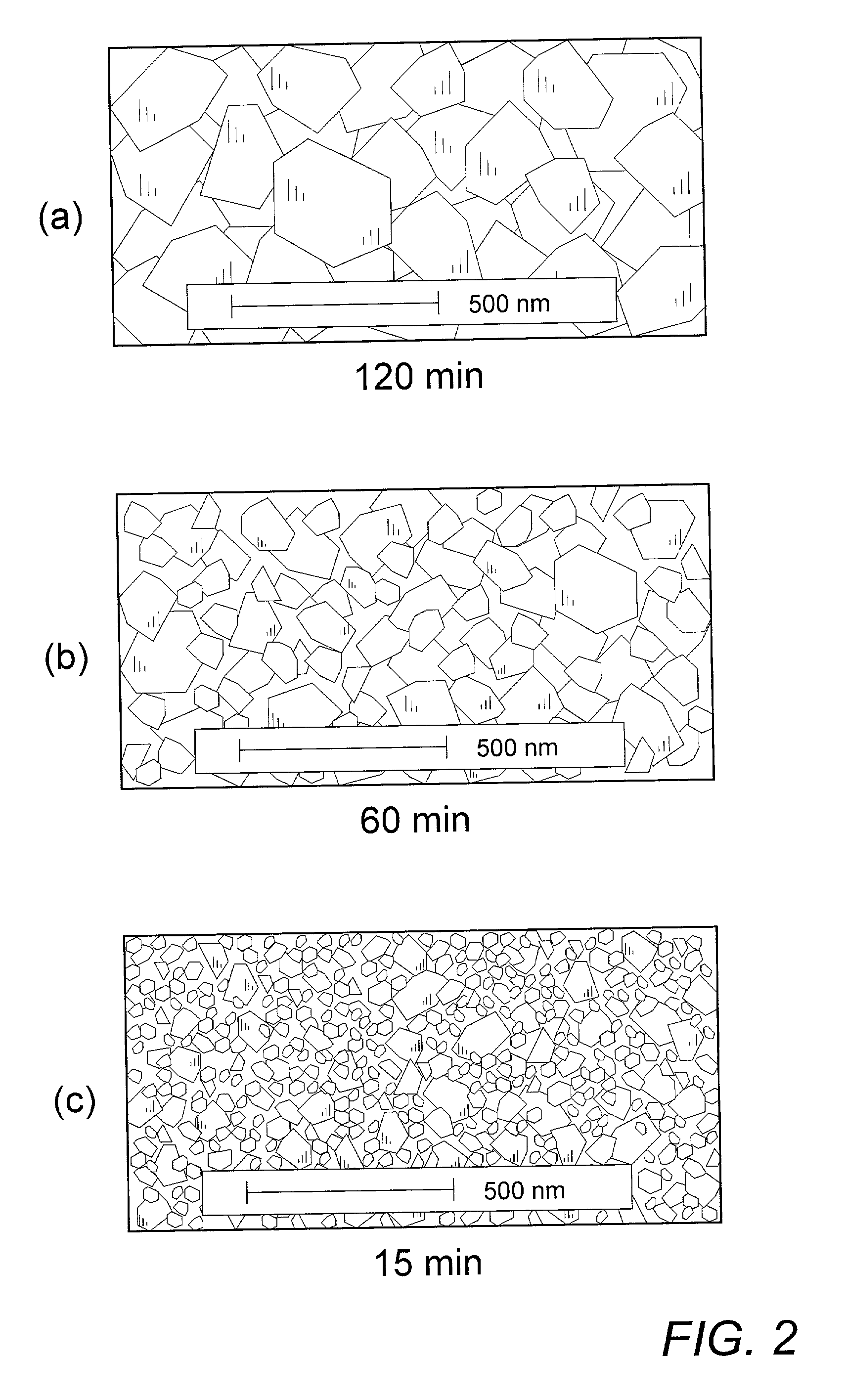
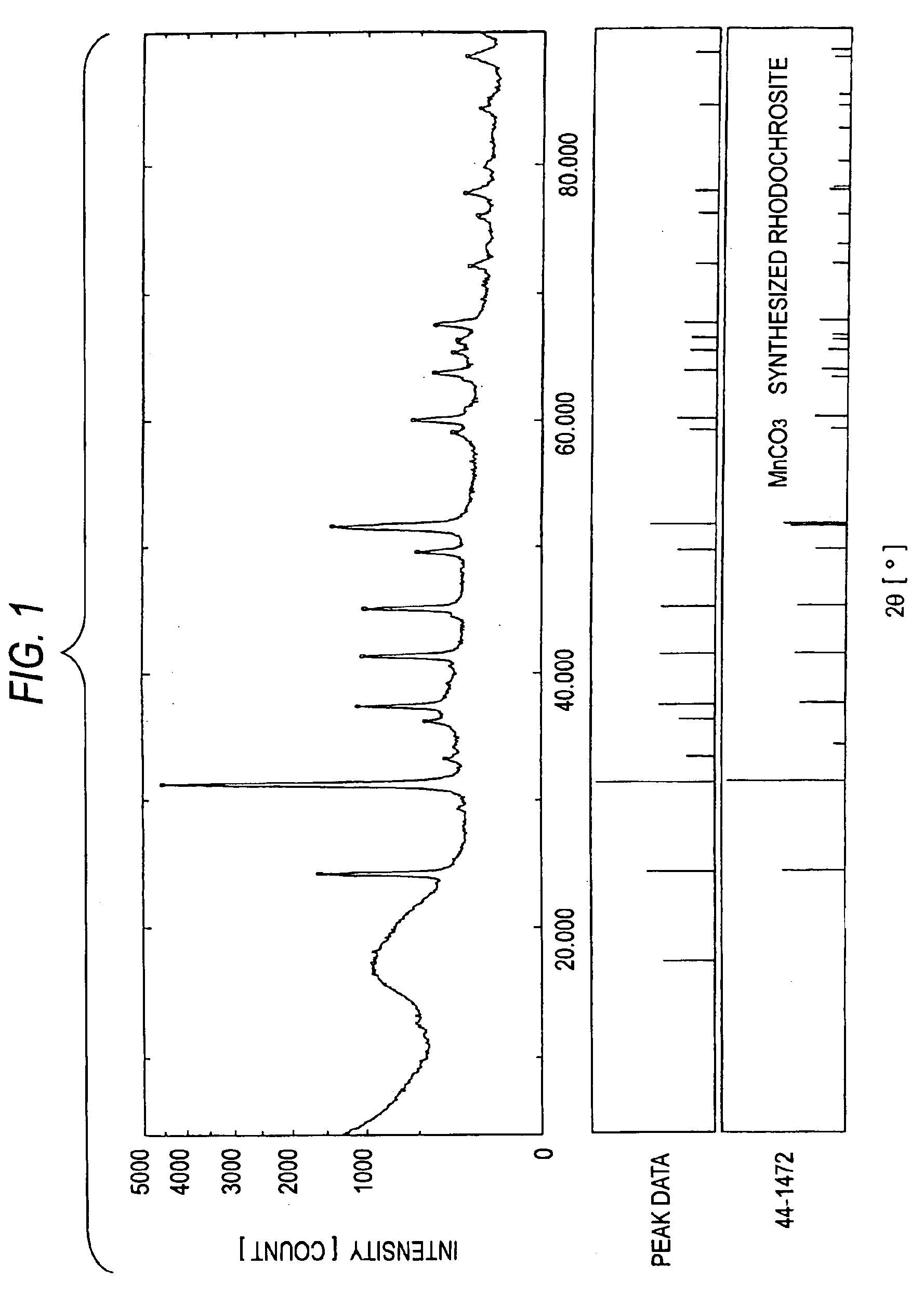
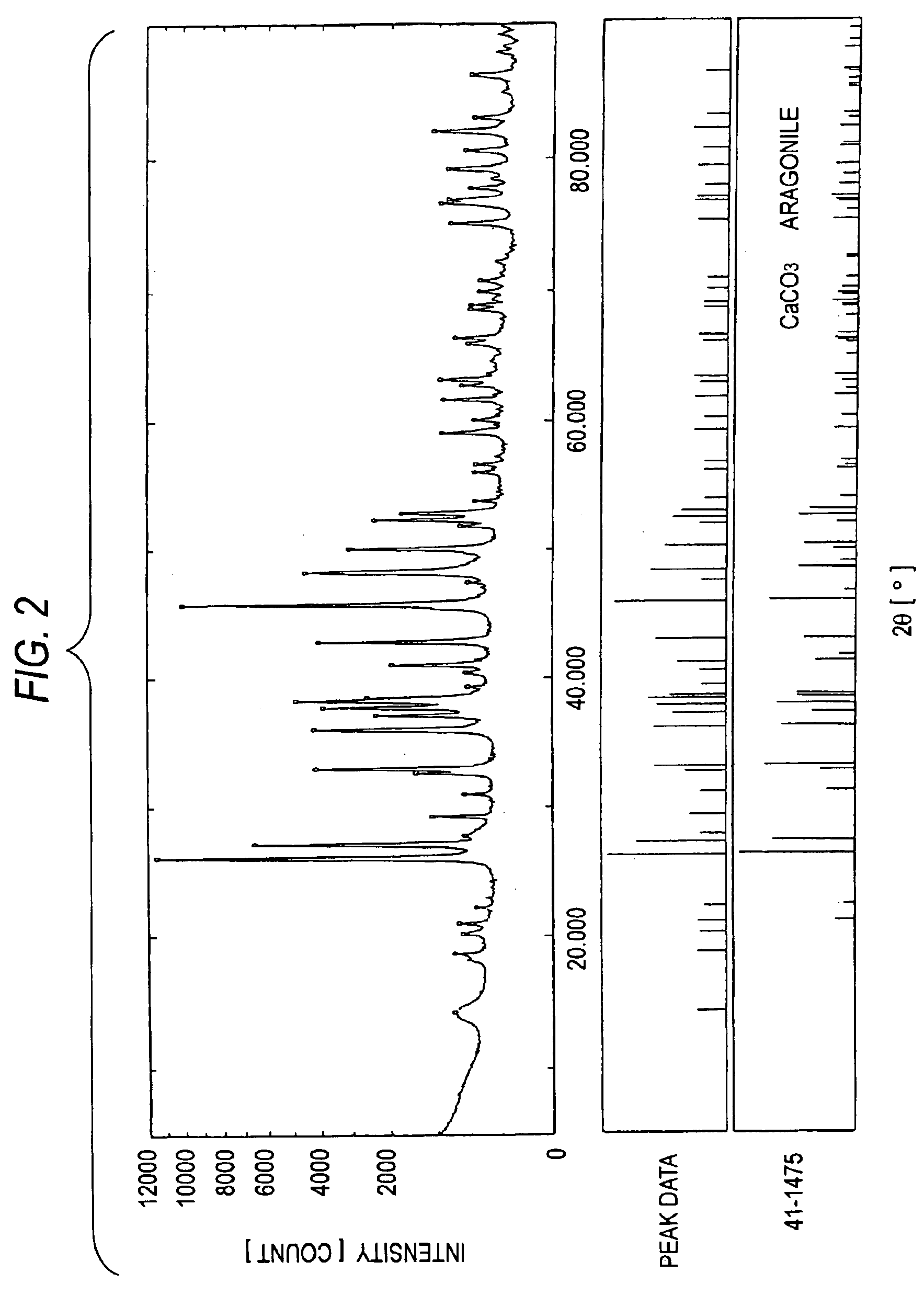
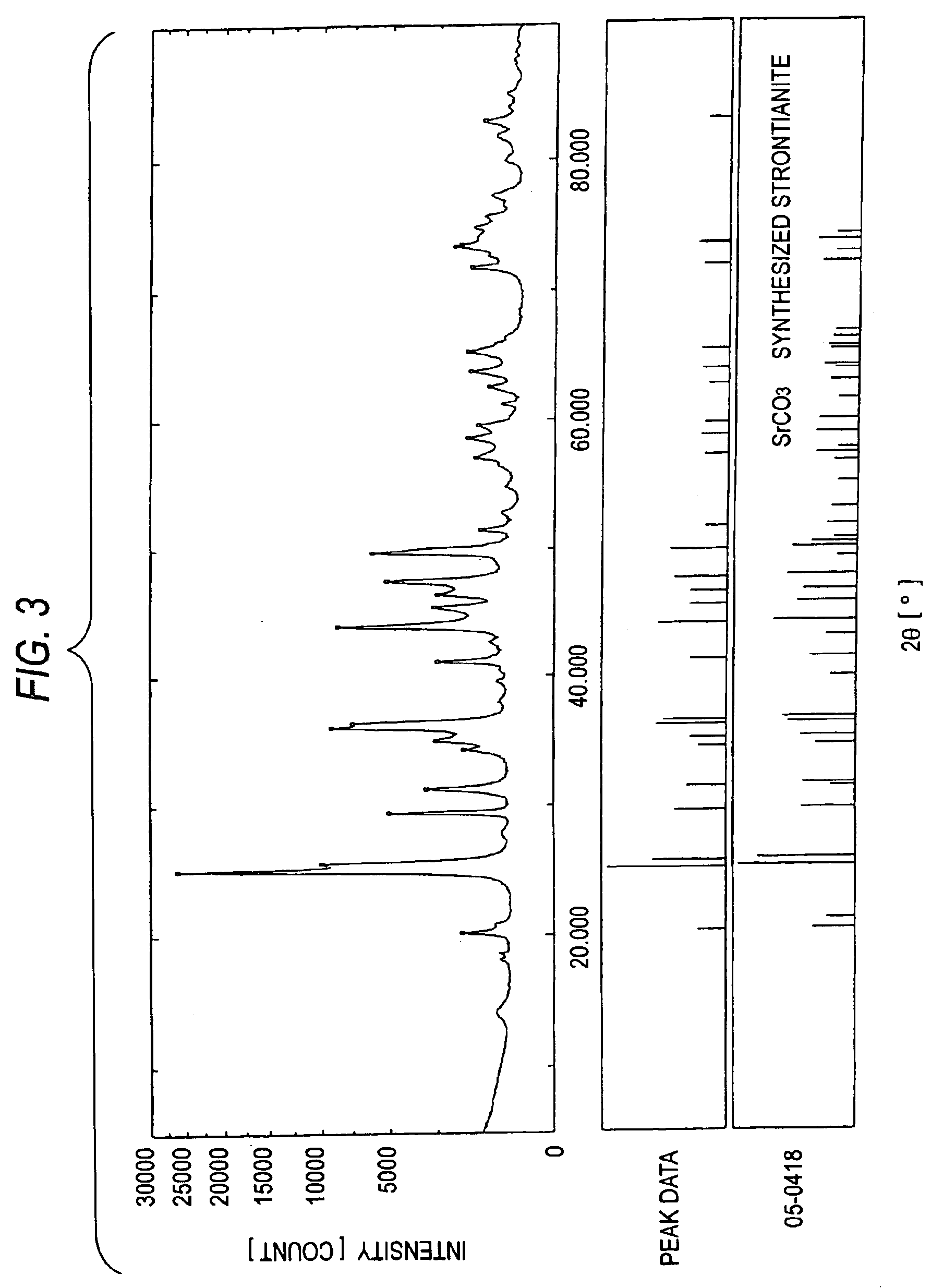
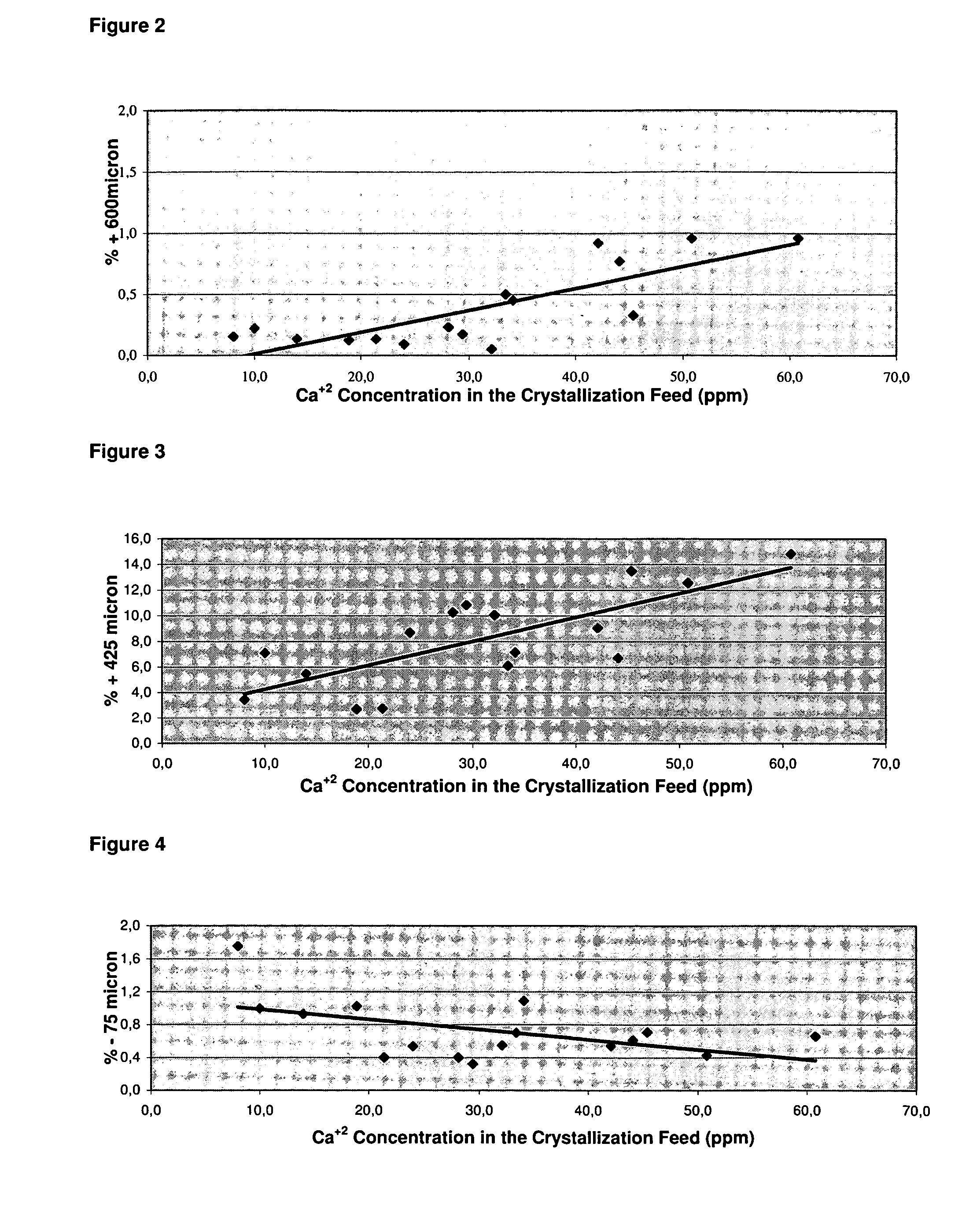
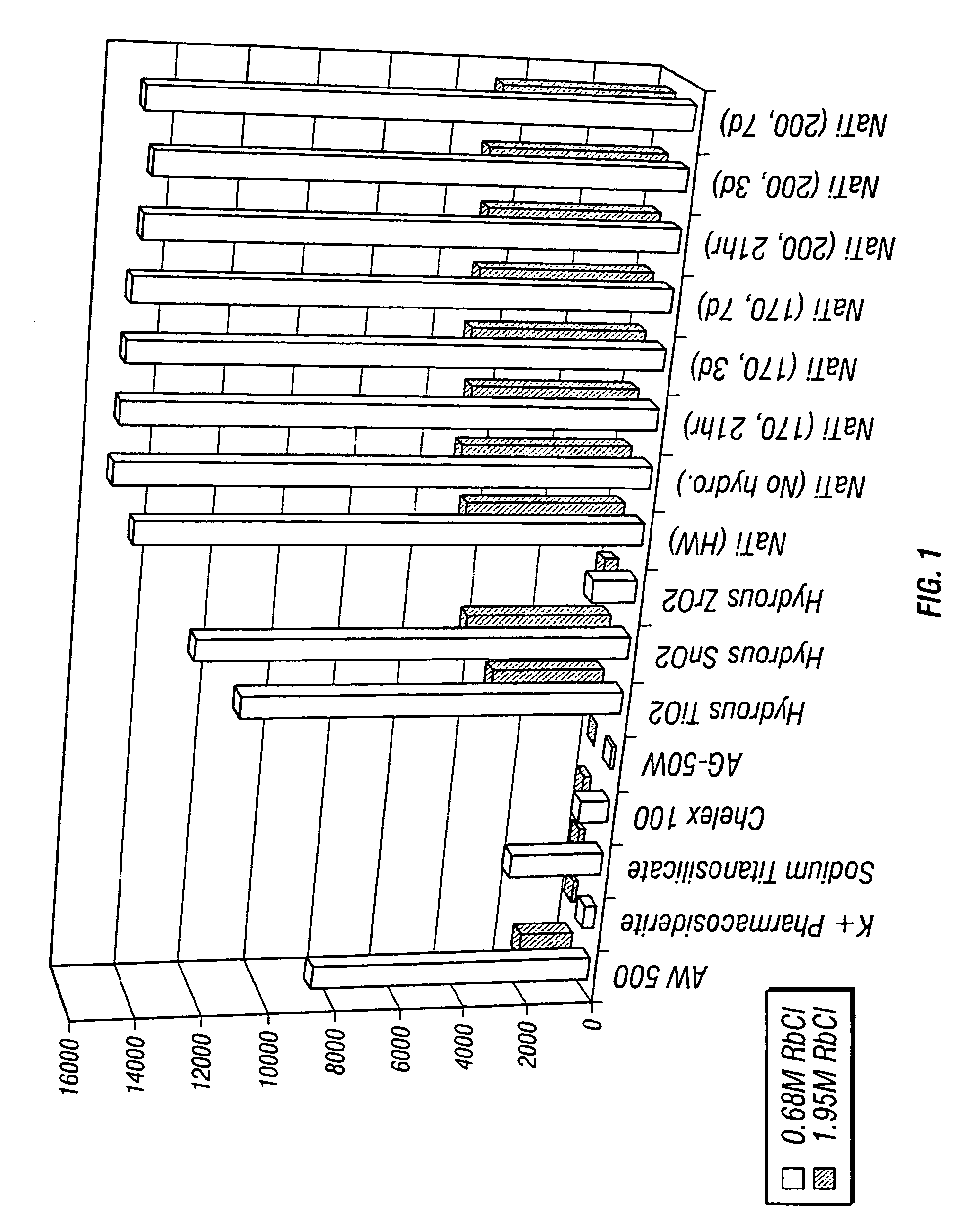
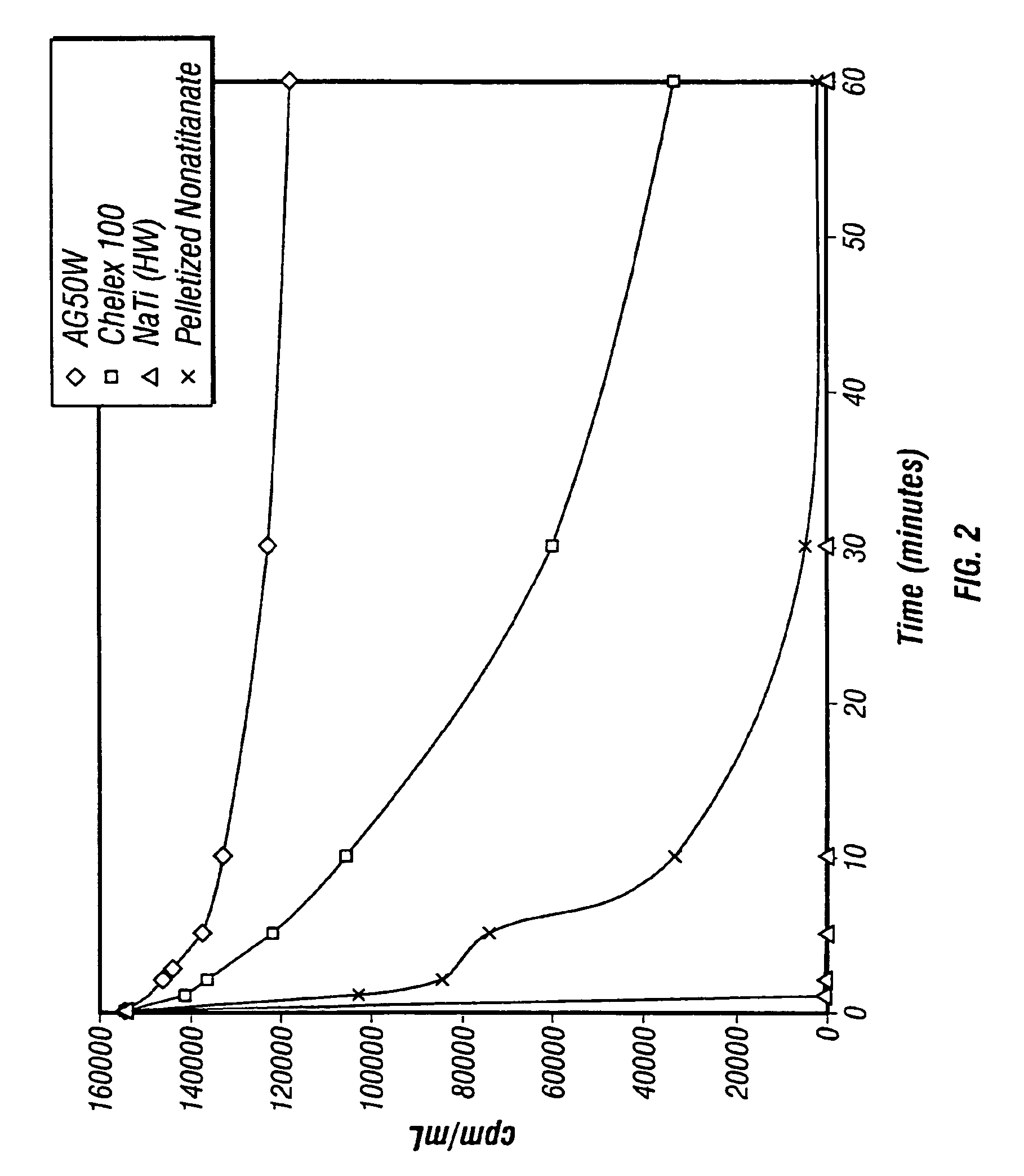
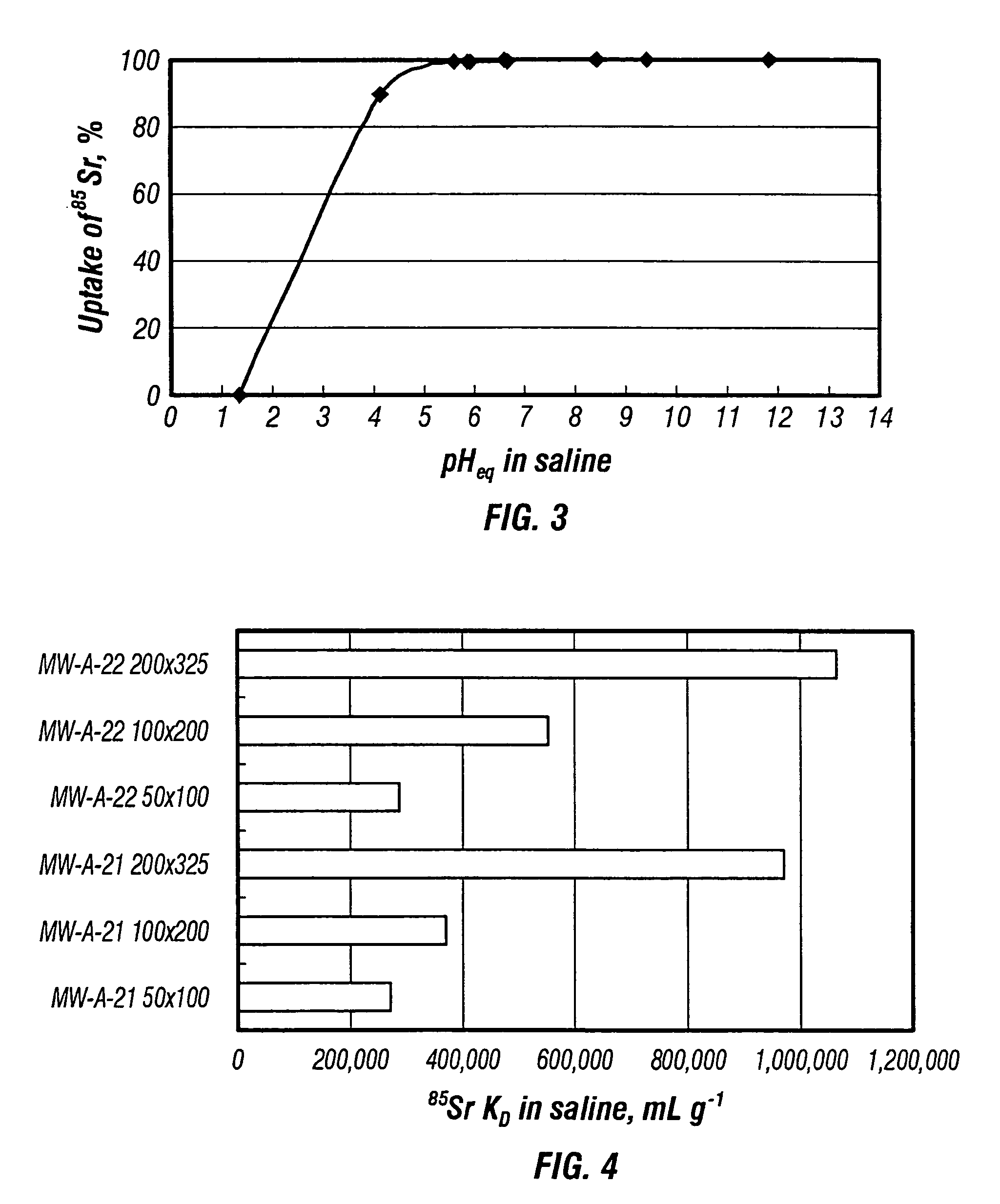
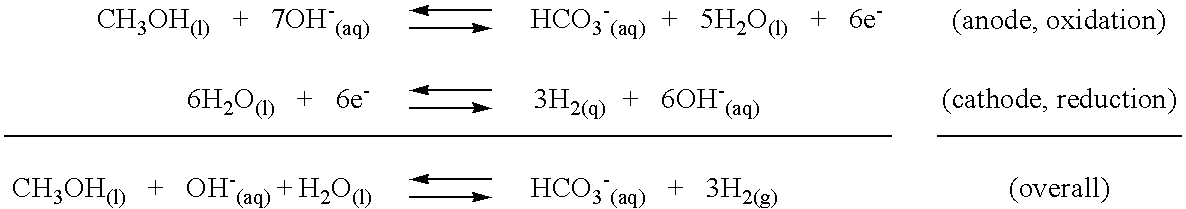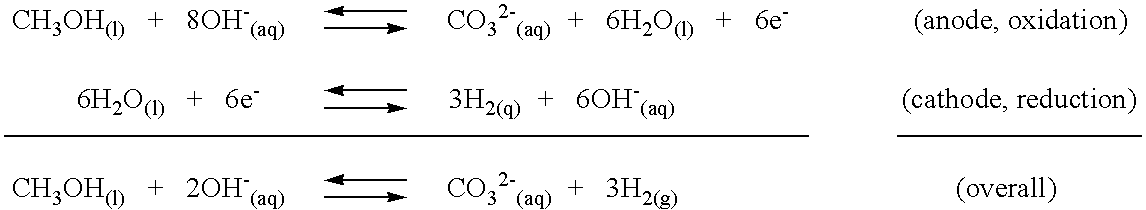Patents
Literature
401results about "Rubidium/caesium/francium compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
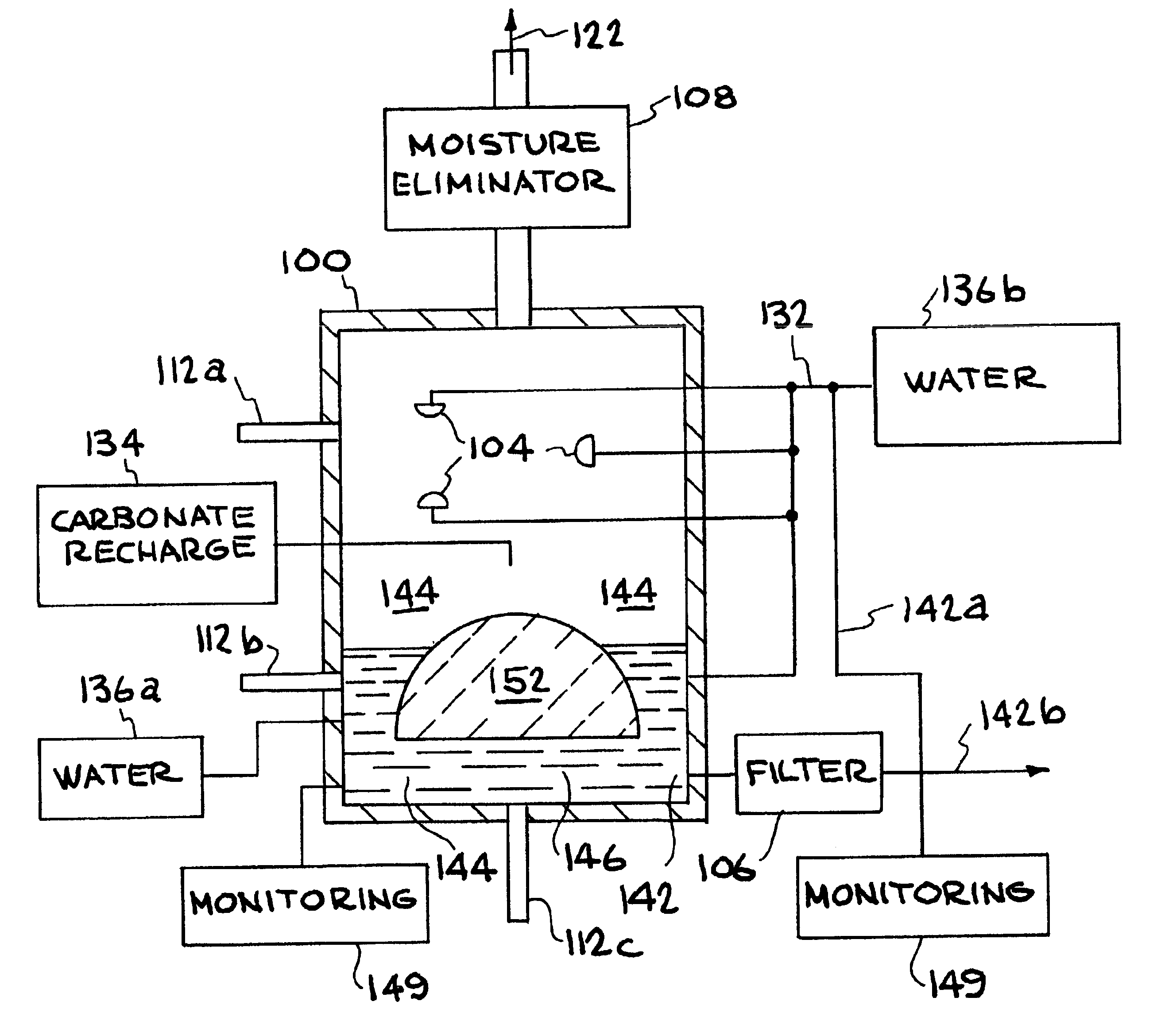
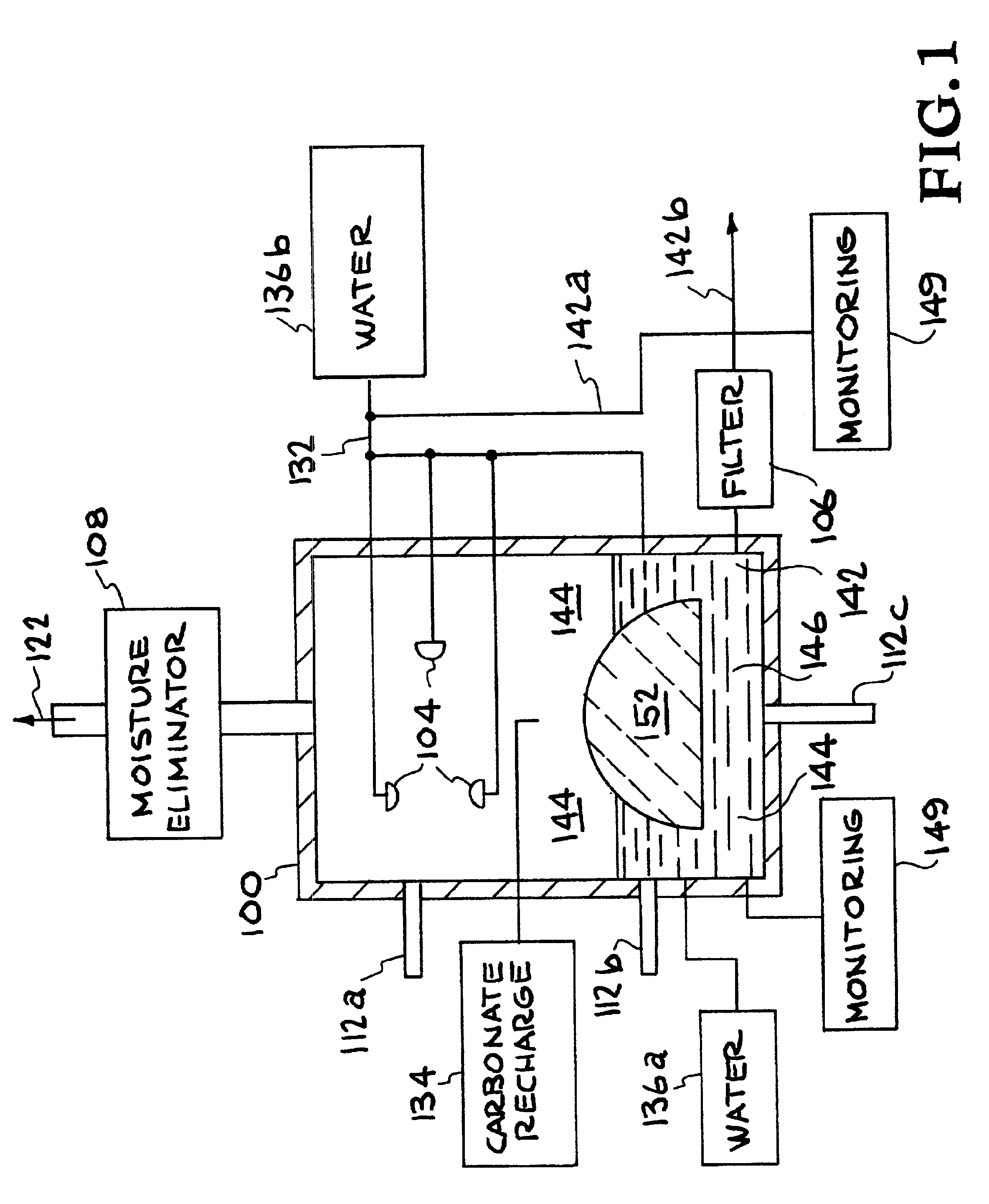
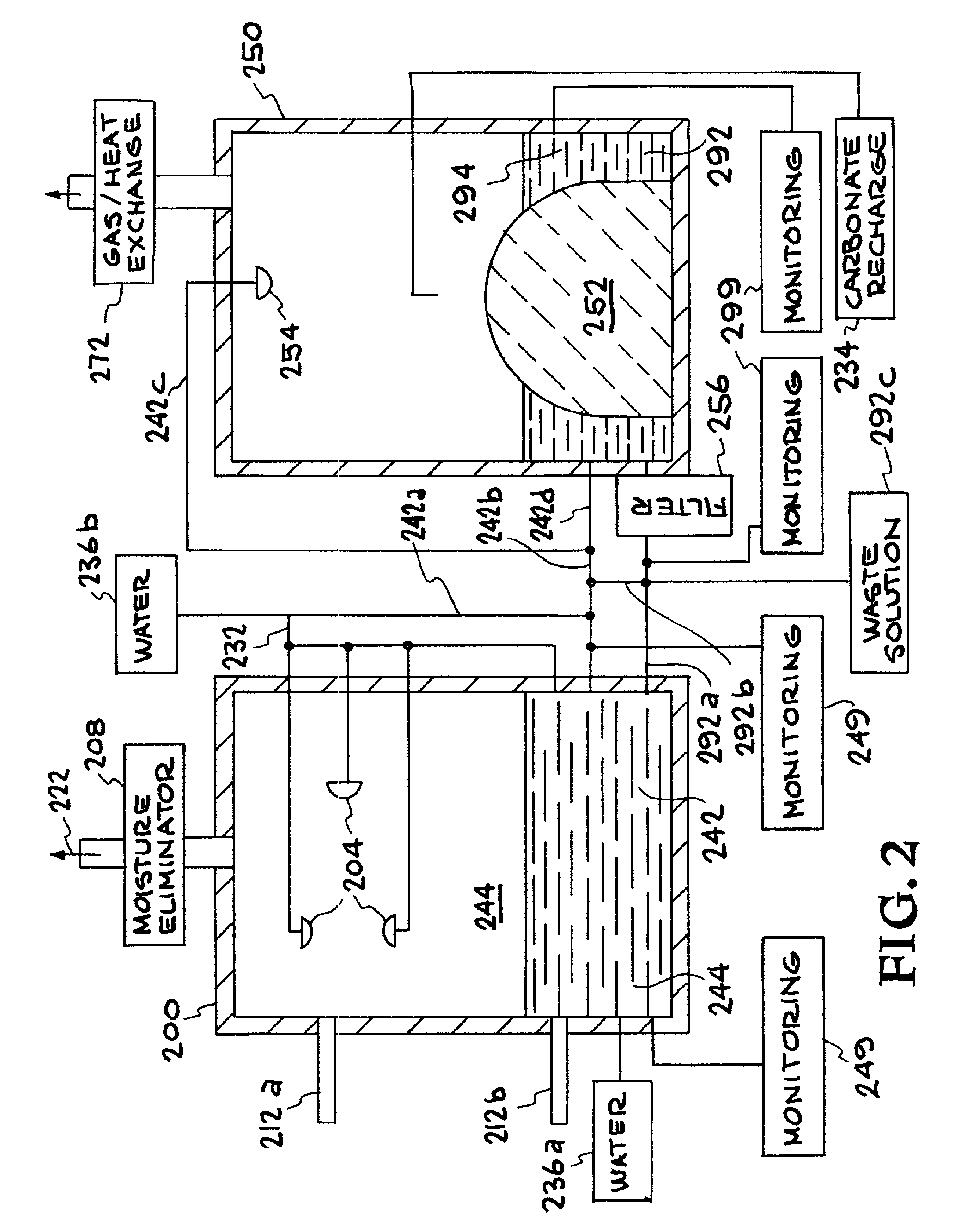
Method for extracting and sequestering carbon dioxide
InactiveUS6890497B2Reduce CO burdenWithout significant expenditureCalcium/strontium/barium carbonatesCombination devicesDicarbonateAlkaline earth metal
A method and apparatus to extract and sequester carbon dioxide (CO2) from a stream or volume of gas wherein said method and apparatus hydrates CO2, and reacts the resulting carbonic acid with carbonate. Suitable carbonates include, but are not limited to, carbonates of alkali metals and alkaline earth metals, preferably carbonates of calcium and magnesium. Waste products are metal cations and bicarbonate in solution or dehydrated metal salts, which when disposed of in a large body of water provide an effective way of sequestering CO2 from a gaseous environment.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Electrocatalyst powders, methods for producing powders and devices fabricated from same
Electrocatalyst powders and methods for producing electrocatalyst powders, such as carbon composite electrocatalyst powders. The powders have a well-controlled microstructure and morphology. The method includes forming the particles from an aerosol of precursors by heating the aerosol to a relatively low temperature, such as not greater than about 400° C.
Owner:CABOT CORP
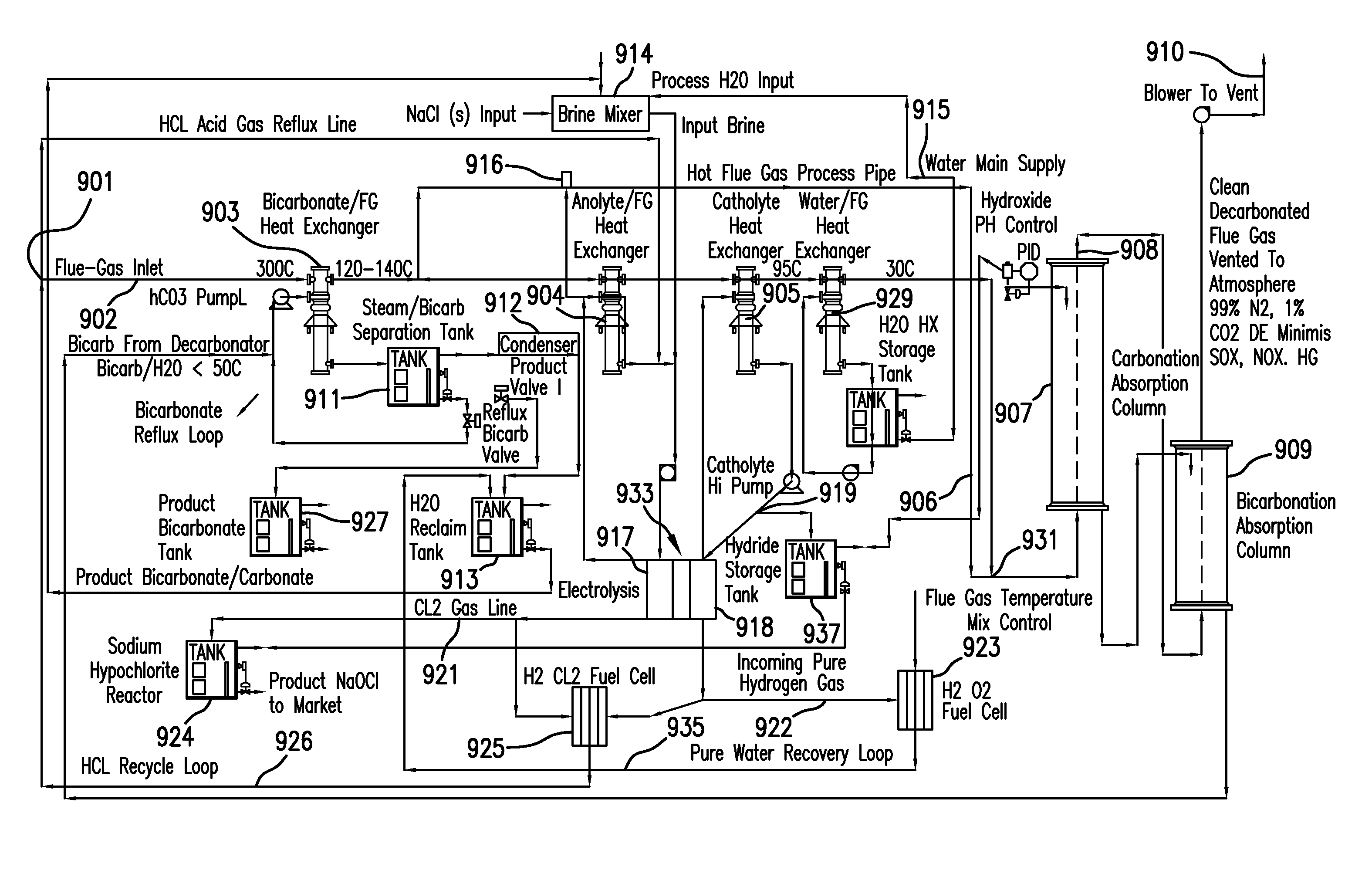
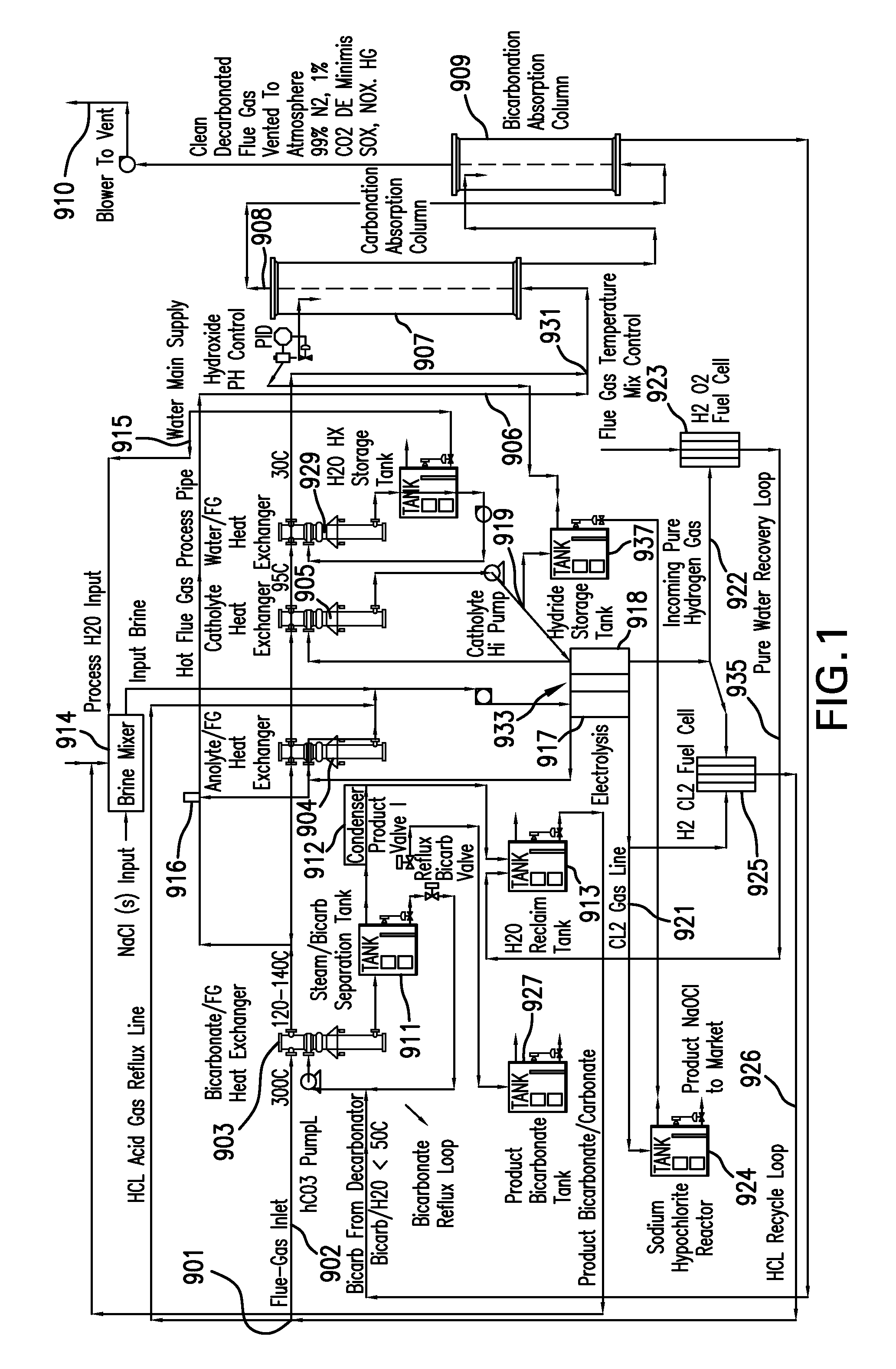
Removing carbon dioxide from waste streams through co-generation of carbonate and/or bicarbonate minerals
ActiveUS20060185985A1Improve ecologic efficiency of processEcologic efficiencyCalcium/strontium/barium carbonatesElectrolysis componentsElectrolysisWaste stream
Apparatuses and methods for removing carbon dioxide and other pollutants from a gas stream are provided. The methods include obtaining hydroxide in an aqueous mixture, and mixing the hydroxide with the gas stream to produce carbonate and / or bicarbonate. Some of the apparatuses of the present invention comprise an electrolysis chamber for providing hydroxide and mixing equipment for mixing the hydroxide with a gas stream including carbon dioxide to form an admixture including carbonate and / or bicarbonate.
Owner:CARBONFREE CHEM HLDG LLC
Flame made metal oxides
ActiveUS7211236B2Add featureWell mixedMaterial nanotechnologyZirconium oxidesSpray pyrolysisCarboxylic acid
Described is a method for the production of metal oxides by flame spray pyrolysis, in particular mixed metal oxides such as ceria / zirconia, and metal oxides obtainable by said method. Due to high enthalpy solvents with a high carboxylic acid content said metal oxides have improved properties. For example ceria / zirconia has excellent oxygen storage capacity at high zirconium levels up to more than 80% of whole metal content.
Owner:EIDGENOSSISCHE TECHN HOCHSCULE ZURICH
Barriers for polymer-coated implantable medical devices and methods for making the same
InactiveUS6953560B1Reduce and prevent and inflammationReduce and prevent proliferationStentsSurgeryHafniumPt element
An implantable medical device and methods for making the implantable medical device are disclosed. The implantable medical device includes a substrate. At least a portion of the substrate is coated with a first layer including a polymer containing a drug. A barrier overlies the first layer. The barrier significantly reduces the rate of release of the drug from the polymer, thereby sustaining release of the drug from the medical device for a longer time.The barrier may be a homogeneous layer overlying the first layer, or a number of discrete deposits over the first layer. Alternatively, the barrier may be intermixed with an outer portion of the first layer. The barrier material is biocompatible, and typically has a thickness ranging from about 50 angstroms to about 20,000 microns. Suitable materials for the barrier include, but are not limited to, inorganic compounds, such as inorganic silicides, oxides, nitrides, carbides, as well as pure metals such as aluminum, chromium, gold, hafnium, iridium, niobium, palladium, platinum, tantalum, titanium, tungsten, zirconium, and alloys of these metals. The barriers disclosed may be applied to the first layer by several techniques, depending on the material being applied. Exemplary deposition techniques include physical vapor deposition, alkoxide hydrolysis, and electroless plating.The implantable device may be a stent or a graft, among other possibilities.
Owner:ABBOTT CARDIOVASCULAR
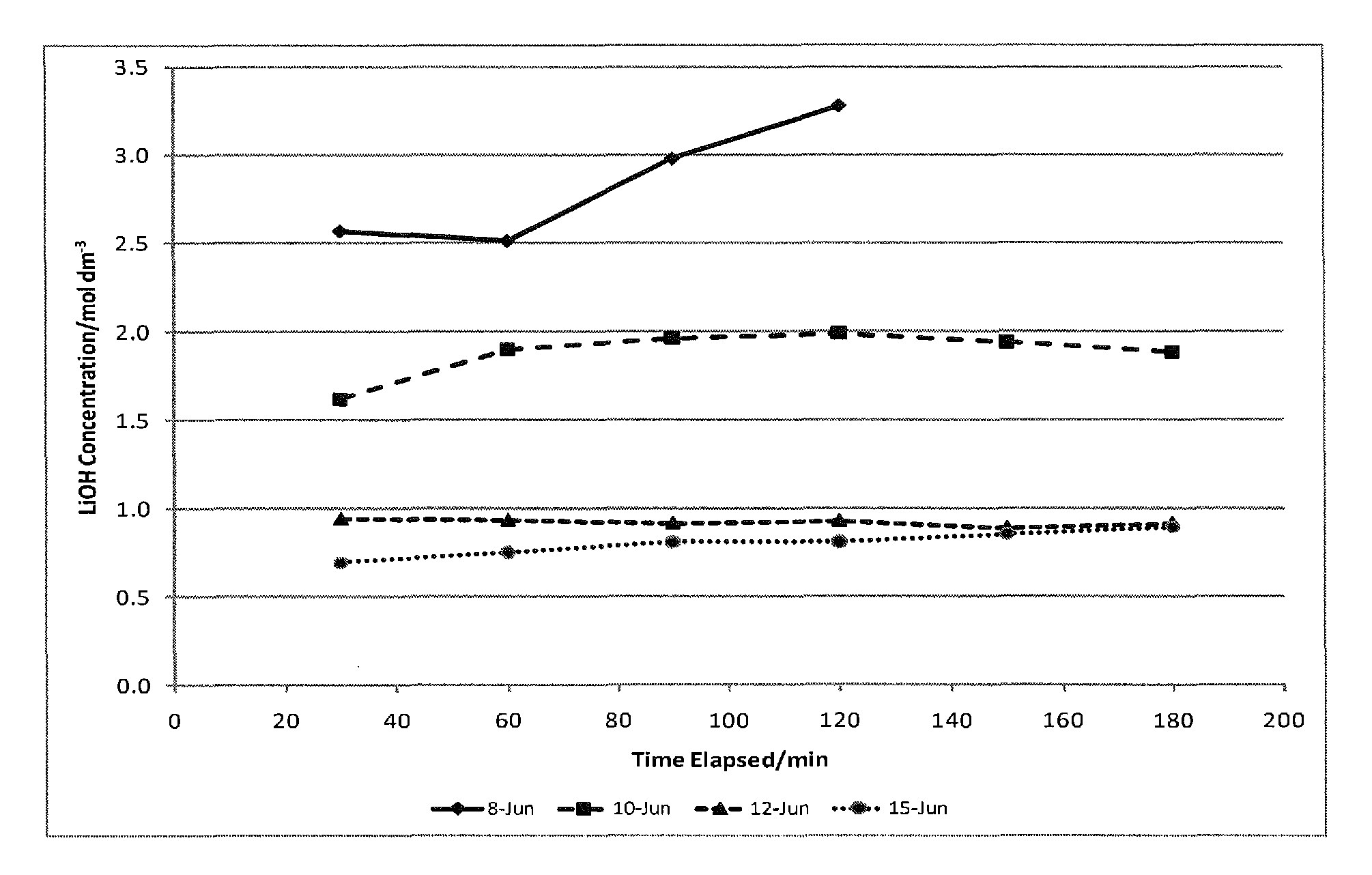
Method of making high purity lithium hydroxide and hydrochloric acid
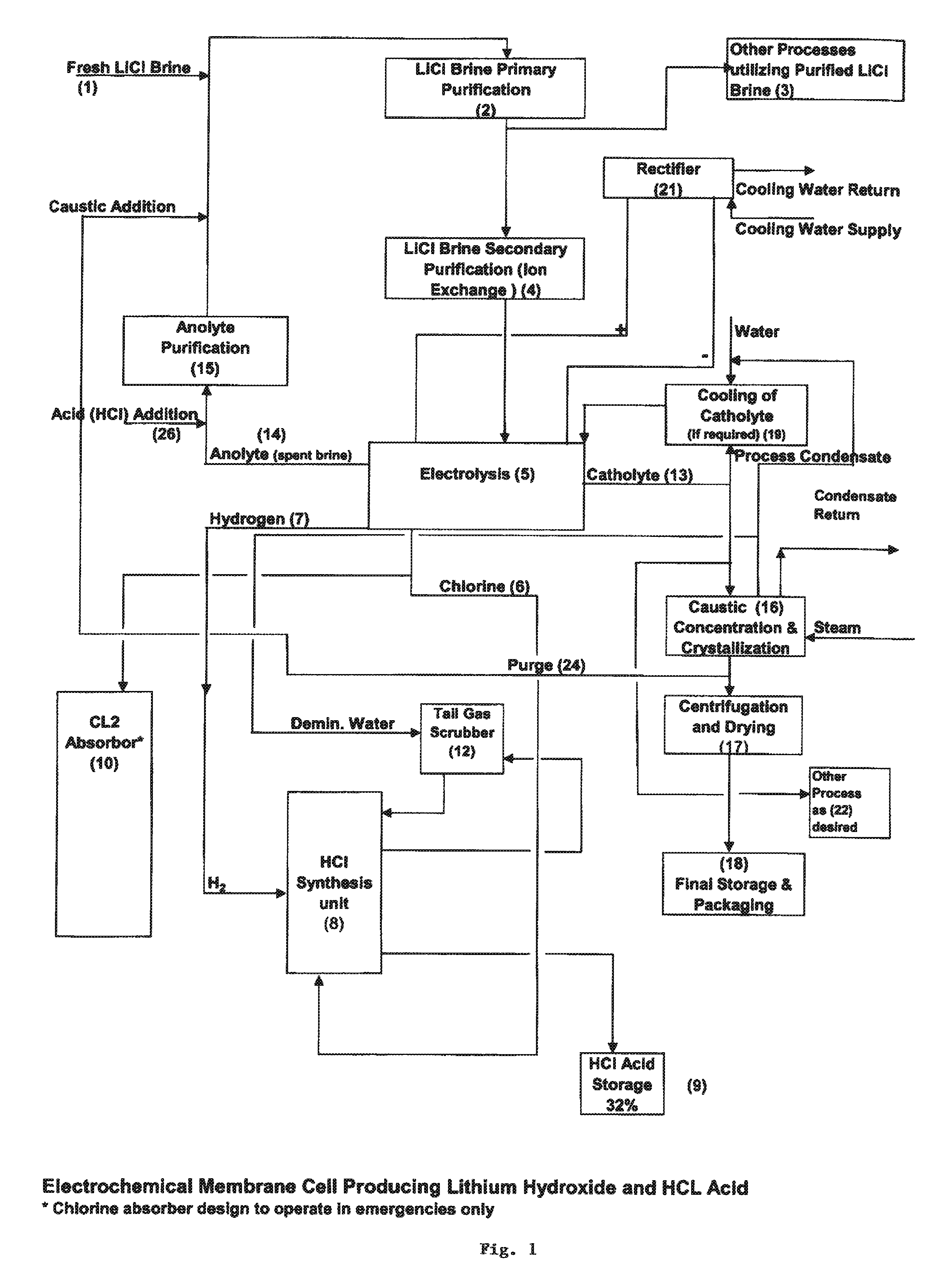
InactiveUS20110044882A1Simple and economical processEasy to convertElectrolysis componentsEnergy inputElectrolysisIon exchange
The present invention relates to a process for producing high purity lithium hydroxide monohydrate, comprising following steps: concentrating a lithium containing brine; purifying the brine to remove or to reduce the concentrations of ions other than lithium; adjusting the pH of the brine to about 10.5 to 11 to further remove cations other than lithium, if necessary; neutralizing the brine with acid; purifying the brine to reduce the total concentration of calcium and magnesium to less than 150 ppb via ion exchange; electrolyzing the brine to generate a lithium hydroxide solution containing less than 150 ppb total calcium and magnesium, with chlorine and hydrogen gas as byproducts; producing hydrochloric acid via combustion of the chlorine gas with excess hydrogen and subsequent scrubbing of the resultant gas stream with purified water, if elected to do so; and concentrating and crystallizing the lithium hydroxide solution to produce lithium hydroxide monohydrate crystals.
Owner:ROCKWOOD LITHIUM INC
Metal oxide processing methods and systems
InactiveUS20050074380A1Move quicklyIncrease load capacityCombination devicesTemperatue controlIndustrial gasBatch processing
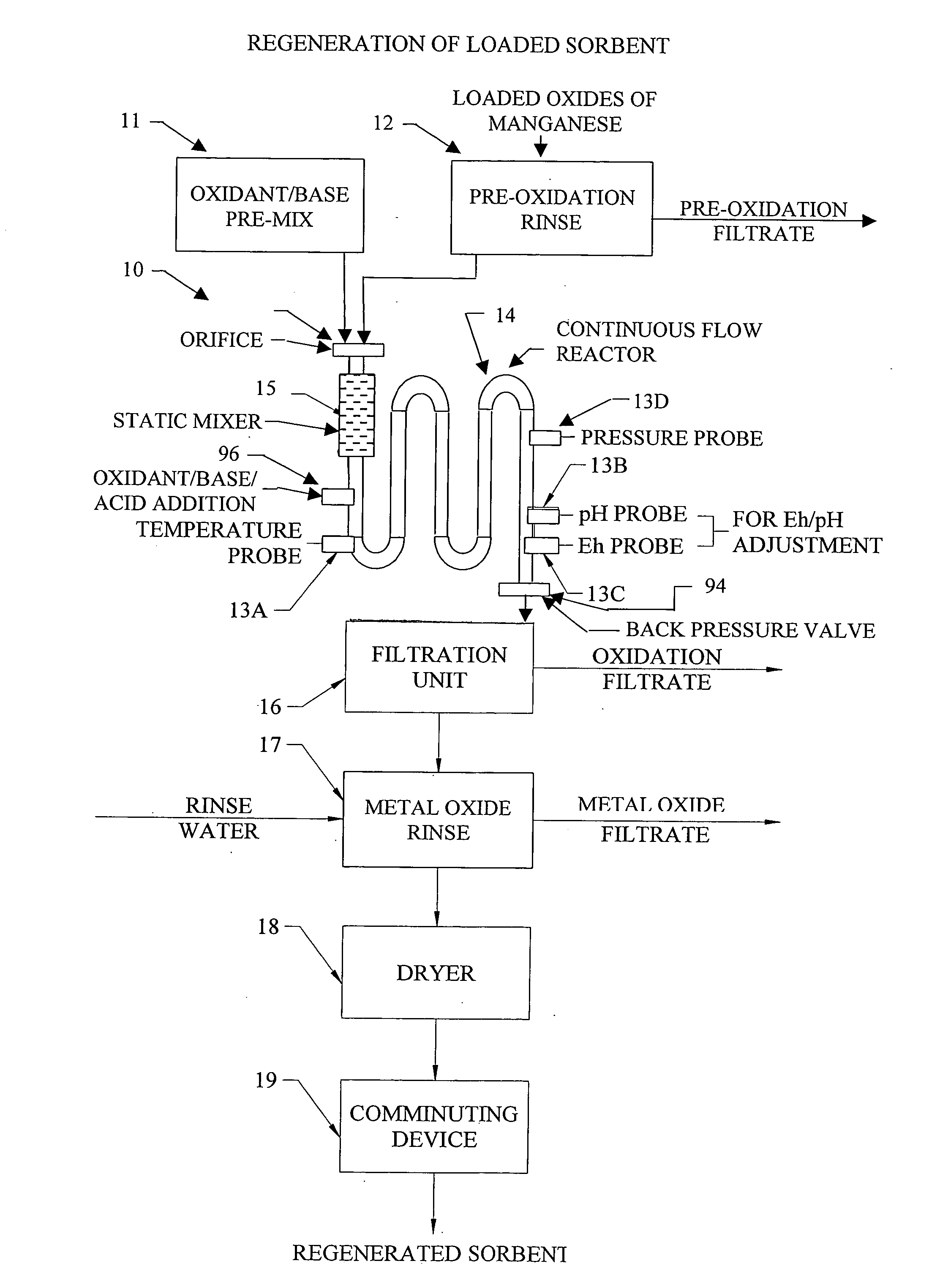
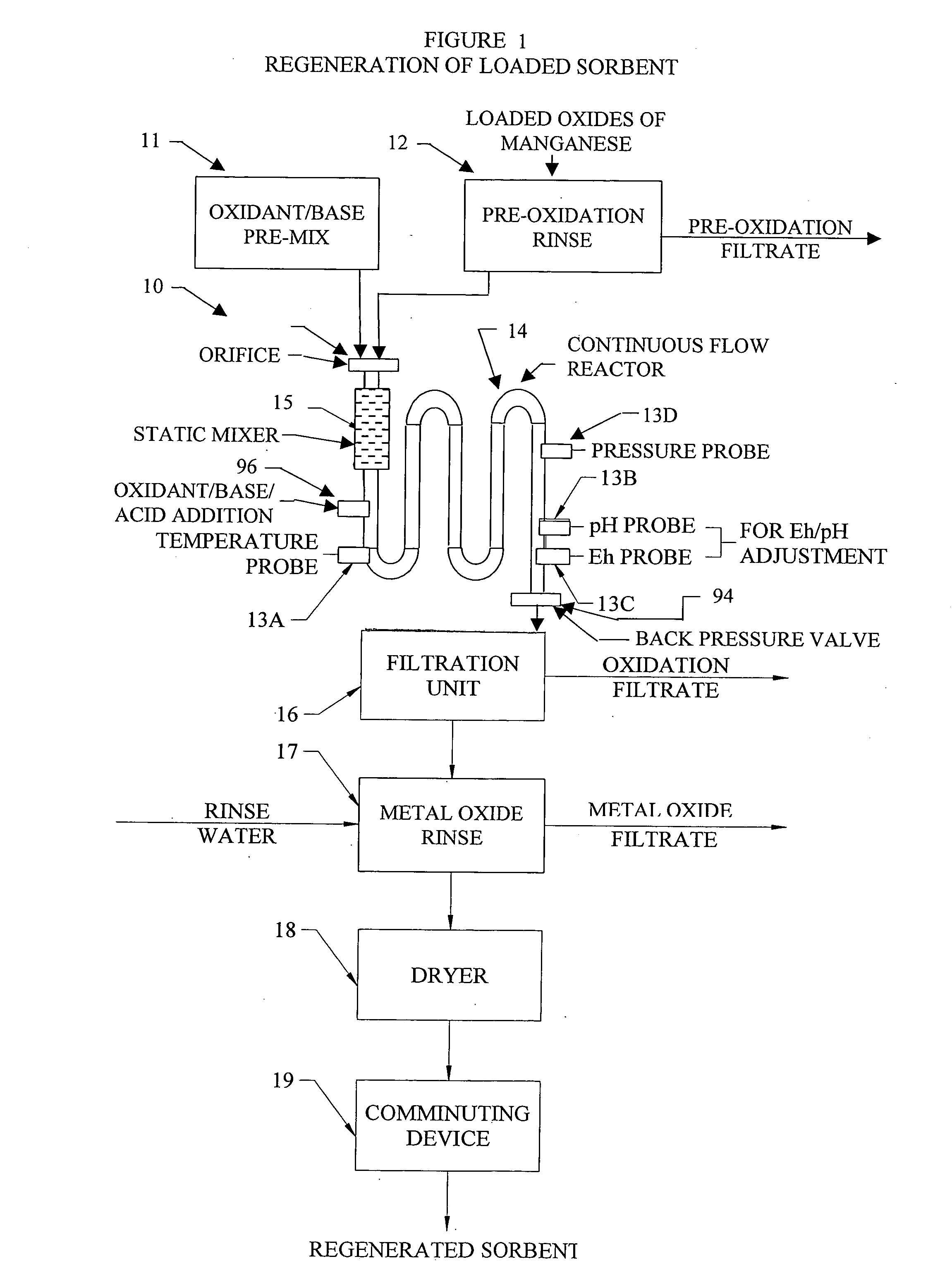
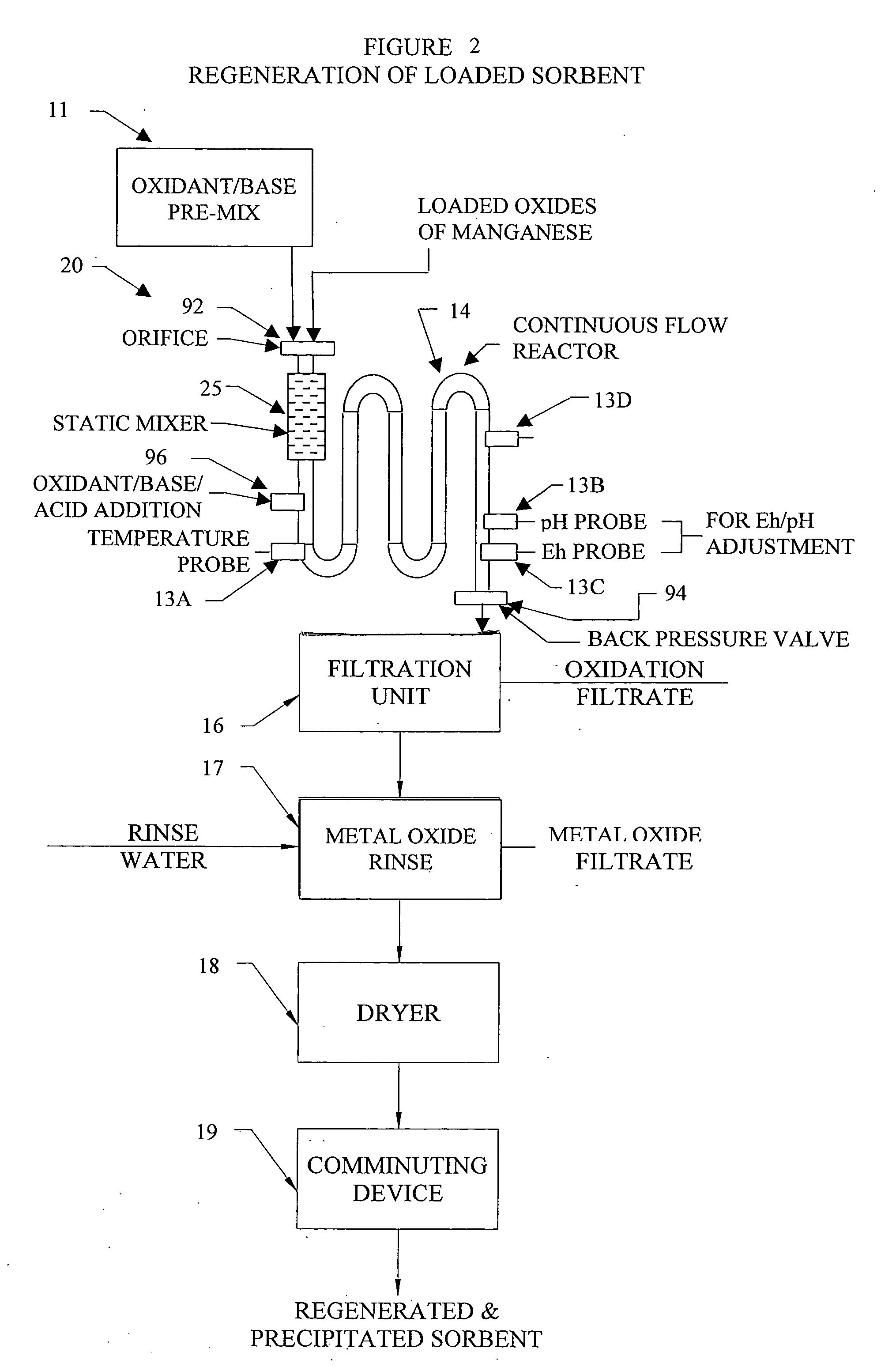
Methods and systems for processing metal oxides from metal containing solutions. Metal containing solutions are mixed with heated aqueous oxidizing solutions and processed in a continuous process reactor or batch processing system. Combinations of temperature, pressure, molarity, Eh value, and pH value of the mixed solution are monitored and adjusted so as to maintain solution conditions within a desired stability area during processing. This results in metal oxides having high or increased pollutant loading capacities and / or oxidation states. These metal oxides may be processed according to the invention to produce co-precipitated oxides of two or more metals, metal oxides incorporating foreign cations, metal oxides precipitated on active and inactive substrates, or combinations of any or all of these forms. Metal oxides thus produced are, amongst other uses; suitable for use as a sorbent for capturing or removing target pollutants from industrial gas streams or drinking water or aqueous streams or for personal protective respirators.
Owner:ENVIROSCRUB TECH CORP
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
ActiveUS20110200508A1Electrolysis componentsLithium organic compoundsLithium carbonateLithium hydroxide
Owner:TERRALITHIUM LLC
Nanostructure lithium titanate electrode for high cycle rate rechargeable electrochemical cell
Rechargeable electrochemical cells, such as lithium batteries and asymmetric hybrid battery / supercapacitor systems, exhibiting exceptional specific capacity levels and stability over extended high-rate recharge cycling comprise nanostructure zero strain Li4Ti5O12 intercalation electrode material synthesized in a short duration process of annealing mixed TiO2 and Li-source precursor compounds at about 800° C. for a time of about 15–30 min which is not substantially longer than that required to effect maximum available reaction between the precursors, thereby substantially eliminating the growth of synthesized Li4Ti5O12 particles beyond nanostructure size. The process reduces by order of magnitude the time and energy required for synthesis of the active electrode material and fabrication of utilizing cell devices, and provides such nanostructure material which enables repeated, high-rate recharge cycling without loss of cell capacity or efficiency.
Owner:RUTGERS THE STATE UNIV
Catalyst for fluidized catalytic cracking of heavy hydrocarbon oil and method of fluidized catalytic cracking
InactiveUS6916762B2Efficient inactivationReduce the amount requiredCalcium/strontium/barium carbonatesAluminium compoundsHydrogenOxide matrix
An FCC catalyst which not only deactivates catalyst poison metals, such as nickel, vanadium and the like, in feedstock oils, inhibits the generation of hydrogen or coke, has excellent cracking activity and bottom oil-treating ability, and can yield a gasoline and LCO fraction in high yields, but also retains the performances on a high level over long and has an improved catalyst life; and an FCC method using the catalyst. The FCC catalyst has a compound of a bivalent metal or of bivalent and trivalent metals showing an XRD pattern of a carbonate of the bivalent metal; an inorganic oxide matrix and the compound dispersed therein; or an inorganic oxide matrix and the compound dispersed therein together with a crystalline aluminosilicate zeolite, and relates to an FCC method in which at least one of the catalysts are used in combination with an FCC catalyst obtained by evenly dispersing a crystalline aluminosilicate zeolite in an inorganic oxide matrix.
Owner:GASOLINEEUM ENERGY CENT FOUND +1
Aqueous dispersion, a process for the preparation and the use thereof
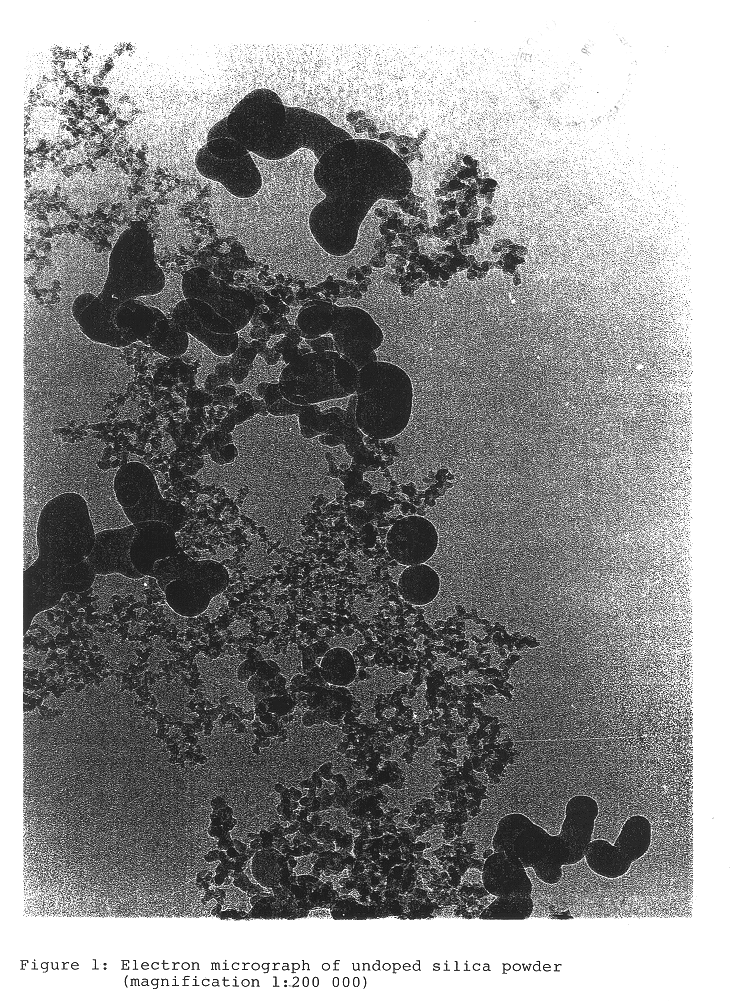
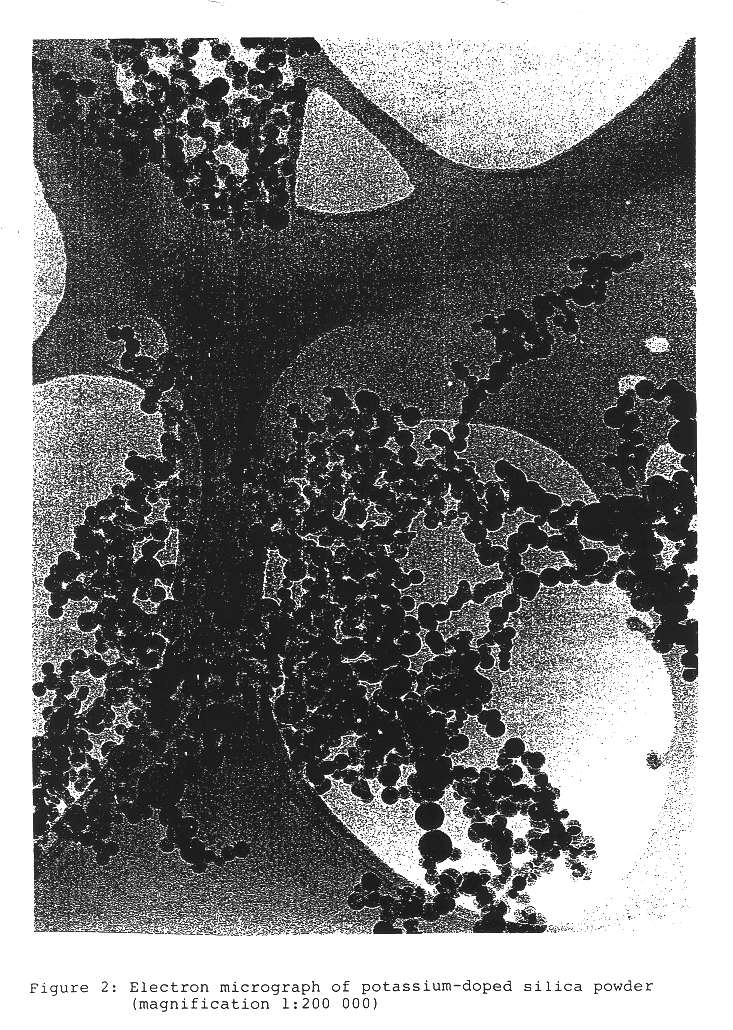
InactiveUS6676719B2Improve stabilityTrend downCosmetic preparationsNon-fibrous pulp additionSilica particleSilicon dioxide
A pyrogenic process is used to prepare alkali-doped silica particles. Particles produced by this process exhibit homogeneous doping, reduced agglomeration, greater stability and higher removal rates. Aqueous dispersions containing alkali-doped pyrogenic silica with average particle size less than 100 nm are used for polishing surfaces (CMP).
Owner:EVONIK DEGUSSA GMBH
Removing carbon dioxide from waste streams through co-generation of carbonate and/or bicarbonate minerals
ActiveUS7727374B2Calcium/strontium/barium carbonatesPhotography auxillary processesElectrolysisWaste stream
Apparatuses and methods for removing carbon dioxide and other pollutants from a gas stream are provided. The methods include obtaining hydroxide in an aqueous mixture, and mixing the hydroxide with the gas stream to produce carbonate and / or bicarbonate. Some of the apparatuses of the present invention comprise an electrolysis chamber for providing hydroxide and mixing equipment for mixing the hydroxide with a gas stream including carbon dioxide to form an admixture including carbonate and / or bicarbonate.
Owner:CARBONFREE CHEM HLDG LLC
Process for producing sodium bicarbonate for flue gas desulphurization
InactiveUS20100290967A1Electrolysis componentsVolume/mass flow measurementSodium bicarbonateFlue gas
Process for producing sodium bicarbonate for purifying flue gases, according to which an aqueous solution containing sodium sulfate is subjected to electrodialysis to produce a sodium hydroxide solution and a sodium bisulfate solution, the sodium hydroxide solution being carbonated in order to obtain sodium bicarbonate.
Owner:SOLVAY SA
Methods of preparing cathode active materials for lithium secondary battery
InactiveUS6071489AEasy to optimizeHigh crystallinityMaterial nanotechnologyNon-aqueous electrolyte accumulatorsManganeseEthyl acetate
The LixMn2O4 powder for cathode active material of a lithium secondary battery of the present invention is prepared by a method of comprising the steps of mixing an acetate aqueous solution using Li acetate and Mn acetate as metal precursors, and a chelating agent aqueous solution using PVB, GA, PAA or GC as a chelating agent; heating the mixed solution at 70 DIFFERENCE 90 DEG C. to form a sol; further heating the sol at 70 DIFFERENCE 90 DEG C. to form a gel precursor; calcining the produced gel precursor at 200 DIFFERENCE 900 DEG C. for 5 DIFFERENCE 30 hours under atmosphere. The cathode active material, LixMn2O4 powder for a lithium secondary battery in accordance with the present invention has a uniform particle size distribution, a high crystallinity and a pure spinel-phase, and a particle size, a specific surface area, a lattice of a cubic structure and the like can be controlled upon the preparing conditions. The present invention also provides a method of preparing LiNi1-xCoxO2 powder, which comprises the steps of providing a gel precursor using PAA as a chelating agent and hydroxide, nitrate or acetate of Li, Co and Ni as metal precursors; heating the gel precursor at 200 DIFFERENCE 900 DEG C. for 5 DIFFERENCE 30 hours to form a powder. The LixMn2O4 and LiNi1-xCoxO2 powder of the present invention can be used for a cathode active material of a lithium secondary battery such as a lithium ion battery or lithium polymer battery.
Owner:SAMSUNG DISPLAY DEVICES CO LTD
System and method for treating a flue gas stream
PendingUS20050201914A1Lower volume resistivityEfficient removalCombination devicesGas treatmentParticulatesDicarbonate
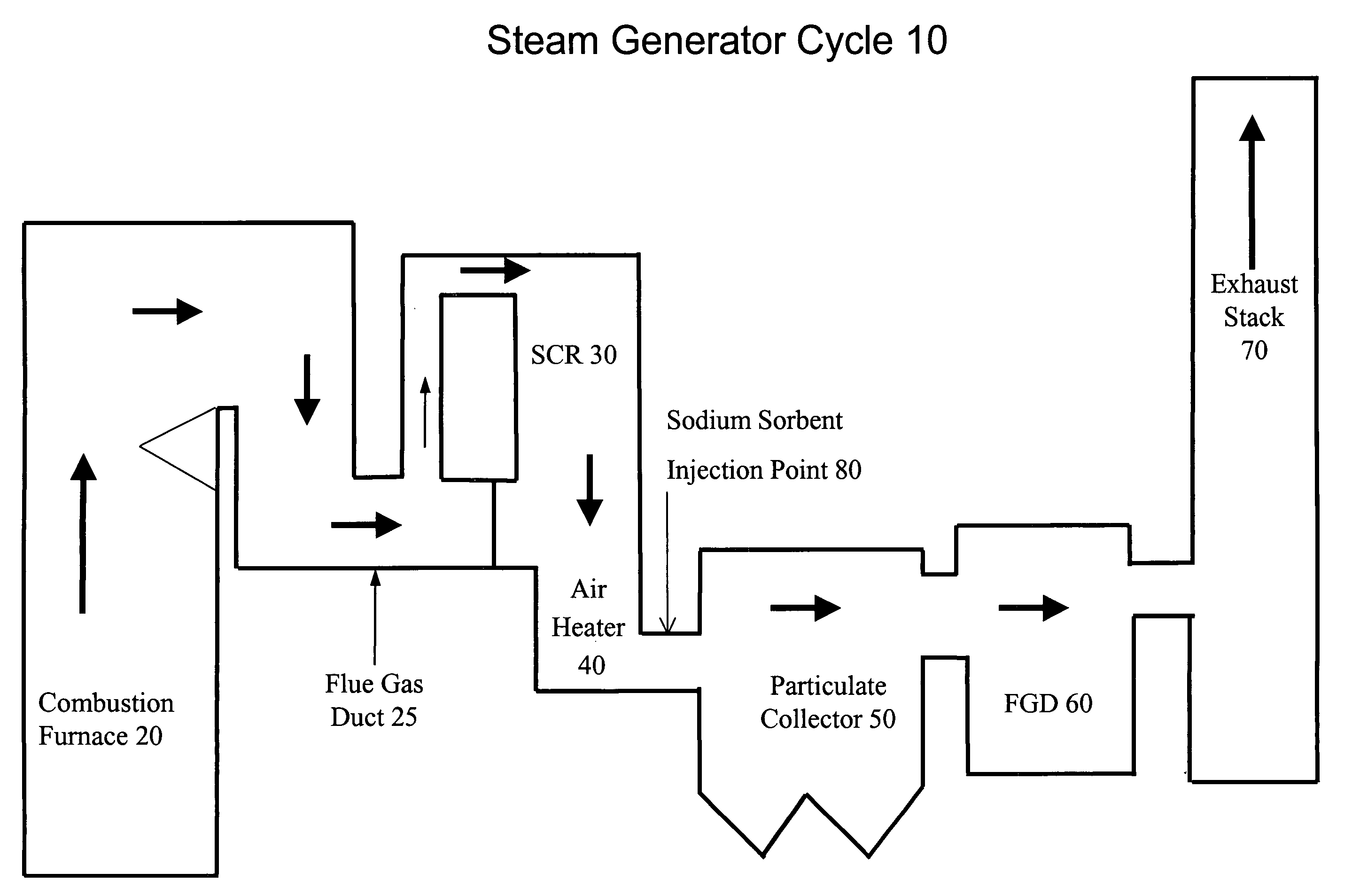
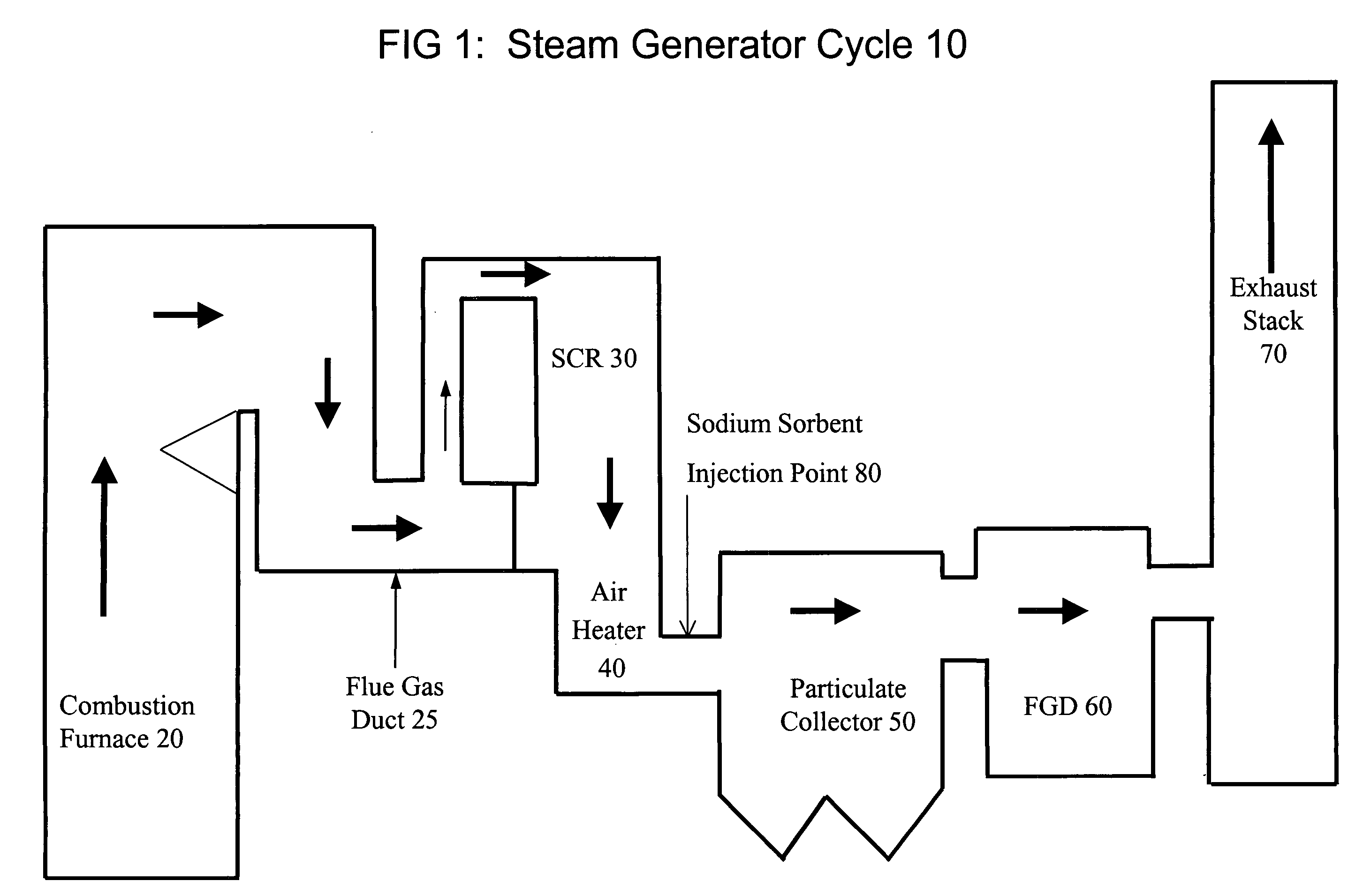
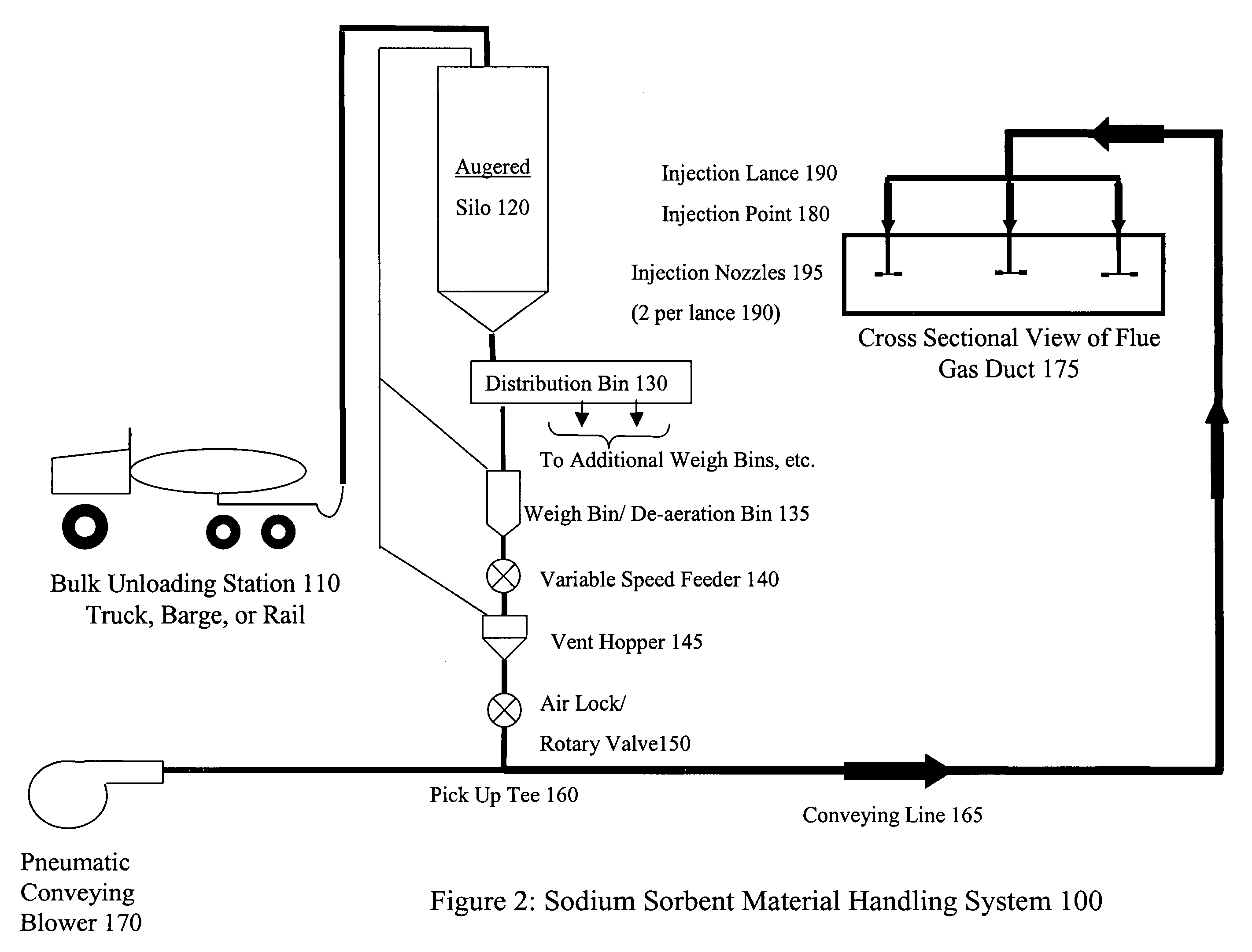
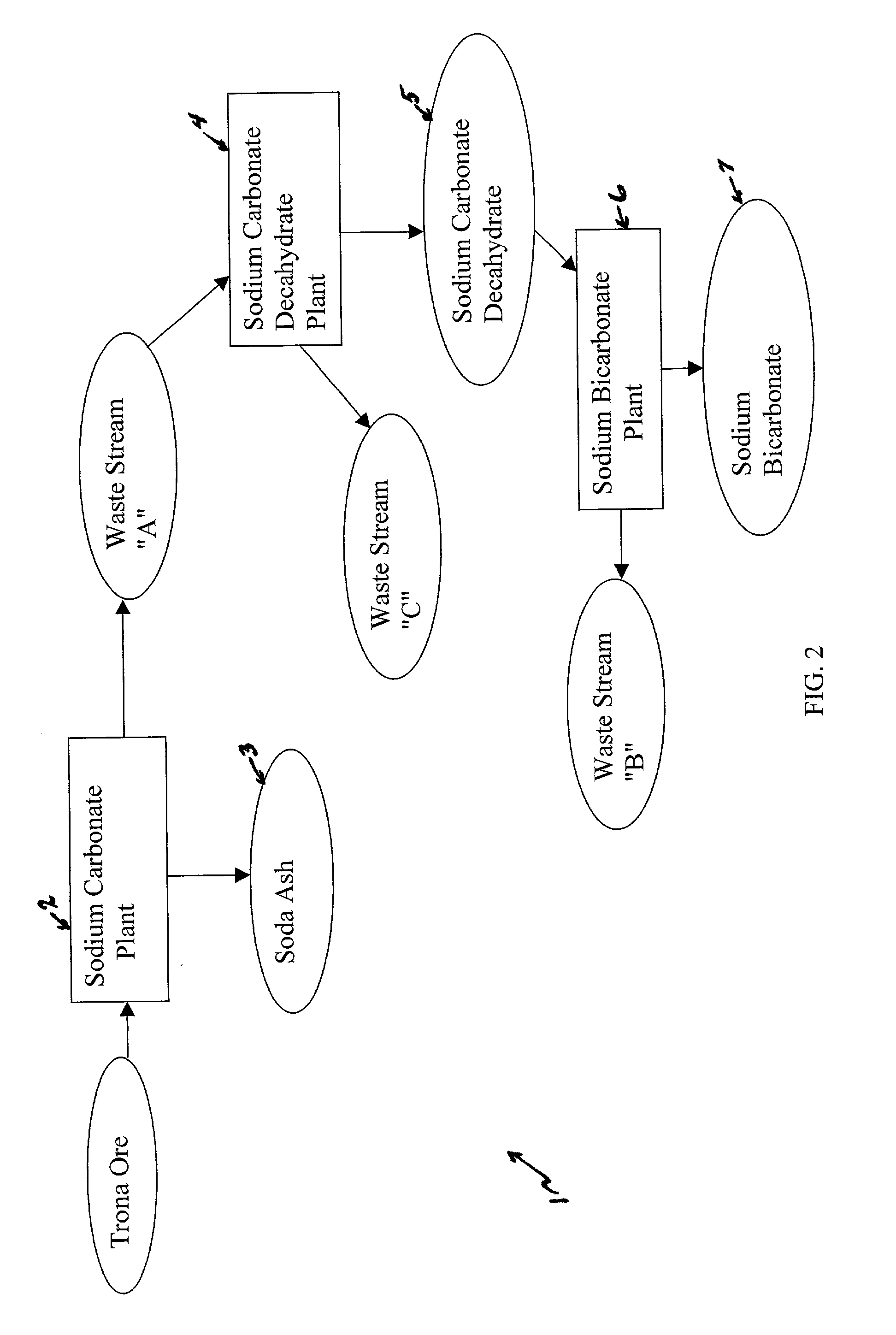
The present invention is a system and method for treating a flue gas stream to remove strong acid compounds selected from the group consisting of hydrofluoric acid (HF), hydrochloric acid (HCl), sulfuric acid (H2SO4), and sulfur trioxide (SO3) by injecting a sodium sorbent selected from the group consisting of sodium sesquicarbonate, sodium carbonate-bicarbonate, trona ore, mechanically refined trona ore, and trona into the flue gas stream, calcining substantially all of the sodium sorbent in the presence of the flue gas stream to form a soda ash, reducing the concentration of the at least one strong acid compound in the flue gas stream by reacting the at least one strong acid compound with the soda ash to form a sodium based by-product; and changing the chemistry of the flue gas stream to reduce the overall average resistivity of the particulate matter.
Owner:AMERICAN ELECTRIC POWER CO INC
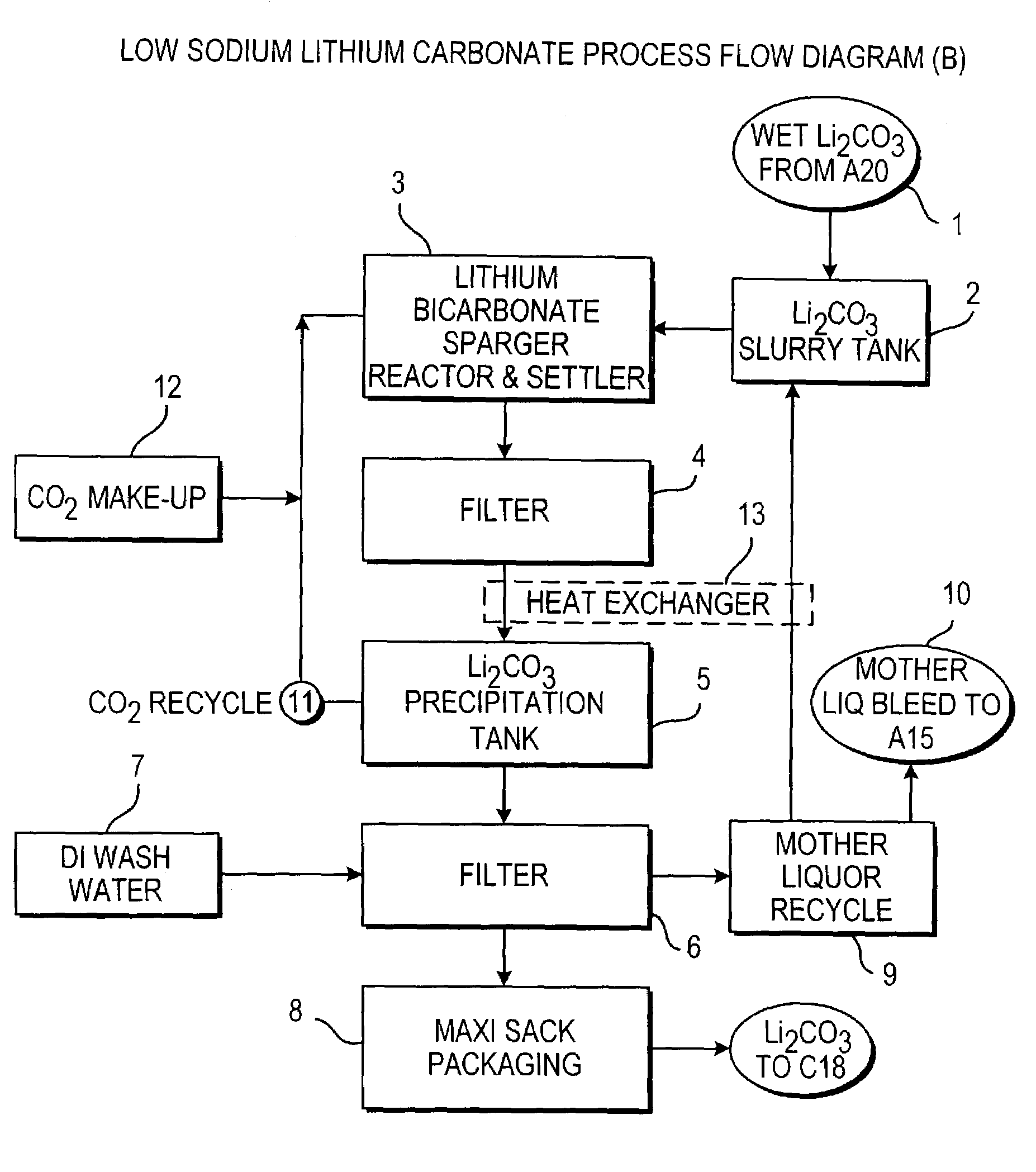
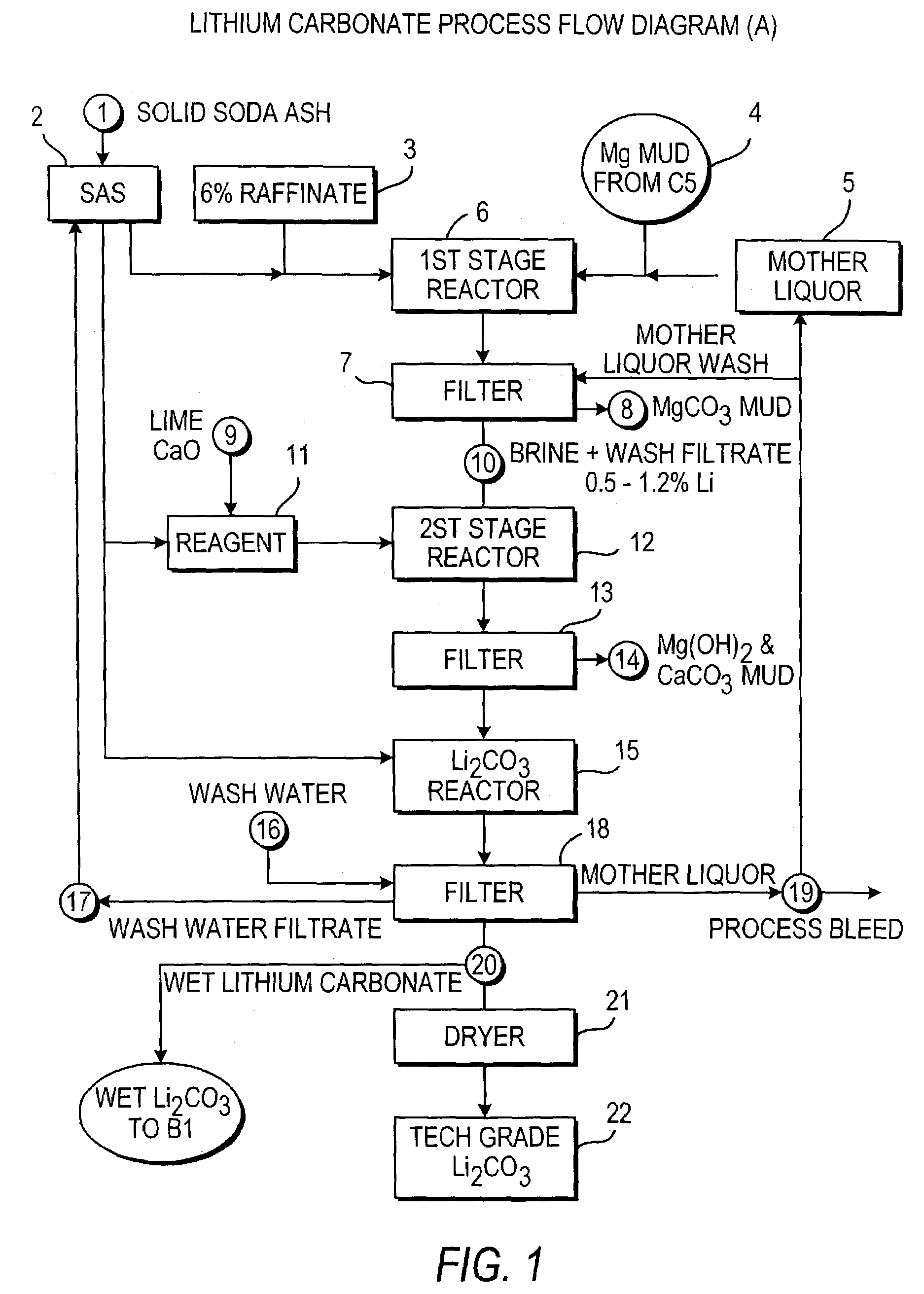
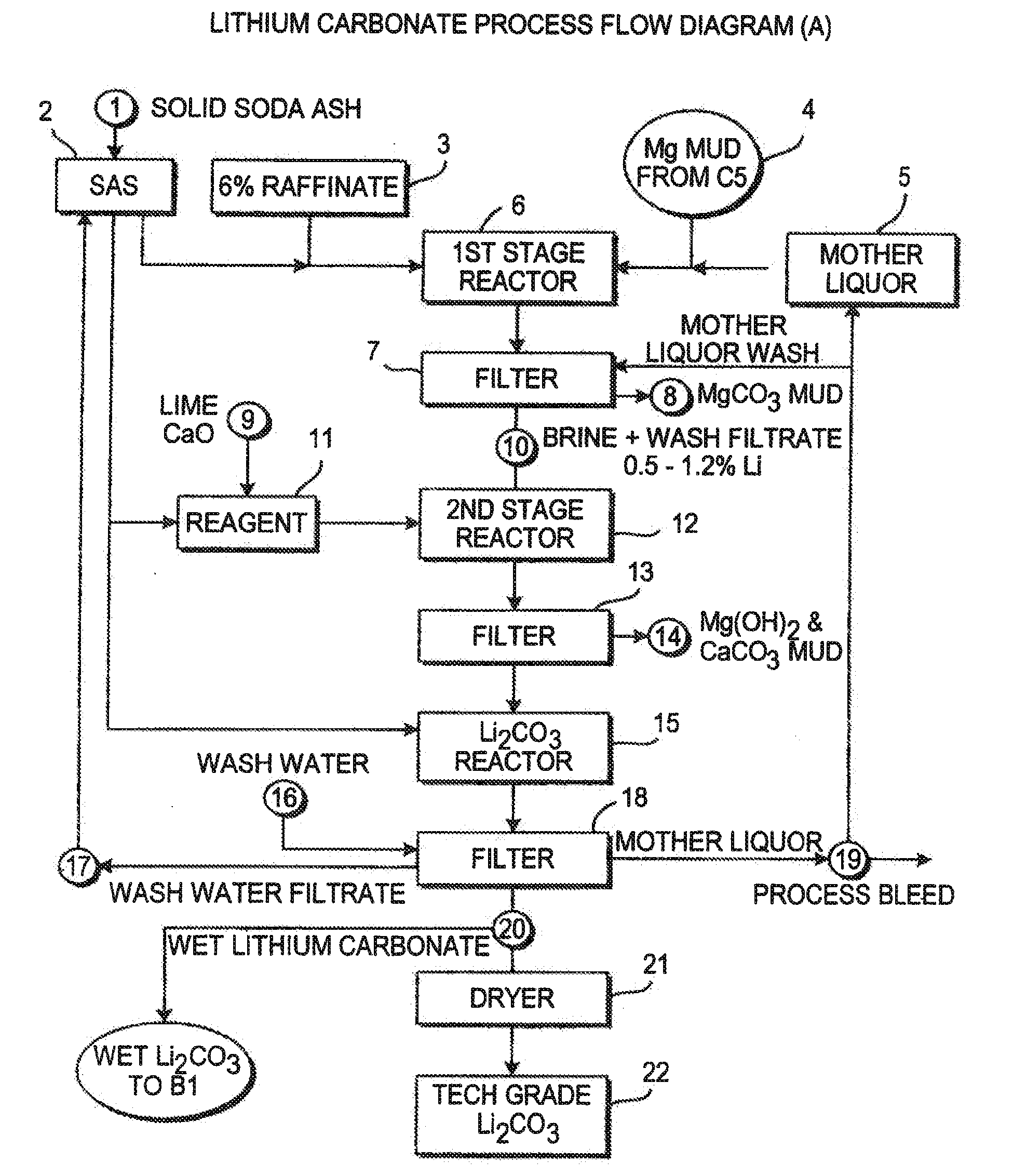
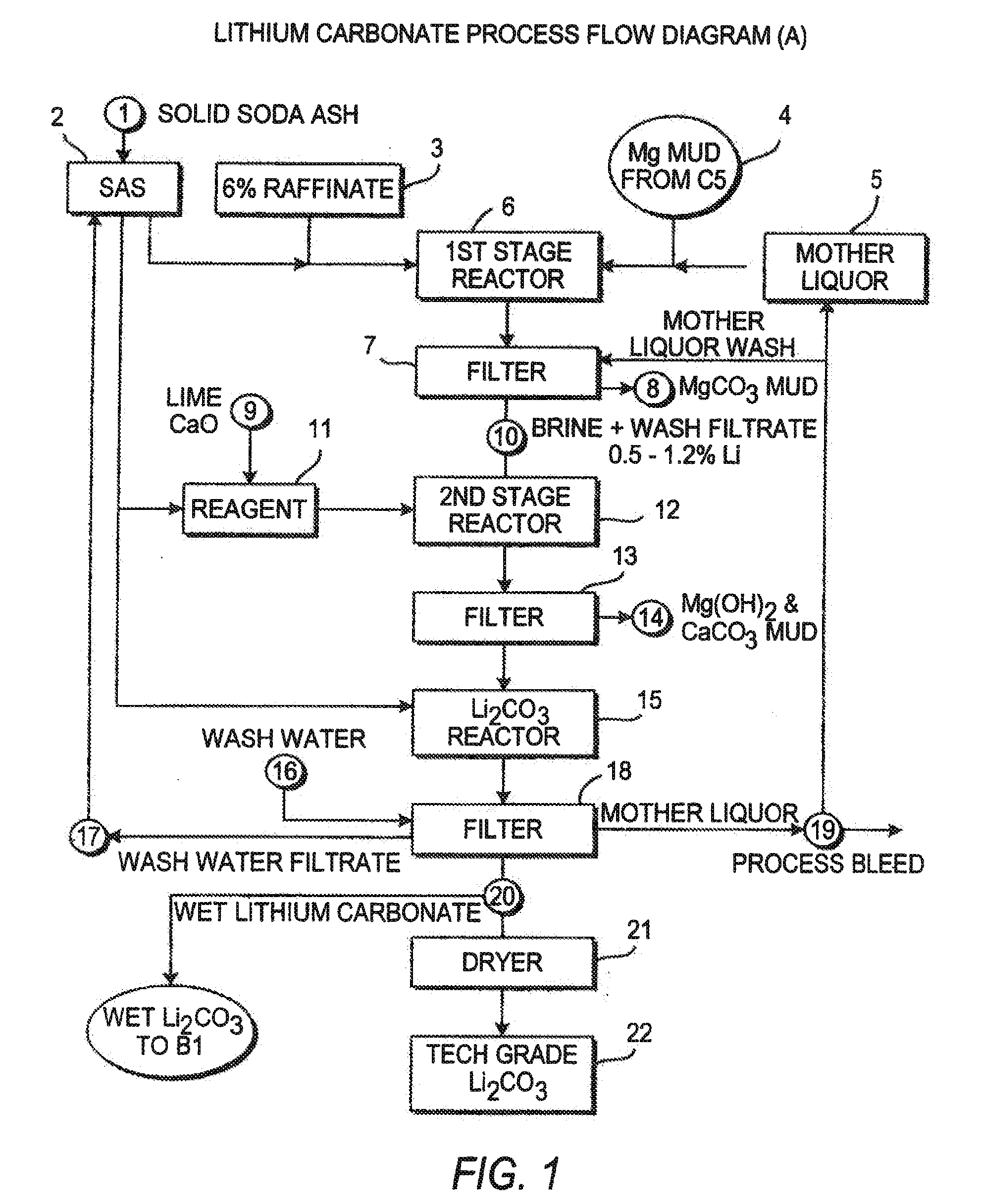
Production of lithium compounds directly from lithium containing brines
InactiveUS7157065B2Reduce in quantityPromote absorptionCalcium/strontium/barium carbonatesChemical/physical/physico-chemical processesSlurryLithium compound
A continuous process for directly preparing high purity lithium carbonate from lithium containing brines by preparing a brine containing about 6.0 wt % lithium and further containing other ions naturally occurring in brines; adding mother liquor containing carbonate to precipitate magnesium; adding a solution of CaO and sodium carbonate to remove calcium and any residual magnesium; precipitating lithium carbonate from the purified brine by adding soda ash solution; filtering to obtain solid lithium carbonate; preparing an aqueous slurry of the lithium carbonate and introducing carbon dioxide gas at a temperature from at least minus 10 to +40° C.; passing the lithium bicarbonate solution through a filter to clarify the solution; introducing said filtered lithium bicarbonate solution into a reactor and adjusting the temperature of the solution to from 60–100° C. to precipitate ultra-pure lithium carbonate.
Owner:ROCKWOOD LITHIUM INC
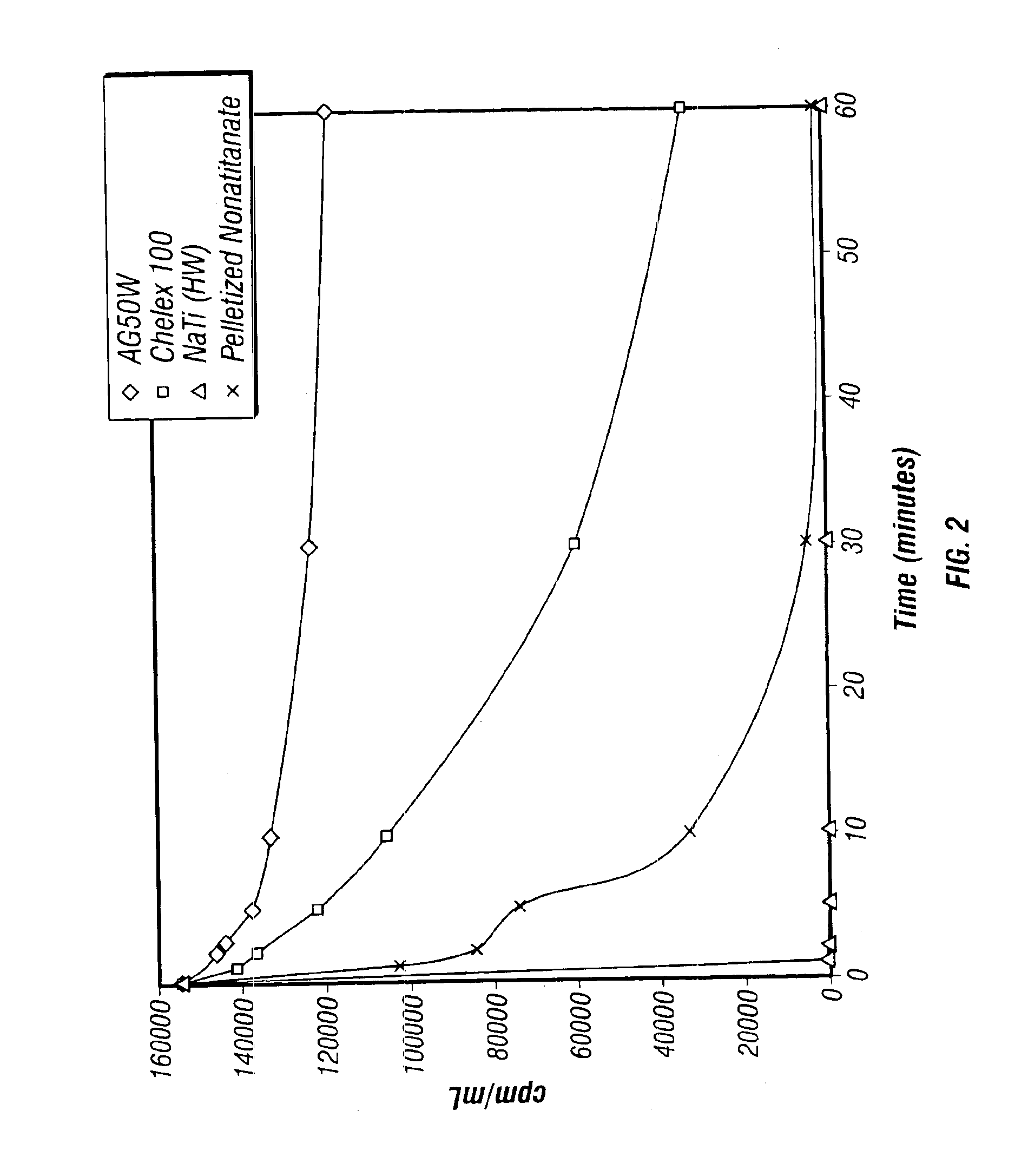
Rubidlum-82 generator based on sodium nonatitanate support, and improved separation methods for the recovery of strontium-82 from irradiated targets
InactiveUS6908598B2Effective recoveryOther chemical processesTransuranic element compoundsLow affinityRubidium
Sodium nonatitanate compositions, a method using the composition for recovery of 82Sr from irradiated targets, and a method using the composition for generating 82Rb. The sodium nonatitanate materials of the invention are highly selective at separating strontium from solutions derived from the dissolution of irradiated target materials, thus reducing target processing times. The compositions also have a very low affinity for rubidium, making it an ideal material for use as a 82Rb generator. Sodium nonatitanate materials of this type both improve the recovery of 82Sr and provide a safer, more effective 82Rb generator system.
Owner:LYNNTECH
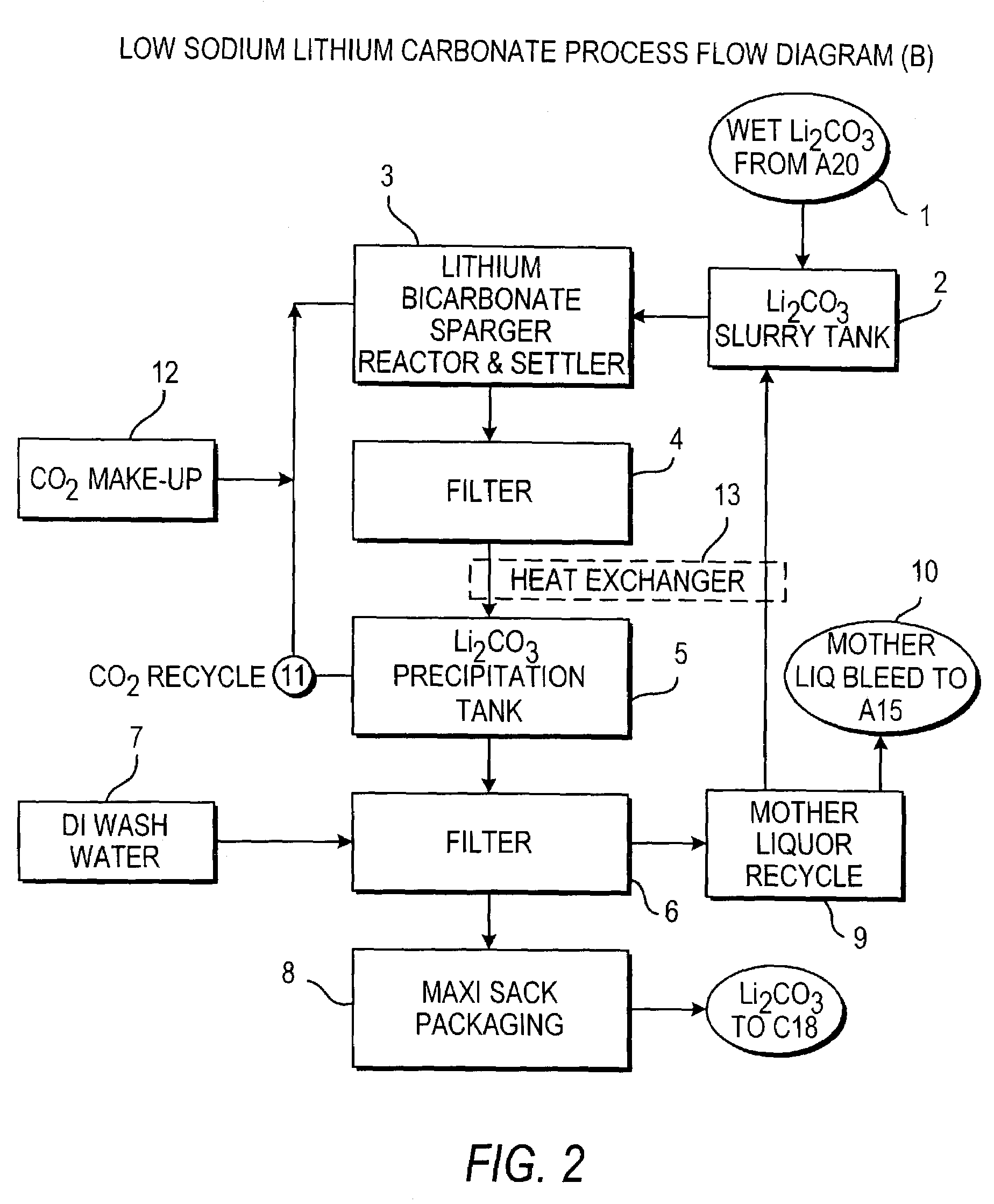
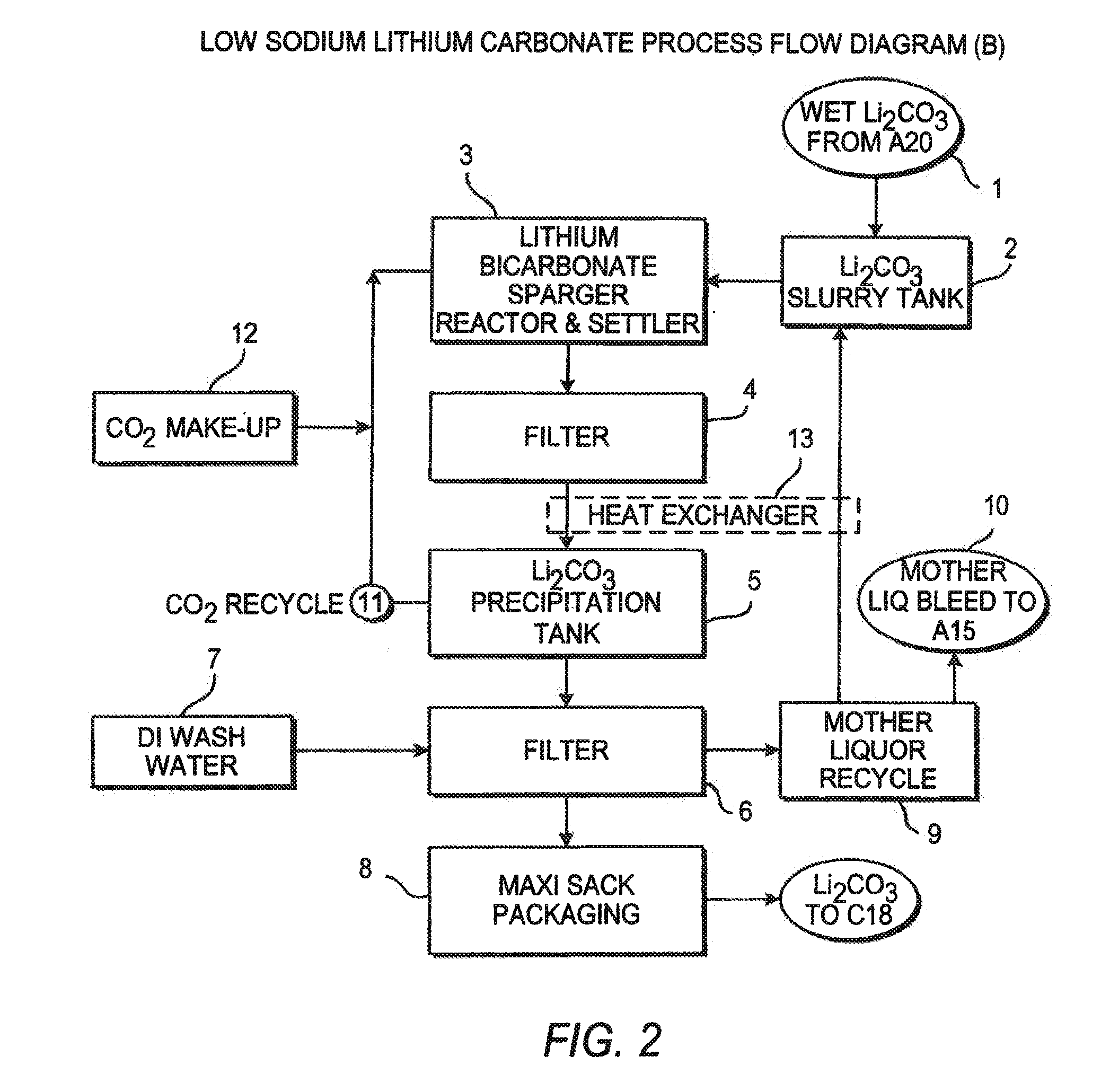
Production of lithium compounds directly from lithium containing brines
InactiveUS20110123427A1Reduce in quantityPromote absorptionVarying alkali metal carbonate water contentRubidium/caesium/francium compoundsLithium chlorideLithium carbonate
Methods and apparatus for the production of low sodium lithium carbonate and lithium chloride from a brine concentrated to about 6.0 wt % lithium are disclosed. Methods and apparatus for direct recovery of technical grade lithium chloride from the concentrated brine are also disclosed.
Owner:ROCKWOOD LITHIUM INC
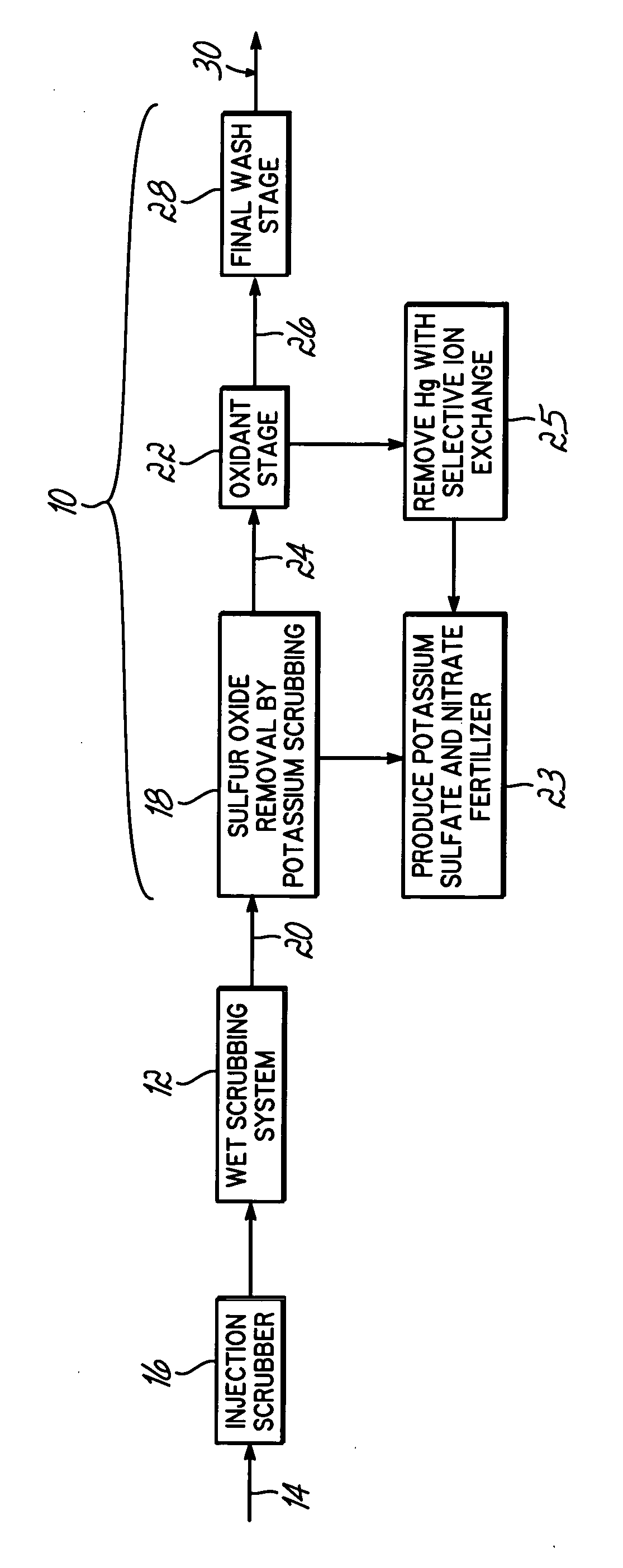
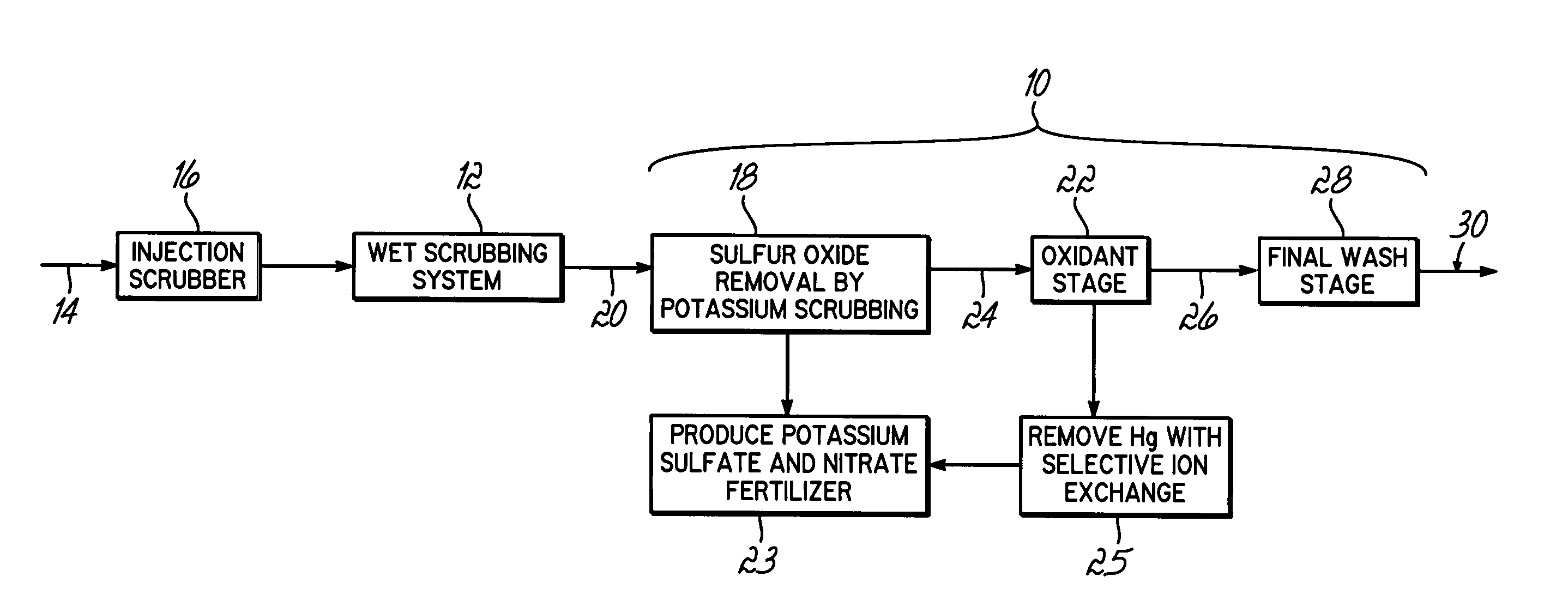
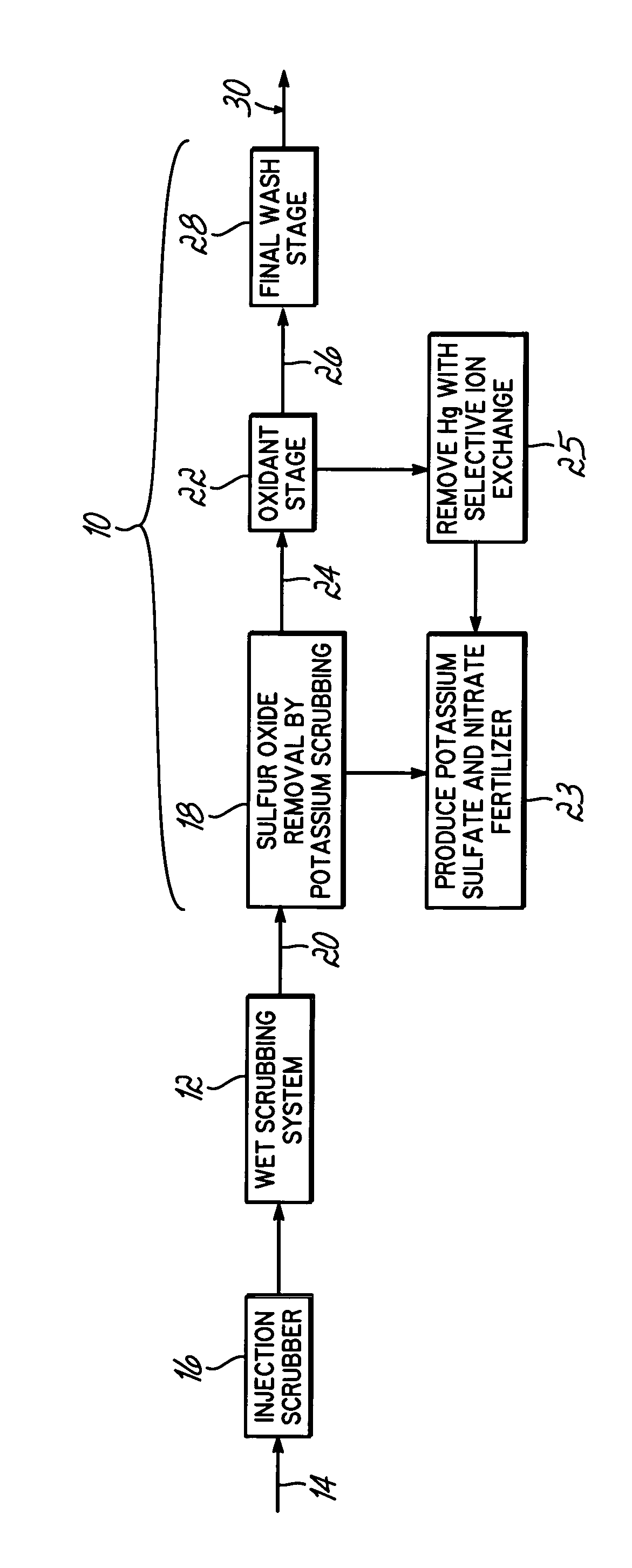
Method for removing sulfur dioxide, mercury, and nitrogen oxides from a gas stream
InactiveUS20060239877A1Low costImprove performanceAmmonium nitratesNitrogen compoundsSorbentPotassium
Methods for scrubbing gas streams to remove acid gases including sulfur dioxide, mercury-containing substances, and / or nitrogen oxides from the gas stream. The gas stream is contacted with a potassium-based sorbent effective for removing at least a portion of the acid gases. The partially cleaned gas stream is then contacted with an oxidant effective to remove at least a portion of the nitrogen oxides and / or mercury-containing substances after partially removing the acid gas substance.
Owner:ENVIROSOLV ENERGY
Method for removing sulfur dioxide, mercury, and nitrogen oxides from a gas stream
InactiveUS7514053B2Improve performanceAvoid pollutionAmmonium nitratesNitrogen compoundsNitrogen oxidesSorbent
Methods for scrubbing gas streams to remove acid gases including sulfur dioxide, mercury-containing substances, and / or nitrogen oxides from the gas stream. The gas stream is contacted with a potassium-based sorbent effective for removing at least a portion of the acid gases. The partially cleaned gas stream is then contacted with an oxidant effective to remove at least a portion of the nitrogen oxides and / or mercury-containing substances after partially removing the acid gas substance.
Owner:ENVIROSOLV ENERGY
Surface and Bulk Modified High Capacity Layered Oxide Cathodes with Low Irreversible Capacity Loss
InactiveUS20090224212A1Large capacityLow costNon-metal conductorsConductive materialCapacity lossCrystal structure
The present invention includes compositions, surface and bulk modifications, and methods of making of (1−x)Li[Li1 / 3Mn2 / 3]O2.xLi[Mn0.5-yNi0.5-yCo2y]O2 cathode materials having an O3 crystal structure with a x value between 0 and 1 and y value between 0 and 0.5, reducing the irreversible capacity loss in the first cycle by surface modification with oxides and bulk modification with cationic and anionic substitutions, and increasing the reversible capacity to close to the theoretical value of insertion / extraction of one lithium per transition metal ion (250-300 mAh / g).
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Comprehensive utilization method for bittern
InactiveCN101691239ARealize closed loopSimple process controlCalcium/strontium/barium carbonatesIodineSimple componentChemistry
The invention relates to a comprehensive utilization method for bittern, which belongs to the technical field of salt chemical engineering. The bittern is a liquid mineral product, and is rich in multiple elements such as potassium, sodium, lithium, boron, bromine, iodine and the like; and at present, in the prior domestic bittern development and utilization, some simple components or components with high additional value in the elements are extracted, and the un-extracted components are discharged along with old bittern to be abandoned so as to cause serious waste of resources and pollute the environment. Through reasonable combination of processes of removing H2S from the bittern, settling magnesium, settling calcium and preparing calcium carbonate, preparing potassium-sodium mixed saltthrough primary salt preparation and secondary salt preparation, extracting potassium chloride through flotation, extracting boron, iodine, bromine, rubidium and cesium through acidification, preparing rubidium chloride and cesium chloride, extracting lithium and the like, the method implements step-by-step ordered extraction of main components; the toil yield of several main components reaches over 95 percent; and the method has the advantages of mutually exclusive loss in component extraction, implementation of closed cycle of processes, no mother liquor discharge, simple process control, low cost, high yield, environmental protection and the like.
Owner:DAZHOU HENGCHENG ENERGY GROUP
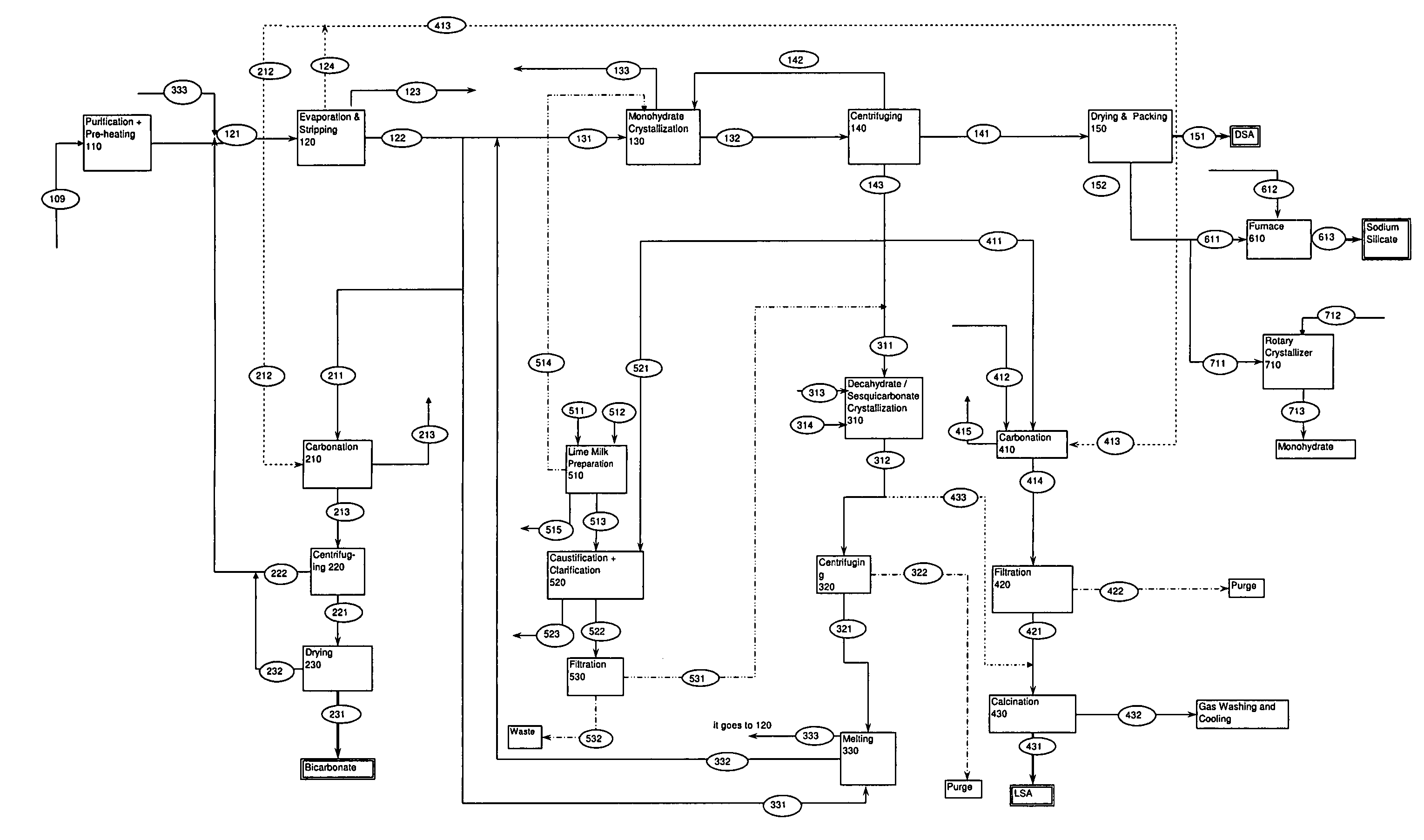
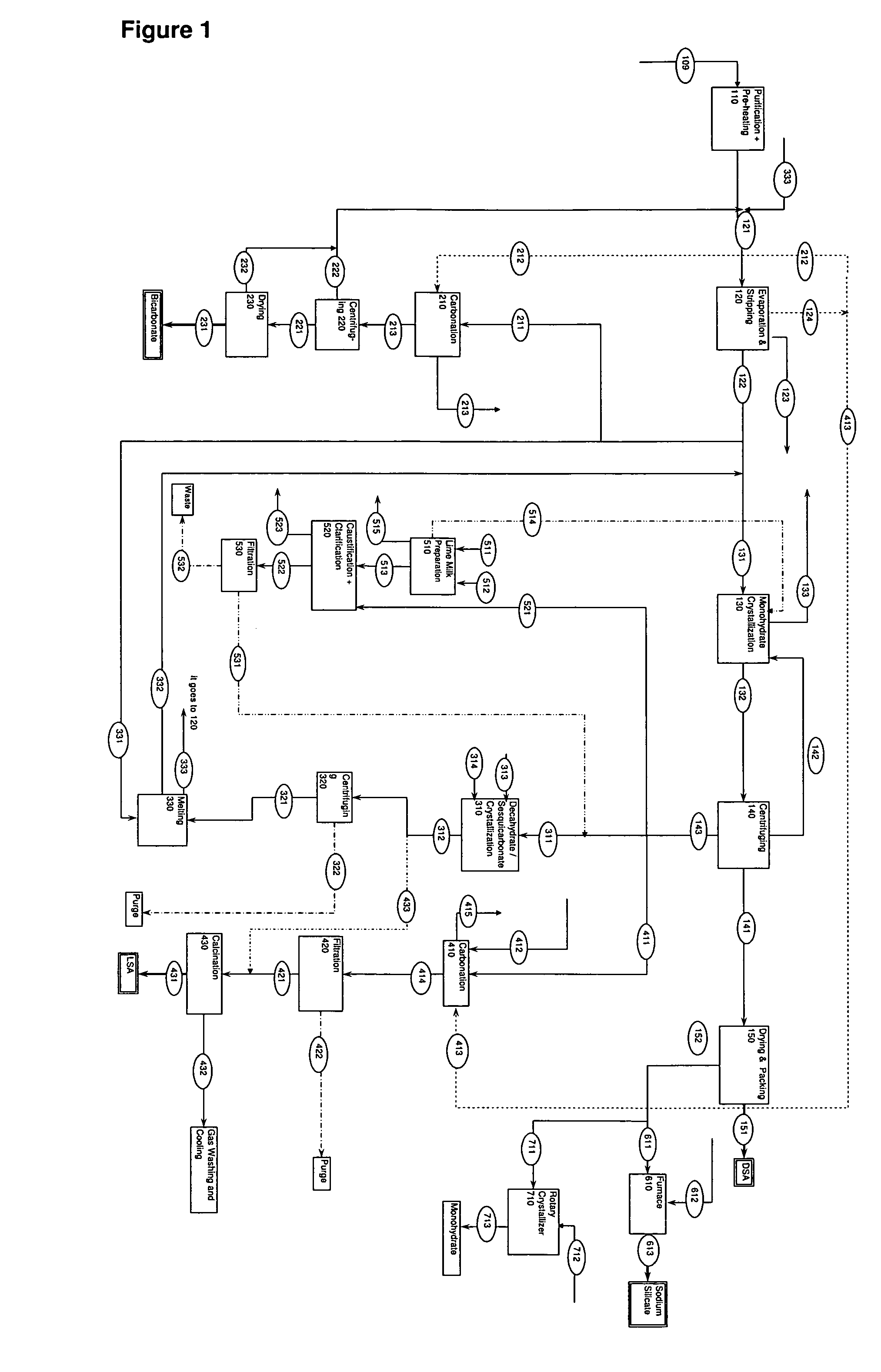
Process for production of dense soda, light soda, sodium bicarbonate and sodium silicate from solutions containing bicarbonate
ActiveUS7507388B2Processing requirementLow production costCrystallization separationAlkali metal silicatesSodium bicarbonateEvaporation
A process related to sodium chemicals production, including the processing of bicarbonate containing solutions obtained by solution mining of trona, nahcolite or wegscheiderite reserves and the lake waters containing bicarbonates, includes the steps of purification, evaporation-decarbonation, crystallization, centrifuging, and drying.
Owner:ETI SODA URETIM PAZARLAMA NAKLIYAT & ELEKTRIK URETIM SANAYI & TICARET A S
Rubidium-82 generator based on sodium nonatitanate support, and improved separation methods for the recovery of strontium-82 from irradiated targets
InactiveUS7476377B2Effective recoveryTransuranic element compoundsOther chemical processesLow affinityRubidium
Sodium nonatitanate compositions, a method using the composition for recovery of 82Sr from irradiated targets, and a method using the composition for generating 82Rb. The sodium nonatitanate materials of the invention are highly selective at separating strontium from solutions derived from the dissolution of irradiated target materials, thus reducing target processing times. The compositions also have a very low affinity for rubidium, making it an ideal material for use as a 82Rb generator. Sodium nonatitanate materials of this type both improve the recovery of 82Sr and provide a safer, more effective 82Rb generator system.
Owner:LYNNTECH
Carbonate recycling in a hydrogen producing reaction
InactiveUS6994839B2Calcium/strontium/barium carbonatesElectrolysis componentsCarbonateMetal hydroxide
A process for producing hydrogen gas from a reaction of an organic substance and a base with a recycling of a carbonate or bicarbonate by-product and a regeneration of the base. In one embodiment, reaction of an organic substance and a base produces hydrogen gas and a metal carbonate. The instant invention provides recycling of the metal carbonate by-product. In a preferred embodiment, the metal carbonate by-product is soluble and recycling includes a three step process. In a first step, the soluble metal carbonate is reacted with a metal hydroxide to form a weakly soluble or insoluble metal carbonate that precipitates in a metathesis reaction. The metal hydroxide reactant of the hydrogen producing reaction is also formed in the metathesis reaction and remains in solution. Precipitation of the carbonate thus permits ready isolation of the carbonate by-product, while leaving behind an aqueous metal hydroxide phase that can be returned to and further utilized in the hydrogen producing reaction. The metal carbonate precipitate of the metathesis reaction is thermally decomposed to form a metal oxide solid in a second step. In a third step, the metal oxide is reacted with water to reform the metal hydroxide reactant of the metathesis reaction. The hydrogen producing reaction and recycling process are sustainable in that the metal hydroxide reactant of each reactant is regenerated in the recycling process. In an alternative embodiment, the hydrogen producing reaction produces a metal carbonate precipitate directly and recycling occurs through thermal decomposition of the metal carbonate to form a metal oxide followed by reaction of the metal oxide with water to reform the metal hydroxide employed in the hydrogen producing reaction. In yet another embodiment, a bicarbonate by-product is formed by a hydrogen producing reaction of an organic substance and a base and bicarbonate recovery occurs by heating the bicarbonate to form a carbonate and recycling according to the instant carbonate recycling process.
Owner:TACTICAL FUEL CELLS
Process for producing lithium-containing composite oxide for positive electrode for lithium secondary battery
ActiveUS20060154146A1Improve featuresSolve the small densityMagnesium halidesCell electrodesAlkaline earth metalNiobium
It is to provide a positive electrode active material for a lithium secondary battery, which has a large volume capacity density and high safety, is excellent in uniform coating properties and is excellent in the charge and discharge cyclic durability and low temperature characteristics even at a high charge voltage. A process for producing a lithium-containing composite oxide represented by the formula LipQqNxMyOzFa (wherein Q is at least one element selected from the group consisting of titanium, zirconium, niobium and tantalum, N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, alkaline earth metal elements and transition metal elements other than the Q element and the N element, 0.9≦p≦1.1, 0<q≦0.03, 0.97≦x<1.00, 0≦y<0.03, 1.9≦z≦2.1, q+x+y=1 and 0≦a≦0.02) from a lithium source, an Q element source and an N element source, and if necessary, at least one source selected from the group consisting of an M element source and a fluorine source, characterized by using as the Q element source an Q element compound aqueous solution having a pH of from 0.5 to 11.
Owner:SUMITOMO CHEM CO LTD
Method for extracting rubidium salt and cesium salt
ActiveCN103787375ASpeed up extractionImprove working environmentLiquid solutions solvent extractionRubidium/caesium/francium compoundsRubidiumSalinity
The invention relates to a method for extracting rubidium salt and cesium salt. The method comprises the steps of 1) regulating a high-salinity solution to obtain an alkaline solution which has a pH value of 11-14; 2) extracting rubidium ions and cesium ions from the alkaline solution obtained from the step 1) in a centrifugal extractor through an organic extracting agent, thus obtaining a loaded organic phase (I) and raffinate; 3) washing the organic phase (I) obtained from the step 2) through washing water to obtain an organic phase (II) and a washing solution; 4) implementing reverse extraction to the loaded organic phase (II) through reverse extraction acid (I) to obtain a rubidium salt reverse extraction solution and an organic phase loaded with cesium ion; and 5) implementing reverse extraction to the organic phase loaded with the cesium salt obtained from the step 4) through reverse extraction acid (II) to obtain a cesium salt reverse extraction solution and a blank organic phase. The extraction method disclosed by the invention is simple and easy to implement, and can be matched with comprehensive development and utilization of a high-salinity medium to produce a rubidium salt and cesium salt product of preset type.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
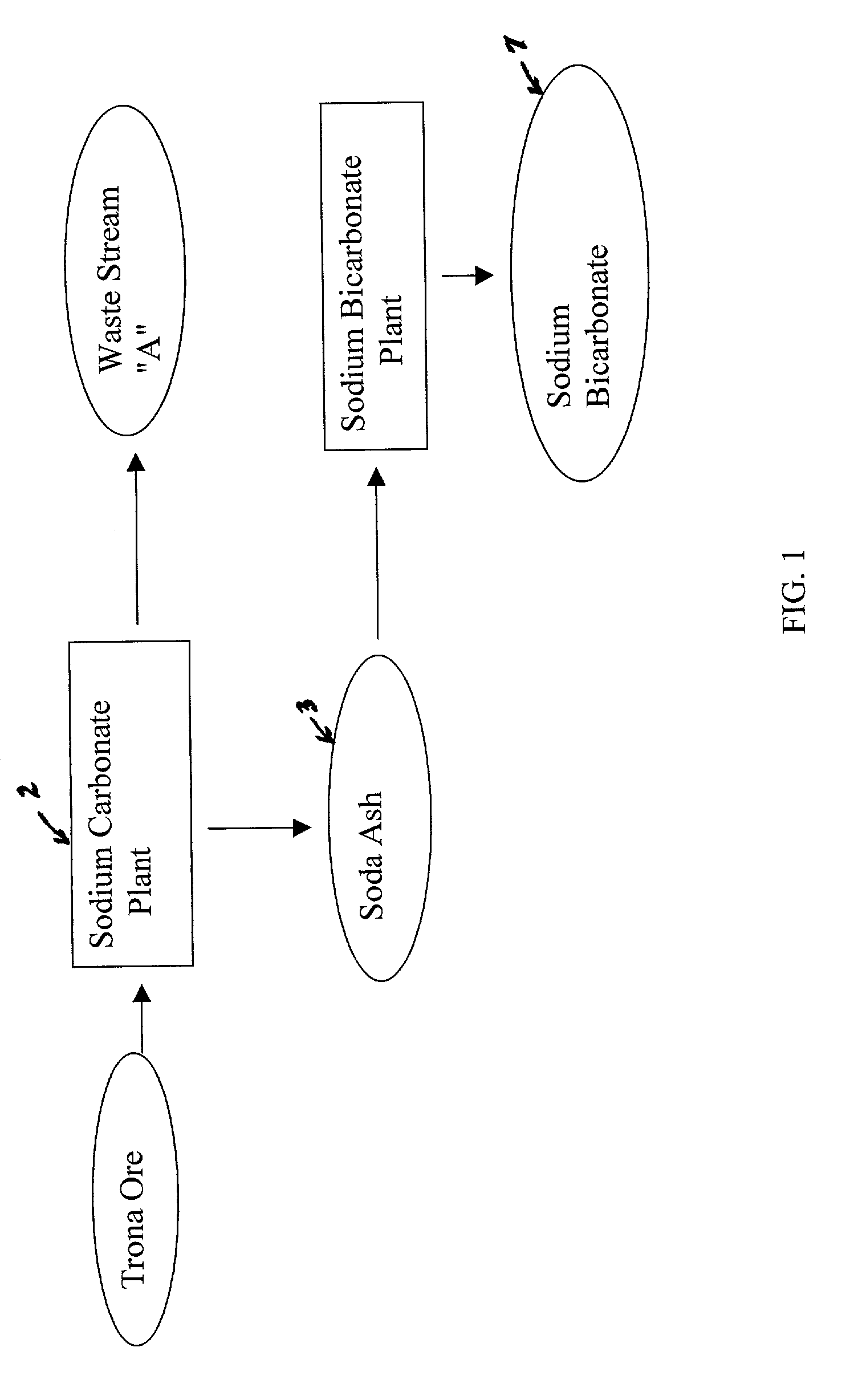
Sodium bicarbonate production method
InactiveUS20030017099A1Improvement in recovered sodium valuePromote recoveryBicarbonate preparationRubidium/caesium/francium compoundsEnvironmental engineeringSodium hydrogencarbonate
A method of producing additional sodium bicarbonate having a high degree of purity and obtaining a net reduction in effluent waste water, as compared to prior processes, when starting from trona ore is disclosed. The process entails utilizing the waste-water effluent stream from the conversion of trona ore to sodium carbonate as the feed for the conversion of sodium carbonate to sodium bicarbonate.
Owner:CHURCH & DWIGHT CO INC
Flame made metal oxides
ActiveUS20040126298A1Improve stabilitySmall crystal sizeMaterial nanotechnologyZirconium oxidesCarboxylic acidSolvent
Described is a method for the production of metal oxides, in particular mixed metal oxides such as ceria / zirconia, and metal oxides obtainable by said method. Due to high enthalpy solvents with a high carboxylic acid content said metal oxides have improved properties. For example ceria / zirconia has excellent oxygen storage capacity at high zirconium levels up to more than 80% of whole metal content.
Owner:EIDGENOSSISCHE TECHN HOCHSCULE ZURICH
Preparation of lithium carbonate from lithium chloride containing brines
ActiveUS9034294B1Chloride preparationSolid sorbent liquid separationLithium carbonateLithium hydroxide
This invention relates to a method for the preparation of lithium carbonate from lithium chloride containing brines. The method can include a silica removal step, capturing lithium chloride, recovering lithium chloride, supplying lithium chloride to an electrochemical cell and producing lithium hydroxide, contacting the lithium hydroxide with carbon dioxide to produce lithium carbonate.
Owner:TERRALITHIUM LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com