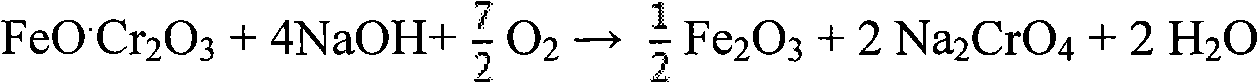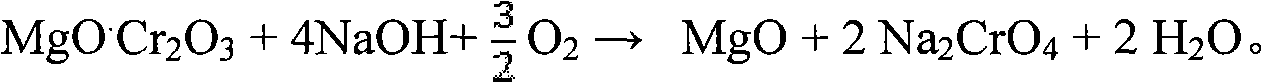Method for pollution-free production of sodium chromate by pressure leaching of chromite
A technology of pressurized leaching and clean production, applied in the direction of chromate/dichromate, can solve the problems of complex extraction process, industrialization constraints, and difficult to remove, and achieves lower reaction temperature, easy comprehensive utilization, and slag discharge. small amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
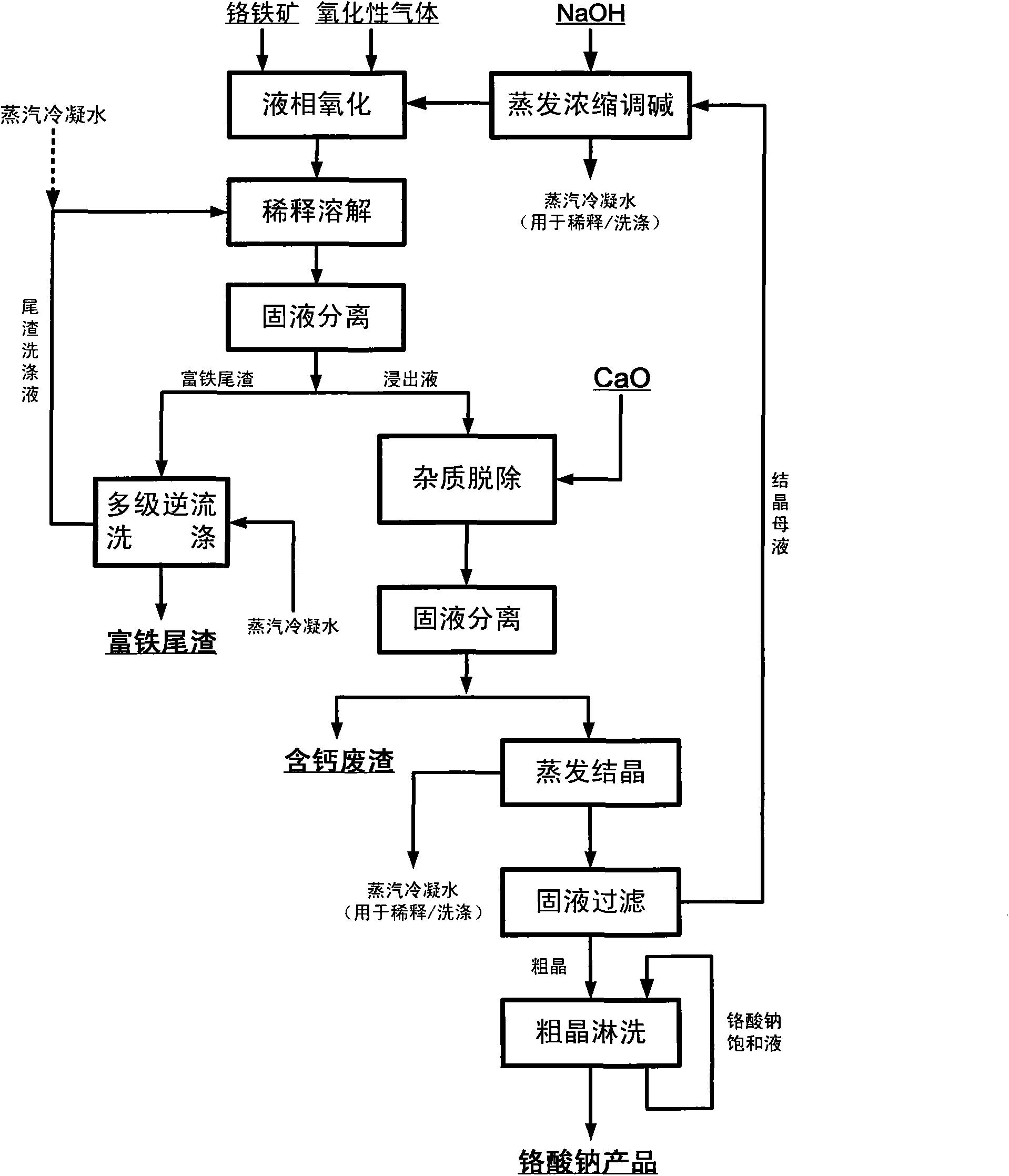
[0040]In the autoclave, add chromite and 30% NaOH solution, the ratio of alkali ore is 10:1, heat to 300 ° C, then pass air into the solution, and stir evenly, so that the chromite and oxygen can fully contact , the total pressure of the control system is 8MPa, and the temperature is kept for 10 hours, so that the chromite ore can fully react with oxygen. Finally get NaOH and Na 2 CrO 4 and other solutions, sodium chromate crystals and iron-rich tailings, the conversion rate of the main element chromium in chromite is above 99%. Add water to dilute the reaction mixture, and then separate the solid and liquid to obtain iron-rich tailings and Na-containing 2 CrO 4 , NaOH and other solutions. Add appropriate amount of calcium oxide to the solution to remove impurities, evaporate and crystallize to obtain sodium chromate crystals. After the sodium chromate crystals are rinsed with a saturated sodium chromate solution and dried, a sodium chromate product with a purity of more ...
Embodiment 2
[0042] In the high-pressure reactor, add chromite and 50wt% NaOH solution, the ratio of alkali ore is 6:1, heat to 280 ° C, then feed oxygen into the solution, and stir evenly, so that the chromite is fully in contact with oxygen , control the total pressure of the system to 6MPa, and keep it warm for 8 hours, so that the chromite ore can fully react with oxygen. Finally get NaOH and Na 2 CrO 4 and other solutions, sodium chromate crystals and iron-rich tailings, the conversion rate of the main element chromium in chromite is above 99%. Add water to dilute the reaction mixture, and then separate the solid and liquid to obtain iron-rich tailings and Na-containing 2 CrO 4 , NaOH etc., the follow-up process is the same as in Example 1.
Embodiment 3
[0044] In the high-pressure reactor, add chromite and 80wt% NaOH solution, the ratio of alkali ore is 2:1, heat to 200 ° C, then pass ozone into the solution, and stir evenly, so that the chromite and ozone are fully contacted , the total pressure of the control system is 0.5MPa, and the temperature is kept for 0.5 hours, so that the chromite ore can fully react with ozone. Finally get NaOH and Na 2 CrO 4 and other solutions, sodium chromate crystals and iron-rich tailings, the conversion rate of the main element chromium in chromite is above 99%. Add the iron-rich tailings washing solution obtained in Example 2 to dilute the reaction mixture, then separate the solid and liquid to obtain iron-rich tailings and Na2CrO-containing 4 , NaOH etc., the follow-up process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com