Process for the rapid leaching of chalcopyrite in the absence of catalysts
Inactive Publication Date: 2001-08-21
MINTEK
View PDF12 Cites 46 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The leaching of sulphide materials using ferric sulphate to oxidise the sulphide mineral usually leads to rapid oxidation and thus dissolution of the required values.
However, in the case of chalcopyrite, leaching using ferric sulphate takes an inordinate length of time, presumably as a consequence of passivation of the exposed surfaces of th
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric potential / voltage | aaaaa | aaaaa |
| Surface potential | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Login to View More
Abstract
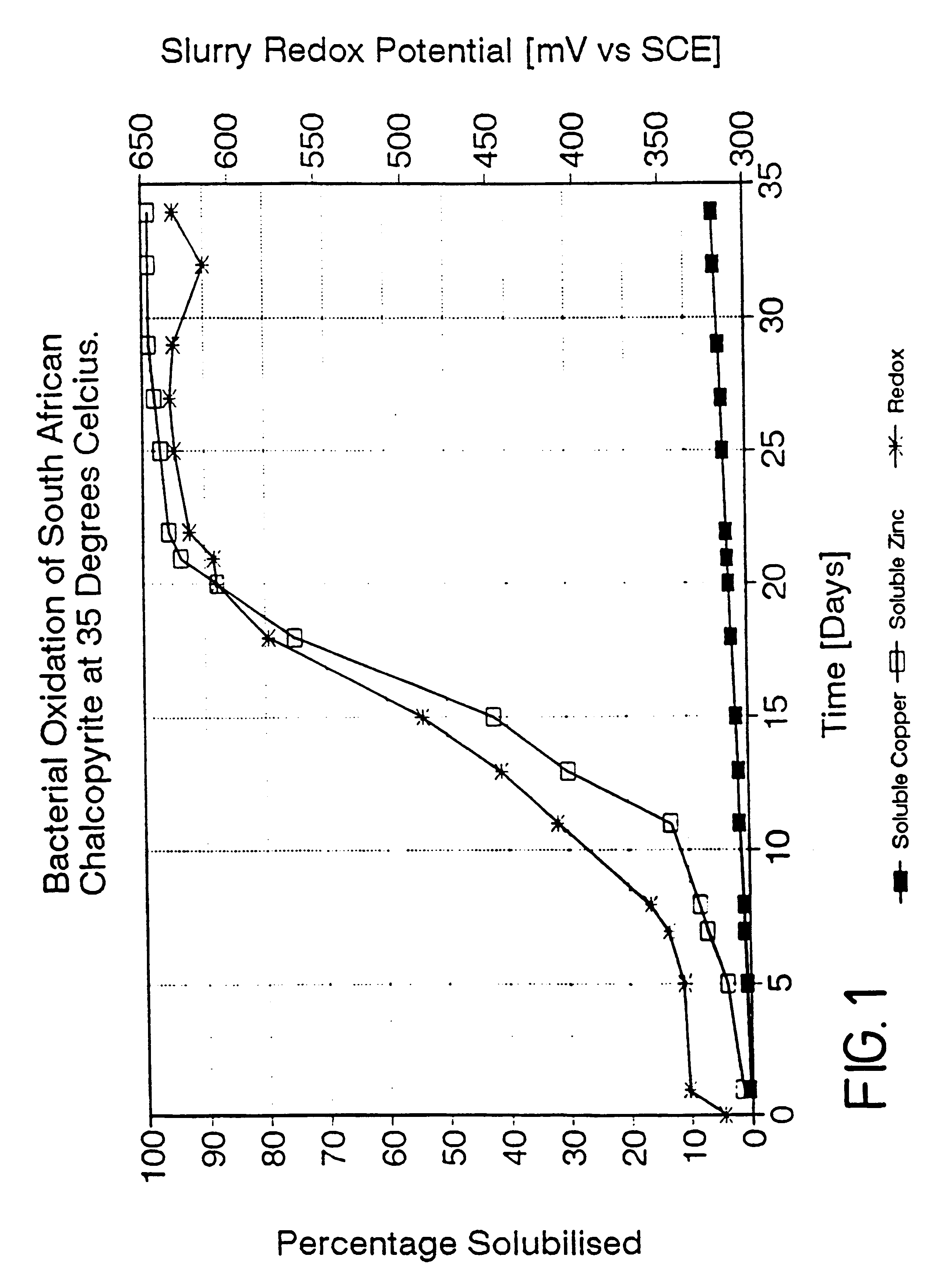
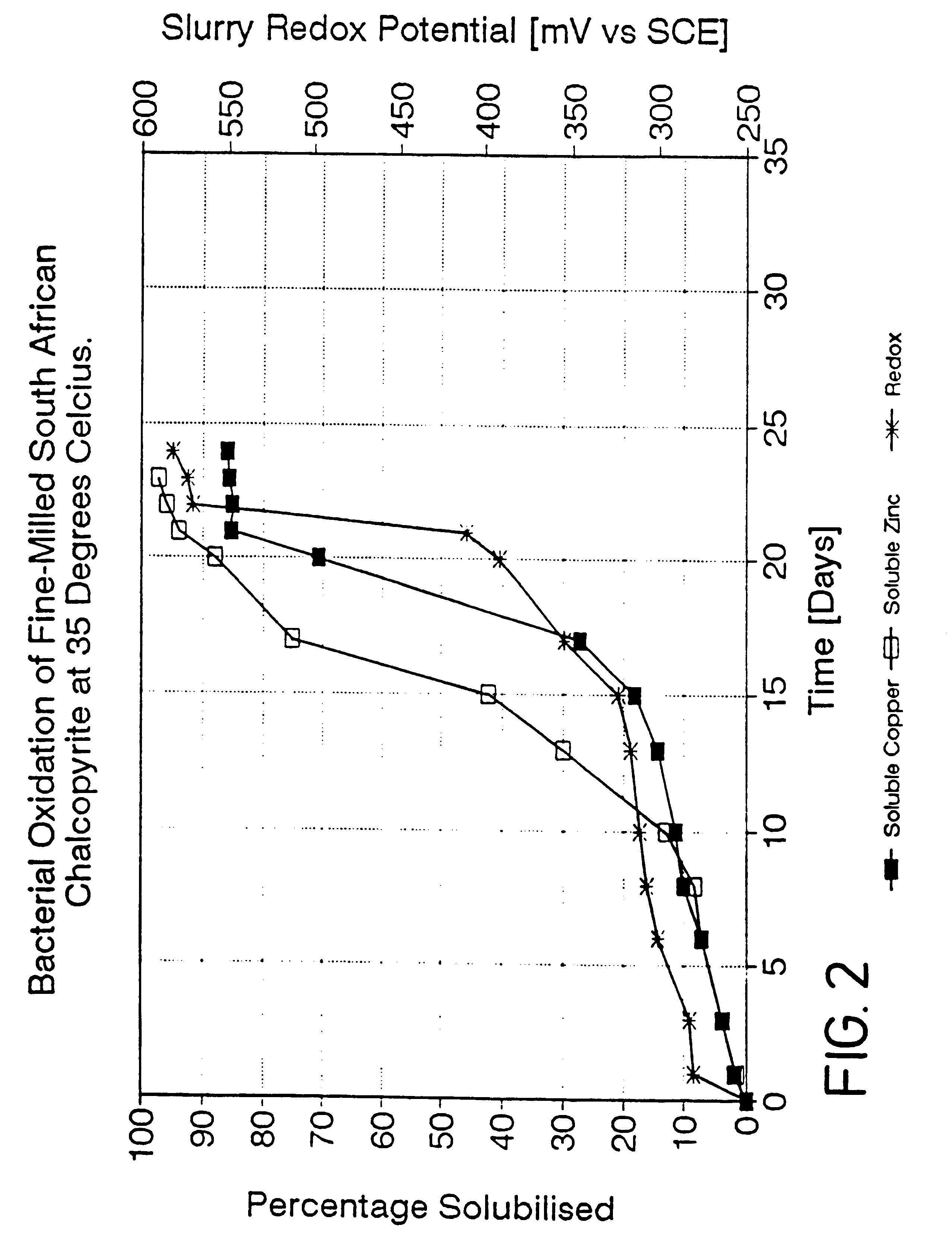
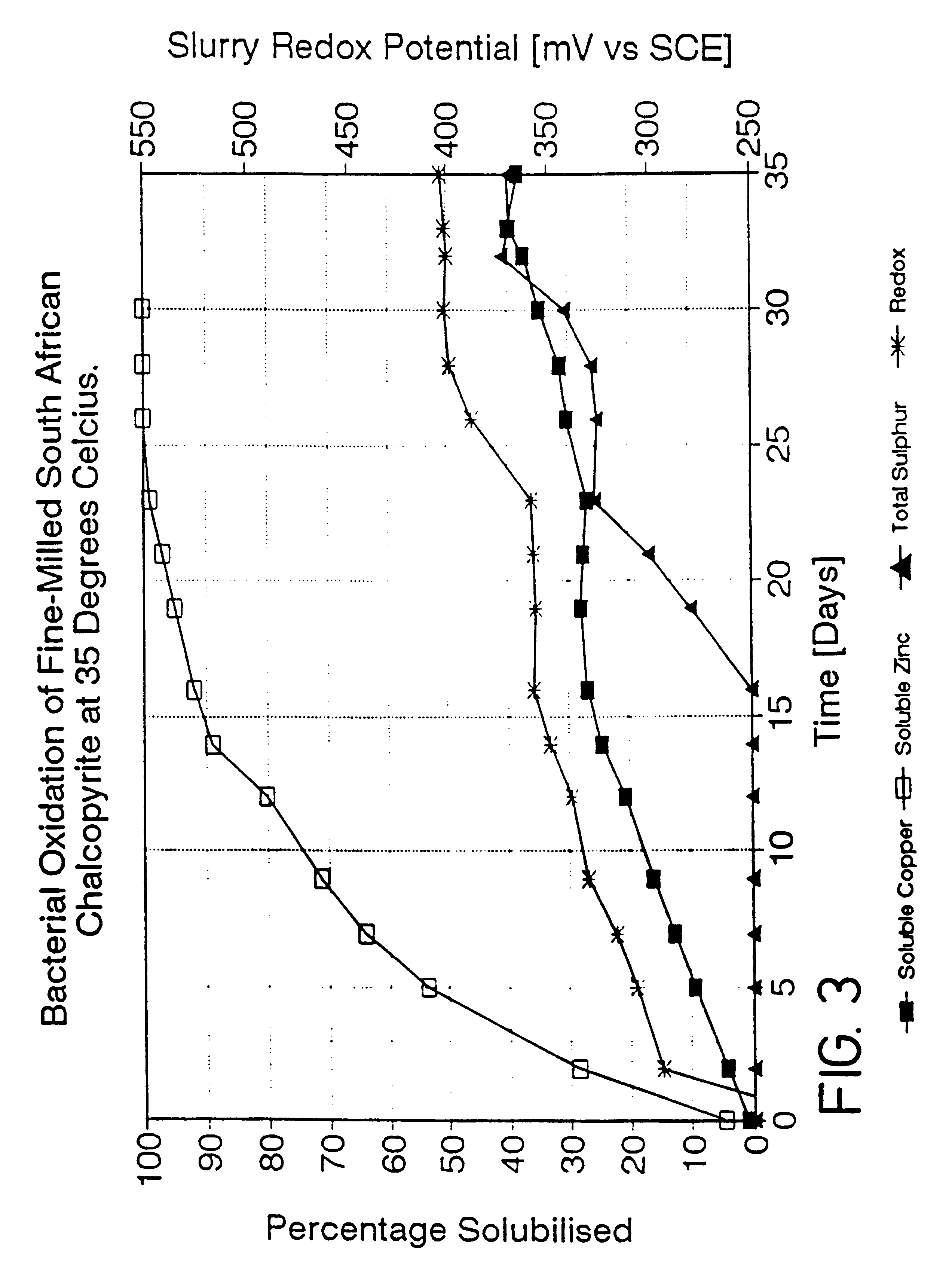
A process is provided for the leaching of copper from chalcopyrite using ferric sulfate in which acceptable rates of leaching are achieved by controlling the surface potential of the chalcopyrite to an empirically determined "window" within the broad range of 350 to 450 mV. The most effective process conditions involve the selection of the said surface potential; the leach temperature; the pH of the leach solution; and the fineness of the grind of the chalcopyrite. The invention may be applied to both tank leaching and heap leaching processes.
Description
This invention relates to the leaching of chalcopyrite in order to recover the copper values therein and more particularly to a process in which ferric ions which are preferably, but not necessarily, bacterially generated, are employed to effect oxidation of the sulphide material in the chalcopyrite.BACKGROUND TO THE INVENTIONThe leaching of sulphide materials using ferric sulphate to oxidise the sulphide mineral usually leads to rapid oxidation and thus dissolution of the required values. The surface potential of the mineral in such a case is usually in the range of 550 to 660 mV and the rate of leaching is henerally expected to increase with increasing potential. This is the case for such sulphide minerals as pyrite (FeS.sub.2); arseno-pyrite (FeAsS); chalcocite (Cu.sub.2 S); and sphalerite (ZnS).However, in the case of chalcopyrite, leaching using ferric sulphate takes an inordinate length of time, presumably as a consequence of passivation of the exposed surfaces of the sulphide...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C22B3/08C22B3/00C22B15/00
CPCC22B15/0071C22B3/08Y02P10/20
Inventor PINCHES, ANTHONYMYBURGH, PETER J.VAN DER MERWE, CHARMAINE
Owner MINTEK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com