Cleaning process for producing sodium vanadate and sodium chromate alkali solution by high chromium vanadium slag
A process method, the technology of alkaline solution, which can be applied to the improvement of process efficiency, chemical instruments and methods, chromate/dichromate, etc., can solve the problems of high energy consumption for evaporation, reduction of overall production capacity, and high reaction temperature, Achieve high resource utilization, shorten follow-up processes, and save energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
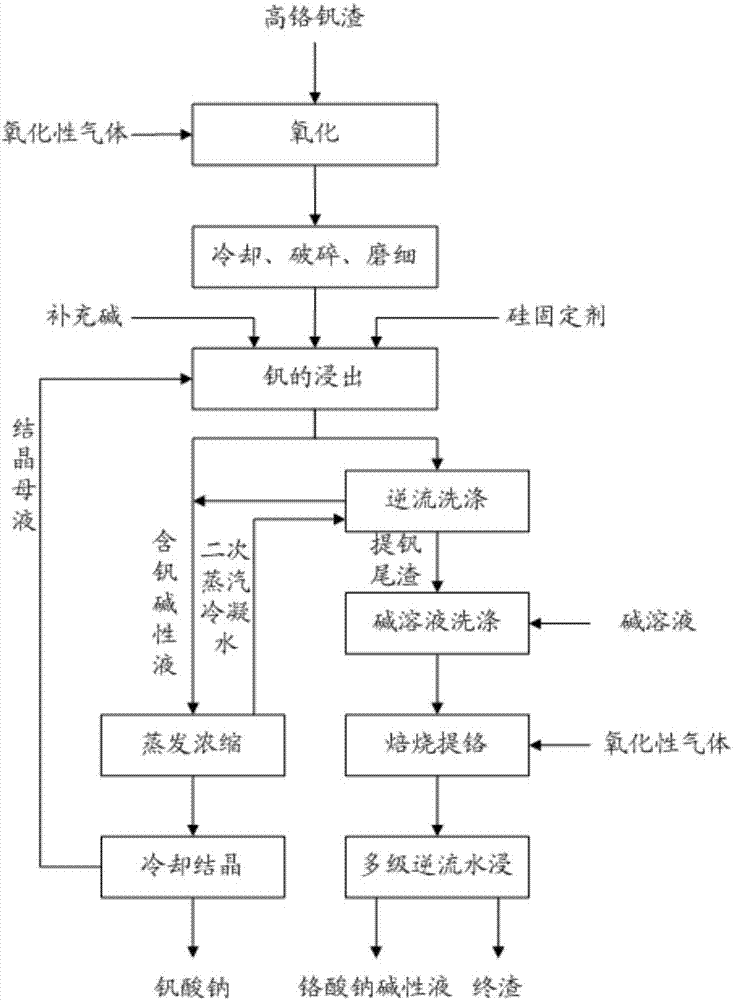
[0057] Oxygen was introduced into the high-temperature high-chromium vanadium slag separated from molten iron, oxidized at 600° C. for 180 minutes, and then crushed, and the high-chromium vanadium slag particles were ground down to below 74 microns. The 10% sodium hydroxide solution is mixed with the ground high chromium vanadium slag and the theoretical amount of alumina to carry out the vanadium leaching reaction, wherein the volume mass ratio (L / kg) of the sodium hydroxide solution to the high chromium vanadium slag is: 10:1, the leaching temperature is 170°C, and the leaching time is 10h. After the leaching is completed, the slurry is filtered, and the slag is washed with two-stage countercurrent. The liquid-solid volume-to-mass ratio in the washing process is 8:1. The recovery rate is 98%, and the chromium is not leached. This process obtains vanadium extraction tailings and vanadium-containing alkaline solution respectively. The vanadium-containing alkaline solution is e...
Embodiment 2
[0059] Oxygen was introduced into the high-temperature high-chromium vanadium slag separated from molten iron, oxidized at 600° C. for 120 minutes, and then crushed, and the high-chromium vanadium slag particles were ground down to less than 74 microns. Mix 20% sodium hydroxide solution with ground high chromium vanadium slag and aluminum hydroxide with twice the theoretical amount to carry out vanadium leaching reaction, wherein the volume mass ratio of sodium hydroxide solution to high chromium vanadium slag (L / kg) was 8:1, the leaching temperature was 150 °C, and the leaching time was 8h. After the leaching was completed, the slurry was filtered, and the slag was washed with 3-stage countercurrent. The liquid-solid volume-to-mass ratio in the washing process was 6:1. The recovery rate of vanadium was calculated to be 98.5%, and the chromium was not leached. The vanadium extraction tailings and the vanadium-containing alkaline solution were obtained in this process. The van...
Embodiment 3
[0061] Air is introduced into the high-temperature high-chromium vanadium slag separated from the molten iron, oxidized at 890°C for 60 minutes, and then crushed, and the high-chromium vanadium slag particles are ground to below 74 microns. The leaching reaction of vanadium is carried out by mixing 40% sodium hydroxide solution with ground high chromium vanadium slag and 1.5 times the theoretical amount of sodium aluminate, wherein the volume mass ratio of sodium hydroxide solution to high chromium vanadium slag (L / kg) was 3:1, the leaching temperature was 100 °C, and the leaching time was 2h. After the leaching was completed, the slurry was filtered, and the filter cake was washed with 6-stage countercurrent. And the recovery rate of vanadium is calculated to be 98%, and the chromium is not leached. This process obtains vanadium extraction tailings and vanadium-containing alkaline solution respectively. The vanadium-containing alkaline solution is evaporated and concentrated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com