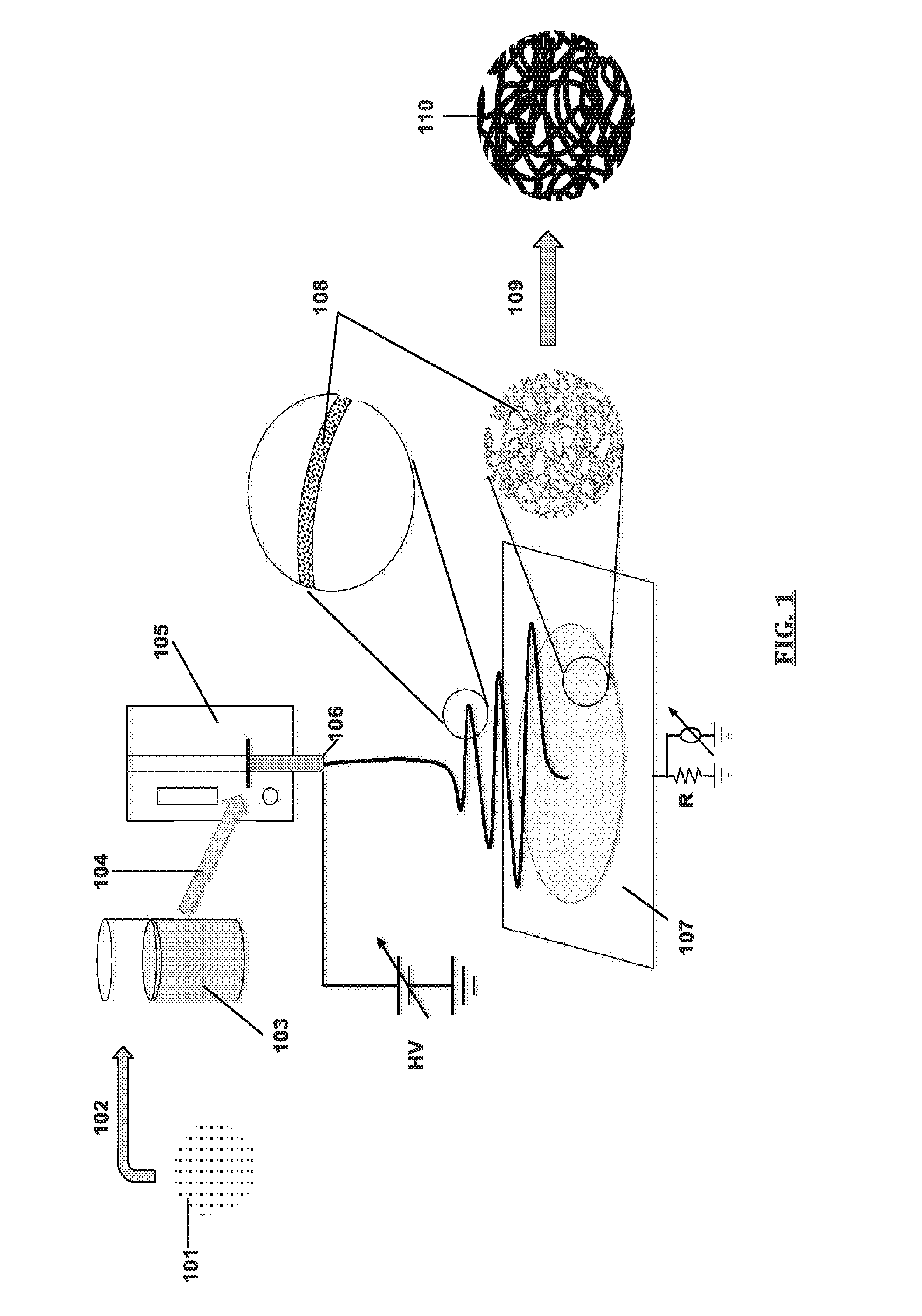
Provided herein are nanofibers and processes of preparing nanofibers. In some instances, the nanofibers are metal and / or ceramic nanofibers. In some embodiments, the nanofibers are high quality, high performance nanofibers, highly coherent nanofibers, highly continuous nanofibers, or the like. In some embodiments, the nanofibers have increased coherence, increased length, few voids and / or defects, and / or other advantageous characteristics. In some instances, the nanofibers are produced by electrospinning a fluid stock having a high loading of nanofiber precursor in the fluid stock. In some instances, the fluid stock comprises well mixed and / or uniformly distributed precursor in the fluid stock. In some instances, the fluid stock is converted into a nanofiber comprising few voids, few defects, long or tunable length, and the like.
Owner:CORNELL UNIVERSITY
Method for producing fine spherical particles of carbonate or hydroxide of nickel, cobalt or copper
InactiveUS6197273B1Amino compound purification/separationOxide/hydroxide preparationSal ammoniacCobalt metal
The invention provides a process for production of fine spherical particles of a carbonate or a hydroxide of nickel, cobalt or copper which comprises: dissolving a carbonate or a hydroxide of nickel, cobalt or copper having the general formula (I)wherein M represents Ni, Co or Cu, and x and y are numerals satisfying the followings: 0<=x<=2, 0<=y<=2 and x+y=2, in aqueous ammonia, converting the resulting solution to a W / O emulsion containing droplets of the solution in a non-aqueous medium, and then removing volatile components including ammonia from within the droplets, thereby precipitating a basic carbonate or a hydroxide of a metal selected from nickel, cobalt and copperin the droplets.The fine spherical particles of a carbonate or a hydroxide of nickel, cobalt or copper obtained according to the process of the invention are especially useful as a precursor for the manufacture of uniform, fine spherical particles of nickel, copper or cobalt metal, as well as useful as themselves as a catalyst for use in organic synthesis, a carrier, a pigment, a filler or a glaze.
Owner:SAKAI CHEM IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com