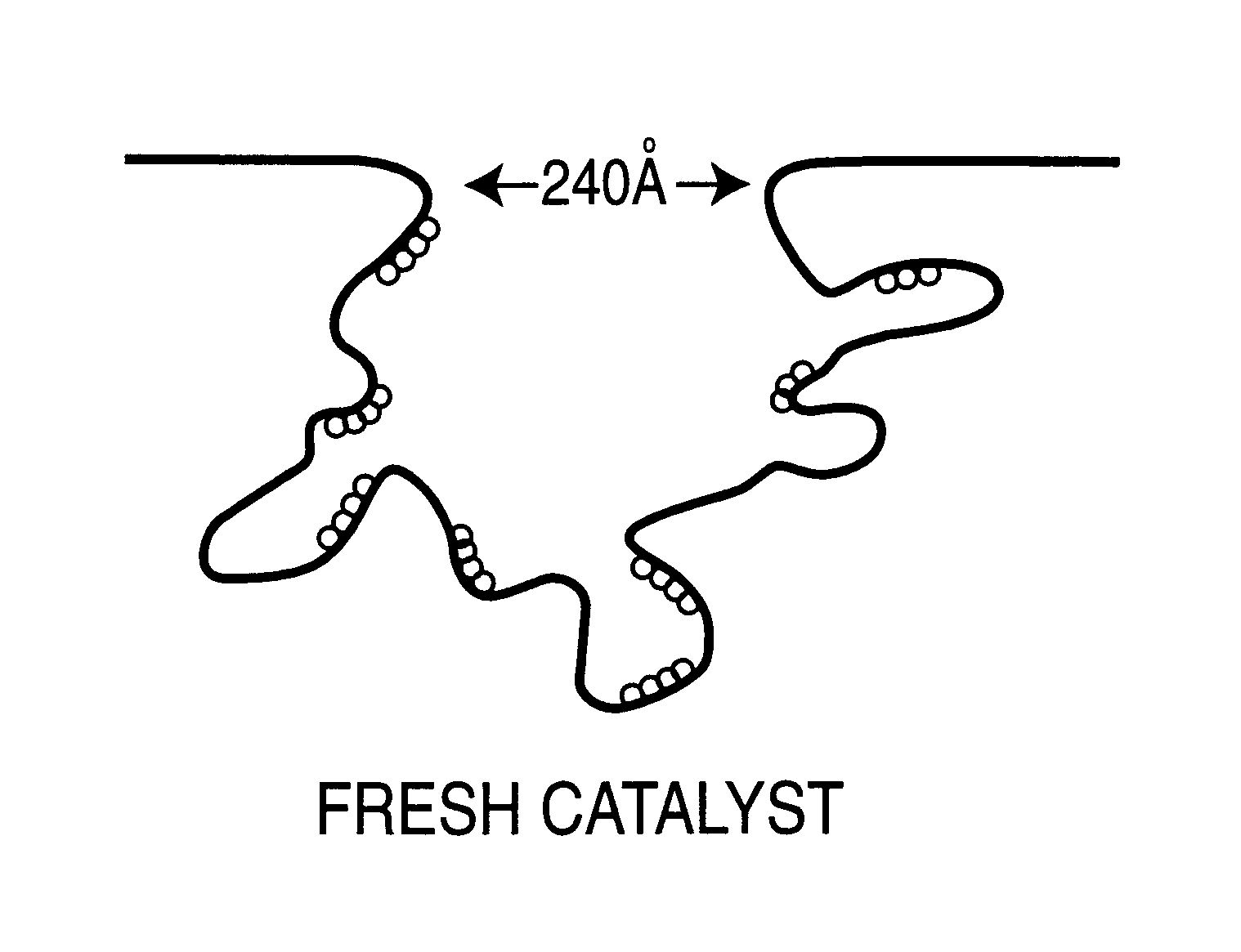
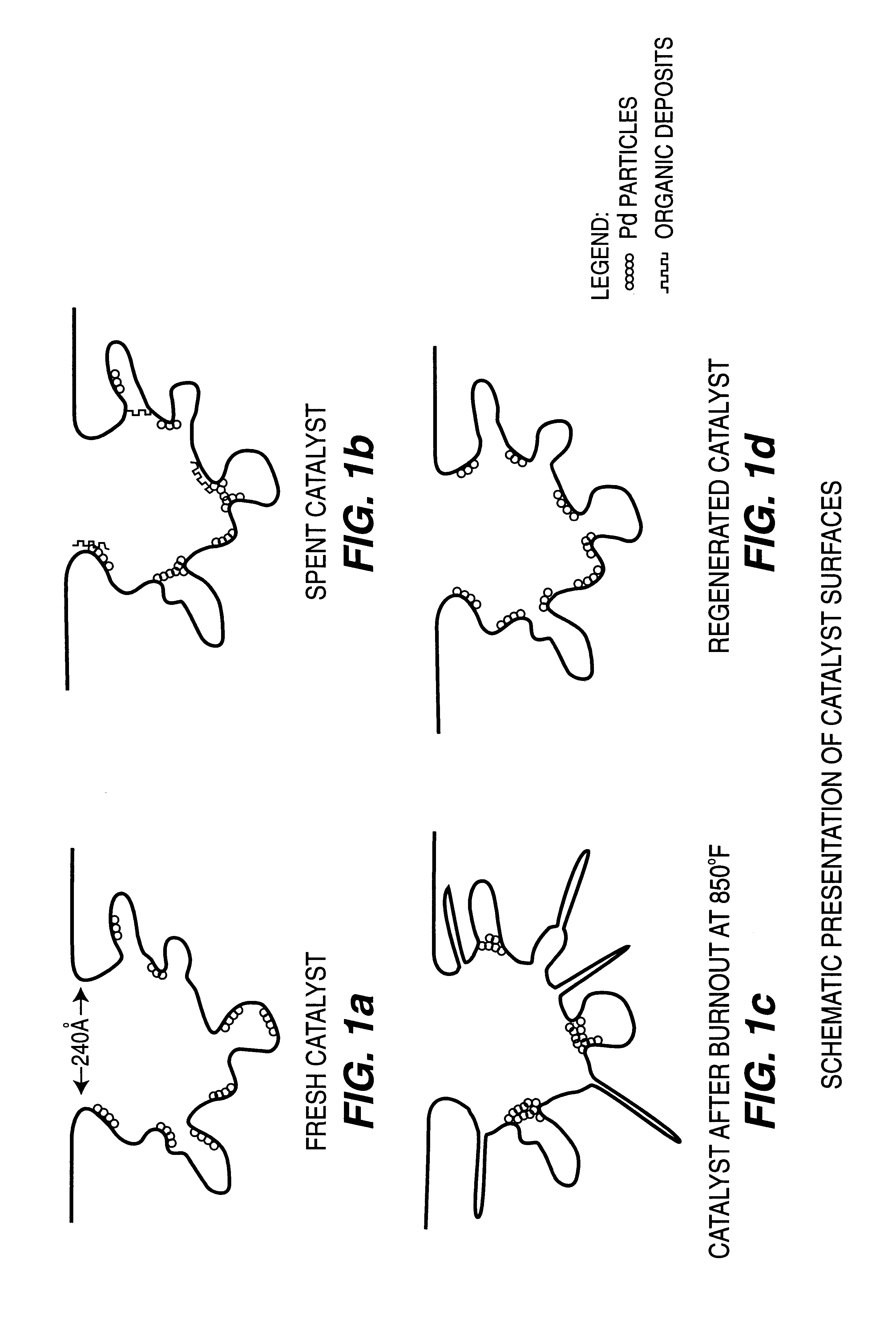
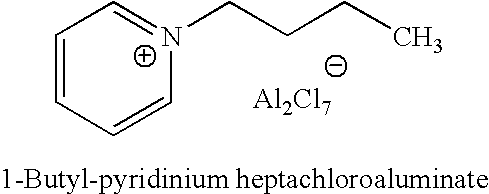Patents
Literature
5181results about "Combustible gas purification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Separation apparatus and separation method
InactiveUS20060000772A1Improve efficiencyHigh activityIon-exchange process apparatusSemi-permeable membranesBuffer solutionAnalytical chemistry
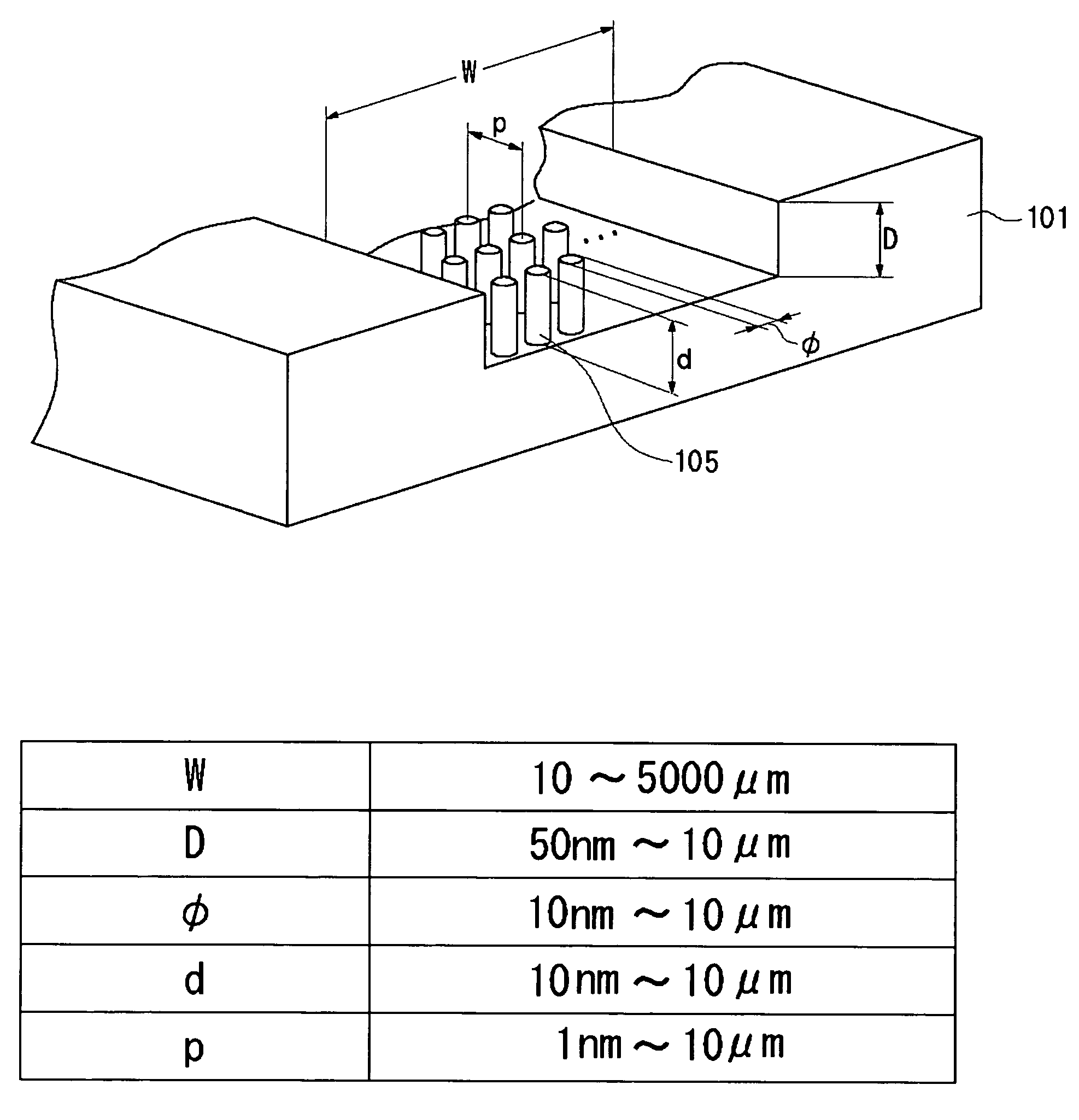
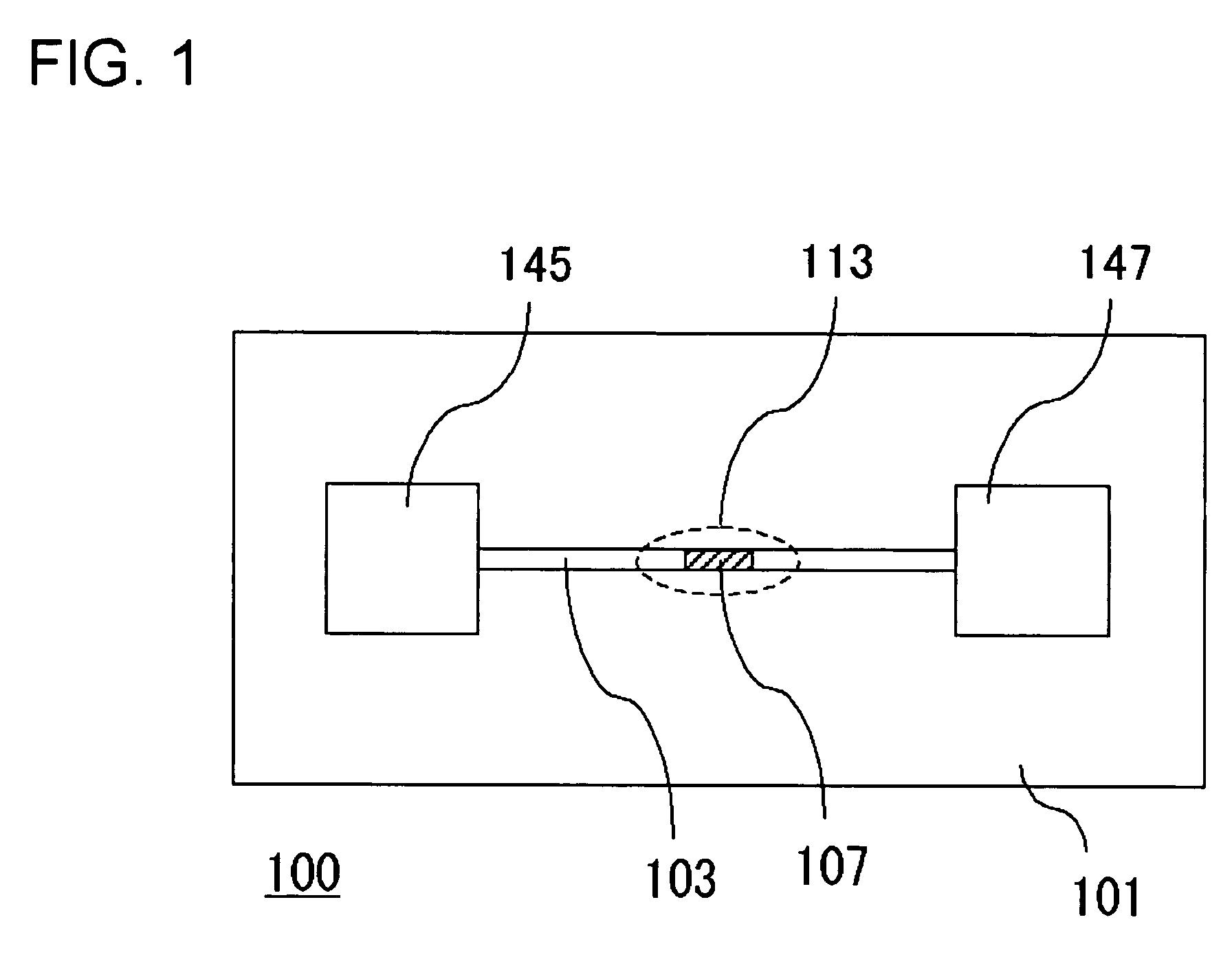
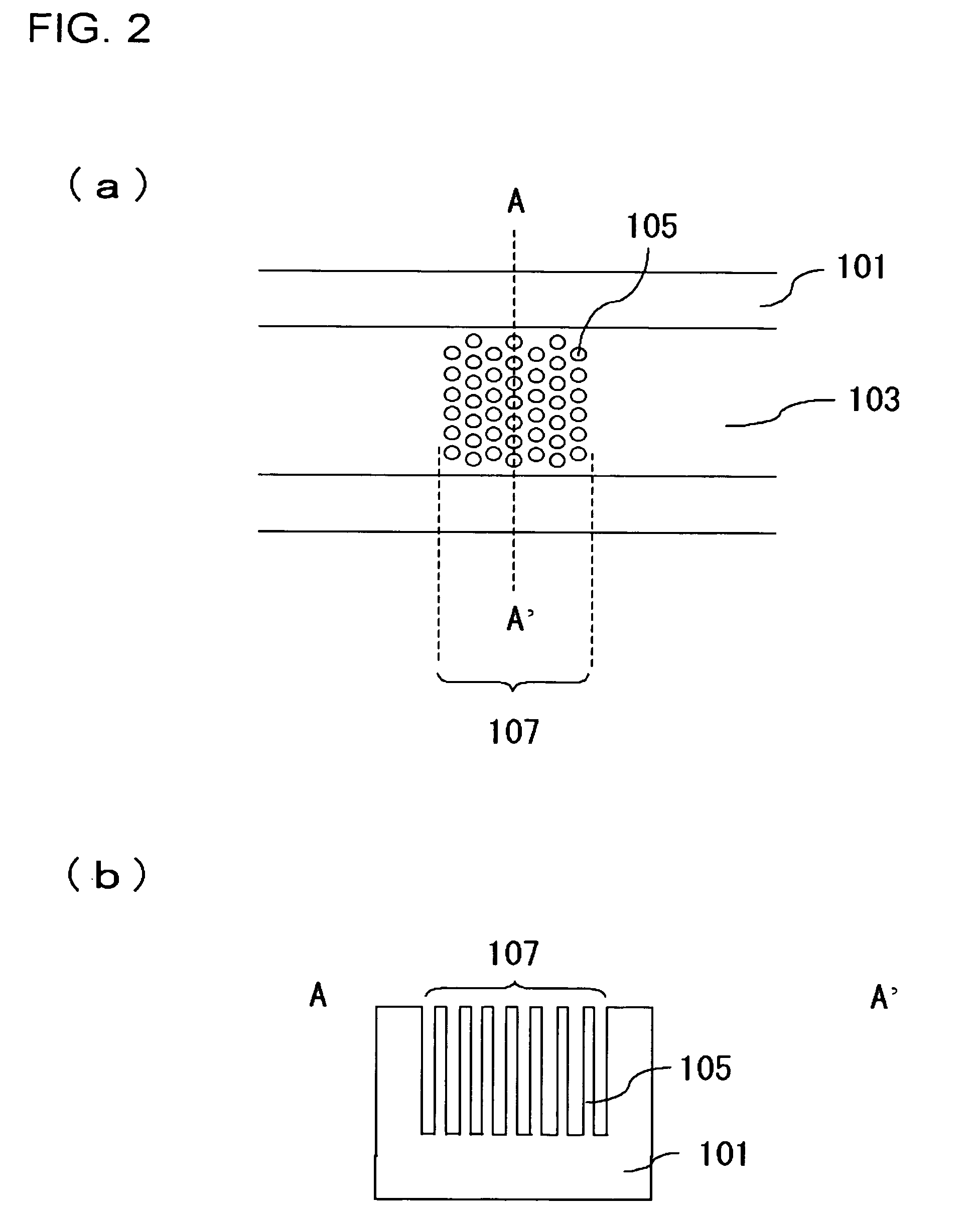
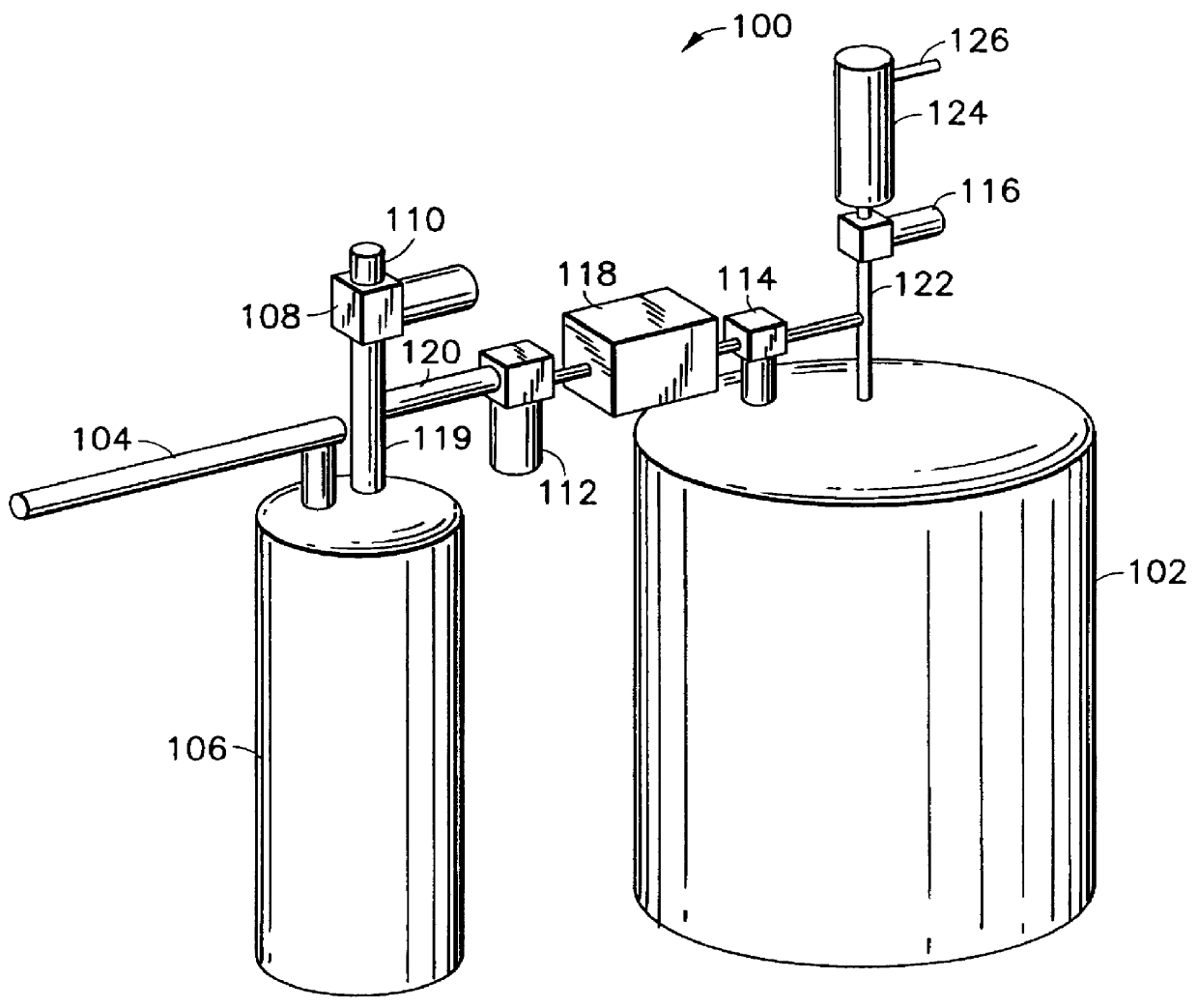
A channel (103) is formed in a substrate (101), and a portion of the channel (103) is provided with a separating portion (107). A number of pillars are formed in the separating portion (107), and an adsorptive substance layer having an adsorptive substance, which exhibits a specific interaction for a specific substance, immobilized on the surface thereof, is formed. Once a sample is introduced into the channel (103), the specific substance is adsorbed on the adsorptive substance layer to be separated from other components. After washing the inside of the channel (103) with a buffer solution, the specific substance is desorbed from the adsorptive substance layer by flowing a eluting solution through the channel (103) and the specific substance is recovered.
Owner:NEC CORP
Layered noble metal-containing exhaust gas catalyst and its preparation
InactiveUS6294140B1Shorten recovery timeImprove conversion efficiencyOrganic chemistryNitrogen compoundsCerium(IV) oxideEngineering
A catalyst for treating exhaust gas from an internal combustion engine includes a carrier body coated with an inner layer and an outer layer. The inner layer includes platinum deposited on a first support material and on a first oxygen storage component, and the outer layer includes platinum and rhodium deposited on a second support material and on a second oxygen storage component. The first and second support materials may be the same or different, and may be selected from the group of: silica, alumina, titania, zirconia, mixed oxides or mixtures thereof, and zirconia-rich zirconia / ceria mixed oxide. The first and second oxygen storage components may include ceria-rich ceria / zirconia mixed oxide compounds, optionally including praseodymia, yttria, neodymia, lanthana or mixtures thereof.
Owner:DMC2 DEGUSSA METALS +1
Catalytic Gasification Process with Recovery of Alkali Metal from Char
ActiveUS20090169448A1Quantity minimizationThermal non-catalytic crackingMuffle furnacesPhysical chemistryAlkali metal
Processes are described for the extraction and recovery of alkali metal from the char that results from catalytic gasification of a carbonaceous material. Among other steps, the processes of the invention include a hydrothermal leaching step in which a slurry of insoluble particulate comprising insoluble alkali metal compounds is treated with carbon dioxide and steam at elevated temperatures and pressures to effect the conversion of insoluble alkali metal compounds to soluble alkali metal compounds. Further, processes are described for the catalytic gasification of a carbonaceous material where a substantial portion of alkali metal is extracted and recovered from the char that results from the catalytic gasification process.
Owner:SURE CHAMPION INVESTMENT LTD
Catalytic Gasification Process with Recovery of Alkali Metal from Char
ActiveUS20090169449A1Quantity minimizationThermal non-catalytic crackingMuffle furnacesParticulatesSlurry
Processes are described for the extraction and recovery of alkali metal from the char that results from catalytic gasification of a carbonaceous material. Among other steps, the processes of the invention include a hydrothermal leaching step in which a slurry of insoluble particulate comprising insoluble alkali metal compounds is treated with carbon dioxide and steam at elevated temperatures and pressures to effect the conversion of insoluble alkali metal compounds to soluble alkali metal compounds. Further, processes are described for the catalytic gasification of a carbonaceous material where a substantial portion of alkali metal is extracted and recovered from the char that results from the catalytic gasification process.
Owner:SURE CHAMPION INVESTMENT LTD
Catalysts, activating agents, support media, and related methodologies useful for making catalyst systems especially when the catalyst is deposited onto the support media using physical vapor deposition
InactiveUS20050095189A1Improve performanceEasy to useMaterial nanotechnologyInternal combustion piston enginesGas phaseAdditive ingredient
Use of physical vapor deposition methodologies to deposit nanoscale gold on activating support media makes the use of catalytically active gold dramatically easier and opens the door to significant improvements associated with developing, making, and using gold-based, catalytic systems. The present invention, therefore, relates to novel features, ingredients, and formulations of gold-based, heterogeneous catalyst systems generally comprising nanoscale gold deposited onto a nanoporous support.
Owner:3M INNOVATIVE PROPERTIES CO
Reclamation of a Titanosilicate, and Reconstitution of an Active Oxidation Catalyst
InactiveUS20080064591A1Acceptable product selectivityAcceptable selectivityMolecular sieve catalystsOther chemical processesCatalytic metalTitanium
A method of reclaiming a titanosilicate from a deactivated or spent oxidation catalyst containing a titanosilicate having deposited thereon one or more catalytic metals, such as gold, and optionally, one or more promoter metals, the method involving treating the deactivated catalyst with an oxidant; contacting the oxidant-treated catalyst with acid, preferably aqua regia; washing the titanosilicate to remove residual acid; and optionally drying and / or calcining. A method of reconstituting an active oxidation catalyst from a spent or deactivated oxidation catalyst, the method involving reclaiming the titanosilicate as noted above, and then depositing one or more catalytic metals and, optionally, one or more promoter metals onto the reclaimed titanosilicate.
Owner:DOW GLOBAL TECH LLC
Method for converting biomass into synthesis gas using a pressurized multi-stage progressively expanding fluidized bed gasifier followed by an oxyblown autothermal reformer to reduce methane and tars
InactiveUS20100040510A1Lower Level RequirementsGasifier mechanical detailsCombustible gas catalytic treatmentSyngasFluidized bed gasifier
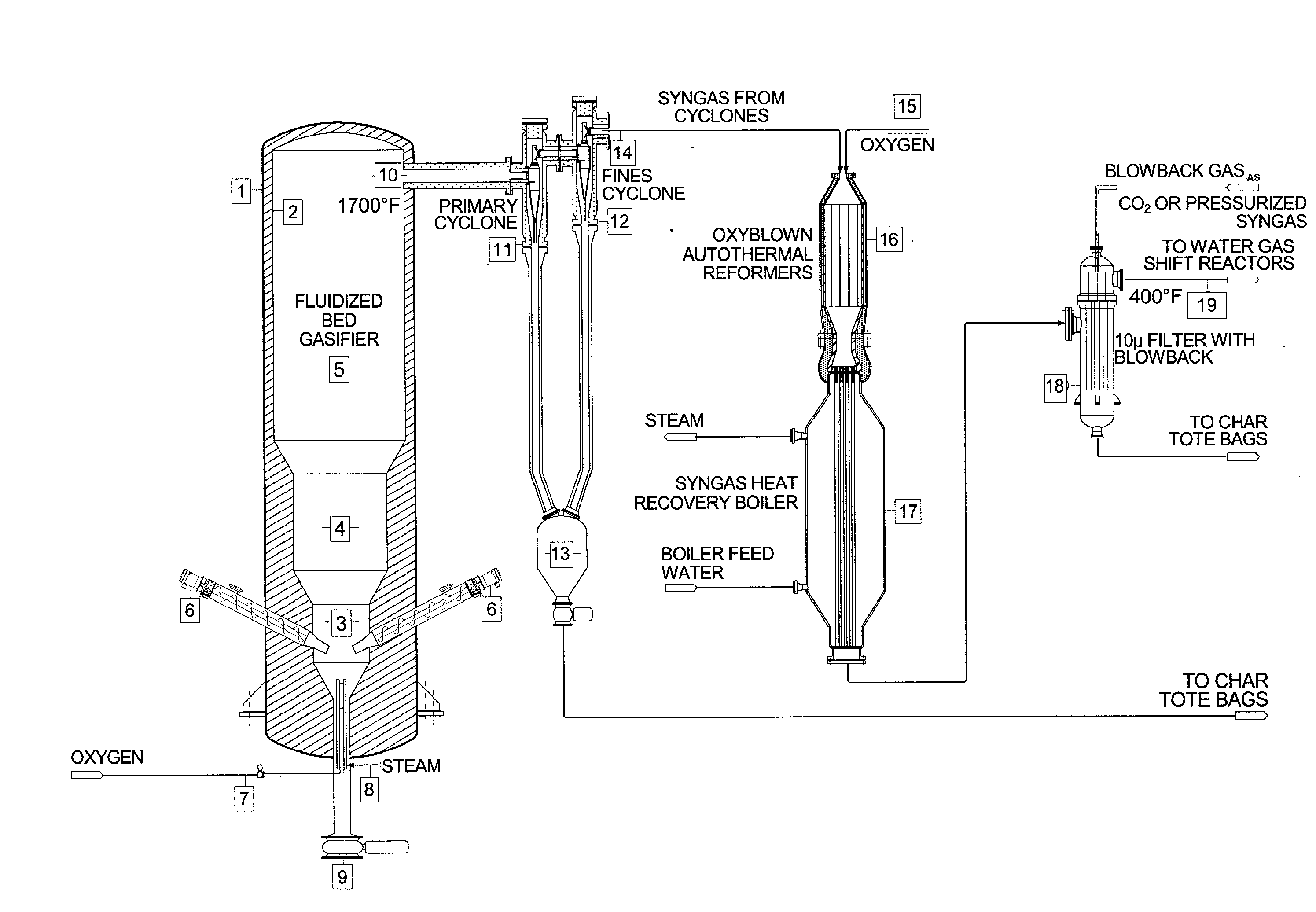
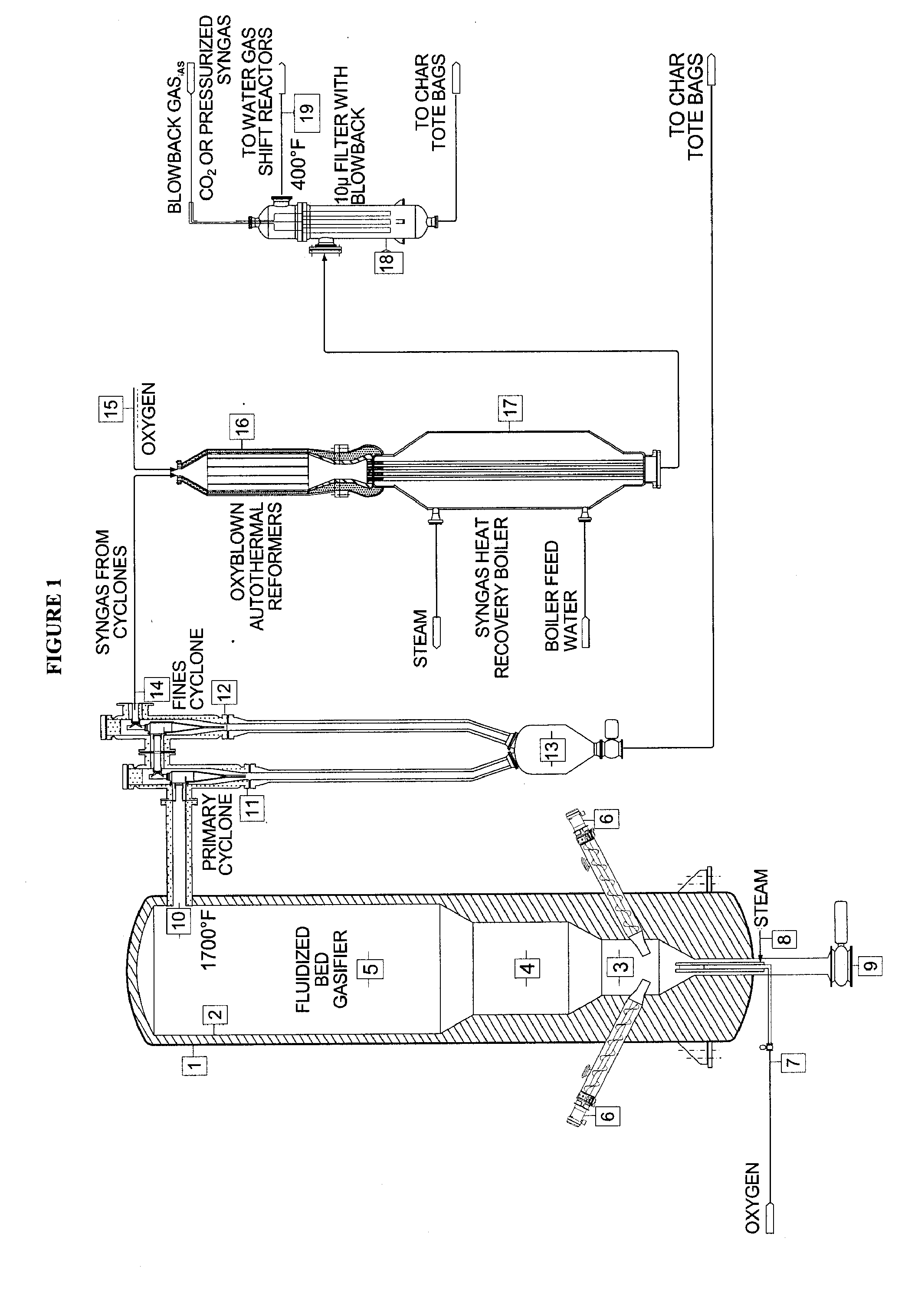
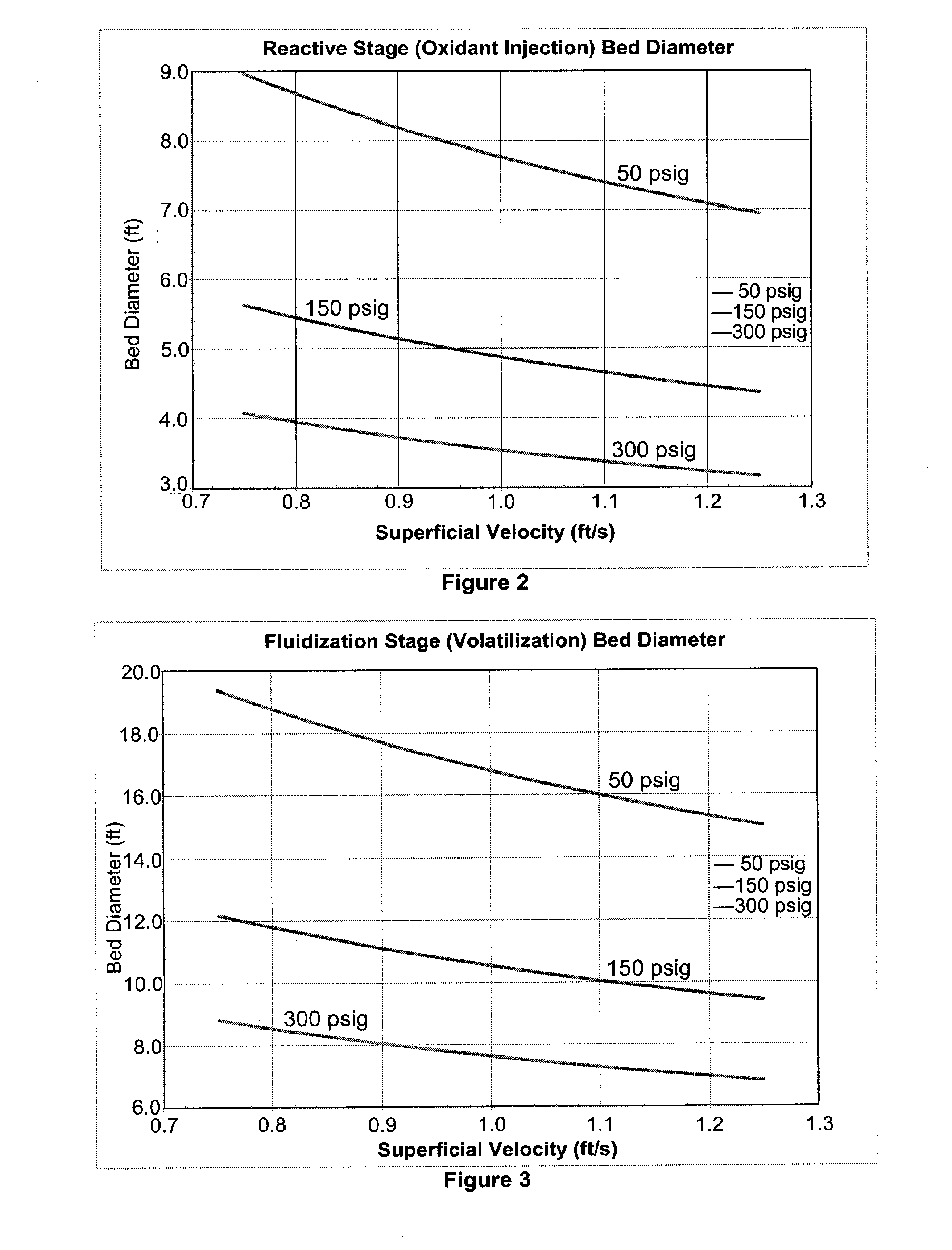
The invention provides systems and methods for converting biomass into syngas using a pressurized multi-stage progressively expanding fluidized bed gasifier to eliminate or reduce the formation of methane, volatiles such as BTX, and tars. The gasifier may include a reactive stage that may receive a biomass feed through a feed line and oxygen through an oxygen feed line. The gasifier may also include a fluidized bed section that may be configured to receive the reaction products from the first stage, mix them and perform fluidized bed activity. A gasifier may also have a disengagement section that may be configured to separate fluidized media and particulate matter from syngas product. A gasification system may also include oxyblown catalytic autothermal reactor and a cryogenic air separation unit.
Owner:SYNT
Separation of carbon dioxide (CO2) from gas mixtures
ActiveUS7618606B2Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentCo2 removalSorbent
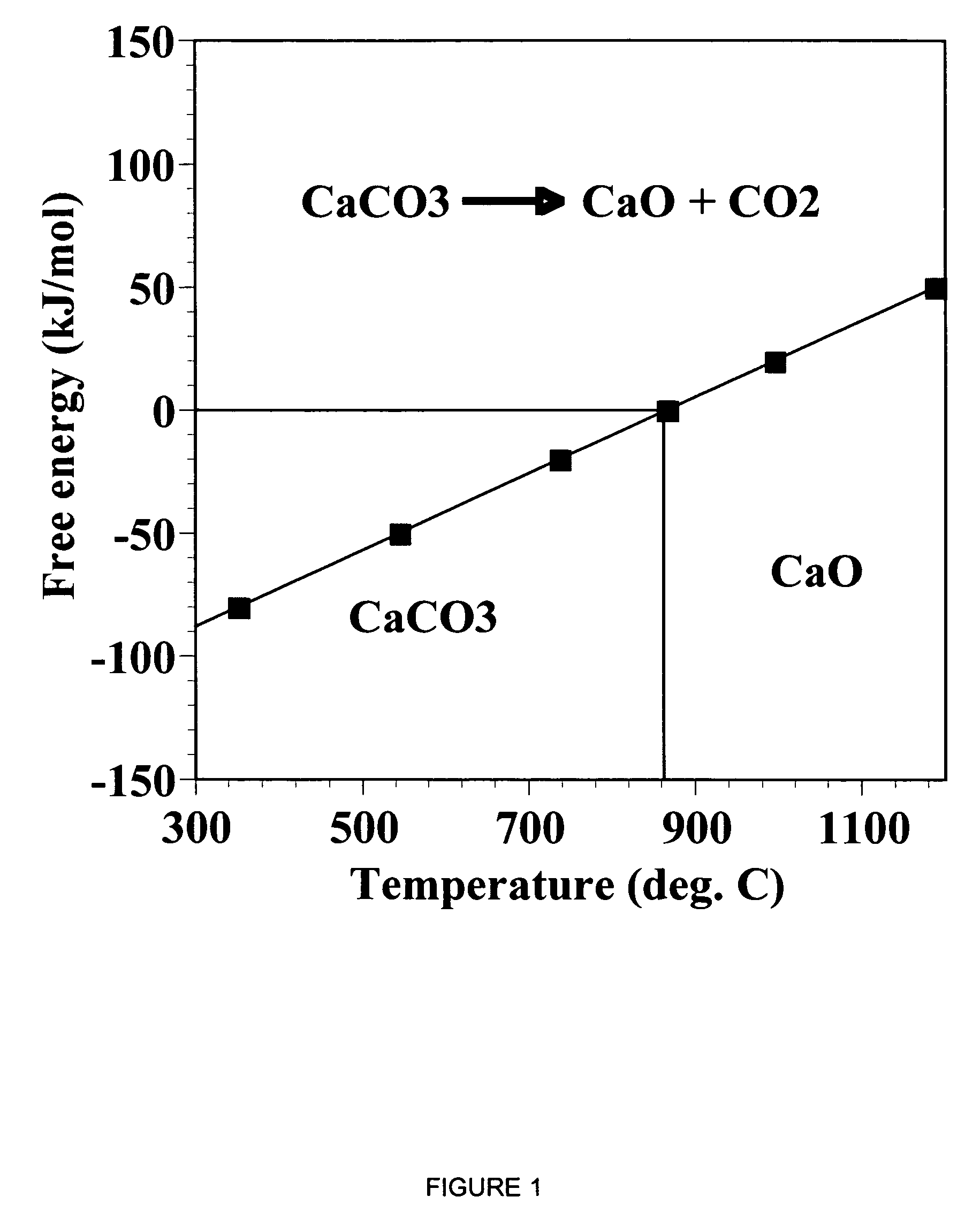
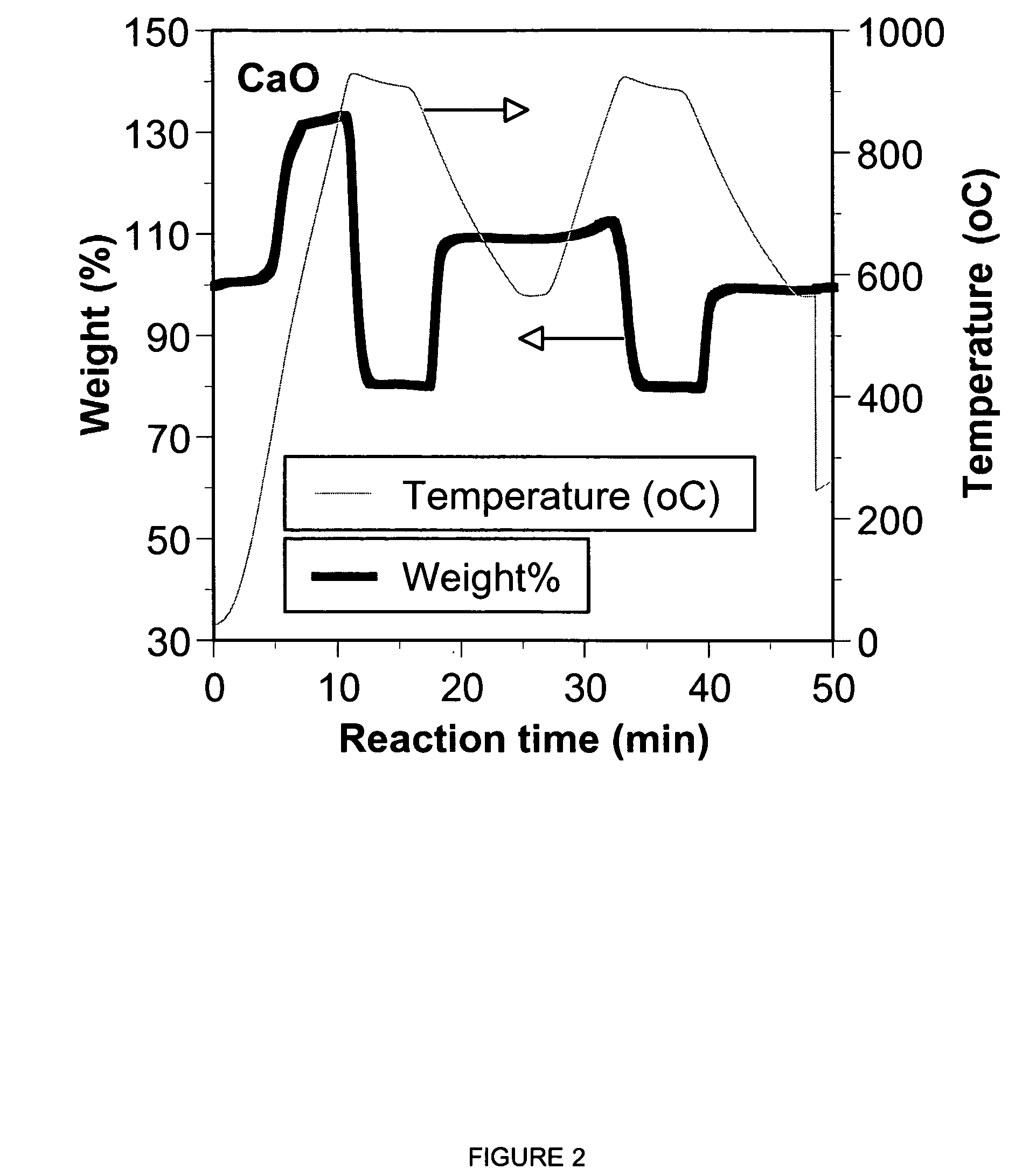
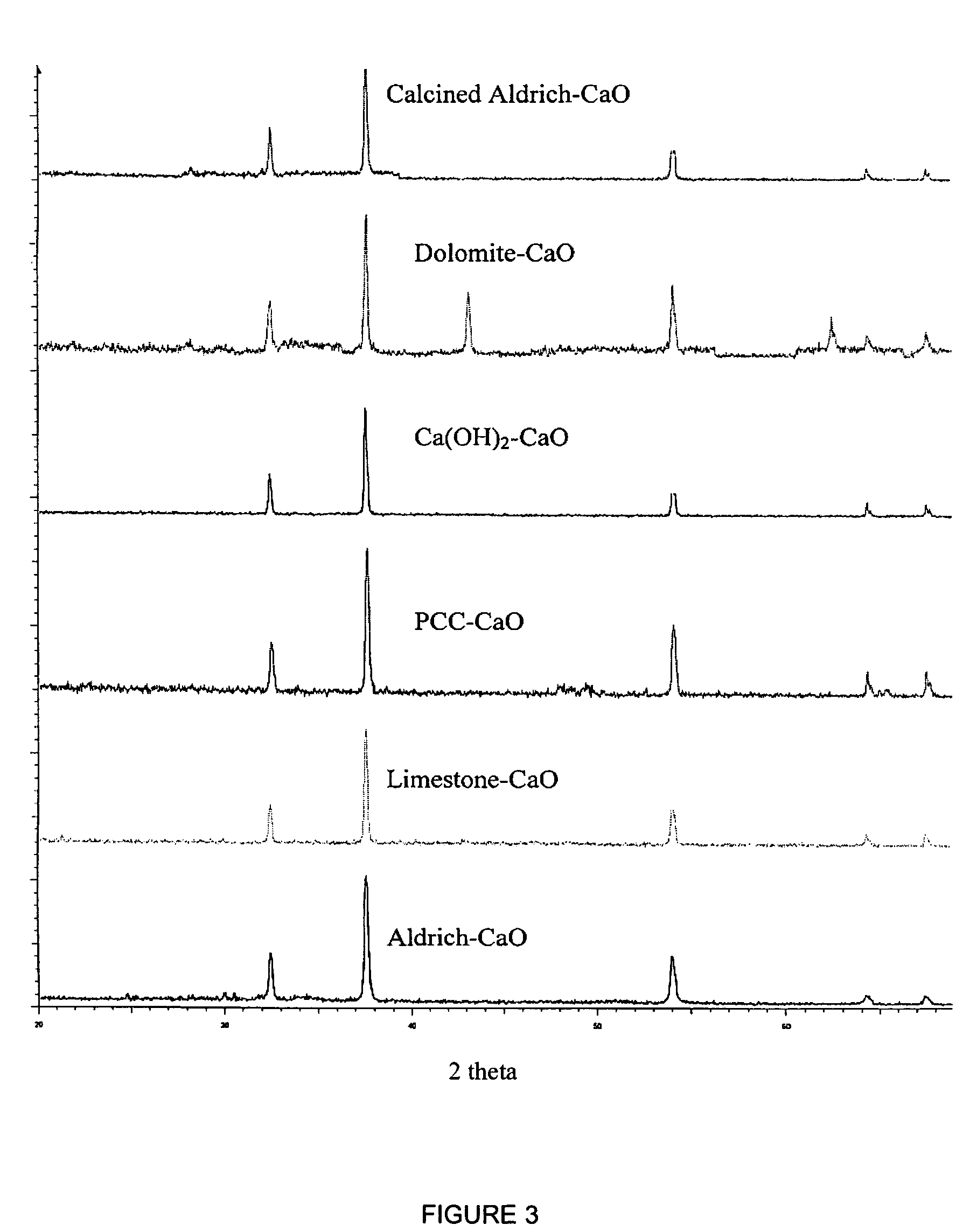
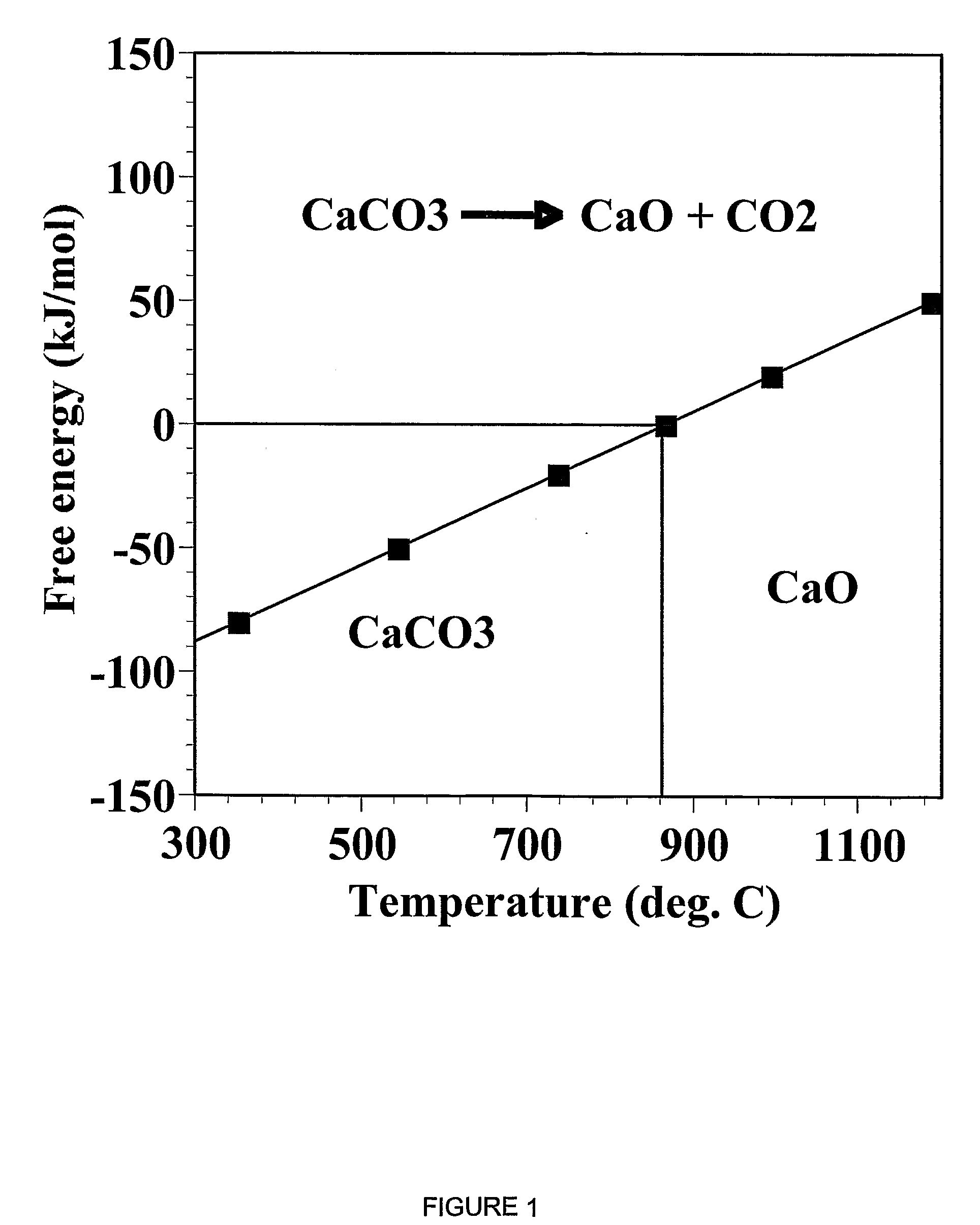
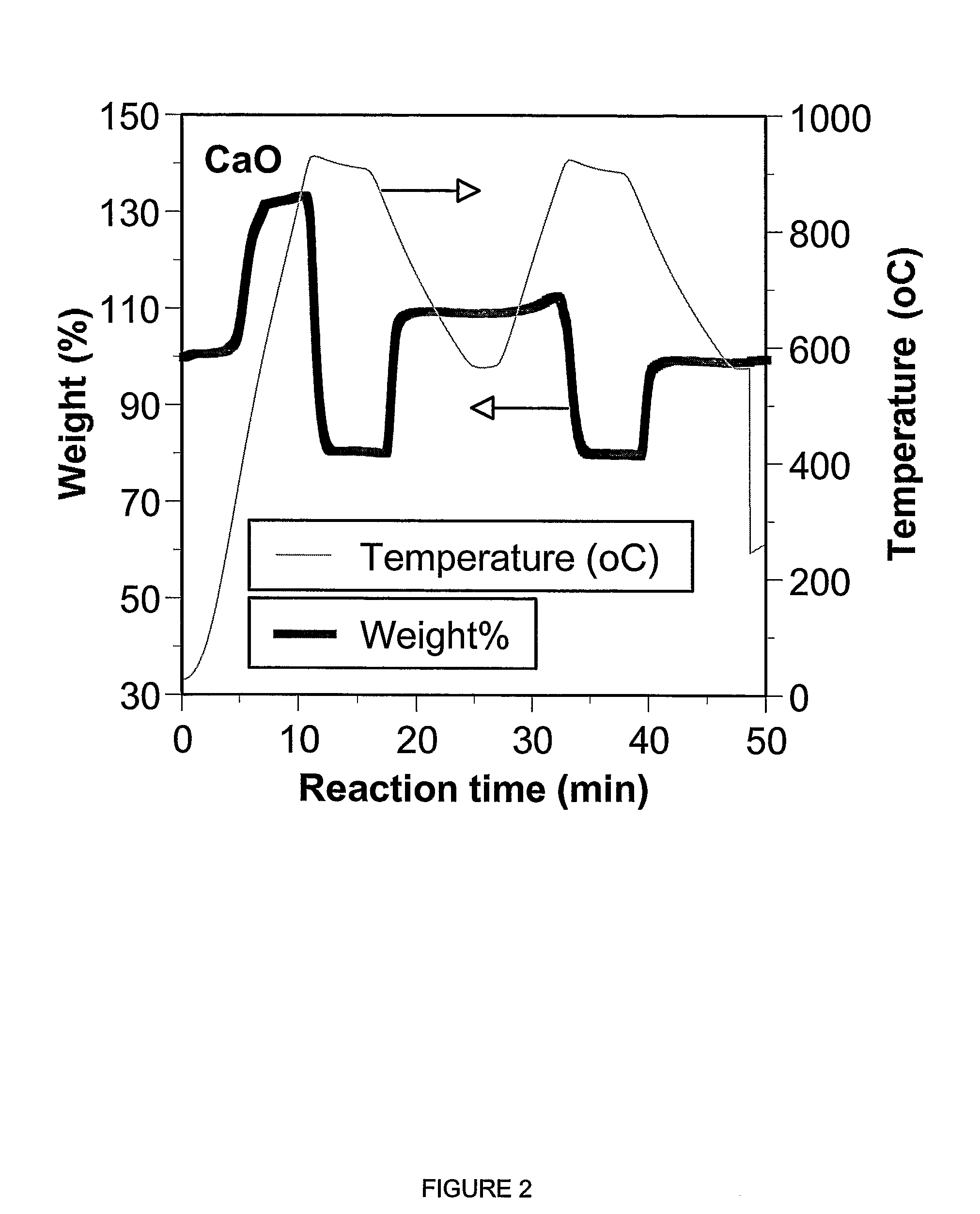
A reaction-based process has been developed for the selective removal of carbon dioxide from a multicomponent gas mixture. The proposed process effects the separation of CO2 from a mixture of gases by its reaction with metal oxides. The Calcium based Reaction Separation for CO2 process consists of contacting a CO2 laden gas with calcium oxide in a reactor such that CaO captures the CO2 by the formation of calcium carbonate. Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent. The “regenerated” CaO is then recycled for the further capture of more CO2. This process also identifies the application of a mesoporous CaCO3 structure, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Method of purifying nanotubes and nanofibers using electromagnetic radiation
Disclosed are methods of purifying mixtures comprising nanofibers and / or nanotubes and residual catalyst particles that are covered by outer layers of the nanotube or nanofiber material. The mixtures are exposed to electromagnetic radiation, which induces localized heating in the residual catalyst particles. The localized heating creates breaches in the outer layers. Thereafter, the residual catalyst particles may be removed under relatively mild conditions that do not significantly affect the structural integrity of the nanotubes or nanofibers. The methods of the invention have been used to particular advantage in the purification of single wall carbon nanotubes (SWNTs) synthesized using metal catalysts. For these SWNTs, microwave radiation is preferably used to induce the localized heating, the outer layers are preferably removed at least in part by carrying out the localized heating under air, and the residual catalyst may be removed by exposure to relatively dilute aqueous acid.
Owner:PENN STATE RES FOUND
System and method for therapeutic application of dissolved oxygen
InactiveUS20050047270A1Rotary stirring mixersUsing liquid separation agentCavitationAqueous solution
A system and method for generating an oxygen enriched aqueous solution for therapeutic application includes a diffuser comprising a first diffusing member having a surface incorporating surface disturbances, and a second diffusing member positioned relative to the first diffusing member to form a channel through which an aqueous solution and oxygen gas may flow. A reservoir containing the aqueous solution is connected to a pump that draws the aqueous solution from the reservoir and inputs the aqueous solution into the diffuser. The aqueous solution is moved through the channel relative to the surface disturbances to create cavitation in the aqueous solution to diffuse the oxygen gas into the aqueous solution to produce an oxygen enriched aqueous solution.
Owner:REVALESIO CORP
Separation of Carbon Dioxide (Co2) From Gas Mixtures By Calcium Based Reaction Separation (Cars-Co2) Process
InactiveUS20080233029A1Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentSorbentTransformation ratio
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS—CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCOa). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS—CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Layered catalyst composite
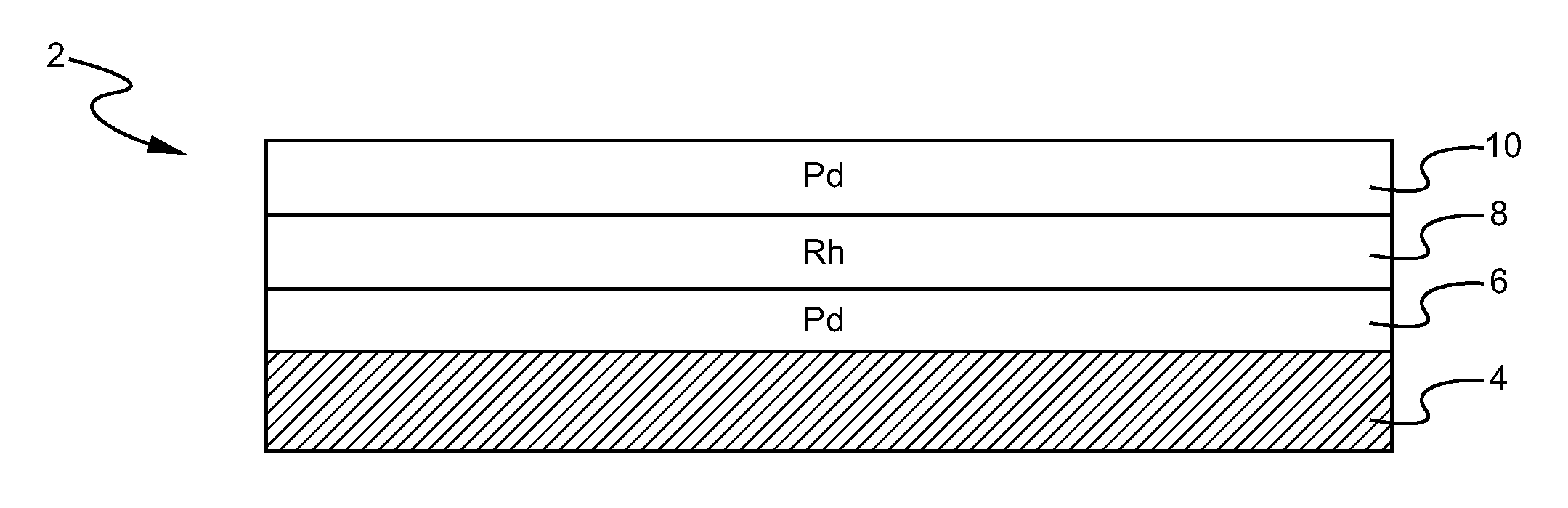
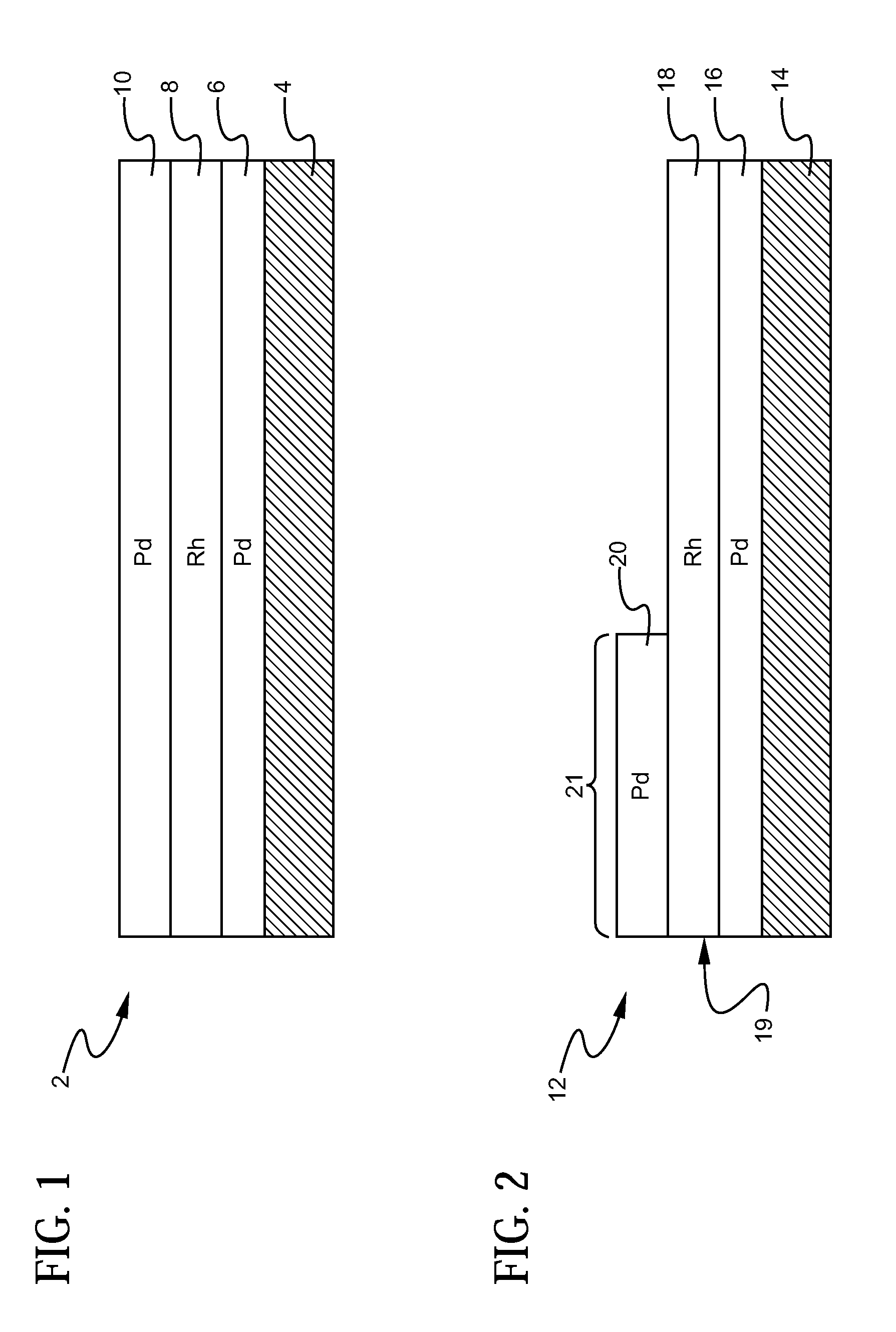
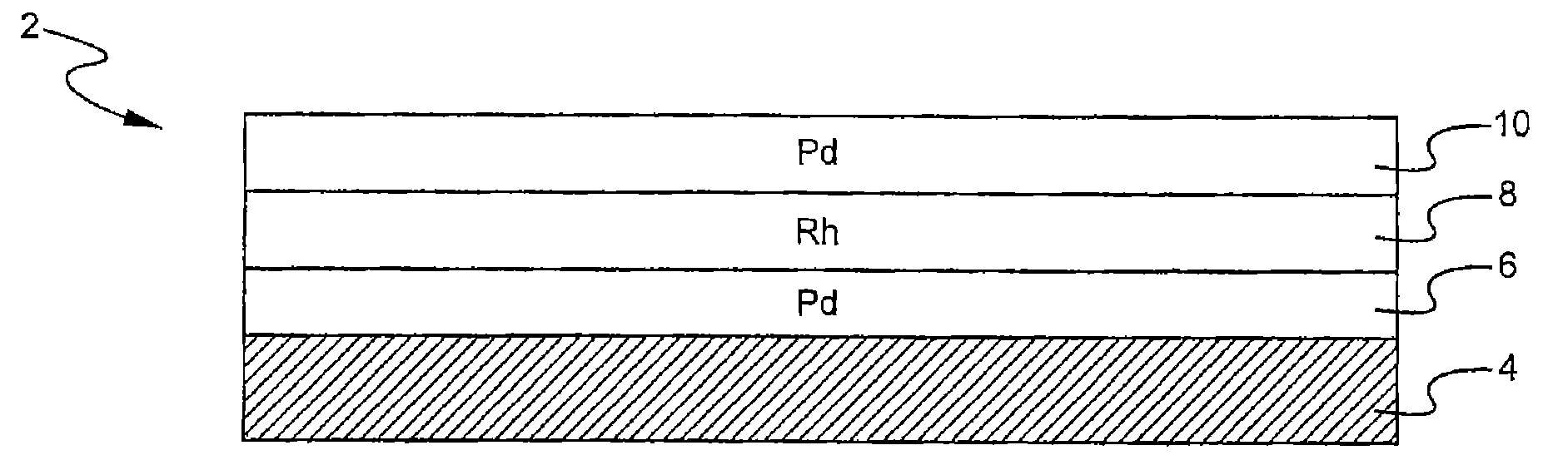
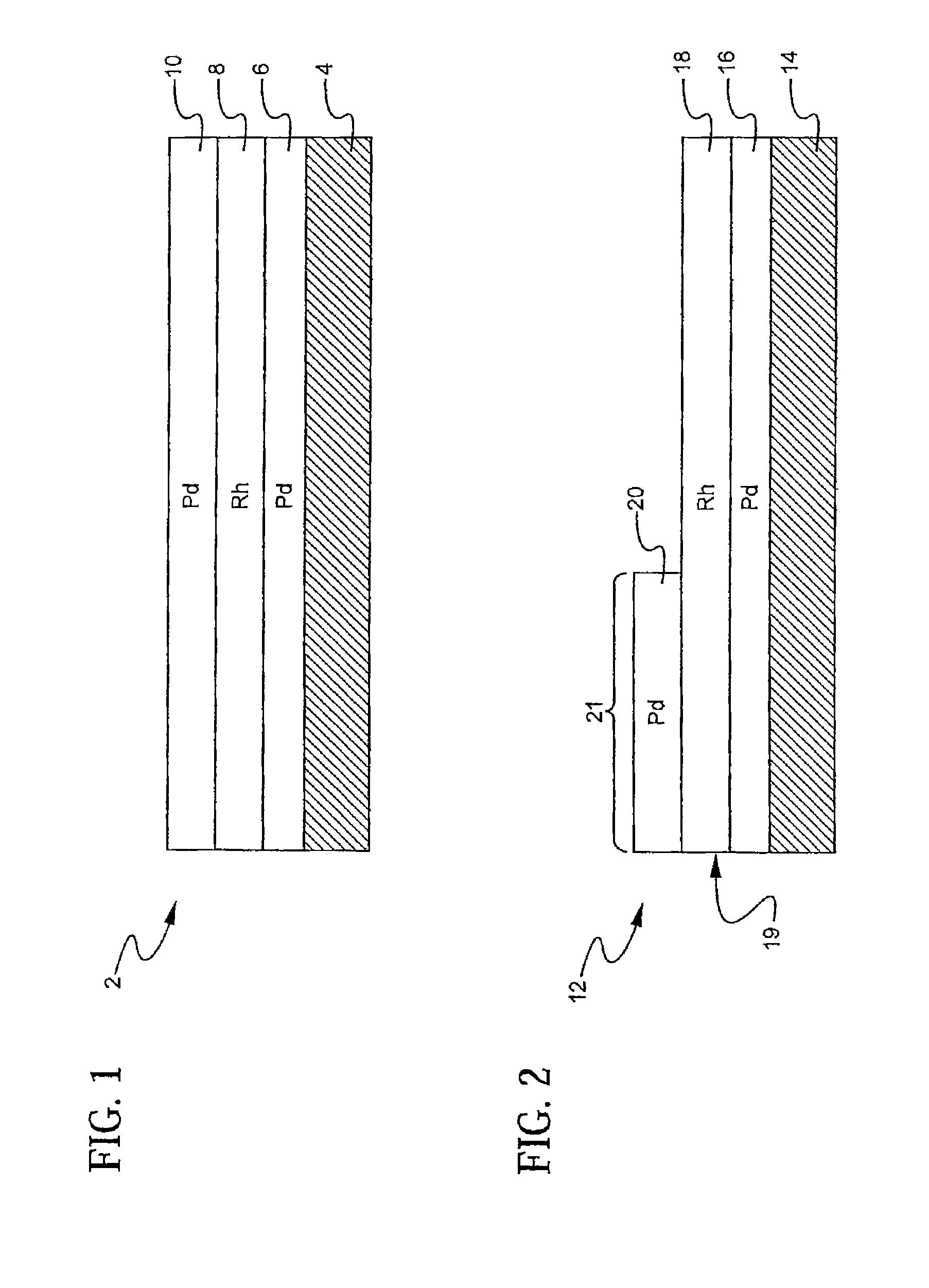
A layered, three-way conversion catalyst having the capability of simultaneously catalyzing the oxidation of hydrocarbons and carbon monoxide and the reduction of nitrogen oxides is disclosed. In one or more embodiments, the catalyst comprises three layers in conjunction with a carrier: a first layer deposited on the carrier and comprising palladium deposited on a refractory metal oxide and an oxygen storage component; a second layer deposited on the first layer and comprising rhodium deposited on a refractory metal oxide and an oxygen storage component; and a third layer deposited on the second layer and comprising palladium deposited on a refractory metal oxide.
Owner:BASF CATALYSTS LLC
Controlled spectrum ultraviolet radiation pollution control process
InactiveUS7498009B2Cost effectiveEasy to adaptCombination devicesOrganic chemistryEnvironmental engineeringNitrogen oxide
A method for reducing or substantially eliminating oxides of nitrogen from an effluent gas stream, that includes providing a source of ultraviolet radiation with a precise wavelength, adding ammonia or an ammonia based reagent to the effluent stream, upstream of the ultraviolet radiation source, controllably operating the ultraviolet radiation source to irradiate the effluent stream flowing in the duct and substantially reducing or eliminating oxides of nitrogen by promotion a reaction of ammonia with the oxides of nitrogen to produce N2 and H2O, and also thereby destroying any surplus ammonia. This process can also be modified to oxidize carbon monoxide and VOC's to CO2 and H2O.
Owner:DANA UV
Catalysts, activating agents, support media, and related methodologies useful for making catalyst systems especially when the catalyst is deposited onto the support media using physical vapor deposition
InactiveUS7727931B2High catalytic activityTendency increaseMaterial nanotechnologyInternal combustion piston enginesGas phasePhysical chemistry
Use of physical vapor deposition methodologies to deposit nanoscale gold on activating support media makes the use of catalytically active gold dramatically easier and opens the door to significant improvements associated with developing, making, and using gold-based, catalytic systems. The present invention, therefore, relates to novel features, ingredients, and formulations of gold-based, heterogeneous catalyst systems generally comprising nanoscale gold deposited onto a nanoporous support.
Owner:3M INNOVATIVE PROPERTIES CO
Layered catalyst composite
A layered, three-way conversion catalyst having the capability of simultaneously catalyzing the oxidation of hydrocarbons and carbon monoxide and the reduction of nitrogen oxides is disclosed. In one or more embodiments, the catalyst comprises three layers in conjunction with a carrier: a first layer deposited on the carrier and comprising palladium deposited on a refractory metal oxide and an oxygen storage component; a second layer deposited on the first layer and comprising rhodium deposited on a refractory metal oxide and an oxygen storage component; and a third layer deposited on the second layer and comprising palladium deposited on a refractory metal oxide.
Owner:BASF CATALYSTS LLC
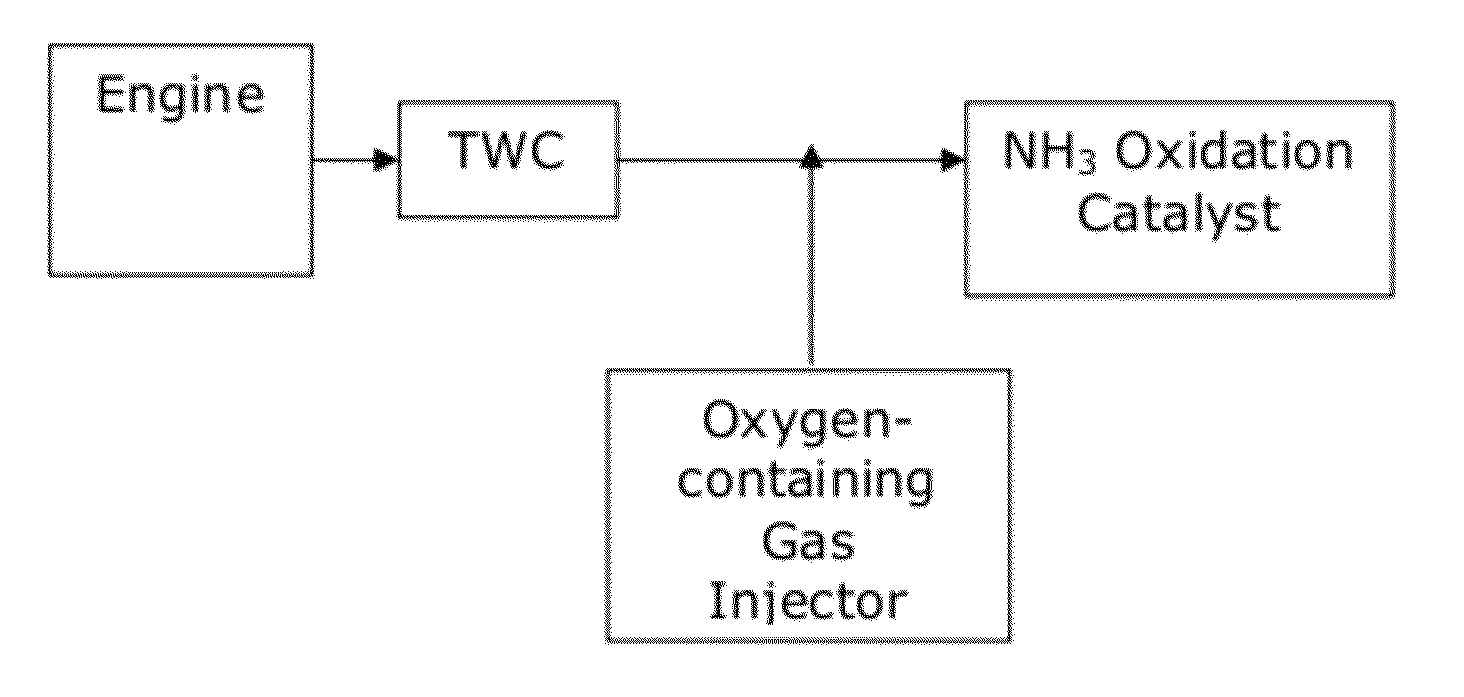
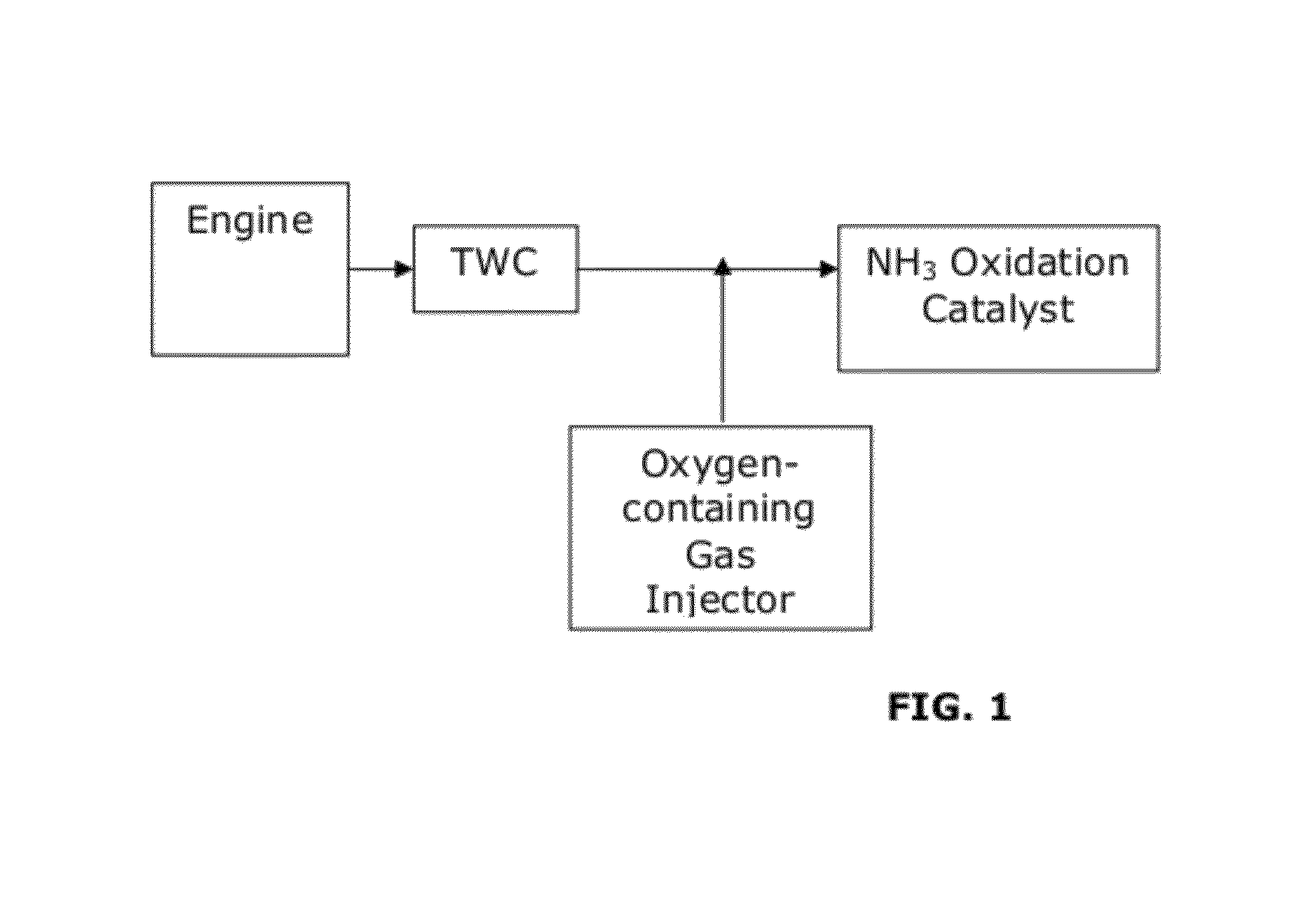
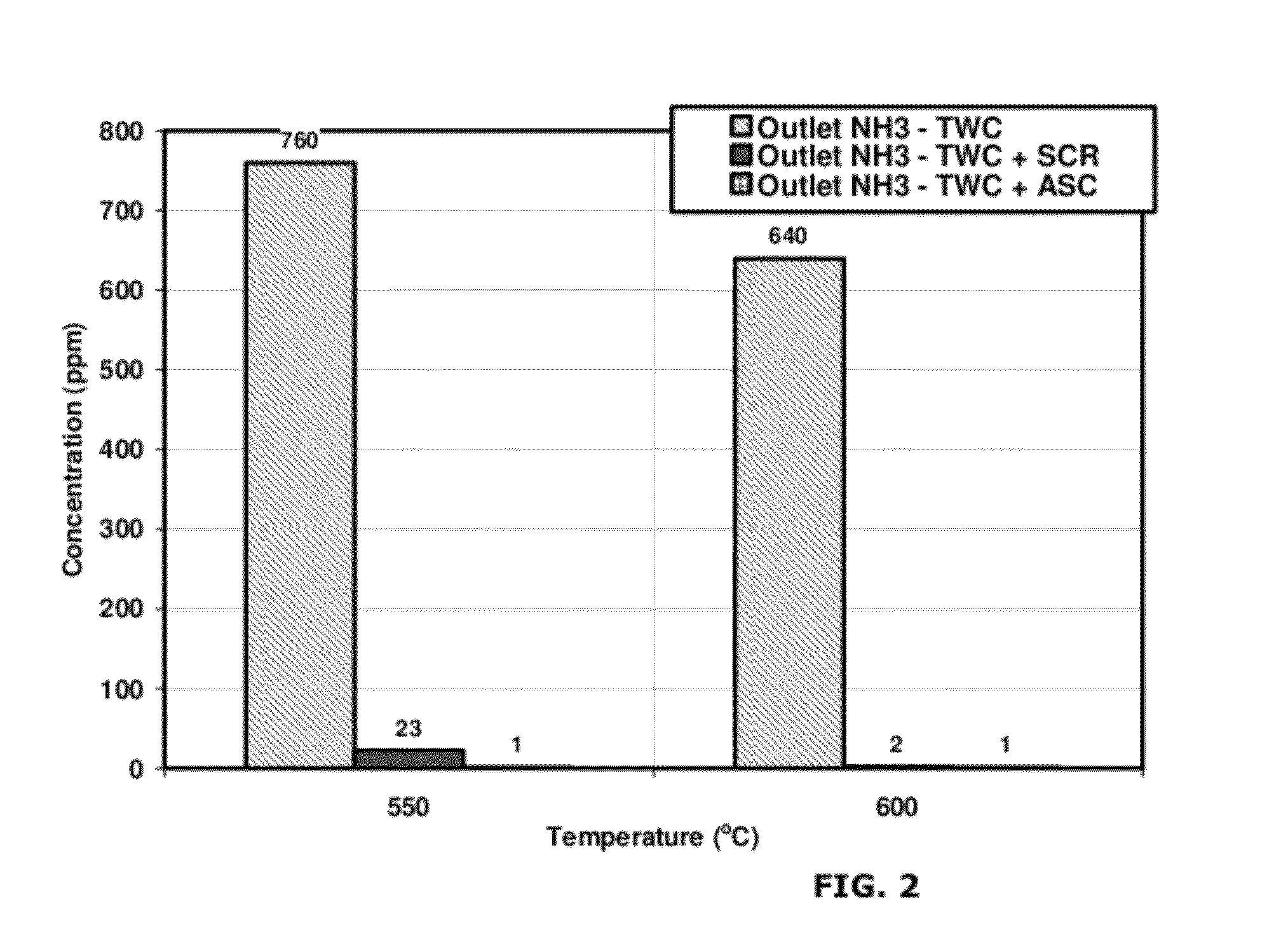
Catalysts for the reduction of ammonia emission from rich-burn exhaust
A system for reducing ammonia (NH3) emissions includes (a) a first component comprising a first substrate containing a three-way catalyst, wherein the first component is disposed upstream of a second component comprising a second substrate containing an ammonia oxidation catalyst, wherein said ammonia oxidation catalyst comprises a small pore molecular sieve supporting at least one transition metal; and (b) an oxygen-containing gas input disposed between the components. For example, a CHA Framework Type small pore molecular sieve may be used. A method for reducing NH3 emission includes introducing an oxygen-containing gas into a gas stream to produce an oxygenated gas stream; and exposing the oxygenated gas stream to an NH3 oxidation catalyst to selectively oxidize at least a portion of the NH3 to N2.
Owner:JOHNSON MATTHEY PLC
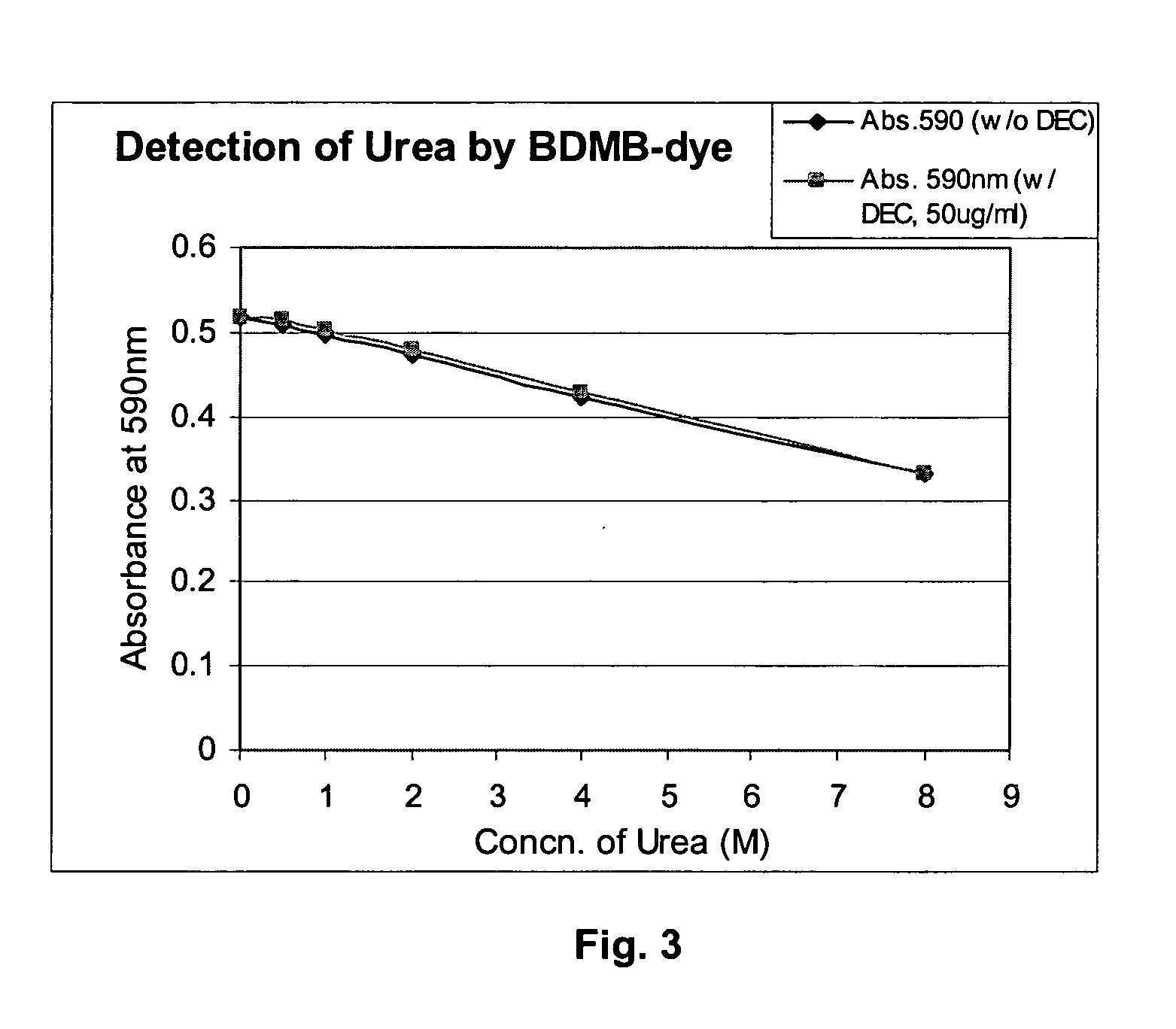
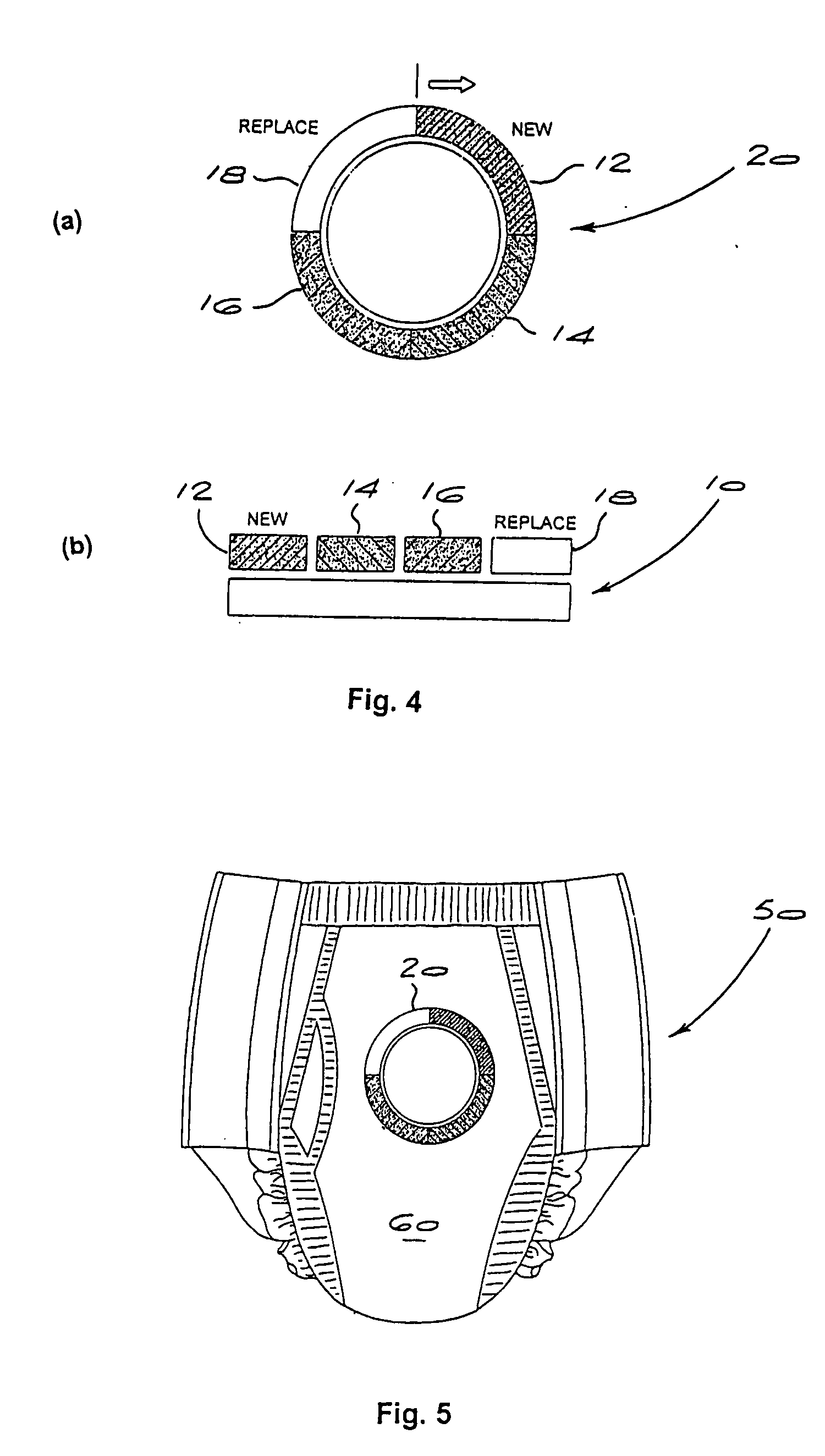
Odor controlling article including a visual indicating device for monitoring odor absorption
The present invention relates to a visual indicating device and an article for controlling odors, in particular foot, garbage, basement, cooking, pet, tobacco, feces and urine odors. The article comprises a visual indicating agent that is color sensitive to the odor, and optionally, an odor absorbing agent. The visual indicating agent changes color when the article has been exposed to a sufficient amount of odor to saturate the article. The indicating agent may be applied in differing concentrations to two or more zones so as to indicate to a user of the article how much of the odor absorbing capacity has been used, or conversely, how much of the odor absorbing capacity remains. Suitable visual indicating agents that change color in response to odors are also described. The article for controlling odors may be a disposable odor absorbing sheet, air freshening product, diaper, undergarment pad, face mask, air filtration device, sanitary napkin, tampon, panty shield or incontinence pad.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Method and apparatus for regenerating an iron-based Fischer-Tropsch catalyst
ActiveUS6838487B1Reduce concentrationMaintain standardOrganic compound preparationOther chemical processesWaxContinuous flow
Solvent extraction is used to remove wax and contaminants from an iron-based Fischer-Tropsch catalyst in a natural circulation continuous-flow system. The wax-free catalyst is then subjected to controlled oxidation to convert the iron to its initial oxidized state, Fe2O3. Reactivation of the oxide catalyst precursor is carried out by addition of synthesis gas.
Owner:RES USA LLC
Gas purifying process and gas purifying apparatus
A method is provided for removing water, carbon monoxide and carbon dioxide out of a gas, such as air, by passing the gas through a packed column so that the gas sequentially contacts a catalyst consisting of platinum or palladium and at least one member selected from the group consisting of iron, cobalt, nickel, manganese, copper, chromium, tin, lead and cerium wherein the catalyst is supported on alumina containing substantially no pores having pore diameters of 110 Angstroms or less under conditions which oxidize the carbon monoxide in the gas into carbon dioxide; an adsorbent selected from the group consisting of silica gel, activated alumina, zeolite and combinations thereof under conditions in which water is adsorbed and removed from the gas and an adsorbent selected from the group consisting of calcium ion exchanged A zeolite; calcium ion exchanged X zeolite; sodium ion exchanged X zeolite and mixtures thereof under conditions which carbon dioxide is adsorbed and removed from the gas. The gas may also be subjected to a catalyst / adsorbent in the packed column to effect oxidation and removal of hydrogen in the gas.
Owner:NIPPON SANSO CORP
Method and device for high-capacity entrained flow gasifier
A method and device for the gasification of pulverized fuels from solid fuels such as bituminous coals, lignite coals, and their cokes, petroleum cokes, coke from peat or biomass, in entrained flow, with an oxidizing medium containing free oxygen, by partial oxidation at pressures between atmospheric pressure and 80 bar, and at temperatures between 1,200 and 1,900° C., at high reactor capacities between 1,000 and 1,500 MW. The method uses the following steps: metering of the fuel, gasification reaction in a gasification reactor with cooled reaction chamber contour, quench-cooling, crude gas scrubbing, and partial condensation.
Owner:SIEMENS AG
Regeneration of acidic catalysts
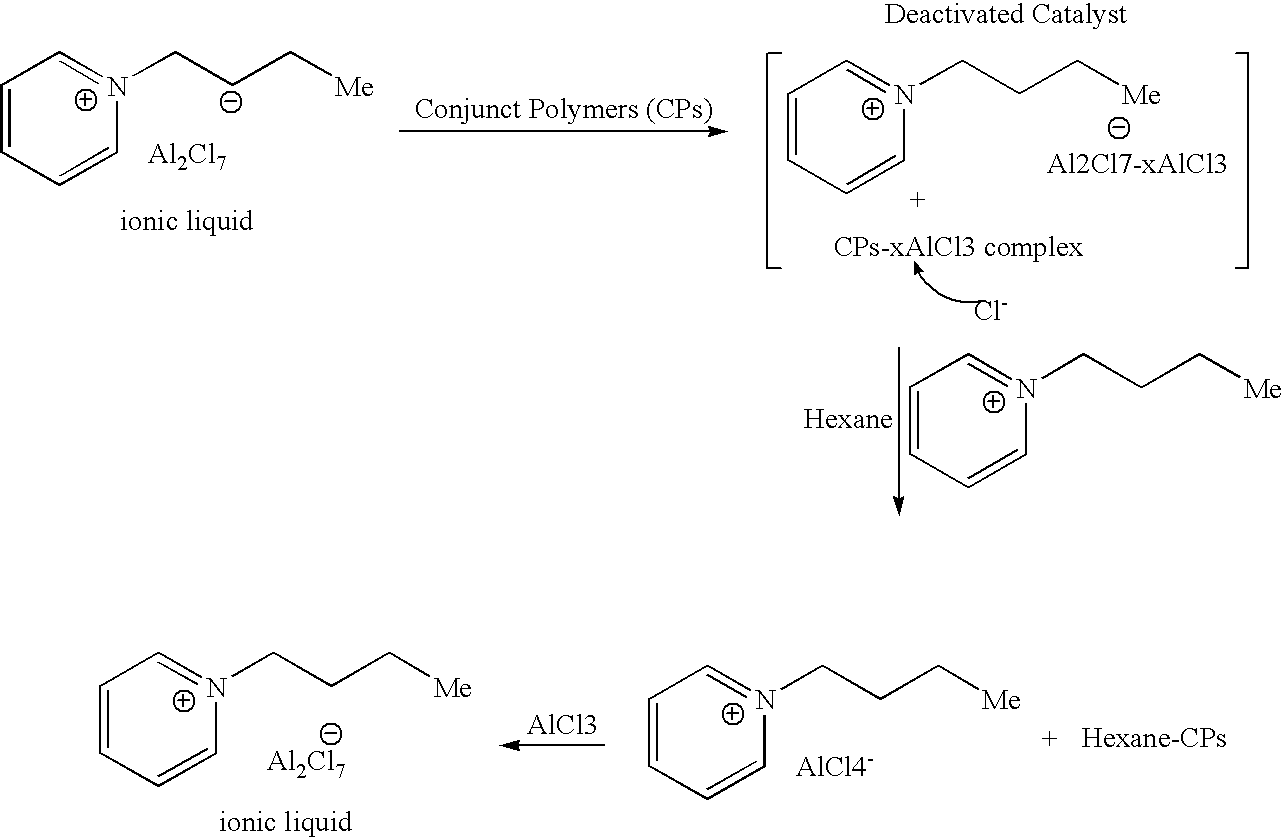
ActiveUS20070142213A1High activityChlorine/hydrogen-chloride purificationSulfur compoundsAlkyl transferIonic liquid
A process for regenerating a used acidic catalyst which has been deactivated by conjunct polymers by removing the conjunct polymers so as to increase the activity of the catalyst is disclosed. Methods for removing the conjunct polymers include hydrogenation, addition of a basic reagent and alkylation. The methods are applicable to all acidic catalysts and are described with reference to certain ionic liquid catalysts.
Owner:CHEVROU USA INC
Shift converter having an improved catalyst composition, and method for its use
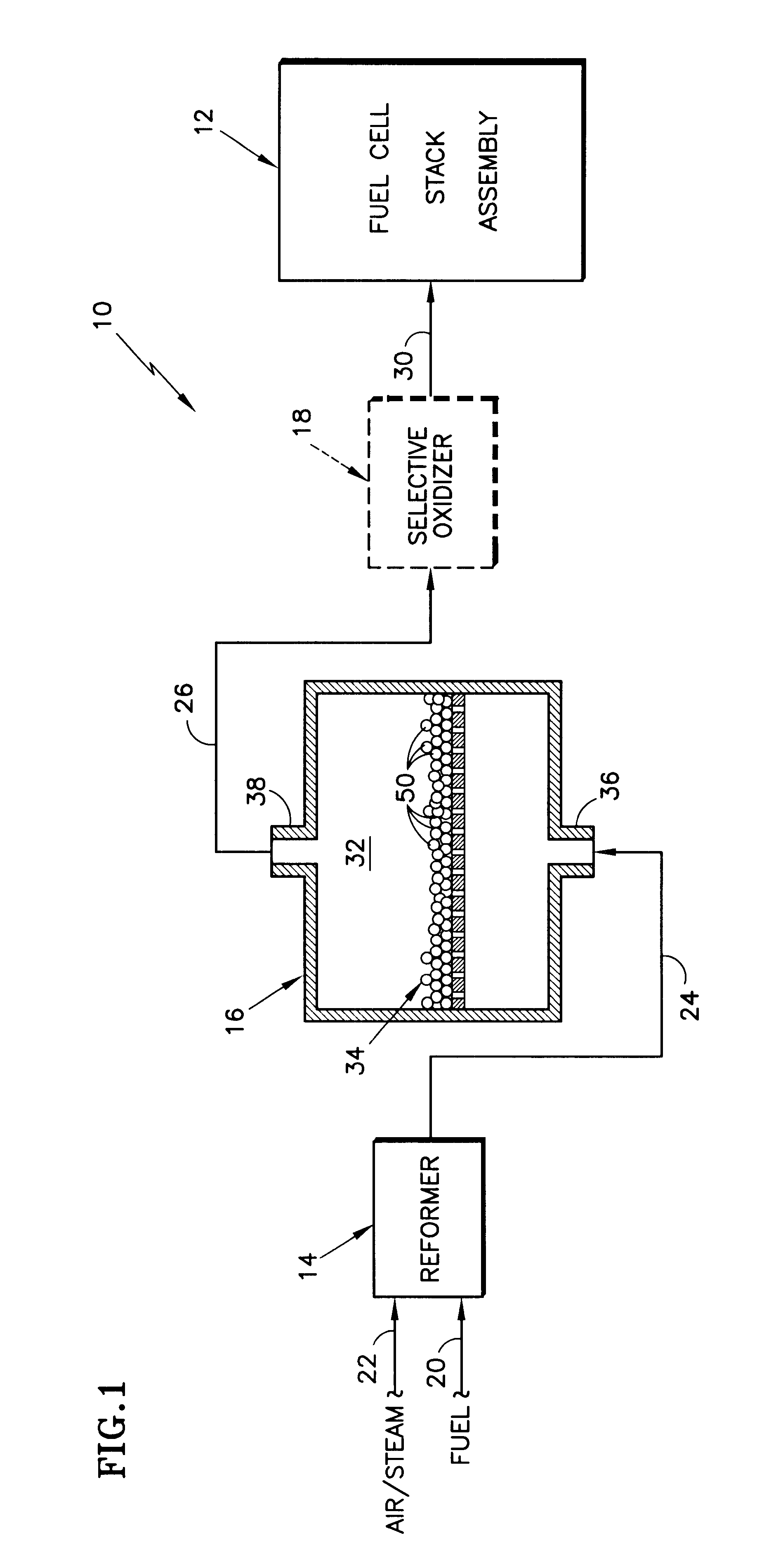
InactiveUS6455182B1Reduce amountImproved catalyst compositionHydrogenCatalyst carriersFuel cellsCerium
A shift converter (16) in a fuel processing subsystem (14, 16, 18) for a fuel cell (12) uses an improved catalyst composition (50) to reduce the amount of carbon monoxide in a process gas for the fuel cell (12). The catalyst composition (50) is a noble metal catalyst having a promoted support of mixed metal oxide, including at least both ceria and zirconia. Cerium is present in the range of 30 to 50 mole %, and zirconium is present in the range of 70 to 50 mole %. Additional metal oxides may also be present. Use of the catalyst composition (50) obviates the requirement for prior reducing of catalysts, and minimizes the need to protect the catalyst from oxygen during operation and / or shutdown.
Owner:HYAXIOM INC
Method and apparatus to protect synthesis gas via flash pyrolysis and gasification in a molten liquid
InactiveUS20080307703A1Improve efficiencyLittle heating lossWaste based fuelRetortsSyngasThermodynamics
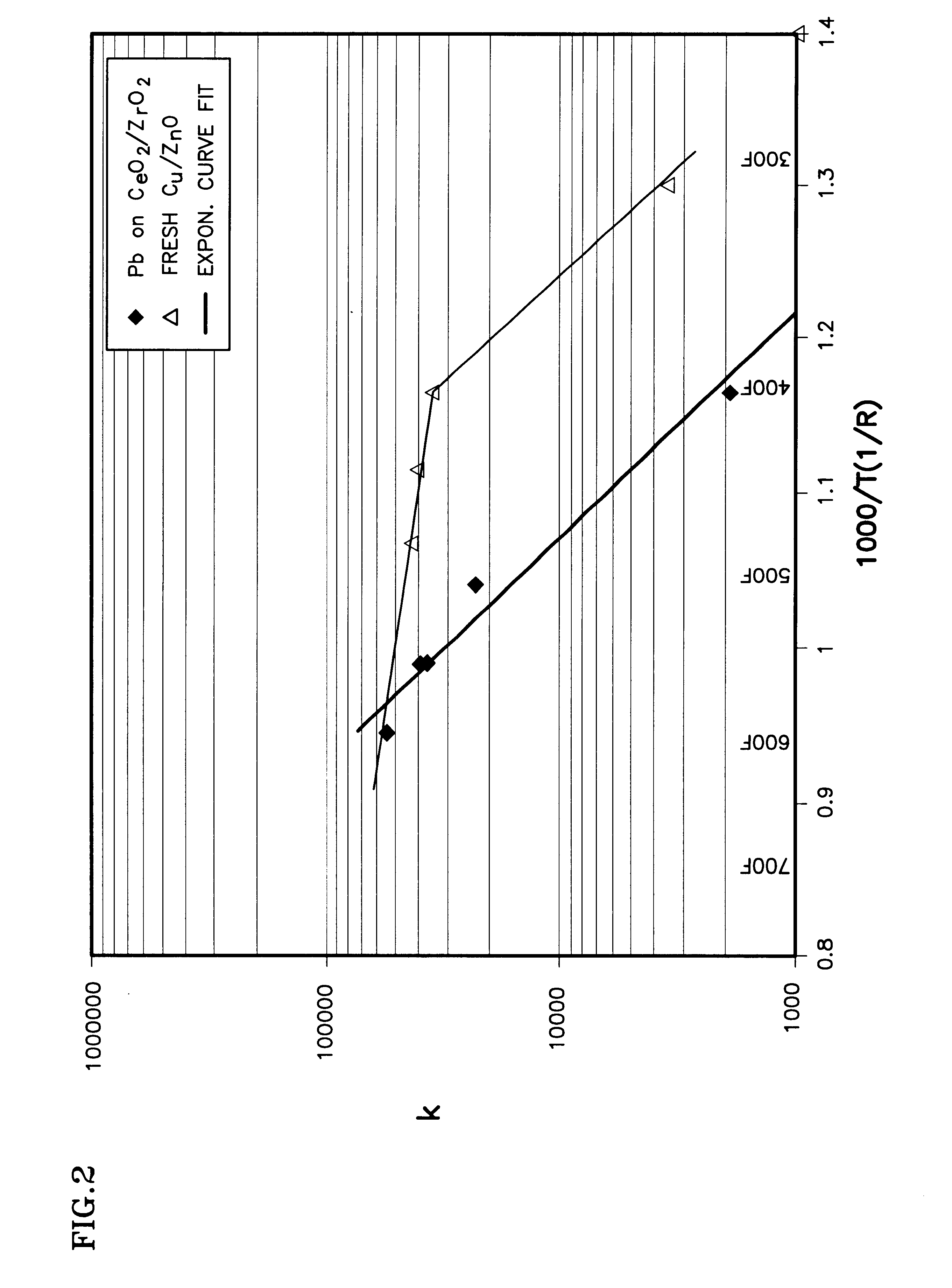
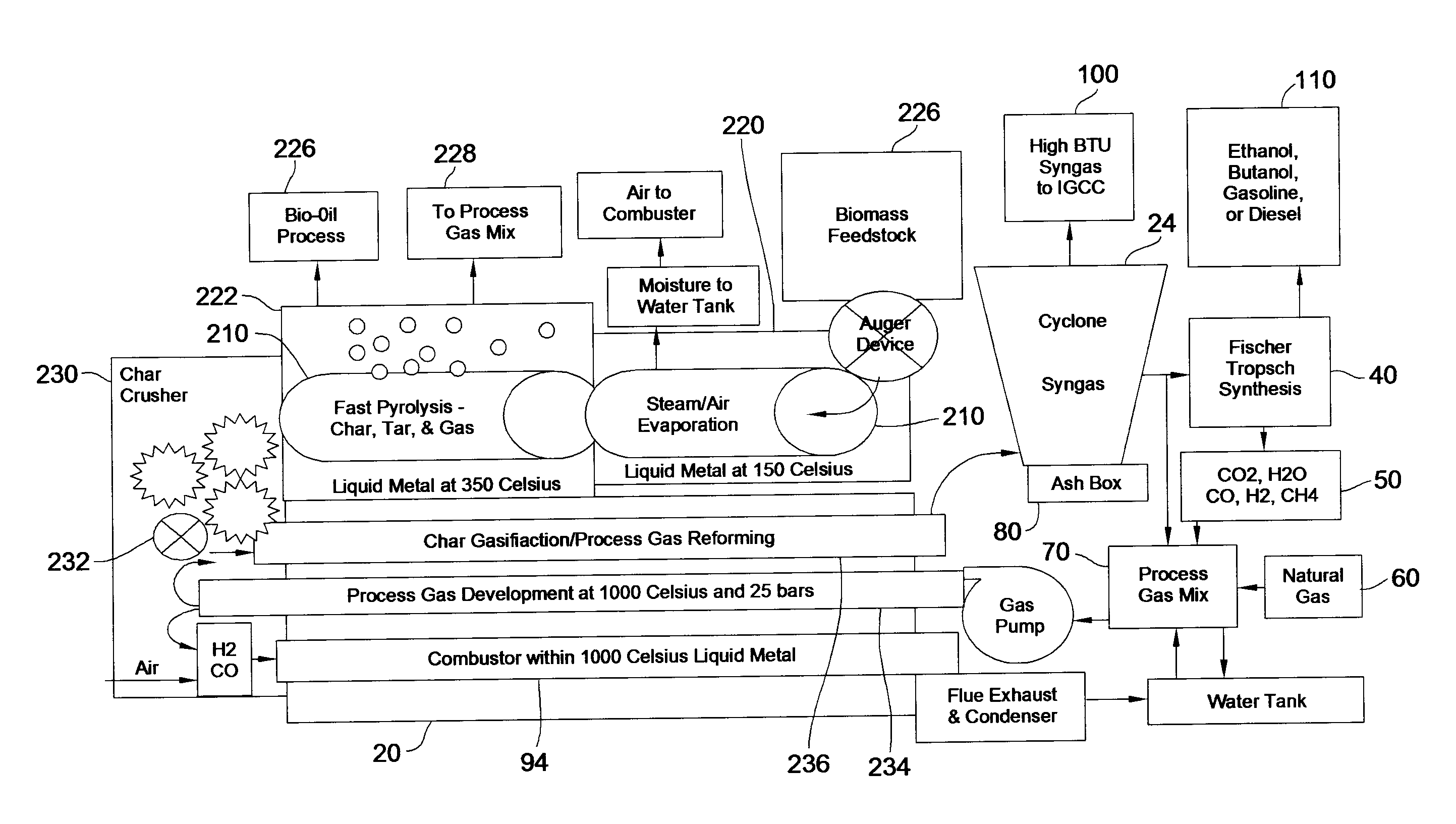
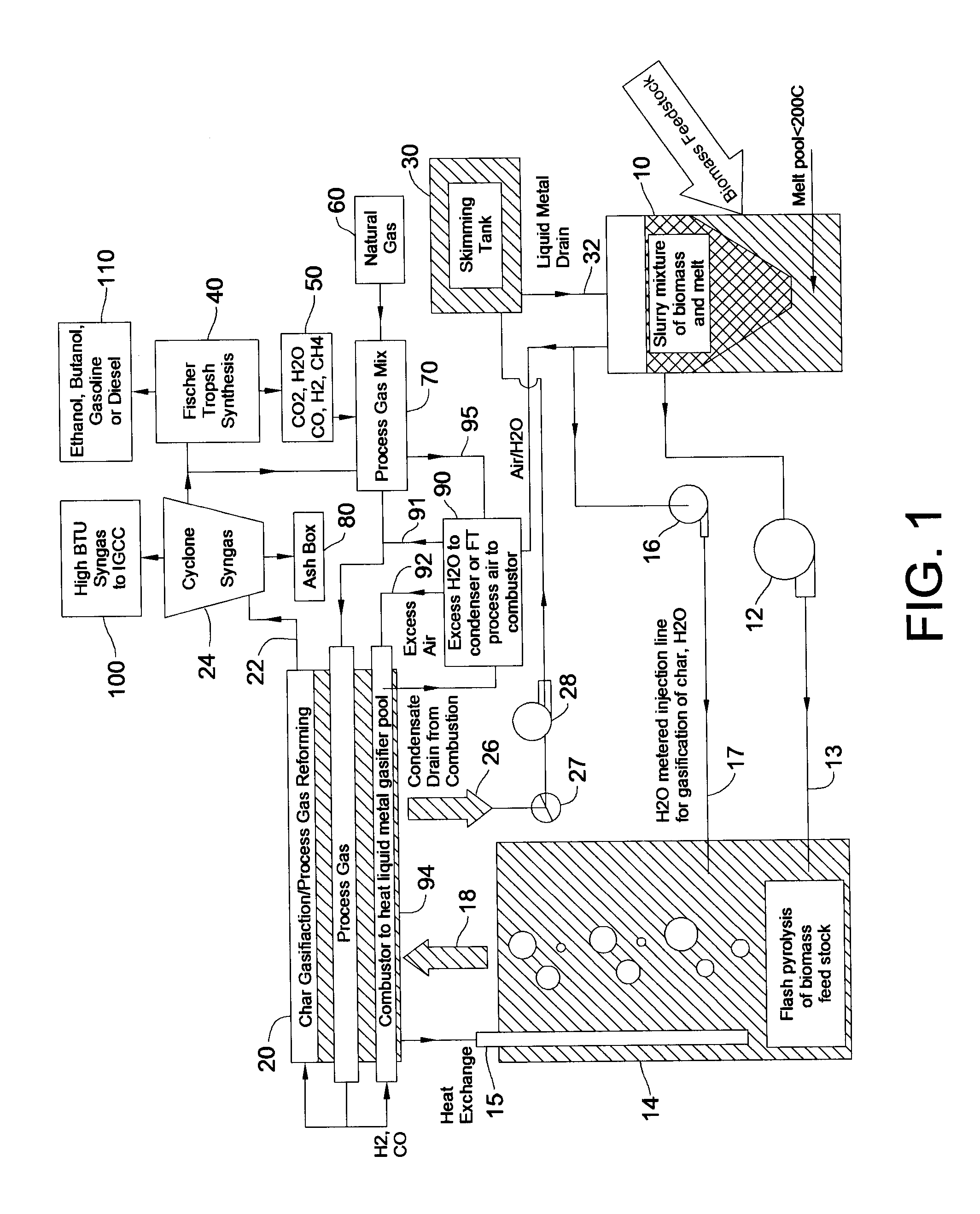
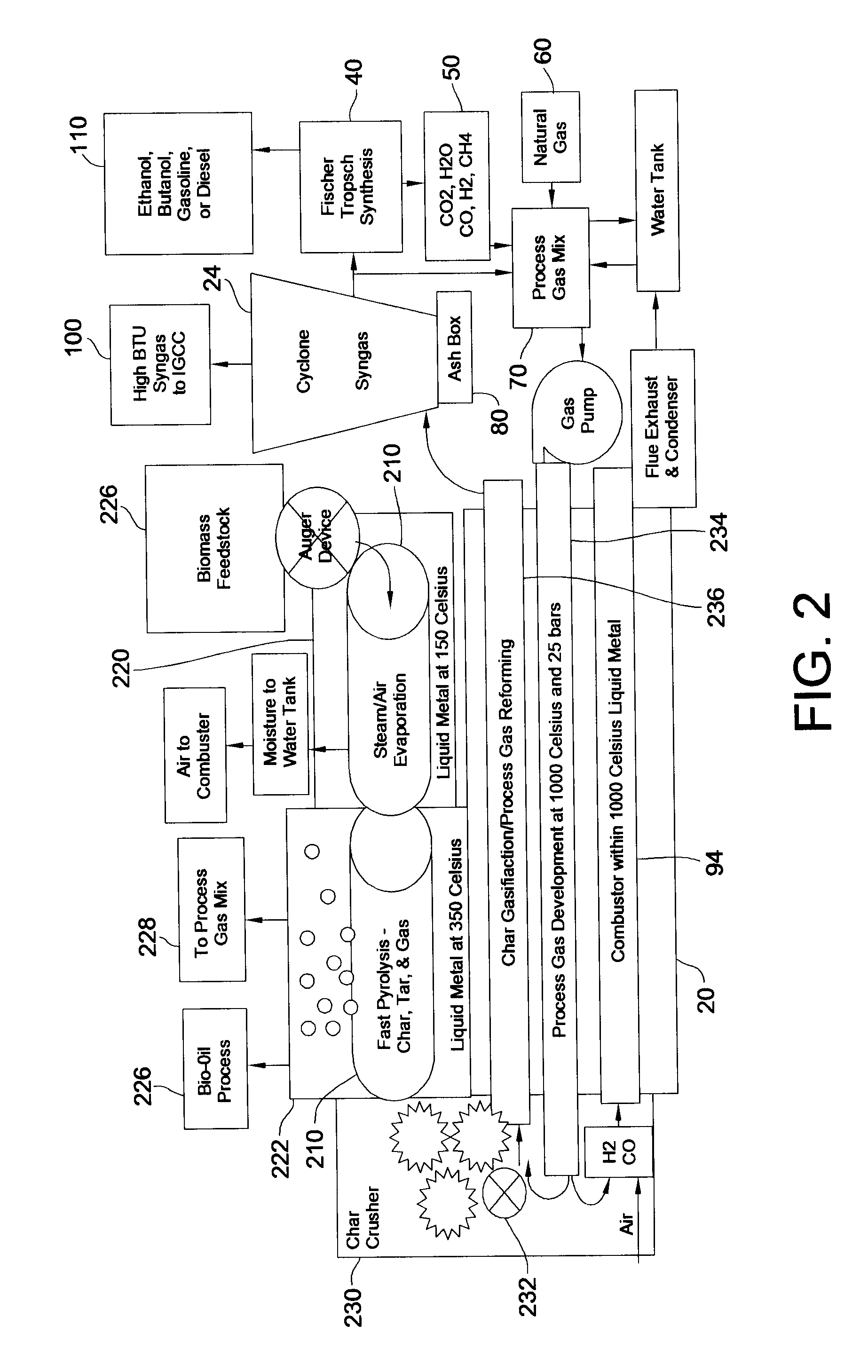
Disclosed are a method and a corresponding apparatus for converting a biomass reactant into synthesis gas. The method includes the steps of (1) heating biomass in a first molten liquid bath at a first temperature, wherein the first temperature is at least about 100° C., but less than the decomposition temperature of the biomass, wherein gas comprising water is evaporated and air is pressed from the biomass, thereby yielding dried biomass with minimal air content. (2) Recapturing the moisture evaporated from the biomass in step 1 for use in the process gas. (3) Heating the dried biomass in a second molten liquid bath at a second temperature, wherein the second temperature is sufficiently high to cause flash pyrolysis of the dried biomass, thereby yielding product gases, tar, and char. (4) Inserting recaptured steam into the process gas, which may optionally include external natural gas or hydrogen gas or recycled syngas for mixing and reforming with tar and non-condensable gases. (5) Further reacting the product gases, tar, and char with the process gas within a third molten liquid bath at a third temperature which is equal to or greater than the second temperature within the second molten liquid bath, thereby yielding high quality and relatively clean synthesis gas after a relatively long residence time needed for char gasification. A portion of the synthesis gas so formed is combusted to heat the first, second, and third molten liquid baths, unless external natural or hydrogen gas is available for this use.
Owner:US SEC AGRI +1
Combined cracking and selective hydrogen combustion for catalytic cracking
A catalyst system and process for combined cracking and selective hydrogen combustion of hydrocarbons are disclosed. The catalyst system contains at least one solid acid component and at least one metal-based component which consists of (a) oxygen and / or sulfur and (b) a metal combination selected from the group consisting of: i) at least one metal from Group 3 and at least one metal from Groups 4-15 of the Periodic Table of the Elements; ii) at least one metal from Groups 5-15 of the Periodic Table of the Elements, and at least one metal from at least one of Groups 1, 2, and 4 of the Periodic Table of the Elements; iii) at least one metal from Groups 1 and 2, at least one metal from Group 3, and at least one metal from Groups 4-15 of the Periodic Table of the Elements; and iv) two or more metals from Groups 4-15 of the Periodic Table of the Elements, wherein the at least one of oxygen and sulfur is chemically bound both within and between the metals and, optionally, (3) at least one of at least one support, at least one filler and at least one binder. The process is such that the yield of hydrogen is less than the yield of hydrogen when contacting the hydrocarbons with the solid acid component alone. Further the emissions of NOx from the regeneration cycle of the catalyst system are reduced.
Owner:EXXONMOBIL CHEM PAT INC
Gas Reformulating System Using Plasma Torch Heat
ActiveUS20070266633A1Gasifier mechanical detailsGas modification by gas mixingControl systemProcess engineering
A method and apparatus is described for reformulating of an input gas from a gasification reaction into a reformulated gas. More specifically, a gas reformulating system having a gas reformulating chamber, one or more plasma torches, one or more oxygen source(s) inputs and control system is provided thereby allowing for the conversion of an input gas from a gasification reaction into a gas of desired composition.
Owner:PLASCO CONVERSION TECH INC
System for hot solids combustion and gasification
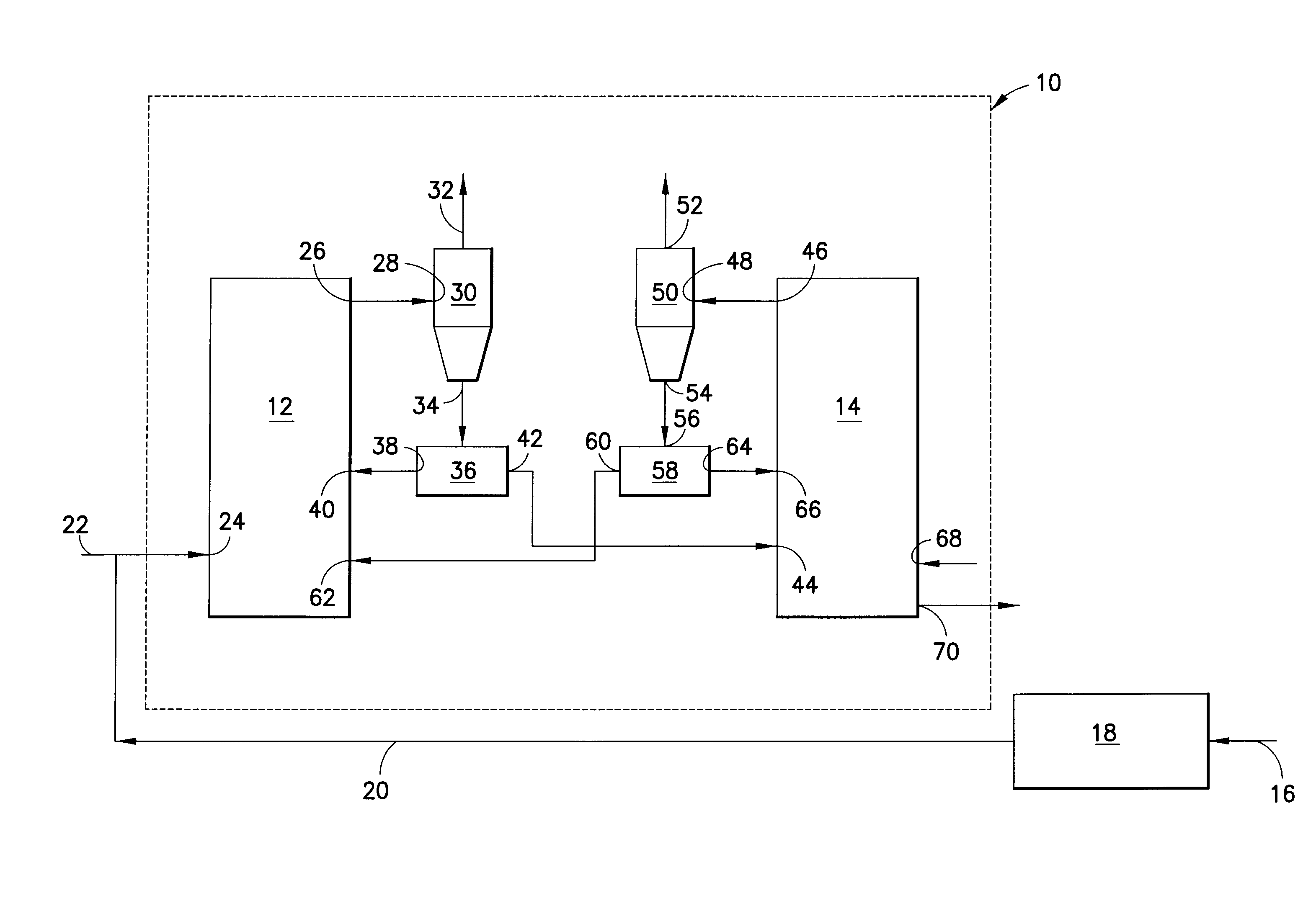
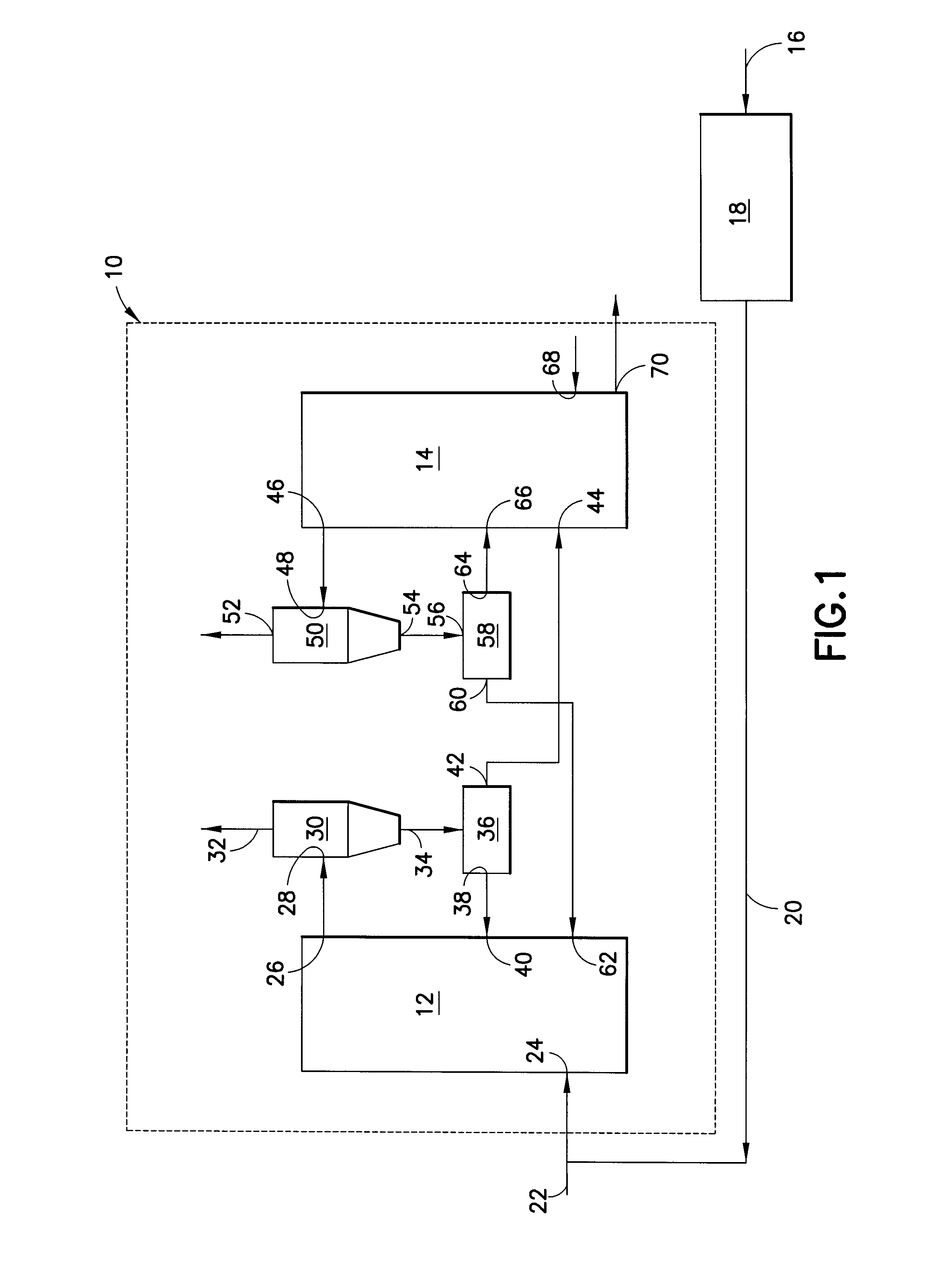
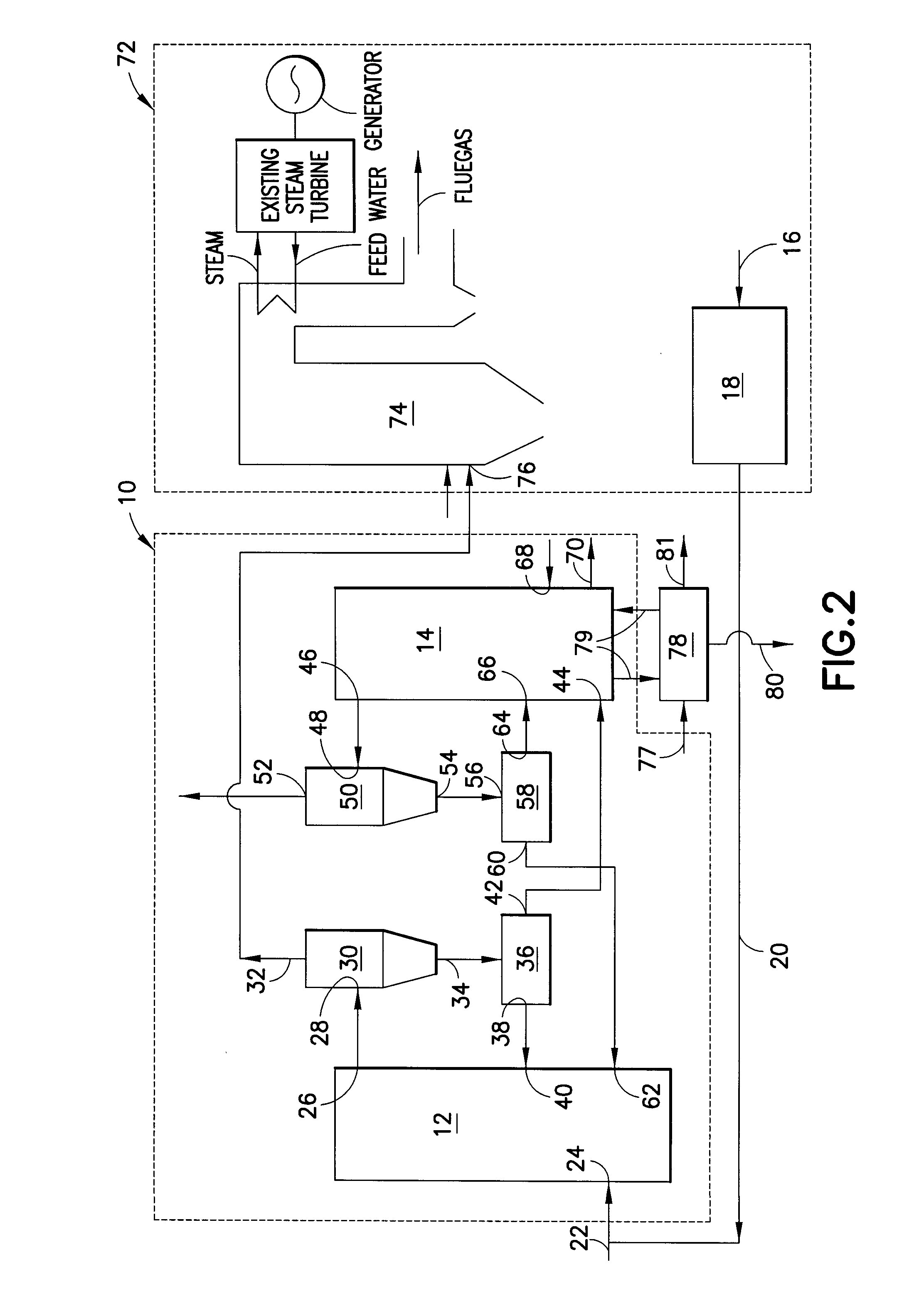
ActiveUS8110012B2Enhanced overall recoveryEliminate needGas modification by gas mixingCombustion enginesCombustionReducer
Owner:AIR PROD & CHEM INC +1
Process for removing and recovering halocarbons from effluent process streams
A process for recovery of fluorocompound gas from an effluent gas stream containing the fluorocompound gas and other gas components, in which at least one of the other gas components is removed, e.g., by oxidation or contacting of the effluent stream with a dry material such as an adsorbent or scrubber medium, to yield a first effluent gas mixture containing the fluorocompound gas. The fluorocompound gas is removed from the first effluent gas mixture and recovered as a concentrated fluorocompound gas, by a process such as cryogenic processing, membrane separation, and / or adsorption.
Owner:ADVANCED TECH MATERIALS INC
Apparatus, Components and Operating Methods for Circulating Fluidized Bed Transport Gasifiers and Reactors
ActiveUS20110146152A1Reduce and prevent reverse flow of gasOvercome problemsHydrogenFluidized bed combustionCycloneFluidized bed
The improvements proposed in this invention provide a reliable apparatus and method to gasify low rank coals in a class of pressurized circulating fluidized bed reactors termed “transport gasifier.” The embodiments overcome a number of operability and reliability problems with existing gasifiers. The systems and methods address issues related to distribution of gasification agent without the use of internals, management of heat release to avoid any agglomeration and clinker formation, specific design of bends to withstand the highly erosive environment due to high solid particles circulation rates, design of a standpipe cyclone to withstand high temperature gasification environment, compact design of seal-leg that can handle high mass solids flux, design of nozzles that eliminate plugging, uniform aeration of large diameter Standpipe, oxidant injection at the cyclone exits to effectively modulate gasifier exit temperature and reduction in overall height of the gasifier with a modified non-mechanical valve.
Owner:SOUTHERN COMPANY SERVICES
Regeneration of used supported noble metal catalysts
InactiveUS6740615B2Efficient removalImproves expositionHydrogen peroxideOther chemical processesPalladium catalystMetal particle
A method for regenerating used supported noble metal catalysts, which method includes solvent cleaning the used catalyst by contact with a suitable organic liquid cleaning solvent such as alcohols, ketones and such to remove organic deposits from the catalyst, followed by drying and calcining at elevated temperature to remove any remaining organic deposits from the catalyst, then treating the catalyst with an organo-metallic complex forming agent having ionization constant pK1 greater than about 2.5, such as glycolic acid and the like. The organic-metallic complex forming agent acts to break down large clusters of noble metal particles such as palladium (Pd) and redistributes the metal particles on the catalyst support such as alumina (Al2O3) in the same or other larger pores, so as to increase catalyst surface area and catalytic activity to provide a catalytic activity level at least 80% or even exceeding that of the fresh catalyst. This regeneration method is particularly useful for regenerating used supported palladium catalysts utilized for hydrogenation of ethyl anthraquinone (EAQ) for producing hydrogen peroxide (H2O2) product.
Owner:POROCEL INT LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com